Penggunaan antibiotik untuk bronkitis akut
Abstract
Background
The benefits and risks of antibiotics for acute bronchitis remain unclear despite it being one of the most common illnesses seen in primary care.
Objectives
To assess the effects of antibiotics in improving outcomes and to assess adverse effects of antibiotic therapy for people with a clinical diagnosis of acute bronchitis.
Search methods
We searched CENTRAL 2016, Issue 11 (accessed 13 January 2017), MEDLINE (1966 to January week 1, 2017), Embase (1974 to 13 January 2017), and LILACS (1982 to 13 January 2017). We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov on 5 April 2017.
Selection criteria
Randomised controlled trials comparing any antibiotic therapy with placebo or no treatment in acute bronchitis or acute productive cough, in people without underlying pulmonary disease.
Data collection and analysis
At least two review authors extracted data and assessed trial quality.
Main results
We did not identify any new trials for inclusion in this 2017 update. We included 17 trials with 5099 participants in the primary analysis. The quality of trials was generally good. At follow‐up there was no difference in participants described as being clinically improved between the antibiotic and placebo groups (11 studies with 3841 participants, risk ratio (RR) 1.07, 95% confidence interval (CI) 0.99 to 1.15). Participants given antibiotics were less likely to have a cough (4 studies with 275 participants, RR 0.64, 95% CI 0.49 to 0.85; number needed to treat for an additional beneficial outcome (NNTB) 6) and a night cough (4 studies with 538 participants, RR 0.67, 95% CI 0.54 to 0.83; NNTB 7). Participants given antibiotics had a shorter mean cough duration (7 studies with 2776 participants, mean difference (MD) ‐0.46 days, 95% CI ‐0.87 to ‐0.04). The differences in presence of a productive cough at follow‐up and MD of productive cough did not reach statistical significance.
Antibiotic‐treated participants were more likely to be improved according to clinician's global assessment (6 studies with 891 participants, RR 0.61, 95% CI 0.48 to 0.79; NNTB 11) and were less likely to have an abnormal lung exam (5 studies with 613 participants, RR 0.54, 95% CI 0.41 to 0.70; NNTB 6). Antibiotic‐treated participants also had a reduction in days feeling ill (5 studies with 809 participants, MD ‐0.64 days, 95% CI ‐1.16 to ‐0.13) and days with impaired activity (6 studies with 767 participants, MD ‐0.49 days, 95% CI ‐0.94 to ‐0.04). The differences in proportions with activity limitations at follow‐up did not reach statistical significance. There was a significant trend towards an increase in adverse effects in the antibiotic group (12 studies with 3496 participants, RR 1.20, 95% CI 1.05 to 1.36; NNT for an additional harmful outcome 24).
Authors' conclusions
There is limited evidence of clinical benefit to support the use of antibiotics in acute bronchitis. Antibiotics may have a modest beneficial effect in some patients such as frail, elderly people with multimorbidity who may not have been included in trials to date. However, the magnitude of this benefit needs to be considered in the broader context of potential side effects, medicalisation for a self limiting condition, increased resistance to respiratory pathogens, and cost of antibiotic treatment.
PICO
Ringkasan bahasa mudah
Rawatan antibiotik untuk mereka yang mengidapi bronkitis akut
Soalan ulasan
Penyelidik ingin mengetahui sama ada antibiotik dapat memperbaiki hasil penghidap bronkitis akut. Kami turut menilai potensi kesan buruk terapi antibiotik.
Latar belakang
Bronkitis akut adalah diagnosis klinikal (berdasarkan tanda‐tanda dan gejala‐gejala pesakit yang dilaporkan) untuk batuk akut, samada menghasilkan kahak ataupun tidak. Bronkitis akut boleh disebabkan oleh virus atau bakteria. Gejala‐gejala biasanya bertahan selama dua minggu tetapi boleh juga bertahan sehingga lapan minggu. Antibiotik biasanya diberikan untuk merawat bronkitis akut, tetapi ia boleh mendatangkan kesan buruk seperti loya dan cirit‐birit serta menyebabkan tindak balas yang lebih serius untuk pesakit yang alah pada antibiotik. Tidak terdapat sebarang ujian yang praktikal untuk membezakan antara bronkitis bakteria dan virus.
Ciri‐ciri kajian
Penyelidik menyertakan kajian rawak terkawal yang membandingkan sebarang terapi antibiotik dengan plasebo atau tanpa rawatan keatas mereka yang mengalami bronkitis akut atau batuk produktif akut dan tidak mempunyai apa‐apa keadaan paru‐paru kronik. Kami telah melibatkan 17 ujian dengan 5099 peserta. Rawatan bersama‐sama dengan ubat‐ubatan lain untuk melegakan gejala‐gejala adalah dibenarkan jika mereka diberikan kepada semua peserta dalam kajian ini.
Keputusan utama
Bukti kami adalah terkini sehingga 13 Januari 2017.
Kami mendapati bukti manfaat klinikal yang terhad untuk menyokong penggunaan antibiotik untuk bronkitis akut. Sesetengah orang dirawat dengan antibiotik pulih lebih cepat dengan hasil berkaitan dengan kekurangan batuk. Walau bagaimanapun, perbezaan ini mungkin tidak penting secara praktikal kerana ia hanya menunjukkan perbezaan setengah hari sahaja dalam tempoh 8 ‐ 10‐hari. Terdapat peningkatan yang kecil tetapi penting dalam kesan‐kesan sampingan yang buruk pada orang yang dirawat dengan antibiotik. Kesan sampingan yang paling kerap dilaporkan termasuk loya, muntah‐muntah, cirit‐birit, sakit kepala, dan ruam.
Ulasan ini menunjukkan bahawa hanya terdapat manfaat yang terhad kepada pesakit yang menggunakan antibiotik untuk bronkitis akut yang tidak menhidapi penyakit‐penyakit lain. Lebih banyak penyelidikan diperlukan tentang kesan penggunaan antibiotik untuk bronkitis akut dalam orang uzur, warga tua dengan beberapa keadaan kronik yang mungkin tidak diambilkira dalam kajian sedia ada. Penggunaan antibiotik perlu dipertimbangkan dalam konteks kesan sampingan, perubatan untuk keadaan diri yang terhad, kos rawatan antibiotik, dan kemudaratan peringkat penduduk khususnya berkaitan dengan peningkatan rintangan antibiotik.
Kualiti bukti
Kualiti ujian ini adalah secara amnya baik, khususnya bagi kajian yang lebih terkini.
Authors' conclusions
Background
Description of the condition
Acute bronchitis is a common illness characterised by fever and cough that is often wheezy in nature and that may or may not be productive. The condition occurs when the bronchi become inflamed due to either viral or bacterial infection. Symptoms generally last for two weeks, but the associated cough can last for up to eight weeks (CDC 2013). Acute bronchitis is the ninth most common outpatient illness recorded by physicians in ambulatory practice in the USA (Delozier 1989), and the fifth most common outpatient illness encountered by Australian general practitioners, for whom it represents 3.5% of encounters and 2.4% of problems seen (Meza 1994). In the UK, there are 300 to 400 consultations for treatment of respiratory tract infections per 1000 registered patients each year, and while antibiotic prescribing for these conditions declined between 1995 and 2000, it has since stabilised (Gulliford 2011). Data provided by the European Centre for Disease Prevention and Control on trends in antimicrobial consumption across Europe suggests that overall antibiotic use varies across Europe, with most countries showing an increase between 1997 and 2010 (ECDC 2013).
Population‐based estimates of the incidence of acute bronchitis range from 33 to 45 cases per 1000 per year (Ayres 1986; Mainous 1996). People with bronchitis miss an average of two to three days off work per episode. The great majority of episodes of acute bronchitis in healthy individuals are presumed to be viral infections, although this has been questioned (Macfarlane 1994). Community‐based studies have isolated viruses in 8% to 23% of cases (Boldy 1990; Macfarlane 1993; Stuart‐Harris 1965). Other pathogens implicated in acute bronchitis are Mycoplasma pneumoniae, Chlamydia pneumoniae, and Bordetella pertussis, each of which has been identified in up to 25% of cases in various populations (Boldy 1990; Falck 1994; Foy 1993; Grayston 1993; Herwaldt 1991; Jonsson 1997; King 1996; Macfarlane 1993; Robertson 1987; Stuart‐Harris 1965; Thom 1994). A more recent study assessing the aetiology and outcome of acute lower respiratory tract infection in 638 adults in UK primary care showed that in 55% of cases viral or bacterial pathogens were identified (Macfarlane 2001).
Description of the intervention
The use of antibiotics in people with acute bronchial infections remains a controversial area in primary healthcare practice (Coenen 2007; Del Mar 2016; Gonzales 1995). Streptococcus pneumoniae,Haemophilus influenzae, andMoraxella catarrhalis have been isolated from sputum samples in up to 45% of people with acute bronchitis (Henry 1995; Macfarlane 1993), but their role is difficult to assess due to potential oropharyngeal colonisation in healthy individuals (Laurenzi 1961; Smith 1986). Unfortunately, there are no clinically useful criteria that accurately help distinguish bacterial from viral bronchial infections, therefore some authors have called for physicians to stop prescribing antibiotics for people with acute bronchitis (Gonzales 1995; Hueston 1997). Nevertheless, antibiotics are prescribed for 60% to 83% of people who present to physicians with the condition (Gonzales 1997; Mainous 1996; Meza 1994; Petersen 2007; Straand 1997).
How the intervention might work
Antibiotics may improve outcomes in acute bronchitis if the disease is caused by a bacterial infection. Antibiotics have no antiviral activity and are therefore not effective in viral bronchitis. In addition, antibiotics can cause harm due to their negative effect on normal bacteria colonising the intestine. The most common adverse effects of antibiotics include gastrointestinal symptoms such as nausea and diarrhoea, but they can also cause more serious reactions related to anaphylaxis in those who are allergic.
Why it is important to do this review
Some estimate of the probable effectiveness of antibiotic therapy for acute bronchitis is needed given the frequent occurrence of the condition. If found to be effective, antibiotics could shorten the course of the disease and consequently reduce the associated loss of productive work time. However, any benefit from antibiotics must be weighed against the possibility that excessive antibiotic use will lead to increases in cost and patient morbidity, as well as the development of resistant strains of common organisms, Coenen 2007; Molstad 1992, and unnecessary medicalisation of individuals with a self limiting illness (Little 2005). If antibiotics are found to be ineffective, then their use should be discontinued.
Objectives
To assess the effects of antibiotics in improving outcomes and to assess adverse effects of antibiotic therapy for people with a clinical diagnosis of acute bronchitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials in people with acute bronchitis assigned to treatment with an antibiotic or to a placebo or no active treatment.
Types of participants
We included trials evaluating people of either sex and any age with a clinical syndrome of cough with or without productive sputum, with a physician's diagnosis of acute bronchitis or cough with persistent cold or flu‐like illness that was not resolving. The term 'acute lower respiratory tract infection when pneumonia is not suspected' is also used to describe this clinical presentation. We excluded trials that included people with pre‐existing chronic bronchitis (i.e. acute exacerbation of chronic bronchitis).
Types of interventions
We included all randomised controlled trials comparing any antibiotic therapy versus no treatment or placebo in the management of acute bronchitis. We excluded trials comparing one antibiotic regimen with another, or trials comparing the use of other active medications (such as bronchodilators) with antibiotic therapy in this review. We included trials that allowed concurrent use of other medications such as analgesics, antitussives, antipyretics, or mucolytics if they allowed equal access to such medications to participants in both the antibiotic and the control group.
Types of outcome measures
We included the following range of cough‐related and general clinical outcomes.
Primary outcomes
-
Cough‐related outcomes including:
-
time to resolution of cough;
-
sputum production, defined as proportion of participants with or without sputum;
-
proportions of participants with cough, night cough, productive cough.
-
-
Global assessment of improvement by clinicians at follow‐up.
-
General clinical outcomes including:
-
severity of symptoms;
-
activity limitations;
-
abnormal lung examination at a designated follow‐up visit.
-
Secondary outcomes
-
Adverse effects.
Search methods for identification of studies
Electronic searches
For this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2016, Issue 11, part of the Cochrane Library (www.cochranelibrary.com/) (accessed 13 January 2017), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to January week 1, 2017), Embase (1974 to 13 January 2017), and LILACS (Latin American and Caribbean Literature in Health Sciences) (1982 to 13 January 2017). We used the search strategy described in Appendix 1 to search MEDLINE and CENTRAL. We adapted the search strategy to search Embase (Appendix 2) and LILACS (Appendix 3). Details of the 2017 update search can be found in Appendix 4.
Searching other resources
We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/) and ClinicalTrials.gov (clinicaltrials.gov/) on 5 April 2017. We also searched the reference lists of relevant trials, and we originally searched review articles and textbook chapters to identify additional trials, including those published prior to 1966. For the original review, we included in our searches articles from the review authors’ personal collections and requested unpublished trials from trial authors. In addition, for the earlier version of this review we also contacted drug companies that manufacture antibiotics. There were no language or publication restrictions.
Data collection and analysis
Selection of studies
One review author (SS) evaluated the titles and abstracts of the identified citations and applied the inclusion criteria. We obtained the full papers of trials deemed potentially relevant for further examination. Two review authors (TF, SS) screened the full‐text papers to determine if they met the inclusion criteria. We discarded reports that were clearly irrelevant. We recorded studies that did not fulfil the inclusion criteria along with the reasons for their exclusion in the Characteristics of excluded studies table..
Data extraction and management
Two or more review authors independently extracted data using a data collection form designed for this review. Any disagreements were resolved by discussion between the review authors. We transferred data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (SS, TF) evaluated the methodological quality of each trial using Risk of Bias domains recommended in the Cochrane Handook as outlined in Figure 1 and Figure 2. Disagreements were resolved by consensus.

'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.

'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Measures of treatment effect
The effect measures of choice were risk ratio (RR) for categorical outcomes and mean difference (MD) for continuous data.
Unit of analysis issues
There were no cluster‐randomised trials included in this review as it involved a simple drug trial with a placebo comparator. Clinicians were generally blinded to the intervention. We identified no unit of analysis errors.
Dealing with missing data
Where data were missing we reported this in the Risk of bias in included studies section. We did not adopt any strategies to deal with missing data such as imputation. In general, missing data did not bias the review findings.
Assessment of heterogeneity
Where we considered clinical heterogeneity to be an issue, we undertook a random‐effects meta‐analysis rather than a fixed‐effect meta‐analysis. This applied in particular to the most recent analysis added to this updated version of the review (Analysis 6.1).
Assessment of reporting biases
We examined funnel plots for each of the analyses conducted and none indicated a significant level of reporting bias.
Data synthesis
All previous versions of this review presented fixed‐effect meta‐analyses. For this update, we included a range of outcomes under the broad definition of 'clinically improved'. These were clinically heterogeneous, so we used a random‐effects meta‐analysis.
GRADE and 'Summary of findings' table
The original review and analyses were conducted prior to the use of GRADE and 'Summary of findings' tables. As we identified no new studies for inclusion in this update, we did not undertake a GRADE assessment or create a 'Summary of findings' table. We made this decision based on time and resource constraints of the author group. The update status is now 'Up to date > No new studies identified with search'.
Subgroup analysis and investigation of heterogeneity
We carried out a subgroup analysis comparing studies using a placebo control or active treatment.
Sensitivity analysis
We included only studies that limited enrolment to people with a clinical diagnosis of acute bronchitis or acute productive cough for the primary analysis. We did a sensitivity analysis that included unpublished data from subgroups of participants with a productive cough and non‐purulent tracheobronchitis from two studies that enrolled people with an influenza‐like illness or a common cold (Howie 1970; Kaiser 1996).
Results
Description of studies
Results of the search
The updated and modified CENTRAL, MEDLINE, Embase, and LILACS searches in 2017 yielded an additional 993 titles. We have identified no new studies since the 2014 update. All of the 17 trials included in the primary analysis enrolled people with a diagnosis of acute cough or acute lower respiratory tract infection. In one study participants were required to produce a sputum sample for analysis as a condition of enrolment (Franks 1984).
Included studies
We included 17 studies in this review. We identified no new studies for this update. The 2014 update added two new studies (Little 2013; Llor 2013). These were important additions, particularly the trial by Little 2013, as it is the largest trial conducted to date, involving 2061 participants recruited across 12 countries. Participants were randomised to receive either amoxicillin or placebo, and there was low risk of bias with more than 80% follow‐up of participants.
Most studies used clinical findings to exclude patients thought to have pneumonia. Four studies included chest radiographs in their protocols: two performed a chest film on all potential participants (Brickfield 1986; Nduba 2008), and in the other two, Scherl 1987 did so on people with rales or fever, and Llor 2013 did so on those with suspected pneumonia (7 of 416 participants). These four studies excluded those with radiological evidence of pneumonia or tuberculosis. One study excluded people with any abnormality noted on examination of the chest (Stott 1976). Four trials also excluded people with a clinical syndrome suggesting sinusitis (Dunlay 1987; King 1996; Verheij 1994; Williamson 1984).
In all trials the duration of illness at entry was less than 30 days. One trial limited enrolment to people who were ill for less than one week (Stott 1976), and in five trials the duration was two weeks or less (Brickfield 1986; Evans 2002; Franks 1984; King 1996; Matthys 2000).
Eight of the trials included only adults (Brickfield 1986; Dunlay 1987; Hueston 1994; Little 2013; Llor 2013; Nduba 2008; Verheij 1994; Williamson 1984). The remaining studies included both adolescents and adults (Franks 1984; Scherl 1987; Stott 1976); people aged three years or older (Little 2005); or eight years or older (King 1996).
Regarding antibiotic treatment, four trials used doxycycline (Scherl 1987; Stott 1976; Verheij 1994; Williamson 1984), four erythromycin (Brickfield 1986; Dunlay 1987; Hueston 1994; King 1996), one trimethoprim/sulfamethoxazole (Franks 1984), one azithromycin (Evans 2002), one cefuroxime (Matthys 2000), one amoxicillin or erythromycin (Little 2005), two amoxicillin (Little 2013; Nduba 2008), and one amoxicillin‐clavulanic acid (Llor 2013).
The majority of studies used a single reassessment visit to evaluate results of the intervention. The timing of this visit varied from study to study, ranging from two to 14 days after the initiation of treatment. Some investigators also asked participants to keep symptom diaries, which were used to determine the duration of symptoms or disability.
Several of the trials provided results of separate analyses of one or more subsets of patients based on characteristics such as cigarette smoking, patient age, duration of symptoms, presence of purulent sputum, or illness severity. All participants enrolled in the study by Nduba 2008 were tested for HIV and a sub‐group analysis was undertaken based on HIV status. The largest study included in the review, (Little 2013), was adequately powered for a subgroup analysis of participants aged over 60 years.
For the sensitivity analyses, we included unpublished data from two trials. In one (Howie 1970), participants began self treatment with dimethyl chlortetracycline or placebo if a cold or influenza‐like illness did not spontaneously resolve after two days. We included data from a subgroup of participants who had a productive cough prior to beginning treatment. The other study randomised participants with the common cold to amoxicillin‐clavulanic acid or placebo (Kaiser 1996). We included data from a subgroup of participants who had a concomitant diagnosis of non‐purulent tracheobronchitis, which incorporates 'acute bronchitis'. Further details on the subgroups of participants included from these studies is provided in the Characteristics of included studies table.
Excluded studies
We excluded studies for a variety of reasons based on study design and intervention criteria. Details of excluded studies are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
Sixteen of the 17 included trials were randomised, double‐ or single‐blind evaluations comparing an antibiotic with a placebo. The study added in the 2011 update was the first equivalence randomised controlled trial included in the review (Nduba 2008). The earlier study by Little 2005 involved three arms comparing immediate antibiotic therapy, no active treatment, or delayed treatment; we only included the two arms comparing immediate antibiotic treatment with no treatment. The study by Llor 2013, involved three arms comparing antibiotic, placebo, and anti‐inflammatory treatment; we only included data from the antibiotic versus placebo arms. Four reports did not clearly state the randomisation method used (Brickfield 1986; Howie 1970; Kaiser 1996; Scherl 1987). Only one of the articles reported a formal evaluation of the effectiveness of the blinding procedures used (Nduba 2008). Six studies measured compliance or adherence with treatment: in five, there were no differences in the number of pills taken in the antibiotic and placebo groups (Dunlay 1987; Hueston 1994; Little 2013; Nduba 2008; Stott 1976); in the study by King 1996, 94% of the participants who returned for follow‐up took at least one‐half of their pills, and Little 2013 reported greater than 90% adherence in both groups by day five. Regarding co‐interventions with other medications, four trials asked participants to record the use of non‐prescription medications and included this as an outcome measure (Dunlay 1987; Franks 1984; Hueston 1994; King 1996); one trial restricted use to aspirin and acetaminophen, but did not have the participants record this (Scherl 1987); and one trial reported adjunctive prescriptions, but not use of over‐the‐counter medications (Verheij 1994). The majority of studies (13 out of 17) followed up more than 80% of participants (details of dropouts are provided in the Characteristics of included studies table). In some cases, no information about withdrawals was available in the paper or from the authors. However, when information was available, we included outcome data from the last point at which the participants remained in the study. To the greatest degree possible, we analysed participants on an intention‐to‐treat basis.
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
Allocation
In general, there was minimal risk of allocation or selection bias: 15 out of 17 studies clearly reported adequate allocation concealment.
Blinding
In general, there was minimal risk of bias relating to lack of blinding, with 14 out of 17 studies clearly reporting adequate blinding of outcome assessors.
Incomplete outcome data
The majority of studies had adequate completion of outcome data with minimal risk of attrition bias.
Selective reporting
Most trials evaluated several different outcome measures. In some cases, the published reports included detailed data for only those outcomes found to be statistically significant. To minimise this reporting bias, we attempted to obtain additional data from the trial authors; five authors provided this information (Howie 1970; Hueston 1994; Kaiser 1996; King 1996; Williamson 1984). However, we were still unable to include data from Stott 1976 for the outcomes of cough, night cough, or activity limitations at follow‐up, which were reported in the published trial as being not significantly different between groups.
Other potential sources of bias
The main concern regarding bias was the relatively small numbers of studies that could be included in individual meta‐analyses. We have attempted to address this by adding a new, broader analysis reflecting clinical improvement. This has been further strengthened by the addition of the largest multi country trial to date (Little 2013). There were no additional concerns regarding other potential sources of bias.
Effects of interventions
All studies did not report the same outcome measures. Some studies reported the presence or absence of various symptoms and signs at a follow‐up visit; others reported the mean duration of symptoms; and still others reported only unique symptom scores. In addition, in some studies explicit data were available only for outcomes that were significantly different between the antibiotic and placebo groups. The number of studies that provided data for the outcomes in this review therefore ranged from three to 11. None of the summary outcomes in the primary analysis exhibited statistically significant heterogeneity apart from the analysis of participants 'clinically improved'. Numbers of studies and participants included in the individual meta‐analyses were generally small, although the meta‐analysis for 'clinically improved' includes 11 studies, and the meta‐analysis for adverse events includes 12 studies.
Primary outcomes
1. Cough‐related outcomes
At the follow‐up visit, participants given antibiotics were less likely to have a cough (4 studies with 275 participants, risk ratio (RR) 0.64, 95% confidence interval (CI) 0.49 to 0.85; number needed to treat for an additional beneficial outcome (NNTB) 6) (Analysis 1.1; Figure 3) or a night cough (4 studies with 538 participants, RR 0.67, 95% CI 0.54 to 0.83; NNTB 7) (Analysis 2.1). The differences in presence of a productive cough at follow‐up and days of productive cough did not reach statistical significance. Antibiotic‐treated participants only had a significant reduction in mean duration of cough when the study by Little 2005, which had a no‐treatment comparison group, was excluded (Figure 4). Llor 2013 also reported no significant difference in median days of cough between the antibiotic and placebo groups. In addition, sensitivity analysis altered the outcome of mean duration of productive cough, which was significantly reduced if the Howie 1970 study relating to upper respiratory tract infection was excluded.

Forest plot of comparison: Cough at follow‐up visit, outcome: number of participants with cough.

Forest plot of comparison: 8 Days of cough, outcome: mean number of days of cough.
2. Global assessment of improvement by clinicians at follow‐up: 'clinically improved'
For the 2011 update of the review, we added an analysis that included a broader outcome 'clinically improved', so that as many studies as possible could be included in a meta‐analysis. This was particularly important following the inclusion of the Nduba 2008 study in 2011, which was of high quality, included a large number of participants, and showed no benefit from antibiotic use. This outcome includes additional data from the authors of the largest included study, (Little 2013). The data from Little 2013 are based on numbers of participants no longer reporting their symptoms being "moderately bad" at one week. The published study presents mean symptom severity scores in the first few days, which indicated no significant difference between the intervention and control groups (Little 2013). This outcome reflects the proportions of participants with clinical improvement and incorporates 'cure' as measured by a greater than 75% reduction in the Acute Bronchitis Severity Score (Nduba 2008), global improvement or being well (Brickfield 1986; Llor 2013; Matthys 2000; Stott 1976; Verheij 1994; Williamson 1984), patient report of no limitations (Dunlay 1987; Evans 2002; Franks 1984), and resolution of symptoms rated as moderately bad, severe, or worsening (Little 2013). This analysis includes 11 studies involving 3841 participants and shows no statistically significant difference between groups (RR 1.07, 95% CI 0.99 to 1.15; NNTB 22) (Analysis 6.1; Figure 5). The addition of data from Little 2013 and Llor 2013 increased the heterogeneity for this analysis. A sensitivity analysis removing the studies reporting 'no limitation' made no difference to this result.

Forest plot of comparison: Clinically improved, outcome: number of participants reporting no limitations or described as cured/well/symptoms resolved or globally improved.
3. General clinical outcomes
Antibiotic‐treated participants also had a reduction in the number of days feeling ill (5 studies with 809 participants, mean difference (MD) ‐0.64, 95% CI ‐1.16 to ‐0.13) (Analysis 8.1; Figure 6) and a reduction in days with impaired activity (6 studies with 767 participants, MD ‐0.49, 95% CI ‐0.94 to ‐0.04) (Analysis 9.1). There was no significant difference in proportions of participants with activity limitations at follow‐up. Participants on antibiotics were more likely to be improved according to clinician's global assessment (6 studies with 891 participants, RR 0.61, 95% CI 0.48 to 0.79; NNTB 11) (Analysis 10.1; Figure 7) and were less likely to have an abnormal lung exam (5 studies with 613 participants, RR 0.54, 95% CI 0.41 to 0.70; NNTB 6) (Analysis 11.1). Additional clinical outcomes were reported by Little 2013, who found no significant difference in mean symptom severity scores on days two to four (intervention score 1.62 (standard deviation (SD) 0.84) versus control score 1.69 (SD 0.84), P = 0.07), and Evans 2002 found that azithromycin had no benefit in terms of health‐related quality of life at day three and day seven follow‐up. Llor 2013 also reported no difference in time to overall symptom resolution between groups.
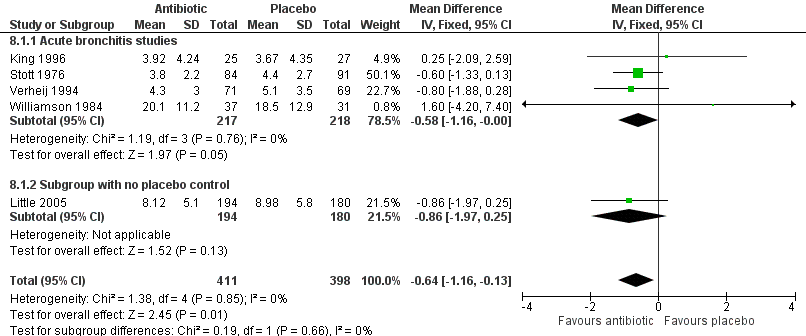
Forest plot of comparison: Days of feeling ill, outcome: mean number of days of feeling ill.

Forest plot of comparison: Not improved by physician's global assessment at follow‐up visit, outcome: number of participants not improved.
Secondary outcomes
1. Adverse effects
With four exceptions (Brickfield 1986; Little 2005; Matthys 2000; Nduba 2008), all of the studies found that participants in the antibiotic group reported more adverse effects than participants receiving a placebo (Figure 8). The RR of adverse effects in the antibiotic‐treated group was statistically significant at 1.20 (95% CI 1.05 to 1.36; number needed to treat for an additional harmful outcome (NNTH) 24; 12 studies with 3496 participants) (Analysis 12.1). The most commonly reported side effects included gastrointestinal symptoms such as nausea, vomiting, or diarrhoea. Headaches, skin rash, and vaginitis also occurred. Side effects seemed to be mild, as only 0% to 13% (overall 3.7%) of participants withdrew because of them, and no deaths were reported.

Forest plot of comparison: Number of participants with adverse effects.
Subgroups
We were not able to obtain enough explicit data from the studies for various patient subgroups, therefore we did not carry out any sensitivity analyses based on patient characteristics (such as age, duration of illness, or smoking status). Little 2013 was adequately powered to assess the effect in the subgroup of participants aged over 60 and found no significant benefit in this group. The results in the individual studies for subgroup analyses were mixed. In one trial, all of the significantly improved outcomes from antibiotics occurred in non‐smokers (Brickfield 1986). The other seven trials reported that they found no differences in antibiotic effectiveness between smokers and non‐smokers, but included no data on these comparisons in their published reports. Verheij 1994, using multiple regression, found that two subsets of patients were more likely to improve with doxycycline than placebo: participants over 55 years and those with very frequent cough who felt ill. Scherl 1987 found that only participants without coryza or sore throat had fewer days of cough or sputum with doxycycline. The only study to use Gram stains reported an earlier return to work for participants with a positive Gram stain who were treated with antibiotics (Franks 1984). Nduba 2008 also examined whether the use of amoxicillin was more effective than placebo in people who had tested positive for HIV and found no difference, though all participants had received a chest X‐ray and those with any abnormal signs were excluded.
Little 2005, which was added to the 2009 update, found no significant difference in outcomes between groups treated with immediate antibiotics compared with no antibiotic treatment. As this study did not involve a placebo control we included it in the analyses, where appropriate data were available, as a subgroup to highlight this difference. The one study added in the 2011 update was powered to detect equivalence between antibiotic and placebo and found no significant difference (Nduba 2008). In fact, the point estimates favoured placebo treatment (84% cured on placebo versus 82.4% cured on amoxicillin). The largest included study, which was added in the 2014 update, was included in the 'clinically improved' and adverse effects meta‐analyses (Little 2013).
Discussion
Summary of main results
We found mixed results across studies, with some suggesting marginal benefits for antibiotics, which are however of doubtful clinical significance. The inclusion of the largest multicentre study of the effectiveness of antibiotics in people with lower respiratory tract infections strengthens the evidence and also highlights a statistically significant increase in adverse events in the antibiotic‐treated groups. However, it is possible that older patients with multimorbidity may not have been recruited to trials, so the evidence guiding decision‐making in this group of patients is less certain.
Overall completeness and applicability of evidence
In general, the available evidence suggests we should not be using antibiotics to treat acute bronchitis or lower respiratory tract infections when pneumonia is not expected. There is a modest benefit from antibiotics for some outcomes, but these are of minimal clinical significance. Any benefit is even less apparent in the sensitivity analysis, which included data from subgroups of patients with productive cough of short duration (two to four days) in conjunction with the common cold. Of the two trials in the primary analysis that limited enrolment to people who had been ill for less than one week, one did not show any benefit from antibiotics (Stott 1976), whilst the other showed modest benefit from antibiotics (Matthys 2000).
It is possible that the overall benefit noted from antibiotics resulted from the inclusion in some trials of people who may have had pneumonia instead of acute bronchitis. There was variation between studies as to whether chest X‐rays were conducted as part of the evaluations. Only one trial obtained chest radiographs on all participants and then excluded those whose films were consistent with pneumonia (Brickfield 1986). In Little 2013, a positive chest X‐ray was not an automatic exclusion criterion, although some participants dropped out following such a finding. All of the remaining studies either excluded or obtained chest radiographs in patients with clinical findings of suspected pneumonia (which in most studies were focal findings on chest examination). Individual signs (such as crackles or fever) are not sensitive (Metlay 1997a), therefore their absence cannot be relied on to rule out pneumonia. On the other hand, since the prevalence of pneumonia in outpatients who present with cough is generally low (less than 5% in the USA) (Metlay 1997b), it is unlikely that a significant number of participants in these trials had pneumonia. In addition, this review was designed to test the effectiveness of treatment for acute bronchitis in clinical practice, and it is not standard practice to confirm the diagnosis of acute bronchitis with a chest X‐ray unless there is a clinical suspicion of underlying pneumonia. Had we only included studies with chest X‐ray confirmation of diagnosis, it would have limited the generalisability of the review findings.
Quality of the evidence
Since there is no gold standard test, the diagnosis of acute bronchitis must be made on clinical grounds. All of the trials excluded people with chronic pulmonary disease and enrolled participants with recent onset of a respiratory illness with a productive cough. The results of the studies in the primary analysis that included participants with a productive cough, without specifically stating that the participants had acute bronchitis, were similar to the studies that used this specific terminology, as one showed some benefits from antibiotics (Verheij 1994), and one did not (Stott 1976). Clinical characteristics of participants regarding the duration of illness and associated symptoms and physical findings did vary somewhat among studies, but were consistent with definitions generally used by primary care physicians (Oeffinger 1997; Verheij 1990). These results would therefore appear to be generalisable to the management of acute bronchitis in community practices.
Potential biases in the review process
This review may also be subject to bias because although we have now included 17 trials and 5099 participants, it is possible that some patient subgroups are under‐represented, as they may not have been recruited into the original trials. Little 2013 points out that while they included a large sample of older people, more severely ill older people with multimorbidities were unlikely to have been approached to participate in the trial, and in these types of patients their results should be interpreted with caution; this applies to the review results also.
Agreements and disagreements with other studies or reviews
In the current update of the review we have included a large multi country trial that shows no benefits from antibiotics even in older patients. Further analyses of the data from this study are ongoing as part of Workpackage 10 of the GRACE program (www.grace‐lrti.org). It should be noted that a recent large observational study examining symptom resolution in 2714 people with acute cough who had been prescribed amoxicillin across 13 European countries found that symptom resolution was quicker in those receiving no antibiotic (Butler 2010).

'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.

'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: Cough at follow‐up visit, outcome: number of participants with cough.

Forest plot of comparison: 8 Days of cough, outcome: mean number of days of cough.

Forest plot of comparison: Clinically improved, outcome: number of participants reporting no limitations or described as cured/well/symptoms resolved or globally improved.

Forest plot of comparison: Days of feeling ill, outcome: mean number of days of feeling ill.

Forest plot of comparison: Not improved by physician's global assessment at follow‐up visit, outcome: number of participants not improved.

Forest plot of comparison: Number of participants with adverse effects.

Comparison 1 Cough at follow‐up visit, Outcome 1 Number of participants with cough.

Comparison 2 Night cough at follow‐up visit, Outcome 1 Number of participants with night cough.

Comparison 3 Productive cough at follow‐up visit, Outcome 1 Number of participants with productive cough.

Comparison 4 Days of cough, Outcome 1 Mean number of days of cough.

Comparison 5 Days of productive cough, Outcome 1 Mean number of days of productive cough.

Comparison 6 Clinically improved, Outcome 1 Number of participants reporting no activity limitations or described as cured/globally improved.

Comparison 7 Limitation in work or activities at follow‐up visit, Outcome 1 Number of participants with limitations.

Comparison 8 Days of feeling ill, Outcome 1 Mean number of days of feeling ill.

Comparison 9 Days of impaired activities, Outcome 1 Mean number of days of impaired activities.

Comparison 10 Not improved by physician's global assessment at follow‐up visit, Outcome 1 Number of participants not improved.

Comparison 11 Abnormal lung exam at follow‐up visit, Outcome 1 Number of participants with abnormal lung exams.

Comparison 12 Adverse effects, Outcome 1 Number of participants with adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with cough Show forest plot | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.49, 0.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with night cough Show forest plot | 4 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with productive cough Show forest plot | 7 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.16] |
| 1.1 Acute bronchitis studies | 6 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 1.2 Subgroup with productive cough from URTI study | 1 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.88, 1.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean number of days of cough Show forest plot | 7 | 2776 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.87, ‐0.04] |
| 1.1 Acute bronchitis studies | 6 | 2350 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [1.00, ‐0.10] |
| 1.2 Subgroup with no placebo control | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐1.01, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean number of days of productive cough Show forest plot | 6 | 699 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.93, 0.07] |
| 1.1 Acute bronchitis studies | 5 | 535 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.03, ‐0.01] |
| 1.2 Subgroup with productive cough from URTI study | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐1.04, 3.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants reporting no activity limitations or described as cured/globally improved Show forest plot | 11 | 3841 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.99, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with limitations Show forest plot | 5 | 478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.46, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean number of days of feeling ill Show forest plot | 5 | 809 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.16, ‐0.13] |
| 1.1 Acute bronchitis studies | 4 | 435 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.16, ‐0.00] |
| 1.2 Subgroup with no placebo control | 1 | 374 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.97, 0.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean number of days of impaired activities Show forest plot | 6 | 767 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.94, ‐0.04] |
| 1.1 Acute bronchitis studies | 5 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.96, 0.01] |
| 1.2 Subgroup with no placebo control | 1 | 374 | Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.75, 0.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants not improved Show forest plot | 6 | 891 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.48, 0.79] |
| 1.1 Acute bronchitis studies | 5 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.65] |
| 1.2 Subgroup with non‐purulent tracheobronchitis from URTI study | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with abnormal lung exams Show forest plot | 5 | 613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.41, 0.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with adverse effects Show forest plot | 12 | 3496 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.05, 1.36] |
| 1.1 Acute bronchitis studies | 11 | 3162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.07, 1.40] |
| 1.2 Subgroup with no placebo control | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.50] |

