Урсодеоксихолевая кислота при заболеваниях печени, связанных с муковисцидозом
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000222.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Katharine Cheng and Rosalind Smyth independently assessed studies for inclusion in the review. Katharine Cheng wrote the text of the review, assisted by Rosalind Smyth, and acts as guarantor of the review.
Deborah Ashby provided statistical advice.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
NHS North West Region R&D Programme, UK.
Declarations of interest
The lead author of this review has been employed by GlaxoSmithKline Research and Development Ltd since late 2004. GlaxoSmithKline does not produce or market any drugs that may fall into the scope of this review.
Acknowledgements
This review was made possible by researchers who kindly provided data and made comments. These include: Sharon O' Brien, Central Middlesex Hospital, London, UK; Manuela Merli, Universita degli Studi di Roma 'La Sapienza', Rome, Italy, Carla Colombo, University of Sassari, Sassary, Italy; Christine Spray, The Children's Hospital, Birmingham, UK; and Carol Seymour, St George's Hospital Medical School, London, UK. It was conducted as an activity of The Cochrane CF and Genetic Disorders Group, supported by grants from North West Region Research and Development and The Cystic Fibrosis Trust. However, these two organisations are not responsible for the contents of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Sep 11 | Ursodeoxycholic acid for cystic fibrosis‐related liver disease | Review | Katharine Cheng, Deborah Ashby, Rosalind L Smyth | |
| 2014 Dec 15 | Ursodeoxycholic acid for cystic fibrosis‐related liver disease | Review | Katharine Cheng, Deborah Ashby, Rosalind L Smyth | |
| 2012 Oct 17 | Ursodeoxycholic acid for cystic fibrosis‐related liver disease | Review | Katharine Cheng, Deborah Ashby, Rosalind L Smyth | |
| 1999 Jul 26 | Ursodeoxycholic acid for cystic fibrosis‐related liver disease | Review | Katharine Cheng, Deborah Ashby, Rosalind L Smyth | |
Notes
Lepage 1997, Kapustina 2000 and Spray 2000 are all studies awaiting assessment. The contact people of these studies have been written to. If addition data are forthcoming from these trialists, the review will be updated accordingly.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Adult; Child; Child, Preschool; Humans;
PICO
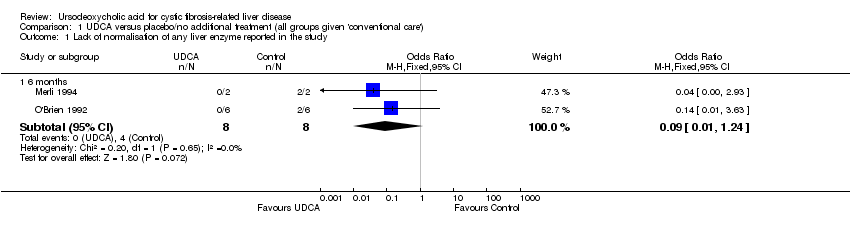
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 1 Lack of normalisation of any liver enzyme reported in the study.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 2 Lack of normalisation of all liver enzymes reported in the study.
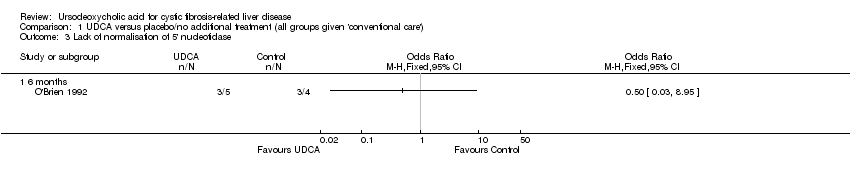
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 3 Lack of normalisation of 5' nucleotidase.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 4 Lack of normalisation of aspartate transaminase.
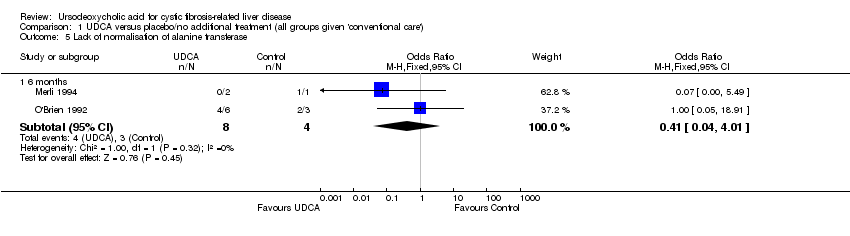
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 5 Lack of normalisation of alanine transferase.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 6 Lack of normalisation of gammaglutamate transferase.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 7 Need for liver transplantation.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 8 Death related to liver disease.
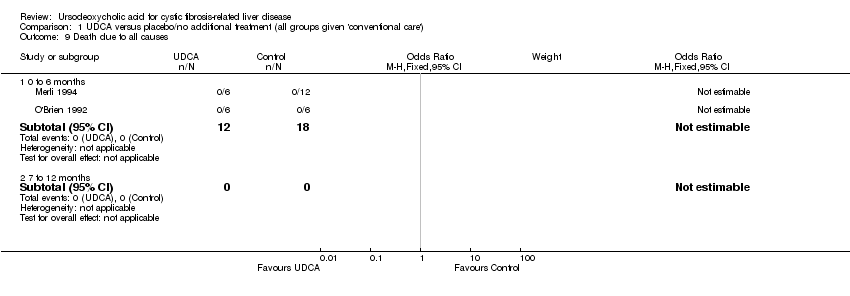
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 9 Death due to all causes.
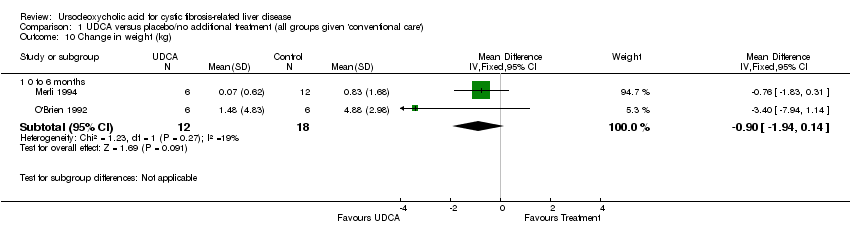
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 10 Change in weight (kg).

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 11 Development of portal hypertension.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 12 Development of complications of portal hypertension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lack of normalisation of any liver enzyme reported in the study Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.24] |
| 2 Lack of normalisation of all liver enzymes reported in the study Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Lack of normalisation of 5' nucleotidase Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Lack of normalisation of aspartate transaminase Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.43, 284.30] |
| 5 Lack of normalisation of alanine transferase Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.04, 4.01] |
| 6 Lack of normalisation of gammaglutamate transferase Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 7 Need for liver transplantation Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 7 to 12 months | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.10, 74.63] |
| 8 Death related to liver disease Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 7 to 12 months | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Death due to all causes Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 7 to 12 months | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Change in weight (kg) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 0 to 6 months | 2 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.94, 0.14] |
| 11 Development of portal hypertension Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 7 to 12 months | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Development of complications of portal hypertension Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 7 to 12 months | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

