극심한 저체중아의 단기 및 장기 질환과 사망을 예방하기 위한 비타민 A 보충제
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Data extraction fields for interventions and outcomes
The authors collected the following data from each included trial.
-
General information
-
-
Supplementation dose
-
Supplementation frequency
-
Time of initiation of supplementation
-
Vitamin A intake of control groups
-
Pre‐randomisation vitamin A concentrations of supplemented and control groups (if available)
-
Post‐randomisation vitamin A concentrations
-
-
Number of the following outcomes in the supplemented and control groups
-
-
Deaths
-
Reported as having a significant patent ductus arteriosus at 2 weeks of age
-
Developing neonatal chronic lung disease in the supplemented and control groups
-
Patent ductus arteriosus
-
Necrotising enterocolitis
-
Intraventricular haemorrhage
-
Periventricular leukomalacia
-
Retinopathy of prematurity
-
≥ 1 episodes of defined sepsis
-
Long‐term neurodevelopmental disability
-
-
Mean, standard deviation, and range of the following outcomes in the supplemented and control groups
-
-
Gestational age
-
Birth weight
-
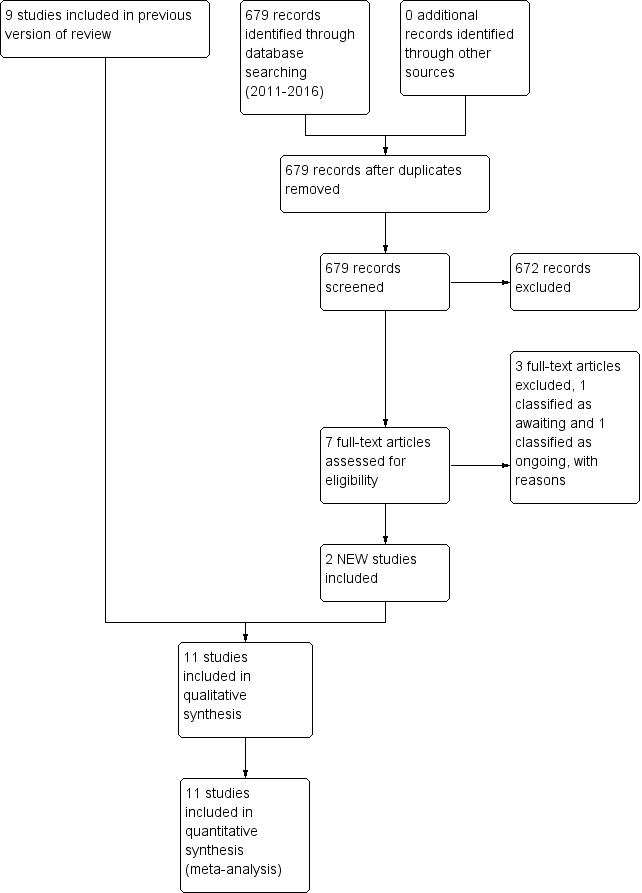
Study flow diagram: review update

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
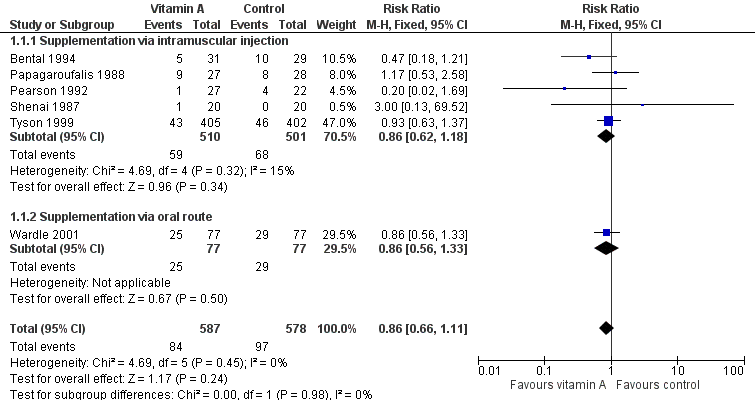
Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.1 Death (before 1 month).

Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.2 Chronic lung disease (oxygen use at 28 days in survivors).
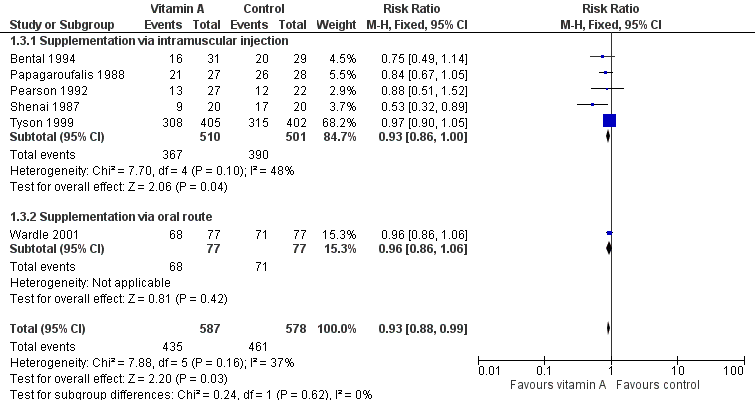
Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.3 Death or chronic lung disease (oxygen use at 28 days).

Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.4 Death before 36 weeks' postmenstrual age.

Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.5 Chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors).
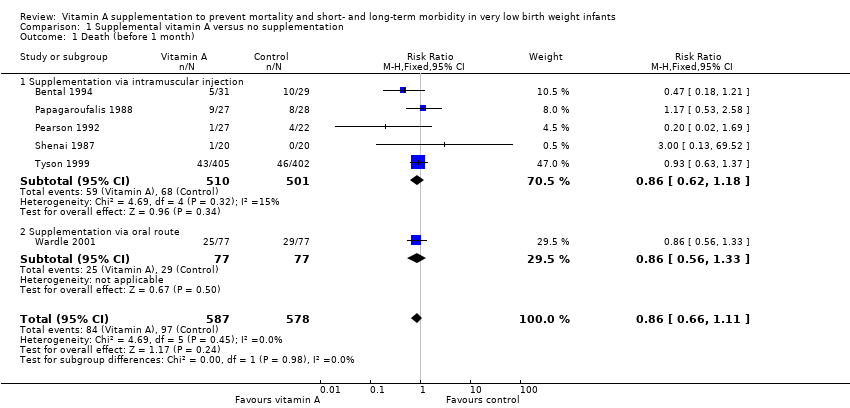
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 1 Death (before 1 month).
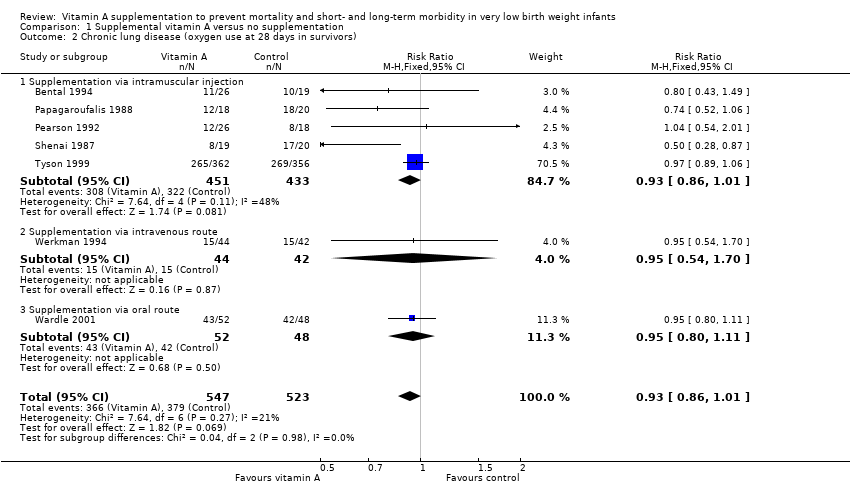
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 2 Chronic lung disease (oxygen use at 28 days in survivors).

Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 3 Death or chronic lung disease (oxygen use at 28 days).

Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 4 Death before 36 weeks' postmenstrual age.

Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 5 Chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors).
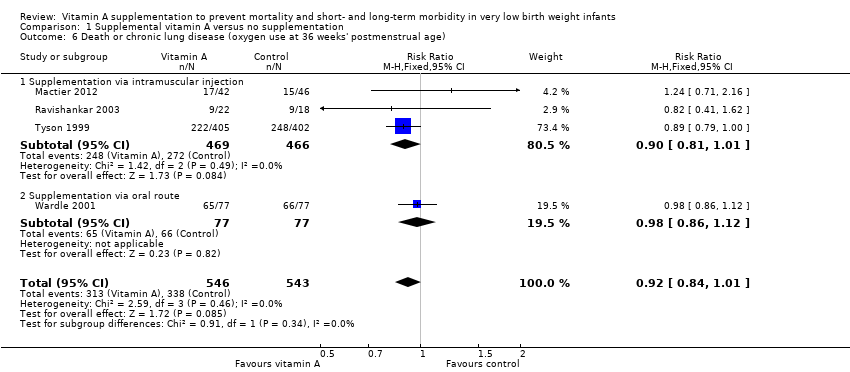
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 6 Death or chronic lung disease (oxygen use at 36 weeks' postmenstrual age).
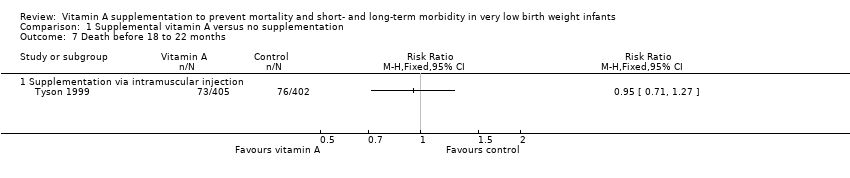
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 7 Death before 18 to 22 months.

Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 8 Neurodevelopmental impairment at 18 to 22 months.

Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 9 Death or neurodevelopmental impairment at 18 to 22 months.
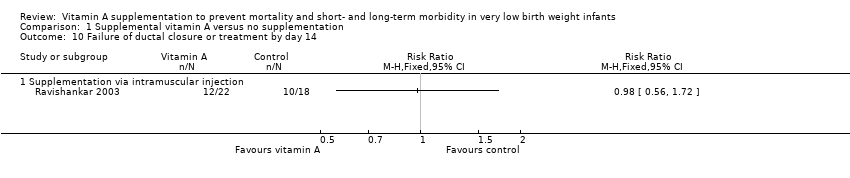
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 10 Failure of ductal closure or treatment by day 14.
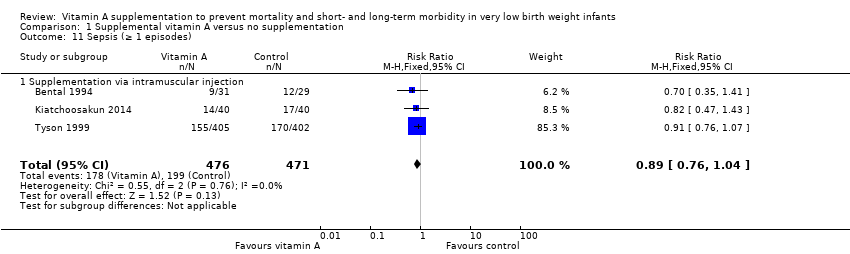
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 11 Sepsis (≥ 1 episodes).

Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 12 Necrotising enterocolitis.

Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 13 Intraventricular haemorrhage.

Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 14 Periventricular leukomalacia.

Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 15 Retinopathy of prematurity (any grade).
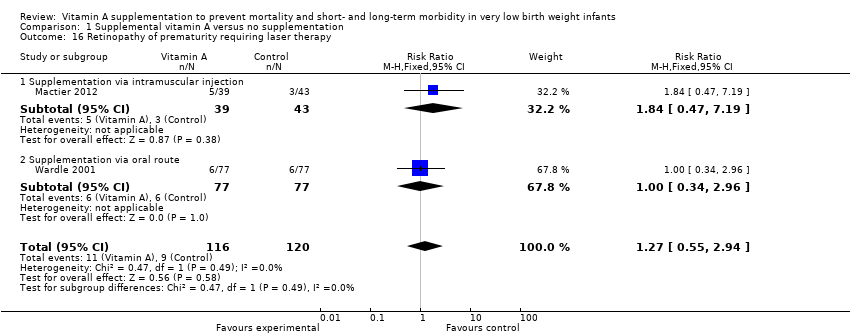
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 16 Retinopathy of prematurity requiring laser therapy.
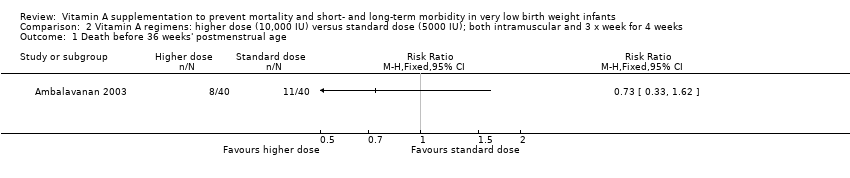
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 1 Death before 36 weeks' postmenstrual age.
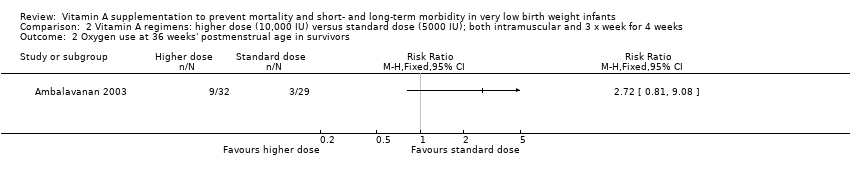
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 2 Oxygen use at 36 weeks' postmenstrual age in survivors.

Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 3 Death or oxygen use at 36 weeks' postmenstrual age.
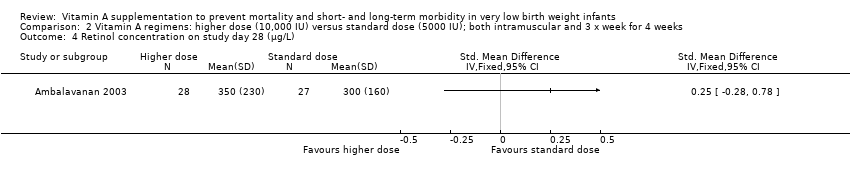
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 4 Retinol concentration on study day 28 (μg/L).

Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 5 Retinol < 200 μg/L on day 28 (%).
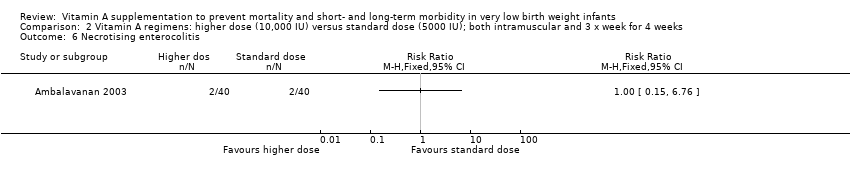
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 6 Necrotising enterocolitis.

Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 7 Retinopathy of prematurity (any grade).
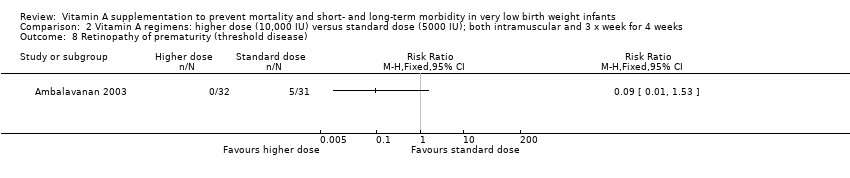
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 8 Retinopathy of prematurity (threshold disease).
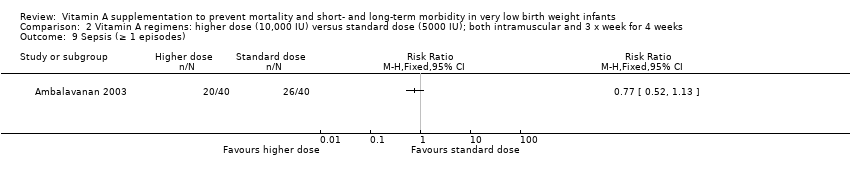
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 9 Sepsis (≥ 1 episodes).

Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 1 Death before 36 weeks' postmenstrual age.
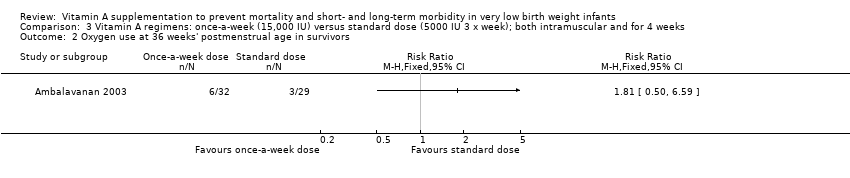
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 2 Oxygen use at 36 weeks' postmenstrual age in survivors.

Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 3 Death or oxygen use at 36 weeks' postmenstrual age.
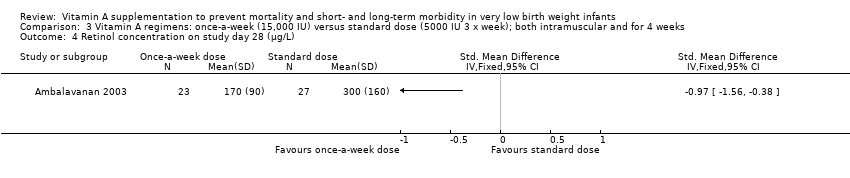
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 4 Retinol concentration on study day 28 (μg/L).

Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 5 Retinol < 200 μg/L on day 28 (%).

Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 6 Necrotising enterocolitis.
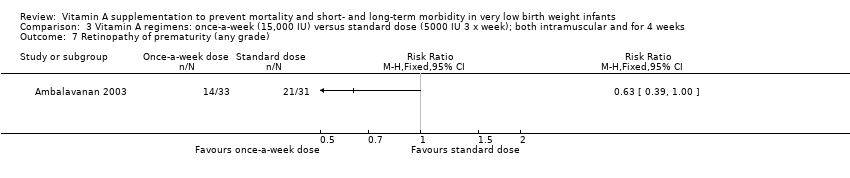
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 7 Retinopathy of prematurity (any grade).

Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 8 Retinopathy of prematurity (threshold disease).

Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 9 One or more episodes of sepsis.
| Supplemental vitamin A compared to no supplementation in very low birth weight infants | ||||||
| Patient or population: very low birth weight infants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| no supplementation | Supplemental vitamin A | |||||
| Neonatal death | 168 per 1000 | 144 per 1000 | RR 0.86 | 1165 | ⊕⊕⊕⊝ | Imprecision: 95% CI are wide and imprecise |
| Chronic lung disease (oxygen use at 28 days in survivors) | 725 per 1000 | 674 per 1000 | RR 0.93 | 1070 | ⊕⊕⊕⊕ | Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect |
| Death or chronic lung disease (oxygen use at 28 days) | 798 per 1000 | 742 per 1000 | RR 0.93 | 1165 | ⊕⊕⊕⊕ | Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect |
| Death before 36 weeks' postmenstrual age | 168 per 1000 | 168 per 1000 | RR 1.00 | 1089 | ⊕⊕⊕⊝ | Imprecision: 95% CI are wide and imprecise |
| Chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors) | 546 per 1000 | 486 per 1000 | RR 0.87 | 986 | ⊕⊕⊕⊝ | Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect |
| Death or chronic lung disease (oxygen use at 36 weeks' postmenstrual age) | 622 per 1000 | 573 per 1000 | RR 0.92 | 1089 | ⊕⊕⊕⊝ | Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect |
| Neurodevelopmental impairment at 18 to 22 months | 481 per 1000 | 428 per 1000 | RR 0.89 | 538 | ⊕⊕⊝⊝ | Concerning imprecision: does not met the optimal information size criteria |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision: 95% CI are wide and imprecise 2 Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect 3 Concerning imprecision; does not met the optimal information size criteria | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death (before 1 month) Show forest plot | 6 | 1165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.66, 1.11] |
| 1.1 Supplementation via intramuscular injection | 5 | 1011 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.18] |
| 1.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.33] |
| 2 Chronic lung disease (oxygen use at 28 days in survivors) Show forest plot | 7 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.01] |
| 2.1 Supplementation via intramuscular injection | 5 | 884 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.01] |
| 2.2 Supplementation via intravenous route | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.54, 1.70] |
| 2.3 Supplementation via oral route | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.11] |
| 3 Death or chronic lung disease (oxygen use at 28 days) Show forest plot | 6 | 1165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.88, 0.99] |
| 3.1 Supplementation via intramuscular injection | 5 | 1011 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.00] |
| 3.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.06] |
| 4 Death before 36 weeks' postmenstrual age Show forest plot | 4 | 1089 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.29] |
| 4.1 Supplementation via intramuscular injection | 3 | 935 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 4.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.33] |
| 5 Chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors) Show forest plot | 5 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.77, 0.99] |
| 5.1 Supplementation via intramuscular injection | 4 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 5.2 Supplementation via oral route | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.24] |
| 6 Death or chronic lung disease (oxygen use at 36 weeks' postmenstrual age) Show forest plot | 4 | 1089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.84, 1.01] |
| 6.1 Supplementation via intramuscular injection | 3 | 935 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 1.01] |
| 6.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.12] |
| 7 Death before 18 to 22 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Supplementation via intramuscular injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Neurodevelopmental impairment at 18 to 22 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Supplementation via intramuscular injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Death or neurodevelopmental impairment at 18 to 22 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Supplementation via intramuscular injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Failure of ductal closure or treatment by day 14 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Supplementation via intramuscular injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Sepsis (≥ 1 episodes) Show forest plot | 3 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 11.1 Supplementation via intramuscular injection | 3 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 12 Necrotising enterocolitis Show forest plot | 4 | 1101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.27] |
| 13 Intraventricular haemorrhage Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Any intraventricular haemorrhage | 3 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 13.2 Severe intraventricular haemorrhage (Grade 3 or 4) | 4 | 1035 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.25] |
| 14 Periventricular leukomalacia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 Retinopathy of prematurity (any grade) Show forest plot | 4 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.65, 1.01] |
| 15.1 Supplementation via intramuscular injection | 3 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.42, 0.99] |
| 15.2 Supplementation via oral route | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.20] |
| 16 Retinopathy of prematurity requiring laser therapy Show forest plot | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.55, 2.94] |
| 16.1 Supplementation via intramuscular injection | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.47, 7.19] |
| 16.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death before 36 weeks' postmenstrual age Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Oxygen use at 36 weeks' postmenstrual age in survivors Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Death or oxygen use at 36 weeks' postmenstrual age Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Retinol concentration on study day 28 (μg/L) Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Retinol < 200 μg/L on day 28 (%) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Necrotising enterocolitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Retinopathy of prematurity (any grade) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Retinopathy of prematurity (threshold disease) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Sepsis (≥ 1 episodes) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death before 36 weeks' postmenstrual age Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Oxygen use at 36 weeks' postmenstrual age in survivors Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Death or oxygen use at 36 weeks' postmenstrual age Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Retinol concentration on study day 28 (μg/L) Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Retinol < 200 μg/L on day 28 (%) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Necrotising enterocolitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Retinopathy of prematurity (any grade) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Retinopathy of prematurity (threshold disease) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 One or more episodes of sepsis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

