Szczepienia w zapobieganiu grypy u zdrowych dorosłych
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind, placebo‐controlled, multicentric RCT performed at 36 centres in the USA assessing effectiveness, reactogenicity and antibodies responses of a Vero cell‐derived, trivalent, split influenza vaccine | |
| Participants | Healthy adults aged 18 to 48 years recruited at 36 centres throughout USA Individuals were excluded if they belong to a CDC risk category for complications of influenza illness, had a history of surgical or functional asplenia, had been treated with any blood product or immune globulin in the previous 90 days, had a history of allergy to vaccine components, had received a live vaccine within 4 weeks or an inactivated vaccine within 2 weeks of study entry, or had dermatological disorders or tattoos that would obscure the assessment of injection‐site reactions. Individuals were not specifically excluded because of egg allergy. Immunisation in previous seasons was not judged to be an exclusion criterion | |
| Interventions | Inactivated, Vero cell‐derived, trivalent split influenza vaccine containing 15 µg haemagglutinin of the following strains, which were recommended by WHO for the season 2008 to 2009 in the Northern hemisphere: A‐H1N1: A/Brisbane/59/2007 A‐H3N2: A/Uruguay/716/2007 (A/Brisbane/10/2007‐like) (A/H3N2) B: B/Florida/4/2006 The vaccine was manufactured by Baxter AG, Vienna. Vaccine strains were egg‐derived wild type strains provided by the National Institute for Biological Standard and Control (NIBSC). Placebo consisted of phosphate buffered saline Participants were randomly allocated to receive one 0.5 ml dose of either vaccine or placebo into the deltoid muscle Vaccinations were performed between 1 and 15 December 2008 | |
| Outcomes | Safety: participants were provided with a diary card, on which they had to record daily the temperature for the first 7 days following immunisation and to report fever and other adverse events for 21 days after immunisation | |
| Notes | Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Individuals were randomly assigned by use of a centralised telephone system" "Randomisation was done in blocks, with block sizes greater than two" |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was generated by Baxter, using an interactive voice response system with the random number generator algorithm of Wichmann and Hill, as modified by Mcleod" |
| Blinding (performance bias and detection bias) | Low risk | "At each study site, an investigator, subinvestigator, or study nurse who was masked to treatment allocation was designated to vaccinate participants, and was then prohibited from participation in data collection or the study. To ensure masking, the participants were enrolled by investigators who were not involved in the randomisation process. Because the syringes containing the test and the control products were different in appearance both studies employed an observational blinding procedure such that study personnel who administered vaccinations were not involved in recording or reviewing study data" |
| Incomplete outcome data (attrition bias) | Low risk | Both efficacy and safety estimates were calculated on ITT study population. We know that all treated participants (3623 to influenza vaccine and 3620 to placebo) had been included in the safety analysis, whereas 3619 and 3617 had been considered for the effectiveness estimate calculation (i.e. those vaccinated and with at least 21 days follow‐up after immunisation). Participants in the per protocol population (completed the study without major protocol deviations) were 3316 and 3318, respectively, in the vaccine and placebo arms |
| Summary assessment | Low risk | Low risk of bias |
| Methods | Randomised, double‐blind, placebo‐controlled study conducted in the Czech Republic during the 2005 to 2006 influenza season. This was defined retrospectively as starting the first week with 2 culture‐confirmed cases in the study area and ending the last week with 1 culture‐confirmed case in the study area. Randomisation was generated by GSK (sponsor) using the SAS program, in a 2:1 blocking scheme using a minimisation procedure (with no explanation of why such a method or the ratio was used). The allocation concealment method was not explicitly mentioned. However, the authors mentioned that placebo and vaccine treatments were indistinguishable in appearance and that blinding to treatment assignment was maintained until study analysis | |
| Participants | Self referred healthy adults (n = 6203), predominately Caucasian (99.8%), aged between 18 and 64 years (mean 35 + 13 years) of both genders (TIV group: female 55.3%, placebo group: female 54.2%) and with no history of influenza vaccination within the last 3 influenza seasons. A subset of participants who were randomly selected for vaccine safety and reactogenicity were given a calibrated thermometer and a diary card to record symptoms. The method of selection of this subset was not explained. Use of antimicrobial/influenza antiviral therapy seem to be allowed but was not quantified | |
| Interventions | TIV vaccine: 0.5 ml single dose by IM injection or placebo (normal saline) administered intramuscularly. Use of more than one lot was not reported TIV contain haemagglutinin antigens of
2 modes of surveillance were used: It is not clear if the surveillance included the entire cohort or just a subset, or why the authors carried out harms surveillance using the 2 surveillance methods already in place | |
| Outcomes | Serological Blood samples were collected for the specified subset and were tested/analysed at GSK Biologicals SSW Dresden, Germany Blood sample obtained prior to vaccination and at 21 days following vaccination. Serum samples were stored at ‐20 °C until blinded analyses were conducted An haemagglutination‐inhibition test was done using chicken red blood cells with the 3 virus strains present in the TIV used as antigens. The serum titre was expressed as the reciprocal of the highest dilution that showed complete inhibition of haemagglutination Serology was not a primary outcome in this study Effectiveness Incidence of culture‐confirmed ILI (primary outcome, reported as the attack rate in the efficacy cohort) Nasal and throat swab collected by a nurse on the same day Swab samples were stored at 28 °C and transferred within 5 days of the onset of ILI symptoms Sample sent to the National Reference Laboratory for Influenza (NRL, Prague, Czech Republic) for conventional influenza virus culture using Madin Darby canine kidney (MDCK) cells Confirmation of influenza A or B was determined using the following:
There were 814 reported ILI episodes, only 46 gave positive culture Clinical Incidence of ILI symptoms (secondary outcome, reported as attack rate in the ATP cohort) IL was defined as fever (oral temperature greater or equal to 37.8 °C) plus cough and/or sore throat. An ILI episode was defined as the period from the first day of ILI symptoms until the last day of ILI symptoms. A new episode was taken into account only after the complete resolution of the previous one. To count as a separate episode at least 7 days free of any symptoms should pass Number of events was 370 reported events (254 in TIV and 120 in placebo) Number of participants reporting at least one event (240 in TIV and 113 in placebo) was used to calculate the attack rate Reasons to exclude from the ATP cohort include:
Immunogenicity: blood sample obtained prior to vaccination and at 21 days following vaccination. Performed only for a subset of patient not all efficacy cohort Safety Data on serious adverse events (SAEs) began at the receipt of vaccine/placebo and continued until the end of the study. However safety was solicited from a subset of participants (no mention of method used to randomly select them, no justification for not collecting SAEs from all participants, especially with the presence of 2 surveillance methods) Reactogenicity: defined as the presence and intensity of the following symptoms within 4 days of vaccination: pain, redness and swelling (found to occur more in the TIV group) other general symptoms of fatigue, fever, headache, muscle aches, shivering and joint pain (found to occur more in the TIV group) The intensities of adverse events were recorded according to a standard 0 to 3 grade scale: "absent", "easily tolerated", "interferes with normal activity" and "prevents normal activity" | |
| Notes | The authors report that due to the atypical nature of the influenza season during this study we were unable to assess TIV efficacy Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomisation list was generated by the sponsor by SAS program and used to number the vaccine and placebo treatments"; "A randomization blocking scheme (2:1) was employed to ensure that balance between treatments was maintained." |
| Allocation concealment (selection bias) | Unclear risk | No explicit description of the method of concealment, authors only mentioned that treatments were numbered and that they were indistinguishable in appearance) |
| Blinding (performance bias and detection bias) | Unclear risk | Authors reported that the blinding assignment was maintained until study analysis Authors mentioned the treatments were indistinguishable in appearance |
| Incomplete outcome data (attrition bias) | Low risk | Exclusion of allocated participants from the analysis of the trial a) Did the report mention explicitly the exclusion of allocated participants from the analysis of trial results? Yes. b) If so did the report mention the reason(s) for exclusion? Yes. Details were reported in the study flow chart |
| Summary assessment | Unclear risk | Unclear risk of bias |
| Methods | A randomised, double‐blind, placebo‐controlled study conducted during the 2006 to 2007 influenza season at 15 centres located in the Czech Republic and Finland. The protocols and study documents were approved by the ethics committee of each country. Participants were randomised to receive 1 dose of TIV (lot 1 or lot 2 of Fluarix) or placebo (normal saline solution) at the first study visit (day 0) by intramuscular injection. Each 0.5 ml dose of TIV contained 15 mg of each of the haemagglutinin antigens of strains A/New Caledonia/20/99(H1N1) IVR‐116, A/Wisconsin/67/2005(H3N2) and B/Malaysia/2506/2004 (from the Victoria lineage) From the day of vaccination, passive and active surveillance (biweekly contact) to detect ILI cases. For each case of suspected ILI, a nasal and throat swab specimen (composed of a swab of both nasal sinuses and a second swab of the throat) was collected for culture (as much as possible on the same day as the ILI report and, at the latest, 5 days after the ILI onset). Each participant was provided with a calibrated thermometer to measure temperature and a diary card to record temperatures and symptoms during the ILI episode. Blinded analysis was carried out at GSK biologicals in Dresden, Germany Blood samples for the evaluation of influenza vaccine immunogenicity were obtained from the randomly selected, planned subset of ? 500 participants just prior to vaccination and 21 to 28 days later. Frozen aliquots of culture supernatants from positive viral cultures were sent to J. Treanor's laboratory University of Rochester Vaccine Evaluation Unit Influenza Serology Laboratory, Rochester, New York) for identification of virus‐matching isolates by conventional haemagglutination‐inhibition testing (using H1 and H3 antisera from the CDC and B/Malaysia antiserum from the WHO) | |
| Participants | Eligible participants were:
WHO provided written informed consent | |
| Interventions | Intervention 1 dose of TIV (lot 1 or lot 2 of Fluarix), IM injection, at the first day of the study (day 0) Comparator placebo (normal saline solution), IM injection, at the first day of the study (day 0) | |
| Outcomes | Serological (only carried out for the TIV group) Effectiveness Evaluate efficacy of TIV versus placebo in the prevention of culture‐confirmed influenza A and/or B due to strains antigenically matched to the vaccine (their primary objective) Secondary objectives
Safety vaccine reactogenicity and immunogenicity in a random subset of participants by obtaining blood samples prior to vaccination and 21 to 28 days later. However, no harms data are reported | |
| Notes | The authors conclude that TIV is efficacious against culture‐confirmed influenza in healthy adults Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) | Unclear risk | There is no mention of appearance of the injection content |
| Incomplete outcome data (attrition bias) | Low risk | Attrition reasons for the whole cohort are provided by the patient flow |
| Summary assessment | Unclear risk | Unclear risk of bias |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1997 to 1998 influenza season. Follow‐up lasted from November to March. Influenza period was defined as the period during which clinical specimens collected from ill participants yielded influenza viruses: 8 December 1997 through 2 March 1998 and lasted 12 weeks. Volunteers were randomly allocated to receive vaccine or placebo using a table of random number. Pharyngeal swab and paired sera were collected from ill people | |
| Participants | 1184 healthy factory employees: 595 treated and 589 placebo. Age of participants was 18 to 64 | |
| Interventions | Commercial trivalent, inactivated, intramuscular vaccine. Schedule and dose were not indicated. Vaccine composition was: A/Johannesburg/82/96, A/Nanchang/933/95 and B/Harbin/7/94. Placebo was sterile saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Influenza‐like illness, influenza, days ill, physician visits, times any drug was prescribed, times antibiotic was prescribed, working days lost, admissions, adverse effects. They were defined as follow: influenza‐like illness: fever = 37.7 °C with cough or sore throat); upper respiratory illness: cough with sore throat or fever = 37.7 °C. Local adverse effects were arm soreness and redness. Systemic adverse effects were: fever, sore throat, coryza, myalgia, headache and fatigue, but authors reported no data. Surveillance was passive | |
| Notes | For analysis we chose the influenza‐like illness definition. ITT was performed. Systemic adverse effects were not reported. Circulating strain was A/Sidney/5/97‐like Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient descriptions |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | Low risk | Attrition reasons for the whole cohort are provided by the patient flow |
| Summary assessment | Low risk | Low risk of bias |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1998 to 1999 influenza season. Follow‐up lasted from November to March. The influenza period was defined as the period during which clinical specimens collected from ill participants yielded influenza viruses: 4 January 1998 through 14 March 1999 and lasted 10 weeks. Volunteers were randomly allocated to receive vaccine or placebo using a table of random numbers. Pharyngeal swabs and paired sera were collected from ill people | |
| Participants | 1191 healthy factory employees: 587 treated and 604 placebo. Age of participants was 19 to 64 | |
| Interventions | Commercial trivalent, inactivated, intramuscular vaccine. Schedule and dose were not indicated. Vaccine composition was: A/Beijing/262/95, A/Sydney/5/97 and B/Harbin/7/94. Placebo was sterile saline for injection. Vaccine was recommended and matched circulating strain | |
| Outcomes | Influenza‐like illness, influenza, days ill, physician visits, times any drug was prescribed, times antibiotic was prescribed, working days lost, admissions, adverse effects. They were defined as follows: influenza‐like illness: fever = 37.7 °C with cough or sore throat); upper respiratory illness: cough with sore throat or fever = 37.7 °C. Local adverse effects were arm soreness and redness. Systemic adverse effect were: fever, sore throat, coryza, myalgia, headache and fatigue, but authors reported no data. Surveillance was passive | |
| Notes | For analysis we chose the influenza‐like illness definition. ITT was performed. Systemic adverse effects were not reported. Circulating strain was A/Sidney/5/97‐like and B/Beijing/184/93‐like Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient descriptions |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | Low risk | Attrition reasons for the whole cohort are provided by the patient flow |
| Summary assessment | Low risk | Low risk of bias |
| Methods | Controlled clinical trial, single‐blind, conducted in South Africa during the 1969 influenza season. Follow‐up lasted from May to July. The first clinical case of influenza appeared on 21 May 1969 and the last 6 weeks later. The epidemic period lasted 6 weeks. The control participants were selected by drawing a 1‐in‐4 systematic sample from a ranked list of the personnel numbers | |
| Participants | 1758 healthy male black African employees: 1254 treated and 413 placebo. Age of participants was 18 to 65 | |
| Interventions | Monovalent inactivated parenteral vaccine. Schedule and dose were single injection, 1 ml. Vaccine composition was: A2/Aichi/2/68 (Hong Kong variant). Placebo was sterile water. Vaccine was recommended and matched circulating strain | |
| Outcomes | Influenza‐like illness, working days lost, days ill. Influenza‐like illness was not defined; case features were generically described in results section. All ill persons were admitted to hospital until recovery. Surveillance was passive | |
| Notes | The word "double blinding" was not used, but the control group received an injection of "dummy vaccine". Poor reporting, poor‐quality study. Circulating strain was A2/Hong Kong/68 virus Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Systematic selection |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding (performance bias and detection bias) | High risk | No descriptions |
| Incomplete outcome data (attrition bias) | High risk | Insufficient description |
| Summary assessment | High risk | High risk of bias |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1986 to 1987 influenza season. Follow‐up lasted the whole epidemic period. The epidemic period in any study year started on the day that the first influenza A virus isolate was obtained in Nashville and ended on the day that the last isolate was obtained and lasted 8 weeks. Participants were recruited from 7 organisations and assigned to 1 of the study groups using a permuted block randomisation scheme that was stratified by treatment centre and age group. Sealed randomisation envelopes contained vaccine codes. Pharyngeal swab and paired sera were collected from ill people | |
| Participants | 1311 healthy children and adults of metropolitan Nashville. 85% of people were older than 16: 872 treated and 439 placebo. Age of participants was 1 to 65 | |
| Interventions | Bivalent, live, cold‐adapted, aerosol‐administered influenza A vaccine and the commercial inactivated intramuscularly administered influenza vaccine. Schedule and dose were: single‐dose; cold‐adapted 107 to 107.6 pfu/ml; inactivated 15 µg each strain. Vaccine composition was: cold‐adapted: Texas/1/85 H1N1 and Bethesda/1/85 H3N2; inactivated: Chile/1/83 H1N1 and Mississippi/1/85 H3N2. Placebo was allantoic fluid. Vaccine was recommended but did not match circulating strain | |
| Outcomes | Influenza‐like illness, influenza. They were defined as follows: fever of abrupt onset with at least one of the following: chills, headache, malaise, myalgia, cough, pharyngitis or other respiratory complaints (only patients who presented for culture were considered); throat culture. Surveillance was passive | |
| Notes | Influenza B strain contained in the commercial and monovalent vaccines was not described. Strains used yearly to develop cold‐adapted and inactivated vaccines were antigenically comparable. Since cold‐adapted influenza B vaccines were not sufficiently characterised to include in the study, the authors used monovalent inactivated influenza B vaccine in all participants in the cold‐adapted arm and as placebo in the control group inactivated arm. Only the cold‐adapted comparison was included in the analysis. The circulating strain was Taiwan/1/86. Effectiveness data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description: "permutated block randomization scheme that was stratified by treatment centre and age group" |
| Allocation concealment (selection bias) | Low risk | Adequate: participants and clinical staff were kept unaware of the assigned vaccine group through the use of sealed randomisation envelopes that contained vaccines codes |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | High risk | Insufficient description |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1987 to 1988 influenza season. Follow‐up lasted the whole epidemic period. The epidemic period in any study year started on the day that the first influenza A virus isolate was obtained in Nashville and ended on the day that the last isolate was obtained and lasted 14 weeks. Participants were recruited from 7 organisations and assigned to 1 of the study groups using a permuted block randomisation scheme that was stratified by treatment centre and age group. Sealed randomisation envelopes contained vaccine codes. Pharyngeal swab and paired sera were collected from ill people | |
| Participants | 1561 healthy children and adults of metropolitan Nashville. 85% of people were older than 16: 1029 treated and 532 placebo. Age of participants was 1 to 65 | |
| Interventions | Bivalent, live, cold‐adapted, aerosol‐administered influenza A vaccine and the commercial inactivated intramuscularly administered influenza vaccine. Schedule and dose were: single dose; cold‐adapted 107 to 107.6 pfu/ml; inactivated 15 µg each strain. Vaccine composition was: cold‐adapted: Kawasaki/9/86 H1N1 and Bethesda/1/85 H3N2; inactivated: Taiwan/1/86 H1N1 and Leningrad/360/86 H3N2. Placebo was allantoic fluid. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Influenza‐like illness, influenza. They were defined as follows: fever of abrupt onset with at least 1 of the following: chills, headache, malaise, myalgia, cough, pharyngitis or other respiratory complaints (ILI retrospectively reported were considered); 4‐fold antibody rise between post‐vaccination and spring sera. Surveillance was passive | |
| Notes | Influenza B strain contained in the commercial and monovalent vaccines was not described. Strains used yearly to develop cold‐adapted and inactivated vaccines were antigenically comparable. Since cold‐adapted influenza B vaccines were not sufficiently characterised to include in the study, the authors used monovalent inactivated influenza B vaccine in all participants in the cold‐adapted arm and as placebo in the control group inactivated arm. Only the cold‐adapted comparison was included in the analysis. The circulating strain was Sichuan/2/87 (H3N2) (antigen drift from vaccine strain) and B/Victoria/2/87 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description: "permutated block randomization scheme that was stratified by treatment centre and age group" |
| Allocation concealment (selection bias) | Low risk | Adequate: participants and clinical staff were kept unaware of the assigned vaccine group through the use of sealed randomisation envelopes that contained vaccines codes |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | High risk | Insufficient description |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1988 to 1989 influenza season. Follow‐up lasted the whole epidemic period. The epidemic period in any study year started on the day that the first influenza A virus isolate was obtained in Nashville and ended on the day that the last isolate was obtained and lasted 11 weeks. Participants were recruited from 7 organisations and assigned to 1 of the study groups using a permuted block randomisation scheme that was stratified by treatment centre and age group. Sealed randomisation envelopes contained vaccine codes. Pharyngeal swab and paired sera were collected from ill people | |
| Participants | 1676 healthy children and adults of metropolitan Nashville. 85% of people were older than 16: 1114 treated and 562 placebo. Age of participants was 1 to 65 | |
| Interventions | Bivalent, live, cold‐adapted, aerosol‐administered influenza A vaccine and the commercial inactivated intramuscularly administered influenza vaccine. Schedule and dose were: single dose; cold‐adapted 107 to 107.6 pfu/ml; inactivated 15 µg each strain. Vaccine composition was: cold‐adapted: Kawasaki/9/86 H1N1 and Los Angeles/2/87 H3N2; inactivated: Taiwan/1/86 H1N1 and Sichuan/2/87 H3N2. Placebo was allantoic fluid. Vaccine was recommended and matched circulating strain | |
| Outcomes | Influenza‐like illness, influenza. They were defined as follows: fever of abrupt onset with at least 1 of the following: chills, headache, malaise, myalgia, cough, pharyngitis or other respiratory complaints (ILI retrospectively reported were considered); 4‐fold antibody rise between postvaccination and spring sera. Surveillance was passive | |
| Notes | Influenza B strain contained in the commercial and monovalent vaccines was not described. Strains used yearly to develop cold‐adapted and inactivated vaccines were antigenically comparable. Since cold‐adapted influenza B vaccines were not sufficiently characterised to include in the study, the authors used monovalent inactivated influenza B vaccine in all participants in the cold‐adapted arm and as placebo in the control group inactivated arm. Only the cold‐adapted comparison was included in the analysis. The circulating strain was Taiwan/1/86 (H1N1) and B/Yamata/16/88. Effectiveness data only were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description: "permutated block randomization scheme that was stratified by treatment centre and age group" |
| Allocation concealment (selection bias) | Low risk | Adequate: participants and clinical staff were kept unaware of the assigned vaccine group through the use of sealed randomisation envelopes that contained vaccines codes |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | High risk | Insufficient description |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1989 to 1990 influenza season. Follow‐up lasted the whole epidemic period. The epidemic period in any study year started on the day that the first influenza A virus isolate was obtained in Nashville and ended on the day that the last isolate was obtained and lasted 11 weeks. Participants were recruited from 7 organisations and assigned to 1 of the study groups using a permuted block randomisation scheme that was stratified by treatment centre and age group. Sealed randomisation envelopes contained vaccine codes. Pharyngeal swab and paired sera were collected from ill people | |
| Participants | 1507 healthy children and adults of metropolitan Nashville. 85% of people were older than 16: 999 treated and 508 placebo. Age of participants was 1 to 65 | |
| Interventions | Bivalent, live, cold‐adapted, aerosol‐administered influenza A vaccine and the commercial inactivated intramuscularly administered influenza vaccine. Schedule and dose were: single‐dose; cold‐adapted 107 to 107.6 pfu/ml; inactivated 15 µg each strain. Vaccine composition was: Kawasaki/9/86 H1N1 and Los Angeles/2/87 H3N2; inactivated: Taiwan/1/86 H1N1 and Shanghai/11/87 H3N2. Placebo was allantoic fluid. Vaccine was recommended and matched circulating strain | |
| Outcomes | Influenza‐like illness, influenza. They were defined as follows: fever of abrupt onset with at least 1 of the following: chills, headache, malaise, myalgia, cough, pharyngitis or other respiratory complaints (ILI retrospectively reported were considered); 4‐fold antibody rise between postvaccination and spring sera. Surveillance was passive | |
| Notes | Influenza B strain contained in the commercial and monovalent vaccines was not described. Strains used yearly to develop cold‐adapted and inactivated vaccines were antigenically comparable. Since cold‐adapted influenza B vaccines were not sufficiently characterised to include in the study, the authors used monovalent inactivated influenza B vaccine in all participants in the cold‐adapted arm and as placebo in the control group inactivated arm. Only the cold‐adapted comparison was included in the analysis. The circulating strain was Shanghai/11/87 (H3N2). Effectiveness data only were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description: "permutated block randomization scheme that was stratified by treatment centre and age group" |
| Allocation concealment (selection bias) | Low risk | Adequate: participants and clinical staff were kept unaware of the assigned vaccine group through the use of sealed randomisation envelopes that contained vaccines codes |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | High risk | Insufficient description |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised, controlled, multicentre, observer‐blind trial assessing effectiveness, immunogenicity and safety of both cell culture‐derived inactivated flu vaccine (CCIV) and trivalent inactivated flu vaccine (TIV) containing the strain recommended by WHO for the current season (2007 to 2008) | |
| Participants | Participants were recruited at 56 centres in the USA, Finland and Poland | |
| Interventions | Individuals aged 18 to 49 years were randomised equally, with use of an interactive voice response system, to receive a single dose of CCIV, TIV or placebo | |
| Outcomes | Safety | |
| Notes | Financial support: "Novartis Vaccines was the funding source and was involved in all stages of the study conduct and analysis" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Low risk | “Individuals...( )..were randomised equally, with use of an interactive voice response system, to receive a single dose of CCIV, TIV, or placebo.” |
| Blinding (performance bias and detection bias) | Unclear risk | “This randomized, placebo‐controlled, observer‐blind trial evaluated....” |
| Incomplete outcome data (attrition bias) | Low risk | Flow of participants during the study is reported and described. Loss to follow‐up amounts to about 5% at study end and is balanced through the 3 arms |
| Summary assessment | Unclear risk | Unclear |
| Methods | Controlled clinical trial, double‐blinded conducted in Australia during the 1976 influenza season. Follow‐up lasted the whole epidemic period. Epidemic influenza was defined by virus isolation and serology tests and lasted from middle of April to middle of August 1976 (17 weeks). Coded identical‐looking vials were sequentially administered to enrolled participants. A throat swab was collected from ill people. Serological confirmation was performed on all participants | |
| Participants | 225 medical students or staff members: 116 treated and 109 placebo. Age of participants was not indicated | |
| Interventions | Trivalent parenteral subunit vaccine. Schedule and dose were: single dose. Vaccine composition was: 250 IU of A/Victoria/3/75, 250 IU of A/Scotland/840/74 and 300 IU of B/Hong Kong/8/73. Placebo was diphtheria and tetanus toxoids. Vaccine was recommended and matched circulating strain | |
| Outcomes | Influenza‐like illness, influenza. Clinical illnesses were not defined. Influenza was defined as respiratory illness which was associated with the isolation of influenza virus, a 4‐fold or greater rise in antibody titre occurring between post‐vaccination and post‐epidemic sera, or both. Surveillance was active | |
| Notes | Clinical illness was not defined and data were included in the analysis as "clinical cases without clear definition". Circulating strain was A/Vic/3/75‐like. Efficacy data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate |
| Allocation concealment (selection bias) | High risk | No description |
| Blinding (performance bias and detection bias) | Unclear risk | No description |
| Incomplete outcome data (attrition bias) | Unclear risk | No description |
| Summary assessment | High risk | No description |
| Methods | Randomised, multicentre, double‐blind, placebo‐controlled trial, assessing the effectiveness and safety of a trivalent inactivated vaccine in preventing confirmed influenza. The study was performed during 2 influenza seasons (2005 to 2006 and 2006 to 2007) in the USA | |
| Participants | Healthy adults aged between 18 and 49 years without significant acute or chronic, or medical or psychiatric illness. Participants with cancer, systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg; belonging to a risk group for which routine influenza vaccination is recommended (chronic pulmonary, cardiovascular, renal, hepatic, haematological or metabolic disorders; immunosuppressive illness, recent/ongoing receipt of immunosuppressive therapy, immunoglobulin, other vaccines, or with human immunodeficiency virus infection were excluded. Participants enrolled for the first season were not included in the second | |
| Interventions | Recruited participants were randomised at the beginning of each season in order to receive 1 dose of trivalent inactivated split influenza vaccine TIV (FluLaval™, a trademark of the GlaxoSmithKline group of companies; manufactured by ID Biomedical Corporation of Quebec (IBD‐Q), Canada), or saline placebo injection | |
| Outcomes | Effectiveness Immunogenicity Safety | |
| Notes | ‐ Per‐protocol (PP): participants who received the treatment to which they were randomised, responded to ≥ 1 post‐vaccination active surveillance telephone call and had no major protocol deviations considered to affect the efficacy or immunogenicity data (determined before unblinding) (for effectiveness estimates) Funding source was pharmaceutical | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Treatment allocation was determined by blocked, stratified randomization with a 1:1 distribution to TIV or placebo; randomization was stratified by study center, age (18‐34 and 35‐49 years), and the subject's report of previous recent receipt (within ≤ 2 years) of TIV.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment “Each study center had a pre‐determined sequence of randomization numbers which were allocated sequentially to eligible participants. Participants were allocated equally among 3 different vaccine lots” |
| Blinding (performance bias and detection bias) | Low risk | “Clinic staff (excluding the nurse giving the vaccine), were blinded to the treatment group until the study was complete.” |
| Incomplete outcome data (attrition bias) | Low risk | Patient flow |
| Summary assessment | Unclear risk | Unclear |
| Methods | See aa Jackson 2010b (the following data refer to the second study season) | |
| Participants | In season II (2006 to 2007), 4144 participants were recruited at 44 centres from 16 October 2006 onwards | |
| Interventions | Recruited participants were randomised at the beginning of each season in order to receive 1 dose of trivalent inactivated split influenza vaccine TIV (FluLaval™, a trademark of the GlaxoSmithKline group of companies; manufactured by ID Biomedical Corporation of Quebec (IBD‐Q), Canada), or saline placebo injection | |
| Outcomes | See aa Jackson 2010b | |
| Notes | See aa Jackson 2010b | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See aa Jackson 2010b |
| Allocation concealment (selection bias) | Unclear risk | See aa Jackson 2010b |
| Blinding (performance bias and detection bias) | Low risk | See aa Jackson 2010b |
| Incomplete outcome data (attrition bias) | Low risk | See aa Jackson 2010b |
| Summary assessment | Unclear risk | See aa Jackson 2010b |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1983 to 1984 influenza season. Follow‐up lasted the whole epidemic period. Influenza period was defined as the interval during which community surveillance recovered influenza viruses from 10% or more of persons with febrile respiratory illness per calendar week (from 8 January to 17 March 1984) and lasted 9 weeks. Volunteers were randomly allocated to receive vaccine or placebo using a table of random numbers according to prior vaccination experience. Specimens for culture and acute‐convalescent blood specimens were obtained from ill people. At spring time volunteers were asked to record any illness that occurred during the epidemic period and blood specimens were collected | |
| Participants | 598 healthy employees working in the Texas Medical Center in Houston, Texas, or in surrounding industrial companies: 300 treated and 298 placebo. Age of participants was 30 to 60 | |
| Interventions | Trivalent, killed, whole, intramuscularly administered vaccine. Schedule and dose were: single dose; 15 µg of haemagglutinin of each influenza strain. Vaccine composition was: A/Philippines/2/82 (H3N2), A/Brazil/11/78 (H1N1) and B/Singapore/222/79. Placebo was sterile saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Outcomes were: ILI, influenza. Illnesses were classified in "any", "flu‐like" (lower respiratory and/or systemic illness) and "febrile" (oral temperature of 37.8 °C or higher). Laboratory confirmation was based on culture and/or 4‐fold or greater rise in antibody titre occurring between post‐vaccination (pre‐epidemic), acute, convalescent and/or spring (post‐epidemic) sera | |
| Notes | Influenza‐like illness and influenza were detected in 3 groups: first vaccinated, multi‐vaccinated and placebo. Febrile illnesses were included in the analysis; the first 2 groups' cases were added up. Circulating strain was A/Victoria/7/83 (H1N1) and B/USSR/100/83. Efficacy data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | No description |
| Incomplete outcome data (attrition bias) | Unclear risk | No description |
| Summary assessment | Unclear risk | No description |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1984 to 1985 influenza season. Follow‐up lasted the whole epidemic period. The influenza period was defined as the interval during which community surveillance recovered influenza viruses from 10% or more of persons with febrile respiratory illness per calendar week (from 6 January to 9 March 1985) and lasted 9 weeks. Volunteers were randomly allocated to receive vaccine or placebo using a table of random numbers according to prior vaccination experience. Specimens for culture and acute‐convalescent blood specimens were obtained from ill people. At spring time volunteers were asked to record any illness that occurred during the epidemic period and blood specimens were collected | |
| Participants | 697 healthy employees working in the Texas Medical Center in Houston, Texas, or in surrounding industrial companies: 456 treated and 241 placebo. Age of participants was 30 to 60 | |
| Interventions | Trivalent, killed, whole, intramuscularly administered vaccine. Schedule and dose were: single dose; 15 µg of haemagglutinin of each influenza strain. Vaccine composition was: A/Philippines/2/82 (H3N2), A/Chile/1/83 (H1N1) and B/USSR/100/83. Placebo was sterile saline for injection. | |
| Outcomes | Outcomes were: ILI, influenza. Illnesses were classified in "any", "flu‐like" (lower respiratory and/or systemic illness) and "febrile" (oral temperature of 37.8 °C or higher). Laboratory confirmation was based on culture and/or 4‐fold or greater rise in antibody titre occurring between postvaccination (pre‐epidemic), acute, convalescent and/or spring (post‐epidemic) sera. Surveillance was passive | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | No description |
| Incomplete outcome data (attrition bias) | Unclear risk | No description |
| Summary assessment | Unclear risk | No description |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1985 to 1986 influenza season. Follow‐up lasted the whole epidemic period. The influenza period was defined by viral surveillance. Volunteers were randomly allocated to receive vaccine or placebo using a table of random numbers according to prior vaccination experience. Specimens for culture and acute‐convalescent blood specimens were obtained from ill people. At spring time, volunteers were asked to record any illness that occurred during the epidemic period and blood specimens were collected | |
| Participants | 830 healthy employees working in the Texas Medical Center in Houston, Texas, or in surrounding industrial companies: 577 treated and 253 placebo. Age of participants was 30 to 60 | |
| Interventions | Trivalent, killed, whole, intramuscularly administered vaccine. Schedule and dose were: single dose; 15 µg of haemagglutinin of each influenza strain. Vaccine composition was: A/Philippines/2/82 (H3N2), A/Chile/1/83 (H1N1) and B/USSR/100/83. Placebo was sterile saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Influenza‐like illness, influenza. Illnesses were classified in "any", "flu‐like" (lower respiratory and/or systemic illness) and "febrile" (oral temperature of 37.8 °C or higher). Laboratory confirmation was based on culture and/or 4‐fold or greater rise in antibody titre occurring between post‐vaccination (pre‐epidemic), acute, convalescent and/or spring (post‐epidemic) sera. Surveillance was active | |
| Notes | Influenza‐like illness and influenza cases were detected in 3 groups: first vaccinated, multi‐vaccinated and placebo. Febrile illnesses were included in the analysis; the first 2 groups cases were added up. Circulating strains were B/Ann Arbor/1/86, A/Mississippi/1/85 Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | No description |
| Incomplete outcome data (attrition bias) | Unclear risk | No description |
| Summary assessment | Unclear risk | No description |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1986 to 1987 influenza season. Follow‐up lasted the whole epidemic period. Influenza period was defined by viral surveillance. Volunteers were randomly allocated to receive vaccine or placebo using a table of random numbers according to prior vaccination experience. Specimens for culture and acute‐convalescent blood specimens were obtained from ill people. At spring time, volunteers were asked to record any illness that occurred during the epidemic period and blood specimens were collected | |
| Participants | 940 healthy employees working in the Texas Medical Center in Houston, Texas, or in surrounding industrial companies: 723 treated and 217 placebo. Age of participants was 30 to 60 | |
| Interventions | Trivalent, killed whole, intramuscularly administered vaccine. Schedule and dose were: 2 doses; 15 µg of haemagglutinin of each influenza strains. Vaccine composition was: A/Mississippi/1/85/H3N2), A/Chile/1/83 (H1N1) and B/Ann Arbor/1/86 plus A/Taiwan/1/86 (H1N1). Placebo was sterile saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Influenza‐like illness, influenza. Illnesses were classified in "any", "flu‐like" (lower respiratory and/or systemic illness) and "febrile" (oral temperature of 37.8 °C or higher). Laboratory confirmation was based on culture and/or 4‐fold or greater rise in antibody titre occurred between postvaccination (pre‐epidemic), acute, convalescent and/or spring (post‐epidemic) sera. Surveillance was passive | |
| Notes | Influenza‐like illness and influenza cases were detected in 3 groups: first vaccinated, multi‐vaccinated and placebo. Febrile illnesses were included in the analysis; the first 2 groups cases were added up. Circulating strain was A/Taiwan/1/86. Effectiveness data only were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | No description |
| Incomplete outcome data (attrition bias) | Unclear risk | No description |
| Summary assessment | Unclear risk | No description |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1987 to 1988 influenza season. Follow‐up lasted the whole epidemic period. Influenza period was defined by viral surveillance. Volunteers were randomly allocated to receive vaccine or placebo using a table of random numbers according to prior vaccination experience. Specimens for culture and acute‐convalescent blood specimens were obtained from ill people. At spring time, volunteers were asked to record any illness that occurred during the epidemic period and blood specimens were collected | |
| Participants | 934 healthy employees working in the Texas Medical Center in Houston, Texas, or in surrounding industrial companies: 789 treated and 145 placebo. Age of participants was 30 to 60 | |
| Interventions | Trivalent, killed, whole, intramuscularly administered vaccine. Schedule and dose were: single dose; 15 µg of haemagglutinin of each influenza strain. Vaccine composition was: A/Leningrad/360/86 (H3N2), A/Taiwan/1/86 (H1N1), B/Ann Arbor/1/86. Placebo was sterile saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Influenza‐like illness, influenza. Illnesses were classified in "any", "flu‐like" (lower respiratory and/or systemic illness) and "febrile" (oral temperature of 37.8 °C or higher). Laboratory confirmation was based on culture and/or 4‐fold or greater rise in antibody titre occurring between postvaccination (pre‐epidemic), acute, convalescent and/or spring (post‐epidemic) sera. Surveillance was passive | |
| Notes | Influenza‐like illness and influenza cases were detected in 3 groups: first vaccinated, multi‐vaccinated and placebo. Febrile illnesses were included in the analysis; the first 2 groups' cases were added up. Circulating strains were A/Sichuan/1/87, B/Victoria/2/87. Effectiveness data only were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | No description |
| Incomplete outcome data (attrition bias) | Unclear risk | No description |
| Summary assessment | Unclear risk | No description |
| Methods | Randomised, placebo‐controlled trial assessing the protective efficacy of a nasally administered meningococcal outer membrane protein adjuvanted trivalent influenza vaccine (OMP‐TIV) against laboratory‐confirmed influenza infection during the 2003 to 2004 influenza season in Canada in healthy adults | |
| Participants | Healthy adults aged 18 to 64 years, who gave informed consent, were eligible to participate (1349 were enrolled at 28 sites in Canada). Exclusion criteria: to belong to groups for which annual influenza vaccination is recommended; presence of significant acute or chronic, uncontrolled medical or psychiatric illness; pregnancy; infection with HIV, hepatitis B or hepatitis C virus; chronic use of any medication or product for symptoms of rhinitis or nasal congestion or any chronic nasopharyngeal complaint or use of such product within 7 days prior to immunisation; asthma; symptoms or diagnosis suggesting gag reflex impairment or predisposition to aspiration; use of systemic glucocorticosteroids or immunosuppressive medications; receipt of investigational drugs in the prior month, presence of febrile or upper respiratory tract illness on the day of immunisation, and known hypersensitivity to mercurials or chicken eggs | |
| Interventions | The vaccine contains equal parts of 3 monovalent egg‐grown, formalin‐inactivated influenza antigens formulated with OMPs of N. meningitidis serogroup B strain 8047 The vaccine tested in this study contained HA from each ‐ A/New Caledonia/20/99 (H1N1) ‐ A/Panama/2007/99 (H3N2) ‐ B/Shangdong/7/97 (H1N1) (recommended for the 2003 to 2004 season) Vaccine was tested in 2 formulations: 1 containing with 75 ± 15 μg/ml of HA from each of the 3 influenza strains and 1 with 150 ± 30 μg HA/ml. Both formulations are sterile, colourless to yellowish opalescent and preserved with 0.01% thimerosal The placebo control was sterile phosphate‐buffered isotonic saline with 0.01% thimerosal and was colourless Participants (n = 1348) were randomised to 1 of the following 3 regimens: | |
| Outcomes | Safety | |
| Notes | Safety and primary endpoint estimates (CCI) were calculated on the intention‐to‐immunise (ITI) population, which included any participant who received at least 1 dose of test article (n = 1348, 455 in Arm 1, 450 in Arm 2, 443 in control arm) Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The study was double‐blind, randomised and placebo controlled.” |
| Allocation concealment (selection bias) | Low risk | “Subjects were assigned centrally within blocks and stratified within each site by age ≤49 and >49 years, and history of prior influenza immunization within 2 years.” |
| Blinding (performance bias and detection bias) | Low risk | “Neither the subject nor the site study team (staff performing clinical safety or efficacy evaluations and investigators) were aware of patient assignment. One research nurse at each site was responsible for randomization, maintenance of the treatment log, test article preparation and administration.” |
| Incomplete outcome data (attrition bias) | Low risk | About 98% of the initially enrolled participants completed the study |
| Summary assessment | Low risk | Low risk of bias |
| Methods | Controlled clinical trial conducted in the USA during the 1969 to 1970 influenza season. The study period was 30 January to 18 May. Follow‐up lasted first 7 weeks of training. Influenza was detected from 11 February to 13 May and lasted 6 weeks. Participants were allocated to vaccine or control group according to the last non‐zero digit of the social security number. Blinding was not mentioned. Specimens for culture and acute‐convalescent blood specimens were obtained from people hospitalised with acute respiratory disease | |
| Participants | 9616 military trainees: 1682 treated and 7934 placebo. Age of participants was 18 to 20 | |
| Interventions | Monovalent inactivated, experimental, intramuscularly administered vaccine. Schedule and dose were: single dose, 556 CCA. Recombinant virus derived from HK/Aichi/68 and A0/PR8/34 was compared against no vaccination. Vaccine was not recommended but matched circulating strain | |
| Outcomes | Outcomes were: hospitalisation for upper respiratory infection (without definition), hospitalisation for influenza. Laboratory confirmation was based on culture and/or 4‐fold or greater rise in antibody titre occurring between acute and convalescent sera. Surveillance was passive | |
| Notes | Recruitment and immunisation period overlapped outbreak period. Most of the illness were due to adenovirus. Illnesses during the first 1 or 2 weeks after vaccination were not excluded, but the authors stated that this fact did not affect the results. Efficacy data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | High risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in Columbia during the 1997 influenza season. Follow‐up lasted from 15 March to 31 August. Influenza period was not defined. Volunteers were randomly allocated to receive vaccine or placebo using a table of random numbers. Double‐blinding was ensured by pre‐labelled, coded, identical‐looking vials. Virological surveillance was not performed | |
| Participants | 493 bank employees: 247 treated and 246 placebo. Age of participants was 18 to 60 | |
| Interventions | Subunit inactivated, intramuscularly administered vaccine. Schedule and dose were: single dose. Vaccine composition was: A/Wahan/359/95, A/Texas/36/91 and B/Beijing/184/93. Placebo was vitamin C. Vaccine was recommended and matched circulating strain | |
| Outcomes | Episodes of clinical illness, working days lost (WDL) and adverse effects. Clinical disease was defined as upper respiratory illness (fever, sore throat and cough lasting more than 24 hours) according to ICD‐IX codes 381, 382, 460, 466, 480 and from 487 to 490. Local adverse effects were oedema, erythema, pain and swelling. Systemic adverse effects were fever, headache and indisposition within 5 days of vaccination. Surveillance was passive | |
| Notes | Circulating strains were not isolated from local cases but by WHO and Columbia surveillance system and matched vaccine components. WDL were detected all the year round, so they were not included in the analysis. Efficacy and safety data were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | Low risk | Adequate |
| Summary assessment | Low risk | Low risk |
| Methods | Randomised controlled trial, double‐blind, conducted in Brazil during the 1997 influenza season. Follow‐up lasted 6 to 7 months. Influenza period was not defined. Authors did not describe the methods used to ensure randomisation and blinding. Virologic surveillance was not performed | |
| Participants | 813 flight crews of an airline company: 405 vaccinated and 408 given placebo. Age of participants was 18 to 64 | |
| Interventions | Split trivalent, intramuscularly administered vaccine. Schedule and dose were: single dose. Vaccine composition was: A/Nanchang/933/95, A/Texas/36/91 and B/Harbin/7/94. Placebo was vaccine diluent. Vaccine was recommended and matched circulating strain | |
| Outcomes | Influenza‐like illness, working days lost. Clinical illness was defined as follow: fever > 37.6 °C and cough, headache, myalgia, rhinorrhea, sore throat lasting at least 24 hours. Surveillance was passive | |
| Notes | Local and systemic effects were reported together and therefore not included in the review. Only 294 treated participants and 299 controls completed follow‐up. Efficacy data were extracted Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | Low |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised study conducted in the USA during the 1968 to 1969 influenza season. Influenza outbreak lasted 9 weeks, from 9 December to 3 February. Randomisation methods were not described. Laboratory confirmation was obtained (by culture or 4‐fold antibody titre increase in acute convalescent sera) for 20 men randomly selected each week from among the ill | |
| Participants | 1402 airmen previously unvaccinated: 881 vaccinated and 521 given placebo. Age of participants was 18 to 21 | |
| Interventions | Monovalent inactivated parenteral influenza A vaccine. Schedule and dose were: single dose. Vaccine composition was: A2/Aichi 2/68 300 CCA. Placebo was saline for injection. Vaccine was recommended and matched circulating strain | |
| Outcomes | Influenza‐like illness and influenza, complications and admissions. All respiratory illnesses were classified as febrile (38.3 °C or greater), afebrile, pharyngitis, bronchitis or pneumonia (complications). Surveillance was passive | |
| Notes | Cases occurring during the first 15 days after vaccination were not included in the analysis. Circulating strain was A2/Hong Kong. Efficacy data were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised study conducted in the USA during the 1968 to 1969 influenza season. Influenza outbreak lasted 9 weeks, from 9 December to 3 February. Randomisation methods were not described. Laboratory confirmation was obtained (by culture or 4‐fold antibody titre increase in acute convalescent sera) for 20 men randomly selected each week from among the ill | |
| Participants | 1551 airmen previously unvaccinated: 1030 vaccinated and 521 given placebo. Age of participants was 18 to 21 | |
| Interventions | Polyvalent inactivated influenza A and B vaccine (the 1967 military formula). Schedule and dose were: single dose. Vaccine composition was: A/Swine/33 100 CCA, A/PR8/34 100 CCA, A1/AA/1/57 100 CCA, A2/Taiwan 1/64 400 CCA, B/Lee/40 100 CCA, B/Mass 3/66 200 CCA. Placebo was saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Influenza‐like illness and influenza cases, complications and admissions. All respiratory illnesses were classified as febrile (38.3 °C or greater), afebrile, pharyngitis, bronchitis or pneumonia (complications). Surveillance was passive | |
| Notes | Cases occurring during the first 15 days after vaccination were not included in the analysis. Circulating strain was A2/Hong Kong. Efficacy data were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised, single‐blind study conducted in the USA during the 1979 to 1980 influenza season. Follow‐up lasted for the whole epidemic period. The epidemic period was defined by first and last isolation (11 February to 18 March) and lasted 5 weeks. Each participant was given a serial number that had previously been assigned randomly by a code to either the vaccine or the placebo group. Specimens for culture were obtained from ill people. At spring time blood specimens were collected | |
| Participants | 306 students: 154 vaccinated and 152 given placebo. Age of participants was not reported | |
| Interventions | Monovalent, live attenuated, intranasal influenza B. Schedule and dose were: single dose. Vaccine composition was: the vaccine virus, cold recombinant, was produced by recombining the attenuated B/Ann Arbor/1/66 with a wild strain B/Hong Kong/8/73. Placebo was vaccine diluent. Vaccine was not recommended and did not match the circulating strain | |
| Outcomes | Clinical and laboratory confirmed cases and adverse effects. Participants suffered a respiratory illness if they had at least 2 respiratory symptoms. Cases were laboratory‐confirmed if they had an increase in antibody titre against 3 influenza B virus antigens, i.e. if there was a 4‐fold increase from an initial sample. Side effects were sore throat, coryza, hoarseness, cough, muscle aches, temperature > 100 °F occurring during the first 3 days after vaccination. Surveillance was active | |
| Notes | Vaccine content was not recommended nor matched. Circulating strain was B/Singapore/79‐like and B/Buenos Aires/79‐like Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | Low risk | Adequate |
| Summary assessment | Low risk | Adequate |
| Methods | Third epidemic season (2007 to 2008) of aa Ohmit 2006 and aa Ohmit 2008 | |
| Participants | A total of 1952 healthy adults between 18 and 49 years were enrolled. Some had been also enrolled in the 2 previous seasons | |
| Interventions | Newly enrolled participants were recruited from the community around 4 university campuses in Michigan. Allocation methods are the same as for aa Ohmit 2006 and aa Ohmit 2008 For the 2007 to 2008 season vaccine composition was the following: ‐ Fluzone (Sanofi Pasteur, inactivated trivalent vaccine intramuscular): 15 μg of haemagglutinin from each of the following strains in a 0.5 ml dose: A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2) and B/Malaysia/2506/2004 (B/Victoria lineage) | |
| Outcomes | Same outcomes as aa Ohmit 2008 | |
| Notes | Funding source ‐ mixed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial conducted in the USA during the 1994 to 1995 influenza season. Follow‐up lasted from 1 December 1994 through to 31 March 1995. Influenza period was not defined. Randomisation was performed according to a computer‐generated randomisation schedule. Double‐blinding was ensured by preloaded, coded, identical‐looking syringes. Virological surveillance was not performed | |
| Participants | 841 full‐time employed: 419 treated and 422 placebo. Age of participants was 18 to 64 | |
| Interventions | Subvirion, trivalent, parenteral influenza A and B vaccine. Schedule and dose were: single dose; 15 µg each strain. Vaccine composition was: A/Texas/36/91, A/Shangdong/9/93, B/Panama/45/90. Placebo was vaccine diluent. Vaccine was recommended and matched circulating strain | |
| Outcomes | Cases (symptom‐defined), working days lost because of respiratory illness, side effects. Participants were defined as cases if they had at least 1 upper respiratory illness (a sore throat associated with either fever or cough that lasted at least 24 hours). Local adverse effects were defined as arm soreness. Systemic adverse effects were defined as fever, tiredness, "feeling under the weather", muscle ache, headache (within a week after vaccination). Surveillance was active | |
| Notes | Circulating strain was not indicated. Efficacy and safety data were extracted Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | Low risk | Adequate |
| Summary assessment | Low risk | Adequate |
| Methods | Randomised controlled trial conducted in the USA during the 1997 to 1998 influenza season. Follow‐up lasted from November to March. Site‐specific peak outbreak period was defined as weeks including 80% of the isolates of a specific area. Total outbreak period lasted from 14 December 1997 through to 21 March 1998. Total outbreak period was included in the analysis and lasted 14 weeks. Participants were recruited from 7 organisations and assigned to 1 of the study groups using a permuted block randomisation scheme that was stratified by treatment centre and age group. Sealed randomisation envelopes contained vaccine codes. Influenza virus surveillance was carried out in the area | |
| Participants | 4561 healthy working adults: 3041 treated and 1520 placebo. Age of participants was 18 to 64 | |
| Interventions | Trivalent, live attenuated influenza A and B vaccine in a single dose. Vaccine composition was: A/Shenzhen/227/95, A/Wuhan/395/95, B/Harbin/7/94‐like. Placebo was egg allantoic fluid. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Clinical cases (symptom‐defined), working days lost and adverse effects. Case definition had 3 specifications: febrile illness (fever for at least 1 day and 2 or more symptoms for at least 2 days: fever, chills, headache, cough, runny nose, sore throat, muscle aches, tiredness); severe febrile illness (3 days of symptoms and 1 day of fever); febrile upper respiratory tract illness (3 days of upper respiratory tract symptoms and 1 day of fever). We chose the febrile illness outcome for analysis. Systemic adverse effects were defined as headache, muscle aches, chills, tiredness and fever. Surveillance was passive | |
| Notes | Complete follow‐up data were obtained for 2874 participants in the treatment arm and for 1433 participants in the placebo arm. The outcome working days lost is presented as a rate ratio; the data are presented in a way that allows us to compute the difference in mean days lost but not to compute the standard error. Circulating strain was A/Sidney/5/97‐like. Efficacy and safety data were extracted Government and industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | Low risk | Adequate |
| Summary assessment | Low risk | Adequate |
| Methods | Multicentre, randomised, placebo‐controlled trial assessing effectiveness of both inactivated and live attenuated vaccines in preventing laboratory‐confirmed influenza in healthy adults aged below 50 | |
| Participants | For enrolment in the first study year (2004 to 2005), participants were recruited at 4 centres (2 university and 2 community sites) in Michigan. Participants were healthy adults between 18 and 46 years, with exclusion of those for whom influenza vaccination was recommended or contraindicated. In all 1247 were enrolled | |
| Interventions | After informed consent was obtained and a first serum sample drawn, enrolled participants were randomly allocated to receive one dose of the following: ‐ Inactivated trivalent vaccine (Fluzone, Sanofi Pasteur) containing 15 μg of haemagglutinin from each of the following strains: A/New Caledonia/20/99 (H1N1), A/Wyoming/3/2003 (H3N2, A/Fujian/411/2002‐like strain) and B/Jiangsu/10/2003 (B/Shanghai/361/2002‐like strain (Yamagata lineage)) in each 0.5 ml dose, as intramuscular injection ‐ Placebo saline administered intramuscularly ‐ Live attenuated trivalent vaccine (FluMist, MedImmune) containing a 106.5‐7.5 median tissue‐culture infective dose of live attenuated influenza virus reassortants of the following strains: A/New Caledonia/20/99 (H1N1), A/Wyoming/ 3/2003 (H3N2 A/Fujian/411/2002‐like strain) and B/Jilin/20/2003 (B/Shanghai/361/2002‐like strain (Yamagata lineage)) in each 0.5 ml dose ‐ Placebo saline administered intranasally Identical syringes were filled on site with the inactivated vaccine or matching placebo (physiologic saline) by study nurses who were aware of the intervention assignments. The live attenuated influenza vaccine and matching placebo (physiologic saline) were preloaded in identical nasal spray devices by the manufacturer. Both vaccines were licensed for use in the 2004 to 2005 influenza season Participants were randomised to vaccine or placebo in ratio of 5:1 using 4 site‐specific randomisation schedules, generated with the use of a random permuted block design with a block size of 12, in order to assign participants sequentially to receive a vaccine or a placebo as they enrolled Since the trial was double‐blind, the participants and the nurses who administered the study vaccine or placebo were unaware of whether the participant was receiving vaccine or placebo but were aware of the route of administration Further serum samples were drawn 3 to 5 weeks after vaccine administration (as participants returned diary cards for local and systemic reactions, preseason sample), and during April to May 2005 (post‐season sample) | |
| Outcomes | ‐ Local and systemic reactions within 7 days from immunisation (self filled questionnaires): fever, chills, runny nose or congestion, cough, sore throat, headache, muscle aches, weakness, abdominal pain, trouble breathing, red eyes, arm soreness, arm redness ‐ Laboratory‐confirmed influenza. Active surveillance was maintained between November 2004 and April 2005. Participants were contacted by phone or e‐mail twice monthly. Symptomatic influenza was described as the presence of at least 1 respiratory symptom (cough or nasal congestion) and at least 1 systemic symptom (fever, feverishness, chills, body aches) occurred during influenza activity and at least 2 weeks after administration. Participants were instructed to contact study staff when 2 at least respiratory and systemic symptoms were observed. Throat swab specimens were collected from all participants with symptomatic influenza Swabs were cultured for identification and all isolates were typed according to strain using the fluorescence antibody assay and evaluated for antigenic relatedness to vaccine strains by the Influenza Branch at the Centers for Disease Control and Prevention (CDC). In addition, all throat‐swab specimens obtained from participants with symptomatic influenza were tested at the University of Michigan by means of real‐time PCR assays using the Taqman system (Applied Biosystems) All collected serum samples were tested with the haemagglutination‐inhibition assay, with the virus strains present in the vaccines used as antigens and against the circulating type A (H3N2) (A/California/07/2004‐like) virus and the circulating type B (B/Hawaii/33/ 2004‐like) virus (i.e. Victoria lineage not included in the vaccine) For effectiveness the following endpoints were used: ‐ On ITT population Laboratory‐confirmed influenza: culture‐positive and/or real‐time PCR‐positive ‐ On per protocol population Laboratory‐confirmed influenza: serologically positive; serologically or culture‐positive | |
| Notes | Intention‐to‐treat analysis: includes all enrolled participants who were randomly assigned to a vaccine or placebo group and received a vaccine or a placebo (TIV = 513; placebo IM = 103; LAIV = 519; placebo IN = 103) Per‐protocol analyses: limited to participants having the post‐intervention (pre‐season) blood specimen collected at least 3 weeks after receipt of a vaccine or a placebo and at least 2 weeks before the beginning of local influenza activity (TIV = 367; placebo IM = 73; LAIV = 363; placebo IN = 73) Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Low |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Low risk | Low |
| Incomplete outcome data (attrition bias) | Low risk | Low |
| Summary assessment | Unclear risk | Unclear |
| Methods | Multicentre, randomised, placebo‐controlled trial assessing the effectiveness of both inactivated and live attenuated vaccines in preventing laboratory‐confirmed influenza in healthy adults aged below 50. Same methods as aa Ohmit 2006 | |
| Participants | For study year 2005 to 2006 healthy men and women, aged 18 to 48 years, were recruited at 6 study sites (4 university sites and 2 community sites) in Michigan. In all 2058 participants were enrolled. Of these, 972 were already enrolled in the 2004 to 2005 season (see aa Ohmit 2006) | |
| Interventions | Participants who were enrolled in the 2005 to 2006 season were randomised (see aa Ohmit 2006) to receive inactivated vaccine (Fluzone; Sanofi Pasteur), live attenuated vaccine (FluMist; MedImmune) or placebo. Participants who had already been enrolled in the 2004 to 2005 season received the same intervention type (i.e. Fluzone, FluMist or placebo) as before ‐ Fluzone (intramuscularly administered) contained 15 g haemagglutinin from each of the following strains: A/New Caledonia/20/99 (H1N1), A/New York/55/2004 (H3N2) (A/California/7/2004‐like) and B/Jiangsu/10/2003 (B/Shanghai/361/2002‐like) ‐ FluMist (intranasally administered) was formulated to contain a median tissue‐culture infective dose of 106.5 to 107.5 live attenuated influenza virus reassortants of the same strains ‐ Intramuscular or intranasal saline placebo | |
| Outcomes | ‐ Local and systemic reactions within 7 days from immunisation (see Ohmit 2006) ‐ Symptomatic laboratory‐confirmed influenza A or B illness (primary efficacy outcome). Symptoms were defined as at least 1 respiratory symptom (cough or nasal congestion) plus at least 1 systemic symptom (fever or feverishness, chills or body aches). Laboratory confirmation was assessed by isolation of the influenza virus in cell culture or by comparison of paired post‐vaccination (pre‐season) and post‐season serum with at least a 4‐fold increase in haemagglutination‐inhibition antibody titre to 1 circulating influenza strain | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial conducted in the USA during the 1993 to 1994 influenza season. Follow‐up was not indicated. Influenza period was not defined. Participants were randomly assigned to receive 1 of the following 5 vaccine preparations in a double‐blinded manner: 15 mg of rHA0, 15 mg of rHA0 plus alum, 90 mg of rHA0, licensed and placebo. Spring sera were collected | |
| Participants | 34 healthy university students: 26 treated and 8 placebo. Age of participants was: 18 to 45 | |
| Interventions | Subvirion licensed trivalent parenteral AB vaccine. Schedule and dose were: single dose; 15 µg each strain. Vaccine composition was: A/Texas/36/91 (H1N1), A/Beijing/32/92 (H3N2) and B/Panama/45/90. Placebo was saline for injection. Vaccine was recommended and matched circulating strain | |
| Outcomes | Clinical and laboratory‐confirmed cases and adverse effects. An "influenza‐like illness" was defined as the presence of any respiratory symptom(s) for >= 2 days, accompanied by fever or systemic symptoms of myalgia or chills. Laboratory evidence of influenza A (H3N2) virus infection was defined as either or both of the isolation of virus from nasopharyngeal secretion and a >= 4‐fold increase in serum HAI antibody titre between the 3‐week post‐vaccination (pre‐season) specimen and the corresponding post‐season specimen collected in the following spring. Local adverse effects were erythema, pain, tenderness, induration, arm stiffness; systemic adverse effects: were headache, generalised myalgia, diarrhoea, nausea, feverishness, temperature > 37.8 °C | |
| Notes | Efficacy and safety data were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Single‐blind randomised controlled trial conducted in the USA during the 1974 to 1975 influenza season. Follow‐up lasted from winter to spring. A 'two‐month' epidemic period was described by the authors with no reference to a definition and lasted 6 weeks. Study participants were randomly assigned into 3 subgroups to receive either 2 doses of the vaccine (n = 47), 1 dose of vaccine and 1 dose of placebo (n = 48) or 2 doses of placebo (n = 48) at 14 days apart. 6‐month sera were collected on all study participants | |
| Participants | 34 healthy university students: 26 treated and 8 placebo. Age of participants was 18 to 45 | |
| Interventions | Subvirion monovalent parenteral vaccine. Schedule and dose were: single dose; 90 µg rHAO. Vaccine composition was: the recombinant HA vaccine contained full‐length uncleaved haemagglutinin (HA0) glycoprotein from the influenza A/Beijing/32/92 (H3N2) virus. Placebo was saline for injection. Vaccine was not recommended but matched circulating strain | |
| Outcomes | Clinical and laboratory‐confirmed cases. An "influenza‐like illness" was defined as the presence of any respiratory symptom(s) for >= 2 days, accompanied by fever or systemic symptoms of myalgia or chills. Laboratory evidence of influenza A (H3N2) virus infection was defined as either or both of the isolation of virus from nasopharyngeal secretion and a >= 4‐fold increase in serum HAI antibody titre between the 3‐week post‐vaccination (pre‐season) specimen and the corresponding post‐season specimen collected in the following spring | |
| Notes | Safety data were not included; effectiveness data were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial conducted in the USA during the 1993 to 1994 influenza season. Follow‐up was not indicated. Influenza period was not defined. Participants were randomly assigned to receive 1 of the following 5 vaccine preparations in a double‐blinded manner: 15 mg of rHA0, 15 mg of rHA0 plus alum, 90 mg of rHA0, licensed and placebo. Spring sera were collected | |
| Participants | 59 healthy university students: 51 treated and 8 placebo. Age of participants was 18 to 45 | |
| Interventions | Subvirion monovalent parenteral vaccine. Schedule and dose were: single dose; 15 µg rHAO. Vaccine composition was: the recombinant HA vaccine contained full‐length uncleaved haemagglutinin (HA0) glycoprotein from the influenza A/Beijing/32/92 (H3N2) virus. Placebo was saline for injection. Vaccine was not recommended but matched circulating strain | |
| Outcomes | Clinical and laboratory confirmed cases. An "influenza‐like illness" was defined as the presence of any respiratory symptom(s) for >= 2 days, accompanied by fever or systemic symptoms of myalgia or chills. Laboratory evidence of influenza A (H3N2) virus infection was defined as either or both of the isolation of virus from nasopharyngeal secretion and a >= 4‐fold increase in serum HAI antibody titre between the 3‐week post‐vaccination (pre‐season) specimen and the corresponding post‐season specimen collected in the following spring | |
| Notes | Efficacy data only were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Single‐blind randomised controlled trial conducted in the USA during the 1974 to 1975 influenza season. Follow‐up lasted from winter to spring. A "two month" epidemic period was described by the authors with no reference to a definition and lasted 6 weeks. Study participants were randomly assigned into 3 subgroups to receive either 2 doses of the vaccine (n = 47), 1 dose of vaccine and 1 dose of placebo (n = 48) or 2 doses of placebo (n = 48) at 14 days apart. 6‐month sera were collected on all study participants | |
| Participants | 143 young adult female student nurse volunteers: 95 treated and 48 placebo. Age of participants was 18 to 35 | |
| Interventions | Live attenuated, bivalent, intranasal influenza A (containing 107,2 EID50) and B (containing 107,8 EID50) vaccines. Schedule and dose were single or double doses. Vaccine composition was: A/England/42/72 (H3N2) and B/Hong Kong/5/72. Placebo was 5% sucrose. Vaccine was not recommended and did not match the circulating strain | |
| Outcomes | Influenza and adverse effects. An influenza case was defined as the presence of an influenza‐like illness (3 or more symptoms of acute respiratory disease and temperature greater then 37.2 °C) and virus isolation and/or 4‐fold rise in antibody titre in sera obtained at 30 days and 6 months following immunisation. Local adverse effects were upper respiratory symptoms and cough. These were subdivided into moderate and severe. A definition of general adverse effects (again distinguished among moderate and severe) was not given | |
| Notes | 1 dose and 2 doses were analysed together. Circulating strain was A/PortChalmers/1/73 (H3N2). Efficacy and safety data extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Field trial conducted in Russia during the 1968 to 1969 influenza season. Follow‐up lasted the whole epidemic period. The epidemic period was defined as the period of highest influenza morbidity and lasted 11 weeks, from the last 10 days of January to the first 10 days of April. Vaccinations were carried out using coded preparation. Sampling virological and serological survey of ill people was performed | |
| Participants | 19,887 population: 9945 treated and 9942 placebo. Age of participants was 13 to 25 | |
| Interventions | Live allantoic intranasal vaccine. Schedule and dose were: 3 doses. Vaccine composition was not indicated. Placebo was not described. Vaccine was not recommended and did not match the circulating strain | |
| Outcomes | Clinical cases, deaths, severity of illness. Clinical outcomes were all the acute respiratory infections. Laboratory confirmation was obtained on a sample of ill participants by virus isolation or demonstration of seroconversion. Bronchitis, otitis and pneumonia were considered as complications. Passive surveillance was carried out | |
| Notes | A first study group with children 3 to 12 years old was excluded. A second study group with participants aged 13 to 25 was included in the analysis. The trial compared 2 live vaccines (allantoic intranasal vaccine and tissue vaccine for oral administration) against placebo. Only intranasal vaccine was included in the analysis. Deaths from flu were not recorded. Circulating strain was A2/Hong Kong/68 Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient description |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient description |
| Summary assessment | Unclear risk | Insufficient description |
| Methods | Controlled clinical trial, double‐blind, conducted in Australia during the 1981 influenza season. Follow‐up lasted from winter to spring. Influenza period was not defined. Voluntary were alternatively allocated to groups in a double‐blind manner. 6‐month sera were collected | |
| Participants | 88 volunteer staff from Newcastle Hospital and the Commonwealth Steel Corporation: 56 treated and 32 placebo. Age of participants was 16 to 64 | |
| Interventions | Trivalent subunit parenteral vaccine. Schedule and dose were: 7 µg each, 1 or 2 doses. Vaccine composition was: A/Brazil/11/78, A/Bangkok/1/79, B/Singapore/222/79. Placebo was saline for injection. Vaccine was recommended and matched circulating strain | |
| Outcomes | Influenza and adverse effects. A case of influenza was defined as a respiratory illness, retrospectively reported, associated with a 4‐fold antibody titre increase between post‐vaccination and post‐epidemic sera. Local side effects were redness, swelling, warmth or irritation, pain on contact, pain with pressure, continuous pain, or restriction of arm movement; systemic reactions were fever, chills, sweating, drowsiness or insomnia | |
| Notes | 1 dose and 2 doses were analysed together; very high drop‐out. Circulating strain was A/Bangkok/1/79. Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Inadequate |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding (performance bias and detection bias) | High risk | Inadequate |
| Incomplete outcome data (attrition bias) | High risk | Inadequate |
| Summary assessment | High risk | Inadequate |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1968 to 1969 influenza season. Follow‐up lasted the whole epidemic period. The epidemic curve was traced by absenteeism in the local industries and schools and virus isolation and lasted 7 weeks. Randomisation methods were not described. One‐half of the volunteers gave serial blood and nasal wash samples | |
| Participants | 524 school teachers: 465 treated and 118 placebo. Age of participants was not indicated | |
| Interventions | Monovalent inactivated intramuscular vaccine. Schedule and dose were: 1 or 2 doses. Vaccine composition was: A/Hong Kong/68. Placebo was saline for injection. Vaccine was recommended and matched circulating strain | |
| Outcomes | Clinical cases and side effects. Clinical case definition was based on the presence of a temperature > 100 °F or a feverish feeling plus any 2 of the following symptoms: sore throat, muscle or joint pain, cough, stuffy or runny nose. Passive surveillance was carried out | |
| Notes | Data concerning adverse effects were only partially reported by graph. Circulating strain was A2/Hong Kong/68. Effectiveness data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1968 to 1969 influenza season. Follow‐up lasted the whole epidemic period. Epidemic curve was traced by absenteeism in the local industries and schools and virus isolation and lasted 7 weeks. Randomisation methods were not described. One‐half of the volunteers gave serial blood and nasal wash samples | |
| Participants | 590 school teachers: 471 treated and 119 placebo. Age of participants was not indicated | |
| Interventions | Polyvalent inactivated intramuscular vaccine. Schedule and dose were: 1 or 2 doses. Vaccine composition was: A2/Japan/170/62 150 CCA, A2/Taiwan/1/64 150 CCA, B/Massachusetts/3/66 300 CCA. Placebo was saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Clinical cases and side effects. Clinical case definition was based on the presence of a temperature > 100 °F or a feverish feeling plus any 2 of the following symptoms: sore throat, muscle or joint pain, cough, stuffy or runny nose. Passive surveillance was carried out | |
| Notes | Data concerning adverse effects were only partially reported by graph. Circulating strain was A2/Hong Kong/68. Efficacy data only were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1968 to 1969 influenza season. Follow‐up lasted the whole epidemic period. The epidemic curve was traced by absenteeism in the local industries and schools and virus isolation and lasted 7 weeks. Randomisation methods were not described. One‐half of the volunteers gave serial blood and nasal wash samples | |
| Participants | 597 school teachers: 479 treated and 118 placebo. Age of participants was not indicated | |
| Interventions | Monovalent inactivated aerosol vaccine. Schedule and dose were: 1 or 2 doses. Vaccine composition was: A/Hong Kong/68. Placebo was saline for injection. Vaccine was recommended and matched circulating strain | |
| Outcomes | Clinical cases and side effects. Clinical case definition was based on the presence of a temperature > 100 °F or a feverish feeling plus any 2 of the following symptoms: sore throat, muscle or joint pain, cough, stuffy or runny nose. Passive surveillance was carried out | |
| Notes | Data concerning adverse effects were only partially reported by graph. Circulating strain was A2/Hong Kong/68. Efficacy data only were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1968 to 1969 influenza season. Follow‐up lasted the whole epidemic period. The epidemic curve was traced by absenteeism in the local industries and schools and virus isolation and lasted 7 weeks. Randomisation methods were not described. One‐half of the volunteers gave serial blood and nasal wash samples | |
| Participants | 590 school teachers: 471 treated and 119 placebo. Age of participants was not indicated | |
| Interventions | Polyvalent inactivated aerosol vaccine. Schedule and dose were: 1 or 2 doses. Vaccine composition was: A2/Japan/170/62 150 CCA, A2/Taiwan/1/64 150 CCA, B/Massachusetts/3/66 300 CCA. Placebo was saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Clinical cases and side effects. Clinical case definition was based on the presence of a temperature > 100 °F or a feverish feeling plus any 2 of the following symptoms: sore throat, muscle or joint pain, cough, stuffy or runny nose. Passive surveillance was carried out | |
| Notes | Data concerning adverse effects were only partially reported by graph. Circulating strain was A2/Hong Kong/68. Efficacy data only were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1968 to 1969 influenza season. Follow‐up lasted the whole epidemic period. The epidemic curve was traced by absenteeism in the local industries and schools and virus isolation and lasted 7 weeks. Identical‐looking coded vials were used to dispense material. Sampling virological and serological survey of ill people was performed. 2 doses were administered but as the outbreak occurred mostly between them, only the effectiveness of the first dose was assessed | |
| Participants | 244 volunteer students and staff members: 195 treated and 49 placebo. Age of participants was not indicated | |
| Interventions | Monovalent A aerosol vaccine. Schedule and dose were: 200 CCA. Vaccine composition was: A2/Aichi/1/68. Placebo was saline for injection. Vaccine was recommended and matched circulating strain | |
| Outcomes | Clinical cases and adverse effects. Clinical cases were defined as febrile respiratory illness with oral temperature higher then 99.5 °F. Local adverse effects were defined as pain and/or tenderness and redness and/or swelling. Systemic adverse effects were defined as general (fever, muscle pain, nausea or vomiting, diarrhoea and malaise) or respiratory (runny and/or stuffy nose, sore throat, cough, shortness of breath). Passive surveillance was carried out | |
| Notes | Illness during the first 1 or 2 weeks after vaccination was not excluded, but the authors stated that this fact did not affect the results. Circulating strain was A2/Aichi/2/68. Efficacy and safety data were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1968 to 1969 influenza season. Follow‐up lasted the whole epidemic period. The epidemic curve was traced by absenteeism in the local industries and schools and virus isolation and lasted 7 weeks. Identical‐looking coded vials were used to dispense material. Sampling virological and serological survey of ill people was performed. 2 doses were administered but as the outbreak occurred mostly between them, only the effectiveness of the first dose was assessed | |
| Participants | 239 volunteer students and staff members: 190 treated and 49 placebo. Age of participants was not indicated | |
| Interventions | Monovalent A subcutaneous vaccine. Schedule and dose were: 200 CCA. Vaccine composition was: A2/Aichi/1/69. Placebo was saline for injection. Vaccine was recommended and matched circulating strain | |
| Outcomes | Clinical cases and adverse effects. Clinical cases were defined as febrile respiratory illness with oral temperature higher then 99.5 °F. Local adverse effects were defined as pain and/or tenderness and redness and/or swelling. Systemic adverse effects were defined as general (fever, muscle pain, nausea or vomiting, diarrhoea and malaise) or respiratory (runny and/or stuffy nose, sore throat, cough, shortness of breath). Passive surveillance was carried out | |
| Notes | Illness during the first 1 or 2 weeks after vaccination was not excluded, but the authors stated that this fact did not affect the results. Circulating strain was A2/Aichi/2/68. Efficacy and safety data were extracted. Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1968 to 1969 influenza season. Follow‐up lasted the whole epidemic period. The epidemic curve was traced by absenteeism in the local industries and schools and virus isolation and lasted 7 weeks. Identical‐looking coded vials were used to dispense material. Sampling virological and serological survey of ill people was performed. 2 doses were administered but as the outbreak occurred mostly between them, only the effectiveness of the first dose was assessed | |
| Participants | 243 volunteer students and staff members: 194 treated and 49 placebo. Age of participants was not indicated | |
| Interventions | Bivalent AB aerosol vaccine. Vaccine composition was: A2/Japan/170/62 150 CCA, A2/Taiwan/1/64 150 CCA and B/Massachusetts/3/66 200 CCA. Placebo was saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Clinical cases and adverse effects. Clinical cases were defined as febrile respiratory illness with oral temperature higher then 99.5 °F. Local adverse effects were defined as pain and/or tenderness and redness and/or swelling. Systemic adverse effects were defined as general (fever, muscle pain, nausea or vomiting, diarrhoea and malaise) or respiratory (runny and/or stuffy nose, sore throat, cough, shortness of breath). Passive surveillance was carried out | |
| Notes | Illness during the first 1 or 2 weeks after vaccination was not excluded but the authors stated that this fact did not affect the results. Circulating strain was A2/Aichi/2/68. Efficacy and safety data were extracted. Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1968 to 1969 influenza season. Follow‐up lasted the whole epidemic period. The epidemic curve was traced by absenteeism in the local industries and schools and virus isolation and lasted 7 weeks. Identical‐looking coded vials were used to dispense material. Sampling virological and serological survey of ill people was performed. 2 doses were administered but as the outbreak occurred mostly between them, only the effectiveness of the first dose was assessed | |
| Participants | 236 volunteer students and staff members: 187 treated and 49 placebo. Age of participants was not indicated | |
| Interventions | Bivalent AB subcutaneous vaccine. Vaccine composition was: A2/Japan/170/62 150 CCA, A2/Taiwan/1/64 150 CCA and B/Massachusetts/3/66 200 CCA. Placebo was saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Clinical cases and adverse effects. Clinical cases were defined as febrile respiratory illness with oral temperature higher then 99.5 °F. Local adverse effects were defined as pain and/or tenderness and redness and/or swelling. Systemic adverse effects were defined as general (fever, muscle pain, nausea or vomiting, diarrhoea and malaise) or respiratory (runny and/or stuffy nose, sore throat, cough, shortness of breath). Passive surveillance was carried out | |
| Notes | Illness during the first 1 or 2 weeks after vaccination was not excluded, but the authors stated that this fact did not affect the results. Circulating strain was A2/Aichi/2/68. Efficacy and safety data were extracted. Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in the USA during the 1985 to 1986 influenza season. Follow‐up was not indicated. Epidemic influenza was defined according to population surveillance data (without better explanation), begun in December 1985 and concluded in February 1986. Participants were assigned using a random number generator to receive either the influenza vaccine or placebo. Virological surveillance was not performed | |
| Participants | 179 healthy volunteer hospital employees: 91 treated and 88 placebo. Age of participants was 21 to 65 | |
| Interventions | Split trivalent intramuscular vaccine. Schedule and dose were: single dose; 15 µg each strain. Vaccine composition was: A/Chile/1/83 (H1N1), A/Philippines/2/82 (H3N2) and B/USSR/100/83. Placebo was saline for injection. Vaccine was recommended but did not match the circulating strain | |
| Outcomes | Clinical cases symptoms defined, WDL regardless of causes and adverse effects. Influenza illness was defined by the CDC case definition: a documented temperature greater than 100 °F and at least the symptoms of cough or sore throat | |
| Notes | Data regarding WDL and adverse effects were not complete and they were not considered. Most of the influenza infections were caused by type B Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Semi‐randomised, double‐blind, placebo‐controlled clinical trial that took place in Leningrad, USSR during the 1981 to 1982 influenza season. The study tested the reactogenicity, safety and effectiveness of an inactivated and a live attenuated vaccine, both administered singly or in combination. Allocation was made on the basis of school classes and it is unclear whether this is a cluster‐randomised or clinical controlled trial. We have opted for the latter as the text mentions random selection to maintain "equivalence". "Double blind" is mentioned in the text. In January to May 1982 there was a rise in the level of ILI due to influenza and other agents | |
| Participants | 3961 participants were enrolled. Participants were healthy "students" aged 18 to 23. Numbers in each of the 4 arms are uneven throughout the trial but no reason is given for this | |
| Interventions | Inactivated vaccine trivalent (Ministry of Health USSR) by subcutaneous injection 0.2 ml once (arm 1), or intranasal live "recombinant" "mono"vaccine 0.5 ml spray 2 to 3 times (Ministry of Health USSR) (arm 2), or combined (arm 3) or subcutaneous and intranasal spray NaCl saline placebo (arm 4). The strains contained were H1N1, H3N2 and B. Vaccine matching was not good | |
| Outcomes | Serological | |
| Notes | The authors conclude that simultaneous inoculation of the vaccines appeared to produce better humoral antibody responses, especially in the last season. However, the correlation between clinical protection and antibody rises is reported as dubious. The authors make the reasonable point that perhaps live attenuated vaccines work better because they stimulate production of secretory antibodies. This is a poorly reported study. No mention is made of how the placebo could have been correctly used in the schedule (i.e. they should have had 6 arms instead of 4 with subcutaneous placebo, spray placebo separately as well combined ‐ maybe this is a problem of translation). Efficacy data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Semi‐randomised, double‐blind, placebo‐controlled clinical trial that took place in Leningrad, USSR during the 1982 to 1983 influenza season. The study tested the reactogenicity, safety and effectiveness of an inactivated and a live attenuated vaccines both administered singly or in combination. Allocation was made on the basis of school classes and it is unclear whether this is a cluster‐randomised or clinical controlled trial. We have opted for the latter as the text mentions random selection to maintain "equivalence". "Double blind" is mentioned in the text. In the season there was an outbreak of A (H3N2) lasting 4 to 5 weeks. However, influenza accounted for only up to 30% of isolates from ill people | |
| Participants | 3944 participants were enrolled. Participants were healthy "students" aged 18 to 23. Numbers in each of the 4 arms are uneven throughout the trial but no reason is given for this | |
| Interventions | Inactivated vaccine trivalent (Ministry of Health USSR) by subcutaneous injection 0.2 ml once (arm 1), or intranasal live "recombinant" "mono" vaccine 0.5 ml spray 2 to 3 times (Ministry of Health USSR) (arm 2), or combined (arm 3) or subcutaneous and intranasal spray NaCl saline placebo (arm 4). The strains contained were H1N1, H3N2 and B | |
| Outcomes | Serological | |
| Notes | The authors conclude that simultaneous inoculation of the vaccines appeared to produce better humoral antibody responses, especially in the last season. However, the correlation between clinical protection and antibody rises is reported as dubious. The authors make the reasonable point that perhaps live attenuated vaccines work better because they stimulate production of secretory antibodies. This is a poorly reported study. No mention is made of how the placebo could have been correctly used in the schedule (i.e. they should have had 6 arms instead of 4 with subcutaneous placebo, spray placebo separately as well combined ‐ maybe this is a problem of translation). Efficacy data only were extracted. Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Double‐blind, placebo‐controlled, randomised trial | |
| Participants | 74 healthy volunteers aged 18 to 40 years (data on 17 asthmatics were not extracted) | |
| Interventions | Cold ‐ recombinant vaccine A (H1N1); n = 16 versus cold ‐ recombinant vaccine A (H3N2); n = 13 versus cold ‐ recombinant vaccine B; n = 17 versus placebo; n = 26 | |
| Outcomes | Pulmonary function tests (performed on day 0, 3 to 4, 7 after vaccination): | |
| Notes | The authors report several non‐significant drops in FEV and FVC up to 7 days post‐inoculation and a higher incidence of ILI (17/46 versus 4/26) in the vaccinated arms. Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial carried out from April 1976 at Rochester University. Vaccine and placebo were randomly administered in a double‐blind manner, but no description of allocation procedure is given. 36 days after immunisation all participants were challenged with wild type virus (A/Victoria/3/75, H3N2) and antibody response was determined from serum and nasal secretions (before vaccination, 36 hours later and 21 days after challenge, not for analysis) | |
| Participants | 47 healthy male and female university students with absent or low HAI titre (i.e. little or no immunity) to both A/Scotland/74 and A/Victoria/3/75 | |
| Interventions | Live attenuated A/Scotland/74 (H3N2) versus placebo, one 0.5 ml dose intranasally. On day 37 after immunisation participants were challenged with A/Victoria/3/75 | |
| Outcomes | A physician examined the participants 1 day and 4 days after they received vaccine or placebo. Temperature was observed only 1 day after. Observed symptoms were: mild sore throat and rhinorrhoea: vaccine 4/23; placebo 3/24; fever (temperature > 37.50 °C): none had it | |
| Notes | Safety data only were extracted Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Not used |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Open‐label/single‐blind randomised controlled trial to assess the safety and immunogenicity of adjuvanted and unadjuvanted subunit influenza vaccine, prepared with the strains recommended for and isolated in the 1997 to 1998 season | |
| Participants | 74 healthy adults aged between 10 and 40 years, who did not receive influenza immunisation during the 6 months preceding the trial | |
| Interventions | 1) M‐59 adjuvanted subunit trivalent flu vaccine (prepared with A/Bayern/795 H1N1, A/Wuhan/359/95 H3N2, B/Beijing/184/93‐like strains, each 15 µg/ 0.5 ml dose) | |
| Outcomes | After each immunisation, participants were observed for 30 minutes, were examined after 2 days and then completed a diary card reporting symptoms that occurred within 7 days after. Local reactions: nasal symptoms, unpleasant taste, bloody nasal discharge, sneezing. Systemic reactions: chills, pulmonary, nausea, malaise, myalgia or arthralgia, urticarial rash, headache, oral temperature >= 38 °C, stay at home, use of analgesic or antipyretic. Data were not given separately for the randomised and open‐label phase of the study | |
| Notes | It is not possible to consider the safety data separately for the two study phases. Safety data only were extracted Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial to assess the reactogenicity and safety of monovalent whole virus and split virus vaccines prepared with strain A/Victoria/3/75 from different US manufacturers | |
| Participants | 208 healthy adult volunteers aged between 18 and 64 years, recruited from the University of Maryland, USA | |
| Interventions | Monovalent whole‐virus vaccine (Merck Sharp & Dohme, Merrell‐National Laboratories) or monovalent split virus vaccine (Parke, Davis and Company; Wyeth Laboratories) administered in different antigen concentrations (200, 400 or 800 CCA) versus placebo. All from A/Victoria75. 1 dose intramuscularly | |
| Outcomes | Temperature >= 100 °F (37.8 °C); feverishness; pain or burning; tenderness; malaise or myalgia; nausea or vomiting; headache; other. 21‐day follow‐up. Safety outcomes are also given as cumulative % for each category: local, systemic, bothersome; febrile; or scores for systemic reactions | |
| Notes | Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial | |
| Participants | 432 healthy participants aged between 18 and 22 years who had not received any influenza immunisation during the previous 2 to 3 years | |
| Interventions | Polymer‐subunit influenza vaccine "Grippol" prepared with the strains A/Victoria/36/88, Wib ‐ 26, B/Panama 45/90. 2 types containing 5 or 2.5 µg haemagglutinin of each strain respectively were compared with whole‐virion inactivated trivalent vaccine (reference preparation, containing 35 µg of haemagglutinin) and placebo (consisting of sterile physiological solution). One 0.5 ml dose was administered subcutaneously | |
| Outcomes | After immunisation participants were placed under medical observation. Fever (48 hours follow‐up): weak (37.1 to 37.5 °C), moderate (37.6 to 38.5 °C), severe (> 38.6 °C). Systemic reactions (3 to 4 days follow‐up): feeling unwell, sore throat, hyperaemia of nasopharynx, head cold, cough, headache, blocked nose, dizziness, shivering, drowsiness, nausea, hoarseness. Local reaction: all (moderate weak); pain at site of injection | |
| Notes | Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial | |
| Participants | 162 healthy participants aged 18 to 61 years | |
| Interventions | Bivalent live attenuated vaccine WRL 105 (recombinant of A/Okuda/57 and A/Finland/4/74) containing 107.0 EID50 virus/ 0.5 ml dose versus placebo. Both preparations were administered intranasally 3 to 4 weeks apart | |
| Outcomes | Reactions to immunisation were observed for 7 days after each dose. Local symptoms (referable to the upper respiratory tract, mainly nasal obstruction, nasal discharge or sore throat) reported as mild moderate or severe. General symptoms (mainly headache fever or myalgia). These 2 are further reported in different intensity classes (mild, moderate, severe, lasting for at least 4 days) reported as mild, moderate or severe. Use of analgesics | |
| Notes | Safety data only were extracted Funding source ‐ mixed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | From this report, only the first phase of the first trial is of interest for the purposes of this review, in which administration of whole virus, oil adjuvanted influenza vaccine Invirin (GSK) was compared with placebo in a semi‐randomised allocation. The trial was performed in November to December 1962 | |
| Participants | Medical students (n = 380) at the Queen's University of Belfast, UK | |
| Interventions | Trivalent aqueous vaccine (Invirin, Glaxo) one 0.25 ml dose IM containing strains A/Singapore/1/57, A/England/1/61, B/England/939/59. Placebo (phosphate‐buffered saline) was administered as control. Participants born on odd days were given placebo (n = 186); those born on even days received the vaccine (n = 194) | |
| Outcomes | Local reactions: pain, erythema, tenderness, bruises. Stratified by means of scores ranging from 0 to 3 depending on their severity. Systemic reactions: coryza, migraine, paroxysmal tachycardia. All assessed at day 0, 1, 3, 7, 21 after inoculation. Data refer to a 3‐day follow‐up | |
| Notes | Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | High risk | Unclear |
| Methods | Randomised controlled trial, double‐blind | |
| Participants | 119 healthy young adults from the Medical and Science Faculties of Sheffield University, UK, aged 18 to 19 years without egg allergy | |
| Interventions | Purified subunit monovalent B/Hong Kong/73 flu vaccine prepared in 4 antigen concentration 40, 20, 10, 5 µg of HA per each 0.5 ml dose versus saline placebo (0.5 ml dose) subcutaneously administered. Participants were divided in 5 groups of equal dimensions (no further description), each group received one of the tested coded preparations. Artificial challenge 1 month later with live attenuated RB77 virus | |
| Outcomes | Local and systemic reactions were assessed by means of questionnaires completed by participants 24 hours after immunisation. Local reactions (including redness, swelling, itching), local pain (including pain on pressure, pain on contact, continuous pain) | |
| Notes | Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, carried out during the season 1976 to 1977 | |
| Participants | 167 students at the technical school in Zagreb, former Republic of Yugoslavia, without sensitivity to egg proteins, pregnancy, acute or chronic diseases | |
| Interventions | Cold‐adapted recombinant A/Victoria/3/75 vaccine administered in 3 different antigen concentrations (107.5, 106.5, 105.5 EID50/0.5 ml) versus placebo. 1 0.5 ml dose intranasal | |
| Outcomes | Participants were medically examined on each of the successive 5 days after immunisation (lasting for at least 1 day). Throat infection, granular palate, oedematous uvula, fever (no cases) as cases and subject‐days. For the following outcomes, authors give the total number of observed cases, without indication of the corresponding arm: malaise, swollen tonsils, fever (1), rhinorrhoea (1), conjunctivitis (7), laryngitis or hoarseness (3), cough (1), swollen tonsils (1), malaise (1). Surveillance was active | |
| Notes | Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | This paper reports the results of 2 randomised controlled trials carried out in the USA | |
| Participants | Healthy volunteers recruited at Texas A&M University and Texas Medical Center, aged between 18 and 40 years | |
| Interventions | 2 0.5 ml doses of cold‐adapted recombinant influenza vaccines, 1 month apart, containing 107.1 TCID50 of each strain/dose. 2 studies were carried out in which 4 groups were formed: 1) placebo 1st and 2nd dose. 2) 1st: A/Kawasaki/9/86 (H1N1, CR 125) + A/Bethesda/1/85 (H3N2, CR90) + B/Ann Arbor/1/86 (B, CRB117) | |
| Outcomes | Mild upper respiratory symptoms. Fever >= 37.8 °C within 1 week after each inoculation | |
| Notes | Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | This paper reports the results of 2 randomised controlled trials carried out in the USA | |
| Participants | Healthy volunteers recruited at Texas A&M University and Texas Medical Center, aged between 18 and 40 years | |
| Interventions | A/Kawasaki/9/86 (H1N1, CR 125, but different lot from 1st) + A/Los Angeles/2/87 (H3N2, CR149) + B/Ann Arbor/1/86 (B, CRB117 but different lot from 1st) 3) 1st: A/Kawasaki/9/86 (H1N1, CR125) + A/Bethesda/1/85 (H3N2, CR90) 2nd: B/Ann Arbor/1/86 (B, CRB117) 4) 1st: B/Ann Arbor/1/86 (B, CRB117) 2nd: A/Kawasaki/9/86 (H1N1, CR125) + A/Los Angeles/2/87 (H3N2, CR149) | |
| Outcomes | Mild upper respiratory symptoms. Fever >= 37.8 °C Within 1 week after each inoculation | |
| Notes | See Keitel 1993a. Safety data only were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial | |
| Participants | Healthy adults aged 18 to 50 years | |
| Interventions | Inactivated A/New Caledonia/20/99 (H1N1) + A/Panama/2007/99 (H3N2) + B/Guangdong/120/2000 non‐covalent associated with outer membrane protein of N. meningitidis. Single nasal dose containing 15, 30, 45 µg versus placebo (phosphate buffered saline) intranasal administered | |
| Outcomes | Local: within 7 days, graphic ‐ rhinorrhoea, congestion, itch/burn, nosebleed, red/puffy eyes, sneezing, sore throat. Systemic: within 7 days ‐ cough, shortness of breath, headache, muscle/joint aches, poor appetite, fatigue within 48 hours, nasal mucosa inflammation, nasal discharge, pharyngeal inflammation, sinusitis, enlarged cervical/post‐auricular nodes | |
| Notes | Safety data only were extracted Government and industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | High risk | High risk |
| Methods | Controlled trial. Randomisation procedure was neither described nor mentioned. Participants were paired according to age and sex, in each pair 1 individual received vaccine, the other placebo. Double‐blind | |
| Participants | 37 volunteers aged 18 to 24 years, with titre of serum neutralising antibodies to A/Hong Kong/8/68 ? 1:16 | |
| Interventions | Live attenuated A/England/ 8/68 grown in presence of heated equine serum. 2 0.5 ml doses containing 104 TCID50 of this strain or placebo (0.85% NaCl) were administered intranasally 2 to 3 weeks apart | |
| Outcomes | Individual observed for 4 days, beginning 24 hours after immunisation. Fever, myalgia, rhinitis, cough, pharyngitis | |
| Notes | Safety data only were extracted Government and industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial | |
| Participants | 43 seronegative healthy adults aged between 22 and 50 years | |
| Interventions | Live attenuated serum inhibitor resistant flu B vaccine R75 (a recombinant of B/Hong Kong/5/72 with B/Russia/69) containing 107.2 EID50 of R75/0.5 ml dose versus placebo (sucrose 5%). Intranasal, 2 doses, 2 weeks apart | |
| Outcomes | Participants were interviewed during the 5 days following each immunisation. Local reaction (defined as immediate complains and comprising bad taste or burning, lasting for a few moments). Systemic reaction (consisting essentially of headache and rhinorrhoea) | |
| Notes | Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial carried out in the 1976 to 1977 season in Finland | |
| Participants | 307 healthy adults | |
| Interventions | 1 of the following 4 preparations were administered to 1 of the 4 groups of participants: live attenuated A/Victoria/3/75; 2 2 ml doses (2 104.5 bivalent subunit vaccine containing 1200 IU of A/Victoria/3/75 (H3N2) and 800 IU of B/Hong Kong/8/73 per dose (0.5 ml) B versus placebo (phosphate buffered saline). Participant received 1 dose administered subcutaneously. Vaccinations were performed between 15 to 23 December 1976; epidemics occurred February to June 1977 | |
| Outcomes | Harms assessed by questionnaires filled out by each participant within 3 days after immunisation. Fever: vaccine 11/151; placebo 9/154 ‐ muscle ache; vaccine 26/151; placebo 12/154 ‐ redness: vaccine 53/151; placebo 3/154 ‐ tenderness at vaccination site: vaccine 89/151; placebo 12/154 ‐ tenderness of axillary glands: vaccine 6/151; placebo 2/154 | |
| Notes | Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial carried out in Wien | |
| Participants | 20 university students aged 20 to 24 years | |
| Interventions | First phase: cold‐recombinant, live flu vaccine II RB‐77 (B/Ann Arbor/1/66 and B/Tecumse/10/77) containing 107.2 EID50 per 0.5 ml dose versus placebo. 1 dose intranasally. During this phase, participants lived under sequestered condition and close contact between vaccine and placebo recipients was possible. 2nd phase: 3 weeks after the 1st dose all participants were immunised with 1 dose of the same vaccine | |
| Outcomes | During the 5 days following immunisation, participants were medically observed and temperature recorded morning and evening. Occurring symptoms were attributed scores (0 to 3) depending on their severity (no, light, moderate, severe). Fever (oral temperature > 38 °C): 0/10; 0/10 sneezing: 1/10; 0/10 stuffy nose: 7/10; 1/10 running nose: 3/10; 0/10 afebrile subjective symptoms: 8/10; 2/10 | |
| Notes | Safety data only were extracted Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Cluster‐randomised controlled trial carried out during the 1976 to 1977 season | |
| Participants | 496 healthy military recruits (aged 18 to 20 years) belonging to 4 different companies from "Scuola Allievi Sottoufficiali" in Viterbo, Italy | |
| Interventions | 1 of the following 4 preparations were administered to 1 of the 4 groups of participants: live attenuated A/Victoria/3/75; 2 2 ml doses (2 104.5 EID50/dose) oral. Live attenuated recombinant A/Puerto Rico/8/34, A/Victoria/3/75; 2 0.5 ml doses intranasally (107 EID50/dose). Inactivated A/Victoria/3/75 (600 IU), B/Hong Kong/5/72 (300 IU) and AlPO4, intramuscular placebo (vaccine diluent) administered intranasally. The 2 doses were administered 2 to 3 weeks apart | |
| Outcomes | Within 15 days after administration of the 1st dose. Malaise, myalgia, headache, sore throat, cough, fever equal to or more than 38.5 °C, fever equal to or more than 37.5 °C, 3 or more symptoms, any symptoms. Surveillance was passive | |
| Notes | Units of randomisation appear to be companies. No description of allocation manner is mentioned. Blind (only for the cases of intranasal administration). Influenza outbreak occurred when the immunisation began (intraepidermic study) Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | As above | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Not used |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised controlled trial, double‐blind, conducted in Finland during the 1996 to 1997 influenza season. Randomisation methods were not described | |
| Participants | 216 healthcare workers: 211 treated and 427 placebo | |
| Interventions | Trivalent inactivated intramuscular vaccine. Schedule and dose were: single dose; 15 µg each strain. Vaccine composition was: A/Wahan/359/95, A/Singapore/6/86 and B/Beijing/184/93. Placebo was saline for injection. Vaccine was recommended | |
| Outcomes | Working days lost because of respiratory infections, episodes of respiratory infections, days ill and antimicrobial prescriptions. Respiratory infection was a common cold; febrile influenza‐like illnesses were not detected. Local adverse effects were defined as local pain. Systemic adverse effects were defined as fever and fatigue | |
| Notes | Efficacy data were not extracted because episodes of respiratory infections were unclearly defined. Safety data only were extracted Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over trial assessing the association between exposure to the vaccine and onset of oculo‐respiratory syndrome (ORS) in healthy adults with no previous history of ORS. The trial took place in 5 centres in Canada in September 2001 and was one of the conditions of registration of the vaccine, given the high incidence of ORS in the previous season. Centralised randomisation and allocation of centrally prepared, coded, opaque syringes took place. Cross‐over to either vaccine or placebo took place 5 to 7 days after the initial injection | |
| Participants | 651 adults with a mean age of 45 took part. 17 participants are unaccounted for | |
| Interventions | Fluviral (Shire) split trivalent containing A/New Caledonia/20/99 (H1N1); A/Panama/2007/99 (H3N2); B/Victoria/504/2000 with additional splitting with Triton X‐100 splitting agent or saline placebo 0.5 ml. Additional splitting was necessary to test the hypothesis that large clumps of virions were responsible for the ORS seen the previous season | |
| Outcomes | ORS (bilateral conjunctivitis, facial swelling ‐ lip, lid or mouth, difficulty in breathing and chest discomfort, including cough, wheeze, dysphagia or sore throat). Local signs/symptoms (redness, swelling, pain). Follow‐up was by phone interview at 24 hours and 6 days after vaccination | |
| Notes | The authors conclude that (mild) ORS is significantly associated with split TIV immunisation (attributable risk 2.9%, 0.6 to 5.2). Other adverse effects associated with TIV are hoarseness (1.3%, 0.3 to 1.3) and coughing 1.2%, 0.2 to 1.6). The study is good quality and the authors conclusions are robust. It is extraordinary that no one has looked for these symptoms before but it may be that the relatively young age of participants and the hypothesis contributed to this. Safety‐only study Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) | Low risk | Adequate |
| Summary assessment | Low risk | Adequate |
| Methods | Controlled trial, single‐blind | |
| Participants | 21 pairs of students and employers at the University of California, aged between 24 and 50 years who lived together or worked in close proximity | |
| Interventions | Recombinant, live attenuated R 75 vaccine (B/Hong Kong/5/72 and B/Russia/69) containing 107.5 EID/dose versus placebo (allantoic fluid). Lyophilised vaccine was supplied by Smith, Kline and French Laboratories and diluted with 2.5 ml of a 5% sucrose solution just before administration. Both preparations were administered intranasally (5 drops/nostril). In each pair 1 individual received vaccine and the other 1 placebo. A second dose was administered 14 days apart | |
| Outcomes | Any clinical symptoms within 7 days after each immunisation (rhinitis, cough, pharyngitis, headache, malaise and myalgia were the prominent observed symptoms, but given as aggregates) | |
| Notes | Reported safety data do not allow quantitative analysis Industry‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study | |
| Participants | Data from Vaccine Safety Datalink (large database of cases of disease following vaccination) in the USA | |
| Interventions | Immunisation with influenza and other vaccines assessed by means of medical records | |
| Outcomes | Cases: physician diagnosis of multiple sclerosis or optic neuritis in medical record | |
| Notes | Rare events (safety) Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | From HMO registry |
| CC ‐ control selection | Low risk | From HMO registry |
| CC ‐ comparability | Unclear risk | Poor matching |
| CC ‐ exposure | Unclear risk | From registry and from telephone interview |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study | |
| Participants | Cases = 145 Guillain‐Barre syndrome (GBS) cases (defined accordingly to the Brighton Collaboration definition) diagnosed in France between 2007 and 2010 | |
| Interventions | Exposure to influenza vaccine. Data about pandemic vaccine has been analysed separately | |
| Outcomes | Association between Guillain‐Barré syndrome and influenza vaccine exposure | |
| Notes | The study has been financial supported by LA‐SER, GSK Biologicals, and Sanofi‐Pasteur | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Unclear risk | From different countries |
| CC ‐ control selection | Unclear risk | Not same population, not sufficient description |
| CC ‐ comparability | Unclear risk | Matching |
| CC ‐ exposure | Unclear risk | Interview |
| Summary assessment | High risk | High risk of bias |
| Methods | Case‐control study | |
| Participants | Cases = 145 Guillain‐Barré syndrome (GBS) cases (defined accordingly to the Brighton Collaboration definition) diagnosed in France between 2007 and 2010 | |
| Interventions | Exposure to influenza vaccine. Data about pandemic vaccine has been analysed separately | |
| Outcomes | Association between Guillain‐Barré syndrome and influenza vaccine exposure | |
| Notes | The study has been financial supported by LA‐SER, GSK Biologicals and Sanofi‐Pasteur | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Low |
| CC ‐ control selection | Unclear risk | Not same population |
| CC ‐ comparability | Unclear risk | Matching |
| CC ‐ exposure | Unclear risk | Interview |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study | |
| Participants | Cases = 145 Guillain‐Barré syndrome (GBS) cases (defined accordingly to the Brighton Collaboration definition) diagnosed in France between 2007 and 2010 | |
| Interventions | Exposure to influenza vaccine. Data about pandemic vaccine has been analysed separately | |
| Outcomes | Association between Guillain‐Barré syndrome and influenza vaccine exposure | |
| Notes | The study has been financial supported by LA‐SER, GSK Biologicals and Sanofi‐Pasteur | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Low |
| CC ‐ control selection | Unclear risk | Not same population |
| CC ‐ comparability | Unclear risk | Matching |
| CC ‐ exposure | Unclear risk | Interview |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study | |
| Participants | Cases = 145 Guillain‐Barré syndrome (GBS) cases (defined accordingly to the Brighton Collaboration definition) diagnosed in France between 2007 and 2010 | |
| Interventions | Exposure to influenza vaccine. Data about pandemic vaccine has been analysed separately | |
| Outcomes | Association between Guillain‐Barré syndrome and influenza vaccine exposure | |
| Notes | The study has been financial supported by LA‐SER, GSK Biologicals and Sanofi‐Pasteur | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Low |
| CC ‐ control selection | Unclear risk | Not same population |
| CC ‐ comparability | Unclear risk | Matching |
| CC ‐ exposure | Unclear risk | Interview |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study | |
| Participants | Cases = 145 Guillain‐Barré syndrome (GBS) cases (defined accordingly to the Brighton Collaboration definition) diagnosed in France between 2007 and 2010 | |
| Interventions | Exposure to influenza vaccine. Data about pandemic vaccine has been analysed separately | |
| Outcomes | Association between Guillain‐Barré syndrome and influenza vaccine exposure | |
| Notes | The study has been financial supported by LA‐SER, GSK Biologicals and Sanofi‐Pasteur | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Low |
| CC ‐ control selection | Unclear risk | Not same population |
| CC ‐ comparability | Unclear risk | Matching |
| CC ‐ exposure | Unclear risk | Interview |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study testing the association between influenza vaccination and GBS | |
| Participants | Cases (n = 140): adults with Guillain‐Barré syndrome (GBS) defined accordingly to the Brighton Collaboration definition (levels 1 to 3) recruited at 121 neurological centres in 7 Italian regions and having symptoms onset between 1 October 2010 and 15 May 2011 Controls (n = 308): were selected from among patients admitted to the Emergency Department of the same hospital as the cases for acute conditions unrelated to chronic diseases (e.g. trauma). Each control was individually matched to a case for admission date (i.e. the same date as the case or up to 30 days afterwards), sex, age (± 5 years) and region of residence | |
| Interventions | Exposure to influenza vaccination (date and brand of vaccine) was verified by contacting patients’ general practitioners (GPs) by telephone. A neurologist (FG) closely verified and queried data quality | |
| Outcomes | Guillain‐Barré syndrome | |
| Notes | The authors also performed data analysis with a controlled case‐series design, considering the 6 weeks following exposure as the risk time Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Consecutive series of cases |
| CC ‐ control selection | Low risk | Hospital control |
| CC ‐ comparability | Unclear risk | Matched analysis only for sex, age, region, admission date |
| CC ‐ exposure | Unclear risk | Unclear if interviewers were blinded to case‐control status |
| Summary assessment | Unclear risk | Unclear risk of bias |
| Methods | Case‐control surveillance study | |
| Participants | Cases (n = 169): patients 18 years of age or older with a diagnosis of certain or probable immune thrombocytopaenia (ITP). Out of the 169 cases included, 130 were outpatients and 39 inpatients | |
| Interventions | Exposure to influenza vaccination during the 28 days preceding the index date. Exposure to other vaccines and drugs has also been considered | |
| Outcomes | Immune thrombocytopaenia (ITP) | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Hospital population |
| CC ‐ control selection | Low risk | Hospital control |
| CC ‐ comparability | Unclear risk | No matching |
| CC ‐ exposure | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Multicentre, case‐control study | |
| Participants | Cases (n = 104): Guillain‐Barré syndrome (GBS) cases (Brighton Collaboration definition, levels 1 to 3) | |
| Interventions | Exposure to monovalent pandemic H1N1 2009 to 2010 influenza vaccine during the 6 months preceding the index date. Vaccination data were obtained from vaccine registries (Denmark and France), from general practitioner records in the (UK and Netherlands) and from structured interviews (Sweden) | |
| Outcomes | Guillain‐Barré syndrome | |
| Notes | This study was funded by the European Centre for Disease Prevention and Control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Neurological clinic registry |
| CC ‐ control selection | Unclear risk | From the same population using only GP registry |
| CC ‐ comparability | Unclear risk | Poor matching |
| CC ‐ exposure | Unclear risk | Interview and record linkage |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study | |
| Participants | Cases (n = 198) were participants with an immune thrombocytopenia (ITP) diagnosis (American Society of Hematology diagnostic criteria) identified with the collaboration of 22 university and major regional hospitals in France participating in the Pharmacoepidemiological General Research on ITP (PGRx‐ITP) registry project Controls (n = 878) matched on age (2 years), sex, region of residence (northern or southern France), index date (date of first symptoms for the cases and date of consultation for the referents 2 months) from a random sample | |
| Interventions | Exposure to influenza vaccine. Assessed by structured interview and confirmed by vaccination records | |
| Outcomes | Immune thrombocytopenia (ITP) | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Multicentre registry consecutive series of cases |
| CC ‐ control selection | Unclear risk | Same population using registry from a sample of GPs |
| CC ‐ comparability | Unclear risk | Matching 1:5 |
| CC ‐ exposure | Unclear risk | Structured interview ‐ confirmation by GPs |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study based on the General Practice Research Database (GPRD) | |
| Participants | Cases (n = 163): patients with confirmed diagnosis of multiple sclerosis between 1 January 1993 and 31 December 2000 Controls (n = 1604): subjects from the GPRD matched to the cases for age, sex, practice, date of joining the practice | |
| Interventions | Exposure to vaccinations (also influenza) as shown from medical records | |
| Outcomes | Association between exposure to influenza vaccine and onset of multiple sclerosis | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Nested case‐control from GPRD registry |
| CC ‐ control selection | Low risk | GPRD registry |
| CC ‐ comparability | Low risk | Matched |
| CC ‐ exposure | Low risk | Registry |
| Summary assessment | Low risk | Low |
| Methods | Case‐control study assessing the association between influenza vaccines and cutaneous melanoma | |
| Participants | 99 cases and 104 controls | |
| Interventions | Influenza vaccine exposure is not described | |
| Outcomes | ||
| Notes | The authors report a protective effect of repeated influenza vaccination on the risk cutaneous melanoma (OR 0.43, 95% CI 0.19 to 1.00). The study is at high risk of bias because of the selective nature of cases (all patients in the authors' hospital), attrition bias (4 cases and 4 controls eliminated because of "failure to collaborate", recall bias (up to 5 years exposure data were based on patients' recollection) and ascertainment bias (non‐blinded exposure survey) Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Low |
| CC ‐ control selection | Unclear risk | Not sufficient information |
| CC ‐ comparability | Unclear risk | Not sufficient information |
| CC ‐ exposure | Low risk | Low |
| Summary assessment | Unclear risk | Unclear |
| Methods | 1 case‐control study and case series based in the German‐speaking regions of Switzerland, which assessed the association between an intranasal inactivated virosomal influenza vaccine and Bell's palsy | |
| Participants | 250 cases that could be evaluated (from an original 773 cases identified) were matched to 722 controls for age and date of clinic visit. All were aged around 50 | |
| Interventions | Immunisation with influenza vaccine took place within 91 days before disease onset | |
| Outcomes | Bells' palsy. | |
| Notes | The study reports a massive increase in risk (adjusted OR 84, 95% CI 20.1 to 351.9) within 1 to 91 days since vaccination. Despite its many limitations (case attrition: 187 cases could not be identified; ascertainment bias: physicians picked controls for their own cases; confounding by indication: different vaccine exposure rate between controls and the reference population) it is unlikely that such a large OR could have been affected significantly by systematic error. The authors called for larger pre‐licence safety trials, given the rarity of Bell's palsy. On the basis of this study the vaccine was withdrawn commercially Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | "All 4891 primary care physicians, ear, nose, and throat specialists, and neurologists in the study area were invited twice to report cases of Bell’s palsy first diagnosed between October 1, 2000, and April 30, 2001." |
| CC ‐ control selection | Low risk | Subsequently, the physicians who had reported cases of Bell’s palsy were asked to document the date of the visit and information pertinent to the study’s inclusion and exclusion criteria and to select, from among their patients without Bell’s palsy, 3 controls sequentially from their registration log Trained study monitors contacted the physicians and reviewed the selection forms regularly to ensure consistency in the selection of controls. At this point, participating physicians had not been made aware of the exposure to be investigated (influenza vaccination) |
| CC ‐ comparability | Unclear risk | The controls were matched with the case patients according to age (within 5 years), date of the clinic visit (within 4 days) and physician |
| CC ‐ exposure | Low risk | Physicians were asked to document the dates of administration and the brand name and type of influenza vaccine (parenteral or intranasal) used during the study period. Other vaccine exposures during the study period and the preceding 2 months were also documented. Since in all 43 sentinel cases reported in the study area the onset of Bell’s palsy occurred within 91 days after intranasal vaccination, we defined the period of 1 to 91 days as the postexposure risk period |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study assessing the association between influenza and other vaccines (data not extracted for this review) and optic neuritis "A matched case‐control study design was used with each optic neuritis case matched to 3 controls based on sex, deployment during the 18 weeks preceding the diagnosis date, and the military component in which the individual served (eg, active or reserve/National Guard). The protocol for this vaccine postmarketing surveillance investigation was approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board and reviewed by the Food and Drug Administration and Department of Defense" | |
| Participants | US military personnel aged at least 18 years | |
| Interventions | Cases (n = 1131) were participants with a diagnosis of optic neuritis between 1 January 1998 and 31 December 2003. The following ICD‐9 codes were considered: 377.30‐32, 377.39 | |
| Outcomes | Date of case diagnosis was ascertained and immunisation status (Anthrax, smallpox, Hepatitis B, influenza) verified by means of electronic records in respect of 3 time intervals: 6, 12 and 18 weeks before onset. For controls, vaccination status was determined for the 3 intervals before the index date. Results were focused on the 18‐week time interval | |
| Notes | Rare events (safety) Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | We defined optic neuritis cases as those having a first‐time diagnosis of the following ICD‐9‐CM codes: optic neuritis, unspecified (377.30); optic papillitis (377.31); retrobulbar neuritis, acute (377.32); and optic neuritis, other (377.39) during the period between 1 January 1998 and 31 December 2003 |
| CC ‐ control selection | Low risk | Controls were selected if their DMSS diagnostic records indicated no history of an optic neuropathy, if they served in the military on the same date of diagnosis as their matched case, and if they had at least 18 weeks of military service preceding this index date |
| CC ‐ comparability | Low risk | Matching |
| CC ‐ exposure | Low risk | We ascertained the date of each case’s first diagnosis of optic neuritis and determined all vaccinations received during each of the following 3 prior study intervals from the electronic record; 6 weeks (42 days), 12 weeks (84 days) and 18 weeks (126 days). For each of the 3 matched controls, we determined all |
| Summary assessment | Low risk | low |
| Methods | Case‐control study | |
| Participants | Cases (n = 415): participants with diagnosis of definite rheumatoid arthritis based on American College of Rheumatology (ACR) criteria Controls (n = 1245) matched for age and number of medical visits before index date | |
| Interventions | Exposure to influenza vaccine. Different times intervals before symptom onset were considered (90, 180, 365 and 730 days). Vaccine exposure status was determined from Kaiser Immunization Tracking System (KITS) and supplemented by chart reviews. Risk of association was, moreover, also determined for tetanus and hepatitis B vaccines | |
| Outcomes | ||
| Notes | This study was funded by the Centers for Disease Control and Prevention Vaccine Safety Datalink Project | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Included as cases the incident cases from the cohort analysis and additional new onset cases identified from the study population whose symptoms began during 1996 |
| CC ‐ control selection | Low risk | Same population |
| CC ‐ comparability | Unclear risk | Poor matching |
| CC ‐ exposure | Low risk | NCKPHP databases |
| Summary assessment | Unclear risk | Unclear |
| Methods | Study assessing the association between influenza vaccination the previous year and the risk of primary (i.e. occurring in people with no previous history of cardiac disease) cardiac arrest. Case‐control study on 360 cases and 418 controls | |
| Participants | Cases: participants who experienced primary cardiac arrest, aged between 25 to 74 years | |
| Interventions | Immunisation with influenza vaccine, assessed by means of questionnaires | |
| Outcomes | Cardiac arrest | |
| Notes | The authors concluded that vaccination is protective against primary cardiac arrest (OR 0.51, 95% CI 0.33 to 0.79). The difficulty of case ascertainment (77% of potential cases had no medical report and/or autopsy), recall bias (spouses provided exposure data for 304 cases, while 56 survivor cases provided data jointly with their spouses) make the conclusions of this study unreliable. It is impossible to judge the reliability of this study because of a lack of detail on the circulation of influenza in the study areas in the 12 months preceding cardiac arrest (the causal hypothesis is based on the effects of influenza infection on the oxygen supply to the myocardium through lung infection and inflammation). Rare events (safety) Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | From paramedic incident reports, cases of out‐of‐hospital PCA attended by paramedics in King County, Washington, from October 1988 to July 1994 were identified. PCA cases were defined by the occurrence of a sudden pulseless condition and the absence of evidence of a non‐cardiac condition as the cause of cardiac arrest |
| CC ‐ control selection | High risk | Selected from the community by using random digit dialling |
| CC ‐ comparability | Unclear risk | For each PCA case, 1 to 2 controls, matched for age (within 7 years) and sex |
| CC ‐ exposure | Unclear risk | Data on the participants’ vaccination status were collected from both case and control spouses by using a standardised questionnaire. For each participant, information was collected on whether they had received an influenza vaccination during the previous 12 months and, if so, when the vaccination |
| Summary assessment | High risk | |
| Methods | Case‐control study | |
| Participants | Cases (n = 140): participants affected by multiple sclerosis (MS) as defined by the International Panel on MS Diagnosis | |
| Interventions | Exposure to influenza vaccination (unspecified). Exposure to many other factors was assessed by means of face‐to‐face structured questionnaires. Time of onset after exposure is probably not mentioned in the text | |
| Outcomes | Multiple sclerosis | |
| Notes | "The study was supported by a grant of the University of Trieste, Italy: MPI 60%, 2001" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Hospital population |
| CC ‐ control selection | High risk | Blood donor population |
| CC ‐ comparability | High risk | Poor matching |
| CC ‐ exposure | High risk | Interview |
| Summary assessment | High risk | High risk |
| Methods | Large, prospective, cohort study assessing the possible association between monovalent, pandemic, H1N1 flu vaccine Pandemrix (GSK) and neurological and/or autoimmune disease | |
| Participants | The study population comprised 1,945,024 people and corresponds to all people registered in Stockholm county on 1 October 2009 and who had lived in the region since 1 January 1998 | |
| Interventions | Monovalent A (H1N1) pandemic vaccine Pandemrix (GlaxoSmithKline, Middlesex, UK) containing adjuvants AS03 and squalene. | |
| Outcomes | Data on vaccination (Vaccinera database) were linked to data on utilisation of inpatient and specialist healthcare (admissions to hospital and visits to specialist care in the county, dates, diagnoses, responsible medical departments and length of hospital stay) contained in the common healthcare registers for Stockholm County Council (GVR) from 1 January 1998 to 31 August 2010 | |
| Notes | ‐ Preliminary assessment (prevalence in vaccination phase I and II): All but 1 (narcolepsy) of the investigated neurological and autoimmune disorders were significantly more prevalent in those vaccinated in the early phase of the campaign (first 45 days) than in the unvaccinated cohort. Comparing the vaccinated in the late phase (> 45 days) with the unvaccinated cohort, the prevalence of the investigated diseases was not statistically relevant, except for inflammatory bowel disease (prevalence odds ratio 1.17, 95% confidence interval 1.12 to 1.22) and also Guillain‐Barré syndrome (OR 0.79, 0.67 to 0.95) and type 1 diabetes (OR 0.77, 0.64 to 0.92, for those born in 1990 and later) Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Low risk | Selected group of users |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Drawn from the same community as the exposed cohort |
| PCS/RCS ‐ comparability | High risk | Not assessed |
| PCS/RCS ‐ assessment of outcome | Low risk | Record linkage |
| Summary assessment | High risk | Unclear |
| Methods | Retrospective cohort study in which the incidence of medical attended events (MAEs) that occurred in participants immunised with live attenuated influenza vaccine (LAIV) through several seasons were compared with that observed in 2 matched control groups (unvaccinated and immunised with inactivated vaccine). Data for the LAIV exposed population were also analysed with a self controlled case series method | |
| Participants | Participants were members of the Kaiser Permanente (KP) Health Plans in Northern California, Hawaii and Colorado. Through KP immunisation registries, approximately 20,000 individuals of 18 to 49 years of age who were immunised from the 2003 to 2004 to 2007 to 2008 influenza seasons with LAIV as part of routine clinical practice were identified | |
| Interventions | Intervention hemi‐cohort: LAIV vaccine provided by MedImmune. Each annual formulation of the vaccines contained the strains recommended for inclusion by the US Public Health Service. Study participants with high‐risk underlying medical conditions such as cancer, organ transplantation, diabetes, endocrine and metabolic disorders, blood disorders, liver disorders, kidney disorders and cardiopulmonary disorders were identified via automated extraction of healthcare databases and excluded from all analysis cohorts. A total of 21,340 participants 18 to 49 years of age were vaccinated with the Ann Arbor strain LAIV during the 5 study seasons | |
| Outcomes | Medical attended adverse events (MAEs) | |
| Notes | Sources of support: "This study was sponsored by MedImmune, LLC. Authors employed by MedImmune were involved in the study design, analysis, and interpretation of data, and in the preparation of the manuscript. Authors employed by | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Selected group of users Participants were screened for underlying medical conditions and provided the appropriate vaccine based on the eligibility criteria in each vaccine’s package insert, physician discretion and patient choice |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | No description of the derivation of the non‐exposed cohort |
| PCS/RCS ‐ comparability | Unclear risk | Matched but not very relevant: "TIV‐vaccinated and unvaccinated participants were matched to LAIV recipients on region (Northern California, Hawaii, Colorado), birth date (within one year), sex, and prior healthcare utilization. Prior utilization was calculated based on the number of clinic visits during the 180 days before vaccination and classified as low (≤ 1 visit) and high (> 1 visit) for matching. In Northern California, participants also were matched on their specific medical clinic, of which there were 48" |
| PCS/RCS ‐ assessment of outcome | Low risk | Record linkage |
| Summary assessment | Unclear risk | Unclear |
| Methods | Surveillance population‐based study conducted in the USA during the 1979 to 1980 and 1980 to 1981 influenza seasons. The study tested the association between influenza vaccination and Guillain‐Barré syndrome. Reports from each case were obtained from neurologists. All case reports were included. The follow‐up period was 1 September 1979 to 31 March 1980 and 1 September 1980 to 31 March 1981 | |
| Participants | USA (minus Maryland), adult population, 18 years or older | |
| Interventions | Seasonal parenteral vaccine | |
| Outcomes | Cases of Guillain‐Barré syndrome. Vaccine‐associated cases were defined as those with onset within the 8‐week period after influenza vaccination | |
| Notes | Vaccination rates in the population were obtained from a national immunisation survey Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | High risk | High risk |
| PCS/RCS ‐ selection non‐exposed cohort | High risk | High risk |
| PCS/RCS ‐ comparability | High risk | High risk |
| PCS/RCS ‐ assessment of outcome | High risk | High risk |
| Summary assessment | High risk | High risk |
| Methods | Surveillance, population‐based study conducted in the USA (4 states: Illinois, Maryland, North Carolina, Washington), during the 1992 to 1993 and 1993 to 1994 influenza seasons. Discharge diagnoses databases were used to identify cases. Hospital charts were reviewed to confirm diagnosis. The follow‐up period was 1 September 1992 to 28 February 1993 and 1 September 1993 to 28 February 1994 | |
| Participants | Approximately 21 million people, 18 years or older | |
| Interventions | Seasonal parenteral vaccine | |
| Outcomes | Cases of Guillain‐Barré syndrome. Vaccine‐associated cases were defined a priori as those with onset within the 6‐week period after influenza vaccination | |
| Notes | Results were stratified by age and adjusted by season and sex. Vaccination rates in population were estimated from a random‐digit dialling telephone survey. Rare events (safety) Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | High risk | High risk |
| PCS/RCS ‐ selection non‐exposed cohort | High risk | High risk |
| PCS/RCS ‐ comparability | High risk | High risk |
| PCS/RCS ‐ assessment of outcome | High risk | High risk |
| Summary assessment | High risk | High risk |
| Methods | Retrospective cohort study evaluating the association between the administration of monovalent pandemic inactivated vaccine H1N1 and severe adverse events | |
| Participants | Participants were identified within several administrative and medical databases of the Italian region Emilia Romagna (about 4.4 million individuals). By data linkage participants immunised with Focetria® in the 2009 to 2010 season (n = 103,642) were identified. From the unvaccinated population (n = 3,967,917) a matched unexposed cohort was selected by using a propensity score | |
| Interventions | Immunisation with MF59‐adjuvanted, monovalent H1N1 vaccine Focetria® (Novartis Vaccines and Diagnostics, Siena, Italy) | |
| Outcomes | Guillain Barré syndrome, paralytic syndromes, encephalitis and encephalomyelitis, Bell’s palsy, demyelinating disease, convulsion, autoimmune hepatitis, vasculitis, immune thrombocytopenia | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Unclear description of the vaccinated population |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Using administrative databases |
| PCS/RCS ‐ comparability | Unclear risk | Propensity score |
| PCS/RCS ‐ assessment of outcome | Low risk | Blind validation process throughout |
| Summary assessment | Unclear risk | Unclear |
| Methods | See bb Ray 2011. Study data were analysed using a cohort design | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ comparability | Unclear risk | Unclear |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Surveillance, population‐based study conducted in the USA during the 1976 to 1977 influenza season. The study tested the association between influenza vaccination and Guillain‐Barré syndrome. Neurologists were directly contacted; physician and hospital records were reviewed. Suspected cases were reported to the CDC directly by patients or medical personnel were included only if accepted by a state health department. Follow‐up period was 1 October 1976 to 31 January 1977 | |
| Participants | USA population | |
| Interventions | Monovalent A/New Jersey/76 or bivalent A/New Jersey/76 and A/Victoria/75 parenteral vaccine | |
| Outcomes | Cases of Guillain‐Barré syndrome | |
| Notes | Results were stratified by age group and vaccine type. Vaccination rates in the population were obtained from a national immunisation survey Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | High risk |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | High risk |
| PCS/RCS ‐ comparability | Unclear risk | High risk |
| PCS/RCS ‐ assessment of outcome | Unclear risk | High risk |
| Summary assessment | Unclear risk | High risk |
| Methods | Case‐control study assessing the effectiveness of influenza vaccination of pregnant women in preventing hospitalisation for influenza in their newborns. Study period ranged from October 2000 to April 2009 | |
| Participants | Cases (n = 113): infants below 12 months hospitalised for influenza between October 2000 and April 2009 who tested positive for influenza with direct fluorescent antibody (DFA) Controls (n = 192): participants hospitalised for influenza during the same time interval as the cases but negative with the DFA test. For each case 1 or 2 controls matched for birth date and date of hospitalisation were randomly selected | |
| Interventions | Immunisation with influenza vaccine during pregnancy (until 14 days before delivery) | |
| Outcomes | DFA confirmed influenza | |
| Notes | This study was supported by the National Center for Research Resources, a component of the National Institutes of Health (NIH) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Infants hospitalised with DFA positive |
| CC ‐ control selection | Low risk | Infant hospitalised with DFA negative |
| CC ‐ comparability | Low risk | Matching |
| CC ‐ exposure | Unclear risk | Structured interview |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study assessing the effectiveness of influenza vaccine administered during pregnancy in preventing influenza in newborns under 6 months | |
| Participants | Children (n = 1510) aged below 6 months, who were hospitalised for fever and/or acute respiratory illness during 7 consecutive epidemic seasons (between 2002 and 2003 and 2008 and 2009). Those with positive laboratory confirmation of influenza were enrolled as cases (n = 151); those whose result was negative were enrolled as controls (n = 1359) | |
| Interventions | Influenza vaccination during pregnancy | |
| Outcomes | Influenza | |
| Notes | This project was supported the Centers for Disease Control and Prevention, National Institute of Allergy and Infectious Diseases,and Wachovia Research Fund. 3 authors had received funding from industry in the past (of these one was on the MedImmune Advisory Board and another was a NexBio consultant Funding source ‐ mixed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Laboratory‐confirmed |
| CC ‐ control selection | Low risk | Infants without laboratory‐confirmed influenza |
| CC ‐ comparability | Unclear risk | No matching, unclear information |
| CC ‐ exposure | Unclear risk | Structured interview |
| Summary assessment | Unclear risk | Unclear |
| Methods | Case‐control study investigating the association between influenza immunisation during pregnancy and spontaneous abortion | |
| Participants | Cases (n = 243) were identified from among the members of 6 Vaccine Safety Datalink organisations. Diagnoses of spontaneous abortion (ICD‐9 code 634) and unspecified abortion (ICD‐9 codes 637) assigned during the 2005 to 2006 and 2006 to 2007 seasons were reviewed and different diagnoses excluded Controls (n = 243) were selected from among women who had confirmed intrauterine pregnancy and delivery after the 20th gestational week by frequency‐matching of last menstrual period (within 2 weeks) and healthcare organisation | |
| Interventions | Immunisation with influenza vaccine. Participants were considered exposed if they were immunised within 28 days before index date. Analysis considering whether vaccine exposure occurred during or before pregnancy was also performed | |
| Outcomes | Spontaneous abortion cases | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC ‐ case selection | Low risk | Consecutive series of cases from electronic databases |
| CC ‐ control selection | Low risk | From the same population |
| CC ‐ comparability | Unclear risk | Matched by LMP ‐ confounders |
| CC ‐ exposure | Unclear risk | Medical record |
| Summary assessment | Unclear risk | Unclear |
| Methods | Retrospective cohort study assessing the effectiveness of flu vaccination for the prevention of ILI or pneumonia in pregnant women and their newborns | |
| Participants | ‐ All women with live births in Kaiser Permanente Northern California (KPNC) between the November and February of 5 subsequent seasons (1997 to 1998 and 2001 to 2002, n = 49,585) excluding cases lacking birth date information and women who were discharged after the end of the flu season | |
| Interventions | Immunisation with flu vaccine (no details about type and composition). Data about immunisation were obtained from the KPNC database. In all, 3707 out of the 49,585 pregnant women included in the study were vaccinated, whereas this was 3652 out of the 48,639 live births | |
| Outcomes | ‐ Hospitalisation for Pneumonia or Influenza: At least 1 inpatient stay during the same flu season as delivery or birth with a principal (first) diagnosis of either influenza or pneumonia. To identify these outcomes the following ICD (9th revision) codes were used to identify inpatient cases: influenza 487 and pneumonia 480, 481, 482, 483, 484, 485 and 486 ‐ Outpatient visits: at least 1 physician visit during the same flu season as delivery or birth with 1 of the following diagnoses: upper respiratory infection, pharyngitis, otitis media, asthma, bronchial asthma, viral infection, pneumonia, fever, cough or wheezing associated with respiratory illness This information was available from the KPNC databases, which include laboratory, hospitalisation and outpatient utilisation information for their members The effect measure (hazard ratio and corresponding 95% confidence interval) was calculated for ILI visits (including and excluding asthma diagnoses) for the mother and hospitalisation for pneumonia or influenza, ILI visits (excluding otitis media) and otitis media visits in newborns ‐ Caesarean section ‐ Preterm delivery (< 37 weeks) | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | From KPNC databases: the influenza vaccination status of women in the cohort was determined through review of the Kaiser Immunization Tracking System database |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | From KPNC databases |
| PCS/RCS ‐ comparability | High risk | No matching |
| PCS/RCS ‐ assessment of outcome | Unclear risk | KPNC maintains administrative databases that include laboratory, hospitalisation and outpatient utilisation information for their members |
| Summary assessment | High risk | High |
| Methods | Prospective cohort study carried out in 6 hospitals located in the Navajo and White Mountain Apache reservation during 3 subsequent epidemic seasons (2002 to 2005) | |
| Participants | Mother‐infant pairs recruited after delivery at Indian Health Service hospitals on the Navajo or White Mountain Apache reservation, either at the hospital or by home visit The study was conducted during 3 influenza seasons from November 2002 to September 2005 The enrolment periods for each year were ‐ 1 December 2002 to 15 March 2003 ‐ 1 November 2003 to 8 March 2004 ‐ 1 November 2004 to 15 March 2005 Inclusion was restricted to mothers who delivered a healthy infant at 36 weeks or later gestation during the enrolment periods. Eligible infants were aged 2 weeks or younger at enrolment. Overall, 1169 mother‐infant pairs were enrolled in the study (241 in 2002 to 2003; 574 in 2003 to 2004; and 354 in 2004 to 2005). Of these, 1160 had at least 1 serum sample and were included | |
| Interventions | Immunisation of the mother with influenza vaccine. Assessed by reviewing of medical record (also in order to obtain information about prenatal visits, illnesses and birth information, in addition to administration and timing of influenza vaccine) or, if missing, by maternal report at enrolment The decision for influenza vaccination was made by the treating clinician and the pregnant woman; personnel had no role in these decisions. Altogether 587 children were born from an unvaccinated mother and 573 from a vaccinated mother during the 3 study seasons | |
| Outcomes | Surveillance for all medically attended illnesses in enrolled infants was conducted at Indian Health Service and nearby private facilities through the influenza season, or until the child reached 6 months of age (whichever came first). It also included review of the clinic, emergency department and inpatient paediatric ward logs. A nasopharyngeal aspirate specimen for viral culture was obtained from infants with ILI within 72 hours of the medical visit ‐ Medically attended influenza‐like illness (ILI): defined as a medical visit with at least 1 of the following signs or symptoms reported: fever of 38.0 ºC or higher, diarrhoea or respiratory symptoms (including cough, runny nose or difficulty breathing) ‐ Laboratory‐confirmed influenza: the first ILI episode with either: | |
| Notes | "Funding/support: "The study was funded by the National Vaccine Program Office, Department of Health and Human Services, the Office of Minority Women’s Health, Centers for Disease Control and Prevention, Aventis‐Pasteur, and Evans‐Powderject." Funding source ‐ mixed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | The study was carried out within Indian reservations |
| PCS/RCS ‐ selection non‐exposed cohort | Low risk | Derived from the same community as the exposed cohort |
| PCS/RCS ‐ comparability | Unclear risk | Reported for some parameters only: sex, presence of household smokers, having wood or coal stove in the house (more frequent among vaccinated), presence of other children in day care, infant breast fed (more frequent among vaccinated), gestational age, mean birthweight |
| PCS/RCS ‐ assessment of outcome | Low risk | Active surveillance and testing for laboratory confirmation for symptomatic ILI cases |
| Summary assessment | Unclear risk | Unclear |
| Methods | Retrospective cohort study based on Vaccine Safety Datalink, assessing the effect of influenza vaccination in pregnant women in preventing respiratory illness in newborns. 6 epidemic seasons were considered | |
| Participants | Infants who were born before or during the influenza season at 4 managed care organisations (MCOs) (Kaiser Permanente Colorado, Denver; Kaiser Permanente Northern California, Oakland; Kaiser Permanente Northwest, Portland, Oregon; and Group Health Cooperative, Seattle, Washington) between 1 October 1995 and 30 September 2001 were eligible for study inclusion | |
| Interventions | An infant was considered exposed if the mother was vaccinated against influenza during the pregnancy and there were at least 28 days from the vaccination date of the mother to the birth date of the infant. Infants of mothers vaccinated within 27 days of birth were excluded from the primary analysis | |
| Outcomes | Medically attended ARI | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | High risk | From MCO databases |
| PCS/RCS ‐ selection non‐exposed cohort | High risk | From MCO databases |
| PCS/RCS ‐ comparability | High risk | Poor matching |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Data link |
| Summary assessment | High risk | High risk |
| Methods | Prospective cohort study assessing the effectiveness of flu vaccination in pregnancy | |
| Participants | Pregnant women (n = 544) recruited from the "hill" district of Pittsburgh | |
| Interventions | ‐ Polyvalent flu vaccine containing 200 units of A2 antigen ‐ Placebo 2 1 ml doses were administered 1 month apart | |
| Outcomes | ‐ Adverse effects following immunisation (pain, malaise) ‐ Influenza‐like illness ‐ Days in bed Assessed by means of questionnaires/phone interviews after epidemic | |
| Notes | Effectiveness follow‐up was available for 59% and 100% of participants in the intervention and placebo arm, respectively Funding source ‐ mixed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | High risk | Unclear |
| PCS/RCS ‐ selection non‐exposed cohort | High risk | Unclear |
| PCS/RCS ‐ comparability | High risk | Unclear ‐ high attrition |
| PCS/RCS ‐ assessment of outcome | High risk | Interview |
| Summary assessment | High risk | Unclear |
| Methods | Retrospective cohort study based on the electronic database of Kelsey‐Seybold Clinic (KSC), a large multispecialty clinic in the metropolitan area of Houston (USA). For the study 5 subsequent flu seasons were taken in account, from 1998 to 2003, considering the time between 1 July and 30 June each year. Approximately 25 obstetricians and 60 paediatricians provided medical care in KSC locations and about 2500 deliveries occurred every year during the time considered for the study | |
| Participants | Exposed cohort (n = 225): women who were immunised with inactivated influenza vaccine within 6 months before delivery and who had an uncomplicated singleton pregnancy, were healthy, had at least 1 prenatal care visit at KSC, and their offspring had at least 1 clinic visit at KSC in their first year of life Comparison (n = 826): for each vaccinated woman a comparison group was selected by matching (KSC database) 3 to 5 women for maternal age at delivery, month of delivery and type of insurance (with the exclusion of both Medicaid or self insurance because of small numbers in this clinic population), who had not received influenza vaccine during pregnancy | |
| Interventions | Influenza vaccines that were used during the study period were Aventis Pasteur or Wyeth products. For the control group the index date ("pseudo vaccination date") corresponds to the same number of days before delivery as the real vaccination date for a matching vaccinated woman | |
| Outcomes | Women ‐ ARI (acute respiratory illness): cases recorded at any time, during each flu season and during each epidemic peak of that season diagnosed with the following ICD‐9 codes: 079, 460‐466, 470‐478, 480‐487. The peak of influenza activity was the period during which the number of laboratory‐confirmed cases included at least 85% of influenza cases for that season ‐ Serious adverse events: hospitalisation (death, cause for hospitalisation and permanently disabling conditions are also included) within 42 days from immunisation identified by ICD‐9 codes ‐ Medical diagnoses occurred between vaccination and delivery with an incidence ≥ 2% among vaccinated women) ‐ Diagnoses different from a “normal newborn infant” given at discharge and within 2 days from delivery ‐ Reason for at least 3 days hospitalisation within 1 week, between 8 and 180 days, and between 6 months and 1 years after delivery In the last 2 categories URTI and respiratory infections are also included | |
| Notes | Little information about characteristics and comparability of the exposed and unexposed cohorts. Outcomes used to assess the effectiveness of vaccination are in some way 'surrogate' and include only hospitalisation and ambulatory diagnoses. For the assessment of effectiveness in mothers, the first 2 weeks after vaccination should have been excluded from follow‐up Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Women were included in the study sample if they had received inactivated influenza vaccine within 6 months before delivery of an uncomplicated singleton pregnancy and were otherwise healthy, had at least 1 prenatal care visit at KSC and their offspring had at least 1 clinic visit at KSC in their first year of life |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | A comparison group was selected by matching of maternal age at delivery, month of delivery and type of insurance (patients with Medicaid or self insurance were excluded because of the small numbers in this clinic population). For each vaccinated woman, they selected 3 to 5 (ratio, 1:3.5) matching healthy women who met all the inclusion criteria but who had not received influenza vaccine during pregnancy |
| PCS/RCS ‐ comparability | Unclear risk | Matching |
| PCS/RCS ‐ assessment of outcome | Unclear risk | The potential protective effect of the vaccine was estimated by recording the occurrence of acute respiratory tract illnesses in vaccinated women from the time of receipt of influenza vaccine to delivery and in unvaccinated women for the equivalent period of time. Specifically, the occurrence of acute respiratory illnesses (ARIs) during the peak of the influenza season was compared between the groups. Diagnostic codes for ARI included 079, 460‐466, 470‐478, 480‐487 |
| Summary assessment | Unclear risk | Unclear |
| Methods | Questionnaire‐based, retrospective cohort study performed at the 121 obstetrical facilities of Hokkaido (Japan) | |
| Participants | All 121 obstetric facilities in Hokkaido were requested to deliver a 12‐item questionnaire to all postpartum women who gave birth between 1 December 2009 and 31 May 2010 during their stay in obstetric facilities. About 1/3 of the women who delivered in Hokkaido during this time answered the questionnaire (n = 7535) | |
| Interventions | Influenza vaccination during pregnancy. Out of the 7535 women who answered the questionnaire, 4921 received pandemic influenza vaccine. Among them, 2212 were also reported to have been vaccinated with seasonal vaccine. A further 270 (considered as unvaccinated) received seasonal vaccine only | |
| Outcomes | Influenza. Definition was not provided. All information was collected by means of a questionnaire, on which items about admission to the intensive care unit, intubation or ventilation, and diagnosis of influenza encephalopathy were also present | |
| Notes | Strongly biased Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | High risk | By interview |
| PCS/RCS ‐ selection non‐exposed cohort | High risk | By interview |
| PCS/RCS ‐ comparability | High risk | No matching |
| PCS/RCS ‐ assessment of outcome | High risk | By interview |
| Summary assessment | High risk | High |
| Methods | Prospective cohort study assessing the safety of monovalent A/NJ/8/76 vaccine administration during pregnancy | |
| Participants | Pregnant women enrolled at several obstetric clinics (Minneapolis) on the occasion of a prenatal visit (n = 706) | |
| Interventions | Flu vaccine containing A/NewJersey/8/76 (split or whole virus formulation) administered during the first, second or third pregnancy semester. Vaccine was administered to 189 women, whereas 517 acted as unvaccinated controls | |
| Outcomes | ‐ Local and systemic reactions observed and reported after vaccine administration (only the vaccinated assessed by questionnaire) ‐ Pregnancy outcomes: maternal mortality, elective abortion, spontaneous abortion, stillbirth, premature live birth ‐ Infant outcomes: deaths, major or minor congenital anomalies, abnormalities during the first 8 days of life | |
| Notes | This study should have been performed without external/private/industry funding Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | High risk | |
| PCS/RCS ‐ selection non‐exposed cohort | High risk | |
| PCS/RCS ‐ comparability | High risk | |
| PCS/RCS ‐ assessment of outcome | High risk | |
| Summary assessment | High risk | |
| Methods | Retrospective cohort assessing the safety of pandemic, monovalent H1N1 vaccine in pregnant women, by using Ontario’s birth record database | |
| Participants | Women with singleton birth in 2009 to 2010 season (n = 55,570) | |
| Interventions | Monovalent pandemic H1N1 influenza vaccine. In all, 23,340 pregnant women were also immunised with seasonal vaccine | |
| Outcomes | Frequency of neonatal outcomes in newborns: ‐ Preterm birth (< 37 weeks or < 32 weeks) | |
| Notes | "This study was funded by the Canadian Institutes of Health Research (grant 218653)" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Low risk | |
| PCS/RCS ‐ selection non‐exposed cohort | Low risk | |
| PCS/RCS ‐ comparability | High risk | |
| PCS/RCS ‐ assessment of outcome | Low risk | |
| Summary assessment | High risk | |
| Methods | Prospective cohort study assessing the safety of pandemic MF‐59 adjuvanted influenza vaccine (Focetria) during pregnancy | |
| Participants | Pregnant women recruited in midwife practices and hospitals in the Netherlands (n = 4281), Argentina (n = 239) and Italy (n = 9). Altogether 4508 pregnant women were included: 2295 were vaccinated and 2213 were not immunised. There were 4522 live births and 18 intrauterine deaths (2310 born from vaccinated and 2213 from unvaccinated mothers). For 4385 babies 3 months follow‐up data were available | |
| Interventions | Monovalent, pandemic, H1N1, MF‐59 adjuvanted flu vaccine Focetria (Novartis Vaccine and Diagnostic, Cambridge, MA). Among the 2295 vaccinated pregnant women, 1724 received 2 doses, 571 received 1 dose | |
| Outcomes | Gestational diabetes | |
| Notes | "This study was supported by Novartis Vaccines and Diagnostics" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ comparability | High risk | |
| PCS/RCS ‐ assessment of outcome | Low risk | |
| Summary assessment | High risk | Unclear |
| Methods | Cohort study assessing the risk of neonatal death following exposure to pandemic monovalent H1N1 influenza vaccine or influenza virus during pregnancy | |
| Participants | A total of 113,331 pregnant women | |
| Interventions | Immunisation with pandemic monovalent H1N1 adjuvanted influenza vaccine Pandemrix (GSK) or Cavaplan (not adjuvanted) | |
| Outcomes | Fetal death | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Data link |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Data link |
| PCS/RCS ‐ comparability | Unclear risk | Multivariate model |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Data link |
| Summary assessment | Unclear risk | Unclear risk of bias |
| Methods | Retrospective cohort study assessing the effect on newborn outcomes of pandemic squalene adjuvanted H1N1 vaccine | |
| Participants | The total number of vaccinated women was 18,612 having 18,844 infants (vaccination group, pandemic H1N1 Pandemrix). These women were compared with 136,914 women having 138,931 infants who gave birth after September 2009 and before the end of 2010 (non‐vaccinated group) and with 83,298 women having 84,484 infants who gave birth in the year 2009 before October (pre‐vaccination group) | |
| Interventions | Pandemrix (GlaxoSmithKline; Brentford, Middlesex, UK) containing inactivated split influenza virus A/California/07/2009), squalene adjuvant and thiomersal preservative | |
| Outcomes | Stillbirth | |
| Notes | "No specific funding was obtained for this study" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ comparability | High risk | |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Unclear |
| Summary assessment | High risk | |
| Methods | Prospective cohort study assessing the effect of immunisation with pandemic monovalent vaccine during pregnancy | |
| Participants | Pregnant women (n = 877) between 12 and 35 weeks of gestation, aged at least 18 years, who were not vaccinated or infected | |
| Interventions | Immunisation with pandemic monovalent influenza vaccine | |
| Outcomes | Delivery before the 37th gestational week, birthweight, death before or during labour | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Low risk | Low |
| PCS/RCS ‐ selection non‐exposed cohort | Low risk | Low |
| PCS/RCS ‐ comparability | Unclear risk | No information was given about possible confounders |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Unclear |
| Summary assessment | Unclear risk | Unclear |
| Methods | Retrospective cohort study | |
| Participants | A total of 396 pregnant Taiwanese women were included in the study. Among them 198 received influenza vaccine during pregnancy | |
| Interventions | Monovalent H1N1 unadjuvanted, inactivated, split‐virus vaccine AdimFlu‐S® (Adimmune Corporation; Taichung, Taiwan)containing 15 g of New York Medical College X‐179A reassortant of the A/California/7/2009 (H1N1)‐like strain in 0.5 ml dose | |
| Outcomes | Systemic and local adverse events in vaccinated mothers In newborns: Hyperbilirubinaemia | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ comparability | High risk | |
| PCS/RCS ‐ assessment of outcome | Low risk | Medical records |
| Summary assessment | High risk | |
| Methods | Retrospective cohort study based on data from Vaccine Safety Datalink (VSD) | |
| Participants | Pregnant women aged between 14 and 49 years (n = 223,898) identified in the VSD, who were pregnant between 1 June 2002 and 31 July 2009 | |
| Interventions | Immunisation with inactivated trivalent influenza vaccine | |
| Outcomes | Demyelinating diseases, neurological events, thrombocytopenia within 42 days after immunisation | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | KP registry |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | KP registry |
| PCS/RCS ‐ comparability | Unclear risk | Matched analysis |
| PCS/RCS ‐ assessment of outcome | Unclear risk | KP registry |
| Summary assessment | Unclear risk | Unclear |
| Methods | Retrospective cohort study based on data from the Georgia Pregnancy Risk Assessment Monitoring System (PRAMS) | |
| Participants | In all 4168 pregnant women were included during 2 consecutive epidemic seasons (2004 to 2005 and 2005 to 2006), 578 received influenza vaccination | |
| Interventions | Influenza vaccination during pregnancy | |
| Outcomes | Small for gestational age (SGA) and preterm births. Periods with different viral circulation were considered in the analysis | |
| Notes | "The study was partially funded through the Emory University, Global Health Institute Faculty of Distinction Fund award (recipient: SBO). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript" Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ comparability | Unclear risk | Unclear |
| PCS/RCS ‐ assessment of outcome | High risk | Interview |
| Summary assessment | Unclear risk | Unclear |
| Methods | Prospective cohort study based data from the Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy (D) carried out during the 2009 to 2010 pandemic | |
| Participants | Pregnant women who received consultation regarding reproductive safety of medical products, planned pregnancy and lactation from the Institute for Clinical Teratology and Drug Risk Assessment. Out of the initial population (n = 16,788), 323 participants received influenza vaccine and completed the follow‐up. A randomly selected control group of 1329 non‐vaccinated women was the control group | |
| Interventions | ‐ Non‐adjuvanted split‐virion vaccine CSL H1N1 Pandemic Influenza Vaccine® (CSL Biotherapies) approved by the responsible national authority (Paul‐Ehrlich‐Institut) in November 2009 exclusively for the vaccination of pregnant women (216/323) | |
| Outcomes | Abortion, preterm birth, malformations | |
| Notes | "This study was supported by the German Federal Institute for Vaccines and Biomedicines (Paul‐Ehrlich‐Institut), Langen, Germany" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ comparability | Unclear risk | Unclear |
| PCS/RCS ‐ assessment of outcome | Low risk | Low |
| Summary assessment | Unclear risk | Unclear |
| Methods | Retrospective cohort study assessing the safety of pandemic H1N1 vaccination | |
| Participants | Danish women who were pregnant during the time interval between November 2009 and September 2010 (n = 58,585). Of these, 7062 received influenza vaccine | |
| Interventions | Monovalent, inactivated, AS03‐adjuvanted split virion influenza A (H1N1) pdm09 vaccine (Pandemrix, GlaxoSmithKline Biologicals) | |
| Outcomes | Abortion cases (retained or spontaneous) | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Low risk | |
| PCS/RCS ‐ selection non‐exposed cohort | Low risk | |
| PCS/RCS ‐ comparability | High risk | |
| PCS/RCS ‐ assessment of outcome | Low risk | |
| Summary assessment | Unclear risk | Unclear |
| Methods | Retrospective cohort study assessing the effect of pandemic H1N1 immunisation during pregnancy on neonatal outcomes | |
| Participants | Eligible pregnant women were identified by means of electronic medical records from Kaiser Permanente (KP) managed care organisation sites in Georgia and Mid‐Atlantic States. A total of 3327 third‐trimester live births to 3236 mothers between 25 May 2009 and 17 April 2010 were included | |
| Interventions | Immunisation with H1N1 pandemic vaccine | |
| Outcomes | Preterm birth (27 to 36 weeks), low birthweight | |
| Notes | Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | KP registry |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | KP registry |
| PCS/RCS ‐ comparability | High risk | Possible residual confounding |
| PCS/RCS ‐ assessment of outcome | Low risk | Low |
| Summary assessment | Unclear risk | Unclear |
| Methods | Retrospective cohort study assessing the safety of seasonal influenza vaccination administered during pregnancy, covering 5 subsequent epidemic seasons (from 2003 to 2004 to 2007 to 2008) | |
| Participants | Women who delivered and received prenatal care at the Southwestern Medical Center of University of Texas and Parkland Health & Hospital System, Dallas, Texas. In all 8690 were vaccinated and 76,153 acted as unvaccinated controls | |
| Interventions | Seasonal influenza vaccination was offered to pregnant women between October through March in each season | |
| Outcomes | ‐ Estimated gestational age *For these outcomes the authors provided effect estimates considering the trimester of administration | |
| Notes | This study should have been performed without external/private/industry funding Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ comparability | High risk | |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Unclear |
| Summary assessment | High risk | |
| Methods | Retrospective cohort study testing the safety of live attenuated influenza vaccine when administered during pregnancy | |
| Participants | Pregnant women (n = 834,999) identified by means of a safety database (LifeLink Health Plan Claims Database, Norwalk, USA) between October 2003 and September 2009. Of these, 138 received immunisation with live attenuated influenza vaccine during their pregnancy | |
| Interventions | Live attenuated influenza vaccine | |
| Outcomes | Hospitalisation and emergency department visits within 42 days after immunisation | |
| Notes | "This research was funded by MedImmune, LLC, Gaithersburg, MD. As part of a consulting agreement with RTI Health Solutions, MedImmune provided funding to support protocol development, data collection, analysis, and manuscript development activities associated with this manuscript. Editorial assistance in formatting the manuscript for submission was provided by Sue Myers, MSc, and Gerard P. Johnson, PhD, of Complete Healthcare Communications, Inc. (Chadds Ford, PA) and was funded by MedImmune, LLC" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ selection exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ selection non‐exposed cohort | Unclear risk | Unclear |
| PCS/RCS ‐ comparability | High risk | |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Unclear |
| Summary assessment | High risk | |
AE = adverse event
ARI = acute respiratory illness
ATP = according to protocol
CDC = Centers for Disease Control and Prevention
CCI = culture‐confirmed influenza illness
CCIV = cell culture‐derived inactivated flu vaccine
CI = confidence interval
DFA = direct fluorescent antibody
FEF = forced expiratory flow
FEV1 = forced respiratory volume in one second
FVC = forced expiratory vital capacity
GBS = Guillain‐Barré syndrome
GMT = geometrical mean titre
GSK = Glaxo‐Smith‐Kline
HA = haemagglutinin
HAO = full‐length uncleaved haemagglutinin
HI = haemagglutination‐inhibiting
HMO = health maintenance organisation
ICD = International Classification of Diseases
ILI = influenza‐like illness
ITI = intention‐to‐immunise
ITT = intention‐to‐treat
IM = intramuscular
IN = intranasal
IU = international units
KP = Kaiser Permanente
KSC = Kelsey‐Seybold Clinic
LAIV = live attenuated influenza vaccine
LCI = laboratory‐confirmed influenza illness
LMP = last menstrual period
MAE = medical attended event
MCO = managed care organisation
MDCK = Madin Darby canine kidney cells
mmHg = millimetres of mercury
NaCl = sodium chloride
NCKPHP = Northern California Kaiser Permanente Health Plan
NICU = neonatal intensive care unit
OMP = outer membrane protein
OR = odds ratio
ORS = oculo‐respiratory syndrome
PCA = primary cardiac arrest
PCR = polymerase chain reaction
PCS/RCS = prospective/retrospective cohort study
PP = per‐protocol
RCT = randomised controlled trial
rHAO = recombinant uncleaved haemagglutinin glycoprotein
RhMK = rhesus macaque kidney cells
RT‐PCR = reverse transcription polymerase chain reaction
SAE = serious adverse event
SAS = statistical analysis systems
TIV = trivalent inactivated vaccine
URTI = upper respiratory tract infection
VMCCI = vaccine matched, culture‐confirmed influenza
WDL = working days lost
WHO = World Health Organization
WRL = Wellcome Research Laboratories (Beckenham, Kent)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No outcomes of interest, differences in cytokine levels between ORS cases and controls after vaccination | |
| Data tables and figure missing | |
| No original data | |
| Randomised controlled trial, single‐blind. Outcomes were clinical cases and adverse effects. Follow‐up data were not reported by arms | |
| Intradermal administration (3 different lots of the same vaccine) versus intramuscular administration. Serologic response and AE at day 21. No adequate placebo/no intervention control | |
| No outcomes of interest | |
| Absence of an adequate control | |
| No design (average days of sick leave in vaccinated and non‐vaccinated subjects during 1996 and 1997 from staff of an international banking institution) | |
| No design (cohort), no safety outcomes | |
| No design: cohort study for effectiveness | |
| A 'head to head trial': "FluBlok (purified HA proteins manufactured in expresSF+® insect cells under serum free conditions using a baculovirus expression system (BEVS). Uncleaved HA produced by this method is referred to as rHA0. Vaccine formulation consisted of 135g total HA protein (45g each) as determined by single radial immunodiffusion assay (SRID) and included rHA0 derived from the following influenza strains A/Solomon Islands/03/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004 VS. The same CDC‐derived vaccine seed viruses were used for the licensed trivalent inactivated vaccine (TIV; Fluzone [2007–2008 formulation; Sanofi Pasteur, Swiftwater, PA), which contained 15g of each HA [45g total])" | |
| No design: controlled case series | |
| Case‐control study, no harm assessment | |
| No original data | |
| Questionnaire survey; non‐comparative analysis | |
| Absence of an adequate control group (quadrivalent versus trivalent inactivated vaccine; low versus normal adjuvant content) | |
| Trial with swine vaccine (Hsw1N1, A/New Jersey/76) | |
| Review | |
| No adequate control, no outcome of interest | |
| Trial with swine vaccine (Hsw1N1, A/New Jersey/76) | |
| Not a randomised controlled trial | |
| No design: cross‐sectional study | |
| No design: case series | |
| No design: case series | |
| Case report | |
| Randomised controlled trial. More than 75% of the study population was out of the age range stated in the protocol | |
| Participants are MS cases | |
| Inadequate comparison and study design: cohort study with pandemic versus seasonal (not exposed) vaccines in women and newborns | |
| Does not contain effectiveness data | |
| Inadequate comparison: all enrolled subjects received LAIV, then they were randomised to either placebo or Lactobacillus GG | |
| Cohort with efficacy outcomes. Experimental and control group were selected separately | |
| Not randomised. Subjects volunteered for immunisation and comparison was made with a randomly selected non‐immunised control group | |
| No comparison, absence of adequate control group | |
| No control | |
| Population at risk of further oculo‐respiratory syndrome episodes | |
| No design: self controlled case series for association between H1N1 and GBS | |
| Trial with swine vaccine (Hsw1N1, A/New Jersey/76) | |
| No design: case‐control study assessing effectiveness in general population | |
| No design: effectiveness cohort study in general population | |
| Influenza B vaccine was used as control | |
| No design: case‐control study assessing effectiveness in general population | |
| Major inconsistencies in the study text | |
| Inadequate comparison (Tetanus Toxoid vaccine) | |
| Randomised controlled trial, double‐blind. 2 bivalent inactivated influenza vaccines, with the same viral composition, differing in purification procedures, were compared | |
| No outcomes of interest (antibody titres only) | |
| Absence of adequate control | |
| No usable safety data (scores) | |
| Conference proceedings | |
| Not a randomised controlled trial | |
| No outcome of interest | |
| Outcome measures outside inclusion criteria | |
| Not a randomised controlled trial | |
| Randomised controlled trial conducted in the USA on 41 cystic fibrosis (CF) patients and 89 family members, recruited through a clinic. Participants were randomly assigned in a double‐blinded fashion by family to receive either intranasal, live, cold‐adapted influenza A vaccine or the recommended intramuscular trivalent inactivated influenza vaccine | |
| No design: self controlled case series | |
| Analysis of temporal trends of Guillain Barré syndrome (GBS) 1990 to 2003, comparison with temporal trends of non‐GBS adverse event reports from the Vaccine Adverse Event Reporting System (VAERS) | |
| Not randomised: all the volunteers were immunised on a single day and the intention to allocate patients randomly was not strictly adhered to | |
| Outcome measures outside inclusion criteria | |
| Participants affected by sickle cell crisis | |
| No design: case‐control study assessing effectiveness in general population | |
| Polyvalent influenza vaccine was used as control | |
| Randomised controlled trial. Clinical outcomes were side effects only | |
| Influenza B vaccine was used as control | |
| Not placebo or 'do nothing' controlled | |
| No control group | |
| Not prospective: appears to be an historical cohort | |
| Report of GBS surveillance 1978 to 1979, non‐comparative study | |
| No adequate control (the same vaccine prepared with different antigenic concentrations was administered to each group) | |
| No design: case‐control study assessing effectiveness in general population | |
| Not a randomised controlled trial | |
| No design: case‐control study assessing effectiveness in general population | |
| Efficacy outcome measures outside inclusion criteria. The safety data are presented in a non‐analysable way | |
| No design: case‐control study assessing effectiveness in general population | |
| Review and cost‐effectiveness analysis | |
| Tables and text show inconsistencies that do not allow data extraction | |
| Surveillance for adverse events | |
| No design: case‐control study assessing effectiveness in general population | |
| No adequate control | |
| Review | |
| No design: self controlled case series | |
| No design: case series | |
| Absence of an adequate control, serological outcomes only | |
| Absence of an adequate control, serological outcomes only | |
| Reported the results of 9 placebo‐controlled clinical trials and 2 field studies, involving a total of about 10,000 participants, carried out in several countries to assess the efficacy of killed influenza spray vaccines. Studies were conducted during the years 1969 to 1971 | |
| Methods for assessing flu vaccine exposure during pregnancy | |
| No design: allocation is arbitrary and groups with different characteristics were formed | |
| Non‐comparative design | |
| Influenza B vaccine was used as control | |
| Influenza B vaccine was used as control | |
| Review | |
| Does not report original results | |
| Review | |
| Review | |
| Review | |
| Total number of AEs observed after administration of each vaccine type | |
| Not a randomised controlled trial | |
| Non‐comparative study | |
| Design is unclear: no standard random allocation. Only 25 out of 30 seem to have been immunised but in the method description 30 were considered for exposure to natural influenza A/Scotland/840/74. One of these was excluded prior because they had tonsillitis | |
| Outcomes were safety only. Absence of adequate control | |
| Not a randomised controlled trial | |
| Not adequate comparison (pregnant versus non‐pregnant women) | |
| Absence of control group, non‐comparative | |
| Same data as Nichol 1995 (included) | |
| Review | |
| Not a randomised controlled trial | |
| Contains data from previous studies | |
| Re‐analysis of Nichol 1999 (included) | |
| Non‐comparative study | |
| No new data: reports data from already published and included studies (aa Ohmit 2006, aa Ohmit 2008, aa Monto 2009) | |
| Absence of adequate control group | |
| No design: case‐control study assessing effectiveness in general population (also children and elderly) | |
| Not outcomes of interest | |
| Not a randomised controlled trial | |
| Safety survey after Celtura (H1N1) administration. Absence of control group | |
| Outcome measures outside inclusion criteria | |
| Very poor reporting, unclear definition, no description of methods | |
| Participants are children | |
| Absence of adequate control | |
| Both arms contained the same vaccine strains | |
| Re‐assessment of Schonberger 1979 (included) | |
| Absence of adequate control | |
| Review of the evidence of the aetiology of GBS, no original data presented | |
| Report about Nichol 1995 (included) | |
| No design: cohort and case‐control study assessing effectiveness in general population | |
| Non‐comparative (survey) | |
| Population at risk of further ORS episodes | |
| Reports a small part of the Hoskins trial. It compared illness occurring among a group of vaccinated boys against non‐vaccinated controls that had no part in the trial | |
| Trial with swine vaccine (Hsw1N1, A/New Jersey/76) | |
| One trial is a 'head to head' (Gc501 versus Fluarix) with serological outcomes only, the other one (safety) has no control | |
| Compares the incidence of GBS cases after tetravalent HPV vaccine with that observed after pneumococcal and flu vaccine administration | |
| Authors did not report crude data on the clinical outcomes | |
| Reporting does not allow one to understand the methods used to allocate subjects and to conceal allocation. Clinical outcome data are not reported | |
| Inadequate control (23v pneumococcal vaccine administered to the control group). Re‐analysis of Zaman 2008 data (excluded) | |
| No outcomes of interest | |
| Data on AEs are not provided in a useful form (bar graphs or cumulatively in the text) | |
| Non‐comparative | |
| No outcomes of interest, rhinovirus vaccine as control | |
| No design: controlled case series | |
| Outcome measures outside inclusion criteria | |
| Outcome measures outside inclusion criteria | |
| No design: case‐control study | |
| Non‐comparative | |
| Same vaccine administered in different pregnancy weeks (inadequate comparison) | |
| None of the 3 studies reported in this paper are includible for the following reasons | |
| Safety data after dose I (seasonal versus placebo) are not extract (bar graph) | |
| Not randomised. Data reporting was not complete | |
| Pneumococcal vaccine was used as control | |
| No placebo or 'do nothing' control | |
| No design: case series | |
| No design | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| No safety data | |
| Non‐comparative study: absence of a control arm | |
| No design: controlled case series | |
| Inadequate control (23v pneumococcal vaccine administered to the control group) |
AEs = adverse events
CDC = Centers for Disease Control and Prevention
CF = cystic fibrosis
GBS =Guillain‐Barré syndrome
HA = haemagglutinin
HPV = human papillomavirus
LAIV = live attenuated influenza vaccine
MS = multiple sclerosis
ORS = oculo‐respiratory syndrome
rHAO = recombinant uncleaved haemagglutinin glycoprotein
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, dose‐escalation study |
| Participants | Healthy adults (n = 48) |
| Interventions | ΔNS1‐H1N1 A/New/Caledonia vaccine (6.4, 6.7, 7.0, 7.4 and 7.7 log10 MTCID) versus placebo |
| Outcomes | Local and systemic reactions, immunogenicity |
| Notes |
| Methods | Phase 2, randomised, double‐blind, placebo‐controlled trial (part A) |
| Participants | Healthy adults between 18 and 64 (n = 1013) |
| Interventions | Monovalent, H1N1, pandemic virus‐like particles (VLP) influenza vaccine (5, 15, 45 μg of VLP/dose or saline placebo, 2 doses administered 21 days apart) |
| Outcomes | Adverse events, immunogenicity |
| Notes |
| Methods | Randomised, placebo‐controlled, cross‐over trial (part B) |
| Participants | Healthy adults between 18 and 64 (n = 3547) |
| Interventions | Monovalent, H1N1, pandemic virus‐like particles (VLP) influenza vaccine (15 μg of VLP/dose) versus saline placebo |
| Outcomes | Adverse events |
| Notes |
| Methods | Randomised, placebo‐controlled trial |
| Participants | Healthy adults aged between 18 and 49 (n = 300) |
| Interventions | Monovalent, pandemic, H1N1 live attenuated vaccine versus placebo. 2 doses administered 28 days apart |
| Outcomes | Local and systemic reactions, immunogenicity |
| Notes |
| Methods | Randomised controlled trial |
| Participants | Healthy adults aged 18 to 64 (n = 849) |
| Interventions | Monovalent, pandemic H1N1, inactivates, split virion vaccine (1 dose 7.5, 15 or 30 μg HA/dose) versus placebo |
| Outcomes | Local and systemic reactions, immunogenicity |
| Notes |
| Methods | Phase 1, multicentre, randomised, double‐blind trial |
| Participants | Healthy adults between 18 and 50 years (n = 266) |
| Interventions | Monovalent, H1N1, inactivated, split vaccine with different antigenic content, with or without adjuvants and placebo (10 arms) |
| Outcomes | Local and systemic reaction, immunogenicity |
| Notes |
| Methods | Dose‐escalation study |
| Participants | Healthy adults aged 18 to 49 (n = 128) |
| Interventions | Recombinant, haemagglutinin influenza‐flagellin fusion vaccine (VAX 125, 0.1, 0.3, 1, 2, 3, 5, 8 μg/dose, placebo) |
| Outcomes | Local and systemic reactions, C‐reactive protein response |
| Notes |
| Methods | Randomised, placebo‐controlled trial |
| Participants | Healthy adults between 18 and 49 (n = 4648) |
| Interventions | Recombinant HA protein vaccine versus placebo |
| Outcomes | Local and systemic reactions, protective efficacy, antibody response |
| Notes |
| Methods | Phase 1, randomised, multicentre trial |
| Participants | Adults aged between 18 to 49 (n = 60) |
| Interventions | Recombinant M2e‐flagellin influenza vaccine (STF2.4xM2e). Different dosages (0.03, 0.1, 0.3, 1, 3, 10 μg/dose) versus placebo |
| Outcomes | Local and systemic reactions, immunogenicity |
| Notes |
| Methods | Randomised, single‐blind, controlled trial |
| Participants | Healthy adults aged between 18 and 49 (n = 60) |
| Interventions | Multimeric‐001 influenza vaccine |
| Outcomes | Local and systemic reactions, immunogenicity |
| Notes |
| Methods | Phase 1, randomised, double‐blind, placebo‐controlled clinical trial |
| Participants | Healthy adults (n = 100) |
| Interventions | Recombinant adjuvanted haemagglutinin‐based influenza vaccine (HAI‐05) administered in 15 μg, 45 μg or 90 μg/dose versus non‐adjuvanted vaccine versus placebo. 2 doses were administered |
| Outcomes | Local and systemic reactions. antibody response. |
| Notes |
| Methods | Randomised controlled trial |
| Participants | Healthy adults aged between 18 and 40 (n = 125) |
| Interventions | Inactivated avian influenza A (H7N7) vaccine containing 7.5, 15, 45 or 90 mg of HA/dose versus placebo. 2 doses were administered 28 days apart |
| Outcomes | Local and systemic reactions, serum antibody response |
| Notes | NCT00546585 |
| Methods | Follow‐up study |
| Participants | Pregnant women (n = 50,897) and newborns (years 1958 to 1966) |
| Interventions | Influenza or polio vaccine during pregnancy |
| Outcomes | Malignancies |
| Notes |
| Methods | Follow‐up study |
| Participants | Pregnant women (n = 14,475) immunised with H1N1 pandemic vaccine in Taiwan (season 2009/2010) |
| Interventions | Administration of adjuvanted or non‐adjuvanted pandemic vaccine during pregnancy |
| Outcomes | Maternal death, gestational age, abortion, neonatal death |
| Notes |
| Methods | Randomised controlled trial |
| Participants | Healthy adults aged between 12 and 75 (n = 363) |
| Interventions | Live attenuated, cold‐adapted, monovalent H1N1 (A/17/CA/2009/38) versus saline placebo, intranasally administered |
| Outcomes | Local and systemic reactions, antibody response |
| Notes |
| Methods | Single‐centre, randomised, double blind trial |
| Participants | Healthy males aged 18 to 40 (n = 48), with body mass index between 18.5 and 28.5 kg/m2, low or non‐smoker |
| Interventions | Administration of synthetic polypeptide vaccine (Flu‐v) containing 250 μg or 500 μg of protein/dose (either with or without adjuvant) versus placebo |
| Outcomes | Local and systemic reactions, antibody response |
| Notes |
| Methods | Randomised controlled trial |
| Participants | Healthy adults (n = 326) who were immunised with pandemic, monovalent H1N1, AS03 adjuvanted influenza vaccine during the 2009‐2010 season in Canada (Arepanrix vaccine) |
| Interventions | Non‐adjuvanted pandemic H1N1 vaccine versus placebo |
| Outcomes | Local and systemic events, antibody response |
| Notes |
| Methods | Randomised, dose‐escalation trial |
| Participants | Healthy adults aged 18 to 49 (n = 112) |
| Interventions | Recombinant haemagglutinin influenza‐flagellin fusion vaccine (VAX128) administered in different antigen concentrations (0.5 to 20 μg/dose) versus buffer placebo |
| Outcomes | Local and systemic reactions, antibody response |
| Notes |
| Methods | Cohort study based on data from the North American Organization of Teratology Information Specialists (OTIS) |
| Participants | Pregnant women (n = 198) |
| Interventions | Exposure to influenza vaccine at different times of gestation |
| Outcomes | Spontaneous abortion |
| Notes |
HA = haemagglutinin
VLP = virus‐like particles
MTCID = median tissue culture infective dose
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 16 | 25795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.78, 0.87] |
| Analysis 1.1  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 1 Influenza‐like illness. | ||||
| 1.1 WHO recommended ‐ matching vaccine | 7 | 4760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.89] |
| 1.2 WHO recommended ‐ vaccine matching absent or unknown | 7 | 20942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.75, 0.90] |
| 1.3 Monovalent not WHO recommended ‐ vaccine matching | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.28, 3.70] |
| 1.4 Monovalent not WHO recommended ‐ vaccine matching ‐ high dose | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.30] |
| 2 Influenza Show forest plot | 22 | 51724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.33, 0.44] |
| Analysis 1.2  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 2 Influenza. | ||||
| 2.1 WHO recommended ‐ matching vaccine | 12 | 26947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.31, 0.45] |
| 2.2 WHO recommended ‐ vaccine matching absent or unknown | 7 | 15068 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.35, 0.56] |
| 2.3 Monovalent not WHO recommended ‐ vaccine matching | 2 | 9675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.10, 0.54] |
| 2.4 Monovalent not WHO recommended ‐ vaccine matching ‐ high dose | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.00, 2.49] |
| 3 Physician visits Show forest plot | 2 | 2308 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.40, 1.89] |
| Analysis 1.3  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 3 Physician visits. | ||||
| 3.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.91] |
| 3.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.90, 1.83] |
| 4 Days ill Show forest plot | 3 | 3133 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.98, 0.56] |
| Analysis 1.4  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 4 Days ill. | ||||
| 4.1 WHO recommended ‐ matching vaccine | 2 | 2003 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.85, ‐0.32] |
| 4.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.66 [0.16, 1.16] |
| 5 Times any drugs were prescribed Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| Analysis 1.5  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 5 Times any drugs were prescribed. | ||||
| 5.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.04, ‐0.00] |
| 5.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 6 Times antibiotic was prescribed Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.03, ‐0.01] |
| Analysis 1.6  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 6 Times antibiotic was prescribed. | ||||
| 6.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.03, ‐0.01] |
| 6.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 7 Working days lost Show forest plot | 4 | 3726 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.14, 0.06] |
| Analysis 1.7  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 7 Working days lost. | ||||
| 7.1 WHO recommended ‐ matching vaccine | 3 | 2596 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.19, 0.02] |
| 7.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.00, 0.18] |
| 8 Hospitalisations Show forest plot | 3 | 11924 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| Analysis 1.8  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 8 Hospitalisations. | ||||
| 8.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [0.12, 70.68] |
| 8.3 Monovalent not WHO recommended ‐ vaccine matching | 1 | 9616 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 9 Clinical cases (clinically defined without clear definition) Show forest plot | 3 | 4259 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.05] |
| Analysis 1.9  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 9 Clinical cases (clinically defined without clear definition). | ||||
| 9.1 WHO recommended ‐ matching vaccine | 2 | 2056 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.25] |
| 9.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 2203 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.69, 0.99] |
| 10 Local harms Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 10 Local harms. | ||||
| 10.1 Local ‐ tenderness/soreness | 20 | 35655 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [2.44, 4.02] |
| 10.2 Local ‐ erythema | 9 | 29499 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.77, 3.78] |
| 10.3 Local ‐ induration | 3 | 7786 | Risk Ratio (M‐H, Random, 95% CI) | 4.28 [1.25, 14.67] |
| 10.4 Local ‐ arm stiffness | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.54, 4.83] |
| 10.5 Local ‐ combined endpoint (any or highest symptom) | 11 | 12307 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.82, 3.28] |
| 11 Systemic harms Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 11 Systemic harms. | ||||
| 11.1 Systemic ‐ myalgia | 10 | 30360 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.40, 2.24] |
| 11.2 Systemic ‐ fever | 12 | 19202 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.22, 1.95] |
| 11.3 Systemic ‐ headache | 13 | 31351 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.01, 1.36] |
| 11.4 Systemic ‐ fatigue or indisposition | 11 | 31140 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.07, 1.42] |
| 11.5 Systemic ‐ nausea/vomiting | 3 | 1667 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [0.55, 13.08] |
| 11.6 Systemic ‐ malaise | 3 | 26111 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.18, 1.92] |
| 11.7 Systemic ‐ combined endpoint (any or highest symptom) | 6 | 2128 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.87, 1.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 6 | 12688 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| Analysis 2.1  Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 1 Influenza‐like illness. | ||||
| 1.1 WHO recommended ‐ matching vaccine | 2 | 4254 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.12] |
| 1.2 WHO recommended ‐ vaccine matching absent or unknown | 3 | 8150 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.97] |
| 1.3 Non WHO recommended ‐ vaccine matching absent or unknown | 1 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 2 Influenza Show forest plot | 9 | 11579 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.35, 0.62] |
| Analysis 2.2  Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 2 Influenza. | ||||
| 2.1 WHO recommended ‐ matching vaccine | 4 | 6584 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.37, 0.82] |
| 2.2 WHO recommended ‐ vaccine matching absent or unknown | 3 | 4568 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.68] |
| 2.3 Non WHO recommended ‐ vaccine matching absent or unknown | 2 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.56] |
| 3 Influenza cases (clinically defined without clear definition) Show forest plot | 3 | 23900 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.71, 1.11] |
| Analysis 2.3  Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 3 Influenza cases (clinically defined without clear definition). | ||||
| 3.1 WHO recommended ‐ matching vaccine | 1 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.80] |
| 3.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 2082 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.25] |
| 3.3 Non WHO recommended ‐ vaccine matching absent or unknown | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4 Local harms Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4 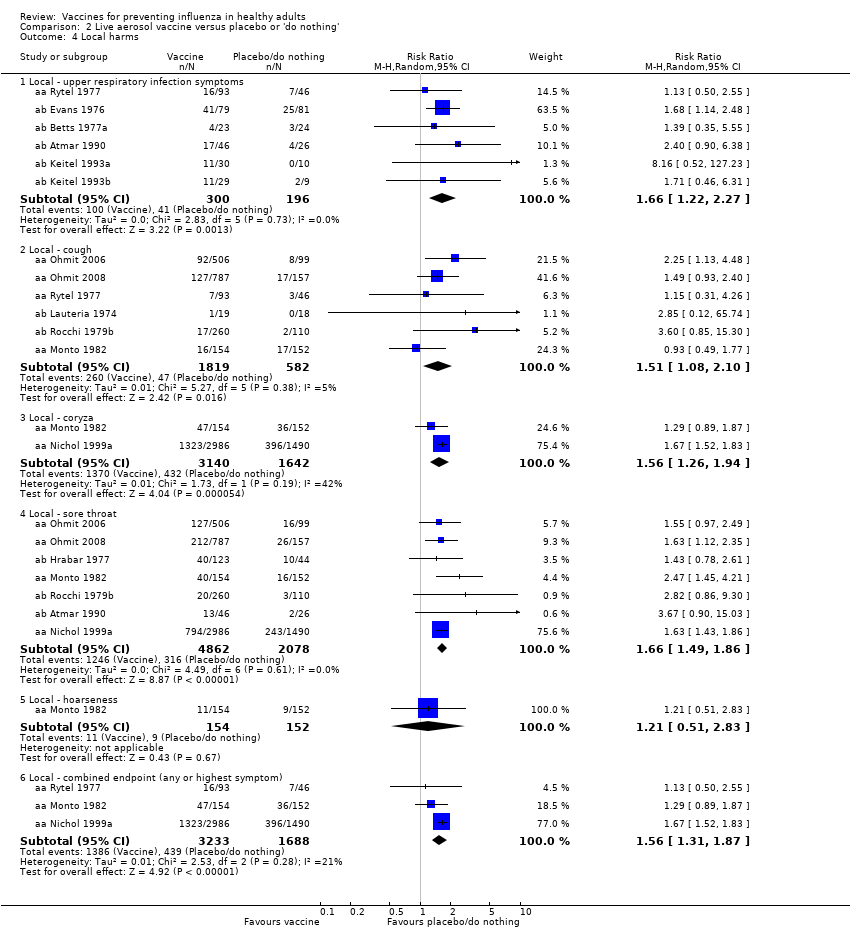 Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 4 Local harms. | ||||
| 4.1 Local ‐ upper respiratory infection symptoms | 6 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.22, 2.27] |
| 4.2 Local ‐ cough | 6 | 2401 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.08, 2.10] |
| 4.3 Local ‐ coryza | 2 | 4782 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.26, 1.94] |
| 4.4 Local ‐ sore throat | 7 | 6940 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.49, 1.86] |
| 4.5 Local ‐ hoarseness | 1 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.51, 2.83] |
| 4.6 Local ‐ combined endpoint (any or highest symptom) | 3 | 4921 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.31, 1.87] |
| 5 Systemic harms Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 5 Systemic harms. | ||||
| 5.1 Systemic ‐ myalgia | 4 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.26, 4.85] |
| 5.2 Systemic ‐ fever | 4 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.54, 1.92] |
| 5.3 Systemic ‐ fatigue or indisposition | 3 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.93, 2.07] |
| 5.4 Systemic ‐ headache | 2 | 975 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.09, 2.18] |
| 5.5 Systemic ‐ combined endpoint (any or highest symptom) | 5 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.82, 2.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza Show forest plot | 1 | 1348 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.02] |
| Analysis 3.1  Comparison 3 Inactivated aerosol vaccine versus placebo or 'do nothing', Outcome 1 Influenza. | ||||
| 1.1 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1348 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.02] |
| 1.2 WHO recommended ‐ matching vaccine | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Local harms Show forest plot | 3 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.71, 1.27] |
| Analysis 3.2  Comparison 3 Inactivated aerosol vaccine versus placebo or 'do nothing', Outcome 2 Local harms. | ||||
| 2.1 Local ‐ sore throat | 3 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.33] |
| 2.2 Local ‐ combined endpoint (any or highest symptom) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.71, 1.48] |
| 3 Systemic harms Show forest plot | 3 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.71, 1.62] |
| Analysis 3.3  Comparison 3 Inactivated aerosol vaccine versus placebo or 'do nothing', Outcome 3 Systemic harms. | ||||
| 3.1 Systemic ‐ myalgia | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.36, 2.25] |
| 3.2 Systemic ‐ fatigue or indisposition | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.52, 3.75] |
| 3.3 Systemic ‐ headache | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.85, 2.72] |
| 3.4 Systemic ‐ fever | 1 | 1349 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.03, 7.80] |
| 3.5 Systemic ‐ combined endpoint (any or highest symptom) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal inactivated vaccine effectiveness in mothers ‐ pregnant women Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 1 Seasonal inactivated vaccine effectiveness in mothers ‐ pregnant women. | ||||
| 1.1 H1N1 ‐ vaccine ‐ effectiveness ILI (unadjusted data) | 1 | 7328 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.06, 0.21] |
| 1.2 Seasonal ‐ vaccine ‐ effectiveness ILI ‐ (unadjusted data) | 2 | 50129 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.22, 1.32] |
| 2 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 2 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women. | ||||
| 2.1 Seasonal vaccine effectiveness ILI (HR adjusted data) | 2 | Hazard Ratio (Random, 95% CI) | 0.96 [0.90, 1.03] | |
| 3 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 3 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women. | ||||
| 3.1 Seasonal vaccine effectiveness ILI (RR adjusted data) | 1 | Risk Ratio (Random, 95% CI) | 0.92 [0.73, 1.16] | |
| 3.2 Seasonal vaccine efficacy influenza ‐ laboratory‐confirmed | 1 | Risk Ratio (Random, 95% CI) | 0.59 [0.37, 0.94] | |
| 4 H1N1 vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women Show forest plot | 9 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 4 H1N1 vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women. | ||||
| 4.1 Abortion (OR ‐ adjusted data) | 5 | Odds Ratio (Random, 95% CI) | 0.75 [0.62, 0.90] | |
| 4.2 Abortion (HR ‐ adjusted data) | 2 | Odds Ratio (Random, 95% CI) | 0.88 [0.67, 1.16] | |
| 4.3 Congenital malformation (OR ‐ adjusted data) | 5 | Odds Ratio (Random, 95% CI) | 1.06 [0.90, 1.25] | |
| 4.4 Prematurity (< 37 weeks) (OR adjusted data) | 8 | Odds Ratio (Random, 95% CI) | 0.86 [0.76, 0.97] | |
| 4.5 Neonatal death (OR adjusted data) | 1 | Odds Ratio (Random, 95% CI) | 1.81 [0.16, 20.35] | |
| 5 Seasonal vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women Show forest plot | 4 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 4.5  Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 5 Seasonal vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women. | ||||
| 5.1 Abortion (OR ‐ unadjusted data) | 1 | Odds Ratio (Random, 95% CI) | 0.60 [0.41, 0.86] | |
| 5.2 Congenital malformation (OR unadjusted data) | 2 | Odds Ratio (Random, 95% CI) | 0.55 [0.08, 3.73] | |
| 5.3 Prematurity (OR unadjusted data) | 4 | Odds Ratio (Random, 95% CI) | 0.96 [0.79, 1.17] | |
| 5.4 Neonatal death (OR unadjusted data) | 1 | Odds Ratio (Random, 95% CI) | 0.55 [0.35, 0.88] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effectiveness in newborns ‐ pregnant women (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.24 [0.04, 1.40] | |
| Analysis 5.1  Comparison 5 Inactivated parenteral vaccine versus placebo ‐ case‐control, Outcome 1 Effectiveness in newborns ‐ pregnant women (adjusted data). | ||||
| 1.1 Seasonal vaccine ‐ effectiveness ‐ ILI ‐ pregnant women | 2 | Odds Ratio (Random, 95% CI) | 0.24 [0.04, 1.40] | |
| 2 Seasonal vaccine safety ‐ pregnancy‐related outcomes (adjusted data) Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 0.80 [0.36, 1.78] | |
| Analysis 5.2  Comparison 5 Inactivated parenteral vaccine versus placebo ‐ case‐control, Outcome 2 Seasonal vaccine safety ‐ pregnancy‐related outcomes (adjusted data). | ||||
| 2.1 Abortion | 1 | Odds Ratio (Random, 95% CI) | 0.80 [0.36, 1.78] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccination and Guillain‐Barré syndrome Show forest plot | 3 | Risk Ratio (Random, 95% CI) | 1.28 [0.85, 1.93] | |
| Analysis 6.1  Comparison 6 Serious adverse events ‐ Guillain‐Barré syndrome ‐ cohort studies, Outcome 1 Seasonal influenza vaccination and Guillain‐Barré syndrome. | ||||
| 1.1 General population (adjusted data) | 2 | Risk Ratio (Random, 95% CI) | 1.29 [0.83, 2.02] | |
| 1.2 Pregnant women (unadjusted data) | 1 | Risk Ratio (Random, 95% CI) | 0.65 [0.03, 15.95] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 2009 to 2010 A/H1N1 ‐ general population (unadjusted data) Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7 Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control, Outcome 1 2009 to 2010 A/H1N1 ‐ general population (unadjusted data). | ||||
| 1.1 < 7 weeks | 6 | 1528 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [1.14, 4.31] |
| 1.2 At any time | 6 | 1656 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.87, 3.29] |
| 2 2009 to 2010 A/H1N1 ‐ general population (adjusted data) Show forest plot | 4 | Odds Ratio (Random, 95% CI) | 0.83 [0.39, 1.75] | |
| Analysis 7.2  Comparison 7 Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control, Outcome 2 2009 to 2010 A/H1N1 ‐ general population (adjusted data). | ||||
| 2.1 < 7 weeks | 4 | Odds Ratio (Random, 95% CI) | 0.92 [0.35, 2.40] | |
| 2.2 > 6 weeks | 3 | Odds Ratio (Random, 95% CI) | 0.71 [0.22, 2.32] | |
| 3 Seasonal influenza vaccination general population (adjusted data) Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 1.38 [0.18, 10.43] | |
| Analysis 7.3  Comparison 7 Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control, Outcome 3 Seasonal influenza vaccination general population (adjusted data). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza vaccination (seasonal) ‐ demyelinating diseases (unadjusted data) Show forest plot | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.25] |
| Analysis 8.1  Comparison 8 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies, Outcome 1 Influenza vaccination (seasonal) ‐ demyelinating diseases (unadjusted data). | ||||
| 1.1 General population | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Pregnant women | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.25] |
| 2 Influenza vaccination (H1N1) ‐ demyelinating diseases (unadjusted) Show forest plot | 1 | 144252 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.51, 8.22] |
| Analysis 8.2  Comparison 8 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies, Outcome 2 Influenza vaccination (H1N1) ‐ demyelinating diseases (unadjusted). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza vaccination (seasonal) ‐ general population ‐ demyelinating diseases (unadjusted data) Show forest plot | 4 | 8009 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.17] |
| Analysis 9.1  Comparison 9 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 1 Influenza vaccination (seasonal) ‐ general population ‐ demyelinating diseases (unadjusted data). | ||||
| 2 Influenza vaccination (seasonal) ‐ general population ‐ multiple sclerosis (adjusted data) Show forest plot | 2 | (Random, 95% CI) | 0.76 [0.54, 1.08] | |
| Analysis 9.2  Comparison 9 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 2 Influenza vaccination (seasonal) ‐ general population ‐ multiple sclerosis (adjusted data). | ||||
| 3 Influenza vaccination (seasonal) ‐ general population ‐ optic neuritis (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.03 [0.82, 1.30] | |
| Analysis 9.3  Comparison 9 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 3 Influenza vaccination (seasonal) ‐ general population ‐ optic neuritis (adjusted data). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccine ‐ HR (adjusted data) Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 10.1  Comparison 10 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ cohort studies, Outcome 1 Seasonal influenza vaccine ‐ HR (adjusted data). | ||||
| 1.1 General population | 0 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pregnant women | 1 | Hazard Ratio (Random, 95% CI) | 0.90 [0.68, 1.19] | |
| 2 Seasonal influenza vaccine (unadjusted data) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 10.2  Comparison 10 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ cohort studies, Outcome 2 Seasonal influenza vaccine (unadjusted data). | ||||
| 2.1 General population | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Pregnant women | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.70, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccine ‐ general population (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 11.1  Comparison 11 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ case‐control studies, Outcome 1 Seasonal influenza vaccine ‐ general population (adjusted data). | ||||
| 1.1 < 2 months | 2 | Odds Ratio (Random, 95% CI) | 1.87 [0.43, 8.06] | |
| 1.2 < 6 months | 1 | Odds Ratio (Random, 95% CI) | 0.90 [0.55, 1.47] | |
| 1.3 < 12 months | 1 | Odds Ratio (Random, 95% CI) | 0.70 [0.47, 1.04] | |
| 2 Seasonal influenza vaccine ‐ general population (unadjusted data) Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 11.2  Comparison 11 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ case‐control studies, Outcome 2 Seasonal influenza vaccine ‐ general population (unadjusted data). | ||||
| 2.1 < 2 months | 2 | 1926 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.48, 6.15] |
| 2.2 < 6 months | 1 | 1065 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.59, 1.43] |
| 2.3 < 12 months | 1 | 1066 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 3 | 3065 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.88] |
| Analysis 12.1  Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 1 Influenza‐like illness. | ||||
| 1.1 Standard recommended parenteral ‐ non‐matching ‐ 1 dose | 3 | 2715 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.57, 0.95] |
| 1.2 Standard recommended parenteral ‐ non‐matching ‐ 2 doses | 1 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.98] |
| 2 Influenza Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.87] |
| Analysis 12.2  Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 2 Influenza. | ||||
| 2.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.87] |
| 3 Hospitalisations Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.68] |
| Analysis 12.3  Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 3 Hospitalisations. | ||||
| 3.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.68] |
| 4 Pneumonia Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.17] |
| Analysis 12.4  Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 4 Pneumonia. | ||||
| 4.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 4 | 4580 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.25, 0.48] |
| Analysis 13.1  Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 1 Influenza‐like illness. | ||||
| 1.1 WHO recommended parenteral ‐ matching vaccine ‐ 1 dose | 4 | 4226 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.23, 0.53] |
| 1.2 WHO recommended parenteral ‐ matching vaccine ‐ 2 doses | 1 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.57] |
| 2 Influenza Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.31] |
| Analysis 13.2  Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 2 Influenza. | ||||
| 2.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.31] |
| 3 Hospitalisations Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.94] |
| Analysis 13.3  Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 3 Hospitalisations. | ||||
| 3.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.94] |
| 4 Pneumonia Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.05, 6.51] |
| Analysis 13.4  Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 4 Pneumonia. | ||||
| 4.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.05, 6.51] |
| 5 Working days lost Show forest plot | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| Analysis 13.5  Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 5 Working days lost. | ||||
| 5.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 6 Days ill Show forest plot | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| Analysis 13.6  Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 6 Days ill. | ||||
| 6.1 WHO recommended ‐ matching vaccine | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 2 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.95] |
| Analysis 14.1  Comparison 14 1968 to 1969 pandemic: inactivated polyvalent aerosol vaccine versus placebo, Outcome 1 Influenza‐like illness. | ||||
| 1.1 Inactivated polyvalent aerosol vaccine versus placebo ‐ non‐matching ‐ 1 dose | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.27] |
| 1.2 Inactivated polyvalent aerosol vaccine versus placebo ‐ non‐matching ‐ 2 doses | 1 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 2 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.32, 0.91] |
| Analysis 15.1  Comparison 15 1968 to 1969 pandemic: inactivated monovalent aerosol vaccine versus placebo, Outcome 1 Influenza‐like illness. | ||||
| 1.1 Inactivated monovalent aerosol vaccine versus placebo ‐ matching ‐ 1 dose | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.41] |
| 1.2 Inactivated monovalent aerosol vaccine versus placebo ‐ matching ‐ 2 doses | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza cases (clinically defined without clear definition) Show forest plot | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| Analysis 16.1  Comparison 16 1968 to 1969 pandemic: live aerosol vaccine versus placebo, Outcome 1 Influenza cases (clinically defined without clear definition). | ||||
| 1.1 Non‐matching | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 2 Complications (bronchitis, otitis, pneumonia) Show forest plot | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |
| Analysis 16.2  Comparison 16 1968 to 1969 pandemic: live aerosol vaccine versus placebo, Outcome 2 Complications (bronchitis, otitis, pneumonia). | ||||
| 2.1 Non‐matching | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |

Study flow diagram

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 1 Influenza‐like illness.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 2 Influenza.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 3 Physician visits.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 4 Days ill.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 5 Times any drugs were prescribed.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 6 Times antibiotic was prescribed.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 7 Working days lost.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 8 Hospitalisations.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 9 Clinical cases (clinically defined without clear definition).

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 10 Local harms.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 11 Systemic harms.

Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 1 Influenza‐like illness.

Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 2 Influenza.

Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 3 Influenza cases (clinically defined without clear definition).

Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 4 Local harms.

Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 5 Systemic harms.

Comparison 3 Inactivated aerosol vaccine versus placebo or 'do nothing', Outcome 1 Influenza.

Comparison 3 Inactivated aerosol vaccine versus placebo or 'do nothing', Outcome 2 Local harms.

Comparison 3 Inactivated aerosol vaccine versus placebo or 'do nothing', Outcome 3 Systemic harms.

Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 1 Seasonal inactivated vaccine effectiveness in mothers ‐ pregnant women.

Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 2 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women.

Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 3 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women.
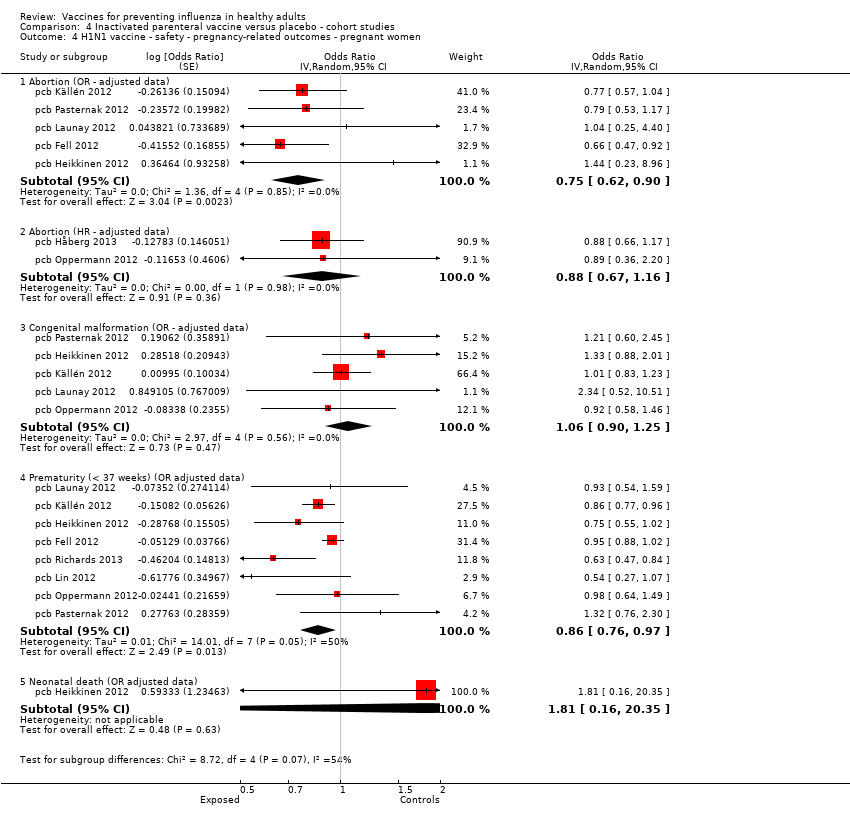
Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 4 H1N1 vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women.

Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 5 Seasonal vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women.

Comparison 5 Inactivated parenteral vaccine versus placebo ‐ case‐control, Outcome 1 Effectiveness in newborns ‐ pregnant women (adjusted data).

Comparison 5 Inactivated parenteral vaccine versus placebo ‐ case‐control, Outcome 2 Seasonal vaccine safety ‐ pregnancy‐related outcomes (adjusted data).

Comparison 6 Serious adverse events ‐ Guillain‐Barré syndrome ‐ cohort studies, Outcome 1 Seasonal influenza vaccination and Guillain‐Barré syndrome.

Comparison 7 Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control, Outcome 1 2009 to 2010 A/H1N1 ‐ general population (unadjusted data).

Comparison 7 Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control, Outcome 2 2009 to 2010 A/H1N1 ‐ general population (adjusted data).

Comparison 7 Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control, Outcome 3 Seasonal influenza vaccination general population (adjusted data).

Comparison 8 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies, Outcome 1 Influenza vaccination (seasonal) ‐ demyelinating diseases (unadjusted data).

Comparison 8 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies, Outcome 2 Influenza vaccination (H1N1) ‐ demyelinating diseases (unadjusted).

Comparison 9 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 1 Influenza vaccination (seasonal) ‐ general population ‐ demyelinating diseases (unadjusted data).

Comparison 9 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 2 Influenza vaccination (seasonal) ‐ general population ‐ multiple sclerosis (adjusted data).

Comparison 9 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 3 Influenza vaccination (seasonal) ‐ general population ‐ optic neuritis (adjusted data).

Comparison 10 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ cohort studies, Outcome 1 Seasonal influenza vaccine ‐ HR (adjusted data).

Comparison 10 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ cohort studies, Outcome 2 Seasonal influenza vaccine (unadjusted data).

Comparison 11 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ case‐control studies, Outcome 1 Seasonal influenza vaccine ‐ general population (adjusted data).

Comparison 11 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ case‐control studies, Outcome 2 Seasonal influenza vaccine ‐ general population (unadjusted data).

Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 2 Influenza.

Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 3 Hospitalisations.

Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 4 Pneumonia.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 2 Influenza.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 3 Hospitalisations.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 4 Pneumonia.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 5 Working days lost.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 6 Days ill.

Comparison 14 1968 to 1969 pandemic: inactivated polyvalent aerosol vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 15 1968 to 1969 pandemic: inactivated monovalent aerosol vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 16 1968 to 1969 pandemic: live aerosol vaccine versus placebo, Outcome 1 Influenza cases (clinically defined without clear definition).

Comparison 16 1968 to 1969 pandemic: live aerosol vaccine versus placebo, Outcome 2 Complications (bronchitis, otitis, pneumonia).
| Study design | High risk | Low risk | Unclear risk | Total |
| Case‐control | 3 | 2 | 15 | 20 |
| Cohort | 14 | 0 | 13 | 27 |
| RCT/CCT | 6 | 9 | 54 | 69 |
| Total | 23 | 11 | 82 | 116 |
| Study design | Government, institutional or public | Industry | Mixed | Total |
| Case‐control | 13 | 1 | 1 | 15 |
| Cohort | 22 | 3 | 2 | 27 |
| RCT/CCT | 31 | 12 | 5 | 48 |
| Total | 66 | 16 | 8 | 90 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 16 | 25795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.78, 0.87] |
| 1.1 WHO recommended ‐ matching vaccine | 7 | 4760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.89] |
| 1.2 WHO recommended ‐ vaccine matching absent or unknown | 7 | 20942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.75, 0.90] |
| 1.3 Monovalent not WHO recommended ‐ vaccine matching | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.28, 3.70] |
| 1.4 Monovalent not WHO recommended ‐ vaccine matching ‐ high dose | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.30] |
| 2 Influenza Show forest plot | 22 | 51724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.33, 0.44] |
| 2.1 WHO recommended ‐ matching vaccine | 12 | 26947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.31, 0.45] |
| 2.2 WHO recommended ‐ vaccine matching absent or unknown | 7 | 15068 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.35, 0.56] |
| 2.3 Monovalent not WHO recommended ‐ vaccine matching | 2 | 9675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.10, 0.54] |
| 2.4 Monovalent not WHO recommended ‐ vaccine matching ‐ high dose | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.00, 2.49] |
| 3 Physician visits Show forest plot | 2 | 2308 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.40, 1.89] |
| 3.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.91] |
| 3.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.90, 1.83] |
| 4 Days ill Show forest plot | 3 | 3133 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.98, 0.56] |
| 4.1 WHO recommended ‐ matching vaccine | 2 | 2003 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.85, ‐0.32] |
| 4.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.66 [0.16, 1.16] |
| 5 Times any drugs were prescribed Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 5.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.04, ‐0.00] |
| 5.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 6 Times antibiotic was prescribed Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.03, ‐0.01] |
| 6.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.03, ‐0.01] |
| 6.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 7 Working days lost Show forest plot | 4 | 3726 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.14, 0.06] |
| 7.1 WHO recommended ‐ matching vaccine | 3 | 2596 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.19, 0.02] |
| 7.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.00, 0.18] |
| 8 Hospitalisations Show forest plot | 3 | 11924 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 8.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [0.12, 70.68] |
| 8.3 Monovalent not WHO recommended ‐ vaccine matching | 1 | 9616 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 9 Clinical cases (clinically defined without clear definition) Show forest plot | 3 | 4259 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.05] |
| 9.1 WHO recommended ‐ matching vaccine | 2 | 2056 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.25] |
| 9.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 2203 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.69, 0.99] |
| 10 Local harms Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Local ‐ tenderness/soreness | 20 | 35655 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [2.44, 4.02] |
| 10.2 Local ‐ erythema | 9 | 29499 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.77, 3.78] |
| 10.3 Local ‐ induration | 3 | 7786 | Risk Ratio (M‐H, Random, 95% CI) | 4.28 [1.25, 14.67] |
| 10.4 Local ‐ arm stiffness | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.54, 4.83] |
| 10.5 Local ‐ combined endpoint (any or highest symptom) | 11 | 12307 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.82, 3.28] |
| 11 Systemic harms Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Systemic ‐ myalgia | 10 | 30360 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.40, 2.24] |
| 11.2 Systemic ‐ fever | 12 | 19202 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.22, 1.95] |
| 11.3 Systemic ‐ headache | 13 | 31351 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.01, 1.36] |
| 11.4 Systemic ‐ fatigue or indisposition | 11 | 31140 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.07, 1.42] |
| 11.5 Systemic ‐ nausea/vomiting | 3 | 1667 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [0.55, 13.08] |
| 11.6 Systemic ‐ malaise | 3 | 26111 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.18, 1.92] |
| 11.7 Systemic ‐ combined endpoint (any or highest symptom) | 6 | 2128 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.87, 1.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 6 | 12688 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 1.1 WHO recommended ‐ matching vaccine | 2 | 4254 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.12] |
| 1.2 WHO recommended ‐ vaccine matching absent or unknown | 3 | 8150 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.97] |
| 1.3 Non WHO recommended ‐ vaccine matching absent or unknown | 1 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 2 Influenza Show forest plot | 9 | 11579 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.35, 0.62] |
| 2.1 WHO recommended ‐ matching vaccine | 4 | 6584 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.37, 0.82] |
| 2.2 WHO recommended ‐ vaccine matching absent or unknown | 3 | 4568 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.68] |
| 2.3 Non WHO recommended ‐ vaccine matching absent or unknown | 2 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.56] |
| 3 Influenza cases (clinically defined without clear definition) Show forest plot | 3 | 23900 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.71, 1.11] |
| 3.1 WHO recommended ‐ matching vaccine | 1 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.80] |
| 3.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 2082 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.25] |
| 3.3 Non WHO recommended ‐ vaccine matching absent or unknown | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4 Local harms Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Local ‐ upper respiratory infection symptoms | 6 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.22, 2.27] |
| 4.2 Local ‐ cough | 6 | 2401 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.08, 2.10] |
| 4.3 Local ‐ coryza | 2 | 4782 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.26, 1.94] |
| 4.4 Local ‐ sore throat | 7 | 6940 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.49, 1.86] |
| 4.5 Local ‐ hoarseness | 1 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.51, 2.83] |
| 4.6 Local ‐ combined endpoint (any or highest symptom) | 3 | 4921 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.31, 1.87] |
| 5 Systemic harms Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Systemic ‐ myalgia | 4 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.26, 4.85] |
| 5.2 Systemic ‐ fever | 4 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.54, 1.92] |
| 5.3 Systemic ‐ fatigue or indisposition | 3 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.93, 2.07] |
| 5.4 Systemic ‐ headache | 2 | 975 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.09, 2.18] |
| 5.5 Systemic ‐ combined endpoint (any or highest symptom) | 5 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.82, 2.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza Show forest plot | 1 | 1348 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.02] |
| 1.1 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1348 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.02] |
| 1.2 WHO recommended ‐ matching vaccine | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Local harms Show forest plot | 3 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.71, 1.27] |
| 2.1 Local ‐ sore throat | 3 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.33] |
| 2.2 Local ‐ combined endpoint (any or highest symptom) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.71, 1.48] |
| 3 Systemic harms Show forest plot | 3 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.71, 1.62] |
| 3.1 Systemic ‐ myalgia | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.36, 2.25] |
| 3.2 Systemic ‐ fatigue or indisposition | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.52, 3.75] |
| 3.3 Systemic ‐ headache | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.85, 2.72] |
| 3.4 Systemic ‐ fever | 1 | 1349 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.03, 7.80] |
| 3.5 Systemic ‐ combined endpoint (any or highest symptom) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal inactivated vaccine effectiveness in mothers ‐ pregnant women Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 H1N1 ‐ vaccine ‐ effectiveness ILI (unadjusted data) | 1 | 7328 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.06, 0.21] |
| 1.2 Seasonal ‐ vaccine ‐ effectiveness ILI ‐ (unadjusted data) | 2 | 50129 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.22, 1.32] |
| 2 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Seasonal vaccine effectiveness ILI (HR adjusted data) | 2 | Hazard Ratio (Random, 95% CI) | 0.96 [0.90, 1.03] | |
| 3 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Seasonal vaccine effectiveness ILI (RR adjusted data) | 1 | Risk Ratio (Random, 95% CI) | 0.92 [0.73, 1.16] | |
| 3.2 Seasonal vaccine efficacy influenza ‐ laboratory‐confirmed | 1 | Risk Ratio (Random, 95% CI) | 0.59 [0.37, 0.94] | |
| 4 H1N1 vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women Show forest plot | 9 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Abortion (OR ‐ adjusted data) | 5 | Odds Ratio (Random, 95% CI) | 0.75 [0.62, 0.90] | |
| 4.2 Abortion (HR ‐ adjusted data) | 2 | Odds Ratio (Random, 95% CI) | 0.88 [0.67, 1.16] | |
| 4.3 Congenital malformation (OR ‐ adjusted data) | 5 | Odds Ratio (Random, 95% CI) | 1.06 [0.90, 1.25] | |
| 4.4 Prematurity (< 37 weeks) (OR adjusted data) | 8 | Odds Ratio (Random, 95% CI) | 0.86 [0.76, 0.97] | |
| 4.5 Neonatal death (OR adjusted data) | 1 | Odds Ratio (Random, 95% CI) | 1.81 [0.16, 20.35] | |
| 5 Seasonal vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women Show forest plot | 4 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Abortion (OR ‐ unadjusted data) | 1 | Odds Ratio (Random, 95% CI) | 0.60 [0.41, 0.86] | |
| 5.2 Congenital malformation (OR unadjusted data) | 2 | Odds Ratio (Random, 95% CI) | 0.55 [0.08, 3.73] | |
| 5.3 Prematurity (OR unadjusted data) | 4 | Odds Ratio (Random, 95% CI) | 0.96 [0.79, 1.17] | |
| 5.4 Neonatal death (OR unadjusted data) | 1 | Odds Ratio (Random, 95% CI) | 0.55 [0.35, 0.88] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effectiveness in newborns ‐ pregnant women (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.24 [0.04, 1.40] | |
| 1.1 Seasonal vaccine ‐ effectiveness ‐ ILI ‐ pregnant women | 2 | Odds Ratio (Random, 95% CI) | 0.24 [0.04, 1.40] | |
| 2 Seasonal vaccine safety ‐ pregnancy‐related outcomes (adjusted data) Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 0.80 [0.36, 1.78] | |
| 2.1 Abortion | 1 | Odds Ratio (Random, 95% CI) | 0.80 [0.36, 1.78] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccination and Guillain‐Barré syndrome Show forest plot | 3 | Risk Ratio (Random, 95% CI) | 1.28 [0.85, 1.93] | |
| 1.1 General population (adjusted data) | 2 | Risk Ratio (Random, 95% CI) | 1.29 [0.83, 2.02] | |
| 1.2 Pregnant women (unadjusted data) | 1 | Risk Ratio (Random, 95% CI) | 0.65 [0.03, 15.95] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 2009 to 2010 A/H1N1 ‐ general population (unadjusted data) Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 < 7 weeks | 6 | 1528 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [1.14, 4.31] |
| 1.2 At any time | 6 | 1656 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.87, 3.29] |
| 2 2009 to 2010 A/H1N1 ‐ general population (adjusted data) Show forest plot | 4 | Odds Ratio (Random, 95% CI) | 0.83 [0.39, 1.75] | |
| 2.1 < 7 weeks | 4 | Odds Ratio (Random, 95% CI) | 0.92 [0.35, 2.40] | |
| 2.2 > 6 weeks | 3 | Odds Ratio (Random, 95% CI) | 0.71 [0.22, 2.32] | |
| 3 Seasonal influenza vaccination general population (adjusted data) Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 1.38 [0.18, 10.43] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza vaccination (seasonal) ‐ demyelinating diseases (unadjusted data) Show forest plot | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.25] |
| 1.1 General population | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Pregnant women | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.25] |
| 2 Influenza vaccination (H1N1) ‐ demyelinating diseases (unadjusted) Show forest plot | 1 | 144252 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.51, 8.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza vaccination (seasonal) ‐ general population ‐ demyelinating diseases (unadjusted data) Show forest plot | 4 | 8009 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.17] |
| 2 Influenza vaccination (seasonal) ‐ general population ‐ multiple sclerosis (adjusted data) Show forest plot | 2 | (Random, 95% CI) | 0.76 [0.54, 1.08] | |
| 3 Influenza vaccination (seasonal) ‐ general population ‐ optic neuritis (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.03 [0.82, 1.30] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccine ‐ HR (adjusted data) Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 General population | 0 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pregnant women | 1 | Hazard Ratio (Random, 95% CI) | 0.90 [0.68, 1.19] | |
| 2 Seasonal influenza vaccine (unadjusted data) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 General population | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Pregnant women | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.70, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccine ‐ general population (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 < 2 months | 2 | Odds Ratio (Random, 95% CI) | 1.87 [0.43, 8.06] | |
| 1.2 < 6 months | 1 | Odds Ratio (Random, 95% CI) | 0.90 [0.55, 1.47] | |
| 1.3 < 12 months | 1 | Odds Ratio (Random, 95% CI) | 0.70 [0.47, 1.04] | |
| 2 Seasonal influenza vaccine ‐ general population (unadjusted data) Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 < 2 months | 2 | 1926 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.48, 6.15] |
| 2.2 < 6 months | 1 | 1065 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.59, 1.43] |
| 2.3 < 12 months | 1 | 1066 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 3 | 3065 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.88] |
| 1.1 Standard recommended parenteral ‐ non‐matching ‐ 1 dose | 3 | 2715 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.57, 0.95] |
| 1.2 Standard recommended parenteral ‐ non‐matching ‐ 2 doses | 1 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.98] |
| 2 Influenza Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.87] |
| 2.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.87] |
| 3 Hospitalisations Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.68] |
| 3.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.68] |
| 4 Pneumonia Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.17] |
| 4.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 4 | 4580 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.25, 0.48] |
| 1.1 WHO recommended parenteral ‐ matching vaccine ‐ 1 dose | 4 | 4226 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.23, 0.53] |
| 1.2 WHO recommended parenteral ‐ matching vaccine ‐ 2 doses | 1 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.57] |
| 2 Influenza Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.31] |
| 2.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.31] |
| 3 Hospitalisations Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.94] |
| 3.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.94] |
| 4 Pneumonia Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.05, 6.51] |
| 4.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.05, 6.51] |
| 5 Working days lost Show forest plot | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 5.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 6 Days ill Show forest plot | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 6.1 WHO recommended ‐ matching vaccine | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 2 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.95] |
| 1.1 Inactivated polyvalent aerosol vaccine versus placebo ‐ non‐matching ‐ 1 dose | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.27] |
| 1.2 Inactivated polyvalent aerosol vaccine versus placebo ‐ non‐matching ‐ 2 doses | 1 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 2 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.32, 0.91] |
| 1.1 Inactivated monovalent aerosol vaccine versus placebo ‐ matching ‐ 1 dose | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.41] |
| 1.2 Inactivated monovalent aerosol vaccine versus placebo ‐ matching ‐ 2 doses | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza cases (clinically defined without clear definition) Show forest plot | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 1.1 Non‐matching | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 2 Complications (bronchitis, otitis, pneumonia) Show forest plot | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |
| 2.1 Non‐matching | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |

