Entrenamiento físico para el asma
Resumen
Antecedentes
Los pacientes con asma pueden mostrar menor tolerancia al ejercicio debido al empeoramiento de los síntomas de la enfermedad durante el ejercicio, o a otros motivos como la falta de condición física debido a la inactividad. Algunos también pueden restringir las actividades por asesoramiento médico o por influencia familiar, lo cual puede dar lugar a una reducción en el buen estado físico. Los programas de entrenamiento físico intentan mejorar la condición física, la coordinación neuromuscular y la seguridad en sí mismos. Subjetivamente, muchos pacientes con asma informan que se sienten sintomáticamente mejor cuando se mantienen en forma, pero los resultados de los ensayos han variado y ha sido difícil compararlos debido a la diferencia en los diseños y protocolos de entrenamiento. Además, como el ejercicio puede inducir asma, debe considerarse la seguridad de los programas de ejercicio.
Objetivos
Comprender mejor el efecto del entrenamiento físico sobre la salud respiratoria y general de los pacientes con asma, a partir de ensayos aleatorios.
Métodos de búsqueda
Se realizaron búsquedas en el registro especializado de ensayos del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group) hasta enero de 2013.
Criterios de selección
Se incluyeron los ensayos aleatorios en pacientes de ocho años de edad o más, con asma, que fueron asignados al azar a entrenamiento físico o ningún entrenamiento. El entrenamiento físico debía realizarse durante al menos 20 minutos, dos veces a la semana, durante al menos cuatro semanas.
Obtención y análisis de los datos
Dos revisores evaluaron de forma independiente la elegibilidad para inclusión y el riesgo de sesgo de los estudios incluidos.
Resultados principales
En esta revisión se incluyeron 21 estudios (772 participantes) y se identificaron dos estudios adicionales de 2012 como "en espera de clasificación". El entrenamiento físico fue bien tolerado y no se informaron efectos adversos. Ninguno de los estudios mencionó el empeoramiento de los síntomas de asma después del entrenamiento físico. El entrenamiento físico mejoró de manera notable el estado cardiopulmonar según lo medido mediante un aumento estadística y clínicamente significativo en la captación máxima de oxígeno (diferencia de medias [DM] 4,92 ml/kg/min; intervalo de confianza [IC] del 95%: 3,98 a 5,87; p < 0,00001; ocho estudios con 267 participantes); sin embargo, no se observaron efectos estadísticamente significativos en el volumen espiratorio forzado en un segundo (VEF1), la capacidad vital forzada (CVF), la ventilación por minuto en el ejercicio máximo (VEmax) o la tasa de flujo espiratorio máximo (TFEM). El metanálisis de cuatro estudios detectó un aumento estadísticamente significativo en la frecuencia cardíaca máxima, y luego de un análisis de sensibilidad y la eliminación de dos estudios se mantuvo la significación (DM 3,67 lpm; IC del 95%: 0,90 a 3,44; p = 0,01). Aunque no hubo datos suficientes para agrupar los resultados debido a las diversas herramientas de informe, hay algunas pruebas disponibles que indican que el entrenamiento físico puede tener efectos positivos sobre la calidad de vida relacionada con la salud, y cuatro de cinco estudios mostraron un beneficio estadística y clínicamente significativo.
Conclusiones de los autores
Esta revisión demostró que el entrenamiento físico produjo una mejoría significativa en la captación máxima de oxígeno, aunque no se observaron efectos en otras medidas de la función pulmonar. Los pacientes con asma en los estudios incluidos toleraron bien el entrenamiento físico, por lo que los pacientes con asma estable deben ser alentados a participar en el entrenamiento regular con ejercicios, sin temor a la exacerbación de los síntomas. Se necesitan más estudios de investigación para comprender los mecanismos por los cuales la actividad física tiene un impacto en el control del asma.
PICO
Resumen en términos sencillos
Entrenamiento físico para el asma
Algunos pacientes con asma pueden mostrar menos tolerancia al ejercicio debido al empeoramiento de los síntomas de su enfermedad cuando realizan ejercicios, o a otros motivos como la falta de una buena condición física. Este empeoramiento puede prevenirse con la práctica de deportes o intentando mantenerse en forma. Se han diseñado programas de entrenamiento físico para pacientes con asma para mejorar la condición física, la coordinación neuromuscular y la seguridad en sí mismos.
La revisión de los ensayos encontró que el entrenamiento con ejercicios (que incluye trote, gimnasia, ciclismo, natación, ejercicios con pesas y caminatas) fue bien tolerado por los pacientes de los estudios. Esta revisión también encontró que el entrenamiento físico mejoró la condición cardiopulmonar y mostró algunos efectos positivos en cuanto a la calidad de vida relacionada con la salud. Sin embargo, el entrenamiento físico no tuvo efectos significativos sobre la función pulmonar en reposo. En resumen, se debe alentar a los pacientes con asma estable a participar en la práctica regular de ejercicios dentro de sus posibilidades, sin temor a un empeoramiento de los síntomas del asma.
Authors' conclusions
Summary of findings
| Physical training for asthma | ||||||
| Patient or population: patients with asthma aged eight years or older | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Physical training | |||||
| Asthma symptoms measured using various techniques. Follow‐up: 6 to 24 weeks | See comment | See comment | N/A | 315 (9 studies) | ⊕⊝⊝⊝ | We were unable to pool data for this outcome due to heterogeneity in the instruments used; 3 studies found symptoms lasted fewer days, 5 studies reported symptoms were unchanged; 1 study reported significant improvement. |
| Quality of life Measured using various scales. Follow‐up: 12 to 18 weeks | See comment | See comment | N/A | 212 (5 studies) | ⊕⊝⊝⊝ | We were unable to pool data for this outcome due to heterogeneity in the quality of life scales used. 4 studies found clinically significant improvement for total scores immediately after physical training,1 study found no significant difference. |
| Exercise tolerance Measured using 6MWD Follow‐up: 18 weeks | See comment | See comment | N/A | 34 (1 study) | ⊕⊕⊝⊝ | There was a statistically insignificant increase in the 6MWD in one study. |
| PEFR | See comment | See comment | N/A | 77 153 (4 studies in total) | ⊕⊝⊝⊝ | We were unable to pool data for this outcome due to heterogeneity between study populations as per the I2 statistic; The results were originally analysed for two of the four studies assessing this outcome using the fixed‐effect model, when the random‐effects model was applied, the statistical significance disappeared. The minimally important difference is estimated to be a mean change of 11.9 (95% CI 7.3 to 16.1) (Karras 2000), which has been met in both these analyses. Data from two studies showed no change in PEFR, however, we were unable to combine data due to a high dropout rate in one study and unsuitable data for imputation in the other. Possible sources of clinical heterogeneity include swimming versus gymnasium activities and 6 versus 12 week intervention duration. |
| VEmax | The mean VEmax ranged across control groups from | The mean VEmax in the intervention groups was | 200 | ⊕⊕⊝⊝ | ||
| VOmax | The mean VOmax ranged across control groups from | The mean VOmax in the intervention groups was | 267 | ⊕⊕⊝⊝ | ||
| HRmax | The mean HRmax ranged across control groups from | The mean HRmax in the intervention groups was | 34 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Methods of randomisation, allocation concealment and/or any attempts to blind outcome assessors were not described for the majority of studies assessing this outcome (limitations of design (‐1)) 5 Single study Abbreviations: 6MWD: six‐minute walking distance; bpm: heart beats per minute; HRmax: maximum heart rate; PEFR: peak expiratory flow rate; VEmax: maximal expiratory volume (the maximum volume of air that can be breathed in 1 min during exercise); VOmax: maximal oxygen consumption (the maximum amount of oxygen in millilitres used while exercising). | ||||||
Background
Description of the condition
Asthma, a chronic respiratory condition affecting 300 million people globally (Masoli 2004), causes inflammation of the lungs as well as structural and functional remodelling of the airways. It is characterised by recurrent attacks of breathlessness and wheezing with varying degrees of frequency and severity, which is caused by swelling of the bronchial tubes resulting in airflow limitation (WHO 2011). Although the causes of asthma are not completely understood, risk factors are known to include inhaling asthma triggers such as allergens, tobacco smoke and chemical irritants. Asthma is incurable and the prevalence is increasing, particularly in children and young adults (Pawankar 2012), however appropriate management can control the disorder and enable people to enjoy a high quality of life (WHO 2011).
Asthma is estimated to account for one in every 250 deaths worldwide, many of which are preventable (Masoli 2004). The prevalence of asthma varies between countries, with prevalence estimates of 18.4% in Scotland, 15.3% in England, 15.1% in New Zealand, 14.1% in Canada, 11.4% in Brazil, 10.9% in the United States of America, 9% in Israel, 8.1% in South Africa, 6.9% in Germany, 6.7% in Japan, 4.5% in Italy, 3.8% in Bangladesh, 2.6% in Taiwan, 2.3% in Switzerland, 1.9% in Greece and 1.1% in Indonesia (EFA 2004; Masoli 2004). Asthma is reported to be the 10th leading contributor to the overall burden of disease in Australia (AIHW 2010), affecting 14.7% of all Australians (AIHW 2008; Braman 2006) and as such it has been a National Health Priority since 1996 (AIHW 2008). Globally, the healthcare costs associated with asthma management are significant and increasing (Bahadori 2009), with estimated costs in developed countries estimated at USD 300 to USD 1300 per patient per year (Braman 2006) and EUR 1583 per patient in Europe (Accordini 2013). With the healthcare burden of asthma increasing, confounding pressures are applied to healthcare systems, governments, families and the patients themselves (Masoli 2004) resulting in sub‐optimal management (Kandane‐Rathayake 2009).
Description of the intervention
Physical activity is comprised of bodily movements produced by the skeletal muscles that result in an increased metabolic rate over that of resting energy expenditure (Bouchard 2012). Physical training is defined by one 2012 publication as "... a form of leisure‐time physical activity that is usually performed repeatedly over an extended period of time (exercise training) with a specific external objective such as the improvement of fitness, physical performance, or health" (Bouchard 2012). For the purpose of improving health outcomes the mode, intensity, frequency and duration of activities need to be considered (Bouchard 2012). Physical training programmes have been designed for people with asthma with the aim of improving physical fitness (Arandelovic 2007), neuromuscular coordination and self confidence, but the reported results have varied and have been difficult to compare because of different study designs and training protocols. Such protocols can include various types of aerobic exercise such as running, jogging, cycling, weight training, swimming, stretching and a combination of these activities amongst many other options (Avallone 2012).
How the intervention might work
A low level of habitual physical activity leads in turn to low levels of physical fitness. Thus a number of studies (Clark 1988; Garfinkel 1992), but not all (Santuz 1997), have reported people with asthma to have lower cardiorespiratory fitness when compared to their peers. For people with asthma, exercise can provoke bronchoconstriction in some individuals resulting in exercise‐induced asthma (EIA). Research has also shown that inactivity as a result of breathlessness can lead to peripheral muscle deconditioning, which is an important factor limiting exercise capacity (Allard 1989; Laveneziana 2006).This is because deconditioning can result in further breathlessness, as atrophied leg muscles for example are more prone to fatigue (Swallow 2007), requiring increased ventilation for exercise to be maintained. Subsequently, this contributes to breathlessness in a spiraling cycle finally resulting in exercise avoidance and therefore further deconditioning of the skeletal muscles (Moxham 2009). Studies have shown that people with asthma are able to exercise and improve their fitness (Scichilone 2012), and that limitations in exercise capacity can sometimes relate more to lack of fitness than to air flow limitation (Sonna 2001). Habitual physical activity is associated with a reduction in asthma prevalence in children (Nystad 2001) and adults (Huovinen 2001), and may lower the minute ventilation of mild and moderate exercise thereby reducing the likelihood of provoking EIA; whilst physical inactivity has been associated with negative health consequences and an increase in asthma‐related difficulties (Avallone 2012). Exercise training may also reduce the perception of breathlessness through a number of mechanisms including strengthening respiratory muscles. There are reports that exercise may have a protective effect against asthma development by reducing airway inflammation (Eijkemans 2012; Ford 2002) and increasing the patency of bronchioles (Eijkemans 2012).
Why it is important to do this review
Regular physical exercise and participation in sports are considered to be important components in the overall management of asthma, especially in children and adolescents (Orenstein 1996). There is some evidence to suggest that regular physical exercise improves asthma symptom management, lung function and mental health (Avallone 2012). However, there is evidence that some people with asthma may avoid participation in exercise and sports due to shortness of breath or worsening asthma symptoms during exercise, or fear of experiencing such symptoms (Avallone 2012; Scott 2013b). Some may have a negative attitude to exercise due to reasons including organisational policies, family beliefs, healthcare advice or inaccurate symptom perception (Williams 2008). Subjectively, many people with asthma report that they are symptomatically better when fit but the physiological basis of this perception has not been systematically investigated.
Objectives
To gain a better understanding of the effect of physical training on the respiratory and general health of people with asthma, from randomised trials.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials of people with asthma undertaking physical training.
Types of participants
We included people with asthma, aged eight years or older, with any degree of asthma severity. We included diagnoses of asthma made by a physician or the use of objective criteria (for example bronchodilator reversibility), or both. We excluded studies reporting results on patients with chronic obstructive pulmonary disease (COPD) but we did include data from studies of mixed populations where separate data were available for people with asthma.
Types of interventions
We included any type of physical training provided it was whole body aerobic exercise lasting at least 20 minutes, undertaken two times a week for a minimum duration of four weeks.
Control groups included no intervention, or co‐interventions such as brief education only (as part of verbal consultations) or asthma medications providing the intervention group also received the same level of education or medication.
Types of outcome measures
Primary outcomes
Asthma symptoms, which included episodes of wheeze or shortness of breath, symptoms score, dyspnoea or number of symptom free days etc.
Secondary outcomes
-
Bronchodilator usage
-
Exercise endurance
-
Work capacity
-
Walking distance
-
Quality of life
-
Physiological measurements (i.e. peak expiratory flow rate (PEFR), forced expiratory volume (FEV), forced vital capacity (FVC), maximal oxygen uptake (VOmax), minute ventilation at maximal exercise (VEmax), maximal heart rate (HRmax), maximal voluntary ventilation (MVV))
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the Specialised Register coded as 'asthma' using the following terms:
"work capacity" OR physical* OR train* OR rehabilitat* OR fitness* or exercis* or aerobic*.
The World Health Organization International Clinical Trials Registry Platform (ICTRP) electronic search portal was also searched for studies using the terms: ['work capacity' OR 'physical' OR 'activit' OR 'train' OR 'rehabilitat' OR 'fitness' OR 'exercis'] AND asthma.
The most recent searches were conducted on the 25th January 2013.
Searching other resources
Two review authors reviewed the reference lists of all primary studies to identify trials not captured by electronic searches.
Data collection and analysis
Selection of studies
Two review authors (KC, MC) screened articles for potential relevance using the title, abstract or descriptors, or both. The same two review authors screened the full text articles from this second comprehensive list based on study design, populations, interventions and outcomes. A third review author checked through all the articles identified as potentially relevant for inclusion (JP).
Data extraction and management
A combination of two authors (KC, MC, MB and JP) independently extracted data for the trials using a standardised data extraction form prior to data entry into RevMan 5. Two review authors (MC and KC) entered data into RevMan 5, and the same two review authors corresponded with authors to obtain missing data and raw data.
Assessment of risk of bias in included studies
Two review authors assessed the risk of bias for all included studies as per the recommendations in the Cochrane Handbook of Systematic Reviews of Interventions, using a domain‐based evaluation (Higgins 2008). We assessed each domain as either high, low or unclear risk of bias using the guidelines from table 8.5.c of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Two review authors (KC and MC, JP or MB) independently assessed the included studies for risk of bias. We resolved conflicts by consensus or by referring to a third party (BS) when disagreement persisted.
Unit of analysis issues
In the case of cluster randomised trials, we planned to perform analyses at the level of individuals whilst accounting for the clustering in the data. We planned to do this using the statistical methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (chapter 16.3.3) (Higgins 2008) and to have our working checked by a statistician. However, no studies were found which used clusters as the unit of randomisation.
In the case of multi‐arm trials where we deemed the studies similar enough to be pooled, we combined the groups using the appropriate formulas in the Cochrane Handbook for Systematic Reviews of Interventions (table 7.7.a for continuous data and chapter 16.5.4 for dichotomous data) (Higgins 2008).
Dealing with missing data
We evaluated missing information about participants using an available case analysis as described in chapter 16.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We addressed missing standard deviations by imputing data from the studies within the same meta‐analysis or from a different meta‐analysis providing these used the same measurement scale, had the same degree of measurement error and the same time periods between baseline and final value measurement (as per chapter 16.1.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2008). Where statistics essential for analysis were missing (for example group means and standard deviations for both groups were not reported) and could not be calculated from other data, we attempted to contact the authors to obtain the data. We also asked study authors to provide data for unreported outcomes. We assumed that the loss of participants prior to performance of baseline measurements had no effect on the eventual outcome data for that study. We excluded studies reporting dropout rates higher than 30% from the meta‐analysis and instead reported the results only in the text.
Assessment of heterogeneity
We expected to encounter some heterogeneity in factors such as baseline asthma severity, participant characteristics (for example age and physical state), intervention methods, time of measurement of results, and tools used to assess outcomes. We used the Chi² and I² statistics to quantify inconsistencies across studies (Higgins 2008). We also explored heterogeneity by visual inspection of the differences between studies (for example types of interventions, participants). We had planned to further explore heterogeneity using subgroup analysis (as per chapter 9.5.3 of the Cochrane Handbook for Systematic Reviews of Interventions), if there had been a sufficient number of studies (Higgins 2008).
Assessment of reporting biases
We had planned to assess reporting biases through visual inspection of a funnel plot, if we had been able to pool 10 or more studies in one meta‐analysis. Although potential reporting, or publication, biases can be assessed using a funnel plot, asymmetry in a funnel plot may also occur due to true heterogeneity, poor methodological design or artefact. If we had found asymmetry in the funnel plots, we planned to include contour lines corresponding to perceived milestones of statistical significance (P = 0.01, 0.05, 0.1 etc), which may help to differentiate between asymmetry due to publication bias from that due to other factors (Higgins 2008).
Data synthesis
We entered data into RevMan 5 software. We pooled continuous data with a fixed‐effect model, however in the presence of significant heterogeneity, as determined by a combination of the I² greater than 60% and Chi² P less than 0.01, we used a random‐effects model to account for expected differences in the intervention and population etc.
We planned to pool dichotomous data using a Peto odds ratio (OR).
Subgroup analysis and investigation of heterogeneity
We had planned to perform subgroup analysis for each classification of intervention (for example swimming, running, cycling) if a sufficient number of included studies had been available.
Sensitivity analysis
We conducted sensitivity analyses by excluding studies with an unclear or high risk of bias for sequence generation or allocation concealment, or both. We also conducted sensitivity analysis by removing studies with participants with significant co‐morbidities from the analyses.
Results
Description of studies
Results of the search
There were 1948 citations identified from the initial search of the pre‐specified databases. Four additional articles were retrieved following handsearching of reference lists of potentially relevant studies and contacting authors, resulting in a total of 1939 citations after the duplicates were removed. From this list, 110 were identified as potentially relevant and full text articles were retrieved for closer inspection. Two review authors independently identified that 21 of these articles (29 citations) fulfilled the inclusion criteria of the review, of which two were newly included studies for the 2013 update (Boyd 2012; Wicher 2010). Two additional studies were also identified as awaiting classification as limited data were presented from scientific abstracts only (Pinto 2012; Pollart 2012). The remaining 79 citations were excluded, 20 of which were considered 'not relevant' to the review, leaving 54 studies (59 citations) that were relevant to the review topic. Reasons for exclusion are reported in the Characteristics of excluded studies table (also see Figure 1).

Study flow diagram.
Included studies
See Characteristics of included studies. Twenty‐one studies were identified as meeting the inclusion criteria, involving randomisation of 772 people. Two of these were new studies published since the previous review. The 21 trials were published between 1980 and 2012. Sample size ranged from 14 to 101 participants. All studies were randomised controlled trials of people with asthma undertaking physical training (whole body aerobic exercise) lasting for at least 20 to 30 minutes, two to three times a week, with a minimum duration of four weeks. The length of the physical training programs varied from six to 16 weeks.
Excluded studies
Fifty‐four studies were excluded based on methodological grounds and we provided the reasons for exclusion in the Characteristics of excluded studies table. Thirty‐four were not randomised, 14 included inadequately designed controls, three had intervention durations too short to meet the pre‐specified criteria, and eight had other reasons for exclusion.
Risk of bias in included studies
Assessment of study quality was difficult due to either the limited availability of data or poor methodological reporting. All studies mentioned randomised allocation though only seven studies provided the exact methods (Fanelli 2007; Mendes 2010; Mendes 2011; Moreira 2008; Turner 2010; Varray 1991; Varray 1995). See the 'Risk of bias' tables (in Characteristics of included studies) for further information and Figure 2 and Figure 3. We did not ask for confirmation regarding 'Risk of bias' characteristics from all authors due to concerns about overburdening the authors with our requests as we were already asking for raw data for meta‐analyses.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation concealment was assessed as adequate in only two studies where we were able to obtain a copy of the protocol (Moreira 2008; Turner 2010). The remaining 19 studies were all unclear.
Blinding
Due to the nature of the study interventions it is understood that blinding of participants was not possible. However, one study (Swann 1983) did mention 'double blinding', though exactly who was part of that blinding process was not described; as such the bias for blinding of participants was unclear in this study. The remaining 20 studies all received a high risk of bias assessment for this domain.
Two studies reported blinding of outcome assessors and were therefore judged to be at low risk of bias (Ahmaidi 1980; Counil 2003), three other studies clearly stated that the outcome assessors were not blinded (Mendes 2010; Mendes 2011; Turner 2010), whilst the remaining 16 studies had an unclear risk of bias.
Incomplete outcome data
Incomplete outcome reporting of data was evident in two studies, which we judged to be at high risk of bias (Girodo 1992; Weisgerber 2003). The other trials were judged to be at low risk of bias (Counil 2003; Fanelli 2007; Gonçalves 2008; Matsumoto 1999; Moreira 2008; Turner 2010; Van Veldhoven 2001; Varray 1991) or unclear for the remaining 11 studies.
Selective reporting
Reporting biases were evident in nine studies (Ahmaidi 1980; Boyd 2012; Fanelli 2007; Girodo 1992; Matsumoto 1999; Mendes 2010; Varray 1991; Wang 2009; Wicher 2010), which included post hoc methods being used and the data presented pictorially or incompletely rather than reported as absolute values (that is the data could not be meta‐analysed). We judged the remaining 12 studies as at unclear risk of selective reporting.
Other potential sources of bias
We identified other potential sources of bias in six studies (Boyd 2012; Fanelli 2007; Gonçalves 2008; Mendes 2010; Mendes 2011; Weisgerber 2003). Within the published abstract of the Boyd 2012 study the authors reported that 20 adults were to be recruited, yet only 19 were reported as being recruited in the primary manuscript with no explanation as to why the 20 participants were not recruited. The Weisgerber 2003 study had significant baseline imbalances (for height and FVC), with no mention of adjustments for these imbalances in the data analysis methods. In addition, the authors stated that the intervention may not have had sufficient intensity and duration to demonstrate a significant result. Similarly, the Fanelli 2007 study also had significant baseline imbalances between groups, which were not reported as being adjusted for in the data analysis methods (more intervention participants had peak VO values < 70% predicted than the controls at baseline, P < 0.05). In addition, the authors reported possible contamination in the control arm through the use of education and written action plans, which could lead to underestimation of the true effect of the intervention. Twenty‐six of 68 participants enrolled into the Mendes 2011 study were simultaneously enrolled into the Mendes 2010 study, however the authors of the studies supplied the raw data excluding the overlapped participants and thus the trials were able to be included in the meta‐analyses.
We identified two studies as having no other potential biases (Counil 2003; Silva 2006), whilst we could not be sure whether there were any other potential biases in the remaining 13 studies and we therefore judged these to be at unclear risk of bias.
Effects of interventions
See: Summary of findings for the main comparison Physical training for asthma
See also summary of findings Table for the main comparison
Asthma symptoms
Nine studies reporting results for 315 participants measured asthma symptoms according to different methods (Boyd 2012; Gonçalves 2008; Mendes 2010; Mendes 2011; Swann 1983; Turner 2010; Varray 1991; Wang 2009; Wicher 2010). The Boyd 2012 study reported asthma control as a composite value according to the Juniper Asthma Control Questionnaire that integrates common indicators of asthma management including the use of bronchodilators, nocturnal symptoms, cough, activity level and pulmonary function. There was no significant difference in asthma control between the groups. One participant in the intervention group did experience an exacerbation during her 12 weeks of exercise, however the authors reported that the exacerbation did not appear to be triggered by the exercise program and that her study data were eventually discarded due to faulty heart rate monitor recordings. Three studies on 151 people (which excluded the known duplications between Mendes 2010 and Mendes 2011) reported on frequency of asthma symptoms, which was assessed by monthly sums of days free of asthma symptoms (Gonçalves 2008; Mendes 2010; Mendes 2011). The intervention groups in all three studies showed improvements in symptom free days over the controls; however, following attempts to meta‐analyse the data, which suggested that it was not normally distributed, meta‐analysis was deemed inappropriate (Gonçalves 2008: intervention 24.8 days (95% confidence interval (CI) 23 to 27) versus control 15.7 days (95% CI 9 to 21), P < 0.02; Mendes 2010, days without symptoms 30 days after study commencement: intervention 21.36 days (95% CI 7 to 27) versus control 14.47 days (95% CI 6 to 24); 60 days after study commencement: intervention 22.71 days (95% CI 11 to 29) versus control 15.51 days (95% CI 10 to 24); 90 days after study commencement: intervention 23.11 days (95% CI 11 to 27) versus control 15.84 days (95% CI 9 to 26), P < 0.001; Mendes 2011, days without symptoms 30 days after study commencement: intervention 23.17 days (95% CI 20 to 25) versus control 14.63 days (95% CI 10 to 18); 60 days after study commencement: intervention 24.33 days (95% CI 22 to 26) versus control 14.25 days (95% CI 10 to 18); 90 days after study commencement: intervention 24.83 days (95% CI 23 to 27) versus control 15.63 days (95% CI 9 to 21)). One study (Turner 2010) reported that asthma control (which was assessed by a six‐point score, Ages and Stages Questionnaire (ASQ)) was unchanged in either group during the intervention period, or after three months follow‐up post‐intervention. Varray 1991 reported that there was no change in frequency of asthma attacks during the intervention period, and the Swann 1983 trial reported no difference between the two groups for the daily score of asthma symptoms during the study. In the study by Wang (Wang 2009), there was a statistically significant improvement in the severity of asthma in the experimental group compared with the control group (daily severity of asthma was monitored at the same time of the day based on National Heart, Lung and Blood Intitute Criteria (NHLBI 2011)). However, this outcome may have been biased by the fact that the intervention group had a greater compliance with controller medications. Authors of the Wicher 2010 study reported that the number of exacerbations for both groups was the same before and during the study, however no numerical data or P values were presented.
Peak expiratory flow rate (PEFR) (L/min)
Four studies reporting results for 153 people contributed data on PEFR (Mendes 2011; Van Veldhoven 2001; Wang 2009; Weisgerber 2003). Two of these studies (Van Veldhoven 2001; Wang 2009) were included in a meta‐analysis, however due to significant heterogeneity as indicated by an I² statistic of 96% they were not able to be pooled (Analysis 1.1). The reason for this heterogeneity is unknown, although possible contributors included: population (Asian versus Dutch), intervention characteristics (swimming versus gymnasium activities) and duration of intervention (six weeks versus 12 weeks) for Wang 2009 and Van Veldhoven 2001 respectively. Better medication compliance in the intervention group of the Wang study may also have contributed. Weisgerber 2003 reported that there was no change in PEFR between the two groups post‐intervention; these data were however excluded from the meta‐analysis due to a dropout rate of over 30%. Data in the Mendes 2011 study showed no difference between groups, but were unable to be meta‐analysed as they were reported as medians and inter‐quartile ranges.
Forced expiratory volume in 1 sec (FEV1) (L)
Sixteen studies with results for 399 people reported on resting FEV1 at the endpoint. However, the results were reported in a number of different ways including: absolute values, per cent predicted, mean of absolute change, or change from baseline. Pooled data from nine studies which reported the absolute value of FEV1 at follow‐up showed no significant effect of physical training on FEV1 (MD ‐0.00 L; 95% CI ‐0.10 to 0.10; Analysis 1.2). Another study (Weisgerber 2003) reported that physical training had no effect on FEV1, however these data could not be used in the analysis due to a dropout rate of greater than 30% (Weisgerber 2003).
Forced vital capacity (FVC) (L)
Thirteen studies reported follow‐up data on resting FVC, however only seven trials involving 301 people provided data that could be pooled (Mendes 2010; Mendes 2011; Moreira 2008; Van Veldhoven 2001; Varray 1991; Wang 2009; Wicher 2010). The pooled results showed that physical training had no effect on FVC (MD 0.00 L; 95% CI ‐0.13 to 0.14; Analysis 1.3). Weisgerber 2003 also reported that physical training had no effect on FVC, however the data were excluded from the meta‐analysis due to a dropout rate of more than 30%.
Minute ventilation at maximal exercise (VEmax) (L/min), maximal oxygen uptake (VOmax) (mL/kg/min), work capacity
Twelve studies performed cardiopulmonary assessment with an attempt to measure either VOmax, work capacity or VEmax post‐intervention. According to the pooled data from five studies involving 200 people, physical training showed no evidence of improvement for VEmax (MD 3.08 L/min; 95% CI ‐0.63 to 6.79; Analysis 1.4). Tests for heterogeneity produced an I² = 64% presenting moderate levels of heterogeneity.
Four studies reported on the VOmax as change from baseline only and therefore could not be meta‐analysed. Data from eight studies involving 267 people could be pooled. These trials compared endpoint VOmax for the two groups of participants and showed that physical training produced a statistically and clinically significant increase in VOmax (MD 4.92 mL; 95% CI 3.98 to 5.87; P < 0.00001; Analysis 1.5). Tests for heterogeneity were not significant (I² = 44%). According to data from four studies (Ahmaidi 1980; Boyd 2012; Matsumoto 1999; Van Veldhoven 2001), physical training resulted in an increase in work capacity, however these results could not be pooled as outcomes were reported using a mixture of change scores and absolute values. A statistically and clinically significant difference in work capacity was evident in two studies (Ahmaidi 1980; Matsumoto 1999), whereas another study showed no significant benefits between intervention and control (Van Veldhoven 2001).
Maximal heart rate (HRmax) (bpm)
Five studies with results for 97 people reported HRmax as an outcome measure with the overall pooled effect for four studies (81 people) being an increase in HRmax (MD 3.16 bpm; 95% CI 0.52 to 5.80). However, this overall mean effect contained significant heterogeneity as determined by a combination of the I² statistic (I² = 93%), visual inspection of the forest plot, and examination of study characteristics. Following a sensitivity analysis, elimination of the Counil 2003 and Van Veldhoven 2001 studies removed the heterogeneity (I² = 46%). There was still a significant increase in overall mean HRmax with two of the studies (MD 3.67 bpm; 95% CI 0.90 to 6.44; Analysis 1.6). Authors for the remaining study (Boyd 2012) reported that HRmax significantly increased in the exercise arm, however the data were not displayed in a way that could be meta‐analysed.
Maximal ventilatory ventilation (MVV)
One study (Wicher 2010) reported MVV for participants of the intervention group only, with improvements observed between the pre‐ and post‐test measurements (56.83 L/min + 18.25 to 66.81 L/min + 23.02; P = 0.001).
Six‐minute walking distance (6MWD)
One study reporting results for 34 people (Turner 2010) measured the effect of physical training on functional exercise capacity as measured by the 6MWD post‐intervention. There was an increase in 6MWD following exercise training of 36 + 37 metres in the exercise group and 6 + 38 metres in the control group, however this difference between the groups did not reach statistical significance.
Quality of life (QoL)
Five studies with results for 212 people reported health‐related quality of life using four different scales: PAQLQ (Paediatric Asthma Quality of Life Questionnaire) (Fanelli 2007; Moreira 2008), AQLQ (Asthma Quality of Life Questionnaire) (Turner 2010), SF‐36 (Short Form‐36) (Turner 2010), and QOL‐EPM (Quality Of Life ‐ Escola Paulista de Medicina) (Gonçalves 2008; Mendes 2010). Four of these trials reported that physical training improved the quality of life scores of asthma participants. Two studies (Gonçalves 2008; Mendes 2010) reported statistically and clinically significant differences between the two groups (intervention and control) for QoL total scores and for physical limitation, symptom frequency and psychosocial subscores immediately following physical training. In the study by Turner (Turner 2010) there was statistically and clinically significant improvement in all domain scores of the health‐related QoL (AQLQ) in the exercise group compared with the control group post‐intervention. The Fanelli study (Fanelli 2007) also reported significant statistical and clinical improvements in health‐related QoL (PAQLQ) total scores as well as all domain scores in the exercise group compared with the controls. In the study by Moreira (Moreira 2008) QoL scores were not statistically significantly different between the exercise and control groups. The authors reported that the number of participants achieving a clinically important improvement in the PAQLQ score from baseline did not differ between groups either (see also Characteristics of included studies).
Bronchodilator usage
There were no usable data available from any of the studies for bronchodilator usage.
Subgroup analyses
We could not perform subgroup analyses to compare different types of physical training because of the small study numbers and missing outcome data.
Discussion
Summary of main results
This systematic review examined the effects of physical training on people with asthma. Twenty‐one randomised controlled trials satisfied the inclusion criteria, with the majority of the studies including small numbers of participants. Although all studies met the inclusion criteria they differed significantly in terms of intervention characteristics (including frequency, duration and type of intervention), reported outcomes and statistical presentation of data. The statistical presentation of results was a particular issue and limited the analysis as data were presented in such a way that they could not be meta‐analysed without raw data being obtained (for example median values instead of means, change scores instead of total scores, and percentage predicted instead of absolute values). None of the studies evaluated longer term benefits of physical training in people with asthma.
According to the available data, physical training was well tolerated by the people with asthma with no adverse effects identified. Data from the available studies suggest that physical training improves asthma symptoms. None of the studies detected a worsening of asthma symptoms following physical training. Exercise‐induced bronchoconstriction (EIB) was assessed in three studies. However, it was not one of our pre‐specified outcomes for this review. Overall, physical training did not seem to change EIB severity.
Physical training improved cardiopulmonary fitness, as measured by an increase in maximum oxygen uptake (VOmax) and the work load achieved by a participant during exercise testing, without having a significant effect on resting lung function. Studies in healthy people have shown that VOmax may increase by up to 20% (Brooks 1996), the extent being dependent upon initial fitness level, type of training and age. Asthma patients respond to physical training in a similar manner and degree to non‐asthma participants (Robinson 1992). Maximum heart rate (HRmax) significantly increased in the group that underwent physical training. A decrease in HRmax is the typical response to training (Brooks 1996). The present finding may indicate that a maximum effort was not achieved in the baseline test or that non‐cardiac factors limited exercise capacity initially. For example, training may result in a decrease in the perception of breathlessness resulting in greater maximum exercise effort.
There were insufficient data to meta‐analyse the effects of physical training on health‐related quality of life. However, this review does provide some limited evidence from available studies that physical training has positive effects on the quality of life of asthma patients. This may also contribute to other health benefits and improved psychosocial well being. Although these positive effects on quality of life could be a translation of improved cardiopulmonary fitness, further studies are required to evaluate this aspect in detail.
Overall completeness and applicability of evidence
The intervention programs in studies which were successful in improving asthma symptoms included aerobic conditioning using a treadmill, other aerobic exercises or swimming. In three out of the four studies that were successful in improving asthma symptoms, exercise was coupled with an asthma education program and breathing exercises (Gonçalves 2008; Mendes 2010; Mendes 2011). Therefore, the effects seen may represent those of a package of care, rather than exercise alone, although this cannot be definitely proven.
Currently, Global Initiative for Asthma guidelines do not provide recommendations for physical training or exercise therapy for asthma management (GINA 2012). It may be appropriate for people with asthma who have stable disease to participate in regular aerobic exercise training programs provided they are educated about prevention and treatment of exercise‐induced asthma. This may improve asthma management and reduce the health risks associated with a sedentary lifestyle.
Quality of the evidence
We were unable to assess reporting biases for outcomes in this review due to insufficient numbers of included studies for each outcome (minimum of 10 included studies required for each outcome). As such, the effect of reporting biases on the outcomes in this review are unknown.
Potential biases in the review process
Selecting only randomised controlled trials is a trade off, allowing higher quality evidence to be meta‐analysed, on which to base future investigations and research. However, this does have the potential of introducing selection bias by excluding relevant studies which do not fulfil the strict criteria for inclusion within the review. In addition, this review, like all others, is potentially susceptible to publication bias, though attempts are made to reduce this as much as possible with the authors searching the relevant databases for published and non‐published studies. Despite numerous attempts to contact study authors for raw data, the inability to obtain all relevant information might introduce a bias that has the potential to alter the outcome of the meta‐analysis. Biases that occur due to poor reporting of trial methodology may not be adequately accounted for, despite the rigorous assessment by two independent review authors.
Agreements and disagreements with other studies or reviews
There are many studies (Dogra 2010; Mancuso 2013; Mendes 2010; Turner 2010) and reviews (Avallone 2012; Morton 2011) now reporting that physical activity does not exacerbate asthma symptoms or compromise asthma control, and associations with improvements in asthma are being observed. Likewise the review by Eijkemans 2012, focusing on longitudinal studies, indicated that physical activity may produce a protective effect against asthma development and that individuals with higher levels of physical activity may have a lower risk of developing asthma. A 2012 review of asthma and aerobic exercise produced similar findings to this review in that regular aerobic exercise improved asthma symptom management, lung function and mental health (Avallone 2012). This review included eight studies examining exercise habits, six examining exercise‐related symptom perception and physiological responses, and nine looking at aerobic exercise as an intervention for asthma. Overall this empirical evidence also suggested that individuals with asthma are less likely to engage in physical activity than those without asthma; individuals with asthma are not biased in their subjective reporting of symptoms during aerobic exercise and physical inactivity among individuals with asthma has an association with negative health consequences and increased asthma‐related difficulties. However, studies examining the mechanisms by which physical training may impact asthma outcomes are lacking (Eijkemans 2012; Scott 2013b). Some studies included in this review have examined several biological indicators (serum IgE, histamine responsiveness, sputum and serum eosinophil cell count, C‐reactive protein (CRP) levels) suggesting that exercise may actually reduce airway inflammation (Boyd 2012; Matsumoto 1999; Mendes 2011; Moreira 2008; Wicher 2010). This paucity of data remains and needs to be addressed in order to advance the understanding of and ability to use physical activity to improve asthma care.
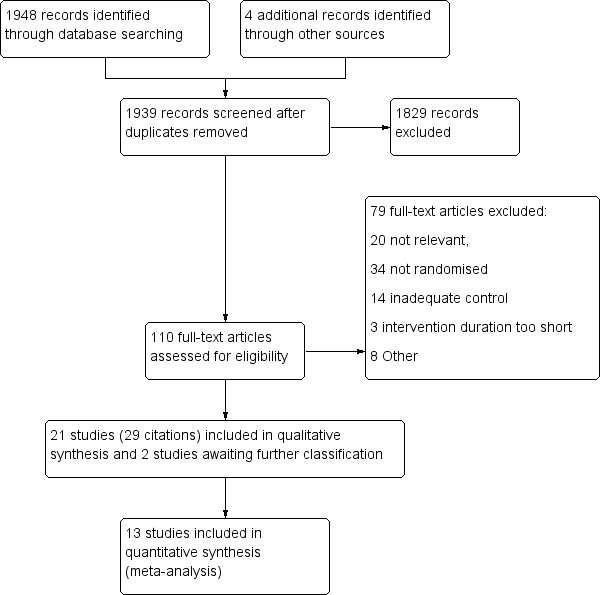
Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Physical training versus control, Outcome 1 PEFR (L/min) ‐ Fixed effect model.
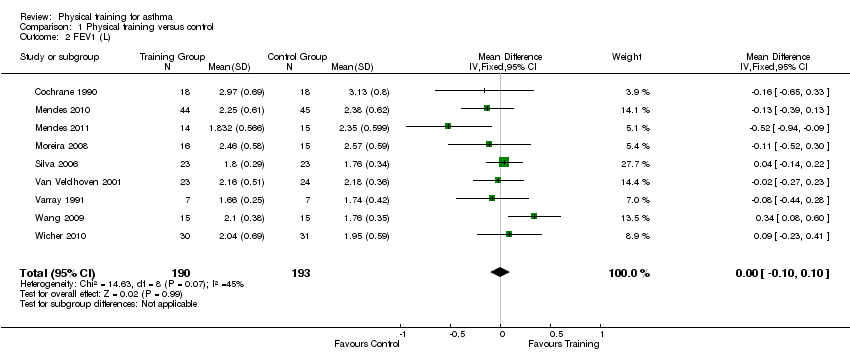
Comparison 1 Physical training versus control, Outcome 2 FEV1 (L).
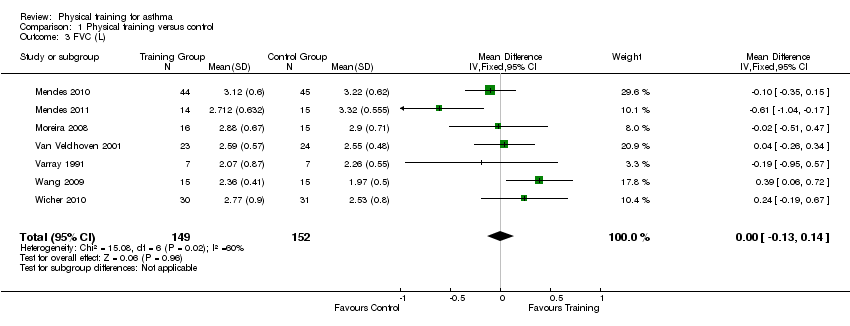
Comparison 1 Physical training versus control, Outcome 3 FVC (L).

Comparison 1 Physical training versus control, Outcome 4 VEmax (L/min).

Comparison 1 Physical training versus control, Outcome 5 VOmax (mL/kg/min).
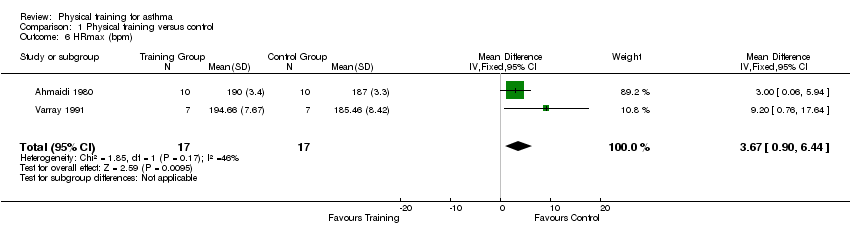
Comparison 1 Physical training versus control, Outcome 6 HRmax (bpm).
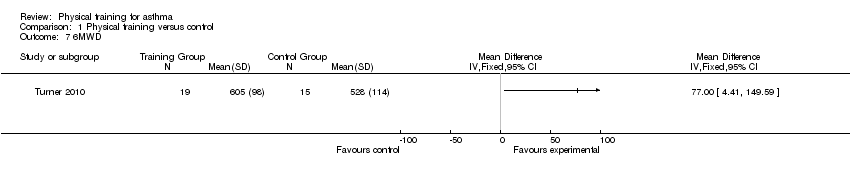
Comparison 1 Physical training versus control, Outcome 7 6MWD.
| Physical training for asthma | ||||||
| Patient or population: patients with asthma aged eight years or older | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Physical training | |||||
| Asthma symptoms measured using various techniques. Follow‐up: 6 to 24 weeks | See comment | See comment | N/A | 315 (9 studies) | ⊕⊝⊝⊝ | We were unable to pool data for this outcome due to heterogeneity in the instruments used; 3 studies found symptoms lasted fewer days, 5 studies reported symptoms were unchanged; 1 study reported significant improvement. |
| Quality of life Measured using various scales. Follow‐up: 12 to 18 weeks | See comment | See comment | N/A | 212 (5 studies) | ⊕⊝⊝⊝ | We were unable to pool data for this outcome due to heterogeneity in the quality of life scales used. 4 studies found clinically significant improvement for total scores immediately after physical training,1 study found no significant difference. |
| Exercise tolerance Measured using 6MWD Follow‐up: 18 weeks | See comment | See comment | N/A | 34 (1 study) | ⊕⊕⊝⊝ | There was a statistically insignificant increase in the 6MWD in one study. |
| PEFR | See comment | See comment | N/A | 77 153 (4 studies in total) | ⊕⊝⊝⊝ | We were unable to pool data for this outcome due to heterogeneity between study populations as per the I2 statistic; The results were originally analysed for two of the four studies assessing this outcome using the fixed‐effect model, when the random‐effects model was applied, the statistical significance disappeared. The minimally important difference is estimated to be a mean change of 11.9 (95% CI 7.3 to 16.1) (Karras 2000), which has been met in both these analyses. Data from two studies showed no change in PEFR, however, we were unable to combine data due to a high dropout rate in one study and unsuitable data for imputation in the other. Possible sources of clinical heterogeneity include swimming versus gymnasium activities and 6 versus 12 week intervention duration. |
| VEmax | The mean VEmax ranged across control groups from | The mean VEmax in the intervention groups was | 200 | ⊕⊕⊝⊝ | ||
| VOmax | The mean VOmax ranged across control groups from | The mean VOmax in the intervention groups was | 267 | ⊕⊕⊝⊝ | ||
| HRmax | The mean HRmax ranged across control groups from | The mean HRmax in the intervention groups was | 34 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Methods of randomisation, allocation concealment and/or any attempts to blind outcome assessors were not described for the majority of studies assessing this outcome (limitations of design (‐1)) 5 Single study Abbreviations: 6MWD: six‐minute walking distance; bpm: heart beats per minute; HRmax: maximum heart rate; PEFR: peak expiratory flow rate; VEmax: maximal expiratory volume (the maximum volume of air that can be breathed in 1 min during exercise); VOmax: maximal oxygen consumption (the maximum amount of oxygen in millilitres used while exercising). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PEFR (L/min) ‐ Fixed effect model Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 FEV1 (L) Show forest plot | 9 | 383 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.10, 0.10] |
| 3 FVC (L) Show forest plot | 7 | 301 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.13, 0.14] |
| 4 VEmax (L/min) Show forest plot | 5 | 200 | Mean Difference (IV, Fixed, 95% CI) | 3.08 [‐0.63, 6.79] |
| 5 VOmax (mL/kg/min) Show forest plot | 8 | 267 | Mean Difference (IV, Fixed, 95% CI) | 4.92 [3.98, 5.87] |
| 6 HRmax (bpm) Show forest plot | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | 3.67 [0.90, 6.44] |
| 7 6MWD Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

