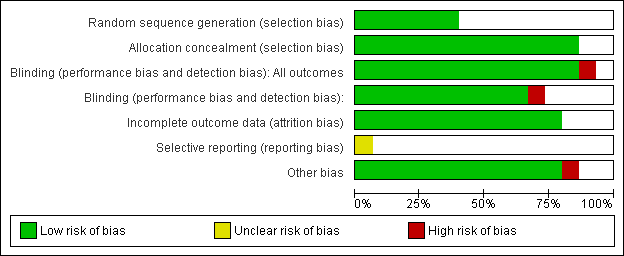
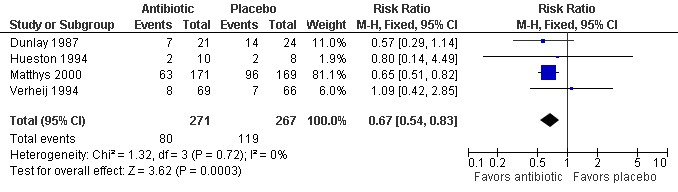
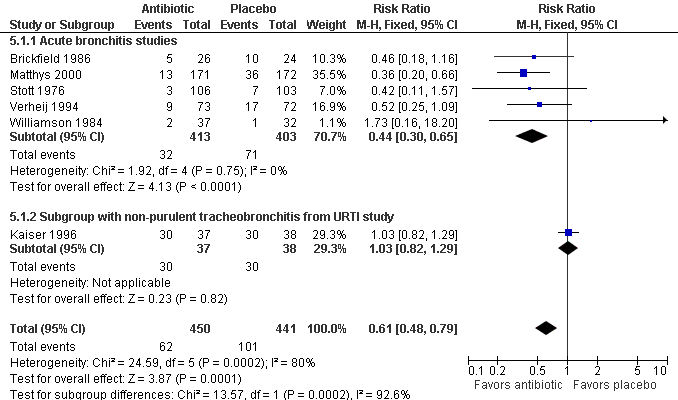
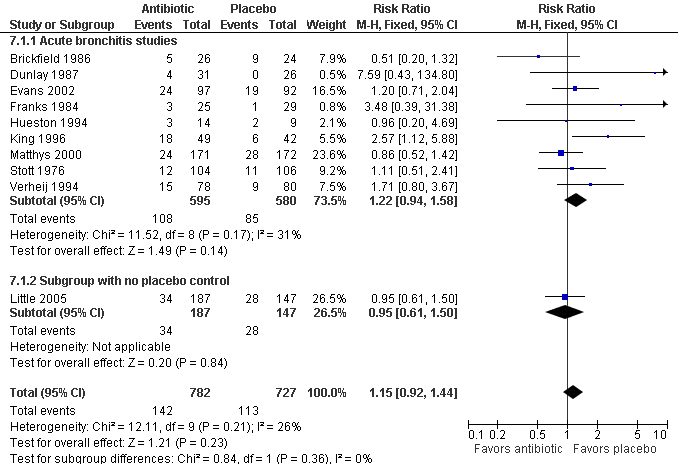
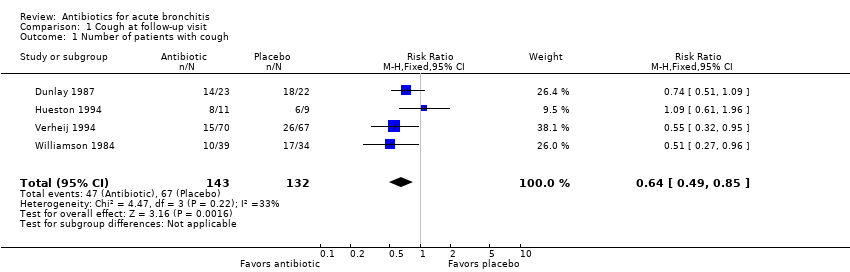
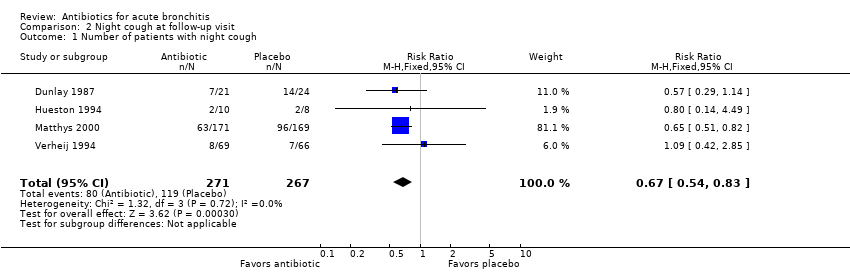
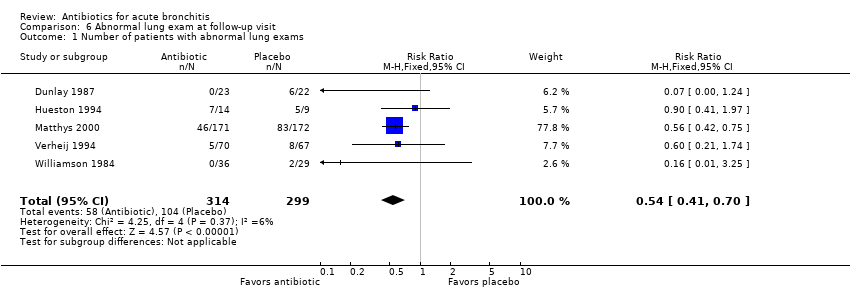
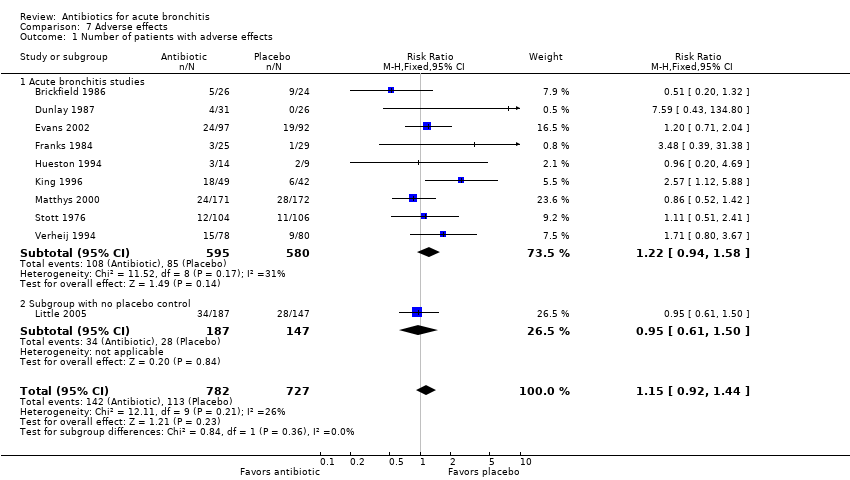
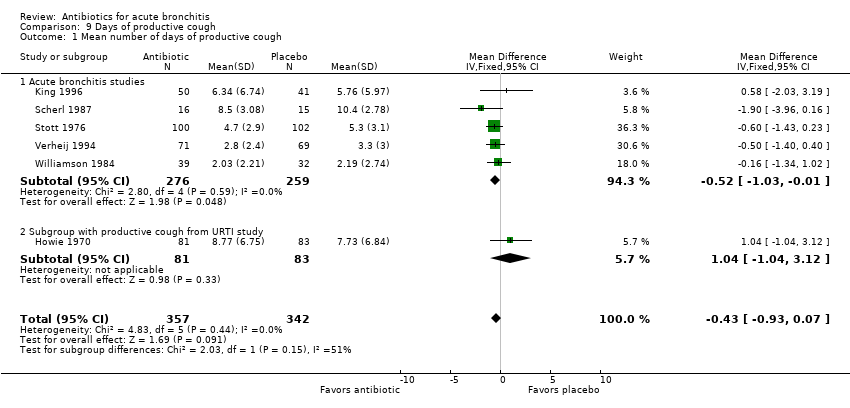
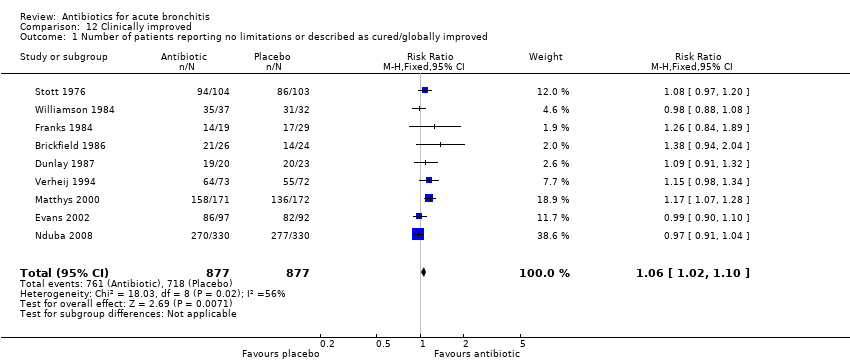
Contenido relacionado
Revisiones y protocolos relacionados
Susan M Smith, Tom Fahey, John Smucny, Lorne A Becker | 19 junio 2017
Anneli Ahovuo‐Saloranta, Ulla‐Maija Rautakorpi, Oleg V Borisenko, Helena Liira, John W Williams Jr, Marjukka Mäkelä | 16 octubre 2015
Ludovic Reveiz, Andrés Felipe Cardona | 23 mayo 2015
Marieke B Lemiengre, Mieke L van Driel, Dan Merenstein, Helena Liira, Marjukka Mäkelä, An IM De Sutter | 10 septiembre 2018
Tim Kenealy, Bruce Arroll | 4 junio 2013
Christina C Chang, Allen C Cheng, Anne B Chang | 10 marzo 2014
Kameshwar Prasad, Amit Kumar, Tarun Singhal, Praveen Kumar Gupta | 17 octubre 2007
Sultan M Altunaiji, Renata H Kukuruzovic, Nigel C Curtis, John Massie | 18 julio 2007
Márcia G Alves Galvão, Marilene Augusta Rocha Crispino Santos, Antonio JL Alves da Cunha | 29 febrero 2016
Amanda J Leach, Peter S Morris | 18 octubre 2006