子宫肌瘤术前的药物治疗
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation method not given. | |
| Participants | Premenopausal women aged over 25 years diagnosed with uterine fibroids confirmed by ultrasound scan and awaiting hysterectomy or myomectomy were recruited from a number of different hospitals in France. | |
| Interventions | Rx: Subcutaneous goserelin 3.6 mg once every month for 3 months followed by hysterectomy (N = 15) or myomectomy (N = 10). | |
| Outcomes | Preoperative haemoglobin | |
| Notes | Groups not comparable at baseline (preoperative uterine and fibroid size greater in the immediate surgery group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomized either to immediate surgery or to treatment” – randomisation method not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were not blinded due to the control group having immediate surgery. It is not stated whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is not stated whether personnel were blinded. |
| Incomplete outcome data (attrition bias) | High risk | 24 of 71 participants withdrew before completion of study. “The results of the study must be viewed in light of these withdrawals”. “The immediate surgery group assessment did not include 7 patients who required intraoperative blood transfusion.” Intention‐to‐treat analysis was not used. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Results did not specify numbers included in the results, so the results could not be included in a meta‐analysis. |
| Other bias | High risk | Groups not comparable at baseline for pre‐operative uterine and fibroid size. “Although the patients not receiving goserelin were scheduled to immediate surgery, the operation took place at a mean of 76 days after randomization” |
| Methods | Method of randomisation not stated. | |
| Participants | Women aged 37 to 52 years with uterine fibroids and menorrhagia, pelvic pain or pressure recruited from Provincial Hospital in Barcelona, Spain. | |
| Interventions | Rx: Intramuscular decapeptyl 3.75 mg every 4 weeks for 2 injections before hysterectomy, N = 23 | |
| Outcomes | Preoperative haemoglobin (g/dL) | |
| Notes | Groups not comparable at baseline (measurements of uterine volume and pretreatment haemoglobin and haematocrit lower in the treatment than in the control group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The patients were randomised to pre‐operative gonadotropin releasing hormone agonist treatment or to immediate hysterectomy”. Did not state allocation method. |
| Allocation concealment (selection bias) | Unclear risk | No details were reported. |
| Blinding of participants and personnel (performance bias) | Low risk | “All ultrasonic measurements of fibroid dimensions were performed by the same blinded ultrasonographer”. “The operations were performed by a staff specialist and a senior gynaecology resident who were blinded as to the treatment groups.” No information was provided regarding participants being blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details relating to outcome assessment were available. |
| Incomplete outcome data (attrition bias) | Low risk | No missing data, no participants withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes of interest were reported. |
| Other bias | Unclear risk | Pretreatment uterine volume and haemoglobin/haematocrit between comparison groups were not comparable at baseline. |
| Methods | Randomisation method not reported. Single centre, parallel group study with no apparent blinding. Number of women randomised: N = 32. Number of women analysed (primary outcomes): N = 32 (6 and 5 women in each group did not proceed to surgery but outcomes measured preoperatively). Power calculation for sample size calculated; 16 subjects per group to detect a difference of 25 cm³ in 3 months fibroid volume chance between groups. Source of funding not reported. | |
| Participants | Inclusion criteria: healthy premenopausal women with regular cycles (ranging from 25 to 35 days); mild fibroid symptoms such as anaemia, pain or pressure symptoms. Exclusion criteria: women requiring emergency surgery due to severe fibroid symptoms and disorders such as anaemia (Hb < 10 mg/dL), pain, dysmenorrhoea, menstrual bleeding and osteoporosis, severe vasomotor symptoms, blood coagulation diseases, history or family history of vascular thrombosis, suspicion of systemic neoplastic and infectious disease, suspicion of uterine malignancies, endometrial abnormalities detected by Pipelle endometrial biopsy and transvaginal ultrasound. Recruited from clinic in Manisa, Turkey. Mean ages: 46.6 years and 45.2 years. | |
| Interventions |
Interventions were compared with a control group (age matched but not randomised) but this group has not been included in comparisons in this review. | |
| Outcomes | Primary: change in fibroid volume between randomised groups. Other outcomes: adverse effects. The other outcomes measured were not included in this review. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Low risk | Closed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding unlikely. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts before surgery ‐ outcomes measured before surgery (substantial attrition from both groups for surgery). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
| Methods | Randomisation scheme controlled by Zeneca and women allocated sequentially as they entered the study. | |
| Participants | Premenopausal women aged over 25 years with menorrhagia or metrorrhagia and anaemia associated with uterine fibroids and awaiting hysterectomy recruited from 30 centres in 10 countries. | |
| Interventions | Rx 1: Goserelin acetate depot 3.6 mg once monthly + iron 600 mg/day before hysterectomy, N = 55 (ITT not performed) | |
| Outcomes | Preoperative haemoglobin (g/dL) | |
| Notes | Groups comparable at baseline for age, weight and height but differences in fibroid volumes, uterine volumes and haemoglobin concentrations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A separate randomization scheme was produced for each centre by the Biometrics group, Zeneca Pharmaceuticals and patients were randomised in a ratio of 1:1:1…strictly sequentially as patients entered the study”. Unclear as to whether quasi‐random. |
| Allocation concealment (selection bias) | Low risk | Central control of allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | “The study was a… double‐blind comparison”. Sham injection given to control group. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported on blinding of assessment. |
| Incomplete outcome data (attrition bias) | Low risk | “All analyses were performed on an intention‐to‐treat basis”. 185 participants were recruited, 17 withdrew with reasons given. |
| Selective reporting (reporting bias) | High risk | The outcomes with suitable data considered in the review were duration and difficulty of surgery, transfusion rate and withdrawal because of adverse effects. For all other outcomes the results were reported incompletely only as P values. |
| Other bias | Unclear risk | Difference in baseline values for fibroid and uterine volumes between groups. |
| Methods | Randomisation method not stated. | |
| Participants | Women aged up to 40 years with diagnosis of uterine fibroids confirmed by clinical examination, ultrasonography and/or laparoscopy and with a desire to preserve their fertility, recruited in Mexico City. | |
| Interventions | Rx: Nafarelin intranasal spray 200 µg twice daily before myomectomy, N = 13. | |
| Outcomes | Preoperative uterine volume (cc) | |
| Notes | Authors contacted but no reply received. Paper translated by Christine Aguilar. Some of the calculations with the raw data did not match the means reported in the tables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised to 2 groups ‐ method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Investigators were blinded but participants were not blinded ‐ however the outcomes could not be influenced by the participants' knowledge of group assignment. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded. |
| Incomplete outcome data (attrition bias) | Low risk | There did not appear to be any dropouts from the study. |
| Selective reporting (reporting bias) | High risk | The outcomes were measured in the intervention group at baseline, 30, 60 and 90 days but were only measured in the control group at baseline so a true comparison between groups could not be made. Postsurgical complication rates were not clearly reported. |
| Other bias | Unclear risk | There appear to be differences between groups at baseline. |
| Methods | Randomisation by alternation and no blinding. | |
| Participants | Women aged 30 to 49 years in good health and with ultrasound evidence of uterine fibroids were recruited from a centre in Italy. | |
| Interventions | Rx: Goserelin depot 3.6 mg every 28 days before surgery, N = 10. | |
| Outcomes | Uterine volume (cc) | |
| Notes | Authors contacted for additional information and reply received. Groups not comparable at baseline (uterine and fibroid volume higher in controls). Type of surgery not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Administered for 6 months to 22 subjects, and for 12 months to 8 subjects. The other 20 subjects… were randomly allocated for 3 months to no treatment or goserelin depot administration.” 20 women were randomised, the other 30 women were in groups of 22 and 8, and it is unclear whether they were randomised. The method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were not blinded. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | None reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | No withdrawals from the study were mentioned, but final numbers were not reported in results. |
| Selective reporting (reporting bias) | High risk | Data were not reported for the 20 women randomised who required surgery. Adverse events were not reported. |
| Other bias | Unclear risk | Baseline variables between groups not reported. |
| Methods | Randomisation according to a computer generated sequence but no description of attempts to conceal allocation and no blinding. | |
| Participants | Women aged 25 to 42 years selected for laparoscopic myomectomy between June 1993 and December 1996 at a clinic in Italy. | |
| Interventions | Rx: Decapeptyl 3.75 mg intramuscularly every 28 days for 3 months before surgery, N = 30. | |
| Outcomes | Duration of surgery (minutes) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients included in the present series were randomized according to a computer‐generated sequence”. |
| Allocation concealment (selection bias) | Unclear risk | No details regarding allocation concealment were provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were not blinded, as they either received immediate surgery or treatment and delayed surgery. It is not stated whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) | Low risk | All women were followed‐up for a minimum 6 months. No missing data. |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest were reported. |
| Other bias | Unclear risk | No details provided of baseline variables between groups. |
| Methods | Randomisation method not specified and no blinding. | |
| Participants | Women with symptomatic fibroids attending the obstetrics and gynaecology department at a hospital in Turkey. | |
| Interventions | Rx: Buserelin intranasally 900 μg/day in 3 doses for 3 months, = 15. | |
| Outcomes | Volume of myomas (cm³) | |
| Notes | The principal review author noted an error in the published paper which was confirmed by the principal study author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Subjects were randomly divided into two groups”. “Prospective, randomised, controlled study”. Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding of participants. Most likely no blinding of personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Ultrasonographic examinations were performed by the same sonographers in all cases”. “All of the myomectomies were performed by the same surgeons”. Did not state whether these personnel were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers included in analysis not stated in results. It appears there were no withdrawals from the study but this is unclear. |
| Selective reporting (reporting bias) | Unclear risk | Mistake was acknowledged in paper by author via past contact. Assuming is related to lack of referenced tables in text. |
| Other bias | Unclear risk | Unclear if baseline variables comparable between groups. |
| Methods | Randomisation method not stated and blinding not clear. | |
| Participants | Premenopausal women aged 36 to 50 years awaiting hysterectomy for uterine fibroids recruited from a clinic in Messina, Italy. | |
| Interventions | Rx: Leuprolide acetate depot 3.75 mg monthly before hysterectomy, N = 15. | |
| Outcomes | Uterine volume (cc) | |
| Notes | Paper translated by Kirsten Duckitt. Groups not comparable at baseline (uterine volume higher in treatment group compared to control group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “In a randomised manner”. Randomisation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | A placebo was used to blind participants, no detail provided as to what the placebo was. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details were reported. |
| Incomplete outcome data (attrition bias) | High risk | 12 dropouts. Adverse events reported with treatment were not compared to adverse events reported with placebo. |
| Selective reporting (reporting bias) | Low risk | No protocol viewed but all outcomes reported in the methods section were reported in the results section. |
| Other bias | Unclear risk | It is not reported whether the difference between the groups of uterine volume pretreatment is statistically significant or not. |
| Methods | Single‐centre (Italy), randomised controlled trial. Sequential numerical allocation to a randomisation list. Number of women randomised: N = 62 Number of withdrawals: none Intention‐to‐treat not mentioned but all participants included in analysis. Power calculation and source of funding not mentioned. | |
| Participants | Inclusion criteria: Premenopausal women with single intramural symptomatic uterine leiomyoma, referred between 2005 and 2007 to the outpatient clinic of the department of Obstetrical‐Gynaecological and Urological Science and Reproductive Medicine of a University. Exclusion criteria: taking hormonal therapy, delivered within 12 months of the study, or had malignant neoplasm. Previous pelvic surgery, uterine malformations, present or past pelvic inflammatory disease, coagulation disorders and unstable general conditions. | |
| Interventions | Treatment: 22 women received 3.75 mg triptorelin subcutaneous depot injection, once a month for 3 months. Surgery carried out at the latest 3 weeks after third injection. Control: 29 women underwent immediate surgery during follicular phase of the menstrual cycle. Duration: 3 months for treatment group. | |
| Outcomes | Fibroid diameter. Total operating time. Intraoperative blood loss. Clear identification of cleavage plane. PCNA expression. CD34 expression. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...patients were randomised using a sequential numerical allocation to a randomisation list prepared before commencing the study" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Control group participants did not receive a placebo injection, and thus participants were aware of study allocation. “Surgeons were blinded to the pre‐surgical medical treatment”. |
| Blinding of outcome assessment (detection bias) | Low risk | "Surgeons were blinded to the pre‐surgical medical treatment". “Sections were examined and immunostaining was graded without previous knowledge of the clinical data of the patients.” |
| Incomplete outcome data (attrition bias) | Low risk | No mention of ITT analysis. Authors did not state whether all participants included in the analyses but it appears there were no dropouts because of the percentages quoted for dichotomous outcomes. |
| Selective reporting (reporting bias) | Low risk | Protocol not viewed. All outcomes described in methods were reported in results section. |
| Other bias | Low risk | In addition to participants enrolled in the study, 20 samples obtained retrospectively were randomly selected from the Pathology Unit database. It was not specified how these samples were randomly selected, or whether these samples were demographically similar to the women enrolled in the study; however, the outcome evaluated was not relevant to this review. Randomised groups appeared similar at baseline. |
| Methods | Women were randomised to one of five groups on a 1:1:1:1:1 basis with 50 participants per group. Placebo group was sub‐randomised to each of the four placebo arms. overall randomisation 4:4:4:4:1:1:1:1:1 Multicentre (Belgium, Spain, Czech Republic, France) trial. Due to nature of injections both medical personnel and participant could not be blinded to the two different (GnRHa and fulvestrant) injections but they were blinded to whether it was placebo or active. Total no randomised N = 313. Total withdrawals N = 12. 4 from fulvestrant 50 mg, 3 from fulvestrant 125 mg,1 from 250 mg and 4 from goserelin group. Intention‐to‐treat analysis was done but not presented. The per protocol analyses had substantial withdrawals for most outcomes. Power calculation done. Supported by Astra Zeneca. | |
| Participants | Inclusion criteria: Premenopausal women with measurable fibroids that required hysterectomy; not involved in night shift work; prepared to use barrier contraception for the study period and could provide signed informed consent. Exclusion criteria: Used GnRHa in the past for > 3 months or had finished the same treatment within 3 months of study entry; used sex hormone therapy, oral contraceptives or danazol within 4 weeks of study entry; had disease effecting bone or steroid metabolism; had changes in menstrual frequency or any changes reflecting the onset of menopause. | |
| Interventions | Rx: Fulvestrant 50 mg IM injection once every 4 weeks for 3 injections, N = 59 Fulvestrant 125 mg IM injection once every 4 weeks for 3 injections, N = 66 Fulvestrant 250 mg IM injection once every 4 weeks for 3 injections, N = 62 Goserelin 3.6 mg SC every 4 weeks for 3 injections, N = 66 Each of the groups had a placebo group which received fulvestrant matched placebo or sham goserelin, N = 60 | |
| Outcomes | Preoperative Primary
Secondary
| |
| Notes | Intraoperative outcomes not assessed. Haematocrit values assessed at base line only and not as outcome. No blinding as regards to fulvestrant and GnRHa but that should not be considered significant as all outcomes except vaginal blood loss were objective. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The treatment received by individual patients was determined centrally, with separate schemes produced for each center" |
| Allocation concealment (selection bias) | Low risk | Central allocation to treatment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants and medical personnel were aware of allocation to fulvestrant or goserelin arm of the trial. However could not distinguish between active drug and placebo. “Because of the differences in the nature of injections, both patients and medical personnel could distinguish between the two medications and, because of the differences in volumes, between the doses of fulvestrant being given. However, it was impossible to distinguish between active agent and placebo (sham) for any of the treatments”. However, most outcomes were objective and unlikely to be influenced by knowledge of treatment. |
| Blinding of outcome assessment (detection bias) | High risk | Medical personnel were aware of drug assignment (fulvestrant or goserelin) but were not aware of placebo vs. active drug. |
| Incomplete outcome data (attrition bias) | High risk | Methods section states that both ITT analysis and per‐protocol analysis carried out, however ITT data not provided in study and the data from per protocol analyses suggested significant attrition. |
| Selective reporting (reporting bias) | Low risk | Protocol not viewed. All outcomes from methods section of paper reported in the results section. |
| Other bias | Low risk | Baseline variables similar between groups. |
| Methods | Multinational (Belgium, Ukraine, France, Romania, Hungary, Czech Republic, Switzerland, UK), multicentre parallel group RCT with double blinding. Number of women randomised: 242 Number of women analysed: 241 (modified ITT) but per protocol analyses also undertaken. Number of withdrawals: 1 (in the 5 mg ulipristal acetate group who was withdrawn before she received the study drug). Power calculation performed for sample size: based on the endpoint of change in fibroid volume. Source of funding: PregLem (data handled by independent data management organisation). | |
| Participants | Women aged 18 to 50 years were recruited between October 2008 and August 2010 from 38 academic centres in 6 countries. Inclusion criteria: PBAC > 100 during days 1 to 8 of menstruation, fibroid related anaemia (Hb ≤ 10.2 g/dL without macrocytosis, fibroid uterus with a size equivalent to a uterus of 16 weeks or less of gestation, at least 1 fibroid ≥ 3.cm in diameter but with no fibroid measuring more than 10.cm in diameter (US), BMI 18 to 40 kg/m². Exclusion criteria: history of uterine surgery, endometrial ablation or uterine artery embolisation, history of current gynaecological cancer, current endometrial hyperplasia, Hb ≤ 6 g/dL or any condition requiring immediate blood transfusion, known haemoglobinopathy, known severe coagulation disorder, large uterine polyp (> 2 cm), one or more ovarian cysts ≥ 4 cm in diameter (U/S), previous or current treatment for fibroids with an SPRM or a GnRHa, treatment with agents known to affect hepatic cytochrome CYP3A4, progestins, acetylsalicylic acid, mefenamic acid, anticoagulants, antifibrinolytic drugs or systemic glucocorticoid treatments. | |
| Interventions | Rx: Ulipristal acetate 5.mg or 10.mg orally once per day, N = 96 and N = 98). Control: placebo (identical pill) orally once per day. Duration: 13 weeks of treatment (before surgery) with follow up at weeks 17, 26, and 38, N = 48. | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Protocol and supplementary data were available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated list". |
| Allocation concealment (selection bias) | Low risk | "Web integrated interactive voice system" under central control. |
| Blinding of participants and personnel (performance bias) | Low risk | Identical treatments, placebo‐controlled study. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were not aware of participant allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Modified ITT analysis (1 participant excluded, withdrawn before taking medication). |
| Selective reporting (reporting bias) | Low risk | Protocol viewed and all predetermined outcomes reported in full. |
| Other bias | Low risk | Participant groups comparable at baseline. |
| Methods | Multinational (Belgium, Poland, Spain, France, Austria, Italy, UK), multicentre parallel group RCT with double dummy design. Number of women randomised: N = 307. Number of women analysed: N = 301 (for safety), N = 297 for modified ITT analysis and N = 281 for per protocol analyses. Number of withdrawals: N = 1 in ulipristal acetate 5 mg group (did not receive study drug), N = 2 in ulipristal acetate10 mg group (did not receive study drug, missing efficacy data), N = 2 in LA group (missing efficacy data). Power calculation for sample size (based on non inferiority of LA with ulipristal acetate). Source of funding: PregLem (supplied ulipristal acetate). | |
| Participants | Inclusion criteria: premenopausal women aged 18 to 50 years, BMI between 18 and 40, heavy uterine bleeding caused by fibroids, at least one fibroid measuring 3 cm or more in diameter (no fibroid measuring > 10 cm), uterine size equivalent to a pregnancy of no more than 18 weeks in gestation; eligible for surgery. Exclusion criteria: history of uterine surgery, endometrial ablation or uterine artery embolisation, history of current gynaecological cancer, current endometrial hyperplasia, Hb ≤ 6 g/dL or any condition requiring immediate blood transfusion, known haemoglobinopathy, known severe coagulation disorder, large uterine polyp (> 2 cm), one or more ovarian cysts ≥ 4 cm in diameter (U/S), previous or current treatment for fibroids with an SPRM or a GnRHa, treatment with agents known to affect hepatic cytochrome CYP3A4, progestins, acetylsalicylic acid, mefenamic acid, anticoagulants, antifibrinolytic drugs or systemic glucocorticoid treatments. | |
| Interventions | Rx 1: ulipristal acetate (SPRM) 5 mg or 10 mg oral tablet daily + intramuscular saline injection once monthly, N = 98 and N = 104. Rx 2: daily oral placebo + intramuscular injection of 3.75 mg leuprolide acetate (GnRHa) once monthly, N = 101. Duration: 13 weeks (before surgery) with follow up at weeks 17, 26 and 38. Iron supplementation could be used at the discretion of the physician. | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Non inferiority trial ‐ PEARL II | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Web integrated voice system under central control. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind, double dummy trial with uterine volume assessed by ultrasound at each centre. |
| Blinding of outcome assessment (detection bias) | Low risk | Biopsy samples were assessed by 3 independent pathologists who were unaware of the study group assignments, the visit sequence and each others assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Modified ITT ‐ did not include 5 participants (2 participants (one in each ulipristal acetate group) who never received the study drug and were not followed and 3 participants (1 who was assigned to receive ulipristal acetate10 mg and 2 in the LA group) with missing efficacy data after baseline. Per protocol analysis also performed (modified ITT population with the exclusion of women with major protocol deviations and a compliance rate of < 80%). |
| Selective reporting (reporting bias) | Low risk | Protocol published and viewed ‐ all outcomes reported. |
| Other bias | Low risk | Groups comparable at baseline. |
| Methods | Single centre, parallel group RCT. Number of women randomised: N = 30. Number of women analysed: N = 28. Number of withdrawals: N = 2 (both from placebo group, with reasons). Power calculation for sample size (at least 10% in % fibroid volume change between groups). Source of funding: Swedish Research Council, Karolinska Institute and Stockholm city. | |
| Participants | Inclusion criteria: healthy non pregnant women referred for evaluation to outpatient clinic due to fibroid related problems indicating surgical intervention. Exclusion criteria: steroid hormonal therapy for a minimum of 3 months before recruitment, any history of breast cancer or other malignancy, uncontrollable bleeding requiring urgent surgical treatment, abnormal mammogram or breast biopsy at baseline, adnexal abnormality or suspicion of leiomyosarcoma upon TVUS, abnormal FSH and LH levels or any other hormonal dysfunction of clinical significance, lab findings that would give suspicion of blood, liver or renal dysfunction, abnormal Pap smear at screening, any other contraindication to mifepristone. Mean age: 41 years Recruited from outpatient clinic at Karolinska University Hospital, Stockholm, Sweden | |
| Interventions |
Duration of treatment 3 months (ended the day before surgery). | |
| Outcomes | Primary: reduction in uterine fibroid size. Other: number of bleeding days, endometrial assessment (from biopsy), symptom scores. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation method. |
| Allocation concealment (selection bias) | Low risk | Central control from pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | Stated as double blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Assumed that assessors were the same as study staff. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition 12% from placebo group (reasons unrelated to intervention). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups were generally comparable at baseline. |
| Methods | Randomisation list on a 1:2 ratio with no blinding. | |
| Participants | Women aged 24 to 38 years (mean 33.6 years) with symptomatic multiple uterine fibroids recruited from a clinic in Milan, Italy. Prevalent symptoms were infertility in 18 and menorrhagia in 6 women. | |
| Interventions | Rx: Intranasal buserelin 1200 µg/day before myomectomy, N = 8. | |
| Outcomes | Preoperative uterine volume (mL) | |
| Notes | Author contacted for additional information on adverse events but no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Using a randomisation list the patients were allocated in a 1:2 ratio”. List unclear whether this was sequential or random. |
| Allocation concealment (selection bias) | Unclear risk | No information pertaining to allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information pertaining to blinding was provided but unlikely as control participants had immediate surgery ‐ however recurrence is an objective outcome. |
| Blinding of outcome assessment (detection bias) | Low risk | “Measurements were performed… in all patients by a physician unaware of the patient’s group allocation”. |
| Incomplete outcome data (attrition bias) | Low risk | There were no withdrawals from the study. Data for all 24 participants were reported. |
| Selective reporting (reporting bias) | Low risk | No previous protocol information was available but all outcomes from methods section were reported in the results section. |
| Other bias | Unclear risk | Insufficient information to determine if groups comparable at baseline |
| Methods | Randomisation by permuted blocks controlled by pharmacy and stratified into 2 groups: moderate (< 600 cm³) or large (≥ 600 cm³). | |
| Participants | Premenopausal women aged 29 to 41 years recruited from Brigham and Womens' Hospital, Massachusetts, USA. | |
| Interventions | Rx: Intramuscular leuprolide acetate depot 3.75 mg monthly for 4 injections before myomectomy, N = 9. | |
| Outcomes | Preoperative uterine volume (cc). | |
| Notes | Author contacted for additional information and reply received. Study population stratified into 2 groups after pretreatment ultrasound: uterine volume < 600 cc and ≥ 600 cc and sensitivity analysis performed in different strata. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...patients were randomised by permuted blocks” |
| Allocation concealment (selection bias) | Low risk | Central control of allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | “…to receive either LA depot 3.75mg or placebo intramuscularly every 4 weeks for four injections” “All patients and examiners were blinded with respect to treatment group throughout the study”. |
| Blinding of outcome assessment (detection bias) | Low risk | Assumed that examiners were also the assessors of outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | “All patients enrolled completed the study protocol and were included in data analysis”. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes appear to have been reported in full. |
| Other bias | Low risk | Groups appear comparable at baseline. |
| Methods | Randomisation method not given. | |
| Participants | Women aged over 25 years recruited from 6 clinics or hospitals in 5 countries. | |
| Interventions | Rx: Subcutaneous goserelin 3.6 mg monthly before hysterectomy, N = 127. | |
| Outcomes | Preoperative uterine volume (cc). | |
| Notes | Author contacted for additional information and request forwarded to Zeneca but no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...patients were randomised.” Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | “Patients were randomized to surgery alone…. Or to Zoladex treatment 3.6mg every month subcutaneously for 3 months prior to surgery” Participants were not blinded. No mention of personnel being blinded to study allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding is not reported and unlikely. |
| Incomplete outcome data (attrition bias) | High risk | Attrition unbalanced between groups ‐ higher in treatment than control group. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes in methods section were reported in full in the results section. |
| Other bias | Unclear risk | The authors acknowledged that the mean uterine volume for the Zoladex group was approximately 50 cm³ bigger than the surgery alone group mainly due to larger fibroids. Also women in surgery alone group had higher mean haemoglobin than the Zoladex group at entry. |
| Methods | Randomisation method not stated and no blinding. | |
| Participants | Women with symptomatology related to uterine fibroids recruited from medical centre in Israel. No other specific inclusion and exclusion criteria specified although all uteri were at least the size of 12 weeks gestation. | |
| Interventions | Rx: Intramuscular D‐Trp LHRH 3.2 mg micro capsules (Decapeptyl) monthly before surgery (hysterectomy, N = 17; myomectomy, N = 12). | |
| Outcomes | Preoperative uterine volume (mL) all participants. | |
| Notes | Author contacted for additional information but unable to supply this information. Each treatment group had a combination of hysterectomy and myomectomy surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The patients were randomly allocated”. Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not reported and unlikely. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details regarding blinding of outcome assessment were recorded. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is not reported whether any participants dropped out during the study |
| Selective reporting (reporting bias) | High risk | No protocol available but all outcomes from methods section were reported in the results section. |
| Other bias | Unclear risk | “Intraoperative blood loss estimated by the senior surgeon based of the volume of aspirated blood by the suction apparatus and the count of soaked abdominal pads”. Not convinced this is an accurate way of measuring blood loss when this was considered to be a primary outcome. This was acknowledged in the discussion. It is also not clear whether the groups were comparable at baseline. |
| Methods | Parallel group single centre RCT. Number of women randomised: 212. Number of women analysed: not clear, assumed it was 212. Number of withdrawals: not reported. Power calculation for sample size not reported. Source of funding: not reported. | |
| Participants | Participants recruited from Gynecological and Obstetric Clinic of Medical Facility of Masaryk University and the University Hospital Brno, Czech Republic. Inclusion criteria: reproductive aged females with uterine symptomatic myomatosis. Exclusion criteria: not reported. | |
| Interventions | Rx: Goserelin acetate 3.6 mg SC 3 times once every 4 weeks, N = 120. Control: No pretreatment before surgery, N = 92. 42.5% of participants had laparoscopic myomectomy and 57.5% of participants had open laparotomic myomectomy. | |
| Outcomes | Perioperative blood loss. Duration of surgery. Length of hospital stay. Perioperative and postoperative complications. | |
| Notes | Translated from Czech by Petr Tomek, Auckland University. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported but stated as ITT. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. SLL outcomes not reported as these were not measured in this review. |
| Other bias | Unclear risk | Czech and English abstracts of the article report different numbers of women treated with open myomectomy (78 vs. 44 respectively). |
| Methods | Parallel group single centre RCT. Number of women randomised: N = 22. Number of women analysed: N = 18. Number of withdrawals: N = 4 (but secondary analysis performed to evaluate whether significant differences existed between dropouts and completers). No power calculation for sample size reported. Source of funding: (in part) Reproductive Biology and Medicine branch (NIH, Bethseda Maryland) and HRA Pharma France. | |
| Participants | Inclusion criteria: healthy non pregnant women aged 33 to 50 years with regular menses (cycles every 24 to 35 days) and one or more leiomyomata > 2 cm in diameter; desiring hysterectomy; haemoglobin > 10 g/dL, current use of non hormonal contraception, BMI < 33 kg/m². Exclusion criteria: inability to complete study requirements, prior uterine artery embolisation, menopausal status (FSH > 20 mU/mL), cervical dysplasia, adnexal mass, genetic cause of rapid growth of leiomyomata, unexplained vaginal bleeding, use of glucocorticoids, progestins or agents that alter ovarian or hepatic function. Mean age: 45, 43 and 44 years Recruitment not clear ‐ study location USA. | |
| Interventions |
Duration: 3 cycles or 90 to 102 days if no menses occurred | |
| Outcomes | Primary: Fibroid volume (determined by MRI). Other: Proportion of amenorrhoea, change in haemoglobin and haematocrit, ovulation inhibition, quality of life. | |
| Notes | Target enrolment was 36 participants but recruitment was terminated after 22 participants were enrolled because of slow recruitment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated blocks of 6. |
| Allocation concealment (selection bias) | Low risk | Authors stated that allocation concealment was "assured". |
| Blinding of participants and personnel (performance bias) | Low risk | "...both patients and health care providers" were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Assumption that assessors were also blinded. |
| Incomplete outcome data (attrition bias) | High risk | Very small study with 18% withdrawals overall (25% withdrawal from 2 of the 3 randomised groups). Quality of life assessments performed in only 50% of original participants. |
| Selective reporting (reporting bias) | Unclear risk | Quality of life assessments not reported in full. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
| Methods | Randomisation by third party who opened the code‐break. | |
| Participants | Premenopausal women with mean age 43 years awaiting total abdominal hysterectomy for uterine fibroids recruited from hospitals in Edinburgh, Glasgow and Newcastle, UK. | |
| Interventions | Rx: Subcutaneous goserelin 3.6 mg monthly before hysterectomy, N = 35. | |
| Outcomes | Preoperative uterine volume (cc). | |
| Notes | Author contacted for additional data who forwarded the request to Zeneca but reply not received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised by a third party who opened the code‐break bearing the next consecutive patient number which contained the randomisation to either goserelin or placebo treatment”. |
| Allocation concealment (selection bias) | Low risk | “Randomisation was performed by the research nurses involved so that the medical staff did not know into which group the patients fell”. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded. “Randomisation was performed by the research nurses involved so that the medical staff did not know into which group the patients fell”. “All applicators (goserelin and sham) were provided with transparent windows covered with a previously coded label so that they look identical, although the sham applicator was actually empty”. “Surgeons were requested not to ask the date of the last menstrual period at the time of the pre‐operative ward round as this would un‐blind the study”. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Randomisation was performed by the research nurses involved so that the medical staff did not know into which group the patients fell”. No further information on whether these medical staff performed outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis, “all randomised patients recruited into the study for whom data were available were included in the efficacy analysis”. Three women in each group withdrew, with details reported on each and it appears they were included in the analyses. |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes of interest were reported. Previous pilot study reported same outcomes as full study. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
| Methods | Single‐centre (UK), prospective, randomised, placebo‐controlled trial. Randomisation carried out by a computer‐generated simple randomisation sequence with opaque sealed envelopes and staff nurses who were not part of the trial. Double‐blind study. Number of women randomised: N = 47, 24 to treatment group and 23 to placebo. Number of withdrawals: 7 women did not undergo planned operation, 3 in treatment group, 4 in placebo. Reasons: allergic reaction in one, two women opted for abdominal myomectomy, four did not attend for operation. Primary outcome was analysed with Intention‐to‐treat. Power calculation carried out. Study supported by Kings College Hospital NHS Foundation Trust. | |
| Participants | Inclusion criteria: history of heavy or irregular menstrual periods. Diagnosis of Type I or Type II submucous fibroid on ultrasound. Type 1 = fibroids with < 50% contained within the myometrium, Type II = ≥ 50% contained within myometrium. No specific exclusion criteria stated. | |
| Interventions | Treatment: 24 women received goserelin 3.6 mg (Zoladex, AstraZeneca) three injections given at 4 weekly intervals. Surgery took place 4 weeks after the last injection. Control: subcutaneous injections of placebo (5 mL 1% lignocaine), three injections given at 4 weekly intervals. Surgery took place 4 weeks after the last injection. Duration: three months with 6 weeks post‐operative follow up. | |
| Outcomes | Completion of fibroid resection. Volume of fluid infusion. Fluid deficit > 1500 mL. Duration of surgery. Complications. Recurrence of myomas. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation carried out by a computer‐generated simple randomisation sequence with staff nurses who were not part of the trial. |
| Allocation concealment (selection bias) | Low risk | "...consecutively numbered, opaque, sealed envelopes". |
| Blinding of participants and personnel (performance bias) | Low risk | "...both patients and clinicians were blinded to the group allocation". |
| Blinding of outcome assessment (detection bias) | Low risk | “Patients underwent hysteroscopic transcervical resection of myoma by a single experienced operator.” "clinicians blinded to group allocation". |
| Incomplete outcome data (attrition bias) | High risk | Substantial attrition. Only postoperative complications were assessed in all participants. |
| Selective reporting (reporting bias) | Low risk | Protocol viewed, all outcomes stated in method and protocol were reported. |
| Other bias | Unclear risk | Participants in treatment group were younger than those in control group. |
| Methods | Randomisation schedule prepared by Biometrics Group, AstraZeneca Pharmaceuticals. Study personnel had to contact the randomisation desk for allocation of treatment. Phase III, multicentre (sites in North America), double blind controlled trial. Number of women randomised: N = 110, 54 to treatment and 56 to control Number of withdrawals: 38 participants dropped out of the study, 20 from treatment group and 18 from control. Reasons include: loss to follow up; adverse event or intercurrent illness; protocol non‐compliance, or withdrawal of informed consent. Power calculation performed, intention‐to‐treat analysis carried out on primary outcome. Study funded by AstraZeneca. | |
| Participants | Premenopausal women aged over 18 years, with a history of excessive menstrual bleeding causing iron‐deficiency anaemia (IDA) who were candidates for hysterectomy or myomectomy. Participants underwent screening to demonstrate uterus ≥ 8 weeks gestation in size and the presence of ≥ 1 non‐calcified leiomyoma of ≥ 3 cm diameter. Participants were required to have a negative cervical smear test within 6 months of trial entry and a negative endometrial biopsy within the 45 day period before randomisation. Exclusion criteria: women with any blood disorder other than IDA (thalassaemia, sickle cell anaemia, folic‐acid deficiency, coagulopathy). Women with renal or hepatic impairment, gynaecological malignancy or pre malignancy, adrenal, pancreatic, ovarian or pituitary tumours, osteoporosis, osteopenia or metabolic bone disease. Women with any other medical condition which might confound the haematologic parameters. Blood transfusion within 8 weeks of randomisation or blood donation within two weeks was not permitted. Women who had received treatment with an LHRH analogue within previous 6 months, or who had a known hypersensitivity to LHRH, LHRH agonists or analogues, or any of the components of the study medication. Pregnant women were excluded. | |
| Interventions | Intervention: Injection of goserelin acetate 10.8 mg depot 12 weeks before planned surgery. Supplied as a pre‐filled sterile delivery device, and dispersed in a cylindrical rod of D,L, lactide glycolide polymer. Control: Sham depot injection containing copolymer only, supplied in a sterile syringe applicator identical to goserelin device, 12 weeks before planned surgery. Every study participant received 325 mg ferrous sulphate taken three times daily, for 12 weeks until surgery. | |
| Outcomes | Haemoglobin concentration at time of surgery (g/dL). Percentage of women achieving an increase in Hb ≥ 2 g/dL. Percentage of women achieving haematologic recovery where Hb ≥ 12 g/dL. Symptoms associated with uterine leiomyomas. Requirement for blood transfusion at pre‐, peri‐, and postoperative visits. Ability to donate blood for autologous transfusion. Fibroid and total uterine volume measured by ultrasound (cm³) | |
| Notes | Author contacted for additional data on 21.11.11 and awaiting reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment group was determined by a randomisation schedule prepared by the Biometrics Group, AstraZeneca Pharmaceuticals." |
| Allocation concealment (selection bias) | Low risk | "Investigators were instructed to contact the randomisation desk for a subject number and allocation". |
| Blinding of participants and personnel (performance bias) | Low risk | "sham injection in a double‐blind manner". “The sham depot was… identical to the goserelin device.” Treatment administrators were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details regarding blinding of surgeons or ultrasonographers. |
| Incomplete outcome data (attrition bias) | High risk | ITT analysis only carried out for Hb levels and adverse events. “Of the 110 subjects treated, 72 completed the trial”. 34.5% of patients withdrew from the study. “Reasons for withdrawal were similar in the 2 groups” although more participants were lost to follow up in the goserelin group than the sham group and more were lost due to protocol noncompliance in the sham group compared to the goserelin group. |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported in the results section. Adverse events reported in full. |
| Other bias | Unclear risk | Unclear if groups comparable at baseline. |
| Methods | Multicentre parallel group RCT. Number of women randomised: N = 39. Number of women analysed: N = 39. No withdrawals. Power calculation for sample size (reduction of 50% in operating time with GnRHa). Source of funding: not reported. | |
| Participants | Inclusion criteria: premenopausal women with submucous fibroids (diagnosed by TVUS) with diameter between 10 mm and 35 mm, grade GO or G1 (fibroids either completely intracavity or with an intramural portion of < 50%), BMI between 18 and 30 kg/m². Exclusion criteria: present or past history of cancer, a preoperative clinical suspicion of associated multiple or large polyps, planned associated non hysteroscopic surgical procedures or > 2 fibroids requiring hysteroscopic resection. Mean age: 42 years. Recruited from 3 tertiary care hospitals in Rome, Italy. | |
| Interventions |
| |
| Outcomes | Operating times, fluid absorption, difficulty of the operation, surgeon satisfaction with the procedure, intraoperative and postoperative complications, postoperative pain, patient satisfaction. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer generated sequence". |
| Allocation concealment (selection bias) | Low risk | "...sealed opaque envelopes". |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes not reported in full. |
| Other bias | Low risk | Groups comparable at baseline. |
| Methods | Randomisation by sealed opaque sequentially numbered identical envelopes. | |
| Participants | Premenopausal women aged 25 to 50 years awaiting surgery for uterine fibroids were recruited from the State Maternity Hospital in Sofia, Bulgaria. | |
| Interventions | Rx: Subcutaneous goserelin 3.6 mg monthly before myomectomy (N = 6) or hysterectomy (N = 11). | |
| Outcomes | Preoperative uterine volume (mL) | |
| Notes | Data given separately for intraoperative outcomes according to whether myomectomy or hysterectomy performed but data entered only for hysterectomy because this was the only surgery performed in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of the method of random sequence generation. "...the women were subdivided into two groups by randomisation principle". |
| Allocation concealment (selection bias) | Low risk | Sealed opaque sequentially numbered identical envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | It seems there were no losses to follow up. In the treatment group 6 women had myomectomy and 11 had hysterectomy, while in the control group all 17 had hysterectomy. |
| Selective reporting (reporting bias) | Unclear risk | For all continuous variables (as outcome measures), the authors did not present the numbers experiencing the event. |
| Other bias | Unclear risk | Unclear if groups similar at baseline. |
| Methods | Single centre, parallel group RCT. Number of women randomised: N = 14. No apparent withdrawals. Power calculation for sample size not reported. Source of funding: not reported. | |
| Participants | Inclusion criteria: not clearly specified ‐ all women had uterine fibroids. Exclusion criteria: not reported. Mean age of participants: not reported. Recruitment source not reported. | |
| Interventions |
| |
| Outcomes | Uterine artery blood flow, uterine volume, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned" but method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported but unlikely. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported but unlikely. |
| Incomplete outcome data (attrition bias) | Unclear risk | Study authors did not report whether there were any withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes not fully reported. |
| Other bias | Unclear risk | Study authors stated that groups were comparable at baseline but no values were reported. |
| Methods | Single centre (Iran), parallel group RCT. Number of women randomised: N = 50. No withdrawals. No power calculation for sample size reported. Source of funding: not reported. | |
| Participants | Inclusion criteria: women with uterine myoma nodules > 5 cm in diameter with irregular menstrual cycle and candidates for myomectomy. Exclusion criteria: > 40 years of age, abnormal uterine pathology, infection, hypersensitivity to any ergot alkaloids, hepatic and renal disorders, history of toxemia of pregnancy, cardiovascular disease, peptic ulcer, taking antipsychotic medications. Mean age: 30 and 32 years. Recruited from Alzahra University Hospital, Tabriz, Iran. | |
| Interventions |
| |
| Outcomes | Reduction in fibroid volume, symptoms, adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...assigned randomly" but no method reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not reported and highly unlikely because of different administration of intervention regimens (injection and tablet). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding not reported and unlikely. |
| Incomplete outcome data (attrition bias) | Low risk | No reported withdrawals. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes fully reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
| Methods | Single centre (Iran) parallel group RCT. Number of women randomised: N = 60. It appears that there are no withdrawals so presumably all participants were analysed. Power calculation for sample size not reported. Source of funding: grant from Tabriz University of Medical Sciences, no funding from drug company. | |
| Participants | Women with uterine fibroids recruited from Iranian hospital between September 2007 and November 2008. Inclusion criteria: women of reproductive age who had abnormal bleeding or infertility with uterine intramural fibroids. Exclusion criteria: submucous or subserous fibroids, abnormal uterine pathology and infection, aged ≥ 43 years. | |
| Interventions | Rx 1: Dipheredine 3.75 mg (GnRHa) 4 times every 28 days, N = 30. Rx 2: Dostinex (Cabergoline) 0.5 mg once per week for 6 weeks, N = 30. Only a proportion of women went on to have surgery. Duration of Rx: 6 weeks to 4 months. | |
| Outcomes | Fibroid volume. Adverse effects. | |
| Notes | Data on adverse effects were inconsistent between table and text so were not extracted. Intraoperative outcomes could not be used in the review as only a small proportion of women went on to have surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Participants were obviously not blinded because of different treatment administration schedules. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported if any participants withdrew. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
| Methods | Randomised controlled single centre (Italy) trial. Number of women randomised: N = 62. Number of withdrawals: not clear. Power calculation not reported and unclear if intention to treat analysis. Source of funding not reported. | |
| Participants | Inclusion criteria: women with symptomatic fibroid, with mobile uterus and vaginal accessibility with uterus size between 16 to 20 weeks clinically and volume between 380 mL and 680 mL ultrasonographically. Exclusion criteria: women with pelvic pathology as prolapse, pelvic floor relaxation, SI, adnexal mass; women with medical conditions requiring monitoring as diabetes, IHD; women who had therapy with GnRHa, danazol or progestational agents in last 6 months; women who had undergone surgery requiring longitudinal laparotomy; women with any contraindication to operative laparoscopy. | |
| Interventions | Treatment group: triptorelin depot 11.25 mg starting in mid luteal phase 3 months before surgery, N = 31. Control group: no therapy, N = 31. | |
| Outcomes | Preoperative Pretreatment: uterine volume and weight, haemoglobin, uterine bleeding, pelvic pain, urinary urgency. Operative Time of operation from skin incision and pneumoperitoneum to closure. Postoperative
| |
| Notes | Age: Treatment group 47.6 ± 3.5 years Control group 48.4 ± 4.6 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were assigned at a ratio 1:1 by random selection” – method of randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Women were randomised to injection or no treatment. No details on personnel blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details regarding outcome assessment were provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether all participants were included in analysis. |
| Selective reporting (reporting bias) | High risk | The study authors only reported adverse events and changes in uterine volume/weight in the intervention group before surgery ‐ no comparison was made with control so this outcome was not relevant to the review. Introduction mentions evaluating “operating time, surgical complications, conversion to laparotomy, blood loss, hospital stay, and costs”. Results report all of these except costs. |
| Other bias | Low risk | Groups appear comparable at baseline. |
| Methods | Method of randomisation not stated. | |
| Participants | Women with large fibroid uteri, from 14 to 30 weeks in gestational size were recruited. | |
| Interventions | Rx: Goserelin depot 3.6 mg before surgery (myomectomy and hysterectomy). | |
| Outcomes | Uterine volume before surgery (mL) (data not given). | |
| Notes | Data not provided for uterine volume before surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “They were randomised to receive Zoladex depot for 4 months or to act as controls with no treatment”. Method of randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants or personnel not reported and unlikely because of the differing treatment regimens. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is not specified who assessed the outcomes or whether the assessor/s was blinded. |
| Incomplete outcome data (attrition bias) | High risk | 32 women were included in the analysis. It is not stated how many were recruited or randomised, or if there were any withdrawals. |
| Selective reporting (reporting bias) | High risk | Uterine volume before surgery was not reported. Intraoperative blood loss was reported, without the numbers of the groups provided. Blood transfusion rate only provided for one group. No adverse effects information was reported. |
| Other bias | Unclear risk | No characteristics of the two assigned groups were provided so we could not confirm if they were comparable at baseline. |
| Methods | Randomisation on a 1:1 basis according to a randomisation schedule controlled by Hoechst and stratified according to uterine size to minimise bias. | |
| Participants | Women aged 29 to 52 years were recruited from 21 medical centres in the UK and 2 in Israel. | |
| Interventions | Rx: Buserelin 3.6 mg monthly (intramuscular), N = 98. | |
| Outcomes | Primary: menstrual blood loss during treatment; blood loss during surgery. | |
| Notes | Unpublished study released by Hoechst. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were randomised to the trial of whom 98 received buserelin and 98 placebo”. Randomisation on a 1:1 basis according to a randomisation schedule controlled by Hoechst and stratified according to uterine size to minimise bias. |
| Allocation concealment (selection bias) | Low risk | Central control. |
| Blinding of participants and personnel (performance bias) | Low risk | “This study was a… double blind comparison”. Participants received either buserelin 3.6 mg monthly or placebo monthly for 3 months. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was not specified who assessed the outcomes or whether the assessment was performed by a blinded party. |
| Incomplete outcome data (attrition bias) | Unclear risk | 210 women randomised, 196 women intention‐to‐treat analysis, 164 subjects per‐protocol analysis. Both analyses presented and reasons given for withdrawals. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | Groups comparable at baseline. |
| Methods | Randomisation by computer generated random number table with no blinding. | |
| Participants | Premenopausal women aged 29 to 51 years with symptomatic uterine fibroids scheduled to undergo hysterectomy were recruited in Tennessee, USA. | |
| Interventions | Rx 1: Either subcutaneous leuprolide acetate 0.5 mg daily or intramuscular depot leuprolide acetate 3.75 mg monthly before hysterectomy, N = 45. Data were only analysed from the subgroup who had gestational size of 14 to 18 weeks. | |
| Outcomes | Preoperative uterine size (gestational weeks). | |
| Notes | Author contacted for additional information but no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised by a computer‐generated random number table”. |
| Allocation concealment (selection bias) | Unclear risk | “Patients were randomised by a computer‐generated random number table”. No other details were given with respect to concealing allocation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Immediate and delayed surgery so participants were unable to be blinded; not clear if personnel blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was not specified who assessed the outcomes or whether the assessment was performed by a blinded party. |
| Incomplete outcome data (attrition bias) | Low risk | It appears there were no withdrawals from the study by checking the percentages recorded for dichotomous outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | High risk | The first 10 participants in group IIB were given leuprolide acetate 0.5 mg subcutaneously daily for 8 weeks, the remaining women received two intramuscular injections of depot leuprolide acetate, 3.75 mg 4 weeks apart ‐ not clear whether this could cause bias. In addition, there was a short‐stay protocol for vaginal hysterectomy resulting in a reduction in hospital stay unrelated to the treatment with GnRHa therapy. |
| Methods | Randomisation method not stated. | |
| Participants | Women aged 23 to 52 years (mean age 39 years) recruited from 50 centres in the USA. | |
| Interventions | Rx 1: Intramuscular leuprolide acetate depot 7.5 mg + iron monthly (results for this treatment group not included in the review), N = 107. | |
| Outcomes | Preoperative haemoglobin (g/dL). | |
| Notes | Study author contacted for additional information but no reply received. Different types of surgery performed (hysterectomy in 137 women (63%), myomectomy in 80 women (37%) and endometrial ablation in 1 woman). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stratified “arbitrarily” into one of two strata based on their pre‐study haematocrit level. “Within each stratum, patients were randomised to one of three treatment arms”. Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment reported. |
| Blinding of participants and personnel (performance bias) | Low risk | “A blinded central reader was used for all bone mineral densitometry scans”. “Each patient received an intramuscular injection of study drug or placebo”. Study stated to be “double‐blinded”. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was reported. |
| Incomplete outcome data (attrition bias) | High risk | 309 women were enrolled and treated, of these only 265 women (86%) were evaluated for efficacy. 47/265 women “decided not to have surgery” so surgical outcomes were not reported. There was also substantial attrition for some other outcomes. All women were included in the adverse event analysis. |
| Selective reporting (reporting bias) | High risk | Data for main outcomes not fully reported. |
| Other bias | Unclear risk | The study authors reported that there were no significant differences between randomised groups but did not clearly report the individual values. |
| Methods | Method of randomisation by computer generated randomisation sequences stratified per centre with consecutively numbered opaque sealed envelopes. | |
| Participants | Premenopausal women with median age 46 years (range 43 to 48 years) were recruited from 4 Italian centres specialising in vaginal surgery. | |
| Interventions | Rx: Intramuscular triptorelin depot injections 3.75 mg (Decapeptyl) monthly before hysterectomy, N = 62. | |
| Outcomes | Preoperative uterine volume (mL). | |
| Notes | Hysterectomy was by both the vaginal and abdominal route but data not provided separately for these groups so separate analysis not possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Performed in a separate setting in accordance with computer‐generated randomisation sequences stratified per centre”. |
| Allocation concealment (selection bias) | Low risk | “Using consecutively numbered opaque, sealed envelopes.” “The evaluator was blinded with regard to treatment allocation”. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Surgeon was not allowed to interview participants, and participants were requested to avoid mention of their last menstrual period. However, participants were not blinded, as they either received immediate surgery or treatment and delayed surgery. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome was vaginal vs. abdominal hysterectomy, no women who were recommended vaginal hysterectomy required conversion to abdominal hysterectomy. The surgeon who evaluated which surgery the woman would have was blinded to the treatment allocation (same surgeon as above), however “it is possible that the evaluator could have recalled examining the same woman three months before” as “only the patients allocated to pre‐operative medical treatment were examined twice.” |
| Incomplete outcome data (attrition bias) | Low risk | Four women withdrew after randomisation and before surgery, two from each arm. These 4 participants were also included in the efficacy analysis. “The inclusion of the four withdrawn patients in the analysis did not modify the appreciably the above estimates”. All women operated on attended the follow‐up evaluation. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported clearly. |
| Other bias | Low risk | Randomised groups appeared comparable at baseline. |
| Methods | Method of randomisation in a proportion of 1:1 by a computer generated randomisation sequence using serially numbered sealed opaque envelopes. Single centre study. Open labelled study (single blind). Number randomised N = 100. Number of withdrawals N = 3, 2 in immediate surgery group (one became pregnant, and one opted for surgery at different hospital,1 in the GnRHa group had to undergo hysterectomy. Power calculation for sample size performed and analysis by intention to treat. | |
| Participants | Premenopausal women aged 18 to 40 years with symptomatic intramural or subserous fibroid > 3 cm were included. Exclusion criteria: If predominantly intracavitary fibroids, previous pelvic surgery for leiomyomas or other genital abnormalities,uterine malformations, present or past pelvic inflammatory diseases, use of GnRHa up to 6 months prior, ultrasonography showing signs of uterine calcifications, coagulation disorders and unstable general conditions. | |
| Interventions | Rx: Intramuscular triptorelin depot injections 3.75 mg (Decapeptyl) on 2 occasions 28 days apart starting during mid luteal phase, N = 50. | |
| Outcomes | No preoperative evaluation. Operative Post operative Duration of hospital stay. | |
| Notes | No preoperative assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment allocation was performed with a computer‐generated randomization sequence". |
| Allocation concealment (selection bias) | Low risk | “...using serially numbered, opaque, sealed envelopes”. |
| Blinding of participants and personnel (performance bias) | High risk | Participants could not be blinded as they either received immediate surgery or treatment and delayed surgery. Study reported as "open label". |
| Blinding of outcome assessment (detection bias) | High risk | Open label trial. |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals were low in number and were evenly distributed, and all available data appeared to be reported. After randomisation and before surgery, 3 women withdrew from the study, 2 from control group and 1 in triptorelin group and were not included in the analysis (reasons given). |
| Selective reporting (reporting bias) | Low risk | It appears that all obvious outcomes were reported. |
| Other bias | Low risk | Groups appear comparable at baseline. |
| Methods | Balanced randomisation list predefined for each centre and balanced after every 4 women. | |
| Participants | Women with symptomatic fibroids indicating surgery (mean age 41.3 years) were recruited from 10 medical centres in France. | |
| Interventions | Rx: Leuprorelin 3.75 mg monthly (subcutaneous), N = 33. | |
| Outcomes | Ultrasound reduction of myoma diameter. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The treatments were allocated according to a randomisation list predefined for each centre as balanced after every four patients”. It is not clear how the balancing worked or whether it had the potential for bias. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) | High risk | “An open‐label study design was used because of the different administration conditions for the two products i.e. leuprorelin is injected subcutaneously and lynestrenol is administered orally. A double‐blind design would have been ideal, but the injection of a placebo for leuprorelin raises ethical problems”. |
| Blinding of outcome assessment (detection bias) | High risk | Open label study. |
| Incomplete outcome data (attrition bias) | Unclear risk | Exploratory intent‐to‐treat analysis, Withdrawals described, but substantial attrition for some outcomes. Details on surgical outcomes were only found for 25/33 participants for the leuprorelin group, and 17/23 in the lynestrenol group. |
| Selective reporting (reporting bias) | Unclear risk | Some outcome details not clearly reported. |
| Other bias | High risk | Significant differences in characteristics between the two groups. Statistically significant difference in distribution by category, more participants with multiple myomas in the leuprorelin group. “Larger number of myomas observed in patients in the leuprorelin group compared to those in the lynestrenol group. Consequently, the group treated with leuprorelin would rather have been put a disadvantage”. In addition, there was poor recruitment, “Three centres had only enrolled the first patient list (leuprorelin group each time).” |
| Methods | Multicentre (4 centres in the UK), parallel group, RCT (phase 2 trial). Number of women randomised: 33. Number of women analysed: 33 (no withdrawals). Power calculation for sample size: 95% power to detect a 0.08 difference between asoprisnil and placebo in RI (resistance index). Source of funding: TAP Pharmaceutical Products Inc (2 authors appear to have major conflicts of interest). | |
| Participants | Premenopausal women scheduled for hysterectomy due to symptomatic fibroids were recruited from 4 UK centres. Inclusion criteria: general good health, menstrual cycle between 17 and 42 days, symptoms related to fibroid size, pressure and/or heavy menstrual bleeding, at least one intramural non‐pedunculated, submucosal or subserosal fibroid with a diameter of at least 2 cm or multiple small fibroids with uterine volume 200 cm³ on ultrasonography, age over 18 years; negative pregnancy test; a washout period of 2 to 12 months for hormonal therapies; serum FSH 30 mIU/mL 21 at commencement; agreement to use double barrier method of contraception (condom/diaphragm/sponge plus spermicide) throughout the study until hysterectomy, unless surgically sterile by bilateral tubal ligation or vasectomy of partner and normal Papanicolaou test. Exclusion criteria: abnormal endometrial biopsy report based on an adequate specimen taken within 3 months of commencement. | |
| Interventions | Rx: Asoprisnil 10 mg or 25 mg orally once daily for 12 weeks, N = 12 and N = 11. Control: placebo, N = 10. All women then proceeded to hysterectomy. | |
| Outcomes | Volume of the largest fibroid and the uterus. Menstrual blood loss (measured by menstrual pictogram). Adverse events. Quality of life (measured by Uterine Fibroid Symptom and Health‐Related Quality of Life Questionnaire (UFS‐QOL). | |
| Notes | The primary outcomes in this study were not measured in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were sequentially assigned to subject numbers in ascending numerical order that encoded the assignment of the woman via a randomisation schedule into one of three arms of the study. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and all study personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and all study personnel were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups comparable at baseline. |
| Methods | Computer generated random assignment to 2 centres in the study, with no blinding. | |
| Participants | Women, aged 24 to 45 years (mean 37.2 years), with symptomatic fibroids, recruited from a university department and a private centre for surgery in Naples, Italy. | |
| Interventions | Rx: Intramuscular leuprolide acetate depot 3.75 mg followed by laparoscopic myomectomy, N = 35. | |
| Outcomes | Main outcomes
Secondary outcomes
| |
| Notes | Attempt made to contact author for additional data but no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The enrolled patients were allocated to one of the two groups according to the same computer‐generated random assignment for both centres”. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants would not be blinded to immediate or delayed surgery. There were no details on whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) | Low risk | “All randomized patients recruited for the study for whom data were available were included in the efficacy analysis”. No loss to follow‐up: “All 67 patients have a clinical follow‐up of at least 6 months”. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not clearly reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
BMI: body mass index
cc: cubic centimetres
CD34: hematopoietic progenitor cell antigen
CDB‐2914: a type of selective progesterone receptor modulator
cm³: cubic centimetres
CYP384: a type of oxidising enzyme
D‐Trp: D‐Tryptophan, an amino acid
FSH: follicle stimulating hormone
g/dL: grams per decilitre
G1: fibroids with intramural portion of < 50%
GnRHa: gonadotropin‐releasing hormone analogues
GO: fibroids completely intracavity
Hb: haemoglobin
IDA: iron deficiency anaemia
IM: intramuscular
kg/m²: kilograms per square metre
LA: leucocyte antigen
LH: luteinizing hormone
LHRH: luteinizing hormone releasing hormone
mg: milligram
mL: millilitre
MRI: magnetic resonance imaging
NIH: National Institute of Health
nmol/L: nanomoles per litre
PBAC: pictorial blood assessment chart
PCNA: anti proliferating cell nuclear antigen
RCT: randomised controlled trial
Ru 486: mifepristone
Rx: treatment
SC: subcutaneous
SHBG: sex hormone binding globulin
SLL: second look laparoscopy
SPRM: selective progesterone receptor modulator
TVUS: transvaginal ultrasound
UFS‐QOL: Uterine Fibroid Symptom and Health‐Related Quality of Life Questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Randomisation was alternate which is subject to bias. | |
| The relevant outcome in the publication, post‐operative complications, was given as a score rather than a proportion as contained in the table of comparisons. Contact was attempted with the principal author for extra information but no reply was received. | |
| Prospective study but not randomised. | |
| Retrospective analysis of a prospectively collected database. | |
| Objectives of trial to measure other outcomes not included in the review. | |
| Intervention was not relevant to this review. | |
| Trial of add‐back therapy which is covered in another Cochrane Review (Moroni 2015). | |
| Intervention was given long term and women participants were trying to avoid surgery. | |
| Participants had either fibroids or adenomyosis and no data provided for only women with fibroids. | |
| Retrospective analysis of a prospectively collected database. | |
| Prospective study but not randomised. | |
| Intervention was misoprostol which is covered in another Cochrane Review (Kongnyuy 2014). | |
| No indication whether the study was randomised ‐ unequal numbers in the two groups. | |
| Not an RCT. | |
| The outcome of this study was not relevant to this review. | |
| Appeared to be a dose finding study of different types of GnRHa without a control arm. | |
| Trial of add‐back therapy which is covered in another Cochrane Review (Moroni 2015). | |
| Wrong participants ‐ no mention of surgery after interventions. | |
| Not RCT ‐ participants could chose their treatment. | |
| The outcomes measured in the trial were not relevant to the review. | |
| RCT, but treatment was not given preoperatively ‐ no indication whether the participants went on to have surgery. | |
| Intervention was misoprostol which is covered in another Cochrane Review (Kongnyuy 2014). | |
| Participants had either endometrial polyps, submucous myoma or septate uterus and results were reported for the whole group. | |
| The women in this study did not have fibroids. | |
| The study author was contacted for extra information not contained in the publication but no reply was received. The study population consisted of women with fibroids and women with menometrorrhagia and pelvic pain and data were not provided separately for the women with fibroids. |
GnRHa: gonadotropin‐hormone releasing analogues; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Parallel group RCT. |
| Participants | N = 58, aged 25 to 51 years, with symptomatic fibroids. |
| Interventions | Goserelin depot 3.6 mg/month for 6 months followed by surgery (unspecified) versus immediate surgery without preoperative medical therapy. |
| Outcomes | Uterine volume, intraoperative blood loss and adverse events. |
| Notes | Study published in Italian, translation pending. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Not reported |
| Methods | Randomised into 5 groups. No other details reported |
| Participants | Women with submucosal fibroids < 3 cm |
| Interventions | 5 separate interventions:
3 month observation period before surgery. Surgery was with intrauterine Bigatti shaver (IBS) |
| Outcomes | Fluid balance Operation time Complications Conversion to bipolar resectoscopy. |
| Starting date | September 2013 ‐ one year later 7 participants had been recruited into the study. |
| Contact information | Not reported. |
| Notes | Abstract presented at European Society of Gynaecological Endoscopy meeting in Belgium 2014. |
| Trial name or title | |
| Methods | Randomised parallel group study. |
| Participants | Premenopausal women aged 18 years to 55 years with submucous fibroids (diagnosed by vaginal ultrasonography and confirmed by diagnostic hysteroscopy). Women with present or past history of cancer, pregnancy, presence of multiple polyps, presence of more than 2 fibroids and with associated non‐hysteroscopic surgical procedures were excluded. |
| Interventions | GnRHa: triptorelin 3.75 mg, intramuscularly, monthly, for 3 months Control: no pharmacological treatment |
| Outcomes | Duration of surgery, fluid absorption during the procedure. |
| Starting date | January 2013, completed August 2015 |
| Contact information | |
| Notes | Principal investigator contacted by email; trial completed and manuscript submitted for publication to Obstetrics and Gynecology journal. Awaiting data. |
| Trial name or title | |
| Methods | Double‐blind parallel group randomised controlled trial. Multicenter (9 in Netherlands) with computer generated randomisation sequences stratified per centre. |
| Participants | Premenopausal women with symptomatic fibroids and eligible for laparoscopic myomectomy. Women were excluded if they were pregnant, had a suspicion of malignancy, used hormonal agents and not willing to discontinue use, used anticoagulants, used NSAIDs impacting bleeding before surgery, had contraindication to laparoscopy, had allergy to leuprolide acetate or ulipristal acetate, had coagulopathy, had any type 0 to 2 fibroids smaller than 5 cm, had more than 2 type 3 to 6 fibroids > 5 cm that needed to be removed (except type 7 fibroids of any size). |
| Interventions | GnRHa and placebo tablets: intramuscular leuprorelin acetate depot 11.25 mg once and placebo tablets for 12 weeks. Ulipristal acetate, 5 mg once daily for 12 weeks plus single saline injection (placebo) at the onset of pretreatment. Control: No pretreatment before laparoscopic myomectomy. |
| Outcomes | Primary: intraoperative blood loss. Secondary: reduction of fibroid volume, haemoglobin levels pre and postoperatively, conversion rate to laparotomy, complication rate, re‐intervention rate, duration of surgery, surgical ease, quality of life during preoperative treatment and postoperatively up until 6 months. |
| Starting date | December 2014 |
| Contact information | |
| Notes | Contacted the contact author and details of the trial from the register were confirmed. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||
| 1 Uterine volume (mL) (preoperative) Show forest plot | 13 | 858 | Mean Difference (IV, Random, 95% CI) | ‐175.34 [‐219.04, ‐131.65] | ||||||||||||||||||||||||||||||||||||||||
| Analysis 1.1  Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 1 Uterine volume (mL) (preoperative). | ||||||||||||||||||||||||||||||||||||||||||||
| 1.1 GnRHa vs. no pretreatment | 11 | 810 | Mean Difference (IV, Random, 95% CI) | ‐178.68 [‐224.63, ‐132.74] | ||||||||||||||||||||||||||||||||||||||||
| 1.2 GnRHa vs. placebo | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐113.76 [‐314.60, 87.08] | ||||||||||||||||||||||||||||||||||||||||
| 2 Uterine volume (preop in data table) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.2
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 2 Uterine volume (preop in data table). | ||||||||||||||||||||||||||||||||||||||||||||
| 2.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| 2.2 GnRHa vs. placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| 3 Fibroid volume (mL) (preoperative) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||
| Analysis 1.3  Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 3 Fibroid volume (mL) (preoperative). | ||||||||||||||||||||||||||||||||||||||||||||
| 3.1 GnRHa vs. no pretreatment | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||
| 3.2 GnRHa vs. placebo | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||
| 4 Fibroid volume (preop in data table) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.4
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 4 Fibroid volume (preop in data table). | ||||||||||||||||||||||||||||||||||||||||||||
| 4.1 GnRHa vs. placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| 5 Haemoglobin (preoperative) Show forest plot | 10 | 834 | Mean Difference (IV, Random, 95% CI) | 0.88 [0.68, 1.08] | ||||||||||||||||||||||||||||||||||||||||
| Analysis 1.5  Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 5 Haemoglobin (preoperative). | ||||||||||||||||||||||||||||||||||||||||||||
| 5.1 GnRHa vs. no pretreatment | 6 | 308 | Mean Difference (IV, Random, 95% CI) | 0.91 [0.52, 1.30] | ||||||||||||||||||||||||||||||||||||||||
| 5.2 GnRHa vs. placebo | 4 | 526 | Mean Difference (IV, Random, 95% CI) | 0.87 [0.62, 1.13] | ||||||||||||||||||||||||||||||||||||||||
| 6 Haemoglobin (preop in data table) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.6
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 6 Haemoglobin (preop in data table). | ||||||||||||||||||||||||||||||||||||||||||||
| 6.1 GnRHa vs. placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| 7 Total frequency of adverse events Show forest plot | 4 | 755 | Odds Ratio (M‐H, Random, 95% CI) | 2.78 [1.77, 4.36] | ||||||||||||||||||||||||||||||||||||||||
| Analysis 1.7  Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 7 Total frequency of adverse events. | ||||||||||||||||||||||||||||||||||||||||||||
| 7.1 GnRHa vs. no pretreatment | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||
| 7.2 GnRHa vs. placebo | 4 | 755 | Odds Ratio (M‐H, Random, 95% CI) | 2.78 [1.77, 4.36] | ||||||||||||||||||||||||||||||||||||||||
| 8 Individual adverse events Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||
| Analysis 1.8  Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 8 Individual adverse events. | ||||||||||||||||||||||||||||||||||||||||||||
| 8.1 Insomnia | 1 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 12.56 [0.68, 232.82] | ||||||||||||||||||||||||||||||||||||||||
| 8.2 Hot flushes | 6 | 877 | Odds Ratio (M‐H, Random, 95% CI) | 7.68 [4.55, 12.96] | ||||||||||||||||||||||||||||||||||||||||
| 8.3 Headache | 6 | 877 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [1.00, 3.03] | ||||||||||||||||||||||||||||||||||||||||
| 8.4 Pain | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.12] | ||||||||||||||||||||||||||||||||||||||||
| 8.5 Nausea | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 2.41 [0.14, 40.59] | ||||||||||||||||||||||||||||||||||||||||
| 8.6 Dizziness | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 2.41 [1.13, 5.14] | ||||||||||||||||||||||||||||||||||||||||
| 8.7 Depression | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 2.12 [0.87, 5.17] | ||||||||||||||||||||||||||||||||||||||||
| 8.8 Arthralgia | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.55, 3.02] | ||||||||||||||||||||||||||||||||||||||||
| 8.9 Asthenia | 3 | 615 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.41, 2.39] | ||||||||||||||||||||||||||||||||||||||||
| 8.10 Vaginitis | 5 | 751 | Odds Ratio (M‐H, Random, 95% CI) | 4.18 [1.58, 11.05] | ||||||||||||||||||||||||||||||||||||||||
| 8.11 Abdominal/pelvic pain | 3 | 615 | Odds Ratio (M‐H, Random, 95% CI) | 2.43 [0.98, 6.05] | ||||||||||||||||||||||||||||||||||||||||
| 8.12 Skin changes | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.48, 3.13] | ||||||||||||||||||||||||||||||||||||||||
| 8.13 Hirsutism | 1 | 65 | Odds Ratio (M‐H, Random, 95% CI) | 6.35 [0.70, 57.72] | ||||||||||||||||||||||||||||||||||||||||
| 8.14 Change in libido | 3 | 332 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.76, 4.46] | ||||||||||||||||||||||||||||||||||||||||
| 8.15 Change in breast size | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 10.87 [1.90, 62.24] | ||||||||||||||||||||||||||||||||||||||||
| 8.16 Sweating | 4 | 497 | Odds Ratio (M‐H, Random, 95% CI) | 14.32 [6.17, 33.27] | ||||||||||||||||||||||||||||||||||||||||
| 8.17 Breast pain/tenderness | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.35, 1.52] | ||||||||||||||||||||||||||||||||||||||||
| 8.18 Uterine haemorrhage | 1 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.78] | ||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||||||||||||
| 1 Duration of surgery (minutes) Show forest plot | 6 | 617 | Mean Difference (IV, Random, 95% CI) | ‐10.11 [‐16.96, ‐3.25] | |||||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 1 Duration of surgery (minutes). | |||||||||||||||||||||||||||||||||||||||
| 1.1 GnRHa vs. no pretreatment | 4 | 321 | Mean Difference (IV, Random, 95% CI) | ‐14.19 [‐25.01, ‐3.38] | |||||||||||||||||||||||||||||||||||
| 1.2 GnRHa vs. placebo | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐5.03 [‐12.17, 2.12] | |||||||||||||||||||||||||||||||||||
| 2 Duration of surgery (data table) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||
| Analysis 2.2
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 2 Duration of surgery (data table). | |||||||||||||||||||||||||||||||||||||||
| 2.1 GnRHa vs. no pretreatment | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||
| 2.2 GnRHa vs. placebo | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||
| 3 Intraoperative blood loss (mL) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||||||||||||||||||||||||||||||||||||
| Analysis 2.3 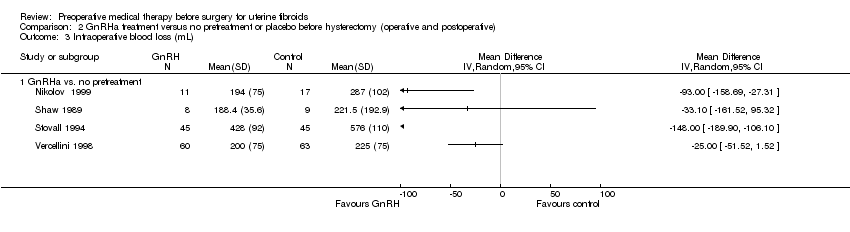 Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 3 Intraoperative blood loss (mL). | |||||||||||||||||||||||||||||||||||||||
| 3.1 GnRHa vs. no pretreatment | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||
| 4 Intraoperative blood loss (data table) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||
| Analysis 2.4
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 4 Intraoperative blood loss (data table). | |||||||||||||||||||||||||||||||||||||||
| 4.1 GnRHa vs. no pretreatment | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||
| 4.2 GnRHa vs. placebo | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||
| 5 Proportion with blood transfusions Show forest plot | 6 | 601 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.29, 1.01] | |||||||||||||||||||||||||||||||||||
| Analysis 2.5  Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 5 Proportion with blood transfusions. | |||||||||||||||||||||||||||||||||||||||
| 5.1 GnRHa vs. no pretreatment | 5 | 487 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.08] | |||||||||||||||||||||||||||||||||||
| 5.2 GnRHa vs. placebo | 1 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.23, 1.78] | |||||||||||||||||||||||||||||||||||
| 6 Proportion with postoperative complications Show forest plot | 7 | 772 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.32, 0.91] | |||||||||||||||||||||||||||||||||||
| Analysis 2.6  Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 6 Proportion with postoperative complications. | |||||||||||||||||||||||||||||||||||||||
| 6.1 GnRHa vs. no treatment | 5 | 507 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.23] | |||||||||||||||||||||||||||||||||||
| 6.2 GnRHa vs. placebo | 2 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.19, 1.11] | |||||||||||||||||||||||||||||||||||
| 7 Proportion with individual complications Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||
| Analysis 2.7 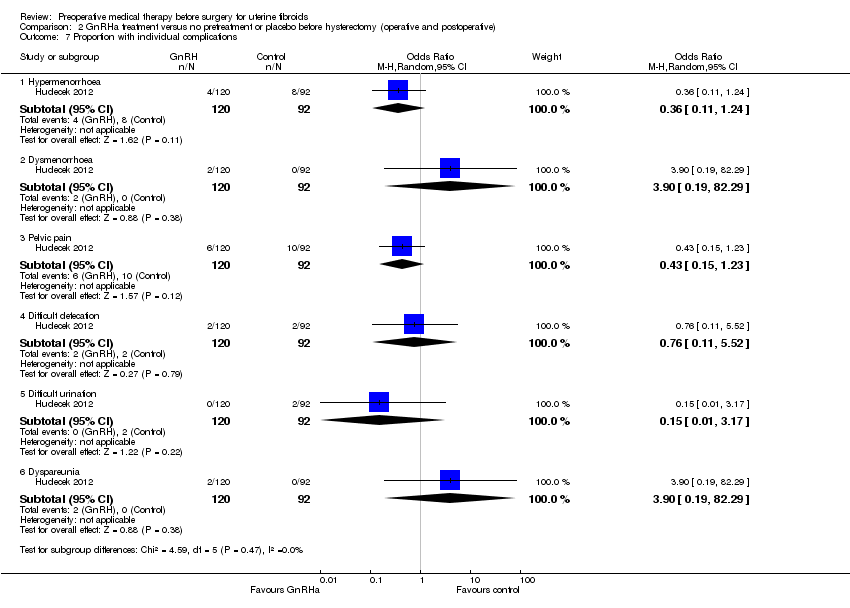 Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 7 Proportion with individual complications. | |||||||||||||||||||||||||||||||||||||||
| 7.1 Hypermenorrhoea | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.11, 1.24] | |||||||||||||||||||||||||||||||||||
| 7.2 Dysmenorrhoea | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 3.90 [0.19, 82.29] | |||||||||||||||||||||||||||||||||||
| 7.3 Pelvic pain | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.15, 1.23] | |||||||||||||||||||||||||||||||||||
| 7.4 Difficult defecation | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.11, 5.52] | |||||||||||||||||||||||||||||||||||
| 7.5 Difficult urination | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 3.17] | |||||||||||||||||||||||||||||||||||
| 7.6 Dyspareunia | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 3.90 [0.19, 82.29] | |||||||||||||||||||||||||||||||||||
| 8 Proportion with difficult surgery Show forest plot | 5 | 712 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.00] | |||||||||||||||||||||||||||||||||||
| Analysis 2.8  Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 8 Proportion with difficult surgery. | |||||||||||||||||||||||||||||||||||||||
| 8.1 GnRH vs. no pretreatment | 2 | 347 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.44] | |||||||||||||||||||||||||||||||||||
| 8.2 GnRH vs. placebo | 3 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.94] | |||||||||||||||||||||||||||||||||||
| 9 Proportion undergoing vaginal rather than abdominal procedure Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | ||||||||||||||||||||||||||||||||||||
| Analysis 2.9  Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 9 Proportion undergoing vaginal rather than abdominal procedure. | |||||||||||||||||||||||||||||||||||||||
| 9.1 GnRHa vs. no treatment | 2 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||
| 9.2 GnRHa vs. placebo | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||
| 10 Proportion with vertical incision Show forest plot | 4 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.21, 0.54] | |||||||||||||||||||||||||||||||||||
| Analysis 2.10  Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 10 Proportion with vertical incision. | |||||||||||||||||||||||||||||||||||||||
| 10.1 GnRHa vs. no pretreatment | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.18, 0.59] | |||||||||||||||||||||||||||||||||||
| 10.2 GnRHa vs. placebo | 2 | 228 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.17, 0.75] | |||||||||||||||||||||||||||||||||||
| 11 Duration of hospital stay (days) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||||||||||||||||||||||||||||||||||||
| Analysis 2.11  Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 11 Duration of hospital stay (days). | |||||||||||||||||||||||||||||||||||||||
| 11.1 GnRHa vs. no pretreatment | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||
| 11.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||
| 12 Duration of hospital stay (data table) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||
| Analysis 2.12
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 12 Duration of hospital stay (data table). | |||||||||||||||||||||||||||||||||||||||
| 12.1 GnRHa vs. no pretreatment | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||
| 13 Postoperative haemoglobin Show forest plot | 3 | 240 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.31, 1.38] | |||||||||||||||||||||||||||||||||||
| Analysis 2.13  Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 13 Postoperative haemoglobin. | |||||||||||||||||||||||||||||||||||||||
| 13.1 GnRHa vs. no pretreatment | 2 | 173 | Mean Difference (IV, Random, 95% CI) | 1.05 [0.39, 1.71] | |||||||||||||||||||||||||||||||||||
| 13.2 GnRHa vs. placebo | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.35, 1.15] | |||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||
| 1 Duration of surgery (minutes) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||||||||||||||||||||||||||
| Analysis 3.1 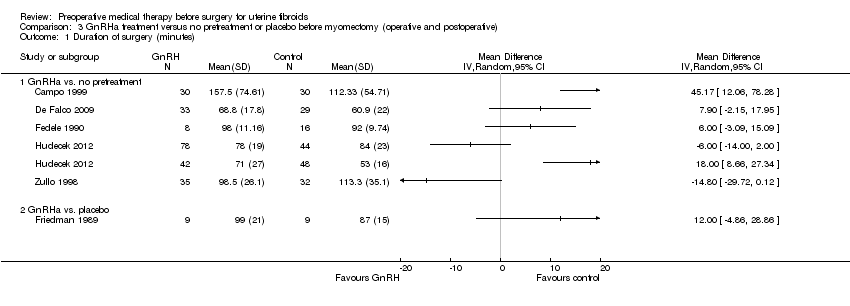 Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 1 Duration of surgery (minutes). | |||||||||||||||||||||||||||||
| 1.1 GnRHa vs. no pretreatment | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||
| 1.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||
| 2 Duration of surgery (descriptive data) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||
| Analysis 3.2
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 2 Duration of surgery (descriptive data). | |||||||||||||||||||||||||||||
| 2.1 GnRHa vs. no pretreatment | Other data | No numeric data | |||||||||||||||||||||||||||
| 3 Intraoperative blood loss (mL) Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||||||||||||||||||||||||||
| Analysis 3.3  Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 3 Intraoperative blood loss (mL). | |||||||||||||||||||||||||||||
| 3.1 GnRHa vs. no pretreatment | 9 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||
| 3.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||
| 4 Proportion with blood transfusions Show forest plot | 4 | 121 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.26, 2.75] | |||||||||||||||||||||||||
| Analysis 3.4  Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 4 Proportion with blood transfusions. | |||||||||||||||||||||||||||||
| 4.1 GnRHa vs. no pretreatment | 3 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.20, 3.19] | |||||||||||||||||||||||||
| 4.2 GnRHa vs. placebo | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.11, 9.23] | |||||||||||||||||||||||||
| 5 Proportion with postoperative complications Show forest plot | 5 | 190 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.43, 2.64] | |||||||||||||||||||||||||
| Analysis 3.5 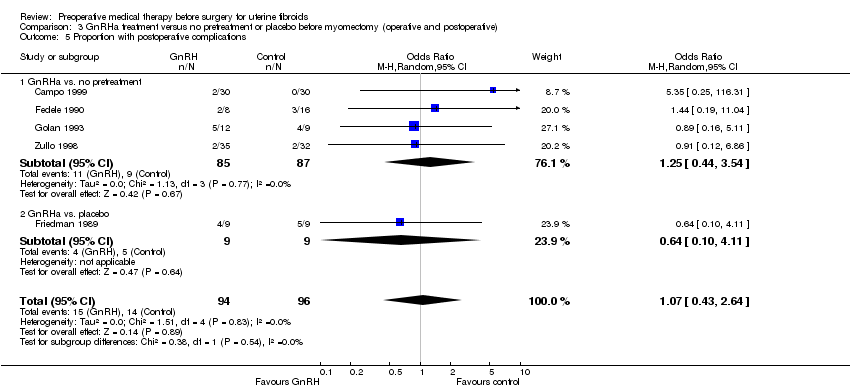 Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 5 Proportion with postoperative complications. | |||||||||||||||||||||||||||||
| 5.1 GnRHa vs. no pretreatment | 4 | 172 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.44, 3.54] | |||||||||||||||||||||||||
| 5.2 GnRHa vs. placebo | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.10, 4.11] | |||||||||||||||||||||||||
| 6 Proportion with vertical incision Show forest plot | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.43] | |||||||||||||||||||||||||
| Analysis 3.6 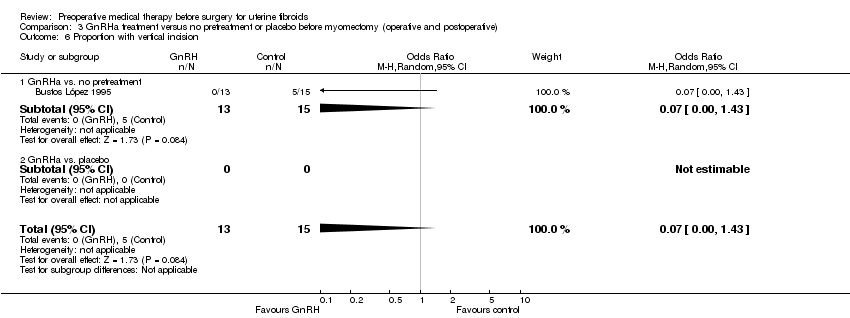 Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 6 Proportion with vertical incision. | |||||||||||||||||||||||||||||
| 6.1 GnRHa vs. no pretreatment | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.43] | |||||||||||||||||||||||||
| 6.2 GnRHa vs. placebo | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||
| 7 Duration of hospital stay (days) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||||||||||||||||||||||||||
| Analysis 3.7  Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 7 Duration of hospital stay (days). | |||||||||||||||||||||||||||||
| 7.1 GnRHa vs. no pretreatment | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||
| 7.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||
| 8 Proportion with postoperative recurrence of myomas Show forest plot | 2 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 4.16 [0.59, 29.09] | |||||||||||||||||||||||||
| Analysis 3.8  Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 8 Proportion with postoperative recurrence of myomas. | |||||||||||||||||||||||||||||
| 8.1 GnRHa vs. no pretreatment | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 11.67 [1.49, 91.54] | |||||||||||||||||||||||||
| 8.2 GnRHa vs. placebo | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 1.6 [0.24, 10.81] | |||||||||||||||||||||||||
| 9 Postoperative haemoglobin Show forest plot | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.22, 1.38] | |||||||||||||||||||||||||
| Analysis 3.9  Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 9 Postoperative haemoglobin. | |||||||||||||||||||||||||||||
| 9.1 GnRHa vs. no pretreatment | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.22, 1.38] | |||||||||||||||||||||||||
| 9.2 GnRHa vs. placebo | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of surgery (minutes) Show forest plot | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐5.4 [‐7.65, ‐3.15] |
| Analysis 4.1 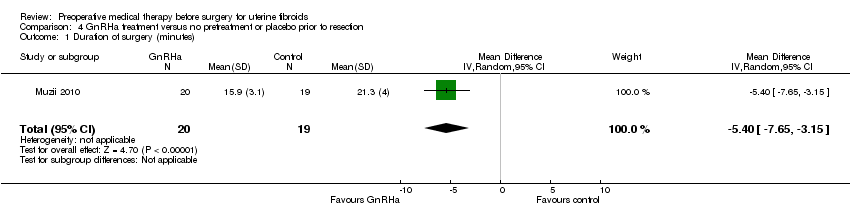 Comparison 4 GnRHa treatment versus no pretreatment or placebo prior to resection, Outcome 1 Duration of surgery (minutes). | ||||
| 2 Difficulty of surgery (VAS) Show forest plot | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐3.05, 0.25] |
| Analysis 4.2  Comparison 4 GnRHa treatment versus no pretreatment or placebo prior to resection, Outcome 2 Difficulty of surgery (VAS). | ||||
| 3 Fibroid recurrence Show forest plot | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.3  Comparison 4 GnRHa treatment versus no pretreatment or placebo prior to resection, Outcome 3 Fibroid recurrence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||
| 1 Uterine volume (descriptive table) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||
| Analysis 5.1
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 1 Uterine volume (descriptive table). | |||||||||||||||||||||||||||||
| 2 Fibroid volume Show forest plot | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 12.71 [‐5.92, 31.34] | |||||||||||||||||||||||||
| Analysis 5.2 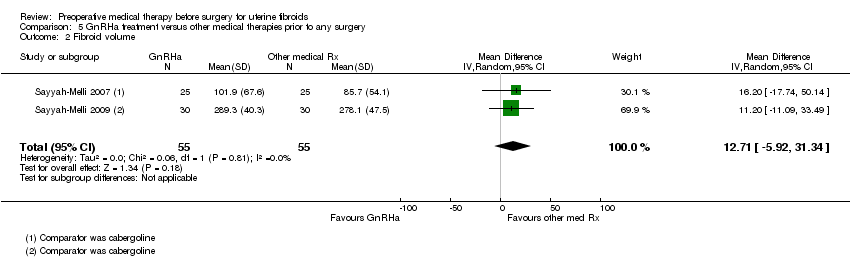 Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 2 Fibroid volume. | |||||||||||||||||||||||||||||
| 3 Fibroid volume (descriptive table) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||
| Analysis 5.3
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 3 Fibroid volume (descriptive table). | |||||||||||||||||||||||||||||
| 4 Haemoglobin at end of preoperative treatment Show forest plot | 1 | 188 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] | |||||||||||||||||||||||||
| Analysis 5.4  Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 4 Haemoglobin at end of preoperative treatment. | |||||||||||||||||||||||||||||
| 5 Reduction in bleeding to PBAC < 75 Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | ||||||||||||||||||||||||||
| Analysis 5.5  Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 5 Reduction in bleeding to PBAC < 75. | |||||||||||||||||||||||||||||
| 5.1 Ulipristal acetate 5 mg | 1 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.30, 1.68] | |||||||||||||||||||||||||
| 5.2 Ulipristal acetate 10 mg | 1 | 203 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.06] | |||||||||||||||||||||||||
| 6 Adverse events Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | ||||||||||||||||||||||||||
| Analysis 5.6  Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 6 Adverse events. | |||||||||||||||||||||||||||||
| 6.1 Hot flushes | 5 | 453 | Odds Ratio (M‐H, Random, 95% CI) | 12.30 [4.04, 37.48] | |||||||||||||||||||||||||
| 6.2 Headache | 4 | 439 | Odds Ratio (M‐H, Random, 95% CI) | 4.51 [1.09, 18.62] | |||||||||||||||||||||||||
| 6.3 Nausea | 3 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.64] | |||||||||||||||||||||||||
| 6.4 Weight gain | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.07, 2.81] | |||||||||||||||||||||||||
| 6.5 Oedema | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 2.2 [0.21, 22.59] | |||||||||||||||||||||||||
| 6.6 Sleep disorder | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 20.71 [2.49, 172.00] | |||||||||||||||||||||||||
| 6.7 Mood disorder or anxiety | 2 | 106 | Odds Ratio (M‐H, Random, 95% CI) | 4.02 [0.02, 727.86] | |||||||||||||||||||||||||
| 6.8 Vaginal dryness | 3 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 31.93 [2.19, 464.89] | |||||||||||||||||||||||||
| 6.9 Cutaneous disorder | 2 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.51, 4.38] | |||||||||||||||||||||||||
| 6.10 Abdominal or pelvic pain | 3 | 383 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.85, 2.91] | |||||||||||||||||||||||||
| 6.11 Procedural pain | 2 | 333 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.21, 6.35] | |||||||||||||||||||||||||
| 6.12 Fatigue | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.14, 1.93] | |||||||||||||||||||||||||
| 6.13 Anaemia | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.40, 3.92] | |||||||||||||||||||||||||
| 6.14 Nasopharyngitis | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.08, 1.79] | |||||||||||||||||||||||||
| 6.15 Breast pain or tenderness | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.10, 2.33] | |||||||||||||||||||||||||
| 6.16 Influenza | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [0.67, 9.72] | |||||||||||||||||||||||||
| 6.17 Insomnia | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [0.67, 9.72] | |||||||||||||||||||||||||
| 6.18 Pharyngitis | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.15, 4.13] | |||||||||||||||||||||||||
| 6.19 Dizziness | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.84] | |||||||||||||||||||||||||
| 6.20 Bone sensitivity | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 125.80 [6.75, 2343.30] | |||||||||||||||||||||||||
| 6.21 Muscular stiffness | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 5.43 [0.25, 118.96] | |||||||||||||||||||||||||
| 7 Quality of life (Uterine Fibroid Symptom and QoL questionnaire) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||
| Analysis 5.7
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 7 Quality of life (Uterine Fibroid Symptom and QoL questionnaire). | |||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||
| 1 Reduction in uterine volume Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||
| Analysis 6.1
Comparison 6 SPRM versus placebo, Outcome 1 Reduction in uterine volume. | ||||||||||||||||||||||||||||||||||
| 2 Reduction in fibroid volume Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||
| Analysis 6.2
Comparison 6 SPRM versus placebo, Outcome 2 Reduction in fibroid volume. | ||||||||||||||||||||||||||||||||||
| 3 Haemoglobin (g/dL) Show forest plot | 2 | 173 | Mean Difference (IV, Random, 95% CI) | 0.93 [0.52, 1.35] | ||||||||||||||||||||||||||||||
| Analysis 6.3 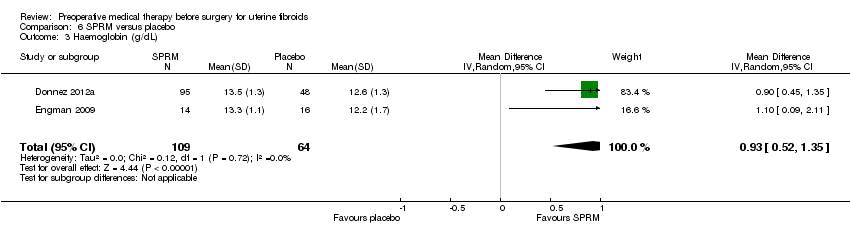 Comparison 6 SPRM versus placebo, Outcome 3 Haemoglobin (g/dL). | ||||||||||||||||||||||||||||||||||
| 4 Reduction in menstrual bleeding (PBAC < 75) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||
| Analysis 6.4 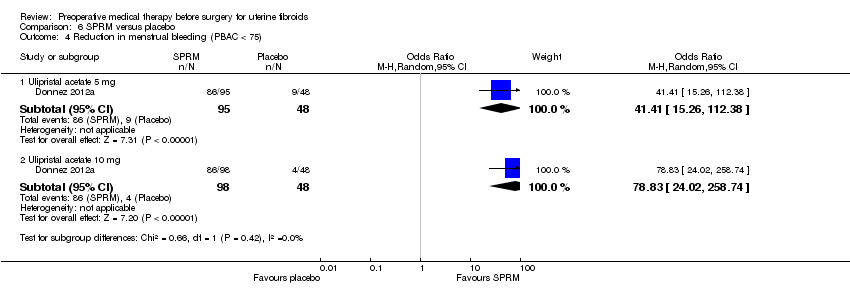 Comparison 6 SPRM versus placebo, Outcome 4 Reduction in menstrual bleeding (PBAC < 75). | ||||||||||||||||||||||||||||||||||
| 4.1 Ulipristal acetate 5 mg | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 41.41 [15.26, 112.38] | ||||||||||||||||||||||||||||||
| 4.2 Ulipristal acetate 10 mg | 1 | 146 | Odds Ratio (M‐H, Random, 95% CI) | 78.83 [24.02, 258.74] | ||||||||||||||||||||||||||||||
| 5 Change in menstrual blood loss from baseline to treatment end Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐166.9 [‐277.60, ‐56.20] | ||||||||||||||||||||||||||||||
| Analysis 6.5 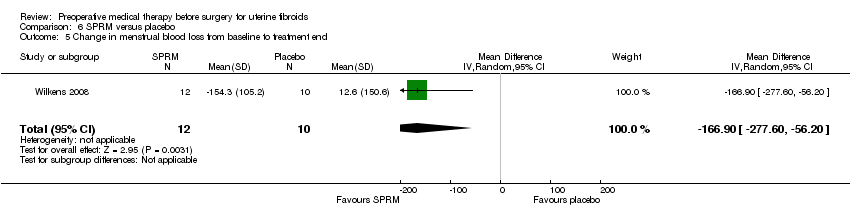 Comparison 6 SPRM versus placebo, Outcome 5 Change in menstrual blood loss from baseline to treatment end. | ||||||||||||||||||||||||||||||||||
| 6 Serious adverse events Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||
| Analysis 6.6  Comparison 6 SPRM versus placebo, Outcome 6 Serious adverse events. | ||||||||||||||||||||||||||||||||||
| 6.1 Breast cancer | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 2.04] | ||||||||||||||||||||||||||||||
| 6.2 Uterine haemorrhage | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.00, 12.70] | ||||||||||||||||||||||||||||||
| 6.3 Ovarian haemorrhage | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.03, 18.84] | ||||||||||||||||||||||||||||||
| 6.4 Fibroid protruding through cervix | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 2.04] | ||||||||||||||||||||||||||||||
| 6.5 Menometrorrhagia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 2.04] | ||||||||||||||||||||||||||||||
| 6.6 Hyperplasia | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 8.38] | ||||||||||||||||||||||||||||||
| 7 Other adverse events Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||
| Analysis 6.7  Comparison 6 SPRM versus placebo, Outcome 7 Other adverse events. | ||||||||||||||||||||||||||||||||||
| 7.1 Headache | 3 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.14, 4.30] | ||||||||||||||||||||||||||||||
| 7.2 Breast pain or tenderness | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.26, 11.70] | ||||||||||||||||||||||||||||||
| 7.3 Abdominal pain | 3 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [0.36, 8.12] | ||||||||||||||||||||||||||||||
| 7.4 Pyrexia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.12, 3.25] | ||||||||||||||||||||||||||||||
| 7.5 Hypercholesterolaemia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.14, 10.96] | ||||||||||||||||||||||||||||||
| 7.6 Hypothyroidism | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 3.36 [0.19, 60.73] | ||||||||||||||||||||||||||||||
| 7.7 Constipation | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.13, 2.79] | ||||||||||||||||||||||||||||||
| 7.8 Hypertriglyceridaemia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.11, 9.11] | ||||||||||||||||||||||||||||||
| 7.9 Influenza | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.11, 9.11] | ||||||||||||||||||||||||||||||
| 7.10 Dizziness | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 2.30 [0.12, 43.52] | ||||||||||||||||||||||||||||||
| 7.11 Nasopharyngitis | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.09, 2.31] | ||||||||||||||||||||||||||||||
| 7.12 Dysmenorrhoea | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 1.02] | ||||||||||||||||||||||||||||||
| 7.13 Bladder pressure | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.64] | ||||||||||||||||||||||||||||||
| 7.14 Micturition problem | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [0.28, 13.23] | ||||||||||||||||||||||||||||||
| 7.15 Lower back pain | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.29, 6.15] | ||||||||||||||||||||||||||||||
| 7.16 Proctodynia | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 3.67 [0.14, 97.49] | ||||||||||||||||||||||||||||||
| 7.17 Coital pain | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 6.6 [0.29, 150.07] | ||||||||||||||||||||||||||||||
| 7.18 Hot flushes | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 25.24 [1.27, 503.38] | ||||||||||||||||||||||||||||||
| 7.19 Nausea | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.97 [0.46, 8.46] | ||||||||||||||||||||||||||||||
| 7.20 Vomiting | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 7.21 Diarrhoea | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 3.62 [0.39, 33.89] | ||||||||||||||||||||||||||||||
| 7.22 Change of mood | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 15.00 [1.54, 146.54] | ||||||||||||||||||||||||||||||
| 7.23 Lowered libido | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 6.0 [0.58, 61.84] | ||||||||||||||||||||||||||||||
| 7.24 Weakness | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 2.8 [0.43, 18.38] | ||||||||||||||||||||||||||||||
| 7.25 Fatigue | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.31, 9.57] | ||||||||||||||||||||||||||||||
| 7.26 Dental pain | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.12, 14.82] | ||||||||||||||||||||||||||||||
| 7.27 Vaginal infections | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 2.44 [0.11, 55.56] | ||||||||||||||||||||||||||||||
| 7.28 Vaginal discharge | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.12, 14.82] | ||||||||||||||||||||||||||||||
| 8 Quality of life (Uterine Fibroid Symptoms and QoL questionnaire) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||
| Analysis 6.8
Comparison 6 SPRM versus placebo, Outcome 8 Quality of life (Uterine Fibroid Symptoms and QoL questionnaire). | ||||||||||||||||||||||||||||||||||

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), outcome: 1.1 Uterine volume (mL) (preoperative).

Forest plot of comparison: 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), outcome: 2.1 Duration of surgery (minutes).

Forest plot of comparison: 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), outcome: 3.1 Duration of surgery (minutes).
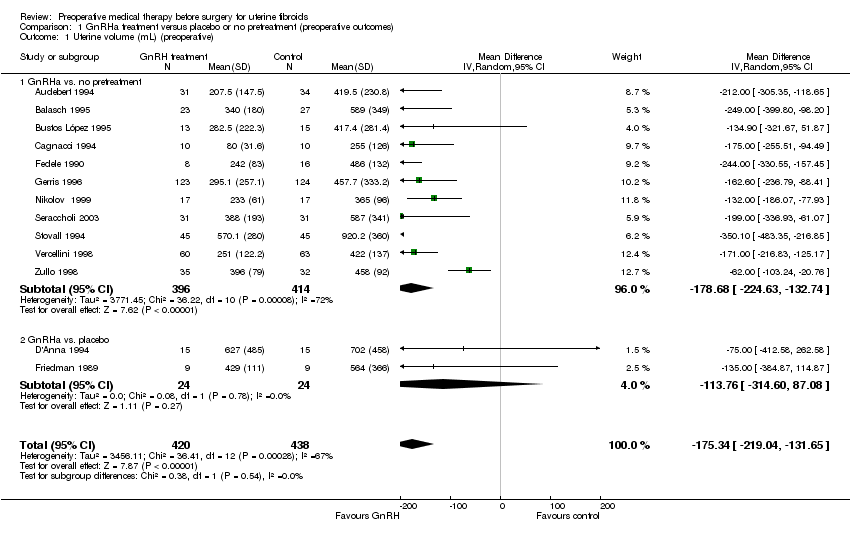
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 1 Uterine volume (mL) (preoperative).
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Golan 1993 | 53 | GnRHa (D‐Trp LHRH) vs. no pre‐treatment | Results reported as "average" with range (likely to be median plus range prior to surgery: D‐Trp: median 380 mL (300 to 400) No pre‐treatment: median 496 mL (370 mL to 600 mL) | Authors did not report whether these the reduction in uterine volume was significantly different between groups |
| GnRHa vs. placebo | ||||
| Lumsden 1994 | 69 | GnRHa (goserelin) vs. placebo | Difference between goserelin and placebo at end of treatment (%): 27.7% (95% CI 10.3 to 45.2), P = 0.002 | Statistical test reported as "calculation of limits of agreement" |
| Muneyyirci‐Delale 2007 | 110 | GnRHa (goserelin) + iron vs. iron + placebo | Goserelin/iron vs iron + placebo: Change in uterine volume: median (IQR): ‐233.1 (IQR NR) vs. +18.9 (IQR NR) cm³ (NS) | Authors reported that there were no significant differences between groups |
| Stovall 1995 | 179 | GnRHa (leuprolide acetate depot) + iron (7.5 mg and 3.75 mg) vs. placebo + iron | Median change from baseline to presurgery: Leuprolide/iron 7.5 mg: ‐31% (no range reported) Leuprolide/iron 3.75 mg: ‐39% (no range reported) Placebo/iron: +10% (no range reported) | Authors reported that the change from baseline in both leuprolide groups was significantly different from placebo (P < 0.01) |
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 2 Uterine volume (preop in data table).

Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 3 Fibroid volume (mL) (preoperative).
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. placebo | ||||
| Muneyyirci‐Delale 2007 | 110 | (GnRHa (goserelin) + iron vs sham injection + iron | Median (range): GnRHa vs placebo: ‐35.4 cm3 (no range reported) vs +3.9 cm3 (no range reported) | Auhors reported that there were no significant between group differences |
| Stovall 1995 | 138 | GnRHa (leuprolide acetate depot 7.5mg and 3.75mg) + iron vs placebo + iron | Median % change from baseline: LA/iron 7.5mg: ‐23% (no range reported) LA/iron 3.75mg: ‐27% (no range reported) Placebo/iron: +8% (no range reported) | Authors reported that both LA groups had significantly greater changes from baseline compared to placebo (p<0.01) |
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 4 Fibroid volume (preop in data table).

Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 5 Haemoglobin (preoperative).
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. placebo | ||||
| Muneyyirci‐Delale 2007 | 110 | GnRHa (goserelin) + iron vs sham injection + iron | Difference of least squares mean: 1.17 g/dL (95% CI 0.7 to 1.7), p<0.001 | Significantly higher in goserelin group |
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 6 Haemoglobin (preop in data table).

Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 7 Total frequency of adverse events.

Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 8 Individual adverse events.

Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 1 Duration of surgery (minutes).
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Balasch 1995 | 50 | GnRHa (triptorelin) vs no preRx | GnRHa vs no preRx: mean (SD) 110 mins (81.5) vs 107 mins (166.3) (NS) | Authors reported that there was no evidence of a significant difference between groups Data reported in table format as it appears skewed |
| Golan 1993 | 32 | GnRHa (triptorelin) vs no preRx | GnRHa vs no preRx: mean (SD): 49 (37.1) vs 70 (131.7) (p<0.05) | Authors reported a significant difference between groups Data reported in table format as it appears skewed |
| GnRHa vs. placebo | ||||
| Lumsden 1994 | 71 | GnRHa (goserelin) vs placebo | GnRHa vs placebo: mean (SD): 61 mins (16) vs 68 mins (208) (NS) | The authors reported that there was no evidence of a significant difference between groups Data reported in table format as it appears skewed |
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 2 Duration of surgery (data table).
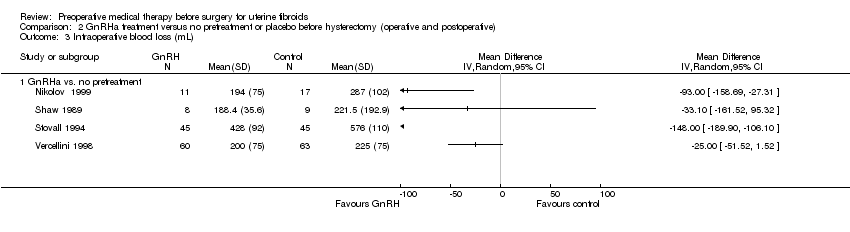
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 3 Intraoperative blood loss (mL).
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Golan 1993 | 32 | GnRHa (triptorelin) vs no preRx | GnRHa vs no preRx: mean (SD) 208 (263.9) vs 309 (313.7) p<0.05 | Authors reported a significant difference between groups Data reported in table format as it appears skewed |
| GnRHa vs. placebo | ||||
| Lumsden 1994 | 69 | GnRHa (goserelin) vs placebo | GnRHa vs placebo: median (range): 187 mls (60 to 600) vs 307.5 (118 to 1000) p<0.05 | Authors reported a significant difference between groups Data reported in table format as it appears skewed |
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 4 Intraoperative blood loss (data table).

Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 5 Proportion with blood transfusions.

Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 6 Proportion with postoperative complications.

Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 7 Proportion with individual complications.

Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 8 Proportion with difficult surgery.

Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 9 Proportion undergoing vaginal rather than abdominal procedure.

Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 10 Proportion with vertical incision.
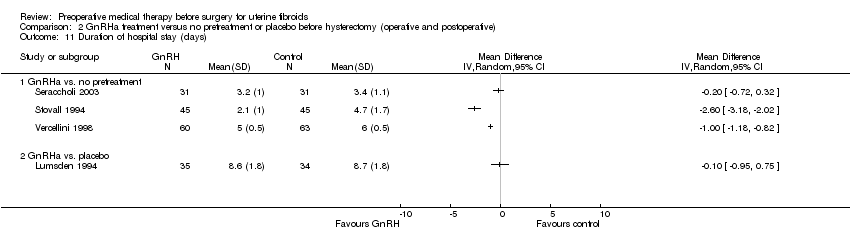
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 11 Duration of hospital stay (days).
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Balasch 1995 | 50 | GnRHa (triptorelin) vs no preRx | GnRHa vs no preRx: mean (SD): 7.2 (2.9) vs 8.6 (18.7) (NS) | Authors reported that there was no evidence of a significant difference between groups Data reported in table format as it appears skewed |
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 12 Duration of hospital stay (data table).

Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 13 Postoperative haemoglobin.

Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 1 Duration of surgery (minutes).
| Study | Number of participants | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Cetin 1995 | 30 | GnRHa (buserelin 900 ugr/day for 3 months) vs no pretreatment (immediate surgery) | GnRHa (mean (SD)): 87 mins (174.3) Control (mean (SD)): 102 mins (135.6) | Authors reported that the difference was not statistically significant, p>0.05 |
| Golan 1993 | 21 | GnRHa (decapeptyl 3.2 mg for 3 months) vs no pretreatment | GnRHa (mean (SD)): 80 mins (145.5 Control (mean (SD)): 96 mins (138.0) | Authors reported that the difference was not statistically significant |
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 2 Duration of surgery (descriptive data).

Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 3 Intraoperative blood loss (mL).
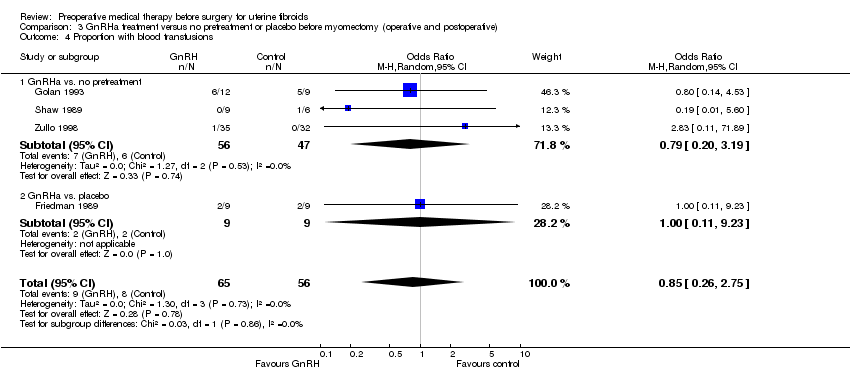
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 4 Proportion with blood transfusions.
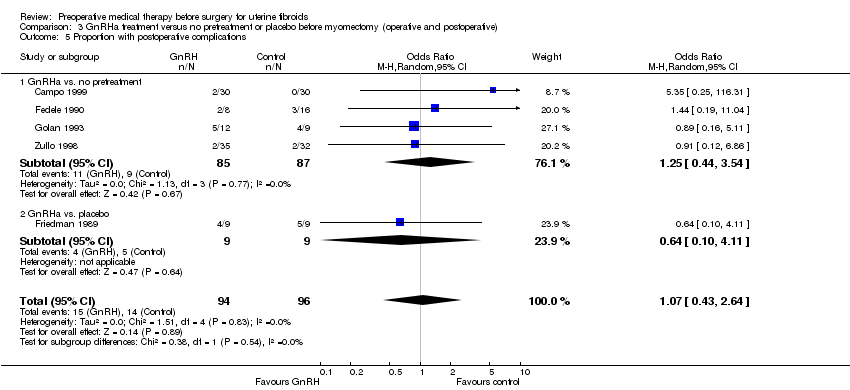
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 5 Proportion with postoperative complications.
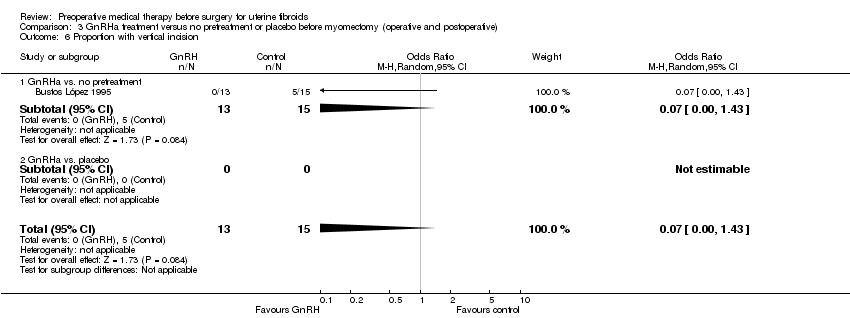
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 6 Proportion with vertical incision.

Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 7 Duration of hospital stay (days).

Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 8 Proportion with postoperative recurrence of myomas.
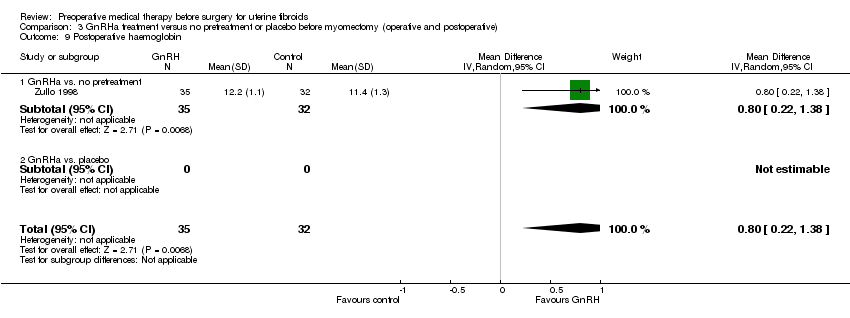
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 9 Postoperative haemoglobin.

Comparison 4 GnRHa treatment versus no pretreatment or placebo prior to resection, Outcome 1 Duration of surgery (minutes).

Comparison 4 GnRHa treatment versus no pretreatment or placebo prior to resection, Outcome 2 Difficulty of surgery (VAS).
Comparison 4 GnRHa treatment versus no pretreatment or placebo prior to resection, Outcome 3 Fibroid recurrence.
| Study | Number in study | Comparison | Results | Comment |
| Baytur 2007 | 32 | GnRHa (goserelin 3.6mg monthly for 3 months) vs SERM (raloxifene 60mg daily orally) | Difference from baseline to after treatment (median (range)): GnRHa: 95cc (37 to 452) Raloxifene: 62.5 (10 to 118) | Not significantly different between groups |
| Donnez 2012b | 307 | GnRHa (leuprolide acetate 3.75mg) vs ulipristal acetate (5mg and 10mg) | Per protocol results: Median percent change from baseline in uterine volume (IQ range): Ulipristal acetate 5mg: ‐20% (‐40 to ‐3) Ulipristal acetate 10mg: ‐22% (‐45 to 0) Leuprolide acetate 3.75mg: ‐47% (‐57 to ‐35) Difference in % points: UA 5mg vs LA 3.75mg: 1.48 (1.25 to 1.74) UA 10mg vs LA 3.75mg: 1.41 (1.19 to 1.66) | Authors reported that LA was associated with a significantly greater reduction in uterine volume than either UA group |
| Reinsch 1994 | 14 | GnRHa (leuprolide acetate 3.75mg) vs mifepristone (RU 486 25mg) | Results reported in the text (median percent reduction and range) Leuprolide acetate 3.75mg: 54% (22 to 84) RU 486 25mg: 32% (1 to 65) | Authors reported that there was no significant change in volume reduction between the 2 groups. |
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 1 Uterine volume (descriptive table).

Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 2 Fibroid volume.
| Study | Number in study | Comparison | Results | Comment |
| Baytur 2007 | 32 | GnRHa (goserelin 3.6mg monthly for 3 months) vs SERM (raloxifene 60mg daily orally) | Difference from baseline to after treatment (median (range)): GnRHa: 30cc (20 to 200) Raloxifene: 18 (12 to 65) | No significant difference between groups |
| Donnez 2003 | 313 | GnRHa (goserelin 3.6mg monthly for 3 months) vs fluvestrant (50mg, 125mg and 250mg for 3 months) ‐ this comparison not blinded | Ratio of the generalised least squares means (fulvestrant or goserelin): Fulvestrant 50mg vs goserelin: 1.88 (95% CI 1.3 to 2.8), p=0.001, n=82 Fulvestrant 125mg vs goserelin: 1.82 (95% CI 1.2 to 2.7), p=0.0002, n=80 Fulvestrant 250mg vs goserelin: 1.56 (95% CI 1.1 to 2.3), p=0.023, n=83 | Authors reported that goserelin was associated with a significantly greater reduction in fibroid volume than any dose of fulvestrant. Note: this analysis was per protocol with significant attrition ‐ the ITT analyses were not reported. |
| Donnez 2012b | 307 | GnRHa (leuprolide acetate 3.75mg) vs ulipristal acetate (5mg and 10mg) | Per protocol results: Percentage change from baseline in 3 largest fibroids (IQ range) Ulipristal acetate 5mg: ‐36% (‐58 to ‐11) Ulipristal acetate 10mg: ‐42% (‐69 to ‐41) Leuprolide acetate 3.75mg: ‐53% (‐69 to ‐36) Difference in % points: UA 5mg vs LA 3.75mg: 1.23 (0.99 to 1.52) UA 10mg vs LA 3.75mg: 1.12 (0.91 to 1.38) | Authors reported that all 3 treatments reduced the volume of the 3 largest fibroids |
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 3 Fibroid volume (descriptive table).

Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 4 Haemoglobin at end of preoperative treatment.

Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 5 Reduction in bleeding to PBAC < 75.

Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 6 Adverse events.
| Study | No of participants | Comparison | Results | Comment |
| Donnez 2012b | 281 | UA 5mg or UA 10mg versus LA 3.75mg | UA 5mg vs LA (change from baseline): 2.5% (‐7.3 to 12.3) UA 10mg vs LA (change from baseline: 5.6% (‐3.9 to 15.1) | No significant difference between groups |
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 7 Quality of life (Uterine Fibroid Symptom and QoL questionnaire).
| Study | Number in study | Comparison | Results | Comment |
| Donnez 2012a | 242 | Ulipristal acetate 5 mg or 10 mg daily vs. placebo | Reduction in uterine volume prior to surgery of 25% or greater: Ulipristal acetate 5 mg: 30/88 (34%) Ulipristal acetate 10 mg: 24/85 (28%) Placebo: 3/47 (6%) Difference UA 5 mg vs. placebo: 28 (11 to 40) Difference UA 10 mg vs. placebo: 22 (6 to 35) | Authors reported that both UA groups had a significantly greater reduction in uterine volume of 25% or greater than the placebo group |
| Wilkens 2008 | 33 | Asoprisnil 10 mg and 25 mg vs. placebo | Median percentage change (from baseline): Asoprisnil 10 mg vs. placebo: 7.9% vs. ‐2.1% (NS) Asoprisnil 25 mg vs placebo: ‐5.1% vs ‐2.1% (NS) | Authors reported no significant difference between groups |
Comparison 6 SPRM versus placebo, Outcome 1 Reduction in uterine volume.
| Study | Number in study | Comparison | Study findings | Comment |
| Donnez 2012a | 242 | Ulipristal acetate 5mg/day and 10mg/day vs placebo | Median change: UA 5mg vs placebo: ‐18.9% vs +1.9% (p=0.002) Difference UA 5mg vs placebo: ‐19.6% (‐31.2 to ‐6.5) UA 10mg vs placebo: ‐6.2% vs +1.9% (p=0.006) Difference UA 10mg vs placebo: ‐14.2% (‐25.9 to ‐2.4) | Clinically and statistically significant differences between UA and placebo in both per protocol and modified ITT analyses |
| Engman 2009 | 30 | Mifepristone 50mg/every other day vs placebo | Mean % change from baseline (CI): MP vs placebo: ‐28% (‐48 to ‐8) vs +6% (‐13 to 25 (p=0.02) | Authors reported that decrease with MP was significantly lower than placebo |
| Levens 2008 | 22 | CDB‐2914 10mg/day and 20mg/day vs placebo | Change: CDB‐2914 (combined dosages) vs placebo: +6% vs ‐29% (p=0.01) | Not clear if mean or median change ‐ no variation measure reported |
| Wilkens 2008 | 33 | Asoprisnil 10mg/day and 25mg/day vs placebo | Median percent change in largest fibroid volume: Asoprisnil 10mg vs placebo: ‐0.4% vs 4.9% (NS) Asoprisnil 25mg vs placebo: ‐25.8% vs 4.9% (0.04) | Authors reported that only the higher dose asoprisnil group reduction was significantly different from placebo (lower dose NS) |
Comparison 6 SPRM versus placebo, Outcome 2 Reduction in fibroid volume.

Comparison 6 SPRM versus placebo, Outcome 3 Haemoglobin (g/dL).

Comparison 6 SPRM versus placebo, Outcome 4 Reduction in menstrual bleeding (PBAC < 75).
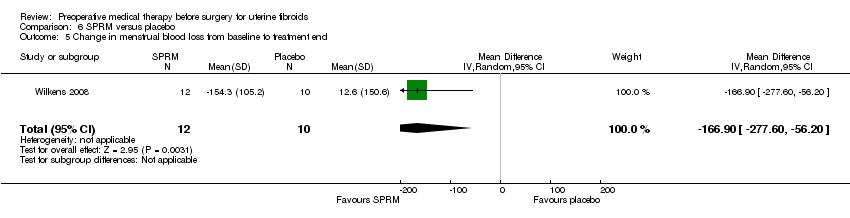
Comparison 6 SPRM versus placebo, Outcome 5 Change in menstrual blood loss from baseline to treatment end.
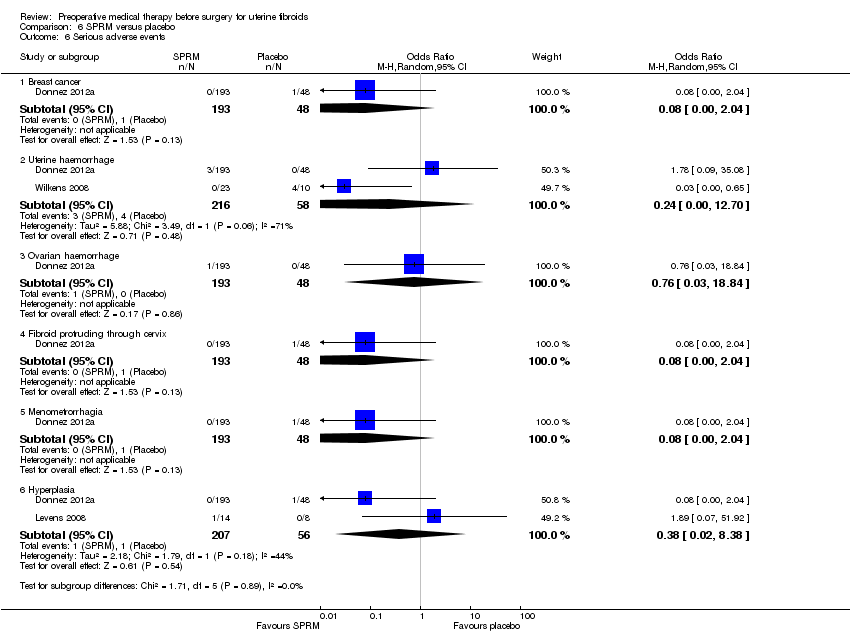
Comparison 6 SPRM versus placebo, Outcome 6 Serious adverse events.

Comparison 6 SPRM versus placebo, Outcome 7 Other adverse events.
| Study | No of participants | Comparison | Results | Comment |
| Donnez 2012a | 239 | UA 5 mg or UA 10 mg versus placebo | Questionnaire assessing discomfort from fibroids (ranging from 0 to 28 points): UA 5 mg vs. placebo (change from baseline): ‐4.0 (‐6.0 to ‐1.0), P = 0.001 UA 10 mg vs. placebo (change from baseline): ‐4.0 (‐7.0 to ‐2.0), P < 0.001 | Differences from placebo group significant |
Comparison 6 SPRM versus placebo, Outcome 8 Quality of life (Uterine Fibroid Symptoms and QoL questionnaire).
| Gonadotropin‐hormone releasing analogue (GnRHa) treatment versus placebo or no pretreatment (preoperative outcomes) for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | GnRHa pretreatment | |||||
| Uterine volume (mL) (preoperative) | Mean uterine volume in control group ranged from 255 mL to 920 mL | Mean uterine volume (mL) (preoperative) in the intervention groups was | ‐ | 858 | ⊕⊕⊝⊝ | This overall estimate assessed effects from studies with two types of control group, either no treatment or placebo |
| Fibroid volume (mL) (preoperative) | See comment | Not estimable | 427 | ⊕⊕⊝⊝ | Estimates were too heterogeneous for pooling. Reduction in fibroid volume ranged from 5 mL to 155 mL in the GnRHa group compared to control | |
| Haemoglobin (g/dL) (preoperative) | Mean haemoglobin ranged from 10.9 g/dL to 13.4 g/dL | Mean haemoglobin (g/dL) (preoperative) in the intervention groups was | ‐ | 834 | ⊕⊕⊝⊝ | This overall estimate assessed effects from studies with two types of control group, either no treatment or placebo |
| Preoperative bleeding | See comment | Not estimable | ‐ | ‐ | This outcome was not measured by validated scales | |
| Adverse events | Study population | OR 2.78 | 755 | ⊕⊕⊕⊝ | ||
| 579 per 1000 | 793 per 1000 | |||||
| Moderate | ||||||
| 608 per 1000 | 812 per 1000 | |||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence quality was downgraded 1 level for serious limitations in study design (few studies had adequate sequence generation, allocation concealment baseline comparability and blinding although lack of blinding was not expected to influence findings. 7 studies had low risk of attrition and reporting bias). 6 Evidence quality downgraded one level because of serious limitations in study design (most trials had low risk of selection, reporting and performance biases, but risk of detection and attrition bias was unclear or high). | ||||||
| Gonadotropin‐hormone releasing analogues (GnRHa) treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative outcome for uterine fibroids) | ||||||
| Patient or population: women with uterine fibroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | GnRHa pretreatment | |||||
| Duration of surgery (minutes) | Mean duration of surgery in the control group ranged from 53 minutes to 115 minutes | Mean duration of surgery (minutes) in the intervention groups was | ‐ | 617 | ⊕⊕⊝⊝ | An additional 3 studies had findings presented in data tables (2 reported no difference between groups and 1 reported a difference of 21 minutes between groups) |
| Intraoperative blood loss (mL) | See comment | Not estimable | 258 | ⊕⊝⊝⊝ | Substantial heterogeneity so estimates could not be pooled. Differences between blood loss (mL) between GnRHa and control group participants ranged from 25 mL to 148 mL | |
| Blood transfusions | Study population | OR 0.54 | 601 | ⊕⊕⊝⊝ | Fixed‐effects model: OR 0.54 (95% CI 0.3 to 0.95) | |
| 104 per 1000 | 59 per 1000 | |||||
| Moderate | ||||||
| 115 per 1000 | 66 per 1000 | |||||
| Postoperative morbidity | Study population | OR 0.54 | 772 | ⊕⊕⊝⊝ | ||
| 195 per 1000 | 116 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 145 per 1000 | |||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence quality downgraded one level because of serious limitations in study design (approximately half of the included studies had unclear or high risk of selection, performance, detection, attrition and reporting bias). | ||||||
| Gonadotropin‐hormone releasing analogue (GnRHa) treatment versus no pretreatment or placebo before myomectomy (operative and postoperative outcomes) for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | GnRHa pretreatment | |||||
| Duration of surgery (minutes) | See comment | Not estimable | 443 | ⊕⊝⊝⊝ | Substantial heterogeneity so estimates could not be pooled. Trial where laparoscopic myomectomy was undertaken indicated that GnRHa was associated with greater duration of surgery than control but no other factors were identified to explain the variation and no estimates could be shown. | |
| Intraoperative blood loss (mL) | See comment | Not estimable | 549 | ⊕⊝⊝⊝ | Substantial heterogeneity so estimates could not be pooled. All trials, except 1, found a difference in intraoperative blood loss between GnRHa and control ranging from 21 mL to 157 mL. A single trial where laparoscopic myomectomy was compared with control found that GnRHa pretreatment was associated with 82 mL greater blood loss than control. | |
| Blood transfusions | Study population | OR 0.85 | 121 | ⊕⊕⊝⊝ | ||
| 143 per 1000 | 124 per 1000 | |||||
| Moderate | ||||||
| 194 per 1000 | 170 per 1000 | |||||
| Postoperative morbidity | Study population | OR 1.07 | 190 | ⊕⊕⊝⊝ | ||
| 146 per 1000 | 154 per 1000 | |||||
| Moderate | ||||||
| 188 per 1000 | 199 per 1000 | |||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence quality downgraded one level because of serious limitations in study design (only 1 study had allocation concealment and blinding of participants or investigators. Half of the studies had low risk of selection and detection bias but most had low risk of reporting and attrition bias). | ||||||
| Gonadotropin‐hormone releasing analogue (GnRHa) treatment versus no pretreatment or placebo before resection for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | GnRHa pretreatment | |||||
| Duration of surgery (minutes) | Mean duration of surgery in the control group was 21 minutes | Mean operating time (minutes) in the intervention groups was | ‐ | 39 | ⊕⊕⊝⊝ | |
| Intraoperative blood loss (mL) | ‐ | No studies measured this outcome | Not estimable | ‐ | ‐ | |
| Blood transfusions | ‐ | No studies measured this outcome | Not estimable | ‐ | ‐ | |
| Postoperative morbidity | ‐ | No studies measured this outcome | Not estimable | ‐ | ‐ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence quality downgraded 1 level for serious limitations in study design (lack of blinding and unclear reporting bias). | ||||||
| Gonadotropin‐hormone releasing analogue (GnRHa) treatment versus other medical therapies before any surgery for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (other medical therapies) | GnRHa pretreatment | |||||
| Uterine volume (cm³) | See comment | Not estimable | ‐ | ‐ | Studies too heterogeneous for pooling. One study comparing a GnRHa with a SERM and another study comparing GnRHA with mifepristone found no difference between groups. One trial comparing GnRHa with ulipristal acetate found a greater reduction with GnRHa (‐47%) compared to 5 mg (‐20%) and 10 mg (‐22%) ulipristal acetate | |
| Fibroid volume (cm³) | Fibroid volume in the other treatment group (cabergoline) ranged from 86 cm³ to 278 cm³ | Mean fibroid volume in the intervention groups was | ‐ | 110 | ⊕⊕⊝⊝ | 2 additional studies with skewed data not suitable for pooling reported no differences between groups (GnRHa vs. raloxifene, GnRHa vs. ulipristal acetate) One additional study found a greater reduction with GnRHa when compared to multiple doses of fulvestrant |
| Preoperative haemoglobin (g/dL) | Mean haemoglobin at end of preoperative treatment in ulipristal acetate group was 12.9 g/dL | Mean haemoglobin at end of preoperative treatment in the intervention groups was | ‐ | 188 | ⊕⊕⊕⊝ | |
| Preoperative bleeding: Reduction in bleeding to PBAC < 75 ulipristal acetate 5 mg | Study population | OR 0.71 | 199 | ⊕⊕⊕⊝ | ||
| 898 per 1000 | 862 per 1000 | |||||
| Moderate | ||||||
| 898 per 1000 | 862 per 1000 | |||||
| Preoperative bleeding: Reduction in bleeding to PBAC < 75 ulipristal acetate 10 mg | Study population | OR 0.39 | 203 | ⊕⊕⊕⊝ | ||
| 941 per 1000 | 862 per 1000 | |||||
| Moderate | ||||||
| 941 per 1000 | 861 per 1000 | |||||
| Adverse events (hot flushes) | 213 per 1000 | 691 per 1000 | OR 12.30 (4.04 to 37.48) | 453 (5 studies) | ⊕⊕⊝⊝ low4 | These findings were for hot flushes (GnRHa compared to raloxifene, ulipristal acetate, mifepristone, cabergoline and lynestrenol). Headache (with comparators raloxifene, ulipristal acetate, cabergoline and lynestrenol), sleep disorder (vs. lynestrenol) and bone sensitivity (vs. cabergoline) were also increased with GnRHa compared to other medical treatments but fewer studies contributed data. There were no other significant differences. No studies compared total numbers of adverse events |
| The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence quality downgraded 1 level because of limitations in study design (unclear risk of selection and attrition bias and lack of blinding). | ||||||
| Selective progesterone‐receptor modulators (SPRM) compared to placebo for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | SPRM | |||||
| Uterine volume (cm³) | See comment | Not estimable | ‐ | ‐ | Two studies could not be pooled. One study found a greater proportion of women taking ulipristal acetate had a reduction of uterine volume > 25% than placebo (34% (ulipristal acetate 5 mg) and 28% (ulipristal acetate 10 mg) vs. placebo 6%). The other study found no difference in this outcome with asoprisnil compared to placebo | |
| Fibroid volume (cm³) | See comment | Not estimable | ‐ | ‐ | Four studies could not be pooled. All studies found a significantly greater reduction with SPRMs (regardless of type) compared to placebo (except for the lower dose of asoprisnil (10 mg)). Reductions with ulipristal acetate, mifepristone, CDB‐2914 and asoprisnil 25 mg ranged from 12% to 29% compared to a range of 3% to 6% with placebo | |
| Preoperative haemoglobin (g/dL) | Mean haemoglobin ranged from 12.2 to 12.6 g/dL | Mean haemoglobin (g/dL) in the intervention groups was | ‐ | 173 | ⊕⊕⊕⊕ | Although one study reported receiving pharmaceutical company funding, results were very similar so funding was unlikely to have influenced the results |
| Preoperative bleeding: (PBAC < 75) ulipristal acetate 5 mg | Study population | OR 41.41 | 143 | ⊕⊕⊝⊝ | Study was funded by the pharmaceutical company that supplied the intervention | |
| 188 per 1000 | 905 per 1000 | |||||
| Moderate | ||||||
| 188 per 1000 | 906 per 1000 | |||||
| Preoperative bleeding: Reduction in menstrual bleeding (PBAC < 75) ulipristal acetate 10 mg | Study population | OR 78.83 | 146 | ⊕⊕⊝⊝ | Study was funded by the pharmaceutical company that supplied the intervention | |
| 83 per 1000 | 878 per 1000 | |||||
| Moderate | ||||||
| 83 per 1000 | 877 per 1000 | |||||
| Preoperative bleeding: Change in menstrual blood loss from baseline to end of treatment | Mean menstrual blood loss change score (menstrual pictogram) increased from baseline of 12.6 (menstrual bleeding score) | Mean change in menstrual blood loss from baseline to end of treatment in the intervention groups was | ‐ | 22 | ⊕⊕⊝⊝ | |
| Adverse events | 42 per 1000 0 per 1000 63 per 1000 | 0 per 1000 429 per 1000 500 per 1000 | OR 0.05 (0.0 to 1.0) OR 15.0 (1.5 to 146.5) | 241 (1 study) (dysmenorrhoea) 30 (1 study) (hot flushes) 30 (1 study) (change in mood) | ⊕⊕⊝⊝ low3 | No evidence of a difference in serious adverse events. For specific less serious adverse events, results were very imprecise. There was no evidence of significant differences for the other individual adverse events. |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence quality downgraded one level because of potential influence from pharmaceutical company funding. 2 Evidence quality downgraded one level for imprecision (wide confidence intervals). | ||||||
| Comparison | Outcomes | |||
| Preoperative | Intra/postoperative + hysterectomy | Intra/postoperative + myomectomy | Intra/postoperative + resection | |
| GnRHa versus no treatment or placebo | Comparison 1 | Comparison 2 | Comparison 3 | Comparison 4 |
| Primary outcomes .Reduction in uterine volume · Reduction in fibroid volume · Preoperative Hb · Preoperative bleeding Secondary outcomes · Adverse events · QoL | Primary outcomes · Duration of operation · Intraoperative blood loss · Frequency of blood transfusions Secondary outcomes · Difficulty of surgery · Proportion of women undergoing vaginal hysterectomy · Type of abdominal incision · Duration of hospital stay · Postoperative morbidity · Postoperative recurrence · Postoperative Hb | Primary outcomes · Duration of operation · Intraoperative blood loss · Frequency of blood transfusions Secondary outcomes · Difficulty of surgery ·Intraoperative hysterectomy · Type of abdominal incision · Duration of hospital stay · Postoperative morbidity · Postoperative recurrence · Postoperative Hb | Primary outcomes · Duration of operation · Intraoperative blood loss · Frequency of blood transfusions Secondary outcomes · Difficulty of surgery · Type of abdominal incision · Duration of hospital stay · Postoperative morbidity · Postoperative recurrence · Postoperative Hb | |
| GnRHa versus other medical treatments | Comparison 5 | |||
| Primary outcomes · Reduction in uterine volume · Reduction in fibroid volume · Preoperative Hb · Preoperative bleeding Secondary outcomes · Adverse events · QoL | not applicable | not applicable | not applicable | |
| SPRMs versus placebo | Comparison 6 | |||
| Primary outcomes · Reduction in uterine volume · Reduction in fibroid volume · Preoperative Hb · Preoperative bleeding Secondary outcomes · Adverse events · QoL | not applicable | ‐ | ‐ | |
| GnRHa: gonadotropin‐releasing hormone analogues Hb: haemoglobin Hb: Haemoglobin QoL: quality of life SPRM: selective progesterone receptor modulator | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine volume (mL) (preoperative) Show forest plot | 13 | 858 | Mean Difference (IV, Random, 95% CI) | ‐175.34 [‐219.04, ‐131.65] |
| 1.1 GnRHa vs. no pretreatment | 11 | 810 | Mean Difference (IV, Random, 95% CI) | ‐178.68 [‐224.63, ‐132.74] |
| 1.2 GnRHa vs. placebo | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐113.76 [‐314.60, 87.08] |
| 2 Uterine volume (preop in data table) Show forest plot | Other data | No numeric data | ||
| 2.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 2.2 GnRHa vs. placebo | Other data | No numeric data | ||
| 3 Fibroid volume (mL) (preoperative) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 GnRHa vs. no pretreatment | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 GnRHa vs. placebo | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fibroid volume (preop in data table) Show forest plot | Other data | No numeric data | ||
| 4.1 GnRHa vs. placebo | Other data | No numeric data | ||
| 5 Haemoglobin (preoperative) Show forest plot | 10 | 834 | Mean Difference (IV, Random, 95% CI) | 0.88 [0.68, 1.08] |
| 5.1 GnRHa vs. no pretreatment | 6 | 308 | Mean Difference (IV, Random, 95% CI) | 0.91 [0.52, 1.30] |
| 5.2 GnRHa vs. placebo | 4 | 526 | Mean Difference (IV, Random, 95% CI) | 0.87 [0.62, 1.13] |
| 6 Haemoglobin (preop in data table) Show forest plot | Other data | No numeric data | ||
| 6.1 GnRHa vs. placebo | Other data | No numeric data | ||
| 7 Total frequency of adverse events Show forest plot | 4 | 755 | Odds Ratio (M‐H, Random, 95% CI) | 2.78 [1.77, 4.36] |
| 7.1 GnRHa vs. no pretreatment | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 GnRHa vs. placebo | 4 | 755 | Odds Ratio (M‐H, Random, 95% CI) | 2.78 [1.77, 4.36] |
| 8 Individual adverse events Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Insomnia | 1 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 12.56 [0.68, 232.82] |
| 8.2 Hot flushes | 6 | 877 | Odds Ratio (M‐H, Random, 95% CI) | 7.68 [4.55, 12.96] |
| 8.3 Headache | 6 | 877 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [1.00, 3.03] |
| 8.4 Pain | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.12] |
| 8.5 Nausea | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 2.41 [0.14, 40.59] |
| 8.6 Dizziness | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 2.41 [1.13, 5.14] |
| 8.7 Depression | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 2.12 [0.87, 5.17] |
| 8.8 Arthralgia | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.55, 3.02] |
| 8.9 Asthenia | 3 | 615 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.41, 2.39] |
| 8.10 Vaginitis | 5 | 751 | Odds Ratio (M‐H, Random, 95% CI) | 4.18 [1.58, 11.05] |
| 8.11 Abdominal/pelvic pain | 3 | 615 | Odds Ratio (M‐H, Random, 95% CI) | 2.43 [0.98, 6.05] |
| 8.12 Skin changes | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.48, 3.13] |
| 8.13 Hirsutism | 1 | 65 | Odds Ratio (M‐H, Random, 95% CI) | 6.35 [0.70, 57.72] |
| 8.14 Change in libido | 3 | 332 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.76, 4.46] |
| 8.15 Change in breast size | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 10.87 [1.90, 62.24] |
| 8.16 Sweating | 4 | 497 | Odds Ratio (M‐H, Random, 95% CI) | 14.32 [6.17, 33.27] |
| 8.17 Breast pain/tenderness | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.35, 1.52] |
| 8.18 Uterine haemorrhage | 1 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of surgery (minutes) Show forest plot | 6 | 617 | Mean Difference (IV, Random, 95% CI) | ‐10.11 [‐16.96, ‐3.25] |
| 1.1 GnRHa vs. no pretreatment | 4 | 321 | Mean Difference (IV, Random, 95% CI) | ‐14.19 [‐25.01, ‐3.38] |
| 1.2 GnRHa vs. placebo | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐5.03 [‐12.17, 2.12] |
| 2 Duration of surgery (data table) Show forest plot | Other data | No numeric data | ||
| 2.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 2.2 GnRHa vs. placebo | Other data | No numeric data | ||
| 3 Intraoperative blood loss (mL) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 GnRHa vs. no pretreatment | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Intraoperative blood loss (data table) Show forest plot | Other data | No numeric data | ||
| 4.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 4.2 GnRHa vs. placebo | Other data | No numeric data | ||
| 5 Proportion with blood transfusions Show forest plot | 6 | 601 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.29, 1.01] |
| 5.1 GnRHa vs. no pretreatment | 5 | 487 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.08] |
| 5.2 GnRHa vs. placebo | 1 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.23, 1.78] |
| 6 Proportion with postoperative complications Show forest plot | 7 | 772 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.32, 0.91] |
| 6.1 GnRHa vs. no treatment | 5 | 507 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.23] |
| 6.2 GnRHa vs. placebo | 2 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.19, 1.11] |
| 7 Proportion with individual complications Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypermenorrhoea | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.11, 1.24] |
| 7.2 Dysmenorrhoea | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 3.90 [0.19, 82.29] |
| 7.3 Pelvic pain | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.15, 1.23] |
| 7.4 Difficult defecation | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.11, 5.52] |
| 7.5 Difficult urination | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 3.17] |
| 7.6 Dyspareunia | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 3.90 [0.19, 82.29] |
| 8 Proportion with difficult surgery Show forest plot | 5 | 712 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.00] |
| 8.1 GnRH vs. no pretreatment | 2 | 347 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.44] |
| 8.2 GnRH vs. placebo | 3 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.94] |
| 9 Proportion undergoing vaginal rather than abdominal procedure Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 GnRHa vs. no treatment | 2 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 GnRHa vs. placebo | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Proportion with vertical incision Show forest plot | 4 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.21, 0.54] |
| 10.1 GnRHa vs. no pretreatment | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.18, 0.59] |
| 10.2 GnRHa vs. placebo | 2 | 228 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.17, 0.75] |
| 11 Duration of hospital stay (days) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 GnRHa vs. no pretreatment | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Duration of hospital stay (data table) Show forest plot | Other data | No numeric data | ||
| 12.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 13 Postoperative haemoglobin Show forest plot | 3 | 240 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.31, 1.38] |
| 13.1 GnRHa vs. no pretreatment | 2 | 173 | Mean Difference (IV, Random, 95% CI) | 1.05 [0.39, 1.71] |
| 13.2 GnRHa vs. placebo | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.35, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of surgery (minutes) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 GnRHa vs. no pretreatment | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Duration of surgery (descriptive data) Show forest plot | Other data | No numeric data | ||
| 2.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 3 Intraoperative blood loss (mL) Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 GnRHa vs. no pretreatment | 9 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Proportion with blood transfusions Show forest plot | 4 | 121 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.26, 2.75] |
| 4.1 GnRHa vs. no pretreatment | 3 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.20, 3.19] |
| 4.2 GnRHa vs. placebo | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.11, 9.23] |
| 5 Proportion with postoperative complications Show forest plot | 5 | 190 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.43, 2.64] |
| 5.1 GnRHa vs. no pretreatment | 4 | 172 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.44, 3.54] |
| 5.2 GnRHa vs. placebo | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.10, 4.11] |
| 6 Proportion with vertical incision Show forest plot | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.43] |
| 6.1 GnRHa vs. no pretreatment | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.43] |
| 6.2 GnRHa vs. placebo | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Duration of hospital stay (days) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 GnRHa vs. no pretreatment | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Proportion with postoperative recurrence of myomas Show forest plot | 2 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 4.16 [0.59, 29.09] |
| 8.1 GnRHa vs. no pretreatment | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 11.67 [1.49, 91.54] |
| 8.2 GnRHa vs. placebo | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 1.6 [0.24, 10.81] |
| 9 Postoperative haemoglobin Show forest plot | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.22, 1.38] |
| 9.1 GnRHa vs. no pretreatment | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.22, 1.38] |
| 9.2 GnRHa vs. placebo | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of surgery (minutes) Show forest plot | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐5.4 [‐7.65, ‐3.15] |
| 2 Difficulty of surgery (VAS) Show forest plot | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐3.05, 0.25] |
| 3 Fibroid recurrence Show forest plot | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine volume (descriptive table) Show forest plot | Other data | No numeric data | ||
| 2 Fibroid volume Show forest plot | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 12.71 [‐5.92, 31.34] |
| 3 Fibroid volume (descriptive table) Show forest plot | Other data | No numeric data | ||
| 4 Haemoglobin at end of preoperative treatment Show forest plot | 1 | 188 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 5 Reduction in bleeding to PBAC < 75 Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Ulipristal acetate 5 mg | 1 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.30, 1.68] |
| 5.2 Ulipristal acetate 10 mg | 1 | 203 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.06] |
| 6 Adverse events Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Hot flushes | 5 | 453 | Odds Ratio (M‐H, Random, 95% CI) | 12.30 [4.04, 37.48] |
| 6.2 Headache | 4 | 439 | Odds Ratio (M‐H, Random, 95% CI) | 4.51 [1.09, 18.62] |
| 6.3 Nausea | 3 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.64] |
| 6.4 Weight gain | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.07, 2.81] |
| 6.5 Oedema | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 2.2 [0.21, 22.59] |
| 6.6 Sleep disorder | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 20.71 [2.49, 172.00] |
| 6.7 Mood disorder or anxiety | 2 | 106 | Odds Ratio (M‐H, Random, 95% CI) | 4.02 [0.02, 727.86] |
| 6.8 Vaginal dryness | 3 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 31.93 [2.19, 464.89] |
| 6.9 Cutaneous disorder | 2 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.51, 4.38] |
| 6.10 Abdominal or pelvic pain | 3 | 383 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.85, 2.91] |
| 6.11 Procedural pain | 2 | 333 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.21, 6.35] |
| 6.12 Fatigue | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.14, 1.93] |
| 6.13 Anaemia | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.40, 3.92] |
| 6.14 Nasopharyngitis | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.08, 1.79] |
| 6.15 Breast pain or tenderness | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.10, 2.33] |
| 6.16 Influenza | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [0.67, 9.72] |
| 6.17 Insomnia | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [0.67, 9.72] |
| 6.18 Pharyngitis | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.15, 4.13] |
| 6.19 Dizziness | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.84] |
| 6.20 Bone sensitivity | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 125.80 [6.75, 2343.30] |
| 6.21 Muscular stiffness | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 5.43 [0.25, 118.96] |
| 7 Quality of life (Uterine Fibroid Symptom and QoL questionnaire) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in uterine volume Show forest plot | Other data | No numeric data | ||
| 2 Reduction in fibroid volume Show forest plot | Other data | No numeric data | ||
| 3 Haemoglobin (g/dL) Show forest plot | 2 | 173 | Mean Difference (IV, Random, 95% CI) | 0.93 [0.52, 1.35] |
| 4 Reduction in menstrual bleeding (PBAC < 75) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Ulipristal acetate 5 mg | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 41.41 [15.26, 112.38] |
| 4.2 Ulipristal acetate 10 mg | 1 | 146 | Odds Ratio (M‐H, Random, 95% CI) | 78.83 [24.02, 258.74] |
| 5 Change in menstrual blood loss from baseline to treatment end Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐166.9 [‐277.60, ‐56.20] |
| 6 Serious adverse events Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Breast cancer | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 2.04] |
| 6.2 Uterine haemorrhage | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.00, 12.70] |
| 6.3 Ovarian haemorrhage | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.03, 18.84] |
| 6.4 Fibroid protruding through cervix | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 2.04] |
| 6.5 Menometrorrhagia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 2.04] |
| 6.6 Hyperplasia | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 8.38] |
| 7 Other adverse events Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Headache | 3 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.14, 4.30] |
| 7.2 Breast pain or tenderness | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.26, 11.70] |
| 7.3 Abdominal pain | 3 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [0.36, 8.12] |
| 7.4 Pyrexia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.12, 3.25] |
| 7.5 Hypercholesterolaemia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.14, 10.96] |
| 7.6 Hypothyroidism | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 3.36 [0.19, 60.73] |
| 7.7 Constipation | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.13, 2.79] |
| 7.8 Hypertriglyceridaemia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.11, 9.11] |
| 7.9 Influenza | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.11, 9.11] |
| 7.10 Dizziness | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 2.30 [0.12, 43.52] |
| 7.11 Nasopharyngitis | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.09, 2.31] |
| 7.12 Dysmenorrhoea | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 1.02] |
| 7.13 Bladder pressure | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.64] |
| 7.14 Micturition problem | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [0.28, 13.23] |
| 7.15 Lower back pain | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.29, 6.15] |
| 7.16 Proctodynia | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 3.67 [0.14, 97.49] |
| 7.17 Coital pain | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 6.6 [0.29, 150.07] |
| 7.18 Hot flushes | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 25.24 [1.27, 503.38] |
| 7.19 Nausea | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.97 [0.46, 8.46] |
| 7.20 Vomiting | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.21 Diarrhoea | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 3.62 [0.39, 33.89] |
| 7.22 Change of mood | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 15.00 [1.54, 146.54] |
| 7.23 Lowered libido | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 6.0 [0.58, 61.84] |
| 7.24 Weakness | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 2.8 [0.43, 18.38] |
| 7.25 Fatigue | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.31, 9.57] |
| 7.26 Dental pain | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.12, 14.82] |
| 7.27 Vaginal infections | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 2.44 [0.11, 55.56] |
| 7.28 Vaginal discharge | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.12, 14.82] |
| 8 Quality of life (Uterine Fibroid Symptoms and QoL questionnaire) Show forest plot | Other data | No numeric data | ||

