Antipsychotic reduction and/or cessation and antipsychotics as specific treatments for tardive dyskinesia
Appendices
Appendix 1. Previous methods
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. We included trials that were described as double‐blind, but that did not mention whether the study was randomised, in a sensitivity analysis. If there was no substantive difference within primary outcomes (see 'Types of outcome measures') when these studies were added, then we included them in the final analysis. If there was a substantive difference, we used only clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
We included people with schizophrenia or any other serious mental illness, diagnosed by any criteria, irrespective of gender, age or nationality who required the use of neuroleptics for more than three months and who developed TD (diagnosed by any criteria) during neuroleptic treatment, and for whom the dose of neuroleptic medication had been stable for one month or more. A post hoc change was made to include studies that did not require neuroleptic medication to have been stable for one month prior to randomisation. We felt it important to include these studies as they will provide additional important information. However, these will be analysed separately, and thus will not change existing outcome data.
Types of interventions
1. Reduction or cessation of the dose of the above drugs compared with the continuation of standard dose of the same compound. For the purposes of this review, these trials were divided into those that aimed to reduce the total dosage of neuroleptic medication, for example reduced dose and intermittent dosage schedule studies, and those that cease neuroleptics (sometimes after variable periods of dose reduction).
2. Specific neuroleptic drugs proposed to have TD lessening qualities compared to placebo or no intervention. A post hoc decision was made to broaden this criteria to also include neuroleptic versus neuroleptic for the treatment of TD.
Types of outcome measures
Clinical efficacy was defined as an improvement in the symptoms of TD of more than 50% on any scale.
The outcomes of interest were:
1. Tardive dyskinesia changes:
1.1. The number of people per treatment group who did not show an improvement of more than 50% on any TD scale
1.2. The number of people per treatment group who did not show any improvement on any TD scale
1.3. The number of people per treatment group who deteriorated on any TD scale
1.4. Treatment group mean change (endpoint ‐ baseline) on any TD scale
1.5 .Treatment group mean change endpoint on any TD scale
2. Global assessment:
2.1. The number of people per treatment group who were defined as relapsed (according to any definition)
2.2. Leaving the study early
3. Adverse effects:
3.1. The number of people per treatment group who had any adverse effect (other than deterioration of symptoms of TD or relapse)
When appropriate, the outcomes were grouped into time periods ‐ short term (less than 6 weeks), medium term (between 6 weeks and 6 months) and long term (over 6 months). Clinical efficacy was defined as an improvement in the symptoms of TD of more than 50%, on any scale.
Primary outcomes
Secondary outcomes
Search methods for identification of studies
1. Electronic searching for update (July 2005).
1.1. We searched The Cochrane Schizophrenia Group's Trials Register (July 2005) using the phrase:
[((dyskine* or diskine*) in title, abstract, index terms of REFERENCE) or ((dyskine* in health care conditions of STUDY)]
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
2. We electronically searched previous versions of the CSG library
2.1. We identified relevant randomised trials by searching several electronic databases (the Cochrane Schizophrenia Group's Register of trials), Biological Abstracts, EMBASE, LILACS, MEDLINE, PsycLIT and SCISEARCH).
2.2. We searched the The Cochrane Schizophrenia Group's Register using the following phrases:
[(dyskine*) and (*amitrex* or *deniban* or *socian* or *solian* or *sulanid* or *sulamid* or *zyprex* or *olanzapin* or *ziprasidone* or *zotepine* or *lodopin* or *nipolept* or *zopite* or *setous* or *majpin* or *cloza* or *leponex* or *71675‐85‐9* or *neuroleptic* or chlorpromazine* or chlorprothixene* or dogmatil* or droperidol* or flupenthixol* or fluperlapine* or fluphenazine* or flunarizine* or haloperidol* or lonidine* or loxapine* or molindone* or penfluridol* or perphenazine* or pimozide* or piperacetazine* or sulpiride* or thiopropazate* or thioridazine* or thiothixene* or trifluoperazine* or clozapine* or zuclopenthixol* or risperidone* or pericyazine in title) or (*reduction* or *cessation* or *withdrawal* or *decrease* or *intermittent* or *target*) and (dyskine*)] and (*amitrex* or *deniban* or *socian* or *solian* or *sulanid* or *sulamid* or *zyprex* or *olanzapin* or *ziprasidone* or *zotepine* or *lodopin* or *nipolept* or *zopite* or *setous* or *majpin* or *cloza* or *leponex* or *71675‐85‐9* or *neuroleptic* or *chlorpromazine* or *chlorprothixene* or *dogmatil* or *droperidol* or *flupenthixol* or *fluperlapine* or *fluphenazine* or *flunarizine* or *haloperidol* or *lonidine* or *loxapine* or *molindone* or *penfluridol* or *perphenazine* or *pimozide* or *piperacetazine* or *sulpiride* or *thiopropazate* or *thioridazine* or *thiothixene* or *trifluoperazine* or *clozapine* or *zuclopenthixol* or *risperidone* or *pericyazine in title, abstract, index terms of REFERENCE) or ((antipsychotics or Targeted Medication* or Treatment Frequency* or Withdrawal* or Dosage of Drug* in interventions of STUDY) and (tardive in health care conditions of STUDY)]
[(dyskine* or #30 = 28) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine or #42 = 500)]
[(reduction or cessation or withdrawal or decrease or intermittent or target*) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
2.3. We searched BIOLOGICAL ABSTRACTS (January 1982 to September 1997) using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with each of the two phrases:
[and ((tardive near (dyskine* or diskine*) or (abnormal near movement* near disorder*) or (involuntar* near movement*)) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
[and (reduction or cessation or withdrawal or decrease or intermittent or target*) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
2.4. We searched EMBASE (January 1980 to September 1997) using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with each of the two phrases:
[and ((tardive dyskinesia in thesaurus ‐subheadings, prevention, drug therapy, side effect and therapy) or (neuroleptic dyskinesia in thesaurus ‐all subheadings) or (tardive or dyskines*) or (movement* or disorder*) or (abnormal or movement* or disorder*)) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
[and (reduction or cessation or withdrawal or decrease or intermittent or target*) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
2.5. We searched LILACS (January 1982 to September 1996) using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with each of the two phrases:
[and ((tardive or (dyskinesia* or diskinesia*)) or (drug induced movement disorders in thesaurus)) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
[and (reduction or cessation or withdrawal or decrease or intermittent or target*) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
2.6. We searched MEDLINE (January 1966 to September 1997) using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with each of the two phrases:
[and ((movement‐disorders in MeSH / explode all subheadings) or (anti‐dyskinesia‐agents in MeSH / explode all subheadings) or (dyskinesia‐drug‐induced in MeSH / explode all subheadings) and (psychosis in MeSH / explode all subheadings) or (schizophrenic disorders in MeSH / explode all subheadings) or (tardive near (dyskine* or diskine*)) or (abnormal* near movement* near disorder*) or (involuntar* near movement*)) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
[and (reduction or cessation or withdrawal or decrease or intermittent or target*) and (antipsychotic‐agents / all subheadings or neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
2.7. We searched PsycLIT (January 1974 to September 1997) using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with each of the two phrases:
[and ((explode movement‐disorders in DE) or (explode tardive‐dyskinesia in DE) or (tardive near (dyskine* or diskine*) or (abnormal* near movement* near disorder*) or (involuntar* near movement*)) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
[and (reduction or cessation or withdrawal or decrease or intermittent or target*) and (neuroleptic* or chlorpromazine or chlorprothixene or dogmatil or droperidol or flupenthixol or fluperlapine or fluphenazine or flunarizine or haloperidol or lonidine or loxapine or molindone or penfluridol or perphenazine or pimozide or piperacetazine or sulpiride or thiopropazate or thioridazine or thiothixene or trifluoperazine or clozapine or zuclopenthixol or risperidone or pericyazine)]
3. Reference searching
3.1. SCISEARCH ‐ Science Citation Index
We sought each of the included studies as a citation on the SCISEARCH database. We inspected reports of articles that had been cited from these studies in order to identify further trials.
3.2. We inspected the references of all identified studies for more studies.
4. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Electronic searches
Searching other resources
Data collection and analysis
[For definitions of terms used in this, and other sections, please refer to the Glossary].
1. Selection of trials
We (KSW and JR) independently inspected citations from the searches and identified relevant abstracts. Where doubts arose, we acquired the full report for more detailed scrutiny. We obtained the full report of the abstracts that met the review criteria. Again, when resolving disputes by discussion was not possible, the article was added to those awaiting assessments and we contacted the authors of the study for clarification.
2. Assessment of methodological quality
KSW allocated trials to three quality criteria as described in the Cochrane Collaboration Handbook (Higgins 2005) and only trials meeting category A or B were included. In addition, we rated trials using the Jadad Scale (Jadad 1996) with a cut‐off of two points used to check the assessment made by the handbook criteria. However, the latter were not used to exclude trials in this review.
3. Data management
3.1. Data extraction
We (KSW and JR) independently extracted data from included studies. Again, any disagreements were discussed, the decisions documented and, where necessary, we contacted the authors of the studies for clarification. Justifications for excluding references from the review were documented. We anticipated that many trials would have inadequate reporting and therefore, for those who dropped out of studies, we assumed they had no change in their TD symptoms. When insufficient data were provided to identify the original group size (prior to drop outs), we contacted the authors and the trials were allocated to the list of those awaiting assessment.
3.2. Intention to treat analysis
We excluded data from studies where more than 50% of participants in any group were lost to follow up (this does not include the outcome of 'leaving the study early'). In studies with less than 50% dropout rate, people leaving early were considered to have had the negative outcome, except for the event of death.
3.3. Dichotomous yes/no ‐ data
We analysed dichotomous outcomes by calculating the relative risk (RR) (Fixed effects) with a 95% confidence interval (CI) to express the uncertainty of each result. The relative risk ratios from the individual trials were combined using appropriate methods of meta‐analysis. When overall results were significant, the number needed to treat (NNT) to produce (or prevent) one outcome was calculated by combining the overall (RR) with an estimate of the prevalence of the event in the control groups of the trials. If heterogeneity was found (see section 5) we used a random effects model.
3.4 Continuous data
3.4.1. Skewed data: continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, the following standards were applied to all data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors; (b) when a scale started from the finite number zero, the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); (c) if a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied to them. When continuous data are presented on a scale which includes a possibility of negative values (such as change on a scale), it is difficult to tell whether data are non‐normally distributed (skewed) or not.
For change data (endpoint minus baseline), the situation is even more problematic. In the absence of individual patient data it is impossible to know if data are skewed, though this is likely. After consulting the ALLSTAT electronic statistics mailing list, we presented change data in MetaView in order to summarise available information. In doing this, it was assumed either that data were not skewed or that the analyses could cope with the unknown degree of skew. Without individual patient data it is impossible to test this assumption. Where both change and endpoint data were available for the same outcome category, we only presented endpoint data. We acknowledge that by doing this much of the published change data were excluded, but argue that endpoint data is more clinically relevant and that if change data were to be presented along with endpoint data, it would be given undeserved equal prominence. We are contacting authors of studies reporting only change data for endpoint figures. Non‐normally distributed data were reported in the 'other data types' tables.
3.4.2. Rating Scales
A wide range of instruments are available to measure outcomes in mental health studies. These instruments vary in quality and many are not validated, or are even ad hoc. As a minimum standard, we did not include data from an instrument in this review unless the instrument and its properties had been published in a peer‐reviewed journal. In addition, the following minimum standards for instruments were set: The instrument should either be (a) a self‐report or (b) completed by an independent rater or relative (not the therapist) and (c) the instrument should be a global assessment of an area of functioning.
3.4.3. Intention to treat analysis
Where possible we analysed data on an intention‐to‐treat basis and assumed that those who had not been accounted for had the negative outcome e.g. global assessment 'not improved' and relapse. This rule did not include the outcomes death or adverse effects. We tested this assumption with a sensitivity analysis. For continuous data it is impossible to manage the data in this way therefore we presented 'completer' data. Where feasible, we converted continuous scores to dichotomous data.
If, for a given outcome, more than 50% of the total numbers randomised were not accounted for, we did not present the results (except for leaving the study early) as such data are impossible to interpret with authority. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we marked data with '*' to indicate that the result may well be prone to bias.
4. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation co‐efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co‐efficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account intra‐class correlation coefficients and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
5. Investigation for heterogeneity
Firstly, we considered all the included studies within any comparison to judge clinical heterogeneity. Then we visually inspected graphs to investigate the possibility of statistical heterogeneity. This was supplemented, primarily, by employing the I‐squared statistic. This provides an estimate of the percentage of inconsistency thought to be due to chance. Where the I‐squared estimate was greater than or equal to 75%, this was interpreted as evidence of high levels of heterogeneity (Higgins 2003). Data were then re‐analysed using a random effects model to see if this made a substantial difference. If it did, and results became more consistent, i.e. falling below 75% in the estimate, the studies were added to the main body of trials. If using the random effects model did not make a difference and inconsistency remained high, data were not summated, but were presented separately and reasons for heterogeneity investigated.
6. Addressing publication bias
We used funnel plots (trial effect versus trial size) for outcomes where more than five trials reported usable data, and visually inspected for asymmetry in an attempt to investigate the likelihood of overt publication bias (Egger 1997).

Message from one of the participants of the public and patient involvement consultation of service user perspectives on tardive dyskinesia research
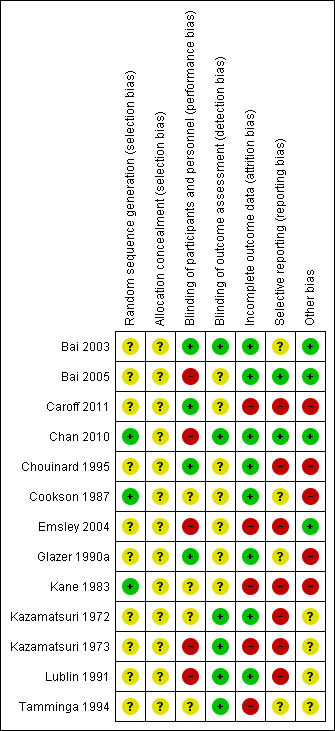
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
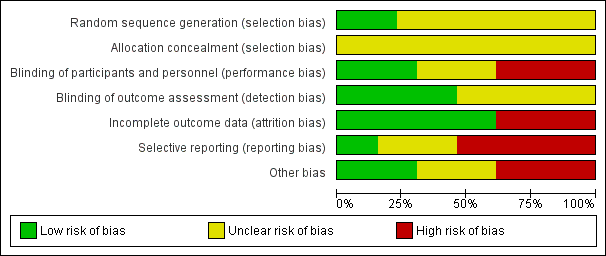
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
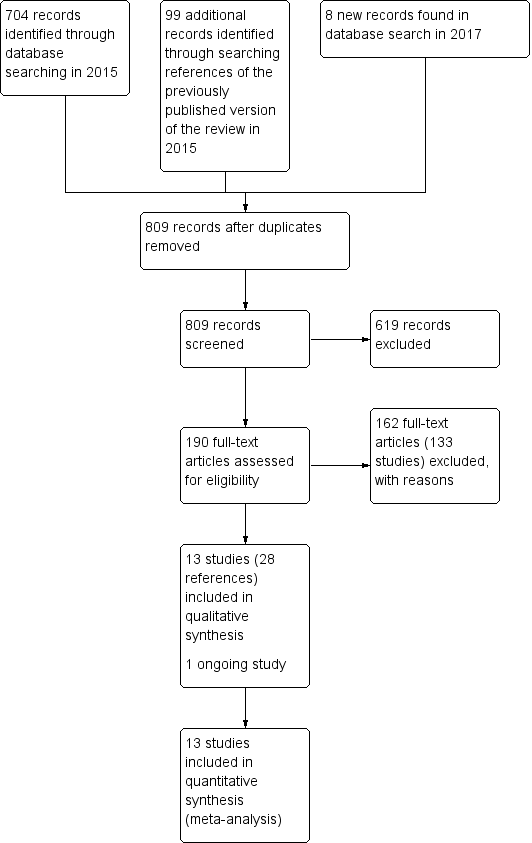
Study flow diagram for 2015 and 2017 searches for this review
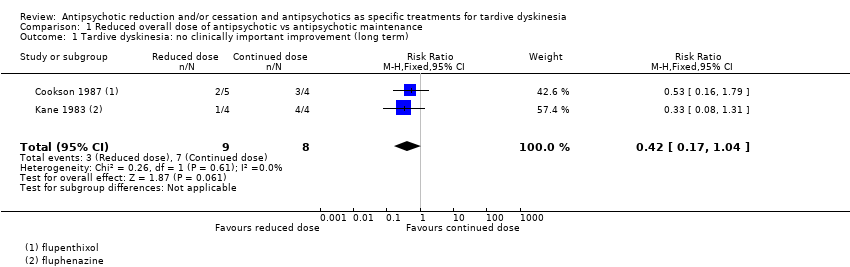
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 1 Tardive dyskinesia: no clinically important improvement (long term).
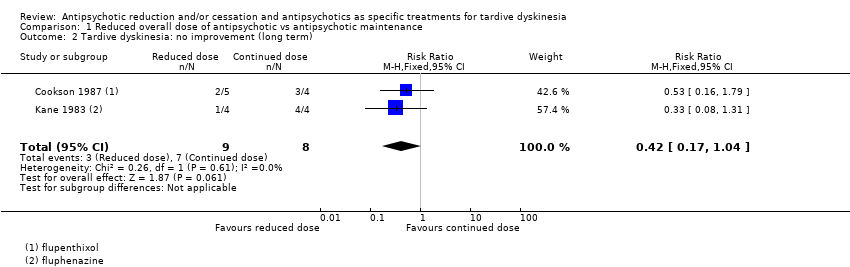
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 2 Tardive dyskinesia: no improvement (long term).
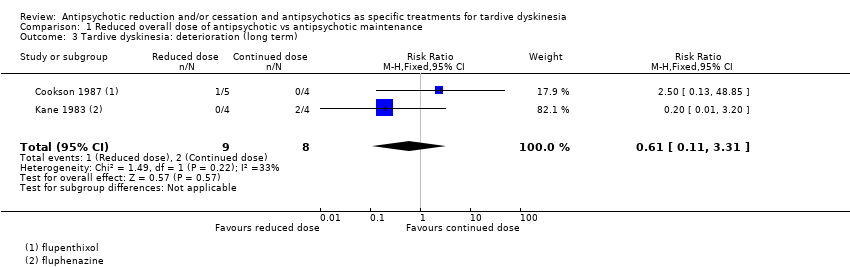
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 3 Tardive dyskinesia: deterioration (long term).
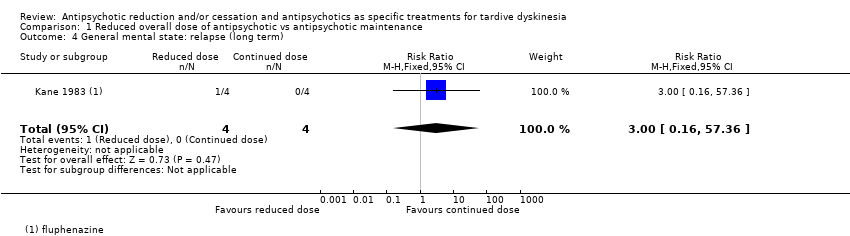
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 4 General mental state: relapse (long term).
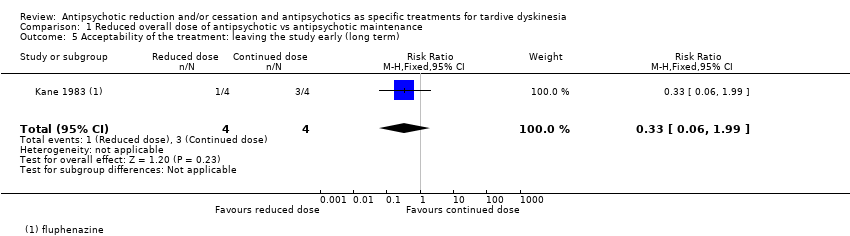
Comparison 1 Reduced overall dose of antipsychotic vs antipsychotic maintenance, Outcome 5 Acceptability of the treatment: leaving the study early (long term).
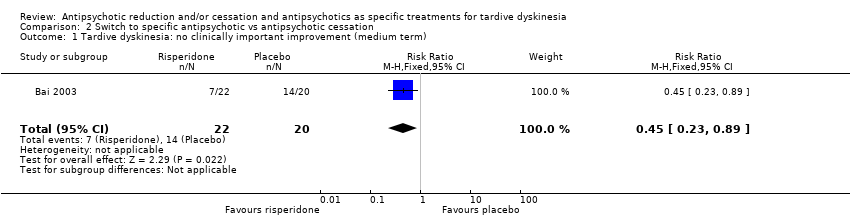
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 1 Tardive dyskinesia: no clinically important improvement (medium term).
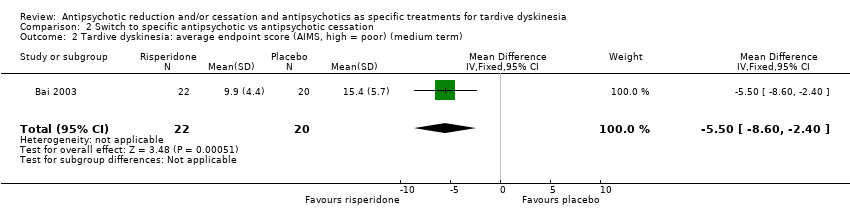
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 2 Tardive dyskinesia: average endpoint score (AIMS, high = poor) (medium term).
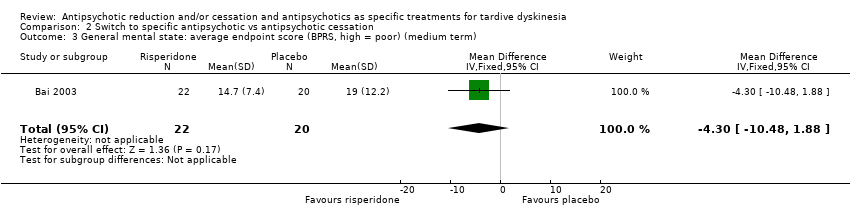
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 3 General mental state: average endpoint score (BPRS, high = poor) (medium term).
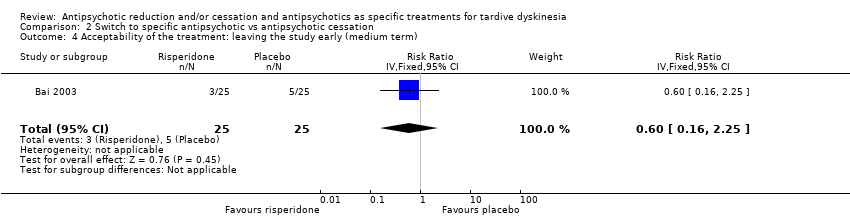
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 4 Acceptability of the treatment: leaving the study early (medium term).
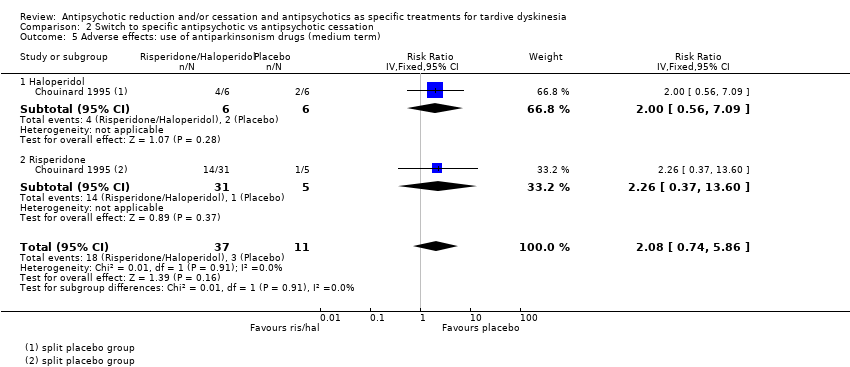
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 5 Adverse effects: use of antiparkinsonism drugs (medium term).
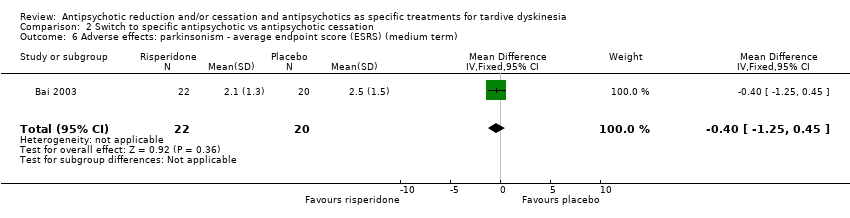
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 6 Adverse effects: parkinsonism ‐ average endpoint score (ESRS) (medium term).
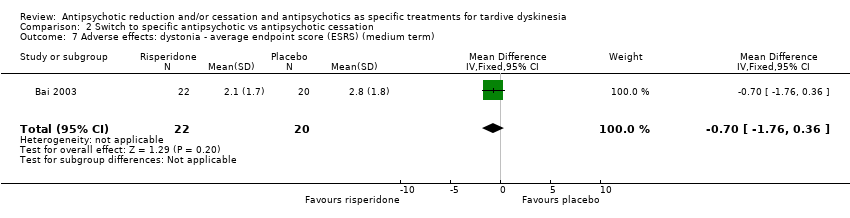
Comparison 2 Switch to specific antipsychotic vs antipsychotic cessation, Outcome 7 Adverse effects: dystonia ‐ average endpoint score (ESRS) (medium term).
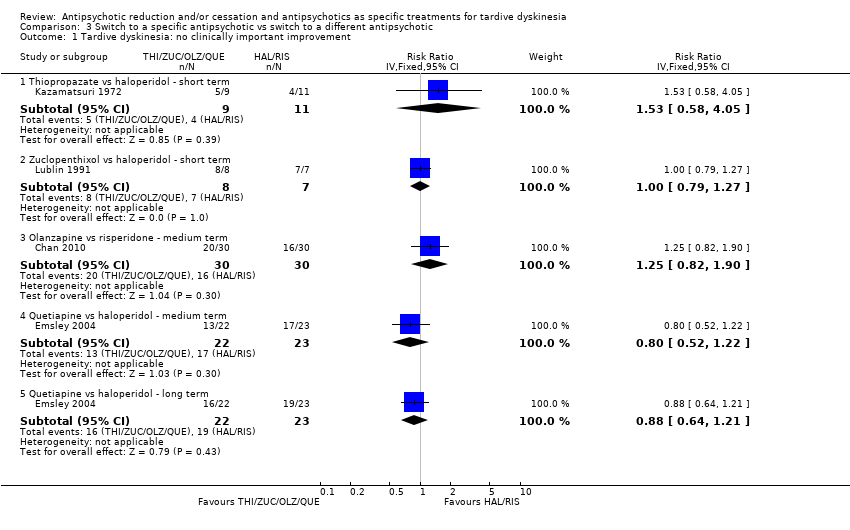
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 1 Tardive dyskinesia: no clinically important improvement.
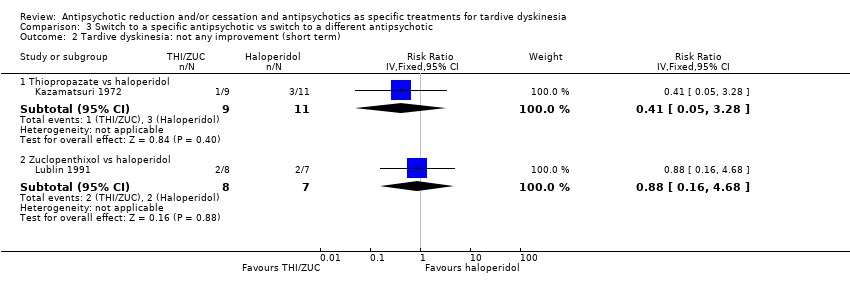
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 2 Tardive dyskinesia: not any improvement (short term).
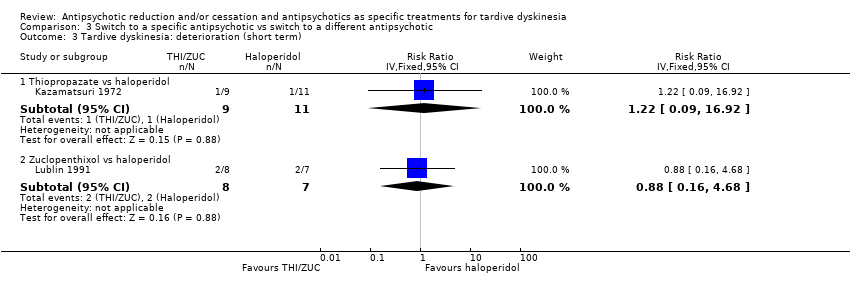
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 3 Tardive dyskinesia: deterioration (short term).
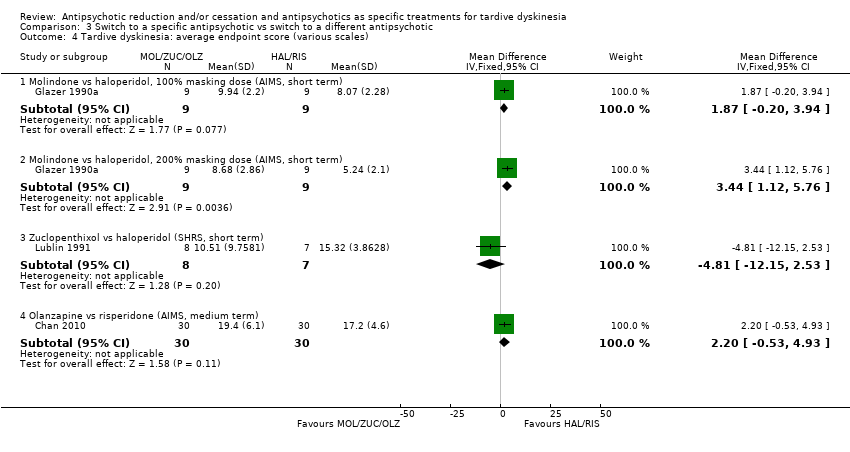
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 4 Tardive dyskinesia: average endpoint score (various scales).
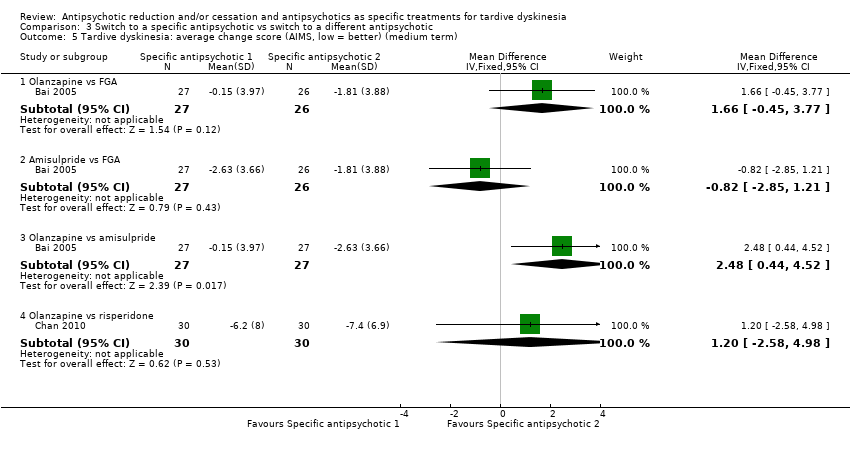
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 5 Tardive dyskinesia: average change score (AIMS, low = better) (medium term).
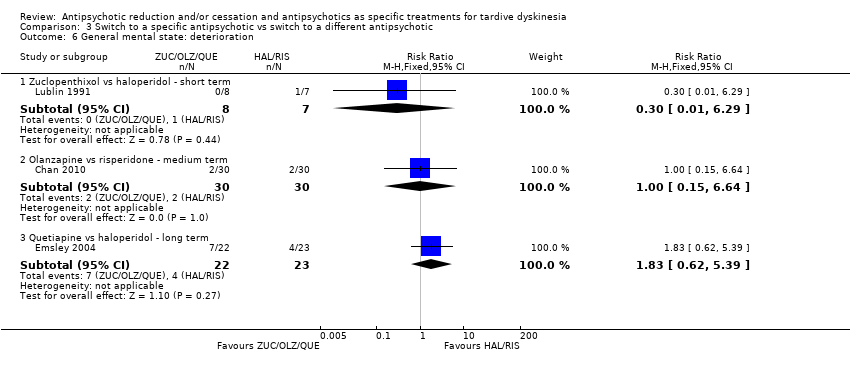
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 6 General mental state: deterioration.
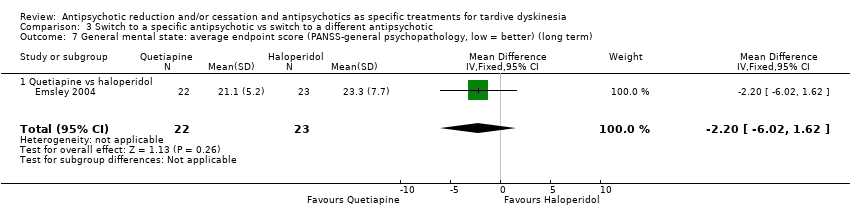
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 7 General mental state: average endpoint score (PANSS‐general psychopathology, low = better) (long term).
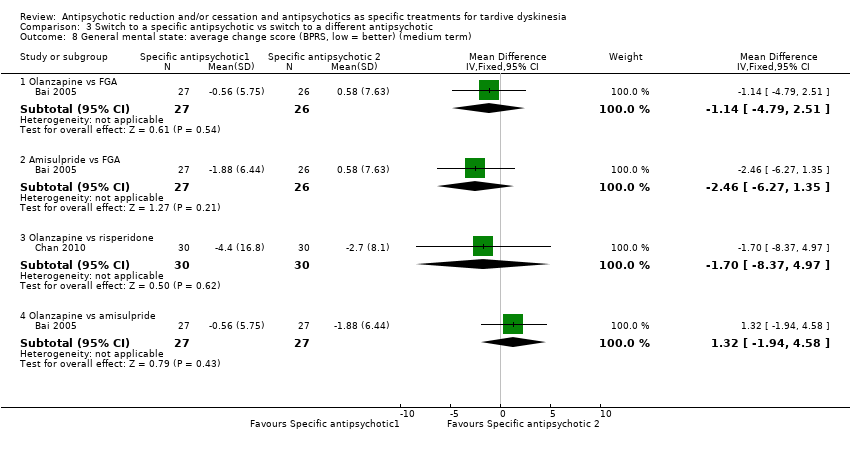
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 8 General mental state: average change score (BPRS, low = better) (medium term).
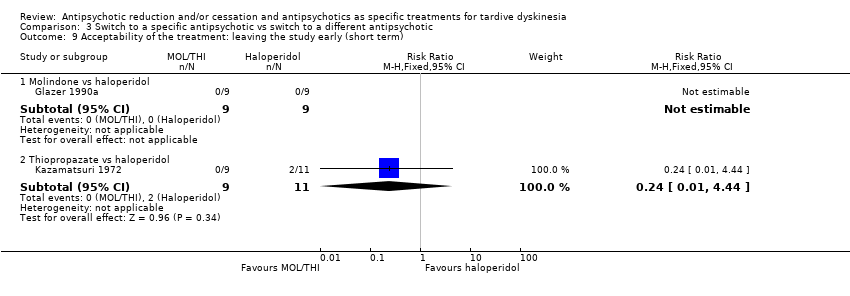
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 9 Acceptability of the treatment: leaving the study early (short term).
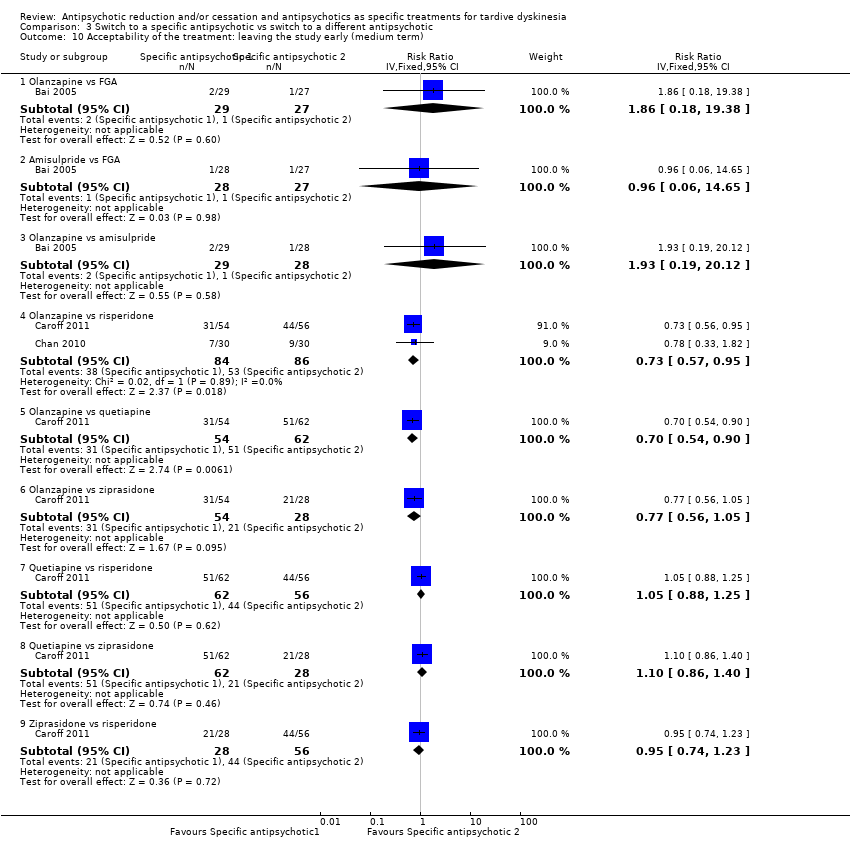
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 10 Acceptability of the treatment: leaving the study early (medium term).
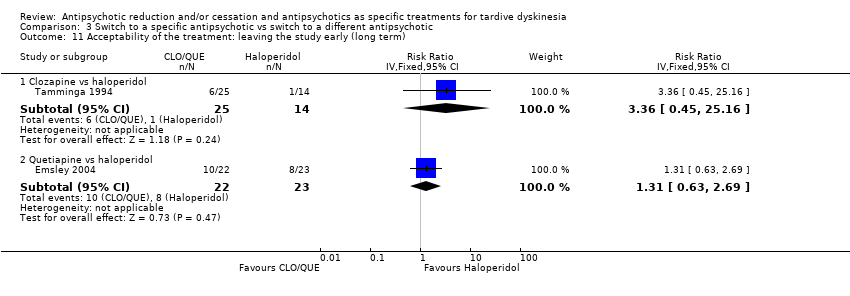
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 11 Acceptability of the treatment: leaving the study early (long term).
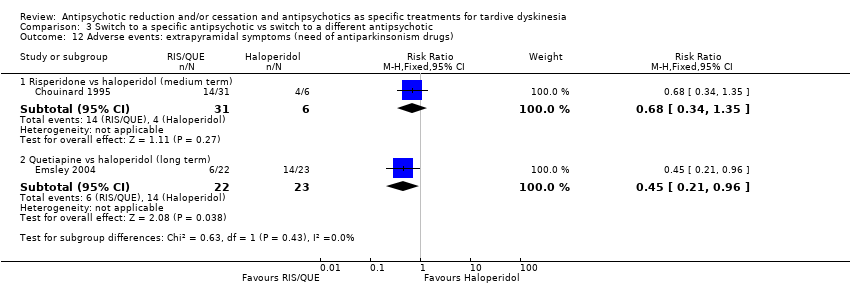
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 12 Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs).
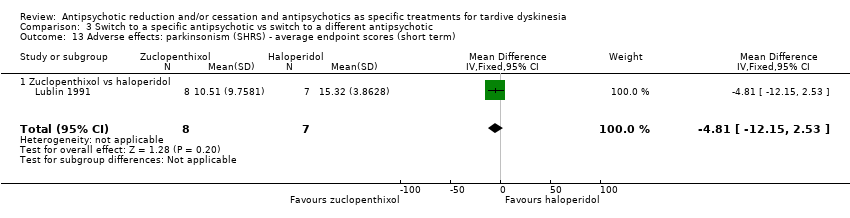
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 13 Adverse effects: parkinsonism (SHRS) ‐ average endpoint scores (short term).
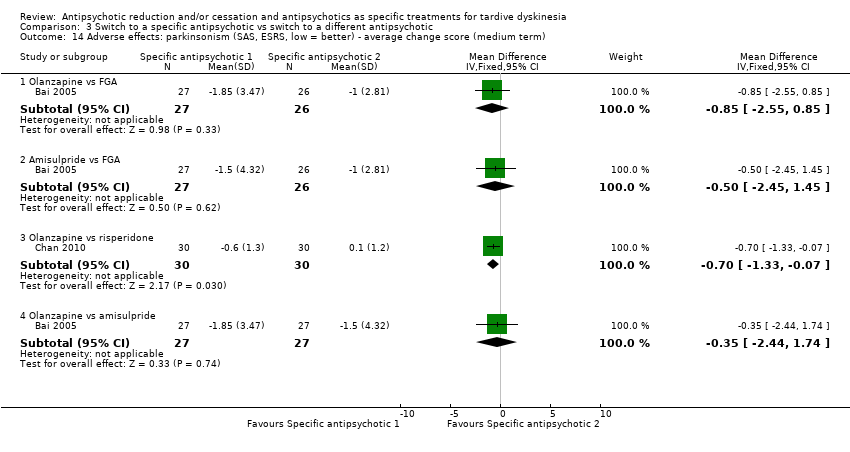
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 14 Adverse effects: parkinsonism (SAS, ESRS, low = better) ‐ average change score (medium term).
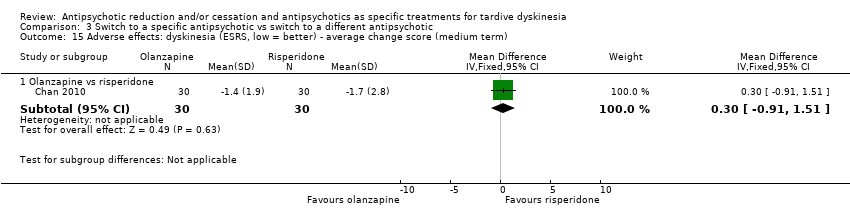
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 15 Adverse effects: dyskinesia (ESRS, low = better) ‐ average change score (medium term).
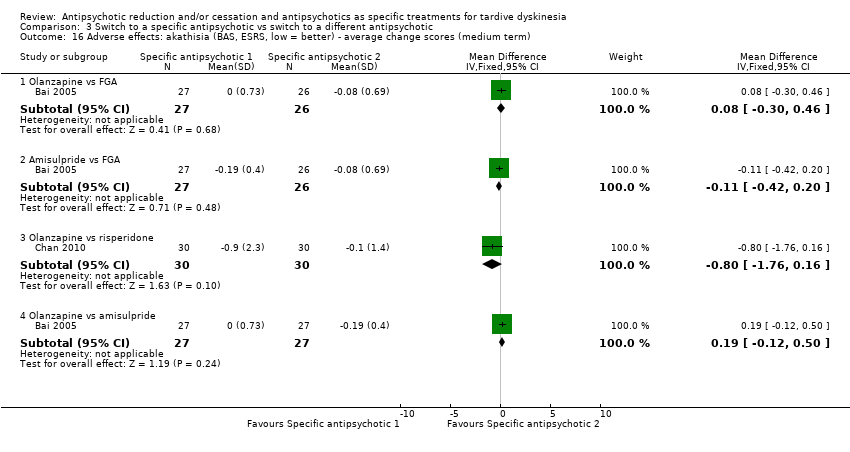
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 16 Adverse effects: akathisia (BAS, ESRS, low = better) ‐ average change scores (medium term).
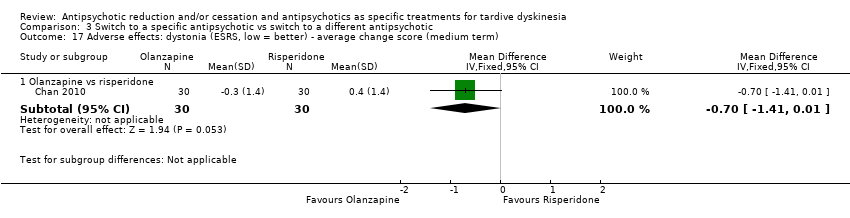
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 17 Adverse effects: dystonia (ESRS, low = better) ‐ average change score (medium term).
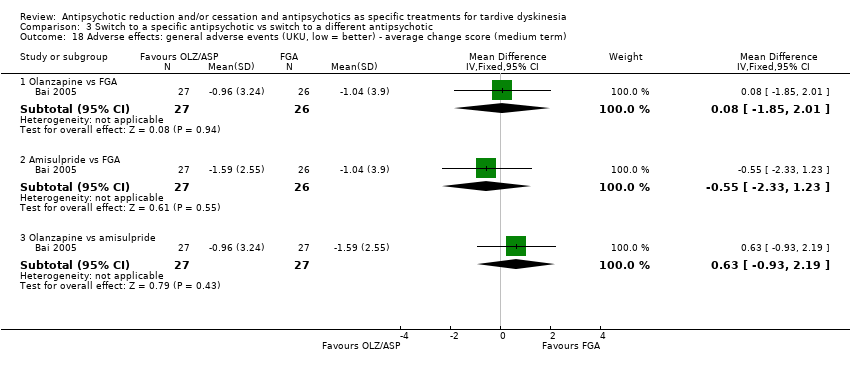
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 18 Adverse effects: general adverse events (UKU, low = better) ‐ average change score (medium term).
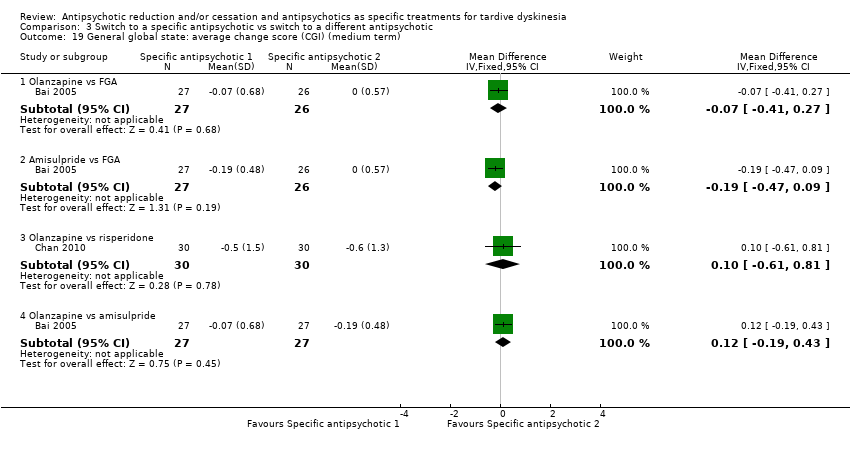
Comparison 3 Switch to a specific antipsychotic vs switch to a different antipsychotic, Outcome 19 General global state: average change score (CGI) (medium term).
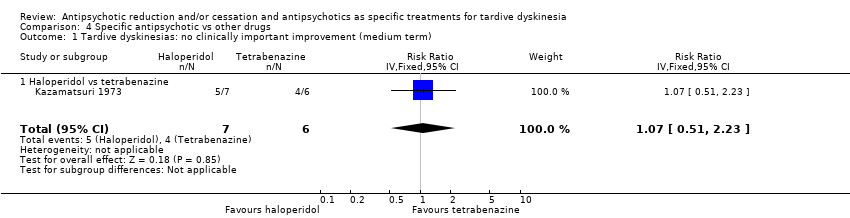
Comparison 4 Specific antipsychotic vs other drugs, Outcome 1 Tardive dyskinesias: no clinically important improvement (medium term).
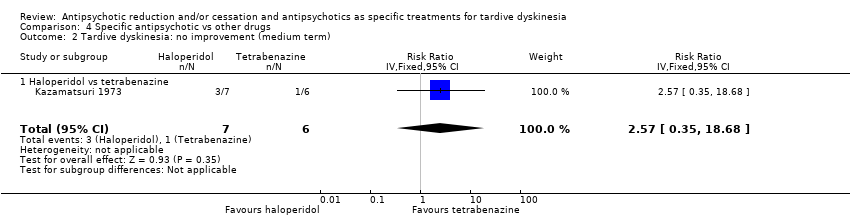
Comparison 4 Specific antipsychotic vs other drugs, Outcome 2 Tardive dyskinesia: no improvement (medium term).
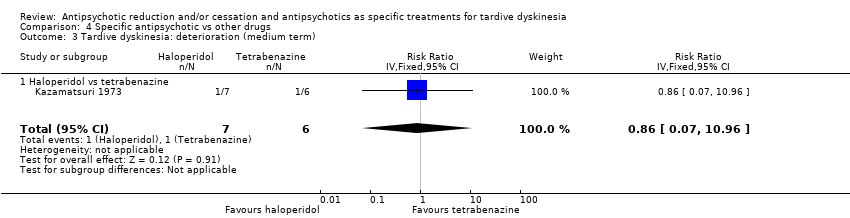
Comparison 4 Specific antipsychotic vs other drugs, Outcome 3 Tardive dyskinesia: deterioration (medium term).
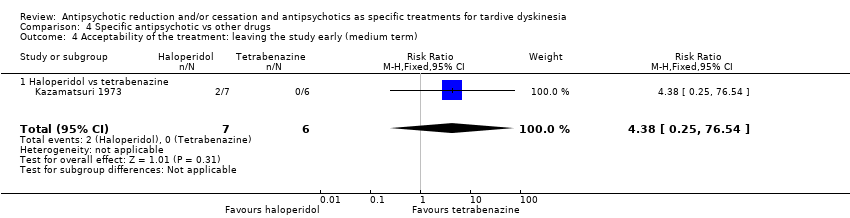
Comparison 4 Specific antipsychotic vs other drugs, Outcome 4 Acceptability of the treatment: leaving the study early (medium term).
| Study ID | Participants – people with: | Intervention | Comparison for review | Cochrane Review |
| Tardive dyskinesia | 1‐stepholidine vs placebo | 1‐stepholidine for schizophrenia | ‐ | |
| Schizophrenia | Amisulpride vs haloperidol | Amisulpride versus haloperidol for schizophrenia | ||
| Tardive dyskinesia | Biperiden vs no treatment | Anticholinergic drugs for tardive dyskinesia | ||
| Chlorprothixene versus haloperidol vs perphenazine vs haloperidol + biperiden | ||||
| Biperiden vs placebo | ||||
| Schizophrenia | Ethopropazine vs benztropine | Anticholinergics for parkinsonism | ||
| Abrupt neuroleptic cessation vs neuroleptic maintenance | Antipsychotic reduction or withdrawal for schizophrenia | |||
| Abrupt neuroleptic cessation vs neuroleptic maintenance | ||||
| Fluphenazine/flupenthixol decanoate continuation vs withdrawal | ||||
| Withdrawal of fluphenazine decanoate vs continuation | ||||
| Trifluoperazine withdrawal vs trifluoperazine continuation | ||||
| Dose reduction vs maintenance (both arms used flupenthixol decanoate) | ||||
| Olanzapine with different timings of dose‐reduction periods | ||||
| Fluphenazine withdrawal vs continuation | ||||
| Low‐ vs conventional‐dose maintenance therapy with fluphenazine decanoate | ||||
| Haloperidol dose reduction vs maintained dose | ||||
| Abrupt neuroleptic cessation vs neuroleptic maintenance | ||||
| Tardive dyskinesia | Flunarizine vs placebo | Calcium channel blockers for neuroleptic‐induced tardive dyskinesia | ||
| Schizophrenia | Chlorpromazine schedule A vs chlorpromazine schedule B | Chlorpromazine timing of dose for schizophrenia. | ||
| Tardive dyskinesia | Chlorprothixene vs haloperidol vs perphenazine vs haloperidol + biperiden | Chlorprothixene for schizophrenia. | ||
| Schizophrenia | Clozapine vs haloperidol | Clozapine versus haloperidol for schizophrenia | ||
| Clozapine vs haloperidol | ||||
| Clozapine vs olanzapine | Clozapine versus olanzapine for schizophrenia | |||
| Clozapine vs olanzapine | ||||
| Gilles de la Tourette's, Huntington's disease and drug‐induced atypical dyskinesia | Clozapine vs placebo | Clozapine versus placebo for schizophrenia. | ||
| Schizophrenia | Clozapine versus risperidone | Clozapine versus risperidone for schizophrenia | ||
| Haloperidol decanoate vs fluphenazine decanoate | Depot fluphenazine for schizophrenia | |||
| Fluphenazine decanoate vs haloperidol decanoate | ||||
| Fluphenazine decanoate vs placebo | ||||
| Fluphenazine decanoate vs intermittent pimozide | ||||
| Fluphenazine decanoate vs vitamin B complex | ||||
| Fluphenazine ethanoate vs pipothiazine palmitate | ||||
| Haloperidol decanoate vs fluphenazine decanoate | Depot haloperidol decanoate for schizophrenia. | |||
| Fluphenazine ethanoate vs pipothiazine palmitate | Depot pipothiazine for schizophrenia. | |||
| Progabide vs placebo | GABA for schizophrenia | |||
| Tardive dyskinesia and psychiatric history | Metoclopramide (10 mg, 20 mg or 40 mg) vs haloperidol (5 mg or 10 mg) | Haloperdiol dose for schizophrenia | ||
| Schizophrenia | Haloperidol vs olanzapine | Haloperidol vs olanzapine for schizophrenia | ||
| Tardive dyskinesia | Chlorprothixene vs haloperidol vs perphenazine vs haloperidol + biperiden | Haloperidol vs perphenazine for schizophrenia | ||
| Schizophrenia | Haloperidol vs risperidone | Haloperidol vs risperidone for schizophrenia | ||
| Brief intermittent antipsychotic treatment vs fluphenazine decanoate | Intermittent antipsychotic treatment for schizophrenia | |||
| Fluphenazine decanoate vs intermittent pimozide | ||||
| Haloperidol with 'drug holiday' vs haloperidol | ||||
| Withdrawal of fluphenazine decanoate vs continuation | ||||
| Lithium vs placebo | Lithium for schizophrenia | |||
| Molidone vs haloperidol | Molidone vs haloperidol for schizophrenia | |||
| Olanzapine 1 mg vs olanzapine 10 mg versus placebo | Olanzapine dose for schizophrenia. | |||
| Olanzapine vs "conventional antipsychotic drugs" | Olanzapine for schizophrenia | |||
| First‐generation antipsychotic vs second‐generation antipsychotic | ||||
| Olanzapine with different timings of dose reduction periods | Olanzapine reduction for schizophrenia | |||
| First‐generation antipsychotic versus second‐generation antipsychotic | Olanzapine vs other atypical antipsychotics for schizophrenia | |||
| Olanzapine 1 mg vs olanzapine 10 mg vs placebo | Olanzapine vs placebo for schizophrenia | |||
| First‐generation antipsychotic vs second‐generation antipsychotic | Perphenazine for schizophrenia | |||
| Fluphenazine decanoate vs intermittent pimozide | Pimozide for schizophrenia | |||
| Quetiapine vs continuation of usual antipsychotic | Quetiapine vs continuation of usual antipsychotic for schizophrenia | |||
| First generation antipsychotic vs second‐generation antipsychotic | Quetiapine vs other atypical antipsychotics for schizophrenia | |||
| Quetiapine vs typical antipsychotic medications for schizophrenia | ||||
| Risperidone vs olanzapine for schizophrenia | ||||
| Risperidone vs other atypical antipsychotics for schizophrenia | ||||
| Quetiapine vs continuation of usual antipsychotic | Switching antipsychotic for schizophrenia. | |||
| Tardive dyskinesia | Thiopropazate vs placebo | Thiopropazate for schizophrenia | ||
| Schizophrenia | Thiopropazine vs trifluoperazine vs placebo | Thiopropazine vs placebo for schizophrenia | ||
| Thiopropazine vs trifluoperazine for schizophrenia | ||||
| Tardive dyskinesia | Thioproperazine and tiapride vs placebo | Thioproperazine for schizophrenia | ||
| Tiapride for schizophrenia | ||||
| Tiapride vs placebo | ||||
| Schizophrenia | Trifluoperazine high‐dose vs trifluoperazine low‐dose vs placebo | Trifluoperazine dose for schizophrenia | ||
| Trifluoperazine vs placebo for schizophrenia | ||||
| Thiopropazine vs trifluoperazine vs placebo | ||||
| Fluphenazine decanoate vs vitamin B complex | Vitamins for schizophrenia | |||
| First‐generation antipsychotic vs second‐generation antipsychotic | Ziprasidone vs other atypical antipsychotics for schizophrenia |
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described |
| Participants | People with antipsychotic‐induced tardive dyskinesiaa |
| Interventions | 1. Antipsychotic reduction/cessation (N = 150) vs antipsychotic maintenance (N = 150) OR 2. Specific antipsychotic (N = 150) vs other specific antipsychotic (N = 150) |
| Outcomes | Tardive dyskinesia: any clinically important improvement in tardive dyskinesia, any improvement, deteriorationc |
| Notes | aThis could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. bSize of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| Reduced dose of antipsychotic compared with antipsychotic maintenance for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (schizophrenia or schizoaffective disorder) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antipsychotic maintenance | Risk with reduced dose of antipsychotic | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 0.42 | 17 | ⊕⊝⊝⊝ | ||
| 875 per 1000 | 368 per 1000 | |||||
| Tardive dyskinesia: deterioration of symptoms | Study population | RR 0.61 | 17 | ⊕⊝⊝⊝ | ||
| 250 per 1000 | 153 per 1000 | |||||
| General mental state: relapse | Study population | RR 3.00 | 8 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effect: any ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: extrapyramidal symptoms ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | Study population | RR 0.33 | 8 | ⊕⊝⊝⊝ | ||
| 750 per 1000 | 248 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for risk of bias: none of the studies adequately described allocation concealment, one study was a subsample from one site of an RCT, and one study's baseline characteristics were not balanced between study groups. | ||||||
| Antipsychotic cessation compared with antipsychotic maintenance for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antipsychotic maintenance | Antipsychotic cessation | |||||
| There is no evidence about the effects of withdrawal of antipsychotics compared with continuation of antipsychotics; none of the included studies evaluated this comparison. | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Switch to another antipsychotic compared with antipsychotic cessation for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antipsychotic cessation (placebo) | Risk with switch to another antipsychotic | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 0.45 | 42 | ⊕⊕⊝⊝ | ||
| 700 per 1000 | 315 per 1000 | |||||
| Tardive dyskinesia: deterioration of symptoms ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| General mental state: average endpoint score (BPRS, high = poor) | The mean general mental state average endpoint score (BPRS, high = poor) was 19 | MD 4.30 lower | ‐ | 42 | ⊕⊝⊝⊝ | |
| Adverse effect: any ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effects: use of antiparkinsonism drugs | Study population | RR 2.08 | 48 | ⊕⊝⊝⊝ | Another study reported ESRS scale data for parkinsonism and also found little or no difference between groups (MD ‐0.4 95% CI ‐1.25 to 0.45, 42 participants). | |
| 273 per 1000 | 567 per 1000 | |||||
| Acceptability of the treatment: leaving the study early | Study population | RR 0.60 | 50 | ⊕⊝⊝⊝ | ||
| 200 per 1000 | 120 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias: generation of random sequence and allocation concealment not adequately described. | ||||||
| Switch to specific antipsychotic compared with switch to a different specific antipsychotic for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with specific antipsychotic 1 | Risk with specific antipsychotic 2 | |||||
| Tardive dyskinesia: no clinically important improvement Follow‐up: 3‐50 weeks | Study population | ‐ | 140 | ⊕⊝⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. The following comparisons found no clinically important improvement: THI vs HAL, ZUC vs HAL, OLZ vs RIS, QUE vs HAL | |
| See comment | See comment | |||||
| Tardive dyskinesia: deterioration Follow‐up: 3‐4 weeks | Study population | ‐ | 35 | ⊕⊝⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. The following comparisons found no difference in deterioration: THI vs HAL, ZUC vs HAL | |
| See comment | See comment | |||||
| General mental state: deterioration Follow‐up: 3‐50 weeks | Study population | ‐ | 120 | ⊕⊝⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. The following comparisons found no difference in mental state deterioration: ZUC vs HAL, OLZ vs RIS, QUE vs HAL | |
| See comment | See comment | |||||
| Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs) Follow‐up: 8‐50 weeks | Study population | ‐ | 53 | ⊕⊕⊝⊝ | No meta‐analysis, studies stratified by antipsychotic. HAL more likely to need antiparkinsonism drugs than QUE (1 RCT, 45 participants, RR 0.45, 95% CI 0.21 to 0.96). No difference: RIS vs HAL | |
| See comment | See comment | |||||
| Adverse effects: general adverse events (UKU Average change score) Follow‐up: 24 weeks | See comment | See comment | ‐ | 80 | ⊕⊝⊝⊝ | No meta‐analysis, 3‐arm study comparing OLZ, ASP and unspecified FGAs found no difference in general adverse events for all pairwise comparisons. |
| Acceptability of the treatment: leaving the study early Follow‐up: 2 weeks ‐ 18 months | Study population | ‐ | 466 | ⊕⊝⊝⊝ | RIS more likely to leave study early than OLZ (2 RCTs, 130 participants, RR 0.73, 95% CI 0.57 to 0.95). Remaining studies no meta‐analysis, no difference (6 RCTs, 450 participants): MOL/THI/CLO/QUE vs HAL, OLZ/ASP vs unspecified FGAs, OLZ vs QUE/ZIP, QUE vs ZIP/RIS, ZIP vs RIS | |
| See comment | See comment | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one step for risk of bias: randomisation procedure, allocation concealment or blinding were not adequately described. | ||||||
| Specific antipsychotic compared with other drugs for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with tetrabenazine | Risk with haloperidol | |||||
| Tardive dyskinesia: not improved to a clinically important extent | Study population | RR 1.07 | 13 | ⊕⊝⊝⊝ | ||
| 667 per 1000 | 713 per 1000 | |||||
| Tardive dyskinesia: deterioration of symptoms | Study population | RR 0.86 | 13 | ⊕⊝⊝⊝ | ||
| 167 per 1000 | 143 per 1000 | |||||
| Mental state ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: any ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: extrapyramidal symptoms ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | Study population | RR 4.38 | 13 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one step for risk of bias: randomisation procedure, allocation concealment and blinding were not adequately described. | ||||||
| Interventions | Current reference (updates underway) |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | This review |
| Non‐neuroleptic catecholaminergic drugs | |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| 2 Tardive dyskinesia: no improvement (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| 3 Tardive dyskinesia: deterioration (long term) Show forest plot | 2 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.11, 3.31] |
| 4 General mental state: relapse (long term) Show forest plot | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.16, 57.36] |
| 5 Acceptability of the treatment: leaving the study early (long term) Show forest plot | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement (medium term) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.23, 0.89] |
| 2 Tardive dyskinesia: average endpoint score (AIMS, high = poor) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐8.60, ‐2.40] |
| 3 General mental state: average endpoint score (BPRS, high = poor) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐10.48, 1.88] |
| 4 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| 5 Adverse effects: use of antiparkinsonism drugs (medium term) Show forest plot | 1 | 48 | Risk Ratio (IV, Fixed, 95% CI) | 2.08 [0.74, 5.86] |
| 5.1 Haloperidol | 1 | 12 | Risk Ratio (IV, Fixed, 95% CI) | 2.0 [0.56, 7.09] |
| 5.2 Risperidone | 1 | 36 | Risk Ratio (IV, Fixed, 95% CI) | 2.26 [0.37, 13.60] |
| 6 Adverse effects: parkinsonism ‐ average endpoint score (ESRS) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.25, 0.45] |
| 7 Adverse effects: dystonia ‐ average endpoint score (ESRS) (medium term) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.76, 0.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: no clinically important improvement Show forest plot | 4 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Thiopropazate vs haloperidol ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 1.53 [0.58, 4.05] |
| 1.2 Zuclopenthixol vs haloperidol ‐ short term | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.79, 1.27] |
| 1.3 Olanzapine vs risperidone ‐ medium term | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.82, 1.90] |
| 1.4 Quetiapine vs haloperidol ‐ medium term | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 1.5 Quetiapine vs haloperidol ‐ long term | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 2 Tardive dyskinesia: not any improvement (short term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.41 [0.05, 3.28] |
| 2.2 Zuclopenthixol vs haloperidol | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
| 3 Tardive dyskinesia: deterioration (short term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 1.22 [0.09, 16.92] |
| 3.2 Zuclopenthixol vs haloperidol | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
| 4 Tardive dyskinesia: average endpoint score (various scales) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Molindone vs haloperidol, 100% masking dose (AIMS, short term) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 1.87 [‐0.20, 3.94] |
| 4.2 Molindone vs haloperidol, 200% masking dose (AIMS, short term) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.44 [1.12, 5.76] |
| 4.3 Zuclopenthixol vs haloperidol (SHRS, short term) | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 4.4 Olanzapine vs risperidone (AIMS, medium term) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐0.53, 4.93] |
| 5 Tardive dyskinesia: average change score (AIMS, low = better) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 1.66 [‐0.45, 3.77] |
| 5.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐2.85, 1.21] |
| 5.3 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 2.48 [0.44, 4.52] |
| 5.4 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.58, 4.98] |
| 6 General mental state: deterioration Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Zuclopenthixol vs haloperidol ‐ short term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.29] |
| 6.2 Olanzapine vs risperidone ‐ medium term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.64] |
| 6.3 Quetiapine vs haloperidol ‐ long term | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.62, 5.39] |
| 7 General mental state: average endpoint score (PANSS‐general psychopathology, low = better) (long term) Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.02, 1.62] |
| 7.1 Quetiapine vs haloperidol | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.02, 1.62] |
| 8 General mental state: average change score (BPRS, low = better) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐4.79, 2.51] |
| 8.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐2.46 [‐6.27, 1.35] |
| 8.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐8.37, 4.97] |
| 8.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [‐1.94, 4.58] |
| 9 Acceptability of the treatment: leaving the study early (short term) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Molindone vs haloperidol | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Thiopropazate vs haloperidol | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.44] |
| 10 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 3 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Olanzapine vs FGA | 1 | 56 | Risk Ratio (IV, Fixed, 95% CI) | 1.86 [0.18, 19.38] |
| 10.2 Amisulpride vs FGA | 1 | 55 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.06, 14.65] |
| 10.3 Olanzapine vs amisulpride | 1 | 57 | Risk Ratio (IV, Fixed, 95% CI) | 1.93 [0.19, 20.12] |
| 10.4 Olanzapine vs risperidone | 2 | 170 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.57, 0.95] |
| 10.5 Olanzapine vs quetiapine | 1 | 116 | Risk Ratio (IV, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 10.6 Olanzapine vs ziprasidone | 1 | 82 | Risk Ratio (IV, Fixed, 95% CI) | 0.77 [0.56, 1.05] |
| 10.7 Quetiapine vs risperidone | 1 | 118 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| 10.8 Quetiapine vs ziprasidone | 1 | 90 | Risk Ratio (IV, Fixed, 95% CI) | 1.10 [0.86, 1.40] |
| 10.9 Ziprasidone vs risperidone | 1 | 84 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 11 Acceptability of the treatment: leaving the study early (long term) Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Clozapine vs haloperidol | 1 | 39 | Risk Ratio (IV, Fixed, 95% CI) | 3.36 [0.45, 25.16] |
| 11.2 Quetiapine vs haloperidol | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 1.31 [0.63, 2.69] |
| 12 Adverse events: extrapyramidal symptoms (need of antiparkinsonism drugs) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Risperidone vs haloperidol (medium term) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.34, 1.35] |
| 12.2 Quetiapine vs haloperidol (long term) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.96] |
| 13 Adverse effects: parkinsonism (SHRS) ‐ average endpoint scores (short term) Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 13.1 Zuclopenthixol vs haloperidol | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐12.15, 2.53] |
| 14 Adverse effects: parkinsonism (SAS, ESRS, low = better) ‐ average change score (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐2.55, 0.85] |
| 14.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.45, 1.45] |
| 14.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.33, ‐0.07] |
| 14.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐2.44, 1.74] |
| 15 Adverse effects: dyskinesia (ESRS, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.91, 1.51] |
| 16 Adverse effects: akathisia (BAS, ESRS, low = better) ‐ average change scores (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.30, 0.46] |
| 16.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.42, 0.20] |
| 16.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.76, 0.16] |
| 16.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.12, 0.50] |
| 17 Adverse effects: dystonia (ESRS, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.41, 0.01] |
| 18 Adverse effects: general adverse events (UKU, low = better) ‐ average change score (medium term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐1.85, 2.01] |
| 18.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.33, 1.23] |
| 18.3 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐0.93, 2.19] |
| 19 General global state: average change score (CGI) (medium term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 Olanzapine vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.41, 0.27] |
| 19.2 Amisulpride vs FGA | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.47, 0.09] |
| 19.3 Olanzapine vs risperidone | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.61, 0.81] |
| 19.4 Olanzapine vs amisulpride | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.19, 0.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesias: no clinically important improvement (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.51, 2.23] |
| 1.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.51, 2.23] |
| 2 Tardive dyskinesia: no improvement (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 2.57 [0.35, 18.68] |
| 2.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 2.57 [0.35, 18.68] |
| 3 Tardive dyskinesia: deterioration (medium term) Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| 3.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.07, 10.96] |
| 4 Acceptability of the treatment: leaving the study early (medium term) Show forest plot | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [0.25, 76.54] |
| 4.1 Haloperidol vs tetrabenazine | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [0.25, 76.54] |

