Acide ursodéoxycholique dans une maladie hépatique liée à la mucoviscidose
Résumé scientifique
Contexte
Une sécrétion biliaire anormale conduit à l'épaississement de la bile et à la formation de bouchons dans les conduits biliaires ; l'obstruction et les anomalies du flux biliaire qui en résultent aboutissent finalement au développement d'une maladie hépatique liée à la mucoviscidose. Cette affection atteint un pic à l'adolescence, avec près de 20 % des adolescents atteints de mucoviscidose développant une maladie hépatique chronique. Des modifications précoces au niveau du foie peuvent finir par provoquer une maladie hépatique en phase terminale chez les personnes ayant besoin d'une greffe. Une option thérapeutique actuellement utilisée est l'acide ursodéoxycholique.
Objectifs
Analyser les preuves selon lesquelles l'acide ursodéoxycholique améliore les indices de la fonction hépatique, réduit les risques de développement d'une maladie hépatique chronique et améliore globalement les résultats concernant la mucoviscidose.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre d'essais du groupe Cochrane sur la mucoviscidose et les autres maladies génétiques qui comprend des références bibliographiques identifiées lors de recherches exhaustives dans des bases de données électroniques et des recherches manuelles dans des journaux et des résumés d'actes de conférence pertinents. Nous avons également contacté des laboratoires pharmaceutiques.
Date de la recherche la plus récente effectuée dans le registre des essais cliniques du groupe : 29 mai 2014.
Critères de sélection
Essais contrôlés randomisés comparant l'administration d'acide ursodéoxycholique pendant une durée d'au moins trois mois à un placebo ou à l'absence de traitement complémentaire chez des personnes atteintes de mucoviscidose.
Recueil et analyse des données
Deux auteurs ont indépendamment évalué l'éligibilité et la qualité méthodologique des essais.
Résultats principaux
Dix essais ont été identifiés, dont trois totalisant 118 participants ont été inclus ; la dose d'acide ursodésoxycholique allait de 10 à 20 mg/kg/jour pendant jusqu'à 12 mois. La conception complexe utilisée dans deux essais signifiait que les données pouvaient uniquement être analysées pour des sous‐ensembles de participants. Il n'y avait aucune différence significative au niveau de la variation pondérale, différence moyenne ‐0,90 kg (intervalle de confiance à 95 % de ‐1,94 à 0,14) chez 30 participants issus de deux essais. Une amélioration de l'excrétion biliaire a été signalée dans un seul essai, mais aucun changement significatif n'a été constaté après le traitement. Aucune donnée n'était disponible pour une analyse des critères de jugement à long terme, comme le besoin d'une greffe hépatique ou la mortalité.
Conclusions des auteurs
Il existe peu d'essais évaluant l'efficacité de l'acide ursodéoxycholique. Les preuves sont insuffisantes pour justifier son administration systématique dans la mucoviscidose.
PICO
Résumé simplifié
Acide ursodéoxycholique dans une maladie hépatique liée à la mucoviscidose
Question de la revue
L'acide ursodéoxycholique permet‐il d'améliorer les mesures de la fonction hépatique, de réduire les risques de développement d'une maladie hépatique chronique et d'améliorer globalement les résultats chez les personnes atteintes de la mucoviscidose ?
Contexte
Les problèmes liés à la consistance de la bile (épaisse) et au flux biliaire provoquent une maladie hépatique chez près de 20 % des jeunes atteints de mucoviscidose. Les voies biliaires peuvent être obstruées et cela peut entraîner la cirrhose dans une ou plusieurs parties du foie. L'acide ursodéoxycholique, un acide biliaire sécrété naturellement, est administré sous forme de comprimés ou de liquide pour tenter d'éviter le développement d'une maladie hépatique chez les personnes atteintes de mucoviscidose. La meilleure réponse semble être obtenue par une dose totale de 20 mg/kg/jour en deux à trois prises séparées, administrée initialement pendant plusieurs mois, et peut‐être indéfiniment. Utilisé à l'origine pour traiter les calculs biliaires, l'acide ursodéoxycholique a été utilisé au cours de ces dernières années pour traiter et prévenir la progression de la maladie hépatique liée à la mucoviscidose.
Date de recherche
Nous avons effectué la dernière recherche de preuves le 29 mai 2014.
Caractéristiques des études
Nous avons recherché des essais sur l'acide ursodésoxycholique ayant duré au moins trois mois, et avons pu en inclure trois dans cette revue. Dans trois autres essais, il n'y avait pas suffisamment d'informations pour pouvoir déterminer leur pertinence. Cette revue porte sur 118 participants âgés de quatre à 32 ans. La dose de médicament administré dans les essais allait de 10 à 20 mg/kg/jour. Dans deux essais, l'acide ursodésoxycholique était comparé à des comprimés sans médicament (placebo), et dans le troisième, au traitement « habituel ». La conception complexe de deux essais signifiait que les données ne pouvaient pas être analysées pour l'ensemble des participants. Les essais ont duré jusqu'à 12 mois au plus ; un essai rapporte cependant quelques données de suivi après neuf ans.
Principaux résultats
Parmi les critères énumérés dans cette revue, seuls quelques‐uns ont été évalués : la prise de poids, l'épaisseur du pli cutané et l'excrétion biliaire. Il n'y a pas eu de véritable différence entre les traitements pour aucun de ces critères d'évaluation. Les critères à long terme que nous avons estimés importants, comme le décès ou la nécessité d'une greffe du foie, n'ont été rapportés que dans le suivi d'un seul essai, et les données ne révèlent pas si les gens décédés ou ayant eu besoin d'une greffe de foie avaient reçu l'acide ursodésoxycholique ou un placebo.
Les recherches actuelles montrent que les effets secondaires de ce traitement sont rares, mais il n'y a pas suffisamment d'information sur son utilisation à long terme pour justifier son administration systématique aux personnes atteints de mucoviscidose. Étant donné qu'il n'existe aucun autre traitement préventif pour la maladie hépatique, des recherches supplémentaires sur l'acide ursodéoxycholique doivent être effectuées.
Qualité des preuves
Les essais semblaient être bien organisés et gérés, mais il n'y avait pas toujours suffisamment d'informations pour les évaluer correctement. Alors que, dans l'ensemble, nous ne pensons pas que des facteurs liés à la façon dont les essais ont été effectués auraient une grande influence sur les résultats, nous avons cependant eu quelques préoccupations à propos d'un essai dans lequel les personnes recevant l'acide ursodésoxycholique n'étaient généralement pas en aussi bonne santé au début de l'essai que le groupe prenant le placebo. En outre, dans un autre essai, certaines personnes se sont retirées et n'ont pas été incluses dans l'analyse finale, mais aucune explication n'a été fournie sur les raisons.
Authors' conclusions
Background
Description of the condition
Cystic fibrosis (CF) is a common inherited disease which invariably leads to progressive lung damage. The medical management of associated chronic chest disease has improved greatly over the last 30 years leading to improvement in survival well into adult life. Clinicians are now examining ways of both treating and delaying the progression of the disease in other affected organs. Among these, CF‐related liver disease is clinically the most significant hepatic complication with a large impact on morbidity and mortality (Leeuwen 2014). A recent review suggested that hepatobiliary disease is the most common non‐pulmonary cause of mortality in CF (the third after pulmonary disease and transplant complications) (Parisi 2013), A recent epidemiological study reported that there was a significantly higher prevalence of CF‐related hepatobiliary abnormalities in CF patients under 18 years of age and 25% of those with CF‐related hepatobiliary abnormalities developed hepatobiliary disease (Bhardwaj 2009).
The mechanism of liver involvement in CF is thought to be due to a chloride channel defect causing abnormal biliary secretion which leads to the thickening of bile and the formation of plugs within the bile ducts. The resulting ductular obstruction and abnormal bile flow ultimately results in the development of bile duct irregularities inside and outside the liver and cirrhosis in one or several parts of the liver. Therefore, therapy has been directed towards attempting to improve biliary secretion and bile acid composition.
Description of the intervention
Ursodeoxycholic acid (UDCA) is a naturally occurring hydrophilic bile acid.
It is usual to take UDCA by mouth twice or three times a day, initially for several months but possibly indefinitely. Side effects are rare but diarrhoea has been reported. In 2003, the cost of six months' (24 weeks) treatment with UDCA for a 10‐year old child weighing 25 kg, at a dose of 20 mg/kg/day, was £131 (RLCH 2003). Colombo demonstrated in a dose‐response study that the biochemical response to UDCA was best with a dose of 20 mg/kg/day (Colombo 1992).
How the intervention might work
In 1990, Erlinger showed that UDCA improves bile acid flow by inducing a bicarbonate‐rich bile flow (Erlinger 1990). This mechanism has potential use for people with CF‐related liver disease in whom the bile ducts are blocked by thick and sticky secretions. Also, UDCA is not as toxic to the liver as other primary bile acids. Initially, UDCA was used in the treatment of gallstones (Roda 1982) and more recently as a possible treatment for other chronic liver diseases such as primary biliary cirrhosis (Poupon 1991) and primary sclerosing cholangitis (Beuers 1992). Over the last few years it has been used in the treatment and prevention of progression of CF‐related liver disease following the observation of its therapeutic effectiveness in these other cholestatic conditions.
Why it is important to do this review
There are a number of debates surrounding the treatment of people with CF who have liver involvement. Both early detection and assessment of progression of liver disease in CF are relatively difficult. This is because by the time liver disease is evident in a person with an enlarged liver or spleen, there is often already raised pressure in the large vein running through the liver (portal hypertension, usually an irreversible event) and end‐stage liver damage (cirrhosis). At this stage the only helpful treatment may be a liver transplant. These problems mean that clinicians are faced with the dilemma of when UDCA should be commenced: early to prevent liver involvement; or later as a therapeutic option.
Another debate is how liver involvement can be evaluated. The important outcomes are death and preventing liver transplantation; other surrogate markers are often used but there are problems associated with these. Biochemical measures of liver function may not be useful because the level of abnormality does not always correlate with the extent of liver involvement (Tanner 1992). Abnormalities of these test results may also be due to an effect other than CF liver disease, such as an effect of a drug treatment (Tanner 1992). Ultrasound can be used to assess the presence and progression of liver disease (Carty 1995). It can show alterations in liver size and texture and can also be used to assess the extent and direction of blood flow in the portal vein. However, results may vary with different operators. Another technique for identifying liver disease is radioisotope scanning (hepatobiliary scintigraphy) (O'Connor 1996). Measuring the hepatic excretion of the compound 99mTc‐HIDA allows an objective measurement of liver function and bile acid secretion. However, these are all intermediate outcomes and their correlations with the outcomes of death and liver transplantation are unknown.
Although UDCA is relatively inexpensive compared to other treatments taken by people with CF (see above), it is yet another treatment of many and it is important that it has been shown to be effective. Therefore, we have undertaken a systematic review assessing its effectiveness in people with CF with liver involvement.
Objectives
To analyse evidence from randomised controlled trials (RCTs) in CF that UDCA improves indices of liver function, reduces the risk of developing chronic liver disease and improves outcomes in general in CF.
Methods
Criteria for considering studies for this review
Types of studies
RCTs (published or unpublished). Trials where pseudo‐randomisation methods are used, such as alternation, will be included.
Types of participants
Children and adults with defined CF, diagnosed clinically and by sweat test or genetic testing, including all ages, all degrees of severity of disease and any degree of liver involvement.
Types of interventions
UDCA compared to a control group receiving either placebo or no additional therapy (i.e. both groups receiving usual CF therapy). UDCA administered orally, at any dose, given for a period of at least three months.
Types of outcome measures
Primary outcomes
-
Change of hepatocellular enzymes from outside the normal range on at least one occasion to within the normal range of the method stated
-
Abnormally large livers reduced to within normal limits, as measured by ultrasound
-
Need for liver transplantation
Secondary outcomes
-
Mortality
-
Weight gain, body mass index, z score (a measure of nutritional status, where weight is expressed as a percentage of ideal for height and then compared to the standard deviation for the population (Frisancho 1990)) and other indices of nutritional improvement, if reported
-
Development of portal hypertension shown by an enlarged spleen (increased by at least 15%), direction of portal vein flow, portal vein flow velocity, oesophageal varices (using ultrasound) or the complications of portal hypertension ‐ these may include haematemesis (vomiting blood), reduction in platelet count or a reduction in white cell count
-
Improved abnormal biliary excretion as documented by isotope scanning (hepatic scintigraphy)
Search methods for identification of studies
Electronic searches
Relevant trials were identified from the Group's Cystic Fibrosis Trials Register using the terms liver AND ursodeoxycholic acid.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), quarterly searches of MEDLINE, a search of EMBASE to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and theJournal of Cystic Fibrosis. Unpublished work is identified by searching through the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the most recent search of the Group's Cystic Fibrosis Trials Register: 29 May 2014.
Searching other resources
Reference sections of any trials identified were checked for any further RCTs. In addition we undertook full text searching of theJournal of Pediatrics from 1988 to 1995. We also contacted the pharmaceutical companies that market UDCA: Hoechst Marion Roussel (Destolit®) and Consolidated Chemicals Ltd (Ursofalk®).
Data collection and analysis
Selection of studies
The two authors (KC and RS) independently applied the inclusion criteria to all potential reports.
Data extraction and management
We attempted to extract data from each RCT from the text, tables and figures. We recorded data on the number of participants with each outcome event, by allocated group, irrespective of compliance and whether or not the participant was later thought to be eligible or otherwise excluded from treatment or follow up. For continuous outcomes we recorded the mean change from baseline for each group and standard error or standard deviation.
Assessment of risk of bias in included studies
In order to assess the risk of bias in the included studies, we considered such aspects as generation of randomisation sequence and allocation concealment. If we regarded these as adequate then there was a low risk of bias to the study; if we regarded these as inadequate, then there was a high risk of bias to the study; and if they were considered unclear then the risk of bias was unclear too. We also considered the degree of blinding and the risk of bias increased as the number of people blinded to the intervention decreased. We also considered other risks of bias e.g. from selective reporting.
Measures of treatment effect
We calculated a pooled estimate of the treatment effect for each outcome across trials. For binary outcomes we calculated, where possible, the odds of an outcome among treatment‐allocated participants to the corresponding odds among controls. For continuous outcomes, where data were available, we calculated a pooled estimate of treatment effect by calculating the mean difference and 95% confidence intervals.
Unit of analysis issues
Although we did not specifically excluded cross‐over trials, we were concerned about the use of a cross‐over design. This was because there may be a carry‐over effect of UDCA in the control arm. We did include one cross‐over study in this review (Merli 1994). The data presented in the published report appeared to be combined from both treatment periods, but the authors attempted to overcome a possible carry‐over effect of UDCA by using a one‐month washout period. However, we considered it appropriate to compare only the first six months of the trial, i.e. UDCA versus placebo. Data from the first period were not available in the published report but the authors kindly provided the raw data. Including data from the first period in cross‐over trials in meta‐analyses is not without problems. Excluding later periods loses some of the information collected. Furthermore, if data from the first period are available in published reports they are likely to represent a biased subset of trials, usually because the authors have found evidence of carry‐over (Elbourne 2002).
Dealing with missing data
Where sufficient data were not available in the published reports or the abstract of the conference proceedings, the review authors attempted to contact the first and last authors of the paper.
We recorded data on the number of participants with each outcome event, by allocated group, irrespective of compliance and whether or not the participant was later thought to be eligible or otherwise excluded from treatment or follow up. This approach permits an intention‐to‐treat analysis.
Assessment of heterogeneity
We tested for heterogeneity between trial results using a standard chi squared test.
Data synthesis
We analysed the data using a fixed‐effect model. If, in future updates of this review, we identify a moderate to large degree of heterogeneity, we will analyse the data using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
For future updates, if heterogeneity is identified and there are sufficient studies included in the review, we plan to investigate heterogeneity by means of examining patients with evidence of liver disease at randomisation separately from those without liver disease.
Sensitivity analysis
We will also examine the robustness of our results using a sensitivity analysis including and excluding studies with a high risk of bias.
Results
Description of studies
Results of the search
Ten trials have been identified as potentially relevant. Four trials were excluded (Bittner 1989; Colombo 1992; Narckewicz 1994; Van de Meeberg 1997). A further three trials are listed under 'Awaiting classification' (Kapustina 2000; Lepage 1997; Spray 2000). Of these three trials, two are as yet only published as abstracts and do not contain sufficient detail for us to make a decision on their eligibility for this review; we will re‐assess these trials when the full papers have been published (Kapustina 2000; Spray 2000). The remaining trial listed as 'Awaiting classification' is a cross‐over trial (Lepage 1997). From the full published paper it is unclear whether there was any washout period employed in the trial and furthermore, data are not published for the first six‐month period of the trial, thus we are unable to treat this as a parallel trial and present data as we have for the Merli trial. Until we are able to obtain such a breakdown of the trial results, we will list this trial as 'Awaiting classification'.Thus, three trials met the inclusion criteria (Colombo 1996; Merli 1994; O'Brien 1992).
Included studies
Three trials involving a total of 118 participants have been included in this review (Colombo 1996; Merli 1994; O'Brien 1992). The ages of the participants ranged from 4 years to 32 years. The dose of UDCA given ranged from 10 to 20 mg/kg/day.
In two trials the comparison was with placebo (Colombo 1996; Merli 1994). In the third trial the comparison was with existing conventional therapy (O'Brien 1992). In two of the trials all of the participants had liver disease (Colombo 1996; O'Brien 1992), whereas in the third trial only 10 out of 51 participants had liver disease (Merli 1994).
The length of follow up was generally short and ranged from 6 months (Merli 1994; O'Brien 1992) to 12 months (Colombo 1996).
Important long‐term outcomes such as death or the need for liver transplant were not reported. Only two of our protocol‐defined outcomes were assessed: the nutritional indices (weight gain and skinfold thickness (Merli 1994; O'Brien 1992)); and biliary excretion (O'Brien 1992).
Study design was complicated in two trials (Colombo 1996; Merli 1994). In the cross‐over trial, 51 participants were randomised to receive UDCA alone or with taurine for six months and then each treatment group was compared with a six‐month placebo period (Merli 1994). The sequence of treatment and placebo was then randomised in a cross‐over design. The data presented in the published report of the cross‐over RCT appeared to be combined from both treatment periods. Although we had not specifically excluded cross‐over trials, we were concerned about the use of a cross‐over design. This was because there may be a carry‐over effect of UDCA in the control arm, although the authors attempted to overcome this by using a one‐month washout period. However, we considered it appropriate to compare only the first six months of the trial, i.e. UDCA versus placebo. Data from the first period were not available in the published report but the authors have now kindly provided the raw data. Including data from the first period in cross‐over trials in meta‐analyses is not without problems. Excluding later periods loses some of the information collected. Furthermore, if data from the first period are available in published reports they are likely to represent a biased subset of trials, usually because the authors have found evidence of carry‐over (Elbourne 2002).
A factorial parallel design was employed in the Colombo trial (Colombo 1996). In this multicentre trial, 55 participants were randomised to receive UDCA or placebo and then each group was further randomised to receive either taurine or a second placebo. In effect, four parallel groups were studied.
In the trial by O'Brien, 12 participants were randomised to UDCA or no additional therapy for six months (other than usual CF treatments, such as pancreatic enzymes and oral calorie supplements, which the UDCA group also received) (O'Brien 1992). Advanced liver disease, as documented by portal hypertension or histological features of fibrosis or cirrhosis or all three, was present in 11 out of 12 participants.
The use of taurine in these two trials also complicated their design and analysis since taurine may affect liver involvement in CF (Colombo 1996; Merli 1994). Although UDCA is known to cause taurine depletion, the combined effect of UDCA and taurine on liver function is unknown.
These possible interactions and the complex study designs caused difficulties when we considered combining the data. We have used subsets of the sample sizes given in the 'Characteristics of included studies' table so the participant numbers evaluated in the data tables and figures do not always tally with the sample sizes. In the cross‐over trial we decided only to use data from the first six months of the UDCA/placebo group and not use the UDCA plus taurine group (Merli 1994). This gave us data on an unbalanced number of participants in the two groups in the first six‐month period: only 6 participants in the UDCA group and 12 participants in the control group (51 were initially randomised). In the factorial, parallel design trial we decided not to use data from participants who received taurine (hence the total number of participants used in the data tables was 28 not 55) (Colombo 1996). However, only 4 of these 18 participants in this subset had abnormal liver enzymes at baseline.
Another issue of the cross‐over RCT was that although weight, height and body mass percentile were measured, we had concerns about this type of trial design (Merli 1994). We would expect there to be a period effect on variables such as weight and height and this would require more subtle analysis. Again, we decided to use data only from participants in the UDCA‐alone group and from the first six months of the trial before cross over.
In 2005, Colombo presented follow‐up survival data (obtained by a data collection form sent to each centre) from the RCT that had been conducted in 1990 (Colombo 1996). Information was obtained from 53 of the original 55 participants (two were lost to follow up) for a median total period of follow up of 13.6 years; follow‐up data for the whole cohort were presented, not by randomised group. The majority of the trial participants had continued open UDCA therapy after the end of the trial (median daily dose 666 mg).
Excluded studies
Four trials were excluded in total. Two trials were excluded because they did not include a placebo arm or a 'no UDCA' arm (Colombo 1992; Van de Meeberg 1997). One trial was not an RCT (Narckewicz 1994) and in another, the duration of follow up was only six weeks (Bittner 1989).
Risk of bias in included studies
Allocation
All three trials were described as randomised, but only one trial stated the method used (Colombo 1996). We therefore judged the Colombo trial to have a low risk of bias (Colombo 1996), and the other two trials to have an unclear risk of bias (Merli 1994; O'Brien 1992).
Two trials described how allocation was concealed and these were judged to be adequate and hence have a low risk of bias (Colombo 1996; O'Brien 1992). The other trial did not discuss allocation concealment and so was judged to have an unclear risk of bias (Merli 1994).
Blinding
One of the trials was described as double‐blinded (Colombo 1996). In the Merli trial, glucose tablets were used as the placebo, so it is probable, although not explicitly stated, that the participants at least were blinded to whether they were in the treatment or control group (Merli 1994). It was not possible to blind the O'Brien trial to participants or clinicians since the participants either received UDCA or no additional treatment (O'Brien 1992).
Incomplete outcome data
An intention‐to‐treat analysis was performed in two trials (Colombo 1996; O'Brien 1992). In one trial 51 participants were initially recruited, but 9 subsequently withdrew (Merli 1994). These participants were not followed up and were not included in the analysis. Data from a further two participants were identified as being lost when the raw data were provided.
Other potential sources of bias
In the Colombo trial the characteristics of the two groups were not equal at baseline; the paper states that all five participants with oesophageal varices and 7 out of 8 participants with abnormal serum bilirubin levels at entry were allocated to the UDCA group (Colombo 1996).
Effects of interventions
The authors of the two six‐month trials have kindly provided us with raw data (personal communication) (Merli 1994; O'Brien 1992). Where possible we have entered quantitative data, but the use of complicated study designs has meant that we have had to use subsets of small sample sizes.
Primary outcomes
1. Reduction of raised hepatocellular enzymes to within normal range of the method stated
We wished to examine the effect of UDCA on abnormal liver biochemistry by comparing the numbers of participants in both groups whose liver enzymes fell to within the normal range of the method stated at various time points. This was not reported as an outcome measure in any of the three RCTs but, serving as a proxy for this, improvement in abnormalities of liver function was measured in all three RCTs. Raw data were available from two of the RCTs to enable us to examine this outcome (personal communication) (Merli 1994; O'Brien 1992). We assessed this outcome in three different ways: normalisation of any liver enzyme reported (odds ratio (OR) 0.09 (95% confidence interval (CI) 0.01 to 1.24)), normalisation of all liver enzymes reported (OR not estimable as there were no participants in either of the two trials with all enzymes normalised) and normalisation of individual liver enzymes (OR less than one for three out of four enzymes but the CIs were very wide). For aspartate transaminase the OR was greater than one, again with a wide CI.
2. Reduction of abnormally large livers as measured by ultrasound
The effect on liver size was not reported in any of the three RCTs.
3. Liver transplantation
Need for liver transplantation was not specifically used as an outcome measure in any of the RCTs. However, one trial reported that one participant, who initially had multilobular cirrhosis and oesophageal varices (advanced liver involvement) and was allocated to treatment with UDCA, was subsequently withdrawn due to further deterioration of liver function (Colombo 1996). This participant proceeded to liver transplantation. However, the confidence interval of the odds ratio generated was very wide and it was not possible to draw any conclusions about the effect of UDCA on the need for transplants.
Need for liver transplantation was reported as an outcome in the long‐term follow‐up data of Colombo (Colombo 1996). Six participants underwent liver transplantation. However, these long‐term data were reported as follow up for the whole cohort, not by randomised group. Therefore, it is not possible to draw any firm conclusions about the effect of UDCA therapy on the need for liver transplantation from these data.
None of the participants in the two six‐month trials required liver transplants (personal communication) (Merli 1994; O'Brien 1992).
Secondary outcomes
1. Mortality
Mortality was not reported in any of the three RCTs, but there were no deaths in the two six‐month trials (personal communication) (Merli 1994; O'Brien 1992).
Mortality was presented in the long‐term follow‐up data of the Colombo trial (Colombo 1996). There were 13 deaths; none of which were due to liver disease. However, these long‐term data were reported as follow up for the whole cohort, not by randomised group. Therefore, it is not possible to draw any firm conclusions about the effect of UDCA therapy on mortality from these data.
2. Change in weight
Nutritional indices were one of only two pre‐defined outcomes reported in the published reports. Weight gain was reported in only one of the RCTs (O'Brien 1992). However, measures of weight before and after six months' treatment were reported in another RCT (Merli 1994). Skinfold thickness was reported in two RCTs (Merli 1994; O'Brien 1992). Body mass percentile, which also takes into account the population mean weight and height rather than body mass index (weight in kilograms divided by height squared in metres), was reported in one trial (Merli 1994).
Using the raw data of weight measurements before and after treatment or control in the two six‐month trials, we calculated the weight change for each participant and then the mean and standard deviation for each trial (using again only 18 out of 51 participants in the cross‐over trial (Merli 1994)). The weighted mean difference was ‐0.90 kg (95%CI ‐1.94 to 0.14) (Merli 1994; O'Brien 1992).
3. Development of portal hypertension (raised pressure in the vein running through the liver) or its complications
These were not reported as outcome measures in any of the three RCTs. However, portal hypertension did not develop in any of the participants in the two six‐month follow‐up RCTs (personal communication) (Merli 1994; O'Brien 1992).
4. Improvement of biliary excretion
This outcome was reported in only one trial (O'Brien 1992). The original trial investigators that they had measured the time (in minutes) from injection of the isotope to maximal hepatic activity and the percentage clearance of isotope from the liver and biliary tree, at 45 and 60 minutes compared with maximal activity. No significant changes in biliary excretion occurred after treatment with UDCA.
Discussion
This first systematic review on the effectiveness of UDCA in CF highlights the paucity of RCTs. Disappointingly, the few RCTs carried out have not adequately examined our pre‐defined outcome measures but do provide important preliminary information which requires further evaluation. There was considerable variation in the outcome measures examined in the three RCTs and in the time points at which they were measured.
There have been no RCTs investigating UDCA for preventing the development of liver disease in people with CF. This review has shown the absence of any significant effects of UDCA treatment on people with CF, apart from a slight effect on the surrogate endpoint of reduction of raised liver enzymes to normal. It is difficult to draw any meaningful conclusions from these results taken from small numbers of participants. Several clinically meaningful outcomes, such as portal hypertension, liver transplantation and survival were not assessed. The information received from the authors of the six‐month trials showed that no participants died, needed liver transplants or developed portal hypertension (Merli 1994; O'Brien 1992). However, these are short‐term trials and there is insufficient evidence to show that UDCA improves survival or reduces the need for liver transplantation. We cannot be sure that those surrogate endpoints used actually correlate with these important endpoints. For example, there is no evidence of a clear correlation between the serum level of hepatocellular enzymes and the degree of liver disease.
We failed to show a significant effect of UDCA on weight change. However, change in weight is not the most appropriate way of assessing change in nutritional status in trials where it was included as an outcome. This is because we would expect children to gain weight over time but the weight of adults to remain stable. It would be more appropriate to use indices such as body mass index or weight for height as a z score (where weight is expressed as a percentage of ideal for height and then compared with the standard deviation for the population). The one trial that assessed effect on biliary excretion failed to demonstrate any significant change after treatment with UDCA (O'Brien 1992).
In this review we have included trials with a non‐homogenous population (Merli 1994) and have, therefore, considered both possible preventative and therapeutic effects of UDCA in the same review. As we cannot be sure how raised levels of certain liver enzymes correlate with liver involvement (or whether absence of raised enzymes indicates a lack of liver involvement), we decided that we would lose important information if this trial were excluded. A future update of this review may be able to address the preventative and therapeutic effects separately.
Although in our quantitative analysis we excluded data on participants who also received taurine, we will briefly mention individual trial results. The Merli trial showed that a six‐month period of UDCA with or without taurine did not significantly affect the nutritional status (Merli 1994), whilst Colombo failed to show an effect of UDCA on liver enzymes (Colombo 1996).
Although UDCA is relatively inexpensive compared to other CF treatments, it would need to be taken on a long‐term basis if it is effective. If it is ineffective then the resources saved by not using it could be used for other aspects of CF care.
We performed only a limited quantitative meta‐analysis due to the lack of data on clinically relevant endpoints and the different time points at which outcomes were measured. However, this systematic review provides an important summary of the information currently available from RCTs on the use of UDCA. This information may be used to inform the design of subsequent RCTs.
A further published trial is currently awaiting assessment (Lepage 1997). There is no information in the published report on methodological quality and the authors did not assess the outcomes assessed in this review. We have contacted the authors for more information, but have not yet received a response.
The most recent new trial identified by the search of the Group's trials register, is one which we knew was ongoing (Spray 2000). This randomised, double‐blind cross‐over trial of UDCA versus placebo recruited 21 participants and examined histological changes in CF‐related liver disease. The authors did not report the specific liver outcomes which we wished to examine in this systematic review (reduction of liver size as measured by ultrasound, development of portal hypertension or the complications of portal hypertension, need for liver transplantation) or mortality. We have contacted the authors and will include these data once they are available.
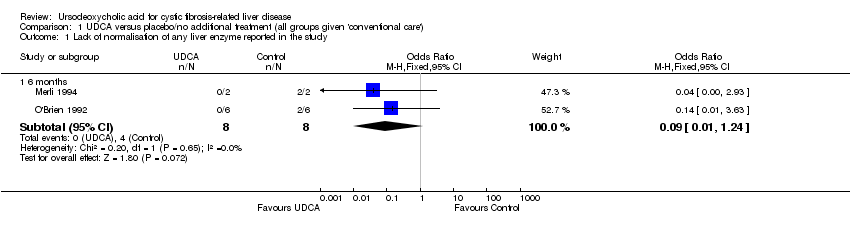
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 1 Lack of normalisation of any liver enzyme reported in the study.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 2 Lack of normalisation of all liver enzymes reported in the study.
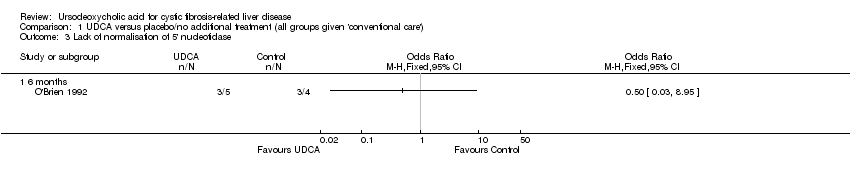
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 3 Lack of normalisation of 5' nucleotidase.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 4 Lack of normalisation of aspartate transaminase.
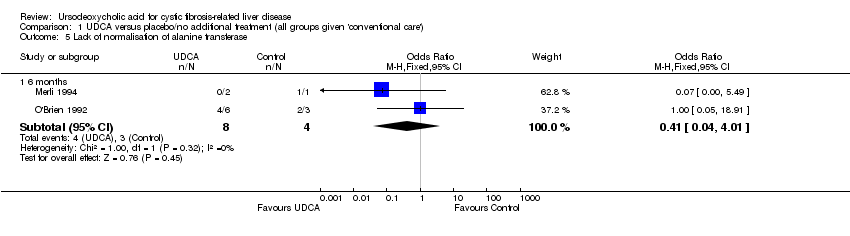
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 5 Lack of normalisation of alanine transferase.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 6 Lack of normalisation of gammaglutamate transferase.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 7 Need for liver transplantation.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 8 Death related to liver disease.
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 9 Death due to all causes.
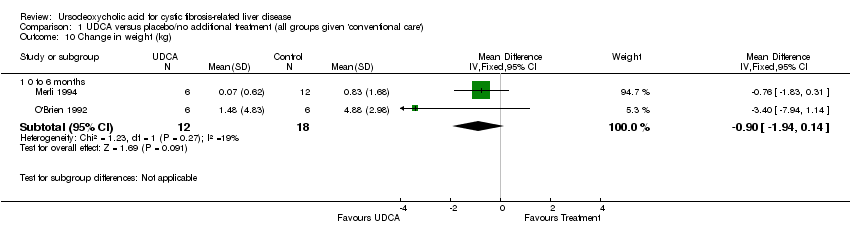
Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 10 Change in weight (kg).

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 11 Development of portal hypertension.

Comparison 1 UDCA versus placebo/no additional treatment (all groups given 'conventional care'), Outcome 12 Development of complications of portal hypertension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lack of normalisation of any liver enzyme reported in the study Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.24] |
| 2 Lack of normalisation of all liver enzymes reported in the study Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Lack of normalisation of 5' nucleotidase Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Lack of normalisation of aspartate transaminase Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.43, 284.30] |
| 5 Lack of normalisation of alanine transferase Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.04, 4.01] |
| 6 Lack of normalisation of gammaglutamate transferase Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 7 Need for liver transplantation Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 7 to 12 months | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.10, 74.63] |
| 8 Death related to liver disease Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 7 to 12 months | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Death due to all causes Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 7 to 12 months | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Change in weight (kg) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 0 to 6 months | 2 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.94, 0.14] |
| 11 Development of portal hypertension Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 7 to 12 months | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Development of complications of portal hypertension Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 0 to 6 months | 2 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 7 to 12 months | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

