Reducción o interrupción de los antipsicóticos y antipsicóticos como tratamientos específicos para la discinesia tardía
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: "randomly assigned", not described Duration: 12 weeks | |
| Participants | Diagnosis: schizophrenia with persistent severe TD (DSM IV, Kane criteria) History: maintenance on conventional antipsychotics for > 1 year with an equivalent dosage of < 200 mg/d of chlorpromazine; duration of TD not reported | |
| Interventions | After a 4‐week washout period with all original conventional antipsychotics discontinued: 1. Risperidone: started at 2 mg/d and increased, with a 2‐mg increase every 2 weeks, to 6 mg/d over 6 weeks; then maintenance dose 6 mg/d for 12 weeks. N = 22 2. Placebo: placebo for 12 weeks. N = 20 Concomitant medication included benzodiazepines (86%‐90%) and antiparkinsonism drugs (50%‐86%) | |
| Outcomes | TD symptoms: AIMS Adverse effects: extrapyramidal symptoms (parkinsonism) (ESRS) Adverse effects: dystonia (ESRS) TD symptoms: clinical efficacy (decrease in AIMS of 3 or 4 = responder) BPRS | |
| Notes | Sponsorship source: supported by Janssen‐Cilag Taiwan, Johnson & Johnson Taiwan Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "subjects were randomly assigned to the risperidone or placebo groups", further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blind" "A placebo with an identical appearance to the risperidone dose was prescribed for the placebo group using the same dose schedule." |
| Blinding of outcome assessment (detection bias) | Low risk | "The TD condition was evaluated blindly by a psychiatrist with the Abnormal Involuntary Movement Scale (AIMS) every 2 weeks" |
| Incomplete outcome data (attrition bias) | Low risk | "Forty‐two patients completed the 12‐week study and 7 subjects withdrew. Four subjects dropped out due to psychotic symptom exacerbation (2 subjects during the washout period: 1 subject in the placebo group and 1 subject in the risperidone group). Another 3 subjects withdrew due to a medical condition (infectious disease, heart condition, and lung carcinoma)." |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all pre‐defined outcomes were reported. A protocol is not available for verification. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
| Methods | Allocation: "randomized", not described Duration: 24 weeks | |
| Participants | Diagnosis: schizophrenia (DSM IV), Schooler and Kane's criteria for persistent TD History: duration of TD not reported; treatment with conventional antipsychotics for > one year | |
| Interventions | No washout period on the discontinuation of all conventional antipsychotics was reported 1. Olanzapine: dose not reported, 24 weeks. N = 27 2. Amisulpride: dose not reported, 24 weeks. N = 27 3. First generation antipsychotic (FGA): dose not reported, 24 weeks. N = 26 | |
| Outcomes | TD symptoms: AIMS Adverse effects: extrapyramidal side effects (SAS) Adverse effects: akathisia (BAS) Adverse effects: general (UKU) General mental state (BPRS) Leaving the study early | |
| Notes | Sponsorship source: the study was supported by grants from National Science Council, Taiwan. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The subjects were randomized to three groups", further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | High risk | "single‐blind and controlled study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "single‐blind and controlled study" Blinding details of outcome assessors not reported |
| Incomplete outcome data (attrition bias) | Low risk | "Finally 76 cases (95%) completed the 24‐week study, 2 cases in the olanzapine groups withdrew due to impaired liver function, 1 case in the amisulpride group due to infectious disease, and 1 case in the FGA controlled groups withdrew due to unstable psychiatric condition" “All data were analyzed on an intent‐to‐treat basis, and endpoint data were generated with the last observation carried forward (LOCF).” |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported |
| Other bias | Low risk | The study seems to have been free of other sources of bias. |
| Methods | Allocation: "randomly assigned", not described Duration: 18 months | |
| Participants | Diagnosis: schizophrenia and TD (DSM IV, Schooler‐Kane criteria) History: duration of TD not reported | |
| Interventions | Overlap in administration of the antipsychotic drugs that participants received before study entry was permitted for the first 4 weeks after randomisation to allow a gradual transition to study medication: 1. Oolanzapine: flexible dose of 7.5 mg each day/twice a day/3 times a day/4 times a day for 18 months. N = 54 2. quetiapine: flexible dose of 200 mg each day/twice a day/3 times a day/4 times a day for 18 months. N = 62 3. Rrisperidone: flexible dose of 1.5 mg each day/twice a day/3 times a day/4 times a day for 18 months. N = 56 4. ziprasidone: flexible dose of 40 mg each day/twice a day/3 times a day/4 times a day for 18 months. N = 28 Medications were flexibly dosed with 1‐4 capsules daily, as judged by the study doctor. Concomitant medications were permitted, except for additional antipsychotic agents. | |
| Outcomes | Leaving the study early Unable to use ‐ AIMS, PANSS, SAS, BAS, cogitive composite score (not reported in means and SDs for the separate intervention groups)* | |
| Notes | Sponsorship source: supported by the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project, National Institute of Mental Health. This article was based on results from the CATIE project, supported by the National Institute of Mental Health. Astra Zeneca Pharmaceuticals LP, Bristol‐Myers Squibb Company, Forest Pharmaceuticals, Inc., Janssen Pharmaceutica Products, L.P., Eli Lilly and Company, Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., and Zenith Goldline Pharmaceuticals, Inc., provided medications for the studies. This material is based upon work also supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research Development, with resources and the use of facilities at the Philadelphia Veterans Affairs Medical Center. *Study author kindly replied to our request for data. At the time of preparing this review no more outcome data were available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were initially randomly assigned", further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "...double‐blind conditions..." "Identical‐appearing capsules contained olanzapine (7.5 mg), quetiapine (200 mg), risperidone (1.5 mg), perphenazine (8 mg), or ziprasidone (40 mg)." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Incomplete outcome data (attrition bias) | High risk | The primary clinical outcome measure was time to all‐cause treatment discontinuation. Total population (N = 200): 74% discontinuation olanzapine: 31/54 (57%); quetiapine: 51/62 (82%); risperidone: 44/56 (79%); zipraidone: 21/28 (75%). Reasons for withdrawal reported |
| Selective reporting (reporting bias) | High risk | Original CATIE study: "The primary clinical outcome measure was time to all‐cause treatment discontinuation. Secondary outcomes included discontinuations for intolerability, inefficacy, and patient decision; rates of discontinuations; mean modal dose; and change from baseline in the PANSS and neurocognitive composite scores/".TD: "The primary outcome measure used to evaluate the course of TD was change from baseline in total AIMS score. Secondary outcome measures included change in global, distress, and impairment of function items on the AIMS; percentage of patients meeting Schooler‐Kane criteria for at least 2 consecutive visits post baseline; percentage of visits at which patients met modified Schooler‐Kane criteria; and percentage of patients with at least a 50% change in AIMS score (excluding month 1). In addition, treatment differences with respect to all cause discontinuation are described for patients with TD at baseline." |
| Other bias | High risk | Post hoc analysis; modified diagnostic criteria for TD were applied at baseline, and a 3‐month history of antipsychotics exposure was not required. |
| Methods | Allocation: "randomly assigned by coin method" Duration: 24 weeks | |
| Participants | Diagnosis: schizophrenia (58) and schizoaffective disorder (2) (DSM‐IV criteria); antipsychotic‐induced TD History: duration of TD not reported. Antipsychotic exposure ˜10 years. All of the participants received FGAs prior to participation in this study. | |
| Interventions | Following a washout period of 3‐7 days: 1. risperidone: flexible dose of 1.9 ± 0.7 (baseline) to 4.1 ± 1.4 (end point) mg/d for 24 weeks. N = 30 2. olanzapine: flexible dose of 8.1 ± 2.0 (baseline) to 12.6 ± 5.4 (end point) mg/d for 24 weeks. N = 30 | |
| Outcomes | TD symptoms: no clinical improvement > 50% (AIMS) TD symptoms: AIMS Adverse effect: dyskinesia Adverse effect: parkinsonism Adverse effect: dystonia Adverse effect: akathisia Adverse effects: general adverse events (39/30 vs 31/30) General mental state: BPRS Leaving the study early | |
| Notes | Sponsorship source: supported by research grant from the Taoyuan Mental Hospital and from the Department of Health, Executive Yuan, Taiwan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned to receive either olanzapine or risperidone with a 1‐to‐1 ratio by coin method with a 6‐block design". |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | High risk | "...primary care physicians and patients were not blinded." |
| Blinding of outcome assessment (detection bias) | Low risk | “Two investigators (C.‐H.C. and J.‐J.C.) served as blinded raters.” "The BPRS, CGI‐S, AIMS and global impression of ESRS were performed at baseline and at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24 or at end point visit by blinded‐rater" |
| Incomplete outcome data (attrition bias) | Low risk | 9/30 in the risperidone and 7/30 in the olanzapine groups dropped out from the study; reasons reported. "All patients who were randomly assigned and had at least 1 post‐baseline assessment were included in the intent‐to‐treat (ITT) analysis. If the ITT subjects withdrew from the study earlier than scheduled, then the last observation carried forward method was employed to extend the end point scores." |
| Selective reporting (reporting bias) | Low risk | Data for all outcomes in the trial registry, NCT00621998, have been reported. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
| Methods | Allocation: "randomly assigned", not described Duration: 8 weeks | |
| Participants | Diagnosis: chronic schizophrenia (DSM‐III R criteria) History: duration TD not reported; the most common pre‐study medications were haloperidol, procyclidine, lorazepam, benztropine and chlorpromazine; the most commonly used depot antipsychotic agents were haloperidol decanoate, fluphenazine decanoate, flupenthixol decanoate and pipothiazine palmitate. | |
| Interventions | Mean duration of washout phase 6 days. 1. Risperidone: dose 2 mg/d for 8 weeks. N = 8 2. Risperidone: dose 6 mg/d for 8 weeks. N = 6 3. Risperidone: dose 10 mg/d for 8 weeks. N = 6 4. Risperidone: dose 16 mg/d for 8 weeks. N = 11 5. Haloperidol: dose 20 mg/d. N = 6 6. Placebo: N = 11 "At the time of selection, all psychotropic and antiparkinsonism medications were discontinued"; "no other psychotropic medication was administered except for chloral hydrate or a benzodiazepine if a sedative/hypnotic was required.", "An antiparkinsonian medication (biperiden or procyclidine) was given in case of the emergence of clinically significant drug‐induced parkinsonism and dystonia" | |
| Outcomes | Adverse events: use of antiparkinsonism medication Unable to use (data does not have variability measures, and only reports differences from baseline to worst scores) ‐ ESRS: dyskinesia symptoms total score, CGI severity dyskinesia, buccolinguomasticatory factor, choreoathetoid factor | |
| Notes | Sponsorship source: not reported. Study author kindly replied to our request for data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "identical tablets" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of raters not reported |
| Incomplete outcome data (attrition bias) | Low risk | 33% of participants terminated the study early due to insufficient therapeutic response. All early terminations were included in the ITT analysis |
| Selective reporting (reporting bias) | High risk | Outcomes not fully reported |
| Other bias | High risk | Subgroup with TD |
| Methods | Allocation: "allocated randomly", not described Duration: 44 weeks | |
| Participants | Diagnosis: hebephrenic or paranoid schizophrenia (ICD‐9 and Feighner criteria) | |
| Interventions | No washout period before study entry 1. Antipsychotic reduction: dose 50% previous dose of cis(z)‐flupenthixol decanoate, bi‐weekly. N = 5 2. Antipsychotic maintenance: dose standard dosage of cis(z)‐flupenthixol decanoate. N = 4 Procyclidine allowed during study. Supplementary antipsychotics allowed were haloperidol (oral) or zuclopenthixol decanoate (depot). Amitriptyline used for depression | |
| Outcomes | TD (AIMS derived) Unable to use ‐ | |
| Notes | Dr Cookson kindly provided additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized in blocks of 4 and stratified by antipsychotic dose and gender", implies adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment details |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double blind", no further details |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double blind", no further details |
| Incomplete outcome data (attrition bias) | Low risk | All participants seem to have completed the study |
| Selective reporting (reporting bias) | Unclear risk | All outcomes proposed in the methods were reported, but some were not presented adequately. No protocol available to check |
| Other bias | High risk | "The randomised allocation of the small number of patients in the pilot study results in inequalities between the 2 groups at entry and confounded comparisons of group mean values during the study" |
| Methods | Allocation: "randomly assigned", not described Setting: inpatients and outpatients, South Africa | |
| Participants | Diagnosis: schizophrenia (DSM IV), TD (Schooler and Kane criteria) | |
| Interventions | After an initial screening visit, subjects were tapered from all psychotropic medication over a 2‐week period. 1. Quetiapine: dose 100 mg/d increased to 400 mg/d. N = 22 2. Haloperidol: dose 5 mg/d increased to 10 mg/d. N = 23 Concomitant medications allowed were benzodiazepines for agitation or insomnia and anticholinergic agents in the event of treatment‐emergent or worsening EPS. Medications not allowed were other antipsychotics or other medication known to improve or exacerbate movement disorders. | |
| Outcomes | TD symptoms: no clinical improvement General mental health PANSS Unable to use ‐ Global assessment: CGI. (data in graphs, no variability) | |
| Notes | Sponsorship source: supported in part by the Medical Research Council of South Africa, Cape Town, and the University of Stellenbosch. Trial medication and monitoring of the study were provided by AstraZeneca, Wilmington, Del. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were then randomly assigned", further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | High risk | "investigator‐blinded", further blinding details not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | "investigator‐blinded", further blinding details not reported |
| Incomplete outcome data (attrition bias) | High risk | 43% dropouts (including the 2 participants excluded in the early stages). 10/22 (45%) quetiapine and 8/23 (35%) haloperidol participants dropped out. |
| Selective reporting (reporting bias) | High risk | Adverse effects: extrapyramidal symptoms (other than dyskinesia) not fully reported |
| Other bias | Low risk | The study seems to be free of other sources of bias. Baseline characteristics were balanced in the compared groups. |
| Methods | Allocation: "randomly assigned", not described Setting: outpatients, USA | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder with TD (operational criteria) and criterion for withdrawal‐exacerbated TD | |
| Interventions | Antipsychotic medications were tapered over a 7‐10 d period and then withdrawn, with single‐blind substitution of placebo for 7‐14 d. Study medication was administered when there was a demonstrable increase in involuntary movements. 1. Molindone: dose 75 mg (mean) during the first week (100% of pre‐trial dose equivalent) and 145 mg (mean) (200% of pre‐trial dose equivalent) during the second week. N = 9 2. Haloperidol: dose 19.3 mg (mean) (100% of pre‐trial dose equivalent) during the first week and 34.3 mg (mean) (200% of pre‐trial dose equivalent) during the second week. N = 9 Concomitant medications: psychoactive medications including antiparkinsonism agents, within 6 months before study entry were not allowed | |
| Outcomes | Clinical improvement: AIMS Unable to use ‐ | |
| Notes | Sponsorship source: supported in part by a grant from E.I. DuPont Pharmaceutical Company and National Institute of Mental Health Grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned to receive either molindone or haloperidol in a double‐blind fashion", further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind" "Medication was supplied in identical‐appearing red capsules containing 25 mg and 5 mg, respectively, of molindone and haloperidol" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind". Details of blinding of outcome assessment not reported |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants completed the trial |
| Selective reporting (reporting bias) | Unclear risk | Unclear if psychiatric symptoms, dyskinetic movements and parkinsonism measured by BPRS and Webster Parkinsonism Rating Scale were defined as outcomes. Only AIMS results were reported. |
| Other bias | High risk | The two groups were comparable except for a greater past hospitalisation duration in the molindone as compared with haloperidol‐treated group |
| Methods | Allocation: randomised using random numbers table Setting: outpatients, USA | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (RDC) | |
| Interventions | 1. Fluphenazine decanoate: low dose 1.25 mg‐5 mg/2 weeks. N = 4 2. Fluphenazine decanoate: antipsychotic maintenance: standard dose 12.5 mg‐50 mg/2 weeks. N = 4 Procyclidine, 5 mg‐20 mg/d, was allowed if needed to treat extrapyramidal side effects. No other psychotropic medication except flurazepam or diazepam was allowed (these benzodiazepines were used sparingly for insomnia). | |
| Outcomes | TD ('no clinical improvement'; 'no improvement'; 'deterioration'), reported as adverse effects Incidence of TD (modified versions of SDS) General mental state: relapse Unable to use ‐ | |
| Notes | Sponsorship source: this investigation was supported in part by grants from the National Institute of Mental Health. Dr Woerner kindly provided unpublished data for one site of the main study and only these are used in this review; the sex ratios are not available. If people in this study developed TD, participation was stopped and they were classified as leaving the study early. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind". Details not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind". Details not reported |
| Incomplete outcome data (attrition bias) | High risk | 4/8 participants left the study early |
| Selective reporting (reporting bias) | High risk | Not all data were reported |
| Other bias | High risk | Only subsample with TD from one site included in this review |
| Methods | Allocation: randomised Setting: inpatients, USA | |
| Participants | Diagnosis: chronic schizophrenia (15), chronic brain syndrome (3), and mental retardation (2) all manifesting typical buccolingual‐masticatory oral dyskinesia due to prolonged antipsychotic medication. N = 20 | |
| Interventions | 4‐week washout using placebo medication, then: 1. haloperidol: dose 2 mg/d, increased to maximum of 16 mg/d. N = 11 2. thiopropazate: dose 10 mg/d, increased to maximum of 80 mg/d. N = 9 Concomitant medication not reported | |
| Outcomes | TD symptoms Leaving the study early Unable to use ‐ | |
| Notes | Sponsorship source: supported in part by Public Health Service Research grant from the National Institute of Mental Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "20 patients were randomly divided", further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants not reported. Ward nurses were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | "Quantitative evaluation of oral dyskinesia... was carried out every two weeks, by a psychiatrist... using a blind basis". Ward nurses were also blind to the treatment. |
| Incomplete outcome data (attrition bias) | Low risk | Two participants dropped out from haloperidol with reasons reported. |
| Selective reporting (reporting bias) | High risk | Adverse effects (except for 2 participants who were discontinued from the study) were not reported. Also, oral dyskinesia and reversible EPS scores reported only as mean for each arm. |
| Other bias | Unclear risk | Insufficient information to make a judgement |
| Methods | Allocation: "randomly" Setting: inpatients, USA | |
| Participants | Diagnosis: chronic psychotic patients: chronic schizophrenia (10), mentally deficient (2), chronic brain syndrome (1); all manifesting typical buccolinguomasticatory oral dyskinesia associated with long‐term antipsychotic medication History: duration of TD not reported | |
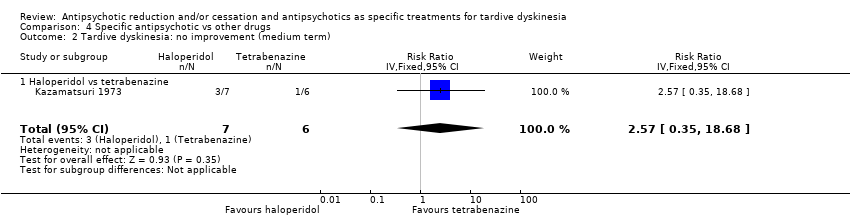
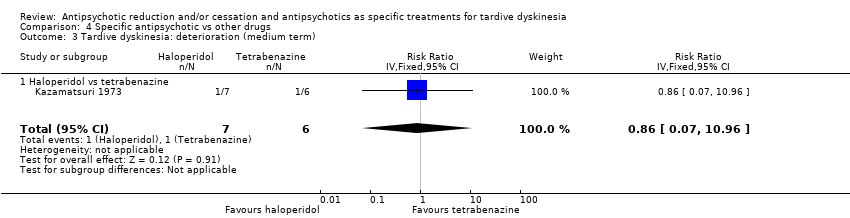
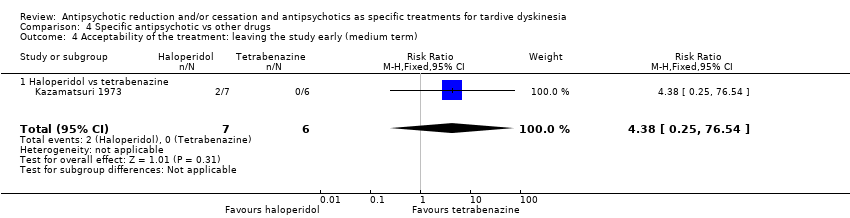
| Interventions | 4 week washout from antiparkinsonism and antipsychotic medication (all replaced by placebo), then:. 1. haloperidol: dose 4 mg twice/d. From week 15 dose was doubled to 16 mg/d. N = 7 2. tetrabenazine: dose 50 mg twice/d. From week 15 onwards, dose was doubled to 200 mg/d. N = 6 Concomitant medications: "Other medications, such as antidiabetic or anticonvulsant drugs, were continued unchanged." | |
| Outcomes | TD symptoms: not clinically improved TD symptoms: no improvement TD symptoms: deterioration Leaving the study early Unable to use ‐ TD scale scores and adverse effects: EPS Ward behaviour (NOSIE) (means, SDs not reported) | |
| Notes | Sponsorship source: supported in part by Public Health Service grant from the National institute of Mental Health. Tetrabenazine and placebo tablets were provided by Hoffman‐La Roche. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The 13 patients were divided randomly into two groups." further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not reported |
| Blinding of outcome assessment (detection bias) | Low risk | "A frequency count of mouth movements (18), done by a psychiatrist blind to the study design was used to assess oral dyskinesia." |
| Incomplete outcome data (attrition bias) | High risk | 2/7 (29%) participants dropped out from the haloperidol group; no further details are provided for addressing the outcomes of these participants. No participants dropped out from the tetrabenazine group. |
| Selective reporting (reporting bias) | High risk | TD scale scores and extrapyramidal symptoms scale scores not fully reported |
| Other bias | Unclear risk | Insufficient information to make a judgement |
| Methods | Allocation: "randomised", no details Setting: inpatients, Denmark and Finland Duration: 18 weeks (3 weeks treatment, followed by 6 weeks washout, then crossed to another 3 weeks treatment followed by 6 weeks washout) | |
| Participants | Diagnosis: psychotic patients with TD History: duration of TD on average 5.1 years (range 0.5‐11 years); duration of antipsychotic treatment on average 18.8 years (range 5‐34 years) | |
| Interventions | Participants were down‐titrated to the lowest possible dose of haloperidol and kept stable for 4 weeks in order to keep them in an optimal mental condition, then: 1. haloperidol: dose 5.4 mg/d‐6.2 mg/d for 3 weeks (followed by 6 weeks washout and 3 weeks zuclopenthixol). N = 15 (7 during first period and 8 during second period) 2. zuclopenthixol: dose of 16.5 mg/d‐zuclopenthixol 26.6 mg/d for 3 weeks (followed by 6 weeks washout and 3 weeks haloperidol). N = 15 (8 during first period and 7 during second period) Concomitant medication: no antiparkinsonism medication was given. 4 participants (2 from each group) received benzodiazepines without changes during the study. "Other neuroleptic drugs, antidepressants and antiparkinsonian medication were not allowed" | |
| Outcomes | TD symptoms: improvement 50% TD symptoms: not any improvement TD symptoms: deterioration Sct. Hans Rating Scale for Extrapyramidal Side Effects: parkinsonism and TD symptoms (data extracted from figure using digitisation software) Unable to use ‐ Leaving the study early (not reported for the phase before crossing over) Adverse events: UKU (not reported) Mental state: BPRS (not reported) | |
| Notes | Sponsorship source: sponsorship source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were then randomized to receive either HAL or ZPT", further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not reported |
| Blinding of outcome assessment (detection bias) | Low risk | "blind evaluation of TD and parkinsonism by means of video recordings."; "All the videotapes were later randomly sequenced and blindly scored by two of the same three raters" |
| Incomplete outcome data (attrition bias) | Low risk | 20 participants entered the study, 4 dropped out during randomised phases with reasons provided |
| Selective reporting (reporting bias) | High risk | Side effects and mental state were measured (UKU, BPRS) but not fully reported |
| Other bias | Unclear risk | Insufficient information to judge |
| Methods | Allocation: randomised Setting: not reported, USA | |
| Participants | Diagnosis: schizophrenia; diagnosis of TD of a moderate or severe degree History: duration of TD not reported; "Before beginning the protocol, each participant was treated with a clinically optimal dose of haloperidol for an initial 1‐ to 6‐month stabilization period" | |
| Interventions | After the stabilisation period, each participant was withdrawn from antipsychotic treatment for 4 weeks to allow an antipsychotic‐free assessment of their dyskinetic symptoms, then: 1. clozapine plus placebo: mean dose at 293.8 ± 171.9 mg/d for 12 months. N = 25 2. haloperidol plus benztropine: mean dose at 28.5 ± 23.8 mg/d for 12 months. N = 14 | |
| Outcomes | Leaving the study early Unable to use ‐ TD symptoms (reported means only in graph) | |
| Notes | Sponsorship source: sponsorship source not reported We contacted study authors for updated data but at the time of preparing this review no more information had been received *49 were recruited for this study but only 32 completed the blind protocol. The study authors reported only on these 32 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were then blindly randomised to two different drug groups," further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Staff, patients, and all raters were blind to the drug group; one non rating physician and one nurse were non blind to dispense medication and monitor safety"; no further details were provided |
| Blinding of outcome assessment (detection bias) | Low risk | "Staff, patients, and all raters were blind to the drug group; one non rating physician and one nurse were non blind to dispense medication and monitor safety"; no further details were provided |
| Incomplete outcome data (attrition bias) | High risk | "Forty‐three patients have entered the 15‐month blinded study, and 4 have not yet finished. Seven participants have been withdrawn from the protocol (6 taking clozapine; one taking haloperidol). One subject from each treatment group was dropped for leukopenia.The other 5 clozapine subjects were dropped for noncompliance (1 patient), decompensation (1 patient), seizure (1 patient), hypotension (1 patient), and ECG changes (1 patient)." Data were reported for completers only. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all predefined outcomes were reported. Efficacy data reported in graphs as means only. A study protocol is needed for firm conclusions. |
| Other bias | Unclear risk | Preliminary results as 4 subjects had not completed the study. |
DSM: Diagnostic and Statistical Manual of Mental Disorders
EPS: Extrapyramidal symptoms
FGA: first‐generation antipsychotic
ICD‐9 ‐ International Classification of Diseases 9th edition
ITT: intention‐to‐treat
RDC ‐ Research Diagnostic Criteria
TD: tardive dyskinesia
Rating Scales:
Global impression
CGI ‐ Clinical Global Impression
Mental state
BPRS ‐ Brief Psychiatric Rating Scale
Adverse events
AIMS ‐ Abnormal Involuntary Movement Scale
EPS ‐ Extrapyramidal Symptoms Scale
ESRS ‐ Extrapyramidal side effect rating scale
GSES ‐ General Side Effects Scale
NOSIE ‐ Nurses Observational Scale of Inpatients Evaluation
SAS ‐ Simpson Angus Scale
SDS ‐ Simpson Dyskinesia Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: randomised Participants: schizophrenia (DSM‐III‐R), N = 26, 14 with TD Intervention: haloperidol vs clozapine Outcomes: no data available for AIMS in clozapine group, the study also reported on plasma homovanillic acid levels, an outcome not relevant for this review We contacted the study authors but no information was received. This study is over 15years old and was excluded. | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: random not mentioned in this short trial registration Participants: elderly patients, many started treatment with antipsychotics at start of study (no baseline TD) | |
| Allocation: random Outcomes: no outcome data was provided for the first period before cross‐over. We were unable to find contact details for the study authors; study is over 35 years old and was excluded | |
| Allocation: random | |
| Allocation: not randomised | |
| Allocation: randomised Outcomes: no usable efficacy data; only P values were reported. We were unable to find contact details for the study authors; study is over 25 years old and was excluded | |
| Allocation: not randomised | |
| Allocation: randomised Participants: people with dementia, not schizophrenia, not TD at baseline | |
| Allocation: not randomised | |
| Allocation: 'randomly assigned' | |
| Allocation: randomised, cross‐over Interventions: tiapride vs placebo Outcomes: doppler ratings, none before cross‐over | |
| Allocation: randomisation not mentioned Intervention: 1‐stepholidine (herbal product that has shown antipsychotic properties in animals) versus placebo Assessed and data extracted by Sai Zhao | |
| Allocation: "allocated by toss of a coin" Interventions: clozapine vs placebo | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: random Interventions: fluphenazine ethanoate vs pipothiazine palmitate | |
| Allocation: random Interventions: ethopropazine vs benztropine | |
| Allocation: random Interventions: haloperidol decanoate vs fluphenazine decanoate | |
| Allocation: random Interventions: clozapine vs risperidone | |
| Allocation: not randomised | |
| Allocation: random Interventions: haloperidol decanoate vs fluphenazine decanoate | |
| Allocation: random Interventions: quetiapine vs continuation of usual antipsychotic | |
| Allocation: not randomised | |
| Allocation: not randomised, review article | |
| Allocation: not randomised | |
| Allocation: random Interventions: trifluoperazine high dose vs trifluoperazine low dose vs placebo | |
| Allocation: not randomised | |
| Allocation: random Interventions: fluphenazine decanoate vs placebo | |
| Allocation: not randomised | |
| Allocation: randomised Interventions: olanzapine vs "conventional antipsychotic drugs" Outcomes: author contacted for data regarding people with TD ‐ data no longer available | |
| Allocation: randomised The study is over 35 years old and we were unable to identify contact details for the author | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: random Participants: schizophrenia, no established, stable TD diagnosis at baseline Interventions: clozapine vs haloperidol | |
| Allocation: the randomisation was just in one arm of the study “Haloperidol + biperiden for 4 weeks (phase 2 and phase 3 in randomized sequence)”. All other arms thioridazine for 3 months, haloperidol for 4 weeks; thioridazine for 4 weeks, clozapine for 4 weeks were not Participants: elderly people with psychiatric history and neuroleptic‐induced TD Interventions: biperiden vs no treatment as an adjunct to haloperidol | |
| Allocation: not randomised, cohort study | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: randomised Interventions: withdrawal of fluphenazine decanoate vs continuation | |
| Allocation: "randomly assigned" | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: randomised Interventions: trifluoperazine withdrawal vs trifluoperazine continuation | |
| Allocation: randomised Dr Herz kindly replied to our request for more information. Unfortunately, individual baseline and endpoint AIMS score are no longer available | |
| Allocation: not randomised | |
| Allocation: quasi‐randomised | |
| Allocation: not randomised | |
| Allocation: Not randomised ("by alternate allocation") | |
| Allocation: random Interventions: clozapine vs olanzapine | |
| Allocation: not randomised Participants: chronic schizophrenia with TD, N = 2 Interventions: chlorpromazine schedule A vs chlorpromazine schedule B. Treatments in the 2 groups were the same except for timing of the doses (frequency and withdrawal) | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: randomised Dr Johnson kindly replied to our letter. No further data available from the first author | |
| Allocation: randomised Interventions: brief intermittent antipsychotic treatment vs fluphenazine decanoate | |
| Allocation: not randomised | |
| Allocation: not randomised, case‐control study Dr Kalachnick kindly provided additional information. After randomisation clinicians reviewed group allocations and re‐assigned selected individuals on clinical grounds | |
| Allocation: not randomised, 2 case series | |
| Allocation: randomised Participants: schizophrenia and TD (Schooler and Kane criteria) Interventions: olanzapine (5 mg‐20 mg/d) with 1 set of intermittent dose‐reduction periods versus olanzapine (5 mg‐20 mg/d) with a different set of intermittent dose reduction periods | |
| Allocation: not randomised | |
| Allocation: random Interventions: haloperidol vs risperidone | |
| Allocation: randomised, cross‐over. Participants: people with schizophrenia. Interventions: thiopropazine vs trifluoperazine vs placebo. Outcomes: no usable data. Dr Lal kindly replied to inquiry. Unable to extract data from the first segment. Jadad score = 4/5 | |
| Allocation: not randomised, cohort study | |
| Allocation: not randomised | |
| Allocation: randomised Interventions: fluphenazine withdrawal vs continuation | |
| Allocation: randomised Intervention: physostigmine vs bromocriptine vs benztropine vs haloperidol for 1 day, then crossed over. Outcomes: no outcome data provided for the first period before cross‐over. We contacted the study author but no information received. Study is over 25 years old years old and was excluded | |
| Allocation: not randomised, cohort study | |
| Allocation: not randomised: naturalistic observational study | |
| Allocation: not randomised | |
| Allocation: "patients were divided into pairs" | |
| Allocation: randomised Interventions: low vs conventional‐dose maintenance therapy with fluphenazine decanoate | |
| Allocation: random Interventions: intermittent pimozide vs fluphenazine decanoate | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: randomised cross‐over Intervention: chlorprothixene vs haloperidol vs perphenazine vs haloperidol + biperiden Outcomes: no outcome data provided for the first period before cross‐over We contacted the study author but no reply. Study is 30 years old and was excluded. | |
| Allocation: randomised Interventions: haloperidol dose reduction vs maintained dose | |
| Allocation: randomised | |
| Allocation: randomisation not mentioned | |
| Allocation: not randomised | |
| Allocation: not randomised Dr Paulsen kindly provided additional information about this double‐blind study | |
| Allocation: not randomised | |
| Allocation: random Interventions: FGA vs second generation antipsychotic | |
| Allocation: randomised | |
| Allocation: not randomised | |
| Allocation: randomised, double‐blind, cross‐over study Participants: people with schizophrenia Intervention: sulpiride (Dogmatil) 300 mg‐ 1200 mg/d Outcomes: no usable data. Drs Marsden and Quinn kindly replied to our letter, but no data suitable for this review could be provided | |
| Allocation: not randomised | |
| Allocation: not described | |
| Allocation: not random | |
| Allocation: random Interventions: haloperidol vs olanzapine | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: randomised Interventions: sulpiride vs placebo Outcomes: no outcome data provided for the first period before cross‐over. We were unable to find contact details for the study authors; this study is over 25 years old and was excluded | |
| Allocation: not randomised | |
| Allocation: not randomised, cohort study | |
| Allocation: randomised Interventions: thiopropazate vs placebo Outcomes: no outcome data provided for the first period before cross‐over. We were unable to find contact details for the study authors; this study is 45 years old and was excluded | |
| Allocation: randomised Intervention: abrupt antipsychotic withdrawal versus continuation of antipsychotic medication | |
| Allocation: not randomised, cohort study | |
| Allocation: not randomised, cohort study | |
| Allocation: not randomised | |
| Allocation: randomised Participants: schizophrenia (DSM‐III‐R), majority with TD Intervention: amisulpride versus haloperidol Outcomes: schizophrenia symptom changes, especially negative symptoms, adverse events, and TD as adverse event. We excluded this reference because, although the majority had TD at baseline and the intervention drugs qualified, the drugs were not examined as a treatment for TD (as our inclusion criteria demand), but for negative symptoms of schizophrenia | |
| Allocation: not randomised, cohort study | |
| Allocation: randomised Dr Spohn kindly replied to our request for further information. Data on baseline and endpoint TD not available | |
| Allocation: randomised Dr Spohn kindly replied to our letter, but no further data were available | |
| Allocation: randomised | |
| Allocation: randomised | |
| Allocation: random | |
| Allocation: random Interventions: olanzapine versus haloperidol | |
| Allocation: not randomised ‐ allocated to treatment group in a "nonsystematic" fashion, but then participants were re‐allocated to alternate groups based on clinical judgement | |
| Allocation: random Interventions: olanzapine 1 mg vs olanzapine 10 mg versus placebo | |
| Allocation: random Interventions: haloperidol vs risperidone | |
| Allocation: randomised Interventions: fluphenazine/flupenthixol decanoate continuation vs withdrawal | |
| Allocation: not randomised, cohort study | |
| Allocation: not randomised | |
| Allocation: not randomised | |
| Allocation: not randomised, cohort study | |
| Allocation: randomised Intervention: flunarizine (calcium channel antagonist) vs placebo Assessed and data extracted by Sai Zhao |
FGA: first‐generation antipsychotic
IV = intravenous
TD: tardive dyskinesia
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A six month, rater blind comparison of quetiapine and risperidone in the treatment of tardive dyskinesia in patients with schizophrenia |
| Methods | Allocation: randomised Blindness: rater blind Design: not reported Setting: not reported Duration: 6 months |
| Participants | People with schizophrenia with TD |
| Interventions | 1. Quetiapine 2. Risperidone |
| Outcomes | Prevalence and severity of abnormal involuntary movements |
| Starting date | |
| Contact information | |
| Notes | Very limited information from two trials registries. We were unable to locate author contact details. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
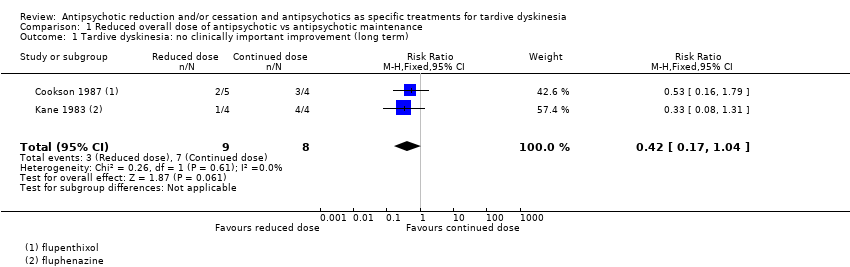
| 1 Tardive dyskinesia: no clinically important improvement (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| Analysis 1.1  Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 1 Tardive dyskinesia: no clinically important improvement (long term). | ||||
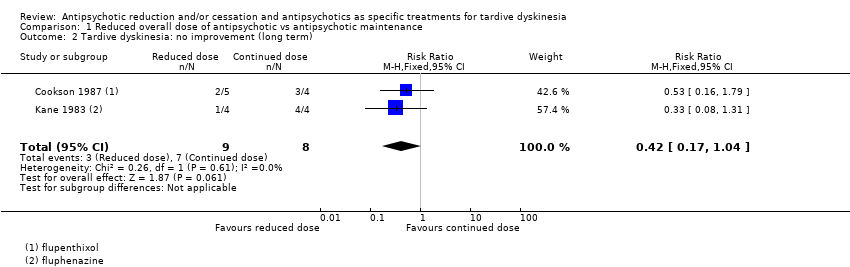
| 2 Tardive dyskinesia: no improvement (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| Analysis 1.2  Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 2 Tardive dyskinesia: no improvement (long term). | ||||
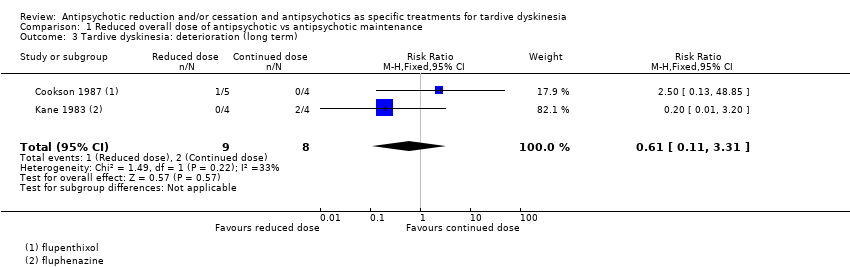
| 3 Tardive dyskinesia: deterioration (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.11, 3.31] |
| Analysis 1.3  Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 3 Tardive dyskinesia: deterioration (long term). | ||||
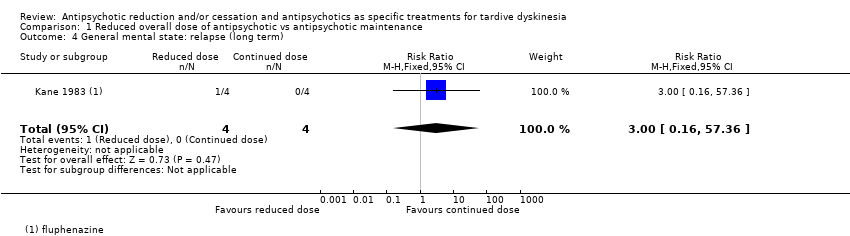
| 4 General mental state: relapse (long term) Show forest plot | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.16, 57.36] |
| Analysis 1.4  Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 4 General mental state: relapse (long term). | ||||
| 5 Acceptability of the treatment: leaving the study early (long term) Show forest plot | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.99] |
| Analysis 1.5  Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 5 Acceptability of the treatment: leaving the study early (long term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement (medium term) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.23, 0.89] |
| Analysis 2.1  Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 1 Tardive dyskinesia: no clinically important improvement (medium term). | ||||
| 2 Tardive dyskinesia: average endpoint score (AIMS, high = poor) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐8.60, ‐2.40] |
| Analysis 2.2  Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 2 Tardive dyskinesia: average endpoint score (AIMS, high = poor) (medium term). | ||||
| 3 General mental state: average endpoint score (BPRS, high = poor) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐10.48, 1.88] |
| Analysis 2.3  Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 3 General mental state: average endpoint score (BPRS, high = poor) (medium term). | ||||
| 4 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| Analysis 2.4  Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 4 Acceptability of the treatment: leaving the study early (medium term). | ||||
| 5 Adverse effects: use of antiparkinsonism drugs (medium term) Show forest plot | 1 | 48 | Risk Ratio (IV, Fixed, 95% CI) | 2.08 [0.74, 5.86] |
| Analysis 2.5  Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 5 Adverse effects: use of antiparkinsonism drugs (medium term). | ||||
| 5.1 Haloperidol | 1 | 12 | Risk Ratio (IV, Fixed, 95% CI) | 2.0 [0.56, 7.09] |
| 5.2 Risperidone | 1 | 36 | Risk Ratio (IV, Fixed, 95% CI) | 2.26 [0.37, 13.60] |
| 6 Adverse effects: parkinsonism ‐ average endpoint score (ESRS) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.25, 0.45] |
| Analysis 2.6  Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 6 Adverse effects: parkinsonism ‐ average endpoint score (ESRS) (medium term). | ||||
| 7 Adverse effects: dystonia ‐ average endpoint score (ESRS) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.76, 0.36] |
| Analysis 2.7  Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 7 Adverse effects: dystonia ‐ average endpoint score (ESRS) (medium term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
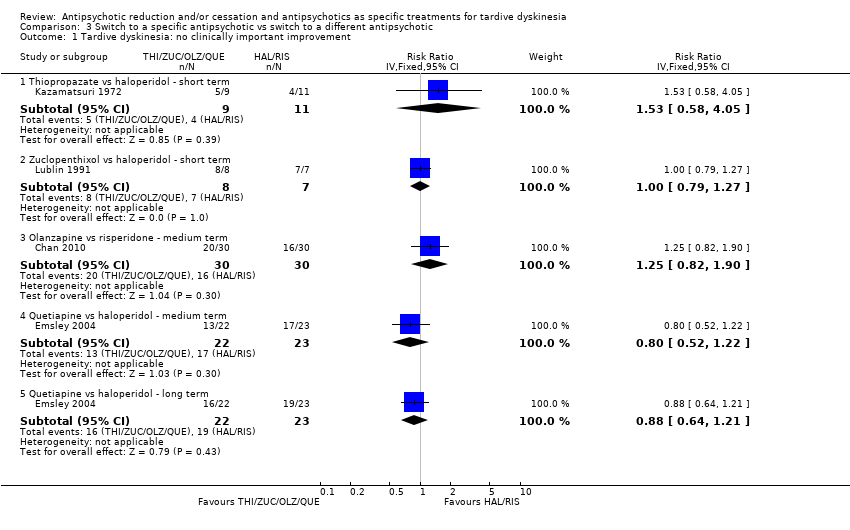
| 1 Tardive dyskinesia: no clinically important improvement Show forest plot | 4 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 1 Tardive dyskinesia: no clinically important improvement. | ||||
| 1.1 Thiopropazate vs haloperidol ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 1.53 [0.58, 4.05] |
| 1.2 Zuclopenthixol vs haloperidol ‐ short term | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.79, 1.27] |
| 1.3 Olanzapine vs risperidone ‐ medium term | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.82, 1.90] |
| 1.4 Quetiapine vs haloperidol ‐ medium term | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 1.5 Quetiapine vs haloperidol ‐ long term | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
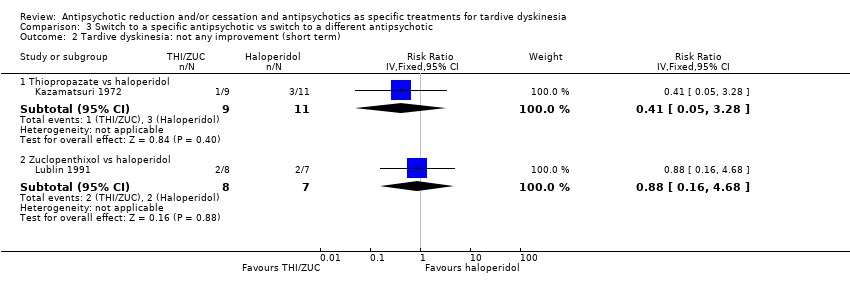
| 2 Tardive dyskinesia: not any improvement (short term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 2 Tardive dyskinesia: not any improvement (short term). | ||||
| 2.1 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.41 [0.05, 3.28] |
| 2.2 Zuclopenthixol vs haloperidol | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
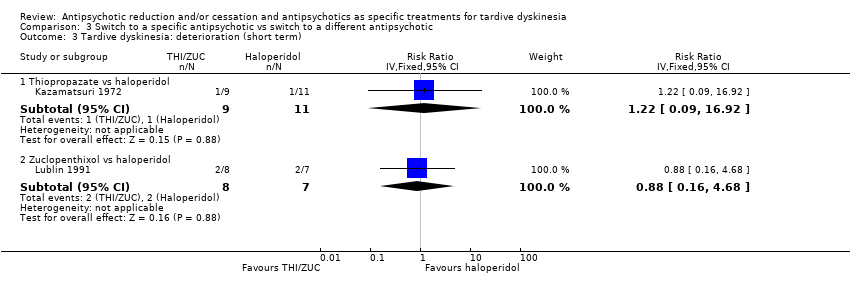
| 3 Tardive dyskinesia: deterioration (short term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 3 Tardive dyskinesia: deterioration (short term). | ||||
| 3.1 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 1.22 [0.09, 16.92] |
| 3.2 Zuclopenthixol vs haloperidol | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
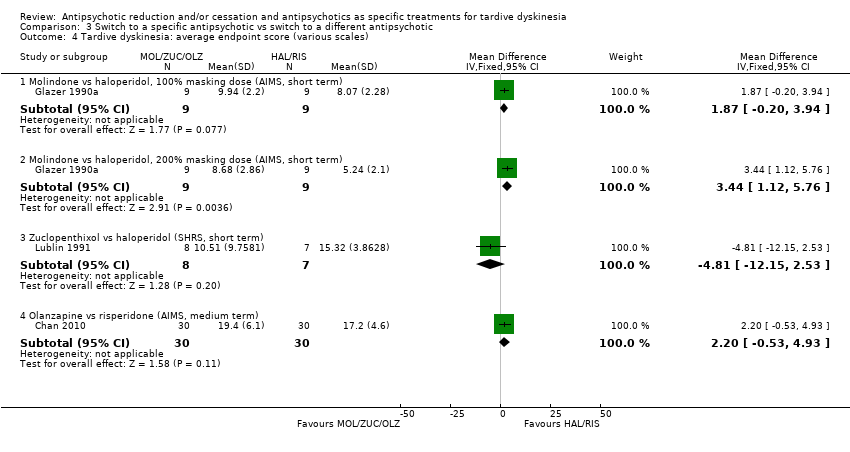
| 4 Tardive dyskinesia: average endpoint score (various scales) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 4 Tardive dyskinesia: average endpoint score (various scales). | ||||
| 4.1 Molindone vs haloperidol, 100% masking dose (AIMS, short term) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 1.87 [‐0.20, 3.94] |
| 4.2 Molindone vs haloperidol, 200% masking dose (AIMS, short term) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.44 [1.12, 5.76] |
| 4.3 Zuclopenthixol vs haloperidol (SHRS, short term) | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 4.4 Olanzapine vs risperidone (AIMS, medium term) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐0.53, 4.93] |
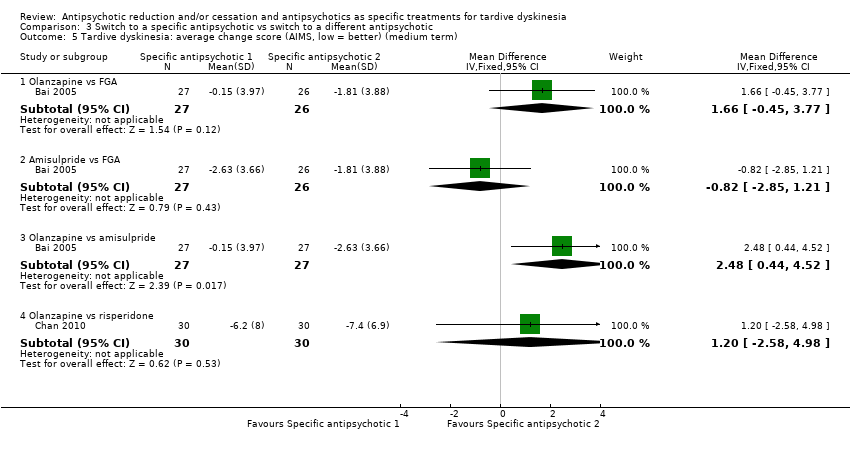
| 5 Tardive dyskinesia: average change score (AIMS, low = better) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5 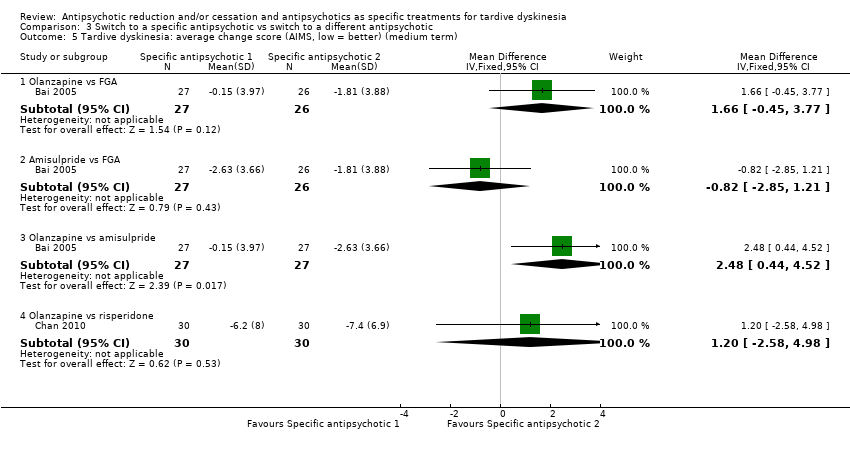 Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 5 Tardive dyskinesia: average change score (AIMS, low = better) (medium term). | ||||
| 5.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 1.66 [‐0.45, 3.77] |
| 5.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐2.85, 1.21] |
| 5.3 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 2.48 [0.44, 4.52] |
| 5.4 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.58, 4.98] |
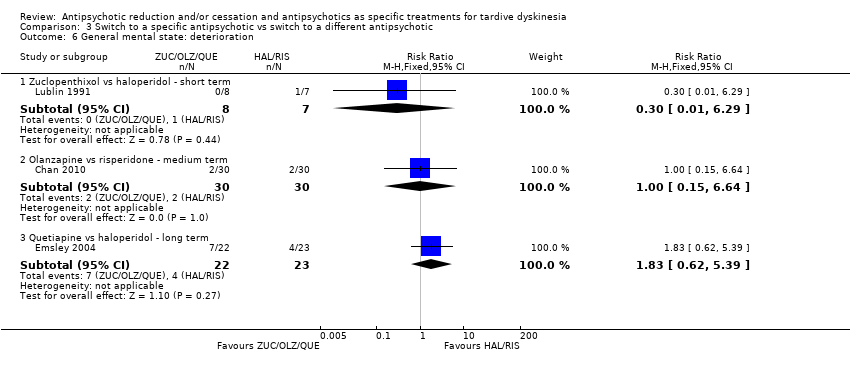
| 6 General mental state: deterioration Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 6 General mental state: deterioration. | ||||
| 6.1 Zuclopenthixol vs haloperidol ‐ short term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.29] |
| 6.2 Olanzapine vs risperidone ‐ medium term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.64] |
| 6.3 Quetiapine vs haloperidol ‐ long term | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.62, 5.39] |
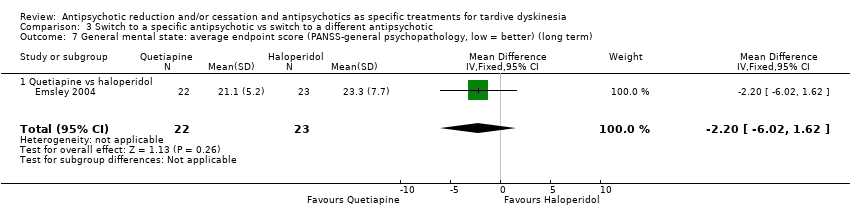
| 7 General mental state: average endpoint score (PANSS‐general psychopathology, low = better) (long term) Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.02, 1.62] |
| Analysis 3.7  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 7 General mental state: average endpoint score (PANSS‐general psychopathology, low = better) (long term). | ||||
| 7.1 Quetiapine vs haloperidol | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.02, 1.62] |
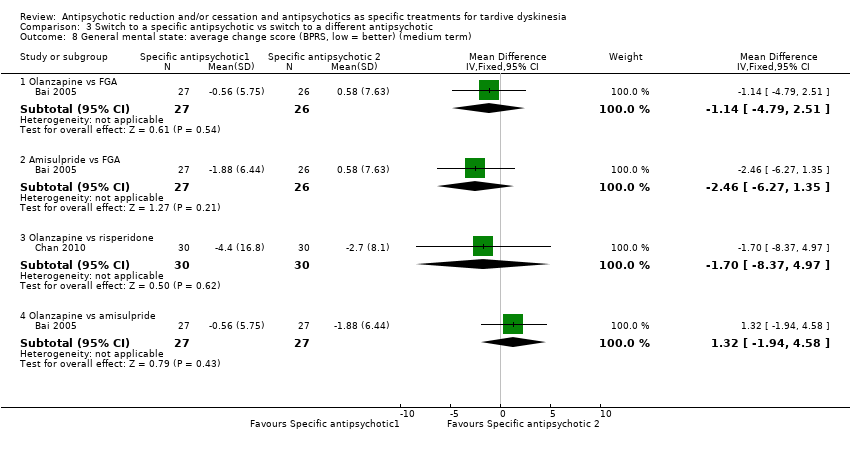
| 8 General mental state: average change score (BPRS, low = better) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.8  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 8 General mental state: average change score (BPRS, low = better) (medium term). | ||||
| 8.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐4.79, 2.51] |
| 8.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐2.46 [‐6.27, 1.35] |
| 8.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐8.37, 4.97] |
| 8.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [‐1.94, 4.58] |
| 9 Acceptability of the treatment: leaving the study early (short term) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.9  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 9 Acceptability of the treatment: leaving the study early (short term). | ||||
| 9.1 Molindone vs haloperidol | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.44] |
| 10 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 3 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.10  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 10 Acceptability of the treatment: leaving the study early (medium term). | ||||
| 10.1 Olanzapine vs FGA | 1 | 56 | Risk Ratio (IV, Fixed, 95% CI) | 1.86 [0.18, 19.38] |
| 10.2 Amisulpride vs FGA | 1 | 55 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.06, 14.65] |
| 10.3 Olanzapine vs amisulpride | 1 | 57 | Risk Ratio (IV, Fixed, 95% CI) | 1.93 [0.19, 20.12] |
| 10.4 Olanzapine vs risperidone | 2 | 170 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.57, 0.95] |
| 10.5 Olanzapine vs quetiapine | 1 | 116 | Risk Ratio (IV, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 10.6 Olanzapine vs ziprasidone | 1 | 82 | Risk Ratio (IV, Fixed, 95% CI) | 0.77 [0.56, 1.05] |
| 10.7 Quetiapine vs risperidone | 1 | 118 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| 10.8 Quetiapine vs ziprasidone | 1 | 90 | Risk Ratio (IV, Fixed, 95% CI) | 1.10 [0.86, 1.40] |
| 10.9 Ziprasidone vs risperidone | 1 | 84 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 11 Acceptability of the treatment: leaving the study early (long term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.11  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 11 Acceptability of the treatment: leaving the study early (long term). | ||||
| 11.1 Clozapine vs haloperidol | 1 | 39 | Risk Ratio (IV, Fixed, 95% CI) | 3.36 [0.45, 25.16] |
| 11.2 Quetiapine vs haloperidol | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 1.31 [0.63, 2.69] |
| 12 Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.12  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 12 Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs). | ||||
| 12.1 Risperidone vs haloperidol (medium term) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.34, 1.35] |
| 12.2 Quetiapine vs haloperidol (long term) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.96] |
| 13 Adverse effects: parkinsonism (SHRS) ‐ average endpoint scores (short term) Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| Analysis 3.13  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 13 Adverse effects: parkinsonism (SHRS) ‐ average endpoint scores (short term). | ||||
| 13.1 Zuclopenthixol vs haloperidol | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 14 Adverse effects: parkinsonism (SAS, ESRS, low = better) ‐ average change score (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.14  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 14 Adverse effects: parkinsonism (SAS, ESRS, low = better) ‐ average change score (medium term). | ||||
| 14.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐2.55, 0.85] |
| 14.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.45, 1.45] |
| 14.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.33, ‐0.07] |
| 14.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐2.44, 1.74] |
| 15 Adverse effects: dyskinesia (ESRS, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.15  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 15 Adverse effects: dyskinesia (ESRS, low = better) ‐ average change score (medium term). | ||||
| 15.1 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.91, 1.51] |
| 16 Adverse effects: akathisia (BAS, ESRS, low = better) ‐ average change scores (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.16  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 16 Adverse effects: akathisia (BAS, ESRS, low = better) ‐ average change scores (medium term). | ||||
| 16.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.30, 0.46] |
| 16.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.42, 0.20] |
| 16.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.76, 0.16] |
| 16.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.12, 0.50] |
| 17 Adverse effects: dystonia (ESRS, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.17  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 17 Adverse effects: dystonia (ESRS, low = better) ‐ average change score (medium term). | ||||
| 17.1 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.41, 0.01] |
| 18 Adverse effects: general adverse events (UKU, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.18  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 18 Adverse effects: general adverse events (UKU, low = better) ‐ average change score (medium term). | ||||
| 18.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐1.85, 2.01] |
| 18.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.33, 1.23] |
| 18.3 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐0.93, 2.19] |
| 19 General global state: average change score (CGI) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.19  Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 19 General global state: average change score (CGI) (medium term). | ||||
| 19.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.41, 0.27] |
| 19.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.47, 0.09] |
| 19.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.61, 0.81] |
| 19.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.19, 0.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesias: no clinically important improvement (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.51, 2.23] |
| Analysis 4.1  Comparison 4 Specific antipsychotic vs other drugs, Outcome 1 Tardive dyskinesias: no clinically important improvement (medium term). | ||||
| 1.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.51, 2.23] |
| 2 Tardive dyskinesia: no improvement (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 2.57 [0.35, 18.68] |
| Analysis 4.2  Comparison 4 Specific antipsychotic vs other drugs, Outcome 2 Tardive dyskinesia: no improvement (medium term). | ||||
| 2.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 2.57 [0.35, 18.68] |
| 3 Tardive dyskinesia: deterioration (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| Analysis 4.3  Comparison 4 Specific antipsychotic vs other drugs, Outcome 3 Tardive dyskinesia: deterioration (medium term). | ||||
| 3.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| 4 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [0.25, 76.54] |
| Analysis 4.4  Comparison 4 Specific antipsychotic vs other drugs, Outcome 4 Acceptability of the treatment: leaving the study early (medium term). | ||||
| 4.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [0.25, 76.54] |

Message from one of the participants of the public and patient involvement consultation of service user perspectives on tardive dyskinesia research
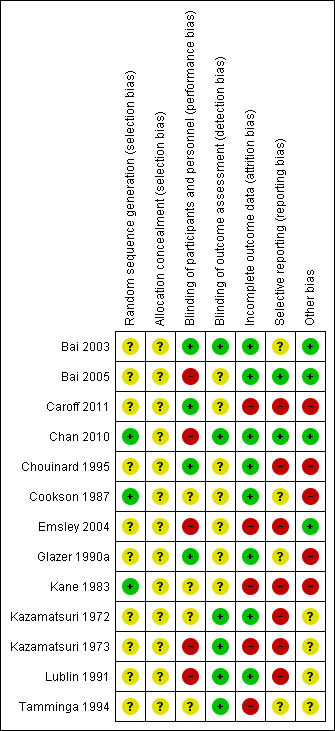
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
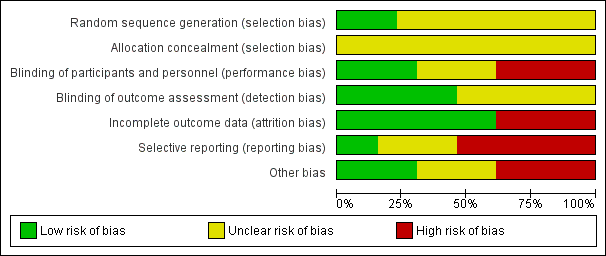
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
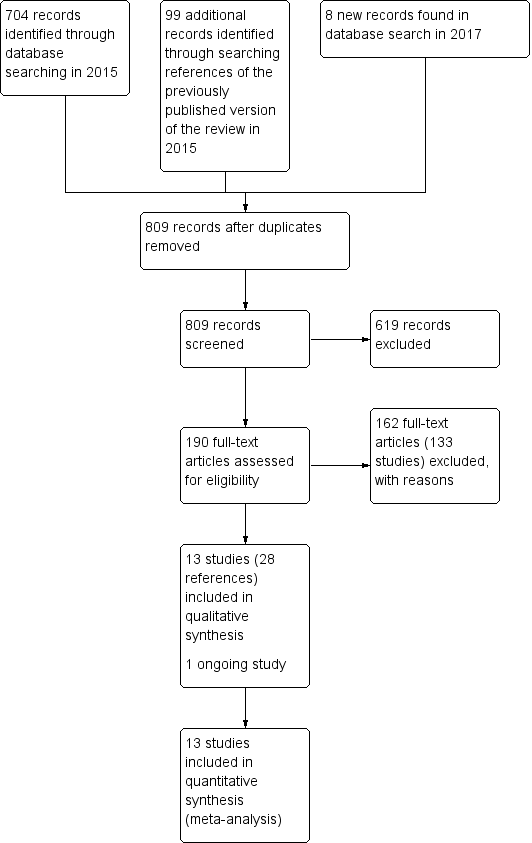
Study flow diagram for 2015 and 2017 searches for this review
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 1 Tardive dyskinesia: no clinically important improvement (long term).
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 2 Tardive dyskinesia: no improvement (long term).
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 3 Tardive dyskinesia: deterioration (long term).
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 4 General mental state: relapse (long term).
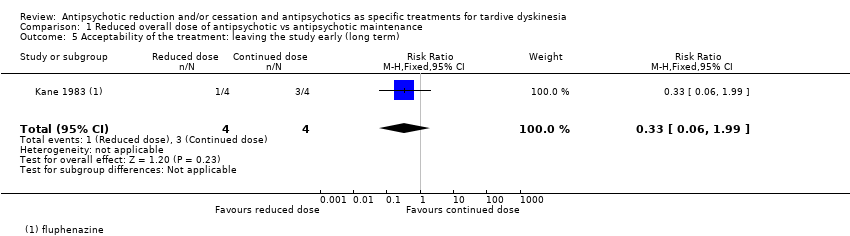
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 5 Acceptability of the treatment: leaving the study early (long term).
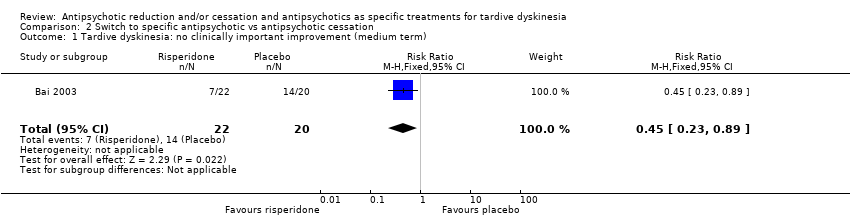
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 1 Tardive dyskinesia: no clinically important improvement (medium term).
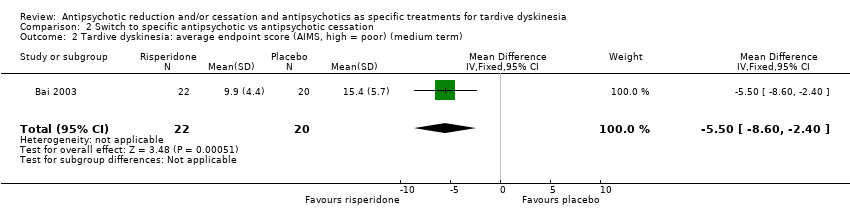
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 2 Tardive dyskinesia: average endpoint score (AIMS, high = poor) (medium term).
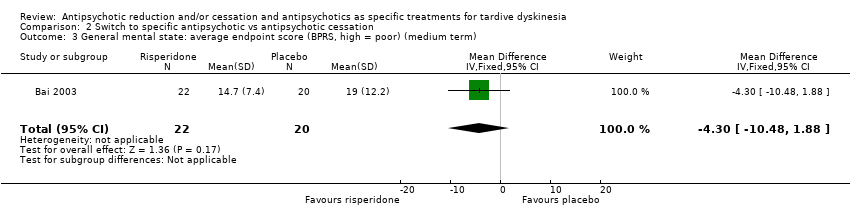
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 3 General mental state: average endpoint score (BPRS, high = poor) (medium term).
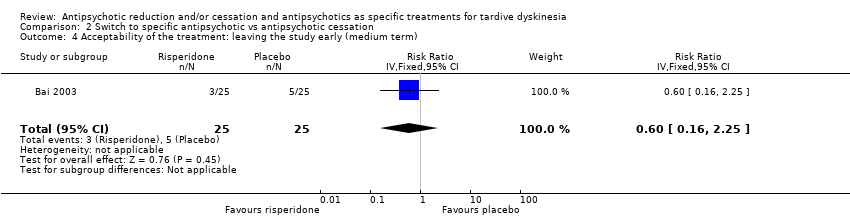
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 4 Acceptability of the treatment: leaving the study early (medium term).
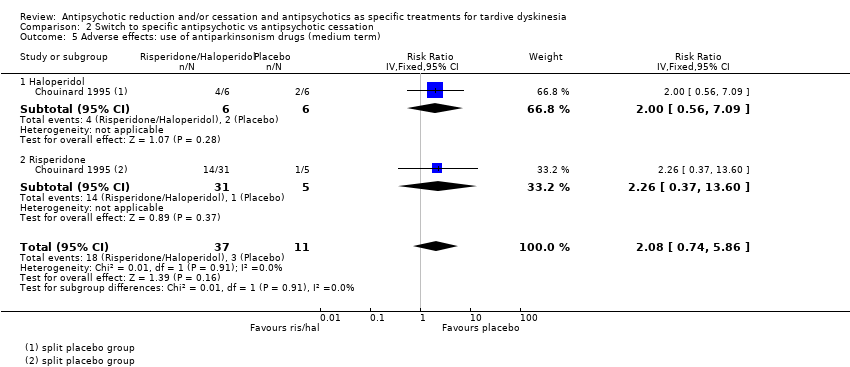
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 5 Adverse effects: use of antiparkinsonism drugs (medium term).
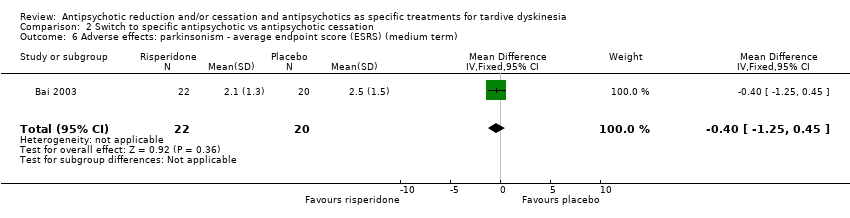
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 6 Adverse effects: parkinsonism ‐ average endpoint score (ESRS) (medium term).
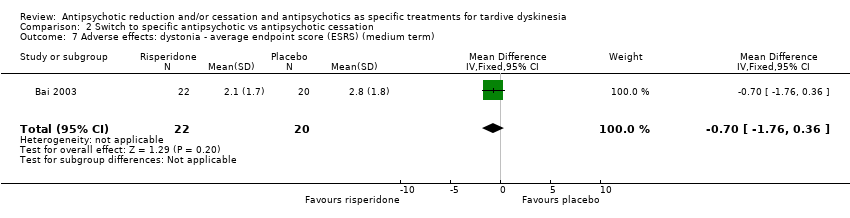
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 7 Adverse effects: dystonia ‐ average endpoint score (ESRS) (medium term).
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 1 Tardive dyskinesia: no clinically important improvement.
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 2 Tardive dyskinesia: not any improvement (short term).
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 3 Tardive dyskinesia: deterioration (short term).
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 4 Tardive dyskinesia: average endpoint score (various scales).
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 5 Tardive dyskinesia: average change score (AIMS, low = better) (medium term).
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 6 General mental state: deterioration.
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 7 General mental state: average endpoint score (PANSS‐general psychopathology, low = better) (long term).
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 8 General mental state: average change score (BPRS, low = better) (medium term).
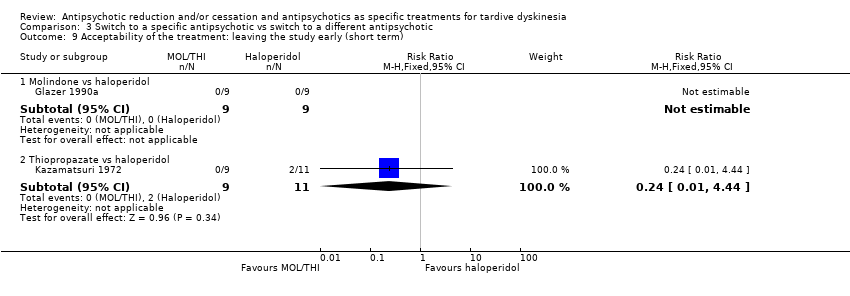
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 9 Acceptability of the treatment: leaving the study early (short term).
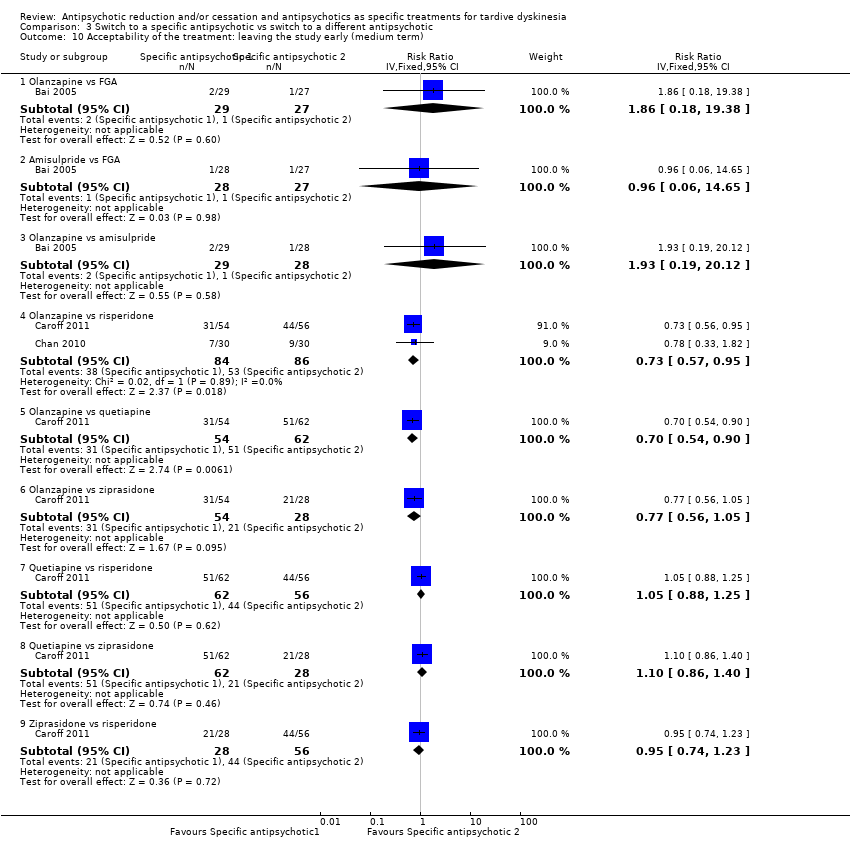
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 10 Acceptability of the treatment: leaving the study early (medium term).
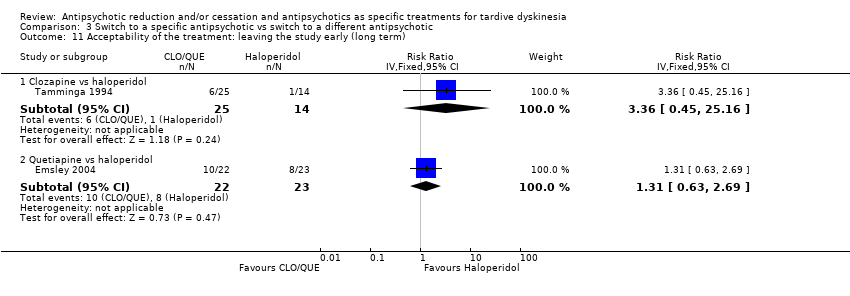
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 11 Acceptability of the treatment: leaving the study early (long term).
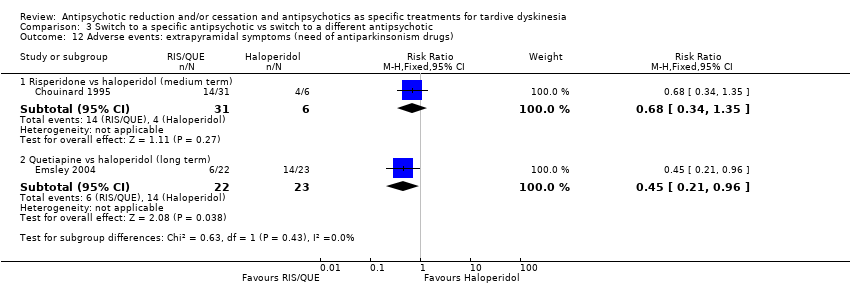
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 12 Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs).
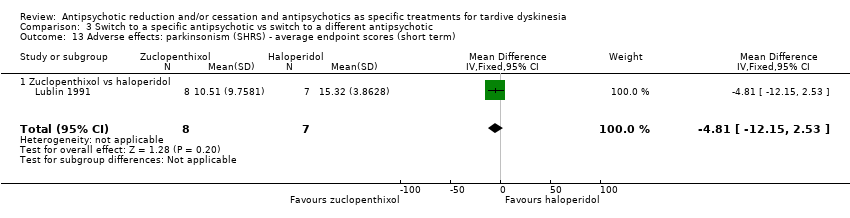
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 13 Adverse effects: parkinsonism (SHRS) ‐ average endpoint scores (short term).
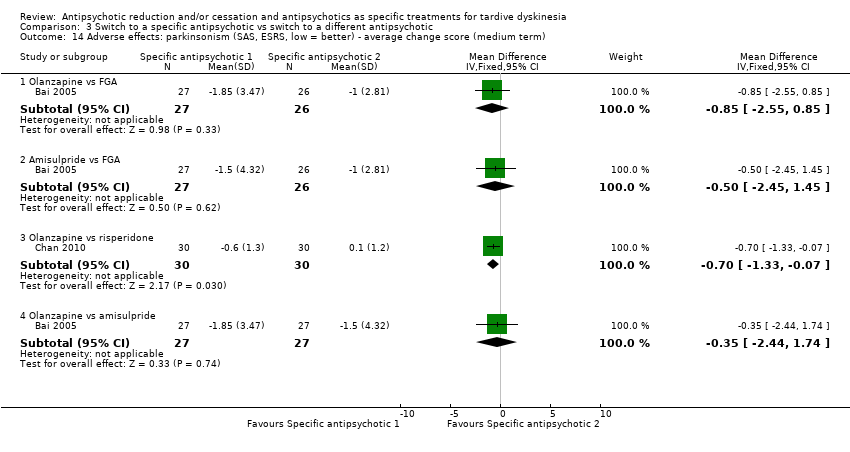
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 14 Adverse effects: parkinsonism (SAS, ESRS, low = better) ‐ average change score (medium term).
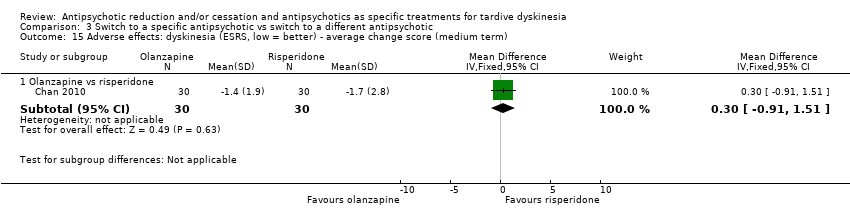
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 15 Adverse effects: dyskinesia (ESRS, low = better) ‐ average change score (medium term).
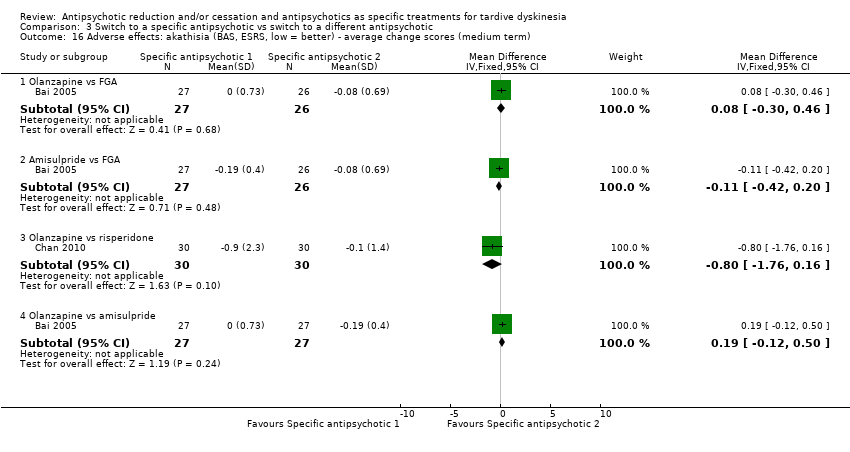
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 16 Adverse effects: akathisia (BAS, ESRS, low = better) ‐ average change scores (medium term).
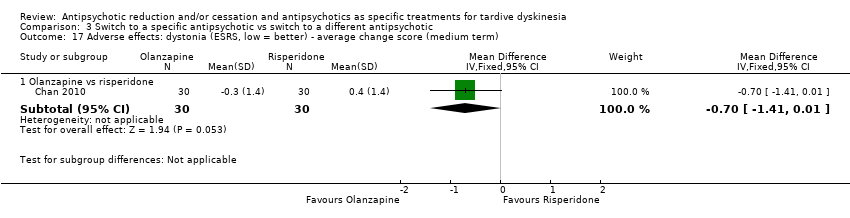
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 17 Adverse effects: dystonia (ESRS, low = better) ‐ average change score (medium term).
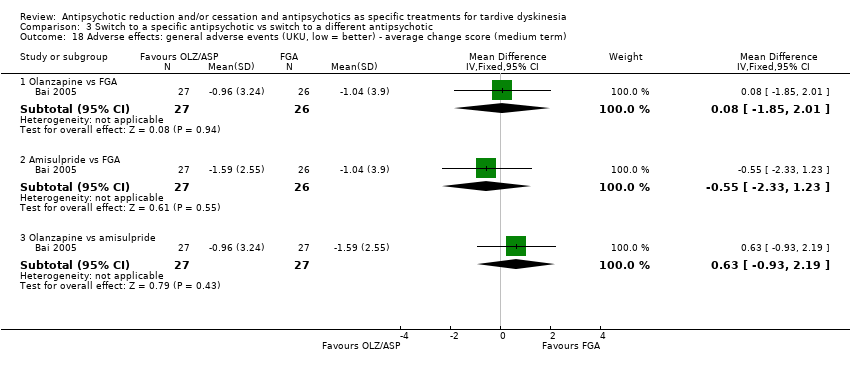
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 18 Adverse effects: general adverse events (UKU, low = better) ‐ average change score (medium term).
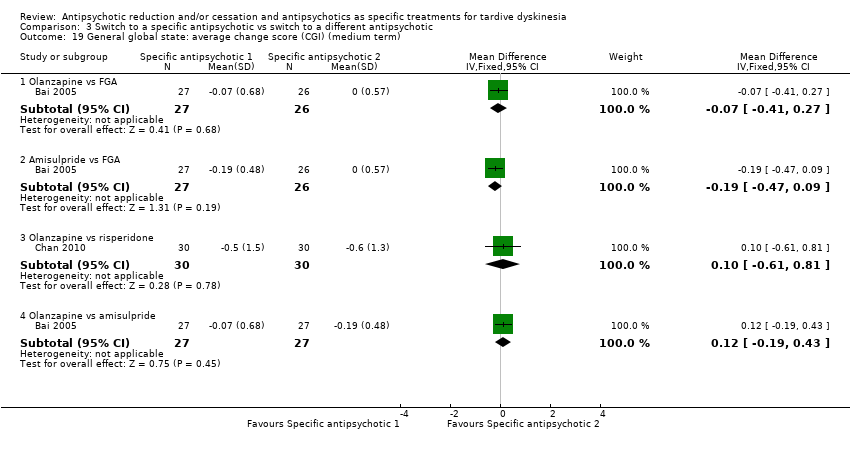
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 19 General global state: average change score (CGI) (medium term).
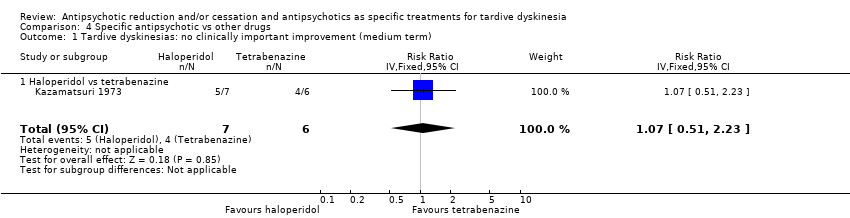
Comparison 4 Specific antipsychotic vs other drugs, Outcome 1 Tardive dyskinesias: no clinically important improvement (medium term).
Comparison 4 Specific antipsychotic vs other drugs, Outcome 2 Tardive dyskinesia: no improvement (medium term).
Comparison 4 Specific antipsychotic vs other drugs, Outcome 3 Tardive dyskinesia: deterioration (medium term).
Comparison 4 Specific antipsychotic vs other drugs, Outcome 4 Acceptability of the treatment: leaving the study early (medium term).
| Study ID | Participants – people with: | Intervention | Comparison for review | Cochrane Review |
| Tardive dyskinesia | 1‐stepholidine vs placebo | 1‐stepholidine for schizophrenia | ‐ | |
| Schizophrenia | Amisulpride vs haloperidol | Amisulpride versus haloperidol for schizophrenia | ||
| Tardive dyskinesia | Biperiden vs no treatment | Anticholinergic drugs for tardive dyskinesia | ||
| Chlorprothixene versus haloperidol vs perphenazine vs haloperidol + biperiden | ||||
| Biperiden vs placebo | ||||
| Schizophrenia | Ethopropazine vs benztropine | Anticholinergics for parkinsonism | ||
| Abrupt neuroleptic cessation vs neuroleptic maintenance | Antipsychotic reduction or withdrawal for schizophrenia | |||
| Abrupt neuroleptic cessation vs neuroleptic maintenance | ||||
| Fluphenazine/flupenthixol decanoate continuation vs withdrawal | ||||
| Withdrawal of fluphenazine decanoate vs continuation | ||||
| Trifluoperazine withdrawal vs trifluoperazine continuation | ||||
| Dose reduction vs maintenance (both arms used flupenthixol decanoate) | ||||
| Olanzapine with different timings of dose‐reduction periods | ||||
| Fluphenazine withdrawal vs continuation | ||||
| Low‐ vs conventional‐dose maintenance therapy with fluphenazine decanoate | ||||
| Haloperidol dose reduction vs maintained dose | ||||
| Abrupt neuroleptic cessation vs neuroleptic maintenance | ||||
| Tardive dyskinesia | Flunarizine vs placebo | Calcium channel blockers for neuroleptic‐induced tardive dyskinesia | ||
| Schizophrenia | Chlorpromazine schedule A vs chlorpromazine schedule B | Chlorpromazine timing of dose for schizophrenia. | ||
| Tardive dyskinesia | Chlorprothixene vs haloperidol vs perphenazine vs haloperidol + biperiden | Chlorprothixene for schizophrenia. | ||
| Schizophrenia | Clozapine vs haloperidol | Clozapine versus haloperidol for schizophrenia | ||
| Clozapine vs haloperidol | ||||
| Clozapine vs olanzapine | Clozapine versus olanzapine for schizophrenia | |||
| Clozapine vs olanzapine | ||||
| Gilles de la Tourette's, Huntington's disease and drug‐induced atypical dyskinesia | Clozapine vs placebo | Clozapine versus placebo for schizophrenia. | ||
| Schizophrenia | Clozapine versus risperidone | Clozapine versus risperidone for schizophrenia | ||
| Haloperidol decanoate vs fluphenazine decanoate | Depot fluphenazine for schizophrenia | |||
| Fluphenazine decanoate vs haloperidol decanoate | ||||
| Fluphenazine decanoate vs placebo | ||||
| Fluphenazine decanoate vs intermittent pimozide | ||||
| Fluphenazine decanoate vs vitamin B complex | ||||
| Fluphenazine ethanoate vs pipothiazine palmitate | ||||
| Haloperidol decanoate vs fluphenazine decanoate | Depot haloperidol decanoate for schizophrenia. | |||
| Fluphenazine ethanoate vs pipothiazine palmitate | Depot pipothiazine for schizophrenia. | |||
| Progabide vs placebo | GABA for schizophrenia | |||
| Tardive dyskinesia and psychiatric history | Metoclopramide (10 mg, 20 mg or 40 mg) vs haloperidol (5 mg or 10 mg) | Haloperdiol dose for schizophrenia | ||
| Schizophrenia | Haloperidol vs olanzapine | Haloperidol vs olanzapine for schizophrenia | ||
| Tardive dyskinesia | Chlorprothixene vs haloperidol vs perphenazine vs haloperidol + biperiden | Haloperidol vs perphenazine for schizophrenia | ||
| Schizophrenia | Haloperidol vs risperidone | Haloperidol vs risperidone for schizophrenia | ||
| Brief intermittent antipsychotic treatment vs fluphenazine decanoate | Intermittent antipsychotic treatment for schizophrenia | |||
| Fluphenazine decanoate vs intermittent pimozide | ||||
| Haloperidol with 'drug holiday' vs haloperidol | ||||
| Withdrawal of fluphenazine decanoate vs continuation | ||||
| Lithium vs placebo | Lithium for schizophrenia | |||
| Molidone vs haloperidol | Molidone vs haloperidol for schizophrenia | |||
| Olanzapine 1 mg vs olanzapine 10 mg versus placebo | Olanzapine dose for schizophrenia. | |||
| Olanzapine vs "conventional antipsychotic drugs" | Olanzapine for schizophrenia | |||
| First‐generation antipsychotic vs second‐generation antipsychotic | ||||
| Olanzapine with different timings of dose reduction periods | Olanzapine reduction for schizophrenia | |||
| First‐generation antipsychotic versus second‐generation antipsychotic | Olanzapine vs other atypical antipsychotics for schizophrenia | |||
| Olanzapine 1 mg vs olanzapine 10 mg vs placebo | Olanzapine vs placebo for schizophrenia | |||
| First‐generation antipsychotic vs second‐generation antipsychotic | Perphenazine for schizophrenia | |||
| Fluphenazine decanoate vs intermittent pimozide | Pimozide for schizophrenia | |||
| Quetiapine vs continuation of usual antipsychotic | Quetiapine vs continuation of usual antipsychotic for schizophrenia | |||
| First generation antipsychotic vs second‐generation antipsychotic | Quetiapine vs other atypical antipsychotics for schizophrenia | |||
| Quetiapine vs typical antipsychotic medications for schizophrenia | ||||
| Risperidone vs olanzapine for schizophrenia | ||||
| Risperidone vs other atypical antipsychotics for schizophrenia | ||||
| Quetiapine vs continuation of usual antipsychotic | Switching antipsychotic for schizophrenia. | |||
| Tardive dyskinesia | Thiopropazate vs placebo | Thiopropazate for schizophrenia | ||
| Schizophrenia | Thiopropazine vs trifluoperazine vs placebo | Thiopropazine vs placebo for schizophrenia | ||
| Thiopropazine vs trifluoperazine for schizophrenia | ||||
| Tardive dyskinesia | Thioproperazine and tiapride vs placebo | Thioproperazine for schizophrenia | ||
| Tiapride for schizophrenia | ||||
| Tiapride vs placebo | ||||
| Schizophrenia | Trifluoperazine high‐dose vs trifluoperazine low‐dose vs placebo | Trifluoperazine dose for schizophrenia | ||
| Trifluoperazine vs placebo for schizophrenia | ||||
| Thiopropazine vs trifluoperazine vs placebo | ||||
| Fluphenazine decanoate vs vitamin B complex | Vitamins for schizophrenia | |||
| First‐generation antipsychotic vs second‐generation antipsychotic | Ziprasidone vs other atypical antipsychotics for schizophrenia |
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described |
| Participants | People with antipsychotic‐induced tardive dyskinesiaa |
| Interventions | 1. Antipsychotic reduction/cessation (N = 150) vs antipsychotic maintenance (N = 150) OR 2. Specific antipsychotic (N = 150) vs other specific antipsychotic (N = 150) |
| Outcomes | Tardive dyskinesia: any clinically important improvement in tardive dyskinesia, any improvement, deteriorationc |
| Notes | aThis could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. bSize of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| Reduced dose of antipsychotic compared with antipsychotic maintenance for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (schizophrenia or schizoaffective disorder) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antipsychotic maintenance | Risk with reduced dose of antipsychotic | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 0.42 | 17 | ⊕⊝⊝⊝ | ||
| 875 per 1000 | 368 per 1000 | |||||
| Tardive dyskinesia: deterioration of symptoms | Study population | RR 0.61 | 17 | ⊕⊝⊝⊝ | ||
| 250 per 1000 | 153 per 1000 | |||||
| General mental state: relapse | Study population | RR 3.00 | 8 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effect: any ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: extrapyramidal symptoms ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | Study population | RR 0.33 | 8 | ⊕⊝⊝⊝ | ||
| 750 per 1000 | 248 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for risk of bias: none of the studies adequately described allocation concealment, one study was a subsample from one site of an RCT, and one study's baseline characteristics were not balanced between study groups. | ||||||
| Antipsychotic cessation compared with antipsychotic maintenance for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antipsychotic maintenance | Antipsychotic cessation | |||||
| There is no evidence about the effects of withdrawal of antipsychotics compared with continuation of antipsychotics; none of the included studies evaluated this comparison. | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Switch to another antipsychotic compared with antipsychotic cessation for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antipsychotic cessation (placebo) | Risk with switch to another antipsychotic | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 0.45 | 42 | ⊕⊕⊝⊝ | ||
| 700 per 1000 | 315 per 1000 | |||||
| Tardive dyskinesia: deterioration of symptoms ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| General mental state: average endpoint score (BPRS, high = poor) | The mean general mental state average endpoint score (BPRS, high = poor) was 19 | MD 4.30 lower | ‐ | 42 | ⊕⊝⊝⊝ | |
| Adverse effect: any ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effects: use of antiparkinsonism drugs | Study population | RR 2.08 | 48 | ⊕⊝⊝⊝ | Another study reported ESRS scale data for parkinsonism and also found little or no difference between groups (MD ‐0.4 95% CI ‐1.25 to 0.45, 42 participants). | |
| 273 per 1000 | 567 per 1000 | |||||
| Acceptability of the treatment: leaving the study early | Study population | RR 0.60 | 50 | ⊕⊝⊝⊝ | ||
| 200 per 1000 | 120 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias: generation of random sequence and allocation concealment not adequately described. | ||||||
| Switch to specific antipsychotic compared with switch to a different specific antipsychotic for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with specific antipsychotic 1 | Risk with specific antipsychotic 2 | |||||
| Tardive dyskinesia: no clinically important improvement Follow‐up: 3‐50 weeks | Study population | ‐ | 140 | ⊕⊝⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. The following comparisons found no clinically important improvement: THI vs HAL, ZUC vs HAL, OLZ vs RIS, QUE vs HAL | |
| See comment | See comment | |||||
| Tardive dyskinesia: deterioration Follow‐up: 3‐4 weeks | Study population | ‐ | 35 | ⊕⊝⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. The following comparisons found no difference in deterioration: THI vs HAL, ZUC vs HAL | |
| See comment | See comment | |||||
| General mental state: deterioration Follow‐up: 3‐50 weeks | Study population | ‐ | 120 | ⊕⊝⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. The following comparisons found no difference in mental state deterioration: ZUC vs HAL, OLZ vs RIS, QUE vs HAL | |
| See comment | See comment | |||||
| Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs) Follow‐up: 8‐50 weeks | Study population | ‐ | 53 | ⊕⊕⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. HAL more likely to need antiparkinsonism drugs than QUE (1 RCT, 45 participants, RR 0.45, 95% CI 0.21 to 0.96). No difference: RIS vs HAL | |
| See comment | See comment | |||||
| Adverse effects: general adverse events (UKU Average change score) Follow‐up: 24 weeks | See comment | See comment | ‐ | 80 | ⊕⊝⊝⊝ | No meta‐analysis, 3‐arm study comparing OLZ, ASP and unspecified FGAs found no difference in general adverse events for all pairwise comparisons. |
| Acceptability of the treatment: leaving the study early Follow‐up: 2 weeks ‐ 18 months | Study population | ‐ | 466 | ⊕⊝⊝⊝ | RIS more likely to leave study early than OLZ (2 RCTs, 130 participants, RR 0.73, 95% CI 0.57 to 0.95). Remaining studies no meta‐analysis, no difference (6 RCTs, 450 participants): MOL/THI/CLO/QUE vs HAL, OLZ/ASP vs unspecified FGAs, OLZ vs QUE/ZIP, QUE vs ZIP/RIS, ZIP vs RIS | |
| See comment | See comment | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one step for risk of bias: randomisation procedure, allocation concealment or blinding were not adequately described. | ||||||
| Specific antipsychotic compared with other drugs for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with tetrabenazine | Risk with haloperidol | |||||
| Tardive dyskinesia: not improved to a clinically important extent | Study population | RR 1.07 | 13 | ⊕⊝⊝⊝ | ||
| 667 per 1000 | 713 per 1000 | |||||
| Tardive dyskinesia: deterioration of symptoms | Study population | RR 0.86 | 13 | ⊕⊝⊝⊝ | ||
| 167 per 1000 | 143 per 1000 | |||||
| Mental state ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: any ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: extrapyramidal symptoms ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | Study population | RR 4.38 | 13 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one step for risk of bias: randomisation procedure, allocation concealment and blinding were not adequately described. | ||||||
| Interventions | Current reference (updates underway) |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | This review |
| Non‐neuroleptic catecholaminergic drugs | |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| 2 Tardive dyskinesia: no improvement (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| 3 Tardive dyskinesia: deterioration (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.11, 3.31] |
| 4 General mental state: relapse (long term) Show forest plot | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.16, 57.36] |
| 5 Acceptability of the treatment: leaving the study early (long term) Show forest plot | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement (medium term) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.23, 0.89] |
| 2 Tardive dyskinesia: average endpoint score (AIMS, high = poor) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐8.60, ‐2.40] |
| 3 General mental state: average endpoint score (BPRS, high = poor) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐10.48, 1.88] |
| 4 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| 5 Adverse effects: use of antiparkinsonism drugs (medium term) Show forest plot | 1 | 48 | Risk Ratio (IV, Fixed, 95% CI) | 2.08 [0.74, 5.86] |
| 5.1 Haloperidol | 1 | 12 | Risk Ratio (IV, Fixed, 95% CI) | 2.0 [0.56, 7.09] |
| 5.2 Risperidone | 1 | 36 | Risk Ratio (IV, Fixed, 95% CI) | 2.26 [0.37, 13.60] |
| 6 Adverse effects: parkinsonism ‐ average endpoint score (ESRS) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.25, 0.45] |
| 7 Adverse effects: dystonia ‐ average endpoint score (ESRS) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.76, 0.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement Show forest plot | 4 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Thiopropazate vs haloperidol ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 1.53 [0.58, 4.05] |
| 1.2 Zuclopenthixol vs haloperidol ‐ short term | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.79, 1.27] |
| 1.3 Olanzapine vs risperidone ‐ medium term | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.82, 1.90] |
| 1.4 Quetiapine vs haloperidol ‐ medium term | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 1.5 Quetiapine vs haloperidol ‐ long term | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 2 Tardive dyskinesia: not any improvement (short term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.41 [0.05, 3.28] |
| 2.2 Zuclopenthixol vs haloperidol | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
| 3 Tardive dyskinesia: deterioration (short term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 1.22 [0.09, 16.92] |
| 3.2 Zuclopenthixol vs haloperidol | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
| 4 Tardive dyskinesia: average endpoint score (various scales) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Molindone vs haloperidol, 100% masking dose (AIMS, short term) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 1.87 [‐0.20, 3.94] |
| 4.2 Molindone vs haloperidol, 200% masking dose (AIMS, short term) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.44 [1.12, 5.76] |
| 4.3 Zuclopenthixol vs haloperidol (SHRS, short term) | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 4.4 Olanzapine vs risperidone (AIMS, medium term) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐0.53, 4.93] |
| 5 Tardive dyskinesia: average change score (AIMS, low = better) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 1.66 [‐0.45, 3.77] |
| 5.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐2.85, 1.21] |
| 5.3 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 2.48 [0.44, 4.52] |
| 5.4 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.58, 4.98] |
| 6 General mental state: deterioration Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Zuclopenthixol vs haloperidol ‐ short term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.29] |
| 6.2 Olanzapine vs risperidone ‐ medium term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.64] |
| 6.3 Quetiapine vs haloperidol ‐ long term | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.62, 5.39] |
| 7 General mental state: average endpoint score (PANSS‐general psychopathology, low = better) (long term) Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.02, 1.62] |
| 7.1 Quetiapine vs haloperidol | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.02, 1.62] |
| 8 General mental state: average change score (BPRS, low = better) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐4.79, 2.51] |
| 8.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐2.46 [‐6.27, 1.35] |
| 8.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐8.37, 4.97] |
| 8.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [‐1.94, 4.58] |
| 9 Acceptability of the treatment: leaving the study early (short term) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Molindone vs haloperidol | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.44] |
| 10 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 3 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Olanzapine vs FGA | 1 | 56 | Risk Ratio (IV, Fixed, 95% CI) | 1.86 [0.18, 19.38] |
| 10.2 Amisulpride vs FGA | 1 | 55 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.06, 14.65] |
| 10.3 Olanzapine vs amisulpride | 1 | 57 | Risk Ratio (IV, Fixed, 95% CI) | 1.93 [0.19, 20.12] |
| 10.4 Olanzapine vs risperidone | 2 | 170 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.57, 0.95] |
| 10.5 Olanzapine vs quetiapine | 1 | 116 | Risk Ratio (IV, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 10.6 Olanzapine vs ziprasidone | 1 | 82 | Risk Ratio (IV, Fixed, 95% CI) | 0.77 [0.56, 1.05] |
| 10.7 Quetiapine vs risperidone | 1 | 118 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| 10.8 Quetiapine vs ziprasidone | 1 | 90 | Risk Ratio (IV, Fixed, 95% CI) | 1.10 [0.86, 1.40] |
| 10.9 Ziprasidone vs risperidone | 1 | 84 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 11 Acceptability of the treatment: leaving the study early (long term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Clozapine vs haloperidol | 1 | 39 | Risk Ratio (IV, Fixed, 95% CI) | 3.36 [0.45, 25.16] |
| 11.2 Quetiapine vs haloperidol | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 1.31 [0.63, 2.69] |
| 12 Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Risperidone vs haloperidol (medium term) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.34, 1.35] |
| 12.2 Quetiapine vs haloperidol (long term) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.96] |
| 13 Adverse effects: parkinsonism (SHRS) ‐ average endpoint scores (short term) Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 13.1 Zuclopenthixol vs haloperidol | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 14 Adverse effects: parkinsonism (SAS, ESRS, low = better) ‐ average change score (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐2.55, 0.85] |
| 14.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.45, 1.45] |
| 14.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.33, ‐0.07] |
| 14.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐2.44, 1.74] |
| 15 Adverse effects: dyskinesia (ESRS, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.91, 1.51] |
| 16 Adverse effects: akathisia (BAS, ESRS, low = better) ‐ average change scores (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.30, 0.46] |
| 16.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.42, 0.20] |
| 16.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.76, 0.16] |
| 16.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.12, 0.50] |
| 17 Adverse effects: dystonia (ESRS, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.41, 0.01] |
| 18 Adverse effects: general adverse events (UKU, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐1.85, 2.01] |
| 18.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.33, 1.23] |
| 18.3 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐0.93, 2.19] |
| 19 General global state: average change score (CGI) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.41, 0.27] |
| 19.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.47, 0.09] |
| 19.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.61, 0.81] |
| 19.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.19, 0.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesias: no clinically important improvement (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.51, 2.23] |
| 1.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.51, 2.23] |
| 2 Tardive dyskinesia: no improvement (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 2.57 [0.35, 18.68] |
| 2.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 2.57 [0.35, 18.68] |
| 3 Tardive dyskinesia: deterioration (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| 3.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| 4 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [0.25, 76.54] |
| 4.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [0.25, 76.54] |

