Óxido nítrico para la insuficiencia respiratoria en recién nacidos a término o casi a término
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Single‐centre randomised study. Masking of allocation: yes. Masking of intervention: no. Completeness of follow‐up: yes. Masking of outcome measurement: no | |
| Participants | 17 near‐term infants ≥ 35 weeks with PaO2 < 100 mmHg on 100% oxygen on ventilator | |
| Interventions | iNO at 20 to 40 ppm increased to 80 if PaO2 stayed < 100 mmHg. Use of iNO allowed in case of failure of control treatment, if PaO2 was (1) < 80 mmHg (10.7 kPa) for longer than 1 hour, (2) < 40 mmHg (5.3 kPa) beyond 1 hour or (3) < 30 mmHg (4 kPa) beyond 30 minutes | |
| Outcomes | Primary outcome: 'treatment failure' or meeting ECMO criteria (defined as PaO2 < 80 mmHg for > 1 hour, < 40 mmHg after 1 hour or < 30 mmHg after 30 minutes) | |
| Notes | Admission OI in control group averaged 26, in treatment group 38 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Low risk | Charitable foundation (March of Dimes) |
| Methods | Single‐centre randomised study. Masking of allocation: yes. Masking of intervention: no. Completeness of follow‐up: yes. Masking of outcome: no | |
| Participants | 42 near‐term infants ≥ 34 weeks with PaO2 < 100 mmHg on 100%oxygen on ventilator. 41 infants after exclusion of 1 case of congenital heart disease. Patients with diaphragmatic hernia were excluded. Some evidence showing increased pulmonary artery pressure on echocardiography required | |
| Interventions | 40 ppm iNO reduced to 20 ppm after 1 hour. Combined therapy with high‐frequency ventilation and iNO allowed | |
| Outcomes | Death before discharge or requirement for ECMO. Secondary outcomes included changes in oxygenation and duration of ventilation and oxygen therapy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | High risk | Study terminated early after ad hoc committee reviewed the data |
| Funding Source(s) | Low risk | Supported by local and government agencies |
| Methods | Multi‐centre randomised trial. Masking of allocation: yes. Masking of intervention: yes. Completeness of follow‐up: yes. Masking of outcome: yes | |
| Participants | 248 near‐term infants, ≥ 34 weeks, ≤ 4 days of age, with OI ≥ 25 | |
| Interventions | 20 ppm iNO or nitrogen placebo. Inhaled NO gas weaned to 5 ppm after 24 hours for a maximum of 96 hours | |
| Outcomes | Death before discharge, need for ECMO, chronic lung disease, neurological injury | |
| Notes | No calculation of sample size for the trial is described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cards on which treatment assignments were written were randomly ordered (shuffled by hand 3 times) and placed in sequentially numbered opaque envelopes in blocks of 8. |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | Uncertain how sample size was determined |
| Funding Source(s) | High risk | Funded "in part" by INOtherapeutics; no other sources listed |
| Methods | Three‐centre randomised trial. Masking of allocation: cannot tell. Masking of intervention: no. Completeness of follow‐up: yes. Masking of outcome: no | |
| Participants | 38 near‐term infants with OI ≥ 25, < 1 week old, with echocardiographically proven pulmonary hypertension | |
| Interventions | Inhaled NO at 2 ppm or no therapy | |
| Outcomes | Primary outcome: failure, defined as OI > 35 after 1 hour of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Low risk | |
| Funding Source(s) | Low risk | Local funds and charitable sources |
| Methods | Multi‐centre randomised trial, with nitrogen used as placebo gas | |
| Participants | 155 term infants with echocardiographic evidence of pulmonary hypertension. PaO2 between 40 and 100 mmHg in 100% oxygen. Randomised equally to each of the 4 groups | |
| Interventions | Inhaled nitric oxide at 5, 20 or 80 ppm or control, gas stopped upon 'failure', defined as PaO2 < 40 mmHg for longer than 30 minutes | |
| Outcomes | Major sequelae index: composite index of death, ECMO, neurological sequelae or bronchopulmonary dysplasia | |
| Notes | Terminated early because of poor enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by a "scratch‐off card" |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | Placebo‐controlled masked trial |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | Terminated early for poor enrolment |
| Funding Source(s) | High risk | Industry funded (Ohmeda) |
| Methods | Single‐centre randomised parallel‐group study | |
| Participants | 22 term or premature infants with OI > 25 and < 40, plus right‐to‐left ductal shunting or estimated peak right ventricular pressure > 75% of systemic systolic pressure | |
| Interventions | 20 ppm iNO. High‐frequency jet ventilation allowed concurrently. Back‐up use of iNO allowed in case of failure of control treatment. Few details of other therapy given | |
| Outcomes | Primary outcomes: oxygenation index, PaO2, echocardiographic Doppler changes | |
| Notes | If the condition of infants in the controlled trial deteriorated to an OI > 40, iNO was given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by "blind draw" |
| Allocation concealment (selection bias) | Unclear risk | Not clearly described |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | No sample size calculation presented |
| Funding Source(s) | Low risk | Local sources |
| Methods | Two‐centre randomised trial | |
| Participants | 56 infants > 34 weeks, < 48 hours old, with OI between 10 and 30 | |
| Interventions | Immediate iNO at 20 ppm, or no iNO unless OI increases to > 40 | |
| Outcomes | Primary outcome was the proportion with OI increasing to > 40. Secondary outcomes were death, days of ventilation and chronic lung disease. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Opaque sealed sequential envelopes |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Unclear risk | Local (university) sources and industry (AGA, SA) |
| Methods | Multi‐centre parallel‐group randomised controlled trial | |
| Participants | 60 full‐term infants ≥ 34 weeks with severe hypoxic respiratory failure for whom attending physician was unsure whether iNO was indicated | |
| Interventions | iNO at 20 ppm or no iNO | |
| Outcomes | Survival without severe disability to 1 year of age | |
| Notes | Sample size determined by limit to trial duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation by random number generator with minimisation |
| Allocation concealment (selection bias) | Low risk | Enrolled before allocation |
| Blinding (performance bias and detection bias) | High risk | Unmasked study |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Low risk | Protocol registered (ISRCTN 17821339). Primary outcome variables consistent with publication |
| Funding Source(s) | Low risk | Government agency |
| Methods | Randomised multi‐centre controlled parallel‐group trial of iNO compared with high‐frequency ventilation | |
| Participants | 205 near‐term infants, OI > 40. Stratified by disease process; infants with diaphragmatic hernia (n = 34) were included as a separate stratum | |
| Interventions | iNO at 40 ppm was compared with high‐frequency ventilation with the SensorMedics oscillator. Initial randomisation was followed by back‐up treatment with alternate therapy in cases of failure. This was followed by further cross‐over to combination treatment with high‐frequency ventilation and iNO if alternate therapy failed. | |
| Outcomes | Sustained PaO2 ≥ 60 mmHg. Failure defined as PaO2 < 60 mmHg after 2 hours of therapy or lack of improvement in PaO2 before 2 hours. Some data on infants with diaphragmatic hernia were presented separately. | |
| Notes | Complex study design; we abstracted only results from the initial randomisation. All infants who failed were exposed to iNO at some stage in the protocol. Study was stopped after interim analysis, suggesting no difference between initial treatment limbs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Low risk | Local and government sources |
| Methods | Multi‐centre randomised controlled trial | |
| Participants | All 302 enrolled (3 excluded, as they turned out to have congential heart disease) infants were ≥ 34 weeks' gestation, with hypoxic respiratory failure and OI between 15 and 25, while receiving ≥ 80% oxygen, on 2 blood gases between 15 minutes and 12 hours apart | |
| Interventions | iNO at 5 ppm; iNO could be increased to 20 ppm in the case of partial response; treated up to 14 days. Controls received nitrogen, or iNO if OI increased to > 25. | |
| Outcomes | Primary outcome: occurrence or death or requirement for ECMO | |
| Notes | Study terminated early because of slowing enrolment; 75% of anticipated sample enrolled; study terminated without knowledge of results at that point | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Randomised by telephone after enrolment |
| Blinding (performance bias and detection bias) | Low risk | Masked gas administration |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | Study registered in 2000 (after start of trial but before completion); NCT00005773. Primary and secondary outcomes match registration documents. |
| Other bias | Unclear risk | Early termination without examination of data |
| Funding Source(s) | Unclear risk | Government agency, partial industry support |
| Methods | Single‐centre randomised trial | |
| Participants | 46 infants with meconium aspiration syndrome, over 36 weeks, > 2.5 kg, OI > 15 | |
| Interventions | iNO at 15 ppm or no additional gas | |
| Outcomes | Primary outcome variable unclear; outcomes reported include changes in OI and in echocardiography, survival, certain medical complications and duration of assisted ventilation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not clearly reported but may be acceptable; 'random number method' |
| Allocation concealment (selection bias) | Unclear risk | No relevant information |
| Blinding (performance bias and detection bias) | High risk | Unmasked study |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes of all participants reported |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Unclear risk | Uncertain |
| Methods | Randomised multi‐centre trial of iNO | |
| Participants | 204 infants; 107 near term, ≥ 33 weeks' gestation. OI 15 to 40, on 2 blood gases 1 hour apart. Congenital diaphragmatic hernia excluded, congenital heart disease excluded, < 7 days of age only | |
| Interventions | iNO at 10 ppm for 2 hours, continued if response. Controls could be treated after 2 hours. | |
| Outcomes | Primary outcome: change in OI at 2 hours after initiation of treatment. Secondary outcomes: death, brain injury, long‐term oxygen therapy, duration of hospitalisation | |
| Notes | ECMO not available as back‐up therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | High risk | Unmasked study gas administration |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | Terminated early for poor enrolment |
| Funding Source(s) | Unclear risk | Government agency and industry support |
| Methods | Randomised multi‐centre study, with oxygen used as placebo gas | |
| Participants | 235 near‐term infants, ≥ 34 weeks' gestation, OI > 25 on 2 blood gases, 15 minutes apart. Congenital diaphragmatic hernia excluded, congenital heart disease excluded, < 14 days of age only | |
| Interventions | iNO at 20 ppm, trial at 80 ppm if no response to 20 ppm (in treatment group). Comparison with control. Both groups received 'maximal therapy' before study entry, including surfactant in the majority, high‐frequency ventilation at experienced centres, muscle relaxation and inotropes. Induction of alkalosis with target pH of 7.45‐7.60 was also used as a guideline. All of these treatment strategies were continued in controls. Investigators were not allowed to start high‐frequency ventilation or to administer surfactant after study entry. | |
| Outcomes | Survival to 120 days or discharge home, without requiring ECMO. Secondary outcomes were oxygenation (OI and PaO2) after 30 minutes, length of hospital stay, days of assisted ventilation and incidence of air leak or bronchopulmonary dysplasia. Neurodevelopment at 18‐24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prepared by study centre |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation system |
| Blinding (performance bias and detection bias) | Low risk | Masked gas administration |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | Study registered, NCT00005776, in 2000 (after completion). Outcomes in registration documents are reported in the main publication. |
| Funding Source(s) | Unclear risk | Started with government agency support, industry support after study commenced |
| Methods | Randomised multi‐centre trial of iNO in infants with diaphragmatic hernia, with oxygen used as placebo gas | |
| Participants | 53 near‐term infants with diaphragmatic hernia, ≥ 34 weeks' gestation, < 14 days of age | |
| Interventions | iNO at 20 ppm, trial at 80 ppm if no response to 20 ppm (in treatment group). Comparison with control. Both groups received 'maximal therapy' before study entry, including surfactant in the majority, high‐frequency ventilation at experienced centres, muscle relaxation and inotropes. Induction of alkalosis with target pH of 7.45‐7.60 was also used as a guideline. All of these treatment strategies were continued in controls. Investigators were not allowed to start high‐frequency ventilation or to administer surfactant after study entry. | |
| Outcomes | Survival to 120 days or discharge home, without requiring ECMO. Secondary outcomes were oxygenation (OI and PaO2) after 30 minutes, length of hospital stay, days of assisted ventilation and incidence of air leak or bronchopulmonary dysplasia. Neurodevelopment at 18‐24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation system |
| Blinding (performance bias and detection bias) | Low risk | Masked gas administration |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Study registered, NCT00005776, in 2000 (after completion). Outcomes as registered are reported. |
| Funding Source(s) | Low risk | Planned and commenced with government agency support; industry support provided after study commenced |
| Methods | Multi‐centre randomised study, with nitrogen used for placebo gas | |
| Participants | 58 'full‐term infants' on FiO2 1.0 with PaO2 < 55 mmHg. All had echocardiographic signs of pulmonary hypertension. | |
| Interventions | iNO at 80 ppm or control. Control patients received conventional ventilation. Surfactant was not allowed during the study. | |
| Outcomes | Primary outcome was 'success', defined as improved OI to < 40, without a fall in PaO2 or hypotension. | |
| Notes | Study was terminated after an interim analysis showed an effect at P < 0.05. Original sample size was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | Low risk | Masked gas administration |
| Incomplete outcome data (attrition bias) | Low risk | Complete outcome assessment |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | High risk | Early termination after examination of data |
| Funding Source(s) | Low risk | Government agency |
| Methods | Multi‐centre randomised trial | |
| Participants | 87 infants > 2 kg birth weight, with A‐aDO2 500‐599 after surfactant on 2 gases 1 hour apart, on 100% oxygen with echocardiographic evidence of PPHN | |
| Interventions | iNO at 10 ppm or control; iNO increased up to 80 ppm until no further increases in arterial PaO2 occurred | |
| Outcomes | Primary outcome variable was progression to severe PPHN, defined as an A‐aDO2 persistently > 600. Secondary outcome variables included death, ECMO rate, length of hospitalisation, amount and duration of mechanical ventilation, number of days of oxygen use and need for supplemental oxygen at 28 days of life. | |
| Notes | Study was terminated early after approval of iNO by the Federal Drug Administration, as this impaired recruitment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation order was determined a priori, in blocks of 10, by coin toss with folded group assignment cards. |
| Allocation concealment (selection bias) | Low risk | Cards were placed in sequentially numbered opaque envelopes for each centre. |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | Early termination, but not because of examination of data |
| Funding Source(s) | Unclear risk | Not described |
| Methods | Single‐centre randomised trial. Masking of allocation: not clear. Masking of intervention: no. Completeness of follow‐up: yes. Masking of outcome: no | |
| Participants | 49 near‐term infants ≥ 34 weeks, PaO2 < 100 mmHg on 100% oxygen; all had evidence of PPHN on echocardiography | |
| Interventions | iNO at 80 ppm, reduced to 40 ppm after 1 hour. All received muscle relaxants and sedation and conventional ventilation. | |
| Outcomes | Primary outcomes were oxygenation as well as death and need for ECMO. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Low risk | Supported by local and charitable sources |
A‐aDO2: alveolar‐arterial oxygen difference.
ECMO: extracorporeal membrane oxygenation.
FiO2: fraction of inspired oxygen.
iNO: inhaled nitric oxide.
NO: nitric oxide.
OI: oxygenation index.
PaO2: partial pressure of arterial oxygen.
PPHN: persistent pulmonary hypertension of the newborn
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Non‐randomised retrospective study; infants were treated or were not treated according to availability of inhaled nitric oxide. The time period over which infants were studied was different between control and inhaled nitric oxide groups. | |
| Randomised comparison of inhaled nitric oxide with intravenous nitroprusside. Study was stopped after enrolment of 25 participants owing to decreasing enrolment. Inhaled nitric oxide produced much greater improvements in oxygenation than were produced by nitroprusside. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or use of ECMO Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Inhaled NO versus control, Outcome 1 Death or use of ECMO. | ||||
| 1.1 Death or use of ECMO; studies that did not allow back‐up use of iNO in controls | 8 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.57, 0.77] |
| 1.2 Death or use of ECMO; studies that allowed back‐up use of iNO in controls | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.34, 4.16] |
| 1.3 Death or use of ECMO; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 2 Death before hospital discharge Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Inhaled NO versus control, Outcome 2 Death before hospital discharge. | ||||
| 2.1 Death; studies that did not allow back‐up use of iNO in controls | 8 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.60, 1.31] |
| 2.2 Death; studies that allowed back‐up use of iNO in controls | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.34, 4.16] |
| 2.3 Death; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.74, 1.96] |
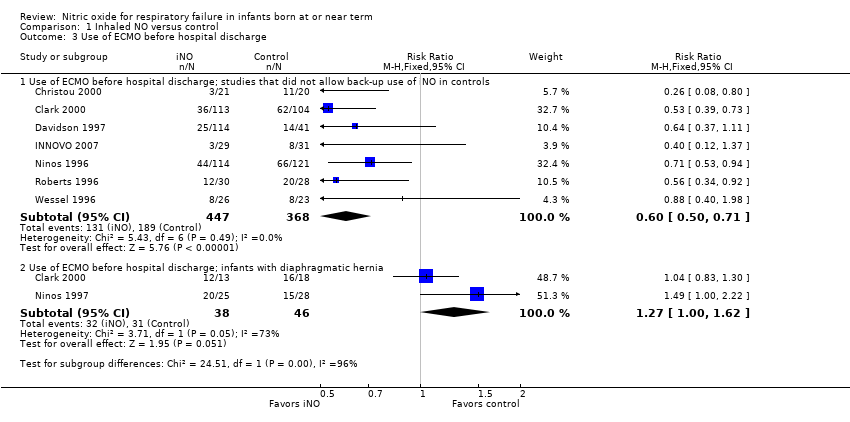
| 3 Use of ECMO before hospital discharge Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Inhaled NO versus control, Outcome 3 Use of ECMO before hospital discharge. | ||||
| 3.1 Use of ECMO before hospital discharge; studies that did not allow back‐up use of iNO in controls | 7 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.50, 0.71] |
| 3.2 Use of ECMO before hospital discharge; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.00, 1.62] |
| 4 Failure to improve oxygenation (PaO2) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Inhaled NO versus control, Outcome 4 Failure to improve oxygenation (PaO2). | ||||
| 4.1 Failure to improve PaO2; studies that did not allow back‐up use of iNO in controls | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Failure to improve PaO2; infants with diaphragmatic hernia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Oxygenation index 30 to 60 minutes after treatment Show forest plot | 6 | 753 | Mean Difference (IV, Fixed, 95% CI) | ‐8.59 [‐11.53, ‐5.65] |
| Analysis 1.5  Comparison 1 Inhaled NO versus control, Outcome 5 Oxygenation index 30 to 60 minutes after treatment. | ||||
| 5.1 OI 30 to 60 minutes after treatment; studies that did not allow back‐up use of iNO in controls | 5 | 709 | Mean Difference (IV, Fixed, 95% CI) | ‐8.45 [‐11.42, ‐5.48] |
| 5.2 OI 30 to 60 minutes after treatment; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐16.1 [‐38.04, 5.84] |
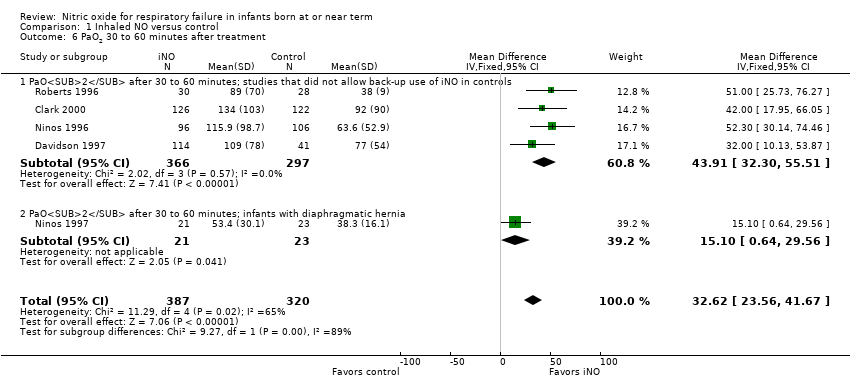
| 6 PaO2 30 to 60 minutes after treatment Show forest plot | 5 | 707 | Mean Difference (IV, Fixed, 95% CI) | 32.62 [23.56, 41.67] |
| Analysis 1.6  Comparison 1 Inhaled NO versus control, Outcome 6 PaO2 30 to 60 minutes after treatment. | ||||
| 6.1 PaO2 after 30 to 60 minutes; studies that did not allow back‐up use of iNO in controls | 4 | 663 | Mean Difference (IV, Fixed, 95% CI) | 43.91 [32.30, 55.51] |
| 6.2 PaO2 after 30 to 60 minutes; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 15.10 [0.64, 29.56] |
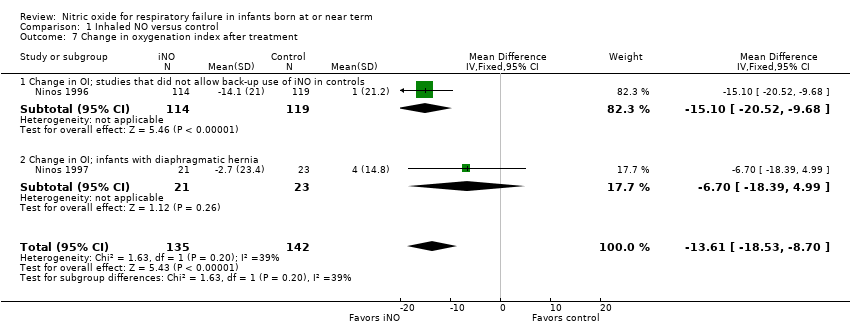
| 7 Change in oxygenation index after treatment Show forest plot | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | ‐13.61 [‐18.53, ‐8.70] |
| Analysis 1.7  Comparison 1 Inhaled NO versus control, Outcome 7 Change in oxygenation index after treatment. | ||||
| 7.1 Change in OI; studies that did not allow back‐up use of iNO in controls | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | ‐15.1 [‐20.52, ‐9.68] |
| 7.2 Change in OI; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐6.7 [‐18.39, 4.99] |
| 8 Change in PaO2 after treatment Show forest plot | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | 15.27 [7.18, 23.36] |
| Analysis 1.8  Comparison 1 Inhaled NO versus control, Outcome 8 Change in PaO2 after treatment. | ||||
| 8.1 Change in PaO2; studies that did not allow back‐up use of iNO in controls | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | 50.4 [32.14, 68.66] |
| 8.2 Change in PaO2; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [‐2.32, 15.72] |
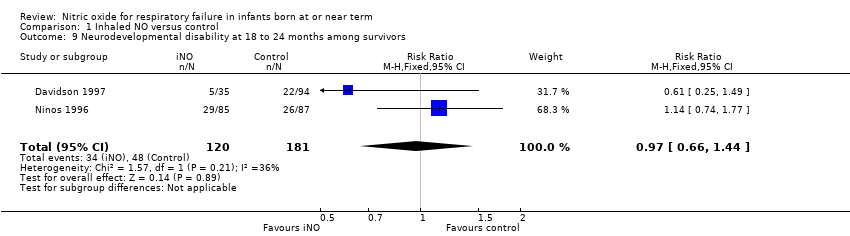
| 9 Neurodevelopmental disability at 18 to 24 months among survivors Show forest plot | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.44] |
| Analysis 1.9  Comparison 1 Inhaled NO versus control, Outcome 9 Neurodevelopmental disability at 18 to 24 months among survivors. | ||||
| 10 Hearing impairment in at least 1 ear among survivors Show forest plot | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.72, 1.68] |
| Analysis 1.10 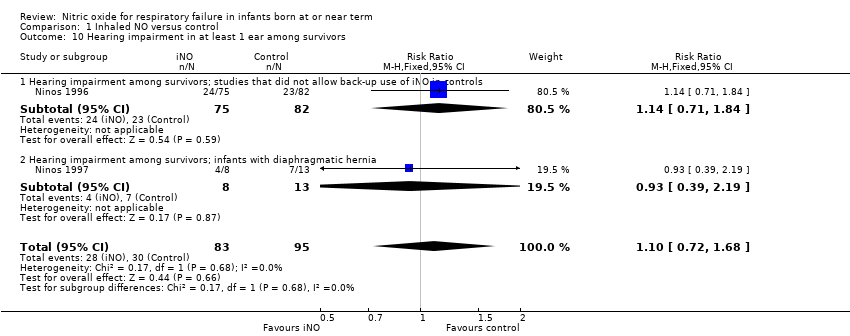 Comparison 1 Inhaled NO versus control, Outcome 10 Hearing impairment in at least 1 ear among survivors. | ||||
| 10.1 Hearing impairment among survivors; studies that did not allow back‐up use of iNO in controls | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.71, 1.84] |
| 10.2 Hearing impairment among survivors; infants with diaphragmatic hernia | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.39, 2.19] |
| 11 Cerebral palsy among survivors Show forest plot | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.62, 2.45] |
| Analysis 1.11  Comparison 1 Inhaled NO versus control, Outcome 11 Cerebral palsy among survivors. | ||||
| 11.1 Cerebral palsy among survivors; studies that did not allow back‐up use of iNO in controls | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.14] |
| 11.2 Cerebral palsy among survivors; infants with diaphragmatic hernia | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [0.45, 154.78] |
| 12 BSID MDI > 2 SD below the mean Show forest plot | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.38, 1.12] |
| Analysis 1.12  Comparison 1 Inhaled NO versus control, Outcome 12 BSID MDI > 2 SD below the mean. | ||||
| 13 BSID PDI > 2 SD below the mean Show forest plot | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.25, 0.94] |
| Analysis 1.13 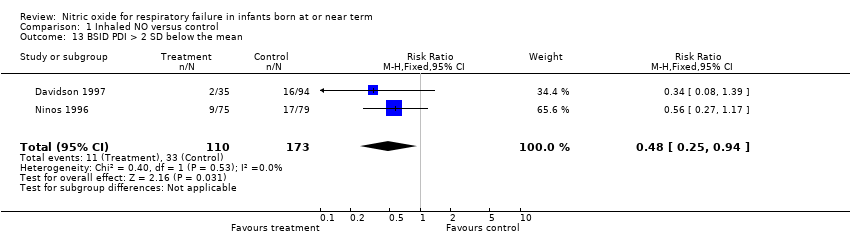 Comparison 1 Inhaled NO versus control, Outcome 13 BSID PDI > 2 SD below the mean. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or use of ECMO Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.27] |
| Analysis 2.1  Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 1 Death or use of ECMO. | ||||
| 2 Death before hospital discharge Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.38, 1.26] |
| Analysis 2.2  Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 2 Death before hospital discharge. | ||||
| 3 Use of ECMO before hospital discharge Show forest plot | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.66, 1.54] |
| Analysis 2.3  Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 3 Use of ECMO before hospital discharge. | ||||
| 4 Progression to severe disease criteria Show forest plot | 6 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.55, 0.79] |
| Analysis 2.4 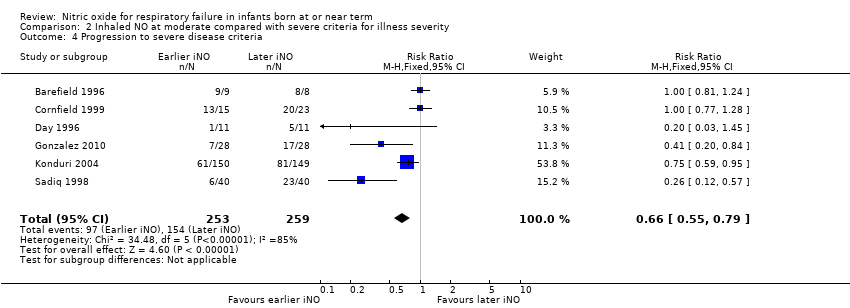 Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 4 Progression to severe disease criteria. | ||||
| 5 Chronic lung disease Show forest plot | 3 | 437 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.53] |
| Analysis 2.5 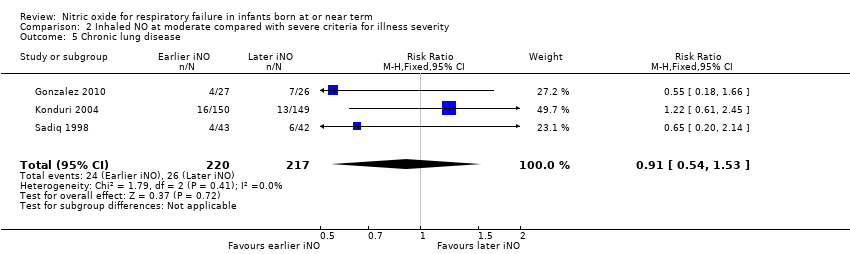 Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 5 Chronic lung disease. | ||||
| 6 Neurodevelopmental disability at 18 to 24 months among survivors Show forest plot | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.74, 1.74] |
| Analysis 2.6 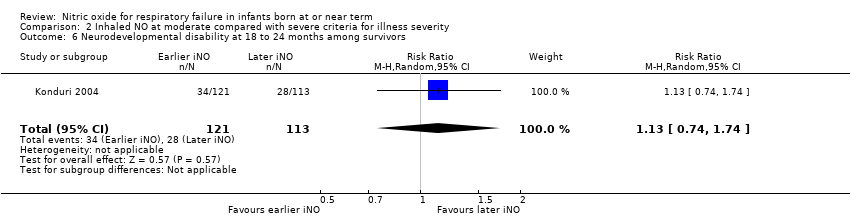 Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 6 Neurodevelopmental disability at 18 to 24 months among survivors. | ||||
| 7 Hearing impairment among survivors Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.95] |
| Analysis 2.7 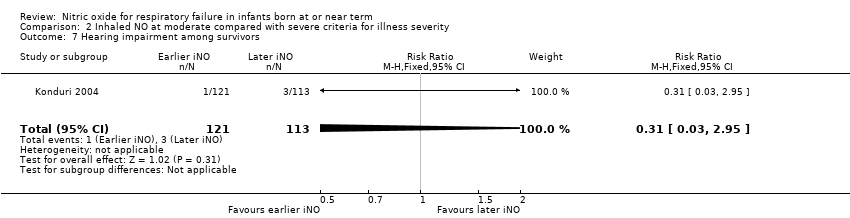 Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 7 Hearing impairment among survivors. | ||||
| 8 Cerebral palsy among survivors Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.53, 3.39] |
| Analysis 2.8  Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 8 Cerebral palsy among survivors. | ||||

Study flow diagram: review update.
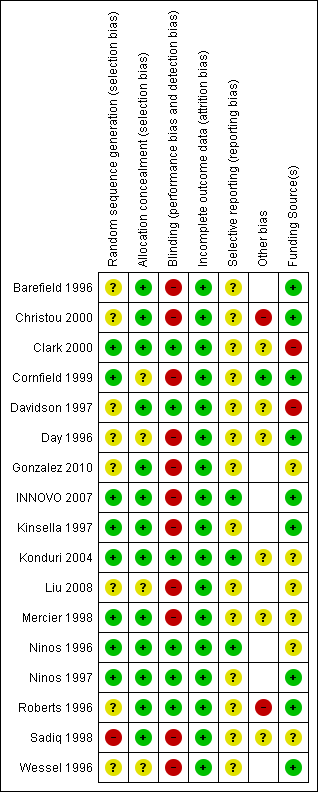
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
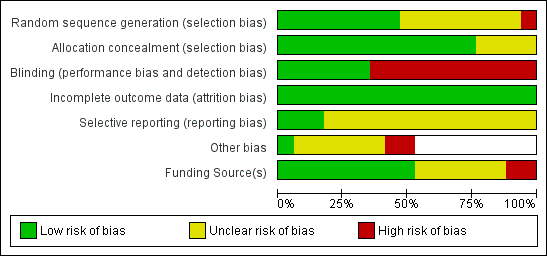
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Inhaled NO versus control, Outcome 1 Death or use of ECMO.

Comparison 1 Inhaled NO versus control, Outcome 2 Death before hospital discharge.
Comparison 1 Inhaled NO versus control, Outcome 3 Use of ECMO before hospital discharge.

Comparison 1 Inhaled NO versus control, Outcome 4 Failure to improve oxygenation (PaO2).

Comparison 1 Inhaled NO versus control, Outcome 5 Oxygenation index 30 to 60 minutes after treatment.
Comparison 1 Inhaled NO versus control, Outcome 6 PaO2 30 to 60 minutes after treatment.
Comparison 1 Inhaled NO versus control, Outcome 7 Change in oxygenation index after treatment.

Comparison 1 Inhaled NO versus control, Outcome 8 Change in PaO2 after treatment.
Comparison 1 Inhaled NO versus control, Outcome 9 Neurodevelopmental disability at 18 to 24 months among survivors.

Comparison 1 Inhaled NO versus control, Outcome 10 Hearing impairment in at least 1 ear among survivors.

Comparison 1 Inhaled NO versus control, Outcome 11 Cerebral palsy among survivors.

Comparison 1 Inhaled NO versus control, Outcome 12 BSID MDI > 2 SD below the mean.
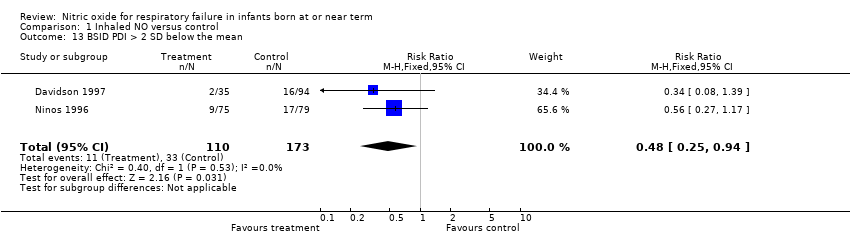
Comparison 1 Inhaled NO versus control, Outcome 13 BSID PDI > 2 SD below the mean.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 1 Death or use of ECMO.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 2 Death before hospital discharge.
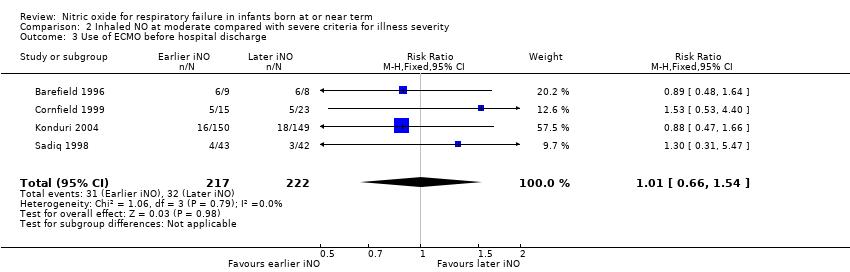
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 3 Use of ECMO before hospital discharge.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 4 Progression to severe disease criteria.
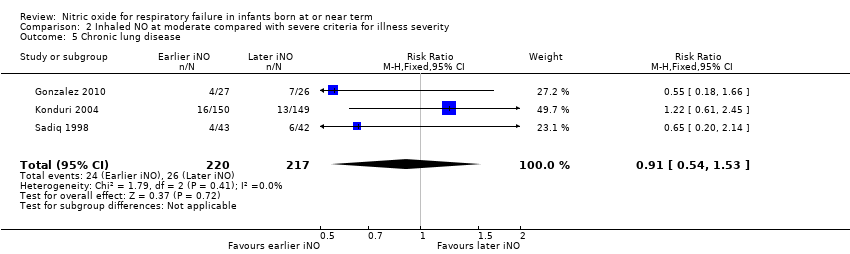
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 5 Chronic lung disease.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 6 Neurodevelopmental disability at 18 to 24 months among survivors.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 7 Hearing impairment among survivors.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 8 Cerebral palsy among survivors.
| Inhaled NO compared with control for respiratory failure in infants born at or near term | ||||||
| Patient or population: respiratory failure in infants born at or near term | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with inhaled NO | |||||
| Death or use of ECMO; studies that did not allow back‐up use of iNO in controls | Study population | RR 0.66 | 859 | ⊕⊕⊕⊕ | ||
| 540 per 1000 | 356 per 1000 | |||||
| Death or use of ECMO; infants with diaphragmatic hernia | Study population | RR 1.09 | 84 | ⊕⊕⊕⊝ | ||
| 870 per 1000 | 948 per 1000 | |||||
| Death before hospital discharge; studies that did not allow back‐up use of iNO in controls | Study population | RR 0.89 | 860 | ⊕⊕⊕⊕ | ||
| 120 per 1000 | 106 per 1000 | |||||
| Death before hospital discharge; infants with diaphragmatic hernia | Study population | RR 1.20 | 84 | ⊕⊕⊕⊝ | ||
| 391 per 1000 | 470 per 1000 | |||||
| Use of ECMO before hospital discharge; studies that did not allow back‐up use of iNO in controls | Study population | RR 0.60 | 815 | ⊕⊕⊕⊕ | ||
| 514 per 1000 | 308 per 1000 | |||||
| Use of ECMO before hospital discharge; infants with diaphragmatic hernia | Study population | RR 1.27 | 84 | ⊕⊕⊕⊝ | ||
| 674 per 1000 | 856 per 1000 | |||||
| Neurodevelopmental disability at 18 to 24 months among survivors | Study population | RR 0.97 | 301 | ⊕⊕⊝⊝ | ||
| 265 per 1000 | 257 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aSmall numbers of participants studied. bSubgroup of participants from only 2 trials evaluated. | ||||||
| Inhaled NO at moderate compared with severe criteria for illness severity in respiratory failure among infants born at or near term | ||||||
| Patient or population: infants born at or near term in respiratory failure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with inhaled NO at severe criteria for illness severity | Risk with Inhaled NO at moderate criteria for illness severity | |||||
| Death or requirement for ECMO | Study population | RR 0.88 | 495 | ⊕⊕⊝⊝ | ||
| 192 per 1000 | 169 per 1000 | |||||
| Death before hospital discharge | Study population | RR 0.69 | 495 | ⊕⊕⊕⊝ | ||
| 100 per 1000 | 69 per 1000 | |||||
| Use of ECMO before hospital discharge | Study population | RR 1.01 | 439 | ⊕⊕⊕⊝ | ||
| 144 per 1000 | 146 per 1000 | |||||
| Progression to severe criteria | Study population | RR 0.66 | 512 | ⊕⊕⊕⊝ | ||
| 595 per 1000 | 392 per 1000 | |||||
| Neurodevelopmental disability at 18 to 24 months among survivors | Study population | RR 1.13 | 234 | ⊕⊕⊕⊝ | ||
| 248 per 1000 | 280 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aHighly variable risk ratio. bVery wide confidence intervals. | ||||||
| Study | Ventilator days | Oxygen days | Hospitalisation days | |
| Gonzalez | Early iNO | Median 6, range 3‐28 | Median 11.5, range 5‐90 | |
| Late iNO | Median 8, range 4‐37 | Median 18, range 6‐142 | ||
| Konduri | Early iNO | Median 8, IQR 6‐12 | Median 13, IQR 9‐19 | Median 17, IQR 12‐22 |
| Late iNO | Median 8, IQR 6‐12 | Median 13, IQR 9‐19 | Median 18, IQR 12‐30 | |
| Sadiq | Early iNO | Mean 8,7, SD 4 | Mean 14, SD 8 | Mean 21, SD 14 |
| Late iNO | Mean 10, SD 6 | Mean 18, SD 17 | Mean 21, SD 11 | |
| IQR: interquartile range; SD: standard deviation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or use of ECMO Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death or use of ECMO; studies that did not allow back‐up use of iNO in controls | 8 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.57, 0.77] |
| 1.2 Death or use of ECMO; studies that allowed back‐up use of iNO in controls | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.34, 4.16] |
| 1.3 Death or use of ECMO; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 2 Death before hospital discharge Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Death; studies that did not allow back‐up use of iNO in controls | 8 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.60, 1.31] |
| 2.2 Death; studies that allowed back‐up use of iNO in controls | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.34, 4.16] |
| 2.3 Death; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.74, 1.96] |
| 3 Use of ECMO before hospital discharge Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Use of ECMO before hospital discharge; studies that did not allow back‐up use of iNO in controls | 7 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.50, 0.71] |
| 3.2 Use of ECMO before hospital discharge; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.00, 1.62] |
| 4 Failure to improve oxygenation (PaO2) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Failure to improve PaO2; studies that did not allow back‐up use of iNO in controls | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Failure to improve PaO2; infants with diaphragmatic hernia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Oxygenation index 30 to 60 minutes after treatment Show forest plot | 6 | 753 | Mean Difference (IV, Fixed, 95% CI) | ‐8.59 [‐11.53, ‐5.65] |
| 5.1 OI 30 to 60 minutes after treatment; studies that did not allow back‐up use of iNO in controls | 5 | 709 | Mean Difference (IV, Fixed, 95% CI) | ‐8.45 [‐11.42, ‐5.48] |
| 5.2 OI 30 to 60 minutes after treatment; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐16.1 [‐38.04, 5.84] |
| 6 PaO2 30 to 60 minutes after treatment Show forest plot | 5 | 707 | Mean Difference (IV, Fixed, 95% CI) | 32.62 [23.56, 41.67] |
| 6.1 PaO2 after 30 to 60 minutes; studies that did not allow back‐up use of iNO in controls | 4 | 663 | Mean Difference (IV, Fixed, 95% CI) | 43.91 [32.30, 55.51] |
| 6.2 PaO2 after 30 to 60 minutes; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 15.10 [0.64, 29.56] |
| 7 Change in oxygenation index after treatment Show forest plot | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | ‐13.61 [‐18.53, ‐8.70] |
| 7.1 Change in OI; studies that did not allow back‐up use of iNO in controls | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | ‐15.1 [‐20.52, ‐9.68] |
| 7.2 Change in OI; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐6.7 [‐18.39, 4.99] |
| 8 Change in PaO2 after treatment Show forest plot | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | 15.27 [7.18, 23.36] |
| 8.1 Change in PaO2; studies that did not allow back‐up use of iNO in controls | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | 50.4 [32.14, 68.66] |
| 8.2 Change in PaO2; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [‐2.32, 15.72] |
| 9 Neurodevelopmental disability at 18 to 24 months among survivors Show forest plot | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.44] |
| 10 Hearing impairment in at least 1 ear among survivors Show forest plot | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.72, 1.68] |
| 10.1 Hearing impairment among survivors; studies that did not allow back‐up use of iNO in controls | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.71, 1.84] |
| 10.2 Hearing impairment among survivors; infants with diaphragmatic hernia | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.39, 2.19] |
| 11 Cerebral palsy among survivors Show forest plot | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.62, 2.45] |
| 11.1 Cerebral palsy among survivors; studies that did not allow back‐up use of iNO in controls | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.14] |
| 11.2 Cerebral palsy among survivors; infants with diaphragmatic hernia | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [0.45, 154.78] |
| 12 BSID MDI > 2 SD below the mean Show forest plot | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.38, 1.12] |
| 13 BSID PDI > 2 SD below the mean Show forest plot | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.25, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or use of ECMO Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.27] |
| 2 Death before hospital discharge Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.38, 1.26] |
| 3 Use of ECMO before hospital discharge Show forest plot | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.66, 1.54] |
| 4 Progression to severe disease criteria Show forest plot | 6 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.55, 0.79] |
| 5 Chronic lung disease Show forest plot | 3 | 437 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.53] |
| 6 Neurodevelopmental disability at 18 to 24 months among survivors Show forest plot | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.74, 1.74] |
| 7 Hearing impairment among survivors Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.95] |
| 8 Cerebral palsy among survivors Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.53, 3.39] |

