Oxígeno ambulatorio para pacientes con enfermedad pulmonar obstructiva crónica que no presentan hipoxemia en reposo
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Country: New Zealand Design: randomised double‐blind cross‐over study Study objective: to assess the short‐term clinical impact of ambulatory oxygen in participants with severe COPD and significant exercise de‐saturation who did not fulfil the criteria for LTOT, and to determine whether baseline characteristics or acute response predicts short‐term response Methods of analysis: Treatment and order of treatment were included in the model with participant as random effect. Logistic regression was used to investigate factors that predict short‐term response to cylinder oxygen Clustering adjustments made: not applicable | |
| Participants | Eligible for study: n = 57 Randomised: intervention n = 25, control n = 25 Completed: n = 41 participants in total (cross‐over) Age: 67.1 ± 9.3 years Gender: male 70% Hypoxemia diagnosis: resting PaO2 9.2 ± 1.0 kPa. SpO2 on exertion and room air 82 ± 5.4; all participants needed to complete 6 weeks of rehabilitation and had to be clinically stable for longer than 2 months with standard optimal medical care Co‐morbidities included: none reported Exclusion criteria: limiting angina or significant musculoskeletal disability | |
| Interventions | Intervention description: prefilled pink‐painted lightweight oxygen cylinder at flow rate of 4 L/min for any activity that would induce dyspnoea Control description: prefilled pink‐painted lightweight air cylinder at flow rate of 4 L/min for any activity that would induce dyspnoea Duration of intervention: 6 weeks for each arm Setting: outpatient clinics and pulmonary rehabilitation referrals to Green Lane Hospital, Auckland, New Zealand; ambulatory participants | |
| Outcomes | Prespecified outcomes: 6‐minute walking distance, SpO2, Borg scale and health‐related quality of life measures Follow‐up period: 12 weeks | |
| Notes | Funding: funded by the Health Research Council of New Zealand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned; however methods not described |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment methods |
| Blinding of participants and personnel (performance bias) | Low risk | All cylinders identical in appearance (painted pink) and prefilled, with unique cylinder numbers for researcher identification purposes |
| Blinding of outcome assessment (detection bias) | Low risk | Study authors report that assessors were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant attrition reported; however potential missing data within questionnaires and outcome collection not mentioned or accounted for in analyses |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of yes or no |
| Other bias | Low risk | No other biases identified |
| Methods | Country: Australia Design: randomised double‐blind cross‐over study Study objective: to assess the short‐term effects of oxygen therapy on exercise capacity and the longer‐term effects on both exercise capacity and quality of life of ambulatory oxygen used during ordinary activities at home in participants with severe COPD, without severe resting arterial hypoxaemia Methods of analysis: paired t‐tests for continuous variables; Wilcoxon test for discrete variables, with carry‐over effects measured by 2‐sample Mann‐Whitney and t‐tests Clustering adjustments made: not applicable | |
| Participants | Eligible for study: not reported Randomised: n = 36 participants in total (cross‐over) Completed: n = 26 participants in total (cross‐over) Age: 73 ± 6 years Gender: male n = 24, female n = 2 Hypoxemia diagnosis: participant PaO2 greater than 60 mmHg; exertional dyspnoea limited daily activities Co‐morbidities included: not reported Exclusion criteria: symptomatic cardiac dysfunction, angina pectoris and locomotor disability | |
| Interventions | Intervention description: oxygen in a portable gas cylinder (Stroller 682; Medical Gases) at flow rate of 4 L/min for any activity that would induce dyspnoea Control description: air in a portable gas cylinder (Stroller 682; Medical Gases) at flow rate of 4 L/min for any activity that would induce dyspnoea Duration of intervention: 6 weeks for each arm Setting: outpatient domiciliary | |
| Outcomes | Prespecified outcomes: exercise capacity (step test and 6‐minute walking distance), quality of life (CRQ), dyspnoea, SaO2 (SaO2 is a direct measurement using a blood sample such as an arterial blood gas analysis). Follow‐up period: 12 weeks | |
| Notes | Funding: supported by the Sir Edward Dunlop Research Foundation and by Medical Gases, Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors report the use of a computer‐generated list of random odd or even numbers |
| Allocation concealment (selection bias) | Low risk | Study authors report concealed allocation, assignment undertaken by the gas cylinder company |
| Blinding of participants and personnel (performance bias) | Low risk | Participants are said to be blinded to the intervention/control through the use of identical apparatus |
| Blinding of outcome assessment (detection bias) | Low risk | Study authors report that assessors performing the tests were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Participant attrition reported with reasons |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of yes or no |
| Other bias | Unclear risk | Insufficient information to permit a judgement of yes or no |
| Methods | Country: Australia Design: prospective parallel, double‐blind, randomised controlled trial Study objective: to perform a large, adequately powered study to determine the effects of domiciliary ambulatory oxygen in participants with COPD and exertional dyspnoea, without severe resting hypoxaemia, with or without exercise de‐saturation. A further aim was to identify factors that might predict any observed benefit Methods of analysis: transition dyspnoea index scores and cylinder utilisation data analysed using t‐tests to compare treatment means; other outcome measures analysed using 2‐way, repeated‐measures analysis of variance; a priori variables selected to identify subgroup differences, which study authors believed may benefit differentially from domiciliary ambulatory oxygen; data analysed using ANCOVA, with week 12 values as the response variable, the corresponding value at baseline as the co‐variate and each of the subgroup variables as an explanatory factor, in addition to treatment (air or oxygen) Clustering adjustments made: not applicable | |
| Participants | Eligible for study: n = 1318 (assessed for eligibility); enrolled n = 160 Randomised: intervention n = 68, control n = 75 Completed: intervention n = 66, control n = 73 Age: intervention 72 ± 9.2, control 72 ± 10.4 years Gender: females enrolled = 44; distribution across arms not reported Hypoxemia diagnosis: PaO2 > 7.3 kPa at rest breathing room air Co‐morbidities included: not reported Exclusion criteria: current smoking, clinically unstable COPD, current participation in a pulmonary rehabilitation programme, current domiciliary oxygen use, significant communication or locomotor difficulties or other severe medical conditions | |
| Interventions | Intervention description: cylinder oxygen, weighing 4.2 kg filled, provided with a trolley/stroller with gas delivered at a flow rate of 6 L/min via the Impulse Elite conservation device (AirSep Corporation, Buffalo, New York, USA) Control description: cylinder air of identical appearance to the intervention Duration of intervention: no recommendations provided regarding duration of use; however end of study was at 12 weeks, following a 2‐week run‐in period Setting: participants recruited through database screening and advertisements; cylinders used inside and outside the home during exertional activities | |
| Outcomes | Prespecified outcomes: dyspnoea, health‐related quality of life, exercise tolerance, activity levels, depression symptoms, service utilisation, mood disturbance, functional status and gas utilisation Follow‐up period: following 2‐week run‐in period, 4‐week (mid‐trial) assessment and 12‐week (end of study) assessment | |
| Notes | Funding: funded by National Health and Medical Research Council, Northern Clinical Research Centre, Victorian Tuberculosis and Lung Association, Austin Hospital Medical Research Foundation, Institute for Breathing and Sleep, Austin Hospital, Australia Finkel Foundation, Air Liquide, Boehringer Ingelheim | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors report the use of a computer‐generated programme for randomisation |
| Allocation concealment (selection bias) | Low risk | Study authors report concealed allocation, assignment undertaken by supplier of the portable cylinders |
| Blinding of participants and personnel (performance bias) | Low risk | Cylinders were of identical appearance, and study authors report that participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Study authors report that study personnel were blinded to group allocation,as assignment was undertaken by supplier of the portable cylinders |
| Incomplete outcome data (attrition bias) | Low risk | Study authors report attrition with reasons, and ‘complete data’ for all participants |
| Selective reporting (reporting bias) | Low risk | Pre‐specified protocol published; outcomes match those in the publication |
| Other bias | Low risk | No other biases identified |
| Methods | Country: Canada Design: N‐of‐1 double‐blind, randomised controlled trial (participants undertook 3 pairs of 2‐week treatment periods) Study objective: to measure the effects of oxygen in participants with COPD who do not meet criteria for mortality reduction with long‐term oxygen therapy Methods of analysis: Analysis of each N‐of‐1 RCT included a paired t–test; effect of oxygen on entire group was measured by analysis of variance; paired t‐tests were used to measure within‐group differences Clustering adjustments made: not applicable | |
| Participants | Eligible for study: n = 178 Randomised: n = 38 Completed: n = 27 Age: 69 ± 10 years Gender: male = 17, female = 10 Hypoxemia diagnosis: participants with COPD who do not meet the criteria for long‐term oxygen therapy for mortality reduction, with symptoms of dyspnoea limiting daily activities and with de‐saturation of 88% or less for 2 continuous minutes during a room air 6MWD Co‐morbidities included: not reported Exclusion criteria: participants with COPD who meet the criteria for long‐term oxygen therapy for mortality reduction (i.e. PaO2 < 55 mmHg at rest or PaO2 of 55 to 60 at rest with right heart failure); participants already on oxygen for palliative care or nocturnal hypoxaemia; inability to provide consent or complete questionnaires | |
| Interventions | Intervention description: oxygen at 1 to 3 L/min. Participants completed walking oximetry while breathing room air, followed by titration of oxygen to establish the flow rate necessary to maintain saturation at 92% or greater during each of the N‐of‐1 RCTs Control description: placebo mixture of air and oxygen (24%) at 2 L/min that provided a fraction of inspired oxygen (FiO2) of approximately 21.2% Duration of intervention: 3 pairs of 2‐week treatment periods Setting: West Park Healthcare Centre; testing occurred in the participant's home | |
| Outcomes | Prespecified outcomes: quality of life (CRQ—dyspnoea, fatigue, mastery and emotional status domains, and SGRQ—dyspnoea as measured by modified Borg scale); exercise capacity as measured by 5MWD Follow‐up period: 3 pairs of 2‐week treatment periods | |
| Notes | Funding: supported by the Ontario Ministry of Health and Long Term Care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned; however methods not described |
| Allocation concealment (selection bias) | Unclear risk | Study authors report that allocation was concealed; however methods not described |
| Blinding of participants and personnel (performance bias) | Low risk | Participants are said to be blinded to the intervention/control through the use of identical apparatus |
| Blinding of outcome assessment (detection bias) | Low risk | Study authors report that assessors were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant attrition reported; however potential missing data within questionnaires and outcome collection not mentioned or accounted for in analyses |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of yes or no |
| Other bias | Low risk | No other biases identified |
5MWD: 5‐Minute Walking Distance test; ANCOVA: Analysis of co‐variance; COPD: Chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Questionnaire; LTOT: Long‐term oxygen therapy; PaO2: Partial pressure of oxygen in arterial blood; RCTs: Randomised controlled trials; SaO2: arterial oxygen saturation; SpO2: Peripheral capillary oxygen saturation.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Short‐term/acute study; not a study of long‐term ambulatory oxygen therapy | |
| Control group E‐cylinders (electronic portable cylinders); not placebo, air or co‐intervention | |
| Short‐term/acute study; not a study of long‐term ambulatory oxygen therapy | |
| Evaluation of the effects of long‐term oxygen therapy (LTOT) in participants with COPD with moderate hypoxia | |
| Short‐term/acute study; not a study of long‐term ambulatory oxygen therapy | |
| Assessment of outcomes in participants who were already receiving long‐term oxygen therapy | |
| Participants already fulfilling criteria for LTOT (PaO2 < 60 mmHg with evidence of cor pulmonale) |
COPD: Chronic obstructive pulmonary disease; LTOT: Long‐term oxygen therapy; PaO2: Partial pressure of oxygen in arterial blood.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 6MWD (cylinder air for 6MWD) Show forest plot | 2 | Odds Ratio (Fixed, 95% CI) | 1.05 [0.62, 1.75] | |
| Analysis 1.1  Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 1 6MWD (cylinder air for 6MWD). | ||||
| 2 6MWD outcome (cylinder oxygen for 6MWD) Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 2 6MWD outcome (cylinder oxygen for 6MWD). | ||||
| 3 Step exercise testing (number of steps) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3 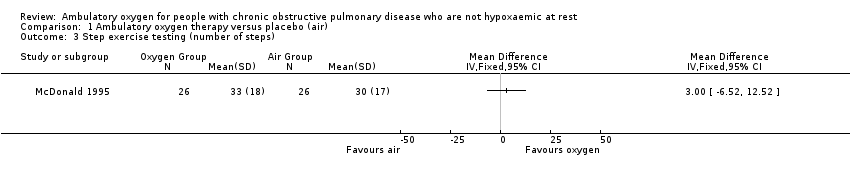 Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 3 Step exercise testing (number of steps). | ||||
| 4 Mortality Show forest plot | 2 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.48, 36.32] |
| Analysis 1.4 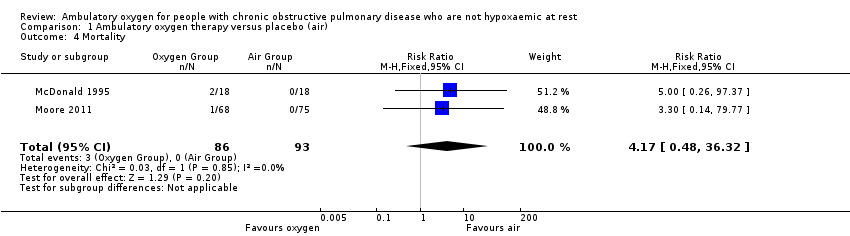 Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 4 Mortality. | ||||
| 5 Borg score—dyspnoea (higher score worse) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5 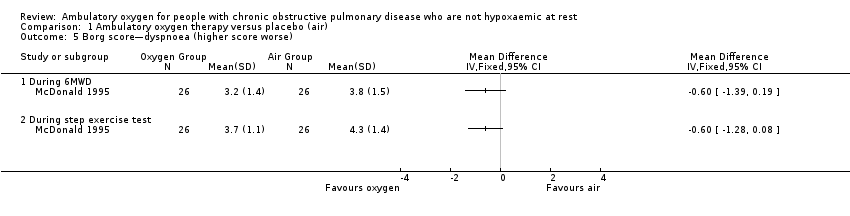 Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 5 Borg score—dyspnoea (higher score worse). | ||||
| 5.1 During 6MWD | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 During step exercise test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Arterial oxygen saturation during exercise Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 6 Arterial oxygen saturation during exercise. | ||||
| 6.1 During 6MWD | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.56, 0.36] |
| 6.2 During step exercise test | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.32, 1.12] |
| 7 Quality of life (Chronic Respiratory Questionnaire) Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 7 Quality of life (Chronic Respiratory Questionnaire). | ||||
| 7.1 CRQ—dyspnoea | 4 | Mean Difference (Fixed, 95% CI) | 0.28 [0.10, 0.45] | |
| 7.2 CRQ—fatigue | 4 | Mean Difference (Fixed, 95% CI) | 0.17 [0.04, 0.31] | |
| 7.3 CRQ—emotional function | 4 | Mean Difference (Fixed, 95% CI) | 0.10 [‐0.05, 0.25] | |
| 7.4 CRQ—mastery | 4 | Mean Difference (Fixed, 95% CI) | 0.13 [‐0.06, 0.33] | |
| 8 SpO2 Show forest plot | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | 6.52 [5.21, 7.83] |
| Analysis 1.8  Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 8 SpO2. | ||||
| 8.1 During exercise | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [4.11, 7.89] |
| 8.2 Post 6MWD | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [5.18, 8.82] |
| 9 Adverse event Show forest plot | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.21, 2.81] |
| Analysis 1.9 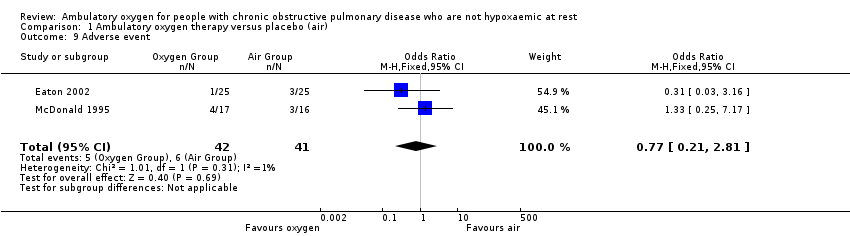 Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 9 Adverse event. | ||||
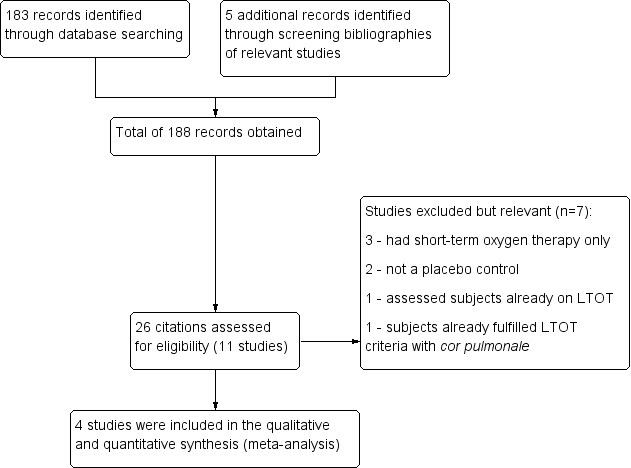
158 Study flow diagram.
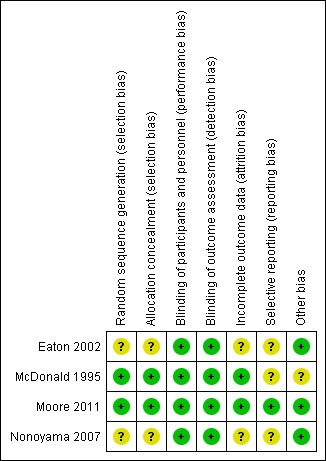
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 1 6MWD (cylinder air for 6MWD).
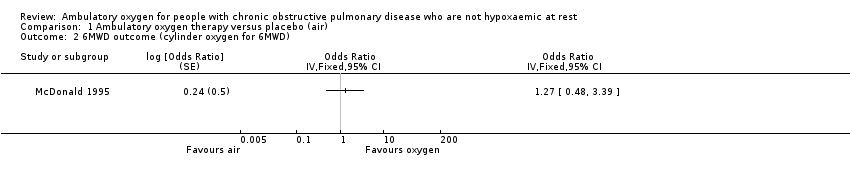
Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 2 6MWD outcome (cylinder oxygen for 6MWD).
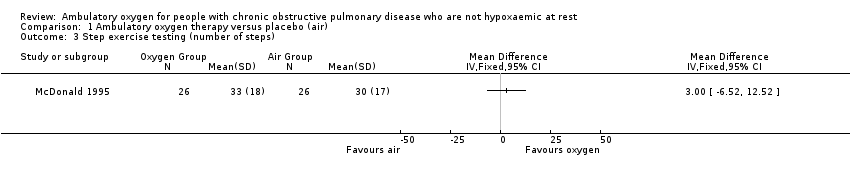
Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 3 Step exercise testing (number of steps).

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 4 Mortality.

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 5 Borg score—dyspnoea (higher score worse).

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 6 Arterial oxygen saturation during exercise.

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 7 Quality of life (Chronic Respiratory Questionnaire).
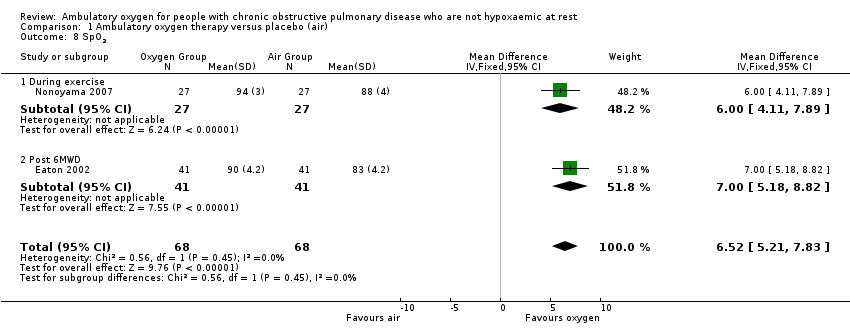
Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 8 SpO2.
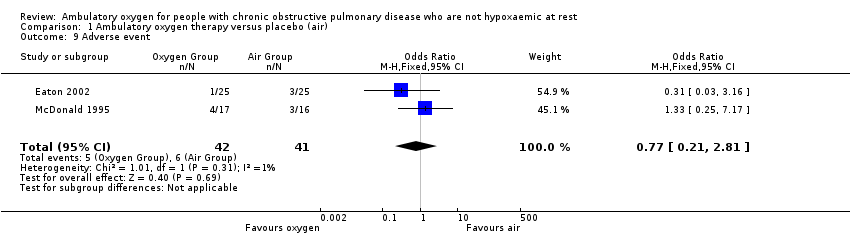
Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 9 Adverse event.
| Ambulatory oxygen for COPD | ||||||
| Patient or population: adults with COPD who had exertional dyspnoea but did not fulfil the criteria for long‐term oxygen treatment Control: placebo/medical air | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Ambulatory oxygen | |||||
| Exercise capacity (5‐ or 6‐minute walking distance on cylinder air) | See comment | See comment | Not estimable | 331 | Not applicable | Meta‐analysis not possible; see effects of interventions for more information |
| Mortality | See comment | See comment | RR 4.17 | 179 | ⊕⊕⊕⊝1 Moderate | Although deaths occurred only in the intervention arm of the study (n = 3), they were not believed to be a direct result of the intervention |
| Quality of life (dyspnoea) Follow‐up: 2 to 12 weeks | Baseline risk in control groups ranged from 2.8 to 3.7 points | Mean quality of life (dyspnoea) in the intervention groups was | MD 0.28 (0.10 to 0.45) | 341 | ⊕⊕⊕⊝2 Moderate | Other CRQ domains were also reported, including fatigue MD 0.14 (95% CI 0.04 to 0.31; P value 0.009), emotional function MD 0.10 (95% CI ‐0.05 to 0.25; P value 0.20) and mastery MD 0.13 (95% CI ‐0.06 to 0.33; P value 0.17) |
| Dyspnoea | See comment | See comment | Not estimable | 198 | Not applicable | Meta‐analysis not possible Dyspnoea was measured in 3 studies using the Borg scale, and 1 study reported dyspnoea during exercise. One study observed improvement in dyspnoea after walking for 6 minutes with cylinder air or oxygen. Another study showed a clinically relevant reduction in dyspnoea scores for the oxygen group post 5MWD compared with placebo |
| Adverse events | 146 per 1000 | 117 per 1000 | OR 0.77 | 83 | ⊕⊕⊝⊝ | Only 1 of the adverse events appeared related to the intervention; this was strain due to carrying the cylinder |
| Hospitalisations | See comment | See comment | Not estimable | 0 | See comment | No studies reported data on hospitalisations |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1 Imprecision 2 Unclear risk for selection bias in 2 studies, attrition bias in 2 studies and selective reporting in 3 studies 3 Unclear risk of selection bias, attrition bias and selective reporting in 1 study | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 6MWD (cylinder air for 6MWD) Show forest plot | 2 | Odds Ratio (Fixed, 95% CI) | 1.05 [0.62, 1.75] | |
| 2 6MWD outcome (cylinder oxygen for 6MWD) Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 3 Step exercise testing (number of steps) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Mortality Show forest plot | 2 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.48, 36.32] |
| 5 Borg score—dyspnoea (higher score worse) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 During 6MWD | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 During step exercise test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Arterial oxygen saturation during exercise Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 During 6MWD | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.56, 0.36] |
| 6.2 During step exercise test | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.32, 1.12] |
| 7 Quality of life (Chronic Respiratory Questionnaire) Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7.1 CRQ—dyspnoea | 4 | Mean Difference (Fixed, 95% CI) | 0.28 [0.10, 0.45] | |
| 7.2 CRQ—fatigue | 4 | Mean Difference (Fixed, 95% CI) | 0.17 [0.04, 0.31] | |
| 7.3 CRQ—emotional function | 4 | Mean Difference (Fixed, 95% CI) | 0.10 [‐0.05, 0.25] | |
| 7.4 CRQ—mastery | 4 | Mean Difference (Fixed, 95% CI) | 0.13 [‐0.06, 0.33] | |
| 8 SpO2 Show forest plot | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | 6.52 [5.21, 7.83] |
| 8.1 During exercise | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [4.11, 7.89] |
| 8.2 Post 6MWD | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [5.18, 8.82] |
| 9 Adverse event Show forest plot | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.21, 2.81] |

