پونکسیون کمری یا بطنی مکرر در نوزادان مبتلا به هموراژی داخل بطنی
چکیده
پیشینه
اگرچه در سالهای اخیر، درصد نوزادان پرهترم مبتلا به هموراژی داخل بطنی (intraventricular haemorrhage; IVH) کاهش یافته، هیدروسفالی بعد از خونریزی (posthaemorrhagic hydrocephalus; PHH) به عنوان یک مشکل جدی همراه با نرخ بالای فلج مغزی باقی مانده و درمان مبتنی بر شواهد نیز ندارد. بازماندگان اغلب باید تحت جراحی شانت بطنی صفاقی (ventriculoperitoneal shunt) قرار بگیرند که کودک را به طور دائم به یک سیستم دریچه (valve) و کاتتر (catheter) وابسته میسازد. این امر، خطر قابل توجه عفونت و نیاز به جراحی تعویض شانت را به دنبال دارد. خارج کردن مکرر مایع مغزی نخانی (cerebrospinal fluid; CSF) از طریق پونکسیون کمری (lumbar puncture)، پونکسیون بطنی (ventricular puncture)، یا از یک مخزن بطنی در نوزادان نارس مبتلا به IVH، به عنوان درمانی برای کاهش خطر ایجاد PHH پیشنهاد شده است.
اهداف
تعیین تاثیر خارج کردن مایع مغزی نخاعی (CSF) مکرر (از راه پونکسیون کمری/بطنی یا برداشت از یک مخزن بطنی) در مقایسه با مدیریت محافظهکارانه که طی آن خارج کردن CSF محدود به زمانی است که علائم افزایش فشار داخل جمجمهای (intracranial pressure; ICP) وجود داشته باشد، بر کاهش خطر وابستگی به شانت دائمی، ناتوانی تکامل سیستم عصبی و مرگومیر در نوزادان مبتلا یا در معرض خطر ابتلا به هیدروسفالی بعد از خونریزی.
روشهای جستوجو
برای جستوجو در پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL؛ شماره 3، 2016)؛ MEDLINE via PubMed (از 1966 تا 24 مارچ 2016)؛ Embase (از 1980 تا 24 مارچ 2016) و CINAHL (از 1982 تا 24 مارچ 2016)، از استراتژی جستوجوی استاندارد گروه نوزادان در کاکرین استفاده کردیم. همچنین بانکهای اطلاعاتی کارآزماییهای بالینی، خلاصه مقالات کنفرانسها و فهرست منابع مقالات بازیابی شده را برای یافتن کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) و شبه‐RCTها جستوجو کردیم.
معیارهای انتخاب
RCTها و شبه‐RCTهایی که خارج کردن مکرر CSF (از طریق پونکسیون کمری، پونکسیون بطنی یا برداشت از یک مخزن بطنی) را با مدیریت محافظهکارانه (خروج CSF فقط در هنگام وجود نشانههای افزایش ICP) مقایسه کرده باشند. همچنین کارآزماییها باید درباره حداقل یکی از پیامدهای مشخص شده مرگومیر، ناتوانی یا جاگذاری شانت گزارش داشته باشند.
گردآوری و تجزیهوتحلیل دادهها
جزئیات انتخاب شرکتکننده، تخصیص شرکتکننده و مداخلات را استخراج کردیم. ما پیامدهای زیر را ارزیابی کردیم: VPS، مرگومیر، مرگومیر یا شانت، ناتوانی، ناتوانی چند‐گانه، مرگومیر یا ناتوانی و عفونت CSF. کیفیت شواهد را با استفاده از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) ارزیابی کردیم.
نتایج اصلی
چهار کارآزمایی (پنج مقاله)، معیارهای ورود به این مرور را داشتند؛ سه مورد RCT و یک مورد شبه‐RCT بودند؛ در مجموع شامل 280 شرکتکننده تحت درمان در بخش مراقبتهای ویژه نوزادان در انگلستان بودند. این کارآزماییها بین سالهای 1980 و 1990 منتشر شده بودند. این مطالعات با توجه به سوال پژوهشی و مداخلاتی که بررسی کرده بودند به اندازه کافی مشابه بودند؛ تا بتوان کارآزماییها را جهت ارزیابی تاثیر این مداخله با یکدیگر ترکیب کرد.
متاآنالیز نشان داد که این مداخله، هیچ تفاوت معناداری در مقایسه با مدیریت محافظهکارانه برای این پیامدها نداشته است: جاگذاری شانت هیدروسفالی (خطر نسبی (RR) معمولی: 0.96؛ 95% فاصله اطمینان (CI): 0.73 تا 1.26؛ 3 کارآزمایی؛ 233 نوزاد؛ I² = 0%؛ شواهد با کیفیت متوسط)، مرگومیر (RR: 0.88؛ 95% CI؛ 0.53 تا 1.44؛ 4 کارآزمایی؛ 280 نوزاد؛ I² = 0%؛ شواهد با کیفیت پائین)، ناتوانی عمده در بازماندگان (RR: 0.98؛ 95% CI؛ 0.81 تا 1.18؛ 2 کارآزمایی؛ 141 نوزاد؛ I² = 11%؛ شواهد با کیفیت بالا)، ناتوانی متعدد در بازماندگان (RR: 0.9؛ 95% CI؛ 066 تا 1.24؛ 2 کارآزمایی؛ 141 نوزاد؛ I² = 0%؛ شواهد با کیفیت بالا)، مرگومیر یا ناتوانی (RR: 0.99؛ 95% CI؛ 0.86 تا 1.14؛ 2 کارآزمایی؛ 180 نوزاد؛ I² = 0%؛ شواهد با کیفیت بالا)، مرگومیر یا شانت (RR: 0.91؛ 95% CI؛ 0.75 تا 1.11؛ 3 کارآزمایی؛ 233 نوزاد؛ I² = 0%؛ شواهد با کیفیت متوسط) و عفونت پیش از جراحی CSF (RR: 1.73؛ 95% CI؛ 0.53 تا 5.67؛ 2 کارآزمایی؛ 195 نوزاد؛ شواهد با کیفیت پائین).
کیفیت شواهد را برای پیامدهای ناتوانی عمده، ناتوانی چند‐گانه و ناتوانی یا مرگومیر، بالا ارزیابی کردیم. شواهد مربوط به پیامدهای جاگذاری شانت و مرگومیر یا جاگذاری شانت را دارای کیفیت متوسط رتبهبندی کردیم؛ زیرا یک کارآزمایی وارد شده از روش تصادفیسازی تناوبی استفاده کرده بود. کیفیت شواهد برای پیامدهای مرگومیر و عفونت پیش از جراحی CSF پائین بود؛ چراکه یک کارآزمایی، از شیوه تناوب بهره برده بود، تعداد شرکتکنندگان برای ارزیابی اهداف با دقت کافی، بسیار کم بود و با توجه به یافتهها در کارآزماییهای وارد شده درباره پیامد عفونت CSF پیش از جراحی، ناهمگونی وجود داشت.
نتیجهگیریهای نویسندگان
شواهدی وجود نداشت که نشان دهد برداشت مکرر CSF از طریق پونکسیون کمری، پونکسیون بطنی یا از یک مخزن بطنی، نسبت به مدیریت محافظهکارانه در نوزادان مبتلا یا در معرض خطر ابتلا به PHH از نظر کاهش ناتوانی، مرگومیر یا نیاز به جاگذاری یک شانت دائمی، برتری دارد.
PICO
خلاصه به زبان ساده
ضربههای مکرر کمری یا بطنی در نوزادان مبتلا به هموراژی داخل بطنی
سوال مطالعه مروری
محققان کاکرین، شواهدی را درباره تاثیر خارج کردن مایع مغزی نخاعی (cerebrospinal fluid; CSF) از راه پونکسیون کمری یا بطنی و تخلیه CSF از طریق یک سوزن که در ستون فقرات یا درون یک حفره پر از مایع در مغز وارد شده مرور کردند تا بهبود نرخ ناتوانی، مرگومیر و نیاز به یک پروسیجر جراحی دائمی را در نوزادان پرهترمی که مبتلا به خونریزی در حفرههای مغز شدهاند (هموراژی داخل بطنی (intraventricular haemorrhage; IVH)، ارزیابی کنند.
پیشینه
کودکانی که نارس به دنیا میآیند، در معرض خطر ابتلا به IVH قرار دارند. این عارضه میتواند موجب تولید CSF اضافی در مغز شود. ممکن است خطر این اتفاق به وسیله خارج کردن خون در CSF از طریق پونکسیونهای کمری یا بطنی کاهش یابد. این موضوع میتواند نیاز به یک پروسیجر جراحی دائمی را به نام شانت بطنی صفاقی (ventriculoperitoneal shunt; VPS) کاهش دهد. VPS مشکلساز است؛ زیرا میتواند به سادگی عفونی شده و اغلب باید جایگزین یا ترمیم شود که خود نیاز به جراحی دارد.
ویژگیهای مطالعه
ما تا 24 مارچ 2016 به جستوجوی کارآزماییهایی پرداختیم که برداشت CSF را از طریق پونکسیونهای کمری یا بطنی در تمام کودکان در معرض خطر ابتلا به افزایش تدریجی مایع در مغز در برابر یک رویکرد محافظهکارانه مقایسه کرده بودند و البته این کار فقط در صورتی انجام میگیرد که شواهدی از فشار زیاد در مغز بر اثر افزایش تدریجی مایع وجود داشته باشد. چهار کارآزمایی را وارد کردیم که در مجموع شامل 280 نوزاد پرهترم تحت درمان در بخش مراقبتهای ویژه نوزادان در انگلستان بود. این کارآزماییها بین سالهای 1980 و 1990 منتشر شده بودند.
نتایج کلیدی
ما هیچ شواهدی نیافتیم که نشان دهد برداشت CSF از طریق پونکسیونهای کمری یا بطنی، نیاز به جاگذاری یک شانت ثابت را کاهش میدهد. همچنین شواهدی وجود نداشت که نشان دهد این عمل، خطر ناتوانی عمده، ناتوانی چند‐گانه یا مرگومیر را کاهش میدهد. شواهد کافی برای تعیین اینکه این رویکرد میتواند منجر به افزایش خطر ابتلا به عفونت در CSF شود یا خیر، وجود نداشت.
کیفیت شواهد
کیفیت شواهد مربوط به پیامدهای ناتوانی عمده، ناتوانی چند‐گانه و ناتوانی یا مرگومیر را بالا ارزیابی کردیم.
کیفیت شواهد مربوط به پیامدهای جاگذاری شانت و مرگومیر یا جاگذاری شانت را پائین رکورد کردیم؛ چراکه مشکلی با شیوه تخصیص تصادفی در یک کارآزمایی که راجع به این پیامد گزارش کرده بود، وجود داشت.
کیفیت شواهد برای پیامدهای مرگومیر و عفونت CSF پیش از جراحی، به دلیل همان مشکل ذکر شده (مشکل مربوط به تخصیص تصادفی)، متوسط بود. به علاوه، این مطالعات دارای بیماران کافی برای پاسخ مناسب به این پرسش نبودند. نتایج در مورد پیامد عفونت CSF پیش از جراحی، بین کارآزماییهای وارد شده ناهمگون بود.
Authors' conclusions
Summary of findings
| Repeated lumbar or ventricular punctures compared to conservative management for infants with intraventricular haemorrhage (IVH) | |||||
| Population: preterm infants less than three months of age with either: a) IVH demonstrated by ultrasound or computed tomography (CT) scan; or b) infants with IVH followed by progressive ventricular dilatation. Settings: neonatal intensive care units. Intervention: serial lumbar puncture, ventricular puncture, or tapping from a subcutaneous reservoir. Comparison: conservative management. | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants | Quality of the evidence | |
| Risk with conservative treatment | Risk with serial lumbar or ventricular punctures | ||||
| Hydrocephalus shunt | Study population | RR 0.96 | 233 | ⊕⊕⊕⊝ | |
| 469 per 1000 | 450 per 1000 | ||||
| Death | Study population | RR 0.88 | 280 | ⊕⊕⊝⊝ | |
| 199 per 1000 | 175 per 1000 | ||||
| Major disability in survivors | Study population | RR 0.98 | 141 | ⊕⊕⊕⊕ | |
| 761 per 1000 | 746 per 1000 | ||||
| Multiple disability in survivors | Study population | RR 0.90 | 141 | ⊕⊕⊕⊕ | |
| 537 per 1000 | 484 per 1000 | ||||
| Death or disability | Study population | RR 0.99 | 180 | ⊕⊕⊕⊕ | |
| 814 per 1000 | 806 per 1000 | ||||
| Death or shunt | Study population | RR 0.91 | 233 | ⊕⊕⊕⊝ | |
| 646 per 1000 | 588 per 1000 | ||||
| Infection of CSF presurgery | Study population | RR 1.73 | 195 | ⊕⊕⊝⊝ | |
| 43 per 1000 | 74 per 1000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 as Mantovani 1980 used an alternation method for random sequence generation. | |||||
Background
Description of the condition
Although many interventions can reduce the risk of intraventricular haemorrhage (IVH), it is still a common consequence of preterm birth. Posthaemorrhagic hydrocephalus (PHH) is the most serious complication of IVH.
PHH is thought to result from the deposition of extracellular matrix proteins, such as fibronectin and laminin, in the channels necessary for circulation and reabsorption of cerebrospinal fluid (CSF) (Cherian 2004). Ventriculoperitoneal shunt (VPS) surgery is the conventional approach for treatment of established hydrocephalus. However, treatment of PHH is more difficult than other types of hydrocephalus because the large amount of blood in the ventricles, combined with the small size and instability of the patient, make an early VPS operation a very high risk procedure. In one series of 19 infants with PHH requiring shunt surgery, there were 29 shunt blockages and 12 infections (Hislop 1988). The risk of shunt blockage was increased if the CSF protein was over 1.5 g/L at shunt insertion. In a series of 36 infants shunt‐operated for PHH, shunt blockage and infection occurred only in those operated on before 35 days of age (Taylor 2001). Repeated shunt revisions and infection are associated with worsening of neurological outcome (Tuli 2003). Furthermore, these infants are nearly always shunt‐dependent for the rest of their lives and will require several later operations even if no other problems occur. Thus it would be advantageous if a treatment could reduce the risk of permanent hydrocephalus after established IVH.
Neurodevelopmental outcome is poor in infants with PHH. Although this is due in part to parenchymal brain lesions present before PHH developed, it is likely that some of the dysfunction is the result of prolonged periods with raised intracranial pressure (ICP) with periventricular oedema and distortion of the developing axonal pathways and their myelination. It is also likely that some of the dysfunction is the result of injury from free radicals and inflammation as free iron, a source of free radicals, has been demonstrated in the CSF of infants with PHH (Savman 2001), as have pro‐inflammatory cytokines (Sävman 2002).
A treatment for PHH remains elusive. A Cochrane Review that assessed drug treatment for reduction of CSF production (acetazolamide and furosemide) found no evidence of benefit (Whitelaw 2001b).
The Drainage, Irrigation and Fibrinolytic therapy (DRIFT) trial was stopped before completion due to an increase in secondary intraventricular bleeding in participants. However, DRIFT reduced severe cognitive disability, severe disability, and overall death in survivors (Whitelaw 2010). A 10‐year follow‐up study is currently in place.
There is now a separate Cochrane Review on intraventricular streptokinase after IVH (Whitelaw 2007). The available evidence suggests that fibrinolytic intervention relatively late (two to four weeks after the IVH) is ineffective.
Further randomised control trials (RCTs) are needed to evaluate the timing of external ventricular drainage, as a retrospective review has suggested that early (less than 25 days) placement is associated with improved cognition (Bassan 2012). Endoscopic choroid plexus cauterisation and ventriculosubgaleal shunt insertion also require investigation as potential treatments.
Description of the intervention
Lumbar punctures (also known as a spinal tap) remove CSF via insertion of a needle into the lower back to drain CSF from the lumbar cistern. In a ventricular puncture the CSF is externally drained directly from the lateral ventricles via a needle. If a ventricular reservoir has been placed (this consists of a catheter leading to the lateral ventricle attached to a reservoir implanted under the scalp), CSF can also be tapped directly from the reservoir without the need for a direct ventricular puncture.
A ventricular or lumbar puncture is indicated in the context of PHH if there is evidence of significant raised ICP. Evidence may include: direct measurement of a CSF pressure over 12 mmHg, decreasing diastolic velocities on cerebral artery Doppler waveforms, deteriorating sensory evoked potentials, and clinical signs of raised ICP. Recently amplitude integrated electroencephalography (aEEG) has been identified as another method that may indicate that increasing pressure is affecting the functioning of the brain. The aEEG trace is described as becoming more discontinuous, followed by a return to a normal pattern after drainage (Olischar 2009; Klebermass‐Schrehof 2013).
In this Cochrane Review we assessed the use of serial lumbar ventricular punctures in infants with, or at risk of, developing PHH but without any signs of raised ICP. We compared this to conservative management, wherein the use of lumbar or ventricular punctures was restricted to where there are signs of raised ICP only.
How the intervention might work
It has been postulated that removal of bloody CSF by serial lumbar or ventricular punctures might improve the prognosis of infants at risk of, or actually developing, PHH. The physical removal of CSF that contains blood and protein might reduce the inflammatory reaction, decrease deposition of extracellular matrix proteins, and re‐establish normal CSF drainage. The infants might benefit in terms of better neurological function because of reduced ICP and less periventricular oedema. Removal of blood and protein might also reduce the need for a permanent shunt.
Why it is important to do this review
There is a clear need to reduce mortality and the burden of disability that arises from this condition. Several trials have attempted to answer the question of whether this approach could produce a clear benefit. A systematic review is required to assess the evidence.
This is an update of the original Cochrane Review, Whitelaw 2001a, in which randomised trials failed to show any benefit of routine removal of CSF via lumbar or ventricular puncture. However, since then a retrospective review of infants with PHH in the Netherlands indicated that infants who received a lumbar puncture or subcutaneous ventricular reservoir “early” (defined as infants who at the decision to remove CSF via lumbar or ventricular puncture had a ventricular width above the 97th centile but below the 97th centile + 4 mm or 2 standard deviations of the mean (Levene 1981)) were less likely to receive a shunt when compared to the “late” group (odds ratio 0.22, 95% confidence interval (CI) 0.08 to 0.62) (de Vries 2002).
This has led to some questioning whether a benefit could be found if CSF removal for infants where there was rapidly increasing ventricular size was completed at an earlier time than is currently used. Subsequently, a RCT that prospectively compares low threshold versus high threshold intervention has started (ISRCTN43171322).
Given the renewed interest in this topic, it was necessary to revise the review and confirm that the assessment of the literature was current and the conclusions still valid.
Objectives
To determine the effect of repeated cerebrospinal fluid (CSF) removal (by lumbar/ventricular puncture or removal from a ventricular reservoir), compared to conservative management, where removal is limited to when there are signs of raised intracranial pressure (ICP), on reduction in the risk of permanent shunt dependence, neurodevelopmental disability, and death in neonates with or at risk of developing posthaemorrhagic hydrocephalus (PHH).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs that compared repeated CSF removal to standard (control) treatment in newborn infants with intraventricular haemorrhage (IVH) or early posthaemorrhagic hydrocephalus (PHH) were to be identified. We defined RCTs as studies that assigned the participants prospectively to one of two (or more) forms of healthcare by using random allocation. A quasi‐RCT was one in which it appeared that the study participants were assigned prospectively to one of two (or more) alternative forms of healthcare by using some quasi‐random method of allocation (such as by alternation, date of birth, or case record number). We excluded trials that did not have a simultaneous control group (for example, those without historical controls).
Types of participants
We included infants younger than three months of age who had either of the following.
-
IVH demonstrated by ultrasound or computed tomography (CT) scan (at risk of PHH).
-
IVH followed by progressive ventricular dilatation.
We excluded infants who had other causes of hydrocephalus (for example, infection, congenital aqueduct stenosis, and tumour).
Types of interventions
Repeated removal of CSF by repeated lumbar puncture, ventricular punctures, or from a subcutaneous ventricular reservoir.
Types of outcome measures
Primary outcomes
-
Placement of a hydrocephalus shunt.
-
Death prior to 12‐month follow‐up.
-
Major disability in survivors.
-
Multiple disability in survivors.
-
Death or disability.
-
Death or shunt.
Secondary outcomes
-
Infection of CSF presurgery.
Search methods for identification of studies
Electronic searches
For the search update in 2016, we used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We conducted a comprehensive search that included the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 3) in the Cochrane Library; MEDLINE via PubMed (1966 to 24 March 2016); Embase (1980 to 24 March 2016); and CINAHL (1982 to 24 March 2016). See Appendix 1 for the full search strategy. We did not apply any language restrictions.
We searched the following clinical trials registries for ongoing or recently completed trials: ClinicalTrials.gov (clinicaltrials.gov); the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.whoint/ictrp/search/en/); the National Institute for Health Research (NIHR) (www.ukctg.nihr.ac.uk/default.aspx); and the ISRCTN Registry (www.isrctn.com/).
Searching other resources
We searched for conference abstracts of the Pediatric Academic Societies (PAS) and the European Society for Paediatric Research (ESPR) from 2009 to 2015, and we searched the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐RCTs.
Previous version of this review
For the previous version of this Cochrane Review, Whitelaw 2001a, we handsearched the following journals from January 1976 (when CT scanning of neonates started) to October 2000: Pediatrics; Journal of Pediatrics; Archives of Disease in Childhood; Pediatric Research; Developmental Medicine and Child Neurology; Acta Paediatrica Scandinavica; European Journal of Pediatrics; Neuropediatrics; Neurosurgery; Journal of Neurosurgery; Pediatric Neurosurgery; Biology of the Neonate; New England Journal of Medicine; The Lancet; and the British Medical Journal (BMJ). We searched MEDLINE (via PubMed), CINAHL, Embase, and the Cochrane Library from 1976 to 2000, and updated the searches in April 2009 using the following MeSH terms: intraventricular haemorrhage, hydrocephalus, lumbar puncture, newborn infant. We handsearched the following conference proceedings from 1988 to October 2000: the Proceedings of the Society for Pediatric Research; the European Society for Pediatric Research; the Neonatal Society; and the British Paediatric Association.
Data collection and analysis
Selection of studies
We employed the standard methods of Cochrane Neonatal. We screened the literature search results by title and abstract, and coded them as either 'retrieve' or 'do not retrieve'. Articles in the 'do not retrieve' category did not fulfil the inclusion criteria. Articles in the 'retrieve' category were articles that either potentially fulfilled the inclusion criteria or articles that we were unsure whether they fulfilled the inclusion criteria or not. We retrieved the full‐text articles of all studies in the 'retrieve' category and assessed them against the inclusion criteria. We listed all studies that we excluded after full‐text assessment and their reasons for exclusion in the 'Characteristics of excluded studies' table. We presented the study selection process in a PRISMA diagram.
Data extraction and management
One review author (RLK) extracted, assessed, and coded all data from the included trials. The second review author, AW, repeated this process to check consistency. We resolved disagreements by discussion. We entered the data into Review Manager 5 (RevMan 5) for analysis and storage (Review Manager 2014).
We extracted data on the following: the number of participants, number of participants allocated to intervention, and primary and secondary outcomes.
We checked the inclusion criteria and therapeutic interventions of each included trial to see how they differed between trials. We examined the outcomes in each trial to see how comparable they were between studies.
We assessed the methodological quality and risk of bias of each included trial.
Assessment of risk of bias in included studies
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We excluded trials that did not have a simultaneous control group (for example, those with historical controls). If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
For this review update, we assessed the included studies using the following key domains for assessing risk of bias (Higgins 2011).
Random sequence generation
-
Low risk of bias: if, for example, the trial used a table of random numbers or computer‐generated random numbers.
-
High risk of bias: if, for example, the trial used alternation, date of birth, day of the week, or case record number.
-
Unclear risk of bias: if insufficient information was provided.
Allocation concealment
-
Low risk of bias: if, for example, numbered or coded identical containers were administered sequentially, by an onsite computer system that could only be accessed after entering the characteristics of an enrolled participant; or serially numbered, opaque, sealed envelopes, were used; or sealed envelopes that were not sequentially numbered or opaque were used.
-
High risk of bias: if, for example, the trial used an open table of random numbers.
-
Unclear risk of bias: if insufficient information was provided.
Blinding
Treatment by removal of CSF through lumbar puncture, ventricular puncture, or from a ventricular reservoir cannot be done 'blind' by the neonatologist but the assessment of outcome could be carried out by individuals blinded to early treatment allocation.
-
Low risk of bias: if there was adequate blinding of outcome assessors to treatment allocation.
-
High risk of bias: if there was no blinding.
-
Unclear risk of bias: if insufficient information was provided.
Incomplete outcome data
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether the study reported attrition and exclusions, the number of participants included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where the trial authors provided sufficient information, we re‐included missing data in the analyses. We categorised the methods as follows.
-
Low risk of bias: no missing data or the proportion of missing data compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
-
High risk of bias: when the proportion of missing data compared with observed event risk was large enough to induce clinically relevant bias in the intervention effect estimate.
-
Unclear risk of bias: if insufficient information was provided.
Selective reporting
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as follows.
-
Low risk: it was clear that the study authors reported all of the study’s prespecified outcomes and all expected outcomes of interest to the review.
-
High risk: not all the study’s prespecified outcomes were reported, one or more reported primary outcomes were not prespecified, outcomes of interest were reported incompletely and so we could not use them; or the study failed to include results of a key outcome that we would have expected to have been reported.
-
Unclear risk: insufficient information is provided.
Other sources of bias
Any other source of bias identified but not part of the previous headings.
Measures of treatment effect
We performed statistical analyses using RevMan 5 (Review Manager 2014). We analysed categorical data using risk ration (RR), risk difference (RD), and the number needed to treat for an additional beneficial outcome (NNTB) for outcomes 1.1 to 1.6, and number needed to treat for an additional harmful outcome (NNTH) for outcome 1.7. For continuous data, we analysed these using weighted mean difference (WMD) values. We reported the 95% confidence interval (CI) on all estimates.
Unit of analysis issues
We made a consideration if an included trial randomised groups of individuals together or if there were repeated observations for the same outcome. In this review we required that the number of observations matched the number of randomised participants.
Dealing with missing data
Where data was missing we attempted to contact the study authors for the original data.
Where the trial authors reported or supplied sufficient information, we re‐included missing data in the analyses. When we were unable to obtain this data, we stated this.
Assessment of heterogeneity
We examined heterogeneity between included trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. If noted, we explored the possible causes of statistical heterogeneity using prespecified subgroup analysis (for example, differences in study quality, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
We tried to obtain the study protocols of all included studies to compare outcomes reported in the protocol versus those reported in the findings for each of the included studies.
When we suspected reporting bias, we intended to contact study authors to ask them to provide missing outcome data. When this was not possible and we suspected that missing data might introduce serious bias, we intended to explore the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Data synthesis
We constructed 2 x 2 tables for each trial for each important outcome, and risk ratio and risk difference with 95% CIs.
We performed meta‐analysis using RevMan 5 (Review Manager 2014). For estimates of typical risk ratio and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. We performed all meta‐analyses using a fixed‐effect model.
Quality of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: insertion of hydrocephalus shunt, death, presence of major disability in survivors, presence of multiple disability in survivors, death or disability, and death or shunt.
Two review authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro Guideline Development Tool (GDT) to create a ‘Summary of findings’ table to report the quality of the evidence (GRADEpro GDT 2014).
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades.
-
High: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
No subgroup analysis was undertaken as part of the review. No subset of participants (i.e. males or females) or studies (i.e. by geographical location) were identified before the review as being heterogenous enough to require a subgroup analysis.
Sensitivity analysis
A sensitivity analysis was not undertaken as the outcome measures of the review were thought to be clearly objective and non‐contentious.
A sensitivity analysis could be performed if missing data was identified during the review and thought sufficient enough to influence the overall assessment of outcomes.
Results
Description of studies
Results of the search
The previous version of this review, Whitelaw 2001a, included four trials (reported in five articles). For this Cochrane Review update, we updated the literature searches without date limit as the search terms were expanded. The searched yielded 771 results, but we did not identify any new studies that satisfied the inclusion criteria of the review (Figure 1). Therefore this Cochrane Review included four trials including 280 preterm infants treated in neonatal intensive care units in the UK. The trials were published between 1980 and 1990.
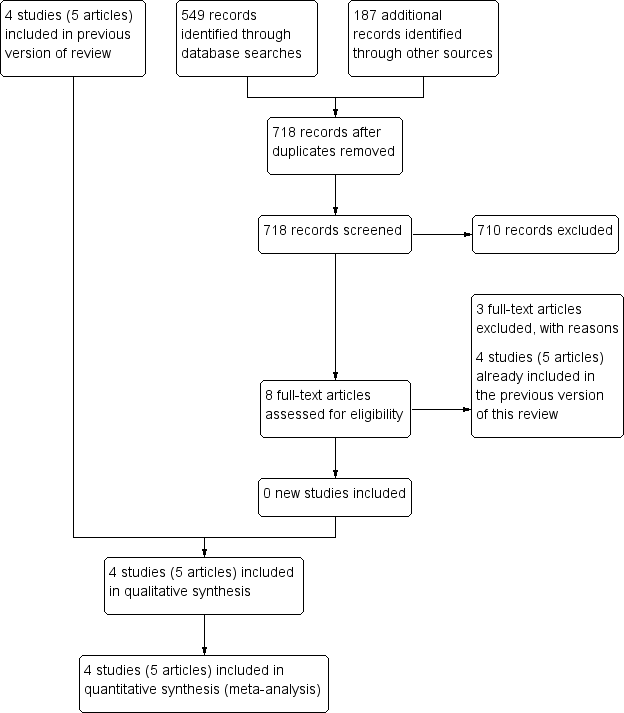
Study flow diagram: review update
Included studies
See the 'Characteristics of included studies' table.
An important issue is the heterogeneity of the populations or intervention, or both, in the included trials. Two trials, Mantovani 1980 and Anwar 1985, enrolled infants with IVH and examined the effect of repeated lumbar puncture in preventing the development of permanent hydrocephalus (as defined by ventriculoperitoneal shunt (VPS) placement). Two trials enrolled neonates with IVH who then went on to show progressive ventricular dilatation (Dykes 1989; Ventriculomegaly 1990). They examined the effect of lumbar punctures (Dykes 1989), or lumbar punctures or ventricular punctures (Ventriculomegaly 1990). The first approach is non‐selective and allows earlier intervention (which might, in theory, offer a better chance of success). The second approach is selective but still means that some babies are treated who would have resolved without shunting anyway. The second approach usually means later treatment because one has to wait and see which IVH infants will show progressive dilatation. A further point is that Ventriculomegaly 1990 used ventricular as well as lumbar puncture to achieve CSF drainage, whereas the other three included trials used only lumbar puncture. Larger volumes of cerebrospinal fluid (CSF) could be taken each time by ventricular puncture than by lumbar puncture but the potential for trauma and infection in the brain is probably greater by the ventricular route. All four included trials tackled the same question: does repeated removal of CSF reduce the risk of hydrocephalus? All four trials attempted in their interventions to drain as much CSF as was practical. For these reasons, we have examined them.
In Dykes 1989, paediatric neurologists and a psychologist assessed developmental outcome at different ages. The study authors did not state whether or not these people were blinded to early treatment allocation. The study classified the children into 'major handicap' and "no major handicap". The study then subdivided those who had major handicap into those with a) 'single system disability'; and b) those with 'multiple handicaps'. We extracted the number of children a) without major disability; b) with a single disability; and c) with multiple disability.
In Ventriculomegaly 1990, one developmental paediatrician who was blinded to early treatment allocation examined virtually all the included children. Children were examined at 12‐months post‐term and at 30‐months post‐term. We extracted the number of children with single‐system disability and those with multiple impairments.
Impairments, disabilities, and handicaps
The term 'handicap' may, in retrospect, have been used in rather an imprecise way and we have avoided it in the analyses. We took disability to mean a disturbance of function severe enough to prevent the child functioning at an age appropriate level. Single‐system disability meant that the findings were confined to one system of the nervous system, for example, a) hemiplegia without mental retardation; or b) sensorineural hearing loss.
We interpreted the terms 'multiple handicap', 'multiple disability', or 'multiple impairments' to mean clinically significant disturbances of function in different domains of the nervous system, for example, the combination of mental retardation, spastic diplegia, cortical blindness, and epilepsy. When we calculated the figures for death or disability, we subtracted the number of infants randomised but lost to follow‐up from the totals originally entered. Death or disability were mutually exclusive and thus we could aggregate them.
Update
For this review update we noted that Anwar 1985 reported the outcome as VPS or placement of ventricular reservoir. As the placement of a reservoir is a much milder outcome than a shunt we were unable to include this data for two outcomes: outcome 1.1 acquiring permanent shunt, or outcome 1.6 death or shunt. We contacted the study authors for the original data in order to obtain information on the number of infants who had only a shunt placement. However, we were unable to obtain this data.
We also combined the outcomes of death and shunt as a new outcome: outcome 1.6 death or shunt. Two trials, Ventriculomegaly 1990 and Anwar 1985, did not report on the breakdown of data to make this analysis in the paper, i.e. they did not provide the number of VPS placements that also died. For Ventriculomegaly 1990 we were able to access the original data to make the new analysis. For Anwar 1985 we contacted the original authors but were unable to obtain the original data. As such, we excluded Anwar 1985 from the analysis of outcome 1.6.
Finally, we added a new outcome: outcome 1.7 presence of CSF infection before surgery. CSF infection (meningitis/ventriculitis) is a serious adverse outcome and repeated lumbar or ventricular punctures in preterm infants carries a theoretical risk of introducing infection. Two trials, Dykes 1989 and Ventriculomegaly 1990, reported incidence of CSF infection prior to surgery and we assessed this as a secondary outcome.
Excluded studies
In this review update we assessed eight full‐text articles. The previous version of this review, Whitelaw 2001a, already included four of these studies. The other three studies were ineligible based on study design (see the 'Characteristics of excluded studies' table).
Risk of bias in included studies
We assessed all included studies for risk of bias and presented the results in 'Risk of bias' tables (Figure 2; Figure 3).
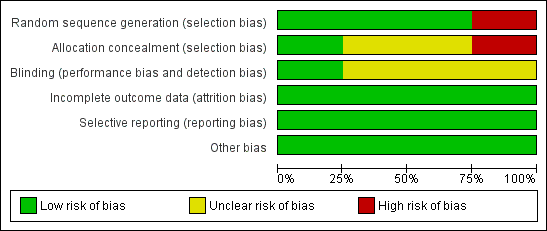
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
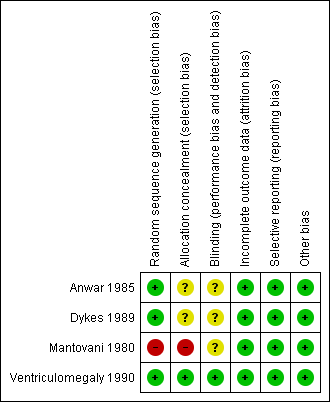
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
We judged Mantovani 1980 to be at high risk of selection bias, as this trial used an alternation method for random sequence generation. All other included studies randomised participants by using a low risk method (random number table or telephone method) and were at low risk.
In Anwar 1985 and Dykes 1989, it was unclear whether or not there was concealment of allocation, another potential source of selection bias. We considered these trials to be at unclear risk of bias.
Blinding
Assessors of neurodevelopmental outcome were blinded to allocation in Ventriculomegaly 1990, but it is unclear if this was the case in Dykes 1989 and Mantovani 1980. For Anwar 1985, the trial authors reported that the ultrasonographers were blinded to study classification. However, the trial defined hydrocephalus as an outcome by ultrasound and clinical signs of raised ICP. The trial authors did not give any information as to whether the assessors of the clinical signs of raised ICP were blinded to treatment allocation.
Incomplete outcome data
Across all included trials there was minimal attrition bias due to loss to follow‐up.
Selective reporting
There was low risk of selective reporting as all included trials reported major outcomes of interest and there was no unexpected omission of key outcomes.
Other potential sources of bias
No other sources of bias were identified.
Effects of interventions
The four trials included a total of 280 infants, with 157 from the Ventriculomegaly 1990 trial alone. There was no evidence of benefit in the treatment group for any of the outcomes (see 'Summary of findings' table 1: summary of findings Table for the main comparison).
Primary outcomes
Placement of a hydrocephalus shunt (outcome 1.1)
Three trials (233 participants) reported on acquiring permanent shunt (Mantovani 1980; Dykes 1989; Ventriculomegaly 1990). These trials showed no benefit to intervention (typical risk ratio 0.96, 95% confidence interval (CI) 0.73 to 1.26; typical risk difference −0.02, 95% CI −0.15 to 0.11; Analysis 1.1).
Death prior to 12‐month follow‐up (outcome 1.2)
All four included trials reported on death prior to 12‐month follow‐up with a total of 280 participants and found no benefit to intervention (typical risk ratio 0.88, 95% CI 0.53 to 1.44; typical risk difference −0.02, 95% CI −0.11 to 0.06; Analysis 1.2).
Major disability in survivors (outcome 1.3) and multiple disability in survivors (outcome 1.4)
Two trials (141 participants) reported on major disability in survivors and multiple disability in survivors (Dykes 1989; Ventriculomegaly 1990). These trials showed no benefit to the treatment group regarding major disability in survivors: typical risk ratio 0.98, 0.81 to 1.18; typical risk difference −0.02 (95% CI −0.16 to 0.12); Analysis 1.3) and multiple disability in survivors (typical risk ratio 0.90, 95% CI 0.66 to 1.24; typical risk difference −0.05, 95% CI −0.21 to 0.11; Analysis 1.4).
Death or disability (outcome 1.5)
Two trial (180 participants) reported on combined death or disability (Dykes 1989; Ventriculomegaly 1990). These trials showed no benefit to the treatment group (typical risk ratio 0.99, 95% CI 0.86 to 1.14; typical risk difference −0.01, 95% CI −0.12 to 0.10; Analysis 1.5).
Death or shunt (outcome 1.6)
Three trials (233 participants) reported on death or shunt (Mantovani 1980; Dykes 1989; Ventriculomegaly 1990). These trials found no benefit to the treatment group (typical risk ratio 0.91, 95% CI 0.75 to 1.11; typical risk difference −0.06, 95% CI −0.19 to 0.06; Analysis 1.6).
Secondary outcome
Infection of CSF presurgery (outcome 1.7)
Two trials (195 participants) reported on the secondary outcome of infection of CSF presurgery (Dykes 1989; Ventriculomegaly 1990). These trials found no significant difference between the treatment groups (typical risk ratio 1.73, 95% CI 0.53 to 5.67; typical risk difference 0.03, 95% CI −0.04 to 0.10; Analysis 1.7).
Discussion
Summary of main results
Although it was a reasonable hypothesis that removal of protein and blood by repeated cerebrospinal fluid (CSF) removal would improve outcome in infants with intraventricular haemorrhage (IVH) but without signs of symptoms of raised intracranial pressure (ICP), meta‐analysis of four included trials did not demonstrate any evidence of benefit in any of the outcome measures assessed (see 'Summary of findings' table 1: summary of findings Table for the main comparison).
For the outcomes of death (Analysis 1.2) and infection of CSF presurgery (Analysis 1.7), the confidence interval (CI) for risk ratio was particularly wide: 0.88 (0.53 to 1.44) and 1.73 (0.53 to 5.67) respectively. This indicates that imprecision is present because the width of CI is consistent with both important benefit and harm. This indicates that the total sample size was insufficiently large for a precise estimate of this outcome.
For the outcome of infection of CSF presurgery (Analysis 1.7), there was inconsistency between the results of the two trials. Dykes 1989 reported no cases of CSF infection and Ventriculomegaly 1990 reported infection in 10/157 cases.
Overall completeness and applicability of evidence
For this Cochrane Review update we re‐conducted the search for completeness. The included randomised controlled trials (RCTs) have outcome measures that are applicable to the review question. We found no ongoing trials that examined this review's question. However, there are ongoing trials that are comparing the timing of CSF removal in response to increasing ventricular size (ISRCTN43171322).
Quality of the evidence
Four RCTs including 208 preterm infants met the inclusion criteria of this review. We downgraded the quality of the evidence due to selection bias, namely the use of a alternation method for randomisation in one trial (Mantovani 1980). Also, it was unclear as to whether there was allocation concealment in two trials (Anwar 1985; Dykes 1989). Other sources of bias were likely to be minimal. In particular there were very low numbers of participants lost to follow‐up and no evidence of selective reporting. There is some evidence that there was insufficient precision to assess the outcomes of death (Analysis 1.2) and infection of CSF presurgery (Analysis 1.7). There were inconsistent results between studies for infection of CSF presurgery (Analysis 1.7). The quality of the evidence was low for the outcomes of death and infection of CSF presurgery; moderate for the outcomes of acquiring permanent shunt, and death or acquiring permanent shunt; and high for the outcomes of major disability in survivors, multiple disability in survivors, and death or disability.
Potential biases in the review process
We were unable to identify any clear sources of bias in the review process. As stated in the declarations of interest, Professor Andrew Whitelaw was an author of one of the included trials (Ventriculomegaly 1990).

Study flow diagram: review update
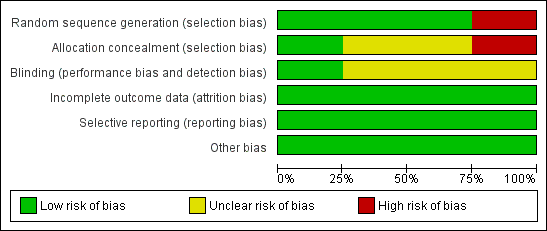
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study

Comparison 1 Lumbar punctures or ventricular punctures versus control, Outcome 1 Placement of a hydrocephalus shunt.

Comparison 1 Lumbar punctures or ventricular punctures versus control, Outcome 2 Death prior to 12‐month follow‐up.

Comparison 1 Lumbar punctures or ventricular punctures versus control, Outcome 3 Major disability in survivors.

Comparison 1 Lumbar punctures or ventricular punctures versus control, Outcome 4 Multiple disability in survivors.

Comparison 1 Lumbar punctures or ventricular punctures versus control, Outcome 5 Death or disability.
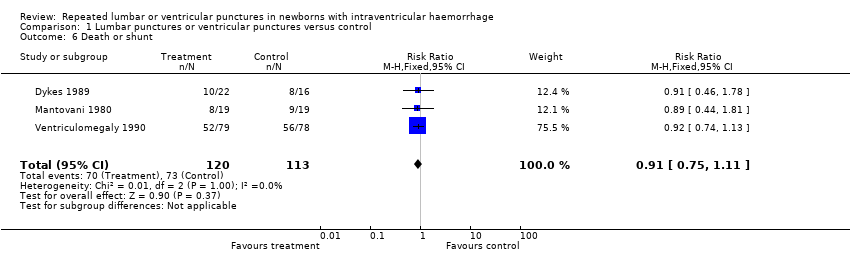
Comparison 1 Lumbar punctures or ventricular punctures versus control, Outcome 6 Death or shunt.

Comparison 1 Lumbar punctures or ventricular punctures versus control, Outcome 7 Infection of CSF presurgery.
| Repeated lumbar or ventricular punctures compared to conservative management for infants with intraventricular haemorrhage (IVH) | |||||
| Population: preterm infants less than three months of age with either: a) IVH demonstrated by ultrasound or computed tomography (CT) scan; or b) infants with IVH followed by progressive ventricular dilatation. Settings: neonatal intensive care units. Intervention: serial lumbar puncture, ventricular puncture, or tapping from a subcutaneous reservoir. Comparison: conservative management. | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants | Quality of the evidence | |
| Risk with conservative treatment | Risk with serial lumbar or ventricular punctures | ||||
| Hydrocephalus shunt | Study population | RR 0.96 | 233 | ⊕⊕⊕⊝ | |
| 469 per 1000 | 450 per 1000 | ||||
| Death | Study population | RR 0.88 | 280 | ⊕⊕⊝⊝ | |
| 199 per 1000 | 175 per 1000 | ||||
| Major disability in survivors | Study population | RR 0.98 | 141 | ⊕⊕⊕⊕ | |
| 761 per 1000 | 746 per 1000 | ||||
| Multiple disability in survivors | Study population | RR 0.90 | 141 | ⊕⊕⊕⊕ | |
| 537 per 1000 | 484 per 1000 | ||||
| Death or disability | Study population | RR 0.99 | 180 | ⊕⊕⊕⊕ | |
| 814 per 1000 | 806 per 1000 | ||||
| Death or shunt | Study population | RR 0.91 | 233 | ⊕⊕⊕⊝ | |
| 646 per 1000 | 588 per 1000 | ||||
| Infection of CSF presurgery | Study population | RR 1.73 | 195 | ⊕⊕⊝⊝ | |
| 43 per 1000 | 74 per 1000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 as Mantovani 1980 used an alternation method for random sequence generation. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Placement of a hydrocephalus shunt Show forest plot | 3 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 2 Death prior to 12‐month follow‐up Show forest plot | 4 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.53, 1.44] |
| 3 Major disability in survivors Show forest plot | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.18] |
| 4 Multiple disability in survivors Show forest plot | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.66, 1.24] |
| 5 Death or disability Show forest plot | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.14] |
| 6 Death or shunt Show forest plot | 3 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.11] |
| 7 Infection of CSF presurgery Show forest plot | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.53, 5.67] |

