Contraceptive orale pentru durerea asociată endometriozei
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register search strategy
Searched 19 October 2017
Procite platform
Keywords CONTAINS "endometriosis" or "Endometriosis‐Symptoms" or "pelvic pain" or "dyspareunia" or "dyschezia" or Title CONTAINS" endometriosis" or "Endometriosis‐Symptoms" or "pelvic pain" or "dyspareunia" or "dyschezia"
AND
Keywords CONTAINS "Oral Contraception" or "oral contraceptive" or "oral contraceptive pill" or "Oral Contraceptive Agent" or "oral contraceptives" or "oral estradiol" or "oral estrogen" or "OCP" or "oestrogen plus progestagen" or "ethinyl estradiol + drospirenone" or "ethinyl estradiol‐cyproterone acetate" or "ethinyl‐estradiol" or "desogestral" or "desogestrel" or "Levonorgestrel" or "Norgestimate" or "Norgestrel" or "Norethisterone" or "noresthisterone" or "combined oral contraceptives" or Title CONTAINS "Oral Contraception" or "oral contraceptive" or "oral contraceptive pill" or "Oral Contraceptive Agent" or "oral contraceptives" or "oral estradiol" or "oral estrogen" or "OCP" or "oestrogen plus progestagen" or "ethinyl estradiol + drospirenone" or "ethinyl estradiol‐cyproterone acetate" or "ethinyl‐estradiol" or "desogestral" or "desogestrel" or "Levonorgestrel" or "Norgestimate" or "Norgestrel" or "Norethisterone" or "noresthisterone" or "combined oral contraceptives" (91 hits)
Appendix 2. CENTRAL CRSO search strategy
Searched 19 October 2017
Web platform
#1MESH DESCRIPTOR Endometriosis EXPLODE ALL TREES (536)
#2Endometrio*:TI,AB,KY (1507)
#3Dyspareunia:TI,AB,KY (616)
#4Dyschezia:TI,AB,KY (25)
#5(pelvic pain):TI,AB,KY 960
#6#1 OR #2 OR #3 OR #4 OR #5 (2656)
#7MESH DESCRIPTOR Contraceptives, Oral EXPLODE ALL TREES (3262)
#8MESH DESCRIPTOR Contraceptives, Oral, Combined EXPLODE ALL TREES (698)
#9MESH DESCRIPTOR Ethinyl Estradiol‐Norgestrel Combination EXPLODE ALL TREES (67)
#10MESH DESCRIPTOR Contraceptives, Oral, Hormonal EXPLODE ALL TREES (242)
#11MESH DESCRIPTOR Contraceptives, Oral, Sequential EXPLODE ALL TREES (32)
#12MESH DESCRIPTOR Contraceptives, Oral, Synthetic EXPLODE ALL TREES (2643)
#13MESH DESCRIPTOR Desogestrel EXPLODE ALL TREES (349)
#14MESH DESCRIPTOR Levonorgestrel EXPLODE ALL TREES (628)
#15MESH DESCRIPTOR Norgestrel EXPLODE ALL TREES (848)
#16(oral contracept*):TI,AB,KY or (contraceptive pill*):TI,AB,KY (2416)
#17OCP*:TI,AB,KY (160)
#18(ethinyl estradiol):TI,AB,KY (1390)
#19desogestrel:TI,AB,KY (534)
#20dienogest:TI,AB,KY (138)
#21levonorgestrel:TI,AB,KY (1218)
#22norgestrel:TI,AB,KY (434)
#23(estrogen* or oestrogen*):TI,AB,KY (10437)
#24(Progestin* or Progest?gen*):TI,AB,KY (2074)
#25MESH DESCRIPTOR Norethindrone EXPLODE ALL TREES (707)
#26Norethindrone:TI,AB,KY (807)
#27norethisterone:TI,AB,KY (731)
#28gestodene:TI,AB,KY (240)
#29MESH DESCRIPTOR Ethinyl Estradiol EXPLODE ALL TREES (1165)
#30MESH DESCRIPTOR Norgestrienone EXPLODE ALL TREES (33)
#31(ethinyl oestradiol or ethinylestradiol):TI,AB,KY (909)
#32#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 (14739)
#33 #6 AND #32 (527)
Appendix 3. MEDLINE search strategy
Searched from 1946 to 19 October 2017
Ovid platform
1 exp contraceptives, oral/ (47054)
2 exp contraceptives, oral, combined/ or exp ethinyl estradiol‐norgestrel combination/ or contraceptives, oral, hormonal/ or contraceptives, oral, sequential/ or exp contraceptives, oral, synthetic/ or exp desogestrel/ or exp levonorgestrel/ or exp norgestrel/ (31520)
3 (oral contracept$ or contraceptive pill*).tw. (27438)
4 OCP$.tw. (3803)
5 ethinyl estradiol.tw. (3985)
6 desogestrel.tw. (1152)
7 dienogest.tw. (397)
8 levonorgestrel.tw. (4445)
9 norgestrel.tw. (1143)
10 (estrogen$ or oestrogen$).tw. (154616)
11 (Progestin$ or Progest?gen$).tw. (18783)
12 exp Norethindrone/ (4456)
13 norethisterone.tw. (2066)
14 gestodene.tw. (801)
15 exp ethinyl estradiol/ or exp norgestrienone/ (11354)
16 (ethinyl oestradiol or ethinylestradiol).tw. (3066)
17 or/1‐16 (213655)
18 exp Endometriosis/ (21265)
19 Endometrio*.tw. (28025)
20 Dyspareunia.tw. (3573)
21 Dyschezia.tw. (250)
22 pelvic pain.tw. (8103)
23 or/18‐22 (40610)
24 17 and 23 (4578)
25 randomized controlled trial.pt. (497191)
26 controlled clinical trial.pt. (99259)
27 randomized.ab. (434049)
28 placebo.tw. (208224)
29 clinical trials as topic.sh. (195576)
30 randomly.ab. (299103)
31 trial.ti. (196021)
32 (crossover or cross‐over or cross over).tw. (80882)
33 or/25‐32 (1240442)
34 exp animals/ not humans.sh. (4679127)
35 33 not 34 (1143190)
36 24 and 35 (529)
Appendix 4. Embase search strategy
Searched from 1946 to 19 October 2017
Ovid platform
1 exp ENDOMETRIOSIS/ (32243)
2 Endometriosis.tw. (27961)
3 Dyspareunia.tw. (5918)
4 Dyschezia.tw. (466)
5 pelvic pain.tw. (12164)
6 or/1‐5 (47558)
7 exp oral contraceptive agent/ (57765)
8 exp ethinylestradiol plus norelgestromin/ or exp ethinylestradiol plus norethisterone/ or exp ethinylestradiol plus norethisterone acetate/ or exp ethinylestradiol plus norgestimate/ or exp ethinylestradiol plus norgestrel/ or exp estrogen/ or exp gestagen/ (312716)
9 exp dienogest/ (1013)
10 exp dienogest plus ethinylestradiol/ (124)
11 dienogest.tw. (674)
12 (oral contracept$ or contraceptive pill*).tw. (27835)
13 OCP$.tw. (4656)
14 ethinyl estradiol.tw. (3159)
15 desogestrel.tw. (1190)
16 levonorgestrel.tw. (5210)
17 norgestrel.tw. (732)
18 (estrogen$ or oestrogen$).tw. (170735)
19 (Progestin$ or Progest?gen$).tw. (19282)
20 exp norethisterone/ (6700)
21 norethisterone.tw. (1939)
22 exp ethinylestradiol/ (16412)
23 ethinylestradiol.tw. (3166)
24 or/7‐23 (411964)
25 6 and 24 (8706)
26 Clinical Trial/ (949969)
27 Randomized Controlled Trial/ (471914)
28 exp randomization/ (75860)
29 Single Blind Procedure/ (29732)
30 Double Blind Procedure/ (140776)
31 Crossover Procedure/ (53437)
32 Placebo/ (300796)
33 Randomi?ed controlled trial$.tw. (168408)
34 Rct.tw. (25850)
35 random allocation.tw. (1695)
36 randomly allocated.tw. (28434)
37 allocated randomly.tw. (2269)
38 (allocated adj2 random).tw. (785)
39 Single blind$.tw. (19880)
40 Double blind$.tw. (175965)
41 ((treble or triple) adj blind$).tw. (717)
42 placebo$.tw. (256628)
43 prospective study/ (405705)
44 or/26‐43 (1812459)
45 case study/ (50227)
46 case report.tw. (340144)
47 abstract report/ or letter/ (1013008)
48 or/45‐47 (1395186)
49 44 not 48 (1766305)
50 25 and 49 (1711)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 19 October 2017
Ovid platform
1 exp Oral Contraceptives/ (871)
2 oral contracept$.tw. (1460)
3 OCP$.tw. (333)
4 ethinyl estradiol.tw. (97)
5 desogestrel.tw. (14)
6 levonorgestrel.tw. (87)
7 norgestrel.tw. (12)
8 (estrogen$ or oestrogen$).tw. (7953)
9 (Progestin$ or Progest?gen$).tw. (789)
10 exp Estradiol/ (2934)
11 Estradiol.tw. (5624)
12 norethisterone.tw. (23)
13 gestodene.tw. (8)
14 ethinylestradiol.tw. (59)
15 or/1‐14 (12986)
16 exp Dyspareunia/ (247)
17 Endometriosis.tw. (222)
18 Dyspareunia.tw. (540)
19 Dyschezia.tw. (7)
20 pelvic pain.tw. (514)
21 or/16‐20 (1190)
22 15 and 21 (78)
Appendix 6. CINAHL search strategy
Searched from 1961 to 19 October 2017
Ebsco platform
| # | Query | Results |
| S36 | S23 AND S35 | 174 |
| S35 | S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 | 1,169,103 |
| S34 | TX allocat* random* | 7,289 |
| S33 | (MH "Quantitative Studies") | 16,561 |
| S32 | (MH "Placebos") | 10,403 |
| S31 | TX placebo* | 47,658 |
| S30 | TX random* allocat* | 7,289 |
| S29 | (MH "Random Assignment") | 44,312 |
| S28 | TX randomi* control* trial* | 132,934 |
| S27 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 912,856 |
| S26 | TX clinic* n1 trial* | 212,303 |
| S25 | PT Clinical trial | 80,036 |
| S24 | (MH "Clinical Trials+") | 222,972 |
| S23 | S16 AND S22 | 598 |
| S22 | S17 OR S18 OR S19 OR S20 OR S21 | 7,342 |
| S21 | TX pelvic pain | 3,154 |
| S20 | TX Dyschezia | 26 |
| S19 | TX Dyspareunia | 1,067 |
| S18 | TX Endometrio* | 3,828 |
| S17 | (MM "Endometriosis") | 1,879 |
| S16 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 | 24,946 |
| S15 | TX ethinylestradiol | 155 |
| S14 | TX gestodene | 39 |
| S13 | TX norethisterone | 128 |
| S12 | TX Norethindrone | 85 |
| S11 | TX Progestin* or TX Progest?gen* | 1,863 |
| S10 | TX estrogen* or oestrogen* | 16,303 |
| S9 | TX norgestrel | 21 |
| S8 | TX levonorgestrel | 1,485 |
| S7 | TX desogestrel or TX dienogest | 163 |
| S6 | TX ethinyl estradiol | 320 |
| S5 | TX OCP* | 356 |
| S4 | TX oral contracept* | 6,785 |
| S3 | (MM "Levonorgestrel") | 679 |
| S2 | (MM "Estrogens") | 3,098 |
| S1 | (MM "Contraceptives, Oral+") OR (MM "Contraceptives, Oral Combined") | 7,394 |
Appendix 7. Trial Registries keyword search
Searched 19 October 2017
Web platform
The keywords 'endometriosis AND oral contraceptive' were used to search:
-
ClinicalTrials.gov (a service of the US National Institutes of Health) (www.clinicaltrials.gov);
-
World Health Organization Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/Default.aspx).

Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
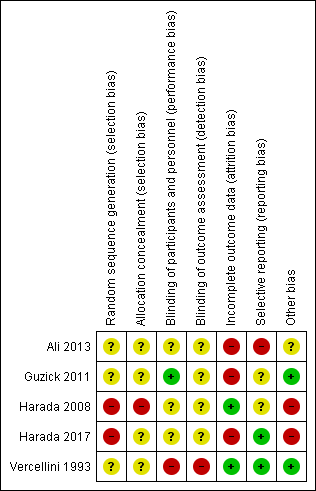
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Combined oral contractive pill (COCP) versus placebo/no treatment, outcome: 1.1 Self‐reported pain (dysmenorrhoea) at end of treatment.

Forest plot of comparison: 2 Combined oral contractive pill (COCP) versus other medical treatment, outcome: 2.1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data).

Forest plot of comparison: 2 Combined oral contractive pill (COCP) versus other medical treatment, outcome: 2.2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data).

Forest plot of comparison: 2 Combined oral contractive pill (COCP) versus other medical treatment, outcome: 2.3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data).
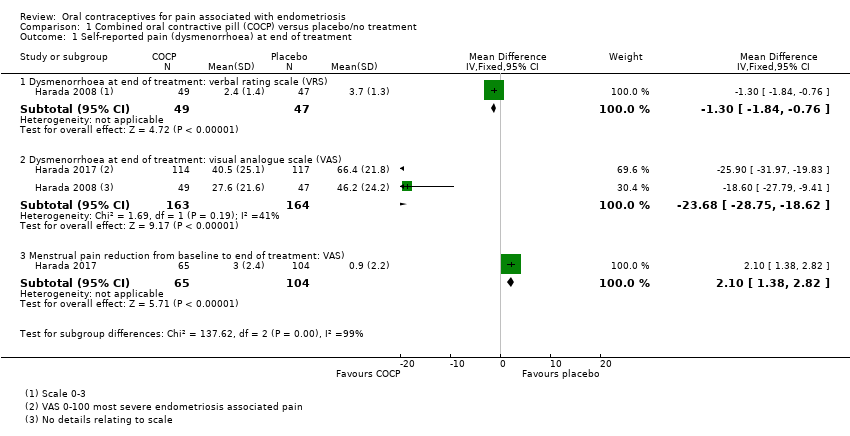
Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 1 Self‐reported pain (dysmenorrhoea) at end of treatment.

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 2 Cyclical pain (non‐menstrual).

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 3 Dyspareunia.

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 4 Dyschezia (pain on defecation).

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 5 Satisfaction (very highly/highly satisfied).

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 6 Withdrawal from treatment.

Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 7 Adverse effects occurring during treatment.

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data).
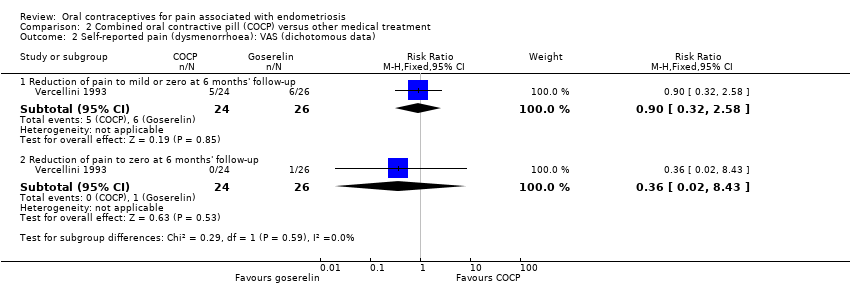
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data).
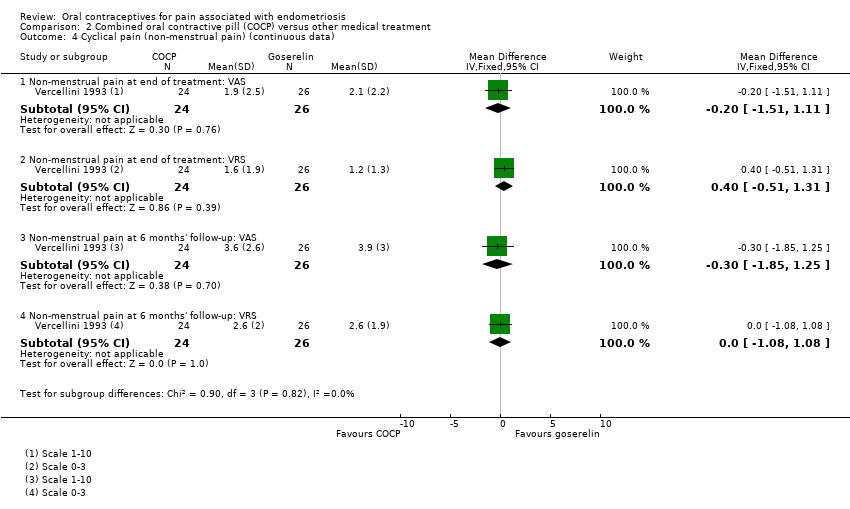
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 4 Cyclical pain (non‐menstrual pain) (continuous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 5 Cyclical pain (non‐menstrual pain) (dichotomous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 6 Dyspareunia (continuous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 7 Dyspareunia (dichotomous data).

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 8 Withdrawal from treatment.

Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 9 Adverse effects.
| Combined oral contraceptive pill (COCP) compared to placebo/no treatment for pain associated with endometriosis | ||||||
| Patient or population: women with endometriosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with COCP | |||||
| Self‐reported pain (dysmenorrhoea) at the end of treatment: dysmenorrhoea VRS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment: Dysmenorrhoea VRS was 3.7 | MD 1.3 lower | ‐ | 96 | ⊕⊝⊝⊝ | VRS ranged from 0 to 3. |
| Self‐reported pain (dysmenorrhoea) at the end of treatment: dysmenorrhoea VAS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment: Dysmenorrhoea VAS was 46.2 | MD 23.68 lower | ‐ | 327 | ⊕⊝⊝⊝ | No details provided for the VAS. |
| Self‐reported pain: menstrual pain reduction from baseline to end of treatment | The mean menstrual pain (reduction from baseline to end of treatment) was 3.00 | MD 2.10 lower (1.38 lower to 2.82 lower) | ‐ | 169 (1 RCT) | ⊕⊝⊝⊝ | Used a VAS from 0 to 10 where 10 was extreme pain. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COCP: combined oral contraceptive pill; MD: mean difference; RCT: randomised controlled trial; VAS: visual analogue scale; VRS: verbal rating scale. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Imprecision: evidence was based on a single small trial; downgraded one level. 2Risk of bias: trial judged to be at high risk of bias; downgraded two levels. 3Imprecision: evidence based on a single trial including 96 women; wide confidence intervals; downgraded two levels. | ||||||
| Combined oral contraceptive pill (COCP) compared to other medical treatment for pain associated with endometriosis | ||||||
| Patient or population: women with endometriosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with other medical treatment | Risk with COCP | |||||
| Self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up: VAS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up was 7.5 | MD 0.1 lower | ‐ | 50 | ⊕⊝⊝⊝ | VAS ranged from 1 to 10. |
| Self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up: VRS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up was 4.8 | MD 0.1 lower | ‐ | 50 | ⊕⊝⊝⊝ | VRS ranged from 0 to 3. |
| Self‐reported pain (dysmenorrhoea) (dichotomous data): reduction of pain to zero at 6 months' follow‐up: VAS | 38 per 1000 | 14 per 1000 | RR 0.36 | 50 | ⊕⊝⊝⊝ | VAS ranged from 1 to 10. |
| Self‐reported pain (dysmenorrhoea) (dichotomous data) ‐ reduction of pain to zero at 6 months' follow‐up: VRS | 1000 per 1000 | 1000 per 1000 | RR 1.00 | 49 | ⊕⊕⊝⊝ | VRS ranged from 0 to 3. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COCP: combined oral contraceptive pill; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale; VRS: verbal rating scale. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: no blinding and randomisation and allocation concealment unclear; downgraded one level. 2Imprecision: evidence from a single small trial including 50 women; wide confidence intervals crossing the line of no effect; downgraded two levels. 3Imprecision: evidence from a single small trial reporting data on 49 women; downgraded one level. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Self‐reported pain (dysmenorrhoea) at end of treatment Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dysmenorrhoea at end of treatment: verbal rating scale (VRS) | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.84, ‐0.76] |
| 1.2 Dysmenorrhoea at end of treatment: visual analogue scale (VAS) | 2 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐23.68 [‐28.75, ‐18.62] |
| 1.3 Menstrual pain reduction from baseline to end of treatment: VAS) | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | 2.1 [1.38, 2.82] |
| 2 Cyclical pain (non‐menstrual) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Non‐menstrual pain at end of treatment: VRS | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.48, 0.68] |
| 2.2 Non‐menstrual pain at end of treatment: VAS | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐11.72, 7.92] |
| 2.3 Non‐menstrual pain reduction from baseline to end of treatment: VAS | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.30, 1.70] |
| 3 Dyspareunia Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.46, 2.34] |
| 4 Dyschezia (pain on defecation) Show forest plot | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.56, 1.84] |
| 5 Satisfaction (very highly/highly satisfied) Show forest plot | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.24 [2.44, 7.37] |
| 6 Withdrawal from treatment Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.83, 2.18] |
| 7 Adverse effects occurring during treatment Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pregnancy | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.12, 68.98] |
| 7.2 Spotting/irregular bleeding/menorrhagia | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.44, 4.15] |
| 7.3 Nausea | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [1.79, 9.54] |
| 7.4 Any treatment‐associated adverse effect | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.00, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dysmenorrhoea at 6 months' follow‐up: visual analogue scale (VAS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.28, 1.08] |
| 1.2 Dysmenorrhoea at 6 months' follow‐up: verbal rating scale (VRS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.99, 0.79] |
| 2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Reduction of pain to mild or zero at 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.32, 2.58] |
| 2.2 Reduction of pain to zero at 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.43] |
| 3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Reduction of pain to mild or zero at 6 months' follow‐up | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.28] |
| 3.2 Reduction of pain to zero at 6 months' follow‐up | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 4 Cyclical pain (non‐menstrual pain) (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Non‐menstrual pain at end of treatment: VAS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.51, 1.11] |
| 4.2 Non‐menstrual pain at end of treatment: VRS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.51, 1.31] |
| 4.3 Non‐menstrual pain at 6 months' follow‐up: VAS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.85, 1.25] |
| 4.4 Non‐menstrual pain at 6 months' follow‐up: VRS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.08, 1.08] |
| 5 Cyclical pain (non‐menstrual pain) (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Reduction of pain to mild or zero at end of treatment: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.21] |
| 5.2 Reduction of pain to zero at end of treatment: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.89] |
| 5.3 Reduction of pain to mild or zero at end of treatment: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.17] |
| 5.4 Reduction to zero at end of treatment: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.89] |
| 5.5 Reduction of pain to mild or zero at 6 months' follow‐up: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.85, 1.53] |
| 5.6 Reduction of pain to zero at 6 months' follow‐up: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.85] |
| 5.7 Reduction of pain to mild or zero at 6 months' follow‐up: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.63, 1.32] |
| 5.8 Reduction of pain to zero at 6 months' follow‐up: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.85] |
| 6 Dyspareunia (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Dyspareunia at end of treatment: VAS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.18, 3.42] |
| 6.2 Dyspareunia at end of treatment: VRS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.41, 0.61] |
| 6.3 Dyspareunia at 6 months' follow‐up: VAS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.30, 2.10] |
| 6.4 Dyspareunia at 6 months' follow‐up: VRS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.47, 0.67] |
| 7 Dyspareunia (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Reduction of pain to mild or zero at end of treatment: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 1.02] |
| 7.2 Reduction of pain to zero at end of treatment: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.48] |
| 7.3 Reduction of pain to mild or zero at end of treatment: VRS | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.56, 1.65] |
| 7.4 Reduction of pain to zero at end of treatment: VRS | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.99, 1.84] |
| 7.5 Reduction of pain to mild or zero at 6 months' follow‐up: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.78] |
| 7.6 Reduction of pain to zero at 6 months' follow‐up: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.77] |
| 7.7 Reduction of pain to mild or zero at 6 months' follow‐up: VRS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.68] |
| 7.8 Reduction of pain to zero at 6 months' follow‐up: VRS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.77] |
| 8 Withdrawal from treatment Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.34, 5.62] |
| 9 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Hot flushes | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.30] |
| 9.2 Insomnia | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.15] |
| 9.3 Spotting/irregular bleeding | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.46, 3.15] |
| 9.4 Decreased libido | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.19] |
| 9.5 Vaginal dryness | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.63] |
| 9.6 Mood change | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.34, 3.19] |
| 9.7 Headache | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.49, 4.92] |
| 9.8 Paraesthesia | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.12] |
| 9.9 Breast tenderness | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.45, 6.55] |
| 9.10 Weight gain | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.41, 10.43] |
| 9.11 Peripheral oedema | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.77] |
| 9.12 Joint pain | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.12] |

