Поддержка принятия решений для помощи людям, которым предстоит принятие решений по лечению или по скринингу
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised to decision aid vs usual care | |
| Participants | 398 + 414 men considering prostate cancer screening in the USA | |
| Interventions | DA: computer tailored program on clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (step by step process for making the decision; interactive computer program: inherently guided the patient through the decision aid and decision making process), tailored print out given to patients to promote discussion with others (practitioner, significant others) COMPARE: received no intervention | |
| Outcomes | decisional status* (pre, post DA), knowledge* (pre, post DA), *decision self‐efficacy(pre, post DA), decisional consistency* (pre, post DA), desire for involvement in decision making (pre, post DA), decisional conflict(pre, post DA), preferred options. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.2173 (Setting): "Sites were blocked on size and percent of male employees and randomly assigned by computer‐generated random numbers to condition within blocks." |
| Allocation concealment (selection bias) | Unclear risk | The study does not address this criterion. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol. |
| Other bias | Low risk | pg.2175 (Intervention delivery): mention of money incentive to complete paperwork, but was judged to have no effect on outcomes measured |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study does not address this criterion. |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes measured were not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 75 + 77 participants considering bariatric surgery in the USA | |
| Interventions | DA: booklet + video on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (list of questions to discuss with clinician) COMPARE: usual care (general information pamphlets on clinical problem) | |
| Outcomes | knowledge* (pre, immediately post and 3 month follow‐up), values (pre, immediately post and 3 month follow‐up), values concordance* (pre, immediately post and 3 month follow‐up), treatment preference (pre and immediately post), decisional conflict (pre and immediately post), decisional self efficacy (pre and immediately post), proportion undecided. | |
| Notes | *primary outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.1670 (Participants and randomization): "used computer‐assisted, block randomisation process to ensure balanced allocation of participants" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment and no mention of impact on study |
| Incomplete outcome data (attrition bias) | Unclear risk | pg.1671 (measures): mentioned 4 choices for treatment preference (surgery, drug therapy, diet and/or exercise program and unsure) but only reported on surgery and unsure options |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol or trial registration; all pre‐specified outcomes included |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg.1670 (Participants and randomization): "study was not blinded"; no mention of impact on study |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subject to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 103 + 100 men newly diagnosed with prostate cancer in Finland | |
| Interventions | DA: pamphlet patient decision aid created for study on options' outcomes, outcome probability, guidance | |
| Outcomes | Uptake of options*, participation in decision making | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Auvinen, 2001, BJU International: pg. 2 "randomized centrally, using software based on a random number generator" No blocking used Auvinen, 2004, BJU International (Primary Study): pg. 1 "randomized using a computer algorithm based on random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Auvinen, 2001, BJU International: pg. 2 ‐ Patients and Methods randomized centrally at the Finnish Cancer Registry Auvinen, 2004, BJU International (Primary Study): pg. 1 ‐ randomized centrally I think central allocation is considered as low risk |
| Incomplete outcome data (attrition bias) | Low risk | Auvinen, 2001, BJU International: pg. 3 flow‐chart; pg. 4 "imbalance in the numbers of patients between the arms within two hospitals. Not expected to affect the results in any way" "some participants refused to give informed consent, health deterioration, not seen by urologist" Auvinen, 2004, BJU International (Primary Study): pg. 2 ‐ flow diagram & results; Baseline data not included. |
| Selective reporting (reporting bias) | Unclear risk | No indication that trial registered in central trials registry. Auvinen, 2001, BJU International: Protocol mentioned pg. 2 "The study protocol was approved by an ethical committee in each participating hospital"; Auvinen, 2004, BJU International (Primary Study): pg. 1 "The study protocol was approved by the institutional review board at each participating hospital" |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | High risk | Auvinen, 2001, BJU International: pg. 3 "recognized carry‐over effect because same physician in charge for intervention and control groups, diminish contrast between groups, as these physicians were more motivated to inform patients than those physicians not participating"; Auvinen, 2004, BJU International (Primary Study): No blinding but primary outcome is choice of treatment for prostate, objectively recorded. But unsure how physicians may have influenced decisions |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding but primary outcome is choice of treatment for prostate, objectively recorded. |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 104 + 123 patients considering benign prostatic hyperplasia treatment in the USA | |
| Interventions | DA: Health Dialog interactive videodisc on options' outcomes, clinical problem, outcome probability, others' opinion | |
| Outcomes | uptake of option; knowledge*; satisfaction with DM process; satisfaction with decision; interest in DM; general health outcomes; condition specific health outcomes | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "Stratified by study site in concealed blocks of 10" |
| Allocation concealment (selection bias) | Low risk | pg. 2 ‐ study coordinator opening serially numbered, opaque, sealed envelopes |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ patient accrual and follow‐up; Reasons for withdrawal mentioned; pg. 4 Baseline characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | No indication that trial registered in central trials registry |
| Other bias | Low risk | Appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | No blinding but phase 1 eliminated risk of contamination |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding but phase 1 eliminated risk of outcome assessor interfering with decision |
| Methods | Randomised to detailed vs routine consultation | |
| Participants | 59 + 58 pregnant women who have received a maternal serum screening positive test result for Down syndrome in the UK | |
| Interventions | DA: decision analysis plus routine consultation on options' outcomes, clinical problem, outcome probability, values clarification, guidance/coaching | |
| Outcomes | uptake of option; knowledge; decisional conflict; anxiety*; informed decision making; satisfaction with consultation; consultation length | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Bekker, 2003, Pt Ed & Counseling: pg. 2 ‐ section 2.3 Sample and Procedure "randomly allocated... using previously numbered... envelopes" Bekker, 2004, Prenat Diagn (Primary Study): pg. 3 "Participants were randomly allocated by previously numbered envelopes"; Does not mention how sequence was generated |
| Allocation concealment (selection bias) | Low risk | Bekker, 2003, Pt Ed & Counseling: pg. 2 ‐ section 2.3 Sample and Procedure "using previously numbered, sealed, opaque envelopes" Bekker, 2004, Prenat Diagn (Primary Study): pg. 3 ‐ previously numbered, sealed, opaque envelopes |
| Incomplete outcome data (attrition bias) | Unclear risk | Bekker, 2003, Pt Ed & Counseling; Bekker, 2004, Prenat Diagn (Primary Study): pg. 4 ‐ results/flow diagram; Baseline characteristics not included. |
| Selective reporting (reporting bias) | Unclear risk | Bekker, 2003, Pt Ed & Counseling: The coding frame was developed from literature. Does not mention protocol. Bekker, 2004, Prenat Diagn (Primary Study): no information provided about central trials registry |
| Other bias | Unclear risk | Bekker, 2003, Pt Ed & Counseling: does not directly address baseline characteristics of participants; Bekker, 2004, Prenat Diagn (Primary Study): appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded, personnel not blinded. Same personnel did control & intervention. Tape recorded sessions to ensure no bias |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 65 + 53 patients with coronary artery disease considering revascularization surgery in the USA | |
| Interventions | DA: Health Dialog video on options' outcomes, clinical problem, outcome probability, others' opinion | |
| Outcomes | uptake of option, knowledge, satisfaction with care, satisfaction with decision and decision making process*, general health outcomes, condition specific health outcomes | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 "Randomization was stratified by study site in blocks of 10" |
| Allocation concealment (selection bias) | Low risk | pg. 3 "randomization performed by a study coordinator opening opaque, sealed envelopes at study headquarters" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ flow diagram; Baseline data comparison included. |
| Selective reporting (reporting bias) | Unclear risk | No information provided indicating trial was included in central trials registry |
| Other bias | Low risk | Appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Neither subjects nor study staff were blinded to treatment assignment ‐ could lead to different satisfaction ratings based on knowing the treatment received |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 266 + 228 men considering prostate cancer treatment in the USA | |
| Interventions | DA: interactive web based video on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (list of questions to ask doctor and automated summary) COMPARE: usual care | |
| Outcomes | decisional conflict*, preferred/actual treatment choice (pre and post DA), proportion undecided | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.3 (Methods section‐ second paragraph) "Participants were randomized automatically by the P3P application to study groups (1:1 using a simple randomization scheme with no blocking") |
| Allocation concealment (selection bias) | Low risk | pg.3 (Methods section) "Participants were randomized automatically by the P3P application to study groups" |
| Incomplete outcome data (attrition bias) | Low risk | pg.4: used ITT analysis and low dropout |
| Selective reporting (reporting bias) | Low risk | Protocol made available |
| Other bias | Unclear risk | Was a multicentre trial which could have lead to contamination, protocol violation and biased questionnaire completion |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were not blinded and study does not address the effect on the results |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear whether outcome assessors are blinded, but outcomes are not subject to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 236 + 247 women less than 11 weeks pregnant considering Down syndrome screening in Sweden | |
| Interventions | DA: linear video on options' outcomes, clinical problem, outcome probabilities, others' opinion, and guidance (step by step process for making the decision) COMPARE: usual care using pamphlet | |
| Outcomes | knowledge* (post DA), values congruent with chosen option (post DA), attitude* (post DA), uptake of CUB* (post DA) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 391: "The midwife allocated the participants randomly by sealed envelopes..." but does not state the actual sequence generation method |
| Allocation concealment (selection bias) | Low risk | pg. 391: used sealed envelopes, "prepared, sequentially coded and distributed to the maternity units by the research group". |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of why some participants' data were excluded in Tables 2, 3 and 4 |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol |
| Other bias | Low risk | Appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | p. 395: 'It was not possible to blind neither [sic] the midwives nor the participants due to the characteristics of the intervention.' |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to DA vs usual care | |
| Participants | 74 + 77 healthcare workers who did not receive the influenza vaccine considering receiving the vaccine in Canada | |
| Interventions | DA: web based DA on options' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance COMPARE: usual care using pamphlet | |
| Outcomes | confidence in decision* (post DA), impact on immunization intent (post DA), proportion undecided. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 199 ‐ "The randomization list was generated using the randomization function in Excel 2002 (version 10.6856.6856 SP3)." |
| Allocation concealment (selection bias) | Low risk | pg. 199 ‐ "The list was imported from Excel into a Microsoft SQL Server |
| Incomplete outcome data (attrition bias) | High risk | 65% completion rate in intervention arm and 77% completion rate in control arm: attrition could be different where the respondents and non‐respondents are different |
| Selective reporting (reporting bias) | Low risk | protocol available |
| Other bias | Unclear risk | Figure 1 numbers for exclusion are not logical |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported whether or not they were blinded during the course of the intervention |
| Blinding of outcome assessment (detection bias) | Low risk | questionnaire scores are objective and not subject to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 753 + 263 Health physicians considering Hep B vaccine in the USA | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probability, explicit values clarification (personal decision analysis), guidance/coaching | |
| Outcomes | uptake of option* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ random numbers table; all incoming residents were assigned to Group 2 (non‐randomised residents identified as Subgroup) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 2 "35 physicians excluded because already received vaccine" Flow chart not included. Baseline characteristics not included. |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | High risk | pg. 287 ‐ potential selection bias ‐ non‐randomised residents were added to group 2 and therefore potential unbalanced distribution; Plus low response rate among those offered decision analysis. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding of participants or personnel. Not clear how this may affect their decision |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but decisions for screening were retrieved from health records |
| Methods | Randomised to decision aid + audio‐taped consultation vs usual care | |
| Participants | 30 + 30 men with prostate cancer considering treatment in Canada | |
| Interventions | DA: written + audiotape consultation of options' outcomes, clinical problem, outcome probability, others' opinion | |
| Outcomes | role in decision making*, anxiety, depression | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 5 ‐ Data Collection "The group to which subjects were assigned was predetermined by a block randomization procedure. This ensured there were an equal number of subjects in both groups for each physician." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned; pg. 5 ‐ group assignment predetermined by block randomisation procedure |
| Incomplete outcome data (attrition bias) | Low risk | no flow diagram; pg. 12 explains why certain men did not listen to audiotape. Baseline characteristics included. All men approached by study investigator agreed to participate, only one man refused to complete the second set of questionnaires. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not mentioned |
| Other bias | Low risk | Appears to be free of other sources of bias. Similar baseline characteristics, |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding. Unclear how participant's willingness to participate was affected by knowing they received the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear blinding, and whether outcomes could be affected by not blinding the assessor |
| Methods | Randomised to detailed vs simple vs usual care | |
| Participants | 70 + 70 + 71 patients diagnosed with knee OA considering OA treatment in the USA | |
| Interventions | COMPLEX DA: videobooklet + interactive conjoint analysis on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (list of questions) COMPARE DA: videobooklet on options' outcomes, clinical problem, outcome probabilities, others' opinion and guidance (list of questions) COMPARE: usual care receiving generic booklet | |
| Outcomes | decisional conflict* (pre and post DA) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 231: computer generated list with uneven blocks |
| Allocation concealment (selection bias) | Low risk | pg. 231 (procedure): numbered, sealed and opaque envelopes |
| Incomplete outcome data (attrition bias) | Low risk | 3 dropouts; missing data effect size unlikely to have significant impact on study outcome |
| Selective reporting (reporting bias) | Unclear risk | protocol not available |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Low risk | pg. 231 (procedures): likely not blinded, but low threat to causality in study |
| Blinding of outcome assessment (detection bias) | Low risk | pg. 231: patients were not blinded but outcome was objectively measured |
| Methods | Randomised to detailed decision aid vs pharmacist consultation | |
| Participants | 67 + 61 women considering hormone replacement therapy in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance/coaching (Ottawa Decision Support Framework) | |
| Outcomes | preferred option*, decisional conflict*, role in decision making*, satisfaction with preparation for decision making, satisfaction with decision*, adherence* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | pg. 4 ‐ flow diagram; pg.3 reasons for attrition mentioned. Baseline characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured |
| Methods | Randomised to detailed vs simple decision aid | |
| Participants | 190 + 203 adults with herniated disc or spinal stenosis considering back surgery in the USA | |
| Interventions | DA: Health Dialog interactive videodisc on options' outcomes, clinical problem, outcome probability, other's opinions | |
| Outcomes | uptake of option*, satisfaction with DM process, satisfaction with care, condition specific health outcomes | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 "computer generated simple randomization sequence"; Phelan ‐ pg. 2 computer generated |
| Allocation concealment (selection bias) | Low risk | pg. 3 "series of numbered opaque envelopes"; Phelan ‐ pg. 2 ‐ concealed in serially marked, opaque envelopes |
| Incomplete outcome data (attrition bias) | Low risk | pg. 5 ‐ flow diagram; Reasons for attrition mentioned and participants balanced across study groups. Baseline data not included; Phelan ‐ Flow of participants not included. Baseline characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | pg. 4 ‐ There were no significant group differences; appears to be free of other potential biases; Phelan ‐ appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured |
| Methods | Randomised to detailed vs simple decision aid | |
| Participants | 52 + 49 women considering hormone replacement therapy in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance/coaching (Ottawa Decision Support Framework) | |
| Outcomes | preferred option, knowledge, decisional conflict*, accurate risk perceptions, congruence between values and choice | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 2 ‐ eligible women randomly assigned ‐ no information on how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | Baseline characteristics included. pg. 3 "Toutes les 101 femmes recrutées ont complété l’étude" [all 101 women recruited completed the study] |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Unclear risk | women of 50‐59 years and married were significantly more numerous in the experimental group |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg. 2 ‐ a research assistant met the women during a debriefing of 20 minutes in small groups of 4‐5 women assigned to the same intervention to avoid inter‐group contact, thus ensuring blinding. Not sure if the physicians were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 50 + 47 average risk for colorectal cancer considering screening in the USA | |
| Interventions | DA: computer with analytic hierarchy process on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance/coaching | |
| Outcomes | uptake of option*, decisional conflict*, role in decision making | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 (Study Interventions) "randomization schedules were created using a computer random number generator" |
| Allocation concealment (selection bias) | Low risk | pg. 2 (Study Interventions) ‐ computer‐based |
| Incomplete outcome data (attrition bias) | Low risk | See flow diagram and description in results |
| Selective reporting (reporting bias) | Unclear risk | Nothing specifically mentioned re: study protocol |
| Other bias | Low risk | Appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding of participants. All patient interviews in both the experimental and control groups were done by the same investigator, unclear on how this could contribute to risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to online decision aid vs paper decision aid vs questionnaire vs usual care | |
| Participants | 129 + 126 + 127 + 132 men considering PSA screening in Wales | |
| Interventions | DA: online program on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (interactive computer program; summary) COMPARE: paper version of online DA on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (interactive computer program; summary) COMPARE: received a questionnaire COMPARE: received nothing | |
| Outcomes | Knowledge* (post DA), attitude (post DA), intention to undergo PSA testing (post DA), anxiety (post DA), uptake of PSA test (post DA), total decisional conflict | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg.4 (recruitment process): "a random sample of 100 men was selected from the list." "The process ensured individual level randomization" |
| Allocation concealment (selection bias) | Low risk | pg.4 (recruitment process): "affirmative consent forms from each practice were transferred to the research officer who allocated each participant with a number provided remotely by the trial statistician to ensure concealment" |
| Incomplete outcome data (attrition bias) | Low risk | see Figure 1 for flow diagram and Table 1 for baseline characteristics of participants |
| Selective reporting (reporting bias) | Low risk | registered as a trial |
| Other bias | Low risk | the study appears free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | the study does not address this outcome |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Decision aid vs delayed intervention vs control | |
| Participants | 382 + 159 + 100 women with an elevated five year risk of breast cancer considering breast cancer prevention medication in the USA | |
| Interventions | DA: tailored DA on options' outcomes, clinical problem, outcome probabilities, and explicit values clarification COMPARE: given DA after 3‐month follow‐up COMPARE: given DA after all outcome measures were taken | |
| Outcomes | decisional conflict (post DA), behavioural intent (post DA), actual behaviour (post DA), proportion undecided, perception of benefits (post DA), perception of risk (post DA). | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random sequence generation was provided by the author |
| Allocation concealment (selection bias) | Low risk | central and web‐based allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | does not report exclusions |
| Selective reporting (reporting bias) | Unclear risk | no mention of study protocol |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | unclear blinding ‐ study does not address this outcome |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 47 + 40 patients with knee pain considering treatment options in the USA | |
| Interventions | DA: interactive computer tool options' outcomes, outcome probability, explicit values clarification COMPARE: usual care using the Arthritis Foundation information pamphlet | |
| Outcomes | Decisional self‐efficacy, preparation for decision making | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | no information provided; computer generated |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ results; baseline characteristics included and balanced |
| Selective reporting (reporting bias) | Unclear risk | no information provided; no indication of trial was registered centrally |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding but unclear if it has impact on the outcomes measured |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs. decision aid + chronic disease trajectory vs chronic disease trajectory vs usual care (Internet information) | |
| Participants | 155 + 152 + 153 + 151 men considering prostate cancer screening | |
| Interventions | DA:information on options' outcomes, clinical problem, outcome probabilities, others' opinions COMPARE: information on options' outcomes, clinical problem, outcome probabilities, others' opinions, explicit values clarification (utilities for outcomes associated with prostate cancer) COMPARE: explicit values clarification (utilities for outcomes associated with prostate cancer) COMPARE: usual care using public information on prostate cancer screening on American Cancer Society and Centers for Disease Control and Prevention websites 2005‐2006 | |
| Outcomes | knowledge*, actual option*, decisional conflict*, concern about prostate cancer, treatment preference if prostate cancer diagnosed | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer algorithm randomly assigned participants to the 4 study groups |
| Allocation concealment (selection bias) | Low risk | revealed after signed consent and completed baseline measures |
| Incomplete outcome data (attrition bias) | Low risk | used intention to treat analysis; imputed missing data for participants who did not complete follow‐up assessments |
| Selective reporting (reporting bias) | Unclear risk | no indication of published protocol |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | accessed a secure Internet site that hosted all study materials; participants had unlimited access to assigned intervention, unclear blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding but outcomes were measured via questionnaires and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 126 + 122 men considering PSA testing in Australia | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probability, explicit values clarification | |
| Outcomes | preferred option, knowledge, decisional conflict, accurate risk perceptions, perceived ability to make an informed choice | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 1 ‐ pre‐randomised code ‐ no further information |
| Allocation concealment (selection bias) | Low risk | pg. 1 ‐ pre‐randomised code unobtrusively marked on envelopes |
| Incomplete outcome data (attrition bias) | Unclear risk | pre‐test characteristics included. Flow chart not included and reasons for attrition not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | consenting men were blinded to allocation, but unclear if personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid booklet vs decision aid video vs usual care | |
| Participants | 140 + 141 + 140 men considering PSA testing in Australia | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probability, explicit values clarification | |
| Outcomes | preferred option, knowledge, decisional conflict, perceived ability to make an informed choice | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ 2.3.1. Unique identification codes assigned to participants according to date and time enrolled into the interventional component of the study. Block randomisation of identification codes then performed via computer software |
| Allocation concealment (selection bias) | Low risk | pg. 2 ‐ 2.3.1. "Allocation concealment was ensured as the interviewers, responsible for enrolling participants onto the trial, were blinded to the randomised study design while one of the |
| Incomplete outcome data (attrition bias) | Low risk | pg. 5 (172) "interviews terminated, call times exhausted, one man with prostate cancer accidentally included, but data is excluded from results" pg. 7 baseline characteristics equally distributed; pg. 6 fig. 1 ‐ flow diagram |
| Selective reporting (reporting bias) | Unclear risk | pg.13 (180)par. 5 "success of study protocol" "limitation to protocol: men not confronted with actual decision to undergo PSA screening; No indication that trial registered in central trials registry |
| Other bias | Low risk | pg. 13 (180) par. 5 "high follow‐up rate and allocation concealment; study not subjected to selection bias" Appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Participants & interviewers were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | At post‐test, it was not possible to blind the interviewers but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to detailed vs simple decision aid Cluster randomised trial | |
| Participants | 86 + 50 women considering surgery for breast cancer (cluster RCT with 57 surgeons randomised) in Canada | |
| Interventions | DA: audiotape + booklet on options' outcomes, clinical problem, outcome probability, values clarification, other's opinions, coaching/guidance (Ottawa Decision Support Framework) | |
| Outcomes | knowledge, decisional conflict*, decisional regret, anxiety | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "blocks of 8 based on a random number generator" |
| Allocation concealment (selection bias) | Unclear risk | pg. 2 ‐ Prerandomisation was done to eliminate opportunities to select into the study intervention arm. The allocation was not revealed to the surgeon until after agreement to participate in the study was obtained. |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ Baseline characteristics included. pg. 3 ‐ results |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg. 2 ‐ unclear on whether participants were blinded. The allocation was not revealed to the surgeon until after agreement to participate in the study was obtained |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid + counselling vs counselling alone vs usual care | |
| Participants | 29 + 14 women with a first degree relative with breast cancer interested in learning about genetic testing in the USA | |
| Interventions | DA: CD‐ROM plus counselling on options' outcomes, clinical problem, others' opinions, guidance/coaching COMPARE: counselling | |
| Outcomes | knowledge, preferred options* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "block randomization schedule to one of 3 groups in a 2:2:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 5 table "Values do not always add up to the number of participants due to missing data" Reasons not mentioned. pg. 4 "Participants' baseline knowledge was reflected in the control group's answers" Participants balanced in study groups. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg. 2 "genetic counsellor blinded to randomization until just prior to the session", unclear if participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to detailed decision aid + genetic counselling vs routine genetic counselling | |
| Participants | 106 + 105 women with first degree relative with breast cancer considering genetic testing in the USA | |
| Interventions | DA: CD‐ROM plus counselling on options' outcomes, clinical problem, others' opinions, guidance/coaching | |
| Outcomes | preferred option, knowledge*, decisional conflict, satisfaction with decision, anxiety, counsellor/participant rating of effectiveness of counselling session, consultation length | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Green, 2004, JAMA (Primary Study):pg. 2 ‐ used separate computer generated randomisation lists for low‐risk and high‐risk individuals at each study site; Green, 2005, Genet Med: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Green, 2004, JAMA (Primary Study): no information provided; Green, 2005, Genet Med: no information provided |
| Incomplete outcome data (attrition bias) | Low risk | Green, 2004, JAMA (Primary Study):pg. 4 figure; flow chart. Reasons for attrition/loss to follow‐up not included. p.5 Baseline characteristics included; Green, 2005, Genet Med:pg. 4 ‐ flow diagram; reasons for attrition mentioned and participants balanced in study groups. Baseline characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | Green, 2004, JAMA (Primary Study): appears to be free of other potential biases; Green, 2005, Genet Med: appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | Green, 2005, Genet Med: pg. 8 ‐ this was not a blinded study "counselor's responses may have been biased" but primary outcome was objective, so it is unlikely to introduce bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Cluster randomised trial of decision aid vs usual care | |
| Participants | 54 + 59 patients with schizophrenia considering treatment options (cluster RCT with 12 wards paired and randomised) in Germany | |
| Interventions | DA: 16‐page booklet on options' outcomes, outcome probabilities, explicit values clarification, coaching/guidance | |
| Outcomes | knowledge, participation in decision making (COMRADE ‐ doctor gave me a chance to decided which treatment I thought was best for me), uptake of psycho education, rehospitalization, adherence, satisfaction with care, severity of illness (baseline only), attitudes about drug use, decision making preference | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p266 "one member of each pair being randomly assigned to the control or to the interventional condition." Sequence generation method was not stated |
| Allocation concealment (selection bias) | Unclear risk | no mention of allocation concealment |
| Incomplete outcome data (attrition bias) | Low risk | reasons for attrition mentioned |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | High risk | clustering was not accounted for in the analysis |
| Blinding of participants and personnel (performance bias) | Unclear risk | no information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | no information provided |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 127 + 129 patients diagnosed with advanced dementia and eating problems considering long term feeding tube placement in the USA | |
| Interventions | DA: booklet or audio recording on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (steps in decision making, worksheet, summary) COMPARE: usual care | |
| Outcomes | surrogate knowledge, risk perceptions, decisional conflict* (3 months post DA), frequency of communication with providers (3 months post DA), feeding treatment use (3, 6 and 9 months post DA), participation in decision making, satisfaction with the decision, decisional regret. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.2010 (randomization): computerized random number generation |
| Allocation concealment (selection bias) | Unclear risk | pg.2010 (randomization): no description of method used to conceal allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | table 3: intervention group missing data for 1 participant, reason for omission not reported |
| Selective reporting (reporting bias) | Low risk | registered with clinicaltrials.gov, protocol on website |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg.2014 (discussion) |
| Blinding of outcome assessment (detection bias) | Low risk | pg.2010 (randomization) "because of cluster randomization, data collectors were not blinded to group assignment", authors believe has little impact on study |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 66 + 67 breast cancer patients eligible for breast reconstruction in the USA | |
| Interventions | DA: interactive software program on options' outcomes, others' opinions | |
| Outcomes | knowledge, anxiety, satisfaction with treatment choice, satisfaction with decision making ability | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ "upon study entry, the participants were randomized (computer generated) to one of two groups" |
| Allocation concealment (selection bias) | Unclear risk | not enough information provided |
| Incomplete outcome data (attrition bias) | Low risk | Baseline anxiety and knowledge included in graphs. pg. 3 Participant numbers between study groups balanced. Reasons for incomplete questionnaires and study withdrawals mentioned. |
| Selective reporting (reporting bias) | Unclear risk | no information provided re: protocol |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | no information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | no information provided |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 103 + 105 patients in the the emergency department with primary symptoms of nontraumatic chest pain and were being considered of admission to the emergency department observation unit for monitoring and cardiac stress testing within 24 hours. | |
| Interventions | DA: one page printout on options' outcomes, clinical problem, and outcome probabilities COMPARE: usual care | |
| Outcomes | knowledge*, risk perceptions, decisional conflict, actual choice, satisfaction with decision making process, patient‐practitioner communication. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg 253 ‐ "Patients were randomized to either usual care or shared decision making through a Web‐based, computer‐generated allocation sequence in a 1:1 concealed fashion" |
| Allocation concealment (selection bias) | Low risk | pg 253 ‐ "Patients were randomized to either usual care or shared decision making through a Web‐based, computer‐generated allocation sequence in a 1:1 concealed fashion" |
| Incomplete outcome data (attrition bias) | Unclear risk | Some of the numbers of patients reported in the results did not match the flow chart |
| Selective reporting (reporting bias) | Low risk | Protocol is available |
| Other bias | Low risk | appears to be free of other biases |
| Blinding of participants and personnel (performance bias) | Low risk | pg 253. outcome measures. Personnel were blinded, but unclear if patients were blinded. However, the primary outcome is unlikely to be biased. |
| Blinding of outcome assessment (detection bias) | Low risk | pg 253. outcome measures. Investigators assessing outcomes were blinded. |
| Methods | Randomised to decision aid with option to speak to genetic counsellor vs individual genetic counselling vs group counselling | |
| Participants | 116 + 126 + 110 women of advanced maternal age considering prenatal diagnostic testing in Canada | |
| Interventions | DA: audiotape workbook on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance/coaching (Ottawa Decision Support Framework) | |
| Outcomes | uptake of option, knowledge*, decisional conflict*, satisfaction with decision making process*, anxiety* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 4 ‐ randomised in blocks of 30, 10 to each intervention group |
| Allocation concealment (selection bias) | Low risk | pg. 4 ‐ "the allocations were provided in opaque envelopes" |
| Incomplete outcome data (attrition bias) | Unclear risk | outcomes reported on fewer participants but no rationale provided for missing data |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 51 + 49 women diagnosed with breast cancer considering surgical treatment in the USA | |
| Interventions | DA: computer program on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (step by step process for making the decision) COMPARE: usual care + breast cancer treatment educational materials normally provided to patients | |
| Outcomes | surgical treatment preference (post DA), breast cancer knowledge (pre, post DA, post DA and consult), satisfaction with surgical decision (post DA), satisfaction with decision making process (post DA), decisional conflict (pre, post DA, post DA and consult), proportional undecided. | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.42 (Methods section): "Patients at each hospital were randomized using permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | not addressed in the study |
| Incomplete outcome data (attrition bias) | Unclear risk | there is no way to know if the plots are including all of the participants' data since they do not specify what was the number of patients used to obtain these mean scores |
| Selective reporting (reporting bias) | Unclear risk | no mention of protocol |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | not addressed in the study |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 32 + 35 patients considering endodontic treatment options in the USA | |
| Interventions | DA: decision board on options' outcomes, clinical problem, outcome probability, guidance | |
| Outcomes | knowledge*, satisfaction with decision making process*, anxiety* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p.3 "four computerized random generation lists to assign to one of two groups" |
| Allocation concealment (selection bias) | Unclear risk | NO for residents: pg. 3 ‐ 4 computer‐generated randomisation lists (1 for each resident) were prepared by the PI; therefore residents would have had pre‐generated lists; but UNCLEAR for patients p.3 "Allocation was concealed from patients" but does not explained how |
| Incomplete outcome data (attrition bias) | Low risk | pg. 6 fig. 3 ‐ flow diagram; pg. 5 ‐ all 40 patients agreed to participate in the study, but only 32 questionnaires were useable several residents did not understand need for entering data on the envelope and placing matched questionnaire in it |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Unclear risk | p.5 "baseline data obtained because possible that clinicians training in the EndoDB would alter usual care discussions" Mentions taking baseline characteristics, but not included in article |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not mentioned. pg. 3 ‐ allocation was concealed from patients only |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 150 + 147 multiple sclerosis patients considering immunotherapy in Germany | |
| Interventions | DA: booklet and worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification (based on IPDAS) COMPARE: information material on immunotherapy (80 pages) | |
| Outcomes | role in decision making*, choice, feeling undecided, helpfulness with making a decision, attitudes toward immunotherapy, expectations of side effects realized at 6 months | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ "allocation using computer generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | pg. 2 ‐ Assignment ‐ randomisation was carried out by concealed allocation, but method of concealment was not described |
| Incomplete outcome data (attrition bias) | Low risk | pg. 2 ‐ Fig 1 flow of participants; baseline data/characteristics included |
| Selective reporting (reporting bias) | Low risk | pg. 2 "The protocol of this study has been published with the trial registration at http://controlled‐trials.com/ ISRCTN25267500" |
| Other bias | Unclear risk | pg. 5 ‐ difference in preferred interaction style between groups at baseline (P value 0.04) |
| Blinding of participants and personnel (performance bias) | Low risk | pg. 3 ‐ Masking ‐ Participants were not told whether the information they received was standard information or the newly developed DA |
| Blinding of outcome assessment (detection bias) | Low risk | pg. 3 ‐ Masking ‐ assessors were not told whether the information they received was standard information or the newly developed DA |
| Methods | Randomised to decision aid + coaching vs decision aid only vs usual care | |
| Participants | 215 + 206 + 204 women considering treatment for menorrhagia in the UK | |
| Interventions | DA: video + booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance/coaching | |
| Outcomes | uptake of option, satisfaction, general quality of life*, menorrhagia severity, cost effectiveness | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 ‐ allocation sequence was generated by computer and stratified by consultant and the age at which the woman left full‐time education |
| Allocation concealment (selection bias) | Low risk | pg. 3 "Secure randomization ensured by using a central telephone randomization system" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 4‐5 ‐ see Table 1 and Figure 1 flow diagram. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free from other risks of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg. 6 possibility of contamination bias, clinicians could have applied the experience gained from consultations with the interventions groups in their consultations with the control group |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear if blinding used but most outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid booklet vs decision aid web‐based vs usual care | |
| Participants | 196 + 226 + 75 patients considering prostate cancer screening in the USA | |
| Interventions | DA: 4 page pamphlet with options' outcomes, clinical problem, outcome probability COMPARE: web‐site with same information as paper based DA COMPARE: usual care | |
| Outcomes | role in decision making*, knowledge, decisional conflict, time spent discussing screening, choice (PSA test ordered) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "coordinator referred to pre‐generated randomisation tables to inform the participant to which arm he was randomised" |
| Allocation concealment (selection bias) | Low risk | pg. 2 ‐ At the time of enrolment, the allocation was concealed from the coordinator |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ results; pg. 4 ‐ flow diagram |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Unclear risk | uneven groups but done intentionally, ration of 1:3:3 but appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | High risk | physicians were not blinded ‐ could affect decision making process and uptake of screening |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs simple decision aid | |
| Participants | 244 + 252 pregnant women considering prenatal testing in the USA | |
| Interventions | DA: computerized tool on options' outcomes, clinical problem, outcome probability, explicit values clarification COMPARE: education booklet on the computer on options' outcomes, clinical problem | |
| Outcomes | knowledge*, decisional conflict*, accurate risk perception* (procedure related miscarriage, DS affected fetus), decision regret, satisfaction with the intervention*, satisfaction with involvement in decision making | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided |
| Allocation concealment (selection bias) | Low risk | pg. 3 ‐ Materials and Methods ‐ interviewer opened an opaque envelope containing the randomisation assignment |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ Fig 1 flow diagram |
| Selective reporting (reporting bias) | Low risk | pg. 1 ‐ Abstract CLINICAL TRIAL REGISTRATION: Clinicaltrials.gov, www.clinicaltrials.gov, NCT00686062 |
| Other bias | Unclear risk | appears to be free of other potential biases p.10 "The effect of these potential selection biases not determined" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs simple decision aid | |
| Participants | 32 + 31 men considering vasectomy as an option for contraception in Quebec, Canada | |
| Interventions | DA: booklet on options outcomes, clinical problem, outcome probability, explicit values clarification, guidance (step by step process for making the decision; one or more questions that asked patients to clarify their preferences; encourages patients to communicate with their practitioners) COMPARE: booklet on options outcomes, clinical problem, outcome probability | |
| Outcomes | knowledge (pre and post DA), decisional conflict* (pre and post DA), preferred option (pre and post DA), proportion undecided. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 557 (section 2.1): "computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | pg. 557 (section 2.1): "Randomization was stratified according to method of recruitment" "Each participant received either the full or the abridged PtDA inside an opaque, sealed, unmarked envelope" |
| Incomplete outcome data (attrition bias) | Low risk | pg.559 (Fig 1 flow diagram): p.560 Table 2 results |
| Selective reporting (reporting bias) | Unclear risk | no mention of examination of selective outcome reporting or study protocol |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | pg. 557 (section 2.1): single‐blinded‐ "researcher in charge of recruitment remained blind to the participants' group assignment". Participants were given an intervention but not aware of the alternative intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | pg.558 (section 2.4): "researcher blinded to the participant's group allocation when conducting the interviews", outcomes were objectively measured. |
| Methods | Randomised to decision aid + pharmacist consultation vs simple decision aid + pharmacist consultation | |
| Participants | 13 + 13 patients considering lifestyle changes and drug therapy to improve cardiovascular health in Canada | |
| Interventions | DA: booklet and worksheet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinion, guidance/coaching (Ottawa Decision Support Framework) | |
| Outcomes | knowledge, risk perception, decisional conflict, satisfaction with decision making process | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p. 2 "research nurse randomly assigned participant" "stratified by community pharmacy" Does not indicate how randomisation occurred. |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | p. 4 does not indicate why participants did not complete post‐intervention interview and post follow‐up interview. Pre‐intervention characteristics included on p. 6 in bar‐graphs. |
| Selective reporting (reporting bias) | Unclear risk | protocol not mentioned |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | unclear blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | unclear blinding, interviews used open‐ended question, do not know whether this contributes to risk of bias |
| Methods | Randomised to decision aid + coaching vs. usual care | |
| Participants | 114 + 108 women pregnant women in their first trimester considering use of contraceptives in the USA | |
| Interventions | DA: double‐sided flip chart on clinical problem, outcome probabilities, guidance (administered by a research assistant), coaching (structured, standardized, non‐directive contraceptive counselling) + usual care COMPARE: usual care | |
| Outcomes | proportion of participants choosing very effective contraceptive method* (post DA and consult), actual choice on day of procedure (post DA and consult), adherence of very effective and/or effective methods at 3 months and at 6 months (post DA and consult) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.363 (Methods‐study procedures): "Using a random‐number table, we determined the sequence for 1:1 allocation constrained by blocks of 10" |
| Allocation concealment (selection bias) | Low risk | pg.363 (Methods‐study procedures): "Randomization assignments were sealed inside numbered, opaque envelopes" |
| Incomplete outcome data (attrition bias) | Unclear risk | for "method initiation on the day of the procedure" it is only said that the "Participants in the intervention group were not more likely to initiate the requested method immediately compared to those in the usual care group"; possible that the results contradicted the hypothesis and were excluded for this reason |
| Selective reporting (reporting bias) | Unclear risk | no mention of study protocol; not enough information to permit judgement |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | pg.363 (Methods‐study procedures): "No blinding of participants or coordinators was feasible due to the nature of the intervention. Physician‐providers did not know the participant's allocation group, did not discuss the study with patients, and were asked not to change their counselling" |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 60 + 60 patients undergoing elective open heart surgery considering pre‐operative autologous blood donation in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance (Ottawa Decision Support Framework) | |
| Outcomes | uptake of option, knowledge*, decisional conflict*, satisfaction with decision making process, satisfaction with decision, accurate risk perceptions | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "Randomization envelopes were prepared centrally by a statistician" |
| Allocation concealment (selection bias) | Low risk | pg. 2 "The envelopes were labeled with identification numbers and contained a card specifying the patient’s group assignment. The envelopes were opened by the interviewer after completion of the baseline interview." |
| Incomplete outcome data (attrition bias) | Low risk | pg. 4 results, fig 1 flow diagram |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | no information provided |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Cluster randomised to decision aid vs simple decision aid | |
| Participants | 97 + 87 post‐menopausal women considering hormone replacement therapy (Cluster RCT with 40 family physicians randomised) in Canada | |
| Interventions | DA: audiotape, booklet and worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) | |
| Outcomes | decisional conflict, satisfaction with decision making process, agreement between physicians' and patients' decisional conflict | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | pg.5 fig 1 flow chart. Reasons for attrition not mentioned. pg.6 Demographic characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 45 + 45 women considering use of natural health products for managing menopausal symptoms | |
| Interventions | DA: booklet with worksheet on options' outcomes, clinical problem, explicit values clarification, guidance/coaching (Ottawa Decision Support Framework) | |
| Outcomes | knowledge of natural health products in general (not specific option outcomes), preferred choice, decisional conflict*, values‐choice agreement, proportion undecided | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schema was carried out by a biostatistician using computer‐generated unequal blocks. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes containing one or the other documents (a PDA in the intervention group and a general information brochure in the control group) were prepared by another individual, external to the study. |
| Incomplete outcome data (attrition bias) | Low risk | See Figure 1 for flow diagram, reason for lost to follow up was described. |
| Selective reporting (reporting bias) | Low risk | trial registration identifier is NCT00325923 |
| Other bias | Low risk | There was no statistically significant difference in women’s characteristics between groups (Table 1) |
| Blinding of participants and personnel (performance bias) | Unclear risk | The investigators were blinded but no mention of blinding of participants |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | randomised to decision aid vs usual care | |
| Participants | 245 + 214 patients with non‐emergent acute respiratory infections considering using antibiotics in Canada | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance and coaching COMPARE: delayed intervention | |
| Outcomes | Patient Outcomes: actual choice* (pre and post DA), perceived decision quality* (pre and post DA), decisional conflict* (pre and post DA), intention to engage in future SDM (pre and post DA), decision regret* (pre and post DA), participation in decision making, general health outcomes*. Practitioner Outcomes: decision*, perceived decision quality*, decisional conflict*, intention to engage in future SDM and comply with clinical practice guidelines | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 99 : "A biostatistician simultaneously randomised all FMGs and allocated them to groups using Internet‐based software." |
| Allocation concealment (selection bias) | Low risk | pg. 99 : "using Internet‐based software." |
| Incomplete outcome data (attrition bias) | Low risk | there appears to be no missing data |
| Selective reporting (reporting bias) | Low risk | no missing pre‐specified outcomes |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | unclear blinding of participants and personnel; pg.99: only biostatistician was blinded |
| Blinding of outcome assessment (detection bias) | Low risk | pg. 99: biostatistician who assesses the outcomes is blinded, outcomes were objectively measured. |
| Methods | Randomised to DA + usual care vs usual care | |
| Participants | 107 + 100 patients diagnosed with metastatic CRC considering advanced chemotherapy in Australia and Canada | |
| Interventions | DA: booklet and audiotape on option' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance (steps in decision making + worksheet) COMPARE: usual care | |
| Outcomes | anxiety (pre and post DA), knowledge* (post DA), satisfaction with consultation (post DA), choice leaning (post DA), decisional conflict (post DA). achievement of their information preference (post DA), participation in decision making (post DA), acceptability (post DA), satisfaction with decision* (post DA), quality of life (post DA) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2078 (study design): computer generated randomised lists |
| Allocation concealment (selection bias) | Low risk | pg. 2078 (study design): code concealed in sealed envelopes until time of random assignment |
| Incomplete outcome data (attrition bias) | Unclear risk | 31% drop out, but similar losses across all groups |
| Selective reporting (reporting bias) | Unclear risk | protocol not available |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | patients not blinded and subjective outcomes may be affected by them knowing their assignment |
| Blinding of outcome assessment (detection bias) | Low risk | all outcomes are not subjected to interpretation |
| Methods | Randomised to decision aid vs waiting list control | |
| Participants | 122 + 114 + 164 women considering BRCA1 gene testing in the USA | |
| Interventions | DA: education and counselling on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance/coaching | |
| Outcomes | preferred option*, knowledge, accurate risk perceptions, perceived personal risk / benefits / limitations, agreement between values and choice | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 2 ‐ of these 440 women, 400 completed 1‐month follow‐up interviews; no reasons provided; Baseline data/characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs simple decision aid | |
| Participants | 100 + 101 women considering prenatal diagnostic testing in China | |
| Interventions | DA: interactive multimedia decision aid on options' outcomes, clinical problem, outcome probability, guidance | |
| Outcomes | Preferred option, proportion remaining undecided, uptake of option* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "women randomised at a 1:1 ratio." "Allocation was made by a nurse specialist opening consecutive, sealed opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | pg. 2 "Allocation was made by a nurse specialist opening consecutive, sealed opaque envelopes" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 2 Baseline demographic data collected. pg. 3 Acknowledges non‐compliant woman. |
| Selective reporting (reporting bias) | Unclear risk | no mention of protocol |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 211 + 232 patients considering CRC screening in the USA | |
| Interventions | DA: web‐based, DVD and VHS videotape formats + stage targeted brochures (and booster kit if patients had not been screened) on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (encouraged patients to communicate with their practitioners by asking questions and sharing preferences; summary) COMPARE: usual care using Aetna annual reminders to obtain CRC screening | |
| Outcomes | knowledge of the age at which screening should begin (post DA), completion of colorectal cancer screening (pre, post DA), intrusive thoughts (pre, post DA), interest in CRC screening (pre, post DA), intent to ask provider about screening (pre, post DA), readiness to be screened (pre, post DA), perceived risk of colon cancer (pre, post DA), general beliefs about colon cancer (pre, post DA), fears about colorectal cancer screening (pre, post DA), perceptions about whether participants had enough information (post DA), whether participants had enough information about specific screening tests (post DA), willingness to pay for screening tests (post), desire to participate in medical decision (post) Practice level measures: assess CRC screening practices (pre, post DA), referrals (pre, post DA), quality improvement initiatives | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 (Practice recruitment and randomisation section): "Randomisation was done using matched pairs and a blocking procedure." |
| Allocation concealment (selection bias) | Unclear risk | pg. 3 (Practice recruitment and randomisation section): "Thus, purposive assignment to treatment group was used, resulting in a hybrid randomisation" There is no mention of the effect of this purposive assignment on the study |
| Incomplete outcome data (attrition bias) | Low risk | There appears to be no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol |
| Other bias | High risk | unadjusted cluster analysis |
| Blinding of participants and personnel (performance bias) | Unclear risk | As mentioned above, staff used purposive assignment and were therefore not blinded, but there is no mention of the effect on the study. |
| Blinding of outcome assessment (detection bias) | Low risk | The study did not address this outcome, but outcomes were objectively measured. |
| Methods | Cluster randomised to decision aid vs usual care | |
| Participants | 263 + 142 patients with physician diagnosed depression (cluster RCT with 30 general practitioners randomised) in Germany | |
| Interventions | DA: decision board on options' outcomes, clinical problem, explicit values clarification, guidance/coaching COMPARE: usual care | |
| Outcomes | participation in decision making, adherence, satisfaction with clinical care, depression severity, consultation length | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 "two‐thirds of the general practitioners were randomly assigned to the intervention group by drawing blinded lots under the supervision of the principal investigator and two researchers" |
| Allocation concealment (selection bias) | Low risk | pg. 3 ‐ 2.1 drawing blinded lots |
| Incomplete outcome data (attrition bias) | Unclear risk | 'pg.5 fig p.3 "Further results resting on the baseline phase of this trial were already presented elsewhere" p. 3 "Unequal distribution of physicians was due to possibility of higher dropout rate in intervention group because of additional time and effort" |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | appears to be free of other potential biases ‐ pg. 5 & 6 details pt and physician baseline characteristics. Stat sig differences were controlled for in outcome analyses |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear blinding, not enough information provided to assess whether this contributes to bias on outcomes not measured by using a scale (e.g. consultation time was documented in minutes by the physicians following each consultation) |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 139 + 148 patients on atrial fibrillation trial considering continuing on aspirin vs change to Warfarin in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) | |
| Outcomes | uptake of options*, help with making a decision, knowledge, accurate risk perceptions, decisional conflict, satisfaction with decision making process, role in decision making, adherence* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ computer‐generated scheme |
| Allocation concealment (selection bias) | Low risk | pg. 2 ‐ administered from a central location |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 4 fig 2 flow chart. Reasons for attrition not mentioned. Baseline data not included. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | no other potential risks of bias |
| Blinding of participants and personnel (performance bias) | High risk | Unclear blinding however, pg. 7 "contamination, physicians may have provided DA information to patients receiving usual care" |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 80 + 70 participants diagnosed with diabetes considering the use of statins to reduce coronary risk | |
| Interventions | DA: healthcare provider led discussion using developed tool (Statin Choice) on options' outcomes,outcome probabilities, guidance (step by step process for making the decision; administered by the physician in the consultation) COMPARE: usual primary care visit + pamphlet | |
| Outcomes | knowledge (post consult and DA), decisional conflict (post consult and DA), risk estimation (post consult and DA), beliefs (post consult and DA), adherence (3 and 6 months post consult and DA) | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p.138 (methods section) told that participants were randomised but there is no mention of method used |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Low risk | Baseline data was provided |
| Selective reporting (reporting bias) | Unclear risk | only reports on improvement (i.e. decisional conflict scale); does not present outcome data to fullest (no numerical data on knowledge results between groups, only describes in words) |
| Other bias | Unclear risk | p.139 (Analysis section): "We did not adjust the clustering of effects given that few participants received care by the same clinicians." No mention of magnitude in change of data due to this choice |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 278 + 139 participants considering diabetes screening in the UK | |
| Interventions | DA: screening invitation on clinical problem, outcome probabilities and explicit values clarification COMPARE: usual care using screening invitation on clinical problem | |
| Outcomes | preferred option* (post DA), whether invitation type impacts on intention (post DA), impact on knowledge (post DA), impact on attitude (post DA), risk perception. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 2‐3 (Methods‐ Participants section): "Invitation taken from the top of a randomly ordered pile (either standard or one of two versions of an informed decision choice invitation). The materials were ordered in a way that the invitation type was hidden until the recruitment process was completed" Unclear how invitation type was hidden |
| Allocation concealment (selection bias) | Low risk | pg. 2‐3 (Methods‐ Participants section): invitation taken from the top of a randomly ordered pile; materials were ordered in a way that the invitation type was hidden until the recruitment process was completed |
| Incomplete outcome data (attrition bias) | Low risk | no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | no mention of protocol; insufficient information to permit judgment |
| Other bias | Unclear risk | pg.6 (Discussion section [end of page]): "present sample was […] not necessarily representative of the highest risk individuals in this age group"; "£5 incentive might have also added a selection bias"; "Lack of anonymity with verbally delivered questionnaire might encourage socially desirable responding" |
| Blinding of participants and personnel (performance bias) | Low risk | pg.3 (methods, participants section): interviewers were not aware of the direction of anticipated effect of materials, and materials were dummy‐coded so that no sense of intervention or control would have been communicated to interviewers or participants |
| Blinding of outcome assessment (detection bias) | Low risk | study did not address this outcome, but outcomes were objectively measured and not subject to interpretation |
| Methods | randomised to decision aid vs usual care | |
| Participants | 633 + 639 patients considering diabetes screening in England | |
| Interventions | DA: screening invitation on clinical problem, outcome probabilities and explicit values clarification COMPARE: usual care using screening invitation on clinical problem | |
| Outcomes | attendance for screening* (post DA and consult), intention to make changes to lifestyle (post DA and consult), satisfaction with decisions made among attenders (post DA and consult) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.2 (randomisation section): "generated simultaneously in a batch by random numbers using Excel spreadsheet software, stratifying by number of participants in household" |
| Allocation concealment (selection bias) | Low risk | pg.2 (randomisation section): "Randomisation […] was undertaken by the study statistician from a central site" |
| Incomplete outcome data (attrition bias) | Low risk | no missing outcome data |
| Selective reporting (reporting bias) | Low risk | pg.2 (methods section): they published a protocol |
| Other bias | Low risk | the study appears free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | pg.2 (randomisation section): personnel were blinded and appears that patients were unaware which arm they were in (members of the same household received the same intervention) |
| Blinding of outcome assessment (detection bias) | Low risk | pg.2 (randomisation section): clinical and trial staff taking measurements and entering data were unaware of the study arm to which participants had been assigned |
| Methods | Randomised to decision aid versus usual care | |
| Participants | 367+367 women aged 70 to 71 years and considering a subsequent screening mammography in Australia | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance with worksheet (Ottawa Decision Support Framework) COMPARE: BreastScreen NSW brochure ‐ includes information for women 70+ but no numeric information about the outcomes of screening | |
| Outcomes | knowledge (includes 5 questions about risk perceptions), anxiety, decisional conflict, breast cancer worry, preference/intension, actual decision*, informed choice*, attitudes about screening, relationship between objective and perceived risk of breast cancer | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ Methods ‐ computer program, which assigned allocations in accordance with a simple randomisation schedule |
| Allocation concealment (selection bias) | Low risk | 'pg. 2 ‐ Methods ‐ randomised by interview staff who accessed a previously concealed computer program |
| Incomplete outcome data (attrition bias) | Low risk | pg. 2 ‐ fig 1 flow diagram |
| Selective reporting (reporting bias) | Low risk | pg. 5 "The trial was registered with the Australian Clinical Trials Registry and the Clinical Trials Registration System" |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | interviewers [at follow‐up] were blinded, outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 189 + 223 women considering mammography screening | |
| Interventions | DA: Internet program + worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance (worksheet with questions relevant to decision making process; one or more questions that asked patients to clarify their preferences; summary) COMPARE: delayed intervention | |
| Outcomes | knowledge* (post DA), intention (post DA), values (post DA), informed choice (post DA), risk perception*, proportion undecided. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 66 (randomization and baseline questions section): "computer generated simple randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | pg. 66 "randomization was conducted in a concealed manner." The method of allocation concealment was not stated |
| Incomplete outcome data (attrition bias) | Low risk | pg.68 (Table 2) all outcomes mentioned in outcome measures section were reported in the results section (information for intention as well as anxiety and acceptability can be found in text format in the secondary outcomes section on pg.67‐68) |
| Selective reporting (reporting bias) | Unclear risk | no mention of protocol |
| Other bias | Low risk | appears to be free of other potential sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were not subjective to interpretation |
| Methods | Cluster randomised to decision aid vs usual care | |
| Participants | 219 + 215 patients considering antithrombotic therapy for nonvalvular atrial fibrillation (Cluster RCT with 102 primary care practices randomised) in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) | |
| Outcomes | uptake of (appropriate) option*, knowledge, decisional conflict, accurate risk perceptions | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "cluster randomization at level of primary care practice to minimize contamination; randomization was done centrally to preserve allocation concealment using a computer generated |
| Allocation concealment (selection bias) | Low risk | pg. 2 ‐ Methods ‐ randomisation was done centrally to preserve allocation concealment |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ Results & Fig 1 ‐ flow diagram |
| Selective reporting (reporting bias) | Low risk | pg. 1 ‐ Methods ‐ DAAFI Trial protocol, including copies of the various questionnaires we employed, has been published |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded, but not sure whether the lack of blinding would affect the outcomes |
| Blinding of outcome assessment (detection bias) | Low risk | outcome assessors blinded |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 289 + 292 peri‐menopausal women considering hormone replacement therapy in the USA | |
| Interventions | DA: options' outcomes, clinical problem, outcome probability, values clarification, others' opinions, guidance/coaching | |
| Outcomes | accurate risk perceptions*, satisfaction with decision, confidence with knowledge & making/discussing decision | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided; Bastian 2002 ‐ no information provided ‐ pg. 4 ‐ Study design is described elsewhere |
| Allocation concealment (selection bias) | Unclear risk | no information provided; Bastian 2002 ‐ no information provided ‐ pg. 4 ‐ Study design is described elsewhere |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 2 Complete data are available for 520 (90%) of the women. Reasons why not mentioned; Bastian ‐ pg. 5 ‐ results; pg. 6 Baseline characteristics/data included |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | appears to be free of other potential biases; Bastian ‐ pg. 8 ‐ Eligible participants were willing to consider HRT and this may have favoured recruitment of women with higher SES and those who had prior experience with HRT |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomized to decision aid + informed choice vs HPV testing vs repeat smear | |
| Participants | 104 + 104 + 106 women screened as HPV indeterminate considering HPV testing in Australia | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (worksheet) COMPARE: no decision support, received immediate HPV testing COMPARE: no decision support, received a repeat cervical smear at 6 months | |
| Outcomes | waiting time anxiety (post DA), quality of life* (post DA), perceived risk (post DA), perceived seriousness of cancer (post DA), worriedness (post DA), intrusive thoughts (post DA), satisfaction with care (post DA), anxiety (post DA), distress and concerns (post DA), self‐esteem (post DA), effect on sexual behaviour (post DA), help seeking behaviour (post DA), knowledge (post DA) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.2 (design): "Participants were randomised centrally by the research team within each clinic in blocks of three" |
| Allocation concealment (selection bias) | Low risk | pg.2 (design): "Participants were randomised centrally by the research team within each clinic in blocks of three" |
| Incomplete outcome data (attrition bias) | Low risk | Figure 3: sensitivity analysis was done to include most of the patients |
| Selective reporting (reporting bias) | Low risk | protocol available |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | patients and staff were unblinded, but objective outcomes were used |
| Blinding of outcome assessment (detection bias) | Low risk | all outcomes are on questionnaires; not subject to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 279 women considering BRCA1 BRCA2 gene testing in the USA | |
| Interventions | DA: educational intervention on options' outcomes, personal family cancer history; clinical problem, outcome probability, explicit values clarification, others' opinions, guidance/coaching | |
| Outcomes | Preferred option, knowledge, perceived risk, satisfaction | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 4 "randomized by the CATI system" Randomized after self initiated telephone contact. |
| Allocation concealment (selection bias) | Low risk | pg. 4 "computerized assisted telephone interview system (CATI)" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 8 reasons stated for initial drop‐out of study participants. Patients contacted offered reasons for dropping‐out. Study protocol allowed patients to be reached up to 13 times at follow‐up; but still not able to be reached. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding was not addressed |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | decision aid vs attention placebo | |
| Participants | 132 + 132 participants considering colon cancer screening in the USA | |
| Interventions | DA: computer‐based web program on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (encourages patient‐practitioner communication, summary) COMPARE: computer‐based web program on prescription drug refills and safety | |
| Outcomes | Receipt of CRC screening* (post DA); ability to state a preference; change in readiness to receive screening (pre and post DA); CRC test ordering (post DA), proportion undecided. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.609 (methods): block randomised, stratified by literacy level |
| Allocation concealment (selection bias) | Unclear risk | study does not address this domain |
| Incomplete outcome data (attrition bias) | Low risk | no missing outcome data |
| Selective reporting (reporting bias) | Low risk | protocol on clinical trials.gov |
| Other bias | Unclear risk | $10 gift card for participation could affect participant pool |
| Blinding of participants and personnel (performance bias) | Low risk | pg.609 (methods): health care providers were not notified of patients' enrolment in the study at any time ; pg.613 (discussion): RAs that administered post‐DA questionnaire were not blinded but believed to be a low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | pg.613 (discussion): "clinical outcome assessors were [blinded]" |
| Methods | Randomised to decision aid + decision analysis vs decision analysis vs decision aid vs usual care | |
| Participants | 51 + 52 + 55 + 59 newly diagnosed hypertensive patients considering drug therapy for blood pressure in the UK | |
| Interventions | DA: decision analysis plus information video and leaflet on options' outcomes, clinical problem, outcome probability, explicit values clarification | |
| Outcomes | uptake of option, knowledge, decisional conflict*, anxiety | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ allocation schedule was computer‐generated by an individual not involved in the study |
| Allocation concealment (selection bias) | Low risk | pg. 2 "allocation was concealed to the author in advance by the nature of the minimization procedure" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 5 ‐ flow diagram |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded ‐ unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid with values clarification vs decision aid without values clarification vs usual care | |
| Participants | 245 + 250 + 247 women with previous caesarean section in the UK | |
| Interventions | DA: options' outcomes, clinical problem, outcome probability, explicit values clarification COMPARE: options' outcomes, clinical problem, outcome probability COMPARE: usual care | |
| Outcomes | decisional conflict*, choice, anxiety, knowledge, satisfaction with decision | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 Methods Randomization: blacked by using randomly permuted and selected blocks of sizes 6, 9, 12, and 15 generated by computer |
| Allocation concealment (selection bias) | Low risk | pg. 2 Methods Randomization: one member of the study team generated the randomisation sequence by computer, and another member of staff with no other involvement in the trial performed the allocation |
| Incomplete outcome data (attrition bias) | Low risk | see flow of women through the study |
| Selective reporting (reporting bias) | Low risk | Trials registry ISRCTN84367722 |
| Other bias | Low risk | Recruited more than planned to account for lost data (Sample Size pg. 4); Baseline characteristics were balanced |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs usual care+booklet | |
| Participants | 52 + 48 women with low bone mass or osteoporosis considering taking bisphosphonates in the USA | |
| Interventions | DA: worksheet on options' outcomes, clinical problem, outcome probabilities, guidance (administered by physician) COMPARE: usual care + general information booklet on osteoporosis | |
| Outcomes | patient knowledge (post DA), satisfaction with knowledge transfer (post DA), decisional conflict (post DA), patient‐clinician communication (OPTION), trust with physician (during intervention), clinician's perception of decision quality (post DA), clinician's satisfaction with knowledge transfer (post DA), uptake(post DA), adherence (post DA), fidelity (post DA), contamination (post DA), risk perception. | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.551(Randomization):"computer generated allocation " |
| Allocation concealment (selection bias) | Low risk | pg.551(Randomization): patients randomised "in a concealed fashion (using a secure study website)" |
| Incomplete outcome data (attrition bias) | Low risk | no missing outcome data |
| Selective reporting (reporting bias) | Low risk | pg.550 (design):"The protocol for this trial has been reported in full" |
| Other bias | Unclear risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg.551 (Randomization): no mention of participants being blinded to their allocation; only mention of data collectors and analysts blinding |
| Blinding of outcome assessment (detection bias) | Low risk | pg.551 (Randomization): "After randomization, data collectors and data analysts were blind to allocation", outcomes were not subject to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 120 + 120 patients with Ischemic heart disease considering revascularization surgery in Canada | |
| Interventions | DA: Health Dialog interactive videodisc on options' outcomes, clinical problem, outcome probability, others' opinions | |
| Outcomes | uptake of option, knowledge, satisfaction with the decision making process* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Morgan, 1997, Thesis: pg. 29 ‐ all randomisation enrolment was performed by telephone at which time the pt was assigned; Morgan, 2000, JGIM (Primary Study):pg. 2 ‐ Methods (Patient Pop) "Only the statistician was privy to the two randomisation schedules and blocking factor used" |
| Allocation concealment (selection bias) | Low risk | Morgan, 1997, Thesis:pg. 29 ‐ only the statistician was privy to the two randomisation schedules and blocking factor; Morgan, 2000, JGIM (Primary Study):pg. 2 ‐ Methods (Patient Pop) "only the statistician was privy to the two randomisation schedules and blocking factor used. All randomization enrolment was performed by telephone" |
| Incomplete outcome data (attrition bias) | Low risk | Morgan, 1997, Thesis: pg. 39 ‐ patient accrual & follow‐up, Baseline characteristics included; Morgan, 2000, JGIM (Primary Study): no reason indicated, but judgement of YES |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Unclear risk | Morgan, 1997, Thesis: pg. 56 ‐ significant number of patients were lost to follow‐up (25%); Morgan, 2000, JGIM (Primary Study): baseline data imbalance (High school grad, income, # of diseased arteries) Drop‐out group reported lower incomes, may have affected results. (discussion par. 6) "Selection bias was minimized by enrolling available consecutive patients" |
| Blinding of participants and personnel (performance bias) | Unclear risk | "due to nature of trial, neither patients or investigators were blinded to the study" ‐ may introduce bias to subjective outcomes such as satisfaction |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Cluster randomised to decision aid vs usual care | |
| Participants | 48 + 37 patients with type 2 diabetes considering treatment options (cluster RCT with 40 clinicians randomised) in the USA | |
| Interventions | DA: decision cards with information on options, outcomes, outcome probability, explicit values clarification Compare: 12‐page pamphlet on oral antihyperglycaemic medications | |
| Outcomes | knowledge, decisional conflict, participation in decision making, acceptability of the information, change in medication, adherence, HA1C levels, trust in physician, OPTION to analyse audio‐taped encounters | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated |
| Allocation concealment (selection bias) | Low risk | central allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | reasons for attrition not included |
| Selective reporting (reporting bias) | Low risk | trial registration # at clinicaltrials.gov reported |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Patients were blinded, the clinicians were not, but each session was recorded |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 57 + 55 men considering treatment for benign prostatic hypertrophy in the UK | |
| Interventions | DA: Health Dialog interactive videodisc on options, outcomes, clinical problem, outcome probability, others' opinions | |
| Outcomes | uptake of option*, decisional conflict, role in decision making, prostate symptoms*, costs, anxiety*, general health status, utility | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 4 "randomisation schedule, stratified according to recruitment centre, was generated by computer" |
| Allocation concealment (selection bias) | Low risk | pg. 4 "Allocation were sealed in opaque numbered envelopes, opened by the study nurse" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 5 ‐ flow diagram; Baseline data/characteristics included and balanced. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded but not sure how this would introduce bias |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 102 + 102 women considering hormone replacement therapy in the UK | |
| Interventions | DA: Health Dialog interactive videodisc on options outcomes, clinical problem, outcome probability, other's opinion | |
| Outcomes | preferred option*, help with making a decision, decisional conflict, role in decision making | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 Methods (Randomization) "randomisation schedule, stratified according to recruitment centre, was generated by computer" |
| Allocation concealment (selection bias) | Low risk | pg. 3 Methods (Randomization) "Allocations were sealed in opaque numbered envelopes, opened by the study nurse after collection of the baseline data" |
| Incomplete outcome data (attrition bias) | Low risk | See page 3 figure for Progress of patients through trial |
| Selective reporting (reporting bias) | Unclear risk | protocol is not mentioned |
| Other bias | Low risk | similar baseline characteristics, appears to be free of other potential biases. Educational achievement was higher in control group. quote "Subsequent analysis showed that educational level not related to use of HRT nor was there an interaction between ed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to detailed decision aid vs simple decision aid | |
| Participants | 121 + 121 African‐American men considering prostate cancer screening in the USA | |
| Interventions | DA: information booklet on options' outcomes + decision education session with clinical problem, explicit values clarification, guidance/coaching | |
| Outcomes | uptake of option* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | pg. 4 ‐ flow diagram; Reasons for not receiving intervention or completing endpoint chart mentioned. Baseline characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Unclear risk | pg. 8 ‐ The mode of delivery of the decision education might have modified a potential intervention effect; baseline characteristics similar except Enhanced Intervention group consisted of more men who were educated beyond high school and who were married |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to detailed vs simple DA | |
| Participants | 156 + 157 men considering prostate cancer screening in the USA | |
| Interventions | DA: 12 page informational brochure (options, outcomes, clinical problem, outcome probability) + coaching (structured decision counselling session with nurse educator) COMPARE: 12 page informational brochure | |
| Outcomes | Participants: knowledge* (pre, post DA and consult), decisional conflict* (post DA and consult), actual choice(post DA and consult), patient‐clinician communication, consult length Physician: knowledge(pre), orientation to talking with patients(pre), preferred role in shared decision making(pre) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention of how sequence was generated |
| Allocation concealment (selection bias) | Low risk | pg.241 (section 2.1): "Using a system of sealed envelopes" |
| Incomplete outcome data (attrition bias) | Unclear risk | pg.243 (section 3.3): does not account for why 24 audio‐recordings were excluded |
| Selective reporting (reporting bias) | Unclear risk | no mention of study protocol; not enough information to permit judgement |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | no mention of blinding of either personnel or participants; all patient charts had a generic note placed in them by the nurse educator to prompt physician to discuss prostate cancer screening |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were not subjective to interpretation |
| Methods | Cluster randomised to decision aid vs usual care | |
| Participants | 167 + 172 women in early pregnancy considering genetic testing (26 + 29 general physicians) (cluster RCT with 60 general practitioners randomised) in Australia | |
| Interventions | DA: 24‐page booklet and worksheet on options, benefits and risks, test limitations, outcomes; clinical problem, outcome probability, explicit values clarification, opinions of others', guidance (Ottawa Decision Support Framework) COMPARE: standard pamphlet on prenatal testing | |
| Outcomes | informed choice*, decisional conflict*, anxiety, depression, attitudes toward pregnancy, acceptability of the intervention, choice | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | pg. 3 ‐ computer‐generated random numbers by an independent statistician; allocation concealment was achieved |
| Incomplete outcome data (attrition bias) | Low risk | pg. 4 ‐ results & pg. 5 fig 1 ‐ flow diagram |
| Selective reporting (reporting bias) | Low risk | pg. 4 ‐ Trial Registration ‐ The ADEPT trial was registered in the UK with Current Controlled Trials [ISRCTN22532458] and with the Australian Clinical Trials Registry (No: 012606000234516). |
| Other bias | Low risk | appears to be free of other potential biases ‐ pg. 8 ‐ selection bias but was adjusted for in analysis |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg. 3 "Due to the nature of the intervention, it was not possible to blind women, GP's or researchers"; unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) | Low risk | researchers were not blinded but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 102 + 98 women diagnosed with a breech presentation from 34 weeks gestation considering external cephalic version in Australia | |
| Interventions | DA: 24‐page booklet, 30‐minute audio‐CD and worksheet; clinical problem, outcome probability, explicit values clarification, opinions of others', guidance (Ottawa Decision Support Framework) COMPARE: usual care counselling and information on the management of breech presentation | |
| Outcomes | knowledge*, decisional conflict*, anxiety*, satisfaction with the decision*, preferred role in decision making, preferred choice | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "randomly generated using computer and stratified by parity and center using random variable block sizes" |
| Allocation concealment (selection bias) | Low risk | pg. 2 "participants were randomized by telephoning a remote, central location" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 Lost to follow‐up because of onset of labour or incomplete data forms. Baseline characteristics are included and equal. Minimum of 84 participants in each study group achieved; pg. 4 ‐ flow diagram |
| Selective reporting (reporting bias) | Low risk | ISRCTN14570598 |
| Other bias | Low risk | pg. 3 Results "Maternal characteristics and baseline measures of cognitive and affective outcomes were comparable between groups (Table 1); pg.6 "Blinding clinicians and employment of a research midwife to interact with women" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Womens were not blinded ‐ unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid with values clarification vs simple decision aid without values clarification | |
| Participants | 101 +100 women considering long term hormone therapy in Canada | |
| Interventions | DA: audiotape booklet on options outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (Ottawa Decision Support Framework) | |
| Outcomes | decisional conflict, congruence with values* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Based on centrally handled randomisation. randomly assigned but sequence generation method was not stated |
| Allocation concealment (selection bias) | Low risk | pg. 4 ‐ Methods (Design) "randomization was handled centrally; RA called research office" |
| Incomplete outcome data (attrition bias) | Low risk | Appears to account for outcome data |
| Selective reporting (reporting bias) | Unclear risk | Does not mention protocol |
| Other bias | Low risk | similar baseline characteristics, appear to be free of any other potential biases. No baseline measures to avoid exposing groups to value measurement before the decision aid |
| Blinding of participants and personnel (performance bias) | Unclear risk | participants were blinded but RA was not (trained to remain neutral) |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding, outcomes were objectively measured and not subjective to to interpretation and data analyst was blinded to assignment of intervention |
| Methods | Randomised to detailed decision aid vs simple decision aid | |
| Participants | 81 + 84 women considering long term hormone therapy in Canada | |
| Interventions | DA: audiotape booklet on options outcomes, clinical problem, outcome probability, explicit values clarification, others' opinion, guidance (Ottawa Decision Support Framework) | |
| Outcomes | preferred option, knowledge, decisional conflict*, accurate risk perceptions* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided; pg.3 "RA called research office with screening info and given participant's id number and intervention assignment" |
| Allocation concealment (selection bias) | Low risk | pg.3 "randomization was handled centrally" |
| Incomplete outcome data (attrition bias) | Unclear risk | pg.8 "in future studies it would be important to collect baseline predispositions" Flow chart not included. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | participants were blinded but RA was not (trained to remain neutral) |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding, outcomes were objectively measured and not subjective to to interpretation and data analyst was blinded to assignment of intervention |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 16 + 17 postmenopausal women with osteoporosis considering treatment options to prevent further bone loss in the UK | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) | |
| Outcomes | satisfaction with information, decisional conflict (intervention group only), improvement in adherence | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided |
| Allocation concealment (selection bias) | Low risk | pg. 1 ‐ group allocation was done by a third party, unconnected to the study and blinded to the identity of the patients |
| Incomplete outcome data (attrition bias) | Unclear risk | Sample characteristics not included. Baseline satisfaction score included. pg. 2 "No evaluation was carried out to determine the reasons for non‐participation" |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Unclear risk | no baseline characteristics; pg. 2 Only 16 patients in intervention group and 17 in control group. Small sample size. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear blinding, some outcomes were assessed by open‐ended questions, do not know whether this contributes to risk of bias |
| Methods | Randomised to decision aid + standard counselling vs usual care (standard counselling) | |
| Participants | 15 + 15 women considering breast cancer prevention in the USA | |
| Interventions | DA: interactive computer decision aid on options outcomes, outcome probability | |
| Outcomes | consultation length*, knowledge, decisional conflict, satisfaction with the decision, acceptability of the decision aid, physician satisfaction with the consultation | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 149 patients were randomised evenly between groups; no information provided about generation |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | demographic data included; reasons for attrition mentioned |
| Selective reporting (reporting bias) | Unclear risk | no reference to study protocol |
| Other bias | Unclear risk | small sample size, does not say how many physicians participated in study, mentions that there were observed changes in physician behaviour (based on doing both intervention and control) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid with others' opinions vs decision aid without others' opinions vs usual care | |
| Participants | 384 + 384 + 384 men considering PSA testing in the USA | |
| Interventions | DA: Health Dialog video on options' outcomes, clinical problem, outcome probability, others' opinions | |
| Outcomes | preferred option, help with making a decision, knowledge*, decisional conflict | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ Using a computer‐generated algorithm |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | pg. 2 ‐ flow diagram; Reasons for attrition mentioned and participants balanced across study groups. Sample characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | pg. 5 "providers were blinded to the fact that their patients were participating in a trial" "coordinator did not have direct contact with subjects" |
| Blinding of outcome assessment (detection bias) | Low risk | "follow‐up interviewers blinded, statisticians were not". Outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 125 + 124 adults considering colon cancer screening in the USA | |
| Interventions | DA: video of options' outcomes, clinical problem, others' opinion | |
| Outcomes | uptake of options* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ Methods (Group Assignment) "computerized random number generator" |
| Allocation concealment (selection bias) | Low risk | pg. 2 ‐ Methods (Group Assignment) "randomization was performed centrally and was not balanced among centers. Assignments were placed in sealed, opaque, sequentially numbered envelopes and were distributed to the three sites" |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 4 Because of an administrative error, 18 controls did not complete the second and third questionnaires. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not mentioned |
| Other bias | Low risk | baseline characteristics similar, appear to be no other potential sources of biases. minimized bias from repeated measurements by administering the same questionnaires to the intervention and control participants |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg 2. "The providers and staff were not blinded to intervention status" "3 to 6 months after, different RA blinded to participant intervention examined clinic records" ‐Does not mention whether patients were blinded; Unclear if lack of blinding contributed to potential risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | a different research assistant who was blinded to participants’ intervention status examined participants’ clinic records in a standardized and validated manner to determine whether colon cancer screening tests were actually completed within 3 months of the index visit. |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 60 + 56 women considering treatment options for menorrhagia in the UK | |
| Interventions | DA: interactive computerized DA on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance | |
| Outcomes | knowledge, decisional conflict*, anxiety, condition specific health outcomes, treatment preference, undecided | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ Methods ‐ computer generated randomisation, stratified by practice and minimized according to age |
| Allocation concealment (selection bias) | Unclear risk | pg. 2 ‐ Methods ‐ Random allocation was concealed from the individual who was making judgments of eligibility, but the method of concealment was not stated |
| Incomplete outcome data (attrition bias) | Low risk | pg. 5 ‐ fig 6 flow diagram; pg. 4 Baseline data/characteristics included and balanced. |
| Selective reporting (reporting bias) | Low risk | ISRCTN72253427 |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to detailed vs simple decision aid | |
| Participants | 395 + 201 primiparous women in final trimester considering pain relief for labour in Australia | |
| Interventions | DA: booklet (and/or audiotape) and worksheet on options' outcomes, clinical problem, outcome probabilities, other's opinion, explicit values clarification, guidance (steps by step process for making the decision; worksheet with questions relevant to decision making process; encouraged patients to communicate with their practitioner by asking questions and sharing preferences; one or more questions that asked patients to clarify their preferences; summary) COMPARE: pamphlet on certain options' outcomes, clinical problem, outcome probabilities. | |
| Outcomes | knowledge* (pre, post DA and consult ), decisional conflict* (pre, post DA, and consult), anxiety* (pre, post DA and consult), satisfaction with decision (post DA and consult), actual choice (post DA and consult), participation in decision making (post DA and consult), condition‐specific health outcomes. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 (procedures section): computer generated; randomly allocated via a remote location |
| Allocation concealment (selection bias) | Low risk | pg.5 (bias section): "we also used remote telephone randomisation", made use of central allocation |
| Incomplete outcome data (attrition bias) | Low risk | all of the aforementioned outcome measures are included in the outcome data |
| Selective reporting (reporting bias) | Low risk | pg. 2 (study setting section): study's protocol was published |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | no blinding, but review authors judge that the outcome is not likely to be influenced by lack of blinding pg. 5 (bias section):"it was not possible to conceal allocation once randomised; however to minimise contamination a number of methods were utilized.", "most women who received the pamphlet were unaware that it was not the intervention" |
| Blinding of outcome assessment (detection bias) | Low risk | pg.5 (bias section):"used forms based on standardised instruments that used highly objective closed ended questions, researchers were kept blinded to women's intervention as much as possible"; authors believe that lack of blinding did not affect the measurements |
| Methods | Randomised to detailed decision aid vs simple decision aid | |
| Participants | 25 + 26 women considering hormone replacement therapy in Canada | |
| Interventions | DA: computer version of same information with feedback to reinforce and correct participant knowledge COMPARE: audiotape booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) | |
| Outcomes | knowledge, accurate risk perceptions*, satisfaction with decision aid | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ randomisation was performed using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | pg. 2 "Allocation concealment was maintained through the use of consecutively numbered sealed envelopes" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 2 ‐ all randomised participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | High risk | pg. 69 ‐ on average, participants in the computer group were more likely to be still menstruating and therefore not taking HRT; see limitations section too |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to detailed decision aid vs simple decision aid | |
| Participants | 83 + 89 peri‐menopausal women considering hormone replacement therapy in the USA | |
| Interventions | DA: lecture with personal decision exercise on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance/coaching | |
| Outcomes | knowledge, decisional conflict, satisfaction with decision, satisfaction with provider, self‐efficacy, adherence, likelihood to take HRT, consistency with values | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 4 "370 were women randomly assigned", no information on method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | pg. 4 Reasons for attrition mentioned. Table 1 demonstrates participant balance in each study group. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Unclear risk | no specific comparisons reported for baseline characteristics |
| Blinding of participants and personnel (performance bias) | Unclear risk | unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | not blinded but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to pretest + decision aid + posttest vs decision aid + posttest vs pretest + posttest vs posttest | |
| Participants | 50 + 50 + 50 + 50 men considering prostate cancer screening in the USA | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probabilities, others' opinions + pretest and posttest COMPARE: booklet on options' outcomes, clinical problem, outcome probabilities, others' opinions + posttest COMPARE: pretest + posttest COMPARE: posttest | |
| Outcomes | knowledge (pre, post DA), decisional anxiety (post DA), decisional conflict (post DA), participation in decision making (pre, post DA), schema for PSA testing (pre, post DA), perception of quality and interpretation of recommendation (post DA) | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.309 (study design section): electronically generated random number sequence |
| Allocation concealment (selection bias) | Low risk | pg.309 (study design section): they were given sealed, sequentially numbered packets |
| Incomplete outcome data (attrition bias) | Low risk | no missing outcome data |
| Selective reporting (reporting bias) | Low risk | pg. 310 (study design section): protocol followed CONSORT checklist |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | no mention of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding, but the outcomes were objectively measured and not subject to interpretation. |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 87 + 87 community dwelling adults not previously screened for CRC in the USA | |
| Interventions | DA: Interactive Web site with information on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinion, guidance COMPARE: Non‐interactive Web site with information on clinical problem | |
| Outcomes | uptake of option* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 "A block randomisation process programmed by the study computer support staff and verified by a statistician was used including two strata, race and gender" |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ flow diagram |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | Both blinded |
| Blinding of outcome assessment (detection bias) | Low risk | The investigators, data collectors, data entry, and data analyst were all blinded to study arm assignment. |
| Methods | Randomised to detailed decision aid vs simple decision aid | |
| Participants | 122 + 135 men considering PSA testing in the USA | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probability | |
| Outcomes | uptake of option*, knowledge, accurate risk perceptions | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3‐4 Reasons for exclusion of participants and not participating mentioned. Participants in study groups balanced. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | acknowledges potential for volunteer bias in limitations section |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to detailed decision aid vs simple decision aid | |
| Participants | 89 + 88 post‐menopausal women considering hormone therapy use in the USA | |
| Interventions | DA: computer‐based decision aid on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinion COMPARE: educational pamphlet on options' outcomes, clinical problem | |
| Outcomes | knowledge*, satisfaction with the decision*, decisional conflict*, choice* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ Assignments were made by randomisation. The allocation sequence and assignments were made at a central site |
| Allocation concealment (selection bias) | Low risk | pg. 2 "Assignments were concealed by an envelope that was opened after informed consent was obtained" ""The allocation sequence and assignments were made at a central site" |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 3 fig 1 Flow chart. Reasons for not completing follow‐up not mentioned. p.4 baseline characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg. 2 ‐ Those administering the intervention and assessing outcomes were not blinded to the group assignment; Unclear if lack of blinding contributed to potential risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Those administering the assessing outcomes were not blinded to the group assignment but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to detailed vs simple decision aid vs control | |
| Participants | 223 + 212 + 231 average‐risk patients considering CRC screening in the USA | |
| Interventions | DETAILED DA: CRC risk assessment + web‐based interactive audio‐visual DA on options' outcomes, clinical problem, outcome probabilities, others' opinion and guidance COMPARE: web‐based decision aid only COMPARE: usual care using pamphlet | |
| Outcomes | knowledge (pre and post DA), satisfaction with decision making process (pre and post DA), preferred choice (pre and post DA) | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | no mention of allocation concealment |
| Incomplete outcome data (attrition bias) | Low risk | no data appears to be missing |
| Selective reporting (reporting bias) | Unclear risk | no mention of examination of selective outcome reporting or study protocol |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Providers were not blinded, subjective outcomes such as satisfaction with decision making process could have been affected, unclear is participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors not blinded but outcome measures not believed to be influenced by it |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 76 + 74 patients undergoing coronary angiography | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance COMPARE: usual care | |
| Outcomes | knowledge, decisional conflict*, risk perception, value congruent with chosen option | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p261 study design ‐ computerized random number generator |
| Allocation concealment (selection bias) | Low risk | p261 study design ‐ sealed envelopes |
| Incomplete outcome data (attrition bias) | Low risk | Did not seem to have incomplete data |
| Selective reporting (reporting bias) | Low risk | Protocol is available |
| Other bias | Low risk | Appeared to be free of other biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | p261 study design ‐ patients and physicians were not blinded to the allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear if DCS score assessed by unblinded individuals, but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 181 + 190 Ashkenazi Jewish women considering genetic testing in the USA | |
| Interventions | DA: 16‐page booklet on genetic testing with options' outcomes, clinical problem | |
| Outcomes | preferred option*, knowledge, accurate risk perceptions | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 ‐ computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | pg. 2 ‐ participants section ‐ high retention rate, baseline data and reasons for lost to follow up were provided |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid + genetic counselling vs genetic counselling alone | |
| Participants | 100 + 114 women considering prophylactic mastectomy for being BRCA1/2 mutation carriers in the USA | |
| Interventions | DA: CD‐Rom on options' outcomes, clinical problem, risk communication with individually tailored risk graphs, explicit values clarification, others' opinion; guidance/counselling ‐ genetic counselling as usual care (Ottawa Decision Support Framework COMPARE: Genetic counselling on benefits and risks of testing, clinical problem (risk assessment, cancer risks associated with mutations, process of testing and interpretation of results) plus written letter outlining all guidelines and recommendations | |
| Outcomes | decisional conflict*, satisfaction with decision*, remaining undecided, actual choice* (risk reduction mastectomy) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 ‐ Procedure ‐ randomised via computer‐generated random number in a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ fig. 1 ‐ flow diagram |
| Selective reporting (reporting bias) | Unclear risk | protocol not mentioned |
| Other bias | Low risk | appears to be free of other sources of bias. p.8 "when variable for not watching DA cd was considered in multivariate models, the results did not change substantively (data not shown) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care (list of risk factors) | |
| Participants | 49 + 38 adults with no history of cardiovascular disease in the USA | |
| Interventions | DA: computerized decision aid on options' outcomes, outcome probabilities | |
| Outcomes | patient‐practitioner communication (e.g. discussion with doctor, specific plan to reduce risk discussed with doctor) | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "computerized random number generator" |
| Allocation concealment (selection bias) | Low risk | pg. 2 "sealed in security envelopes" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 5 ‐ results; pg. 10 ‐ flow diagram; Baseline characteristics/data included |
| Selective reporting (reporting bias) | Low risk | pg. 1 ‐ ClinicalTrials.gov NCT00315978 |
| Other bias | Low risk | appears to have no other potential risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Patients were blinded but the doctors who saw both group were not |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcome was patient reported |
| Methods | Randomised to decision aid + tailored messages vs usual care | |
| Participants | 81 + 79 patients with moderate or high risk for CHD considering CHD prevention strategies in the USA | |
| Interventions | DA: web‐based decision aid on options' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance COMPARE: usual care using computer program | |
| Outcomes | preferred choice (post DA), adherence. | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg.2 "Patients were randomised by study staff who accessed an online randomised schedule." Seqneuce generation method was not stated |
| Allocation concealment (selection bias) | Low risk | pg.2 "Patients were randomised by study staff who accessed an online randomised schedule." |
| Incomplete outcome data (attrition bias) | Low risk | there appears to be no missing data |
| Selective reporting (reporting bias) | Low risk | protocol made available |
| Other bias | Low risk | appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Low risk | patients blinded and physicians unblinded but objective outcomes are not likely affected by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | outcomes deemed objective therefore lack of blinding did not influence assessment |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 85 + 84 pregnant women who have experienced previous cesarean section considering birthing options in Australia | |
| Interventions | DA: decision aid booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (Ottawa Decision Support Framework) | |
| Outcomes | preferred option, help with making a decision, knowledge*, decisional conflict* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 ‐ procedure ‐ computer‐based randomised generation |
| Allocation concealment (selection bias) | Low risk | pg. 3 "opaque envelopes containing a random allocation for each participant code number" |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 4 ‐ results ‐ 16 women were lost to follow‐up from the intervention group and 18 from the control group (no reasons listed) |
| Selective reporting (reporting bias) | Low risk | reference to published protocol |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants/midwives/doctors were blinded to patients' allocation However, women who used the decision‐aid as specified and in a process of consultation with their midwife or doctor would have negated the blinding of their clinicians, and perhaps of the women themselves. For the intervention group, this may have affected the level and type of information exchanged between them and their caregivers. |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to detailed vs simple decision aid vs usual care | |
| Participants | 196 + 188 + 188 socio‐economically disadvantaged participants diagnosed with average or slightly above average risk of bowel cancer considering bowel cancer screening in Australia | |
| Interventions | DA: booklet + DVD + worksheet + question prompt list on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (step‐by‐step process for making the decision; worksheet; encourages patients to communicate with practitioners by asking questions; summary) COMPARE: booklet + DVD + worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (step‐by‐step process for making the decision; worksheet; encourages patients to communicate with practitioners by asking questions; summary) COMPARE: usual care using standard information booklet | |
| Outcomes | values congruent with chosen option* (post DA), knowledge (pre, post DA), attitude, actual choice (post DA), participation in decision making* (pre, post DA), decisional conflict (post DA), decision satisfaction (post DA), confidence in decision making (post DA), general anxiety (post DA), worry about developing bowel cancer (pre, post DA), risk perception. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.3 (Participants and recruitment section): "Participants who verbally consented to take part were then randomised to one of the three groups using random permutated blocks of size 6 and 9 for each sex stratum" |
| Allocation concealment (selection bias) | Low risk | pg.3 (participants and recruitment section): central allocation; "interviewers responsible for recruiting participants were not aware of the randomization sequence or allocation and therefore did not know which intervention respondents would receive" |
| Incomplete outcome data (attrition bias) | Low risk | explanation for the missing data reported at base of tables |
| Selective reporting (reporting bias) | Low risk | study protocol available (ClinicalTrials.gov NCT00765869 and Australian New Zealand Clinical Trials Registry 12608000011381) |
| Other bias | Low risk | appears to be free of other potential sources of bias |
| Blinding of participants and personnel (performance bias) | Low risk | pg.3 (outcome measures section): "It was not possible for the reviewers to be blinded to the group allocation. However, all questions used standardised wording with pre‐coded responses and were asked within a supervised environment, where interviewer performances were regularly monitored to ensure scripts were read as written" |
| Blinding of outcome assessment (detection bias) | Low risk | pg.5 (statistical analysis section): "analyses were by intention to treat and carried out blinded to intervention", outcomes measured were not subject to interpretation |
| Methods | Randomised to detailed vs simple decision aid | |
| Participants | 136 + 164 women diagnosed with uterine fibroids considering treatment options in the USA | |
| Interventions | DA: DVD + booklet + worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (worksheet with questions relevant to decision making process; one or more questions that asked patients to clarify their preferences; summary), coaching (nurse coach access) COMPARE: Simple DA pamphlet on options' outcomes, clinical problem, outcome probabilities, others' opinion. | |
| Outcomes | knowledge (post DA and consult); values congruent with chosen option (post DA and consult); satisfaction with decision (post DA and consult); actual choice (post DA and consult), patient‐clinician communication. Survey of physicians, midwives, nurse practitioners, nurses, and medical assistants to obtain their impressions of the approaches used in their clinic (post DA and consult) | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p.445 (participants and data collection section) random allocation stratified by patient population size and type. First the 2 large central city sites were sorted into opposite study arms, followed by random allocation of the other sites to end up with similar patient sizes and characteristics. Patient allocation to intervention (n = 3 clinics) or control arm (n = 5 clinics) depended on the specific clinic where they received care. |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Low risk | all of the outcomes mentioned in p.446 (measures section) are either found in Tables 2, 3 and 4 or in the text in the results/ staff survey sections |
| Selective reporting (reporting bias) | Unclear risk | no mention of a protocol or a list of pre‐specified outcomes |
| Other bias | Low risk | appears to be free of other potential sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | unclear blinding, but outcomes were objectively measured and not subject to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 785 + 792 patients with no CRC history considering CRC screening in Germany | |
| Interventions | DA: brochure on options' outcomes, clinical problem, and outcome probabilities COMPARE: usual care using pamphlet | |
| Outcomes | values congruent with chosen option* (post DA), knowledge (post DA), combination of actual and planned uptake (post DA), risk perception. | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg.2 (randomisation and blinding): computer generated sequence |
| Allocation concealment (selection bias) | Low risk | pg.2 (randomisation and blinding): Allocation was concealed. Identity numbers were independent of allocation, and study members did not have access to the data. |
| Incomplete outcome data (attrition bias) | Low risk | pg.2 : 12% missing one or both questionnaires in intervention group vs 9.2% in control; judged to have low impact on study outcome |
| Selective reporting (reporting bias) | Low risk | protocol available |
| Other bias | Unclear risk | participants who completed the trial do not add up |
| Blinding of participants and personnel (performance bias) | Low risk | pg.2 (randomisation and blinding): trial staff who sent out questionnaires and reminders were not aware of study arm, unclear if participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | pg.2 (randomisation and blinding): trial staff and statistician who entered data were blinded |
| Methods | Randomised to detailed decision aid vs simple decision aid | |
| Participants | 30 + 30 women considering breast cancer surgery in the USA | |
| Interventions | DA: interactive multimedia on options' outcomes, clinical problem, others' opinion, guidance | |
| Outcomes | uptake of option, *knowledge, *optimism | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 3 "Patients were randomly assigned to one of two pre consultation education conditions"; sequence generation method was not stated |
| Allocation concealment (selection bias) | Unclear risk | Does not mention how allocation occurred and the concealment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Baseline data included. Flow diagram not included. Unsure how/why n = 60. pg. 3 "Only four patients chose not to participate" without reason why. |
| Selective reporting (reporting bias) | Unclear risk | no mention of protocol |
| Other bias | Low risk | pg. 3 ‐ Table 1 & text ‐ no sig. differences between groups; appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Methods | Randomised to decision aid vs usual care by clinical guidelines | |
| Participants | 69 + 67 patients with atrial fibrillation considering treatment options in the UK | |
| Interventions | DA: computerized decision on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance/coaching by physician COMPARE: guidelines applied as direct advice | |
| Outcomes | decisional conflict*, anxiety, knowledge, resource use, choice, health outcomes (stroke, TIA, bleeding events) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ Recruitment and randomisation ‐ "electronically‐generated random permuted blocks via a web‐based randomisation service" |
| Allocation concealment (selection bias) | Low risk | pg. 2 ‐ Recruitment and randomisation ‐ "electronically‐generated random permuted blocks via a web‐based randomisation service" |
| Incomplete outcome data (attrition bias) | Low risk | See flow diagram |
| Selective reporting (reporting bias) | Low risk | ISRCTN24808514 |
| Other bias | Low risk | Baseline characteristics similar, sample size similar, not stopped early |
| Blinding of participants and personnel (performance bias) | Unclear risk | Physicians were blinded. Unclear if patients are blinded and how that may affect the outcome |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to detailed decision aid vs simple decision aid | |
| Participants | 68 + 63 women at increased risk of developing ovarian cancer considering risk management options in Australia | |
| Interventions | DA: decision aid booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance/coaching (Ottawa Decision Support Framework) COMPARE: general education pamphlet with information contained in decision aid but "does not include the strategies commonly used in decision aids, such as a values clarification exercise" | |
| Outcomes | knowledge, decisional conflict, anxiety, depression, choice, acceptability of the intervention, Intrusive thoughts sub‐scale of Impact of Event scale, others influencing the decision | |
| Notes | primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 ‐ Participants ‐ Eligibility criteria and recruitment are outlined in detail elsewhere. Table of random numbers per author. |
| Allocation concealment (selection bias) | Low risk | pg. 3 ‐ Participants ‐ Eligibility criteria and recruitment procedures are outlined in detail elsewhere. Randomisation done centrally per author. |
| Incomplete outcome data (attrition bias) | Unclear risk | Baseline data included. p. 5 flow chart; Reasons for attrition not mentioned. Participants balanced in each study group. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | pg. 3 "Participants blinded to study, told the purpose of the study was to compare two types of education materials, not told how types differed or which one received"; Unclear if lack of blinding of others may have contributed to potential risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care by consumer guidelines | |
| Participants | 157 + 157 patients not previously screened for colorectal cancer in Australia | |
| Interventions | DA: age‐gender‐family history specific DA booklet with information on options, outcome probabilities, explicit values clarification, guidance (personal worksheet with steps in decision making) (Theory of planned behaviour) COMPARE: consumer guidelines recommending faecal occult blood testing | |
| Outcomes | Informed choice*: knowledge, values, screening intention (choice); test uptake, anxiety, acceptability of the intervention, satisfaction with the decision | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 "Sequential ID numbers were randomly assigned by computer program to DA or Guidelines (G) in blocks of four" |
| Allocation concealment (selection bias) | Low risk | pg. 3 "Allocation was concealed via the password‐protected program" |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 3 Baseline characteristics included. pg. 5 fig 2 flow chart. Reasons for loss to follow up not mentioned. |
| Selective reporting (reporting bias) | Low risk | ClinicalTrials.gov ‐ NCT00148226. |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | participants were blinded to the intervention type ‐ not sure about GPs |
| Blinding of outcome assessment (detection bias) | Low risk | Researchers were blinded to allocation for all telephone interviews, outcomes were objectively measured |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 152 + 156 infertile women on wait list for in vitro fertilisation in the Netherlands | |
| Interventions | DA: self‐administered booklet on options' outcomes,clinical problem, outcome probabilities, explicit values clarification, guidance(step by step process for making decision, worksheet with questions relevant to decision making process; one or more questions that asked patients to clarify their preferences; summary to be shared with practitioner), coaching (by trained in vitro fertilization nurse) + standard in vitro fertilisation care COMPARE: standard in vitro fertilisation care, including a session in which the number of embryos transferred was discussed | |
| Outcomes | knowledge (pre, post DA and consult), actual choice* (post DA and consult), empowerment (pre, post DA and consult), participation in decision making, decisional conflict (post DA and consult), levels of anxiety (pre, post DA and consult), depression (pre, post DA and consult), cost evaluation of empowerment strategy (post DA and consult), condition‐specific health outcomes (pregnancies) (post DA and consult) | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 (methods section): computer generated list |
| Allocation concealment (selection bias) | Low risk | pg. 2 (methods section): central allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | pg.3 (Table 1), there are categories in each column where the denominators do not match the number of people in the group and no reason was given to explain why this would be or if this affects the study |
| Selective reporting (reporting bias) | Low risk | outcomes same as those registered with ClinicalTrials.gov |
| Other bias | Low risk | The study appear to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Low risk | pg.2 (methods section): "because of the nature of the intervention it was not possible to blind the participants or in vitro fertilisation doctors to the allocation. Participation in our trial did not change the normal in vitro routine." |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes assessed were not subjective to interpretation |
| Methods | Randomised to detailed decision aid with decision analysis vs simple decision aid | |
| Participants | 44 + 44 women diagnosed with BRCA 1/2 mutation considering prophylactic surgery in the Netherlands | |
| Interventions | DA: video and brochure patient decision with decision analysis on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance/coaching | |
| Outcomes | decision uncertainty*, perceived weighing pros/cons, perceived participation, anxiety, health outcomes | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | van Roosmalen, 2004, J Clin Onco (Primary Study): pg. 4 ‐ randomisation schedule, stratified by medical history of breast/ovarian cancer and by timing of the informative DA, was generated by computer in blocks of 10; van Roosmalen, 2004, Brit J Cancer: p.2 "computer generated in blocks of 10" "stratified by personal medical history of breast/ovarian cancer" |
| Allocation concealment (selection bias) | Unclear risk | Roosmalen, 2004, Brit J Cancer: randomisation was performed after obtaining informed consent, no mention of allocation concealment |
| Incomplete outcome data (attrition bias) | Low risk | van Roosmalen, 2004, J Clin Onco (Primary Study):p.3 fig 1; van Roosmalen, 2004, Brit J Cancer: Fig 1 flow diagram |
| Selective reporting (reporting bias) | Unclear risk | van Roosmalen, 2004, J Clin Onco (Primary Study): no information provided; van Roosmalen, 2004, Brit J Cancer: no information provided |
| Other bias | Unclear risk | van Roosmalen, 2004, J Clin Onco (Primary Study):appears to be free of other potential biases; van Roosmalen, 2004, Brit J Cancer:Between the DA and control groups, significant differences were found for being religiously affiliated, anxiety, and general health; apart from that appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding , unclear if this contributes to bias |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 70 + 79 patients with cystic fibrosis considering referral for lung transplantation in Canada | |
| Interventions | DA: booklet with clinical problem, outcome probability, explicit values clarification, guidance (Ottawa Decision Support Framework) COMPARE: blank pages | |
| Outcomes | knowledge*, accurate risk perceptions*, decisional conflict*, preparation for decision making, choice, durability of decision, undecided | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "computer‐generated random listing of two treatment allocations blocked in blocks of 2 or 4, stratified by site and infection status of Burkholderia cepacia" |
| Allocation concealment (selection bias) | Low risk | pg. 2 ‐ central allocation |
| Incomplete outcome data (attrition bias) | Low risk | pg. 2 ‐ flow diagram; Baseline characteristics included. |
| Selective reporting (reporting bias) | Low risk | Clinical trial registered with www.clinicaltrials.gov (NCT00345449). |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Single blinded RCT; patients and researchers were blinded but physicians were note because they were involved with patients before being randomized. |
| Blinding of outcome assessment (detection bias) | Low risk | research staff, who were blinded to treatment allocation, telephoned each patient and had them complete a follow‐up questionnaire, other outcomes reported are objectively measured |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 74 + 78 women with breast cancer considering treatment options in Germany | |
| Interventions | DA: Decision board and booklet on options' outcomes, clinical problem, outcome probability | |
| Outcomes | decisional conflict*, choice, length of consultation, satisfaction with decision making, participation in decision making | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 2 "Randomisation after the patient gave written informed consent" "Random assignment was performed by means of numbered cards in envelopes" "stratified by age group"; |
| Allocation concealment (selection bias) | Low risk | pg. 2 "numbered cards in envelopes" |
| Incomplete outcome data (attrition bias) | Unclear risk | pg. 5 ‐ flow diagram; Baseline characteristics not included |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded ‐ unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) | Low risk | Not blinded but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 80 + 80 men considering PSA testing in the USA | |
| Interventions | DA: Health Dialog videotape and brochure on options' outcomes, clinical problem, outcome probability, others' opinion | |
| Outcomes | knowledge*, preferred/uptake of option* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Volk, 1999, Arch Fam Med (Primary Study): p.3 "Randomization by permuted blocks" "Each block included the numbers 1 through 4"; Volk, 2003, Ann Fam Med: pg. 2 ‐ Methods ‐ Randomization by permuted blocks was used to balance the number of subjects in each arm of the study. |
| Allocation concealment (selection bias) | Unclear risk | Volk, 1999, Arch Fam Med (Primary Study): no information provided; Volk, 2003, Ann Fam Med: p.2 "Details of the study procedures, subjects, and 2‐week follow‐up results can be found elsewhere" |
| Incomplete outcome data (attrition bias) | Low risk | Volk, 1999, Arch Fam Med (Primary Study): pg. 2 ‐ procedures; Baseline values included. p.4 fig 1 flow chart; Volk, 2003, Ann Fam Med:pg. 4 fig 1 ‐ flow diagram; Baseline data not included |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | Volk, 1999, Arch Fam Med (Primary Study): appears to be free of other potential biases; Volk, 2003, Ann Fam Med: appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Low risk | subjects was not blinded to the treatment assignment, but the physicians were, therefore outcomes were unlikely to be biased. |
| Blinding of outcome assessment (detection bias) | Low risk | Interviewers were not blinded but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to detailed decision aid vs simple decision aid | |
| Participants | 224 + 226 men with no history of prostate cancer in the USA | |
| Interventions | DA: edutainment decision aid with tailored computerized program with information options' outcomes, clinical problem, explicit values clarification, others' opinion, guidance COMPARE: audio‐booklet information on options' outcomes | |
| Outcomes | knowledge*, decisional conflict, self‐advocacy, acceptability of the intervention, length of the intervention | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "randomized separately in each setting using permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | Baseline data not included. pg. 4 reasons for not completing evaluation and recognizes difference in participant characteristics across sites. |
| Selective reporting (reporting bias) | Unclear risk | no mention of protocol |
| Other bias | Unclear risk | Participant imbalance across study sites. Low Literacy = 89 completing follow‐up and High Literacy = 263 completing follow‐up. |
| Blinding of participants and personnel (performance bias) | Unclear risk | does not mention blinding apart from pg. 2 ‐ 2.3 ‐ "Research assistants were not blinded to the study" (but unlikely to introduce bias patient outcomes). Therefore, unclear blinding of participants and physicians |
| Blinding of outcome assessment (detection bias) | Low risk | Research assistants were not blinded to the study but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 184 + 179 women considering treatment for menorrhagia in Finland | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probability | |
| Outcomes | uptake of option*, knowledge, proportion remaining undecided, anxiety, satisfaction, health outcomes, use and cost of healthcare services | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Vuorma, 2003 (Primary Study): pg. 2 ‐ Randomization ‐ computer‐generated; done by a researcher who did not participate in the planning or concealment procedures pg. 2 "done in STAKES, by researcher separately for each hospital in computer‐generated varying clusters"; Vuorma, 2004 no information provided |
| Allocation concealment (selection bias) | Low risk | Vuorma, 2003 (Primary Study):pg. 2 "sequentially numbered, opaque and sealed envelopes"; Vuorma, 2004: pg.2 "sequentially numbered, opaque, sealed envelopes" |
| Incomplete outcome data (attrition bias) | Low risk | Vuorma, 2003 (Primary Study): Flow chart balanced. p. 4‐5 reasons for non‐eligibility. "One women on HRT was randomized by mistake and included in analyses." Baseline characteristics included and balanced across groups; Vuorma, 2004: pg. 3 ‐ flow diagram |
| Selective reporting (reporting bias) | Unclear risk | Vuorma, 2003 (Primary Study): no mention of study protocol; Vuorma, 2004: no information provided |
| Other bias | Low risk | Vuorma, 2003 (Primary Study): pg. 7 "increase in knowledge in both study groups, carry‐over effect; change in decision‐making process of intervention group may have altered physician's negotiation with patients" appears to be free of other potential biases; Vuorma, 2004: p.5 "comparison of the baseline characteristics presented elsewhere" In the pre‐trial group compared with the control group, there was a greater increase in the dimensions of physical role functioning and emotional role functioning of the RAND‐36 |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding, unclear if measurements could be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Study staff were not blinded but outcomes were objectively measured and not subjective to interpretation |
| Methods | Cluster randomised to detailed decision aid vs simple decision aid | |
| Participants | 69 + 84 individuals considering genetic testing for colorectal cancer (cluster RCT with family‐wise randomisation) in Australia | |
| Interventions | DA: detailed 40‐page decision aid booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) COMPARE: simple 4‐page pamphlet with benefits and risks, no values clarification | |
| Outcomes | knowledge, decisional conflict*, informed choice, anxiety, depression, regret, actual choice, family involvement, Impact of event scale | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 3 "agreeing to participate, given a pre‐randomised envelope containing a DA or control pamphlet, a consent form, first questionnaire, and a reply‐paid envelope" ‐unclear how pre‐randomisation was generated |
| Allocation concealment (selection bias) | Low risk | Opaque envelope |
| Incomplete outcome data (attrition bias) | Low risk | Demographic variables similar in both groups. pg. 9 "not possible to collect baseline data"; pg. 5 fig. 2 ‐ flow diagram |
| Selective reporting (reporting bias) | Unclear risk | Protocol not mentioned |
| Other bias | Low risk | No baseline data (pg. 9, however characteristics equally distributed because randomisation) pg. 9 "potential for contamination because lack of blinding clinicians" Participants completed questionnaire before reading info. materials |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded ‐ unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Cluster randomised to detailed decision aid vs simple decision aid | |
| Participants | 73 + 72 women considering genetic testing for breast and ovarian cancer (cluster RCT with family‐wise randomisation) in Australia | |
| Interventions | DA: detailed 40‐page decision aid booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) COMPARE: simple 4‐page pamphlet with benefits and risks, no values clarification | |
| Outcomes | knowledge, decisional conflict*, informed choice, anxiety, depression, genetic testing decision, decision regret, family involvement, impact of event | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 3 "pre‐randomized envelope containing the DA or the control pamphlet, a consent form, the first questionnaire and a reply‐paid envelope" pg. 4 "Participants were randomized according to family‐wise randomization" |
| Allocation concealment (selection bias) | Low risk | pg. 4 ‐ all patients who were the first of their family to attend the clinic were randomly allocated to the control or DA condition. pg. 3 ‐ Those who agreed were given a pre‐randomised opaque envelope containing the DA or the control pamphlet |
| Incomplete outcome data (attrition bias) | Low risk | pg. 11 "It was not possible to collect a baseline assessments" pg. 6 fig 2 Flow chart. Reasons for withdrawal not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | Appears to be free of other sources of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded ‐ unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Cluster randomised to detailed decision aid vs simple decision aid | |
| Participants | 73 + 75 women considering genetic testing for breast and ovarian cancer (cluster RCT with family‐wise randomisation) in Australia | |
| Interventions | DA: detailed 40‐page decision aid booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) COMPARE: simple 4‐page pamphlet with benefits and risks, no values clarification | |
| Outcomes | knowledge, decisional conflict*, informed choice, anxiety, depression, genetic testing decision, decision regret, impact of event, family involvement | |
| Notes | The study procedure was identical for the 2008a trial except that the decision aid was given to women at the beginning of their first consultation with a genetic counsellor, used during counselling, and then taken home by the women *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details provided in Wakefield 2008a: pg. 3 "pre‐randomized envelope containing the DA or the control pamphlet, a consent form, the first questionnaire and a reply‐paid envelope" pg. 4 "Participants were randomized according to family‐wise randomization" |
| Allocation concealment (selection bias) | Low risk | Details provided in Wakefield 2008a pg. 4 ‐ all patients who were the first of their family to attend the clinic were randomly allocated to the control or DA condition. pg. 3 ‐ Those who agreed were given a pre‐randomised opaque envelope containing the DA or the control pamphlet |
| Incomplete outcome data (attrition bias) | Low risk | pg. 5 ‐ fig 2 ‐ flow diagram |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | no baseline characteristics, but author states that due to randomisation should be evenly distributed across both groups |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded ‐ unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 475 + 522 men considering prostate cancer screening in the UK | |
| Interventions | DA: leaflet on options' outcomes, clinical problem, outcome probability COMPARE: usual care | |
| Outcomes | knowledge*, screening intention*, attitudes*, preferred role in decision making | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 3 "random numbers generated centrally by Stata v8.2" |
| Allocation concealment (selection bias) | Low risk | pg. 3 "random numbers generated centrally by Stata v8.2" |
| Incomplete outcome data (attrition bias) | Low risk | pg. 2 ‐ flow diagram; Reason for exclusion from analysis mentioned. Sample characteristics of risk included. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Unclear risk | pg. 3 "Adjustment for multiple testing was not accounted for and hence a degree of caution with interpretation is required, particularly in relation to findings with a P‐value close to 0.05" |
| Blinding of participants and personnel (performance bias) | Unclear risk | no information provided |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Cluster randomised to decision aid vs usual care | |
| Participants | 51 + 46 patients with type 2 diabetes in the USA | |
| Interventions | DA: 1‐page decision aid options' outcomes, clinical problem, tailored outcome probability, guidance/coaching | |
| Outcomes | knowledge*, decisional conflict*, consultation length, acceptability of the intervention, adherence, estimated personal risk, trust, patient participation (OPTION), choice | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 ‐ computer‐generated allocation sequence; Nannenga ‐ no information provided |
| Allocation concealment (selection bias) | Low risk | computer‐generated allocation sequence, unavailable to personnel enrolling patients. Abstract: "with concealed allocation" pg. 5 "maintained allocation concealment" Nannenga ‐ pg. 2 ‐ randomised by concealed central allocation |
| Incomplete outcome data (attrition bias) | Low risk | pg. 3 ‐ flow diagram; Reasons for attrition mentioned. pg.4 Baseline characteristics included; Nannenga ‐ pg. 3 ‐ flow diagram; Reasons for attrition mentioned and study groups balanced. Baseline characteristics included. |
| Selective reporting (reporting bias) | Low risk | clinical trials.gov Identifier: NCT00217061 |
| Other bias | Low risk | Enrollment of patients already receiving statin therapy and limited statin uptake decreased the precision of our results; results should best be interpreted as preliminary and requiring verification; Nannenga ‐ appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and clinicians blinded to the study objectives, providers and patients were naive to this study objective |
| Blinding of outcome assessment (detection bias) | Low risk | Data analysts and statisticians blinded to allocation; intervention and outcomes; adequate blinding wherever possible |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 82 + 93 women with node negative breast cancer considering adjuvant chemotherapy in Canada | |
| Interventions | DA: Decision board and booklet on options' outcomes, clinical problem, outcome probability, guidance/coaching | |
| Outcomes | preferred option, knowledge*, anxiety, accurate risk perceptions, satisfaction of patient*, participation in decision making | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information provided |
| Allocation concealment (selection bias) | Low risk | pg. 3 ‐ randomisation, which was performed at a central location |
| Incomplete outcome data (attrition bias) | Unclear risk | Flow diagram not included. pg. 4 "one patient excluded from analysis, determined by physician not to be candidate for chemotherapy" Baseline data/characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if lack of blinding contributed to potential risk of bias |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | unable to blind participants in our trial for practical reasons, measures were taken to minimize bias in the design of the study and the assessment of outcomes. |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Cluster randomised to decision aid vs usual care | |
| Participants | 94 + 107 women with Stage 1 or 2 breast cancer considering surgery (Cluster RCT with 27 surgeons randomised) in Canada | |
| Interventions | DA: decision board on options' outcomes, outcome probability, guidance/coaching | |
| Outcomes | preferred option*, knowledge*, accurate risk perceptions, decisional conflict*, anxiety, satisfaction* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | does not specify how the sequence was generated ‐ pg. 2 ‐ study design and procedures ‐ a paired cluster randomisation process was used. |
| Allocation concealment (selection bias) | Unclear risk | pg. 2 ‐ study design and procedures ‐ they were then randomly assigned in a concealed fashion, but method of concealment was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Baseline characteristics not included. Reasons given for loss of participants. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | 'appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | p. 6 "chose cluster randomization method to avoid contamination that might have occurred if surgeons used decision board for some patients and not others", unclear if this would introduce bias |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 103 + 102 men considering PSA testing in the USA | |
| Interventions | DA: script of options' outcomes, clinical problem, outcome probability, others' opinions | |
| Outcomes | preferred option* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Wolf, 1996, Arch Intern Med (Primary Study): no information provided Wolf, 1998, J Ger Ser A‐Bio Sc & Med Sc: p.2 "The methodology of the randomized trial has been reported previously" |
| Allocation concealment (selection bias) | Unclear risk | Wolf, 1996, Arch Intern Med (Primary Study): no information provided; Wolf, 1998, J Ger Ser A‐Bio Sc & Med Sc: p.2 "The methodology of the randomized trial has been reported previously" |
| Incomplete outcome data (attrition bias) | Low risk | Wolf, 1996, Arch Intern Med (Primary Study): pg. 2 needed a minimum sample size of 150 participants, and was achieved with total sample size of 205. Reasons for attrition mentioned. Baseline characteristics included; Wolf, 1998, J Ger Ser A‐Bio Sc & Med Sc: 'no information provided pg. 2 ‐ methodology of the randomised trial and the content of the informational intervention have been reported previously p.2 Baseline characteristics included. Flow of participants not included. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | Wolf, 1996, Arch Intern Med (Primary Study): pt population was lower SES therefore external validity of the findings limited, but overall appears to be free of other potential biases; Wolf, 1998, J Ger Ser A‐Bio Sc & Med Sc: appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs usual care | |
| Participants | 266 + 133 elderly (65+) considering CRC screening in the USA | |
| Interventions | DA: script of options' outcomes, clinical problem, outcome probabilities | |
| Outcomes | preferred option*, accurate risk perceptions | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | pg. 2 "patients were randomised" Does not indicate how. |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Baseline data not included; pg. 2 ‐ Results |
| Selective reporting (reporting bias) | Unclear risk | Protocol not mentioned |
| Other bias | Low risk | Appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Methods | Randomised to decision aid vs placebo control leaflet | |
| Participants | 162 + 164 women referred for pregnancy termination in the UK | |
| Interventions | DA: decision aid leaflet on options' outcomes, clinical problem, outcome probability, explicit values clarification | |
| Outcomes | uptake of option*, knowledge*, decisional conflict*, anxiety* | |
| Notes | *primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pg. 2 "1:1 ratio, balanced block of 10" "envelope preparation by drawing slips of paper labelled either control or intervention" "the slip determined leaflet placed into envelope" |
| Allocation concealment (selection bias) | Low risk | pg. 2 ‐ Methods ‐ consecutive numbered, opaque trial envelope |
| Incomplete outcome data (attrition bias) | Unclear risk | Baseline characteristics not included. pg. 3 Reasons for attrition and incompletion mentioned. |
| Selective reporting (reporting bias) | Unclear risk | no information provided |
| Other bias | Low risk | appears to be free of other potential biases |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
CHD: coronary heart disease; CRC: colorectal cancer; DA: decision aid; HPV: human papilloma virus; OA: osteoarthritis; PSA: prostate‐specific antigen; RCT: randomized controlled trial; UK: United Kingdom; USA: United States of American;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| study did not evaluate the decision aid (evaluated clinician use of the decision aid in one arm of a study only) | |
| hypothetical choice, not at a point of decision making | |
| study not focused on making a choice; adhering to medications only | |
| not a patient decision aid | |
| not a randomised controlled trial | |
| not a patient decision aid (not including benefits and harms) | |
| Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid. Additional information requested from author but not provided. | |
| not a randomised controlled trial | |
| hypothetical choice, not at the point of decision making | |
| not a patient decision aid | |
| study did not evaluate the patient decision aid (evaluated SDM process) | |
| not a randomised controlled trial | |
| Not focused on making a choice (no specific decision to be made) | |
| Not a randomised controlled trial | |
| not a patient decision aid (general patient education only) | |
| Hypothetical choice, not at point of decision making | |
| Not a randomised controlled trial | |
| not a patient decision aid | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Unable to ascertain characteristics of the patient decision aid. Additional information requested from author but not provided (e.g. values clarification). | |
| Not a patient decision aid (Describes model of care) | |
| not a randomised controlled trial | |
| Not a randomised controlled trial (editorial) | |
| Not a randomised controlled trial | |
| Unable to ascertain whether intervention meets criteria (values clarification) to qualify as a patient decision aid. | |
| Not a patient decision aid | |
| not a patient decision aid | |
| Not a patient decision aid | |
| not a patient decision aid | |
| Not focused on making a choice | |
| not a patient decision aid | |
| Quasi‐RCT: randomization was by days of the week | |
| not a decision aid (no decision support) | |
| Hypothetical choice, not at point of decision making | |
| end of life decision | |
| Not a randomised controlled trial (Quasiexperimental design); unclear whether at point of decision making | |
| No difference in intervention between arms. Risk communication did not have value clarification. | |
| not a patient decision aid | |
| Not a randomised controlled trial | |
| hypothetical choice, not at point of decision making | |
| Non‐randomized allocation; waiting list control | |
| not a patient decision aid | |
| Not a randomised controlled trial | |
| Same decision aid delivered on the Internet versus on DVD plus booklet | |
| Not a randomised controlled trial | |
| qualitative study for an included RCT | |
| Not a patient decision aid (General information) | |
| hypothetical choice, not at the point of decision making | |
| Not a patient decision aid (Educational intervention) | |
| Not focused on making a choice (Intervention to increase patient involvement in care) | |
| Hypothetical choice, not at the point of decision making | |
| Not a randomised controlled trial | |
| Not a patient decision aid | |
| Not about evaluating a patient decision aid | |
| Not a patient decision aid | |
| not a randomised controlled trial | |
| Not a patient decision aid (education to promote compliance) | |
| Quasi‐RCT: assigned to 1 of 2 alternating groups | |
| Not a patient decision aid; no values clarification | |
| Not a randomised controlled trial (letter) | |
| Not a patient decision aid (General information; no values clarification) | |
| Not a randomised controlled trial | |
| Not a patient decision aid | |
| hypothetical choice, not at the point of decision making | |
| not focused on making a choice (Promotes complying with a recommended option) | |
| not a randomised controlled trial | |
| study does not evaluate a decision aid; evaluation of spiritual versus non‐spiritual framework | |
| Same content | |
| not focused on making a choice (Promotes complying with a recommended option) | |
| Not focused on making a choice (no specific decision) | |
| hypothetical choice, not at point of decision making for all participants | |
| No difference in content of interventions ‐ testing mode of delivery | |
| Not a randomised controlled trial (English paper published in 2008 Urologia Internationals) | |
| not a patient decision aid | |
| Not focused on making a choice ‐ adherence to screening | |
| no comparison outcome data provided (only presents data for intervention group) | |
| not a patient decision aid | |
| not a patient decision aid | |
| hypothetical choice, not at point of decision making ‐ community sample asked to evaluate information booklet on depression | |
| hypothetical choice, not at point of decision making | |
| hypothetical choice, not at point of decision making | |
| not a randomised controlled trial; not a patient decision aid | |
| not a patient decision aid | |
| not a patient decision aid | |
| Not a patient decision aid ‐ no benefits and harms | |
| not a randomised controlled trial; not a patient decision aid | |
| inadequate comparison outcome data provided, Secondary report of pilot study | |
| not a patient decision aid (to increase uptake of screening) | |
| not a patient decision aid | |
| not a patient decision aid (No values clarification) | |
| not a patient decision aid | |
| Hypothetical choice, not at the point of decision making | |
| lifestyle only | |
| not a randomised controlled trial (all patients received DA) | |
| not a patient decision aid (No values clarification) | |
| not about evaluating a patient decision aid | |
| not focused on making a choice (about adherence not decision making) | |
| not focused on making a choice (Promotes complying with a recommended option) | |
| Not a patient decision aid (Review of patient information pamphlets on pre‐operative fasting) | |
| hypothetical choice, not at the point of decision making | |
| Insufficient outcome data provided in publication. Requested from author but not provided. | |
| Not a patient decision aid ‐ genetic counselling only | |
| Hypothetical choice, not at the point of decision making | |
| not a patient decision aid | |
| Not a patient decision aid (No values clarification) | |
| not a patient decision aid | |
| Not a patient decision aid (No risk/benefit information; no values clarification) | |
| not a patient decision aid; hypothetical choice, not at point of decision making | |
| Unable to ascertain whether intervention meets criteria (values clarification) to qualify as a patient decision aid. Additional information requested but author was unable to provide the intervention. | |
| not a patient decision aid (information only) | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial (Editorial) | |
| Not a randomised controlled trial (Editorial) | |
| not a patient decision aid | |
| study protocol, does not appear to be patient decision aid | |
| Not a randomised controlled trial | |
| Suite of 8 decision aids (not an efficacy trial) | |
| No patient decision aid ‐ framing effects | |
| Not patient decision aid | |
| not a patient decision aid | |
| Not a patient decision aid (Focus on provision of information) | |
| Not a patient decision aid (Decision aid only supplies mortality risk information; no risk info; no values clarification) | |
| not a randomised controlled trial, not a patient decision aid (Promotes complying with a recommended option) | |
| Quasi‐RCT: alternating order based on patients' initial appointment sequence | |
| about clinical trial entry | |
| not a patient decision aid | |
| Not a patient decision aid (General patient education resource) | |
| about a lifestyle choice ‐ whether or not to have a child or have another child if I have multiple sclerosis | |
| Not a randomised controlled trial | |
| No difference in intervention content; Comparison of presentation formats; audio‐guided decision aid versus booklet only | |
| Not focused on making a choice (Promotes complying with a recommended option) | |
| Not focused on making a choice (Promotes complying with a recommended option) | |
| Not a randomised controlled trial | |
| not a patient decision aid | |
| Not a patient decision aid | |
| Not focused on making a choice (Promotes complying with a recommended option) | |
| Not a patient decision aid ‐ general information; not a specific decision | |
| Not a randomised controlled trial | |
| hypothetical choice, not at the point of decision making | |
| about do not resuscitate versus initiating cardiopulmonary resuscitation decision | |
| not a patient decision aid (Promotes complying with a recommended option) | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| hypothetical choice, not at point of decision making | |
| hypothetical choice, not at point of decision making | |
| Not a patient decision aid (no discussion of harms) | |
| not a patient decision aid | |
| no decision regarding treatment or screening to be made (decision regarding full disclosure) | |
| Not a randomised controlled trial | |
| not a patient decision aid | |
| Not a randomised controlled trial (about instrument development) | |
| not a patient decision aid (Only effectiveness not cons of options; not at point of decision making) | |
| not a randomised controlled trial | |
| not a patient decision aid | |
| Not focused on making a choice (Promotes complying with a recommended option) | |
| hypothetical choice, not at the point of decision making, not a randomised controlled trial | |
| Not a randomised controlled trial | |
| not a patient decision aid; about stopping medication use | |
| Not a randomised controlled trial; not at point of decision making | |
| Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid. Additional information requested from author but not provided. | |
| Not a randomised controlled trial; not focused on making a choice (complying with a recommended option) | |
| Not a randomised controlled trial | |
| hypothetical choice, not at the point of decision making | |
| not a patient decision aid | |
| advanced care planning options | |
| hypothetical choice, end‐of‐life decision | |
| not a randomised controlled trial (commentary) | |
| Not a randomised controlled trial | |
| Not a patient decision aid ‐ patient preference study | |
| Not a patient decision aid ‐ Intent of intervention to facilitate genetic counselling process, no focused decision. | |
| Same decision aid in both groups | |
| not a patient decision aid | |
| Not a randomised controlled trial | |
| Lifestyle change | |
| Not a randomised controlled trial | |
| not a patient decision aid | |
| Not focused on making a choice ‐ Promotes complying with a recommended option | |
| end‐of‐life decision | |
| Not focused on making a choice ‐ Promotes complying with a recommendation | |
| No difference in intervention content ‐ comparison of presentation of probabilities | |
| not a randomised controlled trial, not a patient decision aid |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | Men considering prostate cancer screening |
| Interventions | 2 decision aids on prostate cancer screening |
| Outcomes | |
| Notes | Weinrich SP, Seger RE, Rao GS, Chan EC, Hamm RM, Godley PA, Moul JW, Powell IJ, Chodak GW, Taylor KL, Weinrich MC. A decision aid for teaching limitations of prostate cancer screening. Journal Natl Black Nurses Association, 2008 Jul, 19(1): 1‐11 There is minimal research regarding men's knowledge of the limitations of prostate cancer screening. This study measured knowledge of prostate cancer screening based on exposure to one of two decision aids that were related to prostate cancer screening (enhanced versus usual care). The sample consisted primarily of low income (54%) African‐American men (81%) (n = 230). The enhanced decision aid was compared against the usual care decision aid that was developed by the American Cancer Society. The enhanced decision aid was associated with higher post‐test knowledge scores, but statistically‐significant differences were observed only in the men who reported having had a previous DRE (P = 0.013) in the multivariable analyses. All the men were screened, regardless of which decision aid they received. This study highlights the impact of previous screening on education of the limitations of prostate screening, and challenges the assumption that increased knowledge of the limitations of prostate cancer screening will lead to decreased screening. |
Unable to obtain a copy of this paper.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Evaluation of DVD and Internet decision aids for hip and knee osteoarthritis: focus on health literacy |
| Methods | RCT |
| Participants | Osteoarthritis patients |
| Interventions | DVD decision aid vs Internet based decision aid |
| Outcomes | Decisional conflict, decision self‐efficacy, knowledge |
| Starting date | January 2012 |
| Contact information | Kelli D Allen, Duke University |
| Notes | Trial #: NCT01618097 |
| Trial name or title | Shared decision making in patients with osteoarthritis of the hip and knee |
| Methods | RCT |
| Participants | Patients with hip or knee osteoarthritis |
| Interventions | Participating in a share decision program with help from trained research nurse versus usual care |
| Outcomes | Choice of treatment, satisfaction, knowledge, length of office visit |
| Starting date | Not yet assessed |
| Contact information | Kevin Bozic |
| Notes | Trial #: NCT01492257 |
| Trial name or title | Effect of a decision aid on decision making for the treatment of pelvic organ prolapse |
| Methods | RCT |
| Participants | Scheduled for consultation visit for pelvic organ prolapse of any type |
| Interventions | Decision aid prior to initial visit vs usual care |
| Outcomes | Decisional conflict, proportion patient that choose surgery or conservative management |
| Starting date | December 2012 |
| Contact information | Hema Brazell, Hartford Hospital |
| Notes | Trial #: NCT01798082 |
| Trial name or title | Development of and feasibility testing of decision support for patients who are candidates for an implantable defibrillator |
| Methods | RCT |
| Participants | Referred for consideration of an ICD(non‐CRT) for a primary prevention indication |
| Interventions | Patient decision aid provided prior to the consultation with the physician, which provides a lay summary that outlines the facts, risks, benefits (including probabilities), specific to the option of an implantable defibrillator or the option of medical management vs usual care |
| Outcomes | Decision aid development and evaluation, decisional conflict and decision quality, sure test, reparation for decision‐making scale, medical outcomes trust short form (SF‐36v2) |
| Starting date | June 2012 |
| Contact information | Sandra Carroll, McMaster University |
| Notes | Trial #: NCT01876173 |
| Trial name or title | ProsCan for Men: Randomized controlled trial of a decision support intervention for men with localised prostate cancer |
| Methods | RCT |
| Participants | 700 men newly diagnosed with localised prostate cancer |
| Interventions | A tele‐based nurse delivered five session decision support/psychosocial intervention vs usual care |
| Outcomes | Cancer threat appraisal; decision‐related distress and bother from treatment side effects; involvement in decision making; satisfaction with health care; heath care utilisation; use of health care resources; and a return to previous activities |
| Starting date | Not yet assessed |
| Contact information | Suzanne K Chambers |
| Notes | Trials # ACTRN012607000233426 |
| Trial name or title | Risk management in patients with diabetes mellitus: development and evaluation of a treatment oriented decision aid [DUTCH] |
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Starting date | Not yet assessed |
| Contact information | P Denig |
| Notes | Trials # NTR1942 |
| Trial name or title | Investigating a training supporting Shared Decision Making (IT'S SDM 2011): study protocol for a randomized controlled trial |
| Methods | RCT |
| Participants | 40 physicians that contribute a sequence of four medical consultations including a diagnostic or treatment decision. |
| Interventions | A training curriculum for the doctors ‐ intend to stimulate efforts to involve their patients in the decision‐making process. |
| Outcomes | Physician‐patient communication, effect of SDM on perceived quality of the decision process and on the elaboration of the decision, decisional conflict |
| Starting date | Not yet assessed |
| Contact information | Friedemann Geiger |
| Notes | Trials #ISRCTN78716079 |
| Trial name or title | Decision aid evaluation by a clinical trial in abdominal aortic aneurysms: Improving decision making |
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Starting date | November 2008 |
| Contact information | A. Goossens |
| Notes | Trials # NTR1524 |
| Trial name or title | Behavioral & social science research on understanding and reducing health disparities: African American preference for knee replacement: a patient‐centred intervention (ACTION) |
| Methods | RCT |
| Participants | African‐American patient referred to orthopedic doctor with presence of knee OA |
| Interventions | Decision aid video + communication, skill‐building intervention vs educational program (an NIH‐developed booklet) that summarizes how to live with knee OA but does not mention joint replacement |
| Outcomes | Recommendation and receipt of knee joint replacement |
| Starting date | July 2010 |
| Contact information | Said A Ibrahim |
| Notes | Trial #: NCT01851785 |
| Trial name or title | A single‐blind randomized controlled trial of the effects of a web‐based decision aid on self‐testing for cholesterol and diabetes. |
| Methods | RCT |
| Participants | Men and women with an intention to use a diabetes and/or cholesterol self‐test |
| Interventions | Patient decision aid versus control pamphlet |
| Outcomes | Knowledge; intention; attitude; ambivalence; psychosocial determinants; behaviour |
| Starting date | Not yet assessed |
| Contact information | Martine Ickenroth |
| Notes | Trial # NTR3149 (Dutch trial register) |
| Trial name or title | Decision aid to technologically enhance shared decision making |
| Methods | RCT |
| Participants | Patients who are not current with colorectal cancer screening |
| Interventions | Web based decision aid + interactive component (preferences and risk assessment) vs web based decision aid only |
| Outcomes | Uptake of screening on patient determinants/preference/intention before the patient‐physician encounter, and on shared decision making, concordance and patient intention during/after the patient‐physician encounter. |
| Starting date | May 2012 |
| Contact information | Mary Rapai |
| Notes | Trial# :NCT01514786 |
| Trial name or title | Improving communication about treatment options for asymptomatic ovarian cancer patients with rising CA125: RCT of patient decision aid |
| Methods | RCT |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Starting date | April 2009 |
| Contact information | Ilona Juraskova |
| Notes | Trials # ACTRN12609001035213 |
| Trial name or title | Development and pilot test of an elective bilateral salpingo‐oophorectomy (BSO) decision support guide |
| Methods | RCT |
| Participants | Patients plans to undergo an elective hysterectomy for symptomatic fibroids, abnormal bleeding, pelvic pain, or pelvic organ prolapse OR hysterectomy via any route |
| Interventions | Not yet assessed |
| Outcomes | Decisional conflict, regret, anxiety |
| Starting date | May 2011 |
| Contact information | Miriam Kuppermann |
| Notes | Trial #: NCT01369654 |
| Trial name or title | Breast cancer metastatic decision aid |
| Methods | Not yet assessed |
| Participants | Women with metastatic breast cancer considering treatment options |
| Interventions | Decision aid versus usual care |
| Outcomes | Treatment decision; satisfaction with decision; knowledge; anxiety; decisional conflict; physician satisfaction with decision‐making |
| Starting date | Sept. 2002 |
| Contact information | Natasha Leighl, Princess Margaret Hospital, 5‐222 610 University Avenue, Toronto, Ontario M5G 2M9, Canada; Telephone; 416‐946‐2399, Fax; 416‐946‐6546, email; [email protected] |
| Notes | Chiew KS, Shepherd H, Vardy J, Tattersall MH, Butow P, Leighl NB. Development and evaluation of a decision aid for patients considering first line chemotherapy for metastatic breast cancer. Health Expectations 2008 March 11(1): 35‐45. Indicates trial in process Trials #ACTRN12607000084482 |
| Trial name or title | Helping patients with spinal stenosis make a treatment decision: a randomized study assessing the benefits of health coaching |
| Methods | RCT |
| Participants | Spinal stenosis patients |
| Interventions | Decision aid + coaching vs decision aid only |
| Outcomes | Decisional conflict, decision self‐efficacy, number of treatment decision‐related clinical contacts, treatment follow‐through and decision regret |
| Starting date | November 2010 |
| Contact information | Jon D Lurie |
| Notes | Trial #: NCT01263678 |
| Trial name or title | Increasing efficacy of primary care‐based counselling for diabetes prevention: rationale and design of the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) trial |
| Methods | RCT |
| Participants | Primary care providers |
| Interventions | Using the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) system to enhance providers' effectiveness to counsel about lifestyle behaviour changes. |
| Outcomes | Outcome measurements are designed to detect changes in patient behaviours that are most likely to result from the use of ADAPT tool: difference between intervention and control patients in the change in mean steps per day at baseline and after six months, and six month difference of differences in haemoglobin A1C and self reported diet between the two groups. |
| Starting date | Not yet assessed |
| Contact information | Devin Mann |
| Notes | Trial #NCT01473654 |
| Trial name or title | Translating comparative effectiveness of depression medications into practice by comparing the depression medication choice decision aid to usual care: study protocol for a randomized controlled trial |
| Methods | RCT |
| Participants | Presumed diagnosis of depression (PHQ‐9 of 10 or greater) and those need to initiate drug treatment for depression as judged by clinician |
| Interventions | DEPRESSION CHOICE decision aid is provided to clinician to share with patient vs usual care |
| Outcomes | Impact of the decision aid on patient involvement in decision making, decision making quality, patient knowledge, and 6‐month measures of medication adherence and mental health compared to usual depression care |
| Starting date | December 2011 |
| Contact information | Victor Montori, Mayo Clinic |
| Notes | Trial #: NCT01502891 |
| Trial name or title | Use of a patient decision aid for gastrologic endoscopy in a paediatric setting |
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Starting date | December 2008 |
| Contact information | Nancy Neilan |
| Notes | Trials # NCT00813033 |
| Trial name or title | Assessing the information desire of patients with advanced cancer by providing information with a decision aid, which is evaluated in a randomized trial: a study protocol |
| Methods | RCT |
| Participants | Patients with advanced colorectal, breast, or ovarian cancer and have started treatment with first‐line palliative chemotherapy |
| Interventions | Patients are randomized to receive either usual care or usual care + decision aid |
| Outcomes | Not yet assessed |
| Starting date | Not yet assessed |
| Contact information | Linda JM Oostendorp |
| Notes | Netherlands Trial Register (NTR): NTR1113 |
| Trial name or title | Testing the helpfulness of 2 decision aids for prostate cancer |
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Starting date | February 2007 |
| Contact information | Julie Parow |
| Notes | Trials # NCT00432601 |
| Trial name or title | Study protocol: Improving patient choice in treating low back pain (IMPACT ‐ LBP): A randomized controlled trial of a decision support package for use in physical therapy |
| Methods | RCT |
| Participants | Physiotherapists |
| Interventions | Physiotherapists are randomized to receive either training for the Decision Support Package or not. Patients are randomly allocated to treatment for non specific low back pain to either a physiotherapist trained in decision support or to receive usual care. |
| Outcomes | Satisfaction with treatment, health‐related quality of life, health utility, anxiety, depression, attitude to movement in pack pain, attendance, satisfaction with decision |
| Starting date | Not yet assessed |
| Contact information | Shilpa Patel |
| Notes | Current Controlled TRials ISRCTN46035546 |
| Trial name or title | Decision aid on radioactive iodine treatment for early stage papillary thyroid cancer‐‐a randomized controlled trial |
| Methods | RCT |
| Participants | Patients with early stage papillary thyroid carcinoma |
| Interventions | Computerized decision aid (DA) relative to a control group receiving usual care |
| Outcomes | Knowledge about papillary thyroid carcinoma and radioactive iodine treatment, decisional conflict, decisional regret, client satisfaction with information received about RAI treatment, final decision to accept or reject adjuvant RAI treatment and rationale |
| Starting date | Not yet assessed |
| Contact information | Not yet assessed |
| Notes | Trials #NCT01083550 |
| Trial name or title | Impact of risk stratification on shared decision making for colorectal cancer screening |
| Methods | RCT |
| Participants | Due for CRC screening based on current recommendations |
| Interventions | Risk Assessment tool + web based decision aid vs web based decision aid only |
| Outcomes | Screening test ordered, test completion rate, concordance between patient and test preference, satisfaction with decision making progress and provider satisfaction |
| Starting date | April 2012 |
| Contact information | Paul C Schroy III, Boston medical center |
| Notes | Trial #: NCT01596582 |
| Trial name or title | Measuring quality of decisions about treatment of menopausal symptoms |
| Methods | RCT |
| Participants | Patients talked with health care provider about ways to manage menopause or seriously considered taking medicine or supplement to manage menopause |
| Interventions | Decision aid (DVD/booklet) vs usual care |
| Outcomes | Knowledge, value concordance |
| Starting date | June 2010 |
| Contact information | Karen R Sepucha |
| Notes | NCT01152294 |
| Trial name or title | Measuring quality of decisions about treatment of depression |
| Methods | RCT |
| Participants | Patients that talked to a health care provider about starting or stopping a treatment (prescription medicine for depression or counselling) |
| Interventions | Decision aid (DVD/booklet) vs usual care |
| Outcomes | Knowledge, value concordance |
| Starting date | June 2010 |
| Contact information | Karen R Sepucha |
| Notes | NCT01152307 |
| Trial name or title | Study to test use of a decision aid in a clinical visit to help patients choose a diabetes medication. Translating Information on Comparative Effectiveness Into Practice (TRICEP) |
| Methods | RCT |
| Participants | Type 2 diabetes mellitus patients |
| Interventions | Diabetes Medication Decision Aid vs usual care |
| Outcomes | Patient satisfaction and knowledge. Physician adoption and satisfaction with the decision aid |
| Starting date | January 2011 |
| Contact information | Nilay D. Shah, Mayo Clinic |
| Notes | NCT01293578 |
| Trial name or title | Evaluating an online decision aid for women considering breast reconstruction following mastectomy |
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Starting date | May 2009 |
| Contact information | Kerry Sherman |
| Notes | Trials # ACTRN12609000363280 |
| Trial name or title | Shared decision making: the effects of a decision aid for Turkish and Morocan mental health care clients with depression on the client‐caregiver relationship |
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Starting date | May 2009 |
| Contact information | C. Smits |
| Notes | RTN 1822 |
| Trial name or title | Comparison of ways to prepare patients for decisions about joint replacement surgery |
| Methods | RCT |
| Participants | Patients considering joint replacement surgery |
| Interventions | Patient decision aid versus usual care |
| Outcomes | Wait times, decision quality, knowledge, choice, disease specific quality of life |
| Starting date | Not yet assessed |
| Contact information | Not yet assessed |
| Notes | Trial Registry NCT00911638 |
| Trial name or title | Decision aid in veterans with PTSD |
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Starting date | May 2009 |
| Contact information | Maha Zayed |
| Notes | Trials # NCT00908440 |
OA: osteoarthritis; RCT: randomized controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knowledge: DA vs usual care ‐ all studies Show forest plot | 42 | 10842 | Mean Difference (IV, Random, 95% CI) | 13.34 [11.17, 15.51] |
| Analysis 1.1  Comparison 1 Knowledge, Outcome 1 Knowledge: DA vs usual care ‐ all studies. | ||||
| 2 Knowledge: DA vs usual care ‐ treatment only Show forest plot | 23 | 3977 | Mean Difference (IV, Random, 95% CI) | 13.75 [11.08, 16.43] |
| Analysis 1.2  Comparison 1 Knowledge, Outcome 2 Knowledge: DA vs usual care ‐ treatment only. | ||||
| 3 Knowledge: DA vs usual care ‐ screening only Show forest plot | 19 | 6865 | Mean Difference (IV, Random, 95% CI) | 12.76 [9.66, 15.86] |
| Analysis 1.3  Comparison 1 Knowledge, Outcome 3 Knowledge: DA vs usual care ‐ screening only. | ||||
| 4 Knowledge: Detailed vs simple decision aids ‐ all studies Show forest plot | 19 | 3531 | Mean Difference (IV, Random, 95% CI) | 5.52 [3.90, 7.15] |
| Analysis 1.4  Comparison 1 Knowledge, Outcome 4 Knowledge: Detailed vs simple decision aids ‐ all studies. | ||||
| 5 Knowledge: Detailed vs simple decision aids ‐ treatment only Show forest plot | 12 | 1930 | Mean Difference (IV, Random, 95% CI) | 4.98 [2.64, 7.33] |
| Analysis 1.5  Comparison 1 Knowledge, Outcome 5 Knowledge: Detailed vs simple decision aids ‐ treatment only. | ||||
| 6 Knowledge: Detailed vs simple decision aids ‐ screening only Show forest plot | 7 | 1601 | Mean Difference (IV, Random, 95% CI) | 6.33 [4.49, 8.17] |
| Analysis 1.6  Comparison 1 Knowledge, Outcome 6 Knowledge: Detailed vs simple decision aids ‐ screening only. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Accurate risk perceptions ‐ all studies Show forest plot | 19 | 5868 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.52, 2.16] |
| Analysis 2.1  Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 1 Accurate risk perceptions ‐ all studies. | ||||
| 2 Accurate risk perceptions ‐ treatments only Show forest plot | 12 | 2435 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.47, 2.01] |
| Analysis 2.2  Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 2 Accurate risk perceptions ‐ treatments only. | ||||
| 3 Accurate risk perceptions ‐ screening only Show forest plot | 7 | 3433 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.40, 2.93] |
| Analysis 2.3  Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 3 Accurate risk perceptions ‐ screening only. | ||||
| 4 Accurate risk perceptions ‐ numbers Show forest plot | 15 | 4776 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.65, 2.43] |
| Analysis 2.4  Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 4 Accurate risk perceptions ‐ numbers. | ||||
| 5 Accurate risk perceptions ‐ words Show forest plot | 4 | 1092 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.13, 1.52] |
| Analysis 2.5  Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 5 Accurate risk perceptions ‐ words. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Values congruent with chosen option ‐ all studies Show forest plot | 13 | 4670 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| Analysis 3.1  Comparison 3 Values congruent with chosen option, Outcome 1 Values congruent with chosen option ‐ all studies. | ||||
| 2 Values congruent with chosen option ‐ treatment only Show forest plot | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.80, 2.30] |
| Analysis 3.2  Comparison 3 Values congruent with chosen option, Outcome 2 Values congruent with chosen option ‐ treatment only. | ||||
| 3 Values congruent with chosen option ‐ screening only Show forest plot | 10 | 4321 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.16, 2.11] |
| Analysis 3.3  Comparison 3 Values congruent with chosen option, Outcome 3 Values congruent with chosen option ‐ screening only. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Decisional conflict: DA vs usual care ‐ all studies Show forest plot | 32 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Decisional conflict, Outcome 1 Decisional conflict: DA vs usual care ‐ all studies. | ||||
| 1.1 Uncertainty sub‐scale | 23 | 4837 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐4.28, ‐0.66] |
| 1.2 Uninformed sub‐scale | 22 | 4343 | Mean Difference (IV, Random, 95% CI) | ‐7.26 [‐9.73, ‐4.78] |
| 1.3 Unclear values sub‐scale | 18 | 3704 | Mean Difference (IV, Random, 95% CI) | ‐6.09 [‐8.50, ‐3.67] |
| 1.4 Unsupported sub‐scale | 19 | 3851 | Mean Difference (IV, Random, 95% CI) | ‐4.77 [‐6.86, ‐2.69] |
| 1.5 Ineffective choice sub‐scale | 19 | 3878 | Mean Difference (IV, Random, 95% CI) | ‐4.86 [‐7.04, ‐2.68] |
| 1.6 Total decisional conflict score | 28 | 5830 | Mean Difference (IV, Random, 95% CI) | ‐6.22 [‐8.00, ‐4.44] |
| 2 Decisional conflict: DA vs usual care ‐ treatment only Show forest plot | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Decisional conflict, Outcome 2 Decisional conflict: DA vs usual care ‐ treatment only. | ||||
| 2.1 Uncertainty sub‐scale | 16 | 3020 | Mean Difference (IV, Random, 95% CI) | ‐3.06 [‐5.33, ‐0.79] |
| 2.2 Uninformed sub‐scale | 17 | 3007 | Mean Difference (IV, Random, 95% CI) | ‐8.06 [‐10.52, ‐5.60] |
| 2.3 Unclear values sub‐scale | 14 | 2474 | Mean Difference (IV, Random, 95% CI) | ‐6.31 [‐9.01, ‐3.61] |
| 2.4 Unsupported sub‐scale | 15 | 2621 | Mean Difference (IV, Random, 95% CI) | ‐5.28 [‐7.74, ‐2.82] |
| 2.5 Ineffective choice sub‐scale | 15 | 2746 | Mean Difference (IV, Random, 95% CI) | ‐6.07 [‐8.41, ‐3.72] |
| 2.6 Total decisional conflict score | 22 | 3783 | Mean Difference (IV, Random, 95% CI) | ‐6.14 [‐7.78, ‐4.50] |
| 3 Decisional conflict: DA vs usual care ‐ screening only Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Decisional conflict, Outcome 3 Decisional conflict: DA vs usual care ‐ screening only. | ||||
| 3.1 Uncertainty sub‐scale | 7 | 1817 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐4.47, 1.83] |
| 3.2 Uninformed sub‐scale | 5 | 1336 | Mean Difference (IV, Random, 95% CI) | ‐4.67 [‐10.61, 1.27] |
| 3.3 Unclear values sub‐scale | 4 | 1230 | Mean Difference (IV, Random, 95% CI) | ‐5.94 [‐13.44, 1.56] |
| 3.4 Unsupported sub‐scale | 4 | 1230 | Mean Difference (IV, Random, 95% CI) | ‐2.94 [‐6.90, 1.02] |
| 3.5 Ineffective choice sub‐scale | 4 | 1132 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐1.88, 1.55] |
| 3.6 Total decisional conflict score | 6 | 2047 | Mean Difference (IV, Random, 95% CI) | ‐6.83 [‐12.64, ‐1.03] |
| 4 Decisional conflict: Detailed vs simple decision aid ‐ all studies Show forest plot | 19 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Decisional conflict, Outcome 4 Decisional conflict: Detailed vs simple decision aid ‐ all studies. | ||||
| 4.1 Uncertainty sub‐scale | 14 | 2130 | Mean Difference (IV, Random, 95% CI) | ‐2.15 [‐4.42, 0.12] |
| 4.2 Uninformed sub‐scale | 10 | 1264 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐4.39, ‐0.39] |
| 4.3 Unclear values sub‐scale | 10 | 1260 | Mean Difference (IV, Random, 95% CI) | ‐2.31 [‐4.67, 0.05] |
| 4.4 Unsupported sub‐scale | 10 | 1268 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐5.37, 1.27] |
| 4.5 Ineffective choice sub‐scale | 9 | 1541 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.83, 0.71] |
| 4.6 Total decisional conflict score | 17 | 3277 | Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.64, ‐0.91] |
| 5 Decisional conflict: Detailed vs simple decision aid ‐ treatment only Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.5  Comparison 4 Decisional conflict, Outcome 5 Decisional conflict: Detailed vs simple decision aid ‐ treatment only. | ||||
| 5.1 Uncertainty sub‐scale | 9 | 1101 | Mean Difference (IV, Random, 95% CI) | ‐2.02 [‐6.65, 2.61] |
| 5.2 Uninformed sub‐scale | 6 | 672 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐4.40, 2.09] |
| 5.3 Unclear values sub‐scale | 6 | 669 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐3.72, 2.80] |
| 5.4 Unsupported sub‐scale | 6 | 674 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐3.09, 1.79] |
| 5.5 Ineffective choice sub‐scale | 7 | 849 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.97, 2.44] |
| 5.6 Total decisional conflict score | 10 | 1732 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐2.30, 0.38] |
| 6 Decisional conflict: Detailed vs simple decision aid ‐ screening only Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.6  Comparison 4 Decisional conflict, Outcome 6 Decisional conflict: Detailed vs simple decision aid ‐ screening only. | ||||
| 6.1 Uncertainty sub‐scale | 5 | 1029 | Mean Difference (IV, Random, 95% CI) | ‐2.16 [‐4.20, ‐0.12] |
| 6.2 Uninformed sub‐scale | 4 | 592 | Mean Difference (IV, Random, 95% CI) | ‐3.42 [‐5.81, ‐1.02] |
| 6.3 Unclear values sub‐scale | 4 | 591 | Mean Difference (IV, Random, 95% CI) | ‐4.54 [‐6.77, ‐2.32] |
| 6.4 Unsupported sub‐scale | 4 | 594 | Mean Difference (IV, Random, 95% CI) | ‐3.65 [‐9.74, 2.44] |
| 6.5 Ineffective choice sub‐scale | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐2.18 [‐3.60, ‐0.75] |
| 6.6 Total decisional conflict score | 7 | 1545 | Mean Difference (IV, Random, 95% CI) | ‐2.26 [‐3.33, ‐1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participation in decision making: DA vs usual care ‐ all studies Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Participation in decision making, Outcome 1 Participation in decision making: DA vs usual care ‐ all studies. | ||||
| 1.1 Patient controlled decision making | 12 | 2438 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.02, 1.60] |
| 1.2 Shared decision making | 12 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.13] |
| 1.3 Practitioner controlled decision making | 14 | 3234 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.53, 0.81] |
| 2 Participation in decision making: DA vs usual care ‐ treatment only Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Participation in decision making, Outcome 2 Participation in decision making: DA vs usual care ‐ treatment only. | ||||
| 2.1 Patient controlled decision making | 10 | 2147 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.05, 1.68] |
| 2.2 Shared decision making | 10 | 2111 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.15] |
| 2.3 Practitioner controlled decision making | 11 | 2318 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.54, 0.90] |
| 3 Participation in decision making: DA vs usual care ‐ screening only Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Participation in decision making, Outcome 3 Participation in decision making: DA vs usual care ‐ screening only. | ||||
| 3.1 Patient controlled decision making | 3 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.82, 1.20] |
| 3.2 Shared decision making | 3 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.89, 1.45] |
| 3.3 Practitioner controlled decision making | 4 | 1512 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.44, 1.01] |
| 4 Participation in decision making: Detailed vs simple decision aid ‐ all studies Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Participation in decision making, Outcome 4 Participation in decision making: Detailed vs simple decision aid ‐ all studies. | ||||
| 4.1 Patient controlled decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.68, 1.64] |
| 4.2 Shared decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.63, 1.81] |
| 4.3 Practitioner controlled decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.56, 2.23] |
| 5 Participation in decision making: Detailed vs simple decision aid ‐ treatment only Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Participation in decision making, Outcome 5 Participation in decision making: Detailed vs simple decision aid ‐ treatment only. | ||||
| 5.1 Patient controlled decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.68, 1.64] |
| 5.2 Shared decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.63, 1.81] |
| 5.3 Practitioner controlled decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.56, 2.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion undecided: DA vs usual care ‐ all studies Show forest plot | 18 | 4753 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.47, 0.72] |
| Analysis 6.1  Comparison 6 Proportion undecided, Outcome 1 Proportion undecided: DA vs usual care ‐ all studies. | ||||
| 2 Proportion undecided: DA vs usual care ‐ treatment only Show forest plot | 14 | 2830 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.51, 0.78] |
| Analysis 6.2  Comparison 6 Proportion undecided, Outcome 2 Proportion undecided: DA vs usual care ‐ treatment only. | ||||
| 3 Proportion undecided: DA vs usual care ‐ screening only Show forest plot | 4 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.90] |
| Analysis 6.3  Comparison 6 Proportion undecided, Outcome 3 Proportion undecided: DA vs usual care ‐ screening only. | ||||
| 4 Proportion undecided: Detailed vs simple decision aids ‐ all studies Show forest plot | 3 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.37] |
| Analysis 6.4  Comparison 6 Proportion undecided, Outcome 4 Proportion undecided: Detailed vs simple decision aids ‐ all studies. | ||||
| 5 Proportion undecided: Detailed vs simple decision aids ‐ treatment only Show forest plot | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.47] |
| Analysis 6.5  Comparison 6 Proportion undecided, Outcome 5 Proportion undecided: Detailed vs simple decision aids ‐ treatment only. | ||||
| 6 Proportion undecided: Detailed vs simple decision aids ‐ screening only Show forest plot | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.21, 1.86] |
| Analysis 6.6  Comparison 6 Proportion undecided, Outcome 6 Proportion undecided: Detailed vs simple decision aids ‐ screening only. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with the choice: DA vs usual care ‐ all studies Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Satisfaction, Outcome 1 Satisfaction with the choice: DA vs usual care ‐ all studies. | ||||
| 2 Satisfaction with the choice: DA vs usual care ‐ treatment only Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Satisfaction, Outcome 2 Satisfaction with the choice: DA vs usual care ‐ treatment only. | ||||
| 3 Satisfaction with the choice: DA vs usual care ‐ screening only Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 Satisfaction, Outcome 3 Satisfaction with the choice: DA vs usual care ‐ screening only. | ||||
| 4 Satisfaction with the choice: Detailed vs simple DA ‐ all studies Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.4  Comparison 7 Satisfaction, Outcome 4 Satisfaction with the choice: Detailed vs simple DA ‐ all studies. | ||||
| 5 Satisfaction with the choice: Detailed vs simple DA ‐ treatment only Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.5  Comparison 7 Satisfaction, Outcome 5 Satisfaction with the choice: Detailed vs simple DA ‐ treatment only. | ||||
| 6 Satisfaction with the decision making process: DA vs usual care ‐ all studies Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.6  Comparison 7 Satisfaction, Outcome 6 Satisfaction with the decision making process: DA vs usual care ‐ all studies. | ||||
| 7 Satisfaction with the decision making process: DA vs usual care ‐ treatment only Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.7  Comparison 7 Satisfaction, Outcome 7 Satisfaction with the decision making process: DA vs usual care ‐ treatment only. | ||||
| 8 Satisfaction with the decision making process: DA vs usual care ‐ screening only Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.8  Comparison 7 Satisfaction, Outcome 8 Satisfaction with the decision making process: DA vs usual care ‐ screening only. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Choice: Surgery over conservative option: DA vs usual care Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.1  Comparison 8 Choice, Outcome 1 Choice: Surgery over conservative option: DA vs usual care. | ||||
| 1.1 As treated analysis | 15 | 2915 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.2 Intention to treat analysis | 15 | 3553 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.68, 0.93] |
| 2 Choice: Surgery over conservative option: Detailed vs simple decision aid Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.2  Comparison 8 Choice, Outcome 2 Choice: Surgery over conservative option: Detailed vs simple decision aid. | ||||
| 2.1 As treated analysis | 3 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.08] |
| 2.2 Intention to treat analysis | 3 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.08] |
| 3 Choice for screening Show forest plot | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.3  Comparison 8 Choice, Outcome 3 Choice for screening. | ||||
| 3.1 PSA screening: DA vs usual care | 9 | 3565 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.98] |
| 3.2 PSA screening: detailed DA vs simple decision aid | 3 | 782 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 3.3 Colorectal cancer screening: DA vs usual care | 10 | 4529 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.31] |
| 3.4 Breast cancer genetic testing: DA vs usual care | 4 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.83, 1.22] |
| 3.5 Prenatal diagnostic testing: Detailed vs simple decision aid | 2 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.90, 1.03] |
| 4 Choice: Diabetes medication (uptake new medication): DA vs usual care Show forest plot | 3 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.77, 4.39] |
| Analysis 8.4  Comparison 8 Choice, Outcome 4 Choice: Diabetes medication (uptake new medication): DA vs usual care. | ||||
| 5 Choice: Menopausal hormone therapy: Detailed vs simple decision aid Show forest plot | 3 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.98] |
| Analysis 8.5  Comparison 8 Choice, Outcome 5 Choice: Menopausal hormone therapy: Detailed vs simple decision aid. | ||||

Study flow diagram.

Risk of bias summary as percentages across all included studies.

Risk of bias summary for each included study.

Funnel plot of comparison: 1 Knowledge, outcome: 1.1 Knowledge: DA vs usual care ‐ all studies.
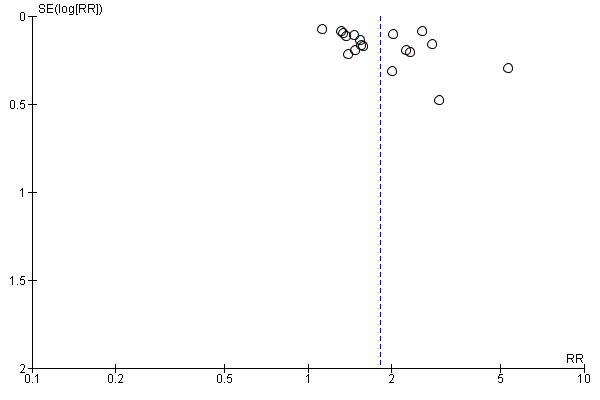
Funnel plot of comparison: 2 Accurate risk perceptions: Decision aid with outcome probabilities vs no outcome probability information, outcome: 2.1 Accurate risk perceptions ‐ all studies.

Funnel plot of comparison: 3 Values congruent with chosen option, outcome: 3.1 Values congruent with chosen option ‐ all studies.

Funnel plot of comparison: 4.1 Decisional conflict: DA vs usual care ‐ all studies, outcome: 4.1.2 Uninformed sub‐scale

Funnel plot of comparison: 4.1 Decisional conflict: DA vs usual care ‐ all studies, outcome: 4.1.3 Unclear sub‐scale
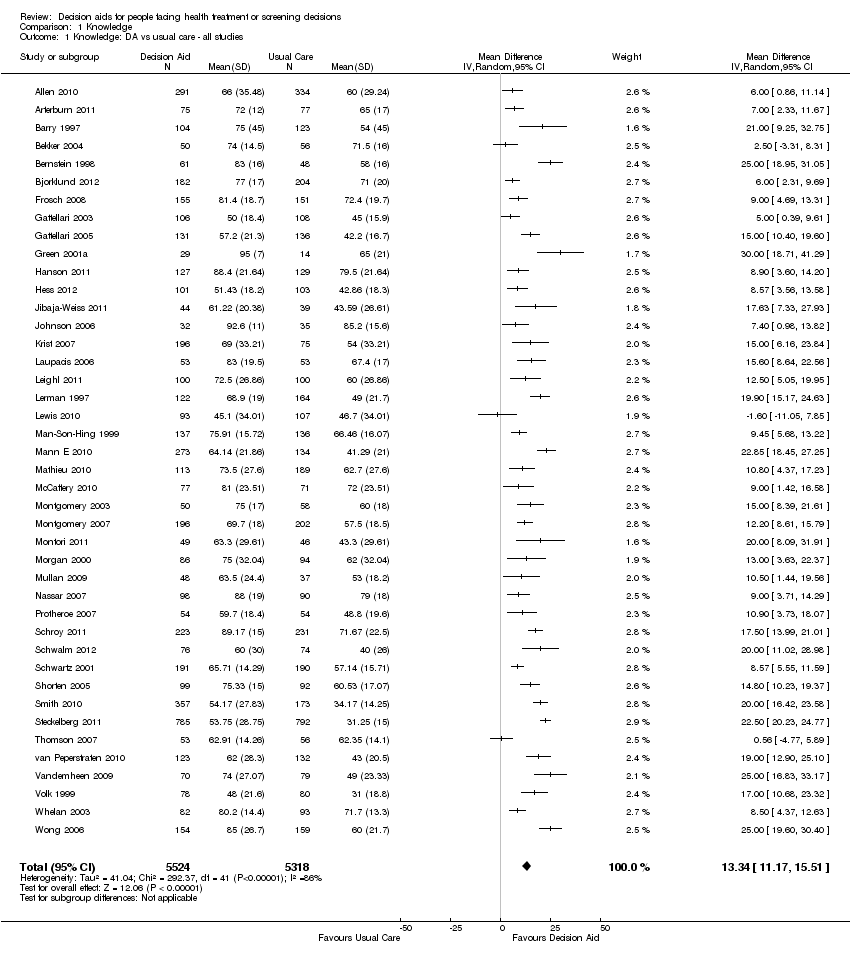
Comparison 1 Knowledge, Outcome 1 Knowledge: DA vs usual care ‐ all studies.

Comparison 1 Knowledge, Outcome 2 Knowledge: DA vs usual care ‐ treatment only.
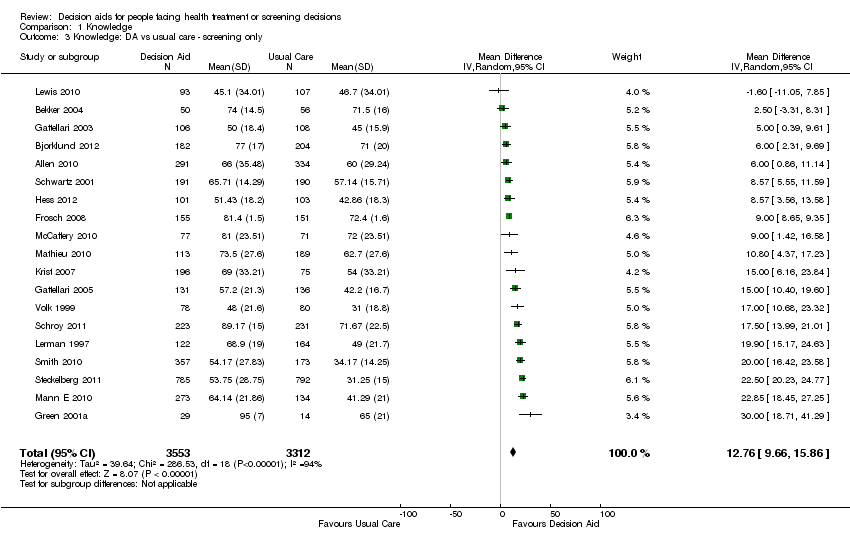
Comparison 1 Knowledge, Outcome 3 Knowledge: DA vs usual care ‐ screening only.

Comparison 1 Knowledge, Outcome 4 Knowledge: Detailed vs simple decision aids ‐ all studies.

Comparison 1 Knowledge, Outcome 5 Knowledge: Detailed vs simple decision aids ‐ treatment only.

Comparison 1 Knowledge, Outcome 6 Knowledge: Detailed vs simple decision aids ‐ screening only.

Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 1 Accurate risk perceptions ‐ all studies.

Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 2 Accurate risk perceptions ‐ treatments only.

Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 3 Accurate risk perceptions ‐ screening only.
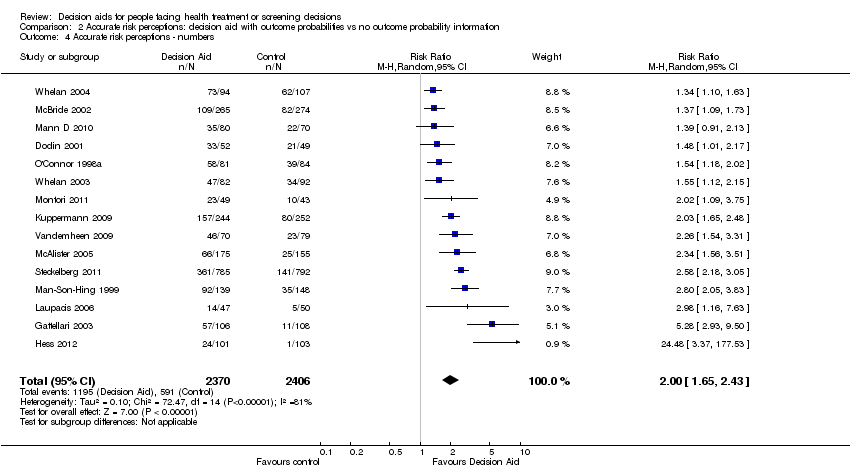
Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 4 Accurate risk perceptions ‐ numbers.

Comparison 2 Accurate risk perceptions: decision aid with outcome probabilities vs no outcome probability information, Outcome 5 Accurate risk perceptions ‐ words.
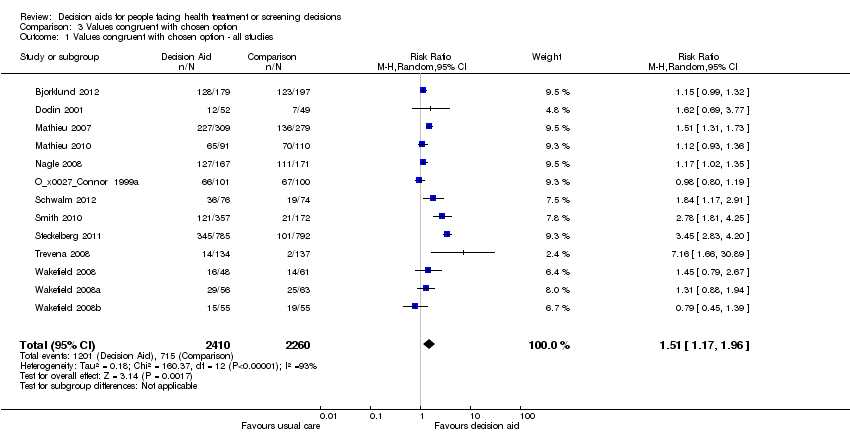
Comparison 3 Values congruent with chosen option, Outcome 1 Values congruent with chosen option ‐ all studies.

Comparison 3 Values congruent with chosen option, Outcome 2 Values congruent with chosen option ‐ treatment only.

Comparison 3 Values congruent with chosen option, Outcome 3 Values congruent with chosen option ‐ screening only.

Comparison 4 Decisional conflict, Outcome 1 Decisional conflict: DA vs usual care ‐ all studies.

Comparison 4 Decisional conflict, Outcome 2 Decisional conflict: DA vs usual care ‐ treatment only.

Comparison 4 Decisional conflict, Outcome 3 Decisional conflict: DA vs usual care ‐ screening only.
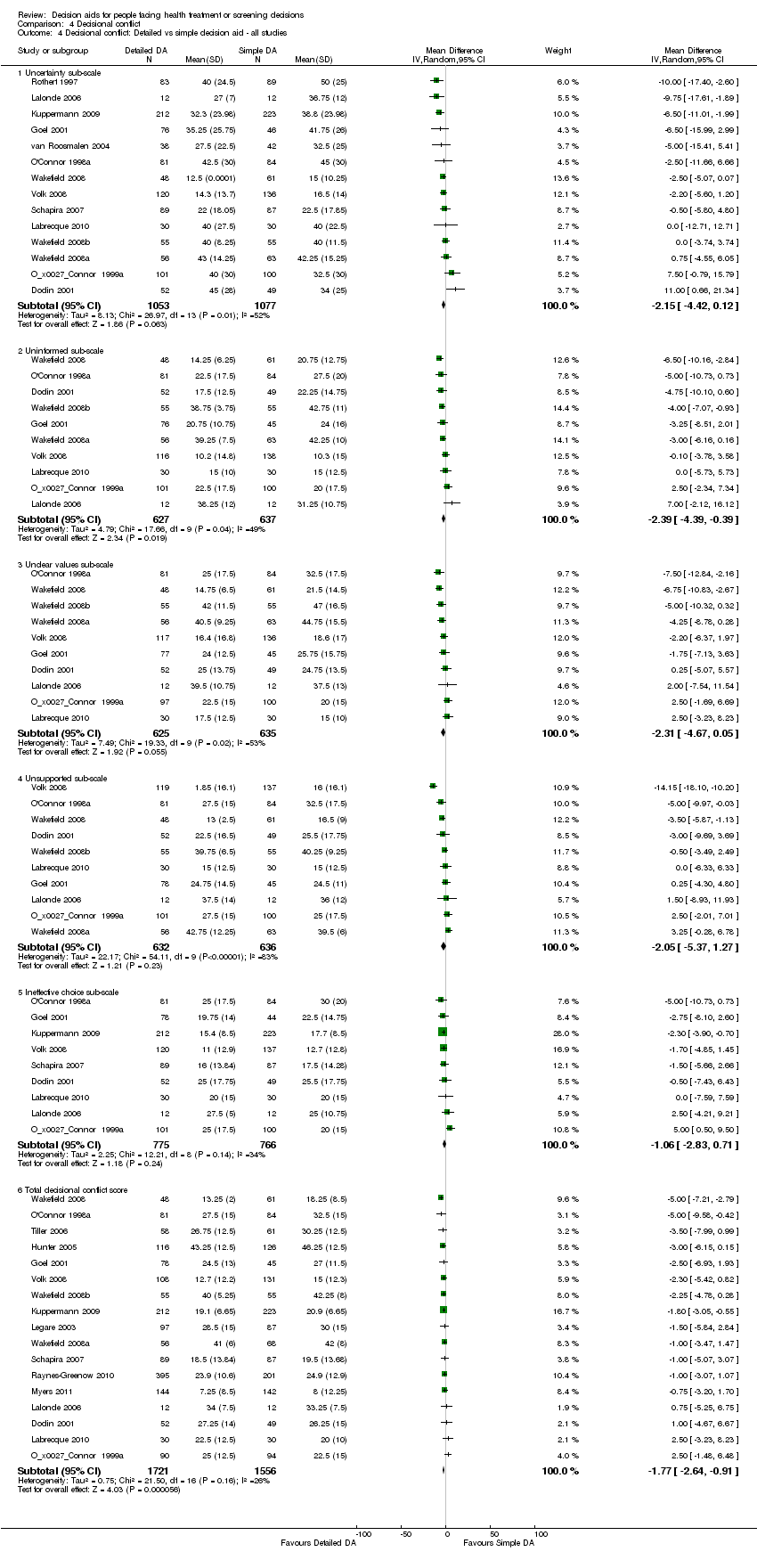
Comparison 4 Decisional conflict, Outcome 4 Decisional conflict: Detailed vs simple decision aid ‐ all studies.

Comparison 4 Decisional conflict, Outcome 5 Decisional conflict: Detailed vs simple decision aid ‐ treatment only.
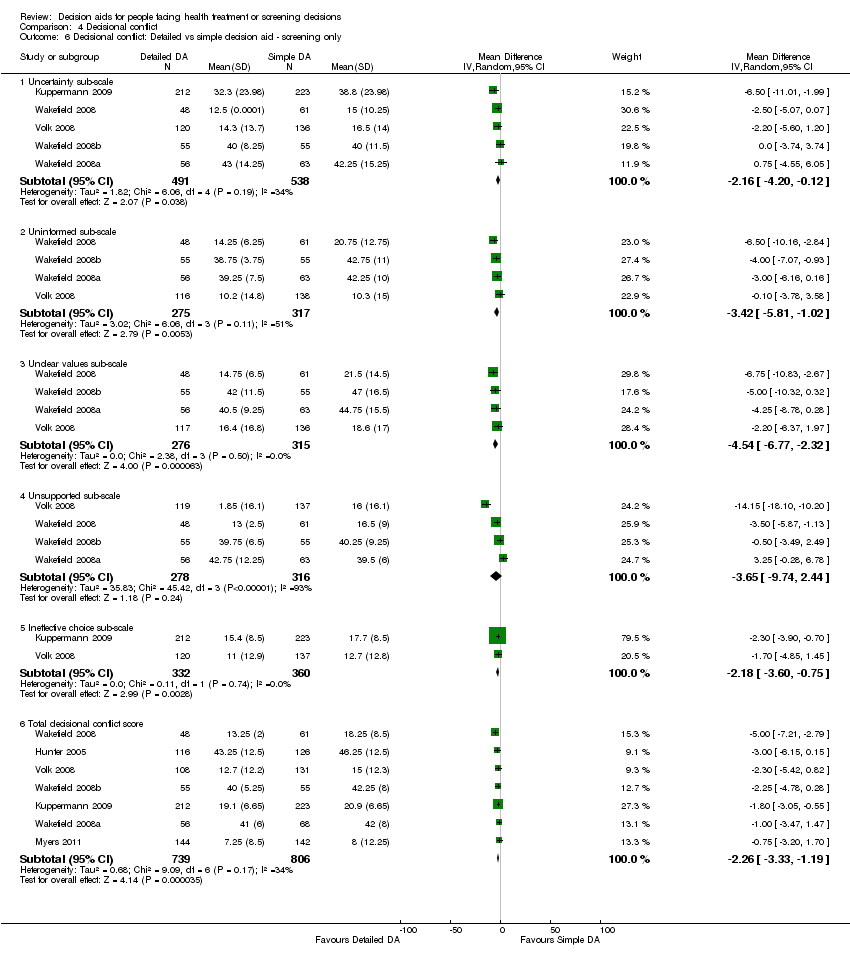
Comparison 4 Decisional conflict, Outcome 6 Decisional conflict: Detailed vs simple decision aid ‐ screening only.
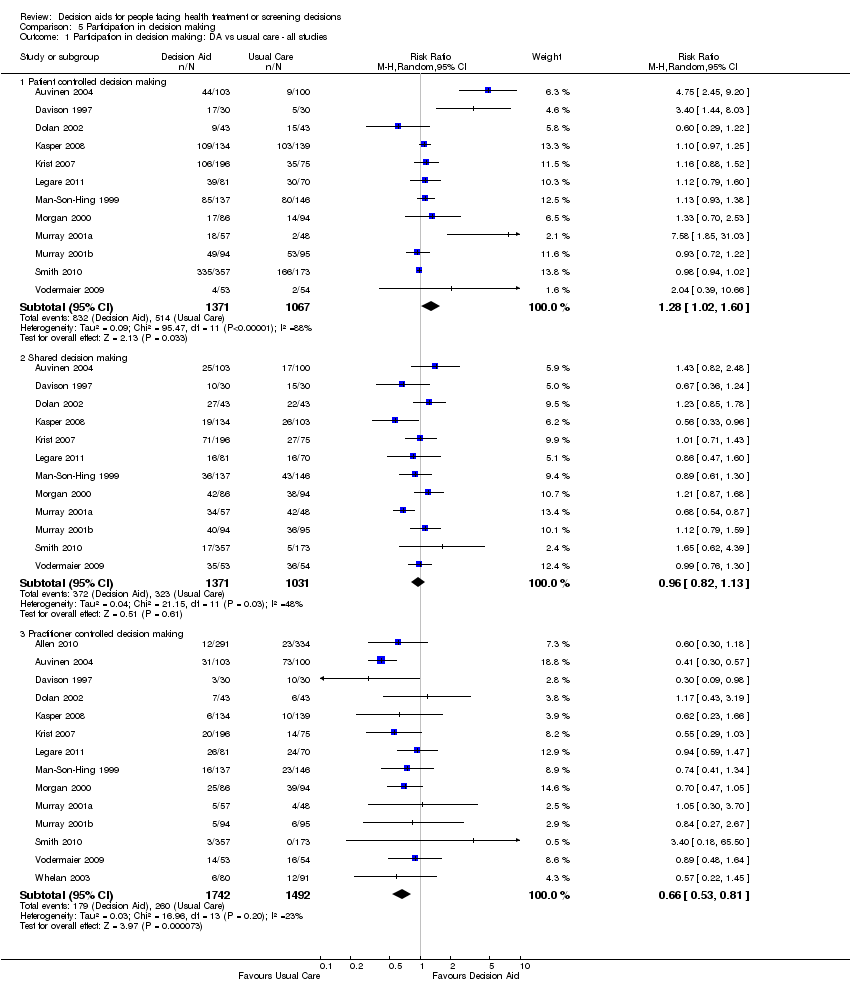
Comparison 5 Participation in decision making, Outcome 1 Participation in decision making: DA vs usual care ‐ all studies.

Comparison 5 Participation in decision making, Outcome 2 Participation in decision making: DA vs usual care ‐ treatment only.

Comparison 5 Participation in decision making, Outcome 3 Participation in decision making: DA vs usual care ‐ screening only.

Comparison 5 Participation in decision making, Outcome 4 Participation in decision making: Detailed vs simple decision aid ‐ all studies.

Comparison 5 Participation in decision making, Outcome 5 Participation in decision making: Detailed vs simple decision aid ‐ treatment only.
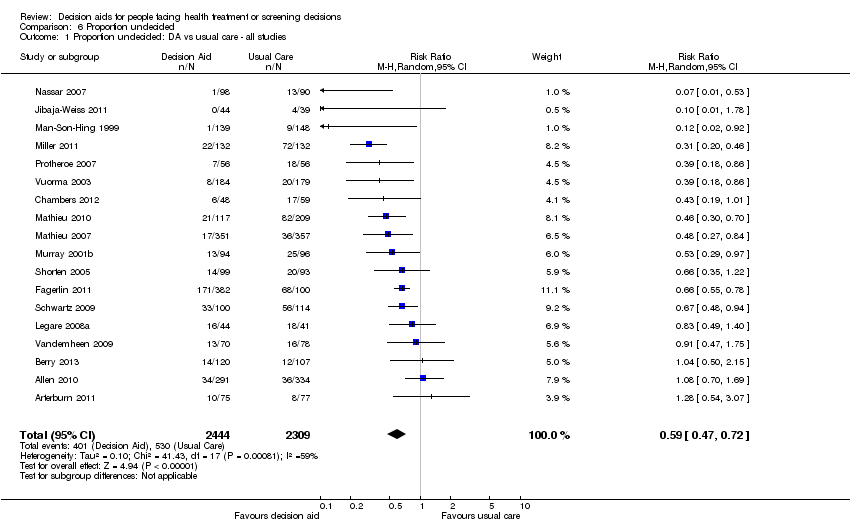
Comparison 6 Proportion undecided, Outcome 1 Proportion undecided: DA vs usual care ‐ all studies.
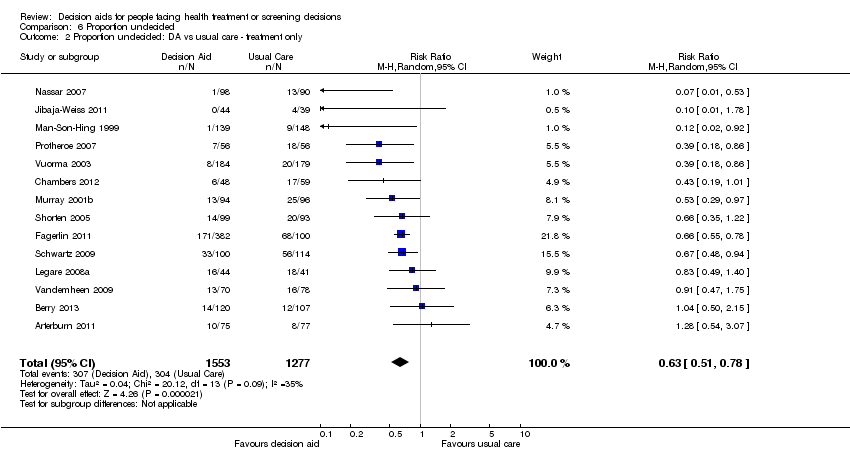
Comparison 6 Proportion undecided, Outcome 2 Proportion undecided: DA vs usual care ‐ treatment only.

Comparison 6 Proportion undecided, Outcome 3 Proportion undecided: DA vs usual care ‐ screening only.
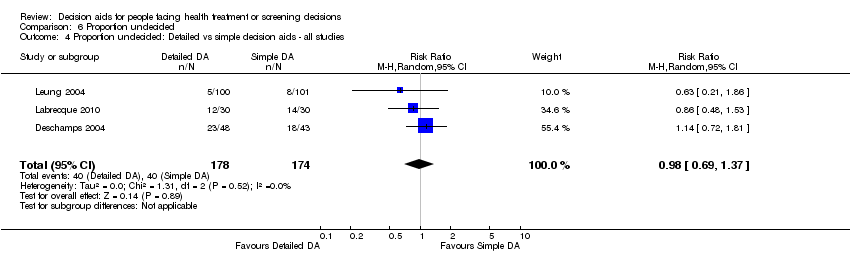
Comparison 6 Proportion undecided, Outcome 4 Proportion undecided: Detailed vs simple decision aids ‐ all studies.
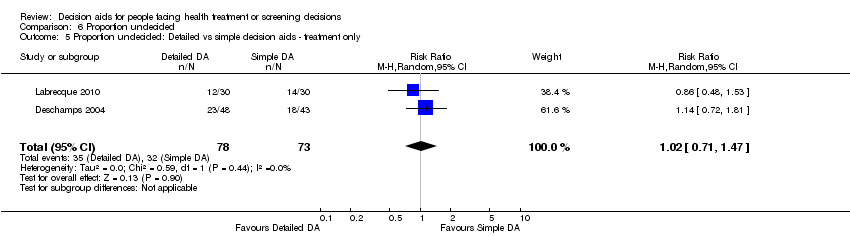
Comparison 6 Proportion undecided, Outcome 5 Proportion undecided: Detailed vs simple decision aids ‐ treatment only.
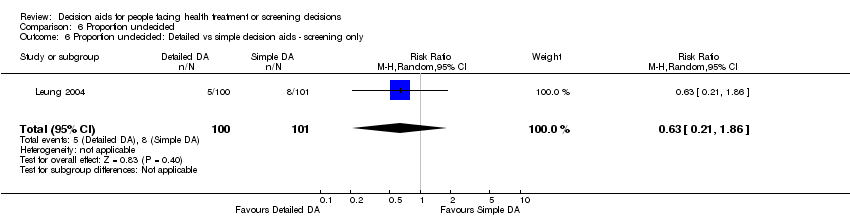
Comparison 6 Proportion undecided, Outcome 6 Proportion undecided: Detailed vs simple decision aids ‐ screening only.

Comparison 7 Satisfaction, Outcome 1 Satisfaction with the choice: DA vs usual care ‐ all studies.

Comparison 7 Satisfaction, Outcome 2 Satisfaction with the choice: DA vs usual care ‐ treatment only.

Comparison 7 Satisfaction, Outcome 3 Satisfaction with the choice: DA vs usual care ‐ screening only.
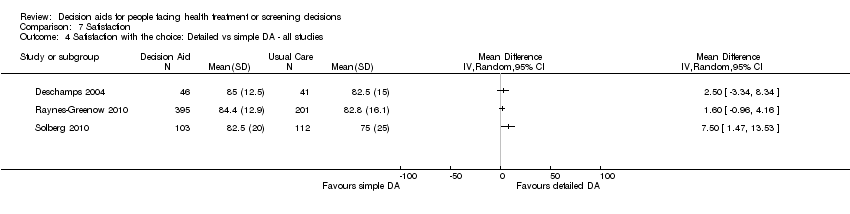
Comparison 7 Satisfaction, Outcome 4 Satisfaction with the choice: Detailed vs simple DA ‐ all studies.
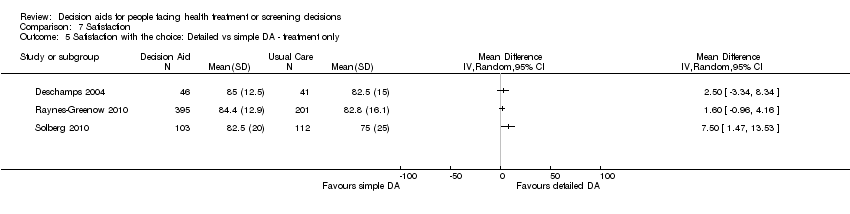
Comparison 7 Satisfaction, Outcome 5 Satisfaction with the choice: Detailed vs simple DA ‐ treatment only.
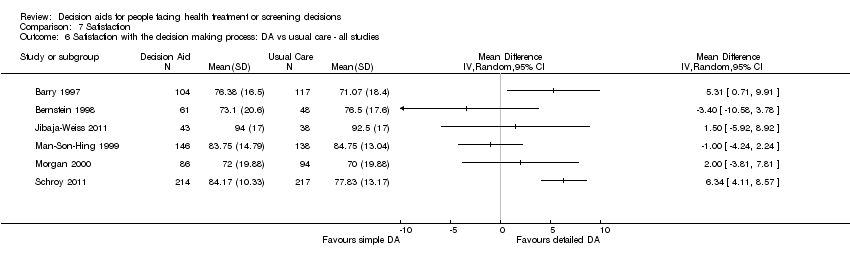
Comparison 7 Satisfaction, Outcome 6 Satisfaction with the decision making process: DA vs usual care ‐ all studies.

Comparison 7 Satisfaction, Outcome 7 Satisfaction with the decision making process: DA vs usual care ‐ treatment only.
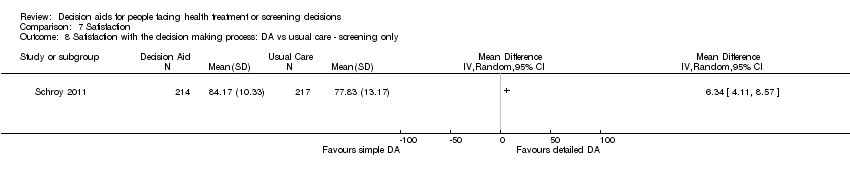
Comparison 7 Satisfaction, Outcome 8 Satisfaction with the decision making process: DA vs usual care ‐ screening only.
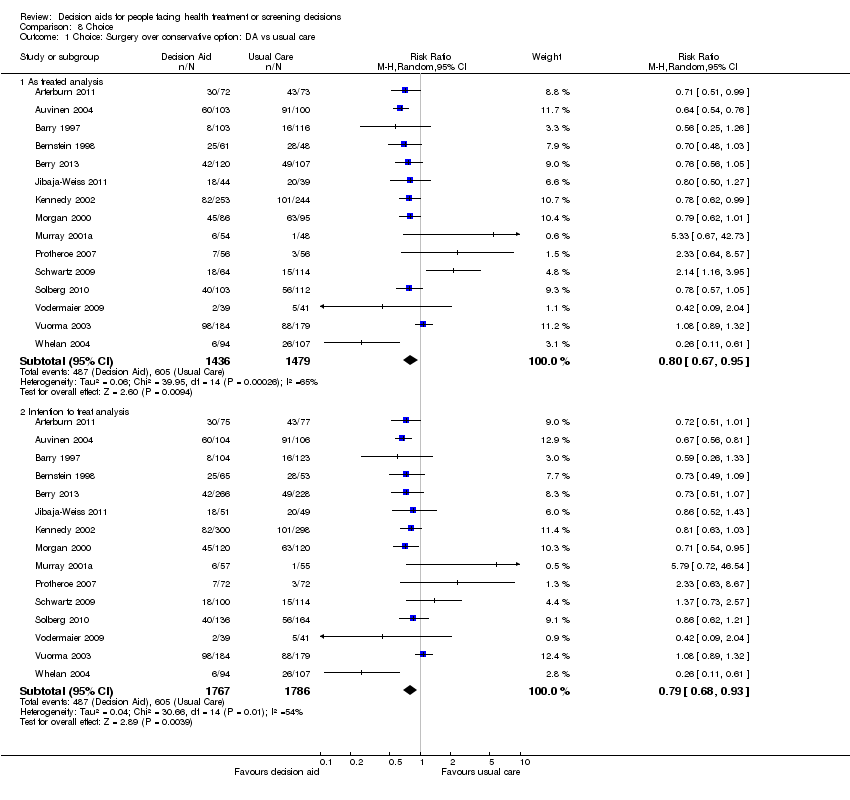
Comparison 8 Choice, Outcome 1 Choice: Surgery over conservative option: DA vs usual care.

Comparison 8 Choice, Outcome 2 Choice: Surgery over conservative option: Detailed vs simple decision aid.
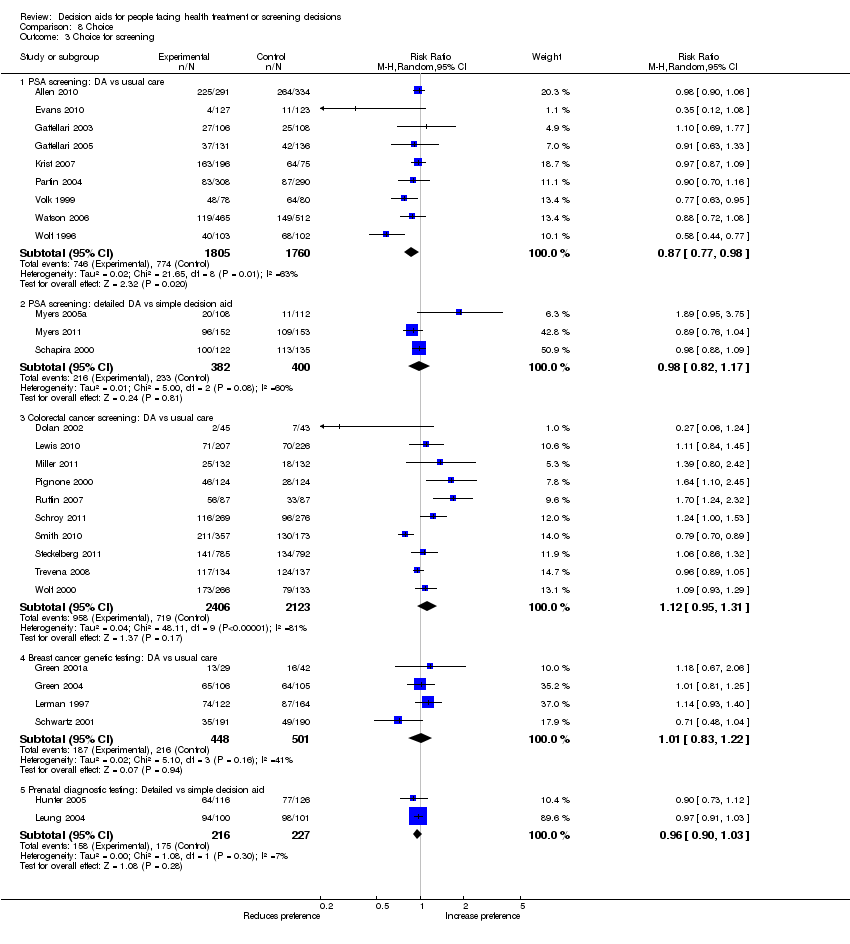
Comparison 8 Choice, Outcome 3 Choice for screening.
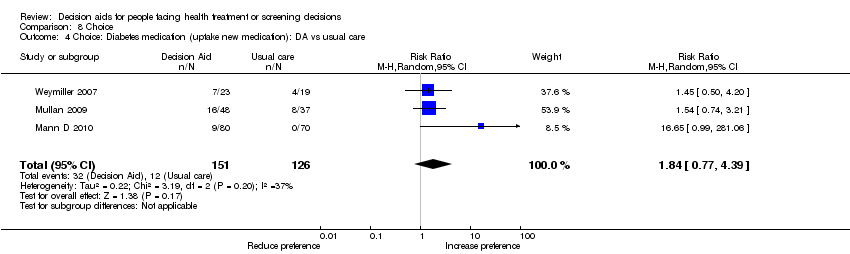
Comparison 8 Choice, Outcome 4 Choice: Diabetes medication (uptake new medication): DA vs usual care.

Comparison 8 Choice, Outcome 5 Choice: Menopausal hormone therapy: Detailed vs simple decision aid.
| Patient decision aids compared with usual care for adults considering treatment or screening decisions | ||||||
| Patient or population: adults considering treatment or screening decisions Settings: all settings Intervention: patient decision aid Comparison: usual care | ||||||
| Outcomes | Illustrative comparative benefits* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed benefit | Corresponding benefit | |||||
| Usual care | Patient decision aid | |||||
| Knowledge: decision aid versus usual care ‐ all studies standardized on score from 0 (no knowledge) to 100 (perfect knowledge) [soon after exposure to the decision aid] | The mean knowledge score was 56.9% ranged across control groups from 31% to 85.2% | The mean knowledge score in the intervention groups was 13.34 higher (11.17 to 15.51 higher) | 10,842 | ⊕⊕⊕⊕ | Higher scores indicate better knowledge. 41 out of 42 studies showed an improvement in knowledge. | |
| Accurate risk perceptions ‐ all studies [soon after exposure to the decision aid] | 296 patients per 1000 | 542 patients per 1000 | RR 1.82 (95% CI: 1.52 to 2.16) | 5868 | ⊕⊕⊕⊝ | |
| Congruence between the chosen option and their values ‐ all studies [soon after exposure to the decision aid] | 316 patients per 1000 | 498 patients per 1000 | RR 1.51 (95% CI: 1.17 to 1.97) | 4670 (13 studies) | ⊕⊕⊝⊝ | |
| Decisional conflict: decision aid versus usual care ‐ all studies ‐ Uninformed sub‐scale standardized on score from 0 (not uninformed) to 100 (uninformed) [soon after exposure to the decision aid] | The mean feeling uninformed ranged across control groups from 12.75 to 49.1. Scores of 25 or lower are associated with follow‐through with decisions; whereas scores that exceed 38 are associated with delay in decision making | The mean feeling uninformed in the intervention groups was 7.26 lower (9.73 to 4.78 lower) | 4343 (22 studies) | ⊕⊕⊕⊕ | Lower scores indicate feeling more informed. | |
| Decisional conflict: decision aid versus usual care ‐ all studies ‐ Unclear values sub‐scale standardized on score from 0 (not unclear) to 100 (unclear) [soon after exposure to the decision aid] | The mean feeling unclear values ranged across control groups from 15.5 to 51.29. Scores of 25 or lower are associated with follow‐through with decisions; whereas scores that exceed 38 are associated with delay in decision making | The mean feeling unclear values in the intervention groups was 6.09 lower (8.50 to 3.67 lower) | 3704 (18 studies) | ⊕⊕⊕⊕ | Lower scores indicate feeling more clear about values | |
| Participation in decision making: decision aid versus usual care ‐ all studies ‐ Practitioner controlled decision making [soon after consultation with practitioner] | 174 patients per 1000 | 103 patients per 1000 | RR 0.66 (95%CI: 0.53 to 0.81) | 3234 | ⊕⊕⊕⊝ | Patient decision aids aim to increase patient involvement in making decisions. Lower proportion of practitioner controlled decision making is better. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The vast majority of studies measuring this outcome were not at high risk of bias. 2.The GRADE rating was downgraded given the lack of precision. 3.The GRADE rating was downgraded given the lack of consistency. 4.The GRADE rating was downgraded given the lack of directness. | ||||||
| Study | Topic | Availability | Source | Contact Information |
| Prostate cancer screening | No | Allen,Center for Community‐Based Research, Dana‐Farber Cancer Institute, Boston, MA, US, 2010 | requested access | |
| Bariatric surgery | Yes | Informed Medical Decisions Foundation, MA,US, 2010 | informedmedicaldecisions.org/imdf_decision_aid/making‐decisions‐about‐weight‐loss‐surgery/ | |
| Prostate cancer treatment | Yes | Auvinen, Helsinki, Finland, 1993 | included in publication | |
| Benign prostate disease treatment | Yes | Informed Medical Decisions Foundation, MA,US, 2001 | informedmedicaldecisions.org/imdf_decision_aid/treatment‐options‐for‐benign‐prostatic‐hyperplasia/ | |
| Prenatal screening | Yes | Bekker, Leeds, UK, 2003 | included in publication | |
| Ischaemic heart disease treatment | Yes | Informed Medical Decisions Foundation, MA,US, 2002 | informedmedicaldecisions.org/imdf_decision_aid/treatment‐choices‐for‐carotid‐artery‐disease/ | |
| Prostate cancer treatment | No | Berry, Phyllis F. Cantor Center, MA, USA, 2011 | ||
| Antenatal Down syndrome screening | Yes | Södersjukhuset, Department of Obstetrics and Gynecology, Stockholm, Sweden | vimeo.com/34600615/ | |
| Healthcare personnel’s influenza immunization | Yes | A McCarthy. Ottawa Influenza Decision Aid Planning Group, CA, 2008 | decisionaid.ohri.ca/decaids.html#oida | |
| Hepatitis B Vaccine | No | Clancy, Richmond VA, US, 1983 | ||
| Prostate cancer treatment | No | Davison, Manitoba CA, 1992‐1996 | ||
| Total | Yes | Informed Medical Decisions Foundation, MA,US | informedmedicaldecisions.org/imdf_decision_aid/treatment‐choices‐for‐knee‐osteoarthritis/ | |
| Hormone replacement therapy | No | O'Connor, Ottawa, CA, 1996 | decisionaid.ohri.ca/decaids‐archive.html | |
| Back surgery | Yes | Informed Medical Decisions Foundation, MA,US, 2001 | informedmedicaldecisions.org/imdf_decision_aid/managing‐chronic‐low‐back‐pain/ | |
| Hormone replacement therapy | No | O'Connor, Ottawa, CA, 1996 | decisionaid.ohri.ca/decaids‐archive.html | |
| Colon cancer screening | No | Dolan, Rochester NY, US, 1999 | ||
| Prostate cancer screening | Yes | Elwyn, Cardiff, UK | www.prosdex.com | |
| Breast cancer prevention | Yes | Fagerlin, Ann Arbor, MI, US | ||
| Osteoarthritis knee treatment | No | Fraenkel, New Haven CT, US | author said DA never fully developed, all info in paper | |
| Prostate cancer screening | No | Frosch, Los Angeles, US | Screenshots from author | |
| Prostate cancer screening | Yes | Gatellari , Sydney, AU, 2003 | included in publication | |
| Prostate cancer screening | Yes | Gatellari , Sydney, AU, 2003 | included in publication | |
| Breast cancer surgery | No | Goel/Sawka, Toronto CAN, 2001 | ||
| Breast cancer genetic testing | Yes | Green, Hershey PA, US, 2000 | 1‐800‐757‐4868 [email protected] | |
| Breast cancer genetic testing | Yes | Green, Hershey PA, US, 2000 | 1‐800‐757‐4868 [email protected] | |
| Schizophrenia treatment | Yes | Hamann, Munich, GER | emailed by author (in German) | |
| Feeding options in advanced | Yes | Mitchell, Tetroe, O'Connor; 2001 (updated 2008) | decisionaid.ohri.ca/decaids.html#feedingtube | |
| Breast reconstruction | Yes | University of Texas M.D. Anderson Cancer Center, Houston TX, US, 2003 | Disc mailed | |
| Stress testing for chest pain | Yes | Hess, Rochester, MN, US, 2012 | Included in publication | |
| Prenatal screening | No | Hunter, Ottawa, CA, 2000 | decisionaid.ohri.ca/decaids‐archive.html | |
| Breast cancer treatment | Yes | Jibaja‐Weiss, Baylor College of Medicine, 2010 | www.bcm.edu/patchworkoflife | |
| Endodontic treatment | Yes | Johnson, Chicago, US, 2004 | Included in publication | |
| Multiple Sclerosis | No | Jürgen Kasper | ||
| Abnormal uterine bleeding treatment | No | Kennedy/Coulter, London UK, 1996 | ||
| Prostate cancer screening | Yes | Krist, Fairfax VA, US | www.familymedicine.vcu.edu/research/misc/psa/index.html | |
| Prenatal screening | No | Kuppermann, San Francisco CA, US | Computerized tool | |
| Vasectomy | Yes | Labrecque, Quebec City, CA, 2010 | www.vasectomie.net (in French) | |
| Cardiovascular health treatment | No | Lalonde, Ottawa, CA, 2002 | decisionaid.ohri.ca/decaids‐archive.html | |
| Contraceptive method choice | Yes | World Health Organization, 2005 | www.who.int/reproductivehealth/publications/family_planning/9241593229index/en/index.html | |
| Pre‐operative autologous blood donation | No | Laupacis, Ottawa, CA, 2001 | decisionaid.ohri.ca/decaids‐archive.html | |
| Hormone replacement therapy | No | O'Connor, Ottawa, CA, 1996 | decisionaid.ohri.ca/decaids‐archive.html | |
| Natural health products | No | Legare, Quebec City, CA, 2006 | ||
| Use of antibiotics for acute | Yes | Legare, Quebec City, CA, 2007 | www.decision.chaire.fmed.ulaval.ca/index.php?id=192&L=2 | |
| Advanced colorectal cancer chemotherapy | Yes | Princess Margaret Hospital, Toronto, 2011 | ||
| Breast cancer genetic testing | No | Lerman/Schwartz, Washington DC, US, 1997 | ||
| Prenatal screening | No | Leung, Hong Kong, China, 2001 | ||
| Colorectal cancer | Yes | Lewis, University of North Carolina, Chapel Hill, NC, USA, 2010 | decisionsupport.unc.edu/CHOICE6/ | |
| Depression treatment | Yes | Loh, Freiburg, GER | (emailed to us by author ‐ in German) | |
| Atrial fibrillation treatment | No | McAlister/Laupacis, Ottawa CA, 2000 | decisionaid.ohri.ca/decaids‐archive.html | |
| Diabetes treatment ‐ statins | Yes | Montori, Rochester MN, US | mayoresearch.mayo.edu/mayo/research/ker_unit/form.cfm | |
| Diabetes | Yes | Marteau, King's College London, London, England, 2010 | Additional file 2 of publication | |
| Diabetes | Yes | Marteau, King's College London, London, England, 2010 | Provided by author, same DA as Mann E 2010 | |
| Mammography | Yes | Mathieu, Sydney, AU, | DA emailed by author | |
| Mammography | Yes | Mathieu, University of Sydney, AUS, 2010 | http://www.psych.usyd.edu.au/cemped/com_decision_aids.shtml | |
| Atrial fibrillation treatment | No | McAlister/Laupacis, Ottawa CAN, 2000 | decisionaid.ohri.ca/decaids‐archive.html | |
| Hormone replacement therapy | Yes, update in progress | Sigler/Bastien, Durham NC, US, 1998 | ||
| Screening after mildly abnormal pap smear | Yes | Screening & test evaluation program, School of public health, University of Sydney 2007 | ||
| BRCA1/BRCA2 gene testing | No | Miller, Fox Chase PA, US | ||
| Colorectal | Yes | University of North Carolina, Chapel Hill, NC, USA, 2007 | intmedweb.wakehealth.edu/choice/choice.html (no longer available) | |
| Hypertension treatment | No | Montgomery, UK, 2000 | ||
| Birthing options after caesarean | Yes | Montgomery, Bristol, UK, last update 2004 | www.computing.dundee.ac.uk/acstaff/cjones/diamond/Information.html | |
| Osteoporosis treatment | Yes | Montori, Mayo Foundation for Medical Education and Research, 2007 | shareddecisions.mayoclinic.org/decision‐aids‐for‐diabetes/other‐decision‐aids/ | |
| Ischaemic heart disease treatment | Yes | Informed Medical Decisions Foundation, MA,US, 2002 | informedmedicaldecisions.org/imdf_decision_aid/treatment‐choices‐for‐carotid‐artery‐disease/ | |
| Diabetes treatment | Yes | Montori or Mayo Foundation?, Rochester MN, US, | included in publication | |
| Benign prostate disease treatment | Yes | Informed Medical Decisions Foundation, MA,US, 2001 | informedmedicaldecisions.org/imdf_decision_aid/treatment‐options‐for‐benign‐prostatic‐hyperplasia/ | |
| Hormone replacement therapy | No, update in progress | Informed Medical Decisions Foundation, MA,US | informedmedicaldecisions.org/imdf_decision_aid/treatment‐choices‐for‐managing‐menopause/ | |
| Prostate cancer screening | No | Myers, Philadelphia PA, US, 1999 | ||
| Prostate cancer screening | Yes | Myers, Philadelphia PA, 1999 | ||
| Prenatal screening | Yes | Nagle, Victoria, AU | www.mcri.edu.au/Downloads/PrenatalTestingDecisionAid.pdf | |
| Birth breech presentation | Yes | Nassar, West Perth WA, AU | sydney.edu.au/medicine/public‐health/shdg/resources/decision_aids.php | |
| Hormone replacement therapy | No | O'Connor, Ottawa CA, 1996 | decisionaid.ohri.ca/decaids‐archive.html | |
| Hormone replacement therapy | No | O'Connor, Ottawa CA, 1996 | decisionaid.ohri.ca/decaids‐archive.html | |
| Osteoporosis treatment | No | Cranney, Ottawa CA, 2002 | decisionaid.ohri.ca/decaids‐archive.html | |
| Breast cancer prevention | No | Ozanne, Boston MA, US, | ||
| Prostate cancer screening | Yes | Informed Medical Decisions Foundation, MA,US, 2001 | informedmedicaldecisions.org/imdf_decision_aid/deciding‐if‐the‐psa‐test‐is‐right‐for‐you/ | |
| Colon cancer screening | Yes | Pignone, Chapel Hill NC, US, 1999 | www.med.unc.edu/medicine/edusrc/colon.htm | |
| Menorrhagia treatment | No | Protheroe, Manchester, UK | computerized decision aid, Clinical Guidance Tree ‐ no longer in existence, author sent chapter in thesis | |
| Labour | Yes | Raynes‐Greenow, Sydney,Australia, 2004 | http://www.psych.usyd.edu.au/cemped/com_decision_aids.shtml | |
| Hormone replacement therapy | No | O'Connor, Ottawa CA, 1996 | decisionaid.ohri.ca/decaids‐archive.html | |
| Hormone replacement therapy | No, update in progress | Rothert, East Lansing MI, US, 1999 | ||
| Prostate cancer screening | No | Centers for Disease Control and Prevention (CDC), US, 2010 | [No longer available] | |
| Colorectal cancer screening | Yes | Regents of the University of Michigan (copyright info), Ann Arbor MI, US, 2006 | colorectalweb.org | |
| Prostate cancer screening | Yes | Schapira, Milwaukee WI, US, 1995 | ||
| Hormone replacement therapy | Yes | Schapira, Milwaukee WI, US | computer‐based DA | |
| Colorectal | Yes | Schroy III, Boston, USA | ||
| Coronary angiogram access site | Yes | Schwalm, Hamilton, ON, Canada, 2009 | http://www.phri.ca/workfiles/studies/presentations/PtDA%20Vascular%20Access%2023‐May‐2012.pdf | |
| Breast cancer genetic testing | No | Schwartz/Lerman, Washington DC, US, 1997 | ||
| BRCA mutation prophylactic surgery | No | Schwartz, Washington DC, US | ||
| Cardiovascular prevention | Yes | Sheridan, Chapel Hill, NC, US | http://www.med‐decisions.com/cvtool/ | |
| Coronary heart | Yes | Sheridan, University of North Carolina at Chapel Hill, Division of General Internal Medicine, North Carolina, US, 2011 | http://www.med‐decisions.com/h2hv3/ | |
| Birthing options after previous caesarean | Yes (updated 2006) | Shorten, Wollongong, AU, 2000 | [email protected] or www.capersbookstore.com.au/product.asp?id=301 | |
| Bowel | Yes | Smith, Sydney, AU 2008 | sydney.edu.au/medicine/public‐health/shdg/resources/decision_aids.php | |
| Uterine fibroid treatment | Yes | Informed Medical Decisions Foundation, MA,US, 2006 | informedmedicaldecisions.org/imdf_decision_aid/treatment‐choices‐for‐uterine‐fibroids/ | |
| Colorectal cancer screening | Yes | Steckelberg, Hamburg, Germany | ||
| Breast cancer surgery | No | Street, College Station TX, US, 1995 | ||
| Atrial fibrillation treatment | Yes | Thomson, Newcastle Upon Thyne, UK | disc sent by mail | |
| Ovarian cancer risk management | No | Tiller, Randwick NSW, AU | ||
| Colorectal cancer screen | Yes | Trevena, Sydney, AU | sydney.edu.au/medicine/public‐health/shdg/resources/decision_aids.php | |
| Embryos transplant | Yes | Radboud University Nijmegen Medical Centre; 2006 | www.umcn.nl/ivfda‐en | |
| Cystic Fibrosis referral transplant | Yes | Aaron, Ottawa ON, CA, 2009 (last update 2011) | decisionaid.ohri.ca/decaids.html#cfda | |
| BRCA1/2 mutation: prophylactic surgery | Yes | vanRoosmalen, Netherlands, 1999 | see publication | |
| Breast cancer surgery | Yes | Vodermaier, Vancouver BC, CA | received by email (in German) | |
| Prostate cancer screening | Yes | Informed Medical Decisions Foundation, MA,US, 1999 | informedmedicaldecisions.org/imdf_decision_aid/deciding‐if‐the‐psa‐test‐is‐right‐for‐you/ | |
| Prostate cancer screening | No | Volk, Houston TX, US | ||
| Menorrhagia treatment | No | Vuorma, Helsinki Finland, 1996 | ||
| Colorectal cancer screening | Yes | Wakefield, Sydney, AU, | www.genetics.edu.au/Information/PublicationsBrochuresandPamphlets/Understanding%20Genetic%20Tests%20for%20Lynch%20Syndrome | |
| Breast cancer genetic testing | Yes | Wakefield, Sydney, AU, | ||
| Breast cancer genetic testing | Yes | Wakefield, Sydney, AU, | ||
| Prostate cancer screening | Yes | Oxford, UK | included in publication | |
| Diabetes mellitus type 2 treatment | Yes | Montori, Rochester MN, US | mayoresearch.mayo.edu/mayo/research/ker_unit/form.cfm | |
| Breast cancer chemotherapy | Yes | Whelan, Hamilton CA, 1995 | included in publication | |
| Breast cancer surgery | Yes | Whelan, Hamilton CA, 1997 | included in publication | |
| Prostate cancer screening | Yes | Wolf, Charlottesville VA, US, 1996 | Script in publication | |
| Colon cancer screening | Yes | Wolf, Charlottesville VA, US, 2000 | Script in publication | |
| Pregnancy termination | No | Bekker, Leeds, UK, 2002 |
| Outcome | Knowledge | Accurate risk perception | Value‐choice agreement | Uninformed | Unclear values | Participation ‐ practitioner controlled | |
| Total studies | n = 42 | n = 19 | n = 13 | n = 22 | n = 18 | n = 14 | |
| Random sequence generation | low | 35 (83.3%) | 8 (42.1%) | 7 (53.8%) | 19 (86.4%) | 17 (94.4%) | 12 (87.7%) |
| unclear | 7 (16.7%) | 11 (57.9%) | 6 (46.2%) | 3 (13.6%) | 1 (5.6%) | 2 (14.3%) | |
| high | 0 | 0 | 0 | 0 | 0 | 0 | |
| Allocation concealment | low | 30 (71.4%) | 12 (63.2%) | 11 (84.6%) | 20 (90.9%) | 17 (94.4%) | 10 (71.4%) |
| unclear | 12 (28.6%) | 7 (36.8%) | 2 (15.4%) | 2 (9.1%) | 1 (5.6%) | 4 (28.6%) | |
| high | 0 | 0 | 0 | 0 | 0 | 0 | |
| Incomplete outcome data | low | 26 (61.9%) | 11 (57.9%) | 11 (84.6%) | 15 (68.2%) | 13 (72.2%) | 10 (71.4%) |
| unclear | 16 (38.1%) | 8 (42.1%) | 2 (15.4%) | 7 (31.8%) | 5 (27.8%) | 4 (28.6%) | |
| high | 0 | 0 | 0 | 0 | 0 | 0 | |
| Selective reporting | low | 15 (35.7%) | 7 (36.8%) | 6 (46.2%) | 9 (40.9%) | 8 (44.4%) | 4 (28.6%) |
| unclear | 27 (64.3%) | 12 (63.2%) | 7 (53.8%) | 13 (59.1%) | 10 (55.6%) | 10 (71.4%) | |
| high | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other bias | low | 34 (81.0%) | 14 (73.7%) | 11 (84.6%) | 19 (86.4%) | 17 (94.4%) | 11 (78.6%) |
| unclear | 7 (16.7%) | 5 (26.3%) | 2 (15.4%) | 3 (13.6%) | 1 (5.6%) | 3 ( 21.4%) | |
| high | 1 (2.4%) | 0 | 0 | 0 | 0 | 0 | |
| Blinding of participants and personnel | low | 9 (21.4%) | 1 (5.3%) | 2 (15.4%) | 3 (13.6%) | 2 (11.1%) | 2 (14.3%) |
| unclear | 31 (73.8%) | 17 (89.5%) | 11 (84.6%) | 18 (81.8%) | 15 (83.3%) | 9 (64.3%) | |
| high | 2 (4.8%) | 1 (5.3%) | 0 | 1 (4.5%) | 1 (5.6%) | 3 (21.4%) | |
| Blinding of outcome assessment | low | 41 (97.6%) | 19 (100%) | 13 (100%) | 22 (100%) | 18 (100%) | 13 (92.9%) |
| unclear | 1 (2.4%) | 0 | 0 | 0 | 0 | 1 (7.1%) | |
| high | 0 | 0 | 0 | 0 | 0 | 0 | |
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| 12 true or false questions; scores ranging from ‐12 to +12 | immediately post | 89 | 4.9 | 103 | 2.17 | P < 0.001 | |
| 7‐item multiple choice knowledge test (unable to standardize results) | on discharge (˜ 1 month) | 49 | 15 (4.4 SD) | 58 | 10.9 (5.4 SD) | P = 0.01 | |
| 12‐item multiple choice | pre‐operatively | 66 | 14%* | 67 | 8%* | *mean increase from baseline P = 0.02 | |
| 10‐item yes/no/unsure general knowledge test about natural health products (not specific to outcomes of options) | change scores from baseline to 2 weeks | 43 | 0.86 ± 1.77 P = 0.002 | 41 | 0.51 ± 1.47 P = 0.031 | No difference between groups (P = 0.162) | |
| 14 items survey | immediately post | No difference in level of knowledge between groups | |||||
| 9 item ‐ 4 concept questions and 5 numeric questions | 351 | 357 | Significantly higher mean increase for the intervention group (2.62 ) compared to control group (0.68) from baseline, P < 0.001 | ||||
| 8 items survey | 2‐week, 2‐month, and 6‐month follow‐ups | Intervention type had no impact on general or specific knowledge | |||||
| Good level knowledge was scored higher than the mid point of the knowledge scale (greater than 4) | 88% (147/167) in DA group compared to 72% (123/171) pamphlet group. Odds ratio (3.43 95%CI 1.79 to 6.58) | ||||||
| Change in knowledge from baseline | post‐test | 15 | 48% to 64% | 15 | 45% to 57% | change in knowledge score was significant for decision aid (P = 0.01) but not control (P = 0.13) | |
| 10‐item knowledge index score | 2 weeks | 308 | 7.44 | 290 | 6.9 | P = 0.001 | |
| 24‐items adapted from existing prostate cancer knowledge measures | immediately post | 100 | 100 | the total mean standardized knowledge score was 84.38 (SD 12.38). | |||
| Adequate knowledge (positive score: understanding benefits/harms) | 1 month | 134 | 28/134 | 137 | 8/137 | P = 0.0001 | |
| 12‐item true/false/don't know | post‐test | 468 | 75% (range 0 to 100) | 522 | 25% (range 0 to 100) | P < 0.0001 | |
| 14‐item ‐ 9 addressed by decision aid; 5 were not | immediately post | 52 | 46 | Mean difference between groups 2.4 (95% CI 1.5 to 3.3) P < 0.05 (when decision aid administered during the consultation only ‐ not if prior to the consultation) | |||
| Detailed versus simple DA | |||||||
| 2 weeks | 233 | 223 | Significant improvement in knowledge with no difference between groups (entertainment decision aid or audio‐booklet) | ||||
| CI: confidence interval; DA: decision aid; SD: standard deviation | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| Expectation of benefit index 11 items score from 1 to 4 with lower score indicating better knowledge | post (after reviewing DA) | 127 | 2.3 | 129 | 2.6 | P = 0.001 | |
| 3 of 8 multiple choice items in the knowledge test (question 4, 5, 7) | 2 weeks post | total knowledge reported only | |||||
| 5 item numerical questions (max = 5) | post | 113 | 3.02 | 189 | 2.45 | P < 0.001 | |
| 2‐week, 2‐month, and 6‐month follow‐ups | Intervention type had no impact on risk perceptions | ||||||
| 8 numerical questions (max = 8) | 357 | 2.93 (SD 2.91) | 173 | 0.58 (SD1.28) | P < 0.001 | ||
| immediately | 52 | 46 | Difference between group OR 22.4 (95% CI 5.9 to 85.8) when decision aid administered during the consultation only (not if prior to) OR 6.7 (95% CI 2.2 to 19.7) when the decision aid administered prior to or during the consultation | ||||
| CI: confidence interval; DA: decision aid; OR: odds ratio; SD: standard deviation | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| Percent match procedures described by Sepucha et al (2007; 2008). For values items were most predictive and used to specify logistic models to estimate predicted probability of selecting surgery > 0.5. | post intervention | 75 | 77 | The intervention group experienced a more rapid early improvement in value concordance immediately after the intervention compared to control, see Figure 2. | |||
| Concordance between patient's preferences and values for potential outcomes related to the decision and the choice made | within weeks | 155 | 151 | Men assigned to the decision aid who chose not to have a PSA test rated their concern about prostate cancer lower than did men who requested a PSA test. Men assigned to usual care provided similar ratings of concern about prostate cancer regardless of their PSA decision. There was no statistically significant difference between groups. | |||
| Women valuing of non chemical aspect of nature health products was positively associated with their choice of nature health products, P = 0.006. | |||||||
| Association between values and choice | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | No difference; between group differences were not reported | |
| Congruence between personal values and decision | 3 weeks | 70 | 70 | Patient choices were consistent with their values across both randomised groups | |||
| Detailed versus simple DA | |||||||
| Correlation between expected utilities and their likelihood of taking hormones | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | Simple DA showed lower correlations between expected value of hormones and likelihood of taking hormones than did more detailed DA | |
| My decision was consistent with my personal values. (Likert Scale, ranged from 1‐5) | 4‐5 weeks after intervention | 103 | 87.5 (SD 20) | 112 | 80 (SD 22.5) | P < 0.01 | |
| multi‐nomial logistic regression analysis | No significant difference between groups | ||||||
| DA: decision aid; SD: standard deviation | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| Total Decisional Conflict‐ change from baseline (standardised values) | immediately post | 75 | mean (‐20) SD (19.44) | 77 | mean (‐11.8) SD (22.83) | P = 0.03 | |
| Decisional conflict scale | uncertainty | ‐3.61 units | P = 0.04 | ||||
| uninformed | No significant difference | ||||||
| unclear values | ‐3.57 units | P = 0.002 | |||||
| unsupported | No significant difference | ||||||
| Ineffective decision | No significant difference | ||||||
| total | ‐1.75 units | P = 0.07 | |||||
| Decisional conflict scale | immediately post | DCS was higher in the intervention group compared to control, P < 0.001. | |||||
| Decisional conflict ‐ sub‐scales only | Feeling uninformed | 155 | 23.37 | 151 | 29.68 | P < 0.05 | |
| Feeling unclear values | 155 | 32.25 | 151 | 37.93 | P < 0.05 | ||
| Feeling supported | 155 | 30.51 | 151 | 35.21 | P < 0.05 | ||
| Feeling uncertain | 155 | 151 | No difference | ||||
| Effective decisions | 155 | 151 | No difference | ||||
| Decisional conflict | immediately after office visit | 196 | 1.54 | 75 | 1.58 | No difference | |
| Decisional conflict scale median (range) | 1‐2 weeks post intervention | 107 | 26 (range 0‐79) | 100 | 26 (range 0‐67) | No difference | |
| Based on approaches suggested by Marteau et al. (informed choice) | immediately after intervention | 91 | 71% | 110 | 64% | P = 0.24 | |
| Decisional conflict | post consultation | 15 | 15 | Both groups showed lower decisional conflict post‐consultation (P < 0.001) but no difference between groups | |||
| Decisional conflict | immediately post | The total mean score was 24.5 with a SD of 15.25 (n=200) | |||||
| Decisional conflict | 12 of 16 items of the original scale | Significant longitudinal impact of the decision aid was moderated by baseline decision status; decision aid led to significant decreases in decisional conflict for those who were undecided at the time of randomisation | |||||
| Decisional conflict | post consultation | 53 | 56 | Difference between decision aid and control group were ‐0.18 (95% CI ‐0.34 to ‐0.01). P = 0.036 | |||
| 3‐months post | 51 | 55 | Difference between decision aid and control group were ‐0.15 (95% CI ‐0.37 to 0.06), no significant difference. | ||||
| 15 item questionnaire (1‐5) ‐ satisfaction‐uncertainty | post intervention, pre IVF | 124 | 72.5 | 128 | 75 | P = 0.76 | |
| 15 item questionnaire (1‐5) ‐ informed (includes some items from DCS). | post intervention, pre IVF | 124 | 77.5 | 128 | 87.5 | P = 0.001 | |
| Decisional conflict | immediately post | 52 | 46 | Mean difference indicates statistically significantly lower decisional conflict for decision aid compared to usual care. Total DCS ‐10.6 (‐15.4 to ‐5.9) Uncertain ‐12.8 (‐18.4 to ‐7.3) Informed ‐17.3 (‐22.6 to ‐12.0) if administered during consult ‐6.6 (‐14.3 to ‐1.1) if administered prior to consult Values clarity ‐8.5 (‐15.7 to ‐1.3) Support ‐9.4 (‐14.8 to ‐3.9) Effective decision ‐10.0 (‐15.0 to ‐5.0) | |||
| CI: confidence interval; DA: decision aid; DCS: decisional conflict scale; IVF: in vitro fertilisation; SD: standard deviation | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| Total DCS | 2 week follow‐up | 357 | 13.63 (SD 20.55) | 173 | 14.91(SD 18.34) | P = 0.02 | |
| Detailed versus simple DA | |||||||
| Uncertainty | 2 weeks | 39 | 5.8 (SD 18.0) | 48 | 6.8 (SD 18.0) | P = 0.80 | |
| Informed | 2 weeks | 39 | 9.1 (SD 26.0) | 46 | 18.8 (SD 26.1) | P = 0.09 | |
| Values | 2 weeks | 40 | 17.4 (SD 36.8) | 48 | 34.9 (SD 36.6) | P = 0.03 | |
| Social Support | 2 weeks | 39 | 17.8 (SD 29.6) | 48 | 27.6 (SD 29.5) | P = 0.12 | |
| Total DCS | 2 weeks | 38 | 12.0 (SD 21.9) | 46 | 21.7 (SD 21.8) | P = 0.04 | |
| DA: decision aid; DCS: decisional conflict scale; SD: standard deviation | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| Discussed feeding with physician, nurse practitioner, or physician's assistant | 3 months | 126 | 46% | 127 | 33% | P = 0.04 | |
| Discussed feeding with other nursing home staff | 3 months | 126 | 64% | 127 | 71% | P = 0.42 | |
| OPTION scale | analysis of the consultation using video‐recorded consultations | 101 | Mean of 26.6 (95% CI 24.9 to 8.2) | 103 | Mean of 7% (95%CI 5.9 to 8.1) | Significantly greater in the intervention arm | |
| DCS / Dolan's Provider DCS | immediately post | Difference 0.26 (95%CI ‐0.06 to 0.53, P = 0.06) | |||||
| OPTION 100 point Scale | analysis of the consultation using video‐recorded consultations | 38 | 49.8 | 32 | 27.3 | P < 0.001 | |
| OPTION Scale | analysis of the consultation using video‐recorded consultations | 48 used decision aid within consultation | 49.7% (SD 17.74) | 37 usual care | 27.7% (SD 11.75 | MD 21.8 (95% CI 13.0, 30.5) for decision aid vs usual care. All but 2 of the 12 items significantly | |
| Discussed CHD with doctor | patient reported immediately post | 16/41 decision aid pre‐consult with summary report to bring to consult |
| 8/34 usual care |
| absolute difference 16%; 95% CI | |
| Plan to reduce CHD risk & discussed with doctor | patient reported immediately post | 15/41 decision aid pre‐consult with summary report to bring to consult |
| 8/34 usual care |
| absolute difference 13%; 95% CI ‐7% to 34%). | |
| Plan to reduce CHD risk & not discussed with doctor | patient reported immediately post | 37/41 decision aid pre‐consult with summary report to bring to consult |
| 25/34 usual care | absolute difference 16%; 95% CI | ||
| OPTION Scale | analysis of the consultation using video‐recorded consultations | 1/2 used decision aid prior to consult and 1/2 used it during consult | usual care | Greater patient participation (MD 4.4; 95% CI 2.9 to 6.0) in decision aid compared to usual care | |||
| Detailed versus simple DA | |||||||
| Agreement between women’s and physicians’ decisional conflict scores | immediately post | 87 | ICC = 0.44 (95% CI 0.25 to 0.59) | 80 | ICC = 0.28 (95% CI 0.06 to 0.47) | Agreement measure was higher for the DA group. | |
| DCS / Dolan's Provider Decision Process Assessment Instrument | immediately post | 97 detailed decision aid pre consult | ICC 0.44 (0.9 SD) | 87 simple decision aid pre consult | ICC 0.28 (1.0 SD) | Agreement measure was higher for the DA group (ICC 0.44; 95% CI 0.25 to 0.59) than for the pamphlet group (ICC 0.28; 95% CI 0.06 to 0.47) | |
| Informed decision making | analysis of the physician–patient encounter using audio‐recordings | 3.0 items | 2.4 items | RR 1.30 (CI 1.03 to 1.64) P = 0.029 | |||
| CHD: coronary heart disease; CI: confidence interval; DA: decision aid; DCS: decisional conflict scale; ICC: intraclass correlation coefficient; OPTION scale: observing patient involvement scale; RR: risk ratio; SD: standard deviation | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| control preferences ‐ patients choosing active/ collaborative decision making | post intervention | 291 | 95% | 334 | 92% | No difference | |
| control preferences did not change | post intervention | 291 | 92% | 334 | 87% | No difference | |
| control preferences changed to passive | post intervention | 291 | 3% | 334 | 5% | No difference | |
| control preferences changed to active/ collaborative | post intervention | 291 | 3% | 334 | 7% | No difference | |
| COMRADE used to measure patients' perceived involvement in decisions | post‐consultation | 49 | 79.5 (SD 18.6) 76.8 (SD 20.9) | 58 | 69.7 (SD 20.0) 73.5 (SD 19.3) | increased patient involvement in decision aid group post intervention compared to usual care at baseline. At discharge there was no difference between groups. | |
| surrogates feeling somewhat or very involved in decision making | post intervention | 83% | 77% | P = 0.18 | |||
| achieved decision involvement | post intervention | 32% | 35% | No difference | |||
| patients' perceived involvement in decision making | post‐consultation | 191 | 26.3 pre 28.0 post | 96 | 24.5 pre 25.5 post | Improved patient participation from baseline to post exposure to the decision aid (P = 0.010) and in comparison to the usual care group (P = 0.003) but there was no change in the control group for the pre‐post comparison | |
| adapted from the Control Preferences Scale | post‐intervention | the total mean scores were: 2.74±1.25 (n=99) pre and 2.83±1.16 (n=199) post, no statistically significant difference. | |||||
| Decision Evaluation scale (15 item questionnaire) Decision control subscale | post‐consultation | 124 | 85 | 128 | 87.5 | P = 0.33 | |
| DA: decision aid; SD: standard deviation | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| single item ‐ ranging from '0 = completely undecided' to '100 = made my decision' | No difference | ||||||
| DA: decision aid | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| 1‐item; pleased with treatment choice | 1 month post‐surgery | 62/66 | 55/67 | P = 0.03 | |||
| satisfaction with decision scale: median (range) | 1 month post intervention | 107 | 22(13‐25) | 100 | 21(15‐25) | No difference | |
| 7‐point scale: ranging from 1‐7 | 4 weeks | 91.17 (14) | 91.33(14.50) | No difference | |||
| 6‐item | 1, 6, 12 months | 100 | 114 | Overall, no difference between groups; decision aid led to significantly increased satisfaction compared to US among those who were undecided at randomisation but not among those who had made a decision before randomisation; (only graph in paper with no raw data) | |||
| satisfaction with the decision | immediately post | 134 | 137 | No difference (P = 0.56) | |||
| Detailed versus simple DA | |||||||
| 6‐item scale (measured on 1 to 5) | 1 day | 83 | 4.0 (0.56) | 89 | 3.8 (0.66) | No difference | |
| 6 months | 63 | 3.8 (0.63) | 75 | 3.8 (0.67) | No difference | ||
| 12 months | 62 | 3.9 (0.62) | 74 | 3.9 (0.67) | No difference | ||
| 6‐item scale | 3 months | No difference | |||||
| DA: decision aid | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| Effectiveness of consultation ‐ patient assessment. Single item 1 (not at all effective) to 7 (extremely effective) | 106 | 6.6 | 105 | 6.6 | No difference | ||
| Effectiveness of consultation ‐ counsellor assessment. Single item 1 to 7 | 5.9 | 5.8 | No difference | ||||
| Satisfaction with decision process (0 for strongly agree to 5 for strongly disagree) | 101 | 103 | Patients in DA group reported greater satisfaction with the DM process (strongly agree, 61% DA vs 40% usual care) | ||||
| Measured satisfaction with opportunities to participate in decision making using a single item | Compared to usual care, women who received the decision aid followed by nurse coaching were statistically significantly more satisfied with the opportunities to participate in decision making (OR 1.5; 95% CI 1.1 to 2.0). | ||||||
| Satisfaction with information received sub‐scale 4‐item (0 to 100; low to high) | average 10 days | 54 | 76 (15.5 SD) | 56 | 59 (23.3 SD) | P = 0.001 | |
| Satisfaction with practitioner treatment during decision process sub‐scale 4‐item (0 to 100; low to high) | average 10 days | 54 | 69 (25.3 SD) | 56 | 54 (26.7 SD) | P = 0.004 | |
| Satisfaction with cancer information service 1‐item (1 to 5; low to high) | 2 weeks | 4.37 (0.84 SD) | 4.38 (0.86 SD) | No difference | |||
| 6 months | 4.51 (0.75 SD) | 4.51 (0.64 SD) | No difference | ||||
| (7 point scales) Participants' satisfaction with knowledge transfer ‐amount of information ‐clarity of information ‐helpfulness of the information ‐would want other decisions ‐recommend to others | post intervention | 49 | 6.6 6 6 6.1 6.4 | 46 | 6.3 6 5.8 5.8 6.2 | P = 0.798 P = 0.296 P = 0.624 P = 0.248 P = 0.435 | |
| Clinicians' satisfaction with knowledge transfer ‐helpfulness of the information ‐would want other decisions ‐recommend to others | post intervention | 39 | 5.8 6.1 5.9 | 33 | 5.2 4.9 4.8 | P = 0.006 P < 0.001 P < 0.001 | |
| Satisfaction with information about medicines | 4 months post | 16 | 10.4 (SD 2.9) | 17 | 10.1 (SD 2.2) | No difference | |
| ‐ physician helped me understand ‐ physician understood important to me ‐ physician answered questions ‐ satisfied with involvement ‐ satisfied with physician's involvement ‐ satisfied with process | 1 week follow‐up | 53 | 49 (92.5%) 47 47 44 36 42 | 56 | 53 (94.6%) 50 51 45 36 50 | High satisfaction with no difference by group | |
| Detailed versus simple DA | |||||||
| Satisfaction with decision making process 7‐item scale (5‐point response) | 3 months | 171 | separate responses provided with no total | 172 | separate responses provided with no total | No difference except DA more likely to report they had as much information as they wanted and less likely to report having relied too much on physician's opinion | |
| Satisfaction with genetic counselling 11‐item short form (range 4 to 44; low to high) | immediately post | 116 | 37.27 (5.74 SD) | 126 | 40.48 (4.26 SD) | P < 0.001 higher satisfaction with individual counselling compared to decision aid | |
| Satisfaction with involvement in decision making (3 questions) | 26 to 30 weeks gestation | 244 | 44.8 44.3 72.6 | 252 | 49.2 48.1 79.9 | P = 0.40 P = 0.45 P = 0.10 | |
| DA: decision aid; SD: standard deviation | |||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA versus usual care | |||||||
| Preparation for Decision Making Scale | Pre‐consultation | 43 | 35 (median) | 40 | 20.5 (median) | P = 0.0001 | |
| Preparation for Decision Making Scale | 3 weeks | 70 | 65.1 (24.9 SD) | 79 | 53.9 (27.1 SD) | P = 0.009 | |
| Detailed versus simple DA | |||||||
| Preparation for Decision Making Scale | Post‐physician consultation | 48 | 28 (6.1 SD) | 42 | 27(5.5 SD) | No difference | |
| DA: decision aid; SD: standard deviation | |||||||
| Study | Type of comparison | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| Other elective surgery ‐ uptake | ||||||
| DA versus usual care | 127 | 1 | 129 | 3 | No difference | |
| DA versus usual care | No difference | |||||
| Other elective surgery ‐ preference | ||||||
| Detailed versus simple DA | 32 | 13 | 31 | 14 | No difference | |
| Screening ‐ Breast cancer genetic testing ‐ uptake | ||||||
| Detailed versus simple DA | No difference | |||||
| Detailed versus simple DA | No difference | |||||
| Screening ‐ Breast cancer genetic testing ‐ preference | ||||||
| DA versus usual care | The intervention decreased intention to obtain genetic testing among women at average risk, but increased in women at high risk | |||||
| Screening ‐ Cardiac stress testing ‐ uptake | ||||||
| DA versus usual care | 101 | 58% | 100 | 77% | P < 0.0001 | |
| Screening ‐ Colorectal cancer genetic testing ‐ uptake | ||||||
| Detailed versus simple DA | No difference | |||||
| Screening ‐ Breast screening ‐ uptake | ||||||
| DA versus usual care | No difference | |||||
| DA versus usual care | 117 | 82% | 209 | 61% | P < 0.001 | |
| Screening ‐ Diabetes ‐ uptake | ||||||
| DA versus usual care | 633 | 353 | 639 | 368 | P = 0.51 | |
| Screening ‐ Diabetes ‐ preference | ||||||
| DA versus usual care | 273 | 134 | No difference | |||
| Screening ‐ Prenatal ‐ uptake | ||||||
| DA versus usual care | No difference | |||||
| DA versus usual care | 184 | 50% | 206 | 53.8% | No difference | |
| DA versus usual care | No difference | |||||
| Screening ‐ PSA ‐ uptake | ||||||
| DA versus usual care | The experimental interventions led to significant reductions in requests for prostate‐specific antigen tests ( ˜2 times greater decline). | |||||
| Medication ‐ Antibiotics for upper respiratory infections ‐ uptake | ||||||
| DA versus usual care | 81 | 33 | 70 | 49 | P = 0.08 | |
| Medication ‐ Cardiovascular disease ‐ preference | ||||||
| DA versus usual care | 79 | 63% | 78 | 42% | P < 0.01 | |
| Medication ‐ Breast cancer prevention ‐ uptake | ||||||
| DA versus usual care | 382 | 0.5% | 100 | 0% | No difference | |
| Medication ‐ Chemotherapy for advanced cancer | ||||||
| DA versus usual care | 107 | 77% | 100 | 71% | No difference | |
| Medication ‐ Hormone replacement therapy ‐ uptake | ||||||
| DA versus usual care | 8% decrease in DA group, not statistically significant | |||||
| Detailed versus simple DA | No difference | |||||
| Medication ‐ Natural heath products ‐ preference | ||||||
| DA versus usual care | 41% | 41% | No difference | |||
| Medication ‐ Anti‐thrombosis ‐ uptake | ||||||
| DA versus usual care | 25% decrease in DA group, not statistically significant | |||||
| DA versus usual care | No difference | |||||
| DA versus usual care | 93.8% | 25% | risk ratio 0.27 (95% CI 0.11 to 0.63) | |||
| Medication ‐ Hypertension ‐ uptake | ||||||
| DA versus usual care | No difference | |||||
| Medication ‐ Chemotherapy for breast cancer ‐ preference | ||||||
| DA versus usual care | No difference | |||||
| Medication ‐ Osteoporosis ‐ uptake | ||||||
| DA versus usual care | 52 | 44% | 48 | 40% | No difference | |
| Medication ‐ Immunotherapy ‐ uptake | ||||||
| DA versus usual care | No difference | |||||
| Medication ‐ Schizophrenia treatment ‐ uptake | ||||||
| Hamann 2006 ‐ prescriptions | DA versus usual care | No difference | ||||
| Hamann 2006 ‐ psycho‐education | DA versus usual care | Higher uptake in DA group (P = 0.003) | ||||
| Obstetrics ‐ Birth control method ‐ preference | ||||||
| DA versus usual care | 114 | 108 | No difference in the methods chosen between groups, participants in the intervention group were not more likely to initiate the requested method immediately compared to those in | |||
| Obstetric ‐ Childbirth procedure ‐ uptake | ||||||
| DA versus usual care | No difference | |||||
| DA versus usual care | No difference | |||||
| Obstetric ‐ Childbirth procedure ‐ preference | ||||||
| DA versus usual care | No difference | |||||
| Obstetric ‐ Embryo transplant ‐ uptake | ||||||
| van Peperstraten 2010 ‐ single embryo transfer | DA versus usual care | 152 | 43% | 156 | 32% | P = 0.05 |
| Obstetric ‐ Pain relief in labour ‐ uptake | ||||||
| Detailed versus simple DA | 308 | 146 | No difference | |||
| Other‐ Lung transplant referral | ||||||
| DA versus usual care | No difference | |||||
| Other ‐ Pre‐operative blood transfusion ‐ uptake | ||||||
| DA versus usual care | No difference | |||||
| Vaccine ‐ Flu shot ‐ uptake | ||||||
| DA versus usual care | 48 | 46% | 59 | 27% | No difference | |
| Vaccine ‐ Hepatitis B ‐ uptake | ||||||
| DA versus usual care | Significant increase of 76% in the DA group | |||||
| DA: decision aid; OR: odds ratio | ||||||
| Reference | Scale used | N Decision aid | Mean (SD) Decision aid | N Comparison | Mean (SD) Comparison | Notes |
| DA versus usual care | ||||||
| 3 months ‐ Using a contraceptive method that was in the same effectiveness group as the method requested at enrolment, 'very effective', as chosen option ‐ eg. if chose sterilization and ended up using an IUD counted as adhering | 48 | 85% | 52 | 77% | P = 0.28 | |
| 3 months ‐ Using a contraceptive method that was in the same effectiveness group, 'effective', as chosen option | 41 | 68% | 31 | 68% | P = 0.96 | |
| 6 to 8 weeks ‐ Patient reported ‐ 5‐point Likert scale on steadiness of following the treatment plan: 1‐very bad to 5‐very good | 191 | 4.3 (0.9) | 96 | 3.9 (1.0) | P = 0.073 | |
| 6 to 8 weeks ‐ Physician reported ‐ 5‐point Likert scale steadiness of following the treatment plan: 1‐very bad to 5‐very good | 191 | 4.8 (0.6) | 96 | 4.3 (1.1) | P = 0.56 | |
| 3 months ‐ telephone administration of the 8‐item Morisky adherence (7 yes/no items and 1 item with 5 point Likert scale to elicit behaviours such as skipping medicines when they have no symptoms) | 70% of participants reported good adherence to statins with no difference between groups | |||||
| 6 months ‐ telephone administration of the 8‐item Morisky adherence (7 yes/no items and 1 item with 5 point Likert scale to elicit behaviours such as skipping medicines when they have no symptoms) | 80% of participants reported good adherence to statins with no difference between groups | |||||
| 6 months ‐ Self reported – Measured % of patients taking therapy initially chosen | 129 | 95.35% | 134 | 93.28% | P = 0.44 | |
| ˜ 3 years ‐ Self reported – 6 item Adherence Questionnaire: from "I take all my tablets at the same time of day" to "I take hardly any of my tablets“ | No difference | |||||
| 6 months ‐ Percentage of participants that self‐reported currently taking medication who have not missed one dose within last week | 17 | 65% | 19 | 63% | P = 0.92 | |
| 6 months ‐ Percentage of participants who opted to take biophosphonates who took their medication on more than 80% of the days for which it was prescribed, based on pharmacy records | 23 | 100% | 19 | 74% | P = 0.009 | |
| 6 months ‐ Pharmacy records ‐ days covered (range) | 48 | 97.5% (range 0 to 100) | 37 | 100 (range 73.9 to 100) | AMD −8.88 (−13.6% to −4.14%) Positive AMD favours decision aid arm. This finding is statistically significant | |
| 6 months ‐ Self reported by telephone call – did not miss a dose in last week | 41 | 76% | 31 | 81% | OR 0.74 (95% CI 0.24‐2.32) | |
| 4 months ‐ Extent to which the patients' behaviour in taking medications coincides with the clinical prescription | 16 | 10.4% (32) [improvement from baseline] | 17 | 2% (26) [improvement from baseline] | Not significant | |
| 3 month ‐ adherence to initial choice post intervention | ||||||
| Any therapy promoted in decision aid | 76 | 45 (59%) | 73 | 25 (34%) | P < 0.01 | |
| any therapy promoted in decision aid + others (eg. diet or physical activity) | 77 | 64 (83%) | 77 | 52 (68%) | P = 0.02 | |
| aspirin | 32 | 30 (94%) | 19 | 11 (58%) | P < 0.01 | |
| cholesterol medicine | 14 | 12 (86%) | 6 | 5 (83%) | The intervention had little effect blood pressure or cholesterol medication, | |
| blood pressure medicine | 9 | 9 (100%) | 12 | 11 (92%) | ||
| stop smoking | 8 | 25% | 5 | 20% | No effect on smoking, although subgroups were small | |
| 3 months ‐ Self reported – mailed surveys & telephone call to non‐respondents on adherence to statin use: missed 1 dose or more within the last week. | 33 | 93.94% | 29 | 79.31% | No difference in adherence when analysis adjusted by sex, cardiovascular disease, and number of medications | |
| Detailed versus simple DA | ||||||
| 12 months ‐ Self reported – Telephone call to patients to ask estimated days missed per week and reasons Response categories: 1) taking medication as prescribed (omitting no more than one day/week) , 2) missing doses occasionally and randomly, 3) systematically deviating from the prescribed directions | 16 | ˜72% | 20 | ˜72% | No difference | |
| 12 months ‐ Self reported – daily adherence recorded on a calendar | 62 | ˜89% | 74 | ˜89% | No difference | |
| 1 month ‐ faecal occult blood test uptake | 134 | 5.2% | 137 | 6.6% | P = 0.64 | |
| DA: decision aid; OR: odds ratio | ||||||
| Reference | Timing | N Decision aid | Mean Decision aid (SD) | Change from baseline | N Comparison | Mean Comparison (SD) | Change from Baseline | Notes |
| General health ‐ DA versus usual care | ||||||||
| Barry 1997 (SF‐36) | Baseline | 104 | 67.2 (19.0) | 123 | 71.1 (17.6) | P = 0.02 | ||
| 3 months | ‐0.96 (1.41) | ‐3.59 (1.57) | ||||||
| 6 months | ‐1.46 (1.41) | ‐4.93 (1.45) | ||||||
| 12 months | 0.61 (1.58) | ‐4.99 (1.44) | ||||||
| Legare 2011 (percentage of people who felt they had a stable and better health, (SF‐12)) | 2 weeks post | not reported | 94 | +7 | not reported | 85 | ‐6 | P = 0.08 |
| Morgan 2000 (SF‐36) | 6 months post | 72 | 62 (23) | +4.0 | 88 | 65 (20) | +7.0 | No difference |
| Kennedy 2002 (SF‐36) | 2 years | 176 | 157 | No difference | ||||
| Vuorma 2003 (RAND‐36) | 1 year | 156 | 2.2 | 159 | 2.8 | No difference | ||
| Physical function ‐ DA versus usual care | ||||||||
| Barry 1997 (SF‐36) | Baseline | 104 | 81.9 (20.0) | 123 | 83.0 (18.9) | P = 0.02 | ||
| 3 months | ‐0.34 (1.61) | ‐1.81 (1.07) | ||||||
| 6 months | 0.10 (1.28) | ‐3.26 (1.37) | ||||||
| 12 months | 0.15 (1.40) | ‐3.74 (1.18) | ||||||
| Morgan 2000 (SF‐36) | 6 months post | 72 | 67 (29) | +7.0 | 88 | 71 (24) | +10.0 | No difference |
| Kennedy 2002 (SF‐36) | 2 years | 176 | 157 | No difference | ||||
| Vuorma 2003 (RAND‐36) | 1 year | 156 | 2.4 | 159 | 2.2 | No difference | ||
| Physical function ‐ Detailed versus simple DA | ||||||||
| Bernstein 1998 (SF‐12) | 3 months post | 61 | 38 (12.1) | +0.6 | 48 | 37.6 (10.6) | +3.8 | No difference |
| Social function ‐ DA versus usual care | ||||||||
| Barry 1997 (SF‐36) | Baseline | 104 | 90.6 (15.5) | 123 | 91.7 (15.7) | P = 0.17 | ||
| 3 months | 0.34 (1.58) | ‐2.26 (1.36) | ||||||
| 6 months | ‐0.05 (1.92) | ‐2.46 (1.45) | ||||||
| 12 months | ‐1.46 (1.85) | ‐3.52 (1.71) | ||||||
| Kennedy 2002 (SF‐36) | 2 years | 176 | 157 | No difference | ||||
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 84.7 | 71 | 82.1 | P = 0.39 | ||
| Vuorma 2003 (RAND‐36) | 1 year | 156 | 5.2 | 159 | 7.1 | No difference | ||
| Mental function ‐ DA versus usual care | ||||||||
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 71.3 | 71 | 71.6 | P = 0.46 | ||
| Kennedy 2002 (SF‐36) | 2 years | 176 | 157 | No difference | ||||
| Vuorma 2003 (RAND‐36) | 1 year | 156 | 4.7 | 159 | 5.3 | No difference | ||
| Mental function ‐ Detailed versus simple DA | ||||||||
| Bernstein 1998 (SF‐12) | 3 months post | 61 | 49.1 (11.4) | 0.0 | 48 | 48.9 (10.8) | +0.9 | No difference |
| Role function ‐ DA versus usual care | ||||||||
| Morgan 2000 (SF‐36) | 6 months post | 72 | 62 (44) | +20.0 | 88 | 58 (43) | +15.0 | No difference |
| Kennedy 2002 (SF‐36) | 2 years | 176 | 157 | P = 0.04 | ||||
| Vuorma 2003 (RAND‐36) | 1 year | 9.2 | 6.3 | No difference | ||||
| Bodily pain ‐ DA versus usual care | ||||||||
| Morgan 2000 (SF‐36) | 6 months post | 72 | 81 (22) | +6.0 | 88 | 77 (24) | +5.0 | No difference |
| Kennedy 2002 (SF‐36) | 2 years | 176 | 157 | No difference | ||||
| Vuorma 2003 (RAND‐36) | 1 year | 156 | 6.5 | 159 | 6.2 | No difference | ||
| Role emotional ‐ DA versus usual care | ||||||||
| Kennedy 2002 (SF‐36) | 2 years | 176 | 157 | No difference | ||||
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 80.3 | 71 | 77.4 | P = 0.61 | ||
| Vuorma 2003 (RAND‐36) | 1 year | 156 | 12.6 | 159 | 1.9 | P = 0.01 | ||
| Energy/vitality ‐ DA versus usual care | ||||||||
| Kennedy 2002 (SF‐36) | 2 years | 176 | 157 | No difference | ||||
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 55.2 | 71 | 54.1 | P = 0.09 | ||
| Vuorma 2003 (RAND‐36) | 1 year | 156 | 8.9 | 159 | 8.8 | No difference | ||
| SF‐36 all dimensions ‐ DA versus usual care | ||||||||
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 47 | 71 | 46.3 | P = 0.35 | ||
| Murray 2001b (SF‐36) | 9 months | 93 | 94 | No difference | ||||
| Murray 2001a (SP‐36) | 9 months | 54 | 48 | No difference | ||||
| Functional status ‐ DA versus usual care | ||||||||
| Deyo 2000 (Roland Disability Questionnaire) | 1 year | 171 | 20.4 | +5.4 | 173 | 20.9 | +5.7 | No difference |
| Leighl 2011 (FACT‐G) median (range) | 1 month post | 74 | 17 (6‐28) | 68 | 17.5 (7‐28) | P = 0.02 | ||
| Health utilities ‐ DA versus usual care | ||||||||
| Murray 2001a (Euroqol EQ‐5D) | No difference | |||||||
| Murray 2001b (Euroqol EQ‐5D) | No difference | |||||||
| DA: decision aid; SF‐36: Medical Outcomes Study 36‐item Short‐Form Health Survey; SF‐12: 12‐item Short‐Form Health Survey; RAND‐36: the 36‐item short form survey from the RAND Medical Outcomes Study FACT‐G: Functional Assessment of Cancer Therapy‐General | ||||||||
| Study | Outcome | Scale used | Timing | N Decision aid | Decision aid mean change | N Comparison | Comparison mean change | Notes |
| DA versus usual care | ||||||||
| Urinary symptoms | AUA Symptom Index (0 to 100) | 3 months | 104 | ‐4.80% (1.74) | 117 | ‐1.40% (1.37) | No difference; trend toward DA | |
| Urinary symptoms | AUA | 6 months | 104 | ‐3.66% (2.06) | 117 | ‐3.17% (1.77) | No difference | |
| Urinary symptoms | AUA | 12 months | 104 | ‐2.51% (2.11) | 117 | ‐4.14% (1.66) | No difference; trend toward control | |
| Impact of symptoms | BPH Impact Index (0 to 100) | 3 months | 104 | ‐6.58% (1.10) | 117 | ‐3.00% (1.05) | No difference; trend toward DA | |
| Impact of symptoms | BPH | 6 months | 104 | ‐4.37% (1.32) | 117 | ‐3.89% (1.16) | No difference; trend toward DA | |
| Impact of symptoms | BPH | 12 months | 104 | ‐5.53% (1.32) | 117 | ‐2.63% (1.32) | No difference; trend toward DA | |
| Satisfaction | SAQ (0 to 100) | 3 months | 61 | +6.2% | 48 | +10.5% | Control significantly more satisfied | |
| Angina stability | SAQ | 3 months | 61 | +17.2% | 48 | +28.3% | No difference | |
| Angina frequency | SAQ | 3 months | 61 | +5.5% | 48 | +15.3% | No difference | |
| Disease Perception | SAQ | 3 months | 61 | +14.1% | 48 | +18.8% | No difference | |
| Physical Capacity | SAQ | 3 months | 61 | ‐0.5% | 48 | +7.1% | No difference | |
| (FACT‐G) median (range) | Physical function at 1 month post | 74 | 21 (0‐28) | 68 | 20 (4‐28) | No difference | ||
| Role emotional at 1 month post | 74 | 17 (0‐20) | 68 | 17(7‐20) | No difference | |||
| No Angina | CCVA | 6 months | 72 | +49% | 88 | +48% | No difference | |
| Class I Angina | CCVA | 6 months | 72 | ‐1% | 88 | +6% | No difference | |
| Class II Angina | CCVA | 6 months | 72 | ‐23% | 88 | ‐26% | No difference | |
| Class III Angina | CCVA | 6 months | 72 | ‐26% | 88 | ‐28% | No difference | |
| Class IV Angina | CCVA | 6 months | 72 | 0% | 88 | 0% | No difference | |
| Urinary symptoms | AUA symptom Index (0 to100) | No difference | ||||||
| Menopausal symptoms | MenQol | No difference | ||||||
| Menorrhagia specific utility scale | (0 to 100) | 6 months | 60 | 59.3 (30.0) | 56 | 50.9 (25.1) | P = 0.03 higher menorrhagia quality of life favouring DA group | |
| Strokes or bleeds requiring admission | 3 months | 51 | 55 | No strokes and no bleeds requiring admission. 1 bleed and 1 transient stroke both in control group that required GP consultation | ||||
| Ongoing pregnancies (> 12 weeks gestation) | after 1st IVF cycle | 152 | 156 | 32% of participants in the intervention group and 38% of participants in the control group had ongoing pregnancies, P = 0.25 | ||||
| Twin pregnancies (> 12 weeks gestation) | after 1st IVF cycle | 152 | 156 | 4% of participants in intervention group and 6% of participants in control group had twin pregnancies, P = 0.33 | ||||
| Inconvenience due to menstrual bleeding | (5 to 25) | 1 year | 156 | 10.4 | 159 | 10.5 | No difference | |
| Menstrual pain | (0 to 12) | 1 year | 156 | 4.7 | 159 | 4.6 | No difference | |
| Detailed versus simple DA | ||||||||
| % working | 1 year | 171 | +17.3% | 173 | +18.3% | No difference | ||
| % missed 1+ day work within past month | 1 year | 171 | ‐38.4% | 173 | ‐35.2% | No difference | ||
| Back pain severity | 1 year | 171 | ‐22.4% | 173 | ‐22% | 1 year scores: DA 27.6% significantly better than control 37.2% | ||
| Leg pain severity | 1 year | 171 | ‐42.1% | 173 | ‐43.9% | No difference | ||
| Seeking compensation | 1 year | 171 | ‐2.9% | 173 | ‐5.9% | No difference | ||
| Satisfied with symptoms | 1 year | 171 | +32.1% | 173 | +32.4% | No difference | ||
| Apgar score | scores > 7 | 1 minute after birth | 395 | 221 (82%) | 201 | 149 (75%) | P = 0.12 | |
| scores > 7 | 5 minutes after birth | 395 | 235 (90%) | 201 | 167 (84%) | P = 0.68 | ||
| Birth weight | in grams | mean (SD) | 395 | 3445 (451) | 201 | 3412 (450) | P = 0.11 | |
| AUA: American Urological Association; CCVA: Canadian Cardiovascular Angina; BPH: benign prostatic hyperplasia; DA: decision aid; SAQ: Seattle Angina Questionnaire; | ||||||||
| Study | Timing | N Decision aid | Mean Decision aid (SD) | Change from baseline | N Comparison | Mean Comparison (SD) | Change from Baseline | Notes |
| State Anxiety Inventory: < 30 days post‐intervention ‐ DA versus usual care | ||||||||
| Bekker 2004; prenatal screening | Immediately post | 50 | 58.9 (16.6) | 56 | 61.2 (13.7) | No difference | ||
| Evans 2010; PSA screening | immediately post DA | 89 | 4.98 | 103 | 4.88 | P = 0.98 | ||
| Green 2004; breast cancer screening (low risk group) | Immediately post | 56 | 29 | ‐4 | 61 | 30 | ‐3 | P = 0.04 (for difference in change score) |
| Green 2004; breast cancer screening (high risk group) | Immediately post | 50 | 30 | ‐3 | 44 | 33 | ‐5 | P = 0.04 (for difference in change score) |
| post consult, 1‐2 weeks and 4 weeks post | No difference; see Figure 3 | |||||||
| Mathieu 2007; mammography screening | immediately after | 321 | 29.61 | 315 | 29.34 | No difference | ||
| McCaffery 2010; HPV screening (state trait anxiety inventory) | 2 weeks | 77 | 10.5 | 71 | 10.6 | P = 0.25 | ||
| Montgomery 2003; hypertension | immediately post DA | 44 | 35.45 (10.52) | 50 | 37.67 (13.92) | No difference | ||
| Montgomery 2007; previous cesarean section | 37 weeks gestation | 196 | 38.7 (12.2) | 195 | 42.1 (12.2) | P = 0.016 | ||
| Nassar 2007; breech presentation | 1 week | 98 | 41.4 (12.5) | 90 | 44.4 (13.9) | No difference | ||
| Protheroe 2007; menorrhagia | 2 weeks | 59 | 11.6 (3.7) | 61 | 12.2 (3.7) | P = 0.016 | ||
| Rubel 2010; PSA screening | immediately after | 20 items adapted from state portion of State‐Trait Anxiety Inventory Scale STAI ‐ Form Y; total mean score= 1.66±0.59 (n=200) for patients in both groups | ||||||
| Smith 2010; bowel cancer screening | 2 week follow‐up | 357 | 13.67 | 173 | 14.05 | P= 0.80 | ||
| Thomson 2007; anti‐thrombotic treatment for atrial fibrillation | immediately after | 53 | 56 | Significant fall in anxiety (‐4.57) but no difference between groups (P = 0.98) | ||||
| Trevena 2008 colorectal cancer screening | immediately after | 134 | 137 | No difference (P = 0.59) | ||||
| van Peperstraten 2010; number of embryos transferred | immediately after | 152 | 27.33% | 156 | 24.5% | P = 0.14 | ||
| Whelan 2004; breast cancer surgery | 7 days post DA | 94 | 42.3 (1.3) | 107 | 41.9 (1.3) | No difference | ||
| Whelan 2003; breast chemotherapy | 7 days post DA | 82 | 45.6 | +2.2 | 93 | 47.4 | +0.8 | No difference |
| Wong 2006; pregnancy termination | Immediately post | 154 | 54 (15.8) | 159 | 54 (16.1) | No difference | ||
| State Anxiety Inventory: < 30 days post‐intervention ‐ Detailed versus simple DA | ||||||||
| Goel 2001; breast cancer surgery | 1 to 3 days post DA | 74 | 51.2 (14.2) | ‐0.7 | 43 | 50.7 (14.8) | ‐0.1 | No difference |
| Hunter 2005; prenatal screening | Immediately post | 116 | 45.50 (9.69) | ‐1.17 | 126 | 47.98 (10.14) | ‐0.37 | No difference |
| Raynes‐Greenow 2010; labour analgesia | 37 weeks gestation | 395 | 33.3 (9.3) | ‐0.6 | 201 | 34.3 (11.0) | 0 | P = 0.32 |
| Tiller 2006; prophylactic ovarian cancer treatment | 2 weeks | 58 | 38.2 (13.4) | 60 | 38.0 (15.2) | No difference | ||
| State Anxiety Inventory: 1 month post‐intervention ‐ DA versus usual care | ||||||||
| Bekker 2004; prenatal screening | 1 month post DA | 29 | 35.3 (12.5) | 39 | 34.7(14.8) | No difference | ||
| Davison 1997; prostate cancer treatment | 5 to 6 weeks post DA | 30 | 35.5 | ‐9.0 | 30 | 34.5 | ‐2.5 | No difference |
| State Anxiety Inventory: 1 month post‐intervention ‐ Detailed versus simple DA | ||||||||
| 1 month post DA | 43 | 35.4 (11.7) | 43 | 37.4 (10.7) | No difference | |||
| State Anxiety Inventory: 3 months post‐intervention ‐ DA vs usual care | ||||||||
| Murray 2001a; benign prostatic hypertrophy | 3 months post DA | 55 | 36.36 (14.99) | +2.4 | 48 | 32.08 (9.836) | +0.7 | No difference |
| Murray 2001b; hormone replacement therapy | 3 months post DA | 93 | 38.42 (10.83) | ‐0.5 | 95 | 40.53 (12.96) | +1.8 | No difference |
| Nagle 2008; prenatal screening | ˜1 to 12 weeks post DA | 167 | 37.2 (12.1) | 171 | 37.36 (12.6) | No difference | ||
| Nassar 2007; breech presentation | 3 months post DA | 86 | 29.2 (9.9) | 84 | 30.8 (10.5) | No difference | ||
| Vuorma 2003; menorrhagia treatment | 3 months post DA | 184 | 37.1 | +1.0 | 179 | 35.9 | ‐1.0 | No difference |
| Whelan 2003; breast chemotherapy | 3 months post DA | 82 | 36.0 | 93 | 37.8 | No difference | ||
| State Anxiety Inventory: 6 months post‐intervention ‐ DA versus usual care | ||||||||
| Protheroe 2007; menorrhagia | 6 months post DA | 47 | 11.2 (4.2) | 52 | 13.3 (4.9) | P = 0.067 | ||
| Whelan 2004; breast cancer surgery | 6 months post DA | 94 | 39.3 (1.3) | 107 | 38.9 (1.6) | No difference | ||
| Whelan 2003; breast chemotherapy | 6 months post DA | 82 | 38.2 | 93 | 38.2 | No difference | ||
| State Anxiety Inventory: 6 months post‐intervention ‐ Detailed versus simple DA | ||||||||
| Goel 2001; breast cancer surgery | 6 months post DA | 59 | 36.6 (12.9) | ‐15.3 | 39 | 34.3 (11.6) | ‐16.5 | No difference |
| Tiller 2006; prophylactic ovarian cancer treatment | 6 months post DA | 53 | 35.7 (9.0) | 55 | 36.2 (13.6) | No difference | ||
| State Anxiety Inventory: 12 months post‐intervention ‐ DA versus usual care | ||||||||
| Whelan 2004; breast cancer surgery | 12 months post DA | 94 | 37.5 (1.4) | 107 | 36.6 (1.5) | No difference | ||
| Whelan 2003; breast chemotherapy | 12 months post DA | 82 | 39.2 | 93 | 40.2 | No difference | ||
| Other ‐ DA versus usual care | ||||||||
| Johnson 2006; endodontic treatment | Immediately post ‐ single question 7‐point Likert scale. | 32 | 3.2 (1.7) | 35 | 3.8 (2.1) | P = 0.27 | ||
| Lewis 2010; colorectal cancer screening | intrusive thoughts ‐ 3 items; 4 point scale ‐ not at all | 139 | 66.2% | 157 | 68.0% | P = 0.92 | ||
| intrusive thoughts ‐ 3 items; 4 point scale ‐ sometimes | 66 | 31.4% | 69 | 29.9% | ||||
| intrusive thoughts ‐ 3 items; 4 point scale ‐ often | 5 | 2.4% | 5 | 2.2% | ||||
| intrusive thoughts ‐ measured using 1 item from the impact of events scale | 77 | 43% | 71 | 32% | No difference | |||
| Worry about developing bowel cancer ‐ quite or very | 357 | 6% | 173 | 8% | P = 0.78 | |||
| Worry about developing bowel cancer ‐ none or a bit | 357 | 94% | 173 | 92% | ||||
| DA: decision aid; HPV: human papilloma virus; PSA: prostate‐specific antigen | ||||||||
| Study | Timing | N Decision aid | Mean Decision aid (SD) | Change from Baseline | N Comparison | MeanComparison (SD) | Change from Baseline | Notes |
| DA versus usual care | ||||||||
| Davison 1997 (20‐item CES‐D) | 5 to 6 weeks | 30 | 29.8 | ‐0.6 | 30 | 29.5 | +1.3 | No difference |
| Loh 2007 (Brief Patient Health Questionnaire‐D) | 6 to 8 weeks | 191 | 29.8 (2.7) | 96 | 27.0 (3.6) | P = 0.236 | ||
| Nagle 2008 (Edinburgh Postnatal Depression Scale) | ˜1 to 12 weeks post DA | 167 | 19 (11.6) | 171 | 19 (11.2) | No difference | ||
| Tiller 2006 (Hospital Anxiety and Depression Scale) | 2 weeks post DA | 58 | 10.9 (5.6) | 61 | 10.7 (6.4) | P = 0.03 | ||
| 6 mos post DA | 50 | 10.1(4.7) | 56 | 10.8 (6.4) | P = 0.12 | |||
| van Peperstraten 2010 (Beck Depression Inventory) | after multifaceted intervention/ before IVF | 126 | 16 (13%) | 136 | 5 (4%) | P = 0.01 | ||
| at uptake of IVF | 147 | 16 (11%) | 151 | 113 (9%) | No difference | |||
| Whelan 2004 (20‐item CES‐D) | 1 week post DA | 94 | 13.8 (1.0) | 107 | 13.4 (1.1) | No difference | ||
| 6 months post DA | 94 | 15.1 (1.1) | 107 | 14.2 (1.2) | No difference | |||
| 12 months post DA | 94 | 13.2 (1.3) | 107 | 12.8 (1.2) | No difference | |||
| Detailed versus simple DA | ||||||||
| Wakefield 2008 (Hospital Anxiety and Depression Scale) | 1 week post | 48 | 61 | No difference | ||||
| Wakefield 2008a (Hospital Anxiety and Depression Scale) | 1 week post | 56 | 63 | No difference | ||||
| Wakefield 2008b (Hospital Anxiety and Depression Scale) | immediately | 55 | 55 | No difference | ||||
| CES‐D: Centre for Epidemiology Studies Depresion Scale; DA: decision aid; IVF: in vitro fertilisation | ||||||||
| Author | Item | N Decision aid | Proportion or Mean (SD) | N Control | Proportion or Mean (SD) | Notes |
| DA vs usual care | ||||||
| 5‐item Decisional Regret Index | 126 | 11.9 | 127 | 14.3 | P = 0.14 | |
| Proportion of patients with decisional regret | 7% | 9% | P=0.91 | |||
| Detailed vs simple DA | ||||||
| Right decision | 63 | 58 (92.06%) | 44 | 42 (95.45%) | No difference | |
| Regret choice | 63 | 8 (12.70%) | 44 | 5 (11.36%) | No difference | |
| Would make same choice | 63 | 54 (85.71%) | 44 | 40 (90.91%) | No difference | |
| Choice did me harm | 63 | 7 (11.11%) | 44 | 3 (6.82%) | No difference | |
| Decision was wise | 63 | 54 (85.71%) | 44 | 41 (93.18%) | No difference | |
| Decisional Regret ‐ 3 items at 26 to 30 weeks gestation | 244 | 9.6 | 252 | 12.8 | P = 0.28 | |
| Decision Regret Scale at 6 months | 41 | 54 | No difference | |||
| Decision Regret Scale | ˜57 | 7.04 (12.12) | ˜63 | 6.39 (13.68) | No difference | |
| Decision Regret Scale | ˜56 | 9.78 (14.49) | ˜49 | 5.13 (10.16) | No difference | |
| DA: decision aid | ||||||
| Study | Scale used | Timing | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Notes |
| DA vs usual care | |||||||
| 11‐item self‐efficacy scale | post intervention | 291 | 83% (40.26% SD) | 334 | 79% (33.08% SD) | No difference | |
| Decisional self efficacy | changes from baseline | 75 | + 3.0 (95% CI 0.6 to 5.4) | 77 | + 2.8 (95%CI 0.9 to 4.8) | P = 0.78 | |
| Mean confidence with decision: scale from 1 (low confidence) to 5 (high confidence) | post intervention | 48 | 4 | 59 | 3.6 | P = 0.02 | |
| Decisional self‐efficacy scale | pre‐consultation | 43 | 32 (median) | 40 | 27 (median) | P = 0.001 | |
| Perceived ability to make an informed choice 1‐item; 5‐point Likert scale | 3 days post | 106 | 108 | P = 0.008; DA group more likely to agree that they could make an informed choice about PSA screening | |||
| Perceived ability to make an informed choice 1‐item; 5‐point Likert scale | Immediately post | 131 | 136 | No difference | |||
| Confidence with ability to understand outcomes of hormone replacement therapy, make a decision, engage in discussion with practitioner 3‐items (0 to 10; low to high confidence) | 1 month post | 273 | 78% (18% SD) | 284 | 70% (19% SD) | P < 0.0001 | |
| 9 months post | 261 | 80% (17%SD) | 278 | 75% (20% SD) | P = 0.0004 | ||
| 3 items adapted from the Decisional self‐efficacy scale | 2 week follow‐up | 357 | 4.67 (0.54 SD) | 173 | 4.61(0.62 SD) | P = 0.26 | |
| Detailed versus simple DA | |||||||
| 8‐items (1 to 10; low to high confidence) | post DA | 83 | 78% (16% SD) | 89 | 80% (19% SD) | No difference | |
| 12 months post | 63 | 78% (15% SD) | 74 | 80% (19% SD) | No difference | ||
| CI: confidence interval; DA: decision aid; SD: standard deviation | |||||||
| Study | Scale used | N Decision aid | Decision aid ‐ mean | N Comparison | Comparison ‐ mean | Difference between groups | Notes |
| Consultation length ‐ DA versus usual care | |||||||
| Consultation length using DA in consult (minutes) | 50 | 32.2 (13.0 SD) | 56 | 26.3 (11.5 SD) | +5.9 minutes | P = 0.01 (longer with decision aid) | |
| Consultation length with practitioner post DA (minutes) | 106 | 82 | 105 | 90 | ‐8 minutes | P = 0.03 (shorter with decision aid) | |
| Time spent discussing prostate cancer with practitioner post DA (minutes) ‐patient reported | 196 | 5.3 | 75 | 5.2 | +0.1 minutes | No difference between groups | |
| Time spent discussing prostate cancer with practitioner post DA (minutes) ‐ physician reported | 196 | 3.8 | 75 | 4.2 | ‐0.4 minutes | No difference between groups but physicians thought they spent less time than patients (P < 0.001) | |
| Consultation length using DA in consult (minutes) | 191 | 29.2 (10.7) | 96 | 26.7 (12.5) | +2.5 minutes | P = 0.681 | |
| Consultation length using DA in consult (minutes) | 15 | 24 | 15 | 21 | +3 minutes | P = 0.42 | |
| Consultation length using DA in consult (minutes) | 8 | 44 (39 to 55) | 10 | 21 (19 to 26) | +23 minutes | P = 0.001 Compared computerized decision aid with standard gamble within the consultation to guideline driven consultation | |
| Consultation length with practitioner post DA | |||||||
| 5 to 10 min | 53 | 6 (11.3%) | 54 | 5 (9.3%) | P = 0.91 | ||
| 10 to 15 min | 17 (32.1%) | 19 (35.2%) | |||||
| 15 to 25 min | 15 (28.3%) | 14 (25.9%) | |||||
| 25 to 35 min | 7 (13.2%) | 5 (9.3%) | |||||
| Above 35 min | 8 (15.1%) | 11 (20.4%) | |||||
| Consultation length using DA in consult (minutes) | 50 | 68.3 | 50 | 65.7 | +2.6 minutes | P = 0.53 | |
| Consultation length using DA in consult (minutes) | 52 | 46 | +3.8 minutes | Not statistically significant 3.8 min longer in DA group (95%CI ‐2.9 to 10.5) | |||
| Consultation length ‐ Detailed versus simple DA | |||||||
| Encounter length with practitioner post DA (minutes) | Median 16 minutes for both groups (range 6 to 44) | ||||||
| Cost and resource use ‐ DA versus usual care | |||||||
| Cost‐consequences analysis | 235 | £2019 (SD £741) | 238 | £2033 (SD £677) | no difference | ||
| Cost effectiveness | 204 | $1556 USD | 215 | $2751 USD | Mean difference $1184 (95%CI $684 to $2110) | ||
| Total costs excluding intervention | 57 | £310.3 (SD £602.0) | 48 | £188.8 (SD £300.4) | Mean difference £121.5 (95% CI £ ‐58.9 to £302.0) | ||
| Total costs including intervention | 57 | £594.10 (SD £602) | 48 | £188.8 (SD £300.4) | Mean difference £405.4 (95% CI £224.9 to £585.8) P < 0.001 | ||
| Total costs excluding intervention | 85 | £90.5 | 84 | £90.9 (SD £39.2) | No difference | ||
| Total costs including intervention | 85 | £306.5 (SD £42.8) | 84 | £90.9 (SD £39.2) | Mean difference £215.5 (95% CI £203.1 to £228.0) P < 0.001 | ||
| GP consultations post intervention | 39/51 | 32/54 | P = 0.35 | ||||
| Hospital appointments post intervention | 29/51 | 10/54 | P = 0.06 | ||||
| Mean total savings per couple | Mean total saving per couple in the intervention group were €169.75 ($219.12 USD) | ||||||
| Cost and productivity losses | 184 | €2760 Euro | 179 | €3094 Euro | P = 0.1 No difference between intervention and control when treatment cost and productivity losses were analysed. | ||
| Cost and resource use ‐ Detailed versus simple DA | |||||||
| Healthcare use at 1 year | 171 | 172 | No difference in most services; DA less surgery for herniated disk | ||||
| CI: confidence interval; DA: decision aid; SD: standard deviation | |||||||
| Outcome | Overall mean effect (95% CI) | Without trials having high risk of bias on at least 1 of 7 criteria | Without trials having high or unclear risk of bias for at least 3 of 7 criteria |
| Knowledge ‐ decision aid versus usual care | 13.29 (11.32 to 15.25) n = 42 | 13.67 (11.60 to 15.74) n = 39 | 14.97 (11.84 to 18.10) n = 21 |
| Knowledge ‐ detailed versus simple decision aid | 5.52 (3.9 to 7.15) n = 19 | 5.48 (3.78 to 7.18) n = 18 | 6.97 (2.39 to 11.55) n = 3 |
| Accurate risk perceptions ‐ with probabilities versus no probabilities | 1.82 (1.52 to 2.16) n = 19 | 1.76 (1.48 to 2.10) n = 18 | 2.28 (1.78 to 2.92) n = 7 |
| Values congruent with chosen option | 1.51 (1.17 to 1.96) n = 13 | 1.52 (1.17 to 1.97) n = 13 | 2.15 (1.35 to 3.44) n = 6 |
| Uninformed sub‐scale of Decisional Conflict Scale ‐ decision aid versus usual care | ‐7.26 (‐9.73 to ‐4.78) n = 22 | ‐7.40 (‐10.06 to ‐4.74) n = 21 | ‐8.26 (‐11.74 to ‐4.78) n = 15 |
| Uninformed sub‐scale of Decisional Conflict Scale ‐ detailed versus simple decision aid | ‐2.39 (‐4.39 to ‐0.39) n = 10 | ‐2.39 (‐4.39 to ‐0.39) n = 10 | too few to assess, n = 1 |
| Unclear values sub‐scale of Decisional Conflict Scale ‐ decision aid versus usual care | ‐6.09 (‐8.5 to ‐3.67) n = 18 | ‐6.40 (‐9.02 to ‐3.79) n = 17 | ‐7.02 (‐10.00 to ‐4.04) n = 14 |
| Unclear values sub‐scale of Decisional Conflict Scale ‐ detailed versus simple decision aid | ‐2.31 (‐4.67 to 0.05) n = 10 | ‐2.31 (‐4.67 to 0.05) n = 10 | too few to assess, n = 1 |
| CI: confidence interval | |||
| Outcome | Overall effect | Treatment decision | Screening decision | Video/computer Decision aid | Audio/pamphlet Decision aid | Base risk control | Removal of Outliers* |
| Knowledge ‐ decision aid versus usual care | 15.2 (11.7 to 18.7) | 16.5 (11.9 to 21.2) | 13.1 (7.7 to 18.5) | 21.3 (16.3 to 26.2) | 11.9 (8.3 to 15.6) | 15.5 (11.3 to 19.8) | 17.3 (13.6 to 20.9) (*Bekker 2004, Gattellari 2003, Johnson 2006) |
| Accurate risk perceptions ‐ probabilities versus no probabilities | 1.6 (1.4 to 1.9) | 1.6 (1.4 to 1.9) | 1.6 (1.1 to 2.3) | No data | 1.6 (1.4 to 1.9) | 1.3 (1.2 to 1.5) (P = 0.3) | 1.5 (1.3 to 1.7) (*Gattellari 2003) |
| Uninformed sub‐scale of the Decisional Conflict Scale ‐ decision aid versus usual care | ‐8.4 (‐11.9 to ‐4.8) | ‐9.4 (‐13.3 to ‐5.5) | ‐3.5 (‐12.9 to 5.8) | ‐12.6 (‐19.5 to ‐5.8) | ‐4.9 (‐7.6 to ‐2.3) (P = 0.06) | ‐5.4 (‐7.7 to ‐3.2) (P = 0.11) | ‐6.2 (‐8.4 to ‐4.1) (P = 0.06) (*Montgomery 2003) |
| Unclear values sub‐scale of the Decisional Conflict Scale ‐ decision aid versus usual care | ‐6.3 (‐10.0 to ‐2.7) | ‐6.0 (‐9.8 to ‐2.3) | Insufficient data | ‐8.0 (‐15.1 to ‐1.0) | ‐4.5 (‐8.4 to ‐0.6) | ‐3.6 (‐6.8 to ‐0.5) | ‐4.0 (‐6.7 to ‐1.3) (*Montgomery 2003) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knowledge: DA vs usual care ‐ all studies Show forest plot | 42 | 10842 | Mean Difference (IV, Random, 95% CI) | 13.34 [11.17, 15.51] |
| 2 Knowledge: DA vs usual care ‐ treatment only Show forest plot | 23 | 3977 | Mean Difference (IV, Random, 95% CI) | 13.75 [11.08, 16.43] |
| 3 Knowledge: DA vs usual care ‐ screening only Show forest plot | 19 | 6865 | Mean Difference (IV, Random, 95% CI) | 12.76 [9.66, 15.86] |
| 4 Knowledge: Detailed vs simple decision aids ‐ all studies Show forest plot | 19 | 3531 | Mean Difference (IV, Random, 95% CI) | 5.52 [3.90, 7.15] |
| 5 Knowledge: Detailed vs simple decision aids ‐ treatment only Show forest plot | 12 | 1930 | Mean Difference (IV, Random, 95% CI) | 4.98 [2.64, 7.33] |
| 6 Knowledge: Detailed vs simple decision aids ‐ screening only Show forest plot | 7 | 1601 | Mean Difference (IV, Random, 95% CI) | 6.33 [4.49, 8.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Accurate risk perceptions ‐ all studies Show forest plot | 19 | 5868 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.52, 2.16] |
| 2 Accurate risk perceptions ‐ treatments only Show forest plot | 12 | 2435 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.47, 2.01] |
| 3 Accurate risk perceptions ‐ screening only Show forest plot | 7 | 3433 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.40, 2.93] |
| 4 Accurate risk perceptions ‐ numbers Show forest plot | 15 | 4776 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.65, 2.43] |
| 5 Accurate risk perceptions ‐ words Show forest plot | 4 | 1092 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.13, 1.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Values congruent with chosen option ‐ all studies Show forest plot | 13 | 4670 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 2 Values congruent with chosen option ‐ treatment only Show forest plot | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.80, 2.30] |
| 3 Values congruent with chosen option ‐ screening only Show forest plot | 10 | 4321 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.16, 2.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Decisional conflict: DA vs usual care ‐ all studies Show forest plot | 32 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Uncertainty sub‐scale | 23 | 4837 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐4.28, ‐0.66] |
| 1.2 Uninformed sub‐scale | 22 | 4343 | Mean Difference (IV, Random, 95% CI) | ‐7.26 [‐9.73, ‐4.78] |
| 1.3 Unclear values sub‐scale | 18 | 3704 | Mean Difference (IV, Random, 95% CI) | ‐6.09 [‐8.50, ‐3.67] |
| 1.4 Unsupported sub‐scale | 19 | 3851 | Mean Difference (IV, Random, 95% CI) | ‐4.77 [‐6.86, ‐2.69] |
| 1.5 Ineffective choice sub‐scale | 19 | 3878 | Mean Difference (IV, Random, 95% CI) | ‐4.86 [‐7.04, ‐2.68] |
| 1.6 Total decisional conflict score | 28 | 5830 | Mean Difference (IV, Random, 95% CI) | ‐6.22 [‐8.00, ‐4.44] |
| 2 Decisional conflict: DA vs usual care ‐ treatment only Show forest plot | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Uncertainty sub‐scale | 16 | 3020 | Mean Difference (IV, Random, 95% CI) | ‐3.06 [‐5.33, ‐0.79] |
| 2.2 Uninformed sub‐scale | 17 | 3007 | Mean Difference (IV, Random, 95% CI) | ‐8.06 [‐10.52, ‐5.60] |
| 2.3 Unclear values sub‐scale | 14 | 2474 | Mean Difference (IV, Random, 95% CI) | ‐6.31 [‐9.01, ‐3.61] |
| 2.4 Unsupported sub‐scale | 15 | 2621 | Mean Difference (IV, Random, 95% CI) | ‐5.28 [‐7.74, ‐2.82] |
| 2.5 Ineffective choice sub‐scale | 15 | 2746 | Mean Difference (IV, Random, 95% CI) | ‐6.07 [‐8.41, ‐3.72] |
| 2.6 Total decisional conflict score | 22 | 3783 | Mean Difference (IV, Random, 95% CI) | ‐6.14 [‐7.78, ‐4.50] |
| 3 Decisional conflict: DA vs usual care ‐ screening only Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Uncertainty sub‐scale | 7 | 1817 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐4.47, 1.83] |
| 3.2 Uninformed sub‐scale | 5 | 1336 | Mean Difference (IV, Random, 95% CI) | ‐4.67 [‐10.61, 1.27] |
| 3.3 Unclear values sub‐scale | 4 | 1230 | Mean Difference (IV, Random, 95% CI) | ‐5.94 [‐13.44, 1.56] |
| 3.4 Unsupported sub‐scale | 4 | 1230 | Mean Difference (IV, Random, 95% CI) | ‐2.94 [‐6.90, 1.02] |
| 3.5 Ineffective choice sub‐scale | 4 | 1132 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐1.88, 1.55] |
| 3.6 Total decisional conflict score | 6 | 2047 | Mean Difference (IV, Random, 95% CI) | ‐6.83 [‐12.64, ‐1.03] |
| 4 Decisional conflict: Detailed vs simple decision aid ‐ all studies Show forest plot | 19 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Uncertainty sub‐scale | 14 | 2130 | Mean Difference (IV, Random, 95% CI) | ‐2.15 [‐4.42, 0.12] |
| 4.2 Uninformed sub‐scale | 10 | 1264 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐4.39, ‐0.39] |
| 4.3 Unclear values sub‐scale | 10 | 1260 | Mean Difference (IV, Random, 95% CI) | ‐2.31 [‐4.67, 0.05] |
| 4.4 Unsupported sub‐scale | 10 | 1268 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐5.37, 1.27] |
| 4.5 Ineffective choice sub‐scale | 9 | 1541 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.83, 0.71] |
| 4.6 Total decisional conflict score | 17 | 3277 | Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.64, ‐0.91] |
| 5 Decisional conflict: Detailed vs simple decision aid ‐ treatment only Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Uncertainty sub‐scale | 9 | 1101 | Mean Difference (IV, Random, 95% CI) | ‐2.02 [‐6.65, 2.61] |
| 5.2 Uninformed sub‐scale | 6 | 672 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐4.40, 2.09] |
| 5.3 Unclear values sub‐scale | 6 | 669 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐3.72, 2.80] |
| 5.4 Unsupported sub‐scale | 6 | 674 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐3.09, 1.79] |
| 5.5 Ineffective choice sub‐scale | 7 | 849 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.97, 2.44] |
| 5.6 Total decisional conflict score | 10 | 1732 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐2.30, 0.38] |
| 6 Decisional conflict: Detailed vs simple decision aid ‐ screening only Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Uncertainty sub‐scale | 5 | 1029 | Mean Difference (IV, Random, 95% CI) | ‐2.16 [‐4.20, ‐0.12] |
| 6.2 Uninformed sub‐scale | 4 | 592 | Mean Difference (IV, Random, 95% CI) | ‐3.42 [‐5.81, ‐1.02] |
| 6.3 Unclear values sub‐scale | 4 | 591 | Mean Difference (IV, Random, 95% CI) | ‐4.54 [‐6.77, ‐2.32] |
| 6.4 Unsupported sub‐scale | 4 | 594 | Mean Difference (IV, Random, 95% CI) | ‐3.65 [‐9.74, 2.44] |
| 6.5 Ineffective choice sub‐scale | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐2.18 [‐3.60, ‐0.75] |
| 6.6 Total decisional conflict score | 7 | 1545 | Mean Difference (IV, Random, 95% CI) | ‐2.26 [‐3.33, ‐1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participation in decision making: DA vs usual care ‐ all studies Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Patient controlled decision making | 12 | 2438 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.02, 1.60] |
| 1.2 Shared decision making | 12 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.13] |
| 1.3 Practitioner controlled decision making | 14 | 3234 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.53, 0.81] |
| 2 Participation in decision making: DA vs usual care ‐ treatment only Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Patient controlled decision making | 10 | 2147 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.05, 1.68] |
| 2.2 Shared decision making | 10 | 2111 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.15] |
| 2.3 Practitioner controlled decision making | 11 | 2318 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.54, 0.90] |
| 3 Participation in decision making: DA vs usual care ‐ screening only Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Patient controlled decision making | 3 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.82, 1.20] |
| 3.2 Shared decision making | 3 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.89, 1.45] |
| 3.3 Practitioner controlled decision making | 4 | 1512 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.44, 1.01] |
| 4 Participation in decision making: Detailed vs simple decision aid ‐ all studies Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Patient controlled decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.68, 1.64] |
| 4.2 Shared decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.63, 1.81] |
| 4.3 Practitioner controlled decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.56, 2.23] |
| 5 Participation in decision making: Detailed vs simple decision aid ‐ treatment only Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Patient controlled decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.68, 1.64] |
| 5.2 Shared decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.63, 1.81] |
| 5.3 Practitioner controlled decision making | 2 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.56, 2.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion undecided: DA vs usual care ‐ all studies Show forest plot | 18 | 4753 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.47, 0.72] |
| 2 Proportion undecided: DA vs usual care ‐ treatment only Show forest plot | 14 | 2830 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.51, 0.78] |
| 3 Proportion undecided: DA vs usual care ‐ screening only Show forest plot | 4 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.90] |
| 4 Proportion undecided: Detailed vs simple decision aids ‐ all studies Show forest plot | 3 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.37] |
| 5 Proportion undecided: Detailed vs simple decision aids ‐ treatment only Show forest plot | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.47] |
| 6 Proportion undecided: Detailed vs simple decision aids ‐ screening only Show forest plot | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.21, 1.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with the choice: DA vs usual care ‐ all studies Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Satisfaction with the choice: DA vs usual care ‐ treatment only Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Satisfaction with the choice: DA vs usual care ‐ screening only Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Satisfaction with the choice: Detailed vs simple DA ‐ all studies Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Satisfaction with the choice: Detailed vs simple DA ‐ treatment only Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Satisfaction with the decision making process: DA vs usual care ‐ all studies Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Satisfaction with the decision making process: DA vs usual care ‐ treatment only Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Satisfaction with the decision making process: DA vs usual care ‐ screening only Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Choice: Surgery over conservative option: DA vs usual care Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 As treated analysis | 15 | 2915 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.2 Intention to treat analysis | 15 | 3553 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.68, 0.93] |
| 2 Choice: Surgery over conservative option: Detailed vs simple decision aid Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 As treated analysis | 3 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.08] |
| 2.2 Intention to treat analysis | 3 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.08] |
| 3 Choice for screening Show forest plot | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 PSA screening: DA vs usual care | 9 | 3565 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.98] |
| 3.2 PSA screening: detailed DA vs simple decision aid | 3 | 782 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 3.3 Colorectal cancer screening: DA vs usual care | 10 | 4529 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.31] |
| 3.4 Breast cancer genetic testing: DA vs usual care | 4 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.83, 1.22] |
| 3.5 Prenatal diagnostic testing: Detailed vs simple decision aid | 2 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.90, 1.03] |
| 4 Choice: Diabetes medication (uptake new medication): DA vs usual care Show forest plot | 3 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.77, 4.39] |
| 5 Choice: Menopausal hormone therapy: Detailed vs simple decision aid Show forest plot | 3 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.98] |

