نقش مصرف فنوباربیتال پس از زایمان در پیشگیری از بروز هموراژی داخل بطنی در نوزادان نارس
چکیده
پیشینه
هموراژی داخل بطنی (intraventricular haemorrhage; IVH) یکی از عوارض اصلی تولد زودرس است. خونریزیهای بزرگ با خطر بالای معلولیت و هیدروسفالی همراه هستند. بیثباتی فشار خون و میزان خون مغزی (cerebral blood) در جریان خون نوزاد به عنوان عوامل ایجاد کننده در نظر گرفته میشوند. مکانیسم دیگر ممکن است به آسیب پرفیوژن مجدد ناشی از رادیکالهای آزاد اکسیژن بازگردد. پیشنهاد شده که فنوباربیتال (phenobarbital) فشار خون را تثبیت کرده و ممکن است در برابر رادیکالهای آزاد محافظت ایجاد کند. این یک نسخه بهروز شده از مروری است که نخستینبار در سال 2001 منتشر شده، و در سالهای 2007 و 2013 بهروز شد.
اهداف
ارزیابی مزایا و آسیبهای تجویز فنوباربیتال پس از زایمان در نوزادان نارس در معرض خطر ابتلا به IVH در مقایسه با گروه کنترل (یعنی عدم مداخله یا دارونما (placebo)).
روشهای جستوجو
در ژانویه 2022، پایگاه مرکزی ثبت کارآزماییهای کنترل شده کاکرین (CENTRAL)؛ Medline؛ Embase؛ CINAHL و پایگاههای ثبت کارآزمایی بالینی را جستوجو کردیم. یک راهبرد جستوجوی جدید و حساستر ایجاد شد، جستوجوها نیز بدون اعمال محدودیت در تاریخ انتشار مقاله انجام شدند.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) یا شبه‐RCTهایی را وارد کردیم که در آنها فنوباربیتال طی 24 ساعت اول زندگی برای نوزادان نارسی تجویز شد که به دلیل سن بارداری کمتر از 34 هفته، وزن هنگام تولد کمتر از 1500 گرم یا نارسایی تنفسی، در معرض خطر ابتلا به IVH قرار داشتند. فنوباربیتال با عدم مداخله یا دارونما مقایسه شد. نوزادان مبتلا به ناهنجاریهای مادرزادی جدی را کنار گذاشتیم.
گردآوری و تجزیهوتحلیل دادهها
از روشهای استاندارد کاکرین بهره بردیم. پیامدهای اولیه، همه درجات IVH و IVH شدید (یعنی درجه III و IV) بود؛ پیامدهای ثانویه عبارت بودند از دیلاتاسیون (dilation) بطنی یا هیدروسفالی (hydrocephalus)، هیپوتانسیون، پنوموتوراکس، هیپرکاپنی (hypercapnia)، اسیدوز (acidosis)، ونتیلاسیون مکانیکی، اختلال در تکامل سیستم عصبی و مرگومیر. از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (Grading of Recommendations Assessment, Development and Evaluation; GRADE) برای ارزیابی قطعیت شواهد برای هر پیامد بهره گرفتیم.
نتایج اصلی
تعداد 10 RCT (792 نوزاد) را وارد کردیم.
شواهد نشان میدهد که فنوباربیتال در مقایسه با کنترل منجر به تفاوتی اندک تا عدم تفاوت در میزان بروز IVH در هر درجهای (خطر نسبی (RR): 1.00؛ 95% فاصله اطمینان (CI): 0.84 تا 1.19، تفاوت خطر (risk difference; RD): 0.00؛ 95% CI؛ 0.06‐ تا 0.07؛ I² برای RD معادل 65%؛ 10 RCT؛ 792 شرکتکننده؛ شواهد با قطعیت پائین) و IVH شدید (RR: 0.88؛ 95% CI؛ 0.64 تا 1.21؛ 10 RCT؛ 792 شرکتکننده؛ شواهد با قطعیت پائین) میشود.
شواهد در مورد تاثیر فنوباربیتال بر دیلاتاسیون بطنی پس از خونریزی یا هیدروسفالی (RR: 0.62؛ 95% CI؛ 0.31 تا 1.26؛ 4 RCT؛ 271 شرکتکننده؛ شواهد با قطعیت بسیار پائین)، اختلال خفیف در تکامل سیستم عصبی (RR: 0.57؛ 95% CI؛ 0.15 تا 2.17؛ 1 RCT؛ 101 شرکتکننده؛ شواهد با قطعیت بسیار پائین)، و اختلال شدید در تکامل سیستم عصبی (RR: 1.12؛ 95% CI؛ 0.44 تا 2.82؛ 2 RCT؛ 153 شرکتکننده؛ شواهد با قطعیت بسیار پائین) بسیار نامطمئن است. فنوباربیتال در مقایسه با کنترل ممکن است منجر به تفاوتی اندک تا عدم تفاوت در مرگومیر پیش از ترخیص (RR: 0.88؛ 95% CI؛ 0.64 تا 1.21؛ 9 RCT؛ 740 شرکتکننده؛ شواهد با قطعیت پائین) و مورتالیتی در طول دوره مطالعه (RR: 0.98؛ 95% CI؛ 0.72 تا 1.33؛ 10 RCT؛ 792 شرکتکننده؛ شواهد با قطعیت پائین) شود.
هیچ کارآزمایی در حال انجامی را شناسایی نکردیم.
نتیجهگیریهای نویسندگان
شواهد حاکی از آن است که فنوباربیتال در مقایسه با کنترل (یعنی عدم مداخله یا دارونما) تفاوتی اندک تا عدم تفاوت در میزان بروز IVH (هر درجه یا شدید) ایجاد میکند. شواهد در مورد تاثیرات فنوباربیتال بر دیلاتاسیون بطنی یا هیدروسفالی و اختلال در تکامل سیستم عصبی بسیار نامطمئن است. شواهد نشان میدهد که فنوباربیتال در مقایسه با کنترل منجر به تفاوتی اندک تا عدم تفاوت در میزان مرگومیر پیش از ترخیص و تمام مرگومیرها در طول دوره مطالعه میشود.
از سال 1993، هیچ مطالعه تصادفیسازی شدهای در مورد نقش فنوباربیتال در پیشگیری از IVH در نوزادان نارس منتشر نشده و هیچ کارآزماییای در حال انجام نیست. تاثیرات تجویز فنوباربیتال پس از زایمان باید در نوزادان مبتلا به تشنجهای نوزادی و IVH در مطالعات تصادفیسازی شده و مشاهدهای ارزیابی شود. ارزیابی مزایا و آسیبها باید شامل پیامدهای طولانیمدت باشد.
PICOs
خلاصه به زبان ساده
مزایا و خطرات فنوباربیتال در پیشگیری از بروز خونریزی مغزی در نوزادانی که خیلی زود به دنیا میآیند، چیست؟
پیامهای کلیدی
• مصرف فنوباربیتال (phenobarbital) (دارویی که برای کنترل تشنجها استفاده میشود) در نوزادانی که خیلی زود به دنیا میآیند، ممکن است تاثیری اندک تا عدم تاثیر بر پیشگیری از بروز هموراژی داخل بطنی (خونریزی مغزی) و مرگومیر داشته باشد.
• شواهد در مورد تاثیر فنوباربیتال در پیشگیری از دیلاتاسیون (dilation) بطنی (فضاهای بزرگ شده در مغز) و رشد طولانیمدت مغز بسیار نامطمئن است.
خونریزی مغزی (هموراژی داخل بطنی) چیست؟
وقوع خونریزیهای بزرگ در مرکز مغز میتوانند باعث ایجاد معلولیت یا مرگومیر در نوزادانی شوند که خیلی زود به دنیا میآیند. اعتقاد بر این است که فشار خون و جریان خون ناپایدار به مغز باعث خونریزی در حفرههای پر از مایع مغز (بطنها) میشود.
ما به دنبال چه یافتهای بودیم؟
اعتقاد بر این است که فنوباربیتال فشار خون را تثبیت کرده و بنابراین بهطور بالقوهای به پیشگیری از خونریزی مغزی کمک میکند. ما میخواستیم بدانیم که مصرف فنوباربیتال برای پیشگیری از خونریزی مغزی بهتر از عدم استفاده از دارو یا دارونما (placebo) (یک درمان «ساختگی» که حاوی هیچ دارویی نیست اما ظاهر یا طعمی مشابه با داروی تست شده دارد) است یا خیر.
ما چه کاری را انجام دادیم؟
در جستوجوی مطالعاتی بودیم که تجویز فنوباربیتال را با عدم استفاده از دارو مقایسه کردند. نتایج این مطالعات را مقایسه و خلاصه کرده و اعتماد خود را نسبت به این شواهد بر اساس عواملی مانند روشهای انجام و حجم نمونه مطالعه رتبهبندی کردیم.
ما به چه نتایجی رسیدیم؟
تعداد 10 مطالعه (792 نوزاد) را وارد کردیم.
شواهد حاکی از آن است که فنوباربیتال، تاثیری اندک تا عدم تاثیر بر پیشگیری از بروز خونریزی مغزی دارد. شواهد در مورد تاثیر فنوباربیتال در پیشگیری از دیلاتاسیون بطنی در مغز و رشد طولانیمدت بسیار نامطمئن است. شواهد نشان میدهد که فنوباربیتال، تاثیری اندک تا عدم تاثیر بر پیشگیری از وقوع مرگومیر دارد. هیچ کارآزمایی در حال انجامی را شناسایی نکردیم.
محدودیتهای شواهد چه هستند؟
ممکن است افراد شرکتکننده در این مطالعات از نوع درمان خود آگاه بوده باشند. همه مطالعات دادههایی را در مورد همه موضوعاتی ارائه نکردند که به آنها علاقهمند بودیم. این مطالعات حجم نمونه بسیار اندکی داشتند.
این شواهد تا چه زمانی بهروز است؟
این مطالعه، مرور قبلی ما را بهروز میکند. شواهد تا ژانویه 2022 بهروز است.
Authors' conclusions
Summary of findings
| Phenobarbital compared to placebo or no intervention for the prevention of IVH in preterm infants | ||||||
| Patient or population: preterm infants with or at risk of IVH | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo or no intervention | Risk with phenobarbital | |||||
| IVH any grade (Papile classification) during hospitalisation | Study population | RR 1.00
RD 0.00 (‐0.06 to 0.07) | 792 | ⊕⊕⊝⊝ | The evidence suggests that phenobarbital results in little to no difference in the incidence of IVH (any grade) compared with control | |
| 388 per 1000 | 388 per 1000 | |||||
| Severe IVH (Papile classification) during hospitalisation | Study population | RR 0.88
RD ‐0.02 (‐0.07 to 0.03) | 792 | ⊕⊕⊝⊝ | The evidence suggests that phenobarbital results in little to no difference in the incidence of severe IVH compared with control | |
| 161 per 1000 | 142 per 1000 | |||||
| Ventricular dilation or hydrocephalus during hospitalisation | Study population | RR 0.62
RD ‐0.05 (‐0.12 to 0.02) | 271 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of phenobarbital on ventricular dilation or hydrocephalus | |
| 129 per 1000 | 80 per 1000 | |||||
| Mild neurodevelopmental impairment at 27 months of age | Study population | RR 0.57
RD ‐0.05 (‐0.16 to 0.06) | 101 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of phenobarbital on mild neurodevelopmental impairment | |
| 111 per 1000 | 63 per 1000 | |||||
| Severe neurodevelopmental impairment at 9 to 27 months of age | Study population | RR 1.12
RD 0.01 (‐0.09 to 0.11) | 153 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of phenobarbital on severe neurodevelopmental impairment | |
| 99 per 1000 | 111 per 1000 | |||||
| Death before discharge | Study population | RR 0.88
RD ‐0.02 (‐0.07 to 0.03) | 740 | ⊕⊕⊝⊝ | The evidence suggests that phenobarbital results in little to no difference in death before discharge compared with control | |
| 173 per 1000 | 152 per 1000 | |||||
| All deaths during the study | Study population | RR 0.98
RD 0.00 (0.05 to 0.05) | 792 | ⊕⊕⊝⊝ | The evidence suggests that phenobarbital results in little to no difference in all deaths during study compared with control | |
| 166 per 1000 | 163 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). IVH: intraventricular haemorrhage RD: risk difference RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for high or unclear risk of bias in all domains of the risk of bias tool. 2 Downgraded by one level for inconsistency (I2 = 58%). 3 Downgraded by one level for imprecision of the estimates. 4 Downgraded by two levels for imprecision of the estimates, due to wide CIs and low sample size. 5 Downgraded by one level for high risk of performance bias and unclear risk for detection and reporting bias. 6 Downgraded by two levels for imprecision for wide CIs in one study with very low sample size and few events. 7 Downgraded by one level for high or unclear risk of bias in most domains (all except attrition bias). | ||||||
Background
Description of the condition
Intraventricular haemorrhage (IVH) is a major complication of preterm birth, and severe haemorrhages (grade 3 or higher) or haemorrhages associated with parenchymal brain lesions (grade 4) have a high rate of disability (Cizmeci 2019; Shankaran 2020; Stoll 2015; Vohr 1989; Younge 2017). Massive IVH may result in death from hypovolaemia, and severe haemorrhages may result in hydrocephalus in infants who survive, causing neurodevelopmental impairment (Cizmeci 2019; Luyt 2020; Shankaran 2020; Stoll 2015; Volpe 1995; Whitelaw 2007). In preterm infants, IVH originates not from an artery, but rather from capillaries of the subependymal germinal matrix (Romantsik 2019). The particular vulnerability of premature infants is thought to result from a subependymal germinal matrix that is rich in immature vessels poorly supported by connective tissue (Ballabh 2014; Gould 1987; Hambleton 1976), marked fluctuations in cerebral blood flow (Mullaart 1994; Pasternak 1983; Perlman 1983) and severe respiratory problems that result in major swings in intrathoracic and venous pressure that are then transmitted to the fragile germinal matrix (Nakamura 1990; Volpe 2008). In addition, there is evidence that ischaemia followed by reperfusion plays a role in the pathogenesis of IVH and that cerebral ischaemia may result from IVH. This may take the form of periventricular haemorrhagic infarction (Volpe 1995; Volpe 2008). Periventricular haemorrhagic infarction lesions are typically unilateral and continuous with the margin of the lateral ventricle. The aetiology is thought to be changes in cerebrospinal fluid (CSF) homeostasis on the one hand due to obstruction of venous drainage by a blood clot and, on the other hand, hypersecretion of CSF through the activation of Toll‐like receptor (TLR)‐4 and the nuclear factor‐κB inflammatory pathway (Aquilina 2012; Karimy 2017). Interventions aimed at the prevention of IVH or its consequences may be targeted at any one (or more) of the above mechanisms.
Diagnosis of IVH by ultrasound
Initially, the diagnosis of IVH was made by cerebral computed tomography (CT). However, now, cranial ultrasound can be conducted cotside and does not expose the infant to any ionising radiation. This enables whole populations of infants to be safely and ethically examined. Indeed, Papile's classification of IVH was originally developed for CT (Papile 1978), but was quickly implemented by ultrasonographers:
-
grade I haemorrhage is confined to the subependymal germinal matrix with no blood clot in the lumen;
-
grade II haemorrhage is a small haemorrhage within the ventricular lumen without ventricular dilation;
-
grade III haemorrhage is a large haemorrhage sufficient to expand the ventricle from the amount of blood
-
grade IV haemorrhage is IVH plus parenchymal haemorrhagic venous infarction (Volpe 1995).
Although ultrasound diagnosis of low‐grade IVH is not perfect, with a sensitivity of 61% and specificity varying between 80% and 100%, the diagnosis of severe IVH with ultrasound shows high sensitivity (90%) and specificity (100%) (Hope 1988; Parodi 2015).
Timing of IVH
Approximately 80% of IVH occurs within 72 hours of birth, but, in a considerable proportion of cases, IVH is visible on the first scan within a few hours of birth (Levene 1982; Volpe 2008). This means that interventions to prevent IVH should ideally start before delivery and should be continued soon after birth.
Description of the intervention
Phenobarbital is a barbiturate that acts on GABAA receptors in the central nervous system. Phenobarbital prolongs and potentiates the action of gamma aminobutyric acid (GABA) on GABAA receptors and may activate the receptors directly. Phenobarbital is frequently used in children as an anticonvulsant.
How the intervention might work
Postnatal phenobarbital
The administration of postnatal phenobarbital for the prevention of IVH in low‐birthweight infants was suggested in the 1980s based on the following data:
-
the observation that phenobarbital may dampen fluctuations in systemic blood pressure in premature infants (Wimberley 1982);
-
evidence that treatment with phenobarbital reduces the incidence of intracranial haemorrhage in newborn beagles made hypertensive with phenylephrine (Goddard 1987);
-
experimental evidence that barbiturates can partially protect the brain against hypoxic–ischaemic damage (Steen 1979);
-
the suggestion that the free radical‐scavenging capacity of phenobarbital may protect the brain after hypoxia‐ischaemia (Ment 1985).
However, very few studies on phenobarbital for the prevention of IVH have been published in the following decades.
Drug side effects
Phenobarbital and other barbiturates may cause respiratory depression, with consequent respiratory acidosis and the need for mechanical ventilation; cardiac depression; and hypotension (Kumar 2021; Maitre 2013; Sharpe 2020). Furthermore, concerns have been raised about the effects of chronic exposure to phenobarbital on long‐term neurodevelopment (Kwan 2004). In addition, data from studies in rats report an increase in apoptosis (cell death) in the immature brain following postnatal administration (Bittigau 2002; Forcelli 2011; Forcelli 2012; Kaushal 2016).
Why it is important to do this review
One previous systematic review on this topic (Horbar 1992), including eight trials, concluded that postnatal phenobarbital did not reduce the frequency or severity of IVH in preterm infants. This Cochrane systematic review was first published in 2013 (Smit 2013) in order to include studies after 1988, as well as outcomes not included in the Horbar 1992 review. This 2023 review is an update of the Smit 2013 review, which was originally titled 'Postnatal phenobarbital for the prevention of intraventricular haemorrhage'.
Objectives
To assess the benefits and harms of postnatal administration of phenobarbital in preterm infants at risk of developing IVH compared to control (i.e. no intervention or placebo).
Methods
Criteria for considering studies for this review
Types of studies
All controlled trials, whether randomised or quasi‐randomised, in which postnatal phenobarbital was compared to control treatment of preterm infants at risk of IVH. We included cross‐over trials and cluster‐randomised trials.
Types of participants
We included:
-
newborn infants (less than 24 hours old) with a gestational age of less than 34 weeks or a birthweight less than 1500 grams;
-
preterm newborn infants with gestational ages of 33 to 36 weeks or birthweights up to 1750 grams if they were mechanically ventilated.
We also planned to include studies reporting on a subset of the aforementioned population, provided results were available for this subset alone.
We excluded infants with serious congenital malformations.
Types of interventions
Phenobarbitone (phenobarbital) by intravenous or intramuscular injection starting within 24 hours of birth, with or without maintenance therapy for up to seven days. The comparator was no intervention or placebo.
We planned to include studies where co‐interventions were administered to both arms (e.g. phenobarbital plus heparin versus placebo plus heparin). We planned to exclude studies where co‐interventions were administered to one arm only (e.g. phenobarbital plus heparin versus placebo without heparin; phenobarbital without heparin versus placebo plus heparin).
Types of outcome measures
The following outcome measures do not form part of the eligibility criteria.
Primary outcomes
-
All grades of IVH (Papile 1978)
-
Severe IVH (i.e. grade III and IV IVH)
Secondary outcomes
-
Ventricular dilation or hydrocephalus
-
Hypotension (mean arterial pressure < 30 mmHg) during the first week of life
-
Pneumothorax or interstitial emphysema during the first week of life
-
Hypercapnia (> 8 kPa or 60 mmHg) during the first week of life
-
Acidosis (pH < 7.2) during the first week of life
-
Mechanical ventilation (including infants who were ventilated at enrolment)
-
Mild neurodevelopmental impairment (developmental quotient (DQ) < 80 or motor abnormality on examination)
-
Severe neurodevelopmental impairment (clinical cerebral palsy or DQ below the range that can be measured)
-
Death before discharge from hospital
-
Death at any time during the study
Search methods for identification of studies
A new, more‐sensitive search strategy was developed for this update and searches were run without date, language or publication type limits. Strategies were written by Information Specialists at Cochrane Sweden and peer reviewed using the PRESS Checklist (McGowan 2016a; McGowan 2016b). A methodological filter was used to restrict retrieval to RCTs and quasi‐randomised trials.
Electronic searches
The following databases were searched without language, publication year, publication type or publication status restrictions in January 2022.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 18 January 2022, Issue 1), via Wiley
-
MEDLINE via EbscoHost (1966 to 18 January 2022)
-
EMBASE via Elsevier (1966 to 18 January 2022)
-
CINAHL Complete via EbscoHost (1966 to 18 January 2022)
Searching other resources
Trial registration records were identified using CENTRAL and by independent searches of the US National Library of Medicine (clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; https://www.who.int/clinical-trials-registry-platform/the-ictrp-search-portal).
We identified conference abstracts using CENTRAL and Embase.
We screened the reference lists of related studies and systematic reviews not identified by the database searches.
We searched for errata or retractions for included studies published on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
For each study included, we collected information regarding the method of randomisation, blinding, intervention and stratification, as well as whether the trial was a single or multi‐centre study. We noted information regarding trial participants, including gestational age and birthweight. We analysed the clinical outcomes listed in the Types of outcome measures.
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises the following three components:
-
known assessments (a service that matches records in the search results to records that have already been screened and labelled as 'RCT' or 'not an RCT' in Cochrane Crowd, Cochrane’s citizen science platform where the Crowd helps identify and describe health evidence);
-
the RCT classifier (a machine‐learning model that distinguishes RCTs from non‐RCTs);
-
Cochrane Crowd (http://crowd.cochrane.org).
For more information about Screen4Me and the evaluations that have been undertaken, please go to the Screen4Me web page on the Cochrane Information Specialist’s portal (https://community.cochrane.org/sites/default/files/uploads/Reporting_Guidance_Screen4Me_FINAL.pdf). Additional detailed information regarding evaluations of the Screen4Me components has been published elsewhere (Marshall 2018; Noel‐Storr 2020; Noel‐Storr 2021; Thomas 2020).
Two review authors (OR, MB) independently screened the titles and abstracts of references remaining after categorisation by Screen4Me. Two review authors independently screened the full text of references included based on title abstract (MB, OR). We resolved disagreements by discussion and, if necessary, by consultation with a third review author (ES). We excluded studies published only in abstract form unless the final results of the trial were reported and all necessary information could be ascertained from the abstract, authors or both. We reviewed studies for relevance by assessing study design, types of participants, interventions provided and outcome measures reported. We have provided details of the studies excluded in the Characteristics of excluded studies table, along with reasons for exclusion. We contacted trial authors if the details of a primary trial were unclear. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009) and 'Characteristics of included studies' table.
We used Covidence for screening (https://www.covidence.org).
Data extraction and management
Two review authors (MB, OR) independently extracted data using a data extraction form integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist (Cochrane EPOC Group 2017). We piloted the form within the review team, using a sample of included studies. We extracted the following characteristics from each included study:
-
administrative details (i.e. study author(s); published or unpublished; year of publication; year in which the study was conducted; presence of vested interest; details of other relevant papers cited);
-
study (study design; type, duration and completeness of follow‐up (e.g. greater than 80%); country and location of study; informed consent; ethics approval);
-
participants (sex; birthweight; gestational age; number of participants);
-
interventions (initiation, dose and duration of administration);
-
outcomes (as mentioned above under Types of outcome measures).
We resolved disagreements by discussion or consultation with a third review author (ES). We described ongoing studies identified by our search, when available, detailing the primary author, research question(s), methods and outcome measures, together with an estimate of the reporting date. This information is reported in the Characteristics of ongoing studies table.
If any queries arose (e.g. discrepancies in the way outcomes were defined in the trials and the definitions in Types of outcome measures), or if additional data would have been required, we contacted the study investigators/authors for clarification. Two review authors (PB, CR) used Cochrane statistical software for data entry (Review Manager 2020). We replaced any standard error of the mean (SEM) with the corresponding standard deviation (SD).
Assessment of risk of bias in included studies
Two review authors (MB, OR) used the Cochrane risk of bias tool to independently assess the risk of bias (low, high or unclear) for all included trials for the following domains (Higgins 2011):
-
sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
blinding of outcome assessment (detection bias);
-
incomplete outcome data (attrition bias);
-
selective reporting (reporting bias);
-
any other bias.
Any disagreements were resolved by discussion or by a third assessor. For a more detailed description of the risk of bias for each domain, see Appendix 1 .
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results using risk ratios (RR) and risk differences (RD) with 95% confidence intervals (CIs). We calculated the number needed to treat for an additional beneficial outcome (NNTB), or the number needed to treat for an additional harmful outcome (NNTH) with 95% CIs if there was a statistically significant reduction (or increase) in RD.
Continuous data
For continuous data, we used the mean difference (MD) when outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measured the same outcome but used different methods. Where trials reported continuous data as the median and interquartile range (IQR) and data passed the test of skewness, we converted the median to mean and estimated the SD as IQR/1.35.
Unit of analysis issues
We performed the primary analysis per individual randomised. We abstracted information on the study design and unit of analysis for each study, indicating whether clustering of observations is present due to allocation to the intervention at the group level or clustering of individually randomised observations (e.g. patients within clinics). Available statistical information needed to account for the implications of clustering on the estimation of outcome variances were abstracted, such as design effects or intracluster correlations, and whether the study adjusted results for correlations in the data. In cases where the study did not account for clustering, we ensured that appropriate adjustments were made to the effective sample size following Cochrane guidance (Higgins 2020). Where possible, we derived the intracluster correlation (ICC) for these adjustments from the trial itself, or from a similar trial. If an appropriate ICC was unavailable, we conducted sensitivity analyses to investigate the potential effect of clustering by imputing a range of ICC values.
If any trials had multiple arms that were compared against the same control condition that was included in the same meta‐analysis, we either combined groups to create a single pair‐wise comparison or selected one pair of interventions and excluded the others.
In the meta‐analysis and data synthesis, we only included first‐phase data from cross‐over trials.
Dealing with missing data
We conducted analyses on an intention‐to‐treat basis for all included outcomes. Whenever possible, we analysed all participants in the treatment group to which they were randomised, regardless of the actual treatment received. If we identified important missing data (in the outcomes) or unclear data, we requested the missing data by contacting the original investigators. We made explicit the assumptions of any methods used to deal with missing data. We planned to perform sensitivity analyses to assess how sensitive the results are to reasonable changes in the assumptions made, but there were no missing data. We planned to address the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We describe the clinical diversity and methodological variability of the evidence in the review text, with study tables describing study characteristics, including design features, population characteristics and intervention details.
To assess statistical heterogeneity, we visually inspected forest plots and describe the direction and magnitude of effects, as well as the degree of overlap between CIs. We also considered the statistics generated in forest plots that measure statistical heterogeneity. We used the I² statistic to quantify inconsistency among the trials in each analysis. We also considered the P value from the Chi² test to assess whether this heterogeneity is significant (P < 0.1). If we identified substantial heterogeneity, we reported the finding and explored possible explanatory factors using prespecified subgroup analysis.
We graded the degree of heterogeneity as follows:
-
0% to 40% may not represent important heterogeneity;
-
30% to 60% may represent moderate heterogeneity;
-
50% to 90% may represent substantial heterogeneity;
-
greater than 75% may represent considerable heterogeneity.
A rough guideline was used to interpret the I2 value rather than a simple threshold, and our interpretation took into account the understanding that measures of heterogeneity (I2 and Tau) are estimated with high uncertainty when the number of studies is small (Deeks 2020).
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary and secondary outcomes against reported outcomes. Where study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias. Studies using the interventions in a potentially eligible infant population but not reporting on any of the primary and secondary outcomes were documented in the 'Characteristics of included studies' tables.
We used the funnel plots to screen for publication bias where there was a sufficient number of studies (> 10) reporting the same outcome. If publication bias was suggested by a significant asymmetry of the funnel plot on visual assessment, we incorporated this in our assessment of certainty of evidence (Egger 1997). If our review includes few studies eligible for meta‐analysis, the ability to detect publication bias will be largely diminished and we would simply note our inability to rule out possible publication bias or small study effects.
Data synthesis
We performed a meta‐analysis using Review Manager 5 (Review Manager 2020). For categorical outcomes, we calculated the typical estimates of RR and RD, each with its 95% CI; for continuous outcomes, we calculated the MD or SMD, each with its 95% CI. We used a fixed‐effect model to combine data where it is reasonable to assume that studies were estimating the same underlying treatment effect. We analysed and interpreted individual trials separately when we judged meta‐analysis to be inappropriate. In case of evidence of clinical heterogeneity, we planned to explain this based on the different study characteristics and subgroup analyses.
Subgroup analysis and investigation of heterogeneity
Tests for subgroup differences in effects are to be interpreted with caution given the potential for confounding with other study characteristics and the observational nature of the comparisons (see Section 10.11.2 Cochrane Handbook version 6). In particular, subgroup analyses with fewer than five studies per category are unlikely to be adequate to ascertain valid differences in effects and, had we been able to conduct subgroup analyses, we would not have highlighted these in our results. If we had performed subgroup analyses, we had planned to conduct stratified meta‐analysis and a formal statistical test for interaction to examine subgroup differences that could account for effect heterogeneity (e.g. Cochran’s Q test, meta‐regression) (Borenstein 2013; Higgins 2020).
Given the potential differences in the intervention effectiveness related to gestational age (extremely preterm infants are more vulnerable) and the need for mechanical ventilation, we planned to conduct subgroup comparisons to see whether the intervention is more effective for certain groups where data were available. We considered the following groups for subgroup analysis, where data were available, and restricted these analyses to the main outcomes:
-
gestational age less than 30 weeks;
-
infants on mechanical ventilation.
These analyses were not conducted because gestational age overlapped across the included studies and all studies included infants on mechanical ventilation.
Sensitivity analysis
We planned to conduct sensitivity analyses to explore the effects of the methodological quality of trials, checking to ascertain whether studies with a high risk of bias (in at least two domains) overestimated the effect of treatment, but this analysis was not conducted because only one of the included trials had an overall low risk of bias (Whitelaw 1983). Differences in study design of included trials may also affect the results of the systematic review. We planned to perform a sensitivity analysis to compare the effects of phenobarbital in truly randomised trials as opposed to quasi‐randomised trials, but this was not done because only one of the included studies was a quasi‐randomised trial (Morgan 1982).
Summary of findings and assessment of the certainty of the evidence
The summary of findings tables (summary of findings Table 1) address the effects of phenobarbital dosage in all enrolled infants. We used the GRADE approach to assess the certainty of evidence for the following (clinically relevant) outcomes (Schünemann 2013):
-
all grades of IVH;
-
severe IVH (i.e. grade III and IV IVH);
-
ventricular dilation or hydrocephalus;
-
mild neurodevelopmental impairment (DQ < 80 or motor abnormality on examination);
-
severe neurodevelopmental impairment (clinical cerebral palsy or DQ below the range that can be measured);
-
death before discharge from hospital;
-
death at any time during the study.
Two review authors (OR, MB) independently assessed the certainty of the evidence for each of the seven outcomes above. We considered evidence from RCTs as high‐certainty, but downgraded the evidence by one level for serious (or two levels for very serious) limitations based on the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and the presence of publication bias. We used GRADEpro GDT to create a summary of findings table to report the certainty of the evidence. The GRADE approach resulted in an assessment of the certainty of a body of evidence in one of the following four grades.
-
High: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
-
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
The search run in January 2022 identified 2060 search results (1731 after de‐duplication). In assessing the studies, we used Cochrane’s Screen4Me workflow to help identify potential reports of randomised trials. The results of the Screen4Me assessment process are shown in Figure 1.
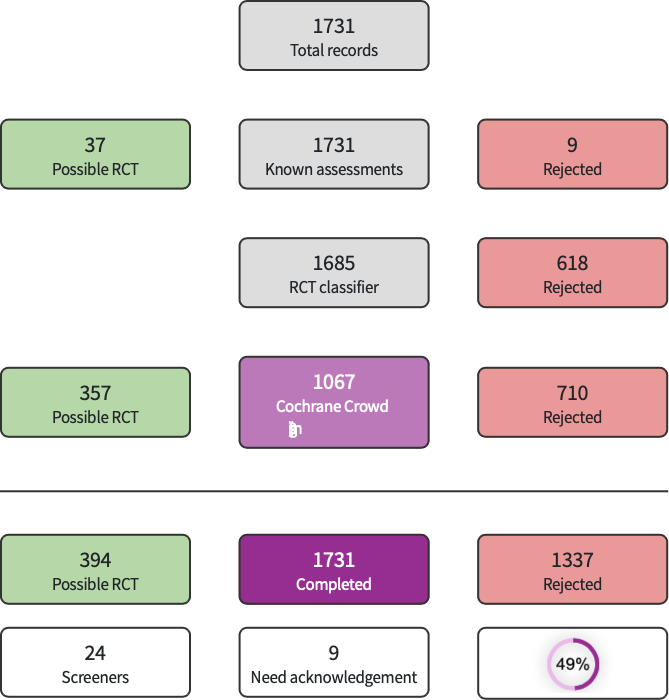
Screen4Me summary diagram.
We then imported the remaining 394 studies left after the Screen4Me assessment to Covidence; 13 additional duplicates were removed by Covidence, leaving 381 studies for assessment. We excluded 369 studies after screening the title and abstract and another two studies after full text screening, leaving 10 RCTs for inclusion in this analysis (Figure 2). We did not identify any ongoing studies.

Prisma flow diagram.
Included studies
We included 10 studies enrolling 792 infants (Anwar 1986; Bedard 1984; Donn 1981; Kuban 1986; Mas‐Munoz 1993; Morgan 1982; Porter 1985; Ruth 1985; Ruth 1988; Whitelaw 1983). One of the studies was funded by a private company (Mead Johnson) (Kuban 1986). Two studies declared funding sources from public institutions (Ruth 1988; Whitelaw 1983). Most studies did not declare whether funding was received.
Participants
The infants in the studies were relatively similar, being preterm infants who were at risk of IVH either because of gestational age below 34 weeks, birthweight below 1500 g, respiratory distress syndrome requiring mechanical ventilation or a combination of these factors (Table 1). Cranial ultrasound was conducted before trial entry in only six trials and infants who already had IVH were thereby excluded. It is likely that some infants in the trials already had IVH before randomisation (Anwar 1986; Donn 1981; Ruth 1985; Ruth 1988). In two trials, infants in the treatment group were older than those in the control group (Bedard 1984; Morgan 1982). In another study, newborns in the phenobarbital group had lower gestational age and birthweight (Kuban 1986). In Porter's trial, newborns in the control group had lower Apgar scores at both 1 and 5 minutes than those in the treatment group (Porter 1985), whereas in Morgan's trial there were more outborn patients in the control group (Morgan 1982). Five studies were conducted in the USA (Anwar 1986; Bedard 1984; Donn 1981; Kuban 1986; Porter 1985), two were conducted in England (Morgan 1982; Whitelaw 1983), two were conducted in Finland (Ruth 1985; Ruth 1988), and one was conducted in Mexico (Mas‐Munoz 1993).
|
|
Birthweight (g) |
GA (weeks) |
Study groups |
Initiation (hours) | |||
|
| Phenobarbital |
Control | Phenobarbital |
Control | Phenobarbital | Control |
|
|---|---|---|---|---|---|---|---|
| 1119 ± 264 | 1120 ± 218 | NR | NR | LD 20 mg/kg, T12 MD 2.5 mg/kg, x12, UD | No intervention | 6 | |
| 1491 ± 421 | 1271 ± 422 | 31.1 ± 2.7 | 32.2 ± 1.7 | LD 10 mg/kg, T12 MD 2.5 mg/kg, x12, for 6 days | No intervention | 6 | |
| 1101 ± 243 | 1037 ± 208 | 28.9 ± 1.9 | 28.6 ± 1.9 | LD 10 mg/kg, T12 MD 2.5 mg/kg, x12, for 7 days | No intervention | 12 | |
| Reported as the number of newborns for four different weight groups: ≤ 1000, 1001 to 1250, 1251 to 1500, 1501 to 1750 g | Reported as the number of newborns for three different GA groups: < 28, ≥28 to < 32 and ≥ 32 to < 37 weeks | LD 10 mg/kg, T12 MD 2.5 mg/kg, x12, UD | Placebo | 6 | |||
| 1544 ± 480 | 1394 ± 430 | 31.5 ± 2 | 31.0 ± 2.0 | LD 20 mg/kg MD 2.5 mg/kg, x12, UD | No intervention | 6 | |
| 1150 ± 200 | 1070 ± 250 | 31.1 ± 2.7 | 28.8 ± 2.8 | LD 20 mg/kg no MD | No intervention | 24 | |
| 1058 ± 269 | 1061 ± 226 | 29.4 ± 2.8 | 28.8 ± 2.2 | LD 30 mg/kg MD 5 mg/kg, x24, for 3 days | No intervention | 6 | |
| 1090 ± 247 | 1180 ± 222 | 28.7 ± 2.1 | 29.3 ± 1.8 | LD 15 mg/kg, T4 MD 5 mg/kg, x24, for 5 days | No intervention | 2 | |
| 1160 ± 210 | 1120 ± 250 | 29.4 ± 1.7 | 29.2 ± 2.1 | LD 15 mg/kg, T4 MD 5 mg/kg, x24, for 5 days | Glucose infusion | 4 | |
| 1116 ± 215 | 1143 ± 238 | 29.7 ± 2.0 | 29.8 ± 2.1 | LD 20 mg/kg no MD | Saline | 4 | |
Unless indicated otherwise, data are given as the mean±SD.
GA: gestational age
LD: loading dose
MD: maintenance dose
NR: not reported
SD: standard deviation
T4: twice 4 hours apart
T12: twice 12 hours apart
UD: unknown duration
x12: every 12 hours
x24: every 24 hours
Variations in the intervention in included studies
The indication for phenobarbital administration was IVH prevention in all 10 studies. Three studies required respiratory support as an obligatory entry criterion (Kuban 1986; Mas‐Munoz 1993; Morgan 1982).
In all trials, treatment started with the injection of a loading dose of phenobarbital, the dose varying between 20 mg/kg (seven trials) and 30 mg/kg (three trials; Table 1). Six of the trials divided the loading dose into two separate injections administered at 90‐minute, four‐hour or 12‐hour intervals. The loading dose of phenobarbital was administered intravenously in eight studies. In one trial, both intravenous and intramuscular routes of administration were used (Whitelaw 1983), whereas in another trial phenobarbital was administered intramuscularly (Morgan 1982). Except for the studies by Morgan 1982 and Whitelaw 1983, the trials used a maintenance dose of phenobarbital: either 2.5 mg/kg every 12 hours (Anwar 1986; Bedard 1984; Donn 1981; Kuban 1986; Mas‐Munoz 1993); or 5 mg/kg every 24 hours (Porter 1985; Ruth 1985; Ruth 1988). In five trials, maintenance therapy with phenobarbital was given for three to seven days, whereas in four studies the duration of phenobarbital treatment was not clear (Anwar 1986; Kuban 1986; Mas‐Munoz 1993; Morgan 1982). Blood concentrations of phenobarbital were measured in all trials except one (Ruth 1985). Placebo was used in three trials (Kuban 1986; Ruth 1988; Whitelaw 1983), but was not revealed to clinicians in the two double‐blind trials (Kuban 1986; Whitelaw 1983).
Outcomes in included studies
The main outcome, IVH, was ascertained by ultrasonography in all 10 trials. Eight studies used the classification of Papile 1978 to grade IVH, whereas the IVH definitions of Shankaran 1982 and Levene 1982 were used in Bedard 1984 and Whitelaw 1983, respectively.
All studies provided some data on mortality. Mortality data from Kuban 1986 were not given in the original publication, but were subsequently reported in the follow‐up paper (Krishnamoorthy 1990). Data on the potential adverse effects of phenobarbital treatment were provided in some studies: hypotension in two (Donn 1981; Kuban 1986); hypercapnia in six (Anwar 1986; Bedard 1984; Donn 1981; Morgan 1982; Porter 1985; Whitelaw 1983); and acidosis in five (Bedard 1984; Kuban 1986; Morgan 1982; Porter 1985; Whitelaw 1983). The rate of mechanical ventilation was reported in all 10 studies, but the duration of mechanical ventilation was reported in only one (Mas‐Munoz 1993). All 10 trials reported the rate of pneumothorax for both groups.
See Characteristics of included studies table.
Excluded studies
Following full text screening, we excluded two studies (Cooke 1982; Saliba 1991). Cooke 1982 is a letter to the editor. Saliba 1991 is described as cross‐over trial, but all infants first received placebo, then phenobarbital. That study reported no relevant effects of phenobarbital on cerebral blood flow velocity, heart rate, mean arterial blood pressure or blood gases (see Characteristics of excluded studies).
Three previously included studies have been moved to Additional references because they were not randomised, as was clear from the titles (Liang 2009; Zhang 2009), or abstract (Sluncheva 2006). As per Cochrane methods, only RCTs and quasi‐RCTs are included in Cochrane reviews, and the full text of these studies should not have been assessed.
Three previously excluded studies have been moved to Additional references: two were not randomised or quasi‐randomised studies, as was clear from the titles and abstracts (Chen 2008; Hope 1982), and one did not meet the gestational age inclusion criteria, as was clear from the abstract (Liu 2010).
Risk of bias in included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Of the nine trials stated to be randomised, the method of randomisation was described only by Bedard 1984 (deck of cards), Donn 1981 (lottery) and Ruth 1988 (lottery). It was not clear how allocation concealment was achieved in any of these nine randomised trials. Morgan 1982 used alternate rather than random allocation with no attempt at allocation concealment (high risk of bias). It was evident in only one of the trials that allocation concealment was achieved (Whitelaw 1983). Two trials used numbered identical vials and were double‐blind (Kuban 1986; Whitelaw 1983).
Blinding
In the open trials by Donn 1981, Morgan 1982, Bedard 1984, Porter 1985, Anwar 1986, Ruth 1988 and Mas‐Munoz 1993, it is likely that the medical and nursing staff knew the treatment allocation. Thus, there is the possibility that the clinical care given to the two groups could have been biased by the knowledge and beliefs of the clinical staff.
Incomplete outcome data
In Kuban 1986, 11 of 291 (4%) infants enrolled were withdrawn after randomisation. In Ruth 1988, 10 of 111 (9%) infants enrolled were excluded because of gestational age less than 25 weeks or congenital anomaly. In Whitelaw 1983, two of 32 (6%) infants were excluded because of congenital anomalies and these two infants were replaced in the randomisation. None of the other trials reported the exclusion of any infants after enrolment. Only Ruth 1988 reported long‐term follow‐up and achieved 100% ascertainment of survivors at 27 months of age.
Selective reporting
All the trials except those by Anwar 1986 and Mas‐Munoz 1993 described the main endpoint, ultrasound or CT diagnosis of IVH, as being determined by ultrasonographers and radiologists who had no knowledge of treatment allocation. In Ruth 1988, the neurologist and psychologist assessing neurodevelopment at 27 months were blinded to treatment allocation.
Other potential sources of bias
We did not identify any other potential sources of bias.
Effects of interventions
Prophylactic administration of phenobarbital in preterm infants at risk of developing IVH
Primary outcomes
All grades of IVH
Data were available from all 10 trials for this outcome. The evidence suggests that phenobarbital results in little to no difference in the incidence of IVH (any grade) compared with control (RR 1.00, 95% CI 0.84 to 1.19; I² for RR = 58%; RD 0.00, 95% CI ‐0.06 to 0.07; I² for RD = 65%; 10 studies, 792 participants; Analysis 1.1). The certainty of the evidence is low for limitations in study design (downgraded by one level) and inconsistency (downgraded by one level).
Severe IVH
Data were available from all 10 trials for this outcome. The evidence suggests that phenobarbital results in little to no difference in the incidence of severe IVH compared with control (RR 0.88, 95% CI 0.64 to 1.21; I² for RR = 37%; RD ‐0.02, 95% CI ‐0.07 to 0.03; I² for RD = 50%; 10 studies, 792 participants; Analysis 1.2). The certainty of the evidence is low for limitations in study design (downgraded by one level) and imprecision of the estimate (downgraded by one level).
Secondary outcomes
Posthaemorrhagic ventricular dilation or hydrocephalus
Four trials reported this outcome. The evidence is very uncertain about the effect of phenobarbital on ventricular dilation or hydrocephalus (RR 0.62, 95% CI 0.31 to 1.26; I² for RR = 21%; RD ‐0.05, 95% CI ‐0.12 to 0.02; I² for RD = 63%; 4 studies, 271 participants; Analysis 1.3 (Anwar 1986; Donn 1981; Ruth 1985; Ruth 1988). The certainty of the evidence is very low for limitations in study design (downgraded by one level) and imprecision of the estimate (downgraded by two levels).
Hypotension
Three trials reported this outcome. The evidence is very uncertain about the effect of phenobarbital on hypotension (RR 1.18, 95% CI 0.97 to 1.43; I² for RR = 0%; RD 0.09, 95% CI ‐0.01 to 0.19; I² for RD = 0%; 3 studies, 382 participants; Analysis 1.4) (Donn 1981; Bedard 1984; Kuban 1986). The certainty of the evidence is very low for limitations in study design (downgraded by one level), and imprecision of the estimate (downgraded by two levels).
Pneumothorax/interstitial emphysema
Eight trials reported this outcome. Phenobarbital may result in little to no difference in pneumothorax/interstitial emphysema (RR 1.28, 95% CI 0.92 to 1.77; I² for RR = 33%; RD 0.04, 95% CI ‐0.01 to 0.10; I² for RD = 49%; 8 studies, 682 participants; Analysis 1.5) (Bedard 1984; Donn 1981; Kuban 1986; Mas‐Munoz 1993; Morgan 1982; Porter 1985; Ruth 1988; Whitelaw 1983). The certainty of the evidence is low for limitations in study design (downgraded by one level) and imprecision of the estimate (downgraded by one level).
Hypercapnia
Five trials reported this outcome. The evidence is very uncertain about the effect of phenobarbital on hypercapnia (RR 1.00, 95% CI 0.73 to 1.37; I² for RR = 0%; RD 0.00, 95% CI ‐0.12 to 0.12; I² for RD = 0%; 5 studies, 241 participants; Analysis 1.6) (Bedard 1984; Donn 1981; Morgan 1982; Porter 1985; Whitelaw 1983). The certainty of the evidence is very low for limitations in study design (downgraded by one level) and imprecision of the estimate (downgraded by two levels).
Acidosis
Six trials reported this outcome. The evidence is very uncertain about the effect of phenobarbital on acidosis (RR 1.16, 95% CI 0.90 to 1.51; I² for RR = 19%; RD 0.04, 95% CI ‐0.03 to 0.12; I² for RD = 0%; 6 studies, 521 participants; Analysis 1.7) (Bedard 1984; Donn 1981; Kuban 1986; Morgan 1982; Porter 1985; Whitelaw 1983). The certainty of the evidence is very low for limitations in study design (downgraded by one level), imprecision of the estimate (downgraded by one level) and inconsistency (downgraded by one level) for different definitions used for acidosis.
Mechanical ventilation
Six trials reported this outcome. Phenobarbital may increase need for mechanical ventilation (RR 1.16, 95% CI 1.04 to 1.28; I² for RR = 7%; RD 0.11, 95% CI 0.04 to 0.19; I² for RD = 0%; 6 studies, NNT = 9375 participants; Analysis 1.8) (Bedard 1984; Donn 1981; Morgan 1982; Ruth 1985; Ruth 1988; Whitelaw 1983). The certainty of the evidence is low for limitations in study design (downgraded by one level) and imprecision of the estimate (downgraded by one level).
Mild neurodevelopmental impairment
One trial reported this outcome. The evidence is very uncertain about the effect of phenobarbital on mild neurodevelopmental impairment (RR 0.57, 95% CI 0.15 to 2.17; RD ‐0.05, 95% CI ‐0.16 to 0.06; I² not applicable; 1 study, 101 participants; Analysis 1.9) (Ruth 1988). The certainty of the evidence is very low for limitations in study design (downgraded by one level) and imprecision of the estimate (downgraded by two levels).
Severe neurodevelopmental impairment
Two trials reported this outcome. The evidence is very uncertain about the effect of phenobarbital on severe neurodevelopmental impairment (RR 1.12, 95% CI 0.44 to 2.82; I² for RR = 0%; RD 0.01, 95% CI ‐0.09 to 0.11; I² for RD = 0%; 2 studies, 153 participants; Analysis 1.10) (Ruth 1985; Ruth 1988). The certainty of the evidence is very low for limitations in study design (downgraded by one level) and imprecision of the estimate (downgraded by two levels).
Mortality prior to hospital discharge
Nine trials reported this outcome. The evidence suggests that phenobarbital results in little to no difference in death before discharge compared with control (RR 0.88, 95% CI 0.64 to 1.21; I² for RR = 6%; RD ‐0.02, 95% CI ‐0.07 to 0.03; I² for RD = 20%; 9 studies, 740 participants; Analysis 1.11) (Anwar 1986; Bedard 1984; Donn 1981; Kuban 1986; Mas‐Munoz 1993; Morgan 1982; Porter 1985; Ruth 1988; Whitelaw 1983). The certainty of the evidence is low for limitations in study design (downgraded by one level) and imprecision of the estimate (downgraded by one level).
Mortality during the study period
Data were available from all 10 trials for this outcome. The evidence suggests that phenobarbital results in little to no difference in all deaths compared with control (RR 0.98, 95% CI 0.72 to 1.33; I² for RR = 23%; RD 0.00, 95% CI ‐0.05 to 0.05; I² for RD = 34%; 10 studies, 792 participants; Analysis 1.12). The certainty of the evidence is low for limitations in study design (downgraded by one level) and imprecision of the estimate (downgraded by one level).
Discussion
Summary of main results
We evaluated the benefits and harms of phenobarbital compared with control (i.e. no intervention or placebo) in preterm infants. Ten studies (corresponding to 792 infants) were included.
Overall, the evidence suggests that phenobarbital results in little to no difference in the incidence of IVH (any grade or severe) compared with control. Among the secondary outcomes of this review, the evidence is very uncertain about the effects of phenobarbital on ventricular dilation or hydrocephalus and on neurodevelopmental impairment. The evidence suggests that phenobarbital results in little to no difference in death before discharge compared with control.
We identified no ongoing trials.
Overall completeness and applicability of evidence
The primary outcomes of this review (i.e. the incidence of IVH of any grade and severe IVH) were reported by all included studies. Eight studies found no difference in the incidence of this outcome between phenobarbital and control, but very few infants were enrolled in those studies; one study reported a reduction in IVH among infants receiving phenobarbital (Donn 1981), whereas another reported an increase in IVH, although in that trial the group receiving phenobarbital was significantly lighter and had a shorter gestation period (Kuban 1986). Because different definitions were used for acidosis, the meta‐analysis for acidosis should be treated with caution. Similarly, prophylactic phenobarbital treatment would, on average, result in one extra infant receiving mechanical ventilation for every nine preterm infants treated, but the certainty of the evidence is low.
Although the dosages of phenobarbital varied, all studies gave plasma phenobarbital concentrations in the recommended anticonvulsant range for 72 hours, the period during which IVH usually occurs. A cause for concern was that four of the trials did not have a normal cranial ultrasound scan as an entry criterion. The trial that found that postnatal phenobarbital reduced IVH was an open trial that lacked a prerandomisation cerebral ultrasound scan (Donn 1981). Some of the IVH reported could have arisen before the administration of phenobarbital. The double‐blind Kuban 1986 trial was planned with adequate sample size; however, randomisation did not result in the two groups having similar risk factors for IVH because the group receiving phenobarbital had a significantly greater risk for IVH than the control group did at the time of randomisation. These factors in the trials of Donn 1981 and Kuban 1986 could contribute to the heterogeneity found for the outcome all grades of IVH.
We noted late timing (e.g. later than 6 hours in 2 studies) of the initial phenobarbital injection and the splitting of loading does in six studies (Table 1). In these situations, it could have been more than 12 hours before anticonvulsant plasma concentrations of phenobarbital were achieved; and yet, many IVHs start by 12 hours of age. The difficulty in achieving therapeutic blood concentrations of phenobarbital before many IVHs have started was one reason for testing antenatal maternal administration of phenobarbital, which has been assessed in a separate Cochrane review (Crowther 2010).
Since the original publication of this review, it has become apparent that administration of anti‐epileptic drugs in the newborn period may have a harmful effect on the developing brain. Phenobarbital has a proapoptotic effect in newborn rat brains (Bittigau 2002). More recently, it has been shown that neonatal rat exposure to a single dose of phenobarbital results in reduced synaptic connectivity in the striatum (Forcelli 2012). It would have been helpful if more of the included studies had monitored neurodevelopment.
Quality of the evidence
According to the GRADE approach, the overall certainty of evidence for critical outcomes for phenobarbital administration for any indication ranged from low to very low (see summary of findings Table 1). All outcomes were downgraded (one level) because of limitations in study design (i.e. unclear high risk of bias in different domains, mainly selection bias, performance bias and reporting bias; Figure 4). Most outcomes were downgraded for imprecision, by either one or two levels, because of few events, low sample size and wide CIs. The outcome 'IVH any grade' was downgraded by one level because of inconsistency (I2 = 58%). Where the certainty of evidence was low included IVH (any grade or severe) and mortality (death before discharge; all deaths during the study period). The evidence for ventricular dilation or hydrocephalus and neurodevelopmental impairment (mild or severe) was rated as very low (i.e. it was downgraded due to limitations in study design (one level) and imprecision of the estimates (one level)). We detected no publication bias using funnel plots (Figure 5; Figure 6).

Funnel plot of comparison: 1 Phenobarbital versus control, Outcome: 1.1 All intraventricular haemorrhage.

Funnel plot of comparison: 1 Phenobarbital versus control, Outcome: 1.2 Severe intraventricular haemorrhage.
Potential biases in the review process
We kept the thresholds for gestational age as defined in Types of participants, although IVH rarely occurs beyond a gestational age of 32 weeks. Two studies were excluded: one non‐randomised trial (Cooke 1982); and one cross‐over, non‐randomised trial in which all infants first received placebo and then phenobarbital (Saliba 1991). Although the authors of this Cochrane Review were not involved in any of the included trials, some of us conducted primary studies (both clinical and preclinical) on IVH in preterm newborns: this may have created an intellectual bias in preparing this review.
Agreements and disagreements with other studies or reviews
A non‐Cochrane review of postnatal phenobarbital for preterm infants included eight trials and noted the heterogeneity between trials concerning any IVH and severe IVH (Horbar 1992). The author of that review concluded that postnatal phenobarbital could not be recommended, but raised the question as to whether, in specific settings, phenobarbital may be beneficial. In addition, that review did not present data on ventricular dilation, neuromotor impairment, mechanical ventilation, hypotension, pneumothorax or acidosis (Horbar 1992).
The previous updates of this Cochrane Review by Whitelaw 2007 and Smit 2013 included one additional study each. In this 2022 update, one new study has been included (Ruth 1985); this is an older study, but it was identified because search strategies were revised to increase sensitivity and the search was conducted without date limits. Three previously included studies have been excluded in this review because they were not randomised (Liang 2009; Sluncheva 2006; Zhang 2009).
This review supports the conclusion of Horbar 1992 that phenobarbital does not reduce the frequency of IVH, severe IVH or death, and suggests that phenobarbital may increase the need for mechanical ventilation, but the certainty of the evidence is low. The data now available do not identify any specific setting in which prophylactic phenobarbital may reduce the risk of IVH.
Prophylactic antenatal phenobarbital is the subject of a separate Cochrane systematic review that concluded that the trials with most reliable methodology showed no evidence that the intervention was effective in reducing IVH (Crowther 2010).

Screen4Me summary diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 Phenobarbital versus control, Outcome: 1.1 All intraventricular haemorrhage.

Funnel plot of comparison: 1 Phenobarbital versus control, Outcome: 1.2 Severe intraventricular haemorrhage.

Comparison 1: Phenobarbital versus control, Outcome 1: All intraventricular haemorrhage

Comparison 1: Phenobarbital versus control, Outcome 2: Severe intraventricular haemorrhage

Comparison 1: Phenobarbital versus control, Outcome 3: Ventricular dilation or hydrocephalus

Comparison 1: Phenobarbital versus control, Outcome 4: Hypotension

Comparison 1: Phenobarbital versus control, Outcome 5: Pneumothorax/interstitial emphysema

Comparison 1: Phenobarbital versus control, Outcome 6: Hypercapnia

Comparison 1: Phenobarbital versus control, Outcome 7: Acidosis

Comparison 1: Phenobarbital versus control, Outcome 8: Use of mechanical ventilation

Comparison 1: Phenobarbital versus control, Outcome 9: Mild neurodevelopmental impairment

Comparison 1: Phenobarbital versus control, Outcome 10: Severe neurodevelopmental impairment

Comparison 1: Phenobarbital versus control, Outcome 11: Death before discharge

Comparison 1: Phenobarbital versus control, Outcome 12: All deaths during study
| Phenobarbital compared to placebo or no intervention for the prevention of IVH in preterm infants | ||||||
| Patient or population: preterm infants with or at risk of IVH | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo or no intervention | Risk with phenobarbital | |||||
| IVH any grade (Papile classification) during hospitalisation | Study population | RR 1.00
RD 0.00 (‐0.06 to 0.07) | 792 | ⊕⊕⊝⊝ | The evidence suggests that phenobarbital results in little to no difference in the incidence of IVH (any grade) compared with control | |
| 388 per 1000 | 388 per 1000 | |||||
| Severe IVH (Papile classification) during hospitalisation | Study population | RR 0.88
RD ‐0.02 (‐0.07 to 0.03) | 792 | ⊕⊕⊝⊝ | The evidence suggests that phenobarbital results in little to no difference in the incidence of severe IVH compared with control | |
| 161 per 1000 | 142 per 1000 | |||||
| Ventricular dilation or hydrocephalus during hospitalisation | Study population | RR 0.62
RD ‐0.05 (‐0.12 to 0.02) | 271 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of phenobarbital on ventricular dilation or hydrocephalus | |
| 129 per 1000 | 80 per 1000 | |||||
| Mild neurodevelopmental impairment at 27 months of age | Study population | RR 0.57
RD ‐0.05 (‐0.16 to 0.06) | 101 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of phenobarbital on mild neurodevelopmental impairment | |
| 111 per 1000 | 63 per 1000 | |||||
| Severe neurodevelopmental impairment at 9 to 27 months of age | Study population | RR 1.12
RD 0.01 (‐0.09 to 0.11) | 153 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of phenobarbital on severe neurodevelopmental impairment | |
| 99 per 1000 | 111 per 1000 | |||||
| Death before discharge | Study population | RR 0.88
RD ‐0.02 (‐0.07 to 0.03) | 740 | ⊕⊕⊝⊝ | The evidence suggests that phenobarbital results in little to no difference in death before discharge compared with control | |
| 173 per 1000 | 152 per 1000 | |||||
| All deaths during the study | Study population | RR 0.98
RD 0.00 (0.05 to 0.05) | 792 | ⊕⊕⊝⊝ | The evidence suggests that phenobarbital results in little to no difference in all deaths during study compared with control | |
| 166 per 1000 | 163 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). IVH: intraventricular haemorrhage RD: risk difference RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for high or unclear risk of bias in all domains of the risk of bias tool. 2 Downgraded by one level for inconsistency (I2 = 58%). 3 Downgraded by one level for imprecision of the estimates. 4 Downgraded by two levels for imprecision of the estimates, due to wide CIs and low sample size. 5 Downgraded by one level for high risk of performance bias and unclear risk for detection and reporting bias. 6 Downgraded by two levels for imprecision for wide CIs in one study with very low sample size and few events. 7 Downgraded by one level for high or unclear risk of bias in most domains (all except attrition bias). | ||||||
|
|
Birthweight (g) |
GA (weeks) |
Study groups |
Initiation (hours) | |||
|
| Phenobarbital |
Control | Phenobarbital |
Control | Phenobarbital | Control |
|
|---|---|---|---|---|---|---|---|
| 1119 ± 264 | 1120 ± 218 | NR | NR | LD 20 mg/kg, T12 MD 2.5 mg/kg, x12, UD | No intervention | 6 | |
| 1491 ± 421 | 1271 ± 422 | 31.1 ± 2.7 | 32.2 ± 1.7 | LD 10 mg/kg, T12 MD 2.5 mg/kg, x12, for 6 days | No intervention | 6 | |
| 1101 ± 243 | 1037 ± 208 | 28.9 ± 1.9 | 28.6 ± 1.9 | LD 10 mg/kg, T12 MD 2.5 mg/kg, x12, for 7 days | No intervention | 12 | |
| Reported as the number of newborns for four different weight groups: ≤ 1000, 1001 to 1250, 1251 to 1500, 1501 to 1750 g | Reported as the number of newborns for three different GA groups: < 28, ≥28 to < 32 and ≥ 32 to < 37 weeks | LD 10 mg/kg, T12 MD 2.5 mg/kg, x12, UD | Placebo | 6 | |||
| 1544 ± 480 | 1394 ± 430 | 31.5 ± 2 | 31.0 ± 2.0 | LD 20 mg/kg MD 2.5 mg/kg, x12, UD | No intervention | 6 | |
| 1150 ± 200 | 1070 ± 250 | 31.1 ± 2.7 | 28.8 ± 2.8 | LD 20 mg/kg no MD | No intervention | 24 | |
| 1058 ± 269 | 1061 ± 226 | 29.4 ± 2.8 | 28.8 ± 2.2 | LD 30 mg/kg MD 5 mg/kg, x24, for 3 days | No intervention | 6 | |
| 1090 ± 247 | 1180 ± 222 | 28.7 ± 2.1 | 29.3 ± 1.8 | LD 15 mg/kg, T4 MD 5 mg/kg, x24, for 5 days | No intervention | 2 | |
| 1160 ± 210 | 1120 ± 250 | 29.4 ± 1.7 | 29.2 ± 2.1 | LD 15 mg/kg, T4 MD 5 mg/kg, x24, for 5 days | Glucose infusion | 4 | |
| 1116 ± 215 | 1143 ± 238 | 29.7 ± 2.0 | 29.8 ± 2.1 | LD 20 mg/kg no MD | Saline | 4 | |
| Unless indicated otherwise, data are given as the mean±SD. GA: gestational age LD: loading dose MD: maintenance dose NR: not reported SD: standard deviation T4: twice 4 hours apart T12: twice 12 hours apart UD: unknown duration x12: every 12 hours x24: every 24 hours | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 All intraventricular haemorrhage Show forest plot | 10 | 792 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 1.2 Severe intraventricular haemorrhage Show forest plot | 10 | 792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 1.3 Ventricular dilation or hydrocephalus Show forest plot | 4 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.31, 1.26] |
| 1.4 Hypotension Show forest plot | 3 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
| 1.5 Pneumothorax/interstitial emphysema Show forest plot | 8 | 682 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.92, 1.77] |
| 1.6 Hypercapnia Show forest plot | 5 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| 1.7 Acidosis Show forest plot | 6 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.90, 1.51] |
| 1.8 Use of mechanical ventilation Show forest plot | 6 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.04, 1.28] |
| 1.9 Mild neurodevelopmental impairment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.10 Severe neurodevelopmental impairment Show forest plot | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.44, 2.82] |
| 1.11 Death before discharge Show forest plot | 9 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 1.12 All deaths during study Show forest plot | 10 | 792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.72, 1.33] |

