Doustne preparaty kwasu 5‐aminosalicylowego w celu utrzymania remisji u chorych na wrzodziejące zapalenie jelita grubego
Abstract
Background
Oral 5‐aminosalicylic (5‐ASA) preparations were intended to avoid the adverse effects of sulfasalazine (SASP) while maintaining its therapeutic benefits. Previously, it was found that 5‐ASA drugs were more effective than placebo but had a statistically significant therapeutic inferiority relative to SASP. This updated review includes more recent studies and evaluates the effectiveness, dose‐responsiveness, and safety of 5‐ASA preparations used for maintenance of remission in quiescent ulcerative colitis.
Objectives
The primary objectives were to assess the efficacy, dose‐responsiveness and safety of oral 5‐ASA compared to placebo, SASP, or 5‐ASA comparators for maintenance of remission in quiescent ulcerative colitis. A secondary objective was to compare the efficacy and safety of once daily dosing of oral 5‐ASA with conventional (two or three times daily) dosing regimens.
Search methods
A literature search for relevant studies (inception to 9 July 2015) was performed using MEDLINE, EMBASE and the Cochrane Library. Review articles and conference proceedings were also searched to identify additional studies.
Selection criteria
Studies were accepted for analysis if they were randomized controlled trials with a minimum treatment duration of six months. Studies of oral 5‐ASA therapy for treatment of patients with quiescent ulcerative colitis compared with placebo, SASP or other 5‐ASA formulations were considered for inclusion. Studies that compared once daily 5‐ASA treatment with conventional dosing of 5‐ASA and 5‐ASA dose ranging studies were also considered for inclusion.
Data collection and analysis
The primary outcome was the failure to maintain clinical or endoscopic remission. Secondary outcomes included adherence, adverse events, withdrawals due to adverse events, and withdrawals or exclusions after entry. Trials were separated into five comparison groups: 5‐ASA versus placebo, 5‐ASA versus sulfasalazine, once daily dosing versus conventional dosing, 5‐ASA versus comparator 5‐ASA formulation, and 5‐ASA dose‐ranging. Placebo‐controlled trials were subgrouped by dosage. Once daily versus conventional dosing studies were subgrouped by formulation. 5‐ASA‐controlled trials were subgrouped by common 5‐ASA comparators (e.g. Asacol and Salofalk). Dose‐ranging studies were subgrouped by 5‐ASA formulation. We calculated the risk ratio (RR) and 95% confidence intervals (95% CI) for each outcome. Data were analyzed on an intention‐to‐treat basis.
Main results
Forty‐one studies (8928 patients) were included. The majority of included studies were rated as low risk of bias. Ten studies were rated at high risk of bias. Seven of these studies were single‐blind and three studies were open‐label. However, two open‐label studies and four of the single‐blind studies utilized investigator performed endoscopy as an endpoint, which may protect against bias. 5‐ASA was significantly superior to placebo for maintenance of clinical or endoscopic remission. Forty‐one per cent of 5‐ASA patients relapsed compared to 58% of placebo patients (7 studies, 1298 patients; RR 0.69, 95% CI 0.62 to 0.77). There was a trend towards greater efficacy with higher doses of 5‐ASA with a statistically significant benefit for the 1 to 1.9 g/day (RR 0.65; 95% CI 0.56 to 0.76) and the > 2 g/day subgroups (RR 0.73, 95% CI 0.60 to 0.89). SASP was significantly superior to 5‐ASA for maintenance of remission. Forty‐eight per cent of 5‐ASA patients relapsed compared to 43% of SASP patients (12 studies, 1655 patients; RR 1.14, 95% CI 1.03 to 1.27). A GRADE analysis indicated that the overall quality of the evidence for the primary outcome for the placebo and SASP‐controlled studies was high. No statistically significant differences in efficacy or adherence were found between once daily and conventionally dosed 5‐ASA. Twenty‐nine per cent of once daily patients relapsed over 12 months compared to 31% of conventionally dosed patients (8 studies, 3127 patients; RR 0.91, 95% CI 0.82 to 1.01). Eleven per cent of patients in the once daily group failed to adhere to their medication regimen compared to 9% of patients in the conventional dosing group (6 studies, 1462 patients; RR 1.22, 95% CI 0.91 to 1.64). There does not appear to be any difference in efficacy among the various 5‐ASA formulations. Forty‐four per cent of patients in the 5‐ASA group relapsed compared to 41% of patients in the 5‐ASA comparator group (6 studies, 707 patients; RR 1.08, 95% CI 0.91 to 1.28). A pooled analysis of two studies showed no statistically significant difference in efficacy between Balsalazide 6 g and 3 g/day. Twenty‐three per cent of patients in the 6 g/day group relapsed compared to 33% of patients in the 3 g/day group (216 patients; RR 0.76; 95% CI 0.45 to 2.79). One study found Balsalazide 4 g to be superior to 2 g/day. Thirty‐seven per cent of patients in the 4 g/day Balsalazide group relapsed compared to 55% of patients in the 2 g/day group (133 patients; RR 0.66; 95% CI 0.45 to 0.97). One study found a statistically significant difference between Salofalk granules 3 g and 1.5 g/day. Twenty‐five per cent of patients in the Salofalk 3 g/day group relapsed compared to 39% of patients in the 1.5 g/day group (429 patients; RR 0.65; 95% CI 0.49 to 0.86). Common adverse events included flatulence, abdominal pain, nausea, diarrhea, headache, dyspepsia, and nasopharyngitis. There were no statistically significant differences in the incidence of adverse events between 5‐ASA and placebo, 5‐ASA and SASP, once daily and conventionally dosed 5‐ASA, 5‐ASA and comparator 5‐ASA formulations and 5‐ASA dose ranging studies. The trials that compared 5‐ASA and SASP may have been biased in favour of SASP because most trials enrolled patients known to be tolerant to SASP which may have minimized SASP‐related adverse events.
Authors' conclusions
5‐ASA was superior to placebo for maintenance therapy in ulcerative colitis. However, 5‐ASA had a statistically significant therapeutic inferiority relative to SASP. Oral 5‐ASA administered once daily is as effective and safe as conventional dosing for maintenance of remission in quiescent ulcerative colitis. There does not appear to be any difference in efficacy or safety between the various formulations of 5‐ASA. Patients with extensive ulcerative colitis or with frequent relapses may benefit from a higher dose of maintenance therapy. High dose therapy appears to be as safe as low dose and is not associated with a higher incidence of adverse events.
PICO
Streszczenie prostym językiem
Doustne preparaty kwasu 5‐aminosalicylowego (5‐ASA) w celu utrzymania remisji u chorych na wrzodziejące zapalenie jelita grubego
Sulfasalazynę od dziesięcioleci stosuje się w leczeniu chorych na wrzodziejące zapalenie jelita grubego. Sulfasalazyna jest zbudowana z kwasu 5‐aminosalicylowego (5‐ASA) połączonego z cząsteczką siarki. Nawet u 1/3 pacjentów leczonych sulfasalazyną zgłasza objawy niepożądane, których występowanie wydaje się wynikać z obecności siarkowej części cząsteczki. Powszechnie występujące działania niepożądane obejmują: nudności, niestrawność, ból głowy, wymioty oraz ból brzucha. Aby uniknąć efektów ubocznych związanych ze stosowaniem sulfasalazyny wyprodukowano leki na bazie 5‐ASA. Niniejszy przegląd obejmuje 41 badań z randomizacją, do których włączono łącznie 8928 uczestników. Podawanie 5‐ASA doustnie okazało się być skuteczniejsze pod względem utrzymania remisji, w porównaniu z placebo (imitacja leku). Pomimo tego, że doustne preparaty 5‐ASA są skuteczne w utrzymywaniu remisji u chorych na wrzodziejące zapalenie jelita grubego, to nie są skuteczniejsze od leczenia sulfasalazyną. Pacjenci, których stan się polepszył, mogą w dalszym ciągu przyjmować którykolwiek z tych leków. Nie ma danych naukowych wskazujących, że działania niepożądane są częstsze w przypadku leczenia któregokolwiek z tych leków. Jednak, częstość występowania objawów niepożądanych może być znacznie mniejsza w przypadku leczenia 5‐ASA, w porównaniu z terapią sulfasalazyną. Powszechne objawy niepożądane związane ze stosowaniem 5‐ASA to: wzdęcia, ból brzucha, nudności, biegunka, ból głowy, niestrawność oraz zapalenie błony śluzowej nosa i gardła. Do większości badań, w których porównywano stosowanie 5‐ASA i sulfasalazyny włączono pacjentów, którzy tolerowali sulfasalazynę. To mogło spowodować mniejszą liczbę działań niepożądanych związanych z leczeniem sulfasalazyną w tych badaniach. Niepłodność męska jest powiązana z leczeniem sulfasalazyną, ale nie z terapią 5‐ASA, zatem preparaty 5‐ASA mogą być preferowane u pacjentów, dla których ważna jest płodność. Leczenie 5‐ASA jest droższe niż terapia sulfasalazyną, dlatego sulfasalazyna wydaje się być preferowaną metodą leczenia w przypadku, gdy koszt jest ważnym czynnikiem. Podawanie 5‐ASA doustnie raz dziennie w celu utrzymania remisji u chorych na wrzodziejące zapalenie jelita grubego jest tak samo skuteczne i bezpieczne jak standardowe dawkowanie (2‐3 razy dziennie). Wydaje się, że nie ma różnicy pod względem skuteczności i bezpieczeństwa stosowania 5‐ASA w różnych postaciach. Chorzy na rozległą postać wrzodziejącego zapalenia jelita grubego lub z częstymi nawrotami mogą odnieść korzyść z zastosowania większej dawki podtrzymującej. Stosowanie dużej dawki 5‐ASA wydaje się być równie bezpieczne jak podawanie małej dawki i nie wiąże się z większą częstością występowania objawów niepożądanych.
Authors' conclusions
Summary of findings
| Oral 5‐ASA versus placebo for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus placebo | |||||
| Failure to maintain clinical or endoscopic remission at study end‐point | 584 per 10001 | 403 per 1000 | RR 0.69 | 1,298 | ⊕⊕⊕⊕ | |
| Any adverse event | 393 per 10001 | 369 per 1000 | RR 0.94 | 774 | ⊕⊕⊕⊝ | |
| Withdrawal due to adverse event | 42 per 10001 | 36 per 1000 | RR 0.86 | 1096 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Oral 5‐ASA versus SASP for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus SASP | |||||
| Failure to maintain clinical or endoscopic remission at study end‐point | 429 per 10001 | 489 per 1000 | RR 1.14 | 1,655 | ⊕⊕⊕⊕ | |
| Any adverse event | 158 per 10001 | 169 per 1000 | RR 1.07 | 1,138 | ⊕⊕⊕⊝ | |
| Withdrawal due to adverse event | 54 per 10001 | 69 per 1000 | RR 1.27 | 1,585 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Once daily dosing versus conventional dosing for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | OD versus conventional | |||||
| Failure to maintain clinical or endoscopic remission at 6 months | 184 per 10001 | 188 per 1000 | RR 1.02 | 1,871 | ⊕⊕⊝⊝ | |
| Failure to maintain clinical or endoscopic remission at 12 months | 314 per 10001 | 286 per 1000 | RR 0.91 | 3,127 | ⊕⊕⊕⊝ | |
| Failure to adhere to study medication regimen | 87 per 10001 | 106 per 1000 | RR 1.22 | 1,462 | ⊕⊕⊕⊝ | |
| Development of any adverse event | 453 per 10001 | 453 per 1000 | RR 1.00 | 2,714 | ⊕⊕⊕⊕ | |
| Withdrawal due to adverse events | 15 per 10001 | 20 per 1000 | RR 1.31 | 3,737 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Oral 5‐ASA versus comparator 5‐ASA formulation for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus 5‐ASA | |||||
| Failure to maintain clinical or endoscopic remission at 12 months | 407 per 10001 | 440 per 1000 | RR 1.08 | 707 | ⊕⊕⊝⊝ | |
| Development of any adverse event | 686 per 10001 | 645 per 1000 | RR 0.94 | 357 | ⊕⊕⊝⊝ | |
| Withdrawal due to adverse events | 44 per 10001 | 55 per 1000 | RR 1.25 | 457 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| High dose oral 5‐ASA versus low dose 5‐ASA for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | High dose 5‐ASA versus low dose 5‐ASA | |||||
| Failure to maintain clinical or endoscopic remission at 12 months | 357 per 10001 | 286 per 1000 | RR 0.80 | 112 | ⊕⊝⊝⊝ | Asacol 4.8 g/day versus 2.4 g/day |
| Failure to maintain clinical or endoscopic remission at 12 months | 330 per 10001 | 251 per 1000 | RR 0.76 | 216 | ⊕⊝⊝⊝ | Balsalazide 6.0 g/day versus 3.0 g/day |
| Failure to maintain clinical or endoscopic remission at 12 months | 554 per 10001 | 366 per 1000 | RR 0.66 | 133 | ⊕⊕⊕⊝ | Balsalazide 4.0 g/day versus 2.0 g/day |
| Failure to maintain clinical or endoscopic remission at 12 months | 392 per 10001 | 255 per 1000 | RR 0.65 | 429 | ⊕⊕⊕⊝ | Salofalk granules 3.0 g OD versus 1.5 g OD |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
Background
The successful management of ulcerative colitis was greatly facilitated after the introduction of sulfasalazine (SASP) by Svartz (Svartz 1942). SASP is composed of 5‐aminosalicylic acid (5‐ASA) linked to sulfapyridine via a diazo bond. This bond is readily cleaved by bacterial azoreductases in the colon (Peppercorn 1972) to yield the two components. Of these, 5‐ASA has been found to be the therapeutically active component, while sulfapyridine, which is primarily absorbed into systemic circulation, is assumed to function solely as a carrier molecule (Azad Khan 1977; Van Hees 1980; Klotz 1980).
Administration of unbound or uncoated 5‐ASA revealed that it was readily absorbed in the upper jejunum and was unable to reach the colon in therapeutic concentrations (Schroeder 1972; Nielsen 1983; Myers 1987). Ingested SASP largely resists such premature absorption and thus is able to serve as a delivery system that transports the 5‐ASA to the affected regions of the lower intestinal tract (Schroeder 1972). While corticosteroid therapy is more effective in the treatment of severe ulcerative colitis (Truelove 1955; Truelove 1959) the use of SASP in maintaining remission (Misiewitz 1965) has been well established.
Despite its benefits, up to 30% of patients receiving SASP have reported adverse side‐effects (Nielsen 1982). It was concluded that many were due to the sulfapyridine moiety, especially those effects found to be dose‐dependent (Das 1973; Myers 1987). This discovery spawned more than a decade of research aimed at finding alternative 5‐ASA delivery systems.
Asacol® (Proctor and Gamble) consists of a pellet of 5‐ASA destined for release in the terminal ileum or colon due to a coating known as Eudragit‐S, a resin that dissolves at a pH greater than 7 (Dew 1982a). Claversal® or Mesasal® (GlaxoSmithKline), Salofalk® (Axcan Pharma, Falk Foundation), and Rowasa® (Reid‐Rowell) are similar delayed‐release preparations of 5‐ASA pellets coated with Eudragit L, a resin that dissolves at a pH greater than 6 (the approximate pH of the ileum/colon) (Hardy 1987; Myers 1987). Pentasa® (Marion‐Merrell‐Dow) is a microsphere formulation that consists of 5‐ASA microgranules enclosed within a semi‐permeable membrane of ethylcellulose. It is designed for controlled release that begins in the duodenum and continues into the affected regions of the lower bowel (Rasmussen 1982). Olsalazine or Dipentum® (Pharmacia & Upjohn) consists of two 5‐ASA molecules linked by a diazo bond (Willoughby 1982; Staerk Laursen 1990). Other formulations, such as benzalazine and balsalazide, are composed of 5‐ASA molecules azo‐bonded to various benzoic acid derivatives (Chan 1983; Fleig 1988). Like SASP, these compounds are poorly absorbed in the upper digestive tract but are readily metabolized by the intestinal flora in the lower bowel. MMX mesalamine (Lialdaa® or Mezavanta®) uses MMX Multi Matrix System (MMX) technology to delay and extend delivery of active drug throughout the colon (Kamm 2008).
The newer 5‐ASA preparations were intended to avoid the adverse effects of SASP while maintaining its therapeutic benefits. These drugs are more costly, however, and have still been shown to cause adverse effects in some patients (Rao 1987). The efficacy and safety of the 5‐ASA preparations have been evaluated in numerous clinical trials that have often lacked sufficient statistical power to arrive at definitive conclusions. In an earlier meta‐analysis, Sutherland 1993 found that the newer 5‐ASA drugs were no more effective than SASP for maintenance of remission in ulcerative colitis. This systematic review is an update of the Cochrane review published in 2012 (Feagan 2012). We proceeded with this updated review, in accordance with the format of the Cochrane Collaboration, in order to include the more recent studies as well as to evaluate the effectiveness, dose‐responsiveness, and safety of the 5‐ASA preparations in terms of more precise outcome measures.
Many patients are non‐adherent with conventional multi‐dose (2 or 3 times daily) treatment regimens which may result in reduced efficacy, and can lead to an increased risk of relapse in patients with quiescent disease (Kane 2001; Kane 2003b), poor long‐term prognosis (Kane 2008b) and increased costs of care (Kane 2008b; Beaulieu 2009). Poor adherence may be particularly problematic in quiescent disease (Kane 2001; Kane 2003b), since patients lack continuing symptoms that incentivize them to take medication. Although multiple factors have been shown to influence medication adherence in patients with ulcerative colitis it is commonly believed that a high pill burden and multi‐dose regimens are major determinants (Ediger 2007; Kane 2008b). Accordingly, it is reasonable to hypothesize that once daily dosing of mesalamine might improve both adherence with maintenance therapy and outcomes.
The efficacy and safety of once daily oral dosing of mesalamine compared to conventional dosing (two or three times daily) for the treatment of ulcerative colitis has been evaluated in numerous clinical trials. These trials have investigated the efficacy of once daily dosing of various formulations of mesalamine compared to conventional dosing schedules of the same drugs or different formulations. Many of these trials were small in size and lacked sufficient statistical power to arrive at definitive conclusions. A secondary objective of this systematic review was to investigate the efficacy and safety of once daily dosing of mesalamine compared to conventional dosing for the treatment quiescent ulcerative colitis.
Objectives
The primary objectives were to assess the efficacy, dose‐responsiveness, and safety of oral 5‐aminosalicylic acid (5‐ASA) compared to placebo, sulfasalazine (SASP), or 5‐ASA comparators (i.e. other formulations of 5‐ASA) for maintenance of remission in quiescent ulcerative colitis. A secondary objective of this systematic review was to compare the efficacy and safety of once daily dosing of oral 5‐ASA with conventional dosing regimens.
Methods
Criteria for considering studies for this review
Types of studies
Prospective, randomized controlled trials of parallel design, with a minimum treatment duration of six months were considered for inclusion.
Types of participants
Patients of any age with mild‐to‐moderate ulcerative colitis in remission as defined by Truelove and Witts (Truelove 1955) were considered for inclusion.
Types of interventions
Trials of oral 5‐ASA therapy for treatment of patients with ulcerative colitis in remission compared with placebo, SASP or other formulations of 5‐ASA were considered for inclusion. Studies that compared once daily 5‐ASA treatment with conventional dosing of 5‐ASA (two or three times daily) and 5‐ASA dose ranging studies were also considered for inclusion.
Types of outcome measures
Outcome measures included endoscopic or clinical relapse, or early withdrawal, as defined by the authors of each study.
Primary outcomes
The primary outcome was endoscopic or clinical relapse as defined by the authors of each study.
Secondary outcomes
Secondary outcomes included:
-
the proportion of patients who failed to adhere with their medication regimen;
-
the proportion of patients who experienced at least one adverse event;
-
the proportion of patients who withdrew due to adverse events; and
-
the proportion of patients excluded or withdrawn after entry.
Search methods for identification of studies
MEDLINE (OvidSP), EMBASE (Ovid SP), and the Cochrane Library were searched from inception to January 20, 2012. No language or document type restrictions were applied. The multipurpose search command for the Ovid SP interface (.mp.) was used to search both text and database subject heading fields. Review articles and conference proceedings were also searched to identify additional studies. The search strategies are listed in Appendix 1.
Data collection and analysis
Study Selection
Relevant studies were selected on the basis of the above criteria. When necessary, the original authors were contacted to clarify points regarding trial methodology. The reasons for exclusion are indicated for each ineligible study.
Data Collection
Each study selected for analysis was independently reviewed by two authors. Data were recorded onto standard data extraction forms by each author. Any discrepancies between authors were settled by consensus. Results were recorded on an intention‐to‐treat basis, regardless of whether the original authors had done so.
Outcome Measures
The primary outcome of interest was the failure to maintain clinical or endoscopic remission as evidenced by disease relapse, the criteria for which were defined by the authors of each trial. Data were also extracted, where possible, to investigate the influence of the dose of 5‐ASA. As well, the numbers of patients who experienced adverse effects, withdrawal from the study due to adverse events, and exclusion or withdrawal from therapy were recorded where the data were available.
Risk of bias assessment
Two authors independently assessed the risk of bias in the included studies using the Cochrane risk of bias tool (Higgins 2011). Factors assessed included:
-
sequence generation (i.e. was the allocation sequence adequately generated?);
-
allocation sequence concealment (i.e. was allocation adequately concealed?);
-
blinding (i.e. was knowledge of the allocated intervention adequately prevented during the study?);
-
incomplete outcome data (i.e. were incomplete outcome data adequately addressed?);
-
selective outcome reporting (i.e. are reports of the study free of suggestion of selective outcome reporting?); and
-
other potential sources of bias (i.e. was the study apparently free of other problems that could put it at a high risk of bias?).
A judgement of 'Yes' indicates low risk of bias, 'No' indicates high risk of bias, and 'Unclear' indicates unclear or unknown risk of bias. Disagreements were resolved by consensus. Study authors were contacted when insufficient information was provided to determine risk of bias.
We used the GRADE approach for rating the overall quality of evidence for primary outcomes and selected secondary outcomes of interest. Randomized trials start as high quality evidence, but may be downgraded due to: (1) limitations in design and implementation (risk of bias), (2) indirectness of evidence, (3) inconsistency (unexplained heterogeneity), (4) imprecision (sparse data), and (5) reporting bias (publication bias). The overall quality of evidence for each outcome was determined after considering each of these elements, and categorized as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); and very low quality (i.e. we are very uncertain about the estimate) (Guyatt 2008; Schünemann 2011).
Statistical Methods
Trials were separated into five comparison groups: 5‐ASA versus placebo, 5‐ASA versus sulfasalazine, once daily dosing versus conventional dosing, 5‐ASA versus comparator 5‐ASA formulation, and 5‐ASA dose‐ranging. Within each group, raw data for every measured outcome were extracted and converted into individual two by two tables. The tables for placebo‐controlled trials were further subgrouped according to the dose of 5‐ASA. The tables for the once daily versus conventional dosing studies were subgrouped by formulation. The tables for 5‐ASA‐controlled trials were subgrouped by common 5‐ASA comparators (e.g. Asacol and Salofalk). The tables for dose‐ranging studies were subgrouped by 5‐ASA formulation. The risk ratio (RR) and 95% confidence intervals (95% CI) derived from each two by two table were individually calculated and plotted. The results for each comparison group were pooled to determine the RR and 95% CI for each outcome resulting from 5‐ASA therapy relative to either placebo, SASP or 5‐ASA comparator formulation and once daily 5‐ASA therapy relative to conventional dosing. A fixed‐effect model was used. Studies were pooled for analysis if patients, outcomes and interventions were similar (determined by consensus among authors). Studies comparing 5‐ASA to comparator 5‐ASA formulations were pooled for analysis if they compared equimolar doses of oral 5‐ASA.
Dose‐responsiveness was analyzed using a Chi2 for trend. Trials were also subgrouped according to the specific 5‐ASA preparation for those outcomes for which there were two or more studies that used a similar drug. Tests for homogeneity among trials within each comparison group were performed. The presence of heterogeneity among studies was assessed using the Chi2 test (a P value of 0.10 was regarded as statistically significant) and the I2 statistic (Higgins 2003). If statistically significant heterogeneity was identified, the RR and 95% CI were calculated using a random‐effects model. We conducted sensitivity analyses as appropriate to investigate heterogeneity. We also conducted sensitivity analyses excluding studies with a high risk of bias. All statistical analyses were performed using the Cochrane Collaboration RevMan 5 software package.
Results
Description of studies
A literature search conducted on July 9, 2015 identified 2525 studies. Eleven additional studies were identified through searching of references. After duplicates were removed a total of 1619 reports remained for review of titles and abstracts. Two authors independently reviewed the titles and abstracts of these studies and 112 reports of oral 5‐ASA maintenance treatment for quiescent ulcerative colitis were selected for full text review (See Figure 1). Thirty reports of 28 studies were excluded (See Characteristics of excluded studies). Eighty‐two reports of 41 studies involving a total of 8928 patients were selected for inclusion (Dew 1983a; Sandberg‐Gertzen 1986; Andreoli 1987; Ireland 1988; McIntyre 1988; Mulder 1988; Riley 1988; Rutgeerts 1989; Courtney 1992; Giaffer 1992a; Green 1992; Kiilerich 1992; Rijk 1992; Wright 1993; Travis 1994; Ardizzone 1995; Fockens 1995; Kruis 1995; Miner 1995; Nilsson 1995; Hanauer 1996; Hawkey 1997; Green 1998; Ardizzone 1999; Deventer 2001; Kruis 2001; Mahmud 2002; Kane 2003a; Paoluzi 2005; Kamm 2008; Kane 2008a; Dignass 2009; Prantera 2009; Ito 2010; Lichtenstein 2010; Sandborn 2010; Kruis 2011; D'Haens 2012; Hawthorne 2012; Pica 2012; Watanabe 2013) (See Characteristics of included studies).

Study flow diagram.
Seven studies were placebo‐controlled (Sandberg‐Gertzen 1986; Wright 1993; Miner 1995; Hanauer 1996; Hawkey 1997; Ardizzone 1999; Lichtenstein 2010). Twelve studies compared 5‐ASA to SASP (Dew 1983a; Andreoli 1987; Ireland 1988; McIntyre 1988; Mulder 1988; Riley 1988; Rutgeerts 1989; Kiilerich 1992; Rijk 1992; Ardizzone 1995; Kruis 1995; Nilsson 1995). Ten studies were maintenance of remission studies comparing once daily dosing of mesalamine with conventional dosing (Kane 2003a; Kamm 2008; Kane 2008a; Dignass 2009; Prantera 2009; Sandborn 2010; Hawthorne 2012; Kruis 2011; D'Haens 2012; Watanabe 2013). Six studies compared the efficacy and safety of various formulations of oral 5‐ASA to other formulations of oral 5‐ASA for maintenance treatment (Courtney 1992; Green 1998; Deventer 2001; Kruis 2001; Mahmud 2002; Ito 2010). Ten trials were dose‐ranging studies of oral 5‐ASA (Giaffer 1992a; Green 1992; Travis 1994; Fockens 1995; Hanauer 1996; Deventer 2001; Kruis 2001; Paoluzi 2005; Kruis 2011; Pica 2012). Six of the included studies were formal non‐inferiority studies (Dignass 2009; Ito 2010; Sandborn 2010; Hawthorne 2012; D'Haens 2012; Watanabe 2013).
Risk of bias in included studies
A summary of the risk of bias assessment is provided in Figure 2. Most of the included studies were of high methodological quality. Ten studies were rated as high risk of bias. Seven of these studies were single‐blind (Courtney 1992; Deventer 2001; Kane 2003a; Kane 2008a; Dignass 2009; Sandborn 2010; Hawthorne 2012). Outcomes were assessed by a blinded investigator in these studies. Three studies were open‐label and investigators and patients were not blinded to treatment assignment (Mahmud 2002; Kamm 2008; Pica 2012). However, two of the open‐label studies (Mahmud 2002; Kamm 2008) and 4 of 7 single‐blind studies (Courtney 1992; Kane 2008a; Dignass 2009; Hawthorne 2012) utilized investigator performed endoscopy as an endpoint, which may protect against bias provided the endoscopist is blinded. The methods used for blinding were not described in one study and this study was rated as unclear (Green 1992). Twenty‐nine of 41 included studies did not describe the method used for randomization and were rated as unclear for this item (Dew 1983a; Sandberg‐Gertzen 1986; Ireland 1988; McIntyre 1988; Mulder 1988; Riley 1988; Rutgeerts 1989; Giaffer 1992a; Green 1992; Rijk 1992; Wright 1993; Travis 1994; Ardizzone 1995; Miner 1995; Nilsson 1995; Hawkey 1997; Green 1998; Ardizzone 1999; Deventer 2001; Kruis 2001; Paoluzi 2005; Kamm 2008; Dignass 2009; Lichtenstein 2010; Sandborn 2010; Hawthorne 2012; D'Haens 2012; Pica 2012; Watanabe 2013). Eighteen studies did not describe methods used for allocation concealment and were rated as unclear for this item (Dew 1983a; Andreoli 1987; Courtney 1992; Giaffer 1992a; Green 1992; Travis 1994; Ardizzone 1995; Fockens 1995; Green 1998; Ardizzone 1999; Deventer 2001; Kruis 2001; Mahmud 2002; Paoluzi 2005; Lichtenstein 2010; Hawthorne 2012; Pica 2012; Watanabe 2013). Thirteen studies were rated as unclear for incomplete outcome data because reasons for withdrawal were not described (Dew 1983a; Sandberg‐Gertzen 1986; Andreoli 1987; Rutgeerts 1989; Rijk 1992; Travis 1994; Miner 1995; Green 1998; Hanauer 1996; Deventer 2001; Kruis 2001; Hawthorne 2012; Pica 2012).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Summary of findings for the main comparison Oral 5‐ASA versus placebo for maintenance of remission in ulcerative colitis; Summary of findings 2 Oral 5‐ASA versus SASP for maintenance of remission in ulcerative colitis; Summary of findings 3 Once daily dosing versus conventional dosing for maintenance of remission in ulcerative colitis; Summary of findings 4 Oral 5‐ASA versus comparator 5‐ASA formulation for maintenance of remission in ulcerative colitis; Summary of findings 5 High dose oral 5‐ASA versus low dose 5‐ASA for maintenance of remission in ulcerative colitis
EFFICACY
5‐ASA versus Placebo
Seven trials (n = 1298 patients) reported treatment outcomes in terms of failure to maintain clinical or endoscopic remission (Sandberg‐Gertzen 1986; Wright 1993; Miner 1995; Hanauer 1996; Hawkey 1997; Ardizzone 1999; Lichtenstein 2010). Forty‐one per cent of 5‐ASA patients relapsed compared to 58% of placebo patients. The pooled RR of failure to maintain clinical or endoscopic remission for all trials was 0.69 (95% CI 0.62 to 0.77; I2 = 15%; P < 0.00001) using a fixed‐effect model. There was a trend towards greater efficacy with higher doses of 5‐ASA with a statistically significant benefit for the 1 to 1.9 g/day (RR 0.65; 95% CI 0.56 to 0.76; I2 = 0%; P < 0.00001) and the > 2 g/day subgroups (RR 0.73; 95% CI 0.60 to 0.89; I2 = 71%; P = 0.002). The GRADE analysis indicated that the overall quality of the evidence for the primary outcome for the placebo‐controlled studies (failure to maintain clinical or endoscopic remission) was high (See summary of findings Table for the main comparison). The pooled RR was similar (RR 0.69; 95% CI 0.57 to 0.84) when calculated exclusively with those trials with endpoints at 12 months (Wright 1993; Miner 1995; Ardizzone 1999). Two of the trials involving olsalazine (Sandberg‐Gertzen 1986; Wright 1993) had a pooled RR of 0.76 (95% CI 0.58 to 0.99). Two trials involving Asacol had a pooled RR of 0.73 (95% CI 0.60 to 0.88).
5‐ASA versus Sulfasalazine
Twelve trials involving a total of 1655 patients compared the efficacy of 5‐ASA and SASP (Dew 1983a; Andreoli 1987; Ireland 1988; McIntyre 1988; Mulder 1988; Riley 1988; Rutgeerts 1989; Kiilerich 1992; Rijk 1992; Ardizzone 1995; Kruis 1995; Nilsson 1995). In 8 of the 11 studies the dose of SASP was limited to 2 g/day; in one trial the dose of SASP was 4 g/day; in one trial the mean dose of SASP was 2.7 g/day and ranged from 2.4 to 4.4 g/day (see Table of Included Studies). When the outcome of interest was defined as the failure to maintain clinical or endoscopic remission (withdrawals and relapses), SASP was significantly superior to 5‐ASA. Forty‐eight per cent of 5‐ASA patients relapsed compared to 43% of SASP patients (RR 1.14, 95% CI 1.03 to 1.27; I2 = 17% P = 0.01). The GRADE analysis indicated that the overall quality of the evidence for the primary outcome for the SASP‐controlled studies (failure to maintain clinical or endoscopic remission) was high (See summary of findings Table 2). The NNT value was found to be negative (‐17), indicating that SASP has a certain degree of therapeutic superiority over the 5‐ASA preparations. When the analysis was limited to those studies with endpoints at 12 months (Andreoli 1987; Mulder 1988; Riley 1988; Rutgeerts 1989; Kiilerich 1992; Rijk 1992; Nilsson 1995; Ardizzone 1995) there was no statistically significant difference between 5‐ASA and SASP (RR 1.10; 95% CI 0.98 to 1.23). Similarly, when the analysis was limited to studies that did not use olsalazine (Dew 1983a; Andreoli 1987; McIntyre 1988; Mulder 1988; Riley 1988; Rutgeerts 1989; Ardizzone 1995) there was no statistically significant difference between 5‐ASA and SASP (RR 1.08; 95% CI 0.92 to 1.26).
Three trials involving Claversal® (Andreoli 1987; Ardizzone 1995; Rutgeerts 1989) had a pooled RR of 1.15 (95% CI 0.95 to 1.40). When the five trials involving olsalazine (Ireland 1988; Kiilerich 1992; Kruis 1995; Nilsson 1995; Rijk 1992) were pooled, the resulting odds ratio was 1.20 (95% CI 1.04 to 1.38), thus demonstrating that SASP was significantly better than olsalazine for maintenance of remission.
Once Daily Dosing versus Conventional Dosing
Three trials (n = 1871 patients) reported treatment outcomes in terms of failure to maintain clinical or endoscopic remission at six months (Kane 2003a; Sandborn 2010; D'Haens 2012). There was no statistically significant difference in relapse rates at six months. Nineteen per cent of once daily patients relapsed compared to 18% of conventionally dosed patients (RR 1.02; 95% CI 0.85 to 1.23). No statistically significant heterogeneity was detected for this comparison (P = 0.76, I2 = 0%). None of the subgroup comparisons by formulation showed any differences in efficacy between once daily dosing and conventional dosing. However, only two formulations were evaluated in this pooled analysis. The GRADE analysis indicated that the overall quality of the evidence for the primary outcome (failure to maintain clinical or endoscopic remission at six months) for the studies comparing once daily with conventional dosing was low due to sparse data and a high risk of bias (single blind) in some studies in the pooled analysis (See summary of findings Table 3).
Eight trials (n = 3127 patients) reported treatment outcomes in terms of failure to maintain clinical or endoscopic remission at 12 months (Kane 2008a; Kamm 2008; Dignass 2009; Prantera 2009; Sandborn 2010; Hawthorne 2012; Kruis 2011; Watanabe 2013). There was no statistically significant difference in relapse rates at 12 months. Twenty‐nine per cent of once daily patients relapsed compared to 31% of conventionally dosed patients (RR 0.91; 95% CI 0.82 to 1.01). No statistically significant heterogeneity was detected for this comparison (P = 0.26, I2 = 22%). The subgroup comparison for Pentasa showed a statistically significant difference in favour of once daily dosing compared to conventional twice daily dosing (RR 0.75; 95% CI 0.60 to 0.93). None of the other subgroup comparisons (by formulation) showed any differences in efficacy between once daily dosing and conventional dosing. The GRADE analysis indicated that the overall quality of the evidence for the primary outcome (failure to maintain clinical or endoscopic remission at 12 months) for the studies comparing once daily with conventional dosing was moderate due to a high risk of bias in some studies (open label and single blind) in the pooled analysis (See summary of findings Table 3).
Eight trials (n = 2126) reported adherence with study medication at study endpoint (Kane 2003a; Kamm 2008; Kane 2008a; Dignass 2009; Prantera 2009; Kruis 2011; Hawthorne 2012; Watanabe 2013). Overall, 9.5% (101/1061) of patients in the once daily group failed to adhere to their medication regimen compared to 7.5% (80/1065) of patients in the conventional dosing group. The pooled RR using a random‐effects model was 1.18 (95% CI 0.69 to 2.03) showing no statistically significant difference in medication adherence between once daily dosing and conventional dosing at study endpoint (6 months for Kane 2003a and 12 months for the other studies in the pooled analysis; P = 0.55). Statistically significant heterogeneity was detected for this comparison (P = 0.009, I2 = 63%). The heterogeneity appears to be a result of the inclusion of two specific trials (Kamm 2008; Hawthorne 2012). Kamm 2008 reported a significantly higher compliance rate of 99.6% in the twice daily dosing group compared to 93.3% in the once daily group. Hawthorne 2012 reported a significantly higher compliance rate of 97.1% in the once daily dosing group compared to 85.5% in the three times daily dosing group. To investigate if these studies were the source of the heterogeneity the analysis was repeated excluding these trials. The pooled analysis of the ITT population now included 6 studies and 1462 patients (Kane 2003a; Kane 2008a; Dignass 2009; Prantera 2009; Kruis 2011; Watanabe 2013). Overall, 11.2% (83/739) of patients in the once daily group failed to adhere to their medication regimen compared to 8.7% (63/723) of patients in the conventional dosing group. The pooled RR using a fixed‐effect model was 1.22 (95% CI 0.91 to 1.64) showing no statistically significant difference in medication adherence between once daily dosing and conventional dosing at study endpoint (P = 0.18). No statistically significant heterogeneity was detected for this comparison (P = 0.55; I2 = 0%). The GRADE analysis indicated that the overall quality of the evidence for the adherence outcome was moderate due to sparse data (See summary of findings Table 3).
5‐ASA versus Comparator 5‐ASA Formulation
Six studies (n = 707 patients) reported treatment outcomes in terms of the failure to maintain clinical or endoscopic remission at 12 months (Courtney 1992; Green 1998; Deventer 2001; Kruis 2001; Mahmud 2002; Ito 2010). The overall pooled RR showed no statistically significant difference in relapse between various formulations of 5‐ASA (including Balsalazide, Pentasa and Olsalazine) and comparator formulations of 5‐ASA (including Asacol and Salofalk). Forty‐four per cent of patients in the 5‐ASA group relapsed compared to 41% of patients in the 5‐ASA comparator group. The pooled RR of relapse was 1.08 (95% CI 0.91 to 1.28; I2 = 31%; P = 0.37) using a fixed‐effect model. The GRADE analysis indicated that the overall quality of the evidence for the primary outcome (failure to maintain clinical or endoscopic remission) was low due to sparse data (300 events) and a high risk of bias (lack of blinding) in two studies in the pooled analysis (See summary of findings Table 4).
5‐ASA Dose Ranging
Several randomized trials have looked at dose‐ranging for various formulations of 5‐ASA including Asacol, Balsalazide, Olsalazine, Salofalk, and Pentasa. Four studies examined the efficacy of various doses of Asacol for maintenance of clinical or endoscopic remission (Hanauer 1996; Deventer 2001; Paoluzi 2005; Pica 2012). Pica 2012 found no statistically significant difference in efficacy between Asacol 4.8 g/day compared to 2.4 g/day. Twenty‐nine per cent of patients in the Asacol 4.8 g/day group relapsed compared to 36% in the 2.4 g/day group (112 patients; RR 0.80; 95% CI 0.46 to 1.38). A GRADE analysis indicated that the overall quality of the evidence for the primary outcome was very low due to very sparse data and risk of bias. Paoluzi 2005 found no statistically significant difference in efficacy between Asacol 2.4 g/day compared to 1.2 g/day. Seventy per cent of patients in the Asacol 2.4 g/day group relapsed compared to 74% in the 1.2 g/day group (156 patients; RR 0.95; 95% CI 0.78 to 1.16). Deventer 2001 found no statistically significant difference in efficacy between Asacol 3.2 g/day compared to 2 g/day. Fifty‐one per cent of patients in the Asacol 3.2 g/day group relapsed compared to 48% of patients in the 2 g/day group (262 patients; RR 1.07; 95% CI 0.83 to 1.37). Hanauer 1996 found no statistically significant difference in efficacy between Asacol 1.6 g/day compared to 0.8 g/day. Fifty‐six per cent of patients relapsed in both dose groups (177 patients; RR 1.01; 95% CI 0.78 to 1.32).
Three studies examined the efficacy of various doses of Balsalazide for maintenance of clinical or endoscopic remission (Giaffer 1992a; Green 1992; Kruis 2001). Two of these studies compared Balsalazide 6 g/day to 3 g/day (Green 1992; Kruis 2001). The pooled analysis showed no statistically significant difference in efficacy between Balsalazide 6 g/day and 3 g/day. Twenty‐three per cent of patients in the 6 g/day group relapsed (216 patients; RR 0.72; 95% CI 0.46 to 1.13). However, these results should be interpreted with caution as statistically significant heterogeneity was detected for this comparison (P = 0.007; I2 = 86%). A GRADE analysis indicated that the overall quality of the evidence for the primary outcome was very low due to sparse data, very wide confidence intervals and inconsistency. Giaffer 1992a compared Balsalazide 4 g/day to 2 g/day and found a statistically significant difference favouring the 4 g/day dose group. Thirty‐seven per cent of patients in the 4 g/day Balsalazide group relapsed compared to 55% of patients in the 2 g/day group (133 patients; RR 0.66; 95% CI 0.45 to 0.97). A GRADE analysis indicated that the overall quality of the evidence for the primary outcome was moderate due to sparse data.
Travis 1994 found no statistically significant difference in efficacy between Olsalazine 2 g/day and 1 g/day. Forty per cent of patients in both dose groups relapsed (127 patients; RR 1.01; 95% CI 0,66 to 1.54). Kruis 2011 found a statistically significant difference between Salofalk granules 3 g/day and 1.5 g/day. Twenty‐five per cent of patients in the Salofalk 3 g/day group relapsed compared to 39% of patients in the 1.5 g/day group (429 patients; RR 0.65; 95% CI 0.49 to 0.86). Fockens 1995 found no statistically significant difference in efficacy between Pentasa 3.0 g/day and 1.5 g/day. Twenty‐eight per cent of patients in the 3.0 g/day group relapsed compared to 38% in the 1.5 g/day group (169 patients; RR 0.74; 95% CI 0.48 to 1.15). A GRADE analysis indicated that the overall quality of the evidence for the primary outcome was moderate due to sparse data.
SAFETY
Three different outcome measures were used to evaluate the safety and clinical utility of 5‐ASA relative to placebo and SASP: the number of patients with adverse events, the number of patients withdrawn due to adverse events, and the total number of patients excluded or withdrawn before completion of the study. Since many studies only reported the total number of adverse events rather than the number of patients who experienced an event, we were often unable to include such data in the analysis.
5‐ASA versus Placebo
Four studies (n = 962 patients) reported the proportion of patients who experienced at least one adverse event (Wright 1993; Miner 1995; Hanauer 1996; Lichtenstein 2010). There was no statistically significant difference in the incidence of adverse events between 5‐ASA and placebo patients. Forty‐one per cent of 5‐ASA patients experienced at least one adverse event compared to 34% of placebo patients (RR 0.98; 95% CI 0.69 to 1.39; P = 0.91). Statistically significant heterogeneity was detected for this comparison (P = 0.04; I2 = 60%). The heterogeneity appears to be a result of the inclusion of one specific trial (Wright 1993). Wright 1993 reported a significantly higher adverse event rate in the 5‐ASA group compared to placebo. This was mostly due to a high rate of olsalazine‐related diarrhea in the 5‐ASA group. To investigate if this study was the source of the heterogeneity the analysis was repeated excluding this trial. The pooled analysis of the ITT population now included 3 studies and 861 patients (Miner 1995; Hanauer 1996; Lichtenstein 2010). There was no statistically significant difference in the incidence of adverse events between 5‐ASA and placebo patients. Overall, 42% of 5‐ASA patients experienced at least one adverse event compared to 39% of placebo patients (RR 0.94; 95% CI 0.77 to 1.15). No statistically significant heterogeneity was detected for this comparison (P = 0.31; I2 = 17%). The GRADE analysis indicated that the overall quality of the evidence for this outcome for the placebo‐controlled studies (the proportion of patients who experienced at least one adverse event) was moderate low due to sparse data (See summary of findings Table for the main comparison).
Six studies (n = 1197 patients) reported the proportion of patients withdrawn due to adverse events (Wright 1993; Miner 1995; Hanauer 1996; Hawkey 1997; Ardizzone 1999; Lichtenstein 2010). There was no statistically significant difference in withdrawal due to adverse events between 5‐ASA and placebo patients. Withdrawals due to adverse events were reported for 5% of 5‐ASA patients compared to 4% of placebo patients (RR 1.34; 95% CI 0.78 to 2.30). Statistically significant heterogeneity was detected for this comparison (P = 0.10; I2 = 44%). Again the heterogeneity appears to be a result of the inclusion of one specific trial (Wright 1993). To investigate if this study was the source of the heterogeneity the analysis was repeated excluding this trial. The pooled analysis of the ITT population now included 5 studies and 1096 patients (Miner 1995; Hanauer 1996; Hawkey 1997; Ardizzone 1999; Lichtenstein 2010). There was no statistically significant difference in withdrawal due to adverse events between 5‐ASA and placebo patients. Overall, 3% of 5‐ASA patients withdrew due to adverse events compared to 4% of placebo patients in the conventional dosing group (RR 0.86; 95% CI 0.46 to 1.63). No statistically significant heterogeneity was detected for this comparison (P = 0.48; I2 = 0%). The GRADE analysis indicated that the overall quality of the evidence for this outcome for the placebo‐controlled studies (the proportion of patients withdrawn due to adverse events) was moderate due to sparse data (See summary of findings Table for the main comparison).
Five studies (n = 1175 patients) reported the proportion of patients excluded or withdrawn after entry (Wright 1993; Miner 1995; Hanauer 1996; Ardizzone 1999; Lichtenstein 2010). There was no statistically significant difference in the proportion of patients withdrawn or excluded after entry. Nineteen per cent of 5‐ASA patients were withdrawn or excluded after entry compared to 18% of placebo patients (RR 1.13; 95% CI 0.88 to 1.44; I2 = 11%).The GRADE analysis indicated that the overall quality of the evidence for this outcome for the placebo‐controlled studies (the proportion of patients withdrawn or excluded after entry) was moderate due to sparse data (202 events; See Summary of findings table 1).
Commonly reported adverse events in the placebo‐controlled trials included: headache (Miner 1995; Hanauer 1996; Hawkey 1997; Lichtenstein 2010), nausea (Miner 1995; Hawkey 1997; Lichtenstein 2010), abdominal pain (Miner 1995; Hanauer 1996; Ardizzone 1999; Lichtenstein 2010), dyspepsia (Miner 1995), bloating (Ardizzone 1999), flu syndrome (Hanauer 1996) rhinitis (Hanauer 1996) and nasopharyngitis (Lichtenstein 2010). Diarrhea was reported in one study involving olsalazine (Wright 1993), two Asacol studies (Hanauer 1996; Ardizzone 1999) and one study of Apriso (Lichtenstein 2010).
5‐ASA versus Sulfasalazine
Seven studies (n = 1138 patients) reported the proportion of patients who experienced at least one adverse event (Andreoli 1987; Ireland 1988; McIntyre 1988; Mulder 1988; Rutgeerts 1989; Kruis 1995; Nilsson 1995). There was no statistically significant difference in the incidence of adverse events. Sixteen per cent of 5‐ASA patients and SASP patients experienced at least one adverse event (RR 1.07; 95% CI 0.82 to 1.40). No statistically significant heterogeneity was detected for this comparison (P = 0.12; I2 = 41%). A GRADE analysis indicated that the overall quality of the evidence for this outcome for the SASP‐controlled studies (the proportion of patients who experienced at least one adverse event) was moderate low due to sparse data (See summary of findings Table 2). Three olsalazine trials including 634 patients (Ireland 1988; Kruis 1995; Nilsson 1995) that were homogeneous (I2 = 0%) had a pooled odds ratio of 1.27 (95% CI, 0.92 to 1.76).
Ten studies (n = 1585 patients) reported the proportion of patients withdrawn due to adverse events (Ireland 1988; McIntyre 1988; Mulder 1988; Riley 1988; Rutgeerts 1989; Kiilerich 1992; Rijk 1992; Ardizzone 1995; Kruis 1995; Nilsson 1995). There was no statistically significant difference in withdrawals due to adverse events. Seven per cent of 5‐ASA patients were withdrawn due to adverse events compared to 5% of SASP patients (RR 1.27; 95% CI 0.87 to 1.87). No statistically significant heterogeneity was detected for this comparison (P = 0.72; I2 = 0%). In five olsalazine trials including 906 patients (Ireland 1988, Kruis 1995, Nilsson 1995, Kiilerich 1992, Rijk 1992), 9.2% of those receiving olsalazine and 6.2% of those receiving SASP were withdrawn because of adverse events (RR 1.61; 95% CI, 1.01 to 2.56). A GRADE analysis indicated that the overall quality of the evidence for this outcome for the SASP‐controlled studies (the proportion of patients withdrawn due to adverse events) was moderate low due to sparse data (See summary of findings Table 2). The results from two Claversal® trials including 422 patients (Ardizzone 1995; Rutgeerts 1989) were not statistically significant (RR 1.10; 95% CI, 0.48 to 2.54).
Nine studies involving 1497 patients (Ireland 1988; McIntyre 1988; Mulder 1988; Riley 1988; Rutgeerts 1989; Kiilerich 1992; Rijk 1992; Kruis 1995; Nilsson 1995) reported the proportion of patients excluded or withdrawn after entry (excluding relapses). Nineteen per cent of 5‐ASA patients were excluded or withdrawn after entry compared to 15% of SASP patients (RR 1.30; 95% CI 1.04 to 1.63). No statistically significant heterogeneity was detected for this comparison (P = 0.18; I2 = 29%). Withdrawals or exclusions after entry were significantly higher in five olsalazine trials (Ireland 1988; Kiilerich 1992; Rijk 1992; Kruis 1995; Nilsson 1995) involving 906 patients. Seventeen per cent of olsalazine patients were withdrawn or excluded after entry compared to 12% of SASP patients (RR 1.51; 95% CI 1.09 to 2.08).
Commonly reported adverse events in the SASP‐controlled trials included: headache (McIntyre 1988; Riley 1988; Kruis 1995), anorexia or appetite loss (Riley 1988; Rutgeerts 1989) nausea (McIntyre 1988; Riley 1988; Rutgeerts 1989; Kiilerich 1992), vomiting (Riley 1988; Rutgeerts 1989; Nilsson 1995), abdominal pain (Ireland 1988; Rutgeerts 1989; Kiilerich 1992; Nilsson 1995), dyspepsia (Ireland 1988; Riley 1988; Kiilerich 1992; Rijk 1992), excessive flatus (McIntyre 1988), bloating (Rutgeerts 1989), urticaria (Kiilerich 1992; Ardizzone 1995) and rash (McIntyre 1988; Mulder 1988; Rijk 1992; Kruis 1995; Nilsson 1995). Diarrhea was reported in five studies involving Olsalazine (Ireland 1988; Kiilerich 1992; Rijk 1992; Kruis 1995; Nilsson 1995), and in two studies involving Claversal (Rutgeerts 1989; Ardizzone 1995).
Once Daily Dosing versus Conventional Dosing
Six studies (n = 2714 patients) reported the proportion of patients who experienced at least one adverse event (Kamm 2008; Dignass 2009; Prantera 2009; Kruis 2011; D'Haens 2012; Watanabe 2013). There was no statistically significant difference in the incidence of adverse events. Approximately 45% of once daily and conventionally dosed patients experienced at least one adverse event (RR 1.00; 95% CI 0.92 to 1.08). No statistically significant heterogeneity was detected for this comparison (P = 0.43; I2 = 0%). Seven studies (n = 3737 patients) reported the proportion of patients who were withdrawn due to adverse events (Kamm 2008; Dignass 2009; Prantera 2009; Sandborn 2010; Kruis 2011; D'Haens 2012; Watanabe 2013). There was no statistically significant difference in withdrawal due to adverse events. Withdrawals due to adverse events were 1.9% (36/1858) in the once daily group compared to 1.5% (28/1879) in the conventionally dosed group (RR 1.31; 95% CI 0.80 to 2.13). No statistically significant heterogeneity was detected for this comparison (P = 0.42; I2 = 1%). Seven studies (n = 3737 patients) reported the proportion of patients who were excluded or withdrawn after entry (Kamm 2008; Dignass 2009; Prantera 2009; Sandborn 2010; Kruis 2011; D'Haens 2012; Watanabe 2013). There was no statistically significant difference in exclusions or withdrawals after entry. Approximately 15% of once daily and conventionally dosed patients were excluded or withdrawn after entry (RR 0.99; 95% CI 0.85 to 1.15). No statistically significant heterogeneity was detected for this comparison (P = 0.48; I2 = 0%).
The most common adverse events reported in the trials assessing once daily dosing included flatulence (Dignass 2009; Prantera 2009), dyspepsia (D'Haens 2012), abdominal pain (Kamm 2008; Dignass 2009; Prantera 2009; D'Haens 2012; Watanabe 2013), nausea (Prantera 2009), diarrhea (Dignass 2009; Prantera 2009; Watanabe 2013), headache (Kamm 2008; Prantera 2009; D'Haens 2012), nasopharyngitis (Kamm 2008; Dignass 2009; Watanabe 2013), inflammation of the upper respiratory tract (Watanabe 2013), gastroenteritis (Watanabe 2013), dental caries (Watanabe 2013), and worsening ulcerative colitis (Kamm 2008; Prantera 2009; Kruis 2011; D'Haens 2012).
5‐ASA versus Comparator 5‐ASA Formulation
Four studies (n = 357 patients) reported the proportion of patients who experienced at least one adverse event (Green 1998; Kruis 2001; Mahmud 2002; Ito 2010). There was no statistically significant difference in the incidence of adverse events between various formulations of 5‐ASA (including Balsalazide, Pentasa and Olsalazine) and comparator formulations of 5‐ASA (including Asacol and Salofalk). Sixty‐four per cent of patients in the 5‐ASA group experienced at least one adverse event compared to 69% of patients in the 5‐ASA comparator group (RR 0.94; 95% CI 0.83 to 1.07). No statistically significant heterogeneity was detected for this comparison (P = 0.35; I2 = 8%).
Five studies (n = 457 patients) reported the proportion of patients who were withdrawn due to adverse events (Courtney 1992; Green 1998; Kruis 2001; Mahmud 2002; Ito 2010). There was no statistically significant difference in withdrawal due to adverse events between various formulations of 5‐ASA (including Balsalazide, Pentasa and Olsalazine) and comparator formulations of 5‐ASA (including Asacol and Salofalk). Six per cent of patients in the 5‐ASA group withdrew due to adverse events compared to 4% of patients in the 5‐ASA comparator group (RR 1.25; 95% CI 0.56 to 2.78). No statistically significant heterogeneity was detected for this comparison (P = 0.76; I2 = 0%).
Five studies (n = 457 patients) reported the proportion of patients who were excluded or withdrawn after entry (Courtney 1992; Green 1998; Kruis 2001; Mahmud 2002; Ito 2010). There was no statistically significant difference in exclusions or withdrawals after entry between various formulations of 5‐ASA (including Balsalazide, Pentasa and Olsalazine) and comparator formulations of 5‐ASA (including Asacol and Salofalk). Twenty‐eight per cent of patients in the 5‐ASA group were excluded or withdrawn after entry compared to 22% of patients in the 5‐ASA comparator group (RR 1.23; 95% CI 0.90 to 1.70). No statistically significant heterogeneity was detected for this comparison (P = 0.52; I2 = 0%).
The most common adverse events reported in these trials included dyspepsia (Mahmud 2002), abdominal pain (Courtney 1992; Green 1998; Kruis 2001; Mahmud 2002), nausea (Courtney 1992; Kruis 2001; Mahmud 2002), distension (Mahmud 2002) diarrhea (Courtney 1992; Green 1998; Kruis 2001; Mahmud 2002; Ito 2010), headache (Green 1998; Kruis 2001), nasopharyngitis or respiratory infections (Green 1998; Ito 2010), flu‐like disorder (Green 1998) and rash (Courtney 1992).
5‐ASA Dose Ranging
Five dose‐ranging studies reported the proportion of patients who experienced at least one adverse event (Travis 1994; Hanauer 1996; Kruis 2001; Paoluzi 2005; Kruis 2011). Kruis 2011 found a statistically significant difference in the proportion of patients who experienced at least one adverse event between Salofalk 3 g/day and 1.5 g/day both dosed once daily. Forty‐one per cent of patients in the 3 g/day group experienced at least one adverse event compared to 55% of patients in the 1.5 g/day group (429 patients; RR 0.74; 95% CI 0.61 to 0.91). Hanauer 1996 found a statistically significant difference in the proportion of patients who experienced at least one adverse event between Asacol 1.6 g/day and 0.8 g/day. Forty‐one per cent of patients in the Asacol 1.6 g/day group experienced at least one adverse event compared to 22% of patients in the 0.8 g/day group (177 patients; RR 1.86; 95% CI 1.18 to 2.95). No statistically significant differences in the incidence of adverse events were found between Asacol 2.4 g/day and 1.2 g/day (RR 2.85; 95% CI 0.12 to 68.95), Balsalazide 6.0 g/day and 3.0 g/day (RR 1.40; 95% CI 0.88 to 2.24), and Olsalazine 2.0 g/day and 1.0 g/day (RR 1.37; 95% CI 0.94 to 1.99).
Seven dose‐ranging studies reported the proportion of patients who were withdrawn due to adverse events (Giaffer 1992a; Green 1992; Fockens 1995; Hanauer 1996; Kruis 2001; Paoluzi 2005; Kruis 2011). No statistically significant differences in withdrawal due to adverse events were found between Asacol 2.4 g/day and 1.2 g/day (1 study, 156 patients, RR 2.85; 95% CI 0.12 to 68.95); Asacol 1.6 g/day and 0.8 g/day (1 study, 177 patients, RR 0.34; 95% CI 0.04 to 3.25); Balsalazide 6.0 g/day and 3.0 g/day (2 studies, 196 patients, RR 0.59; 95% CI 0.21 to 1.70); Balsalazide 4.0 g/day and 2.0 g/day (1 study, 133 patients, RR 1.43; 95% CI 0.54 to 3.80); Salofalk 3.0 g/day and 1.5 g/day (1 study, 429 patients, RR 0.98; 95% CI 0.29 to 3.33); and Pentasa 3.0 g/day and 1.5 g/day (1 study, 169 patients, RR 1.06; 95% CI 0.07 to 16.69).
Eight dose‐ranging studies reported the proportion of patients who were excluded or withdrawn after entry (Giaffer 1992a; Green 1992; Travis 1994; Fockens 1995; Hanauer 1996; Kruis 2001; Paoluzi 2005; Kruis 2011). A statistically significant difference was found between Balsalazide 6.0 g/day and 3.0 g/day (2 studies, 196 patients, RR 0.47; 95% CI 0.26 to 0.84) and between Salofalk 3 g/day and 1.5 g/day (1 study, 429 patients, RR 0.66; 95% CI 0.46 to 0.93). No statistically significant differences were found in exclusions or withdrawals after entry between Asacol 2.4 g/day and 1.2 g/day (1 study, 156 patients, RR 0.95; 95% CI 0.38 to 2.40); Asacol 1.6 g/day and 0.8 g/day (1 study, 177 patients, RR 1.23; 95% CI 0.80 to 1.90); Balsalazide 4.0 g/day and 2.0 g/day (1 study, 133 patients, RR 1.27; 95% CI 0.77 to 2.12); Olsalazine 2.0 g/day and 1.0 g/day (1 study, 127 patients, RR 1.75; 95% CI 0.83 to 3.70); and Pentasa 3.0 g/day and 1.5 g/day (1 study, 169 patients, RR 0.83; 95% CI 0.44 to 1.55).
Discussion
This updated systematic review has largely confirmed the results of previous meta‐analyses (Sutherland 1993; Sutherland 1997; Sutherland 2006; Feagan 2012), but differs from the previous work in a variety of aspects. The updated review includes 41 studies and 8928 patients which greatly increases statistical power. Different quality assessment criteria (i.e. Cochrane risk of bias tool) were also used. The current review also utilized the GRADE criteria (Guyatt 2008; Schünemann 2011). to assess the overall quality of the data obtained from the randomized studies included in the review.
Unfortunately, there are limitations to making general conclusions. Almost every study utilized a unique clinical or endoscopic index. Unlike Crohn's disease, the lack of standard indices in ulcerative colitis prevented the collection of consistent treatment efficacy data and makes comparisons across clinical studies difficult. As well, several studies failed to specify the treatment arm to which certain excluded patients were initially randomized. Despite these and other common factors that must be considered when interpreting meta‐analyses, the data provided strong evidence that pointed towards a number of conclusions.
The effectiveness of oral 5‐ASA preparations for maintenance of remission in quiescent ulcerative colitis was confirmed. Oral 5‐ASA is superior to placebo for maintenance of remission in ulcerative colitis. The quality of the placebo‐controlled trials was assessed using the Cochrane risk of bias tool and the possibility of bias was rated as low for these studies. The outcome failure to maintain clinical or endoscopic remission was rated as 'high' using the GRADE criteria indicating that further research is very unlikely to change our confidence in the point estimates of effect. There was a trend towards greater efficacy with higher doses of 5‐ASA with a statistically significant benefit for the 1 to 1.9 g/day and the > 2 g/day dosage groups.
An interesting result was that SASP was found to have a modest, but statistically significant benefit over 5‐ASA. The quality of the SASP‐controlled trials was assessed using the Cochrane risk of bias tool and the possibility of bias was rated as low for these studies. The outcome failure to maintain clinical or endoscopic remission was rated as 'high' using the GRADE criteria indicating that further research is unlikely to change our confidence in the point estimates of effect. When the pooled analysis was limited to trials with endpoints at 12 months the difference was no longer statistically significant. Nevertheless, certain limitations may have resulted from having combined all trials regardless of whether relapse was defined in terms of clinical or endoscopic criteria. It is possible that the "superiority" of SASP over 5‐ASA is a reflection of the intention‐to‐treat analysis which was employed. This technique considers all patients who received the medication and penalizes medications with high drop‐out rates (for example olsalazine). A "per protocol" analysis which includes those patients who are compliant and who tolerate the medication might not support a "superiority" claim.
When data for maintenance therapy were subgrouped according to the specific 5‐ASA preparation, olsalazine was observed to be significantly inferior to SASP. Firm conclusions regarding other preparations, which have generally been subject to less rigorous clinical evaluation, could not be surmised. In the case of olsalazine, it appeared that the reduced efficacy was influenced by the significantly higher proportions of withdrawals due to adverse events and total exclusions or withdrawals of patients receiving olsalazine compared to those receiving SASP. In fact, if the analysis of efficacy was restricted to relapses as treatment failures, excluding withdrawals for other reasons, there was no significant difference between olsalazine and SASP (data not shown). The difference may also be related to a misclassification bias in which patients who developed diarrhea were falsely classified as treatment relapses rather than having experienced adverse events.
The overall superiority of SASP over 5‐ASA in maintenance therapy may also be attributable to certain pharmacological properties of SASP, including potential therapeutic effects of the sulfapyridine moiety, that are not observed with other 5‐ASA delivery systems. The mechanisms of action of SASP and its metabolites have been reviewed by Greenfield 1993.
It was apparent that the newer 5‐ASA preparations were not entirely innocent of adverse effects in a number of patients. However, the incidence of adverse events and withdrawals due to the 5‐ASA formulations did not significantly differ from that associated with placebo. There was also no apparent difference between the number of adverse events caused by SASP and 5‐ASA. It should be noted that there may have been a bias in favour of SASP since many of the studies involved patients who were known to have tolerated SASP in the past. This may have minimized SASP‐related adverse events in these trials.
In contrast to these results, olsalazine was associated with a significantly higher proportion of withdrawals due to adverse events relative to SASP. The most common adverse event attributed to olsalazine was diarrhea, an effect previously observed to occur in approximately 10% of patients receiving the drug (Ireland 1988b). It has been suggested that protocol alterations may reduce withdrawal rates in future trials since it has been reported that encouraging patients to take olsalazine with meals appears to reduce the incidence of diarrhea to approximately three per cent of patients (Jarnerot 1996). However, four of the five olsalazine‐SASP trials (Kiilerich 1992, Kruis 1995, Nilsson 1995, Rijk 1992) reported that such recommendations were in fact made.
This meta‐analysis indicates that mesalamine administered once daily is as effective as conventional dosing (twice or three times daily) for maintenance of remission over 6 and 12 months periods in patients with quiescent ulcerative colitis. The pooled analyses showed no significant differences between once daily and conventional dosing for maintenance of remission at 6 months (RR 1.02; 95% CI 0.85 to 1.23; P = 0.82) or 12 months (RR 0.91; 95% CI 0.82 to 1.01; P =0.09). With the exception of Pentasa®, subgroup analyses by drug formulation showed no significant differences in efficacy between once daily and conventional dosing for maintenance of remission. Dignass 2009 found that 2 g of Pentasa® dosed once daily was superior to 1 g Pentasa® dosed twice daily for maintenance of remission at 12 months. The other Pentasa® study found no difference between once daily and conventional dosing for maintenance of remission. A plausible biological explanation for the Dignass 2009 finding is not readily apparent to us.
We believe that the methodological basis for these conclusions is relatively sound. The quality of the trials comparing once daily with conventional dosing was assessed using the Cochrane risk of bias tool and the possibility of bias was judged to be low for most items assessed. However, a concern exists regarding blinding. One open‐label study (Kamm 2008) and five studies (Kane 2003a; Kane 2008a; Dignass 2009; Sandborn 2010; Hawthorne 2012) that were single‐blind (investigator‐blind) were rated as having a high risk of bias. However, the open‐label study (Kamm 2008), and 3 of the 5 single‐blind studies (Kane 2008a; Dignass 2009; Hawthorne 2012), included endoscopy as an endpoint which may provide some protection against performance and detection bias. The overall quality of the evidence using the GRADE approach was rated as moderate for the selected primary and secondary outcomes of interest due to sparse data or high risk of bias (due to blinding) in the pooled analyses.
The results of this meta‐analysis suggest that there is no difference in safety between once daily and conventionally dosed mesalamine. No differences between once daily and conventionally dosed mesalamine were observed for safety outcomes including the overall incidence of adverse events or withdrawal from treatment due to an adverse event. In keeping with the well‐established safety profile of mesalamine, most of the adverse events reported in the studies assessing once daily dosing were mild to moderate in intensity. Common adverse events included gastrointestinal symptoms (e.g. flatulence, abdominal pain, nausea, and diarrhea), headache and worsening ulcerative colitis.
Important patient preference and adherence differences may exist between dosing regimens. In the study that measured patient preference the majority of patients preferred once daily dosing to conventional dosing (Sandborn 2010). Although it is generally believed that administration of fewer tablets and less frequent dosing improves both efficacy and adherence, we could not demonstrate the superiority of once daily dosing for either of these outcomes. This result suggests that patient adherence does not appear to be enhanced by once daily dosing in the clinical trial setting. Several possible explanations exist for these observations, however the most plausible one concerns the unique aspects of the clinical trial environment. It is noteworthy that adherence with medication was remarkably high in the studies that measured this outcome (Kamm 2008; Dignass 2009; Prantera 2009; Hawthorne 2012; Kruis 2011). The pooled adherence rate for the maintenance of remission studies was 86% for the once daily dosing group compared to 89% for the conventional dosing group. These rates likely reflect the highly supervised environment in which the studies were conducted. Adherence with medication in clinical trials is generally greater than in clinical practice since participants are highly selected volunteers who are more likely, in general, to be adherent with drug regimens (Andrade 1995; Kane 2001; Kane 2006; Kane 2008b). In addition, adherence is continuously reinforced during the clinical trial process. Thus, it may be difficult to detect differences in adherence between once daily and multiple dose regimens in this setting.
Accordingly, a need exists to compare dosing regimens in large scale community‐based studies. In this regard reported adherence rates in community based studies range from 40 to 60% and are especially poor among patients in remission (Levy 1999; Kane 2001; Kane 2003b; Shale 2003). However, whether once daily dosing regimens improve adherence in the community remains unknown. Although Kane 2003a demonstrated significantly higher adherence among patients receiving once daily dosing compared to conventional dosing at 3 months, no significant differences were found at 6 months. This time‐dependent effect has recently been observed in a larger study (Sandborn 2010). Sandborn 2010 found significantly higher adherence among patients using once daily dosing compared to conventional dosing at 3 months. However no statistically significant difference in adherence was found at 6 and 12 months (Sandborn 2010).
Experience from other indications suggest that factors other than the dosing regimen are important for long‐term compliance (Brixner 2007; Kane 2008b). Long‐term observations in ulcerative colitis patients as well as in other indications indicate that patients and physicians behaviors play a dominant role in adherence (Magowan 2006; Beaulieu 2009). The patient‐physician relationship should reinforce adherence through education, open communication and mutual agreement regarding the value of treatment (Kane 2008b). To ensure continued adherence in a community based setting, Sandborn 2010 have emphasized the importance of health care‐providers evaluating and reinforcing compliance with patients after three months of maintenance therapy.
There does not appear to be any difference in efficacy between the various formulations of oral 5‐ASA. The overall pooled risk ratio (6 studies, n = 707) showed no statistically significant difference in relapse between various formulations of 5‐ASA (including Balsalazide, Pentasa and Olsalazine) and comparator formulations of 5‐ASA (including Asacol and Salofalk). However, a GRADE analysis indicated that the overall quality of the evidence for the primary outcome (failure to maintain clinical or endoscopic remission at 12 months) was low due to a high risk of bias (single blind and open‐label) in three studies in the pooled analysis and sparse data (See summary of findings Table 4). However, the open‐label study (Mahmud 2002) and one of the single‐blind studies (Courtney 1992), included endoscopy as an endpoint which may provide some protection against performance and detection bias.
Pharmacokinetic studies suggest that systemic exposure to 5‐ASA is similar for all oral 5‐ASA formulations and 5‐ASA prodrugs (Sandborn 2002a; Sandborn 2002b; Sandborn 2002c; Sandborn 2003). The excretory function of the kidneys (as measured by the glomerular filtration rate) does not change during maintenance therapy with oral 5‐ASA or olsalazine, and nephrotoxicity is rare for Pentasa or Asacol, suggesting that the systemic exposure to 5‐ASA that occurs for doses used in clinical practice is safe for all drugs in this class (Sandborn 2002a). With the exception of olsalazine‐related diarrhea, there does not appear to be any difference in safety between the various formulations of oral 5‐ASA. The overall pooled risk ratios showed no statistically significant differences in the incidence of adverse events, withdrawal due to adverse events or exclusions or withdrawals after entry. Thus, all of the 5‐ASA formulations can be considered safe and effective for the treatment of active ulcerative colitis, and from a practical standpoint, they can be considered therapeutically equivalent at equimolar doses (Sandborn 2002a). Treatment with sulfasalazine and olsalazine may not be preferable due to the high frequency of adverse events. When deciding which 5‐ASA formulations to use physicians and patients should consider dose‐response data, adherence issues related to dose forms (size of dose form and total number of tablets or capsules per day), and price (Sandborn 2002a).
Few dose ranging maintenance studies were performed which limits the conclusions that can be drawn. Hanauer 1996 compared Asacol at a dosage of 1.6 g/day to 0.8 g/day and found no difference in relapse rates between the dosage groups.Deventer 2001 compared 3.2 g/day to 2 g/day and found no difference in relapse rates between the dosage groups. Paoluzi 2005 compared Asacol at a dosage of 2.4 g/day to 1.2 g/day and found no difference in relapse rates between the dosage groups. However, patients in the higher dosage group remained in remission longer, compared to patients in the low dose group. Paoluzi 2005 recommended a dosage of 2.4 g/day due to the significantly longer time to relapse in the higher dosage group. Pica 2012 compared 4.8 g/day to 2.4 g/day and found no difference in relapse rates between the dosage groups. Further research may be needed to determine the ideal dosage of Asacol for maintenance therapy.
Three studies compared the efficacy of high dose Balsalazide (4.0 to 6.0 g/day) to low dose (2.0 to 3.0 g/day). Giaffer 1992a found a dose of 4.0 g/day to be significantly superior to 2.0 g/day for preventing relapses over a 12 months period. No differences in safety between the dose groups were noted. A pooled analysis of two studies comparing Balsalazide 6.0 g/day to 3.0 g/day found no significant difference in relapse rates. However, these results should be interpreted with caution due to a high degree of heterogeneity and sparse data. The high degree of heterogeneity is due to the fact that the two studies had conflicting results. Green 1992 found no differences in relapse rates between the 6.0 g/day and 3.0 g/day groups at either 6 or 12 months. Green 1992 noted no differences in safety. Kruis 2001 found 6.0 g/day to be superior to 3 g/day for preventing relapse over a 26 week period with no differences in safety. Differences in patient populations may explain these findings. The Green 1992 study included patients with a very distal extent of ulcerative colitis and a large proportion of patients had long term remission (e.g. >1 year) at entry. Kruis 2001 suggested that patients with more extensive ulcerative colitis or with frequent relapses may benefit from a higher dose of maintenance therapy.
Kruis 2011 investigated different doses of once daily Salofalk (3 g or 1.5 g) and found that significantly fewer patients relapsed at 12 months in the group receiving 3 g once daily (25%) compared to patients in the 1.5 g group (39%; P = 0.002). This analysis involved 439 patients and provides moderate evidence (based on GRADE analysis) that 3 g Salofalk once daily is superior to 1.5 g Salofalk once daily for maintenance treatment of ulcerative colitis. No differences in safety were observed among the dose groups. In a post hoc analysis, Kruis 2011 observed that patients with active inflammation at baseline in the 3 g group continued to maintain a higher rate of remission than patients receiving lower doses (i.e. 1.5 g once daily or 0.5 g three times daily). Kruis 2011 concluded that 3 g once daily is an appropriate dose for maintenance of remission, and may be beneficial for patients with signs of inflammation or in whom endoscopic data are not available. No other maintenance studies looked at dose‐ranging for once daily treatment using other 5‐ASA formulations.
Travis 1994 compared the efficacy of Olsalazine 2.0 g/day to 1.0 g/day and 0.5 g/day. No significant differences in relapse at 12 months were found between the 2.0 g and 1.0 g/day groups. A dosage of 2.0 g/day was significantly superior to 0.5 g/day for preventing relapse. No differences in safety were noted. Subgroup analysis showed that patients with proctitis and recent relapse may benefit from a dosage of 2.0 g/day (Travis 1994). Fockens 1995 compared the efficacy of Pentasa 3.0 g/day to 1.5 g/day. Although there was a trend favouring the higher dose there was no statistically significant difference in prevention of relapse over a one year period. The higher dosage was not associated with a higher incidence of adverse events (Fockens 1995).

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
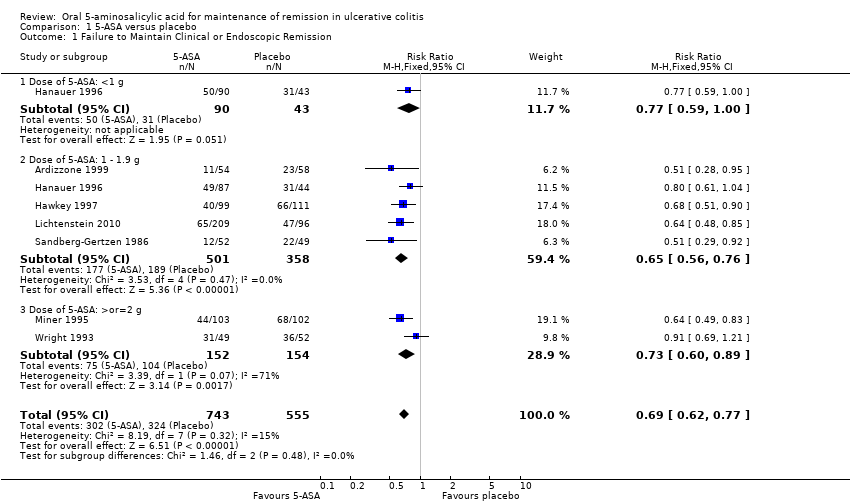
Comparison 1 5‐ASA versus placebo, Outcome 1 Failure to Maintain Clinical or Endoscopic Remission.

Comparison 1 5‐ASA versus placebo, Outcome 2 Development of Any Adverse Event.

Comparison 1 5‐ASA versus placebo, Outcome 3 Development of Any Adverse Event (Sensitivity analysis).

Comparison 1 5‐ASA versus placebo, Outcome 4 Withdrawal from Study due to Adverse Event.

Comparison 1 5‐ASA versus placebo, Outcome 5 Withdrawal from Study due to Adverse Event (Sensitivity analysis).

Comparison 1 5‐ASA versus placebo, Outcome 6 Exclusion/Withdrawal after Entry (not due to relapse).

Comparison 2 5‐ASA versus sulfasalazine, Outcome 1 Failure to Maintain Clinical or Endoscopic Remission.
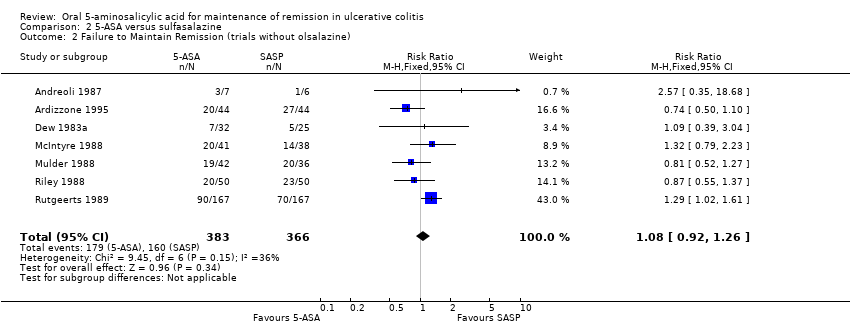
Comparison 2 5‐ASA versus sulfasalazine, Outcome 2 Failure to Maintain Remission (trials without olsalazine).
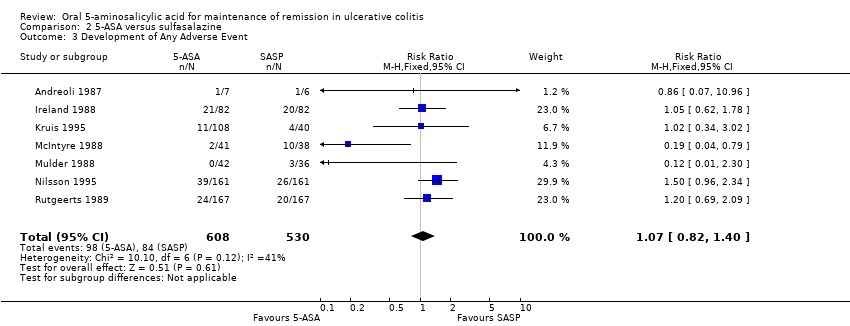
Comparison 2 5‐ASA versus sulfasalazine, Outcome 3 Development of Any Adverse Event.
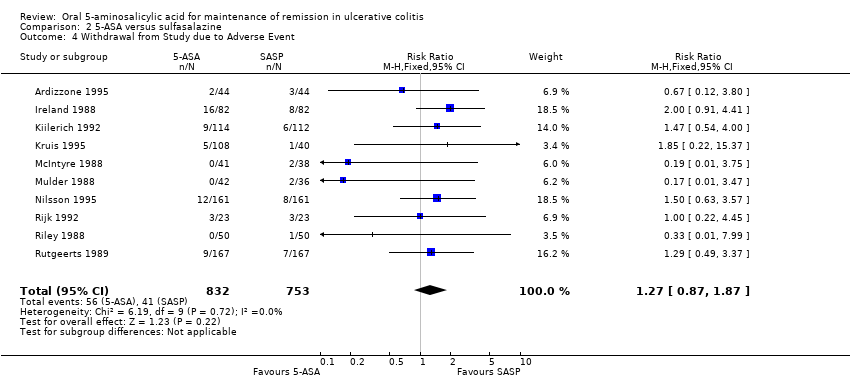
Comparison 2 5‐ASA versus sulfasalazine, Outcome 4 Withdrawal from Study due to Adverse Event.

Comparison 2 5‐ASA versus sulfasalazine, Outcome 5 Exclusion/Withdrawal after Entry (not due to relapse).

Comparison 3 Once daily versus conventional dosing, Outcome 1 Failure to Maintain Clinical or Endoscopic Remission at 6 months.

Comparison 3 Once daily versus conventional dosing, Outcome 2 Failure to Maintain Clinical or Endoscopic Remission at 12 months.
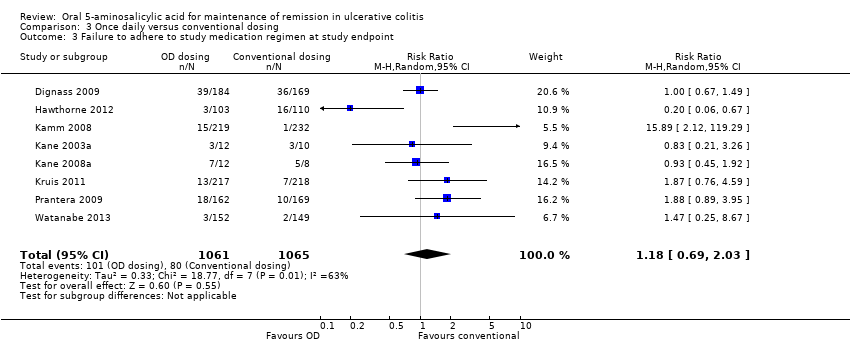
Comparison 3 Once daily versus conventional dosing, Outcome 3 Failure to adhere to study medication regimen at study endpoint.

Comparison 3 Once daily versus conventional dosing, Outcome 4 Failure to adhere to study medication regimen (Sensitivity analysis ‐ excluding outliers).

Comparison 3 Once daily versus conventional dosing, Outcome 5 Development of Any Adverse Event.
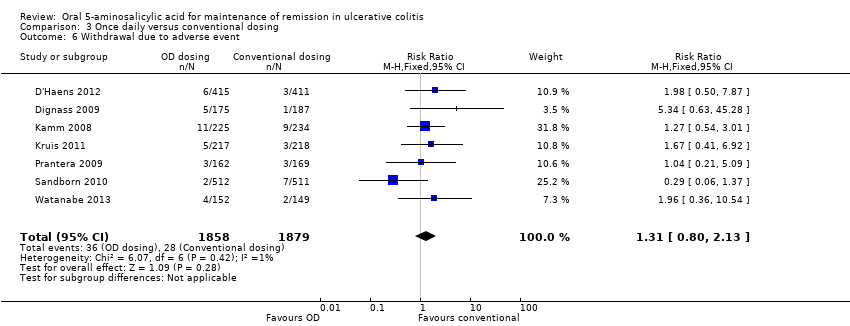
Comparison 3 Once daily versus conventional dosing, Outcome 6 Withdrawal due to adverse event.
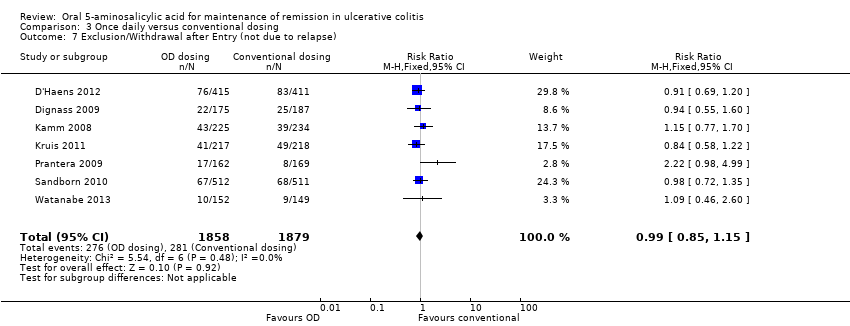
Comparison 3 Once daily versus conventional dosing, Outcome 7 Exclusion/Withdrawal after Entry (not due to relapse).

Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 1 Failure to Maintain Clinical or Endoscopic Remission at 12 months.

Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 2 Development of Any Adverse Event.

Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 3 Withdrawal from Study due to Adverse Event.
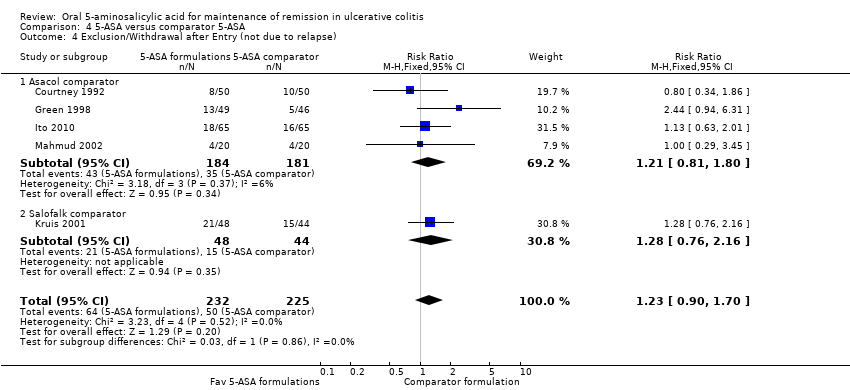
Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 4 Exclusion/Withdrawal after Entry (not due to relapse).

Comparison 5 5‐ASA (dose ranging), Outcome 1 Failure to Maintain Clinical or Endoscopic Remission.

Comparison 5 5‐ASA (dose ranging), Outcome 2 Development of Any Adverse Event.

Comparison 5 5‐ASA (dose ranging), Outcome 3 Withdrawal from Study due to Adverse Event.
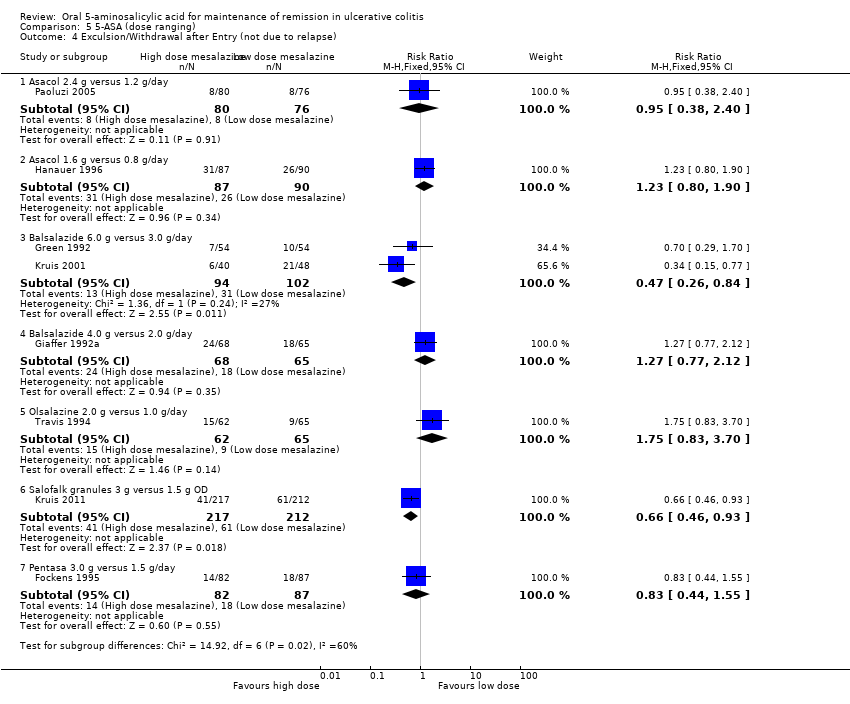
Comparison 5 5‐ASA (dose ranging), Outcome 4 Exculsion/Withdrawal after Entry (not due to relapse).
| Oral 5‐ASA versus placebo for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus placebo | |||||
| Failure to maintain clinical or endoscopic remission at study end‐point | 584 per 10001 | 403 per 1000 | RR 0.69 | 1,298 | ⊕⊕⊕⊕ | |
| Any adverse event | 393 per 10001 | 369 per 1000 | RR 0.94 | 774 | ⊕⊕⊕⊝ | |
| Withdrawal due to adverse event | 42 per 10001 | 36 per 1000 | RR 0.86 | 1096 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Oral 5‐ASA versus SASP for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus SASP | |||||
| Failure to maintain clinical or endoscopic remission at study end‐point | 429 per 10001 | 489 per 1000 | RR 1.14 | 1,655 | ⊕⊕⊕⊕ | |
| Any adverse event | 158 per 10001 | 169 per 1000 | RR 1.07 | 1,138 | ⊕⊕⊕⊝ | |
| Withdrawal due to adverse event | 54 per 10001 | 69 per 1000 | RR 1.27 | 1,585 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Once daily dosing versus conventional dosing for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | OD versus conventional | |||||
| Failure to maintain clinical or endoscopic remission at 6 months | 184 per 10001 | 188 per 1000 | RR 1.02 | 1,871 | ⊕⊕⊝⊝ | |
| Failure to maintain clinical or endoscopic remission at 12 months | 314 per 10001 | 286 per 1000 | RR 0.91 | 3,127 | ⊕⊕⊕⊝ | |
| Failure to adhere to study medication regimen | 87 per 10001 | 106 per 1000 | RR 1.22 | 1,462 | ⊕⊕⊕⊝ | |
| Development of any adverse event | 453 per 10001 | 453 per 1000 | RR 1.00 | 2,714 | ⊕⊕⊕⊕ | |
| Withdrawal due to adverse events | 15 per 10001 | 20 per 1000 | RR 1.31 | 3,737 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Oral 5‐ASA versus comparator 5‐ASA formulation for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus 5‐ASA | |||||
| Failure to maintain clinical or endoscopic remission at 12 months | 407 per 10001 | 440 per 1000 | RR 1.08 | 707 | ⊕⊕⊝⊝ | |
| Development of any adverse event | 686 per 10001 | 645 per 1000 | RR 0.94 | 357 | ⊕⊕⊝⊝ | |
| Withdrawal due to adverse events | 44 per 10001 | 55 per 1000 | RR 1.25 | 457 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| High dose oral 5‐ASA versus low dose 5‐ASA for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | High dose 5‐ASA versus low dose 5‐ASA | |||||
| Failure to maintain clinical or endoscopic remission at 12 months | 357 per 10001 | 286 per 1000 | RR 0.80 | 112 | ⊕⊝⊝⊝ | Asacol 4.8 g/day versus 2.4 g/day |
| Failure to maintain clinical or endoscopic remission at 12 months | 330 per 10001 | 251 per 1000 | RR 0.76 | 216 | ⊕⊝⊝⊝ | Balsalazide 6.0 g/day versus 3.0 g/day |
| Failure to maintain clinical or endoscopic remission at 12 months | 554 per 10001 | 366 per 1000 | RR 0.66 | 133 | ⊕⊕⊕⊝ | Balsalazide 4.0 g/day versus 2.0 g/day |
| Failure to maintain clinical or endoscopic remission at 12 months | 392 per 10001 | 255 per 1000 | RR 0.65 | 429 | ⊕⊕⊕⊝ | Salofalk granules 3.0 g OD versus 1.5 g OD |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission Show forest plot | 7 | 1298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.62, 0.77] |
| 1.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.00] |
| 1.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 5 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.56, 0.76] |
| 1.3 Dose of 5‐ASA: >or=2 g | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.60, 0.89] |
| 2 Development of Any Adverse Event Show forest plot | 4 | 875 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.39] |
| 2.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.51, 1.31] |
| 2.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 2 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.20] |
| 2.3 Dose of 5‐ASA: >or=2 g | 2 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.14, 20.58] |
| 3 Development of Any Adverse Event (Sensitivity analysis) Show forest plot | 3 | 774 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.77, 1.15] |
| 3.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.51, 1.31] |
| 3.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 2 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.20] |
| 3.3 Dose of 5‐ASA: >or=2 g | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.25, 1.12] |
| 4 Withdrawal from Study due to Adverse Event Show forest plot | 6 | 1197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.78, 2.30] |
| 4.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.15, 13.38] |
| 4.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 4 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.50, 2.23] |
| 4.3 Dose of 5‐ASA: >or=2 g | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.78, 4.15] |
| 5 Withdrawal from Study due to Adverse Event (Sensitivity analysis) Show forest plot | 5 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.46, 1.63] |
| 5.1 Dose of 5‐ASA: <1 g | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.15, 13.38] |
| 5.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 4 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.50, 2.23] |
| 5.3 Dose of 5‐ASA: >or=2 g | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.60] |
| 6 Exclusion/Withdrawal after Entry (not due to relapse) Show forest plot | 5 | 1074 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.44] |
| 6.1 Dose of 5‐ASA: <1 g | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.58, 1.40] |
| 6.2 Dose of 5‐ASA: 1 ‐ 1.9 g | 3 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.87, 1.71] |
| 6.3 Dose of 5‐ASA: >or=2 g | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.69, 2.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission Show forest plot | 12 | 1655 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.03, 1.27] |
| 2 Failure to Maintain Remission (trials without olsalazine) Show forest plot | 7 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.26] |
| 3 Development of Any Adverse Event Show forest plot | 7 | 1138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| 4 Withdrawal from Study due to Adverse Event Show forest plot | 10 | 1585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.87, 1.87] |
| 5 Exclusion/Withdrawal after Entry (not due to relapse) Show forest plot | 9 | 1497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.04, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission at 6 months Show forest plot | 3 | 1871 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 1.1 Asacol (OD vs BID or TID) | 2 | 1045 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.83, 1.46] |
| 1.2 MMX (OD) vs Asacol (BID) | 1 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.23] |
| 2 Failure to Maintain Clinical or Endoscopic Remission at 12 months Show forest plot | 8 | 3127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.01] |
| 2.1 Asacol (OD vs BID or TID) | 3 | 1256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.15] |
| 2.2 MMX (OD) vs Asacol (BID) | 1 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.74, 1.33] |
| 2.3 Pentasa (OD vs BID) | 2 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.93] |
| 2.4 MMX (OD vs BID) | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.47] |
| 2.5 Salofalk granules (OD vs TID) | 1 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.10] |
| 3 Failure to adhere to study medication regimen at study endpoint Show forest plot | 8 | 2126 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.69, 2.03] |
| 4 Failure to adhere to study medication regimen (Sensitivity analysis ‐ excluding outliers) Show forest plot | 6 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.91, 1.64] |
| 5 Development of Any Adverse Event Show forest plot | 6 | 2714 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.08] |
| 6 Withdrawal due to adverse event Show forest plot | 7 | 3737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.80, 2.13] |
| 7 Exclusion/Withdrawal after Entry (not due to relapse) Show forest plot | 7 | 3737 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.85, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission at 12 months Show forest plot | 6 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 1.1 Asacol comparator | 5 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.26] |
| 1.2 Salofalk comparator | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.86, 1.98] |
| 2 Development of Any Adverse Event Show forest plot | 4 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 2.1 Asacol comparator | 3 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.08] |
| 2.2 Salofalk comparator | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.34] |
| 3 Withdrawal from Study due to Adverse Event Show forest plot | 5 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.56, 2.78] |
| 3.1 Asacol comparator | 4 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.61, 4.42] |
| 3.2 Salofalk comparator | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.16, 2.90] |
| 4 Exclusion/Withdrawal after Entry (not due to relapse) Show forest plot | 5 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.90, 1.70] |
| 4.1 Asacol comparator | 4 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.80] |
| 4.2 Salofalk comparator | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.76, 2.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to Maintain Clinical or Endoscopic Remission Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Asacol 4.8 g versus 2.4 g/day | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.46, 1.38] |
| 1.2 Asacol 3.2 g versus 2 g/day | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.83, 1.37] |
| 1.3 Asacol 2.4 g versus 1.2 g/day | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 1.4 Asacol 1.6 g versus 0.8 g/day | 1 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.32] |
| 1.5 Balsalazide 6.0 g versus 3.0 g/day | 2 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.21, 2.79] |
| 1.6 Balsalazide 4.0 g versus 2.0 g/day | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.45, 0.97] |
| 1.7 Olsalazine 2.0 g versus 1.0 g/day | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.54] |
| 1.8 Salofalk granules 3 g versus 1.5 g OD | 1 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.49, 0.86] |
| 1.9 Pentasa 3.0 g versus 1.5 g/day | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.48, 1.15] |
| 2 Development of Any Adverse Event Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Asacol 2.4 g versus 1.2 g/day | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 68.95] |
| 2.2 Asacol 1.6 g versus 0.8 g/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.18, 2.95] |
| 2.3 Balsalazide 6.0 g versus 3.0 g/day | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.88, 2.24] |
| 2.4 Olsalazine 2.0 g versus 1.0 g/day | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.94, 1.99] |
| 2.5 Salofalk granules 3 g versus 1.5 g OD | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.61, 0.91] |
| 3 Withdrawal from Study due to Adverse Event Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Asacol 2.4 g versus 1.2 g/day | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 68.95] |
| 3.2 Asacol 1.6 g versus 0.8 g/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.25] |
| 3.3 Balsalazide 6.0 g versus 3.0 g/day | 2 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.21, 1.70] |
| 3.4 Balsalazide 4.0 g versus 2.0 g/day | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.54, 3.80] |
| 3.5 Salofalk granules 3 g versus 1.5 g OD | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.29, 3.33] |
| 3.6 Pentasa 3.0 g versus 1.5 g/day | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.69] |
| 4 Exculsion/Withdrawal after Entry (not due to relapse) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Asacol 2.4 g versus 1.2 g/day | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.38, 2.40] |
| 4.2 Asacol 1.6 g versus 0.8 g/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.80, 1.90] |
| 4.3 Balsalazide 6.0 g versus 3.0 g/day | 2 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.26, 0.84] |
| 4.4 Balsalazide 4.0 g versus 2.0 g/day | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.77, 2.12] |
| 4.5 Olsalazine 2.0 g versus 1.0 g/day | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.83, 3.70] |
| 4.6 Salofalk granules 3 g versus 1.5 g OD | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.93] |
| 4.7 Pentasa 3.0 g versus 1.5 g/day | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.44, 1.55] |

