Apoyo a la lactancia materna en madres sanas con lactantes sanos nacidos a término
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | 2‐arm RCT, single‐site, n = 214 | |
| Participants | Large urban teaching hospital in Toronto, Canada Background rates of breastfeeding initiation: 89% Inclusion criteria: primiparous mothers in the first 2 days postpartum, singleton birth, ≥ 18 years old, ≥ 37 weeks’ gestation at delivery, able to speak and read English, and living with a male partner Exclusion criteria: women sharing a hospital room with a current study participant, a medical problem that could interfere with breastfeeding, infant not discharged from hospital with them, no access to the Internet or a telephone, planning to breastfeed for < 12 weeks, and had a partner who would not be available to participate in the study | |
| Interventions | Intervention: the trial intervention was a multifaceted coparenting breastfeeding support intervention, provided face‐to‐face on the postpartum unit, at which time the couples were provided with breastfeeding information, the information package was reviewed, and couples were given the option of watching a video. The session took ∼15 min in the majority of cases. Couples had a take‐home breastfeeding booklet, developed by Best Start: Ontarios Maternal, Newborn and Early Child Development Resource Centre, access to a secure study website that consisted of extensive information on breastfeeding and coparenting and contained links to related information and resources on the Internet including a copy of the video to watch at home. The couples were followed up at home with emails at 1 and 3 weeks postpartum and a telephone call at 2 weeks postpartum to answer any questions or concerns about the information provided. Control: couples received usual care, which included standard in‐hospital breastfeeding support and any breastfeeding assistance that was proactively sought in the community. | |
| Outcomes | Primary: Exclusive breastfeeding at 6 weeks and 12 weeks postpartum Secondary: Breastfeeding duration at 6 and 12 weeks postpartum Maternal perceptions of breastfeeding support Maternal perception of the coparenting relationship at 12 weeks postpartum | |
| Notes | Dates of the study: recruitment March ‐ July 2012 Funding sources: partial funding from Canadian Institutes of Health Research, Canada Research Chair Program Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Intervention group by sequentially numbered randomly generated numbers |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Envelopes were constructed by a research assistant who was not involved in any other trial procedure. Participants not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Participants in intervention group were known to assessors because they were interviewed. |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were < 25%. Complete follow‐up data were collected from 87.9% (n = 188) of fathers at 6 weeks and 88.3% (n = 189) of mothers at 6 weeks and 91.6% (n = 196) at 12 weeks. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes detailed in the study protocol were reported. |
| Other bias | Unclear risk | There were no significant differences in baseline characteristics between the groups except prenatal education. However, there was a non‐significant difference between the 2 groups in attendance at a prenatal breastfeeding class. |
| Study characteristics | ||
| Methods | 2‐arm cluster RCT, n = 36 clusters, 468 participants | |
| Participants | Rural. Manna district located in Jimma Zone in southwest Ethiopia. Health services are provided through 3 hospitals, 112 health centres and 498 health posts. Background breastfeeding rates: 58% of infants were exclusively breastfed for six months in 2016, giving a national average duration of EBF of 3.1 months Inclusion criteria: women in their second or third trimester of pregnancy, who were willing to participate with no intention of leaving the study area during the intervention period Exclusion criteria: severe mental illness that could interfere with consent and study participation, serious illness or clinical complications warranting hospitalisation, the occurrence of maternal death, abortion, stillbirth, infant death, twin gestation, pre term birth (at <37 weeks gestation), or any child congenital malformation that could interfere with breastfeeding Participant characteristics: 18.5% primiparous, 85.1% in age range 20‐34 years, 53.2% married. Education: Illiterate: 60.2%; can read and write 12.5%; Primary education: 7.63%; Secondary education: 19.7% | |
| Interventions | Peer supporters made home visits to women in the intervention clusters according to a pre‐specified schedule ‐ two home visits in the last trimester of pregnancy and home visits after birth on 1st or 2nd, 6th or 7th and 15th day, and thereafter monthly until the infant was five months. Content of visits by peer supporters: in pregnancy ‐ encouraged delivery at the nearby health centre, emphasised the importance of initiating breastfeeding within 1 h of delivery, feeding colostrum first, discouraging the use of traditional pre‐lacteal foods. The discussions were combined with the use of educational materials and practical demonstrations on proper breastfeeding positioning and latching. First two weeks after delivery, emphasised frequent and on‐demand breastfeeding, encouraged stopping any traditional pre‐lacteal foods or post‐lacteal food items if already given and observed the positioning, latching, and feeding of the newborn, solving any breastfeeding problems and providing appropriate feedback, while encouraging the mothers to continue EBF for six months. From month one, peer‐supporters emphasised techniques for preparing for work and management of breast milk(breast‐milk expression, storing breast milk), discussed the lactation amenorrhoea method, and other family planning options. Mothers were encouraged to ask questions related to any topic discussed. Peer supporters also provided additional visits if women experienced breastfeeding problems. Women also received an emotional, appraisal, and instrumental support. The duration of each visit was typically 20–40 min. Control: women in the control group received the routine care offered by the Health Extension Workers (HEWs) and WDA leaders working in their cluster, similar to that received by women in the intervention group. This included four focused prenatal visits, developing an individualised birth preparedness and complication readiness plan, accompanying a woman to a health facility during delivery, and conducting four postnatal visits. | |
| Outcomes | Exclusive breastfeeding at 6 months Stunting, fever, cough, diarrhoea | |
| Notes | Dates of the study: enroled for the study between May and September 2017. Funding sources: support provided by NORAD(Norwegian Agency for Development Cooperation) under the NORHED‐Programme, the Jimma Zone Health Department and the local MOH office. Declarations of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We used simple randomization with a 1:1 allocation to allocate sub‐districts to either control or intervention. First, the 36 sub‐districts were listed alphabetically and then they were sequentially numbered starting from 01 to 36. Then we generated 18 random numbers from those 01 to 36 using MS Excel 2010 and the districts with the selected random numbers were assigned to the intervention group, while the rest were assigned to the control group. |
| Allocation concealment (selection bias) | Low risk | A statistician that is blinded to study groups and not participating in the research will do the generation of the allocation sequence and the randomization of clusters. |
| Blinding of participants and personnel (performance bias) | High risk | Allocation concealment was not done for study participants, as they would know if they were in the intervention group or not |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reprt data collected from mothers |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐off <20%: A total of 468 pregnant women (n intervention = 249; control = 219) were enrolled at baseline. Outcome data available for 421 (90.0%) mother‐child pairs (n intervention = 221; control = 200) at the one‐month postpartum follow‐up and from 409 (87.4%) mother‐child pairs (n intervention = 212; control = 197) at the six‐month postpartum follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the protocol are reported Protocol included and outcomes section pasted above. Final study agreed "The primary study outcomes were rates of EI and EBF for six months and infant growth." |
| Other bias | Low risk | Funded by NORAD No conflicts of interest declared Baseline characteristics look balanced but statistical significance not reported. Intra‐cluster correlation reported |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation n = 141 | |
| Participants | Three Midwestern hospitals, USA. Background breastfeeding rates: Exclusive breastfeeding rate at 6 months of 11.4% in Indiana Inclusion criteria: ability to read and speak English, ≥ 18 years old, intention to continue breastfeeding after discharge, no serious medical condition, basic knowledge of Internet, and access to electronic mail and the Internet through either a standard PC or a smartphone. Infants ‐ singleton full‐term (≥37 gestational weeks). Exclusion criteria: infants born with cleft lip/palate, congenital heart defects, Down Syndrome, neural tube defects, or other conditions that either require the newborn's admission to a neonatal intensive care unit or interfere with breastfeeding. | |
| Interventions | The intervention group received the same usual care support, but were also given access to an interactive web‐based breastfeeding monitoring system prior to discharge. Mothers were asked to enter breastfeeding and infant output data (along with any problems) for 30 days. The system automatically sent feedback via notifications with tailored interventions if the mother entered data that indicated breastfeeding problems including the inability to latch, latching with nipple shields, difficulty waking up for feedings, jaundice, mother's sore nipples, engorgement, or insufficient feeding (<6 times per day). The system provided positive notifications when a mother breastfed 8 to 10 times per day and read the notifications. Control: standard care which consisted of breastfeeding support and education before discharge, one phone call within the first week after hospital discharge, and a list of community breastfeeding resources. Mothers were encouraged to contact the lactation specialist with any breastfeeding problems. | |
| Outcomes | Any and exclusive breastfeeding at 1,2,3 months Maternal satisfaction with feeding Postnatal depression (EPDS) | |
| Notes | Dates of the study: not reported Funding sources: Indiana CTSI Collaboration in Biomedical/Translational Research (CBR/CTR) Pilot Program Grants. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to the usual care group and intervention group by computer‐generated random numbers using mode of delivery and parity as stratifying factors to control for these variables |
| Allocation concealment (selection bias) | Unclear risk | Unclear – not described |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported breastfeeding |
| Incomplete outcome data (attrition bias) | High risk | 1 month: I= 49/84, C= 55/57 2 month: I= 48/84, C= 54/57 3 month: I= 44/84, C= 52/57 |
| Selective reporting (reporting bias) | Unclear risk | No published protocol to judge outcome |
| Other bias | Low risk | No industry funding reported. No conflicts of interest No significant differences at baseline
|
| Study characteristics | ||
| Methods | 3‐arm RCT, with individual randomisation n = 231 | |
| Participants | The study was carried out in the Tema area of Ghana (sub‐Saharan Africa). Women were recruited in prenatal clinics in 2 hospitals (1 government and 1 private) that served urban areas (an industrial city and a commercial town). Background breastfeeding rates: High baseline prevalence of breastfeeding in Ghana, the median duration of breastfeeding was reported as being 22 months and 53.4% of women with babies < 6 months breastfeed exclusively. It was reported that "almost all" mothers initiated breastfeeding. 231 women randomised (136 eligible at the beginning of the intervention period). Inclusion criteria: pregnant women in the last trimester planning delivery in the study hospitals and to stay in study area for 6 months after delivery. After delivery: singleton babies with normal birthweight (> 2500 g) and Apgar scores ≥ 6 at 1 min and 5 min Exclusion criteria: multiple birth, low Apgar score or planning to move out of area Participant characteristics: 38% of the women had only primary level or no formal education; 90% were married or living with a partner; 46% were primiparous; 73% had vaginal birth; 24% lived in households with access to a car; 74% were described as trader/artisan | |
| Interventions | All 3 groups (intervention 1, intervention 2 and control) were allocated to 2 educational group sessions during pregnancy by trained nurses and 9 proactive home visits by trained nurse counsellors at 1, 2, 4, 6, 8, 12, 16, 20 and 24 weeks postpartum. These were in addition to standard care. The content of the sessions differed between the 3 groups. 63% of intervention 1, 73% of intervention 2 and 65% of the control group women received all 9 scheduled home follow‐up visits. Intervention 1 (n = 74): 43 followed up. Content of sessions was breastfeeding and exclusive breastfeeding. Trained local nurses with experience of breastfeeding gave 2 educational sessions, of approximately 20 min each, to groups of 2‐4 women during their third trimester. At postpartum home visits women received individual counselling and nurses were advised to respond to concerns. Materials were developed from WHO/UNICEF breastfeeding counselling training manual. Intervention 2 (n = 72): 44 followed up. Content of the pregnancy sessions was general health and childcare as for control group. Content of the postpartum home visits was breastfeeding and exclusive breastfeeding as for intervention 1. Control (n = 85): 49 followed up. Content of sessions was general health and childcare topics such as immunisation, HIV/AIDS, nutrition and family planning. | |
| Outcomes | Breastfeeding status at 1, 2, 3, 4, 5, and 6 months, exclusive breastfeeding up to 6 months, infant morbidity and growth | |
| Notes | We have not included data from this study in the review due to high levels of attrition (> 25% loss to follow‐up). Most data were reported in graphs and difficult to interpret. Several measures of exclusive breastfeeding were reported; at 1 and 6 months women were asked about breastfeeding since birth, during previous month and on previous day. In this review we have reported figures for exclusive breastfeeding since birth for both time points. Figures in the paper were expressed as percentage of women still exclusively breastfeeding; in order to use the data we used subtraction to calculate a figure for women who had stopped breastfeeding. Dates of the study: recruitment between May and September 2002 Funding sources: University of Connecticut Research Foundation and LINK‐AGES USAID Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was achieved by writing numbers 1 to 3 on folded pieces of paper." |
| Allocation concealment (selection bias) | High risk | Quote: "The numbers were not viewed by either study staff or mothers and the pieces of paper looked the same on the outside. Before offering papers to mothers, they were shuffled in the interviewer’s palm.” Quote: “The randomisation scheme used was not a formal one. It was one that could be conducted easily in the field. Despite this, it functionally produced balanced groups with no evidence of bias.” |
| Blinding of participants and personnel (performance bias) | High risk | It was stated that women were informed only that they would receive “health education” that would be beneficial to their infants and themselves, but were not aware of their group allocation or of differences in the content of the health education. However, it is not possible to blind. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "It was impossible to keep counsellors unaware of study design.... research assistants [collecting outcome data] were aware of mothers group allocation." |
| Incomplete outcome data (attrition bias) | High risk | 231 women randomized during the third trimester. At delivery 95 women were excluded as they were no longer eligible (41% lost before the intervention). A further 13 women were lost to follow‐up during the intervention period. 123 completed the final follow‐up at 6 months (i.e. 53% of the original randomized sample but 90% of those still eligible at delivery). Results were reported in graphs and percentages and it was not clear how many women commenced breastfeeding, so group denominators are not clear. Loss to follow‐up appeared balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Failure to provide denominators for results means that they are very difficult to interpret. |
| Other bias | Unclear risk | Women in the 3 arms of the trial appeared similar at baseline. Analysis was according to group allocation. |
| Study characteristics | ||
| Methods | RCT, single site, a BFI hospital, n = 66 | |
| Participants | Urban state maternity hospital in Turkey Background rates of breastfeeding initiation: high Inclusion criteria: prima parous, live vaginal birth, healthy term singleton infant, living in study area, able to speak Turkish, no history of chronic diseases, non‐smoker, intending to breastfeed Exclusion criteria: infant birthweight < 2500 g, Apgar score ≤ 7, congenital anomalies, serious disease or needing intensive care Baseline prevalence of "ever breastfed" in Turkey: 96.7% (WHO Global data bank 2010, accessed 6 October 2011). | |
| Interventions | Intervention: women received standard breastfeeding support plus support from trained lay supporters who had undergone WHO/UNICEF 18‐h training. The intervention was a single home visit on day 3 after the birth (in hospital), by 2 lay breastfeeding supporters, that lasted about 30 min and covered the same topics as routine support. Control: at this Baby‐Friendly hospital, a standard breastfeeding education session lasting 20‐30 min was provided to all mothers before standard discharge home at 24 h after the birth. The session included the topics covered by the 18‐h WHO/UNICEF training. | |
| Outcomes | Exclusive breastfeeding at 2 and 6 weeks and 6 months postpartum; breastfeeding duration (any/exclusive) to 18 months; breastfeeding knowledge scores at 2 and 6 weeks postpartum | |
| Notes | Dates of the study: study conducted between March and July 2008 Funding sources: not reported Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information provided to enable a judgement to be made |
| Blinding of outcome assessment (detection bias) | High risk | Women were contacted either through home visits or via the phone and data on breastfeeding was collected, however, not reported whether the assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 82% follow‐up at 18 months. Reasons for loss were explained and were balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Not apparent |
| Other bias | Low risk | Groups appeared similar at baseline. |
| Study characteristics | ||
| Methods | Primary care facilities, recruitment over 5 months, n = 169 | |
| Participants | 3 hospitals in the city of Pelotas, in southern Brazil Background rates of breastfeeding initiation: 88% Ethnic composition not described. Inclusion criteria: term healthy baby, family income ≥ USD 500 per month (no economic constraints to baby's growth), mother intended to breastfeed and did not smoke. Exclusion criteria: multiple birth, gestational age not 37‐42 weeks, significant perinatal morbidity, maternal smoking and family income USD 500 per month. | |
| Interventions | Intervention: hospital visit, home visits at 5, 15, 30, 45, 90 and 120 days, and 24‐h telephone hotline for help or to arrange visits. 2 members of the lactation support team had received the 40‐h WHO lactation support training course. Control: attended paediatric clinics where general advice on advantages of breastfeeding may have been offered, but specific lactation counselling was not provided. | |
| Outcomes | Breastfeeding pattern and duration up to age of 4 months. Breast milk intake for a subgroup of 68 infants at 4 months | |
| Notes | Dates of the study: recruitment from August 1999 to January 2000 Funding sources: not reported Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information provided to enable a judgement to be made |
| Blinding of outcome assessment (detection bias) | High risk | The interviewers were not informed about the intervention or control status of each mother, and did not know the study's objectives. |
| Incomplete outcome data (attrition bias) | High risk | 188 women were randomized. 21 were excluded after 2 weeks as they had introduced formula milk. A further 26 withdrew (some data were available for some of these women). 141 women completed the trial (75%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Excluding women who introduced formula within 2 weeks of randomisation is likely to have introduced bias although similar numbers were excluded from both groups (9 women lost from the intervention group for this reason and 11 women from the control group and an additional control was withdrawn for smoking). |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation n = 135 | |
| Participants | Hartford area of Connecticut, USA in a hospital providing care for predominantly Latina low‐income women Inclusion criteria: age ≥18 years; gestational age < 32 weeks at first approach; healthy, considering breastfeeding, planning delivery at study hospital and resident in area for 3 months after the birth, 185% of the federal poverty level, available for telephone contact and willing to participate Exclusion criteria for mothers: medical conditions such as diabetes or hypertension; drug use that could impair breastfeeding Exclusion criteria for infants: preterm, low birthweight (< 2500 g), any complications requiring admission to special care, Apgar score < 7 at 1 min and 5 min Participant characteristics: at baseline: intervention n = 63; control n = 72 Participant characteristics: Married/cohabiting: intervention 40%; control 26% Hispanic race: intervention 81%; control 64% Education less than high school: intervention 31%; control 38% Received welfare: intervention 31%; control 38% Primiparous: intervention 92%; control 89% Planned breastfeeding duration < 6 months: intervention 20%; control 46% Planned breastfeeding duration 6‐12 months or longer: intervention 80%; control 54% | |
| Interventions | Intervention: in addition to standard care, women received 3 prenatal home visits, daily in‐hospital visits and 9 postpartum home visits from peer counsellors: 3 in first week, 2 in second week and 1 in each week for weeks 3‐6. Women could also phone peer counsellors. Peer counsellors were mothers from the area with experience of successful breastfeeding and training from a lactation consultant (LC). Control: women received what would have been standard care for private patients (these women may not have normally qualified to receive this care as many were participating in welfare programmes). This consisted of: breastfeeding support line open to mothers after delivery staffed by a lactation specialist. Usual in‐patient care and support for breastfeeding was provided by hospital staff. | |
| Outcomes | Infant feeding practices (weekly for first month) breastfeeding and exclusive breastfeeding. Infant morbidity (diarrhoea and ear infection). Breastfeeding outcomes measured in 3 different ways – over the past 24 h, over the past week and since the birth (ever given). | |
| Notes | We have not included data from this study in the review due to high levels of attrition (> 25% loss to follow‐up). Dates of the study: January 2003‐July 2004 Funding sources: Centers for Disease Control and Prevention (Atlanta, Ga) through a subcontract by the Association of Teachers of Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | SPSS software was used to randomly assign subjects to study groups. |
| Allocation concealment (selection bias) | Low risk | Quote: "Recruited subjects were entered into the database at the end of every week” and then random allocation by computer software.
|
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated if women or peer counsellors were blinded, but unlikely. |
| Blinding of outcome assessment (detection bias) | High risk | Not a double‐blind study and the interviewer knew the study hypotheses. Steps were taken to prevent interviewer bias by asking questions regarding peer counsellor contact at the very end of each follow‐up interview session. |
| Incomplete outcome data (attrition bias) | Unclear risk | 182 women were recruited and randomized. 162 were still eligible at delivery and 135 completed the trial (84% of those still eligible at delivery and 74% of the total randomized). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline although women in the control group were more likely (46%) to plan to breastfeed for < 6 months than women in the intervention group (20.4%). This difference in breastfeeding intentions means that the results are more difficult to interpret. |
| Study characteristics | ||
| Methods | 2‐arm RCT, n = 120 | |
| Participants | Four prenatal clinics in West Ahvaz, Iran selected because of its ethnically diverse population and low rates of exclusive breastfeeding. Background breastfeeding rates: 90% of women nationwide initiated breastfeeding and that 44% exclusively breastfeed at 6 months in 2000, decreasing to 27% exclusive breastfeeding at 6 months in 2005. Inclusion criteria: pregnant women who spoke Persian, and were: nulliparous, had singleton fetuses, intended to breastfeed, were between 35 and 37 weeks gestation, and had cell phones. Exclusion criteria: any health condition that could interfere with breastfeeding, such as a high‐risk pregnancy (e.g., antepartum bleeding, low amniotic fluid volume) or any breast surgery. | |
| Interventions | Breastfeeding multifaceted self‐efficacy intervention provided 35 and 37 weeks gestation by a research nurse with extensive breastfeeding knowledge and experience in assisting breastfeeding women. Two 1‐hour small group‐breastfeeding education sessions, an information booklet with breastfeeding images, and biweekly text messages. Control: standard prenatal and postpartum care consisting of eight prenatal visits at a healthcare clinic with a midwife. In the hospital and postnatally, midwives also provide care, including lactation support, for all breastfeeding women. | |
| Outcomes | Any and exclusive breastfeeding at 2 months | |
| Notes | Dates of the study: Recruitment: April 2014 to October 2015. Funding sources: not reported but states no relevant financial relationships. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomiztaion was achieved using sealed, opaque, sequentially numbered envelopes developed from a random number generator.
|
| Allocation concealment (selection bias) | Low risk | Randomiztaion was achieved using sealed, opaque, sequentially numbered envelopes developed from a random number generator. A midwife who was not involved in the recruitment of participants prepared the envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Women and staff were aware of group allocation |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes assessed by questionnaire ‐ self‐report |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐ up rates at 8 weeks postpartum were high, with 56 (93.3%) participants in the intervention group and 54 (90%) participants in the control group who completed the 8‐week postpartum questionnaire. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the protocol are reported
|
| Other bias | Unclear risk | No conflicts of interest declared Funding not reported No baseline imbalance
|
| Study characteristics | ||
| Methods | 2‐arm RCT, individually randomised n = 414 | |
| Participants | Urban Primary Care centres in Barcelona Spain. Background breastfeeding rates: EBF at 6 months in Spain is 28.5% In this region, the prevalence of EBF at 6 months of age is approximately 32%. Inclusion criteria: mothers of healthy infants delivered at term (≥37weeks) in the catchment population of participating centres and who were breastfeeding their children exclusive or partial breastfeeding Exclusion criteria: admission of infant or mother to ICU, multiple pregnancy, severe congenital malformation in the infant, maternal age of 18 years or less, or mother that lacked a phone or had a language barrier. | |
| Interventions | A weekly phone call during the first 2 months and a call every other week between months 2 and 6 post birth by a nurse assigned to the infant who also carried out routine visits. During the first month: position of the newborn for breastfeeding, frequency of feeds, number and consistency of stools, general breast care, normal weight gain, and, in mothers that supplemented feedings, advice and support to try to re‐establish EBF. In months 2‐3: advice on expressing breast milk, with instructions on how to handle and store breast milk. In months 4‐6: how to use stored breast milk, administration of stored milk to infants, importance of maintaining EBF and avoiding administration of other types of milk or foods. Control: mothers in both the control and the experimental groups attended the visits included in the preventive care protocol ‐ postnatal visit with the paediatrician (between days 7 and 15 post birth), check‐ups at 1, 2, 4 and 6 months with the nurse assigned to the patient, under the supervision of the paediatrician. The nurse counselled the mother regarding nutrition during these visits. Mothers could schedule additional appointments or call the nurse on the phone to receive guidance regarding breastfeeding problems. | |
| Outcomes | Any and exclusive breastfeeding at 1, 2, 3 and 6 months | |
| Notes | Dates of the study: data collection between October 2014 and October 2016 Funding sources: not reported. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The mothers that agreed to participate were assigned to the experimental or the control group using a random number table generated by computer software after signing an informed consent form. |
| Allocation concealment (selection bias) | Unclear risk | There is no information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Trial participants self‐reported their breastfeeding experiences. The data was collected by the professional doing the visit. |
| Incomplete outcome data (attrition bias) | Low risk | Intervention group drop‐off = 193/207 = 7% Control group drop‐off = 187/207 = 10% Low risk as symmetrical drop‐off and less than 20% All exclusions appear to have been accounted for |
| Selective reporting (reporting bias) | Unclear risk | Prospective protocol (Clinical Trials number NCT02186613). All outcomes are reported. |
| Other bias | Unclear risk | No industry funding No conflicts of interest No baseline imbalance Study was underpowered ‐ 67% |
| Study characteristics | ||
| Methods | 2‐arm RCT, individually randomised, n = 166 | |
| Participants | Young mothers in seven locations in England: London (two sites), the Midlands (two sites), the North East (one site) and the North West of England (two sites). Background breastfeeding rates: Not reported. Inclusion criteria: expectant mothers with a gestation of 16 to 20 weeks, with expected delivery dates (EDDs) within approximately 10 weeks of each other, for each group in each site. They were either aged below 20 years at their last menstrual period (LMP) with one or more previous live births, or aged 20–24 at LMP with no previous live births and with low educational qualifications, defined as neither mathematics nor English language GCSE at grade C or higher; if both, then no more than four GCSEs at grade C or higher. They had to be able to provide consent and to speak English. Exclusion criteria: women who had previously received FNP and those with psychotic mental illness. | |
| Interventions | Group FNP (gFNP) is designed to run from the first trimester of pregnancy until infants are 12 months old with 44 group meetings in the curriculum, 14 covering pregnancy and 30 covering infancy. Delivered to a group of women living in relatively proximity to each other, with similar expected delivery dates (range 8–10 weeks). Meetings are around 2 h facilitated by two experienced FNP FNs one of whom had notified their intention to practice as a midwife. Includes content to: improve maternal health and pregnancy outcomes, improve child health and development by helping parents provide more sensitive and competent care; and to improve parental life course by helping parents develop effective support networks, plan future pregnancies, complete their education, and find employment. The curriculum domains are: mother’s personal health; the maternal role; maternal life course: family and friends; environmental health; and related health and human services, with referrals made when necessary. Control: usual care ‐ offers every family a program of screening tests, immunisations, developmental reviews, and information and guidance to support parenting and healthy choices. There are core universal elements provided for all families with additional progressive, preventive elements for those with medium or high risk. The universal program includes a neonatal examination, a new baby re‐ view at about 14 days, a 6‐ to 8‐week baby examination and a review by the time the child is 1 year old and at 2 to 2.5 years old. | |
| Outcomes | Any breastfeeding at 6 months Postnatal depression (EPDS) | |
| Notes | Dates of the study: the trial commenced in February 2013, recruitment and baseline data collection commenced in July 2013, continuing to September 2014. Funding sources: UK National Health Service (NHS) National Institute for Health Research (NIHR), Public Health program, grant number 11/3002/02 109425. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to one of two arms was securely computer‐generated and delivered by email to LSHTM which conveyed the information to study participants by post and conveyed to each gFNP team the names and contact details of women allocated to the intervention arm by fax or password‐protected email, receiving confirmation of receipt by email. |
| Allocation concealment (selection bias) | Low risk | Randomization was overseen by the London School of Hygiene and Tropical Medicine Clinical Trials Unit (LSHTM CTU). The unique identification number (which included a site identifier) and age at LMP of eligible consenting mothers‐to‐be were passed to the central randomization service at the Health Service Research Unit (HSRU), University of Aberdeen using an automated telephone procedure. |
| Blinding of participants and personnel (performance bias) | High risk | Participants could not be blind to allocation as they knew whether or not they had been offered gFNP, and gFNP practitioners were not blind to the intervention participants but had no knowledge of the control group. |
| Blinding of outcome assessment (detection bias) | High risk | Assessment of breastfeeding by sel‐report questionnaire |
| Incomplete outcome data (attrition bias) | Low risk | Low and balanced loss to follow‐up at 2 months (85% retained in intervention group and 90% retained in control group) and at 6 months (83% retained in intervention group and 82% retained in control group). Low dropout pertains to breastfeeding outcomes. There was high dropout for the video element assessing mother‐infant interaction (17% refused to be videoed) |
| Selective reporting (reporting bias) | Low risk | Measures used at each time point are presented in Table 1 with full details in the published protocol [ |
| Other bias | Low risk | Funded by NIHR Authors declare they have no competing interests Baseline characteristics balanced |
| Study characteristics | ||
| Methods | 2‐arm RCT, single‐site, n = 900 | |
| Participants | Urban setting in Brazil: in‐patient maternity unit Background rates of breastfeeding initiation: high Ethnic composition not described Inclusion criteria: family income < twice the minimum Brazilian wage; hospital stay < 5 days; wanting to breastfeed: living within the city of Pelotas Baseline prevalence in Pelotas (1993) for any breastfeeding: 85% at 1 month, 66% at 3 months and 38% at 6 months. | |
| Interventions | Intervention: 3 home visits at 5, 10 and 20 days postpartum by a social assistant or nutritionist. The visitor was required to have a personal history of successfully breastfeeding a child and received training in breastfeeding physiology and common breastfeeding problems and their solutions. Control: usual care, a social assistant would not normally make routine home visits but would visit only when requested to do so by the hospital team. | |
| Outcomes | Breastfeeding at monthly intervals to 6 months and median duration of breastfeeding Time to introduction of artificial feeds Difficulties encountered during breastfeeding and reasons for weaning also recorded. | |
| Notes | Dates of the study: January 1988 ‐ March 1989 Funding sources: information not included in translation Declarations of interest: information not included in translation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Record in Portugese and no information in the translation regarding blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | The nurse collecting outcome data was not aware of previous contacts, but the authors stated that s/he may have been made aware of group assignment as women were likely to talk about the intervention. |
| Incomplete outcome data (attrition bias) | Low risk | 900 randomized, approximately 8% lost to follow‐up in the intervention and control groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | No baseline imbalance apparent. Assessment of risk of bias was made from translation notes. The original paper is in Portuguese. |
| Study characteristics | ||
| Methods | 3‐arm RCT, with individual randomisation n = 903 | |
| Participants | Recruited from Maternity Teaching Hospital in Damascus, Syria Background rates of breastfeeding initiation: high Inclusion criteria: consenting women who delivered a healthy newborn by vaginal delivery or caesarean section, who lived within 30 km from hospital, and were available for follow‐up for the next 6 months Exclusion criteria for infants: premature, low birthweight (< 2500 g), with apparent congenital anomalies Participant characteristics: Age not clear. Approximately 37% primiparous 90% had normal labour > 99% of the women were married Home conditions were described as bad (number of rooms, poor sanitation or water, etc.) in 28.5% of control group and approximately 20% of the intervention groups Few of the women (approximately 5%) worked outside the home. | |
| Interventions | Intervention 1 (n = 301): 4 structured home visits from trained midwives at 1, 3 and 7 days and 4 weeks after the birth. Midwives examined mothers and infants and provided and advice and support on a range of healthcare issues including breastfeeding support and education. Intervention 2 (n = 301): a single postnatal visit from a trained midwife at 3 days which included advice and education on breastfeeding. Control (n =301): received standard care in Syria (no postnatal visits). | |
| Outcomes | Primary: Maternal postpartum morbidities, postnatal care uptake, contraceptive uptake and type, infant morbidities, infant immunisation according to the national schedule at 3 months and Infant feeding (specifically exclusive breastfeeding during the first 4 months of life) Secondary: Women’s perceptions of their health, impressions about the home visit and perceptions of its quality | |
| Notes | Some baseline imbalance, women in the control groups were more likely to have poor home conditions and were less likely to have received antenatal care. Dates of the study: June‐December 2004 Funding sources: American University of Beirut Award (Regional Changing Childbirth Re‐search Program at Faculty of Health Sciences supported by Wellcome Trust). Declarations of interest: not reported
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized into blocks to either of the intervention groups (4 home visits or 1 home visit) or to the control group (no home visits). Randomisation was in blocks of 7 where a caseload of 21 eligible deliveries per day was assumed, based on the average daily number of deliveries in the hospital (ranging from 30 to 35) after excluding non‐eligible cases. |
| Allocation concealment (selection bias) | Low risk | Quote: "Numbered, opaque and sealed envelopes..” Group allocation was carried out by a senior midwife not involved in the rest of the study. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The midwives carrying out the intervention were not blinded. It was not stated whether the participants were blinded, but this is unlikely. |
| Blinding of outcome assessment (detection bias) | High risk | The interviewers carrying out outcome assessment were not informed of groups, but would be aware of which group women were in from the interviews. |
| Incomplete outcome data (attrition bias) | Low risk | 903 women met the inclusion criteria. After randomisation (301 in each arm), 27 women were excluded (18 due to lack of address detail and 9 refusals). A total of 876 women were followed up in the 3 study groups: Intervention 1 (285 women), Intervention 2 (294 women) and Control (297 women). Incomplete data were addressed. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Some baseline imbalance, women in the control groups were more likely to have poor home conditions and were less likely to have received antenatal care. Outcome data were collected at 4 months, but it is likely that there may have been recall bias for some outcomes, e.g. breast engorgement – women in the intervention groups would have discussed this and maybe it was recorded at the time it occurred, women in the control group would not have been asked until 4 months postpartum. Outcome data were collected for a large number of variables, so any differences may have occurred by chance. |
| Study characteristics | ||
| Methods | Cluster‐randomised study with 8 sites, n = 1115 | |
| Participants | 8 village communities located 3 km‐5 km from the main highway in Haryana, India Background rates of breastfeeding initiation: high Inclusion criteria: born in a study village within 9 months of start of intervention Exclusion criteria: not reported Baseline breastfeeding prevalence stated to be high | |
| Interventions | Intervention: health and nutrition workers in the intervention communities received training based on Integrated Management of Childhood Illnesses Training Manual on Breastfeeding Counseling (WHO 1997). Messages ‐ feed only breast milk for first 6 months of life; breastfeed the infant day and night, at least 8 times in 24 h; possible adverse effects of other foods and fluids given to breastfeeding infants ‐ given to mothers at birth, plus monthly home visits, immunisation clinics and neighbourhood meetings. Control: at the control sites, the research team provided routine services, in which, according to national policy, workers are required to advise exclusive breastfeeding for 4‐6 months. | |
| Outcomes | Feeding at 3 months | |
| Notes | Dates of the study: 1st January 1998 to 31st March 2002 Funding sources: Department of Child and Adolescent Health and Development of WHO Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communities were paired on the basis of similar scores for socioeconomic, mortality and morbidity indicators. 1 of each pair was allocated to the intervention using a random number table. 8 areas were randomized (4 to each condition). |
| Allocation concealment (selection bias) | Low risk | Statistician independent of project carried out randomisation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated whether participants and peer counsellors were blinded but unlikely. |
| Blinding of outcome assessment (detection bias) | High risk | Authors state "we attempted to keep to a minimum reporting bias by use of a separate team for assessment of outcomes; this team did not take part in the intervention and was unaware of the hypothesis being tested". However, data was self‐report. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for drop‐out recorded. 1151 births within the study period (not clear how many in each area). 588 families received the intervention and 527 no intervention. 895 completed 3 months follow‐up (80%) and 880 6 months (79%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Areas were paired, but it was not clear whether this achieved similar baseline characteristics between groups. Results were reported to have been adjusted for clustering. |
| Study characteristics | ||
| Methods | 2‐arm RCT, n = 382 | |
| Participants | From 2 prenatal care centres in the Bronx, New York (reported to be the county in the USA with the highest poverty rate) Background rates of breastfeeding initiation: low Inclusion criteria: able to speak English or Spanish, singleton or twin pregnancy < 24 weeks (twins subsequently excluded), intending to keep infant and attend for prenatal and postnatal care at centre and affiliated hospital, telephone contact numbers available Exclusion criteria: HIV‐positive status, chronic disease with medication not compatible with breastfeeding, diabetes, serious illness, or breast reduction surgery Participant characteristics: 57% Hispanic, 36% African‐American, 62% multiparous (70% of these had previous breastfeeding experience), mean age 25 years (SD 6.23), 51.5% married or living with a partner, 57% receiving Medicaid | |
| Interventions | Intervention (n = 188): delivered by a trained LC. Women were recruited when < 24 weeks pregnant, and had 2 prenatal LC visits scheduled. During late pregnancy there was telephone contact, and hospital and home visits and telephone support (up to 12 months postpartum) were planned for the postnatal period. In the postnatal period 25% of the intervention group received at least 1 hospital contact; approximately 50% had telephone and/or home visits; but 36% received no home or hospital visits and no telephone support. Control (n = 194): women had no contact with the LC. Standard care varied between the sites and neither site followed an established protocol for breastfeeding. Women enroled in women and child nutrition programmes (WIC) had the opportunity to visit a breastfeeding co‐ordinator. | |
| Outcomes | Infant health outcomes: Duration of breastfeeding and exclusive breastfeeding was presented mostly in graphical form and was difficult to interpret. Breastfeeding was categorised on a 7‐point scale from 7 = exclusive breastfeeding (which was defined as no other milk or food, but infants may have received water and other liquids) through to exclusive formula, between these extremes of the scale there were various 'intensities' of breastfeeding (e.g. > 50% breast milk). This meant that results were complicated and not easy to interpret. Women were followed up for up to 12 months and detailed (graphical) weekly data were reported for weeks 1‐26 postpartum. | |
| Notes | Results estimated from graphs. Dates of the study: Recruitment August 2000 ‐ November 2002 Funding sources: supported by grants from the United States Department of Agriculture, the Maternal and Child Health Bureau, and the Agency for Healthcare Quality and Research. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The project’s biostatistical office generated and maintained a list of random codes for subjects... undisclosed blocking factor and stratification according to center.” |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, numbered and opened sequentially. |
| Blinding of participants and personnel (performance bias) | Unclear risk | It was not stated whether the women were blinded. The LC providing the intervention was not blinded with respect to treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | The research assistant collecting breastfeeding outcome data was not blinded with respect to treatment group. |
| Incomplete outcome data (attrition bias) | Unclear risk | Women were recruited in the antenatal period. 382 women were randomized. Loss to follow‐up included 10 women who miscarried or terminated the pregnancy. 304 women were followed up into the postnatal period (80% of those randomized). There were further missing data for longer term follow‐up. Loss to follow‐up was balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | The intervention did not appear to be standardised and many women in the intervention group (36%) did not receive any postnatal visits. |
| Study characteristics | ||
| Methods | Parallel 2‐arm participant‐level RCT, n = 666 | |
| Participants | Women who attended an urban medical centre providing prenatal care to a low income population in the Bronx, New York City. Background rates of breastfeeding initiation:79% Inclusion criteria: English‐ or Spanish‐speaking women aged ≥18 years, in the first or second trimester of a singleton pregnancy Exclusion criteria: risk factors for premature birth maternal or infant conditions that would preclude or complicate breastfeeding (e.g. mother HIV‐positive, infant congenital anomaly) | |
| Interventions | 666 women were randomised in a 1:3:3:1 ratio to: usual care, electronic prompt (EP) alone, Lactation Consultant (LC) + EP, or LC alone. Only the LC and EP+LC arms are included in this review as the EP arm (n = 253) was antenatal only and therefore does meet the review inclusion criteria for a breastfeeding support intervention. LC intervention (n= 80): Two LCs were allocated to this intervention.The LC protocol included 2 prenatal sessions, a hospital visit, and regular phone calls postpartum for 3 months or until breastfeeding ceased. The prenatal sessions occurred in the examination room, during the 30‐plus min of 'downtime' while waiting for the prenatal care provider. Attempts were made to complete interrupted sessions after the examination. The first session focused on rapport building and education, and the second was on the practical aspects of breastfeeding. The study provided nursing bras and breast pumps to LC group participants as needed. LCs met mothers and their infants at the 1‐week routine paediatric visit, modelling practice on a recent review. Postpartum home visits were optional, based upon participant and LC preference and comfort. LC + EP intervention (n=253): Included the LC protocol detailed above and electronic prompts for healthcare providers to ask three brief open‐ended questions which portrayed breastfeeding as the norm. This was done during pre‐natal care appointments. Control (n=80): usual care | |
| Outcomes | For BINGO, the prespecified primary outcome measure was 3‐month breastfeeding intensity. Quote: "We categorised breastfeeding intensity as < 20% (low), 20% to 80% (medium), and greater than 80% (high) of all feeds from breast milk consistent with previous studies and Infant Feeding Practices Survey II analyses." Other analysis was planned. Power calculations were affected by the finding ‐quote: “we found that breastfeeding intensity was not normally distributed, and most women stopped breastfeeding altogether during follow‐up". Quote: “Study staff assessed infant feeding at 1, 3, and 6 months postpartum during phone interviews using items adapted from the Infant Feeding Practices Survey II quote:”; exclusive breastfeeding, breastfeeding intensity (“defined as the percentage of all feedings in the past 7 days that were breast milk”), breastfeeding initiation, and total duration data collected. | |
| Notes | The paper reported 2 trials that appear in this review (PAIRINGS and BINGO). Dates of the study: 2008‐2011 Funding sources: supported by the National Institute of Child Health and Human Development, and by the National Institute on Minority Health and Health Disparities. Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women were randomized using sequentially numbered opaque sealed envelopes, generated by the study’s biostatistician". Quote: "Randomization incorporated an undisclosed blocking factor and nativity status (US‐born vs foreign‐born)." |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "It was infeasible to blind participants and clinical staff to treatment group." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "we sought to minimize bias by restricting access to allocation assignment, stripping group assignment from study databases to which research staff had access, and omitting group identifiers from participant interview form." |
| Incomplete outcome data (attrition bias) | Low risk | The BINGO analytic sample included 94% of those randomized (628 of 666 participants). |
| Selective reporting (reporting bias) | Low risk | We checked the Clinicaltrials.gov record and the key breastfeeding outcome data seemed to be reported in this paper. |
| Other bias | Low risk | No baseline imbalance No industry funding No conflicts of interest |
| Study characteristics | ||
| Methods | Parallel 2‐arm, participant‐level RCT, (n = 275) | |
| Participants | Women who attended an urban medical centre providing prenatal care for an economically diverse population in the Bronx, New York City. Background rates of breastfeeding initiation:79% Inclusion criteria: English‐ or Spanish‐speaking women aged ≥18 years, in the first or second trimester of a singleton pregnancy Exclusion criteria: risk factors for premature birth maternal or infant conditions that would preclude or complicate breastfeeding (e.g. mother HIV‐positive, infant congenital anomaly) | |
| Interventions | Intervention (n = 136): lactation counselling and electronic pumps. One LC was allocated to this intervention.The LC protocol included 2 prenatal sessions, a hospital visit, and regular phone calls postpartum for 3 months or until breastfeeding ceased. The prenatal sessions occurred in the examination room, during the 30‐plus min of 'downtime' while waiting for the prenatal care provider. Attempts were made to complete interrupted sessions after the examination. The first session focused on rapport building and education, and the second was on the practical aspects of breastfeeding. The study provided nursing bras and breast pumps to LC group participants as needed. LCs met mothers and their infants at the 1‐week routine paediatric visit, modelling practice on a recent review. Postpartum home visits were optional, based upon participant and LC preference and comfort. Electronic prompts for healthcare providers to ask three brief open‐ended questions which portrayed breastfeeding as the norm. This was done during pre‐natal care appointments. Control (n = 139): usual practice. | |
| Outcomes | For PAIRINGS, the prespecified primary outcome was exclusive breastfeeding at 3 months. Other outcomes: Quote: “Study staff assessed infant feeding at 1, 3, and 6 months postpartum during phone interviews using items adapted from the Infant Feeding Practices Survey II”; exclusive breastfeeding, breastfeeding intensity (“defined as the percentage of all feedings in the past 7 days that were breast milk”), breastfeeding initiation, and total duration data collected. | |
| Notes | The paper reported 2 trials that appear in this review (PAIRINGS and BINGO). Dates of the study: 2008‐2011 Funding sources: supported by the National Institute of Child Health and Human Development, and by the National Institute on Minority Health and Health Disparities. Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using sequentially numbered opaque sealed envelopes, generated by the study’s biostatistician. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "It was infeasible to blind participants and clinical staff to treatment group." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "we sought to minimize bias by restricting access to allocation assignment, stripping group assignment from study databases to which research staff had access, and omitting group identifiers from participant interview form." |
| Incomplete outcome data (attrition bias) | Low risk | Analytic sample included 95% of those randomized (262 of 275 participants. |
| Selective reporting (reporting bias) | Unclear risk | Breastfeeding outcomes reported in Clinicaltrials.gov were reported in this paper. Other outcomes on weight and length were not reported in this paper, but they are not included as outcomes in this systematic review. |
| Other bias | Low risk | No baseline imbalance No industry funding No conflicts of interest |
| Study characteristics | ||
| Methods | 2‐arm RCT with individual randomisation, single‐site, duration not stated, n = 115 | |
| Participants | Urban USA ‐ ambulatory care centre and in‐patient maternity unit Background rates of breastfeeding initiation: low Baseline prevalence of breastfeeding at birth in national WIC sample = 33% (1991) Inclusion criteria: English‐speaking; nulliparous Exclusion criteria: separated from child at birth; preterm delivery; child in NICU > 72 h Ethnic composition: described as 71% white 90% of participants were eligible for WIC programmes for those on low income. Study population not limited to those intending to breastfeed. | |
| Interventions | Intervention: package of: 2‐4 prenatal sessions with LC (10 min‐15 min each); telephone call 48 h after discharge; visit to lactation clinic at 1 week postpartum (staffed by paediatrician or LC); contact with LC at each health supervision visit until weaning or 1 year; professional education of nursing and medical staff. Control: women were offered optional prenatal breastfeeding classes, postpartum breastfeeding instruction by nurses and physicians and outpatient follow‐up by nurses and physicians in the paediatric ambulatory department. | |
| Outcomes | Rates of breastfeeding at 2 months and median duration of breastfeeding | |
| Notes | Dates of the study: not reported Funding sources: The Mercy Foundation and by the Care of the Poor Fund, The Mercy Hospital of Pittsburgh Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sample stratified by age with block randomisation in blocks of 8. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Women and LC were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome data were collected by questionnaire administered by the LC who was not blinded to group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Follow‐up 94%. It appeared that 115 women were randomized. It was stated that 7 in the intervention group were excluded as they did not receive the intervention. 8 women in the control group were subsequently excluded from the analysis for at least some outcomes as the treatment they received deviated from protocol. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Potential confounders: women were excluded from intervention group following randomisation if they had received fewer than 2 prenatal lactation consultations; ITT analysis not performed (8 women in control group who met LC excluded); intervention included input by staff caring for both intervention and control groups. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, with add‐on qualitative study, n = 339 | |
| Participants | Denver, USA; a clinic providing care for a predominantly Hispanic, medically underserved population Background rates of breastfeeding initiation: low Inclusion criteria: women ≥ 18 years, primiparous with healthy, term, singleton baby who were willing to consider breastfeeding Exclusion criteria: primary language not English or Spanish, medical complication that interfered with breastfeeding, hospital stay > 72 h following vaginal births or > 96 h following caesarean section, baby with medical problems, admitted to NICU or had a hospital stay > 72 h Participant characteristics: Mean age 22 years; 88% Hispanic or Latino; 77% vaginal delivery Planned to breastfeed only: intervention group 50%, control group 55% (other women planned to combine breastfeeding with formula) > 60% were participating in WIC programmes at 1 month and 74% of these women were provided with formula at WIC clinics. | |
| Interventions | Intervention: daily telephone support, from the day following hospital discharge until 2 weeks postpartum, from trained nurses following a specific protocol covering advantages and disadvantages of breastfeeding, cultural issues, technique, problems and with referral for any lactation or medical problems. Control: usual hospital care (pamphlets on breastfeeding, a breast pump, lanolin cream and a water bottle); usual discharge care (commercial discharge packs) and scheduled healthcare visits at 3‐5 days and at 2 weeks at the local community health centre. | |
| Outcomes | Any breastfeeding or predominantly breastfeeding Maternal satisfaction Healthcare utilisation Reasons for stopping breastfeeding | |
| Notes | Dates of the study: recruitment February 2005 ‐ May 2006 Funding sources: funded by the Center for Disease Control and Prevention, the Children’s Outcomes Research Program, and the Colorado Department of Public Health and Environment. Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block random allocation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | No blinding for participants or caregivers. |
| Blinding of outcome assessment (detection bias) | High risk | Blinding not described for outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | 341 women were randomized. At 1 month there was approximately 8% loss to follow‐up, By 6 months 27% loss. 73% were described as included in the analyses; women in the intervention group that did not receive the intervention as planned were not included. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 251 | |
| Participants | University hospital in the Northeast of Brazil. Background breastfeeding rates: EBF 36.6% at 6 months between 2006 and 2013. Inclusion criteria: mothers were over 18 years of age, knew how to read and write, used the online social network Facebook, and were discharged from the hospital together with their child. Exclusion criteria: mothers who distanced themselves from their child for any reason throughout the study; who were HIV seropositive or had any other diseases that contraindicated breastfeeding; and who gave premature birth with infants with low birth weight, twins, or with congenital problems that prevented or hindered suckling at the breast. | |
| Interventions | Women in the intervention group, in addition to receiving the booklet, joined a closed Facebook group, controlled by moderators of the research team. Each woman received a virtual invitation to allow her inclusion in that group. ‐ [Phone calls were made to each mother who had not accepted the invitation to this group 1 week after hospital discharge]. Women were tagged in a post of the group, corresponding to a topic of the booklet, once each week, for 6 months. Messages were monitored by the project team. Each view by the women was monitored, and when this did not happen in up to 5 days, a private message was sent by the same online social network inviting the woman to view that particular post. Questions and demands of the mothers were answered virtually by the research team as soon as possible and supervised by the coordinators of the project. Control: during their stay at the hospital, mothers received routine guidance from the care team about breastfeeding and the general care for the newborn, in addition to being assisted in accordance with their needs, as is usually done in public health services in Brazil. The educational part of the study was performed with the aid of a booklet with information on breastfeeding developed by the research team and based on the official recommendations of the Brazilian Ministry of Health and the World Health Organization. The messages were illustrated, direct and easy to read and understand. All mothers received the same booklet after birth while they were still in the maternity ward. | |
| Outcomes | Exclusive breastfeeding at 1,2,3 and 6 months | |
| Notes | Dates of the study: August 2016 ‐ August 2017 Funding sources: Not reported. Declarations of interest: None declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | a list of random, binary numbers drawn up before the beginning of the study, using the function “random between 0 and 1” of the Microsoft Excel software. |
| Allocation concealment (selection bias) | Low risk | The academics who performed the recruitment did not participate in the assignment process of the women into groups, and the nutritionist who performed the assignment had no knowledge of or contact with the mothers during the recruitment stage. However no concealment strategies are described. |
| Blinding of participants and personnel (performance bias) | High risk | The participant mothers were not aware that there were two groups in the study and also did not know to which group they had been assigned. However, the HPs who were providing the intervention would not have been blinded. |
| Blinding of outcome assessment (detection bias) | High risk | It is unclear whether the personnel doing the phone interviews would know which group the women were allocated too but the women were self‐reporting their breastfeeding status which gives this a high risk of bia |
| Incomplete outcome data (attrition bias) | Low risk | intervention group 116/123 = 6% drop‐off Control Group 127/128 = 1% drop‐off. |
| Selective reporting (reporting bias) | Low risk | universal registry of clinical trials (UTN: U1111‐1187‐6136) in the International Clinical Trials Registry Platform—ICTRP Outcomes in protocol all reported including primary outcome. |
| Other bias | Low risk | The authors declare that they have no conflicts of interest. No funding source described. The number of prenatal consultations was the only variable with significant statistical difference between the groups |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation; recruitment at an urban USA hospital with BFI accreditation, n = 219 | |
| Participants | Urban USA hospital prenatal clinic serving a low‐income, predominantly Latina population Background rates of breastfeeding initiation: low Antenatal inclusion criteria: low‐income women ≥ 18 years old, at ≤ 26 weeks' gestation, considering breastfeeding, not yet enroled in peer counselling programme, resident in hospital area, available for telephone follow‐up Postnatal inclusion criteria: healthy, full‐term singleton infants, no congenital abnormalities, no maternal history of HIV and no admission to NICU Exclusion criteria: none specified After birth, n = 165 women remained in the study, 90 in the intervention group and 75 controls. Participant characteristics: ethnic composition 80% Hispanic (61% Puerto Rican origin), 9% African American, 3% white, 8% other | |
| Interventions | Intervention: 1 prenatal home visit, daily visits during postpartum hospitalisation, home visit within 24 h and at least 2 more home visits as requested, and telephone/pager contact. Intervention from peer counsellors with 30 h classroom training that covered La Leche League International Peer Counseling Program and Hispanic Health Council's curricula. Peer counsellors had to score 85% in a written exam and work for 3‐6 months with experienced peer counsellors to demonstrate competence before working independently with clients. Peer counsellors had 1 h per month continuing education and were paid for their work. Control: routine breastfeeding education offered by the study hospital, and the same breastfeeding services as women paying privately. A small amount of exposure to peer counsellors among the control group was reported. | |
| Outcomes | Breastfeeding rates at birth and 1, 3 and 6 months postpartum | |
| Notes | Dates of the study: Recruitment 27/07/2000 ‐ 08/08/2002 Funding sources: Funding for this study was received by Dr Pe´rez‐Escamilla from the Centers for Disease Control and Prevention, through a subcontract with the Association of Teachers of Preventive Medicine; Connecticut Family Nutrition Program for Infants, Toddlers, and Children; and the Hartford Hospital Research Foundation. Funding for the program is provided by the University of Connecticut Family Nutrition Program, through a grant from the US Department of Agriculture Food Stamp Family Nutrition Program, and by Hartford Hospital. Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer programme |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No detail provided about whether participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | It was not stated whether outcome assessors were blinded, but to minimise bias, data related to peer counsellor contact were collected at the end of each interview. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up appeared reasonably balanced although there was more loss from the control group. Reasons for loss to follow‐up stated. 219 were randomized, 72% followed up at 1 month, 70% at 3 months and 66% at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups appeared similar at baseline. It was reported that many women in the intervention group received less than half of the planned visits. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 103 | |
| Participants | Setting: two distinct geographical areas in England. Background breastfeeding rates: UK as a whole = 81% initiation and EBF at 6 weeks = 23% Inclusion criteria: women aged ≥16 years who were pregnant with their first child, irrespective of feeding intention. Exclusion criteria: women were excluded if they had had a previous live birth or were aged<16 years. | |
| Interventions | The intervention offered proactive, woman‐centred support using an assets‐based approach and incorporating behaviour change techniques. Based on COM‐B model. IFHs met women at 30‐32 weeks and explored 'Assests for Feeding' which led to the production of a genogram of support. Women were encouraged to discuss feeding with this support network. Women then received monthly calls and to inform the IFH that she had given birth. Postnatally, daily telephone/text message contact was provided for the first two weeks, decreasing in frequency from two to eight weeks, and monthly text messages were sent at 3, 4 and 5 months. During 2‐8 weeks women could request help. Control: routine support from midwives and health visitors. The support for infant feeding that was available and accessed by women, which included local services, such as breastfeeding support groups and peer support, and national breastfeeding helplines. | |
| Outcomes | Any breastfeeding at 2 and 6 months Maternal wellbeing (WEMWBS) | |
| Notes | This study was retrospectively registered. This was queried with study authors and a satisfactory explanation was given, and we were reassured that data collection did not commence prior to registration. Dates of the study: Feb‐Aug 2017 Funding sources: National Institute for Health Research (NIHR) Public Health Research programme Declarations of interest: other sources of funding for research studies (including other breastfeeding studies) declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In site A, a randomisation list was developed by the clinical trials unit, minimised by age group (< 25 years and ≥ 25 years). In Site B the clinical trials unit devised a database to randomize (simultaneously) blocks of women from each sub‐locality, following recruitment. This was performed by an independent researcher. |
| Allocation concealment (selection bias) | Low risk | Randomization performed by a central service. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind women or peers providing intervention. |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data |
| Incomplete outcome data (attrition bias) | Low risk | Overrall follow‐up at 6 months ws 80.5% At 6 months Intervention loss to follow‐up = 22% Control loss to follow‐up = 17% At 2 months Loss to follow‐up Intervention = 18% Loss to follow‐up control = 11% Primary analysis was by modified intention to treat, which included all randomly assigned patients with available data on the primary endpoint
|
| Selective reporting (reporting bias) | Low risk | Outcomes detailed in protocol are reported in the paper.
|
| Other bias | Unclear risk | No industry funding. No conflicts of interest. Baseline imbalances "A visual inspection of the baseline participant demographic and delivery characteristics (see Table 7) revealed some imbalances. Those in the intervention group were more likely to be unemployed, less likely to be educated to degree level and more likely to be single. In addition, there were more premature deliveries and more admissions to the neonatal unit in the intervention group than in the usual care group" |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 49 | |
| Participants | Setting: six low‐income neighbourhoods in Hartford, CT, USA. Background breastfeeding rates: not reported. Inclusion criteria: women who were pregnant or had just delivered a baby, enroled in NFN, English or Spanish‐speaking, singleton birth greater than 34 weeks gestation, any maternal race/ethnicity, no chronic conditions that could affect growth or development of the infant and residence at time of the infant’s birth in a BFF Centre neighbourhood. Exclusion criteria: infants with major malformations, admission to the neonatal intensive care unit or a prolonged hospital stay or infants who were small for gestational age and required special or supplemental nutrition. | |
| Interventions | NFN+ mothers received (i) education and enhanced support regarding breastfeeding; (ii) creation of a Family Wellness Plan that taught mothers goal setting and self‐monitoring skills; (iii) education and skill‐building in behavioural strategies (e.g., problem‐solving and stimulus control) to implement desired changes in infant target areas; (iv) a toolkit with items to support the changes (e.g., 6 oz sippy cup, play mat); and (v) linkages to community programs that support healthy behaviour change including coupons for fruits and vegetables, cooking classes, exercise classes and breastfeeding support resources. Eight teaching modules were developed. Each module consisted of nine elements including an introduction, education and the dispelling of myths, promoting self‐efficacy, goal‐setting, problem‐solving, self‐monitoring, a self monitoring calendar, linkages to community resources and assessment of progress. Module delivery and each completed element were documented by the home visitors. NFN+ mothers also received a weekly $3 coupon to purchase fruits and vegetables and one bonus gift (e.g., a set of pots and pans, bus tokens, running shoes) worth approximately $30. All intervention materials were in English and Spanish and were field‐tested for cultural relevance. Control: standard NFN curriculum using the PAT curricula with weekly 60‐min visits for 2 months and then biweekly home visits by NFN home visitors. The standard NFN curriculum pocuses on the link between child development and parenting and on key developmental topics (i.e., attachment, discipline, health, nutrition, safety, sleep, transitions/routines, healthy births) as well as family well‐being with a focus on family strengths, capabilities, skills and the building of protective factors. | |
| Outcomes | Any breastfeeding at 6 and 12 months | |
| Notes | Dates of the study: June 2013 ‐ January 2016. Funding sources: National Institutes of Health (NICHD R21 HD073966‐A01). Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state that the BFF centers were randomly assigned, but no further information on the sequence generation process provided. |
| Allocation concealment (selection bias) | Unclear risk | There is no information about allocation concealment in either the paper or the protocol. |
| Blinding of participants and personnel (performance bias) | High risk | Open label study, as noted in trial registration at clinicaltrials.gov |
| Blinding of outcome assessment (detection bias) | High risk | Open label study, as noted in trial registration at clinicaltrials.gov therefore not possible as data self‐report |
| Incomplete outcome data (attrition bias) | High risk | Missing data due to loss at follow up high with ≥20% attrition at 6 month and 12 month. An imbalance in numbers reported across groups with greater attrition in the control group at 6 months ( 37% vs 20%) and at 12 months (56% vs 27%). |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be measured as described in protocol. |
| Other bias | High risk | Study is funded by NIH. No conflict of interest was declared. No issues due to clustering reported; however ICC not reported. Baseline imbalance reported with greater number of primigravida women in the control group compared to the intervention group (95% vs 31%, p<0.05) |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation n = 350 | |
| Participants | The study was carried out in 2 hospitals serving urban areas and neighbouring small towns in the interior of the State of Pernambuco, north‐eastern Brazil. Background rates of breastfeeding initiation: high Inclusion criteria: singleton infants Exclusion criteria: infants with congenital anomalies or serious illness necessitating intensive care and those whose mothers had serious disease or mental illness or were planning to leave the area within 6 months Approximately 60% had an income lower than the minimum wage; 33% did not have access to a flush toilet, approximately 35% of the mothers were < 20 years, 39% primiparous, approximately 28% had a caesarean delivery | |
| Interventions | 90% of maternity staff in both hospitals received the 18‐h UNICEF/WHO Baby Friendly Hospital Initiative training course. All participants in the intervention and control groups received their hospital postnatal care from these Baby‐Friendly trained staff. Intervention: women (n = 175) received 10 postnatal home visits (mean duration 30 min); 4 during the first month, 2 during the second month and 1 per month during the third to sixth months. Each mother was given an illustrated booklet. At each visit the home visitors observed the positioning of the infant at the breast, flow of milk and the baby’s satisfaction; encouraged exclusive breastfeeding for 6 months and continued breastfeeding for at least 2 years, and used the booklet as a basis for discussions of key topics relevant to the infant’s age. If there were any difficulties home visitors could not resolve he/she referred the mother for more specialist help at the hospital. If other family members were present, their attitude towards exclusive breastfeeding was assessed and their support was sought, including help with household chores. Control: (n= 175) usual care with no postnatal home visits | |
| Outcomes | Primary outcome: exclusive breastfeeding. Data collected prospectively at 1, 10, 30, 60, 90, 120,150 and 180 days after birth. Any breastfeeding at same time points. The type of other fluids introduced were also recorded at each time point. | |
| Notes | Dates of study: March‐August 2001 Funding sources: the study was funded by the Superintendência para o Desenvolvimento do Nordeste (SUDENE), Brasil (Agency for the Development of the Northeast, Brazil). Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks of 10 per town by use of a random numbers table. The random numbers were generated by the project manager, and enrolment and group assignment were made by 2 maternity‐based research assistants. |
| Allocation concealment (selection bias) | Unclear risk | Concealment was achieved by drawing numbers from envelopes at the time of assignment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Mothers in the trial were not close neighbours, so discussion with other mothers is unlikely, but we did not formally assess whether masking was maintained". It was not stated whether the personnel delivering the intervention were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Data were collected in the trial by 4 researchers who were not aware of group allocation and were unconnected with the delivery of the interventions, however, authors did not formally assess whether masking was maintained. |
| Incomplete outcome data (attrition bias) | Low risk | 350 women were randomized, 175 in each group. 20 women (6%) were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | The random numbers were generated by the project manager and so this may lead to bias. Results were presented in graphs and aggregated results were not simple to interpret. |
| Study characteristics | ||
| Methods | Cluster RCT, 47 communes randomised. | |
| Participants | Setting: Burkina Faso is a low‐income setting with high infant mortality: an early neonatal mortality of 20 deaths per 1000 livebirths, a late neonatal mortality of nine deaths per 1000 livebirths, and a postneonatal mortality of 32 deaths per 1000 livebirths. Background breastfeeding rates: Burkina Faso had a national prevalence of exclusive breastfeeding of 25%, as reported in the 2010 Demographic and HealthSurvey, while a survey by the Ministry of Health found a prevalence of exclusive breastfeeding of 43% in 2016. Inclusion criteria: residing in a village selected for the study (had been members of households identified during household listing for at least 6 months or had the intention to stay there), had a livebirth in the 12 months preceding either of the two surveys and the infant was still alive and living with them, and gave informed consent to participate. The protocol also states inclusion criteria of being aged from 15 to 49 years old (women of reproductive age). Exclusion criteria: not clearly described. | |
| Interventions | The Alive & Thrive initiative with randomisation of two components of the programme: (i) interpersonal communication activities, and (ii) mass communication activities. Interpersonal communication activities aimed to increase mothers’ knowledge about optimal breastfeeding practices and their benefits, to increase mothers’ expertise in breastfeeding, and to improve mothers’ perceptions about social norms relating to breastfeeding. These activities were delivered by existing structures within the public health system. Interpersonal communication activities were delivered by two cadres: government health workers during individual consultations for antenatal, delivery, and postnatal care and during women’s group discussions held at the local health centre; and community health volunteers during home visits that targeted pregnant and postnatal women who, during a visit to a health centre, expressed concerns or mentioned experiencing difficulties with breastfeeding. Community mobilisation activities were conducted to raise awareness of the benefits of breastfeeding primarily among partners, mothers‐in‐law, and grandmothers and to increase the support that they and the community provide to breastfeeding mothers. Community mobilisation activities consisted of community events and facilitated group discussions in public places in the villages to promote recommended breastfeeding practices. Some community health volunteers also received requests to support wives or daughters‐in‐law during their interactions with fathers and grandmothers at these events. The main messages of the Alive & Thrive intervention were: to place the baby to the breast within the first hour of birth, to give colostrum, to not give water, tisanes, or other liquids, and to breastfeed exclusively for 6 months. Control: home visits by community health volunteers to support health promotion in the community. Government health facilities and community health volunteers were also present in the control group, as part of the standard public health system, but did not benefit from any additional training, supervision, or community mobilisation activities of Alive & Thrive. | |
| Outcomes | Exclusive breastfeeding among infants younger than 6 months | |
| Notes | Dates of the study: training May 2016 to June 2017. Community mobilisation activities took place from December, 2015 to July 2017. Funding sources: Alive & Thrive is funded by the Bill & Melinda Gates Foundation and the governments of Canada and Ireland, and managed by FHI 360. This research was supported by funding from the Bill & Melinda Gates Foundation (grant number OPP50838). Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence generation process used a random component of computer generated pseudo‐random numbers through Stata software. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, it was not feasible to blind participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | It was not feasible to blind outcome assessors due to self‐report of outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Four of 41 clusters were removed following randomisation, but prior to baseline data collection. The baseline and endline surveys were conducted with two independent, population‐representative samples within clusters; hence, no attrition due to the cross‐sectional nature of each survey |
| Selective reporting (reporting bias) | Low risk | Outcomes appear as in clinicaltrials.gov trial registration record |
| Other bias | Unclear risk | Clusters were accounted for in the analysis. No industry funding. No competing interests declared. The study design, using two independent, population representative samples of individual participants at baseline and endline may be a source of bias, but this is unclear. No imbalance was observed for the sample of participating women completing the baseline survey, but this was unclear for the sample of participating women completing the endline survey. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 1144 | |
| Participants | Setting: five largest primary health centres in one of three health districts in the city of Bobo‐Dioulasso – each health centre served a predominantly urban population of around 20,000. Background breastfeeding rates: In Burkina Faso, fewer than half of infants are exclusively breastfed 3 months postpartum. Inclusion criteria: eligible women were aged between 15 and 45 years, cohabiting with a man (regardless of marital status), pregnant with an estimated gestational age of 20 to 36 weeks and, based on their obstetric risk profile, expected to be able to give birth in a primary health centre. Exclusion criteria: women who were recommended at the time of recruitment to give birth in a referral hospital. | |
| Interventions | The intervention consisted of three components: (i) an interactive group discussion session for male partners only; (ii) an individual couple counselling session during pregnancy; and (iii) a postnatal couple counselling session before discharge from the facility. Each session lasted approximately 1 hour. Control: routine care‐In this setting, almost all women attended antenatal care at least once. Most do not have regular check‐ups postpartum. Even in urban areas, fewer than half attend the recommended two outpatient postnatal consultations. | |
| Outcomes | Exclusive breastfeeding at 3 months | |
| Notes | Dates of the study: recruiting ran from 16 February until 12 June 2015. Follow‐up ended on 4 July 2016. Funding sources: the study was funded by the Strengthening Evidence for Programming on Unintended Pregnancy (STEP UP) Research Program Consortium (grant code EPIDHC20). STEP UP is funded by UK aid from the Department for International Development. Marina Daniele’s studies at the London School of Hygiene & Tropical Medicine were funded by the Economic and Social Research Council. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We assigned participants to the intervention or control arm of the study on a 1:1 basis by simple, nonstratified randomization according to a sequence generated by the principal investigator using the random integer function of a scientific calculator. |
| Allocation concealment (selection bias) | Unclear risk | At randomization, research assistants invited participants to select a sealed opaque envelope. This seems like it might be open to manipulation as they were not sequentially numbered. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes |
| Incomplete outcome data (attrition bias) | Low risk | At 3 months: Data on 560/583 (96.5%) in intervention group and 541/561 (96.4%) in control group. Reasons for loss to follow up give |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported as per protocol NCT02309489 |
| Other bias | Low risk | No industry funding No competing interests declared No baseline imbalance |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, single‐site study, recruiting over 10 months, n = 258 | |
| Participants | Women at home in Toronto, Canada Background rates of breastfeeding initiation: intermediate Inclusion criteria: English‐speaking, primiparous, ≥ 16 years, single full‐term baby, intending to breastfeed, Exclusion criteria: none specified. | |
| Interventions | Intervention: telephone support by briefly‐trained volunteers (2.5 h session) who had personal breastfeeding experience for at least 6 months. First contact within 48 h of hospital discharge and then as required. Mean number of contacts in those completing log‐books = 5.4. Mean duration of telephone contact = 16.2 min. 97% of contacts by telephone, 3% at home. Control: not described | |
| Outcomes | Breastfeeding (any or exclusive) at 1, 2 and 3 months | |
| Notes | Dates of the study: recruitment September 1997 ‐ June 1998 Funding sources: this research was supported by the University of Toronto Faculty of Nursing and Maternal, Infant, and Reproductive Health Research Unit. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated numbers were provided by a statistician who was not involved in the recruitment process. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not explicitly stated if peer counsellors were blinded but as they were recruited for the study, it is unlikely. No detail provided on blinding participants. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “A research assistant blinded to group allocation telephoned the participants to collect data regarding current infant feeding status, breast problems encountered and health services used.” |
| Incomplete outcome data (attrition bias) | Low risk | Very little loss to follow‐up. 258 women randomized and 2 women lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No apparent differences between groups at baseline. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, methods unclear n = 211 | |
| Participants | From maternity ward of the Hospital de Clinicas de Porto Alegre in Brazil, a university general hospital with Baby Friendly accreditation Background rates of breastfeeding initiation high, however median duration of exclusive breastfeeding 29 days Inclusion criteria: mothers living in the city of Porto Alegre, healthy non‐twin newborns with a birthweight ≥ 2500 g Exclusion criteria: mother‐infant pairs that were unable to stay together due to a health concern for either the mother or infant Participant characteristics: ≥ 20 years old 76%, vaginal delivery 72%, white mothers 70%, > 8 years' education 64%, living with partner 83% | |
| Interventions | Intervention (n =74) in hospital, 2 nurses reinforced the orientation about breastfeeding technique routinely given to mothers, following the WHO breastfeeding counselling principles, in a 30‐min session with no more than 2 mother‐infant pairs. Topics included comfortable and proper mother and infant positioning, correct attachment of the child to the breast and manual milk expression. Pictures, dolls and a model breast were used for demonstrating proper breastfeeding technique. Women also received 2 home visits from the same nurse, when the child reached 7 and 30 days of age. Infant feeding patterns, positioning, attachment, milk expression and breastfeeding problems were discussed, and breast examination performed. Control (n = 137): standard hospital care met Baby‐Friendly standards. The control group appears not to have received home visits. | |
| Outcomes | Primary outcome: number of mothers who breastfed and exclusively breastfed on maternity ward and at 30 days Secondary outcome: frequency of breastfeeding‐related problems | |
| Notes | Dates of study: recruitment June‐November 2003 Funding sources: not reported Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Following interviews and feeding assessments, mother‐infant pairs were randomly assigned by pulling coloured balls from a bag indicating either the control or experimental group. After the number of mothers for the experimental group were selected, all women eligible for the study were added to the control group until the sample was complete. |
| Allocation concealment (selection bias) | High risk | By drawing coloured balls from bags – this could be changed and it was not clear that all women in the control group were randomly allocated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No detail provided regarding whether participants or personnel were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The researchers responsible for the breastfeeding evaluations did not participate in the intervention and were blinded to the group to which the mother infant pairs had been assigned.” |
| Incomplete outcome data (attrition bias) | Unclear risk | 233 women were eligible, 211 followed up. (It was not clear how many were randomized.) |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Groups were described as similar at baseline, although it appeared that more women in the control group that had had previous breastfeeding experience were more likely to feed for 6 months (65%) compared to women in the intervention group (47.5%). Unequal numbers in the intervention and control group. The groups were not balanced (74 in the intervention group and 137 controls). It was not clear that all the women in the control group were randomly allocated. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 78 | |
| Participants | Setting: 2 hospitals in Rochester, NY, USA Background rates of breastfeeding initiation: low Inclusion criteria: maternal age < 20 years, uncomplicated postpartum, breastfeeding singleton infant born at gestational age > 36 weeks and weighing > 2000 g, mothers and infants discharged home together Exclusion criteria: maternal contraindications to breastfeeding (HIV, active substance abuse), postpartum transfusion or intensive care; infants in intensive or special care unit > 6 h, infants with anomalies that interfered with breastfeeding (e.g. cleft lip or palate) Participant characteristics: mean age 18.3 years, approximately half were African Americans, approximately one‐third had private or health maintenance organisation insurance, the rest were on Medicaid or with Medicaid health maintenance organisations, > 80% were first time mothers and gave birth vaginally. | |
| Interventions | Intervention: telephone support from trained peer supporters (teen mothers who had breastfed for > 4 weeks). Peer supporters telephoned the new teen mothers at 2, 4, 7 days and 2, 3, 4 and 5 weeks postdischarge. Peers introduced themselves and talked about the breastfeeding experience. No specific discussion topics were assigned. Peers offered their telephone numbers so that new mothers could call for support. They were advised to refer anyone with a problem to telephone resources for breastfeeding information or to their physician. Peers and women received gift voucher incentives to complete assessments and training. Control: usual care included access to paediatric care providers and hospital LCs. The control group did not receive telephone peer support. | |
| Outcomes | Primary outcome: ‘any breastfeeding’ duration, as measured by the age in days at complete breastfeeding cessation Secondary outcome: exclusive breastfeeding duration, as measured by the time to the first introduction of any other supplement (water, juice, vitamins or formula) | |
| Notes | Dates of study: recruitment September 1996 ‐ June 1997 Funding sources: this project was funded by a Special Projects Grant from the Ambulatory Pediatric Association. Salary support for G.D.M. was provided through fellowship training grants by the National Research Service Award and the Health Research Service Award. Declarations of interest: quote: "No competing financial interests exist" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers
|
| Allocation concealment (selection bias) | Low risk | Quote: “The assignment was recorded in a sealed and numbered envelope. Envelopes were sequentially opened.” |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “In order to blind subjects to the study hypothesis, recruiters explained that this study was about: how young mothers who breastfeed in the hospitals feed their babies at home; how young mothers make feeding decisions and who helps them make those decisions.” Not clear if this attempt was successful. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “a single research assistant conducted all the telephone interviews, using standardised, closed ended questionnaires. The interviewer had no knowledge of the study hypothesis or design.” |
| Incomplete outcome data (attrition bias) | High risk | 78 randomized (38 intervention, 40 control) In intervention group: 6 dropouts before first interview; 3 dropouts before 8‐week interview; 7 dropouts between 8 and 37 weeks In control group: 5 dropouts before first interview; 2 dropouts before 8‐week interview; 9 dropouts between 8 and 21 weeks Overall, 11 women dropped out immediately after recruitment (14%). By 8 weeks 21% lost to follow‐up. 46/78 (61%) were successfully followed up to complete breastfeeding cessation (22 intervention and 24 control). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Of the 5 adolescents who completed peer support training, there was only 1 that remained involved for the entire duration of the study. There was very poor compliance with possibly only half of the intervention group receiving the planned intervention. The analysis is presented in diagrams that are not simple to interpret. Study results published in 2010, data collected 1996‐1997 |
| Study characteristics | ||
| Methods | 2‐arm RCT, single‐site study, mothers recruited March 2000‐December 2001, n = 605 | |
| Participants | Urban Italy Background rates of breastfeeding initiation: intermediate Inclusion criteria: mothers in public maternity ward in Rome, intending to breastfeed Exclusion criteria: mothers who did not speak Italian, had no phone, breastfeeding medically contraindicated, baby in SCBU Ethnic composition not defined. Baseline national breastfeeding initiation rate 70%. | |
| Interventions | Intervention (home visit and telephone contact): home visit, from 1 of the 6 midwives from the maternity ward of the study hospital, took place within 7 days of hospital discharge. Telephone breastfeeding counselling session provided by the same midwife. These midwives had attended the UNICEF 18‐h intensive training course on breastfeeding techniques and management. Control: standard care (not described) | |
| Outcomes | Any breastfeeding up to 60 days | |
| Notes | Extra information about reported numbers requested and received from author. Dates of the study: 08/03/2000 ‐ 08/12/2001 Funding sources: not reported Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sample was stratified "for age and parity, and finally randomly assigned to either the intervention or control group". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details were provided about blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | A trained interviewer conducted the interviews, but was not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 605 women were randomized. Full data were available for 278 women (46%) and partial data available for a further 264 (44%). Follow‐up rates for breastfeeding outcomes collected up to 180 days, but after 60 days follow‐up rates were < 75% so only outcomes up to 60 days are included in this review. Reasons for drop‐out were reported by group. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | Baseline characteristics similar and no apparent differences between those who refused intervention and those who received it, see Table 1. |
| Study characteristics | ||
| Methods | 2‐arm RCT, single site, n = 248 | |
| Participants | A major USA urban university hospital community doula intervention Participant: low‐income, African‐American mothers < 22 years old Background breastfeeding rates: young African‐American mothers continue to breastfeed at low rates, and commonly introduce complementary foods earlier than recommended. In the 2006 National Health and Nutrition Study, for example, only 30% of black adolescent mothers had ever attempted to breastfeed their infants. Background rates of breastfeeding initiation:79% Inclusion criteria: women who were < 34 weeks pregnant, < 21 years of age, and planning to deliver at the affiliated hospital were eligible to participate in the study. Exclusion criteria: mothers who were aware at the time of recruitment that they would require a surgical delivery, who planned to move from the area, or who planned to give up custody of the infant | |
| Interventions | Intervention (n = 124): Women received additional care from doulas who were women from the same communities as the women attending the clinic. During pregnancy women received weekly home visits (average = 10) where the doula focused on building a relationship with the mother and discussed pregnancy health, childbirth preparation, and bonding with the unborn infant.Doulas were present during labour to provide support and help initiate breastfeeding after birth.Doulas continued to provide face‐to‐face breastfeeding support in the post‐natal period (average 12 home visits). Doulas undertook a 20‐week doula training course provided by the Chicago Health Connection (Health Connect One) and a 10‐week breastfeeding peer counsellor training programme from the same organisation. Control (n=124): mothers received usual prenatal care; no doula input | |
| Outcomes | Primary outcomes: data on breastfeeding attempts were collected by mother report at the hospital the second morning after the birth and from review of the nursing notes in the mothers medical chart after the mothers discharge. Mothers were considered to have attempted breastfeeding if breastfeeding was indicated by either self report or nursing notes. At 4 months postpartum, the mothers participated in an interview on feeding practices. Mothers reported on whether they were currently breastfeeding and, if not, when they had stopped breastfeeding. Secondary: mothers were also asked about whether they had started feeding their infants cereal, either in the bottle or by spoon, or other solid foods, and reported the infant age. | |
| Notes | Dates of the study: recruitment Jannuary 2001 ‐ April 2004 Funding sources: Maternal and Child Health Bureau Research Program, HRSA, DHHS, grant R40 MC 00203. The intervention implementation was funded by grants from the Irving B. Harris Foundation, the Blowitz‐Ridgeway Foundation, the Prince Charitable Trusts, the Visiting Nurses Association Foundation, and the Michael Reese Health Trust. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization took place in blocks of 4, 6, or 8, with equal numbers assigned to the intervention and control groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "A biostatistician prepared a set of opaque envelopes, each labelled with a subject ID number and containing a group assignment. Envelopes were opened by the interviewer in the presence of the mother at the completion of the baseline interview." |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants. |
| Blinding of outcome assessment (detection bias) | High risk | Data were collected by research staff through interviews with mothers and by chart review. Not stated whether research staff were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | A total of 221 mothers,113 in the control group and 108 in the doula participated in the 4‐month interview. Attrition < 25% for both groups. |
| Selective reporting (reporting bias) | High risk | Breastfeeding at 12 months was reported as a secondary outcome in the Clinicaltrials.gov record, but this was not reported in the paper. |
| Other bias | Low risk | Mothers in the 2 groups were compared on a variety of demographic, psychological, and health variables measured before randomisation and no significant differences were found. |
| Study characteristics | ||
| Methods | Longitudinal study, 2‐arm cluster‐randomised trial, 10 Swedish municipalities randomised n = 540 | |
| Participants | Setting: Antenatal Centres and Child Health Centres in 10 municipalities in southwest Sweden Background rates of breastfeeding initiation: high Inclusion criteria: Swedish‐speaking mothers who gave birth to singleton, healthy, term infants spontaneously, by vacuum extraction or by caesarean section Participant characteristics: mean age approximately 27 years, married 61%‐69%, vaginal delivery 70%‐75%, university educated 36% | |
| Interventions | Intervention: the intervention included continuity of care at the antenatal and child centres, and a process‐oriented training program of 7 sessions for health professionals. The staff training included reflection on personal experience of breastfeeding and breastfeeding counselling, management and promotion. Staff were encouraged to develop a common breastfeeding policy between the antenatal and child health centres. The family classes were also kept together before and after childbirth. Control: offered standard family classes, usually discontinued at birth | |
| Outcomes | Maternal perceptions of the relationship with the infant, maternal feelings for the infant and duration of exclusive/any breastfeeding | |
| Notes | 10 centres randomised. A total of 540 women took part in the study (intervention group 206 women; 2 control groups 162 + 172 = 334 women). Data collection took place at different times for the 2 control groups. We have included data from 378 women; the intervention group (206 women) and 1 control group (172 women), from whom data were collected at the same time as from the intervention group. Dates of study: study conducted between April 2000 and January 2003 Funding sources: Board of Research for Health and Caring Sciences, Swedish Research Council Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The 10 largest municipalities were classified in pairs that were similar in size and had similar figures of breastfeeding duration. The municipalities were randomized pair‐wise to either an intervention or control group. |
| Allocation concealment (selection bias) | Unclear risk | Trial report did not report. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No detail regarding blinding of participants or personnel, but appears unlikely. |
| Blinding of outcome assessment (detection bias) | High risk | Maternity staff distributed the first questionnaire. Follow‐up questionnaires were sent to women. It was not stated whether there were any blinding procedures. |
| Incomplete outcome data (attrition bias) | Unclear risk | The sample included women cared for in intervention clinics and then 2 control groups. However, data collection in 1 of the control groups was carried out before the intervention period, so in the analyses we have included only the control group data that were collected simultaneously with the intervention group (total 540 women, 378 included in analysis). Response rates at 3 days 84% and 93% in the intervention and control groups, by 9 months postpartum 64% and 73%. There was no adjustment for cluster design. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | No baseline imbalance was apparent, although duration of exclusive breastfeeding was presented as a baseline characteristic. |
| Study characteristics | ||
| Methods | 2‐arm cluster‐RCT, n = 330 in 15 clusters | |
| Participants | NSW, Australia, primary care setting of general practice in rural agricultural settings. Maternity hospitals were not Baby Friendly accredited, although at each hospital an International Board Certified LC and registered midwives encouraged mothers to breastfeed. 35.3% of infants were currently fed solids at 4 months, while 52.9% had received solids, infant formula or other nonhuman milk, at least once, by 4 months. Background rates of breastfeeding initiation:92% Inclusion criteria: all pregnant women who had registered to give birth at 1 of the 3 local hospitals (n = 3127) over 14 months, had reached 24–36 weeks of pregnancy, who planned to have their postnatal care at a participating general practice and who were still breastfeeding at8 weeks were randomised. Exclusion criteria: not reported | |
| Interventions | Intervention (n = 154): a structured conversation to support continuation of breastfeeding following a Conversation Tool flowchart that used a motivational interviewing approach. The Conversation Tool was used with each breastfeeding mother who attended a general practice intervention site for her infant to be immunised at 2, 4 or 6 months. Mothers were informed of the recommendation for breastfeeding exclusively to 6 months and maintenance to 1‐2 years and asked ‘How would that work for you?’ According to the mother’s response, the practice nurse provided a targeted proactive conversational action. Intervention practice nurses attended 2 x 5‐h training workshops that were delivered by a team of a midwife/LC/trainer and a family doctor/breastfeeding counsellor and based on WHO‐based resource that presented breastfeeding maintenance as appropriate and physiological. In addition, training addressed motivational interviewing and reflective practice. Information about local government and community breastfeeding support services, and handout literature for mothers, were provided. Control (n = 176): mothers received usual care from nurses who had not received WHO breastfeeding support training, and who commonly asked whether the mother had any problems. | |
| Outcomes | Outcomes not clearly stated Exclusive and full/predominant (substitution of breast milk with water‐based substances allowed) breastfeeding at 4 and 6 months | |
| Notes | Dates of study:iIntervention run from August 2008 to October 2009 Funding sources: no specific funding other than a PhD scholarship from UNSW Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clusters were coded, computer randomized and assigned to the intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Clusters randomized at same time, so concealment was not an issue. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding was not possible for the practice nurses. Participants were unaware of the group allocation process, but not clear if this was effective. |
| Blinding of outcome assessment (detection bias) | High risk | Research assistants, who were not otherwise associated with the study, collected blinded outcome data by telephone interview. |
| Incomplete outcome data (attrition bias) | Low risk | 2% attrition in intervention group and 3% in control group. |
| Selective reporting (reporting bias) | Unclear risk | The protocol did not state the predefined outcomes clearly. |
| Other bias | High risk | Difference in prenatal intentions to rejoin employment within 12 months between the 2 groups (70% intervention, 56% control). |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation n = 120 | |
| Participants | Setting not clear: women expecting to give birth in an urban maternity unit, Canada. 120 women recruited in late pregnancy. | |
| Interventions | Intervention: in addition to usual care, prenatal breastfeeding class and postnatal drop‐in breastfeeding session. Telephone follow‐up by nurse at 2, 6 and 12 weeks postpartum. Control: usual care in hospital with assistance from nurses who had received breastfeeding education. | |
| Outcomes | Exclusive breastfeeding at 1 and 3 months and any breastfeeding at 3 and 6 months | |
| Notes | We have not included data from this study in the review due to high levels of attrition (> 25% loss to follow‐up). Recruitment to the study took place during pregnancy and by 1 month postpartum there was high loss to follow‐up and loss was not balanced across groups. At 1 month 42% of controls and 22% of the intervention group were not available for follow‐up. The high level and unbalanced attrition means that results from this study were difficult to interpret. Dates of study: not reported Funding sources: not reported Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided about blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | Did not state who collected the data. No details provided about blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | High risk | Recruitment to the study took place during pregnancy and by 1 month postpartum there was high loss to follow‐up and this was not balanced across groups. At 1 month 42% of controls and 22% of the intervention group were not available for follow‐up. The high level and unbalanced attrition means that results from this study are difficult to interpret. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There was very little information about study methods and most of the results in the paper were not reported by randomisation group. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 1157 | |
| Participants | State of Victoria, Australia, relatively disadvantaged communities. Background breastfeeding rates: Victoria state any breastfeeding rate = 50%. Inclusion criteria: first‐time mothers, admitted as public patients to the postnatal units of the participating hospitals, were proficient in English and were intending to breastfeed. Exclusion criteria: serious physical or medical illness, multiple birth, member of the Australian Breastfeeding Association (ABA) prior to the baby's birth (indicative of high motivation to breastfeed and high self‐efficacy), or the infant remained in hospital after the mother's discharge. | |
| Interventions | Proactive telephone‐based support from a peer volunteer. Peers made an initial telephone call to the new mother 24 to 48 h after hospital discharge, with a follow‐up call three to four days after the initial call. Subsequent calls were to be made each week for 12 weeks, then 3‐4 weekly between 12 weeks and 6 months. Calls focused on the new mother's wellbeing and breastfeeding experience, with volunteers referring the mother to existing support services as required. The participant was able to contact the peer volunteer between the scheduled call as needed. Women in the intervention group also received usual care. Control: standard postpartum hospital stay was up to 48h after vaginal birth and 72h after caesarean section, with access to hospital specialist breastfeeding services by lactation consultants if needed. Women were offered one to two postnatal visits in the home from a hospital midwife within the first week after discharge from hospital, after which a Maternal and Child Health Nurse (MCHN) service was provided in the community. All women could also access the ABA telephone helpline service, staffed by trained volunteer breastfeeding counsellors. This free service is available 24h a day seven days per week, but the breastfeeding mother has to access the service herself. | |
| Outcomes | Any and exclusive breastfeeding at 6 months | |
| Notes | Dates of the study: Feb 2013‐December 2015. Funding sources: The Felton Bequest, Australia, philanthropic donation and La Trobe University grant. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by a computerised random number generator in variable block sizes of four to six (to avoid selection bias), and was stratified by site. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was generated and administered by the Clinical Epidemiology and Biostatistics Unit at Murdoch Children's Re‐ search Institute. The program was accessed by research staff who en‐ tered the details of the trial hospital and the woman's birth date, then a randomised allocation was immediately generated, and the woman was informed of the outcome. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind women or peers |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up attrition = 13.2% Loss to follow‐up control = 11.2% |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol reported |
| Other bias | Low risk | No industry funding No conflicts of interest No significant baseline imbalance |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 88 | |
| Participants | Public hospitals in Western Spain. Background breastfeeding rates: For Spain as a whole 77‐88% initiation, EBF at 6 months = 30% to 40%. Inclusion criteria: all women who had given birth by vaginal delivery to healthy babies and had begun to breastfeed within 1hr after birth. Exclusion criteria: mothers whose newborns required admission to the neonatal unit; women with previously diagnosed psychiatric disorder; women with neurological or cognitive damage; women with residence status that did not allow for adequate follow‐up; and women with language or communication barriers. | |
| Interventions | During the immediate postpartum period, the intervention group received a BMI consisting of a 20‐30‐min interview performed by a midwife with previous specific training in BMI provided by psychologists specialising in motivational interviewing. At the first, third, and sixth months postpartum, the women received a phone booster from the same midwife that did not exceed 15 min. Interviews were conducted using the Brief Motivational Interviewing principles of Miller and Rollnick. Control: health education session on breastfeeding during the immediate postpartum period based on the information contained in the leaflet ‘Congratulations on your maternity’ published by the Spanish Association of Paediatrics. The duration of this session was the same as that of the intervention group. Mothers in the control group were contacted by phone in the same periods (first, third, and sixth months postpartum) and were offered information on the benefits of continuing to breastfeed and the opportunity to resolve their questions about breastfeeding. All the women received the usual postpartum care at the hospital and at hospital discharge (a standardised visit in the first postpartum week and at the 6th week) by midwives who were not participating in the study. | |
| Outcomes | Any and exclusive breastfeeding a 1, 3 and 6 months | |
| Notes | Dates of the study: Feb 2018‐March 2019. Funding sources: this study has been awarded in the “International Confederation of Midwives Research Award 2018” funded by Johnson & Johnson. The members of the GISyC group are funded by “Programa Operativo FEDER Extremadura (2014‐2020) y Fondo Europeo Desarrollo Regional (FEDER)” (GR18146). Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomization by minimization was performed to balance the baseline variables associated with breastfeeding success |
| Allocation concealment (selection bias) | Low risk | The randomization sequence remained hidden until the intervention. After recruitment and collection of the baseline data, a midwife contacted the investigator in charge of randomization so the result of the randomization could be communicated to the midwife. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data |
| Incomplete outcome data (attrition bias) | Low risk | Intervention loss to follow‐up = 7% Control loss to follow‐up = 9% Intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes from protocol reported |
| Other bias | High risk | Received funding from Johnson and Johnson No conflicts of interest Significant baseline difference in education level and previous BF experience |
| Study characteristics | ||
| Methods | 4‐arm (factorial design) RCT, single site, recruiting over 17 months, n = 343 | |
| Participants | Urban USA: inpatient maternity unit Background rates of breastfeeding initiation: low Inclusion criteria: breastfed once in hospital; able to speak Spanish or English; baby needed < 48 h on NICU; able to be contacted by telephone after discharge Participant characteristics: 57% primiparous Ethnic composition: black 65%, Hispanic 19%, white 13%, other 4% Socioeconomic status defined by: < 100% poverty level ‐ 69%; 100%‐200% poverty level ‐ 21%; > 200% poverty level ‐ 10% Mean age of participants 25.7 years | |
| Interventions | Intervention: women received postpartum breastfeeding counselling in hospital by trained counsellor (20‐40 min) and by telephone at 5, 7, 14, 21, 28, days and 6, 8 and 12 weeks, also 24‐h advice by pager. Given research discharge pack in English and Spanish. Routine care consisted of postpartum staff nursing contacts (including discharge teaching session on infant care), infrequent breastfeeding classes, written information on breastfeeding management and the opportunity to access a midwife‐run telephone advice line. | |
| Outcomes | Exclusive breastfeeding at 1, 2, 3 and 4 months Any breastfeeding at 4 months Median duration of breastfeeding Time to introduction of formula or solids Rehospitalisation of infants | |
| Notes | Dates of study: study conducted from May 1984 to September 1985 Funding sources: National Institute of Child Health and Human Development grant Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised block design (block size 8) with computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were aware of the overall goal of the interventions but not aware of the study hypotheses. It is not detailed whether personnel were blinded but appears unlikely. |
| Blinding of outcome assessment (detection bias) | High risk | For follow‐up at 4 months it was stated that the investigator was not aware of group assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Participants received a fee to minimise sample attrition. 343 women were recruited. There were a small number of protocol deviations (7 women received the wrong type of discharge pack and were analyzed according to treatment received rather than by randomisation group). 19 women were lost to follow‐up. Attrition and reasons for attrition were described as similar across groups. Follow‐up 94%. Appropriate randomisation procedures. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Low risk | No baseline imbalance apparent. |
| Study characteristics | ||
| Methods | Multicentre, 3‐arm cluster‐RCT, n = 722 (clusters (hospitals) n = 3) | |
| Participants | Mother–infant pairs were recruited from the postnatal units of 3 geographically distributed public hospitals providing obstetrical services in Hong Kong. Participants: 722 primiparous breastfeeding mothers with uncomplicated, full‐term pregnancies Background rates of breastfeeding initiation: 80% In Hong Kong current breastfeeding patterns are similar to those of other developed countries, with > 80% of women initiating breastfeeding, but with only 20% continuing to breastfeed exclusively for 3 months. Inclusion criteria (mother): Hong Kong Chinese primiparas, ≥ 18 years old, intending to breastfeed, and without any major obstetric complications (i.e. severe postpartum haemorrhage) or serious medical problems (i.e. psychiatric illness) Inclusion criteria (baby): gestational age ≥ 37 weeks; birthweight ≥ 2500 g, 5‐min Apgar score ≥ 8, and no physical anomalies that would contraindicate or complicate breastfeeding Exclusion criteria: mothers who were planning to live in mainland China after delivery | |
| Interventions | Intervention 1: standard care plus 3 in‐hospital professional breastfeeding support sessions, of 30–45 min in duration. Intervention 2: standard care plus weekly postdischarge breastfeeding telephone support, of 20–30 min duration, for 4 weeks. Both interventions were delivered by 4 trained research nurses, who were either highly experienced registered midwives or certified LCs. Control: standard postnatal maternity care that consisted of routine perinatal care according to the type of delivery, group postnatal lactation education provided by a midwife or LC, 1‐on‐1 assistance with breastfeeding if problems arose and time permitted, and postdischarge follow‐up, either at the outpatient clinic of the delivery hospital or at the nearest Maternal and Child Health Centre. Information on available peer‐support groups was also provided upon hospital discharge. | |
| Outcomes | Primary: prevalence of any and exclusive breastfeeding at 1, 2, and 3 months postpartum. Classified infant feeding status into 3 categories: exclusive breastfeeding; any breastfeeding; and exclusive formula feeding. Secondary: overall duration of any and exclusive breastfeeding. Measured the duration of any and exclusive breastfeeding as the age of the infant in weeks when the participant completely stopped breastfeeding and first introduced infant formula, respectively. | |
| Notes | Dates of study: participants were recruited between November 2010 and September 2011 Funding sources: Health and Medical Research Fund of the Food and Health Bureau, Government of Hong Kong Special Administration Region. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was generated using an online program (www.randomization.com). All participants at each study site were allocated to the intervention to which the hospital was randomly assigned for that week. Cluster‐randomisation was used, with hospitals being the unit of randomisation. Each week, a study hospital was randomly assigned each study hospital to 1 of the 3 treatment groups. |
| Allocation concealment (selection bias) | Low risk | Conducted by a person not involved in the subject recruitment or data collection. Assignments placed in sequentially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | The blinding of either participants or those delivering the intervention was not possible for this type of study design. For the control and telephone support group, a research nurse not involved with delivering the intervention, recruited the participants. However, authors state that for the inpatients, the same nurse who recruited the participants also delivered the intervention. |
| Blinding of outcome assessment (detection bias) | High risk | A study research assistant, who was blinded to the participants’ treatment allocation, conducted the telephone follow‐up. |
| Incomplete outcome data (attrition bias) | Low risk | 97% of participants had complete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of predefined outcome measures so unable to make a judgement. |
| Other bias | High risk | Not all intervention groups received the full intervention.Of the 191 participants allocated to the in‐hospital support group, 137 (71.7%) received all 3 sessions, 52 (27.2%) received 2 sessions, and 2 (1.0%) received only 1 session before hospital discharge. Of the 268 participants in the telephone support group, 199 (74.3%) received all support sessions for which they were eligible; 27 (10.1%), 24 (9.0%), 13 (4.9%), and 5 (1.9%). Baseline characteristics and maternal and birth data were similar across the 3 groups although there were some minor variations in maternal education, family income, intention to exclusively breastfeed, and antenatal breastfeeding class attendance. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 586, 292 assigned to intervention and 294 to control | |
| Participants | Study conducted at a university teaching hospital and affiliated community health centres in urban Quebec, Canada. Recruitment January 1997‐September 1998 Background rates of breastfeeding initiation: intermediate Inclusion criteria: mothers participating in hospital short‐stay programme Ethnic and socioeconomic composition of sample not reported Baseline prevalence of breastfeeding initiation in Canada (excluding territories) 1994‐5 = 73% | |
| Interventions | Intervention: home visit from community nurse 3‐4 days postpartum. Nurses were Baccalaureate prepared, had a minimum of 3 years' clinical experience in maternal‐child health, and had attended training to ensure assessment skills of maternal‐newborn and breastfeeding support. Contact with the nurse continued if required. Control: usual care was a 48‐h postpartum contact and 1 postpartum hospital clinic visit (day 3) following a standard plan of care and lasting up to 45 min. Referral for continued care was available. | |
| Outcomes | Breastfeeding frequency and infant weight gain assessed at 2 weeks postpartum | |
| Notes | Dates of study: recruitment dates January 1997 to September 1998 Funding sources: Fonds de la recherche en sante du Quebec (FRSQ) Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (block size 8) stratified by parity, by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "masking of the women and health professionals was not possible." |
| Blinding of outcome assessment (detection bias) | High risk | It was reported that outcome data were collected by blind investigators. It was not clear whether planned blinding was effective, although investigators apparently asked women "not to divulge their group status". |
| Incomplete outcome data (attrition bias) | Unclear risk | 586 randomized. 21 protocol deviations, but analysis performed according to randomisation. 499 completed trial and provided information on primary outcome (15% attrition). Some further missing data for some outcomes. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so were unable to evaluate. |
| Other bias | Low risk | Groups described as similar at baseline. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 154. | |
| Participants | Hospital Canaries Spain Background breastfeeding rates: In 2012, 25.7% of women were exclusively breastfeeding at 6 months post‐delivery in the Canary Islands. Inclusion criteria: primiparous women with a singleton pregnancy at term, delivering a healthy baby by spontaneous vertex delivery (SVD) or assisted delivery (forceps or ventouse). Other criteria included hospital admission of no more than 48 hours. Those wishing to breastfeed and voluntarily join the study after signed a consent form prior to recruitment. Exclusion criteria: neonatal admission for hyper‐bilirubinemia, a history of pre‐eclampsia. Not wanting to take part. | |
| Interventions | Extra support through a website to first‐time mothers wishing to breastfeed for 6 months. Mothers had access to the most up‐to‐date information about breastfeeding to answer questions regarding breastfeeding and avoid any unnecessary trips to postnatal clinics. The website allowed one‐to‐one contact between first‐time mothers and peer‐supporters. Each participant was assigned a named supporter, who could be contacted regarding breastfeeding questions. Control: routine support within the hospital or postnatal clinics from health professionals. Women in most parts of Spain are required to attend local clinics for their postnatal checks and feeding advice. Even when women attend clinics, health professionals are not able to spend enough time with them due to time constraints and workload. | |
| Outcomes | Any breastfeeding at 3 and 6 months | |
| Notes | Dates of the study: April to October 2016. Funding sources: Canarian Research Foundation. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It states randomised, but no description was provided on how the allocation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No description was provided on whether the allocation was concealed. |
| Blinding of participants and personnel (performance bias) | High risk | No details provided, but due to the nature of the intervention it was not feasible to blind participants and personnel to group allocation. |
| Blinding of outcome assessment (detection bias) | High risk | No details provided, but as key breastfeeding outcomes were self‐reported by participants, it was not feasible to blind outcome assessors to group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | <20% attrition, with follow up of 75/76 (99%) mothers in the intervention group and 75/78 (96%) mothers in the control group. Four mothers who dropped out (IG=1; CG=3) were replaced. It is unclear how these replacements may have impacted the results, but we judged this as low risk due to small numbers. |
| Selective reporting (reporting bias) | Unclear risk | No publically available protocol. An unpublished protocol was provided from the authors but this was in Spanish. |
| Other bias | Low risk | No significant differences between both groups in any of the selected characteristics at baseline No industry funding declared. No competing interests declared |
| Study characteristics | ||
| Methods | Cluster RCT of 16 health centres. | |
| Participants | 16 health centres in three districts in Lusaka Province, Zambia. Background breastfeeding rates: 73% exclusively breastfeed their infants aged 0–5 months, but 39% of infants aged 4–5 months are given complementary foods. Inclusion criteria: primary caregivers of children younger than 5 years, but the assessment was restricted to two specific populations: mothers of an infant younger than 6 months and primary caregivers of a child younger than 5 years with recent diarrhoea. Exclusion criteria: the follow‐up sample excluded individuals who had moved into the area since baseline. | |
| Interventions | A campaign, developed with creative agency DDB Iris, centred on a group of women known as Adzimayi Bamu Komboni, meaning housewives of the slum community. This group gossiped about women whose behaviour was believed to deviate from the target behaviours, but they ultimately admitted women into their social circle (a reward) when it was proven that they were practising the correct behaviours. The motives of disgust (an adaptive mechanism that helps us to avoid disease) and nurture (a natural instinct to protect and care for one’s off spring) were also used to drive behaviour change through elicitation of strong emotional responses in connection with the targeted behaviours. The campaign had four components: women’s forums delivered in neighbourhoods; roadshows delivered in public gathering spaces; clinic‐based circle of mothers sessions with monthly prize draws; and call‐in programmes on local radio linked to the forums. Control: standard care at clinics. Radio adverts and call in‐show broadcast in control areas too. | |
| Outcomes | Exclusive breastfeeding in all children under 5 months | |
| Notes | Dates of the study: January 2014 to November 2014 Funding sources: Absolute Return for Kids (ARK) and Comic Relief. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clusters were randomly selected within three strata based on district in a 2:1:1 ratio, resulting in a sample that was half peri‐urban (Lusaka district) and half rural (Chongwe and Kafue districts). A statistician unrelated to the study allocated half of the clusters in each district to intervention or control (no intervention, standard care at clinics) using a random number table |
| Allocation concealment (selection bias) | Low risk | Randomisation was done (by an independent statistican) before baseline data collection took place, but cluster allocation was concealed from the study team until after baseline data had been collected. |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, it was not feasible to blind participants or personnel to group allocatio |
| Blinding of outcome assessment (detection bias) | High risk | While authors suggest outcome assessors were blind to group allocation, breastfeeding outcomes were self‐report by the women who were not blind to group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | None of the clusters were lost to follow up. It should be noted that the baseline and endline surveys were conducted with two independent, population‐representative samples within clusters; hence, no attrition due to the cross‐sectional nature of each survey. |
| Selective reporting (reporting bias) | Low risk | The clinicaltrials.gov trial registration record suggests no selective reporting for key breastfeeding outcomes |
| Other bias | Unclear risk | 16 clusters randomised, with 16 clusters participating in baseline and endline surveys. ICC was calculated for each behavioural outcome. Clusters were accounted for in the analysis. No industry funding. Declaration of interests transparent, with statement that three authors have received funding from Unilever, a soap manufacturer. None of their time while working on this study was funded by Unilever. Baseline profiles of participants similar, but there were differences between the study groups in both study profiles with respect to educational level, employment status, prevalence of shared sanitation, awareness of zinc, and use of ORS and zinc. Unclear if this is a source of bias.
|
| Study characteristics | ||
| Methods | Cluster‐randomised trial. 4 clinics were 'randomly assigned' to 4 different interventions n = 548 | |
| Participants | Setting: 4 WIC clinics in Baltimore USA Women were predominantly African American (> 90%) 548 women attending study clinics enroled at between 6 and 24 weeks’ gestation. Women were WIC eligible with singleton pregnancies, planning to keep the baby and to stay in study areas | |
| Interventions | The study was carried out in 4 clinics. Each clinic offered a different intervention. Clinic 1: standard care (usual breastfeeding promotion by clinic staff) Clinic 2: standard care plus a motivational video (encouraging breastfeeding) that was played repeatedly in the clinic waiting area Clinic 3: peer support by a mother who had breastfed and undertaken training. Peer supporters contacted pregnant women and discussed breastfeeding. Women were offered a 1‐h group breastfeeding support session in the WIC clinic before the birth. After the birth, peer counsellors contacted women and remained in contact with breastfeeding women (phone or visits) until 16 weeks after the birth. Clinic 4: standard care plus video plus peer support | |
| Outcomes | Infant feeding method at 8 weeks and 16 weeks postpartum and maternal work status | |
| Notes | We were not able to include data from this study in the review due to very high levels of attrition. The study was at a high risk of bias. This was a cluster trial with 4 clinics each allocated to a different intervention and with no adjustment for study design effect. Women were recruited in the antenatal period. 548 women enroled, but information was only available for 273 women at 7‐10 days postpartum (50%); of the 275 women lost to follow‐up 31% (74) were excluded due to pregnancy complications, the remaining 73% (201 women) refused or could not be contacted – these women represented 37% of the original randomised sample. It was not clear whether loss was similar in the 4 clinics. Dates of study: recruitment between June 1992 and January 1994. Funding sources: Maternal and Chil Health Bureau, Health Resources and Services Administration, Department of Health and Human Services. Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster trial. 4 clinics; method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described
|
| Blinding of participants and personnel (performance bias) | Unclear risk | Did not state whether participants or personnel were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Did not state who the interviewers were or if any attempt at blinding outcome assessment was made. |
| Incomplete outcome data (attrition bias) | High risk | 548 women were enrolled on the study, but information was only available for 273 women at 7 to 10 days postpartum (50%); of the 275 women lost to follow‐up 31% (74) were excluded due to pregnancy complications, the remaining 73% (201 women) refused or could not be contacted – these women represented 37% of the original randomized sample. It was not clear whether loss was similar in the 4 clinics. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not evaluate this. |
| Other bias | Unclear risk | Baseline characteristics: imbalance for educational status, employment and parity ‐ although these were adjusted for in the analysis. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 533. | |
| Participants | Primary care prenatal and paediatric clinics and the postpartum ward of a large urban public hospital and an affiliated satellite neighbourhood health centre in New York City, USA. Background breastfeeding rates: New York State prevalence of exclusive breastfeeding at 3 months is 37% (lower in low‐income and minority women). Inclusion criteria: pregnant women who were: 18 years old or older; self‐identified as Hispanic/Latina; fluent in English/Spanish; with a singleton uncomplicated pregnancy; able to provide phone numbers; and intending to receive care at the study sites. Exclusion criteria: women with: significant medical or psychiatric illness (eg, cardiovascular disease, lupus, neuromuscular disorders, psychosis, drug addiction); homelessness; and severe fetal anomalies on ultrasound (eg, neural tube defects, chromosomal abnormalities). | |
| Interventions | The Starting Early intervention is a family‐c entered primary care‐based early child obesity prevention intervention designed for low‐income Hispanic families beginning in the third trimester of pregnancy and continuing until child age 3 years. The intervention is delivered by registered dieticians (RDs) with maternal‐child health experience who have been trained as certified lactation counsellors. Intervention components were: (1) individual nutrition counselling in the prenatal and postpartum periods; (2) nutrition and parenting support groups coordinated with well‐child visits; (3) plain language handouts; and (4) nutrition education DVDs. All curriculum and materials were developed in English and Spanish. The individual counselling sessions in the prenatal and newborn periods and the support groups for mother‐infant pairs were approximately 45‐60 minutes long. After the infants were born, groups of 4‐8 mother‐infant pairs were formed into a cohort by the infant’s birthdate. These cohorts attended groups together from the 1‐month visit until the children were 3 years old. Control: Routine prenatal, postpartum, and pediatric primary care. Standard prenatal care at the study sites included prenatal visits with an attending or resident obstetrician or nurse midwife. Prenatal visits were scheduled according to American Academy of Obstetrics and Gynecology guidelines with additional visits at the provider’s discretion. An initial individual consultation with a nutritionist and group childbirth and breastfeeding classes were offered to all women | |
| Outcomes | Any and exclusive breastfeeding at 3 months | |
| Notes | Dates of the study: August 2012 ‐ December 2014 Funding sources: supported by the National Institute of Food and Agriculture, US Department of Agriculture (2011‐68001‐ 30207) and the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (Mentored Patient‐Oriented Research Career Development Award (K23HD081077). Declarations of interest: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomized to intervention or control groups at a prenatal visit by a nutritionist who conducted the intervention, using a random number generator, stratified by site. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Personnel collecting follow up data were blind however the women would be aware if they had received the intervention |
| Incomplete outcome data (attrition bias) | Low risk | A total of 456 mother‐infant pairs completed the 3‐month assessment (86.2% of 529 infants born) and were included in these analyses. These analyses included 221 (84.0%) intervention and 235 (88.3%) control mother‐infant pairs |
| Selective reporting (reporting bias) | High risk | Outcomes reported are not listed in the protocol of the study. Protocol outcomes measures the longer term impacts of the programme in relation to childhood obesity |
| Other bias | Unclear risk | No conflicts of interest declared. No industry funding Groups did not for any baseline characteristics expect for lower education in the enrolment sample ‐ significance is not reported for the baseline measures. Unclear if this will impact upon findings. |
| Study characteristics | ||
| Methods | 2‐arm quasi‐RCT, with individual randomisation, single‐site study recruiting over 10 months, n = 97, follow‐up 90%. Quasi‐randomisation via coin toss, with women sharing same room allocated by 1 toss | |
| Participants | Urban USA ‐ inpatient maternity unit Background rates of breastfeeding initiation: low WIC breastfeeding prevalence at birth 1991 = 33% Inclusion criteria: women eligible for WIC programme services for those on low incomes; women intending to breastfeed Participant characteristics: approximately one‐third were primiparous. Ethnic composition described as 54% black. Mean age was 25.4 years. | |
| Interventions | Intervention: package included 30‐45 min face‐to‐face meeting in hospital with LC (a registered nurse) after birth ‐ educational booklet given; telephone contacts on days 2, 4, 7, 10 and 21; a telephone help‐line staffed by a nurse or paediatrician; and back‐up support for those with problems from a lactation clinic Control: routine postnatal teaching on infant care and feeding by obstetric nursing staff | |
| Outcomes | Rates of breastfeeding at 6 weeks and 3 and 6 months Median duration of breastfeeding | |
| Notes | Dates of study: 15th March 1986 and 27th January 1987. Funding sources: Special Projects of Regional and National Significance grant and the Department of Health and Human Services Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Coin toss at the point of randomisation. |
| Allocation concealment (selection bias) | High risk | Coin toss at the point of randomisation, so allocation could be altered. If 2 women occupied the same room they were allocated to the same group. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Did not state whether women or personnel were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Some data were derived from medical records, but telephone outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 97 women randomized, by 6 weeks 4 control group women could not be contacted (> 90% follow‐up but loss not balanced across groups). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not evaluate this. |
| Other bias | Unclear risk | Groups appeared similar at baseline. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 312 | |
| Participants | Illinois, USA, large city and two smaller urban areas. Background breastfeeding rates: Not reported other than to say rates are low in US. Inclusion criteria: women under 26 years, less than 34 weeks gestation, living in the program geographic catchment area, planning to remain the area, and meeting socio demographic risk criteria used by the HFA (Health Families America) or PAT (Parents as Teachers) models. Exclusion criteria: pregnant women under 14 years, involved with the child welfare or juvenile justice systems, or had significant cognitive impairments were excluded from the study. | |
| Interventions | Doula‐home‐visiting programs assigned families a home visitor and a community doula. Doulas and home visitors all had deep roots in their communities. The doula worked with the mother more intensively during pregnancy and the first weeks postpartum, while the home visitor became the primary provider by 6 weeks postpartum. Home visitors focused on the mother‐infant relationship, child development, child safety, and educational‐work planning, as well as screening to make sure that family basic needs were being met. Doulas focused on issues related to pregnancy health, childbirth preparation, breastfeeding, newborn care, postpartum health, and early bonding. Doulas sometimes accompanied mothers to prenatal and postpartum medical visits. Doulas attended births at the hospital where they provided mothers with physical comfort, emotional support, and advocacy during labor and delivery and breastfeeding counselling postpartum. Doulas also offered prenatal classes at the program sites. All programs conducted regular depression screenings and made referrals to mental health consultants. Control: Lay home visitors (also called a Family Support Worker or Parent Educator) and community doulas. | |
| Outcomes | Any breastfeeding at 3 months Hospitalisations Maternal depressive symptoms at 3 weeks and 3 months | |
| Notes | Dates of the study: recruitment 2011‐2015. Funding sources: Award D89MC23146 from the MIECHV competitive grant program from the Health Resources and Services Administration (HRSA) to the State of Illinois Department of Human Services (IDHS). Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization tables were created separately for each community. |
| Allocation concealment (selection bias) | Low risk | At the end of this session, the interviewer opened a sealed opaque envelope that showed whether the participant was assigned to doula‐home‐visiting services (intervention condition) or case management (control condition). These envelopes had been prepared by the principal investigator before the beginning of the study. Randomization tables were created separately for each community. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants or personnel |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Not sure why the data is lower at 37 weeks. Intervention group, 156 recruited, [129 at 37 weeks}. 139 at 3 month follow up. 139/156 = 11% drop=off. Control group 156 recruited, 127 at 37 weeks. 139 at 3 month follow up. 139/156 = 11% drop=off |
| Selective reporting (reporting bias) | High risk | Protocol can be found here: https://clinicaltrials.gov/ct2/show/NCT01947244?term=NCT01947244&draw=2&rank=1 Many of the outcomes in the protocol are not reported in this paper including the primary outcome of mother‐child interaction. The breastfeeding outcomes are reported. |
| Other bias | Low risk | Baseline characteristics nearly all balanced (with exception of co‐residing with a parent). No industry funding The authors declare that they have no conflict of interest." |
| Study characteristics | ||
| Methods | 2‐arm cluster‐RCT with prospective mixed‐method embedded case studies to evaluate implementation processes. 14 localities randomised; recruitment 2002‐2004 n=18858 | |
| Participants | Setting: women registered at GP practices in 14 localities (of 66) in Scotland Background rates of breastfeeding initiation: low In Scotland, in 2005, only 44% of babies had received any breast milk at 6 weeks. 14 clusters randomised, birth records supplied data for n = 9747 in intervention group and n = 9111 in control group. Inclusion criteria: pregnant women and breastfeeding mothers Exclusion criteria: not stated In intervention localities 25.2% of the populations were in the most deprived social groups, compared with 32.1% in the control localities. Mean age of mothers at the first child health record was 28‐29 years. In 7 areas (3 intervention, 4 control) women gave birth at Baby‐Friendly hospitals. | |
| Interventions | Intervention: a policy intervention aimed at locality areas rather than at individual women. The policy aimed to double the number of local breastfeeding support groups and to make weekly support groups open to all pregnant women and breastfeeding mothers. These local breastfeeding support groups were facilitated by health professionals taking a woman‐centred approach and aiming to provide breastfeeding support and social interaction for women. Control: control localities received no additional intervention; however, breastfeeding support groups existed in some control areas. | |
| Outcomes | Primary: number of babies receiving any breast milk at 6‐8 weeks, as reported in routinely collected data for the 2 pretrial years and 2 trial years Secondary: any breastfeeding at birth, 5‐7 days and 8‐9 months, and maternal satisfaction Results were not presented in a way which allowed us to enter them into data and analysis tables, but we have summarised findings in the text. | |
| Notes | When we updated our search in October 2011, Hoddinott 2009 was the only evaluation we found: a) of a policy‐level intervention; b) of breastfeeding in groups; and c) that used routinely collected locality‐level outcome data. Dates of study: study conducted between 1st February 2005 to 31st January 2007 Funding sources: The Chief Scientist's Office of the Scottish Government Health Directorate Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐RCT; 14 localities randomized. Localities varied in size, baseline breastfeeding rates and numbers of pre‐existing groups and how pregnancy and postnatal care were organised. Localities were matched on breastfeeding rates and existing support groups: quote: “An independent statistician used random number tables to randomise locality pairs to either intervention or control”. |
| Allocation concealment (selection bias) | Low risk | Researchers analysing primary and secondary outcomes were blinded to allocation, ensured by coding of localities. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Cluster‐randomised trial, so women may not have been aware of the study although they would be aware of the intervention. Not stated whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Researchers analysing primary and secondary outcomes were reported to be blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | According to flow chart no clusters discontinued the intervention or were lost to follow‐up and there was follow‐up of national data in all localities included in the trial. The amount of data missing varied for different outcomes (e.g. birth and 6 week postpartum records were available for most of the eligible population but child health records at 8‐9 months were only available for approximately a quarter of the children). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Analysis took into account aspects of design effect. It appeared that there were some differences in the localities at baseline. Control localities may have had higher levels of social deprivation. |
| Study characteristics | ||
| Methods | 2‐arn RCT, single‐site study, n = 69 | |
| Participants | Setting: a maternity unit serving a mixed urban and rural population in Scotland Background rates of breastfeeding initiation: at hospital discharge, 54% of babies were exclusively breastfed and 6% were receiving breast and formula milk. In the most disadvantaged areas, 39% exclusively breastfed compared with 63% in the most advantaged areas. Inclusion criteria: women admitted to the ward between 26 July‐18 October 2010 who lived in the 3 most disadvantaged postcode area quintiles for the Scottish Index of Multiple Deprivation (SIMD 1‐3) in 2009 and who were breastfeeding Exclusion criteria: women aged < 16 years with serious medical or psychiatric problems or with insufficient spoken English to communicate by telephone | |
| Interventions | Intervention (n = 35): proactive telephone calls (intervention) daily for 1 week following hospital discharge. Calls were terminated at the woman’s request or if breastfeeding ceased. At 1 week following discharge, women could choose to continue receiving daily calls for a further week, change the frequency of calls, or have no further calls. Women could telephone the feeding team at any point over the 2 weeks following discharge. Text and answer phone messaging was available. All proactive calls stopped 14 days after hospital discharge. Control (n = 34): reactive telephone calls; women could telephone the feeding team at any point over the 2 weeks following discharge. Text and answer‐phone messaging was available. | |
| Outcomes | Any breastfeeding at 6‐8 weeks | |
| Notes | Dates of study: recruitment for RCT 26th July 2010 and 18th October 2010. Cohort study included additional recruits from 3rd May 2010 and 25th July 2010 Funding sources: NHS Grampian through the Scottish Government: nutrition of women of childbearing age, pregnant women and children under 5 years in disadvantaged areas‐funding allocation 200‐2001, NHS Health Scotland. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a website randomisation sequence service set up by an independent statistician. Randomisation was stratified to ensure balance of primiparous and multiparous women across both trial arms." |
| Allocation concealment (selection bias) | Low risk | Performed by an independent statistician. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Although not informed of the randomisation outcome, women knew if they had been randomized to the proactive group as they received a phone call from the feeding team within 24 h of hospital discharge". Healthcare professionals providing intervention would have been aware of allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes were collected by telephone by a researcher who was blind to randomisation and who had no other contact with study women. |
| Incomplete outcome data (attrition bias) | High risk | 3/35 in the intervention group and 8/34 of those in the control group did not have data at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of outcomes being prespecified anywhere, so difficult to judge this. |
| Other bias | High risk | Women in the proactive call group were a year older on average, with more living in the most disadvantaged postcode areas (SIMD 1). Hospital stays were half a day longer on average in the proactive call group; however, data were imbalanced by a small number of women with unusually long hospital stays. Otherwise the randomized groups were similar for parity, method of delivery, gestation and admission to the neonatal special care unit. |
| Study characteristics | ||
| Methods | 2‐arm cluster RCT, n = 2261 | |
| Participants | Five administrative regions in Bavaria, Germany. Background breastfeeding rates: German policy stated but not actual rates. Inclusion criteria: Women with a pre‐pregnancy BMI between ≥ 18.5 kg/m2 and ≤ 40.0 kg/m2, a singleton pregnancy, age between 18 and 43 years, sufficient German language skills and stage of pregnancy before the end of the 12th week of gestation. Exclusion criteria: multiple pregnancy, high risk pregnancy prohibiting study participation (contraindications to exercise e.g. placenta praevia, persistent bleeding, cervical incompetence etc.), pre‐pregnancy diabetes mellitus or early gestational diabetes, uncontrolled chronic diseases (e.g. thyroid dysfunction), psychiatric or psychosomatic diseases, and any other diseases which could interfere with compliance according to the study protocol. | |
| Interventions | Three antenatal (12th–16th, 16th–20th, and 30th–34th week of gestation) and one postpartum (6th–8th week pp) face‐to‐face counselling sessions lasting between 30 and 45 min, by previously trained midwives, medical personnel, or gynaecologists. Women were informed about adequate gestational weight gain and were encouraged to monitor their weight gain weekly using a weight gain chart. Healthy dietary and physical activity behaviour during pregnancy and the postpartum period were addressed. The importance of breastfeeding for both mothers and their offspring was outlined in both the third antenatal counselling (30th–34th week of gestation) and the postpartum session (6th–8th week). Participants were encouraged to exclusively breastfeed their infants until at least the end of the 4th month after delivery and were informed about potential barriers to initiate breastfeeding and strategies to overcome these. Infant hunger and satiety signals were discussed. Control: standard antenatal care and limited information on a healthy antenatal lifestyle and the importance of breastfeeding by means of a flyer. | |
| Outcomes | Exclusive breastfeeding at 3 months Any breastfeeding at 12 months | |
| Notes | Dates of study: recruitment between 2013 and 2015. The last participant completed the12 months follow‐up at the end of 2017. Funding sources: Else Kröner‐Fresenius Foundation (Grant number: 5140889), the Bavarian State Ministry of Food, Agriculture and Forestry, the BavarianState Ministry of Health and Care (Health Initiative “Gesund.Leben.Bayern.”), the AOK Bayern, the large statutory health insurance in Bavaria, as well as the DEDIPAC consortium by the Joint Programming Initiative(JPI) “A Healthy Diet for a Healthy Life”. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation details in an earlier publication although still not fully explained. We perform a paired cluster‐randomization by matching five pairs of regions according to birth figures, sociodemographic and geographic criteria. Randomization is performed within these pairs and each pair is supervised by an expert centre for nutrition run by the Bavarian State Ministry of Food, Agriculture and Forestry. |
| Allocation concealment (selection bias) | Unclear risk | No information given regarding this |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants or personnel |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | The reported drop‐out rate does not match what we calcuated based on figures presented in the paper. We calcuate it to be 20% for the intervention and 21.5% for the control. |
| Selective reporting (reporting bias) | High risk | From the protocol the primary outcome is described as: Proportion of pregnant women showing excessive gestational weight gain according to Institute of Medicine (IOM) guidelines. Breastfeeding was not mentioned in the protocol, nor listed as a secondary outcome in Rauh, 2014 but it is mentioned in the text as an outcome to be assessed at follow‐up. This looks as if the breastfeeding outcome has been added post‐hoc. It was mentioned as part of the intervention in visit 4 at (6th‐8th week postpartum): The fourth counselling session includes dietary advice during breastfeeding and information on infant feeding principles. Full breastfeeding is recommended at least up to the age of 4 months. |
| Other bias | Unclear risk | No industry funding. No conflicts of interest Imbalance in groups re proportion of primiparous women reported Intra‐cluster co‐efficient not mentioned but above statement made. Intra‐cluster coefficaint provided in main paper (Kunath et al 2019): " The intraclass correlation coefficient, reflecting potential systematic differences between the clustered study regions, was low (0.8%). |
| Study characteristics | ||
| Methods | 2‐arm RCT, n = 125 | |
| Participants | Four maternity hospitals in Tokyo and Kanagawa prefectures in Japan. Background breastfeeding rates: In a nationwide survey, most Japanese mothers continued breastfeeding after hospital discharge: 85.9% at 4 months and 81.2% at 6 months postpartum. Inclusion criteria: primiparas and multiparas who were willing to breastfeed their infants, older than 16 years, singleton live birth at a maternity hospital, fluent in Japanese, and expressed (in hospital, after delivery) willingness to breastfeed exclusively or partially after discharge from hospital. Exclusion criteria: serious illness or disability that could significantly interfere with breastfeeding or study participation, infant in the neonatal intensive‐care unit after mother’s hospital discharge, pre‐partum enrolment with La Leche League (LLL). | |
| Interventions | Each peer supporter assisted one to three participants. The contact intervals were chosen to coincide with “frequent days,” when infants undergo growth spurts and want to nurse more frequently. Peer supporters helped participants to continue breastfeeding while adjusting to life with a new infant, listened to participants’ concerns, acknowledged them, and provided information or referrals to LLL Leaders or health care professionals if needed. Participants were also encouraged to call peer supporters any time they wished to talk or when they had breastfeeding concerns. Control: Conventional care, which included breastfeeding support at hospitals and a home visit by a health worker after hospital discharge. After a new mother is discharged from the hospital, she is entitled to a free home visit by a health worker (e.g., midwife, public health nurse) once within 4 months after delivery. The purpose of the visit is to listen to the new mother’s concerns, give necessary information regarding caring for her infant, and assess the child‐rearing environment. | |
| Outcomes | Any and exclusive breastfeeding at 1 and 3 months Maternal satisfaction with feeding | |
| Notes | Dates of the study: October 2016 to September 2017 Funding sources: UT Grants for PhD Researcher Program at the University of Tokyo and research funds from the Department of Community and Global Health, Graduate School of Medicine, University of Tokyo. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the research ID numbers identified the study hospitals, the randomization list of peer‐support participants was dynamically allocated for each hospital (randomization ratio of 1:1, peer support to conventional care), with a block size of four distributed randomly (Forster et al., 2014). The block randomization pattern was created by a biostatistician who was not involved in recruiting participants. |
| Allocation concealment (selection bias) | Low risk | The biostatistician that created the randomisation pattern was not involved in recruiting participants.Randomized allocation was conducted by research assistants who were not involved in the study design or data analysis. However it is not clear if this would conceal allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | The researcher remained blinded to the allocation until after the completion of data cleaning and initial analysis. However outcomes were measured using self‐report questionnaires. |
| Incomplete outcome data (attrition bias) | Low risk | Overall loss to follow up low (IG: n=3/63, 5%; CG: n=8/62, 13%). Reasons for loss to follow up reasonably balanced across the two groups considering the low numbers. |
| Selective reporting (reporting bias) | Low risk | On comparison with the WHO ICTRP trial registration, all key breastfeeding outcomes appear to be reported. |
| Other bias | Low risk | No industry funding. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of the article. No significant baseline imbalance observed between groups. |
| Study characteristics | ||
| Methods | 2‐arm RCT, n = 522 | |
| Participants | A large metropolitan hospital in Houston, Texas, USA, serving predominantly immigrant Hispanic women (85% monolingual Hispanic) Background rates of breastfeeding initiation were high in this study population. Inclusion criteria: mothers of low‐risk infants, mixed feeding in hospitals, had telephones and access to transportation Exclusion criteria: infants with elevated risk for hyperbilirubinaemia (preterm, discharged at < 48 h old, jaundice within 24 h of birth, Rhesus‐incompatibility, cephalohematoma, positive Coombs test, family history of disorders of red blood cell enzyme defects, or defects of red blood cell shape and size) Participant characteristics Mean maternal age: intervention group 26.8 years, control group 27.1 years Mean parity: intervention group 1.5, control group 1.5 Mothers born in the USA: intervention group 2.8%, control group 1.1% (most of the women were born in Mexico or Central America) | |
| Interventions | Intervention: mothers were given an appointment to visit the hospital‐based breastfeeding clinic at 3‐7 days postpartum. At the breastfeeding clinic, peer counselling sessions included a breastfeeding history, breast examination, infant oral‐motor assessment, measurement of infant weight, evaluation of latch and milk transfer, and discussion of the mother's concerns and support system. Additional visits and telephone consultations were provided if deemed necessary by the mother and the clinic staff. Women who missed appointments received a telephone call. Control: received usual care, which included bedside breastfeeding assistance before hospital discharge and the phone number of the hospital breastfeeding clinic with instructions to call if needed. | |
| Outcomes | Primary: exclusive breastfeeding at 1 month Secondary: volume of formula given by mothers who were mixed feeding, and incidence of breastfeeding problems | |
| Notes | Dates of study: recruitment between January and December Funding sources: American Association of Medical Colleges; United States Declarations of interest: nt reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by random number table.
|
| Allocation concealment (selection bias) | Low risk | After participants had given informed consent, the group was determined using opaque, sealed envelopes containing assignments generated by random table number. The envelope was opened by the mother. |
| Blinding of participants and personnel (performance bias) | High risk | Participants were not blinded as the envelope was opened by the mother. Caregivers were also not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | For outcome assessors, outcomes were determined by telephone survey at 4 weeks postpartum by interviewers blinded to group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | 522 randomized (255 in intervention group and 267 in control group. 55 women were lost to follow‐up at 4 weeks (10.5%). Loss was balanced in the 2 groups. Issues around incomplete data were addressed. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol so could not judge this. |
| Other bias | Low risk | There were slight baseline differences between the control group and the experimental group. Women in the intervention group were more likely to have an emergency caesarean, and were taller. Due to low compliance with the intervention, secondary analysis was carried out, but we have reported data from the primary analysis (unadjusted). |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 228 | |
| Participants | Inclusion criteria: healthy pregnant women aged between 20 and 35 years, first‐time mother with a singleton pregnancy from 26 to 29 weeks of gestation and pre‐pregnancy BMI <25.0 kg/m2. Background breastfeeding rates: not reported. Exclusion criteria: smokers, known to be allergic or intolerant to any ingredient in the supplement, had known gestational diabetes and/or a diagnosis of pre‐eclampsia, or adverse maternal or fetal conditions that could have potential effects on child’s growth and/or development. Women and their infants were also discontinued from the study if the infant was preterm defined as gestational age <37 weeks, had a birth weight < 2500 g, or had conditions for the mothers or infants after delivery, which required intensive care admission. | |
| Interventions | Two servings of micronutrient supplements daily starting from the last trimester until12 weeks postpartum, and breastfeeding support consists of one prenatal breastfeeding class, one breast‐feeding consultant visit within 48‐h of delivery, one telephone call at one week postpartum, and one face‐to‐face follow‐up session at week 4 postpartum. Control: continued to take folic acid and iron supplement until delivery and received breastfeeding advice during pre‐natal visits only if this was part of standard of care at the sites. Breastfeeding advice consisting of messages on the benefits of breastfeeding and the encouragement of EBF during the first 6 months was conducted by health care staff or health workers. Breastfeeding promotion as a part of standard of care was also provided through social media such as radio and television. | |
| Outcomes | Exclusive breastfeeding at 1, 2 and 3 months | |
| Notes | Dates of the study: October 2013 and April 2015 Funding sources: Abbott Nutrition was responsible for the study design, monitoring, data analysis and manuscript preparation and submission. Declarations of interest: authors are employees of Abbott Nutrition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization schedules were computer‐ generated using a pseudo‐random permuted blocks algorithm. |
| Allocation concealment (selection bias) | Low risk | For each study site, sealed envelopes containing the study group assignment were prepared for both mother and her infant |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data from unblinded participants |
| Incomplete outcome data (attrition bias) | Low risk | For all time points: Intervention loss to follow‐up = 9% Control loss to follow‐up = 12% Intention to treat analysis |
| Selective reporting (reporting bias) | High risk | There are outcomes reported in the paper that are not in the protocol: infant's breast milk intake and maternal energy and macronutrient intake. There are outcomes in the protocol that are not reported in the paper: lactation assessment and infant anthropometrics at 4,8,12 weeks (instead the growth over 12 weeks is presented. Breastfeeding rate is reported as per protocol. A follow‐up paper by Zhang include additional outcomes on child neurodevelopment not detailed in the protocol. |
| Other bias | High risk | Abbott Nutrition was responsible for the study design, monitoring, data analysis and manuscript preparation and submission. |
| Study characteristics | ||
| Methods | 2‐arm cluster‐controlled trial, n = 1267 | |
| Participants | Primary Care Trust health district (PCT) in Birmingham, UK Background rates of breastfeeding initiation: 58%, with continuation rates poorly collected, but considered to be lower than national average Inclusion criteria: all pregnant women registered with a GP within the PCT, with an estimated delivery date between 1 February 2007‐31 July 2007 Exclusion criteria: none stated | |
| Interventions | Intervention (n = 1267): antenatal peer support offered to all women in intervention clusters to encourage breastfeeding initiation, and postnatal peer support for women who initiated breastfeeding to increase continuation. Community peer support workers were employed and trained by the breastfeeding personnel in the PCT in line with WHO/UNICEF Baby Friendly breastfeeding management course. Antenatal support was aimed to be 2 support sessions (at least 1 at home, although almost all actually took place in the clinic/Children’s Centre setting). The support workers were informed when the women were discharged from hospital so that they could contact and visit them within 24 h–48 h. Further contact would be needs‐based, but with a minimum of 1 more contact in the first week. Additional needs‐based contacts could be by telephone or home visits. Number randomised = 1267 (416 consented to follow‐up at 6 months, and 271 of these responded at 6 months) Control (n = 1370): routine maternity care. Number randomised = 1370 (432 consented to follow‐up at 6 months, 301 responded at 6 months) | |
| Outcomes | Any breastfeeding and exclusive breastfeeding at 10–14 days, 6 weeks and 6 months and 6 months | |
| Notes | The numbers randomised in this paper do match the MacArthur paper which states: Intervention: n = 1140 deliveries Women with data on initiation of breastfeeding n = 1083 Women who initiated breastfeeding n = 747 Comparison: n = 1371 deliveries Women with data on initiation of breastfeeding n = 1315 Women who initiated breastfeeding n = 896 Dates of study: Recruitment of women with an estimated due date between February 1st 2007 and July 31st 2007. Funding sources: The Heart of Birmingham Teaching Primary Care Trust. Authors KJ, CMacA, LI are part‐funded by the National Institute for Health Declarations of interest: the authors from HoBtPCT (RH,JB,JC) were involved in the employment and management of the peer support workers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Practices were "randomized by the trial statistician with stratification by midwifery team and numbers of deliveries per clinic". Unclear how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No details provided to allow us to assess this. |
| Blinding of participants and personnel (performance bias) | High risk | Women were aware of allocation at recruitment. |
| Blinding of outcome assessment (detection bias) | High risk | Women’s options for 6 month follow‐up were by postal questionnaire in English, or by telephone in their language of choice by a researcher blinded to the trial allocation. Unclear whether the questionnaire would have contained identification related to allocation. |
| Incomplete outcome data (attrition bias) | High risk | When based upon the number of women who consented to be followed up at 6 months, the follow‐up was 69.7% in the intervention group and 65.1% in control group. When based on the number actually in the clusters this number is 21.4% in intervention group and 20.5% in the control group. |
| Selective reporting (reporting bias) | Low risk | Outcomes in paper match those prespecified in ISRCTN registry. |
| Other bias | Low risk | None identified |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 64 | |
| Participants | Prenatal outpatient department of a tertiary hospital in Wuhan, central China. Background breastfeeding rates: Previous breastfeeding research in Wuhan showed a 17.6% exclusive breastfeeding rate at 6 months for women who accepted standardised care. Inclusion criteria: pregnant women aged 20 years or older; 32 to 34 weeks’ gestation; primigravida with single fetus; available for a 6‐month postpartum period following delivery; read and understood Mandarin; and resided with important family members (infant’s father and grandmother/grandmother‐in‐law). Exclusion criteria: women with severe physical diseases (hepatitis B virus, active tuberculosis, human immunodeficiency virus, mammary defect/deformity) and/or mental illness and suspected fetal congenital malformations (Trisomy 21, cleft lip/palate, hydrocephalus, and heart disease). | |
| Interventions | Two prenatal breastfeeding education lectures, three home visits during the 1st month postpartum when fathers and grandmothers were present in the home, and eight telephone calls or text messages with video/audio interactions every 2 weeks from 2 to 6 months postpartum by a researcher who had completed 90 hours of education in human lactation and breastfeeding. Participants in the intervention group could discuss breastfeeding‐related issues at any time via text messaging/WeChat and had access to information on Internet/public information platforms. Control: received in‐hospital care and one follow‐up at 14 days postpartum by community nurses after discharge. | |
| Outcomes | Any and exclusive breastfeeding at 1,3,6 months | |
| Notes | Dates of the study: Recruited August to September 2015. Funding sources: the authors received no financial support for the research, authorship, and/or publication of this article. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | They were then assigned to either the intervention group or control group using random numbers without blinding. This part may have a low risk of bias although more detail would add more certainty. |
| Allocation concealment (selection bias) | High risk | They were then assigned to either the intervention group or control group using random numbers without blinding. We assume 'without blinding' in this context means lack of concealment ‐also authors describe the study as 'quasi‐experimental |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Low and balanced loss to follow‐up: Overall 5/64 (7.8%); Intervention group: 3/32 (9.4%); Control 2/32 ( Drop‐off symmetrical and within 10% Intervention group 29/32 = 9% Control group 30/32 = 6% |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available to judge this outcome. |
| Other bias | Low risk | No industry funding The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. No baselineline imabalance |
| Study characteristics | ||
| Methods | Cluster RCT, 14 clusters. | |
| Participants | Korogocho and Viwandani slums in Nairobi. Background breastfeeding rates: Using data obtained through a parallel observation study on comparable women who gave birth in the surveillance area, but were not recruited into this study,EBF rates for 6 months were about 3%. In the urban slums of Nairobi, a study conducted in 2007 found that barely 2% of infants were exclusively breastfed for the first six months. Inclusion criteria: pregnant girls and women aged between 12 and 49 years, who were resident within the defined study area. Girls aged 12– 14 years were included because close to 10% of girls below 15 years are sexually active. Exclusion criteria: recruited women who gave birth before receiving the intervention; women with disability that would make delivery of the intervention difficult e.g. intellectual impairment, or who bore a child with a disability that would make feeding difficult; women who lost the pregnancy and/or had a still‐birth after being recruited in the intervention; and pregnant women who were lost to follow‐up before they delivered. | |
| Interventions | Home‐based nutritional counselling during scheduled visits by community health workers trained to provide specific maternal infant and young child nutrition messages and standard care. Personalised home‐based nutritional counselling of women from the time of recruitment until the baby attained one year. Nutritional counselling messages encompassed maternal nutrition, immediate initiation of breastfeeding after birth, breast positioning and attachment, exclusive breastfeeding, frequency and duration of breastfeeding, expressing breast milk, storage, handling and feeding of expressed breast milk and lactation management. It also included age‐appropriate complementary feeding. Counselling was also informed by the stages of change model. Control: community health workers were trained (through the regular government facilitated training) on standard care, which included antenatal and postnatal care, family planning, water, sanitation and hygiene, delivery with skilled attendance, immunisation and community nutrition. Optimised standard care by ensuring that the intended standard care happened. | |
| Outcomes | Exclusive breastfeeding at 2,3,6 months | |
| Notes | Dates of the study: recruitment September 2012 to February 2014. Funding sources: Wellcome Trust, Grant 097146/Z/11/Z. Core funding for APHRC from The William and Flora Hewlett Foundation and the Swedish International Cooperation Agency (SIDA); and funding for the NUHDSS from the Bill and Melinda Gates Foundation. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization of the community units (CUs) to the intervention or control arm was computer‐generated by a data analyst who was not a primary member of the study team |
| Allocation concealment (selection bias) | Unclear risk | Authors suggest that the data analyst was concealed from group allocation, as they were not a primary member of the study team; however, unclear if participating cluster units or study team enrolling clusters were concealed from group allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, blinding of participants and personnel was not feasible. |
| Blinding of outcome assessment (detection bias) | High risk | Data was collected from mothers who were not blinded |
| Incomplete outcome data (attrition bias) | High risk | None of the 14 clusters dropped out of the study. However, overall exclusion/loss to follow‐up of mothers from recruitment to final follow up at infant age 6 months was 42.1% (IG: 356/771, 46.2%; CG: 299/784; 38.1%). Unclear if reasons for missing outcome data were balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Key breastfeeding outcomes reported in the paper are outlined in the published protocol. Trial registration and protocol publication were retrospective. |
| Other bias | Low risk | No industry funding reported. The authors had no competing interests to declare. No baseline imbalance in socioeconomic and demographic characteristics of mothers observed across the groups. Sample size calculation took into account clustering of women in the community units. The cluster study design was also accounted for in the analyses. |
| Study characteristics | ||
| Methods | 2‐arm Cluster RCT, 13 clusters, n = 823 | |
| Participants | 13 community units in Koibatek/Eldama Ravine sub‐county, one of the six sub‐counties in Baringo County located in the North Rift region of Kenya. Background breastfeeding rates: In Kenya, EBF rates are at 61%. In 2014, the median duration of EBF in the Rift Valley was 3.1 months and half of women delivered at home. The EBF rate for under 6 months in 2015 was 37%. Inclusion criteria: any pregnant woman (15–49 years) in their first or second trimester residing in the 13 community units who intended to stay for 6 months or more after delivery. Exclusion criteria: after initial recruitment, participants were excluded from the analysis if they declined to participate in the study, did not receive a visit by a community health volunteer before delivery, migrated out of the study area or had a miscarriage or a still birth. | |
| Interventions | The package aims at equipping primary healthcare workers with skills to empower mothers to optimally breastfeed and feed their infants and young children. The primary healthcare workers and the community health visitors were provided with counselling aids that included brightly coloured cards depicting key infant and young child feeding concepts and behaviours to share with mothers, fathers and other caregivers. The package was adapted to include maternal nutrition and health counselling messages such as good maternal nutrition and early antenatal care, importance of EBF and the key processes including early initiation of breastfeeding, feeding of colostrum and attachment and positioning. Mothers were also counselled on ways of preventing mother to child transmission of HIV, solving breastfeeding difficulties and obtaining family support. Twenty‐three mother‐to‐mother support groups were also established. Members of these support groups met every month for peer support. They consisted of pregnant and breastfeeding mothers who came together to learn about and discuss issues regarding pregnancy, infant and young child nutrition and other health related issues. Each support group had 9–15 members. When the membership exceeded 15, a new mother support group was established. Some groups also incorporated partners and spouses of the women, traditional herbalists and grandmothers. Control: Standard care package, including routine services offered to mothers and their children through the healthcare system (information materials on nutrition and infant feeding, standard counselling on antenatal and postnatal care, appropriate tests during pregnancy, health facility delivery, general nutrition, hygiene and immunisation). Routine visits from community HVs. Mothers also received information on hygiene practices, breastfeeding and complementary feeding. | |
| Outcomes | Exclusive breastfeeding at 2 and 6 months | |
| Notes | Dates of the study: April 2015 to May 2016. Funding sources: British Academy, Grant/Award Number: MD120048; Wellcome Trust, Grant/Award Numbers: 208791/Z/17/Z, 097146/Z/11/Z; National Institutes of Health (NIH) and United States Agency for International Development (USAID) administered through the National Academy of Sciences (NAS), Grant/Award Number: PGA2000003677/8. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence of allocation of the CUs to either BFCI or control group was computer‐generated and was performed by a biostatistician, who was not a member of the research team. |
| Allocation concealment (selection bias) | Unclear risk | No detail provided on allocation concealment of community units to clusters |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, it was not feasible to blind personnel. Women were not told of the different treatment groups, but it is unlikely they were blind to the interventions. |
| Blinding of outcome assessment (detection bias) | High risk | The investigators were aware of the interventions given to the experimental groups. Self‐report of breastfeeding outcomes were used. |
| Incomplete outcome data (attrition bias) | High risk | No clusters withdrew from the trial; however there was >20% attrition at the 3rd and final follow up of participants (IG: 145/351, 41.3%; CG: 258/472; 54.7%). Unclear if reasons for loss to follow up were balanced across the groups. |
| Selective reporting (reporting bias) | Unclear risk | During subsequent follow‐ups, a 3‐day dietary recall was used because by omission; the translated tool did not have the 24‐h recall section. EBF was determined using two variables: (1) what foods has the child been offered over the last 24 h and (2) has the child been given solids/liquids other than breast milk. The trial registration record was checked and key breastfeeding outcomes seem to be reported in this paper. However, it is unclear how much the change in outcome measurement and the timing might have caused bias. |
| Other bias | Low risk | No industry funding reported. The authors declared that they had no conflicts of interest. No imbalance at baseline across group. No clusters withdrew. Clustering was accounted for in the analysis. |
| Study characteristics | ||
| Methods | 2‐arm cluster‐randomised study with 10 sites, divided into 2 groups, which had similar numbers of births and breastfeeding rates. Recruitment December 2000‐December 2002, n = 781, 408 women in sites assigned to the intervention and 373 in sites assigned to the control | |
| Participants | Child healthcare centres in Limbourg province, the Netherlands Background rates of breastfeeding initiation: high Inclusion criteria: mothers applying for maternity care at any of the 10 centres Exclusion criteria: birthweight < 2000 g Ethnic composition not defined. Baseline prevalence of breastfeeding initiation was 80% in the Netherlands in 2002. | |
| Interventions | Intervention: programme with 3 elements: structured health counselling by maternity and child healthcare nurses and physicians; booklet to transfer information between caregivers and between mother and caregivers and used at each consultation; lactation consultancy available via caregiver faxing consultant with details of problem (LC would then contact the caregiver or mother within 24 h of receiving the fax). Control: not specified | |
| Outcomes | Exclusive and complementary breastfeeding rates at 3 months; determinants of breastfeeding at 3 months | |
| Notes | Dates of study: recruitment between December 2000 and December 2002. Funding sources: The National Prevention Program of ZONMw from the Netherlands Organization for Health Research and Development. Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By coin flip |
| Allocation concealment (selection bias) | Low risk | Clusters were randomized after sites were paired for similarity of breastfeeding rates and the number of births in each centre. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided about whether women and personnel were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | No details provided about whether the caregivers (who collected some of the data) or those responsible for conducting the questionnaires were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 20 centres and 781 women were randomized. Data available for 701 for the first follow‐up (90%) and 683 (87%) at 6 months postpartum. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Low risk | Analyses adjusted for cluster effect by multi‐level analysis. No baseline imbalance apparent. |
| Study characteristics | ||
| Methods | Multi‐site cluster‐randomised study, recruitment period 19 months, n = 17,046 | |
| Participants | Urban and rural sites within Belarus Background rates of breastfeeding initiation: high Inclusion criteria: intention to breastfeed, healthy mother, child ≥ 2500 g at term, Apgar ≥ 5 at 5 min Baseline breastfeeding prevalence 50% at 3 months | |
| Interventions | Intervention: WHO/UNICEF BFI training for all staff dealing with mothers and babies in hospitals and community polyclinics. Infants seen monthly for polyclinic well‐child visits and whenever ill. Control: staff did not receive the training | |
| Outcomes | Any breastfeeding at 3, 6, 9 and 12 months Incidence of respiratory, gastrointestinal infection, and atopic eczema in first year | |
| Notes | Dates of study: recruitment between June 1996 to the end of December 1997 Funding sources: The Thrasher Research Fund, the National Health Research and Development Program (Health Canada), UNICEF and the European Regional Office of WHO. Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomised trial with double randomisation procedure. Random number tables were used to ascribe numbers to sites and higher/lower numbers were used to allocate sites to A or B interventions. Then later, in public, a coin flip was used to determine whether A or B would be intervention or control sites. |
| Allocation concealment (selection bias) | Low risk | 2‐stage randomisation procedure |
| Blinding of participants and personnel (performance bias) | High risk | Personnel working in the hospital were not blinded. It was not stated whether the women were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | The paediatricians carrying out the interventions were aware of the status of the study infants. An audit was carried to assess data validity, but it was not clear what this identified. |
| Incomplete outcome data (attrition bias) | Unclear risk | 34 sites randomized, 2 of which refused to carry out allocated intervention and 1 clinic falsified outcome data and was excluded. 31 sites contributed data. In addition, follow‐up data were missing for 3.3% of women. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this |
| Other bias | Unclear risk | A steering group ensured that "control sites did not institute any changes that would render their maternity hospitals or polyclinics more baby friendly". Analysis took account of cluster design. Groups appeared similar at baseline. |
| Study characteristics | ||
| Methods | Cluster‐randomised, 2‐community‐based trial; 22 municipalities randomised to intervention and control clusters n= 109 Health visitors (HVs) and 1588 women | |
| Participants | Western Denmark, urban and agricultural areas Background rates of breastfeeding initiation: high. The 5 hospitals serving the area had adopted UNICEF/ WHO Baby Friendly Hospital Initiative standards, and 3 of the 5 were accredited at the time of the study. Inclusion criteria: Danish mothers who lived in the 22 municipalities and gave birth to a single child with gestational age of ≥ 37 completed weeks Participant characteristics: 36% primiparous, 7.5% multiparous with previous short breastfeeding experience | |
| Interventions | Usual care included hospital care at hospitals working to Baby‐Friendly standards, and an existing Health Visitor service in all municipalities. Intervention cluster: 1‐3 structured home visits within the first 5 postnatal weeks from Health Visitors with additional training. Main topics for the first visit were effective breastfeeding technique and learning to know the baby; for the second visit, self‐regulated feeding and interpretation of the baby's cues; for the third visit, sufficient milk and interaction with the baby. Mothers were also given a booklet about how to breastfeed and how to read the baby's cues. Control cluster: Health Visitors' usual practice consisting of 1 or more non‐standardised visits | |
| Outcomes | Duration of exclusive breastfeeding and mother’s satisfaction with breastfeeding | |
| Notes | The authors did not adjust for cluster design effect. In our data and analysis tables we have adjusted the sample size and event rates to take account of the design effect. We calculated an effective sample size by dividing figures by the design effect – calculated using the ICC for breastfeeding cessation given in the paper: ICC = 0.02. Dates of study: intervention ran from February 2006 to August 2006 Funding sources: The Health Insurance Foundation, The Lundbeck Foundation and The Counties od Ribe and Ringkjobing in Denmark Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 22 clusters were stratified according to their number of births the year before the trial, and within 3 strata, 11 municipalities were randomized to the intervention group and 11 to the comparison group. The randomisation was computerised and done independently of the investigators. |
| Allocation concealment (selection bias) | Low risk | As for the sequence generation |
| Blinding of participants and personnel (performance bias) | High risk | Participants and caregivers were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | The health visitors provided the mothers with questionnaires which appear to have been self‐completed as the mothers were asked to return the questionnaire in a stamped addressed envelope. The identity of the health visitors was blinded to the investigators and it is not clear whether this partial blinding was effective. |
| Incomplete outcome data (attrition bias) | Unclear risk | 22 municipalities were randomized. 2186 women had babies during the study time period. 1760 women were followed up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Reported that there were no significant differences between groups at baseline. This was a cluster‐randomised trial and authors stated they did not make allowance for clustering in the sample size calculation as the cluster effect was expected to be small. Elsewhere in the paper an ICC value was provided, which the authors said indicated that cluster effect was small. It was not clear that the cluster design effect was taken into account in any of the analyses. |
| Study characteristics | ||
| Methods | 2‐arm RCT | |
| Participants | Inclusion criteria: pregnant women more than 32 weeks’ gestation, healthy, delivery of full term healthy infants, no disease or contraindications to breastfeeding, no nipple abnormalities, and infants who had no sucking problems. They also had to be able to communicate with others, and had to have a telephone line. Exclusion criteria: pregnant women with high‐risk and multifetal pregnancies | |
| Interventions | Knowledge sharing practices with empowerment strategies (KSPES programme). Women received the KSPES programme via antenatal classes. This involved discussing knowledge, storytelling, sharing experiences and thoughts. Programme was based on Gibson’s theory. Women also received postnatal support strategies at days 7, 14 and months 1, 2, 3, 4, 5,6. Intervention was delivered by the Principal Investigator who seems to be a HCP. Control/Comparison intervention: Two routine antenatal check‐ups where women received breastfeeding education. Women received follow‐up at 1,24 and 6 months at the Paediatrics clinic. | |
| Outcomes | Any and exclusive breastfeeding at 1,2,3 and 6 months | |
| Notes | Study dates: Bbtween January and March 2009 No sponsorship details given No conflicts of interests declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization scheme was generated using a random number table |
| Allocation concealment (selection bias) | Low risk | The co‐investigator generated the allocation sequence, and principal investigator enrolled participants and assigned participants to their groups. When a woman met the present study inclusion criteria, the principal investigator picked up a sequentially numbered opaque envelope that contained a ticket identifying the group. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data. Not possible to blind. |
| Incomplete outcome data (attrition bias) | Low risk | I: 93% follow‐up C: 85% follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol available to judge item |
| Other bias | Unclear risk | No conflicts of interest. Funding source not described |
| Study characteristics | ||
| Methods | 2‐arm, cluster RCT, 190 communities. | |
| Participants | Three districts in Al Hodeidah governorate, Yemen. Background breastfeeding rates: not reported. Inclusion criteria: The Cash for Nutrition programme targeted female relatives of the mostly male and elderly beneficiaries of the Social Welfare Fund (Yemen's main social protection programme) who had children under 2 years or were pregnant at the time of enrolment in January 2015. Exclusion criteria: Not reported. | |
| Interventions | Cash for Nutrition participants received monthly cash transfers of 10,000 Yemeni riyals (25% of the value of average monthly food spending) conditional on attendance at monthly nutritional training sessions led by locally recruited community health volunteers. Cash transfers were not labelled for any specific purpose, most participants reported using them for food, medical expenses, and debt repayment. The conditionality was not strictly enforced. Community health volunteers met individually with mothers who were unable to attend the training sessions to find solutions or give individual training sessions. Monthly nutrition and health education sessions for participant women included training on EBF until 6 months, complementary feeding from 6 to 24 months, nutritious meals, handwashing, treatment of drinking water, and how to treat diarrhoea. Additional quarterly sessions targeting pregnant and lactating women covered breastfeeding initiation and the importance of colostrum as well as the consequences of chewing qat and smoking during pregnancy. Monthly education sessions, the community health volunteers carried out quarterly screening sessions during home visits to detect and refer cases of malnutrition to health centres for treatment. Control: Control households remained untreated. In spite of the randomisation, some control households benefitedfrom the programme and self‐reported being part of the programme at the follow‐up survey. Of the households assigned to control, 220 (23%) reported having been enroled in the programme, likely due to local programme administration including them by mistake or due to pressure from the household. | |
| Outcomes | Exclusive breastfeeding in all children under 6 months | |
| Notes | Dates of the study: enrolment in January 2015. The programme was suspended January to September 2016 due to the conflict and resumed in October 2016 to August 2017. Funding sources: UNDP; World Bank Group; Gesellschaft für Internationale Zusammenarbeit (GIZ); CGIAR Research Program on Policies, Institutions, and Markets; Nordic Trust Fund. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clusters were randomly selected within three strata based on district in a 2:1:1 ratio, resulting in a sample that was half peri‐urban (Lusaka district) and half rural (Chongwe and Kafue districts). A statistician unrelated to the study allocated half of the clusters in each district to intervention or control (no intervention, standard care at clinics) using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was done before baseline data collection took place, but cluster allocation was concealed from the study team until after baseline data had been collected. |
| Blinding of participants and personnel (performance bias) | High risk | Participants could not be masked to the intervention. |
| Blinding of outcome assessment (detection bias) | High risk | While authors suggest outcome assessors were blind to group allocation, breastfeeding outcomes were self‐report by the women who were not blind to group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | None of the clusters were lost to follow up. It should be noted that the baseline and endline surveys were conducted with two independent, population‐representative samples within clusters; hence, no attrition due to the cross‐sectional nature of each survey. |
| Selective reporting (reporting bias) | Unclear risk | The clinicaltrials.gov trial registration record suggests no selective reporting |
| Other bias | High risk | 16 clusters randomised, with 16 clusters participating in baseline and endline surveys. ICC was calculated for each behavioural outcome. Clusters were accounted for in the analysis. No industry funding. Declaration of interests transparent, with statement that three authors have received funding from Unilever, a soap manufacturer. None of their time while working on this study was funded by Unilever. Baseline profiles of participants similar, but there were differences between the study groups in both study profiles with respect to educational level, employment status, prevalence of shared sanitation, awareness of zinc, and use of ORS and zinc. Unclear if this is a source of bias. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, recruitment October 2001‐May 2002, n = 231 | |
| Participants | Setting: the maternity section at the Chambery Teaching hospital in Chambery, France Background rates of breastfeeding initiation: intermediate. Breastfeeding prevalence rates were 70.8% in hospital and 58.1% at 1 month of infant age. Inclusion criteria: mothers of healthy singleton infants (gestational age: > 37 completed weeks), breastfeeding on the day of discharge and consenting to participate in the study. Exclusion criteria: infants admitted to neonatal unit, mothers transferred to ICU, mothers < 18 years old, living outside the area, unable to speak French, or unable to complete follow‐up monitoring because of psychosocial problems such as homelessness Participant characteristics: Age: mean (SD): intervention: 29.3 years (4.1); control: 29.7 years (4.8) Education beyond high school graduate level: intervention: 87 (75.0); control 84 (73.0) White collar worker: intervention: 92 (79.3); control 87 (75.6) Primiparous: intervention: 58 (50.0); control: 63 (54.8) | |
| Interventions | Intervention (n = 116): in addition to standard care, mothers were invited to an outpatient visit in a primary care physician’s office within 2 weeks of the birth to see a primary care doctor who had received special breastfeeding education. Topics covered included general health assessment, lactation physiology, feeding position and latch on assessment, management of common lactation problems (nipple pain, nipple cracks, sore nipples, mastitis, and maternal concern regarding low milk supply), management of infant problems (insufficient weight gain, breastfeeding jaundice, diarrhoea and dehydration), maternal medication use while breastfeeding and sources of support. The physicians' training programme was delivered through lectures, panel discussions, role playing exercises and printed educational materials. Control (n = 115): standard care; mothers received verbal encouragement from maternity ward staff to maintain breastfeeding. On discharge, the infant was examined by the paediatrician working in the department, for a general health assessment and an evaluation for evidence of successful breastfeeding behaviour. The mothers were also provided with the telephone number of a peer support group that they could call to ask questions and request help. The postdischarge follow‐up monitoring consisted of routine, preventive, outpatient visits in a primary care physician’s office at 1, 2, 3, 4, 5 and 6 months of infant age. | |
| Outcomes | Primary: exclusive breastfeeding at 4 weeks (exclusive breastfeeding defined as giving maternal milk as the only food source, with no other liquids or foods) Secondary: any breastfeeding at 4 weeks, median duration of breastfeeding, breastfeeding difficulties and maternal satisfaction with the infant feeding experience | |
| Notes | Dates of study: recruitment between between October 1, 2001, and May 31, 2002 Funding sources: Grants from the Union Professionnelle des Me´decins Libe´ raux de la Region Rhone Alpes (Lyon, France); the De´le´gation Re´gionale a la Recherche Clinique, Centre Hospitalier Universitaire (Grenoble, France). J.L. was supported by a grant from the Egide Foundation (Program Lavoisier). Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was generated by the statistical adviser of the study with random permuted blocks with a block size of 8. |
| Allocation concealment (selection bias) | Low risk | The randomisation assignments were unknown to any of the investigators and were concealed in consecutively numbered, sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes were assessed using self‐completed questionnaires, however, it was not stated whether the investigators analysing the data were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | (1080 women assessed for eligibility, 849 deemed not eligible) 231 women randomized, outcome data were available for all but 5 of the woman randomized, and a sensitivity analysis was carried out where the most conservative values were assumed for those women lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not judge this |
| Other bias | Unclear risk | The majority of women assessed were not eligible for inclusion in this trial and so the results may not be generalisable. |
| Study characteristics | ||
| Methods | 2‐arm, RCT, single site, n = 472 | |
| Participants | Ottawa, Canada Background breastfeeding rates: not stated Inclusion criteria: women ≥ 18 years, with no diagnosed medical problems, with a healthy singleton infant at a gestational age of over 36 weeks and 6 days who were breastfeeding their baby and intended to continue upon discharge, and could be contacted by phone or email after hospital discharge Exclusion criteria: women who did not speak English or French, were unable to present to the clinic (transport not available), with multiple, preterm or adopted babies, with no plan or desire to breastfeed, women who had breast surgery or a psychological risk that might impede their ability to attend the first appointment at the clinic. Out‐of‐province women were also excluded given the geographic distance and difference in social services. | |
| Interventions | Intervention (n = 315): within 48 h of discharge, women attended the postpartum clinic. Clinic staff followed up with participants if they failed to keep the mandatory follow‐up appointment. The first appointment included maternal assessment and care (e.g. wound care, prescriptions), neonatal care (e.g. weight gain assessment, jaundice screening), blood work including total serum bilirubin (TSB), and breastfeeding assessment and support. Family physicians were available for on‐site consultations in the mornings, and LCs and registered nurses were at the clinic throughout the day. Additional follow‐up visits were offered to participants as clinically indicated and as many times as they desired up to a maximum of 6 weeks following the birth of their baby. Control (n= 157): after hospital discharge, participants were entitled to receive follow‐up care and seek breastfeeding support currently available in the community (e.g. through their family doctor, Public Health Unit or private services), but could not attend the postpartum clinic. | |
| Outcomes | Primary: exclusive breastfeeding at 12 weeks post birth (additional breastfeeding information regarding partial breastfeeding, expressed breast milk and formula feeding was also collected) Secondary: Mother Satisfaction Survey Breastfeeding self‐efficacy Postpartum depression Use of healthcare resources | |
| Notes | All phases of this study were supported by the Ontario Ministry of Health and Long‐Term Care. Dates of study: recruitment between January and July 2014. Funding sources: Ontario Ministry of Health and Long‐Term Care Declerations of interest: Nnne declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group designation was given from a randomisation list, generated using a permuted randomized block design, with permutation block sizes of 3, 6, and 9 units, prior to study initiation by an external statistician. |
| Allocation concealment (selection bias) | Low risk | Study researchers, recruiters, and participants were blinded to the randomisation allocations prior to patient randomisation and enrolment into the trial. |
| Blinding of participants and personnel (performance bias) | High risk | Women were informed of their randomisation group. Clinicans were also aware of group allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Study staff were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition to 6 months was 14% in the intervention group and 12% in control group. |
| Selective reporting (reporting bias) | High risk | Breastfeeding at 24 weeks is not identified as an outcome in the paper or the protocol, but was reported in table 4. |
| Other bias | Low risk | No baseline imbalance No industry funding No conflicts of interest |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 1003. Participants recruited from 8 public health maternity units, duration of recruitment 6 months | |
| Participants | Urban Brazil Background rates of breastfeeding initiation: high Inclusion criteria: healthy babies, weighing < 3000 g Exclusion criteria: twins, important health problems in mother or child | |
| Interventions | Peer counsellor home visits lasting 30‐40 min at 5, 15, 30, 60, 90 and 120 days. Counsellors were from same social group as women they supported, had personal experience of breastfeeding and had been associated with maternity unit milk bank for a minimum of 5 years. Trained with adapted WHO breastfeeding counselling course (20 h). Paid BRL4 per visit. Each counsellor supported 25 mothers. | |
| Outcomes | Rates of exclusive, predominant, partial and artificial feeding at 4 months | |
| Notes | This is the only study in this review that targeted babies with birthweight below 3000 g. We considered excluding it from this review as the paper did not state the babies had to be born at term and did not specify a lower limit for birthweight. However, as the babies had to be 'free of important health problems' we considered them to be healthy and therefore included this study. Dates of study: data were collected between November 1996 and April 1997 Funding sources: National Institute of Food and Nutrition (INAM—Ministry of Health), the Public Health School of Ceara´ and by the Division of Child Health and Development of the World Health Organization (WHO). Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Study secretary opened a sealed envelope that contained the study code. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Did not describe whether mothers, lay workers or health professionals were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Authors state that the "interviewers had not had any prior contact with the mothers and were also unaware as to the objectives of the research (blinding)". |
| Incomplete outcome data (attrition bias) | Unclear risk | 1003 women randomized. 14% lost to follow‐up by the end of 4 months. Reasons for loss to follow‐up were not described but the loss appeared balanced across the 2 groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Low risk | No baseline imbalance apparent. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 59 | |
| Participants | Kentucky, USA. Background breastfeeding rates: not reported. Inclusion criteria: self‐identify as Immigrant Hispanic women; pregnant at or beyond 30 weeks of gestation; intention to at least try to breastfeed; planning to deliver at a local birthing hospital; and planning to remain in the area for at least 6 months after the birth of their child. There were no age limits for participation. Exclusion criteria: prior or current participation in any study to enhance BF; pregnant with twins; history of breast surgery; contraindication to BF (e.g., HIV‐positive status, chronic therapy with medications incompatible with BF, alcohol dependence or other substance abuse); and presumed or known congenital fetus defects. | |
| Interventions | Intensive peer counselor/professional support (1–2 prenatal visits, one in‐hospital visit, two home postpartum visits, and pre‐/post‐natal follow‐up phone calls as needed) conducted individually with each mother until six months after the birth of the infant. Key components of the intervention were: Informational material with the goals of 1) raising consciousness—through seeking and processing awareness of benefits of adopting a healthy behaviour (i.e., EBF for six months, delaying introduction of solid food) or discontinuing a risky behaviour (e.g., supplement with formula, infant solid food before six months); 2) anticipating barriers to EBF—perceptions concerning the unavailability, inconvenience, difficulty, or time‐consuming nature of the action; and 3) promoting self‐efficacy. BF self‐efficacy was acknowledged and referred to beliefs about being able to carry out progressively more demanding levels of EBF, and to overcome barriers to engage in the behaviour (empower). Second, individual home‐visit sessions were designed to increase trust to enhance the benefits of change (e.g., reinforcing motivation, benefit and positive outcomes derived from the behaviour), and self‐efficacy to control interpersonal and situational influences. Third, a commitment to a plan of action was developed. Control: regular education on BF that was given to all women during their prenatal care visit in the clinic. Additionally, women from both groups gave birth in a “Baby Friendly Hospital” that allowed them to receive support from a clinical IBCLC from the birthing hospital. Women in the control group did not have any contact with the IBCLC/PC study team. | |
| Outcomes | Exclusive breastfeeding at 1,3 and 6 months | |
| Notes | Dates of the study: Not reported. Funding sources: IH National Center for Advancing Translational Sciences through grant number UL1TR000117 and UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given about the randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | No details given about the randomisation process. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants or personnel |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow up was high at 6 month follow‐up 10.39 (25.6%) and higher in the intervention group 6/20 (30%) compared to the control group 4/19 (21%). |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported as per protocol (NB retrospectively registered) |
| Other bias | Low risk | No industry funding No conflict of interest No baselien imbalance |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 20 | |
| Participants | Community setting, Hong Kong. Background breastfeeding rates. Inclusion criteria: primiparous women, intending to breastfeed, singleton pregnancy, term infant, speak Cantonese, Hong Kong resident, no serious medical or obstetrical complications Exclusion criteria: Infant with Apgar score < 8 at 5 min, Infant with birthweight < 2500 g, Infant with any severe medical condition or congenital malformation, Infant was placed in the special care baby unit for more than 48 h after birth, Infant was placed in the neonatal intensive care unit at any time after birth. | |
| Interventions | Peer supporters conducted 5 home visits. Two visits were conducted during the first month postpartum, with the first visit made within the first week postpartum and the second within the third week postpartum. Thereafter, three subsequent visits were conducted at 2, 4, and 6 months postpartum. Peer supporters established phone contact with participants within the first 48 h postpartum to schedule visits. Between visits, WhatsApp and telephone support were proactively initiated by the peer supporter to the participant or vice versa when needed. Control: routine care ‐ postnatal lactation education provided by a midwife or LC, one‐on‐one assistance with breast‐feeding if problems arise, and post‐discharge follow‐up either at an outpatient clinic in the maternity hospital. | |
| Outcomes | Exclusive breastfeeding at 4‐6 weeks and 6 months | |
| Notes | Dates of the study: Feb to March 2019. Funding sources: HKU‐KCL Strategic Partnership Fund. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The group randomisation sequence was gen‐ erated by a computer using Stata version 16 (9) and held by an independent researcher outside the research team who did not participate in participant recruitment, data collection, or analysis |
| Allocation concealment (selection bias) | Low risk | Three different individuals are in‐ volved to maintain allocation concealment. The inde‐ pendent researcher informed the practice nurse at the postnatal ward of allocation via telephone, after the study’s research assistant completed baseline participant data collection of demographic information and other study measures at study entry. |
| Blinding of participants and personnel (performance bias) | High risk | No possible |
| Blinding of outcome assessment (detection bias) | High risk | Women were not blinded and self‐report data was collected from them |
| Incomplete outcome data (attrition bias) | High risk | 1 month Loss to follow‐up in intervention group = 20% Loss to follow‐up in control group = 0% 2 months Loss to follow‐up in intervention group = 40% Loss to follow‐up in control group = 20% Big % difference between the two groups (NB actual numbers are very small with only ten in each group). 4 Months Loss to follow‐up in intervention group = 60% Loss to follow‐up in control group = 20% |
| Selective reporting (reporting bias) | High risk | Outcomes in protocol included number of participants who EBF at 1, 2, 4 and 6 months. This is not reported. Attitudes towards breastfeeding is not reported. Breastfeeding self‐efficacy was reported be measured at 2 and 4 months but only 4 month data provided. NCT03705494 |
| Other bias | Low risk | No industry funding No conflicts of interest No baseline imbalance |
| Study characteristics | ||
| Methods | Parallel 2‐arm RCT, single site, n = 770 | |
| Participants | Maternity ward at the Sotero del Rio Hospital, Santiago, Chile. Programme delivered by South East Metropolitan Health Service in conjunction with the Catholic University of Chile. Background breastfeeding initiation rates: on this ward 79.4% of the live births were fed with exclusive breastfeeding (EBF) up to 1 month of age and 67.3% were fed in this manner until 3 months of age. This was similar to national figures. Inclusion criteria: pregnancy without illness or risk factors which required more intensive maternal and/or perinatal monitoring during the process of labour and delivery, spontaneous initiation of labour, gestational age between 37 + 0 and 41 + 0 weeks, single pregnancy, live fetus and cephalic presentation Exclusion criteria: women with illnesses or risk factors that required more intensive maternal and/or perinatal monitoring | |
| Interventions | Intervention (n = 384): 'comprehensive care' consisting of family member who accompanied the woman from admission to discharge, 24 h/day, and who participated actively throughout the period. Labour took place in a comprehensive room with constant care, with early skin‐to‐skin contact of at least 1 h and encouragement of early initiation of breastfeeding (positive covariates for EBF). During the immediate postpartum period personalised educational support was delivered by the healthcare team. Early discharge with comprehensive intervention (after 24 h) was complemented by a home visit (after 48 h) where the mother's and baby’s care was reinforced, as well as the breastfeeding technique in a family setting. Control (n = 386): 'traditional care', i.e. standard care from the public health system. This involved labour management interventions (negative covariates for EBF) with intermittent and passive family participation. Early skin‐to‐skin contact was performed without any standardised guidelines and mother and child room‐sharing began once the newborn had received immediate care. During the postpartum period, professional and technical support was given for the start of breastfeeding in the postnatal unit. | |
| Outcomes | Prevalence of exclusive breastfeeding at 8, 16 and 24 weeks | |
| Notes | Dates of study: 2017 Funding sources: not reported Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The pregnant women were assigned randomly to both forms of intervention, using a randomized block design of 6‐8 women, so that in each block an equal number of women were assigned to each group but not clear how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No details provided to enable us to judge this. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Did not state whether women or staff providing intervention were blinded, but unlikely. |
| Blinding of outcome assessment (detection bias) | High risk | It was not stated whether the data collectors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up was 85.9% in the intervention group and 82.6% in the control group. There were no significant differences between the women lost to follow‐up and those who remained in the study. The number of cases lost to follow‐up (15.7%) was mainly due to a change of telephone number and address (Figure 1) For this reason it was expected that those lost to follow‐up were 'missing at random'. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or document with predefined outcomes available. This paper focused on data collected at 8 weeks. Data collected at 16 and 24 weeks were not reported and no explanation was given. |
| Other bias | High risk | Intervention contained other components which may influence breastfeeding, including 24‐h family participation during hospital stay and these different birth experiences are also important. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 188 | |
| Participants | Tennessee, USA. Background breastfeeding rates: not reported. Inclusion criteria: women eligible to receive Maternal Infant Health Outreach Worker services; self‐identify as Hispanic; provide written confirmation of pregnancy ≤26 weeks gestation; reside within 30 miles of the study offices; and be willing to be randomised into one of two study groups. Exclusion criteria: previously received Maternal Infant Health Outreach Worker services; had a severe mental or physical disability; or were under 18 years of age. | |
| Interventions | Maternal Infant Health Outreach Workers were recruited from the local Hispanic community and completed 40 h of training. Recognising family strengths and utilising those to address their own family needs; relationships begin in pregnancy and consist of monthly home visits and periodic group gatherings. Listening to maternal concerns, educating about objectives relevant to the woman’s stage of pregnancy or the age of the child, such as healthy eating, developmental milestones, attachment, and breastfeeding, and helping provide links to needed medical and social services. Home visits typically last approximately 1 h. Provided during pregnancy through to 6 months of age. Control: Distribution of printed educational materials about maternal and infant health and development at the end of each data collection interview to all study participants. Materials were available in Spanish or English. | |
| Outcomes | Any and exclusive breastfeeding at 2 and 6 months Postnatal depression (EPDS 2M and 6M) | |
| Notes | Dates of the study: Data collected between July 2014 and September 2016. Funding sources: Affordable Care Act Maternal, Infant and Early Child‐hood Home Visiting Program under Award Number D89MC23542 and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000445. Declarations of interest: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group assignments were generated by the study statistician via a computer‐generated, permuted block program. |
| Allocation concealment (selection bias) | Low risk | Participants received their group assignment after the enrollment interview was completed. Protocol provided by author states that sealed envelopes will be used and stored in a locked cabinent. After taking concent the assistant will provide the women with teh sealed envelope. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Data was collected from women who self‐reported breastfeeding |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant flow chart is difficult to interpret as it looks as if women came in and out of the study. At six months follow‐up it appears that 10/188 (5.3%) were lost to follow‐up Intervention 3/ 94 (3.2%) and control 7/94 (7.4%) |
| Selective reporting (reporting bias) | Unclear risk | No published protocol, however, study protocol provided by study authors. The breastfeeding outcomes in the protocol do not match the paper (ie no mention of 6 month data in protocol). |
| Other bias | Unclear risk | No mention of conflict of interests No industry funding Baseline characteristics balanced |
| Study characteristics | ||
| Methods | Cluster randomised controlled trial | |
| Participants | Background breastfeeding rates: The rate of EBF in the study area was 18.6%, which was lower than national rate of 32% at the time of the study. Currently, the Kenyan national rate is 61% but with high regional variability. Inclusion criteria: Pregnant mothers in their third trimester (33‐37 weeks gestation) attending ANC clinics. Mothers who were HIV negative b) been residents in the study area for at least 6 months c) planning to continue staying in the study site for at least 7 months from the time of recruitment into the study; and d) no history of complications of the current pregnancy based on medical records Exclusion criteria: Not reported | |
| Interventions | MES group: mothers in this study group received breastfeeding education (with focus on EBF) and support such as infant attachment to the breast, positioning, rooming in and breastfeeding on demand. The group was composed of 6 MTMSGs of at most 15 mothers each to facilitate easy sharing of breastfeeding information and support for each other. Each discussion session lasted one hour as per the MTMSG Facilitator’s Manual which was used as a standard for all MTMSGs.(25) At each meeting, one topic on breastfeeding was discussed in a session moderated by a trained facilitator. The MTMSGs groups met on a monthly basis; once prenatally and 6 times postnatally. The discussion topics included: advantages of EBF; breastfeeding myths; early initiation and sustenance of breastfeeding as well as management of common breastfeeding challenges. Each topic was discussed at a different MTMSG meeting. Participants did not receive any other education or counselling on EBF after the MTMSG meetings, but the group members could consult one another or the facilitators any time they encountered challenges on breastfeeding. All group meetings were held at the nearest health facility as agreed by each group members. MESIGA. Same as BF support arm 1 + an income generating activity following the mother support groups. | |
| Outcomes | Exclusive breastfeeding at 1,2,3 and 6 months | |
| Notes | Dates of the study: not reported Funding sources: The National Council of Science and Innovation‐ Kenya, Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was computer generated on a 1:1:1 ratio using Micro‐Soft Excel 2003 Software by an independent biostatistician without knowledge of the study area and the study hypotheses. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data from women so not possible |
| Incomplete outcome data (attrition bias) | High risk | Follow‐up: MES = 61% MESIGA = 83% CG = 52% Big differences in follow‐up between groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as per protocol PACTR201910846018049. |
| Other bias | High risk | One cluster randomized to each study design so risk of confounding that cannot be adjusted for. No industry funding No conflicts of interest |
| Study characteristics | ||
| Methods | 2 arm RCT, with individual randomisation, n = 212 | |
| Participants | Four WIC BFPC sites in Connecticut participated in the study, representing a federally qualified health center, 2 community‐based agencies, and a teaching hospital, all serving low‐income and minority women. Background breastfeeding rates: In the United States (USA) rates of breastfeeding initiation and duration among low‐income women who attend the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) are dramatically lower than women who do not participate in WIC, regardless of income. Inclusion criteria: Pregnant women aged ≥18 years who attended the BFPC program at 1 of the 4 study sites were eligible to participate. Women also had to be ≤28 weeks gestation, have conceived a singleton, have unlimited text messaging on their mobile phone, and have expressed the intention to breastfeed Exclusion criteria: miscarriage; premature birth (< 37 weeks gestation), withdrew from the BFPC program, multiple pregnancy; if the baby weighed less than 5 pounds; spent more than 3 days in the neonatal intensive care unit, or if medication given to mother or baby precluded breastfeeding | |
| Interventions | SMS 2‐way communication. Content ‐ benefits of BF for mothers and children, showed examples of proper positioning, explained how to tell whether the baby was getting enough milk, debunked BF myths, and reinforced the BFpeer counsellor's' supportive role. The theoretical basis of the text messaging intervention was the Health Action Process Approach to behaviour change. Message content was designed to address specific social cognitive constructs of the Health Action Process Approach, including phase‐specific self‐efficacy and planning. Text messages were sent with increasing frequency prenatally and decreasing frequency postpartum. Control:sStandard of care WIC Loving Support BF peer counselling program By study design, control group participants did not exchange text messages with their peer counsellors via MC and were instructed not to text their BF peer counsellors via their personal cell phones. Mothers were still able to make contact. | |
| Outcomes | Exclusive breastfeeding at 3 months | |
| Notes | Dates of the study: August 2014 to January 2016. Funding sources: Federal funds from the US Department of Agriculture, Food and Nutrition Service through Grant WIC NEI‐12‐TX Declarations of interest: not reported (states is online but link not working). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Both participants and peer counsellors were unblinded |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data from women |
| Incomplete outcome data (attrition bias) | High risk | Intervention group 17.5% lost at baseline, 37% LTF time point 1, 41% LTF time point 2 Control group 18% lost at baseline, 43% LTF time point 1, 37% LTF time point 2 |
| Selective reporting (reporting bias) | High risk | Papers aims are to report time to first contact and exclusive BF however does not report BF rates at 6 months which was a primary outcome instead reports planned breastfeeding at 6 month. Also secondary outcomes listed are not reported in this paper such as self efficacy. |
| Other bias | Unclear risk | The authors’ conflict of interest disclosures can be found online with this article on www.jneb.org. However we were not able to access this. No industry funding Baseline characteristics balanced |
| Study characteristics | ||
| Methods | 2‐arm RCT, recruitment March 2000‐October 2001 n = 849 | |
| Participants | Large university teaching hospital in Victoria, Australia Background rates of breastfeeding initiation: high. Baseline prevalence of breastfeeding in Australia = 83% at hospital discharge Participants were women intending to breastfeed their term infants, and were stratified by tertiary education and parity | |
| Interventions | Intervention (n = 425): Extended Midwifery Support (EMS); women received an in‐hospital postnatal education session. Postdischarge, they were offered home support visits with a research midwife once a week and telephone contact at least twice a week for 6 weeks Control (n = 424): Standard Midwifery Support (SMS); women received routine midwifery support and information according to the hospital protocol. The study hospital was working towards Baby‐Friendly accreditation during data collection (achieved in 2004) | |
| Outcomes | Any breastfeeding and exclusive breastfeeding at 6 months | |
| Notes | Dates of study: recruitment was conducted between March 2000 and October 2001. Funding sources: grants from Healthway, Women and Infants Research Foundation, and King Edward Memorial Hospital, Perth, Western Australia. Declerations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sample stratified by educational level and parity. Methods not clear. |
| Allocation concealment (selection bias) | Unclear risk | Paper stated "Women were asked to select an envelope from a group of at least 6 sealed, opaque envelopes, replenished in blocks of 12. The envelopes contained the allocation to either the intervention or control group". |
| Blinding of participants and personnel (performance bias) | Unclear risk | No detail provided on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No detail provided on data collection so judgement not possible. |
| Incomplete outcome data (attrition bias) | Unclear risk | 849 women randomized. Loss to follow‐up was reported by group at 2 months (intervention 83/425, 19.5% vs control 124/424, 29.2%) and at 6 months (intervention 8/425, 1.9% vs. control 4/424, 0.9%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Abstract did not include details of allocation concealment, outcome assessment or loss to follow‐up. Outcomes included in the abstract were reported by ITT. |
| Study characteristics | ||
| Methods | 2‐arm RCT, individual randomisation, n = 101 | |
| Participants | Setting: study carried out in Canada Inclusion criteria: live, singleton, term or near term infant delivered in 12 h before recruitment; women ≥ 21 years residing in defined study area, intending to breastfeed and with satisfactory home circumstances (assessed by postpartum nurses) Exclusions: non‐English‐speaking women, caesarean delivery, postpartum complications, infants with congenital abnormalities or morbidity | |
| Interventions | Intervention: planned early discharge from hospital (24 h‐36 h postpartum) and up to 3 home visits by community nurse LCs. Content of support unclear. The study aimed to compare of breastfeeding support in home and hospital settings. Control: planned hospital discharge 48 h‐60 h postpartum (usual care) with hospital based support for breastfeeding | |
| Outcomes | Exclusive breastfeeding at 5‐10 days postpartum and satisfaction with care | |
| Notes | We have not included data from this study in the review. Outcomes were not assessed at the same time in the intervention and control groups (mean day of follow‐up was 8.4 days in the intervention group vs 7.8 days for controls) and there was high attrition (26% overall, with 33% loss to follow‐up in the control group). Dates of study: July 1999 to December 2000. Funding sources: The Health Transition Fund,Health Canada,Ottawa, and by a financial contribution from The Hospital for Sick Children Foundation, Toronto, Ontario, Canada. Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation by staff not concerned with the study. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Blinding was not possible for the mothers or nurses as the experimental treatment (i.e. discharge to the home‐based lactation support) was known." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Interviewers were originally blinded to group status. However, in the course of answering questions about postpartum care and satisfaction, mothers inadvertently revealed their group status." |
| Incomplete outcome data (attrition bias) | Unclear risk | Outcomes were not assessed at the same time in both groups and there was high attrition (26% overall, with 33% loss to follow‐up in the control group). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Quote: "At baseline, no differences in maternal age, parity or gestational age were found in the two groups." |
| Study characteristics | ||
| Methods | 3‐arm cluster‐controlled trial, single site, n = 9675 | |
| Participants | Local government authorities (LGA) in Victoria, Australia ‐ community‐based maternal and child health centres Background rates of breastfeeding initiation: only rates at 6 months detailed. Ranged from 32% to 68% in different LGAs in Victoria. Inclusion criteria: LGAs in Victoria with a lower rate of any breastfeeding at discharge from hospital than the Victorian state average; and > 450 births per year. For the postal survey women were recruited on the basis of giving birth during the intervention time‐frame in all participating LGAs. Exclusion criteria: LGAs with breastfeeding initiatives in place similar to the proposed interventions. Women living in participating LGAs were not sent an invitation to take part in th postal survey if it was known that either they or the infant died, they had moved to another LGA since the birth, or they were not enroled in the Maternal and Child Health Service. | |
| Interventions | Intervention 1 (n = 3335): home visiting only (HV) ‐ early home‐based visiting by a maternal and child health nurse (MCHN) to women identified at risk of breastfeeding cessation. Aimed to provide proactive breastfeeding assistance as early as possible after birth. The focus of the visits were the normalisation of breastfeeding, building women’s confidence to breastfeed, reassurance, development of an infant feeding plan (where needed), and provision of a list of useful websites and telephone numbers. The topics covered at individual visits were driven by the specific needs of the woman. Intervention 2 (n = 2891): HV + access to a drop‐in centre ‐ women received home visit service as above and could attend local community breastfeeding drop‐in centre staffed by a MCHN, and where possible with a trained peer supporter or community educator or counsellor. Also provided mothers with the opportunity to meet and learn from other mothers. The centre was widely advertised. Control (n=3449): usual care. Midwife visit 1‐2 days after discharge and then MCHN visit 10‐12 days after discharge (breastfeeding assessment, support and advice a core component of care). Then MCH centre based care thereafter. | |
| Outcomes | Primary: any breastfeeding at 4 months Secondary: any breastfeeding at 3 and 6 months | |
| Notes | Dates of study: Intrvention ran from July 2012 to March 2013 Funding sources: The study was funded by the Department of Education and Early Childhood Development, Victoria, Australia Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes shuffled |
| Allocation concealment (selection bias) | Low risk | Allocation to trial arms took place using opaque envelopes at a state‐wide MCH forum. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding "was not possible at the LGA (randomisation) or cluster levels; however, individual women in the LGAs were not aware of the intervention allocation—the intention was that any trial arm allocation was ‘standard’ care within the LGA during the intervention period". However it is not clear if this was successful and whether staff were blinded or not. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment by participant‐completed questionnaire sent by mail, but not stated if those analysing the data were blinded to allocation. |
| Incomplete outcome data (attrition bias) | High risk | High loss to follow‐up at 4 months in control group (69% of women followed‐up) and home visiting group (68% of women followed up), Follow‐up was higher in home visiting plus group, with 81% of women followed up. |
| Selective reporting (reporting bias) | Low risk | Not all secondary outcomes listed in protocol were reported, but these were not outcomes of interest in this review so we marked this trial as being at low risk of bias. |
| Other bias | Unclear risk | Significant differences in proportion of Australian‐born women in across the groups (69% in comparison LGAs; 58% in home‐visiting LGAs; 73% in home‐visiting plus drop‐in centre LGAs). Unclear whether this could have an impact. |
| Study characteristics | ||
| Methods | 2‐arm parallel RCT, single site study, n = 460 | |
| Participants | 20 municipalities in the Netherlands, demographics not described Background rates of breastfeeding initiation: not described Inclusion criteria: ≤ 25 years, low educational level (primary school or prevocational secondary school), maximum 28 weeks of gestation, no previous live birth, understood Dutch, and at least 1 of the following additional risk factors: no social support, previously or currently experiencing domestic violence, psychosocial symptoms, unwanted and/or unplanned pregnancy, financial problems, housing difficulties, no education and/or employment and alcohol and/or drug use Exclusion criteria: previous live births, no additional risk factor as detailed above | |
| Interventions | Intervention (n = 237): the VoorZorg programme ‐ a home visitation programme translated and culturally adapted from the Nurse Family Partnership (NFP) programme. In addition to usual care, women received approximately 10 home visits during pregnancy, 20 during the first life year of the child and 20 during the second by trained, specialised VoorZorg nurses. 6 domains were discussed during the home visits: 1) the health status of the mother, 2) the child's health and safety, 3) the personal development of the mother, 4) the role of the mother, 5) the mother's relation with her partner, family and friends, and 6) the use of (health) care organisations. During pregnancy, women receiving the VoorZorg intervention were encouraged to initiate and continue breastfeeding after childbirth. The VoorZorg nurse also discussed the problems women encountered when breastfeeding their child and worked together with the mother to seek solutions to continue breastfeeding. The VoorZorg nurses also aimed to reduce smoking with the V‐MIS smoking cessation programme. Control (n = 223): usual care provided by the Dutch Youth Health Care Organizations. Every pregnant woman received care by a midwife including health education, physical examination and monitoring fetal development. The baby was automatically registered at an ambulatory well baby clinic for monitoring after birth and the parents were supported in parenthood. | |
| Outcomes | Primary: Prevalence of cigarette smoking (percentage of smokers and average number of cigarettes smoked a day) Average number of cigarettes smoked a day near the baby Birthweight Weeks of gestation Adverse pregnancy outcome (LBW, prematurity) Breastfeeding initiation Breastfeeding at 6 months Secondary: Any breastfeeding at 3 and 6 months | |
| Notes | The Netherlands Organisation for Health Research and Development (ZonMw), Academic Collaborative Centre, Child Health Care‐North HollandVU University Medical Center, participating Youth Health Care organizations and ZonMw Geestkracht, and participating city councils provided funding for the implementation of this study Dates of study: Study dates 2007 to 2009 Funding sources: Netherlands Organisation for Health Research and Development, Academic Collaborative Centre, Child Health Care‐North Holland‐VU University Medical Centre, participating Youth Health Care organisations and ZonMx Geestkracht, and participating city councils. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Independent randomisation procedure performed by a researcher at VU university |
| Blinding of participants and personnel (performance bias) | High risk | Women and staff were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | The interviewers were blinded regarding allocation but this may have been disclosed during interview |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up in control group was 18% and 8.4% in intervention group; authors stated baseline characteristics of women who were lost to follow‐up in each measurement were similar to women who remained in the study. |
| Selective reporting (reporting bias) | Low risk | Protocol included domestic violence, child development and child abuse as primary outcomes, but these were not reported in the results section. Breastfeeding was not reported as an outcome in the protocol, but was included as a primary outcome in the results paper. Authors stated that prevalence of babies with low birthweight, being premature or being small for gestational age, was similar in both groups. |
| Other bias | Low risk | None identified |
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Background breastfeeding rates: not reported Inclusion criteria: clusters: In Bangladesh, 100 sub‐districts, across five divisions, were selected by BRAC as possible A&T intensive areas based on high poverty, stunting levels in excess of 30% among children < 5 y of age, and non‐inclusion of the sub‐district in the government National Nutrition Program. Exclusion criteria: mothers who had a clear mental disability, defined as inability to answer basic questions about their name or willingness to be interviewed that would prevent them from understanding and answering the questions. | |
| Interventions | BANGLADESH INTENSIVE: Interpersonal counselling: Standard care (as described in non‐intensive), plus added intensified counselling: additional emphasis on early initiation of BF, no prelacteal, and EBF during ANC and PNC services provided by the SK; facilitation of initiation of BF within 1 h of birth, incentivised by SS; incentivised counselling on IYCF by SS during monthly door‐to‐door visits to all households with children under 2 y of age; counselling by full‐time dedicated IYCF worker, called Pushti Kormi (one per 2,000±2,500 households), to address difficulties, complete volunteer records of home visits, train mothers at home on complementary feeding at 6 mo, and ensure the following schedule of contacts with mothers during the ®rst 2 y: monthly from 0 to 8 mo, every other month to 12 mo, and one more visit each between 15±18 and 23±24 mo; additional emphasis given to IYCF at community meetings facilitated by BRAC's ANC/PNC provider in the community with pregnant and recently delivered women. More information:( http://aliveandthrive.org/resources/ manual‐implementation‐of‐community‐basedinterventions‐ for‐infant‐and‐young‐child‐feedingprogram‐ in‐bangladesh/; http://aliveandthrive.org/ wp‐content/uploads/2014/11/BRAC‐Final‐report‐8. 28.2014.pdf.) Community mobilisation: Local meetings organised by BRAC managers to introduce the A&T program and activities of FLWsÐ targeted to school teachers, adolescents, religious leaders, village doctors, district hospital/clinic doctors, community elites on the topic of nutrition and IYCF; fathers meetings added, with emphasis on handwashing linked to complementary feeding. Village theatre shows for the public to generate discussion about the importance of nutrition and IYCF and the role of the FLWs. Mass media and policy advocacy were the same across both arms of the trials.
| |
| Outcomes | Exclusive breastfeeding in all children under 6 months | |
| Notes | BANGLADESH Dates of the study:Baseline between 28th April ‐ 26th June 2010 to endpoint between 20th April ‐ 23rd June 2014 Funding sources: Funding for this evaluation and the implementation of the interventions was provided by the Bill & Melinda Gates Foundation, through Alive & Thrive, managed by FHI360; additional financial support to the evaluation study was provided by the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH), led by the International Food Policy Research Institute (IFPRI). The funders provided inputs into the study design and reviewed the manuscript, but had no influence over the data collection and analysis. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization process was carried out using a simple public lottery system in the presence of local, district, and provincial health authorities as well as the pro‐ gram evaluators. |
| Allocation concealment (selection bias) | High risk | program evaluators and health providers were present at the randomisation. No attempts at concealment described. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Not possible due to self‐reported data |
| Incomplete outcome data (attrition bias) | Low risk | No clusters were lost. As this is repeat cross‐sectional data it is not possible to assess loss to follow‐up of the women. |
| Selective reporting (reporting bias) | Low risk | All breastfeeding related outcomes in the protocols have been reported. ClinicalTrials.gov NCT01678716 (Bangladesh) and NCT01676623 (Viet Nam) |
| Other bias | Low risk | Authors with involvement in the development of Alive and Thrive did not contribute to the analysis. ICCs reported No industry funding. The study was generally well balanced for main variables. |
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Background breastfeeding rates: not reported Inclusion criteria: Clusters: 15 provinces were selected for program implementation based on stunting levels, absence of other large organisations working in nutrition, population density, and representation of the different ecological regions covered by the initiative. Four rural provinces, representing four distinct ecological zones, were then selected for inclusion in the evaluation sample, and within these provinces, ten rural districts; two to six communes per district were selected for the evaluation sample based on the presence of a health centre that met the eligibility criteria for the A&T franchise model. Exclusion criteria: mothers who had a clear mental disability, defined as inability to answer basic questions about their name or willingness to be interviewed that would prevent them from understanding and answering the questions. | |
| Interventions | VIETNAM INTENSIVE: interpersonal counselling: Standard government health services (same as non‐intensive)., plus added intensive structured package of eight BF counselling sessions (either group or individual) at social franchise set up at government health facilities addressing the following: preparation for EBF through three counselling sessions during the third trimester of pregnancy; personalised support for initiation of BF at the time of delivery (one contact); support and management of EBF through four counselling sessions during first 4 mo of infancy. Counselling sessions included leaflets, mother‐and child booklets, and distribution of promotional items to mothers who attended counselling sessions (face cloths, raincoats). More information: http:// aliveandthrive.org/wp‐content/uploads/2014/11/ Overview‐of‐the‐Social‐Franchise‐Model.pdf. Community mobilisation: Village health workers visit homes to deliver invitation cards to the counselling sessions. Mass media and policy advocacy were the same across both arms of the trials. | |
| Outcomes | Exclusive breastfeeding in all children under 6 months | |
| Notes | VIETNAM Dates of the Study: baseline (June 7 – August 29, 2010) and end‐line (June 16 – August 30, 2014) Funding sources: funding for this evaluation and the implementation of the interventions was provided by the Bill & Melinda Gates Foundation, through Alive & Thrive, managed by FHI360; additional financial support to the evaluation study was provided by the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH), led by the International Food Policy Research Institute (IFPRI). The funders provided inputs into the study design and reviewed the manuscript, but had no influence over the data collection and analysis. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization process was carried out using a simple public lottery system in the presence of local, district, and provincial health authorities as well as the program evaluators. |
| Allocation concealment (selection bias) | High risk | program evaluators and health providers were present at the randomisation. No attempts at concealment described. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Self report data so not possible |
| Incomplete outcome data (attrition bias) | Low risk | No clusters were lost. As this is repeat cross‐sectional data it is not possible to assess loss to follow‐up of the women. |
| Selective reporting (reporting bias) | Low risk | All breastfeeding related outcomes in the protocols have been reported. ClinicalTrials.gov NCT01678716 (Bangladesh) and NCT01676623 (Viet Nam) |
| Other bias | Low risk | Authors with involvement in the development of Alive and Thrive did not contribute to the analysis. ICCs reported No industry funding. The study was generally well balanced for main variables. |
| Study characteristics | ||
| Methods | 2‐arm Cluster RCT | |
| Participants | Background breastfeeding rates: rates of exclusive breastfeeding in the resource‐restricted setting is very low at 2% Inclusion criteria: over 18years old; less than six (< 6) months gestation; without a history of chronic disorders such as hypertension, diabetes, HIV, and tuberculosis. Postnatal inclusion criteria included; term delivery, singleton births, and a birth outcome more than 2.5kg. Exclusion criteria: not described | |
| Interventions | The study nutritionists educated mothers (twice prenatally) in the intervention group at the health centres to create a link between the CHWs and the health centre. The CHWs educated participants in the IG during home visits, (two times before delivery, during the first week of delivery and thereafter every month until the sixth month postpartum). Mothers received breastfeeding support from CHWs who helped solve breastfeeding problems, guided mothers on positioning techniques during breastfeeding and continually encouraged them to EBF their infants up to six months. | |
| Outcomes | Exclusive breastfeeding at 1,2,3 and 6 months | |
| Notes | Dates of the study: September 2013 and October 2014 Funding sources: National Commission of Science Technology and Innovation of Kenya and from Egerton University Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The Microsoft ® excel function was used to randomize sixteen out of the seventeen villages into either intervention or comparison groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Participants will be aware they are IG or CG |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data |
| Incomplete outcome data (attrition bias) | High risk | Eighty‐seven (33%) from the IG were lost to follow‐up while only eight (3%) in the CG. |
| Selective reporting (reporting bias) | High risk | Breastfeeding at 1 week and perceptions towards breastfeeding are detailed in the protocol but are not reported in the papers. |
| Other bias | Low risk | No industry funding, no conflicts of interest described; no baseline imbalance |
| Study characteristics | ||
| Methods | Quasi‐RCT (drawing numbered tickets), single site, duration of recruitment not stated, n = 200, follow‐up 97% | |
| Participants | Urban Canada Background rates of breastfeeding initiation: intermediate Inclusion criteria: women who wished to breastfeed and who had not previously breastfed Participant characteristics: 97% primiparous, ethnic composition not stated, 57% had received education to college or university level, no specific socioeconomic classification used | |
| Interventions | Intervention: home visit by volunteer during last month of pregnancy followed by telephone contacts weekly for 6 weeks and then 2 weekly to 5 months or until weaning. Volunteers were women who had breastfed themselves and had received 3 training sessions of 3 h duration followed by ongoing monthly supervision sessions. Average caseload was 1‐3 cases at any 1 time. Control: received home visit from public health nurse during the first month after birth followed by other contacts (face‐to‐face or by telephone) as determined by the mother | |
| Outcomes | Breastfeeding rates at 1, 2, 3, 4 and 6 months | |
| Notes | Dates of study: recruitment between 1984 and 1985 Funding sources: not in translation Declarations of interest: not in translation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by "drawing numbered papers" |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Article in French, unable to judge blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | There was an attempt to blind outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for drop‐out recorded; 200 randomized, 3 babies died and 3 other women lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this |
| Other bias | Unclear risk | Not clear over what time period women were recruited or whether controls and intervention women were recruited at the same time. Quote: "Subjects were recruited during various periods of time, depending on the availability of volunteers" |
| Study characteristics | ||
| Methods | 2‐arm RCT, individual randomisation, single‐site study recruiting over 14 months, n = 632 | |
| Participants | Urban UK Background rates of breastfeeding initiation: intermediate. National baseline prevalence 66% breastfeeding at birth and 42% at 4 months. Exclusive breastfeeding 21% at 4 months. Inclusion criteria: English‐speaking women, ≥ 17 years, who gave birth at the study hospital Exclusion criteria: baby spent > 48 h on the SCBU | |
| Interventions | Intervention: community postnatal support worker with 8 weeks' training provided home‐based support of up to 10 visits in the first 28 days (maximum of 3 h/visit) Control: standard UK care (includes postnatal home visits from midwives and health visitors) | |
| Outcomes | Exclusive or any breastfeeding at 6 weeks and 6 months | |
| Notes | Study population not limited to those intending to breastfeed Women consenting to participation more likely to be white and have had a CS Dates of study: recruitment between October 1996 and November 1997 Funding sources: NHS Research and Development, Health Technology Assessment programme. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided about blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No details provided about blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | 623 women randomized; stated that analysis was by ITT; 30 women who declined visits were included in the analysis; 78% follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | There was some baseline imbalance between groups. Women in the intervention group were more likely to have twins (9 vs 1), to have another adult resident in their household and to have used TENS in labour. |
| Study characteristics | ||
| Methods | Community‐based cluster‐randomised study; recruitment over 18 months, n = 130 | |
| Participants | Peri‐urban Mexican community Background rates of breastfeeding initiation: high All pregnant or postnatal women were in 39 geographical clusters. Perinatal death only clinical exclusion criterion: baseline breastfeeding prevalence: 92% initiation; 4% exclusivity at 2 weeks and 3 months; 50% cessation by 6 months | |
| Interventions | Home visits were conducted by peer‐counsellors trained by La Leche League (7 days theoretical teaching/2 months in lactation clinics and with mother‐to‐mother support groups), personal breastfeeding experience was not essential. Intervention 1: 6 visits (mid and late pregnancy and at 1, 2, 4 and 8 weeks) Intervention 2: 3 visits (late pregnancy and 1 and 2 weeks) Control: not specified | |
| Outcomes | Breastfeeding at 3 and 6 months Incidence of diarrhoea in infants 0‐3 months | |
| Notes | Subgroup analysis: antenatal and postpartum support; proactive intervention with scheduled contacts at home; initial face‐to‐face contact; intervention delivered by trained counsellors Dates of study: Recruitment between March 1995 and September 1996 Funding sources: Wellstart International's Expanded Promotion of Breastfeeding Program (USAID) cooperative agreement and the US National Institute of Child health and Human Development. Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomisation, clusters stratified by area, randomisation schedule generated by computer. |
| Allocation concealment (selection bias) | Low risk | Clusters randomized by computer. |
| Blinding of participants and personnel (performance bias) | High risk | Clusters randomized to avoid contamination, but women and counsellors would have been aware of intervention. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome measurement was by staff who were aware of group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | 130 women from 31 cluster areas randomized; 125 followed up at 3 months and 104 at 6 months (20% attrition at 6 months). |
| Selective reporting (reporting bias) | Unclear risk | How cluster design was taken into account was not clear. It was stated that ICC values were 'negligible' and the authors stated "these results show that the cluster‐randomisation design achieved the equivalent of individual randomisation". |
| Other bias | Unclear risk | No baseline imbalance apparent, although group size was uneven (this may have been due to chance). |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 225 | |
| Participants | Setting: general practice in Ayrshire, Scotland Background rates of breastfeeding initiation: low Participant characteristics: Mean age of intervention group: 28.5 years; SD 5.2; range 17‐43. Mean age of control group: 27.8 years; SD 5.5; range 16‐40 Parity: 53% primiparous | |
| Interventions | Intervention (n = 112): women were assigned 2 peer supporters (women with previous breastfeeding experience) who contacted them at least once in the antenatal period and provided further antenatal support on request. In the postnatal period after hospital discharge peer supporters contacted women who were still breastfeeding at least every 2 days by phone or by home visit up until 28 days, and further support was available up to 16 weeks postpartum. Control (n = 113): standard care that included visits from community midwife for the first 10 days, health visitor after 10 days; breastfeeding support groups and breastfeeding workshops were available. | |
| Outcomes | Initiation of breastfeeding, any and exclusive breastfeeding at 6 weeks and 6 months, median breastfeeding duration and reasons for giving up breastfeeding | |
| Notes | The researchers noted that "health professionals varied in their commitment to breastfeeding and also in their acceptance of lay assistance, such as peer support" Dates of study: recruitment between July 1997 and March 2002. Funding sources: Ayrshire and Arran Health Board Declarations of interest: Authors are of the option that breastfeeding is best for babies in most circumstances but otherwise all authors declare that they have no competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequences for each stratum (primagravidae, previous formula feeding, previously breastfed < 6 weeks, previously breastfed > 6 weeks) were generated at the start of the trial by computer in blocks of 10 (that is, 5 random allocations to each of the peer support and control groups in each different block of 10) to give approximate numerical balance between groups. |
| Allocation concealment (selection bias) | Low risk | Allocation to control or peer support group was by post‐recruitment concealed allocation, with a telephone call for the next allocation on the list. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "There was no post‐allocation concealment as once a woman was allocated to the peer support or control group this was known to the peer supporters and others associated with the trial.” |
| Blinding of outcome assessment (detection bias) | High risk | The questionnaire were completed in the presence of a GP or practice nurse. It is also stated that trial team were not involved in the questionnaire completion. Unclear if the GP or practice nurse would have been aware of group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Low loss to follow‐up. Peer support group (intervention group) (n = 112): at 16 week follow‐up, n = 110; control group (n = 113): at 16 week follow‐up, n = 110 |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Planned recruitment was for 320 women but ended after 225 women recruited, therefore the study had reduced power to detect differences between groups. Few demographic data were reported so it was not clear whether or not there was baseline imbalance, although recruitment was balanced for parity by stratification. |
| Study characteristics | ||
| Methods | RCT | |
| Participants | Background breastfeeding rates: 96% initiation and 2% EBF at 6 months Inclusion criteria: healthy pregnancy in the first or second trimester and intention to attempt breastfeeding after delivery Exclusion criteria: pregnancy beyond the second trimester, maternal chronic medical condition such as hypertension or diabetes, abnormal fetal screen at 20–22 weeks, determined not to breastfeed, twin pregnancy, not residing in Lebanon for at least six months after delivery, or delivery before 37 weeks of gestation Participant characteristics: participants in the experimental group had higher monthly income, fewer children, shorter duration of previous breastfeeding, and fewer children who were breastfed (p=0.001). Higher proportion of women in the experimental group had female paediatricians (P = 0.01) | |
| Interventions | a) prenatal breastfeeding education to address common community misconceptions about breastfeeding and improve maternal knowledge and expectations, b) postpartum professional lactation support to avoid, and/or overcome technical breastfeeding challenges that mothers experience, and improve maternal self‐efficacy through empowerment, c) postpartum peer (lay) support to provide emotional support, and build maternal social capital. Our multi‐component intervention was based on the Social Network and Social Support Theory that offers a framework of pathways through which social ties can influence health. Peers provided a minimum of 10 scheduled calls/visits starting with the antenatal class, then at the sixth and ninth months of gestation, the expected week of delivery, the first day postpartum, 48 hours from hospital discharge, one, two, and four weeks postpartum, and monthly until 6 months. Lactation consultants visited the participants on the first postpartum day in the hospital, and continued with home visits on days three, seven, and fifteen postpartum, and then monthly for six months Control: Standard obstetric and paediatric care. Care is mainly focused on obstetrics. Information relating to breastfeeding is not currently part of prenatal care in any region of the country. Advice on infant feeding is provided by paediatric physicians and nurses or midwives, usually after delivery. Moreover, hospitals and maternities do not have lactation consultants on their staff. | |
| Outcomes | Any and exclusive breastfeeding at 1,3,6 months | |
| Notes | Dates of the study: Dec 2013 ‐ Jan 2016 Funding sources: Medical Dean’s Program Projects in Biomedical Research, Medical Dean’s Innovative Fund, American University of Beirut. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation was computer‐ generated by one of the co‐investigators (HT) who was not involved in recruitment. Stratified block randomization was carried out. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by using a set of sequentially numbered opaque sealed envelopes specifying group allocation |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Women were interviewed about breastfeeding status and would have known if they were in the control or intervention group. |
| Incomplete outcome data (attrition bias) | High risk | I = 73% C = 79% |
| Selective reporting (reporting bias) | Low risk | Protocol outcomes all reported |
| Other bias | High risk | No industry funding No conflict of interest Participants in the experimental group had higher monthly income, fewer children, shorter duration of previous breastfeeding, and fewer children who were breastfed (p < 0.001). It is unclear if this would bias findings. |
| Study characteristics | ||
| Methods | 2‐arm cluster RCT, 120 clusters | |
| Participants | Rural communities West Singhbhum and Kendujhar, two adjoining rural districts of Jharkhand and Odisha in eastern India. Background breastfeeding rates: not reported. Inclusion criteria: attempted to recruit all pregnant women and their children in the study areas. Exclusion criteria:sStillbirths and neonatal deaths, infants whose mothers died, those with congenital abnormalities, multiple births, and mother and infant pairs who migrated out of the study area permanently during the trial period. Permanent migrants were defined as those missing two consecutive interviews and the final interview at 18 months, or missing interviews at 12 and 18 months. Migration was assumed to have occurred at the point of the earliest missed consecutive interview. | |
| Interventions | Recruited community‐based workers called Su‐Poshan Karyakarta (SPK), meaning good nutrition worker, in consultation with local village health sanitation and nutrition committees and existing Anganwadi workers. Each worked in her own village and any nearby hamlets, and covered around 1000 people. She had a minimum of 10 years’ schooling, was married, preferably from a tribal community, and was paid a monthly stipend of INR3000. Six supervisors recruited by the study team supported ten SPKs each. SPKs and supervisors received 14 days of training during the intervention period, and attended supervision meetings twice a month. The SPK was responsible for two main activities: conducting a single home visit to each pregnant woman in the third trimester of pregnancy for counselling on maternal nutrition, followed by monthly home visits to all children younger than 2 years with counselling for growth promotion; and the facilitation of two to three participatory meetings with local women’s groups per month (the exact number depended on the size of her working area and number of hamlets). Home visits ‐ immediate causes of undernutrition through counselling for infant and young child feeding practices, illness prevention, and support for referrals in case of illness or acute malnutrition. Participatory group meetings reinforced actions linked to immediate causes and began to address underlying causes of undernutrition, including birth spacing, nutrition in pregnancy, water, sanitation, and women’s agency. At each visit to a mother and child pair, she asked about current or recent illness, took a mid‐upper arm circumference measurement for children older than 6 months, and engaged the mother in a discussion about feeding, hygiene, care, and stimulation. This began with the mothers’ immediate concerns with illness, feeding, and care. Age‐appropriate picture cards that depicted good practices were used. The SPKsalso facilitated a cycle of 29 participatory meetings with women’s groups. These monthly meetings targeted pregnant women and mothers of children younger than 2 years and adolescent girls, but were open to all community members. Control: Ekjut coordinators held five participatory meetings with village health sanitation and nutrition committees in between the committees’ regular monthly meetings for 2 years as a minimum common benefit to villages in both trial arms. Meetings aimed to strengthen the capacity of village health sanitation and nutrition committees to assess community health needs, prepare and implement village health plans, and monitor the provision of local health and nutrition services. This was the only activity implemented in control clusters besides routine government services. | |
| Outcomes | Exclusive breastfeeding at 6 months Infant morbidity (composite measure) | |
| Notes | Dates of the study: recruitment October 2013 to December 2015. Funding sources: UK Medical Research Council, Wellcome Trust, UK Department for International Development (DFID). Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Meeting participants put numbered balls corresponding to clusters in each stratum in a local tombola (lottery device), then sequentially allocated each ball (cluster) to the intervention or control arms. |
| Allocation concealment (selection bias) | Unclear risk | The use of the tombola with each ball sequentially allocated suggests a form of concealment, but not enough information provided. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not feasible. |
| Blinding of outcome assessment (detection bias) | High risk | We documented dietary practices using 24‐h recall through interviews with all mothers |
| Incomplete outcome data (attrition bias) | Low risk | Intervention 1338/1541 = 86.55% Control 1295/1460 = 88.7% |
| Selective reporting (reporting bias) | Unclear risk | The number of secondary outcomes was later reduced in the online trial registration form after feedback from the data monitoring committee . Unclear why this was done. |
| Other bias | Low risk | No industry funding obtained. No competing interests reported. No clusters dropped out. No issues due to clustering reported. No imbalance across groups apparent at baseline on maternal characteristics |
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Background breastfeeding rates: not reported Inclusion criteria: a household census was conducted at baseline and endline to create a list of pregnant women and mothers with infants <6 mo of age. Households were selected for surveys by using systematic sampling beginning with a random seed to yield the desired sample size per cluster. Exclusion criteria: women who could not understand and answer questions were excluded. | |
| Interventions | In the nutrition‐focused MNCH model, the health worker conducted monthly home visits and one‐on‐one ANC sessions for all pregnant women to deliver the following interventions: 1) demonstration of a specific diet plan (both quality and quantity), 2) provision of free supplements [IFA (60 mg elemental Fe and 400 mg folic acid) and calcium (500 mg) tablets] and advising on their use, 3) measurement of weight and explaining weight‐gain patterns, 4) counselling on resting, and 5) engaging other family members to ensure enough foods and supplements and support for the pregnant women. During the postpartum period, health workers counselled mothers on a specific diet plan during lactation and promoted optimal breastfeeding practices. Health workers were tasked with conducting 7 visits during pregnancy and 5 visits during the postpartum period. Health volunteers conducted 2 visits per household per month and provided follow‐up messages to reinforce the demonstrations and counselling given by health workers. Breastfeeding counselling included Core counselling package Frontline workers provided with refresher training on the topic every month More frequent counselling More frequent reinforcing messages by frontline workers Provided support and problem‐solving for any issues that occurred. In the nutrition‐focused MNCH program, regular monitoring and supervision by BRAC staff, district managers, headquarters staff, and an independent team of 5 monitors were provided to track the performance of frontline workers (through direct observation) and practices of mothers (through interview and observation). Each monitor visited ;70 randomly selected households each month. CONTROL: services provided by the standard MNCH program included family planning, identification of pregnancies, ANC, delivery and postnatal care, essential neonatal care, management of neonatal and childhood illnesses, vaccination, and referral for complications | |
| Outcomes | Exclusive breastfeeding in all children under 6 months | |
| Notes | Dates of the study: August 2015 to December 2016 Funding sources: supported by the Bill & Melinda Gates Foundation, the Canadian Department of Foreign Affairs, Trade, and Development, through Alive & Thrive, managed by FHI 360, and the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH), led by the International Food Policy Research Institute. Data collection was provided by Data Analysis and Technical Assistance, Ltd., Dhaka Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Face‐to‐face interviews so data self‐report |
| Incomplete outcome data (attrition bias) | High risk | No loss of clusters. However, this is a repat cross‐sectional study so not possible to judge the loss to follow‐up of the women |
| Selective reporting (reporting bias) | Low risk | Outcomes in the protocol are reported (NCT02745249) |
| Other bias | Low risk | Adjustments were made for clustering effect. Conflicts of interests ‐ authors involved in the development of Alive and Thrive were not directly involved in the analysis of the results No baseline imbalance |
| Study characteristics | ||
| Methods | 2‐arm cluster RCT, 12 clusters, n = 2301 | |
| Participants | Primary Health Centres in the capital city, Burkina Faso. Background breastfeeding rates: 51.4% of children aged <6 months did not receive colostrum at birth. Inclusion criteria: pregnant women living in the catchment areas of the 12 selected health centres for at least 6 months, and not planning to leave in the next 2 years. After birth, singleton babies without major birth defects were included in the study and followed quarterly until 18 months of age. Exclusion criteria: non‐singleton deliveries, malformation that hampered child growth, or stillbirth. | |
| Interventions | Multifaceted child and maternal health programme. Included healthy eating for pregnant women and complementary feeding after 6 months. During the prenatal period, healthcare providers provided counselling on appropriate breastfeeding practices, such as early initiation of breastfeeding, feeding colostrum, exclusive breastfeeding up to the age of 6 months, and timely introduction of complementary foods. After delivery, a patient‐centered approach was used to identify specific individual needs and problems surrounding child feeding. Thereafter, tailored counselling was provided to promote appropriate feeding practices and offer solutions to any nutrition‐related problems. Counselling on breastfeeding practices continued during early infancy. Additional focus areas were the timing of the introduction of complementary foods. Control: Routine preventive, promotional, and curative services were provided to pregnant and lactating women, and children aged <5 years as per national policy. | |
| Outcomes | Exclusive breastfeeding at 6 months Childhood illness: diarrhoea, fever, respiratory infection Stunting | |
| Notes | Dates of the study: Aug 2009 ‐ Dec 2011. Funding sources: Nutrition Third World and Belgian Ministry of Development. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was conducted publicly. For each pair of health centers, two identical pieces of paper were numbered corresponding to each health center and put into a basket. A volunteer not involved in the study was asked to choose a paper for the intervention center. After the first choice for the intervention center, the second center in that pair was systematically allocated to the control arm |
| Allocation concealment (selection bias) | High risk | No allocation concealment and blinding were possible |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Data were collected by trained field workers not involved in the intervention delivery, and who were not blinded to the intervention. |
| Incomplete outcome data (attrition bias) | High risk | Overall, 1,521 children (67.5%) were classified as lost to follow‐up at one point during the fol‐ low‐up period; 803 (68.6%) in the intervention arm and 718 (66.3%) in the control arm |
| Selective reporting (reporting bias) | High risk | The paper includes outcomes not listed in the protocol: prenatal diet; early initiation of breastfeeding; fed colostrum; received something else in first 72 hours; timely introduction of foods; meal frequency; child weight and height; wasting; stunting. The paper reports exclusive breastfeeding up to 6 months but the protocol states this outcome is measured until 18 months |
| Other bias | Low risk | No industry funding. No conflicts of interest. ICC reported. Baseline characteristics comparable. |
| Study characteristics | ||
| Methods | 2‐arm cluster RCT, 10 clusters with 663 health professionals randomised. | |
| Participants | Hospital birth facilities, Denmark. Background breastfeeding rates: not reported but described as having a strong breastfeeding tradition. Inclusion criteria: single infant, intended to breastfeed, able to read information in Danish, and were not expected to be discharged later than 50 hr postnatally due to complications in pregnancy or clinical disease. Exclusion criteria: women expected to be discharged later than 50 hr postnatally due to complications in pregnancy or clinical disease. | |
| Interventions | Participatory approach to increase the new parents self‐efficacy. Included extended skin‐to‐skin contact, frequent breastfeeding, identifying cues and ensuring sufficient milk intake. Good positioning of the mother including laid‐back breastfeeding after birth and experience of pain and relaxation for positional changes. Acknowledgement of the mother and father as equals with different roles in relation to breastfeeding. Mothers were introduced orally to the components. They were also given a postcard and supported postnatally according to the manual and a pamphlet which was used during counselling. The parents were supposed to adhere with the programme during the first 3 days while the infant went through the metabolic adaptation and the mother’s milk production increased or until the first home visit by the HV 3–5 days postnatally. Parents received a phone call 24 hrs after discharge. Control: unclear. It is stated that "Breastfeeding support may vary depending on hospital routines". | |
| Outcomes | Exclusive breastfeeding at 4‐6 weeks and 6 months Readmission due to nutritional problems (breastfeeding, jaundice, dehydration, weight loss) | |
| Notes | Dates of the study: April 2013 to August 2014 Funding sources: Trygfonden and The Danish Nurses’ Organization Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The hospitals were computer randomized to either the intervention or reference group. We stratified the 10 participating birth facilities according to number of births per year (<1,200, 1,200–3,000, >3,000) and the medium size hospitals by geography (mid‐eastern Jutland, other). |
| Allocation concealment (selection bias) | High risk | The midwives doing the recruiting were not blinded. There were differences between the women selected and those not selected. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | The women were not blinded and data was self‐report. |
| Incomplete outcome data (attrition bias) | High risk | We performed intention‐to‐treat analyses (ITT), including all mother–infant dyads, and complete‐case analyses (CC) restricted to mothers and infants with available information on the specific out‐ comes 1 month Intervention loss to follow‐up = 37% 1 month Control loss to follow‐up = 43% 6 month intervention loss to follow‐up = 49% 6 month control loss to follow‐up = 53% |
| Selective reporting (reporting bias) | High risk | There are several outcomes detailed in the protocol which are not reported on: number of stools; milk coming in before 3rd day pp, baby's swallowing of milk. Skin‐to‐skin is included as an outcome in the paper but not detailed in the paper. Breastfeeding problems at 7 days, 30 days and 6 months were included as outcomes in the protocol but in the paper there is only one value given and it is not stated at what time point. Rationale for changes not described. |
| Other bias | High risk | 12/22 clusters declined the invitation to take part in the study. Hospitals had to fund the training of the intervention which may impact on their ability to take part. Women were recruited after the clusters had been randomized which could lead to recruitment bias. No industry funding. No conflicts of interest. |
| Study characteristics | ||
| Methods | 3‐arm cluster‐controlled trial, single‐site study, n = 360 (note only 2 arms included in analysis) | |
| Participants | Kiberia slum, Nairobi, Kenya ‐ a densely populated area that was not well served with basic services such as health facilities, adequate safe water and sanitation services. Background rates of breastfeeding initiation: no data on initiation, but for Kenya the exclusive breastfeeding rate for infants under 6 months was 32.0%. Inclusion criteria: in the third trimester of pregnancy (34–36 weeks’ gestation), HIV‐negative, intention to stay in Kibera for at least 6 months after delivery, willing to be visited at home, willing to be included in the study Exclusion criteria: documented chronic diseases such as diabetes mellitus, renal disease, heart disease or any other chronic disease, and eclampsia in a previous pregnancy | |
| Interventions | Intervention 1 (n = 120): home‐based intensive counselling group (HBICG); mothers received 7 counselling sessions: prenatally, the first week after delivery and then monthly up to 5 months postpartum. The content was similar to the facility‐based semi‐intensive counselling group (FBSICG; see Intervention 2) but was more tailored to the mother’s needs and more detailed. Women also had more practical exposure with regard to supporting breastfeeding (e.g. positioning, attachment, expression of milk). Counsellor training was the same as for FBSICG. Intervention 2 (n = 120): FBSICG; note this intervention was an antenatal one only, so not included in this review). Consisted of 1 session of 1‐to‐1 counselling at the health centre conducted by the investigator and breastfeeding counsellors. The breastfeeding counsellors were 3 local women trained in accordance with the WHO/UNICEF counselling course (40 h). The counselling content was structured around the benefits of exclusive breastfeeding; preparation for breastfeeding initiation and sustainability of breastfeeding; positioning and attachment of baby to the breast during feeding; and prevention and management of breastfeeding challenges. The single session took place after enrolment into the study. Control (n = 120): usual standard health and nutrition education offered at the health centre. This was a group‐based education programme which covered breastfeeding and a range of other topics. | |
| Outcomes | Primary: exclusive breastfeeding at 1, 3 and 6 months Secondary: cumulative (since birth exclusive breastfeeding at 6 months). | |
| Notes | Dates of study: study conducted between April 2006 and April 2008. Funding sources: funded by Nestle. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated using Excel for randomisation of the clusters. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Paper stated that only the investigator and peer counsellors were aware of the treatment given and knew the hypothesis. The nurse in charge was blinded to the intervention allocation. It was not clear if the women were aware of the allocation. |
| Blinding of outcome assessment (detection bias) | High risk | The enumerators conducting the interviews to determine breastfeeding practices were blinded to the study hypotheses to avoid any likelihood of bias in the way they asked questions, even though they were trained to ask questions in a standard way. There was no contact between the enumerators and the breastfeeding counsellors. |
| Incomplete outcome data (attrition bias) | High risk | Follow‐up in both intervention and control groups was 74.2%. The analysis was as‐treated and not ITT. Younger women were significantly more likely to be lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. Unpublished protocol provided by study authors but the outcome timing is not clear. |
| Other bias | High risk | Funded by Nestle. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 150 | |
| Participants | University of Port Harcourt Teaching Hospital (UPTH) in Rivers State, Nigeria. Background breastfeeding rates: the national EBF rate of 25% at the 6th month. Inclusion criteria: mothers who delivered in the hospital, are resident in Port Harcourt metropolis (where hospital is located), intends to and have commenced breastfeeding after delivery. They were also expected to have personal or shared mobile cellular phone device with at least one active subscriber identification module (SIM card). Exclusion criteria: mothers with serious medical problems or have babies with life‐threatening conditions | |
| Interventions | Women received mobile phone‐based advisory support service from the same paediatrician. The participants were contacted on the 7th and 14th day of the first month and subsequently once within 2–7 days of the infant monthly birthdays, until 6 months. An average of eight calls were made to each mother, and they could call back anytime they so desired. A structured instruction package was used to remind mothers of the benefits of EBF for their babies and all questions related to breastfeeding and the wellbeing of the mother and baby were entertained during each telephone chat. Control: 'Usual care’ consisting of standard care during visits to the post‐natal clinic, infant nutrition clinic and routine immunisation unit in the hospital. A breastfeeding session was observed for all mothers in both intervention and control groups to teach them how to properly latch their babies on the breast. Difficulties with establishing breastfeeding such as engorged breast were managed and mothers were assisted to fully establish breastfeeding. | |
| Outcomes | Exclusive breastfeeding at 1,2,3,6 months | |
| Notes | Dates of the study: March ‐ December 2014 Funding sources: not reported. Declarations of interest: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random assignment using balloting. After enrolment, each participant picked a sealed envelope which were numbered and contained a tag for either intervention or control group. The name of the participant and the number on the envelope was recorded in the study register. The allocation was done consecutively until all the study partici‐ pants were randomized into the two groups |
| Allocation concealment (selection bias) | Low risk | random assignment using balloting. After enrolment, each participant picked a sealed envelope which were numbered and contained a tag for either intervention or control group. The name of the participant and the number on the envelope was recorded in the study register. The allocation was done consecutively until all the study partici‐ pants were randomized into the two groups |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants or personnel |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported breastfeeding outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Overall attrition 19/150 (12.7%); Intervention group 8/75 1(10.7%) and control group 11/75 (14.7%). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available to judge outcomes |
| Other bias | Unclear risk | No report of funding or conflicts of interest. Baseline characteristics balanced. |
| Study characteristics | ||
| Methods | 2‐arm cluster RCT, 4 clusters | |
| Participants | Four urban, public, maternity hospitals in Nagpur, India Background breastfeeding rates: The Indian National Family Health Survey 2005–06 reported timely initiation of breastfeeding rates of 24.5%, exclusive breastfeeding rates at 6 months of 46.4%, and only 56.7% of 6–9‐month‐old being fed complementary foods. Inclusion criteria: the participating hospitals (two in intervention and two in control) had to have annual deliveries of above 5000 and catered to women belonging to poor socio‐economic background. Inclusion criteria ‐ Women in their third trimester (32–36 weeks), registered for antenatal clinics, planning to deliver at the same hospital and willing to give follow‐up till 6 months of infant age were considered eligible. Exclusion criteria: women with presence of complications in pregnancy that could affect exclusive breastfeeding such as severe anaemia (Hb < 6 g/dL), at the risk of eclampsia or pre‐eclampsia, consuming drugs contraindicated in pregnancy or HIV positivity. | |
| Interventions | Cell phone counselling provided by certified lactation counsellors once a week, starting in the third trimester of pregnancy until a week after the infant was 6 months old. Counsellors were auxiliary nurse midwives with additional training for counselling over the phone. Provided advice on importance of antenatal care, iron‐folic acid supplementation, maternal nutrition, appropriate infant and young child feeding practices, avoiding of pre‐lacteal feeds (additional liquid supplements prior to initiation of breastfeeding), how to deal with problems regarding breastfeeding and infant immunisations. Counsellors also facilitated seeking of care at the hospitals if the mother or infant reported ill. Additionally, women received a text message daily, in the regional language to augment appropriate feeding practices. These women were also provided cell phones, seven free recharge vouchers and subsidised prepaid calling cards. Also, they could call the counsellors as and when needed, using a speed dial facility. During the study, if a mother lost her study cell phone, she was asked to use her personal or family cell phone. Control: routine healthcare services, which included 6 hospital visits from delivery to 6 months postpartum for postnatal care and child immunisation. | |
| Outcomes | Exclusive breastfeeding at 2,3 and 6 months Maternal satisfaction with care Infant morbidity | |
| Notes | Dates of the study: August 2010 to June 2012 Funding sources: World Bank‐ SARDM (South Asia Region Development Marketplace Grant ID ‐ 806410) and subsequently by Alive and Thrive initiative, The Bill and Melinda Gates Foundation(Grant ID–09‐000076‐AT10‐4LMR). Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors describe a random component (coin tossing) in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided on allocation concealment before or during enrolment. |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, blinding of participants and personnel was not feasible. |
| Blinding of outcome assessment (detection bias) | High risk | This was an unblinded study with outcomes self‐reported by participants. |
| Incomplete outcome data (attrition bias) | Low risk | At the final study visit, at one week and 6 months after delivery, attrition was <20% (IG: 42/518, 8.1%; CG: 44/519, 8.5%). Reasons for loss to follow up at each follow‐up time point were balanced across the two groups. |
| Selective reporting (reporting bias) | Unclear risk | Protocol suggests measurement of exclusive breastfeeding at different time points as follows: One week after enrollment 2. 24 hours after delivery 3. 6th week after delivery 4. 10th week after delivery 5. 14th week after delivery 6. 24th week after delivery 7. 26th week after delivery However, the paper presents exclusive breastfeeding rates within 24 hours after delivery, at 6th, 10th, 14th week and at one time point of 6 months. No explanation as to whether data from the 24th and 26th week are combined, or if one of these was discarded. |
| Other bias | High risk | A small number of clusters (n=4) and baseline imbalance observed across groups. Unclustered analyses were conducted and no ICC reported. No industry funding. The authors declare that they had no competing interests. |
| Study characteristics | ||
| Methods | 2‐arm RCT, n = 1154 | |
| Participants | Pennsylvania, USA Background rates of breastfeeding initiation: no details provided Inclusion criteria: singletons and twins born after at least 34 weeks’ gestation to English‐speaking mothers attempting to breastfeed during the maternity stay and with intent to continue breastfeeding after discharge Exclusion criteria: atypical stays characterised by: 1) a 2‐night or longer stay after a vaginal delivery; 2) a 4‐night stay or longer after a caesarean section; 3) a hospital course with atypical complications (e.g. ambiguous genitalia, endometritis); or 4) newborn hyperbilirubinemia requiring phototherapy during the nursery stay. Mothers were also excluded for major morbidities and/or pre‐existing conditions that would affect postpartum care, lack of a telephone number, previous study participation, residence outside the coverage region of the Visiting Nurse Association of Central Pennsylvania (VNA), or if a home nursing visit was specifically requested by a hospital social worker or child protective services owing to social concerns. | |
| Interventions | Intervention (n = 576): 1 home nursing visit within 48 h of hospital discharge (typically 3‐5 days post birth). All nurses received continuing education related to breastfeeding support and cultural competency prior to study initiation. All newborns in intervention group were scheduled for an office‐based visit 1 week after the visit to assess weight and recovery. Control (n = 578): office‐base care; postdischarge visit timing for newborns was determined by the newborn nursery physician | |
| Outcomes | Primary: maternal and infant use of unplanned health care services in the 14 days after delivery Secondary: Breastfeeding duration and exclusivity Maternal postpartum depression State of anxiety Perceived social support Parenting self‐efficacy | |
| Notes | Dates of study: recruitment between12th September 2006 to 1st August 2009. Funding sources: Maternal Child Health Bureau, Health Resources and Services Administration, Department of Health and Human Services. Additional support was provided by the Children's Miracle Network Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | No information provided about allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not detailed whether mothers and/or home visiting nurses were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Telephone interviews with mothers conducted by study co‐ordinators blinded to study group. |
| Incomplete outcome data (attrition bias) | Unclear risk | 8% attrition by 2‐week telephone interview; 13% attrition at 2‐month telephone interview. However, at 6 months attrition was 31% in the home nursing visit group and 38% in the office‐based care group. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not stated in Clinicaltrials.gov record. Unclear whether ‘any breastfeeding’ or ‘exclusive breastfeeding’ were reported, but both should have been, however, additional information received from author included both. |
| Other bias | Low risk | No baseline imbalance No industry funding No conflicts of interest |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 104 | |
| Participants | Setting: maternal and paediatric clinic for low‐income inner‐city population (New Jersey, USA) Background rates of breastfeeding initiation in this population: high Inclusion criteria: WIC program‐qualified pregnant women in the third trimester of a singleton pregnancy without HIV, cancer, or illegal drug use Participant characteristics: 87.5% of the women were of Hispanic origin, 89% spoke Spanish at home, 30% were single, approximately 70% were educated to less than high school level. 37% of the intervention group, compared with 42% of controls, were expecting their first child. | |
| Interventions | Intervention (n = 52): in addition to routine care, allocated to 2 individual educational/support sessions with an LC in the third trimester of pregnancy lasting 15‐20 min. After birth the LC provided support at the hospital or by phone soon after discharge, with further phone support after the first or second week then after 1 and 2 months. The participants were asked to contact the LC if they experienced any breastfeeding problems. Control (n = 52): routine breastfeeding education and support during the pregnancy and postpartum. LC services were available for all postpartum women if any breastfeeding problems arose during the hospital stay. | |
| Outcomes | Exclusive and any breastfeeding at 7 days and 1, 2 and 3 months postpartum | |
| Notes | Among multiparous participants, 27/29 (93%) in the intervention group had previously breastfed, compared with 17/25 (68%) in the control group. Dates of study: recruitment between March 2006 to December 2006 Funding sources: CDC/AAMC Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We used computer generated random numbers to assign women to the control and intervention groups. Each random number was related to an ordinal number that was assigned to the woman once she assigned the informed consent." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided to enable a judgement. |
| Blinding of outcome assessment (detection bias) | High risk | No details provided to enable a judgement. |
| Incomplete outcome data (attrition bias) | High risk | 104 women randomized. 82% available to follow‐up at 1 month (data included in the review) 70% of women followed up for 3 months (35/52 in intervention group completed the 3‐month follow‐up (loss of 17); 38/52 in the control group completed the 3‐month follow‐up (loss of 14)). High attrition, but reasons for loss were given and balanced across groups (e.g. phone disconnected; women did not answer phone; some women did not notify the research team about their delivery). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | There was some baseline imbalance between groups that meant that differences between groups were difficult to interpret. Of the multiparous women 93% in the intervention group had previous breastfeeding experience compared with 68% in the control group. More women in the control group had a CS (40% vs 14%). Both of these differences possibly relate to breastfeeding outcomes. |
| Study characteristics | ||
| Methods | 2‐arm RCT with individual randomisation, single‐site study, recruiting over 3 months, n = 52 | |
| Participants | Urban Canada Background rates of breastfeeding initiation: intermediate Baseline breastfeeding prevalence at 4 months: approximately 33% Inclusion criteria: singleton pregnancy, healthy mother and child, vaginal delivery, self‐identified on breastfeeding questionnaire as unsupported | |
| Interventions | Intervention: breastfeeding support from the researcher, a community midwife, consisting of daily visits in hospital, telephone call within 72 h of discharge and weekly through the fourth week postpartum, and at least 1 home visit (in the first week), with further home visits as required. Home visits lasted 60‐90 min. Control: hospital care from any member of the mother‐child nursing team | |
| Outcomes | Exclusive and partial breastfeeding at 4 weeks | |
| Notes | Dates of study: recruitment between 1st June 1991 to 31st August 1991 Funding sources: Faculty of Community Services (Schilarly, Research or Creative Activities), Ryerson Polytechnic University, Toronto, Ontario, Canada Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation procedure (stratified by planned length of breastfeeding, parity and education). |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | High risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | 52 randomized, 51 appeared to complete the study, follow‐up was 98%. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Recruitment limited by availability of investigator. No baseline imbalance apparent. |
| Study characteristics | ||
| Methods | 2‐arm RCT, n = 97 | |
| Participants | University hospital, urban in northern Thailand. Background breastfeeding rates: The United Nations Children’s Fund (UNICEF) reported that globally, only 41% of infants aged 0 to 6 months were exclusively breastfed in 2018, but in Thailand the total was only 23.1%. Inclusion criteria: pregnant; age 18 years or more; at 36‐37 weeks of gestational age; expected to have a first child as a singleton pregnancy; intending to breastfeed; having normal breasts and nipples; able to understand Thai; and contactable by phone. Exclusion criteria: a contra‐indication to BF; planning to have a caesarean section; unable to attend the entire program; undergoing a caesarean section during the program implementation; mother and infant were separated; and mother or the newborn developing health complications. | |
| Interventions | Antenatal and postnatal sessions: Session 1 (36‐37 weeks.) Providing knowledge and discussing the content on BF benefits, proper positioning and correct latch‐on, BF problems, and solutions. Demonstrating and BF practising with details on correct latch on and proper positions by using a life‐size breast model and a baby doll. Session 2 (following week) Providing knowledge and discussing the content of methods of hand expressing and storing breast milk. Reviewing the knowledge of proper BF positioning and correct latch‐on. Demonstrating and BF skills practising with details on hand expression techniques and repeating BF skills practice on the correct latch‐on and proper position. At postpartum unit Session 3 (within 24 hours after birth) Encouraging to breastfeed and providing assistance helping to adjust BF positions, giving advice and encouragement to ensure correct practice with strong verbal encouragement. Providing the information about the baby’s early feeding cues, and signs that the baby was satisfied at the end of the feeding. Providing the opportunity to significant persons in the mothers’ life learn how to BF support and encouraged to participate in practice in assisting mothers to breastfeed and take care of the infants. Session 4 (day 2 after birth) Observing the participant breastfed and providing assistance helping to adjust BF positions, giving advice and encouragement to ensure correct practice with strong verbal encouragement. Encouraging to begin hand expressing and providing assistance helping to ensure correct practice. Encouraging the significant persons in the mothers’ life practising in assisting mothers and take care of the infants. Session 5 (day 3 after birth) Observing the participant breastfed and providing assistance helping to adjust BF positions, giving advice and encouragement to ensure correct practice with strong verbal encouragement. Encouraging the significant persons in the mothers’ life practising in assisting mothers and take care of the infants. Reviewing the knowledge and skills and providing feedback for self‐evaluation Making an appointment for telephone call At Home Session 6, 7 (Evening of the discharge day, day 7 and 1 month after birth) Telephone support for counselling about BF problems and the way to solve the problem, and monitoring the EBF (10‐20 min for each call) At Hospital Session 8 (6 weeks after birth, the participants were routinely followed‐up) Counselling about BF problems and the way to solve the problem, and monitoring the EBF. (20 min) At Home Session 9‐12 (3‐6 month, once a month) Telephone support for counselling about BF problems and the way to solve the problem, and monitoring the EBF. Control: Usual care refers to the routine care provided to pregnant women and postpartum mothers by the hospital staff and midwives. Midwives in the ANC inform pregnant women about healthy behaviours during pregnancy and the benefits of BF in one session. In the postpartum unit, midwives provide support for mothers and inform mothers about the techniques of BF and newborn care. They offer a group postnatal education session with the contents covering a variety of topics such as perineum care, breast care, activities and rest, family planning, expressing and storing breast milk. | |
| Outcomes | Exclusive breastfeeding at 6 months | |
| Notes | Dates of the study: not reported. Funding sources: not reported Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | participants joined the study and were randomly assigned either to the experimental or the control groups using simple random sampling. Method of sampling not clearly described. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Not possible for self‐report breastfeeding data |
| Incomplete outcome data (attrition bias) | Low risk | Control LTF = 14% Intervention LTF = 15% |
| Selective reporting (reporting bias) | Unclear risk | No protocol available to judge outcomes |
| Other bias | Unclear risk | Conflict of interest not reported Source of funding not reported There were no statistically significant differences in demographic characteristics between the two groups |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 328 | |
| Participants | Setting: 2 hospitals (1 university and 1 community hospital) serving urban areas in Baltimore, Maryland, USA Background rates of breastfeeding initiation: intermediate Inclusion criteria: mother English‐speaking, with phone access and living within 25 miles of the hospital, intending to breastfeed, family eligible for WIC program, singleton term infant (> 37 weeks’ gestation) Exclusion criteria: infants or mothers with positive drug screen, infants with craniofacial abnormalities, infants admitted to NICU Participant characteristics: all enroled in WIC program; mean age 23.1 years; 87% African Americans; 26.5% with less than high school education; 79.6% single; 17.4% not employed or in school; 26.6% caesarean births; 50.6% first time mothers; 32.3% with previous breastfeeding experience | |
| Interventions | Intervention (n = 168): in addition to usual care, a structured programme of education and support comprising postnatal visits by a breastfeeding team (community nurse and peer counsellor) daily in hospital, 2 home visits in the first week after discharge, a third visit at approximately 4 weeks, then scheduled phone calls by the peer counsellor at least fortnightly until 24 weeks and phone access to the community nurse (24 h) for 24 weeks. Home visits lasted approximately 45‐60 min and the average length of phone calls was approximately 20 min. Control (n = 160): usual care included access to an LC in hospital and phone access after discharge home | |
| Outcomes | Any breastfeeding (breastfed at least once during the previous 24 h) at 6, 12, and 24 weeks postpartum | |
| Notes | Baseline variables were measured using established valid instruments and were used as covariates to adjust for differences between randomisation groups in some of the analyses in the paper. In our analyses we have reported unadjusted figures. Dates of study: study conducted between October 2003 and December 2005. Funding sources: National Institut of Health‐national Institute of Nursing Research Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. Block randomisation (block size 10). |
| Allocation concealment (selection bias) | Low risk | “sealed envelope technique” ... not entirely clear, not described in detail but probably adequate. |
| Blinding of participants and personnel (performance bias) | High risk | Baseline data were collected before randomisation therefore this was collected in a blind fashion, however following randomisation women and staff would be aware of group assignment. |
| Blinding of outcome assessment (detection bias) | High risk | There was a serious risk of bias associated with the lack of blinding of outcome assessors. In the intervention group outcome data were collected by the staff carrying out the intervention whereas in the control group outcome data were collected by a research interviewer who the women will not have met. |
| Incomplete outcome data (attrition bias) | Low risk | 70% of those approached randomized. 328 randomized and followed up, 29% lost to follow‐up by 24 weeks but all women included in the analyses. Women who withdrew from the study early in the project were assumed not to be breastfeeding and those who were lost subsequently were assumed not to be breastfeeding since their last contact. Both I and C groups were treated in the same way and loss was similar in the 2 groups. The numbers recorded as still breastfeeding therefore represent a conservative estimate. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol. |
| Other bias | Unclear risk | There was no apparent baseline imbalance although baseline characteristics were used in regression analysis to determine adjusted treatment effect. In our results we have reported the unadjusted data. |
| Study characteristics | ||
| Methods | 3‐arm RCT, n = 400 | |
| Participants | Obstetric practices in Split‐Dalmatia County, Croatia. Background breastfeeding rates: In Croatia 96% of women initiate BF and EBF at 6 months is 8%. Inclusion criteria: primigravidae, with a singleton pregnancy, who attended their primary care obstetrician between 20 and 32 weeks of pregnancy. Required to speak Croatian and reside within the territory of the Republic of Croatia for at least a year. Exclusion criteria: unable to communicate in Croatian by phone, planning to leave the country within a year or had a severe medical or psychiatric problem. | |
| Interventions | Received a breastfeeding booklet and a general, pregnancy booklet, followed by four proactive telephone calls–one in pregnancy and three after delivery, at 2, 6 and10 weeks. Telephone support aimed to provide women with relevant information, support and encouragement, using Michie's behaviour change technique. Booklet had information from session 3 of the BFHI course. Active Control: received a general, pregnancy booklet, followed by four proactive telephone calls–one in pregnancy and three after delivery, at 2, 6 and 10 weeks. Control: received standard care | |
| Outcomes | Exclusive breastfeeding at 3 and 6 months Maternal satisfaction with feeding method | |
| Notes | Dates of the study: Nov 2013‐ Dec 2016 Funding sources: not reported. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | the lead investigator who randomized each participant to one of three arms of the study, using a computer random number list pregenerated by a member of the research team. |
| Allocation concealment (selection bias) | Unclear risk | None described |
| Blinding of participants and personnel (performance bias) | High risk | It would be difficult for the women to remain unblinded. The nurse carrying out the intervention would not have been blinded. |
| Blinding of outcome assessment (detection bias) | High risk | It is unlikely that the women could have remained blinded and this is self‐report data. |
| Incomplete outcome data (attrition bias) | Low risk | Intervention loss to follow‐up = 5% Control loss to follow‐up =20% The ITT will have underestimated treatment effect size |
| Selective reporting (reporting bias) | Low risk | Additionally, although we did not per protocol plan to collect data on predominant breastfeeding, participants provided information for it, so we also present those results in Table A2. Other outcomes collected per protocol. Minor deviation from protocol is reported. NCT01998087. |
| Other bias | Unclear risk | Funding source not described No conflict of interest Result of baseline testing not reported
|
| Study characteristics | ||
| Methods | 2‐arm RCT, single‐site study, recruitment July 1998‐December 2000, n = 136 | |
| Participants | Urban Australia Background rates of breastfeeding initiation: high. Baseline prevalence of breastfeeding in Australia = 83% at hospital discharge Participants were recruited at a teenage pregnancy clinic serving mostly disadvantaged young women. The intervention was offered regardless of feeding intention or practice. Inclusion criteria: teenagers aged < 18 years; attending first antenatal appointment at public‐care teenage pregnancy clinic for first‐time mothers; English‐speaking; intending to continue with the pregnancy and not relinquish the infant Exclusion criteria: residence > 150 km from the study hospital; known fetal abnormality Participant characteristics: Ethnic composition of sample: 24% indigenous Australian Socioeconomic status: 86.5% of sample scored low or destitute on score derived from educational level of participant and her parents, and family income | |
| Interventions | Intervention: structured home visits in weeks 1 and 2 by certified nurse‐midwives to teach feeding and maternal‐infant bonding skills. Further visits at months 1, 2, 3 and 4 to provide advice and support. Control: routine postnatal support, counselling and information services provided by the hospital including access to routine hospital domiciliary home‐visiting services | |
| Outcomes | Adverse neonatal outcomes (infant death, severe non‐accidental injury and non‐voluntary foster care); knowledge and practice of contraception, vaccination schedules and breastfeeding | |
| Notes | Dates of study: study conducted between July 1998 and December 2000 Funding sources: Innovative Funding for Homeless Youth Support Services Grants Scheme administered by the Health Department of Australia and a National Health and Medical Research Council (Australia) Postgraduate Research Scholarship. Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer‐generated randomized allocation schedule |
| Allocation concealment (selection bias) | Low risk | Concealed in numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Women and staff aware of intervention group. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors aware of intervention group. |
| Incomplete outcome data (attrition bias) | Unclear risk | 65 assigned to the intervention and 71 to the control group. Reasons for drop out recorded, 124 completed trial (91%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | It was not clear how the intervention related to some of the outcomes (e.g. early infant death). No baseline imbalance apparent with similar numbers of women in the 2 groups initiating breastfeeding. |
| Study characteristics | ||
| Methods | 2‐arm quasi‐RCT, recruitment 1989‐1992 n = 408 | |
| Participants | Setting: study in a hospital in Zambia 408 women recruited 1 h following delivery at the study hospital Inclusion criteria: normal birth, term, singleton, Apgar score > 7 at 1 min, no visible malformation and mother and baby assessed as healthy | |
| Interventions | Intervention (n = 208): home visits by a midwife at 3, 7, 28 and 42 days. Home visits lasted about 1 h. Midwives examined women and infants and asked about their health; any health problems and related actions; breastfeeding patterns; social support (if any). If indicated, midwives referred women for medical help. Control (n = 200): home visit by a midwife at 42 days only | |
| Outcomes | Maternal and infant health problems | |
| Notes | We have not included data from this study in the review as they were not reported in a way that allowed for meta‐analysis. Numbers of breastfeeding women were not reported by randomisation group. Dates of study: study was conducted between May 1989 and February 1992. Funding sources: the study was supported by the Swedish Agency for Research Collaboration and Developing Countries (SAREC), The Norwegian Aid Development (NORAD), The Ministry of Health in Zambia, University Teaching Hospital, Lusaka, School of Medicine, University of Zambia and Stockholm University College of Health Sciences. Declaration of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 2‐stage randomisation process with recruitment on certain days, when women were randomly selected to be randomized to treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | It was not clear whether the person carrying out the randomisation had any control over the sample selection and the randomisation process. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Hospital staff were unaware of group allocation. Unclear if midwife delivering intervention and women were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Data were collected by research midwives but unclear if they were blinded to group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Participants seen at follow‐up for the intervention group‐ 98.5% at day 3, 97.5% at day 7, 87% at day 28 and 89% at day 42. Participants seen at follow‐up for the control group ‐ 87% at day 42. Loss to follow‐up < 20% at each follow‐up visit. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Baseline characteristics were similar. |
| Study characteristics | ||
| Methods | 2‐arm RCT n = 235 | |
| Participants | Setting: 235 eligible and consenting women booked for delivery at an Australian hospital in 1989 Inclusion criteria: primiparous women who expressed a wish to breastfeed, who booked for delivery before 20 weeks’ gestation, aged between 18‐35 years and lived within 20 km of the hospital Exclusion criteria: women who received additional care from independent midwives | |
| Interventions | Intervention: programme of care based on health belief model and cognitive‐behavioural principles, including a 3‐h group teaching session in the antenatal period and a visit by an LC shortly after hospital birth, phone support 2‐3 weeks later and at 3 months, with a home visit if needed. The LC was available to provide telephone support at other times. Control: usual breastfeeding care and advice along with routine antenatal classes | |
| Outcomes | Breastfeeding at 6 weeks and 4 months postdelivery, reasons for stopping breastfeeding, satisfaction with the intervention | |
| Notes | We have not included data from this study in the review due to very high attrition rates which meant results were difficult to interpret. In this study women were recruited in the antenatal period. 235 women were randomised; 30% were lost to follow‐up by 6 weeks postpartum (and full interview data were available for only 56% of the sample). Dates of study: recruitment between the middle of August 1989 to the end of November 1989 Funding sources: New South Wales Department of Health Declaration of interest: Nnt reported
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate, by odd or even numbered consent forms. It was stated that forms were given out sequentially. |
| Allocation concealment (selection bias) | Unclear risk | Odd or evenly numbered consent forms. It was stated that those carrying out recruitment and the women were not aware of the code for allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Women and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Data were collected using a self‐completed questionnaire. it is not detailed if the questionnaire contained any information that could identify allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | High loss to follow‐up with interview data at 6 weeks for only 56% of the sample randomized. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Baseline characteristics similar ‐ no significant differences between control and intervention groups on any of these variables. |
| Study characteristics | ||
| Methods | 3‐arm, parallel RCT, n = 1948 | |
| Participants | English‐ or Spanish‐speaking recipients of the Supplemental Nutrition Program for Women, Infants, and Children (WIC) in Oregon, USA Background breastfeeding imitation rates: 90% Inclusion criteria: English‐ and Spanish‐speaking women attending a new pregnancy appointment for the Supplemental Nutrition Program for Women, Infants, and Children (WIC) programme between July 2005 and July 2007; intending to breastfeed or undecided about breastfeeding Exclusion criteria: no exclusions on the basis of age, multiple gestations, known risk factors or previous birth history | |
| Interventions | Intervention 1 (n = 646): low frequency peer counselling; women received 4 planned, peer‐initiated contacts: the first after initial prenatal assignment, the second 2 weeks before the expected due date, and the third and fourth at 1 and 2 weeks postpartum Intervention 2: (n = 645): high frequency peer counselling; women received 8 scheduled calls. The first 4 calls were the same as those in the low‐frequency treatment group and the last 4 calls were scheduled at months 1, 2, 3, and 4. | |
| Outcomes | Breastfeeding imitation Exclusive breastfeeding at 1, 3 and 6 months Any breastfeeding at 1, 3 and 6 months | |
| Notes | Authors contacted for data 21 July 2016. Dates of study: between July 2005 and July 2007 Funding sources: US Department of Agriculture, Food and Nutrition Service, WIC Special Project Grant WISP‐04‐OR‐01 and grant R03 HD072991‐01 from the National Institutes of Child Health and Human Development to the Research Foundation of the City University of New York. Funded by the National Institutes of Health (NIH). Declaration of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomly allocated to 1 of 3 study arms by using a computer‐generated random number function. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Peer counsellors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data "mothers were queried" |
| Incomplete outcome data (attrition bias) | Low risk | Control FU: 97% Low intensity support: 97% High intensity support: 97% |
| Selective reporting (reporting bias) | High risk | Actual data on breastfeeding at 1, 3and 6 months is not reported and cannot be calculated from what is provided. Author contacted but no response. |
| Other bias | Low risk | There was no evidence of pretreatment covariate imbalance among women assigned to a treatment arm No conflicts of interest or industry funding |
| Study characteristics | ||
| Methods | RCT | |
| Participants | Background breastfeeding rates: not reported in translation Inclusion criteria: Iranian nationality, primiparous, at least 31 weeks of gestation (based on LMP or sonography in the first trimester), mothers being 15‐35 years old, lack of any acute or chronic physical or mental disorder in the mother (self‐reporting), non‐smoker, non‐drug user, singleton, having adequate education to read and write, having telephone number for follow‐ups using telephone, and mother’s tendency to participate in the study Exclusion criteria: congenital disease and disorder in the infant, infant’s hospitalisation, mother’s hospitalisation after the delivery, severe abscess in the breast, depression after delivery, consumption of medicines that are contraindicated in lactation, infant’s death, and breastfeeding cessation with the physician’s approval | |
| Interventions | Women were in groups of 8‐12 participants. Motivational interviewing sessions were held at 31‐34 weeks, 35‐37 weeks, 38‐39 weeks, 3‐5 day post‐partum and prior to the infant being 2 months. Sessions lasted 2hrs and were conducted by the researchers. Women also received telephone calls at discharge, infant being 1 month and 4 months. The purpose of the call was to support lactation. A channel in telegram was formed for this group and topics that were related to exclusive lactation success and the answer to the frequently asked questions by the mothers were submitted there. The content of the sessions was organised based on the scientific literature by the researchers. Control: active control group. Women received a 2hr speech (?education) session which lasted 2hrs and included question and answer. This was based on literature on lactation and took place at 31‐34 weeks. | |
| Outcomes | Exclusive breastfeeding at 6 months | |
| Notes | Dates of the study: not reported Funding sources: not reported in translation Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The random allocation sequence is determined using the block method to volume 6 using the software. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Women aware of their group allocation. |
| Blinding of outcome assessment (detection bias) | High risk | The researcher performed the intervention so was not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up is 95% but it is not clear if there were differences between the groups. |
| Selective reporting (reporting bias) | High risk | Protocol was registered during recruitment phase. Self‐efficacy was a variable listed in the protocol which was not reported in the paper. Protocol states breastfeeding data was collected at 4 months but in the paper it is reported to be 3 months. |
| Other bias | Unclear risk | Full transcription of paper not available therefore unable to make clear judgement. No information in translation on funding or conflicts of interest. No baseline imbalance. |
| Study characteristics | ||
| Methods | 3‐arm RCT, with individual randomisation, single‐site study, recruitment August 2000‐July 2002, n = 101 | |
| Participants | Urban setting in Minas Gerais, Brazil Background rates of breastfeeding initiation: high. Baseline prevalence of breastfeeding in Brazil in the first 30 days = 88% Inclusion criteria: mother breastfeeding her well, term baby when appointment for paediatric clinic made; first clinic consultation took place at ≤ 30 days Exclusion criteria: mothers who expressed a preference to see a particular paediatrician; babies no longer breastfed at the first appointment Ethnic composition: 62% of babies white | |
| Interventions | Intervention 1: babies were monitored by a paediatrician working with a multidisciplinary breastfeeding team. The paediatrician and team had all received training to promote exclusive breastfeeding (PNIAM: Programa de Incentivo ao Aleitamento Materno, Brazil). Intervention 2: babies were monitored by the same paediatrician, in individual consultations. Control: babies were monitored by a paediatrician who did not have formal training to promote exclusive breastfeeding. | |
| Outcomes | Exclusive breastfeeding to 4 months | |
| Notes | Dates of study: study conducted between August 2000 and July 2002 Funding sources: CAPES (doctoral grant to L.B. Santiago) Declaration of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | (Risk of bias assessment from translation notes.) Random assignment by drawing lots. |
| Allocation concealment (selection bias) | Unclear risk | Random assignment by drawing lots. Described as simple randomisation in translation notes. |
| Blinding of participants and personnel (performance bias) | High risk | It was stated that staff were aware of group assignment. It is unclear if women were aware of allocation. |
| Blinding of outcome assessment (detection bias) | High risk | It is stated that "information was collected by the author of each child's medical record. it is unclear if it was 1 of the paediatricians that completed or if it was someone who was blinded to allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not clear at what time randomisation took place or the number randomized to each group "the exclusion percentages were similar in the three groups". 190 were eligible and 101 completed the study. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | No baseline imbalance apparent. It was not clear how many women were randomized. |
| Study characteristics | ||
| Methods | 3‐arm, parallel RCT, n = 505 | |
| Participants | Low‐income women attending for antenatal care at a large hospital in Kenya Background rates of breastfeeding imitation: 56.1% No details about inclusion and exclusion criteria provided. | |
| Interventions | Intervention 1: continuous cell phone‐based peer support (CPS); support was provided by trained peer support leaders from late pregnancy (32‐36 weeks) until 3 months postpartum Intervention 2: monthly peer support group (PSG). Support was provided by trained peer support leaders from late pregnancy (32‐36 weeks) until 3 months postpartum. Control: standard care by existing facility‐based support No details provided about the total number randomised in each group. | |
| Outcomes | Exclusive breastfeeding at 3 months | |
| Notes | Conference abstract only. SM contacted authors for more information 20 July 2016. Dates of the study: NR Funding sources: Bill and Melinda Gates Foundation to FHI 360, through the Alive & Thrive Small Grants Program managed by UC Davis; Global Alliance for Improved Nutrition; Canada Research Chair program award. Declarations of interest: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “We randomized at third trimester”Method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data so not possible |
| Incomplete outcome data (attrition bias) | High risk | 81% of enrolled women were eligible, 66.6% of enrolled women completed |
| Selective reporting (reporting bias) | High risk | No published protocol. We did recieve a protocol from study authors which stated that EBF at 7 days was an outcome however the data for this is not provided. |
| Other bias | Unclear risk | No industry funding Conficts of interest not reported |
| Study characteristics | ||
| Methods | 2‐arm cluster‐RCT, n = 452 | |
| Participants | Union Councils in a rural, resource‐poor district in the northwest province of Pakistan with high infant mortality Background rates of breastfeeding initiation: Pakistan has 8% exclusive breastfeeding before 6 months. Inclusion criteria: women aged 17‐40 years, married, in their third trimester of pregnancy, and intending to reside in the study area for the duration of the study Exclusion criteria: women with diagnosed serious medical/psychiatric condition requiring treatment, pregnancy‐related illness (except for common conditions, such as anaemia), and substantial physical/learning disability | |
| Interventions | Intervention (n = 224): 7 psycho‐educational sessions integrated into the routine work of lady health workers (LHWs) and delivered to all women in their Union Council catchment areas. First session delivered before birth, second session immediately after birth, and the remaining 5 sessions monthly thereafter. The intervention had 6 components: developing an empathic relationship: a trusting, safe, alliance with the mother and other family members; collaborating with the family in an equal partnership; using guided discovery: a style of engagement to gently probe for the individual and family’s health beliefs, and also to stimulate alternative ideas; putting knowledge into practice and behavioural activation; and problem‐solving. LHWs underwent 2‐day (12 h) training in simplified cognitive behavioural therapy principles using participatory approaches. Control (n = 228): women received an equal number of visits in exactly the same way as those in the intervention arm, but by routinely trained LHWs. | |
| Outcomes | Primary: rate and duration of exclusive breastfeeding in the first 6 months Secondary: impact on traditional practices impeding exclusive breastfeeding | |
| Notes | Dates of study: study conducted between May 2009 and April 2010 Funding sources: PRIDE, Pakistan (Primary Health care Revitalisation, Integration and Decentralisation in Earthquake Affected Areas), a project funded by the US Agency for International Development. Declaration of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Simple unmatched randomization" |
| Allocation concealment (selection bias) | Low risk | Randomisation by an independent researcher |
| Blinding of participants and personnel (performance bias) | High risk | Participants and LHWs were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | The assessors were blind to the allocation status of the mother |
| Incomplete outcome data (attrition bias) | Low risk | 19% attrition in intervention group and 22% attrition in control group in 6 months after birth. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported on. |
| Other bias | Low risk | None identified |
| Study characteristics | ||
| Methods | 2‐arm RCT, n = 182 | |
| Participants | Suburban hospital and community health and social services trust that served both urban and rural areas in Northern Ireland Background rates of breastfeeding initiation: not detailed Inclusion criteria: primigravid women who intended to have their baby within the trust and who attended the routine 20‐week antenatal appointment during the recruitment phase Exclusion criteria: women who did not speak English (or had interpretation services available), women who experienced infant‐maternal separation and incidences of newborn abnormalities that required additional infant feeding support, or teenagers who had already attended a breastfeeding workshop | |
| Interventions | Intervention (n = 93): motivationally‐enhanced version of midwife instruction as a means of increasing women's expectancy for successful breastfeeding, compared to best practice. The intervention had 4 components: antenatal feeding class (32‐36 weeks' gestation), a breastfeeding information book (provided in the antenatal phase), a breastfeeding CD‐ROM, postnatal instructional support provided by midwives (up to 3 weeks postnatal) and additional lactation consultancy on request. The postnatal midwives who supported the intervention attended an additional 1‐day training session that focused on the role of human motivation and the use of effective strategies to increase participants' expectancy for success. Control (n = 89): local best practice | |
| Outcomes | Primary: women's motivation towards breastfeeding Secondary: breastfeeding on discharge from hospital and at 3 weeks | |
| Notes | Dates of study: recruitment from December 2005 to March 2006 Funding sources: Research and Development Office of Northern Ireland Declaration of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned using computer‐generated random numbers to the intervention or control groups. |
| Allocation concealment (selection bias) | Unclear risk | No details provided to enable judgement of this. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were blinded to group membership but unclear if this was successful. Midwives were informed of the allocation through a colour‐coded sticker on the women‐held records. |
| Blinding of outcome assessment (detection bias) | High risk | Not stated whether researcher or parent education co‐ordinator who collected the data were blinded. |
| Incomplete outcome data (attrition bias) | High risk | Attrition in intervention group was 26% and in control group was 16%. The withdrawal rate was higher in the intervention group (n = 13) compared to the control group (n = 2). |
| Selective reporting (reporting bias) | Unclear risk | No evidence of prespecified outcomes to judge this. |
| Other bias | Low risk | None identified |
| Study characteristics | ||
| Methods | 3‐arm RCT, with individual randomisation, n = 450 | |
| Participants | National University Hospital, Singapore Background rates of breastfeeding initiation: high Inclusion criteria: healthy pregnant women attending antenatal clinics at the study hospital, with no illness that would contraindicate breastfeeding or severely compromise its success; intending to breastfeed; birth at 34 weeks' gestation or later Exclusion criteria: women with high risk and multiple pregnancies Participant characteristics: 40% primiparous, mixed ethnicity (Chinese 31%‐44%, Malay 46%‐54%, Indian and other), approximately a third educated beyond secondary school, approximately half employed outside the home, 56% had previously breastfed | |
| Interventions | Intervention 1: antenatal education: in addition to routine care, women received 1 session of antenatal breastfeeding education and printed guides on breastfeeding. Intervention 2: postnatal lactation support: in addition to routine care, women received 2 postnatal sessions with an LC, 1 in hospital within the first 3 postnatal days (when they received the same printed guides on breastfeeding as the antenatal education group) and 1 during the first routine postnatal visit 1 to 2 weeks after the birth. Each session lasted about 30 min and covered latching on, proper positioning and other techniques to avoid common breastfeeding complications. Control: women received routine antenatal, intrapartum and postnatal care, including optional antenatal classes and postnatal visits by an LC should any problems with breastfeeding arise. | |
| Outcomes | Exclusive and any breastfeeding at hospital discharge and 2 weeks, 6 weeks, 3 and 6 months after the birth. Exclusive breastfeeding was defined as giving breast milk as the only food source, with no other foods or liquids, other than vitamins and minerals being given. | |
| Notes | Intervention group 1, who received the antenatal intervention, are not included in the analysis in this review.
Dates of study: recruitment from February 2004 to September 2005 Funding sources: National Healthcare Group Declaration of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence by external clinical trials unit |
| Allocation concealment (selection bias) | Low risk | Telephone allocation by external trials unit (with envelope back up used only on 4 occasions) |
| Blinding of participants and personnel (performance bias) | High risk | For participants, blinding was not mentioned, but women would be aware of allocation. The caregivers who delivered the intervention would be aware of allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Data collection was on standard forms and was entered by remote unit, therefore outcome assessment may have been partially blinded. Not clear who conducted the actual interviews. |
| Incomplete outcome data (attrition bias) | Unclear risk | Low attrition in all arms. In total 450 randomized 347 completed follow‐up at 6 months (82%). In the data and analyses, 2 arms included 299 randomized, 245 followed up at 6 months (82%).
|
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | There was an imbalance in the groups due to 4 women being randomized by using back up envelopes because of dysfunction in web randomisation, but groups appeared similar at baseline. Some of the data were based on assumptions. Sensitivity analyses were based on the assumption that none of the women lost to follow‐up were exclusively breastfeeding at any time point. |
| Study characteristics | ||
| Methods | 2‐arm RCT, n = 357 | |
| Participants | Public maternity hospital in Kuala Lumpur Background rates of breastfeeding initiation: 92.2% of mothers were exclusively breastfeeding at the study site before discharge. Inclusion criteria: 18 years of age or older; of Malaysian nationality; delivered a single infant at ≥ 37 weeks' gestation; an intention to breastfeed and the ability to understand and communicate in spoken Malay or English; had received a prenatal breastfeeding education programme at least once; had telephone access; and gave informed consent Exclusion criteria: women with multiple pregnancies or medical problems that might hinder breastfeeding; women that delivered via caesarean section; or women whose baby subsequently required prolonged care in a Special Care Nursery | |
| Interventions | Intervention (n = 179): lactation counselling given by certified LCs via telephone twice monthly to each lactating mother, in addition to the current conventional care (as descried below). Each mother was expected to receive 12 lactation counselling sessions by the end of the study. Contact was discontinued any time that a mother decided to stop breastfeeding completely. Contact was also discontinued if the mother had given the baby up for foster care and/or had no physical contact to enable her to breastfeed. LCs in this study were registered nurses from the Maternity Hospital Kuala Lumpur who had post‐basic training in midwifery and were certified as LCs. All 12 LCs had undergone a 40‐h lactation management and counselling course based on the WHO module. Control (n = 178): mothers received current conventional care for postnatal breastfeeding promotion or support from their own public healthcare provider. This conventional care included breastfeeding talks during immunisation follow‐ups, a mothers’ communication with the LCs through information or pamphlets received during antenatal or postnatal follow‐ups, and advice regarding breastfeeding received at any time from any healthcare workers, the media, peer counsellors, family members or friends. | |
| Outcomes | Exclusive breastfeeding at 1, 4 and 6 months Stopped any breastfeeding at 1, 4 and 6 months | |
| Notes | Dates of study: recruitment between April 2010 and July 2010. Follow completed in February 2011 Funding sources: Institute of Research Management and Consultancy, University of Malaya Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Generation of the group assignments was conducted using a blocked randomisation method with a block size of four by a random allocation software program" |
| Allocation concealment (selection bias) | Unclear risk | No details provided to enable judgement of this. |
| Blinding of participants and personnel (performance bias) | High risk | The women and LCs were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Only the Research Enumerator who collected the breastfeeding outcome data was blinded with respect to the treatment group" |
| Incomplete outcome data (attrition bias) | Low risk | Attrition in intervention and control group was 89.4% and 88.8% respectively. |
| Selective reporting (reporting bias) | Low risk | Outcomes are reported per protocol ACTRN12611001014943 |
| Other bias | Low risk | No industry funding No conflicts of interest No baseline imbalance in key variables |
| Study characteristics | ||
| Methods | 4‐arm RCT, 1 study site, n = 802 | |
| Participants | Maternity hospital in Dunedin, New Zealand Background rates of breastfeeding initiation: not specified. Inclusion criteria: all mothers who had booked into the single maternity hospital (> 97% of all births) serving the city of Dunedin, New Zealand, between May 2009‐November 2010, as well as mothers who planned to give birth at home and were invited to participate by their midwife. Mothers were invited to participate at 28‐30 weeks gestation and an 'opt out' recruitment strategy (eligible participants were contacted and excluded only when they said they were unwilling to participate) was used. Exclusion criteria before birth: home address outside the greater Dunedin area, planning to move away from Dunedin in the next 2 years, booked into the maternity centre after 34‐week gestation, or unable to communicate in English or Te Reo Maori [language of the indigenous (Maori) ethnic group of New Zealand]. Exclusion criteria after birth: identification of a congenital abnormality that was likely to affect feeding or growth, or the infant being born before 36.5 weeks gestation. When a mother delivered twins, the oldest child was recruited into the study. There were no triplets born during the study recruitment period. | |
| Interventions | Interventions: various types of support: 1) infant sleep education only intervention (sleep); 2) Food, physical activity and breastfeeding (FAB) intervention: LC providing food, activity and breastfeeding help intervention; 3) combination of both 1 and 2 (Combo), participants received both the sleep and FAB interventions. Total number randomised: n = 802 (sleep 192, FAB 205, Combo 196) Control: usual care n = 209 | |
| Outcomes | Outcomes: delayed introduction of complementary foods at 5 months and preferably until 6 months Complementary foods were defined as foods other than breast milk or infant formula (p 1483) Exclusive and any BF at 6 weeks, 8 weeks, 13 weeks and 26 weeks. | |
| Notes | Dates of study: not reported Funding sources: Health Research Council of New Zealand Declarations of interest: not reported Additional study data on BF outcomes provided by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computerised random‐number generator, which assigned blocks of participants to the 4 arms. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed and performed after application of the prebirth exclusion criteria, stratified by socioeconomic status with use of the New Zealand Deprivation Index 2006. |
| Blinding of participants and personnel (performance bias) | High risk | Group allocation was revealed to the participant after consent to participate had been obtained. |
| Blinding of outcome assessment (detection bias) | High risk | Most of the breastfeeding and other data were collected by a researcher who was not aware of the participants' group, and no data were collected by the LC who delivered the intervention. The statistician remained blinded to group allocation codes until primary analyses were conducted. However, the breastfeeding data was self‐report and women were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up C = 85.6% Follow‐up FAB = 86.3% Follow‐up Combination = 85% |
| Selective reporting (reporting bias) | High risk | Breastfeeding data not reported in paper: "No significant differences in full or any breastfeeding, family quality of life, or the proportion of children watching television were observed (data not shown)". Data was obtained from the author. |
| Other bias | Low risk | Appears to be free of demographic variables, looks comparable across the groups. No conflict of interest or industry funding. |
| Study characteristics | ||
| Methods | One of the 3 country sites that completed this cluster‐randomised trial ‐ Burkino Faso in French‐speaking West Africa (24 clusters: 12 intervention, 12 control). Mother‐infant pairs enroled: 392 intervention and 402 control (794 total). Followed up at 24 weeks: 359/392 (92%) intervention and 372/402 (93%) control | |
| Participants | Rural area where main source of income was farming. 60 primary care facilities and a regional hospital Baseline prevalence of breastfeeding initiation: high (98.4%). Exclusive breastfeeding for babies under 6 months estimated at 16% Inclusion criteria: women living in trial area at least 7 months pregnant and intending to breastfeed, singleton live birth, no serious congenital malformations Exclusion criteria: mothers or infants who died were not included in the analysis Participant characteristics: Mean age of women 25 years. None of the women had any formal education and more than half had had a previous child death. 99% of women had no toilet or an open toilet and < 1% had piped water in yard or home. Monthly income was approximately EUR 3. | |
| Interventions | Intervention: peer counselling by supporters who received a modified version of WHO/UNICEF training (1‐week training). Women were given information about breastfeeding and peers provided support and addressed problems or referred women for specialist help. The intervention involved a minimum of 5 home visits, 1 in the third trimester and at least 4 in the postnatal period up to 6 months postpartum. The supporters were local residents, literate, able to travel to visit women in their homes and had a good reputation in the community. Peer counsellors visited the same women each time to achieve continuity of care. The intervention varied in the 3 study areas and was adapted to local circumstances. Control: mothers and infants in control clusters in Burkina Faso were given standard healthcare only. | |
| Outcomes | Prevalence of exclusive breastfeeding and prevalence of diarrhoea, reported by mothers for infants aged 12 weeks and 24 weeks | |
| Notes | The paper reported that current breastfeeding was assessed at all scheduled postpartum visits using past 24‐h and 7‐day recalls. Babies who were reported to have received no other food or liquids than breast milk (they may have been administered drugs) were classified as exclusively breastfed. This may have been during the last 24 h or 7 days rather than since birth. Prevalence of diarrhoea was based on the mothers’ reports of the past 2 weeks. Dates of Study: Recruitment between May 2006 and July 2008 Funding sources: European Union Sixth Framework International Cooperation–Developing Countries ; Research Council of Norway; Swedish International Development Cooperation Agency; Norwegian Programme for Development, Research and Education; Rockefeller Brothers Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (different procedures in different areas and the procedure in 1 of the 4 areas was not clear) . |
| Allocation concealment (selection bias) | Unclear risk | There was no allocation concealment within clusters and participants would be aware of assignment. |
| Blinding of participants and personnel (performance bias) | High risk | There was no participant or staff blinding. |
| Blinding of outcome assessment (detection bias) | High risk | There was an attempt to mask/blind outcome assessors to randomisation group, although it is possible women would have revealed whether or not they received support. The success of attempted blinding was not formally evaluated. |
| Incomplete outcome data (attrition bias) | Low risk | There was flooding in 1 of the 4 original study area and no results were reported for this area. For the remaining 82 clusters in 3 countries for primary outcomes the authors carried out an ITT analysis (i.e. those that were missing were recorded as non‐events, i.e. NOT exclusive breastfeeding and no diarrhoea). 2579 women enrolled. Missing data and missed visits at various data collection points. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent |
| Other bias | Unclear risk | Recruitment procedures and intervention delivery was slightly different in each of the study countries which meant that results were difficult to interpret. It was reported that the ICC in each country for primary outcomes varied considerably and therefore results were reported separately for each country. Authors stated, “The community‐based approach could possibly have resulted in socially desirable answers, and the results were based on self‐reports. A bias towards desirable answers and thereby an increased effect size cannot be ruled out. We also noted some questionnaire fatigue in the Ugandan site—i.e. reluctance to fully engage in answering similar questions after a few interviews". |
| Study characteristics | ||
| Methods | Second of 3 country sites that completed the cluster‐randomised trial ‐ Mbale district in Eastern Uganda (24 clusters: 12 intervention, 12 control). Mother‐infant pairs enroled: 396 intervention and 369 control (765 total). Followed up at 24 weeks: 368/396 (93%) intervention and 329/369 (89%) control | |
| Participants | Urban and rural areas: urban area included “large slum migrant settlements”. Background rates of breastfeeding initiation: high (> 95%) Inclusion criteria: women living in trial area at least 7 months pregnant and intending to breastfeed, singleton live birth, no serious congenital malformations Exclusion criteria: mothers or infants who died were not included in the analysis Participant characteristics: HIV prevalence for fertile women was 6.2%. 26% of women had no toilet or an open toilet and 5% had piped water in yard or home. Mean age 25 years. Women had approximately 6 years of formal education and approximately a third had had a previous child death. Monthly income was approximately EUR 12. | |
| Interventions | Intervention: peer counselling as in Burkina Faso (Tylleskar 2011a). Paper stated the intervention varied in the 3 study areas and was adapted to local circumstances. Control: mothers and infants in control clusters in Uganda were given standard healthcare only. | |
| Outcomes | Prevalence of exclusive breastfeeding and prevalence of diarrhoea, reported by mothers for infants aged 12 weeks and 24 weeks | |
| Notes | The paper stated that current breastfeeding was assessed at all scheduled postpartum visits using past 24‐h and 7‐day recalls. Babies reported to have received no other food or liquids than breast milk (they may have been administered drugs) were classified as exclusively breastfed. This may have been during the last 24 h or 7 days rather than since birth. Prevalence of diarrhoea was based on the mothers’ reports of the past 2 weeks. Dates of Study: Recruitment between May 2006 and July 2008 Funding sources: European Union Sixth Framework International Cooperation–Developing Countries ; Research Council of Norway; Swedish International Development Cooperation Agency; Norwegian Programme for Development, Research and Education; Rockefeller Brothers Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (different procedures in different areas and procedure in 1 of the 4 areas was not clear) |
| Allocation concealment (selection bias) | Unclear risk | There was no allocation concealment within clusters and participants would be aware of assignment. |
| Blinding of participants and personnel (performance bias) | High risk | There was no participant or staff blinding. |
| Blinding of outcome assessment (detection bias) | High risk | There was an attempt to mask/blind outcome assessors to randomisation group, although it was possible women would have revealed whether or not they received support. The success of attempted blinding was not formally evaluated. |
| Incomplete outcome data (attrition bias) | Low risk | There was flooding in 1 of the 4 original study area and no results were reported for this area. For the remaining 82 clusters in 3 countries for primary outcomes the authors carried out an ITT analysis (i.e. those that were missing were recorded as non‐events, i.e. NOT exclusive breastfeeding and no diarrhoea). 2579 women enrolled. Missing data and missed visits at various data collection points. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Recruitment procedures and intervention delivery was slightly different in each of the study countries which meant that results were difficult to interpret. It was reported that the ICC in each country for primary outcomes varied considerably and therefore results were reported separately for each country. Authors stated, “The community‐based approach could possibly have resulted in socially desirable answers, and the results were based on self‐reports. A bias towards desirable answers and thereby an increased effect size cannot be ruled out. We also noted some questionnaire fatigue in the Ugandan site—i.e. reluctance to fully engage in answering similar questions after a few interviews". |
| Study characteristics | ||
| Methods | Third of 3 country sites that completed this cluster‐randomised trial ‐ 3 geographically separate sites in South Africa (Paarl, a town at the centre of a farming district near Cape Town; Umlazi, a large periurban township near Durban; and Rietvlei, 1 of the country's poorest rural districts: 34 clusters: 17 intervention, 17 control). Mother‐infant pairs enrolled: 535 intervention and 485 control (1020 total). Followed up at 24 weeks: 461/535 (86%) intervention and 410/485 (85%) control | |
| Participants | South Africa (3 areas including 1 of the poorest rural area in South Africa) Under 5 mortality rate in South Africa was 67/1000 and infant mortality rate was 48/1000. Background rates of breastfeeding initiation: high (> 95%). Exclusive breastfeeding at 6 months was estimated at 8% in 2005‐2009. Inclusion criteria: women living in trial area at least 7 months pregnant and intending to breastfeed, singleton live birth, no serious congenital malformations Exclusion criteria: mothers or infants who died were not included in the analysis Participant characteristics: 16% of women had no toilet or open toilets and 66% had piped water in yard or home. Mean age 23 years. Women had approximately 10 years of formal education and approximately 7% had had a previous child death. Monthly income was approximately EUR 103. | |
| Interventions | Intervention: peer counselling as in Burkina Faso and Uganda (Tylleskar 2011a; Tylleskar 2011b). Paper stated the intervention varied in the 3 study areas and was adapted to local circumstances. Control: control clusters were visited by peer counsellors, with the same schedule as the intervention clusters, but they assisted families in obtaining birth certificates and social welfare grants. The peer counsellors for the intervention and control clusters in South Africa were kept separate during the study. | |
| Outcomes | Prevalence of exclusive breastfeeding and prevalence of diarrhoea, reported by mothers for infants aged 12 weeks and 24 weeks | |
| Notes | The paper stated that current breastfeeding was assessed at all scheduled postpartum visits using past 24‐h and 7‐day recalls. Babies reported to have received no other food or liquids than breast milk (they may have been administered drugs) were classified as exclusively breastfed. This may have been during the last 24 h or 7 days rather than since birth. Prevalence of diarrhoea was based on the mothers’ reports of the past 2 weeks. Dates of Study: Recruitment between May 2006 and July 2008 Funding sources: European Union Sixth Framework International Cooperation–Developing Countries ; Research Council of Norway; Swedish International Development Cooperation Agency; Norwegian Programme for Development, Research and Education; Rockefeller Brothers Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (different procedures in different areas and procedure in 1 of the 4 areas was not clear) |
| Allocation concealment (selection bias) | Unclear risk | There was no allocation concealment within clusters and participants would be aware of assignment. |
| Blinding of participants and personnel (performance bias) | High risk | There was no participant or staff blinding. |
| Blinding of outcome assessment (detection bias) | High risk | There was an attempt to mask/blind outcome assessors to randomisation group although it is possible women would have revealed whether or not they received support. The success of attempted blinding was not formally evaluated. |
| Incomplete outcome data (attrition bias) | Low risk | There was flooding in 1 of the 4 original study area and no results were reported for this area. For the remaining 82 clusters in 3 countries for primary outcomes the authors carried out an ITT analysis (i.e. those that were missing were recorded as non‐events, i.e. NOT exclusive breastfeeding and no diarrhoea). 2579 women enrolled. Missing data and missed visits at various data collection points. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent |
| Other bias | Unclear risk | Recruitment procedures and intervention delivery was slightly different in each of the study countries which meant that results were difficult to interpret. It was reported that the ICC in each country for primary outcomes varied considerably and therefore results were reported separately for each country. Authors stated, “The community‐based approach could possibly have resulted in socially desirable answers, and the results were based on self‐reports. A bias towards desirable answers and thereby an increased effect size cannot be ruled out. We also noted some questionnaire fatigue in the Ugandan site—i.e. reluctance to fully engage in answering similar questions after a few interviews". |
| Study characteristics | ||
| Methods | 3‐arm RCT, with individual randomisation, n = 300 | |
| Participants | A public sector maternal child health (MCH) clinic in Nairobi, Kenya. Background breastfeeding rates: Not reported Inclusion criteria: 14 years of age or older, pregnant and < 36 weeks estimated gestational age, had access to a mobile phone (shared or personal) using the Safaricom Ltd network, were able to communicate via SMS, planned to remain in the area for 6 months postpartum, and were not part of another research study. Exclusion criteria: Unwilling or unable to meet inclusion criteria. | |
| Interventions | Women in the one‐way and two‐way trial arms were registered into the Mobile WACh SMS delivery platform and indicated their preferences for message delivery including their name, language, and day of the week and time for delivery. Participants were classified into tracks (routine, adolescents, first‐time mothers, women with a previous caesarean section, and those with multiple gestations) with messaging tailored to the specific track. Participants received routine messages unless they met criteria for another track. The automated system incorporated a personalised approach that provided gestational age‐appropriate educational and counselling messaging. All messages included participant name, clinic and nurse name, an educational message, and actionable advice targeting one of the main study outcomes. SMS topics included ANC, pregnancy complications, family planning, infant health, EBF, infant immunisation, and visit reminders. All messaging was free of charge to the participant using a reverse billed short code. Women randomised to the one‐way group received weekly ‘push’ educational and motivational SMS. The two‐way group received the same weekly SMS; however, each SMS contained a question related to the content ‐ replies to SMS questions were voluntary. Women were also encouraged to send SMS with concerns or questions. The study nurse was available to answer SMS daily on week‐days. A clinician (JU) reviewed messages twice monthly for quality assurance.SMS were sent from enrolment until 12 weeks postpartum. Control: Routine clinic‐based counselling and care. | |
| Outcomes | Exclusive breastfeeding at 2,3,6 months | |
| Notes | Dates of the study: recruited between August 2013 and April 2014 Funding sources: National Institutes of Health (K12HD001264 to JAU, R01HD080460, K24HD054314 to GJS, and K01AI116298 to ALD), the National Science Foundation (Graduate Research Fellowship to TP and BD), as well as the University of Washington Global Center for Integrated Health of Women Adolescents and Children (Global WACh). Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician generated a com‐ puter‐generated randomisation list using random block sizes. |
| Allocation concealment (selection bias) | Low risk | Thee allocation codes were placed in sequentially numbered, opaque, sealed envelopes and distributed by research staff. Envelopes were sequentially provided to par‐ ticipants at randomisation. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Delivery information was ascertained by self‐ report at the 2‐week visit. |
| Incomplete outcome data (attrition bias) | Low risk | Retention at F1 = Control 86% ; one way SMS 81%; two way SMS 84% Retention at F2 = Control 91%; one way SMS 86%; two way SMS 82% |
| Selective reporting (reporting bias) | Low risk | All primary outcome are reported NCT01894126 |
| Other bias | Low risk | Authors reported no conflicts of interest and no industry funding Significant differences between the groups were not reported on.
|
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 203 | |
| Participants | Rural, North Central Pennsylvania, USA Background breastfeeding rates: breastfeeding initiation rates among the hospital’s patients was (74%) compared to national average of 80%. Inclusion criteria: at least 18 years old, spoke English, had a valid e‐mail address, had a singleton baby at a gestational age of at least 35 weeks, and had initiated breastfeeding and planned to continue after hospital discharge. Exclusion criteria: planned separation from the infant (eg, incarceration), NICU stay, or having a condition where breastfeeding was medically contraindicated (eg, HIV positive). | |
| Interventions | Participants were introduced to the app by nurses whilst in hospital. Participants could request unlimited, on‐demand video calls with IBCLCs through the app for as long as they desired. Women could call and speak to IBCLC 24/7 Control: care as usual. Although they were not exposed to IBCLCs through telelactation, while in the hospital, both control and telelactation arm participants received access to the standard support offered by various healthcare professionals (eg, nurses, obstetricians, and paediatricians) who cared for them during their postpartum hospital stay. After discharge, received support from paediatricians and their staff as a component of routine, outpatient paediatric health maintenance visits, and women enroled in WIC could access WIC breastfeeding services. | |
| Outcomes | Any and exclusive breastfeeding at 3 months Maternal satisfaction with breastfeeding | |
| Notes | Dates of the study: Oct 2016 ‐ May 2018 Funding sources: Health Resources and Services Administration (HRSA) grant Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized in a 1:1 ratio using a computer‐generated schedule prepared by the study’s bio‐ statistician. |
| Allocation concealment (selection bias) | Low risk | Allocation was imple‐mented through an electronic interface that revealed group assignment only after a participant had completed the enrollment process. |
| Blinding of participants and personnel (performance bias) | High risk | While it was infeasible to blind participants and hospi‐ tal staff to the treatment group after enrollment, remote IBCLCs delivering the intervention did not know which women were part of the study versus their general population of patients.
|
| Blinding of outcome assessment (detection bias) | High risk | The primary study outcomes of any breastfeeding and exclusive breastfeeding (ie, infant fed only breastmilk) were self‐reported by participants at 12 weeks. |
| Incomplete outcome data (attrition bias) | Low risk | Intervention loss to follow‐up = 8% Control loss to follow‐up = 8% |
| Selective reporting (reporting bias) | Low risk | Outcomes detailed in protocol are reported in the paper |
| Other bias | Low risk | No industry funding. No conflicts of interest Only baseline difference was in EBF while in hospital with the intervention group significantly less likely to practice EBF. This goes against the hypothesis so probably not important. |
| Study characteristics | ||
| Methods | 3‐arm RCT 3‐arm, with individual randomisation, n = 390 | |
| Participants | The study was carried out in 7 prenatal clinics in the American Midwest. Clinics provided services to low‐income adolescent mothers Baseline prevalence of breastfeeding initiation in country/setting: low Inclusion criteria: age 15‐18 years, in second trimester of pregnancy, expecting first birth, planning to keep baby, able to read and speak English, with access to phone; at birth, only mothers of singleton, term healthy babies were included Exclusion criteria: women who had birth complications that prohibited or delayed breastfeeding beyond 48 h Sample characteristics: mean age 17 years (SD 0.9); 61% African American; 75% low‐income; 74% single and living with their families, and 71% were in school. | |
| Interventions | Intervention (n = 128): 2 antenatal classes (1.5–2 h) on benefits of breastfeeding and practical issues run by the LC and the peer counsellor, followed up by phone calls. After the birth, phone calls made to those who had initiated breastfeeding, at 4, 7, 11, 18 days and 4 weeks to provide support. Control 1 (n = 128): the same contact schedule of classes and phone calls as the intervention group, with content concentrating on more general pregnancy and health issues Control 2 (n = 134): usual care with no special intervention | |
| Outcomes | Data on breastfeeding were available for women who initiated breastfeeding – this meant results were difficult to interpret. | |
| Notes | We have not included outcome data from this study in the review due to very high levels of attrition. This was a study where women were recruited in the second trimester and interventions took place both prenatally and postnatally. For postnatal outcomes only those women who initiated breastfeeding were followed up. There was considerable loss to follow‐up. 390 were randomly assigned. Women who did not attend at least 1 of the study classes were dropped from the study. Follow‐up data on duration of breastfeeding were available for 201 women who initiated breastfeeding (51%). Date of study: not recorded Funding sources: not recorded Declarations of interest: not recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “list of random codes generated by the study bio‐statistician." |
| Allocation concealment (selection bias) | Unclear risk | It was not clear how allocation was concealed at the point of randomisation. |
| Blinding of participants and personnel (performance bias) | High risk | Study described as being unblinded. |
| Blinding of outcome assessment (detection bias) | High risk | Study described as being unblinded. |
| Incomplete outcome data (attrition bias) | High risk | 390 women were randomized and those who did not attend at least 1 of the study classes were excluded. Follow‐up data on duration of breastfeeding was available for 201 who initiated breastfeeding (51%). |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Unclear ‐ data limited ‐ only reported in the form of an abstract. |
| Study characteristics | ||
| Methods | 2‐arm RCT | |
| Participants | Central, North Carolina, USA Background breastfeeding rates: not reported but author states BF rates are low. Inclusion criteria: between the ages of 18–39 with a singleton pregnancy, identified as non‐Hispanic Black, were English‐speaking and were willing to identify a study partner Exclusion criteria: premature birth, low birth weight (<2500 g), an extended hospital stay (>7 days) for mothers or infants after delivery, multiple birth, or the diagnosis of a congenital anomaly or condition significantly affecting feeding or growth (e.g., Down’s syndrome, cleft lip or palate). | |
| Interventions | Participants in the obesity prevention group (OPG) received eight home visits, an information toolkit, and four newsletters designed to provide anticipatory guidance and support for enactment of six targeted infant feeding and care behaviours: breastfeeding (EBF until 6 months, continued BF until 12 months); adoption of a responsive feeding style; use of non‐food soothing techniques for infant crying; appropriate timing and quality of complementary foods; minimisation of TV/media; and, promotion of age‐appropriate infant sleep. Six home visits were delivered by a peer educator at 30‐ and 34‐weeks gestation and 3‐, 6‐, 9‐, and 12 months postpartum. Intervention families could receive up to two additional home visits by an International Board Certified Lactation Consultant after hospital discharge, at any time of their choosing. Control: participants in the injury prevention group received 6 home visits, a toolkit, and newsletters. While women in both groups identified a study partner, IPG partners only completed study assessments; they were not encouraged to attend home visits or given their own set of study materials. | |
| Outcomes | Any breastfeeding at 3 months Depressive symptoms | |
| Notes | Dates of the study: November 2013 and December 2017. Funding sources: University of North Carolina, Chapel Hill Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Declarations of interest: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using a computer‐generated sequence, block size of 50, and 1:1 allocation ratio. |
| Allocation concealment (selection bias) | Low risk | The project director, who had no direct contact with participants, was responsible for generating the random number table and uploading it to a secure, online database maintained by a clinical trials unit. After completing the baseline assessment, the PE randomized the participant using the randomization functionality in the database. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible |
| Blinding of outcome assessment (detection bias) | High risk | Self‐report data so not possible |
| Incomplete outcome data (attrition bias) | High risk | LTF at this point was 47% in IG and 46% in CG |
| Selective reporting (reporting bias) | High risk | 3 month breastfeeding data is not reported by group |
| Other bias | Low risk | No industry funding. No conflicts of interest |
| Study characteristics | ||
| Methods | 2‐arm RCT, n = 667 | |
| Participants | The trial was conducted in socially and economically disadvantaged areas of Sydney, Australia, during 2007‐2010. Background rates of breastfeeding initiation: no information provided Inclusion criteria: ≥16 years old, expecting first child, between weeks 24‐34 of pregnancy, able to communicate in English, and lived in the local area Exclusion criteria: women were excluded from the study if they had severe medical conditions as evaluated by their physicians | |
| Interventions | Intervention (n = 337): 5 or 6 home visits from a specifically trained research nurse delivering a staged home–based intervention in the antenatal period and at 1, 3, 5, 9 and 12 months. At each visit the research nurse spent 1 h‐2 h with the mother and infant. (The nurse addressed 4 key areas: infant feeding practices, infant nutrition and active play, family physical activity and nutrition, as well as social support). The was delivered by trained research nurses in accordance with a protocol (www.healthybeginnings.net.au/). Each visit involved standard information with key discussion points, and appropriate resources to reinforce the information. Control (n = 330): received the usual childhood nursing service, comprising 1 home visit within a month of birth if needed. Additional visits at baseline and 12 months were conducted by a research assistant for the purpose of data collection only. | |
| Outcomes | Exclusive breastfeeding at 6 months Prevalence of any breastfeeding at 6 months and 12 months Median breastfeeding duration Time at introduction of solids | |
| Notes | Dates of study: the full trial ran from the 1st January 2007 to the 31st December 2010. Recruitment for this study took place between 1st July 2007 and the 30th June 2008. Funding source: Healthy Beginnings Trial funded by the Australian National Health and Medical Research Council Declaration of interest: no financial disclosure declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "group allocation, which was determined by a computer‐generated random number" |
| Allocation concealment (selection bias) | Low risk | Random allocation was concealed by sequentially numbered, sealed, opaque envelopes containing the group allocation. A research assistant who had no direct contact with participating mothers was responsible for generating the random numbers and preparing the envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and those delivering the intervention were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | The outcome data were collected by telephone at 6 months and by face‐to‐face interview in the home at 12 months. The data collectors and the research staff who dealt with data entry and analysis were masked to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up in intervention group was 82.5% at 6 months and 85.8% in the control group. "Those lost to follow‐up were significantly younger and less educated and were more likely to be unemployed or have low income (Table 1). The main reasons for loss to follow‐up were as follows: could not be contacted (67.8%), moved out of the area (14.2%), no longer interested (8.9%), too busy (4.0%), and illness or death (5.0%). This was similar across both groups" |
| Selective reporting (reporting bias) | Low risk | The breastfeeding outcomes were prespecified in the trial registry record. |
| Other bias | Unclear risk | Authors noted that they were unable to complete the baseline assessment and randomisation before birth, as planned, for 190 women (93 in the intervention group and 97 in the control group). There was no significant difference between these 190 and the 337 who were assessed and randomized before birth (175 in the intervention group and 162 in the control group) for any of the characteristics. Of the 268 participating mothers remaining in the intervention group at 12 months, 34.7% received 5 home visits after giving birth and 35.3% received 6 home visits, including an antenatal visit |
| Study characteristics | ||
| Methods | 2‐arm quasi‐RCT (even numbers to intervention and odd numbers to control group), single‐site, recruitment April 1999‐February 2000, n = 186, with 79 assigned to the intervention and 107 to the control group | |
| Participants | Urban USA ‐ military hospital in Texas Background rates of breastfeeding initiation: intermediate. Baseline breastfeeding rate in Texas at hospital discharge = 67% in 1999. Inclusion criteria: mothers on postpartum ward of study hospital; aged > 18 years, primiparous, uncomplicated delivery and postpartum, healthy baby, mother planned to breastfeed for at least 6 weeks Exclusion criteria: hospitalisation of mother or baby for > 4 days; mothers who did not speak English Ethnic composition of sample: 63% white, 11% black, 20% Hispanic, 2% Asian, 3% other All participants were members of the armed forces or their dependents. | |
| Interventions | Intervention: breastfeeding support in hospital visit lasting approximately 30 min, home visit 2‐4 days after discharge lasting 45‐60 min, and phone call 10‐14 days after the home visit Control: standard care (not described) | |
| Outcomes | Breastfeeding attrition to 6 weeks | |
| Notes | Dates of study: not recorded Funding sources: Sponsorship by United States Air Force Declaration of interest: not recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by odd and even numbers in groups of 10 |
| Allocation concealment (selection bias) | High risk | Could be anticipated |
| Blinding of participants and personnel (performance bias) | Unclear risk | The person delivering the intervention would have been aware of allocation. |
| Blinding of outcome assessment (detection bias) | High risk | The person delivering the intervention seems to have collected outcome data. |
| Incomplete outcome data (attrition bias) | High risk | Information on drop‐outs incomplete and loss to follow‐up not balanced across groups. 79 in intervention group, 5 were lost to follow‐up, data at 6 weeks from 68. Outcome data were not obtained from 32 women in the control group at 6 weeks so more women were enrolled (107 enrolled to this group). Some breastfeeding duration data were obtained from drop‐outs by phone. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the trial registration or protocol, so could not assess this. |
| Other bias | Unclear risk | Replacing women lost to follow‐up in the control group means that this study is at high risk of bias. |
| Study characteristics | ||
| Methods | 2‐arm RCT, with individual randomisation, n = 344 | |
| Participants | Rural areas, Huzhu County China. Background breastfeeding rates: according to the Chinese national data, the exclusive breastfeeding rate under 6 months was 27.6% in 2008 [6] and 20.7% in 2013 (the weighted exclusive breastfeeding rate was 18.6%). Inclusion criteria: first‐time mothers aged 18 years old or above; 14‐36 weeks pregnant and conceiving a singleton fetus; no known illness that limits breastfeeding after childbirth; no long‐term plan to go out of the county in the following year; no plan to change their mobile phone number in the following year; can read and communicate in Mandarin, use WeChat through smartphones, and have access to the Internet. Exclusion criteria: pregnant women with a severe disease and complications of pregnancy, such as cerebrovascular diseases, pneumonia and threatened abortion; pregnant women with HIV. Postpartum exclusion criteria: mothers developing health complications after birth, such as acute uterine inversion, postpartum haemorrhage or postpartum depression; infants who are admitted to the neonatal intensive care unit, have a 1‐min Apgar score < 6, 5‐min Apgar score < 6, low birth weight (< 2500 g), or were born prematurely (< 37 weeks of gestation); infants who are diagnosed with a cleft palate or other congenital abnormality that will prohibit breastfeeding; mothers and infants who move out or do not live in Huzhu County after birth. | |
| Interventions | WeChat account on smartphone and asked to register with the Ke Xue Wei Yang module. There were 4 components in the module: feeding messages, a feeding knowledge competition, a baby growth chart, and an online forum. Feeding messages, provided key breastfeeding knowledge and relevant infant feeding advice, breastfeeding problems encountered for both mother and child, and preparation for both breastfeeding and complementary feeding. Additional messages at 3 key stages: late pregnancy (37 weeks or above), the first month postpartum, and 4 months postpartum on Monday, Wednesday, and Friday every week. Content ‐ 1 month postpartum, key breastfeeding recommendations and common breastfeeding problems; 4 months ‐ starting complementary feeding by 6 months of age. Women could participate in the feeding knowledge competition component to test their breastfeeding knowledge. Moreover, women could enter their children's weight and height in the baby growth chart component whenever they want to monitor their children’s growth and ask breastfeeding related questions on the online forum component. | |
| Outcomes | Any and exclusive breastfeeding at 1,3,6 months | |
| Notes | Dates of the study: May 2019 to April 2020 Funding sources: UNICEF Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | a random number generated in advance. Unclear how it was actually generated. |
| Allocation concealment (selection bias) | Low risk | After agreeing to participate and signing the written consent form, a researcher gave each |
| Blinding of participants and personnel (performance bias) | High risk | participants would be aware they received the intervention |
| Blinding of outcome assessment (detection bias) | High risk | Data were collected by face‐to‐face at baseline and by telephone during follow‐up interviews. |
| Incomplete outcome data (attrition bias) | High risk | total of 32, 15, and 8 participants could not be contacted by phone for unknown reasons at the first, second, and third follow‐ups, respectively. Moreover, 94 mothers missed the follow‐up deadline (children <180 days age) at the 4‐5 months postpartum follow‐up because of the long holiday of Chinese Lunar New Year and the coronavirus (COVID‐19) outbreak in January 2020 in China. No ITT reported for lost participants
|
| Selective reporting (reporting bias) | Low risk | Reported outcomes match protocol
|
| Other bias | High risk | Compared with those in the control group, more participants in the intervention group had their first pregnancy (intervention: 46/161, 28.6%; control: 26/158, 16.5%; P=.01) or primipara (intervention: 48/161, 29.8%; control: 28/158, 17.7%; P=.01) Unclear if this will impact on findings. No industry funding No conflicts described. |
| Study characteristics | ||
| Methods | 3‐arm cluster‐controlled trial, n = 975 | |
| Participants | Healthcare clinics in Kinshasa, DR Congo Background rates of breastfeeding initiation: near‐universal initiation of breastfeeding 90% breastfeeding at age 1 year; 69% of babies aged 0–1 month and 35% of those aged 2–3 months (about 10–14 weeks) were exclusively breastfed. Inclusion criteria: all mothers who gave birth to 1 healthy child in 1 of the participating facilities between 24 May‐25 August 2012 and who intended to attend well‐baby clinic visits in the same facility Exclusion criteria: intended to attend well‐baby clinic visits in a different health facility, or to travel before the child was aged at least 6 months | |
| Interventions | Intervention 1 (n = 363): Baby Friendly Hospital Imitative (BFHI) steps 1‐9; healthcare staff from antenatal and maternity care (i.e. delivery rooms and postpartum wards) in the intervention facilities were trained using the WHO/UNICEF course. Session 14 of the training on 'Ongoing support for mothers' was limited only to 'Describe how to prepare a mother for discharge'. Session 15 on 'Making your hospital baby friendly' was not covered. Additional material in French developed as part of a different project was distributed to staff in clinics. Implementation of steps 1–9 was assessed at the end of the study using the hospital self‐appraisal questionnaire and each of the clinics randomised to intervention groups met at least 80% of the global criteria for each step. Intervention 2 (n = 308): BFHI steps 1‐10; staff training as for Intervention 1 and staff from well‐child clinics also received the same training. Flyers distributed to mothers before discharge from the postpartum ward and during well‐child clinic visits. These were developed locally and contained culturally appropriate messages addressing behaviours that had been identified as the main contributors to suboptimum breastfeeding practices (such as giving the baby water in the first 6 months of life) in a pretrial survey. These were published in 2 languages (French and local language). Additional material in French that had been developed as part of a different project was distributed to staff in clinics Implementation of steps 1–10 was assessed at the end of the study using the hospital self‐appraisal questionnaire and each of the clinics randomised to intervention groups met at least 80% of the global criteria for each step. Control (n = 304): standard care | |
| Outcomes | Primary Breastfeeding initiation within 1 h Exclusive breastfeeding at 14 and 24 weeks Secondary Prevalence of infants with reported diarrhoea between 10‐14 weeks postpartum and 18‐24 weeks postpartum Prevalence of infants with respiratory illness between 10‐14 weeks postpartum and 18‐24 weeks postpartum | |
| Notes | Dates of study: recruitment between May 24th and August 25th 2012 Funding Source: Bill & Melinda Gates Foundation and the Carolina Global Breastfeeding Initiative at the University of North Carolina (OH, USA). Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 3 pairs of facilities were ranked alphabetically and a computer was used to generate 3 random numbers |
| Allocation concealment (selection bias) | Low risk | The randomisation was done by the study statisticians who had no involvement in enrolment or follow‐up of participants |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Staff in participating clinics could not be masked to the interventions to group assignments because of the nature of the interventions". Mothers were masked to group assignment and this worked "quite well". |
| Blinding of outcome assessment (detection bias) | High risk | Attempts were made to blind interviewers but this "did not work so well". |
| Incomplete outcome data (attrition bias) | Low risk | 12% of total participants randomized lost to follow‐up by 24 weeks postpartum. |
| Selective reporting (reporting bias) | Low risk | Breasteeding outcomes were prespecified in study protocol. |
| Other bias | Low risk | None identified |
Abbreviations
AIDS: acquired immunodeficency syndrome
ANC: antenatal care
BFI: Baby Friendly Initiative (UNICEF)
BFHI:Baby Friendly Hospital Initiative
BFPC: Breastfeeding Peer Counselling
BINGO: Best Infant Nutrition for Good Outcomes
BMI: body mass index
CS: caesarean section
d: day(s)
EBF: exclusive breastfeeding
EP: electronic prompt
EPDS: Edinburgh Postnatal Depression Scale
FAB: Food, physical activity and breastfeeding
FBSICG: Facility‐based semi‐intensive counselling group
GP: general practitioner
h: hour(s)
Hb: haemoglobin
HBICG: Home‐based semi‐intensive counselling group
HCP: Healthcare Professional
HIV: human immunodeficiency virus
HV: Health Visitor
ICC: intra‐cluster correlation coefficient
ICU: intensive care unit
ITT: intention‐to‐treat analysis
LBW: low birth weigh
LC: lactation consultant
LHW: lady health worker
LMP: last menstrual period
MB training: maternal breastfeeding training
MCH: Maternal and Child Health
MCHN: Maternal and Child Health Nurse
min: minute(s)
MTMSGs: Mother‐to‐Mother Support Groups
NFN: Nurturing Families Network
NICU: neonatal intensive care unit
PAIRINGS: Provider Approaches to Improved Rates of Infant Nutrition & Growth Study
PC: Primary Care
PCT: Primary CareTtrust
RCT: randomised controlled trial
RG: Registrar General
SCBU: special care baby unit
SD: standard deviation
SPSS: Statistical Package for the Social Sciences
TENS: transcutaneous electrical nerve stimulation
UNICEF: the United Nations Children's Fund
vs: versus
WHO: World Health Organization
WIC: Special Supplemental Nutrition Programme for Women, Infants and Children (US Department of Agriculture, Food and Nutrition Service)
yrs: years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong comparator | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| WrongiIntervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong comparator | |
| Wrong intervention | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong intervention. | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| WrongiIntervention | |
| Wrong study design | |
| Wrong intervention | |
| WrongiIntervention. | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong comparator | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong Intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong study design | |
| Wrong intervention | |
| Wrong study design | |
| Wrong Intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention
| |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention. | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong comparator | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong study design | |
| Wrong study design | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong comparator | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong intervention | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong comparator | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong comparator | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong comparator | |
| Wrong study design | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong study design | |
| Wrong study design | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong patient population | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention |
Abbreviations
Characteristics of studies awaiting classification [ordered by study ID]
| Methods | Cluster RCT |
| Participants | Mother‐infant pairs |
| Interventions | Peer counselling from locally recruited, trained community female volunteers starting in third trimester of pregnancy until 6 months after delivery |
| Outcomes | Early initiation of breastfeeding (EIBF) and exclusive breastfeeding (EBF) rates at 5 months |
| Notes | Awaiting classification reason: retrospective registration and no response from Author |
| Methods | RCT |
| Participants | Mother‐infant pairs |
| Interventions | 2 peer counselling sessions Women could contact their peer counsellor up to 3 months postnatal for additional support. |
| Outcomes | Breastfeeding behaviour |
| Notes | None of our included outcomes were reported in this study. Study authors to be contacted in next update to check if other outcomes were measured. |
| Methods | 2‐arm cluster‐RCT, n = 422 |
| Participants | Kinshasa, Democratic Repbulic of the Congo Background rates of breastfeeding initiation: 52.4% No details about inclusion and exclusion criteria available in English abstract. |
| Interventions | Intervention: training of healthcare providers through the Baby Friendly Hospital Initative using the "20 hour course for Maternity Staff". Total number randomised: details not provided in English abstract Control: details not provided in English abstract |
| Outcomes | Exclusive breastfeeding at 6 months Median duration of breastfeeding |
| Notes | Needs to be translated from French |
| Methods | 3‐arm, parallel RCT, n = 90 |
| Participants | Health centres in Gonbad, Iran Background rates of breastfeeding imitation: > 90% Inclusion criteria: pregnant women. No further details provided in English abstract. Exclusion criteria: no details provided in English abstract. |
| Interventions | Intervention 1 (n = 30): 3‐h workshop on breastfeeding training Intervention 2 (n = 30): booklet about breastfeeding provided. Control (n = 30): no special training on breastfeeding |
| Outcomes | Knowledge about breastfeeding Health beliefs about postpartum breastfeeding Breastfeeding behaviour in first 24 h after delivery |
| Notes | Needs to be translated from Arabic |
| Methods | Controlled clinical trial |
| Participants | Breastfeeding mothers |
| Interventions | Initial education session at Healthcare Centre. Mothers contacted via telephone prior to each visit of the house at the end of the first and sixth month (a total of 6 visits of the house). Home visits included the father and others supporting the mother. |
| Outcomes | Number of months of exclusive breastfeeding |
| Notes | Awaiting classification reason: No loss to follow‐up and concerns over randomisation process. No response from author |
| Methods | 2‐arm RCT, with individual randomisation (although the study also included a non‐randomised comparison group) |
| Participants | 100 breastfeeding mothers randomised; recruited 3 days after the birth Inclusion criteria: married, primiparous with healthy infants born at a maternity hospital in Nova Scotia, Canada Exclusion criteria: infants with birthweight < 2500 g, with Apgar scores < 5, twins, women having operative deliveries, women who did not speak English |
| Interventions | Women in both groups received a pamphlet on breastfeeding. Intervention: weekly telephone calls beginning 10 days after the birth made by a nurse interviewer, offering support and advice and referral if necessary. Calls lasted 5‐10 min and were described as friendly. Women received up to 3 calls up to 6 weeks postpartum. Calls ceased when women discontinued breastfeeding. Control: women received usual care (not specified) |
| Outcomes | Interviews at 6 weeks postpartum. Women were asked about infant behaviour and infant feeding and breastfeeding duration. |
| Notes | Awaiting classification reason: Lack of baseline characteristics data, no loss to follow‐up and randomisation process not explained‐* Unable to contact Author* We have not included data from this study, because results in this paper were not reported in a form in which we could use them in the review. Most of the results were not reported according to randomisation group (rather authors described factors and associations with, e.g. breastfeeding). Breastfeeding in the randomised groups at 6 weeks was not reported, and it was not possible to contact the authors to obtain this information. It was stated that average breastfeeding duration was 28.6 days in the intervention group vs 21.0 days for controls, but no SDs were reported. It was not clear when or how breastfeeding duration data were collected; if at the 6‐week postpartum interviews this suggests that figures for average breastfeeding duration only apply to those women who had discontinued breastfeeding and denominators are therefore not clear. Dates of the study: not reported Funding sources: Social Sciences and Humanities Research council of Canada Declarations of interest: not reported |
| Methods | 2‐arm RCT, with individual randomisation, n = 500 |
| Participants | Setting: urban, a low‐income area of the city of São Leopoldo, Rio Grande do Sul, Brazil. Recruitment from maternity wards of the city's only publicly funded hospital, which mainly serves the low‐income population. Background rates of breastfeeding initiation: high Inclusion criteria: low‐income mothers with healthy, singleton, full‐term (> 37 week) babies with birthweight > 2500 g Exclusion criteria: impediments to breastfeeding, HIV/AIDS, or congenital malformation Demographics: 57% male children; 60% of intervention and 52% of control mothers had less than 8 years schooling; 73% of intervention and 67% of controls had low annual incomes (< USD 3000); 34% of mothers were not in paid work; 70% of children were living with mother and father; almost half of the mothers were overweight |
| Interventions | Both groups received routine assistance from paediatricians in the health service. Intervention (n = 200): dietary advice about breastfeeding and the adequate introduction of complementary foods, given monthly for 6 months in home visits starting within 10 days of the child’s birth then at 8, 10, and 12 months by 12 trained field‐workers (undergraduate students in groups of 2) who counselled mothers on the Ten Steps for Healthy Feeding Children from Birth to Two Years of Age (Brazilian Ministry of Health). Control (n = 300): standard care (not described) |
| Outcomes | Exclusive breastfeeding at 4 and 6 months; any breastfeeding at 12 months; also diarrhoea, respiratory problems, dental caries, anaemia, hospitalisation and nutritional status at 12‐16 months |
| Notes | Awaiting classification reasons: Retrospective registration and randomisation process unclear, no response from author Although the paper called this intervention 'dietary counselling', we have included it as a breastfeeding support intervention because its main purpose was to promote exclusive breastfeeding for 6 months followed by healthy complementary foods, and it involved regular visits during the first year of life. Dates of study: Recruitment between 2001 and 2002, no further details given Funding source: Brazil CNPq (National Funding for Research) and Capes Foundation, Ministry of Education Declarations of interest: not recorded |
| Methods | RCT |
| Participants | Mother‐infant dyads, babies under |
| Interventions | The intervention consisted in providing interpersonal counselling. The information provided included brochures and/or posters (depending on the topic) that a nurse explained to the mother before the infant was immunised. |
| Outcomes | Any breastfeeding at time points up to 12 months, Exclusive breastfeeding at time points up to 6 months (5‐7 months). Maternal satisfaction with care, Maternal satisfaction with feeding method, All cause infant or neonatal morbidity (including infectious illness), Post‐natal depression. |
| Notes | Awaiting classification reason: retrospective registration, implausible results and similarities of baseline characteristics. |
| Methods | 2‐arm, parallel RCT, n = 220 |
| Participants | Women receiving care from the Sexual and Reproductive Health Centre in Barcelona, Spain Background rates of breastfeeding imitation: 77% Inclusion criteria: low‐risk pregnancy and being cared for in the Sexual and Reproductive Health Centre Exclusion criteria: not specified |
| Interventions | Intervention: usual care plus telephone support from a community midwife. Control: usual care Total number randomised: not specified for either group. |
| Outcomes | Frequency of difficulties that women experienced breastfeeding Satisfaction with telephone support |
| Notes | Conference abstract. Unable to locate authors. |
| Methods | Study methods were not clear. This appeared to be a cluster‐randomised trial in 35 clinics. The intervention was carried out with healthcare workers. Results were for women attending intervention and control clinics before and after the intervention period. |
| Participants | Setting: family healthcare teams from Montes Claros city in South East Brazil Baseline prevalence of breastfeeding initiation in country/setting: not clear 1423 women recruited (unclear). Follow‐up for 12 months Inclusion criteria: mothers with children between 0 and 2 years old registered with the family health teams Participant characteristics: Approximately 20% under 20 years, 38% primiparous, 27% vaginal deliveries, 90% with > 4 years' education |
| Interventions | Intervention: 20 healthcare teams received staff training to promote breastfeeding, based on the Baby Friendly Hospital Initiative. Duration of the intervention was unclear; there was an initial interview before the study and a second interview 12 months after the start of training. Control: healthcare teams (n = 15 ‐ unclear) in control clinics did not receive the training. |
| Outcomes | Number of exclusive breastfeeding days; survival curves
|
| Notes | Awaiting classification reason: Lack of data of loss to follow‐up provided. We have not included data from this study. Data were not reported in a way in which we could incorporate results into the review. Authors reported the number of days, not the number of participants, for exclusive breastfeeding. It is reported that the median duration of exclusive breastfeeding was 106 days before and 107 days after the intervention period for the control group. For the intervention group the median duration of exclusive breastfeeding was reported to be104 days before and 125 days after the intervention period; the difference was reported to be statistically significant. Dates of the study: information not included in translation Funding sources: information not included in translation Declarations of interest: information not included in translation |
| Methods | RCT |
| Participants | Setting Turkey, one state hospital setting in Usak, western Turkey. 100 pregnant women Primiparous women, mean age 22.62, SE status: Low (n = 5, 14.7%); Medium (n = 24, 70.6%); High (n = 5, 14.7%) |
| Interventions | Breastfeeding motivation programme (BMP) based on PePener's health promotion model (HPM) Participants in the study were allocated into either the BMP group or control group through systematic sampling and randomisation. The BMP was structured based on the HPM and was carried out with the BMP study group four times: during the antenatal period, on the first postnatal day, between the fourth and sixth postnatal weeks, and during the fourth postnatal month‐ |
| Outcomes | Exclusive breastfeeding at 4‐6 weeks and 6 months |
| Notes | Awaiting classification reason: no protocol and no response from author The authors received no financial support for the research, authorship, and/or publication of this article. No competing financial interests exist. |
| Methods | RCT, parallel group |
| Participants | Primiparous, majority Chinese, mean age 32.6 years |
| Interventions | The self‐efficacy‐based breastfeeding educational programme (SEBEP) comprised a 2.5 hour breastfeeding workshop provided between 28 and 38 weeks of gestation, with small groups of six–eight mothers at each interactive session. A comfortable lecture room facilitated the group discussion and the sharing of experience with multimedia equipment, including a computer, LCD projector and a DVD player. Life‐like dolls and blankets were provided to each participant for practice. The interaction motivated the participant to acquire more information about breastfeeding. At home, the participants were encouraged to practice what they learned from the breast feeding workshop.The breastfeeding workshop protocol is shown in Table 1. Telephone counselling was provided to the participants at two weeks post‐partum, focusing on evaluating their emotional/ physiological condition and breastfeeding status. Each call lasted for 30–60 minutes. The researcher addressed problems, such as fear and pain, with the aim of correcting misconceptions. Coping strategies were reinforced, and emotional support was provided to the participants. The researcher evaluated the participants based on their description of positioning, infant cues of hunger, and frequency of breastfeeding. Appropriate advice was given and breastfeeding practices were encouraged. The telephone counselling protocol is shown in Table 2. |
| Outcomes | Any and exclusivebreastfeeding at 4‐6 weeks; 2 months; 6 months |
| Notes | Awaiting classification reason: no protocol provided. This study was supported by a grant from the Association of Hong Kong Nursing Staff (23/Reply/PD Fund/CEU). The authors declared no potential conflicts of interest with respect to the research, authorship, and/ or publication of this article. |
| Methods | RCT |
| Participants | Setting: A District Hospital in the city of Fortaleza, Ceará., Brazil 132 women, Intervention group primiparous n = 34/66 (51.5%); Age Median (SD) 24.5 ± 7.4 Control group: primiparous n=3 = 40/66 (60.6%); Age Median (SD) 22.0 ± 6.4 |
| Interventions | Telephone educational intervention. In addition to the assistance and routine individual service activities provided by the child‐friendly hospital professionals, the women received an educational intervention by telephone. The intervention consisted of a telephone call lasting seven minutes, on average, made by an experienced nurse and lactation educator, in which she initially introduced herself and recalled the approach in the rooming‐in, in order to establish a bond with the infant. Subsequently, using a form that followed the principles of the Motivational Interview (MI), the evoking‐informing‐evoking technique was used, which is recommended to change patients’ behaviours in a collaborative way, based on their motivation(8). At each call, guidance was given on two items on the scale to which women showed lower self‐efficacy in the rooming‐in; these guidelines were based on the instrument created by the researcher, based on the Breastfeeding Self‐efficacy Scale ‐ Short Form (BSES‐SF)(9) and in the Serial Album “I can breastfeed my child”(10), which addressed issues on technique and interpersonal thinking on breastfeeding. The doubts of the women were solved and, when necessary, they were guided to seek the institution’s milk bank. |
| Outcomes | Any and exclusive breastfeeding at 2 months and 3‐4 months |
| Notes | Awaiting classification reason: retrospective registration |
| Methods | 3‐arm quasi‐RCT, with sequential allocation, n = 180? |
| Participants | 180 women (not clear) attending a hospital in Southern Taiwan Inclusion criteria: breastfeeding at hospital discharge, term, healthy infant, able to read Chinese (hospital discharge at approximately 5 days) |
| Interventions | Intervention 1 ‐ telephone support: weekly phone calls for 2 weeks after hospital discharge then at 4 and 8 weeks postpartum by maternity nurse. The calls were to increase women’s self‐confidence. Intervention 2 ‐ home visits intervention: same schedule as phone support group with visits at home by the maternity nurse Control: usual care |
| Outcomes | Breastfeeding duration and analysis of factors affecting duration of breastfeeding |
| Notes | Awaiting classification reason: Loss to follow‐up not reported and concerns over randomisation process ‐*Unable to contact Author* We have not included data from this study in the review as data were not reported in a way that allowed us to enter them into RevMan 2014 for meta‐analysis. Dates of study: not reported Funding sources: The work reported here was supported bv a grant from the National Science Council (Taipei, Republic of China). Grant # NSC 79‐0412‐8037‐44. Declarations of interest: not reported |
| Methods | RCT (and subsequent retrospective analysis) |
| Participants | First time pregnant women intending to breastfeed recruited between 13 and 25 weeks of pregnancy |
| Interventions | The MILK intervention (SMS text messages) incorporated several automated interactivity and personalisation features as well as an option to receive one‐on‐one assistance from an on‐call study lactation consultant. |
| Outcomes | Participant interactions with the MILK system. Participant feedback on MILK content, delivery preferences, and overall satisfaction with the system via interviews and a remote survey at 8 weeks postpartum. |
| Notes | Awaiting classification reason: no outcome data available as results pending (delayed due to COVID) |
| Methods | RCT |
| Participants | 124 women |
| Interventions | In the last trimester of pregnancy, mothers were interviewed and asked about socioeconomic data, breastfeeding intention, and expectations, and breastfeeding self‐efficacy, in the waiting room of the prenatal consultation at the health service. Afterwards, all mothers received a pamphlet and watched an orientation video on encouraging the practice of breastfeeding and establishing healthy habits, developed based on national and international protocols (Brasil, 2019; Philipp et al., 2010; Wood et al., 2009). Self‐efficacy or confidence in breastfeeding was assessed by the instrument Breastfeeding Self‐Efficacy Scale–Short Form (BSES‐SF) adapted to the study. This scale was used for intervention planning (Supplement). The BSES‐SF consisted of 14 items in two domain categories: Technique (8 items) and Intrapersonal Thoughts (intrapersonal communication??) (6 items). Each item was evaluated according to an agreement scale (Likert type) with the following score: 1. Totally disagree, 2. Partially disagree, 3. Agree sometimes, 4. Partially agree and 5. Totally agree, with score variation from 14 to 70 points (Dennis, 2003). The protocol proposed in this study and performed with the intervention group, in addition to traditional health education carried out during pregnancy, included a home visit by health professionals up to 5 days after the baby's birth to carry out a motivational interview about breastfeeding and record data on childbirth and breastfeeding, and to apply the BSES‐SF scale to orientate the guidelines. All the mothers' doubts must be resolved and guidance given on the promotion of the practice of breastfeeding In the 2nd and 14th week after the baby is born, mothers were contacted by telephone to reinforce the motivational interview and to do a follow‐up by health professionals, and, if necessary, another visit was carried out at home. At the sixth month, mothers were visited at home for counselling on the practice and to give orientation on complementary breastfeeding (Table 1). |
| Outcomes | Breastfeeding at 6 months |
| Notes | Awaiting classification reason: retrospective registration and no response from Author. |
| Methods | Parallel 2‐arm RCT, single‐site study, n = 298 |
| Participants | Community health centres in Los Angeles County, USA Participants: low‐income, Hispanic women Breastfeeding rates: local breastfeeding rates not reported but authors state that within the Hispanic population the exclusive breastfeeding rate in hospital is 27.9% and at 1 week postpartum 33% of breastfeeding Hispanic women also give their babies formula. Inclusion criteria: women 26–34 weeks pregnant, Medicaid recipient, self‐identified Hispanic, available via telephone, not assigned to a WIC peer counsellor, gave birth to a healthy full‐term singleton, absence of congenital abnormality, the infant was not admitted to a NICU Exclusion criteria: participants whose babies had medical conditions that could significantly interfere with breastfeeding. The researchers also avoided recruiting participants from health clinics located near WIC sites that offered peer support. |
| Interventions | Intervention (n = 146): WIC Supplemental Nutrition Programmes. The standard WIC programmes provide monthly food vouchers, nutrition education and breastfeeding support to women, infants and children aged ≤ 5 years. Breastfeeding support includes breastfeeding classes, access to a free breastfeeding helpline, breast pumps and LC service. Some programmes also offered breastfeeding and support and education using peer counsellors. The intervention group received additional support from LCs who were undergraduate students who had completed a semester‐long lactation education course and 10 h of post course training. The lactation education course included content knowledge on the normal breastfeeding process and cultural sensitivity training. The intervention entailed 4 prenatal and 17 postpartum phone calls (first call initiated when mothers were in the third trimester of pregnancy and the last call when mother was 6 months postpartum). The intervention participants were also provided with the lactation educator’s phone number so they could contact her more frequently if need be. On occasion, text messages were used to implement phone contacts with participants. Control (n = 143): standard WIC programme |
| Outcomes | Primary and secondary outcomes not distinguished. Exclusive breastfeeding at 72 h Any breastfeeding at 72 h Exclusive breastfeeding at 1 month Any breastfeeding at 1 month Exclusive breastfeeding at 3 months Any breastfeeding at 3 months Exclusive breastfeeding at 6 months Any breastfeeding at 6 months |
| Notes | Awaiting classification reason: No Protocol and none supplied by Author on contact Dates of study: randomisation between July 2011 and July 2012 Funding sources: Centers for Medicare and Medicaid Services; Hispanic‐Serving Institutions Education Grants Program; NIH Research Infrastructure in Minority Institutions from the National Institute of Minority Health and Health Disparitites, NIH Minority Biomedical Research Support Research Initiative for Scientific Enhancement and California State University Sponsored Projects Declarations of interest: none declared |
| Methods | Single‐site study recruiting over 7 months, n = 134 |
| Participants | Urban Iran Background rates of breastfeeding initiation: high Inclusion criteria: women without breastfeeding experience or chronic disease giving birth normally at term to a healthy baby ≥ 2.5 kg |
| Interventions | Intervention: nutritionist trained using WHO Breastfeeding Counselling training course (40 h). Contact in hospital immediately after birth, between 10 and 15 days, after 30 days and monthly to the fourth month at home or in a lactation clinic Control: standard care (not described) |
| Outcomes | EBF at 1, 2, 3 and 4 months Mean number of days illness with diarrhoea |
| Notes | Awaiting classification reason: Implausible results‐*Unable to contact Author* Dates of study: study conducted March to September 1994 Funding sources: not reported Declarations of interest: not reported |
| Methods | Cluster RCT |
| Participants | Setting: Midlands Province, Zimbabwe Intervention: age 26.6; SEstatus: Formal/informal 46 (25.9%). Unemployed 132 (74.4%) Control: age 27; SEstatus: Formal/informal 44 (24.6%). Unemployed 135 (75.4%) |
| Interventions | At the intervention clusters, mothers received the routine services provided according to the MOH&CC national policy as outlined in the control group. In addition, from August 2012 to November 2012, 214 village health workers (VHWs) were trained on cIYCF using training materials adapted from the community infant and young child feeding counselling package developed by UNICEF. The training was conducted in clusters and covered a period of five days per group. However, all mothers in the intervention sites received a t‐shirt with breastfeeding messages at the end of assessment (at 20 weeks). |
| Outcomes | Exclusive breastfeeding at 3‐4 months, 6 months |
| Notes | Awaiting classification reason: no protocol, implausible results, lack of baseline characteristics and concerns over randomisation process. No response from author Competing interests statement, but no role of funder stated |
| Methods | A nested randomised intervention design within a longitudinal cohort |
| Participants | Breastfeeding women living in regional Western Australia. |
| Interventions | Breastfeeding support offered via an Internet support website |
| Outcomes | Rates of exclusive breastfeeding at 6 months postpartum |
| Notes | Insufficient information on baseline characteristics. No response from study authors. |
| Methods | 2‐arm RCT with individual randomisation, conducted in 32 general practices in the UK; recruitment April 1995‐August 1998, n = 720; 363 assigned to intervention and 357 to control |
| Participants | Urban south‐east England Background rates of breastfeeding initiation: intermediate Inclusion criteria: mothers considering breastfeeding who had not breastfed a previous child for 6 weeks after birth Exclusion criteria: planning to contact a breastfeeding counsellor, address considered unsafe to visit, baby born before 36 weeks' gestation Ethnic composition of sample: 59% white (UK) participants, 11% white (other) participants, 16% African or Caribbean, 8% Indian subcontinent, 6% other Socioeconomic status on RG classification: 10% I, 26% II, 19% IIINM, 26% IIIM, 12% IV, 3% V, 5% other First baby: 74% National baseline prevalence 66% breastfeeding at birth |
| Interventions | Intervention: women received 1 antenatal visit from a National Childbirth Trust trained breastfeeding counsellor, who offered postnatal support by telephone or further visits if the mother requested this after the birth Control: standard care (UK standard care includes postnatal home visits from midwives and health visitors) |
| Outcomes | Prevalence of any breastfeeding to 6 weeks; duration of any breastfeeding to 4 months; time to introduction of formula feeds; maternal satisfaction and common feeding problems; mothers' perspectives on support from counsellors; association between counselling uptake and feeding behaviour |
| Notes | Awaiting classification reason: loss to follow up‐same numbers lost at each stage between control and intervention. No response from author Dates of study: recruitment was between April 1995 and August 1998 Funding sources: The College of General Practitioners Scientific Foundation Board and NHS North Thames responsive funding programme. NHS research and development support through the East London and Essex Network of Researchers. Declarations of interest: Author AW works in a voluntary role as a professional advisor to the national Childbirth Trust and other organisations engaged in breastfeeding support. Author JT is a member of the National Childbirth Trust. |
| Methods | RCT |
| Participants | 352 primiparous women, mean age 29 years |
| Interventions | The TPB‐based intervention programme comprised one individual instruction and two group educations at hospital and continued telephone counselling at home during postpartum period. First three days post‐partum at hospital: – Individual instruction and support: practising correct breastfeeding techniques at day 1 post‐partum (even though not enough breast milk came out) and solving EBF problems every day during the 2 or 3 days of hospitalisation. – Group education: a 60‐minute session was provided at day 2 post‐partum, including 30 minutes of lecture and video watching for information about EBF, emphasising the benefit of EBF, modifying the wrong idea on the nutrition needs for baby, 15 minutes of practice for breastfeeding position and latching‐on, and 15 minutes of discussion on EBF plan and sharing with other experienced mothers or nursing staff for successful breastfeeding experience. The participants’ significant others were invited to the session. During s days to 6 weeks post‐partum at home: Telephone counselling to follow up BF practice and solve any EBF problems: twice a week within the first 2 weeks and once a week during week 3 to week 6 post‐partum. – If there are major problems, researchers will provide a home visit or ask the mother to come to the hospital for further help. At 6 weeks post‐partum at hospital: – A 30‐minute group session of discussion on EBF during the regular check‐up. – Mothers and their significant others were re‐informed about the long term benefits and importance of EBF. During 6 weeks to 3 months at home: – Individual EBF advice by telephone support: once every two weeks from 6 weeks until 3 months. During 3 to 6 months post‐partum at home: A series of individual advice via telephone, focusing on techniques for preparing for work and management of breast milk such as breast milk expression, storing breast milk in ice box, giving breast milk to babies by cup, and dealing with various BF problems at the workplace. – Once a week within 2 weeks before working; – Once during the first 2 days of work; – Once a week within a month after working; – Once every 2 weeks during the 4th and the 6th months. |
| Outcomes | Exclusive breastfeeding at 6 weeks, 4 months and 6 months |
| Notes | Awaiting classification reason: No protocol and no response from author |
| Methods | RCT |
| Participants | 300 healthy pregnant women from an urban Population attending the antenatal clinic at Jawaharlal Nehru Medical College, Aligarh Muslim University were recruited for the study |
| Interventions | Participants were equally assigned randomly to the intervention (2 antenatal and 8 post‐partum home‐counselling visits by the counsellors) and control (co‐counselling) group |
| Outcomes | Exclusive breastfeeding at 6 months |
| Notes | Awaiting classification reason: Implausible results and no protocol. *Unable to contact Author* The study was part of an international project for breastfeeding protection, promotion and support with financial grant by Swedish Agency for International Development Agency (SIDA) |
| Methods | Community‐based cluster‐randomised study (40 adjacent areas randomised), recruitment over 10 months, n = 726 |
| Participants | Setting: Dakka, Bangladesh Background rates of breastfeeding initiation: high Socioeconomic status: mainly lower‐middle and low Inclusion criteria: women aged 16‐35 years with ≤ 3 children (or ≤ 6 pregnancies) and no serious illness Exclusion criteria: multiple births, children with congenital abnormalities, and those weighing < 1800 g National baseline prevalence reported in paper was similar to the control group rates; UNICEF quoted higher rates ‐ 53% exclusive breastfeeding at 0‐3 months |
| Interventions | Intervention: peer counselling by women with personal breastfeeding experience trained over 40 h with the WHO/UNICEF Breastfeeding Counselling course. Paid honorarium. Supervised caseload of 12‐25 mothers. 15 home visits: 2 in last trimester/4 in month 1/2‐weekly in months 2‐5. Duration of visits 20‐40 min. Control:not specified |
| Outcomes | Exclusive breastfeeding at birth, 4 days, 4 weeks, 2, 3, 4 and 5 months. |
| Notes | Awaiting classification reason: Implausible results ‐*Unable to contact Author* Dates of study: recruitment between February 1996 and December 1996 Funding sources: Swiss Agency for Development and Cooperation Grant Declarations of interest: not reported |
| Methods | 3‐arm RCT n = 49 |
| Participants | 49 women giving birth in a small community hospital in the USA planning to breastfeed for at least 6 weeks and breastfeeding for the first time. All women had healthy babies. Women were described as married and middle class aged 17–31 years |
| Interventions | 3 groups: Intervention 1: 15 randomised, 13 followed up (not clear): usual care plus an educational session Intervention 2: 16 randomised, 15 followed up (not clear): usual care plus education plus daily visits by nurse while in hospital and telephone support 2 days after discharge and 1 week later and further support if necessary (up to 5 weeks post‐partum). Control: 18 randomised, 12 followed up (not clear) |
| Outcomes | Outcomes were unclear, but included breastfeeding at 6 weeks and breastfeeding problems. |
| Notes | Awaiting classification reason: baseline characteristics not reported and randomisation process not clear‐ *Unable to contact Author* We have not included data from this study in the review due to methodological weakness and high and unbalanced levels of attrition. More than 30% of the control group were lost to follow‐up and results were therefore difficult to interpret. Most results were not reported according to randomisation group and the only result for breastfeeding duration was approximate, stating: quote: “Approximately 50% of the control group and 50% of the group which received the teaching unit were still nursing at 6 weeks. Of the group who received the teaching plus support 80% were still nursing at 6 weeks.” Dates of study: Not reported Funding sources: Division of Nursing, Bureau of Health Manpower, U.S, Public health Service. Declarations of interest: Author JH, teaches childbirth classes and has spoken on breastfeeding to the ICEA Convention in Seattle. |
| Methods | Cluster RCT |
| Participants | Pregnant women living in 132 wards (3‐4 villages) in 6 rural districts of Southern Tanzania |
| Interventions | Home based counselling service. Women received 3 home visits during pregnancy and 2 postnatally |
| Outcomes | Neonatal mortality Newborn care practices including breastfeeding |
| Notes | None of our included outcomes were reported in this study. Study authors to be contacted in next update to check if other outcomes were measured. |
| Methods | 2‐arm RCT, single site, n = 540 |
| Participants | Black and Latina women who had delivered at a large tertiary hospital located in New York City, USA Background rates of breastfeeding initiation: not stated Inclusion criteria: participants were black/African American or Latina/Hispanic, spoke English or Spanish, had a working telephone, ≥ 18 years old, and had infants with birth weights > 2500 g and 5‐min Apgar scores > 7 Exclusion criteria: not specified |
| Interventions | Intervention (n = 270): The intervention used a behavioural educational approach and aimed to prepare and educate mothers about postpartum symptoms and experiences provide social support, and develop self‐management skills. The intervention was delivered in two parts. The first part was delivered whilst the women was in hospital by a social worker who reviewed an education pamphlet and partner summary sheet with each mother. Education materials provided information about post‐partum care and included information on breastfeeding and breast/nipple pain. Additional information was provided on social support. The second part was a 2‐week postdelivery call, where the social worker assessed patients’ symptoms, skills in symptom management, and other needs. Patients and the social worker created action plans to address current needs that included assessment of community resources. Control: enhanced usual care; participants received a list of community resources and received a 2‐week control call |
| Outcomes | Duration and exclusivity of breastfeeding at 6 months |
| Notes | Awaiting classification reason: retrospective registration and concerns over randomisation process‐ *Unable to contact Author* Dates of study: recruitment between April 2009 and March Funding sources: National Institute on Minority Health and Health Disparities and the National Institute of Mental Health Declarations of interest: None declared |
| Methods | RCT |
| Participants | Setting: public maternity hospital in Chengdu, China 346 caesarean section women: Intervention ‐primiparous 47.4%, mean age 33.47, SE status Monthly income (RMB): <3000 = 2.9%; 3001‐500 = 18.1%; 5001‐10,000 = 36.3%, >10,000= 42.7%, Education level Junior High = 4.7%; Senior High = 9.4%; Junior College = 25.7%; University = 60.2% Control: primiparous 55.9%, mean age 32.55, SE status Monthly income (RMB): <3000 = 2.9%; 3001‐500 = 16.5%; 5001‐10,000 = 37.1%, >10,000= 43.5%, Education level Junior High = 4.7%; Senior High = 5.3%; Junionr College = 16.5%; University = 73.5% |
| Interventions | Routine care plus intervention. At the time of admission, the researcher investigated the breastfeeding knowledge of mothers in the intervention group and corrected their existing breastfeeding misconceptions according to the findings. And targeted health education based on the health belief model was conducted for each mother. The researcher created a WeChat (a social application widely used in China) group to answer the mothers’ questions on breastfeeding at any time. After birth the researcher provided guidance on establishing breastfeeding and treatment of problems. Before discharge the BF knowledge and satisfaction scores were completed and women with low scores received targeted interventions. For 4 months women received WeChat messages and could ask the researchers questions. |
| Outcomes | Exclusive breastfeeding 4‐6 weeks and 6 months |
| Notes | Awaiting classification reason: retrospective registration and no response from Author No funding information or conflict of interest information provided. |
| Methods | RCT |
| Participants | Setting Obstetric admission office, China 352 women Intervention group: Primiparous 64.8%, age 31.7, SE status income (RMB): <3000 = 8%; 3001‐5000 = 15.9%; 5001‐10000 = 65.9%; >10,000 = 10.2%, C‐section 77.8%, Education High school = 11.4%; some college = 19.9%; college graduate = 68.7% Control: Primiparous 62%, age |
| Interventions | Routine care. At the time of admission, the researcher used the breastfeeding attrition prediction scale and the breastfeeding knowledge scale to understand and analysis the problems of breastfeeding knowledge of subjects. Afterwards, on the basis of the results, the researcher provides individualised intervention by face to face. After delivery, researchers provided professional breastfeeding guidance for mothers. At discharge, researchers used breast‐feeding assessment scale and breastfeeding knowledge scale to analyse maternal breastfeeding problems and give intervention. Researchers monthly asked for details of breast‐feeding situation, and gave guidance by telephone follow‐up after maternal hospital discharge to postpartum 4 months. |
| Outcomes | Exclusive breastfeeding 4‐8 weeks and 3‐4 months |
| Notes | Awaiting classification reason: no protocol and concerns over randomisation process. No response from author No conflicts of interest. No funding source described. |
| Methods | Quasi‐RCT, recruitment location/duration not stated, n = 38 |
| Participants | UK white, working‐class women 19‐32 years old, living with partner and intending to breastfeed Background rates of breastfeeding initiation: intermediate: prevalence of breastfeeding in 1985 = 64% at birth and 26% at 4 months |
| Interventions | Intervention: 3 antenatal home visits/1 hospital visit/1 'immediate' home visit and 1 or 2 further home visits 'in the early weeks'; plus face‐to‐face and telephone support by a single lay supporter (mother/previous breastfeeding experience, but no indication of training) Control: 1 antenatal home visit and 1 postnatal hospital visit |
| Outcomes | Breastfeeding at 3 months. Partial breastfeeding grouped with formula feeding as 'breastfeeding failure' |
| Notes | Awaiting classification reason: Implausible results, no loss to follow‐up reported, lack of baseline characteristics given and concerns over randomisation process. *Unable to contact Author* Moderate‐to‐high risk of bias Dates of study: not reported Funding sources: not reported Declarations of interest: not reported |
| Methods | Quasi‐RCT, individual randomisation, single‐site study; recruitment period 18 months, n = 678 |
| Participants | Maternity department of UK district general hospital Background rates of breastfeeding initiation: intermediate Inclusion criteria: all women who attempted at least 1 breastfeed Exclusion criteria: birth of child overlapped intervention and control periods 55% of the sample were primiparous. Ethnic composition not stated. Socioeconomic status defined by UK census categories (I and II 22%, III 46%, IV and V 13%) |
| Interventions | Intervention (n = 228): individual support and problem‐solving by lactation nurse in hospital and at home. Duration of the intervention not specified. Control (n = 355): not specified |
| Outcomes | Breastfeeding rates at 4 weeks, and 3, 6 and 12 months Satisfaction with care and intention to breastfeed after next pregnancy |
| Notes | Awaiting classification reason: lack of baseline characteristics provided. *Unable to contact Author* Dates of study: not reported but states studies was conducted over 18 months Funding sources: not reported Declarations of interest: not reported |
| Methods | RCT |
| Participants | 3188 women Setting Matlab, a rural sub‐district 57 km south‐east of Dhaka, the capital of Bangladesh. Intervention: age 25.8, SE status Asset scores: Lowest quintile 291/1417 (20.5%) the highest quintile 260/1417 (18.3%), C‐section 4.4%, Education level 7.1 years Control: age 25.7, SE status Asset scores: Lowest quintile 278/1428 (19.5)the highest quintile 303/1428 (21.2%), C‐section 4.1%, Education level 7.2 years |
| Interventions | The women allocated to breastfeeding counselling received eight sessions: two sessions during the last trimester of pregnancy, one session in the seven days after delivery and five sessions at monthly intervals up to six months after childbirth. Counsellors were free to make additional contact if required. Counselling was given individually at home, but key family members were also included. The duration of each counselling visit was typically 20–40 minutes, depending upon the mother’s lactation stage and her individual needs. Usually, the first two to three visits were longer, to build the mothers’ confidence, and the later counselling visits were shorter as they aimed to just give support to mothers and reinforce the health messages |
| Outcomes | Exclusive breastfeeding 3‐4 months and 6 months |
| Notes | Awaiting classification reason: retrospective registration and no reply fromaAuthor The author report no conflicts of interest. No concerns with funding source |
| Methods | 2‐arm RCT, recruited August 2008‐April 2009, n = 140 |
| Participants | Recruitment from postnatal wards of 2 hospitals in South Jordan Prevalence of 'ever breastfed' in country: 93% (WHO Global Data Bank on Breastfeeding, accessed 12 Oct 2011). Paper stated that traditionally most women initiate breastfeeding and breastfeed for up to 2 years, with 32% fully breastfeeding for > 6 months. Inclusion criteria: primiparous women following vaginal delivery with term infants Exclusion criteria: women who lived outside the study area or who could not be contacted by phone |
| Interventions | Intervention: women received a 1‐h education session approximately 2 h after the birth. The session included demonstrations of breastfeeding. Mothers were encouraged to ask questions and were given a pamphlet on breastfeeding. At 2 and 4 months postpartum women were contacted by phone by the same researcher/nurse. The purpose of calls was to offer support, monitor breastfeeding practices and identify any problems. Control: usual care; women were given an appointment for 6 weeks after discharge to attend the maternal and child health services for support and follow‐up. Paper states most women did not return for these appointments and were not receiving any postnatal care. Control group women did not receive postnatal home visits from a midwife or a child health nurse. |
| Outcomes | Primary: exclusive breastfeeding at 6 months and breastfeeding knowledge Secondary: infant hospital admissions for diarrhoea and vomiting or respiratory tract infections |
| Notes | Awaiting classification reason:no protocol and author unable to provide on contact. Dates of study: recruitment was conducted between August 2008 and Funding sources: This research was supported by grant from Mutah Declarations of interest: none declared |
| Methods | RCT |
| Participants | 70 pregnant women over 18 years old, all primiparous Setting ‐ Iran Intervention: mean age: 27.25 Control: mean age: 27.73
|
| Interventions | In the intervention group, two 2‐hour sessions of breastfeeding education for two weeks during gestational age of 32 to 36 weeks were held for mother and a member of the family who plays a key role in breastfeeding. These sessions were held in the form of lectures, questions and answers and shows with puppets about the benefits of breastfeeding, the correct techniques of breastfeeding, signs of breastfeeding adequacy, the method of milking and storing milk, nutrition during of breastfeeding and increasing milk, common problems of breastfeeding, baby care and the support and involvement of family in breastfeeding. The questions were answered at the end of each session and the participants were provided with booklets and educational software on breastfeeding so that the mothers and their families can study at home. Then, 3 to 5 days after delivery, when mothers referred for neonatal thyroid screening, 1 session of breastfeeding consultation was held for the breastfeeding woman, her husband and a key person in the family, mother's breastfeeding technique was observed, and signs of breastfeeding adequacy, the method of drawing a child's growth curve and its analysis was taught. The address of breastfeeding counselling centres was given to mothers. In addition, the researchers’ phone number was given to the participants so that they could contact them if they needed support and counselling at any time. |
| Outcomes | Exclusive breastfeeding at 2 months |
| Notes | Awaiting classification reason: concerns with randomisation process, baseline characteristics and implausible results. No reply from author Funding from University, no report on conflicts of interest. |
| Methods | Not clear; described as"'case control randomized trial", 2‐armed n=120 |
| Participants | Setting: Tehran, Iran; mothers and babies recruited in a Baby Friendly accredited hospital. 120 women (baseline characteristics not described) Inclusion criteria: women giving birth to singletons by caesarean section only Exclusion criteria: infants with congenital abnormalities or serious illness necessitating intensive care, and mothers who had a serious illness or were planning to leave the area within 6 months, infants weighing < 2500 g at birth |
| Interventions | Intervention: 4 postnatal home visits (not clear) Control: standard care (not clear) |
| Outcomes | Follow‐up interviews by telephone on days 90, 120, 150 and 180 Results were not reported in a way in which we can include them in the review. Authors reported that quote: "the patterns of exclusive breastfeeding in the 2 groups for days 3 to 180 differed significantly (P < 0.0001) with a mean aggregated of 67.72% among the group assigned home visits compared with 31.78% for the group assigned none". |
| Notes | Awaiting classification reason: concerns with baseline characteristics and randomisation process. Loss to follow up not reported. *Unable to contact author* Dates of study: not reported Funding sources: not reported Declarations of interest: not reported |
| Methods | RCT |
| Participants | 50 women ‐ no further details reported Setting: Krishnagiri District |
| Interventions | Healthy Children Brighter Futures (HCBF) is a pilot program in Krishnagiri District, India that provides home health visits to infants < 12 months of age. Community Health Nurses (CHNs) make monthly visits to the homes of these infants to assess breastfeeding, immunisation status, growth developmental milestones, and to provide anticipatory guidance. CHNs also identify acute healthcare needs and refer to tertiary care as appropriate. The ‘ intervention ’group received visits at one week, one month, and two months of life and nurses provided counselling services during these visits. |
| Outcomes | Exclusive breastfeeding at 4 ‐6 weeks and 2 months |
| Notes | Awaiting classification reason: No protocol, no loss to follow‐up, implausible results. Also concerns with baseline characteristics and randomisation process. No reply from author |
| Methods | 2‐arm, cluster randomised trial, n = 308 |
| Participants | Women attending community health clinics in Shanghai at 11‐22 weeks gestation Background rates of breastfeeding imitation: 41.0% No details provided in abstract about inclusion/exclusion criteria. |
| Interventions | Intervention: weekly SMS messages from 28 weeks gestation until the children were 1 year old. ‘Message bank’ development was based on literature review and in‐depth interviews/focus group discussion with pregnant women, new mothers and healthcare providers. Control: no details provided in abstract No details provided about the total number randomised in each group. |
| Outcomes | Exclusive breastfeeding at 4 months |
| Notes | Conference abstract only. SM contacted authors for more information 21 July 2016. |
| Methods | RCT |
| Participants | 100 primiparous women Setting: Intervention: Age 28, SE Status Family income (dollars) <450 13 (26%) 450‐750 20 (40%) >750 17/50 (34%), C‐Section 44%, Education High school or less 21 (42%) College or above 29 (58%) Control: Age 27.3, SE Status Family income (dollars) <450 11 (22%) 450‐750 21 (42%) >750 18 (36%), C‐Section 48%, Education High school or less: 23; College or above: 27 |
| Interventions | Information support: the breastfeeding knowledge and the request of pregnant women was evaluated after admission. According to the assessment results, the information support scheme was developed [7]. Health education for breastfeeding knowledge and information was provided, including the propaganda column in ward, breastfeeding guide booklets, breastfeeding class, and one‐to‐one breast‐feeding advice. The good relationship and effective communication with pregnant women was established and maintained. The confidence of breastfeeding and psychological state was understood timely. The health education was strengthened when less confidence of participant was appeared. The benefits and approaches of breastfeeding were explained. The mothers who owned experience of breastfeeding were invited and gave talks to improve the confidence of participants. When anxiety, depression and other negative emotions appeared, specific help and psychological counselling was performed. Psychotherapist was participated for intervention when necessary [8]. The mobile discussion group for breastfeeding was built and maintained, which consisted of obstetrical staff and participants. After continually pushing the breastfeeding knowledge in mobile group, it facilitated the communication, counselling and learning for participants. The problems of breastfeeding could be discussed and solved by certain specialists in time [7]. The public account on mobile website was built. The breastfeeding knowledge and new progress were published regularly. It provided a quality learning platform for participants. Behaviour intervention: The guidance of the breastfeeding was taught as follows. Early exposure between maternal and infant was performed after birth. Within 30 min after birth, the nurse demonstrated the correct breast‐feeding method to help the infant intake the breast milk, and promote the lactation of the mother. The breastfeeding skills (such as cor‐rect posture) of the mother were guided. The nurse also helped the infant to learn the correct and effective action for ingestion breast milk [8]. The guidance of getting out the bed at early stage, and moderate activity of primiparas were proceeded. To improve the postpartum recovery and gastrointestinal peristalsis, the primiparas were encouraged to get out the bed at early stage, and perform moderate activity. It would improve the ingestion foods of primiparas, and promote the milk secretion [6]. To feel more joy of motherhood, and build the confidence for taking care of infant, the primiparas were encouraged to participate in neo‐natal care [10]. The guidance of daily life for primiparas were carried out as follows. The instruction of dietary nutrition was performed to improve the breast milk secretion. Protein‐rich and easily absorbed diet (such as fish and chicken soup) was recommended. To uptake various nutrients, fresh fruits were also ingested in the diet [8]. Meanwhile, adequate sleep and rest was executed during the treatment.The instruction of breast cleaning and massage was guided to prepare the lactation. After hot compress of wet towel for 20 min, the longitudinal massage was performed along the mammary ducts through the thumb, index and middle fingers. For the mammary areola, longer time (10 min for each massage) was recommended to promote mammary gland tube patency, which would convenient the infant for uptake the breast milk [6]. To overcome the breastfeeding issues (including breast engorge‐ment and bloating, lactiferous duct blocking, mastitis, and nipple pain), appropriate solutions were instructed for the primiparas. |
| Outcomes | Any and exclusive breastfeeding at 4‐6 weeks |
| Notes | Awaiting classification reason: no protocol, no loss to follow‐up and concerns over randomisation process. no reply from author Authors declared no conflicts of interest Funding source not reported |
| Methods | 2‐arm RCT, with individual randomisation, single‐site study, duration of recruitment not stated, n = 270 |
| Participants | Urban Canada ‐ maternity unit of regional general hospital Background rates of breastfeeding initiation: intermediate. Baseline prevalence (1984) = 69% breastfeeding initiation (75% stopping by 6 months) Inclusion criteria: intending to breastfeed; English‐speaking Exclusion criteria: multiple births; birthweight < 2500 g; birth before 37 weeks Participant characteristics: 41% were primiparous; ethnic composition not described; socioeconomic status defined by Blishen scale for husband's occupation (62% groups 2‐3) |
| Interventions | Intervention: combination of home visit by breastfeeding consultant within 5 days of hospital discharge (duration 2 h) and weekly telephone calls by the consultant for 1 month, then monthly from 2‐6 months Control: postpartum home visit by public health nurse who gave breastfeeding advice determined largely by the questions and concerns of the mother |
| Outcomes | Duration of breastfeeding |
| Notes | Awaiting classification reason: concerns over randomisation process. *Unable to contact Author* Dates of study: Spring of 1984 Funding sources: not reported Declarations of interest: not reported |
| Methods | Randomized controlled trial |
| Participants | 178 women Setting a large public hospital or a community clinic with a birthing centre in southern Quito, Ecuador Intervention: PRIMIPAROUS 24.5%, age 27, C‐section 21.6%, Education Highest level of education: none ‐ 3 (2.9); Primary ‐ 22 (21.6); Some secondary ‐ 26 (25.5); graduated from secondary ‐ 34 (33.3); some education beyond secondary ‐ 11 (10.8); graduated from university ‐ 6 (5.9) Control: PRIMIPAROUS 23.7%, age 25.5, C‐section 25%, Education Highest level of education: none ‐ 5 (6.6); primary ‐ 18 (23.7); some secondary ‐ 18 (23.7); graduated from secondary ‐ 21 (27.6); some education beyond secondary ‐ 11 (14.5); graduated from university ‐ 3 (3.9) |
| Interventions | Mothers assigned to the intervention group received a two‐part intervention in addition to the standard treatment. Both parts of the intervention were delivered by one bachelor‐degree‐level, lichenised Ecuadorian nurse with more than 15 years of clinical experience. Part 1 consisted of an educational session administered by the nurse via phone within 48 h of hospital discharge. The nurse followed a semi‐structured patient education protocol, guided by a checklist of topics to cover and bullet points detailing the information to be provided, to ensure that all participants received the same information (Table 1). This information was developed by the study team in conjunction with local physicians and nurses using best practices information disseminated by the Ecuadorian Ministry of Health. Participants were encouraged to ask questions as needed during the education session. Part 2 of the intervention consisted of access to a nurse on‐call during the first 30 days of the newborn's life. Patients were instructed that they could call the nurse during their 30‐day follow‐up period to ask questions regarding their own or their newborn's health and care, as needed. The nurse provided medical advice, information, and support, and triaged patients to determine whether a clinic visit was needed to address each complaint. The nurse was available via phone from 8 am to 5 pm, Monday to Friday. Patients were cautioned that the nurse on‐call service was not intended for use in emergency situations. In case of emergency, they were instructed to call the hospital or health center emergency phone numbers, or to go to the emergency room as per the usual process. |
| Outcomes | Exclusive bf at 3‐4 months |
| Notes | No conflicts of interest. Grant funding not industry Awaiting classification reason: no protocol. *Unable to contact Author* |
| Methods | 2‐arm RCT, single site, n = 150 |
| Participants | Conducted at a tertiary care centre located in Northwestern Ontario, Canada. Background breastfeeding initiation rates: 87.3% Inclusion criteria: English‐speaking, primiparous mothers who gave birth to a single, healthy, term infant whom they were planning on breastfeeding Exclusion criteria: any condition that could significantly interfere with breastfeeding, such as a serious illness, an infant with a congenital anomaly, or requiring special care that would not be discharged home with the mother |
| Interventions | Intervention (n = 69): participants received 3 individualised, self‐efficacy enhancing sessions with the researcher: 2 in‐hospital and 1 by telephone in the early postpartum period. The first session occurred after randomisation and within 24 h of delivery. The second session also took place in‐hospital, ideally within 24 h of the first session. In addition, observation of breastfeeding at 1 of the 2 in‐hospital sessions was planned to try to maximise performance accomplishment (successful breastfeeding). The third session occurred via telephone within 1 week of hospital discharge. Control (n = 81): standard in‐hospital and community care |
| Outcomes | Primary: feasibility, compliance, and the acceptability of the breastfeeding self‐efficacy intervention Secondary: breastfeeding self‐efficacy, duration, and exclusivity |
| Notes | Awaiting classification reason: no protocol and none given by Author on contact due to University closure with COVID. Dates of study: recruitment between March 3, 2008, to July 23, 2008 Funding sources: not reported Declarations of interest: not reported |
| Methods | Pilot RCT (n = 150), March‐July 2008 |
| Participants | Recruitment from 1 hospital in Northwestern Ontario, Canada, the sole provider of maternity care for the city and regional referral centre Background rates of breastfeeding initiation for Canada: intermediate, however, baseline prevalence of 'ever breastfed' in Ontario 90.6% (WHO Global Data Bank on Infant and Young Child Feeding accessed 12 Oct 2011) Inclusion criteria: English‐speaking, primiparous, planning on breastfeeding, with single, healthy, term infants Exclusion criteria: conditions that could significantly interfere with breastfeeding such as serious illness, infant with congenital anomaly or admitted to special care |
| Interventions | Intervention: standard in‐hospital and community postpartum care plus a 1‐to‐1 self‐efficacy intervention from the researcher (a Registered Nurse with practice, education, and research experience working with breastfeeding mothers). The intervention included assessment of the mother’s breastfeeding goals and breastfeeding self‐efficacy and her general physiologic and affective state; strategies to increase breastfeeding self‐efficacy; evaluation, and planning the next session. There were 3 contacts, 2 face‐to‐face in hospital on days 1 and 2 after the birth, and 1 phone call up to 7 days after hospital discharge. Control: standard in‐hospital and community postpartum care, which included a visit by a public health nurse after hospital discharge |
| Outcomes | Feasibility, compliance, and acceptability of the intervention, breastfeeding confidence (self‐efficacy scores), any and exclusive breastfeeding at 4 and 8 weeks |
| Notes | Awaiting classification reason: No protocol and none given by Author on contact due to University closure with COVID. Paper stated "Observation of breastfeeding at 1 of the 2 in‐hospital sessions was planned, to try to maximise performance accomplishment (successful breastfeeding)”. Dates of study: between March 2008 and July 2008 Funding sources: not reported Declarations of interest: none declared |
| Methods | Cluster rRCT |
| Participants | All health providers (Accredited Social Health Activists (ASHAs;) ANMs, medical officers and Primary Health Centre (PHC) support staff) in the study area were participants All pregnant women; neonates and infants living in the study area were participants |
| Interventions | Mobile phone job aid for staff Women and infants were registered by ASHAs on the system and the software created a schedule of home visits. Reminders of the visits due were sent to staff. Staff recorded information on the system during home visits. Software included decision support systems to aid development of management care plans, suggest medications and home remedies, referrals and to call emergency transport |
| Outcomes | Number of home visits conducted Improvement in coverage of home visits conducted by ASHA (antenatal and postnatal) Breastfeeding initiation Infant and neonatal death |
| Notes | None of our included outcomes were reported in this study. Study authors to be contacted in next update to check if other outcomes were measured. |
| Methods | 2‐arm RCT, n = 186 |
| Participants | Women attending health centres in Sabzevar, Iran Background rates of exclusive breastfeeding at 6 months: 53.1% Inclusion criteria: wanted pregnancy and primi gravidity Exclusion criteria: no details provided in English abstract |
| Interventions | Intervention: husbands attended prenatal care Control: women attended prenatal care alone No details provided about total number randomised in each group |
| Outcomes | Satisfaction of husband involvement Husband taking care of baby in absence of mother Husband's support of breastfeeding |
| Notes | Needs to be translated from Arabic. |
| Methods | RCT |
| Participants | Immigrant pregnant women |
| Interventions | Visit One: last trimester Visit Two: between 2nd and 5th day postnatal Visit Three: 15th day postnatal Visit Four: 40th day postnatal At each visit breastfeeding is assessed and advice given. |
| Outcomes | Breastfeeding Self‐Efficacy Scale Latch Breastfeeding Assessment tool |
| Notes | None of our included outcomes were reported in this study. Study authors to be contacted in next update to check if other outcomes were measured. |
| Methods | RCT |
| Participants | First time mothers and their partners |
| Interventions | 2 40‐minute sessions First visit is when the infant is 3‐5 days old, the second visit is one week later. During the sessions the mother given information on the benefits of breastfeeding and assistance to breastfeed, including positioning and latch. Partners were given advice on how to encourage and support their wife breastfeed. |
| Outcomes | Breastfeeding awareness and attitudes of mothers and fathers |
| Notes | None of our included outcomes were reported in this study. Study authors to be contacted in next update to check if other outcomes were measured. |
| Methods | RCT |
| Participants | 104 primiparous women Setting: Hamadan city, Iran |
| Interventions | Lactation counselling using a mobile phone |
| Outcomes | Exclusive bf at 3‐4 months |
| Notes | Awaiting classification reason: No protocol, no baseline characteristics and concerns over randomisation process. No reply from author
|
| Methods | RCT |
| Participants | Primiparous women giving birth via Caesarean section |
| Interventions | Consultation based on Bristol tool on self‐efficacy and continuation of breast‐feeding |
| Outcomes | Exclusive breastfeeding at 1 and 4 months |
| Notes | Awaiting classification reason: No loss to follow‐up and concerns over randomisation process. *Unable to contact author* |
| Methods | 2‐arm RCT, with individual randomisation n = 60 |
| Participants | Women were recruited at a community hospital in the USA and had diverse socioeconomic status. Background rates of breastfeeding initiation: intermediate Inclusion criteria: women who experienced vaginal deliveries after full‐term pregnancies Exclusion criteria: not stated Participant characteristics: mean age: 24.4 years; married n = 47 (78%); white n = 55 (93%); completed high school n = 58 (97%); income of USD ≤ 20,000 n = 13 (22%) |
| Interventions | Standard care included routine breastfeeding support in hospital following delivery. Intervention: 2 home visits by a professional community health nurse and phone call from a qualified LC. The nurse provided a structured teaching and support protocol. The focus of the first visit was to enhance breastfeeding. For the second visit, of up to 2 h duration, mothers could choose the content from options including help with dishes or laundry. Most chose education or infant assessment; 2 asked for child care help so they could rest and/or spend time with a partner. Control: home visit on day 3 or 4 by a hospital nurse (not specifically about breastfeeding). |
| Outcomes | Primary: duration of breastfeeding Secondary: fatigue, symptoms of anxiety and depression |
| Notes | Awaiting classification reason: lack of baseline characteristics, no loss to follow up and concerns over randomisation process. *Unable to contact author* Dates of study: not reported Funding sources: Mead Johnson Perinatal Nutritionals through Sigma Theta Tau Declarations of interest: not reported |
| Methods | 2‐arm RCT, single‐site study, recruitment April 1999‐February 2000, n = 41; 21 assigned to intervention and 20 to control group |
| Participants | Community intervention in urban USA Background rates of breastfeeding initiation: low Inclusion criteria: low‐income women receiving financial medical assistance Exclusion criteria: not stated Ethnic composition: 95.2% African American |
| Interventions | Intervention: breastfeeding support visits by community health nurse/peer counsellor team. Support offered daily when in hospital, and at home during weeks 1, 2 and 4 and at the team's discretion. Telephone support from peer counsellor twice‐weekly through to week 8 and monthly through to month 6. Control: usual breastfeeding support consisted of support from hospital nurses, assistance by means of a telephone 'warm line' and if mothers gave birth on a weekday, 1 hospital visit from an LC. |
| Outcomes | Duration of breastfeeding to 6 months; healthcare services use by infants; costs |
| Notes | Awaiting classification reason: no loss to follow up. *Unable to contact Author* Dates of study: study conducted between April 1999 and February 2000 Funding sources: The National Institute of Nursing Research, Bethesda, Maryland. Declarations of interest: not reported |
| Methods | RCT |
| Participants | Mother of infants born in March 2011 from four villages surrounding Bethlehem |
| Interventions | CHW targeted intervention group with key messages and support during home visits |
| Outcomes | Change in breastfeeding rates at 6 months and 1 year |
| Notes | This is a conference abstract and was previously excluded on the grounds it was not a paper. As it potentially meets the inclusion criteria we have moved to awaiting classification. Study authors will be contacted in the next update to clarify details on attrition, methods of randomisation and if these data are the final study data. |
| Methods | 2‐arm., parallel RCT, n = 140 |
| Participants | Primiparous women attending the selected health centres of Tehran University of Medical Sciences, Iran Background rates of breastfeeding imitation: > 90% Details of inclusion/exclusion criteria not provided in English abstract. |
| Interventions | Intervention (n = 70): telephone counselling on breastfeeding provided by one of the researchers Control (n = 70): routine care |
| Outcomes | Exclusive breastfeeding at 1 and 3 months Duration of continuity and exclusivity of breastfeeding |
| Notes | Needs to be translated from Arabic |
| Methods | RCT |
| Participants | 203 women Setting The PicoPico‐Union area of Los Angeles Age 28.1, SE Status: receiving social welfare 16.7%, Education ≤ some high school n= 12,863.1%; ≥ High school graduate/GED n= 75,36.9% |
| Interventions | Mentor Mothers (MM) were local parents who were perceived to be socially skilled and positive role models in their community. MM were trained in foundational skills (called practice elements) common across EBP (Chorpita and Daleiden 2009) for 1 week. MM then received a month of training, which included educational information about how to achieve each targeted outcome (e.g., tips about breastfeeding). For each issue, the MM practised how to: frame each targeted behaviour (e.g., breastfeeding protects your child early and has lifelong benefits);•provide information that had to be applied to the participant’s life (breastfeeding will hurt in the beginning, but you can prepare your breasts and know that the tender‐ness will stop in about a week);•build the skills and routines that would help the mother consistently implement a new behaviour;•provide social support for new routines; and•eliminate environmental barriers to achieving the targeted outcome behaviour. |
| Outcomes | Any breastfeeding at 2 months and 6 months |
| Notes | Awaiting classification reason: no loss to follow‐up and concerns over randomisation process. No reply from author Study funded by Kellogg Foundation and National Institutes of Health |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | We have not been able to access this abstract and have no further information. |
| Methods | RCT |
| Participants | Women giving birth via Caesarean at Iran University Medical Sciences Hospital |
| Interventions | Additional support for women giving birth via Caesarean in the form of home visits |
| Outcomes | Exclusive breastfeeding at 6 months |
| Notes | Requires translation |
| Methods | 2‐arm RCT, with individual randomisation, recruitment 1986‐1987, n = 52 |
| Participants | Volunteers attending prenatal clinics in Massachusetts USA who intended to breastfeed their babies for 2 months or longer Background rates of breastfeeding initiation: low Inclusion criteria: breastfeeding for the first time, or unsuccessful previous attempts; English‐speaking |
| Interventions | All women received prenatal breastfeeding information. Intervention (n = 26): home visits and telephone contacts up to 2 months postpartum from an experienced breastfeeding counsellor (who also recruited women to the study). Women received 5‐8 visits lasting 30‐60 min. Control (n = 26): usual care; women were given contact details for the clinic nutritionist to use if problems arose. |
| Outcomes | Breastfeeding at 2 months postpartum and 6 months postpartum |
| Notes | Awaiting classification reason: No loss to follow up and concerns over randomisation process. *Unable to contact author* Dates of study: Study conducted between February 1986 and April 1987 Funding sources: not reported Declaration of interest: not reported
|
| Methods | RCT |
| Participants | Pregnant women |
| Interventions | 4 breastfeeding counselling groups each lasting between 60 and 90 minutes All participants in the intervention group received a booklet Face to face or telephone support was available until 4 months postnatal |
| Outcomes | Breastfeeding self‐efficacy Frequency of reported breastfeeding problems |
| Notes | None of our included outcomes were reported in this study. Study authors to be contacted in next update to check if other outcomes were measured. |
| Methods | RCT |
| Participants | 129 primiparous women Setting Vali‐e‐Asr Hospital in Tehran, Iran. Intervention: age 28.6, C‐section 73.5%, Education Maternal education: Below high school diploma: 24 (37.5%), high school diploma: 27 (42.2%), above high school diploma: 12 (18.8%). Control: age 28.45, C‐section 75.4%, education Maternal education: below high school diploma: 24(36.9%), high school diploma: 27 (41.5%), Above high school diploma: 7 (10.8%) |
| Interventions | The mothers in the intervention group received one training session of breastfeeding self‐efficacy as the intervention, in addition to psychological therapies in case of suffering from anxiety, stress, or depression. The mothers were also provided with breastfeeding training pamphlets and CDs, and one session was held using the training package to make mothers aware of parenting methods, including postpartum care and the relationship with infants. |
| Outcomes | Exclusive bf at 6 months and any breastfeeding at 12 months |
| Notes | Awaiting classification reason: No loss to follow up reported. No reply from author No industry funding and no conflicts of interest declared |
| Methods | RCT |
| Participants | First time mothers attending health centres in Hamadan |
| Interventions | Two group‐counselling sessions lasting around 30 to 45 minutes during the first two weeks after birth. Telephone call follow‐up |
| Outcomes | Breastfeeding self‐efficacy |
| Notes | None of our included outcomes were reported in this study. Study authors to be contacted in next update to check if other outcomes were measured. |
| Methods | 2‐arm RCT, n = 114 |
| Participants | Public maternity hospital in Italy Background rates of breastfeeding initiation: not stated Inclusion criteria: healthy primiparous women without breastfeeding problems, with a healthy baby born at full term (37–41 weeks, birthweight > 2500 g) and who agreed to be enroled Exclusion criteria: multiparous women, premature baby (born before the 37th week), low birth weight baby (< 2500 g), admission to NICU or transfer to another hospital, medical condition which could permanently or temporarily counter‐indicate breastfeeding (e.g. acute tuberculosis, psychosis, acute phase hepatitis A or B, hepatitis C, HIV), women who did not speak Italian, and women who could not be contacted by telephone). |
| Interventions | Intervention (n = 55): structured telephonic counselling (STC); each mother received telephone calls during the first 6 weeks after delivery. The phone call timing was planned by both the mother and the incensed midwife (LM) with at least one a week; in addition, mothers were invited to call the LM when necessary to solve any breastfeeding problem. During every phone call, the LM gave support and all information on fully breastfeeding. No weekly calls were missed. Control (n = 59): conventional counselling ‐ consisting of programmed periodical visits with the physician at 1, 3 and 5 months after delivery. Participants were also invited to call the LM in case of breastfeeding problems. |
| Outcomes | Primary: breastfeeding at 1,3 and 5 months after delivery Secondary: influence of mother’s educational level and employment status on exclusive breastfeeding |
| Notes | Awaiting classification reason: no protocol and no loss to follow‐up Dates of study: study conducted between February and March 2009 Funding sources: not reported Declaration of interest: not reported |
| Methods | Quasi‐RCT, single site, duration 12 months, n = 146 |
| Participants | Urban Sweden ‐ maternity ward of University Hospital Background rates of breastfeeding initiation: high. Baseline prevalence (1972): 4% breastfeeding at 24 weeks Inclusion criteria: resident in Uppsala; normal birth; healthy babies weighing > 3 kg Exclusion criteria: none specified Ethnic composition not stated. 28% of mothers had completed college or university education |
| Interventions | Intervention: 'Interview' with paediatrician in hospital on days 1 and 4, and at home at 2 and 6 weeks and 3 months; telephone contact weekly while breastfeeding followed by home visit if problem noted. Control. Usual care, |
| Outcomes | Partial and exclusive breastfeeding at 2, 4, 8, 12, 16, 20 and 24 weeks |
| Notes | Awaiting classification reason: no baseline characteristics provided. *Unable to contact Author* Primarily designed as a study of the reasons for breastfeeding difficulties and the cessation of breastfeeding. Recruitment halted during holidays. Dates of study: recruitment from November 1973 to November 1974 Funding sources: not reported Declaration of interest: not reported |
| Methods | 2‐arm RCT, n = 120 |
| Participants | Westown Physician Center (WPC), a hospital‐affiliated urban clinic, USA, where most patients received public insurance or charity care Background rates of breastfeeding initiation: data from an inner‐city Cleveland clinic with a similar population reported lower rates with any and exclusive breastfeeding at 5 days at 40.8% and 22.0%, respectively. Inclusion criteria: aged18 years or older, with no contraindications to breastfeeding, ≥ 28 weeks’ gestation at recruitment stage Exclusion criteria: women < 18 years old, non‐English speakers, and those with a diagnosis that was an absolute contraindication to breastfeeding (HIV/AIDS, herpes simplex on the breast, tuberculous lesions of the breast) |
| Interventions | Intervention (n = 50): peer counselling WIC support programme; WIC definition of a breastfeeding peer counsellor: a woman who breastfed her own infant(s) to 1 year with exclusive breastfeeding for 6 months or was currently breastfeeding an infant following recommended practice, who received 20 h training. Counsellor was resident in the local area and received care herself from WPC. Antenatal: peer counsellor initiated contact once during third trimester of pregnancy with additional contacts at mother's request (mostly by telephone). Postnatal: peer counsellor contact within 3‐5 days of birth weekly to 1 month, every 2 weeks up to 3 months, and once at 4 months, in person during clinic visits or via telephone. No home visits for safety reasons. Control (n = 53): standard care (available to both intervention and control group), included access in hospital to International Board Certified LCs and outpatient lactation support from the clinic paediatricians and the WIC nutritionist. The in‐office WIC site had a peer helper available less than once a month. |
| Outcomes | Breastfeeding initiation Any breastfeeding at 1 month and 6 months Exclusive breastfeeding at hospital discharge, 1 month, 6 months Breastfeeding attitude and self‐efficacy Perception of breastfeeding support |
| Notes | Awaiting classification reason: no protocol and concerns over randomisation process. *Unable to contact Author* Dates of study: Study conducted between January 2011 to June 2012. Recruitment between February 2011 to March 2012. Funding sources: Project Implementation Grant from the AAP Community Access to Child Health Project Implementation Grant Program and a CareSource Foundation Responsive Grant Declaration of interest: none declared |
| Methods | RCT |
| Participants | 126 women Setting ‐ a regional teaching hospital in Taiwan |
| Interventions | Breastfeeding support programme |
| Outcomes | Breastfeeding rates at 4‐6 weeks, 2 months, 3‐4 months and 6 months |
| Notes | Awaiting classification reason: no protocol and concerns with randomisation process. *Unable to contact author* |
| Methods | RCT |
| Participants | 120 teenage mothers Setting ‐ hospital in Thailand Primiparous 98.5%, Age 17, SE status: no income 18.3%mMother’s own family 30.8% husband’s family 5.8% husband 22.5% own income 10%; C‐section 30%, education Junior high school 29/93 (24.2%) Senior high school/technical college 18/93 (15%) |
| Interventions | All 120 teenage mothers received the hospital’s standard postpartum service before their discharge from Siriraj Hospital; this included family planning counselling and services, breastfeeding and the setting of their first postpartum follow‐up appointment for 6 weeks after delivery. In contrast, the mothers in the experimental group received a reminder phone call from the research assistant 1–3 days before their scheduled 6‐week, 1‐year and 2‐year postpartum appointments. While they were provided the same standard postpartum care that was given to the control group, they also undertook the mother‐role development program, which was delivered by a paediatrician at the Young Family Clinic. This 2‐year program focused on a number of key areas: the role a mother has in the care of their babies, risk behaviour reduction, mental health assessment, breastfeeding counselling and planning of the mother’s further education and future occupation. During the 2‐year training period, the teen mothers were asked to attend six sessions at the Young Family Clinic: at 2, 4, 6, 9, 12 and 24 months after delivery. As with the babies in the control group, the Well‐Baby Clinic of the Pediatric Department provided standard care to the babies in the experimental group. |
| Outcomes | Any breastfeeding at 4‐6 weeks and 9 months |
| Notes | Awaiting classification reason: no protocol. No reply from author Conflicts of interest ‐ none declared. Funding not reported |
| Methods | 2‐arm RCT, n = 100 |
| Participants | Setting not clear. "Our research was conducted by the Association for a healthy and happy childhood‐Counseling center for mother and child in Bjelovar, Croatia”– not obvious what type of setting this is, but we infer it is a community‐based setting of some sort. Background rates of breastfeeding initiation: 50% of women give‐up breastfeeding after 6 months in Croatia Inclusion criteria: "the criterion for inclusion in the study was that the mother was nursing her child and the child had up to two months" Exclusion criteria: none stated |
| Interventions | Intervention (n = 50): “six basic exercises of autogenic training". Not clear what this is but article states "every two weeks mothers were practicing a new exercise. The 6 basic exercises of autogenic training were taught for 12 weeks in small groups to 10 members". "After mothers have learned all the exercises of autogenic training, they have continued to practice until their child reached six months of life". The exercises seem to be delivered in a group setting and promoted breastfeeding. Control (n = 50): unclear; Quote "mothers of both groups were advised to successful breastfeeding up to 6 months of age" |
| Outcomes | Attitude, decision and duration of breastfeeding Mother’s level of confidence Motivation for successful breastfeeding Motivation for autogenic training Possible factors influencing breastfeeding Risk factors for postpartum mental disorders, anxiety and postpartal depression Degree of satisfaction with practising autogenic training and its possible role in promoting successful breastfeeding in the examined group |
| Notes | Awaiting classification reason: no protocol, no loss to follow‐up, implausible results, baseline characteristics not fully reported and concerns over randomisation process. No reply from author Dates of study: Recruitment recorded as 2010, no other information provided Funding sources: not recorded Declarations of interest: not recorded |
| Methods | 2‐arm RCT, n = 206 |
| Participants | Mother‐baby dyads attending a 2‐week well‐baby visit. Study mothers were mainly white, non‐Hispanic, highly educated, married, of higher socioeconomic status, planned to return to work or school after their baby’s birth, and reported good to excellent baseline confidence in breastfeeding. No details provided about inclusion/exclusion criteria. |
| Interventions | Intervention (n = 100): online breastfeeding tutorial and maternal needs assessment administered at 2‐week, 2‐, 4‐, and 6‐month well‐baby visits with provider counselling targeted to the mother’s needs Control (n = 106): usual care |
| Outcomes | Exclusive breastfeeding at 2 months Exclusive breastfeeding at 2 months Any breastfeeding at 2 months |
| Notes | Awaiting classification reason: no protocol, not enough data including lack of baseline characteristics and concerns over randomisation process. *Unable to contact author* Conference abstract only. SM contacted authors for further details 21 July 2016. |
| Methods | 2‐arm RCT, n = 53 |
| Participants | Mexican–American women (American women of Mexican ethnicity/ancestry) residing in rural western Nebraska in the central USA Background rates of breastfeeding initiation: initiation 80% in Hispanic/Latino women. Duration and exclusivity of breastfeeding at 6 months was 45.2% and 14%, respectively. Inclusion criteria: self‐identified Mexican‐American mothers between the ages of 15‐50 years who were breastfeeding at the time of recruitment/consent Exclusion criteria: admission of the mother to the ICU, multiple births, congenital abnormalities in the infant, or infant admitted to NICU |
| Interventions | Intervention (n = 26): motivational interviewing (MI); MI was operationalised by asking the participant to rank the importance of breastfeeding for 6 months (1–10 scale) and her confidence in her ability to continue breastfeeding (1–10 scale). The researcher focused on the lower score and asked the woman why she did not choose a higher number and what she thought it would take to increase the number. Initial intervention delivered at day 3 visit, MI booster sessions delivered at week 2 and week 6 visits to promote behavioural change. Control (n = 27): attention control (AC); mothers in the AC group were given educational information about infant safety including information on fall prevention, poisoning, fires, and burns during the first visit, about choking/aspiration, suffocation, drowning, and smoking during the second visit, and about car seat safety during the final visit. The principal investigator conducted all AC sessions. |
| Outcomes | Intention to breastfeed for 6 months Breastfeeding self‐efficacy Duration of breastfeeding |
| Notes | Awaiting classification reason: no protocol and lack of baseline characteristics. *Unable to contact author* Feasibility study Dates of study: recruitment from December 2008 to March 2010 Funding sources: Small Dean's Grant from the University of Nebraska Medical Centre, College of Nursing Declaration of interest: none declared |
| Methods | Single‐site study, duration of recruitment not reported, n = 72; 30 allocated to the intervention and 42 to the control group |
| Participants | Community study in North Trent, England, UK Background rates of breastfeeding initiation: intermediate. National baseline prevalence 66% breastfeeding at birth Inclusion criteria: mothers attending for antenatal care on 1 area. Other details not reported. |
| Interventions | Intervention: the midwife asked mothers during their pregnancy to identify a close female confidante who could support them to breastfeed, and visited the mother and confidante together during the third trimester to discuss breastfeeding. |
| Outcomes | Duration of breastfeeding to 3 months; women's satisfaction with the intervention; midwives' assessments of the intervention |
| Notes | Awaiting classification reason: Not enough data provided, unsure of loss to follow‐up numbers. *Unable to contactaAuthor* Numerical outcome data were provided by the researcher. Dates of study: not recorded Funding sources: Department of Health Declarations of interest: not recorded |
| Methods | 2‐arm RCT, with individual randomisation; few details of study methods reported |
| Participants | Partners of women attending for antenatal care at Baltimore Hospital USA 2001‐2002 (567 pregnant women were approached) |
| Interventions | Intervention: 1 group session for fathers, lasting 2 h, to encourage them to support their partners to breastfeed Control: usual care; fathers received classes on child safety and baby care |
| Outcomes | Breastfeeding at 4, 6 and 8 weeks and breastfeeding duration |
| Notes | Awaiting classification reason: concerns over randomisation process. *Unable to contact author* Dates of study: recruitment from March 2001 to August 2002 Funding source: Centers for Disease Control and Prevention, coordinated by the Association of Teachers of Preventive Medicine Declaration of interest: not reported |
| Methods | 2‐arm quasi‐RCT, n = 74 |
| Participants | Participants were recruited from the maternity department of a tertiary hospital in a major city of central China, Wuhan Background rates of breastfeeding initiation: not reported for Wuhan but 95.6% in Shanghai. In Whuhan 67% of mothers have stopped breastfeeding by 4‐6 months. Inclusion criteria: ≥ 18 years of age, able to read and understand Mandarin, new mother with a single, healthy term infant, and intending to breastfeed Exclusion criteria: any condition that would interfere with breastfeeding, such as a serious illness, mental illness, or an infant requiring special care that could not be discharged with the mother |
| Interventions | Intervention (n = 37): self‐efficacy intervention.Women received three sessions post‐partum: one within 1 day of delivery, 1 the next day and third 1 week after discharge. The sessions involved assessment of breastfeeding goals and self‐efficacy, self‐efficacy‐enhancing strategies, and evaluation. Assessment enabled individualisation of the intervention to meet the woman's needs. The self‐efficacy strategies were informed by the WHO breastfeeding counselling course. At the end of each session women completed an evaluation form which was used to identify any changes needed and plan the following session. Control (n = 37): standard care that included in‐hospital care and follow‐up by a community nurse after discharge |
| Outcomes | Breastfeeding self‐efficacy Breastfeeding duration and exclusivity at 4 and 8 weeks postpartum |
| Notes | Awaiting classification reason: no protocol and concerns over randomisation process. *Unable to contact author* Dates of study: recruitment was June to October 2012 Funding source: no funding grant received Declarations of interest: none declared |
| Methods | RRCT |
| Participants | 120 primiparous women Setting Kayseri Province, Turkey Intervention: age 23, SE status Family income (TL): 1400.0 (975.0–2000.0). US$1=1.67 Turkish Liras (TL) in 2011, C‐section 29.4%, Education level Primary school: 1 (2.9%) Secondary school: 4 (11.8%) High school: 9 (55.9%) University: 10 (29.4%) Control: age 24.5, SE status Family income (TL): 1500.0 (775.25–2625.0). US$1=1.67 Turkish Liras (TL) in 2011, C‐section 10%, Education level Primary school: 2 (6.7%) Secondary school: 3(10.0%) High school: 17 (56.7%) University: 8 (26.6%) |
| Interventions | The training group received breastfeeding education during the prenatal and postnatal periods from the researcher. Prenatal training was provided to each pregnant woman in two lessons in a room allocated for training within the hospital. Each group consisted of 8–14 participants. Both groups were monitored through home visits in the 1st and 24th weeks postpartum. . |
| Outcomes | Exclusive breastfeeding at 6 months |
| Notes | Awaiting classification reason: no protocol. *Unable to contact Author* No potential conflict of interest was reported by the authors This study was supported by Erciyes University Coordination of Scientific Research Projects with TSD‐11–3732 pro‐ ject code. |
Abbreviations
BMI: body mass index
EBF: exclusive breastfeeding
EBG: extended breastfeeding
FAB: Food, physical activity and breastfeeding
h: hour
LC: lactation consultant
min: minute
NICU: neonatal intensive care unit
RCT: randomised controlled trial
SD: standard deviation
WIC: Special Supplemental Nutrition Programme for Women, Infants and Children (US Department of Agriculture, Food and Nutrition Service)
Characteristics of ongoing studies [ordered by study ID]
| Study name | An early intervention to promote maternal sensitivity in the perinatal period for women with psychosocial vulnerabilities |
| Methods | RCT |
| Participants | Psychologically or socially vulnerable women in the Capital Region of Denmark |
| Interventions | The intervention consists of four components: 1) detecting symptoms of mental illness in vulnerable pregnant women and initiating treatment if indicated, 2) strengthening parenting skills using the Circle of Security Parenting program, 3) supporting breastfeeding, and 4) sharing knowledge and organising treatment pathways for families across sectors |
| Outcomes | The primary outcome is maternal sensitivity. Secondary outcomes include infant’s socio‐emotional development, parents’ mentalisation, breastfeeding status, parental stress, depressive symptoms, and parental wellbeing. |
| Starting date | Not specified |
| Contact information | |
| Notes |
| Study name | Does post‐natal breastfeeding support improve rates of breastfeeding? |
| Methods | RCT |
| Participants | Participants must: |
| Interventions | Support from a lactation consultant |
| Outcomes | Any and exclusive breastfeeding at 1,3 and 6 months |
| Starting date | 26/2/2018 |
| Contact information | |
| Notes |
| Study name | Evaluating bundling of nutrition‐specific Interventions |
| Methods | Cluster RCT |
| Participants | Pregnant women living in intervention clusters |
| Interventions | Door‐to‐door counselling on nutrition during pregnancy and exclusive breastfeeding from enrolment into study till birth; exclusive breastfeeding and nutrition of lactating mothers during post‐natal 6 months; timely and appropriate complementary feeding with continued breastfeeding until 2 years of child's age, and nutrition of lactating mothers during post‐natal 6‐24 months; all counselling would be supplemented with associated issues including hygiene and sanitation, mother's sleep and rest, maternal and child healthcare seeking etc. |
| Outcomes | Change in length for age Z scores. EBF at 1, 2,3,4,5 and 6 months Nutritional intake in pregnancy Early initiation of breastfeeding Birth weight IYCF practices Change in weight for length and age Z scores |
| Starting date | November 2015 |
| Contact information | Unspecified |
| Notes |
| Study name | Effectiveness an online nursing consultation in the support and establishment of breastfeeding |
| Methods | RCT |
| Participants | Postpartum women hospitalised in the University Hospital “La Paz” |
| Interventions | Online nursing consultation using a computer based platform “Red Sinapsis" |
| Outcomes | "Needs to the beginning of the breastfeeding, paper of the sanitary personnel, abandon and complications of the breastfeeding and satisfaction degree of the platform" |
| Starting date | Unspecified |
| Contact information | |
| Notes | Protocol in Spanish |
| Study name | A community based integrated intervention to improve postpartum health care: a cluster randomized controlled trial |
| Methods | Cluster RCT |
| Participants | 1000 Inclusion criteria: Inclusion criteria for community health service centre: at least 400 person maternal and child health care services per year, with maternal and child health care service team composed of obstetricians and gynaecologists, public health doctors, postpartum visiting nurses and child health care doctors. |
| Interventions | Intervention group:Integrated postnatal health care;control:routine maternal and child health care service; |
| Outcomes | Exclusive breastfeeding rates |
| Starting date | 2017‐08‐0 |
| Contact information | LIANG JI Address: 130 Dong'an Road, Xuhui District, Shanghai, China Telephone: +86 13621693855 Email: Affiliation: Public Health School, Fudan University |
| Notes |
| Study name | Role of phones in improving breast feeding rates |
| Methods | Randomised, parallel group trial |
| Participants | 350 Key inclusion & exclusion criteria Inclusion criteria: primigravida in the third trimester |
| Interventions | ntervention1: Intervention group: baseline session covering information about benefits of breastfeeding, myths around breastfeeding, correct latching and breastfeeding issues.This will be followed by SMS or Whatsapp messages reminding them of the importance of breastfeeding. The intervention group mothers would be encouraged to call the ANM on a dedicated phone number for any clarifications related to breastfeeding |
| Outcomes | First hour initiation of breastfeeding |
| Starting date | 17‐02‐2016 |
| Contact information | Dr Bhavneet Bharti Address: Advanced Pediatrics Center Postgraduate Institute of Medical Education and Research Chandigarh Advanced Pediatrics Center Postgraduate Institute of Medical Education and Research Chandigarh 160012 Chandigarh, CHANDIGARH India Telephone: 9914208327 Email: Affiliation: PGIMER |
| Notes |
| Study name | Effect of antenatal breastfeeding counselling and support in the perinatal period on development of children in first year of life |
| Methods | Randomised, parallel group trial |
| Participants | 180 Inclusion criteria:baby born vaginally having birth weight more than 1800 g and gestational age more than 34 weeks |
| Interventions | Intervention1: antenatal breastfeeding counselling and perinatal support: one to one breastfeeding counselling will be provided to expectant mothers in antenatal period, and breastfeeding support will be provided after birth also |
| Outcomes | Comparison of Feeding practices in both the groups |
| Starting date | 28‐06‐2019 |
| Contact information | Dr Anurag singh Address: Department of pediatric MDM hospital, Jodhpur 342005 Jodhpur, RAJASTHAN India Telephone: 9414133692 Email: Affiliation: Dr S N Medical college jodhpur |
| Notes |
| Study name | Educating Mothers regarding technique of breastfeeding and assessment of exclusive breastfeeding |
| Methods | Randomised, parallel group trial |
| Participants | 64 Inclusion criteria 1)Primigravida Mothers above 18years of age, with no high‐risk factors (pre‐eclampsia and eclampsia, uncontrolled sugar with Hba1c > 6.5%,uncompensated cardiovascular disease with NYHA Grade3 and above)admitted for safe confinement, and not in labour 1)Multiple pregnancy |
| Interventions | Intervention 1: Videobased educational Intervention about breastfeeding technique and its effect on early infant feeding pattern both in study and IEC education for control group: Educational video of 2min 20sec duration in kannada language regarding technique of breastfeeding global health media project video |
| Outcomes | Effect of antenatal video education intervention on breastfeeding technique and re‐enforcement in early puerperium in primigravida mothers on exclusive breastfeeding rate short term at 2 months and long term at 6 months Time point: effect of antenatal video education intervention on breastfeeding technique and re‐enforcement in early puerperium in primigravida mothers on exclusive breastfeeding rate short term at 2 months and long term at 6 months |
| Starting date | 07‐11‐2019 |
| Contact information | Dr Nutan Kamath Address: Dr Nutan Kamath Associate Dean Kasturba Medical college 203,lighthouse hill road Hampankatta Mangalore Karnataka. Kasturba Medical college 203,lighthouse hill road Hampankatta Mangalore Karnataka. 575001 Dakshina Kannada, KARNATAKA India Telephone: 9448147687 Email: Affiliation: Kasturba Medical college Mangalore Manipal Academy of higher education |
| Notes |
| Study name | Evaluation of a package of behaviour change interventions (Baduta Program) to improve maternal and child nutrition in East Java, Indonesia |
| Methods | cluster rRCT |
| Participants | The sample size for each cross‐sectional survey is 1400 mothers and their children aged < 2 years and 200 pregnant women in each treatment group. |
| Interventions | The first intervention will be health system strengthening, including the Baby‐Friendly Hospital Initiative, and training on counselling for appropriate infant and young child feeding (IYCF). The second intervention will be nutrition behaviour change that includes Emo‐Demos; a national television (TV) advertising campaign; local screening TV spots; a free, text message service; and promotion of low‐cost water filters and hygiene practices. |
| Outcomes | The primary study outcome is child stunting (low length‐for‐age), and secondary outcomes include length‐for‐age Z scores, wasting (low weight‐for‐length), anaemia, child morbidity, IYCF indicators, and maternal and child nutrient intakes. |
| Starting date | February 201 |
| Contact information |
|
| Notes |
| Study name | A smartphone application to support breastfeeding for new mothers in Vietnam |
| Methods | RCT |
| Participants | 1000 Key inclusion & exclusion criteria Inclusion criteria: pregnant women, at around 28 weeks of gestation, will be recruited at the hospital antenatal clinic. They will be eligible to participate if they: own a smartphone, aged over 18 years, have sufficient language skills (completed junior high school education) and carrying a singleton fetus. |
| Interventions | The Vietnamese Breastfeeding (VBF) app is a theory‐based yet user‐friendly smartphone application developed to support breastfeeding, with specific focus at different postnatal stages. Its purpose is to provide support and knowledge that will empower new mothers to exclusively breastfeed their babies for six months. The app content is tailored to the context of breastfeeding in Vietnam, addressing known barriers such as prelacteal feeds and emphasising early initiation of breastfeeding after birth. The VBF app will support and educate mothers about the importance of exclusive breastfeeding, build confidence in breastfeeding (e.g. correct positioning, feeding on demand) and motivate them to continue breastfeeding, by providing evidence‐based information and reinforcing regular communication with health professionals to address any problem. All app materials will be culturally appropriate and sensitive to the needs of Vietnamese mothers. |
| Outcomes | ny breastfeeding duration, as assessed by a self‐reported questionnaire (Duong, Lee, Binns 2005, Journal of Paediatrics & Child Health)[6 months postpartum] Exclusive breastfeeding duration, as assessed by a self‐reported questionnaire (Duong, Lee, Binns 2005, Journal of Paediatrics & Child Health)[6 months postpartum] |
| Starting date | 09/03/2020 |
| Contact information | Prof Colin Binns Address: School of Public Health, Curtin University, GPO Box U 1987, Perth, WA 6845 Australia Telephone: +61 8 9266 2952 Email: |
| Notes |
| Study name | Effect of telephone counseling on exclusive breastfeeding and postpartum depression |
| Methods | RCT |
| Participants | 366 Inclusion criteria: being resident of Tehran; married; education more than six years; willing to have exclusive breastfeeding; giving birth to a single healthy baby with Apgar score of 7 or more in the fifth minute; easy access by telephone; no problem in speech and hearing; not suffering from chronic disorders (diabetes, hypertension, thyroid disorders, asthma, or any other diseases) requiring prescription of drug (based on individual’s record); no history of mental disorders requiring drug use including postpartum depression; not being smoker or drug user and no history of still birth or child death. |
| Interventions | Intervention 1: Intervention group: Regular counselling by telephone. The first counselling will be within 48 hours after hospital discharge. Next, there will be telephone contacts 2 times in the first week and once a week in 2‐6 weeks. Each counselling will take about 20 minutes, which can vary upon requirement. Each session has two parts: first, the counsellor will ask the mother about their problems experienced during previous week and will note them in the checklist. Then counselling will be done about the problems. The second part of the counselling will be about support of exclusive breastfeeding and general recommendations for prevention of postpartum depression. Counsellor is a midwifery Master of Science student with special short‐term training in postpartum care. The participants in intervention group can also contact with the counsellor by telephone. Both groups can receive routine care from the health centres (three times in the 6 weeks). Control group: no intervention by the research team. Both groups can receive routine care from the health centres (three times in the 6 weeks).
|
| Outcomes | Exclusive breast‐feeding during the last 24 hours. Time point: Once in 60th to 66th days postpartum. |
| Starting date | 2011‐07‐21 |
| Contact information | Sakineh Mohammad‐Alizadeh‐Charandabi Address: Nursing & Midwifery Faculty, South Shariati Tabriz Iran (Islamic Republic of) Telephone: +98 41 1479 0364 Email: [email protected]; [email protected] Affiliation: Tabriz University of Medical Sciences |
| Notes |
| Study name | The effect of peer support on the pattern of breastfeeding among mothers with a history of unsuccessful breastfeeding |
| Methods | RCT |
| Participants | 168 Inclusion criteria: participants including major criteria: Inclusion in the study group: the desire to be together, living in Gorgan; be available; at least be literate; with a singleton pregnancy is unsuccessful in her last lactation; must be 37 weeks gestational age; the mother has to be a low‐risk pregnancy (no illness, surgery, and midwifery is not known); fetal abnormality is not known. Inclusion of Sponsor: : Must be a resident of Gorgan; be literate, have a successful breastfeeding experience; is not a debilitating disease; might be willing to cooperate in the project. Excluded mothers who are available for telephone consultation; any problems for the mother during labour or after delivery that the baby needs to stay in the hospital. |
| Interventions | Intervention 1: the control group program included the routine trainings. Case group program included the routine trainings plus the support from 1mother with positive breastfeeding experience during the first 3months after delivery. Breastfeeding patterns were evaluated by the end of the first, second and third months. Intervention 2: phone number, address and name of each sponsor several of the mothers who were matched for age, socioeconomic status, education and living there will be similarities and the rest of the calls will be made as needed. Telephone Support Group provides research, monitoring and support will be put up in case of problems breastfeeding, should be referred to medical |
| Outcomes | Primary Outcome(s) Breastfeeding pattern. Time point: One, two and three month after sampling |
| Starting date | 2013‐05‐15 |
| Contact information | Roya Sadat Etemadi Address: No 19, near Hemmat highway, Tavanir Avenue, Tehran Tehran Iran (Islamic Republic of) Telephone: +98 17 1552 7275 Email: [email protected]; [email protected] Affiliation: International branch of Shahid Beheshti University |
| Notes |
| Study name | The effect of education to improve performance in exclusive breastfeeding mothers |
| Methods | RCT |
| Participants | 112 Inclusion criteria: : nulliparous women; women in eighth month of pregnancy; their consent to participate in the study and a medical records of the study centre a Exclusion criteria: infant mortality; migration; death of mother; postpartum depression; maternal or infant illness; lack of satisfactory pregnant women participated in the study and the absence of ongoing training sessions (absences of more than one session) |
| Interventions | In the experimental group held two sessions, each lasting 120 minutes and an instructional booklet on breast milk be given to participants. Intervention 1: in the experimental group held two sessions, each lasting 120 minutes and an instructional booklet on breast milk be given to participants. Intervention 2: the control group will receive training hospital. |
| Outcomes | Do not use other foods until 6 months of age. Time point: Until 6 months after delivery. Method of measurement: Czech List and Questionnaire Performance. Not using dry milk feeding up to 6 months. Time point: Until 6 months after delivery. Method of measurement: Czech List and Questionnaire Performance. |
| Starting date | 2014‐04‐26 |
| Contact information | Dr nasrin roozbahani Address: Mustafa Khomeini, Golestan Koi, Department of Public Health 00988633684615 Arak Iran (Islamic Republic of) Telephone: +98 918 863 1816 Email: [email protected]; [email protected] Affiliation: Arak University of Medical Sciences |
| Notes |
| Study name | Effect of a maternal role education program on maternal role attainment in nulliparous women with unplanned pregnancy |
| Methods | Unclear |
| Participants | Nulliparous women with an unplanned pregnancy |
| Interventions | Various education including visualisation of motherhood role, attachment to fetus and newborn, newborn care and breastfeeding in 34,35,36 weeks gestational age and before discharge hospital and then every week to 4‐week post‐natal. |
| Outcomes | Maternal role satisfaction |
| Starting date | June 2014 |
| Contact information | Maryam Fasanghari ([email protected]), |
| Notes |
| Study name | Effect of couple centered counseling comparison of person centered counseling on self‐efficacy and success of lactation in breastfeed mothers |
| Methods | RCT |
| Participants | 170 Inclusion criteria: women with a first pregnancy, lack of systemic disease, non‐use of some medications, such as antidepressants, lack of antenatal classes and written consent to participate in the study. Exclusion criteria: women who are pregnant with twins or more, continued to participate in counselling sessions and contraindications in breastfeeding and infant hospitalised after childbirth. |
| Interventions | Intervention 1: Group of couples Breastfeeding counselling for 20 to 30 minutes in 4 sessions Intervention 2: Group of mothers Breastfeeding counselling for 20 to 30 minutes in 7 sessions. Intervention 3: The control group received routine care.
|
| Outcomes | Breastfeeding self – efficacy. Time point: before the intervention, immediately after intervention, two hours after delivery, time of discharge and first, second, third and fourth months after delivery. Method of measurement: breastfeeding self‐efficacy questionnaire. Breastfeeding success. Time point: before discharge and then monthly until the end of four months. Method of measurement: Exclusive breastfeeding Monthly form. |
| Starting date | 2015‐09‐23 |
| Contact information | afsaneh farhadi Address: Central Building University of Shahroud Medical Sciences, Hafte‐Tir Square Shahroud Iran (Islamic Republic of) Telephone: +98 23 3234 3024 Email: Affiliation: Shahroud University of Medical Science |
| Notes |
| Study name | Studying the effect of education based on Pender's health promotion models on successful breastfeeding in pregnant women |
| Methods | RCT |
| Participants | 40 Inclusion criteria: pregnant women whose duration of their pregnancy is more than 32 weeks. |
| Interventions | The implementation of 6 sessions. |
| Outcomes | Successful breastfeeding. Time point: Pre‐ intervention, Post‐ intervention, Two months after the intervention. |
| Starting date | 2018‐10‐07 |
| Contact information | Maryam Saberi Address: Namazi Square ‐ School of Nursing and Midwifery‐ Shiraz‐ Iran 7193613119 Shiraz Iran (Islamic Republic of) Telephone: +98 71 3647 4254 Email: Affiliation: Shiraz University of Medical Sciences |
| Notes |
| Study name | Effect attendance and non‐attendance education on Knowledge, Attitude and Practice of females about Breastfeeding |
| Methods | Interventional |
| Participants | 360 Inclusion criteria: having at least one child of 0‐24 months of age, being at least in the 36th week of pregnancy, having a singleton and healthy baby (without major anomalies such as heart problem, Down syndrome, cleft palate, etc.), lack of specific diseases, and willingness to participate in the study
|
| Interventions | Intervention 1: Intervention group: attendance education on breastfeeding. Intervention 2: Intervention group: non‐attendance education on breastfeeding. |
| Outcomes | Attitude. Time point: one month post intervention. Method of measurement: questionnaire. Knowledge. Time point: one month post intervention. Method of measurement: questionnaire. Practice. Time point: one month post intervention. Method of measurement: questionnaire. |
| Starting date | 2017‐03‐21 |
| Contact information | Leila Ghavami Address: Central building Shiraz University of Medical Sciences, Zend Ave ????? ‐ ????? Shiraz Iran (Islamic Republic of) Telephone: +98 71 3230 5410 Email: Affiliation: Shiraz University of Medical Sciences |
| Notes |
| Study name | Effect of message framing on breastfeeding self efficacy and behavior among women |
| Methods | interventional |
| Participants | 210 Inclusion criteria:First time pregnancy; Giving birth to a healthy baby; No psychological distress in mothers; Having the intention to breastfeed; Agree to take part in the study; Being able to communicate easily with mother using SMS; Fluent in Persian(Farsi); Lack of abnormality in the breast tissue; Having at least able to read; and singleton. |
| Interventions | Intervention 1: Intervention groups (Gain and loss group): Gain and loss‐framed messages were sent to both groups for 8 weeks. Each day, a positive (Such as breastfeeding increases the child's IQ) and a negative message (Such as symptoms such as diarrhoea and intestinal bleeding have mostly observed in infants who are fed from other milk other than breast milk) was sent to the gain and loss groups, respectively. Then, from the fourth to the eighth week on a day in between, a positive and negative message was sent to the gain and loss group, respectively. Intervention 2: Control group: No educational intervention. Intervention groups (Gain and loss group): Gain and loss‐framed messages were sent to both groups for 8 weeks. Each day, a positive (Such as breastfeeding increases the child's IQ) and a negative message (Such as symptoms such as diarrhoea and intestinal bleeding have mostly observed in infants who are fed from other milk other than breast milk) was sent to the gain and loss groups, respectively. Then, from the fourth to the eighth week on a day in between, a positive and negative message was sent to the gain and loss group, respectively. |
| Outcomes | Breastfeeding self‐efficacy. Timepoint: At 3‐5 days after giving birth, one month following intervention and 2 months after intervention. Method of measurement: Dennis' Breastfeeding Self‐Efficacy Scale‐Short Form. Breastfeeding behavior. Timepoint: At 3‐5 days after giving birth, One month following intervention and 2 months after intervention. Method of measurement: Researcher‐made Questionnaire. |
| Starting date | 2015‐08‐23 |
| Contact information | Marzieh Araban Address: Social Determinants of Health Research Center, Jondishapour University of Medical Sciences, Golestan Road, Ahvaz, Khuzestan, Iran 159 Ahvaz Iran (Islamic Republic of) Telephone: +98 61336754360 Email: Affiliation: Ahvaz Jundishapur University of Medical Sciences |
| Notes |
| Study name | Effect Postpartum care at home on postpartum outcome |
| Methods | interventional |
| Participants | 62 Inclusion criteria: (Inclusion criteria: accessibility to the residents of the participants; providing written consent form; having a natural delivery with no complications; having a healthy norm neonate; does not require any special care; mother’s, families and house’s appropriate condition; the presence of one of the family members during home visits and exclusion criteria: The lack of care on two occasions completely). |
| Interventions | Intervention 1: The postpartum cares will present at intervention group: Folder forming; folder investigation and familiarity with mother’s situation; history taking of mother and her newborn due to the aims of the study; physical examination of mothers and newborn and postpartum involution situation; prescription of nutritious complements; training and consulting in the fields of postpartum cares of mother and her newborn and safe reproductive consulting; investigating of mother’s awareness level and their environment; necessary education about mother and newborn due to their need to mother and family; exercise after birth; breastfeeding method will run to do simulation. The care of the intervention group was conducted at three stages by two expert midwives for 45 to 90 minutes. The educational package contained physical examination of the mother and the infant; evaluating the condition of getting back to normal after delivery and necessary educations about the mother; the infant and the family members. The correct method of performing postpartum exercises and breastfeeding were educated through simulation to the mother and her companion. Intervention 2: The postpartum cares will do at control group according to hospitals prior process and phone number will be placed them to will respond. According to routine care, the first care hospital care in the second and third care were performed by midwives in health centres in accordance with the national standards. |
| Outcomes | Primary Outcome(s) Postpartum care at home. Time point: In three‐stage visits (1st to 3rd day, 10th to 15th day, and 42 to 60 day). Method of measurement: Using researcher‐made questionnaires that include breastfeeding pattern (1 questions), awareness of maternal health (16 questions), awareness of child health (5 questions), husband’s behaviour (13 items), and mother’s satisfied. Breastfeeding pattern, awareness of maternal health, awareness of child health, husband’s behaviour, and mother’s satisfied. Time point: In three‐stage visits (1st to 3rd day, 10th to 15th day, and 42 to 60 day). Method of measurement: Using researcher‐made questionnaires that include breastfeeding pattern (1 questions), awareness of maternal health (16 questions), awareness of child health (5 questions), husband’s behaviour (13 items), and mother’s satisfied. |
| Starting date | 2017‐05‐11 |
| Contact information | Parvin Bahadoran Address: Nursing and Midwifery Care Research Center, Faculty of Nursing and Midwifery, Isfahan University of Medical Sciences, Isfahan, Iran. Isfahan Iran (Islamic Republic of) Telephone: +98 31 3792 2937 Email: Affiliation: Nursing and Midwifery Care Research Center, Faculty of Nursing and Midwifery, Isfahan University of |
| Notes |
| Study name | Comparison of the effect of acupressure, OKETANI massage and lactation training |
| Methods | interventional |
| Participants | 120 Inclusion criteria: Age 18 t0 35 years |
| Interventions | Intervention 1: Intervention group 1: Recipient of acupressure. The points used in this group are three points GB21 (at the top of the sternocleidomastoid muscle), point CV17, 4 fingers above the sternum, approximately in the centre of the chest) and point CO18 (at the bottom of the indentation) is. Mothers are taught to do these points three times a day for 2 to 5 minutes each time for a total of 14 days. Intervention 2: Intervention group2: Recipients of OKETANI massage for 30 minutes once a day for 14 days (the first day is done by the researcher and the next days by the mother after the necessary training was given to the mother) in both right and left breasts. In total, it includes 8 hand techniques. Intervention 3: Intervention group 3: Breastfeeding counselling and training group that receives breastfeeding‐related training as soon as possible after delivery and communicating with the baby, as well as for 14 days after delivery by phone or in person if necessary. Intervention 4: Control group: They only receive routine postpartum care. |
| Outcomes | Breastfeeding. Time point: Before the intervention Two weeks after the intervention and one month after the intervention. Method of measurement: Breastfeeding self‐efficacy questionnaires, evaluation of infant breastfeeding quality, continuation of exclusive breastfeeding. |
| Starting date | Pending |
| Contact information | Niloofar Rabiee Address: Shahrood University of Medical Sciences 9717419582 Shahrood Iran (Islamic Republic of) Telephone: +98 21 3235 0513 Email: Affiliation: Shahroud University of Medical Sciences |
| Notes |
| Study name | Effect of tele counseling on the continuity and duration of breastfeeding in women In Minudasht health center on summer 2014 |
| Methods | interventional |
| Participants | 154 Inclusion criteria: Iranian nationality |
| Interventions | Intervention 1: mothers in the control group will receive only routine care (including postpartum breastfeeding in the hospital, first, second, and third care after delivery by Beverz at the health centre based on the current training program for the slave). Intervention 2: In the intervention group, mothers receive tele‐counselling in form of weekly on the first month and twice in second month and once in the third month. Tele‐counselling is based on the WHO guidelines (2009). Then the duration Exclusive breastfeeding is recorded in both groups 3 months after delivery and the continuation of exclusive breastfeeding at the end of the first and third months. |
| Outcomes | Primary Outcome(s) Breast feeding (continuing and duration). Time point: Before intervention and 3 months after intervention. Method of measurement: questionnaire made by researcher. |
| Starting date | 2015‐06‐22 |
| Contact information | Sarayloo Khadijeh Address: Health center, Pasdaran Street,Minoodasht,Golestan 4983153847 Minoodasht Iran (Islamic Republic of) Telephone: +98 17 3523 1518 Email: Affiliation: Golestan university of medical scienc |
| Notes |
| Study name | Effect of mother to mother support on breast feeding pattern and breast feeding duration among nulliparous women |
| Methods | Interventional |
| Participants | 240 Key inclusion & exclusion criteria Inclusion criteria: The inclusion criteria for the mothers were as follows: willingness to participate, nulliparity, singleton pregnancy, residing in Ilam, being available and literate, gestational age of over 37 weeks at the time of delivery, no diagnosed debilitating disease in the mother or neonate, no fetal disorders during pregnancy, not requiring NICU stay.The inclusion criteria for the peer support were as follows: willingness to participate, residing in Ilam, being available and literate, Have a successful breastfeeding history, no diagnosed debilitating disease. Exclusion criteria: Mothers not available for telephone counselling. |
| Interventions | Intervention 1: The phone number, address and name of at most 8 nulliparous mothers were given to two supporters with similar age, education status, socio‐economic level, and residential area. The supporter called the mothers within 48 hours after discharge to introduce herself. Then, in the next phone calls and during the next 3 months. The mother’s call was unlimited and based on her need and she could call the supporter at any desired time. Moreover, the supporter called the mothers every week and encouraged them to continue exclusive breastfeeding. Question and answer sessions were also held in the presence of the supporters and the authors every two weeks during the first, second, and third months of study in the Red Crescent Society office in Ilam. Intervention 2: The control group program included the Routine trainings. The intervention group program included the Routine trainings plus the support from the 30 mothers with positive breastfeeding experience during the 3 months after delivery. The control group program included the Routine trainings. The intervention group program included the Routine trainings plus the support from the 30 mothers with positive breastfeeding experience during the 3 months after delivery The phone number, address and name of at most 8 nulliparous mothers were given to two supporters with similar age, education status, socio‐economic level, and residential area. The supporter called the mothers within 48 hours after discharge to introduce herself. Then, in the next phone calls and during the next 3 months. The mother’s call was unlimited and based on her need and she could call the supporter at any desired time. Moreover, the supporter called the mothers every week and encouraged them to continue exclusive breastfeeding. Question and answer sessions were also held in the presence of the supporters and the authors every two weeks during the first, second, and third months of study in the Red Crescent Society office in Ilam. |
| Outcomes | Primary Outcome(s) Breastfeeding Patterns and Duration. Time point: The end of the first, second and third months. Method of measurement: evaluation of Breastfeeding Patterns and Duration.
|
| Starting date | 2009‐06‐22 |
| Contact information | zolaykha karamolahi Address: Banjangnab, Central Hospital of Medical Sciences University, Nursing Midwifery Faculty, Ilam 6931343500 Ilam Iran (Islamic Republic of) Telephone: +98 84 3224 4224 Email: Affiliation: Ilam University of Medical Science |
| Notes |
| Study name | Impact of Peer Education on Breastfeeding Self‐Efficacy in Primiparous Women with hospitalized neonate in neonatal ward |
| Methods | RCT |
| Participants | Primiparous Women with hospitalised neonate in neonatal ward |
| Interventions | Two sessions of one hour of other breastfeeding training that will be held by the peer in the first week after the delivery. |
| Outcomes | Mean score Of Breastfeeding Self‐Efficacy |
| Starting date | Not clear. Registered in November 2018. |
| Contact information | Parvin Aziznejadroshan ([email protected]), Babol University of Medical Sciences |
| Notes |
| Study name | Effectiveness of the Distance Education Program on the Mothers' Empowerment in Breast‐Feeding |
| Methods | interventional |
| Participants | 72 Inclusion criteria: Informed consent |
| Interventions | Intervention 1: Intervention group: The lactation training package provided In accordance with the training curriculum Ministry of Health of the country will Send Via a telegram message from week 32 of gestation weekly up to 6 weeks, fallow‐up to revise of lactation training package via message at Intervention group. Intervention 2: Control group: Only the routine pregnancy and postpartum education Will be taken (face‐to‐face training provided by health care workers during maternity care and training provided by postpartum healthcare staff to client, face‐to‐face). |
| Outcomes | Primary Outcome(s) The Mothers' Empowerment in Breastfeeding. Time point: before intervention, 4 and 8 weeks after childbirth. Method of measurement: The breastfeeding empowerment questionnaire. Secondary Outcome(s) Duration of exclusive breastfeeding after childbirth. Time point: 4 and 8 weeks after childbirth. Method of measurement: Follow‐up questionnaire on fertility variables. |
| Starting date | 2018‐06‐22 |
| Contact information | Fatemeh bakouei Address: Babol University of Medical Sciences, Ganj Afroz Ave, Babol ?????‐????? Babol Iran (Islamic Republic of) Telephone: +98 11 3219 5313 Email: Affiliation: Babol University of Medical Sciences |
| Notes |
| Study name | Effect of postpartum education to mother about breastfeeding position /latch on newborn feeding behavior at discharge |
| Methods | RCT |
| Participants | Women with singleton pregnancies and vaginal or caesarean delivery |
| Interventions | Face‐to‐face education about lactation and then had their baby's milking behaviour checked. |
| Outcomes | Newborn breastfeeding behaviour |
| Starting date | Unclear. Registered in Sept. 2019 |
| Contact information | Zahra Gholipour Soleimani ([email protected]) |
| Notes |
| Study name | Survey on the effect of educational intervention using mobile application on breastfeeding self‐Efficacy of mothers attending to the Health Center of Rasht |
| Methods | RCT |
| Participants | Breastfeeding mothers living in Rasht |
| Interventions | Mobile app ‐ breastfeeding knowledge |
| Outcomes | Awareness of mothers in breastfeeding |
| Starting date | Unclear. Registered in Sept 2019 |
| Contact information | A'azam Seddighi ([email protected]) |
| Notes |
| Study name | Assessing the impact of an educational intervention based on a smartphone application for improving maternal breastfeeding of infants in their first six months of life in Urmia, Iran. |
| Methods | RCT |
| Participants | New mothers experiencing their firstborn child with less than three months of age were selected |
| Interventions | Smartphone educational intervention |
| Outcomes | Maternal breastfeeding self‐efficacy KAP (Knowledge, Attitude, Practice) |
| Starting date | January 2021 |
| Contact information | Bahlol Rahimi ([email protected]), Oroumia University of Medical Sciences |
| Notes |
| Study name | The effect of support through telephone counseling on breastfeeding problems and exclusive breastfeeding |
| Methods | interventional |
| Participants | 85 Key inclusion & exclusion criteria Inclusion criteria: Iranian nationality |
| Interventions | Intervention 1: The intervention group will receive regular telephone counselling. The first phone call will be made within the first 48 hours after hospital discharge (day 3 to 5 after delivery). According to WHO studies and information, these days are the most important time, and mothers have the least access to health centres and hospitals because of their problems and child support.The second consultation is at 13‐15 days postpartum.It takes an average of 20 minutes depending on the individual's needs. Each telephone consultation has two parts: In the first section, the counsellor questions the mother during the week and notes in the checklist, based on the existing problem to the extent that it is in line with the research objectives. The second part is dedicated advice on supporting exclusive breastfeeding. Counselling is provided by a midwifery graduate student with specialised short‐term training in postpartum counselling. The telephone is also provided to mothers so that they can contact the counsellor if needed.Both groups will receive routine postpartum education and care (usually three times in 42‐60 days) from the delivery site and the health care centres covered on a routine basis. Intervention 2: Control group: Care can be received from health centres according to the protocol of the Ministry of Health. |
| Outcomes | Primary Outcome(s) Cracked nipple. Time point: 13‐15 days after childbirth. Method of measurement: Checklist, Ask the mother. Exclusive breastfeeding In the last 24 hours (human milk intake by infant without any supplements (water, fruit, non‐human milk and food) except vitamins, minerals and medicines). Time point: 60 days after childbirth. Method of measurement: Checklist, Ask the mother. Mastitis. Time point: 13‐15 days after childbirth. Method of measurement: Checklist, Ask the mother. |
| Starting date | 2017‐09‐01 |
| Contact information | Parastoo Amiri Address: Tohidshahr Blvd 9617913112 Sabzevar Iran (Islamic Republic of) Telephone: +98 51 4401 8319 Email: Affiliation: Sabzevar University of Medical Sciences |
| Notes |
| Study name | The effect of education on self‐efficacy and social support and breastfeeding continuity in lactating mothers |
| Methods | interventional |
| Participants | 261 Key inclusion & exclusion criteria Inclusion criteria: Primiparous mothers by natural delivery method, with smartphone with social messaging capability
|
| Interventions | Intervention 1: Intervention group 1: Telephone One of the intervention groups is the telephone group, which will include mothers who received educational materials in the form of files, video booklets and educational videos, and on the first, third, fifth and seventh days after discharge by phone with questions and answers will be discussed. The researcher will contact the members of the group and each member will be given an average of 20 minutes to present educational materials. Intervention 2: Intervention group 2: Virtual The virtual group will include mothers who, after giving birth, are members of a group created in one of the social messengers (Telegram, Whats App, etc.) and educational materials in the form of files, video booklets and educational videos in the first days. , Third, fifth and seventh will be sent to their personal page after discharge and will be shared and discussed in the created group. |
| Outcomes | Primary Outcome(s) Mothers' awareness of breastfeeding. Time point: before Intervention, 4 Months after delivery. Method of measurement: Demographic Questionnaire, Denis Short Self‐efficacy Questionnaire, which can Measure Mothers' knowledge through a Self‐efficacy Questionnaire where it is based. Secondary Outcome(s) Breastfeeding Continuity. Time point: birth time, 4 Months after delivery. Method of measurement: Lactation checklist. Breastfeeding Self‐efficacy. Time point: Once before the Intervention and then 10 Days, 1 Month and 4 months after delivery. Method of measurement: Dennis Short Self‐efficacy Questionnaire. Social Support. Time point: Once before the Intervention and then 10 days, 1 month and 4 months after delivery. Method of measurement: Perceived Social Support Questionnaire. |
| Starting date | 2020‐11‐18 |
| Contact information | Fatemeh Valizadeh Address: 6814993165, Lorestan University of Medical Sciences, km 4 of Khorramabad Borujerd Highway, Khorramabad, Lorestan Province,Iran 6814993165 Khorramabad Iran (Islamic Republic of) Telephone: +98 66 3312 0155 Email: Affiliation: Khoram‐Abad University of Medical Sciences |
| Notes |
| Study name | The effect of postpartum home visit on mothers' knowledge and attitude about exclusive breastfeeding, incidence of some breastfeeding complications |
| Methods | Interventional |
| Participants | 100 Key inclusion & exclusion criteria Inclusion criteria: 1‐ Willingness to participate in the study Exclusion criteria: |
| Interventions | Intervention 1: Intervention group: Five visits on day 1 (before hospital discharge) 3 ‐ 7 ‐ 14 ‐ 42 Postpartum receive. Intervention 2: Control group: phone calls are made to them on days 3‐7 ‐ 14 ‐ 42, their status is checked and questionnaires are completed by telephone. question is asked. |
| Outcomes | Primary Outcome(s) Mean score of knowledge and attitude about exclusive breastfeeding. Time point: Before study and day 42 postpartum. Method of measurement: Knowledge and Attitude QuestionnaireIowa infant feeding attitude scale). Secondary Outcome(s) Breast engorgement rate?nipple sore?mastitis. Time point: ‐Days 3,7,14 and 42 postpartum. Method of measurement: Questionnaire. Exclusive breastfeeding rates. Time point: Day 42 postpartum. Method of measurement: Questionnaire. Indicators of physical growth include height, weight and head circumference. Time point: Day 42 postpartum. Method of measurement: Tachometer, Tape Meter, Weight. The incidence of physiological jaundice. Time point: days 3 and 7 postpartum. Method of measurement: Questionnaire and researcher‐made checklist |
| Starting date | 2020‐02‐04 |
| Contact information | Mahnaz Zarshenas Address: School of Nursing and Midwifery, Fatemeh Faculty of Nursing and Midwifery, Namazi Hospital, Shiraz, Iran 7193613119 Shiraz Iran (Islamic Republic of) Telephone: +98 71 3647 4254 Email: Affiliation: Shiraz University of Medical Sciences |
| Notes |
| Study name | Evaluation of the impact of breastfeeding support groups in primary health centers in Andalusia, Spain |
| Methods | Interventional |
| Participants | 511 Key inclusion & exclusion criteria Inclusion criteria: |
| Interventions | Participants will be randomised without blinding due to the impossibility of achieving it with patients or researchers (open, masking not used). Women will be allocated by clusters (primary health centres) by simple random sampling. |
| Outcomes | Primary Outcome(s) Percentage of exclusive breastfeeding, mixed breastfeeding, or formula, measured using study app or online questionnaire completed by the mother at 10 days after birth, 2, 4, 6 months after birth Secondary Outcome(s) 1. Socio demographic outcomes (mother's age, level of education, marital status, employment status, country of origin) measured with closed‐ended questions using study app or online questionnaire completed by the mother and the midwife before birth |
| Starting date | 01/10/2021 |
| Contact information | Fatima Leon‐Larios Address: Avenzoar, 6 41009 Seville Spain Telephone: +34 (0)954556097 Email: |
| Notes |
| Study name | Dreams: a community‐based integrated early life intervention program to help babies survive and thrive |
| Methods | Cluster rRCT |
| Participants | 1056 Inclusion criteria: 1. All pregnant women who are identified in the first, second or early third trimester, and reside in the village of identification till the baby is at least 6 months of age will be considered as eligible for analysis of trial outcomes. |
| Interventions | Intervention(s) 24 clusters (village administrative units) were randomly allocated equally to intervention or control prior to initiation of enrolment. A restricted randomisation approach, balancing the population of clusters and proportion of lower castes, was adopted to ensure balanced allocation. The two allocation sequences generated through the restricted randomisation scheme were allocated to intervention and control by a community member through the toss of a coin. Due to the nature of the intervention (home visitations by special workers called 'life coaches', etc), it was not possible to mask the intervention clusters. However, the entire evaluation was conducted by a separate set of workers, following the same process in both intervention and control clusters. The intervention package included counselling and hand holding support for pregnant women and their families on nutrition (during pregnancy, lactation and complementary feeding), hygiene, early child stimulation including massage and play, early initiation of skin‐to‐skin contact for all babies, and care‐seeking. The intervention was designed around 6 domains (Nutrition, Hygiene & Infection Prevention, Infant Massage, Loving and Responsive Care Practices, Active Stimulation & Interaction and Care Seeking). |
| Outcomes | Primary Outcome(s) 1. Infant mortality rate Secondary Outcome(s) 1. Neonatal mortality rate |
| Starting date | 01/10/2015 |
| Contact information | Aarti Kumar Address: Community Empowerment Lab 226001 Lucknow India Telephone: +91‐9450607023 Email: Affiliation:
|
| Notes |
| Study name | Women who are breastfeeding: increasing Self‐Efficacy to improve outcomes (WISE) Trial |
| Methods | Multi‐centre RCT |
| Participants | 956 Inclusion criteria: In‐hospital breastfeeding mothers who meet the following criteria: 1. Maternal/infant health condition that could interfere with breastfeeding (e.g., severe illness, major congenital anomaly) |
| Interventions | Current interventions as of 19/01/2018: |
| Outcomes | Primary Outcome(s) Breastfeeding exclusivity as identified by the Infant Feeding Questionnaire administered at 6 months postpartum Secondary Outcome(s) What is the effect of the breastfeeding self‐efficacy intervention (BSEI) on: |
| Starting date | 31/01/2014 |
| Contact information | Cindy‐Lee Dennis Address: Lawrence S. Bloomberg Faculty of Nursing University of Toronto 130‐155 College Street M5T 1P8 Toronto Canada Telephone: +01 Email: |
| Notes |
| Study name | Healthy future: a community health worker program to improve maternal, newborn and child health in rural china. |
| Methods | Cluster non‐blinded rRCT |
| Participants | 1200 Inclusion criteria: |
| Interventions | Control group: no intervention |
| Outcomes | Primary Outcome(s) At baseline, 6 months, and 12 months: Secondary Outcome(s) At baseline, 6 months, and 12 months: |
| Starting date | 04/04/2019 |
| Contact information | Alexis Medina Address: 616 Serra Street 94305 Stanford United States of America Telephone: 650‐736‐0972 Email: |
| Notes |
| Study name | A community‐based intervention to prevent obesity beginning at birth among American Indian children: study design and rationale for the PTOTS study |
| Methods | A cluster‐RCT |
| Participants | A birth cohort of 577 children (infants and toddlers aged 0‐2 years) from 5 American Indian tribes randomised by tribe to either the intervention (3 tribes) or the comparison condition (control; 2 tribes) |
| Interventions | Intervention: includes nutrition and physical activity goals, and consists of a community‐wide component coupled with an individualised family‐counselling component to improve nutrition and physical activity in infants and toddlers. The nutrition goals are presented in 4 modules: 1) breastfeeding, 2) curtailment of sugar sweetened beverage consumption, 3) introduction of healthy solid foods, and 4) parental management of feeding behaviours. Control: parents and guardians in the control tribes consent to provide study data for their children. Nondiagnostic dental screenings are offered to children aged 1–5 years as a service to these comparison communities. |
| Outcomes | Breastfeeding initiation and duration rates |
| Starting date | Unclear |
| Contact information | N Karanja |
| Notes |
| Study name | Ghana’s Ensure Mothers and Babies Regular Access to Care (EMBRACE) programme: study protocol for a cluster‐randomised controlled trial |
| Methods | Cluster‐RCT using an effectiveness‐implementation hybrid design in Dodowa, Kintampo, and Navrongo, Ghana |
| Participants | The study population is women of reproductive age between the ages of 15 and 49 years. |
| Interventions | The provision of an intervention package to women living in randomly allocated intervention clusters. The package includes: 1) use of a new continuum of care card, 2) continuum of care orientation for health workers, 3) 24‐h health facility retention of mothers and newborns after delivery, and 4) postnatal care by home visits. |
| Outcomes | Maternal, newborn, and child health outcomes for both intervention and implementation impacts. Intervention outcomes: continuum of care completion rate, rate of postnatal care within 48 h, complication rate requiring mothers' and newborns' hospitalisations, and perinatal and neonatal mortality Implementation outcomes: intervention coverage of the target population, intervention adoption and fidelity, implementation cost, and sustainability |
| Starting date | Unclear |
| Contact information | Current Controlled Trials ISRCTN90618993. Registered on 3 September 2014. |
| Notes |
| Study name | How does a combined nutrition counselling and cash transfer intervention impact women and their level of empowerment? A study protocol from rural Bangladesh |
| Methods | Cluster RCT |
| Participants | Women living in rural Bangladesh |
| Interventions | Nutrition counselling and cash transfer |
| Outcomes | Women's empowerment |
| Starting date | Not stated |
| Contact information | Elizabeth Kirkwood, University of Sydney. |
| Notes |
| Study name | Stage‐based health information messages |
| Methods | Individually‐randomised controlled trial |
| Participants | Five thousand pregnant women will be enroled from four districts of Madhya Pradesh and randomised to an intervention or control arm. |
| Interventions | The women in the intervention arm will receive Kilkari messages while the control group will not receive any Kilkari messages as part of the study. |
| Outcomes | Differences in primary outcomes across study arms including early and exclusive breastfeeding and the adoption of modern contraception at 1 year postpartum |
| Starting date | Not reported |
| Contact information | Amnesty LeFevre
|
| Notes |
| Study name | Peer Conselling Infant Feeding Education Program |
| Methods | Cluster RCT |
| Participants | The sample size required would be 1950 mother‐infant pairs (975 in each treatment group) from 50 Mallahas clusters with 39 mother‐infant dyads per community cluster recruited over 3 months. |
| Interventions | The approach to promoting appropriate infant and young child feeding will be through a program of home‐based peer counselling by trained, local women from the mothers' community. This approach will reach mothers who deliver at home and will also allow the messages to reach other key family members who may play a role in supporting breastfeeding and influence the foods choices for the infant. The main messages will be directed at encouraging early initiation of breastfeeding, promoting exclusive breastfeeding during the first 6 months of life, promoting appropriate timing of the introduction of complementary feeds, and ensuring an adequate frequency of feeds and diversity of foods used in their preparation. |
| Outcomes | Reduction of stunting (HAZ) [Time Frame: At 18 months] Secondary objectives
|
| Starting date | June 2010 |
| Contact information | Not provided |
| Notes |
| Study name | A Mobile‐health Pilot Experiment Targeting Mothers With Newborns in Rural Areas of San Juan Sacatepequez, Guatemala |
| Methods | RCT |
| Participants | Mothers with babies less than 4 months of age or in 8th month of pregnancy |
| Interventions | Participants were made part of virtual communities in which could exchange about infant's health as groups, via SMS, following the SHM Foundation's (http://www.shmfoundation.org/) m‐health methodology. |
| Outcomes | Breastfeeding knowledge Mean change in weight for age Z score |
| Starting date | January 2014 |
| Contact information | Jorge Tulio Rodriguez, Universidad Francisco Marroquin, Guatemala |
| Notes |
| Study name | Best beginnings for babies birth sister program evaluation |
| Methods | Interventional, randomised |
| Participants | 411 recruited Inclusion criteria:
Exclusion criteria
|
| Interventions |
Birth Sisters Best Beginnings for Babies support during Pregnancy, labour and delivery and six weeks postpartum. Participants assigned to the Birth Sister group receive community doula services with consultation from the Medical Legal Partnership when indicated beginning at 24 weeks. Participants receive up to 8 two‐hour prenatal home visits; continuous support through labour and birth, and up to 4 two‐hour postpartum home visits through 6‐8 weeks postpartum. |
| Outcomes | Rates of caesarean sections [Time Frame: at delivery] Total caesareans/total births as assessed by medical record abstraction Rates of preterm birth [Time Frame: at delivery] Total preterm births/total births as assessed by medical record abstraction
Total births< 2500 g/total births as assessed by medical record abstraction
Number of mothers initiating breastfeeding/total # mothers by medical record abstraction
Number of mothers with depression scale scores>9/total # mothers
Medical centre costs reported through hospital accounting system
Per cent of participants breastfeeding at 8 weeks postpartum by interview
Per cent of participants exclusively breastfeeding at 8 weeks pp by interview |
| Starting date | August 2015 |
| Contact information | |
| Notes |
| Study name | Alive & Thrive Nigeria Impact Evaluation |
| Methods | Interventional ‐ randomised |
| Participants | 15169 participants Inclusion Criteria:
|
| Interventions | (1) Interpersonal communication through frontline workers/volunteers to increase mothers' knowledge and practice of optimal infant and young child feeding (IYCF) behaviours. Interpersonal communication will involve multiple contacts with mothers and an array of IYCF messages; (2) Community mobilisation activities to raise awareness of the benefits of optimal IYCF practices among opinion leaders and family members, and increase their support to mother for IYCF; (3) Training of facility and community‐based health workers on IYCF to improve their ability to support mothers and provide timely information on IYCF; and (4) Mass media communication on IYCF. |
| Outcomes | Primary Outcome Measures :
The proportion of infants 0‐5 months who were exclusively breastfed on the previous day. Secondary Outcome Measures :
The proportion of children who were exclusively breastfed from 0‐5 months.
The proportion of children 0‐23 months who were breastfed within 1 hour of birth.
The proportion of children 6‐23 months who were fed the minimum number of food groups on the previous day based on the WHO infant and young child feeding guidelines.
Description: The proportion of children 6‐23 months who were fed the minimum number of meals on the previous day based on the World Health Organization infant and young child feeding guidelines.
Mothers' accurate knowledge of:
Providers' accurate knowledge of:
Primary Outcome Measures :
The proportion of infants 0‐5 months who were exclusively breastfed on the previous day. Secondary Outcome Measures :
The proportion of children who were exclusively breastfed from 0‐5 months.
The proportion of children 0‐23 months who were breastfed within 1 hour of birth.
The proportion of children 6‐23 months who were fed the minimum number of food groups on the previous day based on the WHO infant and young child feeding guidelines.
Description: The proportion of children 6‐23 months who were fed the minimum number of meals on the previous day based on the World Health Organization infant and young child feeding guidelines.
Mothers' accurate knowledge of:
Health providers' knowledge of optimal infant and young child feeding practices. [Time Frame: 2 years] Providers' accurate knowledge of:
|
| Starting date | January 19, 2017 |
| Contact information | Principal Investigator:Valerie FlaxRTI InternationalPrincipal Investigator:Mariam FagbemiTNS RMS Nigeria, Ltd. |
| Notes |
| Study name | Orientation Effects of Breastfeeding for Mothers |
| Methods | Intervention, randomised |
| Participants | 110 pairs of mother / baby Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Guidelines and review of the breastfeeding techniques. |
| Outcomes | Effects of Breastfeeding for Mothers measured by structured questionnaire [Time Frame: Six months] |
| Starting date | March 2012 |
| Contact information | Not provided |
| Notes |
| Study name | Novel Approach To Improving Lactation Support With Mobile Health Technology |
| Methods | RCT |
| Participants | 218 Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | We developed an algorithm using the Epharmix platform, an automated toll‐free phone and text message‐based system that can programmatically query patients via their personal phones and subsequently collect response data, allowing clinically‐relevant responses to trigger alerts to designated healthcare providers. The intervention for breastfeeding, hereafter referred to as EpxBreastfeeding, was built using significant clinical and patient input to only ask the most clinically‐relevant questions for breastfeeding in a multiple‐choice manner, such as "In the past [x] days, have you fed your baby 1) breast milk only, 2) breast milk and formula or 3) formula only?". These communications elicit patient‐reports of breastfeeding at intervals of interest for the provider, which is, on average, every 2 days in the first three weeks postpartum and every 5 days subsequently. All data isare filtered by clinician‐designed algorithms to stratify patients into categories. Other: Baby book survey We developed a "baby book" template that will be given to mothers allowing them to make note of dates related to their child's development during the first year. Examples include: When was baby's first appointment with his/her pediatrician? When did you add formula into baby's feeding? When did you start feeding baby only formula? When did you introduce solid food into baby's diet? When did baby first smile? When did you start reading to baby? What was the first book you read to baby? |
| Outcomes | Primary Outcome Measures :
The primary outcome is the length of time mothers exclusively breastfeed (i.e. continuous length of time for which mothers only give breast milk). Secondary Outcome Measures :
Time to transition from exclusive breastfeeding to partial breastfeeding (supplementing breast milk with formula) as well as supplemental nursing only.
Time to reported problems with: latching, concern regarding deficiency in milk production, concern for inadequate child's weight gain Other Outcome Measures:
Time from reported lactation/nursing problem to provider intervention. Time to nursing status change [Time Frame: Six months] Time from provider intervention to change in nursing status (exploratory analysis of each problem reported) Engagement [Time Frame: Six months] Weekly, monthly, and gross response rate to text messages
Patient satisfaction with provider, communication with provider, frequency of messages, and how likely to recommend the service to a friend measured by automated quality surveys sent each month, with quality ratings of 1‐9.
Compare the proportion of mothers exclusively breastfeeding, mixed feeding, and formula feeding at 6 weeks postpartum between control and intervention arms
Compare the proportion of mothers exclusively breastfeeding, mixed feeding, and formula feeding at 3 months postpartum between control and intervention arms
Compare the proportion of mother exclusively breastfeeding at 6 months postpartum between control and intervention arms
|
| Starting date | September 1, 2017 |
| Contact information | Washington University School of Medicine |
| Notes |
| Study name | Breastfeeding Support and Weight Management for Black Women |
| Methods | Randomised clinical trial |
| Participants | 53 Inclusion Criteria:
|
| Interventions | The intervention is based on the Loving Support peer counselling breastfeeding model developed by the Special Supplemental Program for Women Infants and Children (WIC) with added components to promote postpartum weight loss. |
| Outcomes | Primary Outcome Measures :
Count of women who report any breastfeeding at 20 weeks postpartum
Weight at 20 weeks postpartum minus pre‐pregnancy weight.
|
| Starting date | March 22, 2017 |
| Contact information | Michigan State University |
| Notes |
| Study name | Improving Exclusive Breastfeeding Via Mobile Phone Text Messages |
| Methods | RCT |
| Participants | 201 Inclusion Criteria:
|
| Interventions | Promotional Exclusive breastfeeding text messages Promotional Exclusive breastfeeding text messages will be sent to women via mobile phone |
| Outcomes | Primary Outcome Measures :
The primary outcome is the rate of EBF at 1 to 6 months of the infants' age measured at monthly intervals after delivery Secondary Outcome Measures :
Duration of exclusive breastfeeding
Rates of early initiation of breastfeeding |
| Starting date | January 1, 2018 |
| Contact information | Reham Mohammad Khresheh, Mutah University |
| Notes |
| Study name | Effectiveness of Midwife Phone Support With the to Reduce the Early Abandonment of Breastfeeding |
| Methods | RCT |
| Participants | 220 Inclusion Criteria:
|
| Interventions | Phone care support by midwife Mothers can call the midwife's telephone every working day from 8 a.m. to 8 p.m. |
| Outcomes | Primary Outcome Measures :
Women who still breastfeed 3 months after the intervention with respect to the control arm |
| Starting date | January 1, 2012 |
| Contact information | Jordi Gol i Gurina Foundation |
| Notes |
| Study name | Lactation achievement with texts at home |
| Methods | RCT |
| Participants | 124 Inclusion Criteria:
|
| Interventions | Text‐Based Support ‐ Women randomised to this arm will receive text‐based support via the Way to Health platform as described below. Supportive texts ‐ Encouraging text messages with prompts to ask questions will be sent twice‐weekly during the first four weeks postpartum and once weekly thereafter for the remaining two weeks of the program Enquiry texts ‐ Questions regarding infant feeding with prompts to answer will be sent three times weekly during the first two weeks postpartum and twice‐weekly thereafter for the remaining 4 weeks of the program. PHQ2 text ‐ Women will be sent the PHQ2 at 2 weeks and 6 weeks postpartum to assess mood symptoms. Women in this group will also have the option to send a text with a question or concern at any time during the study. Weekdays from 8am to 5 pm these will be fielded by a trained obstetrician. If a text is received after‐hours or on the weekend, women will be instructed to reach out to their primary OBGYN provider. |
| Outcomes | Primary Outcome Measures :
Exclusive breastfeeding Secondary Outcome Measures :
Any reported breastfeeding
Duration of breastfeeding
Rate of formula use
Compliance with postpartum visit
Mood assessment using PHQ‐2 |
| Starting date | January 6, 202 |
| Contact information | Celeste Durnwald, University of Pennsylvania |
| Notes |
| Study name | Online Intervention to Prevent Perinatal Depression and Promote Breastfeeding |
| Methods | Randomised clinical trial |
| Participants | 22 Inclusion Criteria:
|
| Interventions | The Sunnyside intervention is an online intervention (an interactive website with didactic material and interactive tools) targeting skills to manage mood during and after pregnancy. The Sunnyside intervention will consist of 6weeks of online lessons during pregnancy and booster sessions at 2 weeks, 4 weeks, and 6 weeks postpartum. Each lesson takes approximately 10 minutes to complete. |
| Outcomes | Primary Outcome Measures :
The PHQ‐9 is composed of 9 scored items and 1 unscored item which reflect overall functioning and impairment due to the depressive symptoms. PHQ‐9 scoring interpretations are as follows: 1‐4: minimal depressive symptoms; 5‐9 mild depressive symptoms; 10‐14: moderate depressive symptoms; 15‐19: moderately severe depressive symptoms: 20‐27: severe depressive symptoms.
Breastfeeding initiation, exclusivity, and duration will be assessed in the postpartum period. |
| Starting date | June 15, 2020 |
| Contact information | Jennifer Duffecy, University of Illinois at Chicago |
| Notes |
| Study name | The Impact of a Daily Smartphone‐based Communication Among Postpartum Women on Breastfeeding Rates |
| Methods | Randomised clinical trial |
| Participants | 224 Inclusion Criteria:
|
| Interventions | Each patient will document each evening her lactation performance during the day, and the App will generate a daily report transmitted by email every evening to our computerised research database. Every evening the patient will receive via email an individualised feedback from our team regarding her lactation. This feedback could include: reassurance and positive feedback and lactation tips in attempt to optimise specific obstacles. |
| Outcomes | Primary Outcome Measures :
Full or partial breastfeeding at fixed intervals. |
| Starting date | October 30, 2019 |
| Contact information | https://clinicaltrials.gov/show/NCT04135612 |
| Notes |
| Study name | Engaging Women in Postpartum Care |
| Methods | Randomised clinical trial |
| Participants | 273 Inclusion Criteria:
|
| Interventions | Healthy Beyond Pregnancy is a web platform with an electronic survey that assesses a participant's self‐identified postpartum concerns. Participants are then presented with 3 educational videos that reflect their self‐identified needs from the survey. The Healthy Beyond Pregnancy generates an individualised passport for postpartum care. This passport for care lists the women's self‐identified postpartum needs as well as issues that should be prioritised based on her health history.The individualised passport for postpartum care will be printed out and given to participants. After the participant schedules her postpartum visit, she will receive a print‐out that includes the date and time of her appointment, a copy of the commitment statement as well as a reminder of the monetary incentive she will receive if she fulfils her commitment and attends her postpartum visit. |
| Outcomes | Primary Outcome Measures :
Effective contraception (non‐barrier method)
Effective contraception (non‐barrier method) Secondary Outcome Measures :
Rates of breastfeeding
Rates of breastfeeding
Rates of breastfeeding
Rates of breastfeeding
Diabetes screening includes either 2 hour GTT OR fasting blood sugar and Hgba1c
Follow up includes documented resolution or treatment of persistent hypertension |
| Starting date | February 3, 2020 |
| Contact information | Katherine Himes, University of Pittsburgh |
| Notes |
| Study name | Breastfeeding Support Provided to Mothers Through WhatsApp Messaging Application |
| Methods | RCT |
| Participants | 150 Inclusion Criteria: For the mother;
|
| Interventions | Breastfeeding support provided to mothers through WhatsApp messaging application will be maintained for the first 6 months after birth. In the first 1 month, standard information sharing will be made through whatsApp. Also, for the first 6 months, solutions generating shares will be made according to the problems and questions of the mother regarding breastfeeding. |
| Outcomes | Primary Outcome Measures :
This scale assesses various aspects of attitudes that direct mothers' breastfeeding behaviour. Examines breastfeeding as behaviour. The scale is a five‐point Likert type consisting of 46 items, easy to apply, and which people can answer on their own, and it can be applied to all mothers. Some items in the scale (3, 4, 6, 7, 8, 11, 13, 15, 19, 23, 24, 26, 27, 28, 29, 30, 31, 32, 37, 38, 42, 43) As a positive attitude, I agree with the statement "I totally disagree" to 4‐3‐2‐1‐0, while some items (1, 2, 5, 9, 10, 12, 14, 16, 17, 18, 20, 21 (22, 25, 33, 34, 35, 36, 39, 40, 41, 44, 45, 46) are considered negative, and the opposite score is scored as 0‐1‐2‐3‐4 from the statement entirely agree. In this way, the total score that can be obtained from the scale is between 0 and 184. As the score increases, breastfeeding attitude is evaluated as positive.
This scale evaluates postpartum breastfeeding motivation level in primiparous. The scale consists of 24 items in total. After applying to the sample group, the factor analysis of the scale was made, and it was determined to have 5 factors.
Mirrored editing External regulation is examined in two sub‐dimensions:
Evaluates the satisfaction of mothers from breastfeeding support provided through WhatsApp messaging application. It is a form consisting of 10 questions by the researcher to evaluate the satisfaction of the mothers from the breastfeeding support given through the social media group (WhatsApp) by making use of the literature information. The answer to each question will be evaluated as yes / no. In the tenth question, he will be asked to score the application. There will be a minimum of 1 and a maximum of 10 points. |
| Starting date | December 1, 2020 |
| Contact information | Sevda KORKUT ÖKSÜZ, Ahi Evran University Education and Research Hospital |
| Notes |
| Study name | Hospital‐Based Breastfeeding Training İn The Early Postpartum Period |
| Methods | Randomised clinical trial |
| Participants | 80 Inclusion Criteria:
|
| Interventions | Breastfeeding education The importance of breastfeeding Benefits of breastfeeding for mother and baby Structure of the breast and milk production Breastfeeding techniques and positions Breastfeeding problems and breast care |
| Outcomes | Primary Outcome Measures :
This scale was developed by Cindy‐Lee Dennis to measure breastfeeding competence and consists of 2 subscales and 33 items. The Turkish validity and reliability of the breastfeeding proficiency scale was made by Ekşioğlu and Çeber (2011). Breastfeeding self‐efficacy increases as the total score increases in the 5‐point Likert‐type scale with a Cronbach Alpha coefficient of 0.91. The lowest score is 33, the highest score is 165.
It is a question form consisting of 25 questions in order to evaluate the knowledge about breastfeeding and breast milk before and after the training. The first application was done before the training and the second application was carried out in the 12th week.
The exclusive breastfeeding levels of their babies in the fourth and twelfth weeks of the mothers who received and did not receive education were evaluated. |
| Starting date | August 1, 2015 |
| Contact information | YEŞİM YEŞİL, Ege University |
| Notes |
| Study name | Effect of Lactation Management Model on Breastfeeding Process |
| Methods | Randomised clinical trial |
| Participants | 66 Inclusion Criteria:
|
| Interventions | The main components of the Lactation Management Model; breastfeeding training in the prenatal period (from the 30th week of pregnancy), early postpartum period (first 48 hours) skin‐to‐skin contact, early breastfeeding, relaxation using a dreaming technique, warm application to the breast, breast massage and for the first 48 hours after postpartum 6 months of continuous support (face to face, by phone, via social media). |
| Outcomes | Primary Outcome Measures :
Women who are applied lactation management model feed their babies with only breast milk at a higher rate (Measured using Breastfeeding and breast milk monitoring form.It is a form developed by the researcher, consisting of 4 open‐ended questions for the purpose of continuing breastfeeding and breastfeeding and determining additional nutrients other than breast milk).
It was determined that women who were applied the Lactation Management Model were able to breastfeed successfully with the appropriate technique (Measured using the Breastfeeding observation form developed by Armstrong). The form consists of 25 parameters under 5 main titles. For each parameter, "Percentage of Success" is calculated by taking the frequencies of 0, 1 and 2 scores. "Successful Technique" is indicated as between 46‐50 points and "Insufficient Technique" as 45 and below).
Secondary Outcome Measures :
It was found that problems related to the nipple develop less in women who were applied lactation management model Measured using A breastfeeding charting system and documentation tool. Developed by Jensen et al. scale consists of 5 assessment criteria. Each item has a score of between 0 and 2, and highest score is 10).
Breastfeeding self‐efficacy was higher in women who were applied the Lactation Management Model (Measured using Breastfeeding Self‐Efficacy Scale Scores. It was developed by Dennis and is a 5‐point Likert type scale. Minimum 14 points and maximum 70 points can be obtained from the scale. A higher score indicates that an increase in breastfeeding self‐efficacy. |
| Starting date | November 11, 2017 |
| Contact information | Aslı EKER, Mersin University |
| Notes |
| Study name | Home Based Peer Support Program for Mothers With Low Breastfeeding Self‐efficacy |
| Methods | Randomised clinical trial |
| Participants | 428 Inclusion Criteria:
|
| Interventions | Home‐based peer support will be provided to support participants' breastfeeding. There will be a minimum of 2 and maximum of 3 home visits between trained peer counsellors and participants. Each session will last approximately 30 minutes. |
| Outcomes | Primary Outcome Measures :
Exclusive breastfeeding duration
Exclusive breastfeeding duration
Exclusive breastfeeding duration
Exclusive breastfeeding duration Secondary Outcome Measures :
Breastfeeding Self‐efficacy Scale Short Form is used to measure maternal breastfeeding self‐efficacy. The 14‐item scale is on a 5‐point Likert scale. Total score ranged from 14 to 70 with a higher score indicating higher breastfeeding confidence and self‐efficacy.
The Chinese version of the Edinburgh Postnatal Depression Scale is used to measure the severity of postnatal depressive symptoms. It is a ten‐item scale rated from 0 to 3. The total score could range from 0 to 30. In general, a higher score indicates more severe depressive symptoms. |
| Starting date | August 13, 2021 |
| Contact information | The University of Hong Kong |
| Notes |
| Study name | The Effect of Telephone Support for Breastfeeding Follow‐up on Infantile Colic and Maternal Breastfeeding Self‐efficacy |
| Methods | Randomised clinical trial |
| Participants | 70 Inclusion Criteria:
|
| Interventions | The researcher, after the mother is discharged, by making a video call by phone every day for the first week, providing counselling to the mother on matters that she needs and recording it in the Baby Monitoring Form The researcher gives the mother a video |
| Outcomes | Primary Outcome Measures :
LATCH Breastfeeding Assessment Scale: It is a measurement tool developed to evaluate breastfeeding. It was created to diagnose breastfeeding, to identify problems, to determine training accordingly, to create a common language among health professionals and to be used in studies. This measurement tool consists of the English initials of five evaluation criteria. Each item is scored between 0‐2 points. The highest score is 10.
Infantile Colic Scale: It was developed to diagnose and evaluate colic. The validity and reliability study of the scale was done in Turkey. 12 Scale items are graded with Likert type scoring ranging from 1 to 6.
Breastfeeding Self‐Efficacy Scale‐Short Form: It is a 33‐item scale to evaluate breastfeeding self‐efficacy levels of mothers. Later, a 14‐item short form of the scale was developed in 2003. It is applied more easily and evaluates self‐efficacy correctly. Breastfeeding Self‐Efficacy Short Form Scale is a 5 point Likert type scale.
Physiological parameter of jaundice
exclusive breastfeeding for 6 months |
| Starting date | October 14, 2020 |
| Contact information | Gülçin Özalp Gerçeker, Dokuz Eylul University |
| Notes |
| Study name | The impact of breastfeeding education on breastfeeding behavior and the use of traditional practices |
| Methods | Randomised clinical trial |
| Participants | 304 Inclusion Criteria:
|
| Interventions | Breastfeeding Education The mothers were asked to remember the pseudonyms they used on the pretest and to use the same pseudonym on the posttest. Following the pretest, the mothers in the study group were taken into a separate room at the FHC and asked to breastfeed their infants. The mothers' breastfeeding behaviours were observed. After the breastfeeding, each mother was provided an average 30‐minute session of individual education. All of the mothers in the study group received the education from the same researcher. Both audio and visual materials were used in the mothers' training. |
| Outcomes | Primary Outcome Measures :
The Breastfeeding Behaviors Form was created in line with the literature. The questionnaires were filled out by the researchers using the face‐to‐face interview method. The questionnaire for Breastfeeding Behaviors included the following questions: Education about mother's milk and breastfeeding, When did you first breastfeed your baby after birth? Have you given your baby the first milk from your breast? Has your baby been given any food other than breast milk after birth? Why are you giving other food to your baby? Have you continued to feed your baby any other food? Do you give your baby water after breastfeeding? Breastfeeding Length (Daily)‐Frequency (Daily)‐Position‐Latching, Having Problems with Breastfeeding, b. How long do you intend to breastfeed your baby?
Traditional Practices Assessment Form was created in line with the literature. The questionnaires were filled out by the researchers using the face‐to‐face interview method. The Mother's Traditional Breastfeeding Practices Assessment Form included the following questions: Are there any traditional practices you use for breastfeeding? Do you think the traditional Practices you use for breastfeeding are useful? Who suggested traditional practices regarding breastfeeding? Do you voluntarily use traditional breastfeeding practices? Are There Foods You Consume to Increase Breast Milk? Who recommended the foods you consume to increase breast milk? Do you think the Food You Consume to Increase Breast Milk is beneficial? Do you voluntarily consume the foods you consume to increase breast milk? Secondary Outcome Measures :
The Breastfeeding Behaviors Form was created in line with the literature. The questionnaires were filled out by the researchers using the face‐to‐face interview method. The questionnaire for Breastfeeding Behaviors included the following questions: How are you feeding your baby now? Has your baby been given any food other than breast milk after birth? Breastfeeding Length (Daily)‐Frequency (Daily)‐Position‐Latching, Having Problems with Breastfeeding, What do you pay attention to avoid cracked nipples in your breast? Why are you giving other food to your baby? Do you give your baby water after breastfeeding? How long do you intend to breastfeed your baby? Does your partner support your breastfeeding?
Traditional Practices Assessment Form was created in line with the literature. The questionnaires were filled out by the researchers using the face‐to‐face interview method. The Mother's Traditional Breastfeeding Practices Assessment Form included the following questions: Are there any traditional practices you use for breastfeeding? Do you think the traditional Practices you use for breastfeeding are useful? Who suggested traditional practices regarding breastfeeding? Do you voluntarily use traditional breastfeeding practices? Are There Foods You Consume to Increase Breast Milk? Who recommended the foods you consume to increase breast milk? Do you think the Food You Consume to Increase Breast Milk is beneficial? Do you voluntarily consume the foods you consume to increase breast milk?
|
| Starting date | February 1, 2018 |
| Contact information | Aysegul Durmaz, Kutahya Health Sciences University |
| Notes |
| Study name | Online Theory‐based Educational Programme for Primiparous Women on Improving Breastfeeding Related Outcomes |
| Methods | Randomised clinical trial |
| Participants | 190 Inclusion Criteria:
|
| Interventions | REST intervention The breastfeeding talk will be conducted by Zoom at the third trimester about breastfeeding benefits and other practical advices. There is also a sharing session by a successful breastfeeding mother and a group discussion with different breastfeeding scenarios. Once the mothers return home after delivery, individualised breastfeeding coaching will be provided by PI through a daily Zoom videoconference within 24 hours upon hospital discharge till Day 7 after delivery. During the last breastfeeding videoconference, mothers will be reminded to have weekly telephone follow‐ups by PI from week 2 to 2 months postpartum. Women who are randomised into the intervention group will receive REST in addition to the standard antenatal and postnatal care (described at below) by the Obstetrics Department of their delivery hospitals. |
| Outcomes | Primary Outcome Measures :
The self‐developed postnatal questionnaires are reviewed and commented by 3 lactation consultants and 2 midwives and revised accordingly. Exclusive breastfeeding rate (number of Infants who have only received breast milk or expressed breast milk, and no other liquids or solids except vitamins, mineral supplement and medicines) by self‐developed postnatal questionnaires [Time Frame: At 6 months postpartum] The self‐developed postnatal questionnaires are reviewed and commented by 3 lactation consultants and 2 midwives and revised accordingly.
BSES‐SF is a 14‐item scale with 5‐point Likert scale. Total score ranged from 14 to 70 and higher score indicates higher breastfeeding self‐efficacy and the results will be presented as BSES‐SF mean scores which are measured by Breastfeeding Self‐Efficacy Scale‐Short Form (BSES‐SF) (Dennis, 2003) Secondary Outcome Measures :
The self‐developed postnatal questionnaires are reviewed and commented by 3 lactation consultants and 2 midwives and revised accordingly. Breastfeeding initiation rate (number of mothers initiate breastfeeding within 1st hour of delivery) by self‐developed postnatal questionnaire [Time Frame: At 2 months postpartum] The self‐developed postnatal questionnaire are reviewed and commented by 3 lactation consultants and 2 midwives and revised accordingly.
The self‐developed postnatal questionnaire are reviewed and commented by 3 lactation consultants and 2 midwives and revised accordingly.
Maternal postnatal depression is one kind of mood disorder, which commonly occurs after the birth of a baby. In EPDS, there are 10 questions with a total score of 0 to 30. Risk of postnatal depression was defined as EPDS score ≥10, and higher score indicates higher risk of a woman suffering from postnatal depression.
The medical diseases to be estimated for these morbidities are limited to infectious diseases including respiratory, ear and gastrointestinal infection. The self‐developed postnatal questionnaire are reviewed and commented by 3 lactation consultants and 2 midwives and revised accordingly. |
| Starting date | February 9, 2021 |
| Contact information | WONG Mei Sze, Chinese University of Hong Kong |
| Notes |
| Study name | Web‐based Educational Intervention on Breastfeeding Self‐efficacy and Breastfeeding Outcome |
| Methods | Randomised clinical trial |
| Participants | 448 Inclusion Criteria:
|
| Interventions | Web‐based educational program The mothers and fathers will be provided a website that consist of 6 sessions. At the end of the session, the lactation consultant will do video call to the mothers to discuss the questions from mothers and fathers. after delivery, telephone counselling will be conducted by lactation consultant to mothers at 2, 4, and 6. weeks postpartum |
| Outcomes | Primary Outcome Measures :
Confidence of a mother in her ability to feed the baby and fathers confident in assisting breastfeeding mother that will be measured using BSES‐SF. This questionnaire consist of 14 items.
confidence of a mother in her ability to feed the baby and fathers confident in assisting breastfeeding mother that will be measured using BSES‐SF. This questionnaire consist of 14 items.
Confidence of a mother in her ability to feed the baby and fathers confident in assisting breastfeeding mother that will be measured using BSES‐SF. This questionnaire consist of 14 items.
Confidence of a mother in her ability to feed the baby and fathers confident in assisting breastfeeding mother that will be measured using BSES‐SF. This questionnaire consist of 14 items. Maternal breastfeeding self and paternal breastfeeding self‐efficacy [Time Frame: 3 months after delivery] Confidence of a mother in her ability to feed the baby and fathers confident in assisting breastfeeding mother that will be measured using BSES‐SF. This questionnaire consist of 14 items.
Confidence of a mother in her ability to feed the baby and fathers confident in assisting breastfeeding mother that will be measured using BSES‐SF. This questionnaire consist of 14 items. Secondary Outcome Measures :
EPDS consist of 10‐items which comprises a 4‐point scale ranging from 0 ("no") to 3 ("most of the time"). The total EPDS score ranges from 0‐30, the highest score indicates a higher depression level. Anxiety [Time Frame: baseline, 38 weeks of pregnancy, 1 week, 1, 3, and 6 months after delivery] Anxiety will be measured using The Zung Self‐Rating Anxiety Scale (SAS) that consists of 20 items with 4‐point scale ranging from 0 ("no") to 3 ("most of the time"). Iowa Infant Feeding Attitude Scale (IIFAS) [Time Frame: baseline, 38 weeks of pregnancy, 1 week, 1, 3, and 6 months after delivery] Consists of 16‐items questionnaire to measure attitudes of mothers. Participants will respond for each item on a 5‐point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). Breastfeeding rate [Time Frame: 1 week, 1, 3, and 6 months of infant age] A self‐report questionnaire will use to assess the mothers' breastfeeding rate. A self‐report questionnaire will use to assess the mothers' breastfeeding rate. |
| Starting date | April 6, 2021 |
| Contact information | Shu Yu Kuo, Taipei Medical University |
| Notes |
| Study name | Feasibility and Effectiveness of WhatsApp Online Group on Breastfeeding by Peer Counsellors |
| Methods | Randomised clinical trial |
| Participants | 40 Inclusion Criteria:
|
| Interventions | WhatsApp peer support Trained peer supporters will provide breastfeeding and emotional support for participants in the WhatsApp group. |
| Outcomes | Primary Outcome Measures :
The number of participants who are exclusively breastfeeding at each time point.
The number of participants who are any breastfeeding at each time point. Secondary Outcome Measures :
Breastfeeding efficacy measured using Breastfeeding Self‐Efficacy Scale ‐ Short Form (BSES‐SF). BSES‐SF is a 14‐item scale with total score ranging from 14 to 70. A higher score indicates higher breastfeeding self‐efficacy.
Attitude towards breastfeeding measured using the Iowa Infant Feeding Attitude Scale (IIFAS). IIFAS is a 17‐item scale with total score ranging from 17 to 85. A higher score indicates a more favourable attitude towards breastfeeding. |
| Starting date | March 5, 2021 |
| Contact information | The University of Hong Kong |
| Notes |
| Study name | evaluating the impact of breastfeeding support and information on rates of exclusive breastfeeding among mothers in Kiambu County, Kenya |
| Methods | Randomised clinical trial |
| Participants | We plan to enrol 5916 participants, of whom half will be contacted for follow‐up. |
| Interventions | The intervention is a package of 26 informative and encouraging push‐messages about breastfeeding and monthly responsive surveys that offer mothers an opportunity to receive additional information about common challenges such as pain while breastfeeding, concerns about low milk supply, and breastfeeding through illness. |
| Outcomes | Primary Outcomes (end points) Our primary outcomes of interest are (1) women exclusively breastfeeding their babies, (2) women engaged in any breastfeeding at any point. We further explore engagement with the program, seeing whether the SMS intervention has differential effects |
| Starting date | 2019‐12‐01 |
| Contact information | Not provided |
| Notes |
| Study name | Mobile health solutions to help community providers promote maternal and infant nutrition and health using a community‐based cluster RCT in rural India: a study protocol |
| Methods | cluster RCT |
| Participants | .A total of 297 ASHAs, five trained counsellors, and 2,501 participants from 244 villages are participating in this study. |
| Interventions | mHealth intervention “Mobile Solutions Aiding Knowledge for Health Improvement” (M‐SAKHI) to be delivered by rural community health workers or Accredited Social Health Activists (ASHAs) for rural women, below or up to 20 weeks of pregnancy through delivery until their infant is 12 months of age |
| Outcomes | The primary objective of the trial is to reduce the prevalence of stunting (height‐for‐age < −2 z‐score) in children at 18 months of age by 8% in the intervention as compared with control. The secondary objectives include evaluating the impact on maternal dietary diversity, birth weight, infant and young child feeding practices, infant development, and child morbidity, along with a range of intermediate outcomes for maternal, neonatal, and infant health |
| Starting date | Not stated |
| Contact information | Priyanka Kuhite, Lata Medical Research Foundation, Vasant Nagar, Nagpur 440022, India. Email: [email protected] |
| Notes |
| Study name | The influence of counseling on the duration of exclusive breastfeeding |
| Methods | Clinical trial of prevention, effectiveness, randomised, controlled parallel, open, two arms, phase 3. |
| Participants | Setting Brazil 232 women Inclusion criteria: GESTANT: That intends to breastfeed; That you accept to participate in the research in prenatal and later in the puerperium. |
| Interventions | Intervention women will receive home‐based breastfeeding counselling from Community Health Agents (CHA) and women from the Control will not receive intervention proposed by the study, but possibly from the Family Health Unit staff will receive the usual activity of the first week after delivery, recommended by the Brazilian Ministry of Health, dispensed at the Family Health Strategy units. |
| Outcomes | Primary Outcome(s) The primary outcome measure will be exclusive breastfeeding from birth to the child's 120 days of life, collecting data longitudinally during this period. Secondary Outcome(s) The secondary outcome measure will be the duration of any breastfeeding from birth to the child's 120 days of life, collecting the data longitudinally during that period |
| Starting date | 15/01/2019 |
| Contact information | Eveline Do Amor Divino Address: Rua Nossa Senhora da Guia, 261 apto 202 78043‐605 Cuiabá Brazil Telephone: +55‐065‐999872300 Email: Affiliation: Universidade Federal de Mato Grosso |
| Notes |
| Study name | BReastfeeding Attitude and Volume Optimization (BRAVO) |
| Methods | Randomised trial |
| Participants | Setting: Indonesia 1000 Inclusion criteria
|
| Interventions | Breast feeding optimisation program extends from late pregnancy period to 6 months after birth. Prenatal intervention consists of individual and group counselling/education; perinatal intervention consists of in‐hospital lactation support; postnatal intervention consists of home visit, counselling session, reminding telephone call and bulk SMS, special counselling for working mothers, breast pump rental, occupational‐related support |
| Outcomes | Primary Outcome Measures :
Abdominal aortic/carotid intima media thickness, distensibility, elastic modulus; pulse wave velocity, stiffness index, blood pressure; echocardiography: ejection fraction (%), fractional shortening (%), TAPSE (cm), cardiac index (L/min/m2), LV mass (grams)
Breastfeeding practice: Y/N, night/day, frequency (time interval between breastfeeding, e.g 2 hours) Supplementary formula: frequency/day, volume per feeding Complementary feeding: Y/N, type of foods; frequency/day Employer and employee satisfaction
Secondary Outcome Measures :
Body weight, height, head circumference, abdominal circumference
Resistance, compliance, time constant, FVC (litres), FEV1 (litres) measured by spirometry Microbiome [[Time Frame: 1 years] Infant nasopharyngeal & oral flora, digestive tract/faeces flora, maternal oropharyngeal evaluated using PCR and culture.
Bayley Infant Scales, IQ
Infection/fever, allergic symptoms, wheezing, upper/lower respiratory disease, gastrointestinal symptoms
Serum hs‐CRP, Fibrinogen |
| Starting date | June 2012 |
| Contact information | Nikmah Salamia Idris, Indonesia University |
| Notes |
| Study name | A WeChat‐based Intervention to Support Breastfeeding |
| Methods | A multicentre RCT |
| Participants | Setting: China 1000 Inclusion Criteria:
|
| Interventions | Participants in the intervention group will be asked to follow our WeChat public account immoderately after randomisation. They will receive breastfeeding related messages three times a week from WeChat, including preparation for breastfeeding after birth and the health benefits of breastfeeding, from baseline until childbirth. After childbirth, intervention group mothers will continue to receive information, which is mainly about breastfeeding, once a week for 6 months. WeChat will send push notifications to remind mothers about the importance of exclusive breastfeeding, build confidence and motivate them to continue exclusive breastfeeding. |
| Outcomes | Primary Outcome Measures :
Breastfeeding while giving no other food or liquid, not even water, with the exception of drops or syrups consisting of vitamins, mineral supplements or medicines.
Infants who are receiving almost all of their nutrients from breast milk but take some other liquids such as water, water‐based drinks, oral rehydration solutions, ritual fluids, and drops or syrups. Secondary Outcome Measures :
% of infants fed with breast milk as their first feed
Breastfeeding while giving no other food or liquid, not even water, with the exception of drops or syrups consisting of vitamins, mineral supplements or medicines.
Breastfeeding while giving no other food or liquid, not even water, with the exception of drops or syrups consisting of vitamins, mineral supplements or medicines.
Complementary feeding is defined as feeding infants with solid foods and liquids other than breast milk or infant formula.
The child has received breastmilk (direct from the breast or expressed) with or without other drink, formula or other infant food |
| Starting date | June 28, 2020 |
| Contact information | Li Tang, Chengdu Jinjiang Maternity and Child Health Hospital |
| Notes |
| Study name | Effectiveness of theory based health education intervention on self‐efficacy of breastfeeding among pregnant mothers in hulu langat district, selangor |
| Methods | Randomised clinical trial |
| Participants | Setting: Malaysia Inclusion criteria: 1.Pregnant mother 34 to 37 weeks pregnancy attending maternal child health clinic in Hulu Langat District. |
| Interventions | `SeBF Programme' comprises multicomponent educational interventional program comprises educational (health talk, demonstration, practical video and group discussion) with WhatssApp application by using Social Cognitive Theory constructs. |
| Outcomes | Primary Outcome(s) Self‐Efficacy in Breastfeeding Baseline, immediate post intervention,4 weeks postpartum, 8 weeks postpartum self‐administered questionnaire Secondary Outcome(s) Knowledge in Breastfeeding Baseline, immediate post intervention,4 weeks postpartum, 8 weeks postpartum Self‐administered questionnaire, Attitude in Breastfeeding Baseline, immediate post intervention,4 weeks postpartum, 8 weeks postpartum Self‐administered questionnaire |
| Starting date | 24/02/2020 |
| Contact information | Farahana Mohamad Pilus Address: aculty of Medicine and Health Sciences,Universiti Putra Malaysia 43400 UPM Serdang Selangor Darul Eh 43400 Selangor Malaysia Telephone: +60126787418 Email: Affiliation: Universiti Putra Malaysia |
| Notes |
| Study name | BOOST: breastfeeding Onset and Onward With Support Tools |
| Methods | Two‐group parallel randomised controlled trial |
| Participants | Setting: USA 168 Inclusion Criteria:
|
| Interventions | Participants randomised into SC+BFI will receive the same services as the Standard Care Control (SC) group (standard breastfeeding services from Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) plus monthly home visits that facilitate navigation to as‐needed resources and referrals to services that support breastfeeding and problem‐solving) plus financial incentives (Breastfeeding Incentives, BFI) contingent on observed breastfeeding. |
| Outcomes | Primary Outcome Measures :
Identify breastfeeding behaviour via standardised observational method: Identifying audible swallowing, regular suck/swallow/breathe pattern, or visible milk in the infant's mouth.
Identify breastfeeding behaviour via standardised observational method: Identifying audible swallowing, regular suck/swallow/breathe pattern, or visible milk in the infant's mouth.
Identify breastfeeding behaviour via standardised observational method: Identifying audible swallowing, regular suck/swallow/breathe pattern, or visible milk in the infant's mouth.
Identify breastfeeding behaviour via standardised observational method: Identifying audible swallowing, regular suck/swallow/breathe pattern, or visible milk in the infant's mouth.
Identify breastfeeding behaviour via standardised observational method: Identifying audible swallowing, regular suck/swallow/breathe pattern, or visible milk in the infant's mouth.
For pumping, observed pumping combined with observed resulting milk being fed to the infant.
For pumping, observed pumping combined with observed resulting milk being fed to the infant.
For pumping, observed pumping combined with observed resulting milk being fed to the infant.
For pumping, observed pumping combined with observed resulting milk being fed to the infant.
For pumping, observed pumping combined with observed resulting milk being fed to the infant. Secondary Outcome Measures :
Measured with a portable digital infant scale. Infant weight will be measured before and after each breastfeeding observation in case of breastfeeding mothers to also measure the breast milk in the stomach
Measured with a portable digital infant scale. Infant weight will be measured before and after each breastfeeding observation in case of breastfeeding mothers to also measure the breast milk in the stomach
Measured with a portable digital infant scale. Infant weight will be measured before and after each breastfeeding observation in case of breastfeeding mothers to also measure the breast milk in the stomach
Measured with a portable digital infant scale. Infant weight will be measured before and after each breastfeeding observation in case of breastfeeding mothers to also measure the breast milk in the stomach
Measured with a portable digital infant scale. Infant weight will be measured before and after each breastfeeding observation in case of breastfeeding mothers to also measure the breast milk in the stomach
Self‐reported and medical records of infant health issues with the numbers and reason of medical visits
Self‐reported and medical records of infant health issues with the numbers and reason of medical visits
Self‐reported and medical records of infant health issues with the numbers and reason of medical visits
Self‐reported and medical records of infant health issues with the numbers and reason of medical visits
Self‐reported and medical records of infant health issues with the numbers and reason of medical visits |
| Starting date | June 19, 2019 |
| Contact information | Yukiko Washio, PhD (919) 485‐2794 |
| Notes |
| Study name | Communicating Healthy Beginnings Advice by Telephone (CHAT) to mothers with infants to prevent childhood obesity. |
| Methods | Three‐‐armRCT |
| Participants | Setting: NSW Australia Women will be eligible to participate if they are aged 16 years and over, at third trimester, are able to communicate in English (or can communicate with written information in Chinese or Arabic), have a mobile phone and live in the recruitment areas. |
| Interventions | The proposed interventions are based upon the principles of the successful HBT intervention, to be delivered starting in the late antenatal period and over the first 12 months after birth (14 months). The timing of the staged intervention corresponds to milestones in early childhood development, in particular with regard to healthy feeding practices, nutrition and play as well as parent‐child interactions. Below are the stages and contents of the interventions as well as intervention approaches: |
| Outcomes | Primary outcome [1] Children's BMI Timepoint [1] at 12 and 24 months of age Primary outcome [2] Breastfeeding duration self‐reported by mother in study‐specific survey Timepoint [2] at 6 months and 12 months of age Primary outcome [3] timing of introduction of solids self‐reported by mother in study‐specific survey Timepoint [3] at 6 months of age Secondary outcome [1] time of starting tummy time and frequency of tummy time self‐reported by mother in study‐specific survey Timepoint [1] at 6 months of age whether child starts drinking from a cup self‐reported by mother in study‐specific survey at 12 months of age Secondary outcome [3] screen‐time self‐reported by mother in study‐specific survey Timepoint [3] at 12 and 24 months of age Secondary outcome [4] outdoor playtime on a typical weekday and a weekend day self‐reported by mother in study‐specific survey Timepoint [4] at 12 and 24 months of age Secondary outcome [5] dietary/nutrition (daily or weekly vegetable, fruit, soft drink, fast food consumption) self‐reported by mother in study‐specific survey Timepoint [5] at 12 and 24 months of age Secondary outcome [6] Whether use food for reward self‐reported by mother in study‐specific survey at 12 and 24 months of age Secondary outcome [7] Whether using bottle to go to bed self‐reported by mother in study‐specific survey at 12 months of age |
| Starting date | 16/01/2017 |
| Contact information | A/Prof Li Ming Wen Address Sydney Local Health District. Level 9 North, King George V Building, Missenden Rd. Camperdown. NSW. 2050. Australia Phone +61 2 95159055 Fax +61 2 95159056 |
| Notes |
Abbreviations
BFCI: Baby Friendly Community Initiative
BFHI: Baby Friendly Hospital Initiative
BMI: body, mass index
CU: Community Unit
EBF: exclusive breastfeeding
g: gram(s)
h: hour(s)
ICU: intensive care unit
LGA: large for gestational age
MIYCN: Maternal and Young Child Nutrition
NICU: neonatal intensive care unit
RCT: randomised controlled trial
SGA: small for gestational age
UNICEF: the United Nations Children's Fund
WHO: World Health Organization
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Stopping breastfeeding (any) at 6 months Show forest plot | 30 | 14610 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.89, 0.97] |
| Analysis 1.1  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 1: Stopping breastfeeding (any) at 6 months | ||||
| 1.2 Stopping exclusive breastfeeding at 6 months Show forest plot | 40 | 16332 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.88, 0.93] |
| Analysis 1.2  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 2: Stopping exclusive breastfeeding at 6 months | ||||
| 1.3 Stopping breastfeeding (any) at 4‐6 weeks Show forest plot | 36 | 11413 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.97] |
| Analysis 1.3  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 3: Stopping breastfeeding (any) at 4‐6 weeks | ||||
| 1.4 Stopping exclusive breastfeeding at 4‐6 weeks Show forest plot | 42 | 14544 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.76, 0.90] |
| Analysis 1.4  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 4: Stopping exclusive breastfeeding at 4‐6 weeks | ||||
| 1.5 Stopping breastfeeding (any) at 2 months Show forest plot | 13 | 3169 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.77, 1.11] |
| Analysis 1.5  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 5: Stopping breastfeeding (any) at 2 months | ||||
| 1.6 Stopping exclusive breastfeeding at 2 months Show forest plot | 17 | 4317 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.74, 0.89] |
| Analysis 1.6  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 6: Stopping exclusive breastfeeding at 2 months | ||||
| 1.7 Stopping breastfeeding (any) at 3‐4 months Show forest plot | 32 | 12054 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.81, 0.93] |
| Analysis 1.7  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 7: Stopping breastfeeding (any) at 3‐4 months | ||||
| 1.8 Stopping exclusive breastfeeding at 3‐4 months Show forest plot | 43 | 11575 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.77, 0.85] |
| Analysis 1.8  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 8: Stopping exclusive breastfeeding at 3‐4 months | ||||
| 1.9 Stopping any breastfeeding at 9 months Show forest plot | 1 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.78, 0.97] |
| Analysis 1.9  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 9: Stopping any breastfeeding at 9 months | ||||
| 1.10 Stopping any breastfeeding at 12 months Show forest plot | 2 | 1311 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.00] |
| Analysis 1.10  Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 10: Stopping any breastfeeding at 12 months | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Stopping breastfeeding (any) at 6 months Show forest plot | 11 | 4879 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.91, 0.97] |
| Analysis 2.1  Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 1: Stopping breastfeeding (any) at 6 months | ||||
| 2.2 Stopping exclusive breastfeeding at 6 months Show forest plot | 13 | 7650 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.70, 0.90] |
| Analysis 2.2  Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 2: Stopping exclusive breastfeeding at 6 months | ||||
| 2.3 Stopping breastfeeding (any) at 4‐6 weeks Show forest plot | 6 | 2325 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.08] |
| Analysis 2.3  Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 3: Stopping breastfeeding (any) at 4‐6 weeks | ||||
| 2.4 Stopping exclusive breastfeeding at 4‐6 weeks Show forest plot | 6 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.57, 0.95] |
| Analysis 2.4  Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 4: Stopping exclusive breastfeeding at 4‐6 weeks | ||||
| 2.5 Stopping breastfeeding (any) at 2 months Show forest plot | 4 | 2089 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.07] |
| Analysis 2.5  Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 5: Stopping breastfeeding (any) at 2 months | ||||
| 2.6 Stopping exclusive breastfeeding at 2 months Show forest plot | 9 | 4537 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.03] |
| Analysis 2.6  Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 6: Stopping exclusive breastfeeding at 2 months | ||||
| 2.7 Stopping breastfeeding (any) at 3‐4 months Show forest plot | 5 | 2064 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.15] |
| Analysis 2.7  Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 7: Stopping breastfeeding (any) at 3‐4 months | ||||
| 2.8 Stopping exclusive breastfeeding at 3‐4 months Show forest plot | 10 | 4766 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 1.00] |
| Analysis 2.8  Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 8: Stopping exclusive breastfeeding at 3‐4 months | ||||
| 2.9 Stopping any breastfeeding at 12 months Show forest plot | 2 | 1431 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.91, 1.00] |
| Analysis 2.9  Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 9: Stopping any breastfeeding at 12 months | ||||
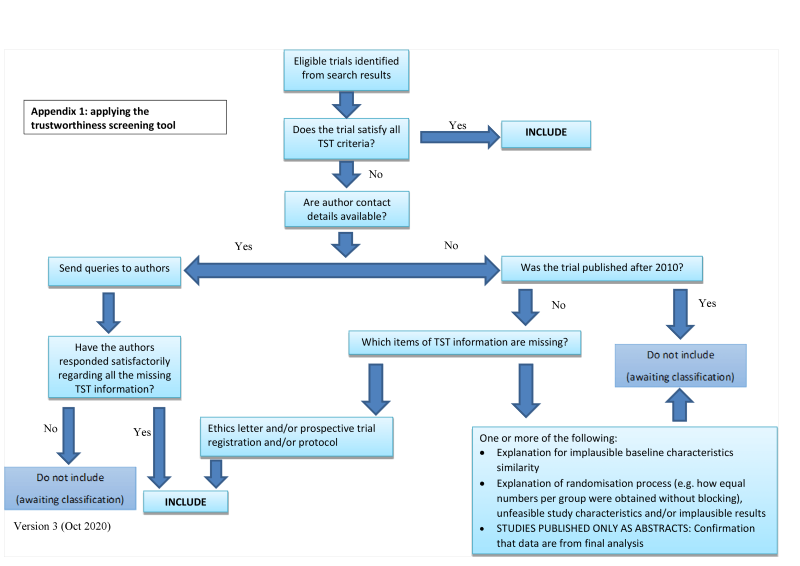
Applying the trustworthiness screening tool criteria

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
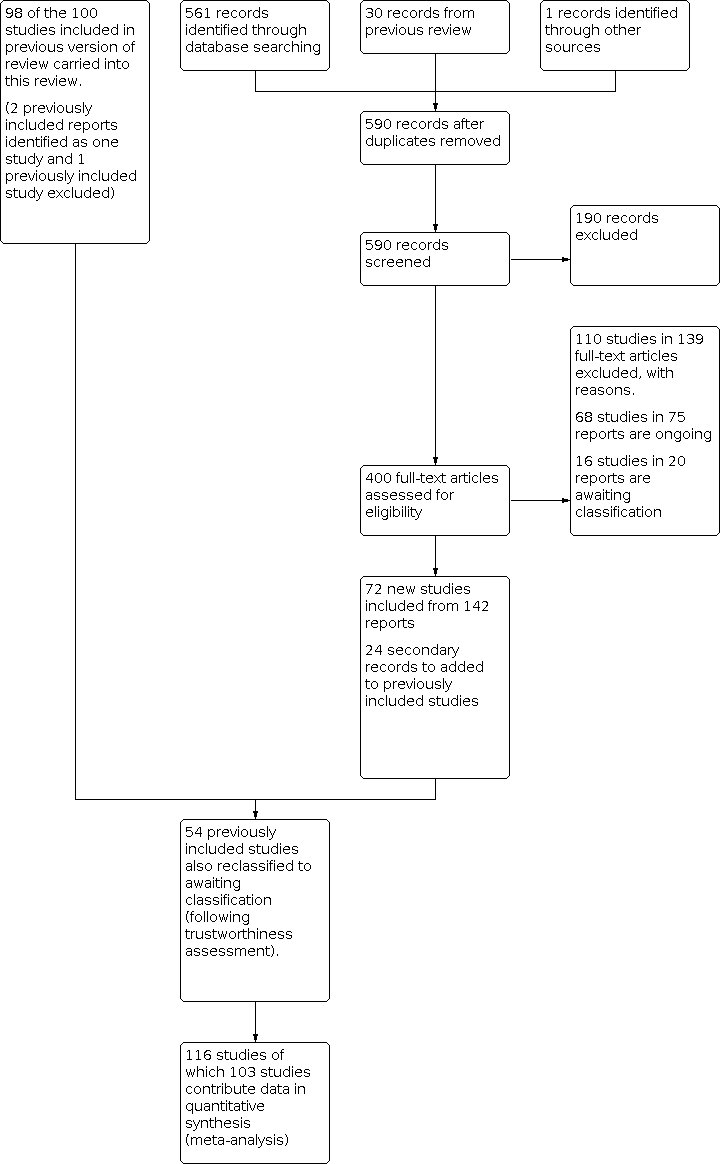
Study flow diagram

Funnel plot of comparison: 1 Breastfeeding only support versus usual care, outcome: 1.1 Stopping breastfeeding (any) at 6 months

Funnel plot of comparison 1: Breastfeeding only support versus usual care, outcome: 1.2 Stopping exclusive breastfeeding at 6 months

Funnel plot of comparison 1: Breastfeeding only support versus usual care, outcome: 1.3 Stopping breastfeeding (any) at 4‐6 weeks

Funnel plot of comparison: Breastfeeding only support versus usual care, outcome: 1.4 Stopping exclusive breastfeeding at 4‐6 weeks

Funnel plot of comparison 1: Breastfeeding only support versus usual care, outcome: 1.5Stopping breastfeeding (any) at 2 months

Funnel plot of comparison 1: Breastfeeding only support versus usual care, outcome: 1.6 Stopping exclusive breastfeeding at 2 months

Funnel plot of comparison 1: Breastfeeding only support versus usual care, outcome: 1.7 Stopping breastfeeding (any) at 3‐4 months

Funnel plot of comparison 1: Breastfeeding only support versus usual care, outcome: 1.8 Stopping breastfeeding at 3‐4 months

Funnel plot of comparison 2: Breastfeeding plus support versus usual care, outcome: 2.1 Stopping breastfeeding (any) at 6 months

Funnel plot of comparison 2: Breastfeeding plus support versus usual care, outcome: 2.2 Stopping exclusive breastfeeding at 6 months

Funnel plot of comparison 2: Breastfeeding plus support versus usual care, outcome: 2.6 Stopping exclusive breastfeeding at 2 months

Funnel Plot for Outcome 2.8 Stopping exclusive breastfeeding at 3‐4 months

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 1: Stopping breastfeeding (any) at 6 months

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 2: Stopping exclusive breastfeeding at 6 months

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 3: Stopping breastfeeding (any) at 4‐6 weeks

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 4: Stopping exclusive breastfeeding at 4‐6 weeks

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 5: Stopping breastfeeding (any) at 2 months

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 6: Stopping exclusive breastfeeding at 2 months

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 7: Stopping breastfeeding (any) at 3‐4 months

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 8: Stopping exclusive breastfeeding at 3‐4 months

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 9: Stopping any breastfeeding at 9 months

Comparison 1: Breastfeeding only support versus usual care 2022, Outcome 10: Stopping any breastfeeding at 12 months

Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 1: Stopping breastfeeding (any) at 6 months

Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 2: Stopping exclusive breastfeeding at 6 months

Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 3: Stopping breastfeeding (any) at 4‐6 weeks

Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 4: Stopping exclusive breastfeeding at 4‐6 weeks

Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 5: Stopping breastfeeding (any) at 2 months

Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 6: Stopping exclusive breastfeeding at 2 months

Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 7: Stopping breastfeeding (any) at 3‐4 months

Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 8: Stopping exclusive breastfeeding at 3‐4 months

Comparison 2: Breastfeeding plus support versus usual care 2022, Outcome 9: Stopping any breastfeeding at 12 months
| Breastfeeding support only compared to usual care | ||||||
| Patient or population: healthy breastfeeding women with healthy term babies | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with Usual care | Risk with Support | |||||
| Stopping breastfeeding (any) at 6 months | 600 per 1000 | 558 per 1000 | RR 0.93 | 14610 | ⊕⊕⊕⊝ | |
| Stopping exclusive breastfeeding at 6 months | 847 per 1000 | 763 per 1000 | RR 0.90 | 16332 | ⊕⊕⊕⊝ | |
| Stopping breastfeeding (any) at 4‐6 weeks | 308 per 1000 | 271 per 1000 | RR 0.88 | 11413 | ⊕⊕⊕⊝ | |
| Stopping exclusive breastfeeding at 4‐6 weeks | 518 per 1000 | 430 per 1000 | RR 0.83 | 14544 | ⊕⊕⊕⊝ | |
| Stopping breastfeeding (any) at 2 months | 384 per 1000 | 357 per 1000 | RR 0.93 | 3169 | ⊕⊕⊝⊝ | |
| Stopping exclusive breastfeeding at 2 months | 607 per 1000 | 491 per 1000 | RR 0.81 | 4317 | ⊕⊕⊕⊝ | |
| Stopping breastfeeding (any) at 3‐4 months | 462 per 1000 | 402 per 1000 | RR 0.87 | 12054 | ⊕⊕⊕⊝ | |
| Stopping exclusive breastfeeding at 3‐4 months | 731 per 1000 | 592 per 1000 | RR 0.81 | 11575 | ⊕⊕⊕⊝ | |
| Stopping breastfeeding at 9 months | 758 per 1000 | 660 per 1000 | RR 0.87 | 552 | ⊕⊕⊝⊝ | |
| Stopping breastfeeding at 12 months | 891 per 1000 | 846 per 1000 | RR 0.95 | 1311 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431686173574519163. | ||||||
| a We downgraded 1 level for serious concerns about inconsistency. Evidence of substantial unexplained heterogeneity. | ||||||
| Support plus compared to usual care for healthy breastfeeding women with healthy term babies | ||||||
| Patient or population: healthy breastfeeding women with healthy term babies | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care | Risk with Support plus | |||||
| Stopping breastfeeding (any) at 6 months | 541 per 1000 | 508 per 1000 | RR 0.94 | 4879 | ⊕⊕⊕⊝ | |
| Stopping exclusive breastfeeding at 6 months | 685 per 1000 | 541 per 1000 | RR 0.79 | 7650 | ⊕⊕⊝⊝ | |
| Stopping breastfeeding (any) at 4‐6 weeks | 433 per 1000 | 407 per 1000 | RR 0.94 | 2325 | ⊕⊕⊕⊝ | |
| Stopping exclusive breastfeeding at 4‐6 weeks | 542 per 1000 | 396 per 1000 | RR 0.73 | 2402 | ⊕⊝⊝⊝ | |
| Stopping breastfeeding (any) at 2 months | 363 per 1000 | 334 per 1000 | RR 0.92 | 2089 | ⊕⊕⊕⊝ | |
| Stopping exclusive breastfeeding at 2 months | 425 per 1000 | 382 per 1000 | RR 0.90 | 4537 | ⊕⊝⊝⊝ | |
| Stopping breastfeeding (any) at 3‐4 months | 386 per 1000 | 374 per 1000 | RR 0.97 | 2064 | ⊕⊕⊝⊝ | |
| Stopping exclusive breastfeeding at 3‐4 months | 587 per 1000 | 505 per 1000 | RR 0.86 | 4766 | ⊕⊕⊝⊝ | |
| Stopping breastfeeding (any) at 12 months | 858 per 1000 | 823 per 1000 | RR 0.96 | 1431 | ⊕⊕⊕⊝ | |
| Stopping breastfeeding (any) at 9 months ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431754481798286092. | ||||||
| a We downgraded 1 level for serious concerns on risk of bias. Studies at risk of selection bias due to unclear allocation concealment. | ||||||
| Factor | Number of studies | Number of women | RR (95% CI) | P value | Total model statistics | ||||
| Tau2 | Chi2 | df | P value | I2 | |||||
|
| 33 |
|
|
|
|
|
|
|
|
| PERSON: |
|
|
|
| 0.0074 | 83.1435 | 30 | <0.0001 | 58.56 |
| Professional | 22 | 10214 | 1 |
|
|
|
|
|
|
| Non‐Professional | 8 | 3366 | 0.958 (0.866, 1.060) | 0.406 |
|
|
|
|
|
| Prof + Non‐prof | 3 | 1030 | 1.037 (0.911, 1.181) | 0.583 |
|
|
|
|
|
| Unspecified | 0 | ‐ | ‐ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INTENSITY: |
|
|
|
| 0.0060 | 74.3190 | 29 | <0.0001 | 52.73 |
| Low | 5 | 2159 | 1 |
|
|
|
|
|
|
| Moderate | 5 | 962 | 0.913 (0.796, 1.047) | 0.194 |
|
|
|
|
|
| High | 17 | 6276 | 0.939 (0.841, 1.048) | 0.262 |
|
|
|
|
|
| Unspecified | 6 | 5213 | 0.989 (0.877, 1.115) | 0.857 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SUPPORT: |
|
|
|
| 0.0072 | 81.8609 | 28 | <0.0001 | 61.13 |
| F2F | 11 | 7508 | 1 |
|
|
|
|
|
|
| F2F and phone | 13 | 3681 | 1.009 (0.918, 1.109) | 0.857 |
|
|
|
|
|
| F2F, phone and digital | 1 | 103 | 0.984 (0.710, 1.364) | 0.924 |
|
|
|
|
|
| Phone | 6 | 2820 | 1.002 (0.888, 1.132) | 0.972 |
|
|
|
|
|
| Digital | 2 | 498 | 0.845 (0.648, 1.102) | 0.213 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INCOME: |
|
|
|
|
|
|
|
|
|
| LMIC | 7 | 3037 | 1 |
| 0.0052 | 81.7673 | 31 | <0.0001 | 53.32 |
| HMIC | 26 | 11573 | 1.069 (0.967, 1.183) | 0.193 |
|
|
|
|
|
| CI: confidence interval;RR: risk ratio. | |||||||||
|
|
|
|
|
| Total model statistics |
| |||
| Factor | Number of interventions | Number of women | RR (95% CI) | P value | Tau2 | Chi2 | P value | I2 |
|
|---|---|---|---|---|---|---|---|---|---|
|
| 44 |
|
|
|
|
|
|
|
|
| PERSON: |
|
|
|
| 0.0251 | 233.3179 | 40 | <0.0001 | 97.31 |
| Professional | 28 | 11780 | 1 |
|
|
|
|
|
|
| Non‐Professional | 12 | 3663 | 0.966 (0.853, 1.093) | 0.583 |
|
|
|
|
|
| Prof + Non‐prof | 3 | 749 | 1.024 (0.832, 1.259) | 0.826 |
|
|
|
|
|
| Unspecified | 1 | 140 | 0.345 (0.149, 0.799) | 0.013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INTENSITY: |
|
|
|
| 0.0233 | 244.6064 | 40 | <0.0001 | 96.02 |
| Low | 7 | 5603 | 1 |
|
|
|
|
|
|
| Moderate | 14 | 2896 | 0.815 (0.696, 0.953) | 0.010 |
|
|
|
|
|
| High | 18 | 6315 | 0.949 (0.822, 1.096) | 0.476 |
|
|
|
|
|
| Unspecified | 5 | 1518 | 0.952 (0.789, 1.147) | 0.603 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SUPPORT: |
|
|
|
| 0.0245 | 218.5338 | 39 | <0.0001 | 96.37 |
| F2F | 20 | 6027 | 1 |
|
|
|
|
|
|
| F2F and phone | 13 | 6647 | 1.113 (0.982, 1.260) | 0.093 |
|
|
|
|
|
| F2F, phone and digital | 1 | 20 | 1.199 (0.779, 1.845) | 0.409 |
|
|
|
|
|
| Phone | 8 | 3043 | 1.059 (0.919, 1.220) | 0.425 |
|
|
|
|
|
| Digital | 2 | 595 | 1.016 (0.793, 1.303) | 0.898 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INCOME: |
|
|
|
| 0.0187 | 230.0321 | 42 | <0.0001 | 96.31 |
| LMIC | 22 | 5622 | 1 |
|
|
|
|
|
|
| HMIC | 22 | 10710 | 1.151 (1.047, 1.265) | 0.003 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CI: confidence interval;RR: risk ratio. | |||||||||
| Factor | Number of studies | Number of women | RR (95% CI) | P value | Total model statistics | ||||
| Tau2 | Chi2 | df | P value | I2 | |||||
|
| 37 |
|
|
|
|
|
|
|
|
| PERSON: |
|
|
|
| 0.0909 | 110.94 | 34 | <0.0001 | 76.55 |
| Professional | 27 | 7526 | 1 |
|
|
|
|
|
|
| Non‐Professional | 8 | 2882 | 0.963 (0.717, 1.293) | 0.801 |
|
|
|
|
|
| Prof + Non‐prof | 2 | 774 | 1.182 (0.723, 1.934) | 0.505 |
|
|
|
|
|
| Unspecified | 0 | ‐ | ‐ | ‐ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INTENSITY: |
|
|
|
| 0.0966 | 100.86 | 33 | <0.0001 | 75.74 |
| Low | 11 | 4548 | 1 |
|
|
|
|
|
|
| Moderate | 8 | 1323 | 0.749 (0.504, 1.115) | 0.155 |
|
|
|
|
|
| High | 15 | 4019 | 0.991 (0.732, 1.342) | 0.953 |
|
|
|
|
|
| Unspecified | 3 | 1292 | 1.349 (0.839, 2.168) | 0.217 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SUPPORT/TYPE: |
|
|
|
| 0.1056 | 108.26 | 33 | <0.0001 | 79.59 |
| F2F | 11 | 4631 | 1 |
|
|
|
|
|
|
| F2F and phone | 17 | 3893 | 1.027 (0.754, 1.398) | 0.866 |
|
|
|
|
|
| F2F, phone and digital | 0 | ‐ | ‐ | ‐ |
|
|
|
|
|
| Phone | 7 | 2173 | 0.923 (0.624, 1.364) | 0.687 |
|
|
|
|
|
| Digital | 2 | 485 | 1.437 (0.707, 2.919) | 0.316 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INCOME: |
|
|
|
| 0.0770 | 104.47 | 35 | <0.0001 | 76.01 |
| LMIC | 7 | 2688 | 1 |
|
|
|
|
|
|
| HMIC | 30 | 8494 | 1.257 (0.903, 1.750) | 0.175 |
|
|
|
|
|
| CI: confidence interval;RR: risk ratio. | |||||||||
| Factor | Number of studies | Number of women | RR (95% CI) | P value | Total model statistics | ||||
| Tau2 | Chi2 | df | P value | I2 | |||||
|
| 45 |
|
|
|
|
|
|
|
|
| PERSON: |
|
|
|
|
|
|
|
|
|
| Professional | 31 | 11266 | 1 |
| 0.0480 | 231.253 | 42 | <00001 | 91.75 |
| Non‐Professional | 11 | 2529 | 0.853 (0.710, 1.025) | 0.09 |
|
|
|
|
|
| Prof + Non‐prof | 3 | 749 | 1.288 (0.939, 1.766) | 0.116 |
|
|
|
|
|
| Unspecified | 0 | ‐ | ‐ | ‐ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INTENSITY: |
|
|
|
|
|
|
|
|
|
| Low | 12 | 7327 | 1 |
| 0.0516 | 197.13 | 41 | <0.0001 | 89.54 |
| Moderate | 13 | 2113 | 0.790 (0.633, 0.986) | 0.037 |
|
|
|
|
|
| High | 17 | 4330 | 1.015 (0.836, 1.232) | 0.879 |
|
|
|
|
|
| Unspecified | 3 | 774 | 1.067 (0.754, 1.511) | 0.714 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SUPPORT: |
|
|
|
| 0.0612 | 207.04 | 40 | <0.0001 | 90.91 |
| F2F | 17 | 4679 | 1 |
|
|
|
|
|
|
| F2F and phone | 17 | 6797 | 1.016 (0.837, 1.24) | 0.869 |
|
|
|
|
|
| F2F, phone and digital | 1 | 20 | 1.193 (0.285, 4.991) | 0.809 |
|
|
|
|
|
| Phone | 7 | 2312 | 0.925 (0.714, 1.199) | 0.556 |
|
|
|
|
|
| Digital | 3 | 736 | 0.867 (0.591, 1.272) | 0.464 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INCOME: |
|
|
|
| 0.0508 | 223.312 | 43 | <0.0001 | 93.12 |
| LMIC | 15 | 3223 | 1 |
|
|
|
|
|
|
| HMIC | 30 | 11321 | 1.126 (0.942, 1.347) | 0.193 |
|
|
|
|
|
| Factor | Number of studies | Number of women | RR (95% CI) | P value | Total model statistics | ||||
| Tau2 | Chi2 | df | P value | I2 | |||||
|
| 12 |
|
|
|
|
|
|
|
|
| PERSON: |
|
|
|
| <0.00005 | 4.5171 | 9 | 0.8742 | <0.001 |
| Professional | 8 | 3601 | 1 |
|
|
|
|
|
|
| Non‐Professional | 3 | 1221 | 1.045 (0.969, 1.127) | 0.25 |
|
|
|
|
|
| Prof + Non‐prof | 0 | ‐ |
|
|
|
|
|
|
|
| Unspecified | 1 | 57 | 0.775 (0.605, 0.994) | 0.044 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INTENSITY: |
|
|
|
| <0.00005 | 9.0329 | 8 | 0.3395 | 0.76 |
| Low | 1 | 1154 | 1 |
|
|
|
|
|
|
| Moderate | 4 | 1236 | 1.037 (0.879, 1.225) | 0.664 |
|
|
|
|
|
| High | 5 | 1607 | 0.990 (0.891, 1.100) | 0.855 |
|
|
|
|
|
| Unspecified | 2 | 882 | 0.884 (0.667, 1.171) | 0.391 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SUPPORT: |
|
|
|
| <0.00005 | 10.2209 | 10 | 0.4213 | 0.12 |
| F2F | 11 | 4469 | 1 |
|
|
|
|
|
|
| F2F and phone | 1 | 410 | 0.969 (0.606, 1.548) | 0.895 |
|
|
|
|
|
| F2F, phone and digital | 0 | ‐ |
|
|
|
|
|
|
|
| Phone | 0 | ‐ |
|
|
|
|
|
|
|
| Digital | 0 | ‐ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INCOME: |
|
|
|
|
|
|
|
|
|
| LMIC | 2 | 1012 | 1 |
| <0.00005 | 9.537 | 10 | 0.4820 | <0.005 |
| HMIC | 10 | 3867 | 0.872 (0.633, 1.202) | 0.402 |
|
|
|
|
|
| Factor | Number of studies | Number of women | RR (95% CI) | P value | Total model statistics | ||||
| Tau2 | Chi2 | df | P value | I2 | |||||
|
| 14 |
|
|
|
|
|
|
|
|
| PERSON: |
|
|
|
| 0.2225 | 213.847 | 12 | <0.0001 | 99.03 |
| Professional | 9 | 4671 | 1 |
|
|
|
|
|
|
| Non‐Professional | 5 | 2979 | 1.033 (0.612, 1.744) | 0.903 |
|
|
|
|
|
| Prof + Non‐prof | 0 | ‐ | ‐ |
|
|
|
|
|
|
| Unspecified | 0 | ‐ | ‐ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INTENSITY: |
|
|
|
| 0.487 | 189.8528 | 10 | <0.0001 | 99.02 |
| Low | 1 | 1154 | 1 |
|
|
|
|
|
|
| Moderate | 3 | 1165 | 0.993 (0.332, 2.969) | 0.990 |
|
|
|
|
|
| High | 8 | 4449 | 0.692 (0.253, 1.898) | 0.475 |
|
|
|
|
|
| Unspecified | 2 | 882 | 0.683 (0.212, 2.200) | 0.523 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SUPPORT: |
|
|
|
| 0.0584 | 68.6107 | 11 | <0.0001 | 97.25 |
| F2F | 11 | 6004 | 1 |
|
|
|
|
|
|
| F2F and phone | 2 | 1447 | 0.338 (0.225, 0.508) | <0.005 |
|
|
|
|
|
| F2F, phone and digital | 0 | ‐ | ‐ |
|
|
|
|
|
|
| Phone | 0 | ‐ | ‐ |
|
|
|
|
|
|
| Digital | 1 | 199 | 0.776 (0.440, 1.369) | 0.382 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INCOME: |
|
|
|
| 0.1712 | 181.2251 | 12 | <0.0001 | 98.91 |
| LMIC | 6 | 3807 | 1 |
|
|
|
|
|
|
| HMIC | 8 | 3843 | 1.517 (0.968, 2.377) | 0.069 |
|
|
|
|
|
| CI: confidence interval;RR: risk ratio. | |||||||||
| Factor | Number of studies | Number of women | RR (95% CI) | P value | Total model statistics | ||||
| Tau2 | Chi2 | df | P value | I2 | |||||
|
| 7 |
|
|
|
|
|
|
|
|
| PERSON: |
|
|
|
| <0.00005 | 9.8833 | 5 | 0.0786 | 0.01 |
| Professional | 5 | 1454 | 1 |
|
|
|
|
|
|
| Non‐Professional | 2 | 871 | 1.046 (0.849, 1.290) | 0.67 |
|
|
|
|
|
| Prof + Non‐prof | 0 | ‐ | ‐ |
|
|
|
|
|
|
| Unspecified | 0 | ‐ | ‐ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INTENSITY: |
|
|
|
| <0.00005 | 4.3241 | 4 | 0.3639 | 0.01 |
| Low | 0 | ‐ | ** NOTE baseline ‘Moderate’ |
|
|
|
|
|
|
| Moderate | 3 | 681 | 1 |
|
|
|
|
|
|
| High | 3 | 1172 | 1.015 (0.799, 1.289) |
|
|
|
|
|
|
| Unspecified | 1 | 472 | Not included in model |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SUPPORT: |
|
|
|
| <0.00005 | 9.9954 | 5 | 0.0754 | 0.02 |
| F2F | 6 | 2090 | 1 |
|
|
|
|
|
|
| F2F and phone | 1 | 235 | 1.042 (0.769, 1.411) | 0.793 |
|
|
|
|
|
| F2F, phone and digital | 0 | ‐ | ‐ |
|
|
|
|
|
|
| Phone | 0 | ‐ | ‐ |
|
|
|
|
|
|
| Digital | 0 | ‐ | ‐ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INCOME: |
|
|
|
| N/A | N/A | N/A | N/A | N/A |
| LMIC | 0 | ‐ |
|
|
|
|
|
|
|
| HMIC | 7 | 2325 | N/A |
|
|
|
|
|
|
| CI: confidence interval;RR: risk ratio. | |||||||||
| Factor | Number of studies | Number of women | RR (95% CI) | P value | Total model statistics | ||||
| Tau2 | Chi2 | df | P value | I2 | |||||
|
| 7 |
|
|
|
|
|
|
|
|
| PERSON: |
|
|
|
| 0.457 | 45.2117 | 4 | <0.0001 | 94.89 |
| Professional | 5 | 1651 | 1 |
|
|
|
|
|
|
| Non‐Professional | 2 | 751 | 0.697 (0.213, 2.280) |
|
|
|
|
|
|
| Prof + Non‐prof | 0 | ‐ | ‐ |
|
|
|
|
|
|
| Unspecified | 0 | ‐ | ‐ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INTENSITY: |
|
|
|
| 0.4011 | 45.0465 | 4 | <0.0001 | 95.05 |
| Low | 0 | ‐ | ** NOTE baseline ‘Moderate’ |
|
|
|
|
|
|
| Moderate | 2 | 529 | 1 |
|
|
|
|
|
|
| High | 4 | 1401 | 0.561 (0.185, 1.701) | 0.307 |
|
|
|
|
|
| Unspecified | 1 | 472 | Not included in model |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SUPPORT: |
|
|
|
| 0.3455 | 44.1268 | 5 | 0.0001 | 95.79 |
| F2F | 5 | 1834 | 1 |
|
|
|
|
|
|
| F2F and phone | 2 | 568 | 0.659 (0.236, 1.839) | 0.426 |
|
|
|
|
|
| F2F, phone and digital | 0 | ‐ | ‐ |
|
|
|
|
|
|
| Phone | 0 | ‐ | ‐ |
|
|
|
|
|
|
| Digital | 0 | ‐ | ‐ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| INCOME: |
|
|
|
| 0.4544 | 42.7192 | 4 | <0.0001 | 95.86 |
| LMIC | 2 | 568 | 1 |
|
|
|
|
|
|
| HMIC | 5 | 1834 | 1.517 (0.457, 5.040) | 0.496 |
|
|
|
|
|
| CI: confidence interval;RR: risk ratio. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Stopping breastfeeding (any) at 6 months Show forest plot | 30 | 14610 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.89, 0.97] |
| 1.2 Stopping exclusive breastfeeding at 6 months Show forest plot | 40 | 16332 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.88, 0.93] |
| 1.3 Stopping breastfeeding (any) at 4‐6 weeks Show forest plot | 36 | 11413 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.97] |
| 1.4 Stopping exclusive breastfeeding at 4‐6 weeks Show forest plot | 42 | 14544 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.76, 0.90] |
| 1.5 Stopping breastfeeding (any) at 2 months Show forest plot | 13 | 3169 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.77, 1.11] |
| 1.6 Stopping exclusive breastfeeding at 2 months Show forest plot | 17 | 4317 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.74, 0.89] |
| 1.7 Stopping breastfeeding (any) at 3‐4 months Show forest plot | 32 | 12054 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.81, 0.93] |
| 1.8 Stopping exclusive breastfeeding at 3‐4 months Show forest plot | 43 | 11575 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.77, 0.85] |
| 1.9 Stopping any breastfeeding at 9 months Show forest plot | 1 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.78, 0.97] |
| 1.10 Stopping any breastfeeding at 12 months Show forest plot | 2 | 1311 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Stopping breastfeeding (any) at 6 months Show forest plot | 11 | 4879 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.91, 0.97] |
| 2.2 Stopping exclusive breastfeeding at 6 months Show forest plot | 13 | 7650 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.70, 0.90] |
| 2.3 Stopping breastfeeding (any) at 4‐6 weeks Show forest plot | 6 | 2325 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.08] |
| 2.4 Stopping exclusive breastfeeding at 4‐6 weeks Show forest plot | 6 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.57, 0.95] |
| 2.5 Stopping breastfeeding (any) at 2 months Show forest plot | 4 | 2089 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.07] |
| 2.6 Stopping exclusive breastfeeding at 2 months Show forest plot | 9 | 4537 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.03] |
| 2.7 Stopping breastfeeding (any) at 3‐4 months Show forest plot | 5 | 2064 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.15] |
| 2.8 Stopping exclusive breastfeeding at 3‐4 months Show forest plot | 10 | 4766 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 1.00] |
| 2.9 Stopping any breastfeeding at 12 months Show forest plot | 2 | 1431 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.91, 1.00] |

