Entrenamiento físico para el asma
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Country: France Design: Randomised controlled trial Objectives: To assess the validity of the 20‐minute shuttle test (20‐MST) to estimate maximal oxygen uptake (VO max) and its ability to register cardiorespiratory modifications over the course of an individualised aerobic training program for mild to moderately severe asthma in children acclimatised to moderate altitude Study Site: Not stated Methods of Analysis: Students paired t‐test, linear regression analysis, Bland and Altman procedure to calculate bias, two‐way analysis of variance | |
| Participants | Randomised: 20 in total; Intervention n = 10; Control n = 10 Age: Intervention mean = 14.1 +1.8 years; Control mean = 13.8 + 2.1 years Gender: Not given Asthma diagnosis criteria: All were known to have had recurrent reversible wheezing episodes and were required to fulfil at least three of the following criteria (1) clinical: family history of asthma or personal history of eczema, conjunctivitis, or rhinitis caused by a known allergen or both; (2) allergic: all the children had a cutaneous hypersensitivity to one or several allergens; (3) immunologic: blood IgE levels were determined by the paper radio immunoabsorbent test (4) functional: improvement 15% at least in the FEV by inhaling bronchodilator Recruitment means: Not stated Co‐morbidities included: None mentioned Participant exclusion reasons: Not stated | |
| Interventions | Setting: Outdoor track for intervention group Intervention description: The training group participated in 36 sessions (3 d/wk for 3 months) of running on an outdoor track; each session lasted 1 hour during which the children ran for 10 min, 3 times, at their own predetermined ventilatory threshold Control description: Served to determine whether testing had an effect on VO max values in the event that the training program was without effect Duration of intervention: 36 sessions; 3 days per week for 3 months Intervention delivered by: Not explicitly stated | |
| Outcomes | Pre‐specified outcomes: VOmax, Vth, HRmax, Wmax, maximum oxygen pulse Follow‐up period: 3 months | |
| Notes | Lung function testing was done for the whole sample (of all possible 48 participants) after the run‐in period and before training but not after training | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods for allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Low risk | Determination of ventilatory threshold during maximal exercise test was done independently by two reviewers without knowledge of other results or participant identities, The shuttle test was accompanied and encouraged by an investigator who did not know to which group they belonged |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective reporting (reporting bias) | High risk | Post hoc methods of comparison for the intra‐ and inter‐group comparisons were made when the analysis of variance‐F ratio was significant |
| Other bias | Unclear risk | Some potential concerns mentioned by the authors regarding outcome measurements (e.g. incidents of premature stopping of the laboratory test causing lower HR values; authors state “large number of participants performing the 20‐MST at once may have made it difficult to evaluate accurately each individual”) Baseline characteristics not given separately for the 2 groups (given together for the entire population), thus unable to assess for baseline imbalance |
| Methods | Country: United States of America Design: Randomised Controlled Trial; Parallel group proof of concept study Objective (Aim): To examine the effect of moderate intensity aerobic exercise on asthmatic responses in adult patients Study Site: University of Alabama at Birmingham Methods of analysis: Outcomes reported before and after protocol completion; Baseline characteristics compared, Paired comparisons were made using Fisher's exact test for nominal characteristics and Wilcoxon Rank Sum for continuous measures; Repeated measures analysis of variance techniques were applied to examine changes over time and to determine if the changes differed by group; Distributional properties of residuals from the repeated measures analysis of variance models were examined with only minor deviations observed for all outcomes | |
| Participants | Randomised: 19 adults Age: Intervention 53 (38‐62) years; Control 54 (33‐78) years Gender: Males and females Asthma diagnosis criteria: Mild‐moderate persistent asthma defined by the NAEPP guidelines with at least a 12% FEV1 reversibility; Physician diagnosis of asthma was also documented with evidence of reversible airflow obstruction Recruitment: By the study coordinator from the Universtiy of Alabama at Birmingham Lung Health Center's Asthma Clinical Research Database Co‐morbidities: None reported; Individuals with major illnesses were excluded Subject exclusion criteria: Individuals who smoked within six months from the start of the exercise protocol or with greater than a 10 pack year smoking history were excluded to reduce the inclusion of patients with COPD; Individuals with major illnesses including coronary artery disease, congestive heart failure, stroke, severe hypertension, immunodeficiency states or other conditions that would have interfered with participation in the study or collection of proposed outcome measures were also excluded; Individuals who were unable or unwilling to provide consent, perform the exercise protocol, provide pre‐ and post‐study measurements, be contacted via telephone or who intended to move out of the area within six months were also excluded | |
| Interventions | Setting: University of Alabama at Birmingham clinical exercise facility Intervention descriptions: 12‐week protocol of moderate intensity aerobic exercise plus usual care with a frequency of three times per week, 30 minutes each session at a steady intensity that achieved 60‐75% of maximum heart rate (HRmax); A mandated graded treadmill test was used to determine each subject's HRmax; Recommended exercise prescription included a five minute warm up, 30 minutes of steady state exercise via walking and a five minute cool‐down; In addition, study subjects randomised to the moderate intensity aerobic exercise group received a three month free membership to a local exercise facility at the time of the initial visit Control descriptions: Usual care alone (standard patient education); To control for interaction/attention within the exercise group, individuals receiving usual care also received weekly phone calls from the study coordinator; During these brief phone calls the study coordinator asked the subject how he/she was doing and if there was anything related to his/her respective program with which he/she needed assistance Duration of intervention: 12 weeks Intervention delivered by: Staff instructed subjects in the use of the heart rate monitor at the initial visit; No other information provided | |
| Outcomes | Pre‐specified outcomes: Asthma control (Juniper Asthma Control Questionniare ACQ), pro‐inflammatory targets in peripheral blood and nasal lavage (eosinophilic cationic protein, serum cytokines, peripheral blood immune cell populations), lung function parameters (FEV, FEV/FVC,) and fitness measures (VO peak, HRmax, RER, total treadmill time) Follow‐up period: 12 weeks (post‐intervention) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised mentioned as developed by a biostatistician, however methods not described |
| Allocation concealment (selection bias) | Unclear risk | Biostatistician developed permuted variable size block randomisation to allocate subjects to the two study arms, thus the block size prevented exact knowledge of the next randomisation assignment, however, no mention of specific allocation blinding or allocation methodology |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention subjects were aware of their group assignment
|
| Blinding (performance bias and detection bias) | Unclear risk | No mention of blinding for outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective reporting (reporting bias) | High risk | Data not reported in a way that can be meta‐analysed, visual representation only; Attrition reported (19 to 16), however reasons not described |
| Other bias | High risk | Abstract states that twenty adults will be recruited, yet only 19 are reported as being recruited in the primary manuscript with no explanation as to why the 20 subjects were not recruited |
| Methods | Country: Scotland Design: Randomised controlled trial with a six‐week run‐in period Objectives: To assess clinical and physiological effects of a medically supervised indoor physical training program for people with asthma Study Site: Indoor facility not explicitly defined Methods of Analysis: Students t‐test; variables adjusted for; linear association between pairs of continuous variables measured with the Pearsons coefficient of correlation; paired t‐test | |
| Participants | Randomised: 36 adults Age: Range 16 to 40 years Gender: Total population only: n = 14 male n = 22 female Asthma diagnosis criteria: Chronic asthma of mild to moderate severity as defined by a requirement for regular prophylactic treatment and reproducible airways obstruction when treatment withdrawn Recruitment means: Following initial evaluation patients were randomly assigned to intervention and control. No further details provided Co‐morbidities included: Participants were free from any concomitant illness Participant exclusion reasons: Not explicitly stated | |
| Interventions | Setting: Indoor facility not explicitly defined Intervention description: 30‐minute training sessions, 3 times a week for 3 months; educational sessions separate from the control group Control description: Attended similar but separate educational sessions to the intervention group only Duration of intervention: 30‐minute training sessions, 3 times a week for 3 months Intervention delivered by: Medical supervision was provided during all hospital training sessions; Audio tape instructions for home use were available for patients unable to attend any of the hospital sessions | |
| Outcomes | Pre‐specified outcomes: FEV, VOmax, VEmax, maximum oxygen pulse, HRmax, RR, Vth and VE/VOmax Follow‐up period: 3 months | |
| Notes | The mean number of training sessions undertaken by each patient was 36 (range was 19‐42 sessions) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Unclear risk | Outcomes (particularly FEV) may have been affected in the 9 participants whom had their treatment altered during the study period |
| Methods | Country: France Design: Randomised controlled trial with no run‐in period Objectives: To assess the effect of a training protocol on aerobic and anaerobic fitness in children with asthma Study Site: The study took place in a small city in the Pyrenees Mountains Methods of Analysis: Mann‐Whitney Wilcoxon rank test, 2‐way analysis of variance, multiple regression models | |
| Participants | Randomised: 16 total; 14 completed the study; Intervention n = 9 (completed n = 7); Control n = 7 (completed n = 7) Age: Intervention mean = 14.0 + 0.6 years; Control mean = 13.9 + 0.8 years; Range 10 to 16 years Gender: Only males in both arms Asthma diagnosis criteria: 1) personal or familial history of allergy, 2) personal history of acute wheezing 3) reversible airway obstruction documented by lung function testing i.e. improvement of 15%, at least in FEV and/or 30% in forced expiratory flow 25‐75 by inhaling a bronchodilator, 4) positive specific immunoglobulin E to inhaled allergens by a multi‐allergen allergosorbent test and/or cutaneous hypersensitivity to one or several allergens, and 5) no evidence of other lung disease Recruitment means: Through pulmonary rehabilitation clinics Co‐morbidities included: None mentioned Participant exclusion reasons: Not stated | |
| Interventions | Setting: A laboratory in France Intervention description: The training group exercised by continuous cycling 3 times weekly for 6 weeks, 45 minutes each session; The target heart rate was individualised and corresponded to the anaerobic threshold level; Training sessions were supervised Control description: Not explicitly defined Duration of intervention: 6 weeks; 3 times a week Intervention delivered by: Training instructor and a pulmonologist | |
| Outcomes | Pre‐specified outcomes: Chronic asthma of mild to moderate severity as defined by a requirement for regular prophylactic treatment and reproducible airways obstruction when treatment withdrawn Follow‐up period: Nothing beyond 6 weeks intervention period PEFR, FEV, FVC, FRC, VEmax, HRmax, VO, episodes of wheeze (days), work capacity W, FRC%, maximal aerobic power, ventilatory reserve, aerobic threshold | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Low risk | Authors state "testing was done blindly" |
| Incomplete outcome data (attrition bias) | Low risk | All incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Country: Brazil Design: Randomised controlled trial with a 2‐week run‐in period Objective: Evaluate whether exercise training would improve HQoL and reduce EIB (exercise‐induced bronchoconstriction) severity in children with moderate to severe persistent asthma. Secondly, assess the effects of training on aerobic fitness and daily use of inhaled steroids Study site: Recruited from a tertiary centre specialising in paediatric asthma ‐ study site not otherwise specified Methods of Analysis: Kolmogorov‐Smirnov test for normality; non‐paired t‐test or Mann‐Whitney test for variables with parametric and non‐parametric distributions for between group baseline comparisons. Chi² or Fisher test to evaluate between group changes in clinical and functional outcomes and response to training; Sign test to determine changes on categorical variables (e.g. level of aerobic impairment); Spearman's ranked correlation coefficient for associations between variables | |
| Participants | Randomised: 38 in total; Intervention n = 21; Control n = 17 Age: Intervention mean= 11 + 2 years; Control mean = 10 + 2 years Gender: Intervention n = 12 males/9 females; Control n = 11 males/6 females Asthma diagnosis criteria: 1) Global Initiative for Asthma (GINA) guidelines 2) under medical treatment for at least 6 months before study 3) in a stable phase of the disease, that is, without any recent disease exacerbation or change in medication usage Recruitment means: From a tertiary centre specialising in paediatric asthma Co‐morbidities included: Patients with other cardiopulmonary and/or musculoskeletal diseases were excluded Participant exclusion reasons: Patients with other cardiopulmonary and/or musculoskeletal diseases; Under medical treatment <6 months before the study; recent (15 day) exacerbations or changes in medication usage | |
| Interventions | Setting: Tertiary centre specialising in paediatric asthma (no further information provided) Intervention description: Education program: in asthma control; 2 once‐a‐week classes, each lasting 2 hours including: education video‐tape, interactive classes to clarify doubts, lessons on disease pathophysiology, use of medication (relief and maintenance), WAP of action in case of worsening of symptoms Physical training program: twice a week for 90 min during 16 wk; four parts: warm‐up/stretching, aerobic exercise, upper‐ and lower‐limb and abdomen endurance exercises, cooling down/stretching/relaxing Initial 8 sessions of PT program were a build‐up‐period in which training intensity was gradually increased. Control description: Non‐exercising control group Education program: in asthma control; 2 once‐a‐week classes, each lasting 2 hours including: education video‐tape, interactive classes to clarify doubts, lessons on disease pathophysiology, use of Rx (relief and maintenance), WAP of action in case of worsening of symptoms Duration of intervention: 16 weeks; Education program: twice a week for 2 hours; Physical training program: twice a week for 90 min Intervention delivered by: Not explicitly stated | |
| Outcomes | Pre‐specified outcomes: QoL using Paediatric asthma quality‐of‐life questionnaire (PAQLQ); pulmonary function test; incremental cardiopulmonary exercise tests (CPET); exercise challenges with post‐effort breathlessness measurements (Borg scale ‐ Dyspnoea) Follow‐up period: Four months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | “A single physician who was blinded to patient’s group allocation was in charge of the medical follow‐up.” However, it is not clear if this is follow‐up of outcomes or general follow‐up |
| Incomplete outcome data (attrition bias) | Low risk | All incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | High risk | Results for PQALQ and FEV are reported as change scores and graphically. Other results are only reported as baseline or change scores only and as such can not be meta‐analysed. |
| Other bias | High risk | Baseline imbalances between groups ‐ more intervention participants had peak VO values < 70% predicted than controls (15/21 versus 9/17, respectively P < 0.05); possible ineffective reporting mechanisms for observed reductions in corticosteroid use through increased use of rescue medication however, participants did not report increased use; possible contamination through education and written action plans in the control group which could underestimate the true effect of the intervention |
| Methods | Country: Iran Design: Randomised controlled trial Objectives: To examine the effects of a course of aerobic exercise on pulmonary function and tolerance of activity in asthma patients Study Site: Not explicitly stated Methods of Analysis: Not reported | |
| Participants | Randomised: 36 in total; Intervention n = 18; Control n = 18 Age: Intervention mean = 27 years; Control mean = 29 years Gender: Intervention n = 8 males/10 females; Control n = 8 males/10 females Asthma diagnosis criteria: Confirmation by investigator using clinical examinations, pulmonary function tests, skin prick test for aeroallergen and 6‐minute walk test Recruitment means: Allergy clinic Co‐morbidities included: None mentioned Participant exclusion reasons: None explicitly stated | |
| Interventions | Setting: Not explicitly stated Intervention description: Aerobic exercise plan, 15 minutes of warming up and tensile exercise before 20 minutes of aerobic practice. No further description provided Control description: No plan of exercise Duration of intervention: Three sessions a week for 8 weeks Intervention delivered by: Not reported | |
| Outcomes | Pre‐specified outcomes: Spirometry (FEV, FVC, FEV/FVC, PEF, FEF 25%‐75%, MVV); RF (respiratory frequency) and 6‐minute Walk Test (6MWT) Follow‐up period: Two months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors state "The patients were randomly put into two groups" but no further details provided |
| Allocation concealment (selection bias) | Unclear risk | Details not provided |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Details not provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Unclear risk | Few methodological details are reported which make it difficult to determine whether the study might have had other problems leading to bias |
| Methods | Country: Canada Design: Randomised controlled trial with a run‐in period Objectives: To examine effects of deep diaphragmatic breathing in terms of symptomatic and behavioural characteristics of asthma patients Study Site: Not explicitly stated Methods of Analysis: ANOVA | |
| Participants | Randomised: 67 were randomly allocated to one of three groups Age: Mean age varied from 28‐33 years Gender: Not stated Asthma diagnosis criteria: Doctor's diagnosis Recruitment means: Media solicitations for people with asthma to volunteer for an experimental breathing study yielded 274 respondents; 150 eliminated, eventually 92 volunteers remained; of these 67 were randomly allocated to one of three groups Co‐morbidities included: Not explicitly stated Participant exclusion reasons: history of allergies, asthma so severe as to preclude participation, chest disease or diabetes, or inability to make a 26‐week commitment to the program | |
| Interventions | Setting: Not explicitly stated Intervention description: No details provided in published paper, written to author for information; Intensity level not mentioned Control description: Wait‐list control Duration of intervention: 16 weeks Intervention delivered by: Group DDB1 was led by a woman trainer; group DDB2 was taught by a 25‐year‐old man who had asthma and obtained significant therapeutic benefits from the use of the technique himself; group PE was led by a female medical student also experienced in physical education. No details provided in published paper, Intensity level not mentioned | |
| Outcomes | Pre‐specified outcomes: Symptomatic and behavioural characteristics including medication use, frequency and intensity of asthma symptoms, asthma symptom checklist, physical activity inventory Follow‐up period: 6 months No details provided in published paper | |
| Notes | We ignored the deep diaphragmatic breathing data and only used the control and physical training data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Details not provided |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Details not provided |
| Incomplete outcome data (attrition bias) | High risk | No details provided for attrition of participants, larger number of dropouts from the intervention group |
| Selective reporting (reporting bias) | High risk | Post hoc analysis was conducted for 'time spent in physical activities between groups' |
| Other bias | Unclear risk | No mention of participant comparability between groups for characteristics or outcomes at baseline |
| Methods | Country: Brazil Design: Randomised controlled trial Objectives: To evaluate the role of an aerobic physical training program on psychosocial characteristics, QOL, symptoms and exhaled nitric oxide in individuals moderate or severe asthma Study Site: Not stated Methods of Analysis: ANOVA, Student t‐test, Kolmogorov‐Smirnov test | |
| Participants | Randomised: 23 total; 20 completed the study; Intervention n = 11 (completed n = 10); Control n = 12 (completed n = 10) Age: (Only for those participants completing the study) Intervention median = 34.6 years (95% CI 21.0 ‐ 47); Control median = 34.6 years (95% CI 21.0 ‐ 47.0) Gender: Intervention n = 3 males/7 females; Control n = 4 males/6 females Asthma diagnosis criteria: Global Initiative for Asthma (GINA) guidelines Recruitment means: Recruited after a medical consultation; no details provided Co‐morbidities included: None reported | |
| Interventions | Setting: Not reported Intervention description: Education programme: Four hour education program which comprised of 2 interactive classes which aimed at explaining disease physiopathology, correct use of medications, and an action plan in case of worsening symptoms Respiratory exercise program: Commenced the week after education program; 30 min bi‐weekly yoga over 3 months. Aerobic conditioning programme: The program started the week after the education program, 30 min bi‐weekly aerobic training on a treadmill. The training intensity was 70% of maximum power obtained in the cardiopulmonary effort test carried out before the beginning of training Control description: Same as for intervention for education program and respiratory exercise program only Duration of intervention: 12 weeks; 30 min bi‐weekly respiratory exercise program and 30 min bi‐weekly aerobic conditioning program for 12 weeks Intervention delivered by: Not reported | |
| Outcomes | Pre‐specified outcomes: Pulmonary function, Maximum aerobic capacity, QoL (used a 4 domain QoL Questionnaire, QQL‐EPM), anxiety and depression levels, asthma symptoms, exhaled nitric oxide levels Follow‐up period: 12 weeks (nothing beyond 12‐week intervention period) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods for allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) | Low risk | All incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | Unclear risk | All stated outcomes were addressed, however, protocol was not available to us |
| Other bias | High risk | Participants within this study may be a subset of those participating in the Mendes 2010 and Mendes 2011 studies, for this reason outcomes reported across these three studies have not been pooled together in meta‐analyses |
| Methods | Country: Japan Design: Randomised controlled trial Objectives: To assess effects of swimming training on aerobic capacity and exercise‐induced bronchoconstriction and bronchial responsiveness to inhaled histamine in children with bronchial asthma Study Site: Not explicitly stated Methods of Analysis: Paired t‐tests to detect differences within a group, Unpaired t‐tests were used to detect differences between groups | |
| Participants | Randomised: 16 in total; Intervention n = 8; Control n = 8 Age: Range 8 to 12 years; Intervention mean = 10.5 years; Control mean = 9.9 years Gender: Intervention males n= 7, females n = 1; Control males n = 7, females n = 1 Asthma diagnosis criteria: ATS criteria Recruitment means: Children admitted to hospital for treatment of asthma were recruited Co‐morbidities included: Not described Participant exclusion reasons: Not explicitly stated | |
| Interventions | Setting: Heated (30°C) indoor pool Intervention description: Training took place for 6 weeks in a heated indoor pool for 2 periods of 15 minutes on 6 days each week; A 10 minute break was taken between the two 15 minute training periods; A swimming ergometer was used to assess work rate and corresponding heart rate at 125% of the lactate threshold; the training intensity was set to 125% of the lactate threshold for each participant individually. Control description: Not explicitly defined; assumed no intervention Duration of intervention: 6 weeks; 30 min each day for 6 days a week Intervention delivered by: Not stated A swimming ergometer was used to assess work rate and corresponding heart rate at 125% of the lactate threshold; The training intensity was set to 125% of the lactate threshold for each participant individually; Training took place for 6 weeks in a heated indoor pool for 2 periods of 15 minutes on 6 days each week; A 10 minute break was taken between the two 15 min training periods; Training intensity was increased as necessary to remain at 125% of the lactate threshold | |
| Outcomes | Pre‐specified outcomes: Aerobic capacity ( work load at LT), exercise‐induced bronchoconstriction, histamine responsiveness Follow‐up period: 6 weeks Aerobic capacity of the participants in both training and control groups was assessed again after the training period; Histamine responsiveness was also reassessed; Outcomes include change in work load during cycle test, % fall in FEV during swimming and cycle tests, changes in concentrations of histamine required to provoke a fall in FEV of 20% or more | |
| Notes | The mean duration of swimming training was 31.4 days (SD 3.2) and the mean distance swum per day was 851.5m (SD 52.2). The mean total distance achieved during the entire training period was 26,675m (SD 2827.6). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, methods not described |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Low risk | All incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | High risk | Data presented in a way which cannot be meta‐analysed |
| Other bias | Unclear risk | Insufficient information to permit judgement of yes or no |
| Methods | Country: Brazil Design: Randomised controlled trial Objectives: To evaluate the effects of an aerobic training programme on asthma‐specific HRQoL and anxiety and depression scores and asthma symptoms in patients with moderate or severe asthma Study Site: Hospital clinics, School of Medicine, University of Sao Paulo, Brazil Methods of Analysis: Kolmogorov‐Smirnov test to evaluate normality; Mann‐Whitney U test to compare baseline nonparametric data; Chi2 test for gender and bronchodilator response at baseline. Two‐way repeated measure analysis of variance followed by a Holm‐Sidak post hoc test for HRQoL and asthma symptoms; McNemar test for anxiety and depression; Spearman test for linear correlation analysis | |
| Participants | Randomised: 101 total; 89 completed the study; Intervention n = 50 (completed n = 44); Control n = 51 (completed n = 45) Age: (Only for those participants completing the study) Intervention median = 39 years (95% CI 22.0 ‐ 47.9); Control median = 39.5 years (95% CI 23.5 ‐ 47.0) Gender: Intervention n = 5 males/ 39 females; Control n = 10 males/35 females Asthma diagnosis criteria: Global Initiative for Asthma (GINA) guideline (moderate or severe persistent asthma) Recruitment means: Recruited at a University Hospital (University of Sao Paulo) after a medical consultation Co‐morbidities included: None reported Participant exclusion reasons: Cardiovascular, pulmonary or musculoskeletal disease that would impair exercise training | |
| Interventions | Setting: Not reported Intervention description: Four‐hour education program which included the teaching of breathing exercises. Education programme: based on a videotape 'ABC of Asthma' which included information about asthma pathophysiology, medication skills, self‐monitoring techniques, and environmental control and avoidance strategies. Patient doubts were elucidated with an interactive discussion. Breathing exercises: based on yoga. Included were Kapalabhati (fast expiratory breathing followed by passive inhalation); Uddhiyana (full exhalation followed by a forced inspiration performed without air inhalation (apnoea)) and Agnisara (full exhalation followed by a sequence of retractions and protrusions of the abdominal wall in apnoea) Control description: Four hour education program which included the teaching of breathing exercises described above for the intervention description. Did not take part in the aerobic training programme. Duration of intervention: 12 weeks; Education programme: two classes held once a week, each 2 hours; Breathing exercise programme: 30 minute session performed twice a week for 3 months; Aerobic training programme: 30 minutes per session, twice a week for 12 weeks Intervention delivered by: Not reported | |
| Outcomes | Pre‐specified outcomes: Asthma specific HRQoL (used a 4 domain QoL Questionnaire, QQL‐EPM); anxiety and depression scores; asthma symptoms; spirometry FEV, FVC, VOMax Follow‐up period: Three months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | High risk | Rehabilitation program and evaluation of outcomes done by same investigators; No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for missing data are described in general terms only. No mention of any missing outcome data or how they would be handled |
| Selective reporting (reporting bias) | High risk | Data for VOMax given only in graphic forms, full data for asthma free days not presented, therefore, could not be meta‐analysed |
| Other bias | High risk | Participants within this study may be a subset of those participating in the Gonçalves 2008 and Mendes 2011 studies, for this reason outcomes reported across these three studies have not been pooled together in meta‐analyses |
| Methods | Country: Brazil Design: Randomised controlled trial Objectives: To evaluate the effects of an aerobic training program on eosinophil inflammation (primary aim) and nitric oxide (secondary aim) in patients with moderate or severe persistent asthma Study Site: University of Sao Paulo Hospital, Brazil Methods of Analysis: Statistical power normality evaluated by Kolmogorov‐Smirnov test and presented as medians and 95% confidence intervals. The Mann‐Whitney test was used to compare non‐parametric data and the Chi2 test to evaluate gender and bronchodilator response between groups at baseline. A two‐way repeated measures ANOVA followed by a Holm‐Sidak post hoc test used to measure induced sputum cellularity, FeNO and other outcomes | |
| Participants | Randomised: n= 68; Intervention n = 34, Control n = 34; Completed: Intervention n = 27, Control n = 24. Age: Range 20 to 50 years; Intervention median = 37.9 (25.7 ‐ 47.3) years; Control median = 36.0 (22.0 ‐ 47.5) years Gender: Intervention n = 24 female, n = 3 male; Control n = 18 female, n = 6 male Asthma diagnosis criteria: Global Initiative for Asthma (GINA) guidelines Recruitment means: Recruited at a University hospital – no other information provided Co‐morbidities included: Not specified, though patients were under medical treatment for at least 6 months and were considered clinically stable Participant exclusion reasons: Patients diagnosed with cardiovascular, pulmonary, or musculoskeletal diseases that would impair exercise training were excluded | |
| Interventions | Setting: University hospital – Sao Paulo: between two medical consultants Intervention description:Educational program: consisting of two classes (once a week) lasting 2 hours. The core activity was based on an educational videotape titled the ‘ABC of Asthma’. Interactive discussions to address patient’s doubts also occurred. Breathing exercises (yoga): including Kapalabhati (fast expiratory breathing followed by passive inhalation), Uddhiyana (full exhalation followed by forced inspiration without air inhalation (apnoea)) and Agnisara (full exhalation followed by a sequence of retractions and protrusions of the abdominal wall). This was performed as 30 minute sessions, twice a week for 3 months. Exercises were executed in sets of three with 2 minutes of exercise with 60 seconds of rest. Aerobic training program: involving indoor treadmill training for 30 minutes twice a week for 3 months. Exercises intensity started at 60% of VO2max and increased by 5% of cardiac frequency until a maximum of 80% maximal cardiac frequency Control description: Same as the educational program and breathing exercise (yoga) above Duration of intervention: 2 weeks; Education programme: two classes held once a week, each 2 hours; Breathing exercise programme: 30 minute session performed twice a week for 3 months; Aerobic training programme: 30 minutes per session, twice a week for 12 weeks Intervention delivered by: Not specified | |
| Outcomes | Pre‐specified outcomes: Induced sputum for eosinophil cell count, fractional exhaled nitric oxide (FeNO), pulmonary function, cardiopulmonary exercise testing, asthma symptom‐free days and asthma exacerbations Follow‐up period: Three months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | High risk | Sputum eosinophil counts and FeNO levels were determined by a blinded investigator but pulmonary function and cardiopulmonary tests were conducted by the same investigator in charge of aerobic training |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of how incomplete outcome data were addressed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | High risk | 26 of 68 participants within this study are a subset of those participating in the Mendes 2010 study. In addition, there is a possibility that participants in the Gonçalves 2008 study are also a subset of those in Mendes 2010. For this reason outcomes reported across these three studies have not been pooled together in meta‐analyses |
| Methods | Country: Portugal Design: Randomised controlled trial Objectives: To determine a rationale for exercise and sporting guidance for children and their parents Study Site: Outpatient clinic of University Hospital Sao Joao, Porto, Portugal Methods of Analysis: Fisher's exact test for categorical variables, unpaired t‐test for numerical variables, changes within groups were compared using a paired t‐test, differences between the exercise and control groups were compared by ANCOVA, with the baseline value as covariate | |
| Participants | Randomised: 34 total; 32 completed the study; Intervention n = 17 (completed n = 16); control n = 17 (completed n = 16) Age: Intervention mean = 12.9 + 3.4 years; Control mean = 12.5 + 3.5 years Gender: Intervention n = 11 males/6 females; Control n = 9 males/8 females Asthma diagnosis criteria: "...controlled asthma, treated with a small‐to‐moderate dose of inhaled corticosteroids (ICSs) for a period of > 1 yr and followed in the outpatient clinic University Hospital of Sao Joao..." Recruitment means: Outpatient clinic of University Hospital Sao Joao, Porto, Portugal Co‐morbidities included: Not specified Participant exclusion reasons: Not explicitly stated | |
| Interventions | Setting: Indoor gymnasium Intervention description: Twelve week, bi‐weekly 50 minute sessions of submaximal aerobic exercise designed as moderately intensive training programme including both lower and upper extremity activities. Typical session consisted of warm‐up (10 minutes) with arm and leg exercise, submaximal training (30‐35 minutes) including aerobic exercises, strength training, and some balance and coordination exercises, and a cool‐down period (7‐10 minutes) Control description: Continued usual daily routine Duration of intervention: Twelve weeks; bi‐weekly for 50 minutes Intervention delivered by: Not specified in methods through in discussion it states "...supervised by health professional..." | |
| Outcomes | Pre‐specified outcomes: Lung volumes (PEF) and bronchial responsiveness to methacholine, Paediatric Asthma Quality‐of‐Life Questionnaire and Paediatric Asthma Caregiver's Quality of Life Questionnaire (PAQLQ and PACQLQ), Exhaled nitric oxide (eNO), CRP levels (C‐reactive protein), Physical activity measure using an Actigraph monitor Follow‐up period: Three months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blinded computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Allocation numbers were encoded on labels placed in each case report form by an outside researcher, and patients were assigned the next available allocation number in sequence |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | No mention of attempted blinding for outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | All incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Unclear risk | Insufficient information to permit judgement of yes or no |
| Methods | Country: Brazil Design: Randomised controlled study with three arms (2 intervention arms combined for this analysis) Objectives: To investigate whether time of the day influences the effects of physical exercise training for children with asthma Study Site: Not explicitly stated, but included an outdoor swimming pool Methods of Analysis: Normality of data distribution determined by Kolmogorov‐Smirnov test, Intra‐group and between group comparisons made by ANOVA followed by the Tukey‐Kramer multiple comparisons test | |
| Participants | Randomised: 69 in total; Morning intervention n = 23; Afternoon intervention n = 23; Control n = 23. Age: Morning intervention mean = 9.5 + 0.2 years; Afternoon intervention n = 9.2 + 0.2 years Control mean = 9.5 + 0.2 years Gender: Morning intervention n = 12 males/11 females; Afternoon intervention n = 12 males/11 females; Control n = 11 males/12 females Asthma diagnosis criteria: Physician diagnosed asthma according to GINA guidelines Recruitment means: Not reported Co‐morbidities included: None mentioned Participant exclusion reasons: Use of oral steroids in the previous 8 weeks, physical disability, and other pulmonary or systemic disease | |
| Interventions | Setting: Not reported other than for the swimming which took place in an outdoor pool Intervention description: Twice‐weekly 90 min sessions over 4 months, morning training group and the afternoon training group followed the same training program Circuit training 45 min: first 5 min no running allowed (to avoid triggering EIB). Tasks were walking for 5 min, running for 10‐15 min, Upper and lower limb exercises, training on a bar, Individual and team games, postural and stretching exercises followed by swimming pool exercises 45 min Control description: Non training group received regular asthma treatment and education Duration of intervention: Twice‐weekly 90 min sessions for 4 months Intervention delivered by: A physical educator and a physiotherapist | |
| Outcomes | Pre‐specified outcomes: 9 min running distance, resting heart rate, spirometry, exercise challenge tests, abdominal muscle strength (number of sit‐ups in 60 seconds) Follow‐up period: Nothing beyond the 4 months intervention period | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods for allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Low risk | No other biases identified |
| Methods | Country: United Kingdom Design: Randomised controlled trial with a run‐in period Objectives: To assess the effect of a physical training program on children with asthma, known to have exercise‐induced bronchoconstriction Study Site: An asthma clinic, no other details provided Methods of Analysis: Not stated | |
| Participants | Randomised: n = 27 children; only n = 21 completed the study Age: Control range: 7‐14 years (mean 10.3); Intervention range 8‐13 years (mean 11.1) Gender: Not stated Asthma diagnosis criteria: Not explicitly defined; assumed doctor diagnosis Recruitment means: Children attending the asthma clinic with proven exercise‐induced bronchospasm (> 20% fall in PEFR after exercise) Co‐morbidities included: None stated Participant exclusion reasons: n = 5 children dropped out of the relaxation group; details not provided | |
| Interventions | Setting: Not explicitly stated Intervention description: Graduated physical training program twice per week and repeated daily at home: warm‐ups, squat thrusts, star jumps, sit‐ups and press‐ups; exercise loads were increased at each session Control description: Relaxation classes supervised by the same physiotherapist once per week for three months Duration of intervention: Twice a week for 3 months Intervention delivered by: Sessions were supervised twice weekly by paediatric physiotherapist | |
| Outcomes | Pre‐specified outcomes: Daily PEFR, % fall in PEFR after exercise, asthma symptom scores Follow‐up period: 3 months | |
| Notes | All children were given sodium cromoglycate by Spinhaler, 15 minutes before exercise. Wrote to author to find out the duration of the training, received no reply to date | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind study mentioned, however who was actually blinded was not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind study mentioned, however who was actually blinded was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Larger number of drop‐outs from the control group (5/12), no characteristics or results provided for attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Unclear risk | Statistical methods not described |
| Methods | Country: Australia Design: Randomised controlled trial with 3 week run‐in period Objectives: To investigate whether exercise training improves functional capacity and QOL in middle‐aged and older adults with fixed airway obstruction asthma Study Site: Physiotherapy department of Sir Charles Gairdner Hospital Methods of Analysis: Unpaired t‐tests, Mann‐Whitney tests, chi squared analysis, ANOVA | |
| Participants | Randomised: 35 in total; Intervention n = 20; Control n = 15 Age: Intervention mean= 65.3 + 10.8 years; Control mean = 71.0 + 9.7 years Gender: Intervention n= 8 males, n = 11 females; Control n = 7 males, n = 8 females Asthma diagnosis criteria: Diagnosed by respiratory physician based upon reported patterns of disease variability, trigger factors, atopy and responsiveness to medications. In addition moderate/severe asthma with fixed airflow obstruction was defined by at least two of the following criteria; FEV < 80% predicted, FEV/FVC < 80% of predicted or RV > 120% predicted Recruitment means: Through a metropolitan hospital and private clinic where patients were managed by either of 2 respiratory physicians Co‐morbidities included: None reported Participant exclusion reasons: Co‐existing respiratory conditions, respiratory tract infection in the previous 4 weeks, current smoker or ex‐smokers who ceased within the previous 2 yrs, smoking history > 15 yrs, co‐morbid conditions likely to reduce exercise capacity, current participation in a > 30 min/day of moderate or vigorous exercise, participation in a Pulmonary Rehabilitation Program in the previous 12 months | |
| Interventions | Setting: Physiotherapy Department Intervention description: Three 80‐90 min exercise classes each week for 6 weeks, this consisted of 10‐15 min warm‐up, 20 min walking training, 5‐10 min cool‐down period followed by exercise circuit comprising 10 min cycle ergometry training, approximately 45 min step‐ups, wall squats, and upper limb endurance training Control description: Standard medical care only Duration of intervention: Three 80‐90 min sessions a week for 6 weeks Intervention delivered by: Supervised by physiotherapists | |
| Outcomes | Pre‐specified outcomes: Health‐related QOL (self complete version of the AQLQ), functional exercise capacity, health status (SF‐36), (6MWT), health status (SF‐36), anxiety and depression, peripheral muscle strength, asthma control Follow‐up period: 3 months post‐6 weeks intervention period | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using http://www.randomizer.org into the two groups |
| Allocation concealment (selection bias) | Low risk | An independent researcher allocated participants at time of consent; investigators and patients were blinded to allocation until after the 3 weeks run‐in period |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | High risk | Author states blinding was not possible for outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete or missing outcome data were replaced using the last observation carried forward method |
| Selective reporting (reporting bias) | Unclear risk | Some post hoc analysis was performed |
| Other bias | Unclear risk | Authors mention some potential contamination of control groups as some individuals stated they had been exercising more than regularly, however the effect of this is unknown |
| Methods | Country: the Netherlands Design: Randomised controlled trial Objectives: To evaluate the effects of a physical exercise programme for children with asthma on an outpatient basis Study Site: Heideheuvel Asthma Centre in Hilversum Methods of Analysis: Chi2 test, analysis of variance (ANOVA), multivariate analysis of variance (MANOVA) | |
| Participants | Randomised: 47 in total; Intervention n = 23; Control n = 24 Age: Range 8 to 13 years Gender: Intervention male n = 16, female n = 7; Control male n = 18, female n = 6 Asthma diagnosis criteria: Severity of asthma was diagnosed using the questionnaire of the classification of the Dutch Central Advisory Committee for Peer Review Recruitment means: Through an asthma centre (n = 9), following an advertisement in a local paper (n = 19), and from a special school (n = 20) Co‐morbidities included: None mentioned Participant exclusion reasons: One child in the experimental group dropped out because of a physical problem not related to asthma and was omitted from further analysis | |
| Interventions | Setting: Not explicitly stated. Intervention description: The 3 month exercise programme consisted of group exercises twice a week for one hour in a gymnasium and one 20 minute exercise session per week at home. The gym sessions started with 10 minutes warming up, 20 minutes of fitness training then 15‐20 minutes different physical activities followed. The training group also received information about asthma and exercise to improve coping behaviour with asthma Control description: Children in the control group did not receive an extra care or treatment Duration of intervention: 3 months Intervention delivered by: Not stated | |
| Outcomes | Pre‐specified outcomes: Exercise test (Wmax, VE, VO, Vco, O pulse), Psychosocial indices (Self Perception Profile for children ‐ CBCK), Asthma Coping Test (ACBT), PEFR, FEV, FVC, EIB; A maximum incremental exercise test was used to determine maximum workload, HR, minute ventilation, oxygen uptake, CO production and a treadmill endurance test at submaximal heart rate was also taken; A translated version of the Self‐Perception Profile for Children was used to measure perceived competence and the Asthma Coping Test was also administered Follow‐up period: 3 months | |
| Notes | Assessments took place immediately before and after the intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods for allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) | Low risk | All incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | Unclear risk | All stated outcomes were addressed, however protocol was not available |
| Other bias | Unclear risk | Insufficient information to permit judgement of yes or no |
| Methods | Country: France Design: Randomised controlled trial Objectives: To determine the impact of different individualised training intensities on cardiorespiratory fitness, and beyond that, on the underlying disease in children with asthma Study Site: Not explicitly stated Methods of Analysis: Unpaired and paired Student t‐test, two way and one way analysis of variance, Hortogonal contrast method Randomised controlled trial with no run‐in period. Authors update: randomisation was ensured by drawing lots | |
| Participants | Randomised: 14 in total; Intervention n = 7; Control n = 7 Age: Intervention mean 11.4 + 1.8 years; Control mean 11.4 + 1.5 Gender: Intervention male n = 6, female n = 1; Control male n = 6, female n = 1 Asthma diagnosis criteria: All 14 participants were known to have recurrent reversible wheezing episodes and were required to fulfil at least 3 of the 4: clinical, allergic, immunological or functional (improvement of > 15% in FEV after bronchodilator) criteria Recruitment means: Not stated Co‐morbidities included: None mentioned Participant exclusion reasons: None mentioned | |
| Interventions | Setting: Indoor swimming pool for intervention group Intervention description: Indoor swimming pool training, twice a week for 3 months. Each session lasted for an hour (i.e. 10 minutes on and 10 minutes off) Control description: Not explicitly stated, assumed no intervention Duration of intervention: 3 months; twice a week 1 hour sessions Intervention delivered by: Training was supervised by a physical education teacher | |
| Outcomes | Pre‐specified outcomes: Clinical benefit (wheezing attack frequency, any modification observed by the parents), exercise test (VOmax, ventilatory threshold Vth), FEV, FVC Follow‐up period: 6 months | |
| Notes | Study had 2 stages and went for 6 months but we only used the first 3 months data, because the second 3 months was specialised high intensity training | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was ensured by drawing lots as per author update |
| Allocation concealment (selection bias) | Unclear risk | Methods for allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) | Low risk | All incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | High risk | Incomplete reporting of outcome data (exercise testing outcome), data reported incompletely and could not be meta‐analysed |
| Other bias | Unclear risk | Potential bias with the study design. Unsure if intervention program could have different effects due to the different training programs |
| Methods | Country: France Design: Randomised controlled trial Objectives: To determine whether individualised aerobic training at ventilatory threshold decreases the exercise hyperventilation and modifies breathing pattern at all exercise intensities Study Site: Not stated Methods of Analysis: ANOVA, contrast method, stepwise regression with a ridge procedure, Bland and Altman procedure | |
| Participants | Randomised: 18 in total; Intervention n = 9; Control n = 9 Age: Intervention mean=1 0.3 years; Control mean = 11.7 years Gender: Intervention males n = 7, females n = 2; Control males n = 7, females n = 2 Asthma diagnosis criteria: Participants included presented a functional improvement of 15% at least in FEV by inhaling a bronchodilator. In addition all participants were required to fulfil: clinical, allergic and immunological criteria Recruitment means: Not reported Co‐morbidities included: None mentioned Participant exclusion reasons: None mentioned | |
| Interventions | Setting: Indoor swimming pool for the intervention group Intervention description: Twice a week for 3 months with each session lasting for total 30 minutes for an hour (i.e. 10 minutes on and 10 minutes off). Individualised training intensity used during study for each participant Control description: Not explicitly stated, assumed no intervention Duration of intervention: 3 months Intervention delivered by: Physical education teacher | |
| Outcomes | Pre‐specified outcomes: Exercise testing (VOmax and Vth) Follow‐up period: 3 months | |
| Notes | Authors update: randomisation was ensured by drawing lots. Authors update: age range in both groups was 9‐13 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation ensured by drawing lots (according to contact with authors by original review authors) |
| Allocation concealment (selection bias) | Unclear risk | Methods for allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Unclear risk | Insufficient information to permit judgement of yes or no |
| Methods | Country: Taiwan Design: Randomised controlled trial Objectives: To investigate the benefits of a 6 week swimming intervention on pulmonary function tests and severity of asthma in children Study Site: Intervention in outdoor swimming pool, study site not otherwise specified Methods of Analysis: Two‐tailed students t‐test for differences in continuous variables between groups Chi2 test for differences in categorical variables between groups | |
| Participants | Randomised: 30 in total; Intervention n = 15; Control n = 15 Age: Intervention mean = 10 (range 9 ‐ 11) years; Control mean = 10 (range 9‐11) years Gender: Intervention n = 10 males/5 females; Control n = 10 males/5 females Asthma diagnosis criteria: ATS criteria Recruitment means: Not stated Co‐morbidities included: None mentioned Participant exclusion reasons: Not stated | |
| Interventions | Setting: Outdoor swimming pool for intervention group Intervention description: 10 min warm‐up including breathing exercise in water, 30 min swimming training (beginners ‐ kicking, experienced swimmers ‐ freestyle and breaststroke); Physical work capacity was set at 65% of peak heart rate, 10 min cool‐down; Regular treatment for asthma continued unchanged Control description: Received no specific treatment, regular treatment for asthma continued unchanged Duration of intervention: Three 50 min sessions a week for 6 weeks Intervention delivered by: Supervised by certified swimming instructors | |
| Outcomes | Pre‐specified outcomes: PFT (FEV, FVC, FEV/FVC, FEF50,FEF25‐75), daily PEF, daily assessment of severity of asthma (NHLBI criteria) Follow‐up period: Nothing beyond the 6 week intervention period | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods for allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective reporting (reporting bias) | High risk | Numerical outcome data for severity of asthma not presented, only P values provided, therefore, cannot be meta‐analysed |
| Other bias | Unclear risk | Author states the swimming group may have greater compliance with controller medications leading to improvement |
| Methods | Country: USA Design: Randomised controlled trial Objectives: To determine whether swimming improved symptoms and PFTs in children with asthma Study Site: Not stated Methods of Analysis: Not stated | |
| Participants | Randomised: 26 children; Completed intervention n = 5, control n = 3 Age: Total population only: range 7 to 14 years Gender: Intervention male n = 3, female n = 2; Control male n = 1, female n = 2 Asthma diagnosis criteria: Criteria for inclusion was moderate persistent asthma according to symptom criteria and a need for preventive daily asthma therapy Recruitment means: From the Medical College of Georgia Pediatric Pulmonary, Allergy/Immunology, and General Pediatric clinics Co‐morbidities included: Participants excluded for other co‐morbidities that would make swimming unsafe or complicate the analysis of their performance Participant exclusion reasons: Eight children could not be reached after consent because of disconnected phones and never began the study. Two children performed initial PFTs and completed swim lessons but missed follow‐up PFTs and could not be reached to reschedule. One child in the control group performed initial PFTs and was then lost to follow‐up. One child withdrew because of a conflict with a school sport. Three children withdrew secondary to transportation difficulties. One child had to reschedule his swimming lessons to a date that would not be finished in time to be included in this analysis. Two in the control group were excluded from analysis due to a change in their asthma therapy. Presence of other co‐morbidities that would make training unsafe or difficult, asthma therapy changed during trial duration or if an exacerbation occurred during the trial period. Children were also excluded from the study if they did not attend at least 80% of the lessons. However, all children assigned to the swim group met this attendance requirement. | |
| Interventions | Setting: Family swimming school Intervention description: Swimming training according to the child's ability. Lessons were conducted twice per week for 5 to 6 weeks, depending on the time of the year, for 45 minutes each Control description: Not explicitly stated; "...an equivalent observation period for the control group" Duration of intervention: 6 weeks Intervention delivered by: Certified swimming instructors Swimming training according to the child's ability; Lessons were conducted twice per week for 5 to 6 weeks, depending on the time of the year, for 45 minutes each; Lessons were taught by certified swimming lesson instructors who were not aware of the child's involvement in the study. Children were excluded from the study if the did not attend at least 80% of the lessons, however, all children assigned to the swim group met this attendance requirement | |
| Outcomes | Pre‐specified outcomes: FEV, FVC, PEFR, child's asthma score Follow‐up period: 6 weeks | |
| Notes | Randomisation was conducted using random number table | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation conducted using a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Investigators unaware as to order of randomisation |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) | High risk | Significantly large number of dropouts for total population (12/26) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | High risk | Baseline imbalances (for height and FVC); Methods of analysis not disclosed; authors state intervention may not have had the intensity and duration to demonstrate a significant result |
| Methods | Country: Brazil Design: Randomised prospective controlled trial Objectives: To investigate the medium‐term benefits of a swimming program in school children and adolescents with moderate persistent atopic asthma and to assess and compare spirometric parameters and bronchial hyper‐responsiveness in two groups of children and adolescents Study Site: Pulmonary physiology laboratory (Laboratrio de Fisiologia Pulmonar, LAFIP) of the Hospital de Clínicas da Universidade Estadual de Campinas (UNICAMP) Division of Pediatric Pulmonology Methods of Analysis: Chi2, Wilcoxon, Mann‐Whitney and Spearman’s rank correlation tests were performed; Categorical study variables for sample profiles were presented in frequency tables (absolute values and percentages) and as descriptive statistics (mean, standard deviation, minimum, maximum and medial vales) for continuous data; Progression of variables in both groups pre and post‐treatment were analysed using the Wilcoxon signed‐rank test; Mann‐Whitney U test was used for comparing participant ages, anthropometric parameters, FEV1 and PC20 between the groups; Spearman’s rank correlation coefficient was used for analysis of the relationship between numeric variables | |
| Participants | Randomised: 71 in total with 61 completing (30 in the swimming group and 31 in the control) Age: Intervention group mean 10.35 years + 3.13; Control group mean 10.90 years + 2.63 Gender: Intervention group 18 females and 12 males; Control group 16 females and 15 males Asthma diagnosis criteria: All participants had moderate persistent atopic asthmas diagnosed according to Global Initiative for Asthma (GINA) criteria and a clinical history of reversible, recurrent symptoms of airway obstruction Recruitment means: Patients from the Hospital de Clínicas da Universidade Estadual de Campinas (UNICAMP) Division of Pediatric Pulmonology Co‐morbidities included: None reported Participant exclusion reasons: Children who did not attend at least 80% of the classes were excluded | |
| Interventions | Setting: Swimming pool and hospital Intervention description: Swimming sessions lasted 60 minutes over a period of three months, twice weekly producing a maximum of 24 lessons; Before exercise children underwent peak expiratory flow to detect any possible bronchial obstruction at time of swimming; This was followed by light stretching exercises, lower and upper limb warm‐ups with global postural exercises and awareness of diaphragmatic breathing whilst subjects were lying on mats (approximately 15 minutes); Subjects were then taken to the pool where training was divided by skill level being: Level 1 – adaptation to the water environment, total immersion breathing, floating/treading water, moving underwater and elementary diving; Level 2 – Where children had already acquired the aforementioned skills and mastered body control in the water; Lessons included mastering the front crawl and backstroke Control description: Control group of non‐swimmers Duration of intervention: 12 weeks Intervention delivered by: Trained by an instructor however details not reported | |
| Outcomes | Pre‐specified outcomes: Demographics, spirometry, bronchial challenge with methacholine, allergy skin testing and serum IgE measurement Follow‐up period: Post‐test 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, however, methods not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding (performance bias and detection bias) | High risk | Due to the nature of the intervention it was not possible to blind participants
|
| Blinding (performance bias and detection bias) | Unclear risk | No mention of attempted blinding for outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition with reasons reported, however, it is unclear if there was any missing data and if so how it was addressed |
| Selective reporting (reporting bias) | High risk | Pre‐specified protocol not available; Data reported in a way that cannot be meta‐analysed such as number of exacerbations in each group |
| Other bias | Low risk | No other biases identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Quasi‐experimental study only; not randomised | |
| Intervention group had swimming plus education through an asthma school that the control group did not receive | |
| Quasi‐experimental study only; not randomised ‐ participants were 'randomised' based on admission order | |
| Study does not included physical training using whole body | |
| Quasi‐experimental study only; not randomised | |
| Inadequate control group ‐ both groups received physical exercise | |
| Inadequate control group ‐ both the groups were trained and the only difference was the intensity of training with no difference in duration or frequency of training | |
| Study included a composite intervention and included both participants with asthma and COPD. A physiotherapist run program included breathing retraining, mucus evacuation and exercise | |
| Quasi‐experimental study only; not randomised | |
| Data for asthma patients not separately reported | |
| Intervention duration too short, being only for 5 days | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised | |
| Intervention duration too short, participants only exercised once per week | |
| Inadequate control group ‐ follow‐up analysis where either all groups had physical training or where study was a before & after assessment; also includes retrospective interview results | |
| Review of physical training evaluations ‐ not an investigational intervention | |
| Inadequate control group ‐ two intervention arms only | |
| Original inclusion now excluded; Authors state volunteers "...were selected to participate in the study." Quasi‐experimental study only; not randomised | |
| All patients had already undergone an 8 week rehabilitation program training prior to being included in the current trial | |
| Quasi‐experimental study only; not randomised ‐ allocation was based on who lived closer to the gymnasium and this group being included in the exercise training arm | |
| Inadequate control group ‐ health control participants used, not participants with asthma | |
| Quasi‐experimental study only; not randomised ‐ participants are said to be randomly chosen but the intervention group of 28 were chosen from a total of 42 because they were inactive in sports and related physical games and had poor physical fitness. Control groups were more physically active than the participants in the intervention group | |
| Inadequate control group ‐ aquatic training program only | |
| Quasi‐experimental study only; not randomised ‐ mentioned as randomised, but all patients who were in hospital were assigned to the control group; participants who had severe asthma were assigned to the control group | |
| Quasi‐experimental study only; not randomised ‐ original inclusion now excluded. Control group "...randomly but prospectively selected..." No randomisation mentioned for the intervention group | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised ‐ but a long term observational study | |
| Inadequate control group ‐ comparison of two types of intervention (interval and continuous running training) | |
| Quasi‐experimental study only; not randomised, but a questionnaire‐based study | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised, but a questionnaire‐based study | |
| Quasi‐experimental study only; not randomised. Composite patient group and not able to obtain data for asthma patients only | |
| Quasi‐experimental study only; not randomised. Participants were consecutively allocated to the training and placebo groups, where the first 26 participants were allocated to the training group and the next 16 to the placebo group | |
| Quasi‐experimental study only; not randomised, participants were assigned to groups according to the availability of transport | |
| Inadequate control group ‐ both study groups underwent physical training. One had intermittent training and the other group had aerobic training | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised | |
| Exercise prescription is of too short a duration | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised ‐ comparison of two intervention groups (swimming and inline skating) | |
| Quasi‐experimental study only; not randomised ‐ participants were randomised on the basis of distance from training centre | |
| Inadequate control group ‐ three arm trial: exercise intervention, dietary intervention or combination of the two | |
| Quasi‐experimental study only; not randomised ‐ original inclusion now excluded due to insufficient randomisation. Participants were 'selected' for the study. No mention of randomisation | |
| Not an investigational intervention ‐ recommendations for sports training in asthma | |
| Quasi‐experimental study only; not randomised ‐ not randomised since the participants could choose which one of the four groups they would like to be in | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised, but a before‐and‐after study | |
| Inadequate control group ‐ comparison of two intervention groups (swimming and golf) | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised | |
| Quasi‐experimental study only; not randomised ‐ participants randomly arranged according to admission order | |
| No outcomes related to asthma patients reported |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Country: Brazil (Sao Paulo) Design: Randomised controlled trial Objective (Aim): To evaluate the effects of aerobic training on bronchial hyper‐responsiveness, airway inflammation and health‐related quality of life in patients with moderate and severe asthma Study Site: Not reported Methods of analysis: Two‐way ANOVA test was used and a significance level of 5% was set (P<0.05) |
| Participants | Randomised: Twenty‐five subjects Completed to date: Twenty‐five subjects Age: 41.6 + 10.5 years Gender: Not reported Asthma diagnosis criteria: Not reported; only described as asthmatic patients Recruitment: Not reported Co‐morbidities: Not reported Subject exclusion criteria: Not reported |
| Interventions | Setting: Patients were studied between two medical consultations, no other information provided Intervention descriptions: Aerobic training performed twice a week over three months; In addition to intervention arm performed an educational program and received placebo treatment Control descriptions: Performed an educational program and received placebo treatment Duration of intervention: 12 weeks Intervention delivered by: Not reported |
| Outcomes | Pre‐specified outcomes: Health‐related quality of life (AQLQ), histamine and airway inflammation by exhaled nitric oxide levels Follow‐up period: 12 weeks (post‐intervention) |
| Notes |
| Methods | Country: United States of America Design: Randomised controlled trial Objective (Aim): To examine if non‐exercising asthmatic subjects enrolled in a structured exercise program will have better quality of life than non‐exercising asthmatic subjects enrolled in an education program only Study Site: Not reported Methods of analysis: Binary data were analysed using random permutation procedures to examine the presence of imbalances at baseline between groups |
| Participants | Randomised: Fourteen subjects Completed to date: Fourteen subjects Age: Not reported Gender: Not reported Asthma diagnosis criteria: Not reported; only described as asthmatic subjects Recruitment: Not reported Co‐morbidities: Not reported Subject exclusion criteria: Not reported |
| Interventions | Setting: Not reported Intervention descriptions: Structured four month exercise program or exercise education (it is unclear from the abstract if the intervention group also received exercise education) Control descriptions: Exercise education Duration of intervention: 16 weeks Intervention delivered by: Not reported |
| Outcomes | Pre‐specified outcomes: Asthma quality of life Follow‐up period: 16 weeks (post‐intervention) in addition to four, eight and 12 weeks |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PEFR (L/min) ‐ Fixed effect model Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Physical training versus control, Outcome 1 PEFR (L/min) ‐ Fixed effect model. | ||||
| 2 FEV1 (L) Show forest plot | 9 | 383 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.10, 0.10] |
| Analysis 1.2  Comparison 1 Physical training versus control, Outcome 2 FEV1 (L). | ||||
| 3 FVC (L) Show forest plot | 7 | 301 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.13, 0.14] |
| Analysis 1.3  Comparison 1 Physical training versus control, Outcome 3 FVC (L). | ||||
| 4 VEmax (L/min) Show forest plot | 5 | 200 | Mean Difference (IV, Fixed, 95% CI) | 3.08 [‐0.63, 6.79] |
| Analysis 1.4  Comparison 1 Physical training versus control, Outcome 4 VEmax (L/min). | ||||
| 5 VOmax (mL/kg/min) Show forest plot | 8 | 267 | Mean Difference (IV, Fixed, 95% CI) | 4.92 [3.98, 5.87] |
| Analysis 1.5  Comparison 1 Physical training versus control, Outcome 5 VOmax (mL/kg/min). | ||||
| 6 HRmax (bpm) Show forest plot | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | 3.67 [0.90, 6.44] |
| Analysis 1.6  Comparison 1 Physical training versus control, Outcome 6 HRmax (bpm). | ||||
| 7 6MWD Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Physical training versus control, Outcome 7 6MWD. | ||||
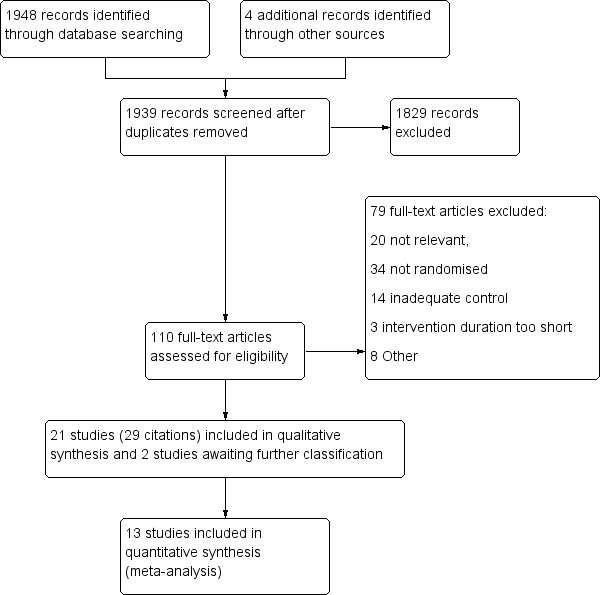
Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Physical training versus control, Outcome 1 PEFR (L/min) ‐ Fixed effect model.
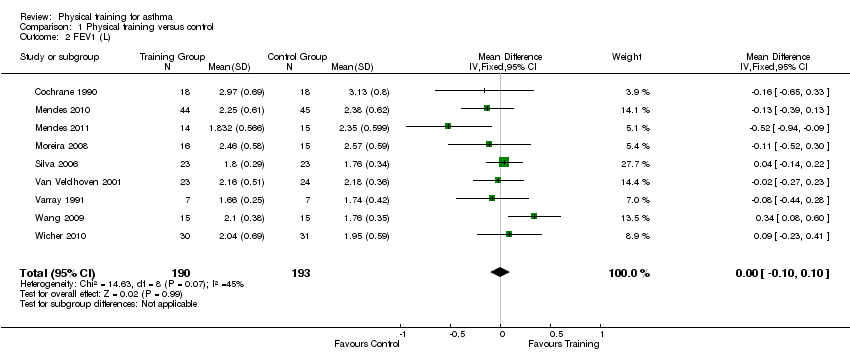
Comparison 1 Physical training versus control, Outcome 2 FEV1 (L).
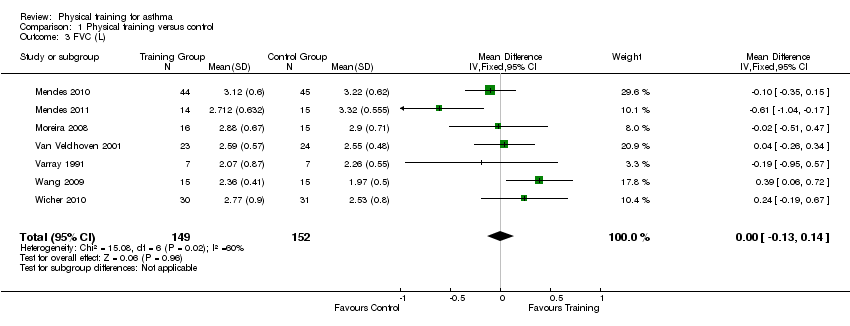
Comparison 1 Physical training versus control, Outcome 3 FVC (L).

Comparison 1 Physical training versus control, Outcome 4 VEmax (L/min).

Comparison 1 Physical training versus control, Outcome 5 VOmax (mL/kg/min).
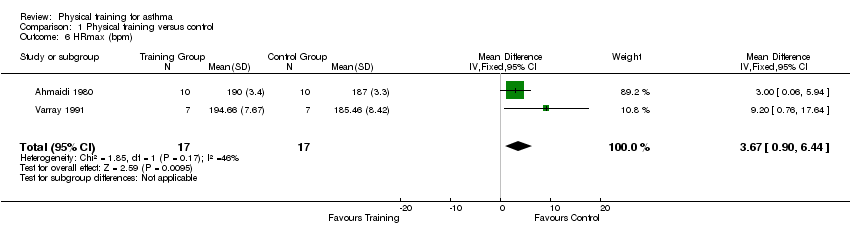
Comparison 1 Physical training versus control, Outcome 6 HRmax (bpm).
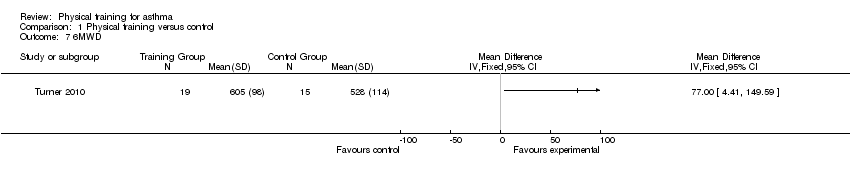
Comparison 1 Physical training versus control, Outcome 7 6MWD.
| Physical training for asthma | ||||||
| Patient or population: patients with asthma aged eight years or older | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Physical training | |||||
| Asthma symptoms measured using various techniques. Follow‐up: 6 to 24 weeks | See comment | See comment | N/A | 315 (9 studies) | ⊕⊝⊝⊝ | We were unable to pool data for this outcome due to heterogeneity in the instruments used; 3 studies found symptoms lasted fewer days, 5 studies reported symptoms were unchanged; 1 study reported significant improvement. |
| Quality of life Measured using various scales. Follow‐up: 12 to 18 weeks | See comment | See comment | N/A | 212 (5 studies) | ⊕⊝⊝⊝ | We were unable to pool data for this outcome due to heterogeneity in the quality of life scales used. 4 studies found clinically significant improvement for total scores immediately after physical training,1 study found no significant difference. |
| Exercise tolerance Measured using 6MWD Follow‐up: 18 weeks | See comment | See comment | N/A | 34 (1 study) | ⊕⊕⊝⊝ | There was a statistically insignificant increase in the 6MWD in one study. |
| PEFR | See comment | See comment | N/A | 77 153 (4 studies in total) | ⊕⊝⊝⊝ | We were unable to pool data for this outcome due to heterogeneity between study populations as per the I2 statistic; The results were originally analysed for two of the four studies assessing this outcome using the fixed‐effect model, when the random‐effects model was applied, the statistical significance disappeared. The minimally important difference is estimated to be a mean change of 11.9 (95% CI 7.3 to 16.1) (Karras 2000), which has been met in both these analyses. Data from two studies showed no change in PEFR, however, we were unable to combine data due to a high dropout rate in one study and unsuitable data for imputation in the other. Possible sources of clinical heterogeneity include swimming versus gymnasium activities and 6 versus 12 week intervention duration. |
| VEmax | The mean VEmax ranged across control groups from | The mean VEmax in the intervention groups was | 200 | ⊕⊕⊝⊝ | ||
| VOmax | The mean VOmax ranged across control groups from | The mean VOmax in the intervention groups was | 267 | ⊕⊕⊝⊝ | ||
| HRmax | The mean HRmax ranged across control groups from | The mean HRmax in the intervention groups was | 34 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Methods of randomisation, allocation concealment and/or any attempts to blind outcome assessors were not described for the majority of studies assessing this outcome (limitations of design (‐1)) 5 Single study Abbreviations: 6MWD: six‐minute walking distance; bpm: heart beats per minute; HRmax: maximum heart rate; PEFR: peak expiratory flow rate; VEmax: maximal expiratory volume (the maximum volume of air that can be breathed in 1 min during exercise); VOmax: maximal oxygen consumption (the maximum amount of oxygen in millilitres used while exercising). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PEFR (L/min) ‐ Fixed effect model Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 FEV1 (L) Show forest plot | 9 | 383 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.10, 0.10] |
| 3 FVC (L) Show forest plot | 7 | 301 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.13, 0.14] |
| 4 VEmax (L/min) Show forest plot | 5 | 200 | Mean Difference (IV, Fixed, 95% CI) | 3.08 [‐0.63, 6.79] |
| 5 VOmax (mL/kg/min) Show forest plot | 8 | 267 | Mean Difference (IV, Fixed, 95% CI) | 4.92 [3.98, 5.87] |
| 6 HRmax (bpm) Show forest plot | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | 3.67 [0.90, 6.44] |
| 7 6MWD Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

