Agents fibrinolytiques contre l'occlusion artérielle périphérique
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: RCT parallel group. | |
| Participants | Country: United Kingdom. Setting: hospital. No of patients: 60; 20 i.v. rt‐PA, 20 i.a. SK, 20 i.a. rt‐PA. Mean age: 71 years. Gender: 39 M; 21 F. Inclusion criteria: peripheral lower limb ischaemia <30 days duration. Exclusion criteria: clinically apparent arterial emboli treated with surgical embolectomy. | |
| Interventions | Treatment: 3 infusion protocols. (i) i.a. streptokinase infused at 5000 U/hr with 250 U/hr heparin (20pts); Duration of treatment: complete lysis achieved or patient deterioration or lytic stagnation after a 12 hr period. Follow up: 30 days and 3 months. | |
| Outcomes | Primary: limb salvage, amputation, death. Secondary: death, major haemorrhage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Information on allocation concealment not provided. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Exclusions post‐randomisation: 6 not included in the analysis. Lost to follow up: none. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Study financially supported by Boehringer Ingelheim who supplied recombinant tissue plasminogen activator. |
| Methods | Study design: prospective RCT. | |
| Participants | Country: Germany and Switzerland. Setting: hospital. No of patients: 234. Mean age: 69.5 years rt‐PA; 70.1 years UK. Gender: M 81 (65%) rt‐PA; M 64 (58%) UK. Inclusion criteria: angiographically documented thrombotic or embolic occlusions five to 40 cm length. Exclusion criteria: thrombi > 6 months old or emboli > 6 weeks. Standard exclusions for increased bleeding risk from thrombolysis. | |
| Interventions | (i) rt‐PA; (ii) UK; Either end hole catheter or microporous balloon. End hole catheter 2.5 mg/hr rt‐PA or 100,000 IU/hr UK. Microporous balloon 0.5 mg/cm thrombus length rt‐PA or 20,000 IU/cm thrombus length. Duration of treatment: end hole catheter serially advanced. Microporous balloon one hour if complete lysis not achieved then infusion lysis and secondary intervention. Follow up: end of lysis and at six months; 81% reached six month follow up. | |
| Outcomes | Primary: vessel patency, time to lysis, amputation, death. Secondary: complications including bleeding and cerebral haemorrhage. | |
| Notes | Techniques not randomised ‐ dependant on local practice ‐ but use of rt‐PA or UK was randomised. Complex trial as two infusion techniques used; the microporous balloon more rapid pharmacomechanical action but no difference in outcomes by technique or drug at follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks of 10. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 55 patients lost to follow up but no information on statistical handling of missing data. Exclusion post‐randomisation: none recorded. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported. |
| Other bias | Unclear risk | Supported by a grant from Boehringer Ingelheim who manufactured the Alteplase. |
| Methods | Study design: RCT. | |
| Participants | Country: USA. Setting: hospital. No of patients: 32. Mean age: 58 years. Gender: 22 M and 10 F. Inclusion criteria: native/bypass occlusion < 90. Exclusion criteria: standard exclusions for increased bleeding risk from thrombolysis. | |
| Interventions | (i) rt‐PA 10 mg intrathrombic dose then 5 mg/hr up to 24 hours. (ii) UK dose 60,000 IU intrathrombic bolus and 240,000 IU/h for two hrs, 120,000 IU/h for two hrs and 60,000 IU for up to 20 hours. Duration of treatment: 24 hours. Follow up: end of lysis for degree of lysis and surgery, angioplasty, death, limb loss within 30 days. | |
| Outcomes | Primary: 95% lysis and restoration of flow, time to lysis. Secondary: surgery, angioplasty, death, limb loss within 30 days, blood transfusion (major haemorrhage) within 72 hours. | |
| Notes | Lysis continued for 18 to 72 hours in 6 urokinase (4 successful). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by means of consecutive numbers. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Exclusions post‐randomisation: none recorded. No losses to follow up. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Supported in part by Genentech Inc (Activase manufacturer) and Abbott Laboratories (Abbokinase manufacturer). |
| Methods | Phase II randomised, multicentre double blind study. | |
| Participants | Country: USA. Setting: hospital. No of patients: 241. Mean age: 65.1 years. Gender: M 143 (62.7%); F 85 (37.3%). Inclusion criteria: patients with lower extremity native artery or graft occlusion of less than or equal to 14 days duration. Exclusion criteria: standard exclusions for increased bleeding risk from thrombolysis. | |
| Interventions | (i) pro‐urokinase 2 mg, 4 mg, 8 mg/hr for 8 hours followed by 0.5 mg/hr in each group. (ii) urokinase group 4000 IU/min for 4 hours followed by 2000 IU/min. Thrombus lacing dose based on length of occlusion. Duration of treatment: > 95% clot lysis or 24 hours. Follow up: 30 days. | |
| Outcomes | Primary: vessel patency, time to lysis, dose of study drug, amputation, death. Secondary: complications including bleeding. | |
| Notes | Dose response curve for pro‐urokinase derived. Fibrin specificity of prourokinase lost at equipotent doses with urokinase. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stratified by type of occlusion; native artery or bypass graft. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) | Low risk | All analyses based on participants who received their randomised treatment. Of 241 patients randomised 13 patients were entered into the trial but never received the specified therapy (reasons given). No losses to follow up recorded. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Some authors were affiliated to Abbott Laboratories manufacturers of Abbokinase. |
| Methods | Study design: RCT. | |
| Participants | Country: Germany. Setting: hospital. No of patients: 120; 60 rt‐PA, 60 UK. Mean age: 65 years. Gender: M 60%, F 40% UK; M 57%, F 43% rt‐PA. Inclusion criteria: < 3 months occlusion of femoropopliteal segment. Exclusion criteria: not recorded. | |
| Interventions | (i) rt‐PA 5 mg intrathrombic bolus followed by infusion at 5 mg/hr plus heparin 750 IU/hr. Treatment duration: rt‐PA 1 to 4 hrs (mean 2 hrs); UK 6 to 72 hrs (mean 24 hours). Follow up: 6 months follow up. | |
| Outcomes | Primary: amputation, Fontaine, ABPI, vessel patency. Secondary: death. | |
| Notes | Fontaine IIb claudication 49% of study population. Short occlusion lengths 6 cm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but method not given. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Exclusions post‐randomisation: none recorded. Two patients lost to follow‐up but no information on statistical handling of missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | No declarations of interest stated, no information given regarding sponsorship. |
i.a. intra‐arterial
IU international units
i.v. intravenous
RCT randomised controlled trial
rt‐PA recombinant tissue plasminogen activator
SK streptokinase
UK urokinase
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Comparison of dose regimes for high and low dose rt‐PA | |
| Comparison of rt‐Pa versus urokinase but non‐randomised selection by surgeons preference | |
| Non‐randomised streptokinase vs rt‐PA | |
| Non‐randomised | |
| Comparison of angioplasty versus rt‐PA and angioplasty | |
| Only partially randomised 10 of 40 patients; insufficient for analysis | |
| Not a true comparison of agents; use of abxicimab as an adjunctive agent studied. Complex randomisation 5:2 to support complication rate as major finding may have impact on study findings | |
| Not a comparison of thrombolytic agents | |
| Paper reports NAPA II/III trials. Not a comparison of thrombolytic agents ‐ comparison with placebo. Safety and efficacy trial with primary end point of avoidance of open vascular surgery within 30 days of treatment | |
| Non‐comparative and non‐randomised | |
| Not a comparison of agents | |
| Randomised but 11 patients in one group and 6 in the other group ‐ method of randomisation not stated, clinical enrolment criteria not accurately described | |
| Not arterial thrombolysis | |
| Venous thromboembolism | |
| Subset of patients comparing urokinase and rt‐PA but insufficient detail reported to permit analysis. Authors report no difference between rt‐PA and urokinase | |
| Comparison is of the effect of the addition of abxicimab to thrombolytic agents and not a true comparison of agents. More appropriately considered under infusion techniques | |
| Staphylokinase. Pilot study. No apparent randomisation. Not blinded. No control group ‐ study compared two different infusion protocols | |
| Comparison of urokinase and danshen root |
rt‐PA recombinant tissue plasminogen activator
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vessel Patency immediately post lysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 1 Vessel Patency immediately post lysis. | ||||
| 2 Asymptomatic Limb salvage at 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 2 Asymptomatic Limb salvage at 30 days. | ||||
| 3 Amputation at 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 3 Amputation at 30 days. | ||||
| 4 Death Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 4 Death. | ||||
| 5 Complications‐ major haemorrhage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5 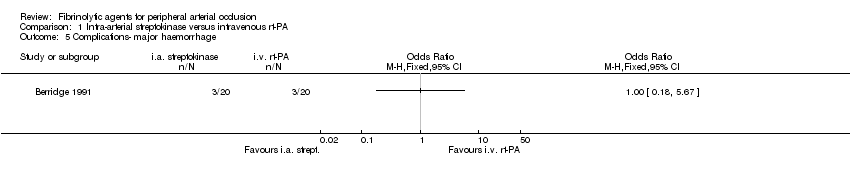 Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 5 Complications‐ major haemorrhage. | ||||
| 6 Complications‐ minor haemorrhage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 6 Complications‐ minor haemorrhage. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vessel patency immediately post lysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 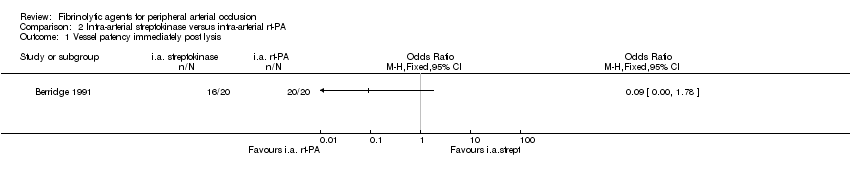 Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 1 Vessel patency immediately post lysis. | ||||
| 2 Asymptomatic Limb salvage at 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 2 Asymptomatic Limb salvage at 30 days. | ||||
| 3 Amputation at 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 3 Amputation at 30 days. | ||||
| 4 Death Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 4 Death. | ||||
| 5 Complications‐ major haemorrhage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 5 Complications‐ major haemorrhage. | ||||
| 6 Complications‐ minor haemorrhage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 6 Complications‐ minor haemorrhage. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vessel patency immediately post lysis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 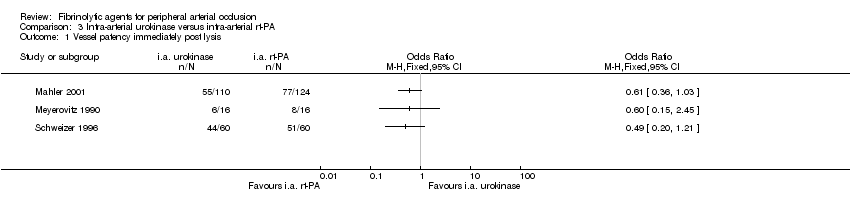 Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 1 Vessel patency immediately post lysis. | ||||
| 2 Limb salvage Show forest plot | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.68, 4.15] |
| Analysis 3.2 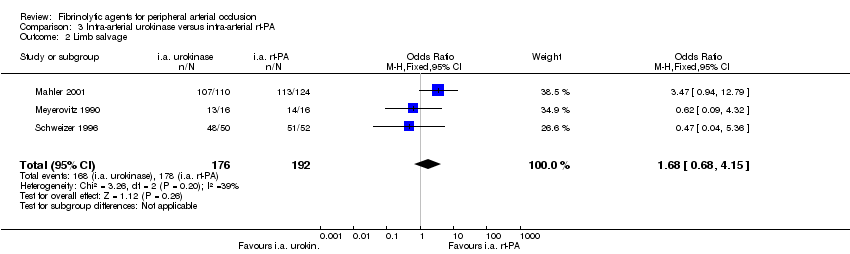 Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 2 Limb salvage. | ||||
| 3 Major amputation at 30 days‐6 months Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 3 Major amputation at 30 days‐6 months. | ||||
| 4 Death Show forest plot | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.24, 2.54] |
| Analysis 3.4  Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 4 Death. | ||||
| 5 Complications ‐ major haemorrhage Show forest plot | 3 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.19, 2.40] |
| Analysis 3.5  Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 5 Complications ‐ major haemorrhage. | ||||
| 6 Complications ‐ minor haemorrhage Show forest plot | 3 | 386 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.26] |
| Analysis 3.6 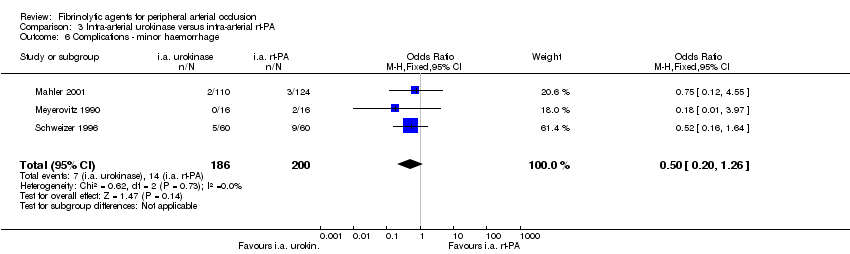 Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 6 Complications ‐ minor haemorrhage. | ||||
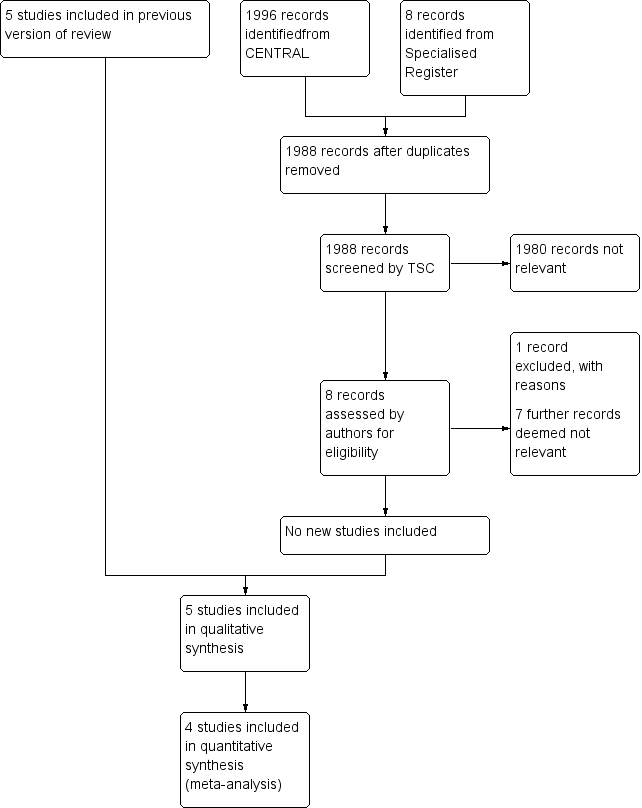
Study flow diagram.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
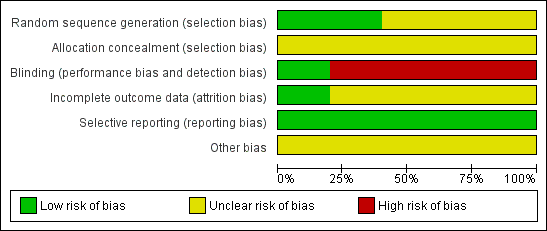
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 1 Vessel Patency immediately post lysis.
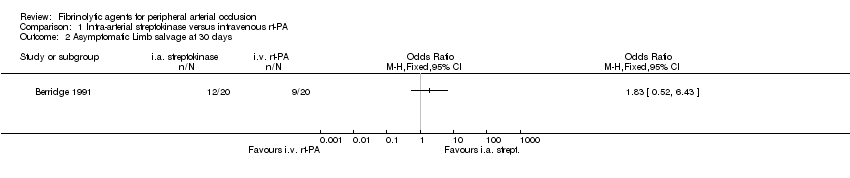
Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 2 Asymptomatic Limb salvage at 30 days.
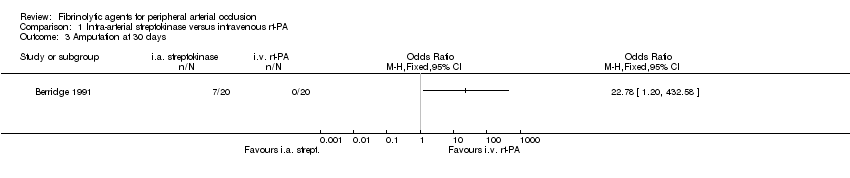
Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 3 Amputation at 30 days.
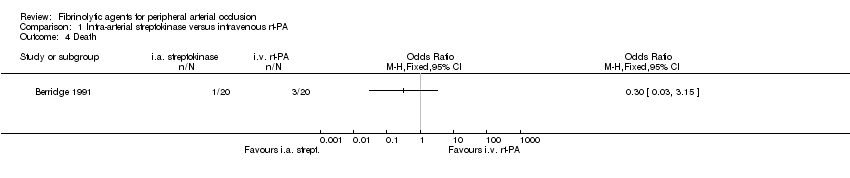
Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 4 Death.
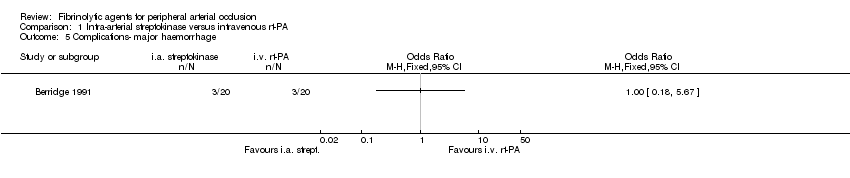
Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 5 Complications‐ major haemorrhage.

Comparison 1 Intra‐arterial streptokinase versus intravenous rt‐PA, Outcome 6 Complications‐ minor haemorrhage.

Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 1 Vessel patency immediately post lysis.

Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 2 Asymptomatic Limb salvage at 30 days.

Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 3 Amputation at 30 days.
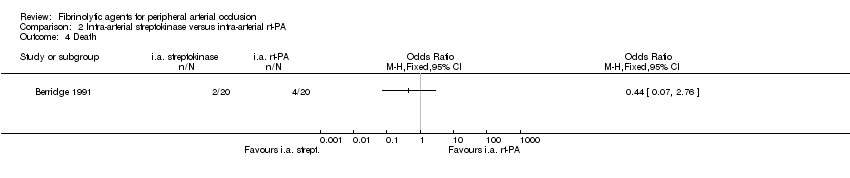
Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 4 Death.

Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 5 Complications‐ major haemorrhage.

Comparison 2 Intra‐arterial streptokinase versus intra‐arterial rt‐PA, Outcome 6 Complications‐ minor haemorrhage.

Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 1 Vessel patency immediately post lysis.
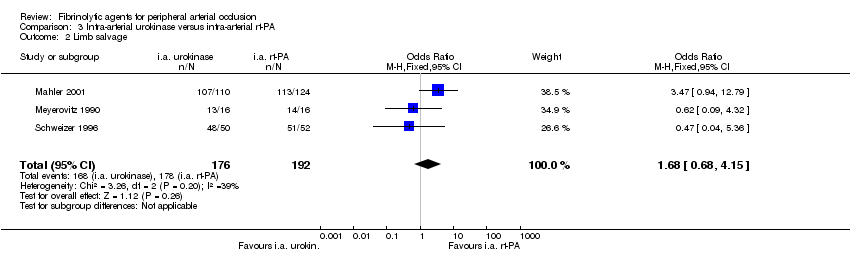
Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 2 Limb salvage.

Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 3 Major amputation at 30 days‐6 months.

Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 4 Death.
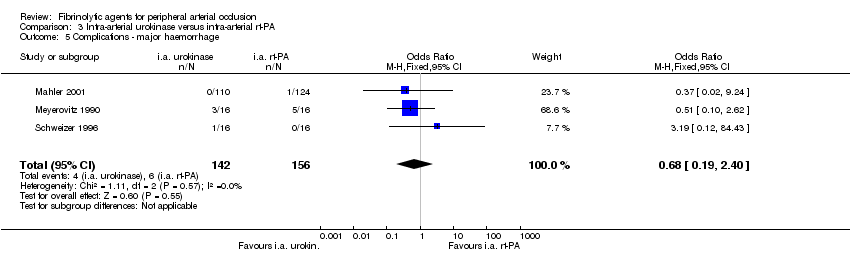
Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 5 Complications ‐ major haemorrhage.

Comparison 3 Intra‐arterial urokinase versus intra‐arterial rt‐PA, Outcome 6 Complications ‐ minor haemorrhage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vessel Patency immediately post lysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Asymptomatic Limb salvage at 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Amputation at 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Death Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Complications‐ major haemorrhage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Complications‐ minor haemorrhage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vessel patency immediately post lysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Asymptomatic Limb salvage at 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Amputation at 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Death Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Complications‐ major haemorrhage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Complications‐ minor haemorrhage Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vessel patency immediately post lysis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Limb salvage Show forest plot | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.68, 4.15] |
| 3 Major amputation at 30 days‐6 months Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Death Show forest plot | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.24, 2.54] |
| 5 Complications ‐ major haemorrhage Show forest plot | 3 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.19, 2.40] |
| 6 Complications ‐ minor haemorrhage Show forest plot | 3 | 386 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.26] |

