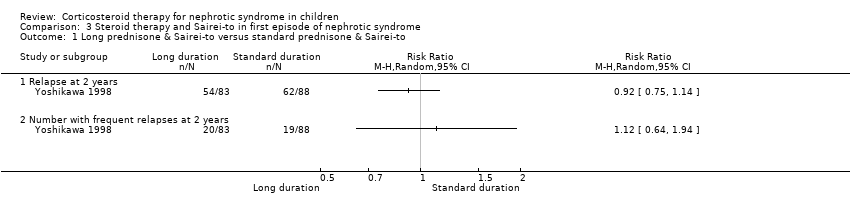
| 1 Number of children relapsing by 6 months Show forest plot | 7 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 1.1 Duration: 1 month versus 2 months (standard) | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.01, 2.54] |
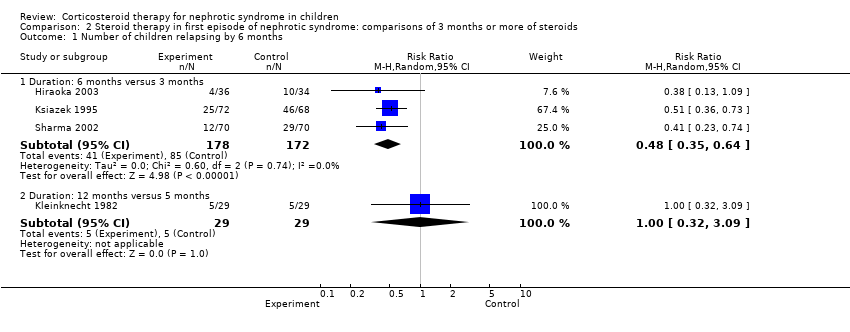
| 1.2 Duration: 3 months or more versus 2 months (standard) | 5 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.28, 0.75] |
| 1.3 Increased dose of prednisone versus standard dose | 6 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.32, 0.71] |
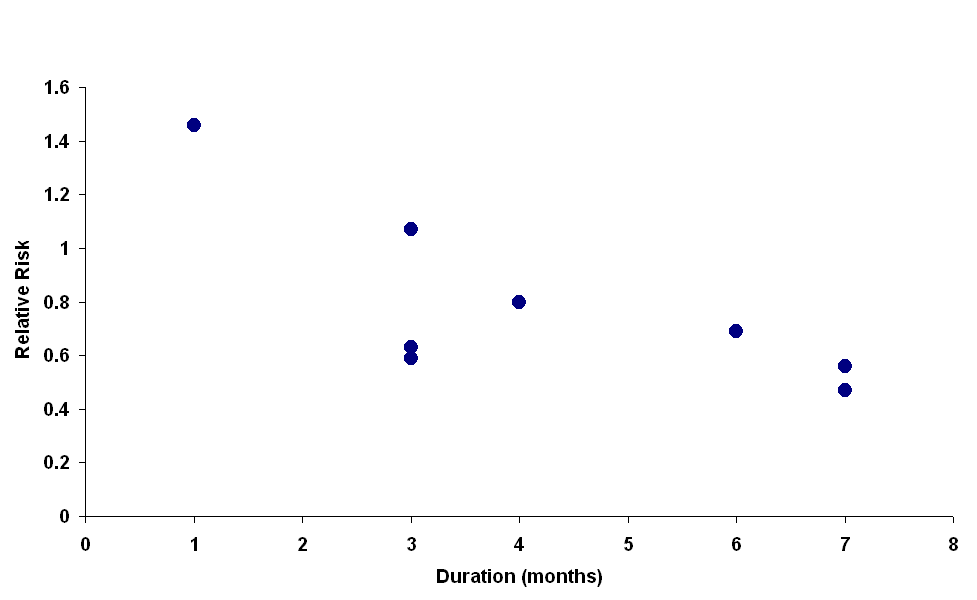
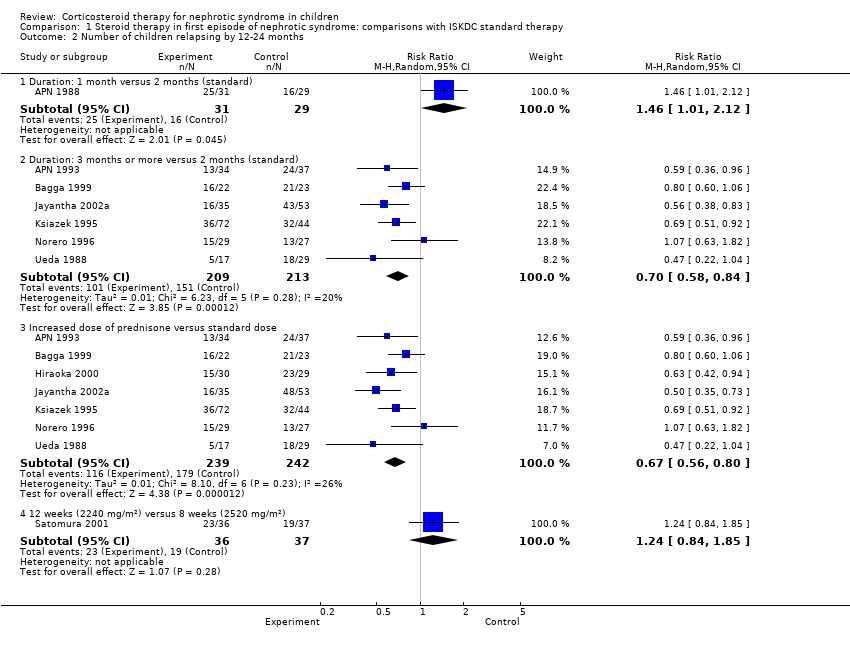
| 2 Number of children relapsing by 12‐24 months Show forest plot | 9 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 2.1 Duration: 1 month versus 2 months (standard) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.01, 2.12] |
| 2.2 Duration: 3 months or more versus 2 months (standard) | 6 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.58, 0.84] |
| 2.3 Increased dose of prednisone versus standard dose | 7 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.56, 0.80] |
| 2.4 12 weeks (2240 mg/m²) versus 8 weeks (2520 mg/m²) | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.84, 1.85] |
| 3 Mean relapse rate/patient/year Show forest plot | 4 | | Mean Difference (IV, Random, 95% CI) | Subtotals only |
|
| 3.1 Duration: 3 months or more versus 2 months (standard) | 4 | 295 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.29, ‐0.00] |
| 4 Number with frequent relapses Show forest plot | 8 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 4.1 Duration: 1 month versus 2 months (standard) | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.85, 2.59] |
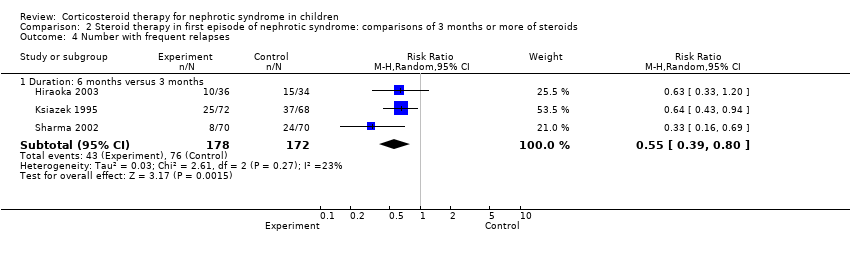
| 4.2 Duration: 3 months or more versus 2 months (standard) | 6 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.46, 0.84] |
| 4.3 Increased dose of prednisone versus standard dose (2240 mg/m²) | 7 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.48, 0.84] |
| 5 Cumulative steroid dose in g/m² Show forest plot | 4 | | Mean Difference (IV, Random, 95% CI) | Subtotals only |
|
| 5.1 Duration: 1 month versus 2 months (standard) | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.28, ‐0.68] |
| 5.2 Duration: 3 months or more versus 2 months (standard) | 3 | 245 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐0.67, 2.09] |
| 6 Adverse events: increased prednisone dose versus standard duration Show forest plot | 7 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 6.1 Psychological disorders | 4 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.66, 9.06] |
| 6.2 Hypertension | 7 | 526 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.60, 4.28] |
| 6.3 Opthalmological disorders | 6 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.15, 4.42] |
| 6.4 Retarded growth | 4 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.25, 1.18] |
| 6.5 Cushing's syndrome | 4 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.78, 1.96] |
| 6.6 Infections | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.17] |
| 6.7 Osteoporosis | 3 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.38] |
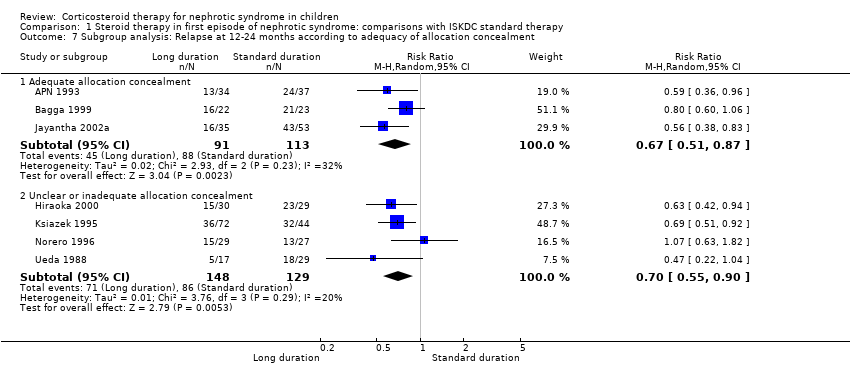
| 7 Subgroup analysis: Relapse at 12‐24 months according to adequacy of allocation concealment Show forest plot | 7 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 7.1 Adequate allocation concealment | 3 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.51, 0.87] |
| 7.2 Unclear or inadequate allocation concealment | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.55, 0.90] |