Монотерапия ламотриджином в сравнении с монотерапией карбамазепином при эпилепсии: обзор индивидуальных данных участников
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 8 centres in the UK 2 treatment arms: LTG and CBZ | |
| Participants | Adults and children over the age of 13 with newly diagnosed epilepsy Number randomised: LTG = 70, CBZ = 66; 56 males (41%) 82 with focal seizures (60%) None had received previous AED treatment Mean age (range): 34 (13 to 71) years | |
| Interventions | Monotherapy with LTG or CBZ for 48 weeks 4‐week escalation phase leading to LTG = 150 mg/day, CBZ = 600 mg/day Range of follow‐up: 0 to 398 days | |
| Outcomes | Time to first seizure after 6 weeks of treatment Time to treatment withdrawal Proportion of randomised patients remaining seizure‐free during the last 40 and 24 weeks of trial Percentages of patients who reported adverse events | |
| Notes | IPD provided by trial sponsor GlaxoSmithKline for time to treatment failure, time to first seizure and time to 6‐month remission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random sequence (information provided by drug manufacturer). Stratification by seizure type |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by individual sealed opaque envelopes (information provided by drug manufacturer) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind achieved using LTG tablets formulated to be identical in appearance to CBZ tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Trial investigator blinded, not stated if other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 8 centres in the UK 2 treatment arms: LTG and CBZ | |
| Participants | Adults and children over the age of 13 with newly diagnosed epilepsy Number randomised: LTG = 61, CBZ = 63 56 males (45%) 62 with focal seizures (50%) None had received previous AED treatment Mean age (range): 30 (14 to 86) years | |
| Interventions | Monotherapy with LTG or CBZ for 48 weeks 4‐week escalation phase leading to LTG = 150 mg/day, CBZ = 600 mg/day Range of follow‐up: 0 to 398 days | |
| Outcomes | Time to first seizure after 6 weeks of treatment Time to treatment withdrawal Proportion of randomised patients remaining seizure‐free during the last 40 and 24 weeks of trial Percentages of patients who reported adverse events | |
| Notes | IPD provided by trial sponsor GlaxoSmithKline for time to treatment failure, time to first seizure and time to 6‐month remission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random sequence (information provided by drug manufacturer). Stratification by seizure type |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by individual sealed opaque envelopes (information provided by drug manufacturer) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind achieved using LTG tablets formulated to be identical in appearance to CBZ tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Trial investigator blinded, not stated if other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, double‐blind, parallel‐group trial conducted in the UK 2 treatment arms: LTG and CBZ randomised in a 2:1 ratio | |
| Participants | Adults over the age of 65 with newly diagnosed epilepsy with 2 or more seizures in the previous year with at least 1 seizure in the last 6 months Number randomised: LTG = 102, CBZ = 48 83 males (55%) 105 with focal seizures (70%) Not stated if any participants had received previous AED treatment Mean age (range): 77 (65 to 94) years | |
| Interventions | Monotherapy with LTG or CBZ for 24 weeks 4‐week escalation phase leading to LTG = 100 mg/day, CBZ = 400 mg/day Range of follow‐up = 0 to 280 days | |
| Outcomes | Time to first seizure after 6 weeks of treatment Time to treatment withdrawal Percentage of patients reporting an adverse event Proportion of patients who were both seizure‐free in the last 16 weeks of the trial and did not discontinue treatment | |
| Notes | IPD provided by trial sponsor GlaxoSmithKline for time to treatment failure and time to first seizure (plus seizure freedom rates at 24 weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence (information provided by drug manufacturer). Participants randomised in a 2:1 ratio (LTG:CBZ) |
| Allocation concealment (selection bias) | Low risk | Allocation concealed with pharmacy‐dispensed treatment packs labelled with participant's trial number |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind achieved using LTG tablets formulated to be identical in appearance to CBZ tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Trial investigator blinded, not stated if other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, open‐label, parallel‐group trial conducted in 7 hospitals in the Republic of Korea 2 treatment arms: LTG and CBZ | |
| Participants | Children between the ages of 6 and 12 with a new diagnosis of focal epilepsy and at least 2 seizures in the last 6 months Number randomised: LTG = 43, CBZ = 41 48 males (57%) 100% focal epilepsy Not stated if any participants had received previous AED treatment Mean age (range): 9 (5 to 13) years | |
| Interventions | Monotherapy with LTG or CBZ for 32 weeks 8‐week escalation phase leading to LTG = 3 to 6 mg/kg/day, CBZ = 10 to 20 mg/kg/day Range of follow‐up: 12 to 788 days | |
| Outcomes | Seizure‐free rate over 6 months (maintenance period) by treatment group Change in cognition (neuropsychological), behaviour and quality of life from screening to the end of the maintenance phase by treatment group Incidence of adverse events | |
| Notes | IPD provided by trial author for time to treatment failure, time to first seizure and time to 6‐month remission No source of funding stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each centre received a separate and independent computer‐generated random code list |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised single‐centre, open‐label, parallel‐group trial conducted at Tel Aviv University and Medical Centre, Israel 2 treatment arms: LTG and CBZ | |
| Participants | Adults admitted to the neurological department with a first seizure event after an ischaemic stroke Number randomised: LTG = 32, CBZ = 32 46 males (72%) 100% focal seizures Unclear if any participants had received previous AED treatment Mean age (range): 67.5 (38 to 90) years | |
| Interventions | Monotherapy with LTG or CBZ for 12 months Dose escalation phase (length not stated) leading to LTG 100 mg/day, CBZ 300 mg/day Range of follow‐up: not stated | |
| Outcomes | The appearance of a second seizure under treatment or by finishing the 12‐month follow‐up without seizures Tolerability: incidence of adverse events Treatment withdrawals due to adverse events | |
| Notes | Contact made with trial author who was willing to provide IPD but data never received. Aggregate data extracted from graphs in the publication. Stated in the title of the paper that LTG and CBZ were monotherapy treatments but Table 1 of the paper refers to total no. AED; unclear if all participants were receiving monotherapy treatment. No source of funding stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in a 1:1 ratio, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rate reported; all randomised participants included in analysis |
| Selective reporting (reporting bias) | Low risk | No protocol available. Seizure outcomes and adverse events well reported |
| Other bias | Unclear risk | Unclear if all participants were receiving monotherapy treatment |
| Methods | Phase IV, open label, randomised, multicentre trial conducted in 21 Centres in Korea Two treatment arms: CBZ and LTG | |
| Participants | Participants were untreated epileptics who had at least 2 unprovoked seizures (focal or generalised tonic clonic) during the last 24 weeks before the study start, more than 24 hours apart. Number randomised: CBZ=129, LTG=264 (ITT population) 154 male participants (39%); 288 participants (73%) with focal epilepsy Mean age (SD): CBZ=37.6 (15.8), LTG=34.2 (16.3) years | |
| Interventions | Monotherapy with CBZ or LTG Permitted doses LTG: 100mg/day – 500mg/day for LTG , CBZ: 400mg/day – 1200mg/day. | |
| Outcomes | Retention Rate at Study End Terminal 24 week seizure free rate and time interval from the end of dose titration phase to the first seizure | |
| Notes | Full text of the trial published in Korean. Abstract and clinical trial summary available in English. IPD requested from trial sponsor Glaxo Smith Kline but data could not be located | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as randomised, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | High risk | Attrition rate reported, not all participants included in analysis, which is not an ITT approach |
| Selective reporting (reporting bias) | Low risk | Results for all outcomes summarised for all listed outcomes |
| Other bias | Unclear risk | None identified |
| Methods | Randomised, multicentre, open‐label, parallel‐group trial conducted in the Republic of Korea 2 treatment arms: LTG and CBZ | |
| Participants | Adults over the age of 16 with newly diagnosed focal epilepsy or untreated focal epilepsy for at least 1 year Number randomised: LTG = 57, CBZ = 53 57 males (52%) 95 focal seizures (86%) Not stated how many participants had received previous AED treatment Mean age (range): 36 (16 to 60) years | |
| Interventions | Monotherapy with LTG or CBZ for 48 weeks 8‐week escalation phase leading to LTG = 200 mg/day, CBZ = 600 mg/day Range of follow‐up: 14 to 337 days | |
| Outcomes | Change of neuropsychological and cognitive scores from baseline: general intellectual ability, learning and memory, attention and executive function (group‐by‐time interaction) Frequency of psychological and health‐related quality of life symptoms Proportion with seizure freedom during the maintenance period | |
| Notes | IPD provided by trial author for time to treatment failure, time to first seizure and time to 6‐month remission This trial was supported by a grant from GlaxoSmithKline Korea. No other funding sources stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (block size 4) via a computer randomisation program (information provided by trial author) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, open‐label, parallel‐group trial conducted in Europe and Mexico 2 treatment arms: LTG and CBZ randomised in a 2:1 ratio | |
| Participants | Adults and children over the age of 2 with newly diagnosed or currently untreated focal epilepsy with 2 or more seizures in the previous 6 months and with at least 1 seizure in the last 3 months Number randomised: LTG = 420, CBZ = 202 329 males (53%) 619 with focal seizures (99.5%) Not stated how many participants had received previous AED treatment Mean age (range): 27 (2 to 84) years | |
| Interventions | Monotherapy with LTG or CBZ for 24 weeks 6‐week escalation phase leading to minimum of LTG 2 mg/kg/day age range 2 to 12 years, 200 mg/day age range 13 to 64 years and 100 mg/day age > 65 years. CBZ aged 2 to 12 years 5 to 40 mg/kg, age > 12 years 100 to 1500 mg/day Range of follow‐up: 0 to 245 days | |
| Outcomes | Proportion of patients seizure‐free during the last 16 weeks of treatment Efficacy success: proportion of patients who did not withdraw before the end of week 18 and were seizure‐free in the last 16 weeks of the trial Time to withdrawal from the trial (proportion of patients completing the trial) Proportion of patients experiencing adverse events Treatment withdrawals due to adverse events | |
| Notes | IPD provided by trial sponsor GlaxoSmithKline for time to treatment failure and time to first seizure (plus seizure freedom rates at 24 weeks) Dates of seizures during the first 4 weeks not provided with individual participant data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. Participants randomised in a 2:1 ratio (LTG:CBZ), stratified by age group and country |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by individual sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 56 centres in Europe and Australia 3 treatment arms: LTG (200 mg/day), LTG (100 mg/day) and CBZ | |
| Participants | Adults and children over the age of 12 with newly diagnosed, currently untreated or recurrent epilepsy with 2 or more seizures in the previous 6 months and with at least 1 seizure in the last 3 months. Participants must not have taken antiepileptic medication in the previous 6 months. Number randomised: LTG (200 mg) = 115, LTG (100 mg) = 116, CBZ = 121 188 males (54%) 237 with focal seizures (68%) Not stated how many participants had received previous AED treatment Mean age (range): 32 (12 to 71) years | |
| Interventions | Monotherapy with LTG or CBZ for 30 weeks 4‐week escalation phase leading to LTG = 100 mg/day, LTG = 200 mg/day, CBZ = 600 mg/day Range of follow‐up: 0 to 378 days | |
| Outcomes | Proportion seizure‐free after the first 6 weeks of treatment Time to first seizure Time to treatment withdrawal Frequency of adverse events with at least 5% incidence in any treatment group | |
| Notes | IPD provided by trial sponsor GlaxoSmithKline for time to treatment failure, time to first seizure and time to 6‐month remission Participants considered to complete the trial if they experienced a seizure after the first 6 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence (information provided by drug manufacturer) |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by individual sealed, opaque envelopes (information provided by drug manufacturer) |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 18 Veterans Affairs Medical Centres in the United States 3 treatment arms: LTG, CBZ and gabapentin (GBP) | |
| Participants | Adults over the age of 60 with newly diagnosed seizures, untreated or treated with sub‐therapeutic AED levels, with at least 1 seizure in the previous 3 months Number randomised: LTG = 200, CBZ = 198 378 males (95%) 299 with focal seizures (75%) Not stated how many participants had received previous AED treatment Mean age: 72 years, range not stated | |
| Interventions | Monotherapy with LTG or CBZ for 12 months 6‐week escalation phase leading to LTG = 150 mg/day, CBZ = 600 mg/day Range of follow‐up: not stated | |
| Outcomes | Retention in the trial for 12 months Seizure freedom at 12 months Time to 1st, 2nd, 5th and 10th seizure (time to seizures) Drug toxicity (incidence of systemic and neurologic toxicities) Serum drug levels and compliance Seizure‐free retention rates | |
| Notes | IPD requested from trial sponsor, the Department of Veterans Affairs, USA. At the time of review, IPD have not been received. Aggregate data extracted from graphs in the publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (varying sizes) performed by site via a computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation used and pharmacy dispensed a prescription of the allocated drug (part of a blinded drug kit) to participants |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinding achieved with double dummy tablets; doses of both increased and decreased simultaneously |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specifically stated |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported. Most of the randomised participants included in analysis; 3 excluded due to site closure (not related to treatment) |
| Selective reporting (reporting bias) | Low risk | No protocol available but case report forms of data collected provided by the sponsor. Seizure outcomes and adverse events well reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 29 centres across Croatia, Finland, France, Finland and Norway. 2 treatment arms: LTG, CBZ | |
| Participants | Adults over the age of 65 with newly diagnosed seizures, with a history of at least 2 seizures and at least 1 seizure in the previous 6 months. Participants must not have taken antiepileptic medication for more than 2 weeks in the previous 6 months and never taken CBZ or LTG. Number randomised: LTG = 94, CBZ = 92 102 males (54%) Proportion with focal seizures not stated Not stated how many participants had received previous AED treatment Mean age: 74 (65 to 91) years | |
| Interventions | Monotherapy with LTG or CBZ for 40 weeks 4‐week escalation phase leading to LTG = 100 mg/day, CBZ = 400 mg/day Range of follow‐up: not stated | |
| Outcomes | Retention in the trial (time to treatment withdrawal for any cause) Seizure freedom after week 4 Seizure freedom after week 20 Time to first seizure Adverse event reports Tolerability according to the Liverpool Adverse Event profile (AEP) | |
| Notes | IPD requested from trial sponsor Glaxo Smith Kline but data could not be located Aggregate summary data extracted from the publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinding achieved with double dummy tablets, packaged together |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specifically stated |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all participants who received trial treatment were included in an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No protocol available but clinical trial summary provided by the sponsor. Seizure outcomes and adverse events well reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, multicentre, open‐label, parallel‐group trial conducted in the UK 5 treatment arms: LTG, CBZ, GBP, topiramate (TPM) and oxcarbazepine (OXC) | |
| Participants | Adults and children over the age of 4 years with newly diagnosed focal epilepsy, relapsed focal epilepsy or failed treatment with a previous drug not used in this trial Number randomised: LTG = 378, CBZ = 378 409 males (54%) 662 focal epilepsy (88%) 139 had received previous AED treatment (18%) Mean age (range): 38 (5 to 83) years | |
| Interventions | Monotherapy for LTG or CBZ (no fixed trial duration) Titration doses and maintenance doses decided by treating clinician Range of follow‐up: 17 to 2420 days | |
| Outcomes | Time to treatment failure Time to 1‐year (12‐month) remission Time to 2‐year remission Time to first seizure Health‐related quality of life via the NEWQOL (Newly Diagnosed Epilepsy Quality of Life Battery) Health economic assessment and cost‐effectiveness of the drugs (cost per QALY gained and cost per seizure avoided) Frequency of clinically important adverse events | |
| Notes | IPD provided for time to treatment failure, time to first seizure, time to 6‐month, time to 12‐month and time to 24‐month remission (trial conducted at our site and sponsored by the Health Technology Assessment programme of the National Institute of Health Research) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer minimisation program stratified by centre, sex and treatment history |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation to a central randomisation allocation service |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
| Methods | Randomised, open‐label, parallel‐group trial conducted in 24 centres across Germany 4 treatment arms: LTG (2 arms), CBZ and sodium valproate (SV) Participants with focal and generalised epilepsy randomised separately to LTG or CBZ and LTG or SV respectively | |
| Participants | Adults and children over the age of 12 with newly diagnosed epilepsy; at least 1 seizure and electroencephalographic imaging suggesting epilepsy Number randomised not stated; number included in analysis: LTG = 88, CBZ = 88 106 males (64%) 100% focal seizures Not stated how many participants had received previous AED treatment Mean age: 47.5 years, range not stated | |
| Interventions | Monotherapy with LTG or CBZ for 22 to 26 weeks 4‐week escalation phase leading to LTG = 100 to 200 mg/day, CBZ = 600 to 1200 mg/day in adults and 600 to 1000 mg/day in children aged 11 to 15 Range of follow‐up: not stated | |
| Outcomes | Number of seizure‐free patients during trial weeks 17 to 24 "Leaving the study" (retention rates) Adverse event rates | |
| Notes | IPD requested from trial sponsor GlaxoSmithKline but data could not be provided due to restrictions over the de‐identification of datasets from trials conducted in Germany Aggregate data extracted from graphs in the publication Data from participants with focal seizures only included as this is the randomised comparison of LTG and CBZ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) | High risk | Number of participants randomised to each group not reported (254 randomised and 239 analysed in the 4 arms of the trial). Reasons for exclusion stated but not to which drug these participants were randomised. |
| Selective reporting (reporting bias) | Low risk | No protocol available but clinical trial summary provided by the sponsor. Seizure outcomes and adverse events well reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, parallel‐group trial conducted in 47 centres across Germany, Austria and Switzerland 3 treatment arms: LTG, CBZ and levetiracetam (LEV) | |
| Participants | Adults over the age of 60 with newly diagnosed focal seizures, with a history of at least 2 seizures and at least 1 seizure in the previous 6 months. Participants must not have taken antiepileptic medication for more than 4 weeks. Number randomised: LTG = 118, CBZ = 121 135 males (56%) 100% focal epilepsy Not stated how many participants had received previous AED treatment Mean age (range): 71 (60 to 89) years | |
| Interventions | Monotherapy with LTG or CBZ for 58 weeks 6‐week escalation phase leading to LTG = 100 mg/day, CBZ = 400 mg/day Range of follow‐up: 0 to 1508 days | |
| Outcomes | Retention rate at week 58 Time to discontinuation from randomisation Seizure freedom rates at week 30 and week 58 Time to first seizure from randomisation Time to first drug‐related adverse event Adverse events (by severity) | |
| Notes | IPD provided by trial author for time to treatment failure, time to first seizure, time to 6‐month and time to 12‐month remission Trial was sponsored by UCB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list for each centre (random permuted blocks) was prepared by the Interdisciplinary Centre for Clinical Trials (IZKS), Mainz, Germany |
| Allocation concealment (selection bias) | Low risk | The pharmacy of the University Hospital Mainz encapsulated the trial drugs and labelled the blinded medication including the randomisation number |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and trial investigator blinded by the use of matching capsules |
| Blinding of outcome assessment (detection bias) | Unclear risk | Trial investigator blinded; not stated if other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | Protocol provided. All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | None identified |
1Abbreviations
AED: antiepileptic drug
CBZ: carbamazepine
IPD: individual participant data
ITT: intention‐to‐treat
LTG: lamotrigine
QALY: quality‐adjusted life year
2For trials for which IPD were provided attrition and reporting bias are reduced as attrition rates and unpublished outcome data are requested (Brodie 1995 A; Brodie 1995 B; Brodie 1999Nieto‐Barrera 2001; Reunanen 1996).
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Participants randomised to lamotrigine and physician's choice of carbamazepine or valproate. No fully randomised comparison between lamotrigine and carbamazepine | |
| Not monotherapy | |
| Wrong drug comparison | |
| Conference abstract for full publication Eun 2012 | |
| Withdrawn to monotherapy. Design excluded. | |
| Wrong drug comparison | |
| Not monotherapy | |
| Conference abstract for full publication Lee 2011 | |
| Not randomised | |
| Wrong drug comparison | |
| Abstract of full publication Rowan 2005 | |
| Conference abstract for full publication Saetre 2007 | |
| Subset of Saetre 2007 | |
| Subset of Saetre 2010 | |
| Wrong drug comparison | |
| Abstract of full publication Steinhoff 2005 | |
| Wrong drug comparison | |
| Not randomised |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to treatment failure (any reason related to the treatment) Show forest plot | 9 | 2569 | Hazard Ratio (Fixed, 95% CI) | 0.73 [0.64, 0.82] |
| Analysis 1.1 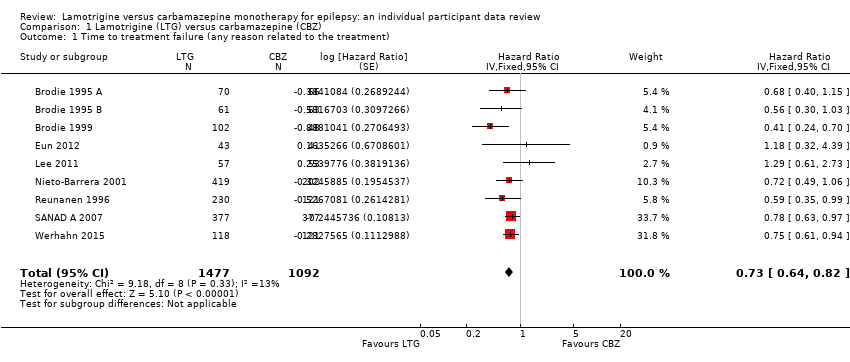 Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 1 Time to treatment failure (any reason related to the treatment). | ||||
| 2 Time to treatment failure due to adverse events Show forest plot | 9 | 2569 | Hazard Ratio (Fixed, 95% CI) | 0.54 [0.45, 0.65] |
| Analysis 1.2  Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 2 Time to treatment failure due to adverse events. | ||||
| 3 Time to treatment failure due to lack of efficacy Show forest plot | 5 | 1874 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.75, 1.41] |
| Analysis 1.3 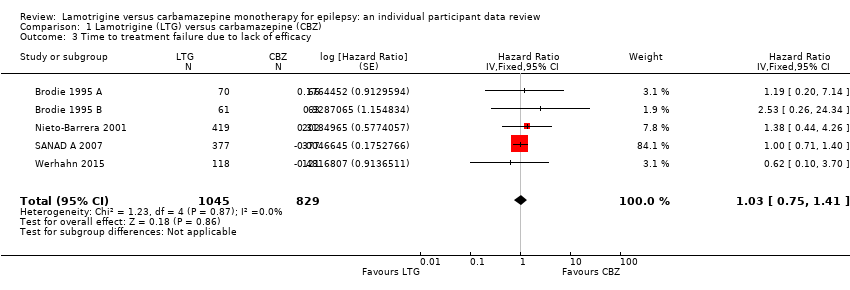 Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 3 Time to treatment failure due to lack of efficacy. | ||||
| 4 Time to treatment failure (any reason related to the treatment) ‐ by seizure type Show forest plot | 9 | 2481 | Hazard Ratio (Fixed, 95% CI) | 0.71 [0.62, 0.82] |
| Analysis 1.4 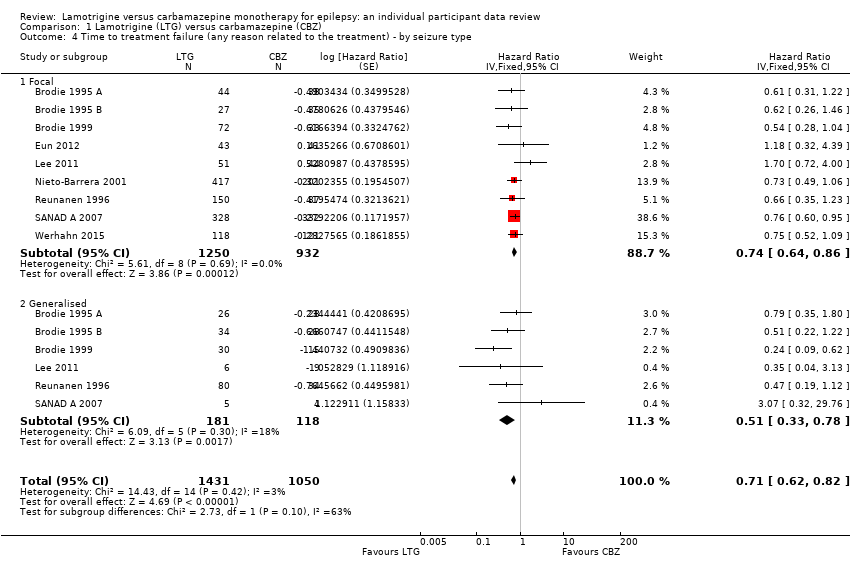 Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 4 Time to treatment failure (any reason related to the treatment) ‐ by seizure type. | ||||
| 4.1 Focal | 9 | 2182 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.64, 0.86] |
| 4.2 Generalised | 6 | 299 | Hazard Ratio (Fixed, 95% CI) | 0.51 [0.33, 0.78] |
| 5 Time to treatment failure due to adverse events ‐ by seizure type Show forest plot | 9 | 2466 | Hazard Ratio (Fixed, 95% CI) | 0.55 [0.45, 0.66] |
| Analysis 1.5  Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 5 Time to treatment failure due to adverse events ‐ by seizure type. | ||||
| 5.1 Focal | 9 | 2182 | Hazard Ratio (Fixed, 95% CI) | 0.56 [0.45, 0.68] |
| 5.2 Generalised | 5 | 284 | Hazard Ratio (Fixed, 95% CI) | 0.49 [0.27, 0.88] |
| 6 Time to treatment failure (any reason related to the treatment, with aggregate data) Show forest plot | 13 | 3391 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.63, 0.78] |
| Analysis 1.6  Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 6 Time to treatment failure (any reason related to the treatment, with aggregate data). | ||||
| 7 Time to treatment failure (any reason related to the treatment) ‐ subgroup analysis (blinding) Show forest plot | 13 | 3391 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.63, 0.78] |
| Analysis 1.7 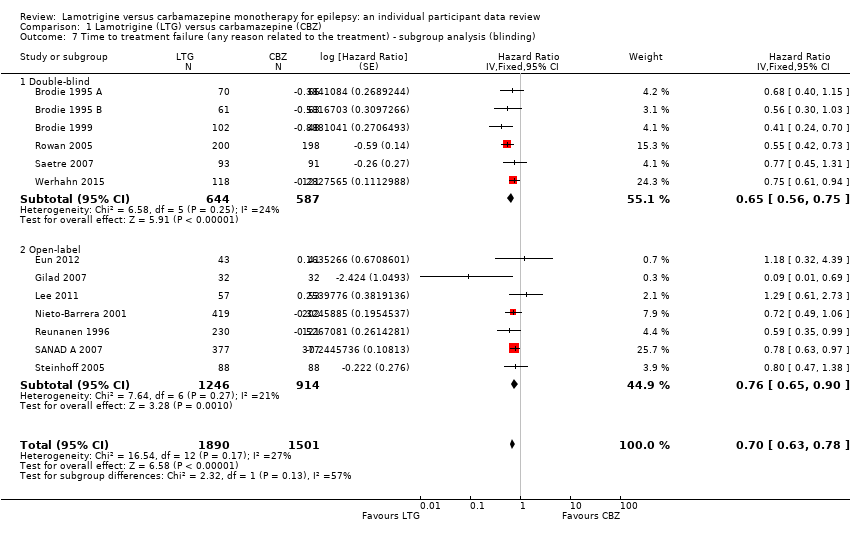 Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 7 Time to treatment failure (any reason related to the treatment) ‐ subgroup analysis (blinding). | ||||
| 7.1 Double‐blind | 6 | 1231 | Hazard Ratio (Fixed, 95% CI) | 0.65 [0.56, 0.75] |
| 7.2 Open‐label | 7 | 2160 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.65, 0.90] |
| 8 Time to first seizure Show forest plot | 9 | 2564 | Hazard Ratio (Fixed, 95% CI) | 1.22 [1.09, 1.37] |
| Analysis 1.8  Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 8 Time to first seizure. | ||||
| 9 Time to first seizure by seizure type Show forest plot | 9 | 2476 | Hazard Ratio (Fixed, 95% CI) | 1.26 [1.12, 1.41] |
| Analysis 1.9 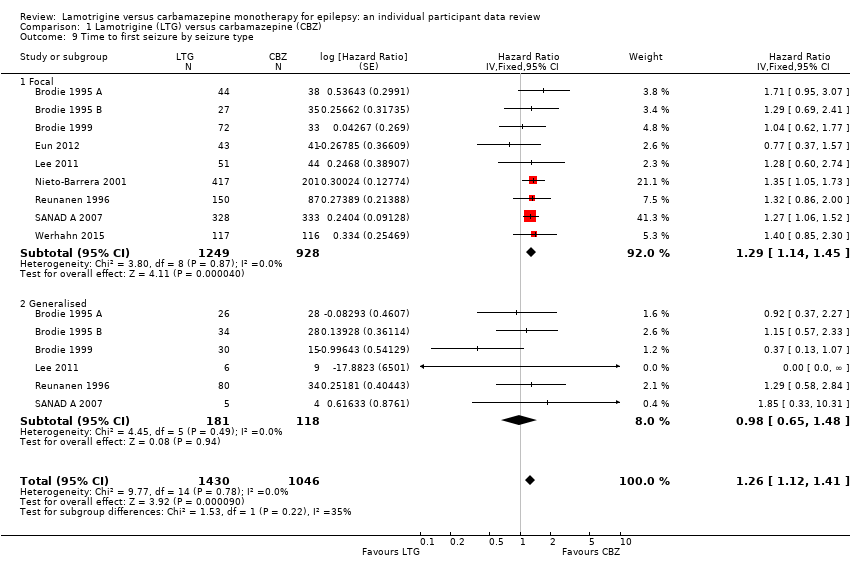 Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 9 Time to first seizure by seizure type. | ||||
| 9.1 Focal | 9 | 2177 | Hazard Ratio (Fixed, 95% CI) | 1.29 [1.14, 1.45] |
| 9.2 Generalised | 6 | 299 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.65, 1.48] |
| 10 Time to first seizure (with aggregate data) Show forest plot | 12 | 3216 | Hazard Ratio (Fixed, 95% CI) | 1.24 [1.12, 1.37] |
| Analysis 1.10 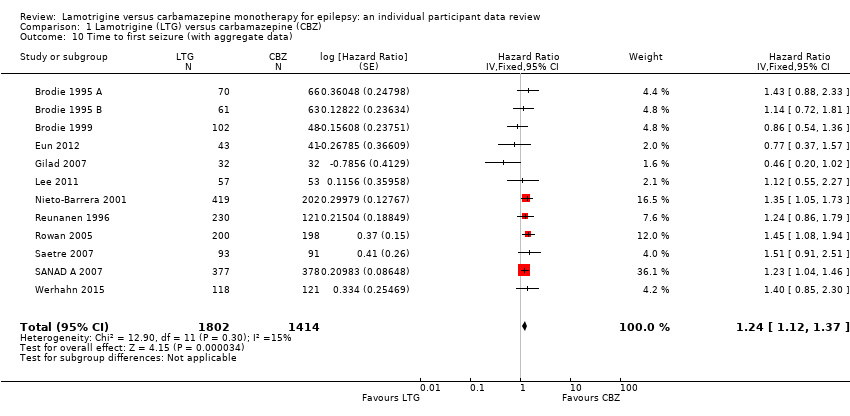 Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 10 Time to first seizure (with aggregate data). | ||||
| 11 Seizure freedom (whole study) Show forest plot | 14 | 3760 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.04, 1.18] |
| Analysis 1.11  Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 11 Seizure freedom (whole study). | ||||
| 12 Time to 6‐month remission Show forest plot | 7 | 1793 | Hazard Ratio (Fixed, 95% CI) | 0.84 [0.74, 0.94] |
| Analysis 1.12  Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 12 Time to 6‐month remission. | ||||
| 13 Time to 6‐month remission by seizure type Show forest plot | 7 | 1708 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.76, 0.97] |
| Analysis 1.13 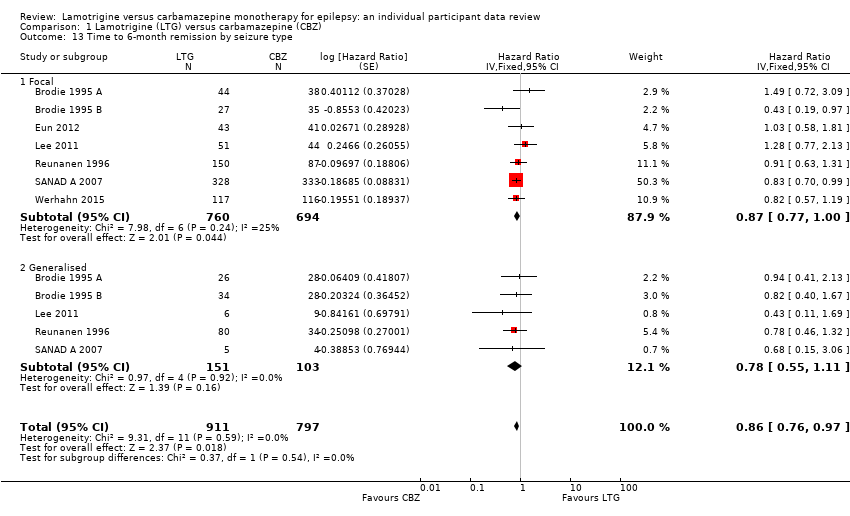 Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 13 Time to 6‐month remission by seizure type. | ||||
| 13.1 Focal | 7 | 1454 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.77, 1.00] |
| 13.2 Generalised | 5 | 254 | Hazard Ratio (Fixed, 95% CI) | 0.78 [0.55, 1.11] |
| 14 Seizure freedom at 6 months Show forest plot | 14 | 3760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.03] |
| Analysis 1.14  Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 14 Seizure freedom at 6 months. | ||||
| 15 Time to 12‐month remission Show forest plot | 2 | 988 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.77, 1.07] |
| Analysis 1.15  Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 15 Time to 12‐month remission. | ||||
| 16 Time to 12‐month remission by seizure type Show forest plot | 2 | 988 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.76, 1.07] |
| Analysis 1.16 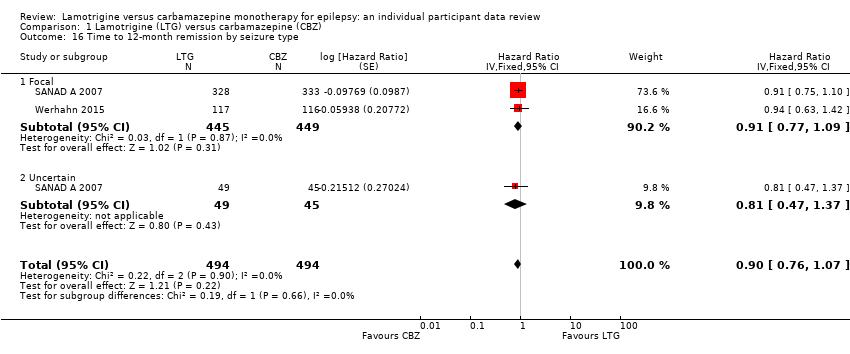 Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 16 Time to 12‐month remission by seizure type. | ||||
| 16.1 Focal | 2 | 894 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.77, 1.09] |
| 16.2 Uncertain | 1 | 94 | Hazard Ratio (Fixed, 95% CI) | 0.81 [0.47, 1.37] |
| 17 Time to 24‐month remission Show forest plot | 1 | 755 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.80, 1.25] |
| Analysis 1.17 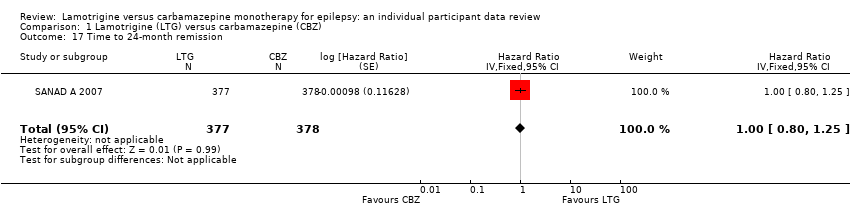 Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 17 Time to 24‐month remission. | ||||
| 18 Time to 24‐month remission by seizure type Show forest plot | 1 | 755 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.82, 1.30] |
| Analysis 1.18  Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 18 Time to 24‐month remission by seizure type. | ||||
| 18.1 Focal | 1 | 661 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.83, 1.35] |
| 18.2 Uncertain | 1 | 94 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.44, 1.67] |
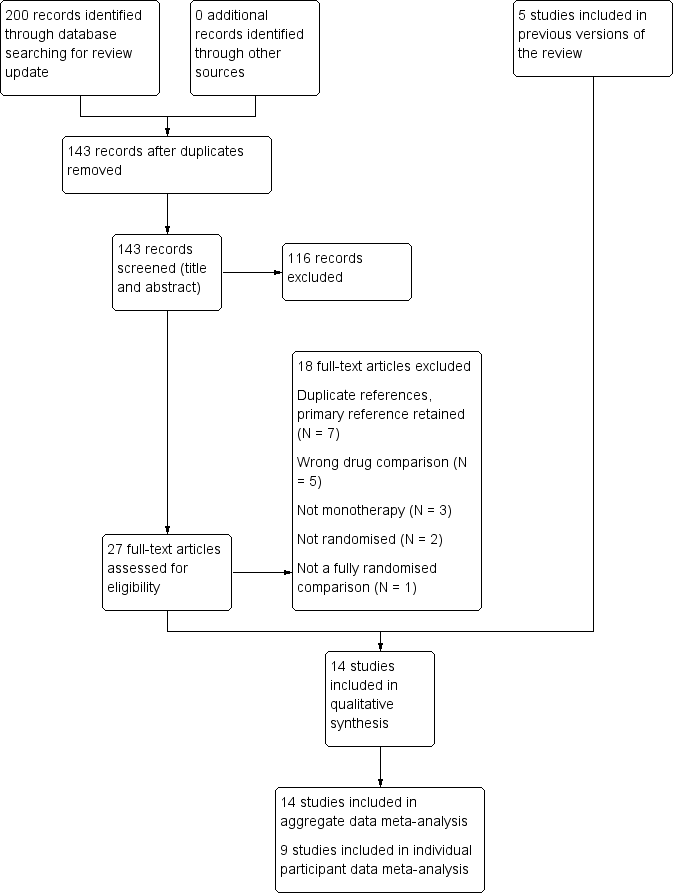
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
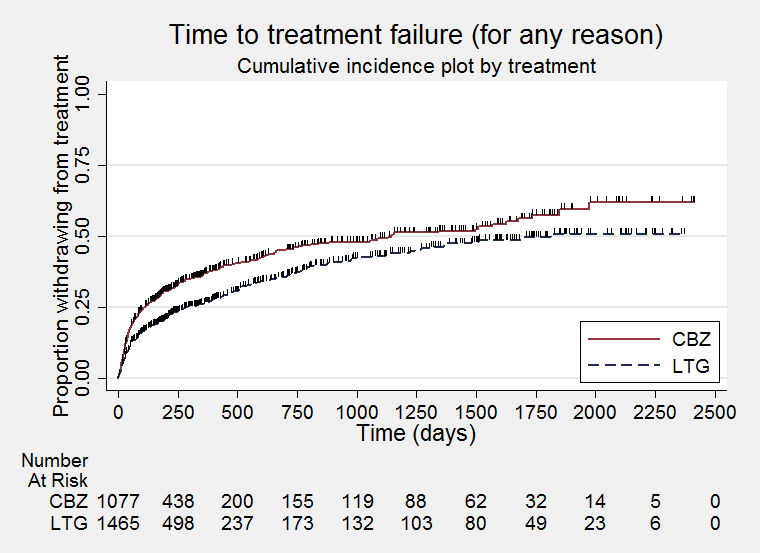
Time to treatment failure for any reason related to treatment (CBZ: Carbamazepine; LTG: Lamotrigine)
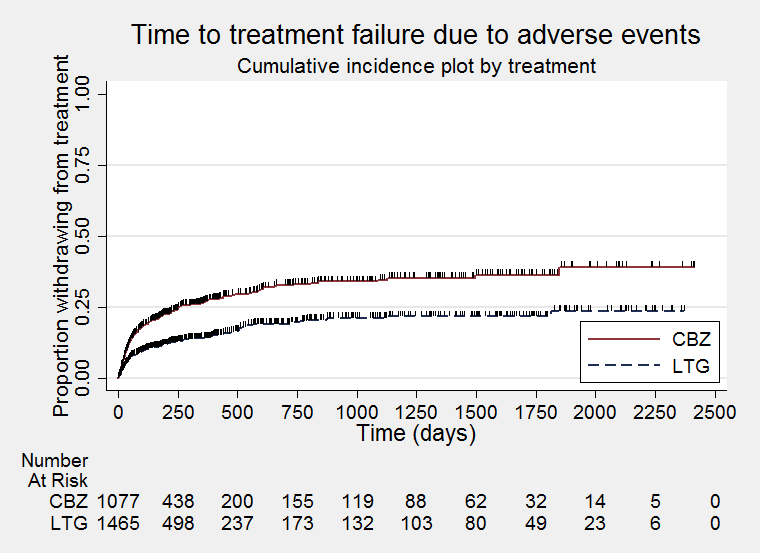
Time to treatment failure due to adverse effects (CBZ: Carbamazepine; LTG: Lamotrigine)

Time to treatment failure due to lack of efficacy (CBZ: Carbamazepine; LTG: Lamotrigine)

Time to treatment failure for any reason related to treatment ‐ by seizure type (CBZ: Carbamazepine; LTG: Lamotrigine)

Time to treatment failure for due to adverse events ‐ by seizure type (CBZ: Carbamazepine; LTG: Lamotrigine)
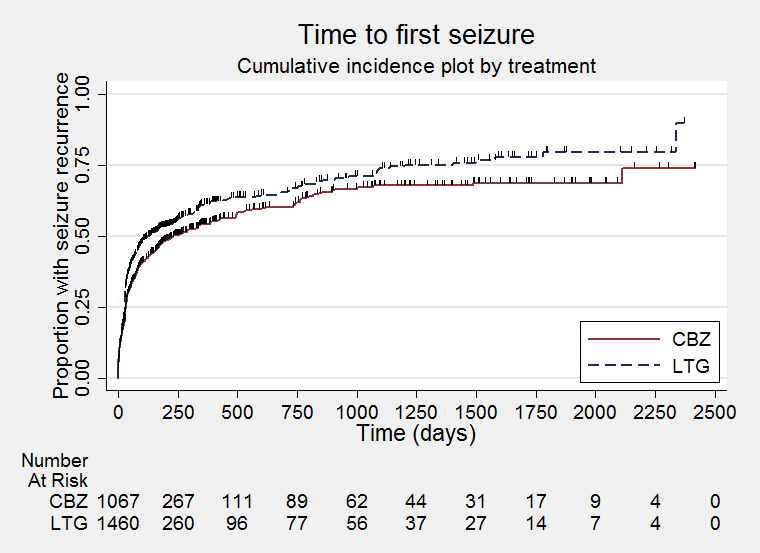
Time to first seizure (CBZ: Carbamazepine; LTG: Lamotrigine)
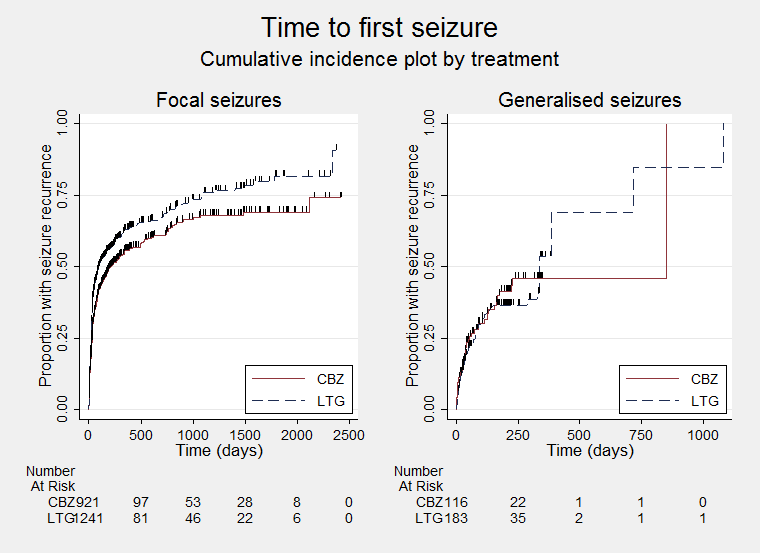
Time to first seizure ‐ by seizure type (CBZ: Carbamazepine; LTG: Lamotrigine)
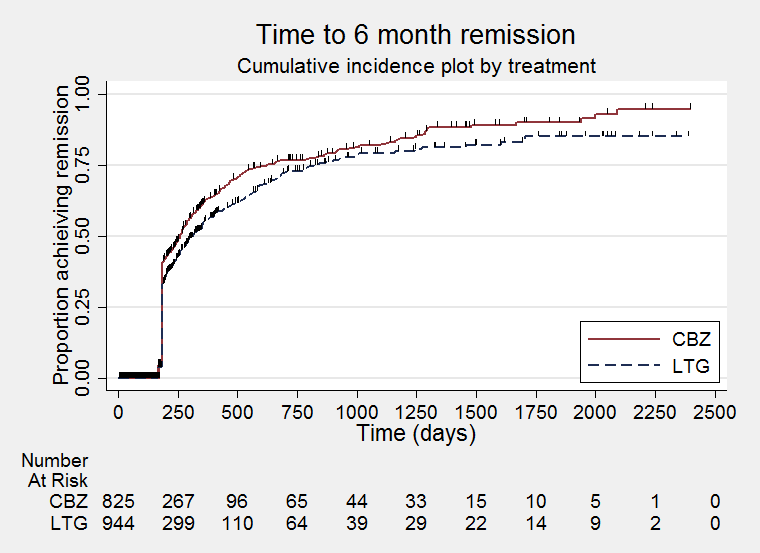
Time to six‐month remission (CBZ: Carbamazepine; LTG: Lamotrigine)

Time to six‐month remission ‐ by seizure type (CBZ: Carbamazepine; LTG: Lamotrigine)
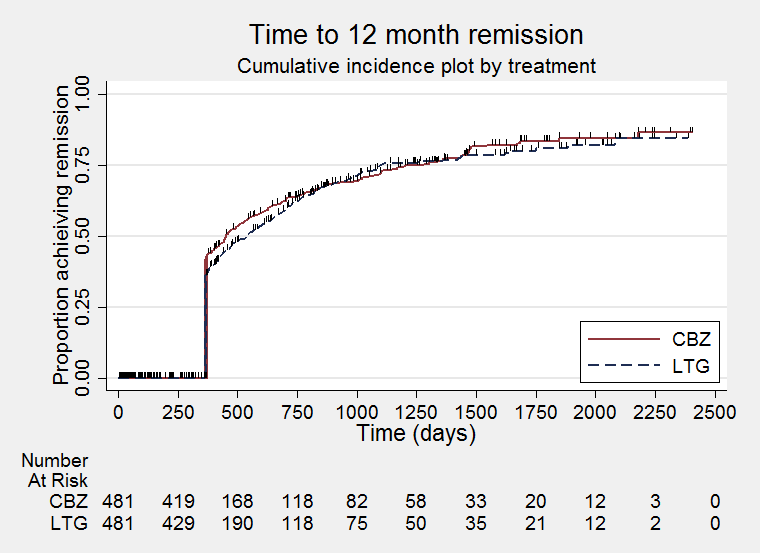
Time to 12‐month remission (CBZ: Carbamazepine; LTG: Lamotrigine)

Time to 24‐month remission (CBZ: Carbamazepine; LTG: Lamotrigine)

Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 1 Time to treatment failure (any reason related to the treatment).

Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 2 Time to treatment failure due to adverse events.
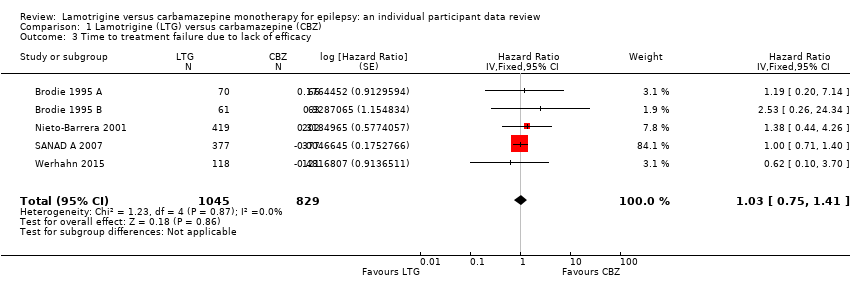
Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 3 Time to treatment failure due to lack of efficacy.
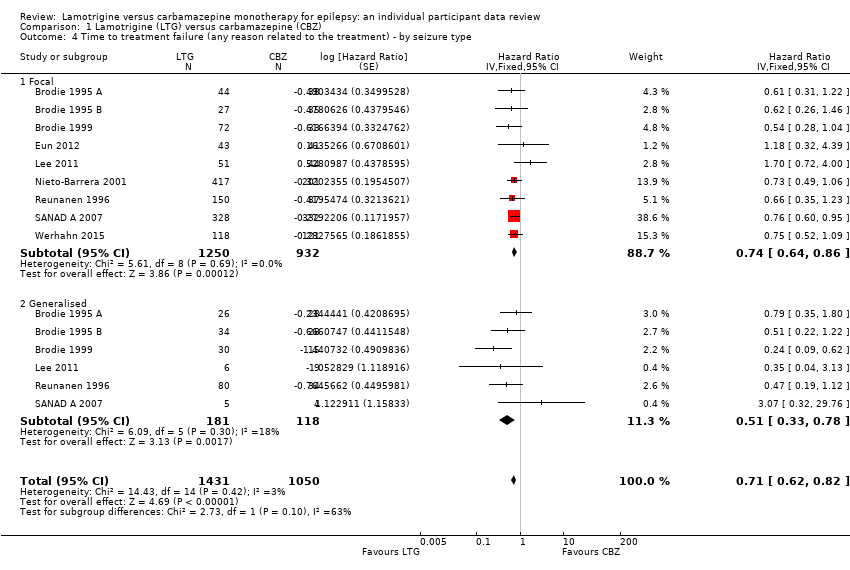
Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 4 Time to treatment failure (any reason related to the treatment) ‐ by seizure type.
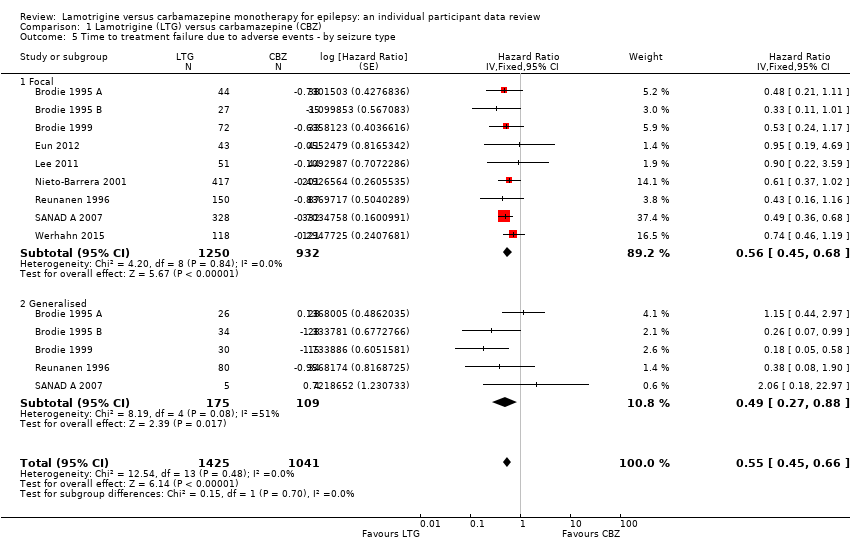
Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 5 Time to treatment failure due to adverse events ‐ by seizure type.
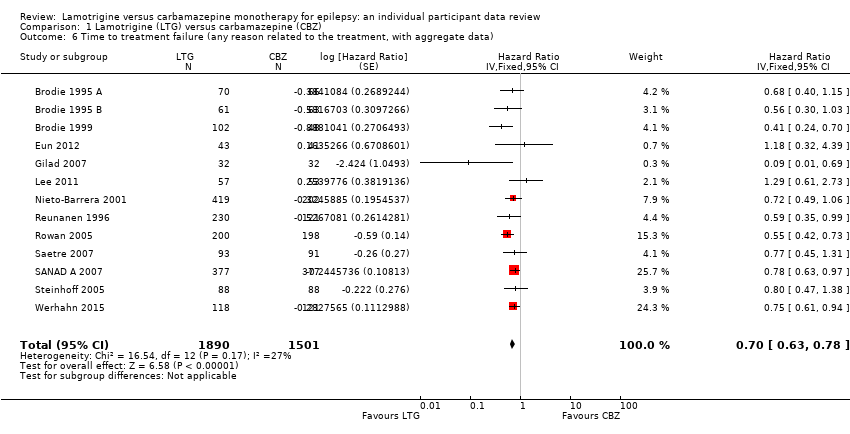
Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 6 Time to treatment failure (any reason related to the treatment, with aggregate data).

Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 7 Time to treatment failure (any reason related to the treatment) ‐ subgroup analysis (blinding).

Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 8 Time to first seizure.

Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 9 Time to first seizure by seizure type.
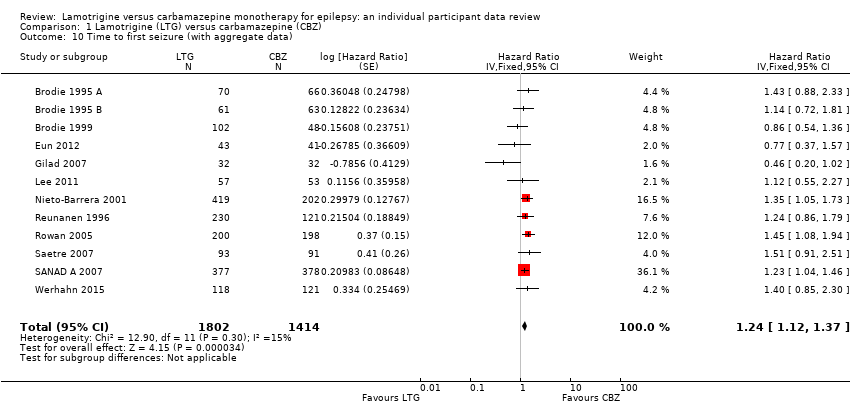
Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 10 Time to first seizure (with aggregate data).
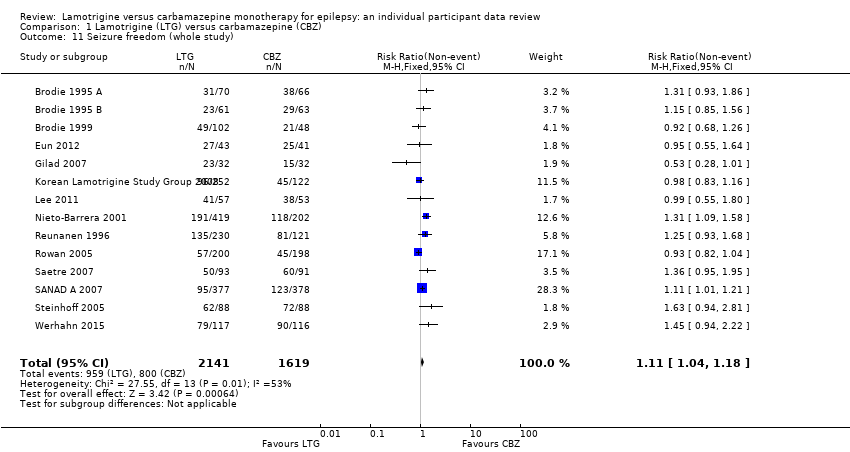
Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 11 Seizure freedom (whole study).

Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 12 Time to 6‐month remission.
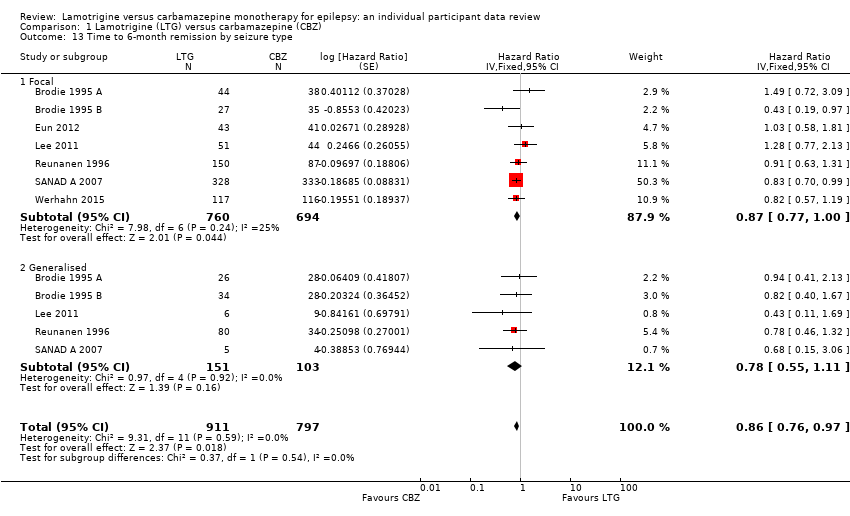
Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 13 Time to 6‐month remission by seizure type.

Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 14 Seizure freedom at 6 months.
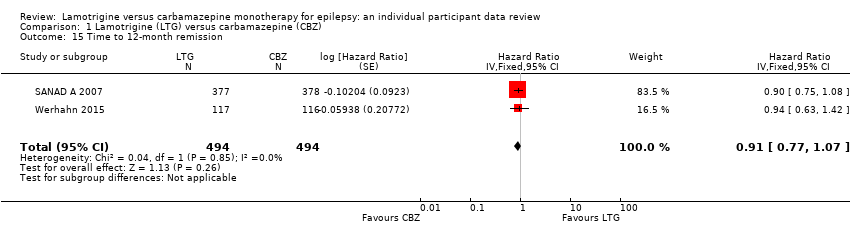
Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 15 Time to 12‐month remission.

Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 16 Time to 12‐month remission by seizure type.

Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 17 Time to 24‐month remission.
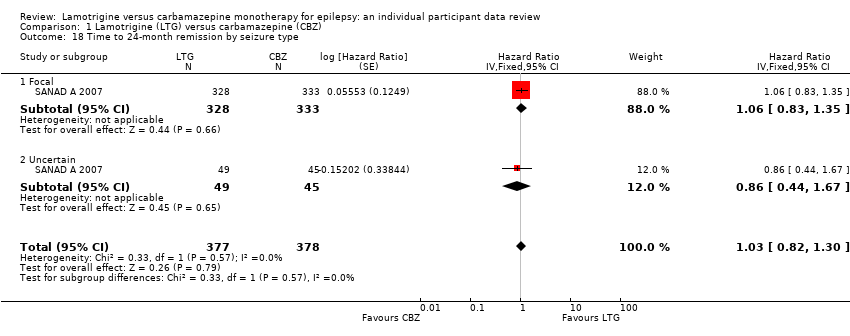
Comparison 1 Lamotrigine (LTG) versus carbamazepine (CBZ), Outcome 18 Time to 24‐month remission by seizure type.
| Lamotrigine compared with carbamazepine for epilepsy | ||||||
| Patient or population: adults and children with focal onset or generalised onset seizures (generalised tonic‐clonic with or without other generalised seizure types) Settings: outpatients Intervention: lamotrigine Comparison: carbamazepine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Carbamazepine | Lamotrigine | |||||
| Time to treatment failure (any reason related to treatment) All participants Range of follow‐up: 0 to 2420 days | The median time to treatment failure was 1144 days in the carbamazepine group | The median time to treatment failure was 1813 days (669 days longer) in the lamotrigine group | HR 0.71 (0.62 to 0.82)a | 2481 (9 trials) | ⊕⊕⊕⊝ | HR of less than 1 indicates an advantage for lamotrigine. Treatment failure due to adverse events also occurred significantly earlier on carbamazepine compared to lamotrigine: HR 0.54 (95% CI 0.45 to 0.65, P<0.00001). There was no difference between lamotrigine and carbamazepine in terms of treatment failure due to lack of efficacy: HR 1.03 (95% CI 0.75 to 1.41, P=0.86) |
| Time to treatment failure (any reason related to treatment) Subgroup: focal onset seizures Range of follow‐up: 0 to 2420 days | The median time to treatment failure was 1149 days in the carbamazepine group | The median time to treatment failure was 1699 days (550 days longer) in the lamotrigine group | HR 0.74 (0.64 to 0.86) | 2182 (9 trials) | ⊕⊕⊕⊝ | HR of less than 1 indicates an advantage for lamotrigine. Treatment failure due to adverse events also occurred significantly earlier on carbamazepine compared to lamotrigine: HR 0.56 (95% CI 0.45 to 0.68, P<0.00001). Treatment failure due to lack of efficacy was not calculated for focal onset seizures subgroup due to small numbers of individuals withdrawing from treatment for lack of efficacy. |
| Time to treatment failure (any reason related to treatment) Subgroup: generalised onset seizures Range of follow‐up: 0 to 1446 days | The 25th percentile** of time to treatment failure was 57 days in the carbamazepine group | The 25th percentile** of time to treatment failure was 510 days (453 days longer) in the lamotrigine group | HR 0.51 (0.33 to 0.78) | 299 (6 trials) | ⊕⊕⊝⊝ | HR of less than 1 indicates an advantage for lamotrigine Treatment failure due to adverse events also occurred significantly earlier on carbamazepine compared to lamotrigine: HR 0.49 (95% CI 0.27 to 0.88, P=0.02). Treatment failure due to lack of efficacy was not calculated for focal onset seizures subgroup due to small numbers of individuals withdrawing from treatment for lack of efficacy. |
| * Illustrative risks in the carbamazepine and lamotrigine groups are calculated at the median time to treatment failure (i.e. the time to 50% of participants failing or withdrawing from allocated treatment) within each group across all trials. The relative effect (pooled hazard ratio) shows the comparison of 'time to treatment failure' between the treatment groups. ** The 25th percentile of time to treatment failure (i.e. the time to 50% of participants failing or withdrawing from allocated treatment) is presented for the subgroup with generalised seizures as less than 50% of participants failed / withdrew from treatment, therefore the median time could not be calculated. Abbreviations: 95% CI: 95% confidence interval; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| a. Pooled hazard ratio for all participants adjusted for seizure type. b. Downgraded once due to high risk of bias due to the open‐label design of five trials included in the analysis (Eun 2012; Lee 2011; Nieto‐Barrera 2001; Reunanen 1996; SANAD A 2007); the design of the trial may have influenced the withdrawal rates. c. Downgraded once due to high risk of bias due to the open‐label design of three trials included in the analysis (Lee 2011; Reunanen 1996; SANAD A 2007); the design of the trial may have influenced the withdrawal rates. d. Downgraded once due to potential misclassification of generalised onset seizures in up to 50% of participants in the trials. | ||||||
| Lamotrigine compared with carbamazepine for epilepsy | ||||||
| Patient or population: adults and children with focal onset or generalised onset seizures (generalised tonic‐clonic with or without other generalised seizure types) Settings: outpatients Intervention: lamotrigine Comparison: carbamazepine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Carbamazepine | Lamotrigine | |||||
| Time to first seizure All participants Range of follow‐up: 0 to 2420 days | The median time to first seizure was 232 days in the carbamazepine group | The median time to first seizure was 134 days (98 days shorter) in the lamotrigine group | HR 1.26 (1.12 to 1.41)a | 2476 (9 trials) | ⊕⊕⊕⊕ | HR of less than 1 indicates an advantage for lamotrigine |
| Time to first seizure Subgroup: focal onset seizures Range of follow‐up: 0 to 2420 days | The median time to first seizure was 208 days in the carbamazepine group | The median time to first seizure was 96 days (112 days shorter) in the lamotrigine group | HR 1.29 (1.14 to 1.45) | 2177 (9 trials) | ⊕⊕⊕⊕ | HR of less than 1 indicates an advantage for lamotrigine |
| Time to first seizure Subgroup: generalised onset seizures Range of follow‐up: 0 to 853 days | The median time to first seizure was 853 days in the carbamazepine group | The median time to first seizure was 337 days (516 days longer) in the lamotrigine group | HR 0.98 (0.65 to 1.48) | 277 (6 trials) | ⊕⊕⊝⊝ | HR of less than 1 indicates an advantage for lamotrigine |
| Time to 12‐month remission All participants Range of follow‐up: 0 to 2420 days | The median time to 12‐month remission was 452 days in the carbamazepine group | The median time to 12‐month remission was 538 days (86 days longer) in the lamotrigine group | HR 0.91 (0.77 to 1.07) | 988 (2 trials) | ⊕⊕⊕⊕ | HR of less than 1 indicates an advantage for carbamazepine Time to 12‐month remission not presented by seizure type due to small numbers of participants with generalised onset seizures in the two trials |
| * Illustrative risks in the carbamazepine and lamotrigine groups are calculated at the median time to first seizure or time to 12‐month remission (i.e. the time to 50% of participants experiencing a first seizure or 12‐months of remission) within each group across all trials. The relative effect (pooled hazard ratio) shows the comparison of 'time to first seizure' or 'time to 12‐month remission' between the treatment groups. Abbreviations: 95% CI: 95% confidence interval; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| a. Pooled hazard ratio for all participants adjusted for seizure type. b. High risk of bias due to the open‐label design in some of the included trials, however outcomes are objective and unlikely to be influenced by knowledge of drug allocation. No downgrade made. c. Downgraded once due to potential misclassification of generalised onset seizures in up to 50% of participants in the trials. | ||||||
| Focal seizures: n (%) | Male gender: n (%) | Age at entry (years): Mean (SD), range | Aged > 30 and generalised seizures: n (%) | Epilepsy duration (years): Mean (SD), range | Number of seizures in prior 6 months: median (range) | |||||||||||||
| LTG | CBZ | Missing | LTG | CBZ | Missing | LTG | CBZ | Missing | LTG | CBZ | Missing | LTG | CBZ | Missing | LTG | CBZ | Missing | |
| 44 (63%) | 38 (58%) | 0 | 28 (40%) | 28 (42%) | 0 | 35.3 (17.1), 15 to 71 | 32.5 (14.4), 13 to 69 | 0 | 11 | 9 | 0 | 2.2 (3.3), 0 to 17.9 | 1.8 (2.3), 0.3 to 11.0 | 0 | 4 (1 to 490) | 3 (1 to 960) | 0 | |
| 27 (44%) | 35 (56%) | 0 | 26 (43%) | 30 (48%) | 0 | 30.9 (14.5), 14 to 86 | 29.1 (13.9), 14 to 81 | 0 | 12 | 11 | 0 | 1.4 (3.2), 0 to 19.4 | 1.2 (1.8), 0 to 7.1 | 0 | 3 (1 to 1020) | 3 (2 to 122) | 0 | |
| 72 (71%) | 33 (69%) | 0 | 55 (54%) | 28 (58%) | 0 | 77.3 (6.1), 65 to 94 | 76.2 (5.9), 66 to 88 | 0 | 30 | 15 | 0 | NA | NA | 150 | 3 (1 to 163) | 4.5 (1 to 108) | 0 | |
| 43 (100%) | 41 (100%) | 0 | 24 (56%) | 24 (59%) | 0 | 9.2 (2.0), 6 to 13 | 8.3 (2.1), 5 to 12 | 0 | 0 | 0 | 0 | 0.6 (0.9), 0 to 4.5 | 0.5 (0.3), 0 to 1.4 | 1 | 3(2 to 11) | 3 (2 to 11) | 0 | |
| 51 (89%) | 44 (83%) | 0 | 24 (42%) | 33 (62%) | 0 | 33.6 (12.6), 16 to 60 | 38.3 (11.5), 16 to 60 | 0 | 2 | 7 | 0 | NA | NA | 110 | 2(0 to 60) | 2 (0 to 200) | 0 | |
| 418 (99.5%) | 201 (99.5%) | 0 | 222 (53%) | 107 (53%) | 0 | 27.1 (21.7), 2 to 84 | 27.5 (21.0), 2 to 77 | 1 | 1 | 1 | 0 | NA | NA | 622 | 4 (1 to 9000) | 3 (1 to 3600) | 0 | |
| 150 (65%) | 87 (72%) | 0 | 127 (55%) | 61 (50%) | 0 | 31.8 (14.0), 12 to 71 | 32.7 (14.6), 13 to 71 | 2 | 31 | 12 | 0 | 2.2 (3.2), 0 to 17.1 | 2.2 (3.7), 0.26 to 8 | 3 | 3(1 to 133) | 3 (1 to 145) | 1 | |
| 329 (99%) | 333 (99%) | 85 | 205 (55%) | 204 (55%) | 18 | 36.8 (18.4), 6 to 83 | 39.3 (18.4), 5 to 82 | 18 | 46 | 42 | 0 | NA | NA | 727 | 2(0 to 1185) | 4 (0 to 466) | 19 | |
| 118 (100%) | 121 (100%) | 0 | 69 (59%) | 65 (54%) | 0 | 70.8 (7.5), 60 to 88 | 71.8 (6.7), 60 to 89 | 0 | 0 | 0 | 0 | NA | NA | 239 | 2 (1 to 20) | 2 (1 to 90) | 6 | |
| CBZ = carbamazepine, LTG = lamotrigine; n = number of participants; NA = not applicable; SD = standard deviation | ||||||||||||||||||
| EEG normal: n (%) | CT scan normal: n (%) | Neurological exam normal: n (%) | |||||||
| LTG | CBZ | Missing | LTG | CBZ | Missing | LTG | CBZ | Missing | |
| 32 (46%) | 30 (46%) | 2 | 38 (84%) | 44 (90%) | 42 | 62 (89%) | 61 (92%) | 0 | |
| 42 (73%) | 34 (56%) | 6 | 34 (77%) | 38 (79%) | 32 | 56 (92%) | 52 (83%) | 0 | |
| NA | NA | 150 | 39 (39%) | 23 (48%) | 1 | 59 (58%) | 31 (65%) | 0 | |
| 3 (7%) | 3 (7%) | 0 | 38 (88%) | 37(90%) | 0 | 43 (100%) | 40 (98%) | 0 | |
| 31 (54%) | 27 (51%) | 0 | 36 (63%) | 38 (72%) | 0 | 57 (100%) | 53 (100%) | 0 | |
| NA | NA | 622 | NA | NA | 622 | NA | NA | 622 | |
| 9 (53%) | 4 (44%) | 325 | 11 (73%) | 5 (83%) | 330 | 202 (89%) | 103 (85%) | 0 | |
| NA | NA | 756 | NA | NA | 756 | 277 (75%) | 281 (76%) | 18 | |
| 45 (38%) | 37 (31%) | 1 | 26 (22%) | 26 (21%) | 1 | NA | NA | 239 | |
| CBZ = carbamazepine; CT = computerised tomography; EEG = electroencephalogram; LTG = lamotrigine; n = number of participants; NA = not applicable | |||||||||
| Number randomised | Time to treatment failure | Time to first seizure | Time to 6‐ month remission1 | Time to 12‐ month remission | Time to 24‐ month remission | |||||||||||||
| LTG | CBZ | Total | LTG | CBZ | Total | LTG | CBZ | Total | LTG | CBZ | Total | LTG | CBZ | Total | LTG | CBZ | Total | |
| 70 | 66 | 136 | 70 | 66 | 136 | 70 | 66 | 136 | 70 | 66 | 136 | NA | NA | NA | NA | NA | NA | |
| 61 | 63 | 124 | 61 | 63 | 124 | 61 | 63 | 124 | 61 | 63 | 124 | NA | NA | NA | NA | NA | NA | |
| 102 | 48 | 150 | 102 | 48 | 150 | 102 | 48 | 150 | 102 | 48 | 150 | NA | NA | NA | NA | NA | NA | |
| 43 | 41 | 84 | 43 | 41 | 84 | 43 | 41 | 84 | 43 | 41 | 84 | NA | NA | NA | NA | NA | NA | |
| 57 | 53 | 110 | 57 | 53 | 110 | 57 | 53 | 110 | 57 | 53 | 110 | NA | NA | NA | NA | NA | NA | |
| 420 | 202 | 622 | 419 | 202 | 621 | 419 | 202 | 621 | 419 | 202 | 621 | NA | NA | NA | NA | NA | NA | |
| 230 | 121 | 351 | 230 | 121 | 351 | 230 | 121 | 351 | 230 | 121 | 351 | NA | NA | NA | NA | NA | NA | |
| 378 | 378 | 756 | 377 | 377 | 754 | 377 | 378 | 755 | 377 | 378 | 755 | 377 | 378 | 755 | 377 | 378 | 755 | |
| 118 | 121 | 239 | 118 | 121 | 239 | 117 | 116 | 233 | 117 | 116 | 233 | 117 | 116 | 233 | NA | NA | NA | |
| Total | 1479 | 1093 | 2572 | 1477 | 1092 | 2569 | 1476 | 1088 | 2564 | 1476 | 1088 | 2564 | 494 | 494 | 988 | 377 | 378 | 755 |
| CBZ = carbamazepine; LTG = lamotrigine; NA: not applicable (trial duration not sufficient to measure the outcome). 1. Brodie 1999, and Nieto‐Barrera 2001, are of 24 weeks duration (approximately six months). The two trials are not included in the analyses of time to six‐month remission but are included in sensitivity analysis of seizure freedom at six months. 2. Follow‐up data are missing for one participant in Nieto‐Barrera 2001. 3. Treatment failure time missing for two participants and seizure data after follow‐up missing for one participant in SANAD A 2007. 4. Seizure data after follow‐up missing for six participants in Werhahn 2015. | ||||||||||||||||||
| Reason for early termination1 | Classification in time‐to‐event analyses: Event | Classification in time‐to‐event analyses: Censored | Total | ||||||||||
| Adverse events | Inadequate response/seizure recurrence | Both adverse events and inadequate response | Protocol violation/non‐compliance | Withdrew consent/participant choice3 | Other (treatment‐related)4 | Illness or death (not treatment‐related) | Remission of seizures | Lost to follow‐up | Other (not treatment‐related)5 | Completed trial | |||
| LTG | 18 | 3 | 0 | 3 | 1 | 0 | 1 | 0 | 1 | 0 | 43 | 70 | |
| CBZ | 22 | 2 | 0 | 6 | 1 | 0 | 0 | 0 | 2 | 0 | 33 | 66 | |
| LTG | 7 | 3 | 0 | 5 | 2 | 0 | 0 | 0 | 1 | 0 | 43 | 61 | |
| CBZ | 23 | 1 | 0 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 34 | 63 | |
| LTG | 18 | 0 | 0 | 7 | 3 | 0 | 1 | 0 | 2 | 0 | 71 | 102 | |
| CBZ | 20 | 0 | 0 | 3 | 2 | 2 | 0 | 0 | 1 | 0 | 20 | 48 | |
| LTG | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 34 | 43 | |
| CBZ | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 35 | 41 | |
| LTG | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31 | 32 | |
| CBZ | 10 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 21 | 32 | |
| LTG | 24 | 11 | 0 | 0 | 0 | 52 | 0 | 0 | 0 | 0 | 165 | 252 | |
| CBZ | 13 | 2 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | 85 | 122 | |
| LTG | 4 | 3 | 0 | 2 | 7 | 0 | 0 | 0 | 2 | 0 | 39 | 57 | |
| CBZ | 7 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 7 | 0 | 34 | 53 | |
| LTG | 34 | 12 | 0 | 6 | 16 | 0 | 0 | 0 | 13 | 0 | 339 | 420 | |
| CBZ | 26 | 4 | 0 | 11 | 2 | 0 | 0 | 0 | 3 | 0 | 156 | 202 | |
| LTG | 10 | 1 | 0 | 17 | 3 | 3 | 4 | 0 | 0 | 0 | 192 | 230 | |
| CBZ | 12 | 0 | 0 | 11 | 6 | 0 | 2 | 0 | 0 | 0 | 90 | 121 | |
| LTG | 20 | 7 | 0 | 15 | 24 | 0 | 7 | 0 | 10 | 5 | 112 | 200 | |
| CBZ | 54 | 3 | 0 | 14 | 28 | 0 | 14 | 0 | 4 | 10 | 71 | 198 | |
| LTG | 13 | 0 | 0 | 2 | 0 | 10 | 0 | 0 | 0 | 0 | 68 | 93 | |
| CBZ | 23 | 0 | 0 | 1 | 0 | 6 | 0 | 0 | 0 | 0 | 61 | 91 | |
| LTG | 61 | 60 | 11 | 1 | 4 | 16 | 7 | 23 | 0 | 14 | 181 | 378 | |
| CBZ | 104 | 43 | 20 | 2 | 1 | 7 | 10 | 25 | 0 | 15 | 151 | 378 | |
| LTG | 7 | 1 | 0 | 0 | 13 | 3 | 0 | 0 | 0 | 0 | 64 | 88 | |
| CBZ | 17 | 0 | 0 | 0 | 7 | 5 | 0 | 0 | 0 | 0 | 59 | 88 | |
| LTG | 31 | 2 | 0 | 6 | 13 | 1 | 0 | 0 | 0 | 0 | 65 | 118 | |
| CBZ | 39 | 3 | 0 | 4 | 20 | 0 | 0 | 0 | 0 | 0 | 55 | 121 | |
| Total LTG | 251 | 105 | 11 | 64 | 86 | 85 | 20 | 23 | 33 | 19 | 1447 | 2144 | |
| Total CBZ | 373 | 58 | 20 | 58 | 71 | 42 | 27 | 25 | 20 | 25 | 905 | 1624 | |
| Total (all) | 624 | 163 | 31 | 122 | 157 | 127 | 47 | 48 | 53 | 44 | 2352 | 3768 | |
| 1. Primary reason for discontinuation specified ‐ participants may have withdrawn from allocated treatment for a combination of reasons. 2. Reasons for treatment failure extracted from trial publications for Gilad 2007, Korean Lamotrigine Study Group 2008; Rowan 2005, Saetre 2007, and Steinhoff 2005. Individual participant data for reasons for treatment failure provided for other trials. 3. Withdrawal of consent/participant choice classified as an event in this review but censored in included trial (SANAD A 2007). Sensitivity analysis classifying withdrawal of consent as a censored observation did not change the conclusions (results available on request). 4. Other treatment‐related reasons: investigator choice (Werhahn 2015), drug‐related death, pregnancy or perceived remission (SANAD A 2007). Specified only as 'other reason' for Brodie 1999; Reunanen 1996; Korean Lamotrigine Study Group 2008; Saetre 2007 and Steinhoff 2005. 5. Other reasons (not treatment‐related): epilepsy diagnosis changed (SANAD A 2007). Specified only as 'other reason' for Rowan 2005, and for seven participants in SANAD A 2007. 6. No information on whether participants withdrew from treatment or completed the study available for 19 participants 7. One participant (randomised to LTG) with date and reason for treatment failure missing. 8. Two participants with date of treatment failure missing so not included in analysis of time to treatment failure but with reasons for treatment failure provided (both censored: one withdrew from LTG due to remission of seizures, one withdrew from CBZ due to 'other' non‐treatment‐related reason). | |||||||||||||
| Treatment | N | Comparator | N | Total | Time to treatment failure | Time to first seizure | Time to 6‐month remission | |||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Lamotrigine (both arms) | 230 | Carbamazepine | 121 | 351 | 0.59 (0.35 to 0.99) | 0.04 | 1.24 (0.86 to 1.79) | 0.25 | 0.84 (0.36 to 1.95) | 0.68 |
| Lamotrigine 200 mg | 115 | Lamotrigine 100 mg + carbamazepine | 236 | 351 | 0.47 (0.25 to 0.86) | 0.02 | 0.96 (0.67 to 1.36) | 0.8 | 0.62 (0.24 to 1.58) | 0.32 |
| Lamotrigine 100 mg | 115 | Lamotrigine 200 mg + carbamazepine | 236 | 351 | 1.05 (0.63 to 1.75) | 0.85 | 1.29 (0.91 to 1.83) | 0.15 | 1.33 (0.56 to 3.17) | 0.52 |
| Lamotrigine 200 mg | 115 | Carbamazepine | 121 | 236 | 0.41 (0.21 to 0.78) | 0.007 | 1.12 (0.73 to 1.72) | 0.59 | 0.63 (0.22 to 1.78) | 0.39 |
| Lamotrigine 100 mg | 115 | Carbamazepine | 121 | 236 | 0.73 (0.43 to 1.26) | 0.26 | 1.37 (0.90 to 2.07) | 0.14 | 1.10 (0.41 to 2.92) | 0.86 |
| mg= milligrams per day; HR = hazard ratio; 95% CI = 95% confidence interval | ||||||||||
| Time to treatment failure | Time to first seizure | Time to 6‐month remission | |
| Original analysis | F: HR 0.74, 95% CI (0.64 to 0.86) G: HR 0.51, 95% CI (0.33 to 0.78) O: HR 0.71, 95% CI (0.62 to 0.82) | F: HR 1.29, 95% CI (1.14 to 1.45) G: HR 0.98, 95% CI (0.65 to 1.48) O: HR 1.26, 95% CI (1.12 to 1.41) | F: HR 0.87, 95% CI (0.77 to 1.00) G: HR 0.78, 95% CI (0.55 to 1.11) O: HR 0.86, 95% CI (0.76 to 0.97) |
| Test of subgroup differences | Chi² = 2.73, df = 1 (P = 0.10), I² = 63.4% | Chi² = 1.53, df = 1 (P = 0.22), I² = 34.5% | Chi² = 0.37, df = 1 (P = 0.54), I² = 0% |
| Generalised onset and age at onset > 30 reclassified | F: HR 0.72, 95% CI (0.62 to 0.83) G: HR 0.58, 95% CI (0.32 to 1.06) O: HR 0.71, 95% CI (0.62 to 0.82) | F: HR 1.25, 95% CI (1.11 to 1.41) G: HR 1.17, 95% CI (0.67 to 2.04) O: HR 1.25, 95% CI (1.11 to 1.40) | F: HR 0.85, 95% CI (0.75 to 0.97) G: HR 0.69, 95% CI (0.44 to 1.08) O: HR 0.84, 95% CI (0.74 to 0.95) |
| Test of subgroup differences | Chi² = 0.45, df = 1 (P = 0.50), I² = 0% | Chi² = 0.06, df = 1 (P = 0.81), I² = 0% | Chi² = 0.80, df = 1 (P = 0.37), I² = 0% |
| Generalised onset and age at onset > 30 reclassified | F: HR 0.74, 95% CI (0.64 to 0.86) G: HR 0.58, 95% CI (0.32 to 1.06) U: HR 0.62, 95% CI (0.39 to 0.97) O: HR 0.72, 95% CI (0.63 to 0.83) | F: HR 1.29, 95% CI (1.14 to 1.45) G: HR 1.17, 95% CI (0.67 to 2.04) U: HR 0.88, 95% CI (0.58 to 1.33) O: HR 1.24, 95% CI (1.11 to 1.39) | F: HR 0.87, 95% CI (0.77 to 1.00) G: HR 0.69, 95% CI (0.44 to 1.08) U: HR 0.89, 95% CI (0.60 to 1.31) O: HR 0.86, 95% CI (0.76 to 0.97) |
| Test of subgroup differences | Chi² = 1.15, df = 2 (P = 0.56), I² = 0% | Chi² = 3.03, df = 2 (P = 0.22), I² = 33.9% | Chi² = 1.02, df = 2 (P = 0.60), I² = 0% |
| CI = confidence interval; F = focal onset seizures; G = generalised onset seizures; HR = hazard ratio; O = overall pooled result adjusted by seizure type; U = uncertain seizure type. | |||
| Trial | Number experiencing adverse events | Number of adverse events | Number of adverse events per person (range) | Number of drug‐related adverse events1 | Number of adverse events requiring action/ treatment change | Number of patients needing a treatment change/dose change | |||||||||||
| LTG | CBZ | Total | LTG | CBZ | Total | LTG | CBZ | LTG | CBZ | Total | LTG | CBZ | Total | LTG | CBZ | Total | |
| 62 | 58 | 120 | 388 | 322 | 710 | 1 to 30 | 1 to 17 | 94 | 124 | 218 | 167 | 111 | 278 | 22 | 32 | 54 | |
| 54 | 58 | 112 | 285 | 291 | 576 | 1 to 14 | 1 to 18 | 81 | 125 | 206 | 98 | 81 | 179 | 20 | 40 | 60 | |
| 91 | 41 | 132 | 338 | 173 | 511 | 1 to 12 | 1 to 10 | 109 | 73 | 182 | 92 | 66 | 158 | 39 | 27 | 66 | |
| 3 | 6 | 9 | 5 | 8 | 13 | 1 to 30 | 1 to 2 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 4 | 6 | 10 | NA | NA | NA | NA | NA | 4 | 5 | 9 | NA | NA | NA | NA | NA | NA | |
| 218 | 120 | 338 | 524 | 277 | 801 | 1 to 10 | 1 to 11 | 238 | 152 | 390 | 116 | 82 | 198 | 70 | 54 | 124 | |
| 124 | 77 | 201 | 451 | 243 | 694 | 1 to 14 | 1 to 8 | 138 | 169 | 307 | 156 | 52 | 208 | 23 | 36 | 59 | |
| 229 | 260 | 489 | 1038 | 1339 | 2377 | 1 to 25 | 1 to 37 | NA | NA | NA | 447 | 665 | 1112 | 120 | 173 | 293 | |
| 120 | 110 | 230 | 779 | 770 | 1549 | 1 to 53 | 1 to 30 | 291 | 382 | 673 | 147 | 159 | 306 | 64 | 65 | 129 | |
| CBZ = carbamazepine; LTG = lamotrigine; NA = information not available. 1. In Brodie 1995 A, Brodie 1995 B and Reunanen 1996 adverse events that are "definitely related", in Brodie 1999 and Nieto‐Barrera 2001 "a reasonable possibility" that adverse events are treatment‐related and in Werhahn 2015 adverse events are "related, probably related or possibility related". | |||||||||||||||||
| Most commonly occurring adverse events | ||||||||||||||||
| LTG | CBZ | LTG | CBZ | LTG | CBZ | LTG | CBZ | |||||||||
| Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | |
| Accidental injury/fracture | 2 | 2 | 1 | 1 | 3 | 3 | 2 | 2 | 19 | 12 | 4 | 3 | 7 | 7 | 1 | 1 |
| Aggression | 8 | 6 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 3 | 3 | 2 | 2 |
| Anorexia/weight loss | 2 | 2 | 0 | 0 | 6 | 4 | 0 | 0 | 2 | 2 | 1 | 1 | 6 | 5 | 0 | 0 |
| Anxiety/depression | 12 | 5 | 7 | 5 | 6 | 3 | 10 | 7 | 3 | 3 | 0 | 0 | 8 | 8 | 2 | 2 |
| Aphasia | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 1 | 1 | 0 | 0 |
| Ataxia | 2 | 2 | 6 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 3 | 3 |
| Chest infection/bronchitis | 11 | 6 | 12 | 8 | 3 | 3 | 1 | 1 | 16 | 12 | 4 | 4 | 18 | 15 | 8 | 8 |
| Cold/influenza | 17 | 15 | 4 | 4 | 8 | 8 | 10 | 9 | 7 | 7 | 1 | 1 | 25 | 19 | 11 | 11 |
| Concentration | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 4 | 4 | 1 | 1 |
| Confusion | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 |
| Cough/wheeze | 5 | 5 | 5 | 5 | 2 | 2 | 1 | 1 | 6 | 5 | 1 | 1 | 6 | 5 | 6 | 5 |
| Dental | 3 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 6 | 3 | 3 |
| Dizzy/faint | 16 | 9 | 16 | 11 | 12 | 9 | 22 | 14 | 26 | 18 | 16 | 14 | 43 | 34 | 16 | 15 |
| Drowsy/fatigued | 32 | 21 | 52 | 31 | 34 | 20 | 49 | 36 | 25 | 17 | 21 | 15 | 36 | 34 | 45 | 40 |
| Gastrointestinal disturbances | 14 | 7 | 10 | 8 | 6 | 6 | 7 | 5 | 29 | 22 | 14 | 11 | 36 | 28 | 17 | 17 |
| Hair loss | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Headache/migraine | 77 | 27 | 31 | 17 | 48 | 24 | 52 | 22 | 14 | 10 | 8 | 8 | 56 | 46 | 16 | 14 |
| Impotence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Increased/worsened seizures | 1 | 1 | 2 | 2 | 1 | 1 | 0 | 0 | 4 | 4 | 2 | 2 | 14 | 12 | 4 | 4 |
| Kidney/urinary problems | 3 | 2 | 2 | 2 | 4 | 4 | 1 | 1 | 12 | 10 | 4 | 4 | 4 | 4 | 1 | 1 |
| Memory problems | 7 | 5 | 2 | 2 | 5 | 3 | 3 | 2 | 4 | 4 | 0 | 0 | 2 | 2 | 1 | 1 |
| Menstrual problems | 3 | 3 | 16 | 12 | 0 | 0 | 4 | 4 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Mood/behavioural change | 9 | 5 | 6 | 5 | 1 | 1 | 6 | 6 | 5 | 4 | 0 | 0 | 7 | 7 | 4 | 4 |
| Nausea/vomiting | 17 | 13 | 15 | 11 | 26 | 18 | 21 | 9 | 21 | 17 | 8 | 6 | 26 | 23 | 13 | 11 |
| Pain | 19 | 13 | 9 | 6 | 23 | 13 | 7 | 5 | 20 | 17 | 7 | 7 | 13 | 8 | 4 | 2 |
| Pins and needles/tingling | 2 | 1 | 3 | 2 | 3 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Rash/skin problems | 25 | 21 | 20 | 13 | 32 | 15 | 32 | 23 | 31 | 19 | 30 | 14 | 49 | 46 | 32 | 30 |
| Sleep problems/dreams | 4 | 3 | 4 | 4 | 8 | 5 | 12 | 5 | 9 | 8 | 0 | 0 | 19 | 19 | 1 | 1 |
| Throat/tonsil infection | 11 | 7 | 7 | 6 | 6 | 5 | 3 | 3 | 1 | 1 | 0 | 0 | 15 | 14 | 7 | 7 |
| Tremor/twitch | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 2 |
| Visual disturbance/nystagmus | 8 | 4 | 6 | 5 | 2 | 2 | 9 | 6 | 1 | 1 | 4 | 3 | 7 | 7 | 3 | 2 |
| Weight gain | 3 | 3 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 3 | 3 |
| Table of most commonly occurring adverse events split into two for formatting reasons. Events = number of adverse events reported; Ppts = number of participants reporting the adverse event (a participant could report the same type of adverse event multiple times). LTG = lamotrigine; CBZ = carbamazepine Most common adverse events are defined as events reported 10 or more times in at least one of the seven trials (Brodie 1995 A; Brodie 1995 B; Brodie 1999; Nieto‐Barrera 2001; Reunanen 1996; SANAD A 2007; Werhahn 2015). Less commonly reported adverse events are not summarised in this table but details are available on request from the review authors. General terminology for the type of adverse events was defined by the review authors based on the individual participant data provided. | ||||||||||||||||
| Most commonly occurring adverse events | Total (across seven studies) | |||||||||||||||
| LTG | CBZ | LTG | CBZ | LTG | CBZ | LTG | CBZ | |||||||||
| Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | Events | Ppts | |
| Accidental injury/fracture | 2 | 2 | 0 | 0 | 29 | 19 | 10 | 10 | 16 | 15 | 14 | 7 | 78 | 60 | 32 | 24 |
| Aggression | 1 | 1 | 0 | 0 | 25 | 18 | 41 | 21 | 1 | 1 | 1 | 1 | 40 | 31 | 46 | 26 |
| Anorexia/weight loss | 3 | 2 | 0 | 0 | 12 | 11 | 16 | 13 | 1 | 1 | 0 | 0 | 32 | 27 | 17 | 14 |
| Anxiety/depression | 4 | 4 | 2 | 2 | 48 | 34 | 46 | 34 | 17 | 14 | 17 | 10 | 98 | 71 | 84 | 60 |
| Aphasia | 1 | 1 | 0 | 0 | 7 | 4 | 11 | 8 | 1 | 1 | 7 | 5 | 11 | 8 | 23 | 18 |
| Ataxia | 0 | 0 | 3 | 3 | 38 | 20 | 30 | 22 | 1 | 1 | 0 | 0 | 43 | 25 | 42 | 33 |
| Chest infection/bronchitis | 3 | 3 | 1 | 1 | 2 | 1 | 6 | 5 | 8 | 8 | 3 | 3 | 61 | 48 | 35 | 30 |
| Cold/influenza | 9 | 8 | 2 | 2 | 1 | 1 | 3 | 3 | 11 | 9 | 20 | 15 | 78 | 67 | 51 | 45 |
| Concentration | 3 | 3 | 4 | 3 | 8 | 7 | 11 | 11 | 5 | 5 | 3 | 3 | 20 | 19 | 21 | 20 |
| Confusion | 0 | 0 | 0 | 0 | 30 | 19 | 33 | 22 | 4 | 4 | 5 | 5 | 37 | 26 | 39 | 28 |
| Cough/wheeze | 3 | 3 | 0 | 0 | 4 | 4 | 1 | 1 | 14 | 11 | 13 | 11 | 40 | 35 | 27 | 24 |
| Dental | 6 | 5 | 0 | 0 | 7 | 7 | 16 | 11 | 3 | 2 | 2 | 2 | 27 | 25 | 25 | 20 |
| Dizzy/faint | 17 | 13 | 20 | 13 | 55 | 32 | 64 | 37 | 74 | 46 | 62 | 41 | 243 | 161 | 216 | 145 |
| Drowsy/fatigued | 56 | 40 | 77 | 47 | 125 | 72 | 267 | 123 | 30 | 24 | 51 | 46 | 338 | 228 | 562 | 338 |
| Gastrointestinal disturbances | 21 | 17 | 10 | 8 | 48 | 31 | 49 | 35 | 45 | 34 | 65 | 42 | 199 | 145 | 172 | 126 |
| Hair loss | 0 | 0 | 0 | 0 | 6 | 4 | 15 | 6 | 3 | 3 | 3 | 3 | 10 | 8 | 20 | 11 |
| Headache/migraine | 74 | 42 | 20 | 13 | 95 | 49 | 97 | 43 | 48 | 31 | 40 | 29 | 412 | 229 | 264 | 146 |
| Impotence | 1 | 1 | 0 | 0 | 5 | 4 | 17 | 5 | 0 | 0 | 0 | 0 | 6 | 5 | 17 | 5 |
| Increased/worsened seizures | 1 | 1 | 0 | 0 | 29 | 21 | 41 | 25 | 86 | 35 | 58 | 27 | 136 | 75 | 107 | 60 |
| Kidney/urinary problems | 4 | 3 | 2 | 2 | 4 | 3 | 10 | 8 | 16 | 16 | 18 | 17 | 47 | 42 | 38 | 35 |
| Memory problems | 4 | 4 | 3 | 3 | 38 | 23 | 71 | 34 | 7 | 6 | 7 | 7 | 67 | 47 | 87 | 49 |
| Menstrual problems | 15 | 9 | 13 | 7 | 4 | 4 | 3 | 2 | 0 | 0 | 0 | 0 | 22 | 16 | 37 | 26 |
| Mood/behavioural change | 5 | 5 | 4 | 1 | 32 | 22 | 56 | 34 | 2 | 2 | 6 | 5 | 61 | 46 | 82 | 55 |
| Nausea/vomiting | 21 | 15 | 15 | 11 | 38 | 23 | 54 | 35 | 30 | 23 | 37 | 24 | 179 | 132 | 163 | 107 |
| Pain | 18 | 15 | 1 | 1 | 14 | 9 | 15 | 12 | 55 | 28 | 28 | 20 | 162 | 103 | 71 | 53 |
| Pins and needles/tingling | 3 | 2 | 0 | 0 | 13 | 13 | 23 | 13 | 4 | 4 | 3 | 3 | 27 | 25 | 29 | 18 |
| Rash/skin problems | 33 | 26 | 17 | 14 | 65 | 36 | 99 | 65 | 23 | 20 | 39 | 32 | 258 | 183 | 269 | 191 |
| Sleep problems/dreams | 27 | 19 | 3 | 2 | 46 | 32 | 24 | 12 | 19 | 18 | 10 | 9 | 132 | 104 | 54 | 33 |
| Throat/tonsil infection | 13 | 10 | 1 | 1 | 2 | 2 | 1 | 1 | 6 | 4 | 4 | 3 | 54 | 43 | 23 | 21 |
| Tremor/twitch | 7 | 6 | 0 | 0 | 28 | 12 | 13 | 10 | 16 | 8 | 10 | 9 | 53 | 28 | 27 | 22 |
| Visual disturbance/nystagmus | 6 | 4 | 7 | 5 | 34 | 22 | 33 | 22 | 13 | 10 | 8 | 4 | 71 | 50 | 70 | 47 |
| Weight gain | 1 | 1 | 0 | 0 | 21 | 13 | 42 | 21 | 4 | 4 | 3 | 3 | 34 | 25 | 49 | 28 |
| Table of most commonly occurring adverse events split into two for formatting reasons. Events = number of adverse events reported; Ppts = number of participants reporting the adverse event (a participant could report the same type of adverse event multiple times). LTG = lamotrigine; CBZ = carbamazepine Most common adverse events are defined as events reported 10 or more times in at least one of the seven trials (Brodie 1995 A; Brodie 1995 B; Brodie 1999; Nieto‐Barrera 2001; Reunanen 1996; SANAD A 2007; Werhahn 2015). Less commonly reported adverse events are not summarised in this table but details are available on request from the review authors. General terminology for the type of adverse events was defined by the review authors based on the individual participant data provided. | ||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to treatment failure (any reason related to the treatment) Show forest plot | 9 | 2569 | Hazard Ratio (Fixed, 95% CI) | 0.73 [0.64, 0.82] |
| 2 Time to treatment failure due to adverse events Show forest plot | 9 | 2569 | Hazard Ratio (Fixed, 95% CI) | 0.54 [0.45, 0.65] |
| 3 Time to treatment failure due to lack of efficacy Show forest plot | 5 | 1874 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.75, 1.41] |
| 4 Time to treatment failure (any reason related to the treatment) ‐ by seizure type Show forest plot | 9 | 2481 | Hazard Ratio (Fixed, 95% CI) | 0.71 [0.62, 0.82] |
| 4.1 Focal | 9 | 2182 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.64, 0.86] |
| 4.2 Generalised | 6 | 299 | Hazard Ratio (Fixed, 95% CI) | 0.51 [0.33, 0.78] |
| 5 Time to treatment failure due to adverse events ‐ by seizure type Show forest plot | 9 | 2466 | Hazard Ratio (Fixed, 95% CI) | 0.55 [0.45, 0.66] |
| 5.1 Focal | 9 | 2182 | Hazard Ratio (Fixed, 95% CI) | 0.56 [0.45, 0.68] |
| 5.2 Generalised | 5 | 284 | Hazard Ratio (Fixed, 95% CI) | 0.49 [0.27, 0.88] |
| 6 Time to treatment failure (any reason related to the treatment, with aggregate data) Show forest plot | 13 | 3391 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.63, 0.78] |
| 7 Time to treatment failure (any reason related to the treatment) ‐ subgroup analysis (blinding) Show forest plot | 13 | 3391 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.63, 0.78] |
| 7.1 Double‐blind | 6 | 1231 | Hazard Ratio (Fixed, 95% CI) | 0.65 [0.56, 0.75] |
| 7.2 Open‐label | 7 | 2160 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.65, 0.90] |
| 8 Time to first seizure Show forest plot | 9 | 2564 | Hazard Ratio (Fixed, 95% CI) | 1.22 [1.09, 1.37] |
| 9 Time to first seizure by seizure type Show forest plot | 9 | 2476 | Hazard Ratio (Fixed, 95% CI) | 1.26 [1.12, 1.41] |
| 9.1 Focal | 9 | 2177 | Hazard Ratio (Fixed, 95% CI) | 1.29 [1.14, 1.45] |
| 9.2 Generalised | 6 | 299 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.65, 1.48] |
| 10 Time to first seizure (with aggregate data) Show forest plot | 12 | 3216 | Hazard Ratio (Fixed, 95% CI) | 1.24 [1.12, 1.37] |
| 11 Seizure freedom (whole study) Show forest plot | 14 | 3760 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.04, 1.18] |
| 12 Time to 6‐month remission Show forest plot | 7 | 1793 | Hazard Ratio (Fixed, 95% CI) | 0.84 [0.74, 0.94] |
| 13 Time to 6‐month remission by seizure type Show forest plot | 7 | 1708 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.76, 0.97] |
| 13.1 Focal | 7 | 1454 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.77, 1.00] |
| 13.2 Generalised | 5 | 254 | Hazard Ratio (Fixed, 95% CI) | 0.78 [0.55, 1.11] |
| 14 Seizure freedom at 6 months Show forest plot | 14 | 3760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.03] |
| 15 Time to 12‐month remission Show forest plot | 2 | 988 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.77, 1.07] |
| 16 Time to 12‐month remission by seizure type Show forest plot | 2 | 988 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.76, 1.07] |
| 16.1 Focal | 2 | 894 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.77, 1.09] |
| 16.2 Uncertain | 1 | 94 | Hazard Ratio (Fixed, 95% CI) | 0.81 [0.47, 1.37] |
| 17 Time to 24‐month remission Show forest plot | 1 | 755 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.80, 1.25] |
| 18 Time to 24‐month remission by seizure type Show forest plot | 1 | 755 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.82, 1.30] |
| 18.1 Focal | 1 | 661 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.83, 1.35] |
| 18.2 Uncertain | 1 | 94 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.44, 1.67] |

