Coloides versus cristaloides para ressuscitação volêmica em pacientes graves
Resumo
Introdução
Pacientes graves podem perder líquidos devido a condições ou infecções graves (como sepse), traumas ou queimaduras, e precisam urgentemente de líquidos adicionais para prevenir a desidratação ou insuficiência renal. Soluções coloidais ou cristaloides podem ser usadas para este fim. Os cristaloides contêm moléculas pequenas, são baratos, fáceis de usar e proporcionam ressuscitação líquida imediata, porém podem aumentar o edema. Os coloides contêm moléculas maiores, são mais caros e podem proporcionar expansão mais rápida do volume no espaço intravascular. Porém, eles podem induzir reações alérgicas, distúrbios de coagulação e insuficiência renal. Esta é uma atualização de uma revisão Cochrane publicada pela última vez em 2013.
Objetivos
Avaliar os efeitos do uso de coloides versus cristaloides em pacientes críticos que necessitam de reposição volêmica sobre a mortalidade, a necessidade de transfusão de sangue ou de terapia de substituição renal (TSR), e a taxa de eventos adversos (reações alérgicas, prurido e erupções cutâneas).
Métodos de busca
Em 23 de fevereiro de 2018 fizemos buscas nas seguintes bases de dados: CENTRAL, MEDLINE, Embase e duas outras bases. Também fizemos buscas em plataformas de registros de ensaios clínicos.
Critério de seleção
Incluímos ensaios clínicos randomizados (ECRs) e quasi‐randomizados que avaliaram pacientes graves com necessidade de reposição de volume no hospital ou durante atendimento de emergência extra‐hospitalar. Os participantes dos estudos poderiam ter traumas, queimaduras ou patologias clínicas como sepse. Excluímos os estudos que recrutaram recém‐nascidos, pacientes submetidos a cirurgias eletivas e a cesarianas. Os estudos deveriam comparar o uso de um coloide (suspenso em qualquer solução cristaloide) versus o uso de uma solução cristaloide (isotônica ou hipertônica).
Coleta dos dados e análises
Dois revisores, trabalhando de forma independente, selecionaram os estudos a serem incluídos, fizeram a extração dos dados, avaliaram o risco de viés dos estudos e fizeram a síntese dos resultados. Usamos o GRADE para avaliar a qualidade da evidência.
Principais resultados
Incluímos 69 estudos (65 ECRs, 4 quasi‐ECRs) com 30.020 participantes. Vinte e oito estudos avaliaram soluções de amido, 20 dextranos, 7 gelatinas e 22 estudos avaliaram albumina ou plasma fresco congelado (PFC). Os diversos tipos de coloides foram comparados aos cristaloides.
Os participantes tinham várias condições típicas de doenças críticas. Dez estudos foram realizados em ambientes extra‐hospitalares. Alguns estudos tinham risco de viés de seleção. A maioria dos estudos tinha risco de viés de relato seletivo pois não registraram seus protocolos antes do início do estudo. Em 14 estudos, os participantes do grupo cristaloide também receberam ou podem ter recebido coloides. Isso pode ter influenciado os resultados.
Comparamos quatro tipos de coloides (amidos; dextranos; gelatinas; e albumina ou PFC) versus cristaloides.
Amidos versus cristaloides
Existe evidência de qualidade moderada de que provavelmente há pouca ou nenhuma diferença entre o uso de amidos versus cristaloides na mortalidade medida no final do período de acompanhamento (razão de risco (RR) 0,97, intervalo de confiança (IC) 95% 0,86 a 1,09; 11.177 participantes; 24 estudos); em 90 dias (RR 1,01, IC 95% 0,90 a 1,14; 10.415 participantes; 15 estudos); ou em 30 dias (RR 0,99, IC 95% 0,90 a 1,09; 10.135 participantes; 11 estudos).
Existe evidência de qualidade moderada de que os amidos provavelmente aumentam ligeiramente a necessidade de transfusão de sangue (RR 1,19, IC 95% 1,02 a 1,39; 1917 participantes; 8 estudos), e TSR (RR 1,30, IC 95% 1,14 a 1,48; 8527 participantes; 9 estudos). Existe evidência de qualidade muito baixa sobre eventos adversos. Isso significa que não temos certeza se há diferença no risco de eventos adversos entre os dois tipos de soluções. Parece haver pouca ou nenhuma diferença no risco de reações alérgicas (RR 2,59, IC 95% 0,27 a 24,91; 7757 participantes; 3 estudos), menor incidência de prurido com cristaloides (RR 1,38, IC 95% 1,05 a 1,82; 6946 participantes; 2 estudos), e menor incidência de erupção cutânea com cristaloides (RR 1,61, IC 95% 0,90 a 2,89; 7007 participantes; 2 estudos).
Dextranos versus cristaloides
Existe evidência de qualidade moderada de que provavelmente há pouca ou nenhuma diferença entre o uso de dextranos versus cristaloides na mortalidade ao final do período de acompanhamento (RR 0,99, IC 95% 0,88 a 1,11; 4736 participantes; 19 estudos), ou com 90 ou 30 dias (RR 0,99, IC 95% 0,87 a 1,12; 3353 participantes; 10 estudos). Existe incerteza se o uso de dextranos ou cristaloides reduz a necessidade de transfusão de sangue. Há pouca ou nenhuma diferença na necessidade de transfusões de sangue entre as intervenções (RR 0,92, IC 95% 0,77 a 1,10; 1272 participantes, 3 estudos; evidência de qualidade muito baixa). Há pouca ou nenhuma diferença na ocorrência de reações alérgicas (RR 6,00, 95% IC 0,25 a 144,93; 739 participantes; 4 estudos; evidência de qualidade muito baixa). Nenhum estudo avaliou a TSR.
Gelatinas versus cristaloides
Existe evidência de baixa qualidade de que pode haver pouca ou nenhuma diferença entre gelatinas versus cristaloides na mortalidade ao final do período de acompanhamento (RR 0,89, IC 95% 0,74 a 1,08; 1698 participantes; 6 estudos), em 90 dias (RR 0,89, IC 95% 0,73 a 1,09; 1388 participantes; 1 estudo), ou em 30 dias (RR 0,92, IC 95% 0,74 a 1,16; 1388 participantes; 1 estudo). A qualidade da evidência para transfusão de sangue foi muito baixa. Os dados foram provenientes de 3 estudos que tiveram poucos eventos ou que não relataram os dados por tipo de intervenção. Os dados para o TSR não foram reportados separadamente para o uso de gelatinas (1 estudo). Existe pouca ou nenhuma diferença entre os grupos em relação à ocorrência de reações alérgicas (evidência de qualidade muito baixa).
Albumina ou PFC versus cristaloides
Existe evidência de qualidade moderada de que provavelmente há pouca ou nenhuma diferença entre o uso de albumina ou PFC versus o uso de cristaloides na mortalidade ao final do acompanhamento (RR 0,98, IC 95% 0,92 a 1,06; 13.047 participantes; 20 estudos), em 90 dias (RR 0,98, IC 95% 0,92 a 1,04; 12.492 participantes; 10 estudos), ou em 30 dias (RR 0,99, IC 95% 0,93 a 1,06; 12.506 participantes; 10 estudos). Existe incerteza se um ou outro tipo de líquido reduz a necessidade de transfusão de sangue (RR 1,31, IC 95% 0,95 a 1,80; 290 participantes; 3 estudos; evidência de qualidade muito baixa). O uso de albumina ou PFC versus cristaloides pode fazer pouca ou nenhuma diferença na necessidade de TSR (RR 1,11, IC 95% 0,96 a 1,27; 3028 participantes; 2 estudos; evidência de qualidade muito baixa), ou na ocorrência de reações alérgicas (RR 0,75, IC 95% 0,17 a 3,33; 2097 participantes, 1 estudo; evidência de qualidade muito baixa).
Conclusão dos autores
O uso de amidos, dextranos, albumina ou PFC (evidência de qualidade moderada) ou gelatinas (evidência de baixa qualidade) versus cristaloides provavelmente faz pouca ou nenhuma diferença no risco de morte. O uso de amidos provavelmente aumenta ligeiramente a necessidade de transfusão de sangue e TSR (evidência de qualidade moderada), e o uso de albumina ou PFC faz pouca ou nenhuma diferença na necessidade de terapia de substituição renal (evidência de baixa qualidade). Existe incerteza quanto ao efeito do uso de dextranos e albumina ou PFC sobre a necessidade de transfusão sanguínea. Também existe incerteza sobre os eventos adversos. A qualidade da evidência pode melhorar na próxima atualização desta revisão com a inclusão dos resultados de três estudos em andamento e de sete estudos que aguardam classificação.
PICO
Resumo para leigos
Coloides ou cristaloides para reposição de líquidos em pessoas gravemente doentes
Introdução
Pessoas gravemente doentes podem perder grandes quantidades de sangue (devido a trauma ou queimaduras) ou apresentar problemas ou infecções graves (por exemplo, sepse) e necessitar urgentemente de líquidos adicionais para prevenir a desidratação ou falência renal. Os coloides e os cristaloides são tipos de líquidos usados para repor o volume que a pessoa perdeu. Geralmente eles são dados por via intravenosa (usando um tubo que injeta o líquido diretamente na corrente sanguínea).
Os cristaloides são soluções salinas de baixo custo (por exemplo, o soro fisiológico). Os cristaloides têm água e pequenas moléculas que podem se deslocar facilmente quando introduzidas no corpo.
Os coloides podem conter produtos criados em laboratório (por exemplo, amidos, dextranos ou gelatinas) ou substâncias naturais (por exemplo, albumina ou plasma fresco congelado‐ PFC). Os coloides têm água e moléculas maiores. Por isso, os coloides ficam mais tempo dentro das veias, antes de irem para outras partes do corpo. Os coloides são mais caros do que os cristaloides. Não sabemos se dar coloides seria melhor do que dar cristaloides para pessoas gravemente doentes que necessitam de reposição de líquidos. Não sabemos se os coloides seriam melhores para reduzir os riscos de a pessoa morrer, necessitar de transfusão de sangue, ou precisar de tratamento para substituir a função renal (filtragem do sangue, com ou sem máquinas de diálise, no caso de falência renal).
Características do estudo
Buscamos todas as evidências disponíveis até fevereiro de 2018. Nós pesquisamos a literatura médica e identificamos 69 estudos relevantes com 30.020 participantes gravemente doentes que receberam reposição de líquidos no hospital ou durante um atendimento de emergência fora do hospital. Esses estudos compararam o uso de coloides (amidos, dextranos, gelatinas, albumina ou PFC) versus cristaloides.
Resultados principais
Existe evidência de qualidade (certeza) moderada de que o uso de coloides (amidos; dextranos; ou albumina ou PFC), comparado ao uso de cristaloides, não muda ou muda pouco o risco de pessoas gravemente doentes morrerem dentro de 30 ou 90 dias, ou até o final do período de acompanhamento do estudo. Também existe evidência de baixa qualidade de que o uso de gelatinas, comparado ao uso de cristaloides, não muda ou muda pouco o número de mortes durante esses períodos.
Existe evidência de qualidade moderada de que o uso de amidos provavelmente aumenta um pouco a necessidade de transfusão de sangue. Existe evidência de qualidade muito baixa para a comparação entre os outros tipos de coloides versus cristaloides. Por isso, não temos certeza se o uso desses outros tipos de coloides modifica a necessidade de transfusão de sangue.
Existe evidência de qualidade moderada de que o uso de amidos para a reposição de líquidos provavelmente aumenta um pouco a necessidade de terapia de substituição renal. O uso de albumina ou PFC comparado aos cristaloides faz pouca ou nenhuma diferença na necessidade de terapia de substituição renal. Um estudo que comparou o uso de gelatinas não avaliou a necessidade de terapia de substituição renal entre os tipos de líquido administrados. Nenhum dos estudos que comparou o uso de dextranos avaliou a necessidade de terapia de substituição renal.
Poucos estudos avaliaram a ocorrência de eventos adversos (reações alérgicas, coceira ou manchas na pele). Consequentemente não temos certeza se um ou outro tipo de líquido causa menos eventos adversos (evidência de qualidade muito baixa). Existe pouca ou nenhuma diferença entre amidos versus cristaloides na ocorrência de reações alérgicas. Porém, no grupo que recebeu cristaloides houve menos pessoas com queixas de coceira ou manchas na pele. Existe pouca ou nenhuma diferença nas reações alérgicas associadas ao uso dos dextranos (quatro estudos), das gelatinas (um estudo) e da albumina ou do PFC (um estudo).
Certeza da evidência
Alguns autores não relataram claramente os métodos utilizados para a realização dos seus estudos. Muitos pesquisadores não registraram os estudos antes do seu início. Isso é um problema pois não podemos ter certeza se os pesquisadores decidiram quais seriam os desfechos avaliados antes ou depois deles terem visto os resultados. Houve também alguns estudos em que algumas pessoas que receberam cristaloides podem também ter recebido coloides, fato que pode afetar os resultados. Houve poucos estudos para alguns desfechos. Isso diminuiu nossa confiança na evidência.
Conclusões
Em pessoas gravemente doentes, existe pouca ou nenhuma diferença no risco de morte com o uso de coloides (amidos, dextranos, albumina, ou PFC) comparado ao uso de cristaloides para reposição de líquidos. Também existe pouca ou nenhuma diferença no risco de morte com o uso de gelatinas versus cristaloides para reposição de líquidos.
O uso de amidos provavelmente aumenta um pouco a necessidade de transfusão de sangue e de terapia de substituição renal. Usar albumina ou PFC pode fazer pouca ou nenhuma diferença na necessidade de terapia de substituição renal. Existe incerteza quanto ao efeito do uso de dextranos, albumina ou PFC versus cristaloides sobre a necessidade de transfusão de sangue. Também existe incerteza se os coloides ou cristaloides aumentam o número de eventos adversos. No futuro, os resultados de estudos em andamento podem aumentar a nossa confiança nas evidências.
Authors' conclusions
Summary of findings
| Starches compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with starches | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.97 | 11,177 | ⊕⊕⊕⊝ Moderatea | We excluded data from 1 study because we could not be certain whether it accounted for attrition | |
| 233 per 1000 | 226 per 1000 | |||||
| All‐cause mortality (at 90 days) | Study population | RR 1.01 | 10,415 | ⊕⊕⊕⊝ Moderateb | We excluded data from 1 study because we could not be certain whether it accounted for attrition | |
| 238 per 1000 | 241 per 1000 | |||||
| All‐cause mortality (within 30 days) | Study population | RR 0.99 | 10,135 | ⊕⊕⊕⊝ Moderateb | We excluded data from 1 study because we could not be certain whether it accounted for attrition | |
| 191 per 1000 | 189 per 1000 | |||||
| Transfusion of blood products | Study population | RR 1.19 | 1917 | ⊕⊕⊕⊝ Moderatea | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only starches; we noted little or no difference between groups in need for transfusion of blood products in this study | |
| 299 per 1000 | 356 per 1000 | |||||
| Renal replacement therapy | Study population | RR 1.30 | 8527 | ⊕⊕⊕⊝ Moderateb | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only starches; we noted little or no difference between groups in need for renal replacement therapy in this study | |
| 82 per 1000 | 106 per 1000 | |||||
| Adverse events | Allergic reaction | ⊕⊝⊝⊝ Very lowc | ||||
| Study population | RR 2.59 (0.27 to 24.91) | 7757 (3 studies) | ||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Itching | ||||||
| Study population | RR 1.38 (1.05 to 1.82) | 6946 (2 studies) | ||||
| 26 per 1000 | 35 per 1000 (27 to 46) | |||||
| Rashes | ||||||
| Study population | RR 1.61 (0.90 to 2.89) | 7007 (2 studies) | ||||
| 5 per 1000 | 9 per 1000 (5 to 15) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; some included studies had unclear risk of selection bias, one small study had a high risk of selection bias, and we were often unable to assess risk of selective reporting bias because many included studies did not have prospective clinical trials registration. | ||||||
| Dextrans compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with dextrans | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.99 | 4736 | ⊕⊕⊕⊝ Moderatea | ||
| 237 per 1000 | 235 per 1000 | |||||
| All‐cause mortality (within 90 days and within 30 days) | Study population | RR 0.99 | 3353 | ⊕⊕⊕⊝ Moderatea | ||
| 258 per 1000 | 256 per 1000 | |||||
| Transfusion of blood products | Study population | RR 0.92 (0.77 to 1.10) | 1272 (3 studies) | ⊕⊝⊝⊝ Very lowb | ||
| 332 per 1000 | 305 per 1000 (255 to 365) | |||||
| Renal replacement therapy | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse events | Allergic reactions | |||||
| Study population | RR 6.00 (0.25 to 144.93) | 739 (4 studies) | ⊕⊝⊝⊝ Very lowc | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Itching | ||||||
| Study population | Not measured | ‐ | ||||
| ‐ | ‐ | |||||
| Rashes | ||||||
| Study population | Not measured | ‐ | ||||
| ‐ | ‐ | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; some included studies had unclear risk of selection bias and we were often unable to assess risk of selective reporting bias because many included studies did not have prospective clinical trials registration. | ||||||
| Gelatins compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with gelatins | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.89 | 1698 | ⊕⊕⊝⊝ Lowa | ||
| 301 per 1000 | 268 per 1000 | |||||
| All‐cause mortality (within 90 days) | Study population | RR 0.89 (0.73 to 1.09) | 1388 (1 study) | ⊕⊕⊝⊝ Lowb | ||
| 334 per 1000 | 298 per 1000 (244 to 364) | |||||
| All‐cause mortality (within 30 days) | Study population | RR 0.92 (0.74 to 1.16) | 1388 (1 study) | ⊕⊕⊝⊝ Lowb | ||
| 266 per 1000 | 244 per 1000 (197 to 308) | |||||
| Transfusion of blood products | Study population | RR 5.89 (0.24 to 142.41) | 167 (1 study) | ⊕⊝⊝⊝ Very lowc | We calculated an effect estimate for one small study, with one event in the gelatin group. 1 study reported transfusion of blood products but data were not reported by group. 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only gelatins. We noted little or no difference between groups in need for transfusion of blood products | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Renal replacement therapy | ‐ | ‐ | ‐ | ‐ | ‐ | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only gelatins. We noted little or no difference between groups in need for renal replacement therapy |
| Adverse events | Allergic reaction | ⊕⊝⊝⊝ Very lowc | We calculated an effect estimate for one small study, with five incidences of allergic reactions in the gelatin group | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | RR 21.61 (1.22 to 384.05) | 167 (1 study) | |||
| Itching | ||||||
| ‐ | ‐ | ‐ | ||||
| Rashes | ||||||
| ‐ | ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; risk of selection bias was unclear in some studies, and because we were unable to assess risk of selective outcome reporting bias in some studies. We downgraded by one level for imprecision; evidence was from few studies, and we could not be certain of time points for data collection. | ||||||
| Albumin and fresh frozen plasma compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with albumin and FFP | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.98 | 13,047 | ⊕⊕⊕⊝ Moderatea | One study also reported mortality but not by group, and so could not be included in analysis | |
| 254 per 1000 | 249 per 1000 | |||||
| All‐cause mortality (within 90 days) | Study population | RR 0.98 | 12,492 | ⊕⊕⊕⊝ Moderatea | One study also reported mortality but not by group, and so could not be included in analysis | |
| 259 per 1000 | 254 per 1000 | |||||
| All‐cause mortality (within 30 days) | Study population | RR 0.99 | 12,506 | ⊕⊕⊕⊝ Moderatea | One study also reported mortality but not by group, and so could not be included in analysis | |
| 234 per 1000 | 231 per 1000 | |||||
| Transfusion of blood products | Study population | RR 1.31 | 290 | ⊕⊝⊝⊝ Very lowb | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only albumins or FFP; we noted little or no difference between groups in need for transfusion of blood products | |
| 281 per 1000 | 368 per 1000 | |||||
| Renal replacement therapy | 201 per 1000 | 223 per 1000 (193 to 255) | RR 1.11 (0.96 to 1.27) | 3028 (2 studies) | ⊕⊕⊝⊝ Lowc | One study stated that renal replacement data were measured but it was not reported in the study report (abstract) 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only albumin and FFP. We noted little or no difference between groups in need for renal replacement therapy |
| Adverse events | Allergic reactions | ⊕⊝⊝⊝ Very lowd | ||||
| Study population | RR 0.75 (0.17 to 3.33) | 2097 (1 study) | ||||
| 4 per 1000 | 3 per 1000 (1 to 13) | |||||
| Itching | ||||||
| ‐ | ‐ | ‐ | ‐ | |||
| Rashes | ||||||
| ‐ | ‐ | ‐ | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; some included studies had unclear risk of selection bias, and we were often unable to assess risk of selective reporting bias because many included studies did not have prospective clinical trials registration. | ||||||
Background
Description of the condition
Critically ill people may experience excessive fluid loss, and hypovolaemia, because of haemorrhage from serious injury or burns, or because of critical illnesses, which lead to dehydration, vomiting, or diarrhoea. Fluid loss may lead to mortality and morbidity, for example, haemorrhage accounts for almost half of deaths in the first 24 hours after traumatic injury (Geeraedts 2009; Kauvar 2006), and, worldwide, traumatic injury is a leading cause of death (Peden 2002). Changes in body fluid balance may also lead to acute kidney injury or failure.
Description of the intervention
Fluid resuscitation is one of the most important strategies for early management of critically ill people (Rhodes 2016; Rossaint 2016). Fluids used for this purpose are crystalloids or colloids.
Crystalloids, such as saline and Ringer's lactate, are solutions of salt, water and minerals, and are commonly used in the clinical setting. They have small molecules, and, when used intravenously, they are effective as volume expanders. They may have an isotonic or hypertonic composition, which could affect the distribution of fluid in the body; for example, because hypertonic crystalloids lower plasma osmolality they cause water movement from the intravascular to the extravascular space, and a lower volume may be required for fluid resuscitation (Coppola 2014). They are cheap and easy to use, with few side effects. However, because they move more easily into the extravascular space, their use may increase oedema (Coppola 2014). The composition of the crystalloid may not affect clinical outcomes; recent reviews have examined the possible effect of hypertonic solutions (Shrum 2016), and compared buffered with non‐buffered fluids (Bampoe 2017), but have not found important clinical differences.
Colloids, which are suspended in crystalloid solutions, are similarly given for the purpose of volume expansion. Different types of colloids may be grouped as synthetic or semi‐synthetic, for example: starches, dextrans, gelatins; or naturally occurring, such as human albumin or fresh frozen plasma (FFP). These colloid solutions have different pharmacokinetic properties that may affect plasma expansion in different ways (Orbegozo 2015). All colloids have a larger molecular weight than crystalloids and do not cross the endothelium into the interstitial fluid easily. This means that they stay in the intervascular space for longer than crystalloids, provide the benefit of rapid plasma expansion, and can correct colloidal osmotic pressure (McClelland 1998). Colloids are a more expensive fluid replacement option, and they may have adverse effects such as allergic reactions, blood clotting disorders, and kidney failure (Bailey 2010).
Why it is important to do this review
This is an update of a Cochrane Review that was first published in 1997 and has been updated several times since. The most recent published version of this Cochrane Review looked at the effect of colloids and crystalloids on mortality at the end of study follow‐up (Perel 2013). Meta‐analysis demonstrated no evidence of a difference in mortality when participants were given dextrans, gelatins, albumin or FFP, versus crystalloids. However, the review found evidence of an increase in mortality with the use of starches. Whilst some advise against using starches as a first line of resuscitation (Reinhart 2012), this is not consistent with findings from large randomised trials (Myburgh 2012; Perner 2012), nor with some other systematic reviews (He 2015; Qureshi 2016).
It is possible that results from Perel 2013 could have been confounded by the inclusion of a wider variety of participants in need of fluid resuscitation. In this review, we have sought to reduce heterogeneity in a critically ill population as much as possible by excluding participants who were scheduled for elective surgery; whilst these participants may require fluid replacement during perioperative management to reduce the risk of hypovolaemia, they are less likely to be critically ill at the point of randomisation ‐ even elderly people undergoing semi‐urgent surgery can seldom be seen as critically ill (Lewis 2016).
Also, our aim was to explore other effects of colloids or crystalloids on resuscitation. In particular we aimed to consider whether colloids or crystalloids affect the number of people who require blood transfusion, and the effect on renal function by assessing whether more or fewer critically ill people are likely to need renal replacement therapy after fluid resuscitation interventions, because evidence suggests that use of some types of fluids may increase these risks (Zarychanski 2013). In addition, we considered the effect of type of fluids on adverse events (allergic reactions, itching or pruritis, and rashes) that have been reported in trials (e.g. in Myburgh 2012).
Objectives
To assess the effect of using colloids versus crystalloids in critically ill people requiring fluid volume replacement on mortality, need for blood transfusion or renal replacement therapy, and adverse events (specifically: allergic reactions, itching, rashes).
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐design randomised controlled trials (RCTs), and quasi‐randomised studies (e.g. studies in which the method of assignment is based on alternation, date of birth or medical record number). We excluded randomised cross‐over trials. We excluded study reports that had been retracted after publication.
Types of participants
We included participants who required fluid volume replacement in hospital or in an emergency out‐of‐hospital setting. We included participants who were described as critically ill, and participants who required fluid volume replacement as a result of trauma, burns, or medical conditions such as sepsis.
We excluded studies of participants undergoing elective surgical procedures. We excluded neonates, and women undergoing caesarean section.
Types of interventions
We included studies that compared a colloid (suspended in any crystalloid solution) versus a crystalloid. We excluded studies in which a colloid was given in both groups of participants.
We included the following colloids: starches; dextrans; gelatins; albumin or fresh frozen plasma (FFP). We included crystalloids of different electrolyte compositions (isotonic or hypertonic).
We considered each colloid type as a separate comparison group. Therefore, we compared:
-
starches versus crystalloids;
-
dextrans versus crystalloids;
-
gelatins versus crystalloids;
-
albumin or FFP versus crystalloids.
We excluded studies in which the colloid was given to replace a known nutritional deficiency (for example, given for hypoalbuminaemia), or was given as a preloading solution before surgery. We excluded studies in which fluids were given to people with head injury to control intracranial pressure.
Types of outcome measures
We did not exclude studies that did not measure or report review outcomes.
We collected outcome data for mortality from any cause at end‐of‐study follow‐up; we included data for this outcome for which the time point was not reported, and for which the time point was reported as 'before hospital discharge', 'within the ICU', or within 30 days, 60 days, or 90 days. In addition, we collected mortality data that were clearly reported within 90 days, or within 30 days. Our secondary outcomes assessed the effectiveness of the resuscitation fluids and included need for transfusion of any blood product, and need for renal replacement therapy. In addition, we collected data for outcomes of adverse events, specifically: allergic reactions, itching/pruritis, and rashes.
Primary outcomes
-
All‐cause mortality (at end of follow‐up)
-
All‐cause mortality (within 90 days)
-
All‐cause mortality (within 30 days)
Secondary outcomes
-
Transfusion of blood products
-
Renal replacement therapy
-
Adverse events (allergic reactions, itching, and rashes)
Search methods for identification of studies
Electronic searches
We developed subject‐specific search strategies in consultation with the Cochrane Injuries Group Information Specialist. We identified RCTs through literature searching of the following electronic databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 2) (which contains the Cochrane Injuries Trials Register) in the Cochrane Library (searched 23 February 2018) (Appendix 1);
-
MEDLINE Ovid (1946 to 23 February 2018) (Appendix 2);
-
Embase Ovid (1974 to 23 February 2018) (Appendix 3);
-
PubMed (1948 to 23 February 2018) (Appendix 4);
-
Web of Science (Core Collection, 1970 to 23 February 2018) (Appendix 5);
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 13 April 2018) (Appendix 6);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 13 April 2018) (Appendix 7)
-
OpenGrey (System for Information on Grey Literature in Europe) (www.opengrey.eu; searched 12 April 2018) (Appendix 8).
This review was an update of a previous Cochrane Review (Perel 2013). However, because we made changes to the inclusion criteria and increased the outcome measures, we ran all the searches from database inception.
Searching other resources
We conducted citation searching of identified included studies published from 2013 onwards in Web of Science (apps.webofknowledge.com) (12 April 2018). We scanned reference lists of relevant systematic reviews (identified during database searches) to search for additional trials.
Data collection and analysis
Two review authors (Sharon Lewis (SL) and either: Michael Pritchard (MP), Andrew Butler (AB), or David Evans (DE)) independently completed all data collection and analyses before comparing results and reaching consensus. We consulted a third review author (Andrew Smith (AS)) to resolve conflicts if necessary.
Selection of studies
We used Endnote reference management software to collate the results of the searches and to remove duplicates. We used Covidence software to screen titles and abstracts and identify potentially relevant studies. We sourced the full texts of all potentially relevant studies and assessed whether the studies met the review inclusion criteria (see Criteria for considering studies for this review). We reviewed abstracts at this stage and included these in the review only if they provided sufficient information to assess eligibility.
We reassessed eligibility of studies included in the last version of the review (Perel 2013), because of changes made to review inclusion criteria.
We recorded the number of papers retrieved at each stage and reported this in a PRISMA flow chart (Liberati 2009; Figure 1). We reported in the review brief details of closely related but excluded papers.

Study flow diagram
Data extraction and management
We used Covidence software to extract data from individual studies. A basic template for data extraction forms is available at www.covidence.org. We adapted this template to include the following information.
-
Methods ‐ type of study design; setting; country; dates of study; funding sources
-
Participants ‐ number of participants randomised to each group, number of lost participants, and number of analysed participants, participant condition or reason for fluid resuscitation. Baseline characteristics to include: age, gender, weight or body mass index, blood pressure, prognostic or illness severity scores (American Society of Anaesthesiologists (ASA), Acute Physiology and Chronic Health Evaluation (APACHE) I or II, Simplified Acute Physiology Score (SAPS), Sequential Organ Failure Assessment (SOFA), Glasgow Coma Scale (GCS))
-
Interventions ‐ details of colloid and crystalloid (concentration of solution, volume, and rate of administration), additional relevant patient management
-
Outcomes ‐ all outcomes reported by study authors, relevant outcomes (including time of measurement for mortality)
-
Outcome data ‐ results of outcome data
Because of changes in reporting expectations in Cochrane Reviews ‐ the Methodological Expectations of Cochrane Intervention Reviews (MECIR) (Higgins 2016) ‐ since the last version of the review (Perel 2013), we also used Covidence to re‐conduct data extraction on studies included in the last version of the review.
We considered the applicability of information from individual studies and the generalisability of data to our intended study population (i.e. the potential for indirectness in the review). If we found associated publications from the same study, we created a composite data set based on all eligible publications.
Assessment of risk of bias in included studies
Two review authors (SL and MP, AB, or DE) independently assessed study quality, study limitations, and the extent of potential bias using the Cochrane 'Risk of bias' tool (Higgins 2017). We completed 'Risk of bias' assessment only for studies that reported the review outcomes.
We assessed the following domains.
-
Sequence generation (selection bias)
-
Allocation concealment (selection bias)
-
Blinding of participants, personnel, and outcome assessors (performance bias and detection bias)
-
Incomplete outcome data (attrition bias)
-
Selective outcome reporting (reporting bias)
-
Baseline characteristics
-
Other bias
We made separate judgements for performance and detection bias for mortality and for blood transfusion/renal replacement therapy/adverse events.
For each domain, we judged whether study authors had made sufficient attempts to minimise bias in their study design. We made judgements using three measures, high, low and unclear risk of bias. We recorded this decision in 'Risk of bias' tables and present a 'Risk of bias' graph and summary figure (Figure 2; Figure 3).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. We did not make judgements for studies that did not report outcomes of interest in the review, which are indicated by blank spaces

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. We did not make judgements for studies that did not report outcomes of interest in the review, which are indicated by blank spaces
Because of changes in reporting expectations in Cochrane Reviews (MECIR; Higgins 2016) since the last version of the review, we also completed a 'Risk of bias' assessment on all studies included in Perel 2013.
Measures of treatment effect
We collected dichotomous data for each outcome measure (the number of participants who had died, the number of participants who required transfusion of blood products, the number of participants who required renal replacement therapy, and the number of participants who had adverse events).
Unit of analysis issues
We reported data separately according to type of colloid (starches; dextrans; gelatins; albumin or FFP).
For multi‐arm studies that included more than one of the same type of study fluid (e.g. two groups of starches combined with an isotonic or a hypertonic crystalloid), we combined data from study groups in the same analysis only when it was appropriate and when it did not include double‐counting of participants.
In subgroup analysis, in which studies were grouped by different types of crystalloid solution, it was not always appropriate to combine data from multi‐arm study groups. If we had included multi‐arm studies in subgroup analysis, we planned to use the halving method to avoid unit of analysis issues (Deeks 2017).
Dealing with missing data
We assessed whether all measured outcomes had been reported by study authors by comparing, when possible, published reports with protocols or clinical trials register documents that had been prospectively published.
We assessed whether all randomised participants had been included in outcome data. In the absence of an explanation for loss of data, we used the 'Risk of bias' tool to judge whether a study was at high risk of attrition bias.
Assessment of heterogeneity
We assessed whether evidence of inconsistency was apparent in our results by considering heterogeneity. We assessed clinical and methodological heterogeneity by comparing similarities in our included studies between study designs, participants, and interventions, using data collected during data extraction (Data extraction and management). We assessed statistical heterogeneity by calculating the Chi² test and I² statistic (Higgins 2003), and judged any heterogeneity using values of I² greater than 60% and Chi² P value of 0.05 or less to indicate moderate to substantial statistical heterogeneity (Deeks 2017).
As well as looking at statistical results, we considered point estimates and overlap of confidence intervals (CIs). If CIs overlap, then results are more consistent. Combined studies may show a large consistent effect but with significant heterogeneity. Therefore, we planned to interpret heterogeneity with caution (Guyatt 2011a).
Assessment of reporting biases
We attempted to source published protocols for each of our included studies by using clinical trials registers. We compared protocols or clinical trials register documents that had been prospectively published with study results to assess the risk of selective reporting. We generated a funnel plot to assess risk of publication bias in the review, for outcomes in which we identified more than 10 studies (Sterne 2017). An asymmetrical funnel plot may suggest publication of only positive results (Egger 1997). We included funnel plot figures for the primary outcome: all‐cause mortality (at the end of follow‐up) (Figure 4; Figure 5; Figure 6).

Funnel plot of comparison 1. Starches vs crystalloid, outcome: 1.1 mortality at end of follow‐up

Funnel plot of comparison 2. Dextrans vs crystalloid, outcome: 2.1 mortality at end of follow‐up

Funnel plot of comparison 4. Albumin and FFP vs crystalloid, outcome: 4.1 mortality at end of follow‐up
Data synthesis
We completed meta‐analysis of outcomes in which we had comparable effect measures for more than one study, and when measures of clinical and methodological heterogeneity indicated that pooling was appropriate.
We presented results according to type of colloid (starches; dextrans; gelatins; albumin or FFP) as four separate comparisons (see Types of interventions).
We used the statistical calculator in Review Manager 5 (RevMan 5) to calculate risk ratios (RR) using the Mantel‐Haenszel model (Review Manager 2014). We used a random‐effects statistical model that accounted for the variation amongst participant groups in the review. We calculated CIs at 95% and used a P value of 0.05 or less to judge whether a result was statistically significant. We considered imprecision in the results of analyses by assessing the CI around an effect measure; a wide CI would suggest a higher level of imprecision in our results. A small number of identified studies may also reduce precision (Guyatt 2011b).
Subgroup analysis and investigation of heterogeneity
We explored potential differences in the tonicity of crystalloid solutions that had been used with colloids or used as the comparative crystalloid. This was an a priori subgroup analysis included in the previous version of the review (Perel 2013). We used the calculator in RevMan 5 to perform subgroup analysis, comparing the Chi² and P value for the test for subgroup differences; we interpreted a P value of less than 0.05 as being indicative of a difference between subgroups. We conducted subgroup analysis when data were available for more than 10 studies (Deeks 2017). We considered subgroup analysis only for the primary outcome (all‐cause mortality (at end of follow‐up)) for each of our comparisons (starches; dextrans; gelatins; albumin or FFP). Subgroups were as follows.
-
Tonicity of crystalloid solution:
-
colloid + isotonic crystalloid versus isotonic crystalloid;
-
colloid + hypertonic crystalloid versus isotonic crystalloid;
-
colloid + isotonic crystalloid versus hypertonic crystalloid;
-
colloid + hypertonic crystalloid versus hypertonic crystalloid.
-
Sensitivity analysis
We explored the potential effects of decisions made as part of the review process as follows.
-
We excluded all studies that we judged to be at high or unclear risk of selection bias.
-
We excluded studies in which we noted that some participants in the crystalloid group were given, or may have been given, additional colloids.
-
We conducted meta‐analysis using the alternative meta‐analytical effects model (fixed‐effect).
-
We used alternative data for individual studies in which we noted discrepancies in reported data.
We conducted sensitivity analysis on the primary outcome: all‐cause mortality (at end of follow‐up).
'Summary of findings' table and GRADE
We used the GRADE system to assess the certainty of the body of evidence associated with the following outcomes (Guyatt 2008).
-
All‐cause mortality (at end of follow‐up)
-
All‐cause mortality (within 90 days)
-
All‐cause mortality (within 30 days)
-
Transfusion of blood products
-
Renal replacement therapy
-
Adverse events (allergic reactions, itching, rashes)
The GRADE approach appraises the certainty of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias, directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
We constructed four 'Summary of findings' tables using the GRADEpro GDT software to create 'Summary of findings' tables for the following comparisons in this review (GRADEpro GDT 2015).
-
Starches versus crystalloids
-
Dextrans versus crystalloids
-
Gelatins versus crystalloids
-
Albumin or FFP versus crystalloids
One review author (SL) completed the table in consultation with a second author (MP).
Results
Description of studies
Results of the search
We screened 7920 titles and abstracts from database searches, forward and backward citation searches, and clinical trials register searches. We assessed 248 full‐text reports for eligibility. See Figure 1.
Included studies
See Characteristics of included studies.
We included 69 studies; 42 of these had been included in the previous version of the review (Perel 2013), and 27 were included for the first time in this update.
These 69 studies comprised a total of 114 publications, and included 30,020 participants (Alpar 2004; Annane 2013; Baker 2009; Bechir 2013; Bentsen 2006; Brunkhorst 2008; Bulger 2008; Bulger 2010; Bulger 2011; Caironi 2014; Chavez‐Negrete 1991; Cifra 2003; Cooper 2006; Du 2011; Dubin 2010; Dung 1999; Ernest 1999; Evans 1996; Finfer 2004; Goodwin 1983; Grba‐Bujevic 2012; Guidet 2012; Hall 1978; Heradstveit 2010; James 2011; Jelenko 1979; Jie 2015; Kumar 2017; Li 2008; Lowe 1977; Lu 2012; Lucas 1978; Mahrous 2013; Maitland 2005; Maitland 2011; Martin 2005; Masoumi 2016; Mattox 1991; McIntyre 2008; McIntyre 2012; Metildi 1984; Modig 1986; Morrison 2011; Myburgh 2012; Nagy 1993; Ngo 2001; O'Mara 2005; Oliveira 2002; Park 2015; Perner 2012; Philips 2015; Pockaj 1994; Quinlan 2004; Rackow 1983; Shah 1977; Upadhyay 2005; Van der Heijden 2009; Vassar 1990; Vassar 1991; Vassar 1993a; Vassar 1993b; Vlachou 2010; Wills 2005; Wu 2001; Younes 1992; Younes 1997; Younes 1998; Zhao 2013; Zhu 2011).
Four studies were quasi‐randomised (Alpar 2004; Cifra 2003; Lucas 1978; Modig 1986), and the remaining studies were RCTs.
We included three studies for which we could only source the abstract (Mahrous 2013; Park 2015; Philips 2015); we sourced the full text of all remaining studies.
Study population
Participants had a wide variety of diagnoses for which fluid volume resuscitation was required, including: trauma, burns, and medical conditions such as sepsis and hypovolaemic shock. We have listed each study with the primary participant conditions in Table 1.
| Participant condition | Study ID |
| Admission to an ICU with any condition (which included trauma, sepsis, ARDS, head injury) | |
| Trauma (includes studies of 'any trauma admissions', and head, chest, and abdominal injuries, and trauma with haemorrhagic or hypovolaemic shock) | Annane 2013*; Alpar 2004; Baker 2009; Bulger 2008; Bulger 2010; Bulger 2011; Evans 1996; Grba‐Bujevic 2012; James 2011; Lowe 1977; Lucas 1978; Masoumi 2016; Mattox 1991; Morrison 2011; Shah 1977; Vassar 1990; Vassar 1991; Vassar 1993a; Vassar 1993b; Wu 2001 |
| Sepsis or septic shock | Annane 2013*; Brunkhorst 2008; Caironi 2014; Dubin 2010; Ernest 1999; Guidet 2012; Jie 2015; Li 2008; Lu 2012; Mahrous 2013; McIntyre 2008; McIntyre 2012; Modig 1986; Oliveira 2002; Park 2015 (cancer with sepsis); Perner 2012; Rackow 1983*; Upadhyay 2005; Zhu 2011 |
| Hypovolaemia, hypovolaemic shock, haemorrhagic shock | Annane 2013*; Chavez‐Negrete 1991; Nagy 1993; Rackow 1983*; Van der Heijden 2009; Younes 1992; Younes 1997; Younes 1998 |
| Burns | Bechir 2013; Cooper 2006; Goodwin 1983; Hall 1978; Jelenko 1979; O'Mara 2005; Vlachou 2010 |
| ALI, ARDS | |
| Spontaneous subarachnoid haemorrhage | |
| Dengue shock syndrome | |
| Postcardiac arrest | |
| Perforation peritonitis | |
| Severe malaria | |
| Severe febrile illness | |
| Severe pulmonary insufficiency | |
| Vascular leak syndrome (cancer patients) | |
| Cirrhosis and septic induced hypotension | |
| Severe acute pancreatitis |
* included for more than one type of condition
ALI: acute lung injury
ARDS: acute respiratory distress syndrome
ICU: intensive care unit
Seven studies recruited only children (Cifra 2003; Dung 1999; Maitland 2005; Maitland 2011; Ngo 2001; Upadhyay 2005; Wills 2005), and two studies recruited children and adults (Hall 1978; Wu 2001). We noted that some studies reported an inclusion criteria of over 15 years of age (Bulger 2010; Bulger 2011), over 16 years of age (Baker 2009; Bechir 2013; Evans 1996; Masoumi 2016; Mattox 1991; Morrison 2011), or over 17 years of age (Bulger 2008); using mean ages reported by study authors, most participants in these studies were adults over 18 years of age. All remaining studies included only adult participants.
Study setting
Nineteen studies were multicentre studies (Annane 2013; Baker 2009; Brunkhorst 2008; Bulger 2010; Bulger 2011; Caironi 2014; Cooper 2006; Dubin 2010; Finfer 2004; Guidet 2012; Maitland 2011; Martin 2005; Mattox 1991; McIntyre 2008; McIntyre 2012; Morrison 2011; Myburgh 2012; Perner 2012; Quinlan 2004); the remaining studies were single‐centre studies.
Ten studies were based in an out‐of‐hospital setting before transition to an emergency or trauma department within a hospital (Baker 2009; Bulger 2008; Bulger 2010; Caironi 2014; Grba‐Bujevic 2012; Mattox 1991; Morrison 2011; Vassar 1991; Vassar 1993a; Vassar 1993b); the remaining studies were based in a hospital.
Most single‐ or multicentre studies were conducted in one of the following countries: the USA (Bulger 2008; Goodwin 1983; Jelenko 1979; Lowe 1977; Lucas 1978; Martin 2005; Mattox 1991; Metildi 1984; Nagy 1993; O'Mara 2005; Pockaj 1994; Quinlan 2004; Rackow 1983; Shah 1977; Vassar 1990; Vassar 1991; Vassar 1993a; Vassar 1993b); Canada (Baker 2009; Cooper 2006; Ernest 1999; McIntyre 2008; McIntyre 2012; Morrison 2011); China (Du 2011; Jie 2015; Li 2008; Lu 2012; Zhao 2013; Zhu 2011); Brazil (Oliveira 2002; Park 2015; Younes 1992; Younes 1997; Younes 1998); India (Kumar 2017; Philips 2015; Upadhyay 2005); Vietnam (Dung 1999; Ngo 2001; Wills 2005); Norway (Bentsen 2006; Heradstveit 2010); South Africa (Evans 1996; James 2011); the UK (Alpar 2004; Vlachou 2010); Argentina (Dubin 2010); Croatia (Grba‐Bujevic 2012); Denmark (Hall 1978); Germany (Brunkhorst 2008); Iran (Masoumi 2016); Italy (Caironi 2014); Kenya (Maitland 2005); Mexico (Chavez‐Negrete 1991); the Netherlands (Van der Heijden 2009); the Philippines (Cifra 2003); Saudi Arabia (Mahrous 2013); Sweden (Modig 1986); Switzerland (Bechir 2013); Taiwan (Wu 2001).
Eight multicentre studies were conducted in more than one country (Annane 2013: France, Belgium, Canada, Algeria and Tunisia; Perner 2012: Denmark, Finland, Iceland and Norway; Maitland 2011: Kenya, Tanzania and Uganda; Bulger 2010 and Bulger 2011: USA and Canada; Finfer 2004 and Myburgh 2012: Australia and New Zealand; Guidet 2012: France and Germany).
Interventions and comparison
Nine studies were multi‐arm studies that included more than one colloid solution or more than one crystalloid solution or more than one of each type of solution (Dung 1999; Li 2008; Ngo 2001; Rackow 1983; Van der Heijden 2009; Vassar 1993b; Wills 2005; Zhao 2013; Zhu 2011). One study compared colloids with crystalloids and the type of colloid or crystalloid was at the discretion of the physician (Annane 2013); types of colloids in this study were starches, gelatins, and albumin.
Colloids
Twenty‐eight studies used a starch solution (hydroxyethyl starch, hetastarch, or pentastarch) for fluid resuscitation (Annane 2013; Bechir 2013; Bentsen 2006; Brunkhorst 2008; Cifra 2003; Du 2011; Dubin 2010; Grba‐Bujevic 2012; Guidet 2012; Heradstveit 2010; James 2011; Jie 2015; Kumar 2017; Li 2008; Lu 2012; Mahrous 2013; Masoumi 2016; McIntyre 2008; Myburgh 2012; Nagy 1993; Perner 2012; Rackow 1983; Van der Heijden 2009; Vlachou 2010; Wills 2005; Younes 1998; Zhao 2013; Zhu 2011). Of these, sixteen studies did not describe what they used as a suspension solution (Annane 2013; Cifra 2003; Dubin 2010; James 2011; Jie 2015; Li 2008; Lu 2012; Mahrous 2013; Nagy 1993; Perner 2012; Rackow 1983; Van der Heijden 2009; Vlachou 2010; Younes 1998; Zhao 2013; Zhu 2011). Five studies used a starch solution combined with an isotonic crystalloid solution, which was normal saline (Brunkhorst 2008; Masoumi 2016; McIntyre 2008; Myburgh 2012; Wills 2005), and seven studies used a starch solution combined with a hypertonic crystalloid solution, which was hypertonic saline (Bentsen 2006; Grba‐Bujevic 2012; Heradstveit 2010; Li 2008; Zhu 2011), or Ringer's lactate (Bechir 2013; Du 2011). Two studies did not specify the type of crystalloid solution that was combined with a starch (Guidet 2012; Kumar 2017), and one multi‐arm study also included a starch combined with glutamine (Zhao 2013).
Twenty studies used dextrans for fluid resuscitation (Alpar 2004; Baker 2009; Bulger 2008; Bulger 2010; Bulger 2011; Chavez‐Negrete 1991; Dung 1999; Hall 1978; Mattox 1991; Modig 1986; Morrison 2011; Ngo 2001; Oliveira 2002; Vassar 1990; Vassar 1991; Vassar 1993a; Vassar 1993b; Wills 2005; Younes 1992; Younes 1997). Two studies did not describe what they used as a suspension solution in dextran 70 (Modig 1986; Ngo 2001); Ngo 2001 gave Ringer's lactate to all participants after an initial infusion of dextran 70. Three studies used dextran 70 (which has relative molecular mass of 70,000) combined with an isotonic crystalloid solution which was normal saline (Dung 1999; Hall 1978; Wills 2005). Eleven studies used hypertonic saline with 6% dextran 70 solution (HSD 6%) (Baker 2009; Bulger 2008; Bulger 2010; Bulger 2011; Mattox 1991; Morrison 2011; Vassar 1990; Vassar 1993a; Vassar 1993b; Younes 1992; Younes 1997). Three studies used hypertonic saline with dextran 70; Vassar 1993b used it at 12%, while Alpar 2004 used it at 4.2% and Oliveira 2002 used it at 8%. One study used hypertonic saline with dextran 60 (a relative molecular mass of 60,000 (HSD 6%)) (Chavez‐Negrete 1991). One study changed concentration of HSD during the study period; participants were initially given HSD 4.2% with dextran 70 before a protocol change to HSD 6% with dextran 70 (Vassar 1991).
Seven studies used a succinylated gelatin solution (of an isotonic composition) for fluid resuscitation (Annane 2013; Dung 1999; Evans 1996; Ngo 2001Upadhyay 2005; Van der Heijden 2009; Wu 2001).
Twenty‐two studies used albumin or FFP for fluid resuscitation. Thirteen studies used albumin (Annane 2013; Caironi 2014; Ernest 1999; Finfer 2004; Lucas 1978; Maitland 2005; Maitland 2011; Martin 2005; McIntyre 2012; Park 2015; Philips 2015; Quinlan 2004; Rackow 1983). Three studies used albumin combined with an isotonic crystalloid, which was normal saline (Cooper 2006; Pockaj 1994; Van der Heijden 2009), and five studies used albumin combined with a hypertonic crystalloid, which was hypertonic saline (Jelenko 1979), or Ringer's lactate (Goodwin 1983; Lowe 1977; Metildi 1984; Shah 1977). One study used FFP with Ringer's lactate (O'Mara 2005).
Individual study protocols for the concentration, quantity, and timing of administration of each type of study colloid varied. We were not able to establish volume ratios of colloid solutions to crystalloid solutions in most studies; we found that study authors often reported that fluids were provided by the pharmacist and manufacturers in pre‐packaged bags, which we assumed contained fluids in clinically appropriate volume ratios.
Crystalloids
Thirty‐four studies used isotonic solutions as the comparative crystalloid fluid, which was normal saline (Annane 2013; Baker 2009; Bentsen 2006; Bulger 2010; Bulger 2011; Dubin 2010; Dung 1999; Ernest 1999; Finfer 2004; Grba‐Bujevic 2012; Guidet 2012; James 2011; Jie 2015; Maitland 2005; Maitland 2011; Martin 2005; Masoumi 2016; McIntyre 2008; McIntyre 2012; Morrison 2011; Myburgh 2012; Ngo 2001; Oliveira 2002; Philips 2015; Pockaj 1994; Quinlan 2004; Rackow 1983; Upadhyay 2005; Van der Heijden 2009; Vassar 1993a; Younes 1992; Younes 1997; Younes 1998; Zhao 2013).
Forty‐one studies used a hypertonic solution, which was Ringer's lactate (Alpar 2004; Annane 2013; Bechir 2013; Brunkhorst 2008; Bulger 2008; Chavez‐Negrete 1991; Cifra 2003; Cooper 2006; Du 2011; Dung 1999; Evans 1996; Goodwin 1983; Hall 1978; Jelenko 1979; Jie 2015; Kumar 2017; Lowe 1977; Lu 2012; Mahrous 2013; Metildi 1984; Nagy 1993; Ngo 2001; O'Mara 2005; Park 2015; Shah 1977; Vassar 1990; Vassar 1991; Vassar 1993b; Vlachou 2010; Wills 2005; Wu 2001; Zhu 2011), Ringer's acetate (Modig 1986; Perner 2012), or hypertonic saline (Bulger 2010; Bulger 2011; Jelenko 1979; Li 2008; Vassar 1993a; Vassar 1993b; Younes 1992).
One study used Ringer's acetate and normal saline (Heradstveit 2010), and three studies did not specify the type of crystalloid (Caironi 2014; Lucas 1978; Mattox 1991).
Individual study protocols for the quantity and timing of administration of each type of study crystalloid varied.
Outcomes
Only five studies did not report mortality data (Bentsen 2006; Dung 1999; Ernest 1999; Grba‐Bujevic 2012; Masoumi 2016); these five studies did not report any of our review outcomes. Fourteen studies reported number of participants who required transfusion of blood products (Annane 2013; Brunkhorst 2008; Bulger 2011; Cifra 2003; Cooper 2006; Guidet 2012; Lowe 1977; McIntyre 2008; Nagy 1993; Ngo 2001; Perner 2012; Pockaj 1994; Vlachou 2010; Wills 2005). Thirteen studies reported number of participants who required renal replacement therapy (Annane 2013; Bechir 2013; Brunkhorst 2008; Caironi 2014; Finfer 2004; Guidet 2012; James 2011; Mahrous 2013; McIntyre 2008; Myburgh 2012; Park 2015; Perner 2012; Vlachou 2010).
Nine studies reported data for adverse events (Bulger 2008; Guidet 2012; Mattox 1991; Myburgh 2012; Ngo 2001; Perner 2012; Vassar 1990; Vassar 1991; Wills 2005); seven reported incidences of allergic reaction (Bulger 2008; Mattox 1991; Myburgh 2012; Ngo 2001; Perner 2012; Vassar 1990; Vassar 1991), two reported incidences of itching (Guidet 2012; Myburgh 2012), and two reported incidences of rashes (Myburgh 2012; Wills 2005).
Funding sources
Thirty‐nine studies reported funding from departments or other sources that we judged to be independent (Annane 2013; Baker 2009; Brunkhorst 2008; Bulger 2008; Bulger 2010; Bulger 2011; Caironi 2014; Du 2011; Dubin 2010; Dung 1999; Evans 1996; Finfer 2004; Goodwin 1983; Hall 1978; Heradstveit 2010; James 2011; Jelenko 1979; Lowe 1977; Lucas 1978; Maitland 2005; Maitland 2011; Martin 2005; McIntyre 2012; Metildi 1984; Modig 1986; Morrison 2011; Myburgh 2012; Nagy 1993; Oliveira 2002; Perner 2012; Quinlan 2004; Rackow 1983; Shah 1977; Van der Heijden 2009; Vassar 1990; Vassar 1991; Vassar 1993a; Wills 2005; Zhao 2013). Nineteen studies reported funding from pharmaceutical companies, which may have supplied study fluids (Bechir 2013; Brunkhorst 2008; Cooper 2006; Dung 1999; James 2011; Guidet 2012; Maitland 2011; Martin 2005; Mattox 1991; McIntyre 2008; Morrison 2011; Myburgh 2012; Ngo 2001; Perner 2012; Van der Heijden 2009; Vassar 1991; Vassar 1993a; Vassar 1993b; Younes 1992). We noted that one study with pharmaceutical funding reported that funders were involved in the study design, analysis and preparation of the report (Guidet 2012).
The remaining studies did not report funding sources or declare conflicts of interest.
Excluded studies
See Characteristics of excluded studies.
We excluded 127 studies following consideration of the full‐text reports. Ninety‐three reports were of an ineligible study design (studies that were not RCTs, or were commentaries or editorial reports), nine studies had an ineligible participant group, and 25 studies used ineligible interventions (did not compare a colloid versus crystalloid, or fluids given at the wrong time). See Figure 1.
We have not included references and details of all 127 studies excluded during full‐text review, only the 31 that we considered to be key excluded studies (Higgins 2011).
Because of changes to the criteria for considering studies since the last version of the review (Perel 2013), we excluded 31 studies that were previously included and have listed these in the review. Reasons for excluding these studies were: in 28 studies fluid resuscitation was given as part of perioperative management of people undergoing elective surgery (Boutros 1979; Dawidson 1991; Dehne 2001; Eleftheriadis 1995; Evans 2003; Fries 2004; Gallagher 1985; Guo 2003; Hartmann 1993; Hondebrink 1997; Karanko 1987; Lee 2011; Ley 1990; Mazher 1998; McNulty 1993; Moretti 2003; Nielsen 1985; Prien 1990; Shires 1983; Sirieix 1999; Skillman 1975; Tollusfrud 1995; Tollusfrud 1998; Verheij 2006; Virgilio 1979; Wahba 1996; Zetterstorm 1981a; Zetterstorm 1981b); two studies were not RCTs (Bowser‐Wallace 1986; Grundmann 1982); and one study was an abstract of a study protocol where the full study was never published (Rocha e Silva 1994). In addition, we excluded five studies because the publications have been retracted; we have not listed references for these retracted publications.
See Criteria for considering studies for this review and Differences between protocol and review.
Studies awaiting classification
Seven studies are awaiting classification (Halim 2016; Bulanov 2004; Charpentier 2011; NCT00890383; NCT01337934; NCT02064075; Protsenko 2009).
We found three studies during the searches of clinical trials registers (NCT00890383; NCT01337934; NCT02064075). These studies were described as completed but study results were not available; we await publication of the full reports to assess their eligibility for inclusion in the review. One study compared tetrastarch versus an unspecified crystalloid for fluid resuscitation following trauma (NCT00890383); one study compared albumin versus Ringer's lactate for fluid resuscitation for sepsis and septic shock (NCT01337934); and one study compared hydroxyethyl starch versus Ringer's lactate for fluid resuscitation following subarachnoid haemorrhage (NCT02064075). Two studies were published only as abstracts with insufficient information; one compared gelatin versus normal saline for fluid resuscitation for sepsis and septic shock (Halim 2016), and one compared albumin versus normal saline for fluid resuscitation for septic shock (Charpentier 2011). Two studies were published in Russian and require translation to assess eligibility: one compared starches with normal saline (Bulanov 2004), and no details are known about the other study (Protsenko 2009). See Characteristics of studies awaiting classification.
Ongoing studies
We found three ongoing studies during searches of clinical trial registers (NCT01763853; NCT02721238; NCT02782819). One study compares 4% albumin versus an unspecified crystalloid in people with acute respiratory distress syndrome (NCT01763853); one study compares 20% albumin versus plasmalyte in people with cirrhosis‐ and sepsis‐induced hypotension (NCT02721238); and the last study compares 5% albumin or gelatin versus Ringer's lactate or normal saline for treatment of shock (NCT02782819). See Characteristics of ongoing studies.
Risk of bias in included studies
We did not complete 'Risk of bias' assessments for studies that reported none of our review outcomes (Bentsen 2006; Dung 1999; Ernest 1999; Grba‐Bujevic 2012; Masoumi 2016).
We did not seek translation of studies that were published in Chinese (Jie 2015; Li 2008; Lu 2012; Zhu 2011). We made 'Risk of bias' assessments from details available in the English abstracts, and from the baseline characteristics tables.
Allocation
All studies were described as randomised. Thirty studies reported adequate methods of randomisation and we judged these to have a low risk of bias for random sequence generation (Annane 2013; Baker 2009; Bechir 2013; Bulger 2008; Bulger 2010; Bulger 2011; Caironi 2014; Cooper 2006; Du 2011; Finfer 2004; Goodwin 1983; Guidet 2012; James 2011; Kumar 2017; Maitland 2011; Martin 2005; Mattox 1991; McIntyre 2008; Morrison 2011; Myburgh 2012; Ngo 2001; O'Mara 2005; Oliveira 2002; Perner 2012; Upadhyay 2005; Vassar 1991; Vassar 1993a; Vassar 1993b; Vlachou 2010; Wills 2005). Twenty‐four studies reported adequate methods of allocation concealment and we judged these to have a low risk of bias (Annane 2013; Baker 2009; Bechir 2013; Bulger 2008; Bulger 2010; Bulger 2011; Caironi 2014; Cooper 2006; Finfer 2004; Guidet 2012; James 2011; Maitland 2011; Martin 2005; Mattox 1991; McIntyre 2008; Morrison 2011; Ngo 2001; Perner 2012; Upadhyay 2005; Van der Heijden 2009; Vassar 1991; Vassar 1993a; Vassar 1993b; Wills 2005).
Four studies were quasi‐randomised studies, and we believed that methods for random sequence generation and random allocation concealment were at high risk of selection bias (Alpar 2004; Cifra 2003; Lucas 1978; Modig 1986). Two studies were described as randomised but because of differences noted in the baseline characteristics table (Jelenko 1979), and unexplained differences in participant numbers (Lowe 1977), we judged them to be at high risk of bias for random sequence generation. One study described "use of lots" to allocate participants to groups and, without additional details, we were uncertain whether this method was adequate and so assessed risk of bias of random sequence generation as unclear (Hall 1978).
The remaining studies reported insufficient details of random sequence generation (Brunkhorst 2008; Chavez‐Negrete 1991; Dubin 2010; Evans 1996; Hall 1978; Heradstveit 2010; Jie 2015; Li 2008; Lu 2012; Mahrous 2013; Maitland 2005; McIntyre 2012; Metildi 1984; Nagy 1993; Park 2015; Philips 2015; Pockaj 1994; Quinlan 2004; Rackow 1983; Shah 1977; Van der Heijden 2009; Vassar 1990; Wu 2001; Younes 1992; Younes 1997; Younes 1998; Zhao 2013; Zhu 2011), and random allocation concealment (Brunkhorst 2008; Chavez‐Negrete 1991; Du 2011; Dubin 2010; Evans 1996; Goodwin 1983; Hall 1978; Heradstveit 2010; Jelenko 1979; Jie 2015; Kumar 2017; Li 2008; Lowe 1977; Lu 2012; Mahrous 2013; Maitland 2005; McIntyre 2012; Metildi 1984; Myburgh 2012; Nagy 1993; O'Mara 2005; Oliveira 2002; Park 2015; Philips 2015; Pockaj 1994; Quinlan 2004; Rackow 1983; Shah 1977; Vassar 1990; Vlachou 2010; Wu 2001; Younes 1992; Younes 1997; Younes 1998; Zhao 2013; Zhu 2011), and we judged these to have an unclear risk of selection bias.
Blinding
For the mortality outcome, we believed that lack of blinding was unlikely to influence performance, or influence outcome assessment, therefore, we judged all studies that reported mortality data as having a low risk of performance bias and a low risk of detection bias for mortality.
For the remaining outcomes (transfusion of blood products, renal replacement therapy, and adverse events), we assessed whether methods had been used to disguise fluid types from clinicians, and from outcome assessors. Nine studies reported sufficient methods of blinding and we judged these to have low risk of performance bias (Bechir 2013; Bulger 2011; Guidet 2012; Finfer 2004; James 2011; McIntyre 2008; Ngo 2001; Perner 2012; Wills 2005). Two studies described methods of fluid administration as open‐label, in which differences between study fluids would be apparent to personnel; we judged these to have a high risk of performance bias (Brunkhorst 2008; Cooper 2006). Study authors in Annane 2013 reported that clinicians were not blinded because of the immediate need for resuscitation; we judged this study to have a high risk of performance bias. We judged the remaining studies as having an unclear risk of performance bias because methods of blinding were not described (Caironi 2014; Cifra 2003; Lowe 1977; Mahrous 2013; Nagy 1993; Pockaj 1994; Vlachou 2010).
Six studies reported sufficient methods of blinding of outcome assessors and we judged these to have a low risk of detection bias (Bechir 2013; Bulger 2011; Guidet 2012; McIntyre 2008; Perner 2012; Wills 2005). We judged the remaining studies to have an unclear risk of detection bias because study authors reported insufficient methods of blinding of outcome assessors (Brunkhorst 2008; Caironi 2014; Cifra 2003; Cooper 2006; Finfer 2004; James 2011; Lowe 1977; Mahrous 2013; Nagy 1993; Ngo 2001; Pockaj 1994; Vlachou 2010).
Incomplete outcome data
Two studies, published only as abstracts, appeared to have some discrepancies in mortality data and we could not be certain whether this was because of loss of participant data; we judged these studies to have unclear risk of attrition bias (Mahrous 2013; Park 2015).
One study had an apparent loss of analysed participants for mortality, but not for transfusion of blood products, and we could not explain this difference in loss; we judged this study to have a high risk of attrition bias (Pockaj 1994). One study excluded three participants because of protocol deviations; because the study was small this represented a high loss and we judged the study to have an unclear risk of attrition bias (Vlachou 2010). One study noted that approximately 10% of participants did not meet eligibility criteria after randomisation, however these were included in an intention‐to‐treat (ITT) analysis; we judged this study to have an unclear risk of attrition bias because this was a large number of participants in an ITT analysis (Bulger 2008).
The remaining studies had no losses, or few losses that were explained, and we judged them all to have low risk of attrition bias.
Selective reporting
We found prospective clinical trials registration reports for nine studies (Annane 2013; Bechir 2013; Bulger 2008; Bulger 2010; Caironi 2014; Finfer 2004; Guidet 2012; Myburgh 2012; Perner 2012). Outcomes were reported according to these trial registration documents in six studies and we judged these to have a low risk of selective reporting bias (Annane 2013; Bulger 2010; Caironi 2014; Finfer 2004; Myburgh 2012; Perner 2012). In one study, we noted that outcomes were added to the trials register documents after the start of the study, and we could not be certain whether selective reporting bias was introduced because of this (Bulger 2008). In two studies, we noted that outcomes in the study report were not listed as outcomes in the clinical trials registration documents, and we judged these studies to have a high risk of selective reporting bias (Bechir 2013; Guidet 2012).
Three studies were registered retrospectively with clinical trials registers (Dubin 2010; James 2011; Maitland 2011); it was not feasible to use information from these clinical trials documents to assess risk of selective reporting bias.
We could not be certain whether Philips 2015 was prospectively registered because the available abstract report included the clinical trials register identification number but not the study dates; we judged this to have an unclear risk of selective reporting bias.
All other studies did not provide clinical trials registration information, or references for published study protocols, and we were unable to assess risk of selective reporting bias for these studies.
Baseline characteristics
We noted no differences in baseline characteristics that we believed could introduce bias in 46 studies, and we judged these studies to have a low risk of bias (Annane 2013; Baker 2009; Bechir 2013; Brunkhorst 2008; Bulger 2010; Bulger 2011; Chavez‐Negrete 1991; Cifra 2003; Du 2011; Dubin 2010; Evans 1996; Goodwin 1983; Guidet 2012; Hall 1978; Jie 2015; Li 2008; Lowe 1977; Lu 2012; Lucas 1978; Maitland 2011; Martin 2005; Metildi 1984; Modig 1986; Morrison 2011; Myburgh 2012; Nagy 1993; Ngo 2001; O'Mara 2005; Perner 2012; Philips 2015; Pockaj 1994; Rackow 1983; Shah 1977; Upadhyay 2005; Van der Heijden 2009; Vassar 1990; Vassar 1991; Vassar 1993a; Vassar 1993b; Vlachou 2010; Wills 2005; Wu 2001; Younes 1992; Younes 1997; Younes 1998; Zhu 2011).
We noted an imbalance in some baseline characteristics in eleven studies (Alpar 2004; Bulger 2008; Caironi 2014; Cooper 2006; Finfer 2004; James 2011; Kumar 2017; Maitland 2005; McIntyre 2008; Oliveira 2002; Quinlan 2004). We could not be certain whether these imbalances could influence results and we judged these studies to have an unclear risk of bias. We noted differences in several baseline characteristics in one study and judged this to have a high risk of bias (Jelenko 1979).
We could not assess comparability of baseline characteristics in four studies because these were either not reported or not reported by group (Mattox 1991; Mahrous 2013; McIntyre 2012; Park 2015).
Other potential sources of bias
We noted that in 14 studies some participants were given, or may have been given, additional colloids in the crystalloid arm either before or during the study (Annane 2013; Baker 2009; Brunkhorst 2008; Bulger 2011; Chavez‐Negrete 1991; Cifra 2003; Du 2011; Finfer 2004; Goodwin 1983; Myburgh 2012; Ngo 2001; Perner 2012; Vassar 1991; Wills 2005); we judged all these studies to have a high risk of other bias.
We noted that one study was published by a single author, and time between completion of the study and publication of the report was longer than expected (Kumar 2017). We could not be certain whether this study was a primary publication, or a secondary publication of an existing or unknown study, and we judged it to have a high risk of bias. We noted differences in the reported number of deaths in Lucas 1978 according to different study reports, and these differences were unexplained; we judged this study to have a high risk of other bias.
We could not be certain of other risks of bias in the Chinese studies for which we did not seek translation (Jie 2015; Li 2008; Lu 2012; Zhu 2011), nor in studies that were published only as abstracts (Mahrous 2013; Park 2015; Philips 2015); and we assessed these studies to have an unclear risk of other bias.
We noted no other sources of bias in the remaining studies, and judged these all to have a low risk of other bias.
Effects of interventions
See: Summary of findings for the main comparison Starches compared to crystalloid for fluid resuscitation in critically ill patients; Summary of findings 2 Dextrans compared to crystalloid for fluid resuscitation in critically ill patients; Summary of findings 3 Gelatins compared to crystalloid for fluid resuscitation in critically ill patients; Summary of findings 4 Albumin and fresh frozen plasma compared to crystalloid for fluid resuscitation in critically ill patients
1. Starches versus crystalloids
All‐cause mortality at end of follow‐up
Twenty‐five studies measured mortality (Annane 2013; Bechir 2013; Brunkhorst 2008; Cifra 2003; Du 2011; Dubin 2010; Guidet 2012; Heradstveit 2010; James 2011; Jie 2015; Kumar 2017; Li 2008; Lu 2012; Mahrous 2013; McIntyre 2008; Myburgh 2012; Nagy 1993; Perner 2012; Rackow 1983; Van der Heijden 2009; Vlachou 2010; Wills 2005; Younes 1998; Zhao 2013; Zhu 2011).
We included 24 in this analysis, in which the time of the assessment point was: within 24 hours (Dubin 2010; Rackow 1983; Younes 1998); within the ICU or hospital stay (Du 2011; Van der Heijden 2009; Vlachou 2010); up to 30 days from hospital discharge (Kumar 2017); within 28 or 30 days (Guidet 2012; Li 2008; McIntyre 2008); within 60 days (Zhao 2013); within 90 days (Annane 2013; Bechir 2013; Brunkhorst 2008; Myburgh 2012; Perner 2012); at 12 months (Heradstveit 2010); and studies in which the time point was unknown (Cifra 2003; James 2011; Jie 2015; Lu 2012; Nagy 1993; Wills 2005; Zhu 2011). We did not include mortality data reported in Mahrous 2013, the data were reported as percentages in the abstract and we could not be certain whether the data were for all randomised participants or whether some participant data were lost.
Three studies were multi‐arm studies. We combined data for both colloid groups in two studies (Zhao 2013; Zhu 2011); and for both colloid groups and both crystalloid groups in Li 2008. One study, which allowed type of colloid or crystalloid to be at the discretion of the clinician, reported mortality outcome data for participants who received only one type of fluid (Annane 2013); we included data for participants who received only hydroxyethyl starch in the colloid group, and combined data for two crystalloid groups (isotonic saline, and Ringer's lactate).
We found little or no difference in the number of participants who died at the end of follow‐up according to whether fluid resuscitation was with a starch or with a crystalloid (RR 0.97 95% CI 0.86 to 1.09; 11,177 participants; 24 studies; I² = 34%; Analysis 1.1).
We generated a funnel plot to assess risk of publication bias and did not interpret this to indicate high risk (Figure 4).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had an unclear risk of selection bias, one small study had a high risk of selection bias, and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table for the main comparison.
All‐cause mortality within 90 days
Sixteen studies measured mortality within 90 days (Annane 2013; Bechir 2013; Brunkhorst 2008; Dubin 2010; Guidet 2012; Kumar 2017; Li 2008; Mahrous 2013; McIntyre 2008; Myburgh 2012; Perner 2012; Rackow 1983; Van der Heijden 2009; Vlachou 2010; Younes 1998; Zhao 2013).
We included mortality data in this analysis in which the time point was: within 24 hours (Dubin 2010; Rackow 1983; Younes 1998); within the ICU or hospital stay (Van der Heijden 2009; Vlachou 2010); up to 30 days from hospital discharge (Kumar 2017); within 28 or 30 days (Guidet 2012; Li 2008; McIntyre 2008); within 60 days (Zhao 2013); or within 90 days (Annane 2013; Bechir 2013; Brunkhorst 2008; Myburgh 2012; Perner 2012). We did not include the mortality data reported in Mahrous 2013, as the data were not clearly reported in the abstract.
Two studies were multi‐arm studies. We combined data for both colloid groups in Zhao 2013, and for both colloid groups and both crystalloid groups in Li 2008. One study, which allowed type of colloid or crystalloid to be at the discretion of the clinician, reported mortality outcome data for participants who received only one type of fluid (Annane 2013). We included data for participants who received only hydroxyethyl starch in the colloid group, and combined data for two crystalloid groups (isotonic saline, and Ringer's lactate).
We found little or no difference in the number of participants who died within 90 days according to whether fluid resuscitation was with a starch or with a crystalloid (RR 1.01, 95% CI 0.90 to 1.14; 10,415 participants; 15 studies; I² = 36%; Analysis 1.2).
We generated a funnel plot to assess risk of publication bias and did not interpret this as indicating high risk.
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had an unclear risk of selection bias and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table for the main comparison.
All‐cause mortality within 30 days
Twelve studies measured mortality within 30 days (Annane 2013; Bechir 2013; Brunkhorst 2008; Dubin 2010; Guidet 2012; Li 2008; Mahrous 2013; McIntyre 2008; Myburgh 2012; Perner 2012; Rackow 1983; Younes 1998). We did not include mortality data reported in Mahrous 2013, as the data were not clearly reported in the abstract.
One study was a multi‐arm study (Li 2008); we combined data for both colloid groups and both crystalloid groups in this study.
We included mortality data in this analysis in which the time point was: within 24 hours (Dubin 2010; Rackow 1983; Younes 1998); and within 28 or 30 days (Annane 2013; Bechir 2013; Brunkhorst 2008; Guidet 2012; Li 2008; McIntyre 2008; Myburgh 2012; Perner 2012).
We found little or no difference in the number of participants who died within 30 days according to whether fluid resuscitation was with a starch or with a crystalloid (RR 0.99, 95% CI 0.90 to 1.09; 10,135 participants; 11 studies; I² = 12%; Analysis 1.3).
We generated a funnel plot to assess risk of publication bias and did not interpret this as indicating high risk.
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had unclear risk of selection bias and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table for the main comparison.
Transfusion of blood products
Nine studies reported the number of participants who required transfusion of blood products (Annane 2013; Brunkhorst 2008; Cifra 2003; Guidet 2012; McIntyre 2008; Nagy 1993; Perner 2012; Vlachou 2010; Wills 2005).
One study, which allowed type of colloid or crystalloid to be at the discretion of the clinician, combined data for all types of colloids (hydroxyethyl starch, gelatins, or albumin), and we could not include these data in the analysis of starches (Annane 2013). We reported data for transfusion of blood products for this study in Table 2; we noted little or no difference between groups in the need for blood products according to type of fluid.
| Study ID | Outcome | Events in colloid group: n/N | Events in crystalloid group: n/N | Effect estimate |
| Colloids (at the discretion of the clinician: HES, gelatins, or albumin) versus crystalloids | ||||
| Transfusion of blood products | 377/1414 | 358/1443 | RR 1.07, 95% CI 0.95 to 1.22; 2857 participants | |
| Renal replacement therapy | 156/1414 | 181/1443 | RR 0.88, 95% CI 0.72 to 1.08; 2857 participants | |
| Gelatin versus crystalloids | ||||
| Mortality (within 90 days) | 84/281 | 346/1035 | RR 0.89, 95% CI 0.73 to 1.09; 1388 participants | |
| Mortality (within 30 days) | 69/281 | 275/1035 | RR 0.92, 95% CI 0.74 to 1.16; 1388 participants | |
| Albumin versus crystalloid | ||||
| Adverse events: allergic reactions | 3/1050 | 4/1047 | RR 0.75, 95% CI 0.17 to 3.33; 2097 participants | |
CI: confidence interval
HES: hydroxyethyl starch
n: number of participants with an event
N: number of participants randomised to group
RR: risk ratio
For the remaining eight studies, we found that more participants required a transfusion of blood product when starches were given (RR 1.19, 95% CI 1.02 to 1.39; 1917 participants; 8 studies; I² = 14%; Analysis 1.4).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had unclear risk of selection bias, one small study had a high risk of selection bias, and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table for the main comparison.
Renal replacement therapy
Ten studies reported the number of participants who required renal replacement therapy or dialysis (Annane 2013; Bechir 2013; Brunkhorst 2008; Guidet 2012; James 2011; Mahrous 2013; McIntyre 2008; Myburgh 2012; Perner 2012; Vlachou 2010).
One study, which allowed type of colloid or crystalloid to be at the discretion of the clinician, combined data for all types of colloids (hydroxyethyl starch, gelatins, or albumin), and we could not include these data in analysis of starches (Annane 2013). We reported data for renal replacement therapy for this study in Table 2; we noted little or no difference between groups in the need for renal replacement therapy according to type of fluid.
We found that fewer participants were given renal replacement therapy when fluid resuscitation was with a crystalloid (RR 1.30, 95% CI 1.14 to 1.48; 8527 participants; 9 studies; I² = 0%; Analysis 1.5).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had unclear risk of selection bias and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table for the main comparison.
Adverse events (allergic reaction, itching, rashes)
Six studies reported adverse event data for allergic reaction, itching, or rashes (Bulger 2008; Guidet 2012; Myburgh 2012; Ngo 2001; Perner 2012; Wills 2005).
Allergic reaction
We found little or no difference in allergic reaction according to whether starches or crystalloids were used (RR 2.59, 95% CI 0.27 to 24.91; 7757 participants; 3 studies; I² = 0%; Analysis 1.6).
Itching
We found fewer incidences of itching when participants were given crystalloids (RR 1.38, 95% CI 1.05 to 1.82; 6946 participants; 2 studies; I² = 0%; Analysis 1.7).
Rashes
We found little or no difference in incidences of rashes (RR 1.61, 95% CI 0.90 to 2.89; 7007 participants; 2 studies; I² = 0%; Analysis 1.8).
We used GRADE, and assessed the level of certainty of the evidence for adverse events as very low. We downgraded the evidence by one level for study limitations because some studies had unclear risk of selection bias, and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. We downgraded the evidence by two levels for imprecision because few of our included studies reported data for these outcomes. See summary of findings Table for the main comparison.
Subgroup analysis
Tonicity of crystalloid solution
We found that many studies did not report the solution in which the colloid was suspended. Two studies compared a starch and isotonic crystalloid versus an isotonic crystalloid and reported mortality outcome data (McIntyre 2008; Myburgh 2012), two studies compared a starch and isotonic crystalloid versus a hypertonic crystalloid (Brunkhorst 2008; Wills 2005), and three studies compared a starch and hypertonic crystalloid versus a hypertonic crystalloid (Bechir 2013; Du 2011; Heradstveit 2010). We did not perform subgroup analysis on all‐cause mortality (at end of follow‐up) for this comparison because we had insufficient studies to do so meaningfully.
Sensitivity analysis
Studies at high or unclear risk of selection bias
We excluded 10 studies that we judged to have unclear risk of selection bias (Brunkhorst 2008; Du 2011; Dubin 2010; Jie 2015; Li 2008; Lu 2012; Nagy 1993; Van der Heijden 2009; Younes 1998; Zhu 2011), and one study that we judged to have high risk of selection bias from analysis of the primary outcome (Cifra 2003). This did not alter interpretation of the effect, with little or no difference between groups in all‐cause mortality (at end of follow‐up) when these studies were excluded (RR 1.03, 95% CI 0.91 to 1.17; 10,139 participants; 13 studies; I² = 34%).
Studies in which some participants in the crystalloid group were given, or may have been given, additional colloids
Some studies were at risk of bias because some participants in the crystalloid group were given, or may have been given, additional colloids. We excluded seven studies from analysis of the primary outcome (Annane 2013; Brunkhorst 2008; Cifra 2003; Du 2011; Myburgh 2012; Perner 2012; Wills 2005). Although excluding these studies did not alter interpretation of the effect for analysis of all‐cause mortality (at end of follow‐up), we noted that without these studies statistical heterogeneity was reduced from 34% to 0% (RR 0.84, 95% CI 0.70 to 1.01; 1115 participants; 17 studies; I² = 0%).
Alternative meta‐analytical effects model (fixed‐effect)
Using the alternative meta‐analytical effects model (fixed‐effect), we found no difference in interpretation of the effect, with little or no difference between groups in all‐cause mortality (at end of follow‐up) (RR 1.01, 95% CI 0.95 to 1.08; 11,177 participants; 24 studies; I² = 34%).
Studies with discrepancies in data
In one study we noted discrepancies in mortality data within the study report (Dubin 2010). We removed this study from analysis and found that it made no difference to interpretation of the effect, with little or no difference between groups in all‐cause mortality (at end of follow‐up) (RR 0.98, 95% CI 0.87 to 1.10; 11,152 participants; 23 studies; I² = 33%).
2. Dextrans versus crystalloids
All‐cause mortality at end of follow‐up
Nineteen studies measured outcome data for mortality (Alpar 2004; Baker 2009; Bulger 2008; Bulger 2010; Bulger 2011; Chavez‐Negrete 1991; Hall 1978; Mattox 1991; Modig 1986; Morrison 2011; Ngo 2001; Oliveira 2002; Vassar 1990; Vassar 1991; Vassar 1993a; Vassar 1993b; Wills 2005; Younes 1992; Younes 1997).
Six studies were multi‐arm studies. We combined data in analysis for both crystalloid groups in Bulger 2010, Bulger 2011, Ngo 2001, Vassar 1993b, and Younes 1992, and we combined data in analysis for both colloid groups and both crystalloid groups in Vassar 1993a.
We included mortality data in this analysis in which the time point was: within 24 hours (Chavez‐Negrete 1991); within 48 hours (Hall 1978); until hospital discharge (Vassar 1991; Vassar 1993a; Vassar 1993b; Younes 1992); or was unknown (Alpar 2004; Modig 1986; Ngo 2001; Oliveira 2002; Wills 2005). The remaining studies reported data at 28 or 30 days (Baker 2009; Bulger 2008; Bulger 2010; Bulger 2011; Mattox 1991; Morrison 2011; Vassar 1990; Younes 1997).
We found little or no difference in the number of participants who died at end of follow‐up according to whether fluid resuscitation was with dextran or with a crystalloid (RR 0.99, 95% CI 0.88 to 1.11; 4736 participants; 19 studies; I² = 7%; Analysis 2.1).
We generated a funnel plot to assess risk of publication bias. One study was an outlier in this plot, which we could not explain, but, because the only outlier was a small study from 1991 (Chavez‐Negrete 1991), we did not believe this was evidence of a high risk of publication bias. See Figure 5.
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had unclear risk of selection bias and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table 2.
All‐cause mortality within 90 days and within 30 days
Ten studies measured mortality within 30 days (Baker 2009; Bulger 2008; Bulger 2010; Bulger 2011; Chavez‐Negrete 1991; Hall 1978; Mattox 1991; Morrison 2011; Vassar 1990; Younes 1997). No studies reported mortality within 90 days, and we included the same data for both outcome time points for this comparison.
Two studies were multi‐arm studies (Bulger 2010; Bulger 2011). We combined the data in analysis for both crystalloid groups.
We included mortality data in this analysis in which the time point was: within 24 hours (Chavez‐Negrete 1991); within 48 hours (Hall 1978); and within 28 or 30 days (Baker 2009; Bulger 2008; Bulger 2010; Bulger 2011; Mattox 1991; Morrison 2011; Vassar 1990; Younes 1997).
We found little or no difference in the number of participants who died within 90 days and within 30 days according to whether fluid resuscitation was with dextran or with a crystalloid (RR 0.99, 95% CI 0.87 to 1.12; 3353 participants; 10 studies; I² = 0%; Analysis 2.2).
We generated a funnel plot to assess risk of publication bias. One study was an outlier in this plot, which we could not explain, but, because the only outlier was a small study from 1991 (Chavez‐Negrete 1991), we did not believe this was evidence of a high risk of publication bias.
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had unclear risk of selection bias and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table 2.
Transfusion of blood products
Three studies reported the number of participants requiring a blood transfusion (Bulger 2011; Ngo 2001; Wills 2005). Bulger 2011, a multi‐arm study, reported blood transfusion of 9 units or fewer of blood, and 10 units or fewer of blood. In analysis, we combined data in the two crystalloids groups in Bulger 2011 for 9 units or fewer of blood.
We found little or no difference in participants requiring a transfusion of blood products according to whether participants were given dextran or a crystalloid (RR 0.92, 95% CI 0.77 to 1.10; 1272 participants; 3 studies; I² = 0%; Analysis 2.3).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as very low. We downgraded the evidence by two levels for study limitations because we noted that in two studies some participants were given additional colloids in the crystalloid group, and in another study we could not be certain whether some participants in the crystalloids groups had also received up to 2000 mL colloid resuscitation prior to randomisation. In addition, we were unable to assess risk of selective reporting bias because, for many studies there was a lack of prospective clinical trials registration. See summary of findings Table 2.
Renal replacement therapy
No studies reported data for this outcome.
Adverse events (allergic reaction, itching, rashes)
Four studies reported allergic reactions (Mattox 1991; Ngo 2001; Vassar 1990; Vassar 1991), with event data in only one study (Ngo 2001).
We found little or no difference between study fluids in cases of allergic reaction (RR 6.00, 95% CI 0.25 to 144.93; 739 participants; 4 studies; Analysis 2.4).
We used the GRADE approach to downgrade the certainty of the evidence for adverse events to very low. We downgraded by one level for study limitations because one study had an unclear risk of selection bias and we were unable to assess risk of selective outcome reporting bias in all studies. We downgraded by two levels for imprecision because evidence was from few studies with few events.
Subgroup analysis
Tonicity of crystalloid solution
Eight studies used a dextran solution with hypertonic saline (HSD) versus an isotonic crystalloid (which was normal saline) and reported mortality outcome data (Baker 2009; Bulger 2010; Bulger 2011; Morrison 2011; Oliveira 2002; Vassar 1993a; Younes 1992; Younes 1997); two studies used a dextran solution with an isotonic crystalloid versus a hypertonic crystalloid (Ringer's lactate) and reported mortality outcome data (Hall 1978; Wills 2005); and five studies used HSD versus Ringer's lactate and reported mortality outcome data (Alpar 2004; Bulger 2008; Chavez‐Negrete 1991; Vassar 1990; Vassar 1991). One multi‐arm study used two concentrations of HSD that were appropriate to combine in subgroup analysis versus two types of hypertonic crystalloid (hypertonic saline and Ringer's lactate), which were also appropriate to combine in subgroup analysis (Vassar 1993b). We did not include three studies in subgroup analysis because the type of crystalloid in which the dextran was suspended was not reported (Modig 1986; Ngo 2001), or a variety of crystalloids was used in the comparison group (Mattox 1991). We found no evidence of a difference between studies in use of isotonic or hypertonic crystalloid solutions for all‐cause mortality (at end of follow‐up) (P = 0.92). See Analysis 5.1.
Sensitivity analysis
Studies at high or unclear risk of selection bias
We judged two studies to have high risk of selection bias (Alpar 2004; Modig 1986), and five studies to have unclear risk of selection bias (Chavez‐Negrete 1991; Hall 1978; Vassar 1990; Younes 1992; Younes 1997), and excluded them from analysis of mortality. This did not alter interpretation of the effect for all‐cause mortality (at the end of follow‐up); there was little or no difference between groups when these studies were excluded (RR 1.03, 95% CI 0.91 to 1.16; 3940 participants; 12 studies; I² = 6%).
Studies in which some participants in the crystalloid group were given, or may have been given, additional colloids
Some studies were at risk of bias because some participants in the crystalloid group were given, or may have been given additional colloids. We excluded six studies from analysis of mortality at end of follow‐up (Baker 2009; Bulger 2011; Chavez‐Negrete 1991; Ngo 2001; Vassar 1991; Wills 2005). This did not alter interpretation of the effect on all‐cause mortality (at end of follow‐up); there was little or no difference between groups when these studies were excluded (RR 1.00, 95% CI 0.88 to 1.15; 3185 participants; 13 studies; I² = 11%).
Alternative meta‐analytical effects model (fixed‐effect)
Using the alternative meta‐analytical effects model (fixed‐effect), did not alter interpretation of the effect on all‐cause mortality (at end of follow‐up); there was little or no difference between groups when we used the fixed‐effect model (RR 0.99, 95% CI 0.89 to 1.10; 4570 participants; 18 studies; I² = 7%).
Studies with discrepancies in data
We included no studies with serious discrepancies in data.
3. Gelatins versus crystalloids
All‐cause mortality at end of follow‐up
Six studies reported outcome data for mortality (Annane 2013; Evans 1996; Ngo 2001; Upadhyay 2005; Van der Heijden 2009; Wu 2001). One study reported the time point as within the ICU or hospital stay (Van der Heijden 2009), one study was at 90 days (Annane 2013), and the remaining time points were unknown.
One study was a multi‐arm study (Ngo 2001); we combined data in analysis for both crystalloid groups.
One study, which allowed type of colloid or crystalloid to be at the discretion of the clinician, reported mortality outcome data for participants who received only one type of fluid (Annane 2013). We included data for participants who received only gelatins in the colloid group, and combined data for two crystalloid groups (isotonic saline and Ringer's lactate).
We found little or no difference in the number of participants who had died from any cause at the end of follow‐up according to whether fluid resuscitation was with gelatins or with a crystalloid (RR 0.89, 95% CI 0.74 to 1.08; 1698 participants; 6 studies; I² = 0%; Analysis 3.1).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as low. We downgraded the evidence by one level for study limitations because risk of selection bias was unclear in some studies and we were unable to assess risk of selective outcome reporting bias in some studies that were not registered with clinical trials registers. We downgraded by one level for imprecision because evidence was from few studies, and we could not be certain of time points for data collection. summary of findings Table 3.
All‐cause mortality within 90 days
One study reported mortality data at 90 days (Annane 2013). This study allowed the type of colloid or crystalloid to be at the discretion of the clinician and reported mortality outcome data for participants who received only one type of fluid. We combined data for the two crystalloid groups (normal saline and Ringer's lactate) and used RevMan 5 to calculate an effect estimate (Review Manager 2014). Study data are reported in Table 2.
We found little or no difference in the number of participants who died from any cause within 90 days according to whether fluid resuscitation was with gelatins or with a crystalloid (RR 0.89, 95% CI 0.73 to 1.09; 1388 participants; 1 study).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as low. We downgraded by two levels for imprecision because evidence was from a single study.
All‐cause mortality within 30 days
One study reported mortality data at 28 days (Annane 2013). This study allowed type of colloid or crystalloid to be at the discretion of the clinician and reported mortality outcome data for participants who received only one type of fluid. We combined data for the two crystalloid groups (isotonic saline and Ringer's lactate) and used the RevMan 5 to calculate an effect estimate (Review Manager 2014). Study data are reported in Table 2.
We found little or no difference in the number of participants who died from any cause within 30 days according to whether fluid resuscitation was with gelatins or with a crystalloid (RR 0.92, 95% CI 0.74 to 1.16; 1388 participants; 1 study).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as low. We downgraded by two levels for imprecision because evidence was from a single study.
Transfusion of blood products
Three studies measured the number of participants who needed a transfusion of blood products (Annane 2013; Ngo 2001; Wu 2001). However, we could not use the data in Wu 2001, because it was not reported by group (five participants overall required blood transfusion), and we could not report the data in Annane 2013, because it was not reported separately for participants who received only gelatins (we noted little or no difference between people receiving either hydroxyethyl starch, gelatins, or albumin; Table 2).
The remaining study reported one participant in the gelatins group who required a blood transfusion following a severe epistaxis (Ngo 2001). We used the calculator in RevMan 5 (Review Manager 2014), and found little or no difference between groups in need for blood transfusion (RR 5.89, 95% CI 0.24 to 142.41; 167 participants; 1 study).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as very low. We downgraded by one level for study limitations because we were unable to assess risk of selective outcome reporting bias due to lack of prospective clinical trials registration, and some participants in the crystalloid groups also received colloids. We downgraded two levels for imprecision because evidence was from a single small study with very few events.
Renal replacement therapy
One study, which allowed type of colloid or crystalloid to be at the discretion of the clinician, reported number of participants who required renal replacement therapy but did not report these data according to type of colloid received (Annane 2013). We did not include these data in our analysis of gelatins because the types of colloid used were either hydroxyethyl starch, gelatins, or albumin. We included data for renal replacement therapy for Annane 2013 in Table 2; we noted little or no difference between groups in the need for renal replacement therapy according to whether a colloid (hydroxyethyl starch, gelatins, or albumin) or a crystalloid was used.
Adverse events (allergic reaction, itching, rashes)
One study reported that five participants in the gelatins group had an allergic reaction (Ngo 2001). We used the calculator in RevMan 5 (Review Manager 2014), and found little or no difference between groups in incidences of allergic reactions (RR 21.61, 95% CI 1.22 to 384.05; 167 participants; 1 study).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as very low. We downgraded by one level for study limitations because we were unable to assess risk of selective outcome reporting bias due to lack of prospective clinical trials registration, and because some participants in the crystalloid groups also received colloids. We downgraded two levels for imprecision because evidence was from a single small study with very few events.
Subgroup analysis
Tonicity of crystalloid solution
We found insufficient studies to conduct meaningful subgroup analysis. Of the six studies that reported mortality outcome data, five studies reported using a modified gelatin solution suspended in isotonic crystalloid solution. Two studies used Haemaccel (Evans 1996; Upadhyay 2005), two studies used Gelofusine (Van der Heijden 2009; Wu 2001), and one study used Gelafundin (Ngo 2001). The remaining study, in which type of colloid solution was at the discretion of the clinician, did not specify the gelatin solution (Annane 2013).
Sensitivity analysis
Studies at high or unclear risk of selection bias
We excluded one study that we judged to have an unclear risk of selection bias from analysis of mortality (Evans 1996). This did not alter interpretation of the effect for all‐cause mortality (at the end of follow‐up), with little or no difference between groups (RR 0.90, 95% CI 0.74 to 1.08; 1673 participants; 5 studies; I² = 0%).
Studies in which some participants in the crystalloid group were given, or may have been given, additional colloids
Some studies were at risk of bias because some participants in the crystalloid group were given, or may have been given additional colloids. We excluded two studies from analysis of the primary outcome (Annane 2013; Ngo 2001). This did not alter interpretation of the effect for all‐cause mortality (at the end of follow‐up), with little or no difference between groups (RR 0.94, 95% CI 0.52 to 1.72; 143 participants; 4 studies; I² = 0%).
Alternative meta‐analytical effects model (fixed‐effect)
Using the alternative meta‐analytical effects model (fixed‐effect) did not alter interpretation of the effect for all‐cause mortality (at the end of follow‐up), with little or no difference between groups (RR 0.89, 95% CI 0.74 to 1.08; 1689 participants; 6 studies; I² = 0%).
Studies with discrepancies in data
We included no studies with serious discrepancies in data.
4. Albumin or FFP versus crystalloids
All‐cause mortality at end of follow‐up
Twenty‐one studies reported mortality (Annane 2013; Caironi 2014; Cooper 2006; Finfer 2004; Goodwin 1983; Jelenko 1979; Lowe 1977; Lucas 1978; Maitland 2005; Maitland 2011; Martin 2005; McIntyre 2012; Metildi 1984; O'Mara 2005; Park 2015; Philips 2015; Pockaj 1994; Quinlan 2004; Rackow 1983; Shah 1977; Van der Heijden 2009). One study was a multi‐arm study and we combined data in analysis for both crystalloid groups (Jelenko 1979).
We did not include outcome data from one study (McIntyre 2012), as mortality data were reported overall, not by group (12 of 50 participants died).
We included mortality data in this analysis in which the time point was: within 24 hours (Rackow 1983); within seven days (Philips 2015); within the ICU or hospital stay (Van der Heijden 2009); within 90 days (Annane 2013; Caironi 2014); or was unknown (Goodwin 1983; Jelenko 1979; Lowe 1977; Lucas 1978; Maitland 2005; Metildi 1984; O'Mara 2005; Pockaj 1994; Shah 1977). The remaining studies reported data at 28 or 30 days.
We found little or no difference in the number of participants who had died from any cause at the end of follow‐up according to whether fluid resuscitation was with albumin or FFP compared to a crystalloid (RR 0.98, 95% CI 0.92 to 1.06; 13,047 participants; 20 studies; I² = 7%; Analysis 4.1).
We generated a funnel plot to assess risk of publication bias. One study was an outlier in this plot, which we could not explain, but, because the only outlier was a small study from 1978, we did not believe this was evidence of a high risk of publication bias. See Figure 6.
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had unclear risk of selection bias, and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table 4.
All‐cause mortality within 90 days
Eleven studies measured mortality within 90 days (Annane 2013; Caironi 2014; Cooper 2006; Finfer 2004; Maitland 2011; Martin 2005; McIntyre 2012; Park 2015; Philips 2015; Quinlan 2004; Rackow 1983). We did not include outcome data from one study (McIntyre 2012), as mortality data were reported overall, not by group (12 of 50 participants died).
We included mortality data in this analysis in which the time point was: within 24 hours (Rackow 1983); within seven days (Philips 2015); within 30 days (Cooper 2006; Finfer 2004; Maitland 2011; Martin 2005; Park 2015; Quinlan 2004); and within 90 days (Annane 2013; Caironi 2014).
We found little or no difference in the number of participants who died from any cause within 90 days according to whether fluid resuscitation was with albumin or FFP compared to a crystalloid (RR 0.98, 95% CI 0.92 to 1.04; 12,492 participants; 10 studies; I² = 0%; Analysis 4.2).
We generated a funnel plot to assess risk of publication bias. One study was an outlier in this plot, which we could not explain; we could not be certain whether this indicated risk of publication bias.
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had unclear risk of selection bias, and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table 4.
All‐cause mortality within 30 days
Eleven studies measured mortality within 30 days (Annane 2013; Caironi 2014; Cooper 2006; Finfer 2004; Maitland 2011; Martin 2005; McIntyre 2012; Park 2015; Philips 2015; Quinlan 2004; Rackow 1983). We did not include outcome data from one study (McIntyre 2012), as mortality data were reported overall, not by group (12 of 50 participants died).
We included mortality data in this analysis in which the time point was: within 24 hours (Rackow 1983); within seven days (Philips 2015); or within 28 or 30 days (Annane 2013; Caironi 2014; Cooper 2006; Finfer 2004; Maitland 2011; Martin 2005; Park 2015; Quinlan 2004).
We found little or no difference in the number of participants who died from any cause within 30 days according to whether fluid resuscitation was with albumin or FFP compared to a crystalloid (RR 0.99, 95% CI 0.93 to 1.06; 12,506 participants; 10 studies; I² = 0%; Analysis 4.3).
We generated a funnel plot to assess risk of publication bias. One study was an outlier in this plot, which we could not explain; we could not be certain whether this indicated risk of publication bias.
We used GRADE, and assessed the level of certainty of the evidence for this outcome as moderate. We downgraded the evidence by one level for study limitations because some studies had unclear risk of selection bias, and because, for many studies, we were unable to assess risk of selective reporting bias due to lack of prospective clinical trials registration. See summary of findings Table 4.
Transfusion of blood products
Four studies reported outcome data for transfusion of blood products (Annane 2013; Cooper 2006; Lowe 1977; Pockaj 1994).
One study, which allowed type of colloid or crystalloid to be at the discretion of the clinician, reported the number of participants who received a blood product, but these data were not reported according to type of colloid received (Annane 2013). We did not include these data in analysis of albumin or FFP because the types of colloid used were either hydroxyethyl starch, gelatins, or albumin. We included data for transfusion of blood products for Annane 2013 in Table 2; we noted little or no difference between groups in the need for blood products according to whether participants were given a colloid (hydroxyethyl starch, gelatins, or albumin) or a crystalloid.
We found little or no difference in the number of participants who had transfusion of blood products according to whether fluid resuscitation was with albumin or FFP compared to a crystalloid (RR 1.31, 95% CI 0.95 to 1.80; 290 participants; 3 studies; I² = 0%; Analysis 4.4).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as very low. We downgraded the evidence by two levels for study limitations because some studies had unclear risk of selection bias, and we noted baseline imbalances in one study. We downgraded by one level for imprecision because analysis included few studies with few participants. See summary of findings Table 4.
Renal replacement therapy
Four studies collected outcome data related to renal replacement therapy (Annane 2013; Caironi 2014; Finfer 2004; Park 2015).
One study, which allowed type of colloid or crystalloid to be at the discretion of the clinician, reported the number of participants who required renal replacement therapy, but these data were not reported according to type of colloid received (Annane 2013). We did not include these data in analysis of albumin or FFP because the types of colloid used were either hydroxyethyl starch, gelatins, or albumin. We included data for renal replacement therapy for Annane 2013 in Table 2; we noted little or no difference between groups in the need for renal replacement therapy according to type of fluid. The study report for Park 2015 was an abstract that stated that renal replacement therapy was a secondary outcome, but outcome data were not reported in the abstract. Data in Finfer 2004 were reported for a smaller subgroup of participants who had severe sepsis; we included these data in the analysis.
We noted little or no difference according to type of fluid resuscitation in the number of participants who received renal replacement therapy (RR 1.11, 95% CI 0.96 to 1.27; 3028 participants; 2 studies; I² = 0%; Analysis 4.5).
We used GRADE, and assessed the level of certainty of the evidence for this outcome as low. We downgraded the evidence by two levels for study limitations because we noted baseline imbalances and because we could not be certain whether participants in the crystalloids group in one study may have received colloids. See summary of findings Table 4.
Adverse events (allergic reaction, itching, rashes)
One study reported incidences of allergic reaction (Maitland 2011). We used RevMan 5 to calculate an effect estimate (Review Manager 2014); we noted little or no difference between groups in allergic reactions (RR 0.75, 95% CI 0.17 to 3.33; 2097 participants; 1 study; Table 2).
We used the GRADE approach to downgrade the certainty of the evidence to very low. We downgraded by one level for study limitations because we were unable to assess the risk of selective outcome reporting bias since the study authors did not report clinical trials registration. We downgraded by two levels because evidence was from one study with few events. See summary of findings Table 4.
Subgroup analysis
Tonicity of crystalloid solution
We found that many studies did not report the solution in which the colloid was suspended. One study used albumin with an isotonic crystalloid (suspended in normal saline) versus an isotonic crystalloid (normal saline) (Pockaj 1994), and one study used albumin with an isotonic crystalloid (normal saline) versus a hypertonic crystalloid (Ringer's lactate) (Cooper 2006). One study used albumin with a hypertonic crystalloid (hypertonic saline) versus a hypertonic crystalloid (Ringer's lactate) (Jelenko 1979), and five studies used albumin with a hypertonic crystalloid (Ringer's lactate) versus a hypertonic crystalloid (Ringer's lactate) (Goodwin 1983; Lowe 1977; Metildi 1984; O'Mara 2005; Shah 1977). We found insufficient studies to conduct meaningful subgroup analysis.
Sensitivity analysis
Studies at high or unclear risk of selection bias
We excluded two studies that we judged to have high risk of selection bias (Lowe 1977; Lucas 1978), and nine studies that we judged to have unclear risk of selection bias from analysis of mortality (Goodwin 1983; Maitland 2005; Metildi 1984; Park 2015; Philips 2015; Pockaj 1994; Quinlan 2004; Rackow 1983; Shah 1977). This did not alter interpretation of the effect for all‐cause mortality (at end of follow‐up), with little or no difference between groups (RR 0.98, 95% CI 0.91 to 1.04; 12,111 participants; 9 studies; I² = 0%).
Studies in which some participants in the crystalloid group were given, or may have been given, additional colloids
Some studies were at risk of bias because some participants in the crystalloid group were given, or may have been given, additional colloids. We excluded three studies from analysis of mortality (Annane 2013; Finfer 2004; Goodwin 1983). This did not alter interpretation of the effect for all‐cause mortality (at end of follow‐up), with little or no difference between groups (RR 0.96, 95% CI 0.88 to 1.04; 4970 participants; 17 studies; I² = 0%).
Alternative meta‐analytical effects model (fixed‐effect)
Using the alternative meta‐analytical effects model (fixed‐effect) did not alter interpretation of the effect for all‐cause mortality (at end of follow‐up), with little or no difference between groups (RR 0.99, 95% CI 0.93 to 1.05; 13,047 participants; 20 studies; I² = 7%).
Studies with discrepancies in data
We noted a discrepancy in mortality outcome data in different published reports for Lucas 1978. In sensitivity analysis, we used alternative data reported in a later publication, Lucas 1980, which is cited as part of Lucas 1978. This did not alter interpretation of the effect for all‐cause mortality (at end of follow‐up), with little or no difference between groups (RR 0.98, 95% CI 0.92 to 1.04; 13,047 participants; 20 studies; I² = 0%).
Discussion
Summary of main results
We included 69 studies comparing colloids (suspended in any solution) versus crystalloids (isotonic or hypertonic) in critically ill people who required fluid resuscitation. In addition, we identified seven studies that are awaiting classification (two studies were published only as abstracts with insufficient information, three completed studies are listed on clinical trials register sites without publication of full reports, and two studies require translation from Russian), and three ongoing studies.
We reported four comparisons for each type of colloid (starches; dextrans; gelatins; and albumin or FFP) versus crystalloids. We collected outcome data for all‐cause mortality at end of follow‐up, within 90 days, and within 30 days; need for transfusion of blood products; need for renal replacement therapy; and adverse events (allergic reaction, itching, and rashes).
We found moderate‐certainty evidence that there is probably little or no difference in all‐cause mortality at the end of follow‐up, within 90 days, or within 30 days between colloids (which are: starches; dextrans; or albumin or FFP) or crystalloids for fluid resuscitation. We found low‐certainty evidence that there may be little or no difference in all‐cause mortality at the end of follow‐up, within 90 days, or within 30 days between gelatins or crystalloids for fluid resuscitation.
We found moderate‐certainty evidence that using starches probably slightly increases the need for transfusion of blood products. Studies comparing dextrans, gelatins, and albumin or FFP to crystalloids, found little or no difference in the need for transfusion of blood products but certainty of this evidence was very low.
We found moderate‐certainty evidence that using starches probably slightly increases the need for renal replacement therapy. We found low‐certainty evidence from two studies that albumin or FFP versus crystalloids may make little or no difference to the need for renal replacement therapy. We could not use data from renal replacement therapy from one study of gelatins because data were not reported by type of colloid solution, and no studies of dextrans measured this outcome.
Evidence for adverse events (allergic reactions, itching, or rashes) is very low certainty because studies often did not report events. For starches, we found little or no difference between either fluid group in allergic reactions in three studies, but we found more incidences of itching and rashes in two studies. For dextrans, gelatins, and for albumin or FFP, we found little or no difference between groups in allergic reactions.
Overall completeness and applicability of evidence
We identified 69 studies with 30,020 participants who were undergoing fluid resuscitation for conditions that indicated that they were critically ill. The conditions being managed with fluid resuscitation varied, and settings also varied; 10 studies were based in an out‐of‐hospital setting.
All studies compared colloids versus crystalloids. We found 28 studies using starch solutions, 20 studies using dextran solutions, seven studies using gelatins, and 22 studies using albumin or FFP. Some study authors did not report the specific nature of the solution the colloid was suspended in, and other studies reported the use of either an isotonic or hypertonic crystalloid suspension solution. Because of the different use of crystalloid solutions for this purpose, and the different compositions of the comparative crystalloids, we could not be certain whether comparisons by type of colloid were always equivalent. We were unable to perform meaningful subgroup analysis for most types of colloids because of limitations in reporting of suspension solutions. Also, individual study protocols for the concentration, quantity, and timing of administration of fluids varied.
We also noted that studies ranged in date of publication from 1977 to 2016, and, while we did not consider the potential influence of date on our results, it is possible that changes in management of critically ill people may mean that some study data may not be generalisable to the current clinical context.
Quality of the evidence
We used GRADE to consider the effect of study limitations on our outcomes. We found many studies did not report adequate methods of randomisation or allocation concealment, and we could not be certain of the risk of selection bias. We noted that some studies did not report whether clinicians were blinded to the type of study fluids they were giving to participants, or whether outcome assessors were blinded. However, we did not consider risk of performance or detection bias to be likely for mortality, and we did not believe lack of performance or detection bias for our remaining outcomes (transfusion of blood products, renal replacement therapy, or adverse events) were important reasons to downgrade the evidence for this review. We noted that few studies were registered prospectively with clinical trials registers, and although many studies predate the expectation of clinical trials registration, we could not rule out the risk of selective outcome reporting in this review. We included some studies in which some participants in the crystalloid groups were given, or may have been given, additional colloids. Because we could not be certain of the influence of this additional colloid use on the results, we judged these studies to have a high risk of bias and downgraded the certainty of the evidence accordingly. We downgraded the certainty of the evidence for some of our outcomes because of imprecision; for these outcomes, we found evidence from few studies.
Potential biases in the review process
We conducted a thorough search and used two review authors independently to assess study eligibility, extract data, and assess risk of bias in included studies, and believe that this reduced potential bias in the review process. However, we made a post hoc decision to change criteria for considering studies in this review update from the previous version of the review (Perel 2013). This decision led to the exclusion of 36 previously included studies. Our intention was to create a more focused review, with a more comparable participant group, once we had excluded participants scheduled for a wide range of elective surgical procedures; we acknowledge that the exclusion of this large number of studies may also have influenced a change in results since the previous review publication.
We included a number of studies in the review in which participants in the crystalloid group may have received additional colloids. It is possible that our decision to include these studies in our primary analysis may have introduced clinical differences, or bias, between studies, and subsequently influenced our results. We assessed this decision during sensitivity analysis for our primary outcome (all‐cause mortality (at end of follow‐up)) and found that the interpretation of our effect estimates was the same regardless of whether we included these studies. However, we noted that in our comparison of starches versus crystalloids, inclusion of these studies increased statistical heterogeneity (I² = 34%); we did not explore this further in the review.
We included additional outcomes in this review; we intended to explore other effects of colloids and crystalloids for fluid resuscitation. We limited these additional outcomes to need for blood transfusion, need for renal replacement therapy, and three possible adverse events (allergic reactions, itching, and rashes). We acknowledge that our review is limited to only eight outcomes in four types of colloid solutions, and therefore does not explore all the potential risks and benefits of using either colloids or crystalloids in the critically ill setting.
The review does not include seven studies that are awaiting classification (Halim 2016; Bulanov 2004; Charpentier 2011; NCT00890383; NCT01337934; NCT02064075; Protsenko 2009). We did not seek translation of the full study reports for four studies that were reported in Chinese (Jie 2015; Li 2008; Lu 2012; Zhu 2011); our judgements and data were limited to information available in the abstract, or the tables.
Agreements and disagreements with other studies or reviews
The results of this review differ from those of the previous version (Perel 2013), which found an increase in mortality when participants were given starches rather than crystalloids for fluid resuscitation. For this 2018 update, because of changes in the criteria for considering studies in this review, we excluded studies of elective surgical patients. However, because of a decision to include additional outcomes, we re‐ran searches from database inception and included 27 new studies in the review, 13 of which compared starches to crystalloids. Our moderate‐certainty evidence, which demonstrates little or no difference in all‐cause mortality for starches, includes a large number of studies, but we cannot be certain whether the difference in our results is because we excluded elective surgical patients. Results for mortality for dextrans, gelatins, and albumin or FFP were the same as those in Perel 2013.
Whilst other systematic reviews may concentrate on particular types of colloids, or particular participant groups, our findings for mortality appear relatively comparable. He 2015 found no increase in mortality with hydroxyethyl starch for non‐septic patients in the intensive care unit, as did Haase 2013 for patients with sepsis. However, Gattas 2013, which included participants undergoing surgical procedures, reported a non‐statistically significant increase in mortality when starches were used. In reviews of other colloids, de Crescenzo 2017 found no effect on mortality of trauma patients treated in a prehospital setting with dextrans; Qureshi 2016 found no increase in mortality of critically ill, trauma, and surgical patients with any type of colloid; and Eljaiek 2017 found no difference in mortality of burn patients who were given albumin for fluid replacement.
Also, we found some comparable results for renal replacement and blood transfusion. Haase 2013 and Gattas 2013 found that more participants given starches required renal replacement therapy, whilst Haase 2013 also found this effect with starches for transfusion of red blood cells. Similarly, Qureshi 2016 found an increase in acute kidney failure requiring renal replacement that was more pronounced for those who were given fluid resuscitation with starches, but this result was not replicated by He 2015, who found no difference in incidence of renal replacement therapy with use of starches.

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. We did not make judgements for studies that did not report outcomes of interest in the review, which are indicated by blank spaces

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. We did not make judgements for studies that did not report outcomes of interest in the review, which are indicated by blank spaces

Funnel plot of comparison 1. Starches vs crystalloid, outcome: 1.1 mortality at end of follow‐up

Funnel plot of comparison 2. Dextrans vs crystalloid, outcome: 2.1 mortality at end of follow‐up

Funnel plot of comparison 4. Albumin and FFP vs crystalloid, outcome: 4.1 mortality at end of follow‐up

Comparison 1 Starches vs crystalloid, Outcome 1 Mortality at end of follow‐up.

Comparison 1 Starches vs crystalloid, Outcome 2 Mortality within 90 days.

Comparison 1 Starches vs crystalloid, Outcome 3 Mortality within 30 days.

Comparison 1 Starches vs crystalloid, Outcome 4 Transfusion of blood product.
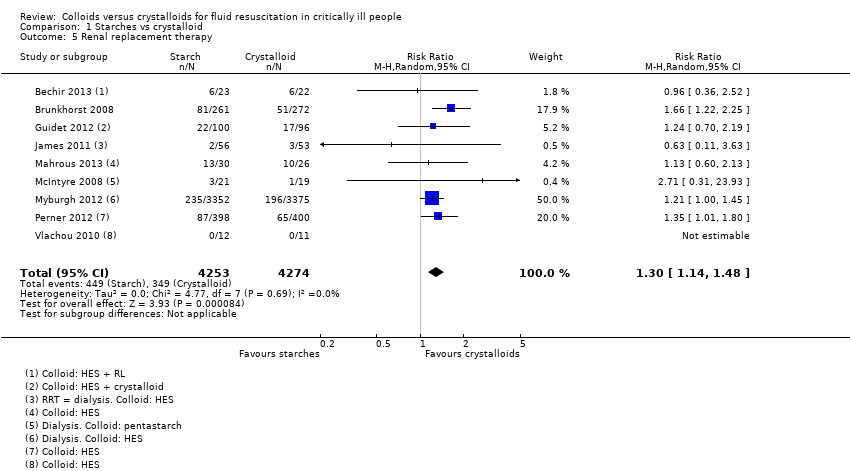
Comparison 1 Starches vs crystalloid, Outcome 5 Renal replacement therapy.

Comparison 1 Starches vs crystalloid, Outcome 6 Adverse event: allergic reaction.

Comparison 1 Starches vs crystalloid, Outcome 7 Adverse event: itching.

Comparison 1 Starches vs crystalloid, Outcome 8 Adverse event: rash.

Comparison 2 Dextrans vs crystalloid, Outcome 1 Mortality at end of follow‐up.

Comparison 2 Dextrans vs crystalloid, Outcome 2 Mortality within 90 days and 30 days.
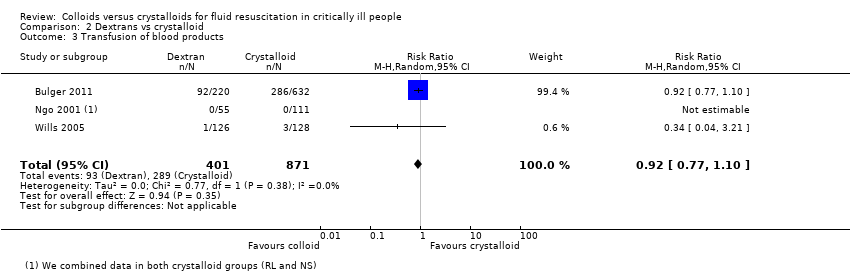
Comparison 2 Dextrans vs crystalloid, Outcome 3 Transfusion of blood products.

Comparison 2 Dextrans vs crystalloid, Outcome 4 Adverse events: allergic reaction.

Comparison 3 Gelatins vs crystalloid, Outcome 1 Mortality at end of follow‐up.

Comparison 4 Albumin or FFP vs crystalloid, Outcome 1 Mortality at end of follow‐up.

Comparison 4 Albumin or FFP vs crystalloid, Outcome 2 Mortality within 90 days.

Comparison 4 Albumin or FFP vs crystalloid, Outcome 3 Mortality within 30 days.
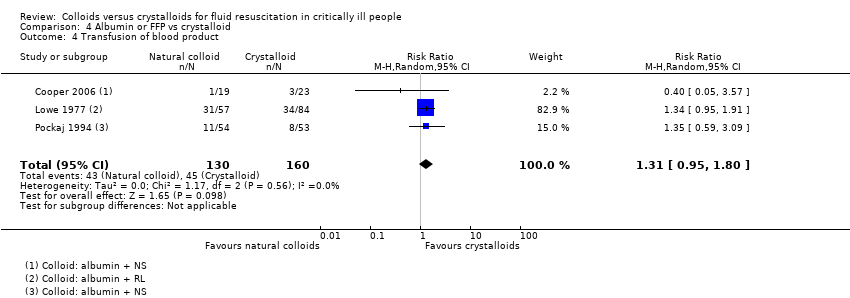
Comparison 4 Albumin or FFP vs crystalloid, Outcome 4 Transfusion of blood product.
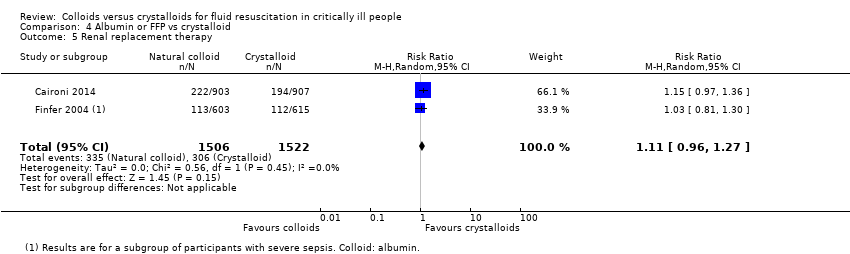
Comparison 4 Albumin or FFP vs crystalloid, Outcome 5 Renal replacement therapy.
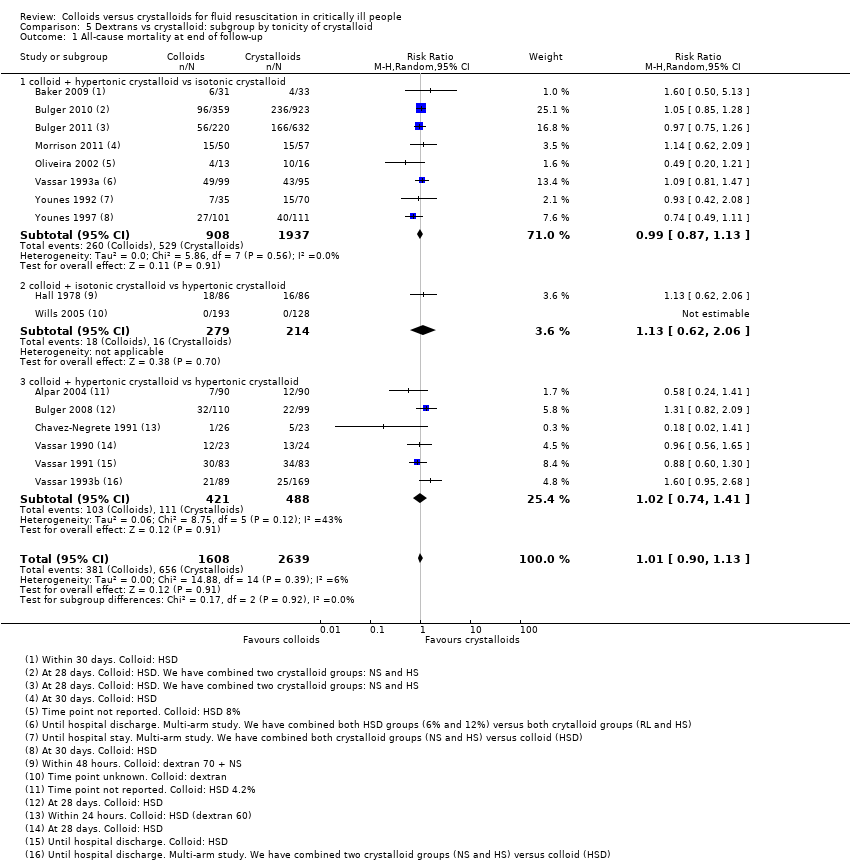
Comparison 5 Dextrans vs crystalloid: subgroup by tonicity of crystalloid, Outcome 1 All‐cause mortality at end of follow‐up.
| Starches compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with starches | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.97 | 11,177 | ⊕⊕⊕⊝ Moderatea | We excluded data from 1 study because we could not be certain whether it accounted for attrition | |
| 233 per 1000 | 226 per 1000 | |||||
| All‐cause mortality (at 90 days) | Study population | RR 1.01 | 10,415 | ⊕⊕⊕⊝ Moderateb | We excluded data from 1 study because we could not be certain whether it accounted for attrition | |
| 238 per 1000 | 241 per 1000 | |||||
| All‐cause mortality (within 30 days) | Study population | RR 0.99 | 10,135 | ⊕⊕⊕⊝ Moderateb | We excluded data from 1 study because we could not be certain whether it accounted for attrition | |
| 191 per 1000 | 189 per 1000 | |||||
| Transfusion of blood products | Study population | RR 1.19 | 1917 | ⊕⊕⊕⊝ Moderatea | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only starches; we noted little or no difference between groups in need for transfusion of blood products in this study | |
| 299 per 1000 | 356 per 1000 | |||||
| Renal replacement therapy | Study population | RR 1.30 | 8527 | ⊕⊕⊕⊝ Moderateb | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only starches; we noted little or no difference between groups in need for renal replacement therapy in this study | |
| 82 per 1000 | 106 per 1000 | |||||
| Adverse events | Allergic reaction | ⊕⊝⊝⊝ Very lowc | ||||
| Study population | RR 2.59 (0.27 to 24.91) | 7757 (3 studies) | ||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Itching | ||||||
| Study population | RR 1.38 (1.05 to 1.82) | 6946 (2 studies) | ||||
| 26 per 1000 | 35 per 1000 (27 to 46) | |||||
| Rashes | ||||||
| Study population | RR 1.61 (0.90 to 2.89) | 7007 (2 studies) | ||||
| 5 per 1000 | 9 per 1000 (5 to 15) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; some included studies had unclear risk of selection bias, one small study had a high risk of selection bias, and we were often unable to assess risk of selective reporting bias because many included studies did not have prospective clinical trials registration. | ||||||
| Dextrans compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with dextrans | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.99 | 4736 | ⊕⊕⊕⊝ Moderatea | ||
| 237 per 1000 | 235 per 1000 | |||||
| All‐cause mortality (within 90 days and within 30 days) | Study population | RR 0.99 | 3353 | ⊕⊕⊕⊝ Moderatea | ||
| 258 per 1000 | 256 per 1000 | |||||
| Transfusion of blood products | Study population | RR 0.92 (0.77 to 1.10) | 1272 (3 studies) | ⊕⊝⊝⊝ Very lowb | ||
| 332 per 1000 | 305 per 1000 (255 to 365) | |||||
| Renal replacement therapy | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse events | Allergic reactions | |||||
| Study population | RR 6.00 (0.25 to 144.93) | 739 (4 studies) | ⊕⊝⊝⊝ Very lowc | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Itching | ||||||
| Study population | Not measured | ‐ | ||||
| ‐ | ‐ | |||||
| Rashes | ||||||
| Study population | Not measured | ‐ | ||||
| ‐ | ‐ | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; some included studies had unclear risk of selection bias and we were often unable to assess risk of selective reporting bias because many included studies did not have prospective clinical trials registration. | ||||||
| Gelatins compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with gelatins | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.89 | 1698 | ⊕⊕⊝⊝ Lowa | ||
| 301 per 1000 | 268 per 1000 | |||||
| All‐cause mortality (within 90 days) | Study population | RR 0.89 (0.73 to 1.09) | 1388 (1 study) | ⊕⊕⊝⊝ Lowb | ||
| 334 per 1000 | 298 per 1000 (244 to 364) | |||||
| All‐cause mortality (within 30 days) | Study population | RR 0.92 (0.74 to 1.16) | 1388 (1 study) | ⊕⊕⊝⊝ Lowb | ||
| 266 per 1000 | 244 per 1000 (197 to 308) | |||||
| Transfusion of blood products | Study population | RR 5.89 (0.24 to 142.41) | 167 (1 study) | ⊕⊝⊝⊝ Very lowc | We calculated an effect estimate for one small study, with one event in the gelatin group. 1 study reported transfusion of blood products but data were not reported by group. 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only gelatins. We noted little or no difference between groups in need for transfusion of blood products | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Renal replacement therapy | ‐ | ‐ | ‐ | ‐ | ‐ | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only gelatins. We noted little or no difference between groups in need for renal replacement therapy |
| Adverse events | Allergic reaction | ⊕⊝⊝⊝ Very lowc | We calculated an effect estimate for one small study, with five incidences of allergic reactions in the gelatin group | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | RR 21.61 (1.22 to 384.05) | 167 (1 study) | |||
| Itching | ||||||
| ‐ | ‐ | ‐ | ||||
| Rashes | ||||||
| ‐ | ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; risk of selection bias was unclear in some studies, and because we were unable to assess risk of selective outcome reporting bias in some studies. We downgraded by one level for imprecision; evidence was from few studies, and we could not be certain of time points for data collection. | ||||||
| Albumin and fresh frozen plasma compared to crystalloid for fluid resuscitation in critically ill patients | ||||||
| Participants: critically ill people requiring fluid resuscitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with crystalloids | Risk with albumin and FFP | |||||
| All‐cause mortality (at end of follow‐up) | Study population | RR 0.98 | 13,047 | ⊕⊕⊕⊝ Moderatea | One study also reported mortality but not by group, and so could not be included in analysis | |
| 254 per 1000 | 249 per 1000 | |||||
| All‐cause mortality (within 90 days) | Study population | RR 0.98 | 12,492 | ⊕⊕⊕⊝ Moderatea | One study also reported mortality but not by group, and so could not be included in analysis | |
| 259 per 1000 | 254 per 1000 | |||||
| All‐cause mortality (within 30 days) | Study population | RR 0.99 | 12,506 | ⊕⊕⊕⊝ Moderatea | One study also reported mortality but not by group, and so could not be included in analysis | |
| 234 per 1000 | 231 per 1000 | |||||
| Transfusion of blood products | Study population | RR 1.31 | 290 | ⊕⊝⊝⊝ Very lowb | 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only albumins or FFP; we noted little or no difference between groups in need for transfusion of blood products | |
| 281 per 1000 | 368 per 1000 | |||||
| Renal replacement therapy | 201 per 1000 | 223 per 1000 (193 to 255) | RR 1.11 (0.96 to 1.27) | 3028 (2 studies) | ⊕⊕⊝⊝ Lowc | One study stated that renal replacement data were measured but it was not reported in the study report (abstract) 1 study included different types of colloids (HES, gelatins, or albumin). We did not include this in analysis because study authors did not report data for only albumin and FFP. We noted little or no difference between groups in need for renal replacement therapy |
| Adverse events | Allergic reactions | ⊕⊝⊝⊝ Very lowd | ||||
| Study population | RR 0.75 (0.17 to 3.33) | 2097 (1 study) | ||||
| 4 per 1000 | 3 per 1000 (1 to 13) | |||||
| Itching | ||||||
| ‐ | ‐ | ‐ | ‐ | |||
| Rashes | ||||||
| ‐ | ‐ | ‐ | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations; some included studies had unclear risk of selection bias, and we were often unable to assess risk of selective reporting bias because many included studies did not have prospective clinical trials registration. | ||||||
| Participant condition | Study ID |
| Admission to an ICU with any condition (which included trauma, sepsis, ARDS, head injury) | |
| Trauma (includes studies of 'any trauma admissions', and head, chest, and abdominal injuries, and trauma with haemorrhagic or hypovolaemic shock) | Annane 2013*; Alpar 2004; Baker 2009; Bulger 2008; Bulger 2010; Bulger 2011; Evans 1996; Grba‐Bujevic 2012; James 2011; Lowe 1977; Lucas 1978; Masoumi 2016; Mattox 1991; Morrison 2011; Shah 1977; Vassar 1990; Vassar 1991; Vassar 1993a; Vassar 1993b; Wu 2001 |
| Sepsis or septic shock | Annane 2013*; Brunkhorst 2008; Caironi 2014; Dubin 2010; Ernest 1999; Guidet 2012; Jie 2015; Li 2008; Lu 2012; Mahrous 2013; McIntyre 2008; McIntyre 2012; Modig 1986; Oliveira 2002; Park 2015 (cancer with sepsis); Perner 2012; Rackow 1983*; Upadhyay 2005; Zhu 2011 |
| Hypovolaemia, hypovolaemic shock, haemorrhagic shock | Annane 2013*; Chavez‐Negrete 1991; Nagy 1993; Rackow 1983*; Van der Heijden 2009; Younes 1992; Younes 1997; Younes 1998 |
| Burns | Bechir 2013; Cooper 2006; Goodwin 1983; Hall 1978; Jelenko 1979; O'Mara 2005; Vlachou 2010 |
| ALI, ARDS | |
| Spontaneous subarachnoid haemorrhage | |
| Dengue shock syndrome | |
| Postcardiac arrest | |
| Perforation peritonitis | |
| Severe malaria | |
| Severe febrile illness | |
| Severe pulmonary insufficiency | |
| Vascular leak syndrome (cancer patients) | |
| Cirrhosis and septic induced hypotension | |
| Severe acute pancreatitis | |
| * included for more than one type of condition ALI: acute lung injury | |
| Study ID | Outcome | Events in colloid group: n/N | Events in crystalloid group: n/N | Effect estimate |
| Colloids (at the discretion of the clinician: HES, gelatins, or albumin) versus crystalloids | ||||
| Transfusion of blood products | 377/1414 | 358/1443 | RR 1.07, 95% CI 0.95 to 1.22; 2857 participants | |
| Renal replacement therapy | 156/1414 | 181/1443 | RR 0.88, 95% CI 0.72 to 1.08; 2857 participants | |
| Gelatin versus crystalloids | ||||
| Mortality (within 90 days) | 84/281 | 346/1035 | RR 0.89, 95% CI 0.73 to 1.09; 1388 participants | |
| Mortality (within 30 days) | 69/281 | 275/1035 | RR 0.92, 95% CI 0.74 to 1.16; 1388 participants | |
| Albumin versus crystalloid | ||||
| Adverse events: allergic reactions | 3/1050 | 4/1047 | RR 0.75, 95% CI 0.17 to 3.33; 2097 participants | |
| CI: confidence interval | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 24 | 11177 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
| 2 Mortality within 90 days Show forest plot | 15 | 10415 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| 3 Mortality within 30 days Show forest plot | 11 | 10135 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.09] |
| 4 Transfusion of blood product Show forest plot | 8 | 1917 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.02, 1.39] |
| 5 Renal replacement therapy Show forest plot | 9 | 8527 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.14, 1.48] |
| 6 Adverse event: allergic reaction Show forest plot | 3 | 7757 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.27, 24.91] |
| 7 Adverse event: itching Show forest plot | 2 | 6946 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.05, 1.82] |
| 8 Adverse event: rash Show forest plot | 2 | 7007 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.90, 2.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 19 | 4736 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.11] |
| 2 Mortality within 90 days and 30 days Show forest plot | 10 | 3353 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 3 Transfusion of blood products Show forest plot | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.10] |
| 4 Adverse events: allergic reaction Show forest plot | 4 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 6.0 [0.25, 144.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 6 | 1698 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at end of follow‐up Show forest plot | 20 | 13047 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.06] |
| 2 Mortality within 90 days Show forest plot | 10 | 12492 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| 3 Mortality within 30 days Show forest plot | 10 | 12506 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.06] |
| 4 Transfusion of blood product Show forest plot | 3 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.95, 1.80] |
| 5 Renal replacement therapy Show forest plot | 2 | 3028 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.96, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality at end of follow‐up Show forest plot | 16 | 4247 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.13] |
| 1.1 colloid + hypertonic crystalloid vs isotonic crystalloid | 8 | 2845 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.13] |
| 1.2 colloid + isotonic crystalloid vs hypertonic crystalloid | 2 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.06] |
| 1.3 colloid + hypertonic crystalloid vs hypertonic crystalloid | 6 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.74, 1.41] |

