急性虚血性脳卒中に対する低分子量ヘパリンまたはヘパリノイドと標準的な未分画ヘパリンの比較
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | R = sequentially numbered identical containers | |
| Participants | Europe | |
| Interventions | Rx: Org 10172 sc (1250 anti‐Xa units 24‐hourly) | |
| Outcomes | Death + cause of death | |
| Notes | Ex: BP greater than 200/120, bleeding risk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope, but no details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Treatment and control arms both involved 2 x daily injections. Manuscript states "patients, physicians and hospital staff were unaware of treatment allocation" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Treatment and control arms both involved 2 x daily injections. Manuscript states "patients, physicians and hospital staff were unaware of treatment allocation" |
| Incomplete outcome data (attrition bias) | Unclear risk | Number lost to follow‐up not stated |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, study report does not give full details of data collected during follow‐up |
| Methods | R = sequentially numbered containers | |
| Participants | Europe | |
| Interventions | Rx: Org 10172 sc (375 anti‐Xa units 24‐hourly); Org 10172 sc (750 anti‐Xa units 24‐hourly); Org 10172 sc (1250 anti‐Xa units 24‐hourly) | |
| Outcomes | Death + cause of death | |
| Notes | Ex: BP greater than 200/120, bleeding risk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | No details provided |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, study report does not give full details of data collected during follow‐up |
| Methods | R = sequentially numbered containers | |
| Participants | Finland | |
| Interventions | Rx: enoxaparin (40 mg once daily) | |
| Outcomes | Death | |
| Notes | Ex: specified by protocol ‐ includes bleeding risk; GCS < 9; pre‐existing DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule had a block size of 4 and was generated by computer programme AC/BIOM/STAT |
| Allocation concealment (selection bias) | Unclear risk | Method for the participating doctor to obtain the treatment allocation not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Experimental and control treatments supplied in prefilled syringes of identical appearance |
| Blinding of outcome assessment (detection bias) | Unclear risk | Steps to blind assessors not stated |
| Incomplete outcome data (attrition bias) | Low risk | Manuscript states no patients lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, study report does not give full details of data collected during follow‐up |
| Methods | R = blocked and stratified randomisation, telephone to central randomisation system | |
| Participants | International | |
| Interventions | Rx: enoxaparin 40 mg sc once daily | |
| Outcomes | Death | |
| Notes | Ex: specified by protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sponsor generated the randomisation schedule in permuted blocks of 4, stratified by baseline stroke severity that was implemented centrally by an independent interactive voice‐response system |
| Allocation concealment (selection bias) | Low risk | The randomisation schedule was implemented centrally by an independent interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not blinded, but all major outcome events were reviewed blind to treatment allocation by an adjudication committee |
| Incomplete outcome data (attrition bias) | Unclear risk | Primary outcome data not available for 32 participants, number with missing modified Rankin Scale status not stated |
| Selective reporting (reporting bias) | Low risk | Trial registered NCT00077805, protocol‐specified outcomes all reported |
| Methods | R = computer‐generated randomisation list | |
| Participants | European Union | |
| Interventions | Rx: certoparin sc (3000 U once daily) plus 2 injections of placebo | |
| Outcomes | Death related to DVT | |
| Notes | Ex: specified by protocol ‐ includes bleeding risk, body weight < 55 kg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by computer |
| Allocation concealment (selection bias) | Low risk | Manuscript states "Treatment allocation kept strictly confidential and available only to authorised persons" |
| Blinding of participants and personnel (performance bias) | Low risk | Experimental and control treatments identical in appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Experimental and control treatments identical in appearance |
| Incomplete outcome data (attrition bias) | High risk | 64 (10%) participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, study report does not give full details of data collected during follow‐up |
| Methods | R = sequentially numbered containers | |
| Participants | Europe | |
| Interventions | Rx: loading dose 1000 anti‐Xa units iv, then Org 10172 sc (1250 anti‐Xa units 12‐hourly) or Org 10172 sc (750 anti‐Xa units 12‐hourly) | |
| Outcomes | Death + cause of death | |
| Notes | Ex: BP > 200/120, bleeding risk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Report to company describes this as an open trial, published abstract states double blind |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up not stated |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, study report does not give full details of data collected during follow‐up |
| Methods | R = not described | |
| Participants | Germany | |
| Interventions | Rx: enoxaparin 1 mg/kg sc twice daily (100 Anti‐Xa units 12‐hourly) | |
| Outcomes | Death | |
| Notes | Ex: specified by protocol ‐ includes bleeding risk, severe organic cerebral disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, study report does not give full details of data collected during follow‐up |
| Methods | R = sequentially numbered identical containers | |
| Participants | Canada | |
| Interventions | Rx: Org 10172 sc (750 anti‐Xa units 12‐hourly) | |
| Outcomes | Death | |
| Notes | Ex: bleeding risk; pre‐existing DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method for concealment not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Participamts, trial nurses, and physicians were all unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Participamts, trial nurses, and physicians were all unaware of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, study report does not give full details of data collected during follow‐up |
| Methods | R = not stated Loss to follow‐up not stated | |
| Participants | Taiwan | |
| Interventions | Rx: unspecified LMWH sc (0.4 mL 4100 anti‐Xa IU twice daily) | |
| Outcomes | Haemorrhagic transformation (systematic CT on day 10 or symptomatic before day 10) | |
| Notes | Ex: progression due to brain oedema or intracranial haemorrhage | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, study report does not give full details of data collected during follow‐up |
APTT: activated partial thromboplastin time
BP: blood pressure
CT: computerised tomography
DVT: deep venous thrombosis
Ex: exclusion criteria
FU: follow up
GCS: Glasgow coma scale
ITT: intention‐to‐treat
iv: intravenously
LMWH: low‐molecular‐weight heparin
MRI: magnetic resonance imaging
mRS: ???
NIHSS: National Institutes of Health stroke Scale
PE: pulmonary embolism
R: randomisation method
Rx: treatment
sc: subcutaneously
TCD: transcranial doppler
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Target participants were not people with acute ischaemic stroke | |
| Intervention did not include unfractionated heparin | |
| Enoxaparin 20 mg subcutaneously once daily versus standard unfractionated heparin 5000 IU subcutaneously twice daily for 10 days in immobile people (38 strokes) | |
| Comparison of different doses of heparinoid, no UFH group | |
| Target participants were not people with acute ischaemic stroke | |
| 2 treatment groups confounded by co‐administration of different warfarin regimens | |
| Intervention did not include UFH | |
| Enoxaparin versus heparin for prophylaxis of thromboembolic events in people with medical conditions | |
| Ongoing trial of heparin versus nadroparin versus placebo. Complex confounded regimens | |
| About 150 (19%) participants had neurological disease | |
| Comparison of LMWH with aspirin instead of UFH | |
| Intervention did not include UFH | |
| Data have not been reported | |
| Intervention did not include UFH | |
| Enoxaparin/CY216 versus standard UFH | |
| Target participants were not people with acute ischaemic stroke | |
| Intervention did not include UFH | |
| Not acute stroke, method of treatment allocation not random | |
| Uncontrolled study | |
| Published in China and only available as an abstract. Attempted to contact authors as well as seek help from colleagues from China to obtain the full text article but to no avail | |
| Intervention did not include UFH | |
| Intervention did not include LMWH | |
| Published in China and only available as an abstract. Attempted to contact authors as well as seek help from colleagues from China to obtain the full text article but to no avail | |
| Intervention did not include UFH |
LMWH: low‐molecular‐weight heparin
UFH: unfractionated heparin
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | An open‐label, randomised, parallel group, multicentre study to evaluate the efficacy and safety of enoxaparin versus unfractionated heparin in the prevention of venous thromboembolism in people following acute ischaemic stroke |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | Ms S Wellington, Senior Clinical Project Leader, Aventis Pharma, Aventis House, 50 Kings Hill Avenue, Kings Hill, West Malling, Kent, UK |
| Notes |
| Trial name or title | Low‐molecular‐weight heparin (enoxaparin) in anticoagulation transition to oral warfarin in ischaemic cerebral vascular accident or transient ischaemic attack |
| Methods | |
| Participants | Acute ischaemic stroke |
| Interventions | Enoxaparin sc + oral warfarin versus UFH sc + oral warfarin |
| Outcomes | |
| Starting date | |
| Contact information | Dr WD Young, North Mississippi Medical Centre, 830 S Gloster Street, Tupelo MS 38801, USA |
| Notes |
sc: subcutaneously
UFH: unfractionated heparin
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dead or dependent at the end of follow‐up | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Death from all causes during treatment period Show forest plot | 8 | 3102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.78, 1.46] |
| Analysis 1.2  Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 2 Death from all causes during treatment period. | ||||
| 2.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.62, 2.26] |
| 2.2 LMWH versus standard UFH | 4 | 2609 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.72, 1.47] |
| 3 Death from all causes during follow‐up Show forest plot | 8 | 3102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.79, 1.23] |
| Analysis 1.3 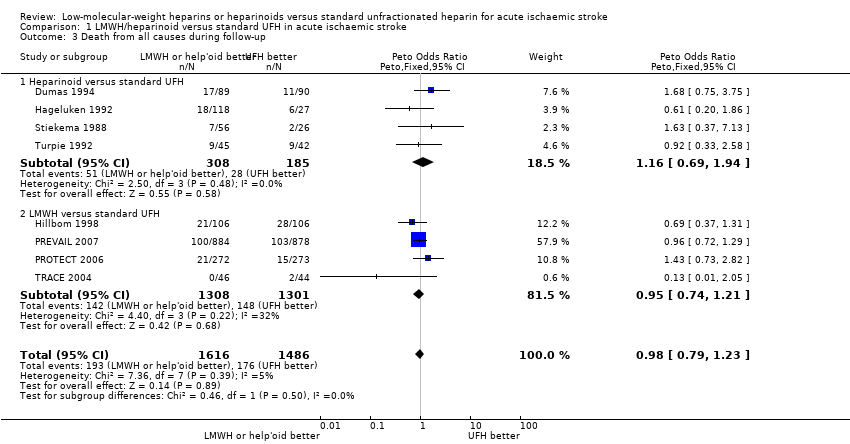 Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 3 Death from all causes during follow‐up. | ||||
| 3.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.69, 1.94] |
| 3.2 LMWH versus standard UFH | 4 | 2609 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.74, 1.21] |
| 4 Vascular death during follow‐up Show forest plot | 5 | 1038 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.72, 1.85] |
| Analysis 1.4  Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 4 Vascular death during follow‐up. | ||||
| 4.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.68, 1.94] |
| 4.2 LMWH versus standard UFH | 1 | 545 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.39, 3.53] |
| 5 Deep venous thrombosis during treatment period Show forest plot | 7 | 2585 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.44, 0.70] |
| Analysis 1.5 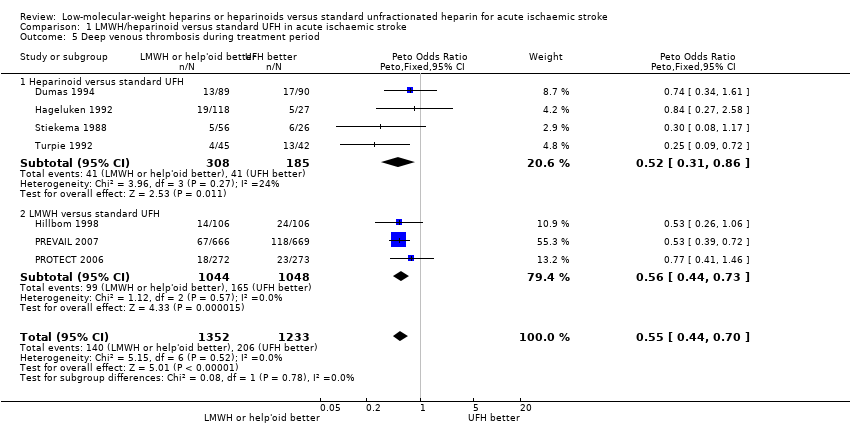 Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 5 Deep venous thrombosis during treatment period. | ||||
| 5.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.31, 0.86] |
| 5.2 LMWH versus standard UFH | 3 | 2092 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.44, 0.73] |
| 6 Pulmonary embolism during follow‐up Show forest plot | 6 | 1250 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.23, 1.41] |
| Analysis 1.6 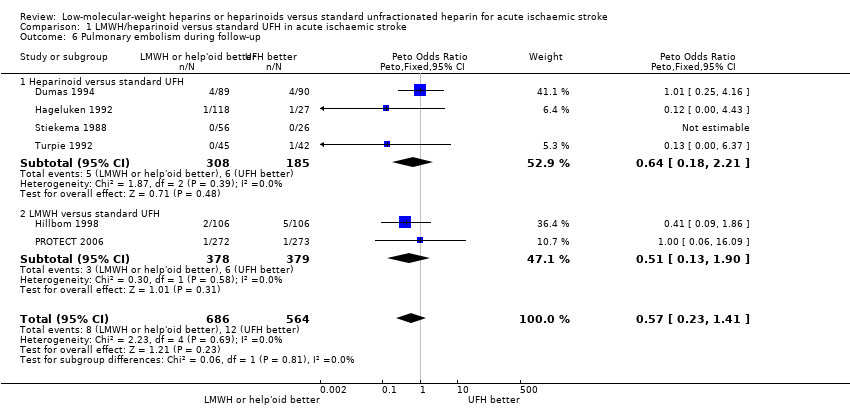 Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 6 Pulmonary embolism during follow‐up. | ||||
| 6.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.18, 2.21] |
| 6.2 LMWH versus standard UFH | 2 | 757 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.13, 1.90] |
| 7 Any intracranial haemorrhage/haemorrhagic transformation of the cerebral infarct during treatment period Show forest plot | 9 | 3137 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.46, 1.23] |
| Analysis 1.7  Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 7 Any intracranial haemorrhage/haemorrhagic transformation of the cerebral infarct during treatment period. | ||||
| 7.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.43, 2.94] |
| 7.2 LMWH versus standard UFH | 5 | 2644 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.37, 1.15] |
| 8 Symptomatic intracranial haemorrhage/haemorrhagic transformation of the infarct during treatment period Show forest plot | 8 | 3102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.35, 1.54] |
| Analysis 1.8 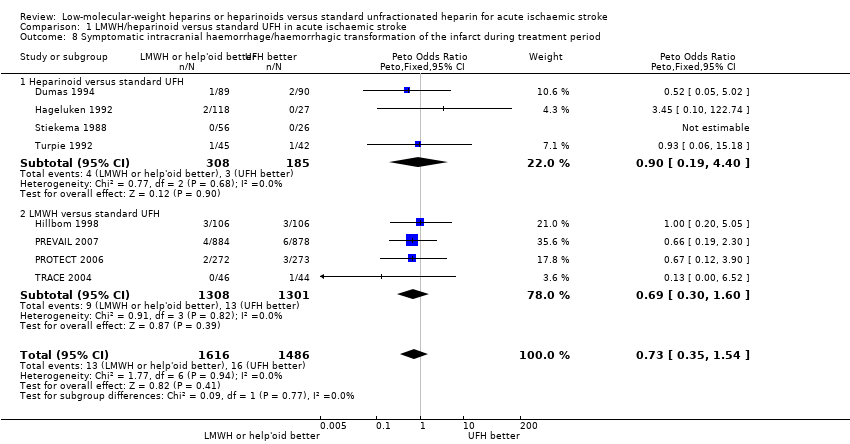 Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 8 Symptomatic intracranial haemorrhage/haemorrhagic transformation of the infarct during treatment period. | ||||
| 8.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.19, 4.40] |
| 8.2 LMWH versus standard UFH | 4 | 2609 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.30, 1.60] |
| 9 Extracranial haemorrhage during treatment period Show forest plot | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 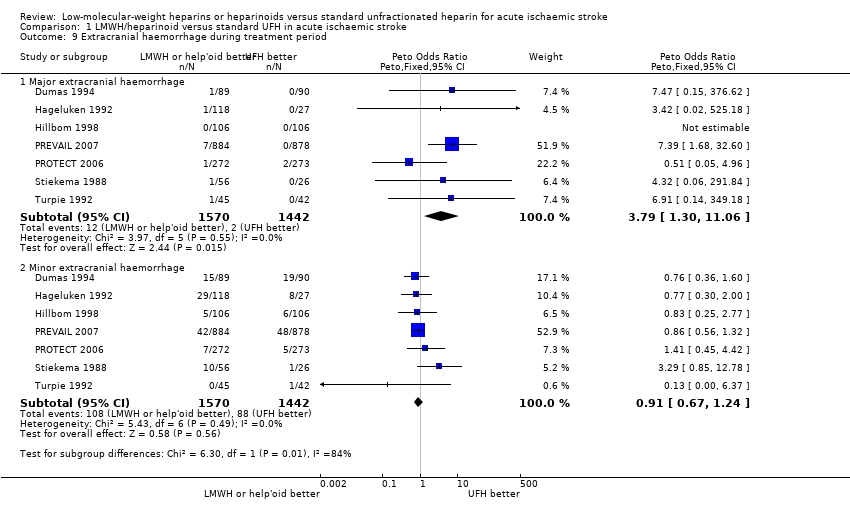 Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 9 Extracranial haemorrhage during treatment period. | ||||
| 9.1 Major extracranial haemorrhage | 7 | 3012 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.79 [1.30, 11.06] |
| 9.2 Minor extracranial haemorrhage | 7 | 3012 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.67, 1.24] |
| 10 Effect of recurrent ischaemic stroke or recurrent stroke of unknown pathological type during treatment period Show forest plot | 2 | 1839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [0.61, 6.11] |
| Analysis 1.10  Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 10 Effect of recurrent ischaemic stroke or recurrent stroke of unknown pathological type during treatment period. | ||||
| 10.1 LMWH versus UFH | 2 | 1839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [0.61, 6.11] |
| 11 Deep venous thrombosis according to heparinoid dosage regimen Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11 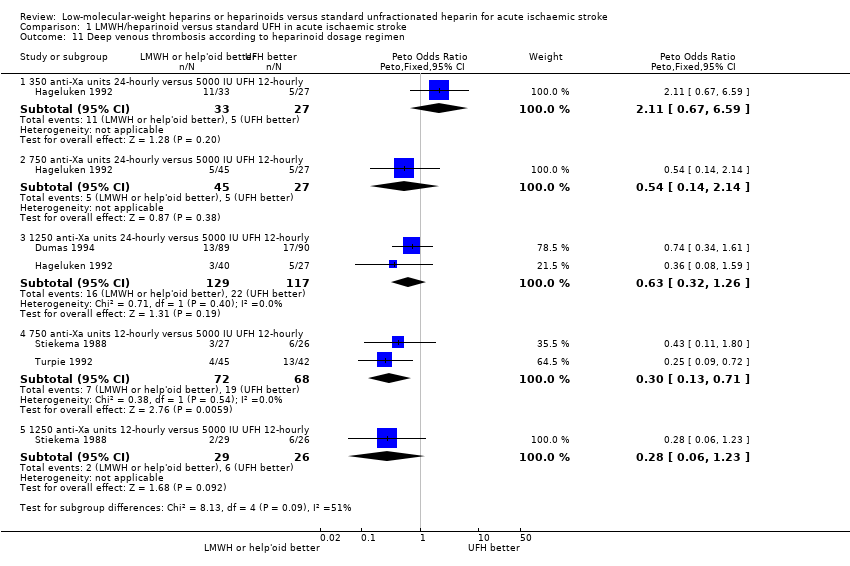 Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 11 Deep venous thrombosis according to heparinoid dosage regimen. | ||||
| 11.1 350 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [0.67, 6.59] |
| 11.2 750 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.14, 2.14] |
| 11.3 1250 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 2 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.32, 1.26] |
| 11.4 750 anti‐Xa units 12‐hourly versus 5000 IU UFH 12‐hourly | 2 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.13, 0.71] |
| 11.5 1250 anti‐Xa units 12‐hourly versus 5000 IU UFH 12‐hourly | 1 | 55 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.06, 1.23] |
| 12 Intracranial and extracranial haemorrhage during treatment according to dosage regimen Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12 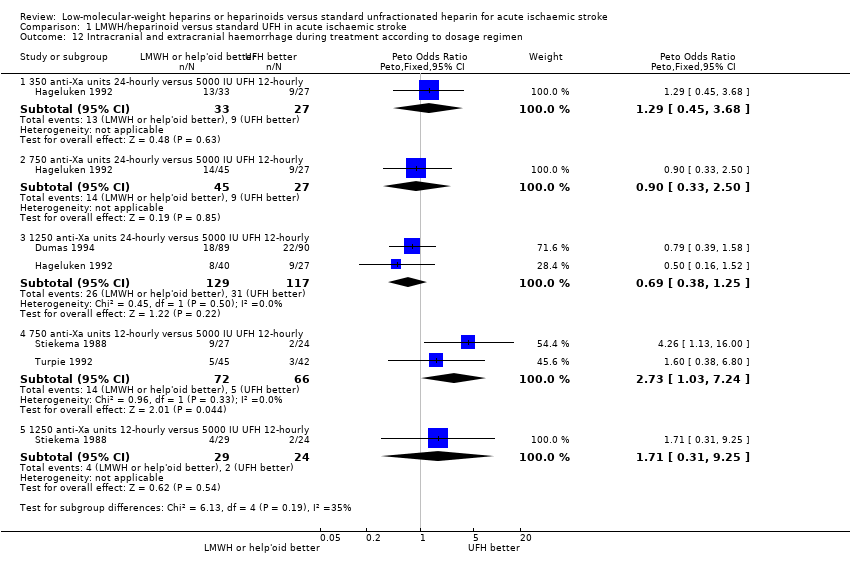 Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 12 Intracranial and extracranial haemorrhage during treatment according to dosage regimen. | ||||
| 12.1 350 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.45, 3.68] |
| 12.2 750 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.33, 2.50] |
| 12.3 1250 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 2 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.38, 1.25] |
| 12.4 750 anti‐Xa units 12‐hourly versus 5000 IU UFH 12‐hourly | 2 | 138 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.73 [1.03, 7.24] |
| 12.5 1250 anti‐Xa units 12‐hourly versus 5000 IU UFH 12‐hourly | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.31, 9.25] |
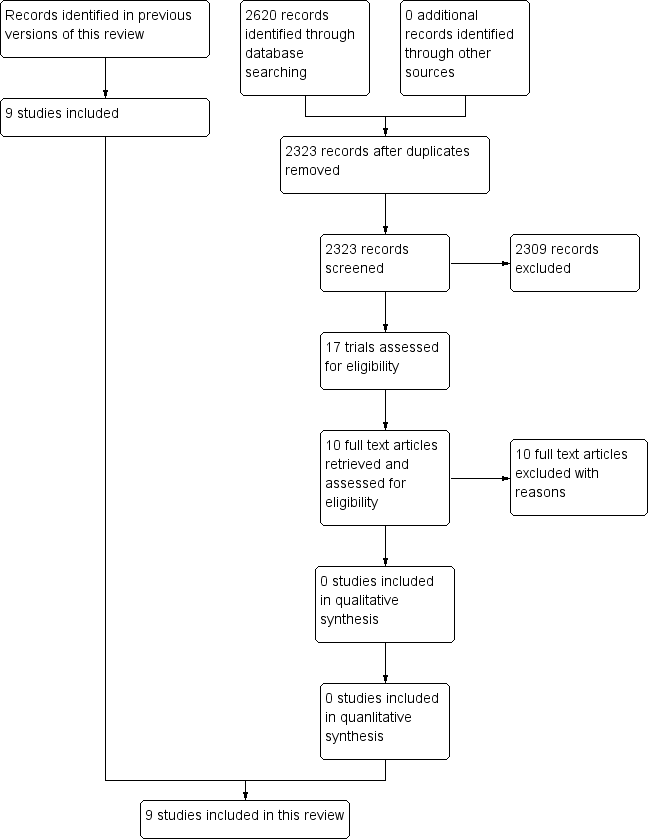
PRISMA flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, outcome: death from all causes during treatment period

Funnel plot of comparison: 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, outcome: death from all causes during follow‐up
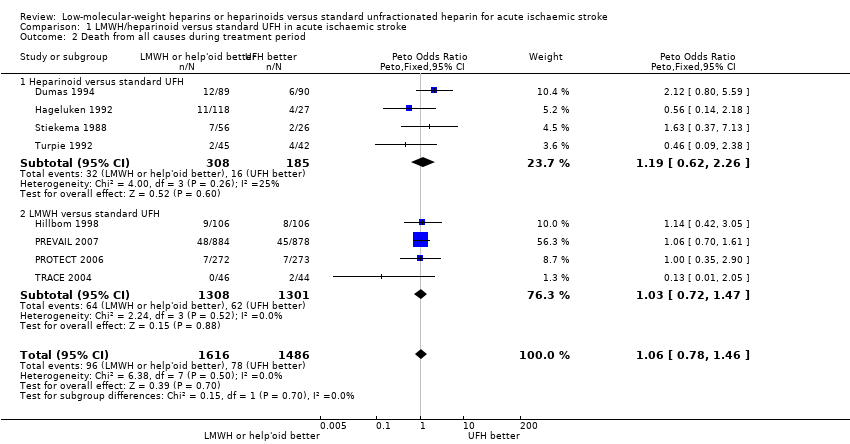
Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 2 Death from all causes during treatment period.
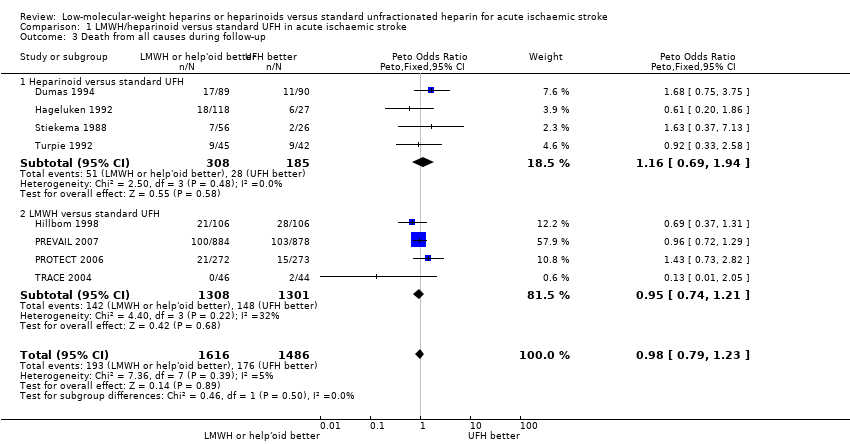
Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 3 Death from all causes during follow‐up.

Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 4 Vascular death during follow‐up.

Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 5 Deep venous thrombosis during treatment period.

Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 6 Pulmonary embolism during follow‐up.

Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 7 Any intracranial haemorrhage/haemorrhagic transformation of the cerebral infarct during treatment period.
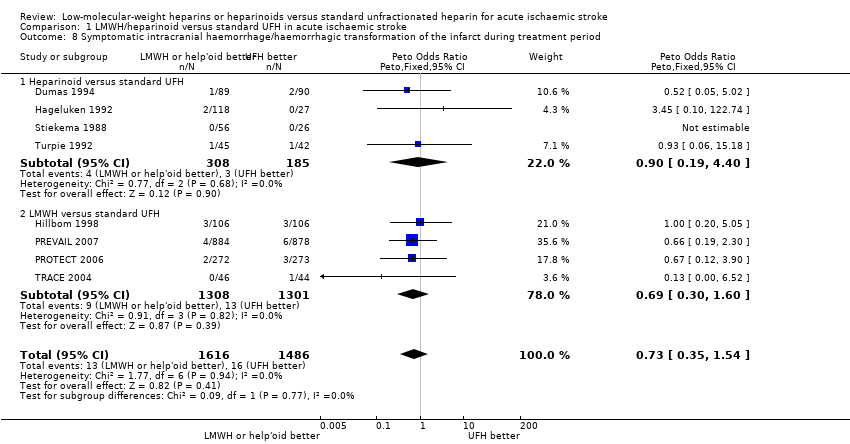
Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 8 Symptomatic intracranial haemorrhage/haemorrhagic transformation of the infarct during treatment period.

Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 9 Extracranial haemorrhage during treatment period.

Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 10 Effect of recurrent ischaemic stroke or recurrent stroke of unknown pathological type during treatment period.
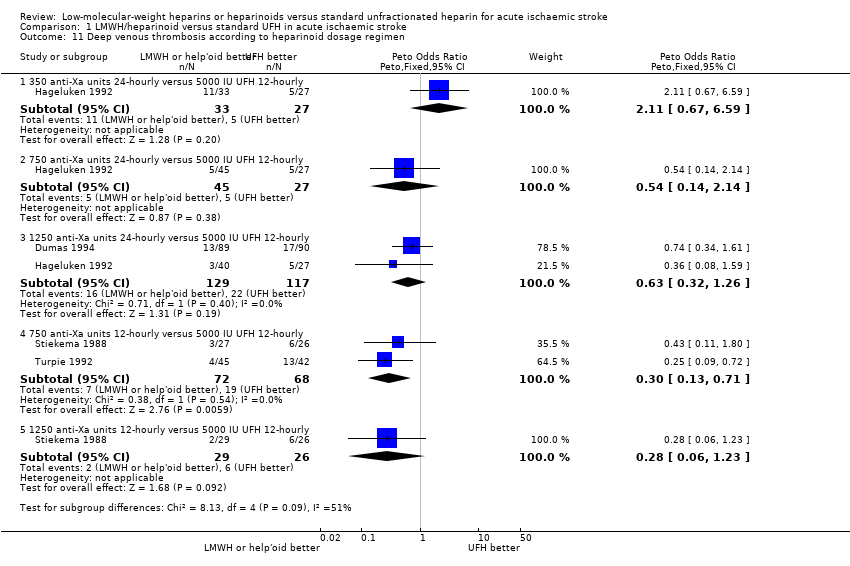
Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 11 Deep venous thrombosis according to heparinoid dosage regimen.
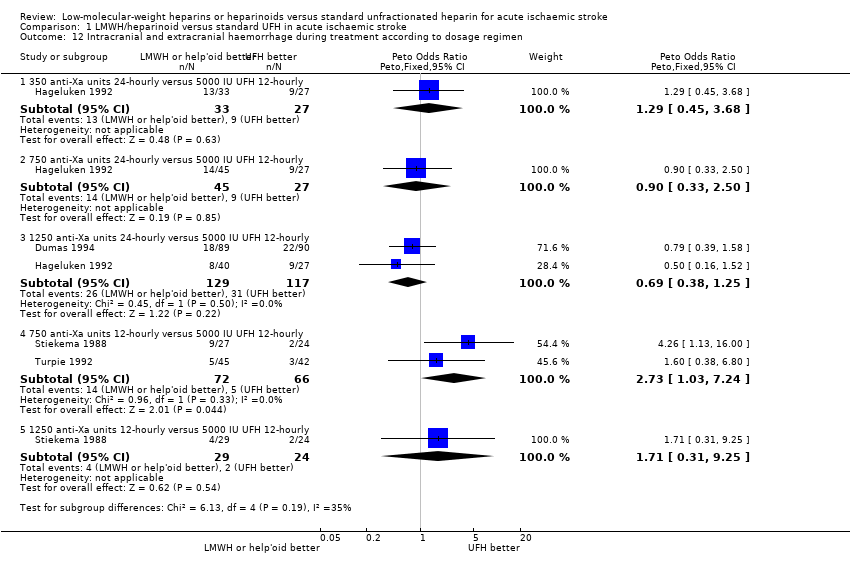
Comparison 1 LMWH/heparinoid versus standard UFH in acute ischaemic stroke, Outcome 12 Intracranial and extracranial haemorrhage during treatment according to dosage regimen.
| Low‐molecular‐weight heparins (LMWH) or heparinoids compared with unfractionated heparin (UFH) for acute ischaemic stroke | ||||||
| Patient or population: acute ischaemic stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with UFH | Risk with LMWH/heparinoids | |||||
| Death from all causes during treatment (range 6 days to 16 days) | Moderate risk population | OR 1.06 | 3102 | ⊕⊕⊝⊝ | ||
| 90 per 1000³ | 95 per 1000 | |||||
| High risk population | ||||||
| 131 per 1000⁴ | 138 per 1000 | |||||
| Death from all causes during follow up (range 2 weeks to 12 weeks) | Moderate risk population | OR 0.98 | 3102 | ⊕⊕⊝⊝ | ||
| 225 per 1000³ | 221 per 1000 | |||||
| High risk population | ||||||
| 251 per 1000⁴ | 247 per 1000 | |||||
| Deep vein thrombosis during treatment period (range 6 days to 16 days) | Moderate risk population | OR 0.55 | 2585 | ⊕⊕⊝⊝ | ||
| 189 per 1000⁶ | 114 per 1000 | |||||
| High risk population | ||||||
| 211 per 1000⁴ | 128 per 1000 | |||||
| Pulmonary embolism during treatment period (range 6 days to 16 days) | Moderate | OR 0.57 | 1250 | ⊕⊕⊝⊝ | ||
| 5 per 1000³ | 3 per 1000 | |||||
| High risk population | ||||||
| 24 per 1000⁴ | 14 per 1000 | |||||
| Symptomatic intracranial haemorrhage/haemorrhagic transformation of the cerebral infarct during treatment period (range 6 days to 16 days)⁹ | Moderate risk population | OR 0.73 | 3102 | ⊕⊕⊝⊝ | ||
| 12 per 1000³ | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ There were unclear risk of selection bias in Hageluken 1992, Dumas 1994, Stiekema 1988, TRACE 2004, Turpie 1992 and Wong 2000. Hageluken 1992 and Stiekema 1988 were single blinded studies; PREVAIL 2007 was an open label study. Hence, making all these study high risk of performance and detection bias (downgraded 1 level). ² Small number of deaths were recorded throughout studies (downgraded 1 level). ³ Calculated based on control event rate from IST 1997 where based on the inclusion criteria, it was interpreted that people are of average risk (hence, they are classified as 'moderate risk population') of developing complications such as deep vein thrombosis (DVT), pulmonary embolism (PE), cranial haemorrhages etc. that resulted in death or disability. ⁴ Calculated based on control event rate from CLOTS3 2015 trial where based on the inclusion criteria, people are of high risk (hence they are classified as 'high risk population') of developing complications such as DVT, PE, cranial haemorrhages that resulted in death or disability. ⁵ Methods of detection of detection of DVT were variable across the studies (downgraded 1 level). ⁶ Calculated based on mean baseline risk from the studies of this Cochrane Review because IST 1997 did not include this outcome data. ⁷ Small number of PEs across the studies. ⁸ Small number of symptomatic intracranial haemorrhage across studies. ⁹ High risk population not available as CLOTS3 2015 trial did not include this outcome data. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dead or dependent at the end of follow‐up | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Death from all causes during treatment period Show forest plot | 8 | 3102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.78, 1.46] |
| 2.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.62, 2.26] |
| 2.2 LMWH versus standard UFH | 4 | 2609 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.72, 1.47] |
| 3 Death from all causes during follow‐up Show forest plot | 8 | 3102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.79, 1.23] |
| 3.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.69, 1.94] |
| 3.2 LMWH versus standard UFH | 4 | 2609 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.74, 1.21] |
| 4 Vascular death during follow‐up Show forest plot | 5 | 1038 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.72, 1.85] |
| 4.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.68, 1.94] |
| 4.2 LMWH versus standard UFH | 1 | 545 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.39, 3.53] |
| 5 Deep venous thrombosis during treatment period Show forest plot | 7 | 2585 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.44, 0.70] |
| 5.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.31, 0.86] |
| 5.2 LMWH versus standard UFH | 3 | 2092 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.44, 0.73] |
| 6 Pulmonary embolism during follow‐up Show forest plot | 6 | 1250 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.23, 1.41] |
| 6.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.18, 2.21] |
| 6.2 LMWH versus standard UFH | 2 | 757 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.13, 1.90] |
| 7 Any intracranial haemorrhage/haemorrhagic transformation of the cerebral infarct during treatment period Show forest plot | 9 | 3137 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.46, 1.23] |
| 7.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.43, 2.94] |
| 7.2 LMWH versus standard UFH | 5 | 2644 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.37, 1.15] |
| 8 Symptomatic intracranial haemorrhage/haemorrhagic transformation of the infarct during treatment period Show forest plot | 8 | 3102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.35, 1.54] |
| 8.1 Heparinoid versus standard UFH | 4 | 493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.19, 4.40] |
| 8.2 LMWH versus standard UFH | 4 | 2609 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.30, 1.60] |
| 9 Extracranial haemorrhage during treatment period Show forest plot | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 Major extracranial haemorrhage | 7 | 3012 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.79 [1.30, 11.06] |
| 9.2 Minor extracranial haemorrhage | 7 | 3012 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.67, 1.24] |
| 10 Effect of recurrent ischaemic stroke or recurrent stroke of unknown pathological type during treatment period Show forest plot | 2 | 1839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [0.61, 6.11] |
| 10.1 LMWH versus UFH | 2 | 1839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [0.61, 6.11] |
| 11 Deep venous thrombosis according to heparinoid dosage regimen Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 11.1 350 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [0.67, 6.59] |
| 11.2 750 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.14, 2.14] |
| 11.3 1250 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 2 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.32, 1.26] |
| 11.4 750 anti‐Xa units 12‐hourly versus 5000 IU UFH 12‐hourly | 2 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.13, 0.71] |
| 11.5 1250 anti‐Xa units 12‐hourly versus 5000 IU UFH 12‐hourly | 1 | 55 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.06, 1.23] |
| 12 Intracranial and extracranial haemorrhage during treatment according to dosage regimen Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 12.1 350 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.45, 3.68] |
| 12.2 750 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.33, 2.50] |
| 12.3 1250 anti‐Xa units 24‐hourly versus 5000 IU UFH 12‐hourly | 2 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.38, 1.25] |
| 12.4 750 anti‐Xa units 12‐hourly versus 5000 IU UFH 12‐hourly | 2 | 138 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.73 [1.03, 7.24] |
| 12.5 1250 anti‐Xa units 12‐hourly versus 5000 IU UFH 12‐hourly | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.31, 9.25] |

