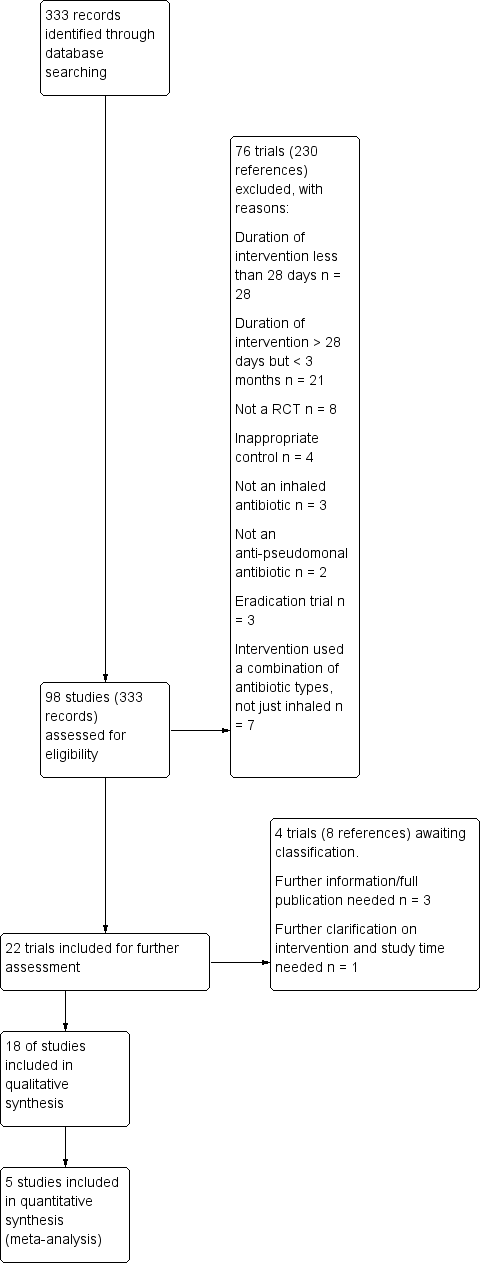
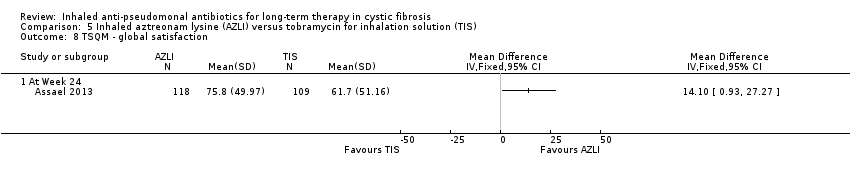
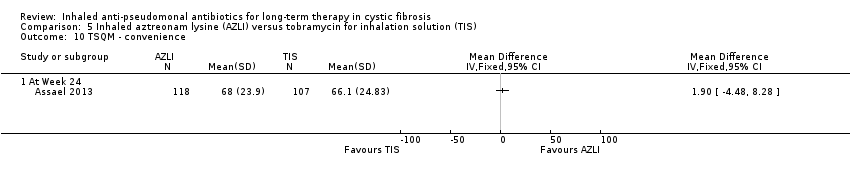
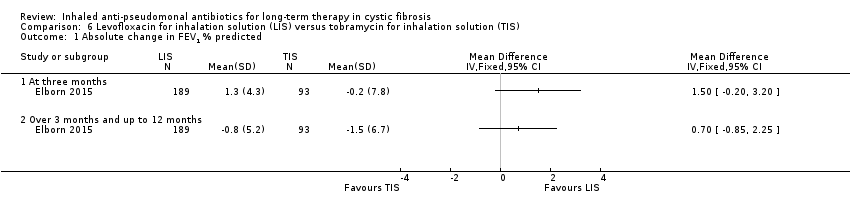
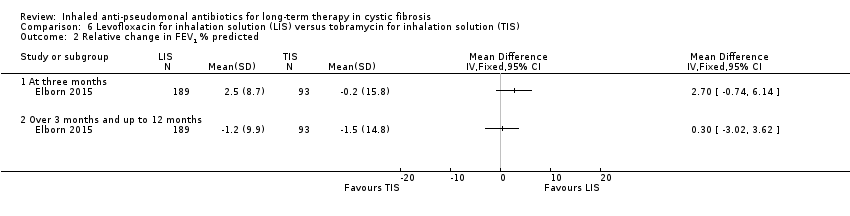
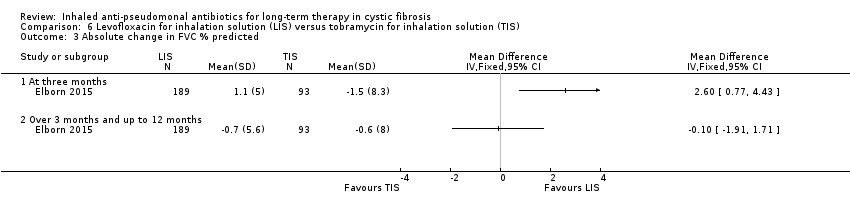
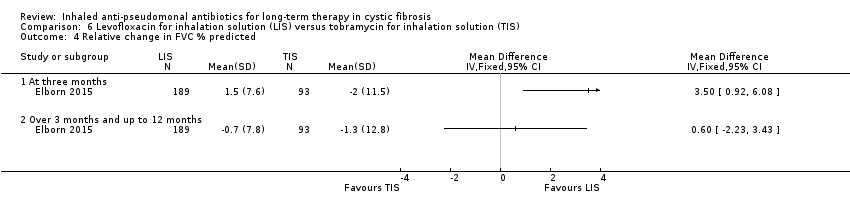
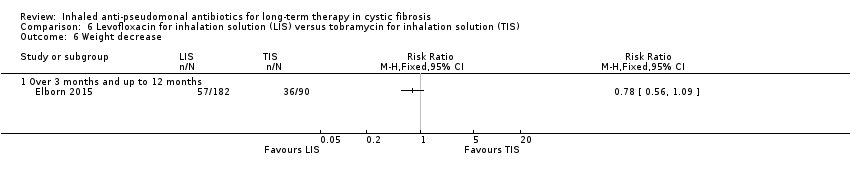
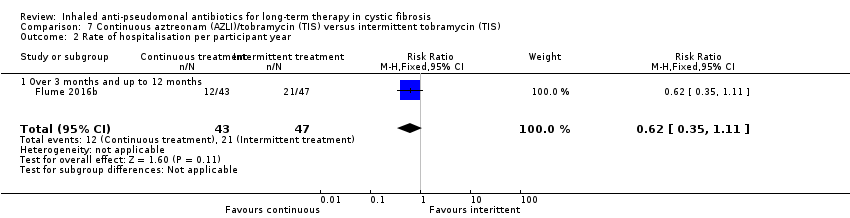
| Trial | Trial characteristics | Participants | Interventions | Summary of results |
| Dorkin 2015 | Duration: 28 days. Design: double‐blind, placebo‐controlled parallel RCT. Location: multicentre ‐ 73 sites in 9 countries (USA, Australia and Europe). Clinical trials identifier: NCT00645788 | Number: estimated enrolment 245, 288 randomised but only 286 received 1 of the 4 treatments. Age: 12 years and older (split children 12 ‐ 17 years and adults 18 years and over). Gender: males or females. Disease status: chronic colonisation with P aeruginosa, clinically stable. | Intervention 1: 32.5 mg ciprofloxacin betaine corresponding to 50 mg ciprofloxacin Pulmonsphere inhalation powder 2x daily. Intervention 2: placebo (50 mg matching placebo powder formulation) 2x daily. Intervention 3: 48.75 mg ciprofloxacin betaine corresponding to 75 mg ciprofloxacin Pulmonsphere inhalation powder 2x daily. Intervention 4: placebo (75 mg matching placebo powder formulation) 2x daily. Interventions 3 and 4 were introduced after amendment 2. | No significant difference in change in FEV1 between ciprofloxacin dry powder inhalation at either dose (P = 0.154). In pooled analyses, FEV1 decline from baseline to treatment end was significantly lower with ciprofloxacin
DPI than with placebo (pooled data; P = 0.02). There were positive effects on sputum bacterial load and quality of life which weren't maintained in the 4‐week follow‐up. There were no significant
differences in type/incidence of treatment‐emergent
adverse events by treatment group (P = 0.115). |
| Dupont 2008 | Duration: 28 days. Design: placebo‐controlled phase IIa parallel RCT (stratified by baseline FEV1 (% predicted) and randomised 2:1 to Arikace™ or placebo). Location: multicentre ‐ 13 centres in Europe. | Number: 66 participants enrolled. Age: 23 adults, 25 adolescents (13 ‐ 18 years) and 18 children (6 ‐ 12 years). Gender: no details. Disease status: chronic P aeruginosa infection; baseline FEV1 (% predicted) 40 ‐ 75% in 43 participants; >75% in 23 participants. | Cohort 1: (n = 32) 280 mg Arikace™ or placebo (hypertonic saline solution (1.5% NaCl)) once daily.
Cohort 2: (n = 34) 560 mg Arikace™ or placebo (hypertonic saline solution (1.5% NaCl)) once daily. Inhaled with PARI eFlow® nebuliser. | Relative change in FEV1 was higher in the 560 mg group at day 28 (P = 0.033) compared to placebo. The adverse event profile was similar among Arikace™ and placebo groups. |
| Galeva 2011 | Duration: 28 days. Design: placebo‐controlled parallel RCT. Location: multicentre ‐ 17 centres in 8 countries. | Number: 62 randomised (target was 100). Age: 6 to 21 years. Gender: no details. Disease status: diagnosed with CF by at least 1 clinical feature plus sweat test, FEV1 of 25 ‐ 80% predicted. | Intervention 1: TIP (n = 32) 112 mg 2x daily. Intervention 2: placebo (n = 30) 2x daily. | Mean treatment difference in absolute change in FEV1 between TIP ‐ placebo was 4.4 % (P < 0.05). Mean treatment difference in relative change in FEV1 between TIP ‐ placebo was 5.9 % (P < 0.0.184). TIP significantly reduced sputum density. |
| Geller 2011b | Duration: 28 days. Design: double‐blind, placebo‐controlled parallel RCT (3 arms). Location: multicentre ‐ 51 centres across USA and Europe. | Number: 151 randomised. Age: mean age 29 years. Gender: 85 males, 66 females. Disease status: diagnosed CF, chronic P aeruginosa airways infection, FEV1 between 25 ‐ 85% predicted, and 3 courses of inhaled antibiotics over the past 12 months. | Intervention 1: (n = 38) MP‐376 120 mg daily. Intervention 2: (n = 37) MP‐376 240 mg daily. Intervention 3: (n = 39) MP‐376 240 mg 2x daily. Intervention 4: (n = 37) placebo. Delivered by a customized investigational PARI eFlow nebulizer. | All doses of MP‐376 resulted in reduced sputum density at day 28 (240 mg twice a day showed a 0.96 log difference compared with placebo P = 0.001) There was a dose‐dependent increase in FEV1 for MP‐376. There was a difference of 8.7 % in FEV1 between MP‐376 240 mg twice a day and placebo (P = 0.003). There was a significant reduction in the need for other anti‐pseudomonal antibiotics compared to placebo. |
| Gibson 2003 | Duration: 28 days. Design: double‐blind, placebo‐controlled parallel RCT. Early termination due to poor recruitment. | Number: 21 randomised (planned 98). Age: 6 months ‐ 6 years. Gender: 11 males, 10 females. Disease status: positive P aeruginosa culture. | Intervention 1: (n = 8) tobramycin 300 mg 2x daily. Intervention 2: (n = 13) placebo 2x daily. | There was a significant difference between treatment groups and placebo in the reduction in P aeruginosa density (no P aeruginosa was detected at day 28 in 8 out of 8 active group patients compared to 1 out of 13 placebo patients). There were no significant differences between treatment groups for clinical indices or adverse events. |
| Goss 2009 | Duration: 28 days (with 28‐day follow‐up). Design: placebo‐controlled parallel phase 2 RCT (stratified by baseline FEV1 (% predicted) and randomised 2:1 to Arikace™ or placebo). Location: multicentre ‐ 18 centres across USA. | Number: 46 randomised. Age: mean (SD) Arikace™ 70 mg: 33.1 (9.7) years. Arikace™ 140 mg: 35.4 (6.0) years. Placebo 70 mg and 140 mg: 24.4 (6.3) years Arikace™ 560 mg: 31.5 (14.5) years. Placebo 560 mg: 26.3 (6.7) years. Gender: 27 males, 19 females. Disease status: Cohorts 1 and 2: baseline FEV1 % predicted 40 ‐ 75% n = 16 and > 75% n = 5. Cohort 3: baseline FEV1 % predicted 40 ‐ 75% n = 19 and > 75% n = 6. More details on lung function and BMI in supplementary papers | Arikace™ or placebo (hypertonic saline (1.5% NaCl). Cohort 1: (n = 14) 70 mg Arikace™ or placebo 1x daily. Cohort 2: (n = 12) 40 mg Arikace™ or placebo 1x daily. Cohort 3: (n = 22) 560 mg Arikace™ or placebo 1x daily. Inhaled using eFlow nebulizer system (PARI Pharma GmbH). Follow‐up for 28 days after trial finish. Review of interim data in combination with data from similar European trial led to addition of Cohort 3 for a further 28 days with follow‐up of 56 days after trial finish. | Arikace™ was well tolerated at doses of 70 mg, 140 mg and 560 mg. |
| Hodson 2002 | Duration: 28 days. Design: open‐label parallel RCT (stratified by age and centre).
Location: multcentre. | Number: 126 randomised, 11 withdrew before treatment, 115 treated. Age: range 7 ‐ 50 years. Gender: males 45% of total. Disease status: criteria for diagnosis abnormal sweat electrolytes, gene mutation. | Intervention 1: tobramycin 300 mg in 5 mL 2x daily, delivered by Pari LC plus nebuliser with CR50 compressor. Intervention 2: colistin 1MU in 3 mL in saline 2x daily, delivered by Ventstream nebuliser with CR50 compressor. | Tobramycin significantly improved lung function (mean improvement in FEV1 % predicted from baseline to week 4 was 6.7 % P = 0.006). The mean change in FEV1 % predicted was not significant in the colistin group (0.37 %). Both antibiotic regimes produced a significant decrease in sputum density, there was no development of highly resistant strains and the safety profile for both antibiotics was good. |
| Konstan 2010a | Duration: total of 24 weeks, 3 cycles each of 28 days on treatment followed by 28 days off treatment (only cycle 1 was double‐blind and randomised, cycles 2 and 3 were open‐label extension phases in which all participants received the same treatment). Design: double‐blind, placebo‐controlled parallel RCT. Location; multicentre ‐ 38 centres in Europe, Latin America and USA. Clinical trials identifier: NCT00125346. Known as the EVOLVE Trial. Trial terminated after showing a statistically significant benefit of TIP. | Number: 102 randomised, 95 received intended treatment, unclear in which group 7 withdrawals were from. Age: mean (SD): TIP 13.4 (4.42) years; placebo 13.2 (3.91) years. Gender: 42 males, 53 females. Disease status: baseline lung function (FEV1 % predicted) (mean (SD)): TIP 54.7 (18.89)%; placebo 58.5 (20.03)%. | Intervention 1: (n = 46) TIP 112 mg 2x daily. Intervention 2: (n = 49) placebo 2x daily. Cycle 1 (28 days on and 28 days off treatment or placebo). Cycles 2 and 3: open‐label cycles of TIP for all participants. | TIP significantly improved FEV1 % predicted from baseline to day 28 (difference 13.3, 95% CI 5.31 to 21.28 P = 0.0016). TIP reduced sputum P aeruginosa density, respiratory related hospitalisation and anti‐pseudomonal antibiotic use. The most common adverse event was cough but the frequency was higher in the placebo group (26.5 %) versus TIP (13.0%). No evidence of ototoxicity or nephrotoxicity. |
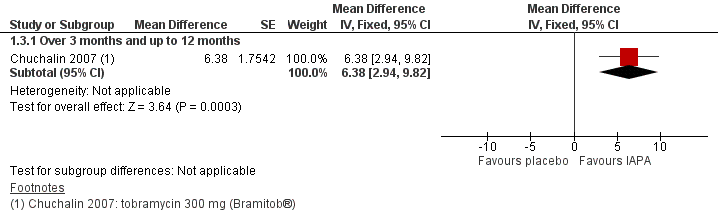
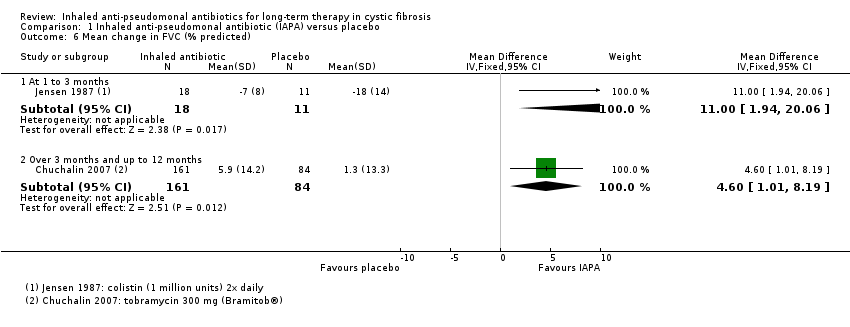
| Lenoir 2007 | Duration: 4 weeks followed by a 4‐week run‐out phase. Design: double‐blind, placebo‐controlled parallel RCT. Location: multicentre ‐ 13 sites in 4 countries. | Number: 59 participants. Age: range 6 ‐ 30 years. Gender: 32 males, 27 females. Disease status: participants diagnosed with CF and P aeruginosa. | Intervention 1: tobramycin 300 mg (Bramitob®) 2x daily. Intervention 2: placebo 2x daily. Active drug and placebo both delivered by Pari LC Plus nebuliser and Pari TurboBoy compressor. | There was a significant increase in FEV1 from baseline in the tobramycin group but not in the placebo group (absolute difference 13.3% P = 0.003). Similar improvements were also seen for FVC in the tobramycin group. Adverse events were lower in the in the tobramycin group. Microbiological outcomes were significantly improved. |
| Mainz 2014 | Duration: 28 days. Design: double‐blind placebo‐controlled parallel RCT. Location: multicentre ‐ 2 centres in Germany (Jena and Tuebingen). | Number: 9 participants. Age: mean (SD): 22.4 (7.6) years; range 10.6 to 38.7 years. Gender: 6 males, 3 females. Disease status: diagnosed with CF by 2 positive sweat tests or genetic analysis (or both) and with chronic P aeruginosa colonisation. | Intervention 1: 80 mg tobramycin daily. Intervention 2: placebo (isotonic saline). Sinonasal inhalation using PARI Sinus™ compressor with a PARI LC SPRINT STAR™ nebuliser. Drug administered to each nostril for 4 minutes with the other nostril occluded, maximum volume of 1 mL per nostril. | P aeruginosa quantity decreased in 4 out of 6 (67%) participants receiving tobramycin and in none of the placebo group. Sinonasal inhalation was well tolerated. |
| Mazurek 2014 | Duration: single cycle of 28 days on and 28 days off (8 weeks total duration). Design: parallel RCT (non‐inferiority trial). Location: multicentre ‐ 38 centres in Europe. Clinical trials identifier: NCT00885365. Follow‐on 48 week extension of TNS4 only: ClinicalTrials ID: NCT01111383. | Number: 406 individuals screened, 324 participants randomised. Age: mean (SD): TNS4 15.89 (6.25) years; TNS5 15.58 (7.31) years. Gender: no details. Disease status: diagnosed with CF. Chronic P aeruginosa infection and FEV1 ≥ 40% and ≤ 80% predicted. | Intervention 1: (n = 156) TNS4 (Bramitob®) 300 mg/4 mL 2x daily. Intervention 2: (n = 168) TNS5 (TOBI®) 300 mg/5 mL 2x daily. Both interventions delivered via PARI Boy N® compressor and the PARI LC Plus® nebuliser. Other standard therapies allowed. | TNS4 showed similar short‐term clinical benefits to TNS5. Adverse event reporting was similar between the 2 treatment groups. |
| McCoy 2008 | Duration: 4 weeks. Design: double‐blind, placebo‐controlled parallel RCT. Location: multicentre ‐ 56 centres in USA. | Number: 246 participants randomised; 173 completed 28‐day treatment phase; and 90 completed open‐label follow‐up for 56 days. Age: 7 to 65 years. Gender: 121 males. Disease status: documented diagnosis of CF and P aeruginosa, 3 or more courses of tobramycin in previous year, FEV1 between 25 and 75% predicted. | Intervention 1: aztreonam 75 mg for 4 weeks, 2x or 3xdaily. Intervention 2: placebo (5 mg lactose in 1mL 0.17% NaCl) for 4 weeks, 2x or 3x daily. | AZLI treatment increased the median time to need for additional anti‐pseudomonal antibiotics by 21 days compared to placebo (AZLI 92 days; placebo 71 days P = 0.007). AZLI improved mean CFQ‐R respiratory scores (P = 0.02) and sputum density (P = 0.006. Adverse events were reported in both groups but were consistent with CF lung disease. |
| Nasr 2004 | Duration: 28 days. Design: double‐blind, placebo‐controlled parallel RCT. Location: single centre in USA. | Number: 32 people with CF (31 completed). Age: mean (SD) and range ‐ TSI group 11.81 (7.46) years, 6.0 to 34.7 years; placebo group 15.86 (7.25) years, 7.4 to 28.8 years. Gender: 12 males, 20 females ‐ TSI group 6 males and 10 females, placebo group 6 males and 10 females. Disease status: CF diagnosis by sweat test or genotype testing. Colonised with P aeruginosa. Lung function FEV1 % predicted mean (SD) and range: TSI group 95.73 (17.21)%, 55.0% to 134.1%; placebo group 83.71 (21.07)%, 45.0% to 108.73%. | Intervention 1: (n = 16) TSI 5 mL (solution of 300 mg tobramycin and 11.25 mg sodium chloride in sterile water) 2x daily. Intervention 2: (n = 16) placebo (solution of 1.25 quinine sulphate in normal saline) 2x daily. Interventions both administered using PARI LC Plus™ jet nebuliser and PulmoAide compressor. | % predicted FEV1 increased slightly for both groups by mean (SD) 1.29 (3.33) for TSI and 1.17 (1.4) for placebo. |
| Ramsey 1993 | Duration: 3x 28‐day periods (only results of first 28‐day parallel group comparison suitable for analysis). Design: double‐blind placebo‐controlled 3‐period cross‐over RCT. | Number: 71 participants. Age: mean (SD): 17.7 (1.25) years and 16.6 (1.24) years in 2 groups. Gender: 37 males, 34 females. Disease status: CF diagnosed by sweat test. Sputum culture of P aeruginosa susceptible to tobramycin. Mean baseline FEV1 55% (SE 3.7) and 60% (SE 3.2) predicted in 2 treatment arms. | Intervention 1: tobramycin 600 mg 3x daily for 28 days, then cross‐over for 2 further 28‐day periods. Intervention 2: placebo (0.5 normal saline) 3x daily for 28 days, then cross‐over for 2 further 28‐day periods. Delivered by Ultrasonic (Ultraneb 100/99) nebuliser with 30 mL solution and 200 inhalations. | In the first 28‐day period there was an increase in % predicted FEV1 compared to placebo (P < 0.001) and FVC (P = 0.014). There was a decrease in the density of P aeruginosa in sputum (P < 0.001). |
| Retsch‐Bogart 2007 | Duration: 28 days. Design: double‐blind, placebo‐controlled parallel Phase III RCT. Location: multicentre: 53 centres in USA, Canada, Australia and New Zealand. Clinical trials identifier: NCT00112359. Known as AIR‐CF1 Trial. | Number: 164 participants. Age: mean (range)): AZLI 27.4 (7 – 54) years; placebo 31.7 (11 – 74) years. Gender: 93 males, 71 females. Disease status: stable condition. P aeruginosa in sputum or throat swab. No use of anti‐pseudomonal antibiotics in previous 14 days. Baseline lung function (FEV1 % predicted) (mean (SD)): AZLI 54.4 (13.4)%; placebo 54.8 (14.0)%. | Intervention 1: AZLI 75 mg 3x daily. Intervention 2: placebo 3x daily. Doses administered at least 4 hours apart using PARI eFlow™ Electronic Nebuliser after pre‐treatment with bronchodilator. Concommitant standard CF therapies allowed except anti‐pseudomonal antibiotics, azithromycin or hypertonic saline. | AZLI improved FEV1 % predicted (P < 0.001), CFQ‐R respiratory score (P < 0.001) and sputum P aeruginosa density (P < 0.001) compared to placebo. Adverse events were comparable between groups with the exception of productive cough. This outcome was reduced by half in AZLI‐treated participants. |
| Rietschel 2009 | Duration: 20 weeks in total (8 weeks intervention 1, followed by 4 week washout, followed by 8 weeks intervention 2). Design: cross‐over. Location: multicentre in Germany. | Number: 35 stated as randomised in first abstract, but 29 randomised and 24/29 as having completed in second abstract. Age: 6 years and over, mean (SD) age 19.8 (6.3) years, range 8 ‐ 35 years. Disease status: chronically infected with P aeruginosa. | Intervention 1: continuous TIS 300 mg/d 1x daily. Intervention 2: continuous TIS 300 mg/d 2x daily. | Mean FEV1 was not markedly different between treatment periods or from baseline. No audiological or nephrotoxic side effects were noted. Once or twice daily dose was shown to be safe and tolerable. |
| Sands 2014 | Duration: 3 months in total, but only 4 weeks taking each intervention (4 weeks intervention 1, 4 weeks washout period, 4 weeks intervention 2). Design: Cross‐over. Location: multicentre in Poland. | Number: 58 randomised, 54 in ITT population Age: 4 years and older. Mean (SD) age 15.4 (6.81) years, range 7 to 36 years. Gender: 25 males, 33 females. Disease status: mean (SD) FEV1 % predicted: VANTOBRA group 63.8 (17.1)%, range 30.0% to 82.8%; TIS group 64.2 (17.7)%, range 28.0% to 83.9%. | Intervention 1 (n = 28): T100 also known as VANTOBRA (170 mg tobramycin in 1.7 mL solution) via drug‐specific eFlow nebuliser Tolero with an eBase controller 2x daily. Intervention 2 (n = 30): TOBI (300 mg tobramycin in 5 mL solution) via PARI LC Plus nebuliser with PARI BOY SX compressor 2x daily. | Treatment with both products were comparable in terms of clinical efficacy (reduction of P aeruginosa density and improvement in lung function. Safety profiles were also comparable. |
| Trapnell 2012 | Duration: 28 days. Design: placebo‐controlled parallel RCT. Location: multicentre ‐ 33 sites in the USA. | Number: 119 participants randomised. Age: mean (SD)): FTI 80/20mg 35 (10.9) years; FTI 160/40mg 31 (10.2) years; placebo 31 (8.8) years. Gender: 68 males, 51 females. Disease status: lung function (FEV1 % predicted) (mean (SD)): FTI 80/20mg 50 (13.4)%; FTI 160/40mg 21 (51)%; placebo 48 (13.6)%. | Intervention 1: (n = 38) FTI 80/20 mg 2x daily. Intervention 2: (n = 41) FTI 160/40 mg 2x daily. Intervention 3: (n = 40) placebo 2x daily. | Improvements in mean FEV1 % predicted achieved in the AZLI run‐in period were maintained in the FTI group compared with placebo (P = 0.002). The treatment effect on P aeruginosa sputum density significantly favoured FTI compared to placebo. Respiratory symptoms were less common in the FTI group. |
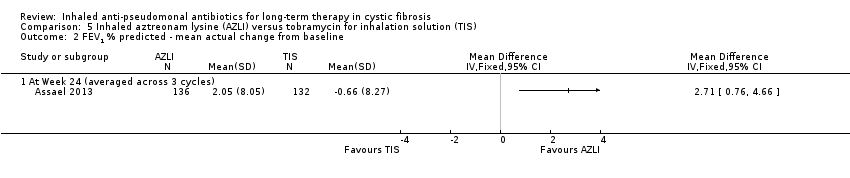
| Wainwright 2011 | Duration: 28 days. Design: placebo‐controlled parallel RCT. Location: multicentre ‐ 40 centres in USA, Canada and Australia. | Number: 160 people randomised, 157 received treatment. Age: mean (SD): AZLI 19.5 (9.1) years; placebo 18.9 (9.1) years. Gender: 90 males, 70 females. Disease status: FEV1 % predicted: AZLI 95.5 (12.7)%; placebo 94.7 (12.9)%. | Intervention 1: (n = 76; 75 analysed, 1 discontinued trial) AZLI (75 mg aztreonam, 52.5 mg lysine monohydrate diluted in 0.17% saline (1 mL)) 3x daily. Intervention 2: (n = 81) placebo (5 mg lactose, 7.3 mg NaCl diluted in 0.17% saline (1 mL)) 3x daily. Both interventions self‐administered with the investigational eFlow® electronic nebulizer (PARI GmbH, Starnberg, Germany). | Treatment effect at 28 days for relative FEV1 % predicted was 2.7 % (P = 0.021 favouring AZLI). Treatment effect for CFQ‐R respiratory symptom score at day 28 was modest at 1.8 points (95% CI ‐2.8 to 6.4 P = 0.443). Sputum density was improved in the AZLI group (P = 0.016). |