نقش نیتریک اکسید در درمان نارسایی تنفسی در نوزادان متولد شده در دوران ترم یا نزدیک ترم
Appendices
Appendix 1. Search strategy 2016
(Nitric OR Nitrix Oxide) with database specific terms:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Clinicaltrials.gov: (infant)
Controlled‐trials.com: (infant)
WHO Trials database: (infant OR neonate)

Study flow diagram: review update.
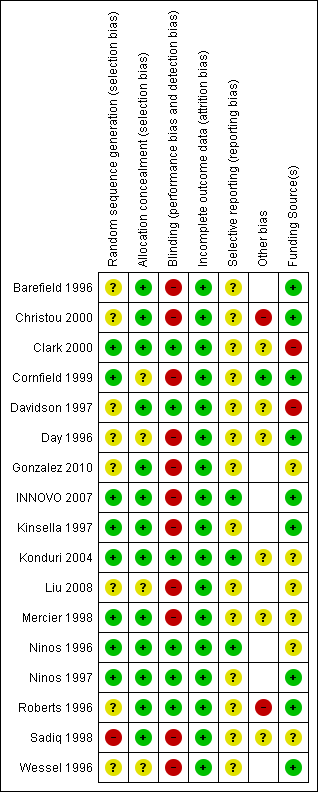
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
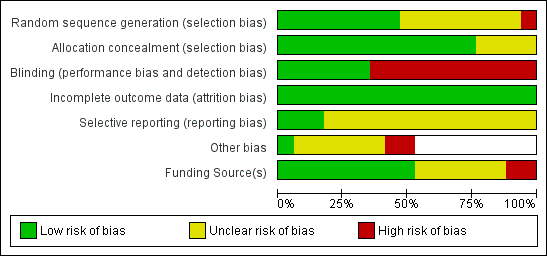
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Inhaled NO versus control, Outcome 1 Death or use of ECMO.

Comparison 1 Inhaled NO versus control, Outcome 2 Death before hospital discharge.
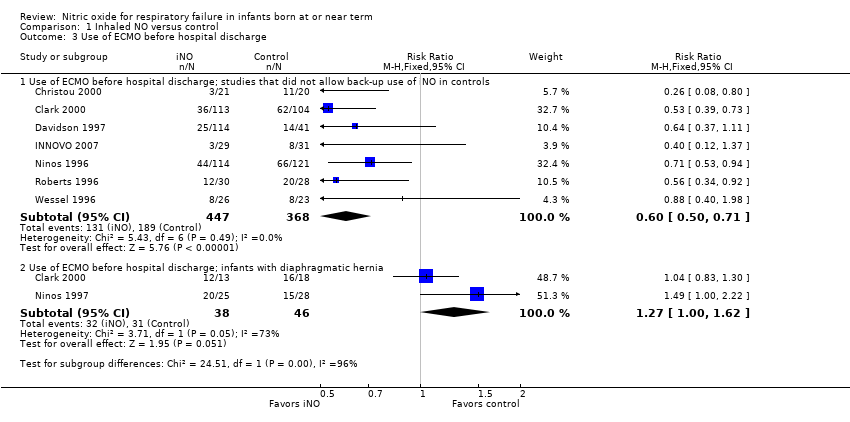
Comparison 1 Inhaled NO versus control, Outcome 3 Use of ECMO before hospital discharge.

Comparison 1 Inhaled NO versus control, Outcome 4 Failure to improve oxygenation (PaO2).

Comparison 1 Inhaled NO versus control, Outcome 5 Oxygenation index 30 to 60 minutes after treatment.
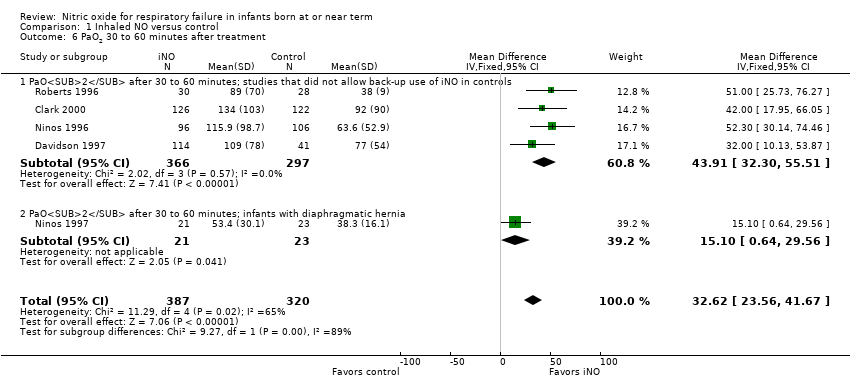
Comparison 1 Inhaled NO versus control, Outcome 6 PaO2 30 to 60 minutes after treatment.
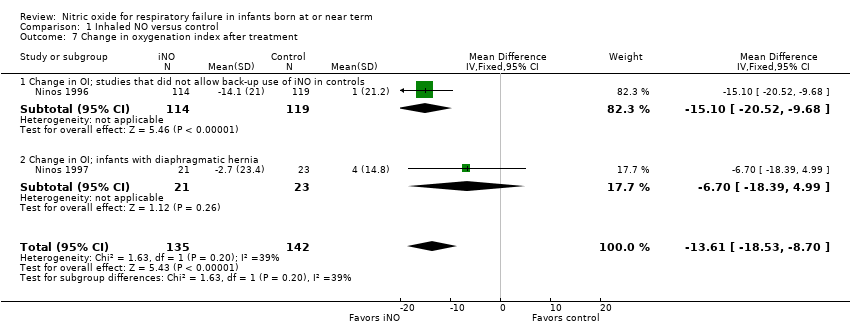
Comparison 1 Inhaled NO versus control, Outcome 7 Change in oxygenation index after treatment.

Comparison 1 Inhaled NO versus control, Outcome 8 Change in PaO2 after treatment.
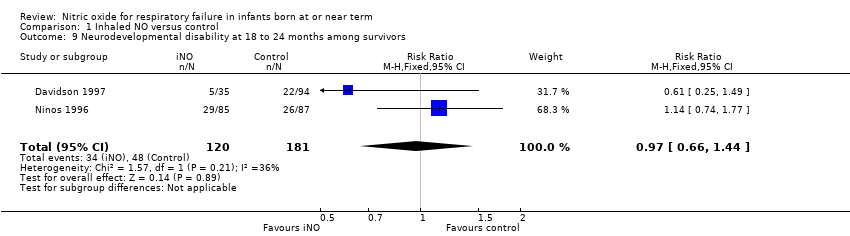
Comparison 1 Inhaled NO versus control, Outcome 9 Neurodevelopmental disability at 18 to 24 months among survivors.

Comparison 1 Inhaled NO versus control, Outcome 10 Hearing impairment in at least 1 ear among survivors.

Comparison 1 Inhaled NO versus control, Outcome 11 Cerebral palsy among survivors.

Comparison 1 Inhaled NO versus control, Outcome 12 BSID MDI > 2 SD below the mean.
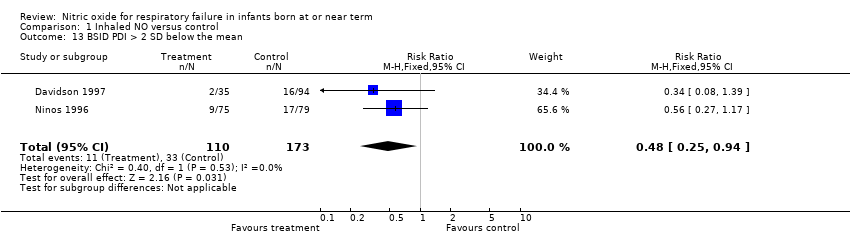
Comparison 1 Inhaled NO versus control, Outcome 13 BSID PDI > 2 SD below the mean.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 1 Death or use of ECMO.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 2 Death before hospital discharge.
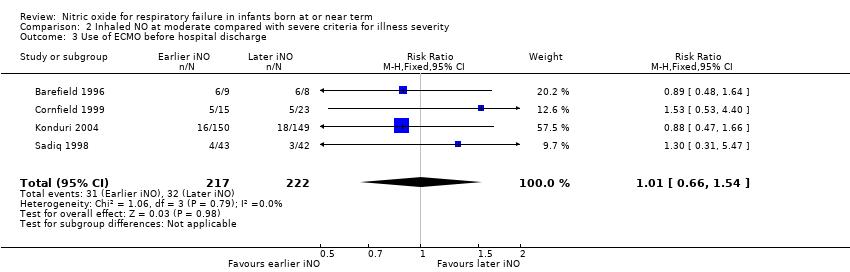
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 3 Use of ECMO before hospital discharge.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 4 Progression to severe disease criteria.
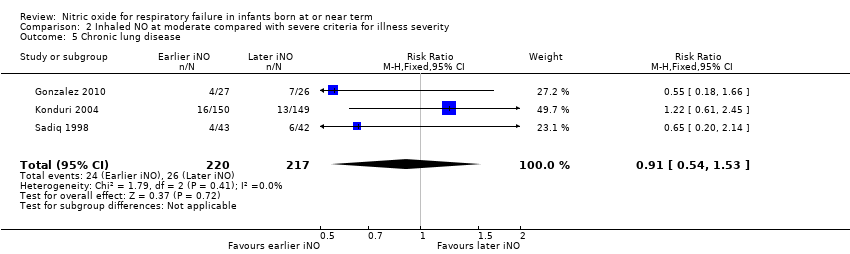
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 5 Chronic lung disease.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 6 Neurodevelopmental disability at 18 to 24 months among survivors.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 7 Hearing impairment among survivors.

Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 8 Cerebral palsy among survivors.
| Inhaled NO compared with control for respiratory failure in infants born at or near term | ||||||
| Patient or population: respiratory failure in infants born at or near term | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with inhaled NO | |||||
| Death or use of ECMO; studies that did not allow back‐up use of iNO in controls | Study population | RR 0.66 | 859 | ⊕⊕⊕⊕ | ||
| 540 per 1000 | 356 per 1000 | |||||
| Death or use of ECMO; infants with diaphragmatic hernia | Study population | RR 1.09 | 84 | ⊕⊕⊕⊝ | ||
| 870 per 1000 | 948 per 1000 | |||||
| Death before hospital discharge; studies that did not allow back‐up use of iNO in controls | Study population | RR 0.89 | 860 | ⊕⊕⊕⊕ | ||
| 120 per 1000 | 106 per 1000 | |||||
| Death before hospital discharge; infants with diaphragmatic hernia | Study population | RR 1.20 | 84 | ⊕⊕⊕⊝ | ||
| 391 per 1000 | 470 per 1000 | |||||
| Use of ECMO before hospital discharge; studies that did not allow back‐up use of iNO in controls | Study population | RR 0.60 | 815 | ⊕⊕⊕⊕ | ||
| 514 per 1000 | 308 per 1000 | |||||
| Use of ECMO before hospital discharge; infants with diaphragmatic hernia | Study population | RR 1.27 | 84 | ⊕⊕⊕⊝ | ||
| 674 per 1000 | 856 per 1000 | |||||
| Neurodevelopmental disability at 18 to 24 months among survivors | Study population | RR 0.97 | 301 | ⊕⊕⊝⊝ | ||
| 265 per 1000 | 257 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aSmall numbers of participants studied. bSubgroup of participants from only 2 trials evaluated. | ||||||
| Inhaled NO at moderate compared with severe criteria for illness severity in respiratory failure among infants born at or near term | ||||||
| Patient or population: infants born at or near term in respiratory failure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with inhaled NO at severe criteria for illness severity | Risk with Inhaled NO at moderate criteria for illness severity | |||||
| Death or requirement for ECMO | Study population | RR 0.88 | 495 | ⊕⊕⊝⊝ | ||
| 192 per 1000 | 169 per 1000 | |||||
| Death before hospital discharge | Study population | RR 0.69 | 495 | ⊕⊕⊕⊝ | ||
| 100 per 1000 | 69 per 1000 | |||||
| Use of ECMO before hospital discharge | Study population | RR 1.01 | 439 | ⊕⊕⊕⊝ | ||
| 144 per 1000 | 146 per 1000 | |||||
| Progression to severe criteria | Study population | RR 0.66 | 512 | ⊕⊕⊕⊝ | ||
| 595 per 1000 | 392 per 1000 | |||||
| Neurodevelopmental disability at 18 to 24 months among survivors | Study population | RR 1.13 | 234 | ⊕⊕⊕⊝ | ||
| 248 per 1000 | 280 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aHighly variable risk ratio. bVery wide confidence intervals. | ||||||
| Study | Ventilator days | Oxygen days | Hospitalisation days | |
| Gonzalez | Early iNO | Median 6, range 3‐28 | Median 11.5, range 5‐90 | |
| Late iNO | Median 8, range 4‐37 | Median 18, range 6‐142 | ||
| Konduri | Early iNO | Median 8, IQR 6‐12 | Median 13, IQR 9‐19 | Median 17, IQR 12‐22 |
| Late iNO | Median 8, IQR 6‐12 | Median 13, IQR 9‐19 | Median 18, IQR 12‐30 | |
| Sadiq | Early iNO | Mean 8,7, SD 4 | Mean 14, SD 8 | Mean 21, SD 14 |
| Late iNO | Mean 10, SD 6 | Mean 18, SD 17 | Mean 21, SD 11 | |
| IQR: interquartile range; SD: standard deviation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or use of ECMO Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death or use of ECMO; studies that did not allow back‐up use of iNO in controls | 8 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.57, 0.77] |
| 1.2 Death or use of ECMO; studies that allowed back‐up use of iNO in controls | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.34, 4.16] |
| 1.3 Death or use of ECMO; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 2 Death before hospital discharge Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Death; studies that did not allow back‐up use of iNO in controls | 8 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.60, 1.31] |
| 2.2 Death; studies that allowed back‐up use of iNO in controls | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.34, 4.16] |
| 2.3 Death; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.74, 1.96] |
| 3 Use of ECMO before hospital discharge Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Use of ECMO before hospital discharge; studies that did not allow back‐up use of iNO in controls | 7 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.50, 0.71] |
| 3.2 Use of ECMO before hospital discharge; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.00, 1.62] |
| 4 Failure to improve oxygenation (PaO2) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Failure to improve PaO2; studies that did not allow back‐up use of iNO in controls | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Failure to improve PaO2; infants with diaphragmatic hernia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Oxygenation index 30 to 60 minutes after treatment Show forest plot | 6 | 753 | Mean Difference (IV, Fixed, 95% CI) | ‐8.59 [‐11.53, ‐5.65] |
| 5.1 OI 30 to 60 minutes after treatment; studies that did not allow back‐up use of iNO in controls | 5 | 709 | Mean Difference (IV, Fixed, 95% CI) | ‐8.45 [‐11.42, ‐5.48] |
| 5.2 OI 30 to 60 minutes after treatment; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐16.1 [‐38.04, 5.84] |
| 6 PaO2 30 to 60 minutes after treatment Show forest plot | 5 | 707 | Mean Difference (IV, Fixed, 95% CI) | 32.62 [23.56, 41.67] |
| 6.1 PaO2 after 30 to 60 minutes; studies that did not allow back‐up use of iNO in controls | 4 | 663 | Mean Difference (IV, Fixed, 95% CI) | 43.91 [32.30, 55.51] |
| 6.2 PaO2 after 30 to 60 minutes; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 15.10 [0.64, 29.56] |
| 7 Change in oxygenation index after treatment Show forest plot | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | ‐13.61 [‐18.53, ‐8.70] |
| 7.1 Change in OI; studies that did not allow back‐up use of iNO in controls | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | ‐15.1 [‐20.52, ‐9.68] |
| 7.2 Change in OI; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐6.7 [‐18.39, 4.99] |
| 8 Change in PaO2 after treatment Show forest plot | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | 15.27 [7.18, 23.36] |
| 8.1 Change in PaO2; studies that did not allow back‐up use of iNO in controls | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | 50.4 [32.14, 68.66] |
| 8.2 Change in PaO2; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [‐2.32, 15.72] |
| 9 Neurodevelopmental disability at 18 to 24 months among survivors Show forest plot | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.44] |
| 10 Hearing impairment in at least 1 ear among survivors Show forest plot | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.72, 1.68] |
| 10.1 Hearing impairment among survivors; studies that did not allow back‐up use of iNO in controls | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.71, 1.84] |
| 10.2 Hearing impairment among survivors; infants with diaphragmatic hernia | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.39, 2.19] |
| 11 Cerebral palsy among survivors Show forest plot | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.62, 2.45] |
| 11.1 Cerebral palsy among survivors; studies that did not allow back‐up use of iNO in controls | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.14] |
| 11.2 Cerebral palsy among survivors; infants with diaphragmatic hernia | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [0.45, 154.78] |
| 12 BSID MDI > 2 SD below the mean Show forest plot | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.38, 1.12] |
| 13 BSID PDI > 2 SD below the mean Show forest plot | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.25, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or use of ECMO Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.27] |
| 2 Death before hospital discharge Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.38, 1.26] |
| 3 Use of ECMO before hospital discharge Show forest plot | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.66, 1.54] |
| 4 Progression to severe disease criteria Show forest plot | 6 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.55, 0.79] |
| 5 Chronic lung disease Show forest plot | 3 | 437 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.53] |
| 6 Neurodevelopmental disability at 18 to 24 months among survivors Show forest plot | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.74, 1.74] |
| 7 Hearing impairment among survivors Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.95] |
| 8 Cerebral palsy among survivors Show forest plot | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.53, 3.39] |

