Agentes antiplaquetarios para la claudicación intermitente
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blinded, controlled trial, stratified by centre. Multicentre (120 Italian centres) study. Enrolment started in January 1989. Power calculation: Based on detection of 25% reduction of "major and minor events" compared with untreated patients (estimated as 14% in 18 months from PACK study), the number of patients required would be 1100 for α = 0.05 and β = 0.20, one‐tailed test. The number of patients enrolled increased by 10% to account for lost to follow‐up. ITT analysis: performed. Single‐blinded run‐in period of one month on placebo treatment. Follow‐up length: 18 months. | |
| Participants | Number of patients randomised: 2304. 1150 (picotamide) versus 1154 (placebo). Male : female ratio = 84.9% male (picotamide); 83.6% male in (placebo). Age (mean with SD): 63.4 ± 7.31 (picotamide); 62.9 ± 7.45 (placebo). Inclusion criteria: consecutive patients up to age of 80 suffering from PVD screened. PVD was defined according to one or more of the following criteria: patients with claudication defined as leg pain on walking that disappeared in five minutes on standing and an ABPI by Doppler ultrasonography ≤0.85 in the posterior and anterior tibial artery of one foot; or patients with claudication with previous amputation or reconstructive vascular surgery. Exclusion criteria: treatment with antiplatelet drugs such as aspirin, ticlopidine, dipyridamole, indobufen and other NSAIDs, anticoagulants; pain at rest; skin lesions; myocardial infarction, stroke, or survival intervention in the previous three months; stable or unstable angina requiring aortocoronary bypass or angioplasty; liver insufficiency (prothrombin activity ≤ 40%); serious renal disorders (serum creatinine ≥2.8 mg%) and other conditions resulting in a life expectancy of < 2 years. | |
| Interventions | Intervention: picotamide 300 mg tds. Control: placebo tds. | |
| Outcomes | All cause mortality. Cardiovascular mortality. Myocardial infarction (fatal and non‐fatal). Stroke (fatal and non‐fatal). Adverse events leading to cessation of therapy. Progression of disease resulting in need for revascularisation. Amputation (above ankle). Adverse events: adverse events leading to cessation of therapy, gastrointestinal symptoms. | |
| Notes | Source of funding: Samil S.p.A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation lists were generated by an automatic procedure developed expressly to have two balanced groups in each centre". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind study", "placebo used", "appearance and taste of the capsules were identical". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind study", "qualitative evaluation of selected events validated under blinded conditions by an independent review committee". Comment: it was not stated explicitly if assessors were blinded to the randomisation arm. Probably done. |
| Incomplete outcome data (attrition bias) | High risk | Lost to follow‐up: 57/1150(4.9%) (picotamide), 59/1154 (5.1%) (placebo). Withdrawal from study: 48/1150 (4.2%) (picotamide), 29/1154 (2.5%) (placebo). Comment: rate of withdrawal is higher in picotamide group (P = 0.02, Chi2 test). Rate of patients lost to follow‐up were comparable (P = 0.86, Chi2 test). |
| Selective reporting (reporting bias) | Low risk | Outcomes clearly listed and defined, all reported clearly in a table. |
| Methods | Randomised, double blinded, placebo controlled trial. Multicentre (29 French and 1 Swiss) study. Study period: December 1982 ‐ October 1985. Power calculation: 204 patients required, based on a "success" rate of 30% with placebo increased to 50% with ticlopidine. The hypothesis under investigation was set one‐tailed with a type I error of 0.05 and a statistical power of 0.90. ITT analysis: performed. Four week, single‐blind placebo run in. Follow‐up length: 24 weeks. | |
| Participants | Number of patients: 169. 83 (ticlopidine) versus 86 (placebo). Male : female ratio = 154 : 15. Age (mean): 59.87 ± 1.03 (ticlopidine); 58.53 ± 0.98 (placebo). Inclusion criteria: symptomatic intermittent claudication for at least 12 months and had to present a walking distance assessed by treadmill testing (3.2 km/h, 10% slope, ambient temperature 20‐24 oC) of between 50 and 300 meters. Patient was included if the relative variation of the walking distance was within 25% of initial values at the end of the run‐in period. A recent confirmation of obstructive arterial disease was required by either angiography (< 6 months) or Doppler studies (< 3 months). Exclusion criteria: patient less than 35 or older than 75 years old, stage III or IV Fontaine disease, purely diabetic arteriopathy, vascular surgery within the past six months or planned within the next six months, severe hypertension not adequately controlled by treatment, need for treatment with anti‐inflammatory or anti‐coagulant or vasodilating agents, any contra‐indication to ticlopidine, hepatic or renal disease, and poor vital prognosis. | |
| Interventions | Intervention: ticlopidine 250 mg bd. Control: placebo bd. | |
| Outcomes | All cause mortality. Cardiovascular mortality. Cardiovascular events (MI, stroke or TIA). Walking distance: total and pain free walking distance (data presented in graphical form). Adverse events: adverse events leading to cessation of therapy; GI symptoms (dyspepsia). Need for revascularisation. | |
| Notes | Trial enrolment terminated early because of persistence of a much slower recruitment rate than expected. Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An academic audit centre...issued the randomisation list". Comment: However, it was unclear how sequence generation was conducted. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "An academic audit centre, independent of the sponsor and clinical investigators, was established, to implement an external quality control. It participated in all administrative commitment. It issued the randomisation list". Comment: Unclear how list was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "An academic audit centre, independent of the sponsor and clinical investigators...carried out the labelling of the treatment containers", "matching placebo". Comment: most likely participants were blinded to the treatment that they received. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "validation committee, blinded to the allocated treatment, had to validate and classify the patients' critical events and evaluate all the files to assign a final result of 'success' or 'failure'". |
| Incomplete outcome data (attrition bias) | High risk | Patients discontinued treatment early 17/83 (20.5%) ticlopidine group, 12/86 (14.0%) placebo group, mostly (14/83 (16.9%) ticlopidine, 11/86 (12.8%) placebo) due to occurrence of critical events. Comment: All patients fully accounted for, including those who had early withdrawals of treatment. The numbers were also balanced between arms (P = 0.26, Chi2 test) and events reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to the method stated in the paper, and consistent with objectives of paper (earlier protocol published in French not accessed). |
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Single centre (UK) study. Power calculation: not performed. ITT analysis: not performed. Placebo run‐in period of four weeks. Follow‐up length: 12 months. | |
| Participants | Number of patients enrolled: 65. Number of patients evaluated: 51. 25 (ticlopidine) versus 26 (placebo). All male. Age: Mean 59 (range 40 to 75). Inclusion criteria: intermittent claudication for at least one year, no change in claudication distance for at least three months, no rest pain, and patients not considered for arterial surgery. Exclusion criteria: not specified. | |
| Interventions | Intervention: ticlopidine 250 mg bd. Control: placebo. | |
| Outcomes | Need for revascularisation. Adverse events: GI symptoms. | |
| Notes | 17 patients had undergone previous vascular reconstructive surgery. 16 patients had history of angina or myocardial infarction. Source of funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "one year randomised trial". Comment: randomisation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind, one year randomised trial". Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind, one year randomised trial". Comment: probably done. |
| Incomplete outcome data (attrition bias) | High risk | Drop out rates: 8/33 (24.2%) ticlopidine, 6/32 (19.0%) placebo Comment: drop out rates comparable (P = 0.59, Chi2 test) between groups. four withdrawals, four defaulters, two deaths, and three with non‐thrombotic medical conditions. |
| Selective reporting (reporting bias) | High risk | Did not present ABPI or walking results for placebo group. |
| Methods | Randomised, double blinded, placebo controlled trial. Multicentre (seven Italian centres) study. Power calculation: based on a 5% risk of error of the first type (α) and a 10% risk of error of the second type (β), on the hypothesis of a 60% frequency of success (40% increase of total walking distance over the basal level after 24 weeks of treatment with triflusal) and 30% target difference versus placebo. ITT analysis: not performed. Follow‐up length: 24 weeks. | |
| Participants | Number of patients: 122. 59 (triflusal) versus 63 (placebo). Male : female ratio = 112 : 16. Age (mean and SD): 64 ± 7.7 (range 40 ‐ 75). Inclusion criteria: stage II Fontaine claudicants. Exclusion criteria: allergy or hypersensitivity to cyclo‐oxygenase inhibitors, assuming drugs that might affect coagulation or fibrinolysis, affected by hypertension, recent myocardial infarction, stroke, cranial trauma or other conditions for active bleeding, peptic ulcers or active organic dyspeptic syndromes, coagulation disorders or other conditions with haemorrhagic, neutropaenia, insulin dependent diabetes, lung, kidney, liver, neurological, psychiatric or haematological disorders or other systemic diseases of clinical importance, any type of neoplasia, surgery during the three antecedent months and use of narcotics. | |
| Interventions | Intervention: triflusal 300 mg bd. Control: placebo bd. | |
| Outcomes | Walking distance: total and pain free walking distance. Adverse events: adverse events leading to cessation of therapy, GI symptoms. | |
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random oral administration". Comment: unclear how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind versus placebo". Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind versus placebo". Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Early cessation of treatment:: 4/59 (6.8%) triflusal group, 1/63 (1.6%) placebo group. Comment: All patients who stopped treatment early were fully accounted for. Drop out rates comparable between groups (P = 0.20, Fisher's exact test). |
| Selective reporting (reporting bias) | Unclear risk | Original study protocol cannot be obtained, but matches methods section. Comment: type of outcomes reported in paper is rational compared with the objectives and length of treatment in the study. |
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Multicentre (European) study. Power calculation: not performed. Three month single‐blind placebo run‐in period. ITT analysis: performed. Follow‐up length: 21 months. | |
| Participants | Number of patients: 151. 76 (ticlopidine) versus 75 (placebo). Male : female ratio = 110 : 42. Age (mean): 59.5 (range 35 ‐ 75) (ticlopidine); 59.9 (range 40 ‐ 75) (placebo). Inclusion criteria: typical intermittent claudication for at least six months, pain free walking distance of 300 m or less as determined on a treadmill (4 kph, no inclination), ankle‐arm systolic blood pressure ratio ≤0.9 at rest in the claudicating leg and further decreased three minutes after the end of the walking test. Exclusion criteria: age > 75 years, treatment in the last six months with angioplasty, thrombolytic drugs or vascular surgery; presence of rest pain or ischaemic skin ulcer; unable to walk on the treadmill, absent ultrasound signals at any foot arteries; concomitant use of oral anti‐coagulants, aspirin, buflomedil, clofibrate, dipyridamole, ditazol, flunarizine, nicergoline, pentoxifylline, suloctidil and sulphinpyrazone. | |
| Interventions | Intervention: ticlopidine 250 mg bd. Control: placebo bd. | |
| Outcomes | All cause mortality. Vascular mortality (stroke or MI related death). Vascular events (fatal and non‐fatal MI or stroke). Walking distance: total and pain free walking distance (data could not be extracted). Revascularisation. Amputation. ABPI: data could not be extracted. Adverse events: adverse events resulting in cessation of study treatment; major bleeding, GI symptoms (dyspepsia). | |
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned". Comment: randomisation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind study", "all tablets were indistinguishable in appearance and taste". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind study". Comment: probably done. |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out rate: 17/76 (22.3%) ticlopidine group, 14/75 (18.7%) placebo group. Comment: drop‐out rate comparable between groups (P = 0.57, Chi2 test). Other reasons of drop out were not clear. |
| Selective reporting (reporting bias) | Unclear risk | Comment: type of outcomes reported in paper is rational compared with the objectives and length of treatment in the study. |
| Methods | Randomised, double‐blinded, controlled trial Multicentre (384 centres in 16 countries: US, Canada and Europe) study. Power calculation: 5000 patients required in PAD subgroup, assuming a three‐year event rate of 14% for PAD, and 25% for patients with stroke or MI, study was expected to have 90% power to detect an overall relative risk reduction of 11.6%, based on the ITT analysis with a two sided α = 0.05. ITT analysis: performed. Study period: March 1992 to February 1996. Follow‐up length: Varied for patients, common stop date for all. (Mean: 1.91 years). | |
| Participants | Number of patients: 6452. 3223 (clopidogrel) versus 3229 (aspirin). Male : female ratio = 73% male (clopidogrel); 72% male (aspirin). Age: (mean with SD): 64.2 ± 9.6 (clopidogrel); 64.4 ± 9.7 (aspirin). Inclusion criteria: for PAD patients ‐ intermittent claudication (WHO: leg pain on walking, disappearing in <10 min on standing) of presumed atherosclerotic origin; and ABPI ≤ 0.85 in either leg at rest (two assessments on separate days); or history of intermittent claudication with previous leg amputation, reconstructive surgery, or angioplasty with no persisting complications from intervention. Exclusion criteria: age <21 years, scheduled for major surgery, severe co‐morbidity likely to limit life expectancy to less than three years, uncontrolled hypertension, contraindication to study drugs (severe renal or hepatic insufficiency; history of haemostatic disorder or systemic bleeding, thrombocytopaenia or neutropaenia, drug induced haematological or hepatic abnormalities; abnormal WCC, differential or platelet count; anticipated requirement for long term anticoagulant, non‐study antiplatelet drugs or NSAIDs affecting platelet function; history of aspirin sensitivity), women of childbearing age not using reliable contraception, currently receiving investigation drug, previous participation in other clopidogrel studies and geographic or other factors making study participation impractical. | |
| Interventions | Intervention: clopidogrel 75 mg plus aspirin placebo od. Control: aspirin 325 mg plus clopidogrel placebo od. | |
| Outcomes | All cause mortality. Vascular mortality. Cardiovascular events (fatal or non‐fatal MI and ischaemic stroke). Vascular events: amputation ‐ results could not be inferred (for PAD). Adverse events: gastrointestinal and intracranial haemorrhage (data for PAD could not be inferred). | |
| Notes | Source of funding: Sanofi‐Aventis and Bristol‐Myers Squibb. Included patients with ischaemic stroke and myocardial infarction. Data was available for patients with PAD. PAD patients included patients with previous leg amputation, reconstructive surgery or angioplasty with no persisting complications from intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated blocks of four treatments, stratified by disease subgroup and treatment centre". |
| Allocation concealment (selection bias) | Low risk | Quote: "access to the study code restricted to Independent Statistical Centre, Chairman of External Safety and Efficacy Monitoring Committee and two independent companies responsible for preparing the study drug". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "patients allocated study drugs sequentially from supplies at the clinical centre", "supplies were in identical blister packs containing either 75 mg tablets of clopidogrel plus aspirin placebo or 325 mg aspirin plus clopidogrel placebo tablets", "initial supply of study drug had a sealed treatment code label attached which once opened, could not be resealed in its original form. This was retained by centre for emergency purposes". "At the close of the study, a representative sample of 3358 code‐break labels were retrieved...to verify that there were no unreported code‐break labels". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "external validation committee used"' Comment: most likely done. |
| Incomplete outcome data (attrition bias) | Low risk | 42 in whole study lost to follow‐up: 22/9599(0.22%) clopidogrel group, 20/9586 (0.21%) aspirin group. Search company hired to look for patients lost to follow‐up. 4059 (21.2%) patients in whole study had study drug permanently discontinued: 21.3% (clopidogrel) and 21.1% (aspirin). |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported consistent with protocol. |
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Single centre (Italy) study. Power calculation: not performed. ITT analysis: not performed. 20 day placebo washout period. Follow‐up length: six months. | |
| Participants | Number of patients: 40. 19 (picotamide) versus 21 (placebo). Male : female ratio = 25 : 15. Age: 40 to 70 (average 63 ± 10.7). Inclusion criteria: functional stage II Fontaine, intermittent claudication for at least six months, a pain free walking distance of less than 300 metres, and an ABPI < 0.8 at rest. Exclusion criteria: congenital or acquired haemorrhagic diseases and uncontrolled hypertension. Patients with diabetes and with severe hepatic or renal impairment, or both, were also excluded. | |
| Interventions | Intervention: picotamide 300mg tds. Control: placebo tds. | |
| Outcomes | Cardiovascular events. Walking distance: pain free walking distance. ABPI. Adverse events: adverse events leading to cessation of therapy, GI symptoms (dyspepsia). | |
| Notes | Placebo given to all patients during a washout and stabilisation period of 20 days. Only patients who had less than 20% variability in pain free walking distance and ABPI during this period were included. Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised into two groups". Comment: method not clearly stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind fashion". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double‐blind fashion". |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐out rates: 2/19 (10.5%) picotamide, 3/21 (14.3%) placebo group (P = 0.55, Fisher's exact test). Number of evaluable patient: 35 (five withdrawals: two in picotamide group due to gastric discomfort; three in placebo group ‐ two kept on smoking and one had gastric discomfort). |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available, and methods section in paper was brief. However, the type of outcomes was appropriate after taking into account the objectives, length of study and sample size. |
| Methods | Randomised, double blinded trial. Multicentre (86 centres in Italy) study. Study enrolment: February 1996 to October 1998. Power calculation: 584 patients per group were required to detect an absolute reduction of mortality under picotamide of 3.5% in two years (assuming a two‐year mortality rate of 6.5% in the aspirin group), with a power of 80% and a significance level set at 0.05 (two‐sided). ITT analysis: performed. Follow‐up length: 24 months (IQR 1.9 to 2.1 years). | |
| Participants | Number of patients: 1209. 603 (picotamide) versus 606 (aspirin). Male : female ratio = 878 : 331. Age (mean with SD): 63.8 ± 7.2 (picotamide), 64.6 ± 7.3 (aspirin). Inclusion criteria: aged between 40 and 75 years old, and with history of type II diabetes for five years or more and PAD. PAD defined as the presence of two or more of the following: 1) history of intermittent claudication lasting more than two months; 2) loss of posterior tibial pulse in the foot; 3) ABPI < 0.90 or > 1.30 in the posterior or anterior tibial artery of the foot; 4) amputation or reconstructive surgery in patients with previous history of intermittent claudication; 5) angioplasty with no persisting complication from intervention. Exclusion criteria: myocardial infarction, stroke or unstable angina in the six months prior to enrolment; severe neurological or mental deficits likely to make the patient non‐compliant; severe co‐morbidity likely to limit patient's life expectancy to less than two years; serum creatinine > 2.0 mg/dL; high risk of endo‐ocular bleeding; alanine aminotransferase or aspartate aminotransferase over three times the upper limit of normal; platelets < 100,000 per mm3; active peptic ulcer or gastro‐enteric bleeding in the six months prior to enrolment; pregnancy; severe, uncontrolled hypertension; total cholesterol level ≥ 300 mg/dL; picotamide or aspirin sensitivity. Patients scheduled for major surgery or requiring long term anti‐coagulant treatment were also excluded. | |
| Interventions | Intervention: picotamide 600 mg bd. Control: aspirin 320 mg (every morning) plus placebo (evening). | |
| Outcomes | All cause mortality. Cardiovascular mortality: MI or stroke related death. Cardiovascular events: fatal and non‐fatal MI or stroke. Amputation. Adverse events: adverse events leading to cessation of therapy; major bleeding (requiring hospitalisation), GI symptoms. | |
| Notes | Source of funding: Novartis S.p.a. Number of patients available for data differ between data points. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation list was performed stratified by centre with treatment in balanced blocks of four patients within each centre, a 1:1 treatment allocation was used”. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "blinding was maintained by the use of indistinguishable active drugs and placebo tablets in separate bottles labelled for morning and evening intake". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) | High risk | Around 20% of patients in each group was lost to follow‐up for the secondary end‐points (mortality plus non‐fatal vascular events). Lost to follow‐up : 32/603(5.3%) picotamide, 26/606 (4.3%) aspirin (P = 0.41, Chi2 test). Treatment discontinued early: 159/603 in picotamide and 160/606 in aspirin
|
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported consistent with protocol. |
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Multicentre (21 centres in Argentina) study. Power calculation: based on a 10% rate of outcome events in the placebo group, with an expected rate of 3.5% in the ticlopidine group, it was proposed to recruit 600 patients. This would give a 95% chance of demonstrating a significant difference in outcome between the two treatment groups at the one‐sided significance level of 5%. ITT analysis: performed. Lost to follow‐up considered as failure, for example, occurrence of an outcome event at the date of the last visit except if investigation proves that patient was event free at the end of his planned study period. Patients stratified into diabetic and non‐diabetic groups. Follow‐up length: 24 weeks. | |
| Participants | Number of patients: 615 (out of 771 patients screened). 304 (ticlopidine) versus 311 (placebo). Male : female ratio = 521 : 94. Patients in "diabetic" stratum: 88/304 ticlopidine, 95/311 placebo Age (mean, range): 63.3 (ticlopidine); 62.5 (placebo) (range 42 to 76 years). Inclusion criteria: obstructive arterial disease of the upper part (popliteal or above) of the lower limbs for at least 12 months, confirmed by angiography (< 6 months) or doppler studies (< 3 months) and intermittent claudication with a walking distance assessed by treadmill testing (3.2 km/h, 10% slope, ambient temperature 22 ± 2 oC) of between 50 and 300 m. Exclusion criteria: < 35 or > 75 years old, disease of stage III or IV Fontaine's classification, insulin treated diabetes, vascular surgery within the past 12 months or planned for the following six months, myocardial infarction or acute stroke within the last three months, severe hypertension not adequately controlled, treatment with anti‐inflammatory or anti‐coagulant agents, contraindications to ticlopidine, aspartate aminotransferase > 50 iu/L, serum creatinine >270 μmol/L, platelets < 150 x 109 /L and granulocytes < 1.8 x 109 /L. | |
| Interventions | Intervention: ticlopidine 250 mg bd (ratio of patients given sugar to film coated tablets 1:1). Control: placebo bd. | |
| Outcomes | All cause mortality. Vascular mortality: stroke or MI related death. Cardiovascular events: fatal and non‐fatal MI or stroke. Walking distance: graphical data only. Need for revascularisation. Adverse events: adverse events leading to cessation of therapy, GI symptoms. | |
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised, stratified, placebo controlled, double‐blind study", "randomisation scheme predetermined for each centre". Comment: method of sequence generation not described. Probably done. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "each (treatment) box was clearly labelled with random treatment number", "no codes were reported as broken". Comment: treatment identity contained in "sealed envelopes" (? opaque) for emergency purposes. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind trial", "placebo tablets were identical in appearance and packaging", "treatment was balanced in blocks of four tablets: ticlopidine film coated, placebo film coated, ticlopidine sugar coated, placebo sugar coated". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind study", "validated committee unaware of the nature of the treatment allocated validated outcomes and adverse events". |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "a lost to follow‐up will be considered as a failure". 17/304 (5.6%) ticlopidine versus 17/311 (5.5%) placebo did not turn up for the last follow‐up visit, but: Quote: "it was possible after telephone or family contact or through their general practitioner to assert that none of these patients had experienced a critical event from their last visit". Comment: this is probably low risk due to balanced rate of not turning up, and small number. Patients were also eventually followed up. Treatment discontinued early: 42/304 (13.8%) ticlopidine group, 38/311 (12.3%) placebo group (P = 0.56, Chi2 test) due to adverse events: 20/304 (6.6%) ticlopidine, 14/311 (4.5%) placebo (P = 0.26, Chi2 test). Comment: comparable drop out rate, low risk overall. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported consistent with protocol. |
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Single centre (Italy) study. Power calculation: not performed. ITT analysis: not performed. Follow‐up length: six months | |
| Participants | Number of patients: 52. 28 (Indobufen) versus 24 (placebo), six patients dropped out "for reasons unrelated to treatment" Male : female ratio = 39 : 13. Age: Mean 60 (range 46 to 70). Inclusion criteria: stage II Fontaine with disease of one to five years. Exclusion criteria: Occlusive thromboangiitis (Buerger), congential or acquired haemorrhagic diseases, diabetes, hyperlipoproteinaemia, smokers, angina and severe hypertension requiring continuous therapy, or both. Patients were excluded if they had concomitant diseases that would have required other associated therapies. | |
| Interventions | Intervention: indobufen 200 mg bd. Control: placebo bd. | |
| Outcomes | Walking distance: pain free walking distance (assessed on a treadmill at constant slope and speed (10o and 4 km/h). Adverse events: gastrointestinal symptoms. | |
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Preset random design". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Preset random design". Comment: did not specify. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind study". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double‐blind study". |
| Incomplete outcome data (attrition bias) | High risk | Quote: "6 patients dropped out of the trial during the first month for reasons unrelated to the treatment". Comment: did not specify attrition rate of groups. |
| Selective reporting (reporting bias) | Unclear risk | Brief methods section in paper Comment: Outcomes reported is as expected from the objectives and design of the study. |
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Multicentre (six Swedish centres) study. Power calculation: 90% power at 5% significance levels to detect a 50% reduction of primary end points. ITT analysis: performed. Study period: November 1980 to December 1987. Stratified into presence or absence of previous leg vascular surgery. Follow‐up length: seven years (median 5.6 years). | |
| Participants | Number of patients: 687. 346 (ticlopidine) versus 341 (placebo). Male : female ratio = 525 : 162. Age (mean with SD): 60.5 ± 6.0 (ticlopidine), 60.2 ± 6.9 (placebo). Inclusion criteria: history of intermittent claudication. Exclusion criteria: age > 70 years; pregnant and fertile women; patients receiving treatment known to affect platelet function or anticoagulant drugs; platelet count below 100 X 109 /L; patients receiving lipid lowering drugs other than clofibrate; myocardial infarction within last three months; major surgery within the last month; diabetics who were treated with insulin or who showed evidence of advanced proliferative retinopathy or renal failure; patients with chronic disease such as malignancy signs of polyneuritis of obscure origin, uncontrolled hypertension (> 190/110 mmHg), rheumatic valvular disease with atrial fibrillation, previous bleeding peptic ulcer, or a stroke which prevented them from walking to the extent that claudication could not be experienced; renal dysfunction (serum creatinine > 150 mmol/L); or liver dysfunction (aspartate aminotransferase > 2 kat/L, alanine aminotransferase > 2 μkat/L, gamma glutamyl transferase > 3 μkat/L; 1 μkat = 1 μmol of reaction product per second). | |
| Interventions | Intervention: ticlopidine 250 mg bd. Control: placebo bd. | |
| Outcomes | All cause mortality. Vascular mortality: stroke or MI related death. Cardiovascular events: fatal and non‐fatal MI, stroke or TIA. Need for vascular interventions and amputations. Adverse events: major bleeding and adverse events requiring cessation of therapy. | |
| Notes | Patients who had undergone reconstructive surgery or amputation were also eligible for this study. Source of funding: Sanofi‐Winthrop. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "minimisation procedure was used to balance the placebo and ticlopidine treatment group". |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stated. Comment: probably used. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind placebo controlled trial", "placebo tablets were identical in appearance and packaging". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double‐blind placebo controlled trial". |
| Incomplete outcome data (attrition bias) | Low risk | Drop out rate: 5/346 (1.4%) ticlopidine; 2/341 (0.59%) placebo (P = 0.26, Chi2 test) are comparable between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported was as expected from the objectives and design of the study. |
| Methods | Randomised, double blinded, placebo controlled trial. Multi‐centre (10 multinational centres ‐ Argentina, Brazil, Denmark, England, Germany, Spain and Yugoslavia) study. Power calculation: 144 evaluable patients per study arm were required to detect an expected improvement of 30% for the placebo group and 50% for the indobufen group with a power of 0.8 using a two‐tailed test at an α level of 0.05. ITT analysis: performed. Single‐blinded placebo run‐in period for one month. Follow‐up length: six months. | |
| Participants | Number of patients: 302. 148 (indobufen) versus 154 (placebo). Male : female ratio = 216 : 100. Age: Median 62 years (range 43 to 78). Inclusion criteria: stable symptomatic, moderately severe chronic occlusive PAD of the lower extremities due to atherosclerosis. Claudication was considered stable if no significant change in the severity of symptoms had occurred in the six months prior to patient enrolment. After enrolment, single blinded placebo run in was conducted for one month, and only patients who still remain within inclusion criteria were randomised. Inclusion: intermitted claudication for at least six months prior to enrolment, ability to walk at least 50 m before complaining of pain in leg (initial claudication distance, ICD) , but no more than 300 m, as assessed on a motorised treadmill set at a slope of 8 degrees, at a speed of 3.2 km/hour. Absolute claudication distance (ACD) less than 500 m, resting ankle/arm systolic blood pressure in the arteries of the worse leg between 0.5 to 0.9. Exclusion criteria: diabetics. Use of antiplatelets, analgesic and anti‐inflammatory drugs other than paracetamol were disallowed. | |
| Interventions | Intervention: indobufen 200 mg bd. Control: placebo bd. | |
| Outcomes | Walking distance: ICD and ACD. Adverse events: adverse events leading to cessation of therapy, GI symptoms including dyspepsia and discomfort. | |
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients who qualified for the double‐blind phase were randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients who qualified for the double‐blind phase were randomly allocated". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "all the patients were randomly allocated to either indobufen or placebo treatment under double‐blind conditions." Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double blinded study". Comment: no further description. |
| Incomplete outcome data (attrition bias) | Unclear risk | All randomised patients included in data analysis‐ however there were some patients who were unaccounted for in adverse event reports. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported was as expected from the objectives and design of the study. |
ABPI: ankle brachial pressure index
bd: twice a day
GI: gastrointestinal
ITT: intention‐to‐treat
IQR: interquartile range
MI: myocardial infarction
NSAID: non‐steroidal anti‐inflammatory drug
od: once a day
SD: standard deviation
tds: three times a day
TIA: transient ischaemic attack
WCC: white cell count
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Cross‐over study. Patient given antiplatelet for two weeks only. No primary or secondary outcomes reported. | |
| Used suloctidil and dihydroergotoxine as drugs of investigation. | |
| Compared antiplatelet agent against surgery. | |
| Compared antiplatelet agent against low dose heparin. See Allegra 1994. | |
| Compared antiplatelet agent against low dose heparin. | |
| Compared antiplatelet against low dose heparin. | |
| No suitable results for primary and secondary outcomes. | |
| No mention of randomisation process. No placebo used. Control group was made up of patients who were unable to follow any kind of treatment (either because they refused it or because they had important gastrointestinal symptoms). | |
| Part of CAPRIE study. Outcome for PAD patients specifically not reported. | |
| Trial to assess dose of clopidogrel only. No primary or secondary outcomes reported. | |
| Used vasodilator (NM‐702) as drug of investigation. | |
| Cross‐over trial. | |
| Recruited patients undergoing angioplasty. | |
| Recruited patients undergoing angioplasty, see Cassar 2005. | |
| Ex‐vivo measure of platelet activation. | |
| Used policosanol as drug of investigation. | |
| Used policosanol as drug of investigation. | |
| Used policosanol as drug of investigation. | |
| Used policosanol as drug of investigation. | |
| Unclear patient grouping. | |
| Used defibrotide as comparator. | |
| Included asymptomatic PAD participants. | |
| Used pentoxifylline as comparator. | |
| Included asymptomatic PAD participants. | |
| Recruited patients undergoing angioplasty. | |
| Cross‐over trial. Patients received treatment for 60 days only. | |
| Proposal for trial. | |
| Used thrombolysis agent as comparator. | |
| Patients had undergone recent revascularisation. | |
| Cross‐over trial with no primary or secondary outcomes reported. | |
| Used cilostazol as drug of investigation. | |
| Included patients with Fontaine stage III. | |
| Non‐randomised study. | |
| Included asymptomatic PAD participants. | |
| Unusual dosing ‐ patient received antiplatelet therapy every third day. | |
| Used suloctidil as drug of investigation. | |
| Used suloctidil as drug of investigation. | |
| Used clomicromene, an antithrombotic as part of comparator. | |
| Used beraprost as drug of investigation. | |
| Part of CAPRIE study. Outcomes for PAD patients specifically, was not reported. | |
| No primary or secondary outcomes reported. | |
| No primary or secondary outcomes reported. | |
| Used nifedipine as comparator. | |
| Used urokinase as drug of investigation. | |
| Used suloctidil as drug of investigation. | |
| Used cilostazol as drug of investigation. Patients also received concomitant antiplatelet. | |
| Patients only received 16 days of treatment. | |
| Used suloctidil as drug of investigation. | |
| Used suloctidil as drug of investigation. | |
| Included patients with ischaemic ulcers. | |
| Used beraprost as drug of investigation at varying doses. | |
| Patients followed up for one month only. | |
| Treatment duration was only two months. | |
| No primary or secondary outcomes reported. | |
| No primary or secondary outcomes reported. | |
| Used beraprost as main drug of investigation. | |
| Used beraprost as main drug of investigation. | |
| Four week treatment duration only. Used prostaglandin as drug of investigation. | |
| Compared antiplatelet to exercise. | |
| No comparator used. | |
| Used suloctidil as drug of investigation. | |
| Used defibrotide as drug of investigation. | |
| Used defibrotide as drug of investigation. | |
| Used sarpogrelate as drug of investigation. | |
| Used beraprost as drug of investigation. | |
| No primary or secondary outcomes reported. | |
| Cross‐over trial. | |
| Cross‐over trial. | |
| Used sarpogrelate as drug of investigation. | |
| Cross‐over trial. Used captopril as comparator. | |
| Used cilostazol as drug of investigation. | |
| Used cilostazol as drug of investigation. | |
| Used vasodilator as drug of investigation. | |
| Used pentoxifylline as drug of investigation. | |
| Only assessed walking distance after day 2 to 5 of treatment. | |
| Used trapidil as drug of investigation. | |
| Included participants with asymptomatic PAD. | |
| Not RCT. Included stage III and IV Fontaine. | |
| No primary or secondary outcomes reported. | |
| Recruited patients following angioplasty. | |
| Recruited patients following angioplasty. | |
| Recruited patients following angioplasty. | |
| Used trapidil and pentoxifylline as drugs of investigation. | |
| Used heparan sulphate as comparator. | |
| Compared antiplatelet against hydroxyethylrutoside. | |
| No primary or secondary outcomes reported | |
| No primary or secondary outcomes reported. | |
| Recruited patients with acute arterial thrombosis. | |
| Trial proposal only. | |
| Not RCT. | |
| Used angiography to determine outcome. | |
| Trial proposal only. | |
| Included patients with coronary heart disease and cerebrovascular disease. Data for PAD patients could not be obtained. | |
| Used warfarin as comparator. | |
| Cross‐over trial. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality Show forest plot | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.98] |
| Analysis 1.1  Comparison 1 Antiplatelet agent versus placebo, Outcome 1 All cause mortality. | ||||
| 1.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.66] |
| 1.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.96] |
| 2 Cardiovascular mortality (fatal stroke or MI) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.32, 0.93] |
| Analysis 1.2  Comparison 1 Antiplatelet agent versus placebo, Outcome 2 Cardiovascular mortality (fatal stroke or MI). | ||||
| 2.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.32, 0.93] |
| 3 Cardiovascular event (fatal and non‐fatal MI or stroke) Show forest plot | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| Analysis 1.3  Comparison 1 Antiplatelet agent versus placebo, Outcome 3 Cardiovascular event (fatal and non‐fatal MI or stroke). | ||||
| 3.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.50] |
| 3.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.02] |
| 4 Myocardial infarction (fatal and non‐fatal) Show forest plot | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.12] |
| Analysis 1.4  Comparison 1 Antiplatelet agent versus placebo, Outcome 4 Myocardial infarction (fatal and non‐fatal). | ||||
| 4.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.39, 1.69] |
| 4.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.16] |
| 5 Myocardial infarction (fatal) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.31, 1.05] |
| Analysis 1.5  Comparison 1 Antiplatelet agent versus placebo, Outcome 5 Myocardial infarction (fatal). | ||||
| 5.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.31, 1.05] |
| 6 Myocardial infarction (non‐fatal) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.48] |
| Analysis 1.6  Comparison 1 Antiplatelet agent versus placebo, Outcome 6 Myocardial infarction (non‐fatal). | ||||
| 6.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.48] |
| 7 Stroke (fatal and non‐fatal) Show forest plot | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.45, 1.13] |
| Analysis 1.7  Comparison 1 Antiplatelet agent versus placebo, Outcome 7 Stroke (fatal and non‐fatal). | ||||
| 7.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.35, 2.30] |
| 7.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.12] |
| 8 Stroke (fatal) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.64] |
| Analysis 1.8  Comparison 1 Antiplatelet agent versus placebo, Outcome 8 Stroke (fatal). | ||||
| 8.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.64] |
| 9 Stroke (non‐fatal) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.30] |
| Analysis 1.9  Comparison 1 Antiplatelet agent versus placebo, Outcome 9 Stroke (non‐fatal). | ||||
| 9.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.30] |
| 10 Major bleeding Show forest plot | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.51, 5.83] |
| Analysis 1.10  Comparison 1 Antiplatelet agent versus placebo, Outcome 10 Major bleeding. | ||||
| 10.1 ticlopidine | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.51, 5.83] |
| 11 GI symptoms (dyspepsia) Show forest plot | 9 | 3818 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.23, 3.61] |
| Analysis 1.11  Comparison 1 Antiplatelet agent versus placebo, Outcome 11 GI symptoms (dyspepsia). | ||||
| 11.1 indobufen | 2 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [1.17, 9.27] |
| 11.2 picotamide | 2 | 2344 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.18] |
| 11.3 ticlopidine | 4 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.82, 3.17] |
| 11.4 trifusal | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 6.41 [0.79, 51.64] |
| 12 Early cessation of treatment Show forest plot | 8 | 4388 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.53, 2.75] |
| Analysis 1.12  Comparison 1 Antiplatelet agent versus placebo, Outcome 12 Early cessation of treatment. | ||||
| 12.1 indobufen | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.47, 4.49] |
| 12.2 picotamide | 2 | 2344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.73, 2.84] |
| 12.3 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.66, 3.31] |
| 12.4 trifusal | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 16.69] |
| 13 Pain Free Walking Distance Show forest plot | 3 | 329 | Mean Difference (IV, Random, 95% CI) | 78.09 [12.24, 143.95] |
| Analysis 1.13  Comparison 1 Antiplatelet agent versus placebo, Outcome 13 Pain Free Walking Distance. | ||||
| 13.1 indobufen | 2 | 294 | Mean Difference (IV, Random, 95% CI) | 196.87 [‐85.58, 479.32] |
| 13.2 picotamide | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 38.70 [2.68, 74.72] |
| 14 Revascularisation Show forest plot | 5 | 3304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.43, 0.97] |
| Analysis 1.14  Comparison 1 Antiplatelet agent versus placebo, Outcome 14 Revascularisation. | ||||
| 14.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.23] |
| 14.2 ticlopidine | 4 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.13, 0.84] |
| 15 Limb amputations Show forest plot | 2 | 2991 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| Analysis 1.15  Comparison 1 Antiplatelet agent versus placebo, Outcome 15 Limb amputations. | ||||
| 15.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.22, 2.98] |
| 15.2 ticlopidine | 1 | 687 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.32, 2.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.93] |
| Analysis 2.1  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 1 All cause mortality. | ||||
| 1.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.60, 1.02] |
| 1.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.31, 0.98] |
| 2 Cardiovascular mortality (fatal stroke or MI) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.15] |
| Analysis 2.2  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 2 Cardiovascular mortality (fatal stroke or MI). | ||||
| 2.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.35] |
| 2.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.16, 1.31] |
| 3 Cardiovascular event (fatal and non‐fatal MI or stroke) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.98] |
| Analysis 2.3  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 3 Cardiovascular event (fatal and non‐fatal MI or stroke). | ||||
| 3.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.97] |
| 3.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.57, 1.54] |
| 4 Myocardial infarction (fatal and non‐fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.86] |
| Analysis 2.4  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 4 Myocardial infarction (fatal and non‐fatal). | ||||
| 4.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.85] |
| 4.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.41, 1.55] |
| 5 Myocardial infarction (fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.15] |
| Analysis 2.5  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 5 Myocardial infarction (fatal). | ||||
| 5.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.21] |
| 5.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.23, 2.25] |
| 6 Myocardial infarction (non‐fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.47, 0.89] |
| Analysis 2.6  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 6 Myocardial infarction (non‐fatal). | ||||
| 6.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.88] |
| 6.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.36, 1.92] |
| 7 Stroke (fatal and non‐fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| Analysis 2.7  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 7 Stroke (fatal and non‐fatal). | ||||
| 7.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |
| 7.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 8 Stroke (fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.05, 6.44] |
| Analysis 2.8  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 8 Stroke (fatal). | ||||
| 8.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.55, 3.42] |
| 8.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 9 Stroke (non‐fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.39] |
| Analysis 2.9  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 9 Stroke (non‐fatal). | ||||
| 9.1 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.74, 4.16] |
| 9.2 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.31] |
| 10 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.10  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 10 Major bleeding. | ||||
| 10.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 GI symptoms (dyspepsia) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.11  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 11 GI symptoms (dyspepsia). | ||||
| 11.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Early cessation of treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.12  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 12 Early cessation of treatment. | ||||
| 12.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Amputation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.13  Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 13 Amputation. | ||||
| 13.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
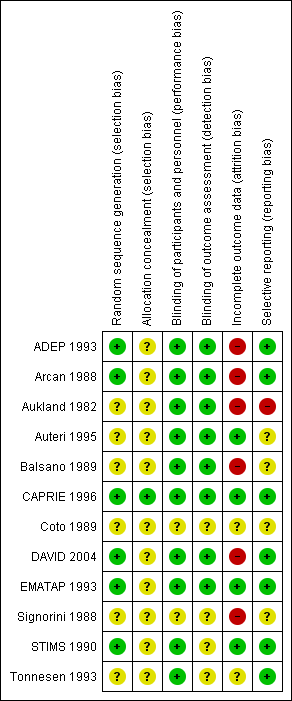
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antiplatelet agent versus placebo, Outcome 1 All cause mortality.

Comparison 1 Antiplatelet agent versus placebo, Outcome 2 Cardiovascular mortality (fatal stroke or MI).

Comparison 1 Antiplatelet agent versus placebo, Outcome 3 Cardiovascular event (fatal and non‐fatal MI or stroke).

Comparison 1 Antiplatelet agent versus placebo, Outcome 4 Myocardial infarction (fatal and non‐fatal).

Comparison 1 Antiplatelet agent versus placebo, Outcome 5 Myocardial infarction (fatal).

Comparison 1 Antiplatelet agent versus placebo, Outcome 6 Myocardial infarction (non‐fatal).

Comparison 1 Antiplatelet agent versus placebo, Outcome 7 Stroke (fatal and non‐fatal).
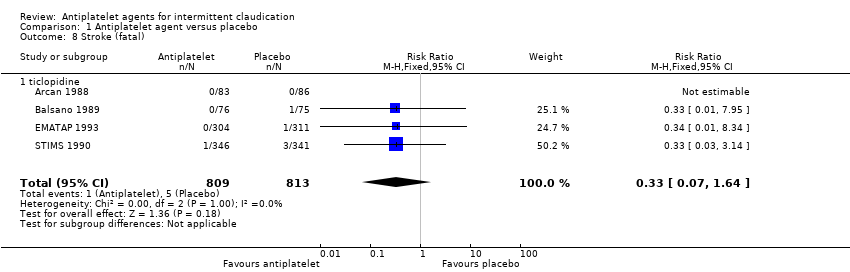
Comparison 1 Antiplatelet agent versus placebo, Outcome 8 Stroke (fatal).

Comparison 1 Antiplatelet agent versus placebo, Outcome 9 Stroke (non‐fatal).

Comparison 1 Antiplatelet agent versus placebo, Outcome 10 Major bleeding.

Comparison 1 Antiplatelet agent versus placebo, Outcome 11 GI symptoms (dyspepsia).
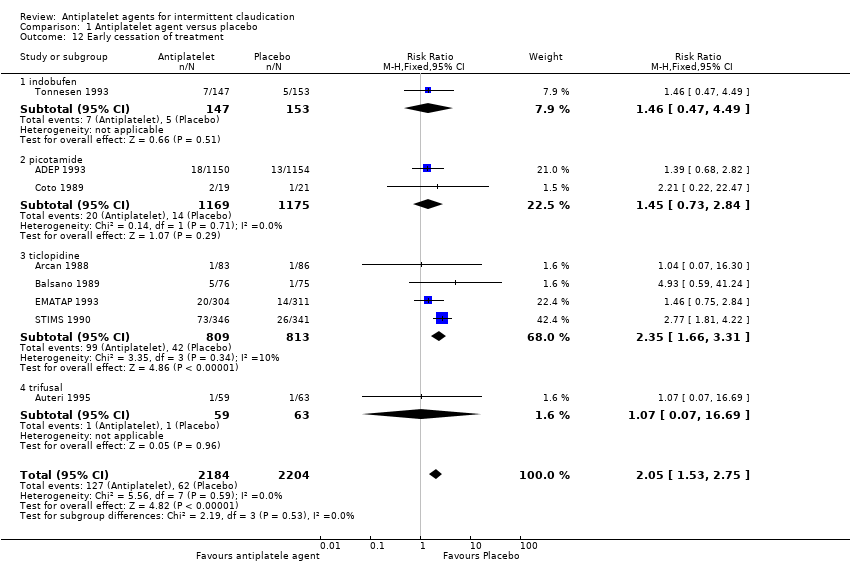
Comparison 1 Antiplatelet agent versus placebo, Outcome 12 Early cessation of treatment.

Comparison 1 Antiplatelet agent versus placebo, Outcome 13 Pain Free Walking Distance.

Comparison 1 Antiplatelet agent versus placebo, Outcome 14 Revascularisation.

Comparison 1 Antiplatelet agent versus placebo, Outcome 15 Limb amputations.
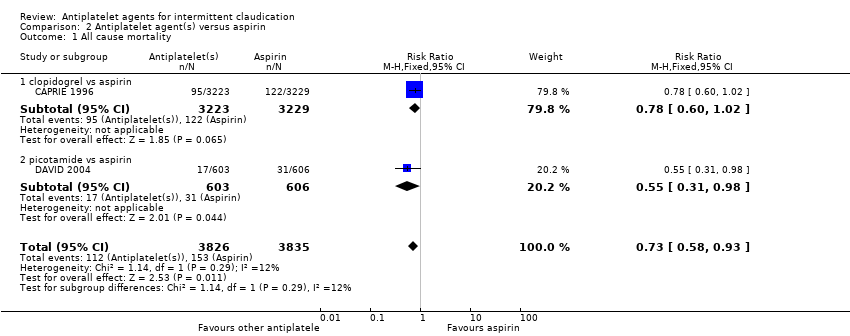
Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 1 All cause mortality.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 2 Cardiovascular mortality (fatal stroke or MI).

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 3 Cardiovascular event (fatal and non‐fatal MI or stroke).
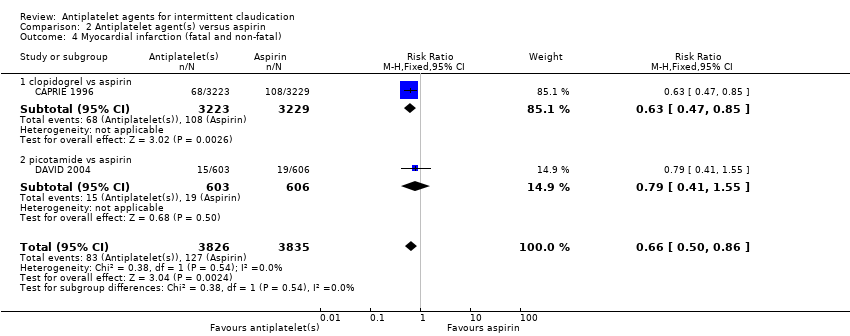
Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 4 Myocardial infarction (fatal and non‐fatal).

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 5 Myocardial infarction (fatal).

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 6 Myocardial infarction (non‐fatal).

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 7 Stroke (fatal and non‐fatal).
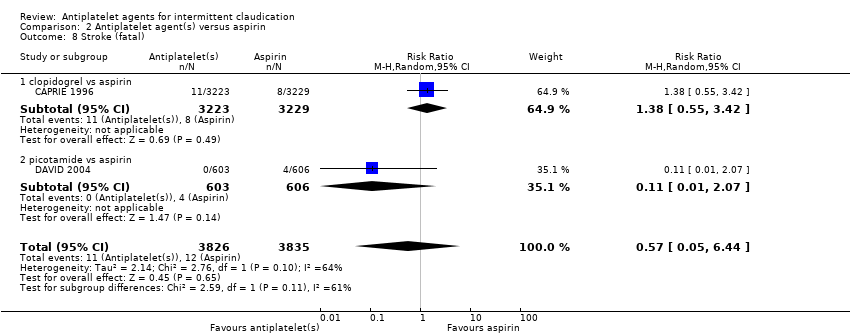
Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 8 Stroke (fatal).

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 9 Stroke (non‐fatal).

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 10 Major bleeding.
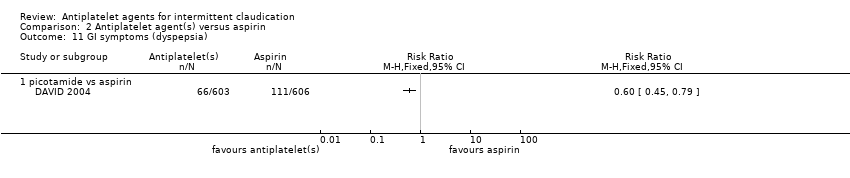
Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 11 GI symptoms (dyspepsia).

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 12 Early cessation of treatment.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 13 Amputation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality Show forest plot | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.98] |
| 1.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.66] |
| 1.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.96] |
| 2 Cardiovascular mortality (fatal stroke or MI) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.32, 0.93] |
| 2.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.32, 0.93] |
| 3 Cardiovascular event (fatal and non‐fatal MI or stroke) Show forest plot | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| 3.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.50] |
| 3.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.02] |
| 4 Myocardial infarction (fatal and non‐fatal) Show forest plot | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.12] |
| 4.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.39, 1.69] |
| 4.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.16] |
| 5 Myocardial infarction (fatal) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.31, 1.05] |
| 5.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.31, 1.05] |
| 6 Myocardial infarction (non‐fatal) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.48] |
| 6.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.48] |
| 7 Stroke (fatal and non‐fatal) Show forest plot | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.45, 1.13] |
| 7.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.35, 2.30] |
| 7.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.12] |
| 8 Stroke (fatal) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.64] |
| 8.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.64] |
| 9 Stroke (non‐fatal) Show forest plot | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.30] |
| 9.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.30] |
| 10 Major bleeding Show forest plot | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.51, 5.83] |
| 10.1 ticlopidine | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.51, 5.83] |
| 11 GI symptoms (dyspepsia) Show forest plot | 9 | 3818 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.23, 3.61] |
| 11.1 indobufen | 2 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [1.17, 9.27] |
| 11.2 picotamide | 2 | 2344 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.18] |
| 11.3 ticlopidine | 4 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.82, 3.17] |
| 11.4 trifusal | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 6.41 [0.79, 51.64] |
| 12 Early cessation of treatment Show forest plot | 8 | 4388 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.53, 2.75] |
| 12.1 indobufen | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.47, 4.49] |
| 12.2 picotamide | 2 | 2344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.73, 2.84] |
| 12.3 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.66, 3.31] |
| 12.4 trifusal | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 16.69] |
| 13 Pain Free Walking Distance Show forest plot | 3 | 329 | Mean Difference (IV, Random, 95% CI) | 78.09 [12.24, 143.95] |
| 13.1 indobufen | 2 | 294 | Mean Difference (IV, Random, 95% CI) | 196.87 [‐85.58, 479.32] |
| 13.2 picotamide | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 38.70 [2.68, 74.72] |
| 14 Revascularisation Show forest plot | 5 | 3304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.43, 0.97] |
| 14.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.23] |
| 14.2 ticlopidine | 4 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.13, 0.84] |
| 15 Limb amputations Show forest plot | 2 | 2991 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| 15.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.22, 2.98] |
| 15.2 ticlopidine | 1 | 687 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.32, 2.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.93] |
| 1.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.60, 1.02] |
| 1.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.31, 0.98] |
| 2 Cardiovascular mortality (fatal stroke or MI) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.15] |
| 2.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.35] |
| 2.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.16, 1.31] |
| 3 Cardiovascular event (fatal and non‐fatal MI or stroke) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.98] |
| 3.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.97] |
| 3.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.57, 1.54] |
| 4 Myocardial infarction (fatal and non‐fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.86] |
| 4.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.85] |
| 4.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.41, 1.55] |
| 5 Myocardial infarction (fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.15] |
| 5.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.21] |
| 5.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.23, 2.25] |
| 6 Myocardial infarction (non‐fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.47, 0.89] |
| 6.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.88] |
| 6.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.36, 1.92] |
| 7 Stroke (fatal and non‐fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 7.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |
| 7.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 8 Stroke (fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.05, 6.44] |
| 8.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.55, 3.42] |
| 8.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 9 Stroke (non‐fatal) Show forest plot | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.39] |
| 9.1 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.74, 4.16] |
| 9.2 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.31] |
| 10 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 GI symptoms (dyspepsia) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Early cessation of treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Amputation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

