Anticholinergika bei Neuroleptika‐induzierten Spätdyskinesien
Appendices
Appendix 1. Previous methods and searches
Criteria for considering studies for this review
Types of studies
We attempted to identify all relevant randomized controlled trials.
Types of participants
People with schizophrenia or schizoaffective disorder or any other chronic mental illnesses, diagnosed by any criteria, irrespective of gender, age or nationality who;
i. required the use of neuroleptics for more than three months;
ii. developed tardive dyskinesia (diagnosed by any criteria at baseline and at least one other occasion) during neuroleptic treatment; and
iii. for whom the dose of neuroleptic medication had been stable for one month or more (the same applies for those free of neuroleptics).
Types of interventions
i. Anticholinergic drugs (benzhexol, benztropine, biperiden, dexetimide, orphenadrine, procyclidine, scopolamine, trihexylphenidyl) compared to placebo, or no intervention; or
ii. the withdrawal of the above anticholinergic drugs compared with the continuation of the treatment.
Types of outcome measures
Clinical efficacy was defined as an improvement in the symptoms of TD of more than 50%, on any scale, after at least six weeks of intervention.
The outcomes of interest were:
A. Tardive dyskinesia changes
i. The number of people per treatment group that did not show an improvement in the symptoms of individuals of more than 50% on any TD scale;
ii. the number of people per treatment group that did not show any improvement in the symptoms of individuals on any TD scale, as opposed to some improvement;
iii. deterioration in the symptoms of individuals, defined as any deleterious change on any TD scale;
iv. any adverse effect, other than deterioration of symptoms of TD, as reported in the trials;
v. average change in severity of TD during the trial period; and
vi. average difference in severity of TD at the end of the trial.
B. General mental state changes
i. Deterioration in general psychiatric symptoms (such as delusions and hallucinations) defined as any deleterious change on any scale; and
ii. average difference in severity of psychiatric symptoms at the end of the trial.
C. Acceptability of the treatment
Acceptability of the intervention to the participant group as measured by numbers of people dropping out during the trial.
When feasible, the outcomes were grouped into time periods ‐ short term (less than 6 weeks), medium term (between 6 weeks and 6 months) and long term (over 6 months).
Search methods for identification of studies
1. Electronic searching
Relevant randomized trials were identified by searching the following electronic databases:
1.1 Biological Abstracts on Silverplatter WinSPIRS 4.0 (January 1982 to March 2000) was searched using the Cochrane Schizophrenia Group's phrase for randomized controlled trials (see Group search strategy) combined with:
[AND (tardive near (dyskine* or diskine*)) or (abnormal near movement* near disorder*) or (involuntar* near movement*))]
This downloaded set of reports was handsearched for possible trials and researched, within the bibliographic package ProCite, with the phrase [anticholinergic* or antiparkinsonian* or benzhexol or benztropine or biperiden or dexetimide or orphenadrine or procyclidine or scopolamine or trihexyphenidyl]
1.2 CINAHL on Silverplatter WinSPIRS 4.0 (1982 ‐ April 2000) was searched using the Cochrane Schizophrenia Group's phrase for randomized controlled trials (see Group search strategy) combined with:
[AND (explode "Movement‐Disorders"/ all topical subheadings / all age subheadings) or (explode "Movement‐Disorders"/ all topical subheadings / all age subheadings) or (tardive near (dyskine* or diskine*)) or (abnormal* near movement* near disorder*) or (involuntar* near movement*)
This downloaded set of reports was handsearched for possible trials and researched, within the bibliographic package ProCite, with the phrase [anticholinergic* or antiparkinsonian* or benzhexol or benztropine or biperiden or dexetimide or orphenadrine or procyclidine or scopolamine or trihexyphenidyl]
1.3 CCTR/CENTRAL of the Cochrane Library (Issue 2, 2000) was searched using the phrase:
[((movement‐disorders*:me) or (dyskinesia‐drug‐induced:me) or (anti‐dyskinesia‐agents*:me) or (tardive near (dyskine* or dyskine*)) or (neuroleptic near dyskine*) or (abnormal* near movement* near disorder*) or (involuntar* near movement*)) and
(anticholinergic* or antiparkinsonian* or benzhexol* or benztroprine or biperiden or dexetimide or orphenadrine or procyclidine or scopolamine or trihexyphenidyl)]
1.4 Cochrane Schizophrenia Group's Register was searched using the phrase:
[anticholinergic* or antiparkinsonian* or benzhexol or benztropine or biperiden or dexetimide or orphenadrine or procyclidine or scopolamine or trihexylphenidyl]
1.5 EMBASE on Silverplatter WinSPIRS 4.0 (January 1980 to May 2000) was searched using the Cochrane Schizophrenia Group's phrase for randomized controlled trials (see Group search strategy) combined with:
[AND (("tardive‐dyskinesia"/ all subheadings) or ("dyskinesia"/ all subheadings) or ("motor dysfunction"/ all subheadings) or (tardive near (dyskines* or diskines*)) or (abnormal* near movement* near disorder*) or involuntar* near movement*))]
This downloaded set of reports was handsearched for possible trials and researched, within the bibliographic package ProCite, with the phrase [anticholinergic* or antiparkinsonian* or benzhexol or benztropine or biperiden or dexetimide or orphenadrine or procyclidine or scopolamine or trihexyphenidyl]
1.6 LILACS (January 1982 to September 1996) was searched using the CSG's phrase for randomized controlled trials (see Group search strategy) combined with the phrase:
[AND ((tardive or (dyskinesia* or diskinesia*)) or (drug induced movement disorders in thesaurus))]
This downloaded set of reports was handsearched for possible trials and researched, within the bibliographic package ProCite, with the phrase [anticholinergic* or antiparkinsonian* or benzhexol or benztropine or biperiden or dexetimide or orphenadrine or procyclidine or scopolamine or trihexylphenidyl]
1.7 MEDLINE on Silverplatter WinSPIRS 4.0 (January 1966 to June 2000) was searched using the Cochrane Schizophrenia Group's phrase for randomized controlled trials (see Group search strategy) combined with:
[AND ((explode "movement‐disorders" in MeSH / all subheadings) or (explode "anti‐dyskinesia‐agents" in MeSH / all subheadings) or (explode "dyskinesia‐drug‐induced" in MeSH / all subheadings) and
(explode "psychotic‐disorders" in MeSH / all subheadings) or (explode "schizophrenic disorders" in MeSH / all subheadings) or (tardive near (dyskine* or diskine*)) or (abnormal* near movement* near disorder*) or (involuntar* near movement*))]
This downloaded set of reports was handsearched for possible trials and researched, within the bibliographic package ProCite, with the phrase [anticholinergic* or antiparkinsonian* or benzhexol or benztropine or biperiden or dexetimide or orphenadrine or procyclidine or scopolamine or trihexyphenidyl]
1.8 PsycLIT on Silverplatter WinSPIRS 4.0 (January 1974 to March 2000) was searched using the Cochrane Schizophrenia Group's phrase for randomized controlled trials (see Group search strategy) combined with the phrase:
[AND ((explode "movement‐disorders") or ("tardive‐dyskinesia" in DE) or (tardive near (dyskine* or diskine*)) or (abnormal* near movement* near disorder*) or (involuntar* near movement*))]
This downloaded set of reports was handsearched for possible trials and researched, within the bibliographic package ProCite, with the phrase [anticholinergic* or antiparkinsonian* or benzhexol or benztropine or biperiden or dexetimide or orphenadrine or procyclidine or scopolamine or trihexyphenidyl]
1.9 SCISEARCH ‐ Science Citation Index
Each of the included studies was sought as a citation on the SCISEARCH database. Reports of articles that had cited these studies were inspected in order to identify further trials.
1.10 Cochrane Schizophrenia Group Trials Register
The Trials Search Co‐ordinator searched the Cochrane Schizophrenia Group’s Trials Register (November 11, 2013)
[((*Akineton* or *Anticholinergic* or *Antimuscarinic* or *Aparkan* or *Arpicolin* or *Artane* or *Atropine* or *Banflex* or *Benapr* or *Benzatropine* or *Benzhexol* or *Benztropine* or *Biperiden* or *Biorphen* or *Bornaprine* or *Broflex* or *Buscopan or *Cholinergic* or *Cogentin* or *Cycrimine* or *Darifenacin* or *Dicycloverine* or *Diphenhydramine* or *Disipal* or *Elantrine* Or *Ethopropazine* or *Flavoxate* or *Flexon* or *Hyoscine* or *Kemadrin* or *Mephenamin* or *Muscarinic* or *Norflex* or *Oxybutynin* or *Parkin* or *Parsid* or *Orphenadrine* or *Pro‐banthine* or *Procyclidine* or *Profenamine* or *Propantheline* or *Propiverine* or *Scopolamine* or *Solifenacin* or *Tolterodine* or *Trihexyphenidyl* or *Trospium*) in title abstract or index terms of REFERENCE)) and ((*dyskinesia* in title abstract or index terms of REFERENCE) and (Tardive dyskinesia in health care conditions of STUDY))]
The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches of journals and conference proceedings (see Group Module). Incoming trials are assigned to existing or new review titles.
2. Reference searching
The references of all identified studies were also inspected for more studies.
3. Personal contact
The first author of each included study was contacted for information regarding unpublished trials.
Data collection and analysis
[For definitions of terms used in this, and other sections, please refer to the Glossary.]
The abstract of each reference identified by the search was inspected, independently, by both reviewers, to see if the study was likely to be relevant. For articles that could possible have been RCTs, or in cases of disagreement between the two reviewers, the full article was obtained. In turn, these articles were then inspected, independently, to assess their relevance to this review. Again, where resolving disagreement by discussion was not possible, the article was added to those awaiting assessment and the authors of the study were contacted for clarification.
The reviewers independently evaluated the quality of all included trials. A rating was given for each trial based on the three quality categories as described in the Cochrane Collaboration Handbook (Mulrow 1999). Only trials stated to be randomized (category A or B of the Handbook) were included in this review.
Data were independently extracted by both reviewers. Again, any disagreement was discussed, the decisions documented and, where necessary, the authors of the studies were contacted for clarification. Justification for excluding references from the review was documented. If insufficient information could be extracted from the publication, a letter was sent to the first author for further clarification. There references were allocated to the 'Awaiting assessment' list. In the absence of specific details, the reviewers assumed that subjects dropped out because they had no improvement in their TD. The results were recalculated without these assumptions in order to test the sensitivity of the results.
We expected that many trials would use a crossover design. In order to exclude the potential additive effect in the second or more stages on these trials, only data from the first stage were analysed.
Dichotomous outcomes were analysed by calculating odds ratios for each trial with the uncertainty in each result being expressed using confidence intervals. The odds ratios from the individual trials were combined using appropriate methods of meta‐analysis. When overall results were significant the number needed to treat to produce (or prevent) one outcome was calculated by combining the overall odds ratio with an estimate of the prevalence of the event in the control groups of the trials.
Continuous outcomes were analysed according to their difference in mean treatment effects and its standard deviation. Meta‐analytical methods for continuous data assume that the underlying distribution of the measurements is Normal. The ratio of the mean to its standard deviation gives a crude way of assessing skew: if this ratio was less than 1.65 for any group in a trial and this can be resolved by log‐transformation, the data then becoming normally distributed. It was proposed to undertake this procedure before including skewed data from any study in the analysis.
Heterogeneity in the results of the trials was assessed both by inspection of graphical presentations and by calculating a test of heterogeneity. Two possible reasons for heterogeneity were pre‐specified: (i) that response differs according to different lengths of follow‐up; (ii) that response differs according to the different drugs. These were assessed by looking at separate subgroups of trials [(i) and (ii)].

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram for 2015 and 2017 searches.
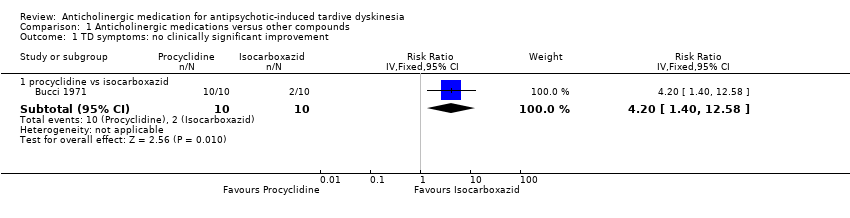
Comparison 1 Anticholinergic medications versus other compounds, Outcome 1 TD symptoms: no clinically significant improvement.
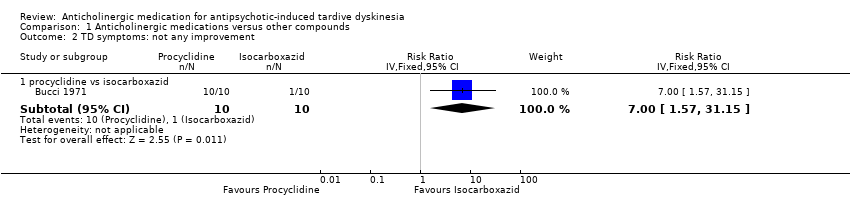
Comparison 1 Anticholinergic medications versus other compounds, Outcome 2 TD symptoms: not any improvement.

Comparison 1 Anticholinergic medications versus other compounds, Outcome 3 Adverse effects.

Comparison 1 Anticholinergic medications versus other compounds, Outcome 4 Leaving the study early.

Comparison 2 Continuation versus withdrawal of anticholinergic medications, Outcome 1 Leaving the study early.
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | 1. Anticholinergic withdrawal (N = 150) versus anticholinergic continuation (N = 150). OR 2. Specific anticholinergic (N = 150) versus placebo (N = 150). |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting, all participants could be screened using operational criteria; otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| Anticholinergic medication compared with other treatments for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia Settings: anywhere. Intervention: any anticholinergic Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| risk with placebo | risk with anticholinergic drugs | |||||
| Tardive dyskinesia: not improved to a clinically important extent | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Tardive dyskinesia: deterioration of symptoms | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Mental state | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: any adverse effects | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Social confidence, social inclusion, social networks, or personalised quality of life | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias: the included study did not adequately describe randomisation procedure or allocation concealment, and there was no mention of the study being blinded. 2 Downgraded two levels for imprecision: very small sample size (n = 20). 3 Downgraded two levels for imprecision: very wide CI that includes appreciable benefit for both groups; very small sample size (n = 20). 4 Downgraded one level for indirectness: leaving the study early can give an indication, but is not a direct measurement, of treatment acceptability. | ||||||
| Anticholinergic medication compared with other treatments for antipsychotic‐induced tardive dyskiesia | ||||||
| Patient or population: chronic schizophrenia patients with antipsychotic‐induced tardive dyskinesia Settings: outpatients in the USA. Intervention: procyclidine (anticholinergic), 5 mg twice/day Comparison: isocarboxazid (MAO‐inhibitor), 10 mg twice/day | ||||||
| Outcomes | Illustrative comparative risks* (CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| risk with MAO‐inhibitor | risk with anticholinergic drugs | |||||
| Tardive dyskinesia: Not improved to a clinically important extent | 200 per 1000 | 840 per 1000 | RR 4.20 (1.40 to 12.58) | 20 | ⊕⊝⊝⊝ | |
| Tardive dyskinesia: deterioration of symptoms | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Mental state | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: any adverse effects | 100 per 1000 | 33 per 1000 | RR 0.33 (0.02 to 7.32) | 20 | ⊕⊝⊝⊝ | |
| Acceptability of the treatment: leaving the study early | 100 per 1000 | 33 per 1000 | RR 0.33 (0.02 to 7.32) | 20 | ⊕⊝⊝⊝ | |
| Social confidence, social inclusion, social networks, or personalised quality of life | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias: the included study did not adequately describe randomisation procedure or allocation concealment, and there was no mention of the study being blinded. 2 Downgraded two levels for imprecision: very small sample size (n = 20). 3 Downgraded two levels for imprecision: very wide CI that includes appreciable benefit for both groups; very small sample size (n = 20). 4 Downgraded one level for indirectness: leaving the study early can give an indication, but is not a direct measurement, of treatment acceptability. | ||||||
| Withdrawal of anticholinergic medication compared to continuing anticholigergic medication for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: chronic schizophrenia patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with continuation of anticholinergic drugs | Risk with withdrawal of anticholinergic drugs | |||||
| Tardive dyskinesia: not improved to a clinically important extent | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Tardive dyskinesia: deterioration of symptoms | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Mental state | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effects | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | 0 per 1,000 | 0 per 1,000 | RR 2.14 | 10 | ⊕⊝⊝⊝ | |
| Social confidence, social inclusion, social networks, or personalised quality of life | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias: the included study did not adequately describe randomisation procedure or allocation concealment. 2 Downgraded one level for indirectness: leaving the study early can give an indication, but is not a direct measurement, of treatment acceptability. In addition, the continuation of anticholinergic medication group stopped biperiden after 4 weeks but the results were measured after 7 weeks. 3 Downgraded two levels for imprecision: very wide CI that includes appreciable benefit for both groups; very small sample size (n = 10). | ||||||
| Interventions | Reference |
| Anticholinergic medication | This review |
| Benzodiazepines | Bhoopathi 2006; update to be published |
| Calcium channel blockers | Essali 2011; update to be published |
| Cholinergic medication | Tammenmaa 2002; update to be published |
| Gamma‐aminobutyric acid agonists | Alabed 2011; update to be published |
| Miscellaneous treatments | Soares‐Weiser 2003; update to be published |
| Neuroleptic reduction and/or cessation and neuroleptics | Soares‐Weiser 2006; update to be published |
| Non‐neuroleptic catecholaminergic drugs | El‐Sayeh 2006; update to be published |
| Vitamin E | Soares‐Weiser 2011; update to be published |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 TD symptoms: no clinically significant improvement Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 4.2 [1.40, 12.58] |
| 2 TD symptoms: not any improvement Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 7.0 [1.57, 31.15] |
| 3 Adverse effects Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 4 Leaving the study early Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 biperiden: withdrawal after 1 week vs withdrawal after 4 weeks | 1 | 10 | Risk Ratio (IV, Fixed, 95% CI) | 2.14 [0.11, 42.52] |

