Ограниченное против неограниченного потребление воды для профилактики заболеваемости и смертности у недоношенных детей
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Water intake was controlled by study protocol until one of six criteria was met: significant patent ductus arteriosus (PDA), dehydration, death, full enteral feedings, transfer to another hospital, or age 30 days. This was a randomized, unblinded clinical trial. Enrolled infants were divided into eight groups (prognostic stratification) according to three factors thought to influence the risk of PDA: birth weight below or above 1.25 kg, size for gestational age (AGA versus SGA), and respiratory status (presence or absence of significant RDS). Within each of the resulting eight groups, subjects were randomly assigned to either of two treatment groups ('low' and 'high' volume water intake) by opening the next opaque, sealed envelope from the pile for the corresponding prognostic group; the envelope contained the designation of 'low' or 'high' volume group as determined from a table of random numbers prior to enrollment of the first subject in the study. Within each of the eight prognostic groups, the randomization was balanced so that the number of low and high volume infants was equal after every second infant was enrolled into that group. Consecutively enrolled infants in each group were paired for analysis. A two‐sided sequential plan was used, and the outcomes for discordant pairs of infants were plotted on this plan. No confounding variables were identified. No infants were withdrawn from the study. Infants were cared for in unhumidified single‐walled incubators. | |
| Participants | The participants were 170 infants with birth weight ranging from 751 to 2000 g. They were enrolled within the first three days of life. Complete accounting is given for infants in this weight range who contemporaneously were not enrolled in the study. Infants were excluded who by the third day of life had died, were receiving more than half of their water intake enterally, had evidence of PDA or other congenital heart defect, were suspected of having renal anomaly or injury or elevated intracranial pressure, or were clinically dehydrated. Of the 384 consecutive infants admitted with birth weight between 751 and 2000 g, 123 were excluded according to one or more of the aforementioned criteria. Of the remaining 261 eligible infants, consent was not sought in 39 cases and was denied in 52 cases. The remaining 170 infants were enrolled in the study. The mean birth weight was 1.4 kg in both groups, and the mean gestational age was 31 weeks. | |
| Interventions | The subjects' total water intake (enteral plus parenteral) was determined by study protocol. An upper limit was set for the 'low' volume group, and a lower limit was set for the 'high' volume group. These limits depended on birth weight and varied with postnatal age and were raised by 10 ml/kg/d during phototherapy. The mean daily water intake for all subjects throughout the study was 122 ml/kg/d for the low volume group and 169 ml/kg/d for the high volume group. | |
| Outcomes | The outcomes compared between the treatment groups included maximum weight loss, PDA, PDA with signs of congestive heart failure, necrotizing enterocolitis, bronchopulmonary dysplasia, and death | |
| Notes | The results of this study were reported in the New England Journal of Medicine (1980; 302:598‐604) except for the detailed limits for water intake in all subgroups, which were published only in a letter in the Lancet (1979; 2:90) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, unblinded clinical trial Enrolled infants were divided into eight groups (prognostic stratification) according to three factors thought to influence the risk of PDA: birth weight below or above 1.25 kg, size for gestational age (AGA versus SGA), and respiratory status (presence or absence of significant RDS) |
| Allocation concealment (selection bias) | Low risk | Within each of the resulting eight groups, subjects were randomly assigned to either of two treatment groups ('low' and 'high' volume water intake) by opening the next opaque, sealed envelope from the pile for the corresponding prognostic group; the envelope contained the designation of 'low' or 'high' volume group as determined from a table of random numbers prior to enrollment of the first subject in the study. Within each of the eight prognostic groups, the randomization was balanced so that the number of low and high volume infants was equal after every second infant was enrolled into that group. Consecutively enrolled infants in each group were paired for analysis. A two‐sided sequential plan was used, and the outcomes for discordant pairs of infants were plotted on this plan. |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded clinical trial |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded clinical trial |
| Incomplete outcome data (attrition bias) | Low risk | No infants were withdrawn from the study |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Water intake was determined by study protocol for first seven days. This was a randomized, unblinded clinical trial | |
| Participants | The participants were 168 infants with birth weight 1500 g or less with required assisted ventilation within 6 hours of birth | |
| Interventions | Subjects were randomly assigned to receive one of two fluid regimens. The water intake prescribed for the infants in the restricted intake group was lower than the liberal group by 20 to 40 ml/kg/d. The water intake could be adjusted according to specific guidelines if an infant in either group developed renal failure, hypotension, or hyperbilirubinemia requiring phototherapy. Overall, the infants in the restricted intake group received 11% less water than the infants in the liberal group. | |
| Outcomes | The outcomes compared between groups were death or survival, duration of assisted ventilation, duration of supplemental oxygen, oxygen dependence at 28 d, oxygen dependence at 36 weeks postmenstrual age, pneumothorax, pulmonary interstitial emphysema, intracranial hemorrhage, patent ductus arteriosus, necrotizing enterocolitis, renal failure, and treatment with pancuronium, inhaled nitric oxide, high‐frequency ventilation, diuretic drugs, and corticosteroids. | |
| Notes | The results of this study were reported in three papers: European Journal of Pediatrics (1999; 158:917‐22), Acta Paediatrica (2000; 89:237‐41), and Archives of Disease in Childhood Fetal and Neonatal Edition (2000; 83:F91‐6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was a randomized, unblinded clinical trial The methods of allocation and randomization were unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded clinical trial |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded clinical trial |
| Methods | The duration of the study (control of water intake according to study criteria) was for five days after birth. This was a randomized, unblinded clinical trial. The details of randomization are not given, but the subjects were first stratified according to birthweight group (750 to 999 g, 1000 to 1249 g, and 1250 to 1500 g), 5‐minute Apgar score (6 or less versus more than 6), presence of respiratory distress syndrome (RDS), and hospital of birth (inborn versus outborn). No confounding variables were identified in a comparison of demographic features in the two groups. Deviations from the protocol were allowed for infants with patent ductus arteriosus (PDA), but the number for whom this occurred is not stated. Seven of 108 infants were withdrawn from the study. Two infants in the liberal water intake group were subsequently found to have non‐PDA congenital heart defects; two in the restricted water intake group were withdrawn because of intestinal obstruction or perforation requiring surgery; and three infants in the restricted water intake group died within 24 hours of enrollment. In addition, 13 infants were excluded from analysis because they had no matching infant (according to the above stratification criteria) who received the other treatment. Infants were cared for in maximally humidified, single‐walled incubators. | |
| Participants | The participants included in the analysis were 88 AGA infants with birth weight between 750 and 1500 g. The 'exclusion' criteria given in the report were actually withdrawal criteria: non‐PDA congenital heart disease, conditions requiring surgery, and death within 24 hours after entry into the study. The mean birth weight in both groups was 1.2 kg, and the mean gestational age was 29 weeks. Thirty‐four infants had 5‐minute Apgar scores of 6 or less; 64 had RDS; and 30 infants were inborn. The gender distribution is not given. | |
| Interventions | The water intake of infants in the restricted water intake group was managed to allow a 3% to 5% loss of weight per day to a maximum of 15%. Their water intake began at 65 to 70 ml/kg/d and increased to 80 ml/kg/d by day 5. In the liberal water intake group, the water intake was managed to allow a 1% to 2% loss of weight per day to a maximum loss of 10%. The water intake in the liberal intake group began at 80 ml./kg on the first day and increased gradually to 140 ml/kg/d by day 5. The actual mean weight losses were 12.9% and 8.8% in the restricted and liberal groups, respectively. | |
| Outcomes | The outcomes examined were maximum weight loss as a percentage of birth weight, water intake and urine output, sodium intake, serum sodium concentration, hypoglycemia, hyperglycemia, hyponatremia, hypernatremia, significant PDA, bronchopulmonary dysplasia, intracranial hemorrhage, necrotizing enterocolitis, dehydration, acute renal failure, and death. | |
| Notes | The results of this study were published in two papers: Journal of Pediatrics (1982; 101:423‐32) and Pediatric Cardiology (1985; 6:17‐24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, unblinded clinical trial. The details of randomization are not given, but the subjects were first stratified according to birthweight group (750 to 999 g, 1000 to 1249 g, and 1250 to 1500 g), 5‐minute Apgar score (6 or less versus more than 6), presence of respiratory distress syndrome (RDS), and hospital of birth (inborn versus outborn) |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded clinical trial |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded clinical trial |
| Incomplete outcome data (attrition bias) | High risk | Seven of 108 infants were withdrawn from the study. Two infants in the liberal water intake group were subsequently found to have non‐PDA congenital heart defects; two in the restricted water intake group were withdrawn because of intestinal obstruction or perforation requiring surgery; and three infants in the restricted water intake group died within 24 hours of enrollment. In addition, 13 infants were excluded from analysis because they had no matching infant (according to the above stratification criteria) who received the other treatment. |
| Methods | The duration of the study, that is determination of water intake according to study protocol, was for 28 days beginning on the day of birth. This was a randomized, unblinded clinical trial. Randomization was by ordered opening of sealed envelopes containing the assignment to 'dry' or 'control' group as determined from a table of random numbers. There was no prognostic stratification. No confounding variables were identified in a comparison of demographic features in the two groups. No information was given about dropouts or deviations from study protocol except to say that water intake was increased by 10 ml/kg/d for infants in either group who lost more than 5% of their body weight in a day or more than 15% in total since birth. All infants were initially cared for in incubators with 50% relative humidity. | |
| Participants | The participants were 100 infants with birth weight below 1751 g who were admitted to the NICU during the first 24 h of life. During a two‐year period, 100 of 103 consecutive eligible infants were enrolled. Two were excluded because of extreme prematurity (gestational age < 24 weeks), and one was excluded because of failure to obtain parental consent. The mean birthweight in both groups was 1.3 kg, and the mean gestational age was 31 weeks. Thirty‐four infants (34%) were SGA, 31% were delivered by cesarean section, 49% were males, and 91% had endotracheal tubes placed for respiratory assistance. | |
| Interventions | The subjects' total water intake (enteral plus parenteral except replacement of phlebotomy losses with transfused erythrocytes) was determined by the study protocol. The 'dry' group was targeted to receive 50 ml/kg on day 1, 60 ml/kg on day 2, 70 ml/kg on day 3, 80 ml/kg on day 4, 90 ml/kg on day 5, 100 ml/kg on day 6, 120 ml/kg on day 7, and 150 ml/kg thereafter. The 'control' group was targeted to receive 80 ml/kg on day 1, 100 ml/kg on day 2, 120 ml/kg on day 3, 150 ml/kg on days 4 through 7, and 200 ml/kg thereafter. The volumes actually delivered varied slightly from these targets but differed highly significantly between the groups, as planned. | |
| Outcomes | The outcomes compared between the treatment groups included maximum weight loss, age to recovery of birth weight, weight at 28 days (as % of birth weight), hypotension, volume of erythrocytes transfused, hypoglycemia, hyponatremia, hypernatremia, hypokalemia, hyperkalemia, need for phototherapy, patent ductus arteriosus requiring treatment, necrotizing enterocolitis, intraventricular hemorrhage, duration of assisted ventilation, duration of intubation, need for high ventilator pressures, pulmonary air leak, bronchopulmonary dysplasia, and death. | |
| Notes | The results of this study were reported in three published papers: Acta Paediatrica (1992; 81:207‐12) and two identical papers in the European Journal of Pediatrics (1992; 151:295‐99 and 1992; 151:367‐71) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, unblinded clinical trial. Randomization was by ordered opening of sealed envelopes containing the assignment to 'dry' or 'control' group as determined from a table of random numbers. There was no prognostic stratification |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded clinical trial |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded clinical trial |
| Incomplete outcome data (attrition bias) | Unclear risk | No information was given about dropouts or deviations from the study protocol except to say that water intake was increased by 10 ml/kg/d for infants in either group who lost more than 5% of their body weight in a day or more than 15% in total since birth |
| Methods | The duration of the study, that is determination of water intake according to study protocol, was the first three days of life. This was a randomized, unblinded clinical trial. The subjects were randomly assigned to 'low' or 'high' volume of water intake for the first three days of life. The details of randomization are not given, and there was no prognostic stratification. Males outnumbered females in both groups, but the preponderance of males was greater in the low volume group (23/28 versus 17/28). The low group also had slightly higher mean birth weight (2.0 versus 1.9 kg) and gestational age (34.6 versus 34.2 weeks). No information was given about dropouts or deviations from the study protocol. All infants were cared for in incubators with maximal humidity. | |
| Participants | The participants were 56 newborn infants, most of whom were premature, all enrolled on the first day of life. Five of these infants required intermittent positive‐pressure ventilation, and six others required continuous positive airway pressure. No information is given on exclusion criteria. | |
| Interventions | The subjects' total intake was determined by study protocol for the first three days of life. The 'low' volume group was given 60 ml/kg/d, and the 'high' volume group was given 150 ml/kg/d. | |
| Outcomes | The outcomes reported include death, maximum weight loss, urine volume, osmolal clearance, creatinine clearance, free water clearance, net acid excretion, sodium clearance, chloride clearance, and a number of laboratory values, including urinary osmolality, sodium, potassium, chloride, calcium, phosphate, creatinine, urea, and uric acid. Also reported were hematocrit, blood osmolality, and serum concentrations of sodium, chloride, calcium, phosphate, creatinine, urea, and bilirubin. | |
| Notes | No information is given on the incidence of PDA, NEC, or BPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, unblinded clinical trial. The subjects were randomly assigned to 'low' or 'high' volume of water intake for the first three days of life. The details of randomization are not given, and there was no prognostic stratification |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded clinical trial |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded clinical trial |
| Incomplete outcome data (attrition bias) | Unclear risk | No information was given about dropouts or deviations from study protocol |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Prospective randomized controlled trial of 64 late preterm and term neonates diagnosed with TTN at a single tertiary care hospital in the United States. Infants were randomized to receive standard fluid management or mild fluid restriction |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight loss (%) Show forest plot | 3 | 326 | Mean Difference (IV, Fixed, 95% CI) | 1.94 [0.82, 3.07] |
| Analysis 1.1  Comparison 1 Restricted versus liberal water intake, Outcome 1 Weight loss (%). | ||||
| 2 Dehydration Show forest plot | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.71, 8.28] |
| Analysis 1.2  Comparison 1 Restricted versus liberal water intake, Outcome 2 Dehydration. | ||||
| 3 Patent ductus arteriosus Show forest plot | 4 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.37, 0.73] |
| Analysis 1.3  Comparison 1 Restricted versus liberal water intake, Outcome 3 Patent ductus arteriosus. | ||||
| 4 Necrotizing enterocolitis Show forest plot | 4 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.21, 0.87] |
| Analysis 1.4  Comparison 1 Restricted versus liberal water intake, Outcome 4 Necrotizing enterocolitis. | ||||
| 5 Bronchopulmonary dysplasia Show forest plot | 4 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.14] |
| Analysis 1.5  Comparison 1 Restricted versus liberal water intake, Outcome 5 Bronchopulmonary dysplasia. | ||||
| 6 Intraventricular hemorrhage (all grades) Show forest plot | 3 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.14] |
| Analysis 1.6  Comparison 1 Restricted versus liberal water intake, Outcome 6 Intraventricular hemorrhage (all grades). | ||||
| 7 Death Show forest plot | 5 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.23] |
| Analysis 1.7 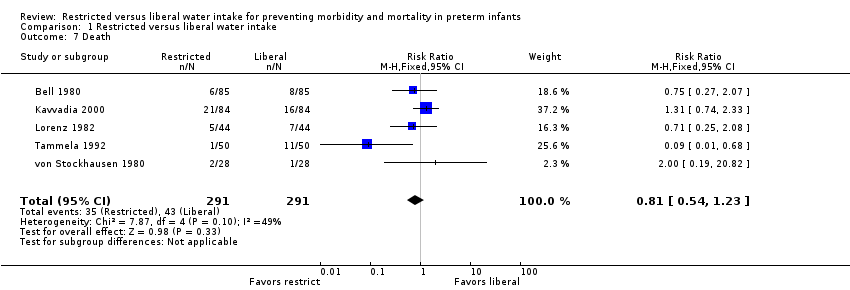 Comparison 1 Restricted versus liberal water intake, Outcome 7 Death. | ||||

Comparison 1 Restricted versus liberal water intake, Outcome 1 Weight loss (%).
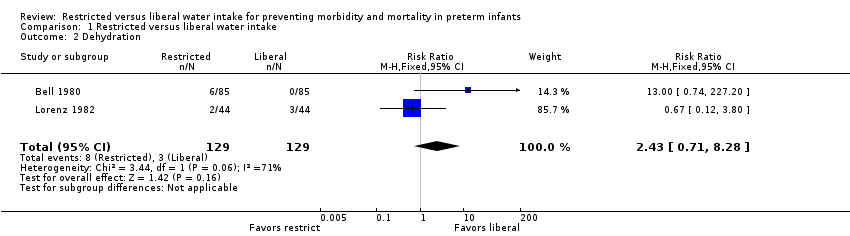
Comparison 1 Restricted versus liberal water intake, Outcome 2 Dehydration.

Comparison 1 Restricted versus liberal water intake, Outcome 3 Patent ductus arteriosus.
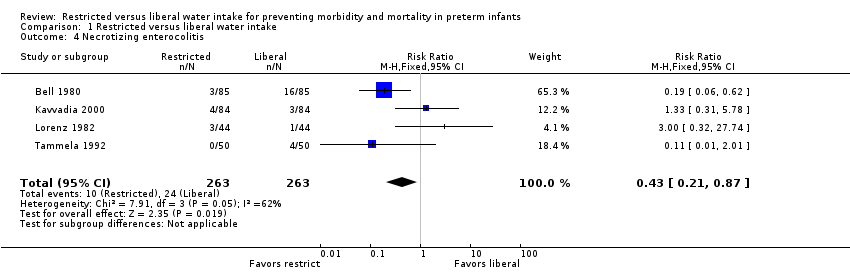
Comparison 1 Restricted versus liberal water intake, Outcome 4 Necrotizing enterocolitis.

Comparison 1 Restricted versus liberal water intake, Outcome 5 Bronchopulmonary dysplasia.
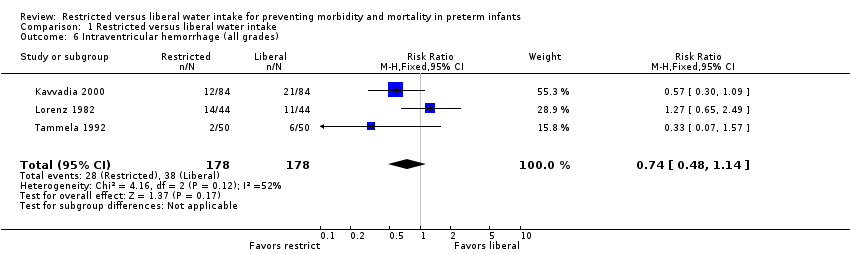
Comparison 1 Restricted versus liberal water intake, Outcome 6 Intraventricular hemorrhage (all grades).

Comparison 1 Restricted versus liberal water intake, Outcome 7 Death.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight loss (%) Show forest plot | 3 | 326 | Mean Difference (IV, Fixed, 95% CI) | 1.94 [0.82, 3.07] |
| 2 Dehydration Show forest plot | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.71, 8.28] |
| 3 Patent ductus arteriosus Show forest plot | 4 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.37, 0.73] |
| 4 Necrotizing enterocolitis Show forest plot | 4 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.21, 0.87] |
| 5 Bronchopulmonary dysplasia Show forest plot | 4 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.14] |
| 6 Intraventricular hemorrhage (all grades) Show forest plot | 3 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.14] |
| 7 Death Show forest plot | 5 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.23] |

