Sonstige Behandlungsmethoden gegen Neuroleptika‐induzierte Spätdyskinesien
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: random, further details not reported. Blindness: not reported. Duration: 40 weeks. Design: parallel. Setting: outpatients, USA (probably). | |
| Participants | Diagnosis: chronic schizophrenia treated with phenothiazine for several years and demonstrating obvious dyskinetic manifestations. N = 20 Sex: 16 female and 4 male Age: range 45‐62 years History: at least two years of TD. | |
| Interventions | 1. Procyclidine (Anticholinergic), 5 mg twice a day + chlorpromazine, 100 mg three times a day N = 10. Continuous phenothiazine‐antiparkisonian treatment for at least 2 years. . Other concomitant medication was not reported. | |
| Outcomes | TD symptoms (clinical evaluation, scale not reported) Leaving the study early Adverse events Unable to use ‐ Mental state (data not reported for both groups) | |
| Notes | Sponsorship source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The patients were divided at random into groups of 10 each”, no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not reported. |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) | Low risk | “One male patient on chlorpromazine and isocarboxazid was discontinued after 6 weeks as he became increasingly tense, apprehensive and sleepless” |
| Selective reporting (reporting bias) | High risk | Adverse effects reported only as those related to treatment. Mental state data not reported for group 2. Unclear if all outcomes have been reported, a protocol is not available for verification. |
| Other bias | Unclear risk | Insufficient information to make a judgement |
| Methods | Allocation: not reported. Design: parallel Setting: not reported. | |
| Participants | Diagnosis: antipsychotic‐induced TD; N = 57 Sex: 33 male and 24 female 24 Age: mean 39.5 (SD 10.3) years old, range 28‐59 History: Duration of TD on average 2.4 (SD 1.8) years. Patients assigned to the treatment group were stable to AP dose from 0.7 to 27 years, whereas patients assigned to the control group were stable to AP dose from 1 to 10 years before start of study. | |
| Interventions | 1. L‐stepholidine (SPD) Group: Management: L‐stepholidine was prescribed two tablets each time, three times per day for 8 weeks. N = 42 2. Placebo Group: The placebo with similar appearance to L‐stepholidine was prescribed two tablets each time, three times per day for 8 weeks. N = 15 All participants received stable AP and concomitant anticholinergic drug. Other concomitant medication is not reported. | |
| Outcomes | Clinical improvement on TD symptoms: no definition. ‐‐Unable to use | |
| Notes | Twenty cases from the SPD group received blood routine examination, urine routine test and liver function test; five cases received electrocardiography. Ten cases in each group received BPRS and TESS measurements. We attempted to contact the study author for information on randomisation, but were informed he had retired. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomized, parallel‐group..." no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind" "The placebo with similar appearance", Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) | Low risk | "double blind" "The placebo with similar appearance", Blinding of participants and key study personnel ensured. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | High risk | The author did not report the outcome: TESS, Blood routine examination, urine routine test and liver function test, electrocardiography |
| Other bias | Unclear risk | No further details reported |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: Psychiatric patients meeting DSM‐IV‐TR diagnostic criteria for the diagnosis of antipsychotic‐induced TD. N = 13. | |
| Interventions | 1. Melatonin: 20 mg/day. N = 7. Duration: 12 weeks. 2. Placebo: N = 6. Duration: 12 weeks. All individuals who participated faithfully complied with antipsychotic treatment and maintained it throughout the study. Other concomitant medication: The treatment with anticholinergics was maintained for ethical reasons. In each of the groups two patients received biperidine. Other treatments applied were, in the melatonin group, valproic acid (1 patient), carbamazepine (1 patient), alprazolam (1 patient), indapamide (1 patient); in the placebo group: carbamazepine (2 patients), chlorimipramine (2 patients) and lithium carbonate (1 patient). During the study there were no dosage adjustments. | |
| Outcomes | Tardive dyskinesia: Brief Psychiatric Rating Scale (BPRS) Tardive dyskinesia: symptoms: AIMS Adverse events: other observed effects. | |
| Notes | This study was supported by the Instituto Venezolano de Investigaciones Científicas (IVIC), Venezuela | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The individuals selected were divided randomly into two groups", no further details. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "A group of 6 patients received placebo capsules, identical in appearance". |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double blind". Blinding of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all pre‐defined outcomes were reported. A protocol is not available for verification. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder with TD (DSM‐IV). | |
| Interventions | 1. Ethyl‐eicosapentaenoic acid (omega‐3 fatty acid eicosapentaenoic acid derivative): dose 2 g/day + antipsychotics. N = 42. Patients who were stabilised on other psychotropic medications (anxiolytic, hypnotic, antidepressant, mood stabilising) before entry to the trial were allowed to continue on these medications; anticholinergic medication for treatment‐emergent extrapyramidal symptoms (EPS); anxiolytic or hypnotic medication for treatment emergent insomnia or acute anxiety; any medication for physical conditions that was taken prior to the commencement of the trial could be continued; medication for other conditions that arose during the course of the trial, at the investigator's discretion. Other omega‐3 fatty acid preparations, additional antipsychotics or antidepressants were not permitted. | |
| Outcomes | Extrapyramidal symptoms: ESRS Mental state deterioration Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomized, parallel‐group..." no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Low risk | "...double‐blind..." "Subjects were randomly assigned to receive either an encapsulated ethyl‐EPA supplement 2 g/day... or an identical capsule containing placebo (medicinal liquid paraffin BP 2 g/day)... Trial supplies were packed by an independent contract clinical trials supplies company (DHP), who prepared the placebo and active packs for the entire trial and assigned the randomization numbers to the packs. The randomization code was broken after completion of the trial". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors for efficacy outcomes (TD symptoms and Mental State) not reported. Adverse events blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate: 35%. 7/84 (8%) participants dropped out before the first post‐randomisation visit. 7/84 (8%) participants were not included in the analysis. "Data from... 77 patients were included in the final analysis". "The number of subjects who discontinued medication prematurely in the ethyl‐EPA group was 8 (19%) (consent withdrawal n=4; non‐compliance n=3; protocol violation n=1), and in the placebo group 14 (33%) (consent withdrawal n=9; non‐compliance n=3; adverse events n=2 (congestive cardiac failure; nose‐bleed) (Chisquare=2.2, df=1, p=0.1)." It seems that these participants were included in the analysis. |
| Selective reporting (reporting bias) | High risk | Data for some outcomes stated in the protocol have not been reported (e.g. remission, CGI severity) |
| Other bias | Unclear risk | "The demographic characteristics and baseline PANSS scores in the two treatment groups were similar, but baseline ESRS dystonia subscale and TD CGI scores differed significantly". Unclear if the differences (confounding variables) may be biased |
| Methods | Allocation: "randomised" unclear. Setting: inpatients, USA | |
| Participants | Diagnosis: psychogeriatric (23) and schizophrenic (18) patients with obvious TD. History: Duration of TD not reported. | |
| Interventions | 1. Papaverine: dose 150 mg/day twice a day for the first week and 300 mg twice a day for the subsequent 5 weeks. N = 21. Concomitant medication: antipsychotics, antidepressants, anxiolytics, or no medication. No changes were made in psychotropic drug administration during the study. | |
| Outcomes | TD symptoms: AIMS (only reported for Boston sample) Unable to use (not reported for first treatment phase before cross‐over) Leaving the study early Adverse effects: Parkinsonism | |
| Notes | Sponsorship source: Supported in part by a grant from the Drug Abuse and Mental Health Administration of HEW. * Authors state that the two samples differed greatly in the incidence and severity of tardive dyskinesia prior to the study. The differences were to a large extent due to the differences in the two populations: Boston patients were older with longer duration of disability and hospitalizations and showed more extensive dyskinesia. In view of the major differences in the populations, data from the two groups were analysed separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random order", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | High risk | "single‐blind". As the participants were randomised to receive papaverine or not, neither the participants nor the study personnel could have been blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | "A single blind design was used with blind raters and a 6‐weeks no drug control condition." |
| Incomplete outcome data (attrition bias) | Unclear risk | Although not clearly reported, it seems that 1/23 participants in the Boston sample and 0/18 participants in the Kentucky sample were withdrawn from the study. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all predefined outcomes have been reported. Also, the reason for not assessing TD symptoms using AIMS in the Kentucky sample is not reported. |
| Other bias | Unclear risk | The two samples were very different in their baseline characteristics. However, this was controlled by reporting data separately. Unclear if there might have been other confounding variables to affect bias. |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: psychiatric disorder (no operational criteria) and Schooler&. Kane's criteria for TD | |
| Interventions | 1. Oestrogen: 1.25 mg/day. N = 6.* Nine of the 10 patients were on medications other than antipsychotics; 7 were on psychiatric medication. Further details not provided. | |
| Outcomes | Tardive dyskinesia: AIMS improved/not improved, deterioration in symptoms, AIMS scale scores . Unable to use ‐ | |
| Notes | Sponsorship source: Supported by HD 13587 and Ayerst Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized". Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind". Details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | TD related and mental state outcomes: "double‐blind". "All ratings were administered without knowledge of the study status of the individual subject. Patients were videotaped during the research nurse's rating on visits 1 and 4. These tapes were subsequently rated by a senior psychiatric resident who administered the AIMS and counted the frequency of abnormal movements of the most severely affected anatomical region as determined by the study psychiatrist during the baseline assessment". Details of blinding not reported. |
| Incomplete outcome data (attrition bias) | Low risk | "Twelve patients were admitted to the study, and 10 completed the 3‐week trial. One patient in the placebo group was hospitalized for congestive heart failure. One patient in the oestrogen group required psychiatric hospitalization after taking an overdose of medications; she had been depressed at the onset of the trial and became worse at the third visit during a period of severe marital discord." Balance between groups, reasons reported. |
| Selective reporting (reporting bias) | High risk | TD symptoms data not reported as mean (SD) but rather as mean only (table 1). Data for Mental state (BPRS) not reported. Adverse effects reported only as adverse events leading to study discontinuation. |
| Other bias | High risk | "Although our study involved randomization and double‐blind drug procedures to prevent bias, the small sample size resulted in some imbalances between groups at the first visit. Thus, the conjugated oestrogen group had less exposure to antipsychotic medication and a shorter duration of TD. They also had higher mean baseline AIMS scores than did the placebo group, thereby leaving more possibility for improvement in scores. The small sample size does not allow statistical analysis to adjust for these differences. Although we found a positive but non‐significant association between duration of TD and decrease in AIMS score between visits 1 and 4, we doubt that TD duration is a confounding factor, since the direction of this association is the opposite of what we would have expected". |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: Diagnosis of chronic schizophrenia; diagnoses of either acute extra pyramidal symptoms, TD, and/or pseudoparkinsonism. | |
| Interventions | 1. Hypnosis: 8 sessions. N = 5. 3. Treatment as usual (control group). 8 sessions. N = 5. Psychotropic medication continued. | |
| Outcomes | Leaving the study early: number of dropouts | |
| Notes | Sponsorship source: Sponsorship source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "There was a total of fifteen subjects randomly selected". "I made the assumption that the order in which patients approached the clinic was not related in any way to their susceptibility or effectiveness of subsequent treatments. Based on these assumptions I assigned the first patient who came into the study to group 1, the second patient '"as assigned to group 2, and the third patient was assigned to Group 3; after every three assignments I started the assignments with group 1 again and continued until each treatment modality had a total of five" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | High risk | As participants in group 1 received hypnosis, those in group 2 received relaxation training, and those in group 3, TAU without any other treatment, blinding could not be achieved |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) | Low risk | There were no refusals, or dropouts among the referrals. |
| Selective reporting (reporting bias) | Low risk | It seems that all outcomes have been reported. However data are not usable. |
| Other bias | Unclear risk | "(Tables 1, 2 and 3). Using a non‐parametric test for testing differences on demographic data between treatment groups yielded no significant difference among these groups (Table 7). With respect to the regimen of medication for each patient (Tables 4, 5, 6), there was close similarity in each group. The majority of patients in each group received either haloperidol or trifluoperazine; therefore any alternative treatment differences could not be influenced by medication. However, due to the formula which is used by physicians in dispensing medication, it was not possible to use a statistical procedure for testing the equality of the three groups in this study. The sample size, sex and marital status variables was so small to preclude a statistical test on these two variables." |
| Methods | Allocation: "randomly assigned" unclear. Setting: outpatients, USA | |
| Participants | Diagnosis: antipsychotic‐induced TD according to DSM‐III‐R, Schooler and Kane criteria with a score of at least 3 (moderate) on a single item or 2 (mild) on at least two items of the AIMS and have a history of at least 6 months of exposure to a antipsychotic prior to onset of dyskinetic movements. Duration TD: not reported. | |
| Interventions | 1. Selengiline (L‐Deprenyl): dose 5 mg (one capsule daily for the first week, then one twice daily thereafter) for 6 weeks. N = 17. Doses of antipsychotics and anticholinergic agents were stable for at least 4 months before the trial. Patients receiving depot antipsychotic were included only if injections were administered on a biweekly schedule. | |
| Outcomes | TD symptoms: not significant clinically improved (defined as an improvement of more than 50%) Leaving the study early Unable to use: Adverse effects: severity of parkinsonian symptoms and akathisia using SAS, Mental state: BPRS for positive, negative and depressive symptoms, Global assessment scale, Average change in severity of TD using AIMS | |
| Notes | Sponsorship source: Supported by PHS grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "identical capsules containing either selegiline 5 mg or placebo." "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | "Examinations with the AIMS were videotaped and scored by a second rater unaware of the temporal sequence of examinations." |
| Incomplete outcome data (attrition bias) | High risk | "Five subjects, all receiving selegiline, dropped out prior to the completion of week 1 and so were not included in analysis of outcome." 29% drop out in the active medications group, not ITT. |
| Selective reporting (reporting bias) | High risk | Data not reported for mental state (BPRS total and sub scales), severity of parkinsonian symptoms (SAS) |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
| Methods | Allocation: "randomly assigned", no further details. Setting: Inpatients, UK (probably) | |
| Participants | Diagnosis: schizophrenia (9), dementia (3 with paranoid features, 3 without), depression (3), pre‐senile dementia (1). and epileptic psychosis (1). History: Duration of TD not reported. Seventeen patients were each receiving one antipsychotic drug, thioridazine (5), promazine (5), chlorpromazine (3), haloperidol (2), trifluoperazine (1) and chlomethiazole (1). Their dosage regimen had remained constant for at least 6 months. Two patients with chronic schizophrenia and one with depression were not taking any antipsychotic drugs for the same period. | |
| Interventions | 1. 4.5 mg co‐dergocrine mesylate once daily for 6 weeks. N = 10. Only two patients had never received anti‐Parkinsonian drugs, the remainder receiving therapy for various periods of time (3 months to 8 years). Two other patients were receiving anti‐Parkinsonian drugs at a constant dose during the study period. Details of medications, not reported. | |
| Outcomes | The abbreviated dyskinesia scale of the Rockland Research Institute (ADS). Death. | |
| Notes | Sponsorship source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double blind", no further details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Details on blinding of outcome assessment were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | One participant from the placebo group died in the second week of the study (run‐in phase) and was not part of the final analyses. |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | Unclear risk | No details. |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). | |
| Interventions | 1. Ceruletine IM: dose 0.8 mcg/kg/week for 3 weeks. N = 43. Previous background medication and treatment held constant throughout trial. | |
| Outcomes | Tardive dyskinesia: AIMS improved/not improved, deterioration in symptoms. | |
| Notes | Data presented for 33 matched pairs only. Sponsorship source: Sponsorship source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...patients were randomized into matched pairs on the basis of age and sex, and of the severity and duration of TD symptoms." Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐ blind", further details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐ blind", further details not reported. |
| Incomplete outcome data (attrition bias) | High risk | "Of the 85 patients who entered the study, eight were excluded from data analysis because of protocol violations (e.g., an insufficient severity of TD symptoms during the baseline period or missing observations)." One patient was discontinued due to adverse event (table 2). Actually, 19 participants were excluded from the analysis (66/85 participants, 33 in each group, were analysed. "Analyses were performed on the 33 pairs (i.e., 33 patients in the ceruletide group vs. 33 patients in the placebo group). p. 131, 1st paragraph. |
| Selective reporting (reporting bias) | High risk | Judgement Comment: "Of the 85 patients who entered the study, eight were excluded from data analysis because of protocol violations (e.g., an insufficient severity of TD symptoms during the baseline period or missing observations)." "Analyses were performed on the 33 pairs (i.e. 33 patients in the ceruletine group versus 33 patients in the placebo group)" |
| Other bias | Unclear risk | Insufficient information. Baseline information reported for participants included in the analyses but not for the participants who were not entered to the analysis due to "protocol violations" |
| Methods | Allocation: "randomly allocated", Details not reported. Blindness: "double‐blind," Details not reported Duration: 4 weeks Design: Parallel Setting: Inpatients in 4 psychiatric hospitals in Japan | |
| Participants | Diagnosis: Schizophrenia (n = 35), others (n = 7), drug‐induced TD N = 42 Sex: Male 13, Female 29 Age: 56.1 (SD: 8.69) History: Duration of TD not reported. | |
| Interventions | 1. Cyproheptadine (12 mg/day to 24 mg/day Flexible, 4 week). N = 21 Concomitant medication not reported. | |
| Outcomes | Tardive dyskinesia: Assessment scale developed by the researchers Leaving the study early. Adverse effects | |
| Notes | Sponsorship source: Cyproheptadine and placebo tablets supplied by Merck‐Banyu Co.Ltd Assessed and data extracted by Yusuke Ogawa. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", Details not reported. |
| Allocation concealment (selection bias) | Low risk | Randomization was conducted by the third person (outside of the research group). Allocation codes were stored until the end of the study |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind," Details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind," Details not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Forty‐two patients were admitted to the study, and 41 completed the 4‐week trial. One patient in the placebo group dropped out due to side effects. Reason reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all pre‐defined outcomes were reported. A protocol is not available. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: "randomly allocated", Details not reported. Blindness: "double‐blind," Details not reported Duration: 6 weeks Design: Parallel Setting: Inpatients in a psychiatric hospital in Japan | |
| Participants | Diagnosis: antipsychotic‐induced TD, Schizophrenia N = 28 Sex: Male 16, Female 12 Age: 59.3 (SD: 8.29) History: Duration of TD not reported. | |
| Interventions | 1. Dihydrogenated Ergot Alkaloids (6 mg/day, 6 week). N = 14 Concomitant medication not reported. | |
| Outcomes | Tardive dyskinesia: Not clinically improved Tardive dyskinesia: Not any improved Tardive dyskinesia: Simpson scale Tardive dyskinesia: Deterioration Mental state: Not any change in general mental state Adverse effects general Leaving the study general Leaving the study Due to side effect | |
| Notes | Sponsorship source: Dihydrogenated Ergot Alkaloids and placebo tablets supplied by Sandoz Assessed and data extracted by Yusuke Ogawa. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", Details not reported. |
| Allocation concealment (selection bias) | Low risk | Randomization was conducted by the third person (outside of the research group). Allocation codes were stored until the end of the study |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind," Details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind," Details not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Twenty‐eight patients were admitted to the study, and all of them completed the 4‐week trial. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all pre‐defined outcomes were reported. A protocol is not available. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder (DSM‐IV). | |
| Interventions | 1. Piracetam: dose 4800 mg/day + conventional antipsychotics. N = 21 Twenty‐four patients received various mood stabilisers (lithium, carbamazepine, or valproate) in combination with antipsychotic agents | |
| Outcomes | Tardive dyskinesia: Extrapyramidal System Rating Scale (ESRS). TD symptoms: not any improvement | |
| Notes | Sponsorship source: Supported by a Clinical Trials Grant from the Stanley Medical Research Institute, Bethesda, Md. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "subjects were randomized to receive either piracetam or placebo." Details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Both patients and raters were blinded to group allocation." further details not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | "raters were blinded to group allocation" |
| Incomplete outcome data (attrition bias) | High risk | "Of the 40 randomly assigned patients, 5 subjects (4 taking placebo and 1 taking piracetam) did not comply with the treatment regimen following the first 2 weeks of the study and were not included in the statistical analysis. Therefore, 35 patients completed phase I, and, of these, 4 patients (2 receiving placebo and 2 receiving piracetam) did not agree to continue to phase II, resulting in 31 patients completing both phases of the crossover protocol. The main reason for patient dropout was the large size and number of the capsules that they were required to take." In the first phase there was a 12.5% dropout while in the completed cross‐over trial 22.5% dropout. Patients who dropped out were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes defined in the Protocol have been reported. NCT00190008 |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: schizophrenia (7), bipolar affective illness (3), depressive psychosis (1) (no diagnostic criteria). | |
| Interventions | 1. Lithium: dose not specified. N = 6. Previous background medication and treatment continued throughout trial, patients were receiving a variety of antipsychotic and anxiolytic drugs: 4 patients received no antipsychotic medication. | |
| Outcomes | Tardive dyskinesia: improved/not improved, deterioration in symptoms, Rockland TD scale score. Unable to use ‐ | |
| Notes | Sponsorship source: Sponsorship source not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A random Li (Camcolit QDS)/placebo cross‐over design", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Raters, nurses and patients were blind to treatment. Two non‐blind 'coordinators'... adjusted the oral dose of Li carbonate to aim at a serum concentration between 0.8 and 1.3 mM. Adjustment of U dosage was mirrored by a similar change for the 'placebo twin'" "At completion of the study, the patients, a psychiatrist and nursing staff involved in ratings were asked to guess the nature of the treatment block. There was a choice of 3 alternative answers: inactive tablets, active drug or uncertain. "Analysis of the guesses made by staff and patients as to the sequence of treatments showed that correct guesses occurred randomly. Thus, despite inevitable cues from side‐effects, it seemed that the double‐blind nature of the study was preserved". |
| Blinding of outcome assessment (detection bias) | Low risk | "Raters, nurses and patients were blind to treatment." "At completion of the study, the patients, a psychiatrist and nursing staff involved in ratings were asked to guess the nature of the treatment block. There was a choice of 3 alternative answers: inactive tablets, active drug or uncertain. "Analysis of the guesses made by staff and patients as to the sequence of treatments showed that correct guesses occurred randomly. Thus, despite inevitable cues from side‐effects, it seemed that the double‐blind nature of the study was preserved". |
| Incomplete outcome data (attrition bias) | Low risk | 1/11 participants (in the lithium first group) did not complete the study. Reason not reported. |
| Selective reporting (reporting bias) | High risk | Mental State and behaviour outcome data not reported. TD symptoms scale scores data not reported. |
| Other bias | Unclear risk | "Patients were divided, as far as possible into matched for age, sex, severity of TD, duration of psychiatric illness and duration of neuroleptic treatment. Matching for sex was exact and the maximum discrepancies between members for any pair for other variables were as follows: age ± 4 years, total Rockland TD scores ±17, duration of illness ±11years, duration of neuroleptic treatment ±5 years" Unclear of a discrepancy of ±17 on the total Rockland TD scores is significant. |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: chronic psychotic inpatients with TD | |
| Interventions | 1. Ceruletide: 0.8 mcg/kg/week. N = 19. Background medication and treatment continued throughout trial. | |
| Outcomes | Tardive dyskinesia: AIMS improved/ not improved, deterioration in symptom. | |
| Notes | Sponsorship source: Shionogi & Co., Ltd., Japan supplied Ceruletide | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were assigned at random". Details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind" Details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double blind" Details not reported. |
| Incomplete outcome data (attrition bias) | High risk | "Of the original 47 patients, one patient dropped out of the study because of fever of unknown origin. Nine patients in whom assessment of TD symptoms was unreliable either due to the erratic variation in symptomatology or to the emotional or situational effect unrelated to the drug treatment, were excluded from the final analyses before opening of the key code. Thus, 37 patients were available for analysis, 19 receiving Ceruletide and 18 receiving placebo". Data not reported for 21% of the participants. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement. A protocol is not available to verify all outcomes defined prior to study. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
| Methods | Allocation: randomised. Setting: inpatients, Italy. | |
| Participants | Diagnosis: Chronic schizophrenia (DSM‐III‐R criteria) and suffering from antipsychotic‐induced TD previously untreated with other drugs. | |
| Interventions | No washout period was reported before trial entry. 1. Ritanserin: dose 10 mg three times a day for 30 days. N = 4. 2. Placebo for 30 days. N = 6. Concomitant medication: The patients continued receiving their basic treatment for the psychosis (i.e. haloperidol, lorazepam, haloperidol decanoate, chlorpromazine, clotiapine, thioridazine) | |
| Outcomes | TD symptoms (AIMS): not clinically improved, not improved, deteriorated Mental state: BPRS | |
| Notes | Sponsorship source: Sponsorship source not reported Results are presented for each phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Six patients, chosen at random (patients 1‐6; group A), first received the placebo and then ritanserin; the other four patients (patients 7 ‐10; group B) received treatment in the reverse order" Further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind" Details not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind" Details not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant flow through the study is not clearly reported. However, full data for all participants have been reported for all visits including post intervention. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes seem to have been reported. However, a protocol is not available for verification. |
| Other bias | Low risk | The study seems to have been free of other sources of bias. |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). | |
| Interventions | 1. Phenylalanine: 100 mg/kg body weight (day 1) followed by placebo (day 2). N = 10. Background medication and treatment continued throughout trial. | |
| Outcomes | Leaving the study early. Unable to use ‐ Cognitive ability: Rey Auditory Verbal Learning Test (AVLT) (not reported for the first treatment phase before crossing over to the next treatment) Mental state: SANS & SAPS (modified version of scales used). | |
| Notes | Sponsorship source: Supported in part by a VA Merit Review grant to one of the authors by The National Institute of Mental Health Grants to a second author, and a NARSAD Young Investigator Award to a third author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized..." Details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Drinks were prepared by an assistant and administered by the first author in a double blind manner". "The fine white L‐phenylalanine powder could not be tasted or detected visually when mixed with the bright orange colored powder." Thus, patients may have been blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "TD ratings were performed double‐blind". No further details are reported. |
| Incomplete outcome data (attrition bias) | Low risk | "One subject in the group who received phenylalanine on the first day, left the hospital against medical advice after completing only 1 day of the study; however, his data were included in the analyses" |
| Selective reporting (reporting bias) | Unclear risk | Not all data are fully reported. Some data are reported partially. No protocol are available |
| Other bias | Low risk | The study seems to be free of other sources of data |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: schizophrenia (DSM‐III). | |
| Interventions | 1. Insulin, 'standard' type: 10 units/day for 15 days administered at 10:00am; weekly for 5 weeks thereafter. N = 10. Background medication and treatment continued throughout trial. Anticholinergics were withdrawn at least 2 weeks prior to the beginning of the study | |
| Outcomes | Tardive dyskinesia: AIMS improved/not improved, deterioration in symptoms, AIMS scale scores. | |
| Notes | Sponsorship source: Standard Insulin or placebo supplied by Novo Industry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were randomly assigned..." Details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Judgement Comment: Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "...double‐blind... " "The patients and the rater were blind to treatment conditions" Details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "...double‐blind... " "The patients and the rater were blind to treatment conditions". Details not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition information has not been reported. However, as end of trial data had been individually reported for all randomised participants, it is assumed that all participants completed the trial |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement. A protocol is not available to verify all outcomes defined prior to study. |
| Other bias | Unclear risk | "At day 0, all 20 patients had severe orofacial dyskinesia and the two groups of patients did not differ in scores, age, duration of disease. Neuroleptic treatment was however significantly (p=0.037) longer in the placebo group than in the insulin group". Unclear if this could have influenced bias. |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder, mood disorder,or gastrointestinal disorder + antipsychotic‐induced TD History: Duration of TD at least 3 months prior to study. Patient stabilisation was minimum of 30 days before study start. Participants who were not using antipsychotic medication had stable psychiatric status. | |
| Interventions | 1. NBI‐98854 (VMAT2 inhibitor): dose 25mg/d‐75mg/d for 6 weeks. N = 39. Concomitant medication not reported. | |
| Outcomes | Tardive dyskinesia: AIMS. CGI‐TD and a patient‐reported scale were also reported for TD symptoms but not included in the review. Leaving the study early | |
| Notes | Sponsorship source: Neurocrine Biosciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation. |
| Allocation concealment (selection bias) | Low risk | Central interactive web response system. |
| Blinding of participants and personnel (performance bias) | Low risk | ""double‐blind, "blinded physician investigator," Personnel blinded, details not reported for participants but we judge that participants were likely to be blinded |
| Blinding of outcome assessment (detection bias) | Low risk | "Movement Disorder Neurologists as blinded central AIMS raters" |
| Incomplete outcome data (attrition bias) | Low risk | "Early discontinuation rates were comparable (5 subjects each, placebo and active)." Active 13% and placebo 10% dropouts. Reasons were reported. |
| Selective reporting (reporting bias) | Unclear risk | Results for some scales to assess safety were not fully reported. |
| Other bias | Low risk | Appears to be free from other sources of bias. |
| Methods | Allocation: "randomly allocated" Setting: Inpatients, UK | |
| Participants | Diagnosis: Schizophrenia (29), dementia (5), depressive psychosis (3), oligophrenia (2), bipolar affective psychosis (1). Presence of persistent involuntary movements predominantly in the orofacial region and unrelated to drug‐induced Parkinsonian movements. History: Duration of TD at least 1 year.. | |
| Interventions | 1. Co‐dergocrine (hydergine): dose 4.5 mg/day for 6 weeks. N = 19. Background antipsychotic and antiparkinsonian medication was held constant throughout the study. | |
| Outcomes | TD symptoms: AIMS combined with abbreviated Rockland Tardive Dyskinesia Rating Scale | |
| Notes | Sponsorship source: Sponsorship source not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to two groups" Further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "[...], one of which received co‐dergocrine, 4.5 mg tablets once daily for 6 weeks, and the other received an identical placebo tablet for the same period. The 2 raters, ward staff and patients were blind to the treatment procedure." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Details not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition information not reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all outcomes have been reported. |
| Other bias | Unclear risk | Baseline characteristics, except for age, sex, and TD scores, not reported per intervention group. |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: Psychiatric patients with long histories of antipsychotic treatment History: Duration of TD: "presumably long‐standing tardive dyskinesia". Patients stable for 2 weeks prior to entry. Patients who began the trial with stable doses of medication but who had changes in antipsychotic antiparkinson, antidepressant, or anticonvulsant drug doses during the trial were dropped from the study. | |
| Interventions | 1. Branched‐chain amino acids: low dose 56 mg/kg of body weight for 3 weeks. N = not reported 3. Branched‐chain amino acids: high dose 222 mg/kg of body weight for 3 weeks. N = 25 4. Placebo for 3 weeks. N = 27 Only results from the high dose and placebo groups were reported. Concomitant medication not reported | |
| Outcomes | Tardive dyskinesia: Simpson Abbreviated Dyskinesia Scale: improvement, deterioration, scale scores. | |
| Notes | Sponsorship source: Supported by NIMH grant MH‐44153, institutional support from the New York State Office of Mental Health, and a grant and product support from Scientific Hospital Supplies International, Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned" Details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind," Details not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | "Movement frequencies were counted from the videotapes by the first author, who was blind to the patients’ treatment status and the chronological order of the videotapes." |
| Incomplete outcome data (attrition bias) | Unclear risk | Participants treated with low and medium dose were not entered to the analysis. ITT was conducted for 41 participants who were treated with high‐dose aminoacid or placebo. |
| Selective reporting (reporting bias) | High risk | Outcome data not reported for low‐ and medium‐dose treatment. Patient recruitment of these two groups was stopped after interim analysis. Full details of interim analysis results not reported. |
| Other bias | Unclear risk | The authors state that there were no differences between participants treated with high dose and placebo. However, details of the two other groups randomised to the trial have not been reported. |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: Chronic schizophrenia (DSM‐IV criteria) History: Duration of TD for a minimum of 5 years. Patient stabilisation duration was unclear but "all patients continued to receive antipsychotic treatment during the trial and treatment was unchanged throughout the study". | |
| Interventions | 1. Melatonin: dose 2 mg/day for 4 weeks. N = 9 2. Placebo for 4 weeks. N = 10 Concomitant medication not reported. | |
| Outcomes | Tardive dyskinesia: AIMS improved/not improved, deterioration in symptoms, AIMS scale scores. Adverse effects | |
| Notes | Sponsorship source: Melatonin (Circadin) and placebo tablets supplied by Neurim Pharmaceuticals, Tel Aviv, Israel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned". Although details of randomisation have not been reported. As allocation was done by the hospital pharmacy, it is assumed that random sequence generation was not biased. |
| Allocation concealment (selection bias) | Low risk | "Both patients and physicians were blinded to the group allocation and all medications were dispensed by the center's pharmacy and added to the patients' regular treatment regimens". |
| Blinding of participants and personnel (performance bias) | Low risk | "The patients were randomly assigned to receive placebo or melatonin 2mg/day, supplied in identical tablet form by Neurim Pharmaceuticals...Both patients and physicians were blinded to the group allocation and all medications were dispensed by the center's pharmacy and added to the patients' regular treatment regimens". |
| Blinding of outcome assessment (detection bias) | Low risk | TD symptoms' outcomes: "The patients were randomly assigned to receive placebo or melatonin 2mg/day, supplied in identical tablet form by Neurim Pharmaceuticals...Both patients and physicians were blinded to the group allocation and all medications were dispensed by the center's pharmacy and added to the patients' regular treatment regimens". "The same investigator... rated the individual patients throughout the trial." |
| Incomplete outcome data (attrition bias) | Low risk | "All 19 patients completed the 10‐week study." |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all outcomes previously stated were reported. A protocol is not available for verification. Also, although stated that "Prior to separating the data into the 2 treatment groups, the data were examined according to order...No carryover effects were demonstrated and baseline values did not differ significantly", data not reported per period. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: Schizophrenia (Structured Clinical Interview for DSM‐IV); antipsychotic‐induced TD (DSM‐IV criteria) History: Duration of TD not reported. Patient stabilisation duration unclear but "the antipsychotic medication regimens remained unchanged throughout the study". | |
| Interventions | 1. Melatonin: dose 10 mg/day for 6 weeks. N = 12 2. Placebo for 6 weeks. N = 10 Concomitant medications: "anticholinergics 12 patients; benzodiazepines, 5 patients; antidepressants, 4 patients; and mood stabilizers, 5 patients. The regimens of these additional medications also remained unchanged throughout the study" | |
| Outcomes | Leaving the study early. Adverse effects Unable to use ‐ Tardive dyskinesia: AIMS improved/not improved, deterioration in symptoms, AIMS scale scores (not reported separately for the first treatment phase before cross‐over to the next treatment). | |
| Notes | Sponsorship source: Sponsorship source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized," Although details of randomisation have not been reported, as allocation was done by the hospital pharmacy, it is assumed that random sequence generation was not biased. |
| Allocation concealment (selection bias) | Low risk | "Medication and placebo were dispensed by the hospital’s pharmacy and added to the patients’ ongoing treatment regimen." |
| Blinding of participants and personnel (performance bias) | Low risk | "using sealed envelopes."...tablets identical in appearance..."...unblinding code following database lock..." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details are provided. |
| Incomplete outcome data (attrition bias) | Low risk | "Two female patients were discharged from the hospital before initiation of the study and are not included in the analysis." Participants were randomised; 2/24(8%) were withdrawn before first dose of medication. It seems that the two withdrawn participants were originally randomised to the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all outcomes previously stated were reported. A protocol is not available for verification. |
| Other bias | Unclear risk | Insufficent information to make a judgement. |
| Methods | Allocation: “randomly assigned” Design: parallel Setting: inpatient (probably) China | |
| Participants | Diagnose: Antipsychotic‐induced TD. N = 76 Sex: male 26, female 50. Age: mean 56.1 (SD 9.12) years old History: Duration of TD not reported. Patient were stabilised before entry to trial but the duration is not reported. | |
| Interventions | 1. Melatonin Group: dose: 9 mg melatonin, oral taken before sleep for 12 weeks. N = 39 2. Control Group: no medication control group for 12 weeks. N = 37 Concomitant medication not reported. | |
| Outcomes | Cognitive function: Wechsler Adult Intelligence Scale (WAIS) ‐‐Unable to use (These outcomes were not predefined by this review and data were translated) VFT subscale‐score: animal, fruits, making words. RBANS, subscale‐score. The author did not report the total score and other subscales scores excluding the subscale‐semantic fluency; because a significant difference was only observed on the latter subscale. | |
| Notes | Funding source: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “ randomly assigned…” The author did not state the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | It’s not possible that the participants and personnel could be blinded. As the control group did not receive intervention in addition to antipsychotics. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about blinding outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | The authors reported all measured outcomes. |
| Other bias | Unclear risk | Insufficent information to make a judgement. |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: antipsychotic‐induced TD | |
| Interventions | 1. Levetiracetam: dose: 1500 mg twice a day (stable dose) for 8 weeks. N = 34. Concomitant medication not reported. | |
| Outcomes | Tardive dyskinesia: mean change of symptoms (hyperkinesia subscale of the St Hans rating scale). Adverse events | |
| Notes | Sponsorship source: Sponsored by UCB Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomized ..." Clinical Study Summary (CSS). Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | " ...double blind..." Clinical Study Summary (CSS). Details not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | " ...double blind..." Clinical Study Summary (CSS). Details not reported |
| Incomplete outcome data (attrition bias) | High risk | "All data are presented on the intent‐to‐Treat population (all randomized subjects who took at least one dose of study medication). ". Clinical Study Summary (CSS) The authors actually performed modified ITT. |
| Selective reporting (reporting bias) | High risk | Outcome data for 2/3 secondary outcomes (antipsychotic‐induced akathisia and other extrapyramidal symptoms, effect on the primary psychiatric disorder) not reported. Only summary data from Sponsor is available. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: schizophrenia* (no operational criteria). | |
| Interventions | 1. Gamma‐Linolenic acid supplementation (oil of evening primrose) 600 mg/day for 6 weeks. N = 8. Background medication and treatment continued throughout trial. 1 patient was on lithium carbonate. Other concomitant medications not reported. | |
| Outcomes | Tardive dyskinesia: AIMS improved/not improved, deterioration in symptoms, AIMS scale scores. | |
| Notes | Sponsorship source: Supported in part by Efamol, Ltd., and by the VA * one participant might have been bipolar. ** assume loss is 0 as all AIMS scores available after 6 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were assigned on a random, double‐blind basis" Details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment details not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind". Details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind". Details not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Although attrition details have not been reported, as end of trial data are reported individually for all randomised participants, it is assumed that all participants completed the trial. |
| Selective reporting (reporting bias) | Unclear risk | Indufficent information to make a judgement. As a protocol is not available, it is not possible to verify if all outcomes defined prior to study have been reported. (e.g. adverse events have not been reported) . |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
| Methods | Allocation: "assigned at random", not described. | |
| Participants | Diagnosis: schizophrenia/schizoaffective disorder (45), affective disorder (4), other psychiatric diagnosis (1) (DSM‐IV); and TD (Glazer‐Morgenstem criteria, i.e., total AIMS score ≥3, with at least 1 body area rated ≥2). History: Duration of TD in the levetiracetam group is 7.5 ± 8.4 years, whereas in the placebo group is 9.0±7.3 years. "sufficiently stable psychiatrically that their CMHC clinician indicated that changes in prescribed antipsychotic medication drug or dosage were not anticipated in the next 3 months". "Changes in the prescribed concomitant antipsychotic medications or their doses during the randomised phase occurred in 3 levetiracetam patients (12%) versus 5 placebo patients (20%)..." | |
| Interventions | 1. Levetiracetam: maximum dose 1500 mg* twice a day for 12 weeks. N = 25 2. Placebo for 12 weeks. N = 25 * side effects permitting and assuming lack of complete response, the dose was recommended to be escalated weekly by 500 mg/day to the maximum dose of 3000 mg/day, given in 2 divided doses. Concomitant medication: baseline anticholinergics (52%, levetiracetam group; 48% placebo group). Changes occurred in 2 placebo patients and none of the active patients. | |
| Outcomes | Tardive dyskinesia: AIMS Adverse effects. Unable to use () ‐ Mental State: Positive and Negative Syndrome Scale (PANSS); Young Mania Scale (YMRS); Montgomery‐Asberg Depression Rating Scale (MADRS); Hamilton Rating Scale for Anxiety (HAM‐A) | |
| Notes | Sponsorship source: Supported by a grant from UCB Pharma. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eligible patients who gave written informed consent were assigned at random", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double blind phase" no further details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double blind phase" no further details. |
| Incomplete outcome data (attrition bias) | Low risk | Completion rates (64% [N = 16] for the levetiracetam group and 80% [N = 20] for the placebo group, P = 0.345), reasons for discontinuation did not differ significantly between treatment groups. Data were reported for all participants randomized to the two groups (mixed‐effects model). |
| Selective reporting (reporting bias) | High risk | Primary outcome, total AIMS scores reported. However the authors stated that: "In addition to the principal analyses of the AIMS total score, we also investigated the relative rates at which subjects achieved remission, defined a priori as no longer meeting the Glazer‐Morgenstern TD entry criteria. " Remission data only reported as "...Group differences did not achieve statistical significance." Also, although not stated in the Protocol, the publication reported that: "At each study visit, patients were weighed and underwent an AIMS examination and symptom ratings using the Positive and Negative Syndrome Scale (PANSS); Young Mania Rating Scale (YMRS), Montgomery Asberg Depression Rating Scale (MADRS), and the Hamilton Rating Scale for Anxiety (HAM‐A) administered via a structured interview guide. Adverse events were rated using the Systematic Assessment for Treatment Emergent Events, general inquiry method. Complete blood counts were obtained at baseline and at 6, 12". Only baseline data for these outcomes have been reported. |
| Other bias | Low risk | "...none of the interactions with baseline were statistically significant...Further analyses revealed that the small age difference at baseline did not confound the levetiracetam treatment effect, nor did the small baseline differences in years of education, antipsychotic chlorpromazine equivalent dose, antipsychotic type at baseline (only atypical vs. any conventional), or gender." |
| Methods | Allocation: "randomly assigned”. Design: parallel. Setting: inpatients, China. Duration: 12 weeks. | |
| Participants | Diagnosis: Antipsychotics‐induced TD (Research Diagnosis Criteria for TD, RD‐TD) N = 36 (2 participants left the study early due to discharge) Sex: male 18 , female 16 Age: mean 50.2 years old, SD 13.4 years old. | |
| Interventions | 1. Promethazine Group: (n = 18) Management: 50 mg promethazine was administrated by IM, twice per day for 2 weeks following with a 2‐week period of IV drip (50 mg promethazine dissolved in 500 mL normal saline, once per day). This 4‐week treatment regimen was conducted three circles in 12 weeks. 2. Placebo Group: (n = 16) Management: The same treatment regimen as promethazine. The placebo drug was 2 mL normal saline with same appearance as promethazine. All participants received antipsychotics as usual but were not allowed to use clozapine, anticholinergic drug, vitamin E or calcium channel blocker. | |
| Outcomes | TD symptoms: improvement, AIMS | |
| Notes | *defined by the 4‐level clinical response standard, recovery, obvious improvement, improvement, no clinical response. Funding source: not reported. Paper in Chinese, assessed and data extracted by Sai Zhao. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ”randomly assigned”, no further details. Although the author did not state the method of randomisation, we have rated selection bias as low: because central allocation was used, it is very likely that an adequate randomisation sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Central allocation, pharmacy‐controlled randomisation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double blind", no details of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | ”The outcome assessor was blinded” |
| Incomplete outcome data (attrition bias) | Low risk | All participants competed the study. |
| Selective reporting (reporting bias) | Low risk | The author reported all measured outcomes. |
| Other bias | Low risk | None obvious. |
| Methods | Allocation: "randomly assigned…" Design: parallel Setting: inpatients | |
| Participants | Diagnose: Antipsychotics‐induced TD Total: N = 42 Sex: male 28, female 14 Age: mean˜32.5 years old, SD˜10.3years old. Length of illness (schizophrenia): mean˜ 7.5 years, SD ˜3.4 years | |
| Interventions | 1. Buspirone Group: (n = 21) Management: The initial dosage, one capsule each day, was titrated to 6‐12 capsules each day within 10 days. 2. Placebo Group: (n = 21) Management: The initial dosage, one capsule each day, was titrated to 6‐12 capsules each day within 10 days. All participants received stable AP and concomitant anticholinergic drug. | |
| Outcomes | Clinical response* Adverse events: dizziness, headache, nausea, vomiting ‐‐Unable to use | |
| Notes | Funding source: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned…" The author did not state the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | The author did not state the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind study , the interventions were coded as intervention A or B by the researcher in pharmacy ". "Participants and personnel did not know the allocation result. The two drugs were contained in capsules with same appearance" Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | All participants competed the study. |
| Selective reporting (reporting bias) | Low risk | The author reported all measured outcomes. |
| Other bias | Low risk | None obvious. |
| Methods | Allocation: "randomly assigned…." Design: parallel Setting: inpatients | |
| Participants | Diagnosis: Antipsychotic induced TD. N = 46 Sex: male 30, female 16. Age: mean ˜33 years old, SD˜10 years old. History: Duration of TD on average 2.2 years (SD˜1.7 years). Patients stabilised prior to study for 5 ± 4 years. | |
| Interventions | 1. Pemoline Group: (n = 23) Management: two capsules per day for six days per week, oral taken before breakfast. 2. Placebo Group: (n = 23) Management: two capsules per day for six days per week, oral taken before breakfast. All participants received stable AP and concomitant anticholinergic drug. | |
| Outcomes | Clinical response* ‐‐Unable to use Blood routine examination, urine routine test and liver function test, electrocardiography, electroencephalogram (the author only stated results of these tests were normal but did not report the data) | |
| Notes | Funding source: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned". The author did not state the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study), the interventions were coded as intervention I or II by the researcher in pharmacy. “Participants and personnel did not know the allocation result. The two drugs were contained in capsules with same appearance.” Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | All participants competed the study. |
| Selective reporting (reporting bias) | Low risk | The author reported all measured outcomes. |
| Other bias | Low risk | None obvious. |
| Methods | Allocation: "randomized", described. | |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia; diagnosis of TD based on the Schooler‐Kane criteria. History: Duration of TD at least 1 year. "A stable dose of antipsychotics for at least 4 weeks prior to trial entry". | |
| Interventions | 1. EGb‐761 (standardised extract of Ginkgo biloba leaves that has antioxidant properties as a free radical scavenger): dose 80 mg, three times a day for 12 weeks. N = 78 2. Placebo for 12 weeks. N = 79 Patient regular antipsychotic medication. Antipsychotics and all other medications remained fixed throughout the double‐blind period (i.e. clozapine, risperidone, aripiprazole, olanzapine, quetiapine, chlorpromazine, haloperidol, sulpiride). Their chlorpromazine‐equivalent doses were 429.3 mg/day and 440.2 mg/day in the EGb‐761 and placebo groups, respectively. Anticholinergics were allowed during the trial. Twenty‐seven patients (12 in the EGb‐761 group and 15 in the placebo group) were treated with anticholinergics for a long time prior to entering the study; however, anticholinergic treatment was stable during the clinical trial. No new use of anticholinergic drugs was allowed. | |
| Outcomes | Tardive dyskinesia: AIMS improved/not improved, deterioration in symptoms, AIMS scale scores. Leaving the study early. | |
| Notes | Sponsorship source: Supported by the National Basic Research Program of China and the National Natural Science Foundation of China. Statistical analysis was funded by Glaxo Smith Kline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients had an equal probability of being assigned to the 2 groups. An independent third party placed them in either the active or placebo group according to a computer‐generated randomization list compiled through simple randomization" |
| Allocation concealment (selection bias) | Low risk | "To ensure the concealment of allocation, this third party used a protected computer database for the randomization list." |
| Blinding of participants and personnel (performance bias) | Low risk | "After the run‐in period, patients were randomly assigned to receive either capsulized EGb‐761... or an identically capsulized placebo in a double‐blind manner". "All of the study personnel and participants were blinded to the treatment assignment for the duration of the double‐blind period, except 1 study staff member in the pharmacy, who remained unblinded to provide the placebo and EGb‐761 treatments" |
| Blinding of outcome assessment (detection bias) | Low risk | All efficacy outcomes: "Each subject was assessed by the same investigator, who was blind to treatment status". Cognitive tests: computer assessments. |
| Incomplete outcome data (attrition bias) | Low risk | "intention‐to‐treat analysis for the 2 groups of randomly assigned patients, with last‐observation‐carried‐forward imputation." |
| Selective reporting (reporting bias) | High risk | Side effects and Adverse Events data not reported. "Systemic side effects were evaluated by means of routine physical and neurologic examinations and laboratory tests and reviewed by applying the UKU Side Effect Rating Scale. These systemic side effects were all mild and brief. For none of the subjects were the routine blood cell count, chemistry, urinalysis, or electrocardiogram parameters significantly affected by the experimental treatment (data not shown)". Also, Change in Simpson‐Angus Rating Scales for EPS not reported. |
| Other bias | Low risk | The study seems to be free from other sources of bias. |
AIMS ‐ Abnormal Involuntary Movement Scale
AVLT ‐ Rey Auditory and Verbal Learning Test
BPRS ‐ Brief Psychiatric Rating Scale
CGI ‐ Clinical Global Impression
DSM ‐ Diagnostic and Statistical Manual of Mental Disorders
EPS ‐ extrapyramidal symptoms
ESRS ‐ Extrapyramidal System Rating Scale
IM ‐ intramuscular
ITT ‐ intention‐to‐treat
IV ‐ intravenous
MAOI ‐ monoamine oxidase inhibitor
mcg ‐ microgram
NOSIE ‐ Nurses Observation Scale for Inpatient Evaluation
PANSS ‐ Positive and Negative Syndrome Scale
Rockland TD ‐ Rockland Tardive Dyskinesia Rating Scale
SANS ‐ Scale for the Assessment of Negative Symptoms
SAPS ‐ Scale for the Assessment of Positive Symptoms
SAS ‐ Simpson Angus Scale
SD ‐ Standard deviation
TAU ‐ treatment as usual
TD ‐ tardive dyskinesiaTESS ‐ Treatment Emergent Symptoms Scale
VMAT ‐ vesicular monoamine transporter 2
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised Participants: TD Intervention: Deanol vs placebo, not relevant for this review, included in Cholinergics review | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. Participants: TD. Interventions: Methylphenidate vs placebo. Outcomes: No outcome data have been provided for the first phase before cross‐over. Author was contacted but no information was received and this over 40 years old study was excluded. | |
| Allocation: randomised. Participants: schizophrenia (DSM‐III‐R) and TD. Interventions: Electromyographic biofeedback vs noncontingent "false" feedback (placebo). Outcomes: No useable data were extractable. Study author was contacted but no information was received and this 25 years old study was excluded. | |
| Allocation: not randomised. | |
| Allocation: randomised. Participants: Psychiatric inpatients with a BLM syndrome induced by antipsychotic treatment; psychiatric‐neurological condition stable and pharmacological treatment unchanged for at least 2 months. Interventions: Lithium vs placebo. Outcomes: All data unusable. Unable to extract from the fist phase of cross‐over. We were unable to find up‐to‐date contact details for the study authors of this over 40 years old study; it was excluded. | |
| Allocation: unclear if randomised. Participants: people with Parkinson's disease and not TD symptoms at baseline | |
| Allocation: randomised Participants: TD Intervention: Alpha‐methyldopa vs. reserpine vs. placebo, not relevant for this review, included in the Non‐antipsychotics review. | |
| Not randomised, controlled clinical trial. | |
| Allocation: randomised. | |
| Allocation: randomised Interventions: Piracetam versus placebo administered IV in 1‐hour intervals. Outcomes: not useable, measured 30 mins after intervention. | |
| Allocation: randomised Interventions: Piracetam 2.4 g/d vs Piracetam 4.8 g/d vs Piracetam 10 g/d vs placebo for 4 weeks. Outcomes: no usable data. We were unable to find up‐to‐date contact details for the study authors of this over 30 years old study; it was excluded. | |
| Allocation: randomised. Participants: Various dyskinesias and movement disorders, including TD. Interventions: Oestrogen vs. placebo Outcomes: No usable data since they are reported in figures with no variability measures. No reply from author. Study is over 30 years old and was excluded. | |
| Allocation: not randomised. | |
| Allocation: randomised | |
| Allocation: randomised Participants: not TD, participants classified as TD or not TD only during measuring outcomes after intervention | |
| Allocation: randomised. Participants: DSM‐III diagnoses were predominantly schizophrenia (N = 16) and included four cases of major affective disorder. All participants had TD for at least 1 year. Interventions: Nalaxone 20g vs Nalaxone 40 g vs placebo. Outcomes: No outcome data have been provided for the first period before cross‐over. Author was contacted but no information was received and this over 25 years old study was excluded. | |
| Allocation: not randomised. | |
| Allocation: randomised. Participants: Psychiatric patients; Schooler and Kane criteria for persistent TD. Interventions: AMPT vs L‐DOPA vs Cholone chloride vs Valproic acid vs Hydroxytryptophan. Outcomes: No outcome data has been provided for the first period before cross‐over. Author was contacted but no information was received and this 30 years old study was excluded. | |
| Allocation: randomised. Participants: people with chronic schizophrenia with multiple hospitalisations and averaged 5.5 years of TD. Interventions: Monosialotetrahexosylganglioside (GM1) versus placebo. Outcomes: All data unusable. Authors were contacted and confirmed data were inaccessible. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. Participants: Parkinson's disease, not TD. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. Participants: chronic schizophrenia patients with symptoms of TD. Interventions: Muscimol vs placebo. Outcomes: No useable data were extractable. Study author was contacted but no information was received and this over 30 years old study was excluded. | |
| Allocation: randomised Interventions: Efamol (essential fatty acid) + Vitamin E vs liquid paraffin + vitamin E. Outcomes: No outcome data have been provided for the first period before cross‐over. Study author was contacted but no information was received and this over 25 years old study was excluded. | |
| Allocation: randomised | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised Interventions: Naltrexone vs placebo and Naltrexone + clonazepam vs placebo + clonazepam. Outcomes: No outcome data have been provided for the first phase before cross‐over. Study author was contacted but no information was received and this over 10 years old study was excluded. |
AIMS: Abnormal involuntary Movement Scale
CGI ‐ Clinical Global Impression
DSM ‐ Diagnostic and Statistical Manual of Mental Disorders
TD = tardive dyskinesia
IV = intravenous
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Allocation: randomised. |
| Participants | Diagnosis: History of using a dopamine receptor antagonist for at least 3 months; clinical diagnosis of tardive dyskinesia and has had symptoms for at least 3 months prior to screening; participants with underlying psychiatric diagnosis are stable and have no change in psychoactive medications N = 117 Sex: male and female Age:18 to 75 years old |
| Interventions | 1. SD‐809 (deutetrabenazine) tablets taken twice daily for 12 weeks, includes a dose titration period and maintenance period. N = 58 2. Placebo tablets taken twice daily for 12 weeks. N = 59 |
| Outcomes | Change in AIMS score from baseline to week 12. Quality of life; Treatment success based on Patient Global Impression of Change (PGIC) questionnaire; Percentage reduction in AIMS score; Proportion of responders based on AIMS change from baseline |
| Notes | Other study ID: SD‐809‐C‐18 Sponsor: Auspex Pharmaceuticals, Inc. From 2017 update search, conference abstract with limited details, awaiting more detailed data. |
| Methods | Allocation: randomised. |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, or mood disorder (for at least 3 months prior to screening); a clinical diagnosis of antipsychotic‐induced TD for at least 3 months prior to screening; if using maintenance medication(s), be on stable doses; negative drug screen for amphetamines, barbiturates, benzodiazepines, phencyclidine, cocaine, opiates, or cannabinoids N = 240 Sex: male and female Age: 18 to 85 years old |
| Interventions | 1. NBI‐98854 administered orally as one 40 mg capsule and one placebo capsule for 6 weeks. At the end of week 6, participants will enter a double‐blind NBI‐98854 treatment period and continue with their current dose N = 80 2. Participants randomised to the NBI‐98854 80 mg dose will receive NBI‐98854 40 mg for the first week (administered orally as one 40 mg capsule and one placebo capsule), followed by NBI‐98854 80 mg administered orally as two 40 mg capsules for 5 weeks. At the end of week 6, participants will enter a double‐blind NBI‐98854 treatment period and continue with their current dose. N = 80 3. Placebo administered orally as two placebo capsules 6 weeks. At the end of Week 6, participants will enter a double‐blind NBI‐98854 treatment period and be randomised to either a 40 mg or 80 mg dose. N = 80 |
| Outcomes | Severity of TD symptoms assessed by AIMS, change from baseline Clinical Global Impression of Change ‐ TD (CGI‐TD); Tardive Dyskinesia Impact Scale (TDIS); Patient Global Impression of Change (PGIC); Percentage of participants classified as responders based on AIMS dyskinesia total score % change from baseline; Percentage of participants classified as responders based on CGI‐TD |
| Notes | Study ID: NCT02274558 From 2017 update search, conference abstracts, poster, and online trial registration record with limited details, awaiting more detailed data. |
| Methods | Allocation: randomised |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder and a clinical diagnosis of antipsychotic‐induced TD (DSM‐IV) for at least 3 months prior to screening; stable dose of antipsychotic medication for a minimum of 30 days before study start; participants not using antipsychotic medication must have stable psychiatric status; the doses of concurrent medications and the conditions being treated be stable for a minimum of 30 days before study start and be expected to remain stable during the study. N = 37 Sex: male and female Age: 18 to 65 years old |
| Interventions | 1. 12.5 mg NBI‐98854 once daily dose for days 1‐14 and placebo once daily dose for days 15‐28 (participants will be randomly assigned to receive one of these treatment sequences) 2. Placebo once daily dose for days 1‐14 and 50 mg NBI‐98854 once daily dose for days 15 mg to 28 mg or 50 mg NBI‐98854 once‐daily dose for days 1‐14 and placebo once‐daily dose for days 15‐28 (participants will be randomly assigned to receive one of these treatment sequences) |
| Outcomes | Treatment efficacy (AIMS) Efficacy of 12.5 mg or 50 mg doses of NBI‐98854 administered once daily for the treatment of TD symptoms using CGI‐TD and PGIC questionnaire Safety and tolerability for adverse events |
| Notes | Other study ID: NBI‐98854‐1101 Online trial registration record, results are provided for combined groups in this cross‐over study, awaiting separate data. |
| Methods | Randomised, double‐blind, sham‐controlled study, 22 months duration. |
| Participants | Diagnosis: psychosis with TD (Schooler and Kane criteria). |
| Interventions | 10 right dorsolateral prefrontal cortex‐repetitive transcranial magnetic stimulation (rTMS) sessions versus 10 sham rTMS sessions, total n = 30. |
| Outcomes | AIMS, MMSE, CPRS. |
| Notes | From 2017 update search, conference abstract with limited details, awaiting more detailed data. |
| Methods | Randomised controlled trial, 12 weeks duration. |
| Participants | Diagnosis: schizophrenia (CHMD‐3) with tardive dyskinesia. |
| Interventions | Ginkgo biloba extract (n = 42) versus standard treatment (n = 40). |
| Outcomes | AIMS, TESS. |
| Notes | From 2017 update search, article in Chinese, awaiting translation. |
AIMS ‐ Abnormal involuntary Movement Scale
CGI ‐ Clinical Global Impression
CHMD‐3 ‐ Chinese Classification of Mental Disorders Version 3
CPRS ‐ Comprehensive Psychopathological Scale
DSM IV ‐ Diagnostic and Statistical Manual of Mental Disorders
MMSE ‐ Mini Mental State Examination
PGIC ‐ Patient Global Impression of Change
rTMS ‐ right dorsolateral prefrontal cortex‐repetitive transcranial magnetic stimulation
TD ‐ tardive dyskinesia
TESS ‐ Treatment Emergent Symptom Scale
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Double‐blind placebo controlled study using buspirone in the treatment of tardive dyskinesia |
| Methods | Allocation: randomised. |
| Participants | Diagnosis: TD patients, criteria not reported. |
| Interventions | 1. Buspirone: Not reported, increasing dose. N = 20. |
| Outcomes | AIMS score |
| Starting date | |
| Contact information | |
| Notes | Abstract of a study protocol, there are no data to be extracted. |
| Trial name or title | Investigation of the potential beneficial effects of cannabidiol in the treatment of tardive dyskinesia |
| Methods | Allocation: randomised Blindness: double‐blind (no further details) Duration: 1 year Design: parallel Setting: Nigeria |
| Participants | Diagnosis: ICD‐10 diagnosis of a psychotic disorder, verified with the MINI‐PLUS questionnaire; clinical diagnosis of TD confirmed with the AIMS; currently receiving treatment for a psychotic disorder and be on either the atypical or conventional antipsychotics. N = 56 Sex: male and female Age: at least 18 years old |
| Interventions | 1. High cannabidiol extract Nabidiolex® (CBD) (300 mg) administered orally in capsules twice a day for six weeks as an adjunctive treatment alongside their usual antipsychotic medication. N = 28 |
| Outcomes | Primary: Improvement in symptoms of TD measured using the Abnormal Involuntary Movement Scale (AIMS). Secondary: Side effects of CBD; improvement in psychotic symptoms. |
| Starting date | December 2015 |
| Contact information | Dr Jaiyeola Kajero, +234 08037140976, [email protected], Nigeria |
| Notes | Sponsor: South Africa Research Chair in PTSD, Stellenbosch University |
| Trial name or title | Efficacy of docosahexaenoic acid on tardive dyskinesia |
| Methods | Allocation: randomised. |
| Participants | Diagnosis: chronic schizophrenia; under long‐term antipsychotic drug treatment, stable for at least 3 months before study entry; presence of TD following Schooler‐Kane research criteria (mild intensity (2/4 points) in at least two body segments, or moderate intensity (3/4 points) for at least one body segment). N = 40 Sex: male and female Age: 30‐75 years |
| Interventions | 1. Omega‐3 fish oil capsules (including docosahexaenoic acid): Fish oil capsules of 1000 mg each, including DHA 460 mg‐540 mg/capsule; dose: 6 capsules per day; N = 20. 2. Placebo: Corn/Soybean placebo 1000 mg capsules; dose: 6 capsules per day; N = 20. |
| Outcomes | Primary: Clinical rating scales (AIMS, St.Hans) Secondary: Quantitative motor testing (kinematic parameters); Monitoring of psychopathology (Neuro‐Psychiatric Inventory, Positive and Negative Syndrome Scale, Calgary Depression Scale for Schizophrenia); Erythrocyte membrane phospholipid profile (gas chromatography) |
| Starting date | February 2008 |
| Contact information | Pierre J. Blanchet, MD, PhD, (514) 890‐8123, [email protected] |
| Notes | Sponsor: Université de Montréal, National Alliance for Research on Schizophrenia and Depression Study IDs: NCT00621634; HD06.067 |
| Trial name or title | Melatonin treatment for tardive dyskinesia in schizophrenia. |
| Methods | Allocation: randomised |
| Participants | Diagnosis: diagnosis of both schizophrenia and TD; duration of TD symptoms longer than 1 year; on stable doses of antipsychotic drug for at least 6 months. N = 120 Sex: male and female Age: 18 to 70 years old |
| Interventions | 1. Melatonin (other Name: APRD00742)10mg/day, 12‐week treatment. N = 60. 2. Placebo 10mg/day, 12‐week treatment. N = 60 |
| Outcomes | Primary: AIMS at 12 weeks Secondary: the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); the Positive and Negative Syndrome Scale (PANSS); the Simpson‐Angus Scale for extrapyramidal side effects (SAS) |
| Starting date | September 2008 |
| Contact information | Lian Y Cao, MD, Beijing Hui‐Long‐Guan Hospital |
| Notes | Sponsor: Beijing HuiLongGuan Hospital, Stanley Medical Research Institute |
| Trial name or title | NBI‐98854 for the for the treatment of tardive dyskinesia in subjects with schizophrenia or schizoaffective disorder (KINECT study) |
| Methods | Allocation: randomised |
| Participants | Diagnosis: Clinical diagnosis of schizophrenia or schizoaffective disorder and a clinical diagnosis of antipsychotic‐induced TD for at least 3 months prior to screening;receiving a stable dose of antipsychotic medication for a minimum of 30 days before study start; participants not using antipsychotic medication must have stable psychiatric status;the doses of concurrent medications and the conditions being treated be stable for a minimum of 30 days before study start and be expected to remain stable during the study. N = 109 Sex: male and female Age: 18 to 65 years old |
| Interventions | 1. NBI‐98854 50 mg administered as two 25 mg capsules by mouth, taken every morning for 6 weeks. N = NR 2. NBI‐98854 100 mg and 50 mg. NBI‐98854 100 mg administered as two 50 mg capsules taken every morning between for 2 weeks. After 2 weeks, NBI‐98854 50 mg administered by two 25 mg capsules by mouth, taken every morning for remaining 4 weeks. N = NR 3. Placebo capsule containing no active substance, manufactured to mimic NBI‐98854 25 mg and 50 mg capsules. N = NR |
| Outcomes | Primary: Severity of TD symptoms assessed by AIMS Secondary: CGI‐TD; Number of participants with adverse events following dosing with NBI‐98854; Evaluation of plasma concentrations of NBI‐98854 and metabolites following repeated daily doses (50 mg and 100 mg) of NBI‐98854 |
| Starting date | September 2012 |
| Contact information | Principal Investigator: Chris O'Brien, MD |
| Notes | Other study ID: NBI‐98854‐1201 |
| Trial name or title | D‐Serine treatment for tardive dyskinesia |
| Methods | Allocation: randomised |
| Participants | Diagnosis: Diagnosis of schizophrenia/schizoaffective disorder according to DSM‐IV criteria; history of at least 3 months antipsychotic drugs treatment and present stable dose antipsychotic treatment for at last 4 weeks; fulfilment of Schooler‐Kane TD research criteria on a first evaluation performed 2‐12 weeks prior to study entrance and on a subsequent evaluation performed prior to allocation to experimental treatment. N = 60 Sex: male and female Age: 18 to 70 years old |
| Interventions | 1. D‐Serine (amino acid) 4 g/dN for 8 weeks. N = 30 2. Placebo adjuvant treatment for 8 weeks. N = 30 |
| Outcomes | Primary: Change in AIMS total score |
| Starting date | January 2013 |
| Contact information | Contact: Uriel Heresco‐Levy, MD, +972‐2‐5316906, [email protected] |
| Notes | Other Study ID Numbers: 1600 |
| Trial name or title | Addressing involuntary movements in tardive dyskinesia (AIM‐TD) |
| Methods | Allocation: randomised. |
| Participants | Diagnosis: History of using a dopamine receptor antagonist for at least 3 months; clinical diagnosis of TD and has had symptoms for at least 3 months prior to screening, participants with underlying psychiatric diagnosis are stable and have no change in psychoactive medications. N = 288 Sex: male and female Age: 18 to 80 years old |
| Interventions | 1. SD‐809 12 mg tablets dose titrated for 4 weeks to target randomised dose. The dose is maintained for an additional 8 weeks. N = 72 2. SD‐809 24 mg tablets dose titrated for 4 weeks to target randomised dose. The dose is maintained for an additional 8 weeks. N = 72 3. SD‐809 36 mg tablets dose titrated for 4 weeks to target randomised dose. The dose is maintained for an additional 8 weeks. N = 72 4. Placebo tablets taken twice daily for 12 weeks. N = 72 |
| Outcomes | Primary: Change in AIMS score from Baseline to Week 12 Secondary:Quality of Life; treatment success based on Patient Global Impression of Change (PGIC) questionnaire; percentage reduction in AIMS score; proportion of responders based on AIMS score change from baseline. |
| Starting date | October 2014 |
| Contact information | Teva US Medical Information (+1‐800‐896‐5855) |
| Notes | Auspex Pharmaceuticals, Inc. |
AIMS ‐ Abnormal Involuntary Movement Scale
CGI ‐ Clinical Global Impression
DHA ‐ docosahexaenoic acid
DSM ‐ Diagnostic and Statistical Manual of Mental Disorders
ICD ‐ International Classification of Diseases
PGIC ‐ Patient Global Impression of Change
TD = tardive dyskinesia
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.97] |
| Analysis 1.1  Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.23, 1.09] |
| Analysis 1.2 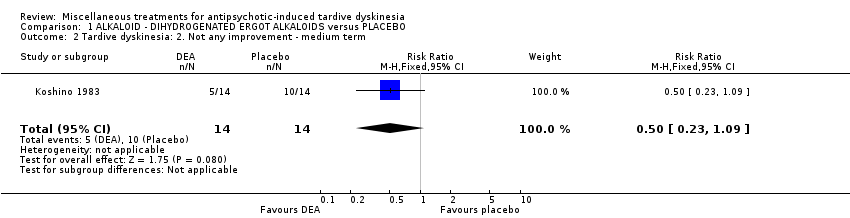 Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term. | ||||
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.55] |
| Analysis 1.3 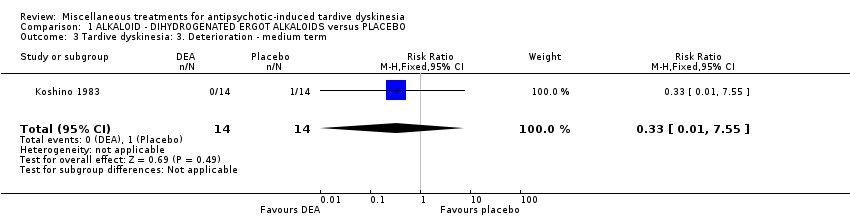 Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term. | ||||
| 4 Tardive dyskinesia: 3. Average endpoint scale score (Simpson scale, high=poor) ‐ medium term Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐12.25, 6.65] |
| Analysis 1.4  Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 4 Tardive dyskinesia: 3. Average endpoint scale score (Simpson scale, high=poor) ‐ medium term. | ||||
| 5 Tardive dyskinesia: Average scale change scores (various scales, high=poor) ‐ medium term Show forest plot | 2 | 59 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.83, 0.20] |
| Analysis 1.5  Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 5 Tardive dyskinesia: Average scale change scores (various scales, high=poor) ‐ medium term. | ||||
| 5.1 AIMS+RTDS | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.93, 0.32] |
| 5.2 ADS | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.24, 0.57] |
| 6 Mental state: Deterioration ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.90] |
| Analysis 1.6  Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 6 Mental state: Deterioration ‐ medium term. | ||||
| 7 Adverse events ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.75, 7.23] |
| Analysis 1.7  Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 7 Adverse events ‐ medium term. | ||||
| 8 Leaving the study early ‐ medium term Show forest plot | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| Analysis 1.8  Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 8 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.35, 0.82] |
| Analysis 2.1  Comparison 2 ALKALOID ‐ L‐STEPHOLIDINE (SPD) versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Mental state: 1. Average endpoint scale score (BPRS, high=poor) ‐ medium term Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐7.60, ‐1.40] |
| Analysis 2.2 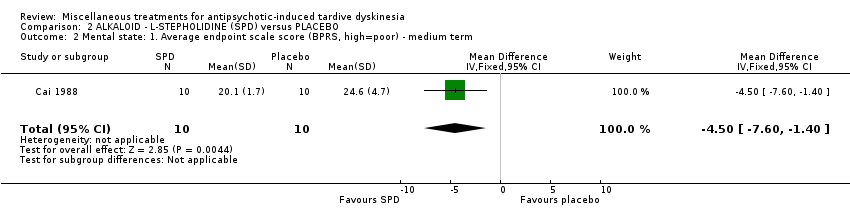 Comparison 2 ALKALOID ‐ L‐STEPHOLIDINE (SPD) versus PLACEBO, Outcome 2 Mental state: 1. Average endpoint scale score (BPRS, high=poor) ‐ medium term. | ||||
| 3 Adverse events: any adverse events ‐ medium term Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.3  Comparison 2 ALKALOID ‐ L‐STEPHOLIDINE (SPD) versus PLACEBO, Outcome 3 Adverse events: any adverse events ‐ medium term. | ||||
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.4  Comparison 2 ALKALOID ‐ L‐STEPHOLIDINE (SPD) versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐1.18, 2.20] |
| Analysis 3.1  Comparison 3 ALKALOID ‐ PAPAVERINE versus PLACEBO, Outcome 1 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.63, 1.00] |
| Analysis 4.1  Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term. | ||||
| 2 Tardive dyskinesia: 1. Not any improvement ‐ short term Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.11] |
| Analysis 4.2  Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 2 Tardive dyskinesia: 1. Not any improvement ‐ short term. | ||||
| 3 Tardive dyskinesia: 2. Deterioration ‐ short term Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.19] |
| Analysis 4.3 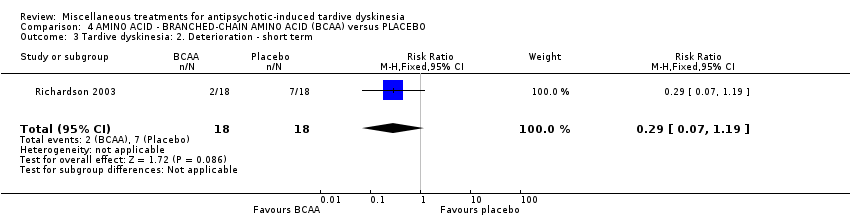 Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 3 Tardive dyskinesia: 2. Deterioration ‐ short term. | ||||
| 4 Tardive dyskinesia: Average endpoint score (Simpson scale, high=poor) ‐ short term Show forest plot | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐92.9 [‐167.57, ‐18.23] |
| Analysis 4.4  Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 4 Tardive dyskinesia: Average endpoint score (Simpson scale, high=poor) ‐ short term. | ||||
| 5 Leaving the study early ‐ short term Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.37, 1.92] |
| Analysis 4.5 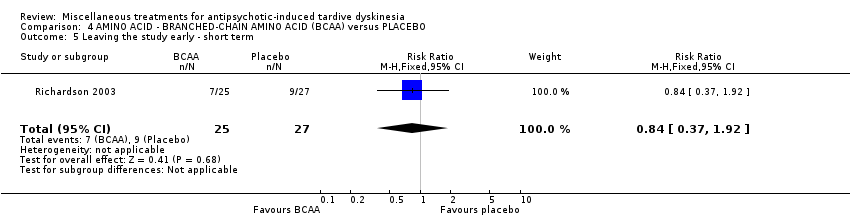 Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 5 Leaving the study early ‐ short term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early ‐ short term Show forest plot | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.11, 53.25] |
| Analysis 5.1  Comparison 5 AMINO ACID ‐ PHENYLALANINE versus PLACEBO, Outcome 1 Leaving the study early ‐ short term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.96, 1.94] |
| Analysis 6.1  Comparison 6 ANTIDEPRESSANT (MAO‐B inhibitor) ‐ SELEGILINE versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Leaving the study early ‐ medium term Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.39 [0.62, 173.97] |
| Analysis 6.2 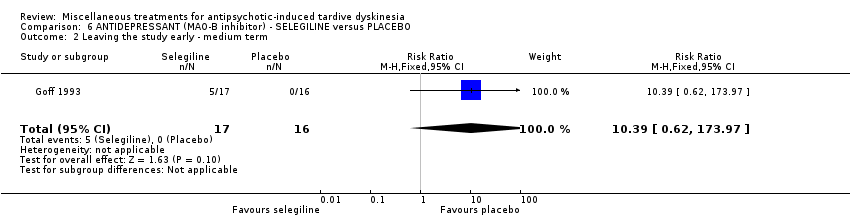 Comparison 6 ANTIDEPRESSANT (MAO‐B inhibitor) ‐ SELEGILINE versus PLACEBO, Outcome 2 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ long term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.08, 0.71] |
| Analysis 7.1 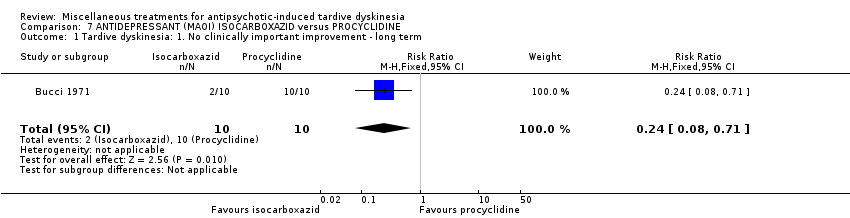 Comparison 7 ANTIDEPRESSANT (MAOI) ISOCARBOXAZID versus PROCYCLIDINE, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ long term. | ||||
| 2 Tardive dyskinesia: 1. Not any improvement ‐ long term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.64] |
| Analysis 7.2  Comparison 7 ANTIDEPRESSANT (MAOI) ISOCARBOXAZID versus PROCYCLIDINE, Outcome 2 Tardive dyskinesia: 1. Not any improvement ‐ long term. | ||||
| 3 Adverse effects ‐ long term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| Analysis 7.3 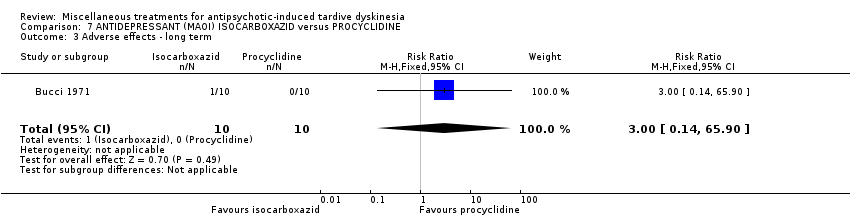 Comparison 7 ANTIDEPRESSANT (MAOI) ISOCARBOXAZID versus PROCYCLIDINE, Outcome 3 Adverse effects ‐ long term. | ||||
| 4 Leaving the study early ‐ long term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| Analysis 7.4  Comparison 7 ANTIDEPRESSANT (MAOI) ISOCARBOXAZID versus PROCYCLIDINE, Outcome 4 Leaving the study early ‐ long term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: No clinically important improvement (short term) Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.70, 1.43] |
| Analysis 8.1  Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 1 Tardive dyskinesia: No clinically important improvement (short term). | ||||
| 2 Tardive dyskinesia: Not any improvement (short term) Show forest plot | 1 | 10 | Risk Ratio (IV, Fixed, 95% CI) | 0.28 [0.02, 4.66] |
| Analysis 8.2 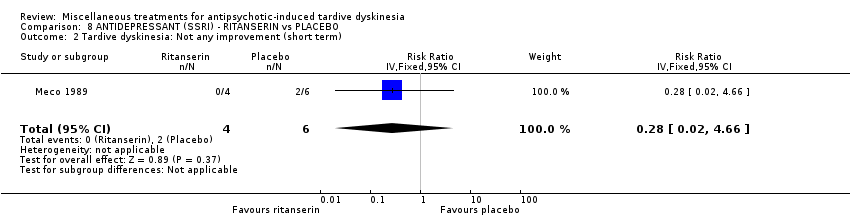 Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 2 Tardive dyskinesia: Not any improvement (short term). | ||||
| 3 Tardive dyskinesia: Deterioration (short term) Show forest plot | 1 | 10 | Risk Ratio (IV, Fixed, 95% CI) | 0.47 [0.02, 9.26] |
| Analysis 8.3 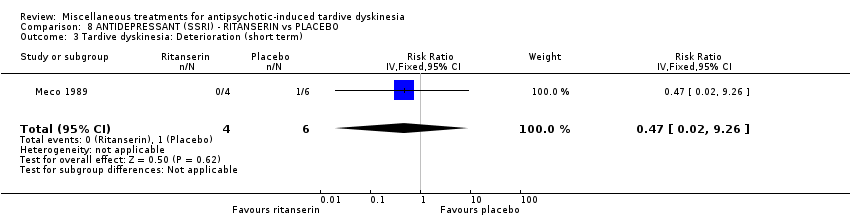 Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 3 Tardive dyskinesia: Deterioration (short term). | ||||
| 4 Tardive dyskinesia: Average change score (AIMS, high=poor) (short term) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.93, 1.93] |
| Analysis 8.4  Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 4 Tardive dyskinesia: Average change score (AIMS, high=poor) (short term). | ||||
| 5 General mental state: Deterioration (short term) Show forest plot | 1 | 10 | Risk Ratio (IV, Fixed, 95% CI) | 0.47 [0.02, 9.26] |
| Analysis 8.5 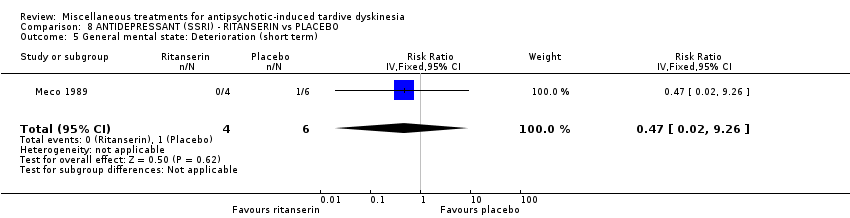 Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 5 General mental state: Deterioration (short term). | ||||
| 6 General mental state: Average change score (BPRS, high=poor) (short term) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐3.10, 1.50] |
| Analysis 8.6 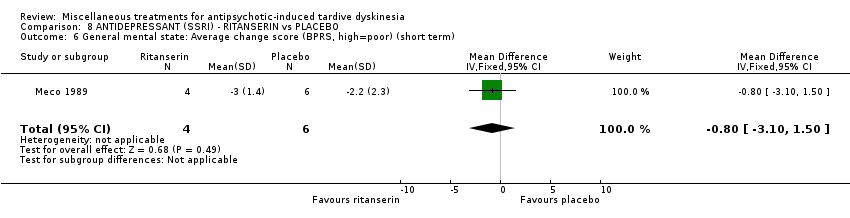 Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 6 General mental state: Average change score (BPRS, high=poor) (short term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. Average endpoint score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.18 [‐3.65, ‐0.71] |
| Analysis 9.1  Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. Average endpoint score (AIMS, high=poor) ‐ medium term. | ||||
| 2 Tardive dyskinesia: 1. Average change score (hyperkinesia subscale of the SHRS , high=poor) ‐ medium term Show forest plot | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.73, 0.99] |
| Analysis 9.2  Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 2 Tardive dyskinesia: 1. Average change score (hyperkinesia subscale of the SHRS , high=poor) ‐ medium term. | ||||
| 3 Leaving the study early ‐ medium term Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.46, 2.22] |
| Analysis 9.3 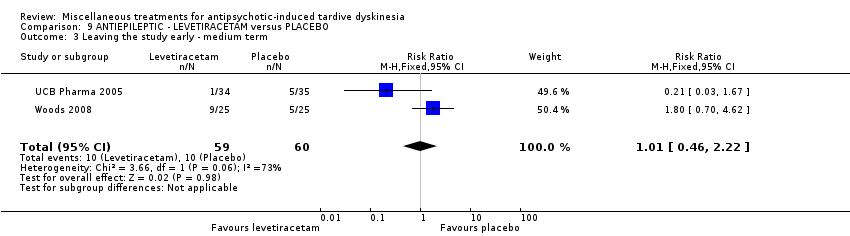 Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 3 Leaving the study early ‐ medium term. | ||||
| 4 Adverse effects ‐ medium term Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.25, 1.04] |
| Analysis 9.4 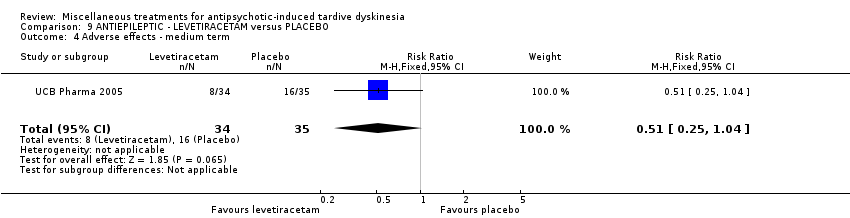 Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 4 Adverse effects ‐ medium term. | ||||
| 5 Mental state: deterioration ‐ medium term Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.65] |
| Analysis 9.5  Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 5 Mental state: deterioration ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.08] |
| Analysis 10.1  Comparison 10 ANTIHISTAMINE ‐ CYPROHEPTADINE versus PLACEBO, Outcome 1 Tardive dyskinesia: 2. Not any improvement ‐ short term. | ||||
| 2 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 10.2  Comparison 10 ANTIHISTAMINE ‐ CYPROHEPTADINE versus PLACEBO, Outcome 2 Tardive dyskinesia: 3. Deterioration ‐ short term. | ||||
| 3 Adverse events ‐ short term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.95] |
| Analysis 10.3  Comparison 10 ANTIHISTAMINE ‐ CYPROHEPTADINE versus PLACEBO, Outcome 3 Adverse events ‐ short term. | ||||
| 4 Leaving the study early ‐ short term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 10.4  Comparison 10 ANTIHISTAMINE ‐ CYPROHEPTADINE versus PLACEBO, Outcome 4 Leaving the study early ‐ short term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: No clinically important improvement (medium term) Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.11, 0.55] |
| Analysis 11.1 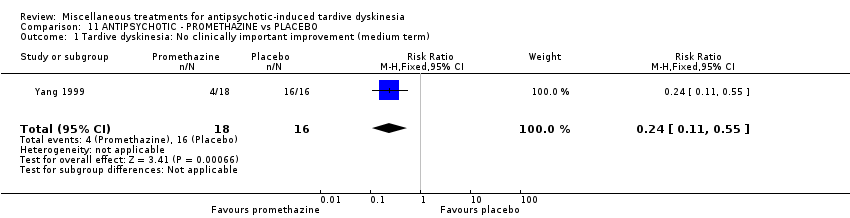 Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 1 Tardive dyskinesia: No clinically important improvement (medium term). | ||||
| 2 Tardive dyskinesia: Not any improvement (medium term) Show forest plot | 1 | 34 | Risk Ratio (IV, Fixed, 95% CI) | 0.06 [0.01, 0.43] |
| Analysis 11.2 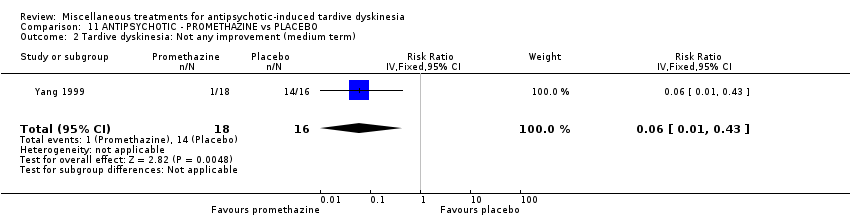 Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 2 Tardive dyskinesia: Not any improvement (medium term). | ||||
| 3 Tardive dyskinesia: Average endpoint score (AIMS, high=poor) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐9.53, ‐4.67] |
| Analysis 11.3 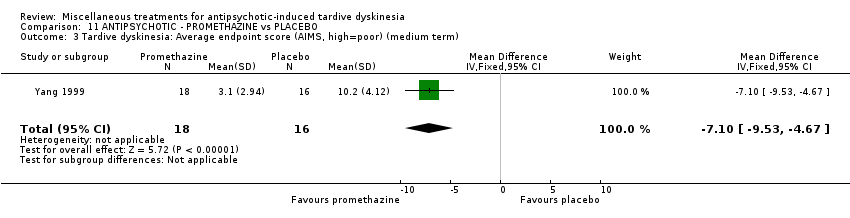 Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 3 Tardive dyskinesia: Average endpoint score (AIMS, high=poor) (medium term). | ||||
| 4 General mental state: Average endpoint score (BPRS, high=poor) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐3.77, 5.17] |
| Analysis 11.4 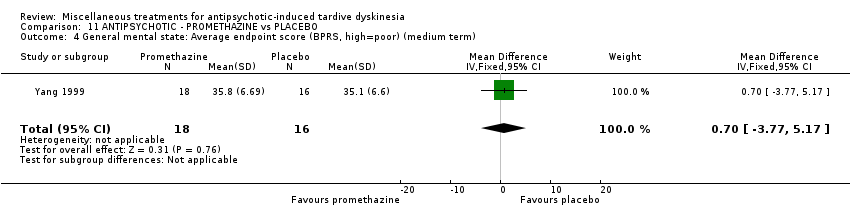 Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 4 General mental state: Average endpoint score (BPRS, high=poor) (medium term). | ||||
| 5 Adverse effects: Any adverse effects (TESS, high=poor) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.53, 0.33] |
| Analysis 11.5 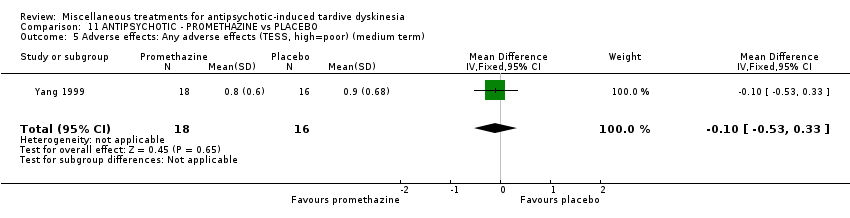 Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 5 Adverse effects: Any adverse effects (TESS, high=poor) (medium term). | ||||
| 6 Adverse effects: Parkinsonism ‐ Average endpoint score (RSESE) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.36, 0.36] |
| Analysis 11.6  Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 6 Adverse effects: Parkinsonism ‐ Average endpoint score (RSESE) (medium term). | ||||
| 7 Global state: Average endpoint score (CGI, high=poor) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐3.78, ‐2.22] |
| Analysis 11.7  Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 7 Global state: Average endpoint score (CGI, high=poor) (medium term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.33, 0.84] |
| Analysis 12.1  Comparison 12 ANXIOLYTIC ‐ BUSPIRONE versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.75] |
| Analysis 12.2  Comparison 12 ANXIOLYTIC ‐ BUSPIRONE versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term. | ||||
| 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.45, 1.45] |
| Analysis 12.3 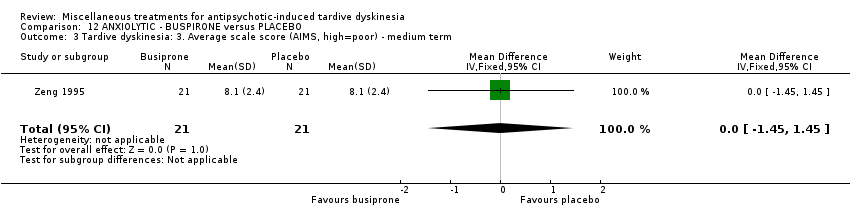 Comparison 12 ANXIOLYTIC ‐ BUSPIRONE versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term. | ||||
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 12.4  Comparison 12 ANXIOLYTIC ‐ BUSPIRONE versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. Average endpoint score (ESRS, high=poor) ‐ short term Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐4.30, 2.90] |
| Analysis 13.1 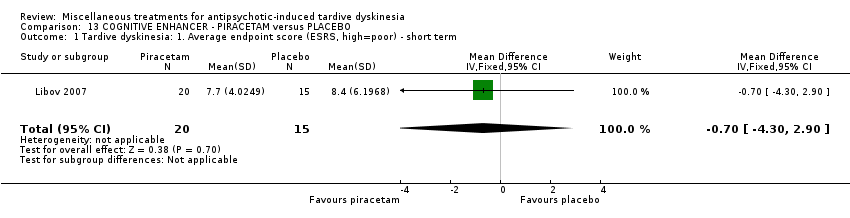 Comparison 13 COGNITIVE ENHANCER ‐ PIRACETAM versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. Average endpoint score (ESRS, high=poor) ‐ short term. | ||||
| 2 Parkinsonism: 1. Average endpoint score (ESRS, high=poor) ‐ short term Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐4.73, 9.73] |
| Analysis 13.2  Comparison 13 COGNITIVE ENHANCER ‐ PIRACETAM versus PLACEBO, Outcome 2 Parkinsonism: 1. Average endpoint score (ESRS, high=poor) ‐ short term. | ||||
| 3 Leaving the study early ‐ short term Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.85] |
| Analysis 13.3 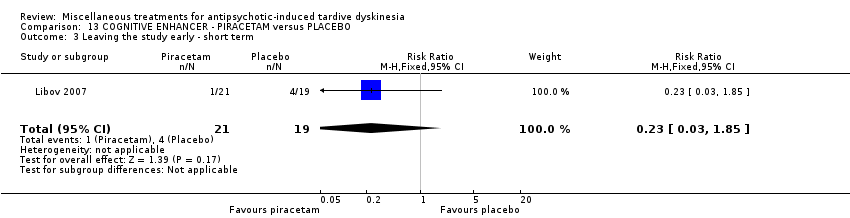 Comparison 13 COGNITIVE ENHANCER ‐ PIRACETAM versus PLACEBO, Outcome 3 Leaving the study early ‐ short term. | ||||
| 4 Global state: Average endpoint score (CGI, high=poor) ‐ short term Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.35, 0.75] |
| Analysis 13.4  Comparison 13 COGNITIVE ENHANCER ‐ PIRACETAM versus PLACEBO, Outcome 4 Global state: Average endpoint score (CGI, high=poor) ‐ short term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.77] |
| Analysis 14.1 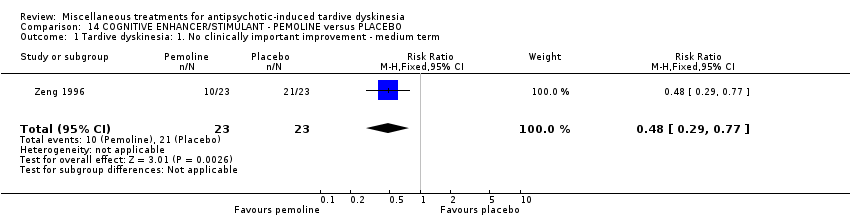 Comparison 14 COGNITIVE ENHANCER/STIMULANT ‐ PEMOLINE versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.66] |
| Analysis 14.2  Comparison 14 COGNITIVE ENHANCER/STIMULANT ‐ PEMOLINE versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term. | ||||
| 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐5.47, ‐2.33] |
| Analysis 14.3 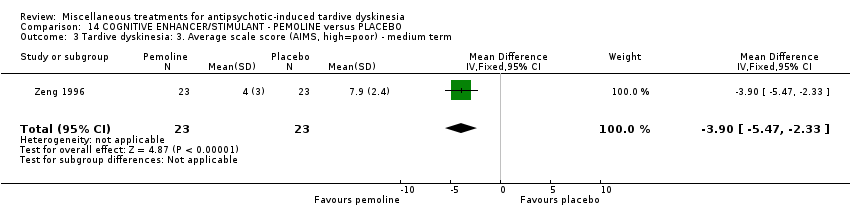 Comparison 14 COGNITIVE ENHANCER/STIMULANT ‐ PEMOLINE versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term. | ||||
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 14.4  Comparison 14 COGNITIVE ENHANCER/STIMULANT ‐ PEMOLINE versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.86] |
| Analysis 15.1 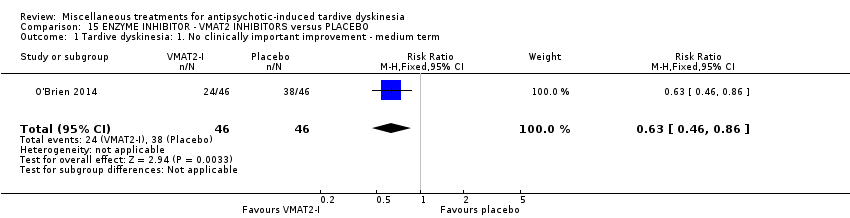 Comparison 15 ENZYME INHIBITOR ‐ VMAT2 INHIBITORS versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Tardive dyskinesia: 3. Average change score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐2.00, ‐1.00] |
| Analysis 15.2 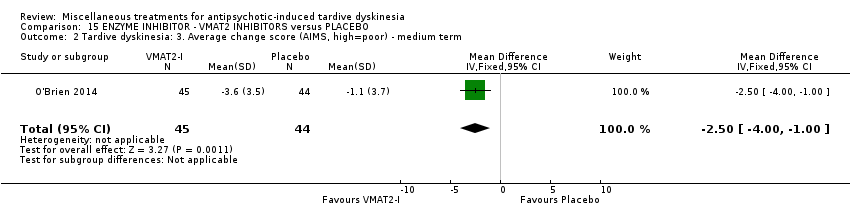 Comparison 15 ENZYME INHIBITOR ‐ VMAT2 INHIBITORS versus PLACEBO, Outcome 2 Tardive dyskinesia: 3. Average change score (AIMS, high=poor) ‐ medium term. | ||||
| 3 Adverse events ‐ medium term Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.92, 2.45] |
| Analysis 15.3  Comparison 15 ENZYME INHIBITOR ‐ VMAT2 INHIBITORS versus PLACEBO, Outcome 3 Adverse events ‐ medium term. | ||||
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.25] |
| Analysis 15.4 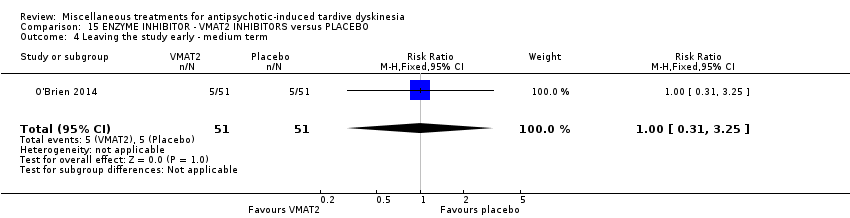 Comparison 15 ENZYME INHIBITOR ‐ VMAT2 INHIBITORS versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 2. No clinically important improvement ‐ medium term Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.18] |
| Analysis 16.1  Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 1 Tardive dyskinesia: 2. No clinically important improvement ‐ medium term. | ||||
| 2 Mental state: deterioration ‐ medium term Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.14] |
| Analysis 16.2  Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 2 Mental state: deterioration ‐ medium term. | ||||
| 3 Adverse events: Parkinsonism ‐ Average change in scale score (ESRS, low=better) ‐ medium term Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.17, 1.77] |
| Analysis 16.3 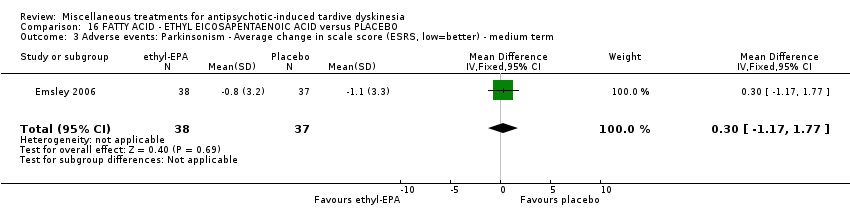 Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 3 Adverse events: Parkinsonism ‐ Average change in scale score (ESRS, low=better) ‐ medium term. | ||||
| 4 Adverse events: Dystonia ‐ Average change in scale score (ESRS, low=better) ‐ medium term Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.58, ‐0.12] |
| Analysis 16.4 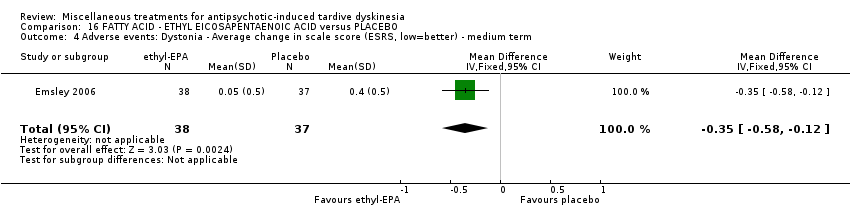 Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 4 Adverse events: Dystonia ‐ Average change in scale score (ESRS, low=better) ‐ medium term. | ||||
| 5 Adverse events: Akathisia ‐ Average change in scale score (ESRS, low=better) ‐ medium term Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.30, 0.22] |
| Analysis 16.5 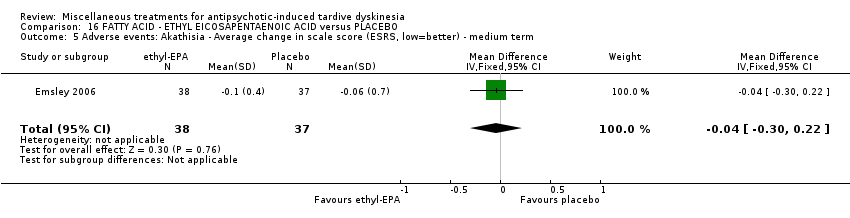 Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 5 Adverse events: Akathisia ‐ Average change in scale score (ESRS, low=better) ‐ medium term. | ||||
| 6 Leaving the study early ‐ medium term Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.27, 1.22] |
| Analysis 16.6  Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 6 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.69, 1.45] |
| Analysis 17.1 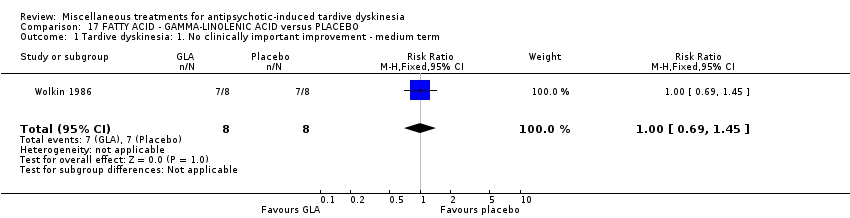 Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.24, 2.33] |
| Analysis 17.2  Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term. | ||||
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.34, 6.70] |
| Analysis 17.3 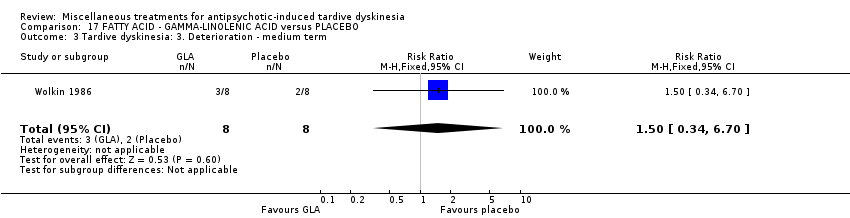 Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term. | ||||
| 4 Tardive dyskinesia: 1. Average change in scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐3.10, 2.70] |
| Analysis 17.4 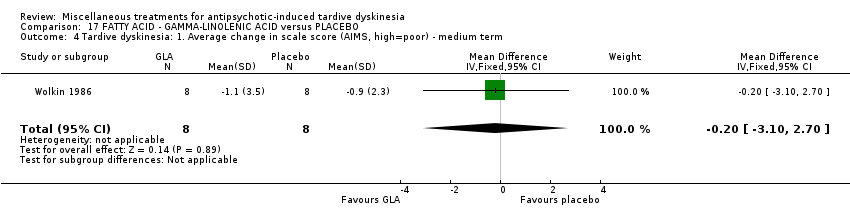 Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 4 Tardive dyskinesia: 1. Average change in scale score (AIMS, high=poor) ‐ medium term. | ||||
| 5 Mental state: 2. Average change in scale score (BPRS, high=poor) ‐ medium term Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐15.99, 3.99] |
| Analysis 17.5  Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 5 Mental state: 2. Average change in scale score (BPRS, high=poor) ‐ medium term. | ||||
| 6 Leaving the study early ‐ medium term Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 17.6  Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 6 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.81, 0.96] |
| Analysis 18.1  Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.41, 0.65] |
| Analysis 18.2 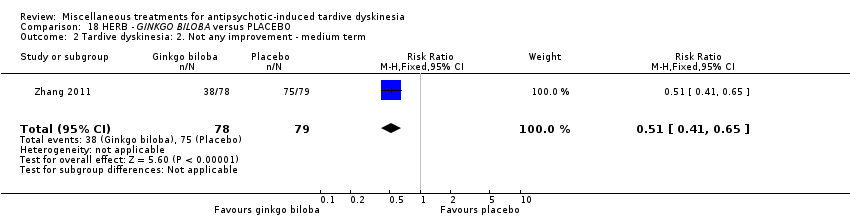 Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term. | ||||
| 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐2.06 [‐2.94, ‐1.18] |
| Analysis 18.3  Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term. | ||||
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.22] |
| Analysis 18.4  Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term. | ||||
| 5 Mental state: deterioration ‐ medium term Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
| Analysis 18.5  Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 5 Mental state: deterioration ‐ medium term. | ||||
| 6 Mental state: 1. Average endpoint scale score (PANSS total, high=poor) ‐ medium term Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.51, ‐0.09] |
| Analysis 18.6  Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 6 Mental state: 1. Average endpoint scale score (PANSS total, high=poor) ‐ medium term. | ||||
| 7 Cognitive function: CPT‐37 ‐ proportion correct responses (high=better) ‐ medium term Show forest plot | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| Analysis 18.7  Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 7 Cognitive function: CPT‐37 ‐ proportion correct responses (high=better) ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.76, 1.83] |
| Analysis 19.1  Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term. | ||||
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.37] |
| Analysis 19.2  Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term. | ||||
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.35] |
| Analysis 19.3 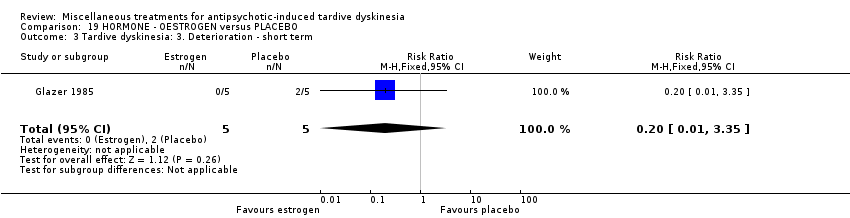 Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term. | ||||
| 4 Tardive dyskinesia: 4. Average scale score (AIMS, high=poor) ‐ short term Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐4.18, 1.78] |
| Analysis 19.4 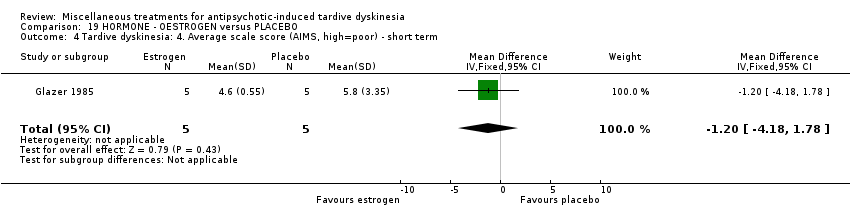 Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 4 Tardive dyskinesia: 4. Average scale score (AIMS, high=poor) ‐ short term. | ||||
| 5 Adverse effects ‐ short term Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.86] |
| Analysis 19.5  Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 5 Adverse effects ‐ short term. | ||||
| 6 Leaving the study early ‐ short term Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.08, 12.56] |
| Analysis 19.6  Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 6 Leaving the study early ‐ short term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| Analysis 20.1  Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.90] |
| Analysis 20.2  Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term. | ||||
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.45] |
| Analysis 20.3  Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term. | ||||
| 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐10.53, ‐1.87] |
| Analysis 20.4  Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term. | ||||
| 5 Leaving the study early ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 20.5 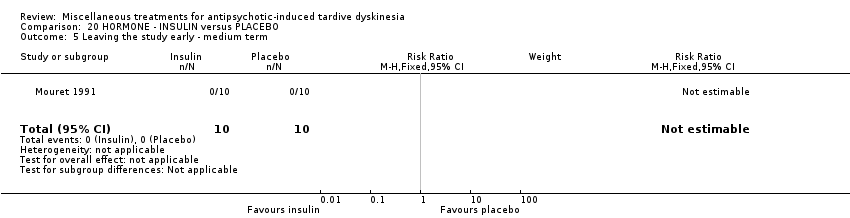 Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 5 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement Show forest plot | 2 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.12] |
| Analysis 21.1  Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement. | ||||
| 1.1 Short term | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.83, 1.21] |
| 1.2 Medium term | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.44, 1.23] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.47, 2.60] |
| Analysis 21.2  Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term. | ||||
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.05] |
| Analysis 21.3  Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term. | ||||
| 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐2.38 [‐6.58, 1.82] |
| Analysis 21.4  Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term. | ||||
| 5 Adverse effects Show forest plot | 3 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 21.5 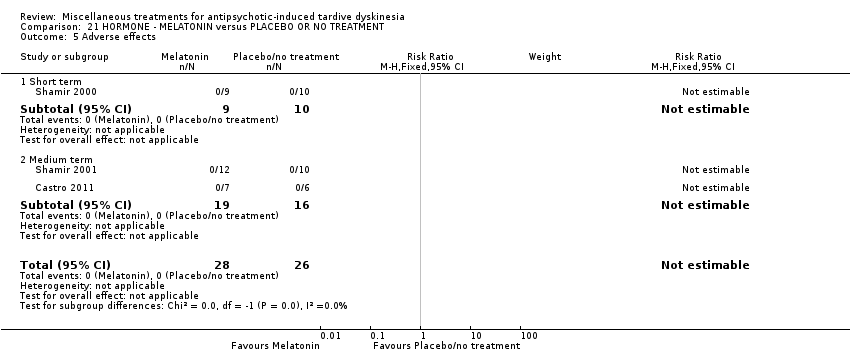 Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 5 Adverse effects. | ||||
| 5.1 Short term | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Medium term | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Leaving the study early Show forest plot | 3 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 21.6  Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 6 Leaving the study early. | ||||
| 6.1 Short term | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Medium term | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Cognitive function: Average scale score ‐ medium term Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 21.7  Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 7 Cognitive function: Average scale score ‐ medium term. | ||||
| 7.1 WAIS | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 15.83 [4.61, 27.05] |
| 7.2 WMS | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 3.77 [‐8.21, 15.75] |
| 8 Mental state: deterioration ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 21.8  Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 8 Mental state: deterioration ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.79, 3.23] |
| Analysis 22.1  Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term. | ||||
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [0.25, 72.90] |
| Analysis 22.2  Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term. | ||||
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [0.25, 72.90] |
| Analysis 22.3 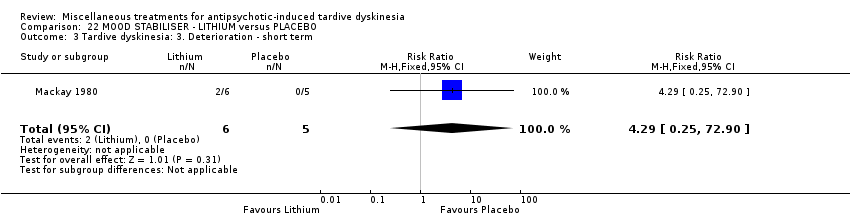 Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term. | ||||
| 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ short term Show forest plot | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐5.23, 6.49] |
| Analysis 22.4 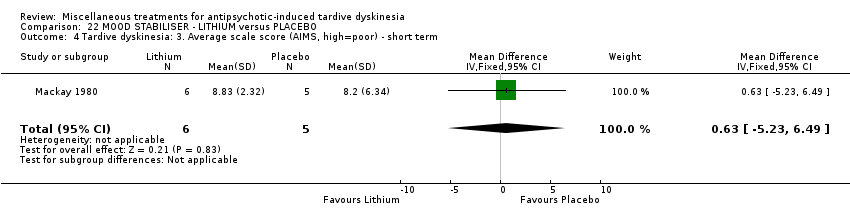 Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ short term. | ||||
| 5 Adverse events ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.38, 94.35] |
| Analysis 22.5 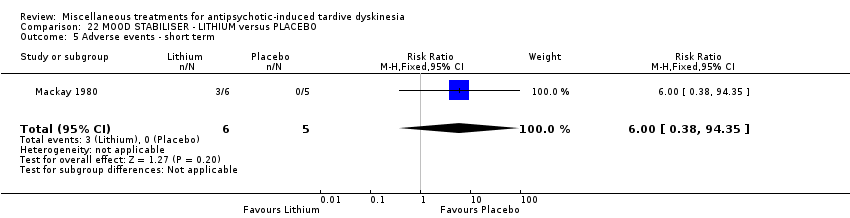 Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 5 Adverse events ‐ short term. | ||||
| 6 Leaving the study early ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.13, 52.12] |
| Analysis 22.6 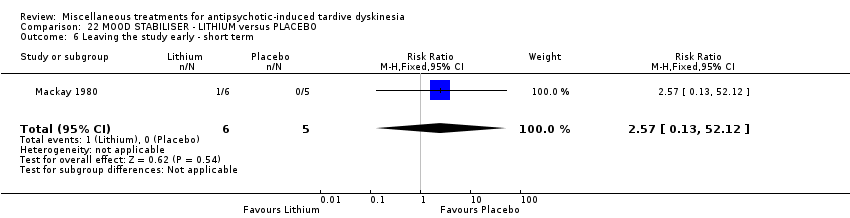 Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 6 Leaving the study early ‐ short term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. Not any improvement Show forest plot | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.65, 1.07] |
| Analysis 23.1 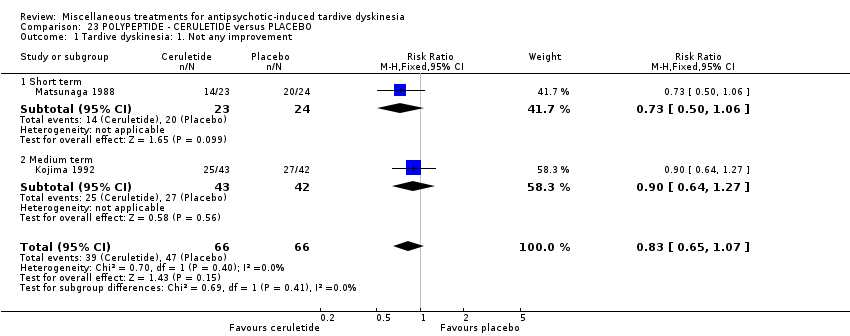 Comparison 23 POLYPEPTIDE ‐ CERULETIDE versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. Not any improvement. | ||||
| 1.1 Short term | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.50, 1.06] |
| 1.2 Medium term | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.64, 1.27] |
| 2 Tardive dyskinesia: 2. Deterioration Show forest plot | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.80] |
| Analysis 23.2  Comparison 23 POLYPEPTIDE ‐ CERULETIDE versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Deterioration. | ||||
| 2.1 Short term | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 65.74] |
| 2.2 Medium term | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.90] |
| 3 Adverse effects Show forest plot | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.74, 2.36] |
| Analysis 23.3  Comparison 23 POLYPEPTIDE ‐ CERULETIDE versus PLACEBO, Outcome 3 Adverse effects. | ||||
| 3.1 Short term | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.79 [0.47, 30.77] |
| 3.2 Medium term | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.61, 2.07] |
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.49, 2.40] |
| Analysis 23.4  Comparison 23 POLYPEPTIDE ‐ CERULETIDE versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.94] |
| Analysis 24.1  Comparison 24 HYPNOSIS OR RELAXATION versus TREATMENT AS USUAL, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term. | ||||
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.81] |
| Analysis 24.2 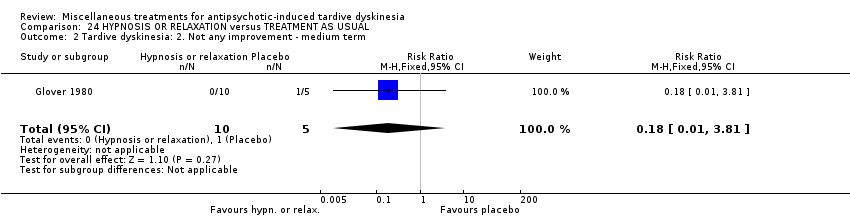 Comparison 24 HYPNOSIS OR RELAXATION versus TREATMENT AS USUAL, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term. | ||||
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.81] |
| Analysis 24.3 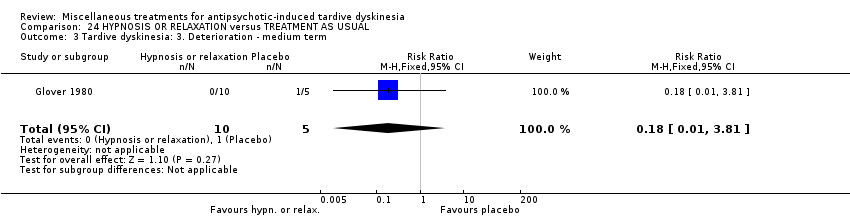 Comparison 24 HYPNOSIS OR RELAXATION versus TREATMENT AS USUAL, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term. | ||||
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 24.4  Comparison 24 HYPNOSIS OR RELAXATION versus TREATMENT AS USUAL, Outcome 4 Leaving the study early ‐ medium term. | ||||
| 4.1 Short term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.
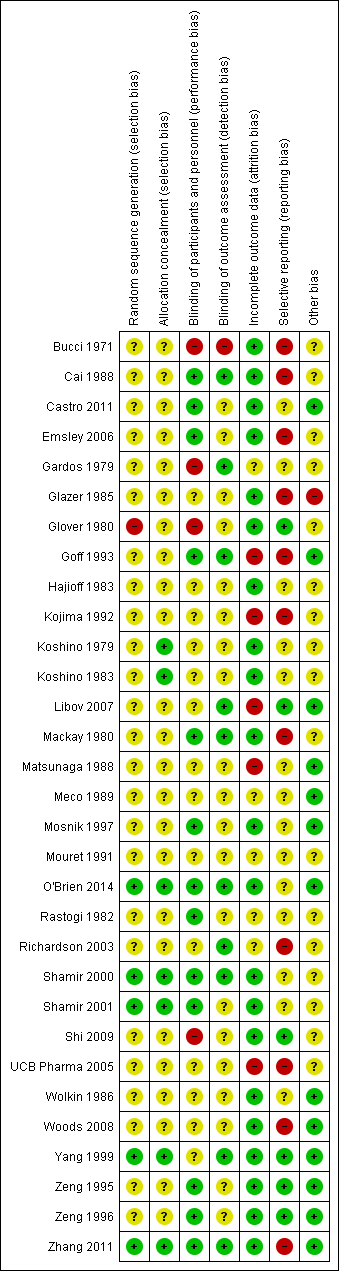
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
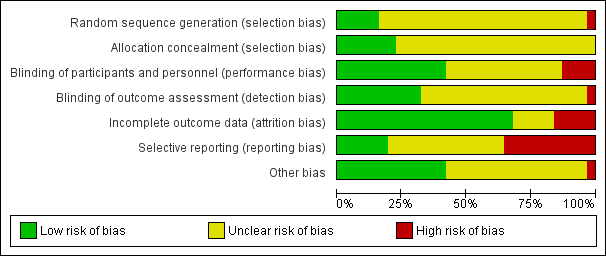
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Study flow diagram for 2015 and 2017 searches.

Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.
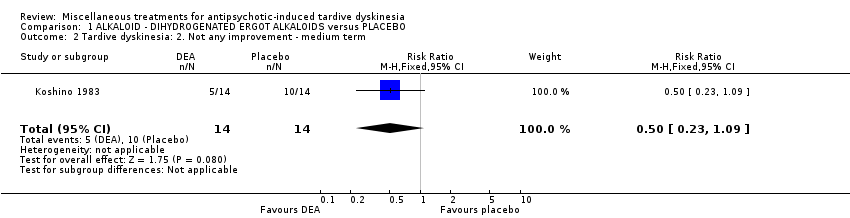
Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.
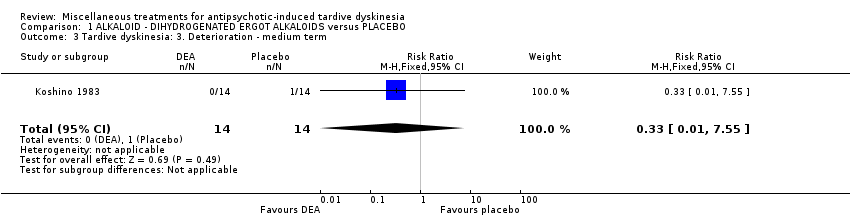
Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term.

Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 4 Tardive dyskinesia: 3. Average endpoint scale score (Simpson scale, high=poor) ‐ medium term.
Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 5 Tardive dyskinesia: Average scale change scores (various scales, high=poor) ‐ medium term.

Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 6 Mental state: Deterioration ‐ medium term.
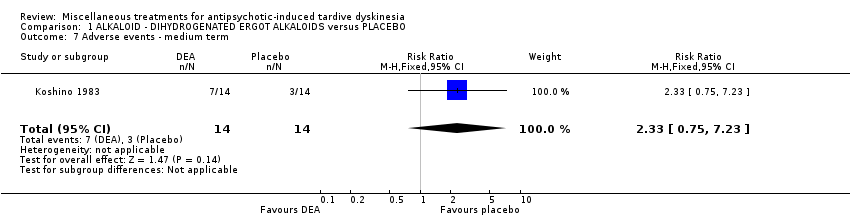
Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 7 Adverse events ‐ medium term.
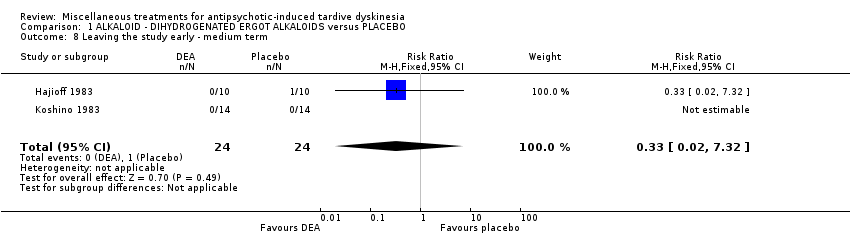
Comparison 1 ALKALOID ‐ DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO, Outcome 8 Leaving the study early ‐ medium term.
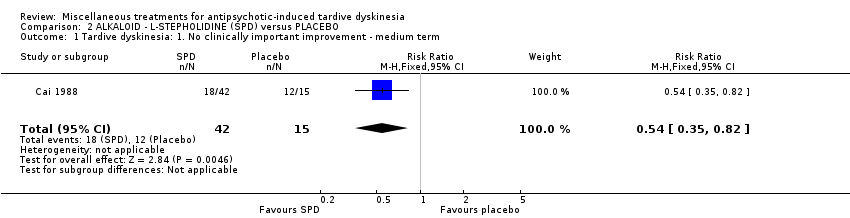
Comparison 2 ALKALOID ‐ L‐STEPHOLIDINE (SPD) versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.

Comparison 2 ALKALOID ‐ L‐STEPHOLIDINE (SPD) versus PLACEBO, Outcome 2 Mental state: 1. Average endpoint scale score (BPRS, high=poor) ‐ medium term.

Comparison 2 ALKALOID ‐ L‐STEPHOLIDINE (SPD) versus PLACEBO, Outcome 3 Adverse events: any adverse events ‐ medium term.
Comparison 2 ALKALOID ‐ L‐STEPHOLIDINE (SPD) versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term.

Comparison 3 ALKALOID ‐ PAPAVERINE versus PLACEBO, Outcome 1 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term.

Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term.
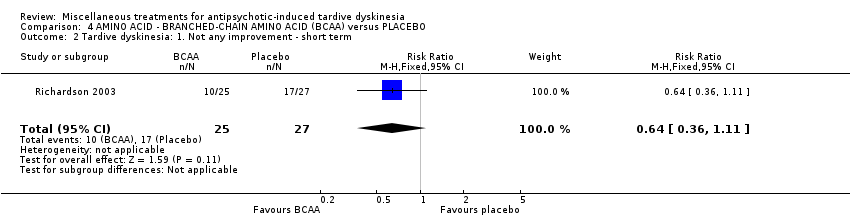
Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 2 Tardive dyskinesia: 1. Not any improvement ‐ short term.

Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 3 Tardive dyskinesia: 2. Deterioration ‐ short term.

Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 4 Tardive dyskinesia: Average endpoint score (Simpson scale, high=poor) ‐ short term.
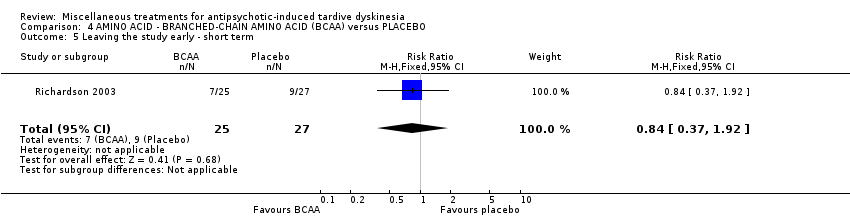
Comparison 4 AMINO ACID ‐ BRANCHED‐CHAIN AMINO ACID (BCAA) versus PLACEBO, Outcome 5 Leaving the study early ‐ short term.
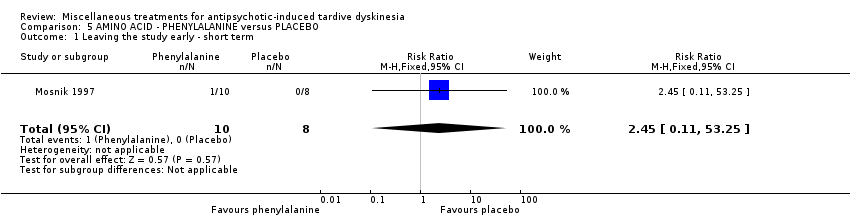
Comparison 5 AMINO ACID ‐ PHENYLALANINE versus PLACEBO, Outcome 1 Leaving the study early ‐ short term.

Comparison 6 ANTIDEPRESSANT (MAO‐B inhibitor) ‐ SELEGILINE versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.
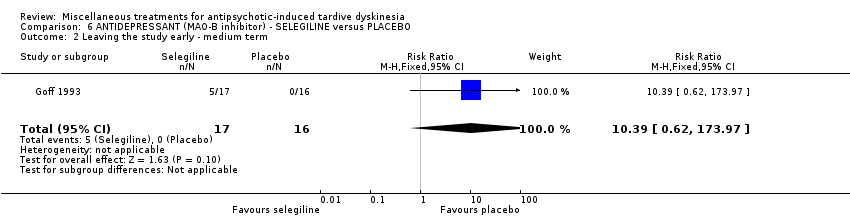
Comparison 6 ANTIDEPRESSANT (MAO‐B inhibitor) ‐ SELEGILINE versus PLACEBO, Outcome 2 Leaving the study early ‐ medium term.

Comparison 7 ANTIDEPRESSANT (MAOI) ISOCARBOXAZID versus PROCYCLIDINE, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ long term.
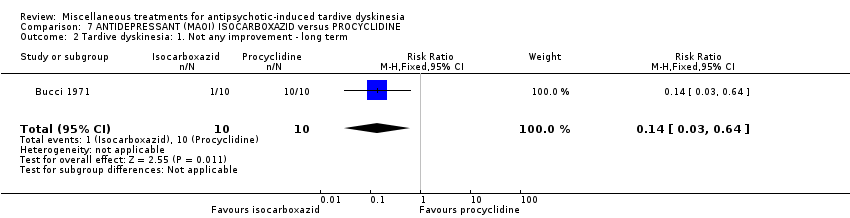
Comparison 7 ANTIDEPRESSANT (MAOI) ISOCARBOXAZID versus PROCYCLIDINE, Outcome 2 Tardive dyskinesia: 1. Not any improvement ‐ long term.
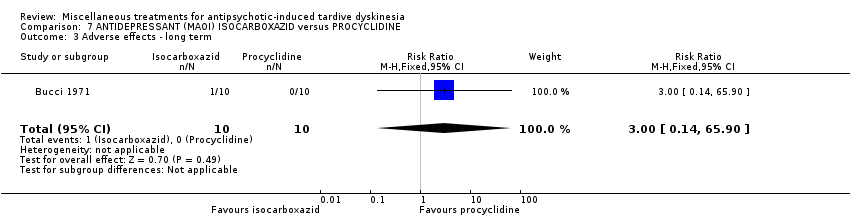
Comparison 7 ANTIDEPRESSANT (MAOI) ISOCARBOXAZID versus PROCYCLIDINE, Outcome 3 Adverse effects ‐ long term.

Comparison 7 ANTIDEPRESSANT (MAOI) ISOCARBOXAZID versus PROCYCLIDINE, Outcome 4 Leaving the study early ‐ long term.

Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 1 Tardive dyskinesia: No clinically important improvement (short term).
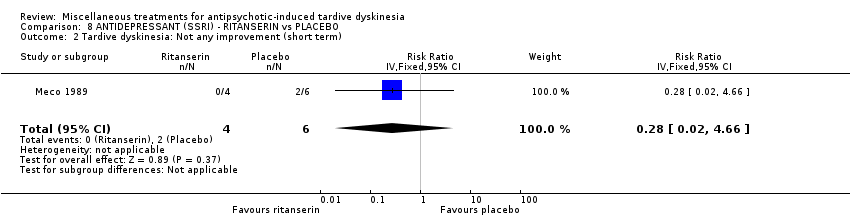
Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 2 Tardive dyskinesia: Not any improvement (short term).
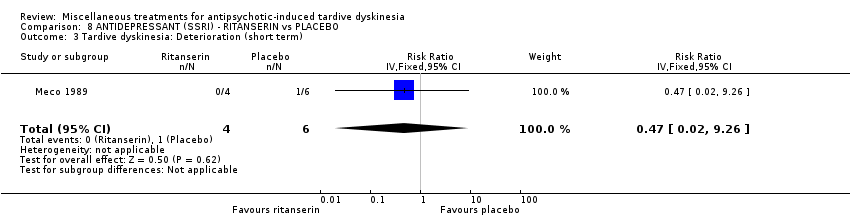
Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 3 Tardive dyskinesia: Deterioration (short term).

Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 4 Tardive dyskinesia: Average change score (AIMS, high=poor) (short term).

Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 5 General mental state: Deterioration (short term).
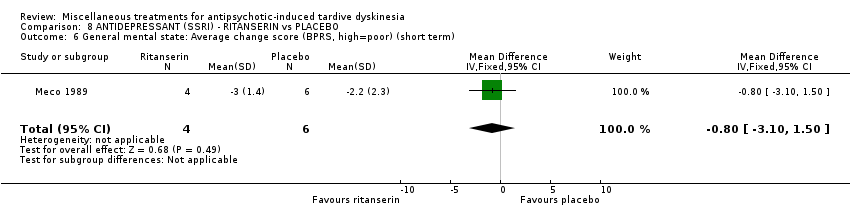
Comparison 8 ANTIDEPRESSANT (SSRI) ‐ RITANSERIN vs PLACEBO, Outcome 6 General mental state: Average change score (BPRS, high=poor) (short term).

Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. Average endpoint score (AIMS, high=poor) ‐ medium term.
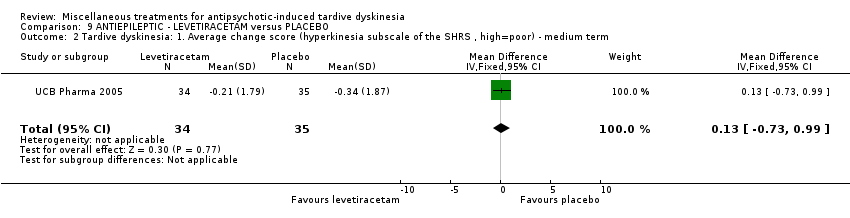
Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 2 Tardive dyskinesia: 1. Average change score (hyperkinesia subscale of the SHRS , high=poor) ‐ medium term.
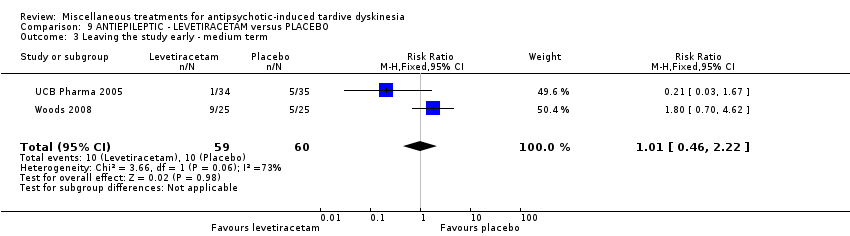
Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 3 Leaving the study early ‐ medium term.

Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 4 Adverse effects ‐ medium term.
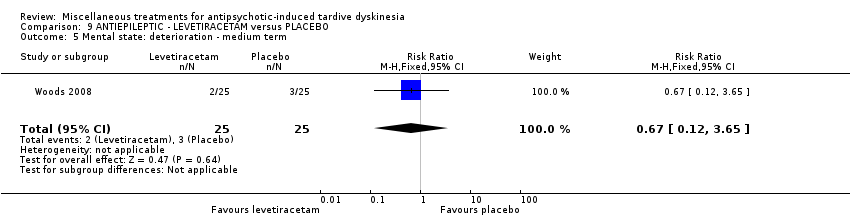
Comparison 9 ANTIEPILEPTIC ‐ LEVETIRACETAM versus PLACEBO, Outcome 5 Mental state: deterioration ‐ medium term.

Comparison 10 ANTIHISTAMINE ‐ CYPROHEPTADINE versus PLACEBO, Outcome 1 Tardive dyskinesia: 2. Not any improvement ‐ short term.
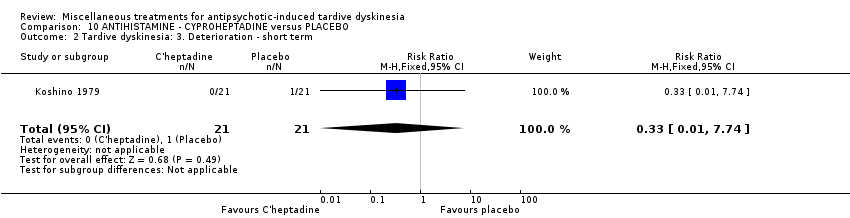
Comparison 10 ANTIHISTAMINE ‐ CYPROHEPTADINE versus PLACEBO, Outcome 2 Tardive dyskinesia: 3. Deterioration ‐ short term.

Comparison 10 ANTIHISTAMINE ‐ CYPROHEPTADINE versus PLACEBO, Outcome 3 Adverse events ‐ short term.

Comparison 10 ANTIHISTAMINE ‐ CYPROHEPTADINE versus PLACEBO, Outcome 4 Leaving the study early ‐ short term.
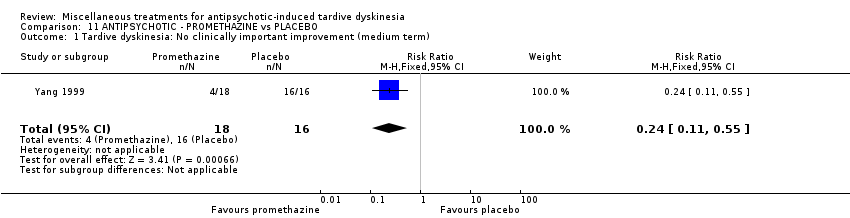
Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 1 Tardive dyskinesia: No clinically important improvement (medium term).

Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 2 Tardive dyskinesia: Not any improvement (medium term).

Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 3 Tardive dyskinesia: Average endpoint score (AIMS, high=poor) (medium term).
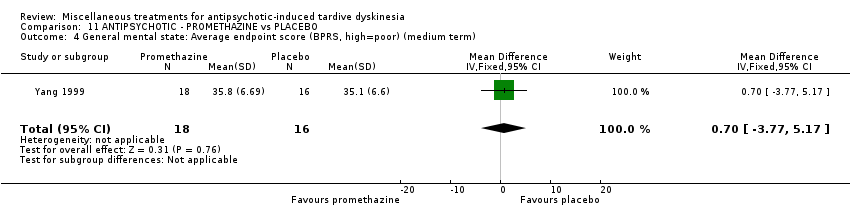
Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 4 General mental state: Average endpoint score (BPRS, high=poor) (medium term).
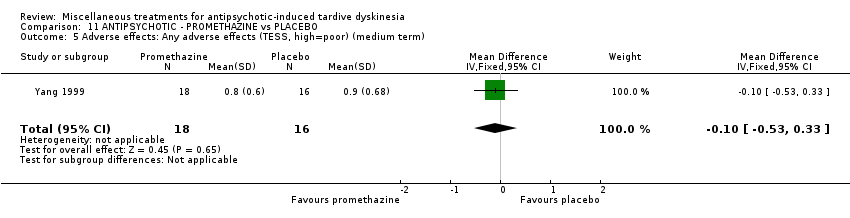
Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 5 Adverse effects: Any adverse effects (TESS, high=poor) (medium term).
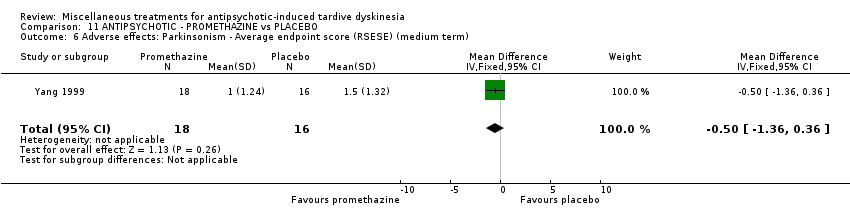
Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 6 Adverse effects: Parkinsonism ‐ Average endpoint score (RSESE) (medium term).
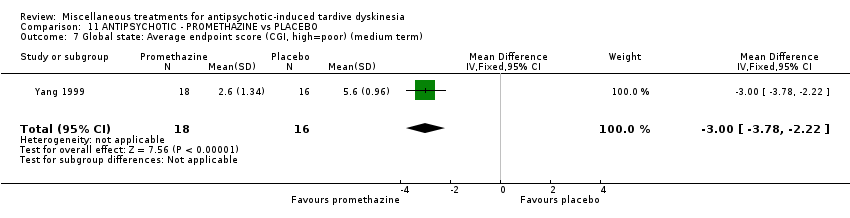
Comparison 11 ANTIPSYCHOTIC ‐ PROMETHAZINE vs PLACEBO, Outcome 7 Global state: Average endpoint score (CGI, high=poor) (medium term).

Comparison 12 ANXIOLYTIC ‐ BUSPIRONE versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.

Comparison 12 ANXIOLYTIC ‐ BUSPIRONE versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.

Comparison 12 ANXIOLYTIC ‐ BUSPIRONE versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term.
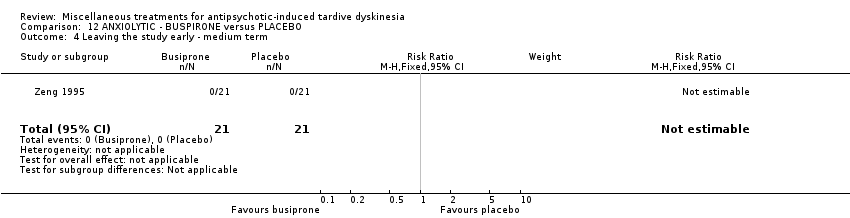
Comparison 12 ANXIOLYTIC ‐ BUSPIRONE versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term.
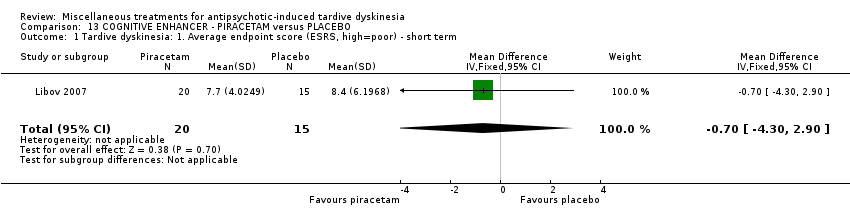
Comparison 13 COGNITIVE ENHANCER ‐ PIRACETAM versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. Average endpoint score (ESRS, high=poor) ‐ short term.

Comparison 13 COGNITIVE ENHANCER ‐ PIRACETAM versus PLACEBO, Outcome 2 Parkinsonism: 1. Average endpoint score (ESRS, high=poor) ‐ short term.
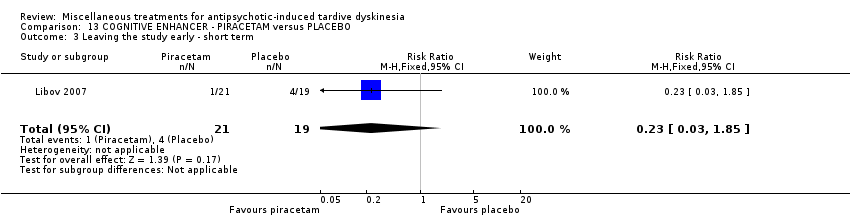
Comparison 13 COGNITIVE ENHANCER ‐ PIRACETAM versus PLACEBO, Outcome 3 Leaving the study early ‐ short term.
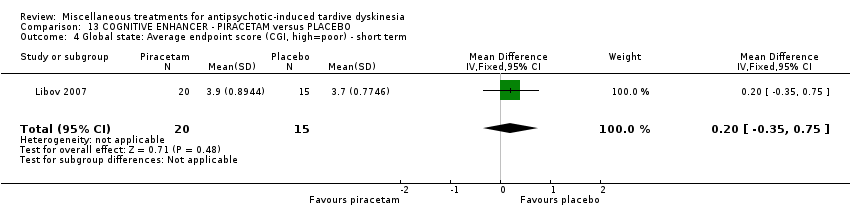
Comparison 13 COGNITIVE ENHANCER ‐ PIRACETAM versus PLACEBO, Outcome 4 Global state: Average endpoint score (CGI, high=poor) ‐ short term.

Comparison 14 COGNITIVE ENHANCER/STIMULANT ‐ PEMOLINE versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.
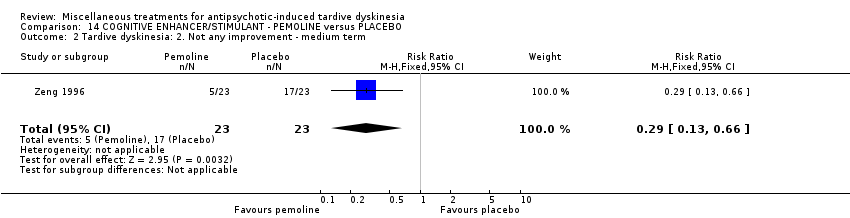
Comparison 14 COGNITIVE ENHANCER/STIMULANT ‐ PEMOLINE versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.

Comparison 14 COGNITIVE ENHANCER/STIMULANT ‐ PEMOLINE versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term.

Comparison 14 COGNITIVE ENHANCER/STIMULANT ‐ PEMOLINE versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term.
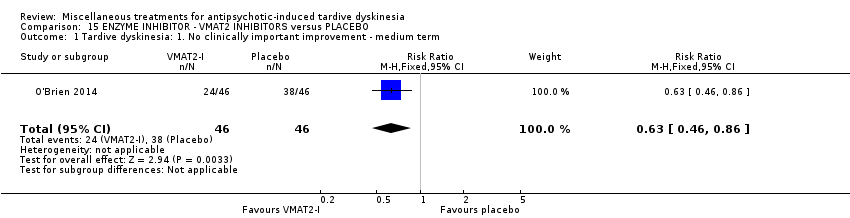
Comparison 15 ENZYME INHIBITOR ‐ VMAT2 INHIBITORS versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.

Comparison 15 ENZYME INHIBITOR ‐ VMAT2 INHIBITORS versus PLACEBO, Outcome 2 Tardive dyskinesia: 3. Average change score (AIMS, high=poor) ‐ medium term.

Comparison 15 ENZYME INHIBITOR ‐ VMAT2 INHIBITORS versus PLACEBO, Outcome 3 Adverse events ‐ medium term.

Comparison 15 ENZYME INHIBITOR ‐ VMAT2 INHIBITORS versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term.
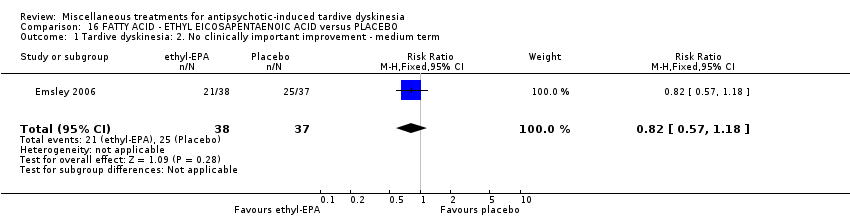
Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 1 Tardive dyskinesia: 2. No clinically important improvement ‐ medium term.

Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 2 Mental state: deterioration ‐ medium term.

Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 3 Adverse events: Parkinsonism ‐ Average change in scale score (ESRS, low=better) ‐ medium term.

Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 4 Adverse events: Dystonia ‐ Average change in scale score (ESRS, low=better) ‐ medium term.

Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 5 Adverse events: Akathisia ‐ Average change in scale score (ESRS, low=better) ‐ medium term.

Comparison 16 FATTY ACID ‐ ETHYL EICOSAPENTAENOIC ACID versus PLACEBO, Outcome 6 Leaving the study early ‐ medium term.

Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.
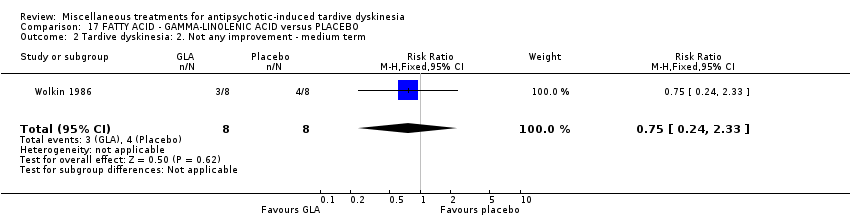
Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.

Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term.
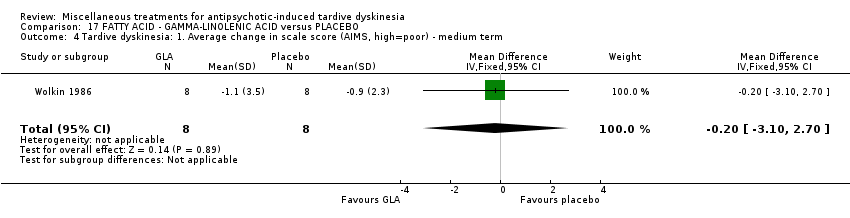
Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 4 Tardive dyskinesia: 1. Average change in scale score (AIMS, high=poor) ‐ medium term.
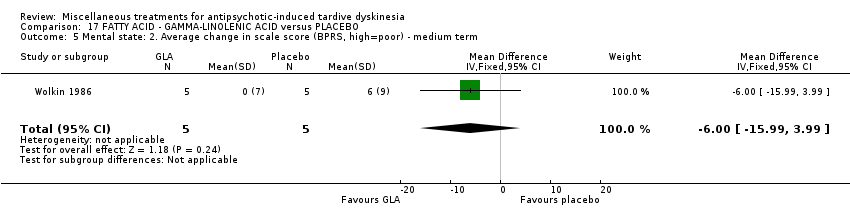
Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 5 Mental state: 2. Average change in scale score (BPRS, high=poor) ‐ medium term.

Comparison 17 FATTY ACID ‐ GAMMA‐LINOLENIC ACID versus PLACEBO, Outcome 6 Leaving the study early ‐ medium term.

Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.
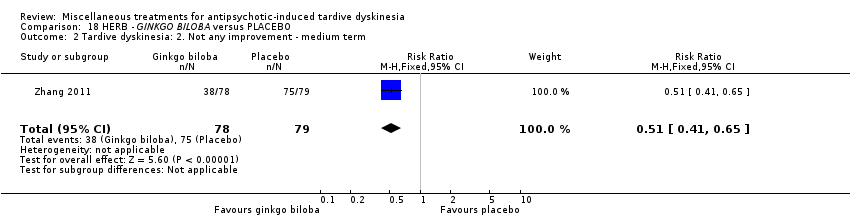
Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.

Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term.

Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term.

Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 5 Mental state: deterioration ‐ medium term.
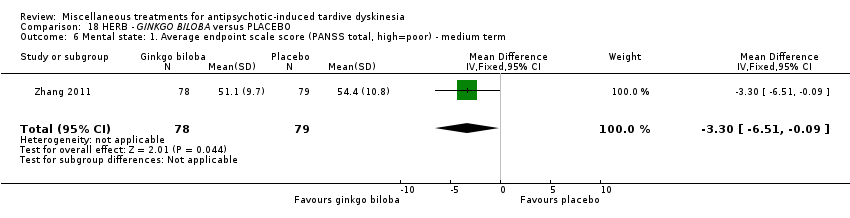
Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 6 Mental state: 1. Average endpoint scale score (PANSS total, high=poor) ‐ medium term.

Comparison 18 HERB ‐ GINKGO BILOBA versus PLACEBO, Outcome 7 Cognitive function: CPT‐37 ‐ proportion correct responses (high=better) ‐ medium term.

Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term.
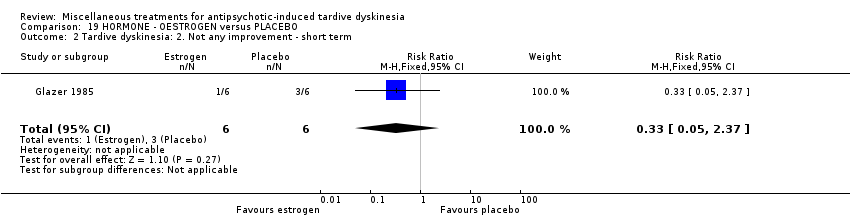
Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term.
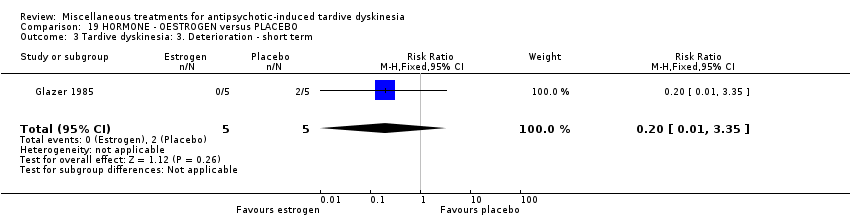
Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term.

Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 4 Tardive dyskinesia: 4. Average scale score (AIMS, high=poor) ‐ short term.

Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 5 Adverse effects ‐ short term.
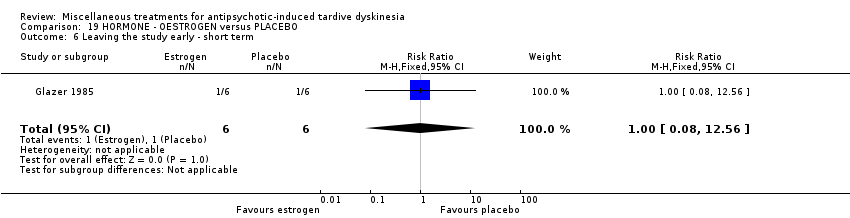
Comparison 19 HORMONE ‐ OESTROGEN versus PLACEBO, Outcome 6 Leaving the study early ‐ short term.
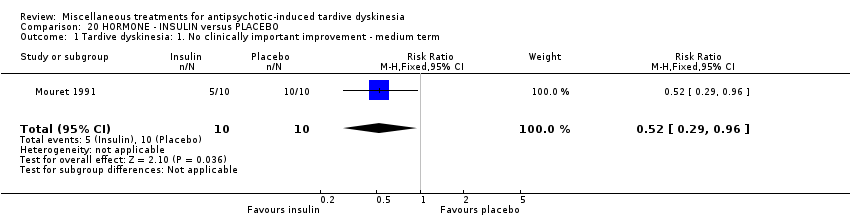
Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.

Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.

Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term.

Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term.
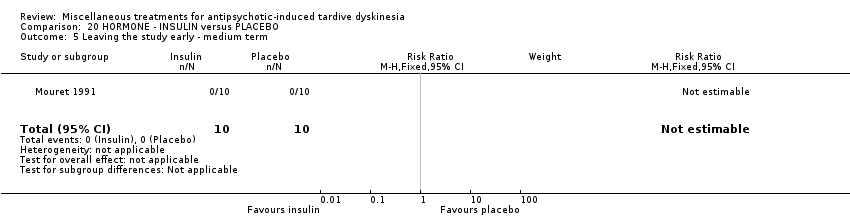
Comparison 20 HORMONE ‐ INSULIN versus PLACEBO, Outcome 5 Leaving the study early ‐ medium term.

Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement.
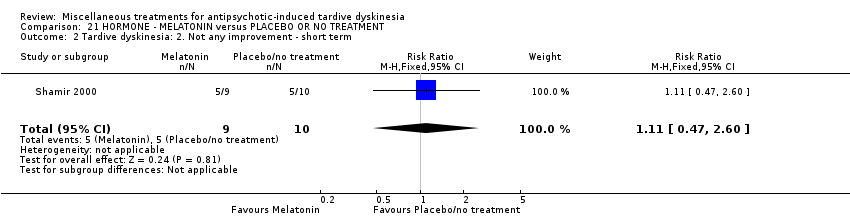
Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term.
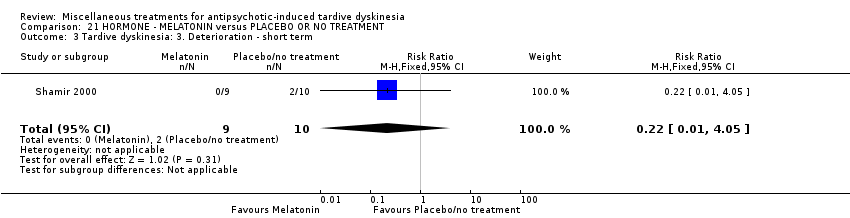
Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term.

Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term.
Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 5 Adverse effects.

Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 6 Leaving the study early.

Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 7 Cognitive function: Average scale score ‐ medium term.
Comparison 21 HORMONE ‐ MELATONIN versus PLACEBO OR NO TREATMENT, Outcome 8 Mental state: deterioration ‐ medium term.
Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term.

Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term.

Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term.

Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ short term.

Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 5 Adverse events ‐ short term.
Comparison 22 MOOD STABILISER ‐ LITHIUM versus PLACEBO, Outcome 6 Leaving the study early ‐ short term.

Comparison 23 POLYPEPTIDE ‐ CERULETIDE versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. Not any improvement.
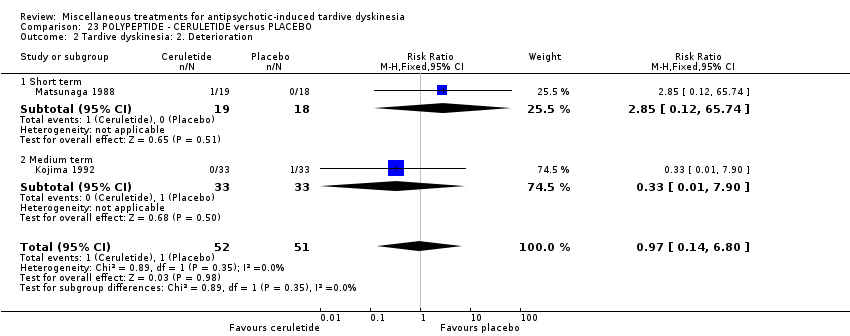
Comparison 23 POLYPEPTIDE ‐ CERULETIDE versus PLACEBO, Outcome 2 Tardive dyskinesia: 2. Deterioration.

Comparison 23 POLYPEPTIDE ‐ CERULETIDE versus PLACEBO, Outcome 3 Adverse effects.

Comparison 23 POLYPEPTIDE ‐ CERULETIDE versus PLACEBO, Outcome 4 Leaving the study early ‐ medium term.

Comparison 24 HYPNOSIS OR RELAXATION versus TREATMENT AS USUAL, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.
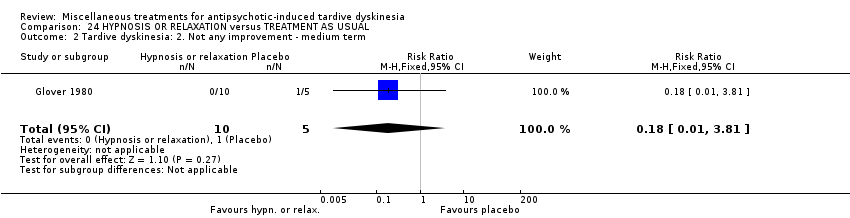
Comparison 24 HYPNOSIS OR RELAXATION versus TREATMENT AS USUAL, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.
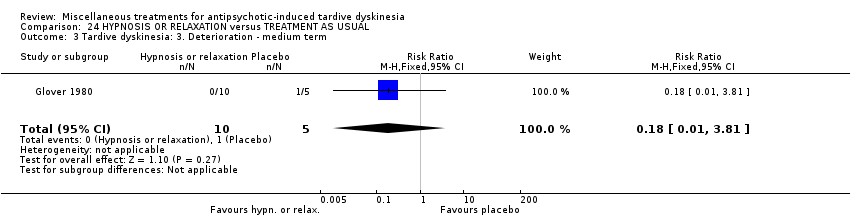
Comparison 24 HYPNOSIS OR RELAXATION versus TREATMENT AS USUAL, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term.

Comparison 24 HYPNOSIS OR RELAXATION versus TREATMENT AS USUAL, Outcome 4 Leaving the study early ‐ medium term.
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | 1. Active intervention. N = 150. |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| DIHYDROGENATED ERGOT ALKALOIDS versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: female and male 59‐80 (mean) years old patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | DIHYDROGENATED ERGOT ALKALOIDS | |||||
| Tardive dyskinesia: No clinically important improvement | 786 per 1000 | 354 per 1000 | RR 0.45 | 28 | ⊕⊕⊝⊝ | |
| Tardive dyskinesia: Deterioration of symptoms follow‐up: 6 weeks | 71 per 1000 | 24 per 1000 | RR 0.33 | 28 | ⊕⊝⊝⊝ | |
| Adverse effects ‐ any adverse effect follow‐up: 6 weeks | 214 per 1000 | 499 per 1000 | RR 2.33 | 28 | ⊕⊝⊝⊝ | |
| Acceptability of treatment (measured by participants leaving the study early) follow‐up: 6 weeks | 42 per 1000 | 14 per 1000 | RR 0.33 | 48 | ⊕⊝⊝⊝ | |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | This outcome was not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: randomisation procedure and allocation concealment were not adequately described. | ||||||
| LEVETIRACETAM versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: female and male 47‐54 years old (mean) patients with various psychiatric conditions and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | LEVETIRACETAM | |||||
| Tardive dyskinesia: No clinically important improvement ‐ not reported | This outcome was not reported. | |||||
| Tardive dyskinesia: Deterioration of symptoms ‐ not reported | This outcome was not reported. | |||||
| Adverse effects ‐ any adverse effect follow‐up: 8 weeks | 457 per 1000 | 233 per 1000 | RR 0.51 | 69 | ⊕⊕⊝⊝ | |
| Acceptability of treatment (measured by participants leaving the study early) | 167 per 1000 | 168 per 1000 | RR 1.01 | 119 | ⊕⊝⊝⊝ | |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | This outcome was not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: randomisation procedure, allocation concealment and blinding were not adequately described. | ||||||
| BUSPIRONE versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: female and male 33‐year old (mean) patients with schizophrenia and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with buspirone | |||||
| Tardive dyskinesia: No clinically important improvement | 905 per 1,000 | 480 per 1,000 | RR 0.53 | 42 | ⊕⊕⊝⊝ | |
| Tardive dyskinesia: Deterioration of symptoms ‐ not reported | This outcome was not reported. | |||||
| Adverse effects ‐ not reported | This outcome was not reported. | |||||
| Acceptability of treatment (measured by participants leaving the study early) | not estimable | not estimable | RR not estimable | 42 | ⊕⊝⊝⊝ | No events were reported. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | This outcome was not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: randomisation procedure, allocation concealment and blinding were not adequately described. 2 Downgraded one step for imprecision: very small sample size and few events reported. 3 Downgraded two steps for imprecision: effect could not be estimated due to very small sample size with no events reported. | ||||||
| VMAT2‐inhibitor compared to placebo for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: 18 to 85 year‐old patients with schizophrenia, schizoaffective disorder, mood disorder, or gastrointestinal disorder + antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | VMAT2‐inhibitor | |||||
| Tardive dyskinesia: 1. No clinically important improvement | 826 per 1000 | 520 per 1000 | RR 0.63 | 92 | ⊕⊕⊕⊝ | |
| Adverse effects ‐ any adverse effect | 327 per 1000 | 490 per 1000 | RR 1.50 | 100 | ⊕⊕⊝⊝ | |
| Leaving the study early | 98 per 1000 | 98 per 1000 | RR 1.00 | 102 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for imprecision: small sample size and few events reported | ||||||
| ETHYL‐EPA versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: female and male 42 (mean) years old patients with schizophrenia or schizoaffective disorder and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | ETHYL‐EPA | |||||
| Tardive dyskinesia: No clinically important improvement ‐ not reported | This outcome was not reported. | |||||
| Tardive dyskinesia: Deterioration of symptoms ‐ not reported | This outcome was not reported. | |||||
| Adverse effects: EPS: parkinsonism measured by average ESRS change scores follow‐up: 12 weeks | mean: ‐1.1 (3.3) | The mean change in ESRS: parkinsonism scale score in the ethyl‐EPA group was | MD 0.30 (‐1.17 to 1.77) | 75 (1 RCT) | ⊕⊕⊝⊝ | |
| Adverse effects: EPS: dystonia measured by average ESRS change scores follow‐up: 12 weeks | mean: 0.4 (0.5) | The mean change in ESRS: dystonia scale score in the ethyl‐EPA group was | MD ‐0.35 (‐0.58 to ‐0.12) | 75 (1 RCT) | ⊕⊕⊝⊝ | |
| Adverse effects: EPS: akathisia measured by average ESRS change scores follow‐up: 12 weeks | mean: ‐0.06 (0.7) | The mean change in ESRS: akathisia scale score in the ethyl‐EPA group was | MD ‐0.04 (‐0.30 to 0.22) | 75 (1 RCT) | ⊕⊝⊝⊝ | |
| Acceptability of treatment (measured by participants leaving the study early) follow‐up: 12 weeks | 548 per 1000 | 214 per 1000 | RR 0. 57 | 84 | ⊕⊝⊝⊝ | |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | This outcome was not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: randomisation procedure, allocation concealment and blinding were not adequately described. | ||||||
| GINKGO BILOBA versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: male 45.2 (mean) years old patients with schizophrenia and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | GINKGO BILOBA | |||||
| Tardive dyskinesia: No clinically important improvement | 987 per 1000 | 869 per 1000 | RR 0.88 | 157 | ⊕⊕⊕⊝ | |
| Tardive dyskinesia: Deterioration of symptoms ‐ not reported | This outcome was not reported. | |||||
| Adverse effects ‐ not reported | This outcome was not reported. | |||||
| Acceptability of treatment (measured by participants leaving the study early) | 51 per 1000 | 13 per 1000 | RR 0.25 | 157 | ⊕⊝⊝⊝ | |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | This outcome was not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for imprecision: small sample size and few events reported. | ||||||
| MELATONIN versus PLACEBO OR NO TREATMENT for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: female and male 28 to 91 years old patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | MELATONIN | |||||
| Tardive dyskinesia: No clinically important improvement | 1000 per 1000 | 890 per 1000 | RR 0.89 | 32 | ⊕⊕⊝⊝ | |
| Tardive dyskinesia: Deterioration of symptoms follow‐up: 3 weeks | 200 per 1000 | 44 per 1000 | RR 0.22 | 19 | ⊕⊕⊝⊝ | |
| Adverse effects follow‐up: 3‐12 weeks | See comment | See comment | Not estimable | 54 | ⊕⊕⊝⊝ | No events were reported. |
| Acceptability of treatment (measured by participants leaving the study early) | See comment | See comment | Not estimable | 54 | ⊕⊝⊝⊝ | No events were reported. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | This outcome was not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for inconsistency: substantial heterogeneity (I2 = 46%). | ||||||
| CERULETIDE versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: female and male 55‐59 year old (mean) patients with various psychiatric conditions and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | CERULETIDE | |||||
| Tardive dyskinesia: No clinically important improvement ‐ not reported | This outcome was not reported. | |||||
| Tardive dyskinesia: Deterioration of symptoms follow‐up: 4‐8 weeks | 20 per 1000 | 19 per 1000 | RR 0.97 | 103 | ⊕⊕⊝⊝ | |
| Adverse effects ‐ any adverse effect follow‐up: 4‐8 weeks | 233 per 1000 | 308 per 1000 | RR 1.32 | 122 | ⊕⊕⊝⊝ | |
| Acceptability of treatment (measured by participants leaving the study early) follow‐up: 8 weeks | 214 per 1000 | 234 per 1000 | RR 1.09 | 85 | ⊕⊝⊝⊝ | |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | This outcome was not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear methods of randomisation, blinding not assessed, and potential introduction of detection bias due to subjective nature of outcome assessments. 3 Downgraded one step for indirectness: leaving the study early can give an indication, but is not a direct measurement, of treatment acceptability. | ||||||
| HYPNOSIS OR RELAXATION versus TAU for antipsychotic‐induced tardive dyskinesia | ||||||
| Patients or population: female and male 35 (mean) years old patients with schizophrenia and tardive dyskinesia, acute extrapyramidal symptoms, and/or pseudoparkinsonism | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with TAU | Risk with hypnosis or relaxation | |||||
| Tardive dyskinesia: No clinically important improvement | 1,000 per 1,000 | 450 per 1,000 | RR 0.45 | 15 | ⊕⊝⊝⊝ | |
| Tardive dyskinesia: Deterioration of symptoms | 200 per 1,000 | 36 per 1,000 | RR 0.18 | 15 | ⊕⊝⊝⊝ | |
| Adverse effects ‐ not reported | This outcome was not reported. | |||||
| Acceptability of treatment (measured by participants leaving the study early) | 0 per 1,000 | 0 per 1,000 | not estimable | 15 | ⊕⊝⊝⊝ | No events were reported. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | This outcome was not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two steps for risk of bias: fully randomised sequence generation and blinding was not achieved. | ||||||
| Review | Citation |
| Anticholinergics | |
| Benzodiazepines | |
| Calcium‐channel blockers | |
| Cholinergics | |
| GABAergic compounds | |
| Neuroleptic medications (including dose reduction and cessation) | |
| Non‐neuroleptic compounds that impact on the dopamine and noradrenaline systems (catecholaminergics) | |
| Vitamin E | |
| This review, Miscellaneous treatments |
| Study | N and setting | Condition | Sex and age | Intervention and duration | Comparison | Outcomes |
| 20 outpatients in the USA | schizophrenia + TD | F+M | Isocarboxazid + AP | Procyclidine + AP | TD symptoms, AEs | |
| 57 participants, setting not reported | TD | F+M | L‐stepholidine + AP | Placebo + AP | TD symptoms, AEs, mental state | |
| 13 in‐ and outpatients in Venezuela | various psychiatric conditions + TD | F+M | Melatonin + AP | Placebo + AP | TD symptoms, AEs, mental state | |
| 84 in‐ and outpatients in South Africa | schizophrenia or schizoaffective disorder + TD | F+M | Omega‐3 fatty acid + AP | Placebo + AP | TD symptoms, AEs, mental state | |
| 22 inpatients in the USA | schizophrenia, dementia + TD | F+M | Papaverine + AP | TAU + AP | TD symptoms | |
| 12 outpatients in the USA | various psychiatric conditions + TD | F | Oestrogen + AP | Placebo + AP | TD symptoms, AEs | |
| Glover 1982 | 15 outpatients in the USA | schizophrenia + TD or EPS | F+M | Hypnosis or relaxation + AP | TAU + AP | TD symptoms |
| 33 outpatients in the USA | TD | F+M | Selengiline + AP | Placebo + AP | TD symptoms | |
| 20 inpatients in the UK | various psychiatric conditions + TD | F+M | Ergoloid mesylates + AP | Placebo + AP | TD symptoms | |
| 85 in‐ and outpatients in Japan | schizophrenia + TD | F+M | Ceruletide + AP | Placebo + AP | TD symptoms, AEs | |
| 42 inpatients in Japan | various psychiatric conditions + TD | F+M | Cyproheptadine + AP | Placebo + AP | TD symptoms, AEs | |
| 28 inpatients in Japan | schizophrenia + TD | F+M | Ergoloid mesylates + AP | Placebo + AP | TD symptoms, AEs, mental state | |
| 40 inpatients in Israel | schizophrenia or schizoaffective disorder + TD | F+M | Piracetam + AP | Placebo + AP | TD symptoms, AEs, global state | |
| 11 inpatients in the UK | various psychiatric conditions + TD | NR | Lithium + AP | Placebo + AP | TD symptoms, AEs | |
| 37 inpatients in Japan | various psychiatric conditions + TD | F+M | Ceruletide + AP | Placebo + AP | TD symptoms, AEs | |
| 10 inpatients in Italy | schizophrenia + TD | F+M | Ritanserin + AP | Placebo + AP | TD symptoms, AEs, mental state | |
| 18 in‐ and outpatients in the USA | schizophrenia + TD | M | Phenylalanine + AP | Placebo + AP | Leaving the study early | |
| 20 inpatients in Morocco | schizophrenia + TD | F+M | Insulin + AP | Placebo + AP | TD symptoms | |
| 88 in‐ and outpatients in the USA | various psychiatric conditions + TD | NR | NBI‐98854 (VMAT2 inhibitor valbenazine) + AP | Placebo + AP | TD symptoms, AEs | |
| 40 inpatients in the UK | various psychiatric conditions + TD | F+M | Ergoloid mesylates + AP | Placebo + AP | TD symptoms | |
| 52 in‐ and outpatients in the USA | various psychiatric conditions + TD | M | Branched‐chain amino acids + AP | Placebo + AP | TD symptoms | |
| 19 inpatients in Israel | schizophrenia + TD | F+M | Melatonin + AP | Placebo + AP | TD symptoms, AEs | |
| 22 inpatients in Israel | schizophrenia + TD | F+M | Melatonin + AP | Placebo + AP | AEs | |
| 76 inpatients in China | TD | F+M | Melatonin + AP | TAU + AP | Cognitive function | |
| 69 inpatients in Belgium and Bulgaria | TD | F+M | Levetiracetam + AP | Placebo + AP | TD symptoms, AEs | |
| 16 in‐ and outpatients in the USA | schizophrenia + TD | M | Evening primrose oil + AP | Placebo + AP | TD symptoms, mental state | |
| 50 outpatients in the USA | various psychiatric conditions + TD | F+M | Levetiracetam + AP | Placebo + AP | TD symptoms, mental state | |
| 34 inpatients in China | schizophrenia + TD | F+M | Promethazine + AP | Placebo + AP | TD symptoms, AEs, mental state, global state | |
| 42 inpatients in China | schizophrenia + TD | F+M | Buspirone + AP | Placebo + AP | TD symptoms | |
| 46 inpatients in China | schizophrenia + TD | F+M | Pemoline + AP | Placebo + AP | TD symptoms | |
| 157 inpatients in China | schizophrenia + TD | M | Ginkgo biloba + AP | Placebo + AP | TD symptoms, mental state, cognitive function | |
| AE = adverse effects, AP = antipsychotics, EPS = extrapyramidal symptoms, F = female, M = male, m = mean, N = number, TAU = treatment as usual, TD = tardive dyskinesia | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.97] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.23, 1.09] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.55] |
| 4 Tardive dyskinesia: 3. Average endpoint scale score (Simpson scale, high=poor) ‐ medium term Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐12.25, 6.65] |
| 5 Tardive dyskinesia: Average scale change scores (various scales, high=poor) ‐ medium term Show forest plot | 2 | 59 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.83, 0.20] |
| 5.1 AIMS+RTDS | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.93, 0.32] |
| 5.2 ADS | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.24, 0.57] |
| 6 Mental state: Deterioration ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.90] |
| 7 Adverse events ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.75, 7.23] |
| 8 Leaving the study early ‐ medium term Show forest plot | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.35, 0.82] |
| 2 Mental state: 1. Average endpoint scale score (BPRS, high=poor) ‐ medium term Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐7.60, ‐1.40] |
| 3 Adverse events: any adverse events ‐ medium term Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐1.18, 2.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.63, 1.00] |
| 2 Tardive dyskinesia: 1. Not any improvement ‐ short term Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.11] |
| 3 Tardive dyskinesia: 2. Deterioration ‐ short term Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.19] |
| 4 Tardive dyskinesia: Average endpoint score (Simpson scale, high=poor) ‐ short term Show forest plot | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐92.9 [‐167.57, ‐18.23] |
| 5 Leaving the study early ‐ short term Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.37, 1.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early ‐ short term Show forest plot | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.11, 53.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.96, 1.94] |
| 2 Leaving the study early ‐ medium term Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.39 [0.62, 173.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ long term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.08, 0.71] |
| 2 Tardive dyskinesia: 1. Not any improvement ‐ long term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.64] |
| 3 Adverse effects ‐ long term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 4 Leaving the study early ‐ long term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: No clinically important improvement (short term) Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.70, 1.43] |
| 2 Tardive dyskinesia: Not any improvement (short term) Show forest plot | 1 | 10 | Risk Ratio (IV, Fixed, 95% CI) | 0.28 [0.02, 4.66] |
| 3 Tardive dyskinesia: Deterioration (short term) Show forest plot | 1 | 10 | Risk Ratio (IV, Fixed, 95% CI) | 0.47 [0.02, 9.26] |
| 4 Tardive dyskinesia: Average change score (AIMS, high=poor) (short term) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.93, 1.93] |
| 5 General mental state: Deterioration (short term) Show forest plot | 1 | 10 | Risk Ratio (IV, Fixed, 95% CI) | 0.47 [0.02, 9.26] |
| 6 General mental state: Average change score (BPRS, high=poor) (short term) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐3.10, 1.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. Average endpoint score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.18 [‐3.65, ‐0.71] |
| 2 Tardive dyskinesia: 1. Average change score (hyperkinesia subscale of the SHRS , high=poor) ‐ medium term Show forest plot | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.73, 0.99] |
| 3 Leaving the study early ‐ medium term Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.46, 2.22] |
| 4 Adverse effects ‐ medium term Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.25, 1.04] |
| 5 Mental state: deterioration ‐ medium term Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.08] |
| 2 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Adverse events ‐ short term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.95] |
| 4 Leaving the study early ‐ short term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: No clinically important improvement (medium term) Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.11, 0.55] |
| 2 Tardive dyskinesia: Not any improvement (medium term) Show forest plot | 1 | 34 | Risk Ratio (IV, Fixed, 95% CI) | 0.06 [0.01, 0.43] |
| 3 Tardive dyskinesia: Average endpoint score (AIMS, high=poor) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐9.53, ‐4.67] |
| 4 General mental state: Average endpoint score (BPRS, high=poor) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐3.77, 5.17] |
| 5 Adverse effects: Any adverse effects (TESS, high=poor) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.53, 0.33] |
| 6 Adverse effects: Parkinsonism ‐ Average endpoint score (RSESE) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.36, 0.36] |
| 7 Global state: Average endpoint score (CGI, high=poor) (medium term) Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐3.78, ‐2.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.33, 0.84] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.75] |
| 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.45, 1.45] |
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. Average endpoint score (ESRS, high=poor) ‐ short term Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐4.30, 2.90] |
| 2 Parkinsonism: 1. Average endpoint score (ESRS, high=poor) ‐ short term Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐4.73, 9.73] |
| 3 Leaving the study early ‐ short term Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.85] |
| 4 Global state: Average endpoint score (CGI, high=poor) ‐ short term Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.35, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.77] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.66] |
| 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐5.47, ‐2.33] |
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.86] |
| 2 Tardive dyskinesia: 3. Average change score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐2.00, ‐1.00] |
| 3 Adverse events ‐ medium term Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.92, 2.45] |
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 2. No clinically important improvement ‐ medium term Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.18] |
| 2 Mental state: deterioration ‐ medium term Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.14] |
| 3 Adverse events: Parkinsonism ‐ Average change in scale score (ESRS, low=better) ‐ medium term Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.17, 1.77] |
| 4 Adverse events: Dystonia ‐ Average change in scale score (ESRS, low=better) ‐ medium term Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.58, ‐0.12] |
| 5 Adverse events: Akathisia ‐ Average change in scale score (ESRS, low=better) ‐ medium term Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.30, 0.22] |
| 6 Leaving the study early ‐ medium term Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.27, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.69, 1.45] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.24, 2.33] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.34, 6.70] |
| 4 Tardive dyskinesia: 1. Average change in scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐3.10, 2.70] |
| 5 Mental state: 2. Average change in scale score (BPRS, high=poor) ‐ medium term Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐15.99, 3.99] |
| 6 Leaving the study early ‐ medium term Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.81, 0.96] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.41, 0.65] |
| 3 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐2.06 [‐2.94, ‐1.18] |
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.22] |
| 5 Mental state: deterioration ‐ medium term Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
| 6 Mental state: 1. Average endpoint scale score (PANSS total, high=poor) ‐ medium term Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.51, ‐0.09] |
| 7 Cognitive function: CPT‐37 ‐ proportion correct responses (high=better) ‐ medium term Show forest plot | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.76, 1.83] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.37] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.35] |
| 4 Tardive dyskinesia: 4. Average scale score (AIMS, high=poor) ‐ short term Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐4.18, 1.78] |
| 5 Adverse effects ‐ short term Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.86] |
| 6 Leaving the study early ‐ short term Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.08, 12.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.90] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.45] |
| 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐10.53, ‐1.87] |
| 5 Leaving the study early ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement Show forest plot | 2 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.12] |
| 1.1 Short term | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.83, 1.21] |
| 1.2 Medium term | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.44, 1.23] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.47, 2.60] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.05] |
| 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ medium term Show forest plot | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐2.38 [‐6.58, 1.82] |
| 5 Adverse effects Show forest plot | 3 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Short term | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Medium term | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Leaving the study early Show forest plot | 3 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Short term | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Medium term | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Cognitive function: Average scale score ‐ medium term Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 WAIS | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 15.83 [4.61, 27.05] |
| 7.2 WMS | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 3.77 [‐8.21, 15.75] |
| 8 Mental state: deterioration ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.79, 3.23] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [0.25, 72.90] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [0.25, 72.90] |
| 4 Tardive dyskinesia: 3. Average scale score (AIMS, high=poor) ‐ short term Show forest plot | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐5.23, 6.49] |
| 5 Adverse events ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.38, 94.35] |
| 6 Leaving the study early ‐ short term Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.13, 52.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. Not any improvement Show forest plot | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.65, 1.07] |
| 1.1 Short term | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.50, 1.06] |
| 1.2 Medium term | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.64, 1.27] |
| 2 Tardive dyskinesia: 2. Deterioration Show forest plot | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.80] |
| 2.1 Short term | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 65.74] |
| 2.2 Medium term | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.90] |
| 3 Adverse effects Show forest plot | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.74, 2.36] |
| 3.1 Short term | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.79 [0.47, 30.77] |
| 3.2 Medium term | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.61, 2.07] |
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.49, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.94] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.81] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.81] |
| 4 Leaving the study early ‐ medium term Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Short term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

