Amnioinfusión para la ruptura prematura de membranas en el tercer trimestre
Resumen
Antecedentes
La ruptura prematura de membranas (RPMP) es una de las principales causas de morbilidad y mortalidad perinatal. La amnioinfusión tiene como objetivo restaurar el volumen del líquido amniótico mediante la infusión de una solución en la cavidad uterina.
Objetivos
El objetivo de esta revisión fue evaluar los efectos de la amnioinfusión para la RPMP en la morbilidad y mortalidad perinatal y materna.
Métodos de búsqueda
Se realizaron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (2 de diciembre de 2013).
Criterios de selección
Ensayos aleatorizados de amnioinfusión comparada con ninguna amnioinfusión en mujeres con RPMP.
Obtención y análisis de los datos
Tres autores de la revisión evaluaron de forma independiente los ensayos para su inclusión. Dos autores de la revisión evaluaron de forma independiente la calidad de los ensayos y extrajeron los datos. Se verificó la exactitud de los datos.
Resultados principales
Se incluyeron cinco ensayos, de calidad moderada, pero sólo se analizaron los datos de cuatro estudios (con un total de 241 participantes). Un ensayo no aportó ningún dato a la revisión.
La amnioinfusión transcervical mejoró el pH de la arteria umbilical fetal en el parto (diferencia de medias 0,11; intervalo de confianza (IC) del 95%: 0,08 a 0,14; un ensayo, 61 participantes) y redujo las desaceleraciones variables persistentes durante el trabajo de parto (cociente de riesgos (RR) 0,52; IC del 95%: 0,30 a 0,91; un ensayo, 86 participantes).
La amnioinfusión transabdominal se asoció con una reducción de la muerte neonatal (RR 0,30; IC del 95%: 0,14 a 0,66; dos ensayos, 94 participantes), la sepsis neonatal (RR 0,26; IC del 95%: 0,11 a 0.61; un ensayo, 60 participantes), hipoplasia pulmonar (RR 0,22; IC del 95%: 0,06 a 0,88; un ensayo, 34 participantes) y sepsis puerperal (RR 0,20; IC del 95%: 0,05 a 0,84; un ensayo, 60 participantes). Las mujeres del grupo de amnioinfusión tuvieron también menos probabilidades de dar a luz dentro de los siete días de la ruptura de la membrana (RR 0,18; IC del 95%: 0,05 a 0,70; un ensayo, 34 participantes). Estos resultados deben tratarse con cautela, ya que los hallazgos positivos se debieron principalmente a un ensayo con una ocultación de la asignación poco clara.
Conclusiones de los autores
Estos resultados son alentadores, pero están limitados por datos insuficientes y la falta de solidez metodológica, por lo que se necesitan más pruebas antes de poder recomendar la amnioinfusión para la RPMP en la práctica clínica habitual.
PICOs
Resumen en términos sencillos
Amnioinfusión para la ruptura prematura de membranas prematuras
Hay algunas pruebas que demuestran que la restauración del volumen del líquido amniótico con solución salina o un líquido similar (amnioinfusión) después de la ruptura prematura de las membranas del prematuro (RPMP) puede ser beneficiosa para los bebés prematuros (al prevenir la infección, el daño pulmonar y la muerte) y para las madres (al prevenir la infección del útero después del parto). Sin embargo, la evidencia actual es insuficiente para recomendar la amnioinfusión para el uso rutinario en la RPMP.
La ruptura prematura de membranas es la causa más identificable del trabajo de parto prematuro. El saco (membranas) que rodea al bebé y el líquido en el útero (útero) suele romperse (ruptura) durante el trabajo de parto. Si las membranas se rompen antes del trabajo de parto y antes de término (antes de las 37 semanas) el bebé tiene un mayor riesgo de infección. La reducción del líquido alrededor del bebé también aumenta la posibilidad de que el cordón umbilical se comprima, lo que puede reducir el suministro de nutrientes y oxígeno al bebé. Además, la cantidad insuficiente de líquido en el útero puede interferir con el desarrollo normal de los pulmones en los bebés muy pequeños y puede causar sufrimiento fetal, con cambios en el ritmo cardíaco. Se puede inyectar líquido adicional al útero a través de la vagina de la mujer (amnioinfusión transcervical) o del abdomen (amnioinfusión transabdominal), proporcionando más líquido para rodear al bebé. La revisión de cinco ensayos controlados aleatorizados (con datos de un total de 241 participantes analizadas) encontró alguna evidencia que demuestran que la amnioinfusión con solución salina puede mejorar los resultados en salud y ser beneficiosa para los bebés y las madres después de la RPMP. Sin embargo, la evidencia actualmente es insuficiente para recomendar su uso rutinario debido al número limitado de ensayos y al bajo número de mujeres incluidas en los mismos.
Authors' conclusions
Summary of findings
| Transabdominal amnioinfusion compared with no amnioinfusion for preterm rupture of membranes (PROM) | ||||
| Patient or population: pregnant women with PROM Settings: hospital Intervention: transabdominal amnioinfusion Comparison: no amnioinfusion | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Neonatal death | RR 0.30 (0.14 ‐ 0.66) | 94 (two studies) | ⊕⊕⊕⊝ | Risk of neonatal death in the amnioinfusion group was 127 per 1000 compared to 426 per 1000 in the control group. |
| Neonatal sepsis/infection | RR 0.26 (0.11 ‐ 0.61) | 60 (one study*) | ⊕⊕⊕⊝ | *Sepsis defined as micro‐erythrocyte sedimentation rate > 5 mm, total leucocyte count < 5000, CRP > 6 mg/dL, platelet count < 100,000 or a positive blood culture within the first 48 hours. |
| Pulmonary hypoplasia | RR 0.22 (0.06 ‐ 0.88) | 34 (one study) | ⊕⊕⊝⊝ | Pulmonary hypoplasia was diagnosed according to strict clinical and radiological criteria, however, this study was small and blinding to group allocation was not described and so we downgraded this evidence from moderate to low. More evidence is needed. |
| Maternal puerperal sepsis | RR 0.20 (0.05 ‐ 0.84) | 60 (one study**) | ⊕⊕⊕⊝ | **Defined as fever > 38° C and a positive high vaginal swab culture. |
| GRADE Working Group grades of evidence CI: confidence interval; CRP: C‐reactive protein; RR: risk ratio | ||||
Background
Description of the condition
Preterm premature rupture of membranes (PPROM) remains the single most identifiable cause of preterm labour and a major contributor to perinatal mortality and morbidity. Oligohydramnios (reduced amniotic fluid volume) following PPROM is associated with a higher risk of chorioamnionitis, neonatal fetal infection and cord compression (Keirse 1989; Vintzileos 1985). Umbilical cord compression may cause persistent variable fetal heart rate decelerations (Gabbe 1976). Oligohydramnios is also the most important predictor of perinatal mortality in very early PPROM and adequate residual amniotic fluid plays a critical role in determining the prevalence of pulmonary hypoplasia, which is a major cause of death in these babies (Vergani 1994; Vintzileos 1985). Abnormal neurological outcomes and postural deformities in the neonate may also occur as a consequence of PPROM (Locatelli 2000).
Description of the intervention
Saline fluid or Ringers lactate/Hartmans is infused transcervically through a catheter into the uterine cavity, or transabdominally, through a narrow gauge needle. Amnioinfusion was first described as a method of preventing or relieving umbilical cord compression during labour (Miyazaki 1983). The technique has since been used prophylactically in various conditions associated with oligohydramnios, including impaired intrauterine growth and PPROM. Amnioinfusion has been shown to prolong the latency period in second trimester PPROM (Locatelli 2000; Ogunyemi 2002; Turhan 2002), improve perinatal survival (Locatelli 2006; Ogunyemi 2002) and decrease rates of pulmonary hypoplasia (Locatelli 2000). Combined with antibiotics and without, it has been used to treat and to prevent infection following premature rupture of membranes (Goodlin 1981; Monahan 1995; Ogita 1988). In a Cochrane review on amnioinfusion for suspected or potential cord compression in labour at term, amnioinfusion improved short‐term measures of neonatal outcome, reduced the use of caesarean section and reduced maternal puerperal sepsis (Hofmeyr 2012). A subcutaneously implanted amniotic fluid replacement port system has been developed for long‐term amnioinfusion in women with preterm premature rupture of the membranes (Tchirikov 2010).
How the intervention might work
Restoring the amniotic fluid volume cushions the fetus, thereby preventing mechanical compression of the umbilical cord and reducing fetal distress. It may prevent fetal lung hypoplasia in PPROM by preventing mechanical compression of the fetal thorax and enabling normal amniotic fluid flow into the fetal lungs (Tranquilli 2005). This effect occurs mainly in the second trimester and is considered in a separate review (Van Teeffelen 2013). Likewise, by preventing mechanical compression of the fetus, amnioinfusion may prevent postural deformities. In addition, the infused fluid may prevent intrauterine infection, possibly by the anti‐bacterial effect of saline, or a diluent effect. Improvements in fetal ductus venosus and umbilical artery flow have been demonstrated following amnioinfusion for PPROM (Hsu 2009).
Why it is important to do this review
Evidence from cohort studies suggests that replenishing the amniotic fluid volume in pregnancies complicated by PPROM is beneficial to mother and child. However, amnioinfusion is an invasive procedure and not without potential risks, thus a thorough evaluation of randomised controlled trials is required.
['Amnioinfusion for cord compression' (Hofmeyr 2012), 'Prophylactic versus therapeutic amnioinfusion' (Novikova 2012), and 'Amnioinfusion for meconium‐stained liquor' (Hofmeyr 2014), are separate reviews.]
Objectives
To assess from the best available evidence the effects of amnioinfusion for preterm premature rupture of membranes on perinatal morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing the effect of amnioinfusion for third trimester preterm premature rupture of the membranes before 37 weeks with a control group (no amnioinfusion); random allocation to treatment and control groups, with adequate allocation concealment; violations of allocated management and exclusions after allocation not sufficient to materially affect outcomes. Quasi‐randomised (alternate allocation), cross‐over and cluster trials were not considered eligible for inclusion.
Types of participants
Pregnant women with preterm premature rupture of membranes.
Types of interventions
Amnioinfusion compared with no amnioinfusion.
Types of outcome measures
Primary outcomes
-
Indicators of fetal condition, e.g. persistent variable decelerations, Apgar scores, cord arterial pH at birth.
-
Neonatal morbidity including infection, lung hypoplasia, abnormal neurological outcomes and postural deformities.
-
Perinatal mortality.
Secondary outcomes
-
Mode of delivery.
-
Indications for delivery.
-
Latency period from amnioinfusion to delivery.
-
Maternal morbidity, e.g. endometritis, postpartum temperature greater than 38°C.
-
Birthweight.
-
Admission to neonatal intensive or high care unit.
We considered outcomes separately for transcervical and transabdominal amnioinfusion.
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (2 December 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
monthly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
No new studies have been included for this update (2014).
For methods used in the previous version of this review, please see Hofmeyr 2011.
For methods to be used in the next update, see Appendix 1.
Selection of studies
T Lawrie (TL), AC Eke (ACE) and GJ Hofmeyr (GJH) independently assessed for inclusion all potential studies. No studies were identified from the updated search. Two studies previously in ongoing were excluded (Roberts: AMIPROM; Vergani 2007). We resolved any disagreement through discussion. We evaluated trials under consideration for methodological quality and appropriateness for inclusion according to the prespecified selection criteria, without consideration of their results.
Results
Description of studies
In total, we have included five studies; three in the transcervical amnioinfusion comparison (Gonzalez 2001; Nageotte 1985; Puertas 2007) and two in the transabdominal comparison (Singla 2010; Tranquilli 2005). One included study contributed no data (Gonzalez 2001). SeeCharacteristics of included studies. The gestational age range for recruitment to the transcervical trials was 26 to 36+ weeks and in the transabdominal trials, 24 to 34 weeks. Transcervical amnioinfusion was performed in a total of 72 women versus 75 controls (excluding unusable data from Gonzalez 2001) and transabdominal amnioinfusion was performed in 47 women versus 47 controls; thus participant numbers for this review are small.
Risk of bias in included studies
All included studies were randomised trials. SeeFigure 1 and Figure 2 for a summary of 'Risk of bias' assessments.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Randomisation was by random number table in Puertas 2007 and was computer‐generated in Tranquilli 2005 and Singla 2010; the method of randomisation and group allocation was not described in Nageotte 1985 and Gonzalez 2001. Allocation concealment was described in two trials (Puertas 2007; Tranquilli 2005).
Blinding
Partial blinding was described in two trials (Nageotte 1985; Puertas 2007).
Incomplete outcome data
Incomplete outcome data were noted in the randomised clinical trial by Gonzalez et al. This is because it is an abstract only publication, hence provided insufficient detail for assessment (Gonzalez 2001). Five post‐randomisation exclusions: three for non‐vertex presentation and two for fetal distress were noted in the randomised trial by Nageotte et al (Nageotte 1985). Otherwise, outcome data were complete for Puertas 2007, Singla 2010 and Tranquilli 2005.
Selective reporting
In the randomised clinical trial by Tranquilli et al, the authors did not report the mode of delivery, Apgar scores or cord arterial pH for the babies delivered (Tranquilli 2005). No selective reporting was found in the Puertas 2007 and Singla 2010 trials. There was insufficient information to assess selective reporting in Gonzalez 2001 and Nageotte 1985.
Other potential sources of bias
Another source of bias was noted in the randomised clinical trial by Puertas et al, where more carriers of group B streptococcal were noted in control group (14 women versus seven women) (Puertas 2007). Even though all these women with Group B streptococcal infection received prophylactic intrapartum antibiotics, the higher incidence of Group B Streptococcus in the control group was a source of bias for the trial. No other biases were reported for Singla 2010 and Tranquilli 2005. There was insufficient information to assess other sources of bias in Gonzalez 2001 and Nageotte 1985.
Effects of interventions
See: Summary of findings for the main comparison
1. Transcervical amnioinfusion
Transcervical amnioinfusion improved fetal umbilical artery pH at delivery (mean difference (MD) 0.11; 95% confidence interval (CI) 0.08 to 0.14; one trial, 61 participants; Analysis 1.6) and reduced persistent variable decelerations during labour (risk ratio (RR) 0.52; 95% CI 0.30 to 0.91; one trial, 86 participants; Analysis 1.1). No significant differences in the rates of caesarean section, low Apgar scores, neonatal death or infectious morbidity were detected.
2. Transabdominal amnioinfusion
Transabdominal amnioinfusion was associated with a reduction in neonatal death (RR 0.30; 95% CI 0.14 to 0.66; two trials, 94 women; Analysis 2.7), neonatal infection/sepsis (RR 0.26; 95% CI 0.11 to 0.61; one trial, 60 participants; Analysis 2.3), and pulmonary hypoplasia (RR 0.22; 95% CI 0.06 to 0.88; one trial, 34 participants; Analysis 2.3). Women in the amnioinfusion group were also less likely to deliver within seven days of membrane rupture (RR 0.18; 95% CI 0.05 to 0.70; one trial, 34 participants; Analysis 2.4) and were less likely to experience puerperal sepsis (RR 0.20; 95% CI 0.05 to 0.84; one trial, 60 participants; Analysis 2.9). There were no significant differences between groups regarding birthweight, gestational age at delivery, admission to neonatal intensive care and neurological sequelae. Fetal distress was diagnosed less frequently in the amnioinfusion group than the control group (RR 0.27; 95% CI 0.08 to 0.88; one trial, 60 participants; Analysis 2.1), but in the absence of blinding this outcome is at risk of bias.
Discussion
Summary of main results
Transabdominal amnioinfusion following preterm premature rupture of the membranes (PPROM) resulted in better neonatal outcomes (decreased sepsis, infection and death) and decreased puerperal sepsis than conventional management. The data contributing to these outcomes were mainly from one trial (Singla 2010). Transcervical amnioinfusion was associated with improved fetal heart rate patterns and umbilical cord blood pH results, but improvements in substantive clinical outcomes were not statistically significant.
Overall completeness and applicability of evidence
These results are not conclusive due to the small numbers of trials and participants. In addition, the numbers are too small to detect rare potential adverse events. The lower threshold of gestational age at which amnioinfusion may be of benefit is not known.
Quality of the evidence
The evidence is of mainly moderate quality (summary of findings Table for the main comparison) and limitations can largely be attributed to the small size of studies and the lack of blinding to group allocation.
Agreements and disagreements with other studies or reviews
This evidence supports the positive findings of the non‐randomised studies conducted to date.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 1 Persistant variable decelerations.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 2 Severe variable decelerations per hour in first stage.
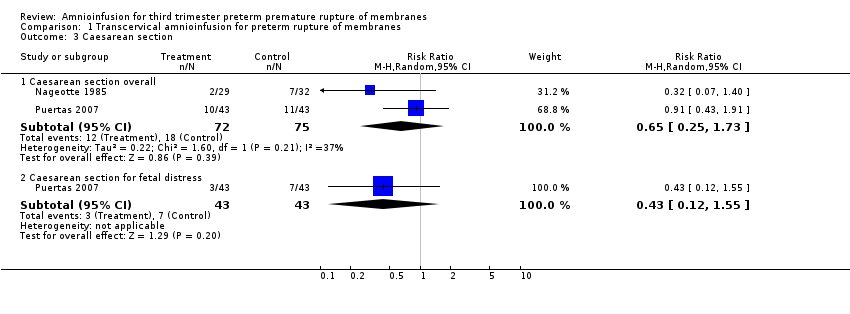
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 3 Caesarean section.
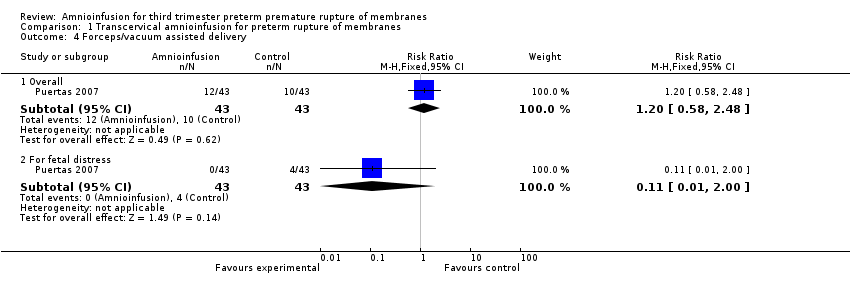
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 4 Forceps/vacuum assisted delivery.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 5 1 minute Apgar score < 4.
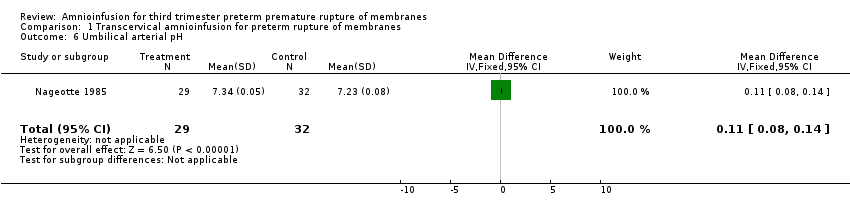
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 6 Umbilical arterial pH.
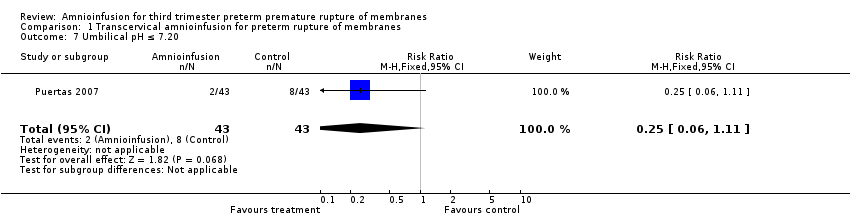
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 7 Umbilical pH ≤ 7.20.
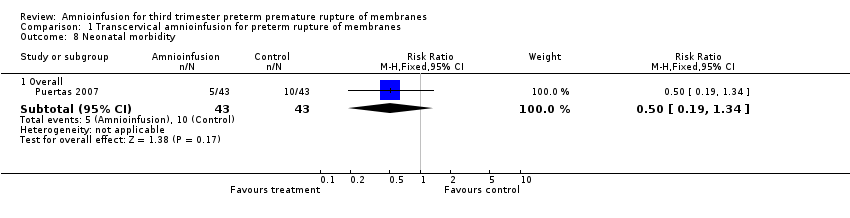
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 8 Neonatal morbidity.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 9 Neonatal death.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 10 Maternal puerperal sepsis.
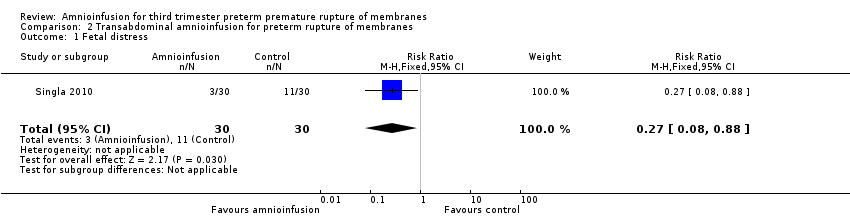
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 1 Fetal distress.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 2 Gestational age at delivery (weeks).
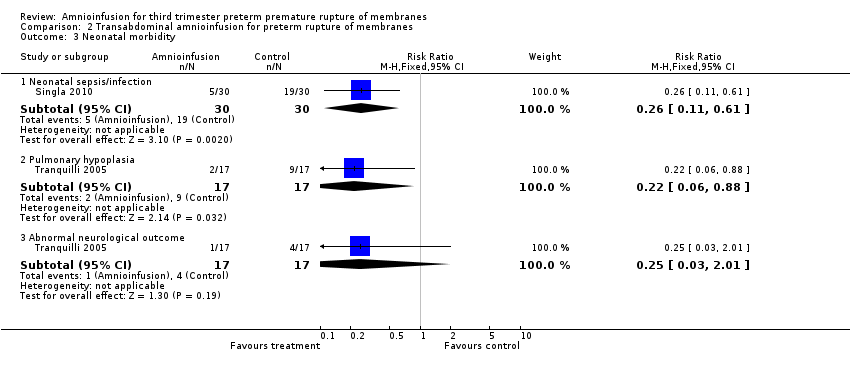
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 3 Neonatal morbidity.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 4 Delivery within 7 days.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 5 Time to delivery (days).
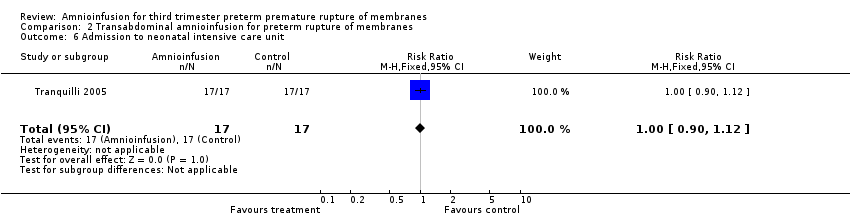
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 6 Admission to neonatal intensive care unit.
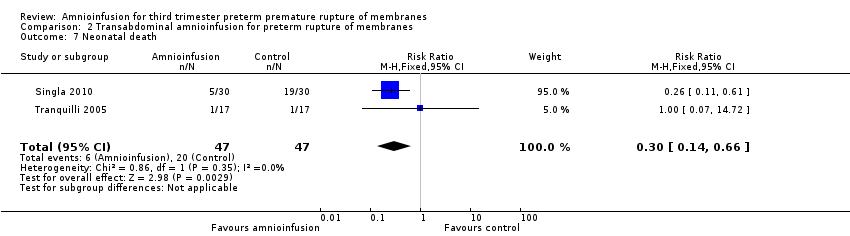
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 7 Neonatal death.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 8 Birthweight (grams).

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 9 Maternal puerperal sepsis.
| Transabdominal amnioinfusion compared with no amnioinfusion for preterm rupture of membranes (PROM) | ||||
| Patient or population: pregnant women with PROM Settings: hospital Intervention: transabdominal amnioinfusion Comparison: no amnioinfusion | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Neonatal death | RR 0.30 (0.14 ‐ 0.66) | 94 (two studies) | ⊕⊕⊕⊝ | Risk of neonatal death in the amnioinfusion group was 127 per 1000 compared to 426 per 1000 in the control group. |
| Neonatal sepsis/infection | RR 0.26 (0.11 ‐ 0.61) | 60 (one study*) | ⊕⊕⊕⊝ | *Sepsis defined as micro‐erythrocyte sedimentation rate > 5 mm, total leucocyte count < 5000, CRP > 6 mg/dL, platelet count < 100,000 or a positive blood culture within the first 48 hours. |
| Pulmonary hypoplasia | RR 0.22 (0.06 ‐ 0.88) | 34 (one study) | ⊕⊕⊝⊝ | Pulmonary hypoplasia was diagnosed according to strict clinical and radiological criteria, however, this study was small and blinding to group allocation was not described and so we downgraded this evidence from moderate to low. More evidence is needed. |
| Maternal puerperal sepsis | RR 0.20 (0.05 ‐ 0.84) | 60 (one study**) | ⊕⊕⊕⊝ | **Defined as fever > 38° C and a positive high vaginal swab culture. |
| GRADE Working Group grades of evidence CI: confidence interval; CRP: C‐reactive protein; RR: risk ratio | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistant variable decelerations Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.91] |
| 2 Severe variable decelerations per hour in first stage Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐1.83, ‐0.57] |
| 3 Caesarean section Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Caesarean section overall | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.25, 1.73] |
| 3.2 Caesarean section for fetal distress | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.12, 1.55] |
| 4 Forceps/vacuum assisted delivery Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Overall | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.58, 2.48] |
| 4.2 For fetal distress | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.00] |
| 5 1 minute Apgar score < 4 Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.33] |
| 6 Umbilical arterial pH Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.08, 0.14] |
| 7 Umbilical pH ≤ 7.20 Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.11] |
| 8 Neonatal morbidity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Overall | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.19, 1.34] |
| 9 Neonatal death Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 5.77] |
| 10 Maternal puerperal sepsis Show forest plot | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.06, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fetal distress Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.08, 0.88] |
| 2 Gestational age at delivery (weeks) Show forest plot | 2 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐2.63, 1.65] |
| 3 Neonatal morbidity Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Neonatal sepsis/infection | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.61] |
| 3.2 Pulmonary hypoplasia | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.88] |
| 3.3 Abnormal neurological outcome | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.01] |
| 4 Delivery within 7 days Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.70] |
| 5 Time to delivery (days) Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐2.86, 4.00] |
| 6 Admission to neonatal intensive care unit Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.90, 1.12] |
| 7 Neonatal death Show forest plot | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.14, 0.66] |
| 8 Birthweight (grams) Show forest plot | 2 | 94 | Mean Difference (IV, Random, 95% CI) | 15.65 [‐254.02, 285.32] |
| 9 Maternal puerperal sepsis Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.84] |

