نقش داروهای کاتکولامینرژیک غیر‐آنتیسایکوتیک در درمان تاردیو دیسکنزی ناشی از آنتیسایکوتیکها
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: randomised, no further details. Raters: blinding of raters not reported. | |
| Participants | Diagnosis: psychiatric disease (no operational criteria) and institutionalised with antipsychotic‐induced tardive dyskinesia. Duration of TD: not reported. | |
| Interventions | 1. Tiapride: dose 100 mg tid/day for 2 weeks. N = 7. 2. Placebo: N = 5. Previous treatment, including that prescribed for the TD, was continued without alterations throughout the trial. No further details on concomitant medications were reported. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | Sponsorship source: Delagrange provided Tiapride. Additional sponsorship details not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to two groups"; further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear, in the introduction it is stated that: "However, the results from these studies seemed to justify a double‐blind controlled cross‐over trial and objective evaluation of the effect of Tiapride on the involuntary movements"; the Methods section does not report blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) | Low risk | "All twelve patients completed the trial". |
| Selective reporting (reporting bias) | High risk | "Besides these quantitative methods, self‐assessment analogue three‐point scales were made by the patients, and subjective analogue ratings were made on a five‐point scale by family, nurses and attendant doctors. At each recording session the patient was asked about possible side‐effects of the treatment. At each investigation motor performance speed was quantified (Schuhfried apparatus) to study possible parkinsonian effect of Tiapride ". "The results of the assessment analogue scales were inaccurate. The patients gave inconsistent answers in 3.1%, the nurses and the attendant doctors even in 37%. Further analysis of the subjective results has been discarded because of the reason outlined above and the fact that statistical analysis on three and five‐point scales does not have enough sensitivity for such a small group of patients." |
| Other bias | High risk | "the randomization has partly failed with respect to the seriousness of the dyskinesia of the patients: the second group consisted of more affected patients." |
| Methods | Allocation: "cross over randomized trial". Duration: 4 weeks. Design: cross‐over. Setting: inpatients, China. Raters: blinding of raters not reported. | |
| Participants | Diagnosis: Antipsychotics‐induced tardive dyskinesia. N = 20*. Sex: 12 M, 8 F. Agemean 34.86 (SD 7.82) years old. Duration of TD: mean 3.52 (SD 2.38) years. | |
| Interventions | 1. Bromocriptine Group: at first phase of the trial, the participants received bromocriptine, 1 capsule each time, twice per day for 4 weeks. The second phase was a 2‐week washout period. At the third phase of the trial, the participants received placebo for 4 weeks. N = 10.* 2. Placebo Group: at first phase of the trial, the participants received placebo for 4 weeks. The second phase was a 2‐week washout period. At the third phase of the trial, the participants received bromocriptine, 1 capsule each time, twice per day for 4 weeks. N = 10.* All participants received stable doses of antipsychotics before and during the study. Other concomitant medication was not reported. | |
| Outcomes | Leaving the study early. Unable to use (data from first phase before cross‐over not reported separately) ‐ Abnormal Involuntary Movement Scale (AIMS). Clinical response of TD.** Adverse events: dizziness, nausea. Study authors were contacted but no more information was received. | |
| Notes | *sequential test method was used; when the 10th participants completed the trial, a significant difference was detected, so they terminated enrolling participants. **clinical improvement defined as the decrease rate of AIMS score ≥ 20%. Data extracted by Sai Zhao from Chinese language report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "cross over randomized trial"; no further details reported. |
| Allocation concealment (selection bias) | Low risk | "the interventions were coded as intervention A or B by the researcher in pharmacy". |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind study, the interventions were coded as intervention A or B by the researcher in pharmacy" "Participants and personnel did not know the allocation result". The 2 drugs were contained in capsules with same appearance. Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all predefined outcomes have been reported. A protocol is not available for verification. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
| Methods | Allocation: randomised, no further details. Setting: psychiatric ward, Austria. | |
| Participants | Diagnosis: symptoms of TD using AIMS. | |
| Interventions | 1. Celiprolol: single dose 200 mg/day. N = 17. 2. Placebo: N = 18. All patients received additional antipsychotic medication. | |
| Outcomes | Improvement in TD symptom using SKAUB (German version of AIMS). Quality of life. Leaving the study early. Unable to use ‐ | |
| Notes | No information on sponsorship. Article in German. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized"; details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind, identical film‐coated tablets. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information is provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Exclusions are reported but no information on whether they were accounted for or discounted from the analysis. |
| Selective reporting (reporting bias) | High risk | Outcome data for adverse events not fully reported. |
| Other bias | Low risk | The study seems to be free from other sources of bias. |
| Methods | Allocation: randomised. Blindness: double blind, identical‐appearing capsules. Duration: each patient was observed for 4 days in a control period before test medication was given. This was followed by a period of 2 weeks of research medication, and a post‐medication period. Design: parallel. Setting: inpatients, USA. Raters: assessments were done subjectively by the same observer at the same time (4:00pm) every day. | |
| Participants | Diagnosis: psychosis (diagnosis details not reported); antipsychotic induced TD. Total number randomised: N = 30. Sex: not reported. Age: 40 to 65 years. Duration of TD: no information. | |
| Interventions | 1. Alpha‐methyldopa (Aldomet)*: 750 to 1500 mg/d. N = 10. 2. Reserpine*: 0.75 to 1.5 mg/d; N = 10. 3. Placebo (lactose): N = 10. Patients were allowed to continue taking antipsychotic and anticholinergic medications throughout this study as required to control persistent psychosis. Antipsychotic and antiparkinsonism medications had been stabilized for more than 1 year and were kept strictly constant. | |
| Outcomes | TD symptoms: improvement and deterioration. Unable to use‐ TD symptoms scale scores, using a tardive dyskinesia rating scale with no published psychometric tests. Adverse effects: sedation, hypotension and mood depression (no usable data). | |
| Notes | Sponsorship source: not reported. *The dose of the research medication was increased during the testing period in order to obtain maximal therapeutic response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Thirty patients were randomly assigned to three medication groups”; no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | “The study was carried out by a double‐blind controlled method. Each identical appearing capsule contained either a‐methyldopa (Aldomet) 250 mg, reserpine 0.25 mg or placebo (lactose)”. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “The severity of... movements were assessed subjectively by the same observer (C. C. Huang) at the same time (4:00pm) every day”, but blinding details of outcome assessor were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | All subjects seem to have completed the 2‐week study. However, attrition information has not been clearly reported. |
| Selective reporting (reporting bias) | High risk | Adverse effects data not reported. Efficacy data reported as ‘medication scores’: “The mean of daily scores recorded during the 7 days in which the highest doses were given was designated as the medication score.” Post medication scores reported for 22/30 subjects: “Post‐medication evaluations were followed in eight patients who received alpha‐methyldopa, nine patients who received placebo and in five patients who received reserpine.” |
| Other bias | Unclear risk | Baseline information available only for the premedication scores per group (groups are balanced). |
| Methods | Allocation: "randomly" ‐ the drugs were given in sealed opaque envelope. Setting: inpatients, Brazil. Rater: not described. | |
| Participants | Diagnosis: 15 participants with schizophrenia, 2 with other associated psychosis, and 2 with effective psychosis and 1 mental retardation. | |
| Interventions | 1. Placebo: starch pill. N = 5. 2. L‐dopa 500 mg: growing dosage per week. From the fourth week the dosage was 500 mg. N = 5. 3‐ L‐dopa 1000 mg: growing dosage per week. From the fourth week the dosage was 1000 mg. N = 5. 4‐ L‐dopa 2000 mg: growing dosage per week. From the fourth week the dosage was 2000 mg. N = 5. All participants were on antipsychotics for a period higher than 6 months, 17 participants were on antipsychotic at the study period, 9 participants were on anticholinergic and 8 had hypnotic or anticonvulsants. | |
| Outcomes | TD symptoms: any improvement. Unable to use ‐ TD symptoms: Bordeleau scale/EBS (only medians reported). | |
| Notes | Sponsorship source: not reported. Article in Portuguese; assessed and data extracted by Antonio Grande. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomly assigned to each group". |
| Allocation concealment (selection bias) | Low risk | "the drugs were given in sealed opaque envelope". |
| Blinding of participants and personnel (performance bias) | Low risk | Each week a number of envelopes were given to the nurse containing a number, so only the researcher knew what was being administered. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not enough information in the study. |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention about loss of follow‐up. |
| Selective reporting (reporting bias) | High risk | Author reported only TD score medians and there is no availability of study protocol. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
| Methods | Allocation: "randomly". Setting: Inpatients, USA. | |
| Participants | Diagnosis: chronic psychotic patients who manifested typical bucco‐linguo‐masticatory oral dyskinesia associated with long‐term antipsychotic medication. Duration of TD: no information available. | |
| Interventions | 1. Haloperidol: dose 4 mg b.i.d. From week 15 dose was doubled to 16 mg/d. N = 7. Pre‐placebo period: initially, all antipsychotic and antiparkinsonian drugs were completely withdrawn and were replaced by placebo for the first 4 weeks. Other medications, such as antidiabetic or anticonvulsant drugs were continued unchanged. | |
| Outcomes | TD symptoms: not improved. TD symptoms: deterioration. Leaving the study early. | |
| Notes | Sponsorship source: supported in part by Public Health Service grant from the National institute of Mental Health. Tetrabenazine and placebo tablets were provided by Hoffman‐La Roche. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The 13 patients were divided randomly into two groups"; further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants and personnel not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | "A frequency count of mouth movements, done by a psychiatrist blind to the study design, was used to assess oral dyskinesia". |
| Incomplete outcome data (attrition bias) | High risk | 2/7 (29%) subjects dropped out from the haloperidol group during the 18th week; no further details are provided for addressing the outcomes of these participants. No participants dropped out from the tetrabenazine group. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all predefined outcomes have been reported. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: "randomly assigned". Setting: outpatients, Greece. | |
| Participants | Diagnosis: schizophrenia and TD (DSM‐4) and stable psychiatric condition. Duration of TD: patients have been ill for 10 (SD 7) years and were receiving stable medical treatment. | |
| Interventions | 1. Amantadine: dose 100 mg/d for 2 weeks (followed by 4‐day washout and 2 weeks of placebo). N = 11. Patients received their usual antipsychotic treatment at the same dosage. | |
| Outcomes | Leaving the study early. Unable to use ‐ changes in TD severity at baseline and endpoint using AIMS. Mental state: BPRS, MMSE, CGI. Adverse effects: insomnia, constipation, dizziness, headache. Study authors were contacted for additional data, no information was received. | |
| Notes | Sponsorship source: there was no financial funding for this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eligible patients were randomly assigned to receive either amantadine or placebo"; further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Participants received identically appearing capsules containing either amantadine (100 mg) or placebo." "double blind", however the authors report that "Those unable to safely tolerate each succeeding dose returned to a lower dose for the remainder of the study or until they were able to tolerate a higher dose". This may have unblinded personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | "Tardive dyskinesia was assessed by means of the Abnormal Involuntary Movements Scale (AIMS) by a blinded, experienced rater". "All safety issues were handled by an unmasked safety officer who was not involved in data collection". |
| Incomplete outcome data (attrition bias) | Low risk | "All 22 enrolled patients completed the study". |
| Selective reporting (reporting bias) | High risk | Many outcomes were not fully reported. TD outcomes: average scores (no SD), range and P for amantadine and placebo at baseline and end of the study have been reported. Mental state outcomes (BPRS, MMSE, CGI): average scores (no SD), range and P for amantadine and placebo reported only for end of study. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: "random". Setting: inpatients, France. | |
| Participants | Diagnosis: schizophrenia (25), organic or affective psychoses, severe personality disorders + dyskinesia (mainly localized to the buccofacial region) induced by long‐term antipsychotic treatment. Duration of TD: in both groups the dyskinesia had been present for an average period of 4 years. | |
| Interventions | 1. Tiapride: dose 400 mg/d for the first 30 days followed by 600 mg/d for the next 30 days. N = 25. Throughout the course of the study the patients continued to take antipsychotics to avoid spontaneous remission or worsening of symptoms. Other associated medication such as anticholinergic drugs was not prescribed during the study. Patients had not been treated previously for their dyskinesia. | |
| Outcomes | Leaving the study early. Unable to use ‐ TD symptoms: Skaub's scale (German version of AIMS) ‐ reduction of symptoms. | |
| Notes | Sponsorship source: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random allocation of either tiapride or placebo for 8 weeks"; further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind". Details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind". Details not reported. |
| Incomplete outcome data (attrition bias) | Low risk | "all patients continued in the study until the end of treatment." |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all predefined outcomes have been reported. Reduction of symptoms not fully reported. |
| Other bias | Unclear risk | Insufficient information to make a judgement. Baseline characteristic not reported per intervention group. Unclear if there were confounding variables. |
| Methods | Allocation: "randomly assigned". Setting: Inpatients from 2 chronic care institutions, USA. | |
| Participants | Diagnosis: tardive dyskinesia in subjects treated with antipsychotics. Duration of TD: no information. | |
| Interventions | 1. Carbidopa/levodopa: full dose: 50/350 mg/d (6 weeks of treatment, and 4 weeks of follow‐up after drug withdrawal). N = 9. 2. Placebo (6 weeks of treatment, and 4 weeks of follow‐up after drug withdrawal). N = 8. "When the appropriate dose was established in the dose finding period, patients received that dose for the next 6 weeks". Concomitant medication: no information. | |
| Outcomes | TD symptoms: improvement and deterioration (AIMS and Simpson Abbreviated Dyskiesia Scale). Leaving the study early. Unable to use ‐ Treatment‐related side‐effects. Mental state: BPRS, SANS (F and P values only). | |
| Notes | Sponsorship source: not reported. Medication and placebo supplied by Merck Sharp and Dohme, Rahway, NJ. (Unclear if medications were supplied free of charge). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned"; further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "active (Sinemet) or placebo tablets (supplied by Merck Sharp and Dohme, Rahway, NJ). Both groups of patients received the same number of identical‐appearing tablets." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind". Details not reported. |
| Incomplete outcome data (attrition bias) | High risk | "Fifteen of the 17 patients completed the trial; there were two dropouts. A female patient experienced "seizures" and the blind was, therefore, broken; a male patient eloped from the hospital. Both patients were found to be in the placebo group." |
| Selective reporting (reporting bias) | High risk | "Because the AIMS and Simpson scale were very highly correlated, only data from the Simpson scale are presented." Also, mental state data (BPRS and SANS) unusable: reported as F and P values. Adverse Events (Treatment Emergent Side Effects Scale) outcome data not reported. |
| Other bias | Unclear risk | Insufficient information reported to make a judgement. |
| Methods | Allocation: "randomly allocated" unclear. Setting: Inpatients in a psychiatric hospital, UK. | |
| Participants | Diagnosis: RDC criteria for chronic schizophrenia and associated TD. Sex: 25 M, 17 F. Duration of TD: TD present for at least 3 consecutive months. | |
| Interventions | 1. Oxypertine: flexible dose 80 mg/d to 240 mg/d for 24 weeks. N = 20. "It was required that their psychiatric condition had been stable on conventional neuroleptic medication for at least 12 months before entry." Anticholinergic antiparkinsonian drugs already prescribed were maintained throughout the trial. The only other drug permitted was nitrazepam for insomnia (10 to 20 mg) but only when required. | |
| Outcomes | Mental state: clinical relapse of psychosis. Leaving the study early. Unable to use ‐ Adverse events: AIMS, EPS (not fully reported). | |
| Notes | Sponsorship source: Sterling Winthrop Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated to either the treatment or the control group"; further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind", matched placebo. Details not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | The AIMS assessment was carried out by the same rater throughout the study and the rater was blind to the treatment. |
| Incomplete outcome data (attrition bias) | High risk | "11 oxypertine and 7 placebo patients has withdrawn..." High overall rate of participants dropping out (45%): oxypertine group (55%) and placebo group (32%). |
| Selective reporting (reporting bias) | High risk | "Table 2 gives the results of only those analyses which showed a statistically significant change: non‐significant results are excluded." Global AIMS scores not reported. EPS data descriptively reported. |
| Other bias | Low risk | The study seems to have been free of other sources of bias. The 2 groups were well matched on specific baseline characteristics. |
General
Acn ‐ anticholinergics
Bz ‐ benzodiazepine
CPE ‐ chlorpromazine equivalent
Scales
AIMS ‐ Abnormal Involuntary Movement
BRS ‐ Barnes & Kidger Rating
GRS ‐ Gerlach Rating
SEPS ‐ Smith Extrapyramidal
SRS ‐ Simpson Rating Scale
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: randomised. | |
| Allocation: randomised. Participants: patients with tardive dyskinesia and at least 2‐year exposure to antipsychotic drugs. Intervention: carbidopa/levodopa 30/300 mg vs carbidopa/levodopa 50/500 mg vs carbidopa/levodopa 75/750 mg. A non‐randomised treatment as usual group was also included. Outcomes: not reported for the pre‐defined randomised groups. 5 subjects were randomised to 3 groups. N per group and baseline characteristics not reported. Data reported for “low dose” and “high dose” participants based on what appears to be a post hoc decision, and not for each intervention group separately. Study authors were contacted for data: no information was received and this over 30 years old study was excluded. | |
| Allocation: randomised. Participants: chronically ill psychiatric inpatients with TD. Interventions: amantadine vs placebo. Outcomes: no usable data, not reported for the first phase before crossing over. No up‐to‐date contact details were found for the study authors of this 19‐year‐old study. | |
| Allocation: not randomised. | |
| Allocation: double blind, cross‐over. No up‐to‐date contact details were found for the study authors of this over 30‐year‐old study. | |
| Allocation: randomised. Participants: people with schizophrenia and antipsychotic induced tardive dyskinesia. Intervention: placebo versus haloperidol versus metoclopramide. Outcomes: no usable data, not reported for the first phase before crossing over. No up‐to‐date contact details were found for the study authors of this over 35‐year‐old study. | |
| Allocation: unclear; "double‐blind crossover". We were unable to identify up‐to‐date study author contact details for this over 25‐year‐old study. | |
| Allocation: randomised. Participants: adult outpatients suffering with antipsychotic‐induced tardive dyskinesia. Intervention: sodium valproate versus oxypertine versus deanol versus placebo. Outcomes: no usable data, not reported for the first phase before crossing over. We were unable to identify up‐to‐date study author contact details for this 30‐year‐old study. | |
| Allocation: randomised. We were unable to identify up‐to‐date study author contact details for this over 35 year‐old‐study. | |
| Allocation: not randomised. | |
| Allocation: randomised. The study is over 35 years old and we were unable to identify contact details for the author. | |
| Allocation: not randomised. Intervention: all participants were started on placebo and then switched to bromocriptine. | |
| Allocation: randomised. Participants: tardive oro‐facial dyskinesia. Intervention: pergolid 0,15 mg/d vs placebo. Outcomes: results not reported for the studied outcomes (irrespective of cross‐over period). Study authors were contacted for data. No information was received and this over 15‐year‐old study was excluded. | |
| Allocation: randomised. | |
| Allocation: randomised. Participants: diagnosis of TD. Interventions: metoclopramide vs placebo. Outcomes: no usable data, not reported for the first phase before crossing over. Study authors were contacted but no information was received. Consequently, this over 30‐year‐old study was excluded. | |
| Allocation: not randomised. | |
| Allocation: randomised. Intervention: amantadine vs trihexyphenidyl. | |
| Allocation: not randomised. | |
| Allocation: randomisation implied. No up‐to‐date contact details were found for the study authors of this over 35 years old study. | |
| Allocation: randomised. Participants: adult inpatients. Intervention: papaverine versus placebo. Outcomes: no usable data, not reported for the first phase before crossing over. No up‐to‐date contact details were found for the study authors of this over 35‐year‐old study. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: randomised. Participants: adult patients with significant antipsychotic‐induced tardive dyskinesia. Intervention: amantadine versus placebo. Outcomes: no usable data, not reported for the first phase before crossing over. No up‐to‐date contact details were found for the study authors of this over 35 year‐old‐study. | |
| Allocation: randomised. Intervention: diazepam vs tetrabenazine. Outcomes: no usable data, not reported for the first phase before crossing over. Study is over 40 years old, we were unable to identify contact details for the authors. | |
| Allocation: randomised. Participants: antipsychotic‐induced tardive dyskinesia according to DSM‐III‐R (SCID), Schooler and Kane criteria. Interventions: selegiline vs placebo. Included in Miscellaneous review. | |
| Allocation: randomised. Participants: psychiatric inpatients with TD. Interventions: pindolol versus placebo. Outcomes: no usable data reported in this brief report. No up‐to‐date contact details were found for the study authors of this over 25‐year‐old study. | |
| Allocation: randomised. | |
| Allocation: randomised. Participants: people with a TD diagnosis. Interventions: metoclopramide 10 mg vs metoclopramide 20 mg vs metoclopramide 40 mg vs placebo. Outcomes: no usable data, not reported for the first phase before crossing over. Study authors were contacted but no information was received. Consequently, this over 30‐year‐old study was excluded. | |
| Allocation: randomised. Participants: various hyperkinetic movement disorders; dose of antipsychotic medication was not stable: "All medications were either discontinued 1 week before the study or continued at the same dosage throughout the study" | |
| Allocation: randomised. Participants: schizophrenia patients (Research Diagnostic Criteria; antipsychotic therapy; good physical condition). 5/11 were diagnosed as having TD. 1 TD patient also had tardive Tourette's syndrome. Interventions: apomorphine vs bromocriptine vs placebo. Outcomes: no usable data, not reported for the first phase before crossing over. Study authors were contacted but no information was received. Consequently, this over 30‐year‐old study was excluded. | |
| Allocation: not randomised. | |
| Allocation: not randomised, controlled clinical trial. Participants: no TD ratings at baseline. Interventions: amantadine vs biperiden. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. Intervention: physostigmine vs bromocriptine vs benztropine vs haloperidol for 1 day, then crossed over. Outcomes: no usable data, not reported for the first phase before crossing over. Author was contacted but no information was received and this over 25 year‐old‐study was excluded. | |
| Allocation: randomised. Intervention: bromocriptive vs placebo. | |
| Allocation: randomised. Intervention: L‐dopa 500 mg + carbidopa 50 mg/d + low dose antipsychotics vs placebo + anticholinergic medication + low dose antipsychotic. Outcomes: no outcome data could be used. The study is over 25 years old and we were unable to identify contact details for the author. | |
| Allocation: randomised, cross‐over design. Interventions: alpha‐methyl‐p‐tyrosine (AMPT) vs L‐dihydroxyphenylalanine vs choline chloride vs valproic acid vs hydroxytryptophan. Outcomes: no usable data, not reported for the first phase before crossing over. Authors were contacted and no reply was received. Consequently, this 30‐year‐old study was excluded. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: unclear. Participants: "psychiatric patients with tardive dyskinesia". Interventions: naloxone versus placebo. Outcomes: no usable data. | |
| Allocation: not randomised. | |
| Allocation: not randomised, double blind. | |
| Allocation: randomised. Participants: people with schizophrenia with and without TD. Interventions: biperiden vs amantadine. Outcomes: unable to use data. No up‐to‐date contact details were found for the study authors of this over 20‐year‐old study. | |
| Allocation: not randomised. | |
| Allocation: randomised. Participants: schizophrenia patients. Interventions: selegiline versus placebo. Outcomes: no usable data, not reported for the first phase before crossing over. We contacted study authors that replied, but no further data were available. | |
| Allocation: randomised. Participants: antipsychotic‐free schizophrenia patients with TD. Interventions: CF 25‐397 vs bromocriptine vs placebo. Outcomes: no usable data, not reported for the first phase before crossing over. Study authors were contacted but no information was received. Consequently, this over 35‐year‐old study was excluded. | |
| Allocation: randomised. Participants: psychogeriatric patients treated with antipsychotics with severe dyskinesia for at least a year. Interventions: methyldopa versus placebo. Outcomes: no usable data, not reported for the first phase before crossing over. Study is over 40 years old, we were unable to identify contact details for the authors. |
GVG ‐ Gamma‐vynil GABA; GAG ‐ Gamma‐acetylenic GABA; THIP ‐ TetrahydroisoxazolopyridinolSCD ‐ Saccadic distractibility; Sz ‐ Schizophrenia; TD ‐ Tardive dyskinesia
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
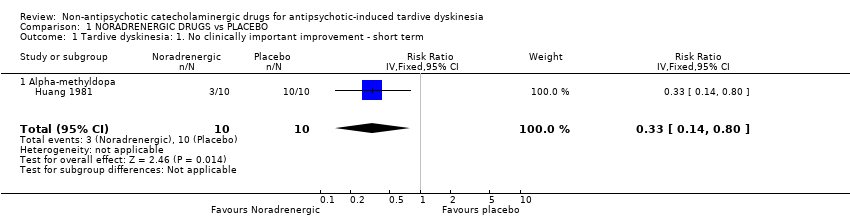
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.14, 0.80] |
| Analysis 1.1 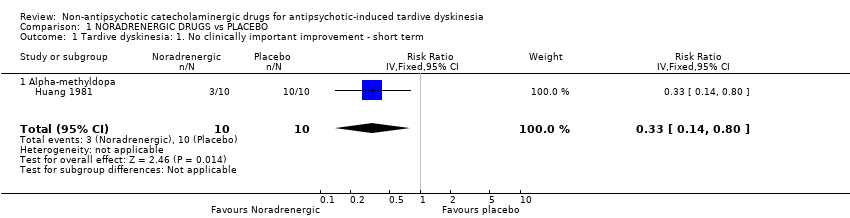 Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term. | ||||
| 1.1 Alpha‐methyldopa | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.14, 0.80] |
| 2 Tardive dyskinesia: 2. Not any improvement Show forest plot | 2 | 55 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| Analysis 1.2  Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement. | ||||
| 2.1 Alpha‐methyldopa ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 2.2 Celiprolol ‐ medium term | 1 | 35 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.66, 1.28] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| Analysis 1.3  Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term. | ||||
| 3.1 Alpha‐methyldopa | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
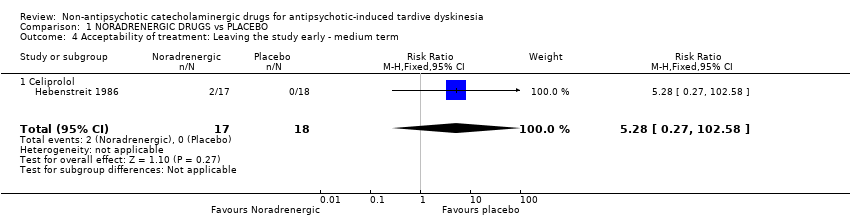
| 4 Acceptability of treatment: Leaving the study early ‐ medium term Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.27, 102.58] |
| Analysis 1.4  Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 4 Acceptability of treatment: Leaving the study early ‐ medium term. | ||||
| 4.1 Celiprolol | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.27, 102.58] |
| 5 Quality of life: No improvement ‐ medium term Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| Analysis 1.5  Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 5 Quality of life: No improvement ‐ medium term. | ||||
| 5.1 Celiprolol | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.19, 1.86] |
| Analysis 2.1  Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term. | ||||
| 1.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.19, 1.86] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.2  Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term. | ||||
| 2.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.3  Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term. | ||||
| 3.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement Show forest plot | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| Analysis 3.1  Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement. | ||||
| 1.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| 2 Tardive dyskinesia: 2. Not any improvement Show forest plot | 3 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.35, 1.03] |
| Analysis 3.2  Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement. | ||||
| 2.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 2.2 L‐DOPA ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.35, 1.27] |
| 2.3 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.26, 1.36] |
| 3 Tardive dyskinesia: 3. Deterioration Show forest plot | 2 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.35, 3.99] |
| Analysis 3.3 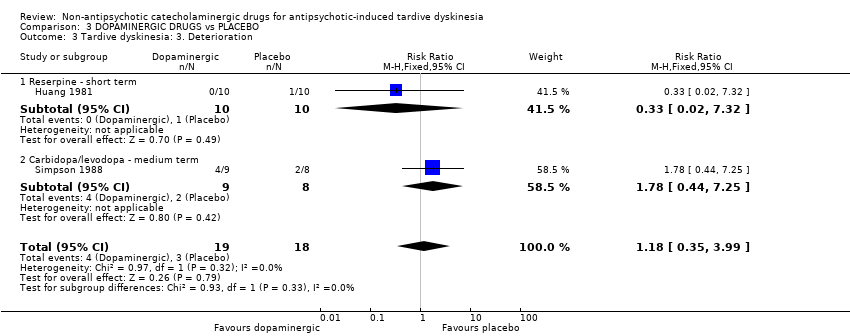 Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration. | ||||
| 3.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3.2 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.44, 7.25] |
| 4 Mental state: Deterioration ‐ medium term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 4 Mental state: Deterioration ‐ medium term. | ||||
| 4.1 Oxypertine | 1 | 42 | Risk Ratio (IV, Fixed, 95% CI) | 2.2 [0.22, 22.45] |
| 5 Acceptability of treatment: Leaving the study early Show forest plot | 6 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.65, 2.54] |
| Analysis 3.5 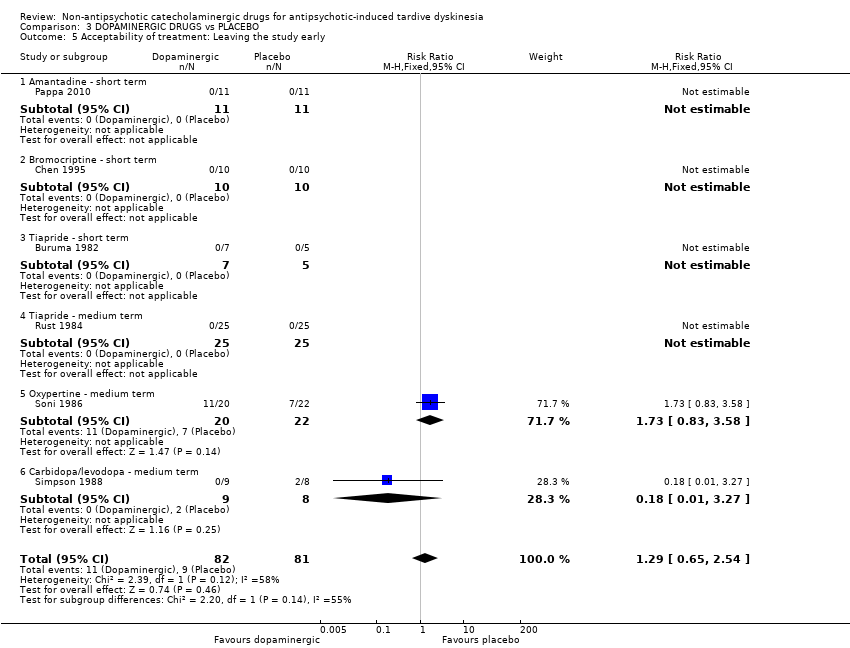 Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 5 Acceptability of treatment: Leaving the study early. | ||||
| 5.1 Amantadine ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Bromocriptine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Tiapride ‐ short term | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Tiapride ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Oxypertine ‐ medium term | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.83, 3.58] |
| 5.6 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.45, 1.95] |
| Analysis 4.1  Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 1.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.45, 1.95] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.05, 2.83] |
| Analysis 4.2  Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term. | ||||
| 2.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.05, 2.83] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| Analysis 4.3 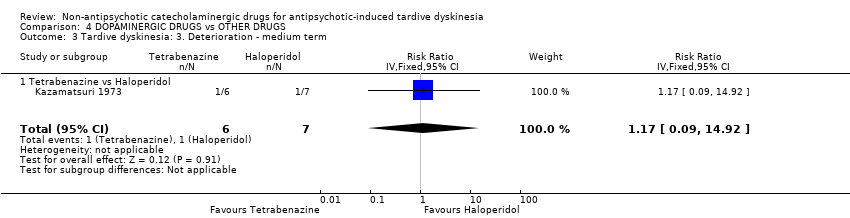 Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term. | ||||
| 3.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 4 Acceptability of treatment: Leaving the study early ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.23 [0.01, 4.00] |
| Analysis 4.4 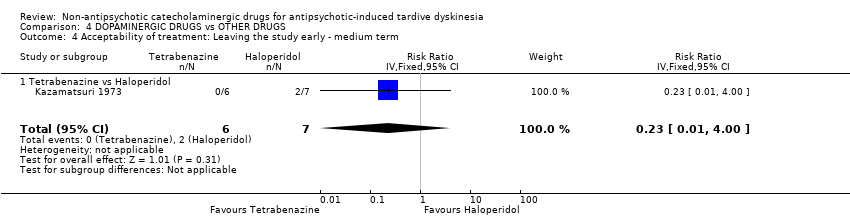 Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 4 Acceptability of treatment: Leaving the study early ‐ medium term. | ||||
| 4.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.23 [0.01, 4.00] |

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
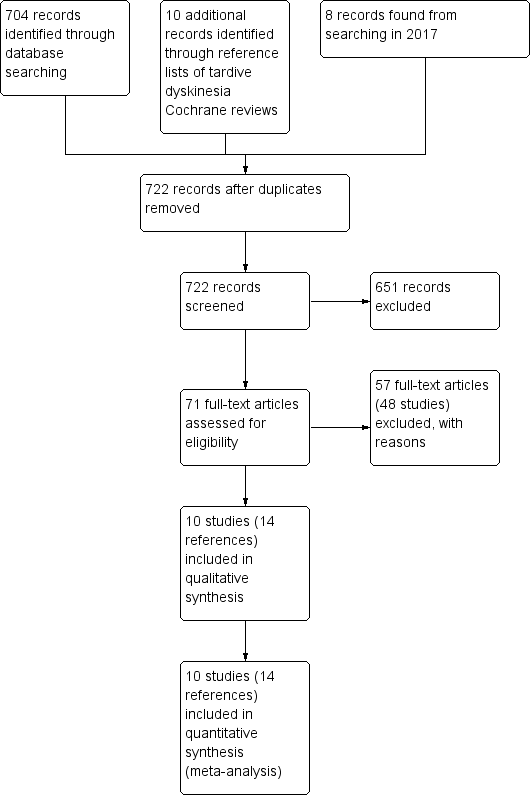
Study flow diagram for 2015 and 2017 searching
Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term.
Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 4 Acceptability of treatment: Leaving the study early ‐ medium term.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 5 Quality of life: No improvement ‐ medium term.

Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term.

Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term.

Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term.

Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement.

Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement.
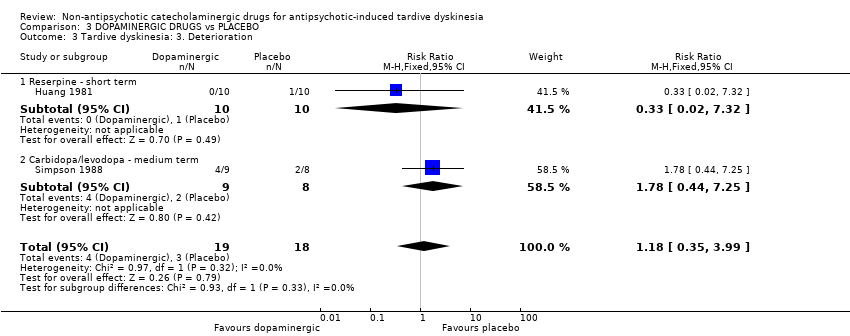
Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration.

Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 4 Mental state: Deterioration ‐ medium term.
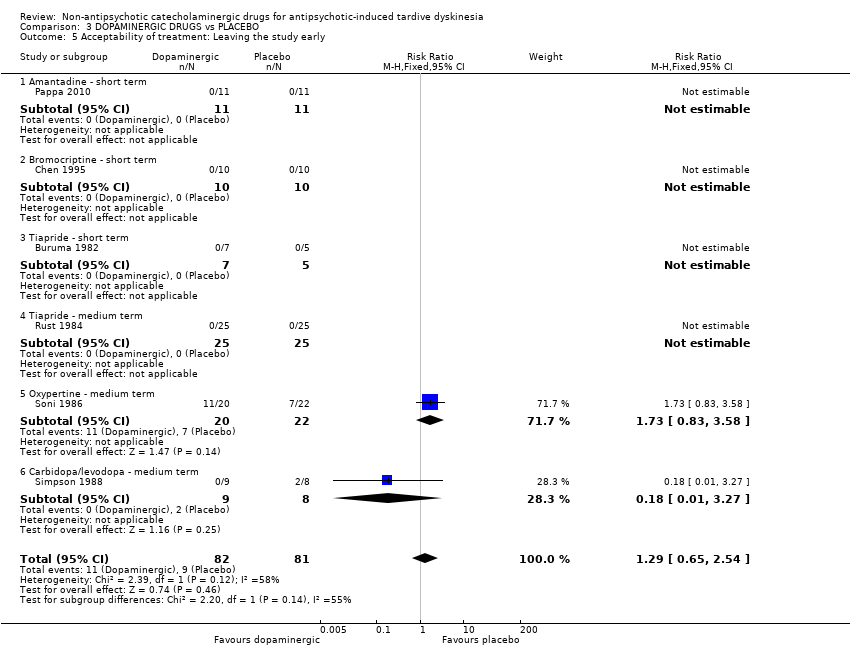
Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 5 Acceptability of treatment: Leaving the study early.

Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.
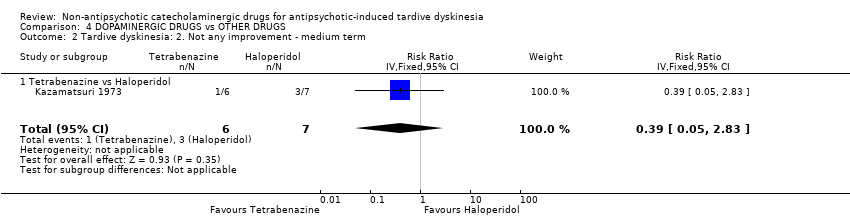
Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.

Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term.

Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 4 Acceptability of treatment: Leaving the study early ‐ medium term.
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | 1. Non‐antipsychotic catecholaminergic compound. N = 150. |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| NORADRENERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | NORADRENERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | 1000 per 1000 | 330 per 1000 | RR 0.33 | 20 | ⊕⊕⊝⊝ | The included study evaluated alpha‐methyldopa. |
| Tardive dyskinesia: deterioration follow‐up: 2 weeks | 100 per 1000 | 33 per 1000 | RR 0.33 | 20 | ⊕⊝⊝⊝ | The included study evaluated alpha‐methyldopa. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| Mental state ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| Acceptability of treatment: Leaving the study early follow‐up: 13 weeks | 0 per 1000 | 0 per 1000 | RR 5.28 | 35 | ⊕⊝⊝⊝ | The included study evaluated celiprolol. |
| No improvement in quality of life follow‐up: 13 weeks | 944 per 1000 | 822 per 1000 | RR 0.87 | 35 | ⊕⊝⊝⊝ | The included study evaluated celiprolol. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately, blinding of outcome assessors was not described. | ||||||
| NORADRENERGIC DRUGS compared to DOPAMINERGIC DRUGS for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with DOPAMINERGIC DRUGS | Risk with NORADRENERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | Study population | RR 0.60 | 20 | ⊕⊝⊝⊝ | ||
| 500 per 1,000 | 300 per 1,000 | |||||
| Tardive dyskinesia: Deterioration follow‐up: 2 weeks | Study population | not estimable | 20 | ⊕⊝⊝⊝ | Among the 20 participants no events were reported. | |
| 0 per 1,000 | 0 per 1,000 | |||||
| Adverse events ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Mental state ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Acceptability of treatment: Leaving the study early | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| DOPAMINERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | DOPAMINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | 1000 per 1000 | 520 per 1000 | RR 0.52 | 20 | ⊕⊕⊝⊝ | The included study evaluated reserpine. |
| Tardive dyskinesia: Deterioration follow‐up: 2‐6 weeks | 167 per 1000 | 197 per 1000 | RR 1.18 | 37 | ⊕⊝⊝⊝ | The included studies evaluated reserpine and carbidopa/levodopa. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| General mental state: Deterioration follow‐up: 24 weeks | 45 per 1000 | 100 per 1000 | RR 2.2 | 42 | ⊕⊝⊝⊝ | The included study evaluated oxypertine. |
| Acceptability of treatment: Leaving the study early follow‐up: 2‐24 weeks | 111 per 1000 | 143 per 1000 | RR 1.29 | 163 | ⊕⊝⊝⊝ | Only two studies (59 participants) evaluating carbidopa/levodopa and oxypertine reported any events for this outcome. 4 studies evaluating amantadine, bromocriptine, and tiapride reported no events and consequently no estimates could be made for these 3 compounds. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | This outcome was designated to be of importance, especially to patients. We found no studies rating this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately, blinding of outcome assessors was not described. | ||||||
| DOPAMINERGIC DRUGS compared to OTHER DRUGS for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER DRUGS | Risk with DOPAMINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 18 weeks | Study population | RR 0.93 | 13 | ⊕⊝⊝⊝ | ||
| 714 per 1000 | 664 per 1000 | |||||
| Tardive dyskinesia: Deterioration follow‐up: 18 weeks | Study population | RR 1.17 | 13 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 167 per 1000 | |||||
| Adverse events ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Mental state ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Acceptability of treatment: Leaving the study early follow‐up: 18 weeks | Study population | RR 0.23 | 13 | ⊕⊝⊝⊝ | ||
| 286 per 1000 | 66 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| Interventions | Reference |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | |
| Non‐neuroleptic catecholaminergic drugs | This review |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.14, 0.80] |
| 1.1 Alpha‐methyldopa | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.14, 0.80] |
| 2 Tardive dyskinesia: 2. Not any improvement Show forest plot | 2 | 55 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| 2.1 Alpha‐methyldopa ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 2.2 Celiprolol ‐ medium term | 1 | 35 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.66, 1.28] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3.1 Alpha‐methyldopa | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 4 Acceptability of treatment: Leaving the study early ‐ medium term Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.27, 102.58] |
| 4.1 Celiprolol | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.27, 102.58] |
| 5 Quality of life: No improvement ‐ medium term Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| 5.1 Celiprolol | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.19, 1.86] |
| 1.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.19, 1.86] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement Show forest plot | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| 1.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| 2 Tardive dyskinesia: 2. Not any improvement Show forest plot | 3 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.35, 1.03] |
| 2.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 2.2 L‐DOPA ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.35, 1.27] |
| 2.3 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.26, 1.36] |
| 3 Tardive dyskinesia: 3. Deterioration Show forest plot | 2 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.35, 3.99] |
| 3.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3.2 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.44, 7.25] |
| 4 Mental state: Deterioration ‐ medium term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oxypertine | 1 | 42 | Risk Ratio (IV, Fixed, 95% CI) | 2.2 [0.22, 22.45] |
| 5 Acceptability of treatment: Leaving the study early Show forest plot | 6 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.65, 2.54] |
| 5.1 Amantadine ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Bromocriptine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Tiapride ‐ short term | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Tiapride ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Oxypertine ‐ medium term | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.83, 3.58] |
| 5.6 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.45, 1.95] |
| 1.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.45, 1.95] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.05, 2.83] |
| 2.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.05, 2.83] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 3.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 4 Acceptability of treatment: Leaving the study early ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.23 [0.01, 4.00] |
| 4.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.23 [0.01, 4.00] |

