Дорназа альфа при муковисцидозе
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Open randomised trial. Cross‐over design. Duration: 4 weeks for each treatment arm with a 2‐week washout period. | |
| Participants | 18 participants (13 female). Age range 8.7 ‐ 25.8 years. | |
| Interventions | Treatment: 2.5 mg rhDNase once daily. Control: 10 ml 6% HS once daily. | |
| Outcomes | Included in this review: FEV1 (% predicted), FVC (% predicted). Not included in review: symptoms score, semi quantitative sputum cultures, in vitro studies of mucociliary transport, cough clearance, acceptance of treatment by participants. | |
| Notes | Details from abstract as well as obtained from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised and information from authors indicates random numbers table used with sequence of treatments kept in the pharmacy in numbered envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Information from authors not clear if investigators were involved in the randomisation. |
| Blinding (performance bias and detection bias) | High risk | Not blinded due to the taste of HS, although technician who performed pulmonary function was blinded and only objective measures were in the included outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if all randomised participants completed both treatments or if there were any withdrawals. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | Cross‐over design with washout period of 2 weeks which should be adequate for lung function to return to baseline. |
| Methods | Randomised, placebo‐controlled trial. Cross‐over design. Duration: 4 weeks of treatment followed by a 4‐week washout before switching to alternate treatment. Single centre. | |
| Participants | 19 randomised, 17 participants (11 females, 8 males) completed. Age 6 ‐ 18 years old; mean (SD) age 10.3 (3.4) years. | |
| Interventions | Treatment: nebulised rhDNase 2.5 mg administered once daily via the PARI LC1 Star® nebuliser. Control: placebo administered once daily via the PARI LC1 Star® nebuliser. | |
| Outcomes | Included in this review: LCI, FEV1 (% predicted, z score), FVC (% predicted, z score), CFQ‐R respiratory and parent respiratory domain, adverse events, exacerbations. | |
| Notes | Visits occurred at 0, 4, 8 and 12 weeks after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by a research pharmacist not otherwise involved in the trial. |
| Blinding (performance bias and detection bias) | Low risk | All participants (solutions indistinguishable from each other), clinicians and outcome assessors blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reported that data analysed according to the intention‐to‐treat principle, however, data only reported on 17 who completed trial compared to the 19 that were randomised. Missing data from 2 participants: the LCI results of 1 participant failed to meet the quality control criteria for 1 of the 4 trial visits; 1 other participant dropped out of the trial after 2 visits because of a pulmonary exacerbation requiring IV antibiotics (protocol identified reason for withdrawal from trial), but not clear what treatment the participant had completed before withdrawal. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Cross‐over design with washout period of 4 weeks which should be adequate for lung function to return to baseline. |
| Methods | Open pilot trial. Cross‐over design. Duration: 2 treatment periods of 3 weeks, with a 3‐week washout period. Participants were assessed before and after each period. | |
| Participants | 14 participants (mean age 13.3 years) with mild to moderate pulmonary involvement. | |
| Interventions | Treatment: 2 puffs salbutamol via a spacer prior to nebulisation of 2.5 mg dornase alfa once daily. Control: 2 puffs salbutamol via a spacer prior to nebulisation of 10 ml 5.85% HS once daily. | |
| Outcomes | Change from baseline for FEV1 (% predicted), not clear if relative or absolute change. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not clear. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | High risk | Not blinded, due to the taste of the hypertonic saline. |
| Incomplete outcome data (attrition bias) | Unclear risk | No discussion of whether ITT analysis performed. Withdrawals were not discussed within the paper. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | Cross‐over design with washout period of 3 weeks which should be adequate for lung function to return to baseline. |
| Methods | Randomised double‐blind placebo‐controlled trial. Cross‐over design. Duration: 6 months for each treatment arm, but no washout period stated. | |
| Participants | 24 infants, clinically well at time of entry into trial. Not stated how many in each group. Gender distribution not stated. | |
| Interventions | Treatment: nebulised rhDNase 2.5 mg once daily. Control: placebo once daily. | |
| Outcomes | Included in this trial: changes in infant PFTs (% predicted and z scores for change in FEV0.5) Not included in this review: FEF25‐75, RV/TLC, change in CT score, change in air trapping, antibiotic treatment days. | |
| Notes | Only data for 19 infants for LFTs and 21 infants for CT scans. Data only available from abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no details given |
| Allocation concealment (selection bias) | Unclear risk | Not stated in abstract. |
| Blinding (performance bias and detection bias) | Low risk | Investigators and parents blinded to treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Follow up lung function data for only 19 of 24 recruited and CT scan data for only 21 of 24 recruited infants were reported. Not clear which groups infants dropped out from. |
| Selective reporting (reporting bias) | Unclear risk | Antibiotic treatment days not reported. |
| Other bias | Unclear risk | Cross‐over design with no stated washout period (abstract only). |
| Methods | Randomised, double‐blind placebo‐controlled trial. Cross‐over design. Duration: 2 treatment periods of 14‐days with a 7‐day wash out period between each period. Measurements were taken at the beginning and end of each treatment period. | |
| Participants | 23 participants randomised. Age: (mean) 27.5 years. | |
| Interventions | Treatment: 2.5 mg rhDNase once daily. Control: 2.5 ml 0.9% saline once daily. | |
| Outcomes | FEV1. | |
| Notes | Raw data provided; however no data legend therefore unable to analyse, FEV1 not reported in abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Unclear risk | No discussion of whether ITT analysis performed. Withdrawals were not discussed within the paper. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | High risk | Cross‐over trial with 7‐day washout period, which is not long enough for lung function to return to baseline; however data from this trial were not available for analysis in this review. |
| Methods | Randomised controlled trial. Parallel design. Duration: 1 year. | |
| Participants | 72 CF participants. Age: range 1.1 ‐ 24.8 years. Gender split: 34 males, 38 females. Exclusion criteria: chronic lung infection, or treatment with rhDNase in previous 2 months. 2 participants excluded, 1 from treatment group, 1 as had been randomised twice (both times to no treatment group). | |
| Interventions | Treatment: aerosolised rhDNase 2.5 mg once daily. Control: no rhDNase treatment. | |
| Outcomes | FEV1. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but process not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Nothing stated in paper. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 participants excluded. One participant was included twice (both times in the untreated group), one from the treated group because he did not take the inhalations for more than 5 months, but it did not state why he discontinued treatment |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Randomised, double‐blind trial. Parallel design with 3 arms. Duration: 24 weeks. Measurements were taken on days 7, 14 and every 14 days thereafter. | |
| Participants | 968 participants randomised, diagnosed CF on genotype, sweat test or clinically. Age: over 5 years. More participants aged 17 ‐ 23 years were in the once daily rhDNase arm. Disease status: FVC > 40 % predicted and clinically stable. | |
| Interventions | Treatment 1: nebulised rhDNase 2.5 mg once daily (n = 322). Treatment 2: nebulised rhDNase 2.5 mg twice daily (n = 321). Control: placebo (n = 325). | |
| Outcomes | Outcomes included in this review: mean % change in FVC and FEV1, number of participants needing IV antibiotics for at least 1 chest exacerbation (protocol defined), mean number of days IV antibiotics used, mean number of days as an inpatient, number of deaths and number experiencing an adverse event. Not included in this review: CF symptom score, dyspnoea score. Cost of treatment is reported by von der Schulenberg (1995), Oster (1995) and Menzin (1996). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Low risk | ITT principle was used. 25 participants withdrew from the trial, 8 in the placebo group and once‐daily group and 9 in the twice‐daily group. |
| Selective reporting (reporting bias) | Unclear risk | Measurements were taken on days 7, 14 and every 14 days thereafter. The published trial reported the end of trial results only. |
| Other bias | Low risk | None identified. |
| Methods | Randomised, double‐blind trial. Parallel design. Duration: 6 days. | |
| Participants | 20 adults with stable stage CF, FVC 35% ‐ 75% predicted and non‐smokers. Age: over 18 years. | |
| Interventions | Treatment: 2.5 mg nebulised rhDNase twice daily (n = 10). Control: placebo twice daily (n = 10). | |
| Outcomes | Included in this review: mean change in % predicted FVC and FEV1. Not included: aerosol distribution homogeneity, changes in mucociliary clearance and changes in cough frequency. | |
| Notes | Measurements were taken on day 6 only and reported in the paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was used in this trial. The published paper stated that there were no withdrawals. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Randomised, double‐blind trial. Parallel design. Duration: 12 weeks. Measurements were taken on days 8, 15, 29, 57 and 85. | |
| Participants | 320 participants with CF diagnosed clinically, by genotype or sweat test. Age: range 7 to 57 years. Disease status: FVC < 40 % predicted. Baseline lung function in the treatment group was lower than that of the control group, P < 0.05. | |
| Interventions | Treatment: nebulised rhDNase 2.5 mg once daily (n = 158). Control: placebo once daily (n = 162). | |
| Outcomes | Included in this review: mean change in % predicted FVC and FEV1, number of deaths and number experiencing adverse event, relative risk of one or more respiratory exacerbation. Not included in this review: mean number of days IV antibiotics used, mean number of days as an inpatient and mean dyspnoea score. | |
| Notes | Mean number of days IV antibiotics used, mean number of days as an inpatient and mean dyspnoea score were said not to differ significantly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Low risk | ITT principle used. 2 participants from the rhDNase arm of the trial did not have lung function recorded. 3 participants inadvertently received rhDNase instead of placebo (the results for these participants for lung function and respiratory exacerbations were analysed on an ITT basis, for safety data the results for these participants were published as if they had been randomised to rhDNase). 40 participants withdrew from the trial, 5 due to adverse events, 10 withdrew consent, 1 did not comply with the trial protocol, 15 died, 2 were unavailable for follow up and 7 stopped for a medical procedure. |
| Selective reporting (reporting bias) | Unclear risk | Measurements were taken on days 8, 15, 29, 57 and 85. The 85‐day mean was reported in the paper. |
| Other bias | Low risk | None identified. |
| Methods | Randomised open‐label trial. Cross‐over design with 3 arms. Total duration 42 weeks; each arm lasted 12 weeks with a 2‐week washout period between treatment blocks where all mucolytics were stopped. Primary endpoint measured at beginning and end of each treatment block. | |
| Participants | 38 children with CF. Age: range 9 ‐ 17 years (mean age 13 years). | |
| Interventions | Treatment 1: 2.5 mg nebulised rhDNase twice daily (n = 21). Treatment 2: combination of 2.5 mg nebulised rhDNase and 400 mg dry powder mannitol via Osmohaler twice daily (n = 23). Control: 400 mg dry powder mannitol via Osmohaler twice daily (n = 23). | |
| Outcomes | Included in this review: FEV1 (L),,FVC (L), pulmonary exacerbations, CFQ‐R respiratory and parent respiratory domain, adverse events Not included in this review: FEF25‐75, sputum microbiology, exercise tolerance, lung inflammation, cost‐effectiveness. | |
| Notes | Pulmonary exacerbation, adverse events and quality of life data not published, although data provided by Pharmaxis. 8 drop outs due to side effects, and these 8 were not included in the final analysis. Outcomes that were part of the original protocol that were not included in any of the provided data included markers of lung inflammation and cost‐effectiveness data. Prior to randomisation 9 out of 38 participants had significant bronchoconstriction to a mannitol challenge and were not randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised, but details of randomisation process not discussed in paper. Dr Minasian provided additional information ‐ participants were allocated a unique randomisation number and treatment schedule with equal probability for assignment to treatment sequences. Randomisation was carried out in balanced blocks with separate schedules created for each of the 2 recruiting centres. |
| Allocation concealment (selection bias) | Unclear risk | Method not clear. |
| Blinding (performance bias and detection bias) | High risk | Open label ‐ not blinded. Outcomes included subjective measures such as quality of life and adverse events therefore risk of bias considered high. |
| Incomplete outcome data (attrition bias) | Low risk | There were 8 withdrawals in total. 21 participants received rhDNase, 23 participants received mannitol and 23 participants received both. Data analysed per protocol on 20 participants who completed all 3 treatments. |
| Selective reporting (reporting bias) | Low risk | Published data only reported FEV1, FVC and FEF25‐75 but unpublished data provided for remainder of outcomes (except exercise tolerance, cost‐effectiveness, lung inflammation). |
| Other bias | Low risk | Cross‐over design with washout period of 2 weeks which should be adequate for lung function to return to baseline. |
| Methods | Randomised controlled trial. Parallel design. Duration: 3 years; participants were evaluated clinically every 3 months. | |
| Participants | 85 participants randomised. Age: range 5 ‐ 37 years. Disease status: normal lung function (FEV1 > 80% predicted) and clinically stable. | |
| Interventions | Treatment: rhDNase 2.5 mg twice daily (n = 46). Control: no rhDNase (n = 39). | |
| Outcomes | Used in this review: FEV1, FVC. Not used in this review: FEF25‐75, inflammatory markers (IL‐8) and microbiology from alveolar lavage samples. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Low risk | Analysis was based on ITT. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Randomised, double‐blind parallel placebo controlled trial. Duration: 96 weeks. Measurements taken at week 4, 12 and every 12 weeks thereafter. Multicentre: 49 CF centres. | |
| Participants | 474 children randomised, 410 completed the trial. 60 participants withdrew from the trial, 472 (out of 474) had follow‐up data. The ITT population was 470. Age: range 6 ‐ 10 years (mean age 8.4 years). Disease status: FVC > 85% predicted. | |
| Interventions | Treatment: 2.5 mg rhDNase once daily (n = 239). Control: placebo once daily (n = 235). | |
| Outcomes | Pulmonary function (FEV1, FVC) and exacerbations, deaths, adverse events, change in weight for age. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer, stratifying by centre using a permuted block design. |
| Allocation concealment (selection bias) | Low risk | Carried out by a pharmacy. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Low risk | ITT approach was used. 60 participants withdrew from the trial, 472 (out of 474) had follow‐up data. The ITT population was 470. |
| Selective reporting (reporting bias) | Unclear risk | Measurements taken at week 4, 12 and every 12 weeks thereafter. The end of trial results were reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomised, double‐blind trial. Parallel design with 4 arms. Duration: 10 days. Participants were followed up for a further 32 days. | |
| Participants | 181 participants diagnosed with CF by genotype or sweat test. Age: range 8 to 65 years. Disease status: stable stage CF, FVC ≥ 40% of predicted. | |
| Interventions | Treatment 1: rhDNase 0.6 mg twice daily (n = 45). Treatment 2: rhDNase 2.5 mg twice daily (n = 44). Treatment 3: rhDNase 10 mg twice daily (n = 44). Control: placebo twice daily (n = 48). | |
| Outcomes | Outcomes included in this review: mean % change in FVC and FEV1, number of deaths and number experiencing adverse event. Not included in this review; airway reactivity to rhDNase, mean rank change in quality of life score and the mean change in dyspnoea score. | |
| Notes | Measurements taken on days 1, 3, 6, 10, with follow‐up data on days 14, 21, 28 and 42. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Low risk | Analysed on an ITT basis. The paper stated that there were no withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Measurements taken on days 1, 3, 6, 10 with follow‐up data on days 14, 21, 28 and 42. Data were reported in the paper on days 3, 10, 21 and 42. |
| Other bias | Low risk | None identified. |
| Methods | Randomised, double‐blind, safety and efficacy trial. Parallel design Duration: 10 days with follow up to 42 days. Measurements taken at days 3, 6 and 10. | |
| Participants | 71 adults with CF diagnosed by genotype, sweat test. Disease status: stable disease and FVC > 40% predicted. | |
| Interventions | Treatment: nebulised rhDNase 2.5 mg twice daily (n = 36). Control: placebo twice daily (n = 35). | |
| Outcomes | Included in this review: relative mean change in % predicted FVC and FEV1 with baseline data calculated from the average of the day ‐3 and day 1 data and the treatment data calculated based on the average of the day 3, 6 and 10 data; number of deaths; and number experiencing an adverse event. Not included in this review: mean number of days of antibiotics used as only recorded at end of 42‐day follow‐up period. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned a carton number based on a randomisation list with a permuted block design, which was generated by Genentech. |
| Allocation concealment (selection bias) | Low risk | Unidentifiable cartons of active drug and placebo were numbered and provided to the pharmacist for dispensing. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT was not discussed. |
| Selective reporting (reporting bias) | Low risk | Measurements taken at days 3,6 and 10 (during treatment) then at day 14, 21, 28 and 42 following treatment. All were included. |
| Other bias | Low risk | None identified. |
| Methods | Randomised double‐blind placebo‐controlled trial. Cross‐over design. Duration: 7 days of treatment for each intervention with 2‐week wash‐out in between. Single centre. | |
| Participants | 15 participants randomised who were rhDNase naive. Age: 18.5 to 38.1 years old. Gender split: 9 males, 4 females. Disease status: clinically stable, mild to severe lung disease (FEV1 27.2% to 103.2% of predicted). | |
| Interventions | Treatment: rhDNase 2.5 mg administered once daily by PARI LC Plus® nebuliser. Control: placebo administered once daily by PARI LC Plus® nebuliser. | |
| Outcomes | Used in review: FEV1 (L), FVC (L). Not used in review: mucociliary clearance, cough clearance, FEF25‐75 (L/s). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described, although both medications were iso‐ismolar and given via the same nebuliser. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind but method not described. |
| Incomplete outcome data (attrition bias) | Low risk | Not ITT. 15 participants randomised and data for 13 participants ‐ 2 participants withdrew because of respiratory exacerbations requiring IV antibiotics (1 from placebo group, 1 from rhDNase group). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Cross‐over design with washout period of 2 weeks which should be adequate for lung function to return to baseline. |
| Methods | Randomised double‐blind, placebo‐controlled trial. Parallel design. Duration: 1 year. Participants evaluated at 3 months and 1 year. | |
| Participants | 25 children randomised. Age: range 6 ‐ 18 years old. Disease status: normal or mildly reduced lung function (FVC ≥ 85%, FEV1 > ˜70%). There were 4 withdrawals, all were for non‐trial drug‐related reasons. | |
| Interventions | Treatment: rhDNase 2.5 mg once daily. Control: normal saline aerosol once daily. | |
| Outcomes | Included in this review: FEV1(% predicted), FVC (% predicted). Not included in this review: FEF25‐75, high resolution CT scores, composite score including high resolution CT and PFT data. | |
| Notes | Measurements were taken at 3 and 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | Low risk | Double blinded (investigators, participants blinded to treatments until trial end). |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis used. 4 withdrawals, all were for non‐trial drug‐related reasons. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Randomised double‐blind trial. Parallel design. Duration: 14 days, with 6‐month open follow up. | |
| Participants | 70 participants with CF diagnosed by sweat test or genotype. Age: 5 years or over. Disease status: severe lung disease (FVC < 40% predicted). Specified 5 dropouts (2 died, 2 withdrew consent, 1 had a heart lung transplant). | |
| Interventions | Treatment: 2.5 mg nebulised rhDNase twice daily (n = 35). Control: placebo twice daily (n = 35). | |
| Outcomes | Included in review: mean change in % predicted FVC and FEV1; number of deaths; number experiencing an adverse event. Not included in the review; dyspnoea score; and quality of life score as data not provided. Reported as not significant. | |
| Notes | 6‐month open‐ended phase not included in review as no control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Low risk | ITT not possible for some outcomes. 5 out of 70 participants did not complete the 14‐day trial period, 1 received a heart‐lung transplant, 2 withdrew consent and 2 from the dornase alfa treated group died. Changes in lung function could therefore not be analysed on an ITT basis, but adverse events and deaths were analysed on this basis. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Open randomised controlled trial. Cross‐over design. Duration: 3 treatment periods of 12 weeks with a 2‐week wash out period between each period. Measurements were taken at the start and end of each 12‐week period. | |
| Participants | 48 children randomised, 45 completed first treatment period, 44 completed the second treatment period and 40 completed the third treatment period. Age: range 7.3 ‐ 17 years. | |
| Interventions | Treatment 1: 2.5 mg rhDNase once daily. Treatment 2: alternate day 2.5 mg rhDNase. Treatment 3: 5 mL 7% HS twice daily. | |
| Outcomes | Primary outcome was FEV1; secondary outcomes were FVC, number of pulmonary exacerbations, weight gain, quality of life, exercise tolerance and the total costs of hospital and community care. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used. Randomisation carried out by telephone to an independent trials co‐ordinating unit, and stratified by hospital and balanced after each group of 12 children. |
| Allocation concealment (selection bias) | Low risk | Independent trials co‐ordinator. |
| Blinding (performance bias and detection bias) | High risk | Not blinded, due to the taste of the HS. Outcomes included subjective measures including quality of life therefore risk of bias considered high. |
| Incomplete outcome data (attrition bias) | Low risk | 48 children randomised, 45 completed 1st treatment period, 44 completed the 2nd treatment period and 40 completed the 3rd treatment period. Data analysed according to ITT principle |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | Cross‐over design with washout period of 2 weeks which should be adequate for lung function to return to baseline. |
| Methods | Randomised double‐blind trial. Parallel design. Duration: 15 days during a respiratory exacerbation. Measurements taken on days 1, 8 and 15. | |
| Participants | 80 participants admitted to hospital for at least 1 night for treatment of a chest exacerbation (protocol defined) with FVC > 35% predicted. CF was diagnosed on genotype, sweat test. Age: over 5 years. No withdrawals mentioned in the paper. | |
| Interventions | Treatment: nebulised rhDNase 2.5 mg twice daily (n = 43) Control: nebulised placebo twice daily (n = 37). | |
| Outcomes | Mean change in % predicted FVC and FEV1, number of deaths and number experiencing an adverse event, quality of life score and dyspnoea score. | |
| Notes | Potential confounder was type of antibiotic used: 8 of 36 placebo participants received an oral antibiotic versus 8 out of the 44 in the treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) | Low risk | ITT. No withdrawals mentioned in the paper. |
| Selective reporting (reporting bias) | Unclear risk | Measurements taken on days 1, 8 and 15, no reported results, graph shown in paper. |
| Other bias | Unclear risk | Potential confounder was type of antibiotic used: 8 of 36 placebo participants received an oral antibiotic versus 8 out of the 44 in the treatment group. |
<: less than
>: greater than
% predicted: percent predicted
CF: cystic fibrosis
CFQ‐R: CF questionnaire‐revised
CI: confidence interval
CT: computer tomography
FEF25‐75: forced expiratory flow at 25 to 75% of the FVC
FEV1: forced expiratory volume at one second
FVC: forced vital capacity
HS: hypertonic saline
ITT: intention‐to‐treat
IV: intravenous
LCI: lung clearance index
PFT: pulmonary function test
rhDNase: recombinant human deoxyribonuclease
RV: residual volume
SD: standard deviation
TLC: total lung capacity
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Trial of timing of rhDNase inhalation in relation to physio (rhDNase dose does not differ between treatment arms). | |
| Trial comparing deposition of rhDNase by different methods of breathing and drug delivery; volume of rhDNase the same in all treatment arms. | |
| This trial was designed with the aim of producing an objective means of selecting those people with CF who would benefit most from dornase alfa. The trial was a cross‐over design. Outcomes such as lung function, symptom scores, oximetry and exercise test response were measured and then scored on a weighted points system which could not be analysed according to our protocol. 3 participants had completed 2 assessment periods; 1 was classed as a responder, having scored 18 or more points out of a total of 27. | |
| Trial of post‐sinus surgery administering rhDNase intranasally. | |
| DNase delivered nasally for sinusitis in people with CF. | |
| Participants currently on rhDNase at entry to trial. | |
| Not a randomised trial. | |
| This is a comparison of two different types of nebuliser. | |
| Not relevant control intervention. | |
| Trial terminated as unable to measure preschool lung function data. | |
| Not a randomised trial. | |
| Participants were already on rhDNase and study was designed as a withdrawal study. | |
| This trial examined the effects of rhDNase on sputum rheology as compared to physiological saline over at least 4 months and did not include relevant clinical outcomes. | |
| Comparison of dispensing methods of rhDNase. | |
| Not a placebo‐controlled trial. | |
| This cross‐over trial in 16 adult participants was not clearly stated to be randomised. 2 of the investigators knew whether participants were allocated to receive placebo or treatment first. | |
| This is a comparison of two different types of nebuliser. | |
| This trial examines the effects of rhDNase and hypertonic saline on sputum rheology in vitro and therefore not relevant to this review. | |
| rhDNase delivered nasally for sinusitis in CF. | |
| This is not a comparison of rhDNase versus another intervention. | |
| Non‐randomised trial. Nasal inhalation. | |
| Nasal inhalation. | |
| This cross‐over trial of 8 people with CF aged 6 to 18 years compared the viscosity of sputum cleared by CCP as compared to HFCC. The participants were randomised to receive either rhDNase or normal saline prior to either CCP or HFCC. | |
| Study using CT scans to measure clinical response to rhDNase and establish how to measure effects of rhDNase not effects themselves. | |
| Comparison of 2 delivery techniques. | |
| Not people with CF. | |
| Study of quantitative HRCT air trapping analysis in people with CF with mild lung disease during a rhDNase intervention. Study to establish how to measure effects of rhDNase not effects themselves. | |
| No placebo group, comparison of two different nebulisers. | |
| Not a randomised controlled trial. | |
| 6‐month open‐label study of rhDNase in stable CF. This was an open‐label extension to a phase II trial where there was no re‐randomisation and all participants received dornase alfa. | |
| Trial comparing two different nebulisers. | |
| Authors contacted and trial does not report on any outcome relevant to this review. | |
| This is a comparison between nebulisation of rhDNase before and after ACT, it is not a comparison of rhDNase versus another intervention. | |
| N‐of‐1 study design. | |
| The comparison in this trial was between the timing of giving rhDNase, which is the subject of a different review. |
ACT: airway clearance techniques
CCP: conventional chest physiotherapy
CF: cystic fibrosis
HFCC: high frequency chest compressions
rhDNase: dornase alfa
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relative mean % change in FEV1 (% predicted) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Dornase alfa versus placebo, Outcome 1 Relative mean % change in FEV1 (% predicted). | ||||
| 1.1 At 1 month | 4 | 248 | Mean Difference (IV, Random, 95% CI) | 9.51 [0.67, 18.35] |
| 1.2 At 3 months | 1 | 320 | Mean Difference (IV, Random, 95% CI) | 7.30 [4.04, 10.56] |
| 1.3 At 6 months | 1 | 647 | Mean Difference (IV, Random, 95% CI) | 5.8 [3.99, 7.61] |
| 1.4 At 12 months | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐11.26, 12.66] |
| 2 Relative mean % change in FEV1 (% predicted) at one month ‐ subgroup analysis by disease severity Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 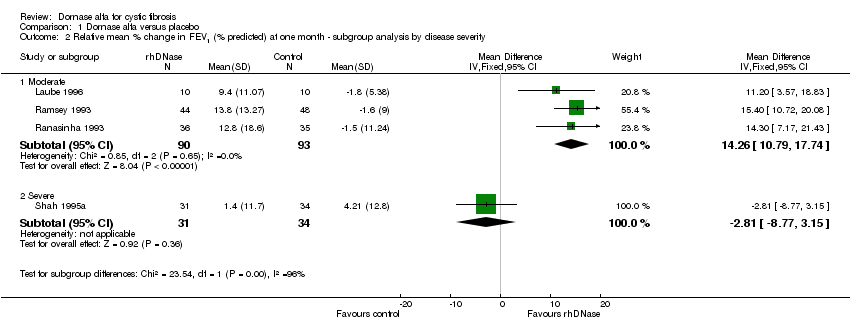 Comparison 1 Dornase alfa versus placebo, Outcome 2 Relative mean % change in FEV1 (% predicted) at one month ‐ subgroup analysis by disease severity. | ||||
| 2.1 Moderate | 3 | 183 | Mean Difference (IV, Fixed, 95% CI) | 14.26 [10.79, 17.74] |
| 2.2 Severe | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐2.81 [‐8.77, 3.15] |
| 3 Absolute mean % change in FEV1 (% predicted) Show forest plot | 1 | Mean difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Dornase alfa versus placebo, Outcome 3 Absolute mean % change in FEV1 (% predicted). | ||||
| 3.1 At 1 month | 1 | Mean difference (Fixed, 95% CI) | 0.08 [‐5.59, 5.74] | |
| 4 Absolute mean % change in FEV1 (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Dornase alfa versus placebo, Outcome 4 Absolute mean % change in FEV1 (% predicted). | ||||
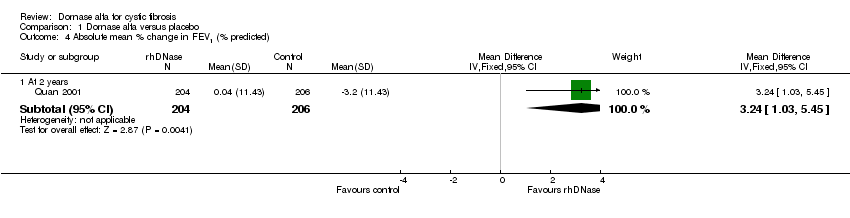
| 4.1 At 2 years | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | 3.24 [1.03, 5.45] |
| 5 Relative mean % change in FEV1 (in participants with acute exacerbations) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Dornase alfa versus placebo, Outcome 5 Relative mean % change in FEV1 (in participants with acute exacerbations). | ||||
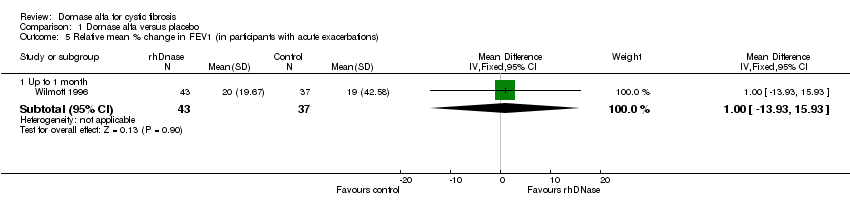
| 5.1 Up to 1 month | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐13.93, 15.93] |
| 6 Relative mean % change in FVC (% predicted) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.6 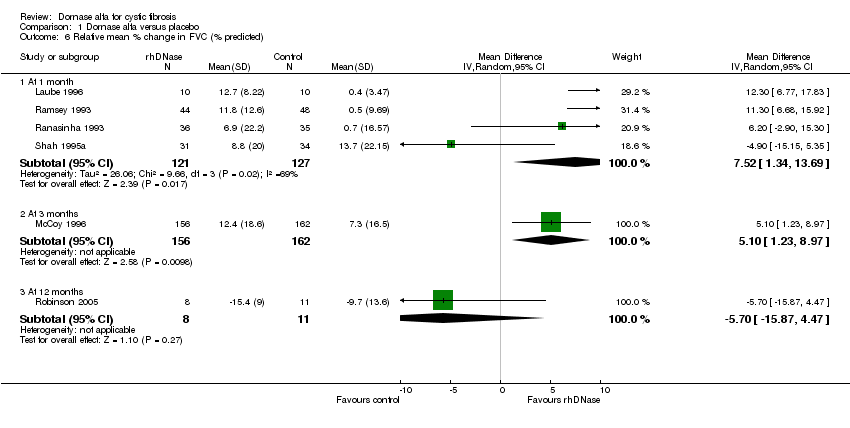 Comparison 1 Dornase alfa versus placebo, Outcome 6 Relative mean % change in FVC (% predicted). | ||||
| 6.1 At 1 month | 4 | 248 | Mean Difference (IV, Random, 95% CI) | 7.52 [1.34, 13.69] |
| 6.2 At 3 months | 1 | 318 | Mean Difference (IV, Random, 95% CI) | 5.10 [1.23, 8.97] |
| 6.3 At 12 months | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐5.70 [‐15.87, 4.47] |
| 7 Relative mean % change in FVC (% predicted) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Dornase alfa versus placebo, Outcome 7 Relative mean % change in FVC (% predicted). | ||||
| 7.1 At 6 months (once daily) | 1 | 2 | Mean Difference (Random, 95% CI) | 3.80 [2.62, 4.98] |
| 7.2 At 6 months (twice daily) | 1 | 2 | Mean Difference (Random, 95% CI) | 3.00 [1.82, 4.18] |
| 8 Relative mean % change in FVC at one month ‐ subgroup analysis by disease severity Show forest plot | 4 | 248 | Mean Difference (IV, Fixed, 95% CI) | 9.49 [6.34, 12.63] |
| Analysis 1.8 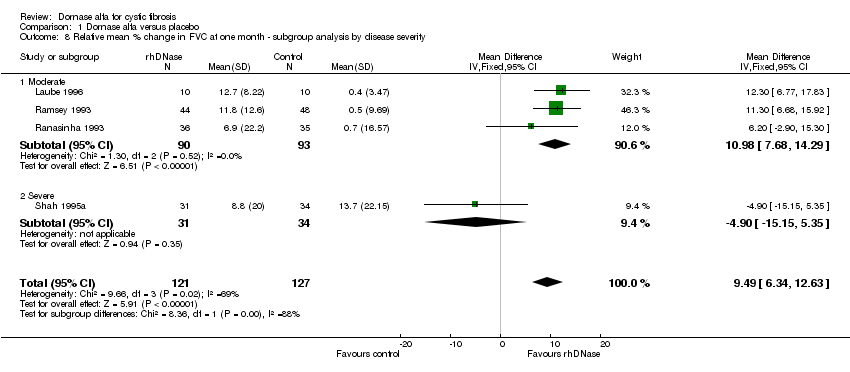 Comparison 1 Dornase alfa versus placebo, Outcome 8 Relative mean % change in FVC at one month ‐ subgroup analysis by disease severity. | ||||
| 8.1 Moderate | 3 | 183 | Mean Difference (IV, Fixed, 95% CI) | 10.98 [7.68, 14.29] |
| 8.2 Severe | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐15.15, 5.35] |
| 9 Absolute mean % change in FVC (% predicted) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 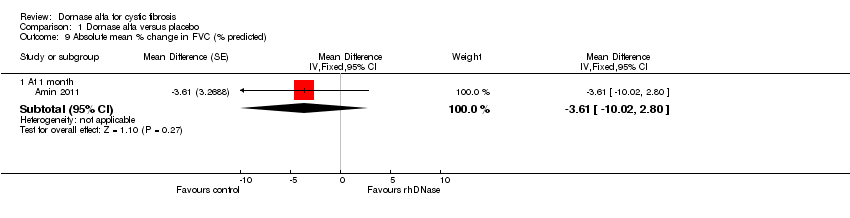 Comparison 1 Dornase alfa versus placebo, Outcome 9 Absolute mean % change in FVC (% predicted). | ||||
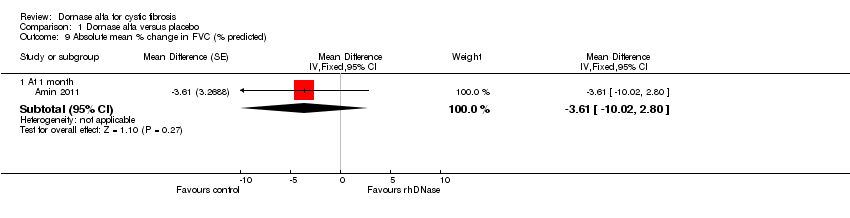
| 9.1 At 1 month | 1 | Mean Difference (Fixed, 95% CI) | ‐3.61 [‐10.02, 2.80] | |
| 10 Absolute mean % change in FVC (% predicted) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.10 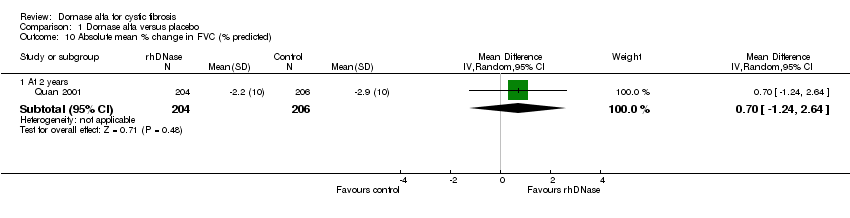 Comparison 1 Dornase alfa versus placebo, Outcome 10 Absolute mean % change in FVC (% predicted). | ||||
| 10.1 At 2 years | 1 | 410 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.24, 2.64] |
| 11 Absolute mean change in LCI Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Dornase alfa versus placebo, Outcome 11 Absolute mean change in LCI. | ||||
| 11.1 At 1 month | 1 | 34 | Mean Difference (Fixed, 95% CI) | ‐0.9 [‐1.87, 0.07] |
| 12 Absolute change in FEV0.5 (z score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Dornase alfa versus placebo, Outcome 12 Absolute change in FEV0.5 (z score). | ||||
| 12.1 At 6 months | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.57, 0.77] |
| 13 Quality of life ‐ CFQ‐R respiratory Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13 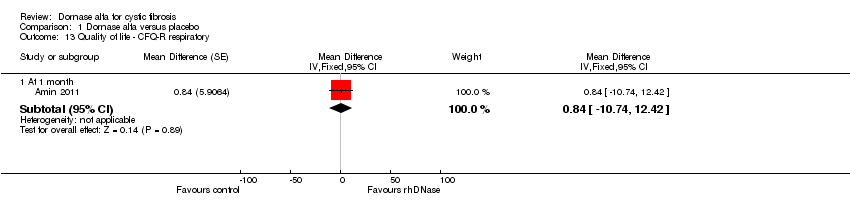 Comparison 1 Dornase alfa versus placebo, Outcome 13 Quality of life ‐ CFQ‐R respiratory. | ||||
| 13.1 At 1 month | 1 | Mean Difference (Fixed, 95% CI) | 0.84 [‐10.74, 12.42] | |
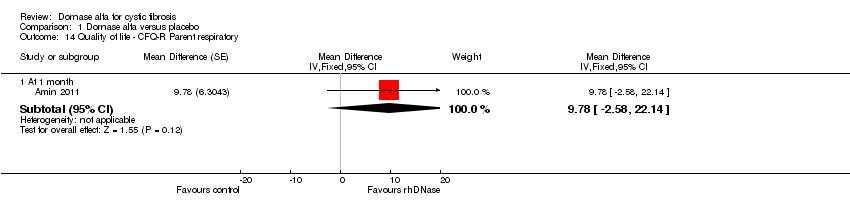
| 14 Quality of life ‐ CFQ‐R Parent respiratory Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 Dornase alfa versus placebo, Outcome 14 Quality of life ‐ CFQ‐R Parent respiratory. | ||||
| 14.1 At 1 month | 1 | Mean Difference (Fixed, 95% CI) | 9.78 [‐2.58, 22.14] | |
| 15 Number of people experiencing exacerbations Show forest plot | 3 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.96] |
| Analysis 1.15 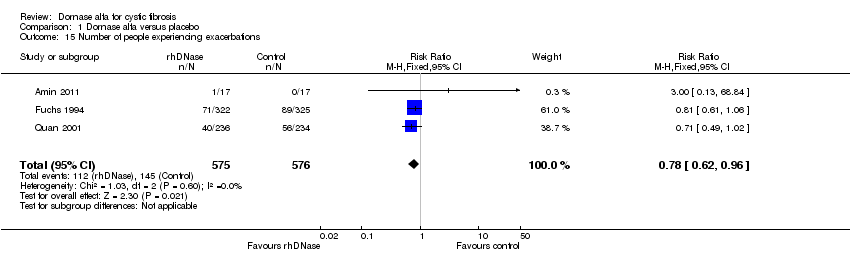 Comparison 1 Dornase alfa versus placebo, Outcome 15 Number of people experiencing exacerbations. | ||||
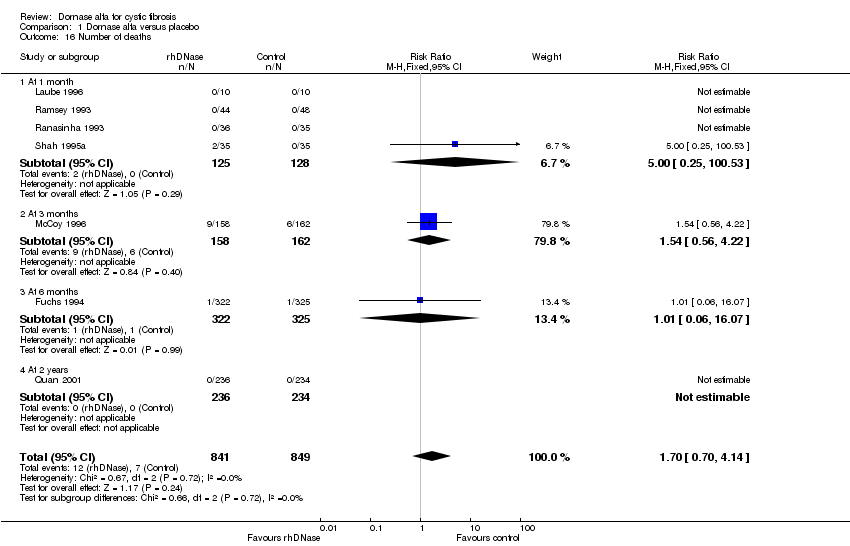
| 16 Number of deaths Show forest plot | 7 | 1690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.70, 4.14] |
| Analysis 1.16  Comparison 1 Dornase alfa versus placebo, Outcome 16 Number of deaths. | ||||
| 16.1 At 1 month | 4 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.53] |
| 16.2 At 3 months | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.56, 4.22] |
| 16.3 At 6 months | 1 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 16.07] |
| 16.4 At 2 years | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Mean number of days IV antibiotics used Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 Dornase alfa versus placebo, Outcome 17 Mean number of days IV antibiotics used. | ||||
| 17.1 At 3 months | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐2.96 [‐7.29, 1.37] |
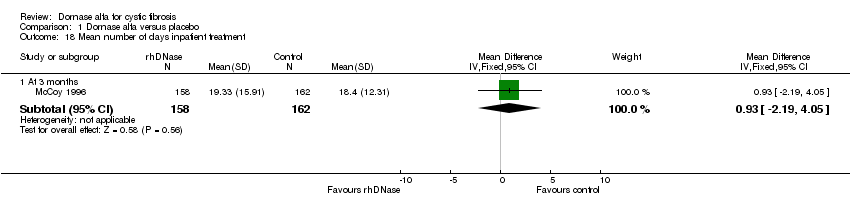
| 18 Mean number of days inpatient treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.18  Comparison 1 Dornase alfa versus placebo, Outcome 18 Mean number of days inpatient treatment. | ||||
| 18.1 At 3 months | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [‐2.19, 4.05] |
| 19 Mean change in weight from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.19 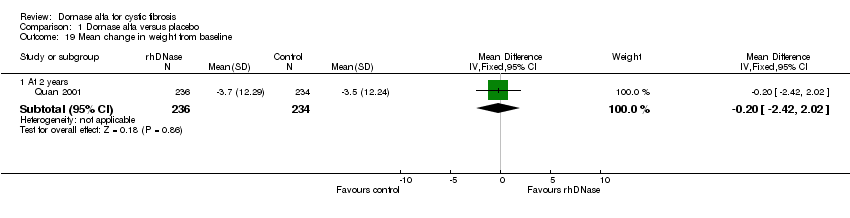 Comparison 1 Dornase alfa versus placebo, Outcome 19 Mean change in weight from baseline. | ||||
| 19.1 At 2 years | 1 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.42, 2.02] |
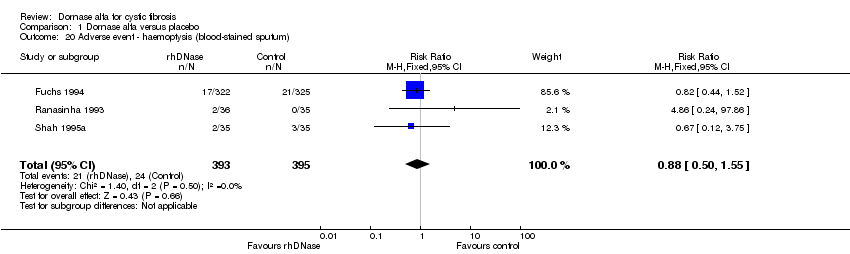
| 20 Adverse event ‐ haemoptysis (blood‐stained sputum) Show forest plot | 3 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.50, 1.55] |
| Analysis 1.20 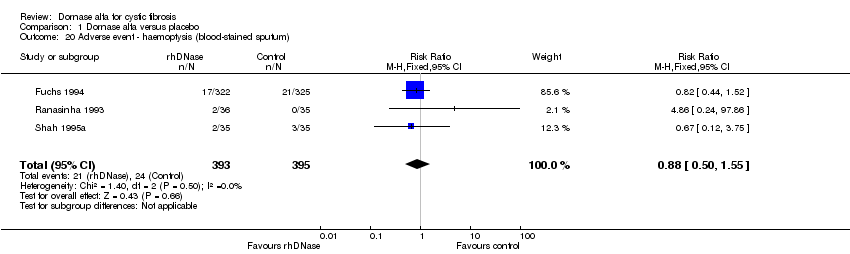 Comparison 1 Dornase alfa versus placebo, Outcome 20 Adverse event ‐ haemoptysis (blood‐stained sputum). | ||||
| 21 Adverse event ‐ dyspnoea (shortness of breath) Show forest plot | 4 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| Analysis 1.21  Comparison 1 Dornase alfa versus placebo, Outcome 21 Adverse event ‐ dyspnoea (shortness of breath). | ||||
| 22 Adverse event ‐ pneumothorax Show forest plot | 3 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.08, 4.50] |
| Analysis 1.22 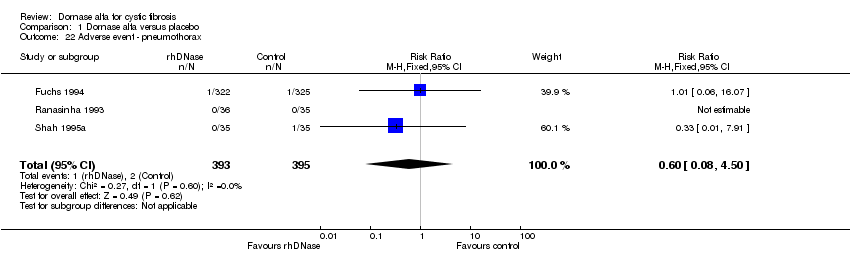 Comparison 1 Dornase alfa versus placebo, Outcome 22 Adverse event ‐ pneumothorax. | ||||
| 23 Adverse event ‐ pneumothorax (in participants with acute exacerbations) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.11, 61.75] |
| Analysis 1.23 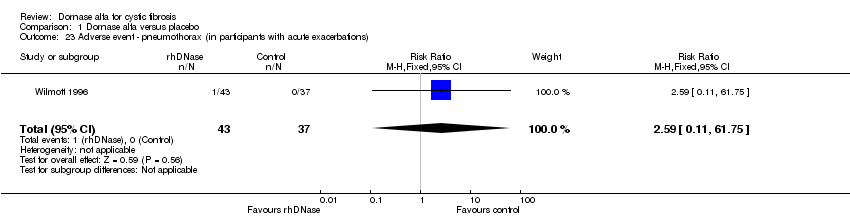 Comparison 1 Dornase alfa versus placebo, Outcome 23 Adverse event ‐ pneumothorax (in participants with acute exacerbations). | ||||
| 24 Adverse event ‐ voice alteration Show forest plot | 6 | 1670 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.20, 2.39] |
| Analysis 1.24 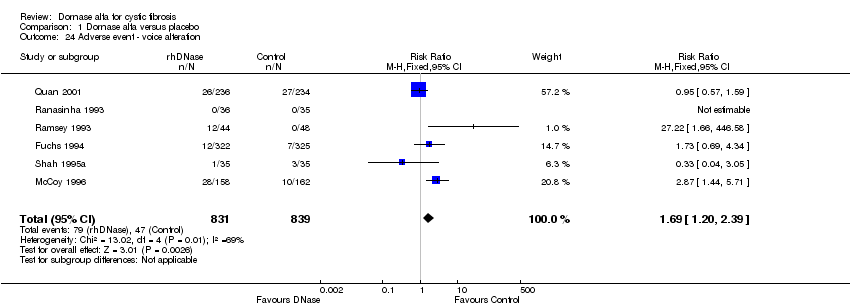 Comparison 1 Dornase alfa versus placebo, Outcome 24 Adverse event ‐ voice alteration. | ||||
| 25 Adverse event ‐ voice alteration (1x versus 2x daily treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.25 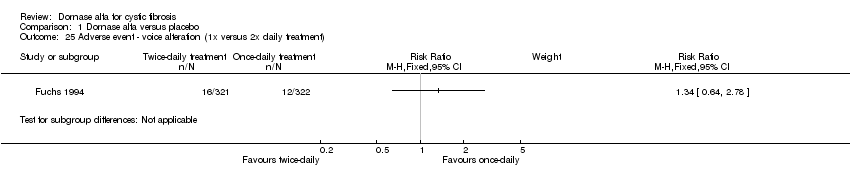 Comparison 1 Dornase alfa versus placebo, Outcome 25 Adverse event ‐ voice alteration (1x versus 2x daily treatment). | ||||
| 26 Adverse event ‐ voice alteration (in participants with acute exacerbations) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.55, 12.03] |
| Analysis 1.26 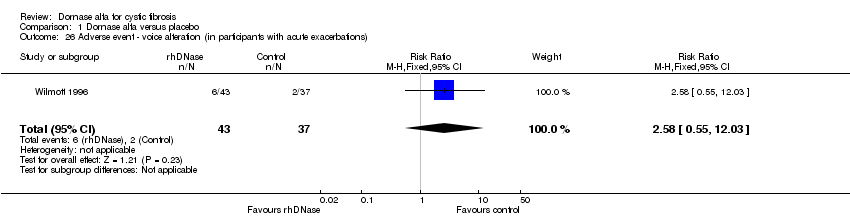 Comparison 1 Dornase alfa versus placebo, Outcome 26 Adverse event ‐ voice alteration (in participants with acute exacerbations). | ||||
| 27 Adverse event ‐ rash Show forest plot | 2 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.16, 4.99] |
| Analysis 1.27  Comparison 1 Dornase alfa versus placebo, Outcome 27 Adverse event ‐ rash. | ||||
| 28 Adverse event ‐ chest pain Show forest plot | 3 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.59, 1.70] |
| Analysis 1.28 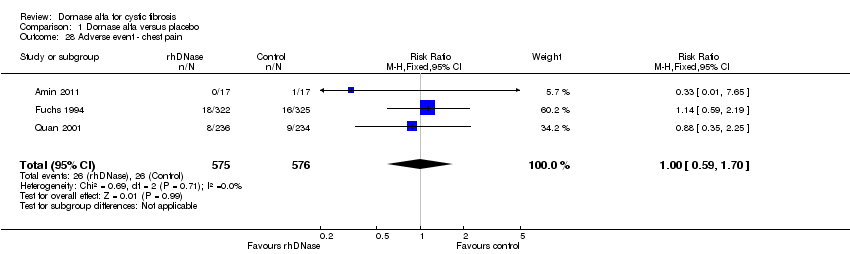 Comparison 1 Dornase alfa versus placebo, Outcome 28 Adverse event ‐ chest pain. | ||||
| 29 Adverse event ‐ cough (new or increased) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.29 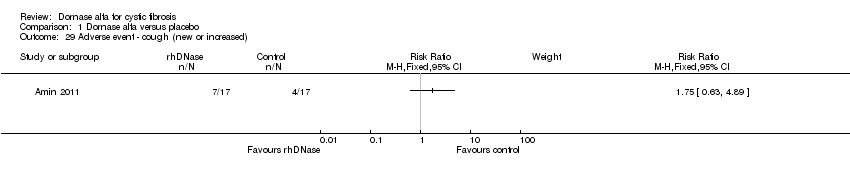 Comparison 1 Dornase alfa versus placebo, Outcome 29 Adverse event ‐ cough (new or increased). | ||||
| 30 Adverse event ‐ increased sputum production Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.30  Comparison 1 Dornase alfa versus placebo, Outcome 30 Adverse event ‐ increased sputum production. | ||||
| 31 Adverse event ‐ dry throat Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.31  Comparison 1 Dornase alfa versus placebo, Outcome 31 Adverse event ‐ dry throat. | ||||
| 32 Adverse event ‐ pharyngitis Show forest plot | 6 | 1612 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.91, 1.46] |
| Analysis 1.32 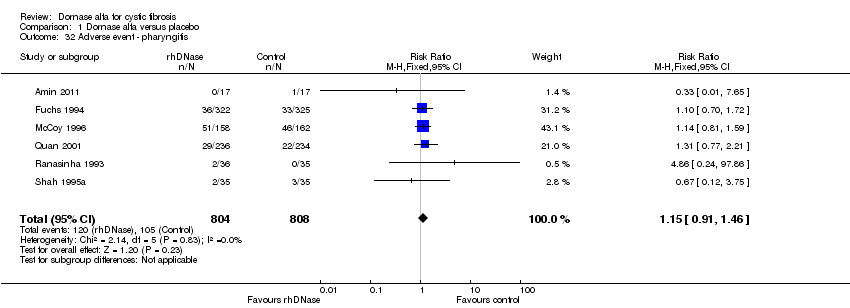 Comparison 1 Dornase alfa versus placebo, Outcome 32 Adverse event ‐ pharyngitis. | ||||
| 33 Adverse event ‐ laryngitis Show forest plot | 3 | 1187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.68, 3.68] |
| Analysis 1.33 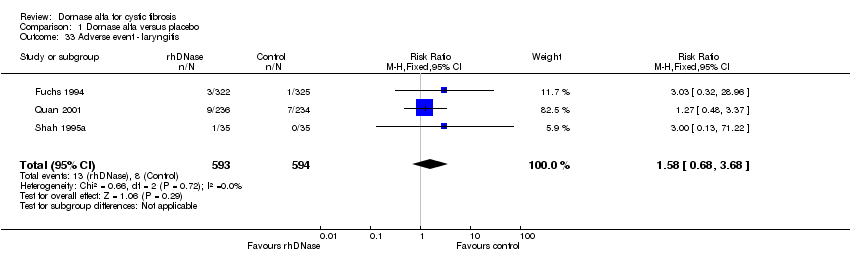 Comparison 1 Dornase alfa versus placebo, Outcome 33 Adverse event ‐ laryngitis. | ||||
| 34 Adverse event ‐ conjunctivitis Show forest plot | 2 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.50, 3.13] |
| Analysis 1.34  Comparison 1 Dornase alfa versus placebo, Outcome 34 Adverse event ‐ conjunctivitis. | ||||
| 35 Adverse event ‐ wheeze Show forest plot | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.15, 2.41] |
| Analysis 1.35 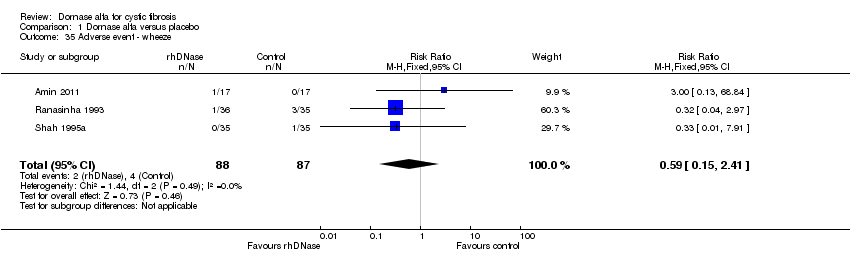 Comparison 1 Dornase alfa versus placebo, Outcome 35 Adverse event ‐ wheeze. | ||||
| 36 Adverse event ‐ facial oedema Show forest plot | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.62 [0.40, 143.52] |
| Analysis 1.36  Comparison 1 Dornase alfa versus placebo, Outcome 36 Adverse event ‐ facial oedema. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean % change in FEV1 Show forest plot | 1 | Mean difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 1 Mean % change in FEV1. | ||||
| 1.1 At 3 months | 1 | Mean difference (Fixed, 95% CI) | 2.0 [‐3.00, 9.00] | |
| 2 Mean % change in FVC Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 2 Mean % change in FVC. | ||||
| 2.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.06, 0.12] | |
| 3 Mean % change in quality of life score Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 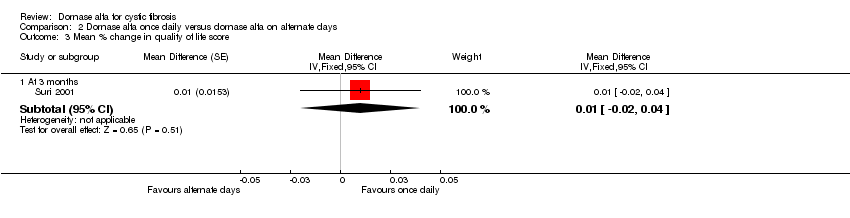 Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 3 Mean % change in quality of life score. | ||||
| 3.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.01 [‐0.02, 0.04] | |
| 4 Mean number of days inpatient treatment Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4 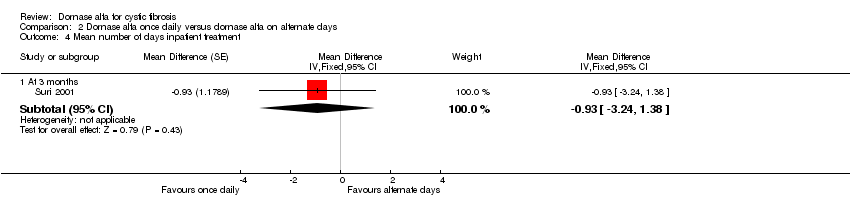 Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 4 Mean number of days inpatient treatment. | ||||
| 4.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.93 [‐3.24, 1.38] | |
| 5 Mean change in weight (kg) from baseline Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5 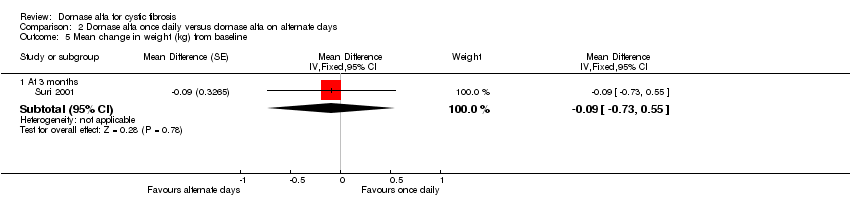 Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 5 Mean change in weight (kg) from baseline. | ||||
| 5.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.09 [‐0.73, 0.55] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean % change in FEV1 Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 1 Mean % change in FEV1. | ||||
| 1.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 8.0 [2.00, 14.00] | |
| 2 Mean % change in FVC Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2 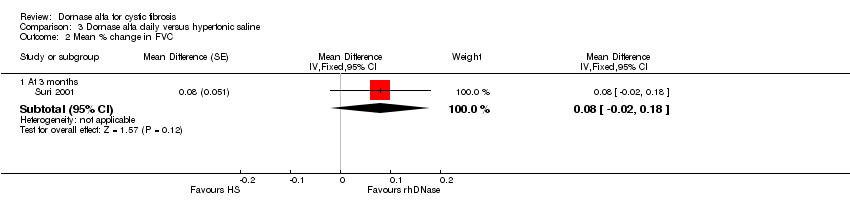 Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 2 Mean % change in FVC. | ||||
| 2.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.08 [‐0.02, 0.18] | |
| 3 Mean % change in quality of life score Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3 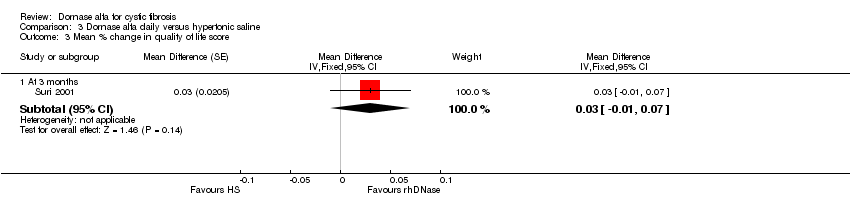 Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 3 Mean % change in quality of life score. | ||||
| 3.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.01, 0.07] | |
| 4 Mean number of days inpatient treatment Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 4 Mean number of days inpatient treatment. | ||||
| 4.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.4 [‐2.32, 1.52] | |
| 5 Mean change in weight (kg) from baseline Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5 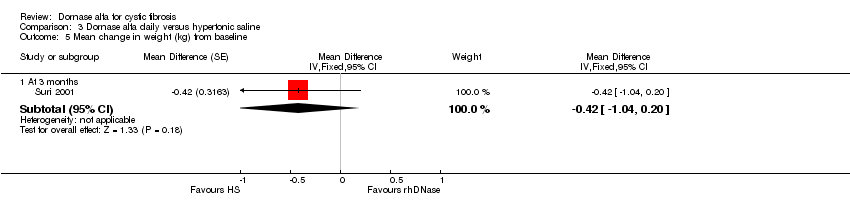 Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 5 Mean change in weight (kg) from baseline. | ||||
| 5.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.42 [‐1.04, 0.20] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean absolute change in FEV1 (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Dornase alfa versus mannitol, Outcome 1 Mean absolute change in FEV1 (L). | ||||
| 1.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.11, 0.16] |
| 2 Mean absolute change in FVC (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Dornase alfa versus mannitol, Outcome 2 Mean absolute change in FVC (L). | ||||
| 2.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.23, 0.19] |
| 3 Quality of life ‐ CFQ‐R Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3 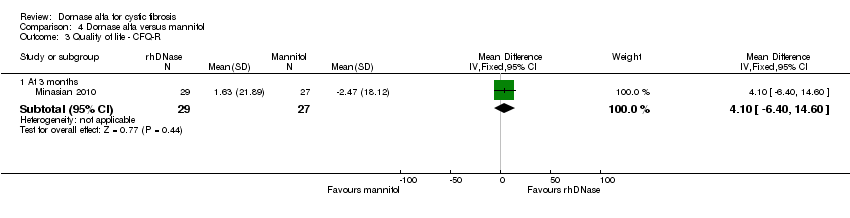 Comparison 4 Dornase alfa versus mannitol, Outcome 3 Quality of life ‐ CFQ‐R. | ||||
| 3.1 At 3 months | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 4.1 [‐6.40, 14.60] |
| 4 Number of people experiencing exacerbations Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Dornase alfa versus mannitol, Outcome 4 Number of people experiencing exacerbations. | ||||
| 4.1 At 3 months | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.25, 4.84] |
| 5 Adverse events at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.5 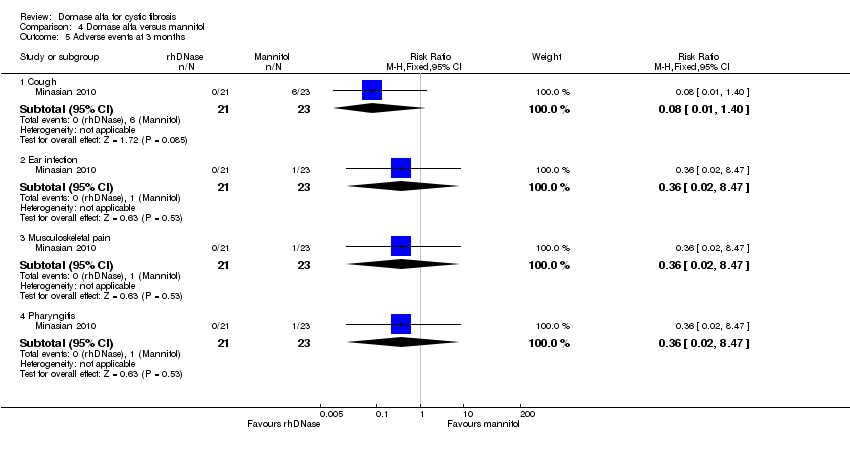 Comparison 4 Dornase alfa versus mannitol, Outcome 5 Adverse events at 3 months. | ||||
| 5.1 Cough | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 1.40] |
| 5.2 Ear infection | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.3 Musculoskeletal pain | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.4 Pharyngitis | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean absolute change in FEV1 (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 1 Mean absolute change in FEV1 (L). | ||||
| 1.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.06, 0.25] |
| 2 Mean absolute change in FVC (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.2 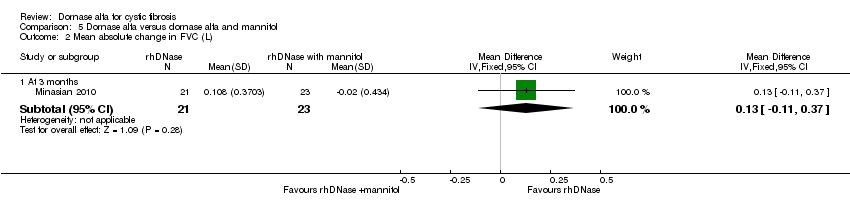 Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 2 Mean absolute change in FVC (L). | ||||
| 2.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.11, 0.37] |
| 3 Quality of life ‐ CFQ‐R Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.3 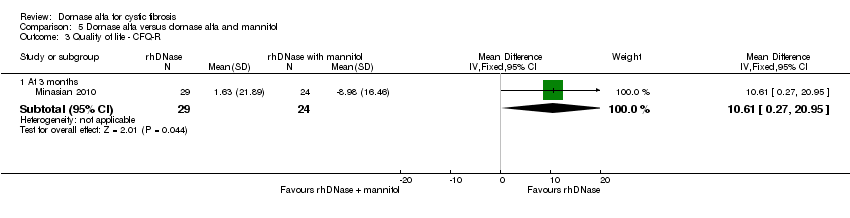 Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 3 Quality of life ‐ CFQ‐R. | ||||
| 3.1 At 3 months | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 10.61 [0.27, 20.95] |
| 4 Number of people experiencing exacerbations Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.4 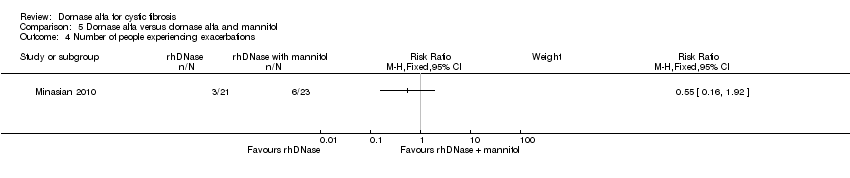 Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 4 Number of people experiencing exacerbations. | ||||
| 5 Adverse events at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 5 Adverse events at 3 months. | ||||
| 5.1 Cough | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 5.2 Headache | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.3 Nausea | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.4 Rash | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
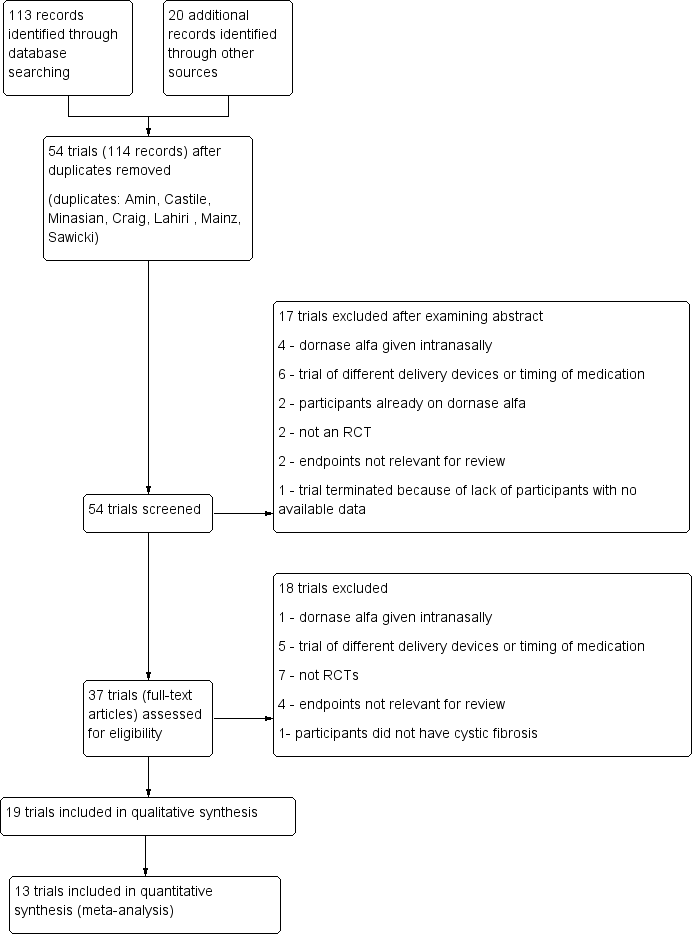
Study flow diagram.
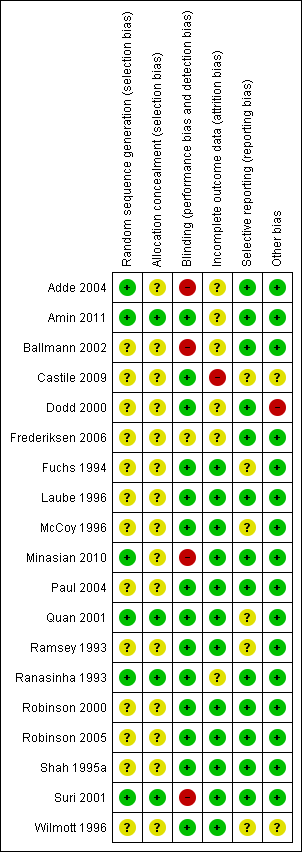
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Dornase alfa versus placebo, Outcome 1 Relative mean % change in FEV1 (% predicted).

Comparison 1 Dornase alfa versus placebo, Outcome 2 Relative mean % change in FEV1 (% predicted) at one month ‐ subgroup analysis by disease severity.

Comparison 1 Dornase alfa versus placebo, Outcome 3 Absolute mean % change in FEV1 (% predicted).
Comparison 1 Dornase alfa versus placebo, Outcome 4 Absolute mean % change in FEV1 (% predicted).
Comparison 1 Dornase alfa versus placebo, Outcome 5 Relative mean % change in FEV1 (in participants with acute exacerbations).

Comparison 1 Dornase alfa versus placebo, Outcome 6 Relative mean % change in FVC (% predicted).

Comparison 1 Dornase alfa versus placebo, Outcome 7 Relative mean % change in FVC (% predicted).

Comparison 1 Dornase alfa versus placebo, Outcome 8 Relative mean % change in FVC at one month ‐ subgroup analysis by disease severity.
Comparison 1 Dornase alfa versus placebo, Outcome 9 Absolute mean % change in FVC (% predicted).

Comparison 1 Dornase alfa versus placebo, Outcome 10 Absolute mean % change in FVC (% predicted).

Comparison 1 Dornase alfa versus placebo, Outcome 11 Absolute mean change in LCI.

Comparison 1 Dornase alfa versus placebo, Outcome 12 Absolute change in FEV0.5 (z score).

Comparison 1 Dornase alfa versus placebo, Outcome 13 Quality of life ‐ CFQ‐R respiratory.
Comparison 1 Dornase alfa versus placebo, Outcome 14 Quality of life ‐ CFQ‐R Parent respiratory.

Comparison 1 Dornase alfa versus placebo, Outcome 15 Number of people experiencing exacerbations.
Comparison 1 Dornase alfa versus placebo, Outcome 16 Number of deaths.

Comparison 1 Dornase alfa versus placebo, Outcome 17 Mean number of days IV antibiotics used.
Comparison 1 Dornase alfa versus placebo, Outcome 18 Mean number of days inpatient treatment.

Comparison 1 Dornase alfa versus placebo, Outcome 19 Mean change in weight from baseline.
Comparison 1 Dornase alfa versus placebo, Outcome 20 Adverse event ‐ haemoptysis (blood‐stained sputum).
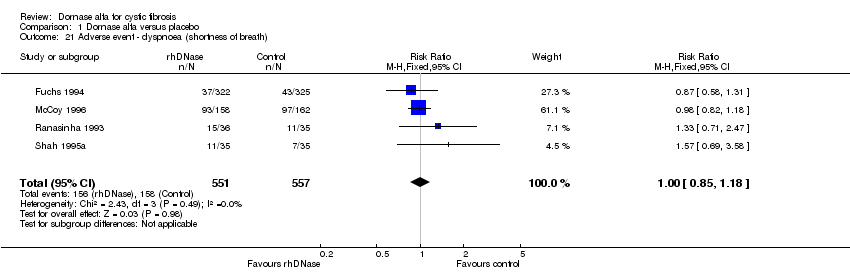
Comparison 1 Dornase alfa versus placebo, Outcome 21 Adverse event ‐ dyspnoea (shortness of breath).

Comparison 1 Dornase alfa versus placebo, Outcome 22 Adverse event ‐ pneumothorax.
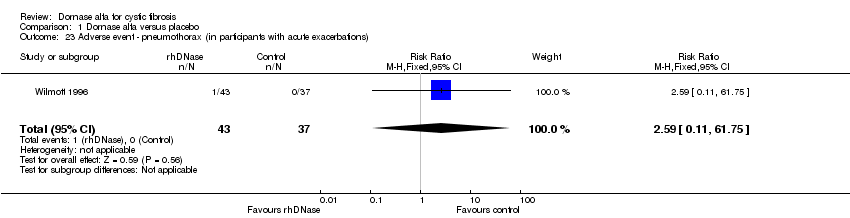
Comparison 1 Dornase alfa versus placebo, Outcome 23 Adverse event ‐ pneumothorax (in participants with acute exacerbations).

Comparison 1 Dornase alfa versus placebo, Outcome 24 Adverse event ‐ voice alteration.

Comparison 1 Dornase alfa versus placebo, Outcome 25 Adverse event ‐ voice alteration (1x versus 2x daily treatment).

Comparison 1 Dornase alfa versus placebo, Outcome 26 Adverse event ‐ voice alteration (in participants with acute exacerbations).

Comparison 1 Dornase alfa versus placebo, Outcome 27 Adverse event ‐ rash.

Comparison 1 Dornase alfa versus placebo, Outcome 28 Adverse event ‐ chest pain.

Comparison 1 Dornase alfa versus placebo, Outcome 29 Adverse event ‐ cough (new or increased).

Comparison 1 Dornase alfa versus placebo, Outcome 30 Adverse event ‐ increased sputum production.
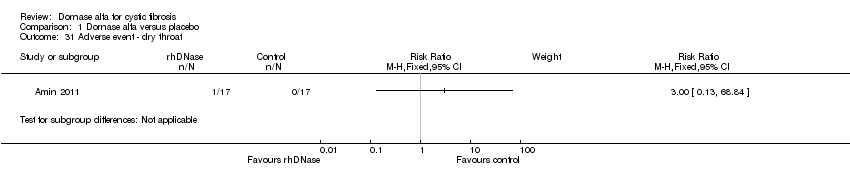
Comparison 1 Dornase alfa versus placebo, Outcome 31 Adverse event ‐ dry throat.
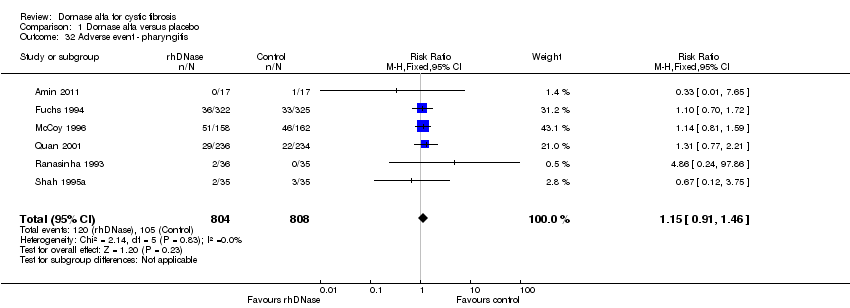
Comparison 1 Dornase alfa versus placebo, Outcome 32 Adverse event ‐ pharyngitis.
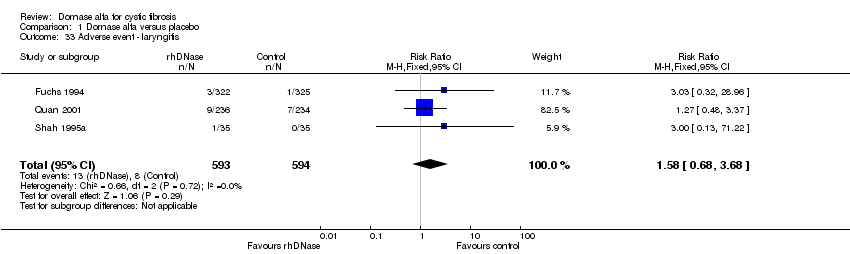
Comparison 1 Dornase alfa versus placebo, Outcome 33 Adverse event ‐ laryngitis.

Comparison 1 Dornase alfa versus placebo, Outcome 34 Adverse event ‐ conjunctivitis.

Comparison 1 Dornase alfa versus placebo, Outcome 35 Adverse event ‐ wheeze.
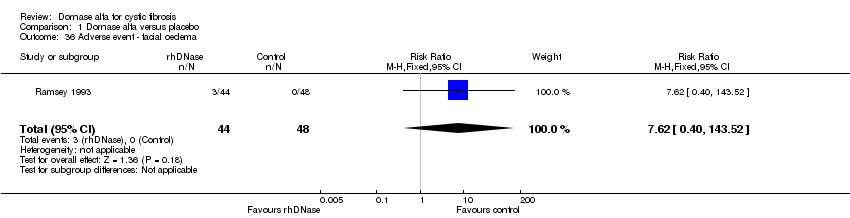
Comparison 1 Dornase alfa versus placebo, Outcome 36 Adverse event ‐ facial oedema.
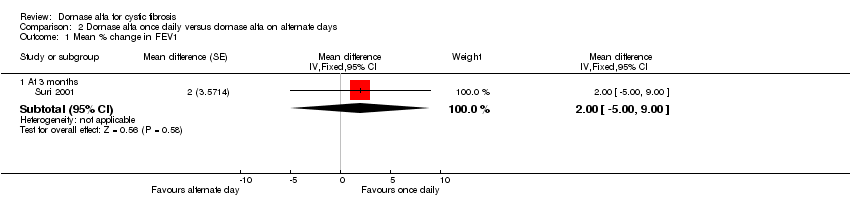
Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 1 Mean % change in FEV1.
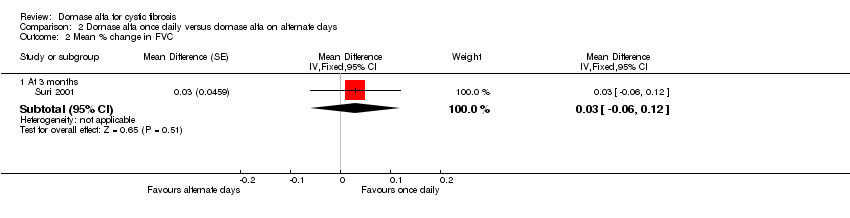
Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 2 Mean % change in FVC.
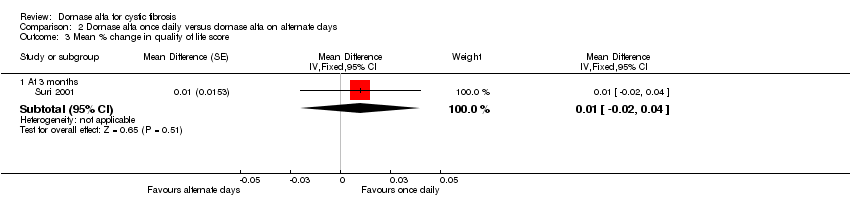
Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 3 Mean % change in quality of life score.

Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 4 Mean number of days inpatient treatment.
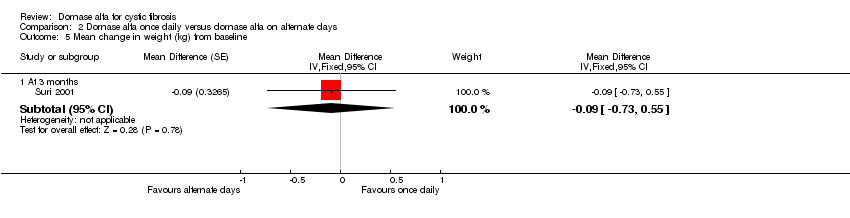
Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 5 Mean change in weight (kg) from baseline.

Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 1 Mean % change in FEV1.
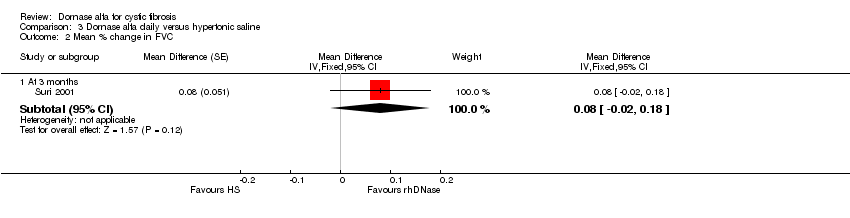
Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 2 Mean % change in FVC.

Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 3 Mean % change in quality of life score.
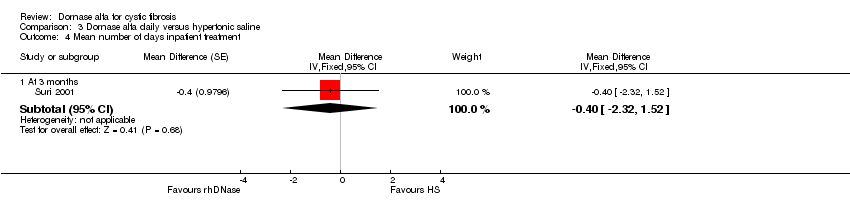
Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 4 Mean number of days inpatient treatment.

Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 5 Mean change in weight (kg) from baseline.

Comparison 4 Dornase alfa versus mannitol, Outcome 1 Mean absolute change in FEV1 (L).

Comparison 4 Dornase alfa versus mannitol, Outcome 2 Mean absolute change in FVC (L).
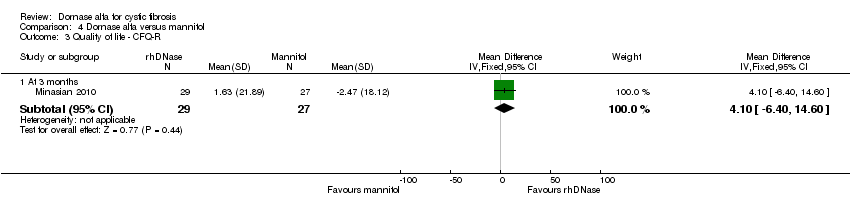
Comparison 4 Dornase alfa versus mannitol, Outcome 3 Quality of life ‐ CFQ‐R.

Comparison 4 Dornase alfa versus mannitol, Outcome 4 Number of people experiencing exacerbations.
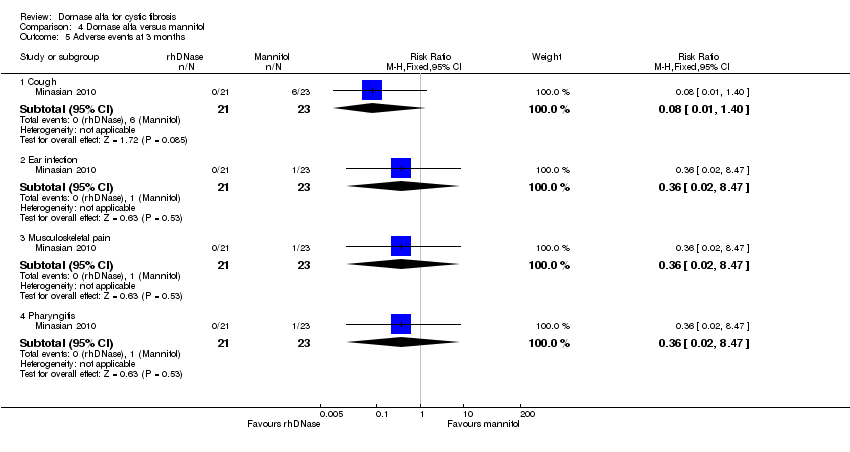
Comparison 4 Dornase alfa versus mannitol, Outcome 5 Adverse events at 3 months.
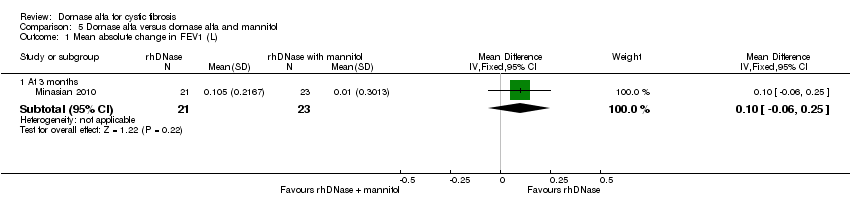
Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 1 Mean absolute change in FEV1 (L).

Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 2 Mean absolute change in FVC (L).

Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 3 Quality of life ‐ CFQ‐R.

Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 4 Number of people experiencing exacerbations.

Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 5 Adverse events at 3 months.
| Dornase alfa compared with placebo or no dornase alfa treatment for cystic fibrosis | ||||||
| Patient or population: Adults and children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no dornase alfa treatment | Dornase alfa | |||||
| Relative mean percentage change in FEV1 (% predicted) at 3 months | The relative mean percentage change in FEV1 (% predicted) was 2.1 | The relative mean percentage change in FEV1 (% predicted) was 7.3 higher (4.04 higher to 10.56 higher) | NA | 320 (1 study)1 | ⊕⊕⊕⊝ | |
| Relative mean percentage change in FEV1 (% predicted) at 6 months | The relative mean percentage change in FEV1 (% predicted) was 0 | The relative mean percentage change in FEV1 (% predicted) was 5.8 higher (3.99 higher to 7.61 higher) | NA | 647 (1 study)1 | ⊕⊕⊕⊕ | Result presented from once‐daily dornase alfa group. Significant benefit for dornase alfa also present in twice‐daily dornase alfa group |
| Relative mean percentage change in FVC (% predicted) at 3 months | The relative mean percentage change in FVC (% predicted) was 7.3 | The relative mean percentage change in FVC (% predicted) was 5.1 higher (1.23 higher to 8.97 higher) | NA | 318 (1 study)4 | ⊕⊕⊕⊝ | |
| Relative mean percentage change in FVC (% predicted) at 3 months | See comment | See comment | MD 3.80 (2.62 to 4.98) | 647 (1 study)1 | ⊕⊕⊕⊕ | Mean difference between groups only presented. Result presented from once‐daily dornase alfa group. Significant benefit for dornase alfa also present in twice‐daily dornase alfa group |
| Change in quality of life ‐ CFQ‐R respiratory at 1 month | See comment | See comment | MD 0.84 (‐10.74 to 12.42) | 19 (1 cross‐over study)5 | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
| Change in quality of life ‐ CFQ‐R respiratory (parent) at 1 month | See comment | See comment | MD 9.78 (‐2.58 to 22.14) | 19 (1 cross‐over study)5 | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
| Number of people experiencing exacerbations at up to 2 years | 252 per 1000 | 196 per 1000 | RR 0.78 (0.62 to 0.96) | 1157 (3 studies)8 | ⊕⊕⊕⊝ | RR <1 indicates an advantage for dornase alfa. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Assumed and corresponding risk not calculated for quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Additionally four trials included in analysis at one month showed a significant advantage to dornase alfa over placebo or no dornase alfa treatment (Laube 1996; Ramsey 1993; Ranasinha 1993; Shah 1995a). Three studies not included in pooled analysis showed no difference between groups in relative FEV1(L) (Robinson 2000) and relative FEV1 (% predicted) (Wilmott 1996) or absolute FEV1 (% predicted) (Amin 2011) at one month. At one year, one study showed a significant advantage to dornase alfa over placebo or no dornase alfa treatment (Frederiksen 2006) and one study showed no difference between treatments (Robinson 2005). At one year, one study showed a significant advantage to dornase alfa over placebo or no dornase alfa treatment (Quan 2001) and at three years, one study showed no significant difference between treatments (Paul 2004). 2. Downgraded due to indirectness: participants in McCoy 1996 had severe lung disease (FVC below 40%). 3. No evidence of imprecision, inconsistency, indirectness, publication bias or serious risk of bias. 4. Additionally four trials included in analysis at one month (Laube 1996; Ramsey 1993; Ranasinha 1993; Shah 1995a) showed a significant advantage to dornase alfa over placebo or no dornase alfa treatment. One study not included in pooled analysis showed a significant advantage in relative FVC (L) to dornase alfa over placebo or no dornase alfa treatment (Robinson 2000) and one study showed no significant different in absolute FVC (% predicted) between groups (Amin 2011) at one month. No significant difference was found between groups at one year (Robinson 2005) and at two years (Quan 2001). 5. Additionally, four studies reported quality of life data which could not be included in pooled analysis. Wilmott 1996 showed no difference between groups in CFQ‐R. Ramsey reported that the frequency and magnitude of improvement across all quality of life questions was greater among participants receiving dornase alfa (Ramsey 1993). Ranasinha reported significant improvements in overall well‐being and significant improvements in general well‐being, cough frequency and chest congestion (Ranasinha 1993) and Fuchs reported significant improvements in well‐being score and dyspnoea score on dornase alfa compared to placebo (Fuchs 1994). 6. Downgraded once for lack of applicability: Amin included children only so results are not applicable to adults (Amin 2011). 7. Downgraded once for imprecision: wide confidence intervals around the effect size due to limited sample size of the trial. 8. Additionally, one study reported an age‐adjusted RR of having more than one respiratory exacerbation, but these data were not included in the pooled analysis (McCoy 1996). No significant difference was found between dornase alfa and control. 9. Downgraded once as data from one cross‐over trial was analysed as parallel data (Amin 2011), which is a conservative approach. | ||||||
| Dornase alfa daily compared with dornase alfa on alternate days for cystic fibrosis | ||||||
| Patient or population: Children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa daily Comparison: Dornase alfa alternate days | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dornase alfa alternate days | Dornase alfa daily | |||||
| Mean relative percentage change in FEV1 (L) at 3 months | See comment | See comment | MD 2.00 (‐5.00 to 9.00) | 43 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
| Mean relative percentage in FVC (L) at 3 months | See comment | See comment | MD 0.03 (‐0.06 to 0.12) | 43 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
| Mean relative percentage in quality of life score at 3 months | See comment | See comment | MD 0.01 (‐0.02 to 0.04) | 43 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
| Number of pulmonary exacerbations at 3 months | 17 exacerbations | 18 exacerbations | NA (see comment) | 43 (1 cross‐over study) | ⊕⊕⊝⊝ | No difference was found in the number of pulmonary exacerbations (no statistical comparison made) |
| *Assumed and corresponding risk not calculated lung function and quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once for lack of applicability: Suri included children only so results are not applicable to adults (Suri 2001). 2. Downgraded once for high risk of bias due to lack of blinding. | ||||||
| Dornase alfa compared with hypertonic saline for cystic fibrosis | ||||||
| Patient or population: Children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa (once daily) Comparison: Hypertonic saline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hypertonic Saline | Dornase alfa | |||||
| Mean relative percentage in FEV1 (L) at 3 months | See comment | See comment | MD 8.00 (2.00 to 14.00) | up to 431,2 (1 cross‐over study) (see comment) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Mean relative percentage in FVC (L) at 3 months | See comment | See comment | MD 0.08, (‐0.02 to 0.18) | up to 431,2 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Mean relative percentage in quality of life score at 3 months | See comment | See comment | MD 0.03, (‐0.01 to 0.07) | up to 431,2 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Number of pulmonary exacerbations at 3 months | 15 exacerbations | 17 exacerbations | NA (see comment) | up to 431,2 (1 cross‐over study) | ⊕⊕⊝⊝ | No difference was found in the number of pulmonary exacerbations (no statistical comparison made) |
| *Assumed and corresponding risk not calculated lung function and quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. In the cross‐over trial, 43 participants completed the dornase alfa arm and 40 completed the hypertonic saline arm (Suri 2001). 2. Two additional cross‐over trials compared dornase alfa and hypertonic saline, no significant differences were found between the treatments for % change in FEV1 and other primary outcomes of the review were not recorded in these trials (Adde 2004; Ballmann 2002). 3. Downgraded once for lack of applicability: Suri included children only so results are not applicable to adults (Suri 2001). 4. Downgraded once for high risk of bias due to lack of blinding. | ||||||
| Dornase alfa compared with mannitol for cystic fibrosis | ||||||
| Patient or population: Children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa Comparison: Mannitol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mannitol | Dornase Alfa | |||||
| Mean absolute change in FEV1 (L) at 3 months | See comment | See comment | MD 0.02 (‐0.11 to 0.16) | up to 231 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Mean absolute change in FVC (L) at 3 months | See comment | See comment | MD ‐0.02, (‐0.23 to 0.19) | up to 231 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Change in quality of life ‐ CFQ‐R at 3 months | See comment | See comment | MD 10.61 (0.27 to 20.95) | up to 231 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Number of people experiencing exacerbations ‐ at 3 months | 130 per 1000 | 143 per 1000 | RR 1.10 (0.25 to 4.84) | up to 231 (1 cross‐over study) | ⊕⊕⊝⊝ | RR <1 indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| *Assumed and corresponding risk not calculated for lung function and quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. In the cross‐over trial, 21 participants completed the dornase alfa arm and 23 participants completed the mannitol arm (Minasian 2010). 2. Downgraded once for lack of applicability: Minasian included children only so results are not applicable to adults (Minasian 2010). 3. Downgraded once for high risk of bias due to lack of blinding. | ||||||
| Dornase alfa compared with dornase alfa and mannitol for cystic fibrosis | ||||||
| Patient or population: Children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa Comparison: Dornase alfa and Mannitol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dornase alfa and mannitol | Dornase alfa | |||||
| Mean absolute change in FEV1 (L) at 3 months | See comment | See comment | MD 0.10 (‐0.06 to 0.25) | up to 231 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Mean absolute change in FVC (L) at 3 months | See comment | See comment | MD 0.13 (‐0.11 to 0.37) | up to 231 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Change in quality of life ‐ CFQ‐R at 3 months | See comment | See comment | MD 10.61 (0.27 to 20.95) | up to 231 (1 cross‐over study) | ⊕⊕⊝⊝ | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Number of people experiencing exacerbations at 3 months | 261 per 1000 | 143 per 1000 | RR 0.55 (0.16 to 1.92) | up to 231 (1 cross‐over study) | ⊕⊕⊝⊝ | RR <1 indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| *Assumed and corresponding risk not calculated lung function and quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. In the crossover trial, 21 participants completed the dornase alfa arm and 23 participants completed the dornase alfa plus mannitol arm (Minasian 2010). 2. Downgraded once for lack of applicability: Minasian included children only so results are not applicable to adults (Minasian 2010). 3. Downgraded once for high risk of bias due to lack of blinding. | ||||||
| Study | Comparison group | Duration of treatment | Frequency of dornase treatment | Study design |
| Placebo | 4 weeks | once daily | cross‐over | |
| Placebo | 6 months | once daily | cross‐over | |
| Placebo | 2 weeks | once daily | cross‐over | |
| No treatment | 1 year | once daily | parallel | |
| Placebo and twice‐daily dornase | 6 months | once or twice daily | parallel | |
| Placebo | 6 days | twice a day | parallel | |
| Placebo | 3 months | once daily | parallel | |
| No treatment | 3 years | twice a day | parallel | |
| Placebo | 2 years | once a day | parallel | |
| Placebo | 10 days | twice a day (0.6 mg, 2.5 mg or 10 mg) | parallel | |
| Placebo | 10 days | twice a day | parallel | |
| Placebo | 7 days | once a day | cross‐over | |
| Placebo | 1 year | once a day | parallel | |
| Placebo | 2 weeks | twice a day | parallel | |
| Placebo | 15 days | twice a day | parallel | |
| Hypertonic saline and alternate day dornase | 3 months | once a day, alternate day | cross‐over | |
| Hypertonic saline | 4 weeks | once daily | cross‐over | |
| Hypertonic saline | 3 weeks | once daily | cross‐over | |
| Mannitol and mannitol plus dornase | 3 months | once daily | cross‐over | |
| *Trial done during acute exacerbation | ||||
| Pre dornase alfa | Post dornase alfa | Pre placebo | Post placebo | |
| FEV1 (L) mean (SD) | 2.63 (0.31) | 2.8 (0.32) | 2.63 (0.32) | 2.70 (0.32) |
| FVC (L) mean (SD) | 4.03 (0.35) | 4.21 (0.35) | 4.12 (0.36) | 4.06 (0.38) |
| FEV1: forced expiratory volume at one second | ||||
| Pre‐hypertonic saline | Post hypertonic saline | Pre dornase alfa | Post dornase alfa | P value | |
| FEV1 (% predicted) mean (SD) | 47 (18) | 46 (18) | 49 (15) | 50 (21) | NS |
| FEV1: forced expiratory volume at one second | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relative mean % change in FEV1 (% predicted) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At 1 month | 4 | 248 | Mean Difference (IV, Random, 95% CI) | 9.51 [0.67, 18.35] |
| 1.2 At 3 months | 1 | 320 | Mean Difference (IV, Random, 95% CI) | 7.30 [4.04, 10.56] |
| 1.3 At 6 months | 1 | 647 | Mean Difference (IV, Random, 95% CI) | 5.8 [3.99, 7.61] |
| 1.4 At 12 months | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐11.26, 12.66] |
| 2 Relative mean % change in FEV1 (% predicted) at one month ‐ subgroup analysis by disease severity Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moderate | 3 | 183 | Mean Difference (IV, Fixed, 95% CI) | 14.26 [10.79, 17.74] |
| 2.2 Severe | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐2.81 [‐8.77, 3.15] |
| 3 Absolute mean % change in FEV1 (% predicted) Show forest plot | 1 | Mean difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 At 1 month | 1 | Mean difference (Fixed, 95% CI) | 0.08 [‐5.59, 5.74] | |
| 4 Absolute mean % change in FEV1 (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 2 years | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | 3.24 [1.03, 5.45] |
| 5 Relative mean % change in FEV1 (in participants with acute exacerbations) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Up to 1 month | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐13.93, 15.93] |
| 6 Relative mean % change in FVC (% predicted) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 At 1 month | 4 | 248 | Mean Difference (IV, Random, 95% CI) | 7.52 [1.34, 13.69] |
| 6.2 At 3 months | 1 | 318 | Mean Difference (IV, Random, 95% CI) | 5.10 [1.23, 8.97] |
| 6.3 At 12 months | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐5.70 [‐15.87, 4.47] |
| 7 Relative mean % change in FVC (% predicted) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 7.1 At 6 months (once daily) | 1 | 2 | Mean Difference (Random, 95% CI) | 3.80 [2.62, 4.98] |
| 7.2 At 6 months (twice daily) | 1 | 2 | Mean Difference (Random, 95% CI) | 3.00 [1.82, 4.18] |
| 8 Relative mean % change in FVC at one month ‐ subgroup analysis by disease severity Show forest plot | 4 | 248 | Mean Difference (IV, Fixed, 95% CI) | 9.49 [6.34, 12.63] |
| 8.1 Moderate | 3 | 183 | Mean Difference (IV, Fixed, 95% CI) | 10.98 [7.68, 14.29] |
| 8.2 Severe | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐15.15, 5.35] |
| 9 Absolute mean % change in FVC (% predicted) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 9.1 At 1 month | 1 | Mean Difference (Fixed, 95% CI) | ‐3.61 [‐10.02, 2.80] | |
| 10 Absolute mean % change in FVC (% predicted) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 At 2 years | 1 | 410 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.24, 2.64] |
| 11 Absolute mean change in LCI Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 11.1 At 1 month | 1 | 34 | Mean Difference (Fixed, 95% CI) | ‐0.9 [‐1.87, 0.07] |
| 12 Absolute change in FEV0.5 (z score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 At 6 months | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.57, 0.77] |
| 13 Quality of life ‐ CFQ‐R respiratory Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 13.1 At 1 month | 1 | Mean Difference (Fixed, 95% CI) | 0.84 [‐10.74, 12.42] | |
| 14 Quality of life ‐ CFQ‐R Parent respiratory Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 14.1 At 1 month | 1 | Mean Difference (Fixed, 95% CI) | 9.78 [‐2.58, 22.14] | |
| 15 Number of people experiencing exacerbations Show forest plot | 3 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.96] |
| 16 Number of deaths Show forest plot | 7 | 1690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.70, 4.14] |
| 16.1 At 1 month | 4 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.53] |
| 16.2 At 3 months | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.56, 4.22] |
| 16.3 At 6 months | 1 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 16.07] |
| 16.4 At 2 years | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Mean number of days IV antibiotics used Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 At 3 months | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐2.96 [‐7.29, 1.37] |
| 18 Mean number of days inpatient treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 At 3 months | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [‐2.19, 4.05] |
| 19 Mean change in weight from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 At 2 years | 1 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.42, 2.02] |
| 20 Adverse event ‐ haemoptysis (blood‐stained sputum) Show forest plot | 3 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.50, 1.55] |
| 21 Adverse event ‐ dyspnoea (shortness of breath) Show forest plot | 4 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| 22 Adverse event ‐ pneumothorax Show forest plot | 3 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.08, 4.50] |
| 23 Adverse event ‐ pneumothorax (in participants with acute exacerbations) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.11, 61.75] |
| 24 Adverse event ‐ voice alteration Show forest plot | 6 | 1670 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.20, 2.39] |
| 25 Adverse event ‐ voice alteration (1x versus 2x daily treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26 Adverse event ‐ voice alteration (in participants with acute exacerbations) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.55, 12.03] |
| 27 Adverse event ‐ rash Show forest plot | 2 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.16, 4.99] |
| 28 Adverse event ‐ chest pain Show forest plot | 3 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.59, 1.70] |
| 29 Adverse event ‐ cough (new or increased) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30 Adverse event ‐ increased sputum production Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31 Adverse event ‐ dry throat Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32 Adverse event ‐ pharyngitis Show forest plot | 6 | 1612 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.91, 1.46] |
| 33 Adverse event ‐ laryngitis Show forest plot | 3 | 1187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.68, 3.68] |
| 34 Adverse event ‐ conjunctivitis Show forest plot | 2 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.50, 3.13] |
| 35 Adverse event ‐ wheeze Show forest plot | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.15, 2.41] |
| 36 Adverse event ‐ facial oedema Show forest plot | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.62 [0.40, 143.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean % change in FEV1 Show forest plot | 1 | Mean difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 At 3 months | 1 | Mean difference (Fixed, 95% CI) | 2.0 [‐3.00, 9.00] | |
| 2 Mean % change in FVC Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.06, 0.12] | |
| 3 Mean % change in quality of life score Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.01 [‐0.02, 0.04] | |
| 4 Mean number of days inpatient treatment Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.93 [‐3.24, 1.38] | |
| 5 Mean change in weight (kg) from baseline Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.09 [‐0.73, 0.55] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean % change in FEV1 Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 8.0 [2.00, 14.00] | |
| 2 Mean % change in FVC Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.08 [‐0.02, 0.18] | |
| 3 Mean % change in quality of life score Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.01, 0.07] | |
| 4 Mean number of days inpatient treatment Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.4 [‐2.32, 1.52] | |
| 5 Mean change in weight (kg) from baseline Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.42 [‐1.04, 0.20] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean absolute change in FEV1 (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.11, 0.16] |
| 2 Mean absolute change in FVC (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.23, 0.19] |
| 3 Quality of life ‐ CFQ‐R Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 3 months | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 4.1 [‐6.40, 14.60] |
| 4 Number of people experiencing exacerbations Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 3 months | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.25, 4.84] |
| 5 Adverse events at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Cough | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 1.40] |
| 5.2 Ear infection | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.3 Musculoskeletal pain | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.4 Pharyngitis | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean absolute change in FEV1 (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.06, 0.25] |
| 2 Mean absolute change in FVC (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.11, 0.37] |
| 3 Quality of life ‐ CFQ‐R Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 3 months | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 10.61 [0.27, 20.95] |
| 4 Number of people experiencing exacerbations Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Adverse events at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Cough | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 5.2 Headache | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.3 Nausea | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.4 Rash | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |

