Benzodiazepine zur Behandlung von Neuroleptika‐induzierter Spätdyskinesie
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: "randomly assigned." Blindness: "double blind." Design: parallel group. Duration: not reported (optimal dose + 2 weeks + taper off). Setting: not reported, USA. | |
| Participants | Diagnosis: psychiatric patients (details not reported). Obvious TD (at least 3 scores of mild or 1 score of moderate on AIMS). Duration of TD: not reported. n = 21. Age: mean 51.6 years; range 36‐63 years. Sex: 16 men and 5 women. | |
| Interventions | 1. Clonazepam: 3.9 ± 2.6 mg/day; optimal dose + 2 weeks + taper. n = 10. 2. Phenobarbital (as active placebo): 88.6 ± 45.7 mg/day, optimal dose + 2 weeks + taper. n = 11. Prior to the trial, 5 participants were taking no antipsychotic drugs and 1 participant was taking homeopathic doses; doses remained stable throughout the study. Concomitant medication was not reported. | |
| Outcomes | TD symptoms: no clinically important improvement, not any improvement (AIMS). Leaving the study early. Adverse effects: any adverse effects. Unable to use ‐ Mental state: POMS: no usable data reported (study reported that "none of the differences was statistically significant"). | |
| Notes | Sponsorship source: supported in part by NIMH grant. Declarations of interest: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind". Details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind". Details not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Although not clearly reported, it seems that all participants completed the double‐blind phase (data reported for all 21 participants). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes seemed to have been reported but not as mean (SD). Protocol not available; impossible to verify that all predefined outcomes were reported. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: "randomly assigned," no details reported. Blindness: "double blind," described. Design: parallel group. Duration: 5‐6 weeks. Setting: outpatients (most) and inpatients from Veterans Administration Medical Center, USA. | |
| Participants | Diagnosis: schizophrenia (RDC criteria). Duration of TD: not reported. n = 17. Sex: not reported. | |
| Interventions | 1. Alprazolam: 7.2 ± 1.8 mg for 5‐6 weeks. n = 5. 2. Diazepam: 48.3 ± 19.4 mg/day for 5‐6 weeks. n = 5. 3. Placebo for 5‐6 weeks: n = 6. Participants were stable for at least 2 weeks prior to study and doses were unchanged during the study. Concomitant medication: 55 participants also received anticholinergic medications. | |
| Outcomes | TD symptoms: no clinically important improvement, not any improvement, deterioration, mean TD score at end of trial (GDS). Leaving study early. Unable to use: Mental state: BPRS, SANS (data not reported for TD subgroup). Adverse effects (data not reported for TD subgroup). | |
| Notes | Sponsorship source: supported by a Public Health Service grant and a grant from the National Institute of Mental Health, a VA Career Development Award to the first author, a grant from the Upjohn Company, and the Research Service of the VA. Participants were extracted post‐hoc from a larger study examining benzodiazepines for the treatment of the negative symptoms of schizophrenia. Data on age, gender, baseline medication doses, adverse effects, and attrition rate for the initial cohort are provided in the parent study (Csernansky 1988). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to the treatment with either Alprozalam [alprazolam], Diadepam [diazepam], or placebo..." Further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Patients were randomly assigned to the treatment with either alprozalam, diadepam, or placebo under double‐blind conditions. Identical capsules contained either 1 mg of alprozalam, 10mg of diazepam, or the drug carrier as placebo." |
| Blinding of outcome assessment (detection bias) | Low risk | "....two independent raters." |
| Incomplete outcome data (attrition bias) | Unclear risk | "Fifty‐five RDC schizophrenic outpatients were rated using the Gerlach Dyskinesia Scale (GDS) before, and at weekly intervals during, treatment... 17 patients were identified with rateable TD symptoms at baseline..." All 17 participants were entered to analysis. However, as 72 participants were enrolled in the original study, it was unclear if relevant data for any of the 17/72 participants that dropped out were missing. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes for the main study seemed to have been reported. Protocol not available for verification. Although mental state and adverse effects were not reported separately for participants with TD symptoms. TD was not an inclusion criterion and thus did not seem to affect bias. "Since TD was not a criterion for inclusion into or exclusion from the trial, it was only by chance that we identified 17 patients with TD symptoms." |
| Other bias | High risk | Participants with TD at baseline were extracted post‐hoc from a larger study examining benzodiazepines for the treatment of the negative symptoms of schizophrenia. |
| Methods | Allocation: randomised. Blindness: single. Design: cross‐over. Duration: 24 weeks (10 weeks + 4 weeks' washout then crossed over to another 10 weeks). Setting: inpatients in a long‐term state psychiatric hospital, USA. | |
| Participants | Diagnosis: schizophrenia (n = 12), organic brain syndrome (n = 1), unknown (n = 2). Baseline AIMS rating ≥ 2 on 1 item, and drug‐induced parkinsonian movements ≤ 6. Duration of TD: 2‐6 years. n = 15. Age: mean 57.4 years, 50‐65 years (among completers). Sex: 10 men and 3 women (among completers). | |
| Interventions | 1. Diazepam + TAU: dose 6‐25 mg/day, mean 12 mg/day. n = 8 (completers). 2. TAU: n = 5 (completers). Participants were on stable doses of both antipsychotic and anticholinergic medication for 2 weeks prior to study and on stable doses throughout the study except 2 participants: medication was altered for 2 participants in the second period of cross‐over. During the study, 10 participants received antipsychotic drugs, while 8 received anticholinergic agents, and 1 received amantadine. | |
| Outcomes | TD symptoms: no clinically important improvement, deterioration, not any improvement, mean TD score at end of trial (AIMS). Mental state: mean score at end of treatment BPRS (sum of 5 features). Leaving study early. | |
| Notes | Sponsorship source: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each patient was assigned randomly..." Further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | High risk | As one of the groups received an intervention and the second standard care, blinding of participants and personnel could not have been possible. |
| Blinding of outcome assessment (detection bias) | Low risk | "rater‐blind." "The rating scales were administered by trained observers who did not know which patients received diazepam." |
| Incomplete outcome data (attrition bias) | Low risk | 13% attrition. "Fifteen patients began the study. Two failed to complete the entire protocol (one because she continued to receive diazepam throughout the study and the other because she was discharged from the hospital)." |
| Selective reporting (reporting bias) | Unclear risk | Outcomes seemed to have been reported. However, protocol was not available for verification. |
| Other bias | Unclear risk | Change in medication for 2 participants may have had a confounding effect; however, both substitutions occurred 4 weeks into the second phase of the study. |
| Methods | Allocation: "randomized controlled trial." Blinding: "double blind." "The two drugs were contained in capsules with same appearance." Duration: 8 weeks. Location: "inpatients," China. Length of follow‐up: 8 weeks. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R) and antipsychotic‐induced TD. Duration of TD: mean 2.7 (SD 1.21) years. n = 24. Age: mean 39.44 (SD 8.43) years. Sex: 15 men and 9 women. | |
| Interventions | 1. Clonazepam: 4‐6 mg/day, mean 5 mg/day. n = 12. 2. Placebo: n = 12. All participants continued previous use of antipsychotic and anticholinergic drugs. | |
| Outcomes | TD: mean TD score at end of trial (AIMS). Leaving study early. | |
| Notes | Sponsorship source: not reported. Participants with stable or aggravating symptoms of TD after suspending antipsychotic drugs for 2 weeks excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized controlled trial." Author did not state the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind." "The two drugs were contained in capsules with same appearance." Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) | Low risk | All participants competed study. |
| Selective reporting (reporting bias) | Low risk | Author reported all measured outcomes. |
| Other bias | Low risk | Free from other bias. |
AIMS: Abnormal Involuntary Movement Side Effects Scale; BPRS: Brief Psychiatric Rating Scale; CCMD‐2‐R: Chinese Clinical Manual for Diagnosis Revised; GDS: Gerlach Dyskinesia Scale; n: number of participants; NIMH: National Institute of Mental Health; POMS: Profile of Mood States; RDC: Research Diagnostic Criteria; SANS: Scale for the Assessment of Negative Symptoms; SD: standard deviation; TAU: treatment as usual; TD: tardive dyskinesia; VA: Veterans Administration.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: not randomised. | |
| Allocation: randomised. Participants: people with moderate‐to‐severe dementia and antipsychotic‐induced TD. Intervention: diazepam vs tetrabenazine. Outcomes: data not reported for first phase of cross‐over. Study dated from 1960s and early 1970s, so we were unable to identify contact details for authors. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: randomised. Participants: people with antipsychotic‐induced akathisia (n = 12). Interventions: clonazepam vs placebo. Outcomes: no data reported for people with TD. | |
| Allocation: randomised. Participants: people with antipsychotic‐induced akathisia. Interventions: benztropine vs propranolol. Outcomes: no data reported for people with TD. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. Participants: people with antipsychotic‐induced TD. Interventions: benzodiazepines vs placebo. Outcomes: data presented in graphs and impossible to extract, first author contacted, original data cannot be provided. | |
| Allocation: randomised. Participants: people with antipsychotic‐induced akathisia. Interventions: benzodiazepines vs artane (trihexyphenidyl hydrochloride). | |
| Allocation: not randomised. | |
| Study 1. Allocation: randomised. Participants: people treated for schizophrenia with antipsychotic‐induced TD. Interventions: naltrexone vs placebo, no benzodiazepines. Study 2. Allocation: randomised. Participants: people treated for schizophrenia with antipsychotic‐induced TD. Interventions: naltrexone + clonazepam vs clonazepam + placebo, benzodiazepines not randomised. |
n: number of participants; TD: tardive dyskinesia.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale) Show forest plot | 2 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.60, 2.09] |
| Analysis 1.1  Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale). | ||||
| 1.1 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.23] |
| 1.2 Diazepam vs TAU ‐ medium term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.71, 3.16] |
| 2 TD symptoms: not any improvement Show forest plot | 2 | 32 | Risk Ratio (IV, Fixed, 95% CI) | 1.49 [0.33, 6.74] |
| Analysis 1.2 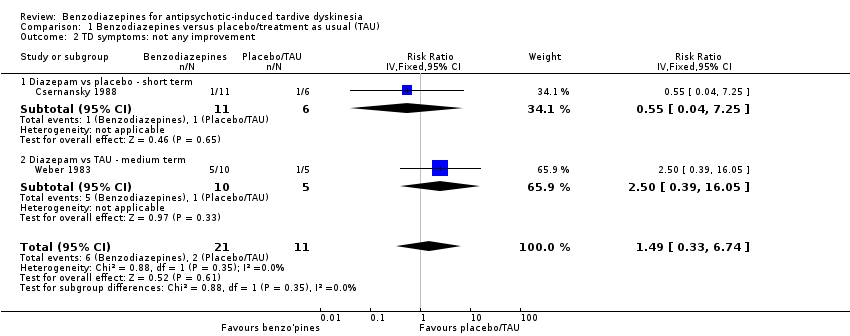 Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 2 TD symptoms: not any improvement. | ||||
| 2.1 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.04, 7.25] |
| 2.2 Diazepam vs TAU ‐ medium term | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 2.5 [0.39, 16.05] |
| 3 TD symptoms: deterioration Show forest plot | 2 | 30 | Risk Ratio (IV, Fixed, 95% CI) | 1.48 [0.22, 9.82] |
| Analysis 1.3 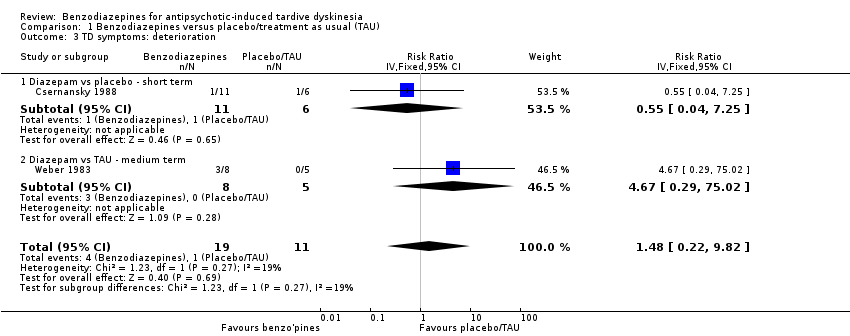 Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 3 TD symptoms: deterioration. | ||||
| 3.1 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.04, 7.25] |
| 3.2 Diazepam vs TAU ‐ medium term | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 4.67 [0.29, 75.02] |
| 4 TD symptoms: mean TD score at the end of treatment Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 4 TD symptoms: mean TD score at the end of treatment. | ||||
| 4.1 Diazepam vs placebo ‐ Gerlach Dyskinesia Scale (GDS) scores (idiopathic Parkinson's disease (IPD), greater = worse) ‐ short term | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.57, 0.99] |
| 4.2 Diazepam vs TAU ‐ Abnormal Involuntary Movement Scale (AIMS) scores (IPD, greater = worse) ‐ medium term | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [0.49, 11.11] |
| 4.3 Clonazepam vs placebo ‐ AIMS scores (IPD, greater = worse) ‐ medium term | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.22 [‐4.63, ‐1.81] |
| 5 Mental state: mean score at the end of treatment (Brief Psychiatric Rating Scale (BPRS), low = best) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5 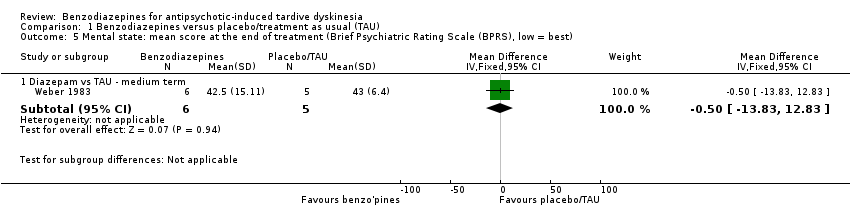 Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 5 Mental state: mean score at the end of treatment (Brief Psychiatric Rating Scale (BPRS), low = best). | ||||
| 5.1 Diazepam vs TAU ‐ medium term | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐13.83, 12.83] |
| 6 Leaving the study early Show forest plot | 3 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.15, 48.04] |
| Analysis 1.6  Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 6 Leaving the study early. | ||||
| 6.1 Clonazepam vs placebo ‐ medium term | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Diazepam vs TAU ‐ medium term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.15, 48.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale) ‐ short term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Benzodiazepines vs other compounds, Outcome 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale) ‐ short term. | ||||
| 1.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Ratio (IV, Fixed, 95% CI) | 0.44 [0.20, 0.96] |
| 2 TD symptoms: not any improvement ‐ short term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Benzodiazepines vs other compounds, Outcome 2 TD symptoms: not any improvement ‐ short term. | ||||
| 2.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.02, 8.03] |
| 3 Adverse events: any adverse events ‐ short term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Benzodiazepines vs other compounds, Outcome 3 Adverse events: any adverse events ‐ short term. | ||||
| 3.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Ratio (IV, Fixed, 95% CI) | 1.53 [0.97, 2.41] |
| 4 Leaving the study early ‐ short term Show forest plot | 1 | Risk Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Benzodiazepines vs other compounds, Outcome 4 Leaving the study early ‐ short term. | ||||
| 4.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Difference (IV, Fixed, 95% CI) | 0.0 [‐0.17, 0.17] |

Message from one of the participants of the public and patient involvement consultation of service user perspectives on tardive dyskinesia research.
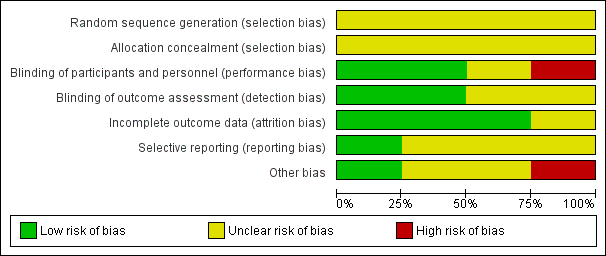
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram.

Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale).

Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 2 TD symptoms: not any improvement.
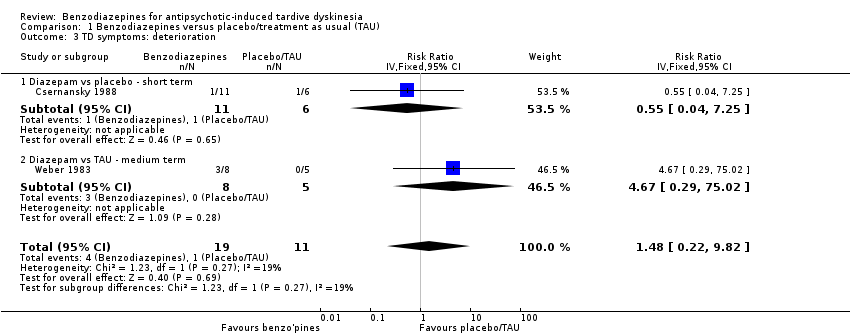
Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 3 TD symptoms: deterioration.
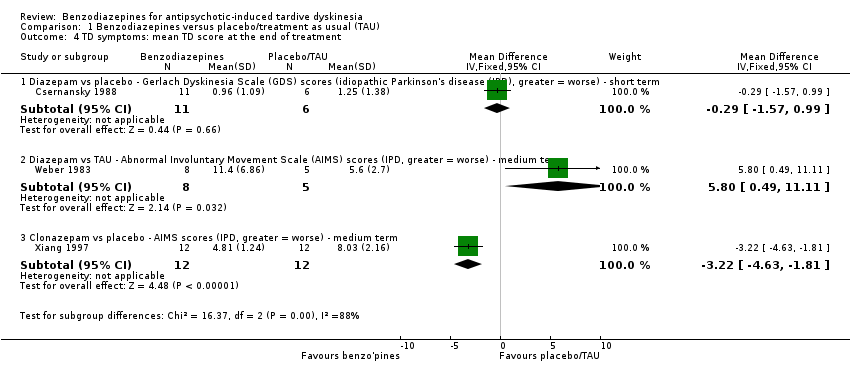
Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 4 TD symptoms: mean TD score at the end of treatment.
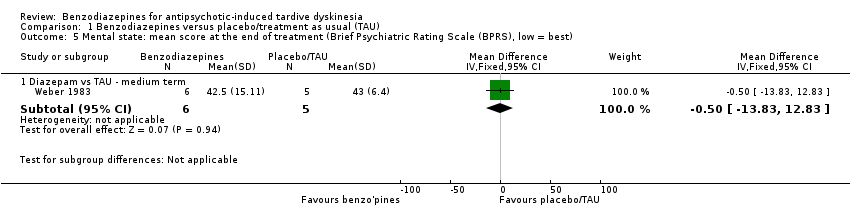
Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 5 Mental state: mean score at the end of treatment (Brief Psychiatric Rating Scale (BPRS), low = best).

Comparison 1 Benzodiazepines versus placebo/treatment as usual (TAU), Outcome 6 Leaving the study early.

Comparison 2 Benzodiazepines vs other compounds, Outcome 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale) ‐ short term.
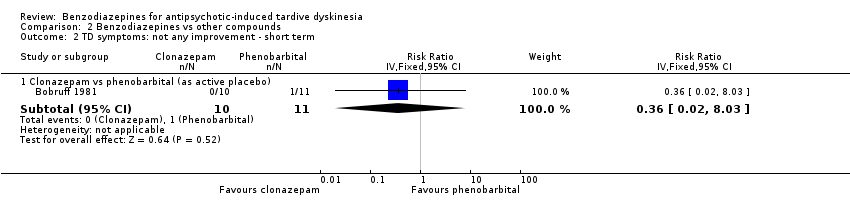
Comparison 2 Benzodiazepines vs other compounds, Outcome 2 TD symptoms: not any improvement ‐ short term.
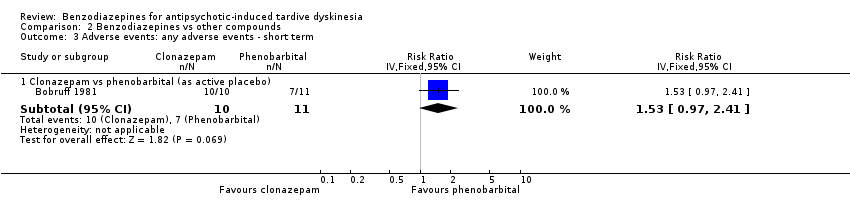
Comparison 2 Benzodiazepines vs other compounds, Outcome 3 Adverse events: any adverse events ‐ short term.

Comparison 2 Benzodiazepines vs other compounds, Outcome 4 Leaving the study early ‐ short term.
| Study tag | Participants | Comparison | Review |
| Antipsychotic‐induced akathisia | Clonazepam vs placebo | ||
| Benztropine vs propranolol | Anticholinergics for neuroleptic‐induced acute akathisia; Central action beta‐blockers versus placebo for neuroleptic‐induced acute akathisia. | ||
| Benzodiazepines vs artane (trihexyphenidyl hydrochloride). | Benzodiazepines for neuroleptic‐induced acute akathisia; Anticholinergics for neuroleptic‐induced acute akathisia. | ||
| Antipsychotic‐induced tardive dyskinesia | Naltrexone vs placebo | Miscellaneous treatments for neuroleptic‐induced tardive dyskinesia. | |
| Naltrexone + clonazepam vs clonazepam + placebo |
| Methods | Allocation: randomised. |
| Participants | Diagnosis: serious mental illness treated by antipsychotic drugs for a protracted period. Tardive dyskinesia.a |
| Interventions | 1. Clonazepam 6‐12 mg oral daily dose. |
| Outcomes | Tardive dyskinesia: any clinically important improvement in tardive dyskinesia, any improvement, deterioration.b |
| aThis could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. bPrimary outcome. The same applies to the measure of primary outcome as for diagnosis. Not everyone may need to have operational criteria applied if clinical impression is proved to be accurate. | |
| n: number of participants. | |
| Benzodiazepines compared with placebo for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with benzodiazepines | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 1.12 | 32 | ⊕⊝⊝⊝ | ‐ | |
| 545 per 1000 | 611 per 1000 | |||||
| Tardive dyskinesia: deterioration in symptoms | Study population | RR 1.48 | 30 | ⊕⊝⊝⊝ | ‐ | |
| 91 per 1000 | 135 per 1000 | |||||
| Adverse effect: any adverse event | None of the included studies reported on these outcomes. | |||||
| Adverse effect: no clinically significant extrapyramidal adverse effects | ||||||
| Acceptability of the treatment (measured by participants leaving the study early) | Study population | RR 2.73 | 56 | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for risk of bias: none of the studies adequately described randomisation procedure or allocation concealment, one study did not blind participants and personnel, and one study was a post hoc subgroup analysis of participants with tardive dyskinesia. 2Downgraded two levels for imprecision: small sample size, and 95% CI of effect estimate includes both appreciable benefit and appreciable harm for benzodiazepines. | ||||||
| Benzodiazepines compared with phenobarbital (as active placebo) for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: psychiatric patients (mainly schizophrenia) with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with phenobarbital (active placebo) | Risk with benzodiazepines | |||||
| Tardive dyskinesia: no clinically important improvement | Study population | RR 0.44 | 21 | ⊕⊝⊝⊝ | ‐ | |
| 909 per 1000 | 400 per 1000 | |||||
| Tardive dyskinesia: deterioration in symptoms ‐ not measured | The included study did not report on this outcome. | |||||
| Adverse events: any | Study population | RR 1.53 | 21 | ⊕⊝⊝⊝ | ‐ | |
| 636 per 1000 | 974 per 1000 | |||||
| Adverse effect: extrapyramidal symptoms ‐ not reported | The included study did not report on this outcome. | |||||
| Acceptability of the treatment (measured by participants leaving the study early) | Study population | Not estimable | 21 | ⊕⊝⊝⊝ | No events were reported; no one left the study early. | |
| 0 per 1000 | 0 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not measured | The included study did not report on this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for risk of bias: the included study did not adequately describe randomisation procedure, allocation concealment, or blinding. 2Downgraded two levels for imprecision: only one study with a very small sample size. | ||||||
| Interventions | Reference |
| Anticholinergic medication | Soares‐Weiser 1997; Soares‐Weiser 2000; 2016 update to be published. |
| Benzodiazepines | This review. |
| Calcium channel blockers | Essali 2011; 2016 update to be published. |
| Cholinergic medication | Tammenmaa 2002; 2016 update to be published. |
| Gamma‐aminobutyric acid agonists | Alabed 2011; 2016 update to be published. |
| Miscellaneous treatments | Soares‐Weiser 2003; 2016 update to be published. |
| Neuroleptic reduction or cessation (or both) and neuroleptics | Soares‐Weiser 2006; 2016 update to be published. |
| Non‐neuroleptic catecholaminergic drugs | El‐Sayeh 2006; 2016 update to be published. |
| Vitamin E | Soares‐Weiser 2011; 2016 update to be published. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale) Show forest plot | 2 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.60, 2.09] |
| 1.1 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.23] |
| 1.2 Diazepam vs TAU ‐ medium term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.71, 3.16] |
| 2 TD symptoms: not any improvement Show forest plot | 2 | 32 | Risk Ratio (IV, Fixed, 95% CI) | 1.49 [0.33, 6.74] |
| 2.1 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.04, 7.25] |
| 2.2 Diazepam vs TAU ‐ medium term | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 2.5 [0.39, 16.05] |
| 3 TD symptoms: deterioration Show forest plot | 2 | 30 | Risk Ratio (IV, Fixed, 95% CI) | 1.48 [0.22, 9.82] |
| 3.1 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.04, 7.25] |
| 3.2 Diazepam vs TAU ‐ medium term | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 4.67 [0.29, 75.02] |
| 4 TD symptoms: mean TD score at the end of treatment Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Diazepam vs placebo ‐ Gerlach Dyskinesia Scale (GDS) scores (idiopathic Parkinson's disease (IPD), greater = worse) ‐ short term | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.57, 0.99] |
| 4.2 Diazepam vs TAU ‐ Abnormal Involuntary Movement Scale (AIMS) scores (IPD, greater = worse) ‐ medium term | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [0.49, 11.11] |
| 4.3 Clonazepam vs placebo ‐ AIMS scores (IPD, greater = worse) ‐ medium term | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.22 [‐4.63, ‐1.81] |
| 5 Mental state: mean score at the end of treatment (Brief Psychiatric Rating Scale (BPRS), low = best) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Diazepam vs TAU ‐ medium term | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐13.83, 12.83] |
| 6 Leaving the study early Show forest plot | 3 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.15, 48.04] |
| 6.1 Clonazepam vs placebo ‐ medium term | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Diazepam vs placebo ‐ short term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Diazepam vs TAU ‐ medium term | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.15, 48.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia (TD) symptoms: no clinically important improvement (> 50% improvement on any TD scale) ‐ short term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Ratio (IV, Fixed, 95% CI) | 0.44 [0.20, 0.96] |
| 2 TD symptoms: not any improvement ‐ short term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.02, 8.03] |
| 3 Adverse events: any adverse events ‐ short term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Ratio (IV, Fixed, 95% CI) | 1.53 [0.97, 2.41] |
| 4 Leaving the study early ‐ short term Show forest plot | 1 | Risk Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Clonazepam vs phenobarbital (as active placebo) | 1 | 21 | Risk Difference (IV, Fixed, 95% CI) | 0.0 [‐0.17, 0.17] |


