Antipsychotic reduction and/or cessation and antipsychotics as specific treatments for tardive dyskinesia
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000459.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 06 febrero 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Esquizofrenia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
HB ‐ study selection, data extraction and assimilation, GRADE and Summary of Findings tables, report writing (2017 update)
JR ‐ study selection, data extraction and assimilation, report writing (original version).
VA ‐ report writing (2017 update).
KSW ‐ protocol development, searching, study selection, data extraction and assimilation, report writing (original version and 2017 update).
Sources of support
Internal sources
-
Queensland Health, Australia.
-
CAPES ‐ Ministry of Education, Brazil.
-
Universidade Federal de São Paulo, Brazil.
-
Enhance Reviews Ltd., UK.
Logistics support for Hanna Bergman
External sources
-
NIHR HTA Project Grant, reference number: 14/27/02, UK.
Salary support for Hanna Bergman.
Support for patient involvement consultation.
Support for accessible, traceable data.
Declarations of interest
HB worked for Enhance Reviews Ltd. during preparation of this review and was paid for her contribution to this review. Enhance Reviews Ltd. is a private company that performs systematic reviews of literature. HB works for Cochrane Response, an evidence consultancy that takes commissions from healthcare guideline developers and policy makers.
JR is not aware of any conflicts of interest for this review.
VA has declared no known conflicts of interest for this review.
KSW is the Deputy Editor‐in‐Chief for Cochrane and Cochrane Innovations. When the NHIR HTA programme grant relevant to this review update was awarded, KSW was the Managing Director of Enhance Reviews Ltd.
One of the earlier reviewers (JJM) is a member of the following advisory boards: Janssen‐Cilag Australia, Eli Lilly Australia, Lundbeck Australia. In addition, JJM has been a co‐investigator on studies of neuroleptic medications produced by the following companies: Astra Zeneca (ICI), Janssen‐Cilag, Eli Lilly, Sandoz, and Pfizer. The same companies have provided travel and accommodation expenses for JJM to attend relevant investigator meetings and scientific symposia. No funds have been paid directly to JJM. Payments related to participation in drug trials and board attendance has been paid to a Government‐audited trust account to support schizophrenia research.
Acknowledgements
In earlier versions of this review (1998 to 2002), John McGrath was a reviewer and made a considerable contribution to the review by developing the protocol, data extracting, data assimilation, and report writing. We are grateful for his substantial input.
We are indebted to Kirsten Mason, Tracey Richardson, Geoff Davies, Carmel Meir, Leanne Roberts, Nicholas Henschke, and Loukia Spineli for assistance with this review. The following authors kindly provided additional information in order to assist this review: Dr D Bateman, Dr M Campbell, Dr S Caroff, Dr G Chouinard, Dr I Cookson, Dr J de Jesus Mari, Dr M Herz, Dr D Johnson, Dr J Kalachnik, Dr J Kane, Dr S Lal, Dr G Paulson, Dr H Spohn, Dr N Quinn, and Dr M Woerner.
We are grateful to Judy Wright (2005) and Farhad Sokraneh (2015, 2017) for the updated trial searches and to Clive Adams for his constant advice.
We thank Rosie Asher and Antonio Grande for screening literature and helping with data extraction for the 2017 update, and Ben Gray for writing the Plain language summary. We are also grateful to Dawn‐Marie Walker, Ruth Sayers, Megan Lees, and Vanessa Pinfold from McPin Foundation for organising and holding the public and patient involvement consultation with TD service users that contributed to selecting outcomes for the 'Summary of findings' tables and to guide future research. Finally, we wish to thank Sai Zhao for assessing articles in Chinese.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Feb 06 | Antipsychotic reduction and/or cessation and antipsychotics as specific treatments for tardive dyskinesia | Review | Hanna Bergman, John Rathbone, Vivek Agarwal, Karla Soares‐Weiser | |
| 2006 Jan 25 | Neuroleptic reduction and/or cessation and neuroleptics as specific treatments for tardive dyskinesia | Review | Karla Soares‐Weiser, John Rathbone | |
| 1998 Apr 27 | Neuroleptic reduction and/or cessation and neuroleptics as specific treatments for tardive dyskinesia | Review | John McGrath, Karla Soares‐Weiser | |
| Interventions | Current reference (updates underway) |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | This review |
| Non‐neuroleptic catecholaminergic drugs | |
| Vitamin E |
Differences between protocol and review
The protocol as published with this review has evolved over time. The revisions of protocol are in line with the development of Review Manager and in keeping with Cochrane guidance. We think the revisions have greatly improved and enhanced this review. We do not think, however, that it has materially affected our conduct of the review or interpretation of the results.
There was a substantial update to the protocol in the 2017 review update. The biggest changes to affect the review were to:
-
broaden the inclusion criteria, and add the comparison 'Specific antipsychotic versus other drug';
-
change the title from 'Neuroleptic reduction and/or cessation and neuroleptics as specific treatments for tardive dyskinesia' to 'Antipsychotic reduction and/or cessation and antipsychotics as specific treatments for tardive dyskinesia';
-
update list of outcomes following consultation with consumers; and
-
add 'Summary of findings' tables.
Previous methods are reproduced in Appendix 1.
Notes
Cochrane Schizophrenia Group internal peer review complete (see Group’s Module).
External peer review scheduled.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antipsychotic Agents [*administration & dosage, *adverse effects];
- Dose‐Response Relationship, Drug;
- Drug Administration Schedule;
- Drug Substitution;
- Dyskinesia, Drug‐Induced [*drug therapy, prevention & control];
- Mental Disorders [drug therapy];
- Randomized Controlled Trials as Topic;
- Schizophrenia [drug therapy];
- Withholding Treatment;
Medical Subject Headings Check Words
Female; Humans; Male; Middle Aged;
PICO

Message from one of the participants of the public and patient involvement consultation of service user perspectives on tardive dyskinesia research
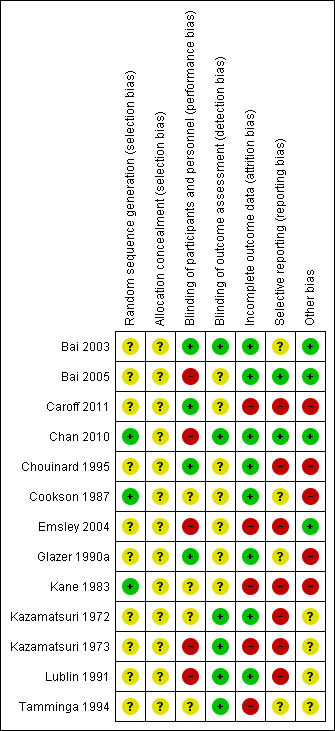
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
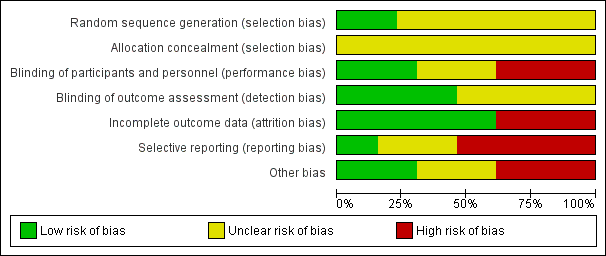
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
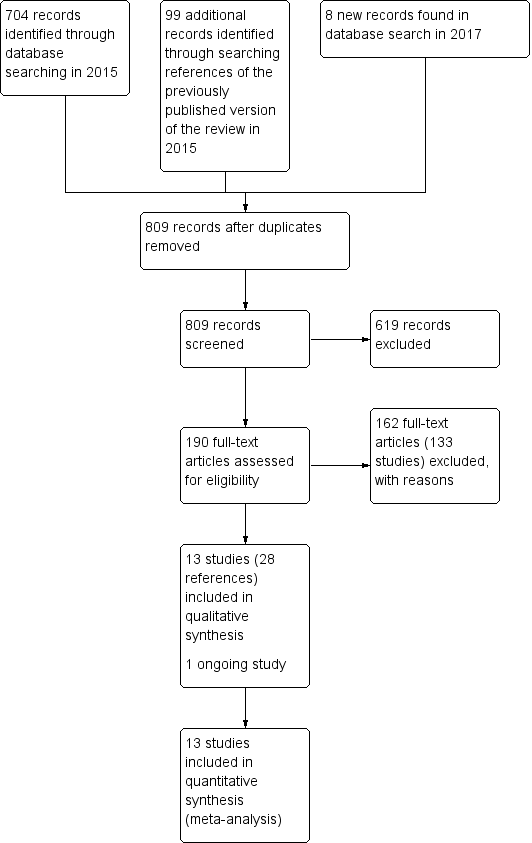
Study flow diagram for 2015 and 2017 searches for this review
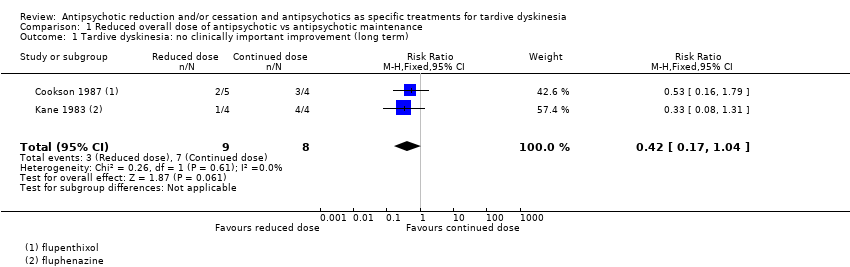
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 1 Tardive dyskinesia: no clinically important improvement (long term).
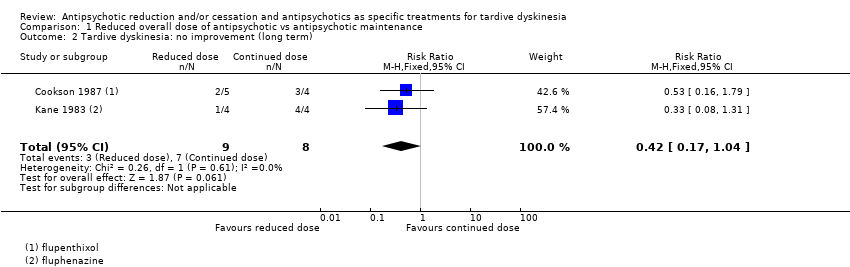
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 2 Tardive dyskinesia: no improvement (long term).
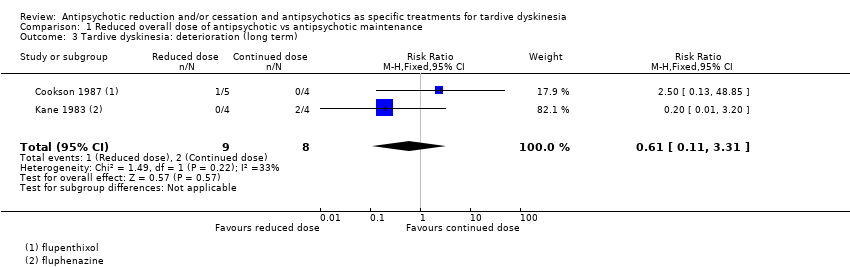
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 3 Tardive dyskinesia: deterioration (long term).
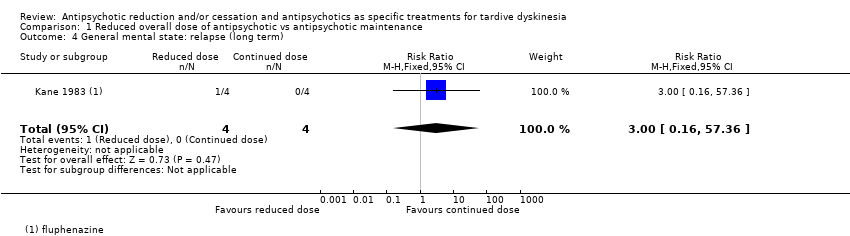
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 4 General mental state: relapse (long term).
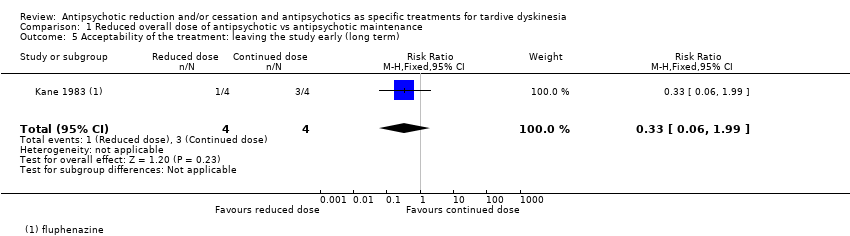
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 5 Acceptability of the treatment: leaving the study early (long term).
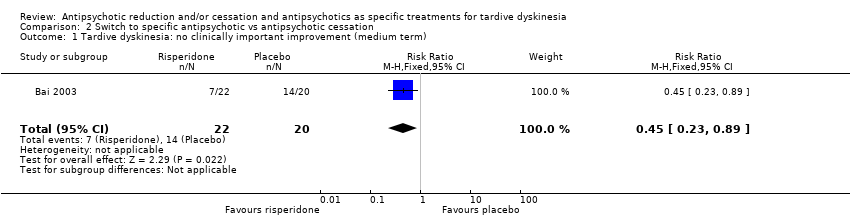
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 1 Tardive dyskinesia: no clinically important improvement (medium term).
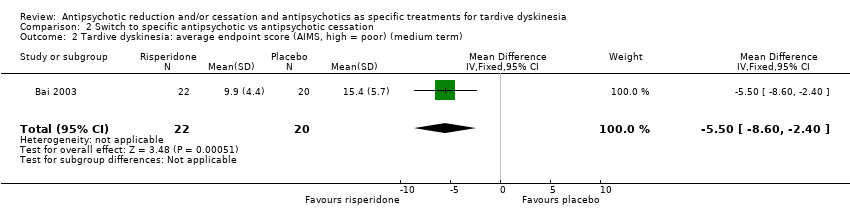
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 2 Tardive dyskinesia: average endpoint score (AIMS, high = poor) (medium term).
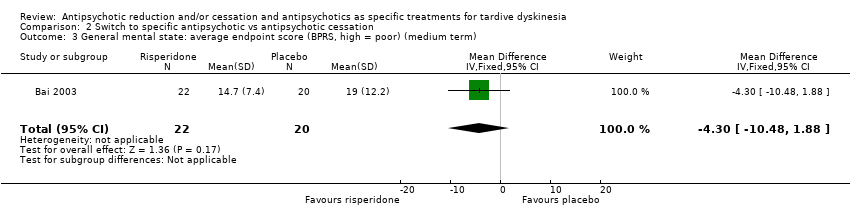
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 3 General mental state: average endpoint score (BPRS, high = poor) (medium term).
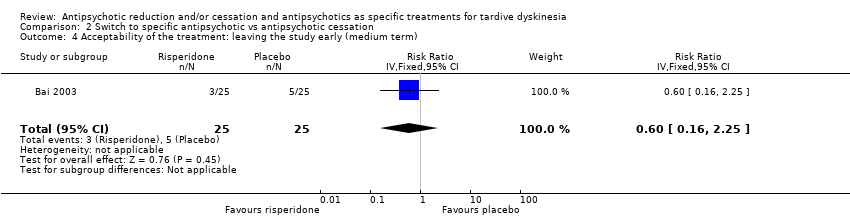
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 4 Acceptability of the treatment: leaving the study early (medium term).
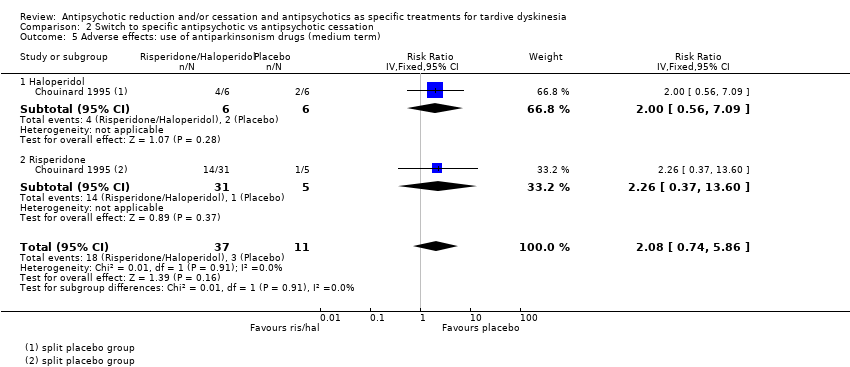
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 5 Adverse effects: use of antiparkinsonism drugs (medium term).
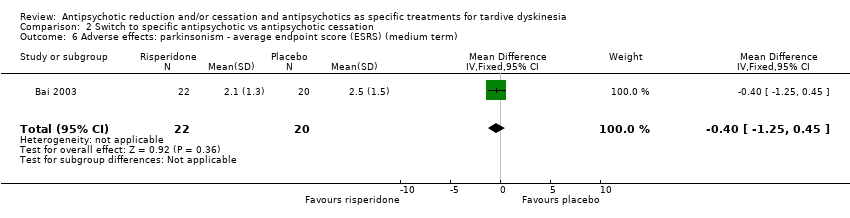
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 6 Adverse effects: parkinsonism ‐ average endpoint score (ESRS) (medium term).
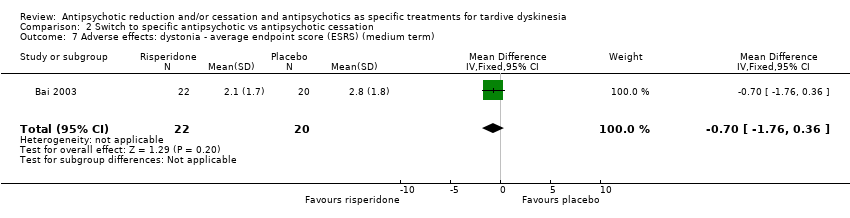
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 7 Adverse effects: dystonia ‐ average endpoint score (ESRS) (medium term).
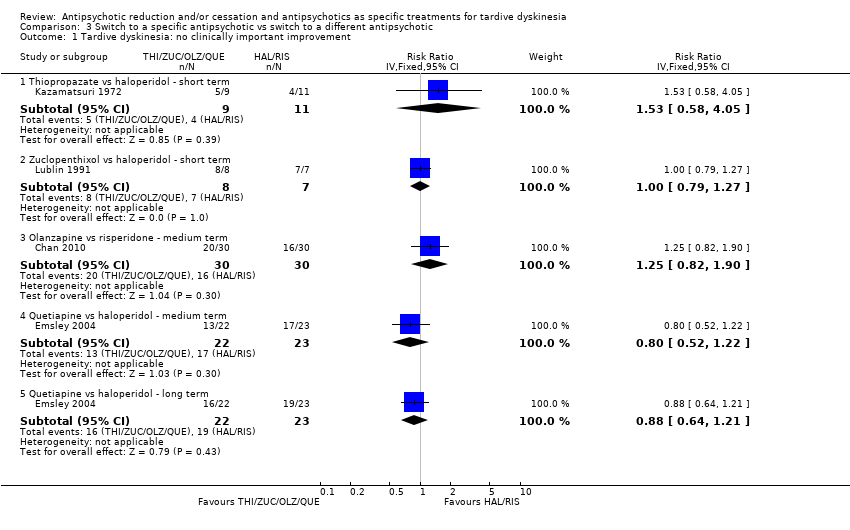
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 1 Tardive dyskinesia: no clinically important improvement.
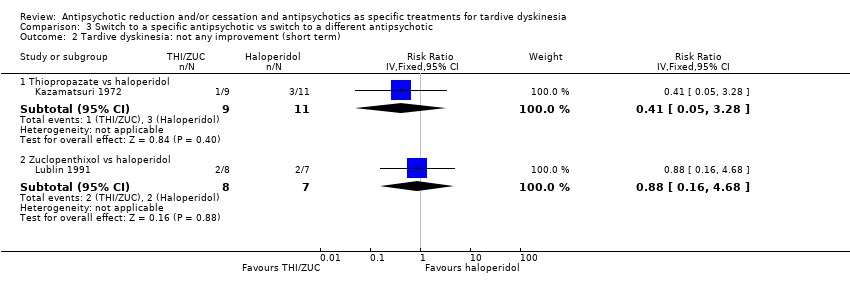
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 2 Tardive dyskinesia: not any improvement (short term).
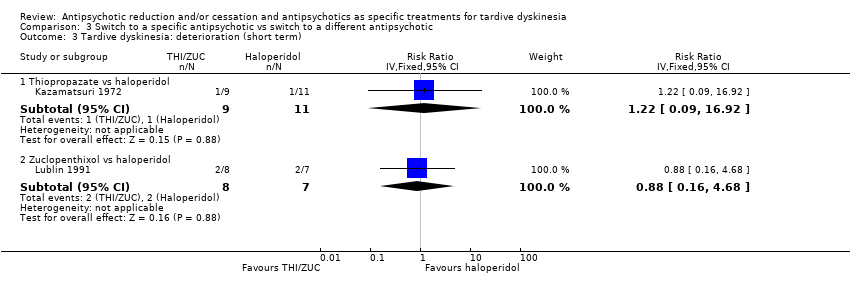
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 3 Tardive dyskinesia: deterioration (short term).
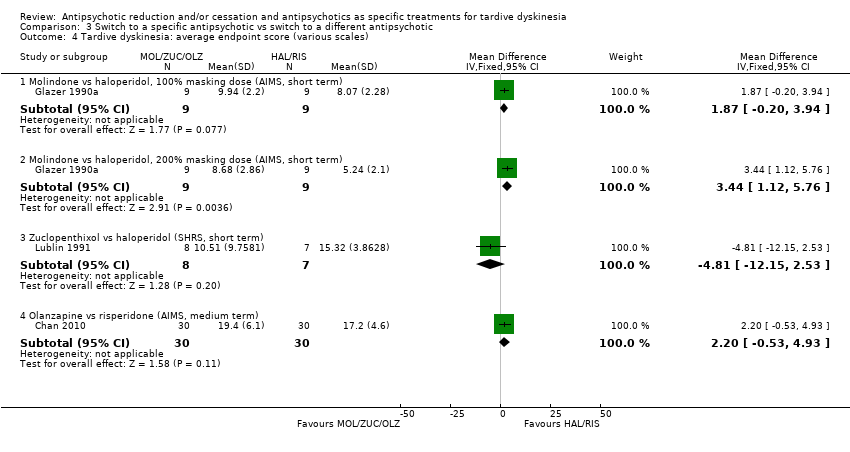
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 4 Tardive dyskinesia: average endpoint score (various scales).
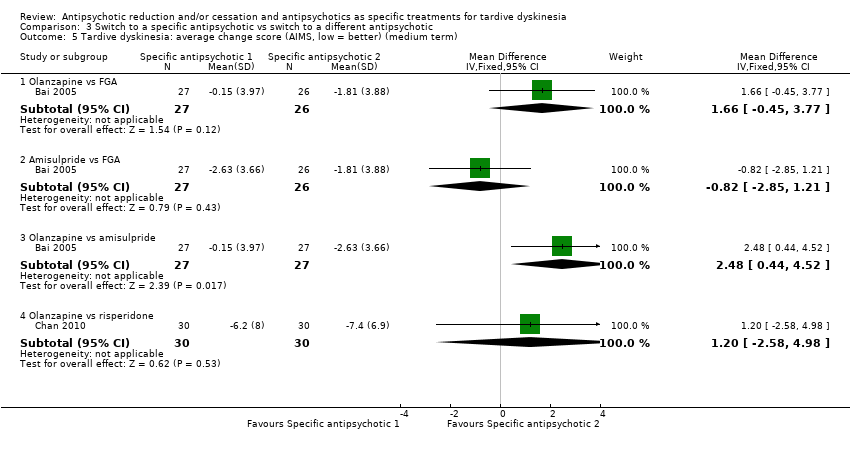
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 5 Tardive dyskinesia: average change score (AIMS, low = better) (medium term).
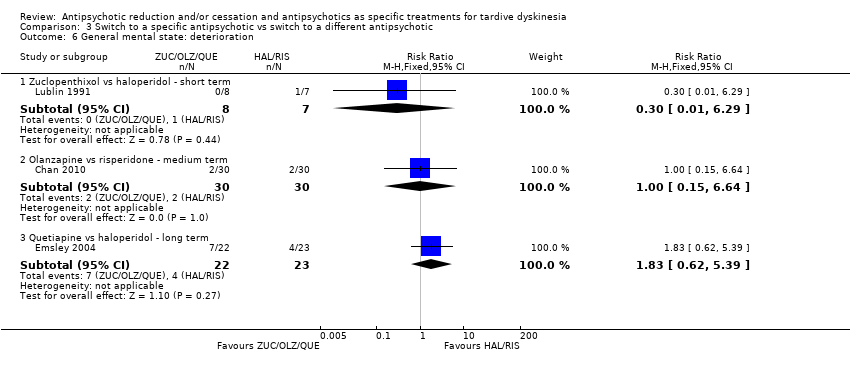
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 6 General mental state: deterioration.
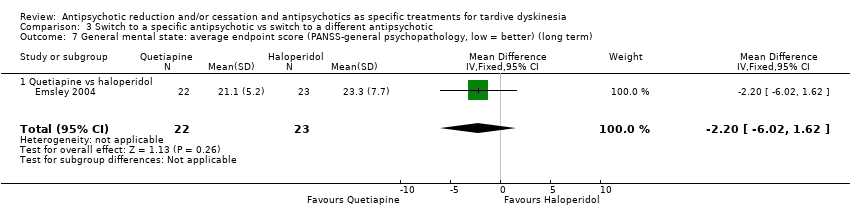
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 7 General mental state: average endpoint score (PANSS‐general psychopathology, low = better) (long term).
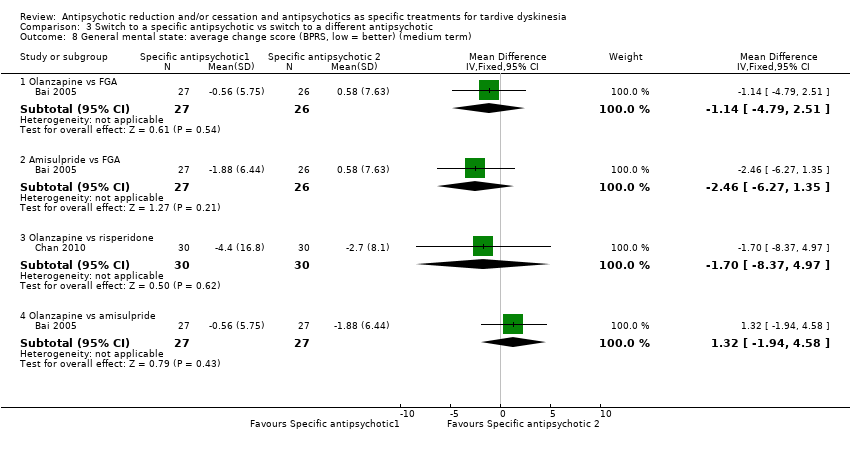
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 8 General mental state: average change score (BPRS, low = better) (medium term).
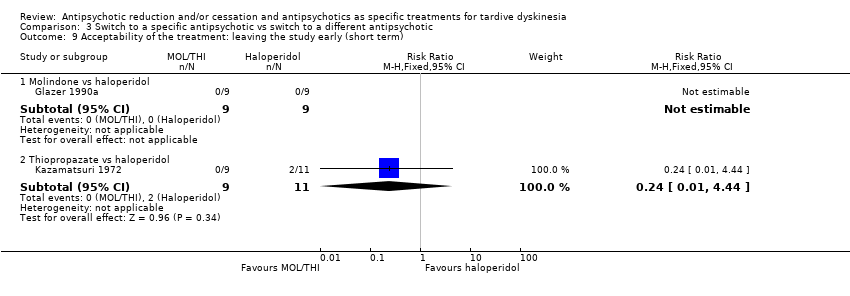
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 9 Acceptability of the treatment: leaving the study early (short term).
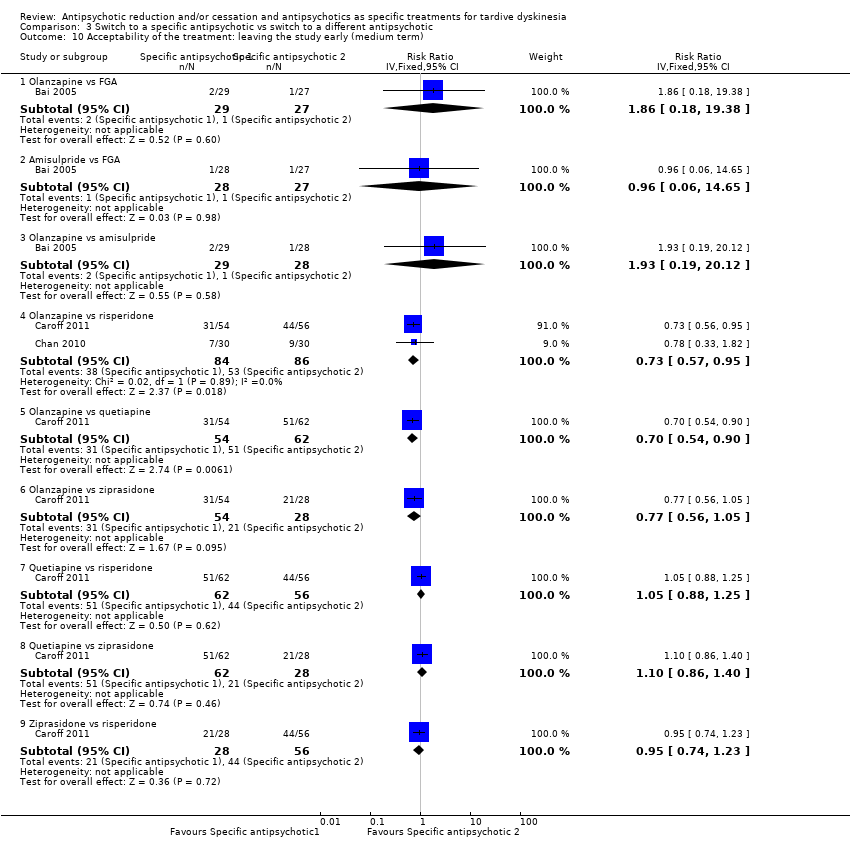
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 10 Acceptability of the treatment: leaving the study early (medium term).
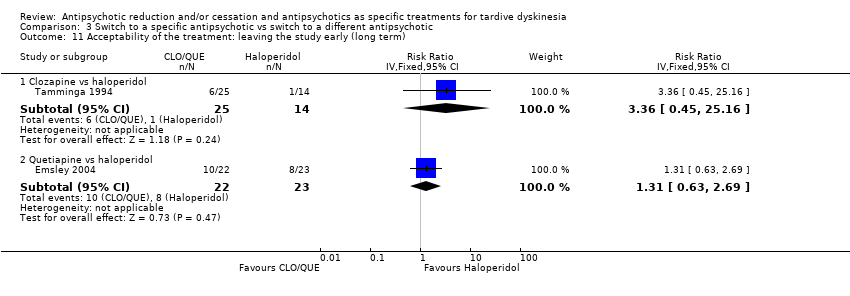
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 11 Acceptability of the treatment: leaving the study early (long term).
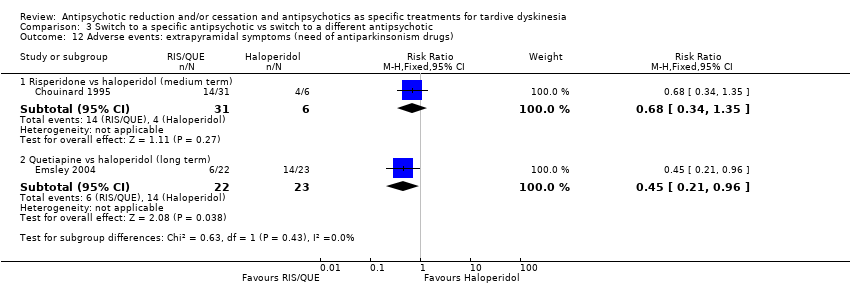
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 12 Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs).
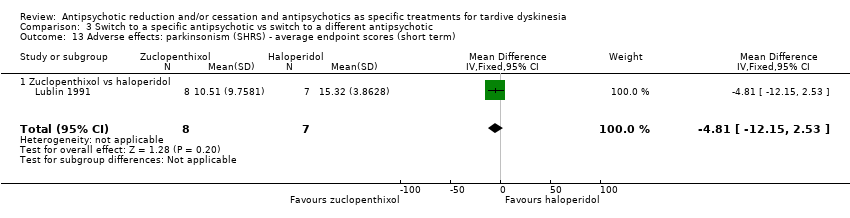
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 13 Adverse effects: parkinsonism (SHRS) ‐ average endpoint scores (short term).
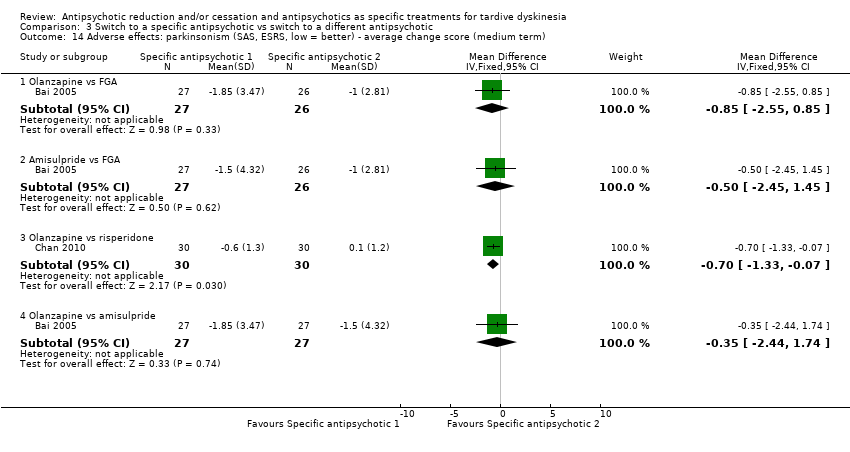
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 14 Adverse effects: parkinsonism (SAS, ESRS, low = better) ‐ average change score (medium term).
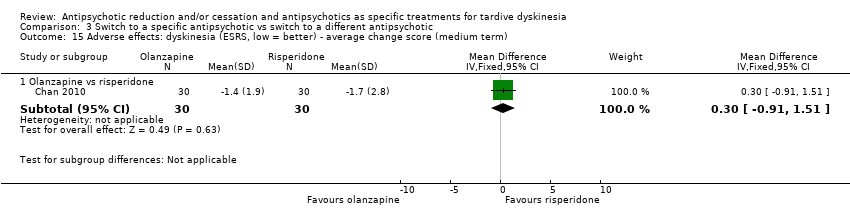
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 15 Adverse effects: dyskinesia (ESRS, low = better) ‐ average change score (medium term).
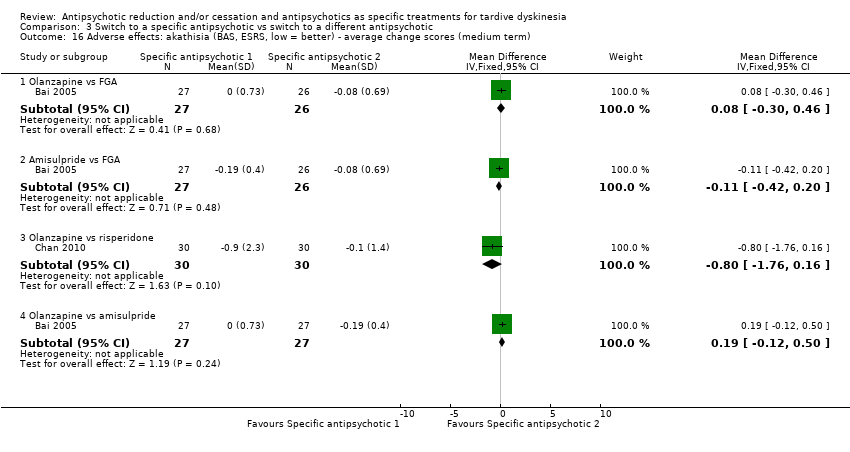
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 16 Adverse effects: akathisia (BAS, ESRS, low = better) ‐ average change scores (medium term).
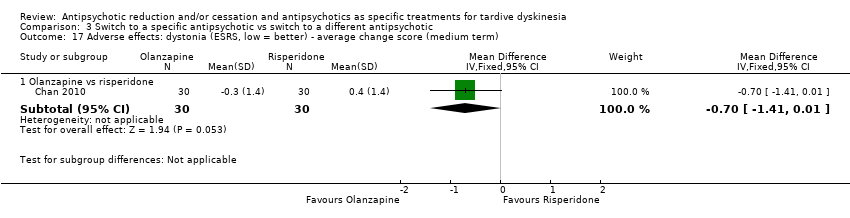
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 17 Adverse effects: dystonia (ESRS, low = better) ‐ average change score (medium term).
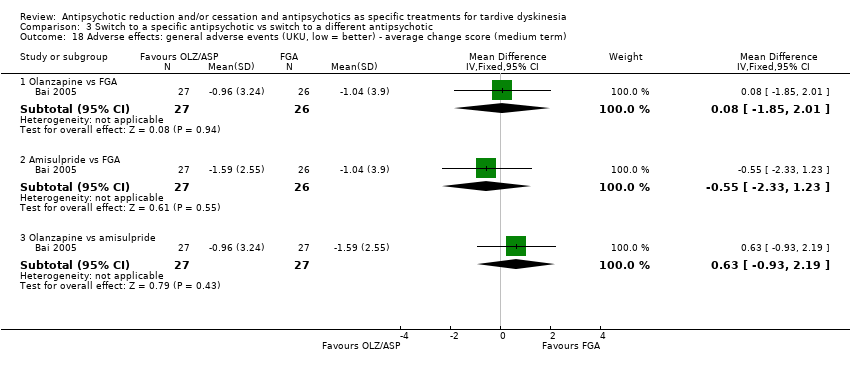
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 18 Adverse effects: general adverse events (UKU, low = better) ‐ average change score (medium term).
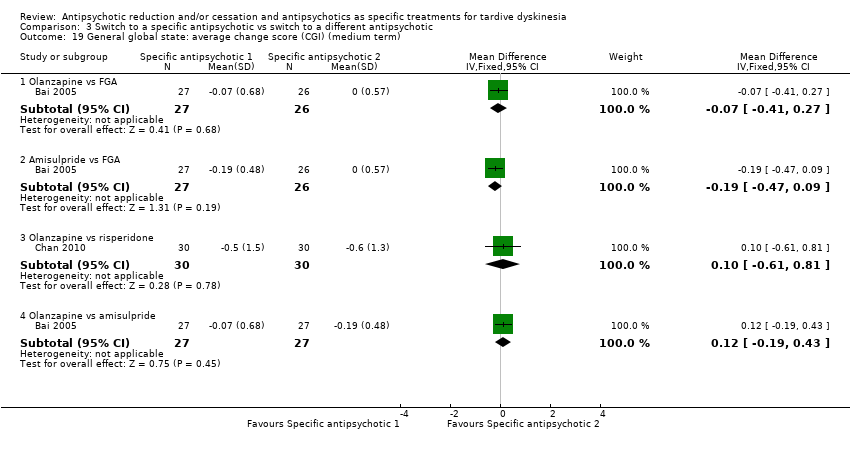
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 19 General global state: average change score (CGI) (medium term).
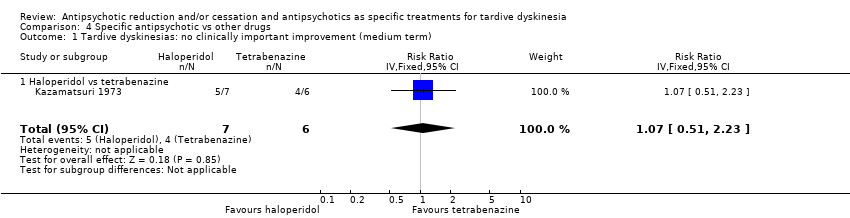
Comparison 4 Specific antipsychotic vs other drugs, Outcome 1 Tardive dyskinesias: no clinically important improvement (medium term).
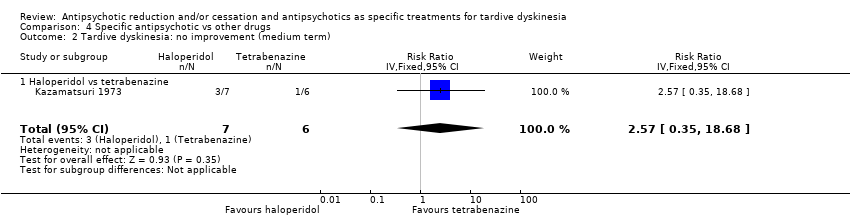
Comparison 4 Specific antipsychotic vs other drugs, Outcome 2 Tardive dyskinesia: no improvement (medium term).
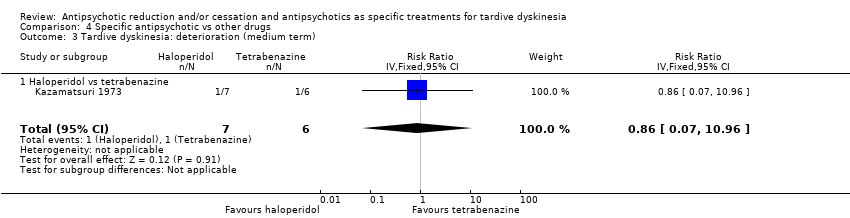
Comparison 4 Specific antipsychotic vs other drugs, Outcome 3 Tardive dyskinesia: deterioration (medium term).
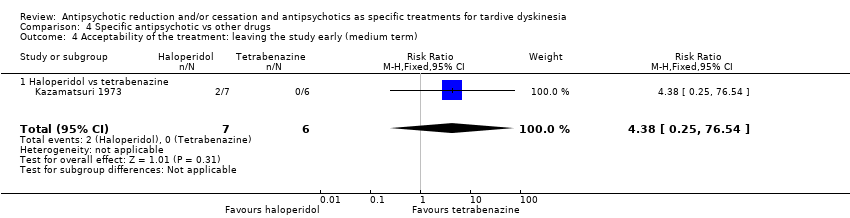
Comparison 4 Specific antipsychotic vs other drugs, Outcome 4 Acceptability of the treatment: leaving the study early (medium term).
| Study ID | Participants – people with: | Intervention | Comparison for review | Cochrane Review |
| Tardive dyskinesia | 1‐stepholidine vs placebo | 1‐stepholidine for schizophrenia | ‐ | |
| Schizophrenia | Amisulpride vs haloperidol | Amisulpride versus haloperidol for schizophrenia | ||
| Tardive dyskinesia | Biperiden vs no treatment | Anticholinergic drugs for tardive dyskinesia | ||
| Chlorprothixene versus haloperidol vs perphenazine vs haloperidol + biperiden | ||||
| Biperiden vs placebo | ||||
| Schizophrenia | Ethopropazine vs benztropine | Anticholinergics for parkinsonism | ||
| Abrupt neuroleptic cessation vs neuroleptic maintenance | Antipsychotic reduction or withdrawal for schizophrenia | |||
| Abrupt neuroleptic cessation vs neuroleptic maintenance | ||||
| Fluphenazine/flupenthixol decanoate continuation vs withdrawal | ||||
| Withdrawal of fluphenazine decanoate vs continuation | ||||
| Trifluoperazine withdrawal vs trifluoperazine continuation | ||||
| Dose reduction vs maintenance (both arms used flupenthixol decanoate) | ||||
| Olanzapine with different timings of dose‐reduction periods | ||||
| Fluphenazine withdrawal vs continuation | ||||
| Low‐ vs conventional‐dose maintenance therapy with fluphenazine decanoate | ||||
| Haloperidol dose reduction vs maintained dose | ||||
| Abrupt neuroleptic cessation vs neuroleptic maintenance | ||||
| Tardive dyskinesia | Flunarizine vs placebo | Calcium channel blockers for neuroleptic‐induced tardive dyskinesia | ||
| Schizophrenia | Chlorpromazine schedule A vs chlorpromazine schedule B | Chlorpromazine timing of dose for schizophrenia. | ||
| Tardive dyskinesia | Chlorprothixene vs haloperidol vs perphenazine vs haloperidol + biperiden | Chlorprothixene for schizophrenia. | ||
| Schizophrenia | Clozapine vs haloperidol | Clozapine versus haloperidol for schizophrenia | ||
| Clozapine vs haloperidol | ||||
| Clozapine vs olanzapine | Clozapine versus olanzapine for schizophrenia | |||
| Clozapine vs olanzapine | ||||
| Gilles de la Tourette's, Huntington's disease and drug‐induced atypical dyskinesia | Clozapine vs placebo | Clozapine versus placebo for schizophrenia. | ||
| Schizophrenia | Clozapine versus risperidone | Clozapine versus risperidone for schizophrenia | ||
| Haloperidol decanoate vs fluphenazine decanoate | Depot fluphenazine for schizophrenia | |||
| Fluphenazine decanoate vs haloperidol decanoate | ||||
| Fluphenazine decanoate vs placebo | ||||
| Fluphenazine decanoate vs intermittent pimozide | ||||
| Fluphenazine decanoate vs vitamin B complex | ||||
| Fluphenazine ethanoate vs pipothiazine palmitate | ||||
| Haloperidol decanoate vs fluphenazine decanoate | Depot haloperidol decanoate for schizophrenia. | |||
| Fluphenazine ethanoate vs pipothiazine palmitate | Depot pipothiazine for schizophrenia. | |||
| Progabide vs placebo | GABA for schizophrenia | |||
| Tardive dyskinesia and psychiatric history | Metoclopramide (10 mg, 20 mg or 40 mg) vs haloperidol (5 mg or 10 mg) | Haloperdiol dose for schizophrenia | ||
| Schizophrenia | Haloperidol vs olanzapine | Haloperidol vs olanzapine for schizophrenia | ||
| Tardive dyskinesia | Chlorprothixene vs haloperidol vs perphenazine vs haloperidol + biperiden | Haloperidol vs perphenazine for schizophrenia | ||
| Schizophrenia | Haloperidol vs risperidone | Haloperidol vs risperidone for schizophrenia | ||
| Brief intermittent antipsychotic treatment vs fluphenazine decanoate | Intermittent antipsychotic treatment for schizophrenia | |||
| Fluphenazine decanoate vs intermittent pimozide | ||||
| Haloperidol with 'drug holiday' vs haloperidol | ||||
| Withdrawal of fluphenazine decanoate vs continuation | ||||
| Lithium vs placebo | Lithium for schizophrenia | |||
| Molidone vs haloperidol | Molidone vs haloperidol for schizophrenia | |||
| Olanzapine 1 mg vs olanzapine 10 mg versus placebo | Olanzapine dose for schizophrenia. | |||
| Olanzapine vs "conventional antipsychotic drugs" | Olanzapine for schizophrenia | |||
| First‐generation antipsychotic vs second‐generation antipsychotic | ||||
| Olanzapine with different timings of dose reduction periods | Olanzapine reduction for schizophrenia | |||
| First‐generation antipsychotic versus second‐generation antipsychotic | Olanzapine vs other atypical antipsychotics for schizophrenia | |||
| Olanzapine 1 mg vs olanzapine 10 mg vs placebo | Olanzapine vs placebo for schizophrenia | |||
| First‐generation antipsychotic vs second‐generation antipsychotic | Perphenazine for schizophrenia | |||
| Fluphenazine decanoate vs intermittent pimozide | Pimozide for schizophrenia | |||
| Quetiapine vs continuation of usual antipsychotic | Quetiapine vs continuation of usual antipsychotic for schizophrenia | |||
| First generation antipsychotic vs second‐generation antipsychotic | Quetiapine vs other atypical antipsychotics for schizophrenia | |||
| Quetiapine vs typical antipsychotic medications for schizophrenia | ||||
| Risperidone vs olanzapine for schizophrenia | ||||
| Risperidone vs other atypical antipsychotics for schizophrenia | ||||
| Quetiapine vs continuation of usual antipsychotic | Switching antipsychotic for schizophrenia. | |||
| Tardive dyskinesia | Thiopropazate vs placebo | Thiopropazate for schizophrenia | ||
| Schizophrenia | Thiopropazine vs trifluoperazine vs placebo | Thiopropazine vs placebo for schizophrenia | ||
| Thiopropazine vs trifluoperazine for schizophrenia | ||||
| Tardive dyskinesia | Thioproperazine and tiapride vs placebo | Thioproperazine for schizophrenia | ||
| Tiapride for schizophrenia | ||||
| Tiapride vs placebo | ||||
| Schizophrenia | Trifluoperazine high‐dose vs trifluoperazine low‐dose vs placebo | Trifluoperazine dose for schizophrenia | ||
| Trifluoperazine vs placebo for schizophrenia | ||||
| Thiopropazine vs trifluoperazine vs placebo | ||||
| Fluphenazine decanoate vs vitamin B complex | Vitamins for schizophrenia | |||
| First‐generation antipsychotic vs second‐generation antipsychotic | Ziprasidone vs other atypical antipsychotics for schizophrenia |
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described |
| Participants | People with antipsychotic‐induced tardive dyskinesiaa |
| Interventions | 1. Antipsychotic reduction/cessation (N = 150) vs antipsychotic maintenance (N = 150) OR 2. Specific antipsychotic (N = 150) vs other specific antipsychotic (N = 150) |
| Outcomes | Tardive dyskinesia: any clinically important improvement in tardive dyskinesia, any improvement, deteriorationc |
| Notes | aThis could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. bSize of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| Reduced dose of antipsychotic compared with antipsychotic maintenance for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (schizophrenia or schizoaffective disorder) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antipsychotic maintenance | Risk with reduced dose of antipsychotic | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 0.42 | 17 | ⊕⊝⊝⊝ | ||
| 875 per 1000 | 368 per 1000 | |||||
| Tardive dyskinesia: deterioration of symptoms | Study population | RR 0.61 | 17 | ⊕⊝⊝⊝ | ||
| 250 per 1000 | 153 per 1000 | |||||
| General mental state: relapse | Study population | RR 3.00 | 8 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effect: any ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: extrapyramidal symptoms ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | Study population | RR 0.33 | 8 | ⊕⊝⊝⊝ | ||
| 750 per 1000 | 248 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for risk of bias: none of the studies adequately described allocation concealment, one study was a subsample from one site of an RCT, and one study's baseline characteristics were not balanced between study groups. | ||||||
| Antipsychotic cessation compared with antipsychotic maintenance for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antipsychotic maintenance | Antipsychotic cessation | |||||
| There is no evidence about the effects of withdrawal of antipsychotics compared with continuation of antipsychotics; none of the included studies evaluated this comparison. | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Switch to another antipsychotic compared with antipsychotic cessation for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antipsychotic cessation (placebo) | Risk with switch to another antipsychotic | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 0.45 | 42 | ⊕⊕⊝⊝ | ||
| 700 per 1000 | 315 per 1000 | |||||
| Tardive dyskinesia: deterioration of symptoms ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| General mental state: average endpoint score (BPRS, high = poor) | The mean general mental state average endpoint score (BPRS, high = poor) was 19 | MD 4.30 lower | ‐ | 42 | ⊕⊝⊝⊝ | |
| Adverse effect: any ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effects: use of antiparkinsonism drugs | Study population | RR 2.08 | 48 | ⊕⊝⊝⊝ | Another study reported ESRS scale data for parkinsonism and also found little or no difference between groups (MD ‐0.4 95% CI ‐1.25 to 0.45, 42 participants). | |
| 273 per 1000 | 567 per 1000 | |||||
| Acceptability of the treatment: leaving the study early | Study population | RR 0.60 | 50 | ⊕⊝⊝⊝ | ||
| 200 per 1000 | 120 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias: generation of random sequence and allocation concealment not adequately described. | ||||||
| Switch to specific antipsychotic compared with switch to a different specific antipsychotic for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with specific antipsychotic 1 | Risk with specific antipsychotic 2 | |||||
| Tardive dyskinesia: no clinically important improvement Follow‐up: 3‐50 weeks | Study population | ‐ | 140 | ⊕⊝⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. The following comparisons found no clinically important improvement: THI vs HAL, ZUC vs HAL, OLZ vs RIS, QUE vs HAL | |
| See comment | See comment | |||||
| Tardive dyskinesia: deterioration Follow‐up: 3‐4 weeks | Study population | ‐ | 35 | ⊕⊝⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. The following comparisons found no difference in deterioration: THI vs HAL, ZUC vs HAL | |
| See comment | See comment | |||||
| General mental state: deterioration Follow‐up: 3‐50 weeks | Study population | ‐ | 120 | ⊕⊝⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. The following comparisons found no difference in mental state deterioration: ZUC vs HAL, OLZ vs RIS, QUE vs HAL | |
| See comment | See comment | |||||
| Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs) Follow‐up: 8‐50 weeks | Study population | ‐ | 53 | ⊕⊕⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. HAL more likely to need antiparkinsonism drugs than QUE (1 RCT, 45 participants, RR 0.45, 95% CI 0.21 to 0.96). No difference: RIS vs HAL | |
| See comment | See comment | |||||
| Adverse effects: general adverse events (UKU Average change score) Follow‐up: 24 weeks | See comment | See comment | ‐ | 80 | ⊕⊝⊝⊝ | No meta‐analysis, 3‐arm study comparing OLZ, ASP and unspecified FGAs found no difference in general adverse events for all pairwise comparisons. |
| Acceptability of the treatment: leaving the study early Follow‐up: 2 weeks ‐ 18 months | Study population | ‐ | 466 | ⊕⊝⊝⊝ | RIS more likely to leave study early than OLZ (2 RCTs, 130 participants, RR 0.73, 95% CI 0.57 to 0.95). Remaining studies no meta‐analysis, no difference (6 RCTs, 450 participants): MOL/THI/CLO/QUE vs HAL, OLZ/ASP vs unspecified FGAs, OLZ vs QUE/ZIP, QUE vs ZIP/RIS, ZIP vs RIS | |
| See comment | See comment | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one step for risk of bias: randomisation procedure, allocation concealment or blinding were not adequately described. | ||||||
| Specific antipsychotic compared with other drugs for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with tetrabenazine | Risk with haloperidol | |||||
| Tardive dyskinesia: not improved to a clinically important extent | Study population | RR 1.07 | 13 | ⊕⊝⊝⊝ | ||
| 667 per 1000 | 713 per 1000 | |||||
| Tardive dyskinesia: deterioration of symptoms | Study population | RR 0.86 | 13 | ⊕⊝⊝⊝ | ||
| 167 per 1000 | 143 per 1000 | |||||
| Mental state ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: any ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: extrapyramidal symptoms ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | Study population | RR 4.38 | 13 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one step for risk of bias: randomisation procedure, allocation concealment and blinding were not adequately described. | ||||||
| Interventions | Current reference (updates underway) |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | This review |
| Non‐neuroleptic catecholaminergic drugs | |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| 2 Tardive dyskinesia: no improvement (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| 3 Tardive dyskinesia: deterioration (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.11, 3.31] |
| 4 General mental state: relapse (long term) Show forest plot | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.16, 57.36] |
| 5 Acceptability of the treatment: leaving the study early (long term) Show forest plot | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement (medium term) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.23, 0.89] |
| 2 Tardive dyskinesia: average endpoint score (AIMS, high = poor) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐8.60, ‐2.40] |
| 3 General mental state: average endpoint score (BPRS, high = poor) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐10.48, 1.88] |
| 4 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| 5 Adverse effects: use of antiparkinsonism drugs (medium term) Show forest plot | 1 | 48 | Risk Ratio (IV, Fixed, 95% CI) | 2.08 [0.74, 5.86] |
| 5.1 Haloperidol | 1 | 12 | Risk Ratio (IV, Fixed, 95% CI) | 2.0 [0.56, 7.09] |
| 5.2 Risperidone | 1 | 36 | Risk Ratio (IV, Fixed, 95% CI) | 2.26 [0.37, 13.60] |
| 6 Adverse effects: parkinsonism ‐ average endpoint score (ESRS) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.25, 0.45] |
| 7 Adverse effects: dystonia ‐ average endpoint score (ESRS) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.76, 0.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement Show forest plot | 4 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Thiopropazate vs haloperidol ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 1.53 [0.58, 4.05] |
| 1.2 Zuclopenthixol vs haloperidol ‐ short term | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.79, 1.27] |
| 1.3 Olanzapine vs risperidone ‐ medium term | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.82, 1.90] |
| 1.4 Quetiapine vs haloperidol ‐ medium term | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 1.5 Quetiapine vs haloperidol ‐ long term | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 2 Tardive dyskinesia: not any improvement (short term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.41 [0.05, 3.28] |
| 2.2 Zuclopenthixol vs haloperidol | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
| 3 Tardive dyskinesia: deterioration (short term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 1.22 [0.09, 16.92] |
| 3.2 Zuclopenthixol vs haloperidol | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
| 4 Tardive dyskinesia: average endpoint score (various scales) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Molindone vs haloperidol, 100% masking dose (AIMS, short term) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 1.87 [‐0.20, 3.94] |
| 4.2 Molindone vs haloperidol, 200% masking dose (AIMS, short term) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.44 [1.12, 5.76] |
| 4.3 Zuclopenthixol vs haloperidol (SHRS, short term) | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 4.4 Olanzapine vs risperidone (AIMS, medium term) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐0.53, 4.93] |
| 5 Tardive dyskinesia: average change score (AIMS, low = better) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 1.66 [‐0.45, 3.77] |
| 5.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐2.85, 1.21] |
| 5.3 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 2.48 [0.44, 4.52] |
| 5.4 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.58, 4.98] |
| 6 General mental state: deterioration Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Zuclopenthixol vs haloperidol ‐ short term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.29] |
| 6.2 Olanzapine vs risperidone ‐ medium term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.64] |
| 6.3 Quetiapine vs haloperidol ‐ long term | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.62, 5.39] |
| 7 General mental state: average endpoint score (PANSS‐general psychopathology, low = better) (long term) Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.02, 1.62] |
| 7.1 Quetiapine vs haloperidol | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.02, 1.62] |
| 8 General mental state: average change score (BPRS, low = better) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐4.79, 2.51] |
| 8.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐2.46 [‐6.27, 1.35] |
| 8.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐8.37, 4.97] |
| 8.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [‐1.94, 4.58] |
| 9 Acceptability of the treatment: leaving the study early (short term) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Molindone vs haloperidol | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.44] |
| 10 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 3 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Olanzapine vs FGA | 1 | 56 | Risk Ratio (IV, Fixed, 95% CI) | 1.86 [0.18, 19.38] |
| 10.2 Amisulpride vs FGA | 1 | 55 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.06, 14.65] |
| 10.3 Olanzapine vs amisulpride | 1 | 57 | Risk Ratio (IV, Fixed, 95% CI) | 1.93 [0.19, 20.12] |
| 10.4 Olanzapine vs risperidone | 2 | 170 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.57, 0.95] |
| 10.5 Olanzapine vs quetiapine | 1 | 116 | Risk Ratio (IV, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 10.6 Olanzapine vs ziprasidone | 1 | 82 | Risk Ratio (IV, Fixed, 95% CI) | 0.77 [0.56, 1.05] |
| 10.7 Quetiapine vs risperidone | 1 | 118 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| 10.8 Quetiapine vs ziprasidone | 1 | 90 | Risk Ratio (IV, Fixed, 95% CI) | 1.10 [0.86, 1.40] |
| 10.9 Ziprasidone vs risperidone | 1 | 84 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 11 Acceptability of the treatment: leaving the study early (long term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Clozapine vs haloperidol | 1 | 39 | Risk Ratio (IV, Fixed, 95% CI) | 3.36 [0.45, 25.16] |
| 11.2 Quetiapine vs haloperidol | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 1.31 [0.63, 2.69] |
| 12 Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Risperidone vs haloperidol (medium term) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.34, 1.35] |
| 12.2 Quetiapine vs haloperidol (long term) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.96] |
| 13 Adverse effects: parkinsonism (SHRS) ‐ average endpoint scores (short term) Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 13.1 Zuclopenthixol vs haloperidol | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 14 Adverse effects: parkinsonism (SAS, ESRS, low = better) ‐ average change score (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐2.55, 0.85] |
| 14.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.45, 1.45] |
| 14.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.33, ‐0.07] |
| 14.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐2.44, 1.74] |
| 15 Adverse effects: dyskinesia (ESRS, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.91, 1.51] |
| 16 Adverse effects: akathisia (BAS, ESRS, low = better) ‐ average change scores (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.30, 0.46] |
| 16.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.42, 0.20] |
| 16.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.76, 0.16] |
| 16.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.12, 0.50] |
| 17 Adverse effects: dystonia (ESRS, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.41, 0.01] |
| 18 Adverse effects: general adverse events (UKU, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐1.85, 2.01] |
| 18.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.33, 1.23] |
| 18.3 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐0.93, 2.19] |
| 19 General global state: average change score (CGI) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.41, 0.27] |
| 19.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.47, 0.09] |
| 19.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.61, 0.81] |
| 19.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.19, 0.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesias: no clinically important improvement (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.51, 2.23] |
| 1.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.51, 2.23] |
| 2 Tardive dyskinesia: no improvement (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 2.57 [0.35, 18.68] |
| 2.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 2.57 [0.35, 18.68] |
| 3 Tardive dyskinesia: deterioration (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| 3.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| 4 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [0.25, 76.54] |
| 4.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [0.25, 76.54] |

