Ubat kolinergik untuk diskinesia tardif cetusan antipsikotik
Appendices
Appendix 1. Previous methods and searches
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
People with schizophrenia or any other serious mental illness, diagnosed by any criteria, irrespective of gender, age or nationality who:
1. Required the use of neuroleptics for more than three months.
2. Developed tardive dyskinesia during neuroleptic treatment (diagnosed by any criteria at baseline of the trial and at least one other occasion).
3. For whom the dose of neuroleptic medication had been stable for one month or more before the trial and during the trial.
Types of interventions
1. The cholinergic drugs arecoline, choline, deanol, lecithin, meclofenoxate, physostigmine, RS 86.
In the first substantial update of the review the following cholinergic compounds were assessed to be relevant and added to the scope of the review: tacrine, 7‐methoxytacrine, ipidacrine, galantamine, donepezil, rivastigmine, eptastigmine, metrifonate, xanomeline and cevimeline.
2. Control condition: Placebo or no intervention.
Types of outcome measures
Clinical efficacy (clinically relevant improvement of tardive dyskinesia symptoms) is defined in this review as an improvement in tardive dyskinesia symptoms of more than 50% on any validated tardive dyskinesia scale.
The outcomes of interest were:
1. Global outcome measures (this category of outcome measures was added in the first substantial up‐date of the review).
1.1 The number of people per treatment group who died for any reason.
1.2 Treatment group mean and standard deviation of endpoint score on any scale of quality of life.
1.3 Treatment group mean and standard deviation of endpoint score on any scale of level of functioning.
2. Tardive dyskinesia changes
2.1 The number of people per treatment group who did not show a clinically relevant improvement (improvement of more than 50% on any validated tardive dyskinesia scale) as assessed by the rater.
2.2 The number of people per treatment group who did not show any improvement by any means of tardive dyskinesia assessment as assessed by the rater.
2.3 The number of people per treatment group who deteriorated by any means of tardive dyskinesia assessment as assessed by the rater.
2.4 The number of people per treatment group who did not experience any improvement in tardive dyskinesia symptoms as rated by self‐assessment (this outcome was added in the first substantial up‐date of the review).
2.5 The number of people per treatment group who deteriorated in tardive dyskinesia symptoms as rated by self‐assessment (this outcome was added in the first substantial up‐date of the review).
2.6 Treatment group mean and standard deviation of endpoint score on any validated tardive dyskinesia scale.
2.7 Treatment group mean and standard deviation of change (baseline minus endpoint) in score on any validated tardive dyskinesia scale.
3. General mental state changes
3.1 The number of people per treatment group who deteriorated in psychiatric symptoms (such as delusions and hallucinations) by any means of assessment of psychiatric symptoms or mental state.
3.2 Treatment group mean and standard deviation of endpoint score on any scale of psychiatric symptoms.
4. Acceptability of the treatment
4.1 The number of people per treatment group who had any adverse effect (other than deterioration of tardive dyskinesia symptoms or change in mental state).
4.2 The number of people per treatment group who dropped out during the trial.
When appropriate, the outcomes were grouped into time periods ‐ short term (less than 6 weeks), medium term (between 6 weeks and 6 months) and long term (over 6 months).
Search methods for identification of studies
1. Electronic searching
1.1 In the original version of the review relevant randomised trials were identified by searching the following electronic databases:
1.1.1 Biological Abstracts (January 1982 to May 1995) was searched using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and ((tardive near (dyskine* or diskine*) or (abnormal near movement* near disorder*) or (involuntar* near movement*))]
The set of reports that resulted from this was hand searched for possible trials and researched, within the bibliographic package ProCite, with the phrase [cholinergic* or arecoline or choline or deanol or lecithin or meclofenoxate or physostigmine or RS?86]
1.1.2 The Cochrane Schizophrenia Group's Register was searched using the phrase:
[cholinergic* or arecoline or choline or (#42 = 12) or deanol or (#42 = 353) or (#42 = 355) or lecithin or (#42 = 151) or meclofenoxate or physostigmine or RS?86]
1.1.3 EMBASE (January 1980 to May 1995) was searched using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and ((tardive dyskinesia in thesaurus ‐subheadings, prevention, drug therapy, side effect and therapy) or (neuroleptic dyskinesia in thesaurus ‐all subheadings) or (tardive or dyskines*) or (movement* or disorder*) or (abnormal or movement* or disorder*))]
The set of reports that resulted from this was hand searched for possible trials and researched, within the bibliographic package ProCite, with the phrase [cholinergic* or arecoline or choline or deanol or lecithin or meclofenoxate or physostigmine or RS?86]
1.1.4 LILACS (January 1982 to September 1996) was searched using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and (tardive or (dyskinesia* or diskinesia*)) or (drug induced movement disorders in thesaurus))]
This downloaded set of reports was hand searched for possible trials and researched, within the bibliographic package ProCite, with the phrase [cholinergic* or arecoline or choline or deanol or lecithin or meclofenoxate or physostigmine or RS?86]
1.1.5 MEDLINE (January 1966 to May 1995) was searched using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and (movement‐disorders in MeSH / explode all subheadings) or (anti‐dyskinesia‐agents in MeSH / explode all subheadings) or (dyskinesia‐drug‐induced in MeSH / explode all subheadings) and
(psychosis in MeSH / explode all subheadings) or (schizophrenic disorders in MeSH / explode all subheadings) or (tardive near (dyskine* or diskine*)) or (abnormal* near movement* near disorder*) or (involuntar* near movement*))]
The set of reports that resulted from was hand searched for possible trials and researched, within the bibliographic package ProCite, with the phrase [cholinergic* or arecoline or choline or deanol or lecithin or meclofenoxate or physostigmine or RS?86]
1.1.6 PsycLIT (January 1974 to May 1995) was searched using the CSG's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and (explode movement‐disorders in DE) or (explode tardive‐dyskinesia in DE) or (tardive near (dyskine* or diskine*) or (abnormal* near movement* near disorder*) or (involuntar* near movement*))]
The set of reports that resulted from this was hand searched for possible trials and researched, within the bibliographic package ProCite, with the phrase [cholinergic* or arecoline or choline or deanol or lecithin or meclofenoxate or physostigmine or RS?86]
1.1.7 SCISEARCH ‐ Science Citation Index. Each of the included studies was sought as a citation on the SCISEARCH database. Reports of articles that had cited these studies were inspected so that further trials could be identified.
1.2 In the first substantial up‐date of the review the Cochrane Schizophrenia Group's Register was searched (October 2001) using the phrase:
[cholinergic* OR arecolin* OR arecholin* OR meclofenoxat* OR meclophenoxat* OR centrofenoxin* OR centrophenoxin* OR 'ANP 235' OR 'EN 1627' OR deanol* OR demanol* OR 'CR 121' OR 'RS 86' OR physostigmin* OR fysostigmin* OR lecithin* OR lecitin* OR choline OR cholin OR coline OR tacrin* OR takrin* OR tetrahydroaminoacridin* OR tetrahydroaminacrin* OR 'CI 970' OR THA OR THAA OR 7‐methoxyacridin* OR 7‐metoxyacridin* OR methoxytacrin* OR metoxytacrin* OR metoxytakrin* OR methoxycrin* OR metoxycrin* OR MEOTA OR ipidacrin* OR amiridin* OR NIK247 OR 'NIK 247' OR donepezil* OR E2020 OR 'E 2020' OR galanthamin* OR galantamin* OR 'CGP 37267' OR rivastigmin* OR ENA713 OR 'ENA 713' OR '212 713' OR eptastigmin* OR heptylstigmin* OR heptylphysostigmin* OR heptylfysostigmin* OR 'L 693 487' OR MF201 OR 'MF 201' OR metrifonat* OR metriphonat* OR trichlorfon* OR trichlorphon* OR trichlorfen* OR trichlorphen* OR 'L 1359' OR 'Bay a 9826' OR 'Bay 1 1359' OR xanomelin* OR 'LY 246708' OR 'FG 10232' OR cevimelin* OR AF102B OR 'AF 102B' OR 'FKS 508' OR 'SND 5008' OR SNK508 OR SNI2011]
The Cochrane Schizophrenia Group's Register is assembled by extensive searches of randomised controlled trials in electronic databases, registers for conference proceedings and dissertations etc. The search strategy of the CSG's Register contains a search strategy for trials on tardive dyskinesia. Please see search strategy in CSG module in Cochrane Library.
2. Reference searching
The references of all identified studies were inspected for more studies.
3. Personal contact
The first author of each included study was contacted for information regarding unpublished trials.
Data collection and analysis
[For definitions of terms used in this, and other sections, please refer to the Glossary.]
1. Selection of trials
The title or abstract of each reference identified by the search was inspected independently by two reviewers (IT and ES) to assess relevance. For articles that could possibly have been RCTs, or in cases of disagreement, the full article was obtained. In turn these articles were independently inspected. There was no disagreement between the two reviewers regarding which trials were relevant.
2. Assessment of methodological quality
The methodological quality of each included trial was assessed independently by two reviewers (IT and ES). Quality was evaluated using criteria described in the Cochrane Reviewers' Handbook (Clarke 2001) and the Jadad Scale (Jadad 1996). The former is based on evidence of a strong relationship between allocation concealment (blinding of random assignment of participants to intervention groups) and the potential for bias in the results (Schulz 1995), i.e. lack of adequate allocation concealment is associated with selection bias (systematic differences in comparison groups). Thus trials can, to a certain extent, be evaluated by their method of allocation concealment. The method for assigning participants to interventions undergoing comparison should be robust against selection bias (i.e. the trialist should not be able to influence which intervention the participant will receive nor should any foreknowledge of treatment assignment influence recruitment) and its description should be clear (Clarke 2001). The risk for bias in the results of a study is defined as below (Clarke 2001):
A. Low risk of bias (adequate allocation concealment)
B. Moderate risk of bias (some doubt about the results)
C. High risk of bias (inadequate allocation concealment)
The Jadad Scale measures a wider range of factors that impact on the quality of a trial. The scale includes three items:
1. Was the study described as randomised?
2. Was the study described as double blind?
3. Was there a description of withdrawals and drop‐outs?
Each item receives 1 point if the answer is positive. In addition: 1 additional point each is given, if randomisation and/or blinding procedures are described and adequate. 1 point each can be deducted if either the randomisation or the blinding/masking procedures described were inadequate. Thus the maximum score is 5 points. (Jadad 1996).
Only trials described as randomised (category A or B) were included in the statistical analysis. The Jadad scale was used as an extra assessment of quality, however Jadad points were not used to exclude trials. If there was not enough information in the publication to assess adequate randomisation and methodological quality, or there was disagreement between the two reviewers, the article was added to those awaiting assessment and authors of the study were contacted for clarification. Justification for excluding trials from the analysis was documented.
3. Data extraction
Data from the included trials were extracted by two researchers. Two kinds of measures were extracted: dichotomous (binary, yes/no) data and continuous (scale) data.
Trials in which a crossover design was used included the risk of carry over effects of a medication in the second or more stages of the trial (after crossover). To exclude potential carry over effects, only data from the first stage of the trial (before crossover) were used in the analysis.
In the case of incomplete data, the article was added to those awaiting assessment and authors were contacted for clarification. If a trial met the criteria for methodological quality, but it was impossible to extract any data or collect unpublished data from the authors, the study had to be excluded from statistical analysis.
4. Data analysis
4.1 Intention to treat analysis
Where possible, all data were analysed using the intention‐to‐treat principle (once randomised, always analyse). Data were excluded from studies where more than 50% of participants in any group were lost to follow up. (This did not include the outcome of 'drop‐outs'.) In studies with less than a 50% drop‐out rate, people leaving early were considered to have had the negative outcome, except for the event of death. When possible and appropriate, the 'Last Observation Carried Forward' technique was used in analysis of continuous outcomes.
4.2 Dichotomous data
For dichotomous outcomes a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI) was calculated. If overall results were significant, the number needed to treat (NNT) was calculated from the absolute risk difference between treatment and control groups. If heterogeneity was found it was investigated and a random effects model used.
4.3 Continuous data
4.3.1 Normally distributed versus skewed data
Continuous data on clinical and social outcomes are often not normally distributed. A reliable statistical analysis of groups undergoing comparison requires that samples tested attain a normal distribution. To avoid including non‐normally distributed samples in the statistical analysis, the following standards were applied to all continuous data before inclusion:
1) Standard deviations and means were reported or derivable from data in the publication, or were obtainable from authors.
2) When a scale started from a finite number (such as zero), the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution ‐ Altman 1996).
3) If a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skewness is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score of the scale. Endpoint scores on scales used in clinical practice often have a definite minimum and maximum on the scale, and so these rules can be applied to them.
4.3.2 Summary statistic
For continuous outcomes a pooled weighted mean difference (WMD) between groups was calculated. If heterogeneity was found it was investigated and a random effects model used.
4.3.3 Valid scales
A wide range of instruments are available to measure mental health outcomes. These instruments vary in quality and many are not valid. Unpublished instruments are more likely to report statistically significant findings than those that have been published (Marshall 2000). The following minimum standards were set for valid scales: 1. The instrument had to have been described in a peer reviewed journal. 2. The instrument had to be either a self report scale or completed by an independent rater.
4.3.4 Endpoint versus change data
When continuous data are presented on a scale which includes the possibility of negative values (such as change on a scale), it is impossible to tell whether data is non‐normally distributed (skewed) or not. It is thus preferable to use end point data of a scale (participants' total scores at the end of study ‐ not change in score from baseline), which typically cannot have negative values. Where possible endpoint data were presented, and if both endpoint and change (from baseline) data were available for the same outcome, then only the former were used.
5. Test for heterogeneity
It is important not to pool heterogeneous studies together, as heterogeneity might reflect differences in study design or sample population, rather than true variation in results of the outcome measured. To investigate the possibility of heterogeneity of trial results, a Mantel‐Haenszel Chi‐square test was used, as well as visual inspection of graphs. A significance level less than 0.10 was pre‐defined as evidence of heterogeneity. If heterogeneity was found, the reasons for it were explored. If no study‐related explaining factor was found, data were tested pooled using the random effects model which takes into account the variation between studies. (The random effects model is more conservative in estimating treatment effect, and takes into account that some trials will produce odd results by chance.) If using the random effects model did not change the statistical significance level of the results, the results remained pooled. If the random effects model did change the statistical significance of the result, studies responsible for heterogeneity were not added to the main body of homogeneous trials, but summated and presented separately and reasons for heterogeneity investigated.
6. Addressing publication bias
Data from all included studies were entered into a funnel graph (trial effect against trial size) in an attempt to investigate the likelihood of overt publication bias.
7. Sensitivity analyses
Four sensitivity analyses were prespecified: 1.Treatment effect differs according to difference in the quality of trials, 2. Treatment effect differs according to different lengths of treatment, 3. Treatment effect differs for the various drugs and 4. Treatment effect differs according to the age of the participants (this sensitivity analysis was added in the first substantial up‐date of the review). These analyses were evaluated by looking at separate subgroups of trials.
8. General
Data were entered into Revman in such a way that the area to the left of the line of no effect in the graph indicated a favourable outcome for cholinergic agents.

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.
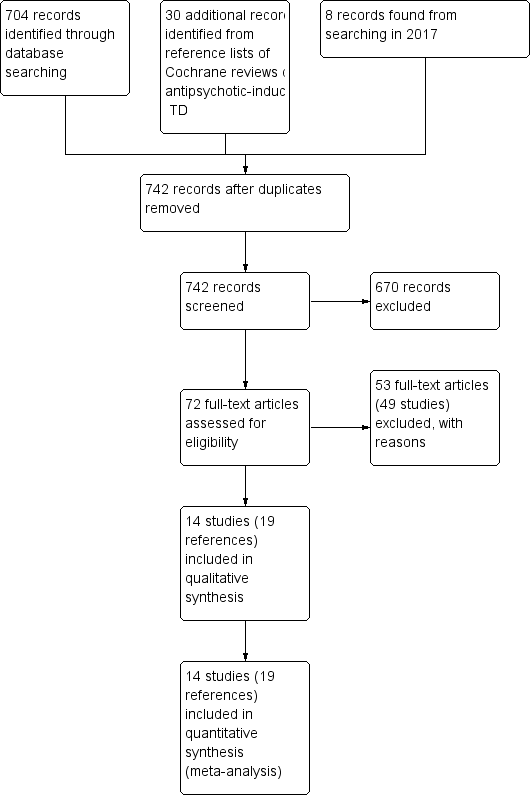
Study flow diagram for 2015 and 2017 searches.

'Risk of bias, summary: review authors' judgements about each risk of bias item for each included study.
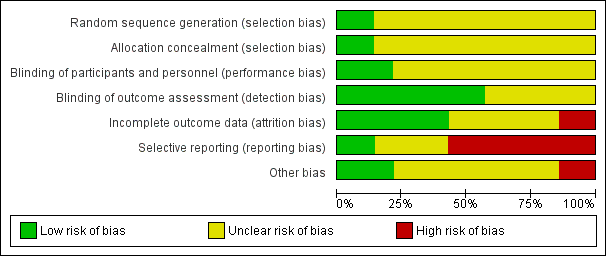
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
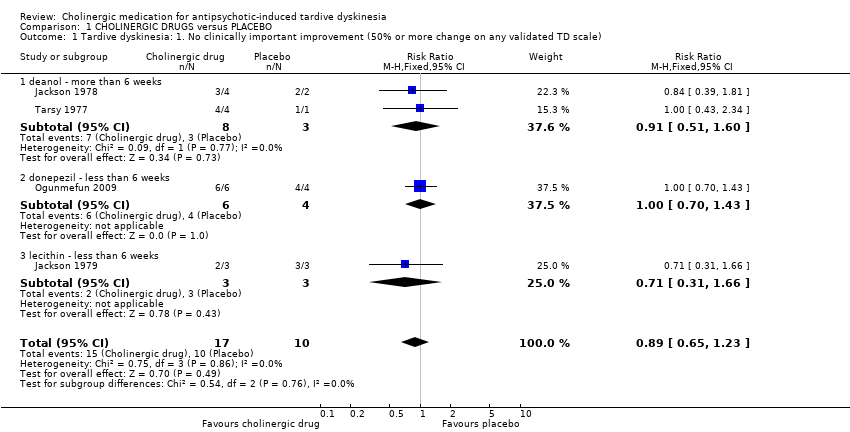
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater).
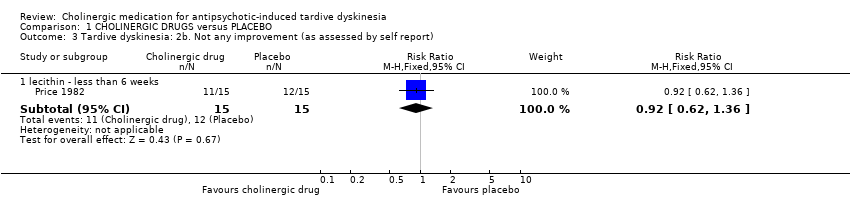
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better).
| Study | Intervention | Mean | SD | N | Comments |
| deanol ‐ more than 6 weeks | |||||
| Tarsy 1977 | Deanol | 10 | 5.48 | 4 | |
| Tarsy 1977 | Placebo | 10 | 0 | 1 | The confidence interval of mean difference was not estimable because the placebo group only had one participant. |
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater).
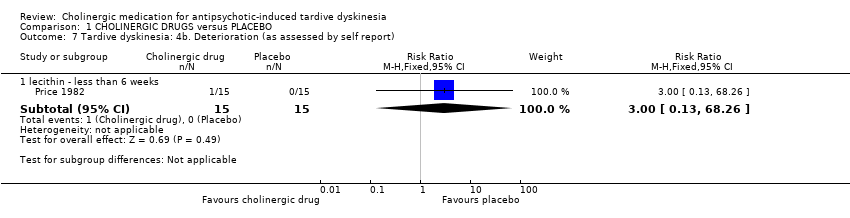
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 8 Global outcome: Death for any reason.
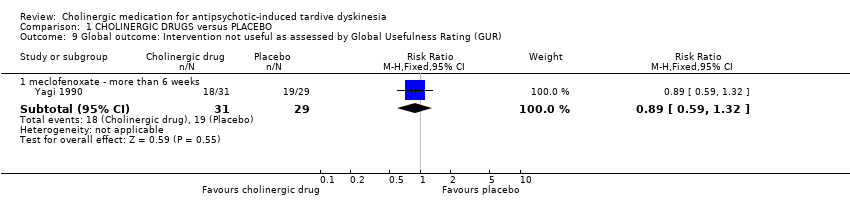
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR).
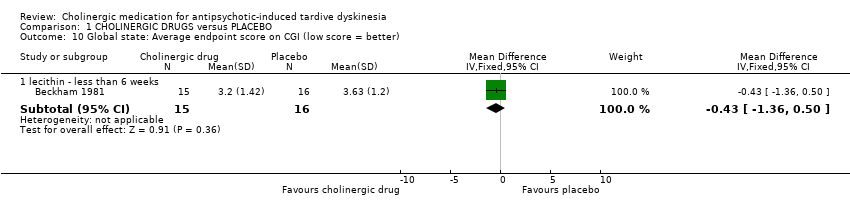
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 10 Global state: Average endpoint score on CGI (low score = better).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 11 Mental state: Deterioration.
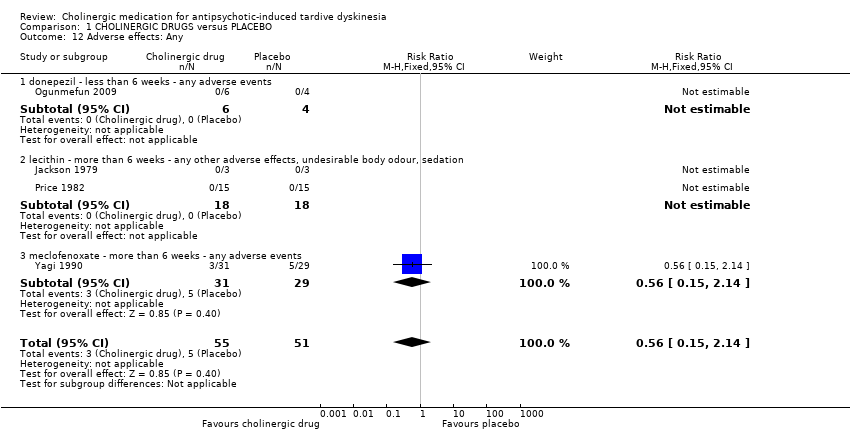
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 12 Adverse effects: Any.

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 13 Adverse effects: Various specific.
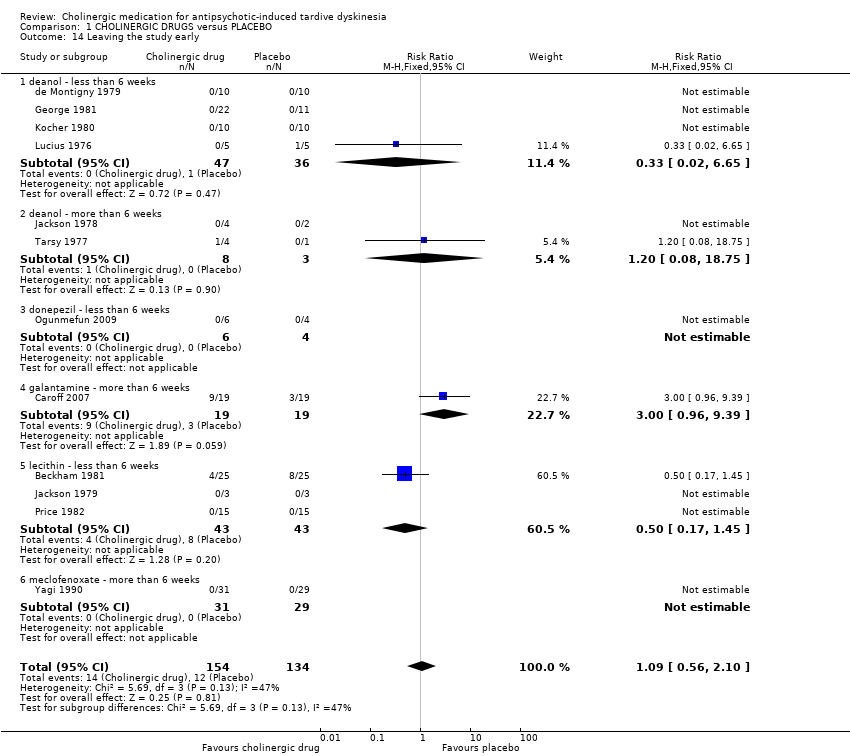
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 14 Leaving the study early.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 2 Global outcome: Death for any reason ‐ less than 6 weeks.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 3 Leaving the study early ‐ less than 6 weeks.
| Excluded study | Comparison | Treatment category | Relevant review | ||||
| #1 | #2 | ||||||
| Alpha‐methyl‐p‐tyrosine (AMPT) | versus choline chloride | Amino acid | Organic salt | ‐ | |||
| versus hydroxytryptophan | Amino acid (serotonin precursor) | ‐ | |||||
| versus valproic acid | Mood stabilisers | ‐ | |||||
| versus L‐DOPA | Amino acid | ‐ | |||||
| Benztropine versus bromocriptine | Anticholinergic | Dopamine agonist | ‐ | ||||
| Bromocriptine versus haloperidol | Dopamine agonist | Antipsychotic | ‐ | ||||
| Choline chloride | versus L‐DOPA | Organic salt | Amino acid | ‐ | |||
| versus hydroxytryptophan | ‐ | ||||||
| versus valproic | Anticonvulsant | ‐ | |||||
| Deanol | versus lithium carbonate | Antidepressant | Organic salt | ‐ | |||
| versus placebo | Placebo | ‐ | |||||
| versus sodium valproate | Anticonvulsant | ‐ | |||||
| versus oxpertine | Antipsychotic | ‐ | |||||
| Hydroxytryptophan versus L‐DOPA | Amino acid | Amino acid | ‐ | ||||
| L‐DOPA versus valproic acid | Anticonvulsant | ‐ | |||||
| Lithium carbonate versus placebo | Mood stabiliser | Placebo | ‐ | ||||
| Oxypertine versus sodium valproate | Antipsychotic | Anticonvulsant | ‐ | ||||
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | Specific cholinergic drug (N = 150) versus placebo (N = 150) |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| CHOLINERGIC DRUGS versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: people with various psychiatric disorders (mainly schizophrenia) and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | CHOLINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 to 12 weeks | 1000 per 1000 | 890 per 1000 | RR 0.89 | 27 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin) found a significant difference between cholinergic drug and placebo. |
| Tardive dyskinesia: Deterioration follow‐up: 9 days to 12 weeks | 116 per 1000 | 129 per 1000 | RR 1.11 | 147 | ⊕⊕⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Mental state: Deterioration follow‐up: 11 days to 12 weeks | 56 per 1000 | 28 per 1000 | RR 0.50 | 77 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, lecithin) found a significant difference between cholinergic drug and placebo. |
| Adverse effects: Any adverse events follow‐up: 9 days to 8 weeks | 98 per 1000 | 55 per 1000 | RR 0.56 | 106 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Acceptability of treatment: Leaving the study early follow‐up: 9 days to 12 weeks | 90 per 1000 | 98 per 1000 | RR 1.09 | 288 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, galantamine, meclofenoxate, lecithin) found a significant difference between cholinergic drug and placebo. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: for many studies it was unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| Interventions | Reference |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | This review |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | |
| Non‐neuroleptic catecholaminergic drugs | |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale) Show forest plot | 4 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.23] |
| 1.1 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.60] |
| 1.2 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.70, 1.43] |
| 1.3 lecithin ‐ less than 6 weeks | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.31, 1.66] |
| 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) Show forest plot | 9 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] |
| 2.1 deanol ‐ less than 6 weeks | 3 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.18] |
| 2.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.26, 2.57] |
| 2.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.72, 2.44] |
| 2.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 2.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.27] |
| 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.36] |
| 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better) Show forest plot | 7 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.44, 0.21] |
| 4.1 deanol ‐ more than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐0.29, 3.13] |
| 4.2 galantamine ‐ more than 6 weeks | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐0.44, 3.44] |
| 4.3 lecithin ‐ less than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐2.21, 0.07] |
| 4.4 lecithin ‐ more than 6 weeks | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.04, 0.84] |
| 4.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.58, 0.20] |
| 4.6 rivastigmine ‐ less than 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐1.16, 5.56] |
| 4.7 donepezil ‐ less than 6 weeks | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐4.22, 6.42] |
| 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better) Show forest plot | Other data | No numeric data | ||
| 5.1 deanol ‐ more than 6 weeks | Other data | No numeric data | ||
| 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater) Show forest plot | 8 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.24] |
| 6.1 deanol ‐ less than 6 weeks | 2 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.48, 5.76] |
| 6.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.20, 2.18] |
| 6.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.06, 7.85] |
| 6.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.31] |
| 6.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.18, 19.55] |
| 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 8 Global outcome: Death for any reason Show forest plot | 11 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.32] |
| 10 Global state: Average endpoint score on CGI (low score = better) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 lecithin ‐ less than 6 weeks | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.36, 0.50] |
| 11 Mental state: Deterioration Show forest plot | 5 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.61] |
| 11.1 deanol ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 11.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] |
| 11.3 lecithin ‐ less than 6 weeks | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 12 Adverse effects: Any Show forest plot | 4 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] |
| 12.1 donepezil ‐ less than 6 weeks ‐ any adverse events | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 lecithin ‐ more than 6 weeks ‐ any other adverse effects, undesirable body odour, sedation | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 meclofenoxate ‐ more than 6 weeks ‐ any adverse events | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] |
| 13 Adverse effects: Various specific Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 deanol ‐ less than 6 weeks ‐ gastric adverse effects | 5 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.55, 147.95] |
| 13.2 deanol ‐ less than 6 weeks ‐ sedation, periferal cholinergic effects, undesirable body odour | 6 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.83 [0.99, 47.25] |
| 13.3 lecithin ‐ less than 6 weeks ‐ GI adverse effects | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Leaving the study early Show forest plot | 12 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.56, 2.10] |
| 14.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 14.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] |
| 14.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.96, 9.39] |
| 14.5 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.17, 1.45] |
| 14.6 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Global outcome: Death for any reason ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Leaving the study early ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

