Support surfaces for pressure ulcer prevention
Abstract
Background
Pressure ulcers (also known as bedsores, pressure sores, decubitus ulcers) are areas of localised damage to the skin and underlying tissue due to pressure, shear or friction. They are common in the elderly and immobile and costly in financial and human terms. Pressure‐relieving beds, mattresses and seat cushions are widely used as aids to prevention in both institutional and non‐institutional settings.
Objectives
This systematic review seeks to answer the following questions:
(1) to what extent do pressure‐relieving cushions, beds, mattress overlays and mattress replacements reduce the incidence of pressure ulcers compared with standard support surfaces?
(2) how effective are different pressure‐relieving surfaces in preventing pressure ulcers, compared to one another?
Search methods
For this second update the Cochrane Wounds Group Specialised Register was searched (28/2/08), The Cochrane Central Register of Controlled Trials (CENTRAL)(2008 Issue 1), Ovid MEDLINE (1950 to February Week 3 2008), Ovid EMBASE (1980 to 2008 Week 08) and Ovid CINAHL (1982 to February Week 3 2008). The reference sections of included studies were searched for further trials.
Selection criteria
Randomised controlled trials (RCTs), published or unpublished, which assessed the effectiveness of beds, mattresses, mattress overlays, and seating cushions for the prevention of pressure ulcers, in any patient group, in any setting. Study selection was undertaken by at least two authors independently with a third author resolving uncertainty. RCTs were eligible for inclusion if they reported an objective, clinical outcome measure such as incidence and severity of new of pressure ulcers developed. Studies which only reported proxy outcome measures such as interface pressure were excluded.
Data collection and analysis
Trial data were extracted by one researcher and checked by a second. The results from each study are presented as relative risk for dichotomous variables. Where deemed appropriate, similar studies were pooled in a meta analysis.
Main results
For this second update 11 trials met the inclusion criteria bringing the total number of RCTs included in the review to 52.
Foam alternatives to the standard hospital foam mattress can reduce the incidence of pressure ulcers in people at risk. The relative merits of alternating and constant low pressure devices are unclear. There is one high quality trial comparing the different alternating pressure devices for pressure ulcer prevention which suggests that alternating pressure mattresses may be more cost effective than alternating pressure overlays.
Pressure‐relieving overlays on the operating table have been shown to reduce postoperative pressure ulcer incidence, although two studies indicated that foam overlays resulted in adverse skin changes. Two trials indicated that Australian standard medical sheepskins prevented pressure ulcers. There is insufficient evidence to draw conclusions on the value of seat cushions, limb protectors and various constant low pressure devices as pressure ulcer prevention strategies.
A study of Accident & Emergency trolley overlays did not identify a reduction in pressure ulcer incidence. There are tentative indications that foot waffle heel elevators, a particular low air loss hydrotherapy mattress and two types of operating theatre overlays are harmful.
Authors' conclusions
In people at high risk of pressure ulcer development higher specification foam mattresses rather than standard hospital foam mattresses should be used. The relative merits of higher‐tech constant low pressure and alternating pressure for prevention are unclear but alternating pressure mattresses may be more cost effective than alternating pressure overlays. Medical grade sheepskins are associated with a decrease in pressure ulcer development. Organisations might consider the use of some forms of pressure relief for high risk patients in the operating theatre. Seat cushions and overlays designed for use in Accident & Emergency settings have not been adequately evaluated.
PICO
Plain language summary
Can pressure ulcers be prevented by using different support surfaces?
Pressure ulcers (also called bed sores) are ulcers on the skin caused by pressure or rubbing at the weight‐bearing, bony points of immobilised people (such as hips, heels and elbows). Different pressure relieving surfaces (e.g. beds, mattresses, mattress overlays and cushions) are used to cushion vulnerable parts of the body and distribute the surface pressure more evenly. The review found that people lying on ordinary foam mattresses are more likely to get pressure ulcers than those on higher specification foam mattresses. Rigorous research comparing different support surfaces is needed.
Authors' conclusions
Background
Description of the condition
Pressure ulcers (also known as pressure sores, decubitus ulcers and bed sores) are areas of localised damage to the skin and underlying tissue, believed to be caused by pressure, shear or friction (Allman 1997). They usually occur over bony prominences such as the base of the spine, hips and heels. Pressure ulcers occur in both hospital and community settings, most often in the elderly and immobile (e.g. orthopaedic patients), those with severe acute illness (e.g. patients in intensive care units) and in people with neurological deficits (e.g. with spinal cord injuries).
The development of pressure ulcers is relatively common. A review of epidemiological studies in the UK, Canada and the USA describes reported pressure ulcer prevalence in the UK of between 4.4% in a community unit up to 37% in palliative care (Kaltenhalter 2001). In the USA and Canada prevalence ranged from 4.7% in hospital patients to 33% in spinal cord injured patients in the community. They represent a major burden of sickness and unmeasured effects on quality of life for patients and their carers, and are costly to health care systems. In the UK the cost of preventing and treating pressure ulcers in a 600‐bedded large general hospital was estimated at between £600,000 and £3 million per year (Clark 1994). The total cost of pressure ulcers to the NHS has been estimated as £1.4–£2.1 billion annually with most of this cost being due to nurse time (Bennett 2004). The extent to which pressure ulcers are preventable is not clear.
Description of the intervention
The aim of pressure ulcer prevention strategies is to reduce the magnitude and/or duration of pressure between a patient and their support surface (the "interface pressure"). This may be achieved by regular manual repositioning (e.g. "two hourly turning"), or by using pressure‐relieving support surfaces such as cushions, mattress overlays, replacement mattresses or whole bed replacements. The cost of these interventions varies widely; from over £30,000 for some bed replacements to less than £100 for some foam overlays. Information on the relative cost‐effectiveness of this equipment is clearly needed to aid rational use.
How the intervention might work
Pressure‐relieving cushions, beds and mattresses either mould around the shape of the patient to distribute the patient's weight over a larger area (constant low pressure devices) (CLP), or mechanically vary the pressure beneath the patient, so reducing the duration of the applied pressure (alternating pressure devices) (AP) (Bliss 1993). CLP devices (either overlays, mattresses or replacement beds) can be grouped according to their construction (foam, foam and air, foam and gel, profiled foam, hammocks, air suspension, water suspension and air‐particulate suspension/air‐fluidised). These devices fit or mould around the body so that the pressure is dispersed over a large area. Alternating pressure devices generate alternating high and low interface pressures between body and support, usually by alternate inflation and deflation of air‐filled cells. Such devices are available as cushions, mattress overlays, and single‐or multi‐layer mattress replacements.
Turning beds, such as turning frames, net beds, and turning/tilting beds move those patients, either manually or automatically, who are unable to turn themselves. Pressure ulcer prevention is often not the reason for using turning and tilting beds; they may be used in Intensive and Critical Care Units for other reasons, e.g. to promote chest drainage.
Why it is important to do this review
Health care professionals attempt to reduce the incidence of severe pressure ulcers by the identification of people at high risk and the use of prevention strategies, such as pressure‐relieving equipment. It is essential that initiatives are based on the best available evidence of clinical and cost‐effectiveness and we have therefore undertaken a systematic review of the evidence for the effectiveness of pressure‐relieving support surfaces such as beds, mattresses, cushions, and repositioning interventions.
Objectives
This systematic review seeks to answer the following questions:
-
to what extent do pressure‐relieving cushions, beds, mattress overlays and mattress replacements reduce the incidence of pressure ulcers compared with standard support surfaces?
-
how effective are different pressure‐relieving surfaces in preventing pressure ulcers, compared to one another?
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing beds, mattresses and cushions which measured the incidence of new pressure ulcers. Studies which used only subjective measures of outcome (e.g., skin condition "better" or "worse") were excluded, as were studies which reported only proxy measures such as interface pressure. There was no restriction on the basis of the language in which the study reports were written, nor publication status.
Types of participants
Patients receiving health care who were deemed to be at risk of pressure ulcer development, in any setting.
Types of interventions
Studies which evaluated the following interventions for pressure ulcer prevention were included:
Low‐tech CLP support surfaces:
-
Standard foam mattresses
-
Alternative foam mattresses/overlays (e.g. convoluted foam, cubed foam): these are conformable and aim to redistribute pressure over a larger contact area
-
Gel‐filled mattresses/overlays: mode of action as above
-
Fibre‐filled mattresses/overlays: mode of action as above
-
Air‐filled mattresses/overlays: mode of action as above
-
Water‐filled mattresses/overlays: mode of action as above
-
Bead‐filled mattresses/overlays: mode of action as above
-
Sheepskins: proposed mode of action unclear.
High‐tech support surfaces:
-
Alternating pressure mattresses/overlays: patient lies on air filled sacs which sequentially inflate and deflate and relieve pressure at different anatomical sites for short periods; may incorporate a pressure sensor (AP).
-
Air fluidised beds: warmed air circulated through fine ceramic beads covered by a permeable sheet; allows support over a larger contact area (CLP).
-
Low air loss beds: patients are supported on a series of air sacs through which warmed air passes (CLP).
Other support surfaces:
-
Turning beds/frames: these work by either aiding manual repositioning of the patient, or by motor driven turning and tilting.
-
Operating table overlays: as above.
-
Wheelchair cushions: may be conforming and therefore reduce contact pressures by increasing surface area in contact, or mechanical e.g. alternating pressure.
-
Limb protectors: pads and cushions of different forms to protect bony prominences.
Types of outcome measures
Primary outcomes
1. Incidence of new pressure ulcers.
Many evaluations have simply measured the pressure on different parts of the body in contact with the support surface (interface pressure). However, interface pressure is an intermediate or surrogate outcome measure which has serious limitations as a proxy for clinical outcome, since the process which leads to the development of a pressure ulcer almost certainly involves the complex interplay of several factors. Unfortunately, because it is relatively simple, quick and inexpensive to measure, most evaluations only compare interface pressure. In this review we have only considered trials which report the clinical outcome measure of pressure ulcer incidence.
Some studies, when reporting outcomes of interventions for prevention, did not differentiate between people developing grade 1 ulcers (in which the skin is unbroken) and those developing more severe ulcers. Studies which compare the incidence of pressure ulcers of grade 2 or greater are more likely to be reliable (see below for details of grading system), however we included all studies irrespective of whether grade 1 ulcers were described separately.
2. Grades of new pressure ulcers.
A range of pressure ulcer grading systems is used in pressure ulcer trials. An example of a commonly used grading system is presented below:
GRADE 1: Persistent discolouration of the skin including non‐blanchable erythema; blue/purple/black discolouration.
GRADE 2: Partial thickness skin loss involving epidermis and dermis.
GRADE 3: Full thickness skin loss involving damage or necrosis of subcutaneous tissues but not through the underlying fascia and not extending to the underlying bone, tendon or joint capsule.
GRADE 4: Full thickness skin loss with extensive destruction and tissue necrosis extending to the underlying bone, tendon or joint capsule.
Secondary outcomes
the following outcomes were also recorded where available:
-
Costs of the devices
-
Patient comfort
-
Durability of the devices
-
Reliability of the devices
-
Acceptability of the devices
Search methods for identification of studies
Electronic searches
For the second update of this review we searched:
Cochrane Wounds Group Specialised Register (Searched 28/2/08)
The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library 2008 Issue 1
Ovid MEDLINE ‐ 1950 to February Week 3 2008
Ovid EMBASE ‐ 1980 to 2008 Week 08
Ovid CINAHL ‐ 1982 to February Week 3 2008
The following search strategy was used for CENTRAL and modified where appropriate for other databases:
#1 MeSH descriptor Beds explode all trees
#2 mattress*
#3 cushion*
#4 "foam" or transfoam
#5 overlay*
#6 "pad" or "pads"
#7 "gel"
#8 pressure NEXT relie*
#9 pressure NEXT reduc*
#10 pressure NEXT alleviat*
#11 "low pressure" NEAR/2 device*
#12 "low pressure" NEAR/2 support
#13 constant NEAR/2 pressure
#14 "static air"
#15 alternat* NEXT pressure
#16 air NEXT suspension*
#17 air NEXT bag*
#18 water NEXT suspension*
#19 elevation NEAR/2 device*
#20 clinifloat or maxifloat or vaperm or therarest or sheepskin or hammock or "foot waffle" or silicore or pegasus or cairwave
#21 (turn* or tilt*) NEXT (bed* or frame*)
#22 kinetic NEXT (therapy or table*)
#23 net NEXT bed*
#24 "positioning" or "repositioning"
#25 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24)
#26 MeSH descriptor Pressure Ulcer explode all trees
#27 pressure NEXT (ulcer* or sore*)
#28 decubitus NEXT (ulcer* or sore*)
#29 (bed NEXT sore*) or bedsore*
#30 (#26 OR #27 OR #28 OR #29)
#31 (#25 AND #30)
The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format (Lefebvre 2008). The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2008). There was no restriction on the basis of the language in which the study reports were written, nor publication status.
See Appendix 1 for the search strategy used for the first update of this review.
Searching other resources
Experts in the field of wound care were originally contacted to enquire about ongoing and recently published trials in the field of wound care. In addition, manufacturers of wound care materials were contacted for details of the trials they are conducting. This process has not been repeated for this update since it was not productive. However citations within obtained reviews and papers were scrutinised to identify additional studies.
Data collection and analysis
Selection of studies
For this update the titles and abstracts of the search results were assessed for relevance by three authors (EMcI, SB‐S, JD), full copies of all potentially relevant studies were obtained. Decisions on final inclusion after retrieval of full papers was made by one author (EMcI) and checked by a second author (RL or JD); disagreements were resolved by discussion with a third author (NC or SB‐S). Rejected studies were checked by a third author (one of SB‐S; NC).
Data extraction and management
Data from included trials were extracted by a single author into pre‐prepared data extraction tables and checked by a second author. The following data were extracted from each study:
-
patient inclusion/exclusion criteria
-
care setting
-
key baseline variables by group, for example, age, sex, baseline risk, baseline area of existing ulcers
-
description of the interventions and numbers of patients randomised to each intervention
-
description of any co‐interventions/standard care
-
duration and extent of follow up
-
outcomes (incidence and severity of new pressure ulcers)
-
acceptability and reliability of equipment if reported
-
description of inclusion and exclusion criteria used to derive the sample from the target population
-
description of a priori sample size calculation
-
incident ulcers described by severity grading as well as frequency (Grade 1 ulcers are not breaks in the skin and are subject to more inter‐rater variation)
-
clear description of main interventions.
Assessment of risk of bias in included studies
The methodological and reporting quality of each trial were assessed by a single author and checked by a second author. The following quality criteria were used:
-
evidence of true randomisation, for example adequate sequence generation is reported using random number tables, computer random number generator, coin tossing, or shuffling.
-
evidence of allocation concealment at randomisation, such as central randomisation; serially numbered, opaque, sealed envelopes.
-
description of baseline comparability of intervention groups
-
outcome assessment stated to be blinded
-
evidence of an intention to treat analysis (ITT), for example specifically reported by authors that ITT was undertaken and this was confirmed on study assessment, or not stated in the trial report but evident from study assessment that ITT was undertaken.
-
percentage of participants for whom data was complete at defined study end‐point
Dealing with missing data
Where study details or data were missing from reports then attempts were made to contact the study authors to complete the information necessary. If studies were published more than once, the most detailed report was used as the basis of the data extraction.
Data synthesis
For each trial, relative risk (RR) was calculated for categorical outcomes such as number of patients developing ulcers. 95% confidence intervals (95% CI) were included when sufficient detail to allow their calculation was provided. The results from replicated studies were plotted on to graphs and discussed by narrative review. Individual study details are presented in structured tables (Characteristics of included studies). Where there was more than one trial comparing similar devices using the same outcome (though possibly differing lengths of follow up), statistical heterogeneity was tested for by I2 (Higgins 2003). In the absence of significant statistical heterogeneity, studies with similar comparisons were pooled using a fixed effects model. If heterogeneity was observed both random and fixed effects models were used to pool the data. For the purpose of meta analysis we assumed that relative risk remained constant for different lengths of follow up, hence we pooled studies which followed participants for different lengths of time. All statistical analysis was performed on RevMan 5.
Results
Description of studies
Fifty two relevant randomised controlled trials met the inclusion criteria for the review (Characteristics of included studies). Thirty trials involved participants without pre‐existing pressure ulcers (intact skin); 4 trials included patients with ulcers greater than stage 1; 5 trials included participants with and without ulcers and in 13 trials the baseline skin status of the participants was unclear.
Study Settings
Five studies evaluated different operating table surfaces (Aronovitch 1999; Feuchtinger 2006; Nixon 1998; Russell 2000; Schultz 1999); eight evaluated different surfaces in intensive care units (ICU) (Cadue 2008; Gentilello 1988; Inman 1993; Laurent 1997; Sideranko 1992; Summer 1989; Takala 1996; Theaker 2005); eight studies confined their evaluation to orthopaedic patients (Cooper 1998; Exton‐Smith 1982; Goldstone 1982; Hofman 1994; McGowan 2000; Price 1999; Santy 1994; Stapleton 1986) and one involved both an accident and emergency and ward setting (Gunningberg 2000). The remaining studies looked at a variety of patients, for example those in nursing homes (n=9) and those on care of the elderly, medical and surgical wards.
Interventions
Five trials evaluated cushions, three evaluated the use of sheepskins, and three looked at turning beds/kinetic therapy. The remaining studies evaluated different mattresses, overlays and beds.
Risk of bias in included studies
A summary of the sample size and methodological quality of each trial is shown in Table 1.
| Trial | Clear inc & excl | Sample size(arms) | A priori calc | True RCT | Baseline comp | Blind outcome assess | Grade 1 sore exclude | Intervent well docum |
| yes | 482(3) | yes | no | yes | no | yes | no | |
| yes | 217(2) | no | no | yes | yes | yes | yes | |
| yes | 98(2) | no | no | yes | no | yes | no | |
| yes | 70/69 (2) | no | yes | yes | unclear | no | yes | |
| yes | 170 (2) | no | unclear | yes | yes | no | yes | |
| yes | 123 (2) | no | yes | no | unclear | no | yes | |
| no | 99(9) | no | yes | no | no | n/a | yes | |
| yes | 187(2) | no | no | yes | yes | yes | no | |
| yes | 288(2) | no | unclear | yes | yes | unclear | yes | |
| yes | 163(2) | no | no | yes | yes | yes | yes | |
| yes | 100(2) | no | yes | yes | no | yes | yes | |
| yes | 32(2) | no | no | yes | no | no | yes | |
| yes | 12(2) | no | yes | yes | no | yes | yes | |
| no | 30(2) | no | no | no | no | no | yes | |
| yes | 66(2) | no | on | yes | no | yes | yes | |
| yes | 175 (2) | yes | Unclear | yes | yes | no | yes | |
| yes | 230(2) | no | no | yes | no | yes | yes | |
| yes | 65(2) | no | yes | yes | no | no | yes | |
| yes | 32 (2) | no | yes | yes | yes | unclear | yes | |
| yes | 338 (2) | yes | yes | no | unclear | no | yes | |
| yes | 75(2) | no | no | yes | no | no | yes | |
| yes | 100(2) | no | yes | yes | yes | yes | no | |
| yes | 170(2) | no | yes | yes | no | yes | yes | |
| yes | 101(2) | yes | yes | yes | yes | yes | yes | |
| yes | 75(2) | no | no | no | no | no | yes | |
| yes | 44(2) | yes | no | yes | no | yes | yes | |
| yes | 100(2) | yes | no | yes | no | yes | no | |
| yes | 539 (2) | Unclear | yes | yes | no | no | yes | |
| yes | 84(2) | no | yes | yes | yes | no | no | |
| yes | 100(2) | yes | yes | yes | unclear | unclear | yes | |
| yes | 312(4) | yes | no | yes | no | yes | yes | |
| yes | 74(2) | no | yes | no | no | yes | no | |
| yes | 62(2) | no | no | yes | yes | yes | yes | |
| yes | 297(2) | yes | no | yes | no | no | yes | |
| yes | 446(2) | yes | yes | yes | yes | yes | yes | |
| yes | 1972 (2) | yes | yes | yes | no | yes | yes | |
| yes | 80(2) | yes | yes | yes | no | yes | no | |
| yes | 198(2) | no | yes | yes | no | no | yes | |
| yes | 1166(2) | yes | yes | yes | no | no | yes | |
| yes | 103 (3) | Unclear | yes | yes | no | no | yes | |
| yes | 505(5) | yes | yes | yes | no | no | yes | |
| yes | 413(2) | yes | yes | yes | yes | no | no | |
| yes | 57(3) | no | no | yes | no | no | no | |
| yes | 100(3) | no | no | no | no | yes | no | |
| yes | 83(2) | no | no | yes | no | no | yes | |
| yes | 40(2) | yes | no | yes | no | yes | yes | |
| yes | 44(2) | yes | unclear | yes | unclear | no | yes | |
| yes | 62 (2) | yes | yes | yes | no | Unclear | yes | |
| yes | 52(2) | yes | no | no | no | yes | yes | |
| yes | 447 (2) | yes | yes | yes | no | yes | yes | |
| yes | 40(2) | no | no | yes | no | yes | yes | |
| no | 51(2) | no | no | no | no | no | no |
Although the majority of trials discussed the criteria for including patients, only approximately 50% of the reports gave information that indicated that patients were randomly allocated with concealed allocation.
Blinded outcome assessment is rarely used in wound care studies and this was certainly the case in these evaluations of pressure relieving surfaces. It can be difficult or impossible to disguise the surface that a patient is on for assessment of outcome, and patients are often too ill to be removed from their bed for assessment of their pressure areas. Nevertheless, some studies minimise bias in outcome assessment by having a second assessor and presenting inter‐rater reliability data, or by presenting photographic evidence of pressure area status which can then be assessed by an assessor blinded to treatment. Of the 52 RCTs in this review, we could be confident that blinded outcome assessment had been used in only 13 trials.
Small sample size was a major limitation of many of the studies; the median sample size was 100 (range 12 to 1972) and only 20 studies described an a priori sample size estimate. High attrition rates and lack of an intention‐to‐treat analysis were also common. For most comparisons there is a lack of replication.
In studies of pressure ulcer prevention it is extremely important for trialists to report on the baseline comparability of the intervention groups for important variables such as baseline risk. Risk of pressure ulcer development is usually reported as one of various risk scores such as Norton, Waterlow, Gosnell or Braden. Some of the studies reviewed here did not present such baseline data nor explain what the various cut‐offs for inclusion in the studies meant in terms of whether study participants were of low, medium or at high risk for the development of pressure ulcers. Another shortcoming was being unclear about whether grade 1 pressure ulcers were included in the study sample and/or analysis.
Effects of interventions
HOW THE RESULTS ARE PRESENTED AND WHAT THE TERMS MEAN
Results of dichotomous variables are presented as relative risk (RR) with 95% confidence intervals (CI). Relative risk has been used rather than odds ratios as event rates are high in these trials and odds ratios would give an inflated impression of the magnitude of effect (Deeks 1998). Relative risk is the pressure ulcer incidence rate in the experimental group divided by the incidence rate in the control group and indicates the likelihood of pressure ulcer development on an experimental device compared with a comparison device. As by definition, the risk of an ulcer developing in the control group is 1, then the relative risk reduction associated with using the experimental bed is 1‐RR. The relative risk indicates the relative benefit of a therapy but not the actual benefit, i.e. it does not take into account the number of people who would have developed an ulcer anyway. The absolute risk reduction (ARR) can be calculated by subtracting the incidence rate in the experimental group from the incidence rate in the control group. The ARR tells us how much the reduction is due to the bed itself, and its inverse is the number needed to treat, or NNT. Thus an incidence rate of 30% on a control mattress reduced to 15% with an experimental mattress translates into an ARR of 30‐15=15% or 0.15, and an NNT of 7, in other words 7 patients would need to receive the experimental mattress to prevent the development of one additional pressure ulcer.
Methods for measuring secondary outcomes such as comfort, durability, reliability and acceptability were not well developed. Where data was presented it appears in the Characteristics of included studies, but not incorporated in the analysis.
'Low‐tech' constant pressure supports
This section considers comparisons of standard foam hospital mattresses with other low‐technology (low‐tech), constant low pressure supports (CLP). We regarded the following as low‐tech CLP: sheepskin, static air‐filled supports; water‐filled supports; contoured or textured foam supports; gel‐filled supports; bead‐filled supports; Silicore‐filled supports. It should be emphasised however that there is no international definition of what constitutes a standard foam hospital mattress and indeed this changes over time within countries and even within hospitals. Where a description of the standard was provided it is included in the Characteristics of included studies. We have assumed that standard mattresses are likely to vary less within than between countries and undertaken subgroup analysis by country, however this was not pre‐specified.
Standard foam hospital mattress compared with other low‐tech CLP.
Eight RCTs compared 'standard' mattresses/surfaces with 'low‐tech' supports for the prevention of pressure ulcers (Andersen 1982; Collier 1996; Goldstone 1982; Gray 1994a; Gunningberg 2000; Hofman 1994; Russell 2002; Santy 1994).
When compared with standard hospital mattresses, the incidence and severity of pressure ulcers in 'high risk' patients were reduced when patients were placed on either the Comfortex DeCube mattress (Hofman 1994)(RR 0.34, 95%CI 0.14 to 0.85); the Beaufort bead bed (Goldstone 1982)(RR 0.32, 95%CI 0.14 to 0.76); the Softform mattress (Gray 1994a) (RR 0.2, 95%CI 0.09 to 0.45); or the water‐filled mattress (Andersen 1982) (RR 0.35, 95%CI 0.15 to 0.79)(Analysis 1.1).
In an unpublished British study of older people with hip fractures admitted to orthopaedic trauma wards, patients allocated to receive the then NHS standard foam mattress (manufactured by Relyon) experienced over three times the rate of pressure ulcers as those using one of a number of foam alternatives (Clinifloat, Therarest, Transfoam and Vaperm) (Santy 1994) (RR 0.36, 95%CI 0.22 to 0.59). Another study found a significant decrease in the incidence of grade I pressure ulcers from 26.3% to 19.9% (p=0.0004) and a non‐significant decrease in the incidence of pressure ulcers grade II to IV from 10.9% to 8.5% in patients allocated to the high‐specification foam mattress/cushion (RR 0.78; 95%CI 0.55 to 1.11) (Russell 2002). No patient developed a pressure ulcer in the Collier 1996 trial. The comparisons were considered too heterogeneous to pool these 7 studies (Analysis 1.1).
Gunningberg 2000 examined the effects of a viscoelastic foam trolley mattress and subsequent overlay on 101 patients with a suspected hip fracture in the A&E and ward setting. There was no significant difference in pressure ulcer incidence between those assigned a visco‐elastic foam trolley mattress on arrival in A&E followed by a viscoelastic foam overlay on the standard ward mattress (4/48, 8%) and those assigned a standard trolley mattress and then a standard hospital mattress on the ward (8/53, 15%).
The five trials comparing foam alternatives with the standard hospital foam mattress (Collier 1996; Gray 1994a; Hofman 1994; Santy 1994; Russell 2002) were pooled using a random effects model (I2 =77%). These trials were of mixed quality; they all provided evidence of allocation concealment but none used blinded outcome assessment. To avoid double counting the control patients in the trials with more than 2 comparisons, and in the absence of major differences between the effects of different foams, the foam alternatives were pooled. This approach maintains the randomisation but results in comparison groups of unequal size. This analysis yielded a pooled relative risk of 0.40 (95%CI 0.21 to 0.74), or a relative reduction in pressure ulcer incidence of 60% (95%CI 26% to 79%)(Analysis 2.1). Concern regarding the heterogeneity in standard hospital mattress between these trials led us to undertake a separate meta analysis of UK based studies (where variation in the standard hospital mattress is likely to be lower). Pooling the 4 studies which compared alternative foam supports with standard foam mattresses in the UK (Collier 1996; Gray 1994a; Russell 2002; Santy 1994) resulted in the significant benefit of alternative foam over standard foam being maintained (RR 0.41, 95%CI 0.19 to 0.87) (Analysis 2.2). Therefore foam alternatives to the standard hospital mattress can reduce the incidence of pressure ulcers in at risk patients, including patients with fractured neck of femur.
Comparisons between Alternative foam mattresses
This section covers results of studies which performed head‐to‐head comparisons of high‐specification foam products (i.e. contoured foam, supports comprising foam of different densities). Five RCTs (Collier 1996; Gray 1994a; Kemp 1993; Santy 1994; Vyhlidal 1997) compared different foam alternatives (Analysis 3.1).
Santy 1994 and colleagues compared 5 alternative foam mattresses (Clinifloat, Vaperm, Therarest, Transfoam, NHS standard foam) and found significant reductions in pressure ulcer incidence associated with Clinifloat, Therarest, Vaperm and Transfoam compared with standard; and Vaperm compared with Clinifloat (RR 0.36, 95%CI 0.22 to 0.59). Vyhlidal 1997 compared a 4 inch thick foam overlay (Iris 3000) with a foam and fibre mattress replacement (Maxifloat) and reported a significant reduction in pressure ulcer incidence (RR 0.42, 95%CI 0.18 to 0.96) with the mattress replacement, however this trial appeared to have used neither allocation concealment nor blinded outcome assessment.
Kemp 1993 compared a convoluted foam overlay with a solid foam overlay in only 84 patients and found no significant difference in pressure ulcer incidence rates however this may be a Type 2 error, in other words the small sample size may have precluded detection of a significant difference (RR 0.66, 95% CI 0.37 to 1.16). Gray 1994b compared the Transfoam and Transfoamwave foam mattresses however only 1 patient in each group developed a ulcer.
Comparisons between 'Low‐tech' Constant Low Pressure Supports:
This section covers head‐to‐head comparisons of the following types of support: foams; static air‐filled supports (including dry flotation); water‐filled supports; gel‐filled supports; Silicore‐filled supports; heel elevators and sheepskins (Analysis 4.1).
Eleven RCTs have compared different low‐tech CLP devices for prevention (Cadue 2008; Cooper 1998; Ewing 1964; Gilcreast 2005; Jolley 2004; Lazzara 1991; McGowan 2000; Sideranko 1992; Stapleton 1986; Takala 1996; Tymec 1997). Most of these trials are underpowered and/or have other methodological flaws.
A trial from Finland (Takala 1996) comparing the Optima (Carital) constant low pressure mattress ‐ which comprises 21 double air bags on a base ‐ with the standard hospital mattress found that significantly more patients (37%) on the standard mattress developed ulcers compared with none on the Optima (RR 0.06; 95%CI 0 to 0.99). The report of this study did not describe either allocation concealment or blinded outcome assessment.
The remaining trials (Cooper 1998; Lazzara 1991; Sideranko 1992; Stapleton 1986) were all unique comparisons with low power and none found statistically significant differences between the surfaces tested (Analysis 4.1).
Heel devices
One trial (52 patients) compared a proprietary heel elevation device (Foot Waffle) comprising a vinyl boot with built in foot cradle, with elevation of the heels using a hospital pillow (Tymec 1997). The study reported that more heel ulcers developed in the group using the Foot Waffle (n=6) compared with the group using a hospital pillow)(n=2) although this difference was not statistically significant and the number of people in each group was not clearly reported.
Gilcreast 2005 assessed three heel pressure relief devices: the Bunny Boot (fleece) high cushion heel protector; the egg‐crate heel lift positioner and the foot waffle air cushion. There were no statistically significant differences between the devices in terms of pressure ulcer incidence (3/77, 4% for the bunny boot; 4/87, 4.6% for the egg crate and 5/76, 6.6% for the foot waffle). However, it was not clear from the trial whether the number of incident ulcers or number of participants with incident ulcers was being reported. Furthermore, the analysis of this trial was not by intention to treat, and 30% of data were not included in the analysis due, in part to non‐compliance.
Sheepskins
Three trials have examined the effects of sheepskins on pressure ulcer incidence. The first (Ewing 1964) comparing the standard hospital mattress with and without sheepskin overlays, was considered too small and poorly designed to detect a difference. The second involving 297 orthopaedic patients (McGowan 2000) found that pressure ulcer incidence was significantly reduced in those assigned an Australian medical sheepskin (RR for sheepskins relative to standard treatment was 0.30 (95% CI 0.17 to 0.52). The third by Jolley 2004 conducted a study on a mixed inpatient population of a metropolitan hospital comparing a sheepskin mattress overlay with ‘usual care’ which included repositioning and any other pressure relieving devices with or without low‐tech constant pressure relieving devices. It seems that analysis by intention to treat was not used as 539 participants were randomised but only 441 analysed. The study states that any patient whose risk increased to high as measured by Braden score <12 for 48 hours was no longer followed up. The rationale for this is not clear. The results for Grade 2 or above pressure ulcers were 12/218 (5.5%) for the sheepskin group and 20/223 (9%) for the ‘usual care’ group (reported denominators). The participant incidence rate ratio for all ulcer grades was 0.58 (95% CI 0.35 to 0.96). Pooling these two trials using a random effects model (I2 = 67%) showed there were statistically significantly fewer pressure ulcers in the group using sheepskins (RR 0.42 95% CI 0.22 to 0.81)(Analysis 4.1).
Body support
One trial with 70 intensive care unit participants (Cadue 2008) compared a foam body support and usual care (half‐seated position, water mattress and preventative massage 6 times a day) with usual care alone for the prevention of heel ulcers. In total 8.6% (3/35) of participants in the support group developed heel ulcers (all grades) compared with 55.4% (19/35) in the control group, this difference was statistically significant (RR: 0.15 95% CI 0.05 to 0.47) (Analysis 4.1).
'High‐tech' pressure supports
Alternating Pressure Supports:
A variety of alternating pressure (AP) supports is used in hospital and community. The depth of the air‐cells, cell cycle time and mechanical robustness vary between devices and these factors may be important in determining effectiveness. It is worth emphasising that most of the RCTs of AP supports did not adequately describe the equipment being evaluated, including the size of the air cells and cell cycle time.
Sixteen RCTs of alternating pressure supports for pressure ulcer prevention were identified: these compared AP and standard hospital mattresses in two studies (Andersen 1982; Sanada 2003); AP and various constant low pressure devices in nine studies such as water (Andersen 1982; Sideranko 1992), static air (Price 1999; Sideranko 1992), Silicore (Conine 1990; Daechsel 1985; Sideranko 1992), foam (Sideranko 1992; Whitney 1984), various (Gebhardt 1994; Laurent 1997); visco‐elastic foam (Vanderwee 2005); continuous low pressure (Cavicchioli 2007), and with other alternating pressure supports in five studies (Exton‐Smith 1982; Hampton 1997; Nixon 2006; Taylor 1999; Theaker 2005).
Alternating Pressure Compared With Standard Hospital Mattress
Andersen 1982 reported that the use of alternating pressure surfaces significantly reduces the incidence of pressure ulcers compared with standard hospital mattresses (RR 0.32, 95% CI 0.14 to 0.74). This report of this large trial, involving 482 patients at 'high‐risk' of pressure ulcers, gave no indication that either allocation concealment or blinded outcome assessment had been used. In an underpowered and unblinded study conducted on patients requiring head elevation, Sanada 2003 compared: the Air Doctor (a single layer air cell overlay); the Tricell (a double‐layer cell overlay), (both with 5‐minute alternating air pressure) and a Paracare (standard hospital mattress). In the Sanada trial both the experimental groups and control group had a two‐hourly change of position and skin care. In the Air Doctor group 4/29 (13.8%) participants developed grade 2 pressure ulcers, in the Tricell group 1/26 (3.8%) participants developed grade 2 pressure ulcers; and in the Paracare group 6/27 (22%) participants developed grade 2 pressure ulcers. The number of grade 1 ulcers was also reported in the study.The denominators are numbers presented by the authors after withdrawals and attrition and the study was not analysed by intention to treat.
These two trials were pooled using a fixed effects model (I2 = 0%), there was a statistically significant reduction in pressure ulcer development with the AP surface compared with the standard hospital mattress (RR 0.31, 95% CI 0.17 to 0.58), however it should be recognised that these trials are of poor quality (Analysis 5.1).
Alternating Pressure Compared With Constant Low Pressure
Ten trials compared AP devices with various constant low pressure devices, however there is conflicting evidence as to their relative effectiveness. One study compared a range of AP supports with a range of CLP supports in a range of specialties in acute care settings (Gebhardt 1994) and reported significantly more pressure ulcers in patients in the CLP group (34% compared with 13% in the AP group) (RR 0.38, 95%CI 0.22 to 0.66)(Analysis 6.1). This trial is difficult to interpret given the wide variety of surfaces used within the study, there is currently insufficient evidence to support a 'class effect' for all alternating pressure devices and all constant low pressure devices.
In contrast, nine RCTs comparing different types of AP supports and a variety of constant low pressure devices such as the Silicore overlay (Conine 1990; Daechsel 1985; Stapleton 1986), a water mattress (Andersen 1982; Sideranko 1992), a foam pad (Stapleton 1986; Whitney 1984), and static air mattresses (Price 1999; Sideranko 1992), a visco‐elastic foam mattress (including 4 hourly turning and a sitting protocol with a cushion)(Vanderwee 2005), continuous pressure mode of the Hill‐Rom Duo mattress (Cavicchioli 2007), individually reported no difference in effectiveness, although many were too small to be able to detect clinically important differences as statistically significant. In the Vanderwee study a sub‐group analysis on the location of pressure ulcers reported there were statistically significantly more heel pressure ulcers in the control group using the viscoelastic mattress (p = 0.008 Fischer's exact test). The study authors also noted that patients nursed on the experimental equipment (Huntleigh APAM, Alpha X‐cell) seemed to develop more severe ulcers (Analysis 6.1).
Four studies which compared AP with Silicore or foam overlays were pooled (Conine 1990; Daechsel 1985; Stapleton 1986; Whitney 1984). To avoid double counting of the patients in the AP arm of the Stapleton 3‐arm trial, and in the absence of obvious heterogeneity in the outcomes for Silicore and foam, the Silicore and foam arms were pooled against the AP arm (maintaining the randomisation, avoiding double counting, but resulting in unequal comparison groups). Overall the pooled relative risk of pressure ulcer development for AP comapred with Silicore or foam overlays (using a fixed effects model; I2 = 0%) was 0.91, (95% CI 0.71 to 1.17) indicating no statistically significant difference between Silicore or foam overlays and AP (Analysis 6.1).
The studies which compared AP with static water or static air mattresses were similarly considered together (Andersen 1982; Price 1999; Sideranko 1992). The Sideranko trial also had 3 comparison groups and for the purposes of the meta‐analysis, the water and static air arms of this study were considered sufficiently similar to pool together against AP to avoid double counting of the AP patients. Pooling these three trials to answer the question of whether AP is associated with fewer incident ulcers than air or water filled mattresses using a random effects model (I2 = 25%) yielded a pooled RR of 1.31 (95% CI 0.51 to 3.35) indicating no statistically significant difference (Analysis 6.3).
It is worth emphasising, however, that all these studies were small, and, even when pooled were too underpowered to detect clinically important differences in effectiveness as statistically significant.
All nine RCTs comparing the various CLP devices and AP devices were pooled to try to answer the question of whether AP is more effective than CLP in pressure ulcer prevention. Double counting was avoided for the Sideranko and Stapleton trials as before. In view of the different devices evaluated in the studies, the I2 of 34% and the Chi‐square of 13.69 (df=9), a random effects model was applied. This yielded an overall relative risk of 0.85 (95% CI 0.64 to 1.13) suggesting no statistically significant difference between the rates of pressure ulcer incidence on AP compared with CLP (Analysis 6.1). Further trials are needed to determine whether the CLP and AP devices are associated with a clinically important difference in risk of pressure ulceration.
One trial used a complex factorial design to compare various combinations of standard, constant low pressure and alternating pressure support in surgical intensive care patients intra‐ and post‐ICU. This trial (which involved only 75 to 80 patients in each group) did not identify any significant benefit associated with using alternating pressure in the ICU (Laurent 1997) (Analysis 7.1).
Comparisons between Different Alternating Pressure Devices
Alternating pressure devices differ somewhat in structure, e.g., the size of the inflatable air cells. One early study of pressure ulcer prevention (Exton‐Smith 1982) compared two large‐celled alternating pressure devices (Pegasus Airwave and the Large Cell Ripple ‐ similar except the Airwave has two layers of cells). The authors reported that the Airwave System was significantly more effective than the Large Cell Ripple in preventing and reducing severity of pressure ulcers in a high risk group of elderly patients. However, the allocation was not truly random, and an intention‐to‐treat analysis would not have shown a statistically significant difference in the rate of pressure ulcers (16% vs 34%, P >0.05).
Hampton 1997 compared the Pegasus Airwave mattress with a new Cairwave Therapy system by the same manufacturer, in 75 patients. No patients developed an ulcer in either arm of this study.
Taylor 1999 compared the Pegasus Trinova 3‐cell alternating pressure air mattress plus a pressure redistributing cushion (intervention) with a 2‐cell alternating pressure air mattress plus a pressure redistributing cushion (control). This study was underpowered to detect important differences (22 patients in each group) and whilst two patients developed a superficial ulcer in the control group and none in the intervention group, this difference was not statistically significant (RR 0.20 95% CI 0.01 to 3.94)(Analysis 8.1).
In an underpowered trial, Theaker 2005 examined two AP devices in an ICU setting. The KCI Therapulse, a stand alone unit that incorporates a mattress into a bed frame and which uses optional pulsation technology and low air loss to reduce tissue interface pressure and the Hill‐Rom Duo mattress (control) which is designed to lay directly onto most standard hospital frames and uses either continuous or alternating low pressure modes. Details of the alternating cycle were not provided. Pressure ulcer incidence (restricted to grade 2 ulcer or greater) was 3/30 (10%) in the experimental group and 6/32 (19%) in the control group (no statistically significant difference).
In a large, high quality trial Nixon 2006 compared an AP overlay with an AP mattress, the primary outcome was pressure ulcer (grade 2 or above) incidence. An intention to treat analysis was conducted on data from 1971 participants (989 in the overlay group and 982 in the mattress group). One hundred and six (10.7%) people in the overlay group and 101 (10.3%) people in the mattress group developed one or more new grade 2 pressure ulcers. The majority of incidence ulcers were grade 2..There was no significant difference between the two groups in terms of development of a new pressure ulcer of grade 2 or greater (RR 1.04, 95% CI 0.81 to 1.35). More participants cared for on the overlay requested a change to another device due to dissatisfaction (23.3%) compared to mattress patients (18.9%), a statistically significant difference.
Nixon 2006 also conducted a full cost effectiveness analysis from the perspective of the UK NHS and Personal Social Service. Cost information was calculated based on length of hospital stay and pressure‐relieving surface used. Benefits were measured as number of pressure ulcer free days. In the base case analysis the mean per patient cost of the AP mattresses was £6509.73 and the mean patient cost of the AP overlays was £6793.33. The mattress cost on average £283.6 less per patient, (95%CI, £377.59 to £976.79) and also conferred greater benefits (a delay in mean time to ulceration of 10.64 days (95% CI, 24.40 to 3.09). Whilst neither the difference in costs or benefits reached statistical significance the assessment of uncertainty around the cost effectiveness decision indicated that, on average, AP mattresses were associated with an 80% probability of being cost saving. This was because the mattress was associated with a delay in ulceration (measured by Kaplan Meier estimates) and reduced costs as a consequence of shorter length of hospital stay. The conclusions of the base case analysis was not altered when challenged in sensitivity analyses.
Low Air‐Loss Beds
One trial reported that low air‐loss beds were more effective at decreasing the incidence of pressure ulcers in critically ill patients than a standard (but poorly described) ICU bed (RR 0.24, 95% CI 0.11 to 0.53) (Inman 1993)(Analysis 9.1). A second trial of 98 participants, compared low air loss hydrotherapy (LAL‐hydro) with standard care (some patients received alternating pressure in this group); more patients developed ulcers of grade 2 ulcer or greater in the LAL‐hydro group (19%) than the standard care group (7%) though this difference was not statistically significant (Bennett 1998) (Analysis 9.1). A third trial with 123 participants recruited from hospital wards and intensive care units compared a low air‐loss bed (KinAir) with a static air overlay in the prevention of pressure ulcers (Cobb 1997). Three grade 1 ulcers developed on the low air‐loss bed (3/62) compared with 1 on the static air overlay (1/61). However, three grade 2 ulcers developed on the low air‐loss bed (3/62) compared with 11 on the static air overlay (11/61). Comparing the incidence of all ulcers showed no statistically significant difference between the two groups (Analysis 9.1). Pooling the two trials which compared low air‐loss beds (Cobb 1997; Inman 1993) showed a statistically significant difference in favour of the low air‐loss bed, RR 0.33 95% CI 0.16 to 0.67 (random effects I2 = 26%) (Analysis 9.2). Inman 1993 also reported that low air‐loss beds reduced the incidence of patients developing multiple pressure ulcers compared with the standard ICU mattress (RR 0.08 95% CI 0.01 to 0.62) (Analysis 9.3).
Air Fluidised Beds compared with Dry Flotation
One small trial in patients after plastic surgical repair of pressure ulcers showed no difference between an air‐fluidised bed and the Roho dry flotation mattress in post‐operative tissue breakdown rates (Economides 1995) (Analysis 10.1).
Other pressure supports
Kinetic Turning Tables
Turning beds contain motors which constantly turn and tilt the patient, and are used in critical care settings primarily to prevent pneumonia and atelectasis. Four RCTs were identified in a meta‐analysis of kinetic therapy (Choi 1992) however full copies of only two of the individual trials could be obtained for this systematic review (Gentilello 1988; Summer 1989). Sample sizes in all the trials was small, and no beneficial effect of kinetic therapy on pressure ulcer incidence was detected (Analysis 11.1).
Profiling Beds
Keogh 2001 recruited 70 participants and found no pressure ulcers developed in either the group assigned the profiling bed with a pressure reducing foam mattress/cushion combination nor the group assigned a flat‐based bed with a pressure‐relieving/redistributing foam mattress/cushion combination.
Operating Table Overlays
Five RCTs have evaluated different methods of pressure relief on the operating table. The first compared a viscoelastic polymer pad with a standard table and found a relative reduction in the incidence of post‐operative pressure ulcers of 47% associated with using the polymer pad for patients undergoing elective major general, gynaecological or vascular surgery (supine or lithotomy) (RR 0.53; 95% CI 0.33 to 0.85) (Nixon 1998)(Analysis 12.1). It is important to note that the majority of incident pressure ulcers were grade 1 (i.e. early ulcers with no break in skin).
Another trial (Feuchtinger 2006) compared an operating theatre table which included a waterfilled warming mattress, a 4cm thermoactive viscoelastic foam overlay with an operating theatre table with waterfilled warming mattress only. The trial was terminated before the full sample was recruited because more patients in the experimental group with the 4‐cm thermoactive viscoelastic foam overlay suffered pressure ulcers (all were Grade 1 to 2), with 13/85 (15%) in the experimental group and 9/90 (10%) in the control group. In terms of grade 2 only pressure ulcers there were 2 in the experimental group and 1 in the control group. There was no statistically significant difference between the two groups at the point at which the trial was terminated.
Two further RCTs compared the Micropulse alternating system (applied both during surgery and post‐operatively) with a gel pad during surgery and standard mattress post‐operatively. We pooled these two trials (I2= 0%) and derived a pooled relative risk (fixed effects) of 0.21, (95% CI 0.06 to 0.7) in favour of the Micropulse system (Aronovitch 1999; Russell 2000). It is not clear from these 2 trials whether the effect is due to the intra‐operative or the post‐operative pressure relief, or both (Analysis 13.1).
Schultz 1999 compared a mattress operating theatre overlay with usual care (which included padding as required, for example gel pads, foam mattresses). People in the overlay group were more likely to experience postoperative skin changes, and six patients in the overlay group developed ulcers of grade 2 or more compared with 3 people with ulcers of grade 2 or more in the control group. No attempt was made to gather information on postoperative skin care of the patient. Details regarding stage of ulcer by group and of the unnamed product have been sought from the study authors with no success. In the absence of this information, the clinical importance of the findings is difficult to assess.
Overlay used on Accident & Emergency trolleys
Gunningberg 2000 examined the effects of a viscoelastic foam trolley mattress and subsequent overlay on 101 patients with a suspected hip fracture in the A&E and ward setting, this trial is dealt with in the review in the section: Standard foam hospital mattress compared with other low‐tech CLP .
Seat Cushions
There have been four RCTs comparing different types of seating cushion for preventing pressure ulcers; one study compared slab foam with bespoke contoured foam and found no difference between the groups (RR 1.06, 95% CI 0.75 to 1.49)(Lim 1988). The second study (Conine 1994) compared the Jay gel and foam wheelchair cushion with a foam cushion in 141 people and found fewer ulcers in the Jay cushion group, though this did not reach statistical significance (RR 0.61, 95% CI 0.37 to 1.00). The third study (Conine 1993) found no difference in pressure ulcer incidence between those assigned a slab foam cushion bevelled at the base and those assigned a contoured foam cushion with a posterior cut out (Graph: Comparison 14, Outcome 1). The fourth study was a small pilot trial of 32 wheelchair users which compared a standard foam (eggcrate) cushion with a pressure reducing wheelchair cushion (Geyer 2001). The trial did not differentiate between patients with grade 1 ulcers or higher grades. In total, 40% of participants on the pressure reducing cushion developed an ulcer (6/15) compared with 58.5% (10/17) on the foam cushion and this difference was not statistically significant (Analysis 14.1).
Summary of Results
Foam alternatives to the standard hospital foam mattress can reduce the incidence of pressure ulcers in people at risk.
The relative merits of alternating and constant low pressure devices, and of the different AP devices for pressure ulcer prevention are unclear. One large, high quality study found no significant differences between an alternating pressure overlay with an AP mattress. However, the AP mattresses were associated with an 80% probability of being cost saving, due to a delay in pressure ulceration and reduced length of stay in hospital.
Pressure‐relieving overlays on the operating table and in the postoperative period have been shown to reduce the postoperative pressure ulcer incidence, although there is some evidence that certain OR overlays may result in post‐operative skin changes
There is insufficient evidence to determine the value of seat cushions, various constant low pressure devices and A&E trolley overlays as pressure ulcer prevention strategies.
Two trials investigating the effectiveness of a specific sheepskin product in preventing pressure ulcers show that sheepskin overlays are effective in reducing the incidence of pressure ulcers.
Discussion
The confidence with which we can draw firm conclusions from the studies detailed in this review is greatly tempered by (a) the poor quality of many of the trials; (b) the lack of replication of most comparisons and (c) that the ‘standard’ mattress is often not clearly defined. The clearest conclusion one can draw is that standard hospital mattresses have been consistently outperformed by a range of foam‐based, low pressure mattresses and overlays, and also by 'higher‐tech' pressure‐relieving beds and mattresses in the prevention of pressure ulcers.
The application of this conclusion to current clinical practice is however hampered by the fact that the "standard" was poorly described in many of these studies, and what is standard varies by hospital, country and over time. This factor leads to major difficulties in interpretation of trial results and the importance of clear descriptions of all interventions in future studies cannot be overemphasised. In view of this and because we thought there would be less variation within a country, a subgroup analysis of UK based studies was undertaken, which showed that the advantage of alternative foam was maintained. Further, the effects of using alternative foam mattresses are noteworthy in their consistency.
Many of the trials reviewed did not provide convincing reassurance that manual repositioning was provided equally to each group of participants. This is a possible confounder as care providers were not blinded to treatment allocation in any of the trials, and may have moved patients in one group more frequently if they perceived a particular mattress to be less effective. As experimental evidence of the effectiveness of manual repositioning is lacking it is difficult to say what impact this has. In addition, in many studies the definitions of ‘pressure ulcer free’, low‐risk, moderate‐risk and high‐risk vary widely. Frequently, it is also often difficult to ascertain whether study participants with Grade 1 ulcers have been accepted into the sample and included in the analyses or not.
The results of 3 of the 5 trials evaluating the use of pressure‐relieving overlays on the operating table suggest that these are beneficial in reducing subsequent pressure ulcer incidence in high risk surgical patients. These 3 trials were of reasonable or good quality; the Nixon 1998 trial particularly was adequately powered with allocation concealment and blinded outcome assessment, lending further weight to the result. At present, the most effective means of pressure relief on the operating table is unclear; Nixon and colleagues found a gel‐filled overlay to be significantly better than a standard operating table, whilst a gel‐filled overlay on the operating table was less effective than an alternating pressure overlay intra‐ and post‐operatively (the Micropulse system) in the other 2 trials. The Micropulse trials are confounded by their provision of a standard mattress post‐operatively in the gel overlay arm, and an alternating pressure overlay post‐operatively in the Micropulse arm. Thus whilst there is clearly a reduction in pressure ulcer incidence associated with the alternating pressure system, it is not clear whether this is merely a result of better postoperative pressure relief. Two other trials (Schultz 1999; Feuchtinger 2006) showed that post‐operative skin changes occurred as a result of different operating theatre overlays but the clinical importance of these results is difficult to ascertain in the absence of further details on the results and products.
One study suggests that low air‐loss beds are more effective than standard foam ICU beds in preventing pressure ulcers for people in ICU beds, however the ICU bed was not described. Another ICU based study found no differences between a low air loss unit and a mattresses that used either continuous or alternative low pressure modes. There are no studies comparing low air‐loss therapy with alternating pressure surfaces and other 'high tech' low pressure supports.
Previously the evidence for different alternating pressure devices was unclear due to the poor quality and small size of existing studies. This update includes a large and robust trial which suggests that AP mattresses are clinically as effective as overlays but likely to be more cost effective and more acceptable to patients (Nixon 2006).
Water‐filled and bead‐filled mattresses were both associated with reductions in the incidence of pressure ulcers compared with standard hospital mattresses, in trials published in the early 1980s. However, the particular products evaluated are no longer available.
There are tentative indications that four interventions may be harmful. Firstly, Foot Waffle heel elevators were associated with a trebling in the incidence of pressure ulcers that did not reach statistical significance due to the small sample size of the study. Secondly low air loss hydrotherapy which was evaluated in a trial in which 19% LAL‐hydro patients developed ulcers compared with 7% of standard care patients ‐ again not a statistically significant difference possibly as a result of the small size of the trial (98 patients in total). Thirdly, Schultz 1999 investigated the effectiveness of an alternative foam overlay used in the operating theatre. Results suggest that patients placed on the intervention devices were significantly more likely to experience postoperative skin changes (i.e. mainly grade 1 pressure ulcers). However, it is difficult to separate out the role of postoperative care and padding which was used as a concomitant intervention, either of which may have caused the skin changes (mainly found on buttock and coccyx). Lastly Feuchtinger 2006 terminated the trial of an operating theatre table which included a waterfilled warming mattress and a 4cm thermoactive viscoelastic foam overlay compared with an operating theatre table with waterfilled warming mattress only. The trial was terminated before the full sample was recruited because more patients in the experimental group with the 4‐cm thermoactive viscoelastic foam overlay suffered pressure ulcers (all were Grade 1 to 2).
Few comparisons have been replicated, and as most of the trials undertaken are under‐powered there is little information from which to draw firm conclusions. For example, air fluidised therapy as a prevention strategy has only been compared with dry flotation, and low air loss only with standard care, in one trial, as an intervention. There remain gaps in the knowledge base to which a rational research agenda could be developed. It is always important to consider publication bias and its potential influence on the population of studies on a topic. Whilst equipment manufacturers appear to have contributed funding to many of the trials identified, it is difficult to see what the impact of this has been. For example, whilst bias in favour of positive results cannot be discounted, most of the studies published did not find a statistically significant difference.
Common methodological flaws include lack of allocation concealment, lack of baseline comparability, high attrition rates, lack of intention to treat analysis, lack of blind or independently verified outcome assessment. Specific to pressure ulcer intervention research, other flaws include failing to report on whether participants were pressure ulcer free or not on study entry and providing an adequate definition for pressure ulcer status. These deficiencies further reduce the confidence with which we can regard many of the individual study findings. It is however, heartening that the recently included studies have improved reporting of some study details to enable quality assessment.
Future trials should continue to address these deficiencies and collect data on aspects of equipment performance such as reliability. It is hoped that future studies will be reported in line with current international standards for trial reporting (Moher 2001).
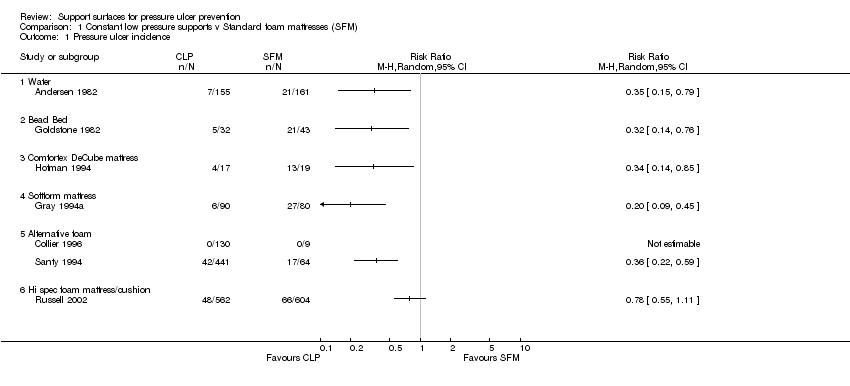
Comparison 1 Constant low pressure supports v Standard foam mattresses (SFM), Outcome 1 Pressure ulcer incidence.

Comparison 2 Alternative Foam Mattress v Standard Foam Mattress, Outcome 1 Pressure ulcer incidence.

Comparison 2 Alternative Foam Mattress v Standard Foam Mattress, Outcome 2 Pressure ulcer incidence UK studies only.

Comparison 3 Comparisons Between Alternative Foam Supports, Outcome 1 Pressure ulcer incidence.

Comparison 4 Comparisons Between CLP Supports, Outcome 1 Pressure ulcer incidence.

Comparison 5 Alternating Pressure v Standard Foam Mattress, Outcome 1 Pressure ulcer incidence.

Comparison 6 Alternating Pressure v Constant Low Pressure, Outcome 1 Pressure ulcer incidence.

Comparison 6 Alternating Pressure v Constant Low Pressure, Outcome 2 AP devices versus silicore or foam overlay.

Comparison 6 Alternating Pressure v Constant Low Pressure, Outcome 3 AP devices versus water or static air mattress.

Comparison 7 AP and CLP in ICU/Post ICU (Factorial Design), Outcome 1 Pressure ulcer incidence.

Comparison 8 Comparisons Between Alternating Pressure Devices, Outcome 1 Pressure ulcer incidence.

Comparison 9 Low Air Loss v Standard Bed, Outcome 1 Pressure ulcer incidence.

Comparison 9 Low Air Loss v Standard Bed, Outcome 2 Pressure incidence pooled.

Comparison 9 Low Air Loss v Standard Bed, Outcome 3 Incidence of patients developing multiple sores.

Comparison 10 Air‐Fluidised Therapy v Dry Flotation, Outcome 1 Rate of wound breakdown.

Comparison 11 Kinetic Treatment Table v Standard, Outcome 1 Pressure ulcer incidence.

Comparison 12 Operating Table Overlay v No Overlay, Outcome 1 Pressure ulcer incidence.

Comparison 13 Micropulse System for Surgical Patients, Outcome 1 Pressure ulcer incidence.

Comparison 14 Seat Cushions, Outcome 1 Pressure ulcer incidence.
| Trial | Clear inc & excl | Sample size(arms) | A priori calc | True RCT | Baseline comp | Blind outcome assess | Grade 1 sore exclude | Intervent well docum |
| yes | 482(3) | yes | no | yes | no | yes | no | |
| yes | 217(2) | no | no | yes | yes | yes | yes | |
| yes | 98(2) | no | no | yes | no | yes | no | |
| yes | 70/69 (2) | no | yes | yes | unclear | no | yes | |
| yes | 170 (2) | no | unclear | yes | yes | no | yes | |
| yes | 123 (2) | no | yes | no | unclear | no | yes | |
| no | 99(9) | no | yes | no | no | n/a | yes | |
| yes | 187(2) | no | no | yes | yes | yes | no | |
| yes | 288(2) | no | unclear | yes | yes | unclear | yes | |
| yes | 163(2) | no | no | yes | yes | yes | yes | |
| yes | 100(2) | no | yes | yes | no | yes | yes | |
| yes | 32(2) | no | no | yes | no | no | yes | |
| yes | 12(2) | no | yes | yes | no | yes | yes | |
| no | 30(2) | no | no | no | no | no | yes | |
| yes | 66(2) | no | on | yes | no | yes | yes | |
| yes | 175 (2) | yes | Unclear | yes | yes | no | yes | |
| yes | 230(2) | no | no | yes | no | yes | yes | |
| yes | 65(2) | no | yes | yes | no | no | yes | |
| yes | 32 (2) | no | yes | yes | yes | unclear | yes | |
| yes | 338 (2) | yes | yes | no | unclear | no | yes | |
| yes | 75(2) | no | no | yes | no | no | yes | |
| yes | 100(2) | no | yes | yes | yes | yes | no | |
| yes | 170(2) | no | yes | yes | no | yes | yes | |
| yes | 101(2) | yes | yes | yes | yes | yes | yes | |
| yes | 75(2) | no | no | no | no | no | yes | |
| yes | 44(2) | yes | no | yes | no | yes | yes | |
| yes | 100(2) | yes | no | yes | no | yes | no | |
| yes | 539 (2) | Unclear | yes | yes | no | no | yes | |
| yes | 84(2) | no | yes | yes | yes | no | no | |
| yes | 100(2) | yes | yes | yes | unclear | unclear | yes | |
| yes | 312(4) | yes | no | yes | no | yes | yes | |
| yes | 74(2) | no | yes | no | no | yes | no | |
| yes | 62(2) | no | no | yes | yes | yes | yes | |
| yes | 297(2) | yes | no | yes | no | no | yes | |
| yes | 446(2) | yes | yes | yes | yes | yes | yes | |
| yes | 1972 (2) | yes | yes | yes | no | yes | yes | |
| yes | 80(2) | yes | yes | yes | no | yes | no | |
| yes | 198(2) | no | yes | yes | no | no | yes | |
| yes | 1166(2) | yes | yes | yes | no | no | yes | |
| yes | 103 (3) | Unclear | yes | yes | no | no | yes | |
| yes | 505(5) | yes | yes | yes | no | no | yes | |
| yes | 413(2) | yes | yes | yes | yes | no | no | |
| yes | 57(3) | no | no | yes | no | no | no | |
| yes | 100(3) | no | no | no | no | yes | no | |
| yes | 83(2) | no | no | yes | no | no | yes | |
| yes | 40(2) | yes | no | yes | no | yes | yes | |
| yes | 44(2) | yes | unclear | yes | unclear | no | yes | |
| yes | 62 (2) | yes | yes | yes | no | Unclear | yes | |
| yes | 52(2) | yes | no | no | no | yes | yes | |
| yes | 447 (2) | yes | yes | yes | no | yes | yes | |
| yes | 40(2) | no | no | yes | no | yes | yes | |
| no | 51(2) | no | no | no | no | no | no |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Water | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Bead Bed | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Comfortex DeCube mattress | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Softform mattress | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Alternative foam | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Hi spec foam mattress/cushion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 5 | 2016 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.74] |
| 1.1 Various alternatives (pooled) | 5 | 2016 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.74] |
| 2 Pressure ulcer incidence UK studies only Show forest plot | 4 | 1980 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.19, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 alternative foam v standard foam | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Maxifloat Foam Mattress v Iris Foam Overlay | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Solid Foam v Convoluted Foam | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Sofflex v ROHO | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.16, 2.47] |
| 1.2 Optima v SFM | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 0.99] |
| 1.3 Gel Mattress v Air‐filled Overlay | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.24, 2.72] |
| 1.4 Static Air Mattress v Water Mattress | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.04, 4.29] |
| 1.5 Foam Overlay v Silicore Overlay | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.64, 2.14] |
| 1.6 Sheepskin v no sheepskin | 2 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.22, 0.81] |
| 1.7 Foam support surface v no support | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.05, 0.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 2 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.17, 0.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 10 | 1606 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 1.1 AP (various) v CLP (various) | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.22, 0.66] |
| 1.2 AP v Silicore or Foam Overlay | 4 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.72, 1.16] |
| 1.3 AP v Water or Static Air Mattress | 3 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.51, 3.35] |
| 1.4 AP v continuous low pressure mattress | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.19, 22.18] |
| 1.5 AP v Visco‐elastic foam mattress | 1 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.64, 1.52] |
| 2 AP devices versus silicore or foam overlay Show forest plot | 4 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.71, 1.17] |
| 3 AP devices versus water or static air mattress Show forest plot | 3 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.51, 3.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Std ICU/SFM post‐ICU v Nimbus AP ICU/SFM post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Std ICU/SFM post‐ICU v Std ICU/Tempur CLP post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Nimbus AP ICU/SFM post‐ICU v Std ICU/Tempur CLP post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Std ICU/SFM post‐ICU v Nimbus AP ICU/Tempur CLP post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Nimbus AP ICU/SFM post‐ICU v Nimbus ICU/Tempur post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Std ICU/Tempur post‐ICU v Nimbus ICU/Tempur post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Airwave v Large Cell Ripple | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Airwave v Pegasus Carewave | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Trinova v control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 AP Overlay v AP Mattress | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 TheraPulse v Duo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Pressure incidence pooled Show forest plot | 2 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.16, 0.67] |
| 3 Incidence of patients developing multiple sores Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of wound breakdown Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.20, 4.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Viscoelastic polymer pad v No overlay | 1 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.85] |
| 1.2 Viscoelastic foam overlay v No overlay | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.69, 3.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pressure ulcer incidence Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Slab Foam v Bespoke Contoured Foam | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Jay Gel Cushion v Foam | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pressure reducing cushion v standard foam cushion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

