Антагонисты гонадотропин‐рилизинг гормона при вспомогательных репродуктивных технологиях
Appendices
Appendix 1. MDSG specialised register
MDSG Search string for HA412 Procite platform
Keywords CONTAINS "GnRH antagonist" or "GnRh antagonists" or "Antagon" or "ceterolix" or "cetrolix" or "cetrorelix" or "cetrotide" or "Ganirelix" or "Luteinising hormone releasing hormone" or "Lutenising hormone releasing hormone" or "LHRH antagonists" or Title CONTAINS "GnRH antagonist" or "GnRh antagonists" or "Antagon" or "ceterolix" or "cetrolix" or "cetrorelix" or "cetrotide" or "Ganirelix" or "Luteinising hormone releasing hormone" or "Lutenising hormone releasing hormone" or "LHRH antagonists"
AND
Keywords CONTAINS "GnRH a", "GnRH agonist" or "GnRH agonist short protocol" or "GnRH agonist vs antagonist" or "GnRH agonists" or "GnRHa" or "GnRHa‐gonadotropin" or"Gonadotrophin releasing agonist" or "buserelin" or "Buserelin Acetate" or "buserelin naferelin" or "busereline" or "Goserelin" or "goserelin acetate" or "Gosereline " or "Leuprolide" or "leuprolide acetate" or "leuprolide depot" or "leuprorelin" or "leuprolin" or "leuprorelin acetate" or "Nafarelin" or "Nafarelin Study Group" or "triptoielin" or "triptoreline" or "triptoreline pamoat" or "triptorelyn" or "triptrolein" or "Lupron" or "Zoladex" or "deslorelin" or "decapeptyl" or "decapeptyl‐daily" or "decapeptyl‐depot" or Title CONTAINS "GnRH a", "GnRH agonist" or "GnRH agonist short protocol" or "GnRH agonist vs antagonist" or "GnRH agonists" or "GnRHa" or "GnRHa‐gonadotropin" or"Gonadotrophin releasing agonist" or "buserelin" or "Buserelin Acetate" or "buserelin naferelin" or "busereline" or "Goserelin" or "goserelin acetate" or "Gosereline " or "Leuprolide" or "leuprolide acetate" or "leuprolide depot" or "leuprorelin" or "leuprolin" or "leuprorelin acetate" or "Nafarelin" or "Nafarelin Study Group" or "triptoielin" or "triptoreline" or "triptoreline pamoat" or "triptorelyn" or "triptrolein" or "Lupron" or "Zoladex" or "deslorelin" or "decapeptyl" or "decapeptyl‐daily" or "decapeptyl‐depot"
Appendix 2. Ovid Cochrane Central Register of Controlled Trials (CENTRAL)
From inception to April 2015
1 Hormone Antagonists/ (305)
2 gonadotropin releasing hormone antagonist$.tw. (87)
3 gonadotrophin releasing hormone antagonist$.tw. (29)
4 GnRH antagonist$.tw. (555)
5 Gn‐RH antagonist$.tw. (0)
6 (Cetrorelix or Cetrotide$).tw. (139)
7 Ganirelix.tw. (81)
8 (Abarelix or Plenaxis).tw. (11)
9 Antagon.tw. (10)
10 Degarelix.tw. (29)
11 or/1‐10 (855)
12 exp gonadotropin‐releasing hormone/ or exp buserelin/ or exp goserelin/ or exp leuprolide/ or exp nafarelin/ or exp triptorelin/ (1885)
13 gonadotropin releasing hormone agonist$.tw. (359)
14 gonadotrophin releasing hormone agonist$.tw. (146)
15 GnRH agonist$.tw. (796)
16 Gn‐RH agonist$.tw. (4)
17 (buserelin or goserelin).tw. (668)
18 (leuprolide or nafarelin).tw. (536)
19 triptorelin.tw. (196)
20 (Lupron or Eligard).tw. (37)
21 (Suprefact or Suprecor).tw. (9)
22 Synarel.tw. (3)
23 Supprelin.tw. (0)
24 Zoladex.tw. (227)
25 deslorelin.tw. (9)
26 Suprelorin.tw. (0)
27 Ovuplant.tw. (0)
28 (decapeptyl or trelstar).tw. (58)
29 (profact or receptal).tw. (4)
30 suprecur.tw. (0)
31 tiloryth.tw. (0)
32 (GNRH‐a or GNRH a).tw. (1393)
33 or/12‐32 (3280)
34 11 and 33 (520)
Appendix 3. Ovid MEDLINE(R)
From inception to April 2015
1 Hormone Antagonists/ (4693)
2 gonadotropin releasing hormone antagonist$.tw. (480)
3 gonadotrophin releasing hormone antagonist$.tw. (125)
4 GnRH antagonist$.tw. (2080)
5 Gn‐RH antagonist$.tw. (7)
6 (Cetrorelix or Cetrotide$).tw. (448)
7 Ganirelix.tw. (136)
8 (Abarelix or Plenaxis).tw. (53)
9 Antagon.tw. (17)
10 Degarelix.tw. (114)
11 or/1‐10 (6692)
12 exp gonadotropin‐releasing hormone/ or exp buserelin/ or exp goserelin/ or exp leuprolide/ or exp nafarelin/ or exp triptorelin/ (29123)
13 gonadotropin releasing hormone agonist$.tw. (1745)
14 gonadotrophin releasing hormone agonist$.tw. (467)
15 GnRH agonist$.tw. (3564)
16 Gn‐RH agonist$.tw. (52)
17 (buserelin or goserelin).tw. (2026)
18 (leuprolide or nafarelin).tw. (1812)
19 triptorelin.tw. (563)
20 (Lupron or Eligard).tw. (163)
21 (Suprefact or Suprecor).tw. (24)
22 Synarel.tw. (12)
23 Supprelin.tw. (2)
24 Zoladex.tw. (373)
25 deslorelin.tw. (204)
26 Suprelorin.tw. (16)
27 Ovuplant.tw. (11)
28 (decapeptyl or trelstar).tw. (208)
29 (profact or receptal).tw. (28)
30 suprecur.tw. (5)
31 tiloryth.tw. (0)
32 (GNRH‐a or GNRH a).tw. (937)
33 or/12‐32 (31184)
34 11 and 33 (2358)
35 randomized controlled trial.pt. (392594)
36 controlled clinical trial.pt. (89288)
37 randomized.ab. (317546)
38 placebo.tw. (165796)
39 clinical trials as topic.sh. (172358)
40 randomly.ab. (229154)
41 trial.ti. (136960)
42 (crossover or cross‐over or cross over).tw. (63745)
43 or/35‐42 (975714)
44 (animals not (humans and animals)).sh. (3933883)
45 43 not 44 (898609)
46 34 and 45 (500)
Appendix 4. Ovid EMBASE
From inception to April 2015
1 Hormone Antagonist/ (1475)
2 Gonadorelin Antagonist/ (4507)
3 gonadotropin releasing hormone antagonist$.tw. (533)
4 Gnrh Antagonist$.tw. (2984)
5 Luteinizing Hormone Releasing Hormone Antagonist$.tw. (88)
6 Lhrh Antagonist$.tw. (354)
7 Cetrorelix.tw. (670)
8 cetrorelix/ or ganirelix/ (2218)
9 ganirelix.tw. (306)
10 Cetrotide.tw. (636)
11 Antagon.tw. (118)
12 Orgalutr?n.tw. (397)
13 Degarelix.tw. (226)
14 or/1‐13 (7917)
15 Gonadorelin Agonist/ (11188)
16 GnRH agonist$.tw. (4929)
17 gonadotropin releasing hormone agonist$.tw. (2015)
18 Lhrh Agonist$.tw. (1323)
19 Luteinizing Hormone Releasing Hormone Agonist$.tw. (562)
20 TRIPTORELIN/ (4176)
21 Triptorelin.tw. (824)
22 (Arvekap or Decapeptyl or Detryptorelin or Trelstar or Tryptorelin).tw. (1772)
23 BUSERELIN/ (4084)
24 Buserelin.tw. (1473)
25 (Bigonist or Busereline or Receptal or Superfact or Suprefact).tw. (1135)
26 (GNRH‐a or GNRH a).tw. (1127)
27 or/15‐26 (19742)
28 14 and 27 (2960)
29 Clinical Trial/ (843206)
30 Randomized Controlled Trial/ (368416)
31 exp randomization/ (66003)
32 Single Blind Procedure/ (20039)
33 Double Blind Procedure/ (119722)
34 Crossover Procedure/ (42461)
35 Placebo/ (254717)
36 Randomi?ed controlled trial$.tw. (114462)
37 Rct.tw. (16650)
38 random allocation.tw. (1399)
39 randomly allocated.tw. (22089)
40 allocated randomly.tw. (2010)
41 (allocated adj2 random).tw. (721)
42 Single blind$.tw. (15600)
43 Double blind$.tw. (149516)
44 ((treble or triple) adj blind$).tw. (439)
45 placebo$.tw. (212288)
46 prospective study/ (286502)
47 or/29‐46 (1449548)
48 case study/ (31233)
49 case report.tw. (279055)
50 abstract report/ or letter/ (920165)
51 or/48‐50 (1224269)
52 47 not 51 (1410589)
53 28 and 52 (929)
Appendix 5. Ovid PsycINFO
PsycINFO <1806 to April 2015>
1 gonadotrop?in releasing hormone antagonist$.tw. (10)
2 GnRH antagonist$.tw. (20)
3 (Cetrorelix or Cetrotide$).tw. (5)
4 (Ganirelix or Degarelix).tw. (3)
5 or/1‐4 (29)
6 gonadotrop?in releasing hormone agonist$.tw. (58)
7 GnRH agonist$.tw. (57)
8 (buserelin or goserelin).tw. (27)
9 (leuprolide or nafarelin).tw. (73)
10 triptorelin.tw. (24)
11 (Lupron or Eligard).tw. (15)
12 Zoladex.tw. (4)
13 deslorelin.tw. (5)
14 (decapeptyl or trelstar).tw. (2)
15 (GnRH‐a or GNRH a).tw. (8)
16 or/6‐15 (195)
17 5 and 16 (7)
18 random.tw. (43340)
19 control.tw. (336514)
20 double‐blind.tw. (18756)
21 clinical trials/ (8577)
22 placebo/ (4049)
23 exp Treatment/ (612193)
24 or/18‐23 (938572)
25 17 and 24 (3)
Appendix 6. EBSCO CINAHL
CINAHL search strategy for HA412 28.04.15
| # | Query | Results |
| S34 | S21 AND S33 | 56 |
| S33 | S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 | 956,465 |
| S32 | TX allocat* random* | 4,252 |
| S31 | (MH "Quantitative Studies") | 13,346 |
| S30 | (MH "Placebos") | 9,191 |
| S29 | TX placebo* | 33,691 |
| S28 | TX random* allocat* | 4,252 |
| S27 | (MH "Random Assignment") | 39,039 |
| S26 | TX randomi* control* trial* | 86,342 |
| S25 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 765,037 |
| S24 | TX clinic* n1 trial* | 171,259 |
| S23 | PT Clinical trial | 77,774 |
| S22 | (MH "Clinical Trials+") | 186,608 |
| S21 | S9 AND S20 | 92 |
| S20 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 | 805 |
| S19 | (MM "Goserelin") | 93 |
| S18 | (MM "Leuprolide") | 124 |
| S17 | TX (GNRH‐a or GNRH a) | 129 |
| S16 | TX (decapeptyl or trelstar) | 8 |
| S15 | TX triptorelin or TX Zoladex | 61 |
| S14 | TX (leuprolide or nafarelin) | 286 |
| S13 | TX (buserelin or goserelin) | 246 |
| S12 | TX GnRH agonist* | 160 |
| S11 | TX gonadotrophin releasing hormone agonist* | 37 |
| S10 | TX gonadotropin releasing hormone agonist* | 190 |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 | 159 |
| S8 | TX Degarelix | 35 |
| S7 | TX Antagon | 2 |
| S6 | TX (Abarelix or Plenaxis) | 8 |
| S5 | TX Ganirelix | 13 |
| S4 | TX (Cetrorelix or Cetrotide) | 14 |
| S3 | TX GnRH antagonist* | 86 |
| S2 | TX gonadotrophin releasing hormone antagonist* | 16 |
| S1 | TX gonadotropin releasing hormone antagonist* | 47 |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
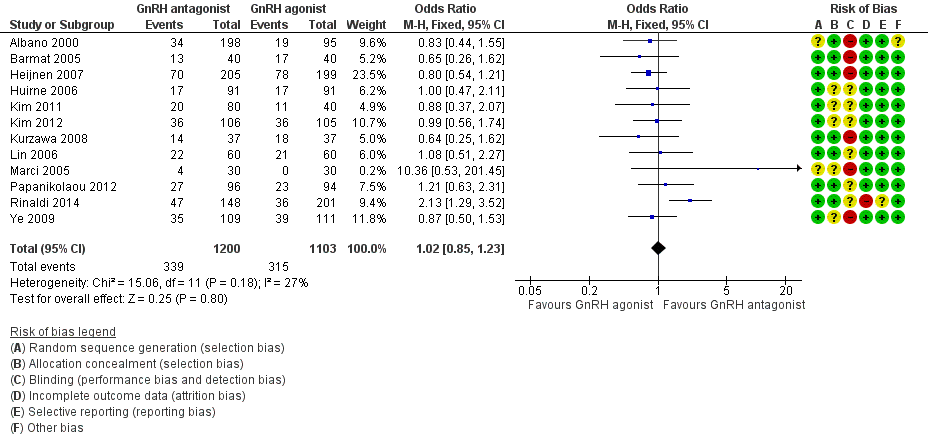
Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.1 Live birth rate per woman randomised.

Funnel plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.1 Live birth rate per woman randomised.
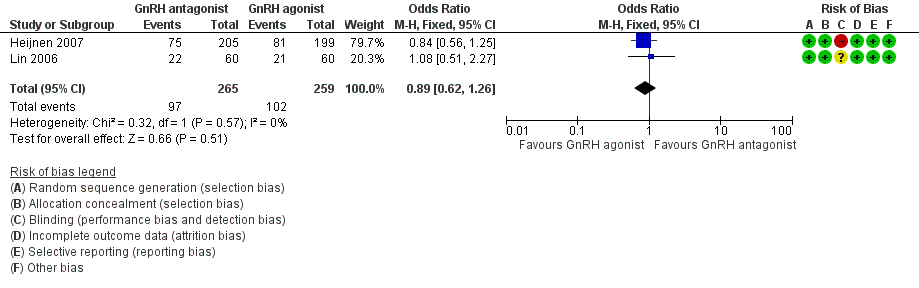
Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.2 Live birth rate per woman randomised ‐ minimal stimulation.

Forest plot of comparison: 1 GnRH antagonist versus long‐course GnRH agonist, outcome: 1.3 Live birth rate per woman randomised ‐ grouped by trigger.
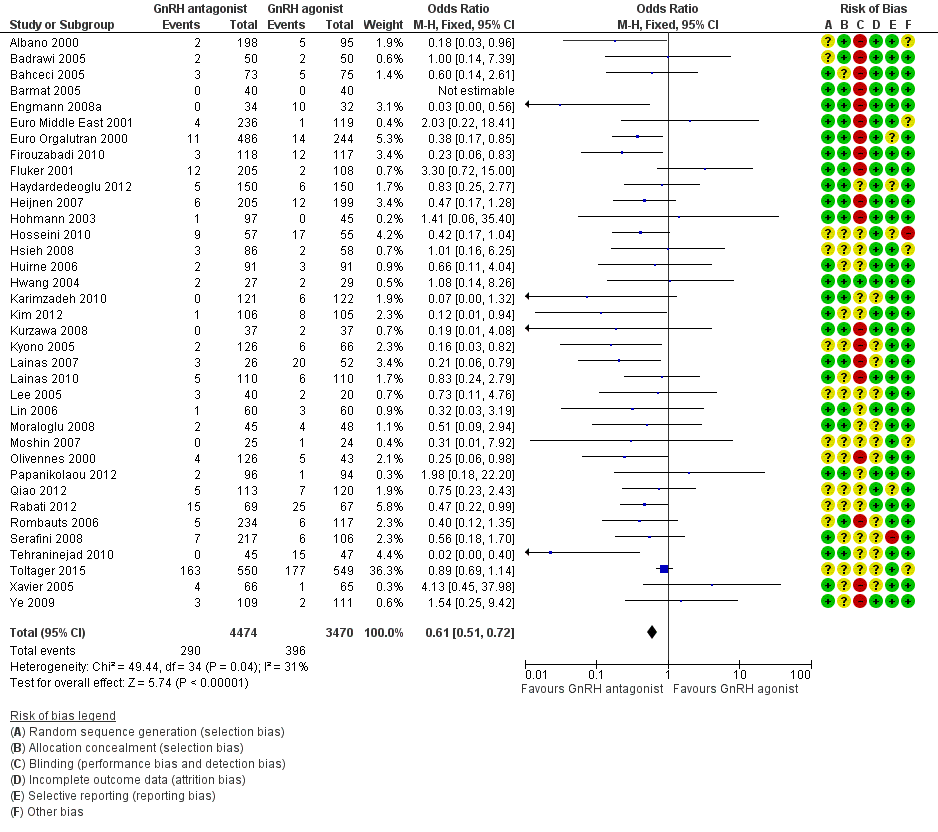
Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.4 Ovarian hyperstimulation per woman randomised ‐ all women.

Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 1 Live birth rate per woman randomised.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 2 Live birth rate per woman randomised ‐ minimal stimulation.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 3 Live birth rate per woman randomised ‐ grouped by trigger.
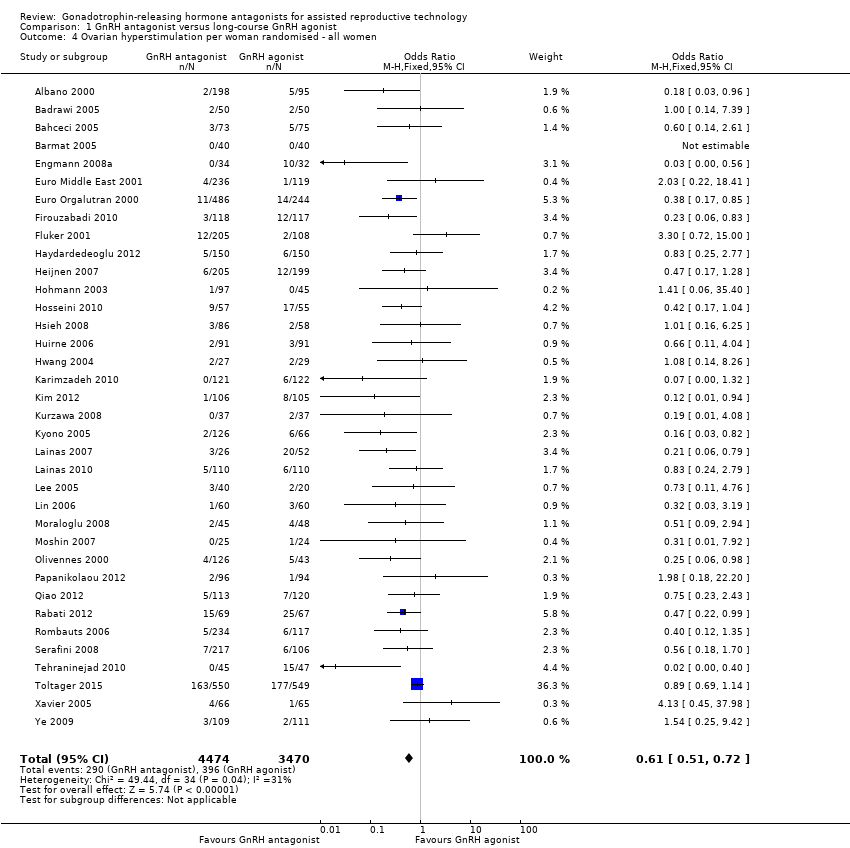
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 4 Ovarian hyperstimulation per woman randomised ‐ all women.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 6 Ongoing pregnancy rate per woman randomised ‐ all women.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 7 Ongoing pregnancy rate per woman randomised ‐ minimal stimulation.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 8 Ongoing pregnancy rate per women randomised ‐ grouped by trigger.
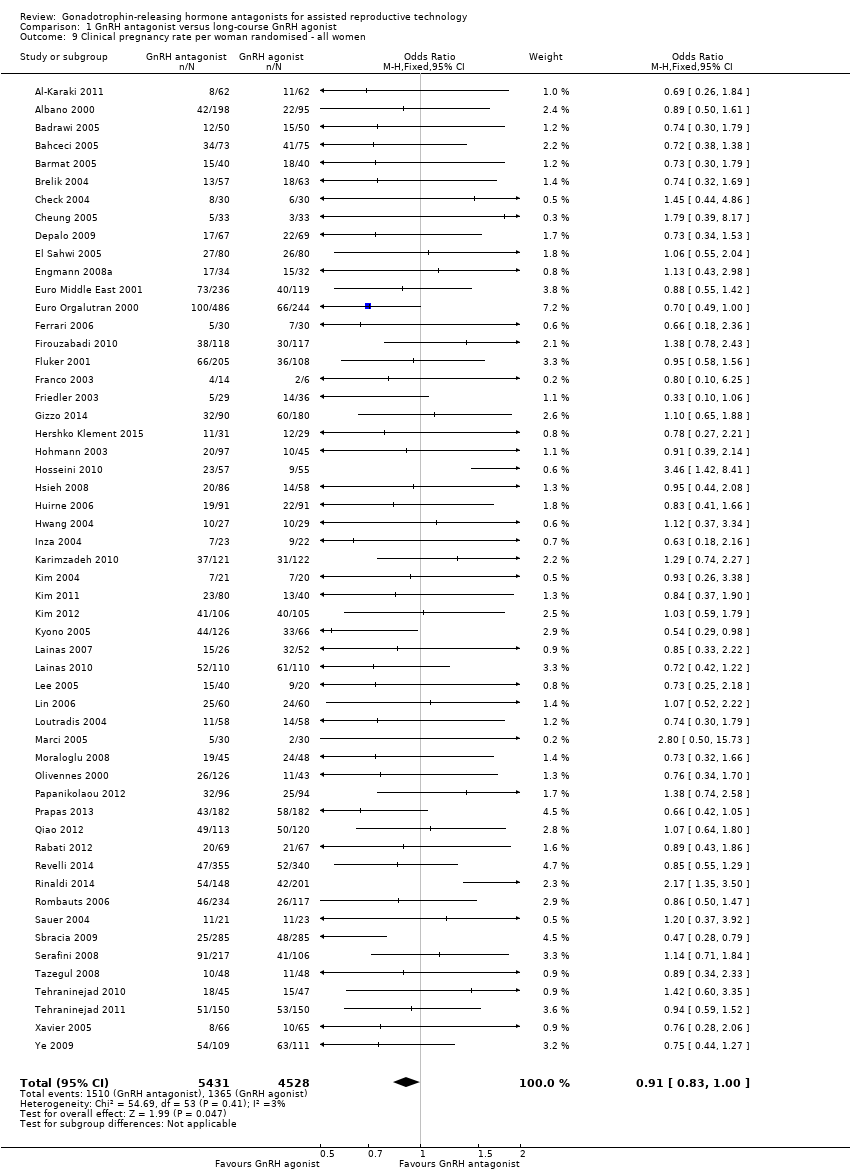
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 9 Clinical pregnancy rate per woman randomised ‐ all women.
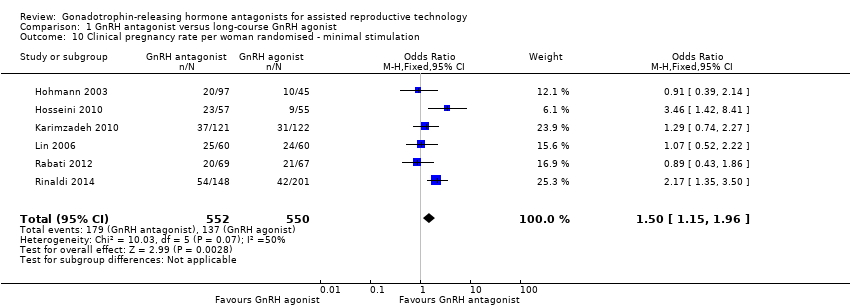
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 10 Clinical pregnancy rate per woman randomised ‐ minimal stimulation.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 11 Miscarriage rate per woman randomised.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 12 Miscarriage rate per clinical pregnancy.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 13 Cycle cancellation rate per woman randomised.
| GnRH antagonist compared to long‐course GnRH agonist for assisted reproductive technology (ART) | ||||||
| Population: women undergoing assisted reproductive technology (ART) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long course GnRH agonist | GnRH antagonist | |||||
| Live birth rate per woman randomised | 286 per 1000 | 290 per 1000 | OR 1.02 | 2303 | ⊕⊕⊕⊝ | |
| OHSS per woman randomised (any grade) | 114 per 1000 | 73 per 1000 | OR 0.61 | 7944 | ⊕⊕⊕⊝ | |
| Ongoing pregnancy rate per woman randomised | 293 per 1000 | 276 per 1000 | OR 0.92 | 8311 | ⊕⊕⊕⊝ | |
| Clinical pregnancy rate per woman randomised | 303 per 1000 | 283 per 1000 | OR 0.91 | 9959 | ⊕⊕⊕⊝ | |
| Miscarriage rate per woman randomised | 48 per 1000 | 49 per 1000 | OR 1.03 | 7082 | ⊕⊕⊕⊝ | |
| Cycle cancellation due to poor ovarian response | 64 per 1000 | 83 per 1000 | OR 1.32 | 5230 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Asymmetry of the funnel plot with small study effects in favour of GnRH antagonist | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman randomised Show forest plot | 12 | 2303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 2 Live birth rate per woman randomised ‐ minimal stimulation Show forest plot | 2 | 524 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.26] |
| 3 Live birth rate per woman randomised ‐ grouped by trigger Show forest plot | 12 | 2303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 3.1 hCG trigger | 11 | 1899 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.34] |
| 3.2 Unknown trigger | 1 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.21] |
| 4 Ovarian hyperstimulation per woman randomised ‐ all women Show forest plot | 36 | 7944 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.51, 0.72] |
| 5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe Show forest plot | 20 | 5141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.40, 0.69] |
| 6 Ongoing pregnancy rate per woman randomised ‐ all women Show forest plot | 37 | 8311 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.01] |
| 7 Ongoing pregnancy rate per woman randomised ‐ minimal stimulation Show forest plot | 7 | 1456 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.18] |
| 8 Ongoing pregnancy rate per women randomised ‐ grouped by trigger Show forest plot | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 hCG trigger | 29 | 5170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.08] |
| 8.2 Mixed trigger (hCG/GnRH agonist) | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.61] |
| 8.3 Unknown trigger | 7 | 3075 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 9 Clinical pregnancy rate per woman randomised ‐ all women Show forest plot | 54 | 9959 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 10 Clinical pregnancy rate per woman randomised ‐ minimal stimulation Show forest plot | 6 | 1102 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.15, 1.96] |
| 11 Miscarriage rate per woman randomised Show forest plot | 34 | 7082 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 12 Miscarriage rate per clinical pregnancy Show forest plot | 34 | 2308 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.37] |
| 13 Cycle cancellation rate per woman randomised Show forest plot | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Cancellation due to high risk of OHSS | 19 | 4256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.69] |
| 13.2 Cancellation due to poor ovarian response | 25 | 5230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.06, 1.65] |

