| Stduy name | Year | N | Country | Setting | Participants | Intervention |
| Avorn 1994 | 1994 | 192 | USA | Nursing homes | Elderly women, mean age 78.5 years | Cranberry juice cocktail: 300 mL/d (30% cranberry concentrate)
Placebo beverage PAC content: NS |
| Haverkorn 1994 | 1994 | 38 | Netherlands | Hospital | Elderly men (9) and women (29), mean age 81 years | Cranberry juice: 15 mL, twice a day (30 mL cranberry juice/d, concentration not specified) PAC content: NS |
| Foda 1995 | 1995 | 40 | Canada | Hospital clinic | Children with neuropathic bladder requiring clean intermittent catheterisation, mean age 9.35 years | Cranberry juice cocktail: 15 mL/kg/d (30% cranberry concentrate) 3‐4 times a day PAC content: NS |
| Walker 1997 | 1997 | 19 | USA | Family practice | Young women with recurrent UTI, median age 37 years | Cranberry capsules: 400 mg of cranberry solids (total amount/d: NS) PAC content: NS |
| Schlager 1999 | 1999 | 15 | USA | Hospital clinic | Children with neuropathic bladder requiring clean intermittent catheterisation, aged 2‐18 years | Cranberry juice cocktail: 300 mL/d (30% cranberry concentrate) PAC content: NS |
| Kontiokari 2001 | 2001 | 150 | Finland | Student health service | Young women (mean age 29‐32 years) with previous UTI | Cranberry‐lingonberry juice: 50 mL once/d, 5 days/week (7.5 g cranberry concentrate) PAC content: NS |
| McGuiness 2002 | 2002 | 135 | Canada | Outpatient clinic for MS patients | Patinets with multiple sclerosis | Cranberry tablet: 8000 mg, once/d (am) for 6 months PAC content: NS |
| Stothers 2002 | 2002 | 150 | Canada | Unclear | Women with recurrent UTI (aged 21‐72 years) | Cranberry juice: 250 mL three times/d or one concentrated cranberry juice tablet twice daily (dose NS apart from 'at least 1:30 parts concentrated juice) PAC content: NS (study authors did not know if the product contained active PAC or not) |
| Linsenmeyer 2004 | 2004 | 21 | USA | Urology rehabilitation clinic | Spinal cord injury patients with neuropathic bladders | Cranberry tablets: 1200 mg/d (3 x 400 mg tablets) PAC content: NS |
| Waites 2004 | 2004 | 48 | USA | Hospital clinic | Spinal cord injury patients with neuropathic bladders | Cranberry juice capsule: 2000 mg/d PAC content: NS |
| McMurdo 2005 | 2005 | 376 | Scotland | Hospital | Elderly inpatients | Cranberry juice: 300 mL once/d PAC concentration: 11.175 µg/g (dry solids basis) |
| Lee 2007 | 2007 | 305 | Australia | Community | Spinal cord injury patients | Cranberry tablets: 1600 mg/d
Methenamine hippurate tablet: 2 mg PAC content: NS |
| Wing 2008 | 2008 | 115 | USA | Pre‐natal clinic | Pregnant women | Cranberry juice ‐ Group 2: 240 mL cranberry drink at breakfast, placebo juice at other meals ‐ Group 3: 240 mL cranberry juice 3 times/d (dosage changed throughout) Mean PAC content: 80 mg/240 mL |
| Hess 2008 | 2008 | 47 | USA | SpInal cord injury patients in Veterans Admin Hospital | Spinal cord injury patients with neurogenic bladders | Cranberry tablet: 1000 mg/d (500 mg tablet) PAC concentration: NS |
| Ferrara 2009 | 2009 | 80 | Italy | Paediatric nephrology ambulatory clinic | Girls with > 1 UTI in past year | Cranberry concentrate, 50 mL in 50 mL water Lactobacillus GG drink: 100 mL PAC content: NS |
| McMurdo 2009 | 2009 | 137 | UK | Scottish primary care research network | Women ≥ 45 years with ≥ 2 UTIs in the previous 12 months | Cranberry tablet: 500 mg Antibiotic: 100 mg TMP PAC content: NS |
| Essadi 2010 | 2010 | 544 | Unsure | Antenatal clinic | Pregnant women | Cranberry juice: 250 mL, 4 times/d PAC content: NS |
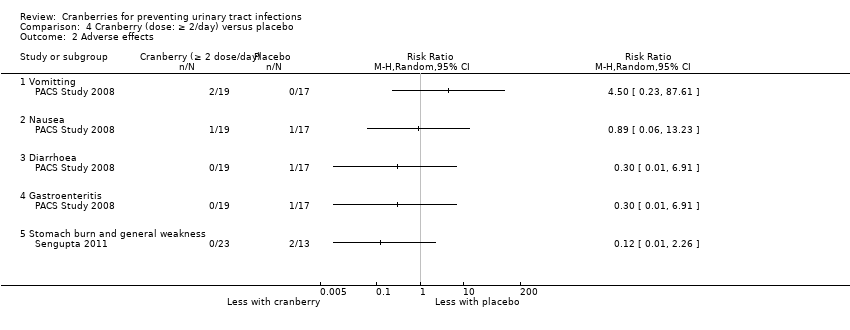
| PACS Study 2008 | 2010 | 56 | USA | Nursing home | Elderly men and women (> 60 years) with dementia | Cranberry tablet: 1 x 650 mg or 2 x 1300 mg PAC content: NS |
| Salo 2010 | 2010 | 252 | Finland | Hospital | Children with UTI | Cranberry juice: 5 mL/kg up to 300 mL PAC concentration: NS |
| Uberos 2010 | 2010 | 51 | Spain | Unclear, possibly hospital | Children with UTI | Cranberry syrup: 0.2 mL/kg Antibiotic: 8 mg/kg TMP 'The concentration guarantees that 5 mL of the syrup contains 36 mg of highly bioactive PAC extracted from the cranberry syrup, measured by the BL‐DMAC method.' |
| Barbosa‐Cesnik 2011 | 2011 | 319 | USA | University Health Service | Adult women with urinary symptoms | Cranberry juice: 2 x 240 mL (480 mL/d) PAC concentration: 112 mg (range 83–136 mg; SD 614.1 mg) |
| NAPRUTI Study 2011 I | 2011 | 199 | Netherlands | Primary care physicians | Adult women (premenopausal) with at least 3 UTIs in previous 12 months | Cranberry tablet: 2 x 500 mg/d Antibiotic: 480 mg TMP‐SMX Type A PAC in cranberry extract: 9.1 mg/g |
| Sengupta 2011 | 2011 | 57 | India | Medical clinic | Adult women | Cranberry tablets: 500 mg/d or 1000 mg/d PAC content: 1.5% |
| Cowan 2012 | 2012 | 128 | UK | Oncology unit | Adults with bladder or cervical cancer | Cranberry juice: twice daily, volume (NS), PAC concentration (NS) |