小兒急性中耳炎之抗生素治療
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000219.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 23 junio 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Chris Del Mar (CDM) and Paul P Glasziou (PPG) prepared the original version of the review.
Sharon L. Sanders (SLS) conducted searches, identified studies, extracted data and prepared the manuscript for the updated reviews in 2003, 2007 and 2008.
Maroeska M. Rovers (MMR) participated in the 2007 update by providing data and information from the individual patient data meta‐analysis that has been included in this update.
Roderick P. Venekamp (RPV) conducted searches, identified studies, extracted data and prepared the manuscript for the updated review in 2012 and 2015.
PPG, CDM, MMR, SLS and RPV have reviewed and provided comments on the updated version of the review.
Declarations of interest
Chris Del Mar (CDM) declares no conflicts of interests in the current work.
Maroeska M. Rovers (MMR) has participated in workshops and educational activities on otitis media organised by GlaxoSmithKline and received a grant from GlaxoSmithKline for a study on the microbiology of otitis media in 2009.
Roderick P. Venekamp (RPV) is an Editor of the Cochrane Acute Respiratory Infections Group.
Sharon L Sanders (SLS) declares no conflicts of interests in the current work.
Paul P Glasziou (PPG) is co‐investigator on NHMRC funded grant Antibiotic Resistance.
Acknowledgements
We would like to thank Professor Charles Bridges‐Webb (deceased) for stimulating initial discussions and for constructive advice on the protocol for this review and Professor Steve Berman for helpful comments on the draft review. We would like to thank Bruce Arroll and Tom Fahey for peer refereeing the 2005 updated review and Dilip Raghavan, Brian Westerberg, Mark Jones and Peter Morris for peer refereeing the 2009 updated review. We also thank Dilip Raghavan, Brian Westerberg, Teresa Neeman and Peter Morris for commenting on the 2012 updated review and Ann Fonfa, Julie Gildie, Conor Teljeur, Bruce Arroll, Brian Westerberg and Peter Morris for peer refereeing the 2015 updated review.
Thank you to David McCormick and his colleagues for allowing us to access raw study data from the trial comparing immediate antibiotics and expectant observation (McCormick 2005).
Thank you to Chris Cates for noticing and advising us of an error in the confidence intervals presented in the review.
We gratefully thank Sarah Thorning for her support with the search strategy and searches.
Version history
| Published | Title | Stage | Authors | Version |
| 2023 Nov 15 | Antibiotics for acute otitis media in children | Review | Roderick P Venekamp, Sharon L Sanders, Paul P Glasziou, Maroeska M Rovers | |
| 2015 Jun 23 | Antibiotics for acute otitis media in children | Review | Roderick P Venekamp, Sharon L Sanders, Paul P Glasziou, Chris B Del Mar, Maroeska M Rovers | |
| 2013 Jan 31 | Antibiotics for acute otitis media in children | Review | Roderick P Venekamp, Sharon Sanders, Paul P Glasziou, Chris B Del Mar, Maroeska M Rovers | |
| 2004 Jan 26 | Antibiotics for acute otitis media in children | Review | Sharon Sanders, Paul P Glasziou, Chris B Del Mar, Maroeska M Rovers | |
| 2000 Apr 28 | Antibiotics for acute otitis media in children | Review | Paul Glasziou, M Hayem, B Del Mar C | |
Differences between protocol and review
In this 2015 updated review, we now provide outcome data on:
-
pain at 24 hours, two to three days, four to seven days and 10 to 14 days (in earlier versions outcome data on pain were presented at 24 hours, two to three days and four to seven days);
-
abnormal tympanometry findings at two to four weeks, six to eight weeks and three months (in earlier versions outcome data on abnormal tympanometry findings were presented at four to six weeks and three months);
-
long‐term effects including number of parent‐reported AOM‐symptom episodes, antibiotic prescriptions and health care utilisation as assessed at least one year after randomisation (in earlier versions no data on long‐term effects were presented).
The outcome 'Adverse effects likely to be related to the use of antibiotics such as vomiting, diarrhoea or rash' has been added to primary outcomes (in earlier versions this outcome was listed as a secondary outcome) according to the recommendations described in Chapter 5.4.2 of the Cochrane Handbook for Systematic Reviews of Interventions ("the primary outcomes should include at least one desirable and at least one undesirable outcome") (Higgins 2011).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Child, Preschool; Humans; Infant;
PICO
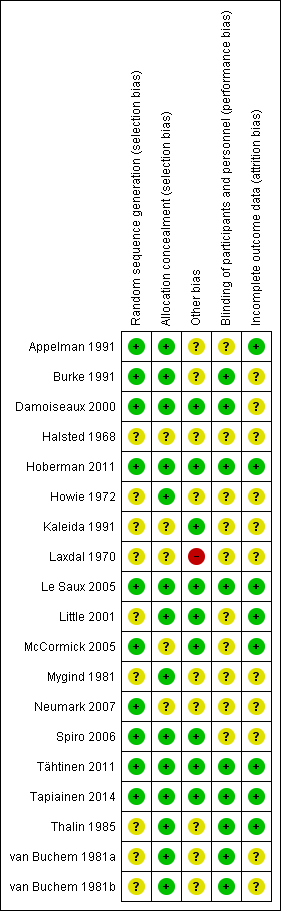
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
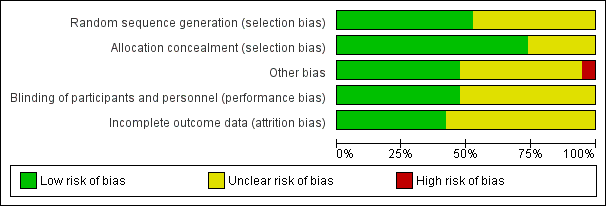
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

L'Abbé plot of the rates of pain at 24 hours for the placebo (control) versus antibiotic (experimental) group.
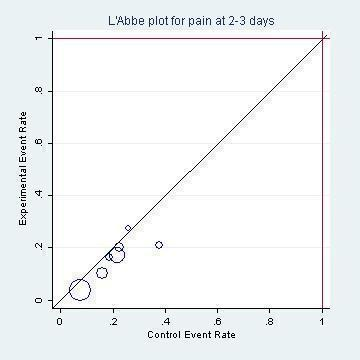
L'Abbé plot of the rates of pain at two to three days for the placebo (control) versus antibiotic (experimental) group.

Funnel plot of comparison: 1 Antibiotic versus placebo, outcome: 1.1 Pain.

Percentage with pain based on the subset of six studies included in the IPD meta‐analysis (Rovers 2006).
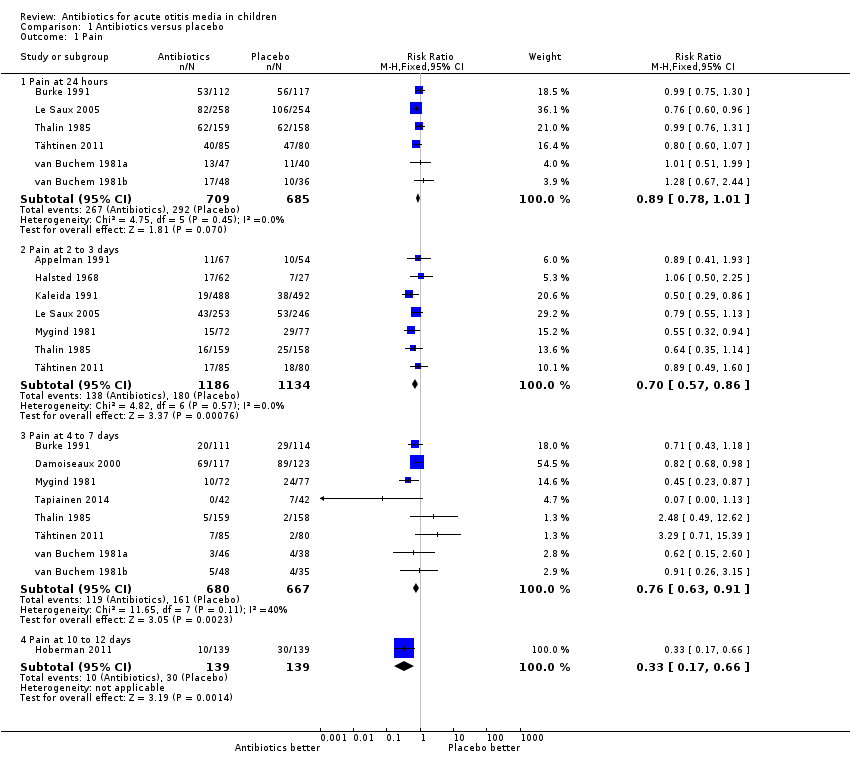
Comparison 1 Antibiotics versus placebo, Outcome 1 Pain.

Comparison 1 Antibiotics versus placebo, Outcome 2 Vomiting, diarrhoea or rash.

Comparison 1 Antibiotics versus placebo, Outcome 3 Abnormal tympanometry.
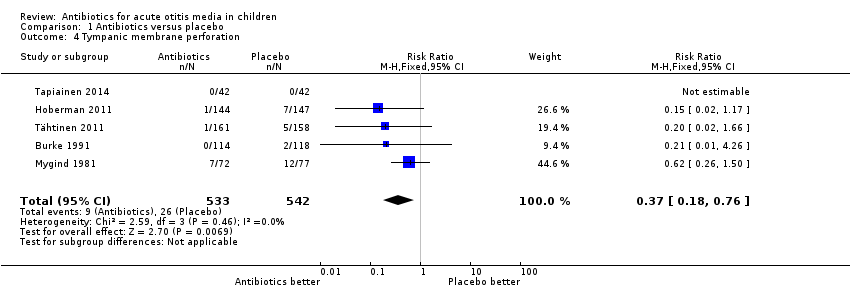
Comparison 1 Antibiotics versus placebo, Outcome 4 Tympanic membrane perforation.
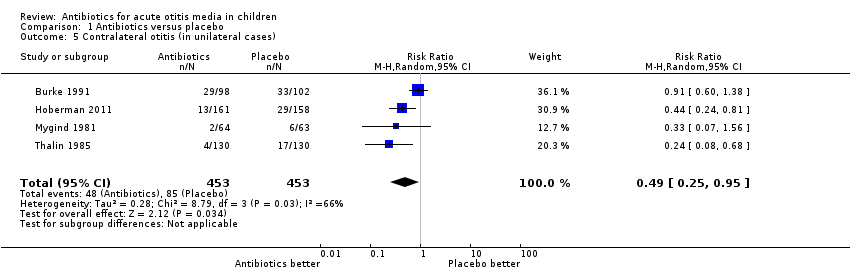
Comparison 1 Antibiotics versus placebo, Outcome 5 Contralateral otitis (in unilateral cases).
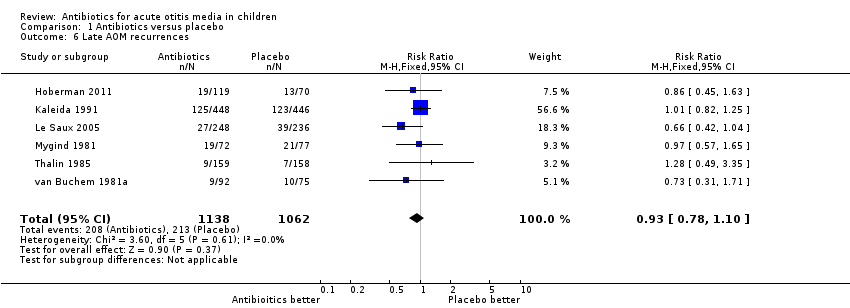
Comparison 1 Antibiotics versus placebo, Outcome 6 Late AOM recurrences.
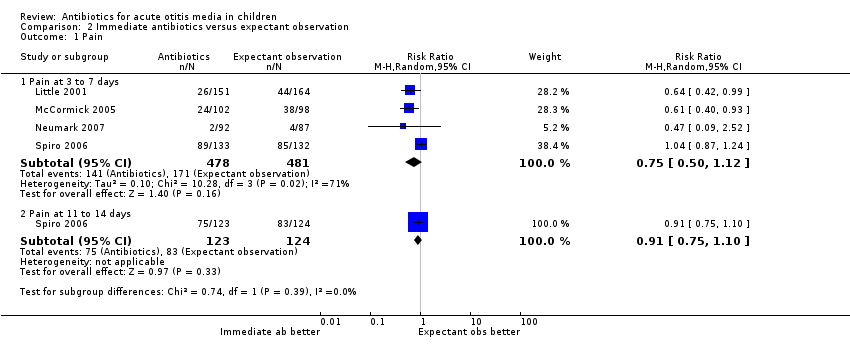
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 1 Pain.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 2 Vomiting, diarrhoea or rash.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 3 Abnormal tympanometry at 4 weeks.
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 4 Tympanic membrane perforation.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 5 AOM recurrences.
| Antibiotics versus placebo for acute otitis media in children | ||||||
| Patient or population: children with acute otitis media | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics versus placebo | |||||
| Pain ‐ pain at 24 hours | Study population | RR 0.89 | 1394 | ⊕⊕⊕⊕ | ||
| 426 per 1000 | 379 per 1000 | |||||
| Pain ‐ pain at 2 to 3 days | Study population | RR 0.70 | 2320 | ⊕⊕⊕⊕ | ||
| 159 per 1000 | 111 per 1000 | |||||
| Pain ‐ pain at 4 to 7 days | Study population | RR 0.76 | 1347 | ⊕⊕⊕⊕ | ||
| 241 per 1000 | 183 per 1000 | |||||
| Pain ‐ pain at 10 to 12 days | Study population | RR 0.33 | 278 | ⊕⊕⊕⊝ | ||
| 216 per 1000 | 71 per 1000 | |||||
| Abnormal tympanometry ‐ 2 to 4 weeks | Study population | RR 0.82 | 2138 | ⊕⊕⊕⊕ | ||
| 481 per 1000 | 395 per 1000 | |||||
| Abnormal tympanometry ‐ 3 months | Study population | RR 0.97 | 809 | ⊕⊕⊕⊕ | ||
| 241 per 1000 | 234 per 1000 | |||||
| Vomiting, diarrhoea or rash | Study population | RR 1.38 | 2107 | ⊕⊕⊕⊕ | ||
| 196 per 1000 | 270 per 1000 | |||||
| *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The number of studies reported in the 'Summary of findings' table for the outcomes 'Pain at 24 hours' and 'Pain at 4 to 7 days' differ slightly from those reported in the Data Analysis Table 1 ‐ Antibiotics versus placebo (five versus six studies and seven versus eight studies, respectively). This is due to the van Buchem trial. This trial is included as one study in our review (and in the 'Summary of findings' table), but we included data from two different comparisons from this 2 x 2 factorial design trial in our analyses (van Buchem 1981a; van Buchem 1981b). 2We downgraded the evidence for pain at days 10 to 12 from high quality as this outcome was not specified a priori in this trial (secondary analysis). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Pain at 24 hours | 6 | 1394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 1.2 Pain at 2 to 3 days | 7 | 2320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.86] |
| 1.3 Pain at 4 to 7 days | 8 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.63, 0.91] |
| 1.4 Pain at 10 to 12 days | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.17, 0.66] |
| 2 Vomiting, diarrhoea or rash Show forest plot | 8 | 2107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.19, 1.59] |
| 3 Abnormal tympanometry Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 2 to 4 weeks | 7 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 3.2 6 to 8 weeks | 3 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 3.3 3 months | 3 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 4 Tympanic membrane perforation Show forest plot | 5 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.76] |
| 5 Contralateral otitis (in unilateral cases) Show forest plot | 4 | 906 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.95] |
| 6 Late AOM recurrences Show forest plot | 6 | 2200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Pain at 3 to 7 days | 4 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.50, 1.12] |
| 1.2 Pain at 11 to 14 days | 1 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 2 Vomiting, diarrhoea or rash Show forest plot | 2 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.24, 2.36] |
| 3 Abnormal tympanometry at 4 weeks Show forest plot | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.35] |
| 4 Tympanic membrane perforation Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 AOM recurrences Show forest plot | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.74, 2.69] |

