Hypothermia for traumatic brain injury
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 48 Baseline characteristics: Therapeutic cooling Age: mean (SD) 6.92 (± 3.09) years Type of injury (closed/open): closed GCS on admission: mean (SD) 5.74 (± 1.39) No cooling Age: mean (SD) 6.86 (± 3.81) years Type of injury (closed/open): closed GCS on admission: mean (SD) 5.64 (± 1.63) Inclusion criteria: children 0 to 156 months of age, with non‐penetrating head injury, GCS ≤ 8, motor score < 6 Exclusion criteria: normal initial CT scan, extended hypotension for > 15 minutes, coagulopathy admission > 6 hours after injury, brain death on initial examination Country: USA Setting: hospital. 6 medical centres | |
| Interventions | Therapeutic cooling Number of participants randomised: 23 Method of cooling: surface cooling using a hypothermia/heating blanket. Sedation and paralysis induced to prevent shivering Target temperature: 32 to 33 °C Time of cooling: < 6 hours Duration of cooling: 48 hours Rewarming details: after 48 hours, passive warming, 1 °C every 3 to 4 hours until 36.5 °C reached. Initiation of warming not stopped due to intracranial hypotension but slowed at times due to elevations in ICP Site of temperature measurement: rectal No cooling Number of participants: 25 Method of temperature management: participants were passively warmed if their initial presenting core temperature was < 36 °C Target temperature: 36.5 to 37.5 °C | |
| Outcomes | Outcomes measured in study: mortality, ventricular arrhythmias, delayed intracranial haemorrhage, intra‐abdominal/thoracic bleeding, coagulopathy infection (pneumonia), GOS (at 3 and 6 months), age‐appropriate neurocognitive tests, intracranial pressure, other secondary clinical outcomes | |
| Notes | Funding/declarations of interest: National Institute of Health grant, General Clinical Research Centre at Children's Hospital of Pittsburgh grant Study dates: July 1999 to December 2003 Note: study authors reported GOS at 3 months as "no difference between treatment groups" with P = 0.799; and GOS at 6 months with P = 0.540 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised and stratification done by age of child at time of injury. No additional details |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details provided |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Not possible to blind personnel; unlikely to influence performance |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Not possible to blind personnel; unclear if this would influence performance |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Not possible to blind personnel; unclear if this would influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Lack of blinding unlikely to influence outcome assessment |
| Blinding of outcome assessment (detection bias): GOS | Low risk | Neurocognitive tests were performed by the site neuropsychologist or technician who were blinded to the therapeutic intervention |
| Incomplete outcome data (attrition bias) | Low risk | Low for mortality and pneumonia. Small loss of follow‐up at 3 and 6 months for GOS data, although not reported by treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 27 Baseline characteristics: Therapeutic cooling Age: mean (SD) 7.17 (± 6.64) years Type of injury (closed/open): closed GCS on admission: mean (SD) 6.42 (± 1.2) No cooling Age: mean (SD) 5.6 (± 5.23) years Type of injury (closed/open): closed GCS on admission: mean (SD) 6.23 (± 1.2) Inclusion criteria: children 0 to 214 months of age, with non‐penetrating head injury, GCS ≤ 8, within 24 hours of admission Exclusion criteria: normal initial CT scan, penetrating brain injury, brain death on admission to emergency department, failure to obtain informed consent 24 hours from admission, uncorrectable coagulopathy (PT/PTT > 16/40 seconds), hypotensive episode for > 5 minutes defined as SBP < fifth percentile for age Country: USA Setting: hospital (single centre) | |
| Interventions | Therapeutic cooling Number of participants: 14 Method of cooling: surface cooling using a hypothermia/heating blanket. Sedation and paralysis induced to prevent shivering Target temperature: 32 to 33 °C Time of cooling: < 6 hours Duration of cooling: 48 hours Rewarming details: after 48 hours, passive warming, 1 °C every 3 to 4 hours until 36.5 °C reached. Initiation of warming not stopped due to intracranial hypotension but slowed at times due to elevations in ICP Site of temperature measurement: rectal No cooling Number of participants: 13 Method of temperature management: participants were passively warmed if their initial presenting core temperature was < 36 °C Target temperature: 36.5 to 37.5 °C | |
| Outcomes | Outcomes measured in study: mortality, ventricular arrhythmias, delayed intracranial haemorrhage, intraabdominal/thoracic bleeding, coagulopathy infection (pneumonia), GOS, Infant Health Questionnaire, Vineland Adaptive Behaviour scale, ICP, other secondary clinical outcomes | |
| Notes | Funding/declarations of interest: National Institute of Health grant, General Clinical Research Centre at Children's Hospital of Pittsburgh grant Study dates: August 2001 to December 2003 Note: HYPO 2 was a single‐centre trial set up later due to recruitment issues in HYPO 1, but running in parallel to HYPO 1 with a different inclusion criteria. All other methods and outcomes were the same as HYPO 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised and stratification done by age of child at time of injury. No additional details |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details provided |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Not possible to blind personnel; unlikely to influence performance |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Not possible to blind personnel; unclear if this would influence performance |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Not possible to blind personnel; unclear if this would influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Lack of blinding unlikely to influence outcome assessment |
| Blinding of outcome assessment (detection bias): GOS | Low risk | Neurocognitive tests (GOS) were performed by the site neuropsychologist or technician who were blinded to the therapeutic intervention |
| Incomplete outcome data (attrition bias) | Low risk | Low for mortality and pneumonia. Small loss of follow‐up at 3 and 6 months for GOS data, not reported by treatment groups. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel design | |
| Participants | Total number of participants: 77 Baseline characteristics: Therapeutic cooling Age: median (IQR) 9.7 (4.2 to 14.5) years Gender: 21/18 Weight (kg): median (IQR) 30 (15.4 to 62) Type of injury (closed/open): closed GCS: median (IQR) 6 (5 to 7) No cooling Age: median (IQR) 12.5 (3.3 to 14.8) years Gender M/F: 27/11 Weight (kg): median (IQR) 38 (16 to 60) Type of injury (closed/open): closed GCS on admission: median (IQR) 6 (5 to 7) Inclusion criteria: 0 to 17 years of age, non‐penetrating brain injury, GCS 3 to 8, motor score on GCS < 6 after resuscitation, within 6 hours of injury Exclusion criteria: normal CT, GCS 3, unreactive pupils, hypotension for > 10 minutes, uncorrectable coagulopathy, hypoxia, abbreviated injury severity score of ≥ 4 for organs other than the brain, suspected pregnancy, or unavailable parent or guardian to consent Country: 15 sites across USA, New Zealand and Australia Setting: emergency department and ICU | |
| Interventions | Therapeutic cooling Number of participants: 39 Method of cooling: cold IV saline 20 to 30 mL/kg followed by surface cooling with a cooling mattress Target temperature: 32 to 33 °C Time of cooling: within 6 hours of injury Duration of cooling: 48 hours Rewarming details: 0.5 to 1 °C every 12 to 24 hours Site of temperature measurement: rectal or brain No cooling Number of participants: 38 Method of temperature management: warming/cooling mattress to maintain normothermia Target temperature: 36.5 to 37.5 °C | |
| Outcomes | Outcomes measured in study: 3 month mortality, GOS, GOS‐E Peds, adverse events, serious adverse events (to include pneumonia) | |
| Notes | Funding/declarations of interest: National Institute of Neurological Disorders and Stroke and National Institutes of Health. Sponsor had no role in data collection, analysis or interpretation, but involved in study design. Study dates: November 2007 to February 2011 Note: trial stopped for futility. Clinical trials registration NCT00222742 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "web‐based random assignment algorithm" |
| Allocation concealment (selection bias) | Low risk | "Randomisation was done by the site study coordinator after screening for eligibility" |
| Blinding of participants and personnel (performance bias): mortality | Low risk | "Emergency service personnel, study nurses involved in randomisation, and personnel who managed the patients were unmasked to treatment" Lack of blinding unlikely to influence performance |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | "Emergency service personnel, study nurses involved in randomisation, and personnel who managed the patients were unmasked to treatment" Unclear if lack of blinding would influence performance |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | "Emergency service personnel, study nurses involved in randomisation, and personnel who managed the patients were unmasked to treatment" Unclear if lack of blinding would influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | "Investigators who assessed outcomes were masked to treatment allocation" |
| Blinding of outcome assessment (detection bias): GOS | Low risk | "Investigators who assessed outcome were masked to treatment allocation" |
| Incomplete outcome data (attrition bias) | Low risk | One participant lost to follow‐up at 3 months for Unfavourable outcome, but small number unlikely to introduce bias |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trials registration (NCT00222742). Outcomes reported at 3 months, not 6 and 12 months as stated in protocol. Use of GCS also not reported nor subgrouped by age |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 26 Baseline characteristics: Therapeutic cooling Age: range 9 to 76 years. Mean (SD) 34 (± 6) years Type of injury: not stated that participants required 'closed' injury for study inclusion. However, participants' diagnoses were for cerebral contusion, epidural haematoma, subdural haematoma, traumatic subarachnoid haemorrhage, intracerebral haematoma, and diffuse axonal injury Gender M/F: 12/3 GCS on admission: mean (SD) 5.7 (± 0.3) No cooling Age: range 4 to 76. Mean (SD) 38 (± 8) years Type of injury: as above Gender M/F: 8/3 GCS on admission: mean (SD) 5.7 (± 0.4) Inclusion criteria: GCS ≤ 8 points on admission to the emergency room and evidence of injury on CT scan Exclusion criteria: patients who had abdominal or chest trauma or those who sustained severe pulmonary infection. Patients who were admitted to the hospital > 8 hours after injury Country: Japan Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 15 Method of cooling: maintained by surface cooling ‐ no further details Target temperature: 32 to 33 °C Time of cooling: within 3 to 4 hours after injury Duration of cooling: normally 3 to 4 days Rewarming details: started if no signs of brain swelling on CT findings and no ICP elevation. Rewarming at rate of 1 °C per day Site of temperature measurement: not stated No cooling Number of participants: 11 Target temperature: 36 to 37 °C | |
| Outcomes | Outcomes measured in study: imbalance of thromboxane B2 (TXB2), 6‐keto prostaglandin F1a (6‐keto PGF1a), GOS at 6 months (to include mortality data at 6 months), complications (to include pneumonia) | |
| Notes | Funding/declarations of interest: not reported Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Not possible to blind personnel; unlikely to influence performance |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Not possible to blind personnel; unclear if this would influence performance |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Not possible to blind personnel; unclear if this would influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Lack of blinding unlikely to influence outcome assessment |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | "Independent neurosurgeons who were not aware of the study presented here determined the neurological outcome 6 months after injury" |
| Incomplete outcome data (attrition bias) | Unclear risk | "Unequal number of patients in each group was the result of exclusion of normothermic patients having chest or abdominal injuries" Assume this is a loss of 4 participants but is not clearly stated |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 387 Baseline characteristics: Therapeutic cooling Age: mean (SD) 37.4 (± 15.4) years Type of injury (closed/open): closed Gender M/F: 157/38 GCS on admission: GCS 3 to 8: 125/195; GCS 9 to 12: 45/195; GCS 12 to 15: 25/195 No cooling Age: mean (SD) 36.7 (± 14.9) years Type of injury (closed/open): closed Gender M/F: 164/28 GCS on admission: GCS 3 to 8: 129/192; GCS 9 to 12: 34/192; GCS 12 to 15: 29/192 Inclusion criteria: primary, closed traumatic brain injury, ICP > 20 mmHg for ≥ 5 minutes after stage 1 treatments, with no obvious reversible cause, initial head injury that had occurred ≤ 10 days earlier, availability of a cooling device or technique for ≥ 48 hours, a core temperature of ≥ 36 °C (at the time of randomisation), and an abnormal CT scan of the brain. Note change to inclusion criteria part way through trial, to remove upper age limit (previously 65 years) and to increase the time from injury from 72 hours to 10 days. Exclusion criteria: participants who were already receiving therapeutic hypothermia or who were unlikely to survive for the next 24 hours, administration of barbiturate infusion before randomisation, a temperature of ≤ 34°C at hospital admission, and pregnancy Country: Multicentre trial. 18 countries to include UK Setting: ICU, hospital | |
| Interventions | Therapeutic cooling Number of participants: 195 Method of cooling: hypothermia was induced by a bolus of intravenous, refrigerated 0.9% sodium chloride (20 to 30 mL/kg of body weight) and thereafter maintained with the usual cooling technique of each site Target temperature: 32 to 35°C Time of cooling: initial study phase was within 72 hours from time of injury, but investigators altered this to within 10 days after initial head injury Duration of cooling: ≥ 48 hours to ensure intracranial pressure maintained Rewarming details: after 48 hours at a rate of 0.25 °C per hour, provided that intracranial pressure was 20 mmHg or less Site of temperature measurement: bladder, oesophageal, pulmonary artery catheter, rectum No cooling Number of participants: 192 | |
| Outcomes | Outcomes measured in study: GOS (at 6 months), 6 month mortality, 28 day mortality (reported in supplementary index) lack of ICP, pneumonia, length of ICU stay, score on modified Oxford Handicap Scale, adverse outcomes | |
| Notes | Funding/declarations of interest: supported by the National Institute for Health Research Health Technology Assessment program, which funded the main phase of the study. The European Society of Intensive Care Medicine funded the pilot phase. The trial sponsors, the University of Edinburgh and NHS Lothian, provided research governance Study dates: November 2009 to October 2014 Note: Some data taken from supplementary protocol and appendix available with New England Journal of Medicine online publication of full study report (Andrews 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central Internet based randomisation service or a telephone randomisation service depending on the available technology at each site |
| Allocation concealment (selection bias) | Low risk | Use of external randomisation service |
| Blinding of participants and personnel (performance bias): mortality | Low risk | "Patients, families, and treating clinicians aware of the study‐group assignments" Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | "Patients, families, and treating clinicians aware of the study‐group assignments" Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | "An investigator who was unaware of the study‐group assignments scored all outcomes according to the standardized approach" |
| Blinding of outcome assessment (detection bias): GOS | Low risk | "An investigator who was unaware of the study‐group assignments scored all outcomes according to the standardized approach" |
| Incomplete outcome data (attrition bias) | Low risk | Some loss of participants not clearly explained, although few in number and unlikely to influence results |
| Selective reporting (reporting bias) | Unclear risk | Registered trial (ISRCTN34555414). Pneumonia reported as outcome in the protocol, but not reported in results. Supplementary protocol published with full study report |
| Other bias | High risk | Inclusion criteria altered part way through study to include participants up to 10 days after injury |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 50 Baseline characteristics: Therapeutic cooling Age: median (IQR) 11.0 (6.9 to 14.2) years Weight kg: median (IQR) 31.5 (24 to 53) Type of injury (closed/open): closed Gender M/F: 11/13 GCS on admission: median (IQR) 5.5 (3.5 to 7) No cooling Age: median (IQR) 9.5 (5.2 to 13.8) years Weight kg: median (IQR) 30 (20 to 50) Type of injury (closed/open): closed Gender M/F: 16/10 GCS on admission: median (IQR) 4.5 (3 to 7) Note: difference between groups, with participants in hypothermia group taking longer to get to study site Inclusion criteria: > 1 year and < 16 years of age, mechanically ventilated, GCS < 9 and an abnormal CT scan (intracranial haemorrhage or contusion, cerebral edema, or diffuse axonal injury) Exclusion criteria: not able to be randomised within 6 hours after injury or had a penetrating brain injury, fixed dilated pupils and a GCS = 3, proven cervical spinal cord injury, more than mild developmental disability prior to injury, an acute epidural hematoma evacuated and were expected to recover rapidly, a post‐traumatic clinical seizure with a normal CT scan, refractory shock (defined as a MAP < 2 SDs below mean for age despite > 80 mL/kg of IV resuscitation fluid), or suspected of having a non‐accidental injury Country: Australia, New Zealand and Canada Setting: PICU | |
| Interventions | Therapeutic cooling Number of participants: 24 Method of cooling: servo‐controlled cooling blanket. Iced IV bolus fluids (if indicated clinically), crushed ice to exposed surfaces, and a second cooling blanket above the child could also be used during induction Target temperature: 32 to 33 °C Time of cooling: No time given however randomisation had to be within 6 hours Duration of cooling: ≤ 72 hours Rewarming details: no faster than 0.5 °C every 3 hours but guided primarily by physiology rather than time. Rewarming slowed or stopped if hypotension or intracranial hypotension were not controlled Site of temperature measurement: oesophageal No cooling Number of participants: 26 Method of temperature management: participants randomised to normothermia were maintained at 36 to 37 °C Target temperature: 36 to 37 °C | |
| Outcomes | Outcomes measured in study: paediatric cerebral performance category, eligibility and recruitment rates, protocol violations, major adverse events, ICP and CCP during first 5 days, duration of mechanical ventilation, length of stay in PICU and hospital, adverse events including infectious complications (ventilator‐associated pneumonia, bacteraemia, cerebral abscess, meningitis, and urinary tract infection), bleeding, pancreatitis, acute respiratory distress syndrome, and arrhythmias (excluding sinus bradycardia), 'bad outcome' and mortality (reported at 12 months) | |
| Notes | Funding/declarations of interest: supported, in part, by grants from the Victorian Transport Accident Commission, the Neuro‐trauma Research Programme of the Western Australian Institute for Medical Research, and the Intensive Care Foundation of Australia and New Zealand. Study dates: November 2006 to May 2010; interruption to recruitment in 2008 due to publication of another study for 2 to 6 months Note: data given for 'bad outcome' using a different scoring system but this is comparable to GOS and therefore we have included it | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was in variable block sizes and was performed by telephone at the co‐ordinating center" |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered opaque envelopes were used" |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of blinding unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | High risk | "There were five major protocol violations (9%), four of which occurred in children randomised to hypothermia" 55 participants recruited but data only reported for 50 due to protocol violations. However this was unevenly spread between groups and reasons for violations are reported |
| Selective reporting (reporting bias) | Low risk | Prospectively registered study (NCT00282269). Reported outcomes are as stated in protocol |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 21 Baseline characteristics: Therapeutic cooling Age: mean (SD) 5.9 (± 2.9) years Type of injury (closed/open): closed GCS on admission: mean (SD) 4.7 (± 1.9) No cooling Age: mean (SD) 6.5 ( ± 3.7) years Type of injury (closed/open): closed GCS on admission: mean (SD) 5.6 (± 1.8) Inclusion criteria: children ≤ 18 years of age who presented with an admission GCS < 8 and admitted to the paediatric ICU within 6 hours of injury Exclusion criteria: GCS 8, evidence of clinical brain death, history of cardiopulmonary arrest at scene or on admission, existing ventriculoperitoneal or ventriculoatrial shunts, marked haemodynamic instability, or admission 6 hours after time of injury, children with significant intra‐abdominal pathology or bleeding diathesis Country: USA Setting: PICU | |
| Interventions | Therapeutic cooling Number of participants: 10 Method of cooling: all participants had cooling blanket placed underneath them Target temperature: 32 to 34 °C Time of cooling: immediately after enrolment over 4 hour period Duration of cooling: 48 hours Rewarming details: over 12 hours, not exceeding 1 °C per hour Site of measurement: rectal No cooling Number of participants: 11 Method of temperature management: all participants had cooling blanket placed underneath them Target temperature: 36.5 to 37.5 °C | |
| Outcomes | Outcomes measured in study: mortality (during study period; see note below), GOS (at 3, 6, 12 months), measurements of ICP, cerebral perfusion pressure, MAP, daily cerebral physiologic data, lactate‐oxygen index | |
| Notes | Funding/declarations of interest: supported, in part, by Cook Critical Care, Bloomington, IN (jugular bulb catheters) and Codman, Johnson & Johnson Professional, Raynham, MA (intracranial pressure monitors) Study dates: March 1998 to April 1999 Note: 2 participants excluded from later data analysis due to death in first 24 hours. These participants were included in this review for the mortality outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After enrollment the children were randomised to either normothermia or hypothermia group" No additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Lack of blinding unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Low risk | "Clinical nurse research co‐ordinator who was blinded to the patients treatment arm performed the evaluation and scoring" |
| Incomplete outcome data (attrition bias) | Low risk | Two participants were excluded from later analysis due to death within 24 hours, but possible to include these for the primary outcome |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | "There were a greater number of patients with Glasgow Coma Scale of 3 in the hypothermia group" Unclear if this may have influenced the results |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 60 Baseline characteristics: Therapeutic cooling Age: 34.1 years (does not state if mean/median) Gender M/F: 24/6 Type of injury (closed/open): closed GCS on admission: 5.01 (does not state if mean/median) No cooling Age: 33.2 years (does not state if mean/median) Gender M/F: 23/7 Type of injury (closed/open): closed GCS on admission: 4.98 (does not state if mean/median) Inclusion criteria: 18 to 60 years of age, closed head injury with GCS averaging 3 to 8, within 6 hours of injury Exclusion criteria: pregnant or breastfeeding women, patients with severe organic disease, psychonosema and hormone‐sensitive history Country: China Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 30 Method of cooling: semiconductor cooling blankets, high‐dose barbiturates Target temperature: 33 to 35 °C Time of cooling: immediately Duration of cooling: 3 to 10 days, until ICP reduced to normal level for 24 hours Rewarming details: not reported Site of measurement: not reported No cooling Number of participants: 30 Method of temperature management: not reported Target temperature: not reported | |
| Outcomes | Outcomes measured in study: mortality, living qualities of survivors and neurobiochemical indexes, GOS at 3 to 6 months | |
| Notes | Funding/declarations of interest: not reported Study dates: September 1997 to January 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details; lack of blinding unlikely to influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of blinding unlikely to influence results |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | Limited detail in paper; not possible to judge if any other sources of bias |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 10 Baseline characteristics: Therapeutic cooling Characteristics poorly reported in study No cooling Characteristics poorly reported in study Inclusion criteria: GCS 4 to 8, traumatic brain injury without major systemic injuries Exclusion criteria: inability to initiate cooling > 6 hours from injury, major injuries to other organ systems, < 15 years of age, likelihood of no family members available for consent within 24 hours of randomisation Country: USA Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 5 Method of cooling: cooling blankets set at 5 °C on participant's dorsal and ventral surfaces, iced saline lavage of the stomach was used to accelerate cooling in most cases Target temperature: 30 to 32 °C Time of cooling: within 6 hours of injury Duration of cooling: 24 hours Rewarming details: gradual warming over 24 hours, no further details Site of temperature measurement: intravascular temperature from Swan‐ganz catheter, oesophageal No cooling Number of participants: 5 | |
| Outcomes | Outcomes measured in study: GOS at 3 months (to include mortality at 3 months), complications | |
| Notes | Funding/declarations of interest: no details Study dates: not reported Note: reported two studies in one paper. One study reported participants undergoing elective craniotomy and one study reported participants with severe brain injury. We only included data for participants from the brain injury group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised to groups and groups stratified by GCS scale; no additional details of methods used for randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Not stated. However, a lack of blinding is unlikely to influence mortality and pneumonia outcomes |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | Lack of blinding could influence assessment of GOS |
| Incomplete outcome data (attrition bias) | Low risk | Severe brain injury section of the study: primary outcome (3 month GOS) reported data for all participants (n = 10) in the first series. Data from second series not available at time of report |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 46 Baseline characteristics: Therapeutic cooling Age and GCS on admission reported as not statistically significantly different between groups No cooling Age and GCS on admission reported as not statistically significantly different between groups Inclusion criteria: 16 to 60 years of age, post‐resuscitation GCS 4 to 7 after non‐penetrating brain injury Exclusion criteria: hypoxia, major systemic injuries requiring laparotomy, pulmonary failure, sustained hypotension; or if cooling could not be initiated within 6 hours of injury Country: USA Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 24 Method of cooling: securely wrapping the participant in cooling blankets set at 5 °C Target temperature: 33 °C Time of cooling: within 6 hours Duration of cooling: 48 hours Rewarming details: 48 hours after a temperature of 33 °C was first reached, participants warmed at 1 °C every 4 hours Site of temperature measurement: intravascular temperatures from the Swan‐Ganz catheter No cooling Number of participants: 22 Method of temperature management: cooling blankets and acetaminophen Target temperature: 37 °C Time of cooling: within 6 hours Duration of cooling: 80 hours after injury Site of temperature measurement: bladder or rectal | |
| Outcomes | Outcomes measured in study: physiological measurements (heart rate, MAP, ICP, CPP), coagulation parameters (PT, PTT), electrolytes, GOS, complications (to include pneumonia), mortality | |
| Notes | Funding/declarations of interest: no details Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, stratified into two groups: GCS 4 and 5; and GCS 6 and 7. No additional details of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding would be unlikely to influence performance |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Lack of blinding unlikely to influence outcome assessment |
| Blinding of outcome assessment (detection bias): GOS | Low risk | GOS assessed by neuropsychologist blinded to treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | Possible loss of 1 participant to GOS assessment (in hypothermia group) ‐ unclearly reported. Otherwise data appears complete |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 392 Baseline characteristics: Therapeutic cooling Age: mean (SD) 31 (± 12) years Type of injury (closed/open): closed GCS on admission: mean (SD) 50.6 (± 1.3) No cooling Age: mean (SD) 32 (± 13) years Type of injury (closed/open): closed GCS on admission: mean (SD) 5.8 (± 1.3) Inclusion criteria: 16 to 65 years of age, non‐penetrating head injury, GCS 3 to 8 after resuscitation Exclusion criteria: GCS score of 3 with unreactive pupils, life‐threatening injury to an organ other than the brain, systolic blood pressure of < 90 mmHg after resuscitation, oxygen saturation < 94 % after resuscitation, bleeding, pregnancy, known pre‐existing medical conditions (e.g. severe heart disease) or if examiners unable to initiate cooling within 6 hours after injury Country: USA Setting: 11 centres, hospital (associated abstract states 9 centres) | |
| Interventions | Therapeutic cooling Number of participants: 199 Method of cooling: cooling procedures included application of ice, gastric lavage with iced fluids, use of room temperature air in the ventilator circuit. Temperature control pads incorporated into a kinetic treatment tables used to maintain temperature once reached Target temperature: 32.5 to 34 °C Time of cooling: within 6 hours after injury Duration of cooling: 48 hours Rewarming details: rate ≥ 0.5 °C per 2 hour period Site of temperature measurement: urinary bladder with use of Foley catheters with thermisters No cooling Number of participants: 193 Target temperature: 37 °C | |
| Outcomes | Outcomes measured in study: GOS at 6 months (to include mortality data at 6 months), time to reach target body temperature, MAP, ICP, therapy intensity level | |
| Notes | Funding/declarations of interest: supported by grants from the National Institute of Neurological Disorders and Stroke and from Kinetic Concept (San Antonio, Texas) Study dates: October 1994 to May 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding would be unlikely to influence performance |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | "Glasgow Outcome Scale was conducted 6 months after the injury by examiners who were unaware of the patients' treatment group assignments. Data on acute care and outcomes were transmitted to the Biostatistics Center at the Medical College of Virginia. Only the study biostatistician was aware of each patient's treatment group assignment but the patient safety and monitoring board had access to data grouped according to treatment" |
| Blinding of outcome assessment (detection bias): GOS | Low risk | "Glasgow Outcome Scale was conducted 6 months after the injury by examiners who were unaware of the patients' treatment group assignments. Data on acute care and outcomes were transmitted to the Biostatistics Center at the Medical College of Virginia. Only the study biostatistician was aware of each patient's treatment group assignment but the patient safety and monitoring board had access to data grouped according to treatment" |
| Incomplete outcome data (attrition bias) | Low risk | "Outcome data were obtained for 385 patients (98%). However, data on age or Glasgow coma score were missing or inaccurate for 17 patients, and therefore outcome data adjusted for age and Glasgow coma score were analysed for 368 patients." Loss of data reported unlikely to affect data ‐ low percentage loss |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel design | |
| Participants | Total number of participants: 97 Baseline characteristics: Therapeutic cooling Age: mean (SD) 26 (± 9) years Type of injury (closed/open): closed GCS on admission: score 5 to 8: 33/52; score 3 to 4: 19/52 No cooling Age: mean (SD) 31 (± 11) years Type of injury (closed/open): closed GCS on admission: score 5 to 8: 22/45; score 3 to 4: 23/45 Inclusion criteria: 16 to 45 years of age, non‐penetrating brain injury, not responsive to instructions, GCS 3 to 8 after resuscitation without life‐threatening associated injuries Exclusion criteria: at randomisation: suspected pregnancy, systolic blood pressure < 110 mmHg, diastolic blood pressure < 60 mmHg, sustained heart rate > 120 beats per minute, or if they could not be reached by study‐affiliated personnel within 2 to 5 hours of injury. After assessment and resuscitation: GCS of 3 with non reactive pupils, GCS 7 to 8 with normal CT scan, inability to measure an accurate GCS score, abbreviated injury severity score of 4 or greater for organs other than the brain, SBP < 110 mmHg, DBP < 60 mmHg, persistent hypoxia, or a positive pregnancy test Country: USA and Canada Setting: participants enrolled during transport to hospital or in emergency department, care then in specialist brain injury setting | |
| Interventions | Therapeutic cooling Number of participants: 52 Method of cooling: initial cooling to 35 °C by infusion of 2 L of cold IV fluid and use of wet sheets. Subseuqent cooling after full assessment using surface cooling, cold IV fluids and cold gastric lavage to 33 °C and maintained for 48 hours Target temperature: 33 °C Time of cooling: stage 1 immediate; stage 2 after full assessment Duration of cooling: initial cooling plus 48 hours of maintenance Rewarming details: 0.5 °C every 2 hours, regardless of levels of ICP Site of temperature measurement: bladder No cooling Number of participants: 45 Method of temperature management: none ‐ although temperatures above 38 °C were managed with paracetamol and cooling blankets Target temperature: 37 °C | |
| Outcomes | Outcomes measured in study: GOS at 6 months adjusted for baseline age and GCS, 58 separate complications | |
| Notes | Funding/declarations of interest: National Institute of Neurological Disorders and Stroke. Sponsor had no role in data collection, analysis and interpretation, but was involved in study design and choosing timing of interim analysis Study dates: December 2005 to June 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated with random number generator by study biostatistician |
| Allocation concealment (selection bias) | Low risk | Secretary at trial co‐ordinating centre placed treatment assignments into numbered opaque envelopes, which were sealed and mailed to each trial centre. Randomisation was done at each centre by study nurses after opening sequentially numbered envelopes |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Personnel were aware of group assignment; unlikely to influence performance |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Personnel were aware of group assignment; unclear if this could influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | "Investigators who assessed the outcome measures were masked to treatment allocation" |
| Blinding of outcome assessment (detection bias): GOS | Low risk | "Investigators who assessed the outcome measures were masked to treatment allocation" |
| Incomplete outcome data (attrition bias) | Low risk | 6 month outcome data were not available for 8/97 participants, and were imputed from earlier 3 month (n = 6), 4 week (n = 1) or 2 week (n = 1) data. The study authors state they conducted a sensitivity analysis using complete outcome data and results were the same |
| Selective reporting (reporting bias) | Unclear risk | Prospective trial registration (NCT00178711). Published study reports data for complications but these are not listed as outcome measures in the trial registration documents. (Also reports on medical interventions, and laboratory data. Trial registration lists secondary outcomes, GOS up to 12 months and neurological assessments, which are not reported in the published study) |
| Other bias | Unclear risk | Some differences in GCS at baseline, and participants in normothermia group were older (P = 0.03). However, it was unclear if these differences were likely to influence results |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 25 Baseline characteristics: Therapeutic cooling Age: mean (SD) 38.1 (± 15.0) years Gender M/F: 11/1 GCS on admission: mean (SD) 3.9 (± 1.7) Type of injury (closed/open): blunt 10; penetrating 2 No cooling Age: mean (SD) 33.2 (± 20.0) Gender M/F: 11/2 GCS on admission: mean (SD) 4.3 (± 2.1) Type of injury (closed/open): blunt 12; penetrating 1 Inclusion criteria: severe traumatic brain injury, GCS score ≤ 8, ≥ 18 years of age, required an ICP monitor and Foley catheter as part of routine treatment, able to receive the Discrete Cerebral Hypothermia cooling cap within 48 hours of hospital admission, participant’s family member or guardian spoke English to ensure proper informed consent Exclusion criteria: patient’s family member or guardian was unwilling or unable to sign an informed consent, physical placement of the cooling cap impeded routine treatment, patient’s core body temperature was ≤ 36°C at the time of initial assessment, treatment could not be initiated within 48 hours of admission Country: USA Setting: Level 1 trauma centre, hospital | |
| Interventions | Therapeutic cooling Number of participants: 12 Method of cooling: Discrete Cerebral Hypothermia System (“the cooling cap”) Target temperature: target intracranial temperature 33 °C, target systemic temperature maintained above 36 °C using heating blankets Time of cooling: mean time in emergency department prior to study enrolment 4.6 (± 4.5) hours. (Note: exclusion criteria suggests could be up to 48 hours after admission) Duration of cooling: 24 hours Rewarming details: 0.5 °C every 3 hours for 24 hours (study hours 25 to 48). Cooling cap was removed after 48 hours. Site of measurement: intracranial and bladder temperatures No cooling Number of participants: 13 Method of temperature management: heating blankets Target temperature: 36°C Time of cooling: mean time in emergency department prior to study enrolment 7.2 (± 3.7) hours | |
| Outcomes | Outcomes measured in study: temperature gradient, mortality (up to day 28), GOS (up to day 28), Functional Independence Measure, complications (respiratory failure, shock, septicaemia, decubitus ulcer, cardiac arrest) | |
| Notes | Funding/declarations of interest: "no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper" Study dates: July 2006 to August 2007 Note: unfavourable outcome was reported as median maximum change in GOS during 28 study period. "No statistically significant intergroup difference in GOS‐determined morbidity" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was determined by the Department of Biostatistics using computer‐generated random numbers" Participants stratified by GCS scores after randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | High risk | Complete outcome data not available for 4 participants, and study has small sample size. Explanations given and patients still included in intention‐to‐treat analysis. Control group has a 23% loss |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | Baseline characteristics comparable. But statistically significant differences in time to study enrolment between two groups |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 17 Baseline characteristics: Therapeutic cooling Age: mean (SD) 29.0 (± 14.9) years Type of injury (closed/open): not clearly reported but baseline data reports participant conditions as: skull base fracture, contusion, epidural haematoma, intracerebral haematoma, subarachnoid haemorrhage, subdural haematoma Gender M/F: 9/0 GCS on admission: mean (SD) 5.4 (± 1.7) No cooling Age: mean (SD) 39.1 (± 13.2) years Type of injury (closed/open): as above Gender M/F: 5/3 GCS on admission: mean (SD) 5.4 (± 2.0) Inclusion criteria: severely head injured patients who required continued infusion of barbiturartes to control intracranial hypotension Exclusion criteria: < 10 years of age, severe life threatening injury to another organ Country: Japan Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 9 Method of cooling: surface cooling, water‐circulating blankets above and below participant Target temperature: 34 °C Time of cooling: deferred until ICP stabilized below 20 mmHg using high dose barbiturate therapy and then cooling started as quickly as possible Duration of cooling: 48 hours Rewarming details: approximately 1 °C per day using barbiturates for 5 days. Aim of 37 °C by day 5. After day 5 maintained at 37.5 to 38.5 °C by surface cooling Site of temperature measurement: intracranial No cooling Number of participants: 8 Target temperature: 36.5 to 37.5 °C Method of temperature management: surface cooling, water‐circulating blankets Duration of temperature management: 5 days | |
| Outcomes | Outcomes measured in study: expression of heat shock proteins in leukocytes, infectious complications within 1 week (to include pneumonia), GCS at 6 months (to include mortality data) | |
| Notes | Funding/declarations of interest: supported by Research on Brain Science grant from Ministry of Health and Welfare of Japan, and a grant from the Marine and Fire Insurance Association of Japan, Inc Study dates: September 1997 to November 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly divided into 2 groups but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 22 Baseline characteristics: Therapeutic cooling. No cooling No baseline characteristics table. Study authors state: "The age, GCS, pupillary reactivity, time of emergency department admission soon after admission, primary diagnosis and initial ICP levels were similar in both groups" Inclusion criteria: participants with severe traumatic brain injury (post resuscitation GCS ≤ 7, closed head injury, arrival within 2 hours of trauma, 18 to 81 years of age Exclusion criteria: not stated Country: Japan Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 12 Method of cooling: cooling blankets Target temperature: 32 to 33 °C Time of cooling: within 6 hours post‐trauma Duration of cooling: 48 hours Rewarming details: 1 °C every 4 hours Site of measurement: not stated No cooling Number of participants: 10 Method of temperature management: "standard management" Target temperature: 37 to 38 °C | |
| Outcomes | Outcomes measured in study: mortality (time point not stated), physiologic data (averaged in 24 hour blocks), GOS at 3 months | |
| Notes | Funding/declarations of interest: not reported Study dates: 1992, one year duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 225 Baseline characteristics: Therapeutic cooling Age: mean (SD) 9.8 (± 4.9) years Weight kg: mean (SD) 39.4 (± 21.2) Type of injury (closed/open): not stated. Injuries include extradural haematoma, intracerebral haematoma, cerebral oedema, midline shift, skull fracture Gender M/F: 70/38 GCS on admission: median (IQR) 5 (4 to 6) No cooling Age: mean (SD) 10.2 (± 4.8) years Weight kg: mean (SD) 40.3 (± 21.1) Type of injury (closed/open): as above Gender M/F: 71/46 GCS on admission: median (IQR) 5 (3 to 6) Inclusion criteria: 1 to 17 years of age, had traumatic brain injury, GCS ≤ 8 at scene of accident or in emergency room, CT scan that showed acute brain injury and need for mechanical ventilation Exclusion criteria: patients who were screened > 8 hours after injury as well as patients with refractory shock, suspected brain death, non‐accidental injury, prolonged cardiac arrest at scene of accident, high cervical spine cord injury, severe neurodevelopmental disability before injury, brain injury due to gunshot wound, acute isolated epidural haematoma or pregnancy Country: Canada Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 108 Method of cooling: use of surface cooling techniques Target temperature: mean (SD) 32.5 (± 0.5) °C Time of cooling: after participant assessment and stabilization (study authors did not provide definition of stabilization), mean time to cooling after injury 6.3 (± 2.3) hours Duration of cooling: 24 hours Rewarming details: temperature increase at a rate of 0.5 °C every two hours. After rewarming, temperature maintained at 37 (± 0.5) °C until intracranial hypertension resolved Site of measurement: oesophageal No cooling Number of participants: 117 Target temperature: 37 (± 0.5) °C until intracranial hypertension resolved | |
| Outcomes | Outcomes measured in study: GOS at 6 months (to include mortality), Paediatric Cerebral Performance Category scale assessments, measures of intelligence, memory functioning and speed of information processing, blood pressure, ICP, co‐interventions, length of stay in ICU and hospital, rates of adverse events (hypotension, infection to include pneumonia, bleeding, arrthymias, electrolyte abnormalities) | |
| Notes | Funding/declarations of interest: supported by grants from Canadian Institutes of Health Research, Ontario Neurotrauma Foundation, the Rick Hansen Institute, the Hospital for Sick Children Foundation, Physicians Services Incorporated, Fonds de la Recherche en Santé du Québec, the Children's Hospital of Eastern Ontario Research Institute, and Direction de la Recherche Clinique, Assistance Publique‐Hôpitaux de Paris. Dr Lacroix reports receiving consulting fees from Johnson & Johnson Study dates: February 1999 to October 2004 Note: includes participants with other injuries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Study physician randomly assigned the patient to a treatment group with the use of central telephone based system that was available 24 hours a day" Independent statistician prepared blocks |
| Allocation concealment (selection bias) | Low risk | "Study physician randomly assigned the patient to a treatment group with the use of central telephone based system that was available 24 hours a day" |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Low risk | Unfavourable outcome assessed by personnel unaware of treatment assignments |
| Incomplete outcome data (attrition bias) | High risk | 20 out of 225 participants were lost to follow‐up but the numbers lost were not balanced between groups (6% in hypothermia versus 12% in normothermic groups) |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration (ISRCTN77393684). However retrospective registration which did not allow for valid comparison of outcomes between protocol and final report |
| Other bias | Unclear risk | Some differences in baseline characteristics, a greater proportion had an extradural haematoma in the normothermic group (19%) than in the hypothermic group (9%). A greater proportion of participants received dopamine, epinephrine or norepinephrine in the hypothermic groups (85%) than in the normothermic group (56%) |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 23 Baseline characteristics: Therapeutic cooling Age: mean (95% CI): 28.9 (17.3 to 40.5) years Type of injury (closed/open): closed Gender M/F: 8/2 GCS on admission: 7 No cooling Age: mean (95% CI): 45.5 (35.0 to 56.1) years Type of injury (closed/open): closed Gender M/F: 10/3 GCS on admission: 6 Inclusion criteria: ≥ 12 years of age and above, severe head injury with GCS 6 to 7, require decompressive craniectomy, able to be followed‐up after 6 months being discharged from the hospital, and consented by next of kin or guardians Exclusion criteria: penetrating brain injury, significant drop in blood pressure and/or significant hypoxia prior to admission, bilateral fixed and dilated pupils, severe injury to other organ systems which may lead to marked morbidity or even mortality, concomitant traumatic spinal cord injury, known pre‐morbid immune or neurological diseases, severe head injury with only extradural haematoma, and known pre‐morbid condition prior to the accident, including history of seizures Country: Malaysia Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 10 Type of cooling method: cold irrigation of Hartmann's solution onto surface of brain Temperature to which body cooled: 30 to 36 °C. Time of cooling: within 7 hours Duration of cooling: for ≥ 24 hours; longer if persistent raised ICP Rewarming details: no details Site of measurement: Licox probe placed in damaged areas of brain No cooling Number of participants: 13 | |
| Outcomes | Outcomes measured in study: GOS at discharge and 6 months, complications (to include pneumonia) | |
| Notes | Funding/declarations of interest: funded by the Short Term Grant of Universiti Sains Malaysia Study dates: January 2010 to January 2012 Note: hypothermia group divided into two groups ‐ deep cooling (20 to 29 °C) and mild cooling (30 to 36 °C). We have only included data for mild cooling group which are more comparable with our other included studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but insufficient details |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes which were blinded to the consenting individuals and clinicians, but insufficient details of how this blinding was ensured |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | Participants in normothermia group were older. Unclear if this would affect results |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 11 Baseline characteristics: Therapeutic cooling Age: no details Gender M/F: no details Weight kg: no details GCS on admission: 3 to 8 Type of injury (closed/open): closed No cooling Age: no details Gender M/F: no details Weight kg: no details GCS on admission: 3 to 8 Type of injury (closed/open): closed Inclusion criteria: severe closed head injuries, GCS 3 to 8 Exclusion criteria: no details Country: Japan Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 7 Method of cooling: no details Target temperature: no details Time of cooling: no details Duration of cooling: no details Rewarming details: no details Site of measurement: no details No cooling Number of participants: 4 | |
| Outcomes | Outcomes measured in study: platelet count, thrombopoietin levels, mortality | |
| Notes | Funding/declarations of interest: no details Study dates:not reported Note: abstract only with very limited detail. Mortality is reported in the hypothermia group; it is not clear from the abstract whether there were no deaths in control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Described as random sampling but no additional details. Uneven number of participants in each group |
| Allocation concealment (selection bias) | Unclear risk | No details. Abstract only |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Lack of blinding unlikely to influence outcome assessment for this outcome |
| Incomplete outcome data (attrition bias) | High risk | Data not clearly reported on mortality for control group |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | No other sources identified but abstract with very limited detail |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 87 Baseline characteristics: Therapeutic cooling Age: mean 42.2 years Gender M/F: 35/8 GCS on admission: 5.04 Type of injury (closed/open): not stated but findings on CT scan of haematoma, contusion and traumatic subarachnoid haemorrhage No cooling Age: mean 40.6 years Gender M/F: 37/7 GCS on admission: 5.14 Type of injury (closed/open): as above Inclusion criteria: patients with severe traumatic brain injury, GCS ≤ 8 Exclusion criteria: no details Country: China Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 43 Method of cooling: cooling blankets beneath body Target temperature: 33 to 35 °C Time of cooling: immediately after admission Duration of cooling: 3 to 14 days (until ICP returned to normal level 15 mmHg) Rewarming details: Once participant's ICP returned to normal levels, passively rewarmed to a temperature of 37 to 38.3 °C at a rate ≤ 1 °C/hour, by gradual adjustment of the blanket thermostat Site of temperature measurement: rectal No cooling Number of participants: 44 Target temperature: 37 to 38 °C Duration of temperature management: 14 days | |
| Outcomes | Outcomes measured in study: complications (including pneumonia at 1 year post injury), ICP, blood glucose values, serum electrolyte levels, mortality at 1 year post injury, GOS at 1 year post injury | |
| Notes | Funding/declarations of interest: no details Study dates: May 1992 to May 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants described as randomly assigned; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Low risk | Neurological outcomes were assessed (GOS) by an expert unaware of treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No apparent other sources of bias |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 31 Baseline characteristics Therapeutic cooling Age: mean (SD) 44.0 (± 15.1) years Gender M/F: 9/6 GCS on admission: mean (SD) 6.3 ( ± 1.2) Type of injury (closed/open): not reported No cooling Age: mean (SD) 43.5 (± 16.4) years Gender M/F: 10/6 GCS on admission: mean (SD) 6.4 (± 1.3) Type of injury (closed/open): not reported Inclusion criteria: a history of traumatic brain injury, GCS of 4 to 8, and brain damage confirmed by sequential CT scanning within 6 hours after trauma Exclusion criteria: pregnant women, patients < 12 years or > 70 years of age, a GCS score of 3, patients with multiple injuries, those with any previous disabling neurologic disease Country: China Setting: university hospital | |
| Interventions | Therapeutic cooling Number of participants: 15 Method of cooling: surface cooling, water‐circulating blankets, ice pillows Target temperature: 33 to 35 ºC Time of cooling: immediately after surgery for participants with evacuated mass lesions and after arrival in the ICU Duration of cooling: assumed to be ≥ 24 hours from information reported in the results section of the full report Rewarming details: no details Site of temperature measurement: brain No cooling Number of participants: 16 | |
| Outcomes | Outcomes measured in study: length of ICU stay, ICP, PtiO2, GOS at 6 months, mortality at 6 months, and complications | |
| Notes | Funding/declarations of interest: grant from China Medical University Hospital Study dates: September 2006 to August 2007 Note: participants were post‐craniotomy. Participants divided into three groups. However comparisons included in this review for just two groups. 'No cooling group' had ICP/CPP‐guided management only and therapeutic cooling group had combined mild hypothermia and ICP/CPP‐guided management | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as a randomised controlled trial; no additional details on methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 43 Baseline characteristics Therapeutic cooling Age: mean (SD) 37.05 (± 15.67) years Type of injury (closed/open): closed Gender M/F: 13/7 GCS on admission: GCS 3 to 5: 7/20; GCS 6 to 8: 13/20 No cooling Age: mean (SD) 38.96 (± 14.18) years Type of injury (closed/open): closed Gender M/F: 17/6 GCS on admission: GCS 3 to 5: 8/23; GCS 6 to 8: 15/23 Inclusion criteria: isolated severe head injury, admission within 4 hours after head injury, GCS ≤ 8, unequivocal contusion and laceration of the brain shown by CT scanning, 16 to 70 years of age, informed consent signed if enrolled in this study, and those who could accept hypothermia therapy immediately after admission Exclusion criteria: combined injury, open craniocerebral injury, traumatic shock, pregnant or menstruating women, patients with hepatic diseases, blood diseases, or diseases affecting blood clotting, anticoagulation therapy, those transferred to other hospitals or withdrawn from this study, surgical operations, patients with severe complication receiving hypothermia therapy, patients who died within 72 hours or accepted component blood transfusion and drug treatment promoting coagulation or fibrinolysis Country: China Setting: neurosurgical ICU | |
| Interventions | Therapeutic cooling Number of participants: 20 Method of cooling: ice blanket, ice cap and ice cubes Target temperature: 32 to 35 °C Time of cooling: immediately Duration of cooling: no details Rewarming details: no details Site of measurement: no details No cooling Number of participants: 23 Target temperature: 37.0 to 38.0 °C | |
| Outcomes | Outcomes measured in study: coagulation variables, GOS at 6 months | |
| Notes | Funding/declarations of interest: grant from the Natural Science Fund of Hainan Province, China Study dates: October 2006 to September 2007 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details; unclear if lack of blinding would influence results |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 22 Baseline characteristics: Therapeutic cooling Age: 8 to 96 months GCS on admission: 6.4 (SD 1.8) Type of injury (closed/open): not stated No cooling Age: 6 to 108 months GCS on admission: 6.5 (SD 1.7) Type of injury (closed/open): not stated Inclusion criteria: children of both genders (6 to 108 months of age) with GCS ≤ 8 Exclusion criteria: Other serious accompanying injuries, history of neurological conditions and/or chronic disease states Country: China Setting: children's hospital | |
| Interventions | Therapeutic cooling Number of participants: 12 Method of cooling: cooling cap Target temperature: 34.5 (± 0.2) ºC Time of cooling: average time taken before initiation of treatment 7.2 (± 1.4) hours after injury Duration of cooling: 72 hours Rewarming details: no details Site of temperature measurement: intracranial No cooling Number of participants: 10 Target temperature: intracranial temperatures were 37.5 to 38.5 ºC | |
| Outcomes | Outcomes measured in study: mortality, heart rate, blood pressure, pH and electrolyte levels, ICP, cerebrospinal fluid, biochemical markers | |
| Notes | Funding/declarations of interest: supported by grant, no conflicting financial interests declared Study dates: January 2006 to August 2007 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "The patients were divided randomly into a moderate hypothermia group (HYPO group; n = 12) and a normothermia group (NORM group; n = 10) on the basis of arrival date (odd number = HYPO; even number = NORM)" Unacceptable randomisation |
| Allocation concealment (selection bias) | Unclear risk | It is unclear whether study investigators revealed randomisation code (odd/even arrival date) to personnel and whether this method of randomisation was conducted externally |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 82 Baseline characteristics: Therapeutic cooling Age: mean (SD) 31 (± 12) years Gender M/F: 36/4 GCS on admission: not reported as mean, balanced between groups Type of injury (closed/open): closed No cooling Age: mean (SD) 35 (± 15) years Gender M/F: 33/9 GCS on admission: not reported as mean, balanced between groups Type of injury (closed/open): closed Inclusion criteria: closed head injury, GCS 3 to 7, 16 to 75 years of age, admission within 6 hours after the injury, and an inability to follow commands Exclusion criteria: clinical brain death, prolonged hypoxia or hypotension, gunshot wound, pregnancy, undetermined time of injury, or normal findings on a CT scan of the head Country: USA Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 40 Method of cooling: cooling blankets placed above and below the participant and nasogastric lavage with iced saline Target temperature: 32 to 33 °C Time of cooling: within 6 hours Duration of cooling: 24 hours Rewarming details: over 12 hours, participants were passively rewarmed to a temperature of 37 to 38.5°C, at a rate ≥ 1°C per hour, by a gradual adjustment of the blanket thermostat. Site of temperature measurement: brain, rectal No cooling Number of participants: 42 Method of temperature management: participants in the normothermia group who had rectal temperatures < 37 °C at admission were passively rewarmed over a period of 12 hours. No further details Target temperature 37 to 38.5 °C Duration of cooling: maintained for 5 days of study period | |
| Outcomes | Outcomes measured in study: cerebrospinal fluid assessment, GOS at 3, 6, 12 months post injury, mortality during stay in trauma centre | |
| Notes | Funding/declarations of interest: grant from the National Institute of Neurological Disorders and Stroke Study dates: February 1991 to September 1994 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a block‐randomization scheme" |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes but no additional details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Low risk | "A specialist in physical medicine and rehabilitation who was unaware of the patients’ treatment assignments determined the neurologic outcome 3, 6, and 12 months after the injury" |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel design | |
| Participants | Patients with severe blunt head injury. Intervention was started within 8 hours of injury | |
| Interventions | Total number of participants: 16 Therapeutic cooling Number of participants: 7 Temperature to which body cooled: 32 to 33 °C Time of cooling (immediately on admission; deferred until intracranial pressure is uncontrollable): within 8 hours Duration of cooling: 48 hours No cooling Number of participants: 9 Target temperature: 36 to 37 °C | |
| Outcomes | Outcomes measured in study: heart rate, mean blood pressure, plasma cortisol | |
| Notes | Funding/declarations of interest: not reported Study dates: not reported Note: abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details. Abstract only. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Abstract only, losses not reported. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | No other sources of bias identified. Limited detail in published abstract |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 24 Baseline characteristics: Therapeutic cooling Age: median 30 years Gender M/F: 9/2 GCS on admission: median 6 Type of injury (closed/open): closed No cooling Age: median 48 years Gender M/F: 12/1 GCS on admission: median 5 Type of injury (closed/open): closed Note: participants in hypothermia group were significantly younger than participants in control group Inclusion criteria: participants with severe head injuries (GCS ≤ 9) who reached the hospital early enough to induce hypothermia, at ≤ 8 hours after trauma Exclusion criteria: patients < 18 years of age, SBP < 90 mmHg, an arterial oxygen saturation < 90 mmHg for > 30 minutes during resuscitation, penetrating head wound Country: Germany Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 11 Method of cooling: external convection and conduction via water blankets and forced air Target temperature: 32 to 33 °C Time of cooling: "As early as possible" Duration of cooling: 24 to 48 hours Rewarming details: rewarming was achieved by isolation and, if necessary, active warming Site of temperature measurement: rectal, bladder No cooling Number of participants: 13 Method of temperature management: temperature was maintained by warming or, in cases of pyrexia, by administration of antipyretics (i.e. metamizol) and/or local cooling with cold compresses Target temperature: 36 to 37 °C | |
| Outcomes | Outcomes measured in study: mortality, serum concentrations | |
| Notes | Funding/declarations of interest: no details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes were not reported for 4 randomised participants ‐ reasons were explained and unlikely to relate to true outcome |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomized controlled trial, parallel group | |
| Participants | Total number of participants: 86 Baseline characteristics: Therapeutic cooling Age: mean 40.0 years Type of injury (closed/open): not reported Gender M/F: 26/17 GCS on admission: apparent typo in study report, therefore not reported in review No cooling Age: mean 42.3 years Type of injury (closed/open): not reported Gender M/F: 30/13 GCS on admission: GCS 3 to 5: 26/43; GCS > 6: 17/43 Inclusion criteria: history of traumatic brain injury, GCS < 8, brain injury having been diagnosed by CT within 24 hours Exclusion criteria: pregnant women, < 14 years and > 65 years of age, those with haemorrhagic shock or haematologic diseases such as leukaemia and other serious organic diseases Country: China Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 43 Method of cooling: water‐circulating cooling blanket and cooling cap. If required refrigerated ice bags were placed around the groin and neck Target temperature: 33.0 to 35.0 °C Time of cooling: immediately after admission or 3 to 5 days post‐craniotomy Duration of cooling: 3 to 5 days Rewarming details: no details Site of measurement: nasopharyngeal, rectal, brain No cooling Number of participants: 43 Target temperature: 38 °C | |
| Outcomes | Outcomes measured in study: haemodynamic and respiratory variables, complications, GOS at 2 years | |
| Notes | Funding/declarations of interest: not reported Study dates: 1998 to 2001 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details; lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | No details; unclear if lack of blinding would influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Lack of blinding unlikely to influence outcome assessment |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details; unclear if lack of blinding would influence assessment of outcome data |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other source of bias identified |
| Methods | Randomized controlled trial, parallel group | |
| Participants | Total number of participants: 80 Baseline characteristics: Therapeutic cooling Age: mean 41.3 years Gender M/F: 25/15 GCS on admission: number of participants < 6 on GCS = 24 Type of injury (closed/open): closed (non‐penetrating). All participants had craniotomies No cooling Age: mean 40.2 years Gender M/F: 27/13 GCS on admission: number of participants < 6 on GCS = 23 Type of injury (closed/open): closed (non‐penetrating). All participants had craniotomies Inclusion criteria: history of traumatic brain injury, GCS scores ≤ 8, brain injury confirmed by sequential CT scanning within 6 hours after trauma Exclusion criteria: pregnant women, patients < 19 or > 65 years of age, patients with multiple injuries, haemorrhagic shock, or without any brain stem reflex on initial examination, previous disabling neurologic disease Country: China Setting: neurological ICU in brain centre | |
| Interventions | Therapeutic cooling Number of participants: 40 Method of cooling method: water‐circulating cooling blanket, cooling cap. For those who failed to achieve target temperature successfully within 2 hours, refrigerated ice bags were placed around the groin and neck Target temperature: brain temperature was maintained between 33 to 35 °C, and the rectal temperature between 34.5 to 36 °C Time of cooling: directly after craniotomy Duration of cooling: over 4 days Rewarming details: spontaneously return to baseline during a period of 10 to 24 hours Site of measurement: brain and rectal No cooling Number of participants: 40 Method of temperature management: routine pharmacologic or physical measures were applied to maintain body temperature ≤ 37.5 °C Target temperature: brain temperature of 36.5 to 37.5 °C, rectal temperature of 37.5 to 38 °C. | |
| Outcomes | Outcomes measured in study: ICP, serum superoxide dismutase level, favourable neurologic outcome 1 year after injury, mortality at 1 year post injury | |
| Notes | Funding/declarations of interest: grant from Health Bureau of Hangzhou, Zheijang Province Study dates: January 2002 to December 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patient was assigned to one of the following 2 groups in the neurologic intensive care unit of our brain center by using a randomisation table" |
| Allocation concealment (selection bias) | Low risk | "Allocation and randomisation was concealed so that the study investigators were not aware to which group the patient would be assigned, and the allocation sequence was protected until assignment." No details on methods of concealment; sufficient information to suggest that an adequate method was used. |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 33 Baseline characteristics: Therapeutic cooling Age: mean (SD) 35.3 (± 15.3) years Gender M/F: 6/10 Type of injury (closed/open): not stated No cooling Age: mean (SD) 35.4 (± 12.6) years Gender M/F: 10/7 Type of injury (closed/open): not stated Inclusion criteria: participants in whom ICP remained > 20 mmHg at 5 to 6 hours after induction of high‐dose barbiturate therapy, GCS on admission ≤ 8 Exclusion criteria: < 10 years, ICP controlled with high‐dose barbiturate therapy alone, ICP equal to MAP before start of study Country: Japan Setting: Department of Traumatology at Osaka University Hospital | |
| Interventions | Therapeutic cooling Number of participants: 16 Method of cooling: surface cooling with water‐circulating blankets above and below the participant Target temperature: 33.5 to 34.5 ºC Time of cooling: no details Duration of cooling: 2 days or until it was considered not to be effective Rewarming details: slowly, core temperature was maintained between 35.5 to 36.5 ºC for 24 hours. If ICP increased > 20 mmHg during rewarming, participant was recooled to 34 ºC. If ICP remained < 20 mmHg for ≥ 24 hours, participants were rewarmed spontaneously to > 37 ºC with continuous infusion of barbiturates at 2 mg/kg to prevent shivering Site of temperature measurement: bladder No cooling Number of participants: 17 | |
| Outcomes | Outcomes measured in study: CPP, ICP, cerebral blood flow and AVDO₂, complications (to include pneumonia), mortality at 6 months, GOS at 6 months | |
| Notes | Funding/declarations of interest: no details Study dates: May 1987 to April 1992 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly divided into two groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Data reported for pneumonia has losses due to death within 48 hours. However, we included total participant numbers in the review data |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 16 Baseline characteristics: Therapeutic cooling Age: mean (SD) 31.4 (± 12.7) years Gender M/F: 8/0 GCS on admission: mean (SD) 5.6 (± 1.8) Type of injury (closed/open): not reported No cooling Age: mean (SD) 40.3 (± 23.1) years Gender M/F: 5/3 GCS on admission: mean (SD) 5.4 (± 2.0) Type of injury (closed/open): not reported Inclusion criteria: participants with severe head injury who required continuous infusion of barbiturate medication to control ICP, GCS ≤ 8 Exclusion criteria: < 10 years of age, ICP equal to MAP before start of study these patients, severe life‐threatening injury to other organs, unable to keep ICP < 20 mmHg by using conventional therapies such as fluid restriction, hyperventilation, and high‐dose barbiturate medications Country: Japan Setting: Department of Traumatology of Osaka University Hospital | |
| Interventions | Therapeutic cooling Number of participants: 8 Method of cooling: water‐circulating blankets above and below the participant Target temperature: 33.5 to 34.5°C Time of cooling: after randomisation Duration of cooling: 48 hours Rewarming details: rewarmed slowly, approximately 1 °C/day, by using continuous infusion of barbiturates at 2 mg/kg/hour to prevent shivering Site of temperature measurement: intracranial, lateral ventricle No cooling Number of participants: 8 Method of temperature management: surface cooling Temperature maintenance details: barbiturates were continuously infused at 6 to 8 mg/kg/hour during the initial 48 hours, followed by continuous infusion of barbiturates at 2 mg/kg/hour to prevent shivering Target temperature: 36.5 to 37.5 °C Duration of cooling: 5 days | |
| Outcomes | Outcomes measured in study: pneumonia, meningitis, diabetes insipidus, GOS at 6 months (to include mortality), daily changes in CSF, concentrations of excitatory amino acids, concentrations of cytokines | |
| Notes | Funding/declarations of interest: supported by a grant from the Ministry of Health and Welfare, Japan Study dates: 1996 to 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No loss of data observed |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 91 Baseline characteristics: Therapeutic cooling Age: mean (SD) 35 (± 20) years Gender M/F: 35/10 GCS on admission: mean (SD) 5.5 (± 1.7) Type of injury (closed/open): not reported No cooling Age: mean (SD) 42 (± 17) years Gender M/F: 31/15 GCS on admission: mean (SD) 5.1 (± 1.9) Type of injury (closed/open): not reported Inclusion criteria: GCS ≤ 8 on admission, no severe life‐threatening injury to another organ, ICP was maintained < 25 mmHg by conventional therapies Exclusion criteria: 84 participants were excluded for one of the following reasons: unable to maintain their ICP < 25 mmHg despite conventional therapies (73 patients), ICP was not measured (9 patients), or informed consent was not obtained (2 patients) Country: Japan Setting: 11 medical centres in Japan | |
| Interventions | Therapeutic cooling Number of participants: 45 Method of cooling: cooling blankets above and below the participant, nasogastric lavage with iced saline Target temperature to which body cooled: 33.5 to 34.5 °C Time of cooling: < 24 hours after randomisation Duration of cooling: 48 hours Rewarming details: slowly, approximately 1 °C/ day, to a temperature of 37 °C after 3 days Site of temperature measurement: at discretion of the investigator, although brain temperature was recommended No cooling Number of participants: 46 Method of temperature management: surface cooling Target temperature: 36.5 to 37.5 °C Duration of temperature management: 5 days | |
| Outcomes | Outcomes measured in study: therapy intensity for control of ICP and CPP, GOS at 3 months (to include mortality), pneumonia. Mortality also reported during hospital stay | |
| Notes | Funding/declarations of interest: grant from the Ministry of Health and Welfare, Japan Study dates: February 1998 to January 2000 Note: some participant losses due to increase in ICP. We included the total number of participants in the review outcome data as some losses were due to death | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of participants and personnel (performance bias): pneumonia | Unclear risk | Unclear if lack of blinding would influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Some inevitable losses to data due to early mortality. Low number, clearly explained |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | Temperature measurement at discretion of physicians |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 72 Baseline characteristics: Baseline characteristics not reported by group. Mean age 41 years; gender M/F: 51/21 Inclusion criteria: Severe brain injury GCS < 8 Exclusion criteria: patients > 60 years of age, those with severe primary brain damage with no possibility of survival (bilateral mydriasis, no reflexes above C1) Country: Czech Republic Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 35 Method of cooling: surface cooling Target temperature: 34 °C Time of cooling: within 15 hours of injury Duration of cooling: to reach 34 °C within 3 hours of cooling for 72 hours Rewarming details: passively warmed after 72 hours Site of measurement: urinary bladder No cooling Number of participants: 37 | |
| Outcomes | Outcomes measured in study: ICP, CCP, jugular bulb oxygen saturation, GOS at 6 months (to include mortality at 6 months) | |
| Notes | Funding/declarations of interest: supported by grant from the Internal Grant Agency of the Czech Ministry of Health Study dates: 2001 to 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding likely to influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other apparent sources of bias |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 40 Baseline characteristics: Baseline characteristics table not reported. Study authors state "no obvious differences in patients' condition such as age, sex, the level of ICP as well as other therapy between two groups" Inclusion criteria: not reported. Severe brain injured participants with GCS ≤ 8 Exclusion criteria: not reported Country: China Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 20 Method of cooling: water‐circulating blankets placed above and below the body Target temperature: 33.5 to 34.5 °C Time of cooling: immediately Duration of cooling: 2 days Rewarming details: approximately 1 °C per day by continuous infusion of barbiturates (2 mg/kg/h) to prevent shivering Site of measurement: intracranial No cooling Number of participants: 20 Methods to maintain normothermia: barbiturates infused during initial 48 hours, followed by continuous infusion of barbiturates to prevent shivering Target temperature: 37 °C | |
| Outcomes | Outcomes measured in study: changes in cerebrospinal fluid, cytokines, GOS at 6 months | |
| Notes | Funding/declarations of interest: not reported Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were described as "randomly divided" into groups. No additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | No details. Unclear if lack of blinding may influence performance |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details. Unclear if lack of blinding may influence outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | Limited detail in paper, not possible to judge other risks of bias |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 44 Baseline characteristics: Therapeutic cooling Age: group A mean (SD) 47.0 (± 18.8) years; group B mean (SD) 34.6 (± 14) years Gender M/F: 16/8 GCS on admission: group A mean (SD) 4.2 (± 0.6); group B mean (SD) 7.2 (± 0.8) Type of injury (closed/open): not stated No cooling Age: group A mean (SD) 43.3 (± 15.8) years; group B mean (SD) 40.5 (± 21) years Gender M/F: 14/6 GCS on admission: group A mean (SD) 4.0 (± 0.9); group B mean (SD) 7.6 (± 0.4) Type of injury (closed/open): not stated Inclusion criteria: GCS < 8 on admission, arrived at hospital < 10 hours after injury Exclusion criteria: additional injuries or heart, lung, liver or kidney diseases Country: China Setting: ICU | |
| Interventions | Therapeutic cooling Number of participants: 24 Method of cooling: cooling body surface with a cooling bed. Sometimes ice bricks placed on participant's neck, axilla or groin to aid cooling Target temperature: 32 to 34 °C Time of cooling: as quickly as possible after admission Duration of cooling: 3 to 5 days Rewarming details: cooling bed stopped and temperature resumed slowly and naturally Site of measurement: rectal No cooling Number of participants: 20 | |
| Outcomes | Outcomes measured in study: evoked neural potentials, mortality | |
| Notes | Funding/declarations of interest: no details Study dates: May 1998 to April 1999 Note: groups initially divided into GCS 3 to 5 (Group A) and GCS 6 to 8 (Group B). Two sets of baseline characteristics reported by Group A and Group B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomly assigned; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 148 Baseline characteristics: Baseline characteristics not reported by group. Overall mean participant age 27.3 years. 106 males, 42 females Inclusion criteria: severe traumatic brain injury (GCS ≤ 8), hospitalised within 10 hours of injury, < 65 years of age, and had no other severe visceral injury, or heart, lung, liver, kidney or other visceral comorbidities Exclusion criteria: no details Country: China Setting: ICU | |
| Interventions | Therapeutic cooling Number of participants: 73 Method of cooling method: medical cooling bed as soon as possible. Ice bags were placed on the neck, axilla, groin, and other regions Target temperature: 32 to 34 ºC Time of cooling: following ICU admission, within 4 to 6 hours Duration of cooling: 3 to 5 days Rewarming details: following hypothermia treatment, the cooling bed was turned off and the participants regained normal body temperature Site of temperature measurement: rectal No cooling Number of participants: 75 Target temperature: 37 (± 0.5) ºC | |
| Outcomes | Outcomes measured in study: GOS (time point not stated, "patients were followed up between 1 and 7 years"; to include mortality data), brain oxygen metabolism monitoring, neuro‐electrophysiological monitoring | |
| Notes | Funding/declarations of interest: supported by the scientific research foundation of Chongqing Municipal Health Bureau (1997 to 2005) Study dates: June 1998 to June 2004 Note: participants divided into subgroups according to GCS. For this review, outcome data for groups have been combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomly assigned; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Unclear if lack of blinding likely to influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | Baseline characteristics not supplied. Unclear if groups are comparable |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 154 Baseline characteristics: Details taken from English abstract only; baseline characteristics not included Inclusion criteria: participants < 65 years of age with traumatic brain injury and a GCS 3 to 8 on admission to hospital Exclusion criteria: no details Country: China Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 77 Target temperature: 32 to 33 ºC Duration of cooling: 3 to 8 days No cooling Number of participants: 77 | |
| Outcomes | Outcomes measured in study: mortality | |
| Notes | Funding/declarations of interest: no details Study dates: not reported in abstract Note: paper in Chinese. All data taken from English abstract. Data for severe head injury separated from data for most severe head injury. In this review only data for 'severe' were recorded. Previous version of review used data from both groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants described as randomly divided; no additional details (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | No details (abstract only) |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Lack of blinding unlikely to influence performance for this outcome |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Lack of outcome assessor blinding is unlikely to influence outcome data |
| Incomplete outcome data (attrition bias) | Low risk | Data presented for all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Unclear risk | Insufficient information to make judgement on other sources of bias (abstract only) |
| Methods | Randomised controlled trial, parallel design | |
| Participants | Total number of participants: 81 Baseline characteristics: Therapeutic cooling Age: mean (SD) 36.9 (± 14.8) years Gender M/F: 29/11 Type of injury (closed/open): closed GCS on admission: GCS score 3 to 5: 15/40; GCS 6 to 8: 25/40 No cooling Age: mean (SD) 37.5 (± 15.2) years Gender M/F: 30/11 Type of injury (closed/open): closed GCS on admission: GCS 3 to 5: 16/41; GCS 6 to 8: 25/41 Inclusion criteria: > 16 years of age, admission within 6 hours of non‐penetrating brain injury, GCS score 3 to 8 after resuscitation, no other chronic illnesses before the injury Exclusion criteria: life‐threatening injuries to other organs, or SBP < 70 mmHg after resuscitation Country: China Setting: hospital, neurosurgical wards | |
| Interventions | Therapeutic cooling Number of participants: 40 Method of cooling: semiconductor hypothermic blanket Target temperature: 33 °C Time of cooling: immediately after randomisation, participants already on neurosurgical ward Duration of cooling: to target within 3 to 5 hours, maintained for 72 hours Rewarming details: "rewarmed spontaneously in room temperature" Site of temperature measurement: rectal No cooling Number of participants: 41 Method of temperature management: standard care Target temperature: 37 °C | |
| Outcomes | Outcomes measured in study: arterial blood glucose and lactate, GOS at 3 months, mortality (as part of GOS) | |
| Notes | Funding/declarations of interest: no details Study dates: January 2006 to June 2009 Note: study authors aimed to investigate blood glucose and lactate levels | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants described as randomly allocated; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Not possible to blind personnel but unlikely to influence performance |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | Not possible to blind personnel but unclear if this would influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | Lack of outcome assessor blinding is unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No apparent loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Randomised controlled trial, parallel group | |
| Participants | Total number of participants: 396 Baseline characteristics: Baseline characteristics table not reported. Study authors report "There was no statistical difference in clinical characteristics between the therapeutic and control group except the mean GCS score was higher in control group." Study report includes GCS for each group which appear comparable Therapeutic cooling Age: mean (SD) 43 (± 17) years GCS on admission: mean (SD) 5.8 (± 1.8) No cooling Age: mean (SD) 42 (± 19) years GCS on admission: mean (SD) 5.9 (± 1.7) Inclusion criteria: participants with acute severe head injury (GCS ≤ 8) within 24 hours of trauma, uncomplicated with major organ damage or functional failure, and without hypotension (SBP ≤ 90 mmHg) Exclusion criteria: not reported Country: China Setting: hospital | |
| Interventions | Therapeutic cooling Number of participants: 198 Method of cooling: water‐circulating cooling blankets Target temperature: 32 to 33 °C, over 3 to 6 hours Time of cooling: immediately or after operation according to type of brain injury Duration of cooling: 1 to 7 days until ICP returned to normal Rewarming details: 1 °C every 4 hours, until rectal temperature returned to about 36.5 to 37.5 °C Site of measurement: rectal No cooling Number of participants: 198 Method of maintaining normothermia: cooling blanket and other approaches (not described) Target temperature: 36.5 to 37 °C | |
| Outcomes | Outcomes measured in study: GOS at 6 months, ICP, blood glucose/lactate, blood gas analysis | |
| Notes | Funding/declarations of interest: not reported Study dates: August 1997 to July 2001 Note: some differences in total number of participants in the intervention group but appears to be a typo in the paper. We have used the number most frequently reported and which matches the number in the abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details; lack of blinding unlikely to influence performance |
| Blinding of participants and personnel (performance bias): GOS | Unclear risk | No details; unclear if lack of blinding would influence performance |
| Blinding of outcome assessment (detection bias): mortality; pneumonia | Low risk | No details; lack of blinding unlikely to influence outcome assessment |
| Blinding of outcome assessment (detection bias): GOS | Unclear risk | No details; unclear if lack of blinding would influence outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or pre‐published protocol not reported. Insufficient information to make judgement |
| Other bias | Low risk | No other source of bias identified |
AVDO₂ = arterio jugular venous difference of oxygen; CPP = cerebral perfusion pressure; CSF = cerebrospinal fluid; CT = computed tomography; DBP = diastolic blood pressure; GCS = Glasgow Coma Scale; GOS = Glasgow Outcome Score; GOS‐E‐Peds = extended paediatric Glasgow Outcome Score; ICP = intracranial pressure; ICU = intensive care unit; IQR = interquartile range; IV = intravenous; MAP = mean arterial pressure; M/F = male/female; n = number of participants; PICU = paediatric intensive care unit; PT/PTT = prothrombin time/partial thromboplastin time; PtiO2 = brain tissue oxygen; SBP = systolic blood pressure; SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Registered ongoing feasibility study. Wrong intervention. Aim to maintain temperature at 36 ºC | |
| Therapeutic hypothermia (32 to 34 °C) compared with 'fever control' (35.5 to 37 °C). No normothermia group | |
| Abstract only. Does not appear to be an RCT | |
| Registered study in clinical trials register. Terminated early due to inadequate accrual. Data for comparison arm with only 3 participants | |
| Registered ongoing study. Traumatised participants, not specifically brain‐injury patients | |
| Participants with intracerebral haemorrhage | |
| Participants with traumatic brain injury randomly divided into hypothermic group or control group. Study objective to assess effect of hypothermia on glucose metabolism and glycerol of brain tissue. No relevant outcomes. Excluded as no details of methods used to induce hypothermia, number of participants per group not reported, and some participants appear not to be included for full study | |
| English abstract does not report any relevant outcomes. We have not sought translation of full report |
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial, parallel group |
| Participants | Patients with GCS 4 to 8; admitted to hospital from 2002 to 2006 |
| Interventions | Therapeutic cooling Number of participants: 47 Method of cooling: water mattress cooling Target temperature: 34 °C Duration of cooling: 72 hours Rewarming details: slowly to temperature of 36.5 °C No cooling Number of participants: 53 Target temperature: 36.5 to 37.5 °C |
| Outcomes | GOS |
| Notes | Abstract. Possibly associated with Smrcka 2005. Email sent to authors for further information |
| Methods | Randomised controlled trial, parallel group |
| Participants | Patients with primary, closed traumatic brain injury, ICP > 20 mmHg after decompressive craniectomy, > 16 years of age, men or nonpregnant women, clear history of head injury, GCS 3 to 8 on admission or after resuscitation, availability of a cooling device or technique for > 72 hours, brain injury confirmed by sequential computed tomography scanning within 6 hours of injury |
| Interventions | Therapeutic cooling Number of participants: 30 Method of cooling: water‐circulating cooling blanket Target temperature: 32 to 35 °C Duration of cooling: 48 hours Rewarming details: after 48 hours at a rate of 0.25 °C per hour No cooling Number of participants: 30 Target temperature: ≥ 36 °C |
| Outcomes | GOS, MAP, CPP, temperature, complications |
| Notes | Study identified during pre‐publication search in June 2017; to be incorporated into review at next formal update |
CPP = cerebral perfusion pressure; GCS = Glasgow Coma Score; GOS = Glasgow Outcome Scale; ICP = intracranial pressure; MAP = mean arterial pressure
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Rationale, methodology, and implementation of a nationwide multicenter randomised controlled trial of long‐term mild hypothermia for severe traumatic brain injury (the LTH‐1 trial) |
| Methods | Randomised controlled trial, parallel group |
| Participants | Inclusion criteria: closed brain injury within 6 hours of arrival at hospital, 18 to 65 years of age, GCS of 4 to 8 on admission or after resuscitation, abnormal images on initial CT scan, ICP > 25 mmHg Exclusion criteria: severely combined injury or life‐threatening injury to other organs, preexisting medical conditions such as severe heart disease, systolic blood pressure < 90 mmHg or oxygen saturation < 94% after resuscitation, pregnant or breastfeeding women, lack of consent |
| Interventions | Therapeutic cooling Method of cooling: cooling blankets Target temperature: 34 °C Time of cooling: immediately Duration of cooling: 5 days Rewarming details: 0.5 °C every 4 hours Site of measurement: rectal No cooling Target temperature: 36 to 37 °C |
| Outcomes | GOS at 6 months, GOS at 1 month, mortality at 6 months, length of ICU and hospital stay, ICP, GCS during 6 months follow‐up, complications (neurological, infectious, cardiovascular, coagulation and bleeding, miscellaneous) |
| Starting date | July 2013 |
| Contact information | |
| Notes | Registered trial ID: NCT01886222 |
| Trial name or title | Protocol for a multicentre randomised controlled trial of early and sustained prophylactic hypothermia in the management of traumatic brain injury |
| Methods | Randomised controlled trial, parallel group |
| Participants | Inclusion criteria: blunt trauma, clinical diagnosis of severe traumatic brain injury, GCS < 9, ≥ 18 and ≤ 60 years of age, patient is intubated or intubation is imminent Exclusion criteria: clinical diagnosis of drug or alcohol intoxication predominant cause of coma, randomisation not possible within 3 hours of injury, able to be intubated without drugs, persistent SBP < 90 mmHg, cardiac arrest, GCS = 3 and unreactive pupils, clinically significant bleeding likely to require haemostatic intervention, penetrating neck or torso injury, pregnancy, current anticoagulant treatment, existing neurological condition, cooling not in the best interest of the patient according to treating clinician |
| Interventions | Therapeutic cooling Method of cooling: bolus infusion of cold IV fluids and exposure, then cooling with refrigerated water blankets and vests Target temperature: 33 °C Time of cooling: within 3 hours of injury Duration of cooling: 72 hours Rewarming details: 0.5 °C every 3 hours No cooling Target temperature: 36.5 to 37.5 °C |
| Outcomes | GOS‐E at 6 months, quality of life assessment, all‐cause mortality at hospital discharge and 6 months, adverse events (bleeding and infections), health economic evaluation |
| Starting date | April 2010 |
| Contact information | |
| Notes | Registered trial ID: NCT00987688 |
CT = computed tomography; GCS = Glasgow Coma Scale; GOS = Glasgow Outcome Score; GOS‐E ‐ Glasgow Outcome Score ‐ Extended; ICP = intracranial pressure; ICU = intensive care unit; IV = intravenous; SBP = systolic blood pressure
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Therapeutic cooling versus no cooling, Outcome 1 Mortality. | ||||
| 2 Unfavourable outcome Show forest plot | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 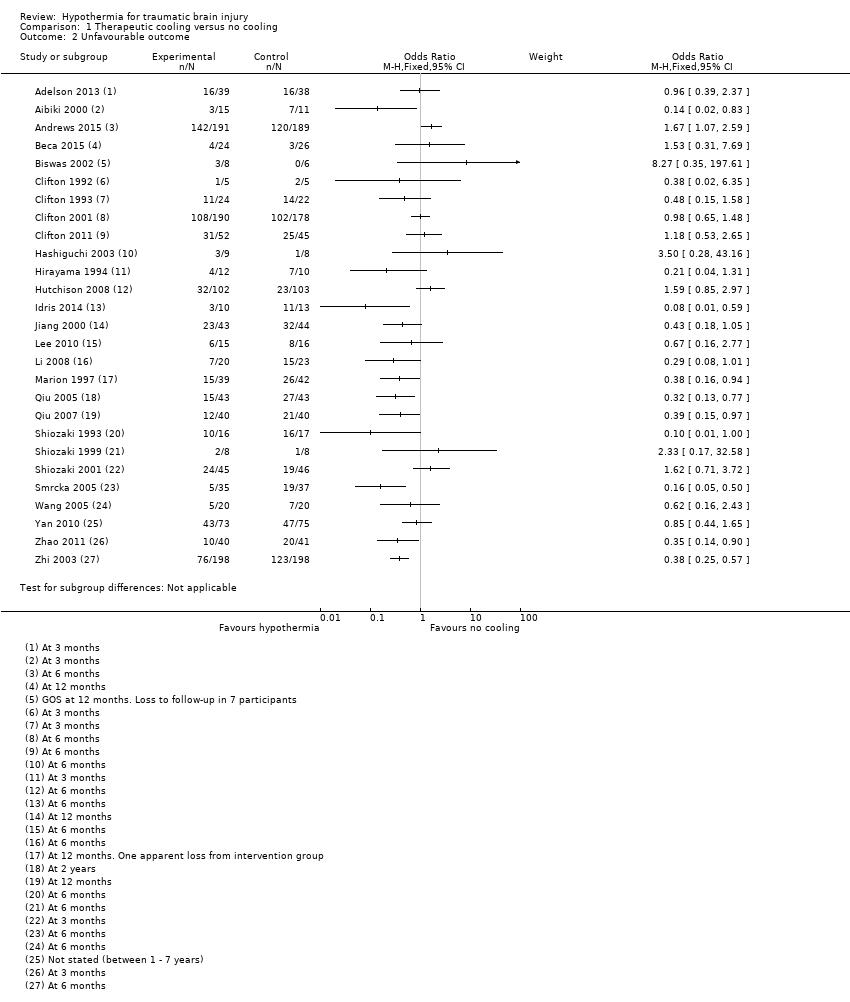 Comparison 1 Therapeutic cooling versus no cooling, Outcome 2 Unfavourable outcome. | ||||
| 3 Pneumonia Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 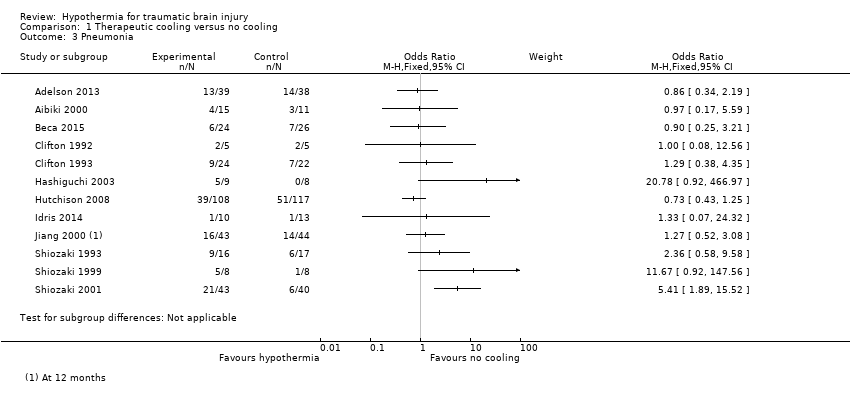 Comparison 1 Therapeutic cooling versus no cooling, Outcome 3 Pneumonia. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Therapeutic cooling versus no cooling: by duration, Outcome 1 Mortality. | ||||
| 1.1 24 hours | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 More than 24 hours | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Unfavourable outcome Show forest plot | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Therapeutic cooling versus no cooling: by duration, Outcome 2 Unfavourable outcome. | ||||
| 2.1 24 hours | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 More than 24 hours | 23 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pneumonia Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Therapeutic cooling versus no cooling: by duration, Outcome 3 Pneumonia. | ||||
| 3.1 24 hours | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 More than 24 hours | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 32 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 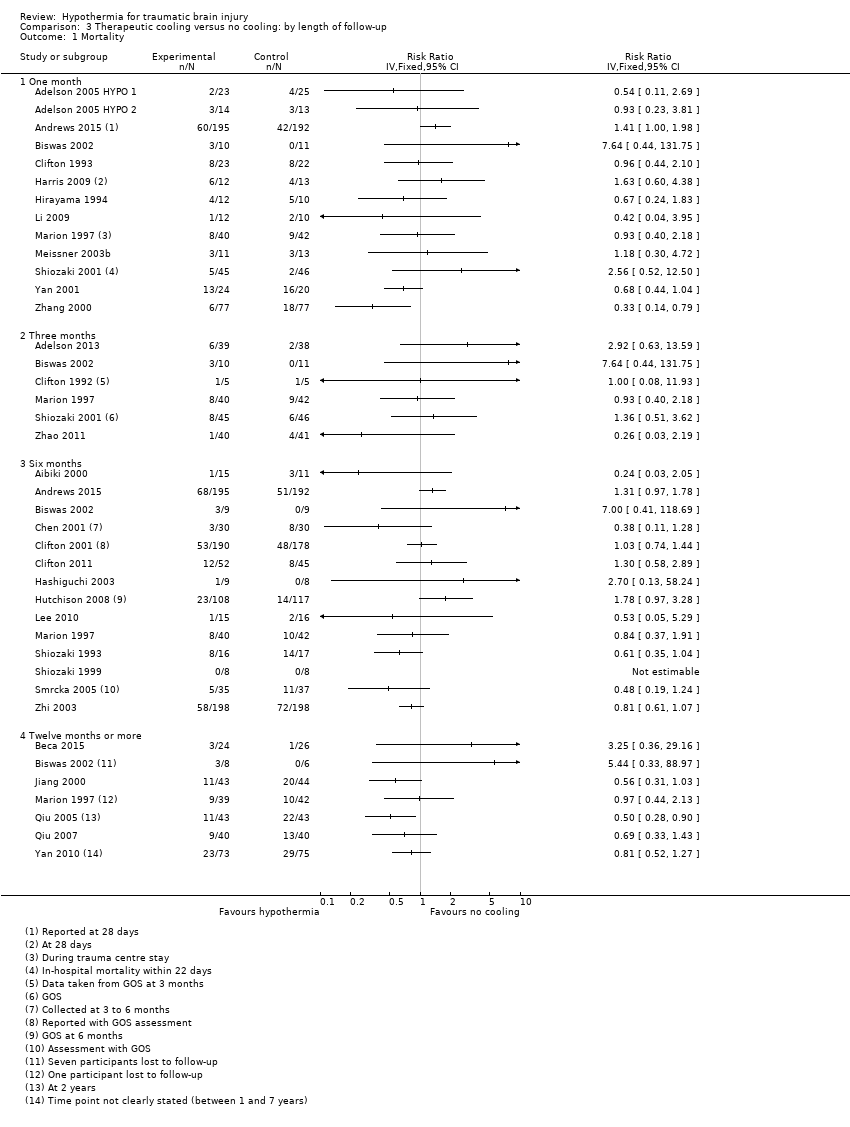 Comparison 3 Therapeutic cooling versus no cooling: by length of follow‐up, Outcome 1 Mortality. | ||||
| 1.1 One month | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Three months | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Six months | 14 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Twelve months or more | 7 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Unfavourable outcome Show forest plot | 26 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2 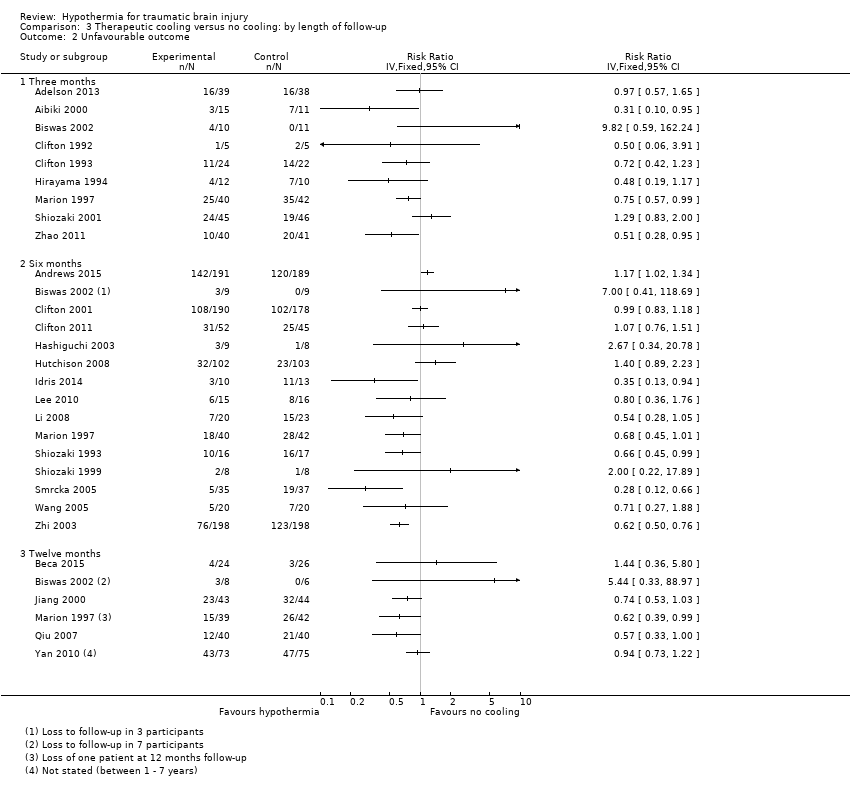 Comparison 3 Therapeutic cooling versus no cooling: by length of follow‐up, Outcome 2 Unfavourable outcome. | ||||
| 2.1 Three months | 9 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Six months | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Twelve months | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
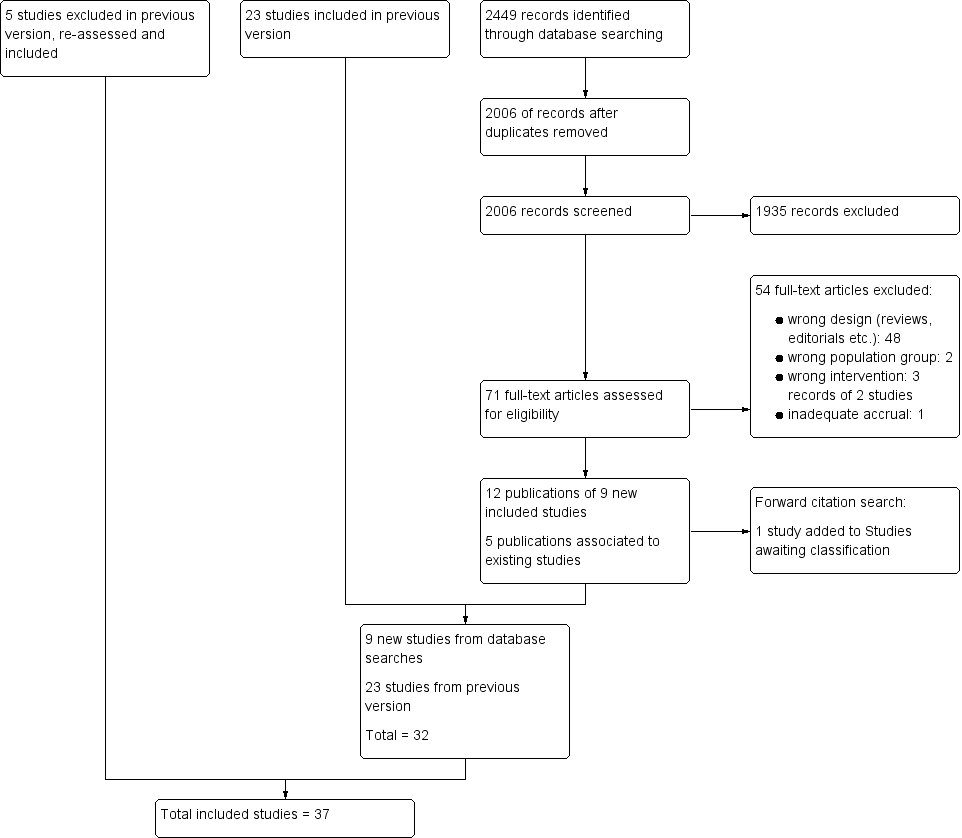
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all 37 included studies.
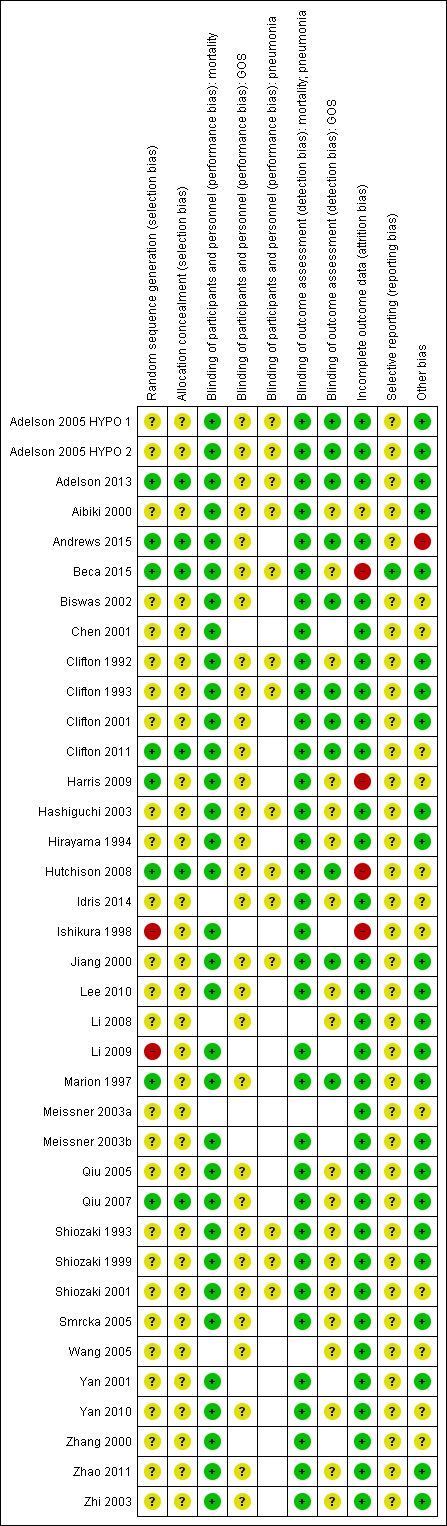
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Note: blank spaces in risk of bias table indicate that the relevant outcome was not reported by study authors

Funnel plot of comparison 1: Therapeutic cooling versus no cooling, outcome: 1.1 Mortality.

Comparison 1 Therapeutic cooling versus no cooling, Outcome 1 Mortality.

Comparison 1 Therapeutic cooling versus no cooling, Outcome 2 Unfavourable outcome.

Comparison 1 Therapeutic cooling versus no cooling, Outcome 3 Pneumonia.

Comparison 2 Therapeutic cooling versus no cooling: by duration, Outcome 1 Mortality.

Comparison 2 Therapeutic cooling versus no cooling: by duration, Outcome 2 Unfavourable outcome.

Comparison 2 Therapeutic cooling versus no cooling: by duration, Outcome 3 Pneumonia.

Comparison 3 Therapeutic cooling versus no cooling: by length of follow‐up, Outcome 1 Mortality.

Comparison 3 Therapeutic cooling versus no cooling: by length of follow‐up, Outcome 2 Unfavourable outcome.
| Hypothermia for traumatic brain injury | |||
| Patient or population: patients with traumatic brain injury | |||
| Outcomes | No of Participants | Quality of the evidence | Comments |
| Mortality at end of follow‐up | 2944 | ⊕⊝⊝⊝ | Data not combined in meta‐analysis. Visual inspection of data showed variation in differences of effect not explained by subgroup analysis |
| Unfavourable outcome at end of follow‐up | 2620 | ⊕⊝⊝⊝ | Data not combined in meta‐analysis. Visual inspection of data showed variation in differences of effect not explained by subgroup analysis |
| Pneumonia | 693 | ⊕⊕⊝⊝ | Data not combined in meta‐analysis. Visual inspection of data showed variation in differences of effect |
| GRADE Working Group grades of evidence | |||
| aWe identified 33 studies that reported data for mortality, with comparable data available for 32 studies | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Unfavourable outcome Show forest plot | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Pneumonia Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 24 hours | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 More than 24 hours | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Unfavourable outcome Show forest plot | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 24 hours | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 More than 24 hours | 23 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pneumonia Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 24 hours | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 More than 24 hours | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 32 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 One month | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Three months | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Six months | 14 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Twelve months or more | 7 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Unfavourable outcome Show forest plot | 26 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Three months | 9 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Six months | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Twelve months | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

