Coloides versus cristaloides para ressuscitação volêmica em pacientes graves
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Quasi‐RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 180 Inclusion criteria: patients admitted to MIU Exclusion criteria: no details Participant condition: head, chest, abdominal injuries Baseline characteristics Colloids group
Crystalloids group
Country: UK Setting: MIU | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic analysis; urine outputs; recovery; LoS Outcomes relevant to the review: mortality (time not reported) | |
| Notes | Funding/declarations of interest: none apparent Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate participants added to each group based on odd/even numbers |
| Allocation concealment (selection bias) | High risk | Alternate allocation used and therefore unlikely to be concealed |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; not likely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Unclear risk | The proportion of participants in each arm with chest injuries differed. It is unclear whether this influenced results. |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 2857 Inclusion criteria: no prior fluid resuscitation in ICU; required fluid resuscitation for acute hypovolaemia Exclusion criteria: received fluid resuscitation in ICU; anaesthesia‐related hypotension; advanced chronic liver disease; acute anaphylactic reaction; inherited coagulation disorders; do‐not‐resuscitate order; pregnant; burned > 20% of TBSA; allergy to study drug; refused consent; dehydrated; brain death or organ donor; other (not specified) Participant condition: acute hypovolaemia, sepsis, and trauma Baseline characteristics Colloids group
Crystalloids group
Country: France, Belgium, Canada, Algeria, Tunisia Setting: ICU | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: mortality at 28 days; mortality at 90 days and at ICU and hospital discharge; number of days alive and not receiving renal replacement therapy, mechanical ventilation or vasopressor therapy; days not in ICU or hospital; days without organ failure Outcomes relevant to the review: mortality (at 28 days, 90 days, and at end of follow‐up); renal replacement therapy; requiring blood transfusion | |
| Notes | Funding/declarations of interest: funded by French Ministry of Health. Study sponsors not involved in design and conduct of study Study dates: Febuary 2003‐November 2012 Note: study was stopped early because study authors noted no difference in 28‐day mortality rates. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used at the bedside to allow randomisation of eligible participants without any delay and was done blinded to block size |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Clinicians were not blinded because of immediate need for resuscitation; unlikely to introduce bias for this outcome |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | High risk | Clinicians were not blinded because of immediate need for resuscitation; could introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Quote: "mortality end‐points were collected and assessed by study members blinded to treatment assignment." Unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration (NCT00318942); all outcomes listed on registration site were reported |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | High risk | 47.5% of participants in the crystalloid group were given colloids within 12 h before the start of the study and this may have influenced study results |
| Methods | RCT Parallel design Multicentre (2 x level 1 adult trauma centres) | |
| Participants | Total number of randomised participants: 64 Inclusion criteria: coma, with a loss of consciousness because of isolated blunt head trauma or a GCS score ≤ 8 Exclusion criteria: primary penetrating injury; previous IV therapy ≥ 50 mL; time of arrival at scene to IV access > 4 h; < 16 years of age; burn or amputation; presumed to be pregnant; vital signs absent prior to randomisation Participant condition: blunt trauma head injury Baseline characteristics Colloids group
Crystalloids group
Country: Canada Setting: ambulatory prior to adult‐designated level 1 trauma centres | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: neurological outcomes at hospital discharge (or 30 days) using various scales; mortality; biomarkers Outcomes relevant to the review: mortality (at 28 days) | |
| Notes | Funding/declarations of interest: funded by Defence Research and Development Canada Study dates: September 2004‐January 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Computer randomisation was used to assign sequentially numbered identical IV bags to the ambulance |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Participants and personnel were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Paramedics, physicians and study co‐ordinators were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | High risk | Study authors report that participants could receive additional fluid resuscitation during standard care and this could influence outcome results for this study |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 48 Inclusion criteria: ≥ 16 years of age with second‐ or third‐degree acute burn injuries and > 15% of body surface area burned Exclusion criteria: expected to die within 24‐36 h (i.e. burn victims with whole body burn trauma); in situations of palliative care; pregnancy; lack of informed consent; known allergy to HES; contraindications for balanced 6% HES 130/0.04; intracerebral bleeding; acute renal failure; severe hypernatraemia and other severe electrolyte disorders; severe von Willebrand Syndrome; acute liver failure Participant condition: burns; TBSA > 15% Baseline characteristics Colloids group
Crystalloids group
Country: Switzerland Setting: tertiary burns unit | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: group difference in administration of fluid with 72 h; creatinine levels; urine output; ARDS; LoS in ICU; LoS in hospital; in‐hospital mortality and at 28 days; post‐hoc 90‐day mortality; RRT Outcomes relevant to the review: mortality (28 days; and 90 days); RRT (collected as a 90‐day post‐hoc analysis) | |
| Notes | Funding/declarations of interest: funding from manufacturer of HES, which supplied study fluids; 2 of the authors have vocationally been members of advisory board meetings. No competing interests declared. Funders reported as having no input in study design and interpretation of results Study dates: November 2009‐January 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation completed using minimisation technique, conducted by a third party |
| Allocation concealment (selection bias) | Low risk | A third party not involved in conduction of study, performed the randomisation process |
| Blinding of participants and personnel (performance bias): mortality | Low risk | All personnel blinded. Fluids prepared externally, and concealed in bags of black plastic |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Low risk | All personnel blinded. Fluids prepared externally, and concealed in bags of black plastic |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details in study report. However, trial registration report states that outcome assessors were blinded |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Low risk | No details in study report. However, trial registration report states that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | 2 participants were retrospectively excluded because of meeting exclusion criteria. Data missing from 1 additional participant because of early discharge. Overall, < 10% dropout/exclusion; data reported for 45/48 randomised participants |
| Selective reporting (reporting bias) | High risk | Prospective clinical trials registration (NCT01012648). Only primary outcome (fluid volume administered) was listed on the trial registration site |
| Baseline characteristics | Low risk | Baseline characteristics comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 22 Inclusion criteria: ICU patients with an acute, spontaneous subarachnoid haemorrhage, with stable ICP in the range of 10 mmHg‐20 mmHg; > 18 years of age; sedated; mechanically ventilated; stable haemodynamics; serum sodium of < 160 mmol/L Exclusion criteria: no details Participant condition: spontaneous subarachnoid haemorrhage Baseline characteristics Colloids group
Crystalloids group
Country: Norway Setting: ICU | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: ICP; CPP; extravascular lung water; serum sodium levels Outcomes relevant to the review: none | |
| Notes | Funding/declarations of interest: not reported Study dates: April 2002‐October 2004 Participant condition not reported by group; "A total of 21 patients had haemorrhaged because of a ruptured aneurysm, and one patient was diagnosed with a fusiform dilation of the left vertebral artery." | |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 537 Inclusion criteria: patients with severe sepsis or septic shock; ≥ 18 years of age; onset of the syndrome < 24 h before admission to the ICU or < 12 h after admission if the condition developed in the ICU Exclusion criteria: treatment with > 1000 mL of HES within 24 h before study inclusion; pre‐existing renal failure requiring dialysis or a serum creatinine level ≥ 320 μmoL/L (3.6 mg/dL); < 18 years of age; pregnancy; known allergy against HES; intra‐cerebral haemorrhage; heart failure with NYHA IV; requirement of an inspiratory oxygen fraction of at least 0.7; immunosuppression from cytostatic chemotherapy; high dosage of steroids or AIDS; participation in another interventional trial; moribund due to coexisting disease; order to withhold or withdraw therapy Participant condition: severe sepsis or septic shock Baseline characteristics Colloids group
Crystalloids group
Country: Germany Setting: ICU; 18 tertiary hospitals | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: mortality (at 28 days and 90 days); morbidity (according to SOFA scores); need for blood transfusion; renal failure (to include need for RRT); time to haemodynamic stabilisation; frequency of vasopressor therapy; need for red‐cell transfusion; duration of mechanical ventilation, LoS in the ICU; adverse events (worsening of oxygenation, bleeding complications, allergic reaction, any event judged to occur in relation to study fluid) Outcomes relevant to the review: mortality (at 28 days and 90 days; need transfusion of a blood product; need for renal replacement therapy | |
| Notes | Funding/declarations of interest: supported by a grant (01 KI 0106) from the German Federal Ministry of Education and Research and by unrestricted grants from B Braun, HemoCue and Novo Nordisk Study dates: April 2003‐June 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Open‐label design; unlikely to introduce bias for this outcome |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | High risk | Open‐label design; could introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | High risk | Note: 26.6% of participants in the crystalloid group were given colloids during the study period and this may have influenced study results |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 209 Inclusion criteria: blunt trauma; > 17 years of age (or adult size if age was unknown); at least 1 prehospital SBP measurement ≤ 90 mmHg; transported directly to a single level 1 trauma centre from the site of injury Exclusion criteria: ongoing cardiopulmonary resuscitation; isolated penetrating trauma; known or suspected pregnancy; receipt of > 2000 mL of crystalloid before availability of study fluid Participant condition: blunt trauma Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: prehospital (ambulatory) prior to admission to a single level 1 trauma centre | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: incidence of ARDS; mortality; multiple organ failure syndrome; nosocomial infections; length of hospital and ICU stay; ventilator‐free days; adverse events; non‐infectious complications Outcomes relevant to the review: mortality (28 days) | |
| Notes | Funding/declarations of interest: grant R01 HL073233‐01 from the National Institutes of Health Study dates: October 2003‐August 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | A random number (computer‐generated by pharmacist) was applied to each bag and kept by the pharmacist. Ambulance crew did not have access to allocation sequence |
| Blinding of participants and personnel (performance bias): mortality | Low risk | All contents of fluid bags were blinded by research pharmacists. Therefore, personnel and participants were blinded to treatment assignment |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | 21 participants did not meet eligibility criteria once randomisation had taken place but remained in the results using ITT analysis. Three participants lost to follow‐up, explanations reported by study authors |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration ID: NCT01012648. All outcomes specified on clinical trials registration site were reported. However, we noted that the outcomes were only added to the trials registration site after the study start date |
| Baseline characteristics | Unclear risk | We noted higher injury severity scores for those in the colloids group, and we could not be certain whether this could influence outcome data |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 1331 Inclusion criteria: blunt mechanism of injury; ≥ 15 years of age; GCS score ≤ 8; ineligibility for enrolment in the haemorrhagic shock cohort Exclusion criteria: known or suspected pregnancy; < 15 years of age; out‐of‐hospital cardiopulmonary resuscitation; administration of > 2000 mL of crystalloid or any amount of colloid or blood products prior to enrolment; severe hypothermia (28 °C); drowning; asphyxia because of hanging; burns on > 20% of TBSA; isolated penetrating head injury; inability to obtain IV access; > 4 h between receipt of dispatch call to study intervention Participant condition: traumatic brain injury Baseline characteristics Colloids group
Crystalloids group (NS)
Crystalloids group (HS)
Country: USA and Canada Setting: 11 regional clinical centres | |
| Interventions | Colloids group
Crystalloids group (NS)
Crystalloids group (HS)
| |
| Outcomes | Outcomes measured/reported: 6‐month neurologic status (Glasgow Outcome Score); 28‐day survival; survival to discharge; ICP; interventions required to manage intracranial hypertension; fluid and bolus requirements in first 24 h; physiologic parameters of organ dysfunction; 28‐day ARDS‐free survival; MODS; nosocomial infections Outcomes relevant to the review: mortality (28 days) | |
| Notes | Funding/declarations of interest: National Heart, Lung and Blood Institute plus partners Study dates: May 2006‐May 2009 Study terminated after futility criteria met at 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated numeric code used at central location |
| Allocation concealment (selection bias) | Low risk | Randomisation scheme conducted externally and all personnel unaware of allocation |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Quote: "Study fluids were provided in identical intravenous bags and shipped to a single distribution center, where they were labelled with a randomly generated numeric code" Participants, caregivers, and outcome assessors were blinded to treatment |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Participants, caregivers, and outcome assessors were blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | Mortality data reported for 359/373 (HSD), 341/355 (HS), and 582/603 (NS). < 5% dropout/loss in each group |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration: NCT00316004. All outcomes were prespecified |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 895 Inclusion criteria: ≥ 15 years of age; in significant haemorrhagic shock (out‐of‐hospital SBP ≤ 70 mmHg or 71‐90 mmHg with concomitant HR ≤ 108 bpm) Exclusion criteria: known or suspected pregnancy; < 15 years of age; out‐of‐hospital cardiopulmonary resuscitation; administration of > 2000 mL crystalloid, colloid, or blood products before enrolment; severe hypothermia (< 28 °C); drowning; asphyxia because of hanging; burns > 20% TBSA; isolated penetrating head injury; inability to obtain IV access; time of dispatch call received to study intervention > 4 h; known prisoners Participant condition: traumatic hypovolaemic shock Baseline characteristics Colloids group
Crystalloids group (NS)
Crystalloids group (HS)
Country: USA and Canada Setting: out‐of‐hospital | |
| Interventions | Colloids group
Crystalloids group (NS)
Crystalloids group (HS)
| |
| Outcomes | Outcomes measured/reported: 28‐day survival; physiologic parameters of organ dysfunction; ARDS criteria met in the first 28 days after injury; MODS; presence of nosocomial infection Outcomes relevant to the review: mortality (28 days); participants having transfusion (0‐9 units); participants having transfusion (> 10 units) | |
| Notes | Funding/declarations of interest: The National Heart, Lung and Blood Institute. Study authors declare no financial conflicts of interest Study dates: May 2006‐August 2008 Note: the previous version of this review did not include participants in the HS group (Perel 2013). We have included outcome data for these participants, and in analysis we have combined both crystalloid groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly generated numeric code was applied to each bag and a randomization list kept by the Data Co‐ordinating Center" Information taken from study protocol |
| Allocation concealment (selection bias) | Low risk | Randomisation list kept by study investigators (taken from study protocol) |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Care providers, investigators, and participants were blinded to treatment assignment, study fluids concealed |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Low risk | Care providers, investigators, and participants were blinded to treatment assignment, study fluids concealed |
| Blinding of outcome assessment (detection bias): mortality | Low risk | All personnel were blinded |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Low risk | All personnel were blinded |
| Incomplete outcome data (attrition bias) | Low risk | 42/895 participants were not included in analysis but reasons were clearly provided (most of these losses were because of inclusion/exclusion criteria) |
| Selective reporting (reporting bias) | Unclear risk | A protocol was published for this study; publication of protocol was retrospective and it was not feasible to use this to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | High risk | We noted that participants may have received up to 2000 mL of crystalloid or colloid before randomisation (as part of exclusion criteria). Study authors did not report how many participants received fluid resuscitation before randomisation, or which fluid was given, and this may influence outcome data for this study |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 1810 Inclusion criteria: ≥ 18 years of age; severe sepsis within previous 24 h Exclusion criteria: < 18 years of age; terminal state; known adverse reaction to albumin administration; severe sepsis or septic shock after proved or suspected head injury; clinically active; congestive heart failure (NYHA class 3 or 4); pathological conditions in which albumin administration was clinically indicated (hepatic cirrhosis with ascites, intestinal malabsorption syndrome, nephrotic syndrome, burns); > 24 h since inclusion criteria were met; religious objection to the administration of human blood products; inclusion in other experimental studies Participant condition: severe sepsis Baseline characteristics Colloids group
Crystalloids group
Country: Italy Setting: ICU | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: death from any cause (28 days); death from any cause (90 days); number of participants with organ dysfunction; length of ICU and hospital stay Outcomes relevant to the review: mortality (at 28 days, and at 90 days); RRT | |
| Notes | Funding/declarations of interest: Italian Medicines Agency Study dates: Aug 2008‐Feb 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally, with the use of the computer‐generated and blinded assignment sequence. Randomization was stratified according to the participating ICU and the interval between the time that the patient met the clinical criteria for severe sepsis and randomization" |
| Allocation concealment (selection bias) | Low risk | Central allocation, blinded |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Open‐label study; lack of blinding unlikely to introduce bias for this outcome |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Few losses; unlikely to affect analysis |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration (NCT00707122). All outcomes listed were reported |
| Baseline characteristics | Unclear risk | Quote: "Baseline characteristics were similar between the two study groups, except for a slight imbalance in the number of patients with organ dysfunction and values of central venous oxygen saturation". It was not reported if these differences between groups were at a level of statistical significance. We were uncertain whether these differences might influence the results |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 49 Inclusion criteria: SBP ≤ 90 mmHg for < 1 h; normal ECG; written consent by participant or first‐degree relative Exclusion criteria: pregnancy; renal, cardiac, or neurological diseases Participant condition: haemorrhagic shock Baseline characteristics Colloids group
Crystalloids group
Country: Mexico Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic variables; urinary output; GCS; mortality (within 24 h) Outcomes relevant to the review: mortality | |
| Notes | Funding/declarations of interest: none reported Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to groups using random numbers but no Additional details provided |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | High risk | Quote: "Dextran 40 was administered to the control group if necessary according to medical judgement." Study authors did not report the number of participants in the crystalloid group who received additional colloids and this may influence outcome data for this study |
| Methods | Quasi‐RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 26 Inclusion criteria: admitted to a children's medical centre; fever lasting 2‐7 days; haemorrhagic manifestations; evidence of consumptive coagulopathy, a fall in platelet count, prolonged bleeding, prolonged prothrombin time, or prolonged partial thromboplastin time; evidence of plasma leakage; evidence of circulatory failure Exclusion criteria: severe infection other than dengue haemorrhagic fever; protein‐deficient abnormalities; bleeding diathesis; given multiple plasma substitutes Participant condition: DSS Baseline characteristics Colloids group
Crystalloids group
Country: Philippines Setting: ICU | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: duration of control of shock, haematocrit level, length of ICU stay, transfusion of blood products, frequency of recurrence of shock, mortality Outcomes relevant to the review: mortality (time point not reported); transfusion of blood products (FFP or packed red blood cells) | |
| Notes | Funding/declarations of interest: not reported Study dates: June 2001‐July 2001 Note: 3 out of 16 participants in the crystalloid group (18.75%) also received colloids during the study period and this may have influenced study results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised method to allocate participants, using alternating allocation to each group |
| Allocation concealment (selection bias) | High risk | Not possible to conceal allocation because of methods used to allocate participants |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Personnel were not blinded; however, unlikely to introduce bias for this outcome |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Four participants were excluded from some analysis. Because mortality data were reported for these participants we included these in analysis. |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | High risk | 3 out of 16 participants in the crystalloid group (18.75%) also received colloids during the study period and this may have influenced study results |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 42 Inclusion criteria: thermal burn of ≥ 20% TBSA; time elapsed since injury ≤ 12 h; written informed consent from the participant or a suitable substitute decision maker; availability of data regarding fluids administered before arrival at the study centre Exclusion criteria: unlikely survival, defined as APACHE II score > 30 or predicted mortality ≥ 90%; ventricular fibrillation; ventricular tachycardia; unstable angina; known congestive heart failure or myocardial infarction within the month before thermal injury; electrical or chemical burn injury; pregnancy Participant condition: burns Baseline characteristics Colloids group
Crystalloids group
Country: Canada Setting: hospital units | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: MODS; mortality; duration of mechanical ventilation; LoS in ICU; local infection events; systemic infection events; percentage of graft take; oxygenation failure (PaO2‐to‐FiO2 ratio) (all evaluated up to and including Day 28) Outcomes relevant to the review: mortality (28 days); blood transfusion | |
| Notes | Funding/declarations of interest: funded by Bayer Biologics, Canada Study dates: June 1999‐June 2001 Trial stopped early due to slow enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | High risk | Quote: "Treatment fluid was given in an open label fashion owing to differences in the physical properties (color, tendency to bubble) and medium of delivery (glass vials vs. polymer bags)" Could introduce bias for blood transfusion outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details; lack of blinding unlikely to influence data for mortality |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Unclear risk | Baseline characteristics and demographics were comparable between groups except for predicted mortality, which was greater in the colloid group (18.6%) compared with the crystalloid group (9.4%) |
| Other bias | Low risk | No other sources of bias |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 41 Inclusion criteria: adults; male and female; with hospital diagnosis of severe acute pancreatitis Exclusion criteria: history of allergy to HES; history of cardiac dysfunction or renal insufficiency; pregnancy, malignancy or immunoinsufficiency; other colloids within 24 h; serum albumin < 25 g/L; likely death within 48 h. Also excluded those who died within 72 h; received surgery during treatment period; severe adverse effects to HES Participant condition: severe acute pancreatitis Baseline characteristics Colloids group
Crystalloids group
Country: China Setting: university hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: mortality (within‐hospital stay); intra‐abdominal pressure; fluid balance; major organ complications; use of respirator; APACHE II score; serum levels of inflammatory mediators Outcomes relevant to the review: mortality (within‐hospital stay) | |
| Notes | Funding/declarations of interest: supported by Sichuan Province of Science and Technology Department Technology Support Project Study dates: January 2008‐November 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐derived random number table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | We have included 1 participant that was excluded from the study as this participant died therefore providing relevant data for this review |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | High risk | 57.1% of participants in the crystalloid group were given colloids during the study period and we noted that this may have influenced study results |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 25 Inclusion criteria: ≥ 18 years of age; confirmed or suspected infection plus ≥ 2 signs of the systemic inflammatory response syndrome (definition of sepsis by American College of Chest Physicians/Society of Critical Care Medicine criteria), and tissue hypoperfusion (MAP < 65 mmHg despite a crystalloid fluid challenge of 20 mL/kg or blood lactate concentration of ≥ 4 mmol/L) Exclusion criteria: impossible to perform sublingual video‐microscopy; < 18 years of age; pregnancy; stroke; acute coronary syndrome; hydrostatic pulmonary oedema; status asthmaticus; cardiac arrhythmias (as a main diagnosis); contraindication for central venous catheterisation; active gastrointestinal haemorrhage; seizures; drug intoxications; burns; trauma; need of immediate surgery; terminal cancer; immunosuppression (organ transplant or systemic illness); no resuscitation order; delayed admission to the intensive care unit from the emergency department (> 4 h); or previous resuscitation with > 1500 mL of fluids Participant condition: sepsis Baseline characteristics Colloids group
Crystalloids group
Country: Argentina Setting: 2 teaching ICUs | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: heart rate; MAP; CVP; central venous gases and oxygen saturations; microcirculatory variables; mortality Outcomes relevant to the review: mortality (time point not reported) | |
| Notes | Funding/declarations of interest: supported by the grant PICT‐2007‐00912, Agencia Nacional de Promoción Científica y Tecnológica, Argentina Study dates: January 2006‐August 2009 Note: data for mortality were not clearly reported in the study report. We have included deaths of participants within 24 h and combined these with deaths reported in the study report outcome table. The previous version of this review did not include mortality outcome data (Perel 2013). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Simple randomization by the use of sealed envelopes" Insufficient details to allow judgement |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, but no mention of opaqueness, or whether they were numbered sequentially |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | Study authors reported a small number of losses because of death. We included these as data for the mortality outcome |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration occurred after the start of the study (NCT00799916); not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 50 Inclusion criteria: 5‐15 years of age; DSS; had not received IV fluid therapy during their current illness Exclusion criteria: no details Participant condition: DSS Baseline characteristics Colloids group (dextran)
Colloids group (gelatin)
Crystalloids group (RL)
Crystalloids group (NS)
Country: Vietnam Setting: hospital | |
| Interventions | Colloids group (dextran)
Colloids group (gelatin)
Crystalloids group (RL)
Crystalloids group (NS)
| |
| Outcomes | Outcomes measured/reported: recovery from shock; duration of shock and number of episodes of shock; improvements in cardiac output and haematocrit values; requirements for further fluid resuscitation Outcomes relevant to the review: none | |
| Notes | Funding/declarations of interest: B Braun provided the fluids used in this study. Financial support from The Wellcome Trust of Great Britain Study dates: all participants admitted between July and November 1995 | |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 18 Inclusion criteria: septic; critically ill; fluid infusion clinically indicated; pulmonary catheter already in place; patient not overtly bleeding Exclusion criteria: no details Participant condition: sepsis Baseline characteristics Colloids group
Crystalloids group
Country: Canada Setting: ICU | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: MAP, PAOP, cardiac index, arterial oxygen content, plasma albumin concentration, PV and ECFV Outcomes relevant to the review: none | |
| Notes | Funding/declarations of interest: none reported Study dates: not reported | |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 25 Inclusion criteria: > 16 years of age; blunt or penetrating trauma; requiring IV fluid resuscitation; arrival at trauma unit within 2 h of injury; RL as the only prehospital infusion; no underlying illness or medication that would affect the patient's coagulating system Exclusion criteria: no details Participant condition: trauma Baseline characteristics Colloids group
Crystalloids group
Country: South Africa Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: bleeding times, prothrombin, thrombin, partial thromboplastin times, platelet count, secondary resuscitation Outcomes relevant to the review: mortality (data from personal communication with study authors; time point unknown) | |
| Notes | Funding/declarations of interest: "Hoechst SA for their independent grant and sponsorship for this research project" Study dates: not reported Note: we used mortality data reported in the previous version of this review (Perel 2013). These data were collected from personal communication with the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 6997 Inclusion criteria: ≥ 18 years of age; treating clinician judged to require fluid administration to maintain or increase intravascular volume Exclusion criteria: people admitted to ICU after cardiac surgery; liver transplantation; treatment of burns Participant condition: various ICU admissions (to include trauma, sepsis, ARDS) Baseline characteristics Colloids group
Crystalloids group
Country: Australia and New Zealand Setting: hospital ‐ 16 ICUs | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: all‐cause mortality within 28 days, survival time during first 28 days, proportion of participants with organ failure, duration of mechanical ventilation, duration of renal‐replacement therapy, duration of ICU and hospital stay Outcomes relevant to the review: mortality (28 days), RRT (for subgroup of participants with severe sepsis) | |
| Notes | Funding/declarations of interest: Auckland District Health Board and the Health Research Council of New Zealand Study dates: November 2001‐June 2003 Note: in the previous version of the review (Perel 2013), this study was called SAFE 2004. This study reports a subgroup of participants who had severe sepsis (1218 participants; 603 in the albumin group, and 615 in the saline group). Data were available for RRT for these participants and we have included this subgroup of participants in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out centrally with the use of a minimisation algorithm; service accessed through a secure website |
| Allocation concealment (selection bias) | Low risk | Used central randomisation by a third party |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Blinding was maintained by use of identical 500 mL bottles and cartons designed to mask fluid type and administration sets |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Low risk | Blinding was maintained by use of identical 500 mL bottles and cartons designed to mask fluid type and administration sets |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding provided; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Data (on vital status) were missing for 1% of randomised participants at 28 days, which is acceptable. Some discrepancies with reported numbers of participants analysed, but not significant |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration (ISRCTN76588266); all outcomes listed on registration site were reported |
| Baseline characteristics | Unclear risk | We noted that the albumin group had a higher CVP at baseline; we could not be certain whether this imbalance might influence results. No other baseline imbalances were noted |
| Other bias | High risk | Study authors reported that 3.9% of participants in the saline group were given albumin in the previous 72 h; this represents few participants and it is not likely to have introduced significant bias. However, some participants were given additional resuscitation fluids during the study period according to clinician preference, and numbers for this were not reported. This may influence outcome data for this study |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 79 Inclusion criteria: control of resuscitation obtained within 4 h of injury; all participants admitted within 12 h of injury Exclusion criteria: none stated Participant condition: burns Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: Brooke Army Medical Center | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic responses; mortality at end of follow‐up Outcomes relevant to the review: mortality at end of follow‐up | |
| Notes | Funding/declarations of interest: study authors state, "the opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense" Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised using random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | No losses reported |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics are comparable |
| Other bias | High risk | All participants in the crystalloid group received colloids after 24 h and this may have influenced study results |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 50 Inclusion criteria: trauma patients who met the criteria for haemorrhagic‐hypovolaemic shock, with definitive signs of external or internal haemorrhage in a prehospital setting; aged 18‐60 years Exclusion criteria: no details Participant condition: haemorrhagic‐hypovolaemic shock Baseline characteristics Colloids group
Crystalloids group
Country: Croatia Setting: prehospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: BP, pulse rate, peripheral oxygen saturation, and respiration rate Outcomes relevant to the review: none | |
| Notes | Funding/declarations of interest: none reported Study dates: not reported | |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 196 Inclusion criteria: ≥ 18 years of age; required fluid resuscitation; clinically defined severe sepsis Exclusion criteria: serum creatinine > 300 µmol/L; chronic renal failure; anuria lasting > 4 h; requirement for renal support Participant condition: severe sepsis Baseline characteristics Colloids group
Crystalloids group
Country: France and Germany Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: amount of study drug to achieve haemodynamic stabilisation; time to achieve initial haemodynamic stabilisation; quantity of study drug infused over 4 consecutive days; LoS in ICU and hospital; SOFA scores; kidney injury (RIFLE and AKIN scores); mortality (28 days and 90 days); blood transfusion; adverse events (itching) Outcomes relevant to the review: mortality (28 days); blood transfusion (red blood cells); RRT (score of 3 using AKIN); adverse events (itching) | |
| Notes | Funding/declarations of interest: supported by grant from Fresenius Kabi, Germany. The pharmaceutical company was involved in the study design, analysis and preparation of the report Study dates: not reported Note: the previous version of this review (Perel 2013) used mortality data at 90 days; in this review we have analysed mortality data at 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Refers to reference from Myburgh 2012 to describe randomisation technique. Used external web‐based randomisation |
| Allocation concealment (selection bias) | Low risk | Use of web‐based system ensured that allocation code was kept concealed |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Participants and personnel were blinded. Study drugs were kept in identical packaging |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Low risk | Participants and personnel were blinded. Study drugs were kept in identical packaging |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Reference from Myburgh 2012 suggests that all personnel were blinded, including outcome assessors |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Low risk | Reference from Myburgh 2012 suggests that all personnel were blinded, including outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | One participant was unaccounted for in saline group for mortality outcome only, unlikely to influence outcome data overall |
| Selective reporting (reporting bias) | High risk | Prospective clinical trials registration (NCT00464204). Clinical trials registration documents do not list mortality, transfusion of blood products, or RRT as study outcomes. Clinical trials registration documents title of the study is "Effects of voluven on hemodynamics and tolerability of enteral nutrition in patients with severe sepsis" and some outcomes relate to assessment of caloric intake |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 172 Inclusion criteria: admitted during acute phase, with burns for which treatment for shock was indicated; adults and children Exclusion criteria: no details Participant condition: burns Baseline characteristics Colloids group
Crystalloids group
Country: Denmark Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: fluid input and output, haemoglobin levels, mortality Outcomes relevant to the review: mortality (48 h) | |
| Notes | Funding/declarations of interest: supported by grant from Danish Medical Research Council Study dates: not reported, the last participant was recruited in December 1975 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were stratified according to burn severity and type and then lots were used to determine which treatment the first participant in each stratum received |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | Data are reported for all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or pre‐published protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 19 Inclusion criteria: witnessed cardiac arrest with probable cardiac cause; advanced medical life support within 15 min; return of spontaneous circulation within 60 min; comatose when admitted to the hospital; aged 18‐80 years Exclusion criteria: terminal illness; strongly in need of nursing; primary coagulopathy; prehospital fluid load > 2000 mL Participant condition: postcardiac arrest Baseline characteristics Colloids group
Crystalloids group
Country: Norway Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: fluid volume required to achieve treatment goals; oedema; haemodynamics; adverse events (to include renal failure); survival after 1 year Outcomes relevant to the review: survival after 1 year | |
| Notes | Funding/declarations of interest: supported by grant from the Regional Centre for Emergency Medical Research and Development and Development and Section of Emergency Medicine, Dept of Anaesthesia and Intensive Care, Haukeland University Hospital Study dates: September 2005‐March 2007 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors report use of stratified randomisation, with allocation generated by study authors. We could not be certain whether this method was sufficient |
| Allocation concealment (selection bias) | Unclear risk | Numbered envelopes were distributed and opened by a physician after participant enrolment. Study authors do not report whether envelopes were sealed or opaque |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding of physician; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics are comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 115 Inclusion criteria: penetrating or blunt trauma; requiring > 3 L of volume resuscitation; 18‐60 years of age Exlusion criteria: fluid overload pulmonary oedema; known allergy to HES; known pre‐existing renal failure with oliguria or anuria; receiving dialysis treatment before the injury; severe hypernatraemia or hyperchloraemia on admission; severe head injury from which recovery was unlikely; severe intracranial bleeding; severe crush injury; arterial pressure unresponsive to 2 L IV fluid loading which could not be recorded; clinically obvious cardiac tamponade; neurogenic shock (high spinal cord injury); known AIDS or AIDS‐related complex; admitted > 6 h after injury; people who had already received any colloid before randomisation; taking part in another clinical trial at the same time; refused consent Participant condition: penetrating or blunt trauma Baseline characteristics Colloids group (penetrating trauma HES)
Crystalloids group (penetrating trauma saline)
Colloids group (blunt trauma HES)
Crystalloids group (blunt trauma saline)
Country: South Africa Setting: hospital, level 1 trauma centre | |
| Interventions | Colloids group (penetrating trauma HES + blunt trauma HES)
Crystalloids group (penetrating trauma saline + blunt trauma saline)
| |
| Outcomes | Outcomes measured/reported: volumes of study fluid in first 24 h; number of participants achieving normal gastrointestinal function by day 5; mortality; serious adverse events; acute renal injury; dialysis; use of blood products; biochemical abnormalities; days in ICU; days on ventilator support, SOFA scores, TEG measurements, skin itching Outcomes relevant to the review: mortality (time point unknown), dialysis | |
| Notes | Funding/declarations of interest: funding from Fresenius‐Kabi, who also supplied study fluids. Funders had no input into study design, analysis, interpretation etc. Also funds from TEG and laboratory investigations derived from Dept of Anaesthesia, UCT, research funds Study dates: not reported Study authors stratified data according to whether participants had penetrating or blunt trauma injuries. We have combined both types of injuries in analysis Note: we used mortality data reported in the previous version of this review (Perel 2013). These data were collected from personal communication with the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random numbers in blocks of 8 for each category of trauma |
| Allocation concealment (selection bias) | Low risk | Fluids prepacked by pharmacy, and we have assumed that, therefore, allocation was concealed from personnel |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Study fluids were presented in identical black bags |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Low risk | Study fluids were presented in identical black bags |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Few losses, and reasons were reported by study authors |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trials registration (ISRCTN 42061860); so not feasible to assess risk of selective reporting bias from these documents |
| Baseline characteristics | Unclear risk | Injury severity scores were higher in the colloids group. We could not be certain whether this could influence outcome data |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 19 Inclusion criteria: 20%‐98% TBSA; selected when, within 15 min, precise time of burn injury and intake and output experienced by patient from time of injury to time of admission was known Exclusion criteria: no details Participant condition: burns Baseline characteristics Colloids group
Crystalloids group (RL)
Crystalloids group (HS)
Country: USA Setting: hospital | |
| Interventions | Colloids group
Crystalloids group (RL)
Crystalloids group (HS)
| |
| Outcomes | Outcomes measured/reported: fluid volume; clinical results; laboratory results; urine variables (including renal failure); serum osmolality; sodium and potassium levels; cardiorespiratory and haemodynamic variables Outcomes relevant to the review: mortality (time point not reported) | |
| Notes | Funding/declarations of interest: supported in part by National Institutes of Health Grant Study dates: January 1977‐March 1978 In the previous version of the review (Perel 2013), the study ID was Jelenko 1978 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Described as randomised. No additional details but significant details in baseline demographics which would suggest an insufficient method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details; lack of blinding unlikely to influence data for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details; lack of blinding unlikely to influence data for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | High risk | Statistically significant differences between groups for baseline characteristics |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 84 Inclusion criteria: 18‐85 years of age; meet criteria for septic shock; resuscitation within 6 h with crystalloid or HES ≥ 30 mL/kg; within 24 h no packed red blood cells, plasma or other blood products that would affect coagulation and fibrinolysis significantly; no unauthorised drugs; no previous coagulation disorders Exclusion criteria: severe heart failure; bleeding occurring during resuscitation and requiring the use of blood products; serious renal insufficiency Participant condition: septic shock Baseline characteristics Colloids group
Crystalloids group
Country: China Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: prothrombin time, tissue factor, tissue factor pathway inhibitor, active protein C, LoS in ICU, mortality Outcomes relevant to the review: mortality (time point unknown) | |
| Notes | Funding/declarations of interest: none reported Study dates: November 2009‐October 2014 Article in Chinese. Data for study characteristics taken from English abstract, and from study report tables, with translation using Google Translate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised. Data for 'Risk of bias' assessment taken from English abstract only |
| Allocation concealment (selection bias) | Unclear risk | No details. Data for 'Risk of bias' assessment taken from English abstract only |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Data for 'Risk of bias' assessment taken from English abstract only. No details of clinical trials registration in English abstract |
| Baseline characteristics | Low risk | Baseline characteristics appeared largely comparable |
| Other bias | Unclear risk | We could not be certain of other risks of bias because 'Risk of bias' assessments were made from the English abstract only |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 105 Inclusion criteria: perforation peritonitis; 18‐60 years of age Exclusion criteria: pregnancy; known allergies or manifesting symptoms of possible anaphylaxis with test dose of HES; major coagulation disorders; renal failure because of medical renal disease; severe hepatic insufficiency; congestive cardiac failure at admission; traumatic perforation cases; < 18 years of age or > 60 years of age; people who had been resuscitated before reaching emergency surgical unit; denied consent Participant condition: perforation peritonitis Baseline characteristics Colloids group
Crystalloids group
Country: India Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: time to achieve goals of fluid resuscitation, morbidity, mortality, length of hospital stay, complications attributable to type of fluid administration Outcomes relevant to the review: mortality (up to 30 days from hospital discharge) | |
| Notes | Funding/declarations of interest: no funding and no conflicts of interest Study dates: October 2006‐April 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised with the help of computer‐generated random table" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Quote: "Administered the fluid therapy according to randomisation without knowledge of the observer" |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Unclear risk | We noted differences in physiological scores between groups. We could not be certain whether this difference could influence the outcome data |
| Other bias | High risk | Note the length of time since completion of trial, and publication of full study report. Also, note that the study was reported by a single author |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 60 Inclusion criteria: not reported in abstract Exclusion criteria: not reported in abstract Participant condition: patients with septic shock Baseline characteristics Colloids group (HES)
Colloids group (HES with HS)
Crystalloids group (NS)
Crystalloids group (HS)
Country: China Setting: hospital | |
| Interventions | Colloids group (HES)
Colloids group (HES with HS)
Crystalloids group (NS)
Crystalloids group (HS)
| |
| Outcomes | Outcomes measured/reported: haemodynamic parameters, blood lactate clearance, mortality (at 28 days) Outcomes relevant to the review: mortality | |
| Notes | Funding/declarations of interest: not reported in abstract Study dates: not reported in abstract Article in Chinese. Data for study characteristics taken from English abstract, and from study report tables, with translation using Google Translate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Risk of bias' assessment made using English abstract only. Described as randomised, no additional detail |
| Allocation concealment (selection bias) | Unclear risk | No details. 'Risk of bias' assessment made using English abstract only |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details. 'Risk of bias' assessment made using English abstract only. However, lack of blinding unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details. 'Risk of bias' assessment made using English abstract only. However, lack of blinding unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias. 'Risk of bias' assessment made using English abstract only |
| Baseline characteristics | Low risk | Baseline characteristics appeared largely comparable |
| Other bias | Unclear risk | We could not be certain about other risks of bias because 'Risk of bias' assessment were made using English abstract only |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 141 Inclusion criteria: people undergoing laparotomy for acute abdominal trauma Exclusion criteria: associated chest injury Participant condition: laparotomy for acute abdominal trauma Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: hospital, trauma unit | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: red blood cell transfusions, urine output, mortality, ventilator support, pulmonary function test variables Outcomes relevant to the review: mortality (at 28 days); blood transfusion (0‐9 units) | |
| Notes | Funding/declarations of interest: supported by a grant form US Army Medical Research and Development Command Study dates: not reported Note: we edited the number of randomised participants in each group as reported in the previous version of this review (Perel 2013); we did not include participants who were excluded because of chest injury. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The use of cards in sealed envelopes is an appropriate method of randomisation but additional details are required. It is unclear why there was a difference in participant numbers between groups once those with chest injuries were excluded. The study author provided an explanation following the discussion but it is possible that the study was not truly randomised |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | Study authors reported exclusion of 30 participants because of chest injury, and the reported results are for the remaining 141 participants. We have assumed that these 30 participants were not 'lost' but were excluded because of prespecified exclusion criteria, We noted missing data in the baseline characteristics for 4 participants; this loss was not explained, but we did not expect it to influence outcome data |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or a prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 42 Inclusion criteria: septic shock; admitted to ICU Exclusion criteria: no details Participant condition: septic shock Baseline characteristics Colloids group
Crystalloids group
Country: China Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: prothrombin time, activated partial thromboplastin time, plasma tissue plasminogen activator, plasminogen activator inhibitor, length of ICU stay, mortality, fluid volume, vasoactive drugs Outcomes relevant to the review: mortality (time point not reported) | |
| Notes | Funding/declarations of interest: not reported in abstract Study dates: September 2009‐June 2011 Article in Chinese. Data for study characteristics taken from English abstract, and from study report tables, with translation using Google Translate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details. 'Risk of bias' assessment made using English abstract only |
| Allocation concealment (selection bias) | Unclear risk | No details. 'Risk of bias' assessment made using English abstract only |
| Blinding of participants and personnel (performance bias): mortality | Low risk | 'Risk of bias' assessment made using English abstract only. No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | 'Risk of bias' assessment made using English abstract only. No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or a prepublished protocol; not feasible to assess risk of selective outcome reporting bias. 'Risk of bias' assessment made using English abstract only |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Unclear risk | We could not be certain about other risks of bias because 'Risk of bias' assessment made using English abstract only |
| Methods | Quasi‐RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 52 Inclusion criteria: serious injuries requiring multiple transfusions Exclusion criteria: no details Participant condition: hypovolaemic shock Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: fluid volumes ‐ input and output, protein variables, serum protein variables Outcomes relevant to the review: mortality (time point not reported) | |
| Notes | Funding/declarations of interest: supported by the Detroit General Hospital Research Corporation Study dates: November 1975‐February 1977 Note: We found a discrepancy between the study reports for Lucas 1978. A later published report (Lucas 1980) covers a longer time period, with a larger number of randomised participants. Lucas 1980 reports 5 deaths (3 in the albumin group and 2 in the crystalloid group). The earlier report, Lucas 1978, is for fewer participants and reports 7 deaths in the albumin group, and no deaths in the crystalloid group. We have used data from the earlier report because this was used in the previous published version of the review (Perel 2013). We assessed this decision in sensitivity analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation decision was based on last digit of each participant's case number |
| Allocation concealment (selection bias) | High risk | Randomisation decision was based on last digit of each participant's case number |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | High risk | We were concerned by differences in the reported number of deaths in the associated publications for this study |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 56 Inclusion criteria: febrile neutropenic patients with severe sepsis and septic shock Exclusion criteria: no details Participant condition: severe sepsis; septic shock Baseline characteristics Colloids group
Crystalloids group
Country: Saudi Arabia Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: acute renal failure, need for RRT, 28‐day mortality Outcomes relevant to the review: mortality (at 28 days), RRT | |
| Notes | Funding/declarations of interest: none reported Study dates: not reported Abstract only. We did not include mortality data from this report, which were reported as percentages; we could not be certain whether the data were for all randomised participants or whether some participant data were lost (crystalloid group: 63.4%; colloid group: 73.3%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, no additional details. Abstract only |
| Allocation concealment (selection bias) | Unclear risk | No details. Abstract only |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Abstract only. However, lack of blinding unlikely to introduce bias for mortality |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details. Abstract only |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Abstract only. However, lack of blinding unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details. Abstract only |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only. We could not be certain whether this study had participant losses for mortality because of apparent discrepancies in reported data in the abstract |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Unclear risk | Not possible to assess baseline characteristics from abstract |
| Other bias | Unclear risk | Not feasible to assess other risks of bias from abstract only |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 117 Inclusion criteria: children with clinical feature of severe malaria; Plasmodium falciparum parasitaemia; metabolic acidosis with base deficit of > 8 mmol/L; haemoglobin concentration of > 50 g/L Exclusion criteria: pulmonary oedema; oedematous malnutrition; papilledema; parental refusal of consent Participant condition: severe malaria Baseline characteristics Colloids group
Crystalloids group
Country: Kenya Setting: hospital (paediatric high‐dependency unit) | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: percentage reduction in base deficit (8 h); requirement for rescue therapies; neurological sequelae; mortality Outcomes relevant to the review: mortality (time point not reported) | |
| Notes | Funding/declarations of interest: supported by a grant from the Wellcome Trust, and from senior fellowship funding Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no additional details |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed cards, but insufficient details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | Nine losses of 159 randomised participants. Losses because of early requirement of randomisation prior to complete diagnoses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Unclear risk | Baseline characteristics reported in moderate and severe acidosis groups. There were no significant clinical differences at the time of hospital admission, although among children in the severe acidosis group who received albumin, seizures, hypotension and hypoglycaemia were more common than among children assigned to the saline group. We could not be certain whether these differences would influence the data |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 2126 (2097 in group A; 29 in group B) Inclusion criteria: between 60 days and 12 years of age; severe febrile illness complicated by impaired consciousness or respiratory distress; impaired perfusion Exclusion criteria: severe malnutrition; gastroenteritis; non‐infectious causes of shock and conditions for which volume expansion is contraindicated Participant condition group A: severe febrile illness, without hypotension Participant condition group B: severe febrile illness with hypotension Baseline characteristics group A Colloids group
Crystalloids group
Baseline characteristics group B Colloids group
Crystalloids group
Country: Kenya, Tanzania, Uganda Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: mortality at 48 h, mortality at 4 weeks, neurologic sequelae at 4 and 24 weeks, episodes of hypertensive shock within 48 h, adverse events Outcomes relevant to the review: mortality (4 weeks) | |
| Notes | Funding/declarations of interest: supported by a grant from Medical Research Council UK; resuscitation fluids donated by Baxter Healthcare. Neither had involvement in study Study dates: January 2009‐January 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in permuted blocks of random sizes and was stratified according to clinical center" |
| Allocation concealment (selection bias) | Low risk | Quote: "Trial numbers were kept inside opaque, sealed envelopes, which were numbered consecutively and opened in numerical order by a study clinician" |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | Few losses, which are clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trials registration (ISRCTN69856593); not feasible to assess risk of selective outcome reporting |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 40 Inclusion criteria: American‐European Consensus Conference definition of ALI; serum protein level < 6.0 g/dL; ongoing nutritional support; mechanical ventilation ≥ 24 h Exclusion criteria: haemodynamic instability; renal disease; clinically documented cirrhosis; allergy to albumin or furosemide; < 18 years of age; pregnancy; serum sodium level > 155 mEq/L or potassium level < 2.5 mEq/L Participant condition: ALI; acute respiratory distress syndrome Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: need for mechanical ventilation, shock, documented nosocomial infections, mortality Outcomes relevant to the review: mortality (30 days) | |
| Notes | Funding/declarations of interest: supported in part by the National Institutes of Health and Bayer Healthcare, Inc. (provision of study drug and an unrestricted grant) Study dates: February 1999‐December 2002 Study also included study of furosemide, given in each group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated four‐subject‐block randomization list held by the investigational pharmacy at each hospital" |
| Allocation concealment (selection bias) | Low risk | Quote: "List held by the investigational pharmacy at each hospital, which was also responsible for study drug preparation, camouflaged, blinding, and dispensation" |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Quote: "Albumin study drug was concealed within a sterile plastic container and infused in opaque intravenous tubing to obscure visual detail" |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 100 Inclusion criteria: traumatic haemorrhagic shock Exclusion criteria: heart failure; people who received blood before study was completed; death; sensitivity to serum; transfer to operating room before study completed; hepatic insufficiency; respiratory failure; renal impairment; sepsis; severe anaemia; non‐haemorrhagic shock; history of sensitivity to intervention fluids; < 16 years of age Participant condition: traumatic haemorrhagic shock Baseline characteristics Colloids group
Crystalloids group
Country: Iran Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: base excess (using measures of arterial blood gas); shock index Outcomes relevant to the review: none (see note below) | |
| Notes | Funding/declarations of interest: none reported Study dates: not reported Note: study authors report, "Five subjects (10% in HES group (Voluven) and seven (14%) in NS group were excluded from the study due to death, blood transfusion, and transfer to the operating room and their info was not included in the final analysis". Number of participants was not reported for each outcome and we were unable to include these data in our analysis | |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 422 Inclusion criteria: ≥ 16 years of age; victim of penetrating or blunt trauma within last hour before randomisation; initial field SBP ≤ 90 mmHg Exclusion criteria: initial trauma score ≤ 2; revised trauma score ≤ 1; pregnancy; history of seizures; coagulopathy; liver or renal disease; application of medical anti‐shock trousers Participant condition: victims of penetrating or blunt trauma Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: out‐of‐hospital. Ambulance paramedic service | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: mortality, change in revised trauma score, complication (to include acute renal failure), fluid and urine output, laboratory variables, adverse events (allergic reaction) Outcomes relevant to the review: mortality (30 days; study authors report that most deaths were within 24 h), adverse events (allergic reaction) | |
| Notes | Funding/declarations of interest: supported by grant from Pharmacia AB, Sweden and Pharmacia, Inc., New Jersey Study dates: October 1987‐November 1988 Note: for mortality data we used data reported for participants that were analysed by study investigators (for 184 participants in colloids group, and 175 participants in the crystalloid group). In the previous version of the review (Perel 2013), review authors used total number randomised (211 in each group) for analysis of mortality data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No details of randomisation method, but completed externally. We have assumed low risk. Fluid bags labelled with consecutive numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence generated externally. Personnel involved in treatment of participants were unlikely to be aware of code |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Blinded. Use of identical, coded treatment bags |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Personnel blinded until end of study |
| Incomplete outcome data (attrition bias) | Low risk | High number of losses postrandomisation. 63 or 424 participants, reasons given were because of eligibility criteria, and being given < 250 mL of allocated fluid. Data reported as per‐protocol data. Study authors reported analysis was performed to compare ITT with per‐protocol, with no difference in results |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Unclear risk | Study authors did not report baseline characteristics |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 40 Inclusion criteria: early septic shock; hypotension; systemic inflammatory response syndrome; a suspected or confirmed infectious source Exclusion criteria: people who received > 500 mL of colloid (5% albumin or pentastarch) or 2000 mL of crystalloid fluid; other forms of shock (haemorrhagic, cardiogenic or obstructive shock); acute myocardial infarction or cardiogenic pulmonary oedema; von Willebrand's disease; previous severe reaction to HES; chronic renal failure requiring dialysis; immediate need for surgery; a contraindication to internal jugular or subclavian line insertion; projected life expectancy < 3 months; < 18 years of age; pregnant or lactating; previous ICU admission with septic shock during the present hospitalisation Participant condition: septic shock Baseline characteristics Colloids group
Crystalloids group
Country: Canada Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: feasibility measure, clinical events such as hospital, 28‐day and 90‐day mortality, ICU and hospital LoS, organ failure Outcomes relevant to the review: mortality (28 days); blood transfusion (any volume); RRT | |
| Notes | Funding/declarations of interest: lead author received unrestricted funds from Bristol Myers Squibb and Edwards Life Sciences to conduct trial. Also unrestricted funds from Abbott Laboratories Study dates: not reported Trial was terminated early because of lower than anticipated recruitment and the results from another similar trial (Brunkhorst 2008). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation using a computerised permuted four‐block randomisation scheme (generated by an independent bio‐statistician) |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the designated research pharmacist at each institution was aware of the treatment allocation for individual patients" |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Quote: "Study fluids were prepared and blinded ahead of time by the site research pharmacist" |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Low risk | Quote: "Study fluids were prepared and blinded ahead of time by the site research pharmacist" |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Only pharmacist aware of group allocation, therefore assume that outcome assessors were blinded |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Low risk | Only pharmacist aware of group allocation, therefore assume that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Data reported for all randomised participants. One participant was excluded post‐randomisation because of meeting exclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias. We noted that 90‐day mortality was listed as an outcome in the methods section of the published report but not included in the results |
| Baseline characteristics | Unclear risk | Baseline characteristics were similar between groups with the exception of the need for organ support at baseline. Fewer patients in the saline group (versus pentastarch group) were on a vasopressor at baseline. We could not be certain whether these differences would influence outcome data |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 50 Inclusion criteria: ≥ 18 years of age; suspected septic shock (refractory hypotension plus ≥ 2 criteria for systemic inflammatory response syndrome) Exclusion criteria: > 8 h passed from the first hypotensive episode; received > 250 mL of colloid fluid (albumin or HES); shock (e.g. haemorrhagic, obstructive, or cardiogenic); previous ICU admission with severe sepsis or septic shock during the current hospitalisation; burn or traumatic brain injury before the current hospitalisation; history of chronic liver disease; religious objection to use of albumin; known previous severe reaction to albumin; lack of commitment of the patient, family, or clinical team to full therapeutic management; pregnant; enrolled in another related interventional trial Participant condition: septic shock Overall baseline characteristics
Country: Canada Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: related to study feasibility; overall mortality (at 28 days) Outcomes relevant to the review: mortality (but number randomised to each group not reported and therefore no available data for the review) | |
| Notes | Funding/declarations of interest: supported by funding from Canadian Institute of Health Research and CSL Behring. Also partial funding from SAFE trial, and unlimited grant from Univerisity of Alberta Study dates: April 2009‐December 2009 Mortality was reported overall, but not by group; 12 out of 50 participants died. This was a feasibility pilot study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used randomisation lists but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Identical glass containers with opaque coverings were used to conceal study fluids from all participants and personnel |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Identical glass containers with opaque coverings were used to conceal study fluids from outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Few losses, which were reported and explained |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Unclear risk | Not reported for each group |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 46 Inclusion criteria: established pulmonary failure; intrapulmonary shunt > 20% and a roentgenogram of the chest demonstrating interstitial and intra‐alveolar oedema Exclusion criteria: no details Participant condition: severe pulmonary insufficiency Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: hospital, surgical ICU | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: colloid osmotic pressure, PCWP, cardiac index, stroke work, intrapulmonary shunt, fluid volume, mortality, length of ICU stay Outcomes relevant to the review: mortality (time point not reported, some deaths were within 48 h) | |
| Notes | Funding/declarations of interest: supported by ONR Contract (definition of ONR not provided in study report) Study dates: June 1978‐May 1979 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assigned by random number. No additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Quasi‐RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 31 Inclusion criteria: severe traumatic shock with a SBP < 70 mmHg Exclusion criteria: < 18 years of age; > 75 years of age; considered to be in a terminal stage; associated major cerebral, thoracic or abdominal injuries; long‐bone fractures requiring major primary anaesthetic and surgical intervention Participant condition: severe traumatic shock Baseline characteristics Colloids group
Crystalloids group
Country: Sweden Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: development of ARDS, complications to include mortality Outcomes relevant to the review: mortality (during study period) | |
| Notes | Funding/declarations of interest: supported by grants from Swedish National Defense Research Institute, Swedish Association against Heart and Chest Diseases, and the Laerdal Foundation Study dates: February 1980‐February 1983 Note: only one author for this study report. In previous version of the review (Perel 2013), the study ID was Modig 1983. Some discrepancies between reports of Modig 1983 and Modig 1986, however they appear to be reports of the same study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation based on even/uneven data of admission to emergency department |
| Allocation concealment (selection bias) | High risk | No concealment. No randomisation sequence |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias. |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 107 Inclusion criteria: ≥ 16 years of age; initial assessment of GCS ≤ 8; blunt traumatic mechanism of injury Exclusion criteria: known pregnancy; primary penetrating injury; vital signs absent before randomisation; previous IV therapy ≥ 50 mL; time interval between arrival at scene and IV access > 4 h; amputation above wrist or ankle; any burn (thermal, chemical, electrical, radiation); suspected environmental hypothermia; asphyxia (strangulation, hanging, choking, suffocation, drowning); fall from height ≤ 1 m or ≤ 5 stairs Participant condition: blunt trauma Baseline characteristics Colloids group
Crystalloids group
Country: Canada Setting: out‐of‐hospital, paramedic service, air and land | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: survival at 30 days, 48‐h survival, cerebral performance at discharge, Functional Independence Measure, Disability Rating Scale, Glasgow Outcome Scale, Extended Glasgow Outcome Scale, neuropsychological assessments Outcomes relevant to the review: mortality (30 days) | |
| Notes | Funding/declarations of interest: Defence Research and Development Canada (DRDC) and Biophausia Sweden provided the study fluid (RescueFlow) free of charge without obligation to the investigators for the duration of the trial Study dates: unclearly reported. Completion date December 2008 (from clinical trials registration documents). Study dates in an associated publication with a subset of participants were September 2004‐January 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used (from Morrison 2009 (see Morrison 2011) ‐ use of computer‐generated random table or block randomisation) |
| Allocation concealment (selection bias) | Low risk | Concealment with use of sealed opaque envelopes |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Personnel remained blinded until after opening of envelopes. Lack of blinding unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Lack of blinding unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study dates are not clearly reported. However, study appears to have retrospective clinical trials registration (NCT00878631), and publication of retrospective protocols. Not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | We noted a higher number of male participants in the crystalloid group, but we did not expect this to influence outcome data |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 7000 Inclusion criteria: requiring fluid resuscitation in the ICU; > 18 years of age Exclusion criteria: > 1000 mL HES before screening; impending or current dialysis‐dependent renal failure; evidence of intracranial haemorrhage on cranial computed tomography Participant condition: requiring fluid resuscitation in the ICU (to include trauma, sepsis, brain injury) Baseline characteristics Colloids group
Crystalloids group
Country: Australia and New Zealand Setting: ICU, 32 hospitals | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: all cause mortality (at 90 days, in the ICU, in hospital, and within 28 days); acute kidney injury (using RIFLE); need for RRT; new organ failure for cardiovascular; respiratory; coagulation; liver systems that were not present at baseline; duration of mechanical ventilation; adverse events (to include allergic reaction, itching, rashes), cause‐specific mortality; duration of ICU and hospital stay; rate of death in the ICU, hospital, and at 28 days Outcomes relevant to the review: mortality (within 28 days, within 90 days); need for RRT (dialysis); adverse events (to include allergic reaction, itching, rashes) | |
| Notes | Funding/declarations of interest: supported by a grant from the National Health and Medical Research Council of Australia, and by unrestricted grants from New South Wales Ministry of Health, and Fresenius Kabi (supplied study fluids and distributed them to sites). Funding agencies had no input into the design, conduct, data collection, statistical analysis, or writing of the manuscript Study dates: December 2009‐January 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used web‐based randomisation program |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | There are an inconsistent number of losses between flow chart and data tables. However, loss of participants is < 10% |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration (NCT00935168). Most outcomes (all review outcomes) were reported according to clinical trials registration |
| Baseline characteristics | Low risk | Baseline characteristics appeared balanced between groups |
| Other bias | High risk | 15% of participants in each group had HES before start of study; this may introduce bias in the crystalloid group |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 41 Inclusion criteria: adults with measurable SBP < 90 mmHg because of haemorrhage Exclusion criteria: no details Participant condition: haemorrhagic shock Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic parameters, arterial blood gases, blood product requirement (transfusion) respiratory measurements Outcomes relevant to the review: mortality (during study), blood transfusion (packed red blood cells) | |
| Notes | Funding/declarations of interest: supported by a grant from American Critical Care, McGaw Park, Illinois Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants described as randomised, but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics not reported. Study authors state "There was no difference between groups with regard to race, age, sex or weight" |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 222 Inclusion criteria: children from 1‐15 years of age; dengue haemorrhagic fever (grade III or IV); had not received any IV fluid therapy; with a parent or guardian who gave consent Exclusion criteria: severe haemorrhagic manifestations for whom transfusion seemed likely; children with chronic disorders Participant condition: DSS Baseline characteristics Colloids group (dextran 70)
Colloids group (gelatins)
Crystalloids group (RL)
Crystalloids group (NS)
Country: Vietnam Setting: ICU, paediatric hospital | |
| Interventions | Colloids group (dextran 70)
Colloids group (gelatins)
Crystalloids group (RL)
Crystalloids group (NS)
| |
| Outcomes | Outcomes measured/reported: initial pulse pressure recovery time, occurrence and timing of subsequent episodes of shock, drop in haematocrit and pulse rate after the first hour, total volume of dextran 70 required after first hour, mortality (time point not reported), adverse events (allergic reactions, severe epistaxis requiring blood transfusion) Outcomes relevant to the review: mortality (time point not reported), transfusion of blood products, adverse events (allergic reactions) | |
| Notes | Funding/declarations of interest: study drugs all supplied by manufacturer (B Braun) Study dates: September 1996‐September 1997 Note: study authors report that 222 children had dengue haemorrhagic fever that was grade III, and 8 children had dengue haemorrhagic fever that was grade IV. Because of the small number of grade IV children, the study authors decided to exclude these from the report. Therefore, analysis is for 222 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done externally in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Use of opaque envelopes containing only a treatment pack number |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Fluid solutions were in bottles covered in opaque black insulating tape to ensure blinding |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Low risk | Fluid solutions were in bottles covered in opaque black insulating tape to ensure blinding |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details; lack of blinding unlikely to influence outcome data |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Appear comparable |
| Other bias | High risk | 36.4% participants in the RL group also received dextran 70 after the first hour; 30.4% participants in the NS group also received dextran 70 after the first hour |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 31 Inclusion criteria: 25% or > TBSA burn with smoke inhalation, or > 40% TBSA burn if inhalation injury was not present Exclusion criteria: patients who had withdrawal of support without efforts of resuscitation; ≤ 16 years of age Participant condition: burns Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: fluid volumes; intra‐abdominal pressure; urine output; renal function; peak airway pressure; mortality Outcomes relevant to the review: mortality (time point not reported) | |
| Notes | Funding/declarations of interest: none reported Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used predetermined randomisation code which was maintained by primary investigator |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 29 Inclusion criteria: newly admitted to ICU; clinically suspected infection; fulfilled ≥ 2 criteria of systemic inflammatory response syndrome; presence of perfusion abnormalities Exclusion criteria: adjustment of catecholamine doses or aggressive volume resuscitation (fluid administration > 200 mL within 30 min) during 180‐min study period; coma after pulmonary cardiocerebral resuscitation; renal failure; hypernatraemia; pregnant Participant condition: severe sepsis Baseline characteristics Colloids group
Crystalloids group
Country: Brazil Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic parameters; PAOP; cardiac index; systemic vascular resistance; stroke volume; metabolic variables; mortality rate Outcomes relevant to the review: mortality (time point not reported) | |
| Notes | Funding/declarations of interest: supported by The Wellcome Trust Study dates: study was completed over 23 months, dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Unclear risk | We noted that participants in the colloids group were younger, with statistically significantly lower APACHE II scores. We could not be certain whether these differences would influence outcome data |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 110 Inclusion criteria: patients with cancer and septic shock Exclusion criteria: no details Participant condition: patients with cancer and septic shock Baseline characteristics Colloids group
Crystalloids group
Country: Brazil Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: mortality (30 days, 90 days, in the ICU), ICU and hospital LoS, daily SOFA scores, rates and duration of mechanical ventilation, renal replacement, need for vasopressor drugs, status performance, fluid balance Outcomes relevant to the review: mortality (30 days), RRT (outcome data not reported in the abstract) | |
| Notes | Funding/declarations of interest: none reported Study dates: start date not reported, recruitment up to November 2014 Available report is from an abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised. No additional details in abstract |
| Allocation concealment (selection bias) | Unclear risk | No details in abstract |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Described as double‐blind but no additional details. However, lack of blinding unlikely to influence outcome data for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Unclear risk | No details. Assume all participants were accounted for (although the percentage data for mortality, which did not give whole numbers, suggests some loss of participants) |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Unclear risk | Baseline characteristics not reported in abstract |
| Other bias | Unclear risk | Insufficient details in abstract to assess other sources of bias |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 800 Inclusion criteria: adults who needed fluid resuscitation in the ICU and who had fulfilled criteria for severe sepsis within the previous 24 h according to the SCCM/ACCP and where informed consent was obtainable either from the patient or by proxy (in Denmark, 2 physicians followed by delayed consent from next of kin and the patient’s general practitioner. In Iceland, Finland and Norway, next of kin) Exclusion criteria: < 18 years of age; previously randomised in the 6S trial; allergy towards HES or malic acid; treatment with > 1000 mL of any synthetic colloid within the last 24 h prior to randomisation; any form of RRT; acute burn injury > 10% TBSA; severe hyperkalaemia, pK > 6 mM; liver or kidney transplantation during current hospital admission; intracranial bleeding within current hospitalisation; enrolment into another ICU trial of drugs with potential action on circulation, renal function or coagulation; withdrawal of active therapy Participant condition: severe sepsis Baseline characteristics Colloids group
Crystalloids group
Country: Denmark, Norway, Finland and Iceland Setting: 26 ICUs | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic parameters; PAOP; cardiac index; systemic vascular resistance; stroke volume; metabolic variables; mortality (at 28 days, at 90 days); transfusion of blood products (packed red blood cells, FFP, platelets; at day 1, day 2, day 3, and cumulative); adverse events (allergic reactions) Outcomes relevant to the review: mortality (at 28 days, and at 90 days); RRT; transfusion of blood products (packed red blood cells at day 1); adverse events (allergic reactions) Note: in order to avoid double of counting of participants we only included transfusion of one type of blood products (red blood cells) and on the first day | |
| Notes | Funding/declarations of interest: Danish Research Council. Study fluids supplied free of charge by B Braun. Neither funders nor B Braun had influence on protocol, trial conduct, data analyses and reporting. Study dates: December 2009‐November 2011 Note: the previous version of this review used mortality data at 90 days (Perel 2013); in this review we have analysed mortality data at 28 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation concealment |
| Allocation concealment (selection bias) | Low risk | Centralised, blinded randomisation |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Used identical fluid bags, covered in black opaque plastic |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Low risk | Used identical fluid bags, covered in black opaque plastic |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Outcome assessors were blinded to treatment groups |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Low risk | Outcome assessors were blinded to treatment groups |
| Incomplete outcome data (attrition bias) | Low risk | Very few losses, which were explained in flow chart (804 participants randomised, but ITT data for only 798) |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration (NCT00962156). Generally all 90‐day outcomes listed in the protocol were well reported in the primary manuscript. Length of hospital stay was not reported in primary publication but was in the long‐term outcomes paper |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | High risk | Most participants in each group received other fluids (study authors listed other fluids as crystalloids, nutrition, water, fluid with medications, synthetic colloids, and albumin); because some participants received additional colloids in both groups, this may influence study results |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 308 Inclusion criteria: patients with cirrhosis and who had sepsis‐induced hypotension Exclusion criteria: no details Participant condition: cirrhosis and sepsis‐induced hypotension Baseline characteristics Colloids group
Crystalloids group
Country: India Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: MAP; HR; lactate; lactate clearance; urine output; survival at 1 week Outcomes relevant to the review: mortality (7 days) | |
| Notes | Funding/declarations of interest: none reported Study dates: not reported Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only with limited detail on randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration (NCT02462902). We do not know if this was prospectively registered; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Unclear risk | Insufficient information in abstract to assess risk of other bias |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 107 Inclusion criteria: adults with metastatic cancer whose standard treatment had failed and had expected survivals of > 3 months Exclusion criteria: no details Participant condition: vascular leak syndrome Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: volume of fluid; number of doses of interleukin‐2; weight gain; pulse; SBP; days in ICU; time to discharge; laboratory changes (haematocrit etc.); blood transfusion; mortality Outcomes relevant to the review: mortality (time point not reported); blood transfusion (any volume) | |
| Notes | Funding/declarations of interest: none reported Study dates: March 1990‐August 1990 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | High risk | Some participants did not complete the full course of treatment and reasons were explained. Outcome data for participants requiring blood transfusion were for all randomised participants, but data for mortality were for 76 participants (loss of 18 participants in colloid group, and loss of 13 participants in crystalloid group) |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Multicentre | |
| Participants | Total number of randomised participants: 20 Inclusion criteria: people fulfilling American‐European Consensus criteria for ALI (including ARDS) Exclusion criteria: no details Participant condition: ALI/ARDS Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: hospital ICU | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: fluid volume; total protein; thiols; antioxidant; iron‐binding anti‐oxidant protection; iron‐oxidising antioxidant protection; mortality Outcomes relevant to the review: mortality (28 days) | |
| Notes | Funding/declarations of interest: supported by grants from the Dunhill Medical Trust, British Lung Foundation, and the Plasma Protein Therapeutics Association Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Unclear risk | We noted that participants in the crystalloid group were younger. We could not be certain whether this might influence outcome data |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 26 Inclusion criteria: included if pretreatment determinations revealed: systolic intra‐arterial pressure of < 90 mmHg; CI < 2.2 L/min/m²; serum arterial lactate > 18 mg/dL; WP < 15 mmHg Exclusion criteria: < 18 years of age; considered to be in a terminal state; manifesting a significant coagulopathy Participant condition: septic or hypovolaemic shock Baseline characteristics Colloids group (HES)
Colloids group (albumin)
Crystalloids group
Country: USA Setting: hospital | |
| Interventions | Colloids group (HES)
Colloids group (albumin)
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic variables; respiratory data; survival (during study period and hospital stay) Outcomes relevant to the review: mortality (within 24 h) | |
| Notes | Funding/declarations of interest: supported by a grant from American Critical Care Study dates: October 1979‐June 1981 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | We noted a larger number of male participants in the colloids group. However, overall numbers of participants were few and we assumed that gender differences would not influence outcome data |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 20 Inclusion criteria: severe multiple trauma and shock; SBP < 90 mmHg Exclusion criteria: no details Participant condition: severe multiple trauma and shock Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: mortality; respiratory and haemodynamic variables Outcomes relevant to the review: mortality (during study period) | |
| Notes | Funding/declarations of interest: supported by grants from National Institute of General Medical Sciences Study dates: not reported Data in baseline characteristics only given for 8 participants in crystalloid group (3 had died because it was not possible to resuscitate them) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised using a sealed envelope technique. Insufficient details |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope containing fluid group. Insufficient details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | 3 participants were excluded from all analyses because of death. However, we have included these mortality data for this review |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 60 Inclusion criteria: 1 month‐12 years of age; septic shock Exclusion criteria: features of multiorgan failure such as disseminated intravascular coagulation with bleeding manifestation; jaundice; acute renal failure; adult respiratory distress syndrome; coma; < 1 month old; underlying immunodeficiency status such as leukaemia; lymphoma; long‐term immunosuppressive therapy Participant condition: paediatric septic shock Baseline characteristics Colloids group
Crystalloids group
Country: India Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: fluid volumes; haemodynamic stability; organ failure; acute respiratory distress syndrome; acute renal failure; mortality Outcomes relevant to the review: mortality (time point not reported) | |
| Notes | Funding/declarations of interest: none reported Study dates: March 1999‐April 2000 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Low risk | Random number generation kept in sealed envelopes by one investigator |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 48 Inclusion criteria: mechanically ventilated and critically ill people with clinical hypovolaemia and at risk for, or with, ALI/ARDS Exclusion criteria: > 78 years of age; pregnant; known anaphylactoid reaction to colloid fluids; life expectancy < 24 h Participant condition: clinical hypovolaemia Baseline characteristics Colloids group (HES)
Colloids group (albumin)
Colloids group (gelatin)
Crystalloids group
Country: the Netherlands Setting: hospital | |
| Interventions | Colloids group (HES)
Colloids group (albumin)
Colloids group (gelatin)
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic variables; respiratory variables; mortality Outcomes relevant to the review: mortality (until discharge from the ICU) | |
| Notes | Funding/declarations of interest: supported in part by B Braun Medical, Melsungen, Germany and the Netherlands Heart Foundation, The Hague Study dates: not reported Patients stratified into septic and non‐septic. We combined these groups. Use of online supplementary information for some data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed by pharmacist; no additional detail on methods used to generate codes |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes prepared by pharmacist |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre (assumed, but not reported by study authors) | |
| Participants | Total number of randomised participants: 47 Inclusion criteria: people attending the emergency department with ≤ SBP 90 mmHg Exclusion criteria: people who appeared to be < 18 years of age; pregnant women; known severe pre‐existing cardiac, hepatic, or renal disease Participant condition: SBP ≤ 80 mmHg Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: emergency department | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic variables; blood chemistry; mortality; adverse events (allergic reactions) Outcomes relevant to the review: mortality (28 days); adverse events (allergic reactions) | |
| Notes | Funding/declarations of interest: supported in part from the National Institutes of Health Study dates: April 1987‐May 1988 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Identical bottles used to conceal study fluids from participants and personnel |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration; not feasible to assess risk of selective reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias detected |
| Methods | RCT Parallel design Single centre (assumed, but not reported by study authors) | |
| Participants | Total number of randomised participants: 166 Inclusion criteria: trauma patients being transported to hospital by helicopter; SBP ≤ 100 mmHg; palpable peripheral pulse or a sinus complex on ECG; ≥ 18 years of age Exclusion criteria: women who appeared to be pregnant; chronically debilitated people with severe hepatic, renal, cardiac, or neurologic disease; peripheral oedema Participant condition: hypovolaemic Baseline characteristics Colloids group
Crystalloids group
Country: USA Setting: out‐of‐hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: survival (to hospital discharge, and in emergency department); haemodynamic parameters; HR; volume of fluid given; volume of surgical blood loss and blood replacement in first 24 h; intracranial bleed in those with head injury; survival in patients with head injury; complications; adverse events (allergic reactions) Outcomes relevant to the review: mortality; adverse events (allergic reactions) | |
| Notes | Funding/declarations of interest: supported in part by a grant from National Institutes of Health and by pharmacia. HSD provided by pharmaceutical company Study dates: June 1986‐February 1988 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number tables |
| Allocation concealment (selection bias) | Low risk | Bags were identical and placed in order by a code established by hospital pharmacy team to be used by helicopter paramedics |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Study solutions were prepared by pharmacist in identical 250 mL bags with codes determined by random number tables |
| Blinding of outcome assessment (detection bias): mortality | Low risk | All personnel involved in participant care were blinded to study groups for at least one month after participants were entered into trial |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | High risk | Study authors changed concentration of HSD during study period. It is unclear whether this could have influenced outcome data. 14 of the 83 participants in the crystalloids group and 15 of the 83 participants in the colloids group were given unspecified resuscitation before flight nurses arrived and this could influence study results |
| Methods | RCT Parallel design Single centre (assumed, but not reported by study authors) | |
| Participants | Total number of randomised participants: 258 Inclusion criteria: SBP < 90 mmHg Exclusion criteria: asystolic or undergoing cardiopulmonary resuscitation; lacked a sinus complex on ECG; appeared to be < 18 years of age; seen > 2 h from time of injury; pregnant; known to have a history of seizures or a bleeding disorder; appeared to have a pre‐existing hepatic cardiac, or renal disease, as indicated by ascites or peripheral oedema; injured as a result of a burn; BP > 90 mmHg by time that IV access was established; lacked IV access Participant condition: trauma Baseline characteristics Colloids group (HSD)
Crystalloids group (NS)
Crystalloids group (HS)
Country: USA Setting: out‐of‐hospital, ambulance service | |
| Interventions | Colloids group (HSD)
Crystalloids group (NS)
Crystalloids group (HS)
| |
| Outcomes | Outcomes measured/reported: BP response; mortality (at hospital discharge); survival compared with that predicted by norms from the MTOS Outcomes relevant to the review: mortality | |
| Notes | Funding/declarations of interest: supported by a grant from national Institutes of Health, and Kabi Pharmaceuticals, Inc. Study dates: September 1988‐July 1991 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Assignment made at pharmacy level, and fluid bag contents concealed from paramedic personnel |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Study fluids were prepared in identical bags, and personnel were blinded to group allocation |
| Blinding of outcome assessment (detection bias): mortality | Low risk | All investigators and personnel were blinded throughout the trial |
| Incomplete outcome data (attrition bias) | Low risk | Large number of exclusions post‐randomisation (36 participants) because these participants did not meet the eligibility. Acceptable loss of participants recruited in a trauma setting (minimal inclusion criteria but large exclusion criteria established once in hospital) |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre (assumed, but not reported by study authors) | |
| Participants | Total number of randomised participants: 194 Inclusion criteria: SBP < 90 mmHg Exclusion criteria: asystolic or undergoing cardiopulmonary resuscitation; lacked a sinus complex on ECG; appeared to be < 18 years of age; > 2 h from the time of injury; thought to be pregnant; known to have a history of seizures or a bleeding disorder; appeared to have pre‐existing hepatic, cardiac, or renal disease, as indicated by ascites or peripheral oedema; were injured as a result of a burn; or lacked IV access Participant condition: various Baseline characteristics Colloids group (HSD)
Colloids group (HSD 12% dextran)
Crystalloids group (RL)
Crystalloids group (HS)
Country: USA Setting: out‐of‐hospital | |
| Interventions | Colloids group (HSD)
Colloids group (HSD 12% dextran)
Crystalloids group (RL)
Crystalloids group (HS)
| |
| Outcomes | Outcomes measured/reported: mortality (until hospital discharge) Outcomes relevant to the review: mortality | |
| Notes | Funding/declarations of interest: supported in part by grant from Kabi‐Pharmacia Study dates: March 1990‐June 1991 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Bags were coded, and allocated sequentially to helicopters |
| Blinding of participants and personnel (performance bias): mortality | Low risk | All personnel were blinded. Used sealed bags with coded identification label |
| Blinding of outcome assessment (detection bias): mortality | Low risk | All investigators kept blinded throughout trial |
| Incomplete outcome data (attrition bias) | Low risk | High number of exclusions after study fluids administered because of late assessment of inclusion/exclusion criteria, but inevitable because of the out‐of‐hospital setting. No additional apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared largely comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 26 Inclusion criteria: adult acute burns admission with injury > 15% TBSA Exclusion criteria: < 16 years of age or > 80 years of age; burn > 80% TBSA; pregnant; transfer delay > 6 h from time of injury; history or biochemical evidence of renal impairment on admission; history or haematological evidence of a bleeding diathesis; failure to obtain consent Participant condition: burns > 15% TBSA Baseline characteristics Colloids group
Crystalloids group
Country: UK Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: fluid intake and balance; weight; urinary albumin; respiratory function; serum C‐reactive protein; mortality; RRT Outcomes relevant to the review: mortality (during hospital stay); RRT; blood transfusion (any volume) | |
| Notes | Funding/declarations of interest: none Study dates: May 2004‐May 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation in blocks of 10 participants |
| Allocation concealment (selection bias) | Unclear risk | Used sealed envelopes, but no additional details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Unclear risk | 3 participants excluded from crystalloid group because they were given colloid. Small study, so this represents a large percentage of losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 383 Inclusion criteria: 2‐15 years of age; presenting directly to the hospital with clinical DSS; parent or guardian provided consent Exclusion criteria: not reported Participant condition: DSS Baseline characteristics Colloids group (dextran)
Colloids group (HES)
Crystalloids group
Country: Vietnam Setting: paediatric ICU | |
| Interventions | Colloids group (dextran)
Colloids group (HES)
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: requirement for supplemental intervention with rescue colloid; time taken to achieve initial and sustained cardiovascular stability; pattern of change in haematocrit; days in hospital; adverse effects (including need for blood transfusion, rashes), mortality Outcomes relevant to the review: need for transfusion of a blood product; mortality (time point not reported); adverse events (rashes) | |
| Notes | Funding/declarations of interest: supported by the Wellcome Trust Study dates: August 1999‐March 2004 Note: this study included a separate arm comparing two colloids for participants with severe shock (pulse pressure, ≤ 10 mm Hg); we did not include these participants because colloids were not compared with a crystalloid | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated random numbers completed by independent research staff |
| Allocation concealment (selection bias) | Low risk | Allocation concealed through treatment packs of fluid prepared in advance, in cardboard containers, and only identifiable by a study number |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Treatment packs of fluid were prepared in advance, in cardboard containers, and only identifiable by a study number |
| Blinding of participants and personnel (performance bias): transfusion/renal replacement therapy/adverse events | Low risk | Treatment packs of fluid were prepared in advance, in cardboard containers, and only identifiable by a study number |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding for assessment of mortality; lack of blinding unlikely to influence data for this outcome |
| Blinding of outcome assessment (detection bias): transfusion/renal replacement therapy/adverse events | Low risk | Blinding reported for assessment of other outcomes, and we assumed that assessment of transfusion data was also blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | High risk | 31% participants in the crystalloid group were also given colloids and this may have influenced study results |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 34 Inclusion criteria: ≥ 16 years of age; MAP < 80 mmHg or SBP < 100 mmHg; impression of haemorrhagic or spinal shock Exclusion criteria: pregnant; history of congestive heart disease; intubated mechanically ventilated patients; refractory to initial fluid challenge Participant condition: hypovolaemic shock Baseline characteristics Colloids group
Crystalloids group
Country: Taiwan Setting: hospital, emergency department | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: haemodynamic variables; haemoglobin and haematocrit levels; survival rates Outcomes relevant to the review: mortality (time point not reported), also reported blood transfusion (although not by group) but these participants were excluded from the study | |
| Notes | Funding/declarations of interest: none reported Study dates: July 1997‐February 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants described as randomly allocated to groups, but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No losses of participants for reporting of mortality data |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | We noted some differences in gender balance between groups; we did not anticipate that these differences would influence outcome data |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 105 Inclusion criteria: > 18 years of age; admitted with haemorrhagic hypovolaemia (SBP < 80 mmHg) with a palpable pulse or positive ECG; not pregnant, and with a previous history of cardiac or metabolic diseases Exclusion criteria: no details Participant condition: hypovolaemic shock Baseline characteristics Colloids group
Crystalloids group (NS)
Crystalloids group (HS)
Country: Brazil Setting: hospital | |
| Interventions | Colloids group
Crystalloids group (NS)
Crystalloids group (HS)
| |
| Outcomes | Outcomes measured/reported: pulmonary complications; renal complications; cardiac complications; infectious complications; haemodynamic variables; mortality Outcomes relevant to the review: mortality (until hospital discharge) | |
| Notes | Funding/declarations of interest: supported by Laboratorios B Braun Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Solutions prepared in similar and unmarked bottles to ensure blinding |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 212 Inclusion criteria: people being treated for haemorrhagic hypovolaemia and requiring blood transfusion Exclusion criteria: < 16 years of age; pregnant; having cardiac or renal failure previous to their acute haemorrhagic episode; arriving with cardiac arrest Participant condition: hypovolaemia Baseline characteristics Colloids group
Crystalloids group
Country: Brazil Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: fluid volumes; survival at 24 h and 30 days; complications (renal failure, cardiac, pulmonary, infectious, and neurologic complications) Outcomes relevant to the review: mortality (30 days) | |
| Notes | Funding/declarations of interest: none reported Study dates: February 1991‐November 1992 Study ID was Younes 1994 in previous version of the review (Perel 2013) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | Fluids in "coded, externally identical vials". Quote: "Neither the investigators nor the ER team had any control or knowledge of the infused solution during the entire study period" |
| Blinding of outcome assessment (detection bias): mortality | Low risk | Quote: "Neither the investigators nor the ER team had any control or knowledge of the infused solution during the entire study period" |
| Incomplete outcome data (attrition bias) | Low risk | Four losses in HSD and 3 in NS group. Explanations for losses given. Few losses; unlikely to introduce significant risk of bias |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 23 Inclusion criteria: people with SBP < 90 mmHg; admitted to emergency department with no previous treatment Exclusion criteria: no details Participant condition: hypovolaemia Baseline characteristics Colloids group
Crystalloids group
Country: Brazil Setting: hospital | |
| Interventions | Colloids group
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: MAP; fluid volumes; transfusion (by volume); complications (not specified); survival (24 h) Outcomes relevant to the review: mortality (24 h) | |
| Notes | Funding/declarations of interest: none reported Study dates: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by closed envelopes. Insufficient details provided |
| Allocation concealment (selection bias) | Unclear risk | Used closed envelopes. Insufficient details provided |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details of blinding; unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 120 Inclusion criteria: 18‐60 years of age; diagnosed with severe acute pancreatitis Exclusion criteria: heart disease; severe renal and hepatic dysfunction; coagulation disturbances; allergy to HES or glutamine; manifestation for > 48 h, or received resuscitation from another hospital Participant condition: severe acute pancreatitis Baseline characteristics Colloids group (HES)
Colloids group (HES and glutamine)
Crystalloids group (NS)
Country: China Setting: hospital | |
| Interventions | Colloids group (HES)
Colloids group (HES and glutamine)
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: respiratory infection; abdominal infection; sepsis; abdominal haemorrhage; intra‐abdominal hypertension; abdominal compartment syndrome; renal failure; acute respiratory distress syndrome; multiple organ dysfunction syndrome; operation intervention; length of ICU and hospital stay; laboratory variables Outcomes relevant to the review: mortality (day 60) | |
| Notes | Funding/declarations of interest: supported by grants from National Science Foundation Committee of China, and Fundamental Research Funds of Central Universities of China Study dates: January 2007‐March 2010 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were described as randomly divided into group; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias): mortality | Low risk | No details; lack of blinding unlikely to influence data for this outcome |
| Blinding of outcome assessment (detection bias): mortality | Low risk | No details; lack of blinding unlikely to influence data for this outcome |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No details of clinical trials registration or prepublished protocol; not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appear comparable |
| Other bias | Low risk | No other sources of bias identified |
| Methods | RCT Parallel design Single centre | |
| Participants | Total number of randomised participants: 135 Inclusion criteria: people with severe sepsis Exclusion criteria: no details Participant condition: severe sepsis Baseline characteristics Colloids group (HES)
Colloids group (HES + HS)
Crystalloids group
Country: China Setting: ICU | |
| Interventions | Colloids group (HES)
Colloids group (HES + HS)
Crystalloids group
| |
| Outcomes | Outcomes measured/reported: MAP; oxygenation; arterial lactate; lactate clearance rate; APACHE I score; fluid infusion volume; urine output; MODS; mortality Outcomes relevant to the review: mortality (time point not reported) | |
| Notes | Funding/declarations of interest: none reported Study dates: not reported in English abstract Note: article in Chinese. Data for study characteristics taken from English abstract, and from study report tables, with translation using Google Translate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Risk of bias' assessment made using English abstract. No details provided |
| Allocation concealment (selection bias) | Unclear risk | 'Risk of bias' assessment made using English abstract. No details provided |
| Blinding of participants and personnel (performance bias): mortality | Low risk | 'Risk of bias' assessment made using English abstract. Lack of blinding unlikely to introduce bias for mortality |
| Blinding of outcome assessment (detection bias): mortality | Low risk | 'Risk of bias' assessment made using English abstract. Lack of blinding unlikely to introduce bias for mortality |
| Incomplete outcome data (attrition bias) | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | 'Risk of bias' assessment made using English abstract. Not feasible to assess risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics appeared comparable |
| Other bias | Unclear risk | We could not be certain of other risks of bias because 'Risk of bias' assessment made using English abstract only |
ACCP: American College of Chest Physicians
AFR: additional fluid rate
AIDS: Acquired Immune Deficiency Syndrome
AKIN: Acute Kidney Injury Network
ALI: acute lung injury
ANH: acute normovolaemic haemodilution
APACHE II: Acute Physiology and Chronic Health Evaluation II
ARDS: acute respiratory deficiency syndrome
ASA: American Society of Anaesthesiologists
ATLSG: Advanced Trauma Life Support Guidelines
BMI: body mass index
BP: blood pressure
bpm: beats per minute
BR: basal rate
CO: cardiac output
CPP: cerebral perfusion pressure
CSL: Central Science Laboratory
CVP: central venous pressure
Da: dalton(s)
DBP: diastolic blood pressure
DSS: Dengue Shock Syndrome
FFP: fresh frozen plasma
ECG: electrocardiogram
EMS: emergency medical services
ER: emergency room
GCS: Glasgow Coma Scale
GDT: goal‐directed therapy
h: hour(s)
HES: hydroxyethyl starch
HR: heart rate
HS: hypertonic saline
HSD: dextran solution with hypertonic saline
ICP: intracranial pressure
ICU: intensive care unit
IQR: interquartile range
ITT: intention‐to‐treat
IV: intravenous infusion
LoS: length of stay
MAP: mean arterial BP
M:F: male:female
MIU: major injuries unit
MMP‐9: matrix metalloproteinase‐9
MMSE: Mini‐Mental State Exam
MODS: Multiple Organ Dysfunction Syndrome
MPA: mega pascal(s)
MTOS: Major Trauma Outcome Study
NS: normal saline
NYHA: New York Heart Association classification
PAOP: pulmonary artery occlusion pressure
PCWP: pulmonary capillary wedge pressure
POCD: postoperative cognitive disorder
RCT: randomised control trial
RL: Ringer's lactate
RRT: renal replacement therapy
SAG M: saline‐adenine‐glucose‐mannitol
SAPS II: Simplified Acute Physiology Score II
SBP: systolic blood pressure
SCCM: Society of Critical Care Medicine
SD: standard deviation
SE: standard error
SEM: standard error of the mean
SOFA: Sequential Organ Failure Assessment
TBSA: total body surface area
TEG: thromboelastography
TFV: tidal flow volume
TIMP‐1: tissue inhibitor of metalloproteinases‐1
TRISS: Trauma Injury Severity Score
WHO: World Health Organization
WP: wedge pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for major abdominal aortic surgery | |
| Included in previous version of review (Perel 2013). Excluded because study was not an RCT | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for abdominal aortic surgery | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for middle ear surgery | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for hip replacement | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for knee replacement | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of the review (Perel 2013). Does not appear to be an RCT, and associated reference does not include crystalloid group; therefore, we have excluded this study from the review | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for cytoreductive surgery | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for major abdominal surgery | |
| Included in previous version of review (Perel 2013); hypoalbuminaemia after major surgery. Study ID was Woittiez 1997 in previous version of the review | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for general, gynaecological, orthopaedic, or urological procedures | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for abdominal aortic surgery | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for modified Whipple's operation | |
| Abstract only. Included in previous version of review (Perel 2013). Protocol for a study that has not been published. We have excluded this study because we no longer expect that results for this study will be published | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for aortic reconstruction surgery | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for abdominal reconstructive surgery | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for abdominal aortic surgery | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective cardiac surgical patients | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for abdominal surgery | |
| Included in previous version of review (Perel 2013). Excluded because participants were elective surgical patients scheduled for abdominal aortic surgery |
RCT: randomised control trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT Parallel design |
| Participants | Number of randomised participants: no details Inclusion criteria: no details Exclusion criteria: no details Participant condition: no details Country: Russia Setting: no details |
| Interventions | Colloids group 1 Details: 6% HES 200/0.5 Colloids group 2 Details: 6% HES 130/0.4 Crystalloids group Details: NS |
| Outcomes | Outcomes measured/reported: no details Outcomes relevant to the review: no details |
| Notes | Funding/declarations of interest: no details Study dates: no details Study report requires translation from Russian to assess eligibility |
| Methods | RCT Parallel design Multicentre |
| Participants | Number of randomised participants: 798 Inclusion criteria: informed consent; any patient with septic shock 6 h after catecholamine introduction Exclusion criteria: overweight; previous severe heart failure; neutropenia; cirrhosis and primary peritonitis and severe burns Participant condition: septic shock Country: France Setting: 29 hospitals |
| Interventions | Colloids group Details: 20% albumin; 100 mL Crystalloids group Details: 0.9% NaCl; 100 mL |
| Outcomes | Outcomes measured/reported: all‐cause mortality (at day 28); SOFA score; LoS in ICU and in hospital Outcomes relevant to the review: mortality |
| Notes | Funding/declarations of interest: none reported Study dates: July 2006 to March 2010 Abstract only. Awaiting publication of the full text for more information to assess eligibility |
| Methods | RCT Parallel design |
| Participants | Number of randomised participants: no details Inclusion criteria: no details Exclusion criteria: no details Participant condition: severe sepsis and septic shock Country: no details Setting: no details |
| Interventions | Colloids group Details: 4% gelatin; 500 mL every 30 min Crystalloids group Details: 0.9% saline; 500 mL every 30 min |
| Outcomes | Outcomes measured/reported: haemodynamic variables Outcomes relevant to the review: no details |
| Notes | Funding/declarations of interest: no details Study dates: no details Report is from a conference abstract only. Awaiting publication of the full text for more information to assess eligibility |
| Methods | RCT Parallel design Multicentre |
| Participants | Estimated number of randomised participants: 50 Inclusion criteria: between 18 and 65 years of age; > 40 kg; onset of trauma ≤ 48 h prior to assessment; clinically judged to be in haemorrhagic shock by the attending surgeon; 2 or more of the following characteristics: penetrating or blunt etiology with haemodynamic instability at ER or intra‐operatively; ISS > 15; hypotension defined as either ≥ 10 mmHg change in SBP or MAP ≤ 65 mmHg or needing vasopressors (dopamine ≥ 5 µg/kg/min or norepinephrine at any dose) at the time of admission; hypoperfusion defined as base deficit ≥ 4 mmol/L Exclusion criteria: known severe congestive heart failure (EF ≤ 35%); chronic renal, liver or pancreatic disease; TB, COPD, asthma; coagulopathy or bleeding tendency; allergy to HES; participation in a clinical drug trial within the last 2 months; pregnancy or lactation; GCS < 9; advanced cancer (stage IV or metastatic disease); receiving immunosuppressive drugs; do‐not‐resuscitate status; advanced directives restricting implementation of the protocol; skeletal deformity, scarring, infection, gross contamination or previous surgery at the CVP insertion site; severe hypoxaemia if the CVP is to be inserted in the subclavian area; active gastrointestinal haemorrhage; concomitant drug poisoning Participant condition: trauma Country: Philippines Setting: 2 × medical centres |
| Interventions | Colloids group Details: tetrastarch (Voluven); goal directed volume therapy for severe trauma resuscitation Crystalloids group Details: crystalloid only; participants will receive crystalloid fluids only for volume therapy for severe trauma |
| Outcomes | Outcomes measured/reported: intra‐abdominal hypertension; abdominal compartment syndrome Outcomes relevant to the review: none |
| Notes | Funding/declarations of interest: sponsored by University of the Philippines and Fresenius Kabi Study dates: May 2009 to December 2009 Study described as completed in clinical trials record. Study results not posted. Awaiting publication of completed study to assess eligibility |
| Methods | RCT Parallel design Single centre |
| Participants | Estimated number of randomised participants: 360 Inclusion criteria: ≥ 18 years of age; severe sepsis or septic shock into 6 h of evolution; written informed consent Exclusion criteria: shock from other causes; adverse reactions to human albumin; previous fluid resuscitation during current disease; previous use of albumin in the last 72 h; religion objection; enrolment in another study; traumatic brain injury; hepatic cirrhosis; end stage renal disease; plasmapheresis; patients receiving end‐of‐life care Participant condition: severe sepsis and septic shock Country: Brazil Setting: medical centre |
| Interventions | Colloids group Details: albumin Crystalloids group Details: RL |
| Outcomes | Outcomes measured/reported: mortality (at 7 days); SOFA score; ICU LoS; hospital LoS; ventilator‐free days; need for RRT (at 28 days); days free of vasopressor; mortality (at 28 days) Outcomes relevant to the review: mortality; need of RRT |
| Notes | Funding/declarations of interest: sponsored by University of Sao Paulo Study dates: October 2013 to December 2017 Study described as completed in clinical trials record. Study results not posted. Awaiting publication of completed study to assess eligibility |
| Methods | RCT Parallel design Single centre |
| Participants | Estimated number of randomised participants: 96 Inclusion criteria: between 18 and 80 years of age; subarachnoid haemorrhage; Hunt‐Hess grade I to III Exclusion criteria: patients with Hunt‐Hess grade IV to V Participant condition: subarachnoid haemorrhage Country: Hungary Setting: medical centre |
| Interventions | Colloids group Details: 15 mL/kg RL and 15 to 50 mL/kg HES Crystalloids group Details: 15 mL/kg to 50 mL/kg RL |
| Outcomes | Outcomes measured/reported: incidence rate of vasospasm; 30‐day survival; neurological status; GOS scores Outcomes relevant to the review: mortality |
| Notes | Funding/declarations of interest: sponsored by University of Debrecen Study dates: February 2013 to October 2013 Study described as completed in clinical trials record. Study results not posted. Awaiting publication of completed study to assess eligibility |
| Methods | RCT Parallel design Multicentre |
| Participants | Number of randomised participants: no details Inclusion criteria: no details Exclusion criteria: no details Participant condition: severe sepsis Country: Russia Setting: no details |
| Interventions | Colloids group Details: no details Crystalloids group Details: no details |
| Outcomes | Outcomes measured/reported: correction of hypovolaemia, and stabilising haemodynamics Outcomes relevant to the review: no details |
| Notes | Funding/declarations of interest: no details Study dates: no details Study report requires translation from Russian to assess eligibility |
COPD: chronic obstructive pulmonary disease
CVP: central venous pressure
EF: ejection fraction
ER: emergency room
GCS: Glasgow Coma Scale
HES: hydroxyethyl starch
ISS: Injury Severity Score
MAP: mean arterial blood pressure
NS: normal saline
RCT: randomised control trial
RL: Ringer's lactate
RRT: renal replacement therapy
SBP: systolic blood pressure
SOFA: Sequential Organ Failure Assessment
TB: tuberculosis
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Impact of fluid resuscitation therapy on pulmonary edema as measured by alveolar fluid clearance in patients with acute respiratory distress syndrome (ARDS) |
| Methods | RCT Parallel design Single centre |
| Participants | Estimated number of randomised participants: 70 Inclusion criteria: ≥ 18 years of age; ICU patients under mechanical ventilation; within the first 24 h after onset of moderate or severe ARDS; hypovolaemia requiring fluid resuscitation therapy Exclusion criteria: pregnancy; < 18 years of age; refusal of the protocol; contraindications for the use of Voluven or RL; contraindications for femoral artery catheterisation or subclavian venous catheterisation Participant condition: ARDS; hypovolaemia; pulmonary oedema Country: France Setting: university hospital |
| Interventions | Colloids group Details: 4% albumin Crystalloids group Details: no details |
| Outcomes | Outcomes measured/reported: rate of alveolar fluid clearance; alveolar oedema fluid resorption; mortality (at 20 days) Outcomes relevant to the review: mortality |
| Starting date | December 2012 |
| Contact information | Patrick LACARIN; email: placarin@chu‐clermontferrand.fr |
| Notes | Funding/declarations of interest: sponsored by University Hospital, Clermont‐Ferrand |
| Trial name or title | Comparison of colloid (20% albumin) versus crystalloid (Plasmalyte) for fluid resuscitation in cirrhotics with sepsis induced hypotension |
| Methods | RCT Parallel design Single centre |
| Participants | Estimated number of randomised participants: 90 Inclusion criteria: between 18 and 75 years of age; cirrhosis with suspected or documented sepsis with MAP < 65 mm Hg Exclusion criteria: already received colloid or 2 L of fluid within the first 12 h of presentation; already on vasopressors and/or inotropes; spontaneous bacterial peritonitis and serum albumin less then 1.5 g/dL; structural heart disease; on maintenance haemodialysis; other causes of hypotension; pregnant or lactating women; in need of emergent surgical interventions; chronic obstructive lung disease and congestive heart failure; previous adverse reaction to human albumin solution Participant condition: cirrhosis with sepsis Country: India Setting: medical centre |
| Interventions | Colloids group Details: 20% albumin Crystalloids group Details: Plasmalyte |
| Outcomes | Outcomes measured/reported: reversal of hypotension; mortality (at 7 and 28 days); proportion of patients with new organ failures; duration of mechanical ventilation; requirement of RRT; length of ICU stay Outcomes relevant to the review: mortality; requirement of RRT |
| Starting date | March 31, 2016 |
| Contact information | Dr Abhinav Verma; email: [email protected] |
| Notes | Funding/declarations of interest: sponsored by Institute of Liver and Biliary Sciences, India |
| Trial name or title | A comparison of crystalloid alone versus crystalloid plus colloid in shock resuscitation |
| Methods | RCT Parallel design Single centre |
| Participants | Estimated number of randomised participants: 320 Inclusion criteria: ≥ 18 years of age; new onset of shock within 24 h; MAP < 65 mmHg or SBP < 60% of baseline BP; evidence of poor tissue perfusion including: urine output < 0.5 mL/kg/h, lactate > 2 mmol/L, alteration of consciousness without other explanation; evidence of fluid inadequacy (CVP < 12 mmHg, PCWP < 18 mmHg) or evidence of fluid responsive (IVC diameter variation > 15%, pulse pressure variation > 15%, positive fluid challenge test) Exclusion criteria: prolonged shock > 24 h; received colloid solution > 1000 mL in previous 72 h; do‐not‐resuscitate order; contraindication for fluid therapy including: suspected cardiogenic shock, evidence of pulmonary oedema, history of anaphylaxis after fluid therapy Participant condition: shock Country: Thailand Setting: hospital |
| Interventions | Colloids group Details: colloid solution resuscitation Crystalloids group Details: isotonic crystalloid solution resuscitation |
| Outcomes | Outcomes measured/reported: proportion of patients who had shock reversal; mortality (at 28 and 90 days); total fluid resuscitation within 24 h; need of RRT Outcomes relevant to the review: mortality; need of RRT |
| Starting date | September 2014 |
| Contact information | Surat Tongyoo, MD; email: [email protected]; Prapan Laophannarai, MD; email: [email protected] |
| Notes | Funding/declarations of interest: sponsored by Mahidol University |
ARDS: Acute Respiratory Distress Syndrome
CVP: central venous pressure
HES: hydroxyethyl starch
ICU: intensive care unit
IVC: inferior vena cava
MAP: mean arterial blood pressure
PCWP: pulmonary capillary wedge pressure
RCT: randomised control trial
RRT: renal replacement therapy
SBP: systolic blood pressure
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 24 | 11177 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
| Analysis 1.1  Comparison 1 Starches vs crystalloid, Outcome 1 Mortality at end of follow‐up. | ||||
| 2 Mortality within 90 days Show forest plot | 15 | 10415 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| Analysis 1.2  Comparison 1 Starches vs crystalloid, Outcome 2 Mortality within 90 days. | ||||
| 3 Mortality within 30 days Show forest plot | 11 | 10135 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.09] |
| Analysis 1.3  Comparison 1 Starches vs crystalloid, Outcome 3 Mortality within 30 days. | ||||
| 4 Transfusion of blood product Show forest plot | 8 | 1917 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.02, 1.39] |
| Analysis 1.4  Comparison 1 Starches vs crystalloid, Outcome 4 Transfusion of blood product. | ||||
| 5 Renal replacement therapy Show forest plot | 9 | 8527 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.14, 1.48] |
| Analysis 1.5  Comparison 1 Starches vs crystalloid, Outcome 5 Renal replacement therapy. | ||||
| 6 Adverse event: allergic reaction Show forest plot | 3 | 7757 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.27, 24.91] |
| Analysis 1.6  Comparison 1 Starches vs crystalloid, Outcome 6 Adverse event: allergic reaction. | ||||
| 7 Adverse event: itching Show forest plot | 2 | 6946 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.05, 1.82] |
| Analysis 1.7  Comparison 1 Starches vs crystalloid, Outcome 7 Adverse event: itching. | ||||
| 8 Adverse event: rash Show forest plot | 2 | 7007 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.90, 2.89] |
| Analysis 1.8  Comparison 1 Starches vs crystalloid, Outcome 8 Adverse event: rash. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 19 | 4736 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.11] |
| Analysis 2.1  Comparison 2 Dextrans vs crystalloid, Outcome 1 Mortality at end of follow‐up. | ||||
| 2 Mortality within 90 days and 30 days Show forest plot | 10 | 3353 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| Analysis 2.2  Comparison 2 Dextrans vs crystalloid, Outcome 2 Mortality within 90 days and 30 days. | ||||
| 3 Transfusion of blood products Show forest plot | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.10] |
| Analysis 2.3  Comparison 2 Dextrans vs crystalloid, Outcome 3 Transfusion of blood products. | ||||
| 4 Adverse events: allergic reaction Show forest plot | 4 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 6.0 [0.25, 144.93] |
| Analysis 2.4  Comparison 2 Dextrans vs crystalloid, Outcome 4 Adverse events: allergic reaction. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 6 | 1698 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| Analysis 3.1  Comparison 3 Gelatins vs crystalloid, Outcome 1 Mortality at end of follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 20 | 13047 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.06] |
| Analysis 4.1  Comparison 4 Albumin or FFP vs crystalloid, Outcome 1 Mortality at end of follow‐up. | ||||
| 2 Mortality within 90 days Show forest plot | 10 | 12492 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| Analysis 4.2  Comparison 4 Albumin or FFP vs crystalloid, Outcome 2 Mortality within 90 days. | ||||
| 3 Mortality within 30 days Show forest plot | 10 | 12506 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.06] |
| Analysis 4.3  Comparison 4 Albumin or FFP vs crystalloid, Outcome 3 Mortality within 30 days. | ||||
| 4 Transfusion of blood product Show forest plot | 3 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.95, 1.80] |
| Analysis 4.4  Comparison 4 Albumin or FFP vs crystalloid, Outcome 4 Transfusion of blood product. | ||||
| 5 Renal replacement therapy Show forest plot | 2 | 3028 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.96, 1.27] |
| Analysis 4.5  Comparison 4 Albumin or FFP vs crystalloid, Outcome 5 Renal replacement therapy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality at end of follow‐up Show forest plot | 16 | 4247 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.13] |
| Analysis 5.1  Comparison 5 Dextrans vs crystalloid: subgroup by tonicity of crystalloid, Outcome 1 All‐cause mortality at end of follow‐up. | ||||
| 1.1 colloid + hypertonic crystalloid vs isotonic crystalloid | 8 | 2845 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.13] |
| 1.2 colloid + isotonic crystalloid vs hypertonic crystalloid | 2 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.06] |
| 1.3 colloid + hypertonic crystalloid vs hypertonic crystalloid | 6 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.74, 1.41] |

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. We did not make judgements for studies that did not report outcomes of interest in the review, which are indicated by blank spaces

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. We did not make judgements for studies that did not report outcomes of interest in the review, which are indicated by blank spaces

Funnel plot of comparison 1. Starches vs crystalloid, outcome: 1.1 mortality at end of follow‐up

Funnel plot of comparison 2. Dextrans vs crystalloid, outcome: 2.1 mortality at end of follow‐up

Funnel plot of comparison 4. Albumin and FFP vs crystalloid, outcome: 4.1 mortality at end of follow‐up

Comparison 1 Starches vs crystalloid, Outcome 1 Mortality at end of follow‐up.

Comparison 1 Starches vs crystalloid, Outcome 2 Mortality within 90 days.

Comparison 1 Starches vs crystalloid, Outcome 3 Mortality within 30 days.

Comparison 1 Starches vs crystalloid, Outcome 4 Transfusion of blood product.
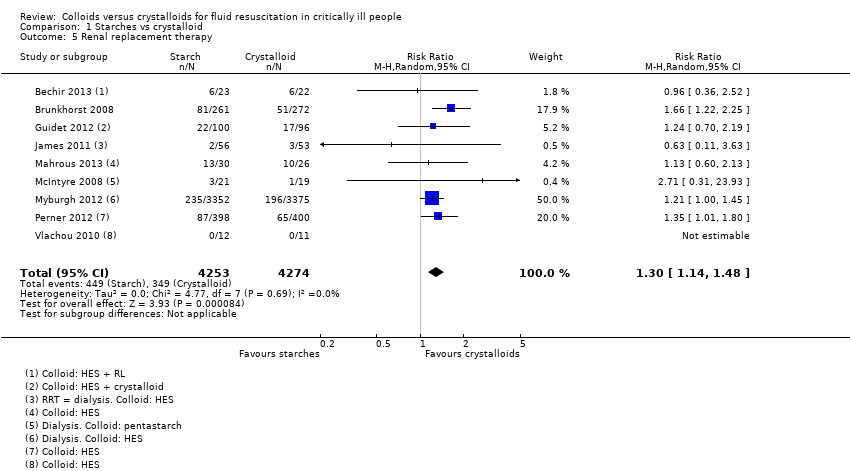
Comparison 1 Starches vs crystalloid, Outcome 5 Renal replacement therapy.

Comparison 1 Starches vs crystalloid, Outcome 6 Adverse event: allergic reaction.

Comparison 1 Starches vs crystalloid, Outcome 7 Adverse event: itching.

Comparison 1 Starches vs crystalloid, Outcome 8 Adverse event: rash.

Comparison 2 Dextrans vs crystalloid, Outcome 1 Mortality at end of follow‐up.

Comparison 2 Dextrans vs crystalloid, Outcome 2 Mortality within 90 days and 30 days.
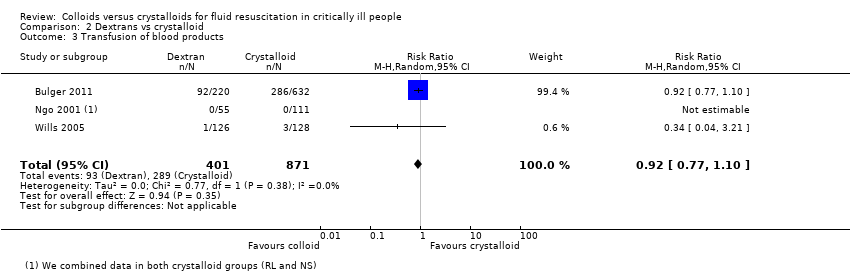
Comparison 2 Dextrans vs crystalloid, Outcome 3 Transfusion of blood products.

Comparison 2 Dextrans vs crystalloid, Outcome 4 Adverse events: allergic reaction.

Comparison 3 Gelatins vs crystalloid, Outcome 1 Mortality at end of follow‐up.

Comparison 4 Albumin or FFP vs crystalloid, Outcome 1 Mortality at end of follow‐up.

Comparison 4 Albumin or FFP vs crystalloid, Outcome 2 Mortality within 90 days.

Comparison 4 Albumin or FFP vs crystalloid, Outcome 3 Mortality within 30 days.
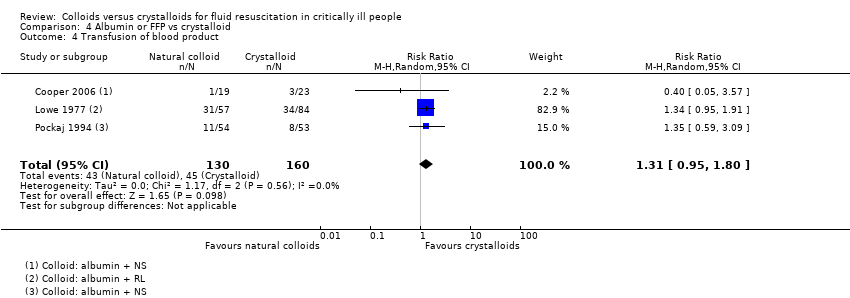
Comparison 4 Albumin or FFP vs crystalloid, Outcome 4 Transfusion of blood product.
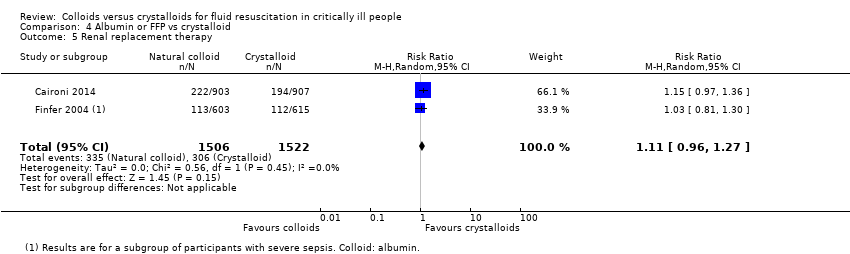
Comparison 4 Albumin or FFP vs crystalloid, Outcome 5 Renal replacement therapy.
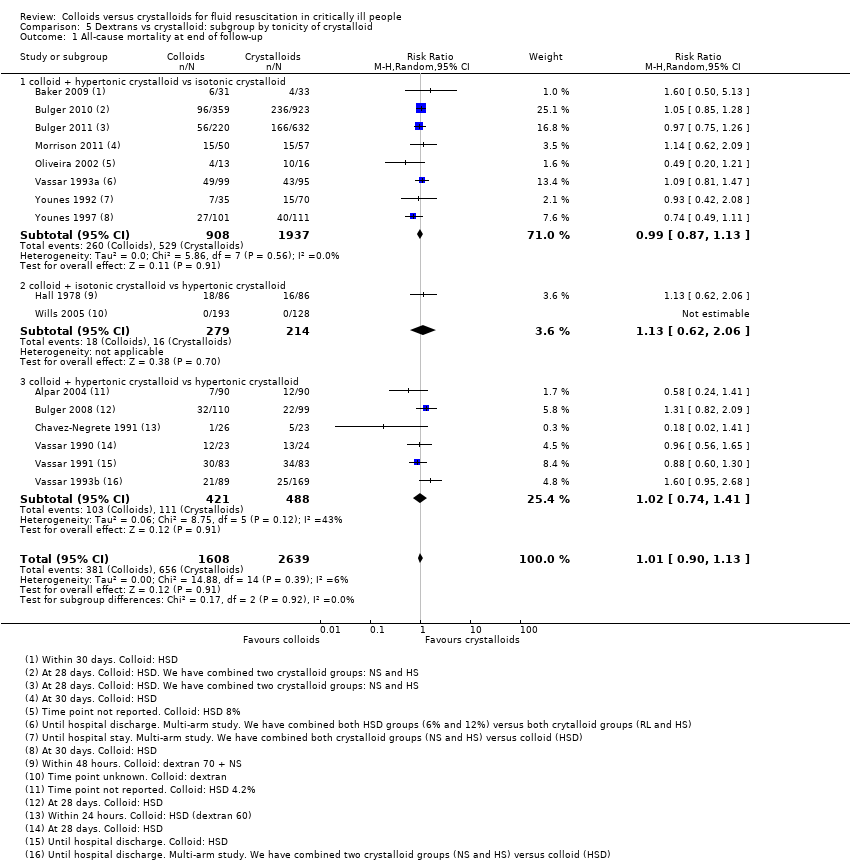
Comparison 5 Dextrans vs crystalloid: subgroup by tonicity of crystalloid, Outcome 1 All‐cause mortality at end of follow‐up.
| Starches compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with starches | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.97 | 11,177 | ⊕⊕⊕⊝ Moderatea | We excluded data from 1 study because we could not be certain whether it accounted for attrition | |
| 233 per 1000 | 226 per 1000 | |||||
| All‐cause mortality (at 90 days) | Study population | RR 1.01 | 10,415 | ⊕⊕⊕⊝ Moderateb | We excluded data from 1 study because we could not be certain whether it accounted for attrition | |
| 238 per 1000 | 241 per 1000 | |||||
| All‐cause mortality (within 30 days) | Study population | RR 0.99 | 10,135 | ⊕⊕⊕⊝ Moderateb | We excluded data from 1 study because we could not be certain whether it accounted for attrition | |
| 191 per 1000 | 189 per 1000 | |||||
| Transfusion of blood products | Study population | RR 1.19 | 1917 | ⊕⊕⊕⊝ Moderatea | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only starches; we noted little or no difference between groups in need for transfusion of blood products in this study | |
| 299 per 1000 | 356 per 1000 | |||||
| Renal replacement therapy | Study population | RR 1.30 | 8527 | ⊕⊕⊕⊝ Moderateb | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only starches; we noted little or no difference between groups in need for renal replacement therapy in this study | |
| 82 per 1000 | 106 per 1000 | |||||
| Adverse events | Allergic reaction | ⊕⊝⊝⊝ Very lowc | ||||
| Study population | RR 2.59 (0.27 to 24.91) | 7757 (3 studies) | ||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Itching | ||||||
| Study population | RR 1.38 (1.05 to 1.82) | 6946 (2 studies) | ||||
| 26 per 1000 | 35 per 1000 (27 to 46) | |||||
| Rashes | ||||||
| Study population | RR 1.61 (0.90 to 2.89) | 7007 (2 studies) | ||||
| 5 per 1000 | 9 per 1000 (5 to 15) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; some included studies had unclear risk of selection bias, one small study had a high risk of selection bias, and we were often unable to assess risk of selective reporting bias because many included studies did not have prospective clinical trials registration. | ||||||
| Dextrans compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with dextrans | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.99 | 4736 | ⊕⊕⊕⊝ Moderatea | ||
| 237 per 1000 | 235 per 1000 | |||||
| All‐cause mortality (within 90 days and within 30 days) | Study population | RR 0.99 | 3353 | ⊕⊕⊕⊝ Moderatea | ||
| 258 per 1000 | 256 per 1000 | |||||
| Transfusion of blood products | Study population | RR 0.92 (0.77 to 1.10) | 1272 (3 studies) | ⊕⊝⊝⊝ Very lowb | ||
| 332 per 1000 | 305 per 1000 (255 to 365) | |||||
| Renal replacement therapy | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse events | Allergic reactions | |||||
| Study population | RR 6.00 (0.25 to 144.93) | 739 (4 studies) | ⊕⊝⊝⊝ Very lowc | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Itching | ||||||
| Study population | Not measured | ‐ | ||||
| ‐ | ‐ | |||||
| Rashes | ||||||
| Study population | Not measured | ‐ | ||||
| ‐ | ‐ | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; some included studies had unclear risk of selection bias and we were often unable to assess risk of selective reporting bias because many included studies did not have prospective clinical trials registration. | ||||||
| Gelatins compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with gelatins | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.89 | 1698 | ⊕⊕⊝⊝ Lowa | ||
| 301 per 1000 | 268 per 1000 | |||||
| All‐cause mortality (within 90 days) | Study population | RR 0.89 (0.73 to 1.09) | 1388 (1 study) | ⊕⊕⊝⊝ Lowb | ||
| 334 per 1000 | 298 per 1000 (244 to 364) | |||||
| All‐cause mortality (within 30 days) | Study population | RR 0.92 (0.74 to 1.16) | 1388 (1 study) | ⊕⊕⊝⊝ Lowb | ||
| 266 per 1000 | 244 per 1000 (197 to 308) | |||||
| Transfusion of blood products | Study population | RR 5.89 (0.24 to 142.41) | 167 (1 study) | ⊕⊝⊝⊝ Very lowc | We calculated an effect estimate for one small study, with one event in the gelatin group. 1 study reported transfusion of blood products but data were not reported by group. 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only gelatins. We noted little or no difference between groups in need for transfusion of blood products | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Renal replacement therapy | ‐ | ‐ | ‐ | ‐ | ‐ | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only gelatins. We noted little or no difference between groups in need for renal replacement therapy |
| Adverse events | Allergic reaction | ⊕⊝⊝⊝ Very lowc | We calculated an effect estimate for one small study, with five incidences of allergic reactions in the gelatin group | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | RR 21.61 (1.22 to 384.05) | 167 (1 study) | |||
| Itching | ||||||
| ‐ | ‐ | ‐ | ||||
| Rashes | ||||||
| ‐ | ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; risk of selection bias was unclear in some studies, and because we were unable to assess risk of selective outcome reporting bias in some studies. We downgraded by one level for imprecision; evidence was from few studies, and we could not be certain of time points for data collection. | ||||||
| Albumin and fresh frozen plasma compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with albumin and FFP | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.98 | 13,047 | ⊕⊕⊕⊝ Moderatea | One study also reported mortality but not by group, and so could not be included in analysis | |
| 254 per 1000 | 249 per 1000 | |||||
| All‐cause mortality (within 90 days) | Study population | RR 0.98 | 12,492 | ⊕⊕⊕⊝ Moderatea | One study also reported mortality but not by group, and so could not be included in analysis | |
| 259 per 1000 | 254 per 1000 | |||||
| All‐cause mortality (within 30 days) | Study population | RR 0.99 | 12,506 | ⊕⊕⊕⊝ Moderatea | One study also reported mortality but not by group, and so could not be included in analysis | |
| 234 per 1000 | 231 per 1000 | |||||
| Transfusion of blood products | Study population | RR 1.31 | 290 | ⊕⊝⊝⊝ Very lowb | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only albumins or FFP; we noted little or no difference between groups in need for transfusion of blood products | |
| 281 per 1000 | 368 per 1000 | |||||
| Renal replacement therapy | 201 per 1000 | 223 per 1000 (193 to 255) | RR 1.11 (0.96 to 1.27) | 3028 (2 studies) | ⊕⊕⊝⊝ Lowc | One study stated that renal replacement data were measured but it was not reported in the study report (abstract) 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only albumin and FFP. We noted little or no difference between groups in need for renal replacement therapy |
| Adverse events | Allergic reactions | ⊕⊝⊝⊝ Very lowd | ||||
| Study population | RR 0.75 (0.17 to 3.33) | 2097 (1 study) | ||||
| 4 per 1000 | 3 per 1000 (1 to 13) | |||||
| Itching | ||||||
| ‐ | ‐ | ‐ | ‐ | |||
| Rashes | ||||||
| ‐ | ‐ | ‐ | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; some included studies had unclear risk of selection bias, and we were often unable to assess risk of selective reporting bias because many included studies did not have prospective clinical trials registration. | ||||||
| Participant condition | Study ID |
| Admission to an ICU with any condition (which included trauma, sepsis, ARDS, head injury) | |
| Trauma (includes studies of 'any trauma admissions', and head, chest, and abdominal injuries, and trauma with haemorrhagic or hypovolaemic shock) | Annane 2013*; Alpar 2004; Baker 2009; Bulger 2008; Bulger 2010; Bulger 2011; Evans 1996; Grba‐Bujevic 2012; James 2011; Lowe 1977; Lucas 1978; Masoumi 2016; Mattox 1991; Morrison 2011; Shah 1977; Vassar 1990; Vassar 1991; Vassar 1993a; Vassar 1993b; Wu 2001 |
| Sepsis or septic shock | Annane 2013*; Brunkhorst 2008; Caironi 2014; Dubin 2010; Ernest 1999; Guidet 2012; Jie 2015; Li 2008; Lu 2012; Mahrous 2013; McIntyre 2008; McIntyre 2012; Modig 1986; Oliveira 2002; Park 2015 (cancer with sepsis); Perner 2012; Rackow 1983*; Upadhyay 2005; Zhu 2011 |
| Hypovolaemia, hypovolaemic shock, haemorrhagic shock | Annane 2013*; Chavez‐Negrete 1991; Nagy 1993; Rackow 1983*; Van der Heijden 2009; Younes 1992; Younes 1997; Younes 1998 |
| Burns | Bechir 2013; Cooper 2006; Goodwin 1983; Hall 1978; Jelenko 1979; O'Mara 2005; Vlachou 2010 |
| ALI, ARDS | |
| Spontaneous subarachnoid haemorrhage | |
| Dengue shock syndrome | |
| Postcardiac arrest | |
| Perforation peritonitis | |
| Severe malaria | |
| Severe febrile illness | |
| Severe pulmonary insufficiency | |
| Vascular leak syndrome (cancer patients) | |
| Cirrhosis and septic induced hypotension | |
| Severe acute pancreatitis | |
| * included for more than one type of condition ALI: acute lung injury | |
| Study ID | Outcome | Events in colloid group: n/N | Events in crystalloid group: n/N | Effect estimate |
| Colloids (at the discretion of the clinician: HES, gelatins, or albumin) versus crystalloids | ||||
| Transfusion of blood products | 377/1414 | 358/1443 | RR 1.07, 95% CI 0.95 to 1.22; 2857 participants | |
| Renal replacement therapy | 156/1414 | 181/1443 | RR 0.88, 95% CI 0.72 to 1.08; 2857 participants | |
| Gelatin versus crystalloids | ||||
| Mortality (within 90 days) | 84/281 | 346/1035 | RR 0.89, 95% CI 0.73 to 1.09; 1388 participants | |
| Mortality (within 30 days) | 69/281 | 275/1035 | RR 0.92, 95% CI 0.74 to 1.16; 1388 participants | |
| Albumin versus crystalloid | ||||
| Adverse events: allergic reactions | 3/1050 | 4/1047 | RR 0.75, 95% CI 0.17 to 3.33; 2097 participants | |
| CI: confidence interval | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 24 | 11177 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
| 2 Mortality within 90 days Show forest plot | 15 | 10415 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| 3 Mortality within 30 days Show forest plot | 11 | 10135 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.09] |
| 4 Transfusion of blood product Show forest plot | 8 | 1917 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.02, 1.39] |
| 5 Renal replacement therapy Show forest plot | 9 | 8527 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.14, 1.48] |
| 6 Adverse event: allergic reaction Show forest plot | 3 | 7757 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.27, 24.91] |
| 7 Adverse event: itching Show forest plot | 2 | 6946 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.05, 1.82] |
| 8 Adverse event: rash Show forest plot | 2 | 7007 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.90, 2.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 19 | 4736 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.11] |
| 2 Mortality within 90 days and 30 days Show forest plot | 10 | 3353 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 3 Transfusion of blood products Show forest plot | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.10] |
| 4 Adverse events: allergic reaction Show forest plot | 4 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 6.0 [0.25, 144.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 6 | 1698 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 20 | 13047 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.06] |
| 2 Mortality within 90 days Show forest plot | 10 | 12492 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| 3 Mortality within 30 days Show forest plot | 10 | 12506 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.06] |
| 4 Transfusion of blood product Show forest plot | 3 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.95, 1.80] |
| 5 Renal replacement therapy Show forest plot | 2 | 3028 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.96, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality at end of follow‐up Show forest plot | 16 | 4247 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.13] |
| 1.1 colloid + hypertonic crystalloid vs isotonic crystalloid | 8 | 2845 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.13] |
| 1.2 colloid + isotonic crystalloid vs hypertonic crystalloid | 2 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.06] |
| 1.3 colloid + hypertonic crystalloid vs hypertonic crystalloid | 6 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.74, 1.41] |

