نقش آنتیبیوتیکها در درمان اوتیت میانی حاد در کودکان
Appendices
Appendix 1. Previous search
Several electronic databases were used to compile relevant published RCTs of antibiotic treatment of AOM in children. The Cochrane Controlled Trials Register, MEDLINE and Current Contents were searched from 1966 to January 2000 by an expert librarian in conjunction with one researcher, using combinations of "OTITIS MEDIA" and a search strategy described by (Dickersin 1994) for optimally identifying controlled trials. In addition, titles in Index Medicus were checked from 1958 to 1965. The references of all relevant retrieved trials were checked to identify other articles.
The search was updated in March 2003, and again in July 2008. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2008, Issue 2), which contains the ARI Group's Specialized Register; MEDLINE (1966 to June week 4 2008); OLDMEDLINE (1958 to 1965); EMBASE (January 1990 to July 2008); and Current Contents (1966 to July 2008). The bibliographies of relevant articles were checked. A forward search of relevant articles was conducted in Web of Science®.
The following search strategy was run on MEDLINE (Ovid) combined with terms from Phase 1 and 2 of the Cochrane highly sensitive search strategy for identifying reports of RCTs (Lefebvre 2011). Modified terms were used to search the other databases:
MEDLINE (Ovid)
#1 exp Otitis Media/
#2 exp Otitis Media with Effusion/
#3 exp Otitis Media, Suppurative/
#4 glue ear.mp.
#5 otitis media.mp.
#6 OME.mp.
#7 AOM.mp.
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 exp Anti‐Bacterial Agents/
#10 exp Drug Therapy/
#11 exp Anti‐Infective Agents/
#12 antibiotic$.mp.
#13 #9 or #10 or #11 or #12
#14 #8 and #13
There were no language or publication restrictions.
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Otitis Media/
2 otitis media.tw.
3 glue ear*.tw.
4 (middle ear adj5 (infect* or inflam*)).tw.
5 (ome or aom).tw.
6 or/1‐5
7 exp Anti‐Bacterial Agents/
8 Drug Therapy/
9 Anti‐Infective Agents/
10 antibiotic*.tw.
11 antibacterial*.tw.
12 exp Ampicillin/
13 exp Cephalosporins/
14 exp Macrolides/
15 exp Penicillins/
16 (ampicillin* or cephalosporin* or macrolide* or penicillin* or amoxicillin* or amoxycillin* or cefdinir or cefpodoxime or cefuroxime or azithromycin or clarithromycin or erythromycin*).tw,nm.
17 or/7‐16
18 6 and 17
Appendix 3. Embase.com search strategy
18 #14 AND #17
17 #15 OR #16
16 random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti
15 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp
14 #4 AND #13
13 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12
12 ampicillin*:ab,ti OR cephalosporin*:ab,ti OR macrolide*:ab,ti OR penicillin*:ab,ti OR amoxycillin*:ab,ti OR amoxicillin*:ab,ti OR cefdinir*:ab,ti OR cefpodoxime*:ab,ti OR cefuroxime*:ab,ti OR
azithromycin*:ab,ti OR clarithromycin*:ab,ti OR erythromycin*:ab,ti
11 'penicillin g'/exp
10 'macrolide'/exp
9 'cephalosporin derivative'/exp
8 'ampicillin'/exp
7 antibiotic*:ab,ti OR antibacterial*:ab,ti
6 'drug therapy'/de OR 'antiinfective agent'/de
5 'antibiotic agent'/exp
4 #1 OR #2 OR #3
3 ('middle ear' NEAR/5 (inflam* OR infect*)):ab,ti
2 'otitis media':ab,ti OR 'glue ear':ab,ti OR 'glue ears':ab,ti OR ome:ab,ti OR aom:ab,ti
1 'otitis media'/exp
Appendix 4. Current Contents search strategy
| # 3 | #2 AND #1 Databases=CM, LS Timespan=All Years Lemmatization=On | |
|
| ||
| # 2 | Topic=(random* or placebo* or crossover* or "cross over" or allocat* or ((doubl* or singl*) NEAR/1 blind*)) OR Title=(trial) Databases=CM, LS Timespan=All Years Lemmatization=On | |
|
| ||
| # 1 | Topic=(otitis or "glue ear" or ("middle ear" NEAR/3 (infect* or inflam*)) or ome or aom) AND Topic=(antibiotic* or antibacterial* or antiinfective* or ampicillin* or cephalosporin* or macrolide* or amoxicillin* or amoxycillin* or penicillin* or cefdinir* or cefpodoxime* or cefuroxime* or azithromycin* or clarithromycin* or erythromycin*) Databases=CM, LS Timespan=All Years Lemmatization=On | |
Appendix 5. CINAHL search strategy
S30 S19 and S29
S29 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28
S28 (MH "Quantitative Studies")
S27 TI placebo* or AB placebo*
S26 (MH "Placebos")
S25 TI random* or AB random*
S24 (MH "Random Assignment")
S23 TI (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) or AB (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*)
S22 TI clinic* N1 trial* or AB clinic* N1 trial*
S21 PT clinical trial
S20 (MH "Clinical Trials+")
S19 S7 and S18
S18 S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17
S17 TI ( ampicillin* or cephalosporin* or macrolide* or amoxicillin* or amoxycillin* or penicillin* or cefdinir* or cefpodoxime* or cefuroxime* or azithromycin* or clarithromycin* or erythromycin* ) or AB ( ampicillin* or cephalosporin* or macrolide* or amoxicillin* or amoxycillin* or penicillin* or cefdinir* or cefpodoxime* or cefuroxime* or azithromycin* or clarithromycin* or erythromycin* )
S16 (MH "Penicillins+")
S15 (MH "Antibiotics, Macrolide+")
S14 (MH "Cephalosporins+")
S13 (MH "Ampicillin+")
S12 TI antibacterial* or AB antibacterial*
S11 TI antibiotic* or AB antibiotic*
S10 (MH "Antiinfective Agents")
S9 (MH "Drug Therapy")
S8 (MH "Antibiotics+")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 TI ( aom or ome ) or AB ( aom or ome )
S5 TI middle ear inflam* or AB middle ear inflam*
S4 TI middle ear infect* or AB middle ear infect*
S3 AB glue ear* or TI glue ear*
S2 TI otitis media or AB otitis media
S1 (MH "Otitis Media+")
Appendix 6. LILACS search strategy
> Search > (MH:"otitis media" OR "otitis media" OR "Otite Média" OR MH:C09.218.705.633$) AND (MH:"Anti‐Bacterial Agents" OR antibiotic$ OR Antibacterianos OR Antibióticos OR MH:"Drug Therapy" OR Quimioterapia OR "Terapia por Drogas" OR Farmacoterapia OR MH:"Anti‐Infective Agents" OR Antiinfecciosos OR MH:ampicillin OR Ampicilina OR ampicillin$ OR MH:D02.065.589.099.750.750.050$ OR MH:D02.886.108.750.750.050$ OR MH:D03.438.460.825.750.050$ OR MH:D03.605.084.737.750.050$ OR D04.075.080.875.099.221.750.750.050$ OR MH:cephalosporins OR cephalosporin$ OR Cefalosporinas OR MH:D02.065.589.099.249$ OR D02.886.665.074$ OR D04.075.080.875.099.221.249$ OR MH:macrolides OR macrolide$ OR Macrólidos OR Macrolídeos OR D02.540.505$ OR D02.540.576.500$ OR D04.345.674.500$ OR MH:penicillins OR penicillin$ OR Penicilinas OR MH:D02.065.589.099.750$ OR D02.886.108.750$ OR D03.438.260.825$ OR D03.605.084.737$ OR D04.075.080.875.099.221.750$ OR amoxicillin$ OR Amoxicilina OR cefdinir OR cefpodoxim$ OR cefuroxim$ OR azithromycin$ OR Azitromicina OR clarithromycin$ OR Claritromicina OR erythromycin OR Eritromicina) > clinical_trials
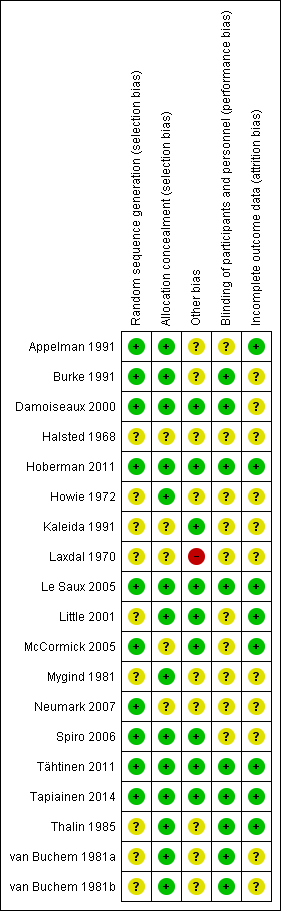
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
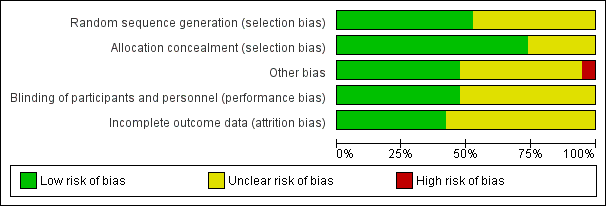
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

L'Abbé plot of the rates of pain at 24 hours for the placebo (control) versus antibiotic (experimental) group.
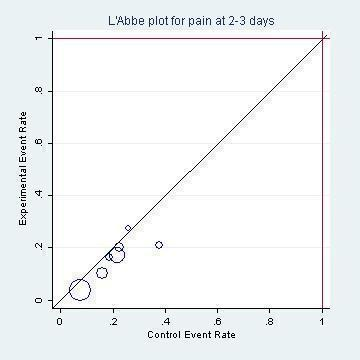
L'Abbé plot of the rates of pain at two to three days for the placebo (control) versus antibiotic (experimental) group.

Funnel plot of comparison: 1 Antibiotic versus placebo, outcome: 1.1 Pain.

Percentage with pain based on the subset of six studies included in the IPD meta‐analysis (Rovers 2006).
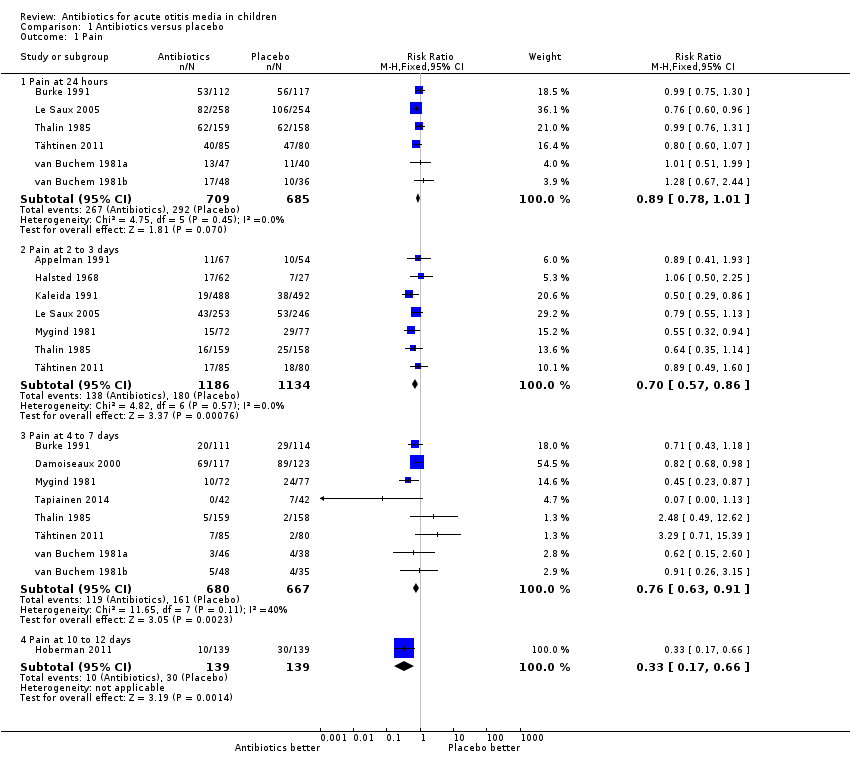
Comparison 1 Antibiotics versus placebo, Outcome 1 Pain.

Comparison 1 Antibiotics versus placebo, Outcome 2 Vomiting, diarrhoea or rash.

Comparison 1 Antibiotics versus placebo, Outcome 3 Abnormal tympanometry.
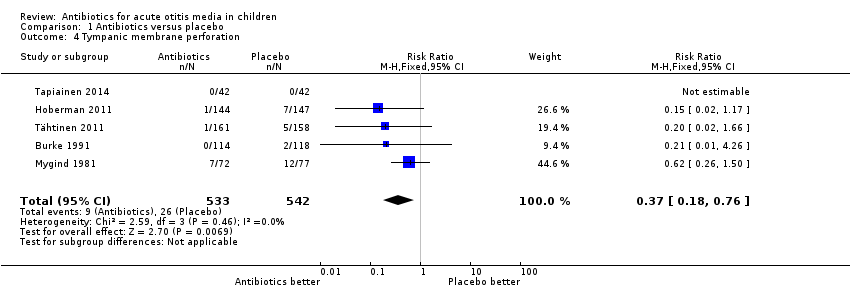
Comparison 1 Antibiotics versus placebo, Outcome 4 Tympanic membrane perforation.
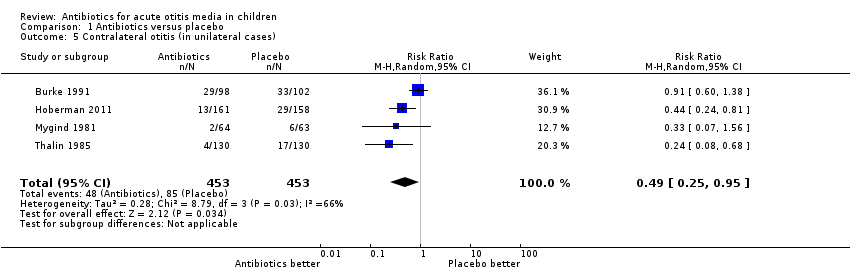
Comparison 1 Antibiotics versus placebo, Outcome 5 Contralateral otitis (in unilateral cases).
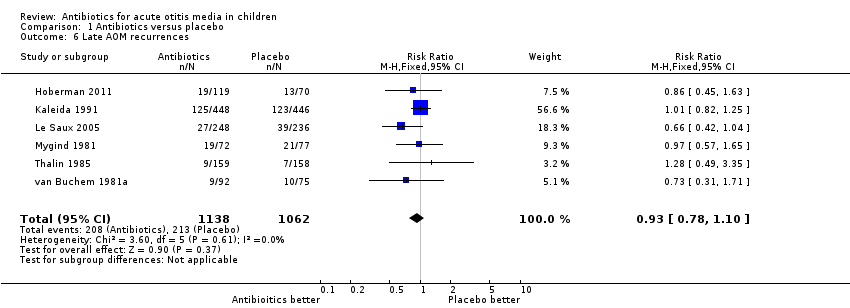
Comparison 1 Antibiotics versus placebo, Outcome 6 Late AOM recurrences.
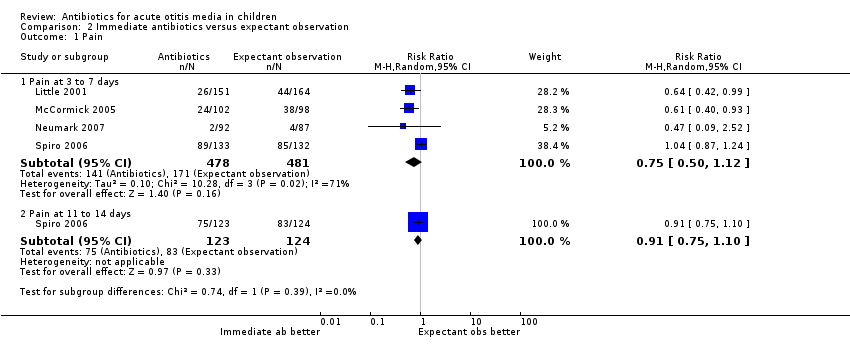
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 1 Pain.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 2 Vomiting, diarrhoea or rash.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 3 Abnormal tympanometry at 4 weeks.
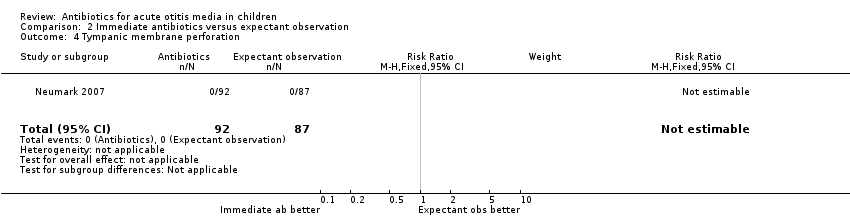
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 4 Tympanic membrane perforation.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 5 AOM recurrences.
| Antibiotics versus placebo for acute otitis media in children | ||||||
| Patient or population: children with acute otitis media | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics versus placebo | |||||
| Pain ‐ pain at 24 hours | Study population | RR 0.89 | 1394 | ⊕⊕⊕⊕ | ||
| 426 per 1000 | 379 per 1000 | |||||
| Pain ‐ pain at 2 to 3 days | Study population | RR 0.70 | 2320 | ⊕⊕⊕⊕ | ||
| 159 per 1000 | 111 per 1000 | |||||
| Pain ‐ pain at 4 to 7 days | Study population | RR 0.76 | 1347 | ⊕⊕⊕⊕ | ||
| 241 per 1000 | 183 per 1000 | |||||
| Pain ‐ pain at 10 to 12 days | Study population | RR 0.33 | 278 | ⊕⊕⊕⊝ | ||
| 216 per 1000 | 71 per 1000 | |||||
| Abnormal tympanometry ‐ 2 to 4 weeks | Study population | RR 0.82 | 2138 | ⊕⊕⊕⊕ | ||
| 481 per 1000 | 395 per 1000 | |||||
| Abnormal tympanometry ‐ 3 months | Study population | RR 0.97 | 809 | ⊕⊕⊕⊕ | ||
| 241 per 1000 | 234 per 1000 | |||||
| Vomiting, diarrhoea or rash | Study population | RR 1.38 | 2107 | ⊕⊕⊕⊕ | ||
| 196 per 1000 | 270 per 1000 | |||||
| *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The number of studies reported in the 'Summary of findings' table for the outcomes 'Pain at 24 hours' and 'Pain at 4 to 7 days' differ slightly from those reported in the Data Analysis Table 1 ‐ Antibiotics versus placebo (five versus six studies and seven versus eight studies, respectively). This is due to the van Buchem trial. This trial is included as one study in our review (and in the 'Summary of findings' table), but we included data from two different comparisons from this 2 x 2 factorial design trial in our analyses (van Buchem 1981a; van Buchem 1981b). 2We downgraded the evidence for pain at days 10 to 12 from high quality as this outcome was not specified a priori in this trial (secondary analysis). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Pain at 24 hours | 6 | 1394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 1.2 Pain at 2 to 3 days | 7 | 2320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.86] |
| 1.3 Pain at 4 to 7 days | 8 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.63, 0.91] |
| 1.4 Pain at 10 to 12 days | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.17, 0.66] |
| 2 Vomiting, diarrhoea or rash Show forest plot | 8 | 2107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.19, 1.59] |
| 3 Abnormal tympanometry Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 2 to 4 weeks | 7 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 3.2 6 to 8 weeks | 3 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 3.3 3 months | 3 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 4 Tympanic membrane perforation Show forest plot | 5 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.76] |
| 5 Contralateral otitis (in unilateral cases) Show forest plot | 4 | 906 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.95] |
| 6 Late AOM recurrences Show forest plot | 6 | 2200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Pain at 3 to 7 days | 4 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.50, 1.12] |
| 1.2 Pain at 11 to 14 days | 1 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 2 Vomiting, diarrhoea or rash Show forest plot | 2 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.24, 2.36] |
| 3 Abnormal tympanometry at 4 weeks Show forest plot | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.35] |
| 4 Tympanic membrane perforation Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 AOM recurrences Show forest plot | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.74, 2.69] |

