Hipotermia por lesión cerebral traumática
Información
- DOI:
- https://doi.org/10.1002/14651858.CD001048.pub5Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 septiembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Lesiones
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
David Signorini wrote the protocol, performed the searches and reviewed the titles and abstracts, extracted the data, performed the analyses and wrote the draft of the review. Phil Alderson (PA) reviewed the manuscripts of potential trials, extracted the data and edited the draft review.
For the 2001 update, the Injuries Group performed the search and screened studies. PA and Chirag Gadkary assessed eligibility, extracted data, performed the analysis and redrafted the text.
For the 2004 update, the Injuries Group performed the search and screened studies. PA and the Injuries Group extracted data, and PA performed the analysis and rewrote the text.
For the 2008 update, the search was carried out by Karen Blackhall (KB) of the Cochrane Injuries Group. Emma Sydenham (ES) and Ian Roberts (IR) assessed trial eligibility and applied the selection criteria. ES extracted data, and IR checked for accuracy. ES updated the text of the review. IR and ES performed the analysis and edited the manuscript. PA checked the final manuscript of the update.
KB performed the search for the January 2009 update. ES and IR assessed trial eligibility and applied the selection criteria. ES extracted the data and IR checked the extracted data for accuracy. ES updated the text of the review. IR and ES performed the analysis and edited the manuscript. PA checked the final manuscript of the update.
In April 2009, KB updated the search for trials. ES and IR assessed trial eligibility and applied the selection criteria. ES and IR re‐assessed all previously included trials against the inclusion criteria. All authors agreed that the Meissner 1998 study should be excluded. ES updated the text of the review. IR and PA checked the final manuscript of the update.
ES included data from the Harris 2009 trial for the July 2009 update. IR and PA checked the extracted data. All authors approved the manuscript for publication.
Sharon R Lewis (SRL) co‐ordinated the 2017 review update. Database searches were carried out by Deidre Beecher. SRL screened titles and abstracts, assessed full‐text publications for eligibility, carried out data extraction and updated the text of the review. Second review authors at each stage were one of: Andrew Butler; David Evans; Oliver Schofield‐Robinson; and PA. PA checked the final manuscript of the update.
Sources of support
Internal sources
-
DFS was supported by MRC project grant G9604637, UK.
-
NHS R&D Programme, UK.
External sources
-
CG was supported by the Doris Duke Research Fellowship, USA.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Phil Alderson: None known
Sharon R Lewis: None known
David JW Evans: None known
Andrew R Butler: None known
Oliver J Schofield‐Robinson: None known
Acknowledgements
Thanks to:
-
Brenda Thomas (Cochrane Stroke) for help and advice with the original Embase search strategy.
-
Ian Whittle, Kate Signorini, Elena Telaro, Yoichi Nagayama, Irene Kwan, Frank Del Vecchio, Lisa Xue and Cynthia To for help with manuscripts in languages other than English.
-
Reinhard Wentz and Irene Kwan of Cochrane Injuries for the original searches.
-
Katharine Ker of Cochrane Injuries for work on previous versions of the review.
-
Karen Blackhall, Information Specialist of Cochrane Injuries for updating the searches in 2003, 2005, 2008 and 2009.
-
Odette Harris and Monique Surles for providing additional data for the Harris 2009 trial.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Sep 21 | Hypothermia for traumatic brain injury | Review | Sharon R Lewis, David JW Evans, Andrew R Butler, Oliver J Schofield‐Robinson, Phil Alderson | |
| 2009 Apr 15 | Hypothermia for traumatic head injury | Review | Emma Sydenham, Ian Roberts, Phil Alderson | |
| 2009 Jan 21 | Hypothermia for traumatic head injury | Review | Emma Sydenham, Ian Roberts, Phil Alderson | |
| 2004 Oct 18 | Therapeutic hypothermia for head injury | Review | Phil Alderson, David Signorini, Chirag Patil | |
| 2002 Jan 21 | Therapeutic hypothermia for head injury | Review | Chirag Gadkary, Phil Alderson, David F Signorini | |
Differences between protocol and review
Differences between the current version and previous version of the review (Sydenham 2009b)
Previous versions of the review were titled Hypothermia for traumatic head injury (Alderson 2004; Gadkary 2002; Sydenham 2009a; Sydenham 2009b). We changed the title to Hypothermia for traumatic brain injury and made appropriate edits throughout the review to reflect this change.
We edited the review Background and Methods sections to incorporate all Methodological Expectations of Cochrane Intervention Reviews (MECIR).
We edited the inclusion criteria to include studies with mixed head injuries if fewer than 10% of participants were described as having an open head injury, as we did not want to exclude important studies on the basis that a very small proportion of participants had an open head injury.
We re‐assessed studies that were excluded due to insufficient information on methods of randomisation in Sydenham 2009b. We included five of these studies; we added the remaining three studies to Excluded studies. We removed the references of studies excluded in previous versions of the review; the studies in Excluded studies relate to searches and decisions made for this update.
We updated the 'Risk of bias' tables to incorporate assessment of all domains for all studies. We updated the Characteristics of included studies tables to incorporate all MECIR requirements.
The 2008 update of this review evaluated study quality by allocation concealment only. The incidence of pneumonia was also stratified by study quality.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
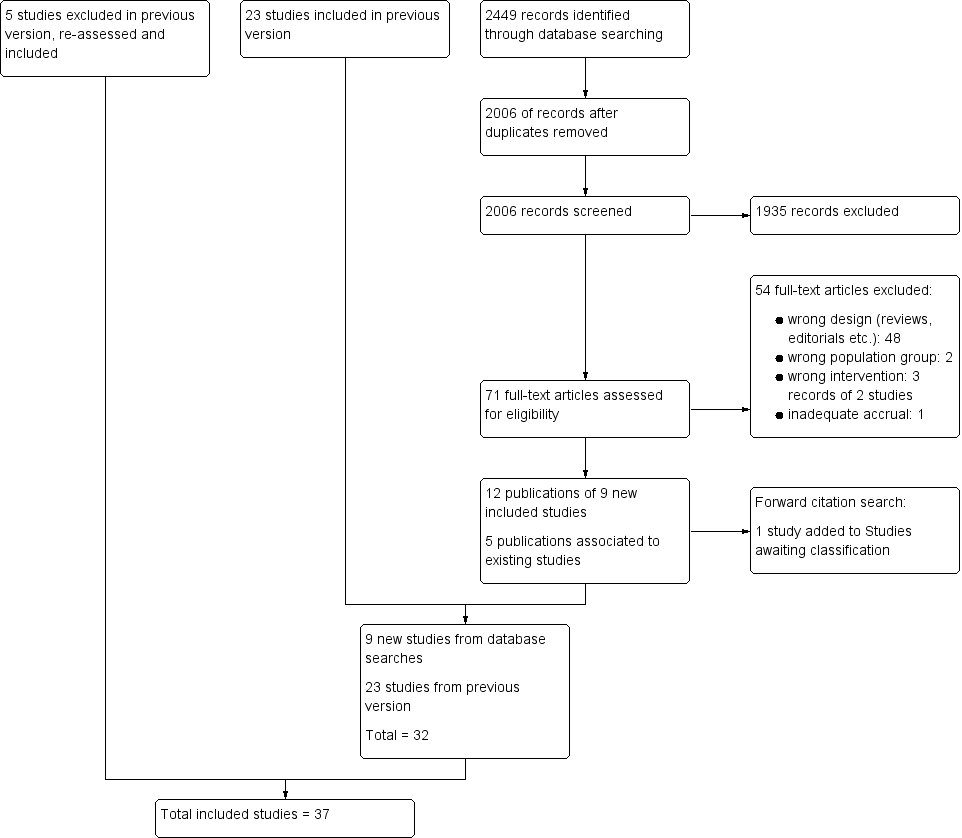
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all 37 included studies.
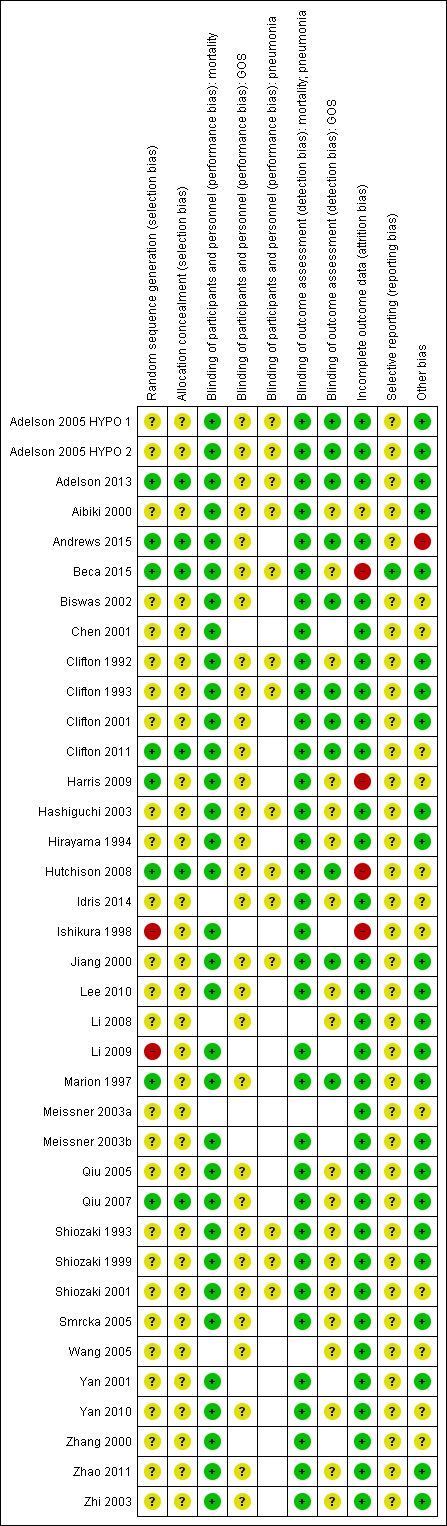
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Note: blank spaces in risk of bias table indicate that the relevant outcome was not reported by study authors

Funnel plot of comparison 1: Therapeutic cooling versus no cooling, outcome: 1.1 Mortality.

Comparison 1 Therapeutic cooling versus no cooling, Outcome 1 Mortality.

Comparison 1 Therapeutic cooling versus no cooling, Outcome 2 Unfavourable outcome.

Comparison 1 Therapeutic cooling versus no cooling, Outcome 3 Pneumonia.

Comparison 2 Therapeutic cooling versus no cooling: by duration, Outcome 1 Mortality.

Comparison 2 Therapeutic cooling versus no cooling: by duration, Outcome 2 Unfavourable outcome.

Comparison 2 Therapeutic cooling versus no cooling: by duration, Outcome 3 Pneumonia.

Comparison 3 Therapeutic cooling versus no cooling: by length of follow‐up, Outcome 1 Mortality.

Comparison 3 Therapeutic cooling versus no cooling: by length of follow‐up, Outcome 2 Unfavourable outcome.
| Hypothermia for traumatic brain injury | |||
| Patient or population: patients with traumatic brain injury | |||
| Outcomes | No of Participants | Quality of the evidence | Comments |
| Mortality at end of follow‐up | 2944 | ⊕⊝⊝⊝ | Data not combined in meta‐analysis. Visual inspection of data showed variation in differences of effect not explained by subgroup analysis |
| Unfavourable outcome at end of follow‐up | 2620 | ⊕⊝⊝⊝ | Data not combined in meta‐analysis. Visual inspection of data showed variation in differences of effect not explained by subgroup analysis |
| Pneumonia | 693 | ⊕⊕⊝⊝ | Data not combined in meta‐analysis. Visual inspection of data showed variation in differences of effect |
| GRADE Working Group grades of evidence | |||
| aWe identified 33 studies that reported data for mortality, with comparable data available for 32 studies | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Unfavourable outcome Show forest plot | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Pneumonia Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 24 hours | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 More than 24 hours | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Unfavourable outcome Show forest plot | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 24 hours | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 More than 24 hours | 23 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pneumonia Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 24 hours | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 More than 24 hours | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 32 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 One month | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Three months | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Six months | 14 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Twelve months or more | 7 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Unfavourable outcome Show forest plot | 26 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Three months | 9 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Six months | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Twelve months | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

