Oxígeno ambulatorio para pacientes con enfermedad pulmonar obstructiva crónica que no presentan hipoxemia en reposo
Resumen
Antecedentes
A menudo los pacientes con enfermedad pulmonar obstructiva crónica (EPOC) presentan hipoxemia (niveles bajos de oxígeno en sangre) transitoria durante el ejercicio y necesitan oxigenoterapia para mejorar la falta de aliento y la capacidad de ejercicio y reducir la discapacidad. La oxigenoterapia ambulatoria consiste en la provisión de oxigenoterapia durante el ejercicio y las actividades cotidianas. Con frecuencia, la oxigenoterapia ambulatoria es utilizada durante el ejercicio por los pacientes que reciben oxigenoterapia a largo plazo (OLP), o por los que no la reciben que presentan o no hipoxemia en reposo, cuando hay evidencia de desaturación durante el ejercicio y muestran que su capacidad para el ejercicio mejora con el uso de suplementos de oxígeno.
Objetivos
Determinar la eficacia a más largo plazo de la oxigenoterapia ambulatoria solamente en pacientes con EPOC que no cumplen los criterios para OLP, con respecto a la mejoría en la capacidad de ejercicio, la mortalidad, la calidad de vida y otras medidas relevantes de mejoría.
Métodos de búsqueda
Se hicieron búsquedas en el registro especializado del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group), que incluyeron MEDLINE, EMBASE y CINAHL. Se revisaron los registros de ensayos clínicos en línea, que incluyeron Controlled Clinical Trials (www.controlled‐trials.com), registros gubernamentales (clinicaltrials.gov) y World Health Organization (WHO) registries (www.who.int/trialsearch), para buscar estudios en curso y recientemente finalizados. En las bibliografías de los estudios incluidos, se buscaron ensayos adicionales que podrían cumplir los criterios de inclusión y no hubieran sido recuperados mediante la estrategia de búsqueda anterior. Se estableció contacto con los autores de los ensayos identificados para que proporcionaran otros estudios publicados y no publicados. Las búsquedas se actualizaron hasta noviembre de 2012.
Criterios de selección
Ensayos controlados aleatorizados (ECA) que compararon oxigenoterapia ambulatoria proporcionada a través de cilindros / dispositivos de pilas portátiles con oxígeno o recipientes con oxígeno líquido versus cilindros con aire placebo, atención médica habitual o cointervención en los participantes del estudio con EPOC que no cumplieran los criterios para OLP.
Obtención y análisis de los datos
Se utilizaron los métodos estándar según lo previsto por la Colaboración Cochrane.
Resultados principales
Cuatro estudios cumplieron los criterios de inclusión (331 participantes), con dos estudios que demostraron un efecto beneficioso estadística y clínicamente significativo a favor de la intervención para la disnea posterior al ejercicio. El dominio calidad de vida de los cuatro estudios incluidos mostró un efecto beneficioso estadísticamente significativo en las subcategorías de disnea y fatiga a favor del grupo de oxígeno (disnea diferencia de medias [DM] 0,28; intervalo de confianza [IC] del 95%: 0,10 a 0,45; valor de P 0,002; fatiga DM 0,17, IC del 95%: 0,04 a 0,31; valor de P 0,009). No se informó evidencia de cualquier efecto para la supervivencia y se observaron efectos beneficiosos limitados para la capacidad de ejercicio (medida según la prueba del paso y la prueba de distancia de caminata), con un estudio que mostró una mejoría estadísticamente significativa en el número de pasos registrados en el grupo de oxígeno para el grupo de estudios N‐de‐1 solamente. No se observaron otros efectos beneficiosos estadísticamente significativos para la capacidad de ejercicio entre los otros ensayos o los estudios individuales N‐de‐1.
Conclusiones de los autores
En los pacientes con EPOC con hipoxia moderada, la evidencia actual sobre la oxigenoterapia ambulatoria muestra mejorías en la disnea posterior al ejercicio y en la disnea y la fatiga del dominio calidad de vida. Sin embargo, en esta revisión no hubo evidencia evidente de la utilidad y la efectividad clínicas del oxígeno ambulatorio para mejorar la mortalidad y la capacidad de ejercicio. Para investigar la función del oxígeno ambulatorio en el tratamiento de la EPOC, se necesitan ECA metodológicamente rigurosos con poder estadístico suficiente para detectar una diferencia.
PICO
Resumen en términos sencillos
Oxígeno portátil para la enfermedad pulmonar obstructiva crónica
Antecedentes
Algunos pacientes con enfermedad pulmonar obstructiva crónica (EPOC) tienen niveles bajos de oxígeno en la sangre cuándo están en reposo o cuando se mueven. Los niveles bajos de oxígeno se conocen como hipoxemia. Estos pacientes pueden moverse con un suministrador de oxígeno (cilindros pequeños con oxígeno, sistemas portátiles con oxígeno líquido o concentradores de oxígeno con pilas) para tener oxígeno para respirar al hacer tareas sencillas como vestirse, salir de la casa, hacer los quehaceres o incluso caminar alrededor del domicilio de una manera más fácil y ayudarles a respirar. Este dispositivo portátil con oxígeno se denomina "oxígeno ambulatorio".
Pregunta de la revisión
Esta revisión se realizó para determinar los efectos beneficiosos a largo plazo de la oxigenoterapia ambulatoria en los pacientes que no presentan hipoxemia grave en reposo.
Características de los estudios
Se analizaron los ensayos controlados aleatorizados que compararon oxígeno ambulatorio versus placebo (aire normal). Se encontraron cuatro estudios con 331 pacientes con una media de edad de 71 años. Dos de los estudios incluidos eran de Australia, uno de Nueva Zelanda y uno de Canadá. El método de suministro de oxígeno y la dosis de oxígeno variaron, aunque en todos los casos el equipo constaba de cilindros livianos o portátiles con un flujo que variaba de 3 l/min a 6 l/min. El final del seguimiento se informó a las 12 semanas en tres estudios y a las dos semanas en el estudio Nonoyama.
Resultados clave
Se encontró que la oxigenoterapia ambulatoria redujo la disnea y el número de pacientes que se sintieron cansados. Sin embargo, no cambió la distancia que los pacientes podrían caminar en cinco a seis minutos ni la supervivencia (tasa de mortalidad).
Calidad de la evidencia
La calidad general de la evidencia de los estudios de esta revisión fue moderada. La forma en que se realizaron los estudios (métodos) no se informó completamente en todos los casos. La mayoría de los estudios carecían del plan de estudio (protocolo) prepublicado.
Conclusión
A partir de esta revisión, no es posible determinar si la oxigenoterapia ambulatoria se debe proporcionar durante el ejercicio o en las actividades cotidianas en los pacientes con EPOC que no presentan hipoxemia grave en reposo.
Este resumen Cochrane en términos sencillos está actualizado hasta noviembre de 2012.
Authors' conclusions
Summary of findings
| Ambulatory oxygen for COPD | ||||||
| Patient or population: adults with COPD who had exertional dyspnoea but did not fulfil the criteria for long‐term oxygen treatment Control: placebo/medical air | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Ambulatory oxygen | |||||
| Exercise capacity (5‐ or 6‐minute walking distance on cylinder air) | See comment | See comment | Not estimable | 331 | Not applicable | Meta‐analysis not possible; see effects of interventions for more information |
| Mortality | See comment | See comment | RR 4.17 | 179 | ⊕⊕⊕⊝1 Moderate | Although deaths occurred only in the intervention arm of the study (n = 3), they were not believed to be a direct result of the intervention |
| Quality of life (dyspnoea) Follow‐up: 2 to 12 weeks | Baseline risk in control groups ranged from 2.8 to 3.7 points | Mean quality of life (dyspnoea) in the intervention groups was | MD 0.28 (0.10 to 0.45) | 341 | ⊕⊕⊕⊝2 Moderate | Other CRQ domains were also reported, including fatigue MD 0.14 (95% CI 0.04 to 0.31; P value 0.009), emotional function MD 0.10 (95% CI ‐0.05 to 0.25; P value 0.20) and mastery MD 0.13 (95% CI ‐0.06 to 0.33; P value 0.17) |
| Dyspnoea | See comment | See comment | Not estimable | 198 | Not applicable | Meta‐analysis not possible Dyspnoea was measured in 3 studies using the Borg scale, and 1 study reported dyspnoea during exercise. One study observed improvement in dyspnoea after walking for 6 minutes with cylinder air or oxygen. Another study showed a clinically relevant reduction in dyspnoea scores for the oxygen group post 5MWD compared with placebo |
| Adverse events | 146 per 1000 | 117 per 1000 | OR 0.77 | 83 | ⊕⊕⊝⊝ | Only 1 of the adverse events appeared related to the intervention; this was strain due to carrying the cylinder |
| Hospitalisations | See comment | See comment | Not estimable | 0 | See comment | No studies reported data on hospitalisations |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1 Imprecision 2 Unclear risk for selection bias in 2 studies, attrition bias in 2 studies and selective reporting in 3 studies 3 Unclear risk of selection bias, attrition bias and selective reporting in 1 study | ||||||
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) primarily consists of chronic bronchitis and emphysema—conditions that are characterised by inflammation of the airways and destruction of pulmonary tissue. The diagnosis of COPD is based on documentation of a postbronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) less than 70% of that predicted in an otherwise healthy person (Rabe 2007). COPD has variable illness trajectories characterised by worsening breathlessness, exercise limitations and progressive deterioration of quality of life (Seamark 2007). Exertional de‐saturation (i.e. oxygen level in the blood decreases during exercise) and dyspnoea (breathlessness) are the markers of poor prognosis (Tham 2011). The mechanisms behind exertional hypoxaemia include ventilation/perfusion mismatch, shunting and limitations in diffusion capacity (Tham 2011). Many patients with COPD identify exertional dyspnoea as the key factor in functional decline (Nonoyama 2007). As the condition progresses, some patients become transiently hypoxaemic with exercise, necessitating oxygen therapy to attempt to improve breathlessness and exercise capacity, and to reduce disability (NICE 2010).
Description of the intervention
Ambulatory oxygen therapy refers to provision of oxygen during exercise, activities of daily living or both. It is often used during exercise by patients on long‐term oxygen treatment (LTOT) or by non‐LTOT users without resting hypoxaemia when they show evidence of oxygen de‐saturation and demonstrate improvement in exercise capacity with supplemental oxygen (BTS 2006). Ambulatory oxygen therapy is usually provided in small oxygen cylinders lasting up to four hours at 2 L/min or through liquid oxygen systems that have a higher oxygen‐carrying capacity lasting around six to 10 hours at 2 L/min (Bradley 2007). Ambulatory oxygen therapy can also be dispensed in an oxygen‐conserving device (OCD) and with battery‐powered portable oxygen concentrators. OCDs deliver bolus amounts of oxygen in response to the patient’s inspiratory effort (or demand), as detected through a nasal cannula (Katsenos 2011). This system of oxygen delivery not only improves the duration of supply, it also improves the cost/benefit ratio of oxygen treatment. The battery‐powered oxygen device weighs approximately 2 kg, which is about half the weight of an oxygen cylinder. The rationale of using these lightweight devices is that they improve compliance and facilitate ambulation; however, no known studies have validated this assumption to date.
How the intervention might work
Ambulatory oxygen has been shown to improve pulmonary hypertension and reduce dynamic hyperinflation in patients with COPD (Fujimoto 2002). Ambulatory oxygen may also delay diaphragmatic muscle fatigue and reduce lactic acidosis (Garrod 2000).
Why it is important to do this review
The prevalence of COPD is increasing worldwide, and COPD is associated with a variety of adverse effects for patients and the healthcare system. Dyspnoea, one of the most common symptoms of COPD, can be present at rest or during exercise, and this can ultimately lead to reduced quality of life. Exercise training and supplemental oxygen (in advanced cases) are generally recommended for control of dyspnoea symptoms during exercise (ATS 1999). LTOT is usually prescribed for patients with severe hypoxia (i.e. partial pressure of oxygen in arterial blood (PaO₂) < 55 mmHg or PaO₂ of 55 to 59 mmHg with evidence of right heart failure). Current evidence suggests that LTOT improves survival and quality of life for these hypoxaemic patients with COPD (Ringbaek 2002; Stoller 2010). However, a significant proportion of patients with COPD do not have severe resting hypoxia but develop symptoms of severe hypoxia with exercise. Some studies suggest that in patients with stable COPD, 4% de‐saturation during the six‐minute walk test may predict long‐term mortality (Casanova 2008). Unfortunately longer‐term effects on survival time or quality of life of ambulatory oxygen in people with COPD with resting mild to moderate hypoxia and exertional dyspnoea or hypoxia remain unclear. Most relevant studies are inconclusive or have provided a short intervention period, used a small sample size or both (Jolly 2001; McDonald 1995). Hence it is important to perform a systematic review of trials of interventions to assess the current level of evidence for long‐term ambulatory oxygen in this particular population.
Objectives
To determine the longer‐term efficacy of ambulatory oxygen therapy only in patients with COPD who do not meet the criteria for LTOT, with respect to improvement in exercise capacity, mortality, quality of life and other relevant measures of improvement.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled parallel and cross‐over trials.
Types of participants
Studies were included only when adult participants with stable COPD were randomly assigned. Participants had chronic hypoxaemia (resting PaO₂ 55 to 59 mmHg) without cor pulmonale (failure of the right side of the heart caused by an increase in blood pressure in the pulmonary artery, the vessel that carries blood from the heart to the lungs) or PaO₂ ≥ 60 mmHg, or they developed hypoxaemia on activity (PaO₂ < 60 mmHg or peripheral capillary oxygen de‐saturation to < 88% SpO₂) with or without cor pulmonale with symptoms on exertion.
Types of interventions
The intervention was long‐term ambulatory oxygen therapy (> two weeks) provided through portable oxygen cylinders or with the use of liquid oxygen canisters or battery‐powered portable oxygen concentrators. Participants must have used ambulatory oxygen while at home. We included studies of greater than two weeks' duration.
Eligible control groups were given placebo air cylinders, usual medical care or other interventions (such as counselling).
Types of outcome measures
Primary outcomes
-
Exercise capacity on room air or on oxygen (e.g. timed walking tests, endurance tests).
-
Mortality.
Secondary outcomes
-
Dyspnoea scores (e.g. Borg scores, visual analogue scores).
-
Arterial oxygen saturation during exercise.
-
Recovery time after exercise.
-
Quality of life (measured using validated questionnaires such as St George's Respiratory Questionnaire (SGRQ) or the Chronic Respiratory Questionnaire (CRQ)).
-
Lung function measurements.
-
Adverse events.
-
Hospitalisation.
-
Length of stay.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). The search terms used in the previous version of the review were (portable* or ambulat*) and (oxygen*). For this version, the search terms were (portable* or ambulat* or mobil* or transport* or travel*) and (oxygen* or 02 or LTOT). The search was run on all records coded as ‘COPD.’
In addition, we conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov), Controlled Clinical Trials (www.controlled‐trials.com) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/).
We searched all databases from their inception to the present, with no restriction on language of publication. The latest searches were conducted in November 2012.
Searching other resources
We searched bibliographies of included studies for potentially relevant trials that were not retrieved by the above search strategy. We contacted authors of included studies to ask if they knew of other relevant published or unpublished studies. We searched online clinical trial registers, including Controlled Clinical Trials (www.controlled‐trials.com), government registries (clinicaltrials.gov) and WHO registries (www.who.int/trialsearch), for ongoing and recently completed studies.
Data collection and analysis
Selection of studies
A combination of three review authors (FA, KC, ZU) independently assessed in duplicate the retrieved citations for inclusion, using titles, abstracts and/or descriptors. We then retrieved full‐text documents, and the same three review authors screened these for inclusion in duplicate. All trials identified as relevant but not meeting the inclusion criteria were re‐discussed before final exclusion. Complete agreement was reached (after discussion) between all review authors working independently on classification of studies for inclusion and exclusion.
Data extraction and management
All study data were extracted by one of the three review authors and were verified by a second review author. Two review authors extracted risk of bias data independently (FA, KC, ZU). Conflicts were resolved by discussion with the third party.
Assessment of risk of bias in included studies
Two review authors (FA, KC, ZU) assessed each study for risk of bias for allocation sequence generation, allocation concealment, blinding of participants and outcome assessors, handling of missing data, selective outcome reporting and other threats to validity in the studies, in line with recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We conducted a retrospective risk of bias assessment using the above method applied to all original studies included in the review for the 2009 update.
Measures of treatment effect
We extracted and analysed continuous and dichotomous outcome data using standard statistical techniques with a fixed‐effect model for all studies deemed similar enough to be pooled. In the presence of significant heterogeneity, we employed a random‐effects model.
For continuous outcomes, we calculated mean differences (MDs) with 95% confidence intervals (CIs) and pooled values as MDs or standardised mean differences (SMDs). For dichotomous outcomes, we calculated risk ratios (RRs) with 95% CIs.
A narrative synthesis was performed for each of the included studies. All trials were combined using Review Manager software.
Unit of analysis issues
A mixture of cross‐over and parallel studies were included in the review, with the potential for unit of analysis issues to occur. We used the generic inverse variance (GIV) method (by entering the effect estimates and their standard errors) to adjust for unit of analysis errors when meta‐analysing the data, as per Section 7.7.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We evaluated missing information regarding participants on an available case analysis basis, as described in Chapter 16.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When statistics essential for analysis were missing (e.g. group means and standard deviations for both groups were not reported) and could not be calculated from other data, we attempted to contact the study authors to obtain the missing data. Loss of participants that occurred before baseline measurements were taken was assumed to have no effect on eventual outcome data provided by the study. Any losses that occurred after baseline measurements had been taken were assessed and discussed on an intention‐to‐treat basis.
Assessment of heterogeneity
Statistical heterogeneity was assessed using a combination of tests, including an I² statistic of 50% or greater and visual inspection of the data. If 10 or more studies had been included, we would also have used funnel plots. The Der‐Simonian and Laird method of analysis presented with a P value less than 0.05 was considered statistically significant.
In the presence of significant heterogeneity (as per the criteria above), we reanalysed data using both fixed‐effect and random‐effects models.
Assessment of reporting biases
We planned to explore potential reporting biases by using a funnel plot if we were able to meta‐analyse 10 or more studies. Instead we extrapolated on this possible risk of bias within the other bias section in the risk of bias tables.
Data synthesis
We analysed data from trials using RevMan 5.1.
Summary of findings tables
We assessed the quality of evidence as high, moderate, low or very low in accordance with recommendations outlined by the GRADE working group for meta‐analyses of randomised trials (GRADE website). We have presented these assessments in a summary of findings table alongside the results of our analyses for the following outcomes: exercise capacity (minute walking distance); mortality; quality of life.
Subgroup analysis and investigation of heterogeneity
If sufficient numbers of studies had been included, subgroup analyses would have been performed for:
-
oxygen therapy type (oxygen cylinder vs liquid oxygen therapy); and
-
duration of follow‐up (short term (≤ six months), medium term (> six months to ≤ 12 months) and long term (> 12 months)).
Sensitivity analysis
We planned to conduct a sensitivity analysis based on risk of bias. However, we did not conduct a sensitivity analysis, as none of the included studies were assessed to be at high risk of bias.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
From the 188 citations identified by the literature search, 26 full‐text articles were considered relevant and were screened for eligibility (Figure 1). Four studies met all of the criteria for inclusion within the review, and seven studies were excluded although still considered relevant.

158 Study flow diagram.
Included studies
Details of the four included studies are reported in the Characteristics of included studies table, and reasons for exclusion of seven studies are reported in the Characteristics of excluded studies table.
Study design
All four included studies investigated the efficacy of ambulatory oxygen for participants with COPD using a randomised cross‐over design (McDonald 1995; Eaton 2002), a parallel randomised controlled design (Moore 2011) or an N‐of‐1 randomised controlled design (Nonoyama 2007) (the definition of an N‐of‐1 randomised controlled trial is one in which a single individual participant acts as the sole unit of observation in a study investigating the efficacy or side effect profiles of different interventions). Included studies were published between 1995 and 2011. Two studies originated from Australia (McDonald 1995; Moore 2011), one from Canada (Nonoyama 2007) and one from New Zealand (Eaton 2002).
Participant characteristics
A total of 331 participants were included across the four included studies, all of whom were adults with a mean age of 71 years. Sample sizes varied from 36 to 166. Mean PaO₂ ranged from 55 mmHg (Moore 2011) to 69 mmHg (Eaton 2002). Characteristics of participants in the McDonald 1995 study were PaO₂ > 60 mmHg (> 8.0 kPa) and exertional dyspnoea limiting their daily activities. For the Eaton 2002 study, PaO₂ was 69 ± 7.5 mmHg (9.2 ± 1.0 kPa) with SpO₂ on exertion and room air 82 ± 5.4, whilst the Moore 2011 study participants had a PaO₂ > 55 mmHg (> 7.3 kPa) at rest on room air. Finally, participants in the Nonoyama 2007 study did not meet the criteria for long‐term oxygen therapy and had exertional de‐saturation of 88% or less; dyspnoea limited their daily activities. All participants across studies had variable degrees of oxygen saturation (refer to the Characteristics of included studies table for additional details).
Intervention characteristics
All included studies compared oxygen cylinders with an identical placebo of air or a composite of air and oxygen. Method of delivery and dose of oxygen varied, although the apparatus in all instances consisted of lightweight or portable cylinders, with flow ranging from 3 L/min to 6 L/min. Final follow‐up was reported as 12 weeks for three studies (Eaton 2002; McDonald 1995; Moore 2011) and two weeks for the remaining study (Nonoyama 2007). The duration of intervention in McDonald and Eaton was six weeks, Moore 12 weeks and Nonoyama two weeks.
Outcomes
Outcome measures included mortality for two studies (McDonald 1995; Moore 2011), exercise capacity (as measured by step test or walk distance test) in all four studies and dyspnoea measured by the Borg scale in participants at rest in one study (McDonald 1995) and after exercise in two others (Eaton 2002; Nonoyama 2007). Quality of life was reported in all four studies using the CRQ (Chronic Respiratory Questionnaire), arterial oxygen saturation during exercise was measured in two studies (McDonald 1995; Nonoyama 2007) and after exercise in one (Eaton 2002) and two studies described adverse events during the study period (Eaton 2002; McDonald 1995). None of the included studies reported data for hospitalisation, length of stay or recovery time post exercise.
Excluded studies
Seven studies appeared relevant from the initial screen but on further investigation did not meet all of the inclusion criteria. These were excluded from the analyses for the following reasons. Three studies (Bradley 2007; Fujimoto 2002; Jolly 2001) evaluated the short‐term effects of oxygen in single‐assessment acute studies. Casaburi 2012 was a head‐to‐head study in which the comparison arm received an electronic portable oxygen concentrator, another study (Lacasse 2005) assessed the effects of ambulatory oxygen on participants who were already receiving LTOT and participants in another study (Lilker 1975) fulfilled the criteria for LTOT (PaO2 < 60 mmHg with evidence of cor pulmonale). Another study (Gorecka 1997) evaluated the effect of LTOT in participants with COPD with moderate hypoxia.
Risk of bias in included studies
Full details of our risk of bias judgements can be found under the risk of bias section at the end of each Characteristics of included studies table. Key features highlighting potential risks of bias are summarised in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation sequence generation
Two studies (Moore 2011; McDonald 1995) reported an adequate method of sequence generation, and the remaining two studies (Eaton 2002; Nonoyama 2007) were unclear. Adequate methods include the use of a computer‐generated programme for randomisation.
Allocation concealment
Allocation concealment was adequately reported in two studies (Moore 2011 ; McDonald 1995), in which allocation was undertaken by the supplier of the cylinder. The remaining two studies (Eaton 2002; Nonoyama 2007) were unclear, as the methods of allocation concealment were not reported.
Blinding
Blinding of both participants and outcome assessors was adequately reported in all four studies.
Incomplete outcome data
Incomplete outcome data were adequately addressed in two studies (Moore 2011 ; McDonald 1995), and the method was unclear in the remaining two studies (Eaton 2002; Nonoyama 2007). The two studies assessed as unclear did not discuss the presence or absence of potential missing data within questionnaires for outcome collection.
Selective reporting
One study was free of selective outcome reporting (Moore 2011) with an online prespecified protocol, whilst the remaining three studies were unclear.
Other potential sources of bias
Other potential sources of bias were not identified in three studies (Eaton 2002; Moore 2011; Nonoyama 2007), whilst this could not be adequately assessed in the McDonald 1995 study.
Effects of interventions
See: Summary of findings for the main comparison Ambulatory oxygen for COPD
See also summary of findings Table for the main comparison for a summary of key comparisons.
Exercise capacity
Four studies on 267 randomly assigned participants (n = 331 when doubling up for cross‐over and N‐of‐1 studies) reported exercise capacity through 'minute walking distance' via six‐minute walking distance (6MWD) (Eaton 2002; McDonald 1995; Moore 2011) or five‐minute walking distance (5MWD) (Nonoyama 2007). In the Eaton study, exercise capacity was assessed as an acute and short‐term response and therefore could not be analysed in this review of 'long‐term' treatment. The manuscript provides P values only for the impact of distance walked on oxygen, depending on long‐term use of cylinder air or oxygen (P value 0.9); therefore we have not included in the meta‐analysis the results of the 6MWD from the Eaton 2002 study. From the remaining three studies, the 6MWD outcome was split into three categories, depending on whether exercise capacity was assessed through 6MWD using cylinder air or oxygen or a cylinder similar to the one participants had used while they were at home. When exercise capacity was assessed through 6MWD using cylinder air (McDonald 1995; Moore 2011) or cylinder oxygen (McDonald 1995), no statistically or clinically significant benefit was seen to favour the intervention group (cylinder air MD 1.19, 95% CI 0.80 to 1.77; P value 0.38; Analysis 1.1; cylinder oxygen MD 1.27, 95% CI 0.48 to 3.39; P value 0.63; Analysis 1.2). Authors of the Nonoyama 2007 study reported a statistically significant improvement in the number of steps taken by participants when on oxygen compared with placebo for group N‐of‐1 RCTs (MD 14.90, 95% CI 0.85 to 28.94; P value 0.04); however, no statistically significant effect was observed for individual N‐of‐1 RCTs. A step exercise test was also performed in the McDonald 1995 study, and no difference was observed between groups (Analysis 1.3).
Mortality
Two studies on 179 participants (of whom 86 were on oxygen and 93 on air) reported deaths during the study periods. Two people in the intervention group of McDonald 1995 died (cerebrovascular accident and overwhelming pneumonia), as did one participant in the intervention group of Moore 2011. Although deaths occurred only in the intervention arms of these studies, they were not believed to be a result of the intervention. When data were pooled in a meta‐analysis, the outcome favoured the control group; however this was not statistically significant (RR 4.17, 95% CI 0.48 to 36.32; Analysis 1.4).
Dyspnoea scores
Dyspnoea was measured in three studies using the Borg scale, with McDonald 1995 (52 participants) reporting on dyspnoea during the 6MWD (MD ‐0.60, 95% CI ‐1.39 to 0.19) and during the step exercise test (MD ‐0.60, 95% CI ‐1.28 to 0.08; Analysis 1.5). A statistically significant improvement in acute post‐6MWD dyspnoea scores was reported with cylinder oxygen by authors for the Eaton 2002 study (cylinder oxygen 4.1 ± 1.8; cylinder air 4.8 ± 1.5; P value 0.005; 39 participants); however, over the six‐week study period, no difference in dyspnoea that was dependent on whether participants were assigned to cylinder air or cylinder oxygen was observed on cylinder oxygen. The Nonoyama 2007 study also produced a statistically significant reduction in dyspnoea scores for the oxygen group post 5MWD compared with placebo (MD ‐0.44, 95% CI ‐0.86 to ‐0.02; P value < 0.05; 27 participants).
Arterial oxygen saturation during exercise
One cross‐over study with 26 participants (McDonald 1995) measured arterial oxygen saturation during exercise for the 6MWD (MD ‐0.60, 95% CI ‐2.32 to 1.12) and for the step exercise test (MD ‐0.60, 95% CI ‐2.32 to 1.12), neither of which produced a statistically or clinically significant difference between groups (Analysis 1.6).
Quality of life
Four studies on 331 participants reported quality of life using the CRQ (Eaton 2002; McDonald 1995; Moore 2011; Nonoyama 2007;). A statistically significant difference favoured the oxygen group for the pooled dyspnoea component (MD 0.28, 95% CI 0.10 to 0.45; P value 0.002) and the fatigue component (MD 0.17, 95% CI 0.01 to 0.32; P value 0.009). However, pooled results for emotional function (MD 0.10, 95% CI ‐0.05 to 0.25) and mastery (MD 0.13, 95% CI ‐0.06 to 0.33) showed no evidence of an effect (Analysis 1.7).
Lung function measurements
Saturation of peripheral oxygen (SpO₂) on oxygen was measured in two studies on 136 participants, one during exercise (Nonoyama 2007) and the other after the 6MWD test (Eaton 2002); both produced a statistically and clinically significant difference in favour of the group that received ambulatory oxygen (MD 6.52, 95% CI 5.21 to 7.83; P value < 0.00001; Analysis 1.8). SpO2 is an indirect measurement using a finger probe, ear sensor or similar device.
Adverse events
Adverse events were not among the prespecified outcomes in the included studies. However, two studies on 83 participants reported adverse events during the intervention period. Eaton 2002 reported the development of co‐morbidities (n = 2) and a cancer diagnosis (n = 1) in one participant during the control phase of the cross‐over, and in another participant (n = 1) in the oxygen phase of the cross‐over. McDonald 1995 reported that three participants withdrew from the study during the first six weeks from the control group as the result of acute gout, muscular pain related to pulling the gas cylinder and unwillingness to continue further in the study; four participants withdrew during the second six weeks from the oxygen group as the result of two deaths (one from a cerebrovascular accident and another from overwhelming pneumonia), hospitalisation due to acute exacerbation of COPD and an incidental injury. No significant difference was observed when these events were pooled (odds ratio (OR) 0.77, 95% CI 0.21 to 2.81; Analysis 1.9). None of the included studies reported oxygen‐related complications such as CO₂ retention and fire hazard.
Hospitalisation
No studies reported data for this outcome.
Length of stay
No studies reported data for this outcome.
Discussion
Summary of main results
The primary aim of this review was to assess the effects of ambulatory oxygen in people with COPD who have exertional hypoxia or are not hypoxic at rest. All of the included studies reported as an outcome the primary outcome for this review, exercise capacity, leading to the overall sample size of 331 participants. All four studies reported this outcome as distance walked during the 6MWT or the 5MWT. Although the meta‐analysis favoured the intervention group, benefit did not reach statistical or clinical significance. One study not included in the meta‐analysis did produce statistically significant improvements in the oxygen group in the 5MWT for group N‐of‐1 studies; however, this effect was not observed for individual N‐of‐1 studies. Mortality, the other primary outcome in our review, was reported by only two studies (McDonald 1995; Moore 2011). Overall, three deaths were reported in the intervention group; however, these events were not statistically significant, and the deaths were unlikely to be caused by the intervention. It is difficult to interpret mortality on the basis of results from cross‐over studies and follow‐up of short duration; therefore, these results are limited and should be interpreted with caution. Two studies (Eaton 2002; Nonoyama 2007) reported dyspnoea post exercise (6MWT or 5MWT), and both studies indicated statistically and clinically significant benefits in favour of oxygen. One study (McDonald 1995) reported dyspnoea and oxygen de‐saturation during 6MWT and exercise testing; however, no significant change was noted. Quality of life was reported by all four included studies using the CRQ. Statistically significant improvement was observed for the dyspnoea and fatigue domains, although no significant improvements were noted for emotional function or mastery. Adverse events were reported by two studies (Eaton 2002; McDonald 1995). None of the included studies reported incidents of oxygen‐related fire or CO₂ retention. Although three participants in one control group reported muscular pain related to pulling cylinders, no significant difference was observed in the pooled analysis. Length of stay, rate of hospitalisation and spirometry were not reported in any of included studies.
Overall completeness and applicability of evidence
Despite an extensive literature search and the high burden of disease in the community, only four studies met our inclusion criteria. The primary aim of this review was to address the possible benefit of supplementary/ambulatory oxygen in mildly to moderately hypoxic patients for exercise capacity and mortality. Although only four studies met the inclusion criteria of this review, the overall sample size for the primary outcome of exercise capacity was moderate (n = 331). Other outcomes such as quality of life, dyspnoea and adverse events were not consistently reported amongst the included studies. We report a statistically significant mean difference of 0.28 (0.1 to 0.45) for the pooled dyspnoea component of the CRQ, favouring the oxygen group. However, this improvement needs to be interpreted in relation to the known minimally important difference (MID)of 0.5.This review provides a reasonable pooled analysis of relevant outcomes for the population with COPD requiring ambulatory oxygen; however, methodologically sound trials of sufficient power to allow confidence in the results are lacking. Good methodological rigour would include publication of a prespecified protocol outlining trial design, adequate sequence generation, randomisation, blinding (for participants and for outcome assessors) and transparent reporting of appropriate outcomes at baseline in a format suitable for meta‐analysis (e.g. mean and corresponding standard deviation and sample size).
Quality of the evidence
The overall quality of included studies in this review was moderate. Methodology not always explicitly reported.Most included studies did report the methods used to blind assessors and participants from allocation; however, not enough information was provided for assessment of incomplete outcome data in two of four studies. Also, most studies did not have a prepublished protocol, which again increases the potential for selective outcome reporting. Sensitivity analysis was not conducted, as none of the included studies were assessed to be at high risk of bias.
Potential biases in the review process
No significant biases were anticipated or were found to occur during the review process. Criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions were strictly followed to limit the potential for bias during screening, data extraction and analyses of included studies. Risk of bias was independently assessed by two review authors, and conflicts were resolved by discussion with a third review author. Corresponding authors of one included study (Nonoyama 2007) were contacted for raw data and clarification of methodological techniques. In response to Feedback 1, a second study author team were contacted for clarification of methodology (Moore 2011). No conflicts of interests, financial or otherwise, were reported for any of the review authors of this meta‐analysis.
Agreements and disagreements with other studies or reviews
The utility of ambulatory oxygen in patients who do not fulfil the criteria for continuous long‐term oxygen therapy is controversial. Similar to this review, the previous Cochrane review (Ram 2002) derived no firm conclusion from available evidence about the effectiveness of this intervention in patients with COPD and mild hypoxaemia. Our review does not fully support the prescription of ambulatory oxygen in patients with moderate hypoxaemia and exercise de‐saturation, per guidelines of the British Thoracic Society (BTS 2006).
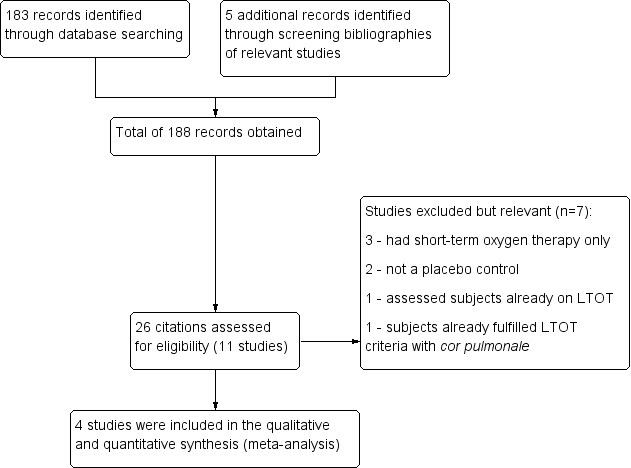
158 Study flow diagram.
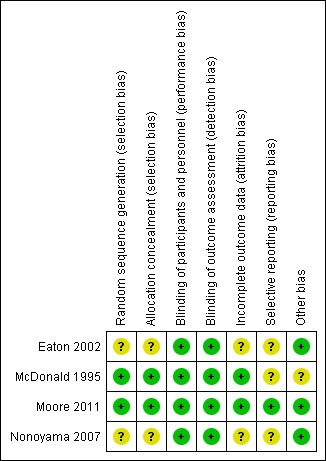
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 1 6MWD (cylinder air for 6MWD).
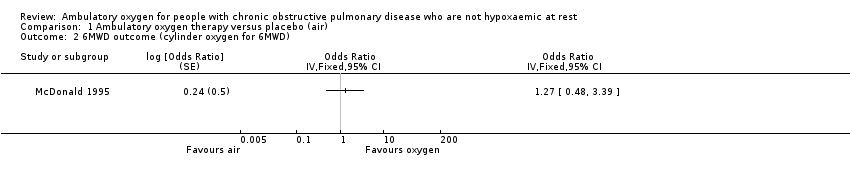
Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 2 6MWD outcome (cylinder oxygen for 6MWD).
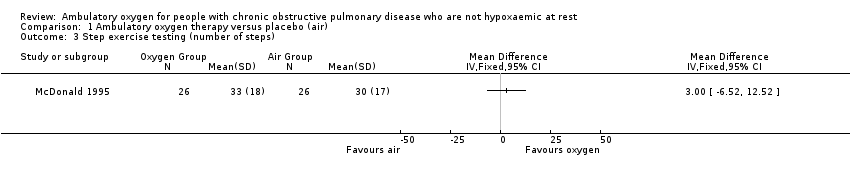
Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 3 Step exercise testing (number of steps).

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 4 Mortality.

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 5 Borg score—dyspnoea (higher score worse).

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 6 Arterial oxygen saturation during exercise.

Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 7 Quality of life (Chronic Respiratory Questionnaire).
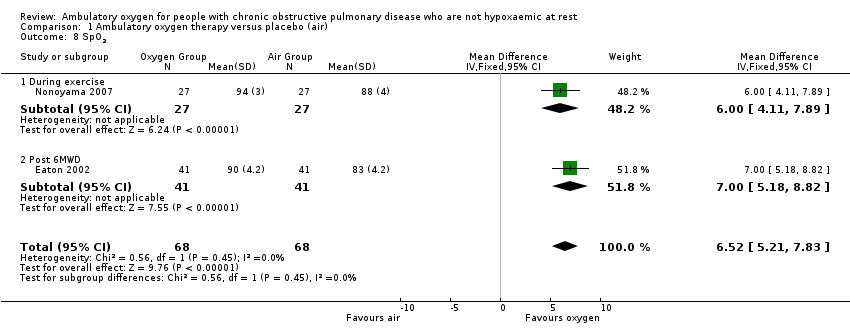
Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 8 SpO2.
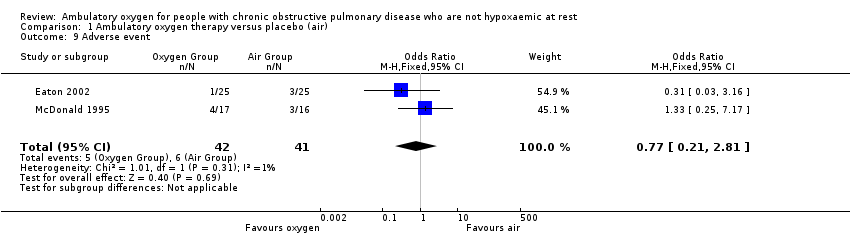
Comparison 1 Ambulatory oxygen therapy versus placebo (air), Outcome 9 Adverse event.
| Ambulatory oxygen for COPD | ||||||
| Patient or population: adults with COPD who had exertional dyspnoea but did not fulfil the criteria for long‐term oxygen treatment Control: placebo/medical air | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Ambulatory oxygen | |||||
| Exercise capacity (5‐ or 6‐minute walking distance on cylinder air) | See comment | See comment | Not estimable | 331 | Not applicable | Meta‐analysis not possible; see effects of interventions for more information |
| Mortality | See comment | See comment | RR 4.17 | 179 | ⊕⊕⊕⊝1 Moderate | Although deaths occurred only in the intervention arm of the study (n = 3), they were not believed to be a direct result of the intervention |
| Quality of life (dyspnoea) Follow‐up: 2 to 12 weeks | Baseline risk in control groups ranged from 2.8 to 3.7 points | Mean quality of life (dyspnoea) in the intervention groups was | MD 0.28 (0.10 to 0.45) | 341 | ⊕⊕⊕⊝2 Moderate | Other CRQ domains were also reported, including fatigue MD 0.14 (95% CI 0.04 to 0.31; P value 0.009), emotional function MD 0.10 (95% CI ‐0.05 to 0.25; P value 0.20) and mastery MD 0.13 (95% CI ‐0.06 to 0.33; P value 0.17) |
| Dyspnoea | See comment | See comment | Not estimable | 198 | Not applicable | Meta‐analysis not possible Dyspnoea was measured in 3 studies using the Borg scale, and 1 study reported dyspnoea during exercise. One study observed improvement in dyspnoea after walking for 6 minutes with cylinder air or oxygen. Another study showed a clinically relevant reduction in dyspnoea scores for the oxygen group post 5MWD compared with placebo |
| Adverse events | 146 per 1000 | 117 per 1000 | OR 0.77 | 83 | ⊕⊕⊝⊝ | Only 1 of the adverse events appeared related to the intervention; this was strain due to carrying the cylinder |
| Hospitalisations | See comment | See comment | Not estimable | 0 | See comment | No studies reported data on hospitalisations |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1 Imprecision 2 Unclear risk for selection bias in 2 studies, attrition bias in 2 studies and selective reporting in 3 studies 3 Unclear risk of selection bias, attrition bias and selective reporting in 1 study | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 6MWD (cylinder air for 6MWD) Show forest plot | 2 | Odds Ratio (Fixed, 95% CI) | 1.05 [0.62, 1.75] | |
| 2 6MWD outcome (cylinder oxygen for 6MWD) Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 3 Step exercise testing (number of steps) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Mortality Show forest plot | 2 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.48, 36.32] |
| 5 Borg score—dyspnoea (higher score worse) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 During 6MWD | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 During step exercise test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Arterial oxygen saturation during exercise Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 During 6MWD | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.56, 0.36] |
| 6.2 During step exercise test | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.32, 1.12] |
| 7 Quality of life (Chronic Respiratory Questionnaire) Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7.1 CRQ—dyspnoea | 4 | Mean Difference (Fixed, 95% CI) | 0.28 [0.10, 0.45] | |
| 7.2 CRQ—fatigue | 4 | Mean Difference (Fixed, 95% CI) | 0.17 [0.04, 0.31] | |
| 7.3 CRQ—emotional function | 4 | Mean Difference (Fixed, 95% CI) | 0.10 [‐0.05, 0.25] | |
| 7.4 CRQ—mastery | 4 | Mean Difference (Fixed, 95% CI) | 0.13 [‐0.06, 0.33] | |
| 8 SpO2 Show forest plot | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | 6.52 [5.21, 7.83] |
| 8.1 During exercise | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [4.11, 7.89] |
| 8.2 Post 6MWD | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [5.18, 8.82] |
| 9 Adverse event Show forest plot | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.21, 2.81] |

