非抗精神病药物儿茶酚胺能药物治疗抗精神病药物引起的迟发性运动障碍
Appendices
Appendix 1. Previous methods
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. Where a trial was described as 'double‐blind' but it was implied that the study was randomised, we included these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes (see types of outcome measures) when these 'implied randomisation' studies were added, then we included these in the final analysis. If there was a substantive difference, we only used clearly randomised trials were and described the results of the sensitivity analysis in the text. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
People with schizophrenia or any other chronic mental illnesses, diagnosed by any criteria, irrespective of gender, age or nationality who:
i. required the use of neuroleptics for more than three months;
ii. developed tardive dyskinesia (diagnosed by any criteria) during neuroleptic treatment; and
iii. for whom the dose of neuroleptic medication had been stable for one month or more before the trial.
Types of interventions
A. Noradrenergic drugs
i. Celiprolol, clonidine, disulfiram, fusaric acid, methyldopa, pindolol, propanolol, oxprenolol or yohimbine, compared with placebo or no intervention.
B. Dopaminergic drugs
i. The dopamine receptor agonists (apomorphine, bromocriptine, CF25‐397, dopamine, hydergine, lisuride);
ii. the dopamine receptor antagonists (AMTP, oxiperomide, metoclopramide, papaverine, tiapride);
iii. the dopamine depleter drugs (oxypertine, reserpine, tetrabenazine);
iv. drugs that increase the release (amantadine, amphetamine) or production (L‐dopa) of dopamine;
all compared with placebo or no intervention.
Types of outcome measures
1. Tardive dyskinesia
1.1. No clinically important change in tardive dyskinesia*
1.2. Not any change in tardive dyskinesia
1.3. Average endpoint tardive dyskinesia score
1.4. Average change in tardive dyskinesia scores
2. Mental state
2.1. No clinically important change in general mental state*
2.2. Not any change in general mental state
2.3. Average endpoint general mental state score
2.4. Average change in general mental state scores
2.5. No clinically important change in specific symptoms
2.6. Not any change in specific symptoms
2.7. Average endpoint specific symptom score
2.8. Average change in specific symptom scores
3. Adverse effects
3.1. Clinically important general adverse effects*
3.2. Any general adverse effects
3.3. Average endpoint general adverse effect score
3.4. Average change in general adverse effect scores
3.5. Clinically important change in specific adverse effects
3.6. Any change in specific adverse effects
3.7. Average endpoint specific adverse effects
3.8. Average change in specific adverse effects
4. Leaving the study early
4.1. For specific reasons
4.2. For general reasons*
* Primary outcomes
When possible, outcomes were grouped into time periods ‐ short term (less than 6 weeks), medium term (between 6 weeks and 6 months) and long term (over 6 months).
Search methods for identification of studies
1. Electronic searching for the update (2005)
1.1. We identified relevant randomised trials by searching the Cochrane Schizophrenia Group's register using the phrase:
SELECT tblStudy.CRGStudyID FROM tblStudy WHERE tblStudy.CRGStudyID In (SELECT tblStudyIntervention.CRGStudyID FROM tblIntervention INNER JOIN tblStudyIntervention ON tblIntervention.InterventionID=tblStudyIntervention.InterventionID WHERE
InterventionDescription Like "*amantadin*" OR InterventionDescription Like "*amphetamin*" OR InterventionDescription Like "*apomorphin*" OR InterventionDescription Like "*bromocriptin*" OR InterventionDescription Like "*celiprolol*" OR InterventionDescription Like "*clonidin*" OR InterventionDescription Like "*dopa*" OR InterventionDescription Like "*disulfiram*" OR InterventionDescription Like "*fusaric*" OR InterventionDescription Like "*hydergin*" OR InterventionDescription Like "*lisurid*" OR InterventionDescription Like "*metoclopramid*" OR InterventionDescription Like "*oxiperomid*" OR InterventionDescription Like "*oxprenolol*" OR InterventionDescription Like "*oxypertin*" OR InterventionDescription Like "*papaverin*" OR InterventionDescription Like "*pindolol*" OR InterventionDescription Like "*propranolol*" OR InterventionDescription Like "*reserpine*" OR InterventionDescription Like "*tetraben*" OR InterventionDescription Like "*tiaprid*" OR InterventionDescription Like "*yohimb*");
2. Details of previous searches:
We identified relevant randomised trials by searching several electronic databases (Biological Abstracts, the Cochrane Schizophrenia Group's Register of trials, EMBASE, LILACS, MEDLINE, PsycLIT and SCISEARCH).
2.1. Biological Abstracts
We searched Biological Abstracts (January 1982 to May 1995) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and ((tardive near (dyskinesia* or disk ine*) or (abnormal near movement* near disorder*) or (involuntary* near movement*)) and (amantadine or amphetamine or AMTP or apomorphine or bromocriptine or celiprolol or CF?25397 or clonidine or *dopa* or disulfiram or fusaric or hydergine or lisuride or methyldopa or metoclopramide or oxiperomide or oxprenolol or oxypertine or papaverine or pindolol or propanolol or reserpine or tetrabenazine or tiapride or yohimbine)]
2.2. The Cochrane Schizophrenia Group's Register (1997)
We searched The Cochrane Schizophrenia Group's register using the phrase:
[(dyskinesia) and (amantadine or amphetamine or AMTP or apomorphine or bromocriptine or celiprolol or CF?25397 or clonidine or *dopa* or disulfiram or fusaric or hydergine or lisuride or methyldopa or metoclopramide or oxiperomide or oxprenolol or oxypertine or papaverine or pindolol or propanolol or reserpine or tetrabenazine or tiapride or yohimbine)]
2.3. EMBASE
We searched EMBASE (January 1980 to May 1995) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and ((tardive dyskinesia in thesaurus ‐subheadings, prevention, drug therapy, side effect and therapy) or (neuroleptic dyskinesia in thesaurus ‐all subheadings) or (tardive or dyskinesia*) or (movement* or disorder*) or (abnormal or movement* or disorder*)) and (amantadine or amphetamine or AMTP or apomorphine or bromocriptine or celiprolol or CF?25397 or clonidine or *dopa* or disulfiram or fusaric or hydergine or lisuride or methyldopa or metoclopramide or oxiperomide or oxprenolol or oxypertine or papaverine or pindolol or propanolol or reserpine or tetrabenazine or tiapride or yohimbine)]
2.4. LILACS
We searched LILACS (January 1982 to September 1996) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and ((tardive or (dyskinesia* or dyskinesia*)) or (drug induced movement disorders in thesaurus)) and (amantadine or amphetamine or AMTP or apomorphine or bromocriptine or celiprolol or CF?25397 or clonidine or *dopa* or disulfiram or fusaric or hydergine or lisuride or methyldopa or metoclopramide or oxiperomide or oxprenolol or oxypertine or papaverine or pindolol or propanolol or reserpine or tetrabenazine or tiapride or yohimbine)]
2.5. MEDLINE
We searched MEDLINE (January 1966 to May 1995) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and ((movement‐disorders in MeSH / explode all subheadings) or (anti‐dyskinesia‐agents in MeSH / explode all subheadings) or (dyskinesia‐drug‐induced in MeSH / explode all subheadings) and (psychosis in MeSH / explode all subheadings) or (schizophrenic disorders in MeSH / explode all subheadings) or (tardive near (dyskine* or diskine*)) or (abnormal* near movement* near disorder*) or (involuntar* near movement*)) and (amantadine or amphetamine or AMTP or apomorphine or bromocriptine or celiprolol or CF?25397 or clonidine or *dopa* or disulfiram or fusaric or hydergine or lisuride or methyldopa or metoclopramide or oxiperomide or oxprenolol or oxypertine or papaverine or pindolol or propanolol or reserpine or tetrabenazine or tiapride or yohimbine)]
2.6. PsycLIT
We searched PsycLIT (January 1974 to May 1995) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and ((explode movement‐disorders in DE) or (explode tardive‐dyskinesia in DE) or (tardive near (dyskine* or diskine*) or (abnormal* near movement* near disorder*) or (involuntar* near movement*)) and (amantadine or amphetamine or AMTP or apomorphine or bromocriptine or celiprolol or CF?25397 or clonidine or *dopa* or disulfiram or fusaric or hydergine or lisuride or methyldopa or metoclopramide or oxiperomide or oxprenolol or oxypertine or papaverine or pindolol or propanolol or reserpine or tetrabenazine or tiapride or yohimbine)]
3. SCISEARCH ‐ Science Citation Index
We sought each of the included studies as a citation on the SCISEARCH database. We inspected reports of articles that had cited these studies in order to identify further trials.
4. Reference searching
We inspected the references of all identified studies for more studies.
5. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
1. Selection of trials
We downloaded citations from electronic sources including details of author, institution or journal of publication. We (HGE) inspected all reports. These were then re‐inspected by (KS and JR) in order to ensure reliable selection. Any disagreement was resolved by discussion, and where there was still doubt we acquired the full article for further inspection. Once the full articles were obtained, we (HGE, KS and JR) decided whether the studies met the review criteria. Whenever we could not resolve any disagreement by discussion, we sought further information and added these trials to the list of those awaiting assessment.
2. Assessment of methodological quality
The methodological quality of all included trials was assessed using the criteria described in the Cochrane Handbook (Higgins 2005) and the Jadad Scale (Jadad 1996). The former is based on the evidence of a strong relationship between allocation concealment and direction of effect (Schulz 1995). The categories are defined below:
A. Low risk of bias (adequate allocation concealment)
B. Moderate risk of bias (some doubt about the results)
C. High risk of bias (inadequate allocation concealment). For the purpose of the analysis in this review, trials were included if they met the Cochrane Handbook criteria A or B.
The Jadad Scale measures a wider range of factors that impact on the quality of a trial. The scale includes three items:
1. Was the study described as randomised?
2. Was the study described as double‐blind?
3. Was there a description of withdrawals and drop outs?
Each item receives one point if the answer is positive. In addition, a point can be deducted if either the randomization or the blinding/masking procedures described were inadequate.
For the purpose of the analysis in this review, in addition to the criteria according to the Cochrane Handbook, a cut‐off of two points was used in the Jadad scale to check the assessment made by the Handbook criteria. However, we did not use the Jadad Scale to exclude trials in this review.
3. Data collection
HGE and JR independently extracted data from selected trials, while KS separately re‐extracted information from two different samples (10%). When disputes arose we attempted resolution by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data but added this outcome of the trial to the list of those awaiting assessment.
4. Data synthesis
4.1 Data types
We assessed outcomes using continuous (for example changes on a behaviour scale), categorical (for example, one of three categories on a symptoms scale, such as 'little change', 'moderate change' or 'much change') or dichotomous measures (for example, either 'no important changes' or 'important changes' in a person's symptoms). Currently RevMan does not support categorical data so we could not analyse them as such.
4.2 Incomplete data
With the exception of the outcome of leaving the study early, we did not include trial outcomes if more than 40% of people were not reported in the final analysis.
4.3 Dichotomous ‐ yes/no ‐ data
We analysed data on an intention to treat analysis. On the condition that more than 60% of people completed the study, we counted everyone allocated to the intervention regardless of whether they completed the follow up. We assumed that those who dropped out had the negative outcome, with the exception of death. Where possible we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut off points on rating scales and dividing subjects accordingly into 'clinically improved' or 'not clinically improved'. If the authors of a study had used a predefined cut off point for determining clinical effectiveness we used the reviewers' criteria where appropriate. Otherwise we generally assumed that if there had been a 50% reduction in a scale‐derived score, this could be considered as a clinically significant response. Similarly, a rating of 'at least much improved' according to the Clinical Global Impression Scale (Guy 1970) could be considered as a clinically significant response.
We calculated the relative risk (RR) and its 95% confidence interval (CI) based on the random effects model, as it takes into account any differences between studies even if there is no statistically significant heterogeneity. It has been shown that RR is more intuitive than odds ratios (Boissel 1999), and also that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. We inspected graphs to see if an analysis using a fixed effects model made any substantive difference in outcomes that were not statistically significantly heterogeneous. When the overall results were significant we calculated the number needed to treat (NNT) and the number‐needed‐to‐harm (NNH) as the inverse of the risk difference.
4.4 Continuous data
4.4.1 Normally distributed data: data on continuous outcomes are frequently skewed, the mean not being the centre of the distribution. The statistics for meta‐analysis are thought to be able to cope with some skew, but were formulated for parametric data. To avoid this potential pitfall we applied the following standards to all data before inclusion: (a) standard deviations and means were reported or obtained from authors and (b) for data with finite limits, such as endpoint scale data, the standard deviation (SD), when multiplied by two, was less than the mean. Otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996). We reported data that did not meet the first or second standard in the 'other data' tables. If a scale starts from a positive value (such as PANSS, which can have values from 30‐210) the calculation described above should be modified to take the scale starting point into account. In these cases skewness is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score.
For change data (endpoint minus baseline), the situation is even more problematic. In the absence of individual patient data it is impossible to know if data are skewed, though this is likely. After consulting the ALLSTAT electronic statistics mailing list, we presented change data in MetaView in order to summarise available information. In doing this, it is assumed either that data were not skewed or that the analyses could cope with the unknown degree of skewness. Without individual patient data it is impossible to test this assumption. Where both change and endpoint data were available for the same outcome category only endpoint data are presented. We acknowledge that by doing this much of the published change data were excluded, but argue that endpoint data is more clinically relevant and that if change data were to be presented along with endpoint data it would be given undeserved equal prominence. We are contacting authors of studies reporting only change data for endpoint figures. We reported non‐normally distributed data in the 'Other data types' tables.
4.4.2 Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation co‐efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co‐efficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
4.4.2 Rating scales: A wide range of instruments is available to measure mental health outcomes. These instruments vary in quality and many are not valid, or even ad hoc. For outcome instruments some minimum standards have to be set. It has been shown that the use of rating scales which had not been described in a peer‐reviewed journal (Marshall 2000) is associated with bias and therefore we excluded the results of such scales. Furthermore, the instrument should either be a self report or be completed by an independent rater or relative (not the therapist), and the instrument could be considered a global assessment of an area of functioning. However, as it was expected that therapists would frequently also be the rater, we did include such data but commented on this data as 'prone to bias'.
4.4.3 Summary statistic
For continuous outcomes we estimated the weighted mean difference (WMD) between groups, again based on the random effects model, as it takes into account any differences between studies even if there is no statistically significant heterogeneity. We inspected data to see if analysis using a fixed effects model made any substantive difference when the results were not statistically significantly heterogeneous. Whenever possible, we took the opportunity to make direct comparisons between trials that used the same measurement instrument to quantify specific outcomes. Where continuous data were presented from different scales rating the same effect, we presented both sets of data and the general direction of effect was inspected.
5. Heterogeneity
Firstly, we considered all of the included studies within any comparison to judge clinical heterogeneity. Then we visually inspected graphs used to investigate the possibility of statistical heterogeneity and supplemented this by using, primarily, the I‐squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I‐squared estimate was greater than or equal to 75%, we interpreted this as indicating the presence of high levels of heterogeneity (Higgins 2003). If inconsistency was high, we did not summate the data, but presented it separately and reasons for heterogeneity were investigated.
6. Addressing publication bias
We entered all data from selected trials into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias.
7. Sensitivity analyses
We analysed the effect of including studies with high attrition rates in the sensitivity analysis.
8. General
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for the treatment groups.

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
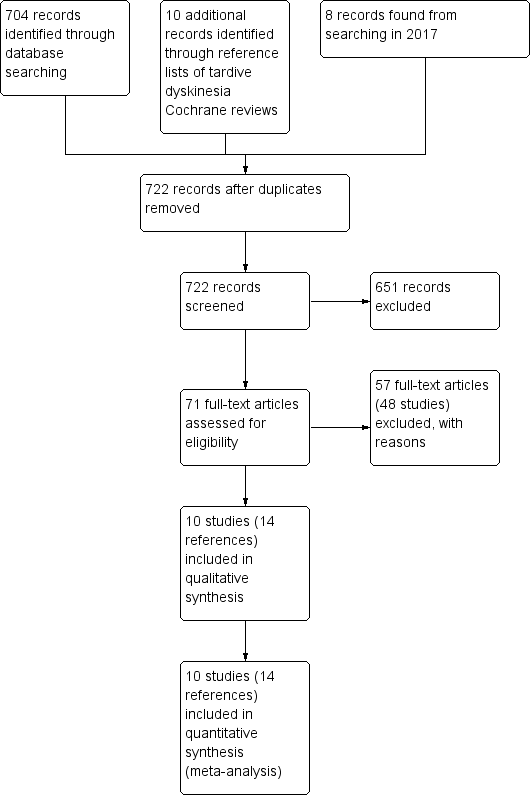
Study flow diagram for 2015 and 2017 searching
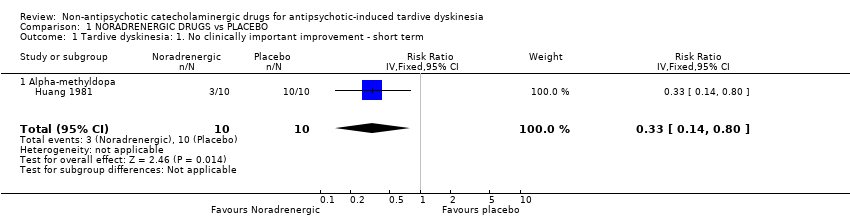
Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term.
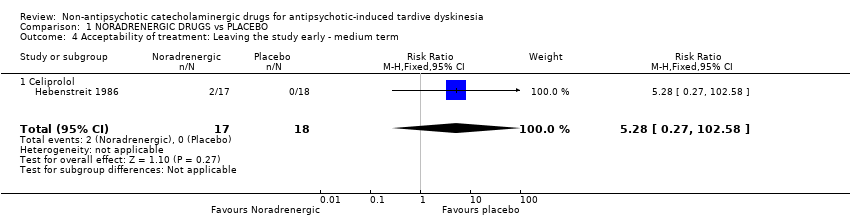
Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 4 Acceptability of treatment: Leaving the study early ‐ medium term.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 5 Quality of life: No improvement ‐ medium term.

Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term.

Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term.

Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term.

Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement.

Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement.
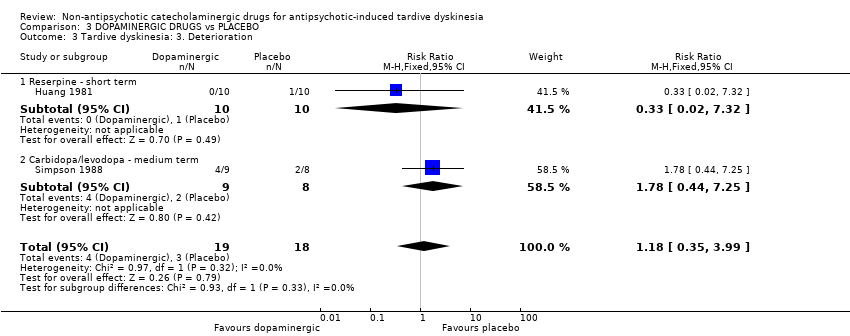
Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration.

Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 4 Mental state: Deterioration ‐ medium term.
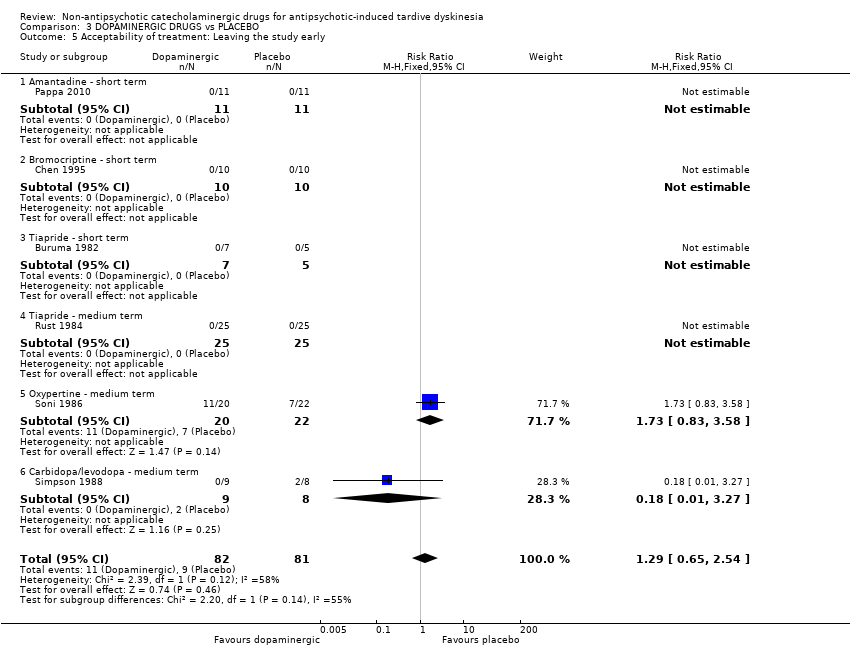
Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 5 Acceptability of treatment: Leaving the study early.

Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.
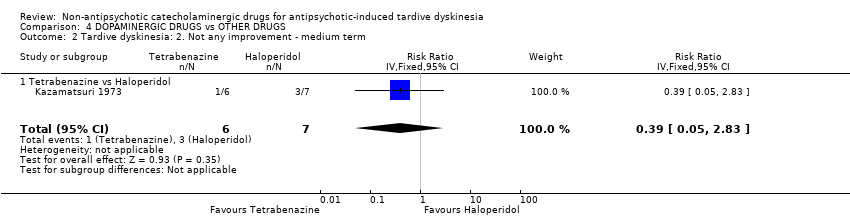
Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.

Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term.

Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 4 Acceptability of treatment: Leaving the study early ‐ medium term.
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | 1. Non‐antipsychotic catecholaminergic compound. N = 150. |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| NORADRENERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | NORADRENERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | 1000 per 1000 | 330 per 1000 | RR 0.33 | 20 | ⊕⊕⊝⊝ | The included study evaluated alpha‐methyldopa. |
| Tardive dyskinesia: deterioration follow‐up: 2 weeks | 100 per 1000 | 33 per 1000 | RR 0.33 | 20 | ⊕⊝⊝⊝ | The included study evaluated alpha‐methyldopa. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| Mental state ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| Acceptability of treatment: Leaving the study early follow‐up: 13 weeks | 0 per 1000 | 0 per 1000 | RR 5.28 | 35 | ⊕⊝⊝⊝ | The included study evaluated celiprolol. |
| No improvement in quality of life follow‐up: 13 weeks | 944 per 1000 | 822 per 1000 | RR 0.87 | 35 | ⊕⊝⊝⊝ | The included study evaluated celiprolol. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately, blinding of outcome assessors was not described. | ||||||
| NORADRENERGIC DRUGS compared to DOPAMINERGIC DRUGS for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with DOPAMINERGIC DRUGS | Risk with NORADRENERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | Study population | RR 0.60 | 20 | ⊕⊝⊝⊝ | ||
| 500 per 1,000 | 300 per 1,000 | |||||
| Tardive dyskinesia: Deterioration follow‐up: 2 weeks | Study population | not estimable | 20 | ⊕⊝⊝⊝ | Among the 20 participants no events were reported. | |
| 0 per 1,000 | 0 per 1,000 | |||||
| Adverse events ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Mental state ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Acceptability of treatment: Leaving the study early | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| DOPAMINERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | DOPAMINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | 1000 per 1000 | 520 per 1000 | RR 0.52 | 20 | ⊕⊕⊝⊝ | The included study evaluated reserpine. |
| Tardive dyskinesia: Deterioration follow‐up: 2‐6 weeks | 167 per 1000 | 197 per 1000 | RR 1.18 | 37 | ⊕⊝⊝⊝ | The included studies evaluated reserpine and carbidopa/levodopa. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| General mental state: Deterioration follow‐up: 24 weeks | 45 per 1000 | 100 per 1000 | RR 2.2 | 42 | ⊕⊝⊝⊝ | The included study evaluated oxypertine. |
| Acceptability of treatment: Leaving the study early follow‐up: 2‐24 weeks | 111 per 1000 | 143 per 1000 | RR 1.29 | 163 | ⊕⊝⊝⊝ | Only two studies (59 participants) evaluating carbidopa/levodopa and oxypertine reported any events for this outcome. 4 studies evaluating amantadine, bromocriptine, and tiapride reported no events and consequently no estimates could be made for these 3 compounds. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | This outcome was designated to be of importance, especially to patients. We found no studies rating this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately, blinding of outcome assessors was not described. | ||||||
| DOPAMINERGIC DRUGS compared to OTHER DRUGS for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER DRUGS | Risk with DOPAMINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 18 weeks | Study population | RR 0.93 | 13 | ⊕⊝⊝⊝ | ||
| 714 per 1000 | 664 per 1000 | |||||
| Tardive dyskinesia: Deterioration follow‐up: 18 weeks | Study population | RR 1.17 | 13 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 167 per 1000 | |||||
| Adverse events ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Mental state ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Acceptability of treatment: Leaving the study early follow‐up: 18 weeks | Study population | RR 0.23 | 13 | ⊕⊝⊝⊝ | ||
| 286 per 1000 | 66 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| Interventions | Reference |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | |
| Non‐neuroleptic catecholaminergic drugs | This review |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.14, 0.80] |
| 1.1 Alpha‐methyldopa | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.14, 0.80] |
| 2 Tardive dyskinesia: 2. Not any improvement Show forest plot | 2 | 55 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| 2.1 Alpha‐methyldopa ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 2.2 Celiprolol ‐ medium term | 1 | 35 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.66, 1.28] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3.1 Alpha‐methyldopa | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 4 Acceptability of treatment: Leaving the study early ‐ medium term Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.27, 102.58] |
| 4.1 Celiprolol | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.27, 102.58] |
| 5 Quality of life: No improvement ‐ medium term Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| 5.1 Celiprolol | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.19, 1.86] |
| 1.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.19, 1.86] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement Show forest plot | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| 1.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| 2 Tardive dyskinesia: 2. Not any improvement Show forest plot | 3 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.35, 1.03] |
| 2.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 2.2 L‐DOPA ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.35, 1.27] |
| 2.3 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.26, 1.36] |
| 3 Tardive dyskinesia: 3. Deterioration Show forest plot | 2 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.35, 3.99] |
| 3.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3.2 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.44, 7.25] |
| 4 Mental state: Deterioration ‐ medium term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oxypertine | 1 | 42 | Risk Ratio (IV, Fixed, 95% CI) | 2.2 [0.22, 22.45] |
| 5 Acceptability of treatment: Leaving the study early Show forest plot | 6 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.65, 2.54] |
| 5.1 Amantadine ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Bromocriptine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Tiapride ‐ short term | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Tiapride ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Oxypertine ‐ medium term | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.83, 3.58] |
| 5.6 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.45, 1.95] |
| 1.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.45, 1.95] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.05, 2.83] |
| 2.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.05, 2.83] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 3.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 4 Acceptability of treatment: Leaving the study early ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.23 [0.01, 4.00] |
| 4.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.23 [0.01, 4.00] |

