Amnioinfusión para la ruptura prematura de membranas en el tercer trimestre
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised trial. Type of randomisation not specified. Abstract only. | |
| Participants | 44 pregnant women with gestational ages from 189‐258 days (24 in amnioinfusion group and 20 controls). | |
| Interventions | Amnioinfusion versus no amnioinfusion. Inclusion and exclusion criteria not specified. | |
| Outcomes | Fetal heart rate variability, mode of delivery, cord arterial pH and perinatal morbidity. | |
| Notes | This is an abstract only; we are not aware of any published final results. The abstract results are presented as percentages only without denominators and so could not be used in this review. Amnioinfusion reduced the percentage of caesarean sections done for fetal distress compared with the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only, insufficient detail provided for assessment. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, insufficient detail provided for assessment. |
| Other bias | Unclear risk | Abstract only, insufficient detail provided for assessment. |
| Methods | 'Assigned in a random fashion.' | |
| Participants | Inclusion criteria: spontaneous premature rupture of membranes; gestational age 26 to 35 weeks; decreased or absent amniotic fluid; vertex presentation; no gross fetal anomalies. Exclusion criteria: premature labour on admission; vaginal bleeding; previous caesarean section and no desire for trial of labour; antepartum fetal distress; consent refused. 1 woman with twin pregnancy and 1 with previous caesarean section were allocated to the amnioinfusion group. Of 66 women allocated, 3 were excluded for a change from the vertex presentation at the time of labour, and 2 for antepartum fetal distress. Of the remainder, 29 were allocated to amnioinfusion and 32 to the control group. | |
| Interventions | Labour commenced spontaneously, or induced for confirmed fetal pulmonary maturity or infection. All women had fetal scalp electrodes and uterine pressure catheters placed as early in labour as possible. Amnioinfusion with warmed saline at 10 mL per minute for 1 hour (repeated if a large volume of fluid was lost), then 3 mL per minute (total volume infused mean 1160, range 735‐1650 mL); compared with no amnioinfusion. Caesarean section was performed for persistent late decelerations; recurrent severe variable decelerations; recurrent or uncorrectable prolonged decelerations (no mention of fetal scalp blood sampling). | |
| Outcomes | Amnionitis (2 of the following: fever 38 degrees celsius or more; leucocytosis > 15,000 per microlitre; foul‐smelling amniotic fluid; uterine tenderness); intrapartum fetal heart rate tracings, evaluated in a blinded manner; endometritis (postpartum fever > 38 degrees celsius twice 4 hours apart, and uterine tenderness or foul‐smelling lochia); Apgar scores; umbilical cord arterial and venous pH; neonatal morbidity and mortality. | |
| Notes | Long Beach, California, USA. March 1984 to March 1985. Of 66 women allocated, 5 (7.6%) were excluded, 3 for a change from the vertex presentation at the time of labour, and 2 for antepartum fetal distress. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Fetal heart rate tracings evaluated in a blinded manner. |
| Incomplete outcome data (attrition bias) | Unclear risk | 5 post‐randomisation exclusions: 3 for non‐vertex presentation and 2 for fetal distress. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | Random allocation to 2 groups using a random number table and opaque sealed envelopes. | |
| Participants | Inclusion criteria: women with preterm premature rupture of membranes between 27 and 35 weeks of gestation; included if labour had begun spontaneously or after induction. | |
| Interventions | Intrapartum transcervical amnioinfusion versus a control group that had an intrauterine pressure catheter inserted but without amnioinfusion. Physiological saline at 37 degree celsius, at a rate of 600 mL/hr during the first hour. After 1 hour, amniotic fluid index was determined, and if amniotic fluid index was greater than 15, amnioinfusion was stopped. In all other women in the study group amnioinfusion continued at a rate of 180 mL/hr until the cervix was completely dilated. Fetal heart rate and uterine activity were recorded continuously throughout labour. All women who were group B streptococcus carriers received prophylactic intrapartum antibiotics. | |
| Outcomes | Mode of delivery, PH and arterial blood concentrations, neonatal morbidity, puerperal morbidity. | |
| Notes | More group B streptococcal carriers in control group (14 women versus 7 women). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Partial blinding. Changes in fetal heart rate analysed with the Cabaniss classification by an independent investigator. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data complete. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Unclear risk | More group B streptococcal carriers in control group (14 women versus 7 women). |
| Methods | Randomised controlled trial conducted in Delhi, India between August 2005 and 2007. | |
| Participants | 60 women with singleton pregnancies (30 in each group) between 26 and 33 + 6 weeks' gestation with amniotic fluid index less than the 5th percentile. Excluded if evidence of chorioamnionitis, placental or fetal anomalies or active labour. Diagnosis of preterm rupture of membranes was made via the litmus paper test and confirmed using ultrasound. | |
| Interventions | Transcervical amnioinfusion with normal saline repeated weekly if amniotic fluid index < 5 versus no amnioinfusion. All women received bed rest, antibiotics and steroids. | |
| Outcomes | Preterm rupture of membranes to delivery interval; neonatal outcome including birthweight, intrapartum fetal distress defined as persistent tachycardia and recurrent, late or severe decelerations; early neonatal sepsis; neonatal mortality; causes of neonatal mortality; mode of delivery; postpartum sepsis. | |
| Notes | No loss to follow‐up. Baseline characteristics were similar between groups. 5 women received amnioinfusion twice, 1 woman received it 3 times. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described: "To exclude observer bias, randomisation was performed ....using a computer generated random number table..." There was no mention of allocation concealment (e.g. sealed opaque envelopes). |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None other bias was noted. |
| Methods | Randomised controlled trial using opaque sealed envelopes and a computer random‐number generator. 44 women met inclusion criteria, 4 refused to participate and 6 delivered before randomisation, leaving 34 women to be randomised. | |
| Participants | Pregnant women included if: singleton pregnancies complicated by preterm premature rupture of membranes, gestational age between 24 and 33 weeks, evidence of preterm premature rupture of membrane within 24 hours of admission, oligohydramnios, absence of uterine contractions at the time of hospitalisation, no placental anomalies or major structural fetal anomalies and normal cardiotocography at the time of admission. | |
| Interventions | 17 women were allocated to amnioinfusion and 17 to no amnioinfusion. All women received bedrest and antibiotic prophylaxis and corticosteroid therapy. Prophylactic tocolytic treatment was administered if there were no clinical signs of chorioamnionitis or placental abruption. Women in the treatment group received weekly serial amnioinfusion if the amniotic fluid index fell below the 5th centile and/or a median pocket of amniotic fluid was < 2 cm. The mean volume infused was 250 mL N/saline (range 120‐350 mL), the aim was to restore the amniotic fluid index to > 10th percentile. The amniotic fluid index was measured after the procedure and it was repeated after 24 hours and at least once weekly. If amniotic fluid index was ≤ 5, the amnioinfusion was repeated weekly until 27 weeks of gestation. A non‐stress test was performed daily. Caesarean section was done for chorioamnionitis and fetal distress (defined as persistent tachycardia with reduced variability, recurrent late or severe variable decelerations). | |
| Outcomes | Birthweight; admission to neonatal intensive care unit; gestational age at delivery (weeks); pulmonary hypoplasia diagnosed on the basis of strict clinical and radiological criteria at 1 and 3 months after delivery; abnormal neurological outcomes. The preterm premature rupture of membrane‐delivery interval was also determined. | |
| Notes | Study conducted at the Salesi Mothers' and Children's Hospital, Ancona, Italy, from January‐December 2002. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were complete. |
| Selective reporting (reporting bias) | Unclear risk | Authors do not report mode of delivery, Apgar scores or cord arterial pH. |
| Other bias | Low risk | No other bias noted. |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| The quality of the randomisation process was poor. Women were by chance admitted into 1 of the 2 divisions of the department of obstetrics and gynaecology. Among the 71 women who were included in the study, 37 underwent serial amnioinfusion and 34 were used as controls. Also included in the study were women who underwent preterm rupture of membrane after amniocentesis for prenatal diagnosis. 10 patients (27.0%) in the amnioinfusion group and 8 patients (23.5%) in the control group. The study is also limited by the long period in which it was performed due to the low prevalence of this disease. The control group was selected without proper randomisation therefore bias could not be excluded during the selection process. | |
| Randomised controlled trial of amnioinfusion versus no amnioinfusion for oligohydramnios. Small numbers (9 controls versus 8 study participants), only 3 study participants who received amnioinfusion had premature rupture of membranes and these outcome data were not extractable from totals. | |
| The study was not a randomised controlled trial. All singleton pregnancies with preterm premature rupture of membranes at < 26 weeks' gestation and lasting > 4 days between January 1991 and June 1998 were included. Consenting women with persistent (> 4 days) oligohydramnios (maximum cord‐free pocket of amniotic fluid volume less or equal to 2 cm) received serial transabdominal amnioinfusions to maintain an amniotic pocket > 2 cm. The pregnancy, neonatal, and long‐term neurological outcomes of the cases that spontaneously maintained a median amniotic fluid pocket > 2 cm (amnioinfusion‐not‐necessary group) were compared with those of women with oligohydramnios who underwent amnioinfusion but continued to have a median amniotic fluid pocket after preterm premature rupture of membranes (amniotic fluid pocket less or equal to 2 cm/persistent oligohydramnios group) and with those of women in whom oligohydramnios was alleviated by amnioinfusion for at least 48 hours (successful amnioinfusion group). The study also incorporated 18 cases that had been previously reported in Vergani 1997. | |
| Study population were women with ruptured membranes at term, not preterm. | |
| Excluded because even though this is a randomised controlled trial involving preterm premature rupture of membrane and amnioinfusion, the gestational ages when the preterm premature rupture of membrane occurred were in the 2nd trimester of pregnancy. There are no reported data for 3rd trimester outcomes. This was a prospective non‐blinded randomised controlled trial with randomisation stratified for pregnancies where the membranes ruptured between 16 + 0 and 19 + 6 weeks' gestation and 20 + 0 and 23 + 6 weeks' gestation to minimise the risk of random imbalance in gestational age distribution between randomised groups. This study was carried out in 4 UK hospital‐based Fetal Medicine Units (Liverpool Women’s NHS Trust, St. Mary’s Hospital Manchester, Birmingham Women’s NHS Foundation Trust, Wirral University Hospitals Trust). Participants were randomly allocated to either serial weekly trans‐abdominal amnioinfusions if the deepest pool of amniotic fluid was < 2 cm or expectant management until 37 weeks of pregnancy. Short‐term maternal, pregnancy and neonatal and long‐term outcomes for the child were studied. Long‐term respiratory morbidity was assessed using validated respiratory questionnaires at 6, 12 and 18 months of age and infant lung function test at around 12 months of age. Neurodevelopment was assessed using Bayley’s Scale of Infant Development II at corrected age of 2. | |
| Excluded because even though this is an randomised controlled trial involving preterm premature rupture of membrane and amnioinfusion, the gestational ages to be considered are preterm premature rupture of membranes that occurred in the 2nd trimester of pregnancy (< 24 weeks). The study will consider singleton pregnancies, early spontaneous preterm premature rupture of membrane < 24.3 weeks, oligohydramnios (deepest vertical pocket < 2 cm) for at least 4 days and no longer than 15 days at enrolment. The study is open and will be run through a dedicated password protected website, and with a minimal number of outcome measures. Primary outcomes include survival until discharge from the neonatal intensive care unit, while secondary outcomes include latency time from preterm premature rupture of membrane to delivery, gestational age at birth, indication for delivery, number of days of ventilatory support, serious neurologic morbidity, neonatal sepsis prevalence, need for oxygen at 36 weeks post‐conception. Statistics: 38 patients in each arm are necessary to show an increase in neonatal survival from 10% to 40%, with a power of 0.80 and alpha = 0.05. An interim analysis after recruitment of 75% of the study population will be held in order to re‐calculate sample size according to the difference between the groups in latency time as the main secondary outcome. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistant variable decelerations Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.91] |
| Analysis 1.1  Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 1 Persistant variable decelerations. | ||||
| 2 Severe variable decelerations per hour in first stage Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐1.83, ‐0.57] |
| Analysis 1.2  Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 2 Severe variable decelerations per hour in first stage. | ||||
| 3 Caesarean section Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3 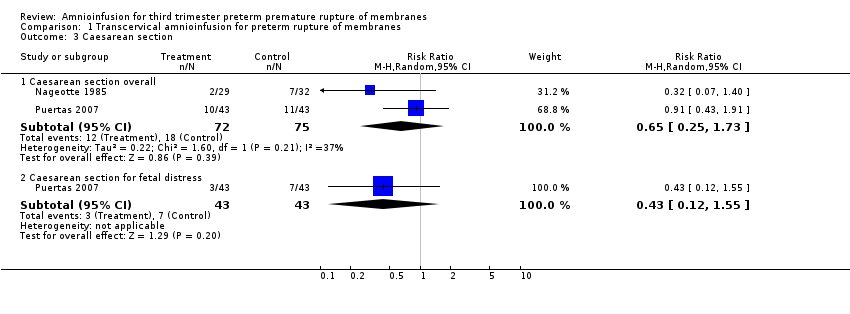 Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 3 Caesarean section. | ||||
| 3.1 Caesarean section overall | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.25, 1.73] |
| 3.2 Caesarean section for fetal distress | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.12, 1.55] |
| 4 Forceps/vacuum assisted delivery Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 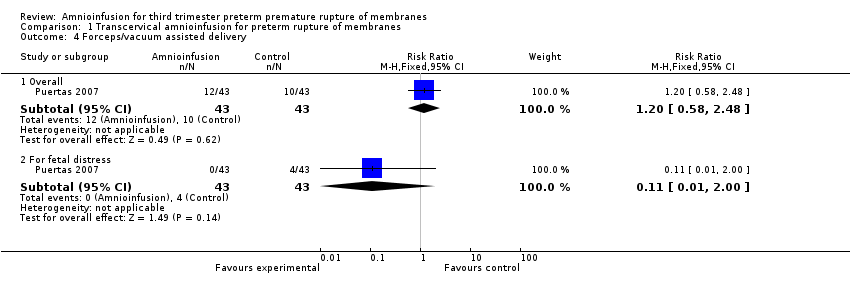 Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 4 Forceps/vacuum assisted delivery. | ||||
| 4.1 Overall | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.58, 2.48] |
| 4.2 For fetal distress | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.00] |
| 5 1 minute Apgar score < 4 Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.33] |
| Analysis 1.5  Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 5 1 minute Apgar score < 4. | ||||
| 6 Umbilical arterial pH Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.08, 0.14] |
| Analysis 1.6 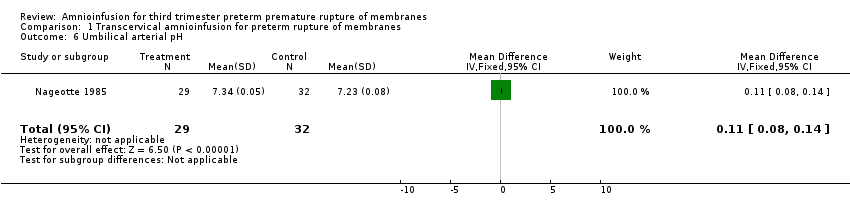 Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 6 Umbilical arterial pH. | ||||
| 7 Umbilical pH ≤ 7.20 Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.11] |
| Analysis 1.7 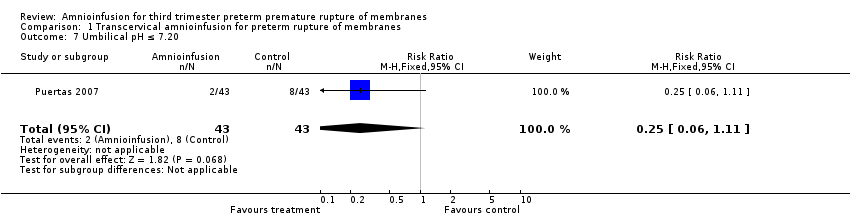 Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 7 Umbilical pH ≤ 7.20. | ||||
| 8 Neonatal morbidity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 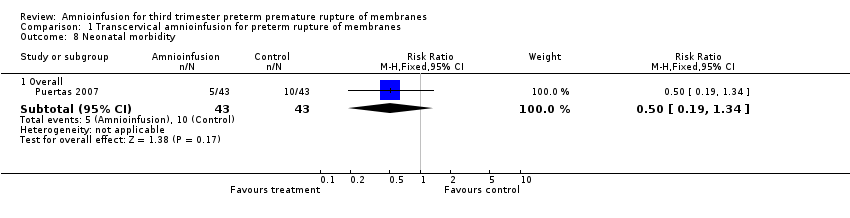 Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 8 Neonatal morbidity. | ||||
| 8.1 Overall | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.19, 1.34] |
| 9 Neonatal death Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 5.77] |
| Analysis 1.9  Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 9 Neonatal death. | ||||
| 10 Maternal puerperal sepsis Show forest plot | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.06, 2.18] |
| Analysis 1.10  Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 10 Maternal puerperal sepsis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fetal distress Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.08, 0.88] |
| Analysis 2.1 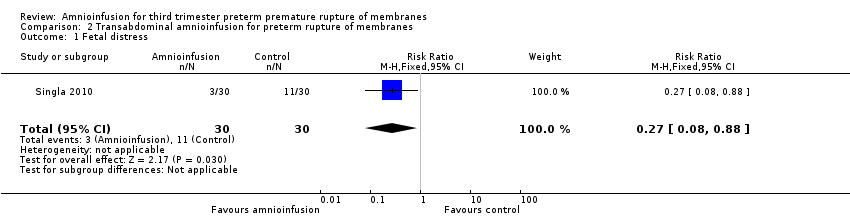 Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 1 Fetal distress. | ||||
| 2 Gestational age at delivery (weeks) Show forest plot | 2 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐2.63, 1.65] |
| Analysis 2.2  Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 2 Gestational age at delivery (weeks). | ||||
| 3 Neonatal morbidity Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 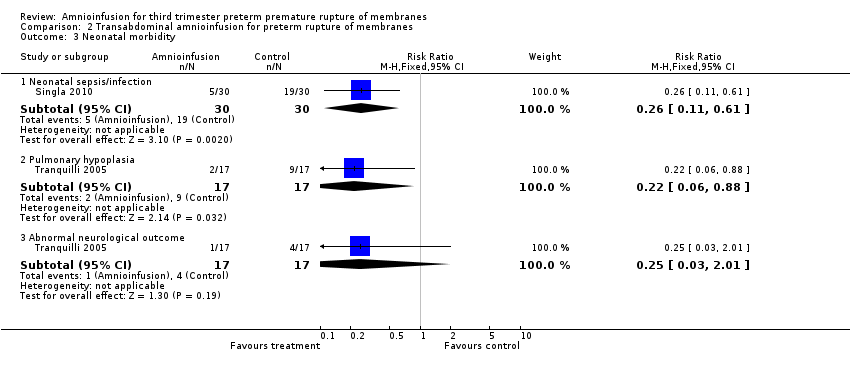 Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 3 Neonatal morbidity. | ||||
| 3.1 Neonatal sepsis/infection | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.61] |
| 3.2 Pulmonary hypoplasia | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.88] |
| 3.3 Abnormal neurological outcome | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.01] |
| 4 Delivery within 7 days Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.70] |
| Analysis 2.4  Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 4 Delivery within 7 days. | ||||
| 5 Time to delivery (days) Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐2.86, 4.00] |
| Analysis 2.5  Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 5 Time to delivery (days). | ||||
| 6 Admission to neonatal intensive care unit Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.90, 1.12] |
| Analysis 2.6 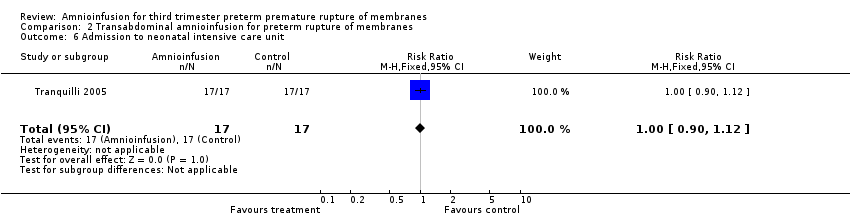 Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 6 Admission to neonatal intensive care unit. | ||||
| 7 Neonatal death Show forest plot | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.14, 0.66] |
| Analysis 2.7 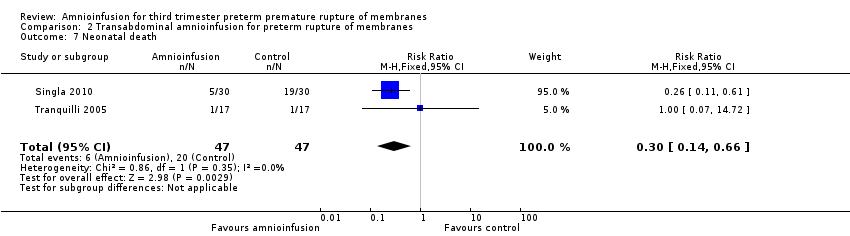 Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 7 Neonatal death. | ||||
| 8 Birthweight (grams) Show forest plot | 2 | 94 | Mean Difference (IV, Random, 95% CI) | 15.65 [‐254.02, 285.32] |
| Analysis 2.8  Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 8 Birthweight (grams). | ||||
| 9 Maternal puerperal sepsis Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.84] |
| Analysis 2.9  Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 9 Maternal puerperal sepsis. | ||||

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 1 Persistant variable decelerations.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 2 Severe variable decelerations per hour in first stage.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 3 Caesarean section.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 4 Forceps/vacuum assisted delivery.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 5 1 minute Apgar score < 4.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 6 Umbilical arterial pH.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 7 Umbilical pH ≤ 7.20.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 8 Neonatal morbidity.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 9 Neonatal death.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 10 Maternal puerperal sepsis.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 1 Fetal distress.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 2 Gestational age at delivery (weeks).

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 3 Neonatal morbidity.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 4 Delivery within 7 days.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 5 Time to delivery (days).

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 6 Admission to neonatal intensive care unit.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 7 Neonatal death.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 8 Birthweight (grams).

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 9 Maternal puerperal sepsis.
| Transabdominal amnioinfusion compared with no amnioinfusion for preterm rupture of membranes (PROM) | ||||
| Patient or population: pregnant women with PROM Settings: hospital Intervention: transabdominal amnioinfusion Comparison: no amnioinfusion | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Neonatal death | RR 0.30 (0.14 ‐ 0.66) | 94 (two studies) | ⊕⊕⊕⊝ | Risk of neonatal death in the amnioinfusion group was 127 per 1000 compared to 426 per 1000 in the control group. |
| Neonatal sepsis/infection | RR 0.26 (0.11 ‐ 0.61) | 60 (one study*) | ⊕⊕⊕⊝ | *Sepsis defined as micro‐erythrocyte sedimentation rate > 5 mm, total leucocyte count < 5000, CRP > 6 mg/dL, platelet count < 100,000 or a positive blood culture within the first 48 hours. |
| Pulmonary hypoplasia | RR 0.22 (0.06 ‐ 0.88) | 34 (one study) | ⊕⊕⊝⊝ | Pulmonary hypoplasia was diagnosed according to strict clinical and radiological criteria, however, this study was small and blinding to group allocation was not described and so we downgraded this evidence from moderate to low. More evidence is needed. |
| Maternal puerperal sepsis | RR 0.20 (0.05 ‐ 0.84) | 60 (one study**) | ⊕⊕⊕⊝ | **Defined as fever > 38° C and a positive high vaginal swab culture. |
| GRADE Working Group grades of evidence CI: confidence interval; CRP: C‐reactive protein; RR: risk ratio | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistant variable decelerations Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.91] |
| 2 Severe variable decelerations per hour in first stage Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐1.83, ‐0.57] |
| 3 Caesarean section Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Caesarean section overall | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.25, 1.73] |
| 3.2 Caesarean section for fetal distress | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.12, 1.55] |
| 4 Forceps/vacuum assisted delivery Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Overall | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.58, 2.48] |
| 4.2 For fetal distress | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.00] |
| 5 1 minute Apgar score < 4 Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.33] |
| 6 Umbilical arterial pH Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.08, 0.14] |
| 7 Umbilical pH ≤ 7.20 Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.11] |
| 8 Neonatal morbidity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Overall | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.19, 1.34] |
| 9 Neonatal death Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 5.77] |
| 10 Maternal puerperal sepsis Show forest plot | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.06, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fetal distress Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.08, 0.88] |
| 2 Gestational age at delivery (weeks) Show forest plot | 2 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐2.63, 1.65] |
| 3 Neonatal morbidity Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Neonatal sepsis/infection | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.61] |
| 3.2 Pulmonary hypoplasia | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.88] |
| 3.3 Abnormal neurological outcome | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.01] |
| 4 Delivery within 7 days Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.70] |
| 5 Time to delivery (days) Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐2.86, 4.00] |
| 6 Admission to neonatal intensive care unit Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.90, 1.12] |
| 7 Neonatal death Show forest plot | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.14, 0.66] |
| 8 Birthweight (grams) Show forest plot | 2 | 94 | Mean Difference (IV, Random, 95% CI) | 15.65 [‐254.02, 285.32] |
| 9 Maternal puerperal sepsis Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.84] |

