مصرف محدود آب در مقابل مصرف آزاد آن در پیشگیری از وقوع عوارض و مرگومیر در نوزادان نارس
چکیده
پیشینه
اکثر نوزادان نارس از نظر فیزیولوژیکی به اندازه کافی کامل نشدهاند تا بتوانند تمام آب و مواد مغذی مورد نیاز خود را به صورت خوراکی مصرف کنند. بنابراین، نوزادان نارس برای تنظیم حجم آب دریافتی خود به مراقبان خود متکی هستند. از این رو، مراقب باید میزان آبی را تعیین کند که هر روز باید به این نوزادان داده شود.
اهداف
تعیین تاثیر مصرف آب بر کاهش وزن پس از تولد نوزاد و بروز خطرات کمآبی بدن، مجرای شریانی باز (patent ductus arteriosus)، انتروکولیت نکروزان (necrotizing enterocolitis)، دیسپلازی برونکوپولمونری (bronchopulmonary dysplasia)، خونریزی داخل جمجمهای (intracranial hemorrhage)، و مرگومیر در نوزادان نارس.
روشهای جستوجو
کارآزماییهای بالینی تصادفیسازی شده که در نسخههای قبلی این مرور شناسایی شدند، دوباره مورد بررسی قرار گرفتند و همگی حفظ شدند. به دنبال کارآزماییهای دیگری بودیم که پیامدهای مورد نظر را در گروههایی از نوزادان نارس با سطوح مختلف مصرف آب طبق یک پروتکل تجربی، مقایسه کردند. چنین کارآزماییهایی در فهرستی از کارآزماییهای ارائهشده توسط گروه مرور نوزادان در کاکرین، در PubMed و در پروندههای شخصی نویسندگان، جستوجو شدند.
این جستوجو در سال 2014 بهروزرسانی شد.
معیارهای انتخاب
فقط RCTهایی از دریافت متفاوت آب در نوزادان نارس وارد شدند. این مرور محدود به کارآزماییهایی شد که شامل نوزادانی بودند که آب دریافتی آنها عمدتا یا بهطور کامل از طریق انفوزیون یا تزریق داخل عروقی تامین شد.
گردآوری و تجزیهوتحلیل دادهها
روشهای استاندارد سازمان همکاری کاکرین (Cochrane Collaboration) استفاده شدند. انتخاب مطالعه، و خلاصه کردن دادهها توسط دو نویسنده مرور بهطور مستقل از هم انجام شدند. نرخ عوارض جانبی برای گروههای مصرف محدود و آزاد آب برای هر پیامد دو حالتی (dichotomous) محاسبه شد، و نسبت خطر (relative risk) و تفاوت خطر (risk difference) به دست آمدند. علاوه بر این، نتایج حداکثر کاهش وزن ثبت شده و تفاوت میانگین وزندهیشده محاسبه شدند.
نتایج اصلی
آنالیز پنج مطالعه با هم نشان داد که مصرف محدود آب کاهش وزن پس از تولد را بهطور قابل توجهی افزایش داده و خطرات وقوع مجرای شریانی باز و انتروکولیت نکروزان را کاهش میدهد. با مصرف محدود آب، روندهایی به سمت افزایش خطر کمآبی بدن و کاهش خطر دیسپلازی برونکوپولمونری، خونریزی داخل جمجمهای، و مرگومیر وجود داشت اما از نظر آماری معنیدار نبودند.
نتیجهگیریهای نویسندگان
بر اساس این آنالیز، به نظر میرسد محتاطانهترین نسخه برای مصرف آب در نوزادان نارس، محدودیت دقیق مصرف آب باشد تا نیازهای فیزیولوژیکی برآورده شود، بدون آنکه کمآبی قابل توجه در بدن ایجاد شود. میتوان انتظار داشت که این عمل خطرات بروز مجرای شریانی باز و انتروکولیت نکروزان را، بدون افزایش قابل توجه در خطر عوارض جانبی، کاهش دهد.
PICO
خلاصه به زبان ساده
مصرف محدود آب در مقابل مصرف آزاد آن در پیشگیری از وقوع عوارض و مرگومیر در نوزادان نارس
اکثر نوزادانی که پیش از هفته 37 بارداری به دنیا میآیند (نوزادان نارس) به اندازه کافی رشد نمیکنند تا تمام آب و مواد مغذی مورد نیاز خود را از طریق دهان دریافت کنند. در نتیجه، نمیتوانند میزان مصرف آب خود را تنظیم کنند. مصرف ناکافی آب میتواند باعث کمآبی بدن نوزاد شود. از سوی دیگر، مصرف بیش از حد آب باعث بروز مشکلات قلبی و ریوی یا آسیب روده میشود. مرور سیستماتیک کارآزماییهای مرتبط با این موضوع منجر به این نتیجهگیری میشود که محدود کردن دقیق مصرف آب برای نوزادان نارس به مقداری که نیازهای فیزیکی آنها را بدون ایجاد کمآبی برآورده کند، خطر بروز برخی عوارض را کاهش میدهد. انجام پژوهش بیشتری در مورد این زمینه مورد نیاز است.
Authors' conclusions
Background
Description of the condition
Premature infants are generally too ill or immature to be fed by the breast or bottle. Therefore, the premature infant depends on his physicians and nurses to determine the rate of water administration by infusion into the infant's veins and arteries or by tube feeding into the stomach or intestine.
Description of the intervention
Estimation of the desirable intake of water each day is based on incomplete knowledge of the consequences of varying the rate of water intake. Moreover, the margin of error is small in managing the premature infant for several reasons. First, the premature infant's water losses to the environment are large (per kg body weight) and highly variable compared to larger, more mature infants or to children and adults. Second, the premature infant's kidneys are limited in their ability to compensate for varying water and solute intake by adjusting the concentration of the urine.
Several clinical trials have been conducted to examine the impact of varying the premature infant's water intake on clinical outcomes. These outcomes have included dehydration, patent ductus arteriosus, necrotizing enterocolitis, bronchopulmonary dysplasia, intracranial hemorrhage, and death. Dehydration may lead to hyperkalemia, cardiac arrhythmia, renal failure, and death. Patent ductus arteriosus, necrotizing enterocolitis, bronchopulmonary dysplasia, and intracranial hemorrhage are serious complications that may lead to death or disability in premature infants. The first two of these have been found by some investigators to be more likely if the water intake is excessive.
Objectives
The objective of this review was to determine the effects of water intake on postnatal weight loss and the risks of dehydration, patent ductus arteriosus, necrotizing enterocolitis, bronchopulmonary dysplasia, intracranial hemorrhage, and death in premature infants. The following questions were examined in premature infants.
-
Does restriction of water intake result in greater maximal postnatal weight loss?
-
Does restricted water intake increase the risk of dehydration?
-
Does restricted water intake decrease (or increase) the risk of patent ductus arteriosus?
-
Does restricted water intake decrease (or increase) the risk of necrotizing enterocolitis?
-
Does restricted water intake decrease (or increase) the risk of bronchopulmonary dysplasia?
-
Does restricted water intake decrease (or increase) the risk of intracranial hemorrhage?
-
Does restricted water intake decrease (or increase) the risk of death?
Methods
Criteria for considering studies for this review
Types of studies
Only randomized clinical trials (RCTs) were included. Quazi‐randomized trials were searched for and eligible for inclusion in the 2014 update.
Types of participants
Only studies where participants consisted entirely or mainly of premature infants (infants born before 37 weeks gestation) were included.
Types of interventions
Studies of varying water intake were included. Trials were excluded if the participants received water mainly or entirely as enteral feedings. Because we wished to examine the effects of water per se, rather than feedings, our review was limited to trials that included infants whose water intake was provided mainly or entirely by parenteral means, that is intravascular infusion.
Types of outcome measures
Primary outcomes
-
Postnatal weight loss
-
Dehydration
Secondary outcomes
-
Patent ductus arteriosus (PDA) (as defined in the study)
-
Necrotizing enterocolitis (Bells Stage II or greater, or as defined by the authors) (Bell 1978)
-
Bronchopulmonary dysplasia
-
Oxygen requirement at 28 to 30 days of life
-
Oxygen requirement at 36 weeks postmenstrual age
-
Intraventricular hemorrhage (IVH), defined using Papiles criteria (any grade or severe (grades III or IV)) (Papile 1978)
-
Death prior to hospital discharge
Search methods for identification of studies
Electronic searches
RCTs identified in previous versions of this review were re‐examined and, in each case, retained. These included trials were identified from multiple sources, including a previous review by one of the authors (Bell 1992) and a MEDLINE search. Additional trials were sought that compared the outcomes of interest in groups of premature infants who were given different levels of water intake according to an experimental protocol. Such trials were sought in a list of trials provided by the Cochrane Neonatal Review Group, in the authors' personal files, and with a PubMed search using the following strategy:
-
infant, low birth weight (18,196 sources identified);
-
infant, premature (40,795 sources identified);
-
1 or 2 (52,832 sources identified);
-
water intake (14,417 sources identified);
-
fluid intake (5979 sources identified);
-
4 or 5 (18,998 sources identified);
-
3 and 6 (176 sources identified).
In April 2010, we updated the search as follows: CENTRAL (The Cochrane Library), MEDLINE (search via PubMed), EMBASE and CINAHL were searched from 2007 to 2010. Search terms: fluid intake OR water intake. Limits: human, newborn infant and clinical trial. No language restrictions were applied.
In October 2014 we updated the search as follows: MEDLINE (search via PubMed), CINAHL, EMBASE and CENTRAL (The Cochrane Library) were searched from 2010 to 2014. Search terms: fluid intake OR water intake. Limits: human, newborn infant and clinical trial. No language restrictions were applied.
Searching other resources
Clinical trials registries were also searched for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp).
Data collection and analysis
The standard methods of The Cochrane Collaboration for conducting a systematic review were used.
Selection of studies
All randomized and quasi‐randomized controlled trials fulfilling the selection criteria described in the previous section were included. Both review authors reviewed the results of the search and separately selected the studies for inclusion. The review authors resolved any disagreement by discussion.
Data extraction and management
The data were then entered into tables using RevMan software.
Assessment of risk of bias in included studies
The standard methods of the Cochrane Neonatal Review Group were employed. The methodological quality of each trial was reviewed independently by the two review authors. Each identified trial was assessed for methodological quality with respect to: a) masking of allocation, b) masking of intervention, c) completeness of follow‐up, and d) masking of outcome assessment. This information is included in the 'Characteristics of included studies' table.
For the updated review in 2010 and 2014, the 'Risk of bias' table was completed. The two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion.
The risk of bias table addressed the following questions.
1. Sequence generation: was the allocation sequence adequately generated?
For each included study, we described the method used to generate the allocation sequence as: adequate (any truly random process for example a random number table, computer random number generator); inadequate (any nonrandom process for example odd or even date of birth, hospital or clinic record number); or unclear.
2. Allocation concealment: was allocation adequately concealed?
For each included study, we described the method used to conceal the allocation sequence as: adequate (for example telephone or central randomization, consecutively numbered sealed opaque envelopes); inadequate (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or unclear.
3. Blinding of participants, personnel and outcome assessors: was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the methods as: adequate, inadequate or unclear for participants; adequate, inadequate or unclear for study personnel; and adequate, inadequate or unclear for outcome assessors and specific outcomes assessed.
4. Incomplete outcome data: were incomplete outcome data adequately addressed?
For each included study, and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as: adequate (< 20% missing data), inadequate (≥ 20% missing data) or unclear.
5. Selective outcome reporting: were reports of the study free of suggestion of selective outcome reporting?
For each included study, we assessed the possibility of selective outcome reporting bias as: adequate (where it was clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported), inadequate (where not all the study's pre‐specified outcomes have been reported, one or more reported primary outcomes were not pre‐specified, outcomes of interest were reported incompletely and so could not be used, study failed to include results of a key outcome that would have been expected to have been reported) or unclear.
6. Other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns regarding other possible sources of bias (for example whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: yes, no or unclear.
Measures of treatment effect
The adverse event rates were calculated for the restricted and liberal water intake groups for each dichotomous outcome; the relative risk and risk difference were computed for each outcome. In addition, the maximal weight loss results were recorded and the weighted mean difference was computed. The analyses, including calculation of relative risk, risk difference, weighted mean difference and tests of heterogeneity were accomplished using RevMan software and a fixed‐effect model.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic.
Data synthesis
Meta‐analysis was carried out using the Review Manager software (RevMan 5, The Cochrane Collaboration). For estimates of typical relative risk and risk difference we used the Mantel‐Haenszel method. For measured quantities we used the inverse variance method. All meta‐analyses were done using the fixed effect model.
Subgroup analysis and investigation of heterogeneity
No subgroup analyses were performed.
Results
Description of studies
Five studies were included in this analysis. All were RCTs of varying water intake in premature infants. Each study compared two groups, one of whom received liberal water intake (this was considered the standard or control therapy) and the other restricted water intake. The principal difference among the studies was the timing and duration of the period when the infants' water intake was determined by the study protocol. In the Bell study (Bell 1980), the prescribed water intake was begun before 72 hours of age and continued up to age 30 days (unless any of certain criteria were met first). In the Kavvadia study (Kavvadia 2000), the prescribed water intake was given only during the first seven days of life; this study was limited to infants who required assisted ventilation starting within six hours of birth. In the Lorenz study (Lorenz 1982), the prescribed water intake was given only during the first five days of life. In the Tammela study (Tammela 1992), the prescribed water intake was begun within 24 hours of birth and continued until age 28 days. In the von Stockhausen study (von Stockhausen 1980), the prescribed water intake was given only during the first three days of life.
Various clinical outcomes were reported for each study, as described in the table 'Characteristics of included studies'.
One study was identified in the search of the literature for the 2014 update and was excluded from the review. Stroustrup 2012 sought to determine the effect of mild fluid restriction on the series of events in hospital (course) of neonates with transient tachypnea of the newborn (TTN). Most infants were late preterm and term neonates diagnosed with TTN. Infants were randomized to receive standard fluid management or mild fluid restriction. Studies in this population will be addressed by the planned review 'Fluid restriction in the management of transient tachypnea of the newborn' by Gupta and colleagues.
Risk of bias in included studies
Bell 1980
Prognostic stratification*?: yes
Pre‐randomization blinding of investigators to allocation?: yes
Blinding of caretakers to treatment?: no
Observer who categorized outcome blinded to treatment?: no
All participants included in the analysis?: yes
Kavvadia 2000
Prognostic stratification?: no
Pre‐randomization blinding of investigators to allocation?: yes
Blinding of caretakers to treatment?: no
Observer who categorized outcome blinded to treatment?: no
All participants included in the analysis?: yes
Lorenz 1982
Prognostic stratification?: yes
Pre‐randomization blinding of investigators to allocation?: could not determine
Blinding of caretakers to treatment?: no
Observer who categorized outcome blinded to treatment?: no
All participants included in the analysis?: no
Tammela 1992
Prognostic stratification?: no
Pre‐randomization blinding of investigators to allocation?: yes
Blinding of caretakers to treatment?: no
Observer who categorized outcome blinded to treatment?: no
All participants included in the analysis?: yes
von Stockhausen 1980
Prognostic stratification?: no
Pre‐randomization blinding of investigators to allocation?: cannot determine
Blinding of caretakers to treatment?: no
Observer who categorized outcome blinded to treatment?: no
All participants included in analysis?: not stated
*Prognostic stratification assures balance between treatment groups of other factors known or suspected to influence the outcomes of interest
Effects of interventions
Restricted versus liberal water intake (Comparison 1)
Weight loss (Outcome 1.1)
Postnatal weight loss (expressed as a percentage of the birth weight) was significantly higher with restricted water intake in the trials of Bell 1980 and Tammela 1992. It was also higher with restricted water intake in the meta‐analysis of the three trials (Bell 1980; Tammela 1992; von Stockhausen 1980) (overall weighted mean difference 1.94% of birth weight, 95% confidence interval (CI) 0.82 to 3.07).
Dehydration (Outcome 1.2)
There was a nonsignificant trend toward increased risk of dehydration with restricted water intake in the trial of Bell 1980. The meta‐analysis, which included the trials of Bell 1980 and Lorenz 1982, revealed a similar trend toward increased dehydration with restricted water intake (typical relative risk (RR) 2.43, 95% CI 0.71 to 8.28; typical risk difference (RD) 0.04, 95% CI ‐0.01 to 0.09) but this trend was not significant.
Patent ductus arteriosus (Outcome 1.3)
The risk of patent ductus arteriosus was significantly lower with restricted water intake in the trial of Bell 1980 and in the meta‐analysis (typical RR 0.52, 95% CI 0.37 to 0.73; typical RD ‐0.14, 95% CI ‐0.21 to ‐0.07), which included four trials (Bell 1980; Kavvadia 2000; Lorenz 1982; Tammela 1992). Based on this analysis, the number needed to treat with restricted water intake to prevent one case of patent ductus arteriosus was 7 (95% CI 5 to 14).
Necrotizing enterocolitis (Outcome 1.4)
The risk of necrotizing enterocolitis was significantly lower with restricted water intake in the trial of Bell 1980 and in the meta‐analysis (typical RR 0.43, 95% CI 0.21 to 0.87; typical RD ‐0.05, 95% CI ‐0.09 to ‐0.01), which included the trials of Bell 1980; Kavvadia 2000; Lorenz 1982 and Tammela 1992. Based on this analysis, the number needed to treat with restricted water intake to prevent one case of necrotizing enterocolitis was 20.0 (95% CI 11 to 100).
Bronchopulmonary dysplasia (Outcome 1.5)
The risk of bronchopulmonary dysplasia was not significantly affected by water intake in any of the four trials in which this was reported (Bell 1980; Kavvadia 2000; Lorenz 1982; Tammela 1992), nor in the meta‐analysis (typical RR 0.85, 95% CI 0.63 to 1.14; typical RD ‐0.04, 95% CI ‐0.11 to 0.03). The direction of effect in all four trials and in the meta‐analysis was toward reduced risk of bronchopulmonary dysplasia with restricted water intake.
Intraventricular hemorrhage (Outcome 1.6)
The risk of intraventricular hemorrhage (all grades) was not significantly affected by restricted water intake in any of the three trials in which this was analyzed (Kavvadia 2000; Lorenz 1982; Tammela 1992) nor in the meta‐analysis (typical RR 0.74, 95% CI 0.48 to 1.14; typical RD ‐0.06, 95% CI ‐0.13 to 0.02). However, the trend in two of the trials (Kavvadia 2000; Tammela 1992) and in the meta‐analysis was toward reduced risk of intracranial hemorrhage with restricted water intake.
Death (Outcome 1.7)
The risk of death was significantly lower with restricted water intake in the trial of Tammela 1992 but not in the other four trials, nor in the meta‐analysis (typical RR 0.81, 95% CI 0.54 to 1.23; typical RD ‐0.03, 95% CI ‐0.08 to 0.03) which included all five trials.
Summary
The analysis of the five studies taken together indicated that restricted water intake significantly increased postnatal weight loss and significantly reduced the risks of patent ductus arteriosus and necrotizing enterocolitis. With restricted water intake there were trends toward increased risk of dehydration and reduced risks of bronchopulmonary dysplasia, intracranial hemorrhage and death, but these trends were not statistically significant.
Discussion
This analysis shows what appear to be significant advantages to a restrictive strategy for managing the water intake of premature infants. When considered collectively using meta‐analysis, the infants in these five trials who were in the restricted groups were at lower risk of patent ductus arteriosus and necrotizing enterocolitis, with no significant increase in adverse effects. There were trends toward increased risk of dehydration and decreased risk of bronchopulmonary dysplasia, intracranial hemorrhage and death with restricted water intake but these trends were not significant. It is important to use caution in extrapolating these results to extremely premature infants, who were under‐represented in these studies.

Comparison 1 Restricted versus liberal water intake, Outcome 1 Weight loss (%).
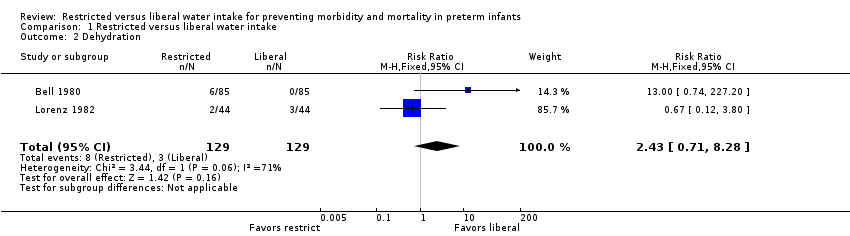
Comparison 1 Restricted versus liberal water intake, Outcome 2 Dehydration.

Comparison 1 Restricted versus liberal water intake, Outcome 3 Patent ductus arteriosus.
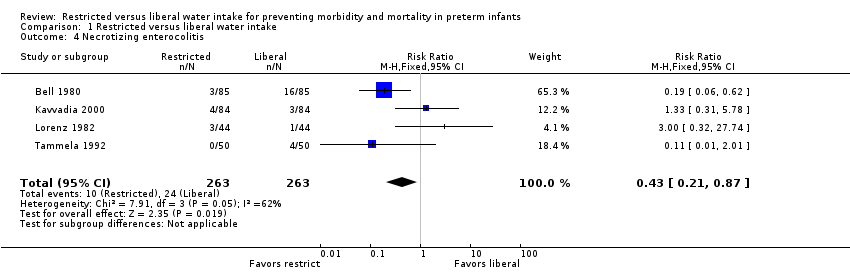
Comparison 1 Restricted versus liberal water intake, Outcome 4 Necrotizing enterocolitis.

Comparison 1 Restricted versus liberal water intake, Outcome 5 Bronchopulmonary dysplasia.
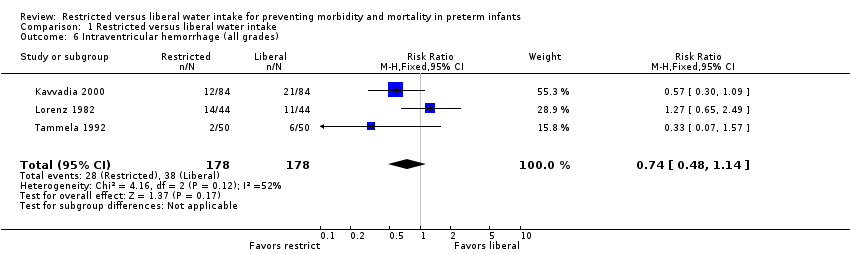
Comparison 1 Restricted versus liberal water intake, Outcome 6 Intraventricular hemorrhage (all grades).

Comparison 1 Restricted versus liberal water intake, Outcome 7 Death.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight loss (%) Show forest plot | 3 | 326 | Mean Difference (IV, Fixed, 95% CI) | 1.94 [0.82, 3.07] |
| 2 Dehydration Show forest plot | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.71, 8.28] |
| 3 Patent ductus arteriosus Show forest plot | 4 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.37, 0.73] |
| 4 Necrotizing enterocolitis Show forest plot | 4 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.21, 0.87] |
| 5 Bronchopulmonary dysplasia Show forest plot | 4 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.14] |
| 6 Intraventricular hemorrhage (all grades) Show forest plot | 3 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.14] |
| 7 Death Show forest plot | 5 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.23] |

