Tratamiento con inyecciones esclerosantes para las venas varicosas
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Hospital‐based study. | |
| Participants | 101 patients. | |
| Interventions | Sclerotherapy versus graduated compression stockings. | |
| Outcomes | 1. Symptomatic improvement and cosmetic result. | |
| Notes | Sclerosant: STD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Hospital‐based study. | |
| Participants | 148 patients: 169 legs. | |
| Interventions | Sclerotherapy with bandaging for 3 weeks versus 6 weeks. | |
| Outcomes | 1. Patient questionnaire: pain, mobility, cosmetic appearance, general satisfaction (3 = best score, 11 = worst). | |
| Notes | Sclerosant: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Multicentre hospital‐based study. | |
| Participants | 534 patients. | |
| Interventions | Sclerotherapy with STD (group A, 1 to 2 ml of 2% or 3%) versus sclerotherapy with high dose STD (group B, 3 to 6 ml of 3%) versus foam sclerotherapy (group E, foam + 3% STD). | |
| Outcomes | 1. Recurrent varicose veins at 5 and 10 years. | |
| Notes | 1. Further 3 treatment groups were excluded as these were surgical: multiple ligations, stab avulsions and surgery followed by sclerotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Hospital‐based study. | |
| Participants | 42 patients. | |
| Interventions | Sclerotherapy with lidocaine/ hypertonic saline (19%) versus hypertonic saline (23.4%). | |
| Outcomes | 1. Patient discomfort from initial injection: no pain (score = 1), mild pain (score = 2), moderate pain (score = 3), severe pain (score = 4). | |
| Notes | Sclerosant: see 'Interventions'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Hospital‐based study. | |
| Participants | 154 patients: 158 legs. | |
| Interventions | Sclerotherapy with Coban bandaging for 6 weeks versus Coban 3 days versus crepe 6 weeks. | |
| Outcomes | 1. Patient symptom score: cosmetic, tiredness, pain, pruritus, cramps, ankle swelling, eczema (7 = worst score). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Clinic‐based study. | |
| Participants | 129 patients. | |
| Interventions | Sclerotherapy with polidocanol (0.5%, 1%, 3%) versus STD (0.25%, 0.5%, 1.5%). | |
| Outcomes | 1. Photographic score: appearance of veins (range from 1 (worse) to 5 (complete disappearance). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Multi‐centre clinic based study. | |
| Participants | 88 patients. | |
| Interventions | Sclerotherapy with polidocanol foam (0.5 ml sclerosant) versus 2.0 to 2.5 ml 3% polidocanol liquid. | |
| Outcomes | 1. Venous spasm, | |
| Notes | Single does of sclerosant only: 2.0 ml if LSV 4 to 6 mm diameter; 2.5 ml if LSV 6 to 8 mm diameter. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Hospital‐based study. | |
| Participants | 30 patients. | |
| Interventions | Sclerotherapy with 3% aethoxysclerol (polidocanol) versus normal saline. | |
| Outcomes | 1. Venous by arterial volume flow. | |
| Notes | 2 layer short stretch bandages in both groups. Duplex scan at 1 and 4 weeks post‐sclerotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Hospital‐based study. | |
| Participants | 1622 patients. | |
| Interventions | Sclerotherapy with aethoxysclerol (Sigg method) vs STD (Fegan method). | |
| Outcomes | 1. Cosmetic appearance, including photographic evidence. | |
| Notes | Third group treated with both aethoxysclerol and STD (Fegan method) not discussed further: relative proportion of 2 sclerosants not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Hospital‐based study. | |
| Participants | 100 patients: 111 legs. | |
| Interventions | Sclerotherapy with bandaging for 1 week versus 6 weeks. | |
| Outcomes | 1. Patient symptoms‐ tolerating bandage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Study setting: not stated. | |
| Participants | 50 patients. | |
| Interventions | Sclerotherapy with 10% hypertonic dextrose versus 0.15% STD. | |
| Outcomes | 1. Patient assessment: disappearance of thread veins. | |
| Notes | Sclerosant: see 'Interventions'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Hospital‐based study. | |
| Participants | 112 patients. | |
| Interventions | Sclerotherapy with bandaging for 8 hours versus 6 weeks. | |
| Outcomes | 1. Patient assessment: cosmetic result and symptomatic improvement. | |
| Notes | Sclerosant: 3% STD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Hospital‐based study. | |
| Participants | 130 patients: 145 legs. | |
| Interventions | Sclerotherapy with bandaging for 1 week versus 3 weeks. | |
| Outcomes | 1. Patient questionnaire: pain, mobility, cosmetic appearance, general satisfaction (4 = best score, 11 = worst). | |
| Notes | Trial 1 refers to Batch 1980 study; trial 2 to Reddy 1986 study. Sclerosant: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Study setting: not stated. | |
| Participants | 30 patients. | |
| Interventions | Sclerotherapy with 4% aetoxisclerol versus 3% Sotradecol (STD). | |
| Outcomes | 1. Venous spasm (75% reduction cross‐sectional diameter) at 3 minutes. | |
| Notes | Sclerosant: see 'Interventions'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Hospital‐based study. | |
| Participants | 42 patients (see Note 2). | |
| Interventions | Sclerotherapy with elastic stocking compression versus conventional bandaging. | |
| Outcomes | 1. Successful sclerosis: 100%, 75 to 99%, 50 to 74%, < 50%. | |
| Notes | Sclerosant: 0.5% ethanolamine. (1) Patients assessed at 3 and 6 weeks but not clearly stated which time results refer to ‐ presume 6 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Hospital‐based study. | |
| Participants | 62 patients. | |
| Interventions | Sclerotherapy with Elastocrepe bandage and elastic stocking compression versus elastic stocking alone. | |
| Outcomes | 1. Patient assessment: discomfort, slipping of dressing. | |
| Notes | Sclerosant: STD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Hospital‐based study. | |
| Participants | 102 patients: 51 each group. | |
| Interventions | Sclerotherapy with Molefoam dressing to injection site versus Sorbo pad. | |
| Outcomes | 1. Successful sclerosis (no further injections required). | |
| Notes | Sclerosant: STD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
LSV long saphenous vein
STD sodium tetradecyl sulphate
VV varicose veins
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| RCT comparing surgery and sclerotherapy to sclerotherapy alone. | |
| RCT comparing local anaesthetic section of varicose veins (dentist's technique), sclerotherapy and 'Section Ambulatoire des Varices avec Sclerotherapie' (SAVAS) (combination of dentist's technique and sclerotherapy). | |
| RCT comparing sclerotherapy with aethoxysclerol versus STD. No details of randomisation, blinding or allocation concealment presented. Number of patients/limbs entering trial not stated, only numbers of limbs assessed at 10‐year follow‐up: drop‐outs impossible to assess. Results presented as percentages: unclear as to the denominator. Abstract states that aethoxysclerol is more effective and better tolerated than STD (Anova p<0.021). | |
| RCT comparing foam sclerotherapy and adjuvant high saphenous ligation under local anaesthetic with conventional surgery. | |
| RCT comparing surgery to sclerotherapy. | |
| RCT comparing surgery (ambulatory phlebectomy) to sclerotherapy. | |
| RCT comparing surgery to sclerotherapy. | |
| RCT comparing surgery to sclerotherapy. | |
| RCT comparing surgery to sclerotherapy. | |
| RCT comparing surgery to sclerotherapy. | |
| RCT comparing surgery with intra‐operative sclerotherapy to surgery with post‐operative sclerotherapy. | |
| RCT comparing surgery to sclerotherapy. | |
| Not an RCT. Controlled study comparing Sotradecol (STD) sclerotherapy with or without heparin. | |
| Not an RCT. Controlled study where 13 patients with bilateral telangiectatic veins (0.2 to 0.4 mm diameter) were treated with 0.25% STD in one leg and 72% glycerine in the contralateral leg. | |
| Not an RCT. Controlled study where 20 women with bilateral telangiectatic veins (0.1 to 1.5 mm diameter) were treated with a long‐pulsed 1064 nm Nd:YAG laser to one leg and sclerotherapy with 0.25% STD in the contralateral leg. Results favoured treatment with sclerotherapy. | |
| Double‐blind trial of sclerotherapy with iodine sodium iodide versus sodium tetradecyl sulphate. No evidence of randomisation in study methods and no results reported of differences between the two sclerosants. | |
| RCT comparing sclerotherapy with 1% STD foam versus liquid formulation. Randomisation method not stated, unable to assess blinding or allocation concealment. End point data unclear. | |
| RCT comparing sclerotherapy with 1% STD as an air‐filled foam versus perfluoropropane‐filled albumin microspheres of STD. Randomisation method not stated, unable to assess blinding or allocation concealment. No numerical data reported, only statistical significance. Trend towards benefit with perfluopropane‐filled albumin microspheres group. | |
| Not an RCT. Controlled study where below‐knee incompetent perforating vein treated with varying doses of 5% ethanolamine and contralateral limb treated with normal saline. | |
| Not an RCT. Alternate assignment of 28 patients with venous ulceration to either Unna's compressive boots alone or in conjunction with sclerotherapy. | |
| RCT comparing surgery to sclerotherapy. | |
| Not an RCT. Double‐blind paired‐comparison study in patients with bilateral starburst telangiectasia and reticular veins. Phase 1: comparison of varying concentrations of hypertonic saline (23.4%, 11.7%, 5.8%) in 600 patients. Phase 2: subgroup of 200 patients treated with hypertonic saline ± heparin. | |
| RCT comparing sclerotherapy of the terminal segment of the long saphenous vein (LSV) with sclerotherapy of LSV tributaries in addition to this. Excluded because no numerical results presented, although results state that treating tributaries prevents early recanalisation of the long saphenous vein. | |
| RCT comparing sclerotherapy to sclerotherapy with post‐operative microthrombectomy. | |
| RCT comparing surgery to sclerotherapy: inadequate method of randomisation (alternate patients assigned to each treatment option). | |
| RCT comparing sclerotherapy with polidocanol foam (Varisolve) with surgery or sclerotherapy. Excluded because no numerical results presented, although abstract states that foam sclerotherapy is as effective as surgery and more effective than conventional sclerotherapy. | |
| RCT comparing sclerotherapy with polidocanol foam to sclerotherapy with polidocanol foam with 0.1 cm2 Gelofusine (a synthetic plasma expander). Excluded because: i) randomization method, blinding and allocation concealment are unclear; ii) no results published on the effect of treatment on venous spasm and reflux, as mentioned in the Methods; iii) it is not clear whether the results indicating number of veins sclerosed apply to immediately post‐treatment or the 1 month follow‐up. |
Surgical treatment of varicose veins is beyond the scope of this Cochrane Review.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 1 Results: venous spasm Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.1  Comparison 1 Sclerotherapy with different sclerosants, Outcome 1 Results: venous spasm. | ||||||||||||||||||||||||
| 2 Results: disappearance of reflux Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.2  Comparison 1 Sclerotherapy with different sclerosants, Outcome 2 Results: disappearance of reflux. | ||||||||||||||||||||||||
| 3 Results: venous by arterial volume flow Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 1.3
Comparison 1 Sclerotherapy with different sclerosants, Outcome 3 Results: venous by arterial volume flow. | ||||||||||||||||||||||||
| 4 Results: disappearance of thread veins Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.4  Comparison 1 Sclerotherapy with different sclerosants, Outcome 4 Results: disappearance of thread veins. | ||||||||||||||||||||||||
| 5 Results: photographic appearance of veins Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.5  Comparison 1 Sclerotherapy with different sclerosants, Outcome 5 Results: photographic appearance of veins. | ||||||||||||||||||||||||
| 6 Results: cosmetic appearance at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.6  Comparison 1 Sclerotherapy with different sclerosants, Outcome 6 Results: cosmetic appearance at 6 months. | ||||||||||||||||||||||||
| 7 Results: cosmetic appearance at 5 years Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.7  Comparison 1 Sclerotherapy with different sclerosants, Outcome 7 Results: cosmetic appearance at 5 years. | ||||||||||||||||||||||||
| 8 Results: symptomatic improvement at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.8  Comparison 1 Sclerotherapy with different sclerosants, Outcome 8 Results: symptomatic improvement at 6 months. | ||||||||||||||||||||||||
| 9 Results: symptomatic improvement at 5 years Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.9  Comparison 1 Sclerotherapy with different sclerosants, Outcome 9 Results: symptomatic improvement at 5 years. | ||||||||||||||||||||||||
| 10 Results: recurrent varicose veins at 5 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.10  Comparison 1 Sclerotherapy with different sclerosants, Outcome 10 Results: recurrent varicose veins at 5 years. | ||||||||||||||||||||||||
| 11 Results: recurrent varicose veins at 10 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.11  Comparison 1 Sclerotherapy with different sclerosants, Outcome 11 Results: recurrent varicose veins at 10 years. | ||||||||||||||||||||||||
| 12 Results: failure at 10 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.12  Comparison 1 Sclerotherapy with different sclerosants, Outcome 12 Results: failure at 10 years. | ||||||||||||||||||||||||
| 13 Complications: allergic reaction Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.13  Comparison 1 Sclerotherapy with different sclerosants, Outcome 13 Complications: allergic reaction. | ||||||||||||||||||||||||
| 14 Complications: pigmentation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.14  Comparison 1 Sclerotherapy with different sclerosants, Outcome 14 Complications: pigmentation. | ||||||||||||||||||||||||
| 15 Complications: pigmentation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.15  Comparison 1 Sclerotherapy with different sclerosants, Outcome 15 Complications: pigmentation. | ||||||||||||||||||||||||
| 16 Complications: skin necrosis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.16  Comparison 1 Sclerotherapy with different sclerosants, Outcome 16 Complications: skin necrosis. | ||||||||||||||||||||||||
| 17 Complications: local urticaria Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.17  Comparison 1 Sclerotherapy with different sclerosants, Outcome 17 Complications: local urticaria. | ||||||||||||||||||||||||
| 18 Complications: pain Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.18  Comparison 1 Sclerotherapy with different sclerosants, Outcome 18 Complications: pain. | ||||||||||||||||||||||||
| 19 Complications: vein thrombosis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.19  Comparison 1 Sclerotherapy with different sclerosants, Outcome 19 Complications: vein thrombosis. | ||||||||||||||||||||||||
| 20 Complications: ecchymosis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.20  Comparison 1 Sclerotherapy with different sclerosants, Outcome 20 Complications: ecchymosis. | ||||||||||||||||||||||||
| 21 Complications: matting Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.21  Comparison 1 Sclerotherapy with different sclerosants, Outcome 21 Complications: matting. | ||||||||||||||||||||||||
| 22 Complications: matting Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 1.22  Comparison 1 Sclerotherapy with different sclerosants, Outcome 22 Complications: matting. | ||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: disappearance of varicosities, pigmentation, neovascularization Show forest plot | 1 | 35 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.1  Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 1 Results: disappearance of varicosities, pigmentation, neovascularization. | ||||
| 2 Complications: moderate or severe pain from sclerotherapy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 2 Complications: moderate or severe pain from sclerotherapy. | ||||
| 3 Complications: microthrombosis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 3 Complications: microthrombosis. | ||||
| 4 Complications: ulceration Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 4 Complications: ulceration. | ||||
| 5 Complications: matting/hyperpigmentation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 5 Complications: matting/hyperpigmentation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: venous spasm Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Sclerotherapy with foam versus liquid, Outcome 1 Results: venous spasm. | ||||
| 2 Results: elimination of reflux Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Sclerotherapy with foam versus liquid, Outcome 2 Results: elimination of reflux. | ||||
| 3 Results: recanalisation at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Sclerotherapy with foam versus liquid, Outcome 3 Results: recanalisation at 6 months. | ||||
| 4 Results: recurrent varicose veins at 10 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Sclerotherapy with foam versus liquid, Outcome 4 Results: recurrent varicose veins at 10 years. | ||||
| 5 Results: failure at 10 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 Sclerotherapy with foam versus liquid, Outcome 5 Results: failure at 10 years. | ||||
| 6 Complications: haematoma Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.6  Comparison 3 Sclerotherapy with foam versus liquid, Outcome 6 Complications: haematoma. | ||||
| 7 Complications: cutaneous inflammation at 3 weeks Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3 Sclerotherapy with foam versus liquid, Outcome 7 Complications: cutaneous inflammation at 3 weeks. | ||||
| 8 Results: recurrent varicose veins at 5 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.8  Comparison 3 Sclerotherapy with foam versus liquid, Outcome 8 Results: recurrent varicose veins at 5 years. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: successful sclerotherapy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Molefoam versus Sorbo pad to injection sites after sclerotherapy, Outcome 1 Results: successful sclerotherapy. | ||||
| 2 Complications: erythema Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Molefoam versus Sorbo pad to injection sites after sclerotherapy, Outcome 2 Complications: erythema. | ||||
| 3 Complications: ulceration Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Molefoam versus Sorbo pad to injection sites after sclerotherapy, Outcome 3 Complications: ulceration. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: disappearance of varicosities Show forest plot | 3 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.71, 2.20] |
| Analysis 5.1  Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 1 Results: disappearance of varicosities. | ||||
| 2 Complications: discomfort Show forest plot | 2 | 168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.65 [1.92, 6.95] |
| Analysis 5.2  Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 2 Complications: discomfort. | ||||
| 3 Complications: slipped stockings Show forest plot | 2 | 168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.24, 1.00] |
| Analysis 5.3  Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 3 Complications: slipped stockings. | ||||
| 4 Complications: thrombophlebitis Show forest plot | 3 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.47, 1.34] |
| Analysis 5.4  Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 4 Complications: thrombophlebitis. | ||||
| 5 Complications: skin staining Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.5  Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 5 Complications: skin staining. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: cosmetic and symptomatic improvement Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 1 Results: cosmetic and symptomatic improvement. | ||||
| 2 Results: pain, mobility, cosmetic appearance, satisfaction at 3 months Show forest plot | 2 | 298 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.2  Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 2 Results: pain, mobility, cosmetic appearance, satisfaction at 3 months. | ||||
| 3 Results: recurrent varicose veins Show forest plot | 3 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.43, 1.10] |
| Analysis 6.3  Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 3 Results: recurrent varicose veins. | ||||
| 4 Results: pain, mobility, cosmetic appearance, satisfaction at 2 years Show forest plot | 1 | 145 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.4  Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 4 Results: pain, mobility, cosmetic appearance, satisfaction at 2 years. | ||||
| 5 Results: pain, mobility, cosmetic appearance, satisfaction at 4 years Show forest plot | 1 | 145 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.5  Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 5 Results: pain, mobility, cosmetic appearance, satisfaction at 4 years. | ||||
| 6 Complications: phlebitis, pigmentation, induration at 3 months Show forest plot | 2 | 298 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.6  Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 6 Complications: phlebitis, pigmentation, induration at 3 months. | ||||
| 7 Complications: phlebitis, staining, pain, blistering, ulceration Show forest plot | 2 | 331 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.66, 1.73] |
| Analysis 6.7  Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 7 Complications: phlebitis, staining, pain, blistering, ulceration. | ||||
| 8 Complications: discomfort, slipping, foot swelling, bandage intolerance Show forest plot | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.40, 0.73] |
| Analysis 6.8  Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 8 Complications: discomfort, slipping, foot swelling, bandage intolerance. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: cosmetic and symptomatic improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Sclerotherapy versus graduated compression stockings, Outcome 1 Results: cosmetic and symptomatic improvement. | ||||

Comparison 1 Sclerotherapy with different sclerosants, Outcome 1 Results: venous spasm.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 2 Results: disappearance of reflux.
| Study | Time point | 3% aethoxysclerol | Sodium chloride |
| Kahle 2003 | Pre‐treatment | 1.39 (95% CI 1.13 to 1.52) | 1.41 (95% CI 1.15 to 1.67) |
| Kahle 2003 | After 1 week | 1.08 (95% CI 0.95 to 1.21) | 1.39 (95% CI 1.16 to 1.62) |
| Kahle 2003 | After 2 weeks | 1.18 (95% CI 0.99 to 1.37) | 1.41 (95% CI 1.11 to 1.71) |
Comparison 1 Sclerotherapy with different sclerosants, Outcome 3 Results: venous by arterial volume flow.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 4 Results: disappearance of thread veins.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 5 Results: photographic appearance of veins.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 6 Results: cosmetic appearance at 6 months.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 7 Results: cosmetic appearance at 5 years.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 8 Results: symptomatic improvement at 6 months.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 9 Results: symptomatic improvement at 5 years.
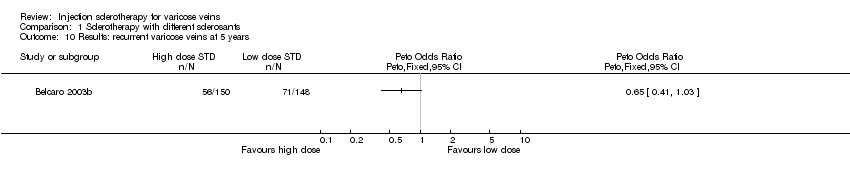
Comparison 1 Sclerotherapy with different sclerosants, Outcome 10 Results: recurrent varicose veins at 5 years.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 11 Results: recurrent varicose veins at 10 years.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 12 Results: failure at 10 years.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 13 Complications: allergic reaction.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 14 Complications: pigmentation.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 15 Complications: pigmentation.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 16 Complications: skin necrosis.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 17 Complications: local urticaria.
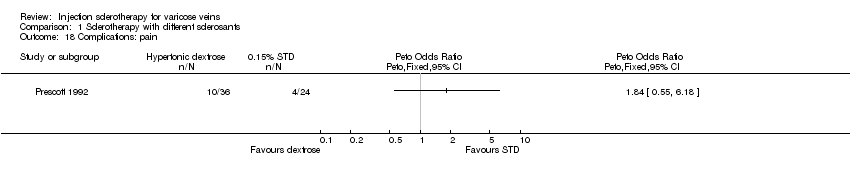
Comparison 1 Sclerotherapy with different sclerosants, Outcome 18 Complications: pain.
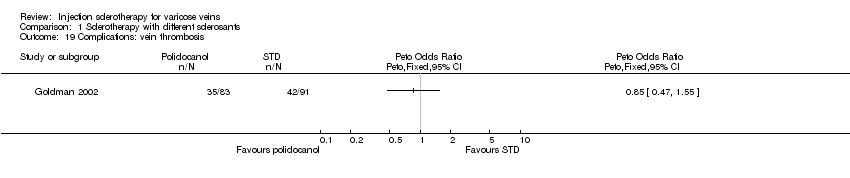
Comparison 1 Sclerotherapy with different sclerosants, Outcome 19 Complications: vein thrombosis.

Comparison 1 Sclerotherapy with different sclerosants, Outcome 20 Complications: ecchymosis.
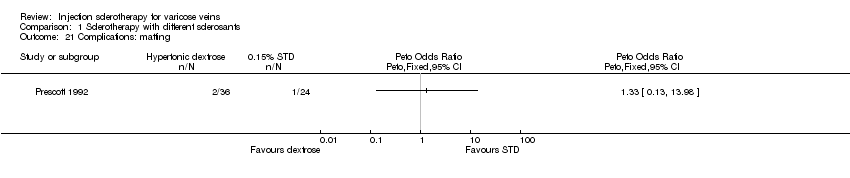
Comparison 1 Sclerotherapy with different sclerosants, Outcome 21 Complications: matting.
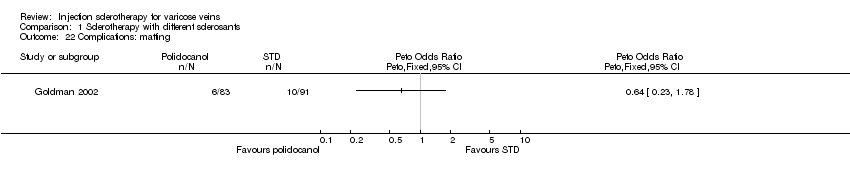
Comparison 1 Sclerotherapy with different sclerosants, Outcome 22 Complications: matting.
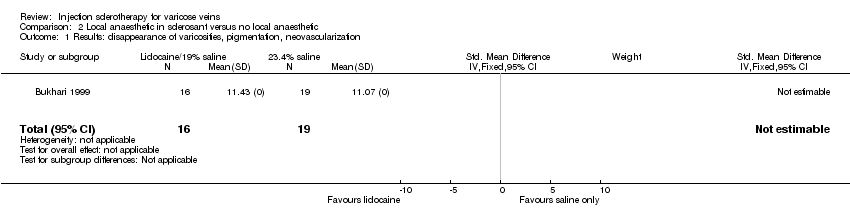
Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 1 Results: disappearance of varicosities, pigmentation, neovascularization.
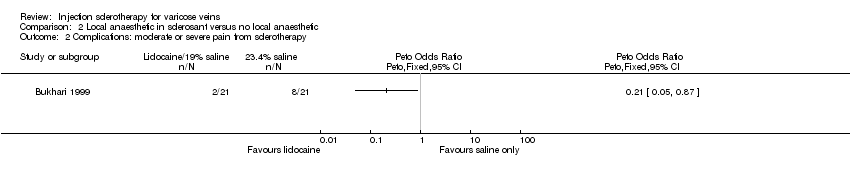
Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 2 Complications: moderate or severe pain from sclerotherapy.

Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 3 Complications: microthrombosis.
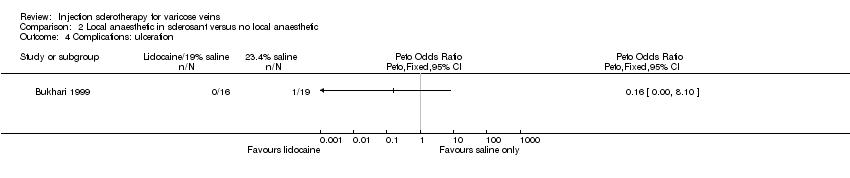
Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 4 Complications: ulceration.

Comparison 2 Local anaesthetic in sclerosant versus no local anaesthetic, Outcome 5 Complications: matting/hyperpigmentation.

Comparison 3 Sclerotherapy with foam versus liquid, Outcome 1 Results: venous spasm.
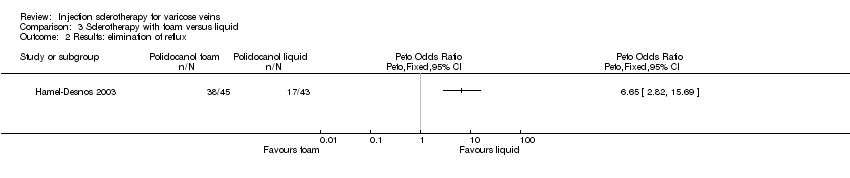
Comparison 3 Sclerotherapy with foam versus liquid, Outcome 2 Results: elimination of reflux.

Comparison 3 Sclerotherapy with foam versus liquid, Outcome 3 Results: recanalisation at 6 months.

Comparison 3 Sclerotherapy with foam versus liquid, Outcome 4 Results: recurrent varicose veins at 10 years.

Comparison 3 Sclerotherapy with foam versus liquid, Outcome 5 Results: failure at 10 years.

Comparison 3 Sclerotherapy with foam versus liquid, Outcome 6 Complications: haematoma.

Comparison 3 Sclerotherapy with foam versus liquid, Outcome 7 Complications: cutaneous inflammation at 3 weeks.

Comparison 3 Sclerotherapy with foam versus liquid, Outcome 8 Results: recurrent varicose veins at 5 years.

Comparison 4 Molefoam versus Sorbo pad to injection sites after sclerotherapy, Outcome 1 Results: successful sclerotherapy.

Comparison 4 Molefoam versus Sorbo pad to injection sites after sclerotherapy, Outcome 2 Complications: erythema.

Comparison 4 Molefoam versus Sorbo pad to injection sites after sclerotherapy, Outcome 3 Complications: ulceration.

Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 1 Results: disappearance of varicosities.

Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 2 Complications: discomfort.
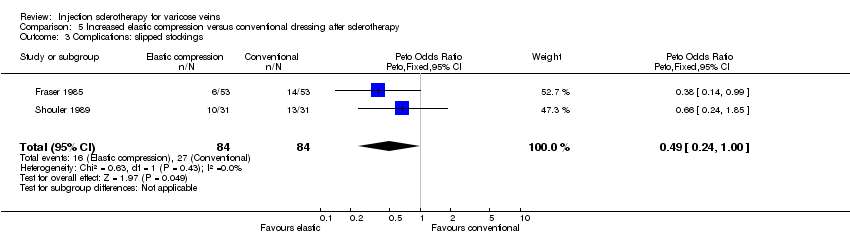
Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 3 Complications: slipped stockings.
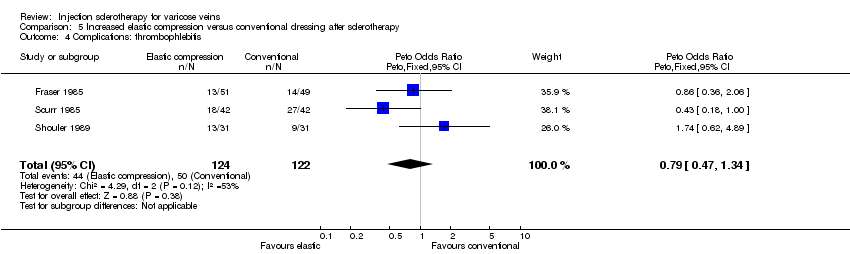
Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 4 Complications: thrombophlebitis.

Comparison 5 Increased elastic compression versus conventional dressing after sclerotherapy, Outcome 5 Complications: skin staining.

Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 1 Results: cosmetic and symptomatic improvement.

Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 2 Results: pain, mobility, cosmetic appearance, satisfaction at 3 months.
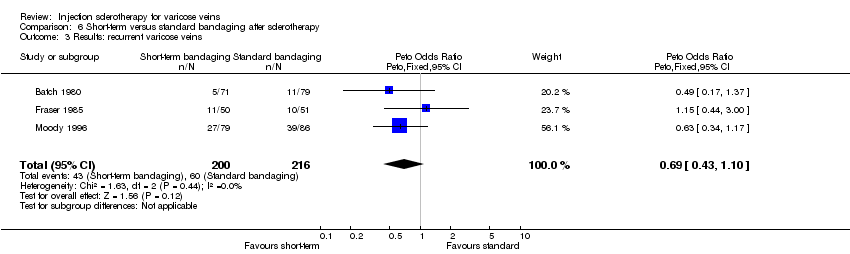
Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 3 Results: recurrent varicose veins.

Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 4 Results: pain, mobility, cosmetic appearance, satisfaction at 2 years.

Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 5 Results: pain, mobility, cosmetic appearance, satisfaction at 4 years.
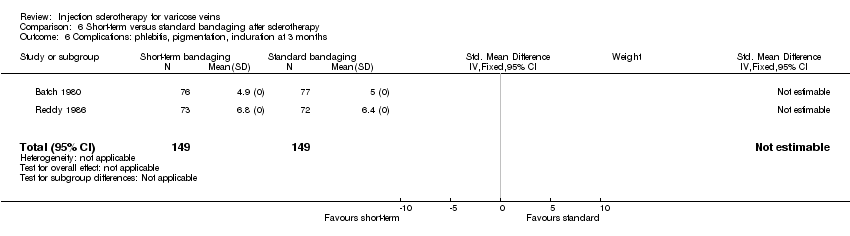
Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 6 Complications: phlebitis, pigmentation, induration at 3 months.

Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 7 Complications: phlebitis, staining, pain, blistering, ulceration.
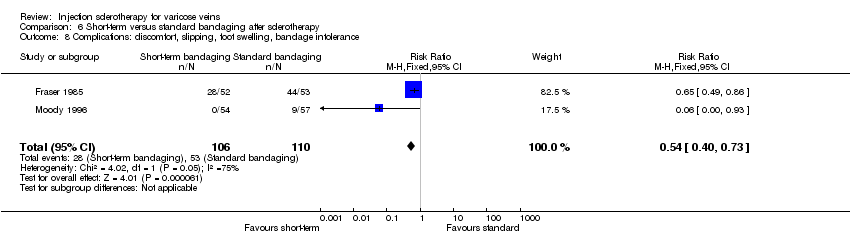
Comparison 6 Short‐term versus standard bandaging after sclerotherapy, Outcome 8 Complications: discomfort, slipping, foot swelling, bandage intolerance.

Comparison 7 Sclerotherapy versus graduated compression stockings, Outcome 1 Results: cosmetic and symptomatic improvement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: venous spasm Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Results: disappearance of reflux Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Results: venous by arterial volume flow Show forest plot | Other data | No numeric data | ||
| 4 Results: disappearance of thread veins Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Results: photographic appearance of veins Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Results: cosmetic appearance at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Results: cosmetic appearance at 5 years Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Results: symptomatic improvement at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Results: symptomatic improvement at 5 years Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Results: recurrent varicose veins at 5 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 11 Results: recurrent varicose veins at 10 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 12 Results: failure at 10 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 13 Complications: allergic reaction Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 14 Complications: pigmentation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 15 Complications: pigmentation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 16 Complications: skin necrosis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 17 Complications: local urticaria Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 18 Complications: pain Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 19 Complications: vein thrombosis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 20 Complications: ecchymosis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 21 Complications: matting Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 22 Complications: matting Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: disappearance of varicosities, pigmentation, neovascularization Show forest plot | 1 | 35 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Complications: moderate or severe pain from sclerotherapy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Complications: microthrombosis Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Complications: ulceration Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Complications: matting/hyperpigmentation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: venous spasm Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Results: elimination of reflux Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Results: recanalisation at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Results: recurrent varicose veins at 10 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Results: failure at 10 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Complications: haematoma Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7 Complications: cutaneous inflammation at 3 weeks Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8 Results: recurrent varicose veins at 5 years Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: successful sclerotherapy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Complications: erythema Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Complications: ulceration Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: disappearance of varicosities Show forest plot | 3 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.71, 2.20] |
| 2 Complications: discomfort Show forest plot | 2 | 168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.65 [1.92, 6.95] |
| 3 Complications: slipped stockings Show forest plot | 2 | 168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.24, 1.00] |
| 4 Complications: thrombophlebitis Show forest plot | 3 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.47, 1.34] |
| 5 Complications: skin staining Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: cosmetic and symptomatic improvement Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Results: pain, mobility, cosmetic appearance, satisfaction at 3 months Show forest plot | 2 | 298 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Results: recurrent varicose veins Show forest plot | 3 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.43, 1.10] |
| 4 Results: pain, mobility, cosmetic appearance, satisfaction at 2 years Show forest plot | 1 | 145 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Results: pain, mobility, cosmetic appearance, satisfaction at 4 years Show forest plot | 1 | 145 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Complications: phlebitis, pigmentation, induration at 3 months Show forest plot | 2 | 298 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Complications: phlebitis, staining, pain, blistering, ulceration Show forest plot | 2 | 331 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.66, 1.73] |
| 8 Complications: discomfort, slipping, foot swelling, bandage intolerance Show forest plot | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.40, 0.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results: cosmetic and symptomatic improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

