การใช้ เอสโตรเจนเฉพาะที่สำหรับช่องคลอดฝ่อในสตรีวัยหมดประจำเดือน
Appendices
Appendix 1. Cochrane Gynaecology and Fertility specialised register
PROCITE platform
From inception until 12.04.16
Keywords CONTAINS "*Vaginitis" or "vaginosis symptoms" or "vaginosis" or "vaginal atrophy" or "vaginal dryness" or "vaginal lubrication" or "vaginal symptoms" or "atrophic vaginitis" or "atrophy" or "dyspareunia" or "uro‐genital symptoms" or "urogenital atrophy" or "urogenital symptoms" or "Vulvar Atrophy" or "vulvo‐vaginal symptoms" or "vulvodynia" or "Vulvovaginal atrophy" or Title CONTAINS "*Vaginitis" or "vaginosis symptoms" or "vaginosis" or "vaginal atrophy" or "vaginal dryness" or "vaginal lubrication" or "vaginal symptoms" or "atrophic vaginitis" or "atrophy" or "dyspareunia" or "uro‐genital symptoms" or "urogenital atrophy" or "urogenital symptoms" or "vulvo‐vaginal symptoms" or "vulvodynia" or "Vulvovaginal atrophy "
AND
Keywords CONTAINS "vaginal capsules" or "vaginal estradiol" or "vaginal gel" or "vaginal pessary" or "vaginal tablet" or "vaginal tablets" or "vaginal ring" or "low dose estradiol" or "oestrodiol" or "oestrogen" or "estradiol" or "estradiol cream" or "Estriol‐" or "estrogen" or "*Estrogens" or "estrogen therapy" or "17‐beta estradiol" or "intravaginal estradiol tablets" or "intravaginal" or Title CONTAINS "vaginal capsules" or "vaginal estradiol" or "vaginal gel" or "vaginal pessary" or "vaginal tablet" or "vaginal tablets" or "vaginal ring" or "low dose estradiol" or "oestrodiol" or "oestrogen" or "estradiol" or "estradiol cream" or "Estriol‐" or "estrogen" or "*Estrogens" or "estrogen therapy" or "17‐beta estradiol" or "intravaginal estradiol tablets" or "intravaginal"
Appendix 2. CENTRAL search strategy
CRSO web platform
From inception until 12.04.16
#1MESH DESCRIPTOR Atrophic Vaginitis EXPLODE ALL TREES (2)
#2MESH DESCRIPTOR Dyspareunia EXPLODE TREES (294)
#3MESH DESCRIPTOR Vaginitis EXPLODE ALL TREES (676)
#4pruritis:TI,AB,KY (150)
#5((vagin* adj2 dry*)):TI,AB,KY (232)
#6(urogenital atroph* or urogenital symptom*):TI,AB,KY (79)
#7(menopaus* adj2 symptom*):TI,AB,KY (745)
#8(postmenopaus* adj2 symptom*):TI,AB,KY (139)
#9Dyspareuni*:TI,AB,KY (442)
#10(urogenital ageing):TI,AB,KY (2)
#11(urogenital disorder*):TI,AB,KY (8)
#12#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 (2220)
#13MESH DESCRIPTOR Estrogens EXPLODE ALL TREES (6147)
#14((*vagina* adj3 administ*)):TI,AB,KY (1464)
#15MESH DESCRIPTOR Vaginal Creams, Foams, and Jellies EXPLODE ALL TREES (302)
#16((*vagin* adj2 cream*)):TI,AB,KY (442)
#17(vaginal ring*):TI,AB,KY (158)
#18(vagina* pessar*):TI,AB,KY (161)
#19(vagina* tablet*):TI,AB,KY (220)
#20vagitories:TI,AB,KY (6)
#21(vagina* gel*):TI,AB,KY (197)
#22(vagina* capsule*):TI,AB,KY (25)
#23ovule*:TI,AB,KY (66)
#24(oestradiol or oestrogen):TI,AB,KY (1845)
#25oestrogenic:TI,AB,KY (80)
#26estradiol:TI,AB,KY (6910)
#2717B‐estradiol:TI,AB,KY (18)
#28((oestriol or estriol)):TI,AB,KY (350)
#29((dienoestrol or replens)):TI,AB,KY (14)
#30#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 (11557)
#31#12 AND #30 (860)
Appendix 3. MEDLINE search strategy
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
OVID platform
From inception until 12.04.16
1 Vaginitis/ (3265)
2 ($vagin$ adj2 atroph$).tw. (619)
3 Sexual Dysfunction, Physiological/ or Dyspareunia/ or vaginism.tw. (9388)
4 pruritis.tw. or Pruritus/ (9794)
5 ($vagin$ adj2 dry$).tw. (789)
6 (urogenital atrophy or urogenital symptom$).tw. (397)
7 (menopaus$ adj2 symptom$).tw. (3660)
8 (postmenopaus$ adj2 symptom$).tw. (697)
9 Dyspareuni$.tw. (2895)
10 urogenital ageing.tw. (11)
11 urogenital disorder$.tw. (96)
12 or/1‐11 (29104)
13 estrogen.tw. or exp Estrogens/ (202231)
14 ($vagina$ adj3 administ$).tw. (1361)
15 "Vaginal Creams, Foams and Jellies"/ (1104)
16 (vagin$ adj2 cream$).tw. (330)
17 vaginal ring$.tw. (730)
18 vaginal pessar$.tw. (309)
19 vagina$ tablet$.tw. (362)
20 vagitories.tw. (11)
21 vagina$ gel$.tw. (372)
22 vagina$ capsule$.tw. (30)
23 ovule$.tw. (2038)
24 (oestradiol or oestrogen).tw. (24894)
25 oestrogenic.tw. (1620)
26 estradiol.tw. (71063)
27 17B‐estradiol.tw. (54)
28 (oestriol or estriol).tw. (4948)
29 (dienoestrol or replens).tw. (77)
30 or/13‐29 (233413)
31 randomized controlled trial.pt. (413263)
32 controlled clinical trial.pt. (90520)
33 randomized.ab. (343103)
34 placebo.tw. (173470)
35 clinical trials as topic.sh. (176075)
36 randomly.ab. (246887)
37 trial.ti. (149165)
38 (crossover or cross‐over or cross over).tw. (66938)
39 or/31‐38 (1033187)
40 exp animals/ not humans.sh. (4224815)
41 39 not 40 (950151)
42 12 and 30 and 41 (1095)
Appendix 4. Embase search strategy
OVID platform
From inception until 12.04.16
1 exp Vaginitis/ (12609)
2 ($vagin$ adj2 atroph$).tw. (983)
3 exp female sexual dysfunction/ (10020)
4 vaginism.tw. (42)
5 pruritis.tw. (1600)
6 exp female genital pruritus/ (37)
7 ($vagin$ adj2 dry$).tw. (1296)
8 (urogenital atrophy or urogenital symptom$).tw. (587)
9 (menopaus$ adj1 symptom$).tw. (4400)
10 (postmenopaus$ adj2 symptom$).tw. (932)
11 Dyspareuni$.tw. (5064)
12 urogenital ageing.tw. (20)
13 urogenital disorder$.tw. (143)
14 or/1‐13 (31094)
15 ($vagina$ adj3 administ$).tw. (1582)
16 vaginal cream$.tw. (327)
17 vaginal ring$.tw. (983)
18 vagina$ pessar$.tw. (425)
19 vagina$ tablet$.tw. (501)
20 vagina$ capsule$.tw. (49)
21 (oestradiol or oestrogen).tw. (25871)
22 estradiol.tw. (77487)
23 oestrogenic.tw. (1656)
24 17B‐estradiol.tw. (392)
25 (oestriol or estriol).tw. (4663)
26 (dienoestrol or replens).tw. (183)
27 exp estrogen/ (230358)
28 estrogen$.tw. (137342)
29 vagitories.tw. (10)
30 ovule$.tw. (1865)
31 vagina$ gel$.tw. (519)
32 or/15‐31 (305624)
33 Clinical Trial/ (855936)
34 Randomized Controlled Trial/ (397801)
35 exp randomization/ (70046)
36 Single Blind Procedure/ (21855)
37 Double Blind Procedure/ (127422)
38 Crossover Procedure/ (46656)
39 Placebo/ (272777)
40 Randomi?ed controlled trial$.tw. (132820)
41 Rct.tw. (19837)
42 random allocation.tw. (1502)
43 randomly allocated.tw. (24326)
44 allocated randomly.tw. (2097)
45 (allocated adj2 random).tw. (750)
46 Single blind$.tw. (17141)
47 Double blind$.tw. (160313)
48 ((treble or triple) adj blind$).tw. (535)
49 placebo$.tw. (229991)
50 prospective study/ (328346)
51 or/33‐50 (1556516)
52 case study/ (37152)
53 case report.tw. (302831)
54 abstract report/ or letter/ (955011)
55 or/52‐54 (1288092)
56 51 not 55 (1515756)
57 14 and 32 and 56 (2651)
Appendix 5. PsycINFO search strategy
OVID platform
From inception until 12.04.16
1 vaginitis.tw. (41)
2 (vagina$ adj2 atroph$).tw. (38)
3 pruritis.tw. (40)
4 (vagina$ adj3 dry$).tw. (125)
5 (urogenital atrophy or urogenital symptom$).tw. (16)
6 (menopaus$ adj1 symptom$).tw. (531)
7 (postmenopaus$ adj1 symptom$).tw. (31)
8 exp Sexual Function Disturbances/ or exp Dyspareunia/ (7800)
9 exp Pruritus/ (196)
10 Sexual Dysfunction.tw. (4376)
11 Dyspareunia.tw. (471)
12 Pruritus.tw. (254)
13 or/1‐12 (10331)
14 exp Estrogens/ (5390)
15 (intravaginal$ adj3 administ$).tw. (7)
16 vaginal cream$.tw. (6)
17 vaginal ring$.tw. (37)
18 (vagina$ adj3 pessar$).tw. (4)
19 (vagina$ adj2 tablet$).tw. (5)
20 vagina$ capsule$.tw. (1)
21 (oestradiol or oestrogen).tw. (796)
22 oestrogenic.tw. (33)
23 17B‐estradiol.tw. (19)
24 oestriol.tw. (3)
25 estrogen$.tw. (6421)
26 (dienoestrol or replens).tw. (2)
27 or/14‐26 (8562)
28 13 and 27 (284)
29 random.tw. (42844)
30 control.tw. (332835)
31 double‐blind.tw. (18599)
32 clinical trials/ (8391)
33 placebo/ (3991)
34 exp Treatment/ (606250)
35 or/29‐34 (929103)
36 28 and 35 (188)
Appendix 6. Data extraction and eligibility form
| Data extraction and eligibility form for JS157 Reviewers: see notes in italics before relevant sections | |||||||||
| Review ID | Date form completed | ||||||||
| Review title | |||||||||
| Review author name / ID | |||||||||
| Co‐reviewer name / ID | |||||||||
| If any other references are found to the same trial, code first paper as A; code any further papers found as B, C, etc.; link any found for listing in RevMan | |||||||||
| Study identifier: Davar 2012 | Author: | Year of publication: | |||||||
| Title | |||||||||
| Contact author details: | |||||||||
| Eligibility | |||||||||
| RCT | [ ] yes [ ] no [ ] unclear | Describe: | |||||||
| Relevant participants | [ ] yes x [ ] no [ ] unclear | Describe: | |||||||
| Relevant interventions | [ ] yes x [ ] no [ ] unclear | Describe: | |||||||
| Relevant outcome measures | [ ] yes [ ] no X [ ] unclear | Describe: | |||||||
| INCLUDE IN REVIEW? | [ ] yes | If no, give reason: | |||||||
| Notes: | |||||||||
| Characteristics of included studies | |||||||||
| Participants | |||||||||
| Diagnostic criteria (definition of eligibility) | Inclusion criteria: Exclusion criteria: | ||||||||
| Group A | Group B | ||||||||
| Number of participants at randomisation | |||||||||
| Number analysed at outcome | |||||||||
| Withdrawals/Exclusions | |||||||||
| Age (mean, SD) | |||||||||
| Setting e.g. fertility clinic | |||||||||
| Country | |||||||||
| Interventions | |||||||||
| Describe these including mode of delivery, route, doses, timing. Quote from paper if possible | |||||||||
| Group A | Group B | ||||||||
| Intervention | |||||||||
| Standard treatment | |||||||||
| Treatment length | |||||||||
| Follow up length | |||||||||
| Loss to follow up | |||||||||
| Other info regarding treatment | |||||||||
| Quality assessment | |||||||||
| Refer to Cochrane Handbook for Systematic Reviews of Interventions, table 8.5a | |||||||||
| Selection bias | |||||||||
| Random sequence generation | [ ] low risk [ ] high risk [ ] unclear risk → more information required | Describe: | |||||||
| Allocation sequence concealment | [ ] low risk [ ] high risk [ ] unclear risk → more information required | Describe: | |||||||
| Performance bias | |||||||||
| Blinding of participants and personnel | [ ] low risk [ ] high risk [ ] unclear risk → more information required | Describe: | |||||||
| Detection bias | |||||||||
| Blinding of outcome assessment (patient‐reported outcomes) | [ ] low risk [ ] high risk [ ] unclear risk → more information required | Describe: | |||||||
| Attrition bias | |||||||||
| Due to amount, nature or handling of incomplete outcome data | [ ] low risk [ ] high risk [ ] unclear risk → more information required | Describe: | |||||||
| Reporting bias | |||||||||
| Selective reporting: | [ ] low risk [ ] high risk [ ] unclear risk → more information required | Describe: | |||||||
| Other sources of bias | |||||||||
| Sources of bias such as differences in demographic characteristics | [ ] low risk [ ] high risk [ ] unclear risk → more information required | Describe: | |||||||
| Is there anything not reported? If information is missing, record this, so that it is apparent that the information is missing, not just not extracted | |||||||||
| Incomplete outcome data | |||||||||
| Description of outcomes (per woman randomised) | |||||||||
| Indicate the reported outcomes and describe as appropriate | |||||||||
| Describe: | |||||||||
| Describe: | |||||||||
| Describe: | |||||||||
| Describe: | |||||||||
| Describe: | |||||||||
| Results | |||||||||
| Record summary data for each intervention group (e.g. 2×2 table for dichotomous data; means and SDs for continuous data). | |||||||||
| Participants (number allocated and completed) | No. in treatment group: | No. in control group: | |||||||
| Dichotomous outcomes | No of events | No of participants | No of events | No of participants | |||||
| Continuous outcomes | Mean, SD | No of participants | Mean; SD | No of participants | |||||
| Note any other results reported but not listed as outcome measures: | |||||||||
| Miscellaneous | |||||||||
| Funding source stated | |||||||||
| Ethical approval obtained | |||||||||
| Written consents obtained from participants | |||||||||
| Key conclusions of the study authors. | |||||||||
| Miscellaneous comments from the study authors. | |||||||||
| References to other relevant studies. | |||||||||
| Correspondence required. | |||||||||
| Miscellaneous comments by the review authors. | |||||||||
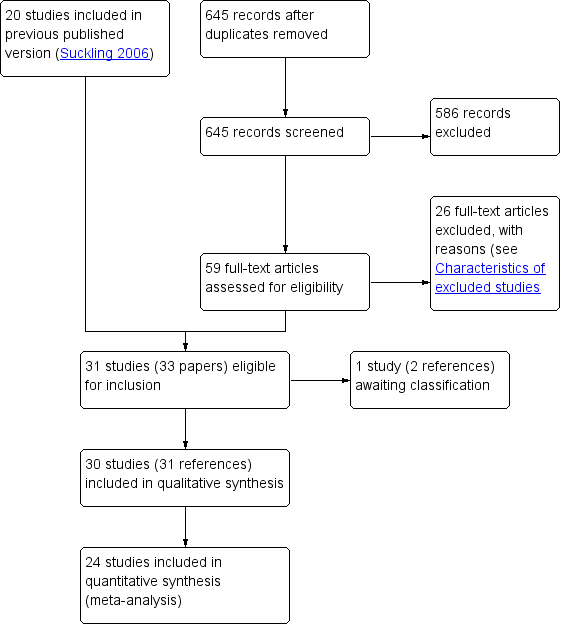
Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
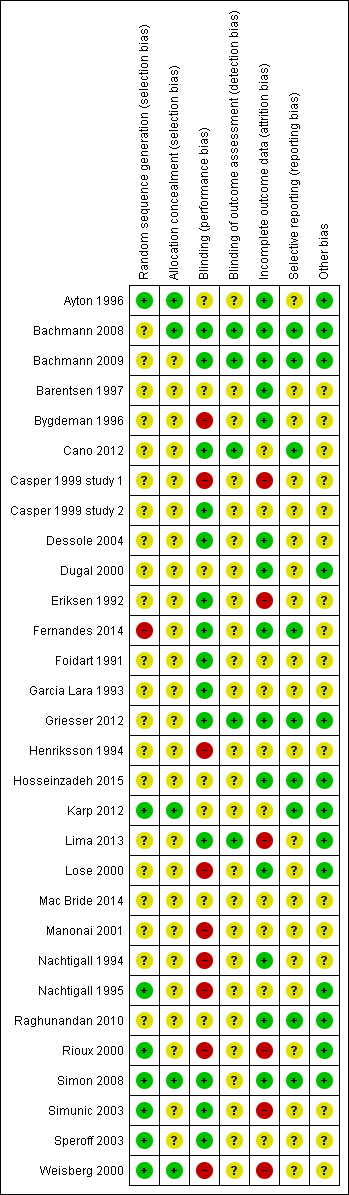
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 1 Oestrogen ring versus placebo or other regimens, outcome: 1.1 Improvement in symptoms (participant‐assessed at end point).

Forest plot of comparison: 2 Oestrogen tablets versus placebo or other regimens, outcome: 2.1 Improvement in symptoms (participant‐assessed at end point).

Forest plot of comparison: 3 Oestrogen cream versus placebo or other regimens, outcome: 3.1 Improvement in symptoms (participant‐assessed at end point).

Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 1 Improvement in symptoms (participant‐assessed at end point).
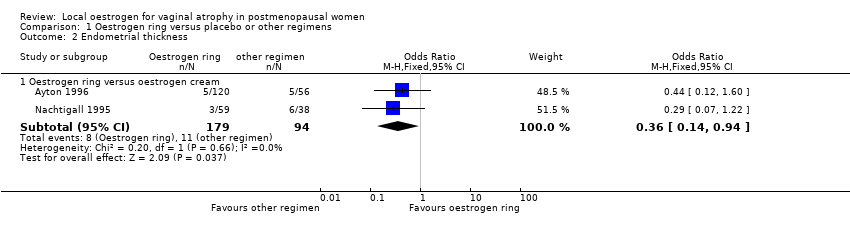
Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 2 Endometrial thickness.

Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 3 Improvement in symptoms (clinician‐assessed at end point).
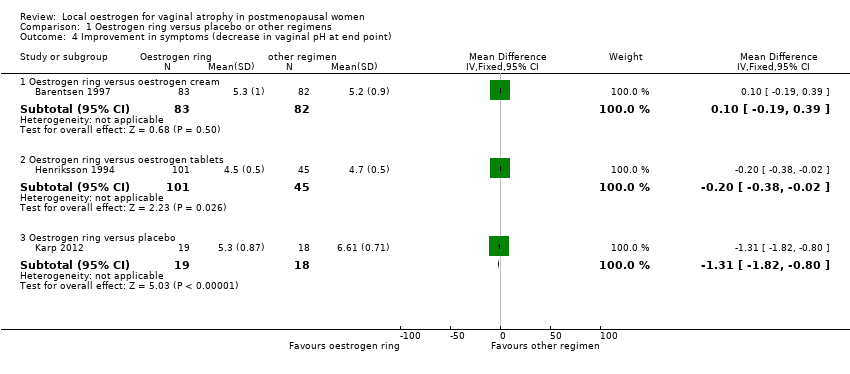
Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 4 Improvement in symptoms (decrease in vaginal pH at end point).

Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 5 Improvement in symptoms (increase in maturation indices at end point).
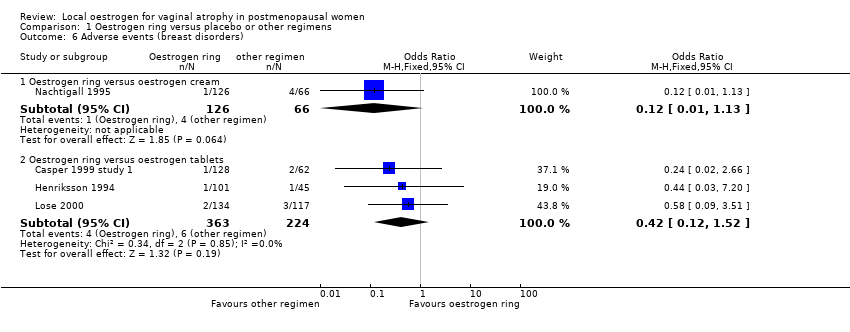
Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 6 Adverse events (breast disorders).
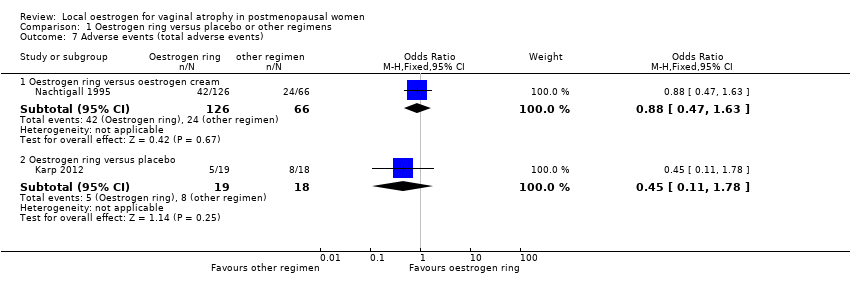
Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 7 Adverse events (total adverse events).

Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 8 Adherence to treatment.
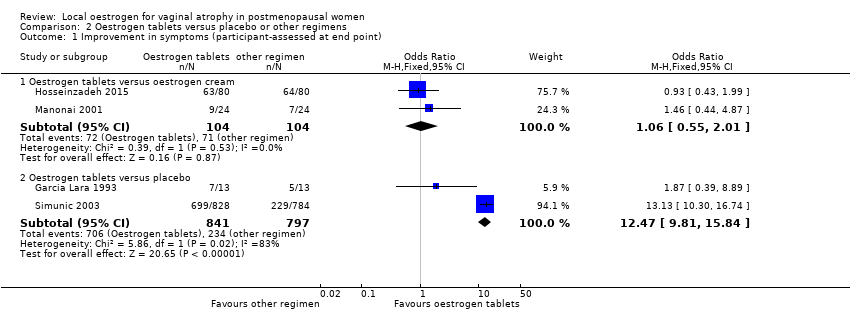
Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 1 Improvement in symptoms (participant‐assessed at end point).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 2 Endometrial thickness.

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 3 Improvement in symptoms (clinician‐assessed at end point).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 4 Improvement in symptoms (decrease in vaginal pH at end point).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 5 Improvement in symptoms (increase in maturation indices at end point).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 6 Adverse events (breast disorders).
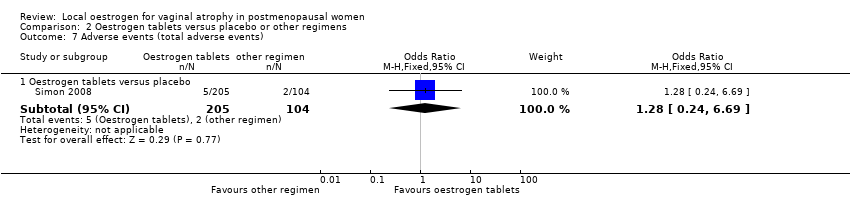
Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 7 Adverse events (total adverse events).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 8 Adherence to treatment.

Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 1 Improvement in symptoms (participant‐assessed at end point).
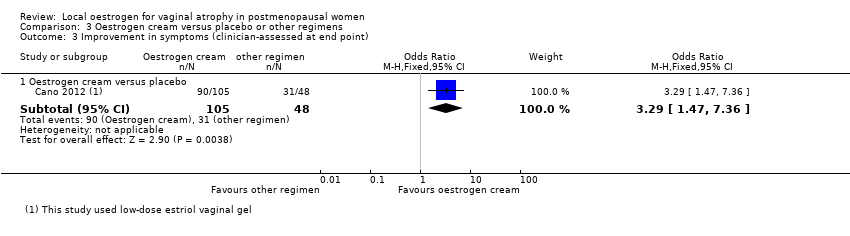
Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 3 Improvement in symptoms (clinician‐assessed at end point).
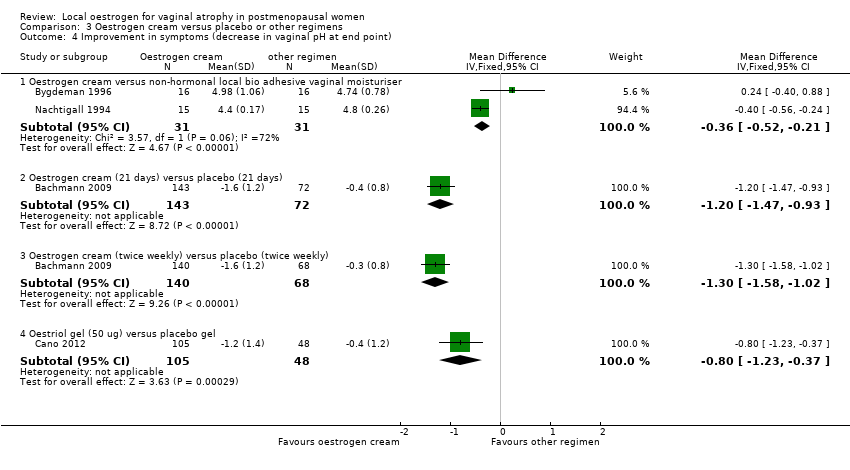
Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 4 Improvement in symptoms (decrease in vaginal pH at end point).

Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 5 Improvement in symptoms (increase in maturation indices at end point).
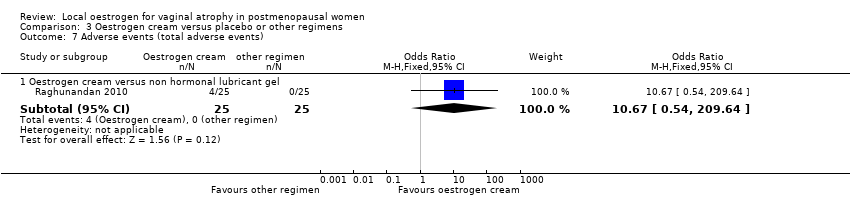
Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 7 Adverse events (total adverse events).
| Oestrogen ring compared to other regimens for vaginal atrophy in postmenopausal women | ||||||
| Patient or population: postmenopausal women with vaginal atrophy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other regimen | Oestrogen ring | |||||
| Improvement in symptoms (participant‐assessed) (oestrogen ring vs oestrogen cream) | 717 per 1000 | 771 per 1000 | OR 1.33 | 341 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (participant‐assessed) (oestrogen ring vs oestrogen tablets) | 582 per 1000 | 521 per 1000 | OR 0.78 | 567 | ⊕⊕⊝⊝ | |
| Endometrial thickness (oestrogen ring vs oestrogen cream) | 117 per 1000 | 46 per 1000 | OR 0.36 | 273 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen ring vs oestrogen cream) | 706 per 1000 | 714 per 1000 | OR 1.04 | 533 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen ring vs oestrogen tablets) | 636 per 1000 | 717 per 1000 | OR 1.45 | 397 | ⊕⊕⊝⊝ | |
| Adverse events (total adverse events) (oestrogen ring vs oestrogen cream) | 364 per 1000 | 335 per 1000 | OR 0.88 | 192 | ⊕⊕⊝⊝ | |
| Adverse events (total adverse events) (oestrogen ring vs placebo) | 444 per 1000 | 264 per 1000 | OR 0.45 | 37 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by 1 level as most risk of bias domains were rated either as unclear or high | ||||||
| Oestrogen tablets compared to other regimens for vaginal atrophy in postmenopausal women | ||||||
| Patient or population: postmenopausal women with vaginal atrophy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other regimen | Oestrogen tablets | |||||
| Improvement in symptoms (participant‐assessed) (oestrogen tablets vs oestrogen cream) | 683 per 1000 | 695 per 1000 | OR 1.06 | 208 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (participant‐assessed) (oestrogen tablets vs placebo) | 294 per 1000 | 839 per 1000 | OR 12.47 | 1638 | ⊕⊕⊝⊝ | Using a random effects model, there was no evidence of a difference in effect: OR 5.80, 95% CI 0.88 to 38.29 |
| Endometrial thickness (oestrogen tablets vs oestrogen cream) | 80 per 1000 | 26 per 1000 | OR 0.31 | 151 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen tablets vs oestrogen cream) | 697 per 1000 | 699 per 1000 | OR 1.03 | 528 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen tablets vs placebo) | 262 per 1000 | 820 per 1000 | OR 12.85 | 2078 | ⊕⊕⊝⊝ | |
| Adverse events (total adverse events) (oestrogen tablets vs placebo) | 19 per 1000 | 24 per 1000 | OR 1.27 | 309 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by 1 level as most risk of bias domains were assessed either as unclear or high | ||||||
| Oestrogen cream compared to other regimens for vaginal atrophy in postmenopausal women | ||||||
| Patient or population: postmenopausal women with vaginal atrophy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other regimen | Oestrogen cream | |||||
| Improvement in symptoms (participant‐assessed) (oestrogen cream vs isoflavone gel) | 967 per 1000 | 984 per 1000 | OR 2.08 | 50 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (participant‐assessed) oestrogen cream vs placebo) | 685 per 1000 | 899 per 1000 | OR 4.10 | 198 | ⊕⊕⊝⊝ | |
| Endometrial thickness not reported | ‐ | ‐ | Not estimable | ‐ | ‐ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen cream vs placebo) | 646 per 1000 | 857 per 1000 | OR 3.29 | 153 | ⊕⊕⊝⊝ | |
| Adverse events (total adverse events) (oestrogen cream vs non‐hormonal lubricant gel) | ‐ | ‐ | OR 10.67 | 50 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by 1 level as most risk of bias domains were assessed either as unclear or high | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in symptoms (participant‐assessed at end point) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen ring versus oestrogen cream | 2 | 341 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.80, 2.19] |
| 1.2 Oestrogen ring versus oestrogen tablets | 3 | 567 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 1.3 Oestrogen ring versus placebo | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.67 [3.23, 49.66] |
| 2 Endometrial thickness Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oestrogen ring versus oestrogen cream | 2 | 273 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.94] |
| 3 Improvement in symptoms (clinician‐assessed at end point) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestrogen ring versus oestrogen cream | 3 | 533 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.70, 1.53] |
| 3.2 Oestrogen ring versus oestrogen tablets | 2 | 397 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.90, 2.32] |
| 3.3 Oestrogen ring versus placebo | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.55, 7.31] |
| 4 Improvement in symptoms (decrease in vaginal pH at end point) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestrogen ring versus oestrogen cream | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 4.2 Oestrogen ring versus oestrogen tablets | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 4.3 Oestrogen ring versus placebo | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐1.31 [‐1.82, ‐0.80] |
| 5 Improvement in symptoms (increase in maturation indices at end point) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestrogen ring versus oestrogen cream | 2 | 341 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [‐1.52, 3.09] |
| 5.2 Oestrogen ring (7.5 ug) versus placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Oestrogen ring (100 ug) versus placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Oestrogen ring (50 ug) versus placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Oestrogen ring (unspecified dose) versus placebo | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 24.4 [15.25, 33.55] |
| 6 Adverse events (breast disorders) Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Oestrogen ring versus oestrogen cream | 1 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 1.13] |
| 6.2 Oestrogen ring versus oestrogen tablets | 3 | 587 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.12, 1.52] |
| 7 Adverse events (total adverse events) Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestrogen ring versus oestrogen cream | 1 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.47, 1.63] |
| 7.2 Oestrogen ring versus placebo | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.11, 1.78] |
| 8 Adherence to treatment Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Oestrogen ring versus oestrogen cream | 2 | 350 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.31, 3.80] |
| 8.2 Oestrogen ring versus oestrogen tablets | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.66, 4.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in symptoms (participant‐assessed at end point) Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen tablets versus oestrogen cream | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.55, 2.01] |
| 1.2 Oestrogen tablets versus placebo | 2 | 1638 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.47 [9.81, 15.84] |
| 2 Endometrial thickness Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oestrogen tablets versus oestrogen cream | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.60] |
| 3 Improvement in symptoms (clinician‐assessed at end point) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestrogen tablets versus oestrogen cream | 3 | 528 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.52] |
| 3.2 Oestrogen tablets versus placebo | 3 | 2078 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.85 [10.39, 15.89] |
| 4 Improvement in symptoms (decrease in vaginal pH at end point) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestrogen tablets versus oestrogen cream | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.12, 0.52] |
| 4.2 Oestrogen tablets versus placebo | 2 | 524 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.10, ‐0.80] |
| 5 Improvement in symptoms (increase in maturation indices at end point) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestrogen tablets versus oestrogen cream | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐4.69 [‐13.58, 4.20] |
| 5.2 Oestrogen tablets versus placebo | 1 | 436 | Mean Difference (IV, Fixed, 95% CI) | 18.63 [14.57, 22.69] |
| 6 Adverse events (breast disorders) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Oestradiol tablets versus oestriol tablets | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.12, 77.09] |
| 7 Adverse events (total adverse events) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestrogen tablets versus placebo | 1 | 309 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.24, 6.69] |
| 8 Adherence to treatment Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Oestrogen tablets versus oestrogen cream | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.41, 8.94] |
| 8.2 Oestradiol tablets versus oestriol tablets | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.15, 6.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in symptoms (participant‐assessed at end point) Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen cream versus isoflavone gel | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.08, 53.76] |
| 1.2 Oestrogen cream versus placebo | 2 | 198 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.10 [1.88, 8.93] |
| 2 Endometrial thickness | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Improvement in symptoms (clinician‐assessed at end point) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestrogen cream versus placebo | 1 | 153 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.47, 7.36] |
| 4 Improvement in symptoms (decrease in vaginal pH at end point) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestrogen cream versus non‐hormonal local bio adhesive vaginal moisturiser | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.52, ‐0.21] |
| 4.2 Oestrogen cream (21 days) versus placebo (21 days) | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.47, ‐0.93] |
| 4.3 Oestrogen cream (twice weekly) versus placebo (twice weekly) | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.58, ‐1.02] |
| 4.4 Oestriol gel (50 ug) versus placebo gel | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.23, ‐0.37] |
| 5 Improvement in symptoms (increase in maturation indices at end point) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestrogen cream versus placebo | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | 23.7 [17.25, 30.15] |
| 6 Adverse events (breast disorders) | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Adverse events (total adverse events) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestrogen cream versus non hormonal lubricant gel | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.67 [0.54, 209.64] |
| 8 Adherence to treatment | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

